Abstract
The sinoatrial node (SAN) is the primary pacemaker of the heart. Normal SAN function is crucial in maintaining proper cardiac rhythm and contraction. Sinus node dysfunction (SND) is due to abnormalities within the SAN, which can affect the heartbeat frequency, regularity, and the propagation of electrical pulses through the cardiac conduction system. As a result, SND often increases the risk of cardiac arrhythmias. SND is most commonly seen as a disease of the elderly given the role of degenerative fibrosis as well as other age-dependent changes in its pathogenesis. Despite the prevalence of SND, current treatment is limited to pacemaker implantation, which is associated with substantial medical costs and complications. Emerging evidence has identified various genetic abnormalities that can cause SND, shedding light on the molecular underpinnings of SND. Identification of these molecular mechanisms and pathways implicated in the pathogenesis of SND is hoped to identify novel therapeutic targets for the development of more effective therapies for this disease. In this review article, we examine the anatomy of the SAN and the pathophysiology and epidemiology of SND. We then discuss in detail the most common genetic mutations correlated with SND and provide our perspectives on future research and therapeutic opportunities in this field.
Keywords: automaticity, pacemaker, sinus node dysfunction
INTRODUCTION
First discovered by Keith and Flack in 1907 (1), the sinoatrial node (SAN) is a key component of the native cardiac conduction system (CCS) and is the primary pacemaker of the heart. The normal function of the SAN is crucial in maintaining proper cardiac rhythm, which affects cardiac performance. Sinus node dysfunction (SND), also historically known as sick sinus syndrome, is due to abnormalities within the SAN, which can affect the heartbeat frequency, regularity, and the propagation of electrical pulses through the CCS. As a result, SND often increases the risk of cardiac arrhythmias. As the most common indication for pacemaker implantations (2, 3), SND affects individuals of all ages and is prevalent not only in the United States but also worldwide. Emerging evidence has also established various genetic abnormalities that can cause SND, which could help us understand the molecular underpinnings of this disease. In this review article, we first briefly explain the anatomy of the SAN and the pathophysiology of SND, followed by the epidemiology of SND. We then discuss in detail the most common genetic mutations correlated with SND and current therapeutic options. We conclude the review with our perspectives on future research and therapeutic opportunities in this field.
ANATOMY AND PHYSIOLOGY OF THE SAN
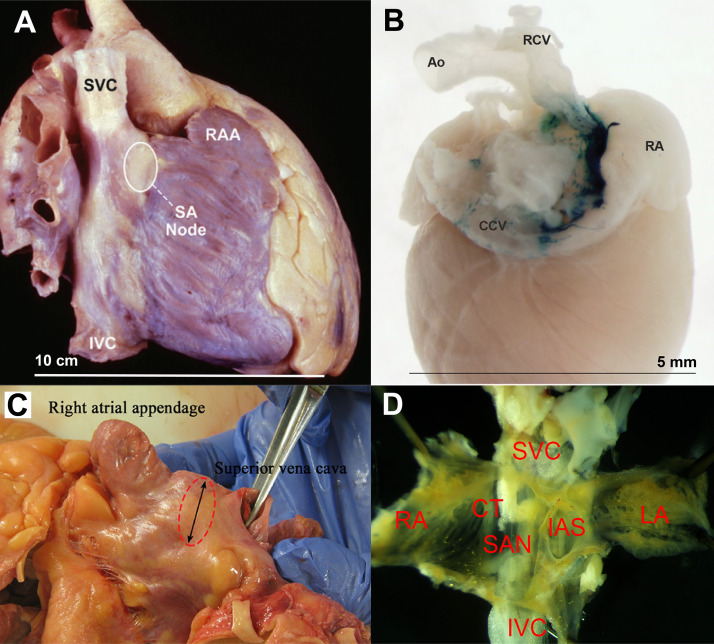
Anatomically, the SAN is located at the anterolateral aspect of the junction of the superior vena cava and the right atrium (RA), lodged immediately subepicardially within the sulcus terminalis (4) (Fig. 1). Grossly, the human SAN has a crescent-like morphology, with a superior head, middle body, and inferior tail portion, although subtle variations in morphology are commonly observed (8). In humans, the mean SAN size is 13.5 mm in length and 1.5 mm in width along its body (8). The SAN body is the most epicardial structure, ∼0.1–1 mm from the epicardial surface, while the SAN tail and head are seen to penetrate into the musculature of the terminal crest, diving closer toward the endocardial surface (8). The distance between the SAN and the endocardium ranges from 2.3 to 4.6 mm in the majority of subjects, with the largest separation seen on the caudal surface of the SAN body, making this location the most resistant to endocardial ablation (8).
Figure 1.
Gross anatomy of human and mouse sinoatrial node (SAN). A: lateral view of a human heart specimen showing location of the SAN. Reproduced from Hayes et al. (5). B: posterior view of a X-Gal-stained mouse heart showing the SA nodal area. Reproduced from Lee et al. (6). C: dissected human heart showing SAN area (dotted circle). Reproduced from Mitrofanova et al. (7). D: dissected SAN tissue from a mouse. Approximate sizes are shown. Ao, aorta; CCV, common carotid vein; CT, crista terminalis; IAS, interatrial septum; IVC, inferior vena cava; LA, left atrium; RCV, right carotid vein; RA, right atrium; RAA, right atrial appendage; SVC, superior vena cava. Reproduced images, as indicated, are used with permission.
The SAN contains the highest density of pacemaker cells, which are histologically and functionally distinct from the surrounding myocytes. Histologically, these pacemaker cells are clear, pale, and relatively empty of the microfilament cytoskeleton and sarcomeric components compared with the surrounding atrial tissue (9). While the nodal cells are contained in a matrix of predominately fibrous tissue, the SAN itself is not isolated from the surrounding atrial myocardium (4). The SAN exhibits several radiations up to 2 mm long along its margins, which then interdigitate with atrial myocardium in a smooth transition, with cells along the peripheral zone seen to be transitional both in structure and function (8, 10). From a developmental point of view, the SAN is derived from the sinus venosus, which expresses Shox2 (encoding short stature homeobox 2), a gene that represses the activation of several genes involved in contractile myocardium (9).
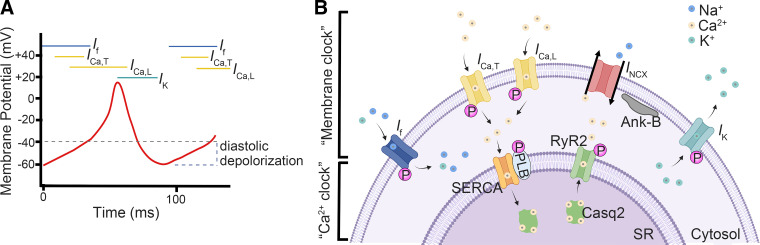
The pacemaker cells are characterized by their automaticity, arising from their spontaneous (phase 4) diastolic depolarization (10) (Fig. 2). The sinoatrial (SA) nodal cells exhibit the most rapid phase 4 depolarization and are thus the dominant pacemakers in a normal heart. Other secondary locations of pacemaker cells include the atrioventricular (AV) node and the ventricular conduction system, which can serve as a backup pacemaker in case of SAN failure. The automaticity of pacemaker cells is the result of a complex interplay between a number of ionic currents, including the inward funny current (If), T-type and L-type Ca2+ currents (ICa,T, ICa,L), outward delayed rectifier K+ current (IK), and electrogenic Na+/Ca2+ exchanger (INCX) (10) (Fig. 2). The relative importance of these currents involved in pacemaking remains controversial (9, 10). At approximately −65 to −40 mV, a cationic current If is conducted through the hyperpolarization-activated cyclic nucleotide-gated (HCN) channels which depolarizes the semipermeable membrane. Once the threshold potential is reached, the voltage-gated T-type Ca2+ channel (TTCC; ICa,T) and the L-type Ca2+ channel (LTCC; ICa,L) are activated, which increases the membrane potential and produce the upstroke of the action potential (AP) in SAN pacemaker cells. Of the two LTCC isoforms in the SAN (Cav1.2 and Cav1.3), Cav1.3 in particular is thought to play a key role in diastolic depolarization. Membrane repolarization starts because of the activation of the rapid (IKr) and slow (IKs) outward rectifier K+ currents along with simultaneous inactivation of the ICa,L current, which reduces the membrane potential of pacemaker cells. This process is known as the “membrane clock.” On the other hand, the automaticity of the pacemaker cells is also influenced by subsarcolemmal Ca2+ induced Ca2+ release (CICR) and membrane-bound Na+/Ca2+ exchanger (NCX) activity, known as the Ca2+ clock (11). The Ca2+ entry during diastolic depolarization can activate ryanodine receptors (RyRs) and initiate Ca2+ release from the sarcoplasmic reticulum (SR), which in turn activates NCX and produces an inward current during diastolic depolarization (12). Spontaneous local SR Ca2+ release that is not triggered by ICa,L during diastolic depolarization has also been observed to occur independent of the CICR mechanism; the relative contribution of this phenomenon to the Ca2+ clock remains unclear however (13). Ca2+ stores in the SR are then replenished by the sarcoplasmic-endoplasmic reticulum Ca2+ ATPase (SERCA) channel on the SR membrane (9). Many of these channels and Ca2+ handling proteins can be phosphorylated by protein kinase A (PKA) or Ca2+/calmodulin-dependent protein kinase II (CaMKII) upon β-adrenergic stimulation (14–16). The synergistic interplay between the two fundamentally different systems provides a robust range of heart rates (HR), with the membrane clock helping to provide a stable minimum HR under increased vagal tone while the Ca2+ clock gives a wide dynamic range to dramatically accelerate the HR when sympathetic tone increases (17).
Figure 2.
Electrophysiology of pacemaker cells. A: ionic current in each phase of an action potential in a pacemaker cell. B: interplay between membrane clock and Ca2+ clock. Casq2, calsequestrin-2; PLB, phospholamban; RyR2, ryanodine receptor type 2; SERCA, sarco(endo)plasmic reticulum Ca2+-ATPase; SR, sarcoplasmic reticulum. B was created with BioRender.com and published with permission.
In addition to the membrane and Ca2+ clock, there is an increasing recognition of a “third clock,” also known as the “mechanics clock,” referring to the phenomenon of SAN mechanosensitivity (18). SAN stretch has long been known to cause an acute increase in HR in most mammals, termed the Bainbridge reflex (19). Interestingly, HR in mice tends to decrease in response to sustained stretch, likely because of differences in the relative contribution of stretch-sensitive channels to the species-specific AP (20). Regardless of the directionality in the stretch-induced changes in HR, it has been recognized that the mechanics clock is a regulator of SAN automaticity (18). While it was initially thought that the extracardiac neurohumoral pathways control the stretch-induced changes in HR, this enhanced automaticity in response to stretch is observed to occur in isolated hearts and even isolated SAN pacemaker cells, suggesting that intrinsic mechanosensitive mechanisms could control the mechanics clock of the SAN (18, 20). Mechanoelectric coupling is an increasingly recognized component of the automaticity of the SAN and perhaps the most important regarding maintaining in vivo global cardiac function. The SAN stretch mechanism is thought to entrain the numerous SAN myocytes by normalizing aberrant electrical activity and promoting synchronized excitation across the entire SAN (18). The molecular mechanisms underlying the mechanics clock remain incompletely understood. Cation nonselective stretch-activated channels (SACNS), which have a reversal potential between −20 and 0 mV, are potential candidates for this mechanism although the molecular identity of these channels remains elusive. Several channels involved in the membrane and Ca2+ clocks have also been demonstrated to be mechanosensitive, such as the HCN (21), LTCC (22), delayed rectifier K+, and NCX channels (23), providing evidence for the interdependent nature of these three clocks in the dynamic modulation of SAN automaticity.
While anatomically the SAN exists as a discrete unit, the site of origin of electrical activity has been suggested to shift within the SAN itself with changes in HR (24). This shift in the leading pacemaker within the SAN is reflected as subtle alterations in P-wave morphology. Recently, optical mapping applied to ex vivo RA tissue isolated from rats and humans revealed the presence of two competing pacemaker sites localized to the superior and inferior regions of the SAN, which preferentially control fast and slow HR, respectively (24). Molecular analysis of these two regions shows unique but similar transcriptional profiles, suggesting inferior SAN dominance when the superior SAN is silent (24). While having multiple pacemaker sites within the SAN may be advantageous from an evolutionary perspective as this system provides a backup site in the case of failure of the primary pacemaker site, further studies are needed to verify these findings.
The majority of our understanding of the molecular mechanisms underlying SAN function has been extrapolated from animal models given their ease of manipulation and practicality for research because of the logistical barriers of obtaining sufficient human SAN specimens. While the overall SAN function bears many similarities among mammalian species, structural and electrophysiological differences unique to each species are important to bear in mind when extrapolating findings from animal models to humans (Table 1). For example, as previously mentioned, mice SAN tissue uniquely demonstrates a reduction in HR in response to stretch, whereas most other mammals (including the dog, cat, rabbit, zebrafish, and guinea pig) demonstrate an increase in HR similar to humans (18). Furthermore, the predominant autonomic tone is seen to differ among species, with rodents, monkeys, and pigs exhibiting a predominant sympathetic tone in comparison to humans and dogs, which exhibit a predominant vagal tone (26). While the molecular mechanisms for these species-specific differences in SAN function remain to be fully elucidated, differences in both density and kinetic behavior of key ion channels including HCN4, the voltage-dependent Na+ channel (Nav1.5), and SERCA are thought to play a significant role (35). The global SAN architecture also varies across species, with the human, dog, and pig SAN surrounded by a layer of atrial muscle between the SAN and endocardium to protect the region against high wall stress, while smaller mammals such as the rabbit and guinea pig lack this layer (36). In addition, the extent of connective tissue within the SAN and the organization of myofilaments in nodal cells are highly variable across species (27). Taken together, these molecular and architectural differences in SAN structure among species are important to consider when extrapolating results from animal models.
Table 1.
SAN characteristics of select animal models compared with humans
| Species | SAN Length, mm | SAN Width, mm | Normal HR, Beats/Min (25) | Cycle Length, ms (26) | Sinoatrial Conduction Time, ms (26) | Action Potential Amplitude, mV (26) | Action Potential Duration, ms (26) | Maximum Diastolic Potential, mV (26) | Diastolic Depolarization Rate, mV/s (26) |
|---|---|---|---|---|---|---|---|---|---|
| Human | 8−21.5 (8) | 1−5 (8, 27) | 60−100 | 828 (28) | 82 (29) | 72 | 309 | −62 | 48.9 (28) |
| Mouse | 1.5 (30) | 450−750 | 144 | 4 | 56 | 82 | −51 | 117 | |
| Rabbit | 6−7 (27) | 2 (27) | 180−350 | 386 | 32 | 66 | 180 | −61 | 83 |
| Dog | 5−15 (27, 31) | 2 (27, 31) | 70−120 | 542 (31) | 45 (31) | 56 (32) | 220 (33) | −56 (32) | 72 (34) |
| Cat | 7.4 (27) | 2.2 (27) | 120−140 | 418 | 29 | 60 | 202 | −59 | 71 |
| Guinea pig | 4 (27) | 1 (27) | 200−300 | 275 | 12 | 67 | 137 | −57 | 121 |
Displayed are data values (reference citation). SAN, sinoatrial node; HR, heart rate.
EPIDEMIOLOGY OF SND
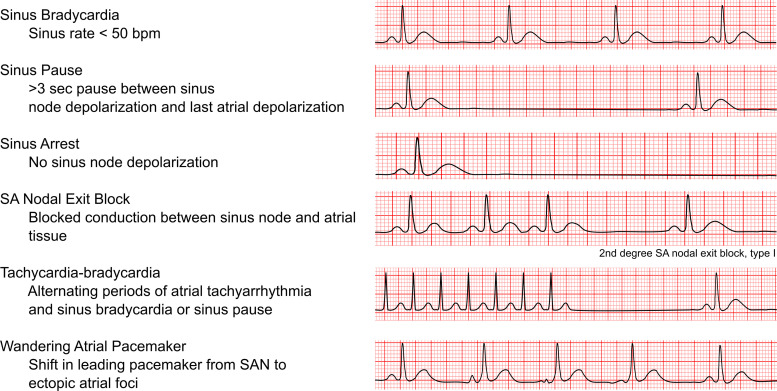
Normal sinus rhythm is initiated by electrical impulses arising from the SAN. In normal sinus rhythm, the P wave is less than 120 ms in duration and 0.25 mV or less in height with a constant P-P interval on ECG. SND encompasses several related conditions that result from abnormalities in the intrinsic SAN impulse formation and/or propagation, leading to inappropriately low HRs, pauses, or irregularities in the heart rhythm (Fig. 3) (2). SND is typically diagnosed based on ECG recording. Electrophysiology studies are not routinely performed to diagnose SND, but they can be used in equivocal cases. The corrected sinus node recovery time (cSNRT) and sinoatrial conduction time (SACT) in response to atrial pacing are two common parameters that can aid the investigation of SND. Regardless of the underlying cause, SND is associated with increased cSNRT, conduction delay along the CCS, a unicentric and caudal shift of the leading pacemaker activity within the SAN, and increased areas of low voltage within the RA due to either atrophy or scar formation (9).
Figure 3.
Classifications of sinus node dysfunction. SAN, sinoatrial node.
Given the role of degenerative fibrosis and other age-related remodeling in the pathogenesis of SND, this disease is most commonly seen as a disease of the elderly and affects one out of 600 cardiac patients above the age of 65 (37). In a study by Jensen et al. (38), among 20,572 patients in the Atherosclerosis Risk In Communities (ARIC) and Cardiovascular Health Study (CHS) cohorts, 291 incident cases of SND (1.4% of the cohort) were reported over an average follow-up time of 17 yr, giving an unadjusted rate of 0.8 per 1,000 person-years. The incidence of SND is seen to steadily increase with age, with the greatest risk in patients in their 70–80s (2, 38). In the randomized Mode Selection Trial in Sinus-Node Dysfunction (MOST) trial, which evaluated the optimal pacemaker device in 2010 patients with SND, the median age was 74 yr old (39). Given the aging population in the United States, the annual number of new SND cases in the United States is projected to increase from 78,000 in 2012 to 172,000 in 2060 (38).
SND is the most common indication for pacemaker implantation, accounting for just over 50% of pacemaker implants in the United States (2, 3). The natural course of untreated SND is highly variable (40). Recurrent syncope is common in patients with previous syncope related to their bradycardia (41). While SND can be highly symptomatic and associated with decreased quality of life, isolated SND in itself does not appear to affect overall survival whether treated or untreated and the incidence of sudden cardiac death is extremely low (40, 42). However, SND can frequently coexist with structural heart disease and heart failure (HF), which also increase in prevalence with age. The increase in comorbidities with age may explain the poorer prognosis observed in some studies of patients with SND (42).
Risk factors associated with SND include greater body mass index, height, hypertension, right bundle branch block, and longer QRS duration (38). AV conduction disturbances can occur in a portion of patients with SND given the similar degenerative process underlying both conditions. The annual incidence of high-grade AV block following pacemaker implantation for SND is around 1.8% (43, 44). This risk is highest among patients with evidence of conduction disturbance (complete bundle branch block or bifascicular block), with an incidence of up to 35% within 5 yr of pacemaker implantation (43). However, the overall low incidence of high-degree AV block in most patients with SND suggests that the degenerative process of the specialized conduction system is slow and does not dominate the clinical course of disease (40).
SND can often coexist with atrial arrhythmias, as the same degenerative changes underlying the development of SND are linked to the development of atrial arrhythmias (2). The combination of sinus bradycardia alternating with periods of atrial tachyarrhythmia is termed tachycardia-bradycardia syndrome. Long-standing atrial arrhythmia in itself leads to fibrotic changes and reduced percentage of nodal cells, predisposing toward SND (45). Up to one out of five patients with atrial fibrillation (AF) are affected by SND, highlighting the close relationship between the two conditions (46). AF and SND also demonstrate similar differences in incidence by race, with white patients having the highest incidence of AF and SND (38). A cross-sectional analysis of the Multi-Ethnic Study of Atherosclerosis (MESA) cohort of 6,814 Americans in 2000–2002 indicated a significantly lower risk of AF in African Americans, Hispanics, and Chinese compared with White Americans (47). These findings coincided with results from the previous study by Jensen et al. (38) that reported increased incidence of SND in white patients as compared with black patients. While the cause of this difference by race is unclear, the similar trends observed with both arrhythmias provide some evidence of correlation in the development of SND and AF.
MOLECULAR MECHANISMS OF SND
Idiopathic SND most often occurs in the elderly and is commonly due to age-related degenerative fibrosis of the SA nodal tissue and surrounding atrial myocardium (2). However, idiopathic SND can also occur in the absence of fibrosis (48). Age-related electrical remodeling and gene expression also have an increasingly recognized role in idiopathic SND, as animal studies have shown age-dependent changes in several ion channels including HCN1, HCN4, Nav1.5, and RyR2 associated with SND physiology (9, 49). In addition to age itself, frailty has been demonstrated to be an important determinant of SND in mouse models via electrophysiological and structural remodeling. Fibrosis, AP morphology, and changes in ion channel expression are correlated with frailty in aging mice (50, 51). Acute myocardial ischemia is another cause of SND and is due to injury to SAN tissue and/or abnormal autonomic tone (9). Although incidence of coronary artery disease and SND increase with age, whether chronic ischemia directly causes SND is unclear (9). Finally, rarer causes of SND include familial SND, inherited cardiomyopathies, or inherited arrhythmia syndromes associated with SND such as catecholaminergic polymorphic ventricular tachycardia (CPVT) and long QT syndrome (LQTS), which are often attributed to a specific gene mutation. While uncommon, such identified gene mutations provide tremendous insight into novel pathways and mechanisms involved in normal SAN function. Below we discuss the molecular mechanisms causing SND by impacting 1) the membrane clock, 2) the Ca2+ clock, or 3) other significant factors that do not strictly fall into these two categories.
Molecular Mechanisms Impacting The Membrane Clock
Hyperpolarization-activated cation currents.
Hyperpolarization-activated cation currents (If) were discovered in heart and nerve cells over 40 yr ago and are key determinants of pacemaker activity. The hyperpolarization-activated cyclic nucleotide-gated (HCN) gene family encodes the channels that underlie If (52). Among the four subtypes of HCN channels (HCN1–4), HCN1, 2, and 4 isoforms are expressed in SAN tissue. HCN4 is the predominant isoform expressed throughout the SAN while HCN1 is restricted to the head region of the SAN (53) and HCN2 shows a confined expression to individual pacemaker cells mainly in the periphery of the SAN (54). HCN channels can be activated upon membrane hyperpolarization with a variable threshold between −50 and −65 mV, leading to Na+ and K+ influx into the SAN pacemaker cells and generating cationic currents during the slow diastolic depolarization phase (55). Ivabradine, a drug used as a HR-reducing agent, has been shown to cause a current-dependent block on open HCN channels (9). When Ivabradine enters the pacemaker cells, it can bind to the open state of HCN channels, which can deactivate the channel during the depolarization phase. During hyperpolarization, the molecules are displaced by a current driving force, removing them from the pores. A HCN4 mutation (p.S672R) located within the cyclic nucleotide-binding domain (CNBD) of HCN4 was the first HCN mutation reported to be associated with a case of familial asymptomatic sinus bradycardia that was inherited in an autosomal-dominant pattern (56). The S672R mutation was seen to modify channel kinetics by shifting the current activation range to hyperpolarized voltages and slowing current deactivation (56). Since then, genetic mutations of HCN channels have been associated with a wide variety of cardiac phenotypes in addition to SND, including ventricular arrhythmias, left ventricular noncompaction, Brugada syndrome, and structural abnormalities of the myocardium (57), suggesting that the function of HCN channels can go beyond pacemaking (58).
Various HCN knockout mice models have been developed for the different HCN isotypes, each of which displays a unique cardiac phenotype. HCN4 plays a fundamental role during cardiac development, with constitutive global Hcn4 knockout mice demonstrating embryonic lethality (59). Isolated hearts with Hcn4 deletion demonstrate bradycardia with a 36.7% reduction in HR compared with controls, in addition to a 75–90% reduction in the If current (60). Inducible Hcn4 knockout mice interestingly demonstrate differing cardiac phenotypes based on the specific mutation and can generally be grouped into two phenotypes: recurrent sinus pauses with normal basal HR and normal response to isoproterenol versus pronounced bradycardia and AV block with decreased response to isoproterenol (61). The reasons for the differing phenotypes among the different Hcn4 knockout models remain unclear (60). Hcn2 knockout mice demonstrate sinus dysrhythmia at rest but normal basal and maximal HR as compared with controls in addition to a reduction of If current by ∼30% (62). Finally, Hcn1 and Hcn3 knockout mice do not appear to demonstrate any appreciable SND phenotype (61).
The activity of HCN channels is controlled by intracellular cyclic AMP (cAMP) levels. Increases in cAMP concentration within the physiological voltage range enhance HCN channel activity (63). A 1-bp deletion in exon 5 of HCN4 was detected in a patient with idiopathic SND, which produced a truncated HCN4 protein (hHCN4-573X) lacking the CNBD (64). Transgenic mice with cardiac-specific overexpression of the dominant-negative CNBD-deficient HCN4 subunit (hHCN4-573X) exhibit the loss of the cAMP sensitivity of If and lowered maximum firing rates of SAN pacemaker cells, although the overall range of SAN frequency regulation (difference between maximum and resting HR) is still preserved (65). On the other hand, heterozygous HCN4-R669Q knockin mice, where the cAMP-binding site of the CNBD was disabled, display normal HRs at rest and during exercise but exhibit SAN block and sinus pauses upon β-adrenergic stimulation (66). In contrast to the hHCN4-573X transgenic mice, the heterozygous HCN4-R669Q mice likely retain some cAMP sensitivity of channels, which may explain the different SND phenotypes observed between the two (65). Nevertheless, these results demonstrate that the cAMP-mediated regulation of If plays an indispensable role in HR adaptation during physical activity, and defects in this process promote SND.
Inward rectifier potassium 3 channel.
The inward rectifier potassium 3 (Kir3) channel is a tetrameric channel that more favorably conducts inward K+ current at hyperpolarized potentials (IKir) (67, 68). Among the four known subunits of the Kir3 family (Kir3.1–Kir3.4), Kir3.1, and Kir3.4 are expressed in the heart and encoded by KCNJ3 and KCNJ5, respectively (68). Kir3.1 and Kir3.4 constitute the heterotetrametric Kir3 channel at 1:1 stoichiometry in cardiomyocytes. The activation of Kir3.1/Kir3.4 channels are voltage independent and directly regulated by levels of membrane phosphatidylinositol 4,5 bisphosphate (PIP2) (69). Additionally, because the Gβγ heterodimeric subunits play a crucial role in modulating the activity of Kir3.1/Kir3.4 channels upon vagal stimulation, Kir3.1 and Kir3.4 channels are also known as the G protein-activated inwardly rectifying K+ channel 1 and 4 (GIRK1, GIRK4) (70, 71). Activation of Kir3.1/Kir3.4 (or GIRK1/4) channels can lead to hyperpolarization of the membrane potential in SAN pacemaker cells, thus slowing HR (72). On the other hand, inactivation of Kir3.1 or Kir3.4 can induce sinus arrhythmias. Mutations of KCNJ5 have been associated with cardiac arrhythmias such as AF, LQTS, SND, and Andersen-Tawil syndrome with symptoms of periodic paralysis, cardiac arrhythmia, and dysmorphic heart features (73). The G→A nonsynonymous substitution at nucleotide 739 affects the composition of the Kir3.4 subunit and, as a result, impacts the activation of the cardiac GIRK channels (71, 74). A gain-of-function (GOF) mutation of KCNJ5 (p.W101C) identified in familial patients with SND is associated with early onset of sinus bradycardia, sinus arrest episodes accompanied with junctional ectopic heartbeats, and periods of AV block, specifically the Mobitz 2 type (71). GIRK4-deficient mice display shorter SNRT and sinus cycle length in the presence of carbachol (75). The mutated Kir3.4 protein can cause a loss of rectification by altering the stoichiometry of the Kir3.1 and Kir3.4 subunits in the channel and increasing K+ efflux and hyperpolarization of SAN pacemaker cells, thereby hampering depolarization and lowering HR (71).
Guanine nucleotide-binding protein.
Guanine nucleotide-binding protein (GNB) or G protein regulates the extracellular signaling pathways between the G protein-coupled receptors and effector proteins (76). As mentioned earlier, βγ-subunits of G proteins directly regulate the activation of GIRK1/4 channels in SAN pacemaker cells. Gehrmann et al. (77) have shown that muscarinic stimulation by carbachol can induce bradycardia and increase HR variability and baroreflex sensitivity in wild-type mice, which can be blunted by cardiac-specific overexpression of the nonprenylated Gγ2-subunits in mice. Among the different subtypes of Gβ subunit, Gβ2 and Gβ5 proteins, encoded by GNB2 and GNB5, respectively, are often involved in the regulation of GIRK1/4 channels or the acetylcholine-activated K+ current (IK,ACh) (76). GOF mutations of GNB2 and GNB5 have been associated with SND (71, 76, 78, 79). Stallmeyer et al. (78) described a novel GNB2 mutation (p.R52L) associated with nonsyndromic SND in a three-generation family with autosomal dominant SND and AV block. The GOF mutation caused sustained activation of GIRK channels, leading to hyperpolarization of the cardiac membrane potential and reduced spontaneous activity (78). Later studies of human induced pluripotent stem cell differentiated cardiomyocytes (iPSC-CMs) lines with a recessive GNB5 mutation (p.S81L) displayed an augmented current density of IK,ACh and a profound decrease of spontaneous activity as compared with isogenic controls (80). Interestingly, the application of the specific IK,ACh blocker XEN-R0703 reversed the cellular phenotypes in the p.S81L iPSC-CMs, positioning it as a potential therapy in patients carrying GNB5 mutations. Additionally, inhibition of IK,Ach by tertiapin-Q peptide can improve the HR and cardiac conduction in various genetic murine models of SND (81). These studies not only suggest the potential usage of the pharmacological IKACh inhibitors for SND management but also further confirm the importance of keeping GIRK or IK,Ach in check, thereby maintaining the repolarization of the membrane in SAN pacemaker cells.
Voltage-gated Ca2+ channels.
Both T-type and L-type voltage-gated Ca2+ channels (TTCCs, LTCCs) contribute to pacemaking activity in the heart. While the LTCCs are widely expressed throughout the myocardium and responsible for the initiation of cardiomyocyte contraction and pacemaker activity, TTCCs are primarily expressed in the CCS (12). The dihydropyridine (DHP)-sensitive LTCCs are constituted by the pore-forming α1-subunit, and several accessory subunits and can be activated between −50 mV and −30 mV. Among the four α1-subunits of LTCCs (Cav1.1–Ca1.4), Cav1.2 (encoded by CACNA1C) and Cav1.3 (encoded by CACNA1D) channels are involved in the automaticity of pacemaker cells. While both Cav1.2 and Cav1.3 channels respond to β-adrenergic stimulation similarly, the Cav1.3-based LTCCs can be activated at more negative voltages (−45 vs. −25 mV) with slower inactivation kinetics compared with the Cav1.2-based LTCC (82, 83). It is believed that Cav1.2 channels contribute to the upstroke phase of the AP and Cav1.3 channels contribute to diastolic depolarization in SAN pacemaker cells. When compared with LTCCs, the characteristics of TTCCs include a negative shift of voltage threshold for activation, a slower activation, and fast voltage-dependent inactivation (84). There are three α1-subunits of TTCCs (Cav3.1–Cav3.3), with Cav3.1 (encoded by CACNA1G) being the most studied TTCC in SAN cells (12). The precise contribution of ICa,T to diastolic depolarization is not completely understood. However, emerging evidence suggests that the activation of TTCCs is involved in the local control of Ca2+ release within SAN pacemaker cells (85, 86). Genetic variants of CACNA1D have been commonly found in patients with sinoatrial node dysfunction and deafness (SANDD) (87, 88). Cacna1d−/− mice exhibit SND and deafness, recapitulating the phenotype in patients with SANDD (89). Genetic variants of CACNA1G in humans have also been linked to bradycardia and congenital heart block (90).
Molecular Mechanisms Impacting the Ca2+ Clock
Na+/Ca2+ exchanger 1.
The Na+/Ca2+ exchanger 1 (NCX1) (encoded by SLC8A1) plays a critical role in maintaining Ca2+ homeostasis and is necessary for maintaining the automaticity of pacemaker cells (86, 91, 92). While at present, there have not been any NCX1 mutations identified in humans with SND, mice models have provided evidence for the critical role of NCX1 in normal SAN function. Mice with genetic inhibition of Slc8a1 (Slc8a1−/−) exhibit a normal resting HR but a blunted response to the β-adrenergic stimulation-induced increase in HR (91). Interestingly, in the absence of fight-or-flight response of HR in Slc8a1−/− mice, If plays an augmented role in β-adrenergic induced HR increases (91). In the study by Gao et al., Slc8a1−/− mice were still seen to retain ∼20% of the control level NCX current, which may explain the normal basal HR observed (91). On the other hand, mice with complete atrial-specific knockout of NCX1 demonstrate a junctional escape rhythm and the complete absence of atrial depolarization (93). Despite the complete lack of atrial depolarization, isolated SAN cells from these knockout mice still demonstrate relatively normal If and intracellular Ca2+ stores, suggesting that the membrane clock (If) is not sufficient to spontaneously cause SAN depolarization in the absence of NCX1 (93). While spontaneous depolarization does not occur in these knockout mice, external pacing is still capable of causing the depolarization and conduction of atrial tissue, indicating intact electrical coupling between cells (93). Finally, NCX1 knockout mice exhibit loss of transverse axial tubules, invaginations of the surface sarcolemma in cardiomyocytes, presumably as a consequence of abnormal Ca2+ regulation and activation of Ca2+-dependent remodeling (94).
Ryanodine receptor type 2.
Ryanodine receptor type 2 (RyR2) protein (encoded by RYR2) can form a homotetramer channel located on the SR (95, 96). RyR2 channels mediate CICR from the SR, a critical step in the electrical contraction coupling process in working cardiomyocytes (97). Although the relative importance of RyR2 channels in maintaining baseline SAN automaticity may vary in different conditions, the involvement of RyR2 in regulating the automaticity of SAN pacemaker cells is well acknowledged. Conceptually, Ca2+ release via RyR2 channels can activate NCX current, potentially facilitating AP firing at the end of diastolic depolarization and supporting the Ca2+ clock. Experimentally, it has been shown that the classic RyR2 inhibitor ryanodine can reduce the firing rate of the SAN (92, 98). Moreover, it is known that PKA-mediated phosphorylation can enhance the activity of RyR2 channels. Because the basal levels of cAMP, an activator of PKA, are high in SAN pacemaker cells, the increased PKA-phosphorylation of RyR2 channels may cause more Ca2+ release and influence pacemaking activity in SAN pacemaker cells (14). Some evidence suggests that the Ca2+ clock and RyR2 may exert greater contribution to pacemaking activity at the periphery of SAN than at the center of the SAN, because of higher protein levels of NCX1, RyR2, and SERCA in the periphery of the SAN (99). There is ample evidence that dysfunctional RyR2 channels, typically with enhanced activity, can cause HF, AF, ventricular tachycardia, and other arrhythmias (100, 101). Genetic variants of RYR2 are often found in patients with CPVT and arrhythmogenic right ventricular cardiomyopathy/dysplasia ((102, 103). The direct association between the genetic variants of RYR2 and idiopathic SND is currently lacking. However, some CPVT patients with RYR2 mutations develop concomitant SND (104, 105). The CPVT-mimicking RyR2-R4496C heterozygous knockin mice display sinus pause following isoproterenol injection. The frequency of Ca2+ transients in isolated SAN cells as an indicator of pacemaking activity is comparable at baseline between wild-type and RyR2-R4496C mutant mice (106, 107). However, there is a blunted chronotropic response to β-adrenergic stimulation and increased frequency of sinus pauses in SAN cells of RyR2-R4496C mice, which are associated with the reduced ICa,L density (106). Mechanistically, it is speculated that the increased Ca2+ release via RyR2 channels can cause a rise in the cytosolic Ca2+ concentration and subsequently inactivate ICa,L, which can lead to impaired diastolic depolarization in SAN pacemaker cells.
Calsequestrin-2.
Calsequestrin-2 (Casq2) (encoded by CASQ2) is a SR Ca2+ binding protein and plays a key role in Ca2+ homeostasis by acting as a Ca2+ reservoir within the SR of cardiomyocytes. CASQ2 loss-of-function (LOF) mutations are associated with an autosomal recessive form of CPVT and represent the 2nd most frequent cause of CPVT after RYR2 mutations (108). Patients with CPVT typically display concomitant sinus bradycardia, which is often the only resting ECG abnormality (104, 109). Casq2 homozygous knockout mice (Casq2−/−) exhibit atrial arrhythmias including atrial ectopic activity and bradycardia under sympathetic stimulation (110). The SAN conduction blocks in Casq2−/− mice are associated with abnormal Ca2+ release and increased interstitial fibrosis within the atrial pacemaker complex, which may disrupt SAN pacemaking and enhance latent pacemaker activity.
Ankyrin-B.
Ankyrins, considered multifunctional proteins, are required to target ion channels and transporters in diverse tissues and cells (111). Ankyrin dysfunction has been linked to abnormalities of ion channels and is implicated in several arrhythmias such as LQTS, HR variability, CPVT, conduction defects, AF, bradycardia, and arrhythmogenic cardiomyopathy (112, 113). Ankyrin-B (AnkB) protein, encoded by Ankyrin-2 (ANK2), is an adaptor and scaffold protein that targets NCX1 and Na+/K+-ATPase in cardiomyocytes (112). Mutations of ANK2 were identified in patients with LQTS with concurrent SND (114, 115) and patients with familial SND and AF (116). In the latter study, severe and highly penetrant SND in two families were linked to LOF mutations in ANK2 (p.E1425G), the first nonion channel protein to be implicated in human SND (116). Individuals with the ANK2 mutation exhibited SND at all ages, including in utero. The Ank2 haploinsufficient mouse model produces a similar SND phenotype (114, 116, 117). Mechanistically, AnkB deficiency can cause a reduction in NCX and Na+/K+-ATPase protein levels, and subsequently lead to an increase in total cellular Ca2+, thereby impacting the Ca2+ clock (117).
Mitochondria-SR communication.
It is known that the high basal levels of cAMP in SAN cells are associated with a high mitochondrial respiratory rate (15, 16). Emerging evidence reveals that proper communication between mitochondria and the SR is important in modulating the Ca2+ and cAMP signaling in SAN cells. Disruption at the mitochondria-SR contact sites due to the reduced level of mitofusin-2 impairs Ca2+ release and cAMP signaling at the mitochondria-SR microdomains, leading to reduced spontaneous firing rate and prolonged AP duration in SAN cells (118). This mechanism contributes to HF-associated SND (118).
Sarcoplasmic-endoplasmic reticulum Ca2+ ATPase.
The sarcoplasmic-endoplasmic reticulum Ca2+ ATPase (SERCA) channel, embedded on the SR surface, serves to transport cytosolic Ca2+ back into the SR and hence plays a key role in cellular Ca2+ homeostasis. cAMP-activated PKA has been shown to phosphorylate SERCA, thereby modulating Ca2+ cycling in SAN cells (119). Inhibition of SERCA with cyclopiazonic acid leads to a dose-dependent suppression in spontaneous SAN firing by up to 50% (120). SERCA2a (the cardiac isoform) knockout mice exhibit normal HR but prolonged PR interval at baseline, as well as a reduced maximal HR during exercise (121). SERCA2a is known to be downregulated in HF, leading to aberrant Ca2+ cycling and a decreased force-frequency response (122). The abnormal expression of SERCA2a underlying both HF and SND may offer another mechanistic link between the two comorbidities.
Other Factors
Voltage-gated sodium channel.
Voltage-gated sodium channel type 5 alpha subunit (SCN5A) is highly expressed in cardiomyocytes and encodes Nav1.5 protein. Nav1.5 channels are responsible for the initiation and transmission of APs in the myocardium and determine the electrical conduction through the heart (123). GOF mutations in SCN5A underlie the development of LQTS because of the increased Na+ influx into cardiomyocytes, while the LOF mutations in SCN5A are often associated with Brugada syndrome and dilated cardiomyopathy (DCM) (123, 124). Mutations of SCN5A have been identified in patients with congenital SND (125, 126). Scn5a heterozygous knockout (Scn5a+/−) mice also display slower SA conduction and overall reduced HR compared with wild-type littermates (127). Nav1.5 is likely absent in the central SAN pacemaker cells but is detectable in peripheral SAN myocytes. The impaired function of the Nav1.5 channels does not directly alter the spontaneous firing of the central SAN pacemaker cells, as the latter is dependent on the If-mediated membrane clock and the NCX-mediated Ca2+ clock as discussed above. However, in Scn5a+/− mice, the peripheral SAN myocytes demonstrate a slowed pacemaker rate as compared with the central SAN myocytes, which contributes to the reduced HR in Scn5a+/− mice. Additionally, the failure of impulses to conduct into adjacent atrial myocardium due to reduced excitability of the cardiomyocytes is likely the mechanism underlying the SCN5A-associated SND (127).
Myosin heavy chain 6.
Cardiac alpha-myosin heavy chain (encoded by MYH6) is an important sarcomeric protein that plays a significant role in cardiac contraction (128). MYH6 is dynamically expressed in cardiomyocytes and is essential for heart development. MYH6 homozygous mice exhibit embryonic lethality at embryonic days 11–12 with gross heart defects and survived heterozygotes mice display severe impairment of cardiac contraction and relaxation due to fibrotic lesions and sarcomeric structure alterations (129). Knockdown of MYH6 can lead to abnormal development of the atrial septum with immature actin filaments and abnormal cardiac looping (130, 131). Genetic variants of MYH6 are a well-known cause of hypertrophic cardiomyopathy and DCM, as well as congenital heart diseases (132, 133). MYH6 variants have also been found in patients with early onset and familial SND, AF, ventricular arrhythmias, and cardiac arrest (134–137). However, the molecular mechanism of MYH6 in SND and other cardiac arrhythmias remains to be elucidated. The available mechanistic studies have suggested that microRNA (miR)-208, a highly conserved cardiac-specific miR encoded by intron 29 of MYH6, is required for proper cardiac conduction. miR-208 can modulate the expression of cardiac gap junction protein connexin 40 (Cx40), and altered miR-208 levels can affect SAN impulse formation and atrial propagation (138, 139).
Short-stature homeobox 2.
Short-stature homeobox 2 Shox2 protein (encoded by SHOX2) is a homeodomain transcription factor, essential for the proper formation and development of the SAN. Several transcription factors, including Tbx18, Tbx3, Isl1, Tbx5, Bmp4, Nkx2.5, and Pitx2c are required for SAN development and function (140–146). Shox2 interacts with these genes to form a fine regulatory network controlling SAN morphogenesis through increasing the expression of Tbx3, Bmp4, and Islet1, being promoted by upstream Tbx5 and repressed by Nkx2.5 and Pitx2c (140). Homozygous deletion of Shox2 in mice leads to embryonic lethality due to severe cardiovascular defects, including bradycardia, severe hypoplastic SAN, and sinus valves owing to a significantly decreased level of cell proliferation (147, 148). The Shox2-deletion can result in reduced expression of HCN4 and Tbx3 (another key regulator of the CCS), along with ectopic activation of the chamber differentiation marker Nppa and atrial myocardium-specific gap junction protein Cx40 in the SAN region (147). On the other hand, overexpression of SHOX2 enhances the differentiation of SAN cells (149). Mutations of SHOX2 are mainly associated with the short stature phenotype seen in patients with Turner’s syndrome (150, 151). A few cases of SHOX2 genetic variants have been found in patients with early onset and familial forms of AF and SND (152–154). However, mechanistic studies are needed to understand the pathogenic mechanism of Shox2-related heart disease.
Lamin A/C.
The LMNA gene encodes A-type nuclear lamins, including lamin A and C (lamin A/C) (155). Lamin A/C proteins constitute the nuclear lamina and play an important role in cell differentiation, lineage specification, and tissue development (156). Nuclear lamin A/C interact with cytoskeleton components in the cytoplasm through the linker of nucleoskeleton and cytoskeleton (LINC) complex and chromatin in the nucleoplasm. These interactions support their function in mechanotransduction, nuclear stability, chromatin organization, gene regulation, DNA replication, cell-cycle regulation, and RNA splicing (157–161). Genomic regions that are bound to nuclear lamins are referred as lamina-associated domains (LADs), which are predominantly located at the nuclear periphery where the repressive chromatin marks are enriched (162, 163). Through LADs, lamin A/C participate in genomic organization (164), recruitment of epigenetic regulators (165), and gene expression (166). Genetic variants of LMNA are associated with a wide spectrum of diseases including DCM, conduction disorders, Hutchinson-Gilford Progeria Syndrome, Emery-Dreifuss muscular dystrophy, and restrictive dermopathy, which are collectively referred to as laminopathies (167). LMNA mutations are the second most common variants associated with familial cardiomyopathy, accounting for ∼6–8% of idiopathic DCM cases and 33% of familial DCM cases with conduction disorders (168–170). Additionally, LMNA mutations have been increasingly described in patients with SND, sinus arrest, AV conduction block, his/bundle disease, AF, supraventricular tachycardia, and ventricular tachycardia/fibrillation (168, 171). Lamin A/C knockout (Lmna−/−) mice demonstrate progressive cardiac dilatation, myocardial fibrosis, and reduced HR with lengthened PR and QRS intervals, leading to premature death by 8 wk of age (172, 173). However, the molecular and cellular mechanisms underlying LMNA mutation-mediated pathogenesis of SND and cardiac conduction disorders remain unknown. Because many genes and pathways are reportedly dysregulated in diverse cell types including cardiomyocytes and cardiac fibroblasts due to lamin A/C deficiency (171, 173–175), it is likely that the pathogenic mechanisms contributing to the LMNA-associated SND and cardiac conduction disorders are multifaceted.
Source-sink mismatch.
For normal SAN function, a delicate source-sink balance must be maintained between the relatively few pacemaker cells (source) and surrounding atrial myocardium (sink) for the SAN to drive the larger surrounding myocardium without being overcome by myocardium’s hyperpolarizing influence (176). Altered connexin function and fibrotic remodeling can often cause a source-sink mismatch, thereby impairing the effectiveness of SAN function.
Connexins form gap junction channels which are responsible for proper AP propagation throughout the heart. Differential expression of connexins within the SAN architecture help maintain the source-sink balance by electrically uncoupling the SAN from the surrounding myocardium except at limited exit sites, reducing the perceived electrical load on the SAN (176). Connexin (Cx)43, the principal connexin of working myocardium, is absent from the center of the SAN and is only present in the periphery of the SAN where strong electrical coupling is needed to drive the atrial muscle (177). The SAN is further uncoupled from the surrounding tissue by the preferential expression of Cx45, which is characterized by its low conductance and is the major isoform expressed in the SAN (176). Altered expression of connexins has been associated with SND. A Cx45 mutation (p.R75H) in the GJC1 gene was described in two unrelated families (a de novo French case and a 3-generation Japanese family) who presented with atrial standstill and congenital AV block in addition to other extracardiac abnormalities including finger deformities, dental dysplasia, and craniofacial abnormalities (178). GJC1 conditional knockout mice also exhibit intermittent atrial pauses and SND but without AV node dysfunction, likely due to species differences in connexin expression in the AV node (178). Age-related decreased expression of Cx43 has also been observed in studies of guinea pigs, with conduction velocity subsequently decreasing with age (179). These age-related changes in connexin expression are thought to contribute to a sink-source mismatch between the SAN and atrial myocardium, providing another potential mechanism for age-related SND.
As mentioned earlier, pacemaker cells are enmeshed in fibrous connective tissue, which provides a structural scaffold and electrical insulation for the pacemaker cells (180). The fibrotic content within the SAN region increases with heart size and age in various species (181–184). Fibroblasts also express stretch-activated channels and can be electronically coupled with SAN myocytes; therefore, the healthy number of fibroblasts within the SAN can meet the contractile demands during the natural growth and prevent the SAN from overstretching (20, 183). However, excessive fibrosis is associated with a spectrum of SND including bradycardia, sinus pause, exit block, tachy-brady syndrome, and reentrant arrhythmia within the SAN. Increased fibrosis can interrupt the coupling between fibroblasts and pacemaker cells, thereby promoting a source-sink mismatch (185). Although mechanistic studies that directly address fibrotic remodeling within the SAN have been limited, it is generally thought that the enhanced crosstalk between pacemaker cells and fibroblasts driven by transforming growth factor (TGF)-β1 signaling can promote myofibroblast transdifferentiation and increase fibrosis in the SAN region (186). A recent transcriptome and proteomics study has shed a light on the fibroblast-specific expression profiles of human SAN in HF hearts compared with non-HF hearts (31). Specifically, the CILP1 (cartilage intermediate layler protein 1)- or POSTN (periostin)-positive fibroblasts were enriched in the SANs of HF hearts but not in non-HF hearts. Moreover, a recent study has shown that mice with CCS-specific deletion of Hippo kinases develop SND, which is associated with increased SAN fibrosis. Chromatin sequencing further revealed that the inactivation of Hippo kinases increases fibroblast proliferation within the SAN region by upregulating Tgfb1 (encoding TGF-β1) and Tgfb3 (encoding TGF-β3) in pacemaker cells (187). Interestingly, the genes involved in cell-cell adhesions, such as ZO-1 and Cdh2, are also altered in the SANs of CCS-specific Hippo knockout mice, suggesting a source-sink mismatch induced by the Hippo inactivation (187).
MANAGEMENT OF SND
In patients symptomatic with SND, assessment of potentially reversible or treatable causes of SND is the first step in management. These include acute myocardial infarction, atrial tachyarrhythmia, electrolyte or metabolic disturbances, hypothyroidism, or medications (2). However, most causes of SND tend to be chronic and irreversible (2). In these cases, there is currently no known cure, and the treatments that do exist for SND, for the most part, aim to manage symptoms from bradycardia attributable to SND. Outlined below are some of the main SND management methods:
Permanent Pacemakers
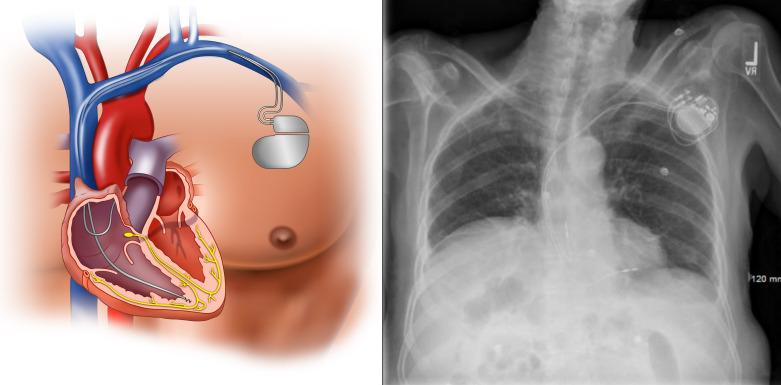
Permanent pacemaker implantation is the primary therapeutic intervention for patients symptomatic with SND without an identifiable reversible cause (2). Either single-chamber atrial pacemakers or dual-chamber pacemakers are used, depending on whether AV conduction abnormalities are present (Fig. 4). While single-chamber ventricular pacing is also feasible for SND, this method is a less physiological form of pacing due to loss of AV synchrony and can lead to chronic left ventricular dysfunction (188). Pacemaker syndrome, or development of signs and symptoms of HF or hypotension, is common with ventricular pacing, occurring in up to 20% of patients (39). While previous randomized controlled trials have failed to demonstrate a clear reduction in hard outcomes of death, stroke, or HF hospitalization with atrial-based pacing modes which preserve AV synchrony compared with ventricular pacing in SND (2), atrial pacing shows a significantly decreased risk of subsequent AF and mild improvement in quality of life (39, 189). Thus current guidelines recommend atrial pacing modes over single-chamber ventricular pacing for SND, primarily because of their decreased risk of subsequent AF (2).
Figure 4.
Dual-chamber pacemaker. Standard dual-chamber pacemaker consisting of a pulse generator in the upper chest and leads implanted into the right atrium and right ventricle.
In patients with SND and intact AV conduction, dual-chamber, and single-chamber atrial pacemakers are equally effective with no difference in long-term mortality, HF, or stroke (190). However, the incidence of AF and risk of pacemaker reoperation is higher with single-chamber atrial pacing (190). While implantation of an additional lead with dual-chamber pacemakers increases procedural risk, this must be weighed against the risk of future development of AV block, which would require reoperation to place an additional ventricular lead (2, 190). The risk of developing AV block after pacemaker implantation can be difficult to predict, with reported rates ranging anywhere from 3 to 35% within 5 yr of follow-up (2). Thus dual-chamber pacemakers are generally used in SND with normal AV conduction unless there is reason to avoid a right ventricular (RV) lead or if strict maintenance of AV synchrony is not deemed necessary (e.g., frailty, limited functional capacity, poor short-term prognosis) (2). Currently, dual-chamber devices are by far the most used pacemaker for SND in the United States, accounting for more than 80% of device implants (191). When dual-chamber pacemakers are used, efforts to minimize RV pacing should be made to further reduce the risk of AF and the long-term deleterious effects of continuous ventricular pacing (192).
There are certain risks associated with permanent pacemaker implantation, including complications associated with the implantation surgery itself as well as complications with the pacemaker over time (193). Pacemaker-related complications occur in up to one in five patients at 5 years and are mainly attributed to the leads (lead dislodgement, fracture, malfunction) or generator pocket (infection, hematoma) (194). Leadless pacemakers are a new technology designed to reduce lead and pocket-related complications by combining the pulse generator and electrode in a self-contained unit that is directly implanted into the RV (195). While only capable of ventricular pacing, leadless pacemakers are a reasonable alternative to atrial or dual-chamber pacing for SND when implantation of a transvenous system is considered difficult or high risk (poor venous access, need to preserve veins for hemodialysis, high risk of infection) (196). Furthermore, leadless pacemakers may be preferable in patients with symptomatic SND that is short-lived or infrequent and the pacing burden is anticipated to be low (2).
Temporary Pacemakers
Temporary pacing can be used acutely to manage bradycardia causing hemodynamic instability refractory to pharmacological therapy. These situations can include prolonged sinus pauses, life-threatening ventricular arrhythmias precipitated by bradycardia, or other severe bradycardia attributable to a reversible cause with the goal to avoid permanent pacemaker implantation (2). However, the use of temporary pacing in SND is uncommon as SND is generally an indolent chronic process that is rarely acutely life-threatening (2). The two main types of temporary pacemaker therapy are transcutaneous cardiac pacing (TCP) and transvenous temporary cardiac pacing (TV-TP).
Transcutaneous cardiac pacing.
TCP is a temporary pacemaking method involving the delivery of electric pulses to the patient’s chest to cause contractions of the heart. It is commonly used during life-threatening cardiac emergencies, such as severe symptomatic or hemodynamically unstable bradyarrhythmias, ventricular, and supraventricular tachycardia, or when episodes of such severe arrhythmias are predicted to occur (197). In patients with bradycardia who are not in full cardiac arrest, the use of TCP has been shown to be an effective measure for increasing HR and is generally tolerable by most patients (198). Studies have also shown mild improvement in survival with prehospital TCP in nonasystolic patients with symptomatic bradycardia (199) although no such benefit is seen in patients with full cardiac arrest (200). TCP is most commonly used as a temporizing measure for pacing until a temporary transvenous pacing wire or permanent pacemaker can be implanted.
Transvenous temporary cardiac pacing.
Transvenous temporary cardiac pacing (TV-TP) is typically performed by inserting a pacing wire into the RV from a central venous access site. The RV is generally targeted because of its ease of access and efficacy for SND or AV block, although temporary pacing of the RA can be performed when strict AV synchrony is necessary and there is no AV block (2). Early studies of TV-TP showed high rates of malfunction of up to 43%, regardless of insertion site (201). Venous thrombosis has also been known to occur in 30 to 45% of patients as a result of electrode insertion (202). Metkus et al. (203) more recently reported that among a large cohort of patients who received TV-TP, 37.9% eventually underwent permanent pacemaker implants. The risk of infection following permanent pacemaker implant is increased in patients with TV-VP before pacemaker implant. Given the risks associated with TV-VP, its use is reserved for severe SND with hemodynamic compromise as a bridge toward permanent pacemaker implant or until the bradycardia resolves (2).
Pharmacological Treatments
A pharmacological approach can be used for the acute management of severe bradycardia attributable to SND. According to current guidelines, atropine, isoproterenol, aminophylline, or theophylline can be used to acutely treat bradycardia that may result from SND (2). Atropine works to block the muscarinic acetylcholine receptor and subsequently reduces the activity of GIRK channels or IK,Ach associated with SND, thereby increasing SAN automaticity (2). Atropine is an effective temporary treatment for bradycardia with minimal risk of ischemia or arrhythmia and is the first-line pharmacological agent in most cases (2). Atropine should be avoided in cases of infranodal conduction block because of the potential worsening of block and bradycardia. Isoproterenol is a nonselective β-receptor agonist with chronotropic and inotropic effects. Primarily used during electrophysiology studies, isoproterenol is useful for increasing HR in unstable bradycardias without vasoconstrictor effects (2). However, isoproterenol should be avoided in cases of suspected coronary ischemia as its nonselective beta-agonist effects increase myocardial oxygen demand while decreasing coronary perfusion (2). In addition, in some patients, isoproterenol may have a vasodilatory effect or a weak effect on HR response (2). Aminophylline and theophylline competitively antagonize adenosine receptors, increasing cardiac automaticity and exerting positive chronotropic effects (204). These drugs have been used for both the acute and chronic management of SND (10). In particular, these drugs are useful for the management of bradycardia following heart transplantation or spinal cord injury, which are refractory to atropine due to cardiac autonomic denervation (2). In SND cases where direct association of symptoms to bradycardia is unclear, a trial of oral theophylline can be considered to help correlate symptoms (2). In this setting, oral theophylline is seen to increase resting HR, decrease the frequency of sinus pauses, and improve subjective symptoms in a majority of patients, although to a lesser degree compared with permanent pacing (205, 206). In patients with symptomatic SND who decline permanent pacemaker implantation or are not candidates otherwise, oral theophylline can also be considered as an alternative treatment (2). While generally well tolerated, caution should be given when using theophylline in patients with tachycardia-bradycardia because of the increased frequency of atrial tachyarrhythmia and in patients with structural heart disease because of the increased risk of ventricular arrhythmias (10).
RESEARCH OPPORTUNITIES
With the goal of developing better and alternative therapeutic strategies for SND, future research is needed to better understand the causative cellular and molecular mechanisms of SND. In this section, we provide our perspective and highlight a few areas of interest for future investigations.
Aging-Related Processes in SND Pathogenesis
It is known that SAN function and pacemaking activity decline with age. There is evidence implicating age-dependent decreases in the expression of genes involved in the membrane clock and Ca2+ clock (45, 49). However, whether the function of certain ion channels and Ca2+ handling proteins presents age-dependent impairment requires experimental investigation. Moreover, whether the relative contribution of the membrane clock and Ca2+ clock to pacemaking activity is altered with aging deserves to be addressed. Naturally, aging is associated with increased atrial fibrosis, more inflammation, mitochondrial dysfunction, epigenetic alterations, and genomic instability. How these processes alter the function of pacemaker cells and the structure of the SAN is another exciting area to be explored.
Sex Impact on SND
There is some evidence to suggest that familial SND may represent a strong male predominance (207). As several risk factors of SND such as AF and HF present sex-dependent differences (208, 209), future studies are needed to understand the impact of sex in the pathogenesis of SND.
Genetic and Epigenetic Mechanisms Underlying SND
Although the genetic association between SHOX2, LMNA, and SND exists, the underlying mechanisms are currently lacking. Examination of the Shox protein level in SAN tissue in humans with and without SND is a prerequisite for future projects in examining the role of Shox2 in SND development. Lamins are very important in many different biological processes. To understand the specific contribution of lamins in SAN and CCS homeostasis and dissect the molecular mechanisms underlying LMNA-associated SND or cardiac conduction disorders, SAN pacemaker cell-specific or CCS-specific LMNA mutant alleles are needed. Findings in these areas can also shed a light on the epigenetic regulation’s involvement in SND development.
Cellular Model of Congenital SND
The association between genetic variants and SND is present. To assess the pathogenicity of any given variants, a cellular model system that can faithfully represent SAN pacemaker cells would be necessary. One classic approach is to reprogram cardiomyocytes cell lines (e.g., HL-1 cells) into SAN-like cells in a dish (210–212) and then introduce the mutated genes by transfection in the heterologous system, followed by electrophysiological assessment. As the techniques in differentiating human iPSCs become mature and commonly available (213), another opportunity is to develop human pacemaker-like cells with the human iPSCs derived from the SND-variant carriers. The advantages of iPSCs in cardiac electrophysiology research include 1) they can be easily scaled up, 2) they can be used for drug screening, and 3) they are helpful in gaining initial insights into monogenic disease (213, 214). While protocols for iPSC differentiation into cardiomyocytes have been optimized over the last decade and become widely available, protocols to differentiate iPSCs into pacemaker cells are still needed in the field. Based on developmental biology, transcription factors such as Tbx3, Tbx18, and Shox2, which are essential for SAN development, are ideal candidates for inducing iPSC-pacemaker cell differentiation. A transgene-independent method of generating SAN-like pacemaker cells from human iPSCs was described recently (215, 216). Through stage-specific modulation of signaling pathways including activation of bone morphogenic protein, retinoic acid, and inhibition of fibroblast growth factor, Protze et al. (215, 216) have shown success in producing enriched populations of SAN-like pacemaker cells with high efficiency. These SAN-like pacemaker cells exhibit typical pacemaker electrophysiological characteristics and are capable of pacing host tissue.
Biological Pacemaker Development
The electronic pacemaker device is the current mainstay treatment for SND. As discussed earlier, limited battery life, infection, and other device-related complications are the major disadvantages associated with the implantable pacemakers. There are ongoing efforts to reproduce a biological pacemaker as an alternative to the electronic pacemaker device (217, 218). Many different gene-based and cell-based approaches to produce biological pacemakers have been described over the years. However, it remains a challenge to translate these studies into clinical trials as the dose of gene expression and the location of delivery need to be optimized and perhaps individualized for SND with different etiologies. For a detailed review on biological pacemaker development, we refer you to a recent review article by Cingolani et al. (219).
SAN Mechanosensitivity
Our understanding of the molecular mechanisms of SAN mechanosensitivity remains limited, with further studies needed to identify the precise pathways implicated in this process. Identified components may offer a novel therapeutic target for the treatment of SND by halting maladaptive stretch-induced changes, thereby normalizing SAN function (18). Changes in mechanical loading observed with HF and AF may offer a mechanistic link between the development of SND and these comorbidities. Finally, consideration of SAN mechano-sensitivity is critical for future mechanistic studies of SAN function. Since many in vitro studies analyze SAN function in the absence of a mechanical load, these nonphysiological conditions may confound extrapolation of findings to in vivo SAN function and further methods to accurately model mechanical stress are needed for future SND studies.
Potential Role of Nonnodal Cells
Recent work has revealed the presence of numerous nonnodal cells within the SAN, including S100 calcium-binding protein B (S100B)+/glial fibrillary acidic protein (GFAP)+ peripheral glial cells, S100B+/GFAP- interstitial cells, telocytes, as well as neuronal fibers projecting from the epicardial ganglionated plexuses directly into the SAN itself (220). S100B+ interstitial cells within the SAN have been shown to modulate rhythmic Ca2+ activity of pacemaker cells and lead to HR variability (220). The nonnodal cells form a unique meshwork of interconnected cells, which are hypothesized to help integrate the multitude of heterogeneous behaviors across the pacemaker cell network to produce the net impulse activity that exits the SAN, in a manner reminiscent of brain neuronal networks (220, 221). This “neuromimetic” framework within the SAN offers a novel conceptualization of SAN structure and highlights the potential role of nonnodal cells in maintaining normal SAN function. Identification of these previously undefined mechanisms of arrhythmia may offer yet another therapeutic target for the treatment of SND.
CONCLUSIONS
Significant advances have been made over the past two decades in understanding the pathophysiology of SND. Identification of genetic abnormalities associated with familial SND has helped the field to gain the current knowledge on the cellular and molecular mechanisms of SND. Nevertheless, abundant research opportunities regarding the pathogenesis of SND remain. Mechanistic insights into how age-dependent changes in metabolism and epigenetic regulation promote the pathogenesis of nonfamilial SND are needed. Biological pacemaker development remains challenging but is an area with tremendous potential as the field of gene therapy and regenerative medicine for cardiovascular diseases is moving forward.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants R01HL136389, R01HL163277, and R01HL147108 (to N. Li); American Heart Association Established Investigator Award 936111 (to N. Li); and Patient-Centered Outcomes Research Institute research support (to M. G. Chelu).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.K., M.G.C., and N.L. conceived and designed research; P.M., J.A.K., S.K., T.L., and M.S. analyzed data; J.A.K., S.K., T.L,. and N.L. interpreted results of experiments; P.M., J.A.K., and N.L. prepared figures; P.M., J.A.K., S.K., and M.S. drafted manuscript; P.M., J.A.K., S.K., T.L., M.G.C., and N.L. edited and revised manuscript; P.M., J.A.K., S.K., T.L., M.S., M.G.C., and N.L. approved final version of manuscript.
REFERENCES
- 1. Keith A, Flack M. The form and nature of the muscular connections between the primary divisions of the vertebrate heart. J Anat Physiol 41: 172–189, 1907. [PMC free article] [PubMed] [Google Scholar]
- 2. Kusumoto FM, Schoenfeld MH, Barrett C, Edgerton JR, Ellenbogen KA, Gold MR, Goldschlager NF, Hamilton RM, Joglar JA, Kim RJ, Lee R, Marine JE, McLeod CJ, Oken KR, Patton KK, Pellegrini CN, Selzman KA, Thompson A, Varosy PD. 2018 ACC/AHA/HRS guideline on the evaluation and management of patients with bradycardia and cardiac conduction delay: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation 140: e382, 2019. [DOI] [PubMed] [Google Scholar]
- 3. Bernstein AD, Parsonnet V. Survey of cardiac pacing and defibrillation in the United States in 1993. Am J Cardiol 78: 187–196, 1996. [PubMed] [Google Scholar]
- 4. Ho SY, Sánchez-Quintana D. Anatomy and pathology of the sinus node. J Interv Card Electrophysiol 46: 3–8, 2016. doi: 10.1007/s10840-015-0049-6. [DOI] [PubMed] [Google Scholar]
- 5. Hayes DL, Asirvatham SJ, Friedman PA. Cardiac Pacing, Defibrillation and Resynchronization a Clinical Approach. Chichester, UK: Wiley-Blackwell, 2013. [Google Scholar]
- 6. Lee FK, Lee JC, Shui B, Reining S, Jibilian M, Small DM, Jones JS, Allan-Rahill NH, Lamont MR, Rizzo MA, Tajada S, Navedo MF, Santana LF, Nishimura N, Kotlikoff MI. Genetically engineered mice for combinatorial cardiovascular optobiology. Elife 10: e67858, 2021. doi: 10.7554/eLife.67858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mitrofanova LB, Gorshkov AN, Konovalov PV, Krylova JS. Telocytes in the human sinoatrial node. J Cell Mol Med 22: 521–532, 2018. doi: 10.1111/jcmm.13340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sánchez-Quintana D, Cabrera JA, Farré J, Climent V, Anderson RH, Ho SY. Sinus node revisited in the era of electroanatomical mapping and catheter ablation. Heart 91: 189–194, 2005. doi: 10.1136/hrt.2003.031542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Choudhury M, Boyett MR, Morris GM. Biology of the sinus node and its disease. Arrhythm Electrophysiol Rev 4: 28–34, 2015. doi: 10.15420/aer.2015.4.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Loscalzo J. Harrison's Cardiovascular Medicine 2/E. New York: McGraw-Hill Education, 2013. [Google Scholar]
- 11. Lyashkov AE, Behar J, Lakatta EG, Yaniv Y, Maltsev VA. Positive feedback mechanisms among local Ca releases, NCX, and I(CaL) ignite pacemaker action potentials. Biophys J 114: 1176–1189, 2018. [Erratum in Biophys J 114: 2024, 2018]. doi: 10.1016/j.bpj.2017.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mangoni ME, Nargeot J. Genesis and regulation of the heart automaticity. Physiol Rev 88: 919–982, 2008. doi: 10.1152/physrev.00018.2007. [DOI] [PubMed] [Google Scholar]
- 13. Capel RA, Terrar DA. The importance of Ca2+-dependent mechanisms for the initiation of the heartbeat. Front Physiol 6: 80, 2015. doi: 10.3389/fphys.2015.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vinogradova TM, Lyashkov AE, Zhu W, Ruknudin AM, Sirenko S, Yang D, Deo S, Barlow M, Johnson S, Caffrey JL, Zhou YY, Xiao RP, Cheng H, Stern MD, Maltsev VA, Lakatta EG. High basal protein kinase A-dependent phosphorylation drives rhythmic internal Ca2+ store oscillations and spontaneous beating of cardiac pacemaker cells. Circ Res 98: 505–514, 2006. doi: 10.1161/01.RES.0000204575.94040.d1. [DOI] [PubMed] [Google Scholar]
- 15. Yaniv Y, Juhaszova M, Lyashkov AE, Spurgeon HA, Sollott SJ, Lakatta EG. Ca2+-regulated-cAMP/PKA signaling in cardiac pacemaker cells links ATP supply to demand. J Mol Cell Cardiol 51: 740–748, 2011. doi: 10.1016/j.yjmcc.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yaniv Y, Spurgeon HA, Ziman BD, Lyashkov AE, Lakatta EG. Mechanisms that match ATP supply to demand in cardiac pacemaker cells during high ATP demand. Am J Physiol Heart Circ Physiol 304: H1428–H1438, 2013. doi: 10.1152/ajpheart.00969.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Weiss JN, Qu Z. The sinus node. JACC Clin Electrophysiol 6: 1841–1843, 2020. doi: 10.1016/j.jacep.2020.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. MacDonald EA, Quinn TA. What keeps us ticking? Sinoatrial node mechano-sensitivity: the grandfather clock of cardiac rhythm. Biophys Rev 13: 707–716, 2021. doi: 10.1007/s12551-021-00831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bainbridge FA. The influence of venous filling upon the rate of the heart. J Physiol 50: 65–84, 1915. doi: 10.1113/jphysiol.1915.sp001736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cooper PJ, Lei M, Cheng LX, Kohl P. Selected contribution: axial stretch increases spontaneous pacemaker activity in rabbit isolated sinoatrial node cells. J Appl Physiol (1985 ) 89: 2099–2104, 2000. doi: 10.1152/jappl.2000.89.5.2099. [DOI] [PubMed] [Google Scholar]
- 21. Calloe K, Elmedyb P, Olesen SP, Jorgensen NK, Grunnet M. Hypoosmotic cell swelling as a novel mechanism for modulation of cloned HCN2 channels. Biophys J 89: 2159–2169, 2005. doi: 10.1529/biophysj.105.063792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lyford GL, Strege PR, Shepard A, Ou Y, Ermilov L, Miller SM, Gibbons SJ, Rae JL, Szurszewski JH, Farrugia G. α1C (CaV1.2) L-type calcium channel mediates mechanosensitive calcium regulation. Am J Physiol Cell Physiol 283: C1001–C1008, 2002. doi: 10.1152/ajpcell.00140.2002. [DOI] [PubMed] [Google Scholar]
- 23. Reeves JP, Abdellatif M, Condrescu M. The sodium-calcium exchanger is a mechanosensitive transporter. J Physiol 586: 1549–1563, 2008. doi: 10.1113/jphysiol.2008.151274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brennan Jaclyn A, Chen Q, Gams A, Dyavanapalli J, Mendelowitz D, Peng W, Efimov IR. Evidence of superior and inferior sinoatrial nodes in the mammalian heart. JACC Clin Electrophysiol 6: 1827–1840, 2020. doi: 10.1016/j.jacep.2020.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Reece WO, Erickson HH, Goff JP, Uemura EE. Dukes' Physiology of Domestic Animals. Hoboken, NJ: Wiley, 2015. [Google Scholar]
- 26. Opthof T. Function and structure of the mouse sinus node: nothing you can see that isn’t shown. Cardiovasc Res 52: 1–4, 2001. doi: 10.1016/s0008-6363(01)00417-5. [DOI] [PubMed] [Google Scholar]
- 27. Opthof T. The mammalian sinoatrial node. Cardiovasc Drugs Ther 1: 573–597, 1988. doi: 10.1007/BF02125744. [DOI] [PubMed] [Google Scholar]
- 28. Verkerk AO, Wilders R, van Borren MM, Peters RJ, Broekhuis E, Lam K, Coronel R, de Bakker JM, Tan HL. Pacemaker current (I(f)) in the human sinoatrial node. Eur Heart J 28: 2472–2478, 2007. doi: 10.1093/eurheartj/ehm339. [DOI] [PubMed] [Google Scholar]
- 29. Breithardt G, Seipel L, Loogen F. Sinus node recovery time and calculated sinoatrial conduction time in normal subjects and patients with sinus node dysfunction. Circulation 56: 43–50, 1977. doi: 10.1161/01.cir.56.1.43. [DOI] [PubMed] [Google Scholar]
- 30. Liu J, Dobrzynski H, Yanni J, Boyett MR, Lei M. Organisation of the mouse sinoatrial node: structure and expression of HCN channels. Cardiovasc Res 73: 729–738, 2007. doi: 10.1016/j.cardiores.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 31. Kalyanasundaram A, Li N, Hansen BJ, Zhao J, Fedorov VV. Canine and human sinoatrial node: differences and similarities in the structure, function, molecular profiles, and arrhythmia. J Vet Cardiol 22: 2–19, 2019. doi: 10.1016/j.jvc.2018.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Woods WT, Urthaler F, James TN. Spontaneous action potentials of cells in the canine sinus node. Circ Res 39: 76–82, 1976. doi: 10.1161/01.RES.39.1.76. doi:. [DOI] [PubMed] [Google Scholar]
- 33. Beaulieu P, Cardinal R, Pagé P, Francoeur F, Tremblay J, Lambert C. Positive chronotropic and inotropic effects of C-type natriuretic peptide in dogs. Am J Physiol Heart Circ Physiol 273: H1933–H1940, 1997. doi: 10.1152/ajpheart.1997.273.4.H1933. [DOI] [PubMed] [Google Scholar]
- 34. Long VP 3rd, Bonilla IM, Baine S, Glynn P, Kumar S, Schober K, Mowrey K, Weiss R, Lee NY, Mohler PJ, Györke S, Hund TJ, Fedorov VV, Carnes CA. Chronic heart failure increases negative chronotropic effects of adenosine in canine sinoatrial cells via A1R stimulation and GIRK-mediated I(Kado). Life Sci 240: 117068, 2020. doi: 10.1016/j.lfs.2019.117068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chandler NJ, Greener ID, Tellez JO, Inada S, Musa H, Molenaar P, DiFrancesco D, Baruscotti M, Longhi R, Anderson RH, Billeter R, Sharma V, Sigg DC, Boyett MR, Dobrzynski H. Molecular architecture of the human sinus node. Circulation 119: 1562–1575, 2009. doi: 10.1161/CIRCULATIONAHA.108.804369. [DOI] [PubMed] [Google Scholar]
- 36. Boyett MR, Honjo H, Kodama I. The sinoatrial node, a heterogeneous pacemaker structure. Cardiovasc Res 47: 658–687, 2000. doi: 10.1016/s0008-6363(00)00135-8. [DOI] [PubMed] [Google Scholar]
- 37. Rodriguez RD, Schocken DD. Update on sick sinus syndrome, a cardiac disorder of aging. Geriatrics 45: 26–30, 1990. [PubMed] [Google Scholar]
- 38. Jensen PN, Gronroos NN, Chen LY, Folsom AR, deFilippi C, Heckbert SR, Alonso A. Incidence of and risk factors for sick sinus syndrome in the general population. J Am Coll Cardiol 64: 531–538, 2014. doi: 10.1016/j.jacc.2014.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lamas GA, Lee KL, Sweeney MO, Silverman R, Leon A, Yee R, Marinchak RA, Flaker G, Schron E, Orav EJ, Hellkamp AS, Greer S, McAnulty J, Ellenbogen K, Ehlert F, Freedman RA, Estes NA 3rd, Greenspon A, Goldman L, Mode Selection Trial in Sinus-Node Dysfunction. Ventricular pacing or dual-chamber pacing for sinus-node dysfunction. N Engl J Med 346: 1854–1862, 2002. doi: 10.1056/NEJMoa013040. [DOI] [PubMed] [Google Scholar]
- 40. Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA, Freedman RA, Gettes LS, Gillinov AM, Gregoratos G, Hammill SC, Hayes DL, Hlatky MA, Newby LK, Page RL, Schoenfeld MH, Silka MJ, Stevenson LW, Sweeney MO, Tracy CM, Epstein AE, Darbar D, DiMarco JP, Dunbar SB, Estes NA, Ferguson TB, Hammill SC, Karasik PE, Link MS, Marine JE, Schoenfeld MH, Shanker AJ, Silka MJ, Stevenson LW, Sweeney MO; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines; Heart Rhythm Society. ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities. Circulation 127: e283–e352, 2013. doi: 10.1161/cir.0b013e318276ce9b. [DOI] [PubMed] [Google Scholar]
- 41. Menozzi C, Brignole M, Alboni P, Boni L, Paparella N, Gaggioli G, Lolli G. The natural course of untreated sick sinus syndrome and identification of the variables predictive of unfavorable outcome. Am J Cardiol 82: 1205–1209, 1998. doi: 10.1016/s0002-9149(98)00605-5. [DOI] [PubMed] [Google Scholar]
- 42. Shaw DB, Holman RR, Gowers JI. Survival in sinoatrial disorder (sick-sinus syndrome). Br Med J 280: 139–141, 1980. doi: 10.1136/bmj.280.6208.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Brandt J, Anderson H, Fåhraeus T, Schüller H. Natural history of sinus node disease treated with atrial pacing in 213 patients: implications for selection of stimulation mode. J Am Coll Cardiol 20: 633–639, 1992. doi: 10.1016/0735-1097(92)90018-I. [DOI] [PubMed] [Google Scholar]
- 44. Höijer CJ, Höglund P, Schüller H, Brandt J. Single chamber atrial pacing: a realistic option in sinus node disease: a long-term follow-up study of 213 patients. Pacing Clin Electrophysiol 30: 740–747, 2007. doi: 10.1111/j.1540-8159.2007.00744.x. [DOI] [PubMed] [Google Scholar]
- 45. Davies MJ, Pomerance A. Quantitative study of ageing changes in the human sinoatrial node and internodal tracts. Br Heart J 34: 150–152, 1972. doi: 10.1136/hrt.34.2.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jackson LR, 2nd, Rathakrishnan B, Campbell K, Thomas KL, Piccini JP, Bahnson T, Stiber JA, Daubert JP. Sinus node dysfunction and atrial fibrillation: a reversible phenomenon? Pacing Clin Electrophysiol 40: 442–450, 2017. doi: 10.1111/pace.13030. [DOI] [PubMed] [Google Scholar]
- 47. Heckbert SR, Austin TR, Jensen PN, Chen LY, Post WS, Floyd JS, Soliman EZ, Kronmal RA, Psaty BM. Differences by race/ethnicity in the prevalence of clinically detected and monitor-detected atrial fibrillation. Circ Arrhythm Electrophysiol 13: e007698, 2020. doi: 10.1161/CIRCEP.119.007698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Thery C, Gosselin B, Lekieffre J, Warembourg H. Pathology of sinoatrial node. Correlations with electrocardiographic findings in 111 patients. Am Heart J 93: 735–740, 1977. doi: 10.1016/s0002-8703(77)80070-7. [DOI] [PubMed] [Google Scholar]
- 49. Tellez JO, McZewski M, Yanni J, Sutyagin P, Mackiewicz U, Atkinson A, Inada S, Beresewicz A, Billeter R, Dobrzynski H, Boyett MR. Ageing-dependent remodelling of ion channel and Ca2+ clock genes underlying sino-atrial node pacemaking. Exp Physiol 96: 1163–1178, 2011. doi: 10.1113/expphysiol.2011.057752. [DOI] [PubMed] [Google Scholar]
- 50. Moghtadaei M, Jansen HJ, Mackasey M, Rafferty SA, Bogachev O, Sapp JL, Howlett SE, Rose RA. The impacts of age and frailty on heart rate and sinoatrial node function. J Physiol 594: 7105–7126, 2016. doi: 10.1113/JP272979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jansen HJ, Moghtadaei M, Rafferty SA, Belke DD, Rose RA. Loss of natriuretic peptide receptor c enhances sinoatrial node dysfunction in aging and frail mice. J Gerontol A Biol Sci Med Sci 77: 902–908, 2022. doi: 10.1093/gerona/glab357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Robinson RB, Siegelbaum SA. Hyperpolarization-activated cation currents: from molecules to physiological function. Annu Rev Physiol 65: 453–480, 2003. doi: 10.1146/annurev.physiol.65.092101.142734. [DOI] [PubMed] [Google Scholar]
- 53. Fenske S, Krause SC, Hassan SI, Becirovic E, Auer F, Bernard R, Kupatt C, Lange P, Ziegler T, Wotjak CT, Zhang H, Hammelmann V, Paparizos C, Biel M, Wahl-Schott CA. Sick sinus syndrome in HCN1-deficient mice. Circulation 128: 2585–2594, 2013. doi: 10.1161/CIRCULATIONAHA.113.003712. [DOI] [PubMed] [Google Scholar]
- 54. Herrmann S, Layh B, Ludwig A. Novel insights into the distribution of cardiac HCN channels: an expression study in the mouse heart. J Mol Cell Cardiol 51: 997–1006, 2011. doi: 10.1016/j.yjmcc.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 55. Wahl-Schott C, Fenske S, Biel M. HCN channels: new roles in sinoatrial node function. Curr Opin Pharmacol 15: 83–90, 2014. doi: 10.1016/j.coph.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 56. Milanesi R, Baruscotti M, Gnecchi-Ruscone T, DiFrancesco D. Familial sinus bradycardia associated with a mutation in the cardiac pacemaker channel. N Engl J Med 354: 151–157, 2006. [Erratum in N Engl J Med 354: 2520, 2006]. doi: 10.1056/NEJMoa052475. [DOI] [PubMed] [Google Scholar]
- 57. Larsson HP. How is the heart rate regulated in the sinoatrial node? Another piece to the puzzle. J Gen Physiol 136: 237–241, 2010. doi: 10.1085/jgp.201010506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hennis K, Biel M, Wahl-Schott C, Fenske S. Beyond pacemaking: HCN channels in sinoatrial node function. Prog Biophys Mol Biol 166: 51–60, 2021. doi: 10.1016/j.pbiomolbio.2021.03.004. [DOI] [PubMed] [Google Scholar]
- 59. Stieber J, Herrmann S, Feil S, Löster J, Feil R, Biel M, Hofmann F, Ludwig A. The hyperpolarization-activated channel HCN4 is required for the generation of pacemaker action potentials in the embryonic heart. Proc Natl Acad Sci U S A 100: 15235–15240, 2003. doi: 10.1073/pnas.2434235100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Baruscotti M, Bucchi A, Barbuti A, DiFrancesco D. Funny current and cardiac rhythm: insights from HCN knockout and transgenic mouse models. Front Physiol 3: 240, 2012. doi: 10.3389/fphys.2012.00240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Herrmann S, Hofmann F, Stieber J, Ludwig A. HCN channels in the heart: lessons from mouse mutants. Br J Pharmacol 166: 501–509, 2012. doi: 10.1111/j.1476-5381.2011.01798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ludwig A, Budde T, Stieber J, Moosmang S, Wahl C, Holthoff K, Langebartels A, Wotjak C, Munsch T, Zong X, Feil S, Feil R, Lancel M, Chien KR, Konnerth A, Pape HC, Biel M, Hofmann F. Absence epilepsy and sinus dysrhythmia in mice lacking the pacemaker channel HCN2. EBMO J 22: 216–224, 2003. doi: 10.1093/emboj/cdg032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. DiFrancesco D, Tortora P. Direct activation of cardiac pacemaker channels by intracellular cyclic AMP. Nature 351: 145–147, 1991. doi: 10.1038/351145a0. [DOI] [PubMed] [Google Scholar]
- 64. Schulze-Bahr E, Neu A, Friederich P, Kaupp UB, Breithardt G, Pongs O, Isbrandt D. Pacemaker channel dysfunction in a patient with sinus node disease. J Clin Invest 111: 1537–1545, 2003. doi: 10.1172/JCI200316387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Alig J, Marger L, Mesirca P, Ehmke H, Mangoni ME, Isbrandt D. Control of heart rate by cAMP sensitivity of HCN channels. Proc Natl Acad Sci U S A 106: 12189–12194, 2009. doi: 10.1073/pnas.0810332106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Harzheim D, Pfeiffer KH, Fabritz L, Kremmer E, Buch T, Waisman A, Kirchhof P, Kaupp UB, Seifert R. Cardiac pacemaker function of HCN4 channels in mice is confined to embryonic development and requires cyclic AMP. EMBO J 27: 692–703, 2008. doi: 10.1038/emboj.2008.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. D'Avanzo N, Cheng WW, Xia X, Dong L, Savitsky P, Nichols CG, Doyle DA. Expression and purification of recombinant human inward rectifier K+ (KCNJ) channels in Saccharomyces cerevisiae. Protein Expr Purif 71: 115–121, 2010. doi: 10.1016/j.pep.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zylbergold P, Ramakrishnan N, Hebert T. The role of G proteins in assembly and function of Kir3 inwardly rectifying potassium channels. Channels (Austin) 4: 411–421, 2010. doi: 10.4161/chan.4.5.13327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Nishida M, Cadene M, Chait BT, MacKinnon R. Crystal structure of a Kir3.1-prokaryotic Kir channel chimera. EMBO J 26: 4005–4015, 2007. doi: 10.1038/sj.emboj.7601828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Claydon TW, Makary SY, Dibb KM, Boyett MR. K+ activation of kir3.1/kir3.4 and kv1.4 K+ channels is regulated by extracellular charges. Biophys J 87: 2407–2418, 2004. doi: 10.1529/biophysj.103.039073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kuß J, Stallmeyer B, Goldstein M, Rinné S, Pees C, Zumhagen S, Seebohm G, Decher N, Pott L, Kienitz MC, Schulze-Bahr E. Familial sinus node disease caused by a gain of GIRK (G-protein activated inwardly rectifying K(+) channel) channel function. Circ Genom Precis Med 12: e002238, 2019. doi: 10.1161/CIRCGEN.118.002238. [DOI] [PubMed] [Google Scholar]
- 72. Mesirca P, Marger L, Toyoda F, Rizzetto R, Audoubert M, Dubel S, Torrente AG, Difrancesco ML, Muller JC, Leoni AL, Couette B, Nargeot J, Clapham DE, Wickman K, Mangoni ME. The G-protein-gated K+ channel, IKACh, is required for regulation of pacemaker activity and recovery of resting heart rate after sympathetic stimulation. J Gen Physiol 142: 113–126, 2013. doi: 10.1085/jgp.201310996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kokunai Y, Nakata T, Furuta M, Sakata S, Kimura H, Aiba T, Yoshinaga M, Osaki Y, Nakamori M, Itoh H, Sato T, Kubota T, Kadota K, Shindo K, Mochizuki H, Shimizu W, Horie M, Okamura Y, Ohno K, Takahashi MP. A Kir3.4 mutation causes Andersen-Tawil syndrome by an inhibitory effect on Kir2.1. Neurology 82: 1058–1064, 2014. doi: 10.1212/WNL.0000000000000239. [DOI] [PubMed] [Google Scholar]
- 74. Calloe K, Ravn LS, Schmitt N, Sui JL, Duno M, Haunso S, Grunnet M, Svendsen JH, Olesen SP. Characterizations of a loss-of-function mutation in the Kir3.4 channel subunit. Biochem Biophys Res Commun 364: 889–895, 2007. doi: 10.1016/j.bbrc.2007.10.106. [DOI] [PubMed] [Google Scholar]
- 75. Kovoor P, Wickman K, Maguire CT, Pu W, Gehrmann J, Berul CI, Clapham DE. Evaluation of the role of I(KACh) in atrial fibrillation using a mouse knockout model. J Am Coll Cardiol 37: 2136–2143, 2001. doi: 10.1016/S0735-1097(01)01304-3. [DOI] [PubMed] [Google Scholar]
- 76. Fukuda T, Hiraide T, Yamoto K, Nakashima M, Kawai T, Yanagi K, Ogata T, Saitsu H. Exome reports A de novo GNB2 variant associated with global developmental delay, intellectual disability, and dysmorphic features. Eur J Med Genet 63: 103804, 2020. doi: 10.1016/j.ejmg.2019.103804. [DOI] [PubMed] [Google Scholar]
- 77. Gehrmann J, Meister M, Maguire CT, Martins DC, Hammer PE, Neer EJ, Berul CI, Mende U. Impaired parasympathetic heart rate control in mice with a reduction of functional G protein βγ-subunits. Am J Physiol Heart Circ Physiol 282: H445–H456, 2002. doi: 10.1152/ajpheart.00565.2001. [DOI] [PubMed] [Google Scholar]
- 78. Stallmeyer B, Kuß J, Kotthoff S, Zumhagen S, Vowinkel K, Rinné S, Matschke LA, Friedrich C, Schulze-Bahr E, Rust S, Seebohm G, Decher N, Schulze-Bahr E. A mutation in the G-protein gene GNB2 causes familial sinus node and atrioventricular conduction dysfunction. Circ Res 120: e33–e44, 2017. doi: 10.1161/CIRCRESAHA.116.310112. [DOI] [PubMed] [Google Scholar]
- 79. Lodder EM, De Nittis P, Koopman CD, Wiszniewski W, Moura de Souza CF, Lahrouchi N, Guex N, Napolioni V, Tessadori F, Beekman L, Nannenberg EA, Boualla L, Blom NA, de Graaff W, Kamermans M, Cocciadiferro D, Malerba N, Mandriani B, Akdemir ZH, Fish RJ, Eldomery MK, Ratbi I, Wilde AA, de Boer T, Simonds WF, Neerman-Arbez M, Sutton VR, Kok F, Lupski JR, Reymond A, Bezzina CR, Bakkers J, Merla G. GNB5 mutations cause an autosomal-recessive multisystem syndrome with sinus bradycardia and cognitive disability. Am J Hum Genet 99: 704–710, 2016. [Erratum in Am J Hum Genet 99: 786, 2016]. doi: 10.1016/j.ajhg.2016.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Veerman CC, Mengarelli I, Koopman CD, Wilders R, van Amersfoorth SC, Bakker D, Wolswinkel R, Hababa M, de Boer TP, Guan K, Milnes J, Lodder EM, Bakkers J, Verkerk AO, Bezzina CR. Genetic variation in GNB5 causes bradycardia by augmenting the cholinergic response via increased acetylcholine-activated potassium current (I K,ACh). Dis Model Mech 12: dmm037994, 2019. doi: 10.1242/dmm.037994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Bidaud I, Chong ACY, Carcouet A, Waard SD, Charpentier F, Ronjat M, Waard MD, Isbrandt D, Wickman K, Vincent A, Mangoni ME, Mesirca P. Inhibition of G protein-gated K+ channels by tertiapin-Q rescues sinus node dysfunction and atrioventricular conduction in mouse models of primary bradycardia. Sci Rep 10: 9835, 2020. doi: 10.1038/s41598-020-66673-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Matthes J, Yildirim L, Wietzorrek G, Reimer D, Striessnig J, Herzig S. Disturbed atrio-ventricular conduction and normal contractile function in isolated hearts from Cav1.3-knockout mice. Naunyn Schmiedebergs Arch Pharmacol 369: 554–562, 2004. doi: 10.1007/s00210-004-0940-7. [DOI] [PubMed] [Google Scholar]
- 83. Striessnig J. Pharmacology, structure and function of cardiac L-type Ca(2+) channels. Cell Physiol Biochem 9: 242–269, 1999. doi: 10.1159/000016320. [DOI] [PubMed] [Google Scholar]
- 84. Perez-Reyes E. Molecular physiology of low-voltage-activated t-type calcium channels. Physiol Rev 83: 117–161, 2003. doi: 10.1152/physrev.00018.2002. [DOI] [PubMed] [Google Scholar]
- 85. Baudot M, Torre E, Bidaud I, Louradour J, Torrente AG, Fossier L, Talssi L, Nargeot J, Barrere-Lemaire S, Mesirca P, Mangoni ME. Concomitant genetic ablation of L-type Cav1.3 (alpha1D) and T-type Cav3.1 (alpha1G) Ca(2+) channels disrupts heart automaticity. Sci Rep 10: 18906, 2020. doi: 10.1038/s41598-020-76049-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Huser J, Blatter LA, Lipsius SL. Intracellular Ca2+ release contributes to automaticity in cat atrial pacemaker cells. J Physiol 524: 415–422, 2000. doi: 10.1111/j.1469-7793.2000.00415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Baig SM, Koschak A, Lieb A, Gebhart M, Dafinger C, Nürnberg G, Ali A, Ahmad I, Sinnegger-Brauns MJ, Brandt N, Engel J, Mangoni ME, Farooq M, Khan HU, Nürnberg P, Striessnig J, Bolz HJ. Loss of Ca(v)1.3 (CACNA1D) function in a human channelopathy with bradycardia and congenital deafness. Nat Neurosci 14: 77–84, 2011. doi: 10.1038/nn.2694. [DOI] [PubMed] [Google Scholar]
- 88. Liaqat K, Schrauwen I, Raza SI, Lee K, Hussain S, Chakchouk I, Nasir A, Acharya A, Abbe I, Umair M, Ansar M, Ullah I, Shah K, Bamshad MJ, Nickerson DA, Ahmad W, Leal SM, University of Washington Center for Mendelian Genomics. Identification of CACNA1D variants associated with sinoatrial node dysfunction and deafness in additional Pakistani families reveals a clinical significance. J Hum Genet 64: 153–160, 2019. doi: 10.1038/s10038-018-0542-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Platzer J, Engel J, Schrott-Fischer A, Stephan K, Bova S, Chen H, Zheng H, Striessnig J. Congenital deafness and sinoatrial node dysfunction in mice lacking class D L-type Ca2+ channels. Cell 102: 89–97, 2000. doi: 10.1016/S0092-8674(00)00013-1. [DOI] [PubMed] [Google Scholar]
- 90. Mesirca P, Fedorov VV, Hund TJ, Torrente AG, Bidaud I, Mohler PJ, Mangoni ME. Pharmacologic approach to sinoatrial node dysfunction. Annu Rev Pharmacol Toxicol 61: 757–778, 2021. doi: 10.1146/annurev-pharmtox-031120-115815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Gao Z, Rasmussen TP, Li Y, Kutschke W, Koval OM, Wu Y, Wu Y, Hall DD, Joiner M-lA, Wu X-Q, Swaminathan PD, Purohit A, Zimmerman K, Weiss RM, Philipson KD, Song L-S, Hund TJ, Anderson ME. Genetic inhibition of Na+-Ca2+ exchanger current disables fight or flight sinoatrial node activity without affecting resting heart rate. Circ Res 112: 309–317, 2013. doi: 10.1161/CIRCRESAHA.111.300193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Bogdanov KY, Vinogradova TM, Lakatta EG. Sinoatrial nodal cell ryanodine receptor and Na(+)-Ca(2+) exchanger: molecular partners in pacemaker regulation. Circ Res 88: 1254–1258, 2001. doi: 10.1161/hh1201.092095. [DOI] [PubMed] [Google Scholar]
- 93. Groenke S, Larson ED, Alber S, Zhang R, Lamp ST, Ren X, Nakano H, Jordan MC, Karagueuzian HS, Roos KP, Nakano A, Proenza C, Philipson KD, Goldhaber JI. Complete atrial-specific knockout of sodium-calcium exchange eliminates sinoatrial node pacemaker activity. PLoS One 8: e81633, 2013. doi: 10.1371/journal.pone.0081633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Yue X, Hazan A, Lotteau S, Zhang R, Torrente AG, Philipson KD, Ottolia M, Goldhaber JI. Na/Ca exchange in the atrium: Role in sinoatrial node pacemaking and excitation-contraction coupling. Cell Calcium 87: 102167, 2020. doi: 10.1016/j.ceca.2020.102167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Liu N, Rizzi N, Boveri L, Priori SG. Ryanodine receptor and calsequestrin in arrhythmogenesis: what we have learnt from genetic diseases and transgenic mice. J Mol Cell Cardiol 46: 149–159, 2009. doi: 10.1016/j.yjmcc.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 96. Dharmawan T, Nakajima T, Ohno S, Iizuka T, Tamura S, Kaneko Y, Horie M, Kurabayashi M. Identification of a novel exon3 deletion of RYR2 in a family with catecholaminergic polymorphic ventricular tachycardia. Ann Noninvasive Electrocardiol 24: e12623, 2019. doi: 10.1111/anec.12623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. 1w?>Dulhunty AF, Casarotto MG, Beard NA. The ryanodine receptor: a pivotal Ca2+ regulatory protein and potential therapeutic drug target. Curr Drug Targets 12: 709–723, 2011. doi: 10.2174/138945011795378595. [DOI] [PubMed] [Google Scholar]
- 98. Rubenstein DS, Lipsius SL. Mechanisms of automaticity in subsidiary pacemakers from cat right atrium. Circ Res 64: 648–657, 1989. doi: 10.1161/01.res.64.4.648. [DOI] [PubMed] [Google Scholar]
- 99. Musa H, Lei M, Honjo H, Jones SA, Dobrzynski H, Lancaster MK, Takagishi Y, Henderson Z, Kodama I, Boyett MR. Heterogeneous expression of Ca(2+) handling proteins in rabbit sinoatrial node. J Histochem Cytochem 50: 311–324, 2002. doi: 10.1177/002215540205000303. [DOI] [PubMed] [Google Scholar]
- 100. Ather S, Respress JL, Li N, Wehrens XH. Alterations in ryanodine receptors and related proteins in heart failure. Biochim Biophys Acta 1832: 2425–2431, 2013. doi: 10.1016/j.bbadis.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Dobrev D, Wehrens XHT. Calcium-mediated cellular triggered activity in atrial fibrillation. J Physiol 595: 4001–4008, 2017. doi: 10.1113/JP273048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Roux-Buisson N, Gandjbakhch E, Donal E, Probst V, Deharo JC, Chevalier P, Klug D, Mansencal N, Delacretaz E, Cosnay P, Scanu P, Extramiana F, Keller D, Hidden-Lucet F, Trapani J, Fouret P, Frank R, Fressart V, Faure J, Lunardi J, Charron P. Prevalence and significance of rare RYR2 variants in arrhythmogenic right ventricular cardiomyopathy/dysplasia: results of a systematic screening. Heart Rhythm 11: 1999–2009, 2014. doi: 10.1016/j.hrthm.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 103. Perez-Riera AR, Barbosa-Barros R, de Rezende Barbosa MPC, Daminello-Raimundo R, de Lucca AA Jr, de Abreu LC. Catecholaminergic polymorphic ventricular tachycardia, an update. Ann Noninvasive Electrocardiol 23: e12512, 2018. doi: 10.1111/anec.12512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Sumitomo N, Sakurada H, Taniguchi K, Matsumura M, Abe O, Miyashita M, Kanamaru H, Karasawa K, Ayusawa M, Fukamizu S, Nagaoka I, Horie M, Harada K, Hiraoka M. Association of atrial arrhythmia and sinus node dysfunction in patients with catecholaminergic polymorphic ventricular tachycardia. Circ J 71: 1606–1609, 2007. doi: 10.1253/circj.71.1606. [DOI] [PubMed] [Google Scholar]
- 105. Miyata K, Ohno S, Itoh H, Horie M. Bradycardia is a specific phenotype of catecholaminergic polymorphic ventricular tachycardia induced by RYR2 mutations. Intern Med 57: 1813–1817, 2018. doi: 10.2169/internalmedicine.9843-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Neco P, Torrente AG, Mesirca P, Zorio E, Liu N, Priori SG, Napolitano C, Richard S, Benitah JP, Mangoni ME, Gomez AM. Paradoxical effect of increased diastolic Ca(2+) release and decreased sinoatrial node activity in a mouse model of catecholaminergic polymorphic ventricular tachycardia. Circulation 126: 392–401, 2012. doi: 10.1161/CIRCULATIONAHA.111.075382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Zhang Y, Wu J, Jeevaratnam K, King JH, Guzadhur L, Ren X, Grace AA, Lei M, Huang CL, Fraser JA. Conduction slowing contributes to spontaneous ventricular arrhythmias in intrinsically active murine RyR2-P2328S hearts. J Cardiovasc Electrophysiol 24: 210–218, 2013. doi: 10.1111/jce.12015. [DOI] [PubMed] [Google Scholar]
- 108. Leenhardt A, Denjoy I, Guicheney P. Catecholaminergic polymorphic ventricular tachycardia. Circ Arrhythm Electrophysiol 5: 1044–1052, 2012. doi: 10.1161/CIRCEP.111.962027. [DOI] [PubMed] [Google Scholar]
- 109. Lahat H, Pras E, Olender T, Avidan N, Ben-Asher E, Man O, Levy-Nissenbaum E, Khoury A, Lorber A, Goldman B, Lancet D, Eldar M. A missense mutation in a highly conserved region of CASQ2 is associated with autosomal recessive catecholamine-induced polymorphic ventricular tachycardia in Bedouin families from Israel. Am J Hum Genet 69: 1378–1384, 2001. doi: 10.1086/324565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Glukhov AV, Kalyanasundaram A, Lou Q, Hage LT, Hansen BJ, Belevych AE, Mohler PJ, Knollmann BC, Periasamy M, Györke S, Fedorov VV. Calsequestrin 2 deletion causes sinoatrial node dysfunction and atrial arrhythmias associated with altered sarcoplasmic reticulum calcium cycling and degenerative fibrosis within the mouse atrial pacemaker complex1. Eur Heart J 36: 686–697, 2015. doi: 10.1093/eurheartj/eht452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Ayalon G, Davis JQ, Scotland PB, Bennett V. An ankyrin-based mechanism for functional organization of dystrophin and dystroglycan. Cell 135: 1189–1200, 2008. doi: 10.1016/j.cell.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 112. Roberts JD, Murphy NP, Hamilton RM, Lubbers ER, James CA, Kline CF, , et al. Ankyrin-B dysfunction predisposes to arrhythmogenic cardiomyopathy and is amenable to therapy. J Clin Invest 129: 3171–3184, 2019. doi: 10.1172/JCI125538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Hashemi SM, Hund TJ, Mohler PJ. Cardiac ankyrins in health and disease. J Mol Cell Cardiol 47: 203–209, 2009. doi: 10.1016/j.yjmcc.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Mohler PJ, Schott JJ, Gramolini AO, Dilly KW, Guatimosim S, duBell WH, Song LS, Haurogné K, Kyndt F, Ali ME, Rogers TB, Lederer WJ, Escande D, Le Marec H, Bennett V. Ankyrin-B mutation causes type 4 long-QT cardiac arrhythmia and sudden cardiac death. Nature 421: 634–639, 2003. doi: 10.1038/nature01335. [DOI] [PubMed] [Google Scholar]
- 115. Schott JJ, Charpentier F, Peltier S, Foley P, Drouin E, Bouhour JB, Donnelly P, Vergnaud G, Bachner L, Moisan JP. Mapping of a gene for long QT syndrome to chromosome 4q25-27. Am J Hum Genet 57: 1114–1122, 1995. [PMC free article] [PubMed] [Google Scholar]
- 116. Le Scouarnec S, Bhasin N, Vieyres C, Hund TJ, Cunha SR, Koval O, Marionneau C, Chen B, Wu Y, Demolombe S, Song LS, Le Marec H, Probst V, Schott JJ, Anderson ME, Mohler PJ. Dysfunction in ankyrin-B-dependent ion channel and transporter targeting causes human sinus node disease. Proc Natl Acad Sci U S A 105: 15617–15622, 2008. doi: 10.1073/pnas.0805500105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Curran J, Mohler PJ. Coordinating electrical activity of the heart: ankyrin polypeptides in human cardiac disease. Expert Opin Ther Targets 15: 789–801, 2011. doi: 10.1517/14728222.2011.575363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Ren L, Gopireddy RR, Perkins G, Zhang H, Timofeyev V, Lyu Y, Diloretto DA, Trinh P, Sirish P, Overton JL, Xu W, Grainger N, Xiang YK, Dedkova EN, Zhang XD, Yamoah EN, Navedo MF, Thai PN, Chiamvimonvat N. Disruption of mitochondria-sarcoplasmic reticulum microdomain connectomics contributes to sinus node dysfunction in heart failure. Proc Natl Acad Sci U S A 119: e2206708119, 2022. doi: 10.1073/pnas.2206708119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Lakatta EG, Maltsev VA, Vinogradova TM. A coupled SYSTEM of intracellular Ca2+ clocks and surface membrane voltage clocks controls the timekeeping mechanism of the heart's pacemaker. Circ Res 106: 659–673, 2010. doi: 10.1161/CIRCRESAHA.109.206078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Vinogradova TM, Brochet DX, Sirenko S, Li Y, Spurgeon H, Lakatta EG. Sarcoplasmic reticulum Ca2+ pumping kinetics regulates timing of local Ca2+ releases and spontaneous beating rate of rabbit sinoatrial node pacemaker cells. Circ Res 107: 767–775, 2010. doi: 10.1161/CIRCRESAHA.110.220517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Logantha SJ, Stokke MK, Atkinson AJ, Kharche SR, Parveen S, Saeed Y, Sjaastad I, Sejersted OM, Dobrzynski H. Ca(2+)-clock-dependent pacemaking in the sinus node is impaired in mice with a cardiac specific reduction in SERCA2 abundance. Front Physiol 7: 197, 2016. doi: 10.3389/fphys.2016.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Schmidt U, Hajjar RJ, Helm PA, Kim CS, Doye AA, Gwathmey JK. Contribution of abnormal sarcoplasmic reticulum ATPase activity to systolic and diastolic dysfunction in human heart failure. J Mol Cell Cardiol 30: 1929–1937, 1998. doi: 10.1006/jmcc.1998.0748. [DOI] [PubMed] [Google Scholar]
- 123. Wilde AAM, Amin AS. Clinical spectrum of SCN5A mutations: long QT syndrome, brugada syndrome, and cardiomyopathy. JACC Clin Electrophysiol 4: 569–579, 2018. doi: 10.1016/j.jacep.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 124. Mizusawa Y, Horie M, Wilde AA. Genetic and clinical advances in congenital long QT syndrome. Circ J 78: 2827–2833, 2014. doi: 10.1253/circj.CJ-14-0905. doi:. [DOI] [PubMed] [Google Scholar]
- 125. Benson DW, Wang DW, Dyment M, Knilans TK, Fish FA, Strieper MJ, Rhodes TH, George AL Jr.. Congenital sick sinus syndrome caused by recessive mutations in the cardiac sodium channel gene (SCN5A). J Clin Invest 112: 1019–1028, 2003. doi: 10.1172/JCI200318062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. VAN DEN Berg MP, Wilde AA, Viersma JW, Brouwer J, Haaksma J, VAN DER Hout AH, Stolte-Dijkstra I, Bezzina CR, VAN Langen IM, Beaufort-Krol GC, Cornel JH, Crijns HJ. Possible bradycardic mode of death and successful pacemaker treatment in a large family with features of long QT syndrome type 3 and Brugada syndrome. J Cardiovasc Electrophysiol 12: 630–636, 2001. doi: 10.1046/j.1540-8167.2001.00630.x. [DOI] [PubMed] [Google Scholar]
- 127. Lei M, Goddard C, Liu J, Léoni AL, Royer A, Fung SS, Xiao G, Ma A, Zhang H, Charpentier F, Vandenberg JI, Colledge WH, Grace AA, Huang CL. Sinus node dysfunction following targeted disruption of the murine cardiac sodium channel gene Scn5a. J Physiol 567: 387–400, 2005. doi: 10.1113/jphysiol.2005.083188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Epp TA, Dixon IM, Wang HY, Sole MJ, Liew CC. Structural organization of the human cardiac alpha-myosin heavy chain gene (MYH6). Genomics 18: 505–509, 1993. doi: 10.1016/s0888-7543(11)80006-6. [DOI] [PubMed] [Google Scholar]
- 129. Jones WK, Grupp IL, Doetschman T, Grupp G, Osinska H, Hewett TE, Boivin G, Gulick J, Ng WA, Robbins J. Ablation of the murine alpha myosin heavy chain gene leads to dosage effects and functional deficits in the heart. J Clin Invest 98: 1906–1917, 1996.doi: 10.1172/JCI118992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Rutland C, Warner L, Thorpe A, Alibhai A, Robinson T, Shaw B, Layfield R, Brook JD, Loughna S. Knockdown of alpha myosin heavy chain disrupts the cytoskeleton and leads to multiple defects during chick cardiogenesis. J Anat 214: 905–915, 2009. doi: 10.1111/j.1469-7580.2009.01079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Berdougo E, Coleman H, Lee DH, Stainier DY, Yelon D. Mutation of weak atrium/atrial myosin heavy chain disrupts atrial function and influences ventricular morphogenesis in zebrafish. Development 130: 6121–6129, 2003. doi: 10.1242/dev.00838. [DOI] [PubMed] [Google Scholar]
- 132. Carniel E, Taylor MR, Sinagra G, Di Lenarda A, Ku L, Fain PR, Boucek MM, Cavanaugh J, Miocic S, Slavov D, Graw SL, Feiger J, Zhu XZ, Dao D, Ferguson DA, Bristow MR, Mestroni L. Alpha-myosin heavy chain: a sarcomeric gene associated with dilated and hypertrophic phenotypes of cardiomyopathy. Circulation 112: 54–59, 2005. doi: 10.1161/CIRCULATIONAHA.104.507699. [DOI] [PubMed] [Google Scholar]
- 133. Granados-Riveron JT, Ghosh TK, Pope M, Bu'Lock F, Thornborough C, Eason J, Kirk EP, Fatkin D, Feneley MP, Harvey RP, Armour JA, David Brook J. Alpha-cardiac myosin heavy chain (MYH6) mutations affecting myofibril formation are associated with congenital heart defects. Hum Mol Genet 19: 4007–4016, 2010. doi: 10.1093/hmg/ddq315. [DOI] [PubMed] [Google Scholar]
- 134. Holm H, Gudbjartsson DF, Sulem P, Masson G, Helgadottir HT, Zanon C, Magnusson OT, Helgason A, Saemundsdottir J, Gylfason A, Stefansdottir H, Gretarsdottir S, Matthiasson SE, Thorgeirsson GM, Jonasdottir A, Sigurdsson A, Stefansson H, Werge T, Rafnar T, Kiemeney LA, Parvez B, Muhammad R, Roden DM, Darbar D, Thorleifsson G, Walters GB, Kong A, Thorsteinsdottir U, Arnar DO, Stefansson K. A rare variant in MYH6 is associated with high risk of sick sinus syndrome. Nat Genet 43: 316–320, 2011. doi: 10.1038/ng.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Ishikawa T, Jou CJ, Nogami A, Kowase S, Arrington CB, Barnett SM, Harrell DT, Arimura T, Tsuji Y, Kimura A, Makita N. Novel mutation in the alpha-myosin heavy chain gene is associated with sick sinus syndrome. Circ Arrhythm Electrophysiol 8: 400–408, 2015. doi: 10.1161/CIRCEP.114.002534. [DOI] [PubMed] [Google Scholar]
- 136. Lam L, Ingles J, Turner C, Kilborn M, Bagnall RD, Semsarian C. Exome sequencing identifies a novel mutation in the MYH6 gene in a family with early-onset sinus node dysfunction, ventricular arrhythmias, and cardiac arrest. HeartRhythm Case Rep 1: 141–145, 2015. doi: 10.1016/j.hrcr.2015.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Yoneda ZT, Anderson KC, Quintana JA, O'Neill MJ, Sims RA, Glazer AM, Shaffer CM, Crawford DM, Stricker T, Ye F, Wells Q, Stevenson LW, Michaud GF, Darbar D, Lubitz SA, Ellinor PT, Roden DM, Shoemaker MB. Early-onset atrial fibrillation and the prevalence of rare variants in cardiomyopathy and arrhythmia genes. JAMA Cardiol 6: 1371–1379, 2021. doi: 10.1001/jamacardio.2021.3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Callis TE, Pandya K, Seok HY, Tang RH, Tatsuguchi M, Huang ZP, Chen JF, Deng Z, Gunn B, Shumate J, Willis MS, Selzman CH, Wang DZ. MicroRNA-208a is a regulator of cardiac hypertrophy and conduction in mice. J Clin Invest 119: 2772–2786, 2009. doi: 10.1172/JCI36154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Oliveira-Carvalho V, Carvalho VO, Bocchi EA. The emerging role of miR-208a in the heart. DNA Cell Biol 32: 8–12, 2013. doi: 10.1089/dna.2012.1787. [DOI] [PubMed] [Google Scholar]
- 140. Liang X, Evans SM, Sun Y. Development of the cardiac pacemaker. Cell Mol Life Sci 74: 1247–1259, 2017. doi: 10.1007/s00018-016-2400-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Wiese C, Grieskamp T, Airik R, Mommersteeg MT, Gardiwal A, de Gier-de Vries C, Schuster-Gossler K, Moorman AF, Kispert A, Christoffels VM. Formation of the sinus node head and differentiation of sinus node myocardium are independently regulated by Tbx18 and Tbx3. Circ Res 104: 388–397, 2009. doi: 10.1161/CIRCRESAHA.108.187062. [DOI] [PubMed] [Google Scholar]
- 142. Liang X, Zhang Q, Cattaneo P, Zhuang S, Gong X, Spann NJ, Jiang C, Cao X, Zhao X, Zhang X, Bu L, Wang G, Chen HS, Zhuang T, Yan J, Geng P, Luo L, Banerjee I, Chen Y, Glass CK, Zambon AC, Chen J, Sun Y, Evans SM. Transcription factor ISL1 is essential for pacemaker development and function. J Clin Invest 125: 3256–3268, 2015. doi: 10.1172/JCI68257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Mommersteeg MT, Hoogaars WM, Prall OW, de Gier-de Vries C, Wiese C, Clout DE, Papaioannou VE, Brown NA, Harvey RP, Moorman AF, Christoffels VM. Molecular pathway for the localized formation of the sinoatrial node. Circ Res 100: 354–362, 2007. doi: 10.1161/01.RES.0000258019.74591.b3. [DOI] [PubMed] [Google Scholar]
- 144. Puskaric S, Schmitteckert S, Mori AD, Glaser A, Schneider KU, Bruneau BG, Blaschke RJ, Steinbeisser H, Rappold G. Shox2 mediates Tbx5 activity by regulating Bmp4 in the pacemaker region of the developing heart. Hum Mol Genet 19: 4625–4633, 2010. doi: 10.1093/hmg/ddq393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Galli D, Domínguez JN, Zaffran S, Munk A, Brown NA, Buckingham ME. Atrial myocardium derives from the posterior region of the second heart field, which acquires left-right identity as Pitx2c is expressed. Development 135: 1157–1167, 2008. doi: 10.1242/dev.014563. [DOI] [PubMed] [Google Scholar]
- 146. Arnolds DE, Liu F, Fahrenbach JP, Kim GH, Schillinger KJ, Smemo S, McNally EM, Nobrega MA, Patel VV, Moskowitz IP. TBX5 drives Scn5a expression to regulate cardiac conduction system function. J Clin Invest 122: 2509–2518, 2012. doi: 10.1172/JCI62617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Espinoza-Lewis RA, Yu L, He F, Liu H, Tang R, Shi J, Sun X, Martin JF, Wang D, Yang J, Chen Y. Shox2 is essential for the differentiation of cardiac pacemaker cells by repressing Nkx2-5. Dev Biol 327: 376–385, 2009. doi: 10.1016/j.ydbio.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Blaschke RJ, Hahurij ND, Kuijper S, Just S, Wisse LJ, Deissler K, Maxelon T, Anastassiadis K, Spitzer J, Hardt SE, Scholer H, Feitsma H, Rottbauer W, Blum M, Meijlink F, Rappold G, Gittenberger-de Groot AC. Targeted mutation reveals essential functions of the homeodomain transcription factor Shox2 in sinoatrial and pacemaking development. Circulation 115: 1830–1838, 2007. doi: 10.1161/CIRCULATIONAHA.106.637819. [DOI] [PubMed] [Google Scholar]
- 149. Ionta V, Liang W, Kim EH, Rafie R, Giacomello A, Marbán E, Cho HC. SHOX2 overexpression favors differentiation of embryonic stem cells into cardiac pacemaker cells, improving biological pacing ability. Stem Cell Reports 4: 129–142, 2015. doi: 10.1016/j.stemcr.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Clement-Jones M, Schiller S, Rao E, Blaschke RJ, Zuniga A, Zeller R, Robson SC, Binder G, Glass I, Strachan T, Lindsay S, Rappold GA. The short stature homeobox gene SHOX is involved in skeletal abnormalities in Turner syndrome. Hum Mol Genet 9: 695–702, 2000. doi: 10.1093/hmg/9.5.695. [DOI] [PubMed] [Google Scholar]
- 151. Clement-Jones M, Schiller S, Rao E, Blaschke RJ, Zuniga A, Zeller R, Robson SC, Gerhard Binder G, Glass I, Strachan T, Lindsay SA, Rappold GS. The short stature homeobox gene SHOX is involved in skeletal abnormalities in Turner syndrome. Human Mol Genetics 9: 695–702, 2000. doi: 10.1093/hmg/9.5.695. [DOI] [PubMed] [Google Scholar]
- 152. Hoffmann S, Clauss S, Berger IM, Weiß B, Montalbano A, Röth R, Bucher M, Klier I, Wakili R, Seitz H, Schulze-Bahr E, Katus HA, Flachsbart F, Nebel A, Guenther SP, Bagaev E, Rottbauer W, Kääb S, Just S, Rappold GA. Coding and non-coding variants in the SHOX2 gene in patients with early-onset atrial fibrillation. Basic Res Cardiol 111: 36, 2016. doi: 10.1007/s00395-016-0557-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Hoffmann S, Paone C, Sumer SA, Diebold S, Weiss B, Roeth R, Clauss S, Klier I, Kaab S, Schulz A, Wild PS, Ghrib A, Zeller T, Schnabel RB, Just S, Rappold GA. Functional Characterization of rare variants in the SHOX2 gene identified in sinus node dysfunction and atrial fibrillation. Front Genet 10: 648, 2019. doi: 10.3389/fgene.2019.00648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Li N, Wang ZS, Wang XH, Xu YJ, Qiao Q, Li XM, Di RM, Guo XJ, Li RG, Zhang M, Qiu XB, Yang YQ. A SHOX2 loss-of-function mutation underlying familial atrial fibrillation. Int J Med Sci 15: 1564–1572, 2018. doi: 10.7150/ijms.27424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Dittmer TA, Misteli T. The lamin protein family. Genome Biol 12: 222, 2011. doi: 10.1186/gb-2011-12-5-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Butin-Israeli V, Adam SA, Goldman AE, Goldman RD. Nuclear lamin functions and disease. Trends Genet 28: 464–471, 2012. doi: 10.1016/j.tig.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Broers JL, Peeters EA, Kuijpers HJ, Endert J, Bouten CV, Oomens CW, Baaijens FP, Ramaekers FC. Decreased mechanical stiffness in LMNA-/- cells is caused by defective nucleo-cytoskeletal integrity: implications for the development of laminopathies. Hum Mol Genet 13: 2567–2580, 2004. doi: 10.1093/hmg/ddh295. [DOI] [PubMed] [Google Scholar]
- 158. Lammerding J, Schulze PC, Takahashi T, Kozlov S, Sullivan T, Kamm RD, Stewart CL, Lee RT. Lamin A/C deficiency causes defective nuclear mechanics and mechanotransduction. J Clin Invest 113: 370–378, 2004. doi: 10.1172/JCI200419670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Dechat T, Pfleghaar K, Sengupta K, Shimi T, Shumaker DK, Solimando L, Goldman RD. Nuclear lamins: major factors in the structural organization and function of the nucleus and chromatin. Genes Dev 22: 832–853, 2008. doi: 10.1101/gad.1652708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Shimi T, Pfleghaar K, Kojima S, Pack CG, Solovei I, Goldman AE, Adam SA, Shumaker DK, Kinjo M, Cremer T, Goldman RD. The A- and B-type nuclear lamin networks: microdomains involved in chromatin organization and transcription. Genes Dev 22: 3409–3421, 2008. doi: 10.1101/gad.1735208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Cho S, Vashisth M, Abbas A, Majkut S, Vogel K, Xia Y, Ivanovska IL, Irianto J, Tewari M, Zhu K, Tichy ED, Mourkioti F, Tang HY, Greenberg RA, Prosser BL, Discher DE. Mechanosensing by the lamina protects against nuclear rupture, DNA damage, and cell-cycle arrest. Dev Cell 49: 920–935.e5, 2019. doi: 10.1016/j.devcel.2019.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Kind J, Pagie L, Ortabozkoyun H, Boyle S, de Vries SS, Janssen H, Amendola M, Nolen LD, Bickmore WA, van Steensel B. Single-cell dynamics of genome-nuclear lamina interactions. Cell 153: 178–192, 2013. doi: 10.1016/j.cell.2013.02.028. [DOI] [PubMed] [Google Scholar]
- 163. Harr JC, Luperchio TR, Wong X, Cohen E, Wheelan SJ, Reddy KL. Directed targeting of chromatin to the nuclear lamina is mediated by chromatin state and A-type lamins. J Cell Biol 208: 33–52, 2015. doi: 10.1083/jcb.201405110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Guerreiro I, Kind J. Spatial chromatin organization and gene regulation at the nuclear lamina. Curr Opin Genet Dev 55: 19–25, 2019. doi: 10.1016/j.gde.2019.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Auguste G, Rouhi L, Matkovich SJ, Coarfa C, Robertson MJ, Czernuszewicz G, Gurha P, Marian AJ. BET bromodomain inhibition attenuates cardiac phenotype in myocyte-specific lamin A/C-deficient mice. J Clin Invest 130: 4740–4758, 2020. doi: 10.1172/JCI135922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. van Steensel B, Belmont AS. Lamina-associated domains: links with chromosome architecture, heterochromatin, and gene repression. Cell 169: 780–791, 2017. doi: 10.1016/j.cell.2017.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Crasto S, My I, Di Pasquale E. The broad spectrum of LMNA cardiac diseases: from molecular mechanisms to clinical phenotype. Front Physiol 11: 761, 2020. doi: 10.3389/fphys.2020.00761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Brodt C, Siegfried JD, Hofmeyer M, Martel J, Rampersaud E, Li D, Morales A, Hershberger RE. Temporal relationship of conduction system disease and ventricular dysfunction in LMNA cardiomyopathy. J Card Fail 19: 233–239, 2013. doi: 10.1016/j.cardfail.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Hershberger RE, Siegfried JD. Update 2011: clinical and genetic issues in familial dilated cardiomyopathy. J Am Coll Cardiol 57: 1641–1649, 2011. doi: 10.1016/j.jacc.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Zaragoza MV, Fung L, Jensen E, Oh F, Cung K, McCarthy LA, Tran CK, Hoang V, Hakim SA, Grosberg A. Exome sequencing identifies a novel LMNA splice-site mutation and multigenic heterozygosity of potential modifiers in a family with sick sinus syndrome, dilated cardiomyopathy, and sudden cardiac death. PLoS One 11: e0155421, 2016. doi: 10.1371/journal.pone.0155421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Captur G, Arbustini E, Bonne G, Syrris P, Mills K, Wahbi K, Mohiddin SA, McKenna WJ, Pettit S, Ho CY, Muchir A, Gissen P, Elliott PM, Moon JC. Lamin and the heart. Heart 104: 468–479, 2018. doi: 10.1136/heartjnl-2017-312338. [DOI] [PubMed] [Google Scholar]
- 172. Nikolova V, Leimena C, McMahon AC, Tan JC, Chandar S, Jogia D, Kesteven SH, Michalicek J, Otway R, Verheyen F, Rainer S, Stewart CL, Martin D, Feneley MP, Fatkin D. Defects in nuclear structure and function promote dilated cardiomyopathy in lamin A/C–deficient mice. J Clin Invest 113: 357–369, 2004. doi: 10.1172/JCI19448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Coste Pradas J, Auguste G, Matkovich SJ, Lombardi R, Chen SN, Garnett T, Chamberlain K, Riyad JM, Weber T, Singh SK, Robertson MJ, Coarfa C, Marian AJ, Gurha P. Identification of genes and pathways regulated by lamin A in heart. J Am Heart Assoc 9: e015690, 2020. doi: 10.1161/JAHA.119.015690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Auguste G, Gurha P, Lombardi R, Coarfa C, Willerson JT, Marian AJ. Suppression of activated FOXO transcription factors in the heart prolongs survival in a mouse model of laminopathies. Circ Res 122: 678–692, 2018. doi: 10.1161/CIRCRESAHA.117.312052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Cheedipudi SM, Matkovich SJ, Coarfa C, Hu X, Robertson MJ, Sweet M, Taylor M, Mestroni L, Cleveland J, Willerson JT, Gurha P, Marian AJ. Genomic reorganization of lamin-associated domains in cardiac myocytes is associated with differential gene expression and DNA methylation in human dilated cardiomyopathy. Circ Res 124: 1198–1213, 2019. doi: 10.1161/CIRCRESAHA.118.314177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Unudurthi SD, Wolf RM, Hund TJ. Role of sinoatrial node architecture in maintaining a balanced source-sink relationship and synchronous cardiac pacemaking. Front Physiol 5: 446, 2014. doi: 10.3389/fphys.2014.00446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Boyett M, Inada S, Yoo S, Li J, Liu J, Tellez J, Greener I, Honjo H, Billeter R, Lei M, Zhang H, Efimov I, Dobrzynski H. Connexins in the sinoatrial and atrioventricular nodes. Adv Cardiol 42: 175–197, 2006. doi: 10.1159/000092569. [DOI] [PubMed] [Google Scholar]
- 178. Seki A, Ishikawa T, Daumy X, Mishima H, Barc J, Sasaki R, Nishii K, Saito K, Urano M, Ohno S, Otsuki S, Kimoto H, Baruteau A-E, Thollet A, Fouchard S, Bonnaud S, Parent P, Shibata Y, Perrin J-P, Le Marec H, Hagiwara N, Mercier S, Horie M, Probst V, Yoshiura K-I, Redon R, Schott J-J, Makita N. Progressive atrial conduction defects associated with bone malformation caused by a connexin-45 mutation. J Am Coll Cardiol 70: 358–370, 2017. doi: 10.1016/j.jacc.2017.05.039. [DOI] [PubMed] [Google Scholar]
- 179. Jones SA, Lancaster MK, Boyett MR. Ageing-related changes of connexins and conduction within the sinoatrial node. J Physiol 560: 429–437, 2004. doi: 10.1113/jphysiol.2004.072108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Csepe TA, Kalyanasundaram A, Hansen BJ, Zhao J, Fedorov VV. Fibrosis: a structural modulator of sinoatrial node physiology and dysfunction. Front Physiol 6: 37, 2015. doi: 10.3389/fphys.2015.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Csepe TA, Zhao J, Sul LV, Wang Y, Hansen BJ, Li N, Ignozzi AJ, Bratasz A, Powell KA, Kilic A, Mohler PJ, Janssen PML, Hummel JD, Simonetti OP, Fedorov VV. Novel application of 3D contrast-enhanced CMR to define fibrotic structure of the human sinoatrial node in vivo. Eur Heart J Cardiovasc Imaging 18: 862–869, 2017. doi: 10.1093/ehjci/jew304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Hao X, Zhang Y, Zhang X, Nirmalan M, Davies L, Konstantinou D, Yin F, Dobrzynski H, Wang X, Grace A, Zhang H, Boyett M, Huang CL, Lei M. TGF-beta1-mediated fibrosis and ion channel remodeling are key mechanisms in producing the sinus node dysfunction associated with SCN5A deficiency and aging. Circ Arrhythm Electrophysiol 4: 397–406, 2011. doi: 10.1161/CIRCEP.110.960807. [DOI] [PubMed] [Google Scholar]
- 183. Alings AM, Abbas RF, Bouman LN. Age-related changes in structure and relative collagen content of the human and feline sinoatrial node. A comparative study. Eur Heart J 16: 1655–1667, 1995. doi: 10.1093/oxfordjournals.eurheartj.a060792. [DOI] [PubMed] [Google Scholar]
- 184. Shiraishi I, Takamatsu T, Minamikawa T, Onouchi Z, Fujita S. Quantitative histological analysis of the human sinoatrial node during growth and aging. Circulation 85: 2176–2184, 1992. doi: 10.1161/01.CIR.85.6.2176. [DOI] [PubMed] [Google Scholar]
- 185. Fahrenbach JP, Mejia-Alvarez R, Banach K. The relevance of non-excitable cells for cardiac pacemaker function. J Physiol 585: 565–578, 2007. doi: 10.1113/jphysiol.2007.144121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Nakajima H, Nakajima HO, Salcher O, Dittie AS, Dembowsky K, Jing S, Field LJ. Atrial but not ventricular fibrosis in mice expressing a mutant transforming growth factor-beta(1) transgene in the heart. Circ Res 86: 571–579, 2000. doi: 10.1161/01.RES.86.5.571. [DOI] [PubMed] [Google Scholar]
- 187. Zheng M, Li RG, Song J, Zhao X, Tang L, Erhardt S, Chen W, Nguyen BH, Li X, Li M, Wang J, Evans SM, Christoffels VM, Li N, Wang J. Hippo-Yap signaling maintains sinoatrial node homeostasis. Circulation 146: 1694–1711, 2022. doi: 10.1161/CIRCULATIONAHA.121.058777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Sweeney MO, Hellkamp AS, Ellenbogen KA, Greenspon AJ, Freedman RA, Lee KL, Lamas GA. Adverse effect of ventricular pacing on heart failure and atrial fibrillation among patients with normal baseline QRS duration in a clinical trial of pacemaker therapy for sinus node dysfunction. Circulation 107: 2932–2937, 2003. doi: 10.1161/01.CIR.0000072769.17295.B1. [DOI] [PubMed] [Google Scholar]
- 189. Connolly SJ, Kerr CR, Gent M, Roberts RS, Yusuf S, Gillis AM, Sami MH, Talajic M, Tang AS, Klein GJ, Lau C, Newman DM. Effects of physiologic pacing versus ventricular pacing on the risk of stroke and death due to cardiovascular causes. Canadian Trial of Physiologic Pacing Investigators. N Engl J Med 342: 1385–1391, 2000. doi: 10.1056/NEJM200005113421902. [DOI] [PubMed] [Google Scholar]
- 190. Nielsen JC, Thomsen PE, Højberg S, Møller M, Vesterlund T, Dalsgaard D, Mortensen LS, Nielsen T, Asklund M, Friis EV, Christensen PD, Simonsen EH, Eriksen UH, Jensen GV, Svendsen JH, Toff WD, Healey JS, Andersen HR; DANPACE Investigators. A comparison of single-lead atrial pacing with dual-chamber pacing in sick sinus syndrome. Eur Heart J 32: 686–696, 2011. doi: 10.1093/eurheartj/ehr022. [DOI] [PubMed] [Google Scholar]
- 191. Greenspon AJ, Patel JD, Lau E, Ochoa JA, Frisch DR, Ho RT, Pavri BB, Kurtz SM. Trends in permanent pacemaker implantation in the United States from 1993 to 2009: increasing complexity of patients and procedures. J Am Coll Cardiol 60: 1540–1545, 2012. doi: 10.1016/j.jacc.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 192. Sweeney MO, Bank AJ, Nsah E, Koullick M, Zeng QC, Hettrick D, Sheldon T, Lamas GA; Search AV Extension and Managed Ventricular Pacing for Promoting Atrioventricular Conduction (SAVE PACe) Trial. Minimizing ventricular pacing to reduce atrial fibrillation in sinus-node disease. N Engl J Med 357: 1000–1008, 2007. doi: 10.1056/NEJMoa071880. [DOI] [PubMed] [Google Scholar]
- 193. Eberhardt F, Bode F, Bonnemeier H, Boguschewski F, Schlei M, Peters W, Wiegand UK. Long term complications in single and dual chamber pacing are influenced by surgical experience and patient morbidity. Heart 91: 500–506, 2005. doi: 10.1136/hrt.2003.025411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Udo EO, Zuithoff NP, van Hemel NM, de Cock CC, Hendriks T, Doevendans PA, Moons KG. Incidence and predictors of short- and long-term complications in pacemaker therapy: the FOLLOWPACE study. Heart Rhythm 9: 728–735, 2012. doi: 10.1016/j.hrthm.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 195. Reynolds D, Duray GZ, Omar R, Soejima K, Neuzil P, Zhang S, Narasimhan C, Steinwender C, Brugada J, Lloyd M, Roberts PR, Sagi V, Hummel J, Bongiorni MG, Knops RE, Ellis CR, Gornick CC, Bernabei MA, Laager V, Stromberg K, Williams ER, Hudnall JH, Ritter P; Micra Transcatheter Pacing Study Group. A leadless intracardiac transcatheter pacing system. N Engl J Med 374: 533–541, 2016. doi: 10.1056/NEJMoa1511643. [DOI] [PubMed] [Google Scholar]
- 196. Khan K, Kim JA, Gurgu A, Khawaja M, Cozma D, Chelu MG. Innovations in cardiac implantable electronic devices. Cardiovasc Drugs Ther 36: 763–775, 2022. doi: 10.1007/s10557-021-07163-5. [DOI] [PubMed] [Google Scholar]
- 197. Doukky R, Bargout R, Kelly RF, Calvin JE. Using transcutaneous cardiac pacing to best advantage: how to ensure successful capture and avoid complications. J Crit Illn 18: 219–225, 2003. [PMC free article] [PubMed] [Google Scholar]
- 198. Zoll PM, Zoll RH, Falk RH, Clinton JE, Eitel DR, Antman EM. External noninvasive temporary cardiac pacing: clinical trials. Circulation 71: 937–944, 1985. doi: 10.1161/01.cir.71.5.937. [DOI] [PubMed] [Google Scholar]
- 199. Sherbino J, Verbeek PR, MacDonald RD, Sawadsky BV, McDonald AC, Morrison LJ. Prehospital transcutaneous cardiac pacing for symptomatic bradycardia or bradyasystolic cardiac arrest: a systematic review. Resuscitation 70: 193–200, 2006. doi: 10.1016/j.resuscitation.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 200. Cummins RO, Graves JR, Larsen MP, Hallstrom AP, Hearne TR, Ciliberti J, Nicola RM, Horan S. Out-of-hospital transcutaneous pacing by emergency medical technicians in patients with asystolic cardiac arrest. N Engl J Med 328: 1377–1382, 1993. doi: 10.1056/NEJM199305133281903. [DOI] [PubMed] [Google Scholar]
- 201. Lumia FJ, Rios JC. Temporary transvenous pacemaker therapy: an analysis of complications. Chest 64: 604–608, 1973. doi: 10.1378/chest.64.5.604. [DOI] [PubMed] [Google Scholar]
- 202. Jafri SM, Kruse JA. Temporary transvenous cardiac pacing. Crit Care Clin 8: 713–725, 1992. doi: 10.1016/S0749-0704(18)30221-5. [DOI] [PubMed] [Google Scholar]
- 203. Metkus TS, Schulman SP, Marine JE, Eid SM. Complications and outcomes of temporary transvenous pacing: an analysis of >360,000 patients from the national inpatient sample. Chest 155: 749–757, 2019. doi: 10.1016/j.chest.2018.11.026. [DOI] [PubMed] [Google Scholar]
- 204. Ogilvie RI. Clinical pharmacokinetics of theophylline. Clin Pharmacokinet 3: 267–293, 1978. doi: 10.2165/00003088-197803040-00002. [DOI] [PubMed] [Google Scholar]
- 205. Alboni P, Menozzi C, Brignole M, Paparella N, Gaggioli G, Lolli G, Cappato R. Effects of permanent pacemaker and oral theophylline in sick sinus syndrome the THEOPACE study: a randomized controlled trial. Circulation 96: 260–266, 1997. doi: 10.1161/01.CIR.96.1.260. [DOI] [PubMed] [Google Scholar]
- 206. Saito D, Matsubara K, Yamanari H, Obayashi N, Uchida S, Maekawa K, Sato T, Mizuo K, Kobayashi H, Haraoka S. Effects of oral theophylline on sick sinus syndrome. J Am Coll Cardiol 21: 1199–1204, 1993. doi: 10.1016/0735-1097(93)90246-w. [DOI] [PubMed] [Google Scholar]
- 207. Abe K, Machida T, Sumitomo N, Yamamoto H, Ohkubo K, Watanabe I, Makiyama T, Fukae S, Kohno M, Harrell DT, Ishikawa T, Tsuji Y, Nogami A, Watabe T, Oginosawa Y, Abe H, Maemura K, Motomura H, Makita N. Sodium channelopathy underlying familial sick sinus syndrome with early onset and predominantly male characteristics. Circ Arrhythm Electrophysiol 7: 511–517, 2014. [Erratum in Circ Arrhythm Electrophysiol 7: 771, 2014]. doi: 10.1161/CIRCEP.113.001340. [DOI] [PubMed] [Google Scholar]
- 208. Magnussen C, Niiranen TJ, Ojeda FM, Gianfagna F, Blankenberg S, Njølstad I, Vartiainen E, Sans S, Pasterkamp G, Hughes M, Costanzo S, Donati MB, Jousilahti P, Linneberg A, Palosaari T, de Gaetano G, Bobak M, den Ruijter HM, Mathiesen E, Jørgensen T, Söderberg S, Kuulasmaa K, Zeller T, Iacoviello L, Salomaa V, Schnabel RB; BiomarCaRE Consortium. Sex differences and similarities in atrial fibrillation epidemiology, risk factors, and mortality in community cohorts. Circulation 136: 1588–1597, 2017. doi: 10.1161/CIRCULATIONAHA.117.028981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Stolfo D, Uijl A, Vedin O, Strömberg A, Faxén UL, Rosano GMC, Sinagra G, Dahlström U, Savarese G. Sex-based differences in heart failure across the ejection fraction spectrum. JACC Heart Fail 7: 505–515, 2019. doi: 10.1016/j.jchf.2019.03.011. [DOI] [PubMed] [Google Scholar]
- 210. Hoogaars WM, Engel A, Brons JF, Verkerk AO, de Lange FJ, Wong LY, Bakker ML, Clout DE, Wakker V, Barnett P, Ravesloot JH, Moorman AF, Verheijck EE, Christoffels VM. Tbx3 controls the sinoatrial node gene program and imposes pacemaker function on the atria. Genes Dev 21: 1098–1112, 2007. doi: 10.1101/gad.416007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Kapoor N, Liang W, Marban E, Cho HC. Direct conversion of quiescent cardiomyocytes to pacemaker cells by expression of Tbx18. Nat Biotechnol 31: 54–62, 2013. doi: 10.1038/nbt.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Raghunathan S, Islas JF, Mistretta B, Iyer D, Shi L, Gunaratne PH, Ko G, Schwartz RJ, McConnell BK. Conversion of human cardiac progenitor cells into cardiac pacemaker-like cells. J Mol Cell Cardiol 138: 12–22, 2020. doi: 10.1016/j.yjmcc.2019.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Wu JC, Garg P, Yoshida Y, Yamanaka S, Gepstein L, Hulot JS, Knollmann BC, Schwartz PJ. Towards precision medicine with human iPSCs for cardiac channelopathies. Circ Res 125: 653–658, 2019. doi: 10.1161/CIRCRESAHA.119.315209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Pozo MR, Meredith GW, Entcheva E. Human iPSC-cardiomyocytes as an experimental model to study epigenetic modifiers of electrophysiology. Cells 11: 200, 2022. doi: 10.3390/cells11020200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Protze SI, Liu J, Nussinovitch U, Ohana L, Backx PH, Gepstein L, Keller GM. Sinoatrial node cardiomyocytes derived from human pluripotent cells function as a biological pacemaker. Nat Biotechnol 35: 56–68, 2017. doi: 10.1038/nbt.3745. [DOI] [PubMed] [Google Scholar]
- 216. Liu F, Fang Y, Hou X, Yan Y, Xiao H, Zuo D, Wen J, Wang L, Zhou Z, Dang X, Zhou R, Liao B. Enrichment differentiation of human induced pluripotent stem cells into sinoatrial node-like cells by combined modulation of BMP, FGF, and RA signaling pathways. Stem Cell Res Ther 11: 284, 2020. doi: 10.1186/s13287-020-01794-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Shlapakova IN, Nearing BD, Lau DH, Boink GJ, Danilo P Jr, Kryukova Y, Robinson RB, Cohen IS, Rosen MR, Verrier RL. Biological pacemakers in canines exhibit positive chronotropic response to emotional arousal. Heart Rhythm 7: 1835–1840, 2010. doi: 10.1016/j.hrthm.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 218. Naumova N, Iop L. Bioengineering the cardiac conduction system: advances in cellular, gene, and tissue engineering for heart rhythm regeneration. Front Bioeng Biotechnol 9: 673477, 2021. doi: 10.3389/fbioe.2021.673477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Cingolani E, Goldhaber JI, Marban E. Next-generation pacemakers: from small devices to biological pacemakers. Nat Rev Cardiol 15: 139–150, 2018. doi: 10.1038/nrcardio.2017.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Bychkov R, Juhaszova M, Calvo-Rubio Barrera M, Donald Lorenzo AH, Coletta C, Shumaker C, Moorman K, Sirenko ST, Maltsev Alexander V, Sollott Steven J, Lakatta Edward G. The heart’s pacemaker mimics brain cytoarchitecture and function. JACC Clin Electrophysiol 8: 1191–1215, 2022. doi: 10.1016/j.jacep.2022.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Grainger N, Santana LF. The central brain of the heart. JACC Clin Electrophysiol 8: 1216–1218, 2022. doi: 10.1016/j.jacep.2022.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]