Abstract
Declines in processing speed performance occur in aging and are a critical marker of functional independence in older adults. Numerous studies suggest that Useful Field of View (UFOV) training may ameliorate cognitive decline in older adults. Despite its efficacy, little is known about the neural correlates of this task. The current study is the first to investigate the coherence of functional connectivity during UFOV task completion. A total of 336 participants completed the UFOV task while undergoing task-based functional magnetic resonance imaging (fMRI). Ten spherical regions of interest (ROIs), selected a priori, were created based on regions with the greatest peak BOLD activation patterns in the UFOV fMRI task and regions that have been shown to significantly relate to UFOV fMRI task performance. We used a weighted ROI-to-ROI connectivity analysis to model task-specific functional connectivity strength between these a priori selected ROIs. We found that our UFOV fMRI network was functionally connected during task performance and was significantly associated to UFOV fMRI task performance. Within-network connectivity of the UFOV fMRI network showed comparable or better predictive power in accounting for UFOV accuracy compared to 7 resting state networks, delineated by Yeo and colleagues. Finally, we demonstrate that the within-network connectivity of UFOV fMRI task accounted for scores on a measure of “near transfer”, the Double Decision task, better than the aforementioned resting state networks. Our data elucidate functional connectivity patterns of the UFOV fMRI task. This may assist in future targeted interventions that aim to improve synchronicity within the UFOV fMRI network.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11357-022-00632-1.
Keywords: Useful field of view, UFOV, Functional connectivity, Cognitive aging
Introduction
The exponential growth and prolonged lifespan of the older adult population have resulted in a vital need to address the detrimental effects of age-related cognitive decline. One cognitive domain that exhibits clear decline within the context of non-pathological aging is processing speed [1]. Processing speed is typically defined as the speed in which an individual can perceive a given stimulus (usually amongst distractors), interpret the information, and produce a response [2, 3]. A substantial body of literature has demonstrated that age-related declines in processing speed occur alongside declines across other cognitive domains and related functional abilities. Specifically, age-related declines in processing speed have been associated with poorer working memory, attention and problem solving, greater medical expenditures, poorer timed instrumental activities of daily living (IADL) scores, higher self-reported difficulties completing IADLs, increased driving accident rates, and greater motor vehicular crash risk [4–10].
Computerized training interventions have proven efficacious in slowing cognitive decline in older adults, particularly within the domain of processing speed. Interventions using the Useful Field of View (UFOV) task have long demonstrated robust, reliable, and durable cognitive training gains [11, 12]. Although several iterations of this task exist, the UFOV task requires participants to identify a central target presented for a brief period of time in addition to a peripheral target. Difficulty is moderated through the presentation time of targets and the addition of distractors in the periphery. Interventions employing UFOV-specific training have shown demonstrable improvement in driving outcomes [13, 14], self-reported cognition [15], and health-related quality of life [5, 16], as well as fewer difficulties in completing IADLs [17, 18].
Despite the fact that UFOV-based interventions have reliably shown deceleration of functional and cognitive declines in processing speed, little is known about the underlying neurocognitive mechanisms of task performance. Recent research suggests that differential patterns of blood-oxygen level dependent (BOLD) activation are associated with UFOV task completion and performance. Following a UFOV-based intervention, Ross and colleagues observed decreased BOLD activation patterns in several regions, including the supplementary motor area (SMA) and dorsolateral prefrontal cortex (DLPFC), suggesting greater neural efficiency in these regions [19]. A cross-sectional study performed by Kraft and colleagues found significant activation of the DLPFC, ventrolateral prefrontal cortex (VLPFC) and SMA, inferior temporal gyrus, precentral gyrus, and superior parietal lobules. Furthermore, these regions were significantly activated when accounting for task accuracy, signifying the importance of these regions in UFOV task performance [20].
Accumulating evidence also suggests that resting-state functional connectivity is associated with UFOV task performance. Functional connectivity refers to concurrent temporal correlations of BOLD signal activation across anatomically remote brain regions via distributed networks [21–24]. Decreased resting-state functional connectivity within-network and increased connectivity between-networks (known as decreased network segregation) have been associated with poorer cognitive performance in an older adult population [25–29]. Within the UFOV literature, resting-state functional connectivity in the cingulo-opercular network (CON) and frontoparietal control network (FPCN) was associated with better performance on an adapted version of the UFOV task, the Double Decision task [30]. Hardcastle and colleagues also found that strengthened resting-state FPCN connectivity was associated with better Double Decision performance following a multidomain cognitive training intervention, suggesting that regions within the FPCN may be important for UFOV task performance [31]. These findings correspond with the findings of Ross and colleagues, who also assessed resting-state functional connectivity following a UFOV intervention. Notably, they found significant increases in resting-state functional connectivity between the anterior insula and anterior cingulate cortex (ACC) and between the dorsolateral prefrontal cortex (DLPFC) and supplementary motor area (SMA) [19].
Although previous research has identified individual regions activated during task performance, as well as resting-state networks associated with UFOV performance, no studies have looked at the engagement of functional networks or functional network segregation during UFOV task performance. Therefore, we aimed to evaluate task-based functional connectivity of the UFOV task, using a modified UFOV task designed for in-scanner presentation, the UFOV fMRI task [32]. This task requires participants to identify targets both in the center of the screen and in the periphery. To assess the viability of a UFOV task-based functional connectivity network in predicting UFOV performance, 10 a priori ROIs were derived from BOLD activation patterns previously implicated in UFOV task performance [20]. Thus, we aimed to assess if regions that were functionally activated in the UFOV fMRI task were temporally correlated via functional connectivity and related to task performance. Furthermore, we compared within-network connectivity of the UFOV functional network to within-network connectivity of 7 resting-state functional connectivity networks (Yeo et al., 2011) in predicting UFOV task performance and a “near-transfer” task, Double Decision performance. We hypothesize that the 10 a priori ROIs delineated in Kraft et al. (2021) will be temporally connected during UFOV task completion and will be associated with UFOV task performance. We further hypothesize that within-network connectivity of these 10 ROIs will predict both UFOV in-scanner performance and Double Decision performance better than the 7 resting-state functional connectivity networks. Additionally, given findings of decreased segregation in older adults, we explored the impact of network segregation between higher-order resting state networks and UFOV performance [25–27].
Materials and methods
Participants
Data from 359 healthy older adults were collected at the University of Florida and University of Arizona as part of an ongoing phase III clinical trial, the Augmenting Cognitive Training in Older Adults (ACT) study (R01AG054077). Participants in this study ranged in age from 65 to 89, had no history of head injury with loss of consciousness greater than 20 min, had no previous diagnosis of neurological or major psychiatric disorders, and were able to undergo an MRI (for further details of inclusion/exclusion criteria, see [32]). Participants were excluded if they met criteria for possible mild cognitive impairment (MCI), which were derived from the Uniform Data Set (UDS), a widely validated tool created by the National Alzheimer’s Coordinating Center (NACC) to assess MCI and Alzheimer’s disease [33]. Possible MCI was defined as 1.5 standard deviations below age-, sex-, and education-adjusted scores in performance of at least one of the five following cognitive domains: general cognition, visuospatial functioning, memory, executive functioning/working memory, and language.
Measures
Useful field of view (UFOV) fMRI task
The UFOV task used in the current study was an adaptation of the third subtask of the original UFOV task and is specific to fMRI presentation [34]. Successful completion of this task requires participants to attend to a target presented in the middle of the display, as well as a target in the periphery amongst distractors. All participants completed the UFOV fMRI task in the scanner, with a total run time of 10 min, 32 s (E-Prime version 2.0; Psychology Software Tools Inc., Pittsburgh, PA, USA).
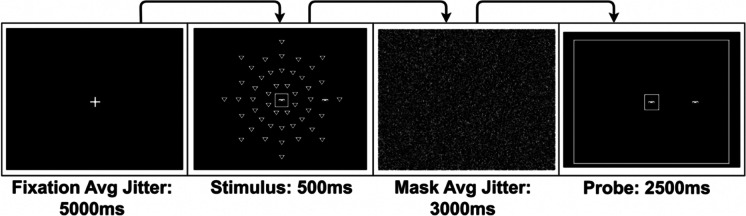
Each trial began with a fixation cross, presented for an average duration of 5000 ms. During the stimulus portion of the task, participants must attend to a target in the center of the screen (either a car or a truck), as well as locate a target (a car) presented in one of eight radial locations in the periphery, amongst distractors. The stimulus screen was presented for 500 ms; a mask screen was then presented for an average of 3000 ms. During the probe portion of the task, the participant performed a forced choice response in which they had to decide if the object in the center of the screen and the location of the car in the periphery were the same as on the previous stimulus screen. All participants received 56 trials of the UFOV fMRI task in-scanner, with 0 to 47 distractors during the stimulus screen (Fig. 1).
Fig. 1.
Useful field of view task [32]
This adapted UFOV task, known as the UFOV fMRI task, differed from the original UFOV task in a number of ways [20]. The stimulus screens and probe screens were presented for static durations of 500 ms and 2500 ms, respectively, to adequately capture hemodynamic responses. This is in contrast to the original UFOV test, in which scores are a function of the briefest display duration in which participants could respond correctly on at least 75% of trials. Additionally, during the probe portion of the task, participants were required to make a forced choice response, as opposed to assessing the radial location of the peripheral target.
POSIT Brain HQ Double Decision
The POSIT Brain HQ Double Decision task is a well-known adaptive cognitive training paradigm, based on the original UFOV task [19, 35, 36]. Like the UFOV fMRI task, the Double Decision task requires participants to identify which central target was presented on the screen (either a car or a truck) and the location of the peripheral target (a Route 66 sign) (Fig. 2). As this is an adaptive training paradigm, the targets were presented for variable lengths of time depending on participant accuracy, with longer presentation times following incorrect trials and shorter presentation times following correct trials. There were 25 presentations total and possible presentation times ranged from 32 to 2600 ms. The task was set at a moderate difficulty to start, with a starting presentation time of 501 ms and 7 distractors. Participants’ scores were calculated as the average presentation time of correct responses in log ms, as reaction time was skewed positively. Lower scores on the task equated to faster processing speed. All participants performed the Double Decision task at their first visit, within 60 days of their MRI visit.
Fig. 2.

Example of the POSIT Double Decision paradigm. Reproduced/adapted from POSIT Brain HQ, used with permission
MRI acquisition
Structural MRI data was collected using T1-weighted magnetization prepared rapid gradient echo (MPRAGE) via a Siemens 3 T Prisma scanner with a 64-channel head coil (University of Florida site, n = 231) or via a Siemens 3 T Skyra scanner with a 32-channel head coil (University of Arizona site, n = 105). T1-weighted structural scans were collected with the following parameters at both sites: repetition time (TR) = 1800 ms; echo time (TE) = 2.26 ms; flip angle = 8°; field of view = 256 mm × 256 mm × 176 mm; voxel size = 1mm3. Task-based fMRI scans were collected using multi-slice interleaved echo planar imaging (EPI-BOLD) with the following parameters: repetition time (TR) = 3000 ms; echo time (TE) = 30.0 ms; flip angle = 70°; field of view = 240 mm × 240 mm × 132 mm; voxel = 3 mm3, for a total of 209 volumes. The total run time of the task-based fMRI sequence was 10 min and 32 s. Scan cards were identical between the two study sites to ensure harmonization of MRI data acquisition.
Functional neuroimaging processing and analyses
Structural and functional MRI images were pre-processed using the CONN toolbox within the MATLAB suite (CONN version 18b, SPM12, MATLAB 2019b) [37]. We used the default pre-processing pipeline within CONN, composed of functional realignment and unwarping, functional centering of the image to (0, 0, 0) coordinates, slice-timing correction, structural centering to (0, 0, 0) coordinates, structural segmentation and normalization to MNI space, functional normalization to MNI space, and spatial smoothing (set at 8 mm full-width half maximum Gaussian smoothing kernel). The Artifact Rejection Toolbox (ART) was used to identify motion outliers (set at the 97th percentile, with motion threshold set at 0.9 mm and global BOLD signal deviation set at z = + / − 5) and to flag outlier scan acquisitions. Following the pre-processing pipeline, the data were run through CONN’s default denoising pipeline, to estimate and account for noise factors. During the denoising process, an anatomical component-based noise correction procedure (aCompCor) extracted five noise components from white matter and CSF, which primarily reflect signal related to cardiac and respiratory fluctuations [38]. Additionally, subject-specific motion parameters were identified, outlier scans were accounted for via scrubbing, and temporal band-pass filtering (0.008–0.09 Hz) was applied to exclude signals not related to BOLD signals, such as scanner frequency drift [39, 40]. In the current study, we chose not to discard the first several volumes unless the volume was identified to be an outlier via ART toolbox. In all instances, we incorporated scrubbing information as a first-level covariates. This method allows for accurate representation of onset time of various components of the UFOV task (particularly given that the fixation cross and mask are jittered for varying lengths of time, Fig. 1). Participants were subsequently excluded if less than 50% of functional volumes remained after motion scrubbing; this demonstrates substantial motion, in accordance with Yeo 2015 recommendations [41].
Of the 359 participants included in the current study, 3 participants were removed due substantial motion, defined above as having less than 50% of functional volumes remaining following scrubbing (104 functional volumes or less). Twelve participants were excluded for responding to less than 50% of trials on the UFOV in-scanner task, and an additional 8 participants who received a 20-channel head coil, as opposed to a 64-channel head coil, were excluded. In total, 336 participants were included in the final analyses (Table 1). All protocol procedures were approved through the University of Florida and University of Arizona Institutional Review Boards. All participants provided written informed consent prior to the start of any study procedures. See Table 1 for demographic information.
Table 1.
Sample demographics
| University of Florida (n = 231) | University of Arizona (n = 105) | Combined (n = 336) | |
|---|---|---|---|
| Age mean (SD) | 71.62 (5.38) | 71.42 (4.42) | 71.56 (5.09) |
| Years of education mean (SD) | 16.30 (2.48) | 16.37 (2.01) | 16.32 (2.34) |
| Sex M:F | 90:141 | 29:76 | 119:217 |
| UFOV % accuracy mean (SD) | 60.10 (13.00) | 62.02 (12.82) | 60.70 (12.96) |
SD standard deviation
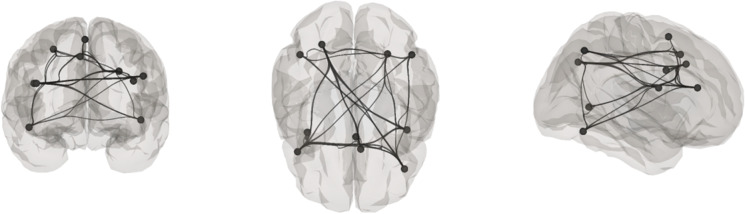
To assess connectivity of regions associated with the UFOV task, we created spherical ROIs using the WFU PickAtlas in SPM12 [42]. These spherical regions were centered on regions with the greatest peak BOLD activation patterns in the UFOV fMRI task, specifically associated with UFOV fMRI task performance [20]. Spherical ROIs were titrated to either 9 mm or 10 mm to capture significant regions of activation without overlapping. The details of the spherical ROIs can be found in Table 2 and visualization in Fig. 3.
Table 2.
Spherical ROIs
| ROI | x | y | z | Volume (mm3) | Radius (mm) | T statistic |
|---|---|---|---|---|---|---|
| L Pars Opercularis | − 38 | 4 | 26 | 729 | 9 | 5.21 |
| L Pars Triangularis | − 46 | 20 | 30 | 729 | 9 | 5.19 |
| L Supplementary Motor Area | 2 | 8 | 60 | 729 | 9 | 4.35 |
| L Inferior Temporal Gyrus | − 44 | − 60 | − 6 | 1000 | 10 | 4.87 |
| L Middle Occipital Gyrus | − 26 | − 68 | 34 | 1000 | 10 | 5.68 |
| R Inferior Temporal Gyrus | 48 | − 60 | − 6 | 1000 | 10 | 4.29 |
| R Pars Triangularis | 42 | 34 | 24 | 1000 | 10 | 4.74 |
| R Precentral Gyrus | 44 | 2 | 24 | 1000 | 10 | 4.77 |
| R Supplementary Motor Area | 6 | 18 | 46 | 1000 | 10 | 5.51 |
| R Superior Parietal Lobule | 28 | − 60 | 52 | 1000 | 10 | 6.12 |
Coordinates refer to x,y,z, location in MNI152 space. T statistic refers to strength of peak BOLD activation, found in Kraft et al. (2021)
Fig. 3.
UFOV network displayed on anterior, superior, and lateral right hemisphere views, displayed on grey matter mask
We used a weighted ROI-to-ROI connectivity analysis to model task-specific functional connectivity strength between a priori ROIs [20]. All task conditions (stimulus, probe, mask, and rest) were modeled. Weighted ROI-to-ROI connectivity was computed via a weighted least squares linear model, with weights defined as a condition-specific timeseries convolved with a canonical hemodynamic response function [43]. Thus, connectivity values in the following analyses are Fisher z-transformed bivariate correlations between ROI’s BOLD time series that quantify associations in activation during task conditions. The following analyses were all corrected at a false discovery rate (FDR) seed-level correction of p = 0.05 [44].
UFOV functional network and UFOV accuracy
We assessed whether within-network task-based functional connectivity of the above ROIs, henceforth called the UFOV functional network, was associated with UFOV fMRI accuracy. Task accuracy was calculated as the number of correct responses divided by 56 total trials. This serves as a confirmatory analysis that connectivity within the UFOV network as a whole did in fact capture activation related to task performance. Average within-network connectivity was calculated as the mean of the pairwise correlations between each of the ROIs of the UFOV functional network throughout completion of the total trial. The total trial was modeled as the onset times of the fixation cross, stimulus, mask, and probe screens for each participant (Fig. 1). General linear regressions were conducted in SPSSv27 to assess the relationship of average connectivity of the UFOV network on UFOV fMRI task performance, controlling for age, sex, years of education, and scanner type.
Resting state networks and UFOV accuracy
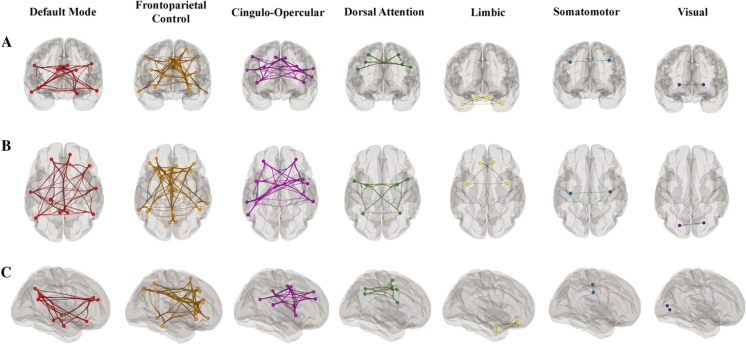
We then compared the efficacy of the UFOV functional network in predicting UFOV fMRI task performance to reliable, well-established resting state networks. To accomplish this, we used a widely validated, publicly available parcellation, composed of 7 resting state networks [45]. Fig. 4 shows the parcellation of the 7 resting-state networks, modeled onto the MNI152 template. Average within-network connectivity values were calculated as the mean of the pairwise correlations between the ROIs within each resting state network. We assessed the relationship of average within-network connectivity of these 7 networks on UFOV fMRI task performance, controlling for age, sex, education, and scanner, via linear regression using an FDR threshold of p = 0.05 for multiple comparisons correction [44].
Fig. 4.
Resting-state networks displayed on grey matter mask with A. anterior B. superior and C. right lateral hemisphere views. Each network is color-coded; however, the colors do not depict correlation strength [28]
UFOV functional network + resting state networks and double decision performance
Additionally, we wanted to evaluate the generalizability of the UFOV functional network as well as the 7 resting state networks to a widely used UFOV training paradigm, the Double Decision task. At present, the Double Decision training paradigm serves as the prevalent implementation for UFOV training interventions in recent and ongoing clinical trials [11, 12, 19, 32]. We assessed the relationship of average network connectivity of the UFOV functional network and the 7 resting state networks to Double Decision performance outside the scanner, controlling for age, sex, education, and scanner type, using an FDR threshold of p = 0.05 for multiple comparisons correction between the 7 network regressions [44].
Higher-order network segregation and UFOV performance
Finally, we aimed to explore the relationship between segregation of higher-order resting state networks and UFOV performance. We calculated network segregation of the dorsal attention network (DAN), default mode network (DMN), FPCN, and CON using the formula delineated by Chan and colleagues:
where is the mean Fisher z-transformed correlation between all nodes within a network and is the mean Fisher z-transformed correlation between the nodes of one network to the nodes of another network. We then ran a general linear regression to assess the relationship of system segregation between the four higher-order resting state network on UFOV fMRI task performance, controlling for age, sex, years of education, and scanner type. We used an FDR threshold of p = 0.05 for multiple comparisons correction between the 12 system segregation comparisons.
Results
UFOV functional network and UFOV accuracy
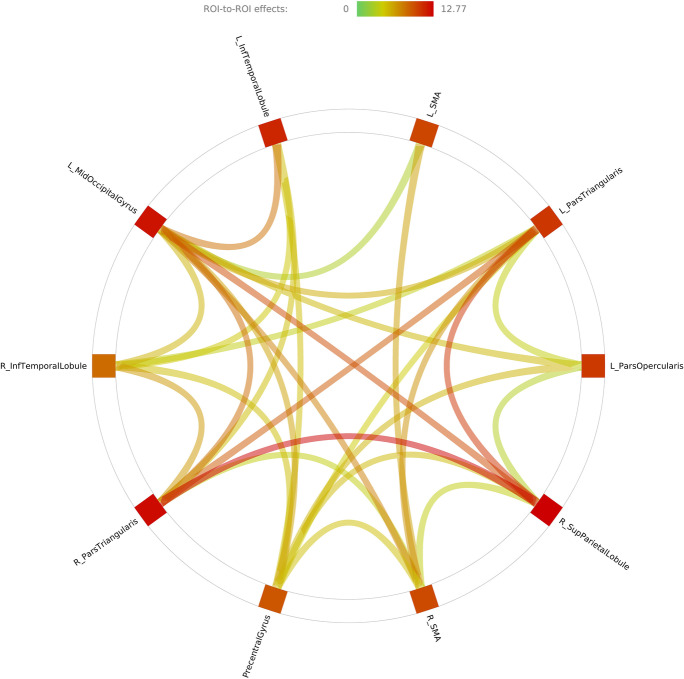
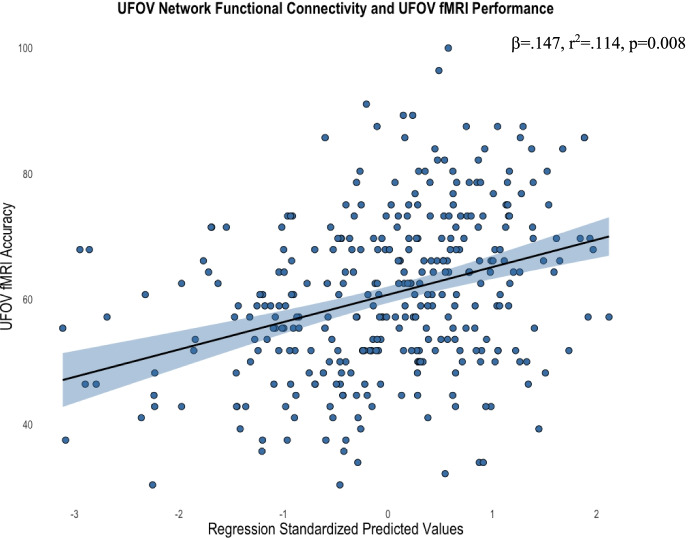
We first aimed to assess whether regions functionally activated during the UFOV fMRI task were (1) functionally connected and (2) subsequently related to UFOV fMRI performance. ROI-to-ROI analysis showed significantly greater functional connectivity between the 10 a priori ROIs during whole trial task completion (all FDR corrected p values < 0.05). No ROIs showed decreased functional connectivity with any other ROI. Fig. 5 shows the strength of connectivity between each of the ROIs within the UFOV functional network. Supplemental Table 1 contains statistical information regarding significant connections between nodes within the UFOV functional network. After confirming that these regions were indeed functionally connected, we evaluated the relationship of within-network functional connectivity of the UFOV network to UFOV fMRI task performance. After controlling for age, sex, years of education, and scanner type, greater within-network connectivity of the UFOV functional network was associated with higher accuracy of the UFOV fMRI task (R2 = 0.114, sr2 = 0.141, β = 0.147, p = 0.008) (Fig. 6).
Fig. 5.
Connectome ring of ROI-to-ROI task-based functional connectivity using the 10 a priori spherical ROIs. The color of the connections and color bar refer to connectivity t-values. Specific p values of ROI-to-ROI connections can be found in Supplemental Table 1
Fig. 6.
Scatterplot depicting the significant relationship between average within-network connectivity of the UFOV network and accuracy on the UFOV fMRI task, controlling for age, sex, years of education, and scanner type (standardized predicted values). Standardized predicted values are calculated by subtracting the mean predicted value of within-network connectivity of the UFOV network (given the mean age, sex, education, and scanner type) from the individual’s predicted value of within-network connectivity of the UFOV network (given an individual’s age, sex, education, and scanner type). This difference is divided by the standard deviation of the predicted values to allow for comparisons between individuals controlling for demographics. Standardized predicted values have a mean of 0 and a standard deviation of 1. Shaded region represents the 95% confidence interval of the regression line
Resting state networks and UFOV accuracy
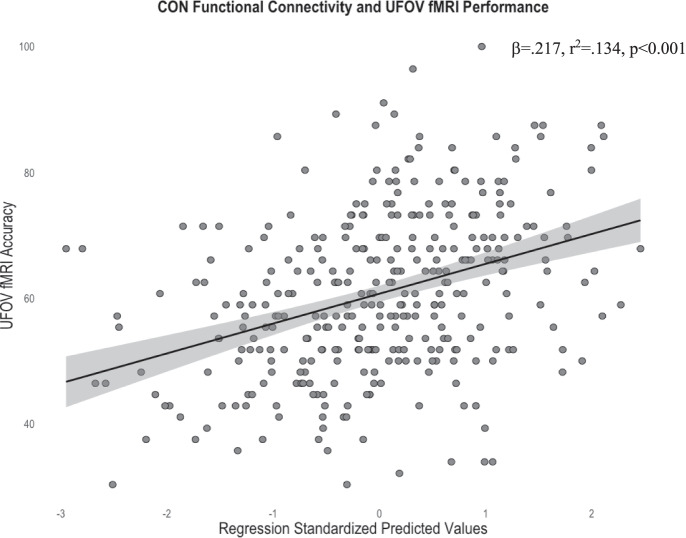
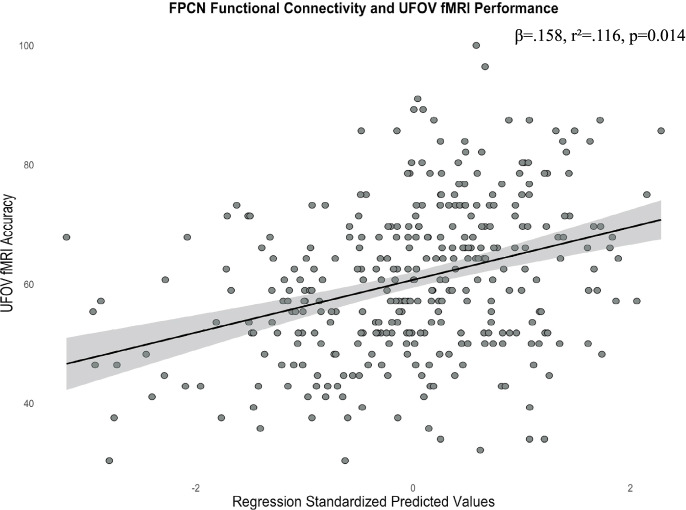
Given our findings of a significant relationship between UFOV fMRI performance and UFOV network functional connectivity, we then sought to compare these results to the 7 resting-state networks. After controlling for covariates and performing FDR corrections, greater within-network connectivity only within the CON (R2 = 0.134, sr2 = 0.202, β = 0.217, pFDR < 0.001) and FPCN (R2 = 0.116, sr2 = 0.150, β = 0.158, pFDR = 0.014) were associated with higher accuracy on the UFOV fMRI task (Figs. 7 and 8). No other resting state networks were significantly related to UFOV fMRI task performance (all pFDR > 0.05).
Fig. 7.
Scatterplot depicting the significant relationship between average within-network connectivity of the CON and accuracy on the UFOV fMRI task, controlling for age, sex, years of education, and scanner type (standardized predicted values). Standardized predicted values are calculated by subtracting the mean predicted value of within-network connectivity of the CON (given the mean age, sex, education, and scanner type) from the individual’s predicted value of within-network connectivity of the CON (given an individual’s age, sex, education, and scanner type). This difference is divided by the standard deviation of the predicted values to allow for comparisons between individuals controlling for demographics. Standardized predicted values have a mean of 0 and a standard deviation of 1. Shaded region represents the 95% confidence interval of the regression line
Fig. 8.
Scatterplot depicting the significant relationship between average within-network connectivity of the FPCN and accuracy on the UFOV fMRI task, controlling for age, sex, years of education, and scanner type (standardized predicted values). Standardized predicted values are calculated by subtracting the mean predicted value of within-network connectivity of the FPCN (given the mean age, sex, education and scanner type) from the individual’s predicted value of within-network connectivity of the FPCN (given an individual’s age, sex, education, and scanner type). This difference is divided by the standard deviation of the predicted values to allow for comparisons between individuals controlling for demographics. Standardized predicted values have a mean of 0 and a standard deviation of 1. Shaded region represents the 95% confidence interval of the regression line
UFOV functional network + resting state networks and double decision performance
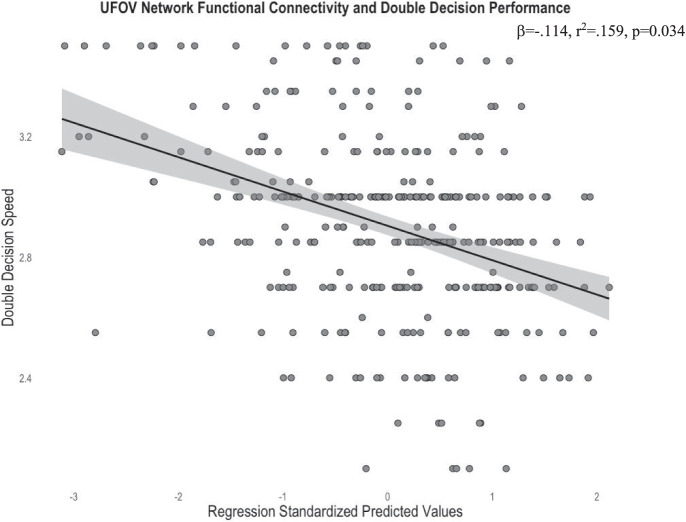
Following the above significant relationships between within-network connectivity of the UFOV functional network, CON, and FPCN to UFOV accuracy, we then evaluated the generalizability of these networks to the Double Decision task, a commonly used cognitive training intervention paradigm based on the original UFOV task. We ran the regressions from both the UFOV functional network and resting-state network analyses, substituting UFOV fMRI performance for Double Decision performance. After controlling for age, sex, years of education, and scanner type, greater within-network connectivity of the UFOV functional network was associated with faster presentation times, and thereby better performance, on the Double Decision task (R2 = 0.159, sr2 = − 0.116, β = − 0.114, p = 0.034) (Fig. 9).
Fig. 9.
Scatterplot depicting the significant relationship between average within-network connectivity of the UFOV network and faster Double Decision presentation, controlling for age, sex, years of education, and scanner type. Standardized predicted values are calculated by subtracting the mean predicted value of within-network connectivity of the UFOV network (given the mean age, sex, education, and scanner type) from the individual’s predicted value of within-network connectivity of the UFOV network (given an individual’s age, sex, education, and scanner type). This difference is divided by the standard deviation of the predicted values to allow for comparisons between individuals controlling for demographics. Standardized predicted values have a mean of 0 and a standard deviation of 1. Shaded region represents the 95% confidence interval of the regression line
Notably, none of the 7 resting state networks were associated with POSIT Double Decision performance (all pFDR > 0.05).
Higher-order network segregation and UFOV performance
Finally, we assessed the relationship between higher-order network segregation and UFOV accuracy. After controlling for age, sex, years of education, and scanner type, no significant relationships between network segregation and UFOV performance remained (all pFDR and p-uncorrected values > 0.05).
Discussion
Prior evidence suggests that UFOV-based interventions provide lasting transfer effects in processing speed, a domain sensitive to age-related cognitive decline. Despite this, little is known about the neurocognitive correlates of the UFOV task. The current study was the first to assess task-based functional connectivity during UFOV performance. We assessed functional connectivity between a priori ROIs derived from BOLD activation patterns previously implicated in UFOV task performance [20]. We further assessed the viability a UFOV functional network by comparing its network connectivity to canonical resting state networks on both UFOV task performance and on a closely related task, the Double Decision task. Such findings may provide insight into potential neural targets to optimize UFOV training gains.
Analyzing the relationship between the UFOV functional network and UFOV accuracy, we observed greater within-network functional connectivity of the UFOV functional network associated with higher accuracy on the UFOV fMRI task. Nodes within the UFOV functional network included the bilateral pars triangularis, inferior temporal gyrus, and supplementary motor areas (SMA), as well as the left pars opercularis, left middle occipital gyrus, right precentral gyrus, and right superior parietal lobule. Several of these regions have previously been associated with out-of-scanner UFOV performance, including the ACC and DLPFC [19]. However, in comparison to established resting-state networks, we were surprised to find that within-network connectivity of the FPCN and CON predicted UFOV task performance equally well or better than our UFOV functional network. This may be due, in part, to the overlap of our ROIs to regions implicated in the FPCN and CON networks.
The FPCN, a functional connectivity resting state network critical in higher-order cognition, is associated with directing attention to relevant stimuli, manipulation of stimuli, and set-shifting abilities [29, 46, 47]. Maintenance of functional connectivity within this network is essential for cognitive control, especially in older adults. Our findings align with the findings of Hardcastle and colleagues, who found evidence of strengthened resting-state frontoparietal control network (FPCN) connectivity following an intervention using the UFOV task [31].
The superior portion of our 9 mm ROIs centered on the pars triangularis and pars opercularis makes up parts of the DLPFC, while the inferior portions comprise much of the VLPFC. This is noteworthy, since the lateral prefrontal cortex, comprising the DLPFC/VLPFC, is a hub region of the FPCN (for graphical depiction of the FCPN, see [41, 46]). The DLPFC’s corresponding ROI in the UFOV functional connectivity network, the pars triangularis, exhibited some of the strongest ROI-to-ROI task-based functional connectivity. The role of the DLPFC in UFOV performance has also been highlighted by Ross and colleagues, who found significant strengthened resting-state functional connectivity between the DLPFC and ACC following a UFOV intervention [19].
The temporal region of the FPCN, centered within our a priori inferior temporal gyrus ROI, demonstrated significant task-based functional connectivity during the UFOV task. In the current study, we saw significant cross-hemispheric connections between the left and right inferior temporal gyrus, as well as widespread cross-hemispheric connections to nearly all other nodes within the UFOV functional network. Our findings suggest that strong cross-hemispheric communications between key regions of the FPCN, particularly the DLPFC and inferior temporal gyrus, are essential in UFOV task performance, and this may be particularly true in aging. Foundational work by Cabeza and colleagues demonstrated that under task-based demands, older adults experience less lateralization of BOLD activation, suggesting a compensatory mechanism in maintaining cognitive performance [48, 49]. It is possible that cross-hemispheric functional connectivity during UFOV completion, particularly within our older adult sample, may support compensation, as described by Cabeza and colleagues.
In addition to the FPCN, we observed overlap of our UFOV functional network ROIs with other established functional connectivity networks [45]. The inferior portion of the 10 mm ROI centered on the right supplementary motor area (SMA) is composed of regions of the anterior cingulate cortex (ACC). Our ROI overlaps with the medial portion of the CON network, centered at the ACC, and this region is a key hub in the CON network. Similar to the FPCN, the CON network is a higher-order resting state network involved in monitoring task performance, maintenance of task rules, and set-shifting abilities [50]. These results again corroborate the findings of strengthened functional connectivity between the ACC and DLPFC following a UFOV intervention, underscoring these regions’ crucial role in UFOV task performance [19]. Moreover, the posterior node of the DAN is encompassed by our 10 mm ROI centered on the right superior parietal lobule. This higher-order resting state network is implicated primarily in orientation to task-relevant stimuli, specifically location of visual stimuli [51, 52]. Previous research has shown that following a visual search intervention, within-network connectivity of the DAN was significantly strengthened [53]. Recent research has further suggested that connections among the superior parietal lobule, ACC, and DLPFC relate to individual performance on a visual search task, all of which are implicated in the current study [54].
We also identified unique regions associated with UFOV task performance not encompassed by the FPCN, CON, or DAN, including ROIs centered on the left SMA, the left middle occipital gyrus, and the right precentral gyrus. Both the SMA and the visual cortex have been implicated in UFOV task performance, and both regions had significant increases in resting-state functional connectivity following a UFOV intervention [19]. The significance of coherent functional connectivity in the middle occipital gyrus is unsurprising, given the visual nature of the UFOV task. The SMA is activated during anticipation and completion of motor tasks and also involved in reaction time maintenance [55–57]. A rapidly growing body of evidence has further suggested the role of the precentral gyrus in UFOV task performance [19]. Consistent with this, the precentral gyrus demonstrated some of the most robust activation patterns when completing the UFOV task in the present study, and structural degradation of this region was associated with poorer performance on an analogous Double Decision task [20, 58]. The motor cortex is contained within the precentral gyrus, a region that plays a critical role in motor planning and completion of motor tasks [59]. These findings suggest that motor planning is an integral part in UFOV task completion and may further explain the role of the precentral gyrus in UFOV task performance.
We then aimed to evaluate the generalizability of within-network connectivity of the UFOV network to scores on a “near-transfer” task, the Double Decision task. We found that the within-network connectivity of UFOV fMRI task predicted scores on the Double Decision task, better than the 7 resting state networks. This finding is significant, given that the Double Decision task is the most prevalent implementation of adaptive UFOV training in clinical trials. The Advanced Cognitive Training for Independent and Vital Elderly (ACTIVE) study was the largest clinical trial of its time to investigate the longitudinal effects of cognitive training using the Double Decision task (n = 2832) [11]. They found that cognitive improvements stemming from a Double Decision based intervention lasted 2 years following cessation of intervention, and resultant work found that these gains can last up to 10 years following intervention [11, 12]. Further studies found that each UFOV training session was associated with up to a 29% reduction in dementia prevalence 10 years following the end of intervention in the ACTIVE study [60]. Such improvements extend beyond cognition—UFOV—and Double Decision-based interventions have been associated with better driving outcomes [13, 14], better self-reported cognition [15], fewer difficulties in completing IADLs [17, 18], and improved health-related quality of life [5, 16]. The overwhelming evidence of efficacy regarding the Double Decision task makes the UFOV functional network an important target for future interventions.
It is interesting to note that the relationship between functional segregation of the four higher-order resting state networks and UFOV task performance was not significant. Functional segregation of networks is essential in cognitive aging, as research has found that increased system segregation is associated with cognition and these relationships exists independent of the aging process [27]. It should be emphasized that we cannot state that system segregation is irrelevant in task performance; rather, within-network connectivity, as opposed to between-network connectivity, may be driving UFOV performance. Future research should investigate how metrics of system segregation, including participation coefficients, influence UFOV task performance.
Limitations and future directions
While this study provides novel insights into task-based functional connectivity of the UFOV cognitive paradigm, results from this work must be interpreted within the context of limitations. Our sample was highly educated, with 69% of our sample achieving a bachelor’s degree or higher; 83.14% of our sample identified as “White/non-Hispanic,” which limits generalizability of our findings to Black, Asian American and Pacific Islander, Indigenous, and Hispanic or Latinx populations. As the aging population becomes more ethnically and racially diverse, we must overcome barriers to research participation that disproportionately impact individuals from marginalized populations to promote more inclusive aging research.
This study was cross-sectional and thus can make no inferences regarding dynamic changes in task-based functional connectivity during the aging process. Future work should analyze longitudinal changes in UFOV task-based functional connectivity, given that both UFOV performance and functional connectivity network segregation are impacted during the cognitive aging process [12, 26, 61]. In the present study, we conducted an ROI-to-ROI task-based analyses using a priori ROIs built from a subset of participants used in the current sample [20]. This method allowed us to focus on functional connectivity metrics among regions that had previously shown activation during the UFOV fMRI task and were related to UFOV task performance. Future studies should replicate these findings using a variety of functional connectivity techniques (e.g., independent components analysis, voxel-wise approaches, graph theory approaches).
Improving replicability and reproducibility of fMRI studies remains essential in the field of cognitive neuroscience. In the current study, we adopted several of the recommendations delineated by Poldrack and colleagues, including the collection of larger samples to improve power, using statistical thresholding procedures such as FDR corrections, and restricting analyses to a priori ROIs [62]. However, limitations using fMRI persist, including temporal resolution limitations, susceptibility to motion and tightly constrained environments. Moreover, MRI contraindications and cost of imaging may further reduce generalizability to a broader population. Functional near-infrared spectroscopy (fNIRS) is an inexpensive, non-invasive neuroimaging technique in which near-infrared light is shined into the head and measures changes in concentrations of oxygenated and deoxygenated hemoglobin (for reviews, see [63] and [64]). Measurements of hemodynamic response in fNIRS and BOLD responses in fMRI have been shown to produce similar results, even within task-based designs [65, 66]. fNIRS has also been shown to be less susceptible to motion, and no contraindications exist for fNIRS [65] Despite the limitations of fNIRS, including spatial resolution and penetration depth, future research should investigate alternative neuroimaging techniques.
Finally, recent studies have investigated the role of non-invasive brain stimulation techniques, such as transcranial direct current stimulation and transcranial alternating current stimulation, in increasing within-network modularity in higher-order resting-state networks [67, 68]. Given the crucial role of within-network synchrony, particularly in the context of aging, future work should explore modulating these networks to offset the deleterious effects of cognitive decline.
Conclusion
To date, this is the first study that has assessed task-based functional connectivity during completion of a widely efficacious paradigm in improving cognition, the UFOV task. Notably, we provide evidence that coherence within 10 a priori ROIs significantly predicts UFOV task performance. Synchronicity in this network, which we termed the UFOV functional network, predicted UFOV performance. Additionally, enhanced within-network connectivity of the UFOV functional network predicted better performance on a measure of “near-transfer,” the Double Decision task. Collectivity, these findings provide insight into potential targets to optimize the efficacy of the UFOV paradigm in slowing cognitive aging.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank all our participants for their time and research assistants for their tireless work and instrumental role in making this manuscript possible.
Author contribution
JK and AW contributed to the conception and design of this specific study. JK extracted data, performed statistical analyses, and wrote the first draft of the manuscript. HH and CH contributed to data analyzing and processing. EP, GH, SW, SD, GA, MM, RC, and AW were involved in project administration. All authors contributed to manuscript revisions, read, and approved the submitted version.
Funding
This work was supported by the National Institute on Aging (NIA R01AG054077, NIA K01AG050707, NIA P30AG019610, T32AG020499), the State of Arizona and Arizona Department of Health Services (ADHS), the University of Florida Center for Cognitive Aging and Memory Clinical Translational Research, the McKnight Brain Research Foundation, and National Heart, Lung, and Blood Institute [T32HL134621].
Data availability
Data are managed under the data sharing agreement established with NIA and the parent R01 clinical trial Data Safety and Monitoring Board in the context of an ongoing Phase III clinical trial (ACT study, R01AG054077). All trial data will be made publicly available 2 years after completion of the parent clinical trial, per NIA and DSMB agreement. Requests for baseline data can be submitted to the ACT Publication and Presentation (P&P) Committee and will require submission of a data use, authorship, and analytic plan for review by the P&P committee (ajwoods@phhp.ufl.edu).
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
References
- 1.Park DC, Lautenschlager G, Hedden T, Davidson NS, Smith AD, Smith PK. Models of visuospatial and verbal memory across the adult life span. Psychol Aging. 2002;17(2):299–320. [PubMed] [Google Scholar]
- 2.Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychol Rev. 1996;103(3):403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- 3.Anderson M, Nettelbeck T, Barlow J. Reaction time measures of speed of processing: speed of response selection increases with age but speed of stimulus categorization does not. Br J Dev Psychol. 1997;15(2):145–157. [Google Scholar]
- 4.Edwards JD, Ruva CL, Brien JLO, Haley CB, Lister JJ. An examination of mediators of the transfer of cognitive speed of processing training to everyday functional performance. Psychol Aging. 2014;28(2):314–321. doi: 10.1037/a0030474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolinsky FD, Mahncke HW, Kosinski M, Unverzagt FW, Smith DM, Jones RN, et al. The ACTIVE cognitive training trial and predicted medical expenditures. BMC Health Serv Res. 2009;9:1–9. doi: 10.1186/1472-6963-9-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edwards JD, Vance DE, Wadley VG, Cissell GM, Roenker DL, Ball KK. Reliability and validity of useful field of view test scores as administered by personal computer. J Clin Exp Neuropsychol. 2005;27(5):529–543. doi: 10.1080/13803390490515432. [DOI] [PubMed] [Google Scholar]
- 7.Aust F, Edwards JD. Incremental validity of Useful Field of view subtests for the prediction of instrumental activities of daily living. J Clin Exp Neuropsychol. 2017;38(5):497–515. doi: 10.1080/13803395.2015.1125453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bezdicek O, Stepankova H, MartinecNovakova L, Kopecek M. Toward the processing speed theory of activities of daily living in healthy aging: normative data of the Functional Activities Questionnaire. Aging Clin Exp Res. 2016;28(2):239–247. doi: 10.1007/s40520-015-0413-5. [DOI] [PubMed] [Google Scholar]
- 9.Goode KT, Ball KK, Sloane M, Roenker DL, Roth DL, Myers RS, et al. Useful Field of view and other neurocognitive indicators of crash risk in older adults. J Clin Psychol Med Settings. 1998;5(4):425–440. [Google Scholar]
- 10.Levitt T, Fugelsang J, Crossley M. Processing speed, attentional capacity, and age-related memory change. Exp Aging Res. 2006;32(3):263–295. doi: 10.1080/03610730600699118. [DOI] [PubMed] [Google Scholar]
- 11.Ball K, Berch DB, Helmers KF, Jobe JB, Leveck MD, Marsiske M, et al. Effects of Cognitive Training Interventions With Older Adults. JAMA. 2002;288(18):2271. doi: 10.1001/jama.288.18.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rebok GW, Ball K, Guey LT, Jones RN, Kim HY, King JW, et al. Ten-year effects of the advanced cognitive training for independent and vital elderly cognitive training trial on cognition and everyday functioning in older adults. J Am Geriatr Soc. 2014;62(1):16–24. doi: 10.1111/jgs.12607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roenker DL, Cissell GM, Ball KK, Wadley VG, Edwards JD. Speed-of-processing and driving simulator training result in improved driving performance. Hum Factors. 2003;45(2):218–233. doi: 10.1518/hfes.45.2.218.27241. [DOI] [PubMed] [Google Scholar]
- 14.Ball K, Edwards JD, Ross LA, McGwin G. Cognitive training decreases motor vehicle collision involvement of older drivers. J Am Geriatr Soc. 2010;58(11):2107–2113. doi: 10.1111/j.1532-5415.2010.03138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith GE, Housen P, Yaffe K, Ruff R, Kennison RF, Mahncke HW, et al. A cognitive training program based on principles of brain plasticity: results from the improvement in memory with plasticity-based adaptive cognitive training (IMPACT) study. J Am Geriatr Soc. 2009;57(4):594–603. doi: 10.1111/j.1532-5415.2008.02167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolinsky FD, Vander Weg MW, Howren MB, Jones MP, Dotson MM. A randomized controlled trial of cognitive training using a visual speed of processing intervention in middle aged and older adults. PLoS One. 2013;8(5):1–11. [DOI] [PMC free article] [PubMed]
- 17.Owsley C, McGwin G, Sloane ME, Stalvey BT, Wells J. Timed instrumental activities of daily living tasks: relationship to visual function in older adults. Optom Vis Sci. 2001;78(5):350–359. doi: 10.1097/00006324-200105000-00019. [DOI] [PubMed] [Google Scholar]
- 18.Valdés EG, Andel R, Lister JJ, Gamaldo A, Edwards JD. Can cognitive speed of processing training improve everyday functioning among older adults with psychometrically defined mild cognitive impairment? J Aging Health [Internet]. 2017;089826431773882. Available from: http://journals.sagepub.com/doi/10.1177/0898264317738828 [DOI] [PMC free article] [PubMed]
- 19.Ross LA, Webb CE, Whitaker C, Hicks JM, Schmidt EL, Samimy S, et al. The effects of useful field of view training on brain activity and connectivity. Journals Gerontol Ser B. 2018;00(00):1–11. doi: 10.1093/geronb/gby041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kraft JN, Albizu A, O’Shea A, Hausman HK, Evangelista ND, Boutzoukas E, et al. Functional neural correlates of a Useful Field of View (UFOV)-based fMRI task in older adults. Cereb Cortex. 2022;32(9):1993–2012. [DOI] [PMC free article] [PubMed]
- 21.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity Echo-Planar MRI. Magn Reson Med [Internet]. 1995;34(4):537–41. Available from: http://onlinelibrary.wiley.com/doi/10.1002/mrm.1910340409/abstract [DOI] [PubMed]
- 22.De Luca M, Beckmann CF, De Stefano N, Matthews PM, Smith SM. fMRI resting state networks define distinct modes of long-distance interactions in the human brain. Neuroimage. 2006;29(4):1359–1367. doi: 10.1016/j.neuroimage.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 23.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8(9):700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 24.van den Heuvel MP, Hulshoff Pol HE. Exploring the brain network: a review on resting-state fMRI functional connectivity. Eur Neuropsychopharmacol [Internet]. 2010;20:519–34. Available from: 10.1016/j.euroneuro.2010.03.008 [DOI] [PubMed]
- 25.Chan MY, Park DC, Savalia NK, Petersen SE, Wig GS. Decreased segregation of brain systems across the healthy adult lifespan. Proc Natl Acad Sci U S A. 2014;111(46):E4997–5006. doi: 10.1073/pnas.1415122111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan MY, Alhazmi FH, Park DC, Savalia NK, Wig GS. Resting-state network topology differentiates task signals across the adult life span. J Neurosci. 2017;37(10):2734–2745. doi: 10.1523/JNEUROSCI.2406-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wig GS. Segregated systems of human brain networks. Trends Cogn Sci [Internet]. 2017;21(12):981–96. Available from: 10.1016/j.tics.2017.09.006 [DOI] [PubMed]
- 28.Hausman HK, O’Shea A, Kraft JN, Boutzoukas EM, Evangelista ND, Van Etten EJ, et al. The role of resting-state network functional connectivity in cognitive aging. Front Aging Neurosci. 2020;12(June):1–10. doi: 10.3389/fnagi.2020.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hausman HK, Hardcastle C, Albizu A, Kraft JN, Evangelista ND, Boutzoukas EM, et al. Cingulo-opercular and frontoparietal control network connectivity and executive functioning in older adults. GeroScience [Internet]. 2021;(0123456789). Available from: 10.1007/s11357-021-00503-1 [DOI] [PMC free article] [PubMed]
- 30.Hardcastle C, Hausman HK, Kraft JN, Albizu A, Evangelista ND, Boutzoukas EM, et al. Higher-order resting state network association with the useful field of view task in older adults. GeroScience [Internet]. 2022;44(1):131–45. Available from: 10.1007/s11357-021-00441-y [DOI] [PMC free article] [PubMed]
- 31.Hardcastle C, Hausman HK, Kraft JN, Albizu A, O’Shea A, Boutzoukas EM, et al. Proximal improvement and higher-order resting state network change after multidomain cognitive training intervention in healthy older adults. GeroScience [Internet]. 2022;44(1):1011–1027. Available from: 10.1007/s11357-022-00535-1 [DOI] [PMC free article] [PubMed]
- 32.Woods AJ, Cohen R, Marsiske M, Alexander GE, Czaja SJ, Wu S. Augmenting cognitive training in older adults (The ACT Study): design and methods of a phase III tDCS and cognitive training trial. Contemp Clin Trials. 2018;65:19–32. doi: 10.1016/j.cct.2017.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weintraub S, Salmon D, Mercaldo N, Ferris S, Graff-radford NR, Chui H, et al. The Alzheimer’s disease centers’ data set (UDS): the neuropsychological test battery. Alzheimer Dis Assoc Disord. 2009;23(2):91–101. doi: 10.1097/WAD.0b013e318191c7dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aust F, Edwards JD. Incremental validity of useful field of view subtests for the prediction of instrumental activities of daily living. J Clin Exp Neuropsychol. 2016;38(5):497–515. doi: 10.1080/13803395.2015.1125453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Edwards JD, Hauser RA, O’Connor ML, Valdés EG, Zesiewicz TA, Uc EY. Randomized trial of cognitive speed of processing training in Parkinson disease. Neurology. 2013;81(15):1284–1290. doi: 10.1212/WNL.0b013e3182a823ba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin F, Heffner KL, Ren P, Tivarus ME, Brasch J, Chen DG, et al. Cognitive and neural effects of vision-based speed-of-processing training in older adults with amnestic mild cognitive impairment: a pilot study. J Am Geriatr Soc. 2016;64(6):1293–1298. doi: 10.1111/jgs.14132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2(3):125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- 38.Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage [Internet]. 2007;37(1):90–101. Available from: 10.1016/j.neuroimage.2007.04.042 [DOI] [PMC free article] [PubMed]
- 39.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage [Internet]. 2012;59(3):2142–54. Available from: 10.1016/j.neuroimage.2011.10.018 [DOI] [PMC free article] [PubMed]
- 40.Friston KJ, Williams S, Howard R, Frackowiak RSJ, Turner R. Movement-related effects in fMRI time-series. Magn Reson Med. 1996;35(3):346–355. doi: 10.1002/mrm.1910350312. [DOI] [PubMed] [Google Scholar]
- 41.Yeo BTT, Tandi J, Chee MWL. Functional connectivity during rested wakefulness predicts vulnerability to sleep deprivation. Neuroimage [Internet]. 2015;111:147–58. Available from: 10.1016/j.neuroimage.2015.02.018 [DOI] [PubMed]
- 42.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19(3):1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 43.Nieto-Castanon A. Handbook of functional connectivity Magnetic Resonance Imaging methods in CONN [Internet]. 2020. p. 108. Available from: https://www.researchgate.net/publication/339460691_Handbook_of_functional_connectivity_Magnetic_Resonance_Imaging_methods_in_CONN. Accessed 14 Jan 2022.
- 44.Benjamini Y, Hochberg Y. Controlling the false discovery rate : a practical and powerful approach to multiple testing. J R Stat Soc. 1995;57(1):289–300. [Google Scholar]
- 45.Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106(3):1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marek S, Dosenbach NUF. The frontoparietal network: function, electrophysiology, and importance of individual precision mapping. Dialogues Clin Neurosci [Internet]. 2018;20:133–40. Available from: 10.31887/DCNS.2018.20.2/smarek [DOI] [PMC free article] [PubMed]
- 47.Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cabeza R. Hemispheric asymmetry reduction in older adults: The HAROLD model. Psychol Aging. 2002;17(1):85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- 49.Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: compensatory brain activity in high-performing older adults. Neuroimage. 2002;17(3):1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- 50.Dosenbach NUF, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RAT, et al. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci U S A. 2007;104(26):11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fox M, Corbetta M, Snyder A, Vincent J, Raichle M. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci U S A [Internet]. 2006;103(26):10046–51. Available from: 10.1073/pnas.0604187103 [DOI] [PMC free article] [PubMed]
- 52.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3(3):201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 53.Bueichekú E, Ávila C, Miró-Padilla A, Sepulcre J. Visual search task immediate training effects on task-related functional connectivity. Brain Imaging Behav. 2019;13(6):1566–1579. doi: 10.1007/s11682-018-9993-y. [DOI] [PubMed] [Google Scholar]
- 54.Bueichekú E, Miró-Padilla A, Ávila C. Functional connectivity at rest captures individual differences in visual search. Brain Struct Funct [Internet]. 2020;225(2):537–49. Available from: 10.1007/s00429-019-02008-2 [DOI] [PubMed]
- 55.Sharma N, Baron JC. Effects of healthy ageing on activation pattern within the primary motor cortex during movement and motor imagery: An fMRI study. PLoS ONE. 2014;9(6):1–8. doi: 10.1371/journal.pone.0088443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bazán PR, Biazoli CE, Sato JR, Amaro E. Motor readiness increases brain connectivity between default-mode network and motor cortex: impact on sampling resting periods from fMRI event-related studies. Brain Connect. 2015;5(10):631–640. doi: 10.1089/brain.2014.0332. [DOI] [PubMed] [Google Scholar]
- 57.Berman BD, Horovitz S, Venkataraman G, Hallet M. Self-modulation of primary motor cortex activity with motor and motor imagery tasks using real-time fMRI-based neurofeedback. Neuroimage. 2012;59(2):9517–925. [DOI] [PMC free article] [PubMed]
- 58.Kraft JN, O’Shea A, Albizu A, Evangelista ND, Hausman HK, Boutzoukas E, et al. Structural neural correlates of double decision performance in older adults. Front Aging Neurosci [Internet]. 2020 Sep 2;12(September):1–16. Available from: https://www.frontiersin.org/article/10.3389/fnagi.2020.00278/full [DOI] [PMC free article] [PubMed]
- 59.Svoboda K, Li N. Neural mechanisms of movement planning: motor cortex and beyond. Curr Opin Neurobiol [Internet]. 2018;49:33–41. Available from: 10.1016/j.conb.2017.10.023 [DOI] [PubMed]
- 60.Edwards JD, Xu H, Clark DO, Guey LT, Ross LA, Unverzagt FW. Speed of processing training results in lower risk of dementia. Alzheimer’s Dement Transl Res Clin Interv. 2017;3(4):603–611. doi: 10.1016/j.trci.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Grady C, Sarraf S, Saverino C, Campbell K. Age differences in the functional interactions among the default, frontoparietal control, and dorsal attention networks. Neurobiol Aging [Internet]. 2016;41:159–72. Available from: 10.1016/j.neurobiolaging.2016.02.020 [DOI] [PubMed]
- 62.Poldrack RA, Baker CI, Durnez J, Gorgolewski KJ, Matthews PM, Munafò MR, et al. Scanning the horizon: Towards transparent and reproducible neuroimaging research. Nat Rev Neurosci. 2017;18(2):115–126. doi: 10.1038/nrn.2016.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Scholkmann F, Kleiser S, Metz AJ, Zimmermann R, Mata Pavia J, Wolf U, et al. A review on continuous wave functional near-infrared spectroscopy and imaging instrumentation and methodology. Neuroimage [Internet]. 2014;85:6–27. Available from: 10.1016/j.neuroimage.2013.05.004 [DOI] [PubMed]
- 64.Ferrari M, Quaresima V. A brief review on the history of human functional near-infrared spectroscopy (fNIRS) development and fields of application. Neuroimage [Internet]. 2012;63(2):921–35. Available from: 10.1016/j.neuroimage.2012.03.049 [DOI] [PubMed]
- 65.Pinti P, Tachtsidis I, Hamilton A, Hirsch J, Aichelburg C, Gilbert S, et al. The present and future use of functional near-infrared spectroscopy (Fnirs) for cognitive neuroscience. Ann N Y Acad Sci. 2020;1464(1):5–29. doi: 10.1111/nyas.13948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cui X, Bray S, Bryant DM, Glover GH, Reiss AL. A quantitative comparison of NIRS and fMRI across multiple cognitive tasks. Neuroimage [Internet]. 2011;54(4):2808–21. Available from: 10.1016/j.neuroimage.2010.10.069 [DOI] [PMC free article] [PubMed]
- 67.Mondino M, Ghumman S, Gane C, Renauld E, Whittingstall K, Fecteau S. Effects of transcranial stimulation with direct and alternating current on resting-state functional connectivity: an exploratory study simultaneously combining stimulation and multiband functional magnetic resonance imaging. Front Hum Neurosci. 2020;13(February):1–8. doi: 10.3389/fnhum.2019.00474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Clancy KJ, Andrzejewski JA, You Y, Rosenberg JT, Ding M, Li W. Transcranial stimulation of alpha oscillations up-regulates the default mode network. Proc Natl Acad Sci U S A. 2022;119(1):1–8. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are managed under the data sharing agreement established with NIA and the parent R01 clinical trial Data Safety and Monitoring Board in the context of an ongoing Phase III clinical trial (ACT study, R01AG054077). All trial data will be made publicly available 2 years after completion of the parent clinical trial, per NIA and DSMB agreement. Requests for baseline data can be submitted to the ACT Publication and Presentation (P&P) Committee and will require submission of a data use, authorship, and analytic plan for review by the P&P committee (ajwoods@phhp.ufl.edu).