Abstract
Background:
Fluoride has been associated with IQ deficits during early brain development, but the period in which children are most sensitive is unknown.
Objective:
We assessed effects of fluoride on IQ scores across prenatal and postnatal exposure windows.
Methods:
We used repeated exposures from 596 mother-child pairs in the Maternal-Infant Research on Environmental Chemicals pregnancy and birth cohort. Fluoride was measured in urine (mg/L) collected from women during pregnancy and in their children between 1.9 and 4.4 years; urinary fluoride was adjusted for specific gravity. We estimated infant fluoride exposure (mg/day) using water fluoride concentration and duration of formula-feeding over the first year of life. Intelligence was assessed at 3–4 years using the Wechsler Preschool and Primary Scale of Intelligence-III. We used generalized estimating equations to examine the associations between fluoride exposures and IQ, adjusting for covariates. We report results based on standardized exposures given their varying units of measurement.
Results:
The association between fluoride and performance IQ (PIQ) significantly differed across prenatal, infancy, and childhood exposure windows collapsing across child sex (p = .001). The strongest association between fluoride and PIQ was during the prenatal window, B = −2.36, 95% CI: −3.63, −1.08; the association was also significant during infancy, B = −2.11, 95% CI: −3.45, −0.76, but weaker in childhood, B = −1.51, 95% CI: −2.90, −0.12. Within sex, the association between fluoride and PIQ significantly differed across the three exposure windows (boys: p = .01; girls: p = .01); among boys, the strongest association was during the prenatal window, B = −3.01, 95% CI: −4.60, −1.42, whereas among girls, the strongest association was during infancy, B = −2.71, 95% CI: −4.59, −0.83. Full-scale IQ estimates were weaker than PIQ estimates for every window. Fluoride was not significantly associated with Verbal IQ across any exposure window.
Conclusion:
Associations between fluoride exposure and PIQ differed based on timing of exposure. The prenatal window may be critical for boys, whereas infancy may be a critical window for girls.
Keywords: community water fluoridation, critical windows of exposure, generalized estimating equations, intelligence quotient, fluoride neurotoxicity
Fluoride has been associated with IQ deficits at water fluoride concentrations >1.2 mg/L (Choi et al., 2012; Dong et al., 2018; Grandjean, 2019; National Toxicology Program, 2020; Seraj et al., 2012; Xiang et al., 2003; Valdez Jiménez et al., 2017). Early-life exposure to optimal levels (i.e., 0.7 mg/L) of fluoride – as defined by levels sufficient to protect against tooth decay and minimize against dental fluorosis – has also been associated with diminished cognitive abilities in prospective studies of children (Bashash et al., 2017; Green et al., 2019; Till et al., 2020). Drinking water is a main source of fluoride for pregnant women (Till et al., 2018) and young children (dela Cruz et al., 2008 ; Green et al., 2020) living in communities with water fluoridation (US EPA, 2010). Therefore, associations between fluoride in pregnancy and child outcomes may be conflated by continuous exposure to fluoride over the lifespan. Few human studies have examined the developmental period of greatest vulnerability to fluoride neurotoxicity (Xu et al., 2020).
Identifying critical windows of vulnerability to fluoride during early brain development is important because the timing of exposure may result in a greater risk of potentially permanent adverse outcomes (Hornung et al., 2009; Selevan et al., 2000). During fetal development, the brain is particularly vulnerable to environmental toxicants (Lanphear, 2015). Still, the brain continues to undergo an orderly sequence of neuronal developmental processes (e.g., synaptogenesis, myelination), and the period of heightened vulnerability may extend for many months after birth (Rice and Barone, 2000). Thus, sensitivity to neurotoxicants may continue into infancy.
The susceptibility of infants to fluoride from drinking water is further amplified by their higher level of water intake than adults on a per bodyweight basis (Snodgrass, 1992) and lower ability to detoxify exogenous compounds than adults. In particular, formula-fed infants whose formula is made with fluoridated water have an approximate 70-fold higher fluoride intake than exclusively breastfed infants (Ekstrand, 1981; Zohoori et al., 2018; US EPA, 2010). Thus, level and timing of fluoride exposure are critical for determining the window of greatest vulnerability for neurodevelopmental outcomes.
We examined the impact of fluoride exposure on children’s intelligence quotient (IQ) scores as a function of exposure timing and sex in the same cohort. Previous studies have used ordinary least-squares linear regression to covary fluoride exposures at timepoints other than those of substantive interest. For example, Bashash et al. (2017) estimated prenatal effects while controlling postnatal effects and Till et al. (2020) estimated neonatal effects while controlling prenatal effects. This approach, however, cannot fully account for non-independent observations due to measurements at different timepoints being nested within mother-infant pairs, nor make formal comparisons of associations across timepoints (Buckley et al., 2019).
To overcome these limitations, we adapted an approach from Sanchez et al. (2011) using generalized estimating equations (GEE) for repeated exposure variables and a single outcome measure of IQ score. Each fluoride exposure measure is treated as a window (i.e., a particular developmental period). We incorporated interactions to estimate sex-specific associations with IQ based on our prior finding that boys may be more susceptible to prenatal fluoride exposure than girls (Green et al., 2019), a recent review of sex effects in animal and human fluoride studies (Green et al., 2020), and literature on other neurotoxins suggesting interactions between sex and exposure timing (Comfort and Re, 2017; Kern et al., 2017; Torres-Rojas and Jones, 2018).
1. Methods
1.1. Study participants
We used data from the Maternal-Infant Research on Environmental Chemical (MIREC) longitudinal cohort, which recruited 2001 pregnant women between 2008 and 2011. Women were recruited from prenatal clinics if they were at least 18 years old, less than 14 weeks gestation, and spoke English or French. Exclusion criteria included fetal abnormalities, medical complications, and illicit drug use during pregnancy; further details have been previously described (Arbuckle et al., 2013; Green et al., 2019; Till et al., 2020). Our sample included 601 mother-child dyads who completed the follow-up phase of the study (MIREC-Child Development Plus) when children’s neurodevelopmental testing was conducted at 3–4 years of age. Data from five mother-child dyads were excluded due to the mothers’ declining prenatal and birth data collection (i.e., trimester fluoride exposures, demographic information, covariates, and offspring date of birth), leaving N = 596 mother-child dyads for our full analytic sample (Fig. 1). Other mother-child pairs missing some data on fluoride exposure, outcomes, or covariates were retained due to the flexibility of GEE to incorporate missing data. On outcomes and covariates, no more than 4.6% of data was missing (M = 1.08, range 0–4.6). Dyads lived in one of six cities that either adhere to community water fluoridation (i.e., Toronto, Halifax, and Hamilton) or do not (i.e., Montreal, Vancouver, and Kingston). About half of all dyads (44%) lived in fluoridated cities.
Fig. 1.
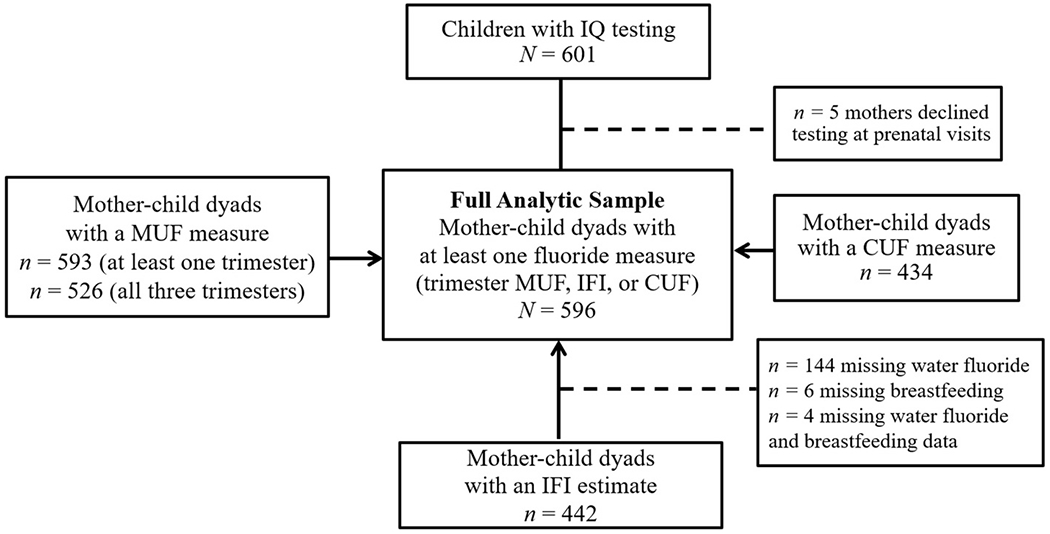
Flowchart of mother-child dyads with neurodevelopmental testing.
Note. MUF = maternal urinary fluoride; IFI = infant fluoride intake; CUF = child urinary fluoride.
1.2. Fluoride exposure measures
Maternal Urinary Fluoride (MUF).
We used MUF (see Till et al., 2018) as a measure of prenatal fluoride exposure. The MIREC study collected spot urine samples in each trimester. To account for urine dilution, concentrations for fluoride in each trimester were adjusted by specific gravity (SG) with
where Pc is the SG-adjusted fluoride concentration, Pi is the observed fluoride concentration, SGi is the specific gravity of the ith urine sample, and SGm is the median SG for the cohort (Duty et al., 2005). Of the 593 women with at least one valid measure of MUF, 526 (88.3%) had a urine sample collected at all three trimesters. Our prenatal fluoride exposure variable was calculated by averaging the trimester-specific MUF measures. We calculated average MUF levels only when valid samples were available for all three trimesters to strengthen reliability of the measure (Till et al., 2018). Because GEE can incorporate missing data, we retained the 67 women (of the N = 596 dyads) for whom an average MUF value was missing; for these participants, trimester MUF measures were used in preliminary trimester-specific analyses, and we included their data on covariates or exposures assessed at other time points in the primary analysis. Urinary fluoride concentrations were analyzed using a modification of the hexamethydisiloxane (Sigma Chemical Co., USA) micro-diffusion procedure (Martinez-Mier et al., 2011).
Infant fluoride intake (IFI).
Following Till et al. (2020), we estimated IFI over the first year of the child’s life using the following equation:
Where water fluoride (mg/L) is the average water fluoride concentration in the community during the first six months of the infant’s life, 0.8 L/day is the approximate amount of water used to reconstitute powdered formula at 3 months of age (Carignan et al., 2015), and 1-(exclusive breastfeeding/11.99) is the proportion of time over the first year of life that the infant was not exclusively breastfed. Water fluoride levels were based on reports from water treatment plants associated with postal codes matching each mother’s residence during the third trimester of her pregnancy. The number of months of exclusive breastfeeding was recoded so that mothers who reported exclusive breastfeeding between 12 and 24 months were assigned a value of 12. Thus, formula-fed infants living in areas with community water fluoridation had IFI values near 1 and exclusively breastfed infants had values near 0. Infants receive very low concentrations of fluoride through breastmilk due to the limited transfer of fluoride from plasma to breast milk (Ekstrand et al., 1984). Mean fluoride concentration in breast milk is < 0.02 μg/mL, with similar levels found among mothers living in fluoridated and non-fluoridated areas (Zohoori et al., 2018).
The type of infant formula used was not reported and thus fluoride from infant formula could not be added to the derivation (see Till et al., 2020). IFI values were available for 440 mother-child dyads (Fig. 1).
Child urinary fluoride (CUF).
We measured CUF as an estimate of childhood fluoride exposure using a spot urine sample taken when children were between 1.86 and 4.40 years old (M = 3.25, SD = 0.54), also adjusted for specific gravity (n = 437) (Fig. 1).
1.3. Child intellectual abilities
Trained research assistants assessed children’s intellectual abilities at the age of 3–4 years using the Wechsler Preschool and Primary Scale of Intelligence-III (WPPSI-III; Canadian norms; Wechsler, 2002). Outcomes included Performance IQ (PIQ), a measure of nonverbal reasoning, Verbal IQ (VIQ), a measure of verbal reasoning and comprehension, and Full-Scale IQ (FSIQ), a measure of overall intellectual ability. Examiners administered the WPPSI between 2012 and 2015, prior to proposing our fluoride research; examiners are therefore considered blinded to exposure status.
1.4. Covariates
We selected covariates consistent with prior work examining fluoride exposure and child intellectual abilities (Green et al., 2019; Till et al., 2020). Covariates included maternal education (dichotomized as Bachelor’s degree or higher; yes/no), maternal race (Caucasian/non-Caucasian), mother-reported exposure to second-hand smoke (yes/no) while pregnant, and a continuous measure of quality of home environment using the Home Observation for Measurement of the Environment (HOME) - Revised Edition (Caldwell and Bradley, 1984) at the 3-4 year-old home visit. We also included child age (in months) at child urine sampling to control for age-related differences in CUF. We did not include child age at IQ testing as the WPPSI-III is age-normed in 2-month intervals. We also did not include city as a covariate in our GEE model based on its redundancy with water fluoride that is used to calculate IFI. There was no collinearity among the covariates or exposures included.
1.5. Statistical analysis
Using the GEE method of Sanchez et al. (2011), we constructed a model to estimate associations between the fluoride exposure variables and IQ scores while adjusting for covariates. To support our decision to combine trimester exposures into a single prenatal measure, we tested whether the associations between MUF and IQ outcomes (FSIQ, PIQ, and VIQ) differed across trimesters of pregnancy by using each trimester as a separate exposure period. For our trimester-specific analysis, we included women with at least one valid MUF value (n = 593).
Our primary model included all fluoride exposure windows (MUF, IFI, and CUF) as predictors of either FSIQ, PIQ, or VIQ. We first present results of a fluoride by time interaction with girls and boys combined (i.e., comparisons of fluoride exposure windows for the overall sample). Each analysis also produced a test of the three-way interaction between fluoride exposure, time, and child sex, which leads to separate fluoride by time interactions for each sex without stratifying the sample. In addition to testing this three-way interaction, we also tested the exposure by time two-way interaction within each sex regardless of the significance of the three-way interaction.
In sensitivity analyses, we removed mother-child dyads if the child’s FSIQ score fell in the intellectual impairment range (i.e. score <70 in three cases) or if removal of a mother-child dyad would change coefficients of exposure variables by at least 0.40 standard deviations according to DFBETAS indices (i.e., the difference in magnitude of an estimated coefficient with and without an observation, scaled by the standard error calculated without the deleted observation; Belsley et al., 1980). Table 5 presents results of GEE analyses for our primary model after excluding influential dyads.
Table 5.
Sensitivity analysis for the effects of standardized average maternal urinary fluoride (MUF), infant fluoride intake (IFI) and child urinary fluoride (CUF) on age-normed IQ scores after excluding influential dyads. B (95% CI reported.
| Males (n = 288)a | Females (n = 302)b | Overall (N = 590)c | |
|---|---|---|---|
| FSIQ | |||
| MUF | −1.22 (−2.41, −0.04) | −1.00 (−2.84, 0.84) | −1.14 (−2.25, −0.04) |
| IFI | 0.10 (−1.55, 1.75) | −1.58 (−3.17, 0.01) | −0.76 (−1.89, 0.38) |
| CUF | 0.40 (−1.14, 1.95) | −0.00 (−1.61, 1.61) | 0.18 (−1.01, 1.38) |
|
| |||
| pint | .19 | .12 | .08 |
|
| |||
| PIQ | |||
| MUF | −2.39 (−4.05, −0.73) | −2.00 (−4.19, 0.20) | −2.24 (−3.56, −0.92) |
| IFI | −1.38 (−3.32, 0.55) | −3.59 (−5.48, −1.70) | −2.51 (−3.86, −1.16) |
| CUF | −1.17 (−3.29, 0.94) | −1.21 (−3.12, 0.71) | −1.19 (−2.61, 0.23) |
|
| |||
| pint | .01 | <.001 | <.0001 |
|
| |||
| VIQ | |||
| MUF | 0.25 (−1.11, 1.61) | 0.33 (−1.47, 2.13) | 0.28 (−0.80, 1.36) |
| IFI | 1.35 (−0.24, 2.93) | 0.64 (−0.91, 2.19) | 0.99 (−0.12, 2.09) |
| CUF | 1.89 (0.16, 3.62) | 0.98 (−0.60, 2.55) | 1.39 (0.23, 2.56) |
|
| |||
| pint | .13 | .36 | .03 |
Note. Covariates include maternal education, maternal race, total HOME score, age that children provided CUF, and prenatal second-hand smoke. Mother-child dyads were influential if DFBETAS indices were >0.40 and/or child FSIQ <70. pint refers to the interaction between exposure timing and fluoride level. Influence analyses were conducted simultaneously for boys, girls, and overall effects for each outcome. Bolded estimates are significant, p < .05 (p values corrected for multiple comparisons using the Benjamini-Hochberg FDR method).
FSIQ n = 287.
PIQ n = 301.
FSIQ and PIQ N = 589.
Given the large number of comparisons, we corrected p values for multiple comparisons using the false discovery rate (FDR) method of Benjamini and Hochberg (1995). A two-tailed FDR correction was implemented using a corrected p value of Q = .05 across the family of 27 coefficients tested in each of our main analyses, sensitivity analyses, and supplemental analyses. We also applied FDR correction to the tests of whether effect estimates differ across exposure windows.
Diagnostic plots of fitted values against residuals did not reveal violation of the assumptions of linearity or constant variance, and residuals were approximately normally distributed. Analyses were conducted in SAS (version 9.4; SAS Institute Inc.). Statistical significance was set at α = .05 for a two-tailed test.
2. Results
Mothers were on average 32.4 years old (SD = 5.1) when they gave birth, predominantly Caucasian (89%), well-educated (66.7% had at least a bachelor’s degree), and very few (2.7%) reported exposure to second-hand smoke during the first trimester of pregnancy (Table 1). Of the 593 mother-child pairs with at least one MUF value, the mean child age at intellectual testing was 3.4 years (SD = 0.3); girls comprised 51.1% of the sample (n = 303). The average Full Scale IQ (FSIQ) score was 106.6 (SD = 13.7) for the study sample, which is consistent for a predominantly educated and middle-to-upper class group. Table 2 shows the descriptive statistics and correlations among fluoride exposure variables.
Table 1.
Characteristics of study participants for the full analytic sample and for samples with complete data on fluoride exposure windows [Mean (SD)/%].
| Characteristic | Samples |
||||
|---|---|---|---|---|---|
| Full Analysis |
Trimester |
MUF |
IFI |
CUF |
|
| N = 596 | n = 593 | n = 526 | n = 442 | n = 434 | |
| Maternal Characteristics | |||||
| Years of age at delivery | 32.4 (5.1) | 32.4 (5.1) | 32.4 (5.1) | 32.5 (4.9) | 32.5 (5.3) |
| Net household income >$70 K CAD | 73.1 | 73.2 | 74.0 | 72.7 | 74.4 |
| Maternal education | |||||
| Trade school/high school | 33.3 | 33.4 | 32.1 | 30.7 | 30.0 |
| Bachelor’s degree or higher | 66.7 | 66.6 | 67.9 | 69.3 | 70.0 |
| Married/common-law at testing | 96.1 | 96.1 | 96.8 | 95.9 | 96.6 |
| Smoked in trimester 1 | 2.7 | 2.7 | 2.5 | 2.5 | 3.0 |
| Child characteristics | |||||
| Years of age at IQ testing | 3.4 (0.3) | 3.4 (0.3) | 3.4 (0.3) | 3.4 (0.3) | 3.5 (0.3) |
| Female sex | 51.2 | 51.1 | 51.7 | 50.7 | 51.3 |
| HOME total score | 47.2 (4.6) | 47.2 (4.6) | 47.2 (4.7) | 47.4 (4.5) | 47.2 (4.8) |
| Second-hand smoke in home | 3.5 | 3.5 | 3.8 | 3.0 | 3.0 |
| Gestational age in weeks | 39.1 (1.8) | 39.1 (1.7) | 39.1 (1.6) | 39.1 (1.8) | 38.9 (2.4) |
| Birth weight (kg) | 3.5 (0.5) | 3.5 (0.5) | 3.5 (0.5) | 3.4 (0.5) | 3.5 (0.5) |
| Full Scale IQ | 106.6 (13.7) | 106.6 (13.8) | 106.9 (13.5) | 107.6 (13.9) | 107.2 (13.3) |
| Verbal IQ | 109.2 (13.7) | 109.2 (13.6) | 109.5 (13.3) | 109.8 (13.6) | 110.1 (13.1) |
| Performance IQ | 102.7 (14.9) | 102.7 (14.9) | 102.7 (14.7) | 103.8 (14.9) | 102.7 (14.6) |
Note. MUF = maternal urinary fluoride; IFI = infant fluoride intake; CUF = child urinary fluoride.
Table 2.
Summary statistics and correlations among fluoride exposure variables.
| N | Median | M (SD) | Range | Pearson correlations |
IFI | ||||
|---|---|---|---|---|---|---|---|---|---|
| MUF | |||||||||
| T1 | T2 | T3 | Average | ||||||
| MUF (mg/L) | |||||||||
| T1 | 578 | 0.31 | 0.44 (0.46) | 0.01–4.29 | – | ||||
| T2 | 566 | 0.37 | 0.51 (0.48) | 0.03–5.28 | .36 | – | |||
| T3 | 552 | 0.49 | 0.65 (0.53) | 0.08–5.56 | .36 | .37 | – | ||
| Average | 526 | 0.44 | 0.53 (0.37) | 0.06–2.48 | .74 | .76 | .77 | – | |
| IFI (mg F) | 442 | 0.09 | 0.14 (0.13) | 0.00–0.61 | .17 | .16 | .30 | .28 | – |
| CUF (mg/L) | 434 | 0.39 | 0.51 (0.39) | 0.05–2.89 | .18 | .12 | .14 | .22 | .25 |
Note. All urinary fluoride values are adjusted based on specific gravity.
Abbreviations: CUF = child urinary fluoride; IFI = infant fluoride intake; MUF = Maternal urinary fluoride; Average = averaged over three trimesters; SD = standard deviation.
2.1. Overall effects of exposure windows
The association between MUF and IQ scores did not differ significantly across trimesters [FSIQ: χ2 (3) = 1.99, p = .57; PIQ: χ2 (3) = 1.08, p = .78; and VIQ: χ2 (3) = 2.21, p = .53] (Table S1). Thus, for the remaining analyses, we used average MUF as a single prenatal exposure. We compared average MUF, IFI, and CUF effects to examine the unique associations of prenatal, infancy, and childhood exposures on IQ scores. Table 3 shows the associations between standardized fluoride exposures (M = 0, SD = 1) and unstandardized IQ scores, whereas Table 4 shows the unstandardized coefficients per 0.5 mg/L MUF and CUF and per 0.1 mg IFI/day (to facilitate comparison of average MUF, IFI, and CUF associations with IQ scores). The standardized coefficient indicates the change in the dependent variable (i.e. age-normed IQ score) per one standard deviation (SD) difference in the fluoride exposure variable; thus, a standardized coefficient of −1.9 means that the IQ score decreases by 1.9 points per one SD increase in the exposure variable, keeping other variables constant. An unstandardized coefficient represents the amount by which the dependent variable changes per one unit change in the fluoride exposure (i.e. per 0.5 mg/L or 0.1 mg/day) variable, keeping other variables constant. Combining across boys and girls, the two-way interaction between fluoride and time was statistically significant for PIQ [χ2 (3) = 18.78, p < .001] and VIQ [χ2 (3) = 8.28 p = .04], but not for FSIQ [χ2 (3) = 4.36, p = .23]. Controlling the FDR, there were significant negative effects of standardized fluoride exposures for overall MUF and overall IFI on PIQ (B = −2.36, 95% CI: −3.63, −1.08; B = −2.11, 95% CI: −3.45, −0.76, respectively). The associations between standardized fluoride exposures and IQ scores are visualized in Fig. 2.
Table 3.
Effects of standardized average maternal urinary fluoride (MUF), infant fluoride intake (IFI) and child urinary fluoride (CUF) on age-normed IQ scores using GEE. B (95% CI) reported.
| Males (n = 291)a | Females (n = 305)b | Overall (N = 596)c | |
|---|---|---|---|
| FSIQ | |||
| MUF | −1.86 (−3.22, −0.49) | −0.23 (−2.06, 1.60) | −1.28 (−2.37, −0.18) |
| IFI | −0.01 (−1.67, 1.65) | −0.72 (−2.34, 0.89) | −0.38 (−1.53, 0.78) |
| CUF | 0.07 (−1.66, 1.80) | −0.41 (−2.07, 1.24) | −0.18 (−1.38, 1.02) |
|
| |||
| pint | .12 | .77 | .23 |
|
| |||
| PIQ | |||
| MUF | −3.01 (−4.60, −1.42) | −1.18 (−3.32, 0.96) | −2.36 (−3.63, −1.08) |
| IFI | −1.45 (−3.40, 0.49) | −2.71 (−4.59, −0.83) | −2.11 (−3.45, −0.76) |
| CUF | −1.49 (−3.50, 0.53) | −1.53 (−3.45, 0.39) | −1.51 (−2.90, −0.12) |
|
| |||
| pint | .01 | .01 | <.001 |
|
| |||
| VIQ | |||
| MUF | −0.25 (−1.57, 1.07) | 0.87 (−0.91, 2.64) | 0.15 (−0.91, 1.20) |
| IFI | 1.22 (−0.39, 2.83) | 1.31 (−0.25, 2.87) | 1.27 (0.15, 2.39) |
| CUF | 1.61 (−0.06, 3.29) | 0.63 (−0.98, 2.23) | 1.10 (−0.06, 2.26) |
|
| |||
| pint | .12 | .30 | .04 |
Note. N = 596. Covariates include maternal education, maternal race, total HOME score, age at urine sampling, and prenatal second-hand smoke. pint refers to the interaction between exposure timing and fluoride level. Estimates in bold are significant, p < .05 (p values corrected for multiple comparisons using the Benjamini-Hochberg FDR method).
Males: IFI n = 218; CUF n = 211.
Females: IFI n = 214; CUF n = 223.
Overall: IFI N = 432; CUF N = 434.
Table 4.
Effect of 0.5 mg/L of average maternal urinary fluoride (MUF)a, 0.1 mg/day of estimated infant fluoride intake (IFI)a and 0.5 mg/L of child urinary fluoride (CUF)a on IQ scores using GEE. Unstandardized B (95% CI reported.
| Males (n = 291)b | Females (n =305)c | Overall Participants (N = 596)d | |
|---|---|---|---|
| FSIQ | |||
| MUF | −2.48 (−4.30, −0.66) | −0.31 (−2.76, 2.14) | −1.71 (−3.17, −0.24) |
| IFI | −0.01 (−1.25, 1.24) | −0.54 (−1.75, 0.66) | −0.28 (−1.15, 0.58) |
| CUF | 0.09 (−2.10, 2.28) | −0.52 (−2.62, 1.58) | −0.23 (−1.75, 1.29) |
|
| |||
| pint | .12 | .77 | .23 |
|
| |||
| PIQ | |||
| MUF | −4.02 (−6.15, −1.89) | −1.58 (−4.43, 1.28) | −3.15 (−4.85, −1.44) |
| IFI | −1.09 (−2.54, 0.37) | −2.03 (−3.43, −0.63) | −1.58 (−2.59, −0.57) |
| CUF | −1.89 (−4.44, 0.67) | −1.94 (−4.37, 0.50) | −1.91 (−3.68, −0.15) |
|
| |||
| pint | .01 | .01 | <.001 |
|
| |||
| VIQ | |||
| MUF | −0.34 (−2.10, 1.43) | 1.16 (−1.22, 3.53) | 0.20 (−1.22, 1.61) |
| IFI | 0.92 (−0.29, 2.12) | 0.98 (−0.19, 2.15) | 0.95 (0.11, 1.79) |
| CUF | 2.05 (−0.08, 4.16) | 0.79 (−1.24, 2.82) | 1.39 (−0.08, 2.86) |
|
| |||
| pint | .12 | .30 | .04 |
Note. The overall N = 596 includes mother-child pairs with at least one measure of (average) MUF, IFI, or CUF. Covariates include maternal education, maternal race, total HOME score, age at urine sampling, and prenatal second-hand smoke. pint refers to the p-value for the interaction between exposure timing and fluoride level. Bolded estimates are significant, p < .05 (p values corrected for multiple comparisons using the Benjamini-Hochberg FDR method).
MUF is presented in 0.5 mg/L units based on the mean MUF = 0.53 mg/L; IFI is presented in 0.1 mg/day units based on the mean IFI = 0.14 mg/day; CUF is presented in 0.5 mg/L units based on the mean CUF = 0.51 mg/L.
Males: IFI n = 218; CUF n = 211.
Females: IFI n = 214; CUF n = 223.
Overall: IFI N = 432; CUF N = 434.
Fig. 2.
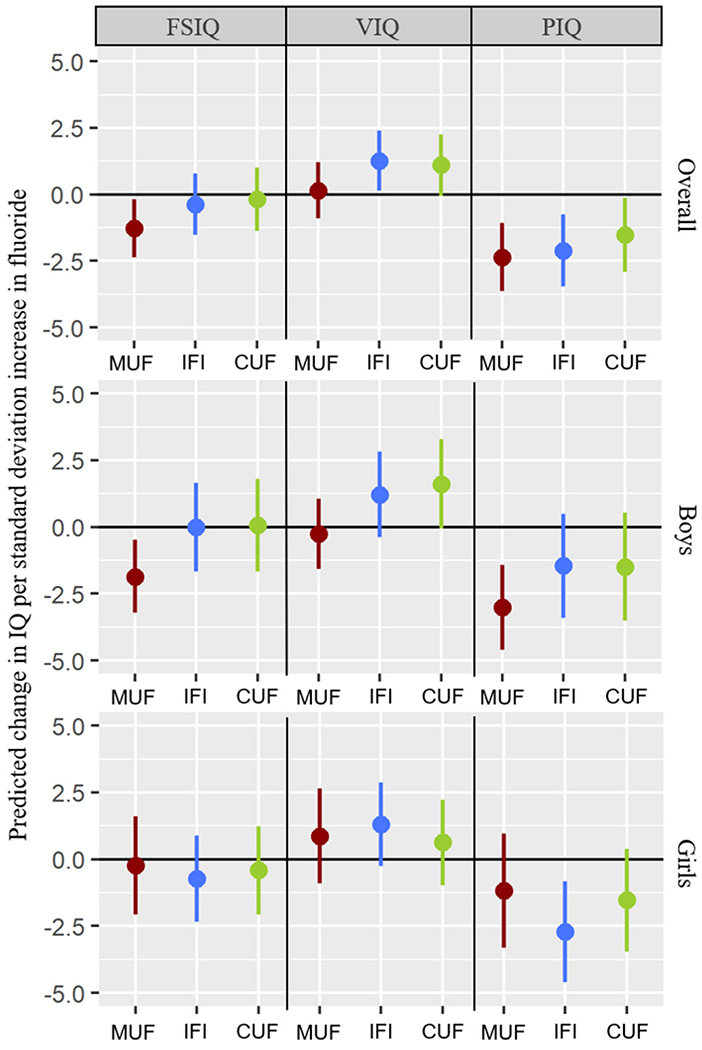
Standardized associations between fluoride exposure windows and IQ outcomes using GEE. Note. Dots represent point estimates and tails represent 95% confidence intervals.
2.2. Effects of exposure windows by sex
The three-way interaction between fluoride, child sex, and time was not statistically significant for FSIQ [χ2 (2) = 2.74, p = .25], PIQ [χ2 (2) = 2.72, p = .26], or VIQ [χ2 (2) = 1.92, p = .38]. However, among boys, the association between fluoride and PIQ significantly differed across windows [χ2 (3) = 11.92, p = .01], but not for FSIQ [χ2 (3) = 5.83, p = .12] or VIQ [χ2 (3) = 5.80, p = .12] (Table 3). Similarly, among girls, the effect of fluoride exposure significantly differed across windows for PIQ [χ2 (3) = 11.69, p = .01], but not for FSIQ [χ2 (3) = 1.15, p = .77] or VIQ [χ2 (3) = 3.63, p = .30] (Table 3). Probing the time (i.e. exposure windows) interaction within boys and girls, significant effects of standardized fluoride exposures after controlling the FDR were as follows: among boys, MUF had stronger negative associations with FSIQ (B = −1.86, 95% CI: −3.22, −0.49) and PIQ (B = −3.01, 95% CI: −4.60, −1.42) than IFI and CUF. Among girls, IFI had a stronger association with PIQ (B = −2.71, 95% CI: −4.59, −0.83) than MUF and CUF (Fig. 2).
2.3. Sensitivity analysis
Removal of six mother-child dyads that were influential (as per DFBETA) on the sex-specific estimates of fluoride exposures on FSIQ made the negative association between MUF and FSIQ among boys weaker and non-significant after adjustment for the FDR (B = −1.22, 95% CI: −2.41, −0.04). All other prenatal and postnatal sex-specific and overall effects remained significant (or non-significant) with removal of influential dyads for FSIQ, PIQ, or VIQ, adjusted for the FDR (Table 5).
3. Discussion
We used data from a prospective pregnancy and birth cohort to compare the associations between fluoride exposures during different developmental windows and preschool aged children’s intellectual abilities. The GEE method advances our understanding of early-life fluoride neurotoxicity by formally comparing strength of associations across windows of exposure. The strongest association between fluoride and child IQ was observed between MUF and age-normed PIQ (for standardized MUF, B = −2.36, 95% CI: −3.63, −1.08); the association was significant during infancy (B = −2.11, 95% CI: −3.45, −0.76), but negligible in childhood. Our results, which show that fetal fluoride exposure is more strongly associated with children’s intelligence than postnatal fluoride exposure, are consistent with a Chinese study examining different susceptibility windows of fluoride exposure; lower IQ was found in children whose mothers were exposed to high fluoride levels in drinking water (>1.0 mg/L) during pregnancy compared to those with high postnatal and low prenatal fluoride exposure (Xu et al., 2020). We did not identify clear differences between the effects of different trimester exposure windows on cognitive outcomes (Supplementary Table 1), and so it may be that the entire prenatal period confers susceptibility.
Critical windows of exposure may also differ by sex; animal and human literature have noted sex differences in response to fluoride exposure (Green et al., 2019; Green et al., 2020, 2020a; Mullenix et al., 1995) as well as several other environmental neurotoxicants (Comfort and Re, 2017; Torres-Rojas and Jones, 2018). When we tested sex differences across windows, our results suggested that prenatal fluoride exposure was a critical developmental window for boys for FSIQ and PIQ, whereas infancy was a critical developmental window for girls for PIQ. Specifically, boys showed a 4-point decrement in PIQ per 0.5 mg/L increase in MUF whereas girls showed a 2-point decrement in PIQ per 0.1 mg increase in IFI (effect estimates are shown based on approximate average values for MUF and IFI in our sample). While the effect of exposure in infancy was greater among girls than boys, the IFI by sex interaction for PIQ was not significant indicating that exposure in infancy is not associated with a statistical difference between boys and girls. After excluding outlying dyads, the adverse association between IFI and PIQ strengthened among girls (from B = −2.0 to B = −3.6), while this association among boys remained about the same (from B = −1.1 to B = −1.4).
Within animal research, a rat experiment similarly demonstrated an interaction between sex and fluoride exposure across developmental windows (Mullenix et al., 1995). Male rat pups were most sensitive to late prenatal exposure whereas female rats were most sensitive to exposure occurring in the postnatal (weanling) period. Exposed adult females also showed a lower threshold for behaviour deficits than exposed adult males. These findings are consistent with some (Bǎran-Poesina et al., 2013; Bera et al., 2007; Flace et al., 2010) but not all (Bartos et al., 2015; Jiang et al., 2014) rat studies examining sex-specific effects of prenatal exposure to fluoride. Further research is needed to examine sex-specific effects of fluoride neurotoxicity, as many of the animal studies conducted to date have been identified as having a high risk of bias (NTP, 2016).
Boys and girls may respond differentially to neurotoxicants. Indeed, studies have shown that boys are often more vulnerable to early-life exposure to neurotoxicants than girls (Brubaker et al., 2010; Desrochers-Couture et al., 2018; Jedrychowski et al., 2009; Kern et al., 2017; Pagalan et al., 2019; Ris et al., 2004; Singh et al., 2018; Torres-Rojas and Jones, 2018). While the biological mechanisms underlying sex-based differences of fluoride neurotoxicity are not well understood, disruption to maternal thyroid or sex hormone levels could potentially contribute to sexually dimorphic effects (Batista and Hensch, 2019). Fluoride may target the hypothalamic-pituitary-thyroid axis (Malin et al., 2018; Bai et al., 2020), though we are not aware of any epidemiologic studies that have measured fluoride-induced changes in thyroid and sex steroid hormone levels in pregnancy. In addition, the timing of neurologic development of specific brain regions differs between the sexes (Lenroot et al., 2007; Perer and Herbstman, 2011), which might increase susceptibility of fluoride exposure during a particular developmental window. In the Mullenix et al. (1995) rat study, fluoride concentrations differed by sex in some brain structures (e.g. hippocampus), which could also contribute to sexually dimorphic changes in behaviour. See Green et al. (2020a, 2020) for further discussion of mechanisms that may contribute to sex-based differences of fluoride neurotoxicity.
The difference in magnitudes and divergence in the direction of some of the associations between verbal and non-verbal intellectual abilities may have several progenitors that reflect these distinct types of cognitive ability. While we would not expect higher fluoride intake in infancy to be beneficial to VIQ, we would expect it to be detrimental to nonverbal (PIQ) intelligence. Fluid (i.e. non-verbal) abilities are more biologically determined whereas crystallized intelligence (i.e. VIQ) is more likely to be shaped by experience (Asbury et al., 2005; Luster and Dubow, 1992). Past studies have suggested that prenatal and early-life exposure to some neurotoxicants, such as lead, is more strongly associated with non-verbal intelligence than verbal intelligence in young children (Bellinger et al., 1991; Dietrich et al., 1991, 1993; Factor-Litvak et al., 1999; Jusko et al., 2008; Wasserman et al., 1997). Consistent with this pattern, our findings showed a decrement of IFI on PIQ (statistically significant decrease of 1.6 points per 0.1 mg/day), but not VIQ (non-significant increase of 1.0 points per 0.1 mg/day).
Our current results are consistent with and extend our previous findings. The effect of MUF on FSIQ was significant for boys (2.48-point decrement in FSIQ per 0.5 mg/L increase in MUF; Table 2), reproducing our prior work (Green et al., 2019) in which we found a 2.2-point decrement in FSIQ per 0.5 mg/L increase in MUF. We note that the current analysis did not include city in the analysis because fluoride intake from formula (i.e. IFI) is a function of residential water fluoride concentration and was therefore deemed redundant. Our finding of a 1.6-point decrement in PIQ per 0.1 mg/day increase in IFI combining boys and girls (B = −1.58, 95% CI: −2.59, −0.57) was also consistent with our prior finding that infancy is a critical period for non-verbal intelligence in boys and girls (Till et al., 2020). Our current results extend our prior work by showing that regardless of child sex or the exclusion of influential dyads, the association of fluoride on PIQ differs across exposure windows.
A 2- to 4-point decrement in PIQ may seem like a small difference at the individual level. However, a small shift in the mean of IQ scores at the population level translates to millions of lost IQ points given the ubiquity of fluoride exposure. The impact of such a shift has a disproportionate effect among vulnerable populations who are at the lower end of the population IQ distribution because the loss in productivity per IQ point is not the same across the entire IQ distribution (i.e. a drop in IQ from 80 to 77 is not the same as 120 to 117) (Rose, 1985). Finally, previous benchmark dose analyses for testing lead and fluoride neurotoxicity have selected 1 IQ point as the benchmark response because of the significant societal and economic burdens of reduced IQ (Budtz--Jorgensen et al., 2004).
Strengths of the present study include the relatively large sample with repeated exposure measures during pregnancy, infancy, and early childhood that resulted in precise estimates of effects, as reflected by narrow confidence intervals. We used the FDR method to guard against false positive conclusions due to multiple comparisons, even though multiplicity control is rarely imposed when evaluating multiple predictors in regression-based models (Cribbie, 2017). We adjusted for numerous potential confounders and avoided problems with collinearity among critical windows of fluoride toxicity by using GEE. Although several epidemiological studies have applied GEE to test critical windows of environmental contaminants on neurobehavioral outcomes (Jackson-Browne et al., 2018; Stacy et al., 2017; Vuong et al., 2017; Zhang et al., 2017), this is the first study to use GEE to model critical windows of fluoride toxicity.
Limitations of our study include modeling marginal effects of fluoride exposures without controlling the effects from other exposure windows or assessing cumulative fluoride exposure, which may be more etiologically relevant. However, it is not of substantive interest to estimate partial effects that vary one exposure window while fixing other exposures. Another limitation is not having MUF, IFI, or CUF levels on all study participants, although we were able to incorporate cases with incomplete data in the GEE analyses. Further, in any research on single neurotoxicants, simultaneous exposure to other environmental contaminants may confound effect estimates. For instance, trace amounts of aluminum can bind fluoride and affect cellular processes (Li, 2003). Moreover, there is always the possibility of residual confounding. We considered many potential confounders in prior research conducted in the same sample examining the association between MUF and child IQ (Green et al., 2019) and they did not meaningfully influence our findings. We also controlled for several other chemicals in our prior analyses including lead, mercury, PFOA, arsenic, and manganese. Controlling for these chemicals did not affect our estimates appreciably. The demographic characteristics of our sample also constrained our ability to test potential fluoride susceptibility in different subpopulations. For example, fewer than 3% of women smoked in the first trimester of pregnancy and 89% of the sample was Caucasian, which limited our ability to assess effect modification by smoking or race. Further, MUF concentration averaged across three trimesters was the strongest predictor of IQ scores among boys and was more reliable than IFI and CUF. Fluoride concentrations measured in single spot urine samples (i.e. trimester-specific MUF and CUF concentrations) suffer from measurement error due to the rapid elimination kinetics of fluoride (half-life in urine < 6 h; Ekstrand, 1983) and lack of control for water/beverage consumption and dental product use prior to urine sampling. IFI may also suffer from measurement error due to the use of mother’s self-reported infant water intake and breastfeeding duration, as well as our reliance on water fluoride measurements made at water treatment plants (as opposed to measuring fluoride directly in household tap water). While we did not have specific information on the type of water used to reconstitute formula (i.e. bottled/filtered versus tap water), we derived IFI only for children of women who reported drinking tap water. However, these possible sources of measurement error are more likely to produce negatively biased effect estimates than positively biased estimates (Budtz-Jorgensen et al., 2004).
Our findings raise the question of whether a decrease in children’s cognitive abilities is worth the benefit that fluoride ingestion provides. To answer this question, we need to consider how and when fluoride works for the developing child and pregnant woman. Fluoride prevents dental decay by being present in the mouth when a decay-inducing acid attack occurs, by precluding minerals from leaving the dental enamel during the attack (prevention of demineralization) and by incorporating into the enamel after the acid attack (promotion of remineralization). These processes only occur after teeth have erupted (CDC, 2001; Ten Cate and Buzalaf, 2019); fluoride incorporated into enamel before eruption has a minimal effect on the prevention of dental decay (CDC, 2001; Takahashi et al., 2017). In contrast, there is potential risk of reduced IQ associated with fluoride exposure during fetal and infant development. Consistent with this conclusion, the Center for Disease Control and Prevention does not recommend the use of fluoride supplements during pregnancy (CDC, 2001). If a pregnant woman chooses to decrease her ingestion of fluoridated water (which accounts for 75% of her fluoride intake; CDC, 2001), common alternatives for minimizing risk of dental decay in pregnancy include reducing sugar intake and using topical fluorides, such as fluoridated toothpastes and varnishes.
Given a heightened sensitivity of the developing brain to environmental toxicants, identifying critical windows of vulnerability to fluoride exposure is essential for promoting child health. Our results suggest the associations of prenatal and postnatal fluoride exposure with cognitive development may be modified by sex, though further replication of this finding is needed. These results indicate that it is important to balance the risks of fluoride exposure during early brain development with its potential to prevent caries, especially for pregnant women and infants.
Supplementary Material
Funding source
This study was funded by a grant from the National Institute of Environmental Health Science (NIEHS) (grant #R21ES027044). The MIREC Study was supported by the Chemicals Management Plan at Health Canada, the Ontario Ministry of the Environment, and the Canadian Institutes for Health Research (grant # MOP-81285).
Abbreviations:
- CI
confidence interval
- HOME
Home Observation for Measurement of the Environment
- FDR
false discovery rate
- FSIQ
Full Scale IQ
- PIQ
Performance Intelligence Quotient
- VIQ
Verbal intelligence Quotient
- IFI
infant fluoride intake
- MIREC
Maternal-Infant Research on Environmental Chemicals
- MUF
maternal urinary fluoride
- SD
standard deviation
Footnotes
Financial disclosure
The authors have no financial disclosures.
Credit author statement
Linda Farmus: Data curation, formal analysis, methodology, software, visualization, writing original draft, writing- review and editing. Christine Till: Conceptualization, Data curation, formal analysis, funding acquisition, methodology, supervision, visualization, writing- review and editing. Rivka Green: Data curation, formal analysis, writing- review and editing. Richard Hornung: methodology, writing- review and editing. E. Angeles Martinez-Mier: writing-review and editing. Pierre Ayotte: writing-review and editing. Gina Muckle: writing- review and editing. Bruce Lanphear: Conceptualization, funding acquisition, writing-review and editing. David Flora: Conceptualization, formal analysis, methodology, software, supervision, writing- review and editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envres.2021.111315.
References
- Arbuckle TE, Fraser WD, Fisher M, Davis K, Liang CL, Lupien N, Bastien S, Velez MP, Von Dadelszen P, Hemmings DG, Wang J, Helewa M, Taback S, Sermer M, Foster W, Ross G, Fredette P, Smith G, Walker M, Shear R, Dodds L, Ettinger AS, Weber JP, D’Amour M, Legrand M, Kumarathasan P, Vincent R, Luo ZC, Platt RW, Mitchell G, Hidiroglou N, Cockell K, Villeneuve M, Rawn DFK, Dabeka R, Cao XL, Becalski A, Ratnayake N, Bondy G, Jin X, Wang Z, Tittlemier S, Julien P, Avard D, Weiler H, Leblanc A, Muckle G, Boivin M, Dionne G, Ayotte P, Lanphear B, Séguin JR, Saint-Amour D, Dewailly É, Monnier P, Koren G, Ouellet E, 2013. Cohort profile: the maternal-infant research on environmental chemicals research platform. Paediatr. Perinat. Epidemiol 27 (4), 415–425. 10.1111/ppe.12061. [DOI] [PubMed] [Google Scholar]
- Asbury K, Wachs TD, Plomin R, 2005. Environmental moderators of genetic influenceon verbal and nonverbal abilities in early childhood. Intelligence 33 (6), 643–661. 10.1016/j.intell.2005.03.008. [DOI] [Google Scholar]
- Bai R, Huang Y, Wang F, Guo J, 2020. Associations of fluoride exposure with sex steroid hormones among U.S. children and adolescents, NHANES 2013–2016. Environ. Pollut 260, 114003. 10.1016/j.envpol.2020.114003. [DOI] [PubMed] [Google Scholar]
- Bǎran-Poesina V, Negreș S, Dobrescu D, Dimcevici-Poesina N, Dimcevici-Poesina A, Feghiu A, et al. , 2013. Experimental pharmacological researches regarding the influence of sodium fluoride in allopathic and homeopathic doses on central nervous system’s performances. A correlation between behavioral response in classic maze test and morphological aspects of ce. Farmacia 61 (4). https://farmaciajournal.com/wp-content/uploads/2013-04-art.18.poesina-781-799small.pdf, 781–99. [Google Scholar]
- Bartos M, Gumilar F, Bras C, Gallegos CE, Giannuzzi L, Cancela LM, et al. , 2015. Neurobehavioural effects of exposure to fluoride in the earliest stages of rat development. Physiol. Behav 147 10.1016/j.physbeh.2015.04.044, 205–12. [DOI] [PubMed] [Google Scholar]
- Bashash M, Thomas D, Hu H, Martinez-Mier EA, Sanchez BN, Basu N, Peterson KE, Ettinger AS, Wright R, Zhang Z, Liu Y, Schnaas L, Mercado-Garcia A, Téllez-Rojo MM, Hernández-Avila M, 2017. Prenatal fluoride exposure and cognitive outcomes in children at 4 and 6 – 12 years of age in Mexico. Environ. Health Perspect 125 (9), 1–12. 10.1289/EHP655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista G, Hensch TK, 2019. Critical period regulation by thyroid hormones: potential mechanisms and sex-specific aspects. Front. Mol. Neurosci 12, 77. 10.3389/fnmol.2019.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger D, Leviton A, Sloman J, Rabinowitz M, Needleman HL, Waternaux C, 1991. Low-level lead exposure and children’s cognitive function in the preschool years. Pediatrics 87 (2), 219–227. [PubMed] [Google Scholar]
- Belsley DA, Kuh E, Welsch RE, 1980. Regression Diagnostics: Identifying Influential Data and Sources of Collinearity. Wiley, New York. [Google Scholar]
- Benjamini Y, Hochberg Y, 1995. Controlling the false-discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Series B Stat. Methodol 57, 289–300. 10.1111/j.2517-6161.1995.tbO2031.x. [DOI] [Google Scholar]
- Bera I, Sabatini R, Auteri P, Flace P, Sisto G, Montagnani M, Potenza MA, Marasciulo FL, Carratu MR, Coluccia A, Borracci P, Tarullo A, Cagiano R, 2007. Neurofunctional effects of developmental sodium fluoride exposure in rats. Eur. Rev. Med. Pharmacol. Sci 11 (4), 211–224. https://pubmed.ncbi.nlm.nih.gov/17876956/. [PubMed] [Google Scholar]
- Brubaker CJ, Dietrich KN, Lanphear BP, Cecil KM, 2010. The influence of age of lead exposure on adult gray matter volume. Neurotoxicology 31 (3), 259–266. 10.1016/j.neuro.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley JP, Hamra GB, Braun JM, 2019. Statistical approaches for investigating periods of susceptibility in children’s environmental health research. Curr. Environ. Health Rep 6, 1–7. 10.1007/s40572-019-0224-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budtz-Jorgensen E, Keiding N, Grandjean P, 2004. Effects of exposure imprecision on estimation of the benchmark dose. Risk Anal. 24 (6), 1689–1696. 10.1111/j.0272-4332.2004.00560.x. [DOI] [PubMed] [Google Scholar]
- Caldwell B, Bradley R, 1984. Home Observation for Measurement of the Environment (HOME) – Revised Edition. University of Arkansas, Little Rock. [Google Scholar]
- Carignan CC, Cottingham KL, Jackson BP, Farzan SF, Gandolfi AJ, Punshon T, Folt CL, Karagas MR, 2015. Estimated exposure to arsenic in breastfed and formula-fed infants in a United States cohort. Environ. Health Perspect 123 (5) 10.1289/ehp.1408789, 500–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC, 2001. Recommendations for using fluoride to prevent and control dental caries in the United States. Centers for Disease Control and Prevention. MMWR Recomm. Rep. (Morb. Mortal. Wkly. Rep.) 50 (RR-14), 1–42. [PubMed] [Google Scholar]
- Choi AL, Sun G, Zhang Y, Grandjean P, 2012. Developmental fluoride neurotoxicity: a systematic review and meta-analysis. Environ. Health Perspect 120 (10), 1362–1368. 10.1289/ehp.1104912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comfort N, Re DB, 2017. Sex-specific neurotoxic effects of organophosphate pesticides across the life course. Curr. Environ. Health Rep 4 (4), 392–404. 10.1007/s40572-017-0171-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cribbie RA, 2017. Multiplicity control, school uniforms, and other perplexing debates. Can. J. Behav. Sci 49 (3), 159–165. 10.1037/cbs0000075. [DOI] [Google Scholar]
- dela Cruz GG, Rozier RG, Bawden JW, 2008. Fluoride concentration in dentin of exfoliated primary teeth as a biomarker for cumulative fluoride exposure. Caries Res. 42 (6), 419–428. 10.1159/000159605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrochers-Couture M, Oulhote Y, Arbuckle TE, Fraser WD, Séguin JR, Ouellet E, Forget-Dubois N, Ayotte P, Boivin M, Lanphear BP, Muckle G, 2018. Prenatal, concurrent, and sex-specific associations between blood lead concentrations and IQ in preschool Canadian children. Environ. Int 121 (Pt 2), 1235–1242. 10.1016/j.envint.2018.10.04. [DOI] [PubMed] [Google Scholar]
- Dietrich KN, Berger O, Succop P, 1993. Lead exposure and the motor developmental status of urban six-year-old children in the Cincinnati Prospective Study. Pediatrics 91, 301–307. [PubMed] [Google Scholar]
- Dietrich KN, Succop PA, Berger OG, Hammond PB, Bornschein RL, 1991. Lead exposure and the cognitive development of urban preschool children: the Cincinnati Lead Study cohort at age 4 years. Neurotoxicol. Teratol 13 (2), 203–211. 10.1016/0892-0362(91)90012-L. [DOI] [PubMed] [Google Scholar]
- Dong L, Yao P, Chen W, Li P, Shi X, 2018. An investigation of dental fluorosis and intelligence levels of children in drinking water-related endemic fluorosis areas of Xi’an. Chin. J. Epidemiol 37 (1), 45–48. 10.3760/cma.j.issn.2095-4255.2018.01.010. [DOI] [Google Scholar]
- Duty SM, Ackerman RM, Calafat AM, Hauser R, 2005. Personal care product use predicts urinary concentrations of some phthalate monoesters. Environ. Health Perspect 113 (11), 1530–1535. 10.1289/ehp.8083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrand J, 1981. No evidence of transfer of fluoride from plasma to breast milk. Br. Med. J 283 (6294), 761–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrand J, Ehrnebo M, 1983. The relationship between plasma fluoride, urinary excretion rate and urine fluoride concentration in man. J. Occup. Med 25 (10), 745–748. 10.1097/00043764-198310000-00014. [DOI] [PubMed] [Google Scholar]
- Ekstrand J, Hardell Li SC, 1984. Fluoride balance studies on infants in a 1-ppm-water-fluoride area. Caries Res. 18, 87–92. [DOI] [PubMed] [Google Scholar]
- Factor-Litvak P, Wasserman G, Kline JK, Graziano J, 1999. The YugoslaviaProspective Study of environmental lead exposure. Environ. Health Perspect 107 (1), 9–15. 10.1289/ehp.991079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flace P, Benagiano V, Vermesan D, Sabatini R, Inchingolo AM, Auteri P, Ambrosi G, Tarullo A, Cagiano R, 2010. Effects of developmental fluoride exposure on rat ultrasonic vocalization, acoustic startle reflex and pre-pulse inhibition. Eur. Rev. Med. Pharmacol. Sci 14 (6), 507–512. https://pubmed.ncbi.nlm.nih.gov/20712257/. [PubMed] [Google Scholar]
- Grandjean P, 2019. Developmental fluoride neurotoxicity: an updated review. Environ. Health 18 (110), 1–17. 10.1186/s12940-019-0551-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green R, Lanphear B, Hornung R, Flora D, Martinez-Mier EA, Neufeld R, Ayotte P, Muckle G, Till C, 2019. Association between maternal fluoride exposure during pregnancy and IQ scores in offspring in Canada. JAMA Pediatr. 173 (10), 940–948. 10.1001/jamapediatrics.2019.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green R, Rubenstein J, Popoli R, Capulong R, Till C, 2020. Sex-specific neurotoxic effects of early-life exposure to fluoride: a review of the epidemiologic and animal literature. Curr. Epidemiol. Rep 1–11. 10.1007/s40471-020-00246-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green R, Till C, Cantoral A, Lanphear B, Martinez-Mier EA, Ayotte P, Wright RO, Tellez-Rojo MM, Malin AJ, 2020a. Associations between urinary, dietary, and water fluoride concentrations among children in Mexico and Canada. Toxics 8 (4), 110. 10.3390/toxics8040110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung RW, Lanphear BP, Dietrich KM, 2009. Age of greatest susceptibility to childhood lead exposure: a new statistical approach. Environ. Health Perspect 117 (8), 1309–1312. 10.1289/ehp.0800426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson-Browne MS, Papandonatos GD, Chen A, Calafat AM, Yolton K, Lanphear BP, Braun JM, 2018. Identifying vulnerable periods of neurotoxicity to triclosan exposure in children. Environ. Health Perspect 126 (5), 1–9. 10.1289/EHP2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedrychowski W, Perera FP, Jankowski J, Mrozek-Budzyn D, Mroz E, Flak E, Edwards S, Skarupa A, Lisowska-Miszczyk I, 2009. Very low prenatal exposure to lead and mental development of children in infancy and early childhood: krakow prospective cohort study. Neuroepidemiology 32 (4), 270–278. 10.1159/000203075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang S, Su J, Yao S, Zhang Y, Cao F, Wang F, Wang H, Li J, Xi S, 2014. Fluoride and Arsenic Exposure Impairs Learning and Memory and Decreases mGluR5 Expression in the Hippocampus and Cortex in Rats. PLoS One 9 (4), e96041. 10.1371/journal.pone.0096041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jusko TA, Henderson CR Jr., Lanphear BP, Cory-Slechta DA, Parsons PJ, Canfield RL, 2008. Blood lead concentrations< 10 μg/dL and child intelligence at 6 years of age. Environ. Health Perspect 116 (2), 243–248. 10.1289/ehp.10424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern JK, Geier DA, Homme KG, King PG, Bjørklund G, Chirumbolo S, Geier MR, 2017. Developmental neurotoxicants and the vulnerable male brain: a systematic review of suspected neurotoxicants that disproportionally affect males. Acta Neurobiol. Exp 77 (4), 269–296. [PubMed] [Google Scholar]
- Lanphear BP, 2015. The impact of toxins on the developing brain. Ann. Rev. Publ. Health 36 (1), 211–230. 10.1146/annurev-publhealth-031912-114413. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Gogtay N, Greenstein DK, Wells EM, Wallace GL, Clasen LS, Blumenthal JD, Lerch J, Zijdenbos AP, Evans AC, Thompson PM, Giedd JN, 2007. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage 36 (4), 1065–1073. 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, 2003. The biochemistry and physiology of metallic fluoride: action, mechanism, and implications. Crit. Rev. Oral Biol. Med 14 (2), 100–114. 10.1177/154411130301400204. [DOI] [PubMed] [Google Scholar]
- Luster T, Dubow E, 1992. Home environment and maternal intelligence as predictors ofverbal intelligence: a comparison of preschool and school-age children. Merrill-Paimer Q. 151–175, 1982-. [Google Scholar]
- Malin AJ, Riddell J, McCague H, Till C, 2018. Fluoride exposure and thyroid function among adults living in Canada: effect modification by iodine status. Environ. Int 21 (Pt 1), 667–674. 10.1016/j.envint.2018.09.026. [DOI] [PubMed] [Google Scholar]
- Martinez-Mier EA, Cury JA, Heilman JR, Katz BP, Levy SM, Li Y, Maguire A, Margineda J, O’Mullane D, Phantumvanit P, Soto-Rojas AE, Stookey GK, Villa A, Wefel JS, Whelton H, Whitford GM, Zero DT, Zhang W, Zohouri V, 2011. Development of gold standard ion-selective electrode-based methods for fluoride analysis. Caries Res. 45 (1), 3–12. 10.1159/000321657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullenix PJ, Denbesten PK, Schunior A, Kernan WJ, 1995. Neurotoxicity of sodium fluoride in rats. Neurotoxicol. Teratol 17 (2), 169–177. 10.1016/0892-0362(94)00070-t. [DOI] [PubMed] [Google Scholar]
- National Toxicology Program, 2020. Revised Draft NTP Monograph on the Systematic Review ofFluoride Exposure and Neurodevelopmental and Cognitive Health Effects. National Institute of Environmental Health Sciences, Research Triangle Park, NC: ). [Google Scholar]
- Pagalan L, Bickford C, Weikum W, Lanphear B, Brauer M, Lanphear N, Hanley GE, Oberlander TF, Winters M, 2019. association of prenatal exposure to air pollution with autism spectrum disorder. JAMA Pediatr 173 (1), 86–92. 10.1001/jamapediatrics.2018.3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perer F, Herbstman J, 2011. Prenatal environmental exposures, epigenetics, and disease. Reprod. Toxicol 31 (3), 363–373. 10.1016/j.reprotox.2010.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice D, Barone S Jr., 2000. Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ. Health Perspect 10 (Suppl. 3), 511–533. 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ris MD, Dietrich KN, Succop PA, Berger OG, 2004. Early exposure to lead and neuropsychological outcome in adolescence. J. Int. Neuropsychol. Soc 10 (2), 261–270. 10.1017/S1355617704102154. [DOI] [PubMed] [Google Scholar]
- Rose G, 1985. Sick individuals and sick populations. Int. J. Epidemiol 14 (1), 32–38. [DOI] [PubMed] [Google Scholar]
- Sanchez BN, Hu H, Litman HJ, Tellez-Rojo MM, 2011. Statistical methods to study timing of vulnerability with sparsely sampled data on environmental toxicants. Environ. Health Perspect 119 (3), 409–415. 10.1289/ehp.1002453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selevan SG, Kimmel CA, Mendola P, 2000. Identifying critical windows of exposure for children’s health. Environ. Health Perspect 108 (Suppl 3), 451–455. 10.1289/ehp.00108s3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seraj B, Shahrabi M, Shadfar M, Ahmadi R, Fallahzadeh M, Eslamlu HF, Kharazifard MJ, 2012. Effect of high water fluoride concentration on the intellectual development of children in Makoo/Iran. J. Dent 9 (3), 221–229. [PMC free article] [PubMed] [Google Scholar]
- Singh G, Singh V, Sobolewski M, Cory-Slechta DA, Schneider JS, 2018. Sex-dependent effects of developmental lead exposure on the brain. Front. Genet 9, 89. 10.3389/fgene.2018.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodgrass SE, 1992. Further effects of role versus gender on interpersonal sensitivity. Journal of Personality and Social Psychology 62 (1), 154–158. 10.1037/0022-3514.62.1.154. [DOI] [Google Scholar]
- Stacy SL, Papandonatos GD, Calafat AM, Chen A, Yolton K, Lanphear BP, Braun JM, 2017. Early life bisphenol A exposure and neurobehavior at 8 years of age: identifying windows of heightened vulnerability. Environ. Int 107, 258–265. 10.1016/j.envint.2017.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi R, et al. , 2017. Fluoride supplementation (with tablets, drops, lozenges or chewing gum) in pregnant women for preventing dental caries in the primary teeth of their children. Cochrane Database Syst. Rev 10, CD011850. 10.1002/14651858.CD011850.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ten Cate JM, Buzalaf MAR, 2019. Jul. Fluoride mode of action: once there was an observant dentist. J. Dent. Res 98 (7), 725–730. 10.1177/0022034519831604. [DOI] [PubMed] [Google Scholar]
- Till C, Green R, Flora D, Hornung R, Martinez-Mier EA, Blazer M, Farmus L, Ayotte P, Muckle G, Lanphear BP, 2020. Fluoride exposure from infant formula and child IQ in a Canadian birth cohort. Environ. Int 135, 105315. 10.1016/j.envint.2019.105315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Till C, Green R, Grundy JG, Hornung R, Neufeld R, Martinez-Mier EA, Ayotte P, Muckle G, Lanphear BP, 2018. Community water fluoridation and urinary fluoride concentrations in a national sample of pregnant women in Canada. Environ. Health Perspect 126 (10), 107001. 10.1289/EHP3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Rojas C, Jones BC, 2018. Sex differences in neurotoxicogenetics. Front. Genet 9 (196) 10.3389/fgene.2018.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Environmental Protection Agency (EPA), 2010. Fluoride: Relative Source Contribution Analysis. Vol. 820-R-10-0. [Google Scholar]
- Valdez Jiménez L, López Guzmán OD, Cervantes Flores M, Costilla-Salazar R, Calderón Hernández J, Alcaraz Contreras Y, Rocha-Amador DO, 2017. In utero exposure to fluoride and cognitive development delay in infants. Neurotoxicology 59, 65–70. 10.1016/j.neuro.2016.12.011. [DOI] [PubMed] [Google Scholar]
- Vuong AM, Braun JM, Yolton K, Xie C, Webster GM, Sjödin A, Dietrich KM, Lanphear BP, Chen A, 2017. Prenatal and postnatal polybrominated diphenyl ether exposure and visual spatial abilities in children. Environ. Res 153, 83–92. 10.1016/j.envres.2016.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman GA, Liu X, Lolacono NJ, Factor-Litvak P, Kline JK, Popovac D, Morina N, Musabegovic A, Vrenezi N, Capuni-Paracka S, Preteni-Redjepi E, Hadzialjevic S, Slakovich V, Graziano JH, 1997. Lead exposure and intelligence in 7-year-old children: the yugoslavia prospective study. Environ. Health Perspect 105 (9), 956–962. 10.1289/ehp.97105956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D, 2002. Wechsler Preschool and Primary Scale of Intelligence, third ed. Pearson Clinical Assessment, Canadian. Toronto, ON, Canada. [Google Scholar]
- Xiang Q, Liang Y, Chen L, Wang C, Chen B, Chen X, Zhou M, 2003. Effect of fluoride in drinking water on children’s intelligence. Fluoride 36 (2), 84–94. [Google Scholar]
- Xu K, An N, Huang H, Duan L, Ma J, Ding J, He T, Zhu J, Li Z, Cheng X, Zhou G, Ba Y, 2020. Fluoride exposure and intelligence in school-age children: evidence from different windows of exposure susceptibility. BMC Publ. Health 20 (1), 1657. 10.1186/s12889-020-09765-4. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zhang H, Yolton K, Webster GM, Ye X, Calafat AM, Dietrich KN, Xu Y, Xi C, Braun JM, Lanphear BP, Chen A, 2017. Prenatal and childhood perfluoroalkyl substances exposures and children’s reading skills at ages 5 and 8 years. Environ. Int 111, 224–231. 10.1016/j.envint.2017.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zohoori FV, Omid N, Sanderson RA, Valentine RA, Maguire A, 2018. Fluoride retention in infants living in fluoridated and non-fluoridated areas: effects of weaning. Br. J. Nutr 121 (1), 74–81. 10.1017/S0007114518003008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


