Abstract
The radiation for therapeutic purposes has shown positive effects in different contexts; however, it can increase the risk of many age-related and neurodegenerative diseases such as Alzheimer’s disease (AD), amyotrophic lateral sclerosis (ALS), Huntington’s disease (HD), and Parkinson’s disease (PD). These different outcomes highlight a dose-response phenomenon called hormesis. Prevailing studies indicate that high doses of radiation could play several destructive roles in triggering oxidative stress, neuroapoptosis, and neuroinflammation in neurodegeneration. However, there is a lack of effective treatments in combating radiation-induced neurodegeneration, and the present drugs suffer from some drawbacks, including side effects and drug resistance. Among natural entities, polyphenols are suggested as multi-target agents affecting the dysregulated pathogenic mechanisms in neurodegenerative disease. This review discusses the destructive effects of radiation on the induction of neurodegenerative diseases by dysregulating oxidative stress, apoptosis, and inflammation. We also describe the promising effects of polyphenols and other candidate phytochemicals in preventing and treating radiation-induced neurodegenerative disorders, aiming to find novel/potential therapeutic compounds against such disorders.
Keywords: Neurodegeneration, polyphenols, phytochemicals, radiation, oxidative stress, inflammation, apoptosis
1. INTRODUCTION
Neurodegenerative diseases are relatively well-known as progressive degeneration of the structure or dysfunction of central nervous system (CNS) neurons. Alzheimer’s disease (AD), amyotrophic lateral sclerosis (ALS), Huntington’s disease (HD), and Parkinson’s disease (PD) are amongst the most common neurodegenerative diseases [1] affected by radiation. Radiation has shown potential applications in various fields of science such as medicine [2]. Although high doses of radiation can exacerbate neurodegenerative diseases, there is no conclusive evidence that low doses in radiotherapy cause neurodegeneration. Besides, several studies have demonstrated the adverse effects of radiation on human health towards neurodegenerative diseases' progression. This procedure highlights the importance of a general biphasic dose-response relationship named hormesis, regarding arriving at suitable doses [3, 4].
From the mechanistic point of view, high doses of radiation induce oxidative stress, inflammation, apoptosis, and mitochondrial dysfunction, which play essential roles in the pathology of neurodegeneration [1, 5]. In this regard, radiation activates oxidative stress via an increase in the levels of reactive oxygen species (ROS) and reactive nitrogen species (RNS) [6, 7]. The relationship between free radicals and radiation has been well-established, such that radiation-induced ROS production causes brain injury and neural cell apoptosis [5]. Apoptosis is yet another critical pathway interconnected with radiation. Radiation leads to cell apoptosis through modifying the caspase-3 and p53 activities during neurodegeneration. Consequently, p53, in turn, controls the levels of produced ROS via different mechanisms such as enhancing the expression of the superoxide dismutase (SOD) gene [8, 9]. The mediators involved in the inflammatory processes are among other pathways that may be stimulated by radiation. Radiation can cause an increase in the expression of tumor necrosis factor-α (TNF-α), intercellular adhesion molecule 1 (ICAM-1), interleukin-1 beta (IL-1β), and cyclooxygenase-2 (COX-2) [10].
Radiation also remarkably increases the concentration and activity of activator protein-1 (AP-1), cyclic adenosine monophosphate (cAMP), cAMP response element-binding protein (CREB), and nuclear factor kappa B (NF-κB). In this line, NF-κB was introduced as an important player in radiation-induced neuronal damage [11, 12].
Nowadays, there are concerns over the effect of radiation on human health [13]. Fortunately, many natural products have shown anti-inflammatory, antioxidative, and anti-apoptotic effects [14-16].
Extensive experiments have introduced polyphenols and some candidate phytochemicals as hopeful radioprotective agents. From the mechanistic point, phytochemicals have shown protective properties in neuronal disorders by involving radical scavenging factors, iron-chelating activities, and anti-inflammatory properties. Besides, these secondary metabolites decline DNA lesions after gamma radiation, decreasing malondialdehyde (MDA) and suppressing caspase-3 activities. Inhibiting the radiation-induced apoptosis and ROS generation, as well as increasing the levels of antioxidant elements, such as glutathione peroxidase (GPx), catalase (CAT), and SOD, are amongst other mechanisms of action of candidate phytochemicals.
Several previous studies have shown miscellaneous radioprotective agents [17], traditional Chinese medicine [18], or natural products in combating radiation. However, there is no focus on the neuroprotective mechanisms of polyphenols and candidate phytochemicals against radiation. This review aims to show major dysregulated signaling pathways in neurodegenerative disorders induced by radiation. The current study also highlights advances in neuroprotective effects of polyphenols and candidate phytochemicals against radiation-induced neurodegeneration.
2. HORMETIC MECHANISMS AND RADIATION HORMESIS
Arriving at the right dose is a major key point towards a successful therapy against neurodegeneration; however, this procedure would be challenging due to human inter-individual variation affected by gender, age, diet, genetics, exercise, and health status [3, 4, 19]. As a general biphasic dose-response relationship, hormesis is a dose-response phenomenon known by low-dose stimulation and a high-dose inhibition of biological responses, represented by either graphical J/U-shaped or an inverted U-shaped dose-response curve [20]. Hormetic responses are triggered by various stimuli, including dietary restriction, chemical exposures, hypoxia, thermal/light/electricity extremes, physical stress, and ionizing radiation [21].
Accordingly, while high doses of radiation indicate cellular damage, decreased doses represent adaptive biological performance, including cognition, growth, longevity, bone density, and other biological activities. Following evaluating the effects of radiation and some other therapeutic agents, similar dose-response curves were reported by researchers. The hormetic dose-response confers a new set of interpretations for the dose-response [3]. Considering the hormetic responses, it would be more accessible to predict desirable drug-induced responses and prevent the associated side effects of therapies. On the other hand, ignoring hermetic responses could fail preclinical studies during the afterward clinical trials. Therefore, there is a need to develop hormetic dose-response for biological systems towards a desirable clinical effect [20].
Accordingly, researchers should modify the sensitivity or susceptibility of the individuals to therapeutic agents regarding the drawbacks of the limitations in hermetic curves. In this line, subjects with resistance to drug therapy encounter a right-shifted dose-response curve in hormetic response. In contrast, individuals with higher drug susceptibility would have hormetic response shifted to the left. Thus, hormetic dose-response could be a critical challenge, especially in the developments of those drugs with concerns of being extrapolated to clinical results.
In this regard, activating hormetic pathways by developing alternative agents without associated risks, as in radiation, could be considered a step forward. These neuroprotective responses of subtoxic doses are termed preconditioning [22] under the hormesis umbrella [23]. Consequently, exposure to low doses of toxicants could protect against the toxicity of subsequent high doses, such named hormesis or preconditioning [24]. Of other examples, exposure of cells to moderate transient stress protects them against more severe stresses [25]. According to that, radiation hormesis hypothesizes that low doses of ionization apply beneficial effects towards stimulating repair mechanisms. Therefore, multiple neuroprotective effects are mediated by pre- /post-conditioning induced by the quantitative features of the hormetic dose-response. The preconditioning concept is variously termed adaptive response in ionizing radiation and rebound effects in phytochemicals [19]. Thus, it has been increasingly clear that hormesis could affect several neurodegenerative endpoints through a direct stimulation of preconditioning [21].
Hormesis could provide central supports for neuroprotective responses by providing a dose-response relationship, mechanistic features, and associated relationship to biological plasticity and developing a bright insight for improving the dose accuracy within the heterogeneous population of humans. Overall, mechanistic details of hormetic dose-responses and endogenous cellular defence pathways integrate preconditioning and adaptive stress responses to prevent neurodegenerative diseases [26, 27]. Furthermore, these hormetic responses are mediated by activating antioxidant mediators (e.g., Nrf2-Keap1/ARE) and inhibiting inflammation/apoptosis and other involved enzymes/mediators [28-30].
The current mechanistic review has highlighted the modulatory roles of phytochemicals against high hermetic doses of radiations.
3. RADIATION TRIGGERS OXIDATIVE STRESS
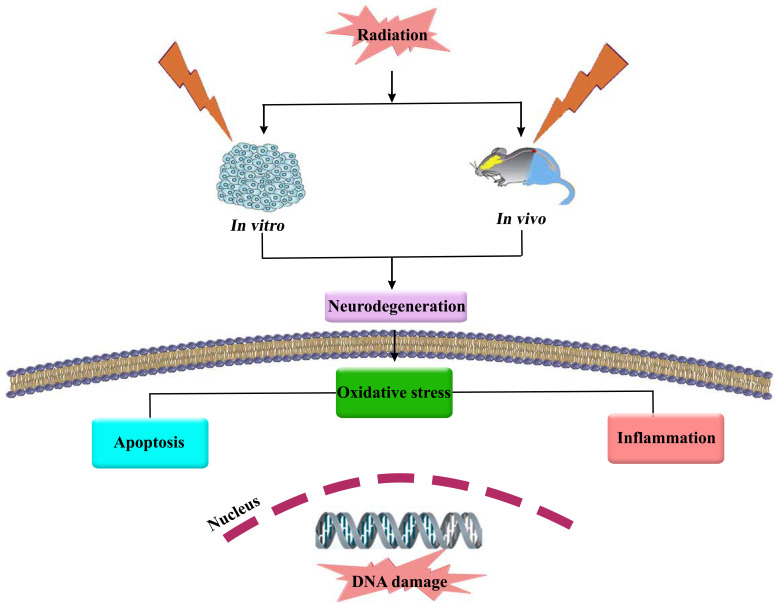
Extensive studies have indicated that radiation can lead to disturbances in learning, memory, and spatial information processing [31]. The injury could result from DNA damages in mammalian cells and the induction of oxidative responses. This response promotes CNS damage and triggers various mechanisms and signaling pathways such as apoptosis and inflammation [32-34] (Fig. 1). Accordingly, radiation affects various signaling mediators such as diacylglycerol formation, protein kinases C (PKC), NF-κB, AP-1, and signal transducer and activator of transcription (STAT) [35]. Limoli et al. suggested ROS as a major key player during the procedure of radiation-induced responses in neural cells [31]. UVA radiation increased the generation of hydrogen peroxide (H2O2), superoxide anion (O2.-), and hydroxyl anion (OH-). The excessive ROS generation by UVA is undesirable and can damage several antioxidant enzymes like SOD, GPx, CAT, and glutathione (GSH) [36]. Furthermore, microwave radiation at 900, 1800, and 2450 megahertz (MHz) alters the levels of MDA, CAT, GSH, and SOD [2]. Bilgici et al. indicated that 900 MHz electromagnetic fields (EMF) increased oxidative stress, lipid peroxidation (LPO), and nitric oxide (NO) levels in serum, as well as a decreased level of SOD and GPx [37]. In a similar study, Heinloth et al. indicated that the mitogen-activated protein kinase (MAPK) pathway is affected by UV radiation [38]. Furthermore, it was observed that radiation had a critical role in precancerous skin lesions (or conditions) and skin cancers, possibly through induction of oxidative stress and apoptosis cross-talk [39]. Research has shown that gamma radiation influences the cell cycle phases by declining the expression of the genes involved in the cell cycle, such as FEN1 (Flap endonuclase1), RFC3 (replication factor C subunit 3), and RFC4 (replication factor C subunit 4) [40]. Lee et al. indicated that ionizing radiation increased c-Jun levels by phosphorylating Ser63 and Ser73 residues [41]. High levels of polyunsaturated fatty acids (PUFAs), such as docosahexaenoic acid and eicosapentaenoic acid, as well as high iron content, increased O2- content, and insufficient antioxidant defence system, cause damages in the brain [2, 42]. There are also close associations between radiation and PUFAs [43], as well as radiation and iron [44] in triggering oxidative damage. Several signaling pathways are activated in response to ionizing radiation, such as PKC, MAPK, p53, NF-κB, ataxia telangiectasia mutated (ATM), and DNA-dependent protein kinase (DNAPK) [45]. It has been observed that a low dose of radiation activates the PKC, thereby leading to increased expression of c-fos, c-jun, c-myc, and c-Ha-ras [46]. Moreover, ionizing radiation activates extracellular signal-regulated kinases (ERK1/2) by activating epidermal growth factor (EGF) receptors, mitogen-activated protein kinase kinase (MEK1), c-Jun N-terminal kinases (JNK), and p38 [46, 47]. Besides, ionizing radiation causes epigenetic changes by interfering with DNA methyltransferases and DNA histones and increasing the generation of ROS [48]. Researchers showed that 1800 MHz radiofrequency radiation-induced oxidative stress is followed by DNA damage. Oxidative stress induced by ionizing radiation, UV radiation, and pathogens promote the generation of ROS. ROS products (e.g., peroxides, superoxide, hydroxyl radical, singlet oxygen, and α-oxygen) damage macromolecules, including lipids, proteins, and DNA, regarding contribution to neurodegenerative disorders [9, 48, 49]. Indeed, radiation induces ceramide (CER) production as one of the important mediators of oxidative stress and apoptosis in neuronal cells, thereby leading to the progression of neurodegenerative diseases [35]. Activation of NADPH oxidase is another mechanism of radiation-induced DNA injury [48, 50], interfering with the expression and function of NF-κB and c-Jun, consequently increasing the level of β-secretase by activated c-Jun amino-terminal kinase and p38MAPK [51-53].
Fig. (1).
Radiation causes neurodegeneration through oxidative stress, inflammation and apoptosis.
As mentioned earlier, oxidative stress, like neuroinflammation, is one of the most important mechanisms in neurodegeneration pathology. Also, extensive evidence shows the key role of radiation in the progress of AD through augmenting oxidative stress. Numerous studies have shown a link between amyloid-beta (Aβ) plaques in the brain and oxidative stress, inflammation, neuronal loss, and neurofibrillary tangles (NFT) formation, as well as increased level of MDA [54-58]. As synaptic flexibility is important and responsible for cognitive deficits resulting from neuron death, research has elucidated that oxidative stress lowers synaptic flexibility in neurodegenerative disorders [59]. Under oxidative stress conditions, as ROS production increases, it enhances the accumulation of Aβ peptides in AD. The resulting amyloid plaques can increase ROS production through the MAPK and result in pathological phenomena such as NFTs formation and enhanced oxidative stress [54-58]. Also, Aβ plaques can deplete the concentration of calcium ions in the endoplasmic reticulum, leading to reduced levels of GSH and increased ROS generation in the neurons. Besides, oxidative stress-activated N-methyl-D-aspartate-type glutamate receptors (NMDARs) lead to increased production of calcium ions, increased intracellular calcium, and instigate mitochondrial damage, which augments ROS production and activates caspases/proteases [60, 61]. Furthermore, ROS targets mitochondrial enzymes, for example, α-ketoglutarate dehydrogenase, aconitase, and JNK/stress-activated protein kinase pathway. This cascade plays a significant role in the phosphorylation of tau protein and Aβ peptides [60, 62]. Besides, it has been found that radiation can mitigate the density of dendritic spines and change spine structure. Also, it can increase amyloid protein precursor/presenilin1 and enhance abnormal tau phosphorylation, which contributes to amyloid-induced pathology in AD patients [63, 64]. It was shown that short and long radiation in different intervals remarkably increased ROS and RNS levels in neural cells. The generation of free radicals and enhanced ROS lead to dopaminergic neuronal loss in PD. It is due to the sensitivity of dopaminergic neurons to oxidative stress.
Mitochondria are amongst the key targets of low-dose radiations. It has been shown that after gamma radiation, antioxidant molecules are increased in PD [65-69]. Neuroepigenetic mechanisms could be viewed as key contributors to radiation-induced brain damage. For instance, increased expression of DNA methyltransferase 1 and 3a, as two associated epigenetic enzymes, can cause cognitive dysfunction and neurodegenerative disorders following radiation [70, 71].
The expression of various genes, such as ATP-binding cassette sub-family A member 7 (ABCA7), Apolipoprotein E (APOE), Myc box-dependent-interacting protein 1 (BIN1), CD2-associated protein (CD2AP), clusterin (CLU), complement receptor type 1 (CR1), membrane-spanning 4-domains A4E (MS4A4E), Membrane-Spanning 4-Domains A6A (MS4A6A), and phosphatidylinositol binding clathrin assembly protein (PICALM), are altered in AD. These genes could also be induced after radiation and activate troponin T1 gene (TNNT1) expression in CNS [72-76]. A similar study confirmed that radiation-induced oxidative stress leads to telomere shortening and an increased mortality rate in AD [77].
ALS is another neurodegenerative disease that results in mutations in SOD1 [78]. SOD is an important enzyme playing a role in various functions such as free radical scavenging, energy metabolism, and post-translational modifications [79]. Dučić and co-workers showed that in a rat model of ALS (hSOD1 G93A), LPO level, spotted SOD, and Copper, Zinc, and Nickle ion levels were increased. The aptitude of the ALS model astrocytes was enhanced to oxidative stress that indicates the particular metabolic changes in these cells, which may be relevant and effective in the progression of the disease [80]. Evidence also indicates that a low dose-radiation increases the levels of vascular endothelial growth factor (VEGF) and growth-associated protein 43 (GAP-43), while high dose-radiation induces demyelination [81]. In this regard, while low doses of radiation lead to cognitive dysfunction, high doses facilitate obvious microscopic alterations such as structural changes [67]. Extensive research has indicated that ionizing radiation can change the morphology of neurons and decrease the length, complexity, and density of dendritic spines, and change spine structure [59]. Belka and co-authors reported increased levels of interferon-gamma (IFN-γ), TNF-α, and adhesion molecules following ionizing radiation [82]. Dynamin-related protein-1 (Drp-1), as a mediator of mitochondrial fission, is implicated in apoptosis and accumulates in mitochondria after radiations [83]. The role of radiation in triggering the redox system, inflammation, and apoptosis has been provided in Fig. (1).
Evidence has also shown a close link between oxidative stress and inflammation/apoptotic pathways/mediators [84].
4. RADIATION CAUSES NEUROINFLAMMATION AND NEUROAPOPTOSIS
Growing reports are revealing the crucial role of radiation in triggering inflammatory/apoptotic pathways in the CNS. Evidence has shown that radiation can induce apoptosis, suppress the activation of caspase-3, and increase the levels/activity of platelet endothelial cell adhesion molecule (PECAM-1), E and P selectins, chemokine (C-X-C motif), ligand 1 (CXCL1), CXCL2, and ICAM-1 [85-89]. ICAM-1, also known as Cluster of Differentiation 54 (CD54), is an important protein with an undeniable role in the immune system in response to radiation [90, 91]. SOD can properly inhibit the radiation-induced inflammation response of other oxidative factors by decreasing oxidative stress and ICAM-1 [91]. Furthermore, protease-activated receptors (PARs), as G-protein coupled receptors, are increased after radiation [89]. In addition, Denning et al. found that UV radiation can activate the death receptors such as Fas (CD95/APO-1) and TNFR to activate caspase-8 and caspase-9 [92, 93]. Moreover, Bax and TNF-α are increased following radiation owing to the enhancement of early growth response protein 1 (EGR-1) [94, 95].
It also has been shown that UV radiation causes phosphorylation in several different serine and threonine residues of the p53 protein [96]. There are four critical phosphorylation sites in p53; Ser392, which is important for p53 oligomerization, Ser15, Ser20, and Ser37, which are important for p53 stabilization as well as the interaction of p53 serine residues with p300/CREB-binding protein (CBP), and P300/CBP-associated factor (PCAF) [96-99]. Studies have indicated that radiation activates p53, stress-activated protein kinase (SAPKs), and the p38 kinase pathways [96]. Chen and colleagues found that two genes, c-Ha-ras, and c-myc, have impressive roles in radiation-induced apoptosis [100]. Also, irradiation increased the level of IFN-γ, an important cytokine with important roles in innate and adaptive immunity [101]. Evidence suggests that radiation enhances and accelerates the production of ROS/RNS, dependent on the modulation of mitochondrial functions or NADPH oxidase-1 (NOX-1) activity that has a key function in ROS generation post-radiation [102, 103]. Furthermore, it induces the expression of high mobility group protein 1 (HMGP1) that can lead to the activation of p53 and some proteins from the HSP70 family, protecting cells from stress in the extracellular space [104]. Researchers also have introduced the MAPK pathway as a critical player in radiation-induced apoptosis. Radiation can also induce apoptosis through the mitochondrial pathway and activation of JNK, increase the level of Bax, and decrease in the level of Bcl-2 [105, 106]. Also, ceramide production as a consequence of sphingomyelin hydrolysis after ionizing radiation can initiate apoptosis and facilitate PKC activation. It has been found that radiation inhibits anti-apoptotic enzymes such as phosphoinositide 3-kinase (PI3K) and protein kinase B (Akt) pathway as well as SAPK or JNK and p38MAPK [107-110]. Results obtained from the study of Shan et al. emphasized that radiation increased the levels of toll-like receptor 4- myeloid differentiation factor 2 (TLR4-MD-2), cluster of differentiation 14 (CD14), and myeloid differentiation primary response 88 (MyD88) as well as pro-inflammatory cytokines [111, 112]. In a similar study, gamma radiation increased the levels of cyclin B1 protein, which regulates radiation-induced apoptosis [113]. Studies have elucidated that radiation could induce the transcription of some factors, including AP-1 and NF-κB, associated with p50, p52, RelA (p65), RelB, and c-Rel [114, 115].
It has been shown that ASK1-JNK/p38MAPK signaling pathways have essential roles in response to UV radiation, cytokines, heat shock, hyperosmotic and ionizing stressors [114, 116, 117]. In addition, evidence has shown that radiation facilitates apoptosis and inflammation processes and induces apoptotic mediators such as caspases through intrinsic and extrinsic pathways [118]. Mitogen-activated protein kinase-4 (MKK4/SEK1), JNK, and AP-1 signaling pathway, as well as caspase-9, caspase-3, Bcl-2, Fas-L, and cytokines such as monocyte chemoattractant protein 1 (MCP-1), and IL-6, are some of the other critical signaling pathways and proteins which may undergo changes and play a role following radiation [119-121]. Radiation can activate NF-κB through MEK/extracellular signal-regulated kinase (ERK)/P90 signaling pathway. Besides, it induces COX-2, TNF-α, IL-1α, IL-1β, IL-6, granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-4, IL-5, IL-10, IL-12, IL-18, and transforming growth factor-beta (TGF-β) [122]. Previous studies have suggested that low-intensity radiation remarkably increases IL-2, IL-6, TNF-α, and IFN-γ [2]. In addition, IL-1β leads to activation of matrix metalloproteinases (MMPs), a group of zinc-dependent enzymes that regulate extracellular matrix (ECM) components. MMP-9 is one of the most important members of the MMP family, which has an essential role in neuroinflammation via the activation of several cytokines and chemokines [123-125]. Evidence has shown that inflammatory cells such as macrophages, lymphocytes, astrocytes, and microglia express and release chemokines, neurotransmitters, cytokines, and ROS, leading to neuronal damage, thereby accelerating the progression of neurodegenerative diseases [123, 126-129].
Studies have suggested that neuroinflammation and neuroapoptosis play important roles in neuronal injury in the context of neurodegenerative diseases [130-132]. Reports have shown that microglia play a prominent role in the pathology of neurodegenerative disorders such as PD, ALS, and AD [133]. Indeed, microglia, activated neurotoxic microenvironment, excitatory amino acids, quinolinic acid, complement proteins, reactive oxygen intermediates, and NO lead to AD progression [130, 131, 134-137]. Activated microglia causes the upregulation of various cell surface markers allegorically macrophage antigen complex-1 (MAC-1). This procedure promotes phagocytosis and the generation of cytotoxic molecules, including ROS, nitric oxide (NO), and several pro-inflammatory cytokines. On such a basis, activated microglia can bring about considerable cell damages with an outstanding role in the pathogenesis of neuronal diseases. Inhibition of microglial activation can prevent dopaminergic neuronal loss and could be considered a treatment option for PD [138, 139]. Additionally, it has been observed that the IL-1/IL-10 ratio is remarkably increased in AD patients [130]. In AD, death receptor 4 (DR 4) and DR5 can be induced by oligomeric Aβ peptides and trigger caspase-3-dependent apoptosis [140]. Investigations have demonstrated that Aβ binds to inflammatory receptors such as TLR-2, TLR-4 for advanced glycation end products (RAGE), NF-κB, TNF-α, COX-2, and inducible nitric oxide synthase (iNOS) [141].
From the mitochondrial point of view, PTEN-induced kinase 1 (PIKN1) is a mitochondrial kinase involved in Ca2+ efflux from mitochondria. Dysfunction of this protein can result in the accumulation of Ca2+ and increased ROS levels, followed by apoptosis in vivo and in vitro [142-145]. Gelders et al. observed that the levels of IL-6, IL-12, and IL-15 were increased during PD. Furthermore, α-synuclein expression was induced in PD via NF-κB, TNF-α, and IL-β [132, 146, 147].
As another inflammatory mediator, C-reactive protein (CRP) is an acute-phase protein augmented at the sites of inflammation/infection. It plays key roles in the inflammatory and related processes such as phagocytosis, apoptosis, NO release, and the production of cytokines, especially IL-6 and TNF-α, during neurodegeneration [148, 149]. In addition to its role in mitochondrial dysfunction, glutamate mediates excitotoxicity, free radical-mediated oxidative cytotoxicity, and neuroinflammation in the pathology of neurodegenerative disorders. The endoplasmic reticulum is also a key factor [150]. It has been found that endoplasmic reticulum stress can induce apoptosis in the microglia and astrocytes and facilities the gliosis reactive that have an important role in the pathology of neurodegenerative diseases [151-153]. The levels of ROS, COX-2, IL-1β, IL-6, and TNF-α are increased with caspases and mutant SOD1/Bcl-2 aggregation [153, 154]. A unique apoptotic pathway in the motor neurons known as Fas-associated protein with death domain (FADD)/caspase-8 pathway has been detected that is activated by FAS receptors and Daxx [155]. A correlation between Daxx and MAPK (ASK1) has been observed in motoneuron cell death. Indeed, ASK1 regulates p38 kinase that increases NO level [156]. Necroptosis pathway is described as a particular type of programmed necrosis whose initiation by death receptors requires receptor-interacting serine/threonine kinase 1 (RIP1) and RIP3. Necroptosis takes part in the pathogenesis of various diseases, such as neurodegenerative disorders, ischemic injury, and viral infection. Thus, it may be an attractive/effective target to prevent unwarranted cell death. Extensive studies highlight the activation of necroptosis pathways in the motor neurons. RIP1, RIP3, and mixed lineage kinase domain-like pseudokinase (MLKL) are other important factors in ALS pathology [157]. Studies also have illustrated that Nod-like receptor family pyrin domain 3 (NLRP3), IL-18, and ASC play a role in neurodegeneration conditions such as ALS [158].
Radiation induces inflammation via upregulating pro-inflammatory molecules such as MCP1, chemokine (C-C motif), ligand 2 (CCL2), and CXCL1 [159]. Also, the expression of several chemokine receptors such as chemokine (C-C motif) ligand 7 (CCL7), CCL8, CCL12, CXCL4, C-C chemokine receptor type 1 (CCR1), and CCR2 are increased after radiation [160]. Radiation also enhances inflammatory gene expression, including IL-6, IL-1β, TNF-α, COX-2, and TGF-β [132]. Low dose radiation induces apoptosis in neuron and glial cells in the subventricular zone covering the olfactory bulbs, neocortex, dentate gyrus, striatum, thalamus, amygdala, brain stem, and the cerebral white matter [159]. It has been found that radiation raises the secretion of chemoattractant molecules and migration of monocytes and enhances BBB permeability [160]. NF-κB, as a transcription factor, has important functions and responds to various stimuli such as cytokines, free radicals, UV, and bacterial or viral antigens [161]. It has been demonstrated that cyclin-dependent kinase 5 (Cdk5) is a proline-directed serine-threonine kinase. It plays a fundamental role in memory formation, hippocampal apoptosis, synaptic flexibility, and adult neurogenesis in neurodegenerative diseases. Thus, the level of Cdk5 is increased after radiation and could be proposed as an important target of radiation-induced damage [162, 163]. Unfolded protein response (UPR) is another cellular stress response that induces apoptosis in neurodegenerative disorders. UPR signaling is mediated by protein kinase RNA-activated (PKR)-like ER kinase (PERK), activating transcription factor 6 (ATF6), and IRE1a [164]. Functional experiments show that Aβ and α-Syn activate the UPR signaling in PD, AD, and ALS [164]. Inositol requiring enzyme 1 alpha (IRE1a), JNK, NF-κB or p38, thioredoxin-interacting protein (TXNIP), and transmembrane E3 ligase RNF183 are a number of the most important factors involved in this pathway [164].
5. NATURAL PRODUCTS TARGET OXIDATIVE STRESS, INFLAMMATION, AND APOPTOSIS INDUCED BY RADIATION IN NEURODEGENERATIVE DISEASES
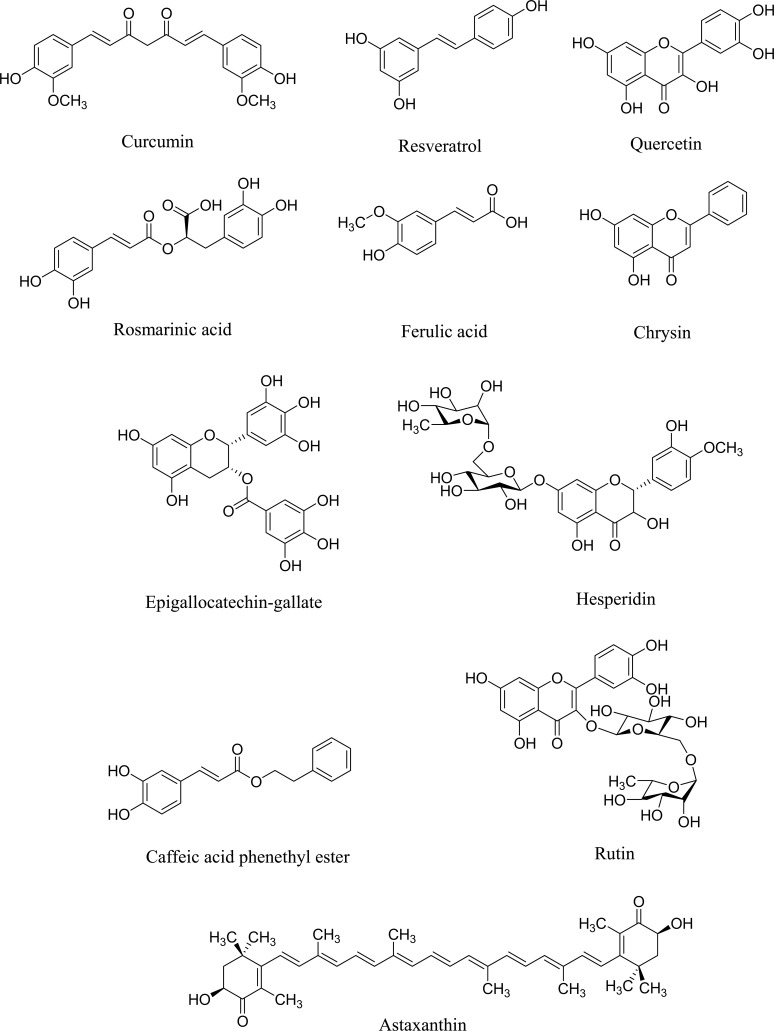
As mentioned in the previous sections, radiation alters cellular antioxidant capacity leading to oxidative stress, increased rate of point mutations, and chromosomal rearrangements [165]. A great deal of evidence has shown that there are different natural products such as polyphenols and candidate phytochemicals with important roles and beneficial effects in preventing, treating, or delaying the progression of neurodegenerative disorders. This event could be induced by radiation exposure by interfering with oxidative stress and other related mechanisms [166]. Besides, inflammation and apoptosis, as two crucial phenomena in close interconnections with oxidative stress, play essential roles in post-radiation neurodegenerative processes. Nowadays, studies have introduced phenolic compounds and other candidate phytochemicals as promising antioxidant, anti-apoptotic, and anti-inflammatory agents in neurodegeneration [167-169]. Fig. (2) illustrates selected polyphenols and candidate phytochemicals against radiation-induced neurodegeneration.
Fig. (2).
Chemical structures of selected polyphenols and candidate phytochemicals against radiation-induced neurodegeneration.
5.1. Curcumin
Over the past decade, evidence has revealed that curcumin possesses various biological effects such as antioxidant, anti-inflammatory, antimicrobial, antimutagenic, and anticarcinogenic activities [166, 170]. Rane et al. showed that curcumin properly reduces oxidative damage and amyloid-mediated pathological symptoms in AD and inhibits the oligomerization/fibrilization of Aβ protein while inducing Aβ disaggregation [171, 172]. In addition, curcumin binds tau protein and inhibits the aggregation of this protein [171].
From a mechanistic point of view, curcumin has shown an inhibitory effect on ionizing radiation-induced NF-κB signaling pathway and decreased various cytokines such as IL-6, IL-1β, and TNF-α. Accordingly, while radiation reduces the levels of glucocorticoid receptor (GR), CAT, and glutathione S-transferase (GST) [36], curcumin increases concentrations and activities of these antioxidative enzymes, including epoxide hydrolase (EH), SOD, heme oxygenase (HO-1), NAD(P)H Quinone Dehydrogenase 1 (NQO1), and other antioxidant enzymes [168, 173-176]. Soltani et al. investigated the beneficial effects of curcumin on the irradiated THP-1 cells. Data points to the improving effects of curcumin on antioxidant defence. It hinders ionizing radiation-induced necrosis by decreasing ROS and LPO levels and increasing CAT and GPx [177]. Besides, curcumin protects against radiation-induced acute/chronic cutaneous toxicity in vivo. This effect is applied through diminishing mRNA and protein expression of inflammatory cytokines, including TNF-α, IL-1, IL-6, IL-18, TGF-β, and lymphotoxin-β [178].
As mentioned earlier, radiation augments ROS generation [48], while curcumin decreases ROS in ALS and other brain damage and inhibits inflammatory mediators such as cytokines, chemokines, and oxidative stress in AD [179]. Additionally, curcumin activates nuclear factor-erythroid factor 2-related factor 2 (Nrf2) and suppresses apoptotic mediators (e.g., Bax, Bcl-2, caspase-3, and caspase-9). Altogether, curcumin could be introduced as a promising radioprotective agent against neurodegenerative diseases.
5.2. Resveratrol
Substantial evidence has corroborated that resveratrol has beneficial effects on AD and other neurodegenerative diseases. Resveratrol exerts its neuroprotective effects via downregulating inflammation/apoptosis, Aβ aggregation, sirtuin 1 (sirt1), and NF-κB. It also decreases ROS, RNS, IFN-γ, TNF-α, IL-6, and IL-1β towards neuroprotective responses [180-183]. As stated, resveratrol inhibits Aβ as one of the inducers of apoptosis in neurons which precipitates neurotoxicity events [184]. Li et al. have shown that resveratrol suppresses apoptosis via activating Sirt1 through Sirt1/PGC-1α in neurological disorders [185]. In several studies, radiation has been shown to upregulate inflammatory mediators [11]. Accordingly, resveratrol inhibits these mediators, including NO, TNF-α, IL-1β, IL-6 [186], MCP-1, and diminishes the production of IL-12 p40 and IL-23 in neurodegenerative disorders [178]. Resveratrol also raises the levels of GSH, SOD, CAT, GPx, and HO-1 and reduces LPO [187] as dysregulated factors following radiation exposure.
Resveratrol has shown a protective function against the cell death triggered by α-syn in PD [187]. Besides, resveratrol meaningfully alleviated DNA damages and cytotoxic effects of megavoltage radiation in the glioblastoma cell line [188]. Studies indicated the inhibitory effects of radiations on the PI3K/Akt pathway [185], while evidence indicated resveratrol as a neuroprotective agent by activating PI3K/Akt/mTOR pathway and inhibiting the apoptosis of neurons [189]. As mentioned above, resveratrol has neuroprotective properties by reducing radiation-induced Bax elevation. Furthermore, it can diminish the in vivo salivary gland dysfunction induced by radiation via enhancing the activity of SOD and reducing the level of MDA [190]. To improve the pharmacokinetics and bioavailability of resveratrol, Yin et al. developed a new nanoformulation of resveratrol that could decrease Aβ or radiation-induced oxidative stress [191]. Reducing Bax, MMP-9, accumulation of tau, BACE1A, amyloidogenic processing and apoptosis of neurons, as well as increasing Sirt-1, AMP-activated protein kinase (AMPK), Bcl-2, claudin 5, and mitochondrial biogenesis are some of the other neuroprotective mechanisms of resveratrol [192].
5.3. Quercetin
Quercetin is a natural flavonoid abundant in many plants possessing pivotal properties, including antioxidant, anti-inflammatory, radical scavenging, antidiabetic, and anticancer activities. It has also shown therapeutic potentials in neurodegenerative disorders [193, 194]. It has been shown that quercetin reduces intracellular tau and gliosis in the amygdala and hippocampus during AD [195]. As mentioned earlier, radiation elevates inflammatory and apoptotic mediators [57], while quercetin ameliorates astrogliosis in AD by inhibiting COX-2, iNOS, NO, IL-1β, TNF-α, and prostaglandin E2. Quercetin also ameliorated the expression of Bax/Bcl-2, SOD, GPx, and CAT in neuronal damages [196, 197]. Kale et al. implemented a study to investigate the in vivo neuroprotective potential of quercetin against radiation-induced brain injury. Findings have demonstrated that quercetin can significantly protect the nervous system against radiation and decrease plasma and tissue concentrations of MDA [198]. Also, quercetin exerts its neuroprotective effects against radiation-induced neuronal endoplasmic reticulum stress by reducing the expression of stress marker genes, C/EBP-homologous protein, TNF-α, JNK, and enhancing the cytoskeletal protein Tuj1 [199].
According to the aforementioned data, quercetin is of potential interest in combating neurodegeneration induced by radiation.
5.4. Ferulic Acid/Hesperidin
Studies have introduced ferulic acid (FA) as a phenolic compound with diverse biological activities, including neuroprotective, anti-inflammatory, antioxidant, antidiabetic, antiaging, and anticancer effects. It has been shown that FA can scavenge or capture free radicals and ROS as well as decrease ERK and p53 levels [200]. Considering the crucial role of radiation in ROS production in CNS [36], FA possesses a notable neuroprotective activity against the Aβ protein. It was reported to decrease the levels of ROS and LPO and increase HO-1, GSH, SOD, and CAT, and heat shock protein (HSP72), leading to the modulation of neuroinflammatory responses [165].
Hesperidin is another polyphenolic compound with significant neuroprotective properties that can considerably modulate brain damages induced by radiation. Hesperidin restored diminished levels/activities of antioxidant enzymes and the parameters such as CAT, GSH, GPx, and SOD, which were decreased in the rats exposed to γ-rays [201]. Furthermore, hesperidin alleviated mitochondrial damage, oxidative stress, and monoamine neurotransmitter alterations in irradiated rats' brains [202]. Moreover, hesperidin exhibited remarkable neuroprotective activities and improved neurological outcomes of radiation-induced brain injury in the rats exposed to a dose of 20 gray (Gy) [203].
Hence, FA and hesperidin employ neuroprotective mechanisms to combat radiation-induced neurodegeneration.
5.5. Other Polyphenols
Evidence has shown that rutin possesses neuroprotective effects via activating PI3K/Akt/glycogen synthase kinase 3 beta (GSK-3β)/Nrf2 and lowering the xanthine oxidase level (XO) that reduces O2-, H2O2, and MDA generation in the brain [204]. Furthermore, monoglucosyl-rutin has been reported to increase cell survival and exhibit in vitro radioprotective activity by attenuating radiation-induced DNA damage in mammalian cells [205]. Oral rutin in Swiss albino rats exposed to irradiation considerably reduced dicentric formation and reduced micronucleated polychromatic, normochromatic erythrocytes [206]. As mentioned earlier, radiation exerts cytogenetic effects, while rutin mitigates DNA damages, a feature that could be attributed to its antioxidant and free radical scavenging properties [207].
Investigations have shown that radiation induces COX-2 mRNA expression in microglia and astrocytes [107], but polyphenolic constituents such as 6-gingerol, zerumbone, 6-shogaol and dehydrozingerone, have shown significant radioprotective activities via inhibition of UVB-induced COX-2 expression and NF-κB, as well as reducing cell apoptosis and DNA damage by activation of Nrf2/keap1/antioxidant response element (ARE) signaling pathway [208-213]. Also, irradiation-induced apoptosis was inhibited by zingerone by reducing Bax and caspase-3 activity and increasing Bcl-2, GST, GSH, SOD, and CAT [214]. In another study, zingerone showed significant in vitro protective advantages against radiation-induced apoptosis, DNA damage, and genotoxicity on the human lymphocytes. Scavenging free radicals and suppressing oxidative stress can also be regarded as other major radioprotective mechanisms of zingerone [215].
As radiation triggers apoptosis, oxidative stress, and inflammation [26], considerable evidence indicates that flavonoids have an important function in preventing and treating neurodegenerative diseases. These compounds reduce iNOS, COX-2, TNF-α, IL-6, IL-1β, iNOS, MMP-9, and COX-2, as well as apoptosis mediators [216]. Besides, evidence has shown that the major flavonoid of Scutellaria baicaleins, baicalein, diminishes DNA damages in humans after gamma radiation [217]. As another flavonoid found in propolis, honey, and other plants, chrysin has also shown advantageous features such as antioxidant activity by reducing MDA and lowering caspase-3 activity post-irradiation [218]. Mansour et al. investigated 5, 7-dihydroxyflavone as a natural flavonoid against gamma irradiation and acrylamide-induced neurotoxicity in rats. Their results demonstrated that 5, 7-dihydroxyflavone could reduce the levels/activities of Aβ, caspase-3, BDNF, MDA, and acetylcholine esterase (AChE) [218]. As demonstrated by investigations, radiation inhibits PI3K/Akt, PKC, MEK1/2-ERK1/2 [107] and increases NF-κB, TNF-α, IL-6, and IL-1β [186]. In this regard, Rehman et al. reported that kaempferol is a natural flavonol capable of inhibiting brain lesion and neuroinflammation by inhibiting STAT3 and NF-κB, as well as alleviating cognitive impairment through increasing the ERK1/2-CREB signaling pathway [219].
Epigallocatechin 3-gallate (EGCG) is another polyphenolic compound with considerable neuroprotective effects inhibiting radiation-induced injuries [220]. EGCG supply this effect via downregulating IL-1β, IL-6, IFN-γ, TNF-α, NF-κB levels, and disaggregation of Aβ protein [221]. In addition, EGCG increases cell survival and preserves human retinal pigment epithelial cells against damages induced by UV irradiation [222]. Epicatechin could properly decline the phosphorylation of MEK1/2, ERK1/2, and c-Jun as well as reduce the expression of p-JNK, p38, and ROS generation after radiation [167]. As mentioned above, EGCG has radioprotective effects through the scavenging of superoxide anions, hydroxyl radicals, and hydrogen peroxide [223, 224]. This phenolic compound can also bind free radicals to intercalate with DNA and inhibit the proteasome as a regulator of inflammation [225].
Furthermore, propolis and its phenolic chemical compound, caffeic acid phenethyl ester (CAPE), could meaningfully increase the activity of SOD and decrease the levels of MDA in the brain of rats exposed to ionizing radiation compared to the control group [226]. CAPE is another flavonoid with anti-inflammatory, immunomodulatory, and free radical scavenging properties [227]. In addition, It has shown radioprotective effects against oxidative damage [228]. While radiation activates oxidative stress through an increase in ROS and RNS levels [36], apigenin, as another phenolic compound [206], was shown to reduce oxidative stress via inhibiting and decreasing iNOS, IL-6, COX-2, and TNF-α [229]. Also, apigenin exhibited cytoprotection as evidenced by its antioxidant and free radical-scavenging activities [230], as well as reduced the number of micronuclei in gamma-irradiation [231].
While radiation induces the expression of COX-2 mRNA [108], silymarin meaningfully declines COX-2 and 5-Lipooxygenase (LOX). Besides, silymarin increases IL-10 toward neuroprotective potentials following radiation [232, 233]. Silymarin also protected the male Balb/c mice exposed to lethal 9 Gy γ-irradiation via increasing the CAT, GPx, glutathione reductase, and SOD activities [234]. In the same way, the neuroprotective potentials of silybin and its analogs (e.g., naringin, naringenin, and hesperetin) have been addressed against X-ray radiation-induced DNA breaks [235]. As previously mentioned, radiation induces oxidative stress [36], and naringin has shown substantial biological effects such as anti-inflammatory, anticarcinogenic, and antioxidant activities leading to a reduction in ROS and an increase in Nrf2/HO-1, and SOD2 levels [236, 237]. Naringin also lowered the expression of several inflammatory proteins and genes like IL-1β, IL-6, cytoplasmic cytochrome C, TNF-α, Bax, p53, caspase-9, caspase-8, caspase-3, Bak, Fas, and FasL [236], thereby alleviating radiation-induced neuroinflammation/neuroapoptosis [186].
As another natural phenol, thymol showed radioprotective activities against ionizing radiation-induced salivary gland dysfunction in a rat model. It has been demonstrated that thymol reduces in vitro gamma ray-induced genotoxicity via decreasing free radicals and inhibiting oxidative stress [238, 239]. Additionally, modulation of the expression of Bax, Bcl-2, and survivin, as well as other apoptotic regulatory molecules, has been proposed as the main radioprotective mechanisms of plumbagin in C33A cells exposed to 2 Gy of radiation. Consequently, gallic acid as another phenolic compound has shown radioprotective properties by reducing radiation-induced DNA damages [240, 241]. Hence, phenolic compounds are of potential importance in combating radiation-induced neurodegeneration. Table 1 provides the radioprotective effects of the selected phenolic compound against neurodegeneration.
Table 1.
Radioprotective effects of selected phytochemicals against neurodegeneration.
| Compounds | Type of Study | Radiation Source | Cell Line/Animal Model | Mechanism of Action | References |
|---|---|---|---|---|---|
| Ferulic acid | In vivo | Gamma irradiation | Male albino rats | ↑CAT, ↑GST, ↑SOD | [165] |
| Rosmarinic acid | In vivo | Wi-Fi radiation | Wistar strain male rats | ↓NO, ↓protein carbonylation, ↓MDA, ↑total antioxidant capacity, ↑GPx, ↑SOD, ↑CAT, ↑GSH | [169] |
| Curcumin | In vivo | Various sources | Microglia | ↑CAT, ↑GST, ↑SOD, ↓Oxidative damage, ↓IL-1β, ↓IL-6, ↓TNF-α |
[18, 259]. |
| Resveratrol | In vivo | Gamma irradiation | Sprague-Dawley rats | ↑Sirt1/PGC-1α, ↓ apoptosis, ↓ ROS production | [185] |
| Quercetin | In vivo | NR | Male Wistar-Albino rats | ↓MDA, ↓LPO | [198] |
| Hesperidin | In vivo | Gamma irradiation | Male albino rats | ↑CAT, ↑GSH, ↑GPx, ↑SOD | [201] |
| Rutin | In vivo | Gamma irradiation | Transgenic mice (APPswe/PS1dE9) | ↑SOD, ↑GSSG, ↓MDA, ↓IL-1β, ↓IL-6, ↓Aβ | [204] |
| Zingerone | In vivo | Gamma irradiation | Microglia and astrocytes | ↑CAT, ↑GST, ↑SOD, ↑GSH, ↓caspase-3, ↑Bcl-2 ↓Bax |
[214] |
| Chrysin | In vivo | Gamma irradiation | Male Wister rats | ↓NF-κB, ↓TNF-α, ↓IL-1β, ↓IL-6, ↓NOS, ↓NO, ↓ROS, ↓COX-2, ↓caspase-3 | [218] |
| 5, 7-Dihydroxyflavone | ↓β-amyloid, ↓caspase-3, ↓BDNF, ↓MDA, ↓AChE | ||||
| EGCG | In vivo | Various sources | Cerebellar granule neurons | ↓ROS, ↓IL-1β, ↓IL-6, ↓IFN-γ, ↓TNF-α, ↓NF-κB, ↓p-JNK, ↓p-38, | [221] |
| Caffeic acid phenethyl ester | In vivo | Gamma irradiation | Male albino Sprague-Dawley rats | ↑SOD, ↓MDA, ↑GPx | [226] |
| Silymarin | In vivo | Gamma irradiation | BALB/c mice | ↑CAT, ↑GPx, ↑Glutathione reductase, ↑SOD | [234] |
| Ursolic acid | In vivo | Gamma irradiation | BALB/c mice | Improves contextual learning and memory, ↑neurogenesis |
[260] |
Abbreviations: AChE: acetylcholinesterase, BDNF: brain-derived neurotrophic factor, CAT: catalase, COX: cyclooxygenase, EGCG: epigallocatechin 3-gallate, GPx: glutathione peroxidase, GSH: glutathione, GSSG: glutathione disulfide, GST: glutathione S-transferases, IFN-γ: interferon-gamma, IL: interleukin, MDA: malondialdehyde, LPO: lipid peroxidation, NF-κB: nuclear factor-κB, NO: nitric oxide, NOS: nitric oxide synthase, NR: not-reported, p-JNK: phosphor c-Jun N-terminal kinase, SOD: superoxide dismutase, Sirt1/PGC-1α: sirtuin 1/peroxisome proliferator-activated receptor-γ coactivator-1alpha, TNF-α: tumor necrosis factor-alpha
Hydroxytyrosol and oleuropein aglycone are other emerging phenolic compounds. They are mainly extracted from olive oil with potential antioxidant effects against radiation-induced neuronal damage. These compounds have shown the potential of reducing α-synuclein accumulation in PD models. Hormesis, antioxidative responses, and activating proteasome/phase II detoxifying enzymes are considered potential underlying mechanisms [242]. In a research study, hydroxytyrosol prevented protein damage induced by long-wave UV radiation through increasing antioxidant responses [243].
5.6. Miscellaneous Candidate Phytochemicals
In addition to polyphenols, other phytochemicals, including alkaloids, terpenes/terpenoids, carotenoids, and organosulfide compounds, also have shown neuroprotective effects [244]. Furthermore, other phytochemicals like phytoestrogens have also shown several useful effects such as neuroprotective, osteoporosis-preventive, antiacne, and anticancer activities. Phytoestrogens like progesterone and 17β-estradiol exert their neuroprotective/radioprotective activities via activation of receptors like estrogen receptors-α (ER-α), ER-β or G-protein coupled receptor-1, ERK1/2, PI3K/Akt, and c-Jun [219]. ER-α can bind to IGF-1 and regulate the signaling cascades involved in neuroprotection as PI3K/Akt/GSK-3β, and upregulate the levels of anti-apoptotic proteins such as Bcl-2, Bcl-xL, and Bcl-W. ER-α could downregulate the levels of Bcl-2-like protein 4 (BCL2L4) and Bcl-2 associated cell death (BAD) as proapoptotic mediators [219].
It has been shown that leafy vegetables rich in alkaloids have beneficial effects on CNS and its related disorders both in vivo and in vitro [245]. Oboh and co-authors have shown that bitter leaf alkaloid-rich extract (BLAE) has several beneficial effects through antioxidant effect and inhibiting the monoamine oxidase (MAO), angiotensin-1 converting enzyme (ACE), ATPdase, and ADPdase [245]. Huperzine is another alkaloid with neuroprotective properties against AD by inhibiting AChE and reducing oxidative stress [246]. In this phytochemical class, kukoamine A also inhibits oxidative stress and apoptosis [247]. Studies have shown that NF-κB and AP-1 trigger radiation side effects; therefore, inhibition of these factors may protect neuronal cells against radiation [247-249]. In the study of Zhang et al., Kukoamine A declined the expression of Bax, and caspase-3 reduced the release of cytochrome C and increased the expression of Bcl-2 [250].
Amongst carotenoids, astaxanthin is a potent antioxidant compound with diverse biological properties. It suppressed radiation-induced cytogenetic impacts and reduced in vivo irradiation-induced hematopoietic system injuries by reducing apoptosis and oxidative stress pathways [251-253]. As stated, radiation increases ROS, oxidative stress, and inflammation [48], while lycopene (a carotenoid) has significant antioxidant, anti-apoptotic and anti-inflammatory effects. In this line, curcumin reduces ROS/LPO and increases the levels of antioxidant enzymes such as SOD and GSH [254], thereby exerting radioprotective responses. It has been shown that p53, as an important factor in the intrinsic apoptotic pathway (Bax/Bcl-2 ratio), is activated by DNA damage [255]. In this line, Cao et al. found that lycopene increased Bcl-2 and decreased p53, Bax, cytochrome C, and caspase-3 [254].
As another emerging miscellaneous phytochemical, sulforaphane induced hormetic activation of Nrf2 to reduce the occurrence and severity of a wide range of human-related pathologies, including PD, AD, stroke, and other age-related damages, while also enhancing stem cell proliferation. Besides, sulforaphane was a wide chemoprotective agent within a hormetic dose-response context. It potentially acts through increasing cell proliferation/viability at low concentrations in multiple tumor cell lines. Interestingly, the mechanistic profile of sulforaphane is similar to that of numerous other hormetic agents in protecting neurons, including activation of Nrf2 and AREs [26]. Of AREs, CAT, SOD, HO-1, NQO1, and GST are predominantly upregulated by sulforaphane to prevent the progression of neurodegeneration [26, 256, 257]. Sulforaphane is also a potential neuroprotective phytochemical acting through suppressing neuroinflammatory mediators (e.g., IL-6, TNF-α, COX-2). So, sulforaphane could be a promising protective agent against high doses of radiation [258].
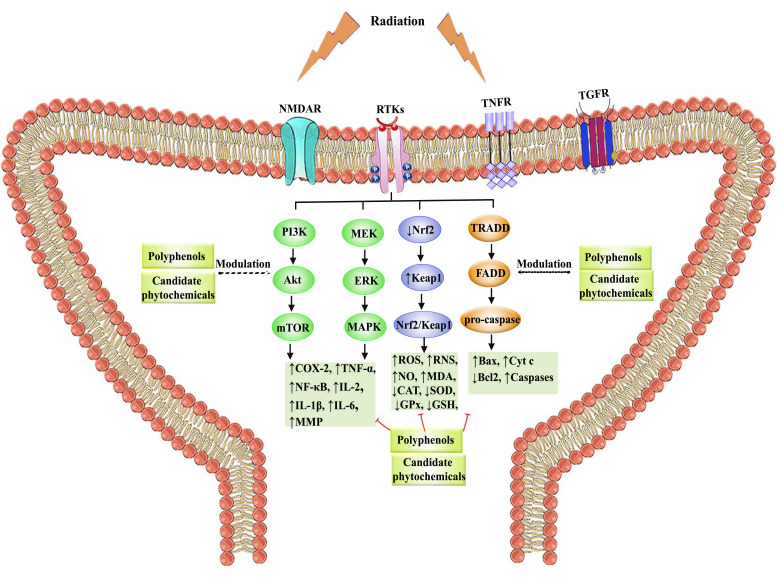
The schematic roles of polyphenols and candidate phytochemicals against radiation-induced neurodegeneration are presented in Fig. (3).
Fig. (3).
The role of polyphenols in modulating neurodegeneration-induced oxidative stress and cross-talking inflammatory/apoptotic mediators. Abbreviations: CAT: catalase, COX: cyclooxygenase, Cyt C: cytochrome C, ERK: extracellular signal-regulated kinase, FADD: Fas Associated Via Death Domain, GLUTR: glutamate receptor, GPx: glutathione peroxidase, GSH: glutathione, IFN γ: interferon-gamma, IL: interleukin, Keap1: Kelch-like ECH-associated protein 1, LPO: lipid peroxidation, MAPK: mitogen-activated protein kinase, MDA: malondialdehyde, MEK: mitogen-activated protein kinase/ERK kinase, MMP: matrix metalloproteinase, mTOR: mammalian target of rapamycin, NF-κB: nuclear factor-κB, NMDAR: N-methyl-D-aspartate receptor, NO: nitric oxide, NOS: nitric oxide synthase, Nrf2: nuclear factor erythroid 2–related factor 2, PI3K: phosphoinositide 3-kinases, RNS: reactive nitrogen species, ROS: reactive oxygen species, RTKs: receptor tyrosine kinases, SOD: superoxide dismutase, TGFR: transforming growth factor-beta receptor, TNF-α: tumor necrosis factor-alpha, TNFR: tumor necrosis factor receptor, TRADD: TNFR1-associated death domain protein.
CONCLUSION
Despite substantial progress in treating neurodegenerative diseases, they have remained a global challenge and a primary cause of disability and death worldwide. Several factors are behind the causative agents of neurodegeneration, among which radiation plays an unavoidable role. Radiation triggers several dysregulated signaling pathways in oxidative stress, inflammation, and apoptosis towards neurodegenerative disorders. Accordingly, there are several signaling pathways behind neurodegeneration, including PI3K/Akt/mTOR, MEK/ERK/MAPK, Nrf2-Keap1, and their cross-talk mediators seem to be of great importance. The complex pathophysiological mechanisms behind radiation-induced neurodegeneration raise the need for providing potential multi-target agents to pave the way in combating radiation-induced neurodegeneration. Nowadays, the plant kingdom is a prominent source of multi-target compounds. Among natural entities, polyphenols and some candidate phytochemicals potentially modulate radiation-induced dysregulated mediators for the treatment/prevention of neurodegeneration. These phytochemicals potentially target multiple neuronal dysregulated pathways following radiation (Fig. 3), thereby envisioning a bright future in the prevention/treatment of neurodegeneration.
Further reports on the destructive mechanisms of radiation-induced neurodegeneration will help to provide related clinical therapies. Future studies should cover wide in vitro and in vivo experiments with regard to hormesis dose-response, related signaling pathways, preconditioning, and revealing radioprotective potentials of phytochemicals against neurodegeneration, followed by well-controlled clinical studies. Such studies play crucial roles in providing more potential therapeutic agents in the prevention, management, and treatment of radiation-induced neurodegeneration.
ACKNOWLEDGEMENTS
Declared none.
AUTHORS’ CONTRIBUTIONS
Conceptualization, S.F., and H.K.; software, S.F., drafting the manuscript, S.F., S.P, and S.Z.M; review, editing, and revising the paper: S.F., and H.K.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Nordvig A.S., Rimmer K.T., Willey J.Z., Thakur K.T., Boehme A.K., Vargas W.S., Smith C.J., Elkind M.S. Potential neurological manifestations of COVID-19. Neurol. Clin. Pract. 2020 doi: 10.1212/CPJ.0000000000000897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Megha K., Deshmukh P.S., Banerjee B.D., Tripathi A.K., Ahmed R., Abegaonkar M.P. Low intensity microwave radiation induced oxidative stress, inflammatory response and DNA damage in rat brain. Neurotoxicology. 2015;51:158–165. doi: 10.1016/j.neuro.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 3.Kruman I., Bruce-Keller A.J., Bredesen D., Waeg G., Mattson M.P. Evidence that 4-hydroxynonenal mediates oxidative stress-induced neuronal apoptosis. J. Neurosci. 1997;17(13):5089–5100. doi: 10.1523/JNEUROSCI.17-13-05089.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belikov A.V. Age-related diseases as vicious cycles. Ageing Res. Rev. 2019;49:11–26. doi: 10.1016/j.arr.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Hao Y-H., Zhao L., Peng R-Y. Effects of microwave radiation on brain energy metabolism and related mechanisms. Mil. Med. Res. 2014;4:9. doi: 10.1186/s40779-015-0033-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fishman K., Baure J., Zou Y., Huang T-T., Andres-Mach M., Rola R., Suarez T., Acharya M., Limoli C.L., Lamborn K.R., Fike J.R. Radiation-induced reductions in neurogenesis are ameliorated in mice deficient in CuZnSOD or MnSOD. Free Radic. Biol. Med. 2009;47(10):1459–1467. doi: 10.1016/j.freeradbiomed.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mulinacci N., Valletta A., Pasqualetti V., Innocenti M., Giuliani C., Bellumori M., De Angelis G., Carnevale A., Locato V., Di Venanzio C., De Gara L., Pasqua G. Effects of ionizing radiation on bio-active plant extracts useful for preventing oxidative damages. Nat. Prod. Res. 2019;33(8):1106–1114. doi: 10.1080/14786419.2018.1457663. [DOI] [PubMed] [Google Scholar]
- 8.Eriksson D., Stigbrand T. Radiation-induced cell death mechanisms. Tumour Biol. 2010;31(4):363–372. doi: 10.1007/s13277-010-0042-8. [DOI] [PubMed] [Google Scholar]
- 9.Szumiel I. Ionizing radiation-induced oxidative stress, epigenetic changes and genomic instability: the pivotal role of mitochondria. Int. J. Radiat. Biol. 2015;91(1):1–12. doi: 10.3109/09553002.2014.934929. [DOI] [PubMed] [Google Scholar]
- 10.Greene-Schloesser D., Robbins M.E., Peiffer A.M., Shaw E.G., Wheeler K.T., Chan M.D. Radiation-induced brain injury: A review. Front. Oncol. 2012;2:73. doi: 10.3389/fonc.2012.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greene-Schloesser D., Robbins M.E. Radiation-induced cognitive impairment--from bench to bedside. Neuro-oncol. 2012;14(Suppl. 4):iv37–iv44. doi: 10.1093/neuonc/nos196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Babini G., Morini J., Baiocco G., Mariotti L., Ottolenghi A. In vitro &-ray-induced inflammatory response is dominated by culturing conditions rather than radiation exposures. Sci. Rep. 2015;5:9343. doi: 10.1038/srep09343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Avci B., Akar A., Bilgici B., Tunçel Ö.K. Oxidative stress induced by 1.8 GHz radio frequency electromagnetic radiation and effects of garlic extract in rats. Int. J. Radiat. Biol. 2012;88(11):799–805. doi: 10.3109/09553002.2012.711504. [DOI] [PubMed] [Google Scholar]
- 14.Battino M., Giampieri F., Cianciosi D., Ansary J., Chen X., Zhang D., Gil E., Forbes-Hernández T. The roles of strawberry and honey phytochemicals on human health: A possible clue on the molecular mechanisms involved in the prevention of oxidative stress and inflammation. Phytomedicine. 2021;86:153170. doi: 10.1016/j.phymed.2020.153170. [DOI] [PubMed] [Google Scholar]
- 15.Abbaszadeh F., Fakhri S., Khan H. Targeting apoptosis and autophagy following spinal cord injury: Therapeutic approaches to polyphenols and candidate phytochemicals. Pharmacol. Res. 2020;160:105069. doi: 10.1016/j.phrs.2020.105069. [DOI] [PubMed] [Google Scholar]
- 16.Fakhri S., Khodamorady M., Naseri M., Farzaei M.H., Khan H. The ameliorating effects of anthocyanins on the cross-linked signaling pathways of cancer dysregulated metabolism. Pharmacol. Res. 2020;159:104895. doi: 10.1016/j.phrs.2020.104895. [DOI] [PubMed] [Google Scholar]
- 17.Smith T.A., Kirkpatrick D.R., Smith S., Smith T.K., Pearson T., Kailasam A., Herrmann K.Z., Schubert J., Agrawal D.K. Radioprotective agents to prevent cellular damage due to ionizing radiation. J. Transl. Med. 2017;15(1):232. doi: 10.1186/s12967-017-1338-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peng X.C., Huang J.R., Wang S.W., Liu L., Liu Z.Z., Sethi G., Ren B.X., Tang F.R. Traditional chinese medicine in neuroprotection after brain insults with special reference to radioprotection. Evid. Based Complement. Alternat. Med. 2018;2018:2767208. doi: 10.1155/2018/2767208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calabrese E.J., Mattson M.P., Dhawan G., Kapoor R., Calabrese V., Giordano J. Hormesis: A potential strategic approach to the treatment of neurodegenerative disease. Int. Rev. Neurobiol. 2020;155:271–301. doi: 10.1016/bs.irn.2020.03.024. [DOI] [PubMed] [Google Scholar]
- 20.Calabrese E.J. Converging concepts: adaptive response, preconditioning, and the Yerkes-Dodson Law are manifestations of hormesis. Ageing Res. Rev. 2008;7(1):8–20. doi: 10.1016/j.arr.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 21.Zhang C., Chen S., Bao J., Zhang Y., Huang B., Jia X., Chen M., Wan J-B., Su H., Wang Y., He C. Low doses of camptothecin induced hormetic and neuroprotective effects in PC12 cells. Dose Response. 2015;13(2):1559325815592606. doi: 10.1177/1559325815592606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dirnagl U., Meisel A. Endogenous neuroprotection: mitochondria as gateways to cerebral preconditioning? Neuropharmacology. 2008;55(3):334–344. doi: 10.1016/j.neuropharm.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 23.Calabrese E.J., Bachmann K.A., Bailer A.J., Bolger P.M., Borak J., Cai L., Cedergreen N., Cherian M.G., Chiueh C.C., Clarkson T.W., Cook R.R., Diamond D.M., Doolittle D.J., Dorato M.A., Duke S.O., Feinendegen L., Gardner D.E., Hart R.W., Hastings K.L., Hayes A.W., Hoffmann G.R., Ives J.A., Jaworowski Z., Johnson T.E., Jonas W.B., Kaminski N.E., Keller J.G., Klaunig J.E., Knudsen T.B., Kozumbo W.J., Lettieri T., Liu S.Z., Maisseu A., Maynard K.I., Masoro E.J., McClellan R.O., Mehendale H.M., Mothersill C., Newlin D.B., Nigg H.N., Oehme F.W., Phalen R.F., Philbert M.A., Rattan S.I., Riviere J.E., Rodricks J., Sapolsky R.M., Scott B.R., Seymour C., Sinclair D.A., Smith-Sonneborn J., Snow E.T., Spear L., Stevenson D.E., Thomas Y., Tubiana M., Williams G.M., Mattson M.P. Biological stress response terminology: Integrating the concepts of adaptive response and preconditioning stress within a hormetic dose-response framework. Toxicol. Appl. Pharmacol. 2007;222(1):122–128. doi: 10.1016/j.taap.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 24.Holtzclaw W.D., Dinkova-Kostova A.T., Talalay P. Protection against electrophile and oxidative stress by induction of phase 2 genes: the quest for the elusive sensor that responds to inducers. Adv. Enzyme Regul. 2004;44(1):335–367. doi: 10.1016/j.advenzreg.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 25.Hyun D.H., Hunt N.D., Emerson S.S., Hernandez J.O., Mattson M.P., de Cabo R. Up-regulation of plasma membrane-associated redox activities in neuronal cells lacking functional mitochondria. J. Neurochem. 2007;100(5):1364–1374. doi: 10.1111/j.1471-4159.2006.04411.x. [DOI] [PubMed] [Google Scholar]
- 26.Calabrese V., Cornelius C., Dinkova-Kostova A.T., Calabrese E.J., Mattson M.P. Cellular stress responses, the hormesis paradigm, and vitagenes: novel targets for therapeutic intervention in neurodegenerative disorders. Antioxid. Redox Signal. 2010;13(11):1763–1811. doi: 10.1089/ars.2009.3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Calabrese E.J., Calabrese V., Giordano J. Demonstrated hormetic mechanisms putatively subserve riluzole-induced effects in neuroprotection against amyotrophic lateral sclerosis (ALS): Implications for research and clinical practice. Ageing Res. Rev. 2021;67:101273. doi: 10.1016/j.arr.2021.101273. [DOI] [PubMed] [Google Scholar]
- 28.Siracusa R., Scuto M., Fusco R., Trovato A., Ontario M.L., Crea R., Di Paola R., Cuzzocrea S., Calabrese V. Anti-inflammatory and anti-oxidant activity of hidrox® in rotenone-induced Parkinson’s disease in mice. Antioxidants. 2020;9(9):824. doi: 10.3390/antiox9090824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Calabrese V., Renis M., Calderone A., Russo A., Barcellona M.L., Rizza V. Stress proteins and SH-groups in oxidant-induced cell damage after acute ethanol administration in rat. Free Radic. Biol. Med. 1996;20(3):391–397. doi: 10.1016/0891-5849(95)02095-0. [DOI] [PubMed] [Google Scholar]
- 30.Ragusa N., Sfogliano L., Calabrese V., Rizza V. Effects of multivitamin treatment on the activity of rat liver tryptophan pyrrolase during ethanol administration. Acta Vitaminol. Enzymol. 1981;3(4):199–204. [PubMed] [Google Scholar]
- 31.Limoli C.L., Giedzinski E., Rola R., Otsuka S., Palmer T.D., Fike J.R. Radiation response of neural precursor cells: linking cellular sensitivity to cell cycle checkpoints, apoptosis and oxidative stress. Radiat. Res. 2004;161(1):17–27. doi: 10.1667/RR3112. [DOI] [PubMed] [Google Scholar]
- 32.Dent P., Yacoub A., Contessa J., Caron R., Amorino G., Valerie K., Hagan M.P., Grant S., Schmidt-Ullrich R. Stress and radiation-induced activation of multiple intracellular signaling pathways. Radiat. Res. 2003;159(3):283–300. doi: 10.1667/0033-7587(2003)159[0283:SARIAO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 33.Cartee L., Vrana J.A., Wang Z., Park J.S., Birrer M., Fisher P.B., Grant S., Dent P. Inhibition of the mitogen activated protein kinase pathway potentiates radiation-induced cell killing via cell cycle arrest at the G2/M transition and independently of increased signaling by the JNK/c-Jun pathway. Int. J. Oncol. 2000;16(2):413–422. doi: 10.3892/ijo.16.2.413. [DOI] [PubMed] [Google Scholar]
- 34.Amundson S.A., Bittner M., Meltzer P., Trent J., Fornace A.J., Jr Induction of gene expression as a monitor of exposure to ionizing radiation. Radiat. Res. 2001;156(5 Pt 2):657–661. doi: 10.1667/0033-7587(2001)156[0657:IOGEAA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 35.Mazière C., Conte M-A., Leborgne L., Levade T., Hornebeck W., Santus R., Mazière J-C. UVA radiation stimulates ceramide production: relationship to oxidative stress and potential role in ERK, JNK, and p38 activation. Biochem. Biophys. Res. Commun. 2001;281(2):289–294. doi: 10.1006/bbrc.2001.4348. [DOI] [PubMed] [Google Scholar]
- 36.Aroun A., Zhong J.L., Tyrrell R.M., Pourzand C. Iron, oxidative stress and the example of solar ultraviolet A radiation. Photochem. Photobiol. Sci. 2012;11(1):118–134. doi: 10.1039/C1PP05204G. [DOI] [PubMed] [Google Scholar]
- 37.Bilgici B., Akar A., Avci B., Tuncel O.K. Effect of 900 MHz radiofrequency radiation on oxidative stress in rat brain and serum. Electromagn. Biol. Med. 2013;32(1):20–29. doi: 10.3109/15368378.2012.699012. [DOI] [PubMed] [Google Scholar]
- 38.Heinloth A.N., Shackelford R.E., Innes C.L., Bennett L., Li L., Amin R.P., Sieber S.O., Flores K.G., Bushel P.R., Paules R.S. Identification of distinct and common gene expression changes after oxidative stress and gamma and ultraviolet radiation. Mol. Carcinog. 2003;37(2):65–82. doi: 10.1002/mc.10122. [DOI] [PubMed] [Google Scholar]
- 39.Birch-Machin M.A., Russell E.V., Latimer J.A. Mitochondrial DNA damage as a biomarker for ultraviolet radiation exposure and oxidative stress. Br. J. Dermatol. 2013;169(Suppl. 2):9–14. doi: 10.1111/bjd.12207. [DOI] [PubMed] [Google Scholar]
- 40.Paunesku T., Mittal S. Proti& M.; Oryhon, J.; Korolev, S.V.; Joachimiak, A.; Woloschak, G.E. Proliferating cell nuclear antigen (PCNA): ringmaster of the genome. Int. J. Radiat. Biol. 2001;77(10):1007–1021. doi: 10.1080/09553000110069335. [DOI] [PubMed] [Google Scholar]
- 41.Lee S.A., Dritschilo A., Jung M. Role of ATM in oxidative stress-mediated c-Jun phosphorylation in response to ionizing radiation and CdCl2. J. Biol. Chem. 2001;276(15):11783–11790. doi: 10.1074/jbc.M004517200. [DOI] [PubMed] [Google Scholar]
- 42.Robbins M.E., Zhao W. Chronic oxidative stress and radiation-induced late normal tissue injury: a review. Int. J. Radiat. Biol. 2004;80(4):251–259. doi: 10.1080/09553000410001692726. [DOI] [PubMed] [Google Scholar]
- 43.Antal O., Hackler L., Jr, Shen J., Mán I., Hideghéty K., Kitajka K., Puskás L.G. Combination of unsaturated fatty acids and ionizing radiation on human glioma cells: cellular, biochemical and gene expression analysis. Lipids Health Dis. 2014;13(1):142. doi: 10.1186/1476-511X-13-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morgan J.L., Ritchie L.E., Crucian B.E., Theriot C., Wu H., Sams C., Smith S.M., Turner N.D., Zwart S.R. Increased dietary iron and radiation in rats promote oxidative stress, induce localized and systemic immune system responses, and alter colon mucosal environment. FASEB J. 2014;28(3):1486–1498. doi: 10.1096/fj.13-239418. [DOI] [PubMed] [Google Scholar]
- 45.Spitz D.R., Azzam E.I., Li J.J., Gius D. Metabolic oxidation/reduction reactions and cellular responses to ionizing radiation: a unifying concept in stress response biology. Cancer Metastasis Rev. 2004;23(3-4):311–322. doi: 10.1023/B:CANC.0000031769.14728.bc. [DOI] [PubMed] [Google Scholar]
- 46.Fukuda A., Tomikawa J., Miura T., Hata K., Nakabayashi K., Eggan K., Akutsu H., Umezawa A. The role of maternal-specific H3K9me3 modification in establishing imprinted X-chromosome inactivation and embryogenesis in mice. Nat. Commun. 2014;5(1):5464. doi: 10.1038/ncomms6464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nazıroglu, M.; Akman, H. Effects of Cellular Phone-and Wi-Fi- Induced Electromagnetic Radiation on Oxidative Stress and Molecular Pathways in Brain. In: Systems biology of free radicals and antioxidants; Springer, . 2014.
- 48.Tharmalingam S., Sreetharan S., Kulesza A.V., Boreham D.R., Tai T. Low-dose ionizing radiation exposure, oxidative stress and epigenetic programing of health and disease. Radiat. Res. 2017;188(4):525–538. doi: 10.1667/RR14587.1. [DOI] [PubMed] [Google Scholar]
- 49.Xu S., Zhou Z., Zhang L., Yu Z., Zhang W., Wang Y., Wang X., Li M., Chen Y., Chen C., He M., Zhang G., Zhong M. Exposure to 1800 MHz radiofrequency radiation induces oxidative damage to mitochondrial DNA in primary cultured neurons. Brain Res. 2010;1311:189–196. doi: 10.1016/j.brainres.2009.10.062. [DOI] [PubMed] [Google Scholar]
- 50.Weyemi U., Redon C.E., Aziz T., Choudhuri R., Maeda D., Parekh P.R., Bonner M.Y., Arbiser J.L., Bonner W.M. Inactivation of NADPH oxidases NOX4 and NOX5 protects human primary fibroblasts from ionizing radiation-induced DNA damage. Radiat. Res. 2015;183(3):262–270. doi: 10.1667/RR13799.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Simone N.L., Soule B.P., Ly D., Saleh A.D., Savage J.E., Degraff W., Cook J., Harris C.C., Gius D., Mitchell J.B. Ionizing radiation-induced oxidative stress alters miRNA expression. PLoS One. 2009;4(7):e6377. doi: 10.1371/journal.pone.0006377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schieven G.L., Ledbetter J.A. Activation of tyrosine kinase signal pathways by radiation and oxidative stress. Trends Endocrinol. Metab. 1994;5(9):383–388. doi: 10.1016/1043-2760(94)90106-6. [DOI] [PubMed] [Google Scholar]
- 53.Lin M.T., Beal M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443(7113):787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 54.Cenini G., Lloret A., Cascella R. Oxidative stress in neurodegenerative diseases: from a mitochondrial point of view. Oxid. Med. Cell. Longev. 2019;2019:2105607. doi: 10.1155/2019/2105607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bhat A.H., Dar K.B., Anees S., Zargar M.A., Masood A., Sofi M.A., Ganie S.A. Oxidative stress, mitochondrial dysfunction and neurodegenerative diseases; a mechanistic insight. Biomed. Pharmacother. 2015;74:101–110. doi: 10.1016/j.biopha.2015.07.025. [DOI] [PubMed] [Google Scholar]
- 56.Sochocka M., Koutsouraki E.S., Gasiorowski K., Leszek J. Vascular oxidative stress and mitochondrial failure in the pathobiology of Alzheimer’s disease: a new approach to therapy. CNS Neurol. Disord. Drug Targets. 2013;12(6):870–881. doi: 10.2174/18715273113129990072. [DOI] [PubMed] [Google Scholar]
- 57.Begum N., Wang B., Mori M., Vares G. Does ionizing radiation influence Alzheimer’s disease risk? J. Radiat. Res. (Tokyo) 2012;53(6):815–822. doi: 10.1093/jrr/rrs036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mancuso C., Bates T.E., Butterfield D.A., Calafato S., Cornelius C., De Lorenzo A., Dinkova Kostova A.T., Calabrese V. Natural antioxidants in Alzheimer’s disease. Expert Opin. Investig. Drugs. 2007;16(12):1921–1931. doi: 10.1517/13543784.16.12.1921. [DOI] [PubMed] [Google Scholar]
- 59.Rodgers C.C. Low-dose X-ray imaging may increase the risk of neurodegenerative diseases. Med. Hypotheses. 2020;142:109726. doi: 10.1016/j.mehy.2020.109726. [DOI] [PubMed] [Google Scholar]
- 60.Liu Z., Zhou T., Ziegler A.C., Dimitrion P., Zuo L. Oxidative stress in neurodegenerative diseases: from molecular mechanisms to clinical applications. Oxid. Med. Cell. Longev. 2017;2017:2525967. doi: 10.1155/2017/2525967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sobel E., Davanipour Z. Electromagnetic field exposure may cause increased production of amyloid beta and eventually lead to Alzheimer’s disease. Neurology. 1996;47(6):1594–1600. doi: 10.1212/WNL.47.6.1594. [DOI] [PubMed] [Google Scholar]
- 62.Wang X., Su B., Perry G., Smith M.A., Zhu X. Insights into amyloid-beta-induced mitochondrial dysfunction in Alzheimer disease. Free Radic. Biol. Med. 2007;43(12):1569–1573. doi: 10.1016/j.freeradbiomed.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 63.Cherry J.D., Liu B., Frost J.L., Lemere C.A., Williams J.P., Olschowka J.A., O’Banion M.K. Galactic cosmic radiation leads to cognitive impairment and increased a& plaque accumulation in a mouse model of Alzheimer’s disease. PLoS One. 2012;7(12):e53275. doi: 10.1371/journal.pone.0053275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li L., Wang W., Welford S., Zhang T., Wang X., Zhu X. Ionizing radiation causes increased tau phosphorylation in primary neurons. J. Neurochem. 2014;131(1):86–93. doi: 10.1111/jnc.12769. [DOI] [PubMed] [Google Scholar]
- 65.Dias V., Junn E., Mouradian M.M. The role of oxidative stress in Parkinson’s disease. J. Parkinsons Dis. 2013;3(4):461–491. doi: 10.3233/JPD-130230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Meiser J., Weindl D., Hiller K. Complexity of dopamine metabolism. Cell Commun. Signal. 2013;11(1):34. doi: 10.1186/1478-811X-11-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sharma N.K., Sharma R., Mathur D., Sharad S., Minhas G., Bhatia K., Anand A., Ghosh S.P. Role of ionizing radiation in neurodegenerative diseases. Front. Aging Neurosci. 2018;10:134. doi: 10.3389/fnagi.2018.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tseng B.P., Giedzinski E., Izadi A., Suarez T., Lan M.L., Tran K.K., Acharya M.M., Nelson G.A., Raber J., Parihar V.K., Limoli C.L. Functional consequences of radiation-induced oxidative stress in cultured neural stem cells and the brain exposed to charged particle irradiation. Antioxid. Redox Signal. 2014;20(9):1410–1422. doi: 10.1089/ars.2012.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kempf S.J., Azimzadeh O., Atkinson M.J., Tapio S. Long-term effects of ionising radiation on the brain: cause for concern? Radiat. Environ. Biophys. 2013;52(1):5–16. doi: 10.1007/s00411-012-0436-7. [DOI] [PubMed] [Google Scholar]
- 70.Wei J., Wang B., Wang H., Meng L., Zhao Q., Li X., Xin Y., Jiang X. Radiation-induced normal tissue damage: Oxidative stress and epigenetic mechanisms. Oxid. Med. Cell. Longev. 2019;2019:3010342. doi: 10.1155/2019/3010342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Acharya M.M., Baddour A.A., Kawashita T., Allen B.D., Syage A.R., Nguyen T.H., Yoon N., Giedzinski E., Yu L., Parihar V.K., Baulch J.E. Epigenetic determinants of space radiation-induced cognitive dysfunction. Sci. Rep. 2017;7:42885. doi: 10.1038/srep42885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hampel H., Prvulovic D., Teipel S., Jessen F., Luckhaus C., Frölich L., Riepe M.W., Dodel R., Leyhe T., Bertram L., Hoffmann W., Faltraco F. The future of Alzheimer’s disease: the next 10 years. Prog. Neurobiol. 2011;95(4):718–728. doi: 10.1016/j.pneurobio.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 73.Bertram L., McQueen M.B., Mullin K., Blacker D., Tanzi R.E. Systematic meta-analyses of Alzheimer disease genetic association studies: the AlzGene database. Nat. Genet. 2007;39(1):17–23. doi: 10.1038/ng1934. [DOI] [PubMed] [Google Scholar]
- 74.Hollingworth P., Harold D., Sims R., Gerrish A., Lambert J.C., Carrasquillo M.M., Abraham R., Hamshere M.L., Pahwa J.S., Moskvina V., Dowzell K., Jones N., Stretton A., Thomas C., Richards A., Ivanov D., Widdowson C., Chapman J., Lovestone S., Powell J., Proitsi P., Lupton M.K., Brayne C., Rubinsztein D.C., Gill M., Lawlor B., Lynch A., Brown K.S., Passmore P.A., Craig D., McGuinness B., Todd S., Holmes C., Mann D., Smith A.D., Beaumont H., Warden D., Wilcock G., Love S., Kehoe P.G., Hooper N.M., Vardy E.R., Hardy J., Mead S., Fox N.C., Rossor M., Collinge J., Maier W., Jessen F., Rüther E., Schürmann B., Heun R., Kölsch H., van den Bussche H., Heuser I., Kornhuber J., Wiltfang J., Dichgans M., Frölich L., Hampel H., Gallacher J., Hüll M., Rujescu D., Giegling I., Goate A.M., Kauwe J.S., Cruchaga C., Nowotny P., Morris J.C., Mayo K., Sleegers K., Bettens K., Engelborghs S., De Deyn P.P., Van Broeckhoven C., Livingston G., Bass N.J., Gurling H., McQuillin A., Gwilliam R., Deloukas P., Al-Chalabi A., Shaw C.E., Tsolaki M., Singleton A.B., Guerreiro R., Mühleisen T.W., Nöthen M.M., Moebus S., Jöckel K.H., Klopp N., Wichmann H.E., Pankratz V.S., Sando S.B., Aasly J.O., Barcikowska M., Wszolek Z.K., Dickson D.W., Graff-Radford N.R., Petersen R.C., van Duijn C.M., Breteler M.M., Ikram M.A., DeStefano A.L., Fitzpatrick A.L., Lopez O., Launer L.J., Seshadri S., Berr C., Campion D., Epelbaum J., Dartigues J.F., Tzourio C., Alpérovitch A., Lathrop M., Feulner T.M., Friedrich P., Riehle C., Krawczak M., Schreiber S., Mayhaus M., Nicolhaus S., Wagenpfeil S., Steinberg S., Stefansson H., Stefansson K., Snaedal J., Björnsson S., Jonsson P.V., Chouraki V., Genier-Boley B., Hiltunen M., Soininen H., Combarros O., Zelenika D., Delepine M., Bullido M.J., Pasquier F., Mateo I., Frank-Garcia A., Porcellini E., Hanon O., Coto E., Alvarez V., Bosco P., Siciliano G., Mancuso M., Panza F., Solfrizzi V., Nacmias B., Sorbi S., Bossù P., Piccardi P., Arosio B., Annoni G., Seripa D., Pilotto A., Scarpini E., Galimberti D., Brice A., Hannequin D., Licastro F., Jones L., Holmans P.A., Jonsson T., Riemenschneider M., Morgan K., Younkin S.G., Owen M.J., O’Donovan M., Amouyel P., Williams J. Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer’s disease. Nat. Genet. 2011;43(5):429–435. doi: 10.1038/ng.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Naj A.C., Jun G., Beecham G.W., Wang L.S., Vardarajan B.N., Buros J., Gallins P.J., Buxbaum J.D., Jarvik G.P., Crane P.K., Larson E.B., Bird T.D., Boeve B.F., Graff-Radford N.R., De Jager P.L., Evans D., Schneider J.A., Carrasquillo M.M., Ertekin-Taner N., Younkin S.G., Cruchaga C., Kauwe J.S., Nowotny P., Kramer P., Hardy J., Huentelman M.J., Myers A.J., Barmada M.M., Demirci F.Y., Baldwin C.T., Green R.C., Rogaeva E., St George-Hyslop P., Arnold S.E., Barber R., Beach T., Bigio E.H., Bowen J.D., Boxer A., Burke J.R., Cairns N.J., Carlson C.S., Carney R.M., Carroll S.L., Chui H.C., Clark D.G., Corneveaux J., Cotman C.W., Cummings J.L., DeCarli C., DeKosky S.T., Diaz-Arrastia R., Dick M., Dickson D.W., Ellis W.G., Faber K.M., Fallon K.B., Farlow M.R., Ferris S., Frosch M.P., Galasko D.R., Ganguli M., Gearing M., Geschwind D.H., Ghetti B., Gilbert J.R., Gilman S., Giordani B., Glass J.D., Growdon J.H., Hamilton R.L., Harrell L.E., Head E., Honig L.S., Hulette C.M., Hyman B.T., Jicha G.A., Jin L.W., Johnson N., Karlawish J., Karydas A., Kaye J.A., Kim R., Koo E.H., Kowall N.W., Lah J.J., Levey A.I., Lieberman A.P., Lopez O.L., Mack W.J., Marson D.C., Martiniuk F., Mash D.C., Masliah E., McCormick W.C., McCurry S.M., McDavid A.N., McKee A.C., Mesulam M., Miller B.L., Miller C.A., Miller J.W., Parisi J.E., Perl D.P., Peskind E., Petersen R.C., Poon W.W., Quinn J.F., Rajbhandary R.A., Raskind M., Reisberg B., Ringman J.M., Roberson E.D., Rosenberg R.N., Sano M., Schneider L.S., Seeley W., Shelanski M.L., Slifer M.A., Smith C.D., Sonnen J.A., Spina S., Stern R.A., Tanzi R.E., Trojanowski J.Q., Troncoso J.C., Van Deerlin V.M., Vinters H.V., Vonsattel J.P., Weintraub S., Welsh-Bohmer K.A., Williamson J., Woltjer R.L., Cantwell L.B., Dombroski B.A., Beekly D., Lunetta K.L., Martin E.R., Kamboh M.I., Saykin A.J., Reiman E.M., Bennett D.A., Morris J.C., Montine T.J., Goate A.M., Blacker D., Tsuang D.W., Hakonarson H., Kukull W.A., Foroud T.M., Haines J.L., Mayeux R., Pericak-Vance M.A., Farrer L.A., Schellenberg G.D. Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer’s disease. Nat. Genet. 2011;43(5):436–441. doi: 10.1038/ng.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lowe X.R., Marchetti F., Lu X., Wyrobek A.J. Molecular stress response in the CNS of mice after systemic exposure to interferon-alpha, ionizing radiation and ketamine. Neurotoxicology. 2009;30(2):261–268. doi: 10.1016/j.neuro.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 77.Rolyan H., Scheffold A., Heinrich A., Begus-Nahrmann Y., Langkopf B.H., Hölter S.M., Vogt-Weisenhorn D.M., Liss B., Wurst W., Lie D.C., Thal D.R., Biber K., Rudolph K.L. Telomere shortening reduces Alzheimer’s disease amyloid pathology in mice. Brain. 2011;134(Pt 7):2044–2056. doi: 10.1093/brain/awr133. [DOI] [PubMed] [Google Scholar]
- 78.Gamez J., Corbera-Bellalta M., Nogales G., Raguer N., García-Arumí E., Badia-Canto M., Lladó-Carbó E., Alvarez-Sabín J. Mutational analysis of the Cu/Zn superoxide dismutase gene in a Catalan ALS population: should all sporadic ALS cases also be screened for SOD1? J. Neurol. Sci. 2006;247(1):21–28. doi: 10.1016/j.jns.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 79.Saccon R.A., Bunton-Stasyshyn R.K., Fisher E.M., Fratta P. Is SOD1 loss of function involved in amyotrophic lateral sclerosis? Brain. 2013;136(Pt 8):2342–2358. doi: 10.1093/brain/awt097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Du&i& T.; Stamenkovi& S.; Lai, B.; Andjus, P.; Lu&i& V. Multimodal synchrotron radiation microscopy of intact astrocytes from the hSOD1 G93A rat model of amyotrophic lateral sclerosis. Anal. Chem. 2019;91(2):1460–1471. doi: 10.1021/acs.analchem.8b04273. [DOI] [PubMed] [Google Scholar]
- 81.Bevelacqua J.J., Mortazavi S.M.J. Alzheimer 's disease: possible mechanisms behind neurohormesis induced by exposure to low doses of ionizing radiation. J. Biomed. Phys. Eng. 2018;8(2):153–156. doi: 10.31661/jbpe.v8i2.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Belka C., Budach W., Kortmann R.D., Bamberg M. Radiation induced CNS toxicity--molecular and cellular mechanisms. Br. J. Cancer. 2001;85(9):1233–1239. doi: 10.1054/bjoc.2001.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kobashigawa S., Suzuki K., Yamashita S. Ionizing radiation accelerates Drp1-dependent mitochondrial fission, which involves delayed mitochondrial reactive oxygen species production in normal human fibroblast-like cells. Biochem. Biophys. Res. Commun. 2011;414(4):795–800. doi: 10.1016/j.bbrc.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 84.El-Missiry M.A., Othman A.I., El-Sawy M.R., Lebede M.F. Neuroprotective effect of epigallocatechin-3-gallate (EGCG) on radiation-induced damage and apoptosis in the rat hippocampus. Int. J. Radiat. Biol. 2018;94(9):798–808. doi: 10.1080/09553002.2018.1492755. [DOI] [PubMed] [Google Scholar]
- 85.Rödel C., Haas J., Groth A., Grabenbauer G.G., Sauer R., Rödel F. Spontaneous and radiation-induced apoptosis in colorectal carcinoma cells with different intrinsic radiosensitivities: survivin as a radioresistance factor. Int. J. Radiat. Oncol. Biol. Phys. 2003;55(5):1341–1347. doi: 10.1016/S0360-3016(02)04618-7. [DOI] [PubMed] [Google Scholar]
- 86.Asanuma K., Moriai R., Yajima T., Yagihashi A., Yamada M., Kobayashi D., Watanabe N. Survivin as a radioresistance factor in pancreatic cancer. Jpn. J. Cancer Res. 2000;91(11):1204–1209. doi: 10.1111/j.1349-7006.2000.tb00906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Philipp J., Azimzadeh O., Subramanian V., Merl-Pham J., Lowe D., Hladik D., Erbeldinger N., Ktitareva S., Fournier C., Atkinson M.J., Raj K., Tapio S. Radiation-induced endothelial inflammation is transferred via the secretome to recipient cells in a STAT-Mediated Process. J. Proteome Res. 2017;16(10):3903–3916. doi: 10.1021/acs.jproteome.7b00536. [DOI] [PubMed] [Google Scholar]
- 88.François A., Milliat F., Guipaud O., Benderitter M. Inflammation and immunity in radiation damage to the gut mucosa. BioMed Res. Int. 2013;2013:123241. doi: 10.1155/2013/123241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Blirando K., Milliat F., Martelly I., Sabourin J-C., Benderitter M., François A. Mast cells are an essential component of human radiation proctitis and contribute to experimental colorectal damage in mice. Am. J. Pathol. 2011;178(2):640–651. doi: 10.1016/j.ajpath.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Boström M., Kalm M., Eriksson Y., Bull C., Ståhlberg A., Björk-Eriksson T., Hellström Erkenstam N., Blomgren K. A role for endothelial cells in radiation-induced inflammation. Int. J. Radiat. Biol. 2018;94(3):259–271. doi: 10.1080/09553002.2018.1431699. [DOI] [PubMed] [Google Scholar]
- 91.Mollà M., Gironella M., Salas A., Closa D., Biete A., Gimeno M., Coronel P., Piqué J.M., Panés J. Protective effect of superoxide dismutase in radiation-induced intestinal inflammation. Int. J. Radiat. Oncol. Biol. Phys. 2005;61(4):1159–1166. doi: 10.1016/j.ijrobp.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 92.Denning M.F., Wang Y., Tibudan S., Alkan S., Nickoloff B.J., Qin J.Z. Caspase activation and disruption of mitochondrial membrane potential during UV radiation-induced apoptosis of human keratinocytes requires activation of protein kinase C. Cell Death Differ. 2002;9(1):40–52. doi: 10.1038/sj.cdd.4400929. [DOI] [PubMed] [Google Scholar]
- 93.Ferrer I. Role of caspases in ionizing radiation-induced apoptosis in the developing cerebellum. J. Neurobiol. 1999;41(4):549–558. doi: 10.1002/(SICI)1097-4695(199912)41:4<549:AID-NEU10>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 94.Ahmed M.M. Regulation of radiation-induced apoptosis by early growth response-1 gene in solid tumors. Curr. Cancer Drug Targets. 2004;4(1):43–52. doi: 10.2174/1568009043481704. [DOI] [PubMed] [Google Scholar]
- 95.Ahmed M.M., Sells S.F., Venkatasubbarao K., Fruitwala S.M., Muthukkumar S., Harp C., Mohiuddin M., Rangnekar V.M. Ionizing radiation-inducible apoptosis in the absence of p53 linked to transcription factor EGR-1. J. Biol. Chem. 1997;272(52):33056–33061. doi: 10.1074/jbc.272.52.33056. [DOI] [PubMed] [Google Scholar]
- 96.Bulavin D.V., Saito S., Hollander M.C., Sakaguchi K., Anderson C.W., Appella E., Fornace A.J. Jr Phosphorylation of human p53 by p38 kinase coordinates N-terminal phosphorylation and apoptosis in response to UV radiation. EMBO J. 1999;18(23):6845–6854. doi: 10.1093/emboj/18.23.6845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ko L.J., Shieh S-Y., Chen X., Jayaraman L., Tamai K., Taya Y., Prives C., Pan Z-Q. p53 is phosphorylated by CDK7-cyclin H in a p36MAT1-dependent manner. Mol. Cell. Biol. 1997;17(12):7220–7229. doi: 10.1128/MCB.17.12.7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Unger T., Juven-Gershon T., Moallem E., Berger M., Vogt Sionov R., Lozano G., Oren M., Haupt Y. Critical role for Ser20 of human p53 in the negative regulation of p53 by Mdm2. EMBO J. 1999;18(7):1805–1814. doi: 10.1093/emboj/18.7.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lambert P.F., Kashanchi F., Radonovich M.F., Shiekhattar R., Brady J.N. Phosphorylation of p53 serine 15 increases interaction with CBP. J. Biol. Chem. 1998;273(49):33048–33053. doi: 10.1074/jbc.273.49.33048. [DOI] [PubMed] [Google Scholar]
- 100.Chen C.H., Zhang J., Ling C.C. Transfected c-myc and c-Ha-ras modulate radiation-induced apoptosis in rat embryo cells. Radiat. Res. 1994;139(3):307–315. doi: 10.2307/3578828. [DOI] [PubMed] [Google Scholar]
- 101.Hekim N., Cetin Z., Nikitaki Z., Cort A., Saygili E.I. Radiation triggering immune response and inflammation. Cancer Lett. 2015;368(2):156–163. doi: 10.1016/j.canlet.2015.04.016. [DOI] [PubMed] [Google Scholar]
- 102.Ivanov V.N., Hei T.K. A role for TRAIL/TRAIL-R2 in radiation-induced apoptosis and radiation-induced bystander response of human neural stem cells. Apoptosis. 2014;19(3):399–413. doi: 10.1007/s10495-013-0925-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yahyapour R., Motevaseli E., Rezaeyan A., Abdollahi H., Farhood B., Cheki M., Rezapoor S., Shabeeb D., Musa A.E., Najafi M., Villa V. Reduction-oxidation (redox) system in radiation-induced normal tissue injury: molecular mechanisms and implications in radiation therapeutics. Clin. Transl. Oncol. 2018;20(8):975–988. doi: 10.1007/s12094-017-1828-6. [DOI] [PubMed] [Google Scholar]
- 104.Jelonek K., Pietrowska M., Widlak P. Systemic effects of ionizing radiation at the proteome and metabolome levels in the blood of cancer patients treated with radiotherapy: the influence of inflammation and radiation toxicity. Int. J. Radiat. Biol. 2017;93(7):683–696. doi: 10.1080/09553002.2017.1304590. [DOI] [PubMed] [Google Scholar]
- 105.Kanzawa T., Iwado E., Aoki H., Iwamaru A., Hollingsworth E.F., Sawaya R., Kondo S., Kondo Y. Ionizing radiation induces apoptosis and inhibits neuronal differentiation in rat neural stem cells via the c-Jun NH2-terminal kinase (JNK) pathway. Oncogene. 2006;25(26):3638–3648. doi: 10.1038/sj.onc.1209414. [DOI] [PubMed] [Google Scholar]
- 106.Kulms D., Zeise E., Pöppelmann B., Schwarz T. DNA damage, death receptor activation and reactive oxygen species contribute to ultraviolet radiation-induced apoptosis in an essential and independent way. Oncogene. 2002;21(38):5844–5851. doi: 10.1038/sj.onc.1205743. [DOI] [PubMed] [Google Scholar]
- 107.Lu F.G., Wong C.S. Radiation-induced apoptosis of oligodendrocytes and its association with increased ceramide and down-regulated protein kinase B/Akt activity. Int. J. Radiat. Biol. 2004;80(1):39–51. doi: 10.1080/09553000310001642876. [DOI] [PubMed] [Google Scholar]
- 108.Verheij M., Ruiter G.A., Zerp S.F., van Blitterswijk W.J., Fuks Z., Haimovitz-Friedman A., Bartelink H. The role of the stress-activated protein kinase (SAPK/JNK) signaling pathway in radiation-induced apoptosis. Radiother. Oncol. 1998;47(3):225–232. doi: 10.1016/S0167-8140(98)00007-3. [DOI] [PubMed] [Google Scholar]
- 109.Willaime S., Vanhoutte P., Caboche J., Lemaigre-Dubreuil Y., Mariani J., Brugg B. Ceramide-induced apoptosis in cortical neurons is mediated by an increase in p38 phosphorylation and not by the decrease in ERK phosphorylation. Eur. J. Neurosci. 2001;13(11):2037–2046. doi: 10.1046/j.0953-816x.2001.01581.x. [DOI] [PubMed] [Google Scholar]
- 110.Lee J.Y., Hannun Y.A., Obeid L.M. Ceramide inactivates cellular protein kinase Calpha. J. Biol. Chem. 1996;271(22):13169–13174. doi: 10.1074/jbc.271.22.13169. [DOI] [PubMed] [Google Scholar]
- 111.Lucas K., Maes M. Role of the Toll Like receptor (TLR) radical cycle in chronic inflammation: possible treatments targeting the TLR4 pathway. Mol. Neurobiol. 2013;48(1):190–204. doi: 10.1007/s12035-013-8425-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shan Y.X., Jin S.Z., Liu X.D., Liu Y., Liu S.Z. Ionizing radiation stimulates secretion of pro-inflammatory cytokines: dose-response relationship, mechanisms and implications. Radiat. Environ. Biophys. 2007;46(1):21–29. doi: 10.1007/s00411-006-0076-x. [DOI] [PubMed] [Google Scholar]
- 113.Porter L.A., Singh G., Lee J.M. Abundance of cyclin B1 regulates gamma-radiation-induced apoptosis. Blood. 2000;95(8):2645–2650. doi: 10.1182/blood.V95.8.2645. [DOI] [PubMed] [Google Scholar]
- 114.Assefa Z., Van Laethem A., Garmyn M., Agostinis P. Ultraviolet radiation-induced apoptosis in keratinocytes: on the role of cytosolic factors. Biochim. Biophys. Acta. 2005;1755(2):90–106. doi: 10.1016/j.bbcan.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 115.Bode A.M., Dong Z. Mitogen-activated protein kinase activation in UV-induced signal transduction. Sci. STKE. 2003;2003(167):RE2. doi: 10.1126/stke.2003.167.re2. [DOI] [PubMed] [Google Scholar]
- 116.Kyrkanides S., Moore A.H., Olschowka J.A., Daeschner J.C., Williams J.P., Hansen J.T., Kerry O’Banion M. Cyclooxygenase-2 modulates brain inflammation-related gene expression in central nervous system radiation injury. Brain Res. Mol. Brain Res. 2002;104(2):159–169. doi: 10.1016/S0169-328X(02)00353-4. [DOI] [PubMed] [Google Scholar]
- 117.Sayama K., Hanakawa Y., Shirakata Y., Yamasaki K., Sawada Y., Sun L., Yamanishi K., Ichijo H., Hashimoto K. Apoptosis signal-regulating kinase 1 (ASK1) is an intracellular inducer of keratinocyte differentiation. J. Biol. Chem. 2001;276(2):999–1004. doi: 10.1074/jbc.M003425200. [DOI] [PubMed] [Google Scholar]
- 118.Lindgren T., Stigbrand T., Riklund K., Johansson L., Eriksson D. Gene expression profiling in MOLT-4 cells during gamma-radiation-induced apoptosis. Tumour Biol. 2012;33(3):689–700. doi: 10.1007/s13277-012-0329-z. [DOI] [PubMed] [Google Scholar]
- 119.Yang J-Y., Xia W., Hu M.C-T. Ionizing radiation activates expression of FOXO3a, Fas ligand, and Bim, and induces cell apoptosis. Int. J. Oncol. 2006;29(3):643–648. doi: 10.3892/ijo.29.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kang K.A., Lee I.K., Zhang R., Piao M.J., Kim K.C., Kim S.Y., Shin T., Kim B.J., Lee N.H., Hyun J.W. Radioprotective effect of geraniin via the inhibition of apoptosis triggered by γ-radiation-induced oxidative stress. Cell Biol. Toxicol. 2011;27(2):83–94. doi: 10.1007/s10565-010-9172-4. [DOI] [PubMed] [Google Scholar]
- 121.Georgakilas A.G., Pavlopoulou A., Louka M., Nikitaki Z., Vorgias C.E., Bagos P.G., Michalopoulos I. Emerging molecular networks common in ionizing radiation, immune and inflammatory responses by employing bioinformatics approaches. Cancer Lett. 2015;368(2):164–172. doi: 10.1016/j.canlet.2015.03.021. [DOI] [PubMed] [Google Scholar]
- 122.Di Maggio F.M., Minafra L., Forte G.I., Cammarata F.P., Lio D., Messa C., Gilardi M.C., Bravatà V. Portrait of inflammatory response to ionizing radiation treatment. J. Inflamm. (Lond.) 2015;12(1):14. doi: 10.1186/s12950-015-0058-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Andrade S., Ramalho M.J., Pereira M.D.C., Loureiro J.A. Resveratrol brain delivery for neurological disorders prevention and treatment. Front. Pharmacol. 2018;9:1261. doi: 10.3389/fphar.2018.01261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Liu W., Ding I., Chen K., Olschowka J., Xu J., Hu D., Morrow G.R., Okunieff P. Interleukin 1β (IL1B) signaling is a critical component of radiation-induced skin fibrosis. Radiat. Res. 2006;165(2):181–191. doi: 10.1667/RR3478.1. [DOI] [PubMed] [Google Scholar]
- 125.Fakhri S., Kiani A., Jalili C., Abbaszadeh F., Piri S., Farzaei M.H., Rastegari-Pouyani M., Mohammadi-Noori E., Khan H. Intrathecal administration of melatonin ameliorates the neuroinflammation-mediated sensory and motor dysfunction in a rat model of compression spinal cord injury. Curr. Mol. Pharmacol. 2020 doi: 10.2174/1874467213666201230101811. [DOI] [PubMed] [Google Scholar]
- 126.Heneka M.T., O’Banion M.K. Inflammatory processes in Alzheimer’s disease. J. Neuroimmunol. 2007;184(1-2):69–91. doi: 10.1016/j.jneuroim.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 127.Tansey M.G., McCoy M.K., Frank-Cannon T.C. Neuroinflammatory mechanisms in Parkinson’s disease: potential environmental triggers, pathways, and targets for early therapeutic intervention. Exp. Neurol. 2007;208(1):1–25. doi: 10.1016/j.expneurol.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ma T., Tan M-S., Yu J-T., Tan L. Resveratrol as a therapeutic agent for Alzheimer’s disease. BioMed Res. Int. 2014;2014:350516. doi: 10.1155/2014/350516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Liu B., Hong J-S. Role of microglia in inflammation-mediated neurodegenerative diseases: mechanisms and strategies for therapeutic intervention. J. Pharmacol. Exp. Ther. 2003;304(1):1–7. doi: 10.1124/jpet.102.035048. [DOI] [PubMed] [Google Scholar]
- 130.Ghavami S. Shojaei, S.; Yeganeh, B.; Ande, S.R.; Jangamreddy, J.R.; Mehrpour, M.; Christoffersson, J.; Chaabane, W.; Moghadam, A.R.; Kashani, H.H.; Hashemi, M.; Owji, A.A.; Łos, M.J. Autophagy and apoptosis dysfunction in neurodegenerative disorders. Prog. Neurobiol. 2014;112:24–49. doi: 10.1016/j.pneurobio.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 131.Akiyama H., Barger S., Barnum S., Bradt B., Bauer J., Cole G.M., Cooper N.R., Eikelenboom P., Emmerling M., Fiebich B.L., Finch C.E., Frautschy S., Griffin W.S., Hampel H., Hull M., Landreth G., Lue L., Mrak R., Mackenzie I.R., McGeer P.L., O’Banion M.K., Pachter J., Pasinetti G., Plata-Salaman C., Rogers J., Rydel R., Shen Y., Streit W., Strohmeyer R., Tooyoma I., Van Muiswinkel F.L., Veerhuis R., Walker D., Webster S., Wegrzyniak B., Wenk G., Wyss-Coray T. Inflammation and Alzheimer’s disease. Neurobiol. Aging. 2000;21(3):383–421. doi: 10.1016/S0197-4580(00)00124-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gelders G., Baekelandt V., Van der Perren A. Linking Neuroinflammation and Neurodegeneration in Parkinson’s Disease. J. Immunol. Res. 2018;2018:4784268. doi: 10.1155/2018/4784268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.McGeer P.L., Itagaki S., Boyes B.E., McGeer E.G. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology. 1988;38(8):1285–1291. doi: 10.1212/WNL.38.8.1285. [DOI] [PubMed] [Google Scholar]
- 134.Cassarino D.S., Fall C.P., Swerdlow R.H., Smith T.S., Halvorsen E.M., Miller S.W., Parks J.P., Parker W.D., Jr, Bennett J.P. Jr Elevated reactive oxygen species and antioxidant enzyme activities in animal and cellular models of Parkinson’s disease. Biochim. Biophys. Acta. 1997;1362(1):77–86. doi: 10.1016/S0925-4439(97)00070-7. [DOI] [PubMed] [Google Scholar]
- 135.Chao C.C., Hu S., Molitor T.W., Shaskan E.G., Peterson P.K. Activated microglia mediate neuronal cell injury via a nitric oxide mechanism. J. Immunol. 1992;149(8):2736–2741. [PubMed] [Google Scholar]
- 136.Gao H-M., Hong J-S., Zhang W., Liu B. Distinct role for microglia in rotenone-induced degeneration of dopaminergic neurons. J. Neurosci. 2002;22(3):782–790. doi: 10.1523/JNEUROSCI.22-03-00782.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.McGuire S.O., Ling Z.D., Lipton J.W., Sortwell C.E., Collier T.J., Carvey P.M. Tumor necrosis factor α is toxic to embryonic mesencephalic dopamine neurons. Exp. Neurol. 2001;169(2):219–230. doi: 10.1006/exnr.2001.7688. [DOI] [PubMed] [Google Scholar]
- 138.Wu D.C., Jackson-Lewis V., Vila M., Tieu K., Teismann P., Vadseth C., Choi D.K., Ischiropoulos H., Przedborski S. Blockade of microglial activation is neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson disease. J. Neurosci. 2002;22(5):1763–1771. doi: 10.1523/JNEUROSCI.22-05-01763.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Stone D.K., Reynolds A.D., Mosley R.L., Gendelman H.E. Innate and adaptive immunity for the pathobiology of Parkinson’s disease. Antioxid. Redox Signal. 2009;11(9):2151–2166. doi: 10.1089/ars.2009.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Fossati S., Ghiso J., Rostagno A. Insights into caspase-mediated apoptotic pathways induced by amyloid-β; in cerebral microvascular endothelial cells. Neurodegener. Dis. 2012;10(1-4):324–328. doi: 10.1159/000332821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Newcombe E.A., Camats-Perna J., Silva M.L., Valmas N., Huat T.J., Medeiros R. Inflammation: the link between comorbidities, genetics, and Alzheimer’s disease. J. Neuroinflammation. 2018;15(1):276. doi: 10.1186/s12974-018-1313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Akundi R.S., Huang Z., Eason J., Pandya J.D., Zhi L., Cass W.A., Sullivan P.G., Büeler H. Increased mitochondrial calcium sensitivity and abnormal expression of innate immunity genes precede dopaminergic defects in Pink1-deficient mice. PLoS One. 2011;6(1):e16038. doi: 10.1371/journal.pone.0016038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gandhi S., Wood-Kaczmar A., Yao Z., Plun-Favreau H., Deas E., Klupsch K., Downward J., Latchman D.S., Tabrizi S.J., Wood N.W., Duchen M.R., Abramov A.Y. PINK1-associated Parkinson’s disease is caused by neuronal vulnerability to calcium-induced cell death. Mol. Cell. 2009;33(5):627–638. doi: 10.1016/j.molcel.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Heeman B., Van den Haute C., Aelvoet S.A., Valsecchi F., Rodenburg R.J., Reumers V., Debyser Z., Callewaert G., Koopman W.J., Willems P.H., Baekelandt V. Depletion of PINK1 affects mitochondrial metabolism, calcium homeostasis and energy maintenance. J. Cell Sci. 2011;124(Pt 7):1115–1125. doi: 10.1242/jcs.078303. [DOI] [PubMed] [Google Scholar]
- 145.Marongiu R., Spencer B., Crews L., Adame A., Patrick C., Trejo M., Dallapiccola B., Valente E.M., Masliah E. Mutant Pink1 induces mitochondrial dysfunction in a neuronal cell model of Parkinson’s disease by disturbing calcium flux. J. Neurochem. 2009;108(6):1561–1574. doi: 10.1111/j.1471-4159.2009.05932.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhang Q.S., Heng Y., Yuan Y.H., Chen N.H. Pathological α-synuclein exacerbates the progression of Parkinson’s disease through microglial activation. Toxicol. Lett. 2017;265:30–37. doi: 10.1016/j.toxlet.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 147.Skaper S.D., Facci L., Zusso M., Giusti P. An inflammation-centric view of neurological disease: Beyond the neuron. Front. Cell. Neurosci. 2018;12(72):72. doi: 10.3389/fncel.2018.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sproston N.R., Ashworth J.J. Role of C-reactive protein at sites of inflammation and infection. Front. Immunol. 2018;9(754):754. doi: 10.3389/fimmu.2018.00754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Luan Y.Y., Yao Y.M. The clinical significance and potential role of C-reactive protein in chronic inflammatory and neurodegenerative diseases. Front. Immunol. 2018;9:1302. doi: 10.3389/fimmu.2018.01302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Atassi N., Xu M., Triantafyllou C., Keil B., Lawson R., Cernasov P., Ratti E., Long C.J., Paganoni S., Murphy A., Salibi N., Seethamraju R., Rosen B., Ratai E.M. Ultra high-field (7tesla) magnetic resonance spectroscopy in Amyotrophic Lateral Sclerosis. PLoS One. 2017;12(5):e0177680. doi: 10.1371/journal.pone.0177680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Heath-Engel H.M., Chang N.C., Shore G.C. The endoplasmic reticulum in apoptosis and autophagy: role of the BCL-2 protein family. Oncogene. 2008;27(50):6419–6433. doi: 10.1038/onc.2008.309. [DOI] [PubMed] [Google Scholar]
- 152.Hetz C.A. ER stress signaling and the BCL-2 family of proteins: from adaptation to irreversible cellular damage. Antioxid. Redox Signal. 2007;9(12):2345–2355. doi: 10.1089/ars.2007.1793. [DOI] [PubMed] [Google Scholar]
- 153.McGeer P.L., McGeer E.G. Inflammatory processes in amyotrophic lateral sclerosis. Muscle Nerve. 2002;26(4):459–470. doi: 10.1002/mus.10191. [DOI] [PubMed] [Google Scholar]
- 154.Morrice J.R., Gregory-Evans C.Y., Shaw C.A. Necroptosis in amyotrophic lateral sclerosis and other neurological disorders. Biochim. Biophys. Acta Mol. Basis Dis. 2017;1863(2):347–353. doi: 10.1016/j.bbadis.2016.11.025. [DOI] [PubMed] [Google Scholar]
- 155.Raoul C., Barthelemy C., Couzinet A., Hancock D., Pettmann B., Hueber A.O. Expression of a dominant negative form of Daxx in vivo rescues motoneurons from Fas (CD95)-induced cell death. J. Neurobiol. 2005;62(2):178–188. doi: 10.1002/neu.20086. [DOI] [PubMed] [Google Scholar]
- 156.Raoul C., Estévez A.G., Nishimune H., Cleveland D.W., deLapeyrière O., Henderson C.E., Haase G., Pettmann B. Motoneuron death triggered by a specific pathway downstream of Fas. potentiation by ALS-linked SOD1 mutations. Neuron. 2002;35(6):1067–1083. doi: 10.1016/S0896-6273(02)00905-4. [DOI] [PubMed] [Google Scholar]
- 157.Oberst A. Death in the fast lane: what’s next for necroptosis? FEBS J. 2016;283(14):2616–2625. doi: 10.1111/febs.13520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Johann S., Heitzer M., Kanagaratnam M., Goswami A., Rizo T., Weis J., Troost D., Beyer C. NLRP3 inflammasome is expressed by astrocytes in the SOD1 mouse model of ALS and in human sporadic ALS patients. Glia. 2015;63(12):2260–2273. doi: 10.1002/glia.22891. [DOI] [PubMed] [Google Scholar]
- 159.Balentova S., Adamkov M. Molecular, cellular and functional effects of radiation-induced brain injury: A review. Int. J. Mol. Sci. 2015;16(11):27796–27815. doi: 10.3390/ijms161126068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Lumniczky K., Szatmári T., Sáfrány G. Ionizing radiation-induced immune and inflammatory reactions in the brain. Front. Immunol. 2017;8:517. doi: 10.3389/fimmu.2017.00517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Mattsson M-O., Simkó M. Is there a relation between extremely low frequency magnetic field exposure, inflammation and neurodegenerative diseases? A review of in vivo and in vitro experimental evidence. Toxicology. 2012;301(1-3):1–12. doi: 10.1016/j.tox.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 162.Sun A-M., Li C-G., Han Y-Q., Liu Q-L., Xia Q., Yuan Y-W. X-ray irradiation promotes apoptosis of hippocampal neurons through up-regulation of Cdk5 and p25. Cancer Cell Int. 2013;13(1):47. doi: 10.1186/1475-2867-13-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zuo H., Lin T., Wang D., Peng R., Wang S., Gao Y., Xu X., Zhao L., Wang S., Su Z. RKIP regulates neural cell apoptosis induced by exposure to microwave radiation partly through the MEK/ERK/CREB pathway. Mol. Neurobiol. 2014:51. doi: 10.1007/s12035-014-8831-5. [DOI] [PubMed] [Google Scholar]
- 164.Su Z., Sheng L., Yu P., Ren N., Li Y., Qin Z. Regulation of microRNAs by IRE1α in apoptosis: implications for the pathomechanism of neurodegenerative diseases. Int. J. Neurosci. 2020;130(12):1230–1236. doi: 10.1080/00207454.2020.1730833. [DOI] [PubMed] [Google Scholar]
- 165.Mansour S.Z., Hanafi N., Noaman E. Aluminium and gamma irradiation induced oxidative damage in brain tissue of male rats - protective role of ferulic acid. J. Radiat. Res. (Tokyo) 2011;4(4A):1163–1188. [Google Scholar]
- 166.Moradi S.Z., Momtaz S., Bayrami Z., Farzaei M.H., Abdollahi M. Nanoformulations of herbal extracts in treatment of neurodegenerative disorders. Front. Bioeng. Biotechnol. 2020;8:238. doi: 10.3389/fbioe.2020.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Costa R.P.O., Lucena L.F., Silva L.M.A., Zocolo G.J., Herrera-Acevedo C., Scotti L., Da-Costa F.B., Ionov N., Poroikov V., Muratov E.N., Scotti M.T. The SistematX web portal of natural products: An update. J. Chem. Inf. Model. 2021;61(6):2516–2522. doi: 10.1021/acs.jcim.1c00083. [DOI] [PubMed] [Google Scholar]
- 168.Wang J., Song Y., Chen Z., Leng S.X. Connection between systemic inflammation and neuroinflammation underlies neuroprotective mechanism of several phytochemicals in neurodegenerative diseases. Oxid. Med. Cell. Longev. 2018;2018:1972714. doi: 10.1155/2018/1972714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Asl J.F., Goudarzi M., Shoghi H. The radio-protective effect of rosmarinic acid against mobile phone and Wi-Fi radiation-induced oxidative stress in the brains of rats. Pharmacol. Rep. 2020;72(4):857–866. doi: 10.1007/s43440-020-00063-9. [DOI] [PubMed] [Google Scholar]
- 170.Fakhri S., Moradi S.Z., Farzaei M.H., Bishayee A. Modulation of dysregulated cancer metabolism by plant secondary metabolites: A mechanistic review. Semin. Cancer Biol., 2020;S1044- 579X(20):30040–7. doi: 10.1016/j.semcancer.2020.02.007. [DOI] [PubMed] [Google Scholar]
- 171.Rane J.S., Bhaumik P., Panda D. Curcumin inhibits tau aggregation and disintegrates preformed tau filaments in vitro. J. Alzheimers Dis. 2017;60(3):999–1014. doi: 10.3233/JAD-170351. [DOI] [PubMed] [Google Scholar]
- 172.Desai P.P., Patravale V.B. Curcumin cocrystal micelles-multifunctional nanocomposites for management of neurodegenerative ailments. J. Pharm. Sci. 2018;107(4):1143–1156. doi: 10.1016/j.xphs.2017.11.014. [DOI] [PubMed] [Google Scholar]
- 173.Xiao L., Ding M., Fernandez A., Zhao P., Jin L., Li X. Curcumin alleviates lumbar radiculopathy by reducing neuroinflammation, oxidative stress and nociceptive factors. Eur. Cell. Mater. 2017;33:279–293. doi: 10.22203/eCM.v033a21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Xie Y., Zhao Q.Y., Li H.Y., Zhou X., Liu Y., Zhang H. Curcumin ameliorates cognitive deficits heavy ion irradiation-induced learning and memory deficits through enhancing of Nrf2 antioxidant signaling pathways. Pharmacol. Biochem. Behav. 2014;126:181–186. doi: 10.1016/j.pbb.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 175.Shen G., Xu C., Hu R., Jain M.R., Gopalkrishnan A., Nair S., Huang M.T., Chan J.Y., Kong A.N. Modulation of nuclear factor E2-related factor 2-mediated gene expression in mice liver and small intestine by cancer chemopreventive agent curcumin. Mol. Cancer Ther. 2006;5(1):39–51. doi: 10.1158/1535-7163.MCT-05-0293. [DOI] [PubMed] [Google Scholar]
- 176.Verma V. Relationship and interactions of curcumin with radiation therapy. World J. Clin. Oncol. 2016;7(3):275–283. doi: 10.5306/wjco.v7.i3.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Soltani B., Ghaemi N., Sadeghizadeh M., Najafi F. Curcumin confers protection to irradiated THP-1 cells while its nanoformulation sensitizes these cells via apoptosis induction. Cell Biol. Toxicol. 2016;32(6):543–561. doi: 10.1007/s10565-016-9354-9. [DOI] [PubMed] [Google Scholar]
- 178.Okunieff P., Xu J., Hu D., Liu W., Zhang L., Morrow G., Pentland A., Ryan J.L., Ding I. Curcumin protects against radiation-induced acute and chronic cutaneous toxicity in mice and decreases mRNA expression of inflammatory and fibrogenic cytokines. Int. J. Radiat. Oncol. Biol. Phys. 2006;65(3):890–898. doi: 10.1016/j.ijrobp.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 179.Sundaram J.R., Poore C.P., Sulaimee N.H.B., Pareek T., Cheong W.F., Wenk M.R., Pant H.C., Frautschy S.A., Low C.M., Kesavapany S. Curcumin ameliorates neuroinflammation, neurodegeneration, and memory deficits in p25 transgenic mouse model that bears hallmarks of Alzheimer’s disease. J. Alzheimers Dis. 2017;60(4):1429–1442. doi: 10.3233/JAD-170093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Wang J., Song Y., Gao M., Bai X., Chen Z. Neuroprotective effect of several phytochemicals and its potential application in the prevention of neurodegenerative diseases. Geriatrics (Basel) 2016;1(4):29. doi: 10.3390/geriatrics1040029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wang H., Jiang T., Li W., Gao N., Zhang T. Resveratrol attenuates oxidative damage through activating mitophagy in an in vitro model of Alzheimer’s disease. Toxicol. Lett. 2018;282:100–108. doi: 10.1016/j.toxlet.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 182.Prager I., Patties I., Himmelbach K., Kendzia E., Merz F., Müller K., Kortmann R.D., Glasow A. Dose-dependent short- and long-term effects of ionizing irradiation on neural stem cells in murine hippocampal tissue cultures: neuroprotective potential of resveratrol. Brain Behav. 2016;6(10):e00548. doi: 10.1002/brb3.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Pannu N., Bhatnagar A. Resveratrol: from enhanced biosynthesis and bioavailability to multitargeting chronic diseases. Biomed. Pharmacother. 2019;109:2237–2251. doi: 10.1016/j.biopha.2018.11.075. [DOI] [PubMed] [Google Scholar]
- 184.Pandey K.B., Rizvi S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009;2(5):270–278. doi: 10.4161/oxim.2.5.9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Li J., Feng L., Xing Y., Wang Y., Du L., Xu C., Cao J., Wang Q., Fan S., Liu Q., Fan F. Radioprotective and antioxidant effect of resveratrol in hippocampus by activating Sirt1. Int. J. Mol. Sci. 2014;15(4):5928–5939. doi: 10.3390/ijms15045928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Lehrer S., Rheinstein P.H., Rosenzweig K.E. Association of radon background and total background ionizing radiation with Alzheimer’s disease deaths in US states. J. Alzheimers Dis. 2017;59(2):737–741. doi: 10.3233/JAD-170308. [DOI] [PubMed] [Google Scholar]
- 187.Arbo B.D., André-Miral C., Nasre-Nasser R.G., Schimith L.E., Santos M.G., Costa-Silva D., Muccillo-Baisch A.L., Hort M.A. Resveratrol derivatives as potential treatments for Alzheimer’s and Parkinson’s disease. Front. Aging Neurosci. 2020;12:103. doi: 10.3389/fnagi.2020.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Firouzi F., Khoei S., Mirzaei H.R. Role of resveratrol on the cytotoxic effects and DNA damages of iododeoxyuridine and megavoltage radiation in spheroid culture of U87MG glioblastoma cell line. Gen. Physiol. Biophys. 2015;34(1):43–50. doi: 10.4149/gpb_2014023. [DOI] [PubMed] [Google Scholar]
- 189.Wiciński, M.; Domanowska, A.; Wódkiewicz, E.; Malinowski, B. Neuroprotective properties of resveratrol and its derivatives-influence on potential mechanisms leading to the development of Alzheimer’s disease. Int. J. Mol. Sci. 2020;21(8):2749. doi: 10.3390/ijms21082749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Xu L., Yang X., Cai J., Ma J., Cheng H., Zhao K., Yang L., Cao Y., Qin Q., Zhang C., Zhang Q., Sun X. Resveratrol attenuates radiation-induced salivary gland dysfunction in mice. Laryngoscope. 2013;123(11):E23–E29. doi: 10.1002/lary.24276. [DOI] [PubMed] [Google Scholar]
- 191.Yin H., Si J., Xu H., Dong J., Zheng D., Lu X., Li X. Resveratrol-loaded nanoparticles reduce oxidative stress induced by radiation or amyloid-beta in transgenic Caenorhabditis elegans. J. Biomed. Nanotechnol. 2014;10(8):1536–1544. doi: 10.1166/jbn.2014.1897. [DOI] [PubMed] [Google Scholar]
- 192.Wang W., Wang S., Liu T., Ma Y., Huang S., Lei L., Wen A., Ding Y. Resveratrol: Multi-targets mechanism on neurodegenerative diseases based on network pharmacology. Front. Pharmacol. 2020;11:694. doi: 10.3389/fphar.2020.00694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Rifaai R.A., Mokhemer S.A., Saber E.A., El-Aleem S.A.A., El-Tahawy N.F.G. Neuroprotective effect of quercetin nanoparticles: A possible prophylactic and therapeutic role in alzheimer’s disease. J. Chem. Neuroanat. 2020;107:101795. doi: 10.1016/j.jchemneu.2020.101795. [DOI] [PubMed] [Google Scholar]
- 194.Kong Y., Li K., Fu T., Wan C., Zhang D., Song H., Zhang Y., Liu N., Gan Z., Yuan L. Quercetin ameliorates Aβ toxicity in Drosophila AD model by modulating cell cycle-related protein expression. Oncotarget. 2016;7(42):67716–67731. doi: 10.18632/oncotarget.11963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Sabogal-Guáqueta A.M., Muñoz-Manco J.I., Ramírez-Pineda J.R., Lamprea-Rodriguez M., Osorio E., Cardona-Gómez G.P. The flavonoid quercetin ameliorates Alzheimer’s disease pathology and protects cognitive and emotional function in aged triple transgenic Alzheimer’s disease model mice. Neuropharmacology. 2015;93:134–145. doi: 10.1016/j.neuropharm.2015.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Bagheri H., Ghasemi F., Barreto G.E., Rafiee R., Sathyapalan T., Sahebkar A. Effects of curcumin on mitochondria in neurodegenerative diseases. Biofactors. 2020;46(1):5–20. doi: 10.1002/biof.1566. [DOI] [PubMed] [Google Scholar]
- 197.Yu X., Li Y., Mu X. Effect of Quercetin on PC12 Alzheimer’s Disease Cell Model Induced by Aβ25-35 and Its Mechanism Based on Sirtuin1/Nrf2/HO-1 Pathway. Biomed Res Int, 2020, . 2020. [DOI] [PMC free article] [PubMed]
- 198.Kale A. Pi&kin, Ö.; Ba& Y.; Aydin, B.G.; Can, M.; Elmas, Ö.; Büyükuysal, Ç. Neuroprotective effects of Quercetin on radiation-induced brain injury in rats. J. Radiat. Res. (Tokyo) 2018;59(4):404–410. doi: 10.1093/jrr/rry032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Chatterjee J., Langhnoja J., Pillai P.P., Mustak M.S. Neuroprotective effect of quercetin against radiation-induced endoplasmic reticulum stress in neurons. J. Biochem. Mol. Toxicol. 2019;33(2):e22242. doi: 10.1002/jbt.22242. [DOI] [PubMed] [Google Scholar]
- 200.Picone P., Nuzzo D., Di Carlo M. Ferulic acid: a natural antioxidant against oxidative stress induced by oligomeric A-beta on sea urchin embryo. Biol. Bull. 2013;224(1):18–28. doi: 10.1086/BBLv224n1p18. [DOI] [PubMed] [Google Scholar]
- 201.Said U.Z., Saada H.N., Amin A.M., Abdalla M.S., Elsayed E.M. Efficacy of hesperidin in modulating radiation-induced brain damage in rats. J. Radiat. Res. (Tokyo) 2012;5(1):71–85. [Google Scholar]
- 202.Said U.Z., Saada H.N., Abd-Alla M.S., Elsayed M.E., Amin A.M. Hesperidin attenuates brain biochemical changes of irradiated rats. Int. J. Radiat. Biol. 2012;88(8):613–618. doi: 10.3109/09553002.2012.694008. [DOI] [PubMed] [Google Scholar]
- 203.Kale A. Pi&kin, Ö.; Ba& Y.; Ayd&n, B.G.; Can, M.; Elmas, Ö.; Büyükuysal, Ç. Ameliorative effects of Hesperidin on radiation induced brain injury in rats. Int. J. Radiat. Biol. 2019;17(2):229–236. [Google Scholar]
- 204.Thabet N.M., Moustafa E.M. Protective effect of rutin against brain injury induced by acrylamide or gamma radiation: role of PI3K/AKT/GSK-3&/NRF-2 signalling pathway. Arch. Physiol. Biochem. 2018;124(2):185–193. doi: 10.1080/13813455.2017.1374978. [DOI] [PubMed] [Google Scholar]
- 205.Sunada S., Fujisawa H., Cartwright I.M., Maeda J., Brents C.A., Mizuno K., Aizawa Y., Kato T.A., Uesaka M. Monoglucosyl-rutin as a potential radioprotector in mammalian cells. Mol. Med. Rep. 2014;10(1):10–14. doi: 10.3892/mmr.2014.2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Fischer N., Seo E-J., Efferth T. Prevention from radiation damage by natural products. Phytomedicine. 2018;47:192–200. doi: 10.1016/j.phymed.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 207.Patil S.L., Rao N.B., Somashekarappa H.M., Rajashekhar K.P. Antigenotoxic potential of rutin and quercetin in Swiss mice exposed to gamma radiation. Biomed. J. 2014;37(5):305–313. doi: 10.4103/2319-4170.132880. [DOI] [PubMed] [Google Scholar]
- 208.Sajjad N., Wani A., Sharma A., Ali R., Hassan S., Hamid R., Habib H., Ganai B.A. Artemisia amygdalina upregulates Nrf2 and protects neurons against oxidative stress in Alzheimer disease. Cell. Mol. Neurobiol. 2019;39(3):387–399. doi: 10.1007/s10571-019-00656-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Alsherbiny M.A., Abd-Elsalam W.H., El Badawy S.A., Taher E., Fares M., Torres A., Chang D., Li C.G. Ameliorative and protective effects of ginger and its main constituents against natural, chemical and radiation-induced toxicities: A comprehensive review. Food Chem. Toxicol. 2019;123:72–97. doi: 10.1016/j.fct.2018.10.048. [DOI] [PubMed] [Google Scholar]
- 210.Tarozzi A., Angeloni C., Malaguti M., Morroni F., Hrelia S., Hrelia P. Sulforaphane as a potential protective phytochemical against neurodegenerative diseases. Oxid. Med. Cell. Longev. 2013;2013:415078. doi: 10.1155/2013/415078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Itoh K., Chiba T., Takahashi S., Ishii T., Igarashi K., Katoh Y., Oyake T., Hayashi N., Satoh K., Hatayama I., Yamamoto M., Nabeshima Y. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun. 1997;236(2):313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- 212.Talalay P., De Long M.J., Prochaska H.J. Identification of a common chemical signal regulating the induction of enzymes that protect against chemical carcinogenesis. Proc. Natl. Acad. Sci. USA. 1988;85(21):8261–8265. doi: 10.1073/pnas.85.21.8261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Fakhri S., Patra J.K., Das S.K., Das G., Majnooni M.B., Farzaei M.H. Ginger and heart health: from mechanisms to therapeutics. Curr. Mol. Pharmacol. 2020 doi: 10.2174/1874467213666201209105005. [DOI] [PubMed] [Google Scholar]
- 214.Rao B.N., Rao B.S., Aithal B.K., Kumar M.R. Radiomodifying and anticlastogenic effect of Zingerone on Swiss albino mice exposed to whole body gamma radiation. Mutat. Res. 2009;677(1-2):33–41. doi: 10.1016/j.mrgentox.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 215.Rao B.N., Archana P.R., Aithal B.K., Rao B.S.S. Protective effect of zingerone, a dietary compound against radiation induced genetic damage and apoptosis in human lymphocytes. Eur. J. Pharmacol. 2011;657(1-3):59–66. doi: 10.1016/j.ejphar.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 216.Spagnuolo C., Moccia S., Russo G.L. Anti-inflammatory effects of flavonoids in neurodegenerative disorders. Eur. J. Med. Chem. 2018;153:105–115. doi: 10.1016/j.ejmech.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 217.Gandhi N.M. Baicalein protects mice against radiation-induced DNA damages and genotoxicity. Mol. Cell. Biochem. 2013;379(1-2):277–281. doi: 10.1007/s11010-013-1649-z. [DOI] [PubMed] [Google Scholar]
- 218.Mansour S.Z., Moawed F.S.M., Elmarkaby S.M. Protective effect of 5, 7-dihydroxyflavone on brain of rats exposed to acrylamide or &-radiation. J. Photochem. Photobiol. B. 2017;175:149–155. doi: 10.1016/j.jphotobiol.2017.08.034. [DOI] [PubMed] [Google Scholar]
- 219.Rehman M.U., Wali A.F., Ahmad A., Shakeel S., Rasool S., Ali R., Rashid S.M., Madkhali H., Ganaie M.A., Khan R. Neuroprotective strategies for neurological disorders by natural products: An update. Curr. Neuropharmacol. 2019;17(3):247–267. doi: 10.2174/1570159X16666180911124605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Zhao H., Zhu W., Jia L., Sun X., Chen G., Zhao X., Li X., Meng X., Kong L., Xing L., Yu J. Phase I study of topical epigallocatechin-3-gallate (EGCG) in patients with breast cancer receiving adjuvant radiotherapy. Br. J. Radiol. 2016;89(1058):20150665. doi: 10.1259/bjr.20150665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Li J. Neuroprotective effect of (-)-epigallocatechin-3-gallate on autoimmune thyroiditis in a rat model by an anti-inflammation effect, anti-apoptosis and inhibition of TRAIL signaling pathway. Exp. Ther. Med. 2018;15(1):1087–1092. doi: 10.3892/etm.2017.5511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Yang S-W., Lee B.R., Koh J-W. Protective effects of epigallocatechin gallate after UV irradiation in cultured human retinal pigment epithelial cells. Korean J. Ophthalmol. 2007;21(4):232–237. doi: 10.3341/kjo.2007.21.4.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Mitrica R., Dumitru I., Ruta L.L., Ofiteru A.M., Farcasanu I.C. The dual action of epigallocatechin gallate (EGCG), the main constituent of green tea, against the deleterious effects of visible light and singlet oxygen-generating conditions as seen in yeast cells. Molecules. 2012;17(9):10355–10369. doi: 10.3390/molecules170910355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Richi B., Kale R.K., Tiku A.B. Radio-modulatory effects of green tea catechin EGCG on pBR322 plasmid DNA and murine splenocytes against gamma-radiation induced damage. Mutat. Res. 2012;747(1):62–70. doi: 10.1016/j.mrgentox.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 225.Nam S., Smith D.M., Dou Q.P. Ester bond-containing tea polyphenols potently inhibit proteasome activity in vitro and in vivo. J. Biol. Chem. 2001;276(16):13322–13330. doi: 10.1074/jbc.M004209200. [DOI] [PubMed] [Google Scholar]
- 226.Alkis H., Kuzhan A., Dirier A., Tarakcioglu M., Demir E., Saricicek E., Demir T., Ahlatci A., Demirci A., Cinar K. Neuroprotective effects of propolis and caffeic acid phenethyl ester (CAPE) on the radiation-injured brain tissue (Neuroprotective effects of propolis and CAPE). Int J Radiat Res. 2015;13(4):297–303. [Google Scholar]
- 227.Calikoglu M., Tamer L., Sucu N., Coskun B., Ercan B., Gul A., Calikoglu I., Kanik A. The effects of caffeic acid phenethyl ester on tissue damage in lung after hindlimb ischemia-reperfusion. Pharmacol. Res. 2003;48(4):397–403. doi: 10.1016/S1043-6618(03)00156-7. [DOI] [PubMed] [Google Scholar]
- 228.Cikman O., Taysi S., Gulsen M.T., Demir E., Akan M., Diril H., Kiraz H.A., Karaayvaz M., Tarakcioglu M. The radio-protective effects of caffeic acid phenethyl ester and thymoquinone in rats exposed to total head irradiation. Wien. Klin. Wochenschr. 2015;127(3-4):103–108. doi: 10.1007/s00508-014-0635-0. [DOI] [PubMed] [Google Scholar]
- 229.Sharman M.J., Verdile G., Kirubakaran S., Parenti C., Singh A., Watt G., Karl T., Chang D., Li C.G., Münch G. Targeting inflammatory pathways in Alzheimer’s disease: A focus on natural products and phytomedicines. CNS Drugs. 2019;33(5):457–480. doi: 10.1007/s40263-019-00619-1. [DOI] [PubMed] [Google Scholar]
- 230.Raškovi& A.; Gigov, S.; &apo, I.; Paut Kusturica, M.; Milijaševi& B.; Koji&-Damjanov, S.; Marti& N. Antioxidative and protective actions of apigenin in a paracetamol-induced hepatotoxicity rat model. Eur. J. Drug Metab. Pharmacokinet. 2017;42(5):849–856. doi: 10.1007/s13318-017-0407-0. [DOI] [PubMed] [Google Scholar]
- 231.Rithidech K.N., Tungjai M., Whorton E.B. Protective effect of apigenin on radiation-induced chromosomal damage in human lymphocytes. Mutat. Res. 2005;585(1-2):96–104. doi: 10.1016/j.mrgentox.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 232.Taheri A., Rostamzadeh A., Gharib A., Fatehi D. Radioprotective effects of Silymarin, a natural medical herb, in modulation and prevention of radiation induced damages. Pharm. Lett. 2016;8(9):146–150. [Google Scholar]
- 233.Son Y., Lee H.J., Rho J.K., Chung S.Y., Lee C.G., Yang K., Kim S.H., Lee M., Shin I.S., Kim J.S. The ameliorative effect of silibinin against radiation-induced lung injury: protection of normal tissue without decreasing therapeutic efficacy in lung cancer. BMC Pulm. Med. 2015;15(1):68. doi: 10.1186/s12890-015-0055-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Adhikari M., Arora R. The flavonolignan-silymarin protects enzymatic, hematological, and immune system against &-radiation-induced toxicity. Environ. Toxicol. 2016;31(6):641–654. doi: 10.1002/tox.22076. [DOI] [PubMed] [Google Scholar]
- 235.Fu H., Lin M., Katsumura Y., Yokoya A., Hata K., Muroya Y., Fujii K., Shikazono N. Protective effects of silybin and analogues against X-ray radiation-induced damage. Acta Biochim. Biophys. Sin. (Shanghai) 2010;42(7):489–495. doi: 10.1093/abbs/gmq045. [DOI] [PubMed] [Google Scholar]
- 236.Wang H., Xu Y.S., Wang M.L., Cheng C., Bian R., Yuan H., Wang Y., Guo T., Zhu L.L., Zhou H. Protective effect of naringin against the LPS-induced apoptosis of PC12 cells: Implications for the treatment of neurodegenerative disorders. Int. J. Mol. Med. 2017;39(4):819–830. doi: 10.3892/ijmm.2017.2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Heidary Moghaddam R., Samimi Z., Moradi S.Z., Little P.J., Xu S., Farzaei M.H. Naringenin and naringin in cardiovascular disease prevention: A preclinical review. Eur. J. Pharmacol. 2020;887:173535. doi: 10.1016/j.ejphar.2020.173535. [DOI] [PubMed] [Google Scholar]
- 238.Archana P.R., Nageshwar Rao B., Satish Rao B.S. Modulation of gamma ray-induced genotoxic effect by thymol, a monoterpene phenol derivative of cymene. Integr. Cancer Ther. 2011;10(4):374–383. doi: 10.1177/1534735410387421. [DOI] [PubMed] [Google Scholar]
- 239.Abedi S.M., Yarmand F., Motallebnejad M., Seyedmajidi M., Moslemi D., Bijani A., Hosseinimehr S.J. Radioprotective effect of thymol against salivary glands dysfunction induced by ionizing radiation in rats. Iran. J. Pharm. Res. 2016;15(4):861–866. [PMC free article] [PubMed] [Google Scholar]
- 240.Nair S., Nair R.R.K., Srinivas P., Srinivas G., Pillai M.R. Radiosensitizing effects of plumbagin in cervical cancer cells is through modulation of apoptotic pathway. Mol. Carcinog. 2008;47(1):22–33. doi: 10.1002/mc.20359. [DOI] [PubMed] [Google Scholar]
- 241.Moradi S.Z., Nowroozi A., Sadrjavadi K., Moradi S., Mansouri K., Hosseinzadeh L., Shahlaei M. Direct evidences for the groove binding of the Clomifene to double stranded DNA. Int. J. Biol. Macromol. 2018;114:40–53. doi: 10.1016/j.ijbiomac.2018.03.040. [DOI] [PubMed] [Google Scholar]
- 242.Brunetti G., Di Rosa G., Scuto M., Leri M., Stefani M., Schmitz-Linneweber C., Calabrese V., Saul N. Healthspan maintenance and prevention of Parkinson’s-like phenotypes with hydroxytyrosol and oleuropein aglycone in C. elegans. Int. J. Mol. Sci. 2020;21(7):2588. doi: 10.3390/ijms21072588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.D’Angelo S., Ingrosso D., Migliardi V., Sorrentino A., Donnarumma G., Baroni A., Masella L., Tufano M.A., Zappia M., Galletti P. Hydroxytyrosol, a natural antioxidant from olive oil, prevents protein damage induced by long-wave ultraviolet radiation in melanoma cells. Free Radic. Biol. Med. 2005;38(7):908–919. doi: 10.1016/j.freeradbiomed.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 244.Alothman M., Bhat R., Karim A. Effects of radiation processing on phytochemicals and antioxidants in plant produce. Trends Food Sci. Technol. 2009;20(5):201–212. doi: 10.1016/j.tifs.2009.02.003. [DOI] [Google Scholar]
- 245.Oboh G., Adedayo B.C., Adetola M.B., Oyeleye I.S., Ogunsuyi O.B. Characterization and neuroprotective properties of alkaloid extract of Vernonia amygdalina Delile in experimental models of Alzheimer’s disease. Drug Chem. Toxicol. 2020:1–10. doi: 10.1080/01480545.2020.1773845. [DOI] [PubMed] [Google Scholar]
- 246.Zhu X.Z., Li X-Y., Liu J. Recent pharmacological studies on natural products in China. Eur. J. Pharmacol. 2004;500(1-3):221–230. doi: 10.1016/j.ejphar.2004.07.027. [DOI] [PubMed] [Google Scholar]
- 247.Zhang Y., Gao L., Cheng Z., Cai J., Niu Y., Meng W., Zhao Q., Kukoamine A. Kukoamine A prevents radiation-induced neuroinflammation and preserves hippocampal neurogenesis in rats by inhibiting activation of NF-&B and AP-1. Neurotox. Res. 2017;31(2):259–268. doi: 10.1007/s12640-016-9679-4. [DOI] [PubMed] [Google Scholar]
- 248.Lee T.C., Greene-Schloesser D., Payne V., Diz D.I., Hsu F.C., Kooshki M., Mustafa R., Riddle D.R., Zhao W., Chan M.D., Robbins M.E. Chronic administration of the angiotensin-converting enzyme inhibitor, ramipril, prevents fractionated whole-brain irradiation-induced perirhinal cortex-dependent cognitive impairment. Radiat. Res. 2012;178(1):46–56. doi: 10.1667/RR2731.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Raju U., Gumin G.J., Tofilon P.J. NF kappa B activity and target gene expression in the rat brain after one and two exposures to ionizing radiation. Radiat. Oncol. Investig. 1999;7(3):145–152. doi: 10.1002/(SICI)1520-6823(1999)7:3<145:AID-ROI2>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 250.Zhang Y., Cheng Z., Wang C., Ma H., Meng W., Zhao Q. Neuroprotective effects of kukoamine a against radiation-induced rat brain injury through inhibition of oxidative stress and neuronal apoptosis. Neurochem. Res. 2016;41(10):2549–2558. doi: 10.1007/s11064-016-1967-0. [DOI] [PubMed] [Google Scholar]
- 251.Kurinnyi D., Rushkovsky S., Demchenko O., Pilinska M. Astaxanthin as a modifier of genome instability after &-radiation. Progress Carotenoid Res. 2018;2018:121–138. doi: 10.5772/intechopen.79341. [DOI] [Google Scholar]
- 252.Xue X-L., Han X-D., Li Y., Chu X-F., Miao W-M., Zhang J-L., Fan S-J. Astaxanthin attenuates total body irradiation-induced hematopoietic system injury in mice via inhibition of oxidative stress and apoptosis. Stem Cell Res. Ther. 2017;8(1):7. doi: 10.1186/s13287-016-0464-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Fakhri S., Nouri Z., Moradi S.Z., Farzaei M.H. Astaxanthin, COVID-19 and immune response: Focus on oxidative stress, apoptosis and autophagy. Phytother. Res. 2020;34(11):2790–2792. doi: 10.1002/ptr.6797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Cao Z., Wang P., Gao X., Shao B., Zhao S., Li Y. Lycopene attenuates aluminum-induced hippocampal lesions by inhibiting oxidative stress-mediated inflammation and apoptosis in the rat. J. Inorg. Biochem. 2019;193:143–151. doi: 10.1016/j.jinorgbio.2019.01.017. [DOI] [PubMed] [Google Scholar]
- 255.Naoi M., Shamoto-Nagai M., Maruyama W. Neuroprotection of multifunctional phytochemicals as novel therapeutic strategy for neurodegenerative disorders: Antiapoptotic and antiamyloidogenic activities by modulation of cellular signal pathways. Future Neurol. 2019;14:FNL9. doi: 10.2217/fnl-2018-0028. [DOI] [Google Scholar]
- 256.Zhao J., Moore A.N., Redell J.B., Dash P.K. Enhancing expression of Nrf2-driven genes protects the blood brain barrier after brain injury. J. Neurosci. 2007;27(38):10240–10248. doi: 10.1523/JNEUROSCI.1683-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Zhao X., Sun G., Zhang J., Strong R., Dash P.K., Kan Y.W., Grotta J.C., Aronowski J. Transcription factor Nrf2 protects the brain from damage produced by intracerebral hemorrhage. Stroke. 2007;38(12):3280–3286. doi: 10.1161/STROKEAHA.107.486506. [DOI] [PubMed] [Google Scholar]
- 258.Talalay P., Fahey J.W., Healy Z.R., Wehage S.L., Benedict A.L., Min C., Dinkova-Kostova A.T. Sulforaphane mobilizes cellular defenses that protect skin against damage by UV radiation. Proc. Natl. Acad. Sci. USA. 2007;104(44):17500–17505. doi: 10.1073/pnas.0708710104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Ullah F., Liang A., Rangel A., Gyengesi E., Niedermayer G., Münch G. High bioavailability curcumin: an anti-inflammatory and neurosupportive bioactive nutrient for neurodegenerative diseases characterized by chronic neuroinflammation. Arch. Toxicol. 2017;91(4):1623–1634. doi: 10.1007/s00204-017-1939-4. [DOI] [PubMed] [Google Scholar]
- 260.Tang F.R., Loke W.K., Wong P., Khoo B.C. Radioprotective effect of ursolic acid in radiation-induced impairment of neurogenesis, learning and memory in adolescent BALB/c mouse. Physiol. Behav. 2017;175:37–46. doi: 10.1016/j.physbeh.2017.03.027. [DOI] [PubMed] [Google Scholar]