Abstract
Interferon-stimulated gene 15 (ISG15) is a 15 kDa protein induced by type I interferons (IFN-α and IFN-β) and is a member of the ubiquitin-like superfamily of proteins. The ISG15 pathway is highly expressed in various malignancies, including pancreatic ductal adenocarcinoma (PDAC), suggesting a potential role of the ISG15 pathway (free ISG15 and ISG15 conjugates) in pancreatic carcinogenesis. However, very little is known about how the ISG15 pathway may contribute to pancreatic tumorigenesis. In the current study, we demonstrate that ISG15 pathway knockdown reverses the KRAS-associated phenotypes of PDAC cells such as increased proliferation and colony formation. Furthermore, clustered regularly interspaced short palindromic repeats (CRISPR)-mediated ISG15 knockdown decreased tumor programmed death ligand-1 (PDL-1) expression leading to increased number of CD8+ tumor-infiltrating lymphocytes and decreased pancreatic tumor growth. In addition, the syngeneic subcutaneous mouse model revealed that knocking down the ISG15 pathway significantly decreased the rate of tumor incidence and increased the survival rate. Interestingly, the ISG15 knockdown-mediated PDL-1 downregulation in pancreatic tumors increased the efficacy of anti-programmed cell death protein-1 (PD-1) treatment. ISG15 knockdown in combination with anti-PD-1 treatment synergistically increased the number of CD8+ tumor-infiltrating lymphocytes. Additionally, ISG15 knockdown alone significantly decreased the number of tumor-infiltrating regulatory T cells (Tregs) compared to wild type tumors treated with anti-PD-1 antibody. Overall, these findings suggest that strategies to target the ISG15 pathway by itself or in combination with immunotherapy may lead to improved survival for patients diagnosed with PDAC.
Electronic supplementary material
The online version of this article (10.1007/s00262-019-02422-9) contains supplementary material, which is available to authorized users.
Keywords: Immunotherapy, ISG15, Pancreatic cancer, Tumor microenvironment
Introduction
Pancreatic cancer is a deadly disease with an average 5-year survival rate around 7% [1]. While pancreatic cancer survival rates have modestly improved over the last decade, this malignancy has been termed “cold” due to its lack of CD8+ lymphocytes and resistance to immunotherapy. The majority (90%) of PDAC tumors have somatic mutations in KRAS, and thus far strategies to target KRAS have failed. Therefore, new approaches are being tested that alter the activity of KRAS through modifying downstream signaling pathways or other regulators of KRAS.
The ISG15 pathway is overexpressed in a variety of cancers, including PDAC [2–4]. Deciphering of the precise role of ISG15 in tumorigenesis is complicated due to dual roles of this protein. Free intracellular ISG15 can be secreted from tumors and immune cells functioning as an extracellular cytokine [5]. In addition, ISG15 can also function as a posttranslational modifier of target intracellular protein through the process of ISGylation [2]. The ISG15 pathway is important in tumorigenesis and its expression can be regulated by oncogenes [6–8] and tumor suppressors [9–11], and the ISG15 pathway, reciprocally, stabilizes oncogenes and cancer-associated proteins via inhibiting proteasomal and lysosomal-mediated protein degradation [7, 12, 13]. An example of this collaboration is demonstrated in breast cancer, where the ISG15 pathway is upregulated by oncogenic KRAS and subsequently, ISG15 stabilizes KRAS protein levels by inhibiting its lysosomal degradation [7].
Studies over the past 10 years have revealed that interferon signaling plays an important role in the upregulation of PDL-1 expression in tumor cells [14–16]. It has been demonstrated that an interferon secretome is required for PDL-1 upregulation in tumor cells [17].
In this study, we investigated the role of the ISG15 pathway in pancreatic cancer cell transformation and tumorigenesis. Using a syngeneic mouse model, we demonstrated that downregulation of the ISG15 pathway interfered with PDAC tumor proliferation and halted the aggressive nature of PDAC. Downregulation of the ISG15 pathway decreased PDAC PDL-1 expression and enhanced CD8+ T cell intratumor infiltration, rendering the tumor susceptible to PD-1 immune checkpoint antibody therapy. Novel strategies to target the ISG15 pathway alone or in conjunction with immunotherapy may improve the survival of PDAC patients and others with ISG15 positive cancers.
Materials and methods
Cells
Panc02 murine pancreatic cancer cells were grown in DMEM supplemented with 10% FBS containing 1% penicillin/streptomycin in humidified air with 5% CO2. Stable ISG15 and UbcH8 knockdown clonal cells were established using CRISPR (pSpCas9 BB-2A-Puro, Genscript, Piscataway, NJ, USA) and Lipofectamine 2000 (Thermo Fisher Scientific, Waltham, MA, USA) following manufacturer’s protocol. Stable clones were selected using puromycin.
RT-PCR
RNA was extracted from cells using the RNeasy Kit (Qiagen, Germantown, MD, USA). RT-PCR was performed with 1 μg of total RNA using a Superscript One-Step RT-PCR kit (Invitrogen, Carlsbad, CA, USA) on a SimpliAmp™ Thermal Cycler (Applied Biosystems, Foster City, CA, USA). The PCR products were analyzed by gel electrophoresis with a 2% agarose gel and ethidium bromide for visualization.
For quantitative PCR, cDNA was synthesized from 1 μg of RNA using qScript cDNA synthesis kit (Quanta Biosciences, Beverly, MA, USA) and transcripts were quantified using PerfeCTa SYBR Green FastMix, ROX (Quanta Biosciences) on a StepOnePlus™ Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). The fold change was calculated using the (2–∆∆Ct) method with hypoxanthine-guanine phosphoribosyltransferase (HPRT) as the reference gene. The primers used are shown in Table 1.
Table 1.
Primers used for RT-PCR and quantitative PCR
| Gene | Forward primer 5′–3′ | Reverse primer 5′–3′ |
|---|---|---|
| ISG15 | CATCTATGAGGTCTTTCTGACGC | TTAGGCCATACTCCCCCAGC |
| UbcH8 | GTGGCGAAAGAGCTGGAGAG | GGGGAAATCAATCCGCACTTG |
| IRF-1 | TCCAAGTCCAGCCGAGACACTA | ACTGCTGTGGTCATCAGGTAGG |
| HPRT | TCAGTCAACGGGGGACATAAA | GGGGCTGTACTGCTTAACCAG |
Immunoblotting
Cells were lysed in lysis buffer containing 50 mM Tris–HCl pH 7.5, 2% SDS, and protease inhibitor cocktail. All lysates were sonicated, boiled, and cleared by centrifugation. The Laemmli sample buffer was then added to the cleared cell lysates, boiled for 10 min, and then separated by gel electrophoresis on 15% polyacrylamide gels. After transfer onto nitrocellulose membrane and blocking in 5% BSA, western blot analysis was carried out using the following antibodies: anti-ISG15 (gift from Dr. Shyamal Desai, Louisana State University, 1:660, overnight at room temperature) [2], anti-ISG15 (gift from Dr. Deborah Lenshow, Washington University, 1:750, overnight at 4 °C) [18], and anti-beta actin (Thermo Fisher Scientific, Cat.# MA1-140, 1:5000, overnight at 4 °C). The blots were then incubated with the corresponding horseradish peroxidase conjugated rabbit or mouse secondary antibody (Cat. # 31466 and # 61-6520, Thermo Fisher Scientific, 1:10,000, overnight at 4 °C). Next, the enhanced chemiluminescence western procedure (Pierce, Rockford, IL, USA) was performed and the signal was detected using a GE Amersham Imager 680 series (GE Healthcare Life Sciences, Pittsburgh, PA, USA).
MTT (tetrazolium dye) proliferation assay
Panc02 cells (N = 7500) were plated into each well of 96-well plates. Two days later, MTT (Trevigen, Gaithersburg, MD, USA) was added to the cell culture media and incubated for 2 h. The cell culture media were then removed and DMSO was added to dissolve formazan (purple precipitate). The absorbance of each well was read at 540 nm on a plate reader.
Clonogenic assay
Panc02 cells (N = 2000) were plated into each well of 6-well plates. Old media were removed and fresh media were applied after 2 days. After 4 days, the cells were stained and fixed with methylene blue (10 mg/ml) in 50% methanol for 10 min and rinsed with distilled water. The plates were scanned and colony intensity % and area % were quantified using ImageJ software.
Conditioned media assay
Conditioned media were created by plating Panc02 wild type or ISG15 knockdown cells (N = 3 × 105) into 60 mm cell culture dishes and collecting and filtering the cell culture media after 3 days of culture. Panc02 ISG15 knockdown cells (N = 1.25 × 105) were plated into each well of 6-well plates, incubated with conditioned media for 72 h, and then collected for qPCR analysis.
Tumor studies
One million cells suspended in PBS were injected subcutaneously into 6-week old female C57BL/6 mice (Charles Rivers). N = 10 for each group.
The antibody against the programmed cell death protein 1 (PD-1-Ab) (Clone RMPI-14, Bio X cell, West Lebanon, NH, USA) was administered intraperitoneally to mice at a dose of 125 μg every 4 days for a total of three treatments.
Immunohistochemistry
Formalin-fixed tumor sections (5 μm) were rehydrated using Leica autostainer program 7 and antigen retrieval was performed using Flex Low-in PT, T/E9 or AR9 in microwave. Next, slides were incubated in 3% hydrogen peroxide for 10 min at room temperature and reacted with antibodies to CD8 (1:40, Cat. #98941, Cell Signaling Technology, Danvers, MA, USA), Foxp3 (1:30, Cat. #14-5773, Thermo Fisher Scientific), and PDL-1 (1:40, Cat. #64988, Cell Signaling) overnight at 4 °C After washing in TBST, the slides were incubated with secondary HRP-conjugated anti-rabbit (ready to use, DAKO Envision kit, Agilent Technologies, Santa Clara, CA, USA) or HRP-conjugated anti-rat (ImmPRESS(RTU), Vector laboratories, Burlingame, CA, USA) antibodies for 30 min at room temperature or biotin-conjugated anti-rabbit (1:200, Vector Vectastain ABC kit, Vector laboratories) for 30 min at room temperature followed by Vectastain ABC-HRP (Vector Vectastain) incubation for 30 min at room temperature. Slides were incubated with 3,3′-diaminobenzidine (DAB) (Vector Vectastain) chromagen for 5 min at room temperature for color development.
Results
ISG15 knockdown decreases pancreatic ductal adenocarcinoma growth
The ISG15 pathway is elevated in PDAC patient tumor samples and not expressed in the normal pancreas [19]. To test the frequency of the ISG15 pathway in PDAC, we screened various PDAC mouse and human cell lines for ISG15 expression. Interestingly, all PDAC cell lines tested contained both free and conjugated ISG15 (Supplemental Fig. 1a). To examine the effect of the ISG15 pathway on PDAC cell proliferation, we treated wild type Panc02 cells with an ISG15 CRISPR construct to knockdown ISG15 expression. The ISG15 CRISPR treated cells had a 50% reduction in ISG15 transcripts and the proliferation rate was significantly reduced compared to wild type cells (p = 0.031) (Supplemental Fig. 1b). To test the effect of ISG15 knockdown on PDAC tumor growth in an immunocompetent mouse model, we performed a syngeneic subcutaneous tumor study. Tumors in the mice inoculated with the ISG15 knockdown cells were significantly smaller compared to wild type cells with a 74% and 78% reduction in tumor volume and weight, respectively (Supplemental Fig. 1c, d). These results suggest that the ISG15 pathway promotes PDAC cell growth and tumorigenesis.
Silencing of ISGylation (ISG15 conjugation) reverses the transformed phenotype of PDAC cells
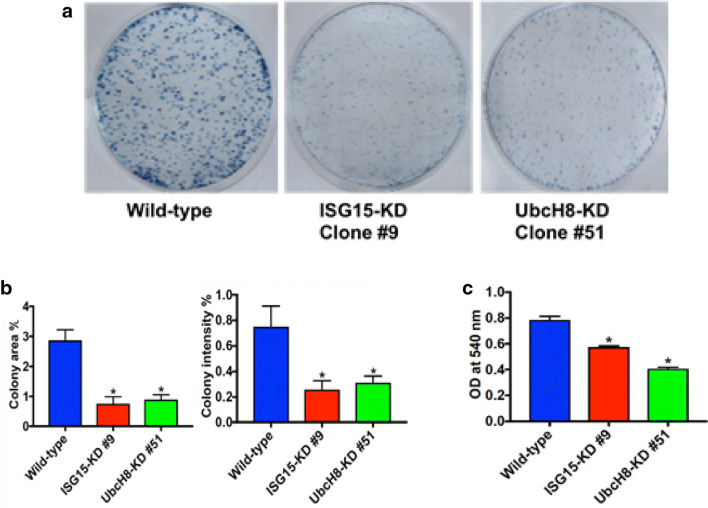
To further investigate the role of the ISG15 pathway in PDAC, we created clonal ISG15 and UbcH8 (ISG15-specific E2 enzyme) knockdown in Panc02 PDAC cells using CRISPR technology. The ISG15 and UbcH8 knockdown clones were first identified by performing RT-PCR with ISG15 and UbcH8 specific primers (Supplemental Fig. 2a). The clones were further characterized by performing qPCR to quantify the level of ISG15 and UbcH8 mRNA knockdown (Supplemental Fig. 2b). Both ISG15 and UbcH8 clones had a 4.5-fold reduction in mRNA transcripts compared to wild type control. Furthermore, to confirm that the protein levels of ISG15 and UbcH8 were knocked down in the clones, we performed a western blot for ISG15 expression (Supplemental Fig. 2c). The advantage of having both ISG15 and UbcH8 knockdown cells is that dual roles of ISG15 functioning as a free protein and in ISGylation can be separated, because ISG15 knockdown cells have decreased levels of conjugates and lack free mature ISG15, while UbcH8 knockdown cells have decreased levels of conjugates, but express free mature ISG15. The ISG15 knockdown cells express proISG15, but it is not functional. Knockdown of the ISG15 pathway significantly altered proliferative characteristics of Panc02 cells. Both ISG15 and UbcH8 clonal cells exhibited decreased colony formation compared to wild type cells (Fig. 1a). The ISG15 and UbcH8 knockdown cells formed significantly fewer colonies compared to wild type cells with a reduction in colony area (p < 0.002) and intensity percent (p < 0.002) (Fig. 1b). Confirmation of role of ISG15 in cell growth was further supported by the MTT (tetrazolium dye) proliferation assay, where the growth of ISG15 and UbcH8 knockdown clones were significantly decreased compared to wild type Panc02 cells (p < 0.0001) (Fig. 1c). Together, the in vitro cell transformation data revealed that knockdown of ISG15 conjugation reverses the malignant phenotype of pancreatic cancer cells.
Fig. 1.
ISG15 pathway knockdown reverses the transformed phenotype of PDAC cells. a Clonogenic assay. Growth of wild type (left), clonal ISG15 (middle), and UbcH8 (right) knockdown cells are shown after 4 days. b Quantitation of clonogenic assay. Colony area % and colony intensity % was measured. (*p value < 0.002, N = 6). c A MTT proliferation assay showing significant decreased cell proliferation with clonal ISG15 and UbcH8 knockdown cells compared to wild type cells (*p value < 0.0001, N = 12)
Knockdown of the ISG15 pathway decreases pancreatic cancer growth in vivo and increases survival rate
In supplemental Fig. 1, we demonstrated that ISG15 knockdown suppresses PDAC tumor growth. This experiment did not allow us the capabilities to test, whether free ISG15 and/or conjugates contribute to pancreatic tumorigenesis. To test the contributions of free ISG15 and conjugates in pancreatic tumorigenesis, we performed a subcutaneous tumor study using syngeneic clonal Panc02 ISG15 and UbcH8 knockdown cells in C57BL/6 mice. Interestingly, the mice inoculated with ISG15 knockdown cells had a significant reduction in tumor take rate compared to both wild type (p = 0.0003) and UbcH8 knockdown (p = 0.04) cells (Fig. 2a). The tumor take rate was decreased by 30% in 7 weeks post tumor cell inoculation in mice injected with UbcH8 knockdown cells compared to wild type cells, but this decrease was not significant. The reduction in tumor take rate in ISG15 pathway knockdown cells prompted us to investigate the effect of the ISG15 pathway on the survival rate of the mice inoculated with PDAC cells. For this analysis, a Kaplan–Meier curve was constructed to examine the survival rate of the mice over an 11-week period (Fig. 2b). The mice inoculated with either ISG15 or UbcH8 knockdown cells had a significant survival advantage compared to wild type cells (p = 0.0004 and p = 0.0020, respectively). The mice inoculated with ISG15 knockdown cells also had a significant survival advantage compared to UbcH8 knockdown cells (p = 0.0054). These tumor studies revealed that both free ISG15 and ISG15 conjugates contribute to pancreatic tumorigenesis since UbcH8 knockdown cells have decreased ISG15 conjugates, but have free ISG15.
Fig. 2.
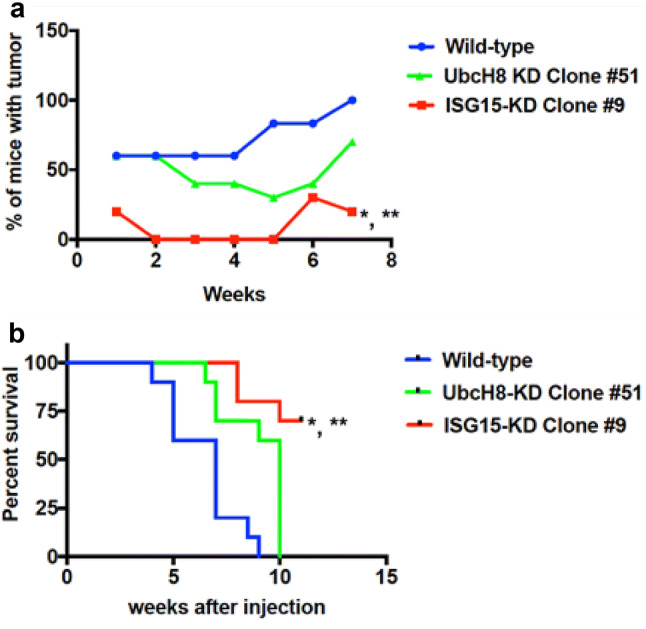
ISG15 pathway knockdown decreases PDAC tumor growth and increases the survival rate. C57BL/6 mice were injected subcutaneously with one million wild type Panc02 cells, ISG15 KD Panc02 cells or with UbcH8 KD Panc02 cells (N = 10 each). a Tumor incidence was significantly less in those injected with ISG15 knockdown cells over a 7-week period compared to wild type (*p value = 0.0003) and UbcH8 knockdown (**p value = 0.04) cells. b Survival analysis. Kaplan–Meier survival analysis confirmed a significant shortened life span in the mice that received wild type or UbcH8 knockdown Panc02 cells as compared to ISG15 knockdown cells (*p value = 0.0004, WT vs. ISG15 KD) and (**p value = 0.0054), UbcH8 KD vs. ISG15 KD)
Free ISG15 upregulates PDL-1 expression in PDAC cells
In a pancreatic cancer retrospective study, the upregulation of PDL-1 was associated with shorter disease-free survival and overall survival [20, 21]. These observations prompted us to test the effect of the ISG15 pathway on PDAC PDL-1 expression in vitro. ISG15 knockdown in PDAC cells was found to have decreased PDL-1 mRNA expression compared to wild type cells (Fig. 3a). The UbcH8 knockdown cells had comparable PDL-1 mRNA levels as wild type cells, suggesting that free ISG15 is the primary driver of PDL-1 expression in PDAC cells, because the UbcH8 knockdown cells lack ISG15 conjugates, but have free ISG15. To further support the role of free ISG15 in regulating PDL-1 expression, we examined the effect of wild type and ISG15 knockdown conditioned media on PDL-1 mRNA levels in ISG15 knockdown PDAC cells. Wild type conditioned media increased PDL-1 mRNA levels twofold compared to regular media control, whereas ISG15 knockdown conditioned media had comparable PDL-1 mRNA levels as regular media control (Fig. 3b). It is known that interferon gamma (IFN-γ) functions through interferon regulatory factor 1 (IRF-1) to upregulate PDL-1 expression on tumor cells [15, 22, 23]. To test if free ISG15 functions through IRF-1 to upregulate PDL-1 expression, we performed quantitative PCR using samples from the conditioned media experiment. Wild type conditioned media increased IRF-1 mRNA levels 1.6-fold compared to regular media control, whereas ISG15 knockdown conditioned media had comparable IRF-1 mRNA levels as regular media control (Fig. 3c). Furthermore, there were no detectable levels of IFN-γ transcripts in ISG15 knockdown PDAC cells treated with either wild type conditioned media or ISG15 knockdown conditioned media (data not shown). Together, the data demonstrate that free ISG15 upregulates PDL-1 expression in PDAC cells independent of IFN-γ.
Fig. 3.
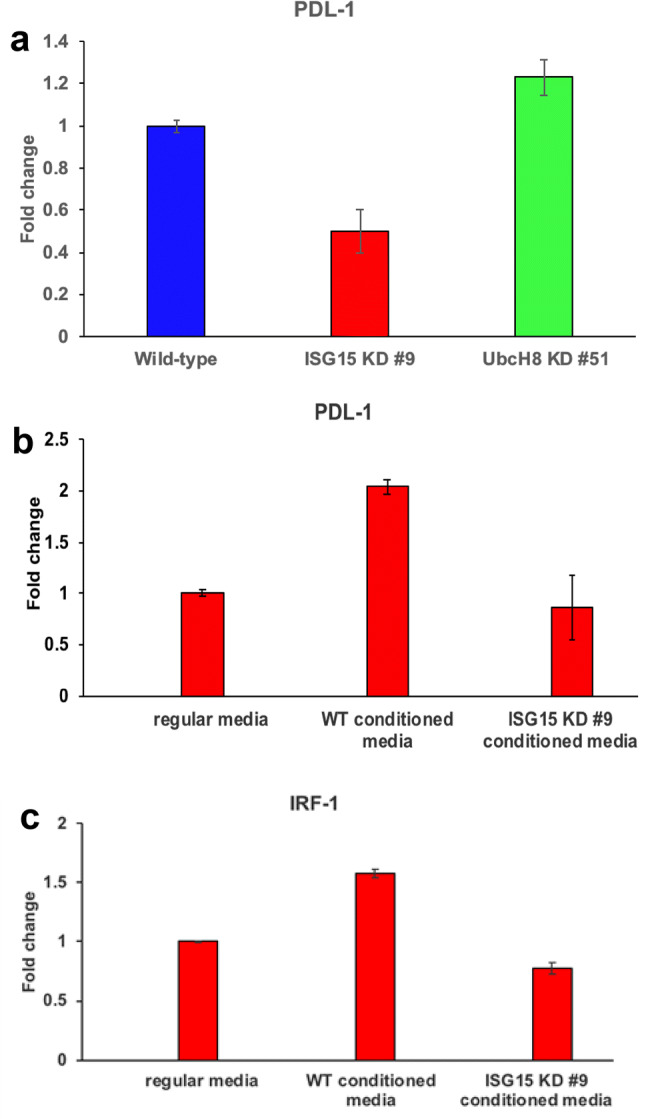
Free ISG15 induces PDL-1 expression on pancreatic cancer cells. a PDL-1 mRNA levels were determined by quantitative PCR in Panc02 wild type, ISG15 knockdown, and UbcH8 knockdown cells. b Wild type Panc02 and ISG15 knockdown cells were cultured for 3 days and the culture media were collected and used as conditioned media. ISG15 knockdown cells were then cultured in the conditioned media for 3 days and quantitative PCR analysis was performed for PDL-1 expression. c IRF-1 quantitative PCR was performed on the same samples as in part b
ISG15 pathway knockdown decreases PDL-1 expression and increases CD8+ T cell intratumor infiltration in PDAC tumors
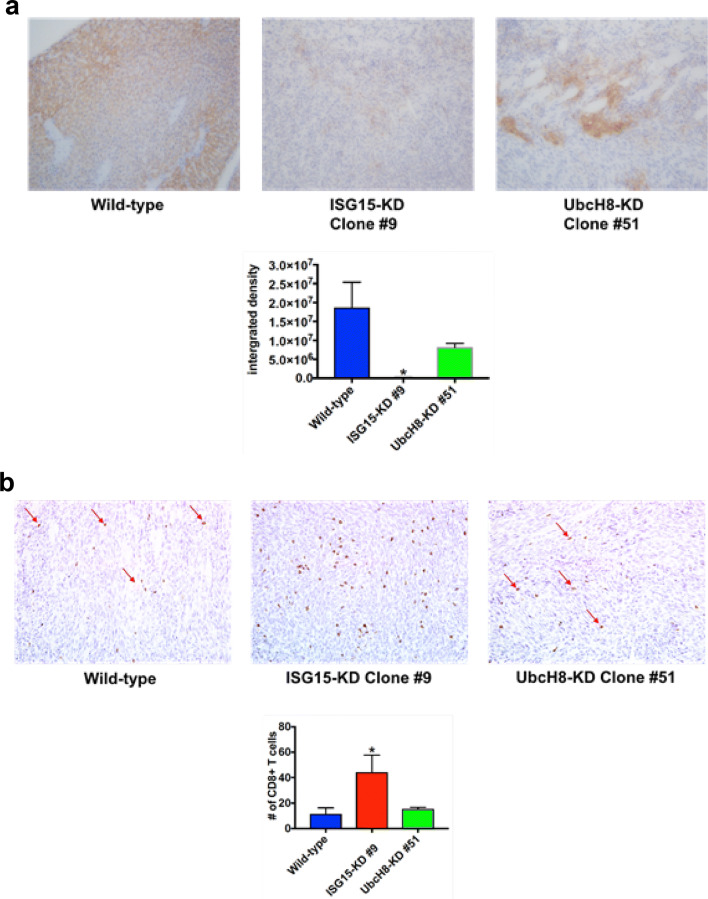
ISG15 pathway knockdown decreases PDAC tumor growth (Supplemental Fig. 1c, d and Fig. 2a) and PDL-1 mRNA expression in pancreatic cancer cell (Fig. 3a). This prompted us to assess the effect of knocking down the ISG15 pathway on PDAC tumor PDL-1 expression and CD8+ T cell intratumor infiltration. ISG15 knockdown tumors had a significant reduction in PDL-1 protein expression compared to wild type tumors (p < 0.001) (Fig. 4a). The UbcH8 knockdown tumors exhibited decreased PDL-1 protein expression compared to wild type tumors, but this decrease was not significant (Fig. 4a). Since tumor PDL-1 expression inhibits CD8+ T cell activation and intratumor infiltration [24, 25], we examined the effect of knocking down the ISG15 pathway on PDAC tumor CD8+ T cell intratumor infiltration. ISG15 knockdown tumors had a significant increase in CD8+ T cell intratumor infiltration compared to wild type tumors (p < 0.04) (Fig. 4b). The UbcH8 knockdown tumors had comparable CD8+ T cell intratumor infiltration as wild type tumors (Fig. 4b). Collectively, our results demonstrate that knocking down the ISG15 pathway in PDAC cells suppresses tumor growth by decreasing PDL-1 expression and subsequently increasing CD8+ T cell intratumor infiltration.
Fig. 4.
ISG15 knockdown decreases PDL-1 expression and increases CD8+ T cell intratumor infiltration in PDAC tumors. a PDL-1 expression. The same tumors from Fig. 2 were stained by immunohistochemistry (IHC) using anti-PDL-1 antibody. Lower panel shows the quantitation of PDL-1 expression was significantly decreased in ISG15 knockdown tumors as compared to wild type (*p < 0.001). b CD8+ T-lymphocyte expression. The same tumors from Fig. 2 were stained by IHC using anti-CD8 antibody. Lower panel. The quantitation of five low power (×10) fields was performed by manually counting positive cells (*p < 0.04 as compared to wild type)
ISG15 knockdown potentiates the efficacy of anti-PD-1 antibody therapy and promotes an anti-tumor immune response
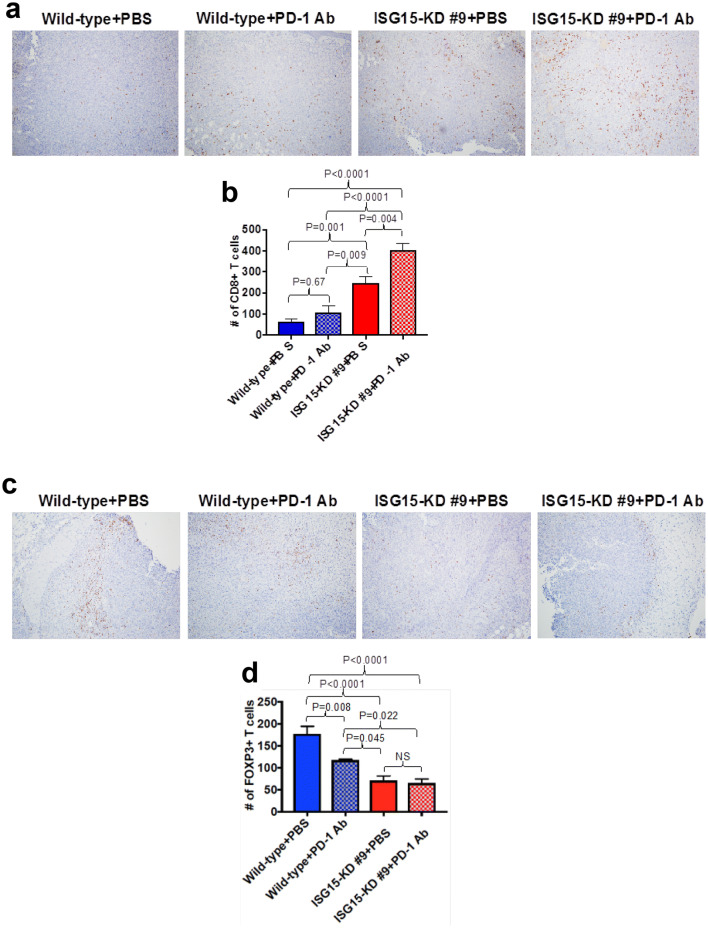
Because ISG15 knockdown decreases PDAC PDL-1 expression and increased the number of tumor-infiltrating CD8+ T cells, we decided to test if ISG15 knockdown would increase the efficacy of anti-PD-1 antibody therapy. In Fig. 5a, we show that ISG15 knockdown tumors that were treated with anti-PD-1 grew significantly smaller than wild type tumors treated with either PBS or anti-PD-1 (p < 0.03). Also, the final tumor weights of the ISG15 knockdown tumors treated with anti-PD-1 were significantly less than wild type tumors treated with either PBS or anti-PD-1 (p < 0.01) (Fig. 5b). The ISG15 knockdown tumors treated with PBS grew significantly smaller compared to wild type tumors treated with either PBS or anti-PD-1 (p < 0.03). Furthermore, the final tumor weights of the ISG15 knockdown tumors that were treated with PBS were significantly less than wild type tumors treated with PBS (p < 0.02). Although not significant, there was a 32% reduction in the final weight of ISG15 knockdown tumors treated with PBS compared to wild type tumors treated with anti-PD-1. There was no significant difference in the growth rate or final tumor weight between ISG15 knockdown tumors treated with or without anti-PD-1 antibody. In contrast, the growth of ISG15 knockdown tumors that were treated with anti-PD-1 stabilized 4 weeks posttreatment, while the PBS-treated ISG15 knockdown tumors continued to grow. Furthermore, there was a 38% decrease in the final tumor weight of the ISG15 knockdown tumors treated with anti-PD-1 compared to PBS-treated ISG15 knockdown tumors. To further investigate the combined effect of ISG15 knockdown and anti-PD-1 treatment, we analyzed the number of tumor-infiltrating CD8+ and Foxp3+ (Tregs) lymphocytes. ISG15 knockdown alone significantly increased the number of tumor-infiltrating CD8+ T cells compared to wild type tumors treated with either PBS (p = 0.001) or anti-PD-1 (p = 0.009) (Fig. 6a, b). Furthermore, the combination of ISG15 knockdown with anti-PD-1 had a synergistic effect on enhancing the number of tumor-infiltrating CD8+ T cell (Fig. 6a, b). Because there is a high correlation between tumor PDL-1 expression and tumor-infiltrating Tregs, we decided to test the effect of ISG15 knockdown on Treg intratumor infiltration. Figure 6c, d demonstrates that ISG15 knockdown tumors had a significant decrease in the number of tumor-infiltrating Tregs compared to wild type tumors treated with either PBS (p = 0.0001) or anti-PD-1 (p = 0.045). The treatment of ISG15 knockdown tumors with anti-PD-1 did not further decrease the number of tumor-infiltrating Tregs (Fig. 6c, d). The results shown in Figs. 4, 5, 6 have revealed that ISG15 knockdown in PDAC has an anti-tumor immune effect and enhances the efficacy of anti-PD-1 treatment.
Fig. 5.
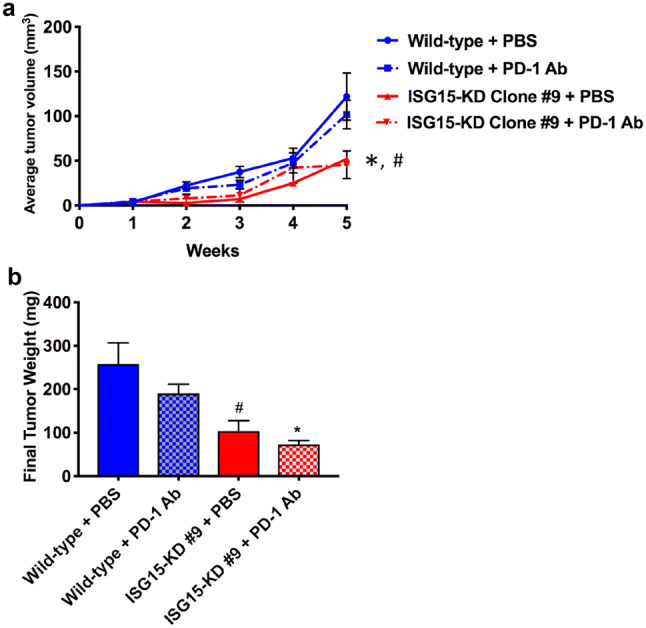
ISG15 knockdown increases the efficacy of PD-1 Ab treatment in PDAC. a Equal sized tumors were treated with anti-PD-1 over a 5-week time period. Tumor volumes were measured weekly using a caliper and the formula L × W2 × 0.5. (*p < 0.03, ISG15 KD + PD-1 Ab vs. wild type + PBS and wild type + PD-1 Ab; #p < 0.03, ISG15 KD + PBS vs. wild type + PBS and wild type + PD-1 Ab). b Final tumor weights after 5 weeks (*p < 0.01, vs. wild type + PBS and wild type + PD-1 Ab; #p < 0.02, vs. wild type + PBS)
Fig. 6.
ISG15 knockdown decreases Foxp3+ T cell intratumor infiltration and in combination with PD-1 Ab treatment synergistically increases CD8+ T cell intratumoral infiltration in PDAC. a The same tumors from Fig. 5 were stained by IHC using anti-CD8 antibody. b The quantitation of five low power (×10) fields was performed by ImageJ analysis. c The same tumors from Fig. 5 were stained by IHC using anti-Foxp3 antibody. d The quantitation of five low power (×10) fields was performed by ImageJ analysis
Discussion
The ISG15 pathway is a unique and dynamic pathway that consists of free ISG15 and ISG15 conjugates. Free or unconjugated ISG15 can be secreted from tumor or immune cells and function as a cytokine [5, 26]. ISG15 also forms conjugates with intracellular proteins and antagonizes the canonical ubiquitin system [2, 27]. It has been shown that ISG15 is aberrantly expressed in most human malignancies, suggesting that it has a pro-tumor function [2, 4, 12]. In vitro studies have revealed that ISGylation has pro-tumor functions and free ISG15 has immunomodulatory effects. However, in vivo studies have been controversial revealing that free ISG15 can have anti-tumor or pro-tumor effects depending on the context of the tumor model [19, 28]. For example, free ISG15 mediates anti-tumor effects by increasing tumor-infiltrating natural killer cells in breast cancer [28]. In contrast, free ISG15 secreted by tumor-associated macrophages supports the growth of PDAC [19]. The interpretation of the function of free ISG15 in these studies is not clear, because immunocompromised mice were used and the immune system plays important roles in anti-tumor and pro-tumor responses.
In the current study, we investigated the role of the ISG15 pathway in pancreatic tumorigenesis using syngeneic mouse models. The advantage of examining both ISG15 and UbcH8 knockdown cells is that dual roles of ISG15 functioning as a free protein and in ISGylation can be separated, because ISG15 knockdown cells have decreased levels of conjugates and lack free mature ISG15, while UbcH8 knockdown cells have decreased levels of conjugates, but express free mature ISG15. Using ISG15 and UbcH8 knockdown PDAC cells, we examined the functions of free and conjugated ISG15 in pancreatic cancer. Our in vitro studies demonstrated that ISGylation promotes KRAS-associated phenotypes of PDAC cells such as increased cell growth and colony formation. These findings are consistent with other studies in breast, hepatocellular, and nasopharyngeal carcinomas [7, 12, 13, 29]. Furthermore, as previously demonstrated in breast cancer cells [7], the ISG15 pathway stabilizes KRAS protein levels in pancreatic cancer cells (data not shown).
Recent studies have demonstrated that type I interferons and an interferon-related secretome are involved in the upregulation of PDL-1 expression in tumors [17, 30, 31]. The current in vitro studies demonstrated that free ISG15 upregulates PDAC PDL-1 expression. We came to this conclusion based on the fact that free ISG15 is a secreted protein and the following findings: (1) ISG15 knockdown cells (reduced levels of ISG15 conjugates and undetectable free ISG15) have decreased levels of PDL-1 transcripts compared to wild type cells, (2) UbcH8 (ISG15 conjugating enzyme) knockdown (reduced levels of ISG15 conjugates but express free ISG15) and wild type cells have comparable levels of PDL-1 transcripts, and (3) wild type conditioned media was able to rescue PDL-1 expression in ISG15 knockdown cells, while regular media and ISG15 knockdown conditioned media could not. In the conditioned media experiment, IRF-1 mRNA levels were increased in ISG15 knockdown cells upon treatment with wild type conditioned media compared to regular media or ISG15 knockdown conditioned media treatments. It has been shown that IFN-γ induced PDL-1 expression is mediated through IRF-1 [15, 22, 23]. In the current study, free ISG15 is upregulating PDL-1 expression independent of IFN-γ since there were no detectable levels of interferon-γ transcripts in the PDAC cells.
In our in vivo studies, ISG15 knockdown significantly reduced the tumor take rate and increased the survival rate compared to mice with wild type and UbcH8 tumors. These results are similar to human studies in glioma, nasopharyngeal carcinoma, breast, colorectal, lung, and bladder cancer, which demonstrated that high ISG15 expression significantly correlated with poor overall survival [19, 29, 32]. Furthermore, UbcH8 knockdown reduced the tumor take rate and increased the survival rate compared to mice with wild type tumors, but it was not significant. These findings suggest that both free ISG15 and conjugates contributes to PDAC tumorigenesis, because ISG15 knockdown cells have deceased levels of ISG15 conjugates and lack free ISG15, while UbcH8 knockdown cells have decreased levels of conjugates, but express free ISG15. The tumor studies also revealed that ISG15 knockdown tumors had decreased expression of PDL-1 and subsequently increased numbers of tumor-infiltrating CD8+ T cells. This effect was synergistically increased by the addition of anti-PD-1 treatment. ISG15 knockdown decreased the number of tumor-infiltrating Tregs and this effect was not enhanced by anti-PD-1 treatment, suggesting that the decreased PDL-1 expression in ISG15 knockdown tumors is directly suppressing the number of tumor-infiltrating Tregs. In agreement with previous studies, tumor PDL-1 expression is associated with regulatory T cell intratumoral infiltration [33, 34]. Furthermore, high ISG15 expression can induce resistance to Treg depletion and promote their recovery [35] suggesting that ISG15 may be regulating Treg intratumoral infiltration by multiple mechanisms.
The current study reveals the role of the ISG15 pathway in pancreatic cancer cell transformation and modulating the tumor microenvironment of PDAC. The pro-tumor responses elicited by the ISG15 pathway make it an ideal candidate for targeted therapy against pancreatic cancer. Furthermore, the synergistic effect of ISG15 knockdown with anti-PD-1 treatment is promising. Strategies to decrease the expression of ISG15 in PDAC tumors may increase the efficacy of immune checkpoint inhibitors and improve survival of the PDAC patients.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Julian Burks would like to thank Dr. Jay Berzofsky at the National Cancer Institute-Vaccine Branch for providing lab space and support for part of the studies.
Abbreviations
- CRISPR
Clustered regularly interspaced short palindromic repeats
- IRF-1
Interferon regulatory factor-1
- ISG15
Interferon-stimulated gene 15
- PDAC
Pancreatic ductal adenocarcinoma
- PD-1
Programmed cell death protein-1
- PDL-1
Programmed death ligand-1
- Tregs
Regulatory T cells
Author contributions
JB conceived and designed the research; JPS, JB, AF, and SL performed the experiments; JB analyzed the data; JPS, JB, AF, and SL interpreted the results of the experiments; JB prepared the figures; JB drafted the manuscript; JPS, JB, AF, and SL edited and revised the manuscript; all the authors approved the content of the final manuscript version.
Funding
This work was supported by the NIH T32CA00968 Grant, a Department of Medicine pilot award and The Ruesch Center for the Cure of Gastrointestinal Cancers, Georgetown University Medical Center. These studies were conducted in part at the Lombardi Comprehensive Cancer Center Histopathology and Tissue Shared resource funded by NIH CA051008.
Compliance with ethical standards
Conflict of interest
The authors have declared that no conflict of interest exists.
Ethical standards
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in the studies involving animals were in accordance with the ethical standards of the Georgetown University. The IACUC Protocol Number was 2016-1193 and the protocol approval date was 10/05/2017.
Animal source
C57BL/6 mice were purchased from Charles River Laboratories (Maryland).
Cell line authentication
Murine pancreatic cancer cells, Panc02, which is syngeneic to C57BL/6 mice, [36] were a gift to Dr. Smith from Professor Corbett (Wayne State University, MI, USA). The Panc02 cell line was donated via the National Cancer Institute cell bank repository and the cell line was authenticated there. IMPACT-III testing was performed by IDEXX BioResearch (Columbia, MO, USA) to ensure the cells were pathogen-free.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med. 2014;371(11):1039–1049. doi: 10.1056/NEJMra1404198. [DOI] [PubMed] [Google Scholar]
- 2.Desai SD, Haas AL, Wood LM, Tsai YC, Pestka S, Rubin EH, Saleem A, Nur EKA, Liu LF. Elevated expression of ISG15 in tumor cells interferes with the ubiquitin/26S proteasome pathway. Can Res. 2006;66(2):921–928. doi: 10.1158/0008-5472.CAN-05-1123. [DOI] [PubMed] [Google Scholar]
- 3.Li XY, Yan J, Sun J, Li C, Jiang JY, Wang JM, Meng XN, Liang JJ. Wang HQ (2019) BAG3 deletion suppresses stem cell-like features of pancreatic ductal adenocarcinoma via translational suppression of ISG15. Biochim Biophys Acta. 1866;5:819–827. doi: 10.1016/j.bbamcr.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 4.Zuo C, Sheng X, Ma M, Xia M, Ouyang L. ISG15 in the tumorigenesis and treatment of cancer: an emerging role in malignancies of the digestive system. Oncotarget. 2016;7(45):74393–74409. doi: 10.18632/oncotarget.11911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D’Cunha J, Ramanujam S, Wagner RJ, Witt PL, Knight E, Jr, Borden EC. In vitro and in vivo secretion of human ISG15, an IFN-induced immunomodulatory cytokine. J Immunol. 1996;157(9):4100–4108. [PubMed] [Google Scholar]
- 6.Andersen JB, Hassel BA. The interferon regulated ubiquitin-like protein, ISG15, in tumorigenesis: friend or foe? Cytokine Growth Factor Rev. 2006;17(6):411–421. doi: 10.1016/j.cytogfr.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Burks J, Reed RE, Desai SD. ISGylation governs the oncogenic function of Ki-Ras in breast cancer. Oncogene. 2013 doi: 10.1038/onc.2012.633. [DOI] [PubMed] [Google Scholar]
- 8.Tsai YC, Pestka S, Wang LH, Runnels LW, Wan S, Lyu YL, Liu LF. Interferon-beta signaling contributes to Ras transformation. PLoS One. 2011;6(8):e24291. doi: 10.1371/journal.pone.0024291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hummer BT, Li XL, Hassel BA. Role for p53 in gene induction by double-stranded RNA. J Virol. 2001;75(16):7774–7777. doi: 10.1128/JVI.75.16.7774-7777.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wood LM, Sankar S, Reed RE, Haas AL, Liu LF, McKinnon P, Desai SD. A novel role for ATM in regulating proteasome-mediated protein degradation through suppression of the ISG15 conjugation pathway. PLoS One. 2011;6(1):e16422. doi: 10.1371/journal.pone.0016422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tripathi MK, Chaudhuri G. Down-regulation of UCRP and UBE2L6 in BRCA2 knocked-down human breast cells. Biochem Biophys Res Commun. 2005;328(1):43–48. doi: 10.1016/j.bbrc.2004.12.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desai SD, Reed RE, Burks J, Wood LM, Pullikuth AK, Haas AL, Liu LF, Breslin JW, Meiners S, Sankar S. ISG15 disrupts cytoskeletal architecture and promotes motility in human breast cancer cells. Exp Biol Med (Maywood) 2012;237(1):38–49. doi: 10.1258/ebm.2011.011236. [DOI] [PubMed] [Google Scholar]
- 13.Li C, Wang J, Zhang H, Zhu M, Chen F, Hu Y, Liu H, Zhu H. Interferon-stimulated gene 15 (ISG15) is a trigger for tumorigenesis and metastasis of hepatocellular carcinoma. Oncotarget. 2014;5(18):8429–8441. doi: 10.18632/oncotarget.2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia-Diaz A, Shin DS, Moreno BH, Saco J, Escuin-Ordinas H, Rodriguez GA, Zaretsky JM, Sun L, Hugo W, Wang X, Parisi G, Saus CP, Torrejon DY, Graeber TG, Comin-Anduix B, Hu-Lieskovan S, Damoiseaux R, Lo RS, Ribas A. Interferon receptor signaling pathways regulating PD-L1 and PD-L2 expression. Cell Rep. 2017;19(6):1189–1201. doi: 10.1016/j.celrep.2017.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee SJ, Jang BC, Lee SW, Yang YI, Suh SI, Park YM, Oh S, Shin JG, Yao S, Chen L, Choi IH. Interferon regulatory factor-1 is prerequisite to the constitutive expression and IFN-gamma-induced upregulation of B7-H1 (CD274) FEBS Lett. 2006;580(3):755–762. doi: 10.1016/j.febslet.2005.12.093. [DOI] [PubMed] [Google Scholar]
- 16.Li Y, Li F, Jiang F, Lv X, Zhang R, Lu A, Zhang G. A mini-review for cancer immunotherapy: molecular understanding of PD-1/PD-L1 pathway and translational blockade of immune checkpoints. Int J Mol Sci. 2016;17:7. doi: 10.3390/ijms17071151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang YQ, Dong WJ, Yin XF, Xu YN, Yang Y, Wang JJ, Yuan SJ, Xiao J, DeLong JH, Chu L, Xu HN, Zhou XM, Wang RW, Fang L, Liu XY, Zhang KJ. Interferon-related secretome from direct interaction between immune cells and tumor cells is required for upregulation of PD-L1 in tumor cells. Protein Cell. 2016;7(7):538–543. doi: 10.1007/s13238-016-0281-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lenschow DJ, Giannakopoulos NV, Gunn LJ, Johnston C, O’Guin AK, Schmidt RE, Levine B, Virgin HWt. Identification of interferon-stimulated gene 15 as an antiviral molecule during Sindbis virus infection in vivo. J Virol. 2005;79(22):13974–13983. doi: 10.1128/jvi.79.22.13974-13983.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sainz B, Jr, Martin B, Tatari M, Heeschen C, Guerra S. ISG15 is a critical microenvironmental factor for pancreatic cancer stem cells. Can Res. 2014;74(24):7309–7320. doi: 10.1158/0008-5472.Can-14-1354. [DOI] [PubMed] [Google Scholar]
- 20.Hu Y, Chen W, Yan Z, Ma J, Zhu F, Huo J. Prognostic value of PD-L1 expression in patients with pancreatic cancer: a PRISMA-compliant meta-analysis. Medicine. 2019;98(3):e14006. doi: 10.1097/md.0000000000014006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao HL, Liu L, Qi ZH, Xu HX, Wang WQ, Wu CT, Zhang SR, Xu JZ, Ni QX, Yu XJ. The clinicopathological and prognostic significance of PD-L1 expression in pancreatic cancer: a meta-analysis. Hepatobil Pancreat Dis Int. 2018;17(2):95–100. doi: 10.1016/j.hbpd.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 22.Moon JW, Kong SK, Kim BS, Kim HJ, Lim H, Noh K, Kim Y, Choi JW, Lee JH, Kim YS. IFNgamma induces PD-L1 overexpression by JAK2/STAT1/IRF-1 signaling in EBV-positive gastric carcinoma. Sci Rep. 2017;7(1):17810. doi: 10.1038/s41598-017-18132-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qian J, Wang C, Wang B, Yang J, Wang Y, Luo F, Xu J, Zhao C, Liu R, Chu Y. The IFN-gamma/PD-L1 axis between T cells and tumor microenvironment: hints for glioma anti-PD-1/PD-L1 therapy. J Neuroinflamm. 2018;15(1):290. doi: 10.1186/s12974-018-1330-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seifert AM, Zeng S, Zhang JQ, Kim TS, Cohen NA, Beckman MJ, Medina BD, Maltbaek JH, Loo JK, Crawley MH, Rossi F, Besmer P, Antonescu CR, DeMatteo RP. PD-1/PD-L1 blockade enhances T-cell activity and antitumor efficacy of imatinib in gastrointestinal stromal tumors. Clin Cancer Res. 2017;23(2):454–465. doi: 10.1158/1078-0432.Ccr-16-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lanitis E, Dangaj D, Irving M, Coukos G. Mechanisms regulating T-cell infiltration and activity in solid tumors. Ann Oncol. 2017;28(suppl_12):xii18–xii32. doi: 10.1093/annonc/mdx238. [DOI] [PubMed] [Google Scholar]
- 26.D’Cunha J, Knight E, Jr, Haas AL, Truitt RL, Borden EC. Immunoregulatory properties of ISG15, an interferon-induced cytokine. Proc Natl Acad Sci USA. 1996;93(1):211–215. doi: 10.1073/pnas.93.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Durfee LA, Huibregtse JM. The ISG15 conjugation system. Methods Mol Boil (Clifton, NJ) 2012;832:141–149. doi: 10.1007/978-1-61779-474-2_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burks J, Reed RE, Desai SD. Free ISG15 triggers an antitumor immune response against breast cancer: a new perspective. Oncotarget. 2015;6(9):7221–7231. doi: 10.18632/oncotarget.3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen RH, Du Y, Han P, Wang HB, Liang FY, Feng GK, Zhou AJ, Cai MY, Zhong Q, Zeng MS, Huang XM. ISG15 predicts poor prognosis and promotes cancer stem cell phenotype in nasopharyngeal carcinoma. Oncotarget. 2016;7(13):16910–16922. doi: 10.18632/oncotarget.7626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bazhin AV, von Ahn K, Fritz J, Werner J, Karakhanova S. Interferon-alpha up-regulates the expression of PD-L1 molecules on immune cells through STAT3 and p38 signaling. Front Immunol. 2018;9:2129. doi: 10.3389/fimmu.2018.02129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morimoto Y, Kishida T, Kotani SI, Takayama K, Mazda O. Interferon-beta signal may up-regulate PD-L1 expression through IRF9-dependent and independent pathways in lung cancer cells. Biochem Biophys Res Commun. 2018;507(1–4):330–336. doi: 10.1016/j.bbrc.2018.11.035. [DOI] [PubMed] [Google Scholar]
- 32.Duarte CW, Willey CD, Zhi D, Cui X, Harris JJ, Vaughan LK, Mehta T, McCubrey RO, Khodarev NN, Weichselbaum RR, Gillespie GY. Expression signature of IFN/STAT1 signaling genes predicts poor survival outcome in glioblastoma multiforme in a subtype-specific manner. PLoS One. 2012;7(1):e29653. doi: 10.1371/journal.pone.0029653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Que Y, Xiao W, Guan YX, Liang Y, Yan SM, Chen HY, Li QQ, Xu BS, Zhou ZW, Zhang X. PD-L1 expression is associated with FOXP3+ regulatory T-cell infiltration of soft tissue sarcoma and poor patient prognosis. J Cancer. 2017;8(11):2018–2025. doi: 10.7150/jca.18683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DiDomenico J, Lamano JB, Oyon D, Li Y, Veliceasa D, Kaur G, Ampie L, Choy W, Lamano JB, Bloch O. The immune checkpoint protein PD-L1 induces and maintains regulatory T cells in glioblastoma. Oncoimmunology. 2018;7(7):e1448329. doi: 10.1080/2162402x.2018.1448329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Francesca Romana Spinelli IP, Piconese Silvia, Ceccarelli Fulvia, Miranda Francesca, Alessandri Cristiano, Barnaba Vincenzo, Valesini Guido, Conti Fabrizio. Interferon stimulated gene 15 protects regulatory T cell of systemic lupus erythematosus patients from interferon alpha-mediated depletion. Ann Rheum Dis. 2019;78:379–380. [Google Scholar]
- 36.Corbett TH, Roberts BJ, Leopold WR, Peckham JC, Wilkoff LJ, Griswold DP, Jr, Schabel FM., Jr Induction and chemotherapeutic response of two transplantable ductal adenocarcinomas of the pancreas in C57BL/6 mice. Can Res. 1984;44(2):717–726. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.