Abstract
Fluorosulfuryl isocyanate (FSI, FSO2NCO) is established as a reliable bis-electrophilic linker for stepwise attachment of an alcohol bearing module to an amine bearing module and thence a new module RO-C(=O)-NH-SO2-NR’R” is created. FSI’s isocyanate motif fuses directly and quickly with alcohols and phenols, affording fluorosulfuryl carbamates in nearly quantitative yield. A new reagent and process to deliver the FSI-derived fluorosulfuryl carbamate fragment to amines are also developed. The resulting SVI-F motifs from step-1 are remarkably stable, given the great structural complexities in diverse products. In the step-2 reaction with amines, the best yield of the S-N linked products arise with water alone. This “on water” interfacial reactivity phenomenon is crucial, revealing the latent reactivity of SVI-F probe for potential covalent capture of proteins in vivo which is important in today’s drug discovery. The scope of the SuFEx chemistry is largely expanded thereby and the facile entry to these phosphate-like connections should prove useful to click chemistry across diverse fields.
Keywords: Click Chemistry, SuFEx reaction, Fluorosulfuryl Isocyanate, on water, Carbamate, Urea
Graphical Abstract
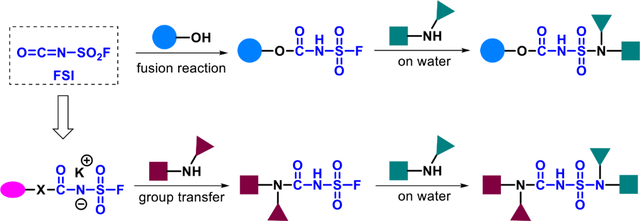
SuFEx Ligation on Water: Fluorosulfuryl isocyanate (FSI, FSO2-NCO) is established as a reliable bis-electrophilic linker for stepwise attachment of alcohol and amine bearing modules. In step-1, FSI’s isocyanate fuses directly and quickly with alcohols and phenols, and aliphatic amines are attached through the sulfuryl carbamate transfer strategy, both with remarkable selectivity. In step-2, best yields of S-N linked products arise with a unique on water process. The scope of SuFEx ligation is greatly expanded in directions which would be especially interesting for drug discovery and chemical biology.
Generating substances through modular synthesis accelerates the screening and discovery of functions. Over the last two decades, the central theme of click chemistry was to explore good reactions that meet the stringent requirements of modular synthesis, such as simple operation, inoffensive product, high selectivity and efficiency.[1] These reactions have found wide applications in materials science,[2] chemical biology,[3] medicinal chemistry, etc.[4] The sulfur(VI) fluoride exchange (SuFEx) click reaction was recently introduced,[5] and quickly its robust utility has been demonstrated.[6] In a typical SuFEx protocol, substrates are modified by a small linker, such as CH2=CHSO2F,[7] SO2F2,[5] SOF4.[8] The resulting SVI-F handles enable a second and even a third ligation through defluorination substitution with another nucleophiles.[5] The success of SuFEx ligations has exquisite requirement for the reaction environment due to the unique reactivity of SVI-F handles. It has been demonstrated in chemical biology events where SuFEx handles serve as “warheads” for covalent capture of proteins.[6c,6d]
To expand the SuFEx platform, we are looking for new linker capable of diverse connections. The fluorosulfuryl isocyanate (FSI) draws our attention because of its versatile chemistry empowered by the “O=C=N” cumulene motif.[9–12] The reaction between isocyanate and nucleophiles R-XH (X = O or NR’) is zero waste-emission. Meanwhile, a “-SO2F” group is delivered to the targeted substrates that allows for further connection. Modules including alcohols (not well suited to previous SuFEx ligations, Figure 1), phenols, and amines are abundant with great structural diversity. The sulfuryl carbamate and urea adducts are also potential phosphate mimics because they are chemically and physically (pKa value) alike, and these features are essential for fundamental functions in life systems (Figure 1).[13]
Figure 1.
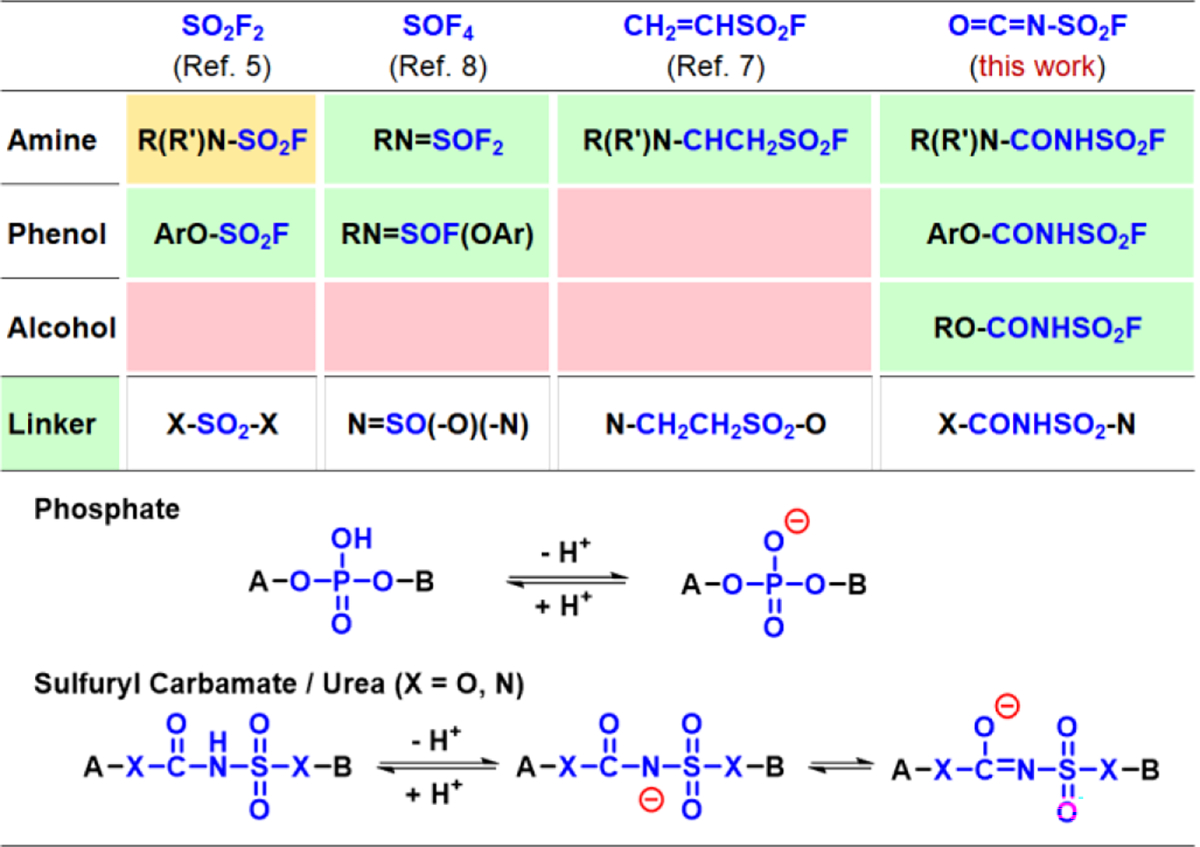
Summary of SuFEx linkers, scopes and their properties (colors of each column reflect the ligation efficiency. Red represents inferior reactivity, green represents excellent, and yellow represents limited substrate scope).
First reported in 1950s, FSI is derived from chlorosulfuryl isocyanate (CSI) through halogen exchange reaction.[14] Subtle reactivity difference between these two molecules could be found in Graf’s early remarks, quote, “It (FSI) can in principle be used in the same way as CSI, but generally reacts a little more slowly. The N-sulfonyl fluorides obtained in the reaction with FSI are generally much more stable to hydrolysis and to heat than the N-sulfonyl chlorides.”[15,16] Indeed, this feature is what SuFEx chemistry appreciates as has been discussed in our 2014 manuscript.[5] Herein, we revisited the reactivity of FSI, aiming to develop a modular SuFEx ligation process.
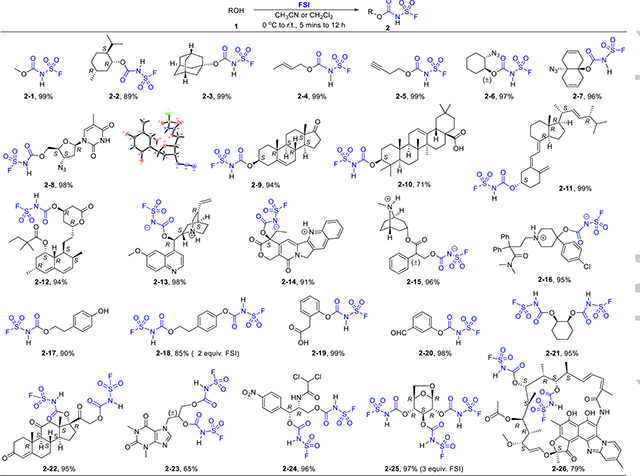
We prepared FSI on half kilogram scale by refluxing CSI with excess sodium fluoride in neat (b.p. 62–65 °C, Supporting information S4).[17] It is moisture sensitive but thermally stable up to 300 °C.[18] Alkyl and phenyl isocyanates could react with alcohols to afford carbamates with the aid of tertiary amines as base and catalyst.[9b,19] But FSI is more reactive because of the strong electron-withdrawing sulfonyl fluoride group, thus direct reaction with alcohols gave nearly quantitative yields within minutes in cold acetonitrile.[14] The selectivity of FSI for aliphatic alcohol groups (1°, 2°, and even 3°, Table 1) over other functional groups we found to be extraordinary. With substrates bearing a tertiary amine, inner salts precipitated instantly upon the addition of FSI, due to the low pKa of the resulting carbamates.[14b] Using this precipitation process, we were able to get a group of bioactive compounds modified and purified simply through filtration (2–13 – 2–16).
Table 1.
Step-1 direct ligation of alcohols and phenols with FSI.
|
Reaction conditions: ROH (1.0 equiv), FSI (1.0 equiv, and 2.0 equiv FSI for diols), CH3CN (or CH2Cl2), 0 °C to r.t., 5 mins to 12 h.
Phenols were reported to react with CSI, giving RO-SO2-NCO as the major product through cleaving the SVI-Cl bond at elevated temperature.[15] But only RO-CONHSO2F carbamate was obtained under identical reaction conditions with FSI, a clear evidence for the special stability of the SVI-F bond. Selective modification of alcohol over phenol gave adduct 2–17 with one equivalent of FSI. On the other hand, the difunctionalized product 2–18 could be obtained when two equivalents of FSI were employed. Full conversion of bioactive diols and triols to the fluorosulfuryl carbamates were also realized with excess amounts of FSI (2–21 – 2–26).
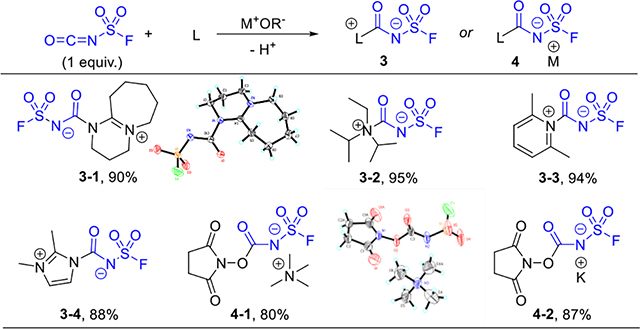
The direct reaction between anilines and FSI worked well and afforded fluorosulfuryl ureas in good to excellent yields (Supporting information S28).[20] But primary and secondary aliphatic amines generally gave mixtures. On the other hand, some tertiary amines afforded zwitterions with isocyanates. These zwitterions were reported as key intermediates in the isocyanate dimerization or polymerization,[17,19] and they were also able to transfer the carbamate fragment to another nucleophiles under certain circumstances.[21] Rolf etc. prepared a few zwitterions from FSI, but did not investigate their synthetic utility.[22] We envisioned these salts might be useful to modify primary and secondary aliphatic amines by taking advantage of the carbamate group transfer event.
A series of addition adducts from FSI and ligands were prepared accordingly (Table 2). Although many decomposed at their melting points, most salts were bench-stable for months. We then evaluated their carbamate transfer ability with primary and secondary amines. The potassium salt 4–2 proved to be the most promising candidate. With slight excess amounts of 4–2 in acetonitrile, various amines were converted to ureas in good to excellent yields (Table 3). The ligation was highly selective for amines over other functional groups, such as carboxylic acid, alcohol, phenol, indole, alkene, and alkyne. The drugs Linagliptin (6–8) and Alogliptin (6–9) were readily modified in this manner.
Table 2.
Salts formed between FSI and ligands (L).[a]
|
FSI (2–5 mmol), L (1 equiv.), 1 mol/L in CH2Cl2 or toluene, 0 °C to r.t., 2 h.
Table 3.
Step-1 amine modification by salt 4-2.[a]
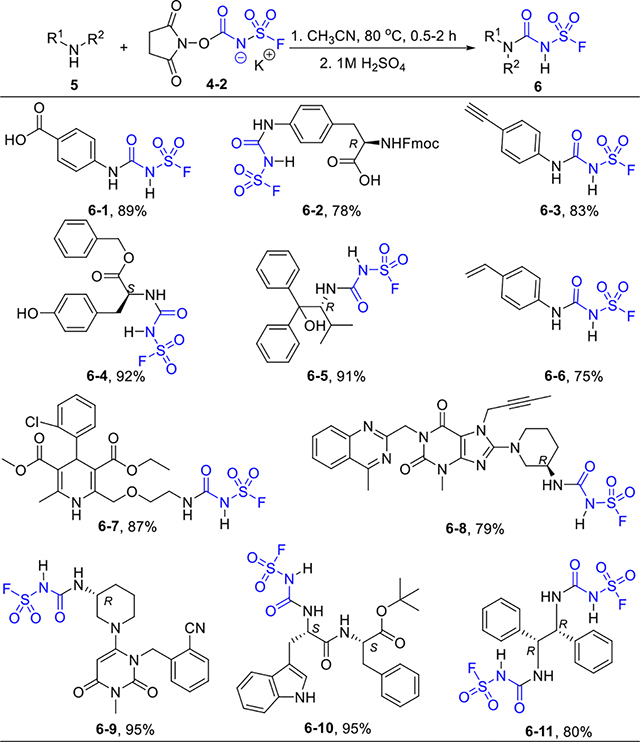
|
5 (0.5–1.0 mmol), 4-2 (1.2 equiv), 0.2 mol/L in CH3CN, 80 °C, 0.5–2 h.
Both sulfuryl carbamate and urea (-CONHSO2X, X = F, Cl) undergo deprotonation in the presence of base due to the low pKa of the N-H bond. The chlorosulfuryl motif would then eliminate chloride to afford an azasulfene (-N=SO2) that is highly reactive and liable to hydrolysis. Therefore, anhydrous reaction conditions are usually used for its step-2 ligation with nucleophiles.[23–25] In contrast, the fluorosulfuryl motif would remain relatively stable. No ligation was observed even when it was treated with ArOTBS and DBU in dry acetonitrile.[5]
After extensive screening of the reaction conditions, we found substitution of the SVI-F with amines could be achieved on water (Table 4).[26,27] The functional group compatibility was excellent on both sides of the substrates, such as the carboxylic acid (9–1), heterocycles (9–2), alcohols (9–2, 9–6), olefins (9–4, 9–5), and the cyano groups (9–10). Aniline (9–6) and hydrazine derivatives (9–9) also afforded the desired ligation with high efficiency. This would be highly valuable for drug conjugation.[28] Meanwhile, fluorosulfuryl carbamates and ureas share similar features with phosphate which are important for fundamental functions in life systems.[13] They were both acidic with pKa values around 2.0,[14b] relatively stable but remained active for further ligation with water as supporting medium. In this context, it is intriguing to further explore the fluorosulfuryl carbamates and ureas as phosphate mimics and covalent probes in drug discovery and chemical biology.[29]
Table 4.
Step-2 SuFEx ligation with amines on water.[a]
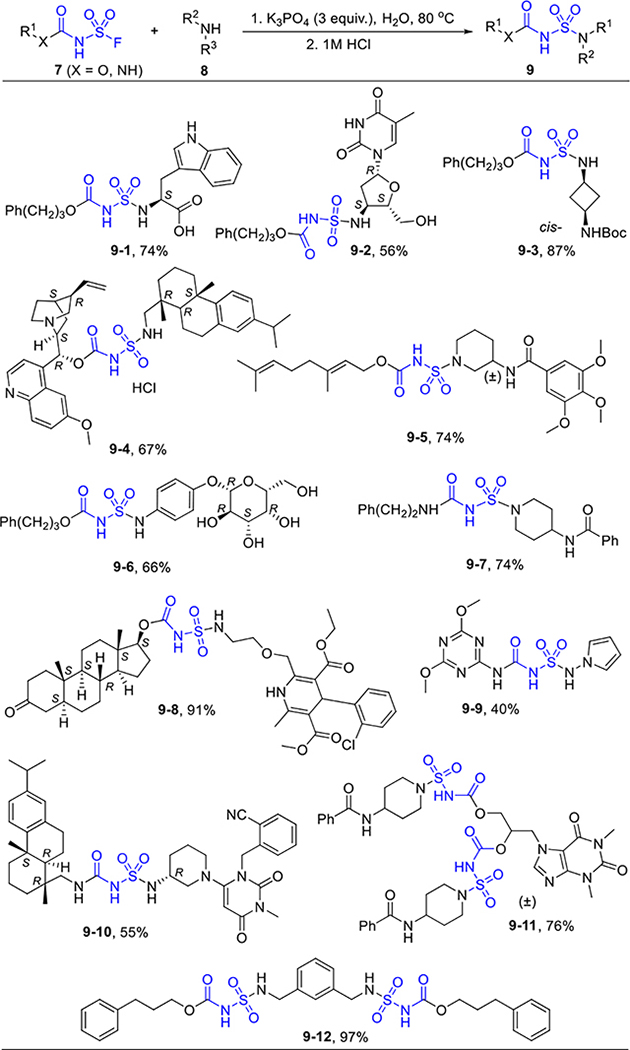
|
7 (0.25–0.5 mmol), 8 (1.5 equiv), K3PO4 (3.0 equiv), H2O (0.25 mol/L), 80 °C, 2–16 h.
To summarize, fluorosulfuryl isocyanate (FSI) is shown to be an outstanding SuFEx linker in stepwise unions of alcohol and amine modules, which are among the most available functional groups. This binary connection sequence exhibits near perfect efficiency complementation to the sulfamide and related libraries made [6c–6f,8] In any case, FSI seems to have been left aside because CSI is so useful already. But the chlorosulfuryl carbamoyl adducts are far less stable than their FSI counterparts. The step-2 defluorinative amminolysis reaction runs on pure water. Implications of these results are listed but not limited to: rapid access to direct-to-screen sulfamide-like chemical libraries, drug conjugation, chemical probes for covalent capture of proteins, and phosphate mimics. All together, we believe the FSI-derived SuFEx ligation would be of general interest in diverse fields.
Supplementary Material
Acknowledgements
The work conducted at Scripps Research Institute by K.B.S. and B.G. was supported by the National Institutes of Health R01GM117145. J.J.D. thank the Shanghai Science and Technology Committee(18JC1415500, 18401933502), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB20020300), the Key Research Program of Frontier Sciences (CAS, Grant No. QYZDB-SSWSLH-028) for financial support.
Footnotes
Conflict of interest
The authors declare the following competing financial interest(s): Shanghai Institute of Organic Chemistry, CAS, has filed a patent application on fluorosulfosuccinic hydroxylimide salt and its applications.
Publisher's Disclaimer: This manuscript has been accepted after peer review and appears as an Accepted Article online prior to editing, proofing, and formal publication of the final Version of Record (VoR). This work is currently citable by using the Digital Object Identifier (DOI) given below. The VoR will be published online in Early View as soon as possible and may be different to this Accepted Article as a result of editing. Readers should obtain the VoR from the journal website shown below when it is published to ensure accuracy of information. The authors are responsible for the content of this Accepted Article.
Contributor Information
Shoujun Sun, Key Laboratory of Organofluorine Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences. University of Chinese Academy of Sciences. 345 Ling-Ling Road, Shanghai 200032 (P. R. China).
Bing Gao, State Key Laboratory of Chemo/Bio-Sensing and Chemometrics, College of Chemistry and Chemical Engineering, Hunan University. Changsha, Hunan 410082 (P. R. China).
Junyu Chen, Key Laboratory of Organofluorine Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences. University of Chinese Academy of Sciences. 345 Ling-Ling Road, Shanghai 200032 (P. R. China).
K. Barry Sharpless, Department of Chemistry, The Scripps Research Institute 10550 North Torrey Pines Road, La Jolla, CA, 92037, USA.
Jiajia Dong, Key Laboratory of Organofluorine Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences. University of Chinese Academy of Sciences. 345 Ling-Ling Road, Shanghai 200032 (P. R. China).
References
- [1].a) Kolb HC, Finn MG, Sharpless KB, Angew. Chem. Int. Ed. 2001, 40, 2004–2021 [DOI] [PubMed] [Google Scholar]; b) Sharpless KB, Angew. Chem. Int. Ed. 2002, 41, 2024–2032. [PubMed] [Google Scholar]
- [2].a) Golas PL, Matyjaszewski K, Chem. Soc. Rev. 2010, 39, 1338–1354 [DOI] [PubMed] [Google Scholar]; b) Kempe K, Krieg A, Becer CR, Schubert US, Chem. Soc. Rev. 2012, 41, 176–191 [DOI] [PubMed] [Google Scholar]; c) Wu P, Feldman AK, Nugent AK, Hawker CJ, Scheel A, Voit B, Pyun J, Fréchet JMJ, Sharpless KB, Fokin VV, Angew. Chem. Int. Ed. 2004, 43, 3928–3932. [DOI] [PubMed] [Google Scholar]
- [3].a) Sletten EM, Bertozzi CR, Angew. Chem. Int. Ed. 2009, 48, 6974–6998 [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Amblard F, Cho JH, Schinazi RF, Chem. Rev. 2009, 109, 4207–4220 [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Tang W, Becker ML, Chem. Soc. Rev. 2014, 43, 7013–7039 [DOI] [PubMed] [Google Scholar]; d) Tiwari VK, Mishra BB, Mishra KB, Mishra N, Singh AS, Chen X, Chem. Rev. 2016, 116, 3086–3240 [DOI] [PubMed] [Google Scholar]; e) McKay Craig S., Finn MG, Chem. & Bio 2014, 21, 1075–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Thirumurugan P, Matosiuk D, Jozwiak K, Chem. Rev. 2013, 113, 4905–4979. [DOI] [PubMed] [Google Scholar]
- [5].Dong J, Krasnova L, Finn MG, Sharpless KB, Angew. Chem. Int. Ed. 2014, 53, 9430–9448. [DOI] [PubMed] [Google Scholar]
- [6].a) Barrow AS, Smedley CJ, Zheng Q, Li S, Dong J, Moses JE, Chem. Soc. Rev. 2019, 48, 4731–4758 [DOI] [PubMed] [Google Scholar]; b) Meng G, Guo T, Ma T, Zhang J, Shen Y, Sharpless KB, Dong J, Nature 2019, 574, 86–89 [DOI] [PubMed] [Google Scholar]; c) Zheng Q, Woehl JL, Kitamura S, Santos-Martins D, Smedley CJ, Li G, Forli S, Moses JE, Wolan DW, Sharpless KB, Proc. Natl. Acad. Sci. 2019, 116, 18808–18814 [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Brighty GJ, Botham RC, Li S, Nelson L, Mortenson DE, Li G, Morisseau C, Wang H, Hammock BD, Sharpless KB, Kelly JW, Nat. Chem. 2020, 12, 906–913 [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Kitamura S, Zheng Q, Woehl JL, Solania A, Chen E, Dillon N, Hull MV, Kotaniguchi M, Cappiello JR, Kitamura S, Nizet V, Sharpless KB, Wolan DW, J. Am. Chem. Soc. 2020, 142, 10899–10904 [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Wortzel LG, Bishop TR, Kitamura S, Milosevich N, Asiaban JN, Zhang X, Zheng Q, Chen E, Ramos AR, Ackerman CJ, Hampton EN, Chatterjee AK, Young TS, Hull MV, Sharpless KB, Cravatt BF, Wolan DW, Erb MA, ACS Cent. Sci. 2021, 7, 815–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Zheng Q, Dong J, Sharpless KB, J. Org. Chem. 2016, 81, 11360–11362. [DOI] [PubMed] [Google Scholar]
- [8].a) Li S, Wu P, Moses JE, Sharpless KB, Angew. Chem. Int. Ed. 2017, 56, 2903–2908 [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Gao B, Li S, Wu P, Moses JE, Sharpless KB, Angew. Chem. Int. Ed. 2018, 57, 1939–1943 [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Liu F, Wang H, Li S, Bare GAL, Chen X, Wang C, Moses JE, Wu P, Sharpless KB, Angew. Chem. Int. Ed. 2019, 58, 8029–8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].a) Saunders JH, Slocombe RJ, Chem. Rev. 1948, 43, 203–218 [DOI] [PubMed] [Google Scholar]; b) Arnold RG, Nelson JA, Verbanc JJ, Chem. Rev. 1957, 57, 47–76. [Google Scholar]
- [10].a) Buchner E, Curtius T, Chem. Ber. 1885, 18, 2371–2377 [Google Scholar]; b) Smith PA S. Curtius Reaction Org. React. 1946, 337–449. [Google Scholar]
- [11].Wallis ES, Lane JF, The Hofmann Reaction Org. React. 2011, 267–306. [Google Scholar]
- [12].Lossen H, Justus Liebigs Ann. Chem. 1869, 150, 314–322. [Google Scholar]
- [13].Westheimer F, Science 1987, 235, 1173–1178. [DOI] [PubMed] [Google Scholar]
- [14].a) Jonas H, Voigt D, Bayer AG. Germany Patent DE1095806B, 1958 [Google Scholar]; b) Roesky HW, Hoff A, Chem. Ber. 1968, 101, 162–173. [Google Scholar]
- [15].Graf R, Angew. Chem. Int. Ed. 1968, 7, 172–182. [Google Scholar]
- [16].Rasmussen JK, Hassner A, Chem. Rev. 1976, 76, 389–408. [Google Scholar]
- [17].Ulrich H, Chem. Rev. 1965, 65, 369–376. [Google Scholar]
- [18].Hoffmann H, Förster H, Monatsh. Chem. 1968, 99, 380–388. [Google Scholar]
- [19].Delebecq E, Pascault J-P, Boutevin B, Ganachaud F, Chem. Rev. 2013, 113, 80–118. [DOI] [PubMed] [Google Scholar]
- [20].a) Tanwar DK, Surendrabhai VR, Gill MS, Synlett 2017, 28, 2495–2498 [Google Scholar]; b) Bae D, Gautam J, Jang H, Banskota S, Lee SY, Jeong M-J, Kim AS, Kim HC, Lee I-H, Nam T.-g., Kim J-A, Jeong B-S, Bioorg. Med. Chem. Lett. 2018, 28, 107–112. [DOI] [PubMed] [Google Scholar]
- [21].a) Aumüller W, and Weyer R, German Patent 1,100,618 1961 [Google Scholar]; b) Chem. Abstr, 1961, 55, 24680. [Google Scholar]; c) Brzozowski Z, and Zacharewicz W, Roczniki Chem, 1962, 36, 291 [Google Scholar]; d) Chem. Abstr. 1962, 57, 16448 [Google Scholar]; e) Graf R, German Patent 1,000,807, 1957; [Google Scholar]; f) Chem. Abstr. 1960, 54, 1555 [Google Scholar]; g) Seefelder M, Chem. Ber. 1963, 96, 3243. [Google Scholar]
- [22].a) Hoffmann H, Förster H, Tor-Poghossian G, Monatsh. Chem. 1969, 100, 311–315 [Google Scholar]; b) Appel R, Montenarh M, Chem. Ber. 1977, 110, 2368–2373. [Google Scholar]
- [23].Dhar DN, Dhar P, The Chemistry of Chlorosulfonyl Isocyanate. World Scientific Publishing Co. Pte. Ltd. Singapore, 2002. [Google Scholar]
- [24].Picard JA, O’Brien PM, Sliskovic DR, Anderson MK, Bousley RF, Hamelehle KL, Krause BR, Stanfield RL, J. Med. Chem. 1996, 39, 1243–1252. [DOI] [PubMed] [Google Scholar]
- [25].a) Atkins GM, Burgess EM, J. Am. Chem. Soc. 1968, 90, 4744–4745 [Google Scholar]; b) Atkins GM, Burgess EM, J. Am. Chem. Soc. 1972, 94, 6135–6141. [DOI] [PubMed] [Google Scholar]
- [26].For the “on water“ reactions:Narayan S, Muldoon J, Finn MG, Forkin VV, Kolb HC, Sharpless KB, Angew. Chem. Int. Ed. 2005, 44, 3275–3279Jung Y, Marcus RA, J. Am. Chem. Soc. 2007, 129, 5492–5502Guo D, Zhu D, Zhou X, Zheng B, Langmuir 2015, 31, 13759–13763.
- [27].The Ca2+ promoted ligation protocol gave moderate yields, see SI.Mukherjee P, Woroch CP, Cleary L, Rusznak M, Franzese RW, Reese MR, Tucker JW, Humphrey JM, Etuk SM, Kwan SC, am Ende CW, Ball ND, Org. Lett. 2018, 20, 3943–3947Mahapatra S, Woroch CP, Butler TW, Carneiro SN, Kwan SC, Khasnavis SR, Gu J, Dutra JK, Vetelino BC, Bellenger J, am Ende CW, Ball ND, Org. Lett. 2020, 22, 4389–4394.
- [28].Verkade JMM, Wijdeven MA, van Geel R, Janssen BMG, van Berkel SS, Del FLV, Antibodies 2018, 7, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Bialy L, Waldmann H, Angew. Chem. Int. Ed. 2005, 44, 3814–3839. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


