Abstract
Background and Aims
In 2016, direct-acting antiviral (DAA) treatment for hepatitis C (HCV) became available through Australia’s universal health care system, with the aim of HCV elimination. We report real-world effectiveness of DAA HCV treatment in Australia from a clinically well-informed cohort, enriched for cirrhosis and prior HCV treatment.
Methods
3413 patients were recruited from 26 hospital liver clinics across Australia from February 2016 to June 2020. Clinical history and sustained viral response (SVR) were obtained from medical records and data linkage to the Australian Pharmaceutical Benefits Scheme. Factors associated with SVR were assessed by multivariable logistic regression (MVR).
Results
At recruitment, 32.2% had cirrhosis (72.9% Child Pugh class B/C), and 19.9% were treatment experienced. Of the 2,939 with data, 93.3% confirmed SVR. 137 patients received second-line therapy. Patients with cirrhosis had lower SVR rate (88.4 vs. 95.8%; p < 0.001). On MVR, failure to achieve SVR was associated with Genotype 3 (adj-OR = 0.42, 95%CI 0.29–0.61), male gender (adj-OR = 0.49, 95%CI 0.31–0.77), fair/poor adherence (adj-OR = 0.52, 95%CI 0.28–0.94), cirrhosis (adj-OR = 0.57, 95%CI 0.36–0.88), FIB-4 > 3.25 (adj-OR = 0.52, 95%CI 0.33–0.83) and MELD score ≥ 20 (adj-OR = 0.25, 95%CI 0.08–0.80). Consistent results were seen in cirrhotic sub-analysis.
Conclusions
Excellent SVR rates were achieved with DAAs in this real-world cohort of patients with chronic HCV infection. More advanced liver disease and clinician impression of poor adherence were associated with HCV treatment failure. Supports to improve liver fibrosis assessment skills for non-specialist DAA prescribers in the community and to optimize patient adherence are likely to enable more effective pursuit of HCV elimination in Australia.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10620-022-07483-y.
Keywords: Cirrhosis, Sustained viral response, Data linkage, Retreatment, Patient adherence, Liver fibrosis
Introduction
In 2016, direct-acting antiviral (DAA) medication for Hepatitis C virus (HCV) became funded through Australia’s universal health care system, providing opportunity for safe, tolerable, and effective treatment of HCV infection [1]. Approximately 307,000 Australians were infected with HCV, like elsewhere in the Asia–Pacific a high proportion of genotype 3 (G3) contributing to the morbidity and mortality of decompensated cirrhosis and hepatocellular carcinoma (HCC) [1, 2]. At a population level, DAAs uptake in Australia has reduced rates of HCC-associated liver-related deaths by a third since 2015, and plateauing in HCV-associated HCC [3].
DAA therapies are associated with very high rates of cure or sustained virological response (SVR, defined as an undetectable HCV viral load at least 12 weeks after completion of DAA therapy) [4–11]. Data are still evolving regarding real-world outcomes in patients with advanced liver disease, multi-treatment failure, and cofactors for progression such as alcohol. Moreover, further data are needed on the efficacy of the adjunctive use of ribavirin for patients with cirrhosis, and how factors such as adherence, socio-demographic disadvantage, and concomitant medical conditions and co-pharmacy may impact outcomes [12]. Population-based data lack clinical detail to inform clinical decision-making, yet cohort studies often suffer from bias, small numbers, and loss to follow-up. Here we report real-world effectiveness of DAA HCV treatment in Australia from a large, clinically well-informed cohort enriched for cirrhosis with population-based data linkage.
Methods
Patient Ascertainment
The OPERA-C is a prospective study of people with HCV who received DAA therapy recruited from 26 hospital-based liver clinics across Australia. Patient inclusion criteria were age ≥ 18 years, HCV diagnosis confirmed on viral RNA using highly sensitive polymerase chain reaction (PCR), and undertaking DAA therapy according to HCV treatment guidelines [13]. The most commonly assay used for diagnosis and monitoring of HCV infection was the Cobas® HCV test for use on the Cobas® 6800/8800 systems with lower limit of detection of 15 IU/ml. After providing informed consent, clinical review occurred prior to initiation, typically at end of treatment (EOT), and 3 months post-treatment to determine SVR. Based on PBS-subsidised HCV medication data, during HCV treatment patients were usually dispensed 4 weeks of medication at initiation with refills dispensed monthly.
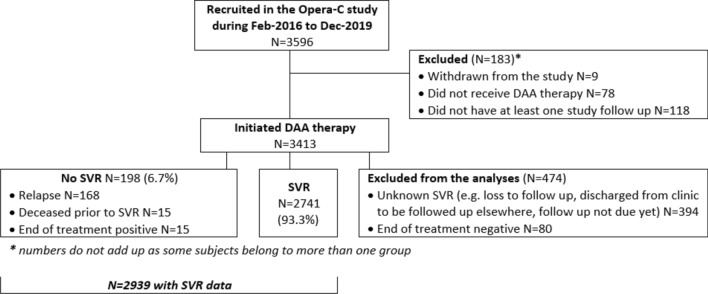
The patient ascertainment flow is represented in Fig. 1. The primary outcome was SVR. In order to assess real-life treatment outcomes, SVR was only assessed in those patients for whom treatment uptake was confirmed, follow-up occurred, and SVR result could be established. Individuals without a definitive result were excluded (n = 183). Patients were considered non-SVR if HCV RNA was positive when tested within 12 weeks after end of treatment (EOT) [14]. For the purposes of the SVR analysis, if a patient died during therapy (n = 15), the patient was considered to not have attained SVR.
Fig. 1.
Flow chart for patient inclusion in the analyses
Data Collection and Measures
At recruitment, demographic and baseline clinical characteristics were obtained from the patient’s medical records. Patients’ residential postcodes at enrolment were used to categorise their place of residence according to rurality of residence [15] and the Australian Bureau of Statistics Index of Relative Socioeconomic Advantage and Disadvantage [16]. Significant alcohol consumption was defined as ≥ 40 g of ethanol per day. Fibrosis evaluation and laboratory results were collected.
Patients were followed up for 2 years to assess HCV treatment and liver-related outcomes. The treating clinician was asked to provide a clinical impression of patient adherence to treatment, categorised as good, fair, or poor. Passive follow-up occurred through data-linkage to the federal Pharmaceutical Benefits Scheme (PBS), the key government agency for subsidised medications in Australia. Complete dispensing histories from May-2015 to Sept-2019 extracted from the PBS were used to identify subsidised use of medications including HCV treatments, diabetes medications, warfarin (to control for erroneous interpretation of INR in cirrhotic patients), and gastric acid-reducing therapies including proton pump inhibitors (PPI) and Histamine-2 receptor blockers (H2-RB).
Liver fibrosis and disease severity were comprehensively assessed using invasive and non-invasive indices. Data on the aspartate aminotransferase to platelet ratio index (APRI), liver stiffness measurement (LSM) by transient elastography, and Fibrosis-4 (FIB-4) were collected [17, 18]. LSM and FIB-4 were least penalised for missing data and utilised in multivariable analysis (MVA). Cirrhosis was defined based on biopsy, clinical (e.g. presence of portal hypertension), and non-invasive criteria. Pre-defined risk thresholds for non-invasive diagnosis of cirrhosis for TE [19] and FIB-4 test [17] were based on prior studies. LSM < 8 kiloPascals (kPa) was considered as no or minimal liver fibrosis, between 8 and 12.5 kPa moderate or advanced fibrosis, and LSM > 12.5 kPa considered cirrhotic. FIB-4 test value of > 3.25 was categorized as advanced fibrosis [17]. Severity of disease was classified at study recruitment using the Child–Pugh and MELD scores [20]. Child–Pugh score, MELD score, and FIB-4 test were calculated from values at the time of recruitment using the most recently available clinical and laboratory data.
Statistical Analysis
Analyses were conducted using Stata/SE (Version 15; Stata Corporation, College Station, TX). Descriptive analyses are presented as frequency (percentages, %), mean (standard deviation, SD), or median (interquartile range, IQR) value depending on data distribution. Unadjusted odds ratios (ORs) and their corresponding 95% confidence interval (CI) were estimated using logistic regression. Forward stepwise selection (p-value for addition < 0.10) was used to create a multivariable logistic regression model for the prediction SVR using variables that were found to have a p < 0.20 in univariate analysis. Final models were calibrated with Hosmer–Lemeshow test and Receiving Operator Characteristic curve, respectively. Sensitivity analyses were conducted by repeating the analyses excluding patients who had HCV treatment prior to DAA-era. Statistical significance was set at alpha = 0.05, and all p values were 2-sided.
Results
Data were available for 3596 patients recruited into the OPERA-C study from Feb-2016 to Jun-2020. We excluded 474 patients (Fig. 1): 183 were excluded due to withdrawals, did not receive DAA therapy, or did not have at least one study follow up, while for 394 SVR was unknown (e.g. loss to follow up) and for 80 EOT was negative but SVR was not confirmed on PCR. Characteristics of the 3413 patients included in this analysis are shown in Table 1. Patients were on average 52 years old (SD = 10.5), predominantly male (66.0%), and 51.1% lived in the lowest two quintiles of area-socioeconomic disadvantage. Diabetes was present in 25.4 and 61.9% were overweight or obese.
Table 1.
Patient demographic and clinical characteristics at recruitment
| Total | ||
|---|---|---|
| N = 3413 | ||
| Age at enrolment (mean, SD) | 51.9 (10.5) | |
| Gender | Female | 1159 (34.0%) |
| Male | 2254 (66.0%) | |
| Country of birth a | Australia | 2720 (81.6%) |
| Overseas | 613 (18.4%) | |
| State (at recruitment) | NSW-ACT | 1504 (44.1%) |
| Qld | 645 (18.9%) | |
| South Australia | 126 (3.7%) | |
| Victoria and Tasmania | 1025 (30.0%) | |
| Western Australia | 113 (3.3%) | |
| Indigenous status b | Indigenous | 94 (2.8%) |
| Non-Indigenous | 3285 (97.2%) | |
| Socioeconomic status c | Q1 most affluent | 658 (19.3%) |
| Q2 | 477 (14.0%) | |
| Q3 | 535 (15.7%) | |
| Q4 | 626 (18.4%) | |
| Q5 most disadvantaged | 1114 (32.7%) | |
| Rurality of residence d | Major city | 2529 (74.1%) |
| Inner regional | 746 (21.9%) | |
| Outer regional/Remote/Very remote | 137 (4.0%) | |
| Hepatitis B surface antigen e | 49 (1.8%) | |
| Hepatitis B surface antibody f | 1115 (44.2%) | |
| Hepatitis B core antibody g | 687 (31.9%) | |
| HIV h | 27 (1.4%) | |
| BMI groupi | Underweight/Normal | 1057 (38.1%) |
| Overweight | 972 (35.1%) | |
| Obese | 744 (26.8%) | |
| Diabetes j | 859 (25.4%) | |
| Prescribed opiate substitute k | 473 (14.7%) | |
| Prescribed PPIs or H2-RBs | 541 (17.1%) | |
| Hepatocellular carcinoma | 69 (2.0%) | |
| Current alcohol consumption l | Zero alcohol | 1507 (58.8%) |
| < 40 g/day | 729 (28.4%) | |
| ≥40 g/day | 327 (12.8%) | |
| Liver fibrosis assessment | 0.6 (0.4–1.3) | |
| APRI (median, IQR) m | ≤1 | 1972 (66.3%) |
| APRI groups | > 1 | 1004 (33.7%) |
| FIB-4 (median, IQR) n | 1.6 (1.0–3.0) | |
| FIB-4 groups | No advanced fibrosis (FIB-4 ≤ 3.25) | 2318 (77.9%) |
| Liver fibrosis FIB-4 > 3.25 | 656 (22.1%) | |
| Liver stiffness (kPa) (median, IQR) o | 7.5 (5.4–13.1) | |
| Liver stiffness groups | < 8.0 kPa (minimal fibrosis) | 1395 (54.7%) |
| 8.0–12.5 kPa (moderate fibrosis) | 487 (19.1%) | |
| > 12.5 kPa (advanced fibrosis/cirrhosis) | 670 (26.3%) | |
| Cirrhosis at enrolment p | 1088 (32.2%) | |
| Child–Pugh class q | A | 264 (28.1%) |
| B | 666 (70.9%) | |
| C | 10 (1.1%) | |
| MELD (median, IQR) r | 7.7 (7.4–9.6) | |
| Presence of complications d | 73 (6.8%) | |
| Ascites s | Absent | 1004 (93.7%) |
| Medically controlled | 55 (5.1%) | |
| Poorly controlled | 12 (1.1%) | |
| Encephalopathy t | Absent | 1050 (98.4%) |
| Medically controlled | 17 (1.6%) |
Data are presented as n (%) unless specified; amissing data for 80 patients; bmissing data for 34 patients; cmissing data for 3 patients; dmissing data for 1 patient; emissing data for 650 patients; fmissing data for 889 patients; gmissing data for 1259 patients; hmissing data for 1500 patients; imissing data for 640 patients; jmissing data for 29 patients; kmissing data for 185 patients; lmissing data for 850 patients; mmissing data for 437 patients; nmissing data for 437 patients; omissing data for 861 patients; pmissing data for 30 patients; qunable to calculate Child–Pugh score for 17 patients (on warfarin) and missing data for 131 patients; rmissing data for 117 patients; smissing data for 17 patients; tmissing data for 21 patients
A third of the cohort had cirrhosis (32.2%) at recruitment, (28.1% had Child–Pugh (CP) class A, 71.9% CP B/C. Key clinical characteristics are listed in Table 2. The laboratory measurements around the time of OPERA-C recruitment are available in Supplementary Table 1.
Table 2.
Hepatitis C virus assessment at recruitment and details of treatment with DAA therapy
| Total | ||
|---|---|---|
| N = 3413 | ||
| Genotype | 1 | 1836 (53.8%) |
| 2 | 161 (4.7%) | |
| 3 | 1279 (37.5%) | |
| Genotype 4, 5 or 6 | 95 (2.8%) | |
| > 1 genotype | 15 (0.4%) | |
| genotype unknown | 27 (0.8%) | |
| Duration of HCV infection in years (mean, SD)a | 22.7 (12.0) | |
| Viral load IU/ml b | 1,258,925 (344,000–3,714,272) | |
| Mode of HCV Acquisition c | ||
| Injecting drug use | 2303 (67.5%) | |
| Tattoo | 579 (17.0%) | |
| Blood transfusion | 295 (8.7%) | |
| Sexual (only) | 25 (0.7%) | |
| Needle stick (only) | 30 (0.9%) | |
| Vaccine (only) | 29 (0.9%) | |
| Medical/dental procedure (only) | 35 (1.0%) | |
| Vertical transmission | 21 (0.6%) | |
| HCV treatment prior to DAA-era | 680 (19.9%) | |
| Regimen | Pegylated or standard interferon ± RBV | 510 (75.0%) |
| First generation PEGIFN/protease inhibitors | 89 (13.1%) | |
| DAA ± RBV | 19 (2.8%) | |
| RCT not brought forward or not otherwise specified | 62 (9.1%) | |
| Treatment response | SVR (presumed re-infection) | 9 (1.3%) |
| Relapse | 265 (39.0%) | |
| Non-responder | 329 (48.4%) | |
| Unknown | 77 (11.3%) | |
| HCV DAA therapy | ||
| Regimen | Sofosbuvir/Ledipasvir | 1348 (39.5%) |
| Sofosbuvir + Daclatasvir | 976 (28.6%) | |
| Sofosbuvir/Velpatasvir | 605 (17.7%) | |
| Sofosbuvir + Ribavirin | 98 (2.9%) | |
| Elbasvir/Grazoprevir | 179 (5.2%) | |
| Glecaprevir/Pibrentasvir | 122 (3.6%) | |
| Ombitasvir/Paritaprevir/Ritonavir/Dasabuvir | 62 (1.8%) | |
| Sofosbuvir/Velpatasvir/Voxilaprevir | 7 (0.2%) | |
| Miscellaneous DAAs | 16 (0.5%) | |
| Treatment included ribavirin d | 90 (2.6%) | |
| SVR/non-SVR confirmation cohort | N = 2939 | |
| Treatment response | SVR | 2741 (93.3%) |
| Relapse | 168 (5.7%) | |
| Deceased prior to SVR | 15 (0.5%) | |
| End of treatment positive | 15 (0.5%) | |
| Treatment adherence e | Good | 2321 (90.0%) |
| Fair/poor | 259 (10.0%) |
aMissing data for 412 patients; bmissing data for 594 patients; cpatients may belong to more than one group unless specified, missing data for 3 patients; dexcluding Sofosbuvir + Ribavirin; emissing data for 359 patients
A fifth of patients had previous HCV treatment prior to DAA-era (n = 680), 75% were treated with pegylated or standard interferon ± RBV. Half were primary non-responders (to pre-DAA therapy), 39% relapsed and nine had documented SVR with reinfection presumed.
DAA therapy reflected regimen registration availability in Australia, with sofosbuvir/ledipasvir the earliest available and most common treatment regimen (39.5%) typically for G1, followed by sofosbuvir/daclatasvir (28.6%, typically for G3), and an increasing proportion of sofosbuvir/velpatasvir (17.7%) during the later recruitment period.
SVR
Of the 3413 patients who received HCV treatment, follow-up data were available for 2,939 (Fig. 1; Table 2). 2741 (93.3%) had a confirmed SVR result during the follow-up period. Failure to achieve SVR included: 168 patients documented as relapse, 15 deceased prior to SVR, and 15 remained positive at the EOT. Analyses of factors associated with SVR are presented in Tables 3 and 4.
Table 3.
Logistic regression analysis of factors associated with SVR among the whole cohort (n = 2939)
| No SVR | SVR | Unadjusted | Adjusted | ||
|---|---|---|---|---|---|
| N = 198 | N = 2741 | OR (95%CI) | OR (95%CI) | ||
| Age at enrolment (years) (mean, SD) | 54.41 (9.11) | 52.61 (10.25) | 0.98 (0.97–0.99) | – | |
| Gender | Female | 42 (4.2%) | 962 (95.8%) | Ref | Ref |
| Male | 156 (8.1%) | 1779 (91.9%) | 0.50 (0.35–0.70) | 0.49 (0.31–0.77) | |
| Country of birth | Australia | 155 (6.7%) | 2159 (93.3%) | Ref | – |
| Overseas | 42 (7.6%) | 514 (92.4%) | 0.88 (0.62–1.25) | ||
| Socioeconomic status | Q1 most affluent | 38 (6.6%) | 539 (93.4%) | Ref | – |
| Q2 | 23 (5.4%) | 401 (94.6%) | 1.23 (0.72–2.10) | ||
| Q3 | 39 (8.5%) | 421 (91.5%) | 0.76 (0.48–1.21) | ||
| Q4 | 50 (9.1%) | 499 (90.9%) | 0.70 (0.45–1.09) | ||
| Q5 most disadvantaged | 48 (5.2%) | 879 (94.8%) | 1.19 (0.83–2.00) | ||
| Rurality of residence | Major city | 158 (7.3%) | 2020 (92.7%) | Ref | – |
| Inner regional | 34 (5.3%) | 603 (94.7%) | 1.39 (0.95–2.03) | ||
| Outer regional to very remote | 6 (4.8%) | 118 (95.2%) | 1.53 (0.67–3.55) | ||
| Genotype | not G3 | 77 (4.2%) | 1776 (95.8%) | Ref | Ref |
| G3 | 121 (11.3%) | 949 (88.7%) | 0.34 (0.25–0.46) | 0.42 (0.29–0.61) | |
| HCV treatment prior to DAA-era | No | 154 (6.6%) | 2163 (93.4%) | Ref | – |
| Yes | 44 (7.1%) | 578 (92.9%) | 0.94 (0.66–1.32) | ||
| Diabetes | Absent | 139 (6.4%) | 2026 (93.6%) | Ref | – |
| Present | 59 (7.8%) | 695 (92.9%) | 0.81 (0.59–1.11) | ||
| Prescribed opiate substitute | No | 155 (6.5%) | 2247 (93.5%) | Ref | – |
| Yes | 27 (7.1%) | 351 (92.9%) | 0.90 (0.59–1.37) | ||
| Prescribed PPIs or H2-RBs | No | 137 (6.0%) | 2141 (94.0%) | Ref | Ref |
| Yes | 49 (10.0%) | 439 (90.0%) | 0.57 (0.41–0.81) | 0.69 (0.45–1.05) | |
| Albumin (g/L) | 37.19 (5.59) | 39.63 (6.31) | 1.08 (1.06–1.11) | – | |
| Platelets (109/L) | 174.65 (90.41) | 207.11 (84.41) | 1.05 (1.03–1.07)* | – | |
| Bilirubin (umol/L) | 17.74 (19.73) | 12.37 (9.74) | 0.78 (0.71–0.85)* | – | |
| Creatinine (umol/L) | 74.04 (25.34) | 74.49 (33.89) | 1.00 (0.86–1.05)* | – | |
| ALT (U/L) | 98.94 (95.48) | 85.62 (82.82) | 0.98 (0.97–0.99)* | – | |
| Hemoglobin(g/L) | 144.60 (17.53) | 147.35 (16.65) | 1.10 (1.01–1.02)* | – | |
| FIB-4 groups | FIB-4 < = 3.25 | 86 (4.4%) | 1873 (95.6%) | Ref | Ref |
| FIB-4 > 3.25 | 77 (13.1%) | 510 (86.9%) | 0.30 (0.22–0.42) | 0.52 (0.33–0.83) | |
| Liver stiffness groups | < 8.0 kPa | 44 (3.7%) | 1131 (96.3%) | Ref | – |
| 8.0–12.5 kPa | 29 (6.8%) | 396 (93.2%) | 0.53 (0.33–0.86) | ||
| > 12.5 kPa | 62 (10.3%) | 538 (89.7%) | 0.34 (0.23–0.50) | ||
| MELD (median, IQR) | 7.50 (6.43–10.20) | 7.21 (6.43–8.07) | 0.87 (0.83–0.91) | 0.94 (0.89–0.99) | |
| Treatment adherence | Good | 91 (3.9%) | 2230 (96.1%) | Ref | Ref |
| Fair/poor | 24 (9.3%) | 235 (90.7%) | 0.40 (0.25–0.64) | 0.52 (0.28–0.94) | |
| Cirrhosis at enrolment | Absent | 82 (4.2%) | 1,849 (95.8%) | Ref | Ref |
| Present | 114 (11.6%) | 872 (88.4%) | 0.34 (0.25–0.46) | 0.57 (0.36–0.88) |
*Odds ratios calculated per 10 unit change in values
Table 4.
Logistic regression analysis of factors associated with SVR among patients with cirrhosis (n = 986)
| No SVR | SVR | Unadjusted | Adjusted | ||
|---|---|---|---|---|---|
| N = 114 | N = 872 | OR (95%CI) | OR (95%CI) | ||
| Age at enrolment (years) (mean, SD) | 56.43 (7.64) | 55.94 (8.25) | 0.99 (0.97–1.02) | – | |
| Gender | Female | 17 (6.4%) | 247 (93.6%) | Ref | Ref |
| Male | 97 (13.4%) | 625 (86.6%) | 0.44 (0.26–0.76) | 0.38 (0.20–0.74) | |
| Country of birth | Australia | 91 (11.6%) | 692 (88.4%) | Ref | – |
| Overseas | 23 (12.2%) | 165 (87.8%) | 0.94 (0.58–1.54) | ||
| Socioeconomic status | Q1 most affluent | 19 (10.6%) | 160 (89.4%) | Ref | – |
| Q2 | 12 (8.9%) | 123 (91.1%) | 1.22 (0.57–2.60) | ||
| Q3 | 23 (14.3%) | 138 (85.7%) | 0.71 (0.37–1.36) | ||
| Q4 | 29 (15.9%) | 153 (84.1%) | 0.63 (0.34–1.16) | ||
| Q5 most disadvantaged | 31 (9.5%) | 297 (90.5%) | 1.14 (0.62–2.08) | ||
| Rurality of residence | Major city | 90 (11.8%) | 673 (88.2%) | Ref | – |
| Inner regional | 21 (11.0%) | 170 (89.0%) | 1.08 (0.65–1.79) | ||
| Outer regional to very remote | 3 (9.4%) | 29 (90.6%) | 1.29 (0.39–4.33) | ||
| Genotype | not G3 | 39 (7.2%) | 500 (92.8%) | Ref | Ref |
| G3 | 75 (16.9%) | 370 (83.1%) | 0.38 (0.26–0.58) | 0.48 (0.30–0.78) | |
| HCV treatment prior to DAA-era | No | 83 (12.0%) | 608 (88.0%) | Ref | – |
| Yes | 31 (10.5%) | 264 (89.5%) | 1.16 (0.75–1.80) | ||
| Diabetes | Absent | 77 (11.7%) | 579 (88.3%) | Ref | – |
| Present | 35 (11.5%) | 286 (88.5%) | 1.03 (0.68–1.56) | ||
| Prescribed opiate substitute | No | 95 (12.0%) | 696 (88.0%) | Ref | – |
| Yes | 16 (10.6%) | 135 (89.4%) | 1.16 (0.66–2.02) | ||
| Prescribed PPIs or H2-RBs | No | 71 (10.3%) | 616 (89.7%) | Ref | Ref |
| Yes | 35 (14.6%) | 205 (85.4%) | 0.68 (0.44–1.04) | 0.60 (0.36–1.00) | |
| Albumin (g/L) | 35.44 (6.22) | 37.32 (7.26) | 1.05 (1.02–1.08) | – | |
| Platelets (109/L) | 131.48 (78.53) | 148.56 (74.87) | 1.04 (1.01–1.07)* | – | |
| Bilirubin (umol/L) | 23.25 (24.06) | 16.49 (12.50) | 0.80 (0.72–0.89)* | – | |
| Creatinine (umol/L) | 71.90 (16.98) | 76.12 (50.07) | 1.04 (0.96–1.12)* | – | |
| ALT (U/L) | 112.69 (87.29) | 96.56 (85.80) | 0.98 (0.96–1.00)* | 0.98 (0.95–1.00) | |
| Hemoglobin(g/L) | 142.38 (18.58) | 144.37 (19.15) | 1.05 (0.95–1.16)* | – | |
| FIB-4 groups | FIB-4 ≤ 3.25 | 28 (7.2%) | 360 (92.8%) | Ref | Ref |
| FIB-4 > 3.25 | 69 (14.6%) | 403 (85.4%) | 0.45 (0.29–0.72) | 0.56 (0.32–0.98) | |
| Liver stiffness groups | ≤12.5 kPa | 9 (9.0%) | 91 (91.0%) | Ref | – |
| > 12.5 kPa | 62 (10.5%) | 530 (89.5%) | 0.84 (0.41–1.76) | ||
| Treatment adherence | Good | 62 (7.9%) | 721 (92.1%) | Ref | Ref |
| Fair/poor | 9 (12.3%) | 64 (87.7%) | 0.61 (0.29–1.29) | 1.06 (0.39–2.85) | |
| Treatment included ribavirin | No | 110 (12.0%) | 809 (88.0%) | Ref | Ref |
| Yes | 4 (6.0%) | 63 (94.0%) | 2.14 (0.76–5.99) | 2.91 (0.97–8.73) | |
| Child–Pugh score (mean, SD) | 6.89 (1.04) | 6.80 (0.70) | 0.86 (0.66–1.12) | – | |
| MELD (median, IQR) | 10.22 (5.04) | 8.78 (2.89) | 0.91 (0.86–0.95) | 0.91 (0.85–0.96) | |
| Presence of complications at enrolment | Absent | 98 (10.8%) | 806 (89.2%) | Ref | – |
| Present | 14 (20.6%) | 54 (79.4%) | 0.47 (0.25–0.87) |
*Odds ratios calculated per 10 unit change in values
In unadjusted analysis, SVR rates were 96.5% for G1, 91.4% for G2, 88.7% for G3, 91.6% for G4, 5 and 5, and 84.6% for patients with mixed genotype (p < 0.001). Patients infected with G3 HCV had lower rates of SVR than non-G3 patients (88.7 vs. 95.8%, OR = 0.34, 95%CI 0.25–0.46; p < 0.001) (Table 3). For the 83 patients with genotypes 4 (n = 42), 5 (n = 1) or 6 (n = 40) for whom SVR data was available, SVR rates were 90.5, 100 and 92.5%, respectively. Patients with mixed genotype infection overall did well (84.6%), although in seven patients with mixed genotype 1/3 SVR was noted to be only 71.4% (all genotype 1/3 patients received sofosbuvir with either velpatasvir or daclatasvir). Regarding HCV treatment, SVR rates vary significantly according to the three main treatment groups, namely Sofosbuvir/Ledispavir (96.1%), Sofosbuvir + Daclastavir (88.1%), Sofosbuvir/Velpatasvir (94.3%), and Glecaprevir/Pibrentasvir (97.6%; p < 0.001).
Patients with cirrhosis experienced lower rates of SVR than those without cirrhosis (88.4 vs. 95.8%; OR = 0.34, 95%CI 0.25–0.46). Likelihood of SVR declined with higher MELD score (MELD ≥ 20, OR = 0.11, 95%CI 0.04–0.27); MELD 10–19 OR = 0.37, 95%CI 0.25–0.56) compared to patients with compensated cirrhosis MELD < 10. Other factors that showed differing rates of SVR on unadjusted analysis included more advanced liver fibrosis (whether measured by higher FIB-4 > 3.25 or LSM > 12.5 kPa), fair/poor adherence, male gender, and history of being recently prescribed PPIs/H2-RBs (Table 3). Prior treatment was not associated with poorer SVR, irrespective of whether IFN-based treatments or not.
Treatment Adherence
The treating clinician’s assessment of the patient’s adherence was good for 90.0% and fair/poor for 10.0%. Patients with fair/poor adherence were younger (mean 49.2 years [SD = 10.4] vs. 52.6 years [SD = 10.2], p < 0.001) and more likely to reside in areas of more socioeconomic disadvantage (63.6% non-adherent vs. 51.3% adherent, p < 0.001), in regional or remote areas (43.9% non-adherent vs. 25.6% adherent, p < 0.001), and have a history of being prescribed opioids (22.2 vs. 13.2%, p < 0.001). Patients who had at least one HCV treatment prior to DAA-era were less likely to have fair/poor adherence (16.0 vs. 20.8%, p = 0.037). Adherence did not vary by gender (p = 0.58), alcohol consumption (p = 0.40), presence of cirrhosis (p = 0.097), and inclusion of RBV in the DAA regimen (p = 0.33).
Of the 183 patients classified as non-responders, 108 had adherence data. Of non-responders with adherence data, 22 patients (20.4%) were noted to have fair/poor adherence, compared with a fair/poor adherence of 9.5% in patients with SVR (p < 0.0001). Interestingly, although poor adherence was over-represented in non-responders, overall SVR rates were still relatively high (90.7%, n = 235), but SVR rates declined along the gradient of good, fair or poor adherence, (SVR = 96.1% [2,230 out of 2,321], 94.9% [206 out of 217], 69.1% [29 out of 42], respectively, p < 0.0001).
Predictors of Virologic Response
In MVA, G3 (adj-OR = 0.42, 95%CI 0.29–0.61), male gender (adj-OR = 0.49, 95%CI 0.31–0.77), fair/poor adherence (adj-OR = 0.52, 95%CI 0.28–0.94), the presence of liver cirrhosis (adj-OR = 0.57, 95%CI 0.36–0.88), markers of more advanced liver disease severity including higher FIB-4 (FIB-4 > 3.25 (adj-OR = 0.52, 95%CI 0.33–0.83) and MELD score (MELD score ≥ 20, adj-OR = 0.25, 95%CI 0.08–0.80), decreased the likelihood of SVR (Table 3). While LSM was strongly associated with SVR in univariate analysis, in it was not associated with SVR (data not shown) on MVA as a continuous, ordinal, or binary variable. Repeating the analysis excluding patients who had HCV treatment prior to DAA-era led to similar results, although the effect sizes were generally smaller (Supplementary Table 2). In MVA, a history of being prescribed PPIs/H2-RBs was below the statistical significance threshold (OR = 0.69; 95%CI 0.45–1.05).
In MVA restricted to patients with cirrhosis, male gender (adj-OR = 0.38, 05%CI 0.20–0.74), G3 (adj-OR = 0.48, 95%CI 0.30–0.78), FIB-4 > 3.25 (adj-OR = 0.56, 95%CI 0.32–0.98) and MELD score (adj-OR = 0.91, 95%CI 0.85–0.96; Table 4) decreased the likelihood of SVR. There was a strong association between MELD ≥ 20 and not achieving SVR (adj-OR = 0.13, 95%CI 0.04–0.50). Other variables of note that did not meet statistical significance were a history of prior PPIs/H2-RBs use (adj-OR = 0.60, 95%CI 0.36–1.00, p = 0.052) and higher ALT (adj-OR = 0.98, 95%CI 0.95–1.00, p = 0.052).
Among cirrhotic patients, there was a trend toward positive association with RBV treatment and SVR (adj-OR = 2.91, 95%CI 0.97–8.73, p = 0.057). The majority of cirrhotic patients taking RBV had G3 infection (64%). To further explore this, the MVA was restricted to G3 patients with cirrhosis adjusting for gender, MELD score, and FIB-4 (variables found significant in the all genotype SVR/cirrhosis model). In this subgroup, RBV use was suggestive of better response though did not achieve statistical significance (adj-OR = 2.91, 0.96–2.81, p = 0.058).
In MVA, SVR rates vary significantly according to the three main treatment groups (p = 0.008). To explore the association between SVR and the somewhat heterogeneous treatment regimens included here, the MVA was repeated also including a variable categorizing treatment regimens as AASLD-approved regimens [21] (Sofosbuvir/Velpatasvir, Sofosbuvir + Ribavirin, Glecaprevir/Pibrentasvir, and Sofosbuvir/Velpatasvir/Voxilaprevir; n = 635) vs non-standard therapy (n = 2304). In these analyses, AASLD-approved regimen use was suggestive of better response among cirrhotic patients though did not achieve statistical significance (adj-OR = 1.78, 0.94–3.35, p = 0.075), with no association with SVR among the whole study cohort (adj-OR = 1.32, 0.83–2.10, p = 0.238).
Retreatment
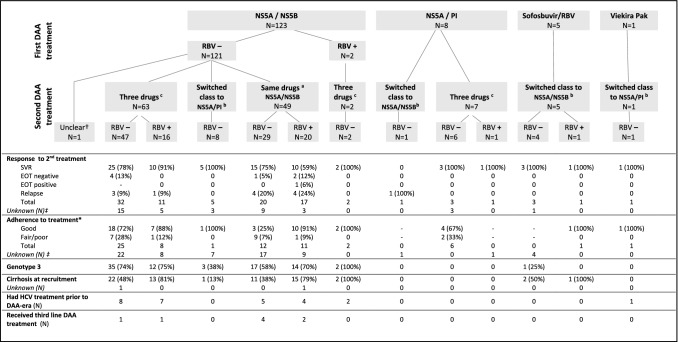
Data about DAA-retreatment were available for 137 patients: 123 did not have SVR after the first treatment, with 114 definitely completing first-line HCV treatment. Twelve patients had SVR after the first HCV DAA treatment and were presumed to have re-infection, for 1 patient SVR was unknown and 1 patient had a HCV PCR at EOT positive. Individuals requiring retreatment were more likely to be male (76.6%; p = 0.007), G3 (62.0%; p < 0.001), those with recognised fair/poor adherence (29.4%; p < 0.001) and cirrhotic (49.6%; p < 0.001). Treatment and retreatment regimens were reflective of PBS-funded availability, with most retreated patients having received first-line NS5A/NS5B combination (N = 123, 89%) such as ledipasvir or daclatasvir or velpatasvir combined with sofosbuvir (Fig. 2).
Fig. 2.
Clinical characteristics and details of HCV treatment for 137 patients who received second-line DAA therapy
Among patients with post-retreatment results, three broad treatment strategies were used with variable SVR rates: (a) Re-use of the same dual agent regime (n = 37 with treatment response data); (b) switch or add a different class of dual therapy agents (n = 11); or (c) triple therapy (n = 49) (Fig. 2). Overall, confirmed SVR for second-line treatment was 78%, 7% were negative at the EOT (but not confirmed SVR), and 15% non-SVR. SVR for the different treatment regimens is available in Supplementary material (e.g. SVR rate reached 75% for patients who received NS5A/NS5B first-line and were retreated with the same NS5A/NS5B regimen).
Discussion
Australia has used universal healthcare funding to improve DAA access for Australians with chronic HCV. Increasingly, HCV treatment is being undertaken by general practitioners rather than liver specialists [22]. These changes provide novel opportunities for HCV elimination, but also new challenges to ensure optimal care for individuals with advanced liver disease. High rates of SVR in DAA treatment and retreatment of patients were demonstrated. Liver fibrosis and/or cirrhosis decreased the likelihood of SVR throughout the spectrum from significant liver fibrosis to decompensated liver disease. While some of the identified predictors of HCV treatment failure have been reported by other real-world DAA treatment studies (e.g. male gender [7, 9] presence of cirrhosis [7–9] G3 [8]), novel findings are discussed below.
A key strategy in Australia’s HCV elimination efforts is the broadening of the DAA prescriber base to include general or family medicine practitioners. To do so safely requires knowledge and accessibility to liver fibrosis assessment to rule out cirrhosis. The presence of more advanced fibrosis (as measured by FIB-4), the presence of cirrhosis, and the severity of cirrhosis (as measured by Child–Pugh or MELD score), all remained important predictors of treatment response in this cohort. While a few real-world HCV cohorts assessed FIB 4 score of patients [8, 9], its effect on the rate of SVR was not reported. Interestingly, the FIB 4 score—a desk-top, non-invasive, inexpensive, and simply calculated score was strongly associated with SVR while transient elastography, after adjustment for clinical factors, was not. Non-specialist family medical practitioners prescribing DAA therapy have free access to such online calculators. Transient elastography, on the other hand, remains restricted to hospital-based clinics and fee-for-service community radiology services. Thus, to improve expansion of general medical practitioner community DAA prescribers, greater emphasis could be placed on developing liver fibrosis assessment skills using accessible tools such as FIB-4. Alternatively, patients with FIB-4 > 3.25 had approximately 50% (adjusted) risk of not achieving SVR compared to patients with lower scores. These patients may benefit from the added inputs of specialist HCV treatment centers or hepatology clinics, where more resources may be available to support adherence, review concomitant medications or exclude cirrhosis more definitively, in order to optimise SVR rates.
Interestingly, cirrhotic patients on gastric alkalinizing therapy (PPI/H2-RB) appeared to have 40% lower odds of achieving SVR, marginally outside statistical significance thresholds in this subgroup analysis (adj-OR = 0.60, 95%CI 0.36–1.00; p = 0.052). Any possible association was less apparent in the overall cohort (adj-OR 0.69, 95%CI 0.45–1.05; p = 0.080). Other real-world cohorts have found limited effect of these agents on HCV treatment outcomes, though, in one study, a similar sub-group analysis found an impact on SVR of higher PPI doses in cirrhotic patients [23]. Another original feature of our study was the use of PBS data to identify a group of patients who have or are at risk of acid reflux symptoms. Through PBS linkage we assessed whether patients had been prescribed these agents within the 5 years of recruitment, not limiting this to noted active prescription at the time of the HCV therapy. This may identify a group of patients who have, or are at risk of acid reflux symptoms and seek over-the-counter alkalizing agent treatments. While most clinics specify the need to defer timing or de-escalate PPI doses, other gastric alkalinizing agents may be “off the radar” and used by patients in order to manage symptoms when avoiding PPI. These may still impair SVR efficacy. Discussion around PPI use on HCV treatment should include a broader discussion of gastric acid symptom management, particularly for cirrhotic patients, to avoid inadvertently affecting treatment outcomes.
Retreatment has poorer SVR outcomes with overall rates of 78%. For a very small group of patients, despite the clinician’s impression of good adherence, multi-exposure DAA therapy results in sub-optimal outcomes at the third or fourth line of therapy. This stresses that key opportunity for cure is the first treatment in DAA-naïve patients, supporting adherence to optimize outcomes. Retreatment with the same NS5A/5B dual therapy resulted in unacceptably low rates of SVR (67%). In environments where triple-agent DAA therapy is not available, class switching and adjunctive therapy may be one strategy to increase SVR. Other studies have suggested benefit from the use of RBV in patients with cirrhosis and G3 infection [24]. These data continue to emerge but do raise the possibility of additional clinical benefit from the use of RBV among this difficult to treat group with G3 or cirrhosis. While limited by small numbers, though ultimately the absence of statistical significance suggests any effect size may be minor.
Other real-world studies of patients with HCV in the Asia–Pacific region [7, 9, 10] and elsewhere [11] did not assess the effect of treatment adherence on SVR rates. One exception was a Taiwanese study [8] reporting that DAA adherence less than 60% was the most important factor associated with treatment failure (add-OR = 117.1, 95%CI 52.4–261.3). In our study, lower adherence was significantly associated with poorer treatment outcomes. A profile of poor adherence includes younger age, socioeconomic disadvantage, residence outside major city areas, and a history of being prescribed opioids. Given poorer SVR results in subsequent treatment, optimising adherence in first-line treatment is vital to ensure the best outcome for patients. Engaging patients with their treatment journey using all available patient supports including nursing staff, family doctors, social workers, and community groups may benefit patients at higher risk of non-adherence. As treatment expands into the community, particularly in high-risk transmission communities, predicting patients at risk of non-adherence risk may enable intensifying supports available for them. Ensuring prescribers are adequately resourced to support patients via nurses and phone supports may be one way to optimize adherence.
The study’s strength included a large multicenter cohort design enriched for patients with cirrhosis and G3 infection, consistent with Asia–Pacific’s high prevalence of G3. Available data included comprehensive assessment for liver fibrosis and cirrhosis using validated invasive and non-invasive tests, and well characterised liver disease with MELD and Child–Pugh score. Additionally, linked population-based prescribing data was used to verify medications. This linkage process demonstrated that acid-neutralising therapy prescription may be associated with lower SVR rates in patients with cirrhosis. Opioid prescription was not associated with lower SVR, but was associated with clinician impression of poorer adherence.
Among the limitations, while this was a large multicenter study, our findings may not be generalizable to all Australians with HCV, in particular patients treated in community clinics or living in more remote areas. Loss to follow-up remains an ongoing challenge, even with population linkage. For a small number of patients, EOT results were used to extrapolate SVR, though conversion to SVR (for EOT positive) and relapse (for EOT negative) is well described. Adherence was assessed using the clinician’s impression. While such subjective method of assessment of medication adherence is less reliable compared to other methods (e.g. quantitative pill-counting assessments), low cost, simplicity, and real-time feedback have contributed to its common use in clinical practice. While the relatively poor sensitivity and specificity of such method can lead to bias, [25] interestingly EOT (e.g. pre-SVR result) impression of adherence had a robust consistent “dose-responsive” association with lower adherence and lower SVR, and is ultimately the only “measure” clinicians have available in practice. A measure of the patient’s impression of adherence would have provided some counterpoint to the clinician’s impression.
A persisting “blind spot” of HCV therapy is demonstrated for patients with G3 and cirrhosis. A role for adjunctive RBV to improve SVR for these patients remains appealing, however, a statistically significant effect was not demonstrated. Interpretation of possible small effect size from RBV should be interpreted with caution due to the small number of patients. Further data in better powered sample size are needed for this important patient group. Identifying a “risk profile” of poor adherence—younger people from lower socio-economic backgrounds, possibly with poorer health literacy and the presence of opioid replacement may be features to help identify those who may benefit from pre-treatment counseling, in order to optimise SVR for first-line therapy. This cohort has been recruiting since the DAA availability through hospital-based liver clinics, however, over this time, a shift to community prescribing has gradually increased [22]. In Australia, HCV treatment for patients with cirrhosis occurs in established in liver clinics. Moving forward, this cohort may capture less of the dynamic epidemiology of HCV incidence and prevalence, as treatments move to the community. Importantly, this cohort is enriched for “hard” end-points of HCV-associated liver disease, such as decompensation and liver cancer.
The OPERA-C study is ongoing and SVR data reported here included only patients for whom follow-up data was available at the time of data extraction. Further follow-up for SVR and other survival outcomes is anticipated to improve when further time for SVR follow-up occurs. After adjustment for other factors on multivariable models, poor adherence was a consistent predictor of non-SVR. Given this, it may be that improving patient targeted supports to help optimize adherence for those most risk (e.g. younger, treatment naïve, and non-cirrhotic patients), could improve SVR outcomes. More advanced liver disease was associated with non-SVR, and as non-specialist DAA prescribers in the community increase, efforts to improve liver fibrosis assessment skills and universal coverage to improve access for liver fibrosis testing maybe enabling at a nation-level for more effective pursuit of HCV elimination in Australia. Longer-term follow-up for outcomes such as survival, liver cancer, and retreatment rate will be available for the OPERA-C cohort through the data-linkage process over time. These will provide further insights into longer implications of universal coverage for HCV therapy including the relative benefits and costs to the community.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgments
Project governance was overseen by the Project Steering Committee, via the GESA Liver Clinical Research Network. QIMR Berghofer was the administering institution. We thank the patients for participating in the study. We also thank David Roche (GESA), Karen Martin (QIMR Berghofer) for their support in coordinating the study, Therese Lawton (QIMR Berghofer) for data management, and the research nurses from each study site for recruitment and data collection.
Abbreviations
- APRI
Aspartate aminotransferase to platelet ratio index
- CI
Confidence interval
- DAAs
Direct-acting antivirals (DAAs)
- EOT
End of treatment
- FIB-4
Fibrosis-4 scores (FIB-4)
- G3
Genotype 3
- HCV
Hepatitis C virus
- H2-RB
Histamine-2 receptor blocker
- INR
International normalised ratio
- LSM
Liver stiffness measurement (LSM)
- LTFU
Lost to follow-up
- MVA
Multivariable analysis
- NS5A
Non-structural protein 5A
- NS5B
Non-structural 5B
- OR
Odds ratio
- OPERA-C
Observational, Prospective Epidemiological Registry in Australia of HCV Liver Disease
- PBS
Pharmaceutical Benefits Scheme
- PCR
Polymerase chain reaction
- PI
Protease inhibitors
- PPI
Proton pump inhibitor
- NS5A
Non-structural protein 5A
- NS5B Ribavirin (RBV)
Non-structural 5B
- SVR
Sustained viral response
Author’s contribution
Study conceptualisation: PJC, SR, SS, AJT, BL, JG, and WS; data curation and analysis: PCV and PJC; patient recruitment: PJC, SIS, MW, AT, ML, BL, AZ, JR, PA, JG, SB, BM, WS, GM, ET, AN, AW, GC, DH, WC, GF, and RS were involved in patient recruitment and/or review of patient data; manuscript drafting and editing: PJC and PCV; manuscript draft revision: all authors revision of the manuscript for important intellectual content and approved the final version of the article, including the authorship list.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. Funding and in-kind support was received from the Commonwealth Department of Health, the Gastroenterological Society of Australia (GESA), and unrestricted research grants from Gilead Sciences, Merck, and Abbvie.
Declarations
Conflict of interest
The authors do not have any disclosures to report.
Consent to participate (ethics)
Informed consent was obtained from all subjects who participated in the study. The Human Ethics Committees of the Royal Brisbane & Women's Hospital (HREC/15/QRBW/183), participating hospitals, and QIMR Berghofer Medical Research Institute (P2126) approved this study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Paul J. Clark and Patricia C. Valery contributed equally.
References
- 1.Deloitte Access Economics . The economic cost and health burden of liver diseases in Australia. Sydney: Gastroenterological Society of Australia/Australian Liver Association; 2013. [Google Scholar]
- 2.Razavi H, Waked I, Sarrazin C, Myers RP, Idilman R, Calinas F, Vogel W, et al. The present and future disease burden of hepatitis C virus (HCV) infection with today's treatment paradigm. J Viral Hepat. 2014;21(Suppl 1):34–59. doi: 10.1111/jvh.12248. [DOI] [PubMed] [Google Scholar]
- 3.Alavi M, Law MG, Valerio H, Grebely J, Amin J, Hajarizadeh B, Selvey C, et al. Declining hepatitis C virus-related liver disease burden in the direct-acting antiviral therapy era in New South Wales. Australia. J Hepatol. 2019;71:281–288. doi: 10.1016/j.jhep.2019.04.014. [DOI] [PubMed] [Google Scholar]
- 4.Lee A, Hanson J, Fox P, Spice G, Russell D, Boyd P. A decentralised, multidisciplinary model of care facilitates treatment of hepatitis C in regional Australia. J Virus Erad. 2018;4:160–164. doi: 10.1016/S2055-6640(20)30270-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta S, Rout G, Patel AH, Mahanta M, Kalra N, Sahu P, Sethia R, et al. Efficacy of generic oral directly acting agents in patients with hepatitis C virus infection. J Viral Hepat. 2018;25:771–778. doi: 10.1111/jvh.12870. [DOI] [PubMed] [Google Scholar]
- 6.Norton BL, Fleming J, Bachhuber MA, Steinman M, DeLuca J, Cunningham CO, Johnson N, et al. High HCV cure rates for people who use drugs treated with direct acting antiviral therapy at an urban primary care clinic. Int J Drug Policy. 2017;47:196–201. doi: 10.1016/j.drugpo.2017.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yee J, Carson JM, Hajarizadeh B, Hanson J, O'Beirne J, Iser D, Read P, et al. High Effectiveness of Broad Access Direct-Acting Antiviral Therapy for Hepatitis C in an Australian Real-World Cohort: The REACH-C Study. Hepatol Commun 2021. [DOI] [PMC free article] [PubMed]
- 8.Chen CY, Huang CF, Cheng PN, Tseng KC, Lo CC, Kuo HT, Huang YH, et al. Factors associated with treatment failure of direct-acting antivirals for chronic hepatitis C: A real-world nationwide hepatitis C virus registry programme in Taiwan. Liver Int. 2021;41:1265–1277. doi: 10.1111/liv.14849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ji F, Li J, Liu L, Liang J, Wang X, Liu J, Cai D, et al. High hepatitis C virus cure rates with approved interferon-free direct-acting antivirals among diverse mainland Chinese patients including genotypes 3a and 3b. J Gastroenterol Hepatol. 2021;36:767–774. doi: 10.1111/jgh.15192. [DOI] [PubMed] [Google Scholar]
- 10.Mushtaq S, Mansoor A, Umar M, Khan A, Siddiqi S, Manzoor S. Direct-acting antiviral agents in the treatment of chronic hepatitis C-Real-life experience from clinical practices in Pakistan. J Med Virol 2020. [DOI] [PubMed]
- 11.Kruger K, Krauth C, Rossol S, Mauss S, Boeker KHW, Muller T, Klinker H, et al. Outcomes and costs of treating hepatitis C patients with second-generation direct-acting antivirals: results from the German Hepatitis C-Registry. Eur J Gastroenterol Hepatol. 2019;31:230–240. doi: 10.1097/MEG.0000000000001283. [DOI] [PubMed] [Google Scholar]
- 12.Rosen HR. "Hep C, where art thou": What are the remaining (fundable) questions in hepatitis C virus research? Hepatology. 2017;65:341–349. doi: 10.1002/hep.28848. [DOI] [PubMed] [Google Scholar]
- 13.Thompson AJ. Australian recommendations for the management of hepatitis C virus infection: a consensus statement. Med J Aust. 2016;204:268–272. doi: 10.5694/mja16.00106. [DOI] [PubMed] [Google Scholar]
- 14.Carver AB, Zuckerman AD, DeClercq J, Choi L, Chastain CA. Incidence and Impact of Persistent Viremia on SVR Rates in Patients Receiving Direct-Acting Antiviral Therapy. Open Forum Infect Dis 2020;7:ofaa569. [DOI] [PMC free article] [PubMed]
- 15.Australian Institute of Health and Welfare (AIHW). Rural, regional and remote health: A guide to remoteness classifications. Canberra, Australia: AIHW; 2004.
- 16.Australian Bureau of Statistics (ABS). Census of Population and Housing: Socio-economic Indexes for Areas (SEIFA), Australia, 2006. Canberra, Australia: ABS; 2008.
- 17.Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, M SS, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006;43:1317–1325. [DOI] [PubMed]
- 18.Lin ZH, Xin YN, Dong QJ, Wang Q, Jiang XJ, Zhan SH, Sun Y, et al. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: an updated meta-analysis. Hepatology. 2011;53:726–736. doi: 10.1002/hep.24105. [DOI] [PubMed] [Google Scholar]
- 19.Bloom S, Kemp W, Nicoll A, Roberts SK, Gow P, Dev A, Bell S, et al. Liver stiffness measurement in the primary care setting detects high rates of advanced fibrosis and predicts liver-related events in hepatitis C. J Hepatol. 2018;69:575–583. doi: 10.1016/j.jhep.2018.04.013. [DOI] [PubMed] [Google Scholar]
- 20.Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31:864–871. doi: 10.1053/he.2000.5852. [DOI] [PubMed] [Google Scholar]
- 21.Ghany MG, Morgan TR, Panel A-IHCG. Hepatitis C Guidance Update: American Association for the Study of Liver Diseases-Infectious Diseases Society of America Recommendations for Testing, Managing, and Treating Hepatitis C Virus Infection. Hepatology. 2019;2020(71):686–721. doi: 10.1002/hep.31060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stafford F, Dore GJ, Clackett S, Martinello M, Matthews GV, Grebely J, Balcomb AC, et al. Prescribing of direct-acting antiviral therapy by general practitioners for people with hepatitis C in an unrestricted treatment program. Med J Aust 2021. [DOI] [PubMed]
- 23.Tapper EB, Bacon BR, Curry MP, Dieterich DT, Flamm SL, Guest LE, Kowdley KV, et al. Evaluation of proton pump inhibitor use on treatment outcomes with ledipasvir and sofosbuvir in a real-world cohort study. Hepatology. 2016;64:1893–1899. doi: 10.1002/hep.28782. [DOI] [PubMed] [Google Scholar]
- 24.Lu M, Wu KH, Li J, Moorman AC, Spradling PR, Teshale EH, Boscarino JA, et al. Adjuvant ribavirin and longer direct-acting antiviral treatment duration improve sustained virological response among hepatitis C patients at risk of treatment failure. J Viral Hepat. 2019;26:1210–1217. doi: 10.1111/jvh.13162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lam WY, Fresco P. Medication Adherence Measures: An Overview. Biomed Res Int 2015;2015:217047. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.