Abstract
E. coli are frequently isolated food-borne pathogens from meat, milk, and their products. Moreover, there has been a significant rise in the antimicrobial resistance patterns of E. coli O157:H7 to commonly used antibiotics. A cross-sectional study was conducted from October 2019 to July 2021 to estimate prevalence and identify associated factors of E. coli and E. coli O157:H7 and to determine antibiotic resistance pattern of E. coli O157:H7 from foods of bovine origin in Dessie and Kombolcha towns. A total of 384 samples were collected. Systematic and simple random sampling techniques were employed for sampling carcasses and milking cows, respectively. E. coli and E. coli O157:H7 were detected according to recommended bacteriological protocols. E. coli O157:H7 strains were evaluated for in vitro antimicrobial susceptibility using agar disk diffusion method. Both descriptive and inferential statistical techniques were applied to analyze the data. Overall prevalence rates of E. coli and E. coli O157:H7 were 54.7% and 6.5%, respectively. Highest prevalence rates of E. coli (79.6%) and E. coli O157:H7 (16.7%) were obtained from carcass swabs and milk tank samples, respectively. Unlike E. coli O157:H7, a statistically significant difference in the E. coli prevalence (P<0.05) was observed among the different sample types. Multidrug resistance was observed among all isolates of E. coli O157:H7. All E. coli O157:H7 isolates (100.0%) were susceptible to Ampicillin, Sulfamethoxazole-trimethoprim, and Norfloxacin. On the contrary, all of the isolates (100%) were resistant to Penicillin G, Vancomycin, and Oxacillin. The current study indicated that different foods of bovine origin in the study area were unsafe for human consumption. Hence, good hygienic production methods should be employed to ensure the safety of foods of bovine origin.
Author summary
Food-producing animals are the major reservoirs for many food-borne pathogens. Milk and meat and their products are important reservoirs for many of the food-borne pathogens. Among food-borne diseases associated with consumption of milk and beef is E. coli O157:H7. On the other hand, the increasing emergence and spread of antibiotic resistant E. coli O157:H7 has become a significant concern globally. Prompt and precise identification of bacterial pathogens in food is critical for tracing bacterial pathogens within the food chain. A total of 384 samples were collected to estimate prevalence and identify associated factors of E. coli and E. coli O157:H7 and to determine antibiotic resistance pattern of E. coli O157:H7 from foods of bovine origin in Dessie and Kombolcha towns. Overall prevalence rates of E. coli and E. coli O157:H7 were 54.7% and 6.5%, respectively. Multidrug resistance was observed among all isolates of E. coli O157:H7. The current study indicated that different foods of bovine origin in the study area were unsafe for human consumption. Hence, preventive measures are required to improve the wholesomeness and safety of foods of bovine origin.
Introduction
Food-borne pathogens are the leading causes of human illness and death in the world [1]. Most microbial pathogens are zoonotic in nature and healthy food animals are reservoirs of many foodborne pathogens [2,3]. In humans, the consumption of foods of animal origin is a major source of exposure to food-borne pathogens [4]. Thus, people are at risk of being infected with pathogens from repository animals through the food chain [5].
Bacteria are the major cause of food-borne infections in humans [6]. Among different food-borne bacteria, Escherichia coli (E. coli) can get access to foods of animal origin from different sources [2], and these bacteria are frequently isolated food-borne pathogens from meat and meat products [7] and milk and dairy products [8].
E. coli are gram-negative, non-spore-forming, facultative anaerobic, and coliform bacteria belonging to the family Enterobacteriaceae that are residing in the intestines of animals and humans as normal microflora [3,8–12]. The detection of E. coli in animal-derived foods is an indicator of fecal contamination and poor hygiene during production, storage, distribution, processing, or preparation of these food items, and the presence of other highly pathogenic microorganisms which can affect food safety and public health [13].
The species E. coli consists of a diverse and large group of bacteria [6]. Most E. coli strains are harmless [9]. However, some strains are pathogenic and can cause severe human illness [14]. Among these pathogenic strains, E. coli O157:H7 is one of the common and virulent food-borne bacterial pathogens [15] which is the subtype of Shiga toxin-producing E. coli strains [16]. This emerging food-borne bacterial strain is the leading cause of acute life-threatening infections such as hemolytic-uremic syndrome, hemorrhagic colitis, and thrombotic thrombocytopenic purpura in humans [1,17,18]. Cattle are the primary reservoirs of E. coli O157:H7 [3,15,18], and foods of bovine origin such as beef, milk, and dairy products are major sources and vehicles of human infection through the food chain [19].
Besides the magnitude of the occurrence of the disease, the increasing emergence and spread of antibiotic-resistant bacteria particularly multi-drug resistant zoonotic foodborne pathogens have become a significant concern globally [20,21]. The antimicrobial-resistant bacteria can be transmitted to humans through the food chain from food animal reservoirs [22]. Studies conducted in different areas indicated that there has been a significant rise in the antimicrobial resistance pattern of E. coli O157:H7 to commonly used antibiotics [23,24].
Analysis of food to detect food-borne pathogens is essential to ensure food safety and to reduce and/or prevent the occurrence of food-borne infections in humans [25–27]. Particularly, the detection of food-borne pathogenic bacteria is critical for the control and prevention of some hazardous points in food production, processing, and/or distribution [28]. However, there is insufficient information related to the occurrence of food-borne infections in developing countries though the burden is high in those countries as compared to developed countries [15]. Despite there is growing tendency of reporting E. coli O157:H7 in beef and dairy products in recent times [1], only few studies have been reported related to the epidemiology and antibiotic resistance pattern of E. coli O157:H7 in Ethiopia [13,15]. Furthermore, in most parts of Ethiopia, cow milk and beef are consumed as raw or undercooked which may prone people to pathogenic and drug-resistant food-borne bacteria. Hence, the objectives of the present study were to estimate the prevalence and identify associated factors of E. coli and E. coli O157:H7 and to determine the antibiotic resistance patterns of E. coli O157:H7 isolates from foods of bovine origin in Dessie and Kombolcha towns.
Materials and methods
Ethics statement
This study was reviewed by the Research Ethics Review Committee of the School of Veterinary Medicine, Wollo University. The committee approved and confirmed that formal ethical approval was not required for conducting this study since it was not an experimental study and there was no risk of harm or injury to the study subjects, dairy and beef cattle, associated with the research. Prior to the investigation, the general procedures and significance of the study were explained to the study participants. Hence, the participants provided their informed verbal consent for their cattle to be included in the study. Moreover, in this study, the best practices of veterinary care were employed and all procedures were done as per the proper guidelines by professionals.
Study area
The study was conducted in Dessie and Kombolcha towns, South Wollo Zone, Eastern Amhara Region, Ethiopia (Fig 1). Dessie is the capital city of South Wollo zone which is located 401km to the northeast of Addis Ababa, the capital city of Ethiopia, and 480 km east of Bahir Dar, the capital city of Amhara Region [29]. The town is located at 11°8’N-11°46’ North latitude and 39°38’E-41013’East longitude. Topographically, Dessie town lies within elevation range of 2,470 and 2,550 meters above sea level. It has a mean annual rainfall of 1100–1200 mm and the mean annual minimum and maximum temperatures of the town are 9°C and 23.7°C, respectively [30]. Administratively, Dessie town is subdivided into 18 urban and 8 rural Kebeles [31].
Fig 1. Map of the study areas.
Kombolcha is an industrial town situated at a distance of 376 km north of Addis Ababa, the capital city of Ethiopia, 23 km south-west of Dessie, the capital city of South Wollo zone, and 505 km from Bahirdar, the capital city of Amhara Region. The town is located at 11°6’ N latitude and 39°45’E longitude with an elevation ranges from 1, 500 to 1, 840 meters above sea level [30]. The mean annual rainfall of Kombolcha town is 1046 mm and its annual minimum and maximum temperatures are 12.9°C and 28.1°C, respectively. Kombolcha town has a total of 11 administrative kebeles, 6 peri-urban and 5 urban [32].
Study population
Udder and tank milk, milk product (yoghurt and cottage cheese), carcass swab, and beef swab samples were collected from dairy farms, milk product shops, municipal and ELFORA abattoirs, and butcher shops and restaurants in the study areas, respectively.
The total number of registered dairy farms in Kombolcha town at the time of sample collection was 164. In these farms, the total milking, dry and pregnant cows were 586, 266, and 386, respectively [33]. According to the document of Dessie Town Animal Production and Health Office [34], seven large-scale and well-organized dairy farms were found in Dessie town. However, 21 additional non-registered dairy farms were found in Dessie town through an assessment conducted prior to sample collection. Therefore, the total number of milking cows in the 28 dairy farms was around 196. During sample collection, only apparently healthy milking dairy cows were included. However, dry cows, heifers, and clinically ill dairy cows were excluded from the sampling.
Besides the dairy cows in the two study areas, beef cattle slaughtered at Dessie and Kombolcha municipal abbatoirs and Kombolcha ELFORA abattoir were included in the study population.
Study design
A cross-sectional study was conducted from October 2019 to July 2021 to estimate the prevalence of E. coli and E. coli O157:H7 and determine the antibiotic resistance pattern of E. coli O157:H7 from foods of bovine origin in Dessie and Kombolcha towns, Amhara, Ethiopia.
Sample size determination
The sample size (n) was determined based on a statistical formula given by Thrusfield [35].
There was no previous published report related to the proportion of E. coli and E. coli O157:H7 from foods of bovine origin in Dessie and Kombolcha towns, hence an expected prevalence (Pexp) of 50% was used for sample size calculation with 95% confidence interval and 0.05 absolute precision (d). According to the above-given formula, the total sample size computed was 384.
After the assessment of the total number of sample sources (dairy farms, milk product shops, butcher shops, and restaurants, and the number of animals slaughtered at abattoirs) in the two study sites, the sample size of each sample type was allocated proportionally. A total of 384 different samples of foods of bovine origin, including carcass swabs (n = 162) from municipal and ELFORA abattoirs, udder milk (n = 146) and milk tank (n = 6) samples from dairy farms, yoghurt (n = 36) and cottage cheese (n = 9) samples from milk product shops, and beef swabs (n = 25) from butcher shops and restaurants were collected in the two selected study settings. With respect to the study site, 203 and 181 samples were collected from Kombolcha and Dessie towns, respectively.
Sampling technique
A systematic random sampling method was employed to select carcass swab samples among cattle slaughtered at municipal and ELFORA abattoirs in study sites and every 3rd cattle was selected. Milking cows from dairy farms in the study sites were selected using simple random sampling technique to collect udder milk samples. Moreover, tank milk, milk products (yoghurt and cottage cheese), and beef swab samples were also collected using simple random sampling technique.
Sample collection
Using sterile labeled screw cupped glass bottles, 25 ml of milk sample was collected from all quarters of the selected individual milking cows after discarding three streams of milk. Tank milk samples, around 25 ml, were also collected from dairy farms using sterile labeled screw cupped glass bottles after the milk was mixed well. From milk product shops in study sites, approximately 25 ml/g yoghurt and cottage cheese samples were collected aseptically using sterile labeled screw-capped glass bottles. At abattoirs, the carcass swab samples were collected using sterile cotton swabs from the outer and internal surface parts of the selected carcass at five separate locations (neck, thorax, abdomen, breast, and crutch). The swab samples taken from different locations of the same carcass were pooled together and dipped into labeled test tubes containing 5 ml of sterile 0.85% NaCl solution. At butcher shops and restaurants, the beef swab samples were collected from different sites of the selected individual beef and placed into labeled test tubes containing 5 ml of sterile 0.85% NaCl solution. During the time of sample collection, all necessary data related to samples such as study site, sample source, sample type, date of collection and condition of the sample sources were recorded in a pre-designed format. The samples were shipped carefully on the day of collection using an ice box containing ice packs and processed within 24 hrs in Veterinary Microbiology Laboratory, School of Veterinary Medicine, Wollo University, Dessie, Ethiopia.
Isolation and identification of E. coli and E. coli O157:H7
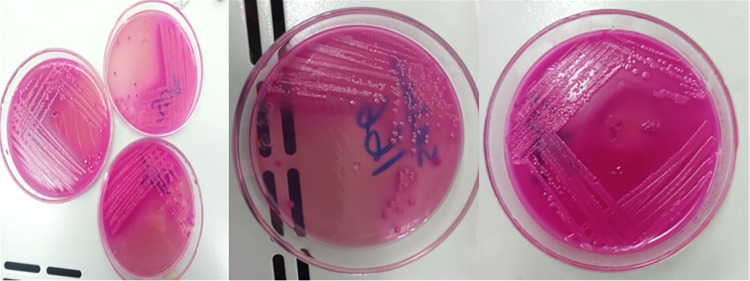
Detection of E. coli and E. coli O157: H7 in all collected samples was conducted according to Quinn et al. [36] with a slight modification. The bacteriological media used for isolation and identification were prepared according to the instructions of the manufacturers. After each original sample was homogenized, 1 ml of the test sample was transferred into 9 ml sterile peptone water (Micromaster, India) and incubated aerobically at 37°C for 24 hrs. The pre-enriched samples were further inoculated into MacConkey broth (Blulux Laboratories Ltd., India) and incubated at 37°C for 24 hrs for selective enrichment. The enrichments were then streaked on MacConkey Agar plates (HiMedia Laboratories Pvt. Ltd., India), and incubated at 37°C for 24 hrs. Pink-colored colonies (Fig 2) were aseptically streaked on nutrient agar plates (HiMedia Laboratories Pvt.Ltd., India) and incubated at 37°C for 24 hrs.
Fig 2. Growth on MacConkey agar plates.
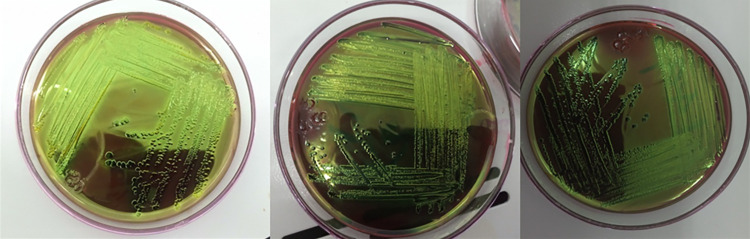
A pure colony was taken from nutrient agar plates and subjected to Gram staining as per procedures described by Merchant and Packer [37]. Gram-negative, pink-colored with rod-shaped appearance and arranged in single or in pairs were suspected as E. coli. A single isolated colony was picked and streaked on Eosin Methylene Blue Agar (EMB) medium (Sisco Research Laboratories Pvt. Ltd., India) and incubated aerobically at 37°C for 24 hrs. The presumptive E. coli colonies that showed greenish metallic sheen [38] (Fig 3) were picked up with a sterile inoculating loop and allowed to grow on nutrient agar plates (HiMedia Laboratories Pvt.Ltd., India) at 37°C for 24 hrs for biochemical examination.
Fig 3. Growth on EMB agar plates.
Standard biochemical tests were used for confirmatory identification of the presumptive E. coli isolates [39,40]. Slide catalase test was performed according to MacFaddin [41]; Indole test was conducted according to Cheesbrough [42]; Methyl Red and Voges Proskauer tests were done according to Cheesbrough [42]; Citrate utilization test was performed according to Simmons [43]; Urease test for bacterial isolates was done according to Chakraborty et al. [44]; and TSI test was carried out according to Vanderzant and Splittstresser [45]. All the biochemical tests were interpreted and isolates which were indole positive, methyl red positive, Voges-Proskauer negative, citrate negative, urease negative, and producing acid with gas and without hydrogen sulfide production on TSI were confirmed to be E. coli.
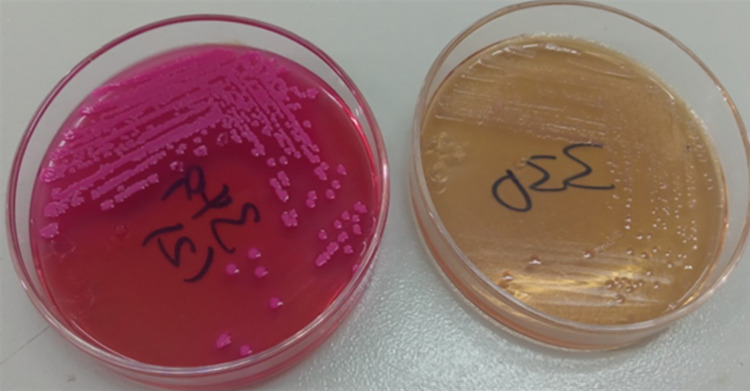
The identified E. coli colonies were further subcultured onto SMAC agar plates (Guangdong Huankai Microbial Sci. & Tech. Co., Ltd., China) at 37°C for 24 hrs to differentiate E. coli O157:H7 strain from other E. coli strains. Sorbitol-fermenters (pinkish colonies) were considered as non-O157:H7 E. coli strains whereas the non-sorbitol-fermenting isolates (colorless or pale colonies) were confirmed as E. coli O157: H7 strains (Fig 4).
Fig 4. Growth on SMAC agar plates.
Antimicrobial susceptibility testing of E. coli O157:H7
All E. coli O157:H7 isolates were evaluated for in vitro antimicrobial susceptibility using the agar disk diffusion method recommended by Bauer et al. [46]. The following sixteen antimicrobial disks (belong to nine classes of antimicrobials) (Mast Group Ltd., Merseyside, U.K) with their concentrations given in parentheses were used in the antibiogram testing: Penicillin class antimicrobials (Amoxicillin (10μg), Ampicillin (25μg), Penicillin G (10IU), and Oxacillin (1μg)); Fluoroquinolones class antimicrobial (Ciprofloxacin (5μg) and Norfloxacin (2μg)); Macrolide class antimicrobial (Erythromycin (15μg)); Aminoglycoside class antimicrobials (Amikacin (30μg), Gentamycin (10μg), and Kanamycin (30μg)); Quinolone class antimicrobial (Nalidixic acid (30μg)); Tetracycline class antimicrobial (Tetracycline (30μg) and Doxycycline (30μg)); Glycopeptides class antimicrobial (Vancomycin (30μg)); Cephalosporin (Ceftriaxone (5μg)); and Sulfonamides (Sulphamethoxazole-trimethoprim (25μg)). The selection of these antibiotics was based on the availability and frequent use of these antimicrobials in the study area both in veterinary and human medicine.
E. coli O157:H7 isolates that had been confirmed biochemically were inoculated onto nutrient agar plates and incubated at 37°C for 24 hrs. After overnight incubation, colonies were transferred and diluted into test tubes containing 5 ml of sterile 0.85% saline solution and mixed thoroughly to generate a homogeneous suspension until the turbidity of the bacterial suspension achieved the 0.5 McFarland turbidity standards. A sterile cotton swab was dipped into the adjusted bacterial suspension and the excess inoculum was removed by lightly pressing the swab against the test tube’s upper inside wall.
To obtain uniform inoculums over the entire surface of the Mueller-Hinton agar plate (HiMedia Laboratories Pvt.Ltd., India), the swab containing the inoculum was spread evenly via a repeated rubbing procedure. The selected antibiotic-impregnated disks were placed at a minimum distance of 24 mm on the surface of the inoculated plate and 10 mm from the edge of the petri dish using sterile thumb forceps after the plates dried for 3 to 5 minutes and gently pressed with the point of a sterile forceps to ensure the complete contact between the disk and the agar surface. Within 15 minutes following the deposit of the disks, the plates were inverted and incubated at 37°C for 24 hrs. After 24 hrs of incubation, the zones of growth inhibition around each of the antibiotic disks were observed. The diameters of inhibition zones were measured using a digital caliper and the findings were recorded in a pre-designed format. The inhibition zone results around individual antibiotic disks were interpreted and the isolates were classified as Sensitive (S), Intermediate (I), and Resistant (R) according to the interpretation tables of the Clinical and Laboratory Standard Institute [47–50], Arabzadeh et al. [51], Reza et al. [52], Tadesse et al. [53], and TMCC [54].
Standard organisms for quality control
To monitor the performance of the laboratory test and ensure accurate test results, the standard strains of E. coli ATCC 25922 obtained from Amhara Public Health Institute (APHI) Dessie branch, were used as control strains.
Data management and analysis
All raw data collected from the study were summarized, compiled, entered, and coded in Microsoft Excel 2007 spreadsheet and imported to STATA Version 12 software for statistical analysis. Both descriptive and inferential statistical techniques were applied to analyze and present the different data types collected from the current study. Among descriptive statistics, frequency and/or percentage were calculated. Chi-square test (χ2) and binary logistic regression were computed to determine the association of different risk factors with contamination of E. coli and E. coli O157: H7 from different foods of bovine origin and the degree of association was determined using Odds ratio (OR) with 95% confidence interval (CI). Statistical significance was considered at P-value less than 0.05.
Results
Overall prevalence
Out of the total of 384 examined samples, 210 (54.7%) and 25 (6.5%) were E. coli and E. coli O157:H7 positive, respectively (Table 1).
Table 1. Prevalence of E. coli and E. coli O157:H7 among the sample types and study sites.
| Variables | No. of examined | No. of positive (%) | χ2-value | P-value | |||
|---|---|---|---|---|---|---|---|
| E. coli | E. coli O157:H7 | E. coli | E. coli O157:H7 | E. coli | E. coli O157:H7 | ||
| Sample type | 82.871 | 6.926 | 0.000 | 0.226 | |||
| Udder milk | 146 | 63 (43.2) | 14 (9.6) | ||||
| Tank milk | 6 | 2 (33.3) | 1 (16.7) | ||||
| Yoghurt | 36 | 5 (13.9) | 0 (0.0) | ||||
| Cheese | 9 | 1 (11.1) | 0 (0.0) | ||||
| Beef swab | 25 | 10 (40.0) | 1 (4.0) | ||||
| Carcass swab | 162 | 129 (79.6) | 9 (5.6) | ||||
| Study site | 0.6695 | 2.4584 | 0.413 | 0.117 | |||
| Dessie | 181 | 95 (52.5) | 8 (4.4) | ||||
| Kombolcha | 203 | 115 (56.7) | 17 (8.4) | ||||
| Overall | 384 | 210 (54.7) | 25 (6.5) | ||||
The sample type based prevalence of E. coli from carcass swab, udder milk, beef swab, tank milk, yoghurt, and cheese samples was 79.6%, 43.2%, 40.0%, 33.3%, 13.9%, and 11.1%, respectively. A statistically significant difference in the E. coli prevalence (P<0.05) was observed among the different sample types of foods of bovine origin. Among the examined sample types, the highest (16.7%) and lowest (0.0%) prevalence rates of E. coli O157:H7 were recorded from tank milk and milk products, respectively. The difference in the prevalence of E. coli O157:H7 among different sample types was not statistically significant (P>0.05) (Table 1).
With respect to the study site, the prevalence rates of E. coli (52.5% and 56.7%) and E. coli O157:H7 (4.4% and 8.4%) were found in Dessie and Kombolcha towns, respectively. There was no statistically significant difference in the prevalence rates of the isolates between the two study sites (P>0.05) (Table 1).
The odds of detection of E. coli were 31.27 times higher among carcass swab samples than in cheese samples and it was statistically significant (P<0.05) (Table 2).
Table 2. Bivariate logistic regression result of E. coli among different sample types.
| Sample type predictor | E. coli | |
|---|---|---|
| OR (95% CI) | P-value | |
| Cheese | Reference | |
| Tank milk | 4.0 (0.27–58.56) | 0.311 |
| Yoghurt | 1.29 (0.13–12.66) | 0.827 |
| Udder milk | 6.07 (0.74–49.81) | 0.093 |
| Beef swab | 5.33 (0.57–49.48) | 0.141 |
| Carcass swab | 31.27 (3.78–258.91) | 0.001 |
Prevalence of E. coli and E. coli O157:H7 among variables of different sample types
The recorded prevalence rate of E. coli O157:H7 in milk samples from cows with previous treatment history was 13.7%. The difference in the prevalence of E. coli O157:H7 among treatment history categories was statistically significant (P<0.05). The prevalence of E. coli O157:H7 from milk samples was higher in Kombolcha town (14.0%) than in Dessie town (1.9%). The difference in the prevalence of E. coli O157:H7 between the two sites was statistically significant (P<0.05) (Table 3).
Table 3. Prevalence of E. coli and E. coli O157:H7 among different variables of milk samples.
| Variables | No. of examined | No. of positive | χ2-value | P-value | |||
|---|---|---|---|---|---|---|---|
| E. coli | E. coli O15:7H | E. coli | E. coli O157:H7 | E. coli | E. coli O157:H7 | ||
| Study site | 0.598 | 5.610 | 0.440 | 0.018 | |||
| Dessie | 52 | 20 (38.5) | 1 (1.9) | ||||
| Kombolcha | 100 | 45 (45.0) | 14 (14.0) | ||||
| Sample type | 0.227 | 0.325 | 0.634 | 0.569 | |||
| Udder milk | 146 | 63 (43.2) | 14 (9.6) | ||||
| Tank milk | 6 | 2 (33.3) | 1 (16.7) | ||||
| Farm System | 0.000 | 0.714 | 0.993 | 0.398 | |||
| Intensive | 131 | 56 (42.7) | 14 (10.7) | ||||
| Semi Intensive | 21 | 9 (42.9) | 1 (4.8) | ||||
| Treatment history | 1.392 | 5.186 | 0.238 | 0.023 | |||
| No | 50 | 18 (36.0) | 1 (2.0) | ||||
| Yes | 102 | 47 (46.1) | 14 (13.7) | ||||
| Milking practice | 6.155 | 0.721 | 0.104 | 0.868 | |||
| Excellent | 3 | 0 (0.0) | 0 (0.0) | ||||
| Very good | 42 | 22 (52.4) | 4 (9.5) | ||||
| Good | 104 | 43 (41.3) | 11 (10.6) | ||||
| Poor | 3 | 0 (0.0) | 0 (0.0) | ||||
| Farm hygiene | 3.708 | 1.711 | 0.295 | 0.635 | |||
| Excellent | 4 | 0 (0.0) | 0 (0.0) | ||||
| Very good | 52 | 25 (48.1) | 6 (11.5) | ||||
| Good | 85 | 35 (41.2) | 7 (8.2) | ||||
| Poor | 11 | 5 (45.5) | 2 (18.2) | ||||
| Total | 152 | 65 (42.8) | 15 (9.9) | ||||
According to the result presented in Table 4, E. coli O157:H7 was not detected in milk products. The difference in the prevalence of E. coli among all hypothesized variables of milk products was not statistically significant (P>0.05).
Table 4. Prevalence of E. coli and E. coli O157:H7 among the variables of milk product samples.
| Variables | No. of examined | No. of positive (%) | χ2-value | P-value | |
|---|---|---|---|---|---|
| E. coli | E. coli O15:7H7 | E. coli | E. coli | ||
| Study site | 0.087 | 0.769 | |||
| Dessie | 20 | 3 (15.0) | 0 (0.0) | ||
| Kombolcha | 25 | 3 (12.0) | 0 (0.0) | ||
| Sample Type | 0.048 | 0.826 | |||
| Yoghurt | 36 | 5 (13.9) | 0 (0.0) | ||
| Cheese | 9 | 1 (11.1) | 0 (0.0) | ||
| Equipment Type | 0.322 | 0.570 | |||
| Aluminum can | 2 | 0 (0.0) | 0 (0.0) | ||
| Plastic | 43 | 6 (14.0) | 0 (0.0) | ||
| Hygiene | 4.350 | 0.114 | |||
| Excellent | 6 | 0 (0.0) | 0 (0.0) | ||
| Very good | 20 | 5 (25.0) | 0 (0.0) | ||
| Good | 19 | 1 (5.3) | 0 (0.0) | ||
| Total | 45 | 6 (13.3) | 0 (0.0) | ||
There was no statistically significant difference in the prevalence of E. coli O157:H7 among all hypothesized variables of carcass swab samples (P>0.05). The prevalence of E. coli from carcass swab samples was higher in Kombolcha town (89.4%) than in Dessie town (72.9%) and the difference was statistically significant (P<0.05) (Table 5).
Table 5. Prevalence of E. coli and E. coli O157:H7 among variables of carcass swab samples.
| Variables | No. of examined | No. of positive (%) | χ2-value | P-value | ||||
|---|---|---|---|---|---|---|---|---|
| E. coli | E. coli O157H7 | E. coli | E. coli O157H7 | E. coli | E. coli O157H7 | |||
| Study site | 6.546 | 0.217 | 0.011 | 0.642 | ||||
| Dessie | 96 | 70 (72.9) | 6 (6.2) | |||||
| Kombolcha | 66 | 59 (89.4) | 3 (4.5) | |||||
| Source | 7.293 | 0.014 | 0.007 | 0.905 | ||||
| Municipal Abattoir | 105 | 77 (73.3) | 6 (5.7) | |||||
| ELFORA | 57 | 52 (91.2) | 3 (5.3) | |||||
| Hygiene of slaughtering process | 0.196 | 1.071 | 0. 907 | 0.585 | ||||
| Very good | 78 | 63 (80.8) | 3 (3.8) | |||||
| Good | 49 | 38 (77.6) | 4 (8.2) | |||||
| Poor | 35 | 28 (80.0) | 2 (5.7) | |||||
| Hygiene of butchers | 0.599 | 1.703 | 0.741 | 0.427 | ||||
| Very good | 78 | 63 (80.8) | 3 (3.8) | |||||
| Good | 55 | 42 (76.4) | 3 (5.5) | |||||
| Poor | 29 | 24 (82.8) | 3 (10.3) | |||||
| Hygiene of slaughtering materials | 8.529 | 0.87 | 0.014 | 0.958 | ||||
| Excellent | 57 | 52 (91.2) | 3 (5.3) | |||||
| Good | 83 | 59 (71.1) | 5 (6.0) | |||||
| Poor | 22 | 18 (81.8) | 1 (4.5) | |||||
| Total | 162 | 129 (79.6) | 9 (5.6) | |||||
The proportion of E. coli in beef swab samples collected from butcher shops and restaurants in Kombolcha town (66.7%) was higher than in Dessie town (15.4%) and the difference was statistically significant (P<0.05). A higher prevalence of E. coli O157:H7 (50.0%) was obtained in beef swab samples collected from butcher shops having poor hygiene and the difference was statistically significant (P<0.05) as presented in Table 6.
Table 6. Prevalence of E. coli and E. coli O157:H7 among the variables of beef swab samples.
| Variables | No. of examined | No. of positive (%) | χ2-value | P-value | |||
|---|---|---|---|---|---|---|---|
| E. coli | E. coli O157H7 | E. coli | E. coli O157H7 | E. coli | E. coli O157H7 | ||
| Study site | 6.838 | 0.962 | 0.009 | 0.327 | |||
| Dessie | 13 | 2 (15.4) | 1 (7.7) | ||||
| Kombolcha | 12 | 8 (66.7) | 0 (0.0) | ||||
| Where get slaughtered | 0.446 | 0.198 | 0.504 | 0.656 | |||
| Abattoir | 21 | 9 (42.9) | 1 (4.8) | ||||
| Field | 4 | 1 (25.0) | 0 (0.0) | ||||
| Hygiene of butchers | 2.778 | 3.299 | 0.249 | 0.192 | |||
| Very good | 18 | 6 (33.3) | 0 (0.0) | ||||
| Good | 6 | 4 (66.7) | 1 (16.7) | ||||
| Poor | 1 | 0 (0.0) | 0 (0.0) | ||||
| Hygiene of cutting utensils | 4.167 | 0.260 | 0.041 | 0.610 | |||
| Very good | 5 | 4 (80) | 0 (0.0) | ||||
| Good | 20 | 6 (30) | 1 (5.0) | ||||
| Hygiene of butcher shops | 3.405 | 11.979 | 0.333 | 0.007 | |||
| Excellent | 2 | 1 (50.0) | 0 (0.0) | ||||
| Very good | 13 | 3 (23.1) | 0 (0.0) | ||||
| Good | 8 | 5 (62.5) | 0 (0.0) | ||||
| Poor | 2 | 1 (50.0) | 1 (50) | ||||
| Total | 25 | 10 (40.0) | 1 (4.0) | ||||
In vitro antimicrobial sensitivity pattern of E. coli O157:H7 isolates
The result of the in vitro antimicrobial sensitivity assay of the 25 E. coli O157:H7 isolates to the sixteen selected antimicrobial agents revealed that all strains (100.0%) were susceptible to Ampicillin, Sulfamethoxazole-trimethoprim, and Norfloxacin. On the contrary, all of the isolates (100%) were resistant to Penicillin G, Vancomycin, and Oxacillin. Moreover, high percentages of the isolates (92.0%) were also resistant to Erythromycin as presented in Table 7.
Table 7. In vitro antimicrobial sensitivity pattern of E. coli O157:H7 isolated from different sample types of foods of bovine origin.
| Antimicrobial agents | Interpretation categories | ||
|---|---|---|---|
| Sensitive | Intermediate | Resistant | |
| Amikacin | 18 (72.0) | 6 (24.0) | 1 (4.0) |
| Erythromycin | 0 (0.0) | 2 (8.0) | 23 (92.0) |
| Gentamicin | 22 (88.0) | 3 (12.0) | 0 (0.0) |
| Kanamycin | 7 (28.0) | 18 (72.0) | 0 (0.0) |
| Nalidixic acid | 22 (88.0) | 3 (12.0) | 0 (0.0) |
| Amoxicillin | 15 (60.0) | 0 (0.0) | 10 (40.0) |
| Ampicillin | 25 (100) | 0 (0.0) | 0 (0.0) |
| Doxycycline | 23 (92.0) | 1 (4.0) | 1 (4.0) |
| Tetracycline | 24 (96.0) | 0 (0.0) | 1 (4.0) |
| Penicillin G | 0 (0.0) | 0 (0.0) | 25 (100) |
| Sulfamethoxazole-trimetoprim | 25 (100) | 0 (0.0) | 0 (0.0) |
| Vancomycin | 0 (0.0) | 0 (0.0) | 25 (100) |
| Norfloxacin | 25 (100) | 0 (0.0) | 0 (0.0) |
| Ceftriaxone | 24 (96.0) | 1 (4.0) | 0 (0.0) |
| Ciprofloxacin | 18 (72.0) | 7 (28.0) | 0 (0.0) |
| Oxacillin | 0 (0.0) | 0 (0.0) | 25 (100) |
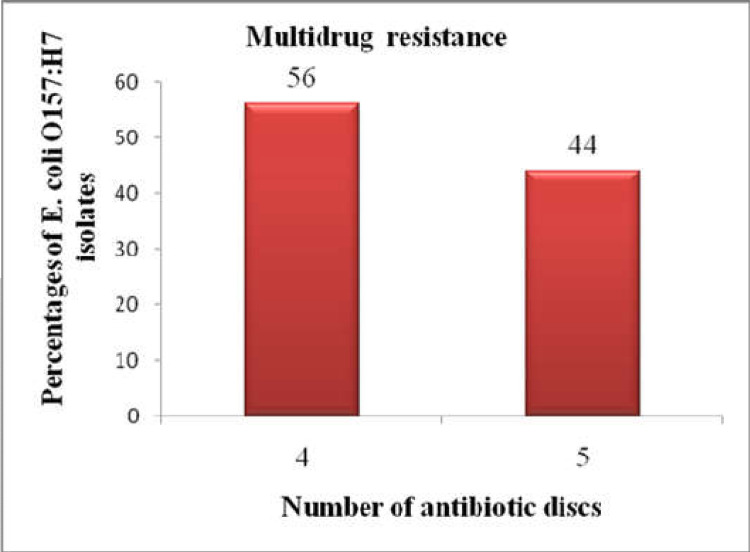
Multidrug resistance to more than three drugs was observed among all isolates of E. coli O157:H7. As shown in Fig 5, 14 (56.0%) and 11 (44.0%) of the isolates showed resistance to four and five drugs, respectively.
Fig 5. Multidrug resistance pattern of E. coli O157:H7 isolates.
Discussion
The present study revealed an overall E. coli prevalence of 54.7% from different foods of bovine origin collected from different sources in the study areas. This prevalence was in agreement with previous studies reported by Limbu et al. [55] (55.0%), Soomro et al.) [56] (55.0%), Atsbha et al. [57] (57.29%), Reta et al. [58] (58.0%), Tadesse et al. [53] (51.2%), and Meshref [59] (52.6%) in Dharan (Nepal), Tandojam (Pakistan), Mekelle town, Jigjiga city, Mekelle town, and Beni-Suef governorate (Egypt), respectively.
However, in comparison to the present study, higher prevalence rates of E. coli were reported by Salauddin et al. [60] (100.0%), Baz et al. [61] (96.0%), Arjyal et al. [62] (92.0%), Balcha et al. [63] (62.5%), Gundogan and Avci [64] (74.0%), Lingathurai and Vellathurai [65] (70.0%), Altalhi and Hassan [66] (66.0%), Chyea et al. [67] (64.5%), and Ali and Abdelgadir [68] (63.0%) in Rangpur Division (Bangladesh), Kars city (Turkey), Kathmandu Valley (Nepal), Mekelle, Turkey, Madurai (South India), Taif region (Western Saudi Arabia), Malaysia, and Khartoum state, respectively.
On the other hand, the prevalence of E. coli in the current study was higher than the reports of Messele et al. [7] in Addis Ababa and Bishoftu towns (5.5%), Messele et al. [12] in central Ethiopia (Sebeta, Burayu, and Holeta towns) (7.1%), Kumar and Prasad [69] in and around Pantnagar (India) (8.14%), Yakubu et al. [70] in Sokoto Metropolis (Nigeria) (9.23%), Mengistu et al. [71] in Eastern Ethiopia (12.41%), Ngaywa et al. [72] in Kenya (13.8%), Mohammed et al. [73] in Dire Dawa city (15.89%), Ababu et al. [17] in Holeta District (19.0%), Hiwot et al. [74] in Arsi and East Shewa Zones (19.8%), Bedasa et al. [19] in Bishoftu town (20.0%), Sebsibe and Asfaw [75] in Jimma town (20.2%), Tadese et al. [76] in Ambo town (23.4%), Abebe et al. [77] in selected districts of Tigray (23.7%), Abayneh et al. [78] in Jimma town (23.9%), Yohannes [79] in Mekelle town (25.0%), Haileselassie et al. [80] in Mekelle city (27.3%), Hiko et al. [81] in Addis Ababa (29.0%), Momtaz et al. [82] in Iran (29.7%), Taye et al. [83] in Haramaya University abattoir (30.97%), Disassa et al. [8] in and around Asosa town (33.9%), Tadesse et al. [53] in Mekelle town (36.63%), Thaker et al. [84] in Anand Gujarat (India) (38.0%), Zerabruk et al. [85] in Addis Ababa (43.75%), Sobeih et al. [86] in Kafr El-Sheikh Governorate (Egypt) (44.44%), and Welde et al. [87] in and around Modjo town (46.26%).
The result obtained from the current bacteriological study revealed that the overall prevalence of E. coli O157:H7 was 6.5%. This finding was consistent with the previous reports of Gutema et al. [88] (6.3%), Ababu et al. [17] (5.2%), Beyi et al. [89] (4.5%), Reuben and Owuna [90] (4.5%), Sebsibe and Asfaw [75] (5.4%), Hiko et al. [91] (8.0%), Rahimi et al. [92] (8.2%), Vanitha et al. [93] (8.8%), and Tadese et al. [76] (9.1%) in Bishoftu town, Holeta District, central Ethiopia, Nasarawa State (Nigeria), Jimma town, Debre-Zeit and Modjo towns, Fars and Khuzestan provinces (Iran), Kerala (India), and Ambo town, respectively. However, the result found in the present study was higher than Dadi et al. [94] (0.0%) in Sebeta town (Ethiopia), Baz et al. [61] in Kars city (Turkey) (0.0%), Swai and Schoonman [95] in Tanga region (Tanzania) (0.0%), Abdissa et al. [15] in Addis Ababa and Debre Berhan cities (0.8%), Yakubu et al. [70] in Sokoto Metropolis (Nigeria) (1.92%), Mengistu et al. [71] in Eastern Ethiopia (2.06%), Geresu and Regassa [96] in the selected study settings of Arsi Zone (2.1%), Atnafie et al. [13] in Hawassa town (2.33%), Meshref [59] in Beni-Suef governorate (Egypt) (2.6%), Taye et al. [83] in Haramaya University abattoir (2.65%), Disassa et al. [8] in and around Asosa town (2.9%), Carney et al. [97] in Ireland (3.0%), Mcevoy et al. [98] in Ireland (3.2%), Ahmed and Shimamoto [99] in Egypt (3.4%), and Bedasa et al. [19] in Bishoftu town (3.5%).
On the other hand, the prevalence of E. coli O157:H7 found in this study was lower than the reports of Lingathurai and Vellathurai [65] (65.0%), Islam et al. [100] (52.4%), Llorente et al. [101] (36.1%), Chyea et al. [67] (33.5%), Bekele et al. [10] (13.3%), Hamid et al. [102] (12.0%), Balcha et al. [63] (11.3%), and Abebe et al. [77] (10.4%) in Madurai (South India), Bangladesh, Buenos Aires (Argentina), Malaysia, Addis Ababa, Addis Ababa, in and around Mekelle, and selected districts of Tigray, respectively. Such variations in E. coli and E. coli O157:H7 prevalence rates between present and other previous studies might be due to differences in management and hygienic practices in dairy and beef farms, standards and furnishings of abattoir and dairy farms, dairy cow herd health status (these bacteria are commonly isolated from mastitic milk), hygienic conditions in slaughterhouses and milking premises, cleanliness of milking and slaughtering utensils, hygienic practices during milking and slaughtering, water quality and its availability, and hygienic conditions of foods of bovine origin during handling, transportation, storage, and distribution up to consumption. Moreover, the variations could also arise from differences in study methods employed by researchers including sample source, sample size, sampling techniques, sample type, and methods of detection in laboratories.
The current study showed that the prevalence of E. coli was highest in carcass swab samples (79.6%) followed by udder milk (43.2%), beef swab (40.0%), tank milk (33.3%), yoghurt (13.9%), and cheese (11.1%). Unlike E. coli O157:H7, a statistically significant difference in the E. coli prevalence (P<0.05) was observed among different sample types of foods of bovine origin. The odds of detection of E. coli were 31.27 times higher among carcass swab samples than in cheese samples and it was statistically significant (P<0.05). At abattoirs, sanitation and hygiene are the crucial factors that contribute to meat contamination [103]. Poor hygienic practices at abattoirs during bleeding, skinning, evisceration, carcass washing, and splitting might be responsible for the contamination and higher magnitude of E. coli in carcass samples. In addition, the prevalence of E. coli O157:H7 in tank milk, udder milk, carcass swab, beef swab, yoghurt, and cheese samples was 16.7%, 9.6%, 5.6%, 4.0%, 0.0%, and 0.0%, respectively. The presence of E. coli in milk is not only regarded as faecal contamination but also an indicator of poor hygiene and sanitary practices during milking and further handling [8,64,66]. The higher proportion of E. coli O157:H7 in tank milk could be from different sources including unhygienic milking practices, cows infected with mastitis, milk handlers with poor hygiene, poor quality water, and inappropriately cleaned milk filtering utensils and tanks.
The prevalence of E. coli O157:H7 from milk samples was higher in Kombolcha town (14.0%) than in Dessie town (1.9%). The statistically significant difference (P<0.05) in the prevalence of E. coli O157:H7 among the two study sites could be associated with variation in hygienic practices in the dairy environment and herd health status of dairy farms. A higher prevalence of E. coli O157:H7 was recorded in milk samples from cows with teat treatment history (13.7%) than non treated cows (2.0%) and the difference was statistically significant (P<0.05). Cows with previous mastitis history are more likely to become infected than those which had never been exposed as they might remain in a carrier state as well as the ineffectiveness of mastitis treatment medicines [104]. The most common serotypes of E. coli recovered from mastitic milk are O157, O55, O111, O124, O119, O114, O26, and O44 [105]. Thus, the relatively high magnitude of E. coli O157:H7 in milk samples from cows with treatment history might be associated with environmental bovine mastitis.
In the present study, E. coli O157:H7 was not detected in milk products. According to Rahimi et al. [106], the survival of E. coli O157:H7 in foods is dependent on the acidity of the sample; when the pH falls below 3.5, the bacteria die. Thus, the absence of E. coli O157:H7 in yogurt and cheese samples in this study might be due to the acidity of these products and the temperature used during the processing of cheese.
The prevalence of E. coli from carcass swab samples was higher in Kombolcha town (89.4%) than in Dessie town (72.9%) and the difference was statistically significant (P<0.05). The variation could be due to the difference in hygienic practices at abattoirs. The proportion of E. coli in beef swab samples collected from butcher shops and restaurants in Kombolcha town (66.7%) was higher than in Dessie town (15.4%) and the difference was statistically significant (P<0.05). The variation could be due to the difference in hygienic practices during the slaughtering process at abattoirs and sanitation at butcher shops and restaurants. Moreover, a higher prevalence of E. coli O157:H7 (50.0%) was obtained in beef swab samples collected from butcher shops having poor hygiene and the difference was statistically significant (P<0.05). The higher occurrence of E. coli O157:H7 in beef swab samples collected from butcher shops having poor hygiene was not surprising since beef contamination is usually associated with poor hygiene.
The occurrence of antimicrobial resistance among foodborne pathogens is increasing [107]. The E. coli O157:H7 strains are heterogeneous with respect to antibiotic resistance [108]. The development of antimicrobial resistance in E. coli O157:H7 strains isolated from animals and humans [90] and the emergence of multidrug-resistant E. coli O157:H7 strains become a universal public health concern [109]. In the present study, multidrug resistance to more than three drugs was observed among all E. coli O157:H7 isolates. In brief, 56.0% and 44.0% of the isolates showed resistance to four and five drugs, respectively.
All isolates of E. coli O157:H7 (100%) were resistant to Penicillin G, Vancomycin, and Oxacillin. Moreover, high percentages of the isolates (92.0%) were also resistant to Erythromycin. The total resistance to Penicillin G was similar to the reports of Igbinosa and Chiadika [110] and Reuben et al. [111] who reported 100.0% resistance to Penicillin G in Benin City (Nigeria) and Nasarawa State (Nigeria), respectively. However, Msolo et al. [112] reported 85.0% resistance to Penicillin G in South Africa. The resistance of all isolates to Vancomycin was comparable to the report of Bedasa et al. [19] who reported 90.0% resistance to Vancomycin in Bishoftu town. The high frequency of resistance to Erythromycin was in agreement with the previous reports of Reuben and Owuna [90] in Nasarawa State, Nigeria, and Igbinosa and Chiadika [110] in Benin City (Nigeria) who reported 94.7% and 89.5% resistance to Erythromycin, respectively. The total resistance of the isolates to Oxacillin was higher than the report of Reuben and Owuna [90] who reported 84.2% resistance to Oxacillin.
On the contrary, all E. coli O157:H7 strains (100.0%) were susceptible to Ampicillin, Sulfamethoxazole-trimethoprim, and Norfloxacin. The total susceptibility to Sulfamethoxazole-trimethoprim was similar to previous findings of Tadese et al. [76], Bekele et al. [10], Beyi et al. [89], and Geresu and Regassa [96] who reported 100.0% sensitivity to Sulfamethoxazole-trimethoprim in Ambo town, Addis Ababa, central Ethiopia, and selected study settings of Arsi Zone, respectively. The total susceptibility to Norfloxacin was similar to the previous finding of Tadese et al. [76] (100.0%) in Ambo town. The 100.0% susceptibility to Ampicillin was similar to the previous report of Osaili et al. [113] who reported 100.0% sensitivity to Ampicillin in Amman City, Jordan.
Higher percentages of the isolates were also sensitive to Doxycycline (92.0%), Tetracycline (96.0%), Ceftriaxone (96.0%), Gentamicin (88.0), Nalidixic acid (88.0%), Amikacin (72.0%), and Ciprofloxacin (72.0%). The sensitivity of isolates to Gentamicin was comparable with the report of Bedasa et al. [19] who reported 82.5% sensitivity to Gentamicin in Bishoftu town. The susceptibility to Amikacin was consistent with Msolo et al. [112] who reported 70.0% sensitivity to Amikacin in South Africa. The sensitivity to Ciprofloxacin was consistent with the reports of Bekele et al. [10] in Addis Ababa and Reuben and Owuna [90] in Nasarawa State, Nigeria who reported 76.5% and 78.9% sensitivity to Ciprofloxacin, respectively. The high sensitivity to Ceftriaxone was consistent with the reports of Bedasa et al. [19], Atnafie et al. [13], and Haile et al. [114] who reported 100% sensitive isolates to Ceftriaxone in Bishoftu, Hawassa, and Jimma towns, respectively. The 96.0% sensitivity to Tetracycline was consistent with Haile et al. [114] in Jimma, Bekele et al. [10] in Addis Ababa, Osaili et al. [113] in Amman City (Jordan), and Bedasa et al. [19] in Bishoftu town who reported 100%, 100.0%, 100.0% and 97.5% sensitivity to Tetracycline. However, Welde et al. [87] reported 77.8% resistance to Tetracycline in and around Modjo town. According to Mokgophi et al. [115] and Qamar et al. [116], the extensive, indiscriminate and injudicious use of antibiotics in both veterinary medicine and public health leads to genetic modification in most bacterial strains for evolving resistance and an increase in the prevalence of resistance among pathogens.
Conclusion and recommendations
The high magnitude of E. coli contamination and finding of multidrug-resistant E. coli O157:H7 in the current study indicated that different foods of bovine origin in the study area were unsafe for human consumption. The multidrug resistance pattern of all E. coli O157:H7 isolates might be due to the injudicious and extensive use of antibiotics in both veterinary and human medicines. In addition, slaughtering of cattle on the floor at municipal abattoirs, unsanitary milk production, and handling, and the community’s consumption habit of raw animal products could expose humans in study sites to multidrug-resistant E. coli O157:H7. However, the current study didn’t address the serotyping and molecular characterization of E. coli O157:H7 and its antimicrobial resistance genes. Hence, good hygienic production methods should be employed to ensure the safety of different foods of bovine origin. Microbiological guidelines mainly the HACCP system and standardized slaughtering operations should be followed to improve meat safety. The emergence and spread of antibiotic-resistant pathogens should be assessed regularly and rational use of antibiotics should be practiced. Moreover, further studies on serotyping and molecular characterization of E. coli O157:H7 should be done at the study sites.
Acknowledgments
We would like to thank the dairy farm owners and/or managers, farm attendants, milk product shop workers, restaurant managers, and owners, butchers, abattoir workers, veterinarians, animal health and production officers, and other inhabitants in the study sites for their voluntariness and cooperation during sample collection.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This research work was funded by Wollo University Research and Community Service Vice President Office and Wollo University Post Graduate Directorate Office. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Assefa A. Prevalence of Escherichia coli O157:H7 in foods of animal origin in Ethiopia: A meta-analysis. Cogent Food Agric. 2019; 5(1): 1–10. [Google Scholar]
- 2.Abebe E, Gugsa G, Ahmed M. Review on major food-borne zoonotic bacterial pathogens. J Trop Med. 2020; 2020: 1–19. doi: 10.1155/2020/4674235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abreham S, Teklu A, Cox E, Tessema ST. Escherichia coli O157:H7: Distribution, molecular characterization, antimicrobial resistance patterns and source of contamination of sheep and goat carcasses at an export abattoir, Mojdo, Ethiopia. BMC Microbiol. 2019; 19: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li M, Havelaar AH, Hoffmann S, Hald T, Kirk MD, Torgerson PR, Devleesschauwer B. Global disease burden of pathogens in animal source foods, 2010. PLoS ONE. 2019; 4(6): 1–18. doi: 10.1371/journal.pone.0216545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arafa AA, Ibrahim ES, Fouad EA, Gaber ES. Antibiotic resistance of Staphylococci concerning strains included in food industry in Egypt. Int J Pharm Clin Res. 2016; 8(12): 1583–9. [Google Scholar]
- 6.Bintsis T. Foodborne pathogens. AIMS Microbiol. 2017; 3(3): 529–63. doi: 10.3934/microbiol.2017.3.529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Messele YE, Abdi RD, Yalew ST, Tegegne DT, Emeru BA, Werid GM. Molecular determination of antimicrobial resistance in Escherichia coli isolated from raw meat in Addis Ababa. Ann Clin Microbiol Antimicrob. 2017; 16(55): 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Disassa N, Sibhat B, Mengistu S, Muktar Y, Belina D. Prevalence and antimicrobial susceptibility pattern of E. coli O157:H7 isolated from traditionally marketed raw cow milk in and around Asosa Town, Western Ethiopia. Vet Med Int. 2017; 2017: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adzitey F. Antibiotic Resistance of Escherichia coli Isolated from Beef and its Related Samples in Techiman Municipality of Ghana. Asian J Anim Sci. 2015; 9(5): 233–40. [Google Scholar]
- 10.Bekele T, Zewde G, Tefera G, Feleke A, Zerom K. Escherichia coli O157:H7 in raw meat in Addis Ababa, Ethiopia: Prevalence at an abattoir and retailers and antimicrobial susceptibility. Int J Food Contam. 2014; 1(1): 1–9. [Google Scholar]
- 11.Dulo F, Feleke A, Szonyi B, Fries R, Baumann MPO, Grace D. Isolation of Multidrug-Resistant Escherichia coli O157 from Goats in the Somali Region of Ethiopia: A Cross-Sectional, Abattoir-Based Study. PLoS ONE. 2015; 10(11): 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Messele EY, Abdi DR, Tegegne TD, Bora KS. Analysis of milk-derived isolates of E. coli indicating drug resistance in central Ethiopia. Trop Anim Health Prod 2018; 51(3): 661–7. [DOI] [PubMed] [Google Scholar]
- 13.Atnafie B, Paulos D, Abera M, Tefera G., Hailu D, Kasaye S, Amenu K. Occurrence of Escherichia coli O157:H7 in cattle feces and contamination of carcass and various contact surfaces in abattoir and butcher shops of Hawassa, Ethiopia. BMC Microbiol. 2017; 17(24): 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stromberg ZR, Goor AV, Redweik GAJ, Brand MJW, Wannemuehler MJ, Mellata M. Pathogenic and non-pathogenic Escherichia coli colonization and host inflammatory response in a defined microbiota mouse model. Dis Model Mech. 2018; 11: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abdissa R, Haile W, Fite TA, Beyi AF, Agga EG, Edao MB, et al. Prevalence of Escherichia coli O157: H7 in beef cattle at slaughter and beef carcasses at retail shops in Ethiopia. BMC Infect Dis. 2017; 17(1): 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Ajmi D, Rahman S, Banu S. Occurrence, virulence genes, and antimicrobial profiles of Escherichia coli O157 isolated from ruminants slaughtered in Al Ain, United Arab Emirates. BMC Microbiol. 2020; 20: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ababu A, Endashaw D, Fesseha H. Isolation and antimicrobial susceptibility profile of Escherichia coli O157:H7 from raw milk of dairy cattle in Holeta District, Central Ethiopia. Int J Microbiol. 2020; 2020: 1–8. [Google Scholar]
- 18.Kwan SY, Chang WS, Loo YY, Nordin Y, Tan CW, Kuan CH, et al. Prevalence and antibiotic profile of Shiga-toxin producing Escherichia coli and Escherichia coli O157:H7 in beef and buffalo. Food Research. 2019; 3 (1): 28–39. [Google Scholar]
- 19.Bedasa S, Shiferaw D, Abraha A, Moges T. Occurrence and antimicrobial susceptibility profile of Escherichia coli O157:H7 from food of animal origin in Bishoftu Town, Central Ethiopia. Int J Food Contam. 2018; 5(1): 1–8. [Google Scholar]
- 20.Okwu MU, Olley M, Akpoka AO, Izevbuwa OE. Methicillin-resistant Staphylococcus aureus (MRSA) and anti-MRSA activities of extracts of some medicinal plants: A brief review. AIMS Microbiol. 2019; 5(2): 117–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Umaru GA, Kabir J, Umoh VJ, Bello M, Kwaga JKP. Methicillin-resistant Staphylococcus aureus (MRSA) in fresh and fermented milk in Zaria And Kaduna, Nigeria. Int J Drug Res Tech. 2013; 3(3): 67–75. [Google Scholar]
- 22.Schroeder CM, Zhao C, DebRoy C, Torcolini J, Zhao S, White DG, Wagner DD, McDermott PF, Walker RD, Meng J. Antimicrobial resistance of Escherichia coli O157 isolated from humans, cattle, swine, and food. Appl Environ Microbiol. 2002; 68(2): 576–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goncuoglu M, Bilir Ormanci FS, Ayaz ND, Erol I. Antibiotic resistance of Escherichia coli O157: H7 isolated from cattle and sheep. Ann Microbiol. 2010; 60(3): 489–94. [Google Scholar]
- 24.Haile AF, Alonso S, Berhe N, Atoma TB, Boyaka PN, Grace D. Prevalence, Antibiogram, and Multidrug-Resistant Profile of E. coli O157: H7 in Retail Raw Beef in Addis Ababa, Ethiopia. Front Vet Sci 2022; 9: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fung F, Wang HS, Menon S. Food safety in the 21st century. Biomed J. 2018; 41(2): 88–95. doi: 10.1016/j.bj.2018.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foddai ACG, Grant IR. Methods for detection of viable foodborne pathogens: Current state-of-art and future prospects. Appl Microbiol Biotechnol. 104(10); 2020: 4281–8. doi: 10.1007/s00253-020-10542-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Law JWF, Ab Mutalib NS, Chan KG, Lee LH. Rapid methods for the detection of foodborne bacterial pathogens: principles, applications, advantages and limitations. Front Microbiol. 2015: 5:1–19. doi: 10.3389/fmicb.2014.00770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baraketi A, Salmieri S, Lacroix M . Foodborne pathogens detection: persevering worldwide challenge. Biosensing technologies for the detection of pathogens-a prospective way for rapid analysis. IntechOpen, Rijeka, Croatia; 2018, pp.53–72. [Google Scholar]
- 29.Ademe S, Edmealem A. Detection of hypertension and its associated factors among Dessie Town Government School Staffs, Amhara Region, Dessie, Ethiopia, 2019. Ann Clin Hypertens. 2019; 4: 009–015. [Google Scholar]
- 30.Ahmed K, Tamir B, Mengistu A. Fattening cattle health problems and fatteners indigenous measures in Dessie and Kombolcha towns, Ethiopia. Agric Biol J N Am. 2017; 8(3): 85–93. [Google Scholar]
- 31.Ahmed K, Tamir B, Mengistu A. Constraints, Opportunities and Challenges of Cattle Fattening Practices in Urban and Peri-Urban Kebeles of Dessie Town, Ethiopia. J Fisheries Livest Prod. 2016; 4(4): 1–10. [Google Scholar]
- 32.Eskinder Z, Eyassu Y, Mitiku H. Assessment of the impact of industrial effluents on the quality of irrigation water and changes on soil characteristics (a case of Kombolcha town), fourteenth international water technology conference, IWTC 14, 2010, Cairo, Egypt; 2010, pp. 711–27. [Google Scholar]
- 33.Kombolcha Town Animal Production and Health Office. Annual report of dairy cattle production in Kombolcha town; 2019. [Google Scholar]
- 34.Dessie Town Animal Production and Health Office. Annual report of dairy cattle production in Dessie town; 2019. [Google Scholar]
- 35.Thrusfield M. Veterinary Epidemiology (3rd Ed.). UK: Black well science Ltd., A Blackwell publishing company; 2005, pp. 230–4. [Google Scholar]
- 36.Quinn PJ, Markey BK, Carter ME, Donelly WJ, Leonard FC. Veterinary microbiology and microbial disease, Blackwell Science Ltd, a Blackwell Publishing Company; 2002, pp. 465–75. [Google Scholar]
- 37.Merchant IA, Packer RA. Veterinary bacteriology and virology. 7th ed, the Iowa State University Press, Ames, Iowa, USA; 1969, pp. 211–305. [Google Scholar]
- 38.Eaton AD, Clesceri LS, Greenberg AE. Standard methods for the examination of water and wastewater, 19th ed. American Public Health Association; Washington, DC;1995. [Google Scholar]
- 39.Jarvis CJ, Kellerman GE, Van-Rensburg WJJ, Whitehead CJ. The bacteriology manual. 2nd edition; 1994. [Google Scholar]
- 40.Brenner DJ, Krieg NR, Staley JT. Bergey’s manual of systematic bacteriology. 2nded, Springer, New York; 2005. [Google Scholar]
- 41.MacFaddin JF. Biochemical tests for identification of medical bacteria. 3rd ed. Philadelphia, Lippincott Williams & Wilkins; 2000, pp. 451–53. [Google Scholar]
- 42.Cheesbrough M. Medical laboratory manual for tropical countries. Microbiol. 1985; 2: 400–80. [Google Scholar]
- 43.Simmons J. Standard methods for the examination of water and wastewater. 11th ed. APHA Inc; New York; 1960, pp. 626. [Google Scholar]
- 44.Chakraborty SP, KarMahapatra S, Roy S. Biochemical characters and antibiotic susceptibility of S. aureus isolates. Asian Pac J Trop Biomed. 2011; 1(3): 192–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vanderzant C, Splittstresser DF. Compendium of methods for the microbiological examination of foods. 3rd ed. American Public Health Association; Washington DC; 1992, pp. 331–8. [Google Scholar]
- 46.Bauer AW, Kirby M, Sheris JD, Turch M. Antibiotic susceptibility testing by standard single disc method. Am J Clin Pathol. 1966; 45(4): 493–6. [PubMed] [Google Scholar]
- 47.CLSI. Performance standards for antimicrobial susceptibility testing; M100-S22 Vol. 32 No.3, twenty-second informational supplement, Wayne PA., USA; 2012, pp. 1–183. [Google Scholar]
- 48.CLSI. Performance standards for antimicrobial susceptibility testing; twenty-fourth informational supplement. m100-s24, an informational supplement for global application through the clinical and laboratory standard institute consensus process, Wayne PA, USA; 2014, pp. 1–226. [Google Scholar]
- 49.CLSI. Performance standards for antimicrobial susceptibility Testing; M100 27th ed., An informational supplement for global application through the clinical and laboratory standard institute consensus process, Wayne PA, USA; 2017, pp. 1–250. [Google Scholar]
- 50.CLSI. Performance standards for antimicrobial susceptibility testing; M100 30th ed., A CLSI supplement for global application. Wayne PA, USA; 2020, pp. 1–294. [Google Scholar]
- 51.Arabzadeh F, Aeini F, Keshavarzi F, Behrvash S. Resistance to Tetracycline and Vancomycin of Staphylococcus aureus isolates from Sanandaj patients by Molecular Genotyping. Ann Clin Lab Res. 2018; 6(4): 1–5. [Google Scholar]
- 52.Reza RH, Shipa SA, Naser MN, Miah F. Surveillance of Escherichia coli in a fish farm of Sylhet, Bangladesh. Bangladesh J Zool. 2020; 48(1): 335–46. [Google Scholar]
- 53.Tadesse HA, Gidey NB, Workelule K, Hailu H, Gidey S, Bsrat A, et al. Antimicrobial resistance profile of E. coli isolated from raw cow milk and fresh fruit juice in Mekelle, Tigray, Ethiopia. Vet Med Int. 2018; 2018: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.TMCC. Microbiology resource center: antimicrobial susceptibility testing; 2021. Accessed from: https://www.tmcc.edu/microbiology-resource-center/lab-protocols/antimicrobial/susceptibility-testing.
- 55.Limbu DS, Bantawa K, Limbu DK, Devkota M, Ghimire M. Microbiological quality and adulteration of pasteurized and raw milk marketed in Dharan, Nepal. Hi J O S T. 2020; 3(4): 37–44. [Google Scholar]
- 56.Soomro AH, Arain MA, Khaskheli M, Bhutto B. Isolation of Escherichia coli from raw milk and milk products in relation to public health sold under market conditions at Tandojam. Pak J Nutr. 2002; 1(3): 151–2. [Google Scholar]
- 57.Atsbha WT, Weldeabezgi TL, Seyoum AK, Tafere G, Kassegn HH. Salmonella and risk factors for the contamination of cattle carcass from abattoir of Mekelle City, Ethiopia. Cogent Food Agric. 2018; 4(1): 1–8. [Google Scholar]
- 58.Reta MA, Bereda TW, Alemu AN. Bacterial contaminations of raw cow’s milk consumed at Jigjiga City of Somali Regional State, Eastern Ethiopia. Int J Food Contam. 2016; 3(4): 1–9. [Google Scholar]
- 59.Meshref AMS. Bacteriological quality and safety of raw cow’s milk and fresh cream. Slov Vet Res. 2013: 50(1): 21–30. [Google Scholar]
- 60.Salauddin M, Rowshan AM, Hossain KM, Nazir KHM NH, Noreddin A, El Zowalaty ME. Molecular detection of multidrug-resistant Staphylococcus aureus isolated from bovine mastitis milk in Bangladesh. Vet Sci. 2020; 7(36): 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Baz E, Gulmez M, Guven A, Sezer C, Duman B, Tarihi G. Examination of coliforms, E. coli and E. coli O157: H7 in raw milk, and ripened white cheese samples sold in Kars-Turkey. Kafkas Univ Vet Fak Derg. 2003; 9(2): 165–7. [Google Scholar]
- 62.Arjyal C, Dahal BN, Khadka B. Microbial quality of milk available in Kathmandu Valley. J Nepal Med Assoc. 2004; 43: 137–40. [Google Scholar]
- 63.Balcha E, Kumar A, Tassew H. Evaluation of safety of beef sold in and around Mekelle with special reference to Enterohemorrhagic Escherichia coli O157: H7. Glob Vet. 2014; 12(4): 569–72. [Google Scholar]
- 64.Gundogan N, Avci E. Occurrence and antibiotic resistance of Escherichia coli, Staphylococcus aureus and Bacillus cereus in raw milk and dairy products in Turkey. Int J Dairy Technol. 2014; 67(4): 562–9. [Google Scholar]
- 65.Lingathurai S, Vellathurai P. Bacteriological quality and safety of raw cow milk in Madurai, South India. Webmed Cent Microbiol. 2011; 1: 1–10. [Google Scholar]
- 66.Altalhi AD, Hassan SA. Bacterial quality of raw milk investigated by Escherichia coli and isolates analysis for specific virulence-gene markers. Food Control. 2009; 20: 913–7. [Google Scholar]
- 67.Chyea FY, Abdullah A, Ayob MK. Bacteriological quality and safety of raw milk in Malaysia. Food Microbiol. 2004; 21: 535–41. [Google Scholar]
- 68.Ali AA, Abdelgadir WS. Incidence of Escherichia coli in raw cow’s milk in Khartoum State. Br J Dairy Sci. 2011; 2(1): 23–26. [Google Scholar]
- 69.Kumar R, Prasad A. Detection of E.coli and Staphylococcus in milk and milk products in and around Pantnagar. Vet. World. 2010; 3(1):495–6. [Google Scholar]
- 70.Yakubu Y, Shuaibu AB, Ibrahim AM, Hassan UL, Nwachukwu RJ. Risk of Shiga toxigenic Escherichia coli O157: H7 infection from raw and fermented milk in Sokoto Metropolis, Nigeria. J Pathog. 2018; 2018: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mengistu S, Abayneh E, Shiferaw D. E. coli O157:H7 and Salmonella Species: public health importance and microbial safety in beef at selected slaughterhouses and retail shops in Eastern Ethiopia. J Vet Sci Technol. 2017; 8(5): 1–8. [Google Scholar]
- 72.Ngaywa C, Aboge GO, Obiero G, Omwenga I, Ngwili N, Wamwere G, et al. Antimicrobial-resistant Escherichia coli isolates detected in raw milk of livestock in pastoral areas of northern Kenya. Food Control. 2019; 102: 173–178. [Google Scholar]
- 73.Mohammed O, Shimelis D, Admasu P, Feyera T. Prevalence and antimicrobial susceptibility pattern of E. coli isolates from raw meat samples obtained from abattoirs in Dire Dawa City, Eastern Ethiopia. Int J Microbiol Res. 2014; 5(1): 35–9. [Google Scholar]
- 74.Hiwot D, Savoinni G, Cattaneo D, Gabriella S, Martino P. Bacteriological quality of milk in raw bovine bulk milk in the selected milk collection centers: smallholder dairy processing Ethiopia. J Vet Sci Ani Husb. 2016; 4(2): 1–5. [Google Scholar]
- 75.Sebsibe AM, Asfaw TE. Occurrence of multi-drug resistant Escherichia coli and Escherichia coli O157:H7 in meat and swab samples of various contact surfaces at abattoir and butcher shops in Jimma Town, Southwest District of Ethiopia. Infect Drug Resist. 2020; 13: 3853–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tadese ND, Gebremedhi EZ, Moges F, Borana BM, Marami LM, Sarba EJ, et al. Occurrence and antibiogram of Escherichia coli O157:H7 in raw beef and hygienic practices in abattoir and retailer shops in Ambo Town, Ethiopia. Vet Med Int. 2021; 2021: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Abebe M, Hailelule A, Abrha B, Nigus A, Birhanu M, Adane H, et al. Antibiogram of Escherichia coli strains isolated from food of bovine origin in selected Woredas of Tigray, Ethiopia. J Bacteriol Res. 2014; 6(3): 17–22. [Google Scholar]
- 78.Abayneh M, Tesfaw G, Woldemichael K, Yohannis M, Abdissa A. Assessment of extended-spectrum β-lactamase (ESBLs) -producing Escherichia coli from minced meat of cattle and swab samples and hygienic status of meat retailer shops in Jimma town, Southwest. BMC Infect Dis. 2019; 19: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yohannes G. Isolation, identification and antimicrobial susceptibility testing of Escherichia coli isolated from selected dairy farms in and around Mekelle, Ethiopia. J Dairy Vet Anim Res. 2018; 7(6): 287–91. [Google Scholar]
- 80.Haileselassie M, Taddele H, Adhana K, Kalayou S. Study on food safety knowledge and practices of abattoir and butchery shops and the microbial profile of meat in Mekelle City, Ethiopia. Asian Pac J Trop Biomed. 2012; 2012: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hiko A, Ameni G, Langkabel N, Fries R. Microbiological load and zoonotic agents in beef mortadella from Addis Ababa City supermarkets. J Food Prot. 2015; 78(5):1043–5. doi: 10.4315/0362-028X.JFP-14-395 [DOI] [PubMed] [Google Scholar]
- 82.Momtaz H, Safarpoor F, Rahimi E, Ezadi H, Arab R. Incidence of Shiga toxin-producing Escherichia coli serogroups in ruminant’s meat. Meat Sci. 2013; 95: 381–8. [DOI] [PubMed] [Google Scholar]
- 83.Taye M, Berhanu T, Berhanu Y, Tamiru F, Terefe D. Study on carcass contaminating Escherichia coli in apparently healthy slaughtered cattle in Haramaya University Slaughter House with special emphasis on Escherichia coli O157:H7, Ethiopia. J Veterinary Sci Technol. 2013; 4(1): 1–3. [Google Scholar]
- 84.Thaker HC, Brahmbhatt MN, Nayak JB. Study on occurrence and antibiogram pattern of Escherichia coli from raw milk samples in Anand, Gujarat, India. Vet World. 2012; 5(9): 556–9. [Google Scholar]
- 85.Zerabruk K, Retta N, Muleta D, Tefera AT. Assessment of microbiological safety and quality of minced meat and meat contact surfaces in selected butcher shops of Addis Ababa. J Food Qual. 2019; 2019: 1–8. [Google Scholar]
- 86.Sobeih AMK, Al-hawary II, Khalifa EM, Ebied NA. Prevalence of Enterobacteriaceae in raw milk and some dairy products. K V M J. 2020; 18 (2): 9–13. [Google Scholar]
- 87.Welde N, Abunna F, Wodajnew B. Isolation, identification and antimicrobial susceptibility profiles of E. coli O157: H7 from raw cow milk in and around Modjo Town, Ethiopia. J Am Sci. 2020; 16(6): 62–79. [Google Scholar]
- 88.Gutema FD, Rasschaert G, Agga GE, Jufare A, Duguma AB, Abdi RD, et al. Occurrence, molecular characteristics, and antimicrobial resistance of Escherichia coli O157 in cattle, beef, and humans in Bishoftu Town, Central Ethiopia. Foodborne Pathog Dis. 2020; XX: 1–7. [DOI] [PubMed] [Google Scholar]
- 89.Beyi FA, Fite TA, Fite E, Tafese A, Genu T, Kaba T, et al. Prevalence and antimicrobial susceptibility of Escherichia coli O157 in beef at butcher shops and restaurants in Central Ethiopia. BMC Microbiol. 2017; 17(1): 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Reuben RC, Owuna G. Antimicrobial resistance patterns of Escherichia coli O157:H7 from Nigerian fermented milk samples in Nasarawa State, Nigeria. Int J Pharm Sci Invent. 2013;. 2(3): 38–44. [Google Scholar]
- 91.Hiko A, Asrat D, Zewde G. Occurrence of Escherichia coli O157:H7 in retail raw meat products in Ethiopia. J Infect Dev Ctries. 2008; 2(5): 389–93. [DOI] [PubMed] [Google Scholar]
- 92.Rahimi E, Chaleshtori SS, Parsaei P. Prevalence and antimicrobial resistance of Escherichia coli O157 isolated from traditional cheese, ice cream and yoghurt in Iran. Afr J Microbiol Res. 2011; 5(22): 3706–10. [Google Scholar]
- 93.Vanitha HD, Sethulekshmi C, Latha C. An epidemiological investigation on occurrence of enterohemorrhagic Escherichia coli in raw milk. Vet World. 2018; 11(8): 1164–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dadi S, Lakew M, Seid M, Koran T, Olani A, Yimesgen L. Isolation of Salmonella and E. coli (E. coli O157:H7) and its antimicrobial resistance pattern from bulk tank raw milk in Sebeta Town, Ethiopia. J Anim Res Vet Sci. 2020; 4: 1–7. [Google Scholar]
- 95.Swai ES, Schoonman L. Microbial quality and associated health risks of raw milk marketed in the Tanga region of Tanzania. Asian Pac J Trop Biomed. 2011; 1(3): 217–22. doi: 10.1016/S2221-1691(11)60030-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Geresu MA, Regassa S. Escherichia coli O157:H7 from food of animal origin in Arsi: occurrence at catering establishments and antimicrobial susceptibility profile. Sci World J. 2021; 2021: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Carney E, Brien SBO, Sheridan JJ, Mcdowell DA, Blair IS, Duffy G. Prevalence and level of Escherichia coli O157 on beef trimmings, carcasses and boned head meat at a beef slaughter plant. Food Microbiol. 2006; 23: 52–9. [DOI] [PubMed] [Google Scholar]
- 98.Mcevoy JM, Doherty AM, Sheridan JJ, Thomson-Carter FM, Garvey P, Mcguire L, et al. The prevalence and spread of Escherichia coli O157: H7 at a commercial beef abattoir. J Appl Microbiol. 2003; 95: 256–66. [DOI] [PubMed] [Google Scholar]
- 99.Ahmed AM, Shimamoto T. International Journal of Food Microbiology Isolation and molecular characterization of Salmonella enterica, Escherichia coli O157:H7 and Shigella spp. from meat and dairy products in Egypt. Int J Food Microbiol. 2014; 168–169: 57–62. [DOI] [PubMed] [Google Scholar]
- 100.Islam MA, Mondol AS, Azmi IJ, de Boer E, Beumer RR, Zwietering MH, et al. Occurrence and characterization of Shiga toxin-producing Escherichia coli in raw meat, raw milk, and street vended juices in Bangladesh. Foodborne Pathog Dis. 2010; 7(11): 1381–5. [DOI] [PubMed] [Google Scholar]
- 101.Llorente P, Barnech L, Irino K, Rumi MV, Bentancor A. Characterization of Shiga toxin-producing Escherichia coli isolated from ground beef collected in different socioeconomic strata markets in Buenos Aires, Argentina. Biomed Res Int. 2014; 2014: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hamid M, Tefera Y, Eguale T, Worku Y. Escherichia coli O157: H7: Prevalence, identification and antimicrobial resistance in cattle slaughter at Addis Ababa Municipal Abattior, Ethiopia. Int J Adv Res Biol Sci. 2018; 5(10): 136–46. [Google Scholar]
- 103.Ncoko P, Jaja IF, Oguttu JW. Microbiological quality of beef, mutton, and water from different abattoirs in the Eastern Cape Province, South Africa. Vet World. 2020; 13(7): 1363–71. doi: 10.14202/vetworld.2020.1363-1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Etifu M, Tilahun M. Prevalence of bovine mastitis, risk factors, isolation and anti-bio gram of major pathogens in Mid Rift valley, Ethiopia. Int J Livest Prod. 2019; 10(1): 14–23. [Google Scholar]
- 105.Momtaz H, Dehkordi FS, Taktaz T, Rezvani A, Yarali S. Shiga toxin-producing Escherichia coli isolated from bovine mastitic milk: serogroups, virulence factors, and antibiotic resistance properties. Sci World J. 2012; 2012: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rahimi E, Kazemeini HR, Salajegheh M. Escherichia coli O157: H7 / NM prevalence in raw beef, camel, sheep, goat, and water buffalo meat in Fars and Khuzestan provinces, Iran. Vet Res Forum. 2012; 3(1):13–7. [PMC free article] [PubMed] [Google Scholar]
- 107.Hassan A, Hiko A, Bogale K, Abera B, Tsegaye B. Antimicrobial resistance profiles of Staphylococcus aureus isolates along Asella Municipal Beef Abattoir Line, South Eastern Ethiopia. J Vet Sci Technol. 2018; 9(3): 1–5. [Google Scholar]
- 108.Arthur TM, Kalchayanand N, Bosilevac JM, Brichta-Harhay DM, Shackelford SD, Bon J L, et al. Comparison of effects of antimicrobial interventions on multidrug-resistant Salmonella, susceptible Salmonella, and Escherichia coli O157:H7. J Food Prot. 2008; 71(11): 2177–81. [DOI] [PubMed] [Google Scholar]
- 109.Su Z, Tong P, Zhang L, Zhang M, Wang D, Ma K, et al. First isolation and molecular characterization of blaCTX-M-121-producing Escherichia coli O157:H7 from cattle in Xinjiang, China. Front Vet Sci. 2021; 8: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Igbinosa IH, Chiadika C. Prevalence, characteristics and antibiogram profile of Escherichia coli O157:H7 isolated from raw and fermented (nono) milk in Benin City, Nigeria. Afr J Clin Exper Microbiol. 2021; 22(2): 223–33. [Google Scholar]
- 111.Reuben CR, Okolocha EC, Bello M, Tanimu H. Occurrence and antibiogram of Escherichia coli O157:H7 in locally fermented milk (Nono) sold under market conditions in Nasarawa State, Nigeria. Int J Sci Res. 2013; 2(2): 591–8. [Google Scholar]
- 112.Msolo L, Igbinosa EO, Okoh AI. Prevalence and antibiogram profiles of Escherichia coli O157:H7 isolates recovered from three selected dairy farms in the Eastern Cape Province, South Africa. Asian Pac J Trop Dis. 2016; 6(12): 990–5. [Google Scholar]
- 113.Osaili TM, Alaboudi AR, Rahahlah M. Prevalence and antimicrobial susceptibility of Escherichia coli O157:H7 on beef cattle slaughtered in Amman abattoir. Meat Sci. 2013; 93: 463–8. [DOI] [PubMed] [Google Scholar]
- 114.Haile FA, Kebede D, Wubshet KA. Prevalence and antibiogram of Escherichia coli O157 isolated from bovine in Jimma, Ethiopia: Abattoirbased Survey. Ethiop Vet J. 2017; 21(2): 109–20. [Google Scholar]
- 115.Mokgophi TM, Gcebe N, Fasina F, Adesiyun AA. Antimicrobial resistance profiles of Salmonella isolates on chickens processed and retailed at outlets of the informal market in Gauteng Province, South Africa. Pathogens. 2021; 10(273): 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Qamar A, Ismail T, Akhtar S. Prevalence and antibiotic resistance of Salmonella spp. in South Punjab-Pakistan. PLoS ONE. 2020;15(11): 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.