Abstract
The complement system and the natural antibody repertoire provide a critical first-line defense against infection. The binding of natural antibodies to microbial surfaces opsonizes invading microorganisms and activates complement via the classical pathway. Both defense systems cooperate within the innate immune response. We studied the role of the complement system in the host defense against experimental polymicrobial peritonitis using mice lacking either C1q or factor B and C2. The C1q-deficient mice lacked the classical pathway of complement activation. The factor B- and C2-deficient mice were known to lack the classical and alternative pathways, and we demonstrate here that these mice also lacked the lectin pathway of complement activation. Using inoculum doses adjusted to cause 42% mortality in the wild-type strain, none of the mice deficient in the three activation routes of complement (factor B and C2 deficient) survived (mortality of 100%). Mortality in mice deficient only in the classical pathway of complement activation (C1q deficient) was 83%. Application of further dilutions of the polymicrobial inoculum showed a dose-dependent decrease of mortality in wild-type controls, whereas no changes in mortality were observed in the two gene-targeted strains. These results demonstrate that the classical activation pathway is required for an effective antimicrobial immune defense in polymicrobial peritonitis and that, in the infection model used, the remaining antibody-independent complement activation routes (alternative and lectin pathways) provide a supporting line of defense to gain residual protection in classical pathway deficiency.
In response to an infection, humoral and cellular components of the innate immune defense interact to contain and eliminate the invading microorganisms. Pattern recognition molecules on phagocytes play a role, as well as chemokines and cytokines, adhesion molecules, and other inflammatory mediators such as histamine, serotonin, leukotrienes, and kinins.
The complement system is an integral part of the innate antimicrobial immune defense and mediates humoral and cellular interactions within the immune response, including chemotaxis, phagocytosis, cell adhesion, and B-cell differentiation (38). Complement may be activated via three different routes: the classical pathway, the alternative pathway, and the recently described lectin pathway.
The classical activation pathway is initiated by the binding of the globular heads of the hexameric recognition molecule C1q to immune complexes via the Fc regions of the antigen-bound immunoglobulins. This binding causes a distortion in the collagenous stalks of C1q, whereby the C1q-associated serine protease dimer of C1r is activated, which in turn activates the coassociated serine protease dimer of C1s. Activated C1s consecutively cleaves C4- and C4b-bound C2 to generate the C3 convertase, C4b2b, which converts native C3 to C3b. The deposition of multiple C3b molecules in close proximity causes a switch in substrate specificity to form the classical pathway C5 convertase, C4b2b(C3b)n, which converts native C5 to C5b. During each of these enzymatic reactions, potent anaphylatoxins (C4a, C3a, and C5a) are produced.
The alternative pathway forms a powerful amplification loop of complement activation (30) and is initiated by binding of the complement activation product C3b (generated either by spontaneous hydrolysis of C3 [“tick-over”] [C3-H2O is assumed to act similarly to C3b] or by C3 convertase-mediated cleavage) to the serine protease zymogen factor B. Upon binding to C3b, factor B is cleaved by factor D to form the alternative pathway C3 convertase, C3bBb. Again, the subsequent binding of multiple C3b molecules in close proximity also induces a switch in the substrate specificity of the alternative pathway C3 convertase from C3 to C5 to form the alternative pathway C5 convertase complex, C3bBb(C3b)n.
The lectin pathway can be activated in the absence of immune complexes and is initiated by the recognition of certain oligosaccharide moieties on the surfaces of pathogens via macromolecular complexes present in body fluids. These complexes are composed of a multivalent pattern recognition subunit and associated serine proteases. To date, two pattern recognition components of the lectin activation pathway have been described, i.e., mannan-binding lectin (MBL) (18) and ficolin p35 (22), which have differing carbohydrate binding specificities (10, 16). Both MBL and ficolin p35 associate with specific serine proteases, termed MBL-associated serine protease-1 (MASP-1) and MASP-2 (22, 34). In vitro, purified recombinant MASP-2 was shown to cleave the fourth and second components of complement (i.e., C4 and C2) in the absence of MASP-1 (20, 34, 36). The analysis of sera of gene-targeted MASP-1-deficient mouse strains showed no impediment in activation of the lectin pathway (32) and thereby underlines that MASP-2 is the effector component that cleaves C4 and C2 also in the absence of MASP-1. The sequential proteolytic cleavage of C4- and C4b-bound C2 to form the C3 convertase, C4b2b, and the C5 convertase, C4b2b(C3b)n, respectively, is the essential step by which the lectin pathway activation complex as well as the classical pathway activation complex initiates further downstream activation of complement (20, 36), yielding the final, nonenzymatic assembly of the bactericidal membrane attack complex.
In order to study the physiological and pathophysiological consequences of selective complement deficiency, mouse strains deficient in the expression of the complement proteins C1q and factor B and C2 were generated by gene-specific targeting as previously reported (3, 33). C1q a−/− mice are deficient in the classical pathway of complement activation (3), while the lectin and alternative pathways are functionally active. H2-Bf/C2−/− mice have been reported to be deficient in the classical and the alternative routes of complement activation (33), and here we show that these mice are also deficient in the lectin pathway activation route. This study reports the impact that selective deficiency of antibody-mediated classical complement activation and complete deficiency of all hitherto-known complement activation pathways have on survival after peritoneal infection in comparison with the wild-type strain using an experimental model of polymicrobial peritonitis and sepsis. This is a model for the postoperative complication of anastomotic leakage. As we have shown previously, animals in this model, in contrast to those in other models of peritonitis (e.g., cecal ligation and puncture), did not die early, indicating that death was not caused by toxic effects but was a consequence of an infectious peritonitis (17).
MATERIALS AND METHODS
Animals.
The study was performed in accordance with German federal regulations. Animals were reared, bred, and health screened according to institutional guidelines and were kept under conventional conditions. C1q a−/− and H2-Bf/C2−/− mice were on a pure 129/Sv genetic background. Adult male transgenic and control (129/Sv) mice (6 to 8 weeks old) were used.
MBL-dependent C3 and C4 cleavage assay.
Mice were sacrificed by CO2 inhalation and bled by cardiac puncture. After centrifugation of blood samples (with 5 mM EDTA added), plasma was stored at −70°C until further use.
An MBL-dependent C4 cleavage assay was established as described by Petersen et al. (27). Microtiter wells (Maxisorp; Nunc, Roskilde, Denmark) were coated with 1 μg of mannan (Sigma-Aldrich, St. Louis, Mo.) in 100 μl of 15 mM Na2CO3–35 mM NaHCO3 (pH 9.6) at room temperature (RT) overnight. Wells were blocked with 200 μl of 0.1% (wt/vol) human serum albumin in 10 mM Tris-HCl–140 mM NaCl–1.5 mM NaN3 (pH 7.4) (TBS) for 1 h and then washed three times with TBS–0.05% (vol/vol) Tween 20–5 mM CaCl2 (TBS-Tw-Ca2+). A human serum sample and pooled mouse plasma samples (reconstituted with 10 mM CaCl2) were diluted in 20 mM Tris-HCl–10 mM CaCl2–1 M NaCl–0.05% (vol/vol) Triton X-100–0.1% (wt/vol) human serum albumin (pH 7.4) (this Ca2+-reconstituting buffer inhibits coagulation of plasma) and added to the mannan-coated wells in duplicate. Wells receiving buffer instead of serum or plasma were used as negative controls. In some experiments, wells received plasma dilutions preincubated with 0.06 mg of mannan per ml (4°C, overnight) to assess the specificity of this assay.
Following overnight incubation at 4°C and washing three times with TBS-Tw-Ca2+, 0.1 μg of human C4 (prepared as previously described [6]) in 100 μl of BBS (4 mM barbital, 145 mM NaCl, 2 mM CaCl2, 1 mM MgCl2, pH 7.4) was added to the wells (90 min, 37°C). It was necessary to add exogenous C4 because the high ionic strength of the buffer, used to allow binding of MBL to mannan, precluded cleavage of C4 present in the sample (27). Following another washing step with TBS-Tw-Ca2+, deposited C4b was detected by addition of 100 μl of biotinylated rabbit anti-human C4c (catalog no. A0065; DAKO, Glostrup, Denmark) at 1 μg/ml in TBS-Tw-Ca2+ (90 min, RT). After washing, alkaline phosphatase-conjugated streptavidin (DAKO; 1:2,000 dilution in TBS-Tw-Ca2+) was added (1 h, RT). After another washing step with TBS-Tw-Ca2+, the presence of alkaline phosphatase was determined by addition of 100 μl of substrate solution (Sigma Fast p-nitrophenyl phosphate tablet sets) (37°C, 30 min). The optical density was measured at 405 nm, using a Dynatech Laboratories MRX plate reader.
To assess further downstream complement activation after MBL pathway activation, namely, C3 cleavage, wells were washed after C4 incubation and then incubated with plasma dilutions made in BBS (3 h, 37°C). Human C3-deficient serum (catalog no. C-8788; Sigma) was added as a negative control. After another three washes with TBS-Tw-Ca2+, wells received 100 μl of peroxidase-conjugated goat anti-human C3 (catalog no. 55237; Cappel, ICN, Basingstoke, England) at 1:2,000 in BBS (90 min, 37°C). Following another three washes in TBS-Tw-Ca2+, wells received 100 μl of tetramethylbenzidine peroxidase enzyme immunoassay substrate (90% solution A and 10% solution B [Bio-Rad]) for 20 to 30 min at 37°C. The reaction was stopped by adding 50 μl of 1 M H2SO4 to each well, and the absorbance was read at 450 nm.
Animal experiment.
An appropriate level of anesthesia was induced with a fentanyl-droperidol mixture (1:1; 12 ml/kg intraperitoneally [i.p.]). Laparotomy (0.5-cm midline incision) was performed aseptically, and dilutions of a standardized polymicrobial human fecal suspension in Ringer's solution (17), or Ringer's solution only where indicated, were inoculated i.p. (0.02 to 0.2 ml, depending on the dilutions made). The microbiological profile of the inoculum, which was used previously to establish a rat model of postoperative abdominal peritonitis (17), included anaerobic, aerobic, gram-positive, and gram-negative bacteria and fungi (17). The peritoneal, muscle, and skin incisions were closed with stitches. Mortality rates and survival times were recorded. The end point of the experiment was defined as the mortality rate at 120 h.
An inoculum of 0.12 ml/kg of body weight was used to challenge 129/Sv and C1q a−/− as well as H2-Bf/C2−/− mice (n = 12 each) in a two-block design performed in two separate experiments.
Statistical evaluation.
Descriptive statistics were expressed as means and standard deviations. Chi-square tests were performed for nominal data. As distributions were unknown, nonparametric tests were used for parameters of location and variance (Mann-Whitney U test for two samples and Kruskal-Wallis test for k samples). Time courses of survival were analyzed by Kaplan-Meier survival curve calculations and the log-rank test for k samples. P values of <0.05 were defined as significant for discrimination. Data were analyzed using SPSS software (version 10).
RESULTS
Mortality was assessed in an experimental model of polymicrobial peritoneal infection to evaluate the effects of targeted deficiencies of the recognition molecule of the classical pathway of complement, C1q, or of the complement serine protease zymogens factor B and C2 and to evaluate their relative contributions to survival in the wild-type strain.
Lectin pathway activity.
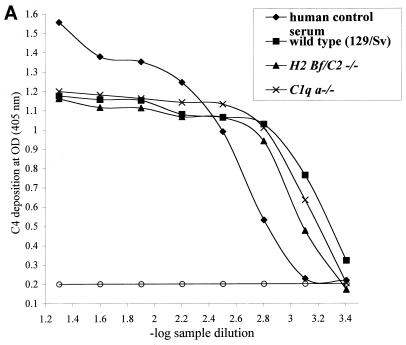
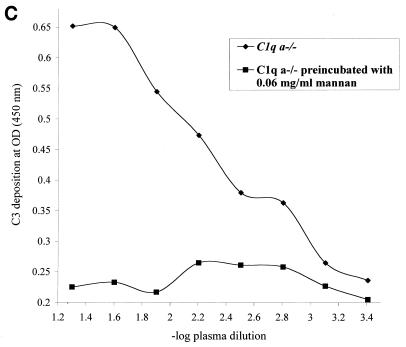
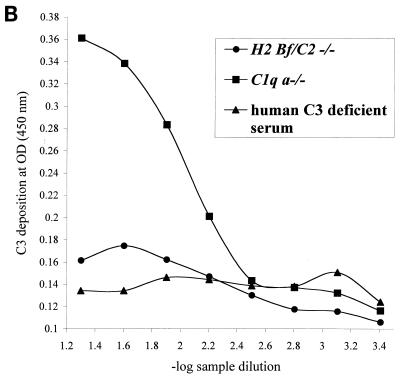
To assess the presence or absence of lectin pathway activation, a complement activation pathway especially relevant to the polymicrobial infection model used, plasma samples from all three experimental mouse strains were assayed in a mannan-dependent C4 cleavage test. After trapping of plasma MBL-MASP complexes on mannan-coated plates, MASP-2 mediated cleavage of C4 was measured by detecting C4b deposition (27). As shown in Fig. 1A, plasma samples from C1q a−/−, H2-Bf/C2−/−, and 129/Sv mice cleaved C4 in this assay and thereby completed the first step of lectin pathway activation. To assess the subsequent cleavage step and the formation of the lectin pathway C3 convertase in C1q a−/− and H2-Bf/C2−/− mice, we determined cleavage of C3. Lectin pathway-dependent C3 cleavage was detected in mice deficient in C1q (Fig. 1B), which could be inhibited by preincubating the plasma with mannan (Fig. 1C), whereas no C3 cleavage was seen in H2-Bf/C2−/− mice (Fig. 1B). These data demonstrate for the first time that C1q a−/− mice have a functional lectin pathway, whereas H2-Bf/C2−/− mice are deficient in all three complement activation pathways.
FIG. 1.
(A) Lectin pathway-dependent C4 cleavage in plasma samples of H2-Bf/C2−/− (pool of n = 12), C1q a−/− (pool of n = 11), and 129/Sv (pool of n = 5) mice. A human control serum (MBL level, 3.6 μg/ml) was assayed in parallel as a positive control. Serial dilutions are expressed on a logarithmic scale (x axis, −log 1/20 = 1.3 to −log 1/2,560 = 3.4). The optical density (OD) (y axis) is the readout of C4b deposition on the well surfaces. Each point is the mean from duplicate experiments. For the negative control, serum or plasma was replaced by buffer only (open circles). The graph represents results from one of two experiments. (B) Lectin pathway-dependent C3 cleavage in plasma samples of H2-Bf/C2−/− (pool of n = 12) and C1q a−/− (pool of n = 11) mice. Human serum deficient in complement component C3 was assayed in parallel as a negative control. Serial dilutions are expressed on a logarithmic scale (x axis, −log 1/20 = 1.3 to −log 1/2,560 = 3.4). The optical density (OD) (y axis) is the readout of C3b deposition on the well surfaces. Each point is the mean from duplicate experiments. The graph represents results from one of two experiments. (C) Lectin pathway-dependent C3 cleavage in C1q a−/− mice was inhibited by preincubation of the plasma with mannan.
Dose responses of control and complement-deficient mice in the polymicrobial abdominal infection and sepsis model.
Wild-type mice (129/Sv) were subjected to increasing doses of a standardized polymicrobial inoculum administered i.p. (six groups with n = 10 per group). There was a dose-dependent response to the amount of inoculum instilled. Using inoculum doses of 0.6 and 0.45 ml/kg of body weight, all 10 of the wild-type mice died (100% mortality); 90% died at a dose of 0.3 ml/kg, 50% died at a dose of 0.15 ml/kg, and all survived (0% mortality) at a dose of 0.08 ml/kg. A control experiment with the electrolyte carrier fluid (Ringer's solution) used to dilute the polymicrobial suspension was performed to exclude an impairment in survival due to anesthesia and the operation technique alone. This showed 0% mortality (n = 5). This sham control experiment was also performed on the two complement-deficient strains (n = 5 each). Both strains survived the operational procedure and instillation of sterile electrolyte solution (0% mortality).
To assess the survival of H2-Bf/C2−/− mice after challenge with lower doses of the polymicrobial inoculum, dilutions of 0.04, 0.02, 0.01, and 0.005 ml/kg of body weight were applied in a parallel experiment. In each of the four groups (n = 5 per group), none of the H2-Bf/C2−/− mice survived the infection with any of these high dilutions of the inoculum (mortality, 100%; 95% confidence interval [CI 95%], 48 to 100%).
Using the same dilutions to challenge C1q a−/− mice and wild-type control mice in parallel, 80% of C1q a−/− mice (CI 95%, 28 to 100%) and none of the wild-type control mice (CI 95%, 0 to 52%) died. Thus, while 129/Sv mice showed a dose-dependent mortality rate, the degree of impairment in the two complement-deficient strains remained constant irrespective of further dilutions (up to 20-fold) of the polymicrobial inoculum chosen (H2-Bf/C2−/− mortality, 100%; C1q a−/− mortality, 80%). For H2-Bf/C2−/− and C1q a−/− mice alike, the survival times at these high dilutions were marginally increased compared to those observed for H2-Bf/C2−/− and C1q a−/− mice, respectively, receiving the high inoculum dose of 0.12 ml/kg. For each strain, there was no significant difference in survival time between the groups receiving dilutions of 0.04, 0.02, 0.01, or 0.005 ml/kg of body weight.
Survival of complement-deficient mice in the polymicrobial abdominal infection and sepsis model.
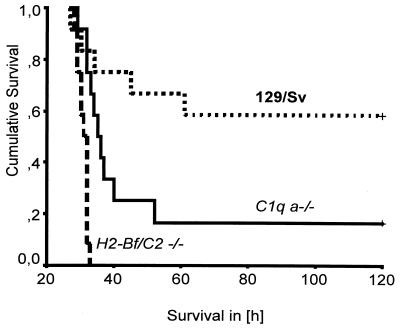
An inoculation dose of 0.12 ml/kg of body weight was chosen to study survival and survival time of 129/Sv, C1q a−/−, and H2-Bf/C2−/− mice. While 5 of 12 (42%; CI 95%, 15 to 72%) wild-type control mice died within the 120-h observation period, twice as many (10 of 12 [83%; CI 95%, 52 to 98%]) C1q a−/− mice succumbed to the infection (P = 0.04 by the chi-square test; df = 1). None of the 12 H2-Bf/C2−/− mice survived i.p. infection (100% mortality; CI 95%, 74 to 100%) (P = 0.002 for wild-type control versus H2-Bf/C2−/− mice by the chi-square test; df = 1). The average survival time of H2-Bf/C2−/− mice was only 31 (±1.6) h, while the average survival time of the C1q a−/− mice was 50 (±33.2) h (P = 0.001 by the Mann-Whitney U test for two samples). The average survival time of the wild-type control mice was 86 (±42.4) h. Kruskal-Wallis analysis was performed between these three groups and showed that the differences between wild-type control mice, C1q a−/− mice, and H2-Bf/C2−/− mice were statistically significant (P = 0.001; df = 2). Analysis of cumulative survival showed that while 58% of 129/Sv control mice were alive at 120 h, only 17% of C1q a−/− mice were alive. H2-Bf/C2−/− mice were even more severely impaired to combat the i.p. infection, and all died within 31 h after infection (Fig. 2). Both C1q a−/− and H2-Bf/C2−/− mice died significantly sooner than the wild-type control mice (log-rank test, P < 0.001).
FIG. 2.
Cumulative survival of C1q a−/− and H2-Bf/C2−/− mice in experimentally induced polymicrobial peritonitis compared with the wild-type mouse strain 129/Sv (Kaplan-Meier analysis). +, end of the observation period of 5 days (120 h), at which time 58% of the 129/Sv mice and 17% of the C1q a−/− mice but none of the H2-Bf/C2−/− mice were still alive. The differences between these three groups were statistically significant (log-rank test, P < 0.001).
DISCUSSION
The classical pathway of complement activation is important for survival following experimentally induced polymicrobial peritonitis and sepsis.
The natural antibody repertoire, upheld mainly by peritoneal B-1 cells, represents an important first line of defense against various pathogens. Additionally, within the first hours of infection after septicemia, T-cell-independent B-cell activation leads to a rapid production of neutralizing antibodies (24). These antibodies (immunoglobulin M [IgM]) may bind to an invading microorganism and support opsonization through the interaction with C1q, thereby initiating complement activation by the classical pathway leading to complement-mediated clearance of the pathogens. Finally, binding of the collagenous regions of C1q to surface binding structures triggers cellular effector mechanisms such as phagocytosis and respiratory burst (1).
This study showed that mice deficient in the expression of the recognition component of the classical pathway of complement activation (C1q) were impaired in their survival following peritoneal infection. In an experimental setting in which 42% of the wild-type control strain succumbed to the infection, 83% of C1q a−/− mice did not survive. Even at high dilutions of the polymicrobial inoculum, this mortality rate did not change. Therefore, the results obtained in this study suggest a significant role for C1-mediated complement activation by antibodies (be they natural antibodies or part of the early adaptive immune response) bound to microorganisms in survival from experimental peritonitis.
C1q a−/− mice, on 129/Sv pure and 129/Sv × C57BL6 mixed genetic backgrounds, have been shown previously to be impaired in their humoral response to T-cell-dependent antigen (5). This T-cell-dependent antibody response, however, takes 4 to 6 days (26) and therefore is outside the observation period used in this study.
The significance of the natural antibody repertoire in in vivo models of peritonitis and septicemia has been documented previously: in a cecal ligation and perforation model, Boes et al. (2) found that the wild-type strain showed a mortality of 20%, while 70% of the secretory IgM−/− strains (on 129/Sv pure and 129/Sv × C57BL6 mixed genetic backgrounds) showed a detrimental outcome. Likewise, recombinase-activating gene-2 (RAG-2)-deficient mice (which are deficient in plasma immunoglobulins and B cells) and mice deficient in Bruton's tyrosine kinase (Btk) (which are deficient in immunoglobulin subclasses and B-1 cells) were significantly impaired in a model of i.p. endotoxin challenge (mortality of wild-type control mice [129/Sv × C57BL6 mixed genetic background], 20%; mortality of RAG-2−/− mice, 94%; and mortality of Btk−/− mice, approximately 60%). Survival and endotoxin clearance were significantly enhanced when RAG-2−/− mice were reconstituted with nonimmune serum and Btk−/− mice were reconstituted with purified IgM (29).
This is the first study to report a severe impairment of C1q a−/− mice in survival of polymicrobial peritonitis and sepsis, and it demonstrates the likely pathway of protection against this infection by antibodies. It shows that the classical pathway of complement activation is significantly involved in survival following peritoneal infection.
Combined deficiency of the classical, lectin, and alternative pathways of complement activation is lethal in an experimental model of polymicrobial peritonitis and sepsis.
The data presented on lectin pathway-dependent activation of C4 and C3 (Fig. 1) were in line with the hypothesis that a deficiency in factor B and C2 would allow formation of the lectin pathway C4 and C2 convertases (via MBL and MASP-2), while the lack of C2 would prevent the formation of the C3 and C5 convertases of the lectin pathway of complement. There was no C3 cleavage in H2-Bf/C2−/− mice. “C2 bypass” activation has been described for C2-deficient individuals to compensate in part for the deficient serine protease of the classical pathway (14, 21, 37), by essentially switching to the alternative pathway using the serine protease factor B (13). Hence, no C2 bypass activity was expected to be present in H2-Bf/C2−/− mice. Nevertheless, the results indicate that the postulated cleavage of C3 via MASP-1 (20) may not represent a significant bypass route under physiological conditions.
In factor B-deficient mice, sensitivity to lethal endotoxic shock (induced by i.p. infection with Salmonella enterica serovar Typhosa lipopolysaccharide) was not significantly altered compared to that in the wild-type control (19). However, all of the H2-Bf/C2−/− mice died from the i.p. contamination described in the present study. Therefore, it may be inferred that activation and cleavage of C3 are most likely the key steps in complement activation in protection against this infection. C3 cleavage products are important in chemotaxis to sites of inflammation. They are also vital in the localization of antigenic targets to the splenic marginal zone. C3−/− mice mounted less than 10% of early T-cell-independent IgM antibody titers (25). It is conceivable that this response was diminished in H2-Bf/C2−/− mice.
Interestingly, C3−/− mice showed 100% mortality in a cecal ligation and puncture model (28). Likewise, C3−/− mice were more susceptible to endotoxin-induced shock (i.p. S. enterica serovar Typhimurium lipopolysaccharide) than wild-type controls (75 and 25% mortality, respectively) (8). More recently, mast cells bearing receptors for C3 activation fragments (CD21 and CD35) were shown to be crucial for the survival from acute septic peritonitis in a cecal ligation and puncture model (100% mortality in mast cell-deficient mice compared to 30% in a strain-matched wild-type control [7, 9]).
This study showed that a combined deficiency of factor B and C2 leads to 100% mortality of the host in a model of polymicrobial i.p. infection. This mortality remained unchanged even at high dilutions (20-fold) of the polymicrobial inoculum. Compared to mortalities of 83% of the C1q-deficient and 42% of the wild-type mice, this absolute impairment of the H2-Bf/C2−/− mice points to a role in survival of either the lectin or alternative pathway apart from the now-established important role of the classical pathway.
In summary, these results show that an intact complement system is crucial to successfully combat a polymicrobial septic event. The classical pathway of complement activation is most important for survival in this experimental model, while the lectin and alternative pathways of complement activation provide an important supporting line of defense. Other components of innate immunity such as scavenger receptors (35), Fc receptors (31), cytokines (4), and antimicrobial peptides (12) may provide residual protection at low infectious doses. In shock research, much work has focused on the negative hyperinflammatory responses of complement (11). However, our data clearly demonstrate a protective role of complement in a clinically relevant model of abdominal contamination and infection (15, 23).
ACKNOWLEDGMENTS
This study was supported by the Deutsche Forschungsgemeinschaft (grants SFB 297 and STO-430) and the Wellcome Trust (grants 049658 and 060574).
We especially thank Heinrich Schnabel for his support throughout the experiments. Oguzkan Sürücü and Selim Sevinc are acknowledged for technical support. We thank K.-U. Hartmann (Department of Experimental Immunology, Philipps University Marburg) for his interest and support and Daniela Männel, Peter Andrew, and Löms Ziegler-Heitbrock for their comments on the manuscript.
REFERENCES
- 1.Alvarez-Dominguez C, Carrasco-Marin E, Lopez-Mato P, Leyva-Cobian F. The contribution of both oxygen and nitrogen intermediates to the intracellular killing mechanisms of C1q-opsonized Listeria monocytogenes by the macrophage-like IC-21 cell line. Immunology. 2000;101:83–89. doi: 10.1046/j.1365-2567.2000.00083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boes M, Prodeus A, Schmidt T, Carroll M C, Chen J. A critical role of natural immunoglobulin M in immediate defense against systemic bacterial infection. J Exp Med. 1998;188:2381–2386. doi: 10.1084/jem.188.12.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Botto M, Dell'Agnola C, Bygrave A E, Thompson E M, Cook H T, Petry F, Loos M, Pandolfi P P, Walport M J. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat Genet. 1998;19:56–59. doi: 10.1038/ng0598-56. [DOI] [PubMed] [Google Scholar]
- 4.Calandra T, Echtenacher B, Roy D L, Pugin J, Metz C N, Hultner L, Heumann D, Männel D, Bucala R, Glauser M P. Protection from septic shock by neutralization of macrophage migration inhibitory factor. Nat Med. 2000;6:164–170. doi: 10.1038/72262. [DOI] [PubMed] [Google Scholar]
- 5.Cutler A J, Botto M, van Essen D, Rivi R, Davies K A, Gray D, Walport M J. T cell-dependent immune response in C1q-deficient mice: defective interferon gamma production by antigen-specific T cells. J Exp Med. 1998;187:1789–1797. doi: 10.1084/jem.187.11.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dodds A W. Small-scale preparation of complement components C3 and C4. Methods Enzymol. 1993;223:46–61. doi: 10.1016/0076-6879(93)23037-n. [DOI] [PubMed] [Google Scholar]
- 7.Echtenacher B, Männel D N, Hultner L. Critical protective role of mast cells in a model of acute septic peritonitis. Nature. 1996;381:75–77. doi: 10.1038/381075a0. [DOI] [PubMed] [Google Scholar]
- 8.Fischer M, Prodeus A, Nicholson-Weller A, Ma M, Murrow J, Reid R, Warren H, Lage A, Moore F, Jr, Rosen F, Carroll M. Increased susceptibility to endotoxin shock in complement C3- and C4-deficient mice is corrected by C1 inhibitor replacement. J Immunol. 1997;159:976–982. [PubMed] [Google Scholar]
- 9.Gommerman J L, Oh D Y, Zhou X, Tedder X, T F, Maurer M, Galli S J, Carroll M C. A role for CD21/CD35 and CD19 in responses to acute septic peritonitis: a potential mechanism for mast cell activation. J Immunol. 2000;165:6915–6921. doi: 10.4049/jimmunol.165.12.6915. [DOI] [PubMed] [Google Scholar]
- 10.Holmkov U L. Collectins and collectin receptors in innate immunity. APMIS. 2000;108:5–59. [PubMed] [Google Scholar]
- 11.Horn K D. Evolving strategies in the treatment of sepsis and systemic inflammatory response syndrome (SIRS) Q J Med. 1998;91:265–277. doi: 10.1093/qjmed/91.4.265. [DOI] [PubMed] [Google Scholar]
- 12.Jin H, Yang R, Marsters S, Ashkenazi A, Bunting S, Marra M N, Scott R W, Baker J B. Protection against endotoxic shock by bactericidal/permeability-increasing protein in rats. J Clin Invest. 1995;95:1947–1952. doi: 10.1172/JCI117877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klint C, Gullstrand B, Sturfelt G, Truedsson L. Binding of immune complexes to erythrocyte CR1 (CD35): difference in requirement of classical pathway components and indication of alternative pathway-mediated binding in C2-deficiency. Scand J Immunol. 2000;52:103–108. doi: 10.1046/j.1365-3083.2000.00752.x. [DOI] [PubMed] [Google Scholar]
- 14.Knutzen Steuer K L, Sloan L B, Oglesby T J, Farries T C, Nickells N W, Densen P, Harley J B, Atkinson J P. Lysis of sensitized sheep erythrocytes in human sera deficient in the second component of complement. J Immunol. 1989;143:2256–2261. [PubMed] [Google Scholar]
- 15.Kuenneke M, Stinner B, Celik I, Lorenz W. Cardiovascular adverse effects of antimicrobials in complex surgical cases. Eur J Surg. 1996;162:24–28. [PubMed] [Google Scholar]
- 16.Le Y, Lee S H, Kon O L, Lu J. Human L-ficolin: plasma levels, sugar specificity, and assignment of its lectin activity to the fibrinogen-like (FBG) domain. FEBS Lett. 1998;425:367–370. doi: 10.1016/s0014-5793(98)00267-1. [DOI] [PubMed] [Google Scholar]
- 17.Lorenz W, Reimund K P, Weitzel F, Celik I, Kurnatowski M, Schneider C, Mannheim W, Heiske A, Neumann K, Sitter H, Rothmund M. Granulocyte colony-stimulating factor prophylaxis before operation protects against lethal consequences of postoperative peritonitis. Surgery. 1994;116:925–934. [PubMed] [Google Scholar]
- 18.Lu J H, Thiel S, Wiedemann H, Timpl R, Reid K B. Binding of the pentamer/hexamer forms of mannan-binding protein to zymosan activates the proenzyme C1r2C1s2 complex, of the classical pathway of complement, without involvement of C1q. J Immunol. 1990;144:2287–2294. [PubMed] [Google Scholar]
- 19.Matsumoto M, Fukuda W, Circolo A, Goellner J, Strauss-Schoenberger J, Wang X, Fujita S, Hidvegi T, Chaplin D D, Colten H R. Abrogation of the alternative complement pathway by targeted deletion of murine factor B. Proc Natl Acad Sci USA. 1997;94:8720–8725. doi: 10.1073/pnas.94.16.8720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsushita M, Thiel S, Jensenius J, Terai I, Fujita T. Proteolytic activities of two types of mannose-binding lectin-associated serine protease. J Immunol. 2000;165:2637–2642. doi: 10.4049/jimmunol.165.5.2637. [DOI] [PubMed] [Google Scholar]
- 21.Matsushita M, Okada H. Alternative complement pathway activation by C4b deposited during classical pathway activation. J Immunol. 1986;136:2994–2998. [PubMed] [Google Scholar]
- 22.Matsushita M, Endo Y, Fujita T. Complement-activating complex of ficolin and mannose-binding lectin-associated serine protease. J Immunol. 2000;164:2281–2284. doi: 10.4049/jimmunol.164.5.2281. [DOI] [PubMed] [Google Scholar]
- 23.Nyström P O. Transition from contamination to infection: implications in colonic surgery. Eur J Surg. 1996;162:42–46. [PubMed] [Google Scholar]
- 24.Ochsenbein A F, Zinkernagel R M. Natural antibodies and complement link innate and acquired immunity. Immunol Today. 2000;21:624–630. doi: 10.1016/s0167-5699(00)01754-0. [DOI] [PubMed] [Google Scholar]
- 25.Ochsenbein A F, Pinschewer D D, Odermatt B, Ciurea A, Hengartner H, Zinkernagel R M. Correlation of T cell independence of antibody responses with antigen dose reaching secondary lymphoid organs: implications for splenectomized patients and vaccine design. J Immunol. 2000;164:6269–6302. doi: 10.4049/jimmunol.164.12.6296. [DOI] [PubMed] [Google Scholar]
- 26.Ochsenbein A F, Pinschewer D D, Odermatt B, Carroll M C, Hengartner H, Zinkernagel R M. Protective T cell-independent antiviral antibody responses are dependent on complement. J Exp Med. 1999;190:1165–1174. doi: 10.1084/jem.190.8.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petersen S V, Thiel S, Jensen L, Steffensen R, Jensenius J C. An assay for the mannan-binding lectin (MBL) pathway of complement activation. J Immunol Methods. 2001;257:107–116. doi: 10.1016/s0022-1759(01)00453-7. [DOI] [PubMed] [Google Scholar]
- 28.Prodeus A P, Zhou X, Maurer M, Galli S J, Carroll M C. Impaired mast cell-dependent natural immunity in complement C3-deficient mice. Nature. 1997;390:172–175. doi: 10.1038/36586. [DOI] [PubMed] [Google Scholar]
- 29.Reid R, Prodeus A P, Khan W, Hsu T, Rosen F S, Carroll M C. Endotoxin shock in antibody-deficient mice. Unraveling the role of natural antibody and complement in the clearance of lipopolysaccharide. J Immunol. 1997;159:970–975. [PubMed] [Google Scholar]
- 30.Schwaeble W, Reid K B M. Does properdin crosslink the cellular and the humoral immune response? Immunol Today. 1999;20:17–21. doi: 10.1016/s0167-5699(98)01376-0. [DOI] [PubMed] [Google Scholar]
- 31.Simms H H, D'Amico R, Burchard K. Untreated intra-abdominal sepsis: lack of synergism between polymorphonuclear leukocyte (PMN) complement receptors CR1/CR3 and IgG receptor FcRIII. J Trauma. 1990;30:1027–1031. doi: 10.1097/00005373-199008000-00013. [DOI] [PubMed] [Google Scholar]
- 32.Takahashi M, Miura S, Ishii N, Matsushita M, Endo Y, Sugamura K, Fujita T. An essential role of MASP-1 in activation of the lectin pathway. Immunopharmacology. 2000;49:3. [Google Scholar]
- 33.Taylor P R, Nash J T, Theodoridis E, Bygrave A E, Walport M J, Botto M. A targeted disruption of the murine complement factor B gene resulting in loss of expression of three genes in close proximity, factor B, C2, and D17H6S45. J Biol Chem. 1998;273:1699–1704. doi: 10.1074/jbc.273.3.1699. [DOI] [PubMed] [Google Scholar]
- 34.Thiel S, Petersen S V, Vorup-Jensen T, Matsushita M, Fujita T, Stover C M, Schwaeble W J, Jensenius J C. Interaction of C1q and mannan-binding lectin (MBL) with C1r, C1s, MBL-associated serine proteases 1 and 2, and the MBL-associated protein MAp19. J Immunol. 2000;165:878–887. doi: 10.4049/jimmunol.165.2.878. [DOI] [PubMed] [Google Scholar]
- 35.Thomas C A, Li Y, Kodama T, Suzuki H, Silverstein S C, El Khoury J. Protection from lethal gram-positive infection by macrophage scavenger receptor-dependent phagocytosis. J Exp Med. 2000;191:147–156. doi: 10.1084/jem.191.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vorup-Jensen T, Petersen S V, Hansen A G, Poulsen K, Schwaeble W, Sim R B, Reid K B, Davis S J, Thiel S, Jensenius J C. Distinct pathways of mannan-binding lectin (MBL)- and C1 complex autoactivation revealed by reconstitution of MBL with recombinant MBL-associated serine protease-2. J Immunol. 2000;165:2093–2100. doi: 10.4049/jimmunol.165.4.2093. [DOI] [PubMed] [Google Scholar]
- 37.Wagner E, Platt J L, Howell D N, Marsh H C, Jr, Frank M M. IgG and complement-mediated tissue damage in the absence of C2: evidence of a functionally active C2-bypass pathway in a guinea pig model. J Immunol. 1999;163:3549–3558. [PubMed] [Google Scholar]
- 38.Whaley K, Schwaeble W. Complement and complement deficiencies. Semin Liver Dis. 1997;17:297–310. doi: 10.1055/s-2007-1007206. [DOI] [PubMed] [Google Scholar]