Abstract
Background
Long-term cocaine exposure leads to dysregulation of the reward system and initiates processes that ultimately weaken its rewarding effects. Here, we studied the influence of an escalating-dose cocaine regimen on drug-associated appetitive behavior after a withdrawal period, along with corresponding molecular changes in plasma and the prefrontal cortex (PFC).
Methods
We applied a 5 day escalating-dose cocaine regimen in rats. We assessed anxiety-like behavior at the beginning of the withdrawal period in the elevated plus maze (EPM) test. The reinforcement properties of cocaine were evaluated in the Conditioned Place Preference (CPP) test along with ultrasonic vocalization (USV) in the appetitive range in a drug-associated context. We assessed corticosterone, proopiomelanocortin (POMC), β-endorphin, CART 55–102 levels in plasma (by ELISA), along with mRNA levels for D2 dopaminergic receptor (D2R), κ-receptor (KOR), orexin 1 receptor (OX1R), CART 55–102, and potential markers of cocaine abuse: miRNA-124 and miRNA-137 levels in the PFC (by PCR).
Results
Rats subjected to the escalating-dose cocaine binge regimen spent less time in the cocaine-paired compartment, and presented a lower number of appetitive USV episodes. These changes were accompanied by a decrease in corticosterone and CART levels, an increase in POMC and β-endorphin levels in plasma, and an increase in the mRNA for D2R and miRNA-124 levels, but a decrease in the mRNA levels for KOR, OX1R, and CART 55–102 in the PFC.
Conclusions
The presented data reflect a part of a bigger picture of a multilevel interplay between neurotransmitter systems and neuromodulators underlying processes associated with cocaine abuse.
Supplementary Information
The online version contains supplementary material available at 10.1007/s43440-022-00443-3.
Keywords: Cocaine binge, HPA axis, Neuromodulators, KOR, OX1R, microRNA
Introduction
A common symptom in cocaine addicts is impairment of reward anticipation. We know so far that long-term dose-escalating psychostimulant (cocaine) administration leads to dysregulation of the reward system and initiates processes that ultimately result in the weakening of the dopaminergic signal reflected by a decrease in the rewarding effect of a drug [1–7]. This phenomenon may lead to increased drug consumption, compulsive drug-seeking, and loss of control over drug use despite its adverse consequences [8, 9].
Cortical regions are implicated in the control of reward-seeking behavior and reinforcement mechanisms associated with cue-potentiated behavior [10–16]. The bidirectional regulation occurs between the ventral tegmental area (VTA) dopaminergic neurons and its target, the medial prefrontal cortex (mPFC), playing an essential role in control over reward system activity [9, 17, 18].
Dysregulation of the hypothalamic–pituitary–adrenal (HPA) axis is also supposed to contribute to craving and early relapse-associated processes [19, 20]. A lot of research points to the role of neuromodulators related to the HPA axis and hypothalamic peptides, the cocaine and amphetamine-regulated transcript (CART 55–102), and orexins, which are highly expressed in structures composing the HPA axis, as modulators of dopaminergic neurotransmission [12–14, 16, 21–23]. Orexin neurons co-express the inhibitory opioid dynorphin, and those two peptides are characterized by opposing actions on motivated behavior, namely, orexin is implicated in states of arousal and reward, whereas dynorphin is implicated in depressive-like states [24]. Orexin signaling in the PFC is implicated in the reinstatement of reward seeking [25–28].
In the current study, we were looking for a molecular pattern associated with an altered appetitive response to cocaine after the withdrawal period following dose-escalating administration. In humans, cocaine is usually consumed in recurrent cycles, which occur over 1–7 days with a period of abstinence accompanied by depressive symptoms and anxiety [29–35]. In the current study, we applied an escalating-dose cocaine regimen in rats, which was modeled to mimic the human pattern of cocaine abuse. In the study, we assessed anxiety-like behavior in the early withdrawal period in the Elevated Plus Maze (EPM) test, as anxiety and depression-related behavior prevails typically during the initial period of abstinence [36]. The reinforcement properties of cocaine were evaluated in the Conditioned Place Preference (CPP) test [37, 38] along with ultrasonic vocalization (USV) in the appetitive range (30–120 kHz, mainly referred to as 50 kHz) in the drug-associated context, often used to measure appetitive response to the context of various rewarding stimuli [39].
In search for a molecular pattern associated with altered response to cocaine we measured neuromodulators related to the HPA axis and hypothalamic peptides, namely corticosterone, proopiomelanocortin (POMC), β-endorphin, along with CART 55–102 levels in plasma, and mRNA levels for D2 dopaminergic receptor, κ-opioid receptor (KOR), orexin 1 receptor (OX1R), CART 55–102, and previously suggested markers of cocaine abuse: microRNA-124 (miRNA-124) and microRNA-137 (miRNA-137) levels, in the PFC [40–42].
Materials and methods
Animals
Male Wistar adult rats (n = 20), 9-week old and weighing 220–250 g at the beginning of the experiment, purchased from a licensed breeder (The Center for Experimental Medicine of the Medical University, 24 A Skłodowskiej-Curie Street, Białystok, Poland) were used in the study. The animals were housed in environmentally enriched laboratory conditions (temperature 20 ± 2 °C; 12 h light/dark cycle, light on at 7 am; 45–55% humidity, the cages were enriched with wood for gnawing). The rats were housed per 4 in opaque plastic cages (55 × 33 cm floor size, H = 19.5 cm) with free access to standard laboratory rat chow and tap water.
The study was conducted following the European Communities Council Directive 2010/63/UE. The Local Committee for Animal Care and Use at the Medical University of Warsaw, Poland, approved this study (Protocol No. WAW2/073/2018). All care was administered to minimize animal discomfort during experimental procedures. We confirm that the animals did not suffer unnecessarily at any stage of the experiments.
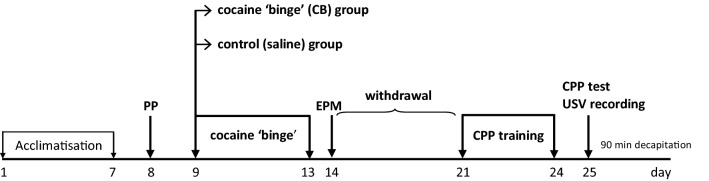
Experimental scheme (Fig. 1)
Fig. 1.
The experimental scheme. PP natural place preference assessment, EPM elevated plus maze test, CPP condition place preference, USV ultrasonic vocalization
After 7 days of acclimatization to the vivarium, the natural place preference of the animals was assessed. Next, the cocaine ‘binge’ regimen was introduced for 5 days. Twenty-four hours after the last cocaine injection, the EPM test was performed, and a withdrawal procedure was carried out for 7 days. Subsequently, the rats were trained for the CPP for 4 days. Two rats were excluded from the experiments for technical reasons. The day after, the place preference in the cocaine-paired compartment was assessed, and 50-kHz USVs were recorded simultaneously. Ninety minutes later, animals were decapitated.
Behavioral assessment
Escalating dose ‘binge’ cocaine regimen
The escalating-dose cocaine regimen consisted of 15 cocaine injections at increasing doses. Cocaine hydrochloride (TRC, Canada) was dissolved in a sterile aqueous 0.9% NaCl solution (Polpharma, Starogard Gdański, Poland) and injected intraperitoneally. Half of the animals (cocaine ‘binge’, CB group, n = 10) were injected with cocaine three times a day (at 7:45 am; 9:45 am; and 11:45 am) for 5 consecutive days in the following scheme: 1st day—three injections, 10 mg/kg, 2nd day—three injections, 15 mg/kg; 3rd day—two injections, 20 mg/kg (an adjusted volume 2 ml/kg) and one injection, 25 mg/kg; 4th day—two injections, 25 mg/kg and one injection, 30 mg/kg; 5th day—three injections, 30 mg/kg. Other animals (control group, n = 10) were given saline (sterile aqueous 0.9% NaCl solution) in the same regimen. The cocaine and saline injections were administered in dark cages (55 × 33 cm floor size, H = 19 cm) with no bedding for 40 min.
EPM test
The EPM apparatus was made of wood and consisted of two opposed open arms, two opposed walled arms (arm floor sizes, 50 × 10 cm), and an open square (10 × 10 cm) in the center, as it was described in our previous study [43]. The rats were transferred individually to the testing room, placed on the central square, facing an open arm, and had 5 min access to explore the maze. The behavior was recorded with a ceiling-mounted EV-650CG video camera (Sony, Japan) connected to a PC equipped with the EthoVision XT VideoTracking System v.7 (Noldus Information Technology B.V., Wageningen, The Netherlands). Video recordings were then used to calculate the total time spent in the open arms by an independent observer.
CPP procedure
The apparatus consisted of a plywood box (with 34 cm high walls) divided into two main compartments (35.5 × 20 cm floor size) separated by a smaller compartment (10 × 20 cm floor size). The apparatus and procedure were previously described by Taracha et al., 2014 [44]. Two CPP apparatuses were used simultaneously, thoroughly cleaned after each rat. The apparatuses were separated with a 1.1 × 0.7 m (L × H) sound-attenuating wall made of a 2-cm thick particle board with a black veneer on both sides. Rats’ behaviors in non-preferred and preferred areas were recorded with a ceiling-mounted EV-650CG video camera (Sony, Japan) and analyzed by the computerised system EthoVision XT VideoTracking System v.7 (Noldus Information Technology B.V., Wageningen, The Netherlands). Natural place preference was determined in a 15 min pre-test at the beginning of the experiment. The CCP training was carried out for 4 consecutive days. On the first day, the rats were given an i.p. saline injection and were instantly confined to the preferred section of the apparatus for 40 min. On the next day, designated rats received a cocaine injection and were immediately confined to the non-preferred section of the apparatus for 40 min. The 10 mg/kg cocaine (an adjusted volume 2 ml/kg) dose was used in all CPP training sessions. The training procedure was repeated over the next two days. The cocaine injections were performed between 7:45 am and 11:45 am. On the 5th day, all the rats were given access to the open CPP apparatus for 20 min (of which the first 15 min were for CPP analysis) to examine place preference and USVs response. The training sessions (conditioning) and the testing were performed at the same time of day. The CPP was calculated as the difference between the times spent in the drug-paired compartment during the test and pre-test [44, 45].
50-kHz USV recording
The USVs were recorded during the CPP test session. The USVs were recorded with a single CM16 condenser microphone (Avisoft Bioacoustics, Berlin, Germany) placed face down 35 cm above the testing box floor and centrally above the rat-accessible area. The microphone was sensitive to frequencies of 15–180 kHz, had a flat response characteristic (± 6 dB) within the 25–140 kHz frequency range and was connected to a custom-made amplifier of 600 Ω input impedance, 16 v/v (12 dB) voltage gain, and ± 0.1 dB (30 Hz–100 kHz) frequency response. The amplified signal was passed to an adjacent (observer-occupied) room, processed with a custom-made antialiasing filter, and then sent to a PC equipped with a model PCI-703-16A (Eagle Technology, Eagle River, WI, USA) acquisition board (14-bit, 400 kHz) and custom-written software (Rat-Rec Pro 5.0), as previously described [45]. The sound proofing method was validated [45, 46]. The recorded data were processed using the RAT-REC PRO 5.0 software (custom-made, Warsaw, Poland) and displayed as colour spectrograms. We did not observe the 22-kHz calls. Frequency-modulated (FM) and non-FM (‘flat’) 50-kHz calls were identified using the characteristics specified in earlier studies by an independent observer. Each 50-kHz signal was manually marked by the section label to be included in the automatic calls’ counting [44–46].
Biochemical and molecular analysis
Tissue preparation for ELISA and real-time PCR
After decapitation, the trunk blood samples were taken and stored at − 20 °C for ELISA assay, while the brains were removed. The PFC (4.7 to 4.2 anterior to bregma), designated for mRNA assessment, was crudely dissected from the brain. The obtained tissue was placed in stayRNA (A@A Biotechnology, Poland), frozen and stored at − 70 °C for further analyses.
ELISA analyses
Plasma corticosterone levels
Plasma corticosterone concentrations were analyzed by Corticosterone rat/mouse ELISA kit (Demeditec Diagnostics GmbH., Germany). We used 10 μl plasma per well for the assay. The sensitivity of the corticosterone assay was 11.83 nmol/l. The corticosterone antibody cross-reactivity with other naturally occurring adrenal steroids was not detectable, except for 11-deoxycorticosterone (2.4%), progesterone (0.7%), cortisol (0.3%), and aldosterone (0.2%). The inter-and intra- assay coefficients of variance were 8.2 and 5.3%, respectively [47].
Plasma CART levels
Plasma CART concentrations were analyzed by CART Enzyme Immunoassay Kit, RayBiotech, sensitivity (minimum detectable concentration was 7.2 pg/ml, Standard curve range was 1–10.000 ng/ml). We used 10 μl of plasma per well, for the assay. All samples were twofold diluted as recommended by the kit manufacturer; the dilution factor was included in the analysis software. The inter-intra assay coefficients of variance were < 15% and 10%, respectively.
Plasma β-endorphin levels
Plasma β-endorphin concentrations were analyzed by Rat Beta-Endorphin, SunRed, sensitivity 3. 127 ng/L, Assay range was 5 ng/L–900 ng/L. We used 10 μl of plasma per well, volume-dissolved 1:5 in PBS for the assay (according to the previous examination).
Plasma POMC levels
Plasma POMC concentrations were analyzed by Rat for Proopiomelanocortin, BioSource, sensitivity 33 pg/mL, Detection (Assay) 78–5.000 pg/mL. We used 10 μl of plasma per well, volume-dissolved 1:2 in PBS for the assay, according to the prescription. The inter-and intra assay coefficients of variance were < 12 and 10%, respectively.
Real-time PCR
The total RNA was extracted using the miRNeasy Mini Kit (Qiagen, Germany), according to the manufacturer’s instructions. The concentration and purity of total RNA were determined using spectrophotometry (NanoDrop 2000/2000c, Thermo Scientific, USA). All samples had Abs 260/280 > 1.9 and 260/230 > 1.4.
Reverse transcription of mRNA was performed using the RevertAid First Strand cDNA Synthesis Kit (Thermo Scientific, USA) in a total volume of 20 μl according to the manufacturer’s instructions. Reverse transcription of miRNA was performed using TaqMan® MicroRNA Reverse Transcription Kit and TaqMan® Gene Expression Assays specific primers (Thermo Scientific, USA). Real-time PCR analysis was performed using PikoReal™ Real-Time PCR System (Thermo Fisher Scientific) with PowerSYBR® Green PCR Master Mix (Applied Biosystems). The PCR-specific primers used in this study are as follows: D2 dopamine receptor mRNA (5′ → 3′, F:CAACAATACAGACCAGAATGAG; R:CAGCAGAGTGACGATGAA), CART mRNA (5′ → 3′, F:GATCGGGAAGCTGTGTGACT; R:ATTTTGAAGCAGCAGGGAAA), KOR mRNA (5′ → 3′, F:GGCAGCAAGTGTGAAGAACA; R:GGTGCCCAGTAAGTTTTGGA), OX1R mRNA (5′ → 3′, F:GCGCGATTATCTCTATCCGAA; R:AAGGCTATGAGAAACACGGCC). Housekeeping reference genes: glyceraldehyde-3-phosphate dehydrogenase (GAPDH, 5′ → 3′, F:ATGACAATGAATATGGCTACA; R:CTCTTGCTCTCAGTATCCTT) and peptidylprolyl isomerase A (PPIA, 5′ → 3′, F:AATGGCACTGGTGGCAAGTC; R:GCCAGGACCTGTATGCTTCAG. cDNA (concentration 5 ng/μl) was amplified for each sample in a total volume of 10 μl.
The amplification reaction included 40 cycles with a 95 °C denaturation step for 5 s and a 61 °C annealing step for 45 s and was preceded by 95 °C initial denaturation for 1 min. A dissociation stage was performed to assess the specificity of primers. Each sample was run in a triplicate. Real-time PCR assays of total RNA were performed to measure the expression levels of miRNA-124 and miRNA-137. Relative levels were normalized to U6 snRNA (Control Sequence: GTGCTCGCTTCGGCAGCACATATACTA AAATTGGAAC GATACAGAGAAGATTAGCATGGCCCCTGCGCAAGGAT GACACGCA ATTCGTGAAGCGTTCCATATTTT) and 4.5S RNA(H) (Control Sequence: GCCGGTTGTGGTGGCGCACACCGGTAGGATTTGC TGAAGGAGGCAGAG GCAGGAGGATCACGAGTTCGAGGCCAGCCTGG GCTACACATTT).
Analysis of miRNAs was also performed using PikoReal™ Real-Time PCR System (Thermo Fisher Scientific, USA) with TaqMan® Universal Master Mix II, no UNG(Applied Biosystems), specific TaqMan® Probes (TaqMan™ miRNA Assays, Termofisher Scientific, in Table 1) and products of reverse transcription. Each sample was run in triplicate. Analysis of all real-time PCR data was performed using the comparative ΔΔCT method [48].
Table 1.
TaqManTM MicroRNA Assays: mmu-miR-124a, hsa-miR-137
| Assay name | miRBase ID | miRBase accession numbers | Mature miRNA sequence |
|---|---|---|---|
| mmu-miR-124a | mmu-miR-124-3p | MIMAT0000134 | UAAGGCACGCGGUGAAUGCC |
| hsa-miR-137 | hsa-miR-137-3p | MIMAT0000429 | UUAUUGCUUAAGAAUACGCGUAG |
Statistical analysis
The statistical analyses were performed using Statistica v.12 software. We used the Shapiro–Wilk test to assess the data distribution. Some variables presented a normal distribution, namely the time spent in the open arms in the EPM test, the total distance in the CPP test, β-endorphin levels, miRNA-124, and miRNA-137 levels in the PFC. We performed statistical analysis using the Student’s t test for variables with normal distribution. The data sets that did not present the normal distribution were analyzed with the Mann–Whitney U test. The data are presented as the mean + the standard error of the mean (SEM).
Results
Behavioral analysis: EPM test and CPP test (Fig. 2)
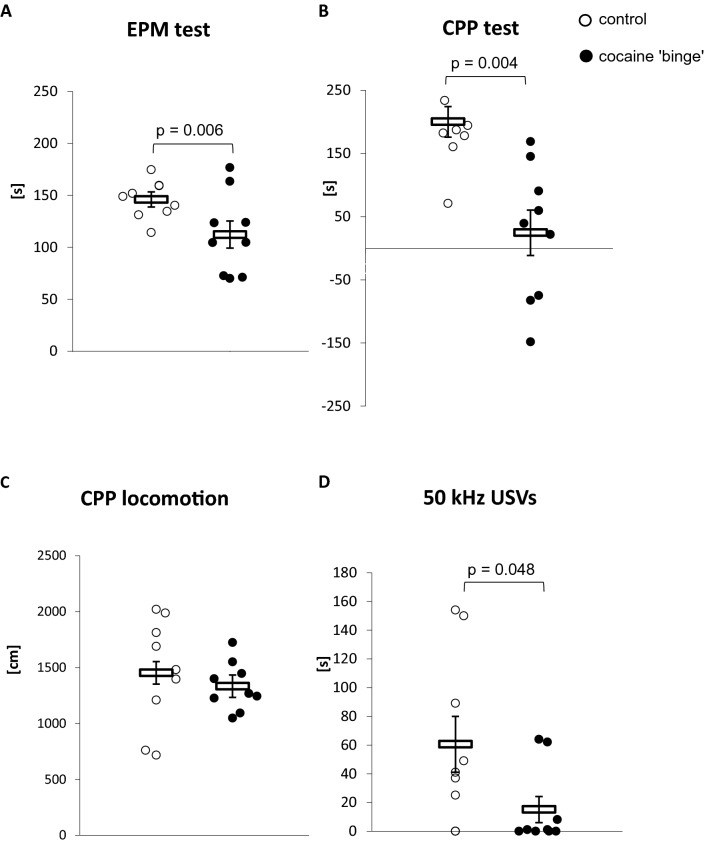
Fig. 2.
A Time spent in open arms in the EPM test after cocaine ‘binge’; B Shift of time spent in the cocaine-associated compartment during the CPP test; C Locomotor activity in the CPP test; D 50-kHz USVs in the cocaine-associated context during the CPP test. CPP conditioned place preference test, EPM elevated plus maze test. The time spent in the open arms in the EPM test and the total distance in the CPP test were analyzed with the Student t test. The shift of time spent in cocaine-associated compartment during the CPP test and the number of 50-kHz USVs in the cocaine-associated context during the CPP test were analyzed by the Mann–Whitney U test. Control-group receiving saline (n = 7–9), cocaine ‘binge’–cocaine ‘binge’ group (n = 9). The data are shown as the means + SEM
The CB rats spent significantly less time in the open arms in the EPM test compared to the control group (t16 = 3.12, p = 0.006), whereas no differences in the locomotor activity measured as the total distance crossed during the test were observed between groups (U = 37, N1 = 9, N2 = 9, p = 0.791).
Mann–Whitney U test revealed that the CB rats spent significantly less time in the cocaine-paired compartment (U = 4.0, N1 = 7, N2 = 9, p = 0.004) and demonstrated less episodes of 50-kHz USV compared to the control rats (U = 15.0, N1 = 8, N2 = 9, p = 0.048). No significant differences between the experimental groups were found in the total distance crossed in the CPP test (t14 = 0.86, p = 0.401).
Corticosterone, POMC, CART 55–102, and β-endorphin levels in the plasma (Fig. 3)
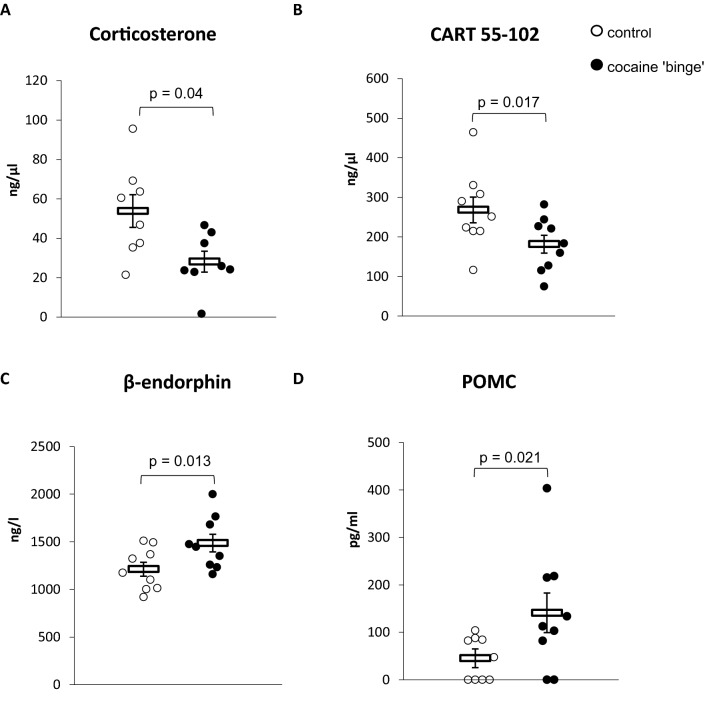
Fig. 3.
A Corticosterone; B CART 55–102; C β-endorphin; D POMC levels in the plasma. CART—cocaine amphetamine regulated transcript 55–102; POMC—proopiomelanocortin peptide. The β-endorphin levels were analyzed with the Student t test. The corticosterone, CART 55–102, and POMC levels were analyzed by the Mann–Whitney U test. Control-group receiving saline (n = 8–9), cocaine ‘binge’–cocaine ‘binge’ group (n = 8–9). The data are shown as the means + SEM
The CB rats showed significantly lower plasma corticosterone concentrations (U = 12, N1 = 8, N2 = 8, p = 0.04) and CART 55–102 levels (U = 13.0, N1 = 9, N2 = 9, p = 0.017), while plasma concentrations of β-endorphin (t16 = -2.8, p = 0.013) and POMC (U = 14, N1 = 9, N2 = 9, p = 0.021) were significantly higher when compared to the control group.
mRNA levels for D2R, OX1R, KOR, and CART 55–102 in the PFC (Fig. 4)
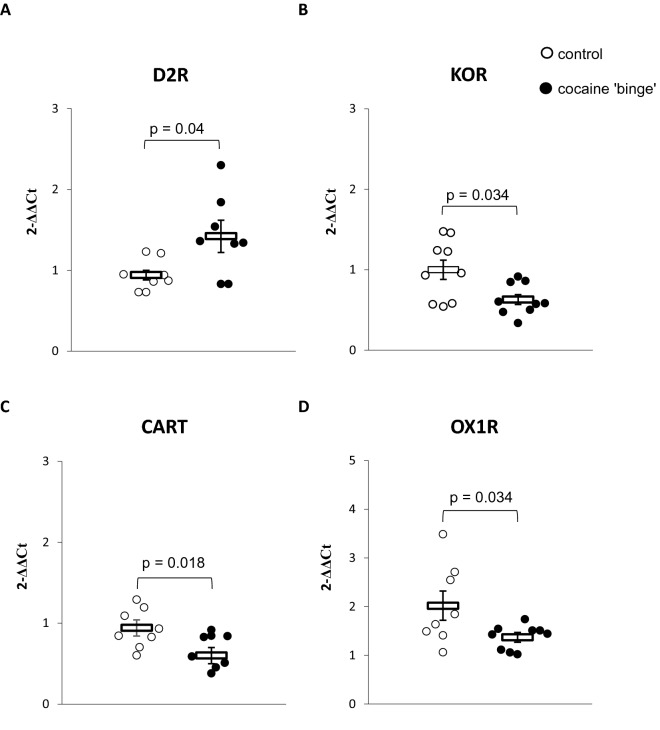
Fig. 4.
mRNA levels in the prefrontal cortex (PFC) for A Dopamine type 2 receptor (D2R); B Kappa opioid receptor (KOR); C Cocaine and amphetamine-regulated transcript 55–102 (CART 55–102); D Orexin type 1 receptor (OX1R). The presented data were analyzed by the Mann–Whitney U test. Control-group receiving saline (n = 8–9), cocaine ‘binge’–cocaine ‘binge’ group (n = 8–9). The data are shown as the means + SEM
Mann–Whitney U test revealed that the CB rats presented higher D2R mRNA (U = 12.0, N1 = 8, N2 = 8, p = 0.04), lower OX1R mRNA (U = 11, N1 = 8, N2 = 9, p = 0.018), lower CART 55–102 mRNA (U = 13.5, N1 = 8, N2 = 9, p = 0.034), and lower KOR mRNA levels (U = 16, N1 = 9, N2 = 9, p = 0.034), compared to the control rats.
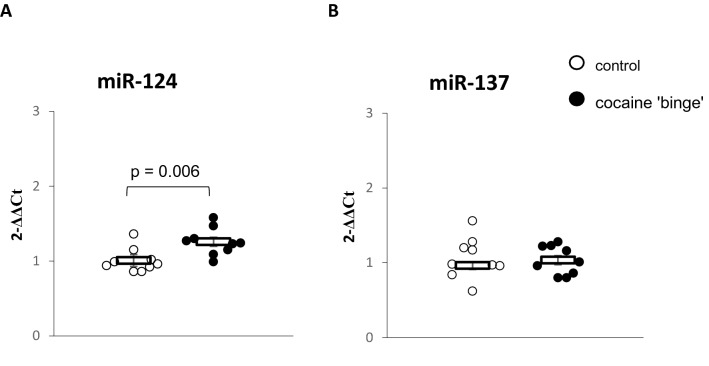
miRNA-124 and miRNA-137 levels in the PFC (Fig. 5)
Fig. 5.
A miRNA-124 and B miRNA-137 levels in the prefrontal cortex (PFC). The presented data were analyzed with Student t-test. Control-group receiving saline (n = 9), cocaine ‘binge’–cocaine ‘binge’ group (n = 9). The data are shown as the means + SEM
The CB rats presented higher miRNA-124 levels compared to the control rats (t16 = 3.12, p = 0.006). No differences were observed between the groups in the miRNA-137 levels (t16 = 0.26, p = 0.8).
Discussion
In our study, we demonstrated that the CB rats exposed to an escalating-dose cocaine regimen showed enhanced anxiety at the beginning of the withdrawal period defined as a decrease in the number of visits in the open arms in the EPM test, and diminished cocaine-associated appetitive response after the withdrawal period measured as less time spent in the cocaine-paired compartment and a lower number of appetitive USV episodes. The changes in appetitive behavior were accompanied by molecular alterations in plasma and the PFC. Specifically, we observed a decrease in corticosterone and CART 55–102 levels, and an increase in β-endorphin and POMC levels in the plasma of the CB rats, along with an increase in the mRNA for D2R, but a decrease in the mRNA levels for OX1R, KOR, and CART55-102 in the PFC. We also found an increase in the miRNA-124 level in the PFC of the CB rats.
At the beginning of the withdrawal period that followed cocaine binge, we observed enhanced anxiety-like behavior in the CB rats, similarly to other studies [49, 50]. Also in human cocaine abusers, discontinuation of drug intake usually produces a variety of adverse withdrawal symptoms among which anxiety and depression-related behaviors are prevailing during the initial period of abstinence [36]. Moreover, the CB rats showed a weaker place preference following cocaine’s 7 day withdrawal period, most possibly because of an increase in reward threshold [51]. In other studies, the rewarding effect of cocaine was reported to have been restored 14 days after cocaine withdrawal [38]. The sensitization process is observed in the early period of cocaine use, while tolerance appears over time of administration, phenomena established in both rodents and humans [52, 53]. Although it is a passive paradigm of cocaine administration compared to self-administration, the escalating dose regimen seems to at least partly mimic the cocaine binge, namely, the increase in cocaine intake. The studies of Calipari et al. [54, 55] regarding differences in dopamine signaling in different patterns of cocaine self-administration, suggest that tolerance depends not on the pattern of administration, but on the total cocaine intake within sessions. Similar patterns of behavior were demonstrated in our previous study regarding amphetamine self-administration [56].
In our study, we observed lower corticosterone concentration accompanied by an increase in POMC and β-endorphin levels in the plasma of the CB rats. The escalating-dose cocaine administration has been proved to lead to dysregulation of the HPA axis [57–59]. An increase in plasma corticosterone level was observed at the beginning of the chronic binge cocaine administration, while it was reduced on the 14th day of the applied regimen followed by a return to its basal level after 10 days of the withdrawal period [57]. In the study by García-Fuster et al. [59] corticosterone levels progressively decreased during the course of withdrawal from extended daily access of cocaine self-administration, and normalized following 28 days of withdrawal. An enhanced secretion of corticotropin-releasing factor (CRF) and adrenocorticotropic hormone (ACTH), and decreased cortisol levels were observed during the withdrawal period in humans and linked to depression and anxiety symptoms [60, 61]. Here, we can assume that lower corticosterone level may be the result of dysregulation of the HPA axis activity in response to cocaine binge and be related to the diminished cocaine-associated appetitive response after the withdrawal period.
POMC is synthesized in the pituitary gland in the brain and in several peripheral tissues. During posttranslational processing, it can be tissue-specifically cleaved to hormones and neuropeptides with very different biological activities [62]. In the anterior pituitary, POMC is processed predominantly to ACTH, β-lipotropin (β-LPH), and to β-endorphin to a lesser extent [62]. In the study by Zhou and Kreek [63], the increased hypothalamic POMC expression was persistent during the withdrawal period along with an increase in β-endorphin biosynthesis and release, which authors associated with enhanced cocaine seeking. However, continuously increased levels of β-endorphin in plasma were reported not only in abstinent human cocaine addicts, but also during cocaine binges [64]. Except for the postulated role of β-endorphin in mediating the rewarding or reinforcing effects of cocaine, it is also known to be released in response to physical stress [65, 66]. In normal human subjects a decline in ACTH and cortisol plasma levels was associated with elevated β-endorphin, which suggests feedback loop inhibition of pituitary ACTH release or suppression of hypothalamic CRF release by β-endorphin [67]. This observation may also partially explain our results, namely lower corticosterone concentrations along with an increase in β-endorphin and POMC levels in the plasma of the CB rats. However, in the current experimental design, it is difficult to assess to what extent effects of these two processes, namely cocaine withdrawal and cocaine re-exposure, may somehow overlap.
CART 55–102 is known for its properties to modulate the activity of the mesolimbic dopaminergic pathway and to affect reward-seeking behavior [68, 69]. Furthermore, compelling evidence has shown that CART 55–102 is involved in the HPA axis regulation associated with stress response [70–75]. CART 55–102 stimulates CRF and glucocorticoid secretion, whereas CRF and glucocorticoids increase the transcriptional activity of the CART gene [76–78]. The administration of CART 55–102 upregulates ACTH and corticosterone levels through a CRF-dependent mechanism [73, 74]. In light of these data, decreased CART 55–102 levels in plasma and corresponding decreased CART 55–102 mRNA levels in the PFC may be linked to the HPA axis dysregulation and diminished cocaine-associated appetitive response. In the study of Rakovska et al. [69], CART 55–102 administration was associated with a decrease in dopamine in the mouse nucleus accumbens (NAc) and attenuation of cocaine-induced effects on dopamine release.
Drug-withdrawal after chronic cocaine administration decreases dopamine signaling, in contrast to the positive reinforcement induced by the substance [51]. Diminished dopaminergic signaling after repeated cocaine intake may increase the risk of anxiety and dysphoria and lead to depressive-like behavior [79–81]. In our experiment, we detected a rise in the D2R mRNA in the PFC of the CB rats, similar to the study by Frankowska et al. [82], which presents an increased D2R expression in the PFC in the withdrawal period after cocaine self-administration. These phenomena may reflect adaptive changes in D2R expression in the cortex (probably following lower dopamine levels in the cortical-limbic system after exposure to the binge cocaine paradigm and withdrawal), and can be associated with lower appetitive response to cocaine [31].
The modulatory control of the dynorphin/KOR system over dopamine signaling in mesolimbic areas contributes to the development of negative affective states and changes in the perception of reinforcing and aversive stimuli [83]. Chronic cocaine exposure elevates the neuropeptide dynorphin levels, an endogenous ligand at KOR that suppresses dopamine release in the NAc and elicits negative affective states upon drug withdrawal [84–87]. Infusion of a KOR agonist into the PFC decreased dopamine levels in wild-type mice but not in KOR-knock-out mice (a model of a specific deletion of KOR in dopaminergic neurons), confirming KOR-mediated control of dopaminergic transmission in the PFC [88].
In our study, we observed a decrease in the KOR mRNA in the PFC of the CB rats. Although we are aware of some limitations of this study, namely changes in the level of transcripts were not confirmed at the protein level, we suggest that the reduced KOR mRNA may be associated with the lower appetitive response to cocaine-associated context. This speculation is supported by the study by Wee et al. [89] on the effects of KOR blockade on cocaine seeking and consumption in different animal models of cocaine exposure. The KOR blockade selectively reduced cocaine seeking but not cocaine consumption in animals with a history of extended cocaine administration, but not in animals that self-administered it on a short access procedure [89]. These data link the decreased KOR function with lower appetitive response upon withdrawal from chronic cocaine exposure.
In the current study, we also observed a decrease in the OX1R mRNA in the PFC of the CB rats. Orexin neurons, localized mainly in the lateral hypothalamus and projecting through the cortex and limbic system, initiate arousal states and modulate reward system activity [90]. Much evidence supports the important role of OX1R in the prelimbic cortex in addiction-related states [10, 14, 22, 24, 91–93], specifically, the significance of signaling at OX1R in relapse to cocaine-seeking [94]. The OX1R gene was induced in the PFC by cocaine exposure [95]. Systemic administration of OX1R antagonist attenuated cue-induced reinstatement of extinguished cocaine-seeking [96], but did not attenuate reinstatement of responding induced by a priming injection of cocaine [97]. In animals that underwent abstinence from chronic self-administration, the OX1R antagonism reduced the reinstatement of cocaine-seeking [98]. Based on those data, we can assume that lower OX1R mRNA levels in the PFC may be related to lower appetitive response to cocaine-associated context and decreased cortical activity following the withdrawal period.
Repeated intake of drugs of abuse, such as cocaine, promotes alterations in gene expression that underlie addiction-related processes [99–101]. Several studies have shown that cocaine can influence the activity of small non-coding RNAs, miRNAs, known to regulate gene expression on posttranscriptional level [102–104]. Among them, miRNA-124 is considered as a promising biomarker of cocaine abuse as it was down-regulated in a dopaminergic neuron-like model after acute cocaine exposure [105], and found elevated in the blood of cocaine-addicted women during the withdrawal period [106]. In our study, the CB rats that presented lower appetitive vocalization in the cocaine-associated context after the withdrawal period showed also an increased miRNA-124 level in the PFC. Chandrasekar et al. [107] found that lentiviral vector (LV)-miRNA-124 expression in the NAc attenuated cocaine CPP, whereas silencing miRNAs by corresponding LV-miRNA silencers inversed this effect. We are aware of the pleiotropic effects of miRNAs as they can target many different molecular pathways in cell- and tissue-specific manner, thus further research is needed to confirm the targets of these miRNAs in this context. So far, other studies identified PARP-1 [108] and BDNF [109] as plausible direct targets of miRNA-124 in neuronal cells.
Another promising marker of diseases associated with dopaminergic dysfunction is miRNA-137, which regulates DAT expression at the post-transcriptional level [40]. Addiction-prone rats showed elevated miRNA-137 expression in the CNS after extinction and relapse testing following cocaine self-administration [41]. However, we observed no differences in miRNA-137 between the CB and control rats in our study.
Conclusions
Our study shows that the escalating-dose cocaine regimen resulted in anxiety-like behavior at the beginning of the withdrawal period and reduced cocaine-associated appetitive response afterwards. This behavioral pattern was accompanied by dysregulation of the HPA axis activity followed by changes in related neuromodulators in the plasma, and alterations in mRNA levels for D2, KOR, OX1R, CART 55–120, and miRNA-124, a postulated marker of cocaine abuse, in the PFC. Our observations are in line to a large extent with results of other studies, thereby confirming the impact of cocaine on the HPA axis activity and systems that mediate reinforcing effects of the drug and related affective states. To conclude, the obtained data reflect a part of a bigger picture of a multilevel interplay between neurotransmitter systems and post-transcriptional regulation of gene expression underlying processes associated with cocaine abuse. However, in-depth characteristics of molecular processes in this model require more detailed “cause-and-effect” exploration.
Limitations
The major limitation of this study is that the levels of transcripts in the PFC were not confirmed at the protein level, however, the obtained data are generally consistent with those presented by other authors. Moreover, target-specific studies regarding plausible miRNA-regulated molecular pathways associated with cocaine biological effects should be undertaken.
Supplementary Information
Below is the link to the electronic supplementary material.
Abbreviations
- ACTH
Adrenocorticotropic hormone
- BDNF
Brain-derived neurotrophic factor
- CART 55–102
Cocaine and amphetamine-regulated transcript 55–102
- CB rats
Cocaine ‘binged’ rats
- CNS
Central nervous system
- CPP test
Conditioned place preference test
- CRF
Corticotropin-releasing factor
- D2R
D2 dopaminergic receptor
- DAT
Dopamine active transporter
- ELISA
Enzyme-linked immunosorbent assay
- EPM test
Elevated plus maze test
- HPA axis
Hypothalamic–pituitary–adrenal axis
- KOR
κ-Opioid receptor
- LV
Lentiviral vector
- mPFC
Medial prefrontal cortex
- NAc
Nucleus accumbens
- OX1R
Orexin 1 receptor
- PARP-1
Poly [ADP-ribose] polymerase 1
- PFC
Prefrontal cortex
- POMC
Proopiomelanocortin
- USV
Ultrasonic vocalization
- VTA
Ventral tegmental area
- miRNA
MicroRNA
Author contributions
Conception and design: ML, K, AWS, AS; supervision—ML, PM, AP; data acquisition—KK, AWS; behavioral analysis—ML, KK, AWS; neurobiological analysis—AG, ASo, DT, MLL, FT, MBM, ML, KK; statistical analysis and interpretation—ML, KK, AWS; contributed to the writing of the manuscript—ML, AWS, KK. All authors have approved the final version of the manuscript.
Funding
The study was supported by Grant No. 501-40003-18017 from the Institute of Psychiatry and Neurology in Warsaw, and Grant No. 2018/28/C/NZ7/00240 from the National Science Centre in Poland. The project was implemented with CePT infrastructure financed by the European Union—The European Regional Development Fund within the operational program “Innovative economy” for 2007–2013.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Małgorzata Lehner: Deceased
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Antelman SM, Eichler AJ, Black CA, Kocan D. Interchangeability of stress and amphetamine in sensitization. Science. 1980;207:329–331. doi: 10.1126/science.7188649. [DOI] [PubMed] [Google Scholar]
- 2.Haile CN, GrandPre T, Kosten TA. Chronic unpredictable stress, but not chronic predictable stress, enhances the sensitivity to the behavioral effects of cocaine in rats. Psychopharmacology. 2001;154:213–220. doi: 10.1007/s002130000650. [DOI] [PubMed] [Google Scholar]
- 3.Haile CN, Hiroi N, Nestler EJ, Kosten TA. Differential behavioral responses to cocaine are associated with dynamics of mesolimbic dopamine proteins in Lewis and Fischer 344 rats. Synapse. 2001;41:179–190. doi: 10.1002/syn.1073. [DOI] [PubMed] [Google Scholar]
- 4.Keralapurath MM, Briggs SB, Wagner JJ. Cocaine self-administration induces changes in synaptic transmission and plasticity in ventral hippocampus. Addict Biol. 2017;22:446–456. doi: 10.1111/adb.12345. [DOI] [PubMed] [Google Scholar]
- 5.Maier EY, Abdalla M, Ahrens AM, Schallert T, Duvauchelle CL. The missing variable: ultrasonic vocalizations reveal hidden sensitization and tolerance-like effects during long-term cocaine administration. Psychopharmacology. 2012;219:1141–1152. doi: 10.1007/s00213-011-2445-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Naranjo CA, Tremblay LK, Busto UE. The role of the brain reward system in depression. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:781–823. doi: 10.1016/s0278-5846(01)00156-7. [DOI] [PubMed] [Google Scholar]
- 7.Shirayama Y, Chaki S. Neurochemistry of the nucleus accumbens and its relevance to depression and antidepressant action in rodents. Curr Neuropharmacol. 2006;4:277–291. doi: 10.2174/157015906778520773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barnea R, Bekker L, Zifman N, Marco A, Yadid G, Weller A. Trait and state binge eating predispose towards cocaine craving. Addict Biol. 2017;22:163–171. doi: 10.1111/adb.12315. [DOI] [PubMed] [Google Scholar]
- 9.Di Chiara GA. Motivational learning hypothesis of the role of mesolimbic dopamine in compulsive drug use. J Psychopharmacol. 1998;12:54–67. doi: 10.1177/026988119801200108. [DOI] [PubMed] [Google Scholar]
- 10.Cole S, Keefer SE, Anderson LC, Petrovich GD. Medial prefrontal cortex neural plasticity, orexin receptor 1 signaling, and connectivity with the lateral hypothalamus are necessary in cue-potentiated feeding. J Neurosci. 2020;40:1744–1755. doi: 10.1523/JNEUROSCI.1803-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalivas PW, McFarland K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology. 2003;168:44–56. doi: 10.1007/s00213-003-1393-2. [DOI] [PubMed] [Google Scholar]
- 12.Koob G, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry. 2007;164:1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kreek MJ. Cocaine, dopamine and the endogenous opioid system. J Addict Dis. 1996;15:73–96. doi: 10.1300/J069v15n04_05. [DOI] [PubMed] [Google Scholar]
- 14.Matzeu A, Kallupi M, George O, Schweitzer P, Martin-Fardon R. Dynorphin counteracts orexin in the paraventricular nucleus of the thalamus: cellular and behavioral evidence. Neuropsychopharmacology. 2018;43:1010–1020. doi: 10.1038/npp.2017.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2001;21:8655–8663. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang B, You ZB, Wise RA. Reinstatement of cocaine seeking by hypocretin (orexin) in the ventral tegmental area: independence from the local corticotropin-releasing factor network. Biol Psychiatry. 2009;65:857–862. doi: 10.1016/j.biopsych.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang JY, Janak PH, Woodward DJ. Comparison of mesocorticolimbic neuronal responses during cocaine and heroin self-administration in freely moving rats. J Neurosci. 1998;18:3098–3115. doi: 10.1523/JNEUROSCI.18-08-03098.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang JY, Sawyer SF, Paris JM, Kirillov A, Woodward DJ. Single neuronal responses in medial prefrontal cortex during cocaine self-administration in freely moving rats. Synapse. 1997;26:22–35. doi: 10.1002/(SICI)1098-2396(199705)26:1<22::AID-SYN3>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 19.Ligabue KP, Schuch JB, Scherer JN, Ornell F, Roglio VS, Assunção V, et al. Increased cortisol levels are associated with low treatment retention in crack cocaine users. Addict Behav. 2020;103:106260. doi: 10.1016/j.addbeh.2019.106260. [DOI] [PubMed] [Google Scholar]
- 20.Mantsch JR, Taves S, Khan T, Katz ES, Sajan T, Tang LC, et al. Restraint-induced corticosterone secretion and hypothalamic CRH mRNA expression are augmented during acute withdrawal from chronic cocaine administration. Neurosci Lett. 2007;415:269–273. doi: 10.1016/j.neulet.2007.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balkan B, Keser A, Gozen O, Koylu EO, Dagci T, Kuhar MJ, et al. Forced swim stress elicits region-specific changes in CART expression in the stress axis and stress regulatory brain areas. Brain Res. 2012;1432:56–65. doi: 10.1016/j.brainres.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 22.James MH, Stopper CM, Zimmer BA, Koll NE, Bowrey HE, Aston-Jones G. Increased number and activity of a lateral subpopulation of hypothalamic orexin.∕hypocretin neurons underlies the expression of an addicted state in rats. Biol Psychiatry. 2019;85:925–935. doi: 10.1016/j.biopsych.2018.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meng Q, Kim HC, Oh S, Lee YM, Hu Z, Oh KW. Cocaine- and amphetamine-regulated transcript (CART) peptide plays critical role in psychostimulant-induced depression. Biomol Ther (Seoul) 2018;26:425–431. doi: 10.4062/biomolther.2018.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muschamp JW, Hollander JA, Thompson JL, Voren G, Hassinger LC, Onvani S, et al. Hypocretin (orexin) facilitates reward by attenuating the antireward effects of its cotransmitter dynorphin in ventral tegmental area. Proc Natl Acad Sci USA. 2014;111:E1648–E1655. doi: 10.1073/pnas.1315542111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baimel C, Bartlett SE, Chiou LC, Lawrence AJ, Muschamp JW, Patkar O, et al. Orexin/hypocretin role in reward: implications for opioid and other addictions. Br J Pharmacol. 2015;172:334–348. doi: 10.1111/bph.12639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- 27.Matzeu A, Martin-Fardon R. Blockade of orexin receptors in the posterior paraventricular nucleus of the thalamus prevents stress-induced reinstatement of reward-seeking behavior in rats with a history of ethanol dependence. Front Integr Neurosci. 2020;14:599710. doi: 10.3389/fnint.2020.599710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 29.Branch AD, Unterwald EM, Lee SE, Kreek MJ. Quantiation of pre-pronkephalin mRNA levels in brain region from male Fischer following chronic cocaine treatment using a recently developed solution hybridization assay. Brain Res Mol Brain Res. 1992;14:231–238. doi: 10.1016/0169-328x(92)90178-e. [DOI] [PubMed] [Google Scholar]
- 30.Daunais JB, McGinty F. The effects of D1or D2dopamine receptors blockade on zif/268 and preprodynorphin gene expression in rat forebrain following a short-term cocaine binge. Brain Res Mol Brain Res. 1996;35:237–248. doi: 10.1016/0169-328x(95)00226-i. [DOI] [PubMed] [Google Scholar]
- 31.Maisonneuve IM, Ho A, Kreek MJ. Chronic administration of a cocaine ‘binge’ alters basal extracellular levels in male rats: an in vivo microdialysis study. J Pharmacol Exp Ther. 1995;272:652–657. [PubMed] [Google Scholar]
- 32.Maisonneuve IM, Kreek MJ. Acute tolerance to the dopamine response induced by a binge pattern of cocaine administration in male rats an in vivo microdialysis study. J Pharmacol Exp Ther. 1994;268:916–921. [PubMed] [Google Scholar]
- 33.Schlussman SD, Ho A, Zhou Y, Curtis AE, Kreek MJ. Effects of “binge” pattern cocaine on stereotypy and locomotor activity in C57BL/6J and 129/J mice. Pharmacol Biochem Behav. 1998;60:593–599. doi: 10.1016/s0091-3057(98)00047-1. [DOI] [PubMed] [Google Scholar]
- 34.Unterwald EM, Ho A, Rubenfeld JM, Kreek MJ. Time course of the development of behavioral sensitization and dopamine D2 receptor up-regulation during binge cocaine administration. J Pharmacol Exp Ther. 1994;270:1387–1396. [PubMed] [Google Scholar]
- 35.Zhang Y, Mantsch JR, Schlussman SD, Ho A, Kreek MJ. Conditioned place preference after single doses or “binge” cocaine in C57BL/6J and 129/J mice. Pharmacol Biochem Behav. 2002;73:655–662. doi: 10.1016/s0091-3057(02)00859-6. [DOI] [PubMed] [Google Scholar]
- 36.Gawin FH. Cocaine addiction: psychology and neurophysiology. Science. 1991;251:1580–1586. doi: 10.1126/science.2011738. [DOI] [PubMed] [Google Scholar]
- 37.Smith LN, Penrod RD, Taniguchi M, Cowan CHW. Assessment of cocaine-induced behavioural sensitization and conditioned place preference in mice. J Vis Exp. 2016;108:e53107. doi: 10.3791/53107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Y, Schlussman SD, Butelman ER, Ho A, Kreek MJ. Effects of withdrawal from chronic escalating-dose binge cocaine on conditioned place preference to cocaine and striatal preproenkephalin mRNA in C57BL/6J mice. Neuropharmacology. 2012;63:322–329. doi: 10.1016/j.neuropharm.2012.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simola N. Rat ultrasonic vocalizations and behavioral neuropharmacology: from the screening of drugs to the study of disease. Curr Neuropharmacol. 2015;13:164–179. doi: 10.2174/1570159X13999150318113800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jia X, Wang F, Han Y, Geng X, Li M, Shi Y, et al. miR-137 and miR-491 negatively regulate dopamine transporter expression and function in neural cells. Neurosci Bull. 2016;32:512–522. doi: 10.1007/s12264-016-0061-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Quinn RK, James MH, Hawkins GE, Brown AL, Heathcote A, Smith DW, et al. Temporally specific miRNA expression patterns in the dorsal and ventral striatum of addiction-prone rats. Addict Biol. 2018;23:631–642. doi: 10.1111/adb.12520. [DOI] [PubMed] [Google Scholar]
- 42.Yang W, Liu M, Zhang Q, Zhang J, Chen J, Chen Q, et al. Knockdown of miR-124 reduces depression-like behavior by targeting CREB1 and BDNF. Curr Neurovasc Res. 2020;17:196–203. doi: 10.2174/1567202617666200319141755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wisłowska-Stanek A, Lehner M, Skórzewska A, Krząścik P, Maciejak P, Szyndler J, et al. Changes in the brain expression of alpha-2 subunits of the GABA-A receptor after chronic restraint stress in ow-and high-anxiety rats. Behav Brain Res. 2013;253:337–345. doi: 10.1016/j.bbr.2013.07.042. [DOI] [PubMed] [Google Scholar]
- 44.Lehner M, Gryz M, Wisłowska-Stanek A, Turzyńska D, Sobolewska A, Skórzewska A, et al. The amphetamine-associated context exerts a stronger motivational effect in low-anxiety rats than in high-anxiety rats. Behav Brain Res. 2017;330:97–107. doi: 10.1016/j.bbr.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 45.Taracha E, Kaniuga E, Chrapusta SJ, Maciejak P, Sliwa L, Hamed A, et al. Diverging frequency-modulated 50-kHz vocalization, locomotor activity and conditioned place preference effects in rats given repeated amphetamine treatment. Neuropharmacology. 2014;83:128–136. doi: 10.1016/j.neuropharm.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 46.Hamed A, Szyndler J, Taracha E, Turzyńska D, Sobolewska A, Lehner M, et al. κ-opioid receptor as a key mediator in the regulation of appetitive 50-kHz ultrasonic vocalizations. Psychopharmacology. 2015;232:1941–1955. doi: 10.1007/s00213-014-3824-7. [DOI] [PubMed] [Google Scholar]
- 47.Skórzewska A, Lehner M, Wisłowska-Stanek A, Turzyńska D, Sobolewska A, Krząścik P, et al. Midazolam treatment before re-exposure to contextual fear reduces freezing behavior and amygdala activity differentially in high- and low-anxiety rats. Pharmacol Biochem Behav. 2015;129:34–44. doi: 10.1016/j.pbb.2014.11.020. [DOI] [PubMed] [Google Scholar]
- 48.Kołosowska K, Gawryluk A, Wisłowska-Stanek A, Liguz-Lęcznar M, Hetmańczyk K, Ługowska A, et al. Stress changes amphetamine response, D2 receptor expression and epigenetic regulation in low-anxiety rats. Prog Neuropsychopharmacol Biol Psychiatry. 2019;93:256–268. doi: 10.1016/j.pnpbp.2019.04.009. [DOI] [PubMed] [Google Scholar]
- 49.de Citó O, Mdo C, da Silva FC, Silva MI, Moura BA, Macêdo DS, Woods DJ, et al. Reversal of cocaine withdrawal-induced anxiety by ondansetron, buspirone and propranolol. Behav Brain Res. 2012;231:116–123. doi: 10.1016/j.bbr.2012.01.056. [DOI] [PubMed] [Google Scholar]
- 50.Philogene-Khalid HL, Hicks C, Reitz AB, Liu-Chen LY, Rawls SM. Synthetic cathinones and stereochemistry: S enantiomer of mephedrone reduces anxiety- and depressant-like effects in cocaine- or MDPV-abstinent rats. Drug Alcohol Depend. 2017;178:119–125. doi: 10.1016/j.drugalcdep.2017.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Koob GF, Ahmed SH, Boutrel B, Chen SA, Kenny PJ, Markou A, et al. Neurobiological mechanisms in the transition from drug use to drug dependence. Neurosci Biobehav Rev. 2004;27:739–749. doi: 10.1016/j.neubiorev.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 52.Mendelson JH, Sholar M, Mello NK, Teoh SK, Sholar JW. Cocaine tolerance: behavioral, cardiovascular, and neuroendocrine function in men. Neuropsychopharmacology. 1998;18:263–271. doi: 10.1016/S0893-133X(97)00146-2. [DOI] [PubMed] [Google Scholar]
- 53.Reed SC, Haney M, Evans SM, Vadhan NP, Rubin E, Foltin RW. Cardiovascular and subjective effects of repeated smoked cocaine administration in experienced cocaine users. Drug Alcohol Depend. 2009;102:102–107. doi: 10.1016/j.drugalcdep.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Calipari ES, Ferris MJ, Jones SR. Extended access of cocaine self-administration results in tolerance to the dopamine-elevating and locomotor-stimulating effects of cocaine. J Neurochem. 2014;128:224–232. doi: 10.1111/jnc.12452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Calipari ES, Ferris MJ, Zimmer BA, Roberts DC, Jones SR. Temporal pattern of cocaine intake determines tolerance vs sensitization of cocaine effects at the dopamine transporter. Neuropsychopharmacology. 2013;38:2385–2392. doi: 10.1038/npp.2013.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kuchniak K, Wyszogrodzka E, Chrapusta SJ, Czarna M, Michalak M, Płaźnik A, et al. Using anticipatory and drug-evoked appetitive ultrasonic vocalization for monitoring the rewarding effect of amphetamine in a rat model of drug self-administration. Behav Brain Res. 2019;376:112187. doi: 10.1016/j.bbr.2019.112187. [DOI] [PubMed] [Google Scholar]
- 57.Zhou Y, Schlussman SD, Ho A, Spangler R, Fienberg AA, Greengard P, Kreek MJ. Effects of chronic 'Binge' cocaine administration on plasma ACTH and corticosterone levels in mice deficient in DARPP-32. Neuroendocrinology. 1999;70(3):196–199. doi: 10.1159/000054476. [DOI] [PubMed] [Google Scholar]
- 58.Zhou Y, Spangler R, LaForge KS, Maggos CE, Ho A, Kreek MJ. Corticotropin-releasing factor and type 1 corticotropin-releasing factor receptor messenger RNAs in rat brain and pituitary during "binge"-pattern cocaine administration and chronic withdrawal. J Pharmacol Exp Ther. 1996;279:351–358. [PubMed] [Google Scholar]
- 59.García-Fuster MJ, Flagel SB, Mahmood ST, Watson SJ, Akil H. Cocaine withdrawal causes delayed dysregulation of stress genes in the hippocampus. PLoS ONE. 2012;7:e42092. doi: 10.1371/journal.pone.0042092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li SX, Yan SY, Bao YP, Lian Z, Qu Z, Wu YP, et al. Depression and alterations in hypothalamic-pituitary-adrenal and hypothalamic-pituitary-thyroid axis function in male abstinent methamphetamine abusers. Hum Psychopharmacol. 2013;28:477–483. doi: 10.1002/hup.2335. [DOI] [PubMed] [Google Scholar]
- 61.Zuloaga DG, Jacobskind JS, Raber J. Methamphetamine and the hypothalamic-pituitary-adrenal axis. Front Neurosci. 2015;9:178. doi: 10.3389/fnins.2015.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Feek CM, Marante DJ, Edwards CR. The hypothalamic-pituitary-adrenal axis. Clin Endocrinol Metab. 1983;12:597–618. doi: 10.1016/s0300-595x(83)80057-7. [DOI] [PubMed] [Google Scholar]
- 63.Zhou Y, Kreek MJ. Persistent increases in rat hypothalamic POMC gene expression following chronic withdrawal from chronic "binge" pattern escalating-dose, but not steady-dose, cocaine. Neuroscience. 2015;289:63–70. doi: 10.1016/j.neuroscience.2014.12.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vescovi PP, Coiro V, Volpi R, Passeri M. Diurnal variations in plasma ACTH, cortisol and beta-endorphin levels in cocaine addicts. Horm Res. 1992;37:221–224. doi: 10.1159/000182316. [DOI] [PubMed] [Google Scholar]
- 65.Pilozzi A, Carro C, Huang X. Roles of β-endorphin in stress, behavior, neuroinflammation, and brain energy metabolism. Int J Mol Sci. 2020;22:338. doi: 10.3390/ijms22010338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Roth-Deri I, Green-Sadan T, Yadid G. Beta-endorphin and drug-induced reward and reinforcement. Prog Neurobiology. 2008;86:1–21. doi: 10.1016/j.pneurobio.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 67.Taylor T, Dluhy RG, Williams GH. Beta-endorphin suppresses adrenocorticotropin and cortisol levels in normal human subjects. J Clin Endocrinol Metab. 1983;57:592–596. doi: 10.1210/jcem-57-3-592. [DOI] [PubMed] [Google Scholar]
- 68.Awathale SN, Choudhary AG, Subhedar NK, Kokare DM. Neuropeptide CART modulates dopamine turnover in the nucleus accumbens: Insights into the anatomy of rewarding circuits. J Neurochem. 2021;158:1172–1185. doi: 10.1111/jnc.15479. [DOI] [PubMed] [Google Scholar]
- 69.Rakovska A, Baranyi M, Windisch K, Petkova-Kirova P, Gagov H, Kalfin R. Neurochemical evidence that cocaine- and amphetamine-regulated transcript (CART) 55–102 peptide modulates the dopaminergic reward system by decreasing the dopamine release in the mouse nucleus accumbens. Brain Res Bull. 2017;134:246–252. doi: 10.1016/j.brainresbull.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 70.Job MO, McNamara IM, Kuhar MJ. CART peptides regulate psychostimulants and may be endogenous antidepressants. Curr Neuropharmacol. 2011;9:12–16. doi: 10.2174/157015911795017074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Koylu EO, Balkan B, Kuhar MJ, Pogun S. Cocaine and amphetamine regulated transcript (CART) and the stress response. Peptides. 2006;27:1956–1969. doi: 10.1016/j.peptides.2006.03.032. [DOI] [PubMed] [Google Scholar]
- 72.Peng Q, Sun X, Liu Z, Yang J, Oh KW, Hu Z. Microinjection of CART (cocaine- and amphetamine-regulated transcript) peptide into the nucleus accumbens inhibits the cocaine-induced upregulation of dopamine receptors and locomotor sensitization. Neurochem Int. 2014;75:105–111. doi: 10.1016/j.neuint.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 73.Smith SM, Vaughan JM, Donaldson CJ, Rivier J, Li C, Chen A, et al. Cocaine- and amphetamine-regulated transcript activates the hypothalamic-pituitary-adrenal axis through a corticotropin-releasing factor receptor-dependent mechanism. Endocrinology. 2004;145:5202–5209. doi: 10.1210/en.2004-0708. [DOI] [PubMed] [Google Scholar]
- 74.Stanley SA, Murphy KG, Bewick GA, Kong WM, Opacka-Juffry J, Gardiner JV, et al. Regulation of rat pituitary cocaine- and amphetamine regulated transcript (CART) by CRH and glucocorticoids. Am J Physiol Endocrinol Metab. 2004;287:E583–E590. doi: 10.1152/ajpendo.00576.2003. [DOI] [PubMed] [Google Scholar]
- 75.Vrang N, Larsen PJ, Kristensen P, Tang-Christensen M. Central administration of cocaine-amphetamine-regulated transcript activates hypothalamic neuroendocrine neurons in the rat. Endocrinology. 2000;141:794–801. doi: 10.1210/endo.141.2.7295. [DOI] [PubMed] [Google Scholar]
- 76.Elias CF, Lee CE, Kelly JF, Ahima RS, Kuhar M, Saper CB, et al. Characterization of CART neurons in the rat and human hypothalamus. J Comp Neurol. 2001;432:1–19. doi: 10.1002/cne.1085. [DOI] [PubMed] [Google Scholar]
- 77.Elias CF, Saper CB, Maratos-Flier E, Tritos NA, Lee C, Kelly J, et al. Chemically defined projections linking the mediobasal hypothalamus and the lateral hypothalamic area. J Comp Neurol. 1998;402:442–459. [PubMed] [Google Scholar]
- 78.Vicentic A. CART peptide diurnal variations in blood and brain. Peptides. 2006;27:1942–1948. doi: 10.1016/j.peptides.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 79.Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- 80.Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- 81.Koob GF, Sanna PP, Bloom FE. Neuroscience of addiction. Neuron. 1998;21:467–476. doi: 10.1016/s0896-6273(00)80557-7. [DOI] [PubMed] [Google Scholar]
- 82.Frankowska M, Miszkiel J, Pomierny-Chamioło L, Pomierny B, Giannotti G, Suder A, et al. Alternation in dopamine D(2)-like and metabotropic glutamate type 5 receptor density caused by differing housing conditions during abstinence from cocaine self-administration in rats. J Psychopharmacol. 2019;33:372–382. doi: 10.1177/0269881118821113. [DOI] [PubMed] [Google Scholar]
- 83.Estave PM, Spodnick MB, Karkhanis AN. KOR control over addiction processing: an exploration of the mesolimbic dopamine pathway. Handb Exp Pharmacol. 2022;271:351–377. doi: 10.1007/164_2020_421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chartoff EH, Ebner SR, Sparrow A, Potter D, Baker PM, Ragozzino ME, et al. Relative timing between kappa opioid receptor activation and cocaine determines the impact on reward and dopamine release. Neuropsychopharmacology. 2016;41:989–1002. doi: 10.1038/npp.2015.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Heijna MH, Padt M, Hogenboom F, Portoghese PS, Mulder AH, Schoffelmeer AN. Opioid receptor-mediated inhibition of dopamine and acetylcholine release from slices of rat nucleus accumbens, olfactory tubercle and frontal cortex. Eur J Pharmacol. 1990;181:267–278. doi: 10.1016/0014-2999(90)90088-n. [DOI] [PubMed] [Google Scholar]
- 86.Karkhanis AN, Huggins KN, Rose JH, Jones SR. Switch from excitatory to inhibitory actions of ethanol on dopamine levels after chronic exposure: role of kappa opioid receptors. Neuropharmacology. 2016;110:190–197. doi: 10.1016/j.neuropharm.2016.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Siciliano CA, Calipari ES, Yorgason JT, Lovinger DM, Mateo Y, Jimenez VA, et al. Increased presynaptic regulation of dopamine neurotransmission in the nucleus accumbens core following chronic ethanol self-administration in female macaques. Psychopharmacology. 2016;233:1435–1443. doi: 10.1007/s00213-016-4239-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tejeda HA, Counotte DS, Oh E, Ramamoorthy S, Schultz-Kuszak KN, Bäckman CM, et al. Prefrontal cortical kappa-opioid receptor modulation of local neurotransmission and conditioned place aversion. Neuropsychopharmacology. 2013;38:1770–1779. doi: 10.1038/npp.2013.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wee S, Koob GF. The role of the dynorphin-kappa opioid system in the reinforcing effects of drugs of abuse. Psychopharmacology. 2010;210:121–135. doi: 10.1007/s00213-010-1825-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schöne C, Burdakov D. Orexin/hypocretin and organizing principles for a diversity of wake-promoting neurons in the brain. Curr Top Behav Neurosci. 2017;33:51–74. doi: 10.1007/7854_2016_45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Brown RM, Lawrence AJ. Ascending orexinergic pathways and alcohol-seeking. Curr Opin Neurobiol. 2013;23:467–472. doi: 10.1016/j.conb.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 92.Hopf FW. Recent perspectives on orexin/hypocretin promotion of addiction-related behaviors. Neuropharmacology. 2020;168:108013. doi: 10.1016/j.neuropharm.2020.108013. [DOI] [PubMed] [Google Scholar]
- 93.Matzeu A, Martin-Fardon R. Drug seeking and relapse: new evidence of a role for orexin and dynorphin co-transmission in the paraventricular nucleus of the thalamus. Front Neurol. 2018;9:720. doi: 10.3389/fneur.2018.00720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Aston-Jones G, Smith RJ, Sartor GC, Moorman DE, Massi L, Tahsili-Fahadan P, et al. Lateral hypothalamic orexin/hypocretin neurons: a role in reward-seeking and addiction. Brain Res. 2010;1314:74–90. doi: 10.1016/j.brainres.2009.09.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Saad L, Sartori M, Pol Bodetto S, Romieu P, Kalsbeek A, Zwiller J, et al. Regulation of brain DNA methylation factors and of the orexinergic system by cocaine and food self-administration. Mol Neurobiol. 2019;56:5315–5331. doi: 10.1007/s12035-018-1453-6. [DOI] [PubMed] [Google Scholar]
- 96.Smith RJ, See RE, Aston-Jones G. Orexin/hypocretin signaling at the orexin 1 receptor regulates cue-elicited cocaine-seeking. Eur J Neurosci. 2009;30:493–503. doi: 10.1111/j.1460-9568.2009.06844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Smith R, See R, Aston-Jones G. The orexin-1 receptor antagonist SB-334867 blocks cue induced reinstatement of cocaine-seeking. Neuroscience Meeting Planner. Program No. 916.1. 2007 Online.
- 98.Smith RJ, Tahsili-Fahadan P, Aston-Jones G. Orexin/hypocretin is necessary for context-driven cocaine-seeking. Neuropharmacology. 2010;58:179–184. doi: 10.1016/j.neuropharm.2009.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kalivas PW. How do we determine which drug-induced neuroplastic changes are important? Nat Neurosci. 2005;8:1440–1441. doi: 10.1038/nn1105-1440. [DOI] [PubMed] [Google Scholar]
- 100.Pierce RC, Kumaresan V. The mesolimbic dopamine system: the final common pathway for the reinforcing effect of drugs of abuse? Neurosci Biobehav Rev. 2006;30:215–238. doi: 10.1016/j.neubiorev.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 101.Shaham Y, Hope BT. The role of neuroadaptations in relapse to drug seeking. Nat Neurosci. 2005;8:1437–1439. doi: 10.1038/nn1105-1437. [DOI] [PubMed] [Google Scholar]
- 102.Kenny PJ. Epigenetic, micro RNA, and addiction. Dialogues Clin Neurosci. 2014;16:335–344. doi: 10.31887/DCNS.2014.16.3/pkenny. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lim LP, Lau NC, Garrett-Engele P, Grimson A, Schelter JM, Castle J, et al. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature. 2005;433:769–773. doi: 10.1038/nature03315. [DOI] [PubMed] [Google Scholar]
- 104.Smith ACW, Kenny PJ. MicroRNAs regulate synaptic plasticity underlying drug addiction. Genes Brain Behav. 2018;17:e12424. doi: 10.1111/gbb.12424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cabana-Domínguez J, Arenas C, Cormand B, Fernàndez-Castillo N. MiR-9, miR-153 and miR-124 are down-regulated by acute exposure to cocaine in a dopaminergic cell model and may contribute to cocaine dependence. Transl Psychiatry. 2018;8:173. doi: 10.1038/s41398-018-0224-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Viola TW, Heberle BA, Zaparte A, Sanvicente-Vieira B, Wainer LM, Fries GR, et al. Peripheral blood microRNA levels in females with cocaine use disorder. J Psychiatr Res. 2019;114:48–54. doi: 10.1016/j.jpsychires.2019.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chandrasekar V, Dreyer JL. Regulation of MiR-124, Let-7d, and MiR-181a in the accumbens affects the expression, extinction, and reinstatement of cocaine-induced conditioned place preference. Neuropsychopharmacology. 2011;36:1149–1164. doi: 10.1038/npp.2010.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dash S, Balasubramaniam M, Martínez-Rivera FJ, Godino A, Peck EG, Patnaik S, et al. Cocaine-regulated microRNA miR-124 controls poly (ADP-ribose) polymerase-1 expression in neuronal cells. Sci Rep. 2020;10:11197. doi: 10.1038/s41598-020-68144-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shi LS, Ji CH, Tang WQ, Liu Y, Zhang W, Guan W. Hippocampal miR-124 participates in the pathogenesis of depression via regulating the expression of BDNF in a Chronic Social Defeat Stress model of depression. Curr Neurovasc Res. 2022;19:210–218. doi: 10.2174/1567202619666220713105306. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.