Abstract
Background
Patients with gastroparesis (GP) and functional dyspepsia (FD) have similar symptoms, but the pathophysiology of postprandial symptoms remains uncertain.
Aims
To compare symptoms and gastric myoelectrical activity (GMA) after liquid and solid test meals in patients with GP and FD.
Methods
Patients enrolled in the Gastroparesis Clinical Research Consortium Registry were studied. Clinical characteristics were measured with standard questionnaires. GP was determined by 4‐h solid‐phase gastric scintigraphy. GMA was measured using electrogastrography before and after ingestion of a water load or nutrient bar on separate days. Symptoms were measured on visual analog scales. GMA responses to the water load for individual patients were also determined.
Results
284 patients with GP and 113 with FD were identified who ingested both test meals. Patients with GP and FD had similar maximal tolerated volumes of water [mean (SD) 378 (218) ml vs. 402 (226) ml, p = 0.23] and reported similar intensity of fullness, nausea, bloating, and abdominal discomfort after the test meals. Twenty‐six percent and 19% of the patients with GP and FD, respectively, ingested subthreshold (<238 ml) volumes of water (p = 0.15). Gastric dysrhythmias were recorded in 66% of the GP and 65% of the FD patients after the water load. Symptoms and GMA were similar in both groups after ingestion of the nutrient bar.
Conclusion
The similarity in GMA responses and symptoms after ingestion of solid or liquid test meals suggests GP and FD are closely related gastric neuromuscular disorders.
Keywords: functional dyspepsia, gastric dysrhythmias, gastroparesis, nutrient bar meal, postprandial distress syndrome, water load satiety test
Patients with gastroparesis (GP) and functional dyspepsia (FD) have similar symptoms, but the pathophysiology of symptoms remains uncertain. Results show symptoms and gastric myoelectrical responses after ingestion of solid or liquid test meals are similar and suggests GP and FD are closely related gastric neuromuscular disorders.

Key Points.
The pathophysiology of symptoms associated with gastroparesis (GP) and functional dyspepsia (FD) remains uncertain.
GP and FD patients reported similar symptoms after the water load or nutrient bar test meals.
Two thirds of the GP and FD patients had dysrhythmic gastric myoelectrical activity (GMA) and 1/3 had normal 3 cpm GMA.
Almost 25% of patients in both groups ingested low volumes of water.
Poor gastric accommodation and gastric dysrhythmias are gastric neuromuscular abnormalities that link GP and FD patients.
1. INTRODUCTION
Symptoms associated with gastroparesis (GP) include early nausea, vomiting, early satiety, prolonged fullness, bloating, and abdominal discomfort or pain in the absence of mechanical obstruction. 1 Symptoms associated with functional dyspepsia (FD) include bothersome early satiation, fullness after meals with supporting symptoms of epigastric burning (not pain), bloating, belching, and nausea and are termed postprandial distress syndrome (PDS), and symptoms associated with patients with unexplained epigastric pain are termed epigastric pain symptoms (EPS). 2 These symptoms are present in the absence of other diagnoses after routine investigations including upper endoscopy. 2 , 3 Most patients with GP also have symptoms that meet the definition of FD, and the majority have PDS. 3 , 4 , 5 , 6 , 7 , 8
The pathophysiological mechanisms of symptoms associated with GP and FD remain uncertain. The symptoms associated with GP do not always correlate with the delayed rate of gastric emptying and the relevance of delayed gastric emptying as the primary pathophysiological mechanism of postprandial symptoms has been questioned. 3 , 5 , 6 , 7 , 8 Transition from delayed gastric emptying status to normal emptying status at 48 weeks of follow‐up did not affect GCSI scores. 9 Thus, other gastric neuromuscular abnormalities in patients with GP and FD include abnormal gastric accommodation, gastric hypersensitivity, and gastric dysrhythmias that may be more relevant to the postprandial symptomatology. 10 , 11 , 12 , 13
Gastric dysrhythmias are present in patients with GP and FD when the numbers of interstitial cells of Cajal (ICCs) per high‐power field (hpf) in the corpus–antrum and normal 3 cycles per min (cpm) gastric myoelectrical activity (GMA) are diminished. 14 , 15 , 16 , 17 , 18 The ICCs, the pacemaker cells of the stomach, are severely depleted in patients with GP 18 and are modestly depleted in patients with chronic unexplained nausea and vomiting and normal gastric emptying. 9 , 19 With loss of ICCs, normal 3 cpm GMA is diminished and gastric dysrhythmias appear. 9 , 19
Postprandial symptoms, gastric dysrhythmias, gastric capacity, and sensitivity to gastric distention after ingestion of test meals may be detected in real time during satiety tests. 10 , 11 , 13 , 20 The aim of this study was to compare GMA and symptoms in response to liquid and solid test meals in patients with GP and FD. We hypothesized that patients with GP would have more intense symptoms and poorer gastric capacity, less 3 cpm GMA, and more gastric dysrhythmias after the water load satiety test (WLST) and after ingestion of a solid nutrient meal compared with FD patients.
2. METHODS
2.1. Patients
Patients in the Registry were 18 years or older, had four‐hour solid‐phase gastric emptying by scintigraphy, and had no structural abnormalities on upper endoscopy. Patients with delayed gastric emptying or with normal gastric emptying were enrolled at six clinical centers from September 2012 to March 2018. In this report, the term FD is used to describe symptomatic patients with normal gastric emptying. Written informed consent was obtained prior to enrollment in the Registry.
2.2. Four‐hour gastric emptying study
Gastric emptying scintigraphy was performed using a low‐fat egg white meal (EggBeaters®) with imaging at 0, 1, 2, and 4 h after meal ingestion. 21 Medications affecting gastrointestinal motility were stopped 3 days prior to the study. Tests were performed after an overnight fast. In patients with diabetes, low blood sugar (hypoglycemia <70 mg/dl) or high blood sugar (hyperglycemia >270 mg/dl) was corrected or the study was rescheduled for another day under better glucose control. Gastric retention of Tc‐99m >60% at 2 h and/or >10% at 4 h was considered delayed gastric emptying of solids. 21
2.3. Electrogastrography and the water load satiety test (WLST) and the nutrient bar meal
Standard electrogastrography methods were used to record GMA in response to the WLST. 11 , 22 EKG‐type electrodes were placed in standard position on the upper abdominal surface after the skin was cleaned with alcohol wipes. Electrodes were connected to the electrogastrogram (EGG) recording device to record GMA (3CPM Company, Towson, MD). The EGG signal was digitized for computer analysis. 11 , 22
Patients stopped proton pump inhibitors, histamine2‐receptor antagonists, prokinetic drugs, opiates, anticholinergics, cannabinoids, over‐the‐counter laxatives, isotonic polyethylene glycol electrolyte preparations, and prescription laxatives for 3 days before the studies. Patients fasted overnight before the test meal. On the morning of the studies, insulin‐requiring patients with diabetes injected half of their usual long‐acting insulin dose.
2.3.1. Water load satiety test
On the day of the WLST (or the nutrient bar test meal), glucose levels over 270 mg/dl in patients with diabetes were treated or the test was rescheduled. Patients were seated in a comfortable chair in a quiet area. A baseline fasting EGG recording was performed for 15 min. For the WLST, patients ingested water until they achieved the sensation of “completely full” during the timed and continuous five‐minute period for water ingestion. 11 , 13 , 22 The volume of water ingested was recorded. The volume of water ingested reflects gastric capacity and gastric accommodation to that volume ingested. Ingestion of <238 ml of water in the five‐minute period is 2 SD below the mean volume ingested by healthy controls and was considered abnormal. 11 The patients indicated the intensity of fullness, hunger, abdominal discomfort, bloating, and nausea on a 100‐mm visual analog scale (VAS) before and 10, 20, and 30 min after the water was ingested. GMA was recorded for 30 min after the water load was ingested.
2.3.2. Nutrient bar test meal
The nutrient bar was consumed with the capsule on a separate day with preparations and placement of electrodes for EGG recordings on the abdomen as described above for the WLST. 23 Each patient ingested one nutrient bar (244 Cal, 66% carbohydrate, 17% protein, 2% fat, and 3% fiber) over a 10‐min period with 50 ml water. GMA was recorded during the 15‐min baseline and for 90 min after the meal. Patients reported intensity of symptoms on the VAS as described above before and at 15, 30, 45, 60, and 90 min after the bar was ingested.
2.4. Analyses of GMA in response to WLST and nutrient bar meal
The raw GMA signal is digitized and subjected to fast Fourier transform and running spectral analysis. The power calculation in the running spectral analysis reflects the amplitude of GMA in four frequency ranges: 1–2.5 cpm (bradygastria), 2.5–3.7 cpm (normal range), 3.7–10.0 cpm (tachygastria) and 10–15.0 cpm (duodenal/respiration range) before and after the WLST or nutrient bar test. The power in each frequency range is divided by the total power in the 1–15 cpm range. This calculation provides the percentage distribution of power for each of the four frequency ranges listed above. 11 , 13 , 22 The percentage distribution of power in the four frequency ranges is plotted over time and compared with controls as shown in Results. The average percentage distributions of GMA from the patients with GP and FD were compared at baseline and for each time from 1–10, 11–20, and 21–30 min after the WLST. 11 , 13 , 22 Similarly, for the nutrient bar meal, the GMA percentages in each frequency range were calculated for baseline and for the 1‐ to 15‐, 16‐ to 30‐, 31‐ to 45‐, 46‐ to 60‐, and 75‐ to 90‐min periods after ingestion of the bar and results from GP and FD cohorts were compared.
In addition, the GMA in response to the WLST was determined for each patient. The patient's results were compared with controls, and individual EGG diagnoses in response to the WLST were determined. The definitions for EGG diagnoses are based on the GMA response to the WLST. The dysrhythmias include tachygastria, bradygastria, mixed gastric dysrhythmia, duodenal–respiration, and hyponormal 3 cpm. The normal 3 cpm diagnoses include normal 3 cpm GMA, hypernormal 3 cpm GMA, and normal 3 cpm GMA with dysrhythmias (Table S1). 11 , 13 , 22 The EGG diagnosis for each patient was determined by one of the authors (KK) who was blinded to the normal or delayed emptying status of the patient and to the clinical site.
2.5. Patient assessment of upper gastrointestinal disorder symptoms (PAGI‐SYM)
Gastroparesis symptom severity was determined by the Gastroparesis Cardinal Symptom Index (GCSI) score, which includes nine questions from the PAGI‐SYM. PAGI‐SYM subscale and individual scores for upper GI symptoms were calculated. 24
2.6. The Nausea Profile
Nausea is one of the most common and debilitating symptoms reported by patients with GP and in subtypes of FD, but nausea and the extent of symptoms associated with nausea can be difficult to describe. The Nausea Profile questionnaire consists of 17 questions that describe the sensation of nausea in words other than the word nausea. 25 Factor analysis of 416 such words yielded three dimensions of nausea that correlated with overall nausea intensity. The three dimensions are GI distress (sick, stomach awareness/discomfort, as if he or she might vomit, ill, and queasy), somatic distress (fatigue, weak, hot, sweaty, lightheaded, and shakiness), and emotional distress (nervous, scared, afraid, worry, upset, panic, and hopeless).
2.7. Statistical methods
Characteristics at enrollment were compared between patients with GP and FD‐PDS using the t test for unequal variance for continuous variables and Fisher's exact test for categorical variables. Symptoms and EGG results from the water load test and nutrient bar test were compared between the FD and GP patients using robust regression, which downweights the effect of outliers. The average of the post‐test time points was used as the outcome for change. The baseline value was regressed on FD versus GP group status, and change was regressed on FD versus GP group status and the baseline value of change. Random‐effects linear regression models were used for analyses of change from pretest levels averaged across all post‐test time points. p‐values were two‐sided and not corrected for multiple comparisons. Analyses were conducted using SAS and Stata. 26 , 27
3. RESULTS
3.1. Clinical characteristics of patients with functional dyspepsia and gastroparesis
Table 1 summarizes demographic and clinical characteristics of the 284 patients with GP and the 113 patients with FD who completed the WLST and ingested the nutrient bar. Eighty‐four percent of the GP patients and 90% of the FD patients were women. The average age of patients in both groups was 44 years, and 90% of the patients with GP versus 93% of the patients with FD were White. Diabetes was present in 33% of the GP patients and 25% of the FD patients (p = 0.08). The onset of symptoms was acute in 40% of GP patients and 34% of FD patients, and duration of symptoms at the time of evaluation was similar in both groups.
TABLE 1.
Characteristics at enrollment of patients with gastroparesis and functional dyspepsia
| Characteristics at enrollment | Gastroparesis (n = 284) | Functional dyspepsia (n = 113) | Total (n = 397) | p‐value |
|---|---|---|---|---|
| Mean (SD)/N (%) | Mean (SD)/N (%) | Mean (SD)/N (%) | ||
| Demographics | ||||
| Gender (female) | 238 (84%) | 102 (90%) | 340 (86%) | 0.11 |
| Age—years | 44 (13) | 44 (16) | 44 (14) | 0.99 |
| Race (White) | 257 (90%) | 105 (93%) | 362 (91%) | 0.56 |
| Ethnicity (Hispanic) | 44 (15%) | 14 (12%) | 58 (15%) | 0.53 |
| Body mass index (kg/m2) | 28 (7) | 28 (10) | 28 (8) | 0.96 |
| Characteristics of gastroparesis | ||||
| Etiology | 0.08 | |||
| Diabetes | 94 (33%) | 28 (25%) | 122 (31%) | |
| Idiopathic | 177 (62%) | 83 (73%) | 260 (65%) | |
| Fundoplication | 13 (5%) | 2 (2%) | 15 (4%) | |
| Acute onset of symptoms | 114 (40%) | 38 (34%) | 152 (38%) | 0.25 |
| Severity | 0.85 | |||
| Grade 1, mild | 56 (20%) | 24 (21%) | 80 (20%) | |
| Grade 2, compensated | 188 (67%) | 76 (67%) | 264 (67%) | |
| Grade 3, gastric failure | 38 (13%) | 13 (12%) | 51 (13%) | |
| Duration of symptoms—years | 6.2 (6.8) | 6.8 (7.9) | 6.3 (7.2) | 0.51 |
| Rome III categorization | 0.38 | |||
| Neither PDS nor EPS | 53 (19%) | 18 (16%) | 71 (18%) | |
| PDS only | 56 (20%) | 28 (25%) | 84 (21%) | |
| EPS only | 27 (10%) | 6 (5%) | 33 (8%) | |
| Both PDS and EPS | 148 (52%) | 61 (54%) | 209 (53%) | |
| Scintigraphy | ||||
| 2‐h solid retention—% | 65 (18) | 31 (16) | 55 (23) | <0.0001 |
| 4‐h solid retention—% | 31 (21) | 4 (3) | 23 (21) | <0.0001 |
| Laboratory results | ||||
| HbA1c—% | 6.3 (1.8) | 6.1 (1.6) | 6.3 (1.7) | 0.22 |
| Erythrocyte sedimentation rate—mm/h | 19.8 (19.2) | 16.3 (15.1) | 18.8 (18.2) | 0.05 |
| C‐reactive protein—mg/dl | 3.7 (9.5) | 2.2 (7.4) | 3.3 (8.9) | 0.10 |
| White blood cells—103 cells/µl | 7.2 (2.2) | 6.7 (2.4) | 7.0 (2.3) | 0.06 |
| Hemoglobin—g/dl | 13.3 (1.5) | 13.2 (1.2) | 13.2 (1.5) | 0.56 |
| Medication use | ||||
| Systemic corticosteroids | 61 (21%) | 25 (22%) | 86 (21%) | 0.89 |
| Narcotic pain medications | 97 (34%) | 35 (31%) | 132 (33%) | 0.56 |
| Neuropathic pain medications | 91 (32%) | 34 (30%) | 125 (31%) | 0.72 |
| Antidepressants | 229 (81%) | 90 (80%) | 319 (80%) | 0.89 |
| Mirtazapine (Remeron) | 14 (5%) | 9 (8%) | 23 (6%) | 0.24 |
| Prokinetics | 95 (33%) | 27 (24%) | 122 (31%) | 0.07 |
| Comorbidities | ||||
| GERD | 187 (66%) | 74 (65%) | 261 (66%) | 1.00 |
| Interstitial cystitis | 8 (3%) | 3 (3%) | 11 (3%) | 1.00 |
| Endometriosis | 39 (14%) | 21 (19%) | 60 (15%) | 0.28 |
| Migraine headaches | 109 (38%) | 42 (37%) | 151 (38%) | 0.91 |
| Chronic fatigue syndrome | 35 (12%) | 8 (7%) | 43 (11%) | 0.15 |
| PCOS | 27 (10%) | 11 (10%) | 38 (10%) | 1.00 |
| Fibromyalgia | 42 (15%) | 28 (25%) | 70 (18%) | 0.03 |
| Eating disorder | 9 (3%) | 2 (2%) | 11 (3%) | 0.74 |
| Anxiety requiring treatment | 75 (26%) | 24 (21%) | 99 (25%) | 0.31 |
| Major depression requiring treatment | 88 (31%) | 29 (26%) | 117 (29%) | 0.33 |
| Bipolar | 16 (6%) | 14 (12%) | 30 (8%) | 0.03 |
| Smoking, drinking, and marijuana use | ||||
| Smoking history | 0.71 | |||
| Never | 176 (62%) | 75 (66%) | 251 (63%) | |
| Former | 67 (24%) | 23 (20%) | 90 (23%) | |
| Current | 41 (14%) | 15 (13%) | 56 (14%) | |
| Alcohol use | 0.40 | |||
| Never | 139 (49%) | 50 (44%) | 189 (48%) | |
| Monthly or less | 96 (34%) | 37 (33%) | 133 (34%) | |
| ≥2 drinks per month | 49 (17%) | 26 (23%) | 75 (19%) | |
| Marijuana use | 34 (12%) | 12 (11%) | 46 (12%) | 0.86 |
| PAGI‐QOL | ||||
| Total score | 2.8 (1.1) | 2.8 (1.1) | 2.8 (1.1) | 0.80 |
| Daily activities subscale | 2.6 (1.2) | 2.5 (1.2) | 2.6 (1.2) | 0.49 |
| Clothing subscale | 2.9 (1.8) | 3.0 (1.8) | 2.9 (1.8) | 0.54 |
| Diet subscale | 1.8 (1.3) | 1.9 (1.4) | 1.8 (1.4) | 0.49 |
| Relationship subscale | 3.3 (1.4) | 3.4 (1.3) | 3.3 (1.4) | 0.86 |
| Psychological well‐being and distress subscale | 3.2 (1.4) | 3.2 (1.3) | 3.2 (1.3) | 1.00 |
| SF‐36 | ||||
| Physical component score | 33 (11) | 34 (12) | 34 (11) | 0.83 |
| Mental component score | 42 (13) | 43 (12) | 42 (13) | 0.88 |
| PAGI‐SYM | ||||
| GCSI total score | 2.9 (1.1) | 2.8 (1.0) | 2.8 (1.1) | 0.48 |
| Nausea/vomiting subscale | 2.1 (1.4) | 1.9 (1.2) | 2.1 (1.4) | 0.15 |
| Nausea | 3.2 (1.5) | 3.1 (1.5) | 3.1 (1.5) | 0.50 |
| Retching | 1.6 (1.7) | 1.5 (1.6) | 1.6 (1.7) | 0.37 |
| Vomiting | 1.6 (1.8) | 1.2 (1.6) | 1.5 (1.8) | 0.06 |
| Postprandial fullness/early satiety subscale | 3.4 (1.2) | 3.3 (1.3) | 3.4 (1.2) | 0.96 |
| Stomach fullness | 3.5 (1.3) | 3.6 (1.4) | 3.5 (1.3) | 0.89 |
| Unable to finish meal | 3.4 (1.6) | 3.4 (1.5) | 3.4 (1.6) | 0.93 |
| Felt full after meals | 3.6 (1.4) | 3.7 (1.5) | 3.6 (1.4) | 0.92 |
| Loss of appetite | 2.9 (1.6) | 2.8 (1.6) | 2.8 (1.6) | 0.65 |
| Bloating subscale | 3.1 (1.6) | 3.1 (1.7) | 3.1 (1.6) | 0.85 |
| Bloating | 3.2 (1.6) | 3.2 (1.7) | 3.2 (1.7) | 0.79 |
| Stomach visibly larger | 3.0 (1.8) | 2.9 (1.9) | 3.0 (1.8) | 0.93 |
| Upper abdominal pain subscale | 3.0 (1.5) | 2.8 (1.5) | 2.9 (1.5) | 0.37 |
| Lower abdominal pain subscale | 2.0 (1.5) | 2.0 (1.5) | 2.0 (1.5) | 0.58 |
| Heartburn/regurgitation subscale | 1.9 (1.4) | 1.7 (1.3) | 1.8 (1.4) | 0.22 |
| Constipation | 2.5 (1.8) | 2.7 (1.7) | 2.6 (1.7) | 0.40 |
| Diarrhea | 1.8 (1.7) | 1.5 (1.6) | 1.7 (1.7) | 0.10 |
| Nausea Profile (0% = none, 100% = severe) | ||||
| Overall nausea score | 49 (22) | 46 (21) | 48 (22) | 0.25 |
| Somatic distress dimension | 49 (27) | 44 (26) | 48 (27) | 0.07 |
| GI distress dimension | 74 (23) | 73 (23) | 73 (23) | 0.78 |
| Emotional distress dimension | 29 (29) | 27 (27) | 28 (28) | 0.53 |
Abbreviations: EPS, epigastric pain syndrome; GERD, gastroesophageal reflux disease; GI, gastrointestinal; PCOS, polycystic ovary syndrome; PDS, postprandial distress syndrome.
The overall severity of symptoms as assessed qualitatively by the investigators was similar in both groups. Laboratory values were similar in the two groups. The general classes of medications that the GP and FP patients received were similar. Fibromyalgia and bipolar disorders were more frequent in FD versus GP (25% vs. 15%, p = 0.03 and 12% vs. 6%, p = 0.03, respectively). The use of tobacco, alcohol, opioids, and marijuana was similar in the two groups. The Rome III questionnaire was used to determine the PDS or EPS designation in patients with normal gastric emptying. Eighty‐five percent of the patients with FD (n = 95) had symptoms associated with PDS, EPS, or both, and fifteen percent had neither PDS nor EPS (n = 18). Details are shown in Table S3.
3.2. Symptoms in patients with GP and FD
The PAGY‐QOL, SF‐36, PAGY‐SYM, and GCSI total scores were similar in the GP and FD patients, and the subscores for vomiting, postprandial fullness, and bloating were also similar in each group as shown in Table 1. Regarding dimensions of nausea from the Nausea Profile, the GI distress scores for GP were 74% and for FD were 73%. Somatic distress trended higher in GP (49%) versus FD (44%) (p = 0.07). Emotional distress symptoms had the lowest scores and were similar at 29% and 27% in the GP and FD patients, respectively.
3.3. Symptoms and GMA elicited by the WLST and nutrient bar meals in GP and FD
Figure 1 shows the maximal tolerated volumes of water ingested in the five‐minute time limit of the WLST. The mean ± SD for volume ingested by patient with GP and FD was 378 ml ± 218 and 402 ml ± 226, respectively (p = 0.23) (Table 2). Abnormally low volumes (<2 SD was 238 ml) were ingested by 26% of the patients with GP and by 19% of the patients with FD (p = 0.15).
FIGURE 1.
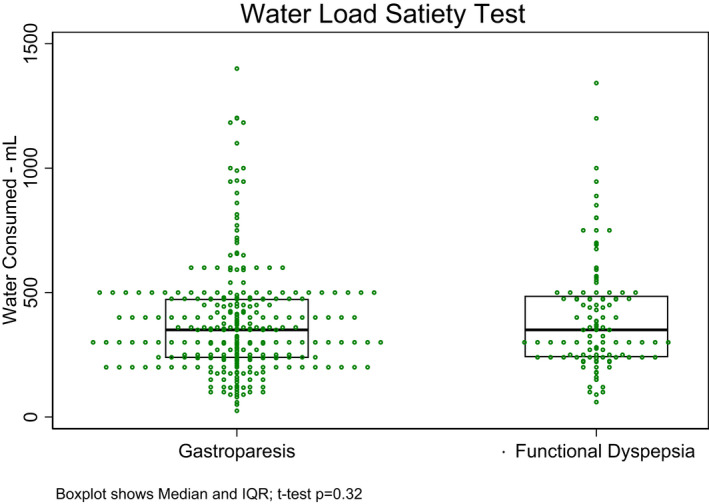
Volume of water consumed by each of the patients with gastroparesis and functional dyspepsia is shown. The volume is the amount in ml consumed until the patient was completely full within the 5‐min time limit. The average volume was 378 ± 218 ml in the GP group and 402 ± 226 ml in the FD group (p = 0.32)
TABLE 2.
Symptoms and GMA before and after water load satiety test in patients with gastroparesis and functional dyspepsia
| Gastroparesis (n = 282) | Functional dyspepsia (n = 112) | p‐value b | |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| Water load test | |||
| Amount—ml | 378 (218) | 402 (226) | 0.23 |
| Abnormal (<238 ml)—n (%) | 74 (26%) | 21 (19%) | 0.15 |
| Test length—min | 4.4 (2.5) | 4.1 (1.7) | 0.86 |
| Symptoms (VAS 0–100) | |||
| Fullness | |||
| Baseline | 28 (30) | 29 (32) | 0.98 |
| Change a | 37 (29) | 38 (32) | 0.44 |
| Hunger | |||
| Baseline | 30 (31) | 33 (32) | 0.43 |
| Change a | −5 (26) | −9 (24) | 0.07 |
| Nausea | |||
| Baseline | 27 (30) | 23 (28) | 0.17 |
| Change a | 5 (16) | 9 (21) | 0.17 |
| Bloating | |||
| Baseline | 28 (31) | 25 (29) | 0.34 |
| Change a | 12 (23) | 11 (20) | 0.54 |
| Abdominal discomfort | |||
| Baseline | 24 (29) | 20 (25) | 0.36 |
| Change a | 6 (16) | 8 (15) | 0.52 |
| GMA (distribution of average—%) | |||
| Bradygastria (1–2.4 cpm) | |||
| Baseline | 49 (21) | 55 (20) | 0.02 |
| Change a | 0.7 (19.0) | −1.8 (19.7) | 0.63 |
| Normogastria (2.5–3.7 cpm) | |||
| Baseline | 20 (14) | 20 (14) | 0.86 |
| Change a | 1.5 (14.3) | 1.8 (15.0) | 0.93 |
| Tachygastria (3.8–10 cpm) | |||
| Baseline | 23 (14) | 19 (11) | 0.01 |
| Change a | −0.7 (11.8) | 1.2 (11.8) | 0.34 |
| Duodenal (>10–15 cpm) | |||
| Baseline | 7 (9) | 6 (10) | 0.08 |
| Change a | −1.5 (7.7) | −1.1 (7.4) | 0.40 |
Abbreviations: GMA, gastric myoelectrical activity; GP, gastroparesis.
Mean of 3 values taken at 10, 20, and 30 min after start of test—baseline.
p‐value derived from robust regression of outcome on GP type for baseline and robust regression of change in outcome on GP type and baseline value of outcome.
The intensity of symptoms and the percentage distribution of power in the four GMA frequency ranges before and after the WLST are compared for the GP and FD groups in Table 2. Baseline and change in symptoms of fullness, bloating, nausea, and abdominal discomfort for the total 1‐ to 30‐min post‐WLST period were similar in the GP and FD groups. At baseline, the average percentage distribution in the tachygastria range was significantly greater in the GP group compared with FD (23% vs. 19%, p = 0.01) and average percentage bradygastria was significantly lower (49% vs. 55%, p = 0.02). After the water load was ingested, changes in the mean percentage distribution of GMA activity in the four frequency ranges in the combined 1‐ to 30‐min time period were similar in both groups.
Table 3 shows symptoms and GMA before and for 90 min after ingestion of the nutrient bar. These data are shown in a format similar to Table 2. Patients with GP ingested approximately 83% of the nutrient bar compared with 90% in patients with FD (p = 0.006), a difference of approximately 18 calories. The average intensity of symptoms before and after ingestion of the nutrient bar was similar in both groups. The GMA percentages in the four frequency ranges, the averaged sum of the five time points after ingestion of the nutrient bar, were similar in patients with GP and FD.
TABLE 3.
Symptoms and GMA before and after the nutrient bar test meal in patients with gastroparesis and functional dyspepsia
| Gastroparesis (n = 283) | Functional dyspepsia (n = 112) | p‐value c | |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| Nutrient meal test | |||
| Water taken with smart bar—ml | 53 (45) | 60 (60) | 0.27 e |
| Smart bar—% consumed | 83 (25) | 90 (20) | 0.006 e |
| Test length—min | 8.5 (2.4) | 8.5 (2.2) | 0.38 |
| Symptoms (VAS 0–100) | |||
| Fullness | |||
| Baseline | 28 (30) | 30 (32) | 0.74 |
| Change a | 30 (31) | 33 (30) | 0.33 |
| Hunger | |||
| Baseline | 34 (30) | 33 (31) | 0.93 |
| Change a | −18 (27) d | −20 (26) d | 0.56 |
| Nausea | |||
| Baseline | 29 (31) | 28 (31) | 0.78 |
| Change a | 2 (19) | 6 (22) | 0.55 |
| Bloating | |||
| Baseline | 29 (31) | 28 (31) | 0.67 |
| Change a | 9 (20) | 9 (21) | 0.66 |
| Abdominal discomfort | |||
| Baseline | 26 (30) | 26 (30) | 0.88 |
| Change a | 4 (19) | 5 (17) | 0.11 |
| GMA (distribution of average—%) | |||
| Bradygastria (1–2.4 cpm) | |||
| Baseline | 51 (20) | 50 (20) | 0.66 |
| Change b | −4 (18) | −3 (18) | 0.50 |
| Normogastria (2.5–3.7 cpm) | |||
| Baseline | 19 (13) | 21 (14) | 0.29 |
| Change b | 4 (13) | 4 (15) | 0.92 |
| Tachygastria (3.8–10 cpm) | |||
| Baseline | 23 (12) | 22 (11) | 0.53 |
| Change b | 2 (11) | 0 (10) | 0.22 |
| Duodenal (>10–15 cpm) | |||
| Baseline | 7 (8) | 7 (8) | 0.98 |
| Change b | −1 (7) | −1 (7) | 0.12 |
Abbreviations: GMA, gastric myoelectrical activity; GP, gastroparesis.
Mean of 6 values taken at 0, 15, 30, 45, 60, and 90 min after start of test—baseline.
Mean of 5 values taken at 15, 30, 45, 60, and 90 min after start of test—baseline.
p‐value derived from robust regression of outcome on GP type for baseline and robust regression of change in outcome on GP type and baseline value of outcome.
p‐value based on Kruskal–Wallis test due to non‐convergence using robust regression.
p‐value based on the t test.
Bold values indicates the significant of 0.006 value.
Figure 2 shows the average symptom scores reported before and during each of the three 10‐min periods after the WLST and before and during the five periods after ingestion of the nutrient bar in the GP and FD patients. The symptoms reported are similar in each group before and after ingestion of the water load and the nutrient bar.
FIGURE 2.
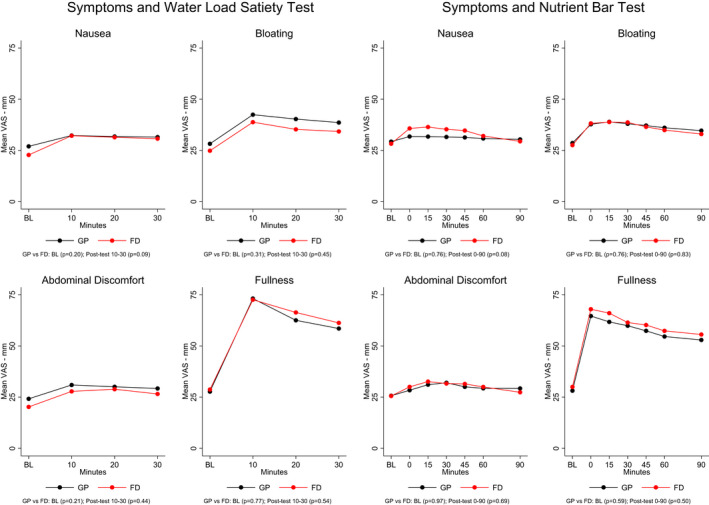
Symptoms before and after the water load satiety test and nutrient bar test are shown. Symptoms were scored on a visual analog scale from 0 to 100 for each symptom. BL indicates the time 15 min before ingestion of the water, and 0 represents symptoms immediately after ingestion of the nutrient bar. Symptoms increased after the water load in both groups, but there were no significant differences in intensity between the GP and FD groups. Symptoms before and after ingestion of the nutrient bar test meal were similar in the GP and FD groups
Figure 3 shows the average GMA percentages in the four frequency ranges before and 10, 20, and 30 min after the WLST. At baseline, the average percentage tachygastria was significantly higher in the GP patients compared with FD patients and average percentage bradygastria was higher at baseline in the FD patients as noted above in Table 1. After the water load was ingested, there were no differences in average GMA in the two groups. Figure 3 also shows the GMA results at baseline and 15, 30, 45, 60, and 90 min after the nutrient bar meal was ingested. Before and after ingestion of the nutrient bar, mean percentage distributions of GMA in the four frequency ranges were similar in the GP and FD cohorts.
FIGURE 3.
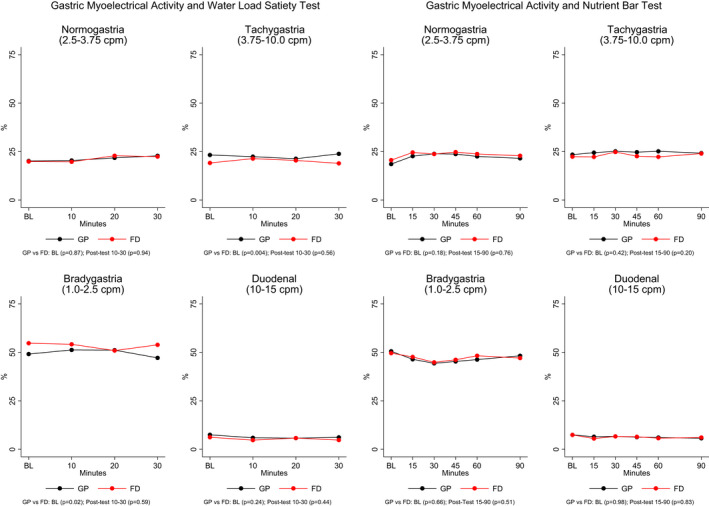
Percentage (%) distribution of gastric myoelectrical activity (GMA) is shown in the four frequency ranges on the y‐axis before and after the water load satiety test and nutrient bar test in the GP and FD groups. On the x‐axis, BL indicates 15 min before the water load or nutrient bar was ingested and the 10, 20, and 30 min after the water load and up to 90 min after the nutrient bar was ingested. There were no differences in the percentage of GMA activity in patients with GP and FD
3.4. Individual GMA responses to the WLST in patients with GP and FD
The individual EGG diagnoses based on the GMA response to the WLST are shown in Table 4. Of the 284 patients with GP, 184 (66%) had gastric dysrhythmias and 96 (34%) had normal 3 cpm GMA. Of the patients with GP, 31% had tachygastria, 17% had bradygastria, 9% had mixed dysrhythmias, and 9% had hyponormal 3 cpm GMA only; of the patients termed normal 3 cpm GMA, there were variable patterns: 15% had normal 3 cpm GMA, 5% had hypernormal 3 cpm, and 14% had normal 3 cpm with dysrhythmias.
TABLE 4.
Summary of gastric myoelectrical activity responses to the water load satiety test (WLST) in patients with gastroparesis and functional dyspepsia
| Gastroparesis (n = 284) | Functional dyspepsia (n = 113) | |
|---|---|---|
| Dysrhythmic GMA response a | ||
| Tachygastria | 87 (31%) | 35 (31%) |
| Bradygastria | 50 (18%) | 23 (20%) |
| Mixed dysrhythmia | 25 (9%) | 1 (1%) |
| Hyponormal 3 cpm GMA b | 26 (9%) | 14 (12%) |
| Normal 3 cpm GMA response | ||
| Normal 3 cpm GMA | 44 (15%) | 21 (19%) |
| Hypernormal 3 cpm GMA | 14 (5%) | 10 (9%) |
| Normal 3 cpm with dysrhythmia c | 38 (14%) | 9 (7%) |
Dysrhythmic GMA and hyponormal 3 cpm occur in responses to WLST.
Includes patients with hyponormal 3 cpm GMA with increased activity in duodenal–respiratory range (2 patients with GP and 1 with FD in dysrhythmic GMA response groups).
Includes 36 GP patients with tachygastria and 2 with mixed dysrhythmias, and 7 FD patients with tachygastria and 2 with mixed gastric dysrhythmias.
In regard to the 113 patients with FD, 31% had tachygastria, 20% had bradygastria, 1% had mixed dysrhythmia, and 13% had hyponormal 3 cpm only; of the patients with overall normal 3 cpm diagnoses, 19% had normal 3 cpm GMA, 9% had hypernormal 3 cpm GMA, and 7% had 3 cpm GMA with dysrhythmia. The distribution of individual diagnoses in patients with GP and FD are generally similar; comparison of the diagnostic categories in the two groups by logistic regression showed that there were significantly fewer patients with mixed gastric dysrhythmias in the FD group compared with the GP group (p = 0.05).
Comparison of characteristics in patents with GP and normal 3 cpm GMA versus patients with GP and dysrhythmic GMA showed that the group with dysrhythmic GMA had significantly higher BMI (29 vs. 26 kg/m2), were prescribed more steroids and anxiolytics, had higher GCSI retching scores, and consumed more water during the WLST. These comparisons for patients with FD showed that patients with dysrhythmic GMA had significantly higher BMI (29 vs. 26 kg/m2, p = 0.05), lower incidence of acute onset of symptoms, longer duration of GP symptoms, and were prescribed more neuropathic pain medications compared with those with normal 3 cpm GMA (Table S3).
Examples of normal 3 cpm GMA and tachygastria in the EGG recordings, running spectral analyses, and percentage distribution of power in the four frequency ranges in individual patients with GP and FD are shown in Figures S4–S7.
4. DISCUSSION
The key findings of this study are (1) patients with GP and FD had similar symptoms in response to liquid and solid test meals and (2) the GMA response to the WLST revealed gastric dysrhythmias in two‐thirds of the patients and normal 3 cpm GMA in one third. In addition, almost 25% of the patients with GP and FD ingested less than 238 ml of water during the WLST, indicating poor gastric accommodation. These findings indicate the similarity of gastric pathophysiological abnormalities in patients with GP and FD.
Dysrhythmic GMA was present in 66% of the patients with GP and may be relevant to the selection and efficacy of drug or device therapies. Gastric electrical stimulation therapy was significantly more effective in reducing symptoms in patients with GP who had 3 cpm GMA, less tachygastria, and greater numbers of ICCs compared with GP patients with tachygastria and fewer ICCs. 28 Domperidone treatment improved symptoms and increased 3 cpm GMA in patients with diabetic GP and gastric dysrhythmias, 29 and cisapride improved symptoms and 3 cpm GMA in 71% of patients with idiopathic GP and gastric dysrhythmias. 30 On the contrary, almost 1/3 of the patients with GP had normal 3 cpm GMA. Normal 3 cpm GMA is present when there are 5 or more ICCs/ hpf in the gastric antrum and corpus. 18 , 19 Normal 3 cpm GMA in patients with GP would seem to be discordant, but 20% and 50% of patients with diabetic and idiopathic GP, respectively, had normal numbers of ICCs by immunohistological studies. 15 , 16 The patients with GP and normal numbers of gastric ICCs had milder delay in gastric emptying at four hours compared with patients with GP and low ICC numbers (22 ± 9.4% vs. 47.6 ± 25.6%, respectively, p < 0.05). 16 Ultrastructural abnormalities in ICCs and enteric neurons on the contrary have been detected by electron microscopy in all patients with GP. 15 Thus, it is possible ICCs and enteric neurons are normal in number but are dysfunctional in some patients with GP and normal 3 cpm GMA.
Patients with GP secondary to pyloric stenosis have normal 3 cpm GMA. 31 In the vast majority of patients with GP and 3 cpm GMA, the pylorus appears normal at endoscopy, 32 but pyloric neuromuscular dysfunction has been appreciated in GP. Mearin et al. reported pylorospasm in patients with diabetic GP. 33 Fisher et al. described premature closure of the terminal antrum before peristaltic waves reached the pylorus in patients with GP. 34 Poor distensibility of the pylorus was found in almost 30% of patients with moderate‐to‐severe GP. 35 Furthermore, ICCs were decreased and fibrosis was increased in the pylorus in 83% of patients with GP. 36 Improvement in symptoms was reported after botulinum toxin injection or balloon dilation of the pylorus in 78% of the patients with GP and normal 3 cpm GMA 32 and in selected patients who subsequently underwent pyloroplasty, symptoms decreased, and gastric emptying was normal or improved six months after the operation. 37
Of the 113 patients with FD, 65% had gastric dysrhythmias and 35% had normal 3 cpm GMA after the WLST, incidences similar to the patients with GP. Gastric dysrhythmias are abnormalities found in patients with FD and GP and link the two entities on the spectrum of gastric neuromuscular disorders. Gastric dysrhythmias may also have a role in the pathophysiology of postprandial symptoms in FD. 9 , 19 Symptoms significantly decreased, and normal 3 cpm GMA increased after treatment with cisapride in children and adults with FD and gastric dysrhythmias. 38 , 39 Patients with chronic nausea (with normal or with delayed gastric emptying) had significantly decreased nausea and decreased tachygastria activity after aprepitant compared with patients who received placebo. 12 Normal 3 cpm GMA was recorded in 35% of the patients with FD. The normal 3 cpm GMA in these patients suggests normal numbers of gastric ICCs were present. Patients with FD and normal 3 cpm GMA may have poor gastric accommodation. These patients may also have non‐gastric diseases that mimic FD such as atypical gastroesophageal reflux disease, 40 rapid gastric emptying (dumping syndrome), 41 gallbladder diseases, 42 small intestinal bacterial overgrowth, and irritable bowel syndrome that contribute to their symptoms. 2
In summary, symptoms and GMA responses after liquid and solid test meals were similar in patients with GP and FD. Poor gastric distension and gastric dysrhythmias are pathophysiological abnormalities that link the similar postprandial symptoms and gastric neuromuscular dysfunction in patients with GP and FD.
DISCLOSURES
Dr. Koch is a shareholder in 3CPM Company, Inc. There are no other conflicts of interest to declare.
AUTHOR CONTRIBUTIONS
Kenneth L. Koch, and Pankaj J. Pasricha, designed the study, enrolled patients, critically revised the manuscript for important intellectual content, supervised the study, and approved the final manuscript. Mark Van Natta, analyzed and interpreted the data, critically revised the manuscript for important intellectual content, and approved the final manuscript. Henry P. Parkman, conceived and designed the study, enrolled patients, analyzed and interpreted the data, wrote the manuscript, supervised the study, and approved the final manuscript. Madhusudan Grover, Hossam Shaltout, Gianrico Farrugia, Robert J. Shulman, and Frank Hamilton, critically revised the manuscript for important intellectual content and approved the final manuscript. Thomas L. Abell, and Irene Sarosiek, enrolled patients, critically revised the manuscript for important intellectual content, and approved the final manuscript. Richard W. McCallum, conceived and designed the study, enrolled patients, critically revised the manuscript for important intellectual content, and approved the final manuscript. James Tonascia, designed the study, analyzed and interpreted the data, critically revised the manuscript for important intellectual content, and approved the final manuscript. Laura Miriel, designed the study, read the manuscript for important intellectual control, and approved the final manuscript.
Supporting information
Table S1
Supplementary Material
Koch KL, Van Natta M, Parkman HP, et al. Effect of liquid and solid test meals on symptoms and gastric myoelectrical activity in patients with gastroparesis and functional dyspepsia. Neurogastroenterology & Motility. 2023;35:e14376. doi: 10.1111/nmo.14376
ClinicalTrials.gov Identifier: NCT01696747.
Funding information
The Gastroparesis Consortium (GpCRC) was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (grants U01DK112193, U01DK112194, U01DK073983, U01DK073975, U01DK074035, U01DK074007, U01DK073985, U01DK073974, and U24DK074008) and the National Center for Advancing Translational Sciences (NCATS) (grants UL1TR000424, UL1TR000135)
REFERENCES
- 1. Kim BJ, Kuo B. Gastroparesis and functional dyspepsia: a blurring distinction of pathophysiology and treatment. J Neurogastroenterol Motil. 2019;25:27‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Talley NJ, Stanghellini V, Chan FKL, et al. Gastroduodenal disorders. In: Drossman DA, Hasler WL, eds. Rome IV. Functional Gastrointestinal Disorders. Rome Foundation; 2016:203‐965. [Google Scholar]
- 3. Carbone F, Vanuytsel T, Tack J. Analysis of postprandial symptom patterns in subgroups of patients with Rome III or IV functional dyspepsia. Clin Gastroenterol Hepatol. 2020;18:838‐846. [DOI] [PubMed] [Google Scholar]
- 4. Jehangir A, Parkman P. Rome IV diagnostic questionnaire complements patient assessment of gastrointestinal symptoms for patients with gastroparesis symptoms. Dig Dis Sci. 2018;63:2231‐2243. [DOI] [PubMed] [Google Scholar]
- 5. Pasricha PJ, Colvin R, Yates K, et al. Characteristics of patients with chronic unexplained nausea and vomiting and normal gastric emptying. Clin Gastroenterol Hepatol. 2011;9:567‐576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lacy BE. Functional dyspepsia and gastroparesis: one disease or two? Am J Gastroenterol. 2012;107:1615‐1620. [DOI] [PubMed] [Google Scholar]
- 7. Stanghellini V, Tack J. Gastroparesis: separate entity or just a part of dyspepsia? Gut. 2014;63:1972‐1978. [DOI] [PubMed] [Google Scholar]
- 8. Tack J, Carbone F. Functional dyspepsia and gastroparesis. Curr Opin Gastroenterol. 2017;33:446‐454. [DOI] [PubMed] [Google Scholar]
- 9. Pasricha PJ, Grover M, Yates KP, GpCRC . Functional dyspepsia and gastroparesis are interchangeable syndromes with common clinical and pathological features. Gastroenterology. 2021. doi: 10.1053/j.gastro.2021.01.230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tack J, Caenepeel P, Piessevaux H, et al. Assessment of meal induced gastric accommodation by a satiety drinking test in health and in severe functional dyspepsia. Gut. 2003;52:1271‐1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Koch KL, Hong S‐P, Xu L. Reproducibility of gastric myoelectrical activity and the water load test in patients with dysmotility‐like dyspepsia symptoms and in control subjects. J Clin Gastroenterol. 2000;31:125‐129. [DOI] [PubMed] [Google Scholar]
- 12. Pasricha PJ, Yates KP, Sarosiek I, et al. Aprepitant has mixed effects on nausea and reduces other symptoms in patients with gastroparesis and related disorders. Gastroenterology. 2018;154:65‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Koch KL, Hasler WL, Van Natta M, et al.; GpCRC . Satiety testing in diabetic gastroparesis: effects of insulin pump therapy and continuous glucose monitoring on upper gastrointestinal symptoms and gastric myoelectrical activity. Neurogastroenterol Motil. 2019;32:e13720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Koch KL. Gastric neuromuscular function and neuromuscular disorders. In: Feldman M, Friedman LS, Brandt LJ, eds. Sleisenger and Fordtran's Gastrointestinal and Liver Disease: Pathophysiology/Diagnosis/Management. Elsevier, Inc.; 2015:811‐838. [Google Scholar]
- 15. Faussone‐Pellegrini MS, Grover M, Pasricha PJ, et al. Ultrastructural differences between diabetic and idiopathic gastroparesis. J Cell Mol Med. 2012;16:1573‐1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Grover M, Bernard CE, Pasricha PJ, et al. Clinical‐histological associations in gastroparesis: results from the Gastroparesis Clinical Research Consortium. Neurogastroenterol Motil. 2012;24:531‐e249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Grover M, Farrugia G, Lurken MS, et al. Cellular changes in diabetic and idiopathic gastroparesis. Gastroenterology. 2011;140:1575‐1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. O'Grady G, Angeli TR, Du P, et al. Abnormal initiation and conduction of slow‐wave activity in gastroparesis, defined by high‐resolution electrical mapping. Gastroenterology. 2012;143:589‐598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Angeli TR, Cheng LK, Du P, et al. Loss of interstitial cells of Cajal and patterns of gastric dysrhythmia in patients with chronic unexplained nausea and vomiting. Gastroenterology. 2015;149:56‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jones MP, Hoffman S, Shah D, et al. The water load test: observations from healthy controls and patients with functional dyspepsia. Am J Physiol Gastrointest Liver Physiol. 2003;284:G896‐G904. [DOI] [PubMed] [Google Scholar]
- 21. Tougas G, Eaker EY, Abell TL, et al. Assessment of gastric emptying using a low‐fat meal: establishment of international control values. Am J Gastroenterol. 2000;95:1456‐1462. [DOI] [PubMed] [Google Scholar]
- 22. Koch KL. Electrogastrography for suspected gastroparesis. In: McCallum R, Parkman H, eds. Gastroparesis: Pathophysiology, Clinical Presentation, Diagnosis and Treatment. Elsevier; 2020:189‐205. [Google Scholar]
- 23. Lee AA, Rao S, Nguyen LA, et al. Validation of diagnostic and performance characteristics of the wireless motility capsule in patients with suspected gastroparesis. Clin Gastroenterol Hepatol. 2019;17:1770‐1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Revicki DA, Rentz AM, Dubois D, et al. Development and validation of a patient‐assessed gastroparesis symptom severity measure: the Gastroparesis Cardinal Symptom Index. Aliment Pharmacol Ther. 2003;18:141‐150. [DOI] [PubMed] [Google Scholar]
- 25. Muth ER, Stern RM, Thayer JF, Koch KL. Assessment of the multiple dimensions of nausea: the nausea profile (NP). J Psychosom Res. 1996;5:511‐520. [DOI] [PubMed] [Google Scholar]
- 26. SAS Institute, Inc . SAS Software, Version 9.3 of the SAS System for Windows. SAS Institute, Inc. 2013. [Google Scholar]
- 27. StataCorp LP . Stata 15.1. Stata Statistical Software: Release 12. StataCorp LP. 2017. [Google Scholar]
- 28. Lin Z, Sarosiek I, Forster J, et al. Association of the status of interstitial cells of Cajal and electrogastrogram parameters, gastric emptying, and symptoms in patients with gastroparesis. Neurogastroenterol Motil. 2010;22:56‐61. [DOI] [PubMed] [Google Scholar]
- 29. Koch KL, Stern RM, Stewart WR, Vasey MW. Gastric emptying and gastric myoelectrical activity in patients with symptomatic diabetic gastroparesis: effects of long‐term domperidone treatment. Am J Gastroenterol. 1989;84:1069‐1075. [PubMed] [Google Scholar]
- 30. Rothstein R, Alavi A, Reynolds JC. Electrogastrography in patients with gastroparesis and effect of long‐term cisapride. Dig Dis Sci. 1993;38:1518‐1524. [DOI] [PubMed] [Google Scholar]
- 31. Brzana RJ, Bingaman S, Koch KL. Gastric myoelectrical activity in patients with gastric outlet obstruction and idiopathic gastroparesis. Am J Gastroenterol. 1998;93:1083‐1089. [DOI] [PubMed] [Google Scholar]
- 32. Wellington J, Scott B, Kundu S, Stuart P, Koch KL. Effect of endoscopic pyloric therapies for patients with nausea and vomiting and functional obstructive gastroparesis. Auton Neurosci. 2017;202:56‐61. [DOI] [PubMed] [Google Scholar]
- 33. Mearin F, Camilleri M, Malagelada JR. Pyloric dysfunction in diabetics with recurrent nausea and vomiting. Gastroenterology. 1986;90:1919‐1925. [DOI] [PubMed] [Google Scholar]
- 34. Fisher RS. Gastroduodenal motility disturbances in man. Scand J Gastroenterol. 1985;109:59‐68. [DOI] [PubMed] [Google Scholar]
- 35. Snape WJ, Lin MS, Agarwal N, Shaw RE. Evaluation of the pylorus with concurrent intraluminal pressure and EndoFLIP in patients with nausea and vomiting. Neurogastroenterol Motil. 2016;28:758‐764. [DOI] [PubMed] [Google Scholar]
- 36. Bashashati B, Moraveji S, Torabi A, et al. Pathological findings of the antrum and pylori smooth muscle in patients with gastroparesis and gastroparesis‐like syndrome compared to gastroparesis: similarities and differences. Dig Dis Sci. 2017;62:2828‐2833. [DOI] [PubMed] [Google Scholar]
- 37. Wellington J, Stuart P, Westcott C, Koch KL. Obstructive gastroparesis: patient selection and effect of laparoscopic pyloroplasty. J Gastrointest Surg. 2020;24:1778‐1784. [DOI] [PubMed] [Google Scholar]
- 38. Cucchiara S, Minella R, Riezzo G, et al. Reversal of gastric electrical dysrhythmias by cisapride in children with functional dyspepsia: report of three cases. Dig Dis Sci. 1992;37:1136‐1140. [DOI] [PubMed] [Google Scholar]
- 39. Besherdas K, Leahy A, Mason I, et al. The effect of cisapride on dyspepsia symptoms and the electrogastrogram in patients with non‐ulcer dyspepsia. Aliment Pharmacol Ther. 1998;12:755‐759. [DOI] [PubMed] [Google Scholar]
- 40. Brzana RJ, Koch KL. Intractable nausea presenting as gastroesophageal reflux disease. Ann Intern Med. 1997;126:704‐707. [DOI] [PubMed] [Google Scholar]
- 41. Wang P, Wellington J, Koch KL. Clinical features and gastric myoelectrical activity in patients with idiopathic and post‐surgical rapid gastric emptying who present with unexplained chronic nausea. Neurogastroenterol Motil. 2021;33(3):e13988. [DOI] [PubMed] [Google Scholar]
- 42. Whitaker LF, Powell MS, Refugia J, et al. Outcomes after laparoscopic cholecystectomy in hyperkinetic biliary dyskinesia. Am Surg. 2021:31348211023390. doi: 10.1177/00031348211023390 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Supplementary Material


