Abstract
Introduction
Exercise is vital to staying well and preventing secondary complications in people with chronic neurological impairments (CNI). Appropriate exercise is often inaccessible to this population. The purpose of the study is to investigate the effects of a seated, virtual exercise programme on heart rate, recovery, fatigue, pain, motivation, enjoyment and quality of life in people with CNI.
Methods and analysis
Individuals with CNI will be screened for eligibility, and 60 participants will be randomised 1:1 into either a live or prerecorded group. There is no geographical limitation to where participants reside, since participation is virtual. The study will be coordinated by one site in White Plains, New York, USA. The live group will exercise with an instructor via Zoom while the prerecorded group will exercise at their chosen time using prerecorded videos, 3×/week for 12 weeks. Primary outcome measures: change in heart rate during exercise/recovery. Secondary outcome measures: fatigue, motivation, level of pain and exertion, physical well-being, enjoyment of physical activity, motivation and quality of life. Outcomes will be assessed at baseline, midpoint, end of study and 1-month poststudy. Adverse events, medication changes and physical activity will be tracked throughout. Within-group and between-group comparisons will be performed by using analysis of covariance and regression.
Ethics and dissemination
BRANY IRB approval: 22 September 2020, protocol #20-08-388-512. All participants will provide written informed consent. Results will be disseminated through presentations, publications and ClinicalTrials.gov.
Trial registration number
Keywords: Rehabilitation medicine, Neurology, Telemedicine
STRENGTHS AND LIMITATIONS OF THIS STUDY.
This study will use a comprehensive set of physiological and behavioural assessments to assess effects of exercise.
The virtual design of the study enables people to participate without commuting to our institute.
For those participants with limited technological experience, difficulty using blood pressure devices and heart rate monitors will cause missing data values.
The virtual design of the study prohibits hands-on evaluations.
Introduction
Exercise is a vital component to staying well and preventing secondary impairments. Exercise is known to positively influence a range of factors including cardiovascular health, cognition, mental health, bone health, sleep and quality of life, among many others.1–8 The benefits of exercise are further amplified in the estimated one billion people worldwide with chronic (>6 months) neurological impairments (CNI), as the injuries often lead to sedentary lifestyles and thus higher rates of obesity and cardiovascular disease.9–11 Incorporating an exercise regimen is vital to preventing secondary complications such as muscular weakness, fatigue, limited mobility, pain, spasticity, bone loss and increased risk of fractures, falls, diabetes, depression and obesity.7 8 12 13 Exercise can be neuroprotective and neuroregenerative by increasing neurotrophic factors, which are involved in neuroprotection, neuroplasticity and maintenance of neuronal health.2 14–18 Exercise can also improve muscle strength and bone integrity in people with CNI.19–21
People with CNI often cannot access community exercise centres, as mobility can be more difficult, insurance payments sparse, centres not welcoming or accessible and assistive exercise equipment too costly for most facilities.22–35
Another key barrier to exercise for people with CNI is transportation.36 Although COVID-19 introduced many new barriers to community participation, it also had some silver linings for people with CNI. COVID-19 resulted in the emergence of many new Zoom or online classes tailored to people with CNI, which removed the barrier of transportation.
Although there has been an explosion of virtual-based exercise opportunities since 2020, much is unknown regarding optimal delivery models to bring exercise to people with CNI. Two general types of virtual exercise delivery models exist. In synchronous exercise, one or more participants join a virtual space via videoconferencing, and interact with the trainer in real time. Synchronous exercise enables the trainer to provide feedback as participants exercise. In contrast, in asynchronous exercise, trainers record instructional videos, which participants complete on their own time, without being in the same virtual space with the trainer at the same time. Asynchronous exercise provides scheduling flexibility to participants but does not allow for real time interaction and feedback from the trainer. There has only been one study directly comparing synchronous versus asynchronous exercise training in people with CNI.
In a recent study, 40 adults with spinal cord injury were randomised to either synchronous or asynchronous tele-exercise. While there was no significant difference in average daily workload between the interventions, the synchronous tele-exercise found significantly higher values for adherence and successful data recording of the exercise, resulting in greater weekly training loads.37 Virtual exercise platforms are part of society even as the pandemic wanes. Thus, it is important to determine optimal delivery models for specific groups of participants.
This study will examine the cardiovascular benefits, as well as differences in compliance, motivation and feelings of socialisation and exertion between synchronous and asynchronous classes. The classes will be held for 12 weeks, 3 times/week using seated exercise via Zoom. The instructor will remain the same and alternate between boxing, high intensity interval training (HIIT) and power posture classes. We will examine the potential benefits of an accessible form of exercise for a population that is in urgent need. Additionally, by comparing synchronous and asynchronous classes, we will compare user compliance, motivation, enjoyment and socialisation.
We hypothesise that individuals in the synchronous class will have better attendance, more robust exertion as measured by heart rate (HR) and better enjoyment than people in the asynchronous class, due to the social component of the synchronous classes. We hypothesise that participants in both groups will show improvement in HR recovery, physical wellness, quality of life, enjoyment and motivation/engagement, and that changes will be greater in the synchronous class due to consistent supervision and contact.
Methods and analysis
Study design
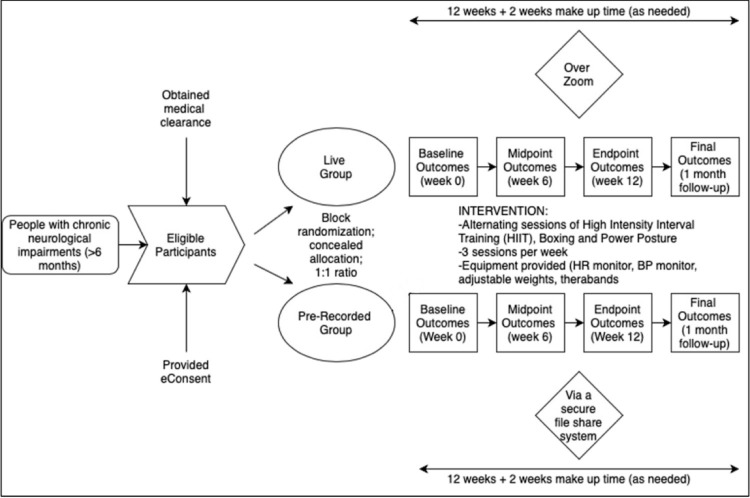
This study is a parallel randomised controlled trial to investigate the effects of a 12-week, 3 times/week, seated tele-exercise programme for adults with CNI (figure 1). All 36 exercise sessions will be completed online via Zoom with the physical exercise instructor (live, synchronous group) or offline using recorded videos (prerecorded, asynchronous group). Participants will be in their own homes as they complete the exercise classes. Participants can continue their medications, physical, occupational, speech therapies and existing exercise routines (two times/week or fewer).
Figure 1.
Study flow diagram. BP, blood pressure; HR, heart rate.
Eligibility criteria and participants
The eligibility criteria will be kept broad to accommodate all neurological impairments.
Inclusion criteria:
Participants diagnosed with chronic (>6 months) neurological impairments.
Ages 18–75 years.
Able to sit for at least 1 hour.
Stable HR and blood pressure (BP)
Medical clearance to participate in moderate intensity exercises.
Can don and doff a wrist HR monitor with or without assistance.
Can maintain daily exercise and physical activity during the study.
No other neurological, medical or cognitive impairments.
Able to access the internet and use the cloud-based Zoom conference platform.
Ability to speak and understand English.
Currently exercising 2 days or less per week.
Presence of caregiver or supervisor during exercise, for safety.
Exclusion criteria:
Unstable or uncontrolled medical conditions.
Medical issues preventing safe participation.
Unable to follow simple two-step commands.
Number of participants
We plan to conduct multiple cohorts of participants, each with different participant populations, for example, multiple sclerosis, cerebral palsy, stroke, spinal cord injury.
For each cohort, with 27 participants in the synchronous and asynchronous groups, we will have 80% power to detect a change in maximum HR during exercise from about 80 bpm to 100 bpm (or greater), from the first session to the last session of the intervention. This calculation assumes a conservative SD of the pre/post differences in HR of 35 bpm. We plan for a 30% drop-out rate, which is an estimate based on our feasibility pilot study (Current status of the study), which means that we will recruit 35 participants per group to achieve 80% power.
Recruitment
Participants will be recruited via the Sabrina Cohen Foundation, which has a network of people with CNI, and by the Burke Neurological Institute (BNI), who has a database of potential participants from prior studies or programmes. We will also post our recruitment flier on our website, social media and will mail it to neurological patient advocacy groups. We will send the study flier to all above mentioned entities, without prioritising any database or group over another. If we cannot recruit appropriate numbers using these methods, we will pursue paid advertising in our community.
Randomisation
A person not affiliated with the study will generate a series of permuted randomised blocks in a computerised spreadsheet with a defined block size of 4. Participants will be allocated to either the live (synchronous) or prerecorded (asynchronous) group, with a 1:1 ratio between groups. Due to the nature of the intervention, it is not possible to conceal allocation from the participants or the study team. However, the two groups will never interact with one another.
Blinding and rigour of data
Due to the nature of the study, blinding of participants and study team members to group allocation will not be possible. All participants will complete the outcome surveys directly online, without input or assistance from the study team. HR data will be collected through a HR monitor that collects quantitative data without input or filtering by the study team.
Intervention
The exercise intervention will be delivered by a trained professional who specialises in adaptive exercise for individuals with CNI. The instructor will structure each class to ensure standardisation in terms of class structure, including warm-up, cool-down, cues for recording HR during class, use of additional equipment and adherence to the general study protocol. The instructor has extensive experience in offering adaptations to exercises in a virtual setting. He offers alternative ways to perform the exercises, such that everyone in class will be able to fully participate safely.
All participants, regardless of allocation to live or prerecorded groups, will participate in 45 min of aerobic exercise intervention, three times a week, for a period of 12 weeks. The overall exercise session will be up to 1 hour long to include measuring vitals, answering questions before/after sessions and rest breaks (if any). Two additional weeks will be provided for making up missed sessions, if applicable. Each intervention will be preceded by a warm up and followed by a cool-down period. All participants will be asked to document any adverse events and changes in medications before each session. The order of classes will be rotated. Individual aerobic interventions for the first cohort are described below and summarised in table 1.
Table 1.
Summary of exercise sessions
| Minutes into session | HIIT | Boxing | Power posture |
| 5 | Warm up | Warm up | Warm up |
| 10 | Intervals of 30 s high intensity movements, 1–2 min lower intensity | Intervals of 30 s high intensity boxing movements, 1–2 min lower intensity | Stretching |
| 15 | Fast paced movements targeting trunk, posture | ||
| 20 | |||
| 25 | |||
| 30 | Stretching | ||
| 35 | |||
| 40 | Cool down | Cool down | Cool down |
| 45 | End | End | End |
HIIT, high intensity interval training.
High intensity interval training
In HIIT sessions, participants will do repetitive bouts of high intensity exercises, with intermittent periods of rest or active recovery. Examples of high intensity movements used in these sessions include fast punching movements, repeatedly rapidly lifting one’s arms overhead (with or without weights, as tolerated by each participant) and abdominal crunches. The duration of each bout of high intensity is about 10–30 s. Since this study will include participants with variable neurological impairments, the upper limit of exercise intensity in HIIT will be dependent on instructor’s judgement, participant feedback, rate of perceived exertion (RPE) and any exercise HR restrictions given by their doctor in the medical screening.
Boxing
In boxing sessions, participants will do a circuit of individual and combinations of various arm strikes and punches. Boxing is a moderate to high intensity aerobic activity by nature. During the session, participants will do bouts of high intensity movements, similar to HIIT, along with periods of lower intensity arm movements such as practising defensive blocks of punches from an imaginary boxing opponent. The exercise intensity for boxing, as in HIIT, will be subjective for participants based on level of perceived exertion as well as exercise HR restrictions, if any.
Power posture
Power posture is a HIIT-style strength and endurance class in which participants will move in ways that train trunk stability and control of sitting with correct posture. In these sessions, there will be a warm up period of 5–10 min, focused on slow, gentle repetitions to prepare the trapezius and other supporting scapular muscles for movement. This workout will target postural form, with fast paced targeted movements for 10–15 min. After the fast-paced movements, a cool-down period will focus on gentle deep neck flexor/extensor chin tuck exercises, range of motion and stretching.
Virtual nature of the study
Each aspect of this study will be performed virtually. Screenings will be performed on the telephone, with a standard list of criteria asked of each person. If deemed eligible, the potential participant will be sent a Zoom link to go through the informed consent on a one-to-one basis. The informed consent will be uploaded to REDCap, a secure web application for building and managing online surveys and databases. REDCap is 21 CFR part 11-ready and HIPAA compliant and is specifically geared to support online and offline data capture for research studies and operations.
To obtain informed consent directly from participants, a study team member will arrange a Zoom call with each participant. The informed consent will be signed virtually by sharing a virtual remote with the participant during the call. If anyone has difficulty, they can be sent the informed consent from REDCap via email and return it to REDCap just as easily. Each participant will be sent a copy of their signed consent form via the same REDCap email system (see online supplemental material for a sample consent form).
bmjopen-2022-065032supp001.pdf (33.1KB, pdf)
Each participant will be mailed a home exercise kit, which will contain a Polar optical HR monitor (OH1), BP monitor, yellow and green resistance TheraBands and wrist weights.
After the home kits have been received by the participants and before the intervention begins, we will hold a series or training videoconferencing calls to acquaint participants with the study procedures and the use of all devices.
Polar OH1 HR monitor: We will train participants in donning/doffing the HR monitor, using the Polar app, using their Polar accounts, syncing the Polar devices with their smartphones and starting/ending their HR tracking during exercise sessions.
BP monitor: We will send each participant a commercially available automatic BP monitor. We will train each person to use it, and we have embedded an instructional video into the REDCap data entry form on which participants will input their BP.
Checking compliance: After each session, a study team member will review each participant’s data entry forms and synching of their HR data with the Polar app. For the synchronous group, this will occur before the participant leaves the session. For the asynchronous group, this will occur within 1 day of when the participant completes the session.
All questionnaires and questions to be answered before and after each class will be sent from and stored on REDCap. All HR data will be transmitted to the Polar website, which can be accessed only by the participant or the researchers. Then, the data will be analysed in MATLAB using custom scripts written by the study team.
Study safety
We will monitor side effects before and after each session by having participants complete surveys on REDCap. If pain or BP are higher than usual, we will ask the participant if they wish to continue. If BP rises near limitations prescribed by a participant’s doctor, we will discontinue the participant. If any injury occurs during or outside of study sessions that may increase the risk of further injury, we will discontinue the participant.
Patient and public involvement
Persons with disabilities were involved in the design of this study, and will be involved in the conduct, reporting and dissemination plans of this research. Our pilot study participants provided constructive feedback on the study, in surveys and in a zoom discussion group after they completed the study. Our class instructor owns and operated an adaptive gym for people with disabilities, and received feedback from his clients when designing the exercise classes. We will continue to involve these people as we complete the study, through regular meetings and surveys.
Strategies to improve adherence
In this study, adherence is defined as the number of sessions a participant completed. The study team will take attendance at each synchronous session and will monitor session completions for the asynchronous group. Completion for the asynchronous group will be monitored via REDCap. Each time a person completes a data entry form before and after the session, it will be automatically saved and time stamped in REDCap.
If a participant misses a class, we will reach out and inquire about their ability to attend future sessions. We have a priori set a minimum number of 30 of 36 classes that a participant must attend to be included in the main analyses of the study. We will offer 2 weeks of makeup sessions at the end of the 12 weeks. If a participant drops out before completing 30 sessions, we will encourage them to complete the poststudy outcome measures and the satisfaction survey. We will use the number of sessions completed as a covariate in the analyses.
Outcome measures
All outcome measures are summarised in table 2.
Table 2.
Outcome measures
| Primary outcome measure | |||
| Measure | Modality assessed | Method of Ascertainment | Time points |
| Peak heart rate | Cardiovascular function | Polar OH1/Polar Beat app | Each session |
| Secondary outcome measures | |||
| Measure | Modality assessed | Method of ascertainment | Time points |
| Demographics | -- | REDCap survey | Before first session |
| Attendance | Adherence | REDCap time stamps | Each session |
| Baseline heart rate | Cardiovascular function | Polar OH1/Polar Beat app | Each session |
| Heart rate at end of session | Cardiovascular function | Polar OH1/Polar Beat app | Each session |
| Heart rate recovery | Cardiovascular function | Polar OH1/Polar Beat app | Each session |
| Blood pressure | Cardiovascular function | Automatic BP device | Before and after each session |
| Rate of perceived exertion | Cardiopulmonary fitness | REDCap survey | Before and after each session |
| Perceived Wellness Survey | Wellness | REDCap survey | Baseline, weeks 6, 12, 16 |
| Physical Activity Enjoyment Scale | Enjoyment of exercise | REDCap survey | Baseline, weeks 6, 12, 16 |
| Short Form-36 | Physical and mental health | REDCap survey | Baseline, weeks 6, 12, 16 |
| Numerical Pain Rating Scale | Pain | REDCap survey | Baseline, weeks 6, 12, 16 |
| Reason for Exercise Inventory | Motivation to exercise | REDCap survey | Baseline, weeks 6, 12, 16 |
| Participant Feedback Survey | Participant satisfaction | REDCap survey | End of study or at dropout |
BP, blood pressure.
Demographics
We will collect the following demographics by having each participant complete a demographics data entry form on REDCap: age, gender, neurological diagnosis, time of diagnosis and comorbid health issues (eg, diabetes, high BP). We will also document whether a person needs assistance with walking or transfers. In addition, we will require each participant to receive written medical clearance by their doctor to participate in the study, and if any exercise restrictions need to be followed.
Primary outcome measure: peak HR during exercise
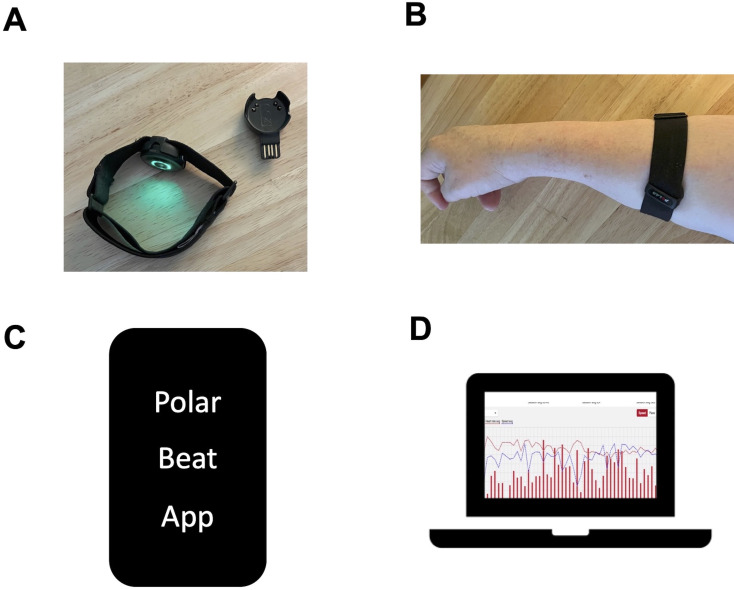
Continuous HR tracking is the primary outcome measure for the study. All participants will receive the OH1 Polar HR monitor as part of their exercise kit for the study (figure 2). The Polar OH1 (Polar Electro, Bethpage, New York, USA) is an optical HR sensor on an armband that records HR activity. It has six LED sensors that record at 1 s intervals.38 HR activity is recorded via Bluetooth to a phone compatible with the Polar Flow or Polar Beat app. It has validity in moderate to vigorous intensity exercises and high endurance sports activities.38–40
Figure 2.
Polar OH1 heart rate monitor. (A) Picture of the monitor and charger. (B) Proper placement of the monitor, with the device on the forearm. (C) The polar beat APP on a smart phone streams data in real time from the OH1 monitor via Bluetooth. (D) After a session, data are Retrievable from the polar coach website, where it is exported to a CSV file and processed in Matlab. CSV, comma-separated values.
Immediately before each exercise session, participants will turn on their OH1 monitor. Then, the participant will open the Polar Beat app on their smart phone and hit ‘start’ when the exercise session begins. The Polar OH1 continuously records HR at 10 Hz when the device is powered on and the Polar Beat app recording has been started. Participants will record HR through the entire exercise session and for 10 min after completion of the session to capture HR during exercise as well as HR recovery for 10 min after the end of exercise sessions. The live group will be supervised by the research team for each session. The prerecorded groups will have the research team available for assistance and troubleshooting. Data are stored on the Polar Coach website, where study staff can export sessions as CSV files that are then processed in MATLAB (MathWorks). In MATLAB, peak HR (primary measure) will be calculated.
Secondary outcome measures
Measures acquired at each exercise session
We will calculate secondary measures of HR in MATLAB using the same Polar comma-separated values (CSV) files mentioned above: baseline HR, HR at end of session, and HR at 30 s intervals for 10 min after the end of the session will be analysed, to measure HR recovery.
At each session, participants will complete an online assessment to RPE, BP, HR and adverse events before and after the exercise session. Additionally, as described above, attendance will be taken at each session as a measure of adherence. After the session is over, participants will complete a lab-developed survey to rate their experience on a 10-point scale from 1 (not a good experience) to 10 (very good experience) on questions related to motivation, energy, satisfaction and performance. In addition, participants will provide information on changes related to health, falls or other adverse events.
Measures acquired at specified times during the intervention
A comprehensive set of secondary outcome measures will be completed by participants at baseline, mid-study (6 weeks), end of study (12 weeks) and follow-up after 1 month. All questionnaires will be self-administered or administered by a trained researcher virtually using REDCap.
Perceived Wellness Survey (PWS): Participants will quantify their perceived health status by completing the PWS. The PWS is a 36-item instrument used to measure an individual’s perceived health status in physical, psychological, emotional, intellectual, spiritual and social wellness constructs. Items under each construct are scored on a scale of 1 (very strongly disagree) to 6 (very strongly agree). Higher scores indicate better perceived wellness. The PWS has good reliability and validity in healthy and neurological populations.41–43
Physical Activity Enjoyment Scale (PACES): The PACES is a self-assessment measure of enjoyment with their current physical activity. It is an 18-item scale with scoring from 1 (I enjoy it) to 7 (I hate it). Scores on the bipolar rating scale are summed with higher scores indicative of more enjoyment. It is reliable and valid in neurological populations.44–47
Short Form-36 Health Survey (SF-36): The SF-36 measures physical health and mental health. There are eight scales-Physical Functioning, Physical Role Limitations, Bodily Pain, General Health, Vitality, Social Functioning, Emotional Role Limitations, and Mental Health. It is easy to use with good validity and reliability in neurological populations.48–51
Numerical Pain Rating (NPR) scale: The NPR is a standardised instrument for pain assessment in clinical and research practice. It is an 11-point scale from 0 (no pain) to 10 (the most intense pain) at rest and during movement. It is simple to use with high sensitivity to changes in chronic pain in adult populations.52 53 It is reliable and valid for pain assessments of neurological populations.54
Borg’s RPE: The Borg RPE is a widely used standardised measure to evaluate perceived intensity of exertion, effort and fatigue during physical exercise. The scale ranges from 6 (no exertion at all) to 20 (absolute maximum). Higher ratings on the scale indicate greater overall body exertion. It is a reliable and valid measure in neurological populations.55–62
Reason for exercise inventory (REI): The REI is a 24-item scale to assess the reason that motivates a person to exercise. The modified version has four subscales: Weight/appearance management, fitness/health management, stress/emotion management, and socialisation. A 7-point scale ranges from 1 (not at all important) to 7 (extremely important). Higher scores represent greater motivation to exercise.63
Participant feedback: On study completion, or when withdrawn/dropped out from the study, participants will complete a study feedback form. The feedback will be based on study acceptance, ease of using HR and BP devices and exercise equipment, and support from the research team. Participants who do not complete the study will also provide their final timepoint measure at the end of their final exercise session.
Statistical methods
Descriptive statistics (including mean, SD, median, IQR, frequency and percent) will be calculated for demographic and clinical characteristics of the study participants. Analysis of covariance (ANCOVA) will be used to evaluate the independent effect of synchronous versus asynchronous group status on change in peak HR (first session vs final session), after controlling for baseline HR and any other factors that may remain imbalanced between groups after randomisation. Adjusted mean differences in peak HR (first session vs final session) between the synchronous and asynchronous groups and 95% CIs for these differences will be estimated from the multivariable ANCOVA model. Similar analyses as described above will be performed for other continuous outcomes (primary and secondary), including Borg’s RPE scale, mood, motivation and pain scales, PWS, SF-36v2 score and PACES score. Categorical variables (eg, safety questions, adverse events, medication changes) will be compared between the synchronous and asynchronous groups (at baseline, 6 weeks and 12 weeks) by the χ2 test or Fisher’s exact test, as appropriate. Generalised estimating equations modelling will also be explored to evaluate between-group differences over repeated assessments, and to account for potential missing data in some of the outcome variables at one or more of the evaluation time points. All estimates from the multivariable models will serve as preliminary data (ie, hypothesis-generating) for future studies. All p values will be two sided with statistical significance evaluated at the 0.05 alpha level. Ninety-five per cent CIs for all parameters of interest will be calculated to assess the precision of the obtained estimates. All analyses will be performed in R and SPSS v.26 by a person blinded to treatment allocation.
Data management
Participant identifying information will be kept confidential by providing a unique identification number (ID number) for the course of the study. To maintain privacy, only authorised research investigators involved in the study will have access to participant information. Password protected laptops, online documents and access to cloud-based servers will prevent leaks in data privacy. All records including informed consent, screening tools, medical records and participant surveys will be completed, and documented on REDCap, a Health Insurance Portability and Accountability Act (HIPAA)-compliant online research database.
Privacy on Zoom platform: Our institutional Zoom account (through Weill Cornell Medicine) is compliant with HIPAA guidelines. Participant profile names will be updated to display their ID. After each session, the recorded video will be uploaded to the password protected WCM cloud-based server. Respective group participants will be given the live Zoom links (synchronous) or passwords to access the asynchronous Zoom sessions.
Polar OH1 HR monitor (Optical HR sensor): Participants’ profiles using allotted ID’s will be created in the Polar Beat App and the Flow for Coach cloud-based online platform. All information uploaded to the platform will be accessible by the investigators using a secured login ID and password.
Adverse events, change in medications and protocol deviation will be documented and updated before and after each class on REDCap. The study team will perform weekly self-audits to ensure data quality and completeness.
Procedure for handling missing data
This study is an intention-to-treat trial. If data are missing, we will use a mixed linear regression model that accounts for uneven numbers of data points across participants.
Data monitoring and stopping rules
Since this is a low-risk study, our IRB determined that a data monitoring committee is not necessary. We will audit our data weekly and assess safety data at each session. If we find an increase in pain by 25% or a decrease in quality of life measures by 25% across participants in a cohort, we will pause the trial and evaluate how to improve the safety of the study.
Current status of the study
We have performed a feasibility cohort, with people with a range of abilities and diagnoses. The first participant was consented on 22 January 2021, for an intervention that began on 24 March 2021 and ended on 28 June 2021. In this feasibility study, we determined that participants could readily complete the online assessments, exercise sessions and could effectively use Zoom, the Polar OH1 sensor and Polar Beat app, and the BP measurement device. Our next step will be to enrol separate cohorts of people with specific neurological diagnoses, such as cerebral palsy, stroke and multiple sclerosis. This will begin in 2023.
Resource sharing plan
We plan to use the concept, methods and analysis as a framework to develop evidence-based protocols for future cohorts and studies. We have made a commitment to publish all relevant scientific information in a timely manner. Unpublished information may be available to interested individuals or organisations by request to the principal investigator.
Ethics and dissemination
The study was approved by the BRANY Institutional Review Board on 22 September 2020, protocol #20-08-388-512. All study participants will provide written informed consent before participation. All protocol deviations, adverse events and protocol modifications will be reported to the IRB immediately. The results of the study will be disseminated to the academic community via publications and presentations. The deidentified study data will be shared on our laboratory website. The trial is registered at Clinicaltrials.gov, number NCT04564495.
Supplementary Material
Acknowledgments
We thank the Sabrina Cohen Foundation for funding and for commitment to assist with study development and recruitment. We thank Devon Palermo at DPI Adaptive Fitness who will serve as the adaptive fitness instructor for this study.
Footnotes
Contributors: KMF is the principal investigator. KMF, AAD and AB were responsible for ethics applications and reporting. AAD, AB, DSK, RMG, LEJC, TC and KMF drafted the final version of this manuscript. All authors have contributed to the writing and critical review of the manuscript and have approved the final version.
Funding: This work is supported by funding from the Sabrina Cohen Foundation and the Burke Foundation. This work is supported by grant number UL1 TR 002384 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH). The Sabrina Cohen Foundation has established the tele-exercise classes we are studying and will assist with recruitment.
Disclaimer: The Sabrina Cohen Foundation and Burke Foundation will have no role in data collection, analyses, interpretation, or publication of the work.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Gkotzamanis V, Magriplis E, Panagiotakos D. The effect of physical activity interventions on cognitive function of older adults: a systematic review of clinical trials. Psychiatriki 2022; 10.22365/jpsych.2022.060 [DOI] [PubMed] [Google Scholar]
- 2.Johansson ME, Cameron IGM, Van der Kolk NM, et al. Aerobic exercise alters brain function and structure in Parkinson’s disease: a randomized controlled trial. Annals of Neurology 2022;91:203–16. 10.1002/ana.26291 Available: https://onlinelibrary.wiley.com/toc/15318249/91/2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Q, Wu C. Social interaction, lifestyle, and depressive status: mediators in the longitudinal relationship between cognitive function and instrumental activities of daily living disability among older adults. IJERPH 2022;19:4235. 10.3390/ijerph19074235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Minto LR, Ellis R, Cherry KE, et al. n.d. Impact of cardiovascular risk factors on the relationships of physical activity with mood and cognitive function in a diverse sample. Aging, Neuropsychology, and Cognition 2022:1–14. 10.1080/13825585.2022.2071414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosado H, Bravo J, Raimundo A, et al. Effects of two 24-week multimodal exercise programs on reaction time, mobility, and Dual-task performance in community-dwelling older adults at risk of falling: a randomized controlled trial. BMC Public Health 2021;21:Suppl 2. 10.1186/s12889-021-10448-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schuch FB, Vancampfort D, Firth J, et al. Physical activity and incident depression: a meta-analysis of prospective cohort studies. Am J Psychiatry 2018;175:631–48. 10.1176/appi.ajp.2018.17111194 [DOI] [PubMed] [Google Scholar]
- 7.Yang PY, Ho KH, Chen HC, et al. Exercise training improves sleep quality in middle-aged and older adults with sleep problems: a systematic review. J Physiother 2012;58:157–63. 10.1016/S1836-9553(12)70106-6 [DOI] [PubMed] [Google Scholar]
- 8.Yuan Y, Chen X, Zhang L, et al. The roles of exercise in bone remodeling and in prevention and treatment of osteoporosis. Prog Biophys Mol Biol 2016;122:122–30. 10.1016/j.pbiomolbio.2015.11.005 [DOI] [PubMed] [Google Scholar]
- 9.Moore SA, Flynn D, Jones S, et al. Feasibility, acceptability, and fidelity of physical activity routines after stroke (PARAS): a multifaceted behaviour change intervention targeting free-living physical activity and sedentary behaviour in community-dwelling adult stroke survivors. Pilot Feasibility Stud 2022;8:197. 10.1186/s40814-022-01139-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ryan JM, Allen E, Gormley J, et al. The risk, burden, and management of non-communicable diseases in cerebral palsy: a scoping review. Dev Med Child Neurol 2018;60:753–64. 10.1111/dmcn.13737 [DOI] [PubMed] [Google Scholar]
- 11.Zwierzchowska A, Rosołek B, Sikora M, et al. Forced sedentariness and sports activity as factors differentiating anthropometric characteristics, indices, and body composition in people with disabilities. Biology 2022;11:906. 10.3390/biology11060906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jasińska-Mikołajczyk A, Drews K, Domaszewska K, et al. The effect of physical activity on neurotrophin concentrations and cognitive control in patients with a depressive episode. Front Psychiatry 2022;13:777394. 10.3389/fpsyt.2022.777394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lampousi AM, Berglind D, Forsell Y. Association of changes in cardiorespiratory fitness with health-related quality of life in young adults with mobility disability: secondary analysis of a randomized controlled trial of mobile APP versus supervised training. BMC Public Health 2020;20:1721. 10.1186/s12889-020-09830-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dimyan MA, Cohen LG. Neuroplasticity in the context of motor rehabilitation after stroke. Nat Rev Neurol 2011;7:76–85. 10.1038/nrneurol.2010.200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knaepen K, Goekint M, Heyman EM, et al. Neuroplasticity - exercise-induced response of peripheral brain-derived neurotrophic factor: a systematic review of experimental studies in human subjects. Sports Med 2010;40:765–801. 10.2165/11534530-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 16.Li C, Ke C, Su Y, et al. Exercise intervention promotes the growth of synapses and regulates neuroplasticity in rats with ischemic stroke through exosomes. Front Neurol 2021;12:752595. 10.3389/fneur.2021.752595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Penna LG, Pinheiro JP, Ramalho SHR, et al. Effects of aerobic physical exercise on neuroplasticity after stroke: systematic review. Arq Neuropsiquiatr 2021;79:832–43. 10.1590/0004-282X-ANP-2020-0551 [DOI] [PubMed] [Google Scholar]
- 18.Phillips C. Lifestyle modulators of neuroplasticity: how physical activity, mental engagement, and diet promote cognitive health during aging. Neural Plast 2017;2017:3589271. 10.1155/2017/3589271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Legerlotz K. The effects of resistance training on health of children and adolescents with disabilities. Am J Lifestyle Med 2020;14:382–96. 10.1177/1559827618759640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Connell ML, Coppinger T, McCarthy AL. The role of nutrition and physical activity in frailty: a review. Clin Nutr ESPEN 2020;35:1–11. 10.1016/j.clnesp.2019.11.003 [DOI] [PubMed] [Google Scholar]
- 21.van der Scheer JW, Martin Ginis KA, Ditor DS, et al. Effects of exercise on fitness and health of adults with spinal cord injury: a systematic review. Neurology 2017;89:736–45. 10.1212/WNL.0000000000004224 [DOI] [PubMed] [Google Scholar]
- 22.Fleming KM, Herring MP, Coote SB, et al. Participant experiences of eight weeks of supervised or home-based Pilates among people with multiple sclerosis: a qualitative analysis. Disabil Rehabil 2022;44:5549–56. 10.1080/09638288.2021.1939446 [DOI] [PubMed] [Google Scholar]
- 23.Forgea MC, Lyons AG, Lorenz RA. Barriers and facilitators to engagement in rehabilitation among stroke survivors: an integrative review. Rehabil Nurs 2021;46:340–7. 10.1097/RNJ.0000000000000340 [DOI] [PubMed] [Google Scholar]
- 24.Giroux EE, Casemore S, Clarke TY, et al. Enhancing participation while aging with spinal cord injury: applying behaviour change frameworks to develop intervention recommendations. Spinal Cord 2021;59:665–74. 10.1038/s41393-020-00555-8 [DOI] [PubMed] [Google Scholar]
- 25.Hickingbotham MR, Wong CJ, Bowling AB. Barriers and facilitators to physical education, sport, and physical activity program participation among children and adolescents with psychiatric disorders: a systematic review. Transl Behav Med 2021;11:1739–50. 10.1093/tbm/ibab085 [DOI] [PubMed] [Google Scholar]
- 26.Karssemeijer EGA, de Klijn FH, Bossers WJR, et al. Ranking barriers, motivators, and facilitators to promote physical activity participation of persons with dementia: an explorative study. J Geriatr Phys Ther 2020;43:71–81. 10.1519/JPT.0000000000000210 [DOI] [PubMed] [Google Scholar]
- 27.Keramat SA, Ahammed B, Mohammed A, et al. Disability, physical activity, and health-related quality of life in Australian adults: an investigation using 19 waves of a longitudinal cohort. PLoS One 2022;17:e0268304. 10.1371/journal.pone.0268304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kirk TN, Haegele JA, Zhu X. Barriers, expectancy-value beliefs, and physical activity engagement among adults with visual impairments. Adapt Phys Activ Q 2021;38:286–306. 10.1123/apaq.2019-0196 [DOI] [PubMed] [Google Scholar]
- 29.Lindsay RK, Carmichael C, Allen PM, et al. Fishing participation, motivators and barriers among UK anglers with disabilities: opportunities and implications for green social prescribing. Int J Environ Res Public Health 2022;19:4730. 10.3390/ijerph19084730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McDermott G, Brick NE, Shannon S, et al. Barriers and facilitators of physical activity in adolescents with intellectual disabilities: an analysis informed by the COM-B model. J Appl Res Intellect Disabil 2022;35:800–25. 10.1111/jar.12985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mckenzie G, Willis C, Shields N. Barriers and facilitators of physical activity participation for young people and adults with childhood-onset physical disability: a mixed methods systematic review. Dev Med Child Neurol 2021;63:914–24. 10.1111/dmcn.14830 [DOI] [PubMed] [Google Scholar]
- 32.Nightingale TE, Heneghan NR, Fenton SAM, et al. Physical activity and health-related quality of life in adults with a neurologically-related mobility disability during the COVID-19 pandemic: an exploratory analysis. Front Neurol 2021;12:699884. 10.3389/fneur.2021.699884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pedersen MRL, Hansen AF, Elmose-Østerlund K. Motives and barriers related to physical activity and sport across social backgrounds: implications for health promotion. Int J Environ Res Public Health 2021;18:5810. 10.3390/ijerph18115810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pinto SM, Newman MA, Hirsch MA. Perceived barriers to exercise in adults with traumatic brain injury vary by age. J Funct Morphol Kinesiol 2018;3:47. 10.3390/jfmk3030047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Porteny T, Alegría M, Del Cueto P, et al. Barriers and strategies for implementing community-based interventions with minority elders: positive minds-strong bodies. Implement Sci Commun 2020;1:41. 10.1186/s43058-020-00034-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schootemeijer S, van der Kolk NM, Ellis T, et al. Barriers and motivators to engage in exercise for persons with Parkinson’s disease. J Parkinsons Dis 2020;10:1293–9. 10.3233/JPD-202247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Costa RRG, Dorneles JR, Veloso JH, et al. n.d. Synchronous and asynchronous tele-exercise during the coronavirus disease 2019 pandemic: comparisons of implementation and training load in individuals with spinal cord injury. J Telemed Telecare 2021:1357633X2098273. 10.1177/1357633X20982732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hermand E, Cassirame J, Ennequin G, et al. Validation of a photoplethysmographic heart rate monitor: polar OH1. Int J Sports Med 2019;40:462–7. 10.1055/a-0875-4033 [DOI] [PubMed] [Google Scholar]
- 39.Hettiarachchi IT, Hanoun S, Nahavandi D, et al. Validation of polar OH1 optical heart rate sensor for moderate and high intensity physical activities. PLoS One 2019;14:e0217288. 10.1371/journal.pone.0217288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schubert MM, Clark A, De La Rosa AB. The polar ® OH1 optical heart rate sensor is valid during moderate-vigorous exercise. Sports Med Int Open 2018;2:E67–70. 10.1055/a-0631-0920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hamdani Y, Yee T, Oake M, et al. Multi-stakeholder perspectives on perceived wellness of special olympics athletes. Disabil Health J 2019;12:422–30. 10.1016/j.dhjo.2019.01.009 [DOI] [PubMed] [Google Scholar]
- 42.Kaveh MH, Ostovarfar J, Keshavarzi S, et al. Validation of perceived wellness survey (PWS) in a sample of Iranian population. Malays J Med Sci 2016;23:46–53. 10.21315/mjms2016.23.4.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sekiguchi Y, Curtis RM, Huggins RA, et al. The relationships between perceived wellness, sleep, and acute: chronic training load in national collegiate athletics association division I male soccer players. J Strength Cond Res 2021;35:1326–30. 10.1519/JSC.0000000000004003 [DOI] [PubMed] [Google Scholar]
- 44.Jekauc D, Voelkle M, Wagner MO, et al. Reliability, validity, and measurement invariance of the German version of the physical activity enjoyment scale. J Pediatr Psychol 2013;38:104–15. 10.1093/jpepsy/jss088 [DOI] [PubMed] [Google Scholar]
- 45.Mullen SP, Olson EA, Phillips SM, et al. Measuring enjoyment of physical activity in older adults: invariance of the physical activity enjoyment scale (paces) across groups and time. Int J Behav Nutr Phys Act 2011;8:103. 10.1186/1479-5868-8-103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murrock CJ, Bekhet A, Zauszniewski JA. Psychometric evaluation of the physical activity enjoyment scale in adults with functional limitations. Issues Ment Health Nurs 2016;37:164–71. 10.3109/01612840.2015.1088904 [DOI] [PubMed] [Google Scholar]
- 47.Teques P, Calmeiro L, Silva C, et al. Validation and adaptation of the physical activity enjoyment scale (PACES) in fitness group exercisers. J Sport Health Sci 2020;9:352–7. 10.1016/j.jshs.2017.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anderson C, Laubscher S, Burns R. Validation of the short form 36 (SF-36) health survey questionnaire among stroke patients. Stroke 1996;27:1812–6. 10.1161/01.str.27.10.1812 [DOI] [PubMed] [Google Scholar]
- 49.Knox SA, King MT. Validation and calibration of the SF-36 health transition question against an external criterion of clinical change in health status. Qual Life Res 2009;18:637–45. 10.1007/s11136-009-9467-1 [DOI] [PubMed] [Google Scholar]
- 50.Rotstein Z, Barak Y, Noy S, et al. Quality of life in multiple sclerosis: development and validation of the “ rays ” scale and comparison with the SF-36. Int J Qual Health Care 2000;12:511–7. 10.1093/intqhc/12.6.511 [DOI] [PubMed] [Google Scholar]
- 51.Wan C, Tu X, Messing S, et al. Development and validation of the general module of the system of quality of life instruments for chronic diseases and its comparison with SF-36. J Pain Symptom Manage 2011;42:93–104. 10.1016/j.jpainsymman.2010.09.024 [DOI] [PubMed] [Google Scholar]
- 52.Farrar JT, Young JP, LaMoreaux L, et al. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 2001;94:149–58. 10.1016/S0304-3959(01)00349-9 [DOI] [PubMed] [Google Scholar]
- 53.Turk DC, Rudy TE, Sorkin BA. Neglected topics in chronic pain treatment outcome studies: determination of success. Pain 1993;53:3–16. 10.1016/0304-3959(93)90049-U [DOI] [PubMed] [Google Scholar]
- 54.Farrar JT, Troxel AB, Stott C, et al. Validity, reliability, and clinical importance of change in a 0-10 numeric rating scale measure of spasticity: a post hoc analysis of a randomized, double-blind, placebo-controlled trial. Clin Ther 2008;30:974–85. 10.1016/j.clinthera.2008.05.011 [DOI] [PubMed] [Google Scholar]
- 55.Abadie BR. Effect of viewing the RPE scale on the ability to make ratings of perceived exertion. Percept Mot Skills 1996;83:317–8. 10.2466/pms.1996.83.1.317 [DOI] [PubMed] [Google Scholar]
- 56.Chung PK, Zhao Y, Liu JD, et al. A brief note on the validity and reliability of the rating of perceived exertion scale in monitoring exercise intensity among Chinese older adults in Hong Kong. Percept Mot Skills 2015;121:805–9. 10.2466/29.PMS.121c24x8 [DOI] [PubMed] [Google Scholar]
- 57.Compagnat M, Salle JY, Mandigout S, et al. Rating of perceived exertion with borg scale in stroke over two common activities of the daily living. Top Stroke Rehabil 2018;25:145–9. 10.1080/10749357.2017.1399229 [DOI] [PubMed] [Google Scholar]
- 58.Morishita S, Tsubaki A, Hotta K, et al. Relationship between the borg scale rating of perceived exertion and leg-muscle deoxygenation during incremental exercise in healthy adults. Adv Exp Med Biol 2021;1269:95–9. 10.1007/978-3-030-48238-1_15 [DOI] [PubMed] [Google Scholar]
- 59.Morishita S, Tsubaki A, Nashimoto S, et al. Face scale rating of perceived exertion during cardiopulmonary exercise test. BMJ Open Sport Exerc Med 2018;4:e000474. 10.1136/bmjsem-2018-000474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Morishita S, Tsubaki A, Takabayashi T, et al. Relationship between the rating of perceived exertion scale and the load intensity of resistance training. Strength Cond J 2018;40:94–109. 10.1519/SSC.0000000000000373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Penko AL, Barkley JE, Koop MM, et al. Borg scale is valid for ratings of perceived exertion for individuals with Parkinson’s disease. Int J Exerc Sci 2017;10:76–86. [PMC free article] [PubMed] [Google Scholar]
- 62.Skinner JS, Hutsler R, Bergsteinová V, et al. The validity and reliability of a rating scale of perceived exertion. Med Sci Sports 1973;5:94–6. [PubMed] [Google Scholar]
- 63.Cash TF, Novy PL, Grant JR. Why do women exercise? factor analysis and further validation of the reasons for exercise inventory. Percept Mot Skills 1994;78:539–44. 10.2466/pms.1994.78.2.539 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-065032supp001.pdf (33.1KB, pdf)