Abstract
Menopause is associated with adverse changes in vascular health coinciding with an increased risk of stroke and vascular cognitive impairment. However, there is significant variation in the age at menopause. The present study examined how the age at natural menopause impacts cerebrovascular reactivity and structural biomarkers of brain aging. Thirty-five healthy postmenopausal women were classified as early-onset menopause (Early; n = 19, age at menopause: 47 ± 2 yr) or later-onset menopause (Late; n = 16, age at menopause: 55 ± 2 yr). Middle cerebral artery blood velocity (MCAv), mean arterial blood pressure (MAP), and end-tidal carbon dioxide (ETCO2) were recorded during a stepped hypercapnia protocol. Reactivity was calculated as the slope of the relationship between ETCO2 and each variable of interest. Brain volumes and white matter hyperintensities (WMHs) were obtained with 3T MRI. Resting MAP was greater in the Early group (99 ± 9 mmHg) compared with the Late group (90 ± 12 mmHg; P = 0.02). Cerebrovascular reactivity, assessed using MCAv, was blunted in the Early group (1.87 ± 0.92 cm/s/mmHg) compared with the Late group (2.37 ± 0.75 cm/s/mmHg; P = 0.02). Total brain volume did not differ between groups (Early: 1.08 ± 0.07 L vs. Late: 1.07 ± 0.06 L; P = 0.66), but the Early group demonstrated greater WMH fraction compared with the Late group (Early: 0.36 ± 0.14% vs. Late: 0.25 ± 0.14%; P = 0.02). These results suggest that age at natural menopause impacts cerebrovascular function and WMH burden in healthy postmenopausal women.
Keywords: brain volume, cerebrovascular reactivity, menopause, white matter hyperintensities
INTRODUCTION
Understanding cerebrovascular dysregulation with aging represents a critical target for improving brain health in older adults, as the risk of cerebrovascular disease and vascular-related cognitive disorders, which represent leading causes of mortality and disability, increases with age. Indeed, an important link exists between cerebrovascular and cognitive health, whereby failure of the cerebral vasculature to adequately regulate blood flow represents the first stage in the Alzheimer’s disease process before neuropathological alterations and declines in cognitive function become evident (1). Importantly, the trajectory of disease risk throughout the adult life span differs by sex (2), whereby older women not only demonstrate greater prevalence of stroke and vascular cognitive impairment (or Alzheimer’s disease) but also exhibit greater disability and mortality associated with these pathologies (3–5). Noticeable increases in vascular risk factors occur in women in their fifth and sixth decades of life, coinciding with menopause, including significant increases in arterial blood pressure (6). Therefore, understanding vascular-related processes that occur with menopause represents a critical avenue for mitigating the effects of menopause on women’s cerebrovascular function and brain health.
The reduction in ovarian sex hormones with menopause is linked to cerebrovascular dysfunction and brain structural changes. Endogenous estrogen exhibits vasoprotective effects largely through promoting vasodilation (7, 8). Indeed, postmenopausal women demonstrate lower cerebral blood velocity (8–10), lower cerebrovascular conductance (9), increased cerebrovascular resistance (10), and increased pulsatility index (11) at rest compared with premenopausal women. In response to hypercapnia, postmenopausal women have lower cerebrovascular reactivity compared with premenopausal women (8, 12, 13). Dysregulation of the cerebral vasculature may cause injury to the small cerebral vessels, known as cerebral small vessel disease, which represents a key underlying mechanism for stroke, Alzheimer’s disease, and dementia (14). Importantly, brain structural changes in the form of white matter hyperintensities (WMHs) represent the most notable clinical finding of cerebral small vessel disease (14). Postmenopausal women demonstrate steeper age-related increases in WMH volume than premenopausal women and men (15). Menopause also appears to accelerate age-related declines in brain volume, whereby steeper inverse associations between age and total brain volume were observed in women compared with men as well as in postmenopausal women relative to premenopausal women (15). However, these findings are not unanimous, with reports that cerebrovascular function (9, 10, 13) and WMH volume (15) were not different between pre- and postmenopausal women. Variations in the age at menopause or the timing of measurement following menopause may be important factors contributing to discrepancies between studies.
Although menopause represents a significant physiological event that produces adverse changes in vascular function and brain structure, a high degree of variability exists regarding the timing of natural menopause. The average age for natural menopause is 51 yr, but it can range from ∼40 to 60 yr (16). Women who experience menopause, whether natural or surgically induced, at a younger age have an increased risk of ischemic stroke, dementia, cognitive decline, cardiovascular disease mortality, and all-cause mortality (16–19) compared with women who experience natural menopause later in life. As such, the timing of natural menopause in healthy women may impact cerebrovascular function and brain structure; yet, this remains to be elucidated.
The objective of this study was to examine the influence of age at natural menopause on 1) cerebrovascular function evaluated as cerebrovascular reactivity to carbon dioxide (CO2) and 2) structural biomarkers of brain aging, including brain volumes and WMH lesions. We tested the hypothesis that postmenopausal women with an earlier onset of menopause would demonstrate lower cerebrovascular reactivity than postmenopausal women with a later onset of menopause. Our exploratory hypothesis was that women with an earlier onset of menopause would have lower brain volume and higher WMH lesions compared with women with a later onset of menopause.
METHODS
Participants
Thirty-five healthy women (age: 55–68 yr) were included in the retrospective analysis and divided into two groups based on self-reported age at onset of natural menopause. Data from women with surgical menopause were excluded. The median age of menopause ranges from 50 to 52 yr (16, 20, 21). Therefore, participants were divided into the Early group (≤49 yr of age at onset of menopause) or the Late group (≥53 yr of age at onset of menopause), with data from women who experienced menopause between the ages of 50 and 52 yr excluded from the analysis. The Early group (n = 19) began natural menopause at 47 ± 2 yr of age (range: 43–49 yr), and the Late group (n = 16) began natural menopause at 55 ± 2 yr of age (range: 53–57 yr). All participants were postmenopausal for >1 yr and were not using oral menopausal hormone therapy. Participants were healthy women free from known cardiovascular or cerebrovascular disease, uncontrolled hypertension, major neurological disorders, or cognitive diagnoses (e.g., mild cognitive impairment, Alzheimer’s disease). All study procedures were approved by the University of Wisconsin-Madison Institutional Review Board (No. 2017-0441) and performed in accordance with the Declaration of Helsinki. All participants provided written informed consent. Some of the participants in this retrospective study were included in a recently published study investigating neurovascular coupling (22).
Laboratory Procedures
Participants arrived at the laboratory having fasted for 4 h and having abstained from caffeine or chocolate for 12 h, exercise and alcohol for 24 h, and nonsteroidal anti-inflammatory drugs for 48 h prior. Participants completed self-report questionnaires regarding physical activity history, including the Godin Leisure-Time Exercise Questionnaire, and height and weight were measured. Participants rested quietly in the supine posture, and brachial blood pressure was taken in triplicate (Cardiocap 5, Datex-Ohmeda, Madison, WI), with 2 min of rest between for measures of resting blood pressure and heart rate (HR). Participants were instrumented with a finger blood pressure cuff to record beat-to-beat blood pressure (Finapres Medical System, Amsterdam, The Netherlands), a 3-lead electrocardiogram to record HR (Cardiocap 5, Datex-Ohmeda, Madison, WI), and a nasal cannula to continuously record end-tidal CO2 (ETCO2). To further characterize vascular health, aortic blood pressure was measured with applanation tonometry (Sphygmocor, AtcorMedical, Sydney, Australia), and common carotid artery ultrasound was performed 1–2 cm below the bifurcation using an 11-L probe with a transmission frequency of 4.5–12 MHz (GE LOGIQ S8, GE Healthcare, Waukesha, WI). Middle cerebral artery blood velocity (MCAv) was recorded with transcranial Doppler ultrasound (ST3, Spencer Technologies, Redmond, WA) and a 2-MHz probe secured at the left transtemporal window. Participants were fitted with a face mask with a one-way valve to prevent rebreathing (7450-V2, Hans Rudolph, Shawnee Mission, KS). Cerebrovascular reactivity was performed as previously described (23). In a stepwise manner, participants breathed room air, 2% CO2, 4% CO2, and 6% CO2 gas mixtures (21% oxygen, balanced nitrogen) for 5 min each. On a separate visit, waist circumference was measured, and a venous blood draw was collected for measures of blood glucose, lipids, and high-sensitivity C-reactive protein.
Magnetic Resonance Imaging
Participants attended a separate session for magnetic resonance imaging (MRI) at the Wisconsin Institutes for Medical Research in Madison, WI. The MRI visit was performed using a 3-T MRI scanner (MR750, GE Healthcare, Waukesha, WI) with a 32-channel head coil (Nova Medical Head Coil, Nova Medical, Wilmington, MA). Brain volumes were acquired with T1-weighted structural brain volume (BRAVO) scans with the following imaging parameters: fast spoiled gradient echo sequence, inversion time = 450 ms, repetition time (TR) = 8.1 ms, echo time (TE) = 3.2 ms, flip angle = 12°, acquisition matrix = 256 × 256, field of view (FOV) = 256 mm, slice thickness = 1.0 mm, and scan time = ∼8 min. WMHs were evaluated from T2 fluid-attenuated inversion retention (FLAIR) images with the following imaging parameters: inversion time = 1,724–1,742 ms, TR = 6,000–6,002 ms, TE = 107–125 ms, flip angle = 90°, acquisition matrix = 256 × 256, FOV = 256 mm, slice thickness = 1.6–2.0 mm, and scan time = ∼4 min.
Data Analysis
Researchers were blinded to menopausal status at the time of data analysis. Ultrasound images used to calculate carotid intima-media thickness (IMT) were analyzed offline (Carotid Analyzer, Medical Imaging Applications, LLC, Coralville, IA), and the average of the far wall IMT is reported. Cardiovascular and cerebrovascular data were collected at 250 Hz and stored for offline analysis (LabChart 8, ADInstruments, Dunedin, New Zealand). For the stepped hypercapnia protocol, mean arterial pressure (MAP), MCAv, and ETCO2 were averaged over 60 s (minute 2 to minute 3) of each stage. Cerebrovascular conductance index (CVCi) was calculated as MCAv/MAP and was also averaged over the same 60 s of each stage. Cerebrovascular reactivity was calculated as the slope of the relationship between ETCO2 and each variable of interest (MAP, MCAv, and CVCi) across all stages, using both raw values and percent change values from room air. Tissue class segmentation of T1-weighted images into gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF) was performed using Statistical Parametric Mapping 12 (SPM12). The sum of GM and WM volumes provided total brain volume, whereas intracranial volume (ICV) was calculated as the sum of GM, WM, and CSF volumes. From T2-FLAIR images, total WMH lesion volume was calculated using the lesion prediction algorithm from the Lesion Segmentation Tool in SPM12. WMH fraction was calculated as the cube root of (WMH lesion volume/ICV) × 100.
Statistical Analysis
Statistical analysis was conducted using IBM SPSS Statistics 28 (SPSS, Inc., Chicago, IL). A χ2 test of association evaluated participants’ race, ethnicity, and medication use between the Early and Late groups. Participant demographics, resting hemodynamics, brain volumes, and WMH between the Early and Late groups were compared using independent t tests or Mann–Whitney tests, where indicated. A two-way ANOVA with repeated measures evaluated differences in MAP, MCAv, CVCi, and ETCO2 between the Early and Late groups (Group) during the stepped hypercapnia protocol, including the room air, 2% CO2, 4% CO2, and 6% CO2 steps (Condition). An independent t test evaluated MAP reactivity, whereas an analysis of covariance (ANCOVA) with baseline MAP as a covariate compared MCAv and CVCi reactivity between groups. The relationships between age at menopause and variables of interest (MAP, WMH) were determined with Spearman correlations, whereas Pearson correlations evaluated the relationships between time since menopause and variables of interest. Partial correlations with baseline MAP as a covariate determined the relationships between MCAv reactivity and years since menopause as well as age at menopause. The relationships between WMH fraction and cerebrovascular reactivity were examined by Pearson correlations (MCAv reactivity and CVCi reactivity) and Spearman correlations (MAP reactivity). A Pearson correlation examined associations between resting MAP and WMH fraction. Statistical significance was set at P < 0.05.
RESULTS
The Early and Late groups did not differ in age at the time of the study (Table 1) and were 14 ± 5 yr and 7 ± 4 yr since the onset of menopause, respectively (P < 0.001). No differences were observed between groups in participant characteristics (Table 1). Participant race and ethnicity did not differ between the Early and Late groups [race: χ2 (3) = 3.27, P = 0.35; ethnicity: χ2 (1) = 0.87, P = 0.35]. Medication use, including blood pressure, depression, cholesterol, and thyroid medications, did not differ between the Early and Late groups [χ2 (3) = 0.69, P = 0.88]. Differences in resting hemodynamics were observed, with the Early group demonstrating greater aortic and brachial blood pressure compared with the Late group (Table 1). Resting MAP was inversely associated with age at menopause (r = −0.3, P = 0.04) and positively associated with time since menopause (r = 0.4, P = 0.03).
Table 1.
Participant characteristics, resting hemodynamics, and brain structural indices
| Early (n = 19) | Late (n = 16) | P Value | |
|---|---|---|---|
| Age at onset of menopause, yr* | 47 ± 2 | 55 ± 2 | <0.001 |
| Age at study, yr | 61 ± 4 | 61 ± 3 | 0.58 |
| Education, yr* | 16 ± 2 | 17 ± 2 | 0.23 |
| Godin Leisure-Time Exercise Score, AU | 43 ± 25 | 33 ± 17 | 0.20 |
| MET-min, min/wk | 1,677 ± 977 | 1,660 ± 765 | 0.96 |
| Height, cm | 164 ± 7 | 165 ± 5 | 0.66 |
| Weight, kg | 72 ± 14 | 70 ± 14 | 0.61 |
| BMI, kg/m2 | 27 ± 4 | 26 ± 5 | 0.46 |
| Waist circumference, cm | 90 ± 12 | 90 ± 13 | 0.83 |
| Glucose, mg/dL | 92 ± 8 | 90 ± 6 | 0.42 |
| Total cholesterol, mg/dL | 215 ± 32 | 217 ± 30 | 0.86 |
| Triglycerides, mg/dL* | 91 ± 49 | 98 ± 39 | 0.40 |
| HDL cholesterol, mg/dL | 70 ± 14 | 70 ± 14 | 0.98 |
| LDL cholesterol, mg/dL | 127 ± 25 | 127 ± 22 | 0.97 |
| C-reactive protein, mg/dL* | 1.5 ± 1.2 | 2.2 ± 3.4 | 0.99 |
| Carotid IMT, mm | 0.70 ± 0.09 | 0.68 ± 0.09 | 0.40 |
| Aortic SBP, mmHg | 131 ± 13 | 117 ± 18 | 0.02 |
| Aortic DBP, mmHg | 81 ± 7 | 74 ± 9 | 0.02 |
| Brachial SBP, mmHg | 136 ± 15 | 123 ± 20 | 0.04 |
| Brachial DBP, mmHg | 80 ± 7 | 73 ± 9 | 0.02 |
| Brachial MAP, mmHg | 99 ± 9 | 90 ± 12 | 0.02 |
| HR, beats/min* | 61 ± 7 | 60 ± 9 | 0.39 |
| Total brain volume, L | 1.08 ± 0.07 | 1.07 ± 0.06 | 0.66 |
| Intracranial volume, L | 1.37 ± 0.10 | 1.40 ± 0.07 | 0.33 |
| GM volume, L | 0.67 ± 0.05 | 0.66 ± 0.04 | 0.37 |
| WM volume, L | 0.41 ± 0.04 | 0.41 ± 0.04 | 0.86 |
| CSF volume, L* | 0.29 ± 0.06 | 0.33 ± 0.06 | 0.01 |
| WMH lesion volume, mL* | 1.00 ± 1.32 | 0.41 ± 0.51 | 0.07 |
Values are presented as means ± SD. BMI, body mass index; CSF, cerebrospinal fluid; DBP, diastolic blood pressure; GM, gray matter; HR, heart rate; IMT, intima-media thickness; MAP, mean arterial pressure; MET, metabolic equivalent of task-minutes; SBP, systolic blood pressure; WM, white matter; WMH, white matter hyperintensity. Boldface terms represent significant P values. Mann–Whitney U test.
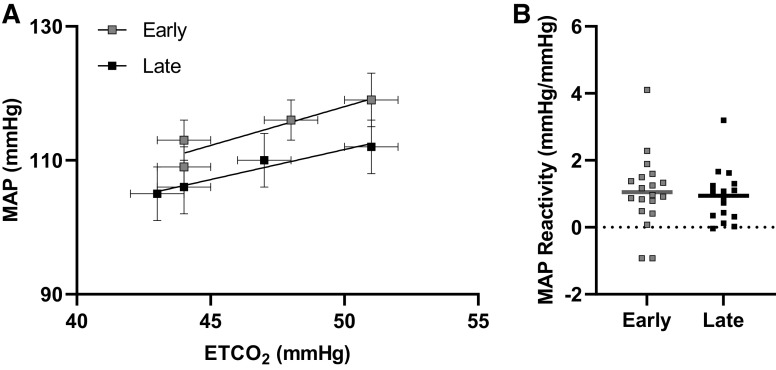
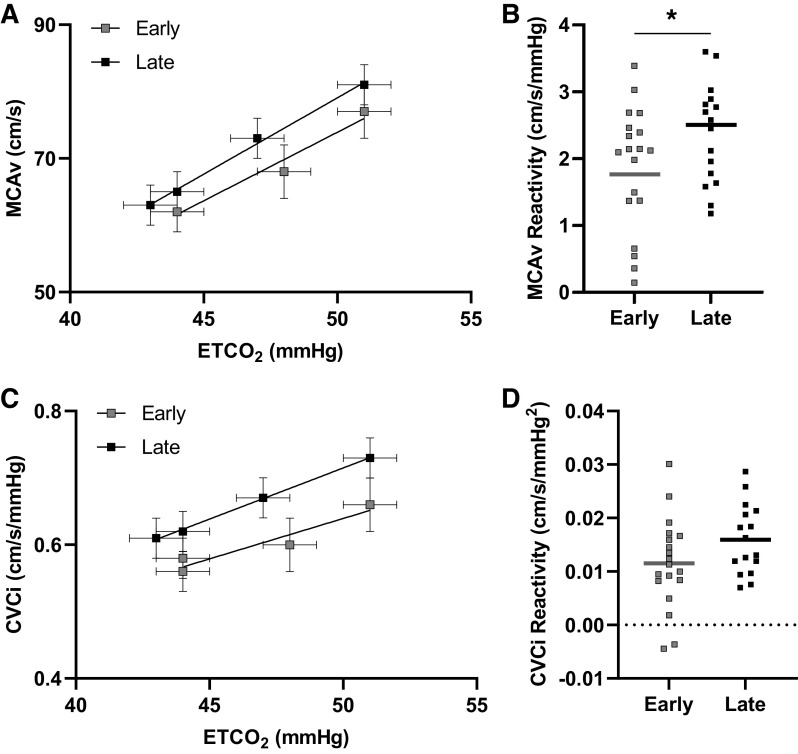
During the hypercapnia protocol, ETCO2 levels during room air, 2% CO2, 4% CO2, and 6% CO2 did not differ between the Early- and Late-onset menopause groups (Table 2). Hypercapnia increased MAP (Table 2, Fig. 1), MCAv (Table 2, Fig. 2), and CVCi (Table 2, Fig. 2), but the responses at each level did not differ between groups. Also, there were no differences between groups in MAP reactivity to hypercapnia when reactivity was calculated using raw values (Early: 1.05 ± 1.10 mmHg/mmHg, Late: 0.95 ± 0.81 mmHg/mmHg, P = 0.75; Fig. 1) or percent change values (Early: 0.39 ± 0.41%mmHg/%mmHg, Late: 0.41 ± 0.37%mmHg/%mmHg, P = 0.88).
Table 2.
Hemodynamic and gas exchange variables during stepped hypercapnia
| Room Air | 2% CO2 | 4% CO2 | 6% CO2 | ANOVA P Values |
|||
|---|---|---|---|---|---|---|---|
| Group | Condition | Interaction | |||||
| ETCO2, mmHg | |||||||
| Early | 44 ± 3 | 44 ± 3 | 48 ± 3 | 51 ± 3 | 0.82 | <0.01 | 0.62 |
| Late | 43 ± 4 | 44 ± 3 | 47 ± 3 | 51 ± 3 | |||
| MAP, mmHg | |||||||
| Early | 109 ± 14 | 113 ± 15 | 116 ± 15 | 119 ± 18 | 0.28 | <0.01 | 0.27 |
| Late | 105 ± 17 | 106 ± 16 | 110 ± 16 | 112 ± 16 | |||
| MCAv, cm/s | |||||||
| Early | 62 ± 14 | 62 ± 15 | 68 ± 17 | 77 ± 19 | 0.47 | <0.01 | 0.23 |
| Late | 63 ± 10 | 65 ± 11 | 73 ± 12 | 81 ± 11 | |||
| CVCi, cm/s/mmHg | |||||||
| Early | 0.58 ± 0.14 | 0.56 ± 0.15 | 0.60 ± 0.17 | 0.66 ± 0.18 | 0.20 | <0.01 | 0.09 |
| Late | 0.61 ± 0.11 | 0.62 ± 0.11 | 0.67 ± 0.13 | 0.73 ± 0.13 | |||
| PI, AU | |||||||
| Early | 0.80 ± 0.10 | 0.81 ± 0.11 | 0.77 ± 0.09 | 0.73 ± 0.09 | 0.88 | <0.01 | 0.42 |
| Late | 0.80 ± 0.09 | 0.79 ± 0.11 | 0.77 ± 0.11 | 0.74 ± 0.11 | |||
Values are presented as means ± SD. CO2, carbon dioxide; CVCi, cerebrovascular conductance index; ETCO2, end-tidal carbon dioxide partial pressure; MAP, mean arterial pressure; MCAv, mean middle cerebral artery blood velocity; PI, pulsatility index. Boldface terms represent significant P values.
Figure 1.
Relationship between end-tidal carbon dioxide (ETCO2) and mean arterial pressure (MAP) by early-onset menopause (n = 19) and late-onset menopause (n = 16; A). Mean and standard errors are shown. MAP reactivity between early-onset menopause and late-onset menopause (B). Mean (line) and individual data shown. An independent t test demonstrated no differences in MAP reactivity between groups (P = 0.75).
Figure 2.
Relationship between end-tidal carbon dioxide (ETco2) and middle cerebral artery blood velocity (MCAv) by early-onset menopause (n = 19) and late-onset menopause (n = 16; A). Mean and standard errors are shown. MCAv reactivity between early-onset menopause and late-onset menopause (B). Mean (line) and individual data shown. An analysis of covariance (ANCOVA) with mean arterial pressure (MAP) as a covariate demonstrated attenuated MCAv reactivity in the Early group compared with the Late group (P = 0.02). Relationship between ETCO2 and cerebrovascular conductance index (CVCi) by early-onset menopause (n = 19) and late-onset menopause (n = 16; C). Mean and standard errors are shown. CVCi reactivity between early-onset menopause and late-onset menopause (D). Mean (line) and individual data shown. An ANCOVA with MAP as a covariate demonstrated no difference in CVCi reactivity between groups (P = 0.15). *P < 0.05 between groups.
For cerebrovascular reactivity, MCAv reactivity was attenuated in the Early group [ANCOVA adjusted mean (MAP as covariate): 1.76 ± 0.19 cm/s/mmHg] relative to the Late group (ANCOVA adjusted mean: 2.50 ± 0.21 cm/s/mmHg; P = 0.02, d = 0.9; Fig. 2) when calculated with raw values. The time since menopause in years was inversely related to MCAv reactivity (r = −0.4, P = 0.04; Fig. 4), whereas the age at menopause was not related (r = 0.3, P = 0.06). When MCAv reactivity was calculated with percent change values, the Early group demonstrated reduced MCAv reactivity compared with the Late group (Early ANCOVA adjusted mean: 1.25 ± 0.14%cm/s/%mmHg; Late ANCOVA adjusted mean: 1.71 ± 0.16%cm/s/%mmHg; P = 0.045, d = 0.9). When cerebrovascular reactivity was evaluated using CVCi, no differences were observed between groups when calculated using raw values (Early ANCOVA adjusted mean: 0.01 ± 0.002 cm/s/mmHg2, Late ANCOVA adjusted mean: 0.02 ± 0.002 cm/s/mmHg2, P = 0.15; Fig. 2) or percent change values (Early ANCOVA adjusted mean: 0.84 ± 0.14%cm/s/%mmHg, Late ANCOVA adjusted mean: 1.20 ± 0.15%cm/s/%mmHg, P = 0.10).
Figure 4.
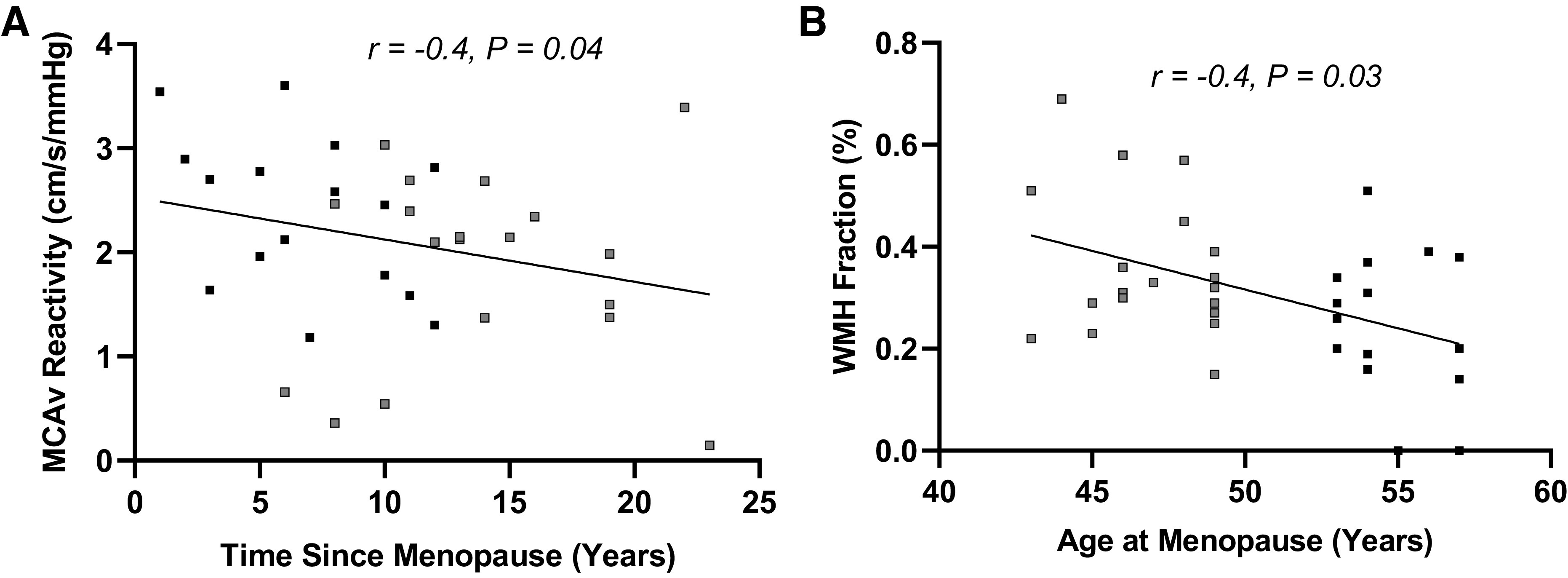
Relationships between time since menopause and middle cerebral artery blood velocity (MCAv) reactivity (A) and age at menopause and white matter hyperintensity (WMH) fraction (B) in the combined group (n = 35, gray squares represent early-onset menopause, black squares represent late-onset menopause). A partial correlation controlling for resting mean arterial pressure (MAP) demonstrated an inverse relationship between MCAv reactivity and time since menopause. A Spearman correlation demonstrated an inverse relationship between WMH fraction and age at menopause.
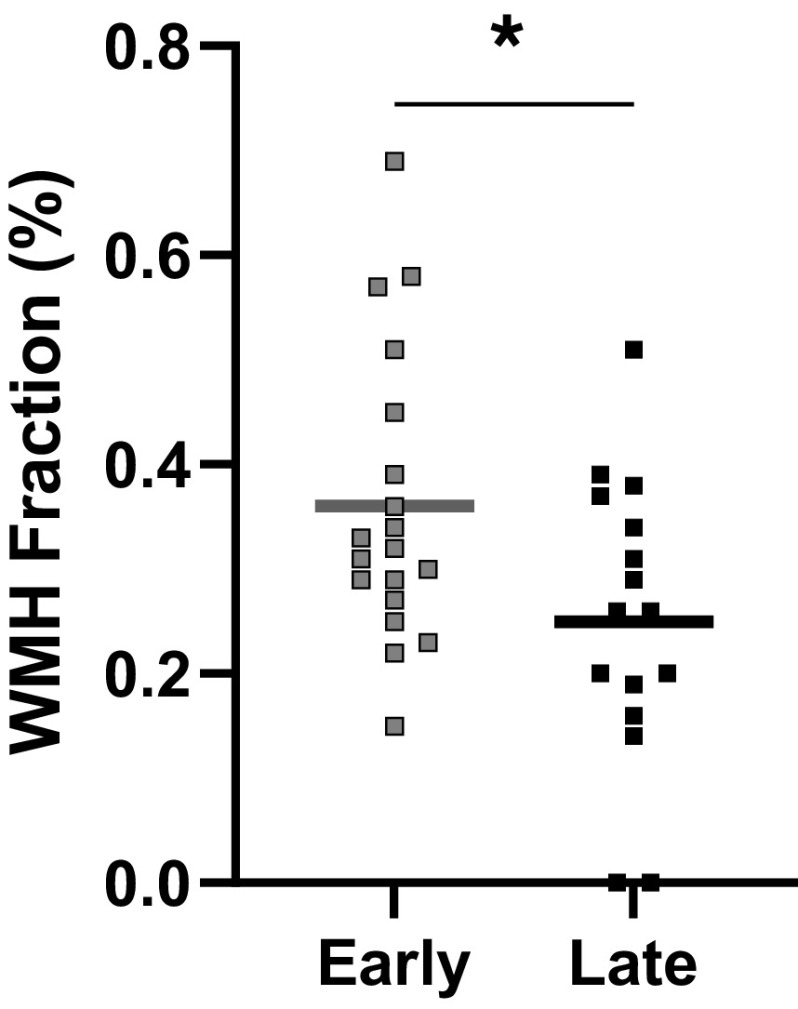
Total brain volume and intracranial volume did not differ between groups (Table 1). In contrast, the Early group demonstrated greater WMH fraction compared with the Late group (P = 0.02; Fig. 3). There was an inverse relationship between the age at onset of menopause and WMH fraction (r = −0.4, P = 0.03; Fig. 4), whereas no association was observed between years since menopause and WMH fraction (P = 0.10). WMH fraction was not associated with cerebrovascular reactivity (MAP reactivity: P = 0.96; MCAv reactivity: P = 0.99; CVCi reactivity: P = 0.48), but resting MAP was positively associated with WMH fraction (r = 0.5, P = 0.01).
Figure 3.
White matter hyperintensity (WMH) fraction in the Early group (n = 19) compared with the Late group (n = 16). Mean (line) and individual data shown. An independent t test demonstrated higher WMH fraction in the Early group compared with the Late group (P = 0.02). *P < 0.05 between groups.
DISCUSSION
The present study provides a novel insight into the impact of the age at onset of menopause and the time since menopause on cerebrovascular function and brain health. To our knowledge, this is the first report demonstrating that healthy women who experienced the onset of natural menopause at a younger age (≤49 yr) had lower cerebrovascular reactivity to CO2 relative to individuals who experienced the onset of natural menopause at a later age (≥53 yr). This was despite the fact that the groups were similar in terms of age at the time of study participation. We also report that years since menopause was inversely associated with MCAv reactivity. Importantly, no differences were observed in total brain volume and intracranial volume between groups, but the Early group demonstrated greater WMH fraction compared with the Late group, and the age at menopause was positively associated with WMH fraction. Greater baseline MAP was observed in the Early group compared with the Late group, and, in the combined group, MAP was positively associated with WMH fraction. Importantly, these data are the first to demonstrate that age at menopause and time since menopause impact cerebrovascular function and white matter structure.
Blood pressure increases with aging, but sex-specific trajectories are observed corresponding with the age of menopause in women (6). Indeed, women experience rapid increases in systolic blood pressure around the age of menopause compared with age-matched men (6, 24), such that postmenopausal women demonstrate higher systolic blood pressure than perimenopausal women, whereas the same trend is not observed for men of similar ages (24). In the present study, postmenopausal women who experienced menopause at an earlier age demonstrated greater resting MAP compared with women who experienced menopause at a later age. Furthermore, an inverse correlation was observed between age at menopause and MAP, whereas a positive correlation was observed between years since menopause and MAP. This finding is consistent with a previous investigation in which systolic and diastolic blood pressures were inversely related to age at menopause and positively related to time since menopause (25). In addition, the prevalence of hypertension in postmenopausal women was reduced by 2% per year that the onset of menopause was delayed (26).
Menopause is characterized by declines in brain health, including structural deterioration (15) and cerebrovascular dysfunction (9, 10, 12, 13). However, the timing of menopause varies greatly in women, from roughly 40 to 60 yr of age (16). Early menopause is associated with long-term health risks such as increased all-cause and cardiovascular mortality as well as increased risk for ischemic stroke, depression, cognitive impairment, and dementia (16–19). To the best of our knowledge, the present study represents the first report on the impact of age at natural menopause and time since menopause on cerebrovascular function and brain structure. Our findings demonstrate that women who experience menopause at an earlier age (≤49 yr) have attenuated cerebrovascular reactivity to CO2 when compared with age-matched women who experienced menopause later in life (≥53 yr). Indeed, years since menopause was inversely associated with MCAv reactivity. Importantly, the difference in cerebrovascular reactivity was observed despite no differences in total brain volume or intracranial volume between the groups. Although there were no differences in brain volume, women who experienced menopause at an earlier age demonstrated greater WMH fraction than women who experienced menopause at a later age, and the age at onset of menopause was positively associated with WMH fraction. The present findings suggest that changes to white matter structure represent early signs of neurodegeneration following menopause that may occur before significant changes in brain volume. In addition, worse cerebrovascular outcomes that are associated with a longer length of time since menopause may contribute to the increased risk of stroke and dementia in early-onset menopause.
Elevated arterial blood pressure after menopause likely represents a key mechanism subserving the association between early-onset menopause and impaired cerebrovascular function as well as deteriorations in white matter structure. Indeed, increased blood pressure impacts the cerebral vasculature’s ability to respond to physiological stimuli. For example, reduced cerebrovascular dilation was observed following acetazolamide infusion in hypertensive adults (27). Furthermore, in our previous study, postmenopausal women with a history of preeclampsia during pregnancy demonstrated lower cerebrovascular reactivity to CO2 compared with postmenopausal women with a history of normotensive pregnancy (28). Increased blood pressure may contribute to reduced cerebrovascular reactivity through multiple mechanisms, including blood vessel stiffening and myogenic-mediated vasoconstriction. Increased blood pressure may also contribute to white matter structural damage. The relationship between blood pressure and WMH has been documented extensively in humans (29–31), and the present investigation provides further support, as resting MAP was positively associated with WMH fraction. Although the mechanisms underlying this association remain unclear, reductions in blood flow to white matter (32), endothelial dysfunction (33), and blood-brain barrier breakdown (33) have been proposed. As such, increases in blood pressure in postmenopausal women appear to contribute to cerebrovascular changes and brain structural alterations.
The present observation that the Early group demonstrated lower cerebrovascular reactivity to CO2 compared with the Late group may also be explained by a legacy effect of estrogen on the cerebral vasculature. More precisely, the vasoprotective effect of estrogen may continue following the reduction in ovarian hormones that occurs with menopause, which may impose a gradual decline, rather than an abrupt change, in cerebrovascular function. The legacy effect of estrogen is supported by our previous data in postmenopausal women who were studied 3 years after the cessation of either hormone replacement therapy or placebo treatment (34). In this previous study, women who had received hormone replacement therapy demonstrated higher cerebrovascular reactivity relative to the women who had received the placebo (34). Therefore, we hypothesize that greater cerebrovascular reactivity observed among the later-onset menopause group was attributed to a legacy effect of estrogen on cerebrovascular function. Indeed, estrogen imposes cerebrovascular dilation through direct effects on vascular smooth muscle cells or indirectly via endothelial pathways including nitric oxide synthase and cyclooxygenase (35, 36).
There are methodological considerations with the present study. First, transcranial Doppler ultrasound assessed cerebrovascular reactivity. Cerebrovascular reactivity calculated using blood velocity underestimates true blood flow responses during hypercapnia (37). Therefore, differences between groups in the present study may have been underestimated with measures of blood velocity. Second, the groups in the present study were divided based on the median age at the onset of natural menopause of 50–52 yr (19, 20, 38). In particular, the Early group were characterized as experiencing natural menopause at ≤49 yr of age. However, some reports have defined early menopause as ≤45 yr of age (19, 38). Nonetheless, defining early menopause in the present study at a later age would likely result in differences between groups being further underestimated. Third, the laboratory and MRI visits were completed on separate days and were scheduled 4 ± 11 mo apart. Previously, the rate of cortical atrophy was found to be ∼0.5% per year in healthy older adults (39). As such, we do not expect that the length of time between visits in the present study would significantly impact MRI outcomes. Finally, this study was retrospective, whereby we leveraged existing data in postmenopausal women to determine the effect of time since natural menopause on cerebrovascular reactivity and brain structure. As such, sex hormones were not measured in the present study. Nevertheless, the results provide a rationale to further investigate the impact of time since menopause on brain health, considering additional factors that can influence age at natural menopause as well as sex hormones in early-onset menopause compared with later-onset menopause. There are several strengths with the present study to acknowledge, including the extensive information that was collected on the participants. The groups were well matched in terms of overall health status, as demonstrated by several markers including body mass index, waist circumference, blood glucose, and lipoproteins. Therefore, the participants were healthy, cognitively unimpaired postmenopausal women, and only women who experienced natural menopause were included.
Perspectives and Significance
Menopause produces significant cerebrovascular and brain structural changes coinciding with a marked increase in the risk of stroke and vascular cognitive impairment, which are leading causes of disability and mortality (3, 40). However, there is a large variation in the timing of menopause, and recent studies have demonstrated that early-onset menopause is associated with increased risk of ischemic stroke, cognitive decline, and dementia (17, 18). The current study, to the best of our knowledge, represents the first report on the impact of age at natural menopause on cerebrovascular function and brain structural indices. The findings demonstrate that women who experienced menopause at an earlier age had reduced cerebrovascular reactivity and greater WMH burden relative to age-matched women who experienced menopause at a later age. As such, worse cerebrovascular outcomes and white matter structure are observed with longer time since the onset of menopause. Attenuation of cerebrovascular function and greater WMH burden in women who experience menopause at an earlier age may contribute to the increased risk of stroke and vascular cognitive impairment in this cohort. As such, understanding the factors that contribute to early menopause is critical to reduce the long-term health consequences associated with the early reduction in ovarian hormones. Many factors are determinants of age at natural menopause, including genetic factors, reproductive factors (menstrual and reproductive history), lifestyle factors (e.g., body mass, smoking history), and childhood factors (e.g., parental divorce) (20). Importantly, some factors are modifiable, and future research should aim to better understand how these modifiable factors may contribute to the timing of natural menopause. In addition, future research on the impact of menopause on brain health should collect information on menopause history and consequently consider age at menopause and/or time since menopause.
DATA AVAILABILITY
Data will be made available upon reasonable request.
GRANTS
This study was supported by the Wisconsin Alzheimer’s Disease Research Center (P30-AG062715), Ruth L. Kirschstein National Research Service Award T32 from the National Institutes of Health (NIH) National Heart Lung and Blood Institute to the University of Wisconsin-Madison Cardiovascular Research Center (HL007936 to K.B.M.), Alzheimer’s Association Research Grant (17–499398 to J.N.B.) ,and NIH (HL118154 to J.N.B.).
DISCLOSURES
S.C.J. has served on advisory panels for Roche and Eisai. He has received an equipment grant from Roche Diagnostics, and a research grant from Cerveau Technologies.
AUTHOR CONTRIBUTIONS
M.E.M., A.T.C., K.A.S., K.B.M., A.G.P., N.A.L., A.J.H., and J.N.B. conceived and designed research; A.T.C., K.A.S., K.B.M., A.G.P., N.A.L., and A.J.H. performed experiments; M.E.M., A.T.C., K.A.S., K.B.M., A.G.P., S.H.A.G., K.A.C., L.B.E., and S.C.J. analyzed data; M.E.M., A.T.C., K.A.S., K.B.M., A.G.P., N.A.L., A.J.H., S.H.A.G., K.A.C., L.B.E., S.C.J., and J.N.B. interpreted results of experiments; M.E.M. prepared figures; M.E.M., A.T.C., K.A.S., and J.N.B. drafted manuscript; M.E.M., A.T.C., K.A.S., K.B.M., A.G.P., N.A.L., A.J.H., S.H.A.G., K.A.C., L.B.E., S.C.J., and J.N.B. edited and revised manuscript; M.E.M., A.T.C., K.A.S., K.B.M., A.G.P., N.A.L., A.J.H., S.H.A.G., K.A.C., L.B.E., S.C.J., and J.N.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the Wisconsin Alzheimer’s Disease Research Center for assistance with this project.
REFERENCES
- 1. Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer's disease. Nat Rev Neurosci 5: 347–360, 2004. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- 2. Miller VM, Garovic VD, Kantarci K, Barnes JN, Jayachandran M, Mielke MM, Joyner MJ, Shuster LT, Rocca WA. Sex-specific risk of cardiovascular disease and cognitive decline: pregnancy and menopause. Biol Sex Differ 4: 6, 2013. doi: 10.1186/2042-6410-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Anstey KJ, Peters R, Mortby ME, Kiely KM, Eramudugolla R, Cherbuin N, Huque MH, Dixon RA. Association of sex differences in dementia risk factors with sex differences in memory decline in a population-based cohort spanning 20–76 years. Sci Rep 11: 7710, 2021. doi: 10.1038/s41598-021-86397-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Oveisgharan S, Arvanitakis Z, Yu L, Farfel J, Schneider JA, Bennett DA. Sex differences in Alzheimer’s disease and common neuropathologies of aging. Acta Neuropathol 136: 887–900, 2018. doi: 10.1007/s00401-018-1920-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Phan HT, Reeves MJ, Blizzard CL, Thrift AG, Cadilhac DA, Sturm J, Otahal P, Rothwell P, Bejot Y, Cabral NL, Appelros P, Kõrv J, Vibo R, Minelli C, Gall SL. Sex differences in severity of stroke in the INSTRUCT study: a meta‐analysis of individual participant data. J Am Heart Assoc 8: e010235, 2019. doi: 10.1161/JAHA.118.010235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ji H, Kim A, Ebinger JE, Niiranen TJ, Claggett BL, Merz CNB, Cheng S. Sex differences in blood pressure trajectories over the life course. JAMA Cardiol 5: 255–262, 2020. [Erratum in JAMA Cardiol 5: 364, 2020]. doi: 10.1001/jamacardio.2019.5306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Raz L. Estrogen and cerebrovascular regulation in menopause. Mol Cell Endocrinol 389: 22–30, 2014. doi: 10.1016/j.mce.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 8. Skinner BD, Davies RJ, Weaver SR, Cable NT, Lucas SJ, Lucas RA. A systematic review and meta-analysis examining whether changing ovarian sex steroid hormone levels influence cerebrovascular function. Front Physiol 12: 687591, 2021. doi: 10.3389/fphys.2021.687591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brislane Á, Low DA, Carter SE, Holder SM, Jones H, Hopkins ND. Cerebral and peripheral vascular differences between pre-and postmenopausal women. Menopause 27: 170–182, 2020. doi: 10.1097/GME.0000000000001442. [DOI] [PubMed] [Google Scholar]
- 10. Edgell H, Robertson AD, Hughson RL. Hemodynamics and brain blood flow during posture change in younger women and postmenopausal women compared with age-matched men. J Appl Physiol (1985) 112: 1482–1493, 2012. doi: 10.1152/japplphysiol.01204.2011. [DOI] [PubMed] [Google Scholar]
- 11. Penotti M, Farina M, Sironi L, Barletta L, Gabrielli L, Vignali M. Cerebral artery blood flow in relation to age and menopausal status. Obstet Gynecol 88: 106–109, 1996. doi: 10.1016/0029-7844(96)00119-6. [DOI] [PubMed] [Google Scholar]
- 12. Kastrup A, Dichgans J, Niemeier M, Schabet M. Changes of cerebrovascular CO2 reactivity during normal aging. Stroke 29: 1311–1314, 1998. doi: 10.1161/01.str.29.7.1311. [DOI] [PubMed] [Google Scholar]
- 13. Matteis M, Troisi E, Monaldo BC, Caltagirone C, Silvestrini M. Age and sex differences in cerebral hemodynamics: a transcranial Doppler study. Stroke 29: 963–967, 1998. doi: 10.1161/01.str.29.5.963. [DOI] [PubMed] [Google Scholar]
- 14. Petersen M, Frey BM, Mayer C, Kühn S, Gallinat J, Hanning U, Fiehler J, Borof K, Jagodzinski A, Gerloff C, Thomalla G, Cheng B. Fixel based analysis of white matter alterations in early stage cerebral small vessel disease. Sci Rep 12: 1581, 2022. doi: 10.1038/s41598-022-05665-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Than S, Moran C, Beare R, Vincent AJ, Collyer TA, Wang W, Callisaya ML, Thomson R, Phan TG, Fornito A, Srikanth VK. Interactions between age, sex, menopause, and brain structure at midlife: a UK Biobank study. J Clin Endocrinol Metab 106: 410–420, 2021. doi: 10.1210/clinem/dgaa847. [DOI] [PubMed] [Google Scholar]
- 16. Muka T, Oliver-Williams C, Kunutsor S, Laven JS, Fauser BC, Chowdhury R, Kavousi M, Franco OH. Association of age at onset of menopause and time since onset of menopause with cardiovascular outcomes, intermediate vascular traits, and all-cause mortality: a systematic review and meta-analysis. JAMA Cardiol 1: 767–776, 2016. [DOI] [PubMed] [Google Scholar]
- 17. Lisabeth LD, Beiser AS, Brown DL, Murabito JM, Kelly-Hayes M, Wolf PA. Age at natural menopause and risk of ischemic stroke: the Framingham Heart Study. Stroke 40: 1044–1049, 2009. doi: 10.1161/STROKEAHA.108.542993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Georgakis MK, Beskou-Kontou T, Theodoridis I, Skalkidou A, Petridou ET. Surgical menopause in association with cognitive function and risk of dementia: a systematic review and meta-analysis. Psychoneuroendocrinology 106: 9–19, 2019. doi: 10.1016/j.psyneuen.2019.03.013. [DOI] [PubMed] [Google Scholar]
- 19. Shuster LT, Rhodes DJ, Gostout BS, Grossardt BR, Rocca WA. Premature menopause or early menopause: long-term health consequences. Maturitas 65: 161–166, 2010. doi: 10.1016/j.maturitas.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gold EB. The timing of the age at which natural menopause occurs. Obstet Gynecol Clin North Am 38: 425–440, 2011. doi: 10.1016/j.ogc.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhu D, Chung H-F, Dobson AJ, Pandeya N, Brunner EJ, Kuh D, Greenwood DC, Hardy R, Cade JE, Giles GG, Bruinsma F, Demakakos P, Simonsen MK, Sandin S, Weiderpass E, Mishra GD. Type of menopause, age of menopause and variations in the risk of incident cardiovascular disease: pooled analysis of individual data from 10 international studies. Hum Reprod 35: 1933–1943, 2020. doi: 10.1093/humrep/deaa124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pearson AG, Miller KB, Corkery AT, Eisenmann NA, Howery AJ, Cody KA, Chin NA, Johnson SC, Barnes JN. Sympathoexcitatory responses to isometric handgrip exercise are associated with white matter hyperintensities in middle-aged and older adults. Front Aging Neurosci 14: 888470, 2022. doi: 10.3389/fnagi.2022.888470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Barnes JN, Schmidt JE, Nicholson WT, Joyner MJ. Cyclooxygenase inhibition abolishes age-related differences in cerebral vasodilator responses to hypercapnia. J Appl Physiol (1985) 112: 1884–1890, 2012. doi: 10.1152/japplphysiol.01270.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Staessen JA, Ginocchio G, Thijs L, Fagard R. Conventional and ambulatory blood pressure and menopause in a prospective population study. J Hum Hypertens 11: 507–514, 1997. doi: 10.1038/sj.jhh.1000476. [DOI] [PubMed] [Google Scholar]
- 25. Izumi Y, Matsumoto K, Ozawa Y, Kasamaki Y, Shinndo A, Ohta M, Jumabay M, Nakayama T, Yokoyama E, Shimabukuro H, Kawamura H, Cheng Z, Ma Y, Mahmut M. Effect of age at menopause on blood pressure in postmenopausal women. Am J Hypertens 20: 1045–1050, 2007. doi: 10.1016/j.amjhyper.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 26. Song L, Shen L, Li H, Liu B, Zheng X, Zhang L, Liang Y, Yuan J, Wang Y. Age at natural menopause and hypertension among middle-aged and older Chinese women. J Hypertens 36: 594–600, 2018. doi: 10.1097/HJH.0000000000001585. [DOI] [PubMed] [Google Scholar]
- 27. Ficzere A, Valikovics A, Fülesdi B, Juhász A, Czuriga I, Csiba L. Cerebrovascular reactivity in hypertensive patients: a transcranial Doppler study. J Clin Ultrasound 25: 383–389, 1997. doi:. [DOI] [PubMed] [Google Scholar]
- 28. Barnes JN, Harvey RE, Miller KB, Jayachandran M, Malterer KR, Lahr BD, Bailey KR, Joyner MJ, Miller VM. Cerebrovascular reactivity and vascular activation in postmenopausal women with histories of preeclampsia. Hypertension 71: 110–117, 2018. doi: 10.1161/HYPERTENSIONAHA.117.10248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wartolowska KA, Webb AJS. Midlife blood pressure is associated with the severity of white matter hyperintensities: analysis of the UK Biobank cohort study. Eur Heart J 42: 750–757, 2021. doi: 10.1093/eurheartj/ehaa756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Marcus J, Gardener H, Rundek T, Elkind MS, Sacco RL, DeCarli C, Wright CB. Baseline and longitudinal increases in diastolic blood pressure are associated with greater white matter hyperintensity volume: the Northern Manhattan study. Stroke 42: 2639–2641, 2011. doi: 10.1161/STROKEAHA.111.617571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Aribisala BS, Morris Z, Eadie E, Thomas A, Gow A, Valdés Hernández MC, Royle NA, Bastin ME, Starr J, Deary IJ, Wardlaw JM. Blood pressure, internal carotid artery flow parameters, and age-related white matter hyperintensities. Hypertension 63: 1011–1018, 2014. doi: 10.1161/HYPERTENSIONAHA.113.02735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang T, Li Y, Guo X, Huang D, Ma L, Wang DJ, Lou X. Reduced perfusion in normal‐appearing white matter in mild to moderate hypertension as revealed by 3D pseudocontinuous arterial spin labeling. J Magn Reson Imaging 43: 635–643, 2016. [DOI] [PubMed] [Google Scholar]
- 33. Wardlaw J, Sandercock P, Dennis M, Starr J. Is breakdown of the blood-brain barrier responsible for lacunar stroke, leukoaraiosis, and dementia? Stroke 34: 806–812, 2003. doi: 10.1161/01.STR.0000058480.77236.B3. [DOI] [PubMed] [Google Scholar]
- 34. Barnes JN, Harvey RE, Eisenmann NA, Miller KB, Johnson MC, Kruse SM, Lahr BD, Joyner MJ, Miller VM. Cerebrovascular reactivity after cessation of menopausal hormone treatment. Climacteric 22: 182–189, 2019. doi: 10.1080/13697137.2018.1538340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Geary GG, Krause DN, Duckles SP. Estrogen reduces mouse cerebral artery tone through endothelial NOS-and cyclooxygenase-dependent mechanisms. Am J Physiol Heart Circ Physiol 279: H511–H519, 2000. [DOI] [PubMed] [Google Scholar]
- 36. Salom JB, Burguete MC, Pérez-Asensio FJ, Torregrosa G, Alborch E. Relaxant effects of 17-β-estradiol in cerebral arteries through Ca2+ entry inhibition. J Cereb Blood Flow Metab 21: 422–429, 2001. doi: 10.1097/00004647-200104000-00011. [DOI] [PubMed] [Google Scholar]
- 37. Coverdale NS, Gati JS, Opalevych O, Perrotta A, Shoemaker JK. Cerebral blood flow velocity underestimates cerebral blood flow during modest hypercapnia and hypocapnia. J Appl Physiol (1985) 117: 1090–1096, 2014. doi: 10.1152/japplphysiol.00285.2014. [DOI] [PubMed] [Google Scholar]
- 38. Rocca WA, Grossardt BR, Miller VM, Shuster LT, Brown RD Jr.. Premature menopause or early menopause and risk of ischemic stroke. Menopause 19: 272–277, 2012. doi: 10.1097/gme.0b013e31822a9937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fjell AM, Walhovd KB, Fennema-Notestine C, McEvoy LK, Hagler DJ, Holland D, Brewer JB, Dale AM. One-year brain atrophy evident in healthy aging. J Neurosci 29: 15223–15231, 2009. doi: 10.1523/JNEUROSCI.3252-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Carcel C, Woodward M, Wang X, Bushnell C, Sandset EC. Sex matters in stroke: a review of recent evidence on the differences between women and men. Front Neuroendocrinol 59: 100870, 2020. doi: 10.1016/j.yfrne.2020.100870. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available upon reasonable request.