Abstract
Thrombosis is a common disorder with a relevant burden of morbidity and mortality worldwide, particularly among elderly patients. Growing evidence demonstrated a direct role of oxidative stress in thrombosis, with various cell types contributing to this process. Among them, erythrocytes produce high quantities of intracellular reactive oxygen species (ROS) by NADPH oxidase activation and haemoglobin autoxidation. Concomitantly, extracellular ROS released by other cells in the blood flow can be uptaken and accumulate within erythrocytes. This oxidative milieu can alter erythrocyte membrane structure, leading to an impaired erythrocyte function, and promoting erythrocytes lysis, binding to endothelial cells, activation of platelet and of coagulation factors, phosphatidylserine exposure and release of microvesicles. Moreover, these abnormal erythrocytes are able to adhere to the vessel wall, contributing to thrombin generation within the thrombus. This process results in accelerated haemolysis and in a hypercoagulable state, in which structurally impaired erythrocytes contribute to increase thrombus size, to reduce its permeability and susceptibility to lysis. However, the wide plethora of mechanisms by which oxidised erythrocytes contribute to thrombosis is not completely elucidated. This review discusses the main biochemical aspects linking erythrocytes, oxidative stress and thrombosis, addressing their potential implication for clinical and therapeutic management.
Key words: Erythrocyte, oxidative damage, oxidative stress, reactive oxygen species, redox regulation, thrombosis, venous thrombosis
Introduction
Thromboembolic events account for around one quarter of deaths worldwide, being the most frequent condition underlying myocardial infarction and ischaemic stroke. The incidence of thrombosis increases with age and its complications are among the major causes of long-term morbidity and poor quality of life, particularly in western countries (Ref. 1). Understanding the pathogenetic mechanisms of thrombosis is a major challenge to set up appropriate prophylactic interventions.
In recent years, many studies have focused on the role of oxidative stress, that is, a condition in which a massive reactive oxygen species (ROS) production overwhelms antioxidant defences, in inducing thrombosis (Refs 2–6). It is known that ROS can stimulate coagulation by increasing the expression of tissue factor in endothelial cells, monocytes and vascular smooth muscle cells, by directly interfering with platelet activation, as well as by inducing oxidative structural and functional modifications to key proteins involved in the coagulation cascade (including tissue factor pathway inhibitor, TFPI, protein C, thrombomodulin, fibrinogen, antithrombin). Moreover, ROS can mediate thrombo-inflammation, also via leucocyte (particularly neutrophil) hyperactivation and extracellular traps release (Ref. 7). Interestingly, while erythrocytes have traditionally been considered as playing a bystander role in haemostasis and thrombosis (Ref. 8), growing evidence suggests a direct involvement of these cells in ROS-induced thrombogenesis (Ref. 9).
Erythrocytes produce high amounts of intracellular ROS by NADPH oxidase activation and haemoglobin autoxidation. Moreover, erythrocytes can uptake extracellular ROS released by other cells in the blood flow. Accumulated ROS can induce structural changes to cell membrane, resulting in an impaired erythrocyte function and in the generation of a hypercoagulable milieu.
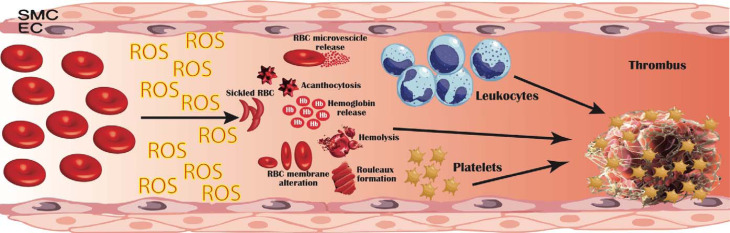
In this review, we aim to connect the dots linking erythrocytes, oxidative stress and thrombosis (Fig. 1), addressing their potential implication for the clinical and therapeutic management of thrombosis.
Fig. 1.
Pathogenetic mechanisms linking erythrocyte oxidative modifications to thrombosis. Erythrocytes produce high quantities of intracellular reactive oxygen species (ROS), mostly by NADPH oxidase activation and haemoglobin autoxidation; furthermore, extracellular ROS released by other cells in the blood flow can be uptaken and accumulate within erythrocytes. This oxidative milieu can alter erythrocyte membrane, leading to an impaired erythrocyte function and promoting erythrocytes lysis, binding to endothelial cells (EC), activation of platelet, coagulation factors and leucocytes. Moreover, structurally altered erythrocytes are able to adhere to the vessel wall, contributing to thrombin generation within thrombus. This process results in an accelerated haemolysis and in a hypercoagulable state, in which structurally impaired erythrocytes contribute to increase thrombus size and to reduce its permeability and susceptibility to lysis. EC, endothelial cells; RBC, red blood cells; ROS, reactive oxygen species; SMC, smooth muscle cells.
Erythrocytes as leading actors in thrombosis
Haematocrit and thrombosis
The concept that erythrocytes contribute to haemostasis was formulated more than a hundred years ago, based on the evidence that bleeding time in anaemic patients was prolonged also in the presence of a normal platelet count (Ref. 10), and that a negative correlation existed between haematocrit and bleeding time (Ref. 11). On the other hand, an abnormally high haematocrit, as observed in patients with polycythaemia vera or taking erythropoietin, has been associated with an increased risk of thrombosis (Ref. 12). Erythrocytes primarily influence blood viscosity, which increases in a nonlinear manner with haematocrit. Increased viscosity decelerates blood flow and is a component of the Virchow's triad leading to a prothrombotic state (Ref. 13). Indeed, haematocrit-related blood viscosity influences the interaction between platelets and blood vessel surfaces, with a remarkable rheological effect. Indeed, erythrocytes generally move down the centre of blood vessels, while platelets occupy marginal positions, to easily adhere at sites of vessel-wall injury (Ref. 14). In the presence of abnormally high haematocrit, platelets tend to accumulate near the vessel wall with arterial shear rates, increasing their interactions with the activated endothelium (Ref. 15). In vessels of small calibre, erythrocytes may aggregate and concentrate along the flow axis, thus further resulting in platelet margination. Moreover, as erythrocytes have a lower viscosity compared to platelets (Ref. 16), an increased haematocrit determines a reduced local viscosity (Ref. 17), which results in a decreased wall shear stress and a lower local nitric oxide (NO) release (Ref. 18). As NO prevents the activation of endothelial cells and platelets, this leads to cellular activation in a pro-thrombotic sense.
Also, at low shear rates, the peculiar erythrocyte morphology allows electrostatic interactions and cell aggregation into piled-up ‘rouleaux’ structures, which cause an increased viscosity and hydrodynamic resistance (Ref. 19). This phenomenon is more common in larger venous vessels at lower shear rates, such as in the lower limbs, which indeed are an elective site of venous thrombosis (Ref. 20). Notably, fibrinogen is essential for the formation of rouleaux under low shear conditions (Ref. 21) as it is able to bridge nearby cells, stimulating aggregates formation; the connection between fibrinogen and erythrocytes seems to be mediated by an integrin receptor on erythrocytes membrane, the β3 integrin (Ref. 22) and/or the integrin-associated protein (CD47) (Ref. 23).
Erythrocyte structure and thrombosis
Even when haematocrit is within physiological ranges, erythrocytes can promote a pro-thrombotic state following structural and functional cell alterations. Erythrocytes are uniquely deformable cells with a characteristic biconcave shape capable of undergoing reversible shape changes into a bullet-like shape each time they pass inside microvessels. This morphology is essential to guarantee oxygen/carbon dioxide exchange between tissues and blood; indeed, by maximising the active contact area between erythrocytes and the vessel wall, as a result of erythrocyte deformation and high surface-to-volume ratio, gas exchange is optimised (Ref. 24).
In some diseases, including sickle cell disease, β-thalassemia, haemolytic anaemias and hereditary stomatocytosis, as well as in chronic conditions such as diabetes, hypertension and coronary heart disease, erythrocytes show more rigid and less deformable structure (Refs 25, 26). This results in a lower ability to squeeze through capillaries and in an increased platelet margination, contributing to a prothrombotic state (Refs 27, 28).
Also, in sickle cell disease and β-thalassemia, the damaged erythrocyte membrane externalises phosphatidylserine, a negatively charged phospholipid which is physiologically located on the cytoplasmic side of the membrane. Phosphatidylserine exposure provides an active surface for prothrombin activation, determining a high thrombotic potential (Ref. 29).
When exposed to high shear rates, inflammation, or in the above-mentioned diseases, erythrocytes can also generate microscopic extracellular membranous structures named microvesicles or microparticles, as a result of apoptosis activation or aging (Ref. 30).
Microparticles enhance thrombin generation via the expression of phosphatidylserine and tissue factor, via the internalisation of free haeme and its transfer to vascular endothelium, as well as via the amplification of systemic inflammation through thrombin-dependent complement activation (Ref. 31).
Erythrocytes and clot structure
Erythrocytes not only influence clot formation but also clot structure. Growing evidence shows that erythrocytes may be integrated into the thrombus, through unique liaisons with activated endothelial cells and/or exhibited subendothelial matrix (Ref. 32). Under normal circumstances, mature erythrocytes are not able to interface with endothelium; conversely, structurally and functionally altered erythrocytes (as observed in sickle cell disease, malaria or diabetes) show an increased stickiness and adhesion to the vascular endothelium, contributing to microvascular occlusions associated with thrombosis (Ref. 33). Incorporation of erythrocytes in the thrombus influences fibrin network by increasing fibre diameter thus impacting on the viscoelastic clot properties (Ref. 34). In contracted clots and thrombi, erythrocytes have been shown to undergo a shape transformation from their native biconcave shape to a close-packed polyhedral structures covered by platelets and fibrin (polyhedrocytes) (Ref. 35). Polyhedrocytes have been reported in coronary arterial thrombi from patients after myocardial infarction (Ref. 35) and in pulmonary embolia (Ref. 36). This structure decreases clot permeability to fibrinolytic agents, thereby increasing its resistance to lysis.
Interestingly, it has been suggested that erythrocytes can display also antithrombotic properties. In particular, haemoglobin deoxygenation is followed by an allosteric transition stimulating NO release from cysteine β93 of haemoglobin, with consequent capillary and postcapillary venules dilatation and inhibition of platelet reactivity (Ref. 37). Moreover, ATP released from erythrocytes at low pH/reduced PO2 conditions or shear stress, can stimulate the activation of endothelial cell purinergic receptors, increasing NO production (Ref. 38). Also, it has been demonstrated that erythrocyte expression of ectoenzyme degrading ADP to AMP exerts antithrombotic properties by suppressing platelet aggregation (Ref. 39).
Therefore, erythrocytes structural and functional integrity displays critical roles in physiological haemostasis and thrombosis.
Erythrocyte and platelet interactions
Erythrocytes interact with platelets via different mechanisms. As previously described, erythrocytes exert a rheological effect, concentrating along the flow axis and causing platelet margination (Refs 16, 17). As a consequence, platelets are in close contact with the vessel wall, where they can interact with other clotting factors.
Moreover, as erythrocytes have a lower viscosity compared to platelets (Ref. 16), an increased haematocrit determines a reduced local viscosity (Fahraeus effect), except in capillaries that are smaller than erythrocytes, where the viscosity increases because of the presence of platelets (Ref. 17).
The reduced viscosity near the vessel wall determines a decreased wall shear stress and a reduced NO release (Ref. 18), leading to cellular activation in a pro-thrombotic sense.
Erythrocytes can interact directly with platelets at venous shear rates, although erythrocyte-platelet binding has been described also in the so-called ‘white’ arterial thrombi mainly composed of activated platelets and fibrin (Ref. 40).
Beside straight adhesive interactions (Refs 41, 42), erythrocytes can stimulate platelet degranulation and aggregation via chemical signalling, (i.e. through the release of ATP and ADP under low pO2 and low pH), as well as through the action of extracellular haemoglobin released from damaged erythrocytes (Ref. 43). Indeed, haemoglobin is a strong NO scavenger, and the release of extracellular haemoglobin from damaged erythrocytes determines a reduction in NO bioavailability, thus preventing the suppressive effect of NO on platelet activation (Ref. 43). Concomitantly, the release of arginase from damaged erythrocytes determines the cleavage of L-arginine, a substrate for NO production (Ref. 43).
Oxidative stress and thrombosis
In the last years, a prominent role of oxidative stress in regulating both endothelial dysfunction and thrombus formation is emerging.
The importance of oxidative stress in thrombogenesis was first demonstrated in an experimental mice model of thrombosis (mice lacking functional eNOS), where NO deficiency was significantly associated to arterial thrombosis. These mice showed lower bleeding times if compared to wild-type animals (Ref. 44). Later on, it has been shown that a moderate iron overburden significantly stimulates thrombus formation, via a defective vasoreactivity as well as via an enhanced ROS production (Ref. 45).
ROS interfere with pro- and anticoagulant molecules
ROS can interfere with the coagulation process via a plethora of multiple, interconnected mechanisms. ROS, mostly generated by NOX enzymes, can directly stimulate the coagulation cascade by increasing the expression of tissue factor in endothelial cells, monocytes and vascular smooth muscle cells (Refs 46–48). ROS can also promote a procoagulant state via oxidative modification of proteins involved in the coagulation pathway, such as the anticoagulant proteins protein C (Ref. 49), thrombomodulin (Ref. 50) and the TFPI, resulting in their inactivation (Ref. 51). Indeed, in mice models lacking superoxide dismutase (SOD-/- mice), larger, rapidly growing venous thrombi were observed, due to an impaired SOD1-mediated protein C activation (Ref. 52). Also, the heparin-binding capacity of antithrombin is decreased following oxidation by hydrogen peroxide (Ref. 53) or lipid peroxide (Ref. 54).
Furthermore, lipid oxidation can inactivate the anticoagulant function of protein Z-dependent protease inhibitor, a specific inhibitor of membrane-associated factor Xa (FXa) (Ref. 55).
Similarly, it has been observed that leucocyte-produced ROS can oxidise fibrinogen, altering its secondary structure and the overall clot architecture, characterised by reduced porosity and by tight fibrin network with filaments of decreased average size. Also, these oxidative alterations result in an impaired fibrinogen function, both in terms of thrombin-catalysed fibrin polymerisation and fibrin susceptibility to plasmin-induced lysis. This mechanism has been linked to increased thrombosis risk in Behcet's syndrome (Refs 4, 56), cirrhosis (Ref. 5), and it has been also described in post-acute myocardial infarction (Ref. 6), pulmonary hypertension (Ref. 57) and pulmonary embolism (Refs 58, 59).
ROS and platelets
Besides affecting the activity of pro- or anti-coagulant molecules through oxidative modification, ROS can also directly interfere with platelets and other cells involved in haemostasis and thrombosis.
Intraplatelet ROS can activate platelets, by oxidising arachidonic acid, generating isoprostanes (Ref. 60); this mechanism has been linked with an increased risk of deep venous thrombosis in patients with hypercholesterolaemia (Ref. 61), diabetes mellitus (Ref. 62), homozygous homocystinuria (Ref. 63) and in obese women (Ref. 64). Concomitantly, ROS can also indirectly promote platelet activation by negatively regulating mechanisms of platelet inhibition, such as NO scavenging (Ref. 65). In hyperhomocysteinemia superoxide formation by hyperactive platelets has been described as one of the key pathways contributing to arterial thrombosis in this condition (Ref. 66).
ROS and leucocytes
ROS also modulate platelet-leucocyte interactions: ROS produced by NOX2 can affect the expression of P-selectin (CD62) and CD40L, that are transferred to the platelet surface upon activation. P-selectin and CD40L promote leucocyte recruitment and activation (Refs 67, 68) and their levels are associated with an increased risk of venous thromboembolism in various conditions (Ref. 69), such as in Behçet's syndrome (Refs 67, 68). Concomitantly, ROS can induce leucocyte recruitment via different complementary mechanisms: they can directly act as a chemoattractant for neutrophils and monocytes, mostly via upregulation of IL-8 (Ref. 70) and of monocyte chemotactic protein-1 (MCP-1) production, respectively (Ref. 71). Moreover, they can increase the expression of leucocyte adhesion molecule expression (such as platelet-endothelial cell adhesion molecules-1, PECAM-1) and promote leucocyte endothelial adhesion (Ref. 72).
Also, ROS can activate mast cells, which on their turn produce ROS, mostly via NOX2, with consequent redox-sensitive calcium channels activation, increase in cytoplasmic calcium concentrations required for the induction of mast cell degranulation (Ref. 73) and leucocyte recruitment and activation (Ref. 74). The leucocyte-ROS axis is particularly relevant in the process of thrombo-inflammation, which sustains thrombotic events in various immune-mediated conditions such as thrombosis in Behçet's syndrome (Ref. 4).
In Behçet's syndrome, ROS have been shown to stimulate neutrophils to release extracellular traps (NETs) (Ref. 7). NETs are structures composed of cell-free DNA, histones, microbicidal proteins and proteases, that are extruded by dead neutrophils, mostly by low-density granulocytes (LDGs), following infective or inflammatory stimuli (Ref. 75). NETs can directly induce thrombogenesis (Ref. 76), by activating the intrinsic and extrinsic coagulation pathways, and by enhancing thrombin production in plasma, probably via histone/polyphosphate triggering (Ref. 76). Concomitantly, NETs can stimulate neutrophils to further produce ROS, in a self-sustaining process.
Also, in Behçet's syndrome, leucocyte ROS levels have been correlated with a peculiar profile of circulating miRNAs (i.e. small non-coding RNAs that act as post-transcriptional regulators of gene expression) affecting pathways related to cell-matrix interaction, oxidative stress and blood coagulation (Refs 77, 78), suggesting a contribution of epigenetic mechanisms in ROS-induced thrombo-inflammation.
Connecting the dots: the erythrocyte-ROS axis in thrombosis
As described in the previous paragraphs, erythrocyte can contribute to thrombogenesis via different mechanisms and growing studies suggest a key role of oxidative stress in linking erythrocytes to thrombosis (Supplementary Table 1).
Erythrocytes have a plethora of enzymatic (e.g. superoxide dismutase, catalase, glutathione peroxidase and peroxiredoxin-2 (PRDX-2)) and non-enzymatic antioxidant defences. Among the latter, reduced glutathione (GSH) is a ubiquitous intracellular antioxidant which inhibits free radical formation and more generally acts as a redox buffer, detoxifier and chemokine scavenger. Erythrocytes can export GSH at a constant rate of ~ 21 nmol/h/ml erythrocytes, contributing to the extracellular GSH reservoir (Ref. 79). GSH is synthesised de novo from cysteine, glycine and glutamate by the enzymes, γ-L-glutamate L-cysteine ligase and glutathione synthetase (Ref. 79). Reduced GSH concentration has been reported in various conditions characterised by an increased cardiovascular risk, such as diabetes mellitus (Ref. 80), hypertension (Ref. 81), haemodialysis and peritoneal dialysis (Ref. 82), and is considered as an indicator of an impaired oxidative stress.
Within erythrocytes, oxidative stress can be sustained by ROS released from neutrophils and macrophages into the plasma and taken up by erythrocytes, particularly in microcirculation, where the erythrocytes are in close contact with the vasculature (Ref. 83). Also, erythrocyte also contains NADPH oxidases, which can generate endogenous ROS (Ref. 84). Endogenous and exogenous ROS induce oxidation of iron contained in haemoglobin, from Fe2+ containing haemoglobin to Fe3+-containing methaemoglobin.
Fe3+ induces iron-dependent free radical generation (Fenton reaction) which causes lipid peroxidation, haemolysis and endothelial perturbation. This triggers a haemolysis/oxidative cycle, which promotes vascular injury, thrombus formation and atherothrombotic events (Ref. 85) as observed in severe haemolytic syndromes (Ref. 85).
The oxidised Fe3+ methaemoglobin can be converted back into the reduced form by a cytochrome b5 reductase. However, if the reducing equivalents for this enzyme are scarce, haeme is further degraded to quaternary compounds with consequent ROS formation (Ref. 86).
ROS damage erythrocyte membrane (Ref. 87), reduce cell deformability and induce cell lysis, by triggering a molecular signalling cascade with the activation of Ca2+ permeable cation channel (Ref. 88). The influx of Ca2+ activates Ca2+-sensitive K+ channels, leading to phosphatidylserine exposure on the erythrocyte membrane (Ref. 88). This provides an active surface for prothrombin activation: it has been postulated that even a small fraction of erythrocytes exposing phosphatidylserine can lead to thrombin generation, accounting for up to 30–40% of the thrombin-generating potential of whole blood (Ref. 89). Notably, in a mouse model of sickle cell disease, reducing erythrocyte ROS production with manganese porphyrins, which suppress erythrocyte NOX activity (Ref. 90) was found to result in a reduced phosphatidylserine exposure and improved eryptosis (Ref. 91).
Beside directly stimulating thrombin generation, phosphatidylserine exposure on the erythrocyte membrane stimulates the release of microvesicles (Ref. 92) with a high thrombotic potential (Ref. 29), as previously described and considered a promising target for the treatment of thrombotic disorders (Ref. 93). Oxidation-induced damage on erythrocyte membrane further induces haemolysis. Under physiological conditions, the release of free haemoglobin and haeme can be inactivated by plasma haptoglobin and hemopexin (Refs 94, 95) leading to their phagocytosis (Ref. 96). Conversely, oxidised haemoglobin has a low affinity for haptoglobin, resulting in an impaired plasma clearance and in an increased release of haeme and iron (Ref. 97). Free redox-active haeme translocate into endothelial cells, triggering H2O2-mediated endothelial damage and overwhelming intracellular antioxidant defences.
Moreover, extracellular haeme derived from lysed erythrocytes mediates additional pro-thrombotic mechanisms: it stimulates neutrophil recruitment and NETosis (Ref. 98), as observed in sicke cell disease (Ref. 98) and promotes NLRP3 inflammasome activation and cytokine and lipid mediator production in macrophages (Ref. 99) which have been shown to potentiate venous thrombosis (Ref. 100). Specifically, free haemoglobin and haeme can stimulate the nuclear factor κB (NF-κB) under the control of a Toll-like receptor (TLR)-signalling pathway (Refs 101, 102) leading to the activation of hypoxia-inducible factor (HIF)-1α and HIF-2α (Ref. 103) which further induce inflammation, vasoconstriction and increase endothelial permeability (Ref. 103).
Furthermore, free haemoglobin can upregulate the expression of functional tissue factors in macrophages and desensitises tissue factor to the effects of antioxidants, such as glutathione or serum (Ref. 104). Also, it can scavenge NO, thereby impairing its regulatory effects on vasocostriction, endothelial adhesion molecule expression and platelet activation and aggregation, in a pro-thrombotic sense (Ref. 105). Free haeme can induce platelet activation also by binding to glycoprotein-1b alpha (GPIbα) on platelets (Ref. 106), as well as through C-type lectin-like receptor-2 (CLEC-2) (Ref. 107).
A direct role of erythrocyte oxidative stress has been described in retinal vein occlusion, a condition characterised by vision loss resulting from hypoperfusion and hypoxia of the retina. Increased erythrocyte oxidative stress levels were found in patients with retinal vein occlusion; also, erythrocyte-derived ROS and erythrocyte lipid peroxidation were found to positively correlate with erythrocyte membrane viscosity and deformability (Ref. 108).
Similarly, in patients with cochlear vascular occlusion leading to sudden sensorineural hearing loss, a significant structural and functional involvement of erythrocyte membrane alterations was found, associated with enhanced levels of membrane lipid peroxidation and intracellular ROS production. Notably, in vitro experiments demonstrated that ROS display a critical role in impairing erythrocyte membrane fluidity (Ref. 109).
Of major note, ROS-induced erythrocyte modifications are particularly relevant during aging. An age-dependent increase in erythrocyte oxidative stress markers paralleled by an age-dependent decline in the total plasma antioxidant capacity has been reported (Refs 110–112). In rat models, an increase in plasma membrane redox system activity, lipid peroxidation and erythrocyte malondialdehyde has been reported in senescent erythrocytes, paired by a reduced L-cysteine influx and a consequent decrease in intracellular GSH (Ref. 113).
Beside erythrocytes, also platelets exhibit a progressive impairment in redox status during aging, with a marked increase in oxidative stress, hyperactivation and apoptotic markers, although this trend is reverted in old subjects (80–100 years) (Ref. 114). Accordingly, erythrocyte and platelet oxidative stress has been suggested as one of the major mechanisms sustaining the pathogenesis of thrombotic events during aging, with potentially relevant implications in terms of thrombotic prophylaxis and treatment (Refs 115, 116). In aging rat models, rapamycin, particularly when combined with metformin, was found to be a promising age-delaying agent, able to restore altered levels of redox biomarkers in erythrocytes (Refs 117, 118).
Therapeutic implications
Understanding the role of the erythrocyte-oxidative stress axis in inducing thrombosis offers the possibility of setting up new prophylactic strategies for cardiovascular preventions (Table 1).
Table 1.
Therapeutic implications
| Therapeutic interventions | Main protective action against ROS-induced thrombosis |
|---|---|
| Pharmacological therapies | |
| Antihypertensive agents: Angiotensin-converting enzyme (ACE) inhibitors | Block the conversion of Ang I to Ang II, which induces endothelial dysfunction via promoting leucocytes recruitment, ROS production, LDL oxidation and NO degradation |
| Lipid-lowering agents: Statins | Block Rho and Rac activation (a major source of ROS production in vasculature), thus reducing endothelial activation, while increasing the expression of eNOS and the endothelial production of the vasorelaxant NO. Contribute to resolution of venous thrombi. |
| Antiplatelets (aspirin) and anticoagulants (Xa inhibitor, rivaroxaban) | Reduce NOX2-mediated platelet ROS production |
| Thioredoxin inhibitors | Attenuate platelet function and thrombus formation |
| Antidiabetic drugs (alogliptin) | Suppress stress-induced free fatty acid release, oxidative stress, adipose tissue inflammation and prothrombotic state in a dose-dependent manner, and improve insulin sensitivity |
| Non-pharmacological interventions | |
| Vitamins | |
| Vitamin E (tocopherols and tocotrienols) | Pleiotropic antithrombotic effects, reduces the expression and release of endothelial adhesion molecules, prevents leucocyte/endothelial cell interactions, inhibits smooth muscle cells proliferation, inhibits the formation of platelet-leucocytes aggregates. Lipoperoxyl radical-scavenging actions. |
| Vitamin C | Enhances endothelium-dependent vasodilation by increasing NO availability. |
| Dietary regimens | |
| Mediterranean diet | Downregulates sNOX2-dp (soluble NOX2-derived peptide) and F2-isoprostanes |
| Antioxidants (beer, red wine, olive oil) | Decrease ROS accumulation and inhibit platelet activation |
| Nattokinase (traditional Japanese food Natto) | Inhibits LPS-mediated TLR-4 and NOX2 signalling in macrophages |
| Butyrate | In Behçet syndrome, reduces ROS production and ROS-mediated fibrinogen structural and functional alternations |
Ang, angiotensin; LDL, low-density lipoprotein; LPS, Lipopolysaccharide; NO, nitric oxide; NOX, NADPH oxidase; ROS, reactive oxygen specifies; TLR, toll-like receptor.
Pharmacological therapies
Angiotensin-converting enzyme (ACE) inhibitors are among guideline-recommended first-line therapies in patients with hypertension to reduce the related risk of atherosclerotic disease and cardiovascular events. Growing evidence suggests that these agents exert cardiovascular effects that go beyond blood pressure reduction (Refs 119–121).
ACE inhibitors block the conversion of Ang I to Ang II, which induces endothelial dysfunction via promoting leucocytes recruitment and ROS production, with consequent enhanced LDL oxidation and NO degradation (Ref. 120).
Similarly, statins are lipid-lowering agents recommended in patients with hypercholesterolemia. In vitro and in vivo studies showed that statins can modulate the atherosclerotic process, through mechanisms additive to blood cholesterol reduction, that include anti-inflammatory and antioxidant actions (Refs 122–124).
Indeed, statins can interfere with leucocyte migration, proliferation and leucocyte/endothelial interactions (Ref. 125). Also, statins (particularly atorvastatin) can block Rho and Rac activation, thus reducing endothelial activation, while increasing the expression of eNOS and the endothelial production of the vasorelaxant NO. As the activation of Rho family members is a major source of ROS production in the vasculature, statins can counteract oxidative stress mechanisms which contribute to an increased risk of thrombotic events (Refs 126–128). Statins were found to contribute also to the resolution of venous thrombi, although the mechanism has not fully clarified (Ref. 129).
Similar effects have been reported for antiplatelets (aspirin), anticoagulants (Xa inhibitor, rivaroxaban), thioredoxin inhibitors (Refs 130–132) and the oral anti-diabetic drug alogliptin (Ref. 133).
Vitamins
Among non-pharmacological agents, vitamins, particularly A, C and E, are known to reduce the risk of atherosclerosis and related complications.
In vitro and in vivo studies report that vitamin E exerts pleiotropic antithrombotic effects by reducing the expression and release of endothelial adhesion molecules, preventing leucocyte/endothelial cell interactions. Also, it counteracts cholesterol-induced atherosclerotic lesions progression by inhibiting smooth muscle cells proliferation and it can inhibit the formation of platelet-leucocytes aggregates and the activation of the clotting system (Refs 134–136). Notably, natural vitamin E consists of a family of eight compounds, four tocopherols and four tocotrienols. All tocopherols and tocotrienols are potent antioxidants with lipoperoxyl radical-scavenging actions able to counteract oxidative stress. In patients with type 2 diabetes mellitus and the haptoglobin 2-2 genotype presenting increased oxidative stress levels, vitamin E was found to reduce the risk of cardiovascular events (Ref. 137); however, the cardioprotective effect of vitamin E supplementation in the general population as well as in other high-risk setting was disappointing (Refs 138–140).
Vitamin C was found to enhance endothelium-dependent vasodilation, both in normotensive and hypertensive subjects (Ref. 141), thanks to its effects on NO availability (Ref. 142). However, contrasting findings were reported on the benefits of vitamin C supplementation for cardiovascular prevention.
In another study, it was shown that vitamin C (0.5–5 mM) increased the procoagulant activity of freshly isolated human erythrocytes, particularly those from cancer patients, via the externalisation of phosphatidylserine and the formation of phosphatidylserine -bearing microvesicles. Also, in rat models, the intravenous injection of vitamin C (0.5–1.0 g/kg) significantly increased thrombosis. (Ref. 143).
Dietary regimens
Diets, especially high-fat or high-carbohydrate diets, can increase oxidative stress by elevating the levels of protein carbonylation and lipid peroxidation while impairing antioxidant defences (Ref. 144). In obese patients, insulin resistance greatly increases oxidative stress, thus contributing to the increased risk of hypertension, dyslipidaemia, type 2 diabetes, atherosclerosis and non-alcoholic fatty liver disease associated with this condition (Ref. 145).
The cardioprotective role of specific nutritional regimens has been widely investigated. In a prospective cohort study on more than seven hundred patients with atrial fibrillation, the cardioprotective role of Mediterranean diet was investigated. Results indicated that adherence to Mediterranean diet could be associated with a reduction of cardiovascular events, through an antioxidant effect, as shown by a downregulation of sNOX2-dp (soluble NOX2-derived peptide) and F2-isoprostanes during this dietary regimen (Ref. 146).
Moreover, xanthohumol contained in beer, was found to prevent arterial and venous thrombosis in mice, by decreasing ROS accumulation and inhibiting platelet activation (Ref. 147). Similar effects were suggested for antioxidants contained in red wine (Refs 148, 149) and olive oil (Ref. 150). Also, nattokinase, a serine protease from the traditional Japanese food Natto, displays anti-inflammatory and anti-oxidative stress activities by inhibiting LPS-mediated TLR-4 and NOX2 signalling in macrophages, thereby exerting a protective effect against inflammation-induced thrombosis (Ref. 151).
More recently, tailored nutritional interventions have been investigated to counteract thrombo-inflammation in peculiar chronic immune-mediated diseases, such as Behçet syndrome. Behçet syndrome displays a peculiar gut microbiota fingerprint, with an impaired production of short-chain fatty acids, especially butyrate (Ref. 152), which can exert protective effects against cardiovascular diseases (Ref. 153). Butyrate-enriched dietary interventions were recently found to reduce ROS production and ROS-mediated fibrinogen structural and functional alternations in these patients (Ref. 154) paving the way for new cardioprotective therapies in this condition.
Concluding remarks
The erythrocyte/ROS axis is involved in the regulation of various processes that promote thrombosis. An impaired redox state induces erythrocyte membrane damage, leading to membrane fluidity alterations and decreased deformability. These changes impair erythrocyte function in the haemostatic process, promoting thrombosis via haemolysis, phosphatidylserine exposure, microvescicle release, induction of platelet activation and aggregation and vascular injury. Oxidised erythrocytes not only promote thrombus formation but also contribute to increase its size and to reduce its permeability and susceptibility to lysis and studies have suggested that the role of erythrocytes is particular once the thrombogenetic process has started and erythrocytes are entrapped within the growing thrombus (Ref. 155). However, the wide plethora of mechanisms by which oxidised erythrocytes contribute to thrombosis is not completely elucidated.
Deeping current knowledge on the mechanisms linking ROS and erythrocytes and their crosstalk with leucocytes, platelets and pro- and anti-coagulant molecules will pave the way to new therapeutic strategies for reducing thrombosis risk, particularly in conditions characterised by a sustained thrombo-inflammatory milieu.
Acknowledgements
All people who contributed to this work are listed as co-authors.
Supplementary material
For supplementary material accompanying this paper visit http://doi.org/10.1017/erm.2022.25.
click here to view supplementary material
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of interest
All authors declare none.
Ethical standards
Not applicable.
References
- 1.Wendelboe AM and Raskob GE (2016) Global burden of thrombosis: epidemiologic aspects. Circulation Research 118, 1340–1347. [DOI] [PubMed] [Google Scholar]
- 2.Emmi G et al. (2015) Thrombosis in vasculitis: from pathogenesis to treatment. Thrombosis Journal 13, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Becatti M et al. (2015) Protection of coronary endothelial cells from cigarette smoke-induced oxidative stress by a new Mn(II)-containing polyamine-polycarboxilate scavenger of superoxide anion. Vascular Pharmacology 75, 19–28. [DOI] [PubMed] [Google Scholar]
- 4.Becatti M et al. (2016) Neutrophil activation promotes fibrinogen oxidation and thrombus formation in Behcet disease. Circulation 133, 302–311. [DOI] [PubMed] [Google Scholar]
- 5.Becatti M et al. (2020) Super-Resolution microscopy reveals an altered fibrin network in cirrhosis: the key role of oxidative stress in fibrinogen structural modifications. Antioxidants (Basel) 9, 737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Becatti M et al. (2014) Oxidative modification of fibrinogen is associated with altered function and structure in the subacute phase of myocardial infarction. Arteriosclerosis Thrombosis and Vascular Biology 34, 1355–1361. [DOI] [PubMed] [Google Scholar]
- 7.Bettiol A et al. (2021) Neutrophil-mediated mechanisms of damage and in-vitro protective effect of colchicine in non-vascular Behcet's syndrome. Clinical and Experimental Immunology 206, 410–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weisel JW and Litvinov RI (2019) Red blood cells: the forgotten player in hemostasis and thrombosis. Journal of Thrombosis and Haemostasis: JTH 17, 271–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barshtein G, Ben-Ami R and Yedgar S (2007) Role of red blood cell flow behavior in hemodynamics and hemostasis. Expert Review of Cardiovascular Therapy 5, 743–752. [DOI] [PubMed] [Google Scholar]
- 10.Duke WW (1983) The relation of blood platelets to hemorrhagic disease. By W.W. Duke. JAMA 250, 1201–1209. [PubMed] [Google Scholar]
- 11.Hellem AJ, Borchgrevink CF and Ames SB (1961) The role of red cells in haemostasis: the relation between haematocrit, bleeding time and platelet adhesiveness. British Journal of Haematology 7, 42–50. [DOI] [PubMed] [Google Scholar]
- 12.Marchioli R et al. (2013) Cardiovascular events and intensity of treatment in polycythemia vera. New England Journal of Medicine 368, 22–33. [DOI] [PubMed] [Google Scholar]
- 13.Wolberg AS et al. (2012) Procoagulant activity in hemostasis and thrombosis: Virchow's triad revisited. Anesthesia & Analgesia 114, 275–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aarts PA et al. (1988) Blood platelets are concentrated near the wall and red blood cells, in the center in flowing blood. Arteriosclerosis (Dallas, Tex.) 8, 819–824. [DOI] [PubMed] [Google Scholar]
- 15.Goldsmith HL et al. (1995) Physical and chemical effects of red cells in the shear-induced aggregation of human platelets. Biophysical Journal 69, 1584–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dintenfass L (1967) Inversion of the Fahraeus-Lindqvist phenomenon in blood flow through capillaries of diminishing radius. Nature 215, 1099–1100. [DOI] [PubMed] [Google Scholar]
- 17.Fahraeus R (1958) The influence of the rouleau formation of the erythrocytes on the rheology of the blood. Acta Medica Scandinavica 161, 151–165. [PubMed] [Google Scholar]
- 18.Baskurt OK et al. (2004) Modulation of endothelial nitric oxide synthase expression by red blood cell aggregation. American Journal of Physiology. Heart and Circulatory Physiology 286, H222–H229. [DOI] [PubMed] [Google Scholar]
- 19.Piety NZ et al. (2016) Shape matters: the effect of red blood cell shape on perfusion of an artificial microvascular network. Transfusion 56, 844–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu FT et al. (2011) A local increase in red blood cell aggregation can trigger deep vein thrombosis: evidence based on quantitative cellular ultrasound imaging. Journal of Thrombosis and Haemostasis: JTH 9, 481–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lominadze D and Dean WL (2002) Involvement of fibrinogen specific binding in erythrocyte aggregation. FEBS Letters 517, 41–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carvalho FA et al. (2010) Atomic force microscopy-based molecular recognition of a fibrinogen receptor on human erythrocytes. ACS Nano 4, 4609–4620. [DOI] [PubMed] [Google Scholar]
- 23.De Oliveira S et al. (2012) Integrin-associated protein (CD47) is a putative mediator for soluble fibrinogen interaction with human red blood cells membrane. Biochimica et Biophysica Acta 1818, 481–490. [DOI] [PubMed] [Google Scholar]
- 24.Danielczok JG et al. (2017) Red blood cell passage of small capillaries is associated with transient Ca(2 + )-mediated adaptations. Frontiers in Physiology 8, 979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vaya A et al. (2011) Rheological red blood cell behaviour in minor alpha-thalassaemia carriers. Clinical Hemorheology and Microcirculation 48, 241–246. [DOI] [PubMed] [Google Scholar]
- 26.Symeonidis A et al. (2001) Impairment of erythrocyte viscoelasticity is correlated with levels of glycosylated haemoglobin in diabetic patients. Clinical and Laboratory Haematology 23, 103–109. [DOI] [PubMed] [Google Scholar]
- 27.Aarts PA et al. (1986) Increased red blood cell deformability due to isoxsuprine administration decreases platelet adherence in a perfusion chamber: a double-blind cross-over study in patients with intermittent claudication. Blood 67, 1474–1481. [PubMed] [Google Scholar]
- 28.van Gelder JM, Nair CH and Dhall DP (1996) Erythrocyte aggregation and erythrocyte deformability modify the permeability of erythrocyte enriched fibrin network. Thrombosis Research 82, 33–42. [DOI] [PubMed] [Google Scholar]
- 29.Ataga KI, Cappellini MD and Rachmilewitz EA (2007) Beta-thalassaemia and sickle cell anaemia as paradigms of hypercoagulability. British Journal of Haematology 139, 3–13. [DOI] [PubMed] [Google Scholar]
- 30.Heijnen HF et al. (1999) Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood 94, 3791–3799. [PubMed] [Google Scholar]
- 31.Zecher D, Cumpelik A and Schifferli JA (2014) Erythrocyte-derived microvesicles amplify systemic inflammation by thrombin-dependent activation of complement. Arteriosclerosis Thrombosis and Vascular Biology 34, 313–320. [DOI] [PubMed] [Google Scholar]
- 32.Silvain J et al. (2014) Impact of red blood cell transfusion on platelet aggregation and inflammatory response in anemic coronary and noncoronary patients: the TRANSFUSION-2 study (impact of transfusion of red blood cell on platelet activation and aggregation studied with flow cytometry use and light transmission aggregometry). Journal of the American College of Cardiology 63, 1289–1296. [DOI] [PubMed] [Google Scholar]
- 33.Smith JD et al. (2013) Malaria's deadly grip: cytoadhesion of Plasmodium falciparum-infected erythrocytes. Cellular Microbiology 15, 1976–1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gersh KC, Nagaswami C and Weisel JW (2009) Fibrin network structure and clot mechanical properties are altered by incorporation of erythrocytes. Thrombosis and Haemostasis 102, 1169–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cines DB et al. (2014) Clot contraction: compression of erythrocytes into tightly packed polyhedra and redistribution of platelets and fibrin. Blood 123, 1596–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Litvinov RI et al. (2018) Morphological signs of intravital contraction (Retraction) of pulmonary thrombotic emboli. BioNanoScience 8, 428–433. [Google Scholar]
- 37.Sun CW et al. (2019) Hemoglobin beta93 cysteine is not required for export of nitric oxide bioactivity from the red blood cell. Circulation 139, 2654–2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kawai Y et al. (2010) Shear stress-induced ATP-mediated endothelial constitutive nitric oxide synthase expression in human lymphatic endothelial cells. American Journal of Physiology. Cell Physiology 298, C647–C655. [DOI] [PubMed] [Google Scholar]
- 39.Netsch P et al. (2018) Human mesenchymal stromal cells inhibit platelet activation and aggregation involving CD73-converted adenosine. Stem Cell Research & Therapy 9, 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Silvain J et al. (2011) Composition of coronary thrombus in acute myocardial infarction. Journal of the American College of Cardiology 57, 1359–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Turitto VT and Baumgartner HR (1975) Platelet interaction with subendothelium in a perfusion system: physical role of red blood cells. Microvascular Research 9, 335–344. [DOI] [PubMed] [Google Scholar]
- 42.Santos MT et al. (1991) Enhancement of platelet reactivity and modulation of eicosanoid production by intact erythrocytes. A new approach to platelet activation and recruitment. Journal of Clinical Investigation 87, 571–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Helms CC et al. (2013) Mechanisms of hemolysis-associated platelet activation. Journal of Thrombosis and Haemostasis: JTH 11, 2148–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Freedman JE et al. (1999) Deficient platelet-derived nitric oxide and enhanced hemostasis in mice lacking the NOSIII gene. Circulation Research 84, 1416–1421. [DOI] [PubMed] [Google Scholar]
- 45.Barr JD et al. (2013) Red blood cells mediate the onset of thrombosis in the ferric chloride murine model. Blood 121, 3733–3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Golino P et al. (1996) Effects of tissue factor induced by oxygen free radicals on coronary flow during reperfusion. Nature Medicine 2, 35–40. [DOI] [PubMed] [Google Scholar]
- 47.Cadroy Y et al. (2000) Polymorphonuclear leukocytes modulate tissue factor production by mononuclear cells: role of reactive oxygen species. Journal of Immunology 164, 3822–3828. [DOI] [PubMed] [Google Scholar]
- 48.Herkert O et al. (2002) NADPH oxidase mediates tissue factor-dependent surface procoagulant activity by thrombin in human vascular smooth muscle cells. Circulation 105, 2030–2036. [DOI] [PubMed] [Google Scholar]
- 49.Nalian A and Iakhiaev AV (2008) Possible mechanisms contributing to oxidative inactivation of activated protein C: molecular dynamics study. Thrombosis and Haemostasis 100, 18–25. [DOI] [PubMed] [Google Scholar]
- 50.Glaser CB et al. (1992) Oxidation of a specific methionine in thrombomodulin by activated neutrophil products blocks cofactor activity. A potential rapid mechanism for modulation of coagulation. Journal of Clinical Investigation 90, 2565–2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ohkura N et al. (2004) Oxidized phospholipids in oxidized low-density lipoprotein reduce the activity of tissue factor pathway inhibitor through association with its carboxy-terminal region. Antioxidants & Redox Signaling 6, 705–712. [DOI] [PubMed] [Google Scholar]
- 52.Dayal S et al. (2015) Deficiency of superoxide dismutase impairs protein C activation and enhances susceptibility to experimental thrombosis. Arteriosclerosis Thrombosis and Vascular Biology 35, 1798–1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Van Patten SM et al. (1999) Oxidation of methionine residues in antithrombin. Effects on biological activity and heparin binding. Journal of Biological Chemistry 274, 10268–10276. [DOI] [PubMed] [Google Scholar]
- 54.Gray E and Barrowcliffe TW (1985) Inhibition of antithrombin III by lipid peroxides. Thrombosis Research 37, 241–250. [DOI] [PubMed] [Google Scholar]
- 55.Huang X et al. (2019) Protein Z-dependent protease inhibitor (ZPI) is a physiologically significant inhibitor of prothrombinase function. Journal of Biological Chemistry 294, 7644–7657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Becatti M et al. (2019) Behcet's syndrome as a tool to dissect the mechanisms of thrombo-inflammation: clinical and pathogenetic aspects. Clinical and Experimental Immunology 195, 322–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Miniati M et al. (2010) Fibrin resistance to lysis in patients with pulmonary hypertension other than thromboembolic. American Journal of Respiratory and Critical Care Medicine 181, 992–996. [DOI] [PubMed] [Google Scholar]
- 58.Cellai AP et al. (2013) Fibrinolytic inhibitors and fibrin characteristics determine a hypofibrinolytic state in patients with pulmonary embolism. Thrombosis and Haemostasis 109, 565–567. [DOI] [PubMed] [Google Scholar]
- 59.Lami D et al. (2014) Residual perfusion defects in patients with pulmonary embolism are related to impaired fibrinolytic capacity. Thrombosis Research 134, 737–741. [DOI] [PubMed] [Google Scholar]
- 60.Pignatelli P et al. (2011) Inherited human gp91phox deficiency is associated with impaired isoprostane formation and platelet dysfunction. Arteriosclerosis Thrombosis and Vascular Biology 31, 423–434. [DOI] [PubMed] [Google Scholar]
- 61.Davi G et al. (1997) In vivo formation of 8-Epi-prostaglandin F2 alpha is increased in hypercholesterolemia. Arteriosclerosis Thrombosis and Vascular Biology 17, 3230–3235. [DOI] [PubMed] [Google Scholar]
- 62.Davi G et al. (1999) In vivo formation of 8-iso-prostaglandin f2alpha and platelet activation in diabetes mellitus: effects of improved metabolic control and vitamin E supplementation. Circulation 99, 224–229. [DOI] [PubMed] [Google Scholar]
- 63.Davi G et al. (2001) Oxidative stress and platelet activation in homozygous homocystinuria. Circulation 104, 1124–1128. [DOI] [PubMed] [Google Scholar]
- 64.Davi G et al. (2002) Platelet activation in obese women: role of inflammation and oxidant stress. JAMA 288, 2008–2014. [DOI] [PubMed] [Google Scholar]
- 65.Tajima M and Sakagami H (2000) Tetrahydrobiopterin impairs the action of endothelial nitric oxide via superoxide derived from platelets. British Journal of Pharmacology 131, 958–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Riba R et al. (2004) Altered platelet reactivity in peripheral vascular disease complicated with elevated plasma homocysteine levels. Atherosclerosis 175, 69–75. [DOI] [PubMed] [Google Scholar]
- 67.Turkoz Y et al. (2005) Serum levels of soluble P-selectin are increased and associated with disease activity in patients with Behcet's syndrome. Mediators of Inflammation 2005, 237–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Martinez M et al. (2007) Platelet activation and red blood cell phosphatidylserine exposure evaluated by flow cytometry in patients with Behcet's disease: are they related to thrombotic events? Pathophysiology of Haemostasis and Thrombosis 36, 18–22. [DOI] [PubMed] [Google Scholar]
- 69.Ay C et al. (2007) High concentrations of soluble P-selectin are associated with risk of venous thromboembolism and the P-selectin Thr715 variant. Clinical Chemistry 53, 1235–1243. [DOI] [PubMed] [Google Scholar]
- 70.Miyoshi T et al. (2010) The role of endothelial interleukin-8/NADPH oxidase 1 axis in sepsis. Immunology 131, 331–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee YW, Lee WH and Kim PH (2010) Role of NADPH oxidase in interleukin-4-induced monocyte chemoattractant protein-1 expression in vascular endothelium. Inflammation Research 59, 755–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rattan V et al. (1997) Oxidant stress-induced transendothelial migration of monocytes is linked to phosphorylation of PECAM-1. American Journal of Physiology 273, E453–E461. [DOI] [PubMed] [Google Scholar]
- 73.Chelombitko MA et al. (2016) Role of reactive oxygen species in mast cell degranulation. Biochemistry (Mosc) 81, 1564–1577. [DOI] [PubMed] [Google Scholar]
- 74.Krystel-Whittemore M, Dileepan KN and Wood JG (2015) Mast cell: a multi-functional master cell. Frontiers in Immunology 6, 620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brinkmann V et al. (2004) Neutrophil extracellular traps kill bacteria. Science (New York, N.Y.) 303, 1532–1535. [DOI] [PubMed] [Google Scholar]
- 76.Folco EJ et al. (2018) Neutrophil extracellular traps induce endothelial cell activation and tissue factor production through interleukin-1alpha and cathepsin G. Arteriosclerosis Thrombosis and Vascular Biology 38, 1901–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bagni G et al. (2021) Circulating miRNome profiling data in Behcet's syndrome. Data in Brief 38, 107435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Emmi G et al. (2022) A unique circulating miRNA profile highlights thrombo-inflammation in Behcet's syndrome. Annals of the Rheumatic Diseases 81, 386–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Giustarini D et al. (2008) Red blood cells as a physiological source of glutathione for extracellular fluids. Blood Cells Molecules and Diseases 40, 174–179. [DOI] [PubMed] [Google Scholar]
- 80.Murakami K et al. (1989) Impairment of glutathione metabolism in erythrocytes from patients with diabetes mellitus. Metabolism: Clinical and Experimental 38, 753–758. [DOI] [PubMed] [Google Scholar]
- 81.Pouvreau C et al. (2018) Inflammation and oxidative stress markers in diabetes and hypertension. Journal of Inflammation Research 11, 61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ross EA, Koo LC and Moberly JB (1997) Low whole blood and erythrocyte levels of glutathione in hemodialysis and peritoneal dialysis patients. American Journal of Kidney Diseases: The Official Journal of the National Kidney Foundation 30, 489–494. [DOI] [PubMed] [Google Scholar]
- 83.Mohanty JG, Nagababu E and Rifkind JM (2014) Red blood cell oxidative stress impairs oxygen delivery and induces red blood cell aging. Frontiers in Physiology 5, 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.George A et al. (2013) Erythrocyte NADPH oxidase activity modulated by Rac GTPases, PKC, and plasma cytokines contributes to oxidative stress in sickle cell disease. Blood 121, 2099–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Woollard KJ et al. (2009) Erythrocyte hemolysis and hemoglobin oxidation promote ferric chloride-induced vascular injury. Journal of Biological Chemistry 284, 13110–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mahdi A et al. (2021) Novel perspectives on redox signaling in red blood cells and platelets in cardiovascular disease. Free Radical Biology & Medicine 168, 95–109. [DOI] [PubMed] [Google Scholar]
- 87.Barodka VM et al. (2014) New insights provided by a comparison of impaired deformability with erythrocyte oxidative stress for sickle cell disease. Blood Cells Molecules and Diseases 52, 230–235. [DOI] [PubMed] [Google Scholar]
- 88.Foller M et al. (2008) TRPC6 contributes to the Ca(2 + ) leak of human erythrocytes. Cellular Physiology and Biochemistry 21, 183–192. [DOI] [PubMed] [Google Scholar]
- 89.Whelihan MF et al. (2012) Prothrombin activation in blood coagulation: the erythrocyte contribution to thrombin generation. Blood 120, 3837–3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.MacKinney A et al. (2019) Disrupting the vicious cycle created by NOX activation in sickle erythrocytes exposed to hypoxia/reoxygenation prevents adhesion and vasoocclusion. Redox Biology 25, 101097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Thamilarasan M et al. (2020) Mn porphyrins as a novel treatment targeting sickle cell NOXs to reverse and prevent acute vaso-occlusion in vivo. Blood Advances 4, 2372–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Morel O et al. (2011) Cellular mechanisms underlying the formation of circulating microparticles. Arteriosclerosis Thrombosis and Vascular Biology 31, 15–26. [DOI] [PubMed] [Google Scholar]
- 93.Van Der Meijden PE et al. (2012) Platelet- and erythrocyte-derived microparticles trigger thrombin generation via factor XIIa. Journal of Thrombosis and Haemostasis: JTH 10, 1355–1362. [DOI] [PubMed] [Google Scholar]
- 94.Smith A and McCulloh RJ (2015) Hemopexin and haptoglobin: allies against heme toxicity from hemoglobin not contenders. Frontiers in Physiology 6, 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cooper CE et al. (2013) Haptoglobin binding stabilizes hemoglobin ferryl iron and the globin radical on tyrosine beta145. Antioxidants & Redox Signaling 18, 2264–2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Thomsen JH et al. (2013) The haptoglobin-CD163-heme oxygenase-1 pathway for hemoglobin scavenging. Oxidative Medicine and Cellular Longevity 2013, 523652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nagy E et al. (2010) Red cells, hemoglobin, heme, iron, and atherogenesis. Arteriosclerosis Thrombosis and Vascular Biology 30, 1347–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chen G et al. (2014) Heme-induced neutrophil extracellular traps contribute to the pathogenesis of sickle cell disease. Blood 123, 3818–3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dutra FF et al. (2014) Hemolysis-induced lethality involves inflammasome activation by heme. Proceedings of the National Academy of Sciences of the United States of America 111, E4110–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gupta N et al. (2017) Activation of NLRP3 inflammasome complex potentiates venous thrombosis in response to hypoxia. Proceedings of the National Academy of Sciences of the United States of America 114, 4763–4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Belcher JD et al. (2014) Heme triggers TLR4 signaling leading to endothelial cell activation and vaso-occlusion in murine sickle cell disease. Blood 123, 377–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ogasawara N et al. (2009) Hemoglobin induces the expression of indoleamine 2,3-dioxygenase in dendritic cells through the activation of PI3K, PKC, and NF-kappaB and the generation of reactive oxygen species. Journal of Cellular Biochemistry 108, 716–725. [DOI] [PubMed] [Google Scholar]
- 103.Lisk C et al. (2013) Hemoglobin-induced endothelial cell permeability is controlled, in part, via a myeloid differentiation primary response gene-88-dependent signaling mechanism. American Journal of Respiratory Cell and Molecular Biology 49, 619–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bahl N et al. (2014) Extracellular haemoglobin upregulates and binds to tissue factor on macrophages: implications for coagulation and oxidative stress. Thrombosis and Haemostasis 111, 67–78. [DOI] [PubMed] [Google Scholar]
- 105.Rother RP et al. (2005) The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: a novel mechanism of human disease. JAMA 293, 1653–1662. [DOI] [PubMed] [Google Scholar]
- 106.Singhal R et al. (2015) Hemoglobin interaction with GP1balpha induces platelet activation and apoptosis: a novel mechanism associated with intravascular hemolysis. Haematologica 100, 1526–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bourne JH et al. (2021) Heme induces human and mouse platelet activation through C-type-lectin-like receptor-2. Haematologica 106, 626–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Becatti M et al. (2016) Erythrocyte oxidative stress is associated with cell deformability in patients with retinal vein occlusion. Journal of Thrombosis and Haemostasis: JTH 14, 2287–2297. [DOI] [PubMed] [Google Scholar]
- 109.Becatti M et al. (2017) Erythrocyte membrane fluidity alterations in sudden sensorineural hearing loss patients: the role of oxidative stress. Thrombosis and Haemostasis 117, 2334–2345. [DOI] [PubMed] [Google Scholar]
- 110.Rizvi SI and Maurya PK (2007) Markers of oxidative stress in erythrocytes during aging in humans. Annals of the New York Academy of Sciences 1100, 373–382. [DOI] [PubMed] [Google Scholar]
- 111.Kasapoglu M and Ozben T (2001) Alterations of antioxidant enzymes and oxidative stress markers in aging. Experimental Gerontology 36, 209–220. [DOI] [PubMed] [Google Scholar]
- 112.Li G et al. (2010) Age-related carbonyl stress and erythrocyte membrane protein carbonylation. Clinical Hemorheology and Microcirculation 46, 305–311. [DOI] [PubMed] [Google Scholar]
- 113.Kumar D and Rizvi SI (2014) Markers of oxidative stress in senescent erythrocytes obtained from young and old age rats. Rejuvenation Research 17, 446–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jain K et al. (2019) Age-associated non-linear regulation of redox homeostasis in the anucleate platelet: implications for CVD risk patients. EBioMedicine 44, 28–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang Q and Zennadi R (2020) Oxidative stress and thrombosis during aging: the roles of oxidative stress in RBCs in venous thrombosis. International Journal of Molecular Sciences 21, 4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fuentes E and Palomo I (2016) Role of oxidative stress on platelet hyperreactivity during aging. Life Sciences 148, 17–23. [DOI] [PubMed] [Google Scholar]
- 117.Singh AK et al. (2018) Rapamycin mitigates erythrocyte membrane transport functions and oxidative stress during aging in rats. Archives of Physiology and Biochemistry 124, 45–53. [DOI] [PubMed] [Google Scholar]
- 118.Singh AK et al. (2017) Synergistic effect of rapamycin and metformin against age-dependent oxidative stress in rat erythrocytes. Rejuvenation Research 20, 420–429. [DOI] [PubMed] [Google Scholar]
- 119.Borghi C et al. (1999) Effects of the administration of an angiotensin-converting enzyme inhibitor during the acute phase of myocardial infarction in patients with arterial hypertension. SMILE study investigators. Survival of myocardial infarction long-term evaluation. American Journal of Hypertension 12, 665–672. [DOI] [PubMed] [Google Scholar]
- 120.Borghi C and Levy BI (2022) Synergistic actions between angiotensin-converting enzyme inhibitors and statins in atherosclerosis. Nutrition, Metabolism, and Cardiovascular Diseases: NMCD 32, 815–826. [DOI] [PubMed] [Google Scholar]
- 121.Borghi C and Omboni S (2020) Angiotensin-converting enzyme inhibition: beyond blood pressure control-the role of zofenopril. Advances in Therapy 37, 4068–4085. [DOI] [PubMed] [Google Scholar]
- 122.Pedersen TR (2010) Pleiotropic effects of statins: evidence against benefits beyond LDL-cholesterol lowering. American Journal of Cardiovascular Drugs: Drugs, Devices, and Other Interventions 10(suppl. 1), 10–17. [DOI] [PubMed] [Google Scholar]
- 123.Verbree-Willemsen L et al. (2018) LDL extracellular vesicle coagulation protein levels change after initiation of statin therapy. Findings from the METEOR trial. International Journal of Cardiology 271, 247–253. [DOI] [PubMed] [Google Scholar]
- 124.Oesterle A, Laufs U and Liao JK (2017) Pleiotropic effects of statins on the cardiovascular system. Circulation Research 120, 229–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Takemoto M and Liao JK (2001) Pleiotropic effects of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors. Arteriosclerosis Thrombosis and Vascular Biology 21, 1712–1719. [DOI] [PubMed] [Google Scholar]
- 126.Dechend R et al. (2001) Modulating angiotensin II-induced inflammation by HMG Co-A reductase inhibition. American Journal of Hypertension 14, 55S–61S. [DOI] [PubMed] [Google Scholar]
- 127.Nakagami H, Jensen KS and Liao JK (2003) A novel pleiotropic effect of statins: prevention of cardiac hypertrophy by cholesterol-independent mechanisms. Annals of Medicine 35, 398–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pignatelli P et al. (2012) Immediate antioxidant and antiplatelet effect of atorvastatin via inhibition of Nox2. Circulation 126, 92–103. [DOI] [PubMed] [Google Scholar]
- 129.Kessinger CW et al. (2015) Statins improve the resolution of established murine venous thrombosis: reductions in thrombus burden and vein wall scarring. PLoS One 10, e0116621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Basili S et al. (2011) Anoxia-reoxygenation enhances platelet thromboxane A2 production via reactive oxygen species-generated NOX2: effect in patients undergoing elective percutaneous coronary intervention. Arteriosclerosis Thrombosis and Vascular Biology 31, 1766–1771. [DOI] [PubMed] [Google Scholar]
- 131.Metcalfe C et al. (2016) Thioredoxin inhibitors attenuate platelet function and thrombus formation. PLoS One 11, e0163006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cammisotto V et al. (2019) Nox2-mediated platelet activation by glycoprotein (GP) VI: effect of rivaroxaban alone and in combination with aspirin. Biochemical Pharmacology 163, 111–118. [DOI] [PubMed] [Google Scholar]
- 133.Yisireyili M et al. (2016) Dipeptidyl peptidase- IV inhibitor alogliptin improves stress-induced insulin resistance and prothrombotic state in a murine model. Psychoneuroendocrinology 73, 186–195. [DOI] [PubMed] [Google Scholar]
- 134.Crutchley DJ and Que BG (1995) Copper-induced tissue factor expression in human monocytic THP-1 cells and its inhibition by antioxidants. Circulation 92, 238–243. [DOI] [PubMed] [Google Scholar]
- 135.Violi F et al. (2022) Interventional study with vitamin E in cardiovascular disease and meta-analysis. Free Radical Biology & Medicine 178, 26–41. [DOI] [PubMed] [Google Scholar]
- 136.Myung SK et al. (2013) Efficacy of vitamin and antioxidant supplements in prevention of cardiovascular disease: systematic review and meta-analysis of randomised controlled trials. BMJ 346, f10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Milman U et al. (2008) Vitamin E supplementation reduces cardiovascular events in a subgroup of middle-aged individuals with both type 2 diabetes mellitus and the haptoglobin 2–2 genotype: a prospective double-blinded clinical trial. Arteriosclerosis Thrombosis and Vascular Biology 28, 341–347. [DOI] [PubMed] [Google Scholar]
- 138.Stephens NG et al. (1996) Randomised controlled trial of vitamin E in patients with coronary disease: Cambridge Heart Antioxidant Study (CHAOS). Lancet (London, England) 347, 781–786. [DOI] [PubMed] [Google Scholar]
- 139.Heart Outcomes Prevention Evaluation Study, I. et al. (2000) Vitamin E supplementation and cardiovascular events in high-risk patients. New England Journal of Medicine 342, 154–160. [DOI] [PubMed] [Google Scholar]
- 140.(1999) Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico. Lancet (London, England) 354, 447–455. [PubMed] [Google Scholar]
- 141.Taddei S et al. (2001) Age-related reduction of NO availability and oxidative stress in humans. Hypertension 38, 274–279. [DOI] [PubMed] [Google Scholar]
- 142.Bendich A (1990) Antioxidant vitamins and their functions in immune responses. Advances in Experimental Medicine and Biology 262, 35–55. [DOI] [PubMed] [Google Scholar]
- 143.Kim K et al. (2015) High-dose vitamin C injection to cancer patients may promote thrombosis through procoagulant activation of erythrocytes. Toxicological Sciences 147, 350–359. [DOI] [PubMed] [Google Scholar]
- 144.Apel K and Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annual Review of Plant Biology 55, 373–399. [DOI] [PubMed] [Google Scholar]
- 145.Jiang S, Liu H and Li C (2021) Dietary regulation of oxidative stress in chronic metabolic diseases. Foods (Basel, Switzerland) 10, 1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Pastori D et al. (2015) Does Mediterranean diet reduce cardiovascular events and oxidative stress in atrial fibrillation? Antioxidants & Redox Signaling 23, 682–687. [DOI] [PubMed] [Google Scholar]
- 147.Xin G et al. (2017) Xanthohumol isolated from Humulus lupulus prevents thrombosis without increased bleeding risk by inhibiting platelet activation and mtDNA release. Free Radical Biology & Medicine 108, 247–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wollny T et al. (1999) Modulation of haemostatic function and prevention of experimental thrombosis by red wine in rats: a role for increased nitric oxide production. British Journal of Pharmacology 127, 747–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gresele P et al. (2008) Resveratrol, at concentrations attainable with moderate wine consumption, stimulates human platelet nitric oxide production. Journal of Nutrition 138, 1602–1608. [DOI] [PubMed] [Google Scholar]
- 150.Carnevale R et al. (2014) Extra virgin olive oil blunt post-prandial oxidative stress via NOX2 down-regulation. Atherosclerosis 235, 649–658. [DOI] [PubMed] [Google Scholar]
- 151.Wu H et al. (2020) Breaking the vicious loop between inflammation, oxidative stress and coagulation, a novel anti-thrombus insight of nattokinase by inhibiting LPS-induced inflammation and oxidative stress. Redox Biology 32, 101500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Consolandi C et al. (2015) Behcet's syndrome patients exhibit specific microbiome signature. Autoimmunity Reviews 14, 269–276. [DOI] [PubMed] [Google Scholar]
- 153.Brown JM and Hazen SL (2018) Microbial modulation of cardiovascular disease. Nature Reviews Microbiology 16, 171–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Emmi G et al. (2021) Butyrate-rich diets improve redox status and fibrin Lysis in Behcet's syndrome. Circulation Research 128, 278–280. [DOI] [PubMed] [Google Scholar]
- 155.Gutmann C et al. (2020) Reactive oxygen species in venous thrombosis. International Journal of Molecular Sciences 21, 1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit http://doi.org/10.1017/erm.2022.25.
click here to view supplementary material