Abstract
Objective
Apoptosis is programmed cell death that occurs by several pathways. Caspase-3 is induced by active caspase-9 via the intrinsic pathway. The aim of this research was to explore the expression of caspase-3 and caspase-9 in schizophrenia patients and healthy samples.
Methods
RNA was isolated from the peripheral blood of 39 schizophrenia patients’ and healthy samples. After cDNA synthesis, real time PCR (RT-PCR) was used to analyse caspase-3 and caspase-9 gene expression. The severity of psychopathological symptoms of schizophrenia was evaluated using the Positive and Negative Symptoms Scale for schizophrenia (PANSS) and Clinical Global Impressions (CGI).
Results
The expression of caspase-3 and caspase-9 genes was higher in schizophrenia patients than in healthy samples (p = 0.012, p = 0.002, respectively). The increase in caspase-3 gene expression was significant with being male, smoking and with a duration of less than 6 years (p = 0.047, p = 0.049, p = 0.034, respectively). On the other hand, the increase in caspase-9 gene expression was significant in patients who is smoke, have children, and are under 33 years old (p = 0.040, p = 0.043, p = 0.045, respectively). A significant positive correlation was detected between the caspase-3 and caspase-9 gene expression (r = 0.3218, p = 0.049).
Conclusion
Our findings indicate that caspase-3 and caspase-9 gene expression may activate cell death mechanisms by intrinsic apoptotic genes. Furthermore, caspase-3 and caspase-9 may play essential roles in different ways in schizophrenia. Hence there is a need to further study the apoptotic mechanism with expanded patient populations.
Keywords: Schizophrenia, Apoptosis, Caspase-3, Caspase-9, Real time PCR, Intrinsic pathway
INTRODUCTION
Schizophrenia is a complex chronic, persistent mental health disorder marked by a wide range of symptoms including delusions, disorganised speech, and cognitive impairment [1]. Schizophrenia affects about 1 of 100 indivi-duals worldwide [2]. Schizophrenia patients with cardio-vascular disease have a high mortality rate [3]. Schizophrenia is often related with an average 20−25 years reduction in life span [2]. Pathological activation of neuronal apoptosis and abnormal expression of apoptotic regulatory proteins have been demonstrated in neurodegenerative disorders [4,5]. Different neurochemical processes and neural networks may be involved in schizophrenia, one of them being is the apoptosis pathway [6]. However, the exact molecular mechanisms of apoptosis in schizophrenia disease remain to be elucidated. It is now known that many diseases, including neurodegenerative diseases, are the result of not only genetic but also epigenetic changes. Transciriptional changes of 157 genes were found to be associated with schizophrenia in a transcription-wide association study [7]. Most of these genes consist of chromatin-associated genes that emphasise epigenetic mechanisms [8]. The epigenetic alterations may be the cause of some conditions that are not explained by genetic mechanisms in diseases. Both genetic and epigenetic studies can be expected to facilitate our understanding of the background of schizophrenia.
The term apoptosis (a-po-toe-sis) was originally used to explain a morphologically distinct form of cell death [9-11]. Apoptosis is a self-ordered cell death triggered by gene regulation, and deficiency of apoptosis is linked to the development of malignant tumours [12]. DNA damage leads to occur DNA fragmentation and these fragments induce genes that are crucial to the apoptotic process [13]. Caspases (cysteine-aspartic protease) are a type of endoprotease with crucial roles in the cell regulatory networks that regulate inflammation and cell death [14].
Apoptosis depending on apoptotic stimuli it is initiated with two different pathways, intrinsic and extrinsic. Intrinsic pathway is activated by the prensence of cell stress, DNA breaks, impairment of cell cycle, or hypoxia. These stimuli induce proapoptotic factors including cytochrome C (CytC) releasing from the permebilised membrane of mitochondria [15]. CytC binds to the apoptotic protease-activating factor 1 (Apaf-1) to form the apoptosome, which targets caspase-9 and activates it. Caspase-9 activates effector caspases including caspase-3 [16]. Caspase-3, the ultimate executor of apoptotic death in mammalian cells, is a crucial protease that can directly cleave a variety of essential structural and functional proteins [17].
The extrinsic pathway is started with extracellular ligands including CD95L/FasL [18]. The Fas-associated protein with death domain, binds to initiator caspases. Activated caspases further activate excecuter caspases including caspase-3 [19]. Both of intrinsic and extrinsic pathways activate caspase-3 and caspase-9 to apoptotic activation.
Thus, caspase-3 and caspase-9 are last step of the apoptotic pathway and evaluating of their expressions may give us clearer results than other proteins. The purpose of this research was to explore the expression of caspase-3 and caspase-9 in schizophrenia patients and determine their significance by comparing to other clinical and demographic results.
METHODS
Patients
Thirty-nine peripheral blood samples from schizophrenia patients and 39 healthy volunteers were included in this research. Samples were collected from Research Hospital Psychiatry Clinic of Atatürk University. This study was approved by the local ethics committee of Ataturk University (decision no: 2021/23). For diagnosis the Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-V) [20] was used by experienced psychiatrists. The severity of psychopathological symptoms of schizophrenia was evaluated using the Positive and Negative Symptoms Scale for schizophrenia (PANSS) and Clinical Global Impressions (CGI) [21]. We excluded healthy volunteers with mental disorders and cancer. Two psychiatrist performed an assesment of the mental state of patients and healthy volunteers.
RNA Isolation, cDNA Synthesis and Gene Expression Analysis
The EcoPURE total RNA isolation kit (cat no: E2075; EcoTech Biotechnology, Erzurum, Turkey) was used to isolate total RNA. For the RNA isolation, 100 ml of non-coagulating fresh blood was used. After adding 400 ml EcoPURE lysis/binding buffer to mix, it was vortexed for 10 seconds. Then, 400 ml absulate ethanol was added to lysate and mixed well by vortexing for 10 seconds. An EcoPURE column was inserted into a collection tube and 700 ml sample was transferred to the EcoPURE coloumns, where it was centrifuged at maximum speed in a tabletop microcentrifuge for 30 seconds at room temperature. After the washing and elution steps, the isolation was successfully completed. The concentration of the RNA samples was measured qualitatively using a NanoDrop instrument Take3 Plate (BioTek, Winooski, VT, USA). The RNA of each sample was stored at −20°C until use for RT-PCR.
The cDNA was then synthesised using the Bio-Rad IscriptTM cDNA kit (cat no: 1708891; Bio-Rad, Hercules, CA, USA). For the cDNA synthesis reaction, 4 ml of 5x iScript reaction mix, 1 ml of iScript reverse transcriptase, 8 ml of RNA and 7 ml nuclease free water were mixed in 20 ml volume. The reaction was performed in the thermalcycler device at the temperatures recommended by the kit (priming: 5 minutes 25°C, reverse transcription: 20 minutes 46°C, RT inactivation: 1 minute 95°C, hold at 4°C). For the RT-PCR synthesis reaction we used 10 ml SsoAdvanced Universal SYBR Green Supermix (cat no: 1725270; Bio-Rad), 5 ml cDNA, 1 ml forward primer, 1 ml reverse primer and 3 ml PCR grade water. Subsequently a PCR reaction was performed in the Bio-Rad CFX RT-PCR device at the temperatures recommended by the kit (30 seconds at 95°C for polymerase activation, 10 seconds at 95°C for denaturation, 20 seconds at 60°C for annealing, 35−40 cycles). Each sample was run twice. The relative expression was determined by the 2−∆∆Ct method:
∆Ct = Ct (gene of interest (caspase 3 or caspase 9) − Ct (Beta actin (housekeeping gene))
∆Ct is the value obtained by subtracting the Ct value of the internal gene from the gene under investigation for a given sample. The internal control gene is not affected by the experiment, and it is used to normalise the value of interested gene. The delta-delta Ct (∆∆Ct) values show the difference between the samples and the controls. The final results of the analysis are then indicated by 2−∆∆Ct as a fold change of the gene expression.
The primer sequences for RT-PCR (housekeeping gene, caspase-3, and caspase-9) are as follows:
Beta actin for humans;
Primer-F: 5-CCTCCTGAGCGCAAGTACT-3
Primer-R: 5-TGCTTGCTGATCCACATCT-3
caspase-9 for humans;
Primer-F: 5-’TGTCTACGGCACAGATGGA-’3
Primer-R: 5-’GGACTCGTCTTCAGGGGA-’3
caspase-3 for humans;
Primer-F: 5-ATGGAAGCGAATCAATGGA-3
Primer-R: 5-TGTACCAGACCGAGATGTC-3
Statistical Analysis
GraphPad Prism 7.04 was used for all statistical analyses. We used mean ± standard deviation (SD) to describe normally distributed variables. The normal distribution of the data was analyzed by the Kolmogorov−Smirnov and Shapiro−Wilk normality test. A ttest was used to see the age difference in patients and healthy individuals. The χ2 test was used to see the sex difference in patients and healthy individuals. The Mann−Whitney Utest was used to see the difference in patients and control group gene expression, as well as to see the difference in patients’ gene expression according to demographic data. The Pearson correlation was used to reveal the correlation of caspase-3 and caspase-9 gene expression. If the result is p < 0.05, it is accepted statistically significantly.
RESULTS
Demographics Characteristics of 39 Patients and Healthy Individuals
The demographic characteristics of the total 39 patients are shown in Table 1. We could not reach the data of some patients. Age ranged from 20 to 65, and the average age of the schizophrenia patients was 37.74 ± 10.80 (mean ± SD). Twenty four (61.5%) of the patients were male and 15 (38.5%) of the patients were female. The mean total score on the PANSS was 93.29 ± 16.73. The mean total score on the CGI was 5.71 ± 1.15. The number of single patients was 24 (61.5%). The number of patients who have children was 11 (28.2%). When the educational status of the patients were examined, 25 (64.1%) of the patients had only elementary or high school education. Among the patients, the number of people with a family history of psychiatric illness was 13 (33.3%).
Table 1.
Characteristics of schizophrenia patients’ and healthy samples’ demographics
| Characteristics | Patients (n = 39) | Healthy (n = 39) | p value |
|---|---|---|---|
| Age (yr) | 37.74 ± 10.80 | 39.26 ± 12.03 | 0.5724a |
| Sex | 0.4917b | ||
| Female | 15 (38.46) | 18 (46.15) | |
| Male | 24 (61.54) | 21 (53.85) | |
| Educational status | |||
| Elementary and high school graduate | 25 (64.10) | - | |
| University or higher graduate | 6 (15.38) | - | - |
| Illiterate | 2 (5.128) | - | |
| Unidentified | 6 (15.38) | ||
| Smoking status | |||
| Yes | 17 (43.59) | - | |
| No | 17 (43.59) | - | - |
| Unidentified | 5 (12.82) | ||
| Duration of illness (yr) | |||
| ≤ 5 | 11 (28.21) | - | - |
| > 5 | 23 (58.97) | - | |
| Unidentified | 5 (12.82) | ||
| Number of children | |||
| Yes | 11 (28.21) | - | - |
| No | 21 (53.84) | - | |
| Unidentified | 7 (17.94) | ||
| Marital status | |||
| Married | 11 (28.21) | - | - |
| Single | 24 (61.54) | - | |
| Unidentified | 4 (10.26) | ||
| Presence of psychiatric disease in relative | |||
| Yes | 13 (33.33) | - | - |
| No | 20 (51.3) | - | |
| Unidentified | 6 (15.38) | ||
| Duration of treatment (yr) | |||
| ≤ 5 | 13 (33.33) | - | - |
| > 5 | 20 (51.3) | - | |
| Unidentified | 6 (15.38) | ||
| Antipsychotic drug use status | |||
| Aripiprazole | 9 (23.07) | - | |
| Clozapine | 7 (17.94) | - | - |
| Paliperidone | 14 (35.90) | - | |
| Unidentified | 5 (12.82) | - | |
| Other drug (risperidone, klorapimine etc.) | 4 (10.26) | ||
Values are presented as mean ± standard deviation or number (%).
attest and bχ2 test were used.
The number of smoking and non-smoking patients was equal (n = 17). The number of patients with the disease duration of more than 5 years was 23 (58.7%), while 20 patients (51.3%) had a duration of treatment of over 5 years. The most commonly used antipsychotic drug among patients was paliperidone 14 (35.9%). Aripiprazole, clozapine and risperodine are other antipsychotic drugs that patients frequently use.
Age ranged from 18 to 63, and the average age of the healthy individuals was 39.26 ± 12.03 (mean ± SD). Twenty-one (53.9%) of the individuals were male and 18 (46.15%) of the individuals were female.
Caspase-3 and Caspase-9 Expression were Shown to be Higher in Schizophrenia Patients and were Related to Some Demographic Characteristics of Patients
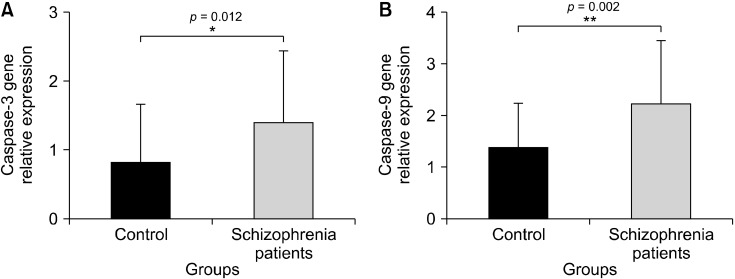
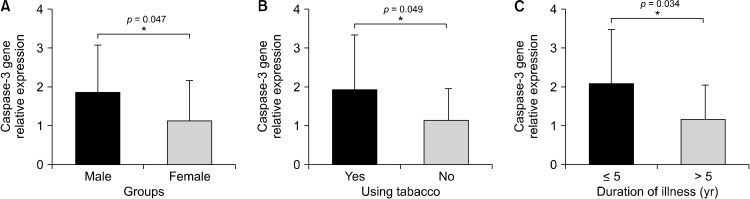
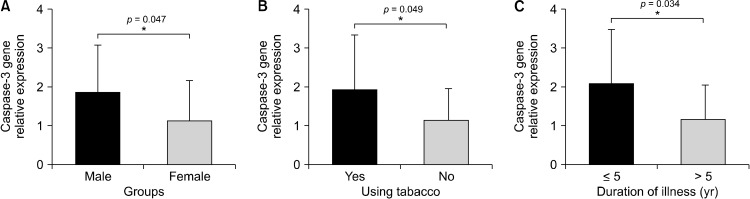
According to the our results, caspase-3 and caspase-9 genes were significantly overexpressed in schizophrenia patients compared with healthy samples (p = 0.012, p = 0.002, respectively) (Fig. 1). In addition, caspase-3 gene expression was higher in male patients, smokers, and those who had the disease for less than 5 years (p = 0.047, p = 0.049, p = 0.034, respectively) (Fig. 2). On the other hand, the increase in caspase-9 gene expression was significant in patients who is smoke and those who are being under 33 years old or have children (p = 0.040, p = 0.045, p = 0.043, respectively) (Fig. 3). Caspase-3 and caspase-9 gene expression levels were not shown to have a significant differences for other demographic and clinical parameters (p > 0.05). The caspase-3 and caspase-9 levels were lower in patients using paliperidone compared to those using other drugs (p = 0.690, p = 0.177, respectively).
Fig. 1.
(A, B) Gene expression levels of caspase-3 and caspase-9 in schizophrenia patients and control. *p < 0.05 is significantly, **Means statically very significant.
Fig. 2.
(A−C) Differrences of caspase-3 gene expression levels depending on patients’ characteristics. *p < 0.05 is significantly.
Fig. 3.
(A−C) Differences of caspase-9 expression levels depend on patients’ characteristics. *p < 0.05 is significantly.
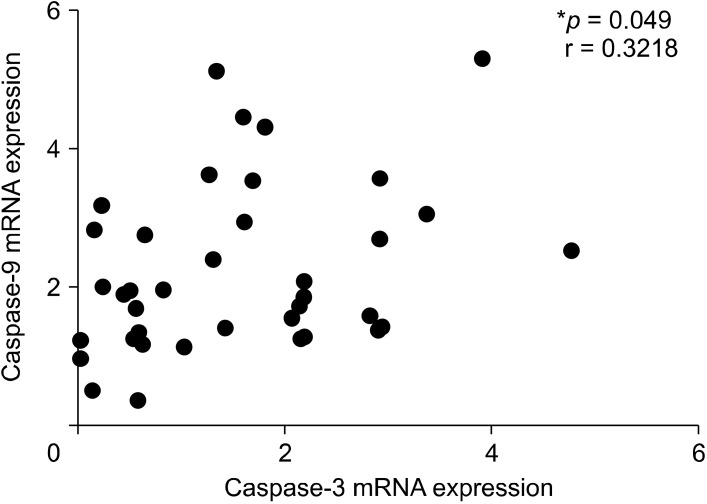
Caspase-3 and caspase-9 gene expression levels were shown to have a significant positive correlation (r = 0.3218, p = 0.049) (Fig. 4). However, caspase-3 and caspase-9 gene expression levels were not shown to have a correlation with PANSS and CGI score (p > 0.05). Caspase-9 activates caspase-3 in the apoptotic pathway, so the existence of positive correlations between these two genes is already expected.
Fig. 4.
Positive correlation between caspase-3 and caspase-9 gene expression in severe schizophrenia patients. *p < 0.05 is significantly.
DISCUSSION
There are several mechanism related to schizophrenia pathophysiology. Beside the enviromental changes genetic and also epigenetic alterations must also considered in order to understand the disease background. Apoptosis is one of the significant mechanisms to be explained in schizophrenia. Apoptosis is important for neural cells, during the process of aging and neurodegeneration, which constitutes the control mechanism of neural development [22]. Upstream apoptotic signaling mechanisms focus on mitochondria to achieve CytC release and procaspases. Apoptotic protease activating factor 1 (Apaf1) is activated by CytC and forms the apoptosome morphology in the cytoplasm [23]. As a consequence, caspase-9 activates executioner caspases like caspase-3, which aids in nuclear DNA break and cytoskeleton and nuclear lamina sequestration, causing cells to assume an apoptotic, spherical form [23]. Caspase-3 is the most commonly active among the effector caspases in neuronal apoptosis [24].
Many genes have been investigated by blood levels of schizophrenia patients. One of them the tumour necrosis factor (TNF)-like weak inducer of apoptosis (TWEAK), is expressed in many tissues including the brain [25]. Kiliç et al. [26] was found higher serum TWEAK levels in schizophrenia patients than control groups. Beyazyüz et al. [27] showed an increased serum apoptosis level of deficit schizophrenia patients and non-deficit schizophrenia patients compared to healthy controls. Catts et al. [28] used dermal fibroblast cells from schizophrenia patients, non- schizophrenic psychosis patients, and healthy subjects and investigated apoptosis susceptibility by the proportion of cells in the sub-G0 cell cycle fraction and pro-apoptotic effector (activate caspase-3). They concluded that there is higher apoptotic susceptibility in schizophrenia than other groups [28]. According to findings of one study, the expression of the Bcl-2 gene and the caspase-3 gene in schizophrenic patients was substantially higher than controls [29].
Apoptotic proteins including caspase-3 and caspase-9 are known and they have special task in different sitiuations [30]. Caspase-3 can be measured for evaluating apoptosis and comparing it to classical neurodegeneration [31]. Because it causes the release of cytochrome c, which initiates the caspase cascade, Bax/Bcl-2 ratios are associated with susceptibility to apoptosis [32]. Caspase-3, Bcl-2 and Bax, which are expressed at different levels in the pathophysiology of schizophrenia, distinguish it make the difference from classical neurodegenerative disorders [33]. A higher rate of Bax/Bcl-2 was observed in the temporal cortex of schizophrenic patients than in healthy controls [33]. However, the amount of caspase-3 did not show significant differences [33]. Changes in Apaf1 complex formation and caspase-9 activation form the fine line between cell survival and death [34]. One study evaluated the altered expression levels of autophagy and apoptosis-related genes in schizophrenia patients receiving treatment with olanzapine [35].
The fact that people with mental problems are more likely to smoke tobacco has raised the idea that patients may be self-medicating. Schizophrenic patients have received special scrutiny when it comes to smoking as a form of self-medication [36]. In present study, the expression of apoptosis genes caspase-3 and caspase-9 was shown to be increased in schizophrenia patients. The fact that the patients are male, smokers, and have a disease duration of less than five years indicates differences in caspase-3 gene expression. Smoking that is related with oxidative stress in schizophrenia patients [37] may be an independent factor to induce caspase activation. Yu et al. [38] showed that smoking increased cell apoptosis, causing an elevated cleaved-caspase 3/pro-caspase 3 ratio, and Bax expressesion but decreased Bcl-2 signals. In this study, caspase-3 and caspase-9 genes were high expressed in smoking schizophrenia patients. As the duration of the disease increases, the duration of medical treatment process of the patient also increases. Prolongation of the treatment period may have contributed to the reduction of caspase-3 expression. On the other hand, the increase in caspase-9 gene expression was significant with patients who smoke, have children or are under 33 years old. Similar to caspase-3, caspase-9 expression may be affected by smoking. Patients younger than 33 showed higher disease severity than those older than 33. Increased disease severity may lead to high gene expression of caspase-9. Also, there was a significant positive correlation between caspase-3 and caspase-9 gene expression levels. Caspase-3 and caspase-9 gene expression levels were not shown to have a correlation with PANSS and CGI score.
The present study has some limitations that should be mentioned. We analysed caspase-3 and caspase-9 gene expressions and demographic parameters from Schizo-phrenic patients. First, we were unable to analyse other apoptotic genes (Bax and Bcl-2 etc.). Second, we did not have the opportunity to work with large numbers of patients. Additional in vivo and in vitro research and studies with larger patient populations are needed to substantiate our findings and explain their relevance. To investigate tissue-specific changes, post-mortem brain tissue studies will be useful to demonstrate apoptotic activity. Investigating the differences between cerebrospinal fluid and peripheral blood may provide more information about tissue-specific expression.
In conclusion, the expression of caspase-3 and caspase-9 genes was significantly higher in schizophrenia patients than healthy samples in this sudy. In addition, several patients characteristics have been associated with the expression of caspase. The levels of caspase-3 and caspase-9 gene expression were found to have a significant positive correlation. Caspase-9 is the activator of caspase-3, so they are expected to show the same directional expression. Caspase-3 and caspase-9 gene expression in schizophrenia patients may be apoptosis activators or regulators. With this study, we have contributed to the discovery of markers that may be used for schizophrenia in the future. Therefore, studies in the larger schizophrenia patient population will reveal the value of apoptotic genes.
Footnotes
Funding
None.
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
Research concept and design: Ebubekir Dirican, Halil Özcan, Sevgi Karabulut Uzunçakmak, Uğur Takım. Collection and/or assembly of data: Ebubekir Dirican, Halil Özcan, Sevgi Karabulut Uzunçakmak, Uğur Takım. Data analysis and interpretation: Ebubekir Dirican, Halil Özcan, Sevgi Karabulut Uzunçakmak, Uğur Takım. Writing the article: Ebubekir Dirican, Halil Özcan, Sevgi Karabulut Uzunçakmak, Uğur Takım. Critical revision of the article: Ebubekir Dirican, Halil Özcan, Sevgi Karabulut Uzunçakmak, Uğur Takım. Final approval of article: Ebubekir Dirican, Halil Özcan, Sevgi Karabulut Uzunçakmak, Uğur Takım.
References
- 1.Patel KR, Cherian J, Gohil K, Atkinson D. Schizophrenia: overview and treatment options. P T. 2014;39:638–645. [PMC free article] [PubMed] [Google Scholar]
- 2.Hu L, Chen Y, Yang CP, Huang Y, Song NN, Chen JY, et al. Ulk4, a newly discovered susceptibility gene for schizophrenia, regulates corticogenesis in mice. Front Cell Dev Biol. 2021;9:645368. doi: 10.3389/fcell.2021.645368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guillen-Aguinaga S, Brugos-Larumbe A, Guillen-Aguinaga L, Guillen-Grima F, Ortuño F, Forga L, et al. Schizophrenia and hospital admissions for cardiovascular events in a large population: the APNA study. J Cardiovasc Dev Dis. 2022;9:25. doi: 10.3390/jcdd9010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bredesen DE. Neural apoptosis. Ann Neurol. 1995;38:839–851. doi: 10.1002/ana.410380604. [DOI] [PubMed] [Google Scholar]
- 5.Su JH, Deng G, Cotman CW. Bax protein expression is increased in Alzheimer's brain: correlations with DNA damage, Bcl-2 expression, and brain pathology. J Neuropathol Exp Neurol. 1997;56:86–93. doi: 10.1097/00005072-199701000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Jarskog LF, Glantz LA, Gilmore JH, Lieberman JA. Apoptotic mechanisms in the pathophysiology of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:846–858. doi: 10.1016/j.pnpbp.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 7.Gusev A, Mancuso N, Won H, Kousi M, Finucane HK, Reshef Y, et al. Transcriptome-wide association study of schizophrenia and chromatin activity yields mechanistic disease insights. Nat Genet. 2018;50:538–548. doi: 10.1038/s41588-018-0092-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Föcking M, Doyle B, Munawar N, Dillon ET, Cotter D, Cagney G. Epigenetic factors in schizophrenia: mechanisms and experimental approaches. Mol Neuropsychiatry. 2019;5:6–12. doi: 10.1159/000495063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paweletz N. Walther Flemming: pioneer of mitosis research. Nat Rev Mol Cell Biol. 2001;2:72–75. doi: 10.1038/35048077. [DOI] [PubMed] [Google Scholar]
- 11.Kerr JF. History of the events leading to the formulation of the apoptosis concept. Toxicology. 2002;181-182:471–474. doi: 10.1016/S0300-483X(02)00457-2. [DOI] [PubMed] [Google Scholar]
- 12.Zerban H, Radig S, Kopp-Schneider A, Bannasch P. Cell proliferation and cell death (apoptosis) in hepatic preneoplasia and neoplasia are closely related to phenotypic cellular diversity and instability. Carcinogenesis. 1994;15:2467–2473. doi: 10.1093/carcin/15.11.2467. [DOI] [PubMed] [Google Scholar]
- 13.Evan G, Littlewood T. A matter of life and cell death. Science. 1998;281:1317–1322. doi: 10.1126/science.281.5381.1317. [DOI] [PubMed] [Google Scholar]
- 14.McIlwain DR, Berger T, Mak TW. Caspase functions in cell death and disease. Cold Spring Harb Perspect Biol. 2013;5:a008656. doi: 10.1101/cshperspect.a026716. Erratum in: Cold Spring Harb Perspect Biol 2015;7: a026716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henry-Mowatt J, Dive C, Martinou JC, James D. Role of mitochondrial membrane permeabilization in apoptosis and cancer. Oncogene. 2004;23:2850–2860. doi: 10.1038/sj.onc.1207534. [DOI] [PubMed] [Google Scholar]
- 16.Zou H, Li Y, Liu X, Wang X. An APAF-1.cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9. J Biol Chem. 1999;274:11549–11556. doi: 10.1074/jbc.274.17.11549. [DOI] [PubMed] [Google Scholar]
- 17.Marks N, Berg MJ, Guidotti A, Saito M. Activation of caspase-3 and apoptosis in cerebellar granule cells. J Neurosci Res. 1998;52:334–341. doi: 10.1002/(SICI)1097-4547(19980501)52:3<334::AID-JNR9>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 18.Wiley SR, Schooley K, Smolak PJ, Din WS, Huang CP, Nicholl JK, et al. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3:673–682. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- 19.Falschlehner C, Emmerich CH, Gerlach B, Walczak H. TRAIL signalling: decisions between life and death. Int J Biochem Cell Biol. 2007;39:1462–1475. doi: 10.1016/j.biocel.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 20.American Psychiatric Association, author. Diagnostic and statistical manual of mental disorders: DSM-5. American Psychiatric Association; Arlington: 2013. [DOI] [Google Scholar]
- 21.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 22.Mazarakis ND, Edwards AD, Mehmet H. Apoptosis in neural development and disease. Arch Dis Child Fetal Neonatal Ed. 1997;77:F165–F170. doi: 10.1136/fn.77.3.F165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clay HB, Sillivan S, Konradi C. Mitochondrial dysfunction and pathology in bipolar disorder and schizophrenia. Int J Dev Neurosci. 2011;29:311–324. doi: 10.1016/j.ijdevneu.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yuan J, Yankner BA. Apoptosis in the nervous system. Nature. 2000;407:802–809. doi: 10.1038/35037739. [DOI] [PubMed] [Google Scholar]
- 25.Yepes M. TWEAK and the central nervous system. Mol Neurobiol. 2007;35:255–265. doi: 10.1007/s12035-007-0024-z. [DOI] [PubMed] [Google Scholar]
- 26.Kiliç F, Işik Ü, Usta A, Demirdaş A. Serum tumor necrosis factor- like weak inducer of apoptosis levels are elevated in schizo-phrenia. Braz J Psychiatry. 2021;43:242–246. doi: 10.1590/1516-4446-2020-0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beyazyüz M, Küfeciler T, Bulut L, Ünsal C, Albayrak Y, Akyol ES, et al. Increased serum levels of apoptosis in deficit syndrome schizophrenia patients: a preliminary study. Neuropsychiatr Dis Treat. 2016;12:1261–1268. doi: 10.2147/NDT.S106993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Catts VS, Catts SV, McGrath JJ, Féron F, McLean D, Coulson EJ, et al. Apoptosis and schizophrenia: a pilot study based on dermal fibroblast cell lines. Schizophr Res. 2006;84:20–28. doi: 10.1016/j.schres.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 29.Szymona K, Dudzińska E, Karakuła-Juchnowicz H, Gil-Kulik P, Chomik P, Świstowska M, et al. Analysis of the expression of BAX, BCL2, BIRC6, CASP3, CASP9 apoptosis genes during the first episode of schizophrenia. Psychiatr Pol. 2019;53:1293–1303. doi: 10.12740/PP/OnlineFirst/99971. [DOI] [PubMed] [Google Scholar]
- 30.López E, Pozas E, Rivera R, Ferrer I. Bcl-2, Bax and Bcl-x expression following kainic acid administration at convulsant doses in the rat. Neuroscience. 1999;91:1461–1470. doi: 10.1016/S0306-4522(98)00704-0. [DOI] [PubMed] [Google Scholar]
- 31.Hartmann A, Hunot S, Michel PP, Muriel MP, Vyas S, Faucheux BA, et al. Caspase-3: a vulnerability factor and final effector in apoptotic death of dopaminergic neurons in Parkinson's disease. Proc Natl Acad Sci U S A. 2000;97:2875–2880. doi: 10.1073/pnas.040556597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609–619. doi: 10.1016/0092-8674(93)90509-O. [DOI] [PubMed] [Google Scholar]
- 33.Jarskog LF, Selinger ES, Lieberman JA, Gilmore JH. Apoptotic proteins in the temporal cortex in schizophrenia: high Bax/Bcl-2 ratio without caspase-3 activation. Am J Psychiatry. 2004;161:109–115. doi: 10.1176/appi.ajp.161.1.109. [DOI] [PubMed] [Google Scholar]
- 34.Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 35.Gu S, Cui F, Yin J, Fang C, Liu L. Altered mRNA expression levels of autophagy- and apoptosis-related genes in the FOXO pathway in schizophrenia patients treated with olanzapine. Neurosci Lett. 2021;746:135669. doi: 10.1016/j.neulet.2021.135669. [DOI] [PubMed] [Google Scholar]
- 36.Manzella F, Maloney SE, Taylor GT. Smoking in schizophrenic patients: a critique of the self-medication hypothesis. World J Psychiatry. 2015;5:35–46. doi: 10.5498/wjp.v5.i1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buosi P, Borghi FA, Lopes AM, Facincani IDS, Fernandes-Ferreira R, Oliveira-Brancati CIF, et al. Oxidative stress biomarkers in treatment-responsive and treatment-resistant schizophrenia patients. Trends Psychiatry Psychother. 2021;43:278–285. doi: 10.47626/2237-6089-2020-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu Q, Yang S, Li Z, Zhu Y, Li Z, Zhang J, et al. The relationship between endoplasmic reticulum stress and autophagy in apoptosis of BEAS-2B cells induced by cigarette smoke condensate. Toxicol Res (Camb) 2021;10:18–28. doi: 10.1093/toxres/tfaa095. [DOI] [PMC free article] [PubMed] [Google Scholar]