Abstract
Diuretics, such as furosemide, are routinely administered to dogs with congestive heart failure (CHF). Traditionally, dose and determination of efficacy primarily are based on clinical signs rather than quantitative measures of drug action. Treatment of human CHF patients increasingly is guided by quantification of urine sodium concentration (uNa) and urine volume after diuretic administration. Use of these and other measures of diuretic responsiveness is associated with decreased duration of hospitalization, complication rates, future rehospitalization, and mortality. At their core, loop diuretics act through natriuresis, and attention to body sodium (Na) stores and handling offers insight into the pathophysiology of CHF and pharmacology of diuretics beyond what is achievable from clinical signs alone. Human patients with low diuretic responsiveness or diuretic resistance are at risk for difficult or incomplete decongestion that requires diuretic intensification or other remedial strategies. Identification of the specific etiology of resistance in a patient can help tailor personalized interventions. In this review, we advance the concept of loop diuretic responsiveness by highlighting Na and natriuresis. Specifically, we review body water homeostasis and congestion in light of the increasingly recognized role of interstitial Na, propose definitions for diuretic responsiveness and resistance in veterinary subjects, review relevant findings of recent studies, explain how the particular cause of resistance can guide treatment, and identify current knowledge gaps. We believe that a quantitative approach to loop diuretic usage primarily involving natriuresis will advance our understanding and care of dogs with CHF.
Keywords: diuretic resistance, furosemide, natriuresis, parenchymal disease, pulmonary edema, respiratory tract
Abbreviations
- ACEI
angiotensin converting enzyme inhibitor
- CHF
congestive heart failure
- Cl
chloride
- FeNa
fractional excretion of sodium
- GAG
glycosaminoglycans
- K
potassium
- Na
sodium
- NKCC
Na‐K‐2Cl cotransporter
- pFur
plasma furosemide concentration
- RAAS
renin‐angiotensin‐aldosterone system
- uFur
urine furosemide concentration
- uK
urine potassium concentration
- uNa
urine sodium concentration
- uNa : K
urinary Na to K ratio
1. INTRODUCTION
Congestive heart failure (CHF) is characterized by increased renal sodium (Na) avidity and volume retention, which leads to the accumulation of fluid in the interstitial space or body cavities. Congestion is associated with morbidity and mortality, and relief using diuretic drugs is a mainstay of treatment in veterinary and human patients. Diuretics increase urine production, causing water and Na loss and resolution of congestion. In animals with CHF, successful diuresis resolves edema or effusion, which improves clinical signs. Loop diuretics, such as furosemide and torsemide, inhibit the Na‐potassium (K)‐2‐chloride (Cl) cotransporter (NKCC) in the ascending limb of the loop of Henle, and urinary excretion of these ions induces water loss as consequence of the resulting osmotic gradient. Thus, loop diuretics rely on an increase in urinary Na (uNa) excretion to be maximally effective. 1 Sodium is particularly important in fluid homeostasis in that, together with Cl, it is the primary osmole that controls the balance of fluid between the extracellular and intracellular space. 2 Heart failure is characterized by increased renal Na avidity secondary to neurohormonal and hemodynamic stimuli. The clinical signs associated with fluid retention are easily appreciated by veterinarians and pet owners, whereas the role of Na and uNa is clinically less obvious. In dogs and cats with CHF, monitoring and success of treatment primarily is guided by assessment of the fluid aspects of diuretics, namely increased urine volume, resolution of congestive signs, hydration status, and secondary effects on renal perfusion. 3 , 4 In comparison, relatively little attention is paid to assessing natriuresis after diuretic administration. In contrast, natriuresis is increasingly recognized as the primary determinant of both short and long‐term decongestion in human patients with CHF, and individualization of CHF treatment guided by natriuresis improves outcomes over standard practice. 5 , 6 , 7 , 8 Specifically, studies in human patients indicate that quantification of diuretic‐induced natriuresis provides unique insight into fluid balance and clinical status above and beyond the presence or absence of congestion. 9 , 10 , 11 , 12 Focusing on natriuresis opens new potential treatment and monitoring strategies. The purpose of this review is to highlight the importance of Na in fluid homeostasis and development of congestion, advance the concept of diuretic responsiveness as a quantitative measure primarily based on uNa, describe how this concept could personalize patient management, and finally, identify gaps in current knowledge that can help inform future veterinary research.
2. PHYSIOLOGY OF FLUID HOMEOSTASIS AND THE IMPORTANCE OF Na
A key premise of this review is the importance of natriuresis when using diuretic treatment for CHF. To this end, it is useful to briefly review the role of Na in fluid homeostasis and CHF. The main route of Na intake is diet, and after a meal, increased serum Na concentration stimulates vasopressin release and resorption of free water in the nephron's collecting ducts in order to maintain serum Na concentration in a tightly controlled range. The body's main route of Na excretion is through urine. In the kidney, Na is filtered into the nephron lumen. In animals with CHF, renal Na avidity is increased because of neurohormonal activation, tubular hypertrophy, and transcriptional changes, among other mechanisms. 13 , 14 , 15 , 16 , 17 , 18 As a result, the fractional excretion of Na (FeNa), that is, the percentage of filtered Na that ultimately is excreted in the urine, decreases. The reabsorbed Na increases the hypertonicity of the renal medullary space and facilitates free water reabsorption from the collecting duct, particularly in the presence of increased vasopressin. In the setting of left‐sided CHF, the increase in intravascular plasma volume and accompanying hydrostatic pressure forces water out of pulmonary capillary beds and into the surrounding extracellular space or interstitium, causing congestion within tissues and organs. Keeping these events in mind, we next look closer at the balance of Na between the intravascular and extravascular and extracellular (ie, interstitial) space and how this relationship sheds new perspective on diuretic treatment.
The majority of extracellular water within the body is interstitial fluid. Sodium chloride makes up >80% of the osmoles within the interstitial space, and along with hydrostatic pressure, the osmotic gradient established by NaCl helps control the movement of water between the vasculature and interstitium. 2 , 19 The structure of the interstitium is such that it serves as a Na reservoir for the body and can hold >3 times the amount of Na found in plasma. 19 , 20 Thus, there exists differential regulation of interstitial Na content as compared to plasma Na concentration. The importance of this feature has been described in human patients with CHF. 19 , 21 , 22 , 23 Within the interstitium, most Na is bound to an extensive network of interstitial glycosaminoglycans (GAGs) as opposed to residing free within the interstitial fluid (Figure 1). 20 Bound Na does not contribute to the tonicity of the surrounding interstitial fluid so that the interstitium acts a body Na buffer capable of storing excess Na. 23 , 24 In this way, the interstitial and intravascular Na concentrations and osmotic pressures remain relatively equal, and Na can be stored without affecting plasma Na concentration or causing accumulation of interstitial water. 20 Another important role of GAGs is to support and give structure to the interstitial tissue. Glycosaminoglycans rich with bound Na provide structural rigidity that makes the interstitium possess relatively low distensibility and compliance. 25 Thus, the network of Na‐bound GAGs helps control not only the osmotic gradient, but also the hydrostatic gradient between surrounding capillaries and the interstitial space. A stiff GAG network decreases the gradient between the intravascular and interstitial space and impairs influx of water from the capillaries into the interstitium. 19 , 26 Important to the pathophysiology of congestion, the Na buffering capacity of the interstitial GAG network has limits, and chronic increased renal Na avidity overloads the network, causing it to break down. 19 , 21 , 23 , 26 As the overloaded interstitial GAG structure fails, the compliance of the interstitium increases, encouraging accumulation of interstitial water along both osmotic and hydrostatic pressure gradients (Figure 1). 19 Thus, changes in both the intravascular and interstitial space contribute to congestion, the latter of which is predominantly affected by interstitial Na content.
FIGURE 1.
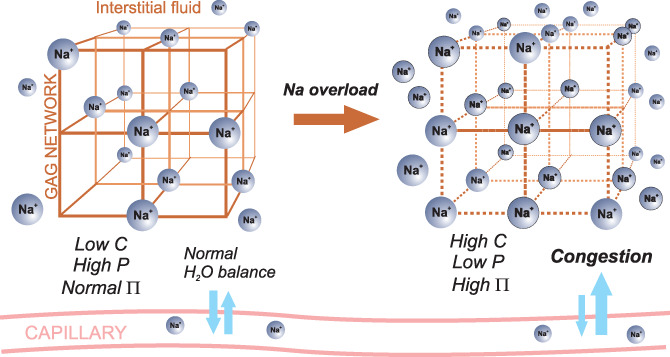
Conceptual representation of the interstitial space comprised of a glycosaminoglycan (GAG) network, which gives the tissue structure and rigidity, and surrounding interstitial fluid. In the presence of normal interstitial sodium (Na) concentration, Na is stored within the negative‐charged GAG network and interstitial Na and osmotic pressure (∏) are similar to that in the capillaries. The rigid GAG network keeps compliance (C) low. The normal osmotic pressure and high hydrostatic pressure (P) of the interstitium maintain normal water balance between the interstitium and capillaries. In situations of Na overload, the GAG network is disrupted, tissue compliance increases, and hydrostatic pressure decreases. Excess Na favors high osmotic pressure in the interstitial fluid and along with the decreased hydrostatic pressure, net water movement occurs from the capillaries into the interstitium
At this point, it is helpful to review the various different physiological compartments that hold the body's water and consider the effect of diuresis and natriuresis on each reservoir. Approximately 2/3 of the body's water is intracellular. The remaining 1/3 is extracellular in the interstitial space and vasculature at a ratio of approximately 3.5 : 1. 27 The ideal goal of diuretic treatment is decongestion, that is, to specifically remove interstitial water and prevent its reaccumulation while avoiding depletion of intravascular or intracellular water (Figure 2). 28 Increasingly, physicians recognize that successful long‐term decongestion of tissues primarily is achieved by removal of excess interstitial Na, without which any decrease in interstitial water content likely will be short‐lived. 29 , 30 This particular view is supported by drug trials of arginine vasopressin antagonists, which caused free water loss without accompanying natriuresis, but failed to improve survival or prevent reccurrence of heart failure. 31 Removal of free water without natriuresis is more likely to produce dehydration, a deficit in total body water shared across the intracellular, intravascular, and extravascular compartments (Figure 2). 32 , 33 The importance of natriuresis on body Na balance has led physicians to increasingly define the actions (and benefits) of loop diuretic drugs in the context of their natriuretic action. 9 For example, the Heart Failure Association of the European Society of Cardiology recommends uNa as one of the primary metrics of treatment efficacy in patients with acute CHF. 34 Such recommendations help establish quantitative definitions for diuretic responsiveness or resistance (Table 1).
FIGURE 2.
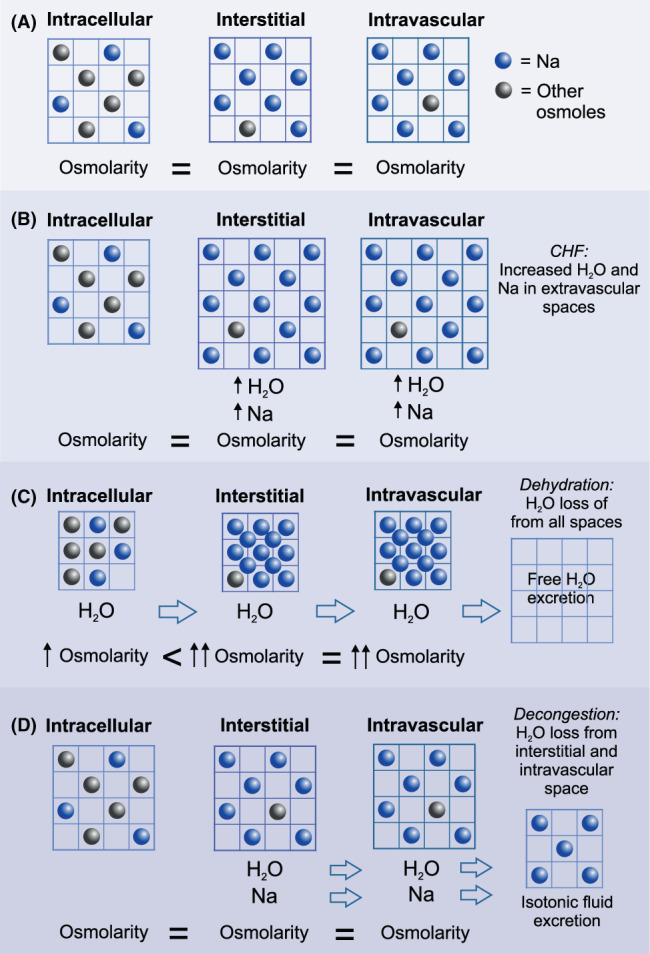
Conceptual representation of the consequences of hypotonic or free water loss (ie, diuresis) vs more balanced or isotonic fluid loss (ie, diuresis and natriuresis) in congestive heart failure (CHF). (A) Body water (grid) is distributed among the intracellular, interstitial, and intravascular spaces, all with similar osmolarity. Movement of water from intracellular to extracellular spaces is influenced by the relative osmolarity between spaces. In the extracellular spaces, Na makes up the majority of osmoles and H2O and Na freely diffuse between the interstitial and intravascular spaces. (B) During CHF, retention of H2O and Na causes expansion of blood volume and congestion in the form of excess H2O in the interstitial and intravascular spaces. (C) Free water excretion without accompanying natriuresis increases the osmolarity of the extracellular spaces, which promotes the undesirable effect of intracellular water loss through osmosis and all body spaces experience H2O loss (ie, dehydration). (D) Excretion of both H2O and Na decongests the interstitial and intravascular spaces while maintaining normal extracellular osmolarity and avoidance of intracellular water loss
TABLE 1.
Proposed definitions related to diuretic therapy
| Term | Definition |
|---|---|
| Diuretic responsiveness | Clinicopathological effects of water and urinary Na loss and subsequent relief of clinical signs secondary to congestion experienced after administration of diuretic drug(s) |
| Diuretic resistance | Quantity of water and urinary Na loss sufficiently less than what typically is expected from appropriate diuretic usage, such that morbidity and mortality are increased as compared to patients with typical responses |
| Decongestion | Removal of excess fluid and Na from interstitial or intracavitary spaces |
| Dehydration | Depletion of total body water stores across the intracellular, intravascular, and interstitial spaces |
3. DIURETIC RESPONSIVENESS AND RESISTANCE BASED ON NATRIURESIS
Diuretic drugs induce a range of effects on water, Na, and clinical signs that can be described as diuretic responsiveness or diuretic resistance. Put simply, quantitative measures of diuretic responsiveness relate the decongestive ability of a diuretic administered to a CHF patient to the amount of diuresis or natriuresis induced. 35 , 36 Diuretic responsiveness (also called diuretic efficiency) has been quantitatively defined in many different ways, including net weight loss, urine volume, fluid loss per mg of loop diuretic, and more recently uNa. 34 , 37 As previously mentioned, assessment of diuretic treatment in veterinary species is qualitative or semiqualitative and based on relief of clinical signs, such as tachypnea and dyspnea, or resolution of radiographic pulmonary edema, rather than direct measures of the pharmacologic effects of the drug. 3 Thus, in veterinary species, existing guidelines about dosage and frequency of loop diuretic treatment primarily are based on evaluation of clinical or radiographic signs rather than quantitative measures of diuresis or natriuresis. For example, furosemide dosage in dogs with acute CHF is largely based on respiratory rate and effort and supported only by expert opinion.
In contrast, in human patients with acute CHF, quantitative measures of diuretic responsiveness, such as uNa, are obtained as soon as 2 hours after IV diuretic administration, and results guide treatment during the subsequent 24‐hour time period. 34 An important goal of this initial assessment is to identify patients with low diuretic responsiveness or diuretic resistance at the outset. 34 On a qualitative basis, diuretic resistance generally is considered to be decreased sensitivity to diuretics, such that the resulting natriuresis and diuresis are insufficient to alleviate congestion or achieve euvolemia. This form of resistance is recognized only after attempts to decongest have been attempted and failed. 34 Recently, quantitative metrics of diuretic resistance that are based on uNa and urine volume, have been proposed in humans and dogs (Table 2). 34 , 38 , 39 , 43 Diuretic resistance based on objective biochemical measures can be assessed earlier in the time course of treatment and offers insight into the relevant pathophysiology. Specifically, quantification of diuretic efficacy helps determine the presence and etiology of resistance, helps stage and assess risk in animals without frank congestion, individualizes management, and provides an objective means to comparatively evaluate new diuretics. The rationale for and use of uNa as a measure of diuretic responsiveness is the subject of the next section.
TABLE 2.
Proposed quantitative definitions of diuretic resistance after loop diuretic administration in humans and dogs 34 , 38 , 39 , 40 , 41 , 42
| Physiologic variable | Criteria |
|---|---|
| uNa | <50‐70 mEq/L in spot urine sample at 2‐3 h (human; dog?) |
| <90‐100 mEq cumulative uNa excretion over first 6 h (human) | |
| uNa : uK | uNa : uK <1 (human; dog?) |
| uVol | <100‐150 mL first 6 h (human) |
| <1.5 mL/kg/h during first 7 h (dog?) | |
| FeNa | <0.2% (human; dog?) |
| Total Na output | <50‐100 mEq over first 6 h (human) |
| <1.0 mEq/kg over first 7 h (dog?) | |
| Weight loss | 4‐day weight loss <0.38‐0.67 kg/40 mg furosemide (human) |
| 5‐day weight loss <0.22 kg/40 mg furosemide (human) |
4. URINARY SODIUM AS A MEASURE OF DIURETIC RESPONSIVENESS
The use of uNa as a quantitative measure of loop diuretic efficacy is based on the fact that inhibition of Na reabsorption in the thick ascending limb of the loop of Henle is central to the drug class effect. 36 , 44 With respect to the 2 main aspects of diuretic action, there are a number of reasons why natriuresis might be a more attractive measure of responsiveness than measures of diuresis, including weight loss and urine volume. Firstly, congestion is not always associated with weight gain, nor is decongestion always associated with weight loss. 38 , 45 , 46 , 47 Volume redistribution between body compartments, such as from venous and splanchnic reservoirs into the interstitium, increasingly is recognized as a component of congestion. 38 , 48 , 49 Redistribution of existing body water helps explain the seemingly contradictory finding in human patients that suffer from acute CHF without premonitory change in body weight or plasma volume.
Urine volume is another potential measure of diuretic responsiveness based on fluid loss, but accurate measurement of urine volume in veterinary patients is challenging. In our experience, noninvasive methods of urine volume quantification, including voiding, weighing of cage bedding, and bladder ultrasound are not practical in routine practice, and true quantification requires placement of urinary catheters. Urine volume alone cannot differentiate between urine with high or low Na content, which as previously mentioned, is an important determinant of long‐term outcome. In the healthy dog, IV furosemide infusion results in urine volume and uNa that are closely correlated, 40 but in dogs with CHF, diuresis and natriuresis, although related, are not interchangeable metrics of responsiveness. 43
Previous studies in humans and dogs measured uNa at specific time points after furosemide dosing and lend support to the concept of uNa‐based measures. 8 , 43 , 50 , 51 , 52 , 53 One study in dogs with compensated CHF showed that uNa measured 3 hours after PO administration of furosemide (3 mg/kg) was significantly and highly correlated to the total amount of urinary Na excreted in response to this dose. 43 The correlation between uNa, total Na excretion, and the total urinary volume, however, was only moderate, indicating the complexity of natriuresis, diuresis, and net water balance in some animals. The same study also found that dogs exhibiting low uNa fulfilled criteria for diuretic resistance that were similar to those described for human patients (Table 2). The inability to use uNa and water loss as equivalent measures of efficacy mirrors other veterinary studies that also reported discordant urine volume and urine Na excretion in response to furosemide and torsemide. 54 , 55 In veterinary species, the use of spot uNa, as opposed to urine volume, is attractive because of its relative simplicity, although a urine sample is still required. The concept of diuretic responsiveness based on uNa has been shown to be pathophysiologically and clinically sound. 6 , 53 Despite the encouraging results of a limited number of studies in veterinary species, more study is needed to define expected responses related to dose, route of administration, timing of sampling, and differences between loop diuretics and other classes of diuretics. In addition, interpretation of uNa in acute CHF likely depends on whether or not multiple doses of diuretics previously were given because of phenomena such as diuretic braking (i.e., decreased natriuretic and diuretic response to subsequent doses). For example, in a study of human patients with acute CHF, spot uNa during the early decongestive stages of CHF treatment was predictive of outcome whereas spot uNa at the time of discharge after multiple doses of diuretic was not. 10 The findings from these and other studies indicate that urinary Na excretion is a distinct and often overlooked variable. In humans with acute CHF, guidelines recommend measurement of spot uNa 2 hours after loop diuretic administration along with determination of urine volume over the first 6 hours as the best means to determine response to treatment and prognosis. 34 We are most interested in uNa as opposed to urine volume for the practical reasons previously mentioned. An important rationale behind diuretic responsiveness is to identify patients with suspected diuretic resistance early in the course of treatment so that alterations can be considered. The concept, causes, and treatment of diuretic resistance are the subjects of the next section.
5. DIURETIC RESISTANCE
Interest in diuretic resistance is piqued by the fact that human patients with resistance suffer from worse outcomes, such as higher rehospitalization and mortality as compared to patients without resistance. 35 , 56 Diuretic resistance is pertinent to both acute and chronic heart failure. In dogs with chronic CHF, identification of diuretic resistance currently is based on the administered dosage relative to clinical signs, and is specifically defined as the need for >8 mg furosemide/kg/d (in conjunction with standard heart failure medications) to control clinical signs of congestion. 3 In the acute setting, resistance can delay CHF resolution and increase duration of hospital stay. 11 In the chronic setting, resistance can increase the risk of recurrence of signs, 12 and in both acute and chronic CHF, resistance portends poor outcome. 5 , 7 , 10
In humans and dogs, various different metrics based on Na have been proposed as indicators of diuretic resistance, including uNa <60 mmol/L 2‐3 hours after PO or IV loop diuretic, 35 , 50 , 51 , 52 , 57 urinary Na : K ratio (uNa : uK) <1, and FeNa <0.2%. 51 , 58 , 59 , 60 Other biochemical variables, including serum electrolyte and blood urea nitrogen concentrations have been proposed as measures of advanced heart failure and, by proxy, as markers of diuretic resistance. 58 , 61 For example, hyponatremia has long been considered evidence of excessive fluid retention and neurohormomal activation unchecked by diuretic administration and neurohormonal blockade. 62 , 63 In our review and others, Na has received most attention, but emerging evidence in humans and dogs suggests that serum chloride (Cl) concentration is also a marker and driver of diuretic resistance (Box 1). 58 , 64 , 65 , 66 , 67 , 68
BOX 1. Spotlight on chloride.
The importance of Cl in heart failure historically has been overlooked. Hypochloremia is a marker of poorly controlled failure as well as a cause of low diuretic responsiveness. Mechanisms of hypochloremia include loop diuretic chloride wasting and volume expansion causing dilution. 58 , 65 , 69 In dogs, hypochloremia is associated with advanced disease and correlates with other markers of diuretic resistance such as uNa : uK <1. 58 , 70 Some studies indicated that hypochloremia was a stronger predictor of poor outcomes than hyponatremia in people with CHF. 64 , 68 , 71 These data support the fact that Cl, rather than Na, modulates the glomerular filtration rate of individual nephrons through a Cl‐specific tubuloglomerular feedback system mediated by the renin‐angiotensin‐aldosterone axis and its effect primarily on afferent arteriolar tone. 66 , 72 The NKCC transporter brings tubular Cl to the macula densa cells; adequate Cl concentrations suppress renin release and low Cl concentrations stimulate renin release. 72 Loop diuretics interrupt this normal feedback mechanism so that although tubular fluid is Cl‐rich secondary to NKCC blockade in the ascending limb of the loop of Henle, the NKCC cotransporter‐mediated transport of Cl into the macula densa cells also is inhibited, thereby stimulating renin release. 64 , 72 Chloride supplementation is viewed as a potential novel therapeutic approach to heart failure, although administration of Cl without sodium is challenging. 68 The counterintuitive application of hypertonic saline administration and the lack of benefit observed with severe salt restriction might be partially explained by Cl. 73 Little is known about hypochloremia in veterinary species. One study found that hypochloremia was present in approximately 20% of dogs with CHF, including diuretic‐naïve dogs with first‐time heart failure. 70 In the same study, hypochloremia correlated with the dosage of administered diuretics suggesting value as a prognostic marker similar to people. 70 Treatment of hypochloremia potentially involves diuretics such as acetazolamide that possess Cl‐retaining properties. 74 , 75
6. CAUSES OF DIURETIC RESISTANCE
There are many different potential causes of diuretic resistance (Table 3). Resistance after chronic diuretic administration can occur secondary to increased NKCC transporter expression as well as distal tubular hypertrophy and increased activity of Na+/K+ ATPase, which occur in response to decreased Na resorption in the loop of Henle. 76 In these instances, distal tubular resorption of Na can substantially exceed the relatively small amount of reabsorption that usually occurs in that nephron segment. Repeated parenteral or PO administration of diuretics can cause resistance because of diuretic braking, which results from stimulation of the renin‐angiotensin‐aldosterone system (RAAS) and sympathetic nervous system, such that diuresis is quickly curtailed as the body attempts to mitigate intravascular volume depletion. 40 , 44 , 77 , 78 The heightened RAAS activity that causes rebound Na and water retention between dose administrations is referred to as a postdiuretic effect. 78 Rebound Na and water retention might be particularly relevant for PO administration of furosemide because of its short duration of action (ie, 3‐6 hours) relative to the typical dosing interval (i.e., q12h). 44
TABLE 3.
Causes and corrective approaches to poor diuretic responsiveness
| Cause | Supportive clinical findings | Potential objective measures in addition to low uNa, urine volume, and FeNa | Treatment approaches |
|---|---|---|---|
| Inadequate dose | Furosemide <6 mg/kg/day with continued congestion | Low serum drug concentration | Increase dose or frequency, change route |
| Poor or delayed gastrointestinal absorption |
Right‐sided CHF Administration with food Drugs with low or variable oral bioavailability Concurrent gastrointestinal disease |
Low serum drug concentration |
Change route, dose, or frequency Administer without food Change to drugs with higher oral bioavailability |
| Decreased delivery to kidney |
Right‐sided CHF (kidney congestion) Poor cardiac output |
Low urine drug concentration |
Resolve CHF Inotropic support |
| Decreased secretion into proximal convoluted tubule |
Coadministration of NSAIDs Hypoalbuminemia Renal failure Metabolic alkalosis |
Low urine drug concentration |
Discontinue competing drugs Correct hypoalbuminemia Increase dosage Correct acid‐base imbalance |
| Binding to urinary proteins | Proteinuria | Treat proteinuria (ie, ACEI) | |
| Enhanced Na absorption in distal nephron |
Decreased diuretic efficacy over time without apparent cause Hypochloremia Inadequate RAAS suppression |
Low uNa : uK |
Add thiazide diuretics Add or increase ACEI Add or increase mineralocorticoid receptor inhibition Add acetazolamide |
| Diuretic braking and reflex neurohormonal activation |
Decreased diuretic efficacy over time (especially IV doses for acute CHF) Hypochloremia Suboptimal concurrent treatment (ie, ACEI, spironolactone) |
Low uNa : uK |
Add or increase ACEI Add or increase mineralocorticoid receptor inhibition Change to diuretic with longer half‐life Constant rate infusion (acute CHF) |
| Rebound Na and water Retention | Use of diuretics with short half‐life |
Change frequency of dosing Change to diuretic with longer half‐life |
|
| Excessive water consumption |
Excessive polydipsia Weight gain after diuretic administration |
Low uNa with high urine volume |
Cautious water restriction Behavior modification Rule out concurrent disease |
| Excessive Na intake | High Na diet | Dietary Na > total uNa | Moderate Na‐restricted diet |
| Noncompliance |
Pill counts Owner history Uncooperative animal |
Decrease number or frequency of drugs Client education 2‐in‐1 pills or compounds |
|
| Nonosmotic vasopressin stimulation |
Hyponatremia Hypochloremia |
Corrected a > measured serum Cl |
Antivasopressin drugs (eg, vaptans) Improve renal perfusion (eg, inotropes) Add or increase RAAS suppression Add acetazolamide Cautious water restriction |
Abbreviations: ACEI, angiotensin converting enzyme inhibitor; CHF, congestive heart failure; Cl, chloride; FeNa, fractional excretion of sodium; Na, sodium; RAAS, renin‐angiotensin‐aldosterone system; uK, urine potassium concentration; uNa, urine sodium concentration.
Corrected Cl = (median value of Na reference range/measured Na) × measured Cl.
A diagnosis of diuretic resistance can be supported by measures such as urine potassium concentration (uK) or plasma and urine diuretic drug concentrations, which might increase suspicion of potential causes, such as aldosterone breakthrough, low PO drug bioavailability, or specific intrarenal mechanisms. For example, low uNa : uK indicates that more Na than K is being reabsorbed from the tubules, which might be caused by excessive aldosterone and up‐regulation of sodium channels in the distal nephron and collecting duct. 57 Low uNa and low plasma furosemide concentration (pFur) after PO administration suggests poor gastrointestinal bioavailability. 76 High pFur but low urine furosemide concentration (uFur) suggests impairment of active secretion of furosemide by the proximal tubular cells into the luminal space that might be the result of concurrent nonsteroidal anti‐inflammatory drug administration or hypoalbuminemia. 79 High uFur but low uNa might be a result of hypertrophy of the distal convoluted tubule and excessive Na reabsorption distal to the Loop of Henle. 76 An important implication is that definition and detection of diuretic resistance opens the potential to employ countermeasures to specifically address the most likely cause, as opposed to the current standard practice of empirically increasing the loop diuretic dose.
7. STRATEGIES TO ADDRESS DIURETIC RESISTANCE
Identification of the underlying cause(s) of diuretic resistance should help guide treatment to restore diuresis and treat or prevent congestion (Table 3). For instance, the bioavailability of PO furosemide is relatively low, inconsistent, delayed with food, and decreased in the presence of gastrointestinal congestion. 36 , 44 , 80 Specific treatments to address this situation might include parenteral administration of furosemide or use of loop diuretics such as torsemide, that have higher and more consistent bioavailability. 55
Because of a high degree of protein‐binding to albumin, furosemide is not filtered at the glomerulus, but rather is secreted from the blood into the proximal renal tubule by organic anion transporters. 44 Efficient transport is dependent on the presence of sufficient albumin and the absence of other drugs, such as nonsteroidal anti‐inflammatory drugs, that compete for these transporters. 79 Resistance associated with poor transport might be particularly amenable to correction of any hypoalbuminemia or temporary cessation of competing drugs. In contrast to the desirable protein binding in plasma, once furosemide is transported into the nephron lumen, it must remain free of protein interactions so that it can bind to the NKCC transporters on the luminal side of the tubular epithelial cells of the loop of Henle. Diseases such as protein‐losing nephropathies can impair the availability of furosemide to bind to these transporters.
The postdiuretic effect caused by rebound Na and water retention during intervals between dosing can be countered by increasing the dosing frequency or using loop diuretics with longer half‐lives, such as torsemide. 81 In the case of intrarenal causes of resistance, such as distal tubular hypertrophy, sequential nephron blockade using distal tubule‐specific drugs such as hydrochlorothiazide or spironolactone, or proximal tubule‐specific drugs, such as acetazolamide, might be particularly effective at restoring responsiveness. 74 , 82 Another potential benefit of objectively measuring diuretic responsiveness is the ability to assess utility of changes in treatment or in patient status without dependence on the presence of clinical signs. For instance, serial measurements of uNa in patients being treated for chronic CHF might detect gradual loss of diuretic efficacy that can be addressed before an episode of congestion occurs. 12 With additional knowledge linking uNa to outcomes in veterinary patients, modifications of existing treatment, such as the reduction or escalation of diuretic dose could be objectively performed. The recommendation that dogs treated for chronic heart failure be prescribed the lowest effective diuretic dose might be more easily followed if based on uNa rather than reccurrence of CHF signs.
8. FUTURE DIRECTIONS AND AREAS OF INTEREST
Currently, treating CHF in veterinary species is often an exercise in trial and error. Diuretic doses are empirically determined, and success or failure is measured by clinical signs related to congestion that often are not known until many hours or days into treatment. We hypothesize that quantitative assessment of diuretic responsiveness and recognition and treatment of specific causes of resistance in dogs and cats will lead to better outcomes as has been observed in humans. The concept of diuretic responsiveness is supported by fundamental pathophysiologic and pharmacologic principles and facilitates assessment of proposed or ongoing treatment without having to wait until congestion occurs. We hypothesize that individualized treatment plans based on diuretic responsiveness and resistance carry the highest chance of both resolution and prevention of congestion as well as decreasing the risk of adverse effects by ensuring that unnecessary or ineffective treatments are not administered to patients that might already have renal insufficiency, dehydration, or electrolyte imbalances. Diuresis in CHF patients, especially those with acute CHF, often raises concern about renal function. Dosing guided by quantification of diuretic responsiveness could proactively adjust medications and predict successful decongestion while using the lowest required dose in order to avoid dehydration, excessive RAAS activation, worsening renal function, and electrolyte imbalances. As previously discussed, with regard to the 2 main metrics of diuretic responsiveness, quantification of urine volume currently is not practical, but spot uNa is simple enough to be routinely employed, and further studies are of interest.
There are important knowledge gaps that must be filled before quantitative diuretic responsiveness can be fully appreciated. Most prominent among these is better understanding of the association between measures, primarily uNa, and short‐ and long‐term outcomes. Longitudinal studies also will help address relevant issues such as the ideal timing of measurement, the effect of individual variation and treatment history, and guiding of treatment. We propose study of uNa and other markers of diuretic responsiveness alongside practices such as monitoring clinical signs, weight loss, hydration status, renal function tests, among others, that currently guide treatment. Diuretic responsiveness must take into account Na and water as 2 separate but interrelated entities. Study of when and why these variables act in opposite directions rather than in concert is of interest. Another area of interest is if and how dogs with CHF balance osmotically active vs inactive Na. We suggest that quantification of diuretic responsiveness can improve our understanding of disease mechanisms, create new evidence‐based treatment guidelines, avoid adverse effects, and improve outcome. Despite existing knowledge gaps, study thus far in humans and dogs gives us reason to believe that advances in diuretic responsiveness and resistance are achievable and will provide a better way to prevent and treat CHF in veterinary species.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest. Article written independent of any connection to industry. We describe physiology of heart failure and standards of care in human medicine that are not affected by our relationship with veterinary industry. The companies we have a relationship with do not promote or possess any products related to the concepts of diuretic responsiveness that the article discusses.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENT
No funding was received for this study.
Oyama MA, Adin D. Toward quantification of loop diuretic responsiveness for congestive heart failure. J Vet Intern Med. 2023;37(1):12‐21. doi: 10.1111/jvim.16590
Mark A. Oyama and Darcy Adin contributed equally.
REFERENCES
- 1. Brater DC. Pharmacokinetics of loop diuretics in congestive heart failure. Br Heart J. 1994;72:S40‐S43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Roumelioti ME, Glew RH, Khitan ZJ, et al. Fluid balance concepts in medicine: principles and practice. World J Nephrol. 2018;7:1‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Keene BW, Atkins CE, Bonagura JD, et al. ACVIM consensus guidelines for the diagnosis and treatment of myxomatous mitral valve disease in dogs. J Vet Intern Med. 2019;33:1127‐1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Luis Fuentes V, Abbott J, Chetboul V, et al. ACVIM consensus statement guidelines for the classification, diagnosis, and management of cardiomyopathies in cats. J Vet Intern Med. 2020;34:1062‐1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Singh D, Shrestha K, Testani JM, et al. Insufficient natriuretic response to continuous intravenous furosemide is associated with poor long‐term outcomes in acute decompensated heart failure. J Card Fail. 2014;20:392‐399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Testani JM, Hanberg JS, Cheng S, et al. Rapid and highly accurate prediction of poor loop diuretic natriuretic response in patients with heart failure. Circ Heart Fail. 2016;9:e002370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brinkley DM Jr, Burpee LJ, Chaudhry SP, et al. Spot urine sodium as triage for effective diuretic infusion in an ambulatory heart failure unit. J Card Fail. 2018;24:349‐354. [DOI] [PubMed] [Google Scholar]
- 8. Rao VS, Ivey‐Miranda JB, Cox ZL, et al. Natriuretic equation to predict loop diuretic response in patients with heart failure. J Am Coll Cardiol. 2021;77:695‐708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Damman K, Ter Maaten JM, Coster JE, et al. Clinical importance of urinary sodium excretion in acute heart failure. Eur J Heart Fail. 2020;22:1438‐1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Biegus J, Zymlinski R, Sokolski M, et al. Serial assessment of spot urine sodium predicts effectiveness of decongestion and outcome in patients with acute heart failure. Eur J Heart Fail. 2019;21:624‐633. [DOI] [PubMed] [Google Scholar]
- 11. Cunningham JW, Sun JL, Mc Causland FR, et al. Lower urine sodium predicts longer length of stay in acute heart failure patients: insights from the ROSE AHF trial. Clin Cardiol. 2020;43:43‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Martens P, Dupont M, Verbrugge FH, et al. Urinary sodium profiling in chronic heart failure to detect development of acute decompensated heart failure. JACC Heart Fail. 2019;7:404‐414. [DOI] [PubMed] [Google Scholar]
- 13. Tidholm A, Haggstrom J, Hansson K. Effects of dilated cardiomyopathy on the renin‐angiotensin‐aldosterone system, atrial natriuretic peptide activity, and thyroid hormone concentrations in dogs. Am J Vet Res. 2001;62:961‐967. [DOI] [PubMed] [Google Scholar]
- 14. Sisson DD. Neuroendocrine evaluation of cardiac disease. Vet Clin North Am Small Anim Pract. 2004;34:1105‐1126. [DOI] [PubMed] [Google Scholar]
- 15. Ichiki T, Huntley BK, Harty GJ, Sangaralingham SJ, Burnett JC Jr. Early activation of deleterious molecular pathways in the kidney in experimental heart failure with atrial remodeling. Physiol Rep. 2017;5:e13283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pieruzzi F, Abassi ZA, Keiser HR. Expression of renin‐angiotensin system components in the heart, kidneys, and lungs of rats with experimental heart failure. Circulation. 1995;92:3105‐3112. [DOI] [PubMed] [Google Scholar]
- 17. Ro WB, Kang MH, Song DW, Lee SH, Park HM. Expression profile of circulating microRNAs in dogs with cardiac hypertrophy: a pilot study. Front Vet Sci. 2021;8:652224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stanton BA, Kaissling B. Adaptation of distal tubule and collecting duct to increased Na delivery. II. Na+ and K+ transport. Am J Physiol. 1988;255:F1269‐F1275. [DOI] [PubMed] [Google Scholar]
- 19. Nijst P, Verbrugge FH, Grieten L, et al. The pathophysiological role of interstitial sodium in heart failure. J Am Coll Cardiol. 2015;65:378‐388. [DOI] [PubMed] [Google Scholar]
- 20. Titze J, Shakibaei M, Schafflhuber M, et al. Glycosaminoglycan polymerization may enable osmotically inactive Na+ storage in the skin. Am J Physiol Heart Circ Physiol. 2004;287:H203‐H208. [DOI] [PubMed] [Google Scholar]
- 21. Nijst P, Olinevich M, Hilkens P, et al. Dermal interstitial alterations in patients with heart failure and reduced ejection fraction: a potential contributor to fluid accumulation? Circ Heart Fail. 2018;11:e004763. [DOI] [PubMed] [Google Scholar]
- 22. Rossitto G, Mary S, Chen JY, et al. Tissue sodium excess is not hypertonic and reflects extracellular volume expansion. Nat Commun. 2020;11:4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sulyok E, Farkas B, Nagy B, Várnagy Á, Kovács K, Bódis J. Tissue sodium accumulation: pathophysiology and clinical implications. Antioxidants (Basel). 2022;11:750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Heer M, Baisch F, Kropp J, Gerzer R, Drummer C. High dietary sodium chloride consumption may not induce body fluid retention in humans. Am J Physiol Renal Physiol. 2000;278:F585‐F595. [DOI] [PubMed] [Google Scholar]
- 25. Titze J, Machnik A. Sodium sensing in the interstitium and relationship to hypertension. Curr Opin Nephrol Hypertens. 2010;19:385‐392. [DOI] [PubMed] [Google Scholar]
- 26. Wiig H, Swartz MA. Interstitial fluid and lymph formation and transport: physiological regulation and roles in inflammation and cancer. Physiol Rev. 2012;92:1005‐1060. [DOI] [PubMed] [Google Scholar]
- 27. Hall JE, Hall ME. Guyton and Hall Textbook of Medical Physiology. Philadelphia, PA: Elsevier; 2021. [Google Scholar]
- 28. Costanzo MR, Jessup M. Treatment of congestion in heart failure with diuretics and extracorporeal therapies: effects on symptoms, renal function, and prognosis. Heart Fail Rev. 2012;17:313‐324. [DOI] [PubMed] [Google Scholar]
- 29. Verbrugge FH. Editor's choice‐diuretic resistance in acute heart failure. Eur Heart J Acute Cardiovasc Care. 2018;7:379‐389. [DOI] [PubMed] [Google Scholar]
- 30. Verbrugge FH, Dupont M, Steels P, et al. The kidney in congestive heart failure: ‘are natriuresis, sodium, and diuretics really the good, the bad and the ugly?’. Eur J Heart Fail. 2014;16:133‐142. [DOI] [PubMed] [Google Scholar]
- 31. Konstam MA, Gheorghiade M, Burnett JC Jr, et al. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST outcome trial. J Am Med Assoc. 2007;297:1319‐1331. [DOI] [PubMed] [Google Scholar]
- 32. Mange K, Matsuura D, Cizman B, et al. Language guiding therapy: the case of dehydration versus volume depletion. Ann Intern Med. 1997;127:848‐853. [DOI] [PubMed] [Google Scholar]
- 33. Thomas DR, Cote TR, Lawhorne L, et al. Understanding clinical dehydration and its treatment. J Am Med Dir Assoc. 2008;9:292‐301. [DOI] [PubMed] [Google Scholar]
- 34. Mullens W, Damman K, Harjola VP, et al. The use of diuretics in heart failure with congestion—a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2019;21:137‐155. [DOI] [PubMed] [Google Scholar]
- 35. Gupta R, Testani J, Collins S. Diuretic resistance in heart failure. Curr Heart Fail Rep. 2019;16:57‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Verbrugge FH, Mullens W, Tang WH. Management of cardio‐renal syndrome and diuretic resistance. Curr Treat Options Cardiovasc Med. 2016;18:11. [DOI] [PubMed] [Google Scholar]
- 37. ter Maaten JM, Valente MA, Damman K, et al. Diuretic response in acute heart failure‐pathophysiology, evaluation, and therapy. Nat Rev Cardiol. 2015;12:184‐192. [DOI] [PubMed] [Google Scholar]
- 38. Testani JM, Brisco MA, Kociol RD, et al. Substantial discrepancy between fluid and weight loss during acute decompensated heart failure treatment. Am J Med. 2015;128:776‐783.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Uechi M, Matsuoka M, Kuwajima E, et al. The effects of the loop diuretics furosemide and torasemide on diuresis in dogs and cats. J Vet Med Sci. 2003;65:1057‐1061. [DOI] [PubMed] [Google Scholar]
- 40. Adin D, Atkins C, Papich MG. Pharmacodynamic assessment of diuretic efficacy and braking in a furosemide continuous infusion model. J Vet Cardiol. 2018;20:92‐101. [DOI] [PubMed] [Google Scholar]
- 41. Feola M, Rossi A, Testa M, et al. Six‐month predictive value of diuretic resistance formulas in discharged heart failure patients after an acute decompensation. J Clin Med. 2020;9:2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Voors AA, Davison BA, Teerlink JR, et al. Diuretic response in patients with acute decompensated heart failure: characteristics and clinical outcome—an analysis from RELAX‐AHF. Eur J Heart Fail. 2014;16:1230‐1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Loughran KA, Larouche‐Lebel E, Huh T, Testani JM, Rao VS, Oyama MA. Prediction and measurement of diuretic responsiveness after oral administration of furosemide to healthy dogs and dogs with congestive heart failure. J Vet Intern Med. 2020;34:2253‐2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Huang X, Dorhout Mees E, Vos P, Hamza S, Braam B. Everything we always wanted to know about furosemide but were afraid to ask. Am J Physiol Renal Physiol. 2016;310:F958‐F971. [DOI] [PubMed] [Google Scholar]
- 45. Arrigo M, Jessup M, Mullens W, et al. Acute heart failure. Nat Rev Dis Primers. 2020;6:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cotter G, Metra M, Milo‐Cotter O, Dittrich HC, Gheorghiade M. Fluid overload in acute heart failure—re‐distribution and other mechanisms beyond fluid accumulation. Eur J Heart Fail. 2008;10:165‐169. [DOI] [PubMed] [Google Scholar]
- 47. Miller WL. Fluid volume overload and congestion in heart failure: time to reconsider pathophysiology and how volume is assessed. Circ Heart Fail. 2016;9:e002922. [DOI] [PubMed] [Google Scholar]
- 48. Chaudhry SI, Wang Y, Concato J, Gill TM, Krumholz HM. Patterns of weight change preceding hospitalization for heart failure. Circulation. 2007;116:1549‐1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fudim M, Hernandez AF, Felker GM. Role of volume redistribution in the congestion of heart failure. J Am Heart Assoc. 2017;6:e006817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Testani JM, Brisco MA, Turner JM, et al. Loop diuretic efficiency: a metric of diuretic responsiveness with prognostic importance in acute decompensated heart failure. Circ Heart Fail. 2014;7:261‐270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Doering A, Jenkins CA, Storrow AB, et al. Markers of diuretic resistance in emergency department patients with acute heart failure. Int J Emerg Med. 2017;10:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Caravaca Perez P, Nuche J, Moran Fernandez L, et al. Potential role of natriuretic response to furosemide stress test during acute heart failure. Circ Heart Fail. 2021;14:e008166. [DOI] [PubMed] [Google Scholar]
- 53. Cobo‐Marcos M, Zegri‐Reiriz I, Remior‐Perez P, et al. Usefulness of natriuresis to predict in‐hospital diuretic resistance. Am J Cardiovasc Dis. 2020;10:350‐355. [PMC free article] [PubMed] [Google Scholar]
- 54. Potter BM, Ames MK, Hess A, Poglitsch M. Comparison between the effects of torsemide and furosemide on the renin‐angiotensin‐aldosterone system of normal dogs. J Vet Cardiol. 2019;26:51‐62. [DOI] [PubMed] [Google Scholar]
- 55. Pelligand L, Guillot E, Geneteau A, et al. Population pharmacokinetics and pharmacodynamics modeling of torasemide and furosemide after oral repeated administration in healthy dogs. Front Vet Sci. 2020;7:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kristjansdottir I, Thorvaldsen T, Lund LH. Congestion and diuretic resistance in acute or worsening heart failure. Card Fail Rev. 2020;6:e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ferreira JP, Girerd N, Medeiros PB, et al. Spot urine sodium excretion as prognostic marker in acutely decompensated heart failure: the spironolactone effect. Clin Res Cardiol. 2016;105:489‐507. [DOI] [PubMed] [Google Scholar]
- 58. Adin D, Kurtz K, Atkins C, Papich MG, Vaden S. Role of electrolyte concentrations and renin‐angiotensin‐aldosterone activation in the staging of canine heart disease. J Vet Intern Med. 2020;34:53‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kumar D, Bagarhatta R. Fractional excretion of sodium and its association with prognosis of decompensated heart failure patients. J Clin Diagn Res. 2015;9:OC01‐03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Shah N, Madanieh R, Alkan M, Dogar MU, Kosmas CE, Vittorio TJ. A perspective on diuretic resistance in chronic congestive heart failure. Ther Adv Cardiovasc Dis. 2017;11:271‐278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. ter Maaten JM, Valente MA, Metra M, et al. A combined clinical and biomarker approach to predict diuretic response in acute heart failure. Clin Res Cardiol. 2016;105:145‐153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Brady CA, Hughes D, Drobatz KJ. Association of hyponatremia and hyperglycemia with outcome in dogs with congestive heart failure. J Vet Emerg Crit Care. 2004;14:177‐182. [Google Scholar]
- 63. Gheorghiade M, Rossi JS, Cotts W, et al. Characterization and prognostic value of persistent hyponatremia in patients with severe heart failure in the ESCAPE trial. Arch Intern Med. 2007;167:1998‐2005. [DOI] [PubMed] [Google Scholar]
- 64. Bellino MC, Massari F, Albanese M, et al. Baseline and incident hypochloremia in chronic heart failure outpatients: clinical correlates and prognostic role. Eur J Intern Med. 2021;84:32‐37. [DOI] [PubMed] [Google Scholar]
- 65. Kataoka H. Chloride in heart failure syndrome: its pathophysiologic role and therapeutic implication. Cardiol Ther. 2021;10:407‐428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kazory A, Ronco C. Emergence of chloride as an overlooked cardiorenal connector in heart failure. Blood Purif. 2020;49:219‐221. [DOI] [PubMed] [Google Scholar]
- 67. Radulovic B, Potocnjak I, Dokoza Teresak S, et al. Hypochloraemia as a predictor of developing hyponatraemia and poor outcome in acute heart failure patients. Int J Cardiol. 2016;212:237‐241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hanberg JS, Rao V, Ter Maaten JM, et al. Hypochloremia and diuretic resistance in heart failure: mechanistic insights. Circ Heart Fail. 2016;9:e003180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Adin D, Atkins C, Londono L, et al. Correction of serum chloride concentration in dogs with congestive heart failure. J Vet Intern Med. 2021;35:51‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Roche‐Catholy M, Van Cappellen I, Locquet L, et al. Clinical relevance of serum electrolytes in dogs and cats with acute heart failure: a retrospective study. J Vet Intern Med. 2021;35:1652‐1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Cuthbert JJ, Bhandari S, Clark AL. Hypochloraemia in patients with heart failure: causes and consequences. Cardiol Ther. 2020;9:333‐347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Rivera FB, Alfonso P, Golbin JM, et al. The role of serum chloride in acute and chronic heart failure: a narrative review. Cardiorenal Med. 2021;11:87‐98. [DOI] [PubMed] [Google Scholar]
- 73. Paterna S, Di Gaudio F, La Rocca V, et al. Hypertonic saline in conjunction with high‐dose furosemide improves dose‐response curves in worsening refractory congestive heart failure. Adv Ther. 2015;32:971‐982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Verbrugge FH, Martens P, Ameloot K, et al. Acetazolamide to increase natriuresis in congestive heart failure at high risk for diuretic resistance. Eur J Heart Fail. 2019;21:1415‐1422. [DOI] [PubMed] [Google Scholar]
- 75. Moffett BS, Moffett TI, Dickerson HA. Acetazolamide therapy for hypochloremic metabolic alkalosis in pediatric patients with heart disease. Am J Ther. 2007;14:331‐335. [DOI] [PubMed] [Google Scholar]
- 76. Ter Maaten JM, Rao VS, Hanberg JS, et al. Renal tubular resistance is the primary driver for loop diuretic resistance in acute heart failure. Eur J Heart Fail. 2017;19:1014‐1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Huang XP, Setola V, Yadav PN, et al. Parallel functional activity profiling reveals valvulopathogens are potent 5‐hydroxytryptamine(2B) receptor agonists: implications for drug safety assessment. Mol Pharmacol. 2009;76:710‐722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Cox ZL, Lenihan DJ. Loop diuretic resistance in heart failure: resistance etiology‐based strategies to restoring diuretic efficacy. J Card Fail. 2014;20:611‐622. [DOI] [PubMed] [Google Scholar]
- 79. Lee TH, Kuo G, Chang CH, et al. Diuretic effect of co‐administration of furosemide and albumin in comparison to furosemide therapy alone: an updated systematic review and meta‐analysis. PLoS One. 2021;16:e0260312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ikeda Y, Ishii S, Maemura K, et al. Association between intestinal oedema and oral loop diuretic resistance in hospitalized patients with acute heart failure. ESC Heart Fail. 2021;8:4067‐4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Hori Y, Takusagawa F, Ikadai H, Uechi M, Hoshi F, Higuchi SI. Effects of oral administration of furosemide and torsemide in healthy dogs. Am J Vet Res. 2007;68:1058‐1063. [DOI] [PubMed] [Google Scholar]
- 82. Ali S, Jung S, Nandkeolyar S, et al. Inpatient diuretic management of acute heart failure: a practical review. Am J Cardiovasc Drugs. 2021;21:595‐608. [DOI] [PubMed] [Google Scholar]


