Abstract
Background: Olfactory training is the only evidence-based treatment for post-viral olfactory dysfunction. Smell disorders after SARS-CoV-2 infection have been attributed to neuroinflammatory events within the olfactory bulb and the central nervous system. Therefore, targeting neuroinflammation is one potential strategy for promoting recovery from post-COVID-19 chronic olfactory dysfunction. Palmitoylethanolamide and luteolin (PEA-LUT) are candidate anti-inflammatory/ neuroprotective agents.
Objective: To investigate recovery of olfactory function in patients treated with PEA-LUT oral supplements plus olfactory training versus olfactory training plus placebo.
Methods: Multicenter double-blinded randomized placebo-controlled clinical trial was held. Eligible subjects had prior COVID-19 and persistent olfactory impairment >6 months after follow-up SARS-CoV-2 negative testing, without prior history of olfactory dysfunction or other sinonasal disorders. Participants were randomized to daily oral supplementation with ultramicronized PEA-LUT 770 mg plus olfactory training (intervention group) or olfactory training with placebo (control). Sniffin’ Sticks assessments were used to test the patients at baseline and 90 days.
Results: A total of 185 patients, including intervention (130) and control (55) were enrolled. The intervention group showed significantly greater improvement in olfactory threshold, discrimination, and identification scores compared to controls (p=0.0001). Overall, 92% of patients in the intervention group improved versus 42% of controls. Magnitude of recovery was significantly greater in the intervention group versus control (12.8 + 8.2 versus mean 3.2 + 3), with >10-fold higher prevalence of anosmia in control versus intervention groups at the 90-day endpoint.
Conclusion: Among individuals with olfactory dysfunction post-COVID-19, combining PEA-LUT with olfactory training resulted in greater recovery of smell than olfactory training alone.
Clinical Trial Registration: (Italian; Clinicaltrials.gov number: NCT04853836)
Keywords: COVID-19, SARS-CoV-2, coronavirus, anosmia, hyposmia, olfactory dysfunction, olfactory training, olfaction, anti-inflammatory
1. INTRODUCTION
Nearly two-thirds of patients with COVID-19 report at least transient anosmia or hyposmia [1-4], and there is growing public health concern regarding COVID-19 chronic olfactory dysfunction (COD). [5] While most of these individuals will spontaneously recover baseline function within weeks [2,3], 10-20% of individuals report persistence of impaired smell or taste long after the acute phase of illness has subsided [3,6]. Persistent loss is particularly common among individuals suffering from post-acute sequelae of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), also term long-haul Covid [7]. The unmet needs of these long haulers are increasingly recognized as a looming public health crisis [8]. Several treatments have been investigated [9], but the results have generally been underwhelming, with treatment recommendations emphasizing safety counseling in anticipation of sustained impairment [10]. To date, the olfactory training is the only disease-specific intervention with consistent evidence of efficacy for treating post-viral olfactory loss, but significant recovery is achieved in fewer than 40% of patients [11, 12]. The rising tide of patients with olfactory loss and the limited treatment options available provide an impetus for novel therapeutic strategies. One potential strategy for achieving recovery of chronic olfactory function involves targeting neuroinflammation.
The rationale for reducing neuroinflammation derives from observations regarding the pathogenesis of COD in patients with COVID. Following SARS-CoV-2 infection, the olfactory bulb and higher olfactory centers demonstrate sustained neuroinflammation [13, 14]. Patients with COVID-19-induced anosmia or hyposmia exhibit activation of pro-inflammatory microglia and associated neuroinflammatory changes along olfactory pathways [11-15]. Although human pathological data are limited, this finding suggests that lingering neuroinflammation can impede olfactory recovery [16]. Olfactory training, which involves repetitive stimulation of peripheral olfactory neurons, relies on the regenerative capacity of superior olfactory pathways [13]. Therefore, counteracting neuroinflammation during olfactory training might support olfactory recovery. Inflammatory changes are potentially more amenable to intervention than permanent neuronal loss, but it is unknown to what extent COVID-19 COD beyond 6 months is reversible.
Although several anti-neuroinflammatory therapies have been proposed for treating disorders of smell and taste [17], clinical data on efficacy of such therapies for COVID-19 COD are lacking. Our study, therefore, investigated a novel anti-neuroinflammatory strategy aimed at improving olfactory function in patients with persistent smell alterations after COVID-19. We hypothesized that administering the neuroprotective and anti-inflammatory agents palmitoylethanolamide (PEA) and luteolin (PEA-LUT) in conjunction with olfactory training would improve olfactory recovery more than placebo plus olfactory training. PEA-LUT has been shown to reduce neuroinflammation by modulating microglia and reducing reactive oxygen species (ROS) [18, 19]. We previously reported results of a feasibility study [19] with a small enrollment and short follow-up period, in which patients tolerated the protocol well and showed improved olfactory recovery at one month.
The objective of this study was to investigate whether the addition of PEA-LUT to olfactory training influences the course of olfactory recovery in patients with post-COVID-19 chronic olfactory dysfunction. In this multicenter double-blinded placebo-controlled randomized trial, patients were administered either PEA-LUT or placebo for 90 consecutive days, and both groups of patients received concomitant olfactory training. Olfactory function was assessed at baseline and 90 days.
2. METHODS
2.1. IRB Approvals and General Considerations
This study adhered to CONSORT guidelines for Clinical Trials (Fig. 1). We performed a multi-center double-blinded placebo-controlled clinical trial. All patients included were recruited, screened, treated, and followed in the otolaryngology clinic of all centers. The study was authorized by the Institutional Review Board of Humanitas University with number 3002 registered at Clinicaltrials.gov in April 2021 with number: 20112020PGFN. It was conducted in accordance with the Declaration of Helsinki. All patients signed a written consent before the inclusion in the study.
Fig. (1).
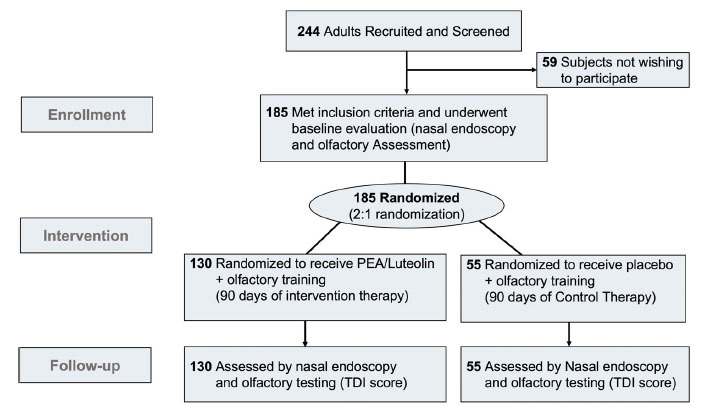
Consort Diagram showing enrollment, intervention, and follow-up of patients who participated in the clinical trial. PEA: Palmitoylethanolamide; TDI: Threshold, Discrimination, and Identification.
2.2. Study Population and Demographic Data
This multicenter double-blinded randomized-clinical trial was conducted in three referral hospitals (Fano, Naples and Sassari) from April 2021 to October 2021. To recruit patients, we used word of mouth communication among clinicians and calls through newspaper, television, and internet (mass media). All centers used the same procedures and protocols. Patients were included or excluded based on the following criteria:
2.2.1. Inclusion Criteria
Outpatients, ages 18 to 80 years, with a confirmed history of COVID-19 (positive nasopharyngeal swab for SARS-CoV-2), and anosmia/hyposmia confirmed with the extended version of the Sniffin’ Sticks psychophysical test (TDI score < 31), persisting ≥ 180 days (6 months) after subsequent negative COVID-19 nasopharyngeal swab and receiving the signature of informed consent with the agreement to participate to the study.
2.2.1. Exclusion Criteria
Previous history of olfactory-gustatory disorders, active chemotherapy or treatment with estrogen inhibitors (aromatase), impaired cognitive function, history of neurodegenerative disease (Alzheimer's and Parkinson's Disease), medical therapy with known detrimental effects on olfactory function, presence of active rhinological disorders (sinusitis, rhinosinusitis, sinonasal polyposis, atrophic rhinitis, allergy) at the moment of the enrollment, history or chemo-radiotherapy of the head and neck region, history of stroke or neurotrauma, severe nasal blockage from stenosis or deformity, severe psychiatric illness (e.g. schizophrenia, bipolar disorder, olfactory hallucination), previous sinonasal or nasopharyngeal tumors, or taking corticosteroid therapy to treat olfactory dysfunction within the previous 30 days. Additionally, any patients who were using medications with anti-inflammatory or immune-modulating effects that could interfere with PeaLut were excluded from the study.
2.3. Demographic Data Extraction
For each patient, the following demographic data were collected: sex, age, major disease, tobacco/alcohol use, medications, prior treatment for olfactory disorder, and time elapsed since negative COVID-19 test. A medical record was created for each patient that included: patient’s general medical and family health history, details on COVID-19 illness (date and symptoms at the onset, date of positive and negative PCR testing, treatments used during the infection, persistent symptoms, treatments used after COVID-19 resolution), information about COVID-19 vaccination. To this medical history record, we added a section for recording detailed data on the identification of smell and taste alterations. After verbally explaining the differences between anosmia, hyposmia, parosmia, and phantosmia to patients, we clarified the difference between taste alteration and sense of smell/flavor. Patients were then queried about their own history. At the end of the study (three months after randomization and initiation of therapy), the data were extracted and analyzed by a statistician, following the procedures stipulated by the study coordinator (ADS).
2.4. Experimental Groups
The two study groups were defined as follows:
2.4.1. Intervention Therapy (Intervention Group)
Daily treatment with PEA-LUT oral supplement and olfactory training. The supplement contained co-ultra-micronized PEA 700 mg and Luteolin 70 mg (Glialia®, Epitech Group SpA, Milano, Italy) and was administered as a single dose, 5-10 minutes before breakfast plus olfactory training. Olfactory training entailed stimulation using four 100% organic essences (Lemon, Rose, Eucalyptus and Cloves) administered three times every day for 6 minutes each session; stimulation consisted in smelling an odor for 4-6 seconds, then 40 seconds of relaxation, and then, new stimulation for 4-6 seconds with another essence. This short duration was used to avoid “saturation” of the olfactory receptors [12]. Subjects performed this regimen for 90 consecutive days.
2.4.2. Conventional Therapy (Control Group)
Daily treatment with placebo and olfactory training. Patients in the control group received olfactory training as noted for the intervention group plus a daily placebo supplement therapy (multivitamin, vitamin D (400 UI), and/or alpha-lipoic acid (120 mg). The 400 UI dosage of vitamin D and 120 mg of alpha-lipoic acid were selected based on an evidence-based literature review documenting that these dosages do not exert significant systemic anti-inflammatory, immunomodulatory, or antioxidant effects [20, 21].
All patients, after adequate training with the physician, performed the olfactory training independently at home (self-administered rehabilitation). The initial training consisted of a face-to-face explanation on how to perform the sniffing exercise, practice performing the exercise, and a written description on how to prepare the sniffing essence. This instruction was reinformed by providing access to an instructional video, which explains in detail how to perform the steps of olfactory training, available on “YouTube” (https://www.youtube.com/watch?v=Ri5YwM6EmWM). The video recommended the clinician was always the same, as previously identified by the study coordinator. Participants in the study were in frequent communication (1 contact every 15 days) with clinic staff and physicians to promote adherence to the study protocol, with interaction via phone calls, electronic communications, and office visits.
All patients (control and intervention) underwent the following assessments at T0 (baseline) and T3 (90 days after treatment):
• Nasal endoscopic examination
• Evaluation of olfactory function using the extended version of the Sniffin’ Sticks test
The T0 nasal endoscopic examination was evaluated for the presence of polyps, masses, anatomic blockage, or other pathology, any of which would result in exclusion from the study. All patients were evaluated for olfactory function by Sniffin’ Sticks (Burghardt®, Medisense, Winschoten, The Netherlands). This initial olfactory evaluation was performed at the outset of the study (T0), before initiating olfactory training, with or without supplement treatment.
Patients in the intervention group were evaluated by Sniffin’ Sticks every 30 days; in this way, we obtained the following observation points: T1, T2, T3. The subjects in the control group were instead re-evaluated after 90 days only because this is the minimum time to observe variation/improvement in the olfactory ability using the olfactory training [12]. This observational point was called T3, because assessments were performed 3 months after treatment. The patients routinely underwent nasal endoscopy before the Sniffin’ sticks assessment to ensure consistency in the therapeutic protocol.
2.5. Assessment of Olfactory Dysfunction
The Sniffin’ Sticks battery was administered following a previously established protocol [18], using standard pen-like devices filled with odorants. Clinicians conducting the scoring were blinded to the experimental group of patients. Three score subtests were conducted to measure olfactory function:
(1) detection threshold (“T”, the lowest concentration at which an odor can be perceived),
(2) odor discrimination (“D”, ability to distinguish between odors), and
(3) odor identification (“I” ability to assign names to odors).
Possible scores ranged from 1–16 for the detection threshold subtest and 0–16 for both the discrimination and identification subtests. Adding these, the subtests yielded a composite threshold/ discrimination/ Identification Sniffin’ Stick score, the “TDI score.” Anosmia was defined as a TDI score of <17, hyposmia by a TDI score 17 to 30.75, and normosmia by a TDI score of ≥31.
The TDI score was calculated based on the patients’ performance on 3 sub-tests. In the first subtest (detection threshold), the odor detection threshold was determined using a triplet option, yes-no staircase, and forced-choice procedure with the odorant n-butanol, as previously described [22]. Briefly, participants were presented with triplets of odorant pens and asked to identify the pen containing n-butanol when presented with two blank distractor pens. In the second subtest (odor discrimination), the discriminative ability was assessed using 16 triplets of odorants: within each triplet, two pens contained the same odorant, while the third pen contained a different odorant. In a forced-choice procedure, the participants were asked to detect the odd pen for each triplet. In the third subtest (odor identification task), participants were presented with 16 common odors. They were asked to select which of 4 odor labels matched the presented odor in multiple-choice answering format. In prior literature, an incremental improvement of 5 points was accepted as the minimum variance for identifying a clinically meaningful response to olfactory training, based on statistical variances in patient outcomes [12]. Therefore, we defined the “recovery score” as <5 points or >5 points to delineate not only mean and median differences between groups but also to compare patients with clinically meaningful recovery.
2.6. Study Outcomes
The primary outcome was the change over the time in TDI scores for the control group versus intervention group. The change in TDI score for any individual was reported as positive if olfaction improved over the 90-day study period, negative if olfaction worsened, and zero if no change, with the caveat that change in TDI score of <5 points from baseline does not necessarily constitute a clinically meaningful difference. The link between months after COVID-19 resolution (based on negative test) and Sniff' score, demographic analysis in relation to olfactory recovery, and examination of the correlation between age and sex in relation to baseline scores or recovery were all secondary outcomes. Clinicians conducting the study were also instructed to alert the principal investigators to any adverse events in patients with attention to intolerance, gastrointestinal symptoms, excessive drowsiness, or heart palpitations.
2.7. Randomization and Blinding
Patients were assigned a number at the time of recruitment, and this reference number was used for tracking during randomization and throughout the study protocol. Using a block randomization within each site, eligible participants were randomly assigned in a 2:1 ratio to achieve approximately twice as many patients allocated to the intervention group as the control group [23, 24]. Randomization with 2:1 (intervention: control) was intended to maximize detection of any adverse effects, assess feasibility across institutions, and reduce the costs of conducting the trial [25-28]. After patient counseling and consent, the physician used computer-generated randomization for the assignment of patients. Participants were assigned to oral supplements plus olfactory training or olfactory training with placebo (Fig. 1). Randomization was performed using an online random number generator, with participants sorted based on a random generation of odd or even numbers until there was a minimum of 55 participants in the control group.
Patients were informed that the purpose of the study was to investigate approaches for treating persistent loss of smell after COVID-19. Patients were informed that after baseline assessment, the treatment would include performing olfactory training and taking a supplement that would remain unknown to them but that had no reported drug interactions or safety-related concerns. Patients were also told that after baseline assessment, they would have a repeat TDI assessment at 90 days, with up to two possible intermediary TDI assessments, with an individual schedule dictated by protocol. Patients were instructed that their participation was voluntary and that they could withdraw from the study at any time.
The study was performed in a double-blinded manner. The patients did not know their status in the control or intervention group (patient-blinding), with both groups receiving treatment with olfactory training and oral supplement. A single physician at each center performed the endoscopy, and a second physician performed olfactory testing. The physician who performed olfactory testing remained blinded to experimental group throughout the study. The physician who performed the nasal endoscopy had knowledge of the experimental groups and did not participate in olfactory assessment. Having these separate physician roles ensured that individuals performing assessments of olfactory recovery were always blinded to the patients’ treatment arm. Patients were blinded throughout the study, as well, and unaware of whether they were in the PEA-LUT or placebo arm of the study. All scoring assessments were performed by a blinded clinician. Data obtained by the olfactory test were anonymized, and results were collected on a protected Excel sheet shared by all the centers [Google (Mountain View, California, USA)].
2.8. Sample Size Calculation
The sample size was calculated as indicated by Wang and Ji [29] and it was specific for Randomized Clinical Trial. The calculation was performed on the https://riskcalc.org. The calculator verified that the proposed sample of 100 in the intervention group and 50 in the control group would suffice to achieve adequate sample size with alpha (α)=0.05 at a statistical power level of 80%, with our design of a superiority trial for answering to our clinical question: “Is daily oral supplementation with ultramicronized PEA-LUT 770 mg plus olfactory training (intervention group) superior to olfactory training with placebo (control) at 90 days based on the assessment of olfactory recovery by TDI testing.” The plan was to enroll to a cutoff of 55 patients in the control group to allow for possible attrition over the course of the study.
2.9. Statistical Analyses
One-way ANOVA for paired measures and Tukey post-hoc (Tph) tests were used to analyze statistical differences in TDI score within the experimental group at T0, T1, T2, T3. Between-group comparisons were performed between control (T0 and T3) and treatment (T0 and T3). These same tests were performed to evaluate any differences by age or sex, at T0 and T3 across groups. Chi-square test was performed to analyze the difference between the control and intervention groups for TDI recovery scores. Point biserial was performed to analyze the effect of sex on recovery scores (difference between T0 and T3). Spearman for correlation between age and recovery score. Finally, we used longitudinal linear regressions to analyze the total effect of the variables (age, sex, comorbidities, and months of olfactory impairment) on the recovery.
Difference in TDI scores from T0 to T3 was defined as “recovery score” and compared between groups. Chi-square test was performed to analyze the difference between control and intervention groups for TDI recovery scores (>5 versus <5) and increase, decrease and unchanged scores. Analysis of association of sex with recovery scores (difference between T0 and T3) was analyzed by point biserial correlation; correlation between age and recovery scores was analyzed with Spearman correlation. Longitudinal linear regressions were performed to analyze the total effect of the variables (TDI baseline, age, sex, comorbidities, and months of olfactory impairment) on the recovery. Cohen’s d test was performed to evaluate the effect of different sample size on the outcome. Chi-Square analysis was used to review the distribution of TDI scores and the likelihood of improvement or worsening over the study period. Shapiro-Wilk test was performed to assess for normal distribution. Results are presented as mean and standard deviation (+).
Statistical significance was set at p<0.05 with two-tailed test. All analyses were performed using Stata®.
3. RESULTS
A total of 185 individuals who met the eligibility criteria were recruited for the study, and all completed the entire course of treatment, with 90-day follow-up (T3). In keeping with the 2:1 target randomization scheme and target recruitment (material and methods section), randomization yielded 130 individuals in the intervention group and 55 individuals in the control group. Groups’ characteristics are shown in Table 1.
Table 1.
Demographic data.
| - | - | Number (%) | - | |
|---|---|---|---|---|
| Characteristics | Total | Treatment | Control | P-value |
| Age, mean (SD) | 43.5 + 14.6 | 42.1 + 14.5 | 47 + 14.6 | 0.04 |
| Sex | - | Number (%) | - | |
| Women | 121 (65.4) | 83 (63.8) | 38 (69) | 0.5 |
| Men | 64 (34.6) | 47 (36.2) | 17 (31) | 0.5 |
| - | - | Mean and SD | - | - |
| Months of olfactory dysfunction | 8.4 + 2.9 | 8.2 + 3 | 8.8 + 2.4 | 0.2 |
| TDI (baseline) | 20 + 8 | 20.6 + 7.9 | 18.3 + 7.9 | 0.7 |
| Comorbidities | Number (%) | |||
| History of nasal Allergies | 4 (3) | 1 (1.8) | 0.7 | |
| Ashma | 1 (0.8) | 1 (1.8) | 0.7 | |
| Thyroid Disease | 5 (3.8) | 3 (5.4) | 0.7 | |
| Diabetes | 1 (0.8) | 1 (1.8) | 0.7 | |
| Hypertension | 8 (6.1) | 5 (9) | 0.7 | |
| Prior systemic Allergy | 3 (2.3) | 3 (5.4) | 0.7 | |
| Multiple Sclerosis | 3 (2.3) | 0 (0) | 0.7 | |
| Dislipidemia | 2 (1.5) | 1 (1.8) | 0.7 | |
| Other | 3 (2.3) | 3 (5.4) | 0.7 | |
All patients had undergone one or more previous treatments for COVID-19 related persistent olfactory disorders: 44.8% (83) patients were treated with nasal steroids only (3 puff /day for 30 days), 7% (13 subjects) with oral steroids (25mg/daily for 7 days, then tapering up to 10 days). The remaining 89 patients (48.2%) performed traditional olfactory training as previously described [11,12] and some of them added oral supplement and vitamins, as described in Table 2. In all patients included in the study, olfactory loss had persisted beyond 6 months (180 days), in keeping with inclusion criteria.
Table 2.
Details about the different treatments previously used in the treatment and control group. The treatments were performed to fight the olfactory loss and were all suspended 30 days at least before the inclusion in the study.
| - | - | Number (%) | - | |
|---|---|---|---|---|
| Previous Treatments for Smell Disorders | Total | Treatment | Control | P-value |
| Cortisone Nasal Spray | 83 (53.5) | 58 (44.8) | 25 (9) | 0.8 |
| Vitamine B | 13 (7) | 10 (7.6) | 3 (5.4) | 0.6 |
| Alpha-Lipoic acid | 11 (5.9) | 8 (6.1) | 3 (5.4) | 0.8 |
| Other | 4 (2.1) | 3 (2.3) | 1 (1.8) | 0.8 |
After 2:1 (intervention: control) randomization, the intervention and control groups were similar in the distribution of sex and comorbidities, with a predominance of women in both groups. None of the patients in the treatment or control group were under treatment with drugs expected to interact with the treatment regimens. PEA-LUT does not have known medication interactions, and no patients were receiving medications with systemic immunomodulant, anti-inflammatory, or antioxidant effects. Mean age was 5 years younger in the intervention group (p=0.04), corresponding to a small effect (Cohen d) on analyses. No patient safety or adverse effects were identified in either group. The data comparison between groups is shown in Figs. (2 and 3).
Fig. (2).
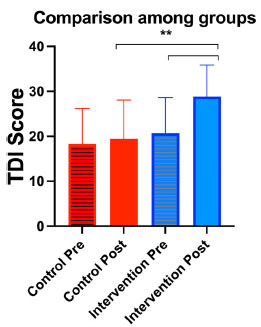
Comparison among groups at the two different observational points. The y axis shows the composite olfactory Threshold, Discrimination, and Identification (TDI) scores of the patients. Standard deviation (SD) is reported. “**” indicates p <0.0001.
Fig. (3).
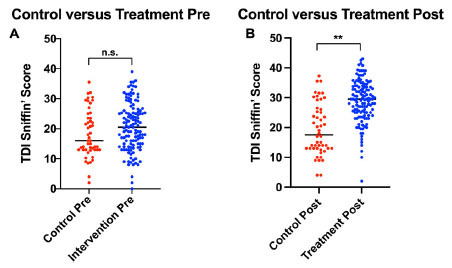
Distribution of the olfactory Threshold, Discrimination, and Identification (TDI) scores and recovery based on the results of the Sniffin’ Sticks test. The graph shows the distribution of the patients’ TDI scores in intervention and control groups. (A) Control group TDI scores at T0 vs. T3 (B) Intervention group TDI scores at T0 vs. T3. Patients in the intervention groups demonstrated improved recovery vs. control, as reflected by increase in TDI scores. “**” indicates p <0.0001. n.s: non statistically significant.
3.1. Intervention Group
The intervention group (n=130) was composed of 64% women and 36% men, with an average age of 42.1 +14.5 years. No differences were observed in the endoscopy findings between T0 and T3. Patients reported either hyposmia or anosmia with mean duration of 8.2 + 3 months (CI 95%: 6-16). Of the 130 patients in the group, from T0 to T3, 120 patients (92%) had improved (higher) TDI score, 9 patients (7%) had worsened (lower) TDI score, and 1 patient (1%) was unchanged. The mean TDI score increased from 20.6 + 7.9 at T0 to 29.8 + 7.5 at T3 (p=0.0001). 80 patients (66.7%) recovered by more than 5 points (mean 12.8 +8.2) of the TDI scores; 40 (33.3%) recovered 5 points or less (mean 3+1.9) (Fig. 4), including 7.8% of patients (10 people) with no recovery or worsening of score (mean point reduction of -3.8 +1.3). TDI scores recovered significantly from T1 to T3 in the intervention group (ANOVA: p< 0.00001). No statistically significant differences were observed between T0 and T1 (Tph: p=0.3), between T1 and T2 (Tph: p=0.2), or T2 and T3 (Tph: p=0.08). Statistically significant differences were observed between T0 and T2 (Tph: p=0.002), T0 and T3 (Tph: p <0.00001), T1 and T3 (Tph: p=0.0001) (Fig. 5).
Fig. (4).
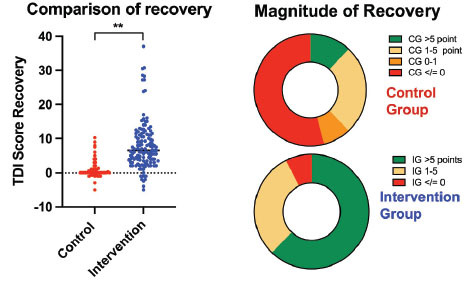
Recovery in Control group (CG) (T3 -T0) vs. Intervention group (IG) (T3 -T0). Patients in the intervention group demonstrated improved recovery vs. patients in the control group, as reflected by increase in Threshold Detection Identification (TDI) scores. “**” indicates p <0.0001. Patients in the intervention group had a larger recovery compared to patients in the control group, as shows by magnitude of recovery.
Fig. (5).
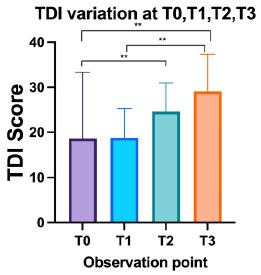
Variation of the Threshold Detection Identification (TDI) scores in the intervention group at T1, T2 and T3, at 30 days, 60 days, and 90 days respectively. “**” indicates p < 0.01.
No statistically significant differences were observed by sex (Point Biserial: p=0.49); however, both women and men each showed statically significant recovery from T0 to T3 (ANOVA: p=0.0001). Age was also not correlated with smell recovery outcomes (Spearman: p=0.12). Prolonged olfactory dysfunction was positively statistically correlated with improvement after treatment (Spearman: p=0.00644).
Multiple regression analyses showed that only TDI at the baseline affected the recovery scores (p<0.0001). None of the other variables influenced the recovery scores.
3.2. Control Group
The control group (n=55) included 69% women and 31% men, with average age of 47 + 14.6 years. No differences were observed in the endoscopy findings between T0 and T3. Mean duration of hyposmia or anosmia was 8.8 + 2.4 months (CI 95%: 6-12). Mean TDI scores did not differ from T0 (18.2 + 7.9) to T3 (19.5 + 7.3) (p=0.4). Within the group, 23 patients (42%) had an increase in the TDI score, 12 patients had a decrease in the TDI score (mean -1.3 + -1.4) and 20 (36%) were unchanged. Among those with an increase in TDI score (mean 3.25 + 3), 17 patients (73.9%) recovered 5 points or less (mean 1.8 + 1.5) and 6 patients (26.1%) had a mean recovery >5 points (8.1 + 1.6) (Fig. 4). No statistically significant differences were identified in demographic analyses.
Multiple regression analyses showed that only the number of months of olfactory disorder influenced the recovery scores (p=0.01).
3.3. Groups Comparison
The comparison among the groups showed statistically significant differences based on pre-and post- TDI scores (ANOVA: p < 0.00001) (F=13.23). No significant differences in TDI scores existed between the groups at baseline, T0 (Tph: p=0.7), but significant differences in TDI scores were present at the 90-day experimental endpoint, T3 (p <0.00001) (Figs. 4 and 6). Chi-square showed statistically significant differences (p <0.00001) in the likelihood of recovery to normal TDI score (>31) at T3, favoring the intervention group over control group, 56% and 10% respectively. Similar statistical differences were observed in “increase, decrease, or unchanged” TDI score among the groups. For analyses of the impact of the different sample sizes (control versus intervention), Cohen’s d was 0.8 (CI95%: 1.046-1.763) pre-treatment and 0.8 (CI 95%: -2.163 to -1.41) post-treatment. This result indicated that the different sample sizes did not affect the difference observed between the groups at T0 and T3.
Fig. (6).
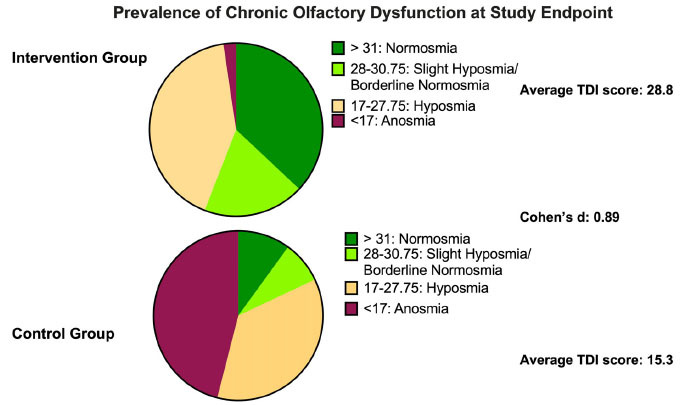
Distribution of patients in the different categories of olfactory impairment as identified by Threshold/Detection/Identification (TDI) score after treatment in the two groups. In the intervention group, a higher percentage of patients recovered normal olfactory function (green) after three months of therapy; the percentage with recovery in the control group was lower. Considering also slight hyposmia/borderline normosmia (bright green) patients in the intervention groups recovered in over 50% of cases, whereas in the control group, fewer than 20% patients exhibited a similar recovery.
4. DISCUSSION
Although persistent olfactory dysfunction affects a growing number of patients with Long-haul COVID, progress in identifying effective therapeutic strategies has been limited. Several therapies, including administration of oral or topical corticosteroids, phosphodiesterase inhibitors, intranasal calcium buffers, and other treatments, are under study, but evidence of benefit is thus far insufficient to guide clinical care [11]. Treatments generally target COVID-19 smell impairment arising from either peripheral damage (injury to nasal neuroepithelium), central inflammation (injury to olfactory bulbs or higher olfactory centers), or both [29]. In this trial, individuals receiving PEA-LUT during olfactory training had a significantly higher likelihood of recovering olfactory function than those receiving placebo; an increase in TDI score was observed in 92.2 percent of those receiving PEA-LUT versus only 42 percent of those receiving placebo, with significantly lower rates of residual anosmia in the PEA-LUT group.
Looking specifically at the quality of the recovery, 56% of patients in the interventional group recovered to a normal TDI (>31) versus 10% only in control (Figs. 4 and 6). Regarding TDI recovery scores, the difference between intervention and control groups was wide, both in terms of likelihood of improvement and magnitude of improvement, as measured by a gain of over > 5 points (66.7% treatment versus 26.1% control). In the control group, the instances of recovery > 5 points could be related to the beneficial effect of olfactory training as previously reported by other authors [11, 12], to a placebo effect, actual benefit of placebo supplements, or a chance result. Alpha lipoic acid has antioxidant properties and plausibly could confer beneficial effects, as could other supplements; however, the placebo used has not been shown to have immunomodulant, anti-inflammatory, or antioxidant effects in the dosages administered [30]. Future work might consider a more inert placebo to remove the possible confounder of subclinical effects.
In the interventional group, some patients (<10%) failed to improve or even had a worsening TDI scores; this outcome could be related to lack of efficacy, nonadherence, permanent injury to olfactory structures, non-neuroinflammatory cause of olfactory loss, or variable pharmacokinetics of PEA-LUT metabolism among patients [31]. Further work is necessary to understand whether a different dosing regimen could benefit these patients. We are currently investigating whether increasing PEA-LUT dosage improves TDI in patients who had only slight recovery and significant residual olfactory loss. For the intervention groups, TDI changes were assessed monthly to guide future research endeavors. We observed that a trend toward efficacy appeared within 30 days of use but was not significant until 60 days, with maximal efficacy after 90 days. This observation raises the question of duration of treatment -- whether therapy extending beyond 90 days might achieve complete recovery and whether benefits persist after treatment cessation. At the end of the therapy, only 56% of patients recovered normal TDI, so additional studies extending the time of PEA-LUT administration over 6 months should be performed. In addition, recovery in the control group was limited during the 90-day period studied, suggesting not only a lack of efficacy of the control treatments but also a lack of spontaneous improvement in patients with post-COVID-19 olfactory impairment that exceeds 6 months’ duration.
The mean age of patients was in the 40’s for both groups, and this relatively young population may have responded better to therapy than older populations for several reasons such as, i) the level of neuroinflammation is thought to be lower in younger individuals than in elderly individuals; ii) senescence of olfactory neurons and age-related olfactory loss is not present in this age range [32]; and iii) neuroregenerative capacity is greater at younger ages [33, 34]. The young age of this cohort limits the generalizability of these findings, and additional studies on elderly patients are necessary to evaluate the effectiveness of interventions for chronic olfactory dysfunction. We also observed that patients affected by the smell alteration for a longer duration (>12 months) were equally likely to benefit from the PEA-LUT treatment, suggesting a role for intervention even after several months of refractory olfactory loss. Many patients had received olfactory training, nasal steroids, or other treatments prior to enrolling in the study at T0, which may explain why further olfactory training with placebo was associated with little improvement in mean scores. An unexpected finding was that the duration of anosmia was positively correlated with olfactory recovery. This result differs from prior studies, which have shown a decrease in the likelihood of recovery of smell over time [10]. Recovery from COD would be unlikely if anosmia were solely attributable to permanent neuronal death rather than neuroinflammation and SARS-CoV-2 related olfactory loss.
The link between olfactory loss and inflammatory neurodegenerative disorders is well documented, and the effects of PEA-LUT in COVID-19 merit future investigation. The role of neuroinflammatory response in frontotemporal dementia is supported by recent studies linking genetic mutations in microglial activation and risk of dementia [35, 36]. Some studies have speculated that COD may portend future emergence of neurodegenerative disease [37, 38], but it will be years before such associations can be assessed. Nonetheless, the available data support the rationale for further study of PEA-LUT in this context. Assays of serum and cerebrospinal fluid of patients with dementia reveal elevated cytokines and pro-inflammatory markers [39]. Pre-clinical studies document that PEA-LUT achieves neuroprotection through control of neuroinflammation [40, 41], and clinical studies suggest that the components of PEA-LUT can suppress neuroinflammatory dysregulation, activation of microglia and astrocytes, and severity of neuroinflammatory diseases such as Alzheimer's disease, Parkinson's disease, and frontolateral dementia, [42-44]. Further studies are needed to understand whether targeting or modulating neuroinflammatory pathways might allow for partial reversal of COD.
Studies of the olfactory system demonstrate that after SARS-CoV-2 enters the olfactory neuroepithelium, it can spread to the olfactory bulb and other areas of the central nervous system [45]. Even after recovery from acute infection, SARS-CoV-2 can persist in the olfactory bulbs of patients, contributing to prolonged olfactory deficits [46]. D’Ascanio et al. [3] reported persistent olfactory alteration in approximately 14% of individuals, although prevalence varies across studies [47]. Patients with altered smell may report headache or brain fog [5, 48-50], findings suggest brain inflammation as a common mediator of symptoms [51]. Studies with MRI have also documented inflammatory alterations to olfactory bulbs with COVID-19 [52, 53].
Such observations are consistent with an integrated explanation involving neuroinflammation. SARS-CoV-2 induces pro-inflammatory microglia within the olfactory bulb [53], diffuse neuro-inflammation [46, 53], and spread to other parts of the brain [54] contributing to the constellation of neurological symptoms comprising long-COVID [6, 48]. The administration of PEA-LUT was intended to support regeneration during olfactory training by reducing degree of SARS-CoV-2 induced neuroinflammation [44, 55]. The PEA component modulates the polarization of microglia to M2 protective phenotype [56], supporting neural regeneration and recovery of smell. Luteolin, in turn, blocks the polarization of pro-inflammatory microglia, inhibiting neural cell degeneration [43]. Reducing neuro-inflammation with PEA-LUT may explain the differences in the outcomes observed among the groups if PEA-LUT complements olfactory training to improve regenerative capacity and neuroplasticity of the olfactory bulb [18].
Very few randomized clinical trials on therapeutics for olfactory loss are available. To our knowledge, this trial is the first to systematically investigate oral supplements combined with olfactory training for post-Covid-19 anosmia or hyposmia. The literature reports the efficacy of olfactory training for treating other post-viral infection smell disorders [57, 58], but few data are specific to COVID-19. Most therapeutic clinical trials treating smell alteration in COVID-19 have focused on topical applications, primarily corticosteroid nasal sprays [59, 60].
Although the results of this clinical trial are consistent with the hypothesis of a potential anti-neuroinflammatory effect of PEA-LUT, no evaluation of inflammatory biomarkers (blood/urine) was performed in this study. Therefore, the mechanism of olfactory recovery observed remains speculative and awaits further investigation.
5. LIMITATIONS OF THE STUDY
Several limitations should be considered when assessing the significance and generalizability of these findings. We hypothesized that neuroinflammation underlies persistent olfactory loss in this population, but we made no assessment of baseline or post-treatment neuroinflammation with biomarkers, nor did we establish dose-response relationships or conduct pharmacokinetic analyses; further work might incorporate neuroimaging, serological measures of inflammation, and detailed assessment of optimal dosing regimens. Second, since both groups received olfactory training, we cannot discern whether improvement in TDI scores in the intervention group was attributable to PEA-LUT alone or interaction with olfactory training, nor did we collect data on either qualitative disorders of smell (parosmia, phantosmia) or disorders of taste. Third, although the unequal randomization scheme increased the opportunities to detect any untoward effects and potential for therapeutic benefit, it added complexity to allocation with a small control versus intervention group and lower statistical power; the failure to detect any demographic associations should be interpreted accordingly. Also, although the mean age of both groups was young, the lower mean age in the intervention group may have improved response to therapy and increased the likelihood of spontaneous improvement. Fourth, patients in the placebo group were only re-evaluated by Sniffin’ Stick after 90 days, because this interval corresponds to the suggested minimum time for assessing the efficacy of treatments aimed at promoting olfactory recovery [11, 12]. To mitigate potential bias relating to the differential assessment schedule, we asked all patients to complete a diary of their treatment, and both groups had regular contact with the research team through follow-up calls and emails. Last, despite a strong treatment effect, the study had heterogeneity in outcomes within each group and had limited follow-up of 90 days, so it is unknown whether the adherence to regimen and recovery would be sustained with a longer duration of therapy. Future work investigating longer durations of therapy may benefit from tracking adherence at standard intervals, although frequent team interactions with subjects prevented attrition during the present study.
CONCLUSION
This randomized trial found that oral supplementation of PEA-LUT, when combined with the olfactory training, is associated with improved olfactory recovery compared to olfactory training with placebo. PEA-LUT should be used for 60 days at least to prove statistically significant efficacy. The multimodal approach is intended to reduce neuroinflammation within the olfactory system and create a regenerative milieu conducive to olfactory recovery. The observation of olfactory recovery in patients with olfactory loss in excess of 12 months suggests that some SARS-CoV-2 related injury is reversible long after the acute illness has subsided. Further longitudinal studies are necessary to clarify the optimal timing and dosing parameters for patients with limited or absent recovery from COVID-19 associated olfactory loss, and to evaluate the effect of the molecule on neuroinflammation using specific neuroinflammatory biomarkers.
ACKNOWLEDGEMENTS
We are grateful to Dr. Francesco Della Valle.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was authorized by the Institutional Review Board of Humanitas University with number 3002 registered at Clinicaltrials.gov in April 2021 with number: 20112020PGFN.
HUMAN AND ANIMAL RIGHTS
No animals were used in this study. All human procedures were followed in accordance with the Declaration of Helsinki.
CONSENT FOR PUBLICATION
All patients signed a written consent before their inclusion in the study.
STANDARDS OF REPORTING
CONSORT guidelines were followed for the study.
AVAILABILITY OF DATA AND MATERIALS
The data that support the findings of this study are available from the corresponding authors upon request.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Tong J.Y., Wong A., Zhu D., Fastenberg J.H., Tham T. The Prevalence of olfactory and gustatory dysfunction in COVID-19 patients: A systematic review and meta-analysis. Otolaryngol. Head Neck Surg. 2020;163(1):3–11. doi: 10.1177/0194599820926473. [DOI] [PubMed] [Google Scholar]
- 2.Gerkin R.C., Ohla K., Veldhuizen M.G., Joseph P.V., Kelly C.E., Bakke A.J., Steele K.E., Farruggia M.C., Pellegrino R., Pepino M.Y., Bouysset C., Soler G.M., Pereda-Loth V., Dibattista M., Cooper K.W., Croijmans I., Di Pizio A., Ozdener M.H., Fjaeldstad A.W., Lin C., Sandell M.A., Singh P.B., Brindha V.E., Olsson S.B., Saraiva L.R., Ahuja G., Alwashahi M.K., Bhutani S., D’errico A., Fornazieri M.A., Golebiowski J., Dar H.L., Öztürk L., Roura E., Spinelli S., Whitcroft K.l., Faraji F., Fischmeister F.P.S., Heinbockel T., Hsieh J.W., Huart C., Konstantinidis I., Menini A., Morini G., Olofsson J.K., Philpott C.M., Pierron D., Shields V.D.C., Voznessenskaya V.V., Albayay J., Altundag A., Bensafi M., Bock M.A., Calcinoni O., Fredborg W., Laudamiel C., Lim J., Lundström J.N., Macchi A., Meyer P., Moein S.T., Santamaría E., Sengupta D., Rohlfs D.P., Yanik H., Hummel T., Hayes J.E., Reed D.R., Niv M.Y., Munger S.D., Parma V. Gccr Group Author. Recent smell loss is the best predictor of Covid-19 Among Individuals With Recent Respiratory Symptoms. Chem. Senses. 2021;46:Bjaa081. doi: 10.1093/chemse/bjaa081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.D’Ascanio L., Pandolfini M., Cingolani C., Latini G., Gradoni P., Capalbo M., Frausini G., Maranzano M., Brenner M.J., Di Stadio A. Olfactory Dysfunction in COVID-19 Patients: Prevalence and Prognosis for Recovering Sense of Smell. Otolaryngol. Head Neck Surg. 2021;164(1):82–86. doi: 10.1177/0194599820943530. [DOI] [PubMed] [Google Scholar]
- 4.Mercante G., Ferreli F., De Virgilio A., Gaino F., Di Bari M., Colombo G., Russo E., Costantino A., Pirola F., Cugini G., Malvezzi L., Morenghi E., Azzolini E., Lagioia M., Spriano G. Prevalence of taste and smell dysfunction in coronavirus disease 2019. JAMA Otolaryngol. Head Neck Surg. 2020;146(8):723–728. doi: 10.1001/jamaoto.2020.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khan A., Kallogjeri D., Piccirillo J. Growing public health concern of Covid-19 chronic olfactory dysfunction. JAMA Otolaryngol. Head Neck Surg. 2021 doi: 10.1001/jamaoto.2021.3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sampaio Rocha-Filho P.A., Voss L. Persistent headache and persistent anosmia associated with COVID-19. Headache. 2020;60(8):1797–1799. doi: 10.1111/head.13941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vaira L.A., Hopkins C., Petrocelli M., Lechien J.R., Cutrupi S., Salzano G., Chiesa-Estomba C.M., Saussez S., De Riu G. Efficacy of corticosteroid therapy in the treatment of long- lasting olfactory disorders in COVID-19 patients. Rhinology. 2021;59(1):21–25. doi: 10.4193/Rhin20.515. [DOI] [PubMed] [Google Scholar]
- 8.Burges Watson D.L., Campbell M., Hopkins C., Smith B., Kelly C., Deary V. Altered smell and taste: Anosmia, parosmia and the impact of long Covid-19. PLoS One. 2021;16(9):E0256998. doi: 10.1371/journal.pone.0256998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Phillips S., Williams M.A. Confronting our next national health disaster - long-haul Covid. N. Engl. J. Med. 2021;385(7):577–579. doi: 10.1056/NEJMp2109285. [DOI] [PubMed] [Google Scholar]
- 10.Whitcroft K.L., Hummel T. Olfactory dysfunction in COVID-19: Diagnosis and management. JAMA. 2020;323(24):2512–2514. doi: 10.1001/jama.2020.8391. [DOI] [PubMed] [Google Scholar]
- 11.Levy J.M. Treatment recommendations for persistent smell and taste dysfunction following COVID-19-the coming deluge. JAMA Otolaryngol. Head Neck Surg. 2020;146(8):733. doi: 10.1001/jamaoto.2020.1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sorokowska A., Drechsler E., Karwowski M., Hummel T. Effects of olfactory training: A meta-analysis. Rhinology. 2017;55(1):17–26. doi: 10.4193/Rhino16.195. [DOI] [PubMed] [Google Scholar]
- 13.Cecchetto C., Di Pizio A., Genovese F., Calcinoni O., Macchi A., Dunkel A., Ohla K., Spinelli S., Farruggia M.C., Joseph P.V., Menini A., Cantone E., Dinnella C., Cecchini M.P., D’Errico A., Mucignat-Caretta C., Parma V., Dibattista M. Assessing the extent and timing of chemosensory impairments during COVID-19 pandemic. Sci. Rep. 2021;11(1):17504. doi: 10.1038/s41598-021-96987-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laurendon T., Radulesco T., Mugnier J., Gérault M., Chagnaud C., El Ahmadi A.A., Varoquaux A. Bilateral transient olfactory bulb edema during COVID-19-related anosmia. Neurology. 2020;95(5):224–225. doi: 10.1212/WNL.0000000000009850. [DOI] [PubMed] [Google Scholar]
- 15.Stoyanov G.S., Petkova L., Dzhenkov D.L., Sapundzhiev N.R., Todorov I. Gross and Histopathology of COVID-19 With First Histology Report of Olfactory Bulb Changes. Cureus. 2020;12(12):e11912. doi: 10.7759/cureus.11912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee M.H., Perl D.P., Nair G., Li W., Maric D., Murray H., Dodd S.J., Koretsky A.P., Watts J.A., Cheung V., Masliah E., Horkayne-Szakaly I., Jones R., Stram M.N., Moncur J., Hefti M., Folkerth R.D., Nath A. Microvascular injury in the brains of patients with Covid-19. N. Engl. J. Med. 2021;384(5):481–483. doi: 10.1056/NEJMc2033369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khani E., Khiali S., Beheshtirouy S., Entezari-Maleki T. Potential pharmacologic treatments for COVID-19 smell and taste loss: A comprehensive review. Eur. J. Pharmacol. 2021;5912:174582. doi: 10.1016/j.ejphar.2021.174582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Skaper S.D., Facci L., Giusti P. Glia and mast cells as targets for palmitoylethanolamide, an anti-inflammatory and neuroprotective lipid mediator. Mol. Neurobiol. 2013;48(2):340–352. doi: 10.1007/s12035-013-8487-6. [DOI] [PubMed] [Google Scholar]
- 19.D’Ascanio L., Vitelli F., Cingolani C., Maranzano M., Brenner M.J., Di Stadio A. Randomized clinical trial “olfactory dysfunction after COVID-19: Olfactory rehabilitation therapy vs. intervention treatment with Palmitoylethanolamide and Luteolin”: Preliminary results. Eur. Rev. Med. Pharmacol. Sci. 2021;25(11):4156–4162. doi: 10.26355/eurrev_202106_26059. [DOI] [PubMed] [Google Scholar]
- 20.Balvers M.G., Brouwer-Brolsma E.M., Endenburg S., de Groot L.C., Kok F.J., Gunnewiek J.K. Recommended intakes of vitamin D to optimise health, associated circulating 25-hydroxyvitamin D concentrations, and dosing regimens to treat deficiency: Workshop report and overview of current literature. J. Nutr. Sci. 2015;4:e23. doi: 10.1017/jns.2015.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salehi B., Berkay Yılmaz Y., Antika G., Boyunegmez Tumer T., Fawzi Mahomoodally M., Lobine D., Akram M., Riaz M., Capanoglu E., Sharopov F., Martins N., Cho W.C., Sharifi-Rad J. Insights on the use of α-lipoic acid for therapeutic purposes. Biomolecules. 2019;9(8):356. doi: 10.3390/biom9080356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.D’Ascanio L., Vitelli F., Cingolani C., Maranzano M., Brenner M.J., Di Stadio A. Randomized clinical trial “olfactory dysfunction after COVID-19: Olfactory rehabilitation therapy vs. intervention treatment with Palmitoylethanolamide and Luteolin”: Preliminary results. Eur. Rev. Med. Pharmacol. Sci. 2021;25(11):4156–4162. doi: 10.26355/eurrev_202106_26059. [DOI] [PubMed] [Google Scholar]
- 23.Kuznetsova O.M., Tymofyeyev Y. Preserving the allocation ratio at every allocation with biased coin randomization and minimization in studies with unequal allocation. Stat. Med. 2012;31(8):701–723. doi: 10.1002/sim.4447. [DOI] [PubMed] [Google Scholar]
- 24.Palmer C.R., Rosenberger W.F. Ethics and practice: Alternative designs for phase III randomized clinical trials. Control. Clin. Trials. 1999;20(2):172–186. doi: 10.1016/S0197-2456(98)00056-7. [DOI] [PubMed] [Google Scholar]
- 25.Dumville J.C., Hahn S., Miles J.N., Torgerson D.J. The use of unequal randomisation ratios in clinical trials: A review. Contemp. Clin. Trials. 2006;27(1):1–12. doi: 10.1016/j.cct.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 26.Torgerson D., Campbell M. Unequal randomisation can improve the economic efficiency of clinical trials. J. Health Serv. Res. Policy. 1997;2(2):81–85. doi: 10.1177/135581969700200205. [DOI] [PubMed] [Google Scholar]
- 27.Peckham E., Brabyn S., Cook L., Devlin T., Dumville J., Torgerson D.J. The use of unequal randomisation in clinical trials-An update. Contemp. Clin. Trials. 2015;45(Pt A):113–122. doi: 10.1016/j.cct.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 28.Dibao-Dina C., Caille A., Sautenet B., Chazelle E., Giraudeau B. Rationale for unequal randomization in clinical trials is rarely reported: A systematic review. J. Clin. Epidemiol. 2014;67(10):1070–1075. doi: 10.1016/j.jclinepi.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 29.Cantone E., Ricciardiello F., Cuofano R., Castagna G., Oliva F., Sequino G., Abate T., Villani R., Iengo M. The human sense of smell. Transl. Med. Reports. 2017;1:2. [Google Scholar]
- 30.Shay K.P., Moreau R.F., Smith E.J., Smith A.R., Hagen T.M. Alpha-lipoic acid as a dietary supplement: Molecular mechanisms and therapeutic potential. Biochim. Biophys. Acta. 2009;1790(10):1149–1160. doi: 10.1016/j.bbagen.2009.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alpers D.H. Vitamins as drugs: The importance of pharmacokinetics in oral dosing. Curr. Opin. Gastroenterol. 2011;27(2):146–151. doi: 10.1097/MOG.0b013e32834172c0. [DOI] [PubMed] [Google Scholar]
- 32.Boyce J.M., Shone G.R. Effects of ageing on smell and taste. Postgrad. Med. J. 2006;82(966):239–241. doi: 10.1136/pgmj.2005.039453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hess J.R., Brenner M.J., Myckatyn T.M., Hunter D.A., Mackinnon S.E. Influence of aging on regeneration in end-to-side neurorrhaphy. Ann. Plast. Surg. 2006;57(2):217–222. doi: 10.1097/01.sap.0000215258.57614.89. [DOI] [PubMed] [Google Scholar]
- 34.Xie Y., Schneider K.J., Ali S.A., Hogikyan N.D., Feldman E.L., Brenner M.J. Current landscape in motoneuron regeneration and reconstruction for motor cranial nerve injuries. Neural Regen. Res. 2020;15(9):1639–1649. doi: 10.4103/1673-5374.276325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barresi M., Ciurleo R., Giacoppo S., Foti Cuzzola V., Celi D., Bramanti P., Marino S. Evaluation of olfactory dysfunction in neurodegenerative diseases. J. Neurol. Sci. 2012;323(1-2):16–24. doi: 10.1016/j.jns.2012.08.028. [DOI] [PubMed] [Google Scholar]
- 36.Doty R.L. Olfactory dysfunction in Parkinson disease. Nat. Rev. Neurol. 2012;8(6):329–339. doi: 10.1038/nrneurol.2012.80. [DOI] [PubMed] [Google Scholar]
- 37.Doty R.L. The mechanisms of smell loss after SARS-CoV-2 infection. Lancet Neurol. 2021;20(9):693–695. doi: 10.1016/S1474-4422(21)00202-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xydakis M.S., Albers M.W., Holbrook E.H., Lyon D.M., Shih R.Y., Frasnelli J.A., Pagenstecher A., Kupke A., Enquist L.W., Perlman S. Post-viral effects of COVID-19 in the olfactory system and their implications. Lancet Neurol. 2021;20(9):753–761. doi: 10.1016/S1474-4422(21)00182-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sjögren M., Folkesson S., Blennow K., Tarkowski E. Increased intrathecal inflammatory activity in frontotemporal dementia: Pathophysiological implications. J. Neurol. Neurosurg. Psychiatry. 2004;75(8):1107–1111. doi: 10.1136/jnnp.2003.019422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paterniti I., Cordaro M., Campolo M., Siracusa R., Cornelius C., Navarra M., Cuzzocrea S., Esposito E. Neuroprotection by association of palmitoylethanolamide with luteolin in experimental Alzheimer’s disease models: The control of neuroinflammation. CNS Neurol. Disord. Drug Targets. 2014;13(9):1530–1541. doi: 10.2174/1871527313666140806124322. [DOI] [PubMed] [Google Scholar]
- 41.Caltagirone C., Cisari C., Schievano C., Di Paola R., Cordaro M., Bruschetta G., Esposito E., Cuzzocrea S. Co-ultramicronized palmitoylethanolamide/luteolin in the treatment of cerebral ischemia: From rodent to man. Transl. Stroke Res. 2016;7(1):54–69. doi: 10.1007/s12975-015-0440-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Assogna M., Casula E.P., Borghi I., Bonnì S., Samà D., Motta C., Di Lorenzo F., D’Acunto A., Porrazzini F., Minei M., Caltagirone C., Martorana A., Koch G. Effects of palmitoylethanolamide combined with luteoline on frontal lobe functions, high frequency oscillations, and GABAergic transmission in patients with frontotemporal dementia. J. Alzheimers Dis. 2020;76(4):1297–1308. doi: 10.3233/JAD-200426. [DOI] [PubMed] [Google Scholar]
- 43.Kempuraj D., Thangavel R., Kempuraj D.D., Ahmed M.E., Selvakumar G.P., Raikwar S.P., Zaheer S.A., Iyer S.S., Govindarajan R., Chandrasekaran P.N., Zaheer A. Neuroprotective effects of flavone luteolin in neuroinflammation and neurotrauma. Biofactors. 2021;47(2):190–197. doi: 10.1002/biof.1687. [DOI] [PubMed] [Google Scholar]
- 44.Cordaro M., Cuzzocrea S., Crupi R. An update of palmitoylethanolamide and luteolin effects in preclinical and clinicalstudies of neuroinflammatory events. Antioxidants. 2020;9(3):216. doi: 10.3390/antiox9030216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meinhardt J., Radke J., Dittmayer C., Franz J., Thomas C., Mothes R., Laue M., Schneider J., Brünink S., Greuel S., Lehmann M., Hassan O., Aschman T., Schumann E., Chua R., Conrad C., Eils R., Stenzel W., Windgassen M., Rößler L., Goebel H.H., Gelderblom H.R., Martin H., Nitsche A., Schulz-Schaeffer W.J., Hakroush S., Winkler M.S., Tampe B., Scheibe F., Körtvélyessy P., Reinhold D., Siegmund B., Kühl A.A., Elezkurtaj S., Horst D., Oesterhelweg L., Tsokos M., Ingold-Heppner B., Stadelmann C., Drosten C., Corman V.M., Radbruch H., Heppner Fl. Olfactory transmucosal Sars-Cov-2 invasion as a port of central nervous system entry in individuals with Covid-19. Nat. Neurosci. 2021;24:168–175. doi: 10.1038/s41593-020-00758-5. [DOI] [PubMed] [Google Scholar]
- 46.De Melo Gd., Lazarini F., Levallois S., Hautefort C., Michel V., Larrous F., Verillaud B., Aparicio C., Wagner S., Gheusi G., Kergoat L., Kornobis E., Donati F., Cokelaer T., Hervochon R., Madec Y., Roze E., Salmon D., Bourhy H., Lecuit M., Lledo Pm. Covid-19-related anosmia is associated with viral persistence and inflammation in human olfactory epithelium and brain infection in hamsters. Sci. Transl. Med. 2021;13(596):Eabf8396. doi: 10.1126/scitranslmed.abf8396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.La V., Hopkins C., Petrocelli M. Lechien, Jr.; Chiesa-Estomba, Cm.; Salzano, G.; Cucurullo, M.; Salzano, Fa; Saussez, S.; Boscolo-Rizzo, P.; Biglioli, F.; De Riu, G. Smell and taste recovery in coronavirus disease 2019 patients: A 60-day objective and prospective study. J. Laryngol. Otol. 2020;134(8):703–709. doi: 10.1017/S0022215120001826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cocco A., Amami P., Desai A., Voza A., Ferreli F., Albanese A. Neurological features in SARS-CoV-2-infected patients with smell and taste disorder. J. Neurol. 2021;268(5):1570–1572. doi: 10.1007/s00415-020-10135-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paterson R.W., Brown R.L., Benjamin L., Nortley R., Wiethoff S., Bharucha T., Jayaseelan D.L., Kumar G., Raftopoulos R.E., Zambreanu L., Vivekanandam V., Khoo A., Geraldes R., Chinthapalli K., Boyd E., Tuzlali H., Price G., Christofi G., Morrow J., McNamara P., McLoughlin B., Lim S.T., Mehta P.R., Levee V., Keddie S., Yong W., Trip S.A., Foulkes A.J.M., Hotton G., Miller T.D., Everitt A.D., Carswell C., Davies N.W.S., Yoong M., Attwell D., Sreedharan J., Silber E., Schott J.M., Chandratheva A., Perry R.J., Simister R., Checkley A., Longley N., Farmer S.F., Carletti F., Houlihan C., Thom M., Lunn M.P., Spillane J., Howard R., Vincent A., Werring D.J., Hoskote C., Jäger H.R., Manji H., Zandi M.S. The emerging spectrum of COVID-19 neurology: Clinical, radiological and laboratory findings. Brain. 2020;143(10):3104–3120. doi: 10.1093/brain/awaa240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kanberg N., Ashton N.J., Andersson L.M., Yilmaz A., Lindh M., Nilsson S., Price R.W., Blennow K., Zetterberg H., Gisslén M. Neurochemical evidence of astrocytic and neuronal injury commonly found in COVID-19. Neurology. 2020;95(12):e1754–e1759. doi: 10.1212/WNL.0000000000010111. [DOI] [PubMed] [Google Scholar]
- 51.Dossantos M.F., Devalle S., Aran V., Capra D., Roque N.R., Coelho-Aguiar J.M., Spohr T., Subilhaga J.G., Pereira C.M., D’andrea Meira I., Niemeyer S.F.P., Moura-Neto V. Neuromechanisms Of Sars-Cov-2: A Review. Front. Neuroanat. 2020;16:37. doi: 10.3389/fnana.2020.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kandemirli S.G., Altundag A., Yildirim D., Tekcan Sanli D.E., Saatci O. Olfactory bulb MRI and paranasal sinus CT findings in persistent COVID-19 anosmia. Acad. Radiol. 2021;28(1):28–35. doi: 10.1016/j.acra.2020.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aragão M.F.V.V., Leal M.C., Cartaxo Filho O.Q., Fonseca T.M., Valença M.M. Anosmia in COVID-19 associated with injury to the olfactory bulbs evident on MRI. AJNR Am. J. Neuroradiol. 2020;41(9):1703–1706. doi: 10.3174/ajnr.A6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morbini P., Benazzo M., Verga L., Pagella F.G., Mojoli F., Bruno R., Marena C. Ultrastructural evidence of direct viral damage to the olfactory complex in patients testing positive for SARS-CoV-2. JAMA Otolaryngol. Head Neck Surg. 2020;146(10):972–973. doi: 10.1001/jamaoto.2020.2366. [DOI] [PubMed] [Google Scholar]
- 55.De Luca P., Scarpa A., Ralli M., Tassone D., Simone M., De Campora L., Cassandro C., Di Stadio A. Auditory disturbances and Sars-Cov-2 infection: Brain inflammation or cochlear affection? Systematic Review and Discussion Of Potential Pathogenesis. Front Neurol. 2021;12:707207. doi: 10.3389/fneur.2021.707207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guida F., Luongo L., Boccella S., Giordano M.E., Romano R., Bellini G., Manzo I., Furiano A., Rizzo A., Imperatore R., Iannotti F.A., D’Aniello E., Piscitelli F., Sca R.F., Cristino L., Di Marzo V., de Novellis V., Maione S. Palmitoylethanolamide induces microglia changes associated with increased migration and phagocytic activity: Involvement of the CB2 receptor. Sci. Rep. 2017;7(1):375. doi: 10.1038/s41598-017-00342-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu D.T., Sabha M., Damm M., Philpott C., Oleszkiewicz A., Hähner A., Hummel T. Parosmia is associated with relevant olfactory recovery after olfactory training. Laryngoscope. 2021;131(3):618–623. doi: 10.1002/lary.29277. [DOI] [PubMed] [Google Scholar]
- 58.Damm M., Pikart L.K., Reimann H., Burkert S., Göktas Ö., Haxel B., Frey S., Charalampakis I., Beule A., Renner B., Hummel T., Hüttenbrink K.B. Olfactory training is helpful in postinfectious olfactory loss: A randomized, controlled, multicenter study. Laryngoscope. 2014;124(4):826–831. doi: 10.1002/lary.24340. [DOI] [PubMed] [Google Scholar]
- 59.Abdelalim A.A., Mohamady A.A., Elsayed R.A., Elawady M.A., Ghallab A.F. Corticosteroid nasal spray for recovery of smell sensation in COVID-19 patients: A randomized controlled trial. Am. J. Otolaryngol. 2021;42(2):102884. doi: 10.1016/j.amjoto.2020.102884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kasiri H., Rouhani N., Salehifar E., Ghazaeian M., Fallah S. Mometasone furoate nasal spray in the treatment of patients with COVID-19 olfactory dysfunction: A randomized, double blind clinical trial. Int. Immunopharmacol. 2021;98:107871. doi: 10.1016/j.intimp.2021.107871. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors upon request.


