Abstract
Background
Nonalcoholic fatty liver disease (NAFLD) is a liver metabolic syndrome and still lacks effective treatments because the molecular mechanism underlying the development of NAFLD is not completely understood. We investigated the role of Hydroxyl CoA dehydrogenase alpha subunit (HADHA) in the pathogenesis of NAFLD.
Methods
HADHA expression was detected both in NAFLD cell and mice, and knockdown of HADHA in free fatty acids (FFA)-treated L02 or overexpression of HADHA in high fat diet (HFD)-fed mice was used to detected the influence of HADHA on hepatic steatosis, mitochondrial dysfunction, and oxidative stress by regulating of MKK3/MAPK signaling.
Results
Our data revealed that HADHA expression was decreased in FFA-treated L02 cells and in HFD-fed mice. Knockdown of HADHA markedly aggravated hepatic steatosis, inflammation and oxidative stress in FFA-treated L02 cells, which was associated with the activation of MKK3/MAPK signalling pathways. Moreover, oxidative stress and liver lesions were improved in NAFLD mice by upregulation of HADHA. Importantly, we demonstrated that overexpression of HADHA inhibited the expression of p-MAPK in NAFLD mice, reducing lipid accumulation and steatosis.
Conclusion
HADHA may function as a protective factor in the progression of NAFLD by alleviating abnormal metabolism and oxidative stress by suppressing MKK3/MAPK signalling pathway activation, providing a new target for the treatment of NAFLD.
Keywords: HADHA, Nonalcoholic fatty liver disease, Lipid metabolism, Oxidative stress, MKK3/MAPK
Introduction
Nonalcoholic fatty liver disease (NAFLD) is initialized by abnormal lipid accumulation in hepatocytes in the absence of alcohol intake 1. According to different developmental processes and pathological changes, NAFLD can be divided into three stages. The earliest stage is simple fatty liver. At this stage, there is excessive fat deposition in liver cells, but it has not yet caused liver cell damage. The second stage is non-alcoholic steatohepatitis (NASH), with liver cell damage and inflammatory cell infiltration. In the third stage, intrahepatic fibrosis or even cirrhosis occurs. Therefore, NAFLD is also the pathophysiological basis of many diseases (steatohepatitis, liver fibrosis, cirrhosis and hepatocellular carcinoma) and threatens human health 2, 3. Aside from lifestyle modification and weight loss, there is no effective treatment for NAFLD 4, 5. The formation of large lipid droplets in hepatocytes is caused by excessive inflammation, endoplasmic reticulum (ER) stress and oxidative stress 6. NAFLD mouse livers show oxidative damage, excessive inflammation and liver damage 7. ER stress triggers the unfolded protein response (UPR), resulting in inflammation and inflammasome activation of hepatocytes 8.
The mitogen-activated protein kinase (MAPK) signalling pathway is fundamental in inflammation and oxidative stress because it regulates nuclear factor E2-related factor 2 (Nrf2) and nuclear factor kappa B (NF-κB), which are involved in liver and metabolic diseases 9, 10. Serum vitamin D deficiency (VDD) patients show upregulation of p-MAPK expression in the serum 11. Activation of MAPK signalling in high fat diet (HFD)-fed mice contributes to lipid accumulation and inflammatory and reactive oxygen species (ROS) production 12. The R4/B subfamily regulators of G protein signalling protein 5 (RGS5) inhibit the activation of JNK/p38 pathways, which is a promising target for NAFLD 13, 14. The inactivation of NF-κB and MAPK cascades reverses hepatic steatosis, inflammation, and abnormal lipid metabolism, representing a potential strategy for NAFLD treatment 15. Lipid accumulation and inflammatory responses are triggered by MAPK signalling to exacerbate NAFLD progression 16. Activation of MAPK modulates lipid metabolism-related gene expression to participate in NAFLD progression 17. Insulin resistance is induced by activation of MAPK to promote NAFLD development 18. Lipid accumulation is induced by an ERK1/2-dependent pathway to promote NAFLD development 19.
Mitogen-activated protein kinase kinase 3 (MKK3) activates MAPK to regulate inflammation 20, oxidative stress 21 and ER stress 22. Phosphorylation of MKK3 (p-MKK3) is increased in the NAFLD mouse liver, and melatonin alleviates NAFLD phenotypes such as body weight gain, hepatic lipid accumulation, and fibrosis by inhibiting p-MKK3 expression 23. MKK3 upregulates TNF-α production to exacerbate inflammation, which results in liver damage 24. However, the regulatory mechanism of the MKK3 pathway in NAFLD progression is still unknown.
Hydroxyl CoA dehydrogenase alpha subunit (HADHA) regulates fatty acid beta-oxidation 25, lipid programming 26 and mitochondrial function 27, which are involved in inflammatory and oxidative stress response. Recent research findings show that acetylation of the mitochondrial β-oxidation enzyme HADHA modulates hepatic fatty acid oxidation activity, and HADHA may be a key regulator during the pathogenesis of fatty liver disease 28. The diabetic myocardium decreases the activity of HADHA to promote lipid droplet accumulation and elevate ER stress 29. The decreased expression of HADHA in NASH liver was verified by western blotting, which was used as a complementary technique to confirm the proteomic results 30. Nevertheless, the beneficial role of HADHA in NAFLD remains unclear.
Here, our study showed that HADHA expression was decreased in both free fatty acid (FFA)-treated L02 cells and NAFLD mouse liver tissues. In addition, HADHA overexpression in NAFLD mice inhibited the progression of hepatic steatosis. Mechanistic studies demonstrated that HADHA inactivated the MAPK pathway. Upregulation of HADHA alleviated lipid accumulation and oxidative stress in NAFLAD mice. Knocking down HADHA aggravated the damage caused by NAFLD. In summary, our findings reveal a previously unappreciated role of HADHA in NAFLD development, which may be a potential therapeutic target for NAFLD.
Materials and methods
Cell line culture and treatment
L02 normal human liver cells were purchased from the ATCC and cultured in RPMI 1640 medium (Thermo Scientific™, # 88,365) with 10% foetal bovine serum (Gibco™, #30,044,333) and 1% penicillin/streptomycin in a humidified atmosphere with 5% CO2 at 37 °C. We used 1 mM FFA (FFA; oleate acid and palmitate acid (2:1)) to treat L02 cells to establish a NAFLD cell model. L02 cells were cultured in RPMI 1640 medium containing FBS until the cells reached 80% confluence and then treated with 1 mM FFA for 24 h (24 h). The cells were transfected with 25 nM siHADHA or siControl for 24 h. The siRNA sequence for HADHA was 5`-TGGTGACAAGATTTGTGAA-3`. The siRNA sequence for the control was 5`-TTCTCCGAACGTGTCACGT-3`.
Animals and treatment
Adenovirus (Adv) vectors were used to drive the expression of GFP (Adv-NC) or HADHA (Adv-HADHA) in mouse livers. Eight-week-old male C57BL/6 mice were randomly divided into 4 groups: the control group was fed a standard chow diet; the NAFLD model group was fed a HFD for 8 weeks (8 w); the HFD + Adv-NC group was fed a HFD for 6 weeks and then administered 2 × 109 ifu Adv-NC by tail vein injection; and the HFD + Adv-HADHA group was fed a HFD for 6 weeks and then administered 2 × 109 ifu Adv-HADHA by tail vein injection. Two weeks after virus injection, the mice were sacrificed using isoflurane. All animal studies were approved by the Animal Care and Use Committee of Zhejiang University in accordance with the Chinese guidelines for the care and use of laboratory animals.
Western blot assay
RIPA lysis buffer (Thermo Scientific™, 89,900) containing PMSF (Thermo Scientific™, #36,978) was used to lyse L02 cells. The BCA method (Thermo Scientific™, #23,225) was used to measure the concentration of the protein lysate. After mixing 60 µg of cell lysate with 5× sample buffer, SDS–PAGE was used to separate the proteins, and the separated proteins were transferred to a PVDF membrane (Bio-Rad, #162–0177). After blocking with 4% milk containing 0.1% Tween, the following antibodies were added, and the membrane was incubated overnight at 4 °C: phosphor-MKK3 (1:2000, Cell Signaling Technology, #9231), MKK3 (1:2000, Cell Signaling Technology, #5674), HADHA (1:2000, Abcam, ab203114), phosphor-MAPK (1:1000, Cell Signaling Technology, #4370), MAPK (1:2000, Cell Signaling Technology, #9102), and GAPDH (1:1500, Abcam, ab9485). After washing the membrane 3 times with PBS solution containing 0.1% Tween, 4% milk containing 0.1% Tween and an HRP secondary antibody (1:4000; Abcam, ab205718)) were added, and the membrane was incubated for 2 h at room temperature. The membrane was removed, and ECL reagent (Bio-Rad, 170–5060) was added to the membrane. The membrane was placed into a Micro-Chemi 4.2 imaging system (Bio-Rad). ImageJ software was used to perform optical density analysis.
qRT–PCR assays
TRIzol reagent (Invitrogen, #12,183,555) was used to extract total RNA. cDNA synthesis was performed using a high-capacity cDNA reverse transcription kit (Applied Biosystems, #4,368,813). The qRT–PCR experiment was performed using a StepOnePlus Real-Time PCR system with the SYBR Green experimental method (Applied Biosystems). The relative expression of genes was calculated by the 2−ΔΔCt method. The qRT–PCR primer sequences and related primer sequences are listed in Table 1.
Table 1.
qRT–PCR primer sequences and related primer sequences
| Gene | Forward Primer (5`-3`) | Reverse Primer (5`-3`) |
|---|---|---|
| Mus-HADHA | TGCATTTGCCGCAGCTTTAC | GTTGGCCCAGATTTCGTTCA |
| Human-HADHA | TCAAGCAGGGGAAGGTCA | CTGGAGGATTCGGATGACTT |
| Human-PPARα | GCGAGCTCGCCTCCCTGTTGTTTCTA | GCGTCGACGGTGGCATCAGTCTTCAT |
| Human-CPT2 | CATACAAGCTACATTTCGGGACC | AGCCCGGAGTGTCTTCAGAA |
| Human-GAPDH | TCAAGAAGGTGGTGAAGCAGG | TCAAAGGTGGAGGAGTGGGT |
| Human-EHHADH | AAACTCAGACCCGGTTGAAGA | TTGCAGAGTCTACGGGATTCT |
| Human-ECHS1 | TGTCCTGTTGAGACACTGGTG | ACAAACGCGGTCATCCCTTC |
| Human-HADHB | CTGTCCAGACCAAAACGAAGAA | CGATGCAACAAACCCGTAAGC |
| Human-HADH | CACACAGTAGTGTTGGTAGACC | TGCCACTTTCCTAAGGCTTTC |
| Human-ACOX1 | TGTCCTATTTGAACGACCTGCCCA | AGGTTCCAAGCTACCTCCTTGCTT |
| Mus-GAPDH | AGGCCGGTGCTGAGTATGTC | TGCCTGCTTCACCACCTTCT |
Oil red O staining in L02 cells and liver tissues
Cells on slides were fixed with 4% paraformaldehyde and Oil red O staining solution (Nanjing Jiancheng Bioengineering Institute (NJBI), D027) for 15 min, washed in 60% isopropanol and counterstained with haematoxylin after rinsing in distilled water.
Detection of mitochondrial membrane potential (MMP) in L02 cells and liver tissues
Mitochondrial depolarization was analysed with an MMP assay kit with JC-1 (Abcam, ab141387) according to the manufacturer’s instructions. Mitochondrial JC-1 monomers (green) and aggregates (red) were detected by fluorescence microscopy and laser confocal microscopy.
Measurement of ROS
L02 cells were inoculated into 6-well plates, transfected with siHADHA or siControl for 24 h, and then treated with 1 mM FFA for 24 h. The cells were incubated with 2ʹ,7ʹ-dichlorofluorescein diacetate in the dark for 0.5 h in the presence or absence of NAC (ROS scavenger N-acetylcysteine, 3 mM). The cells were washed to eliminate extracellular DCFH-DA with PBS, and intracellular DCFH-DA was transformed into fluorescent dichlorofluorescein (DCF). Then, DCF fluorescence of the cells was analysed by flow cytometry at 480 nm/520 nm.
Biochemical analysis
The triglyceride (TG, NJBI, A110-1), alanine aminotransferase (ALT, NJBI, C009-2), aspartate transaminase (AST, NJBI, C101-2), adenosine triphosphate (ATP, NJBI, A095), hydrogen peroxide (H2O2, NJBI, A064-1), catalase (CAT, NJBI, A007-1-1) and total cholesterol (TC, NJBI, A111-1-1) levels in vivo and in vitro were measured according to the manufacturer’s instructions.
HE staining
Tissue sections were stained with Mayer’s haematoxylin staining solution for 5–7 min, washed in ddH2O to turn blue, incubated with 1% hydrochloric acid alcohol for differentiation for 2–5 seconds, and washed with ddH2O. After air-drying, the slides were mounted with neutral gum. Finally, the morphologic changes in the liver tissues were observed under a light microscope.
Statistical analysis
The data were analysed by SPSS 18.0 and are presented as the mean ± SEM (standard error of the mean). Significant differences between groups were determined by two-way ANOVA followed by Tukey post hoc tests. A P value < 0.05 was considered to indicate statistical significance.
Results
The expression of HADHA was decreased in FFA-treated cells and NAFLD mouse liver tissues
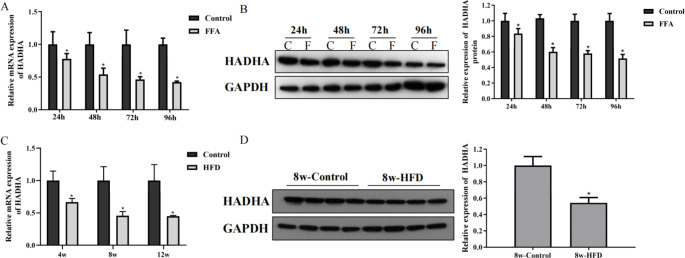
To study the potential role of HADHA in the development of NAFLD, we measured the expression of HADHA in FFA-treated L02 and NAFLD mouse liver tissues. FFA treatments inhibited the expression of HADHA in L02 cells (Fig. 1 A and Fig. 1B). In addition, liver tissues from NAFLD patients showed decreases in HADHA mRNA and protein levels (Fig. 1 C and Fig. 1D). Together, these results demonstrated that HADHA may be related to the progression of NAFLD.
Fig. 1.
The expression of HADHA was decreased in FFA-treated cells and liver tissues of NAFLD mice. A-B: HADHA mRNA and protein levels in FFA-treated L02 cells were examined by qRT–PCR and western blotting. C-D: HADHA mRNA and protein levels in NAFLD mouse liver tissues were examined by qRT–PCR and western blotting. *P < 0.05 compared with the control group
Knockdown of HADHA accelerated hepatic steatosis in FFA-treated L02 cells
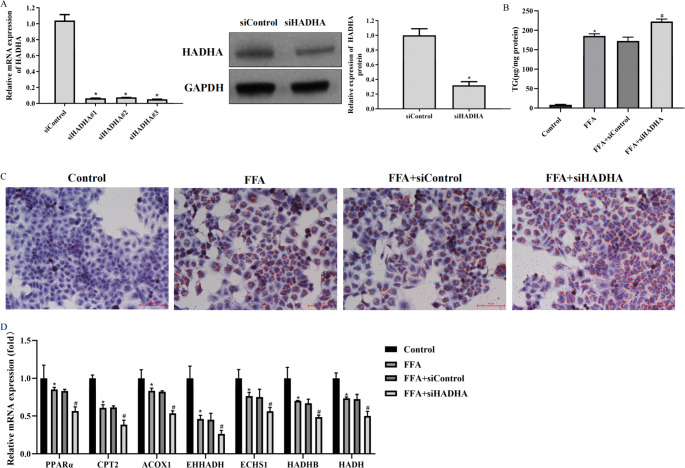
To study the role of HADHA in the pathological process of NAFLD, we successfully constructed siHADHA, and L02 cells transfected with siHADHA had low expression of HADHA protein and mRNA (Fig. 2 A). High levels of TG were observed in FFA-treated L02 cells transfected with siHADHA (Fig. 2B). In addition, Oil red O staining showed increased lipid accumulation in FFA-treated L02 cells transfected with siHADHA (Fig. 2 C). Decreases in the expression of genes associated with lipid metabolism-related factors, including peroxisome proliferator-activated receptor-α (PPARα), carnitine palmitoyl transferase 2 (CPT2), acyl-CoA oxidase 1 (ACOX1), enoyl-CoA hydratase and 3-hydroxyacyl CoA dehydrogenase (EHHADH), enoyl-CoA hydratase, short chain 1 (ECHS1), hydroxyacyl-CoA dehydrogenase trifunctional multienzyme complex subunit beta (HADHB) and hydroxyacyl-CoA dehydrogenase (HADH), were detected in FFA-treated L02 cells transfected with siHADHA (Fig. 2D). Collectively, the findings above indicated that downregulation of HADHA accelerated lipid accumulation in NAFLD cells.
Fig. 2.
Knockdown of HADHA accelerated hepatic steatosis in FFA-treated L02 cells A: L02 cells were transfected with siHADHA or siControl for 24 h, and western blotting and qRT–PCR were used to analyse the protein and mRNA expression of HADHA. B: L02 cells were transfected with siHADHA or siControl for 24 h and then treated with 1 mM FFA for 24 h. TG kits were used to measure the TG content. C: Oil Red O staining assay detected the accumulation of lipid droplets in L02 cells (200×). D: qRT–PCR detected lipid metabolism-related gene expression. *P < 0.05 compared with the control group; #P < 0.05 compared with the FFA + siControl group
Inhibition of HADHA accelerated mitochondrial dysfunction and oxidative stress in L02 cells
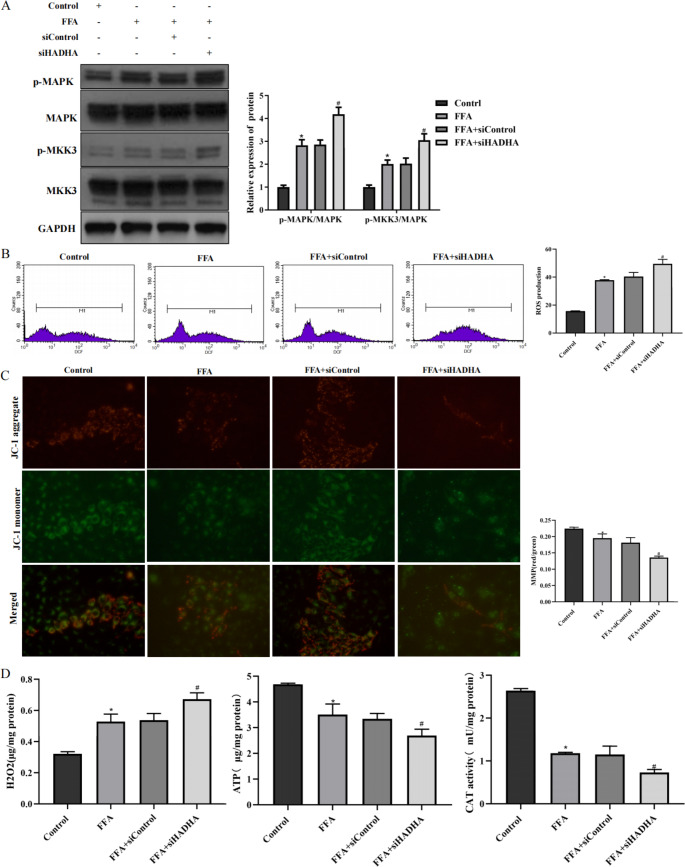
Phosphorylation of MKK3 (p-MKK3) which is the key upstream signal of MAPK, increases in the NAFLD liver and inhibiting p-MKK3 alleviates hepatic lipid accumulation and exacerbate inflammation 31, 32. Therefore, the regulatory mechanism of HADHA on the activation of MKK3 and MAPK was explored. In L02 cells treated with FFA, the expression of p-MKK3 and p-MAPK was increased by HADHA inhibition (Fig. 3 A). MAPK regulates oxidative stress, which is related to NAFLD development 17, 33. Inhibition of HADHA resulted in high ROS production (Fig. 3B). Moreover, MMP decreased when HADHA was inhibited (Fig. 3 C). In addition, ATP content and CAT activity decreased when HADHA was downregulated, while H2O2 levels increased when HADHA was downregulated (Fig. 3D). In summary, the regulation of HDAHA in NAFLD was associated with MKK3/MAPK activation, mitochondrial function, and oxidative stress.
Fig. 3.
Inhibition of HADHA accelerated mitochondrial dysfunction and oxidative stress in L02 cells A: L02 cells were transfected with siHADHA or siControl for 24 h and then treated with 1 mM FFA for 24 h. The proteins p-MAPK, MAPK, MKK3 and p-MKK3 were examined by western blotting. B: DCF fluorescence staining was used to examine ROS production. C: A JC-1 assay was used to examine MMP (400×). D: The levels of H2O2, ATP, and CAT in L02 cells were examined by biochemical tests. *P < 0.05 compared with the control group; #P < 0.05 compared with the FFA + siControl group
Upregulation of HADHA alleviated hepatic steatosis and inflammation in NAFLD mice
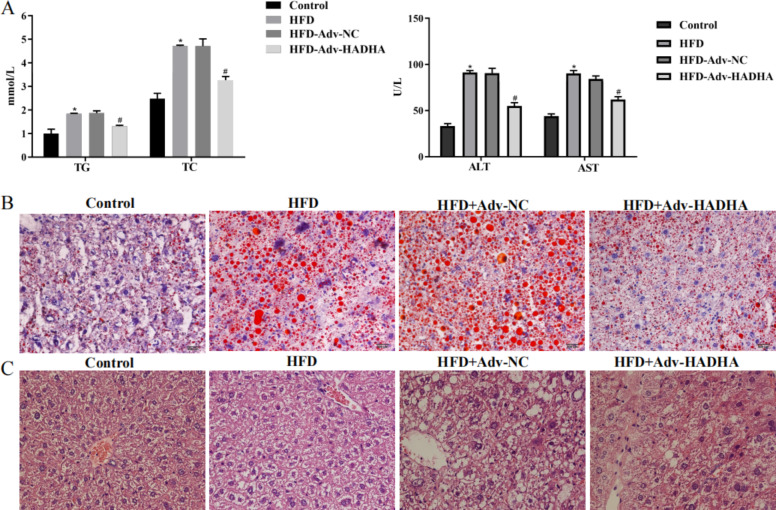
To further verify the regulation of HADHA, NAFLD mice were fed a HFD for 8 weeks, and Adv-HADHA was used for tail vein injection. We found that the TG, TC, ALT and AST levels in NAFLD mouse serum were increased, while HADHA overexpression decreased the TG, TC, ALT and AST levels (Fig. 4 A). Oil Red O staining showed that NAFLD mouse liver tissues had large numbers of red-stained lipid droplets and that overexpression of HADHA decreased the numbers of red-stained lipid droplets in NAFLD mouse liver tissues (Fig. 4B). HE staining showed that the liver tissues of the NAFLD mice had increases in ballooning and degeneration of hepatocytes and visible focal necrosis, while overexpression of HADHA alleviated liver tissue lesions in NAFLD mice (Fig. 4 C). The above results indicate that upregulation of HADHA alleviated the abnormal lipid metabolism and inflammation of NAFLD mice.
Fig. 4.
Upregulation of HADHA alleviated hepatic steatosis and inflammation in NAFLD mice. NAFLD mice were fed a HFD for 8 weeks, and Adv-NC or Adv-HADHA was used for tail vein injection once 2 weeks before sacrifice. A: TG, TC, ALT and AST in NAFLD mouse serum were examined by biochemical tests. B: An Oil Red O staining assay detected the accumulation of lipid droplets in mouse liver tissues (400×). C: An HE staining assay was used to analyse the liver tissue lesions of mice (400×). *P < 0.05 compared with the control group; #P < 0.05 compared with the HFD + Adv-NC group
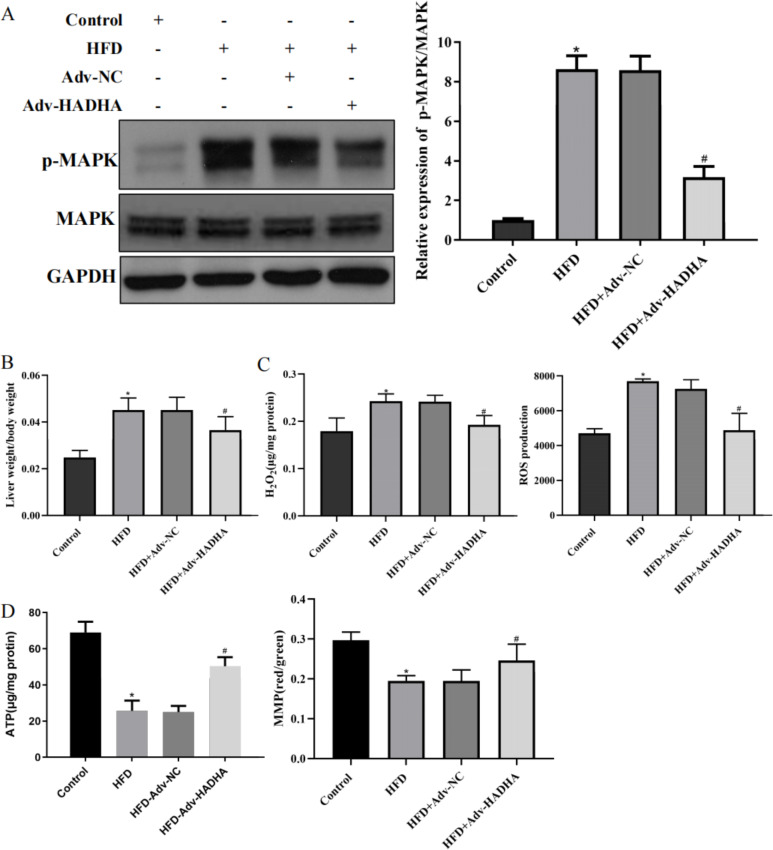
Upregulation of HADHA alleviated mitochondrial dysfunction and oxidative stress in NAFLD mice
Moreover, Adv-HADHA effectively upregulated the expression of HADHA while downregulating p-MAPK in the liver tissue of NAFLD mice (Fig. 5 A). In addition, the liver weight/body weight ratio decreased when HADHA was upregulated (Fig. 5B). Moreover, the levels of H2O2 and ROS in the NAFLD mice were increased, while the ATP activity and MMP were reduced. The H2O2 and ROS levels in Adv-HADHA-treated NAFLD mice were reduced, while the ATP activity and MMP were increased (Fig. 5 C and Fig. 5D). In summary, HADHA improved the pathological changes in NAFLD liver tissue.
Fig. 5.
Upregulation of HADHA alleviated mitochondrial dysfunction and oxidative stress in NAFLD mice. NAFLD mice were fed a HFD for 8 weeks, and Adv-NC or Adv-HADHA was used for tail vein injection once 2 weeks before sacrifice. A: The expression of MAPK and p-MAPK in mouse liver tissues was examined by western blotting. B: The liver weight/body weight ratio was determined. C: H2O2 and ROS production were examined. D: ATP activity and MMP were examined. *P < 0.05 compared with the control group; #P < 0.05 compared with the HFD + Adv-NC group
Discussion
NAFLD, a metabolic disorder with a growing incidence, can progress to cirrhosis and liver cancer to threaten human life and health, but the mechanism of NAFLD is unclear 34. Apart from lifestyle changes and weight loss, there is a lack of an effective treatment strategy for NAFLD. Here, we identified HADHA as a key inhibitor of NAFLD. The expression of HADHA mRNA and protein was decreased in NAFLD mouse livers and human normal hepatocytes L02 stimulated by FFA. Knockdown of HADHA in L02 cells stimulated by FFA markedly promoted lipid accumulation, mitochondrial dysfunction, and oxidative stress. In contrast, overexpression of HADHA alleviated lipid accumulation, liver lesions, and oxidative stress in NAFLD mice. Finally, we showed that HFD feeding or FFA administration activated the MKK3/MAPK pathway, and this activation was reversed by downregulation of HADHA. In summary, our findings strongly suggest that targeting HADHA with the MKK3/MAPK pathway may attenuate NAFLD progression.
Growing evidence has shown that the pathogenesis of NAFLD is complicated and involves lipid accumulation, oxidative stress, mitochondrial dysfunction, and ER stress 35, 36, 37, 38. And the activation of MAPK is related to hepatic lipid deposition in patients with NASH and the pathogenesis of NASH-related fibrosis 37. For example, the activating the MAPK pathway exacerbates liver fibrosis by inducing inflammatory factor secretion and abnormal lipid metabolism 39. Downregulation of MAPK inhibits hepatic steatosis and inflammation in L02 cells 40. IL11 leads to hepatocyte death via NADPH oxidase 4 (NOX4)-derived ROS and activation of MAPK to impair mitochondrial function and inhibit fatty acid oxidation 41. Oleic acid upregulates the expression of hepassocin (HPS) to activate MAPK, leading to lipid accumulation in HepG2 cells 19. Consistent with our research, FFA-treated L02 cells had increased p-MAPK levels upon inhibition of HADHA, and these increases were accompanied by increases in ROS production, TG and H2O2. Moreover, our study showed that the expression of p-MAPK was decreased in HFD-challenged mouse livers when HADHA was overexpressed. MKK3, an upstream activator of p38 MAPK, increases p-MAPK expression, leading to the phosphorylation of MKK-3/6 and upregulation of ERK1/2 in macrophages 42, 43. In addition, the p-MKK3 protein was upregulated by HADHA inhibition in FFA-treated cells. Therefore, HADHA can inactivate MKK3/MAPK pathways to protect against NAFLD.
HADHA, a fatty acid β-oxidation-related factor, mediates lipid programming to regulate multiple cellular programs, such as apoptosis, fat metabolism and mitochondrial function 26, 44, 45, 46, 47. Increasing research has shown the important role of HADHA in metabolic dysfunction in the liver. Recent studies have demonstrated that downregulation of HADHA expression is related to oxidative stress, hepatic steatosis and mitochondrial function in the liver 28, 47. Therefore, we hypothesized that HADHA participates in NAFLD progression to maintain the homeostasis of lipid metabolism. CPT2 mediates the β-oxidation of long-chain acyl-CoA in the mitochondrial matrix 48. Abnormal lipid accumulation of hepatocytes increases ALT and AST content while decreasing ATP content 49,50. Consistent with our research, HADHA showed a positive correlation with the expression of CPT2. However, overexpression of HADHA decreased the content of H2O2, ALT and AST. In summary, HADHA had a protective effect on steatotic cells via inhibition of MKK3 activation and MAPK signalling pathways, subsequently alleviating lipid accumulation, inflammation, ROS production, and ER stress.
Conclusion
In summary, our study revealed a protective effect of HADHA against NAFLD progression. HADHA inhibition significantly accelerated lipid accumulation, oxidative stress, mitochondrial dysfunction, and ER stress by activating MKK3, which subsequently activated the downstream MAPK signalling pathways to promote p-MAPK expression. Finally, overexpression of HADHA alleviated steatosis and liver damage. Therefore, inhibition of MKK3/MAPK pathways by HADHA may be an effective treatment for NAFLD. However, considering the complexity of NAFLD pathogenesis, more studies to determine new targets for NAFLD treatments are needed.
Acknowledgements
We sincerely appreciate the investigators and authors who have contributed to this field. This research was supported by the Zhejiang Provincial Natural Science Foundation of China under Grant No. LQ18H070006 and the Zhejiang Provincial Medical and Health Technology Project (Grant No. 2019RC069).
Authors’ contributions
Jiexia Ding, Lili Wu and Youming Li contributed to study design. Guoxian Zhu contributed to data collection. Jing Zhu and Pingping Luo contributed to data analysis. Jiexia Ding and Lili Wu contributed to drafting of the manuscript. All authors contributed to data interpretation and review/critical revision of the manuscript.
Data Availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
The authors declare there are no competing interests.
Ethical statement
All experiments were conducted according to the guidelines of Zhejiang University.
Footnotes
Jiexia Ding and Lili Wu these authors have contributed equally to this work.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Eslam M, George J. Genetic contributions to NAFLD: leveraging shared genetics to uncover systems biology. Nat Rev Gastroenterol Hepatol. 2019;17:40–52. doi: 10.1038/s41575-019-0212-0. [DOI] [PubMed] [Google Scholar]
- 2.Francque S, Szabo G, Abdelmalek MF, et al. Nonalcoholic steatohepatitis: the role of peroxisome proliferator-activated receptors. Nat Rev Gastroenterol Hepatol. 2020;18:24–39. doi: 10.1038/s41575-020-00366-5. [DOI] [PubMed] [Google Scholar]
- 3.Friedman SL, Neuschwander-Tetri BA, Rinella M, et al. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 2018;24:908–922. doi: 10.1038/s41591-018-0104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sheka AC, Adeyi O, Thompson J, Hameed B, et al. Nonalcoholic Steatohepatitis: A Review Jama. 2020;323:1175–1183. doi: 10.1001/jama.2020.2298. [DOI] [PubMed] [Google Scholar]
- 5.Younes R, Bugianesi E. A spotlight on pathogenesis, interactions and novel therapeutic options in NAFLD. Nat Rev Gastroenterol Hepatol. 2018;16:80–82. doi: 10.1038/s41575-018-0094-6. [DOI] [PubMed] [Google Scholar]
- 6.Gluchowski NL, Becuwe M, Walther TC, et al. Lipid droplets and liver disease: from basic biology to clinical implications. Nat Rev Gastroenterol Hepatol. 2017;14:343–355. doi: 10.1038/nrgastro.2017.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Besse-Patin A, Léveillé M, Oropeza D, et al. Estrogen Signals through PPARG coactivator 1 alpha to Reduce Oxidative Damage Associated with Diet-induced Fatty Liver Disease. Gastroenterology. 2016;152:243–256. doi: 10.1053/j.gastro.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 8.Lebeaupin C, Vallée D, Hazari Y, et al. Endoplasmic Reticulum stress signaling and the pathogenesis of Non-Alcoholic Fatty Liver Disease. J Hepatol. 2018;69:927–947. doi: 10.1016/j.jhep.2018.06.008. [DOI] [PubMed] [Google Scholar]
- 9.Peluso I, Yarla NS, Ambra R, et al. MAPK Signalling Pathway in Cancers: Olive Products as Cancer Preventive and Therapeutic Agents. Semin Cancer Biol. 2017;56:185–195. doi: 10.1016/j.semcancer.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 10.Lawan A, Bennett AM. Mitogen-Activated Protein Kinase Regulation in Hepatic Metabolism. Trends Endocrinol Metab. 2017;28:868–878. doi: 10.1016/j.tem.2017.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nelson JE, Roth CL, Wilson LA et al (2016) Vitamin D Deficiency Is Associated With Increased Risk of Non-alcoholic Steatohepatitis in Adults With Non-alcoholic Fatty Liver Disease: Possible Role for MAPK and NF-κB? The American journal of gastroenterology. 111:852–863 [DOI] [PMC free article] [PubMed]
- 12.Wu L, Liu Y, Zhao Y, Li M, et al. Targeting DUSP7 signaling alleviates hepatic steatosis, inflammation and oxidative stress in high fat diet (HFD)-fed mice via suppression of TAK1. Free Radic Biol Med. 2020;153:140–158. doi: 10.1016/j.freeradbiomed.2020.04.009. [DOI] [PubMed] [Google Scholar]
- 13.Wang J, Ma J, Nie H, et al. Hepatic Regulator of G protein Signaling 5 Ameliorates NAFLD by Suppressing TAK1-JNK/p38 Signaling. Hepatology. 2020;73:104–125. doi: 10.1002/hep.31242. [DOI] [PubMed] [Google Scholar]
- 14.Win S, Than TA, Zhang J, et al. New insights into the role and mechanism of c-Jun-N-terminal kinase signaling in the pathobiology of liver diseases. Hepatology. 2017;67:2013–2024. doi: 10.1002/hep.29689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu Y, Jiang Z, Dai H, et al. Hepatic leukocyte immunoglobulin-like receptor B4 (LILRB4) attenuates nonalcoholic fatty liver disease via SHP1-TRAF6 pathway. Hepatology. 2017;67:1303–1319. doi: 10.1002/hep.29633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang L, Tian R, Yao X, et al. Milk Fat Globule-EGF Factor 8 improves Hepatic Steatosis and Inflammation. Hepatology. 2020;73:586–605. doi: 10.1002/hep.31277. [DOI] [PubMed] [Google Scholar]
- 17.Zai W, Chen W, Wu Z, et al. Targeted Interleukin-22 Gene Delivery in the Liver by Poly-Metformin and Penetratin-Based Hybrid Nanoparticles to Treat Non-Alcoholic Fatty Liver Disease. ACS Appl Mater Interfaces. 2019;11:4842–4857. doi: 10.1021/acsami.8b19717. [DOI] [PubMed] [Google Scholar]
- 18.Wu HT, Ou HY, Hung HC, et al. A novel hepatokine, HFREP1, plays a crucial role in the development of insulin resistance and type 2 diabetes. Diabetologia. 2016;59:1732–1742. doi: 10.1007/s00125-016-3991-7. [DOI] [PubMed] [Google Scholar]
- 19.Wu HT, Lu FH, Ou HY, et al. The role of Hepassocin in the development of non-alcoholic fatty liver disease. J Hepatol. 2013;59:1065–1072. doi: 10.1016/j.jhep.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 20.Ivanov AA, Gonzalez-Pecchi V, Khuri LF, et al. OncoPPi-informed discovery of mitogen-activated protein kinase kinase 3 as a novel binding partner of c-Myc. Oncogene. 2017;36:5852–5860. doi: 10.1038/onc.2017.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patel PH, Pénalva C, Kardorff M, et al. Damage sensing by a Nox-Ask1-MKK3-p38 signaling pathway mediates regeneration in the adult Drosophila midgut. Nat Commun. 2019;10:4365. doi: 10.1038/s41467-019-12336-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Canfrán-Duque A, Rotllan N, Zhang X, et al. Macrophage deficiency of miR-21 promotes apoptosis, plaque necrosis, and vascular inflammation during atherogenesis. EMBO Mol Med. 2017;9:1244–1262. doi: 10.15252/emmm.201607492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li DJ, Tong J, Li YH, et al. Melatonin safeguards against fatty liver by antagonizing TRAFs-mediated ASK1 deubiquitination and stabilization in a β-arrestin-1 dependent manner. J Pineal Res. 2019;67:e12611. doi: 10.1111/jpi.12611. [DOI] [PubMed] [Google Scholar]
- 24.González-Terán B, Cortés JR, Manieri E, et al. Eukaryotic elongation factor 2 controls TNF-α translation in LPS-induced hepatitis. J Clin Invest. 2013;123:164–178. doi: 10.1172/JCI65124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miklas JW, Clark E, Levy S, et al. TFPa/HADHA is required for fatty acid beta-oxidation and cardiolipin re-modeling in human cardiomyocytes. Nat Commun. 2019;10:4671. doi: 10.1038/s41467-019-12482-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Y, Lu LL, Wen D, et al. MiR-612 regulates invadopodia of hepatocellular carcinoma by HADHA-mediated lipid reprogramming. J Hematol Oncol. 2020;13:12. doi: 10.1186/s13045-019-0841-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amoedo ND, Sarlak S, Obre E, et al. Targeting the mitochondrial trifunctional protein restrains tumor growth in oxidative lung carcinomas. J Clin Investig. 2021;131:e133081. doi: 10.1172/JCI133081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Le-Tian Z, Cheng-Zhang H, Xuan Z, et al. Protein acetylation in mitochondria plays critical functions in the pathogenesis of fatty liver disease. BMC Genomics. 2020;21:435. doi: 10.1186/s12864-020-06837-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ljubkovic M, Gressette M, Bulat C, et al. Disturbed Fatty Acid Oxidation, Endoplasmic Reticulum Stress and Apoptosis in Left Ventricle of Patients with Type 2 Diabetes Mellitus. Diabetes. 2019;68:1924–1933. doi: 10.2337/db19-0423. [DOI] [PubMed] [Google Scholar]
- 30.Li L, Lu DZ, Li YM. Proteomic analysis of liver mitochondria from rats with nonalcoholic steatohepatitis. World J Gastroenterol. 2014;20(16):4778–4786. doi: 10.3748/wjg.v20.i16.4778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grit JL, Johnson BK, Dischinger PS, et al. Distinctive epigenomic alterations in NF1-deficient cutaneous and plexiform neurofibromas drive differential MKK/p38 signaling. Epigenetics Chromatin. 2021;14:7. doi: 10.1186/s13072-020-00380-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.James J, Srivastava A, Valuparampil Varghese M, et al. Heme induces rapid endothelial barrier dysfunction via the MKK3/p38MAPK axis. Blood. 2020;136:749–754. doi: 10.1182/blood.2019003986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ding Y, Zhang Z, Yue Z, et al. Rosmarinic acid Ameliorates H2O2-Induced Oxidative Stress On L02 Cells through MAPK and Nrf2 Pathways. Rejuvenation Res. 2018;22:289–298. doi: 10.1089/rej.2018.2107. [DOI] [PubMed] [Google Scholar]
- 34.Foulds CE, Treviño LS, York B, et al. Endocrine-disrupting chemicals and fatty liver disease. Nat reviews Endocrinol. 2017;13:445–457. doi: 10.1038/nrendo.2017.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Byrne CD, Targher G. What’s new in NAFLD pathogenesis, biomarkers and treatment? Nat Rev Gastroenterol Hepatol. 2019;17:70–71. doi: 10.1038/s41575-019-0239-2. [DOI] [PubMed] [Google Scholar]
- 36.Ray K. NAFLD-HCC: target cholesterol. Nat Rev Gastroenterol Hepatol. 2018;15:390. doi: 10.1038/s41575-018-0029-2. [DOI] [PubMed] [Google Scholar]
- 37.Younossi ZM, Karrar A, Pierobon M, et al. An exploratory study examining how nano-liquid chromatography-mass spectrometry and phosphoproteomics can differentiate patients with advanced fibrosis and higher percentage collagen in non-alcoholic fatty liver disease. BMC Med. 2018;16:170. doi: 10.1186/s12916-018-1136-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schuster S, Cabrera D, Arrese M, et al. Triggering and resolution of inflammation in NASH. Nat Rev Gastroenterol Hepatol. 2018;15:349–364. doi: 10.1038/s41575-018-0009-6. [DOI] [PubMed] [Google Scholar]
- 39.Wang Y, Zeng Z, Guan L, et al. GRHL2 induces liver fibrosis and intestinal mucosal barrier dysfunction in non-alcoholic fatty liver disease via microRNA-200 and the MAPK pathway. J Cell Mol Med. 2020;24:6107–6119. doi: 10.1111/jcmm.15212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang Z, Wu LM, Zhang JL, et al. DUSP12 Regulates Hepatic Lipid Metabolism through Inhibition of Lipogenesis and ASK1 Pathways. Hepatology. 2019;70:1099–1118. doi: 10.1002/hep.30597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dong J, Viswanathan S, Adami E, et al. Hepatocyte-specific IL11 cis-signaling drives lipotoxicity and underlies the transition from NAFLD to NASH. Nat Commun. 2021;12:66. doi: 10.1038/s41467-020-20303-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jha MK, Sarode AY, Bodhale N, et al. Development and Characterization of an Avirulent Leishmania major Strain. J Immunol. 2020;204:2734–2753. doi: 10.4049/jimmunol.1901362. [DOI] [PubMed] [Google Scholar]
- 43.Iijima K, Yoshizumi M, Hashimoto M, et al. Red wine polyphenols inhibit vascular smooth muscle cell migration through two distinct signaling pathways. Circulation. 2002;105:2404–2410. doi: 10.1161/01.cir.0000016349.36385.fb. [DOI] [PubMed] [Google Scholar]
- 44.Yang Y, Wang W, Liu X, et al. Probing the effects of hexavalent chromium exposure on histology and fatty acid metabolism in liver of Bufo gargarizans tadpoles. Chemosphere. 2020;243:125437. doi: 10.1016/j.chemosphere.2019.125437. [DOI] [PubMed] [Google Scholar]
- 45.Ali MR, Wu Y, Han T, et al. Simultaneous Time-dependent Surface Enhanced Raman Spectroscopy, Metabolomics and Proteomics Reveal Cancer Cell Death Mechanisms Associated with Au-Nanorod Photo-thermal Therapy. J Am Chem Soc. 2016;138:15434–15442. doi: 10.1021/jacs.6b08787. [DOI] [PubMed] [Google Scholar]
- 46.Margolis LM, Wilson MA, Whitney CC, et al. Exercising with low muscle glycogen content increases fat oxidation and decreases endogenous, but not exogenous carbohydrate oxidation. Metab Clin Exp. 2019;97:1–8. doi: 10.1016/j.metabol.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 47.Khare T, Khare S, Angdisen JJ, et al. Defects in long chain 3-hydroxy acyl-CoA dehydrogenase lead to hepatocellular carcinoma: A novel etiology of hepatocellular carcinoma. Int J Cancer. 2020;147:1461–1473. doi: 10.1002/ijc.32943. [DOI] [PubMed] [Google Scholar]
- 48.Jernberg JN, Bowman CE, Wolfgang MJ, Scafidi S. Developmental regulation and localization of carnitine palmitoyltransferases (CPTs) in rat brain. J Neurochem. 2017;142:407–419. doi: 10.1111/jnc.14072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ioannou GN, Green P, Kerr KF, et al. Models estimating risk of hepatocellular carcinoma in patients with alcohol or NAFLD-related cirrhosis for risk stratification. J Hepatol. 2019;71:523–533. doi: 10.1016/j.jhep.2019.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rao Y, Lu YT, Li C, et al. Bouchardatine analogue alleviates NAFLD/NASH in high fat fed mice via blunting ATP synthase activity. Br J Pharmacol. 2019;176:2877–2893. doi: 10.1111/bph.14713. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.