Abstract
The obligate intracellular pathogen Chlamydophila pneumoniae (Chlamydia pneumoniae) initiates infections in humans via the mucosal epithelia of the respiratory tract. Here, we report that epithelial cells infected with C. pneumoniae are resistant to apoptosis induced by treatment with drugs or by death receptor ligation. The induction of protection from apoptosis depended on the infection conditions since only cells containing large inclusions were protected. The underlying mechanism of infection-induced apoptosis resistance probably involves mitochondria, the major integrators of apoptotic signaling. In the infected cells, mitochondria did not respond to apoptotic stimuli by the release of apoptogenic factors required for the activation of caspases. Consequently, active caspase-3 was absent in infected cells. Our data suggest a direct modulation of apoptotic pathways in epithelial cells by C. pneumoniae.
Apoptosis plays an active role in the control of viral and bacterial infections (39, 42). To establish a successful infection, pathogens developed a variety of strategies for modulating apoptosis of the host cell. Gram-negative bacteria such as Salmonella enterica serovar Typhimurium (27), Shigella flexneri (43), Neisseria gonorrhoeae (29), Yersinia enterocolitica (26, 35), and Legionella pneumophila (31) have been shown to induce apoptosis. In contrast, some obligate intracellular bacteria such as Rickettsia rickettsii (7), Chlamydia trachomatis (10), and Chlamydia psittaci (8) have been shown to actively block apoptosis of their host cells.
Apoptosis is initiated by two major pathways involving either cell surface receptors or mitochondria. In recent years, several death receptors, all of which belong to the tumor necrosis factor (TNF) receptor family, have been identified (2). Ligation of these receptors results in the activation of so-called caspases, a class of cysteine proteases with a specific function in the execution of the apoptotic program. As a result of caspase activation, certain cellular substrates are cleaved and the cells undergo apoptosis (36, 40).
Unlike the ligands of death receptors, which induce apoptosis in a well-defined way, numerous insults, toxic cell metabolites, and cytotoxic and environmental stress initiate apoptotic cell death by a less well understood pathway. Many of the signals induced by these stimuli are integrated by mitochondria. Mitochondria respond by the release of caspases or caspase-activating proteins such as cytochrome c (17). Thus, both pathways to apoptosis finally result in the activation of caspases.
Chlamydiales represents a group of obligate intracellular bacteria that reside in a membrane-bound inclusion. Chlamydia pneumoniae has a unique biphasic developmental cycle involving two functionally and morphologically distinct forms of the bacteria, the invasive elementary bodies (EB) and the noninvasive, metabolically active reticulate bodies (RB). C. pneumoniae, first described in 1986 as a respiratory pathogen (16), has also been implicated in the onset or progression of several nonpulmonary diseases such as atherosclerosis, reactive arthritis, and Alzheimer's disease (3, 15, 37). In all cases of chlamydial infections, the primary site of entry is the mucosal epithelium. In vitro, C. pneumoniae completes its cycle of development in 72 to 96 h. During this time, bacteria require the integrity of host cells to support their intracellular growth and development. Recently, it has been shown that epithelial cells and macrophages infected with C. trachomatis are resistant to apoptosis induced by staurosporine, TNF-α, granzyme B/perforin, and Fas ligand (10). Interestingly, C. psittaci (8, 33) and C. trachomatis (10, 34) have been reported to either induce or inhibit apoptosis in the infected cells, depending on the stage of development. Geng et al. (14) have recently demonstrated that C. pneumoniae could inhibit apoptosis in the infected peripheral blood mononuclear cells, an effect that was attributed to interleukin-10 secreted in response to the chlamydial infection.
To date, whether infection with C. pneumoniae can influence apoptosis in epithelial cells, the likely site of chlamydial entry, has not been investigated. Here we demonstrate that epithelial host cells infected with C. pneumoniae are resistant to apoptosis induced by different stimuli. The potential of C. pneumoniae to prevent apoptosis largely depended on the infection conditions, suggesting a crucial role for bacterial factors produced while the pathogen multiplies in the host cells.
MATERIALS AND METHODS
Host cells and bacterial strain.
The host cells were HEp-2 (ATCC CCL23), a human epithelioid cell line derived from a larynx carcinoma. The strain of C. pneumoniae used in this study was TW-183, obtained from Washington Research Foundation (Seattle, Wash.), propagated in HEp-2 cells, and purified as described previously (1). HEp-2 cells were grown in growth medium (GM) composed of minimal essential medium (MEM) (Life Technologies, Karlsruhe, Germany) supplemented with 1% (vol/vol) nonessential amino acids (Sigma, Steinheim, Germany), 2 mM l-glutamine (Biochrom KG, Berlin, Germany), 10% (vol/vol) fetal bovine serum (FBS), 10 mM HEPES (Sigma), and 10 μg of gentamicin (Sigma)/ml. All experiments involving infection with C. pneumoniae and the induction of apoptosis were performed in the infection medium composed of MEM with the same final concentration of amino acids, l-glutamine, and HEPES as in GM and supplemented with 5% (vol/vol) FBS.
Chlamydial infections.
Host cells grown on coverslips in 12- or 24-well plates (Techno Plastic Products AG, Trasadingen, Switzerland) were infected using different multiplicities of infection (MOI) ranging from 0.5 to 10. The infection was performed as described previously (1). In brief, the suspension of EB diluted in infection medium was added directly to cells and the mixture was centrifuged at 920 × g for 1 h followed by incubation at 37°C for one more hour in the presence of 5% CO2. After the extracellular bacteria were washed away, infected cells were further incubated at 37°C in the presence of 5% CO2 for various times after the beginning of infection. Unless otherwise mentioned, all the infections were performed in the absence of cycloheximide.
Detection of C. pneumoniae.
To confirm the presence of C. pneumoniae in the infected cells, we performed staining with antichlamydia antibodies. The experiments were done at room temperature. HEp-2 cells were fixed with 4% (wt/vol) paraformaldehyde (Merck, Damstadt, Germany) in phosphate-buffered saline (PBS) (pH 7.4) for 30 min, washed twice with PBS, and permeabilized with 0.05% (vol/vol) Triton X-100 and either 2% (wt/vol) bovine serum albumin (BSA) or 10% (vol/vol) goat serum (blocking agents) in PBS. The cells were then incubated for 1 h with either the anti-C. pneumoniae major outer membrane protein (MOMP)-specific mouse monoclonal antibody used at a final dilution of 1:15 (DAKO, Hamburg, Germany) or the anti-Chlamydia genus-specific rabbit polyclonal antibody used at a dilution of 1:60 (Milan Analytica AG, La Roche, Switzerland). Unless otherwise mentioned, all antibodies were diluted in 2% (wt/vol) BSA-PBS solution. After being washed with PBS, the cells were incubated for 45 min with a rabbit anti-mouse (1:100 dilution) or goat anti-rabbit immunoglobulin G antibody coupled with Cy3 (Dianova, Hamburg, Germany) or Alexa red 548 (Molecular Probes, Leiden, The Netherlands) used at a final dilution of 1:100. The sizes of chlamydial inclusions were measured using the confocal microscope with an objective lens of 63× using the Leica TCS NT software options.
Induction of apoptosis.
To induce apoptosis, we used either 1 μM staurosporine (Sigma) or 40 ng of TNF-α (Pharmingen, San Diego, Calif.)/ml, together with 2 μg of cycloheximide/ml. The infected and uninfected HEp-2 cells were treated with staurosporine for 4 to 5 h and with TNF-α for 5 to 6 h. The control cells were incubated with cycloheximide alone. Both the stimulated and control cells were fixed with 4% paraformaldehyde in PBS for 30 min. Fixed specimens were then processed further for the analysis of apoptosis (see below).
Analysis of apoptosis. (i) Detection of chromatin condensation.
The cells that underwent induction of apoptosis were washed twice with PBS followed by permeabilization and blocking with 0.05% (vol/vol) Triton X-100 and 2% (wt/vol) BSA or 10% (vol/vol) goat serum in PBS for 30 min. First, the staining for C. pneumoniae was performed as described above. Next, the specimens were washed twice with PBS and stained with 10 μM Hoechst 33342 (Sigma) for 30 min at room temperature. After being washed three times with PBS, the coverslips were mounted onto microscope slides and evaluated for chromatin condensation and the presence of C. pneumoniae with a Leica fluorescence microscope. For each sample, cells from five random fields were counted under 400× magnification. The percentage of apoptotic cells was calculated as the number of apoptotic cells divided by a total number of cells counted × 100.
(ii) Annexin V assay.
HEp-2 cells cultured in six-well plates were infected with C. pneumoniae EB at various MOI. At 15 h postinfection the cells in the supernatants were collected by centrifugation and counted by hemocytometer. Adherent cells were harvested by Accutase (Innovative Cell Technologies, Inc., San Diego, Calif.) treatment. Cells were washed twice with PBS and suspended in 100 μl of binding buffer (10 mM HEPES-NaOH, 140 mM NaCl, 2.5 mM CaCl2, pH 7.4). Annexin V-fluorescein isothiocyanate (FITC) (BenderMed Systems) was added, and cells were incubated for 15 min in the dark. After one wash, cells were resuspended in 200 μl of binding buffer and counterstained with 1 μg of propidium iodide (PI)/ml for determination of permeable (necrotic) cells. Ten thousand cells per sample were analyzed with a Becton Dickinson FACS Calibur equipped with a 15-mW, 488-nm air-cooled argon laser using FACS Express software.
(iii) Detection of caspase-3 and cytochrome c and TUNEL assay.
Following the induction of apoptosis, the infected and uninfected cells were first subjected to detection of C. pneumoniae. After being washed with PBS, cells were incubated for 1 h with the rabbit polyclonal antibody specific for activated caspase-3, used at a final dilution of 1:100 (a gift from A. Srinivasan, Idun Pharmaceuticals, San Diego, Calif.). This was followed by incubation for 1 h with goat anti-rabbit immunoglobulin G coupled with DTAF (Dianova) diluted 1:100. The terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling (TUNEL) reaction was performed using the Apoptosis Detection System, Fluorescein strictly in accordance with the manufacturer's instructions (Promega, Madison, Wis.). Cytochrome c was detected with the mouse monoclonal antibody diluted 1:100 (Pharmingen).
Quantification of cytochrome c.
HEp-2 cells cultured in 75-cm2 flasks were infected with EB at an MOI of 5. Cells at 36 h postinfection were treated with 40 ng of TNF-α/ml and 2 μg of cycloheximide/ml for 4 to 5 h to induce apoptosis. The cells in the supernatant were collected by centrifugation, and those that were adherent were collected by trypsinization. The pellet was resusupended and washed twice at 400 × g with ice-cold PBS. All subsequent centrifugation steps were performed at 4°C. After another wash with MB buffer (400 mM sucrose, 50 mM Tris, 1 mM EGTA, 5 mM β-mercaptoethanol, 0.2% BSA, 10 mM KH2PO4, pH 7.6) the pellet was resuspended in 5 ml of MB buffer and incubated for 20 min on ice. The cells were then homogenized with 35 strokes with a Teflon homogenizer. Cell debris was removed by centrifugation at 4,000 × g for 1 min, and the supernatant was centrifuged for 10 min at 15,000 × g to precipitate the mitochondria. The amount of cytochrome c in the supernatant was determined using the cytochrome c enzyme-linked immunosorbent assay (ELISA) kit (Qnantikine human cytochrome c immunoassay kit; R&D Systems, Inc., Minneapolis, (Minn.) in accordance with the manufacturer's instructions. Cytochrome c was quantified using an ELISA reader (Spectra Max 250; Molecular Devices, Munich, Germany) and the Softmax Pro, version 3.0, software.
Statistics.
For statistical calculations, graphs, and histograms, Microsoft Excel for Windows 7.0 was used. The error bars represent the standard deviations (SD) of the means. To determine if the observed effect is statistically significant, the Student t test was performed. P values <0.05 were considered statistically significant.
RESULTS
Epithelial cells infected with C. pneumoniae are resistant to staurosporine-induced apoptosis.
To evaluate the influence of chlamydial infection on the process of apoptosis in the epithelium, we treated HEp-2 cells with staurosporine 36 h postinfection (middle stage of infection). Five hours later, chromatin condensation in infected and uninfected cells was assessed by staining with nuclear dye Hoechst 33342. The number of apoptotic cells determined by chromatin condensation was lower in the infected population than in the uninfected one. To determine the infection status of individual cells, double staining with Hoechst 33342 and an antichlamydia antibody was performed. The results demonstrated that the infected cells containing chlamydial inclusions had normal, noncondensed chromatin whereas the majority of noninfected cells had condensed chromatin (Fig. 1A). Quantification and subsequent analyses have determined that the antiapoptotic effect was statistically significant (P = 0.002) (Fig. 1B).
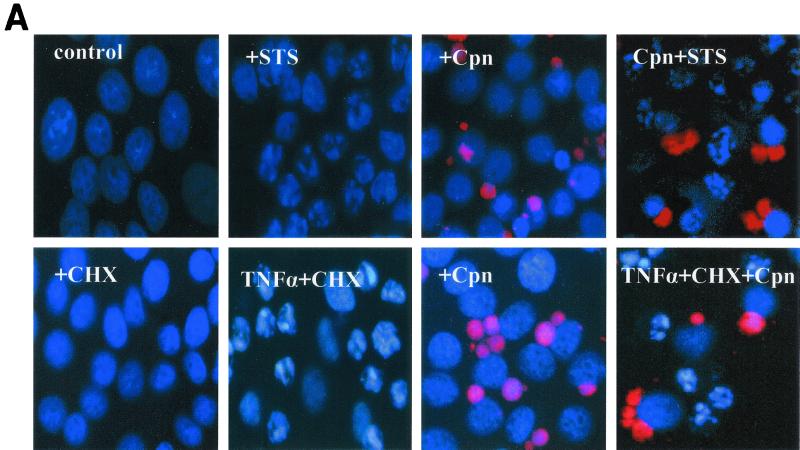
FIG. 1.
(A) HEp-2 cells infected with C. pneumoniae are resistant to apoptotic chromatin condensation induced by staurosporine and TNF-α. HEp-2 cells 36 h postinfection were treated with 1 μM staurosporine (STS) for 4 h (top), and infected and uninfected HEp-2 cells were treated with 40 ng of TNF-α/ml and 2 μg of cycloheximide (CHX)/ml for 5 h (bottom). The cells were examined at ×400 magnification under a fluorescence microscope equipped with a digital camera. Nuclei were stained with Hoechst 33342 (blue), and the chlamydial inclusions (Cpn) were stained in red. (B) Chlamydia-infected HEp-2 cells are resistant to apoptosis induced by staurosporine and TNF-α HEp-2 cells infected with C. pneumoniae (MOI = 5) were treated with staurosporine (STS) or TNF-α, as described in Materials and Methods. Nuclei were stained with Hoechst 33342, and the apoptotic and nonapoptotic cells from the infected and uninfected specimens in five random fields were counted under a fluorescence microscope (×400 magnification). The percentage of apoptotic cells was calculated based on data obtained in two independent experiments. Error bars, SD of the means.
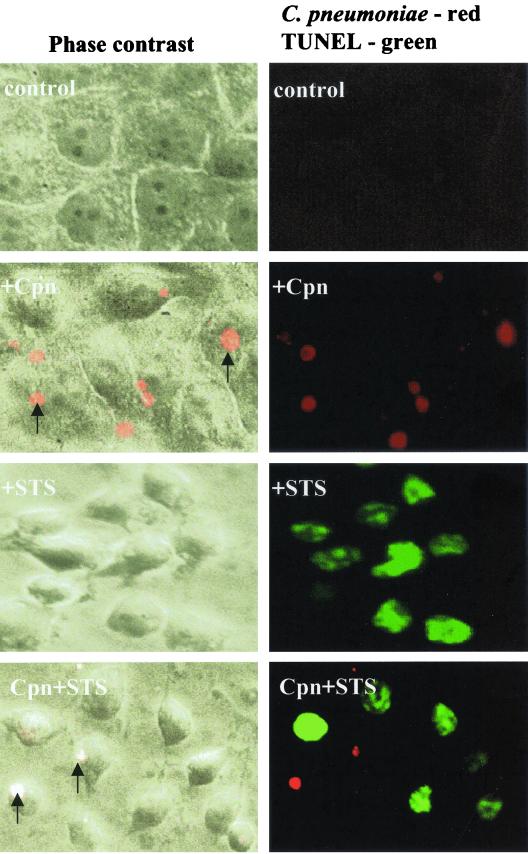
To further confirm this phenomenon, we performed TUNEL, which visualizes the fragmented chromosomal DNA of apoptotic cells by incorporating fluorescein-12-dUTP at 3′ ends of DNA. Using staurosporine as an inducer, we demonstrated a positive TUNEL reaction only in the uninfected cells. In contrast, cells with chlamydial inclusions identified by staining with a monoclonal antibody were TUNEL negative (Fig. 2). Thus, we confirmed the lack of staurosporine-induced DNA fragmentation in the infected cells.
FIG. 2.
HEp-2 cells infected with C. pneumoniae are resistant to staurosporine-induced DNA fragmentation. Infected (Cpn) and uninfected HEp-2 cells were treated with staurosporine (STS) and tested for DNA fragmentation using the TUNEL reaction. Shown are phase-contrast images (left) of the corresponding fluorescent fields (right). The TUNEL reaction is revealed by green fluorescence, and the presence of chlamydial inclusions is shown in red (arrows). The staining shows a mutually exclusive pattern: cells carrying inclusions are negative for the TUNEL reaction, and cells without inclusions are positive for the TUNEL reaction. Microscopic fields shown here were photographed from the samples infected at a MOI of 1 and visualized under ×400 magnification.
Inhibition of apoptosis depends on the infection conditions.
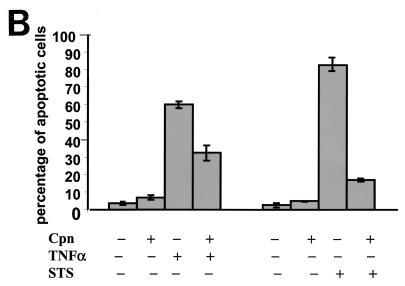
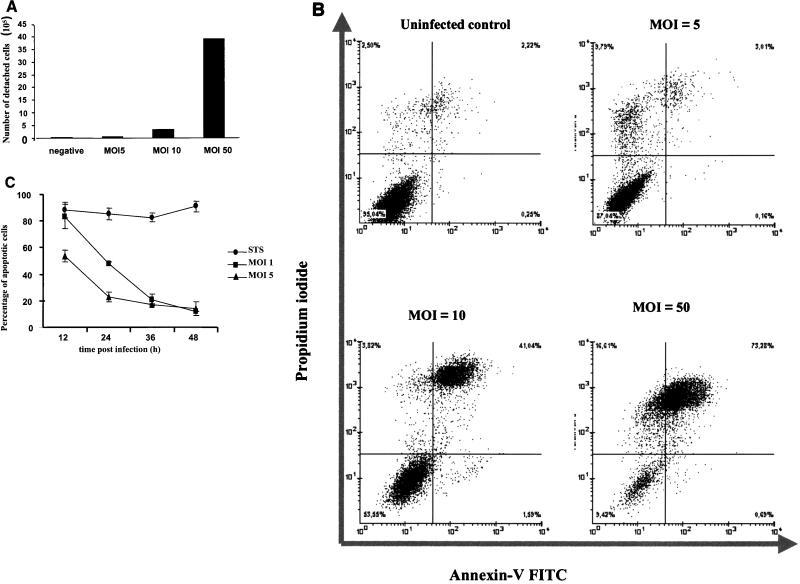
The infection conditions under which apoptosis induced by staurosporine treatment was inhibited were defined. The MOI had a significant influence on the viability of the host cells. Infections at MOI ranging from 0.5 to 5 had no apparent toxic effect on the cells, whereas infections at MOI ≥10 resulted in rounding up and detachment of the cells (Fig. 3A). To investigate whether the rounded cells observed in samples infected at high MOI died by apoptosis or necrosis, propidium iodide (PI) and annexin-V double-labeling assays were performed. From the samples infected at MOI of 10 and 50, 41 and 73%, respectively, stained double positive for PI and annexin-V–FITC (Fig. 3B), suggesting that these cells died by necrosis (4). The number of double-positive cells was not increased in samples infected at a MOI of 5 compared to the uninfected control (Fig. 3B). In no case did we observe annexin-V-positive, PI-negative cells, which would be typical for apoptotic cells (4). Therefore we infected at a MOI of 5 or less in further experiments. To determine whether the antiapoptotic effect depends on the stage of infection, apoptosis was induced 12, 24, 36, and 48 h postinfection. Double staining with Hoechst and an antichlamydia antibody demonstrated inhibition of apoptosis already at 24 h postinfection. However, the resistance to apoptosis at this time point correlated with the size of the inclusion in that generally cells harboring inclusions of 4 μm in diameter or larger were protected, whereas cells with smaller inclusions frequently underwent apoptosis, similar to uninfected cells. Also, infecting HEp-2 cells with either 1, 3, or 5 bacteria per cell resulted in an apparent increase of rescued cells at a given time point. Additionally, at higher MOI more cells carried inclusions larger than 4 μm, suggesting that increasing the dose of infection led to a critical bacterial load in more cells than infections at lower MOI (Fig. 3C). Based on these results, for all subsequent experiments we chose to determine antiapoptotic effects 36 h postinfection in cultures infected at a MOI of 1, 3, or 5.
FIG. 3.
(A) Cells infected at high MOI detach from the culture plate. HEp-2 cells (5 × 106) grown in six-well plates were infected with C. pneumoniae at MOI of 5, 10, and 50. At 15 h postinfection cells in the supernatants were collected by centrifugation and counted in a hemocytometer. Shown are the absolute numbers of detached cells for every sample. (B) Cells detaching upon C. pneumoniae infection are not apoptotic. Supernatants of HEp-2 cells infected with C. pneumoniae at MOI of 5, 10, and 50 were collected at 15 h postinfection. Cells were harvested, stained with annexin-V–FITC, and PI and analyzed by flow cytometry. The dot plots show the FITC and PI fluorescence intensities of 10,000 events. Note that infection at MOI of 10 and 50 increases the necrotic annexin-V and PI double-positive population but not the apoptotic annexin-V single-positive population. (C) MOI used influences the antiapoptotic effect in host cells treated with staurosporine. HEp-2 cells infected with C. pneumoniae at two different MOI (1 and 5) were treated with staurosporine (STS) at different time points postinfection (12, 24, 36, and 48 h). Nuclei were stained with Hoechst 33342, and the apoptotic and nonapoptotic cells from the infected and uninfected specimens were counted in five random fields (total of more than 400 cells) under a fluorescence microscope (×400 magnification). The plot shows the results of two independent experiments. Error bars, SD of the means.
Epithelial cells infected with C. pneumoniae are resistant to TNF-α-induced apoptosis.
The pathway leading to apoptosis induced by staurosporine differs from the one engaged via the TNF-α receptor (TNF-R). Since HEp-2 cells express the TNF-R on their surfaces (4), we used these cells to investigate whether infection blocks apoptosis induced via the TNF-R. Uninfected cells were treated with 2 μg of cycloheximide/ml and 40 ng of TNF-α/ml. Cycloheximide has been shown to suppress survival signals induced by an engagement of TNF-R; thus we used it for efficient induction of apoptosis (18). Six hours after stimulation, about 60% of uninfected control cells displayed typical features of apoptotic cells, whereas the infected cells were unaffected by apoptosis, similar to the untreated control cells (Fig. 1A). However, the protection from apoptosis was restricted to the cells harboring inclusions, as uninfected cells present in the inoculated cultures underwent apoptosis (Fig. 1A). Quantification showed that the inhibition of apoptosis in infected versus uninfected cells is significant (P = 0.027) (Fig. 1B).
Lack of caspase-3 activation in infected cells.
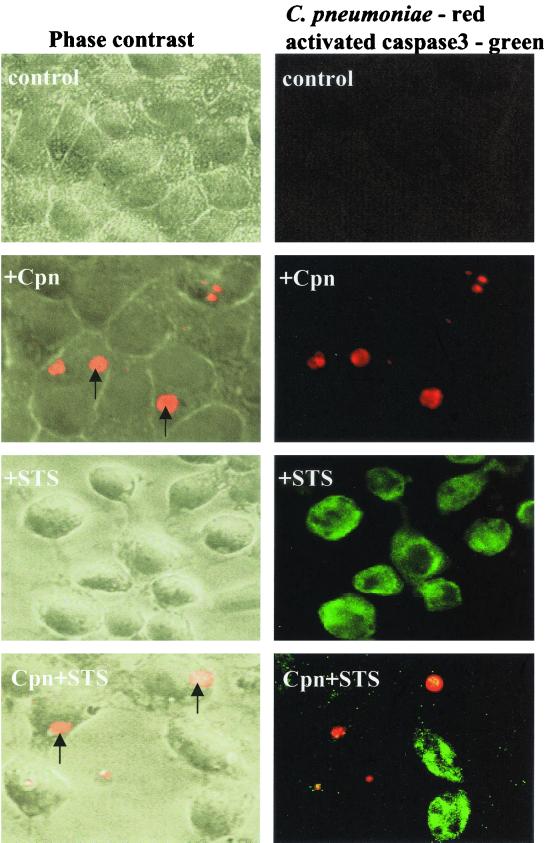
To further analyze the mechanism of apoptosis inhibition occurring during infection with C. pneumoniae, we determined whether the block takes place upstream or downstream of caspase-3 activation. Caspase-3 has been implicated as a general effector of apoptosis that is activated via upstream caspases by a cleavage (21). Staurosporine-treated infected and uninfected HEp-2 cells were subjected to immunostaining with a polyclonal antibody directed against the cleaved and activated form of caspase-3. Only uninfected cells and cells in the infected sample that carried no or small inclusions contained active caspase-3. In contrast, activated caspase-3 was absent in cells that contained chlamydial inclusions (Fig. 4).
FIG. 4.
Caspase-3 activation induced by staurosporine is inhibited in HEp-2 cells infected with C. pneumoniae. Infected (MOI = 1) and uninfected HEp-2 cells were treated with staurosporine (STS) at 36 h postinfection. After 4 to 5 h of treatment with STS, the cells were fixed and stained for chlamydial inclusions (Cpn) and activated caspase-3 as described in Materials and Methods. Activated caspase-3 (green) can only be seen in the uninfected cells or in the individual cells from the infected sample that do not contain chlamydial inclusions. Furthermore, activated caspase-3 is absent in the cells carrying inclusion(s) (red; arrows).
Inhibition of mitochondrial cytochrome c release in infected cells.
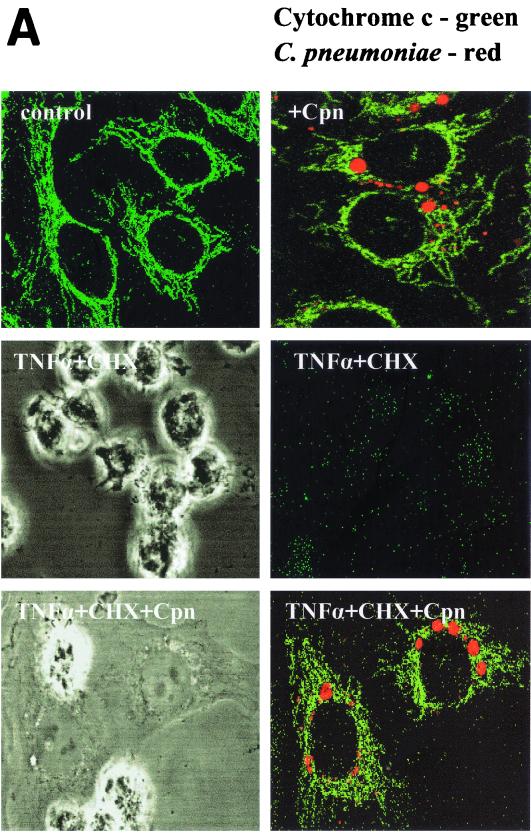
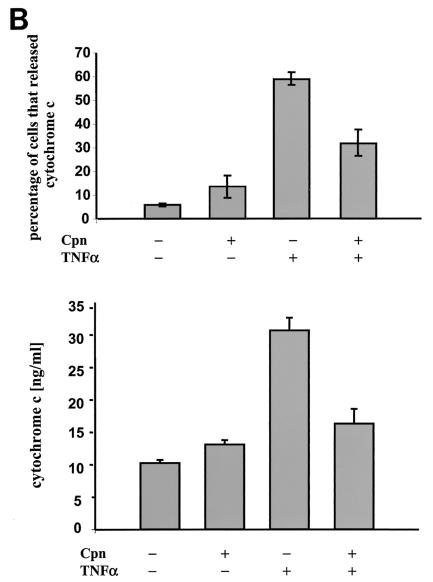
During apoptosis, cytochrome c is rapidly released from the mitochondria and then initiates the activation of caspases in the cytosol (23). To investigate whether TNF-α-induced release of cytochrome c is blocked during infection with C. pneumoniae, infected and uninfected HEp-2 cells were treated with TNF-α in the presence of cycloheximide. Approximately 60% of the control cells released cytochrome c from the mitochondria, as revealed by the absence of cytochrome c staining in the cells (Fig. 5). Within the inoculated culture, in the cells remaining uninfected or having inclusions smaller than 4 μM cytochrome c was not detected (Fig. 5A). In addition, these cells displayed apoptotic morphologies. In contrast, cells carrying inclusions larger than 4 μM retained cytochrome c in the mitochondria and were protected from apoptosis (Fig. 5A). Similar results were obtained by quantifying the amount of cytosolic cytochrome c by ELISA (Fig. 5B). These data suggested, that C. pneumoniae blocks apoptosis in epithelial cells upstream of the mitochondrial cytochrome c release.
FIG. 5.
(A) Cytochrome c release is prevented in cells infected with C. pneumoniae. HEp-2 cells infected for 36 h (MOI = 3) were treated with TNF-α in the presence of cycloheximide (CHX). Cytochrome c (green) and chlamydial inclusions (Cpn) (red) were detected using immunofluorescence. Cells carrying inclusions measuring more than 4 μm in diameter were protected from cytochrome c release as shown by double-positive staining. Cells containing chlamydial inclusions measuring less than 4 μm released cytochrome c upon induction of apoptosis. The phase-contrast images show the presence of apoptotic cells. (B, top) Cells of five random fields in two independent experiments were analyzed. Shown is the relative number of cells which did not stain with the anti-cytochrome c antibody. (Bottom) Mitochondria were separated from the cytosol, and the concentration of cytochrome c in the cytosol of cells was determined by ELISA. The data were normalized by the cell number of each sample.
DISCUSSION
Cells infected with viruses or bacteria frequently undergo apoptosis. Apoptosis in this context is an important antiviral or antibacterial response that prevents the spreading of the pathogen in the host (42). The immune system very efficiently removes apoptotic cells including their harmful load even in the absence of a classical immune response. Therefore, apoptosis of the infected cell is of prime importance when the pathogens are hidden from the immune system, e.g., inside a cell. As a response to this host defense mechanism many viruses and obligate intracellular bacteria have developed strategies to prevent the self-destruction of their host cells. Among the latter are C. trachomatis (10), C. psittaci (8), and R. rickettsii (7).
We have here demonstrated that epithelial cells infected with C. pneumoniae are resistant to apoptosis induced by staurosporine and TNF-α. Since epithelial cells are the prime site of infection with C. pneumoniae, successful replication at this site could be essential for the pathogen in order to penetrate and infect other tissues. Presence of chlamydial inclusions larger than 4 μm in the infected cells was a prerequisite for the inhibition of apoptosis. This implied that the induction of resistance depended on the stage of the chlamydial developmental cycle. It is rather unlikely that the inhibition of apoptosis in epithelial cells requires a soluble factor secreted by the infected host as the neighboring, uninfected epithelial cells were not protected from apoptosis, suggesting that the protective effect was exclusive for the cells inhabited by bacteria.
Our finding is not consistent with what has been shown earlier for inhibition of apoptosis in peripheral blood mononuclear cells infected with C. pneumoniae (14), which could be due to the different types of cells used. It is possible that C. pneumoniae-mediated inhibition of apoptosis in the epithelium depends on a factor produced by chlamydial metabolically active forms (RB) and on the concentration of this factor. The latter could reflect the number of RB inside the inclusion because cells containing inclusions with diameters <4 μM, thus likely having fewer bacteria, were not protected from apoptosis. From the view of C. pneumoniae this offers the advantage that successfully infected cells further support its growth and will not destroy themselves even if heavily infected.
We investigated the influence of C. pneumoniae on two different pathways of apoptosis induction in infected cells. Staurosporine, a cell-permeable potent inhibitor of several protein kinases, was used as inducer of intrinsic apoptotic pathways converging at mitochondria. The inducer of an extrinsic pathway was TNF-α, which cross-links the TNF-R. We have shown that the infection with C. pneumoniae inhibited both staurosporine- and TNF-α-induced apoptosis of epithelial cells. It is still a matter of debate whether receptor-initiated apoptosis requires mitochondrial function. At least in certain cell types this seems to be the case. In so-called type II cells, apoptosis is efficiently blocked by the overexpression of the Bcl-2 protein, an inhibitor of mitochondrial apoptosis (38). If HEp-2 cells are of type II, C. pneumoniae might well prevent mitochondrial apoptosis, the simplest explanation for the effects we observed. There is also a possibility that the inhibitory mechanism in the infected cells is complex and operates on several levels.
An observation consistent with the infection-induced block of apoptosis at the mitochondrial level was the absence of significant alterations in the size or in the membrane potential of the mitochondria in TNF-α-treated cells. For many pathways leading to apoptosis, the release of cytochrome c is the most essential upstream event for the activation of effector caspases (17, 25). Cytochrome c was always retained in the mitochondria of the inclusion-carrying cells treated with TNF-α. Thus, the mitochondria were completely protected and showed features of mitochondria typical for untreated cells. Consequently, active caspase-3 was not observed in cells infected with C. pneumoniae, as also shown for C. trachomatis (10). We have so far no evidence that antiapoptotic proteins such as the Bcl-2 family are upregulated in infected epithelial cells. Moreover, the antiapoptotic nuclear factor κB (NF-κB) is not activated in HEp-2 cells at 20 h postinfection (H. Al-Younes, T. F. Meyer, and T. Rudel, unpublished data) although NF-κB activation has been shown for endothelial cells and the smooth muscle cells infected with C. pneumoniae (9, 24). However, it is still possible that other antiapoptotic host factors besides Bcl-2 and NF-κB are induced by the pathogen.
Many viral and bacterial factors have been shown to interact at various checkpoints of the apoptotic machinery to modulate host cell apoptosis (28). These include baculovirus protein p35 (6), a broad-range inhibitor of caspases, the caspase-8 and -1 inhibitor CrmA from cow pox virus, and the V-FLIPs, virally encoded proteins which interfere with receptor-initiated apoptosis (36). Vpr-1 from human immunodeficiency virus targets mitochondria and prevents mitochondrial signaling probably in a manner similar to that of Bcl-2 (22). Other bacterial factors target mitochondria to modulate apoptosis, such as the pore-forming protein PorB from Neisseria (30) and a fragment of the vacuolating toxin VacA from Helicobacter pylori (13). Thus, it is also possible that C. pneumoniae produces factors that interfere directly with the apoptotic machinery. Many gram-negative bacteria have evolved a highly specialized secretion apparatus to pump proteins into the host cell cytosol, the so-called type III secretion system (20). Proteins injected by the type III system often interfere with host cell signaling (12, 26). A putative locus of the type III secretion apparatus has recently also been identified in the chlamydial genome, and this enables us to speculate that the factor(s) responsible for blocking apoptosis may be a chlamydial protein secreted into the host cell cytosol by the type III secretion apparatus (11, 19).
As C. trachomatis has also been shown to block apoptosis of infected host cells, this phenomenon seemed to be a common strategy developed by Chlamydiaceae. Evidently, obligate intracellular organisms benefit from the survival of their hosts. An apoptosis resistance pattern described in this work would also allow the pathogen to escape the antigen-specific immune effector mechanisms, most importantly cytotoxic T-lymphocyte-mediated killing of infected cells (32). In agreement with intracellular survival strategies is also the down-regulation of major histocompatibility complex class I expression induced by C. pneumoniae in an infected macrophage cell line (5), which would prevent the antigen-specific immune response directed against the infected cell. In summary, C. pneumoniae exploits many ways to carve a safe niche within its host. Identification of bacterial factors will not only enable us to define the pathogenesis of chlamydial infection but might also lead to a deeper understanding of the central mechanisms of host cell apoptosis.
ACKNOWLEDGMENTS
We thank V. Brinkmann for excellent advice with microscopy, A. Popp for valuable discussions, and F. Kühl for technical assistance.
REFERENCES
- 1.AlYounes H M, Rudel T, Meyer T F. Characterization and intracellular trafficking pattern of vacuoles containing Chlamydia pneumoniae in human epithelial cells. Cell Microbiol. 1999;1:237–247. doi: 10.1046/j.1462-5822.1999.00024.x. [DOI] [PubMed] [Google Scholar]
- 2.Ashkenazi A, Dixit V M. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 3.Balin B J, Gerard H C, Arking E J, Appelt D M, Branigan P J, Abrams J T, Whittum-Hudson J A, Hudson A P. Identification and localization of Chlamydia pneumoniae in the Alzheimer's brain. Med Microbiol Immunol. 1998;187:23–42. doi: 10.1007/s004300050071. [DOI] [PubMed] [Google Scholar]
- 4.Berkova N, Lemay A, Korobko V, Shingarova L, Sagaidak L, Goupil S. Tumor necrosis factor mutants with selective cytotoxic activity. Cancer Detect Prev. 1999;23:1–7. doi: 10.1046/j.1525-1500.1999.00067.x. [DOI] [PubMed] [Google Scholar]
- 5.Caspar-Bauguil S, Puissant B, Nazzal D, Lefevre J C, Thomsen M, Salvayre R, Benoist H. Chlamydia pneumoniae induces interleukin-10 production that down-regulates major histocompatibility complex class I expression. J Infect Dis. 2000;182:1394–1401. doi: 10.1086/315856. [DOI] [PubMed] [Google Scholar]
- 6.Clem R J, Fechheimer M, Miller L K. Prevention of apoptosis by a baculovirus gene during infection of insect cells. Science. 1991;254:1388–1390. doi: 10.1126/science.1962198. [DOI] [PubMed] [Google Scholar]
- 7.Clifton D R, Goss R A, Sahni S K, van Antwerp D, Baggs R B, Marder V J, Silverman D J, Sporn L A. NF-kappa B-dependent inhibition of apoptosis is essential for host cell survival during Rickettsia rickettsii infection. Proc Natl Acad Sci USA. 1998;95:4646–4651. doi: 10.1073/pnas.95.8.4646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coutinho-Silva R, Perfettini J L, Persechini P M, Dautry-Varsat A, Ojcius D M. Modulation of P2Z/P2X(7) receptor activity in macrophages infected with chlamydia psittaci. Am J Physiol. 2001;280:C81–C89. doi: 10.1152/ajpcell.2001.280.1.C81. [DOI] [PubMed] [Google Scholar]
- 9.Dechend R, Maass M, Gieffers J, Dietz R, Scheidereit C, Leutz A, Gulba D C. Chlamydia pneumoniae infection of vascular smooth muscle and endothelial cells activates NF-kappaB and induces tissue factor and PAI-1 expression: a potential link to accelerated arteriosclerosis. Circulation. 1999;100:1369–1373. doi: 10.1161/01.cir.100.13.1369. [DOI] [PubMed] [Google Scholar]
- 10.Fan T, Lu H, Hu H, Shi L, McClarty G A, Nance D M, Greenberg A H, Zhong G. Inhibition of apoptosis in chlamydia-infected cells: blockade of mitochondrial cytochrome c release and caspase activation. J Exp Med. 1998;187:487–496. doi: 10.1084/jem.187.4.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fields K A, Hackstadt T. Evidence for the secretion of Chlamydia trachomatis CopN by a type III secretion mechanism. Mol Microbiol. 2000;38:1048–1060. doi: 10.1046/j.1365-2958.2000.02212.x. [DOI] [PubMed] [Google Scholar]
- 12.Galan J E, Collmer A. Type III secretion machines: bacterial devices for protein delivery into host cells. Science. 1999;284:1322–1328. doi: 10.1126/science.284.5418.1322. [DOI] [PubMed] [Google Scholar]
- 13.Galmiche A, Rassow J, Doye A, Cagnol S, Chambard J C, Contamin S, de Thillot V, Just I, Ricci V, Solcia E, Van Obberghen E, Boquet P. The N-terminal 34 kDa fragment of Helicobacter pylori vacuolating cytotoxin targets mitochondria and induces cytochrome c release. EMBO J. 2000;19:6361–6370. doi: 10.1093/emboj/19.23.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geng Y, Shane R B, Berencsi K, Gonczol E, Zaki M H, Margolis D J, Trinchieri G, Rook A H. Chlamydia pneumoniae inhibits apoptosis in human peripheral blood mononuclear cells through induction of IL-10. J Immunol. 2000;164:5522–5529. doi: 10.4049/jimmunol.164.10.5522. [DOI] [PubMed] [Google Scholar]
- 15.Grayston J T. Background and current knowledge of Chlamydia pneumoniae and atherosclerosis. J Infect Dis. 2000;181(Suppl. 3):S402–S410. doi: 10.1086/315596. [DOI] [PubMed] [Google Scholar]
- 16.Grayston J T, Kuo C C, Wang S P, Altman J. A new Chlamydia psittaci strain, TWAR, isolated in acute respiratory tract infections. N Engl J Med. 1986;315:161–168. doi: 10.1056/NEJM198607173150305. [DOI] [PubMed] [Google Scholar]
- 17.Green D R, Reed J C. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 18.Higuchi M, Singh S, Aggarwal B B. Characterization of the apoptotic effects of human tumor necrosis factor: development of highly rapid and specific bioassay for human tumor necrosis factor and lymphotoxin using human target cells. J Immunol Methods. 1995;178:173–181. doi: 10.1016/0022-1759(94)00254-t. [DOI] [PubMed] [Google Scholar]
- 19.Hsia R C, Pannekoek Y, Ingerowski E, Bavoil P M. Type III secretion genes identify a putative virulence locus of Chlamydia. Mol Microbiol. 1997;25:351–359. doi: 10.1046/j.1365-2958.1997.4701834.x. [DOI] [PubMed] [Google Scholar]
- 20.Hueck C J. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol Mol Biol Rev. 1998;62:379–433. doi: 10.1128/mmbr.62.2.379-433.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacobsen M D, Weil M, Raff M C. Role of Ced-3/ICE-family proteases in staurosporine-induced programmed cell death. J Cell Biol. 1996;133:1041–1051. doi: 10.1083/jcb.133.5.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacotot E, Ravagnan L, Loeffler M, Ferri K F, Vieira H L, Zamzami N, Costantini P, Druillennec S, Hoebeke J, Briand J P, Irinopoulou T, Daugas E, Susin S A, Cointe D, Xie Z H, Reed J C, Roques B P, Kroemer G. The HIV-1 viral protein R induces apoptosis via a direct effect on the mitochondrial permeability transition pore. J Exp Med. 2000;191:33–46. doi: 10.1084/jem.191.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kroemer G, Dallaporta B, Resche-Rigon M. The mitochondrial death/life regulator in apoptosis and necrosis. Annu Rev Physiol. 1998;60:619–642. doi: 10.1146/annurev.physiol.60.1.619. [DOI] [PubMed] [Google Scholar]
- 24.Krull M, Klucken A C, Wuppermann F N, Fuhrmann O, Magerl C, Seybold J, Hippenstiel S, Hegemann J H, Jantos C A, Suttorp N. Signal transduction pathways activated in endothelial cells following infection with Chlamydia pneumoniae. J Immunol. 1999;162:4834–4841. [PubMed] [Google Scholar]
- 25.Liu X, Kim C N, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996;86:147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- 26.Mills S D, Boland A, Sory M P, van der Smissen P, Kerbourch C, Finlay B B, Cornelis G R. Yersinia enterocolitica induces apoptosis in macrophages by a process requiring functional type III secretion and translocation mechanisms and involving YopP, presumably acting as an effector protein. Proc Natl Acad Sci USA. 1997;94:12638–12643. doi: 10.1073/pnas.94.23.12638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Monack D M, Raupach B, Hromockyj A E, Falkow S. Salmonella typhimurium invasion induces apoptosis in infected macrophages. Proc Natl Acad Sci USA. 1996;93:9833–9838. doi: 10.1073/pnas.93.18.9833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Müller A, Rudel T. Modification of host cell apoptosis by viral and bacterial pathogens. Int J Med Microbiol. 2001;291:197–207. doi: 10.1078/1438-4221-00125. [DOI] [PubMed] [Google Scholar]
- 29.Muller A, Gunther D, Dux F, Naumann M, Meyer T F, Rudel T. Neisserial porin (PorB) causes rapid calcium influx in target cells and induces apoptosis by the activation of cysteine proteases. EMBO J. 1999;18:339–352. doi: 10.1093/emboj/18.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muller A, Gunther D, Brinkmann V, Hurwitz R, Meyer T F, Rudel T. Targeting of the pro-apoptotic VDAC-like porin (PorB) of neisseria gonorrhoeae to mitochondria of infected cells. EMBO J. 2000;19:5332–5343. doi: 10.1093/emboj/19.20.5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muller A, Hacker J, Brand B C. Evidence for apoptosis of human macrophage-like HL-60 cells by Legionella pneumophila infection. Infect Immun. 1996;64:4900–4906. doi: 10.1128/iai.64.12.4900-4906.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nagata S. Apoptosis by death factor. Cell. 1997;88:355–365. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- 33.Ojcius D M, Souque P, Perfettini J L, Dautry-Varsat A. Apoptosis of epithelial cells and macrophages due to infection with the obligate intracellular pathogen Chlamydia psittaci. J Immunol. 1998;161:4220–4226. [PubMed] [Google Scholar]
- 34.Perfettini J L, Darville T, Gachelin G, Souque P, Huerre M, Dautry-Varsat A, Ojcius D M. Effect of Chlamydia trachomatis infection and subsequent tumor necrosis factor alpha secretion on apoptosis in the murine genital tract. Infect Immun. 2000;68:2237–2244. doi: 10.1128/iai.68.4.2237-2244.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruckdeschel K, Roggenkamp A, Lafont V, Mangeat P, Heesemann J, Rouot B. Interaction of Yersinia enterocolitica with macrophages leads to macrophage cell death through apoptosis. Infect Immun. 1997;65:4813–4821. doi: 10.1128/iai.65.11.4813-4821.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rudel T. Caspase inhibitors in prevention of apoptosis. Herz. 1999;24:236–241. doi: 10.1007/BF03044967. [DOI] [PubMed] [Google Scholar]
- 37.Saario R, Toivanen A. Chlamydia pneumoniae as a cause of reactive arthritis. Br J Rheumatol. 1993;32:1112. doi: 10.1093/rheumatology/32.12.1112. [DOI] [PubMed] [Google Scholar]
- 38.Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli K J, Debatin K M, Krammer P H, Peter M E. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998;17:1675–1687. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shen Y, Shenk T E. Viruses and apoptosis. Curr Opin Genet Dev. 1995;5:105–111. doi: 10.1016/s0959-437x(95)90061-6. [DOI] [PubMed] [Google Scholar]
- 40.Stroh C, Schulze-Osthoff K. Death by a thousand cuts: an ever increasing list of caspase substrates. Cell Death Differ. 1998;5:997–1000. doi: 10.1038/sj.cdd.4400451. [DOI] [PubMed] [Google Scholar]
- 41.van Engeland M, Nieland L J, Ramaekers F C, Schutte B, Reutelingsperger C P. Annexin V-affinity assay: a review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry. 1998;31:1–9. doi: 10.1002/(sici)1097-0320(19980101)31:1<1::aid-cyto1>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 42.Weinrauch Y, Zychlinsky A. The induction of apoptosis by bacterial pathogens. Annu Rev Microbiol. 1999;53:155–187. doi: 10.1146/annurev.micro.53.1.155. [DOI] [PubMed] [Google Scholar]
- 43.Zychlinsky A, Prevost M C, Sansonetti P J. Shigella flexneri induces apoptosis in infected macrophages. Nature. 1992;358:167–169. doi: 10.1038/358167a0. [DOI] [PubMed] [Google Scholar]