Abstract
Ischemic stroke is highly concerning because it often leads to severe long-term neurological disability. Among clinical trials, ischemic stroke and inflammatory bowel disease interactions have been increasingly reported in recent years. Therefore, using bioinformatics approaches to explore novel protein interactions between them is of interest. We performed this exploratory analysis by using bioinformatics tools such as string to analyze gene data downloaded from NHGRI-GWAS data related to ischemic stroke and inflammatory bowel disease. We constructed a prospective protein interaction network for ischemic stroke and inflammatory bowel disease, identifying cytokine and interleukin-related signaling pathways, Spliceosome, Ubiquitin–Proteasome System (UPS), Thrombus, and Anticoagulation pathways as the crucial biological mechanisms of the network. Furthermore, we also used data-independent acquisition mass spectrometry (DIA-MS) to detect differential protein expression in eight samples, which also suggested that immune system, signal transduction, and hemostasis-related pathways are key signaling pathways. These findings may provide a basis for understanding the interaction between these two states and exploring possible molecular and therapeutic studies in the future.
Subject terms: Genetics, Immunology, Neuroscience, Neurology
Introduction
Ischemic stroke is the most common type of cerebrovascular disease, with four high characteristics such as high morbidity, high disability, high recurrence and high mortality, which brings a heavy medical and economic burden to patients' families and society. By 2030, almost 3.9% of the adult population in the United States would have had a stroke, considering that there has been a 20.5% increase in its prevalence since 20121. Of all strokes, 87% are ischemic strokes (ISs)2. In the United States, strokes have caused serious and long-term neurological disability in3 approximately 3% of the males and 2% of the females that have suffered them. It is predicted that the total direct medical costs of stroke-related diseases will increase between 2015 and 2035 from $36.7 billion to $94.3 billion, with much of the anticipated increase arising from affliction of people more than 80 years of age4. The Global Burden of Disease Study 2019 (GBD 2019) showed that the global prevalence of stroke was 104.2 million and that of IS was 82.4 million5.
Considering the high rates of morbidity and mortality, ferreting out the causes and the potential molecular mechanisms of stroke and identifying molecular biomarkers for early diagnosis, preventive treatment, and precise therapy are critically important. Familial epidemiological studies suggest that stroke has a genetic component. Genes modestly associated with stroke have been identified in several studies. Several heritable disorders associated with stroke have also been reported6.
Inflammatory bowel disease (IBD), including ulcerative colitis (UC) and Crohn's disease (CD). It was first identified in the western developed countries, and the cause is still unknown, but the number of patients in Europe and the United States has accounted for 0.5% of the world population. It is an idiopathic inflammatory disease of the intestinal tract involving the ileum, rectum and colon. Clinical manifestations include diarrhea, abdominal pain, and even bloody stools. In the past 20 years, the incidence and prevalence of inflammatory bowel disease (IBD) has risen steeply in developing countries in Asia, South America, the Middle East, and Africa, with the rate of increase particularly significant in South America and East Asia, and has become a global disease A 2017 retrospective study of the incidence and prevalence of IBD worldwide published in The Lancet2 showed that the incidence in Europe and the United States has reached a plateau while non-Western countries are in the first phase of new case growth. More than 2 million Europeans and 1.5 million North Americans currently suffer from inflammatory bowel disease. And according to 2014 data from the Chinese Center for Disease Control and Prevention, the total number of IBD cases in China between 2005 and 2014 was about 350,000. One study estimated that by 2025, the number of people with IBD in China will reach 1.5 million7.
Compared with the general population, there was a significant difference in the risk of stroke in patients with IBD8. Patients with IBD have an abnormal immune response to microbial dysbiosis, leading to a sustained stimulated pro-inflammatory state, and pro-inflammatory Th1, Th17, and γδ T cells are often associated with increased inflammatory injury, while subsets of T cells can help or exacerbate ischemic brain injury9,10. In addition, inflammatory bowel disease increases patients' risk of atrial fibrillation11, predisposes patients to recurrent ischemic cerebrovascular events, and stroke survivors with inflammatory bowel disease are more likely to have a worse prognosis than those without inflammatory bowel disease12. In addition, one study used two-sample bidirectional Mendelian randomization (MR) to analyze the relationship between stroke and osteoarthritis (OA), a common general inflammatory disease. Among the three categories of OA at any site, knee OA and hip OA, hip OA was found to be a potential risk factor for overall stroke, IS and small vessel IS. The mechanisms linking this site-specific OA to stroke remain to be further studied13. Potential mechanisms between inflammatory bowel disease and ischemic stroke may include activation of the innate immune system, systemic inflammation leading to vascular endothelial dysfunction, increased cholesterol biosynthesis, and thrombotic states. All of these may exacerbate atherosclerotic lesions and increase the risk of atherosclerosis, thereby promoting cerebrovascular events, including large- and small-vessel cerebral infarcts. To this end, protein interaction data obtained from a wide range of cellular and biochemical model systems can be used to build protein–protein interaction (PPI) networks from genes associated with inflammatory bowel disease and stroke, which would enable a better understanding of genetics for ischemic stroke pathogenesis in terms of biological pathway and functional analysis, integrating relevant genetic risk factors. As biotechnology continues to advance, PPI network analysis may be a powerful time- and cost-efficient approach to identify potential biological pathways in ischemic stroke, key players or candidate genes involved in the inflammatory bowel disease-stroke linkage.
Therefore, the aim of this study was to identify key proteins and bioregulatory pathways involved in the physiopathology of inflammatory bowel disease and stroke through bioinformatic analysis and application of surveillance-related proteins.
Materials and methods
Data collection
We downloaded data on inflammatory bowel disease and ischemic stroke from the NHGRI-GWAS database by entering the keywords ischemic stroke and inflammatory bowel disease, respectively, in the search field of the NHGRI-GWAS database (https://www.ebi.ac.uk/gwas/, accessed on 27 January 2022). The GWAS-related studies on ischemic stroke included more than 2,951,164 individuals covering Europeans, East Asians, South Asians, African Americans or African Caribbean, Hispanics or Latinos. The GWAS-related studies on inflammatory bowel disease included approximately 640,515 individuals from sub-Saharan African, European, East Asian, South Asian, African American or African Caribbean, Hispanic, or Latino ethnic populations. The GWAS data were all from different populations because none of the included studies combined data from both ischemic stroke and inflammatory bowel disease conditions.
Data organization and analysis
We used Microsoft Office Excel to perform basic processing of the data. After removing duplicates by excel, the total number of genes involved in ischemic stroke was 418, while the number involved in inflammatory bowel disease was 400.We then accessed the string online website (version 11.5) and selected the multi-protein model to construct the protein–protein interaction network. We set the maximum number of interactors displayed to 10 and the minimum required interaction score of 0.4 for the first analysis. Based on the analysis, we then determined if a higher interaction score of 0.7 or 0.9 would be chosen as the cutoff score for the analysis.
Construction and analysis of protein–protein interaction network
The STRINGdb v11.5 package (https://string-db.org/cgi/input.pl?) was used toconstruct a PPI network of the proteins encoded by the genes. Furthermore, Cytoscape v3.7.1 was utilized to construct a PPI network, considering confidence a score ≥ 0.9 and maximum number of interactors = 10 as cut-off criteria, and the interaction of the candidate gene-encoded proteins was analyzed. Moreover, the Network Analyzer plug-in was used to calculate node degrees, i.e., the number of interconnections useful to filter hub genes from the PPI network. The Molecular Complex Detection (MCODE) plug-in of the Cytoscape tool was employed to visualize the significant gene clusters in IS with a degree cutoff = 2, node score cutoff = 0.2, k-core = 2, and max. depth = 100. The criteria for selecting the two most significant clusters were set as MCODE scores ≥ 4 and number of nodes ≥ 4. The corresponding proteins in the central nodes might be key proteins and hub candidate genes with important physiological regulatory functions.
GO and biological pathway enrichment analysis
GO and pathway enrichment analyses of genes were performed using multiple online databases. We submitted the genes to the DAVID online program (https://david.ncifcrf.gov/; version: 6.8) with p < 0.05 as the cut-off criterion. Besides, GO and pathway analyses were carried out using KEGG (http://www.genome.jp/kegg), Reactome (http://www.reactome.org), BioCyc (http://biocyc.org), and PANTHER (http://www.pantherdb.org)14. The enriched GO terms were ranked according to their p-values and visualized as bar charts; p < 0.05 was considered as statistically significant15,16.
Corroboration of key pathways
To further validate the key signaling pathways obtained from the above bioinformatic analysis, we used data-independent acquisition mass spectrometry (DIA-MS), one of the highly regarded mass spectrometry acquisition techniques in recent years, to identify and quantify proteins in samples from 8 blood samples (4 ischemic stroke samples and 4 normal population samples). The main steps were as follows: first, whole blood was collected using a vacuum blood collection tube and gently mixed by inverting up and down 5–6 times; the blood was left to stand for 30 min at 4 °C to allow coagulation; the supernatant was immediately taken by centrifugation at 1700g for 10 min at 4 °C; the upper layer of yellowish serum was transferred to a centrifuge tube using a pipette; liquid nitrogen was snap frozen for 5 min and stored at − 80 °C for backup. Then, protein extraction was performed, and then a portion of the prepared total protein solution was trypsinized, and the enzymatically cleaved peptides were desalted using a SOLA™ SPE 96-well plate for LC–MS/MS high-resolution mass spectrometry detection (Full MS: Resolution = 120,000, AGC target = 3e6, Maximum injection time = 50 ms, Scan range = 350–1250 m/z; MS2: Resolution = 15,000, AGC target = 1e5, Maximum injection time = 35 ms, Scan range = 200–2000 m/z, Isolation window = 1.4 m/z, Normalized Collision Energy = 28), and the high-abundance proteins were removed using a MilliPore de-abundance kit before detection. The LC–MS/MS raw files were imported into Spectronaut Pulsar software for matching, and the machine signals were transformed into peptide and protein sequence information, and then combined with sequence information, peptide retention time, and fragment ion information for the spectral library building to facilitate the subsequent DIA analysis. Based on obtaining plausible proteins, we adopted the following criteria to screen the differential proteins: FoldChange ≥ 1.2, P ≤ 0.05 as up-regulated proteins; FoldChange ≤ 0.833, P ≤ 0.05 as down-regulated proteins. To further understand the biological functional information of differential proteins, we performed GO and pathway enrichment analysis on candidate DEPs using multiple online databases.
Results
Identification of genes in ischemic strokes and inflammatory bowel disease
NHGRI-GWASCatalog supplies a publicly hand-curated collection of published GWAS assaying more than 100,000 single-nucleotide polymorphisms (SNPs) and all SNP-trait associations with p < 1 × 10–5.The Catalog includes 5595 curated publications of 195,610 SNPs and 326,947 associations. In addition to SNP-trait association data, Catalo is a global public source that archives and makes freely available high-throughput functional genomics data submitted by researchers. The GWAS datasets of ischemic stroke (EFO ID: HP_0002140, include 17 reported traits with 314 Associations) and inflammatory bowel disease (EFO ID: EFO_0003767, include 11 reported traits with 526 Associations, excluding child trait data) were taken from this repository, we extracted 418, and 400 genes. After performing an integrated bioinformatical analysis using jvenn (https://www.vandepeerlab.org/?q=tools/venn-diagrams)17, the intersection of these datasets yielded 12 genes, and the total number of unique elements is 806 (Table 1).
Table 1.
Identification of genes in ischemic strokes and inflammatory bowel disease (calculate and draw custom Venn diagrams).
| List names | Number of genes | Number of unique elements |
|---|---|---|
| Inflammatory bowel disease | 400 | 400 |
| Ischemic stroke | 418 | 418 |
| Overall number of unique elements | 806 | |
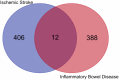
| ||
| Names | Total | Genes |
|---|---|---|
| Inflammatory Bowel Disease Ischemic Stroke | 12 | DELEC1 ERAP2 SOX5 ADGRF1 PHACTR2 ATP6V1G3 SH2B3 F5 Y_RNA ATXN2 PTCH1 PTPRC |
| Ischemic Stroke | 406 | RPTOR LINC01013 KCNMA1 DEFA3 KIF26B NDUFS6 LINC01435 INPP5F COX7A2L ACOT6 FOXP4 GLTP DCHS2 COL4A2 PRDM8 RNU4-64P LINC01893 TECTA MMP7 MC4R R3HCC1L BRWD1P2 HNRNPA1P67 CGNL1 CENPQ CHORDC1 CRYBB2P1 RCN1P1 MCPH1-AS1 PLCB2 STN1 TSPAN15 LATS2 SIGLEC15 TMOD4 KCNK3 GDA HTR4 PRKCZ STARP1 UNQ6494 VWDE MTND1P24 PTPRD RORA NSF GPR37 NFIA CDH13 PTPRN2 TANGO2 LINC02125 ARHGAP21 MIR4277 RPL18P1 RAB19 LINC00923 PLCB4 UBE2V1P16 SLC44A2 MAPKAPK5-AS1 F11-AS1 TERF2IP DSCAML1 A1CF FGGY Metazoa_SRP FAM170B-AS1 LINC01915 SRRM4 USP46 CCDC39 NDUFB10P1 RPS12P23 LINC02267 DAB1 RPL17P35 SLC22A7 GALNT13 ESRRAP2 SGO1P2 COL28A1 LINC02884 CHMP4AP1 NCAM2 VAT1L ALDH2 TRAPPC11 SWAP70 RNU6-560P FGA SLC1A1 PRTFDC1 SEMA3A ULK4 CYSLTR2 WIZ FGG TPT1P9 HELLPAR PMF1 SMARCA4 RNF17 RBFOX1 LINC00924 TTC8 PCDH7 QRICH2 LINC02400 OPCML RNA5SP430 SECISBP2L RN7SL474P AMBN CD36 PRPF8 ENPEP MTND4P33 VN2R10P ELAPOR2 EPG5 HSD17B11 TRAV2 CCL16 SLC4A1AP APOD LINC01438 DNAH11 ADGRL3 KHDRBS3 NLRP5 GRK3 CDKN2B-AS1 ABO COL14A1 OSBPL1A CHD3 OR10J6P RN7SL73P ATPSCKMT RNU7-92P PTPRG GRIK2 TMEM273 MORN1 MIR8084 TNFRSF21 HTR3A ZNF474-AS1 CYP1B1-AS1 OR52B6 DEPDC1-AS1 FGB CCDC83 WNT2B CASZ1 FOXF2-DT B4GALT4-AS1 LINC01765 PPIAP22 PDZRN4 DNM2 OR4E1 HPS4 SDHAP3 C8orf86 PICALM ENAM BCL9L SLC36A2 TMEM74 RUNX1 TMEM170B ZBED1P1 RB1 ALDH1A2 FGF5 NAALADL2 RNU6-102P CXCL8 HNRNPA1P53 POP1 HDAC9 NALCN-AS1 GDI2 LINC01394 KNG1 LINC00640 C17orf102 SORBS2 SH3PXD2A TSPEAR LINC02426 RNU7-165P NDUFB8P1 ZNF626 GOSR2 ADAMTS13 TMEM243 PDE9A TP53TG1 COL4A1 ADAMTS3 GRM7 DNAH8 HTR3B ILF2P2 PITX2 TMEM123 LINC02511 FAF1 CST13P CDK6 SNTG2 SDR16C6P FZD7 LRCH1 RP1 RNU6-380P CADPS2 SRGAP3 FURIN TGFBR3 TMEM14A ANK2 CDKN1A ASTN2 LINC02709 ZNF516 SSPN KCTD13 USP36 MYRIP PRKCZ-AS1 XKR9 PATJ LINC01377 RHAG NTN4 NRCAM DISC1FP1 LINC02882 NYNRIN LINC02537 CDKN2C TTBK1 RN7SL363P EIF4A2 CST8 ICE2P2 COL6A3 LINC00545 STARD3NL ASPHD2 BOLA3P1 LINC00398 SDR16C5 AGMO MIX23P2 CRTAC1 F2 TMEM126B RNA5SP107 ZFHX3 TRAM2-AS1 ZNF77 PLRG1 LMX1B RASAL3 CNTNAP4 IRX1 ABCC1 RNU4ATAC9P DPPA3 SYCE1L VWF DEFA11P VPS33B ATP5MC1P8 PIK3C2G ALKBH8 RNU6-694P NPY1R RPL21P41 PSMB3 DTWD1 SLCO1B1 KCNK1 SFRP4 DDR2 LHFPL3 NRBF2P1 MPRIP ZFAND2A PPP2R5E TMEM132D RNU6-440P RUFY4 AACSP1 CDC5L LMNA PTPRT PDE3A MKRN1 SLC26A11 LYZL6 C10orf143 POU2F1 FUNDC2 GCATP1 LINC01522 TBC1D3P2 LAMA1 GALNT1 TRMT112P7 CDH11 MARCHF1 CPNE8-AS1 LRAT FAM95C MACROD2 MTX2 SLC14A1 RNU6-144P LINC02399 UAP1 USP28 CRISP2 ILF3 LINC01894 AGBL1 ONECUT2 AUTS2 RNU7-159P NRG1 ADAMTSL3 RNA5SP360 MIR4497 SYT9 NIPAL2 THRAP3P1 LEXM CDH4 E2F7 RNU6-284P JPH3 GAPDHP50 LINC01148 KAZN SLC14A2 FGD6 ZBTB46 TWIST1 TNR DPP10 LINC02052 PLAA DINOL PGLYRP3 ZNF292 CRPP1 EFCAB3 OLFML1 TBCEL-TECTA CRLF3 PROCR FUT8 LNX1 C8orf87 CNTN6 EEF1A1P36 KCNS3 ARID1A LINC02406 RNU6-1252P SEMA3C TTC7B RERGL CMAHP KAT2B CYP1B1 ATP2B1 PCNX1 ELMOD1 VIPR2 RPH3A GTF2IP2 RNA5SP63 CELF2-DT RN7SKP242 LINC02772 STT3B RPS6KA2 RNU6-667P PMF1-BGLAP FXR1 MVB12B ZCCHC14 ANP32C AJAP1 RPP40P2 ARNT2 RNF213 ZNHIT6 USP38 RPL7AP30 |
| Inflammatory Bowel Disease | 388 | CCND3P1 RIT2 ERGIC1 ADAM30 IRF4 TRAF3IP2-AS1 HORMAD2 KANK1 LINC01997 INTS11 LINC00604 TUBD1 CRTC3-AS1 LPP KPNA7 FIBP PTPRK DMRT1 OR7E91P RPS21P8 ARPC2 PPIAP9 DPH5 INAVA NCF4 CDC27 SLC17A1 PLAU LINC02745 TRAF3IP2 C10orf55 ADCY7 FOSL2 FGFR1OP2P1 FCGR2A MUC19 P4HA2 SERPINB5 RN7SL72P MIR548H4 GPR65 LINC01882 C17orf67 TMBIM1 SLC22A4 IMPG2 ITLN2 ITLN1 IRF1 PTK2B BANK1 GOT1 C6orf47-AS1 TMEM174 LSP1 IL2RA DUSP22 SLC22A23 PLCG2 FAP TAGAP-AS1 CDYL2 SYN3 HHEX USP15 LINC00698 SMURF1 SHC1 PPP5C LINC02723 CYCSP42 BSN PRKCB LINC00358 AIMP1P2 IL10 CDC14A GPR183 FAM171B RPL23AP12 MAML2 NFATC1 KCNJ4 LINC01620 RASGRP1 BTF3L4P3 FTO GRID2IP SMAD3 LINC01934 SLC2A13 DAP3 CTBP2P2 TYK2 IKZF1 HIF3A DENND1B LINC00484 CTIF RBPJ LRRC8D ZFP36L1 GPBAR1 IRGM UBAC2 GRB7 KLF3-AS1 GYPC PSMG4 IL2 CYP2C18 LINC02128 HDAC7 PLCL1 C5orf66 OSMR PDLIM4 FOS RPL13P2 CDKN2A-DT CCDC26 CD28 CHORDC1P5 OTUD3 RPS6KA4 ZNF300 CAMK2A LINC02341 SPDEF SBNO2 SLAIN2 TWF1 LINC02694 THADA IRF5 ZPBP2 TET2 TNFSF15 HSD17B4 TMEM117 CD6 LINC01989 SP140 DNMT3B NR5A2 FEN1 COMMD7 CPEB4 GPR35 IFNG-AS1 RPL3 RTEL1-TNFRSF6B LINC02132 COX6B1P6 STAT4 MTAP CD40 AQP12B PPBP LRRK2 LACC1 IKZF3 LINC02009 TEX41 ALDH7A1P4 ETS1 SEMA6D OSTF1P1 RNU4ATAC14P RN7SKP211 GCKR LINC00598 IRF6 LINC01082 NUPR1 RELA CCL7 IRF1-AS1 PLA2R1 LPXN NIFKP1 RNA5SP224 GLYAT FLJ31356 FADS2 LTBR TNFRSF14 IL1RL1 KIR3DL2 HLA-DQB1 DUSP29 LINC01475 SLC9A4 BOK-AS1 PTPN2 PDE10A LINC02571 LITAF RIT1 RGS14 CEBPA YBX1P5 LINC01271 CDC37 ACO2 BATF3 RFX6 IL23R MECOM SKAP2 CALM3 MROH3P SUFU SCAMP3 KSR1 EIF2S2P3 LINC02757 RNF186 CARD9 CELSR3 LINC02635 ADCY3 MAGOH3P CRTC3 MAP3K8 PCBP3 BUD13 HLA-DRB1 CIT LINC02773 FERMT1 PLA2G4A DLD LINC01845 BRD2 SMAD7 PARK7 LINC02888 HLA-B TAB2 HLA-DQB3 CUL2 PDGFB IDI1P2 RNASET2 CADPS VSX2 SLC39A11 CXCL5 PITPNM3 SH2B1 DAP ZMIZ1 JAK2 TM9SF4 CD226 RNU4ATAC4P STAT3 FOXP1 CCL20 POFUT1 MAD1L1 OTUD7A PCSK5 RORC IFIH1 IFNGR2 LINC01378 PPIAP34 CDC42SE2 GALC TNFRSF6B NXPE2P1 FCAR SPATA48 EEF1AKMT2 TPTEP2 CTH COL6A1 IL12B DUSP16 POP7 MIR4679-2 SV2C PRXL2B CCDC85B IPMK GRAMD2B LINC02278 NOD2 RN7SL636P C6orf47 PYGO2 TRIB1 NXPE1 NUDT15 TOM1 SPRED2 EEF1A1P27 YWHAQP1 ERRFI1 PSMA2P1 FUT2 LINC01622 RN7SKP144 GNA12 ST7 IL18R1 ANKRD55 SLC7A10 LINC02555 UBE2L3 SNX20 TTC33 ATG16L1 PTGIR HGFAC MST1 MTCO3P1 RPL32P17 GPR18 BACH2 KAT2A ZGPAT ATXN2L GATD3A SERPINB12 LINC02513 LINC02539 BTBD8 NDFIP1 LINC00581 EPO DNAJB12P1 NOTCH1 CDKAL1 MIR3939 RMI2 TSPAN14 CCL2 PUS10 LINC00824 EPHB4 CYLD-AS1 CCND3 IL27 ZNF300P1 HLA-DQA1 RNU6-222P SYNGR1 U2 SATB1-AS1 CAMK2G EMSY THEMIS NHLRC1 MTND4P6 KIF3B IL18RAP SNRPGP8 LINC02357 ZBTB40 CSF2RB RNU6-793P KCP CREM HNRNPA1P41 RPSAP35 PRDM1 ADAD1 DUSP5-DT PNKD PIGCP2 RNU6-925P TAB2-AS1 ZNF831 PLXNA4 LINC01588 CCRL2 CUL1 AMZ1 LINC01075 LINC02791 KIAA1109 EXOC6 PTCD2 MIR3936HG ITGA4 |
Construction and analysis of protein–protein interaction network
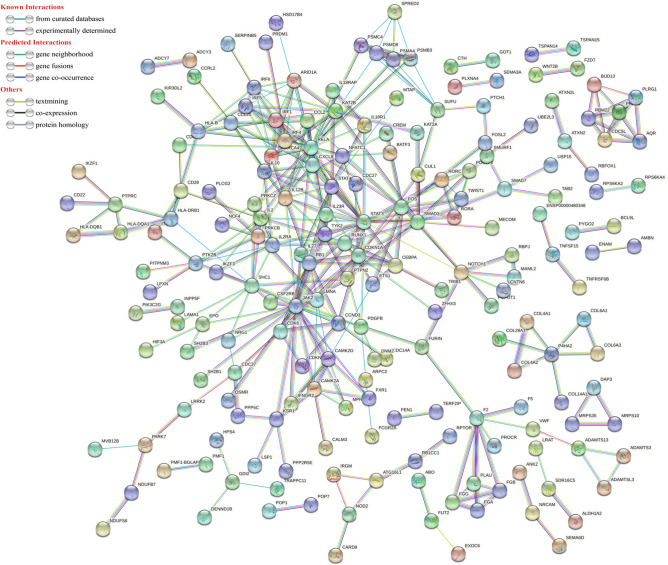
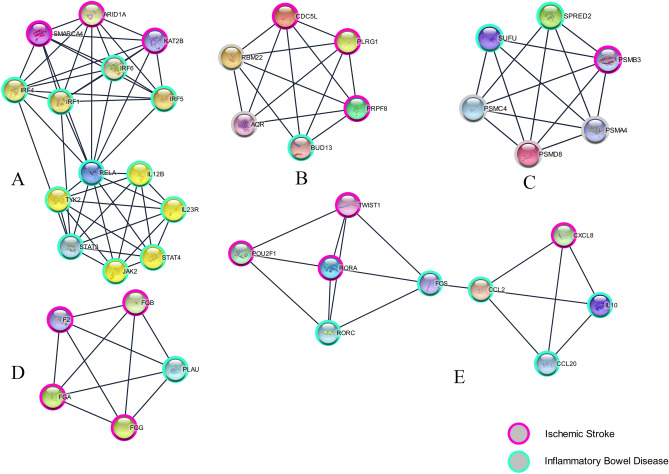
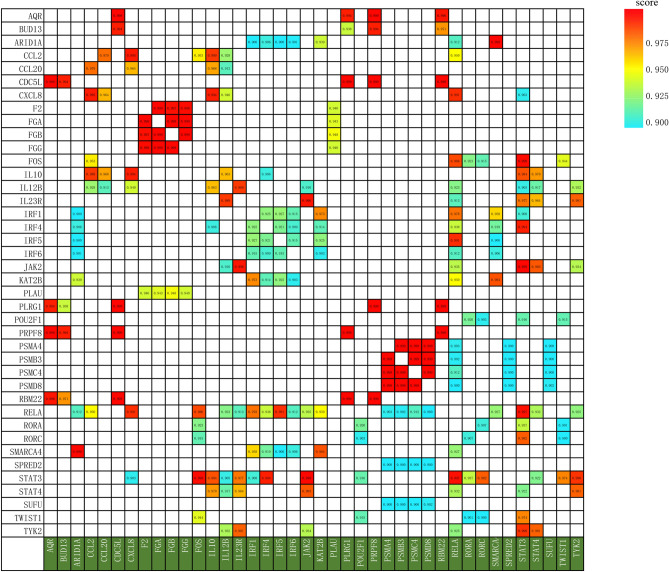
Using the online web tool STRINGdb and the Cytoscape software platform, a total of 589genes out of the 818 identified, were used to construct a PPI network complex that contained 587 nodes and 366 edges (Fig. 1). According to their degree of importance, we chose five significant clusters from the PPI network complex for further analysis using Cytoscape MCODE. Pathway enrichment analysis showed that cluster 1 (Fig. 2A) consisted of 14 genes primarily associated with Cytokine Signaling in Immune system and Interleukin-23 signaling, cluster 2 (Fig. 2B) consisted of 6 genes primarily associated with U2-type spliceosomal complex and Spliceosome, cluster 3 (Fig. 2C) consisted of 6 genes primarily associated with Degradation of GLI1 by the proteasome and Proteasome, cluster 4 (Fig. 2D) consisted of 5 genes primarily associated with Blood clotting cascade and Fibrinolysis, whereas cluster 5 (Fig. 2E) consisted of 9 genes primarily associated with Interleukin-4 and Interleukin-13 signaling and Signaling by Interleukins. Furthermore,The interactions of the 40 hub node genes are visualized using a heatmap plot (Fig. 3).
Figure 1.
Protein–protein interaction (PPI) network between ischemic stroke and inflammatory bowel disease with minimum required interaction score 0.900, the highest confidence as the cut-off criterion.
Figure 2.
Five significant clusters from the PPI network complex for further analysis using Cytoscape MCODE. (A) Cluster 1 consisted of 14 genes primarily associated with Cytokine Signaling in Immune system and Interleukin-23 signaling; (B) cluster 2 consisted of 6 genes primarily associated with U2-type spliceosomal complex and Spliceosome; (C) cluster 3 consisted of 6 genes primarily associated with Degradation of GLI1 by the proteasome and Proteasome; (D) cluster 4 consisted of 5 genes primarily associated with Blood clotting cascade and Fibrinolysis; (E) cluster 5 consisted of 9 genes primarily associated with Interleukin-4 and Interleukin-13 signaling and Signaling by Interleukins.
Figure 3.
The interactions of the 40 hub node genes.
GO and pathway enrichment analysis
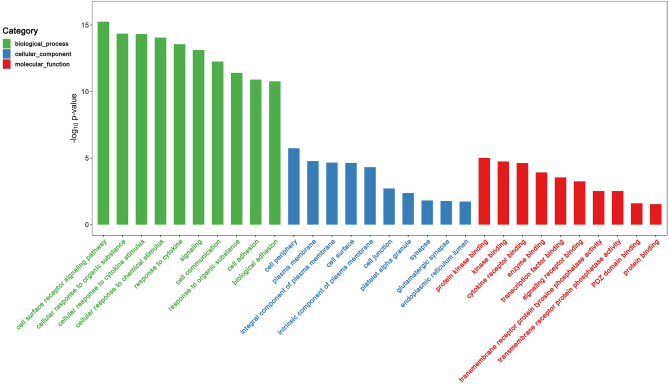
GO and pathway enrichment analyses of these significant genes were performed using multiple online databases. The functional GO terms were classified into three categories: molecular function (MF), biological process (BP), and cellular component (CC). As shown in Fig. 4 and Table 2, in the BP category, the genes were primarily enriched in Cellular response to cytokine stimulus, Cell surface receptor signaling pathway, Response to cytokine, et al. In the MF category, the genes were primarily enriched in Protein binding, Protein kinase binding, Kinase binding, et al. In the CC category, the genes were primarily enriched in Plasma membrane, Cell periphery, Integral component of plasma membrane, et al.. These results show that most of the identified genes were significantly enriched in protein binding, innate immune response, defense response to virus, cytosol, and negative regulation of apoptotic process terms.
Figure 4.
Gene ontology analysis and significant enriched GO terms of genes associated with Ischemic stroke and inflammatory bowel disease. GO analysis classified the DEGs into three groups (i.e., biological process, molecular function and cellular component).
Table 2.
GO and pathway enrichment analyses of significant genes in ischemic strokes and inflammatory bowel disease.
| Term | Category | Observed gene count | Strength | False discovery rate [FDR] |
|---|---|---|---|---|
| Cellular response to cytokine stimulus | BP | 92 | 0.48 | 7.00E−16 |
| Cell surface receptor signaling pathway | BP | 150 | 0.33 | 3.29E−15 |
| Response to cytokine | BP | 94 | 0.45 | 4.04E−15 |
| Signaling | BP | 254 | 0.21 | 8.51E−14 |
| Cellular response to organic substance | BP | 147 | 0.32 | 9.24E−14 |
| Cell communication | BP | 254 | 0.2 | 3.93E−13 |
| Cellular response to chemical stimulus | BP | 167 | 0.28 | 3.93E−13 |
| Cytokine-mediated signaling pathway | BP | 67 | 0.52 | 4.15E−13 |
| Signal transduction | BP | 238 | 0.21 | 4.45E−13 |
| Regulation of response to stimulus | BP | 211 | 0.23 | 4.45E−13 |
| Protein binding | MF | 283 | 0.13 | 6.44E−06 |
| Protein kinase binding | MF | 47 | 0.38 | 0.00026 |
| Kinase binding | MF | 50 | 0.35 | 0.00045 |
| Binding | MF | 433 | 0.06 | 0.0005 |
| Signaling receptor binding | MF | 83 | 0.24 | 0.00091 |
| Cytokine receptor binding | MF | 25 | 0.5 | 0.0012 |
| Enzyme binding | MF | 102 | 0.18 | 0.014 |
| Transcription factor binding | MF | 41 | 0.31 | 0.0172 |
| Immune receptor activity | MF | 15 | 0.58 | 0.0172 |
| Extracellular matrix structural constituent | MF | 14 | 0.59 | 0.018 |
| Plasma membrane | CC | 220 | 0.14 | 7.74E−05 |
| Cell periphery | CC | 225 | 0.14 | 7.74E−05 |
| Integral component of plasma membrane | CC | 88 | 0.26 | 8.33E−05 |
| Cell surface | CC | 54 | 0.34 | 0.00012 |
| Intrinsic component of plasma membrane | CC | 90 | 0.25 | 0.00012 |
| Side of membrane | CC | 36 | 0.35 | 0.0046 |
| Cell junction | CC | 94 | 0.18 | 0.016 |
| Platelet alpha granule | CC | 12 | 0.64 | 0.016 |
| Receptor complex | CC | 27 | 0.37 | 0.0192 |
| Endoplasmic reticulum lumen | CC | 23 | 0.4 | 0.0286 |
Pathway enrichment analysis
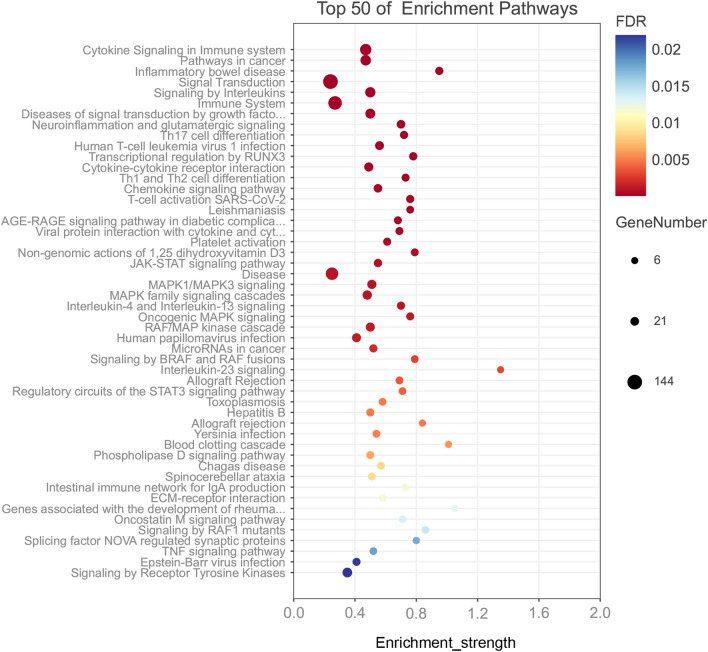
Functional and signaling pathway enrichment analyses of these significant genes were conducted using multiple online databases. By KEGG pathway analysis, these genes were mainly enriched in Pathways in cancer, Inflammatory bowel disease, Th17 cell differentiation, Human T-cell leukemia virus 1 infection, Cytokine-cytokine receptor interaction. Furthermore, We used 'Reactome pathways' to analyze these important genes and calculated the false discovery rate using Fisher's Exact, and the results showed that Cytokine Signaling in Immune system, Signal Transduction, Signaling by Interleukins, Immune System, Diseases of signal transduction by growth factor receptors and second messengers, and Transcriptional regulation by RUNX3 were the significant signaling pathways. In addition, we also used "WikiPathways" analysis as a complement to show that these significant genes aremainly enriched in Neuroinflammation and glutamatergic signaling, T-cell activation SARS-CoV-2, Non-genomic actions of 1,25 dihydroxyvitamin D3, Allograft Rejection, and Regulatory circuits of the STAT3 signaling pathway. Together, these results showed that these significant genes had several pathways in common, including those involved in immune system, cytokine signaling in immune system, adaptive immune system (Fig. 5).
Figure 5.
Significantly enriched pathway terms of genes associated with ischemic stroke and inflammatory bowel disease.
Corroboration of predicted signaling pathways
To further corroborate the key signaling pathways obtained from the above bioinformatic analysis, we used DIA-MS, one of the highly regarded mass spectrometry acquisition techniques in recent years, to identify and quantify proteins in samples from 8 blood samples (4 ischemic stroke samples and 4 normal population samples) (Table 3), 60 down-regulated and 18 up-regulated protein (Tables 4, 5) followed by pathway enrichment analysis with Reactome pathways, and revealed some aspects of over-representation in Complement cascade, Regulation of Complement cascade, Scavenging of heme from plasma, innate immune system and binding and uptake of ligands by scavenger receptors (Table 6).
Table 3.
Clinical characteristics of 4 ischemic stroke (IS) patients and healthy (HE) (n = 4).
| No | Group | Subjects source | Sex | Age | Disease duration (days) | Combined diseases | Crisis | NIHSS score |
|---|---|---|---|---|---|---|---|---|
| 1 | IS | Hospital | Male | 63 | 25 | Hypertension, type 2 diabetes | N | 7 |
| 2 | IS | Hospital | Female | 55 | 30 | Hypertension, Hyperlipidemia, Chronic Bronchitis | N | 10 |
| 3 | IS | Hospital | Female | 55 | 14 | – | N | 8 |
| 4 | IS | Hospital | Female | 67 | 3 | Hypertension, Type 2 diabetes, hyperlipidemia | N | 8 |
| 5 | HE | Community | Female | 37 | – | – | – | – |
| 6 | HE | Community | Male | 35 | – | – | – | – |
| 7 | HE | Community | Female | 20 | – | – | – | – |
| 8 | HE | Community | Male | 28 | – | – | – | – |
Table 4.
78 differentially expressed proteins (DEPs) were identified from four profile datasets, including 18 up-regulated and 60 down-regulated proteins in ischemic stroke.
| Expression | Genes name |
|---|---|
| Down-regulated | IGLV5-45, IGKV3D-15, IGKV6D-21, IGKV1D-13, QSOX1, APOM, ADH1B, F10, A2M, IGLV2-8, IGHA2, KRT14, APOA1, APOC1, AHSG, TF, PROC, APOB, LCAT, GAPDH, IGKV1-16, SERPINA7, APOA4, CTSD, C8G, MBL2, LAMP1, HSP90B1, JUP, CPN1, DSP, ITIH2, GPX3, CPN2, CRHBP, DPP4, KRT9, IGFALS, AFM, MMP16, PLTP, CFHR1, HGFAC, SPP2, ALCAM, SPARCL1, HABP2, PON3, SPDYA, MEGF8, OAF, GGH, NEO1, CNDP1, FUCA2, COLEC11, COG4, MINPP1, LYVE1, FCGBP |
| Up-regulated | IGLV10-54, IGKV2-40, IGKJ1, IGHV3-15, IGHV1-24, IGHV3-64D, CP, IGHV2-5, SLC4A1, LRG1, DBH, IGLL1, PROZ, IGLV3-21, CHIT1, PM20D1, OIT3, CFHR4 |
Table 5.
The gene ontology (GO) enrichment analysis of differentially expressed proteins/genes in ischemic stroke.
| ID | Term | Category | padj | Enrichment score | Genes |
|---|---|---|---|---|---|
| Up-regulated | |||||
| 0006958 | Complement activation, classical pathway | Biological process | 5.69E−08 | 54.46 | IGHV3-15, IGHV1-24, IGHV3-64D, IGHV2-5, IGLL1, IGLV3-21 |
| 0050871 | Positive regulation of B cell activation | Biological process | 1.30E−07 | 74.82 | IGHV3-15, IGHV1-24, IGHV3-64D, IGHV2-5, IGLL1 |
| 0006910 | Phagocytosis, recognition | Biological process | 1.30E−07 | 73.82 | IGHV3-15, IGHV1-24, IGHV3-64D, IGHV2-5, IGLL1 |
| 0006911 | Phagocytosis, engulfment | Biological process | 2.66E−07 | 62.21 | IGHV3-15, IGHV1-24, IGHV3-64D, IGHV2-5, IGLL1 |
| 0050853 | B cell receptor signaling pathway | Biological process | 5.11E−07 | 53.24 | IGHV3-15, IGHV1-24, IGHV3-64D, IGHV2-5, IGLL1 |
| 0042742 | Defense response to bacterium | Biological process | 9.22E−06 | 29.14 | IGHV3-15, IGHV1-24, IGHV3-64D, IGHV2-5, IGLL1 |
| 0006955 | Immune response | Biological process | 0.00017 | 15.21 | IGLV10-54, IGHV2-5, IGLL1, IGLV3-21, CHIT1 |
| 0045087 | Innate immune response | Biological process | 0.00078 | 10.23 | IGHV3-15, IGHV1-24, IGHV3-64D, IGHV2-5, IGLL1 |
| 0050900 | Leukocyte migration | Biological process | 0.00019 | 25.02 | IGHV2-5, DBH, IGLL1, IGLV3-21 |
| 0030449 | Regulation of complement activation | Biological process | 0.00075 | 34.97 | IGHV2-5, IGLV3-21, CFHR4 |
| 0005576 | Extracellular region | Cellular component | 0.00014 | 5.43 | CP, IGHV2-5, LRG1, DBH, , IGLL1, IGLV3-21, CHIT1, CFHR4 |
| 0070062 | Extracellular exosome | Cellular component | 0.01925 | 3.18 | CP, SLC4A1, LRG1, PROZ, IGLV3-21, PM20D1 |
| 0005886 | Plasma membrane | Cellular component | 0.23425 | 1.45 | IGLV10-54, CP, IGHV2-5, SLC4A1, , IGLV3-21 |
| 0042571 | Immunoglobulin complex, circulating | Cellular component | 1.30E−07 | 81.42 | IGHV3-15, IGHV1-24, IGHV3-64D, IGHV2-5, IGLL1 |
| 0019814 | Immunoglobulin complex | Cellular component | 0.00025 | 52.73 | IGLV10-54, , IGLV3-21 |
| 1904724 | Tertiary granule lumen | Cellular component | 0.00612 | 40.27 | LRG1, CHIT1 |
| 0035580 | Specific granule lumen | Cellular component | 0.00748 | 35.72 | LRG1, CHIT1 |
| 0005788 | Endoplasmic reticulum lumen | Cellular component | 0.04555 | 7.43 | CP, PROZ |
| 0043231 | Intracellular membrane-bounded organelle | Cellular component | 0.17757 | 2.80 | LRG1, DBH |
| 0005783 | Endoplasmic reticulum | Cellular component | 0.25882 | 2.13 | DBH, IGLL1 |
| 0003823 | Antigen binding | Molecular function | 4.57E−08 | 63.28 | IGHV3-15, IGHV1-24, IGHV3-64D, IGHV2-5, IGLL1, IGLV3-21 |
| 0034987 | Immunoglobulin receptor binding | Molecular function | 1.30E−07 | 79.10 | IGHV3-15, IGHV1-24, IGHV3-64D, IGHV2-5, IGLL1 |
| 0005507 | Copper ion binding | Molecular function | 0.0059 | 41.79 | CP, DBH |
| 0005509 | Calcium ion binding | Molecular function | 0.15874 | 3.09 | PROZ, OIT3 |
| Down-regulated | |||||
| 0044267 | Cellular protein metabolic process | Biological process | 4.36E−12 | 21.42 | QSOX1, APOA1, AHSG, TF, PROC, APOB, APOA4, HSP90B1, ITIH2, IGFALS, SPP2, SPARCL1, FUCA2 |
| 0043687 | Post-translational protein modification | Biological process | 4.70E−07 | 10.38 | QSOX1, APOA1, AHSG, TF, PROC, APOB, HSP90B1, ITIH2, SPP2, SPARCL1, FUCA2 |
| 0034375 | High-density lipoprotein particle remodeling | Biological process | 1.02E−09 | 108.19 | APOM, APOA1, APOC1, LCAT, APOA4, PLTP |
| 0033344 | Cholesterol efflux | Biological process | 4.70E−07 | 70.56 | APOM, APOA1, APOC1, APOB, APOA4 |
| 0001523 | Retinoid metabolic process | Biological process | 4.92E−05 | 25.76 | APOM, ADH1B, APOA1, APOB, APOA4 |
| 0043691 | Reverse cholesterol transport | Biological process | 9.22E−06 | 76.37 | APOM, APOA1, LCAT, APOA4 |
| 0042157 | Lipoprotein metabolic process | Biological process | 1.89E−05 | 61.82 | APOM, APOA1, APOC1, APOA4 |
| 0042158 | Lipoprotein biosynthetic process | Biological process | 1.27E−05 | 194.74 | APOA1, APOB, LCAT |
| 0010873 | Positive regulation of cholesterol esterification | Biological process | 6.39E−05 | 108.19 | APOA1, APOC1, APOA4 |
| 0034371 | Chylomicron remodeling | Biological process | 6.39E−05 | 108.19 | APOA1, APOB, APOA4 |
| 0005576 | Extracellular region | Cellular component | 2.71E-23 | 6.89 | QSOX1, APOM, F10, A2M, IGLV2-8, IGHA2, APOA1, APOC1, AHSG, TF, PROC, APOB, LCAT, SERPINA7, APOA4, CTSD, C8G, MBL2, HSP90B1, JUP, CPN1, ITIH2, GPX3, CPN2, CRHBP, DPP4, IGFALS, AFM, PLTP, CFHR1, HGFAC, SPP2, SPARCL1, HABP2, PON3, GGH, CNDP1, FUCA2, COLEC11 |
| 0070062 | Extracellular exosome | Cellular component | 5.92E−15 | 5.13 | QSOX1, A2M, IGHA2, KRT14, APOA1, AHSG, TF, APOB, LCAT, GAPDH, SERPINA7, APOA4, CTSD, C8G, LAMP1, HSP90B1, JUP, DSP, ITIH2, GPX3, CPN2, DPP4, KRT9, IGFALS, AFM, ALCAM, PON3, MEGF8, GGH, FUCA2, MINPP1, LYVE1, FCGBP |
| 0005788 | Endoplasmic reticulum lumen | Cellular component | 2.85E−11 | 15.25 | QSOX1, F10, APOA1, AHSG, TF, PROC, APOB, APOA4, HSP90B1, ITIH2, SPP2, SPARCL1, FUCA2, MINPP1 |
| 0062023 | Collagen-containing extracellular matrix | Cellular component | 4.75E−06 | 9.41 | A2M, APOA1, AHSG, APOA4, CTSD, MBL2, HSP90B1, ITIH2, SPP2, SPARCL1 |
| 0034364 | High-density lipoprotein particle | Cellular component | 5.92E−11 | 94.67 | APOM, APOA1, APOC1, APOB, LCAT, APOA4, PLTP |
| 0034361 | Very-low-density lipoprotein particle | Cellular component | 2.67E−07 | 81.14 | APOM, APOA1, APOC1, APOB, APOA4 |
| 0019814 | Immunoglobulin complex | Cellular component | 4.92E−05 | 25.76 | IGLV5-45, IGKV3D-15, IGKV6D-21, IGLV2-8, |
| 0042627 | Chylomicron | Cellular component | 3.26E−06 | 99.87 | APOA1, APOC1, APOB, APOA4 |
| 0034362 | Low-density lipoprotein particle | Cellular component | 0.00017 | 74.90 | APOM, APOA1, APOB |
| 0071682 | Endocytic vesicle lumen | Cellular component | 0.00023 | 64.91 | APOA1, APOB, HSP90B1 |
| 0005543 | Phospholipid binding | Molecular function | 0.00038 | 14.89 | APOM, F10, APOA1, APOB, APOA4 |
| 0004252 | Serine-type endopeptidase activity | Molecular function | 0.00203 | 9.72 | F10, PROC, DPP4, HGFAC, HABP2 |
| 0120020 | Cholesterol transfer activity | Molecular function | 1.89E−05 | 61.82 | APOA1, APOB, APOA4, PLTP |
| 0031210 | Phosphatidylcholine binding | Molecular function | 5.97E−05 | 44.77 | APOA1, APOC1, APOA4, PLTP |
| 0004866 | Endopeptidase inhibitor activity | Molecular function | 0.00016 | 32.46 | A2M, AHSG, ITIH2, SPP2 |
| 0060228 | Phosphatidylcholine-sterol O-acyltransferase activator activity | Molecular function | 2.11E−05 | 162.28 | APOA1, APOC1, APOA4 |
| 0086083 | Cell adhesive protein binding involved in bundle of His cell-Purkinje myocyte communication | Molecular function | 0.0013 | 129.83 | JUP, DSP |
| 0055102 | Lipase inhibitor activity | Molecular function | 0.00177 | 108.19 | APOA1, APOC1 |
| 0008035 | High-density lipoprotein particle binding | Molecular function | 0.00413 | 64.91 | APOA1, PLTP |
| 0005537 | Mannose binding | Molecular function | 0.01148 | 36.06 | MBL2, COLEC11 |
Table 6.
The significant enriched analysis of differentially expressed proteins (DEPs) in ischemic stroke.
| Pathway identifier | Pathway name | #Entities found | #Entities total | Entities ratio | Entities p value | Entities FDR | Submitted entities found |
|---|---|---|---|---|---|---|---|
| Down-regulated | |||||||
| R-HSA-381426 | Regulation of Insulin-like growth factor (IGF) transport and uptake by insulin-like growth factor binding proteins (IGFBPs) | 12 | 127 | 8.43E−03 | 3.89E−12 | 8.53E−10 | TF; ITIH2; PROC; AHSG; FUCA2; SPP2; SPARCL1; APOA1; QSOX1; IGFALS; APOB; HSP90B1 |
| R-HSA-8957275 | Post-translational protein phosphorylation | 11 | 109 | 7.23E−03 | 1.63E−11 | 1.78E−09 | TF; ITIH2; PROC; AHSG; FUCA2; SPP2; SPARCL1; APOA1; QSOX1; APOB; HSP90B1 |
| R-HSA-174824 | Plasma lipoprotein assembly, remodeling, and clearance | 9 | 98 | 6.50E−03 | 2.84E−09 | 2.07E−07 | APOC1; APOA1; LCAT; APOA4; APOB; A2M; PLTP |
| R-HSA-8963899 | Plasma lipoprotein remodeling | 7 | 56 | 3.72E−03 | 2.24E−08 | 1.21E−06 | APOA1; LCAT; APOA4; APOB; PLTP |
| R-HSA-166658 | Complement cascade | 9 | 156 | 1.04E−02 | 1.46E−07 | 6.28E−06 | IGLV2-8; COLEC11; C8G; IGLV5-45; IGKV1-16; CFHR1; CPN2; CPN1; MBL2 |
| R-HSA-168249 | Innate immune system | 23 | 1334 | 8.85E−02 | 2.11E−07 | 7.59E−06 | DSP; COLEC11; JUP; AHSG; FUCA2; GGH; CPN2; CPN1; HSP90B1; IGLV2-8; TF; APOM; C8G; IGLV5-45; IGKV1-16; LAMP1; CFHR1; QSOX1; APOB; CTSD; MBL2 |
| R-HSA-8963898 | Plasma lipoprotein assembly | 5 | 30 | 1.99E−03 | 6.23E−07 | 1.93E−05 | APOC1; APOA1; APOA4; APOB; A2M |
| R-HSA-114608 | Platelet degranulation | 8 | 141 | 9.36E−03 | 8.48E−07 | 2.29E−05 | TF; APOM; LAMP1; AHSG; SPP2; APOA1; QSOX1; A2M |
| R-HSA-76005 | Response to elevated platelet cytosolic Ca2+ | 8 | 148 | 9.82E−03 | 1.21E−06 | 2.91E−05 | TF; APOM; LAMP1; AHSG; SPP2; APOA1; QSOX1; A2M |
| R-HSA-2173782 | Binding and uptake of ligands by scavenger receptors | 8 | 168 | 1.11E−02 | 3.08E−06 | 6.46E−05 | IGLV2-8; COLEC11; IGLV5-45; IGKV1-16; APOA1; APOB; IGHA2; HSP90B1 |
| R-HSA-6798695 | Neutrophil degranulation | 12 | 480 | 3.18E−02 | 7.66E−06 | 1.45E−04 | DSP; TF; APOM; JUP; LAMP1; AHSG; FUCA2; GGH; QSOX1; CTSD; CPN1 |
| R-HSA-8964058 | HDL remodeling | 4 | 24 | 1.59E−03 | 8.78E−06 | 1.47E−04 | APOA1; LCAT; PLTP |
| R-HSA-977606 | Regulation of complement cascade | 7 | 139 | 9.22E−03 | 9.21E−06 | 1.47E−04 | IGLV2-8; C8G; IGLV5-45; IGKV1-16; CFHR1; CPN2; CPN1 |
| R-HSA-109582 | Hemostasis | 15 | 803 | 5.33E−02 | 1.65E−05 | 2.47E−04 | F10; AHSG; APOA1; IGLV2-8; TF; PROC; APOM; IGLV5-45; IGKV1-16; LAMP1; SPP2; QSOX1; APOB; A2M; IGHA2 |
| R-HSA-9029569 | NR1H3 and NR1H2 regulate gene expression linked to cholesterol transport and efflux | 5 | 66 | 4.38E−03 | 2.77E−05 | 3.88E−04 | APOC1; PLTP |
| R-HSA-8963888 | Chylomicron assembly | 3 | 14 | 9.29E−04 | 6.01E−05 | 7.82E−04 | APOA1; APOA4; APOB |
| R-HSA-9024446 | NR1H2 and NR1H3-mediated signaling | 5 | 85 | 5.64E−03 | 9.09E−05 | 1.09E−03 | APOC1; PLTP |
| R-HSA-6809371 | Formation of the cornified envelope | 6 | 138 | 9.16E−03 | 9.25E−05 | 1.11E−03 | DSP; JUP; KRT14; KRT9 |
| R-HSA-8963901 | Chylomicron remodeling | 3 | 17 | 1.13E−03 | 1.06E−04 | 1.17E−03 | APOA1; APOA4; APOB |
| R-HSA-3000480 | Scavenging by Class A receptors | 4 | 49 | 3.25E−03 | 1.38E−04 | 1.38E−03 | COLEC11; APOA1; APOB; HSP90B1 |
| R-HSA-76002 | Platelet activation, signaling and aggregation | 8 | 293 | 1.94E−02 | 1.56E−04 | 1.56E−03 | TF; APOM; LAMP1; AHSG; SPP2; APOA1; QSOX1; A2M |
| R-HSA-2168880 | Scavenging of heme from plasma | 5 | 106 | 7.03E−03 | 2.52E−04 | 2.27E−03 | IGLV2-8; IGLV5-45; IGKV1-16; APOA1; IGHA2 |
| R-HSA-166786 | Creation of C4 and C2 activators | 5 | 111 | 7.37E−03 | 3.11E−04 | 2.80E−03 | IGLV2-8; COLEC11; IGLV5-45; IGKV1-16; MBL2 |
| R-HSA-140837 | Intrinsic pathway of fibrin clot formation | 3 | 26 | 1.73E−03 | 3.68E−04 | 3.31E−03 | PROC; F10; A2M |
| R-HSA-166663 | Initial triggering of complement | 5 | 120 | 7.96E−03 | 4.42E−04 | 3.54E−03 | IGLV2-8; COLEC11; IGLV5-45; IGKV1-16; MBL2 |
| R-HSA-975634 | Retinoid metabolism and transport | 4 | 79 | 5.24E−03 | 8.29E−04 | 6.64E−03 | APOM; APOA1; APOA4; APOB |
| R-HSA-8866423 | VLDL assembly | 2 | 9 | 5.97E−04 | 1.07E−03 | 7.46E−03 | APOC1; APOB |
| R-HSA-159763 | Transport of gamma-carboxylated protein precursors from the endoplasmic reticulum to the Golgi apparatus | 2 | 9 | 5.97E−04 | 1.07E−03 | 7.46E−03 | PROC; F10 |
| R-HSA-6805567 | Keratinization | 6 | 226 | 1.50E−02 | 1.24E−03 | 7.87E−03 | DSP; JUP; KRT14; KRT9 |
| R-HSA-6806667 | Metabolism of fat-soluble vitamins | 4 | 89 | 5.91E−03 | 1.28E−03 | 7.87E−03 | APOM; APOA1; APOA4; APOB |
| R-HSA-8964046 | VLDL clearance | 2 | 10 | 6.64E−04 | 1.31E−03 | 7.87E−03 | APOC1; APOB |
| R-HSA-159782 | Removal of aminoterminal propeptides from gamma-carboxylated proteins | 2 | 10 | 6.64E−04 | 1.31E−03 | 7.87E−03 | PROC; F10 |
| R-HSA-8964043 | Plasma lipoprotein clearance | 3 | 42 | 2.79E−03 | 1.46E−03 | 8.77E−03 | APOC1; APOA1; APOB |
| R-HSA-140877 | Formation of fibrin clot (clotting cascade) | 3 | 43 | 2.85E−03 | 1.56E−03 | 9.37E−03 | PROC; F10; A2M |
| R-HSA-202733 | Cell surface interactions at the vascular wall | 6 | 257 | 1.71E−02 | 2.36E−03 | 1.42E−02 | IGLV2-8; PROC; IGLV5-45; IGKV1-16; APOB; IGHA2 |
| R-HSA-166662 | Lectin pathway of complement activation | 2 | 15 | 9.95E−04 | 2.90E−03 | 1.45E−02 | COLEC11; MBL2 |
| R-HSA-159740 | Gamma-carboxylation of protein precursors | 2 | 15 | 9.95E−04 | 2.90E−03 | 1.45E−02 | PROC; F10 |
| R-HSA-381183 | ATF6 (ATF6-alpha) activates chaperone genes | 2 | 15 | 9.95E−04 | 2.90E−03 | 1.45E−02 | HSP90B1 |
| R-HSA-159854 | Gamma-carboxylation, transport, and amino-terminal cleavage of proteins | 2 | 16 | 1.06E−03 | 3.29E−03 | 1.64E−02 | PROC; F10 |
| R-HSA-381033 | ATF6 (ATF6-alpha) activates chaperones | 2 | 17 | 1.13E−03 | 3.70E−03 | 1.85E−02 | HSP90B1 |
| R-HSA-8963896 | HDL assembly | 2 | 18 | 1.19E−03 | 4.13E−03 | 2.07E−02 | APOA1; A2M |
| R-HSA-9006931 | Signaling by nuclear receptors | 7 | 387 | 2.57E−02 | 4.25E−03 | 2.13E−02 | APOC1; PLTP; CTSD |
| R-HSA-3000471 | Scavenging by Class B receptors | 2 | 21 | 1.39E−03 | 5.57E−03 | 2.23E−02 | APOA1; APOB |
| R-HSA-140875 | Common pathway of fibrin clot formation | 2 | 25 | 1.66E−03 | 7.79E−03 | 3.12E−02 | PROC; F10 |
| R-HSA-392499 | Metabolism of proteins | 20 | 2207 | 1.46E−01 | 8.85E−03 | 3.54E−02 | ITIH2; F10; AHSG; COG4; FUCA2; APOA1; APOA4; HSP90B1; DPP4; TF; PROC; SPP2; SPARCL1; QSOX1; IGFALS; APOB; CTSD; GAPDH |
| R-HSA-168256 | Immune system | 23 | 2684 | 1.78E−01 | 9.29E−03 | 3.71E−02 | DSP; COLEC11; JUP; AHSG; FUCA2; GGH; CPN2; CPN1; HSP90B1; IGLV2-8; TF; APOM; C8G; IGLV5-45; IGKV1-16; LAMP1; CFHR1; QSOX1; APOB; CTSD; MBL2 |
| R-HSA-8963889 | Assembly of active LPL and LIPC lipase complexes | 2 | 30 | 1.99E−03 | 1.10E−02 | 4.41E−02 | APOA4 |
| R-HSA-5653656 | Vesicle-mediated transport | 10 | 827 | 5.49E−02 | 1.11E−02 | 4.45E−02 | IGLV2-8; COLEC11; TF; IGLV5-45; IGKV1-16; COG4; APOA1; APOB; IGHA2; HSP90B1 |
| R-HSA-977225 | Amyloid fiber formation | 3 | 89 | 5.91E−03 | 1.17E−02 | 4.67E−02 | TF; APOA1; APOA4 |
| R-HSA-382551 | Transport of small molecules | 11 | 966 | 6.41E−02 | 1.19E−02 | 4.76E−02 | TF; APOC1; APOA1; LCAT; APOA4; APOB; A2M; NEO1; PLTP |
| R-HSA-2187338 | Visual phototransduction | 4 | 169 | 1.12E−02 | 1.22E−02 | 4.88E−02 | APOM; APOA1; APOA4; APOB |
| Up-regulated | |||||||
| R-HSA-977606 | Regulation of complement cascade | 5 | 135 | 1.16E−02 | 2.87E−06 | 1.55E−04 | IGHV2-5; IGLV3-21; IGLL1; CFHR4; IGLV10-54 |
| R-HSA-166658 | Complement cascade | 5 | 146 | 1.26E−02 | 4.20E−06 | 1.55E−04 | IGHV2-5; IGLV3-21; IGLL1; CFHR4; IGLV10-54 |
| R-HSA-173623 | Classical antibody-mediated complement activation | 4 | 95 | 8.20E−03 | 1.97E−05 | 2.70E−04 | IGHV2-5; IGLV3-21; IGLL1; IGLV10-54 |
| R-HSA-2168880 | Scavenging of heme from plasma | 4 | 99 | 8.54E−03 | 2.31E−05 | 2.70E−04 | IGHV2-5; IGLV3-21; IGLL1; IGLV10-54 |
| R-HSA-2029481 | FCGR activation | 4 | 101 | 8.72E−03 | 2.50E−05 | 2.70E−04 | IGHV2-5; IGLV3-21; IGLL1; IGLV10-54 |
| R-HSA-2730905 | Role of LAT2/NTAL/LAB on calcium mobilization | 4 | 102 | 8.80E−03 | 2.60E−05 | 2.70E−04 | IGHV2-5; IGLV3-21; IGLL1; IGLV10-54 |
| R-HSA-166786 | Creation of C4 and C2 activators | 4 | 103 | 8.89E−03 | 2.70E−05 | 2.70E−04 | IGHV2-5; IGLV3-21; IGLL1; IGLV10-54 |
| R-HSA-166663 | Initial triggering of complement | 4 | 111 | 9.58E−03 | 3.61E−05 | 2.83E−04 | IGHV2-5; IGLV3-21; IGLL1; IGLV10-54 |
| R-HSA-2029485 | Role of phospholipids in phagocytosis | 4 | 114 | 9.84E−03 | 4.00E−05 | 2.83E−04 | IGHV2-5; IGLV3-21; IGLL1; IGLV10-54 |
| R-HSA-2871809 | FCERI mediated Ca + 2 mobilization | 4 | 117 | 1.01E−02 | 4.42E−05 | 2.83E−04 | IGHV2-5; IGLV3-21; IGLL1; IGLV10-54 |
| R-HSA-2871796 | FCERI mediated MAPK activation | 4 | 119 | 1.03E−02 | 4.72E−05 | 2.83E−04 | IGHV2-5; IGLV3-21; IGLL1; IGLV10-54 |
| R-HSA-202733 | Cell surface interactions at the vascular wall | 5 | 246 | 2.12E−02 | 5.12E−05 | 3.07E−04 | IGHV2-5; IGLV3-21; IGLL1; IGLV10-54 |
| R-HSA-9664323 | FCGR3A-mediated IL10 synthesis | 4 | 128 | 1.10E−02 | 6.26E−05 | 3.13E−04 | IGHV2-5; IGLV3-21; IGLL1; IGLV10-54 |
| R-HSA-2173782 | Binding and uptake of ligands by scavenger receptors | 4 | 129 | 1.11E−02 | 6.45E−05 | 3.22E−04 | IGHV2-5; IGLV3-21; IGLL1; IGLV10-54 |
| R-HSA-9664417 | Leishmania phagocytosis | 4 | 149 | 1.29E−02 | 1.12E−04 | 4.49E−04 | IGHV2-5; IGLV3-21; IGLL1; IGLV10-54 |
| R-HSA-9664407 | Parasite infection | 4 | 149 | 1.29E−02 | 1.12E−04 | 4.49E−04 | IGHV2-5; IGLV3-21; IGLL1; IGLV10-54 |
| R-HSA-9664422 | FCGR3A-mediated phagocytosis | 4 | 149 | 1.29E−02 | 1.12E−04 | 4.49E−04 | IGHV2-5; IGLV3-21; IGLL1; IGLV10-54 |
| R-HSA-2029482 | Regulation of actin dynamics for phagocytic cup formation | 4 | 150 | 1.29E−02 | 1.15E−04 | 4.61E−04 | IGHV2-5; IGLV3-21; IGLL1; IGLV10-54 |
| R-HSA-2871837 | FCERI mediated NF-kB activation | 4 | 167 | 1.44E−02 | 1.74E−04 | 5.21E−04 | IGHV2-5; IGLV3-21; IGLL1; IGLV10-54 |
| R-HSA-2029480 | Fcgamma receptor (FCGR) dependent phagocytosis | 4 | 175 | 1.51E−02 | 2.08E−04 | 6.23E−04 | IGHV2-5; IGLV3-21; IGLL1; IGLV10-54 |
| R-HSA-5690714 | CD22 mediated BCR regulation | 3 | 70 | 6.04E−03 | 2.33E−04 | 6.98E−04 | IGHV2-5; IGLV3-21; IGLL1 |
| R-HSA-2454202 | Fc epsilon receptor (FCERI) signaling | 4 | 218 | 1.88E−02 | 4.77E−04 | 1.43E−03 | IGHV2-5; IGLV3-21; IGLL1; IGLV10-54 |
| R-HSA-983695 | Antigen activates B cell receptor (BCR) leading to generation of second messengers | 3 | 95 | 8.20E−03 | 5.66E−04 | 1.70E−03 | IGHV2-5; IGLV3-21; IGLL1 |
| R-HSA-9664433 | Leishmania parasite growth and survival | 4 | 259 | 2.23E−02 | 9.07E−04 | 1.81E−03 | IGHV2-5; IGLV3-21; IGLL1; IGLV10-54 |
| R-HSA-9662851 | Anti-inflammatory response favouring Leishmania parasite infection | 4 | 259 | 2.23E−02 | 9.07E−04 | 1.81E−03 | IGHV2-5; IGLV3-21; IGLL1; IGLV10-54 |
| R-HSA-198933 | Immunoregulatory interactions between a lymphoid and a non-lymphoid cell | 4 | 297 | 2.56E−02 | 1.50E−03 | 3.01E−03 | IGHV2-5; IGLV3-21; IGLL1; IGLV10-54 |
| R-HSA-9673163 | Oleoyl-phe metabolism | 1 | 1 | 8.63E−05 | 1.72E−03 | 3.45E−03 | PM20D1 |
| R-HSA-5619050 | Defective SLC4A1 causes hereditary spherocytosis type 4 (HSP4), distal renal tubular acidosis (dRTA) and dRTA with hemolytic anemia (dRTA-HA) | 1 | 1 | 8.63E−05 | 1.72E−03 | 3.45E−03 | SLC4A1 |
| R-HSA-9658195 | Leishmania infection | 4 | 345 | 2.98E−02 | 2.60E−03 | 5.19E−03 | IGHV2-5; IGLV3-21; IGLL1; IGLV10-54 |
| R-HSA-168249 | Innate immune system | 7 | 1191 | 1.03E−01 | 2.79E−03 | 5.59E−03 | CHIT1; LRG1; IGHV2-5; IGLV3-21; IGLL1; CFHR4; IGLV10-54 |
| R-HSA-983705 | Signaling by the B cell receptor (BCR) | 3 | 176 | 1.52E−02 | 3.29E−03 | 6.58E−03 | IGHV2-5; IGLV3-21; IGLL1 |
| R-HSA-5619049 | Defective SLC40A1 causes hemochromatosis 4 (HFE4) (macrophages) | 1 | 2 | 1.73E−04 | 3.45E−03 | 6.89E−03 | CP |
| R-HSA-5619060 | Defective CP causes aceruloplasminemia (ACERULOP) | 1 | 2 | 1.73E−04 | 3.45E−03 | 6.89E−03 | CP |
| R-HSA-109582 | Hemostasis | 5 | 726 | 6.26E−02 | 6.76E−03 | 1.35E−02 | IGHV2-5; IGLV3-21; IGLL1; IGLV10-54 |
| R-HSA-209905 | Catecholamine biosynthesis | 1 | 4 | 3.45E−04 | 6.88E−03 | 1.37E−02 | DBH |
| R-HSA-166187 | Mitochondrial uncoupling | 1 | 6 | 5.18E−04 | 1.03E−02 | 1.37E−02 | PM20D1 |
| R-HSA-5619102 | SLC transporter disorders | 2 | 103 | 8.89E−03 | 1.35E−02 | 1.37E−02 | SLC4A1; CP |
| R-HSA-1247673 | Erythrocytes take up oxygen and release carbon dioxide | 1 | 8 | 6.90E−04 | 1.37E−02 | 1.37E−02 | SLC4A1 |
| R-HSA-159763 | Transport of gamma-carboxylated protein precursors from the endoplasmic reticulum to the Golgi apparatus | 1 | 9 | 7.77E−04 | 1.54E−02 | 1.54E−02 | PROZ |
| R-HSA-425381 | Bicarbonate transporters | 1 | 10 | 8.63E−04 | 1.71E−02 | 1.71E−02 | SLC4A1 |
| R-HSA-159782 | Removal of aminoterminal propeptides from gamma-carboxylated proteins | 1 | 10 | 8.63E−04 | 1.71E−02 | 1.71E−02 | PROZ |
| R-HSA-159740 | Gamma-carboxylation of protein precursors | 1 | 10 | 8.63E−04 | 1.71E−02 | 1.71E−02 | PROZ |
| R-HSA-2534343 | Interaction with cumulus cells and the zona pellucida | 1 | 11 | 9.49E−04 | 1.88E−02 | 1.88E−02 | CHIT1 |
| R-HSA-159854 | Gamma-carboxylation, transport, and amino-terminal cleavage of proteins | 1 | 11 | 9.49E−04 | 1.88E−02 | 1.88E−02 | PROZ |
| R-HSA-1480926 | O2/CO2 exchange in erythrocytes | 1 | 12 | 1.04E−03 | 2.05E−02 | 2.05E−02 | SLC4A1 |
| R-HSA-1237044 | Erythrocytes take up carbon dioxide and release oxygen | 1 | 12 | 1.04E−03 | 2.05E−02 | 2.05E−02 | SLC4A1 |
| R-HSA-189085 | Digestion of dietary carbohydrate | 1 | 12 | 1.04E−03 | 2.05E−02 | 2.05E−02 | CHIT1 |
| R-HSA-209776 | Metabolism of amine-derived hormones | 1 | 18 | 1.55E−03 | 3.06E−02 | 3.06E−02 | DBH |
| R-HSA-5619115 | Disorders of transmembrane transporters | 2 | 181 | 1.56E−02 | 3.85E−02 | 3.85E−02 | SLC4A1; CP |
| R-HSA-5653656 | Vesicle-mediated transport | 4 | 761 | 6.57E−02 | 3.86E−02 | 3.86E−02 | IGHV2-5; IGLV3-21; IGLL1; IGLV10-54 |
| R-HSA-8935690 | Digestion | 1 | 24 | 2.07E−03 | 4.06E−02 | 4.06E−02 | CHIT1 |
| R-HSA-1187000 | Fertilization | 1 | 26 | 2.24E−03 | 4.39E−02 | 4.39E−02 | CHIT1 |
| R-HSA-425410 | Metal ion SLC transporters | 1 | 26 | 2.24E−03 | 4.39E−02 | 4.39E−02 | CP |
| R-HSA-8963743 | Digestion and absorption | 1 | 29 | 2.50E−03 | 4.89E−02 | 4.89E−02 | CHIT1 |
Discussion
For this exploratory bioinformatics analysis, potential PPI networks between ischemic stroke and inflammatory bowel disease were predicted using the open catalog of human GWAS studies. Although this predicted PPI network requires careful interpretation and preclinical and clinical validation, it shows new evidence that will contribute to new studies on the relationship between ischemic stroke and inflammatory bowel disease. In the following we propose different hypothetical biological scenarios depending on our PPI network, by which inflammatory bowel disease can increase the risk of ischemic stroke.
It has long been known that cardioembolism, large artery atherosclerosis, and small vessel occlusion are three main pathophysiological mechanisms in patients with IS18. Moreover, cerebral ischemia is responsible for producing various immune cell mediators, which can exacerbate ischemic brain injury. CNS inflammation induced by ischemic conditions plays an essential role in stroke pathophysiology and exacerbates infarct formation at the injury site19,20.
As previously mentioned, in our study, the five most significant clusters from the PPI network were identified by integrated bioinformatics analysis. These clusters contained 40 hub genes. The first one consisted of 14 genes (KAT2B, IRF4, IL23R, SMARCA4, IRF1, IRF5, IRF6, RELA, IL12B, STAT4, STAT3, JAK2, TYK2, ARID1A) (Fig. 4B) whose biological functions could be associated with Cytokine Signaling in Immune system, Interleukin-23 signaling, Signaling by Interleukins, Th17 cell differentiation, and JAK-STAT signaling pathway. By analyzing the first cluster, we found that various immune cell mediators influence the various processes of atherosclerosis mainly by affecting inflammation, with cytokines and interleukin-related signaling having a particularly significant impact. IRF5 expression was significantly increased in peripheral blood mononuclear cells (PBMCs) and colonic inflammatory tissue in patients with IBD and was significantly associated with IBD activity21. The activated IRF5 increased macrophage infiltration and lipid accumulation, with concomitant reductions in smooth muscle cells and collagen content, weakening plaque stability and promoting the formation of atherosclerotic plaques 22. KAT2B upregulation promotes acetylation of HMGB1, promotes pro-inflammatory cell polarization and macrophage recruitment, and promotes atherosclerotic progression 23. Dysregulation of KAT2B may lead to inhibition of IL-10, a key anti-inflammatory cytokine, disrupting innate and adaptive inflammatory responses and promoting the development of inflammatory bowel disease24. In peripheral inflammation, interferon regulatory factor-5 (IRF5) and IRF4 regulate M1 and M2 activation of macrophages, respectively, leading to a greater risk of ischemia in the elderly brain25. Traditional T cells coordinate the inflammatory response in atherosclerosis and their function is altered by the lipoprotein milieu and complement activity. Hypercholesterolemic states are associated with upregulation of DAF expression in circulating T cells and increased levels of nuclear factor kappa B (NF-kB) and interferon regulatory factor 4 (IRF4) 26. The inhibition of IRF5 expression and promotion of IRF4 expression in lamina propria monocytes induces macrophage polarization to the M2 phenotype, which improves the inflammatory response in inflammatory bowel disease27, and increased IRF4 expression results in enhanced M2 activation, reduced pro-inflammatory response, and improved stroke prognosis, whereas downregulation of IRF4 results in increased IRF5 expression, enhanced M1 activation, increased pro-inflammatory response, and functional poorer recovery28. The inflammatory response, immune response, and cytokine-cytokine receptor interactions may play an important role in the progression of atherosclerosis. Co-operative inflammatory signaling by TLRs, STAT/IRF, and IFNs leads to M1 polarization of macrophages, which contributes to the development of atherosclerosis29. In response to TNF-α stimulation, Ataxin-10 promoted the cytoplasmic localization of IRF-1, thereby inhibiting VCAM-1 transcription 30. IL23R-dependent gamma-delta T cells can release IL-17 and GM-CSF, which together induce inflammation and necrosis in macrophages. Il-23R + gamma-delta T cells locally promote early lesion formation in the aortic root and contribute to the expansion of the necrotic core, a hallmark of vulnerable atherosclerotic lesions 31. Interferon regulatory factor 1 (IRF-1) contributes to the pathological phenotype of VSMCs during atherosclerosis by increasing CCL19 transcription, whereas silencing IRF-1 inhibits angiotensin proliferation and migration and downregulates CCL19 expression, thereby suppressing atherosclerosis 32. RelA-driven escalation of pro-inflammatory gene responses in intestinal epithelial cells exacerbates inflammatory cell infiltration and colonic lesions, driving inflammation associated with inflammatory bowel disease (IBD) 33. Upregulation of RELA /p65 inhibits Hif-1α, thereby suppressing neuroinflammatory activity after ischemic stroke, and attenuating brain injury caused by cerebral ischemia34. SMARCA4/BAF190A may promote neural stem cell self-renewal/proliferation by enhancing Notch-dependent proliferative signaling while insensitizing neural stem cells to SHH-dependent differentiation cues. Inflammation of endothelial cells induces inflammatory cytokine production and monocyte adhesion, which are key events in initiating atherosclerosis. aberrant activation of STAT3 has been shown to contribute to the development and progression of atherosclerosis, and signal transducer and activator of transcription 3 (STAT3) have key roles in endothelial cell dysfunction, macrophage polarization, inflammation, and immunity during atherosclerosis35. Reduced phosphorylation of signal transducer and activator of transcription 3 (STAT3) and nuclear levels of STAT3 and interferon-regulated transcription factor-1 (IRF1), which in turn inhibits the STAT3/IRF1 pathway in vascular endothelial cells, produce anti-atherosclerotic effects on TNF-α-induced inflammation36. As the primary pathological basis of ischemic stroke disease, its pathogenesis has been shown to involve an imbalance in anti-inflammatory/pro-inflammatory processes. IL-23R can enhance its antigen-presenting capacity through an autocrine pathway, allowing it to infiltrate the lesion site, promoting its secretion of large amounts of inflammatory factors, and upregulating pro-inflammatory DCs and macrophages. The IL-23 binds to IL-23R on the surface of target cells through Janus kinase 2/signal transducer and transcriptional activator channels to deliver signals involved in chronic inflammatory and autoimmune diseases37. Among the nine IRFs, at least three (IRF-1, IRF-5, and IRF-8) are involved in pro-inflammatory M1 commitment, while IRF-3 and IRF-4 control M2 polarization38. IRF6 is a potential causative gene for ischemic stroke39. STAT4 deletion reduces perivascular and visceral AT inflammation, which may reduce atheromatous plaque formation through direct or indirect effects40. The mRNA levels of IL-23 and IL-23R were significantly higher in carotid plaques compared to non-atherosclerotic vessels. The coalition showed co-localization with plaque macrophages. IL-23 accentuated tumor necrosis factor release in monocytes from patients with carotid atherosclerosis, and high plasma levels of IL-23 may increase mortality. IL-23 is associated with disease progression in patients with carotid atherosclerosis and may be involved in IL-17-related mechanisms41. Inhibition of TNF-α-induced phosphorylation of IkappaB-α and nuclear translocation of NF-kappaB P65 (RELA) attenuated TNFα-induced expression of ICAM-1, VCAM-1 and E-selectin, which had a significant benefit in delaying the progression of inflammatory diseases, including atherosclerosis42. ARID1A, a subunit of the SWItch/Sucrose Non-Fermentable (SWI/SNF) chromatin remodeling complex, is localized to promoters and enhancers to influence transcription to affect the progression of atherosclerosis43.
Spliceosome and mRNA Splicing-Major Pathway are the most relevant signaling pathways in the second cluster, and this cluster consists of BUD13, RBM22, CDC5L, PRF8, PLRG1, and AQR. The main molecular events after ischemic stroke are translation-related, which may be caused by subsequent signaling pathway-related effects caused by upregulated cell membrane ribosomal and spliceosomal complex proteins44. BUD13, a subunit of the retention and splicing complex, may significantly increase the risk of ischemic stroke by affecting APOA1 leading to elevated TG and VLDL45,46. PLRG1 is a core component of the complex encoding the cell division cycle 5-like (CDC5L) complex. The CDC5L complex is part of the spliceosome, and both are highly conserved spliceosomal proteins across species. They are both shown to be part of the secondary spliceosomal protein complex and their encoded proteins play a critical role in alternative splice site selection. The interaction between CDC5L and PLRG1 is important for pre-mRNA splicing and essential for human pre-mRNA splicing47. Transcript variants of this gene with alternative splicing have been observed, encoding multiple isoforms48. CDC5L is significantly associated with the diagnosis of atrial fibrillation and ischemic stroke49. Knockdown of AQR in HepG2 facilitates glucose uptake, decreases PCK2 expression levels, increases GSK-3β phosphorylation, and restores insulin sensitivity50. The Innate immune genes are differentially expressed during either adaptation to hypoxia or recovery from H/R stress. Prolyl hydroxylase EGL-9, a known regulator of adaptation to hypoxia and innate immune response, inhibits the rapid recovery from H/R stress by activating AQR activity in O2-sensing neurons51. PPRF8 (pre-mRNA processing factor 8), a core component of the spliceosome, is an important mediator of hypoxia-induced mitosis52, and a high-fat diet upregulates the expression of genes related to the spliceosome53, increasing the risk of ischemic stroke. Adjusting the diet may enhance functional recovery after stroke by modulating insulin resistance and normalizing glucose metabolism54.
The third cluster contains six genes, SPRED2, PSMA4, PSMC4, PSMB3, SUFU, PSMD8, which are mainly involved in Degradation of GLI1, GLI2and GLI3 by the proteasome and Proteasome signaling pathways. Atherosclerosis is the primary pathological basis of ischemic stroke. In addition to its intensive inflammatory character, atherosclerosis also has features of enhanced proliferation, apoptosis, and oxidative stress. Besides playing an essential role in the degradation of dysfunctional and oxidatively damaged proteins, the ubiquitin–proteasome system (UPS), the major intracellular degradation system in eukaryotic cells, is involved in many processes that influence the progression of atherosclerotic disease55, which in turn affects the outcome of ischemic brain injury. SPRED2 is a negative regulator of the extracellular signal-regulated kinase (ERK) pathway and is important for cell proliferation, neuronal differentiation, plasticity, and survival. The decrease in SPRED-2 after injury may be related to the activation of the ERK pathway, and SPRED-2 signaling may play a role in the cell proliferation phase of neural repair in the zebrafish brain after injury56. SPRED2 affects neurological recovery after stroke by reducing cell migration through the ERK/c-Fos/MMPs pathway57. By binding to Gli, Sufu regulates the expression of target genes downstream of the Hedgehog (Hh) signaling pathway, and Sufu alternates between "open" and "closed" conformations, with the "closed" form of Sufu stabilized by Gli binding and inhibited by Hh processing. In contrast, the "open" state of Sufu is facilitated by the separation of Gli and Hh signaling58, which in turn affects neural regeneration in the brain. Down-regulation of three subunits of the proteasome (PSMA2, PSMA3, and PSMA4) genes to regulate cellular functions against oxidative stress, which has the effect of reducing atherosclerosis59. Specific proteasome inhibitor PR957 inhibited LMP7 and significantly attenuated histological damage to the cerebral white matter and the cognitive function in ischemic stroke by suppressing the inflammatory response60.
The fourth cluster is mainly related to thrombosis and coagulation signaling pathways, containing five genes PLAU, FGG, F2, FGB, and FGA. The three genes FGG, FGB, and FGA encode fibrinogen, which is a separate risk factor for ischemic stroke, like hypertension and diabetes mellitus61. In addition, single nucleotide polymorphisms (SNPs) in FGG and FGA mediate an increase in D-dimers62, which are significantly associated with stroke progression63. Fibrinogen (Fg) levels decrease in the colon of inflammatory bowel disease (IBD), which plays a crucial role in the pathogenesis of IBD by regulating vascular permeability (VP) through activation of AKT and subsequent microfilament depolymerization64. The level of intraplatelet PLAU is closely related to the production of intraplatelet plasma proteins and the secondary degradation of α-granulin, which affects fibrinolysis65. Moreover, PLAU may play a role in the treatment of ulcerative colitis66. Binding of recombinant PLAU (uPA) or endogenous uPA to uPAR induces membrane recruitment and activation of β1 integrins via low-density lipoprotein receptor-associated protein-1 (LRP1), causing activation of the Rho family small GTPase Rac1 and Rac1-induced axonal regeneration, which promotes neuronal growth during development and functional improvement after ischemic injury67,68. The F2 gene encodes thrombospondin (also known as coagulation factor II), which is proteolytically cleaved in multiple steps to form activated serine protease thrombin. The activated thrombin plays an essential role in thrombosis and hemostasis by converting fibrinogen to fibrin during clot formation, stimulating platelet aggregation, and animating other coagulation factors.
Interestingly, the fifth cluster also mainly involves the interleukin signaling pathway, and the interaction of FOS and CCL2 genes in this cluster may play a bridging role. FOS is a potential target gene for Crohn's disease (CD)69, where downregulation of FOS significantly enhances oxidative stress levels, accelerates neuronal apoptosis, and inhibits mitochondrial function, increasing the risk of ischemic stroke70. CCL2 is intimately associated with unstable atherosclerosis as upregulated based on inflammatory stimuli. Inhibition of CCL2 expression attenuates the polarization of pro-inflammatory microglia and inhibits the release of inflammatory cytokines such as TNF-α, IFN-γ, IL-1β, IL-6, IL-12, IL-17, and IL-23 while acting as a neuroprotective agent71,72. Overexpression of CCL2 is closely associated with colonic inflammation in inflammatory bowel disease73. IL-23 and IL-17 induce platelet-activating factor receptor (PAF-R) expression on activated T cells, which is invoked by PAF binding to PAF-R. PAF-R is co-expressed with IL-17 and similarly regulated with the Th17 markers IL-17A, IL-17F, IL-22, and RORC74. Through STAT3, IL-6 and IL-21 promote the expression of RORA and RORC genes encoding RORα and RORγt, respectively, RORγ(t) is an essential regulator of Th17 cell differentiation. These two RORs then drive the expression of IL-17A/F and IL-22. Inverse RORγ(t) agonists cause a rebalancing of the Th17/Treg ratio in favor of anti-inflammatory Tregs75. RORA is not only overexpressed in both Crohn's disease (CD) and ulcerative colitis (UC)76, but is also involved in cholesterol metabolism77. RORgammat( +) Treg cells in patients with inflammatory bowel disease are progressively enlarged and have pro-inflammatory properties78. Endothelial-to-mesenchymal transition (EndMT) plays a significant role in atherosclerosis, and Twist-179, a prime regulator of T helper cells adapted to chronic inflammatory metabolism, and its activation induces this process80. Many cytokines and chemokines are involved in the process of atherosclerotic plaque formation, either accelerating the progression of atherosclerotic plaque or slowing down this process. Plasma levels of IL-8/CXCL8 in patients with acute ischemic stroke tend to increase over time81. Serum CXCL8 levels are associated with infarct size and functional outcome in patients with ischemic stroke82. And IL10 causes IL-1beta and TNF-alpha to be down-regulated, which may effectively reduce inflammation in atherosclerotic plaques83. Chemokine CXCL12, on the other hand, exerts a protective role against atherosclerosis84. Furthermore, atherosclerosis is an inflammatory disease of the vessel wall characterized by the activation of the innate immune system with macrophages as the main actors and the adaptive immune system with Th1 as the primary factor. In advanced atherosclerosis, a defect in outflow cells drives disease progression, and the expansion of regulatory T cells (TREG) ameliorates the role of outflow cells. Treg cells secrete interleukin-13 (IL-13), which stimulates macrophage production of IL-10. Endocrine paracrine signaling of IL-10 induces Vav1 in macrophages and promotes phagocytosis by apoptotic cells85. In addition, Treg cells promote macrophage efflux and improve inflammatory conditions by enhancing the transcytotic signaling pathway of apoptotic cell internalization86.
Finally, the results of bioinformatics analysis of proteins significantly differentially expressed in four ischemic stroke patients and four healthy subjects suggested that the Complement cascade related signaling pathways, Innate Immune System, and hemostasis related signaling pathways were the most significantly associated signaling pathways in ischemic stroke. Although this is not entirely consistent with the signaling pathways associated with ischemic stroke and inflammatory bowel disease obtained from previous analyses, it also suggests that the immune system, signal transduction, and hemostasis-related pathways are key signaling pathways in ischemic stroke. There are two main limitations of this study. First, due to the lag in the inclusion of proteins and protein interactions in bioinformatics such as string, many newly discovered proteins are not available with corresponding matching data, and the results may be further updated with the addition of data from subsequent related studies. However, because of the universality of this study, which included more than one million people and involved multiple populations, the results are of good value. Second, we did not perform further protein validation, but since this study used the DIA-MS technique, which has high accuracy, so further validation was carried out in the next phase. Our study may help us understand the relationship between ischemic stroke and inflammatory bowel disease and, with the help of advances in proteomics such as DIA-MS, help further understand the molecules and cytokines involved in inflammatory signaling in the gut-brain axis, which is essential to our understanding of neuroimmune communication and help develop immunomodulatory therapies for ischemic stroke.
Conclusion
In conclusion, by performing bioinformatic analysis of two datasets downloaded from NHGRI-GWAS, including ischemic stroke and inflammatory bowel disease, we constructed a protein interaction network containing 589 genes, identified a core protein-related interaction network composed of 40 hub genes, and determined that cytokine and interleukin-related signaling pathways, spliceosome, ubiquitin–proteasome system (UPS), thrombotic and anticoagulant signaling pathways are closely associated with ischemic stroke and inflammatory bowel disease. And these findings could significantly improve our understanding of the potential molecularly linked events in IS and IBD. Furthermore, these candidate genes and pathways could serve as predictive and therapeutic targets for both states (Supplementary Information S1).
Supplementary Information
Acknowledgements
The authors sincerely acknowledge the anonymous reviewers for their insights and comments to further improve the quality of the manuscript.
Abbreviations
- IS
Ischemic stroke
- IBD
Inflammatory bowel disease
- PPI
Protein–protein interaction
- UPS
Ubiquitin–proteasome system
- DIA
Data independent acquisition
- GO
Gene ontology
- DEPs
Differentially expressed proteins
Author contributions
W.H., N.Z., and S.T. conceived and designed the study; W.H. and P.L. performed data analysis; W.H. and S.T. wrote the paper.
Funding
Natural Science Foundation of Hunan Province, China (Grant No. 2018JJ6035).
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Nianju Zeng, Email: zengnianju@163.com.
Sheng Tan, Email: tansheng18@126.com.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-27459-w.
References
- 1.Ovbiagele B, Goldstein LB, Higashida RT, Howard VJ, Johnston SC, Khavjou OA, Lackland DT, Lichtman JH, Mohl S, Sacco RL, et al. Forecasting the future of stroke in the United States: A policy statement from the American Heart Association and American Stroke Association. Stroke. 2013;44(8):2361–2375. doi: 10.1161/STR.0b013e31829734f2. [DOI] [PubMed] [Google Scholar]
- 2.Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, Boehme AK, Buxton AE, Carson AP, Commodore-Mensah Y, et al. Heart disease and stroke statistics-2022 update: A report from the American heart association. Circulation. 2022;145(8):e153–e639. doi: 10.1161/CIR.0000000000001052. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease C, Prevention. Prevalence and most common causes of disability among adults--United States, 2005. MMWR Morb. Mortal Wkly Rep.58(16), 421–426 (2009). [PubMed]
- 4.Ogawa H, Yamamoto K, Kamisako T, Meguro T. Phytosterol additives increase blood pressure and promote stroke onset in salt-loaded stroke-prone spontaneously hypertensive rats. Clin. Exp. Pharmacol. Physiol. 2003;30(12):919–924. doi: 10.1111/j.1440-1681.2003.03939.x. [DOI] [PubMed] [Google Scholar]
- 5.Collaborators GBDLRoS, Feigin, V.L., Nguyen, G., Cercy, K., Johnson, C. O., Alam, T., Parmar, P.G., Abajobir, A.A., Abate, K.H., Abd-Allah, F., et al. Global, regional, and country-specific lifetime risks of stroke, 1990 and 2016. N. Engl. J. Med.379(25), 2429–2437 (2018). [DOI] [PMC free article] [PubMed]
- 6.Ando M, Mukuda T, Kozaka T. Water metabolism in the eel acclimated to sea water: From mouth to intestine. Comp. Biochem. Physiol. B: Biochem. Mol. Biol. 2003;136(4):621–633. doi: 10.1016/s1096-4959(03)00179-9. [DOI] [PubMed] [Google Scholar]
- 7.Kaplan GG. The global burden of IBD: from 2015 to 2025. Nat. Rev. Gastroenterol. Hepatol. 2015;12(12):720–727. doi: 10.1038/nrgastro.2015.150. [DOI] [PubMed] [Google Scholar]
- 8.Tanislav C, Trommer K, Labenz C, Kostev K. Inflammatory bowel disease as a precondition for stroke or TIA: A matter of Crohn's disease rather than ulcerative colitis. J. Stroke Cerebrovasc. Dis. 2021;30(7):105787. doi: 10.1016/j.jstrokecerebrovasdis.2021.105787. [DOI] [PubMed] [Google Scholar]
- 9.Arya AK, Hu B. Brain-gut axis after stroke. Brain Circ. 2018;4(4):165–173. doi: 10.4103/bc.bc_32_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rhee SH, Pothoulakis C, Mayer EA. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat. Rev. Gastroenterol. Hepatol. 2009;6(5):306–314. doi: 10.1038/nrgastro.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kichloo A, Dahiya DS, Shaka H, Jamal S, Khan MZ, Wani F, Mehboob A, Kanjwal K. Impact of atrial fibrillation on inflammatory bowel disease hospitalizations-a nationwide retrospective study. Proc. (Bayl Univ. Med. Cent.) 2021;34(6):673–677. doi: 10.1080/08998280.2021.1951071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang WS, Tseng CH, Chen PC, Tsai CH, Lin CL, Sung FC, Kao CH. Inflammatory bowel diseases increase future ischemic stroke risk: A Taiwanese population-based retrospective cohort study. Eur. J. Intern. Med. 2014;25(6):561–565. doi: 10.1016/j.ejim.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 13.Zhao H, Zhu J, Ju L, Sun L, Tse LA, Kinra S, Li Y. Osteoarthritis and stroke: A bidirectional mendelian randomization study. Osteoarth. Cartil. 2022;30(10):1390–1397. doi: 10.1016/j.joca.2022.06.006. [DOI] [PubMed] [Google Scholar]
- 14.Mi H, Muruganujan A, Ebert D, Huang X, Thomas PD. PANTHER version 14: More genomes, a new PANTHER GO-slim and improvements in enrichment analysis tools. Nucleic Acids Res. 2019;47(D1):D419–D426. doi: 10.1093/nar/gky1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 2000;25(1):25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gene Ontology C. Gene ontology consortium: Going forward. Nucleic Acids Res.43(Database issue), D1049–1056 (2015). [DOI] [PMC free article] [PubMed]
- 17.Bardou P, Mariette J, Escudie F, Djemiel C, Klopp C. jvenn: an interactive Venn diagram viewer. BMC Bioinf. 2014;15:293. doi: 10.1186/1471-2105-15-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Paiva BR, de Miranda Alves MA, Conforto AB, Rodrigues DLG, Silva GS. Etiological classification of stroke in patients with chagas disease using TOAST, causative classification system TOAST, and ASCOD phenotyping. J. Stroke Cerebrovasc. Dis. 2017;26(12):2864–2869. doi: 10.1016/j.jstrokecerebrovasdis.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 19.Amantea D, Micieli G, Tassorelli C, Cuartero MI, Ballesteros I, Certo M, Moro MA, Lizasoain I, Bagetta G. Rational modulation of the innate immune system for neuroprotection in ischemic stroke. Front. Neurosci. 2015;9:147. doi: 10.3389/fnins.2015.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benakis C, Garcia-Bonilla L, Iadecola C, Anrather J. The role of microglia and myeloid immune cells in acute cerebral ischemia. Front Cell Neurosci. 2014;8:461. doi: 10.3389/fncel.2014.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang Y, Zhang C, Jing D, He H, Li X, Wang Y, Qin Y, Xiao X, Xiong H, Zhou G. IRF5 acts as a potential therapeutic marker in inflammatory bowel diseases. Inflamm. Bowel Dis. 2020;3:3. doi: 10.1093/ibd/izaa200. [DOI] [PubMed] [Google Scholar]
- 22.Wu Z, Geng J, Bai Y, Qi Y, Chang C, Jiao Y, Guo Z. MicroRNA-22 inhibition promotes the development of atherosclerosis via targeting interferon regulator factor 5. Exp. Cell Res. 2021;409(2):112922. doi: 10.1016/j.yexcr.2021.112922. [DOI] [PubMed] [Google Scholar]
- 23.Qi X, Wang H, Xia L, Lin R, Li T, Guan C, Liu T. miR-30b-5p releases HMGB1 via UBE2D2/KAT2B/HMGB1 pathway to promote pro-inflammatory polarization and recruitment of macrophages. Atherosclerosis. 2021;324:38–45. doi: 10.1016/j.atherosclerosis.2021.02.016. [DOI] [PubMed] [Google Scholar]
- 24.Bai AH, Wu WK, Xu L, Wong SH, Go MY, Chan AW, Harbord M, Zhang S, Chen M, Wu JC, et al. Dysregulated lysine acetyltransferase 2B promotes inflammatory bowel disease pathogenesis through transcriptional repression of interleukin-10. J. Crohns. Colitis. 2016;10(6):726–734. doi: 10.1093/ecco-jcc/jjw020. [DOI] [PubMed] [Google Scholar]
- 25.Zhao S-C, Wang C, Xu H, Wu W-Q, Chu Z-H, Ma L-S, Zhang Y-D, Liu F. Age-related differences in interferon regulatory factor-4 and -5 signaling in ischemic brains of mice. Acta Pharmacol. Sin. 2017;38(11):1425–1434. doi: 10.1038/aps.2017.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nyambuya TM, Dludla PV, Mxinwa V, Nkambule BB. The pleotropic effects of fluvastatin on complement-mediated T-cell activation in hypercholesterolemia. Biomed. Pharmacother. 2021;143:112224. doi: 10.1016/j.biopha.2021.112224. [DOI] [PubMed] [Google Scholar]
- 27.Zhu W, Jin Z, Yu J, Liang J, Yang Q, Li F, Shi X, Zhu X, Zhang X. Baicalin ameliorates experimental inflammatory bowel disease through polarization of macrophages to an M2 phenotype. Int. Immunopharmacol. 2016;35:119–126. doi: 10.1016/j.intimp.2016.03.030. [DOI] [PubMed] [Google Scholar]
- 28.Al Mamun A, Chauhan A, Qi S, Ngwa C, Xu Y, Sharmeen R, Hazen AL, Li J, Aronowski JA, McCullough LD, et al. Microglial IRF5-IRF4 regulatory axis regulates neuroinflammation after cerebral ischemia and impacts stroke outcomes. Proc. Natl. Acad. Sci. USA. 2020;117(3):1742–1752. doi: 10.1073/pnas.1914742117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu GF, Chen SC, Xia YP, Ye ZM, Cao F, Hu B. Synergistic inflammatory signaling by cGAS may be involved in the development of atherosclerosis. Aging (Albany NY) 2021;13(4):5650–5673. doi: 10.18632/aging.202491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Y, Zhang Q, Li N, Ding L, Yi J, Xiao Y, Chen S, Huang X. Ataxin-10 inhibits TNF-alpha-induced endothelial inflammation via suppressing interferon regulatory factor-1. Mediat. Inflamm. 2021;2021:7042148. doi: 10.1155/2021/7042148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gil-Pulido J, Amezaga N, Jorgacevic I, Manthey HD, Rosch M, Brand T, Cidlinsky P, Schafer S, Beilhack A, Saliba AE, et al. Interleukin-23 receptor expressing gammadelta T cells locally promote early atherosclerotic lesion formation and plaque necrosis in mice. Cardiovasc. Res. 2021;1:1. doi: 10.1093/cvr/cvab359. [DOI] [PubMed] [Google Scholar]
- 32.Shen Y, Sun Z, Mao S, Zhang Y, Jiang W, Wang H. IRF-1 contributes to the pathological phenotype of VSMCs during atherogenesis by increasing CCL19 transcription. Aging (Albany NY) 2020;13(1):933–943. doi: 10.18632/aging.202204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chawla M, Mukherjee T, Deka A, Chatterjee B, Sarkar UA, Singh AK, Kedia S, Lum J, Dhillon MK, Banoth B, et al. An epithelial Nfkb2 pathway exacerbates intestinal inflammation by supplementing latent RelA dimers to the canonical NF-kappaB module. Proc. Natl. Acad. Sci. U S A. 2021;118(25):1. doi: 10.1073/pnas.2024828118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu R, Liao XY, Pan MX, Tang JC, Chen SF, Zhang Y, Lu PX, Lu LJ, Zou YY, Qin XP, et al. Glycine exhibits neuroprotective effects in ischemic stroke in rats through the inhibition of M1 microglial polarization via the NF-κB p65/Hif-1α signaling pathway. J. Immunol. 2022;202(6):1704–1714. doi: 10.4049/jimmunol.1801166. [DOI] [PubMed] [Google Scholar]
- 35.Chen Q, Lv J, Yang W, Xu B, Wang Z, Yu Z, Wu J, Yang Y, Han Y. Targeted inhibition of STAT3 as a potential treatment strategy for atherosclerosis. Theranostics. 2019;9(22):6424–6442. doi: 10.7150/thno.35528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iwashima T, Kudome Y, Kishimoto Y, Saita E, Tanaka M, Taguchi C, Hirakawa S, Mitani N, Kondo K, Iida K. Aronia berry extract inhibits TNF-alpha-induced vascular endothelial inflammation through the regulation of STAT3. Food Nutr. Res. 2019;63:1. doi: 10.29219/fnr.v63.3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu W, Chang C, Hu H, Yang H. Interleukin-23: A new atherosclerosis target. J. Interferon. Cytokine Res. 2018;38(10):440–444. doi: 10.1089/jir.2018.0006. [DOI] [PubMed] [Google Scholar]
- 38.Chistiakov DA, Myasoedova VA, Revin VV, Orekhov AN, Bobryshev YV. The impact of interferon-regulatory factors to macrophage differentiation and polarization into M1 and M2. Immunobiology. 2018;223(1):101–111. doi: 10.1016/j.imbio.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 39.Mo XB, Lei SF, Zhang YH, Zhang H. Integrative analysis identified IRF6 and NDST1 as potential causal genes for ischemic stroke. Front. Neurol. 2019;10:517. doi: 10.3389/fneur.2019.00517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dobrian AD, Hatcher MA, Brotman JJ, Galkina EV, Taghavie-Moghadam P, Pei H, Haynes BA, Nadler JL. STAT4 contributes to adipose tissue inflammation and atherosclerosis. J. Endocrinol. 2015;227(1):13–24. doi: 10.1530/JOE-15-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abbas A, Gregersen I, Holm S, Daissormont I, Bjerkeli V, Krohg-Sorensen K, Skagen KR, Dahl TB, Russell D, Almas T, et al. Interleukin 23 levels are increased in carotid atherosclerosis: possible role for the interleukin 23/interleukin 17 axis. Stroke. 2015;46(3):793–799. doi: 10.1161/STROKEAHA.114.006516. [DOI] [PubMed] [Google Scholar]
- 42.Jiang Y, Jiang LLI, Maimaitirexiati XMZY, Zhang Y, Wu L. Irbesartan attenuates TNF-α-induced ICAM-1, VCAM-1, and E-selectin expression through suppression of NF-κB pathway in HUVECs. Eur. Rev. Med. Pharmacol. Sci. 2015;19(17):3295. [PubMed] [Google Scholar]
- 43.Wu S, Fatkhutdinov N, Rosin L, Luppino JM, Iwasaki O, Tanizawa H, Tang HY, Kossenkov AV, Gardini A, Noma KI, et al. ARID1A spatially partitions interphase chromosomes. Sci. Adv. 2022;5(5):5294. doi: 10.1126/sciadv.aaw5294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Agarwal A, Park S, Ha S, Kwon JS, Kang SU. Quantitative mass spectrometric analysis of the mouse cerebral cortex after ischemic stroke. PLoS ONE. 2020;15(4):e0231978. doi: 10.1371/journal.pone.0231978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pranavchand R, Reddy BM. Quantitative trait loci at the 11q23.3 chromosomal region related to dyslipidemia in the population of Andhra Pradesh. India. Lipids Health Dis. 2017;16(1):1. doi: 10.1186/s12944-017-0507-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berger JS, Mcginn AP, Howard BV, Kuller L, Manson J, Otvos J, Curb JD, Eaton CB, Kaplan RC, Lynch JK. Lipid and lipoprotein biomarkers and the risk of ischemic stroke in postmenopausal women. Stroke. 2012;43(4):958–966. doi: 10.1161/STROKEAHA.111.641324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ajuh P, Lamond AI. Identification of peptide inhibitors of pre-mRNA splicing derived from the essential interaction domains of CDC5L and PLRG1. Nucleic Acids Res. 2003;31(21):6104–6116. doi: 10.1093/nar/gkg817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Llères D, Denegri M, Biggiogera M, Ajuh P, Lamond AI. Direct interaction between hnRNP-M and CDC5L/PLRG1 proteins affects alternative splice site choice. EMBO Rep. 2010;11(6):445–451. doi: 10.1038/embor.2010.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang YF, Meng LB, Hao ML, Yang JF, Zou T. Identification of co-expressed genes between atrial fibrillation and stroke. Front. Neurol. 2020;11:184. doi: 10.3389/fneur.2020.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Song C, Yan H, Wang HM, Zhang Y, Cao H, Wan Y, Kong L, Chen S, Xu H, Pan BX. AQR is a novel type 2 diabetes-associated gene that regulates signaling pathways critical for glucose metabolism. J. Genet. Genom. 2018;1:111–120. doi: 10.1016/j.jgg.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 51.Zuckerman B, Abergel Z, Zelmanovich V, Romero L, Abergel R, Livshits L, Smith Y, Gross E. Characterization of gene expression associated with the adaptation of the nematode C. elegans to hypoxia and reoxygenation stress reveals an unexpected function of the neuroglobin GLB-5 in innate immunity. Free Radic. Biol. Med. 2017;108:858–873. doi: 10.1016/j.freeradbiomed.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 52.Xu G, Li T, Chen J, Li C, Zhao H, Yao C, Dong H, Wen K, Wang K, Zhao J, et al. Autosomal dominant retinitis pigmentosa-associated gene PRPF8 is essential for hypoxia-induced mitophagy through regulating ULK1 mRNA splicing. Autophagy. 2018;14(10):1818–1830. doi: 10.1080/15548627.2018.1501251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cho YW, Kwon YH. Regulation of gene expression in the development of colitis-associated colon cancer in mice fed a high-fat diet. Biochem. Biophys. Res. Commun. 2022;592:81–86. doi: 10.1016/j.bbrc.2022.01.016. [DOI] [PubMed] [Google Scholar]
- 54.Karampatsi D, Zabala A, Wilhelmsson U, Dekens D, Vercalsteren E, Larsson M, Nystrom T, Pekny M, Patrone C, Darsalia V. Diet-induced weight loss in obese/diabetic mice normalizes glucose metabolism and promotes functional recovery after stroke. Cardiovasc. Diabetol. 2021;20(1):240. doi: 10.1186/s12933-021-01426-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ludwig A, Wilck N. Targeting the ubiquitin-proteasome system in atherosclerosis: Status quo, challenges, and perspectives. Antioxid. Redox Signal. 2014;21:1. doi: 10.1089/ars.2013.5805. [DOI] [PubMed] [Google Scholar]
- 56.Lim FT, Ogawa S, Parhar IS. Spred-2 expression is associated with neural repair of injured adult zebrafish brain. J. Chem. Neuroanat. 2016;77:176–186. doi: 10.1016/j.jchemneu.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 57.Chen KC, Liao YC, Wang JY, Lin YC, Chen CH, Juo SH. Oxidized low-density lipoprotein is a common risk factor for cardiovascular diseases and gastroenterological cancers via epigenomical regulation of microRNA-210. Oncotarget. 2015;6(27):24105–24118. doi: 10.18632/oncotarget.4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang, Y., Fu, L., Qi, X., Zhang, Z., Xia, Y., Jia, J., Jiang, J., Zhao, Y., & Wu, G. Structural insight into the mutual recognition and regulation between Suppressor of Fused and Gli/Ci. Nat. Commun. [DOI] [PMC free article] [PubMed]
- 59.Takabe W, Mataki C, Wada Y, Ishii M, Noguchi N. Gene expression induced by BO-653, probucol and BHQ in human endothelial cells. J. Atheroscler. Thromb. 2000;7(4):223. doi: 10.5551/jat1994.7.223. [DOI] [PubMed] [Google Scholar]
- 60.Chen X, Yao N, Lin Z, Wang Y. Inhibition of the immunoproteasome subunit LMP7 ameliorates cerebral white matter demyelination possibly via TGFbeta/Smad signaling. Evid. Based Complement Altern. Med. 2021;2021:6426225. doi: 10.1155/2021/6426225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chuang SY, Bai CH, Chen WH, Lien LM, Pan WH. Fibrinogen independently predicts the development of ischemic stroke in a Taiwanese population: CVDFACTS study. Stroke J. Cereb. Circ. 2009;40(5):1578–1584. doi: 10.1161/STROKEAHA.108.540492. [DOI] [PubMed] [Google Scholar]
- 62.Abu-Farha M, Al-Sabah S, Hammad MM, Hebbar P, Channanath AM, John SE, Taher I, Almaeen A, Ghazy A, Mohammad A, et al. Prognostic genetic markers for thrombosis in COVID-19 patients: A focused analysis on D-dimer. homocysteine and thromboembolism. Front. Pharmacol. 2020;11:587451. doi: 10.3389/fphar.2020.587451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yushun P, Liu Z, Hou Y, Neurology DO. Relationship between D-dimer and fibrinogen with progressive ischemic stroke. J. Apoplexy Nerv. Dis. 2015;1:1. [Google Scholar]
- 64.Zhang C, Chen H, He Q, Luo Y, He A, Tao A, Yan J. Fibrinogen/AKT/microfilament axis promotes colitis by enhancing vascular permeability. Cell Mol. Gastroenterol. Hepatol. 2021;11(3):683–696. doi: 10.1016/j.jcmgh.2020.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hayward CPM, Rivard GE. Quebec platelet disorder. Expert Rev. Hematol. 2011;4(2):137–141. doi: 10.1586/ehm.11.5. [DOI] [PubMed] [Google Scholar]
- 66.Wei M, Li H, Li Q, Qiao Y, Ma Q, Xie R, Wang R, Liu Y, Wei C, Li B, et al. Based on network pharmacology to explore the molecular targets and mechanisms of Gegen Qinlian decoction for the treatment of ulcerative colitis. Biomed. Res. Int. 2020;2020:5217405. doi: 10.1155/2020/5217405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Merino P, Diaz A, Jeanneret V, Wu F, Torre E, Cheng L, Yepes M. Urokinase-type plasminogen activator (uPA) binding to the uPA receptor (uPAR) promotes axonal regeneration in the central nervous system. J. Biol. Chem. 2022;292(7):2741–2753. doi: 10.1074/jbc.M116.761650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Diaz A, Merino P, Manrique LG, Cheng L, Yepes M. Urokinase-type plasminogen activator (uPA) protects the tripartite synapse in the ischemic brain via ezrin-mediated formation of peripheral astrocytic processes. J. Cereb. Blood Flow Metab. 2022;39(11):2157–2171. doi: 10.1177/0271678X18783653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lin LJ, Zhang Y, Lin Y, Jin Y, Zheng CQ. Identifying candidate genes for discrimination of ulcerative colitis and Crohn's disease. Mol. Biol. Rep. 2014;41(10):6349–6355. doi: 10.1007/s11033-014-3469-y. [DOI] [PubMed] [Google Scholar]
- 70.Mu Q, Zhang Y, Gu L, Gerner ST, Qiu X, Tao Q, Pang J, Dipritu G, Zhang L, Yin S, et al. Transcriptomic profiling reveals the antiapoptosis and antioxidant stress effects of Fos in ischemic stroke. Front. Neurol. 2021;12:728984. doi: 10.3389/fneur.2021.728984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Khyzha N, Khor M, DiStefano PV, Wang L, Matic L, Hedin U, Wilson MD, Maegdefessel L, Fish JE. Regulation of CCL2 expression in human vascular endothelial cells by a neighboring divergently transcribed long noncoding RNA. Proc. Natl. Acad. Sci. USA. 2022;116(33):16410–16419. doi: 10.1073/pnas.1904108116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.He S, Liu R, Li B, Huang L, Fan W, Tembachako CR, Zheng X, Xiong X, Miyata M, Xu B. Propagermanium, a CCR2 inhibitor, attenuates cerebral ischemia/reperfusion injury through inhibiting inflammatory response induced by microglia. Neurochem. Int. 2019;1:1. doi: 10.1016/j.neuint.2019.02.010. [DOI] [PubMed] [Google Scholar]
- 73.Rahabi M, Jacquemin G, Prat M, Meunier E, AlaEddine M, Bertrand B, Lefevre L, Benmoussa K, Batigne P, Aubouy A, et al. Divergent roles for macrophage C-type lectin receptors, dectin-1 and mannose receptors, in the intestinal inflammatory response. Cell Rep. 2020;30(13):4386–4398. doi: 10.1016/j.celrep.2020.03.018. [DOI] [PubMed] [Google Scholar]
- 74.Midgley A, Barakat D, Braitch M, Nichols C, Nebozhyn M, Edwards LJ, Fox SC, Gran B, Robins RA, Showe LC, et al. PAF-R on activated T cells: Role in the IL-23/Th17 pathway and relevance to multiple sclerosis. Immunobiology. 2021;226(1):152023. doi: 10.1016/j.imbio.2020.152023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ladurner A, Schwarz PF, Dirsch VM. Natural products as modulators of retinoic acid receptor-related orphan receptors (RORs) Nat. Prod. Rep. 2021;38(4):757–781. doi: 10.1039/d0np00047g. [DOI] [PubMed] [Google Scholar]
- 76.Palmieri O, Mazzoccoli G, Bossa F, Maglietta R, Palumbo O, Ancona N, Corritore G, Latiano T, Martino G, Rubino R, et al. Systematic analysis of circadian genes using genome-wide cDNA microarrays in the inflammatory bowel disease transcriptome. Chronobiol. Int. 2015;32(7):903–916. doi: 10.3109/07420528.2015.1050726. [DOI] [PubMed] [Google Scholar]
- 77.Baker E, Sims R, Leonenko G, Frizzati A, Harwood JC, Grozeva D, Morgan K, Passmore P, Holmes C, Powell J, et al. Gene-based analysis in HRC imputed genome wide association data identifies three novel genes for Alzheimer's disease. PLoS ONE. 2019;14(7):e0218111. doi: 10.1371/journal.pone.0218111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Quandt J, Arnovitz S, Haghi L, Woehlk J, Mohsin A, Okoreeh M, Mathur PS, Emmanuel AO, Osman A, Krishnan M, et al. Wnt-beta-catenin activation epigenetically reprograms Treg cells in inflammatory bowel disease and dysplastic progression. Nat. Immunol. 2021;22(4):471–484. doi: 10.1038/s41590-021-00889-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hradilkova K, Maschmeyer P, Westendorf K, Schliemann H, Husak O, von Stuckrad ASL, Kallinich T, Minden K, Durek P, Grün JR, et al. Regulation of fatty acid oxidation by twist 1 in the metabolic adaptation of T helper lymphocytes to chronic inflammation. Arthritis Rheumatol. 2022;71(10):1756–1765. doi: 10.1002/art.40939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li Y, Zhang YX, Ning DS, Chen J, Ou ZJ. Simvastatin inhibits POVPC-mediated induction of endothelial to mesenchymal cell transition. J. Lipid Res. 2021;62:100066. doi: 10.1016/j.jlr.2021.100066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zafar A, Farooqui M, Ikram A, Suriya S, Kempuraj D, Khan M, Tasneem N, Qaryouti D, Quadri S, Adams HP, et al. Cytokines, brain proteins, and growth factors in acute stroke patients: A pilot study. Surg. Neurol. Int. 2021;12:366. doi: 10.25259/SNI_569_2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.He Q, Shi X, Zhou B, Teng J, Zhang C, Liu S, Lian J, Luo B, Zhao G, Lu H, et al. Interleukin 8 (CXCL8)-CXC chemokine receptor 2 (CXCR2) axis contributes to MiR-4437-associated recruitment of granulocytes and natural killer cells in ischemic stroke. Mol. Immunol. 2018;101:440–449. doi: 10.1016/j.molimm.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 83.Li J, Ding F, Qian X, Sun J, Ge Z, Yang L, Cheng Z. Anti-inflammatory cytokine IL10 loaded cRGD liposomes for the targeted treatment of atherosclerosis. J. Microencapsul. 2021;38(6):357–364. doi: 10.1080/02652048.2020.1836058. [DOI] [PubMed] [Google Scholar]
- 84.Munjal A, Khandia R. Atherosclerosis: orchestrating cells and biomolecules involved in its activation and inhibition. Adv. Protein Chem. Struct. Biol. 2020;120:85–122. doi: 10.1016/bs.apcsb.2019.11.002. [DOI] [PubMed] [Google Scholar]
- 85.Kusters PJ, Lutgens E. Cytokines and immune responses in murine atherosclerosis. Methods Mol. Biol. 2015;1339:17–40. doi: 10.1007/978-1-4939-2929-0_2. [DOI] [PubMed] [Google Scholar]
- 86.Proto JD, Doran AC, Gusarova G, Yurdagul A, Jr, Sozen E, Subramanian M, Islam MN, Rymond CC, Du J, Hook J, et al. Regulatory T cells promote macrophage efferocytosis during inflammation resolution. Immunity. 2018;49(4):666–677.e666. doi: 10.1016/j.immuni.2018.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.