Abstract
The cell density-dependent control of gene expression is employed by many bacteria for regulating a variety of physiological functions, including the generation of bioluminescence, sporulation, formation of biofilms, and the expression of virulence factors. Although periodontal organisms do not appear to secrete acyl-homoserine lactone signals, several species, e.g., Porphyromonas gingivalis, Prevotella intermedia, and Fusobacterium nucleatum, have recently been shown to secrete a signal related to the autoinducer II (AI-2) of the signal system 2 pathway in Vibrio harveyi. Here, we report that the periodontal pathogen Actinobacillus actinomycetemcomitans expresses a homolog of V. harveyi luxS and secretes an AI-2-like signal. Cell-free conditioned medium from A. actinomycetemcomitans or from a recombinant Escherichia coli strain (E. coli AIS) expressing A. actinomycetemcomitans luxS induced luminescence in V. harveyi BB170 >200-fold over controls. AI-2 levels peaked in mid-exponential-phase cultures of A. actinomycetemcomitans and were significantly reduced in late-log- and stationary-phase cultures. Incubation of early-log-phase A. actinomycetemcomitans cells with conditioned medium from A. actinomycetemcomitans or from E. coli AIS resulted in a threefold induction of leukotoxic activity and a concomitant increase in leukotoxin polypeptide. In contrast, no increase in leukotoxin expression occurred when cells were exposed to sterile medium or to conditioned broth from E. coli AIS−, a recombinant strain in which luxS was insertionally inactivated. A. actinomycetemcomitans AI-2 also induced expression of afuA, encoding a periplasmic iron transport protein, approximately eightfold, suggesting that LuxS-dependent signaling may play a role in the regulation of iron acquisition by A. actinomycetemcomitans. Finally, A. actinomycetemcomitans AI-2 added in trans complemented a luxS knockout mutation in P. gingivalis by modulating the expression of the luxS-regulated genes uvrB and hasF in this organism. Together, these results suggest that LuxS-dependent signaling may modulate aspects of virulence and the uptake of iron by A. actinomycetemcomitans and induce responses in other periodontal organisms in mixed-species oral biofilm.
The regulation of bacterial gene expression in response to changes in cell density is known as quorum sensing. Quorum-sensing bacteria synthesize and secrete extracellular signaling molecules called autoinducers, which accumulate in the environment as the population increases. When a critical threshold concentration of autoinducer is attained, a signal transduction cascade is triggered, resulting in an alteration in gene expression and a change in behavior of the organism (3, 14). For example, two quorum-sensing systems in Vibrio harveyi, signal systems 1 and 2, function in parallel to control the density-dependent expression of bioluminescence (4) and other cellular processes (32). The autoinducer of signal system 1 (AI-1) has been identified as an acyl-homoserine lactone (acyl-HSL) (5, 10), whereas the structure of AI-2 has not yet been fully resolved. The synthesis of the acyl-HSL AI-1 requires two polypeptides encoded by luxL and luxM (4), whereas AI-2 is synthesized by the luxS gene (49). AI-1 and AI-2 are recognized by their cognate sensor kinase proteins, LuxN and LuxQ, respectively, although AI-2 interaction with the LuxQ sensor may also be mediated by a periplasmic-binding protein, designated LuxP (5). At low cell density, and hence low autoinducer concentration, the sensors LuxN and LuxQ function as kinases and autophosphorylate (32). Phosphate is subsequently transferred to a shared integrator protein, LuxU, which in turn donates phosphate to the response regulator protein, LuxO (6, 16, 17). Phosphorylated LuxO is active and presumably induces the expression or activity of an unidentified repressor of the luciferase structural operon, luxCDABE (32). In contrast, at high cell density, LuxN and LuxQ bind their cognate signals and function as phosphatases, which draws phosphate away from LuxO in a LuxU-dependent reaction. Thus, LuxO becomes inactive and the downstream repression of luxCDABE is removed, resulting in the production of light.
Acyl-HSL-dependent quorum-sensing systems exist in many gram-negative bacteria, e.g., Pseudomonas aeruginosa, Agrobacterium tumefaciens, and Ralstonia solanacearum, and control diverse cellular functions, including toxin and alginate production in P. aeruginosa (46), type IV secretion in A. tumefaciens (35), exoenzyme production in Burkholderia cepacia (31), and the expression of other virulence-associated factors (2, 3, 13, 19, 37, 48, 49). However, a recent survey of gram-negative periodontal organisms by Frias et al. (18) suggested that this group of bacteria do not possess acyl-HSL-dependent signaling circuits. Instead, several of these organisms, e.g., Fusobacterium nucleatum, Porphyromonas gingivalis, and Prevotella intermedia, appeared to secrete a signal related to AI-2 of V. harveyi. Indeed, highly conserved homologs of V. harveyi luxS have recently been identified in both gram-negative and gram-positive bacteria (49), including the periodontal pathogen P. gingivalis (11), where inactivation of luxS influenced the expression of several genes encoding virulence factors and proteins which may be involved in the uptake of iron. This is consistent with recent evidence suggesting that LuxS-dependent quorum sensing controls aspects of virulence in Escherichia coli, Salmonella enterica serovar Typhimurium, and Vibrio cholerae (47–49). Interestingly, Frias et al. (18) found no evidence of AI-2 activity in the periodontal pathogen Actinobacillus actinomycetemcomitans, suggesting that this organism may not possess luxS.
A. actinomycetemcomitans is associated with a variety of infectious disease processes, including endocarditis, brain abscesses, osteomyelitis, subcutaneous abscesses, and early-onset periodontal disease (7, 36, 43, 52, 53), but little is known about the mechanisms of A. actinomycetemcomitans pathogenesis. However, the organism produces an array of potential virulence factors that may contribute to pathogenesis. For example, the breakdown of the extracellular matrix and the induction of bone resorption that occur in periodontitis may be facilitated by expression of collagenase (38), lipopolysaccharide (28), and GroEL-like proteins (20). A. actinomycetemcomitans expresses various adherence factors (34) (including fimbriae [39]), invades human epithelial cells (33, 45), produces several toxins which target various components of the immune system, and may play a role in modulating the host response by killing cells of the lymphocytic and monomyelocytic lineages. The best-characterized toxin is leukotoxin (29), a member of the RTX (repeats in toxin) family of gram-negative bacterial toxins (15, 50).
In this study, we report that A. actinomycetemcomitans secretes an AI-2-like signal that stimulates light production in V. harveyi and induces the expression of leukotoxin and a periplasmic iron-transporting protein in early-log-phase A. actinomycetemcomitans cells. In addition, we show that conditioned broth from a recombinant E. coli strain expressing A. actinomycetemcomitans luxS complements a luxS mutation in P. gingivalis. These results show that A. actinomycetemcomitans possesses a LuxS-dependent signal circuit and suggest that LuxS-dependent signaling may mediate intra- and interspecies responses among periodontal pathogens in the human oral cavity.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The bacterial strains used in this study are shown in Table 1. A. actinomycetemcomitans (strain JP2) was grown in brain heart infusion (Difco, Detroit, Mich.) supplemented with 40 mg of NaHCO3 per liter. Cultures were maintained at 37°C in an atmosphere of 5% CO2. E. coli strains were grown in Luria-Bertani (LB) medium (1% tryptone, 0.5% yeast extract, 0.5% NaCl) with aeration at 37°C. V. harveyi BB170 (sensor 1−, sensor 2+) was kindly provided by B. Bassler (Princeton University) and grown in AB medium (47) overnight at 30°C. AB medium consists of 10 mM potassium phosphate (pH 7.0), 0.3 M NaCl, 0.05 M MgSO4, 0.2% vitamin-free Casamino Acids (Difco), 2% glycerol, 1 mM l-arginine, 1 μg of thiamine per ml, and 0.01 μg of riboflavin per ml. For recombinant strains carrying plasmids, antibiotic selection was carried out by supplementing the appropriate medium with ampicillin (100 μg/ml) or chloramphenicol (34 μg/ml). P. gingivalis strains were grown anaerobically at 37°C in Trypticase soy broth (BBL) supplemented with 1 mg of yeast extract per ml, 5 μg of hemin per ml, and 1 μg of menadione per ml.
TABLE 1.
Bacterial strains
| Strain | Reference | Comments |
|---|---|---|
| A. actinomycetemcomitans JP2 | 8 | Highly leukotoxic |
| A. actinomycetemcomitans 652 | 8 | Minimally leukotoxic |
| A. actinomycetemcomitans ATCC 29524 | Minimally leukotoxic | |
| A. actinomycetemcomitans HK890 | 8 | Minimally leukotoxic |
| A. actinomycetemcomitans HK905 | 8 | Minimally leukotoxic |
| A. actinomycetemcomitans Fambo | Clinical isolate; highly leukotoxic | |
| A. actinomycetemcomitans Emory | Clinical isolate; highly leukotoxic | |
| E. coli AIS | This study | Carries A. actinomycetemcomitans luxS on pGEM-Easy |
| E. coli AIS− | This study | luxS insertional mutant of E. coli AIS |
| P. gingivalis ATCC 33277 | ATCC type strain | |
| P. gingivalis PLM1 | 11 | Isogenic luxS insertional mutant of ATCC 33277 |
Cloning of the A. actinomycetemcomitans luxS gene.
A 750-bp DNA fragment containing the luxS gene from A. actinomycetemcomitans JP2 was PCR amplified from genomic DNA using the primers Aa_luxS5 (5′-TAAAGCCTGCGATTTTCCTG-3′) and Aa_luxS3 (5′-CTTATTGTTTTAATAAGCTTTCGTC-3′). Primer sequences were derived from the sequence of the A. actinomycetemcomitans HK1651 genome (B.A. Roe, F. Z. Najar, S. Clifton, T. Ducey, L. Lewis, and D. Dyer, Actinobacillus Genome Sequencing Project, University of Oklahoma). The resulting PCR product was cloned into pGEMT-Easy (Promega) to generate plasmid pGEMT750, which was subsequently modified by insertion of a chloramphenicol resistance marker at the unique ClaI site within the luxS open reading frame to generate pGEMT750C. The chloramphenicol resistance gene was PCR amplified from pACYC184 (New England Biolabs) using the primers Cm-3 (5′-GGCGATCGATCACGGTCACA-3′) and Cm-4 (5′-CGCGATCGATTGAGACGTTG-3′), cloned into pGEMT750, and transformed into competent E. coli DH5α. Plasmid was purified from the resulting recombinant organism and cleaved with ClaI. The insert was purified and ligated with pGEMT750 that had been cut with ClaI, and recombinant organisms were selected for resistance to ampicillin and chloramphenicol. Plasmids pGEMT750 and pGEMT750C were subsequently transformed into competent E. coli DH5α (Gibco BRL) to generate E. coli AIS and AIS−, respectively.
To construct pGEMT1.5K, a 1.5-kbp DNA fragment containing the entire luxS gene was amplified from genomic DNA using primers lux5-1 (5′-CCATCGAAGTTCAAAGTTTG-3′) and lux3-1 (5′-CGCTCCCATCAATTACGCCTGC-3′). Primer sequences were derived from the strain HK1651 genomic sequence as described above. The resulting fragment was cloned into pGEMT to produce pGEMT1.5, which was further modified by the addition of the kanamycin resistance determinant from pUC4K into the unique ClaI site within the luxS open reading frame. To generate an isogenic luxS-deficient strain of JP2, pGEMT1.5K (which does not replicate in A. actinomycetemcomitans) was introduced into A. actinomycetemcomitans JP2 by electroporation, and colonies were selected for resistance to 25-μg of kanamycin per ml, and subsequently counterselected on medium containing kanamycin and 100 μg of ampicillin per ml. Two populations of clones were identified: some clones were resistant to both antibiotics, whereas others were sensitive to ampicillin and resistant to kanamycin. To conform integration of pGEMT1.5K into the genome, genomic DNA was isolated and analyzed by PCR using primers Aa_luxS5 and Aa_luxS3, described above. Clones which had undergone gene replacement were also analyzed for AI-2 production as described below.
AI-2 assay.
The V. harveyi luminescence bioassay was performed essentially as described by Surette and Bassler (47). To obtain cell-free conditioned broth for these assays, an overnight A. actinomycetemcomitans culture was diluted 1:20 into fresh medium and incubated for 2 h (early log phase) to 7 h (late log phase) at 37°C as described above. Cells were removed by centrifugation, and the resulting supernatant was filtered through 0.2-mm-pore-size filters and used immediately or stored at −70°C. For the determination of V. harveyi bioluminescence, an overnight culture of V. harveyi BB170 was diluted 1:5,000 into fresh AB medium, and 90 μl of the diluted cells was added to wells on a 96-well microtiter dish. Cell-free conditioned medium was added to the diluted V. harveyi culture at a 10% (vol/vol) final concentration. Positive control wells contained 10 μl of cell-free conditioned medium from V. harveyi BB170, while negative control wells contained 10 μl of sterile AB growth medium. The microtiter dish was shaken in a rotary shaker at 500 rpm at 30°C. Light production was measured hourly using a Wallac (Gaithersburg, Md.) model 1450 Microbeta Plus liquid scintillation counter in the chemiluminescence mode. The data are reported as the increase in light emission by V. harveyi BB170 exposed to the various conditioned media over the level of luminescence obtained for the negative control containing sterile growth medium alone.
For some experiments, cell-free conditioned media from recombinant E. coli strains containing intact (strain AIS) or inactivated (strain AIS−) A. actinomycetemcomitans luxS were analyzed. Strains AIS and AIS− containing pGEMT750 and pGEMT750C, respectively, were grown at 37°C to mid-logarithmic to late logarithmic phase in LB medium supplemented with 0.5% glucose, while V. harveyi BB170 was grown as described above. Cell-free conditioned media were prepared by centrifuging the bacterial cultures at 8,000 × g, and the supernatant was filtered through 0.2-mm-pore-size filters and stored at −70°C.
Determination of leukotoxic activity.
Leukotoxin-mediated cytolysis of human HL-60 cells was determined by trypan blue exclusion as described previously (8). Previous results had shown that intact A. actinomycetemcomitans cells are leukotoxic and that whole-cell cytotoxicity correlates with the level of leukotoxin polypeptide expressed by the bacterial cell (8). HL-60 cells (12) were cultured at 37°C in an atmosphere of 5% CO2 in RPMI 1640 (Gibco Laboratories) containing 10% heat-inactivated fetal calf serum, penicillin G (100 μg/ml), and streptomycin (100 μg/ml). Prior to use, the cells were washed with RPMI 1640 to remove the antibiotics and were suspended in RPMI 1640 without antibiotics at a density of 4 × 106 cells per ml. Early-log-phase A. actinomycetemcomitans cells (optical density = 0.1 to 0.15) were exposed to cell-free conditioned broth from A. actinomycetemcomitans cultures or from E. coli AIS or AIS− for 15 to 90 min at 37°C. The bacterial cells were then harvested, washed in RPMI 1640, and suspended in the same medium at a density of 108 cells per ml. Bacterial cells (50 μl) were mixed with HL-60 cells (50 μl) in Eppendorf tubes and incubated at 37°C for 15 min. A negative control consisting of HL-60 cells without bacteria was run for each reaction. All reactions were terminated by the addition of 100 μl of 0.4% trypan blue, and surviving cells were counted using a hemocytometer. At least four fields were counted for each sample, and percent lysis was calculated by dividing the number of surviving cells by the number of cells in the negative control. Values are the averages of triplicate assays.
Enzyme-linked immunosorbent assay analysis of A. actinomycetemcomitans leukotoxin.
Early-log-phase A. actinomycetemcomitans cells were incubated at 37°C for 15 to 90 min in cell-free conditioned medium from E. coli strain AIS or AIS− as described above. Cells were harvested, washed in phosphate-buffered saline (PBS; 50 mM sodium phosphate [pH 7.5]–150 mM NaCl), and suspended in the same buffer at a density of 108 cells per ml. Serial twofold dilutions of the cell suspension were spotted onto a nitrocellulose membrane. The membrane was incubated for 1 h at room temperature with gentle agitation in PBS containing 1% bovine serum albumin and then for 1 h in the same buffer containing polyclonal leukotoxin antibody (1:1,000 dilution). Filters were washed three times with PBS, reacted with goat anti-immunoglobulin G–peroxidase conjugate, and developed using diaminobenzidine (0.5 mg/ml in 50 mM Tris [pH 7.5]–0.03% H2O2) as the substrate. The developed filters were scanned on a Hewlett-Packard ScanJet 6100C, and the digital images were analyzed with a Molecular Dynamics personal densitometer.
RNA isolation and RT-PCR.
A. actinomycetemcomitans total RNA was isolated using the RNeasy mini kit (Qiagen) according to the manufacturer's instructions. Reverse transcriptase PCR (RT-PCR) of A. actinomycetemcomitans RNA was performed using the Platinum quantitative RT-PCR Thermoscript one-step system (Gibco BRL) as described by the manufacturer. Reverse transcription was routinely performed at 60°C for 30 min using the afuA2 primer (see below) and 10 ng of total RNA as the template. The resulting cDNA was amplified using the primers afuA1 (5′-CTTGCCGGTCAGTTAAAAGA-3′) and afuA2 (5′-TCCTGCCTGTTCAATCAATT-3′) derived from the published afuI sequence (53), under the following conditions: denaturation at 94°C for 45 s, annealing at 55°C for 45 s, and elongation at 68°C for 45 s for 40 cycles, followed by extension at 72°C for 4 min. Controls without RT were included in all experiments. Products were visualized after electrophoresis in 1% agarose gels.
For complementation of the P. gingivalis luxS knockout mutant (strain PLM1), a fresh culture of P. gingivalis PLM1 was incubated overnight with 1% filtered supernatants of E. coli AIS or with sterile LB medium in an anaerobic chamber at 37°C. Cells were harvested, washed, and suspended at 1010 cells/ml, and total RNA was isolated using the Totally RNA isolation kit (Ambion). Reverse transcription was performed in the presence of 1 μg of total RNA, 50 ng of antisense primer (random hexamers), 50 U of RT (Ambion), 13 U of RNase inhibitor, 10 mM deoxynucleoside triphosphate, and 1× RT buffer. Annealing of primer and template was carried out at 72°C for 2 min and then at 48°C for 1 h. Controls without RT were included in all experiments. The resulting cDNA was amplified, with each 100 μl of PCR mixture containing 1× PCR buffer, 3 μl of cDNA, 1.5 mM MgCl2, 10 mM deoxynucleoside triphosphate, 100 ng of each primer (see below), and 2.5 U of Taq DNA polymerase. The amplification conditions were denaturation at 94°C for 30 s, annealing at 45°C for 30 s, and elongation at 72°C for 2 min for 35 cycles. Primers used in these reactions were 5′-TACAAGGAGCACGCAGACAG-3′ and 5′-TCCCGTGGACGATATGTAGG-3′, specific for P. gingivalis uvrB, and 5′-ATACGGAGGAGGTGAGCGTA-3′ and 5′-AGTGATGCAATGCTCTGACG-3′, specific for P. gingivalis hasF. These primers were previously described by Chung et al. (11).
RESULTS
Secretion of an AI-2-like signal by A. actinomycetemcomitans.
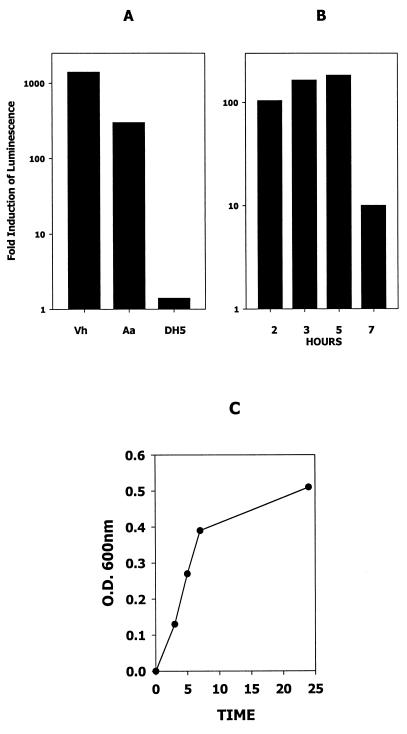
To determine if A. actinomycetemcomitans secretes AI-2 activity, we exposed the V. harveyi reporter strain BB170 (sensor 1−, sensor 2+; kindly supplied by B. Bassler) to cell-free conditioned medium from a mid- to late-log-phase A. actinomycetemcomitans culture (see Materials and Methods) or to conditioned medium from an overnight V. harveyi culture. As shown in Fig. 1A, luminescence of V. harveyi BB170 increased approximately 1,200-fold when cells were exposed to conditioned broth from the overnight V. harveyi culture and increased approximately 250-fold when they were incubated with the A. actinomycetemcomitans broth. In contrast, conditioned medium from E. coli DH5α, which carries a mutation in luxS rendering it inactive (49), induced very little luminescence in V. harveyi BB170. To determine if AI-2 activity varied during the growth of A. actinomycetemcomitans, V. harveyi luminescence was measured after exposure of cells to conditioned medium from early-, mid-, and late-log-phase A. actinomycetemcomitans cultures. As shown in Fig. 1B, AI-2 activity was maximal in early and mid-log phase and deceased significantly in late log phase.
FIG. 1.
AI-2 activity secreted by A. actinomycetemcomitans. (A) AI-2 activity in cell-free conditioned medium from an overnight V. harveyi BB170 culture (Vh) and from mid-exponential-phase A. actinomycetemcomitans (Aa) and E. coli DH5α (DH5) cultures was determined by monitoring the induction of V. harveyi luminescence as described in Materials and Methods. V. harveyi luminescence was determined after incubation of cells with the appropriate conditioned medium for 4 h. A negative control reaction (not shown) consisted of V. harveyi cells incubated with sterile medium. The increase in induction was calculated by dividing the light production of the experimental samples by that of the negative control. (B) AI-2 activity is maximal in mid-exponential-phase A. actinomycetemcomitans cultures. An overnight A. actinomycetemcomitans culture was diluted into fresh medium (1:20) and harvested after incubation at 37°C for 2, 3, 5, and 7 h. Cell-free conditioned medium was prepared from each sample and analyzed for AI-2 activity as described above. (C) Growth curve of A. actinomycetemcomitans JP2. O.D., optical density.
Isolation of A. actinomycetemcomitans luxS.
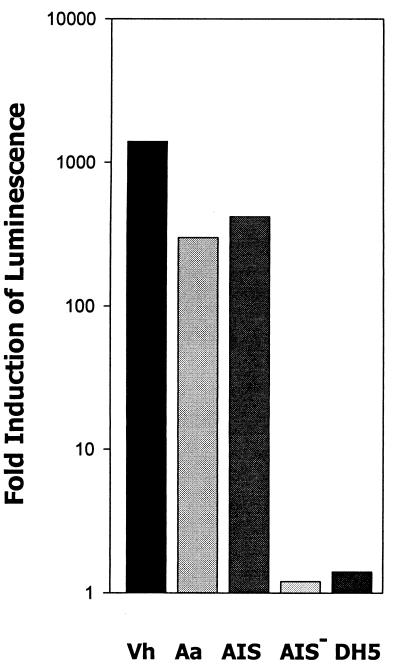
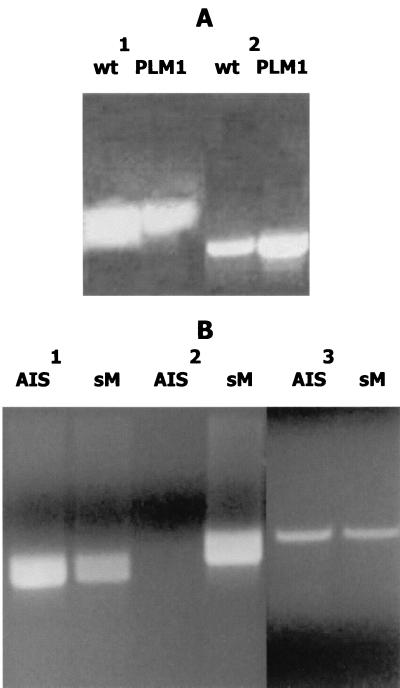
To identify A. actinomycetemcomitans luxS, the partially completed genome of A. actinomycetemcomitans HK1651 (Actinobacillus Genome Sequencing Project) was searched using the E. coli luxS sequence as a probe. These searches yielded an open reading frame that encoded a polypeptide exhibiting significant sequence similarity to LuxS proteins from gram-negative and gram-positive bacteria (Table 2). The A. actinomycetemcomitans sequence was most similar to LuxS from Pasteurella multocida, Neisseria meningitidis, Haemophilus influenzae, and V. harveyi and exhibited significantly lower similarity to the LuxS proteins of Borrelia burgdorferi, P. gingivalis, and several gram-positive organisms. PCR amplification of A. actinomycetemcomitans JP2 genomic DNA using primers designed from the sequence identified above yielded a 750-bp product which encoded a LuxS protein capable of synthesizing AI-2 in E. coli DH5α (see below). Similar 750-bp products were also obtained from PCRs using genomic DNA of A. actinomycetemcomitans 652, 29524, HK890, HK905, Emory, and Fambo (data not shown). The 750-bp product obtained from JP2 genomic DNA was cloned into pGEMT and introduced into E. coli DH5α to generate E. coli AIS. Sequencing of the plasmid insert from E. coli AIS confirmed that it possessed the luxS open reading frame and the putative luxS promoter. This plasmid was then further modified by ligating the chloramphenicol resistance determinant of pACYC184 into a unique ClaI site within the luxS open reading frame to inactivate the gene. The resulting E. coli strain containing the inactivated luxS was designated AIS−. As shown in Fig. 2, conditioned broth from E. coli AIS induced V. harveyi luminescence approximately 400-fold, whereas the induction of light by medium from strain AIS− was minimal, similar to that in the host E. coli DH5α. Together, these results show that A. actinomycetemcomitans luxS is necessary and sufficient for the synthesis of an extracellular signaling molecule that is capable of inducing light production through the V. harveyi AI-2 signaling system.
TABLE 2.
Similarity of selected bacterial LuxS proteins
| Organism with LuxS | % Identity and % similarity with A. actinomycetemcomitans LuxSa |
|---|---|
| P. multocida | 83, 88 |
| H. influenzae | 81, 89 |
| V. harveyi | 72, 86 |
| S. enterica serovar Typhimurium | 70, 82 |
| E. coli | 68, 83 |
| Campylobacter jejuni | 68, 83 |
| Clostridium perfringens | 50, 66 |
| Staphylococcus aureus | 43, 62 |
| H. pylori | 42, 61 |
| S. mutans | 41, 61 |
| Streptococcus pyogenes | 39, 61 |
| B. burgdorferi | 30, 52 |
| P. gingivalis | 31, 46 |
Comparisons were conducted with BLAST2 and include conservative substitutions using the Blosum62 matrix (1).
FIG. 2.
AI-2 activity requires a functional luxS. AI-2 activity in cell-free conditioned medium from an overnight V. harveyi BB170 culture (Vh) and from mid-exponential-phase cultures of A. actinomycetemcomitans (Aa), E. coli DH5α (DH5), E. coli AIS, which expresses A. actinomycetemcomitans luxS in pGEMT-Easy, and E. coli AIS−, in which the plasmid-borne luxS was inactivated by insertion of a chloramphenicol resistance marker, was determined by monitoring the induction of V. harveyi luminescence as described in Materials and Methods. V. harveyi luminescence was determined after incubation of cells with the appropriate conditioned medium for 4 h. A negative control reaction consisted of V. harveyi cells incubated in sterile medium. The increase in induction was calculated by dividing the light production of the experimental samples by that of the negative control.
A. actinomycetemcomitans homologs of V. harveyi LuxP, LuxQ, and LuxO.
Signal transduction in V. harveyi requires the periplasmic protein LuxP, the sensor kinase LuxQ, the phosphorelay protein LuxU, and the response regulator LuxO. To determine if A. actinomycetemcomitans possesses homologs of these proteins, each sequence was used as a probe to search the available genomic sequence database. As shown in Table 3, proteins exhibiting sequence similarity to LuxP, LuxQ, and LuxO were identified in A. actinomycetemcomitans. Consistent with the previously reported results of Bassler et al. (4, 5), A. actinomycetemcomitans LuxP is homologous to the periplasmic ribose binding protein of E. coli and LuxO exhibits similarity to the NtrC family of response regulators (32). No homolog of LuxU was found in A. actinomycetemcomitans.
TABLE 3.
A. actinomycetemcomitans homologs of V. harveyi LuxP, LuxQ, and LuxO
| Protein (accession no.) | Function | % Identity and % similarity of A. actinomycetemcomitans homologa |
|---|---|---|
| LuxP (U07069) | Ribose binding protein | 29, 45 |
| LuxQ (AA20838) | AI-2 sensor kinase | 29, 50 |
| LuxO (S49540) | Response regulator | 39, 52 |
| LuxU (L26221) | Phosphorelay protein | No homolog |
Includes conservative substitutions as defined by BLAST analysis using the Blosum62 matrix (1).
The LuxS-dependent signal influences leukotoxin and iron transport protein expression in A. actinomycetemcomitans.
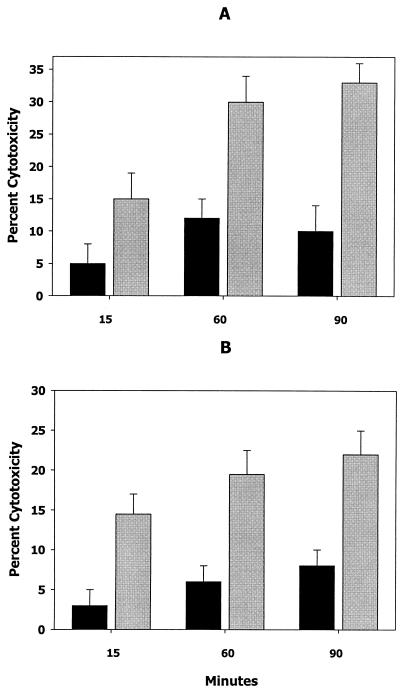
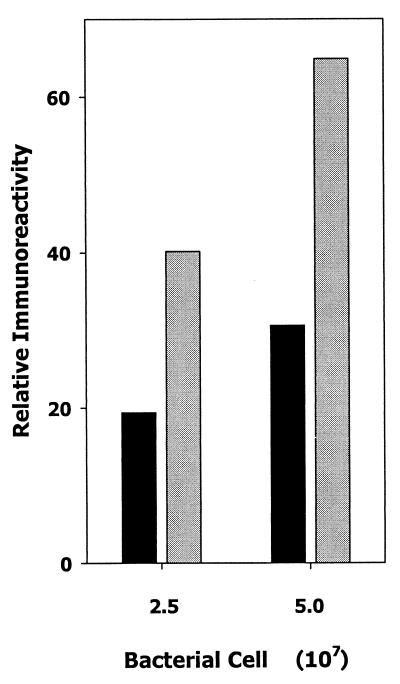
Several reports have suggested that LuxS-dependent signaling controls the expression of virulence determinants in E. coli (44) and P. gingivalis (11). To determine if the LuxS-dependent signal influenced the expression of leukotoxin (a member of the RTX family of gram-negative toxins) in A. actinomycetemcomitans JP2, early-logarithmic-phase bacteria were exposed to cell-free conditioned medium from a mid- to late-log-phase culture or with sterile medium for 15, 30, 60, and 90 min at 37°C. In fresh growth medium, the doubling time for A. actinomycetemcomitans under these conditions is approximately 90 min. An aliquot of each culture containing 108 cells per ml was then analyzed for leukotoxic activity. As shown in Fig. 3A, cells exposed to cell-free conditioned media exhibited a two- to threefold increase in leukotoxic activity relative to the control culture exposed to sterile medium. Furthermore, early-log-phase A. actinomycetemcomitans cells exposed to conditioned medium from E. coli AIS exhibited a similar increase in leukotoxicity compared to the leukotoxicity of cells exposed to conditioned broth obtained from E. coli AIS− (Fig. 3B). These results suggest that the leukotoxicity of A. actinomycetemcomitans cells increases upon exposure to the LuxS-dependent signal. This was further confirmed by spotting aliquots of cells onto nitrocellulose and reacting the filters with polyclonal antileukotoxin antibodies. As shown in Fig. 4, cells exposed to conditioned medium exhibited a >2-fold-greater reactivity with antileukotoxin antibodies than cells exposed to sterile broth, suggesting that the observed increase in whole-cell leukotoxicity arises from a concomitant increase in leukotoxin polypeptide.
FIG. 3.
Induction of leukotoxic activity by AI-2. The cytotoxicity of early-log-phase A. actinomycetemcomitans was determined after cells had been incubated for 15, 60, and 90 min in cell-free conditioned broth from mid-log-phase cultures (A, gray bars), sterile growth medium (A, black bars), conditioned medium from E. coli AIS (B, gray bars), or conditioned medium from E. coli AIS− (B, black bars). Error bars represent standard deviations; n = 3.
FIG. 4.
Induction of leukotoxin protein by AI-2. Early-log-phase A. actinomycetemcomitans cells were incubated for 60 min in cell-free conditioned medium from E. coli AIS (gray bars) or AIS− (black bars) and spotted onto nitrocellulose. The filter was washed with PBS containing 1% bovine serum albumin and reacted with polyclonal antileukotoxin antibodies. Immunoreactivity was determined by measuring the relative intensity of spots on the developed filter using a Molecular Dynamics personal densitometer.
Lilley and Bassler (32) showed that AI-2 influenced siderophore production in V. harveyi, which suggests that signal system 2 may modulate aspects of iron acquisition. In addition, our previous work with P. gingivalis suggested that the expression of genes involved in the acquisition of iron were modulated by LuxS-dependent signaling (11). To determine if luxS may influence iron acquisition in A. actinomycetemcomitans, we examined the expression of AfuA, a periplasmic protein which is highly related to HitA of H. influenzae (21, 51) and a major component involved in the transport of iron by A. actinomycetemcomitans. As shown in Fig. 5A, the expression of afuA, as determined by RT-PCR, was increased approximately eightfold when early-log-phase A. actinomycetemcomitans cells were exposed to conditioned broth from E. coli AIS versus conditioned broth from E. coli AIS−. In contrast, the expression of cdtB, encoding the cytolethal distending toxin protein B of A. actinomycetemcomitans, was unaffected by exposure to the conditioned broth (Fig. 5B). These results suggest that LuxS-dependent signaling may increase the expression of leukotoxin, a virulence-associated protein, and afuA, involved in iron transport.
FIG. 5.
AI-2 stimulates the expression of afuA in A. actinomycetemcomitans. RT-PCR was carried out with primers that were specific for A. actinomycetemcomitans afuA (A) or cdtB (B) using 10 ng of total RNA from early-log-phase A. actinomycetemcomitans cells that had been exposed to conditioned medium from E. coli AIS (lane 1) or AIS− (lane 2). A 10-μl aliquot of the RT-PCR mixture was electrophoresed in 1% agarose. Lanes M, DNA size markers.
Complementation of luxS knockout mutation in P. gingivalis by A. actinomycetemcomitans AI-2.
P. gingivalis and A. actinomycetemcomitans reside in a complex oral microbial biofilm, and we have shown that both organisms possess luxS (11; this paper). We also showed previously that inactivation of luxS influenced the expression of specific genes in P. gingivalis (11). To determine if AI-2 from A. actinomycetemcomitans is capable of complementing the luxS mutation in P. gingivalis, we used RT-PCR to compare the expression of two luxS-regulated genes, hasF and uvrB, in P. gingivalis cells exposed to conditioned medium from E. coli AIS or to sterile growth medium. These genes were chosen because they exhibited differential behavior in response to luxS inactivation (11), in that loss of luxS function in P. gingivalis resulted in a decrease of uvrB expression but increased hasF expression (Fig. 6A). Thus, complementation by A. actinomycetemcomitans AI-2 would require that it reverse the opposing effects exhibited by these genes. As shown in Fig. 6B, exposing strain PLM1 to cell-free conditioned medium from E. coli AIS resulted in an increase in uvrB expression relative to the control cells that were incubated with sterile broth (lane 1). Furthermore, the expression of hasF was turned off in the presence of A. actinomycetemcomitans AI-2 (Fig. 6B, lane 2). Indeed, A. actinomycetemcomitans AI-2 appeared to influence hasF expression to a greater degree than the endogenous signal in wild-type P. gingivalis 33277 cells (Fig. 6A). This may be due to increased signal dosage arising from the expression of luxS from a multicopy plasmid in E. coli AIS. Thus, A. actinomycetemcomitans luxS synthesizes a signal that is capable of modulating the expression of luxS-regulated genes in P. gingivalis, suggesting that the basic structure of the signal molecule and the mechanism for transducing signal information are conserved in these two periodontal organisms.
FIG. 6.
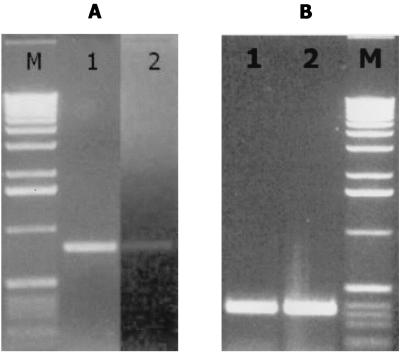
A. actinomycetemcomitans AI-2 complements a luxS knockout mutation in P. gingivalis. The wild-type P. gingivalis strain ATCC 33277 and its isogenic mutant (PLM1) in which luxS has been inactivated have been described (11). (A) RT-PCR using RNA isolated from P. gingivalis ATCC 33277 and PLM1 showed that inactivation of P. gingivalis luxS results in reduced expression of uvrB (lane 1) and increased expression of hasF (lane 2). (B) P. gingivalis PLM1 was subsequently incubated with cell-free conditioned broth from E. coli AIS or with sterile growth medium (sM) as described in Materials and Methods, and RT-PCRs were carried out using the uvrB- and hasF-specific primers used for panel A. Exposure to A. actinomycetemcomitans AI-2 induced the expression of uvrB (lane 1) and turned off hasF expression (lane 2). The fimA gene, which is not regulated by P. gingivalis luxS, was unaffected by exposure to A. actinomycetemcomitans AI-2 (lane 3).
Generation of an isogenic luxS-deficient A. actinomycetemcomitans strain.
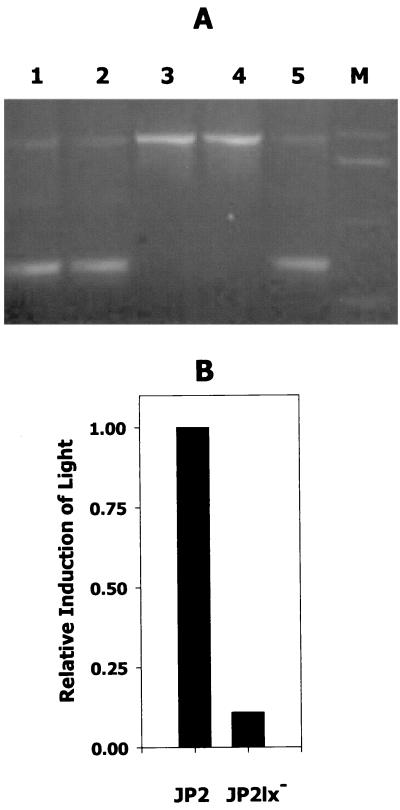
To facilitate further study of the role of luxS-dependent signaling of A. actinomycetemcomitans, an isogenic luxS-deficient mutant was constructed by transforming strain JP2 with pGEMT1.5K and selecting for kanamycin-resistant colonies. Five resistant clones were selected for further analysis by PCR using the luxS primers Aa_luxS5 and Aa_luxS3. As shown in Fig. 7, three clones exhibited amplification products of 750 and 2,000 bp and were resistant to both kanamycin and ampicillin, suggesting that they represent strains arising from single Campbell-type recombination events. The remaining two kanamycin-resistant clones were sensitive to ampicillin and exhibited only the 2,000-bp PCR product, indicating that genomic luxS was replaced by the inactivated copy of the gene from pGEMT1.5K. These results were confirmed by Southern blotting of EcoRI-digested genomic DNA using the 750-bp luxS fragment as a probe (not shown). In addition, the induction of V. harveyi BB170 luminescence by conditioned culture medium from the knockout strains was reduced by 90% relative to wild-type JP2 (Fig. 7B), suggesting that the mutant is incapable of synthesizing AI-2.
FIG. 7.
Isogenic luxS-deficient A. actinomycetemcomitans JP2. (A) Five kanamycin-resistant colonies of strain JP2 that had been transformed with pGEMT1.5K were analyzed by PCR using the primers Aa_luxS5 and Aa_luxS3 as described in Materials and Methods. Three clones (lanes 1, 2, and 5) contained the two amplification products predicted to result from single recombination of genomic luxS with pGEMT1.5K. The remaining clones (lanes 3 and 4) generated the single PCR product predicted to arise from the replacement of genomic luxS with the inactivated gene from pGEMT1.5K. Lane M, DNA size markers. (B) Conditioned culture medium from one of these clones (JP2lx−) was also analyzed and exhibited significantly reduced induction of luminescence from the V. harveyi BB170 reporter strain compared to conditioned medium from strain JP2. The induction of light by JP2 was arbitrarily assigned a value of 1.0.
DISCUSSION
The LuxS-dependent quorum-sensing circuit was originally identified in the marine organism V. harveyi, but recent studies have shown that many gram-negative and gram-positive bacteria possess homologs of the luxS gene (3, 11, 18, 24, 44, 49). Indeed, a recent survey of periodontal pathogens by Frias et al. (18) showed that P. intermedia, F. nucleatum, and P. gingivalis secrete AI-2-like signals that are capable of inducing light in V. harveyi. The luxS gene has also been recently cloned from P. gingivalis and appears to be important for regulating aspects of iron acquisition by this organism (11). Interestingly, Frias et al. (18) did not detect AI-2 in A. actinomycetemcomitans strains. Our results clearly show that A. actinomycetemcomitans possesses luxS and secretes AI-2. Conditioned broth from early- and mid-exponential-phase A. actinomycetemcomitans cultures contained the greatest AI-2 activity, as determined by monitoring the production of light by V. harveyi BB170. Activity decreased significantly in late log and stationary phases. Thus, the expression of AI-2 by A. actinomycetemcomitans is similar to that reported for Helicobacter pylori (24) and S. enterica serovar Typhimurium (48, 49). Furthermore, luxS was present in six additional A. actinomycetemcomitans strains. These strains represented both highly leukotoxic and minimally leukotoxic organisms (Table 1) and were representative of serotypes b (JP2 and ATCC 29524) and c (652). This suggests that the presence of the signaling circuit is independent of leukotoxic phenotype and occurs in at least two of six A. actinomycetemcomitans serotypes. However, in light of the inability of Frias et al. (18) to detect AI-2 activity in a serotype c strain (ATCC 33384), a more extensive analysis will be necessary to determine the distribution of the LuxS-dependent signal system in A. actinomycetemcomitans.
The A. actinomycetemcomitans LuxS polypeptide is similar in sequence to LuxS proteins from a variety of organisms (e.g., P. multocida, H. influenzae, E. coli, and V. harveyi) but exhibits significantly lower similarity to LuxS of other oral organisms (e.g., Streptococcus mutans and P. gingivalis). In addition, homologs of V. harveyi LuxP, LuxQ, and LuxO were identified in A. actinomycetemcomitans, but these proteins did not exhibit the high degree of sequence similarity that was observed between the respective LuxS proteins. Furthermore, no homolog of LuxU was identified in searches of the A. actinomycetemcomitans genome. Although we cannot exclude the possibility that our inability to identify LuxU arises from the incomplete nature of the A. actinomycetemcomitans genome sequence, searches of the completed E. coli, S. enterica serovar Typhi, and P. gingivalis genomes also failed to identify homologs of LuxU but did identify proteins exhibiting similarity to LuxP, LuxQ, and LuxO. This suggests that mechanistic differences may occur in the pathways of AI-2 signal transduction in V. harveyi and A. actinomycetemcomitans. Interestingly, the putative A. actinomycetemcomitans sensor protein corresponding to LuxQ is similar to a family of tripartite sensor kinases which are capable of catalyzing autophosphorylation and two intramolecular phosphotransfer reactions. Thus, the A. actinomycetemcomitans sensor kinase may encompass the function of both LuxQ (autophosphorylation and first phosphotransfer) and LuxU (phosphorelay), raising the possibility that AI-2 signal transduction in A. actinomycetemcomitans may not require an independent phosphorelay protein corresponding to LuxU. Studies are under way to investigate the role of the LuxQ-homologous polypeptide in AI-2 signal transduction by A. actinomycetemcomitans.
The extent of the cellular functions regulated by LuxS-dependent signaling are not known. In V. harveyi, AI-2 controls light production by the lux operon, the expression of genes involved in siderophore production and regulates aspects of colony morphology (32). In addition, Sperandio et al. (44) recently showed that AI-2 regulates the expression of the locus of enterocyte effacement (LEE) operon in E. coli O157:H7, suggesting that LuxS may play a role in modulating virulence. Our results show that the expression of the A. actinomycetemcomitans leukotoxin is influenced by AI-2 and increases by several fold in early-log-phase cells after exposure to conditioned medium from recombinant E. coli cultures expressing luxS. The leukotoxin is an RTX pore-forming toxin that induces apoptosis (at low concentration) or cell lysis (at high concentration) in a defined set of human leukocytes (26, 29, 30). In addition, several recent studies have shown that A. actinomycetemcomitans strains which express high levels of leukotoxin are associated with severe forms of early-onset periodontal diseases (9, 22, 23), suggesting that the toxin is important for A. actinomycetemcomitans pathogenesis. Since A. actinomycetemcomitans thrives in a complex biofilm that exists in the gingival pocket, it is conceivable that the LuxS-dependent induction of leukotoxin by AI-2 may play an important role in the expression of virulence in vivo. Experiments are under way to determine if other potential virulence factors of A. actinomycetemcomitans (e.g., cytolethal distending toxin [29, 40, 41] and adherence factors [25, 34]) are regulated by LuxS-dependent signaling.
Signal system 2 may also control the acquisition of iron. For example, AI-2 regulates the expression of siderophore production in V. harveyi (32) and influences the expression of hemR and rgpA in P. gingivalis (11), both of which encode proteins that may be involved in the acquisition of hemin (27, 42). In A. actinomycetemcomitans, an important mode of iron acquisition and transport involves afuA, which encodes a 35-kDa periplasmic protein related to H. influenzae HitA that is coexpressed with outer membrane proteins corresponding to HitBC (21, 51). Together, these polypeptides function to transport iron across the outer membrane and periplasm of A. actinomycetemcomitans. Our results show that expression of afuA is dramatically increased upon exposure to AI-2, suggesting that LuxS-dependent signaling may stimulate iron acquisition in A. actinomycetemcomitans.
The widespread distribution of luxS and the observations that AI-2 from diverse organisms induce luminescence in V. harveyi has lead to the hypothesis that signal system 2 transcends species barriers and may function to report total bacterial cell density and the metabolic potential of the environment (3, 49). Indeed, such a role for LuxS-dependent signaling may be particularly relevant for organisms in the oral cavity, where there exist many distinct ecological niches inhabited by specific populations of bacteria and where populational shifts in this complex community contribute to the onset and/or progression of disease. Inherent to this hypothesis is that specific luxS-regulated genes in a given species should respond to a heterologous AI-2 that is produced by another organism. Until now, cross-species signaling has been demonstrated only by the induction of luminescence in V. harveyi, and it is not known whether interspecies signaling is widespread or whether V. harveyi is simply promiscuous in responding to AI-2 signals. Our studies show that AI-2-mediated cross talk occurs between A. actinomycetemcomitans and P. gingivalis and that specific luxS-regulated genes involved in diverse physiologic processes in P. gingivalis respond to a heterologous signal generated by A. actinomycetemcomitans. These results suggest that the general structure of AI-2 may be conserved among these two organisms and they support the hypothesis that LuxS-dependent signaling may function in interspecies communication. However, since we have thus far examined relatively few target genes modulated by AI-2, we cannot exclude the possibility that LuxS-dependent signaling may also mediate species-specific responses and that some luxS-regulated genes of P. gingivalis may not respond to a heterologous signal or may exhibit a differential response to cognate and heterologous signals. The identification and analysis of additional luxS-regulated targets will address these issues, and such experiments are being carried out.
Our results also show that AI-2 signal concentration is maximal during mid-exponential-phase growth of A. actinomycetemcomitans and decreases significantly as cells approach stationary phase. This is consistent with previous results reported for E. coli, S. enterica serovar Typhimurium, and H. pylori (25, 48, 49). Thus, LuxS-dependent signaling may function at relatively low cell density in A. actinomycetemcomitans compared to other quorum-sensing bacteria. The basis for this discrepancy among quorum-sensing bacteria has not been fully explained. However, Surette and Bassler have suggested that pathogenic E. coli and S. enterica serovar Typhimurium may never reach stationary phase in vivo (48). A similar situation may occur with A. actinomycetemcomitans in multispecies oral biofilms, where host antimicrobial activities, the constant flow of saliva, and competition for nutrients among the various organisms may prevent A. actinomycetemcomitans from attaining high cell density. Under these adverse conditions, the LuxS-dependent signal system may be adapted to function at lower cell density.
In summary, A. actinomycetemcomitans expresses luxS and secretes a signal related to AI-2 of V. harveyi. LuxS-dependent signaling was shown to induce the expression of leukotoxin and a periplasmic transport protein that may be involved in the acquisition of iron by A. actinomycetemcomitans. The A. actinomycetemcomitans signal also complemented a luxS mutation in P. gingivalis, suggesting that the LuxS-dependent signal circuit of A. actinomycetemcomitans may induce both intra- and interspecies responses in the mixed-species microbial communities that exist in the oral cavity.
ACKNOWLEDGMENTS
We thank Bonnie L. Bassler for kindly providing the V. harveyi BB170 reporter strain.
This work was supported by Public Health Service grants DE10729 and DE12505 from the National Institutes of Health.
REFERENCES
- 1.Altschul S F, Madden T L, Schaffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bainton N J, Bycroft B W, Chhabra S, Stead P, Gledhill L, Hill P J, Rees C E D, Winson M K, Salmond G P C, Stewart G S A B, Williams P. A general role for the lux autoinducer in bacterial cell signaling: control of antibiotic biosynthesis in Erwinia. Gene. 1992;116:87–91. doi: 10.1016/0378-1119(92)90633-z. [DOI] [PubMed] [Google Scholar]
- 3.Bassler B L. How bacteria talk to each other: regulation of gene expression by quorum sensing. Curr Opin Microbiol. 1999;2:582–587. doi: 10.1016/s1369-5274(99)00025-9. [DOI] [PubMed] [Google Scholar]
- 4.Bassler B L, Wright M, Showalter R E, Silverman M R. Intercellular signalling in Vibrio harveyi: sequence and function of genes regulating expression of luminescence. Mol Microbiol. 1993;9:773–786. doi: 10.1111/j.1365-2958.1993.tb01737.x. [DOI] [PubMed] [Google Scholar]
- 5.Bassler B L, Wright M, Silverman M R. Multiple signalling systems controlling expression of luminescence in Vibrio harveyi: sequence and function of genes encoding a second sensory pathway. Mol Microbiol. 1994;13:273–286. doi: 10.1111/j.1365-2958.1994.tb00422.x. [DOI] [PubMed] [Google Scholar]
- 6.Bassler B L, Wright M, Silverman M R. Sequence and function of LuxO, a negative regulator of luminescence in Vibrio harveyi. Mol Microbiol. 1994;12:403–412. doi: 10.1111/j.1365-2958.1994.tb01029.x. [DOI] [PubMed] [Google Scholar]
- 7.Block P J, Fox A C, Yoran C, Kaltman A J. Actinobacillus actinomycetemcomitans endocarditis: report of a case and review of the literature. Am J Med Sci. 1973;276:387–392. doi: 10.1097/00000441-197311000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Brogan J M, Lally E T, Poulsen K, Kilian M, Demuth D R. Regulation of Actinobacillus actinomycetemcomitans expression: analysis of the promoter regions of leukotoxic and minimally leukotoxic strains. Infect Immun. 1994;62:501–508. doi: 10.1128/iai.62.2.501-508.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bueno L C, Mayer M P, DiRienzo J M. Relationship between conversion of localized juvenile periodontitis-susceptible children from health to disease and Actinobacillus actinomycetemcomitans leukotoxin promoter structure. J Periodontol. 1998;69:998–1007. doi: 10.1902/jop.1998.69.9.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao J, Meighen E I. Purification and structural identification of an autoinducer for the luminescence system of Vibrio harveyi. J Biol Chem. 1989;264:21670–21676. [PubMed] [Google Scholar]
- 11.Chung W, Park Y, Lamont R J, McNab R, Barbieri B, Demuth D R. A signaling system in Porphyromonas gingivalis based on a LuxS protein. J Bacteriol. 2001;183:3903–3909. doi: 10.1128/JB.183.13.3903-3909.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collins S, Gallo R C. Human myeloid leukemic cells in vitro (HL-60): growth, differentiation and comparison to Friend cells. In: Rossi G B, editor. In vivo and in vitro erythropoiesis, the Friend system. New York, N.Y: Elsevier/North Holland Biomedical Press; 1980. pp. 477–501. [Google Scholar]
- 13.DeKievit T R, Iglewski B H. Bacterial quorum sensing in pathogenic relationships. Infect Immun. 2000;68:4839–4849. doi: 10.1128/iai.68.9.4839-4849.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Engebrecht J, Nealson K, Silverman M. Bacterial bioluminescence: isolation and genetic analysis of functions from Vibrio fischeri. Cell. 1983;32:773–781. doi: 10.1016/0092-8674(83)90063-6. [DOI] [PubMed] [Google Scholar]
- 15.Fives-Taylor P M, Meyer D H, Mintz K P, Brissette C. Virulence factors of Actinobacillus actinomycetemcomitans. Periodontology. 1999;20:136–167. doi: 10.1111/j.1600-0757.1999.tb00161.x. [DOI] [PubMed] [Google Scholar]
- 16.Freeman J A, Bassler B L. A genetic analysis of the function of LuxO, a two-component response regulator involved in quorum sensing in Vibrio harveyi. Mol Microbiol. 1999;31:665–677. doi: 10.1046/j.1365-2958.1999.01208.x. [DOI] [PubMed] [Google Scholar]
- 17.Freeman J A, Bassler B L. Sequence and function of LuxU: a two-component phosphorelay protein that regulates quorum sensing in Vibrio harveyi. J Bacteriol. 1999;181:899–906. doi: 10.1128/jb.181.3.899-906.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frias J, Olle E, Alsina M. Periodontal pathogens produce quorum sensing signal molecules. Infect Immun. 2001;69:3431–3434. doi: 10.1128/IAI.69.5.3431-3434.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fuqua C, Winans S C, Greenberg E P. Census and consensus in bacterial ecosystems: the LuxR-LuxI family of quorum sensing transcriptional regulators. Annu Rev Microbiol. 1996;50:727–751. doi: 10.1146/annurev.micro.50.1.727. [DOI] [PubMed] [Google Scholar]
- 20.Gouhlen F, Hafezi A, Uitto V-J, Hinode D, Nakamura R, Grenier D, Mayrand D. Subcellular localization and cytotoxic activity of the GroEL-like protein isolated from Actinobacillus actinomycetemcomitans. Infect Immun. 1998;66:5307–5313. doi: 10.1128/iai.66.11.5307-5313.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Graber K R, Smoot L M, Actis L A. Expression of iron binding proteins and hemin binding activity in the dental pathogen Actinobacillus actinomycetemcomitans. FEMS Microbiol Lett. 1998;163:135–142. doi: 10.1111/j.1574-6968.1998.tb13037.x. [DOI] [PubMed] [Google Scholar]
- 22.Guthmiller J M, Lally E T, Korostoff J. Beyond the specific plaque hypothesis: are highly leukotoxic strains of Actinobacillus actinomycetemcomitans a paradigm for periodontal pathogenesis? Crit Rev Oral Biol Med. 2001;12:116–124. doi: 10.1177/10454411010120020201. [DOI] [PubMed] [Google Scholar]
- 23.Haraszthy V I, Hariharan G, Tinoco E M, Cortelli J R, Lally E T, Davis E, Zambon J J. Evidence for the role of highly leukotoxic Actinobacillus actinomycetemcomitans in the pathogenesis of localized juvenile and other forms of early-onset periodontitis. J Periodontol. 2000;71:912–922. doi: 10.1902/jop.2000.71.6.912. [DOI] [PubMed] [Google Scholar]
- 24.Joyce E A, Bassler B L, Wright A. Evidence for a signaling system in Helicobacter pylori: detection of a luxS-encoded autoinducer. J Bacteriol. 2000;182:3638–3643. doi: 10.1128/jb.182.13.3638-3643.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kachlaney S C, Planet P J, Desalle R, Fine D H, Figurski D H, Kaplan J B. flp-1, the first representative of a new pilin gene subfamily, is required for non-specific adherence of Actinobacillus actinomycetemcomitans. Mol Microbiol. 2001;40:542–554. doi: 10.1046/j.1365-2958.2001.02422.x. [DOI] [PubMed] [Google Scholar]
- 26.Karakelian D, Lear D, Lally E T, Tanaka J C. Characterization of Actinobacillus actinomycetemcomitans leukotoxin pore formation in HL60 cells. Biochim Biophys Acta. 1998;1406:175–187. doi: 10.1016/s0925-4439(98)00002-7. [DOI] [PubMed] [Google Scholar]
- 27.Karunakaran T, Madden T, Kuramitsu H. Isolation and characterization of a hemin-regulated gene, hemR, from Porphyromonas gingivalis. J Bacteriol. 1997;179:1898–1908. doi: 10.1128/jb.179.6.1898-1908.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kato T, Honma K, Yamanaka A, Miura T, Okuda K. Heterogeneity in the immune response to serotype b LPS of Actinobacillus actinomycetemcomitans in inbred strains of mice. FEMS Immunol Med Microbiol. 2000;28:67–70. doi: 10.1111/j.1574-695X.2000.tb01458.x. [DOI] [PubMed] [Google Scholar]
- 29.Lally E T, Kieba I R, Sato A, Green C L, Rosenbloom J, Korostoff J, Wang J F, Shenker B S, Ortlepp S, Robinson M K, Billings P C. RTX toxins recognize a β2 integrin on the surface of human target cells. J Biol Chem. 1997;272:30463–30469. doi: 10.1074/jbc.272.48.30463. [DOI] [PubMed] [Google Scholar]
- 30.Lear J D, Karakelian D, Furblur U, Lally E T, Tanaka J C. Conformational studies of Actinobacillus actinomycetemcomitans leukotoxin: partial denaturation enhances toxicity. Biochim Biophys Acta. 2000;1476:350–362. doi: 10.1016/s0167-4838(99)00241-1. [DOI] [PubMed] [Google Scholar]
- 31.Lewenza S, Conway B, Greenberg E P, Sokol P A. Quorum sensing in Burkholderia cepacia: identification of the luxRI homologs cepRI. J Bacteriol. 1999;181:748–756. doi: 10.1128/jb.181.3.748-756.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lilley B N, Bassler B L. Regulation of quorum sensing in Vibrio harveyi by LuxO and sigma-54. Mol Microbiol. 2000;36:940–954. doi: 10.1046/j.1365-2958.2000.01913.x. [DOI] [PubMed] [Google Scholar]
- 33.Meyer D H, Lippman J E, Fives-Taylor P M. Invasion of epithelial cells by Actinobacillus actinomycetemcomitans: a dynamic multistep process. Infect Immun. 1994;64:2988–2997. doi: 10.1128/iai.64.8.2988-2997.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meyer D H, Fives-Taylor P M. Characteristics of adherence of Actinobacillus actinomycetemcomitans to epithelial cells. Infect Immun. 1994;62:928–935. doi: 10.1128/iai.62.3.928-935.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oger P, Kim K S, Sackett R L, Piper K R, Farrand S K. Octopine-type Ti plasmids code for mannopine-inducible dominant-negative allele of TraR, the quorum-sensing activator that regulates Ti plasmid conjugal transfer. Mol Microbiol. 1998;27:277–288. doi: 10.1046/j.1365-2958.1998.00671.x. [DOI] [PubMed] [Google Scholar]
- 36.Page M I, King E O. Infection due to Actinobacillus actinomycetemcomitans and Haemophilus aphrophilus. N Engl J Med. 1966;275:181–188. doi: 10.1056/NEJM196607282750403. [DOI] [PubMed] [Google Scholar]
- 37.Passador L, Cook J M, Gambello M J, Rust L, Iglewski B H. Expression of Pseudomonas aeruginosa virulence genes requires cell to cell communication. Science. 1993;260:1127–1130. doi: 10.1126/science.8493556. [DOI] [PubMed] [Google Scholar]
- 38.Robertson P B, Lantz M, Marucha P T, Kornman K S, Trummel C L, Holt S C. Collagenolytic activity associated with Bacteroides species and Actinobacillus actinomycetemcomitans. J Periodontal Res. 1982;17:275–283. doi: 10.1111/j.1600-0765.1982.tb01154.x. [DOI] [PubMed] [Google Scholar]
- 39.Rosan B, Slots J, Lamont R J, Listgarten M A, Nelson G M. Actinobacillus actinomycetemcomitans fimbriae. Oral Microbiol Immunol. 1988;3:58–63. doi: 10.1111/j.1399-302x.1988.tb00082.x. [DOI] [PubMed] [Google Scholar]
- 40.Shenker B J, McKay T, Datar S, Miller M, Chowden R, Demuth D R. Actinobacillus actinomycetemcomitans immunosuppressive protein is a member of the family of cytolethal distending toxins capable of causing G2 arrest in human T cells. J Immunol. 1999;162:4773–4780. [PubMed] [Google Scholar]
- 41.Shenker B S, Hoffmaster R H, McKay T, Demuth D R. Expression of cytolethal distending toxin (cdt) operon in Actinobacillus actinomycetemcomitans: evidence that the CdtB protein is responsible for G2 arrest of the cell cycle in human T cells. J Immunol. 2000;165:2612–2618. doi: 10.4049/jimmunol.165.5.2612. [DOI] [PubMed] [Google Scholar]
- 42.Shi Y, Ratnayake D B, Okamoto K, Abe N, Yamamoto K, Hakayama K. Genetic analysis of proteolysis, hemoglobin binding, and hemagglutination of Porphyromonas gingivalis. Construction of mutants with a combination of rgpA, rgpB, kgp and hagA. J Biol Chem. 1999;274:17955–17960. doi: 10.1074/jbc.274.25.17955. [DOI] [PubMed] [Google Scholar]
- 43.Slots J, Reynolds H S, Genco R J. Actinobacillus actinomycetemcomitans in human periodontal disease: a cross-sectional microbiological investigation. Infect Immun. 1980;29:1013–1020. doi: 10.1128/iai.29.3.1013-1020.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sperandio V, Mellies J L, Nguyen W, Shin S, Kaper J B. Quorum sensing controls expression of the type III secretion gene transcription and protein secretion on enterohemorrhagic and enteropathogenic Escherichia coli. Proc Natl Acad Sci USA. 1999;96:15196–15201. doi: 10.1073/pnas.96.26.15196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sreenivasan P K, Meyer D H, Fives-Taylor P M. Requirements for invasion of epithelial cells by Actinobacillus actinomycetemcomitans. Infect Immun. 1993;61:1239–1245. doi: 10.1128/iai.61.4.1239-1245.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Storey D G, Ujack E E, Rabin H R, Mitchell I. Pseudomonas aeruginosa lasR transcription correlates with the transcription of lasA, lasB and toxA in chronic lung infections associated with cystic fibrosis. Infect Immun. 1998;66:2521–2528. doi: 10.1128/iai.66.6.2521-2528.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Surette M G, Bassler B L. Quorum sensing in Escherichia coli and Salmonella typhimurium. Proc Natl Acad Sci USA. 1998;95:7046–7050. doi: 10.1073/pnas.95.12.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Surette M G, Bassler B L. Regulation of autoinducer production in Salmonella typhimurium. Mol Microbiol. 1999;31:585–595. doi: 10.1046/j.1365-2958.1999.01199.x. [DOI] [PubMed] [Google Scholar]
- 49.Surette M G, Miller M B, Bassler B L. Quorum sensing in Escherichia coli, Salmonella typhimurium, and Vibrio harveyi: a new family of genes responsible for autoinducer production. Proc Natl Acad Sci USA. 1999;96:1639–1644. doi: 10.1073/pnas.96.4.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tervahartiala B, Uitto V-J, Kari K, Laasko T. Outer membrane vesicles and leukotoxic activity of Actinobacillus actinomycetemcomi-tans from subjects with different periodontal status. J Dent Res. 1989;97:33–42. doi: 10.1111/j.1600-0722.1989.tb01428.x. [DOI] [PubMed] [Google Scholar]
- 51.Willemsen P T, Vulto I, Boxem M, de Graaff J. Characterization of a periplasmic protein involved in iron utilization of Actinobacillus actinomycetemcomitans. J Bacteriol. 1997;179:4949–4952. doi: 10.1128/jb.179.15.4949-4952.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zambon J J. Actinobacillus actinomycetemcomitans in human periodontal disease. J Clin Periodontol. 1985;12:1–20. doi: 10.1111/j.1600-051x.1985.tb01348.x. [DOI] [PubMed] [Google Scholar]
- 53.Zambon J J, Slots J, Genco R J. Serology of oral Actinobacillus actinomycetemcomitans and serotype distribution in human periodontal disease. Infect Immun. 1983;41:19–27. doi: 10.1128/iai.41.1.19-27.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]