Approximately 80% of the world’s population have been infected by cytomegalovirus (CMV) based on seroprevalence data, and immunosuppressed CMV-seropositive patients are at increased risk for CMV reactivation.1 After allogeneic hematopoietic cell transplantation (alloHCT), CMV reactivation occurs in 40-45% of CMV-seropositive recipients and is associated with increased non-relapse mortality.2,3 As in alloHCT, lymphodepleting conditioning is given before CAR T-cell (CAR T) therapy; thus, the incidence of CMV reactivation may also be increased after CAR T therapy.
The incidence and clinical significance of CMV reactivation after CAR T therapy is not well described. CMV viremia (n=14) and end organ disease (pneumonitis [n=2], enteritis [n=1], encephalitis [n=2], and retinitis [n=1)) have been reported after CAR T therapy.4-12 Another study reported that ten of 60 (17%) CMV-seropositive and -seronegative patients receiving CAR T therapy developed CMV reactivation based upon a single test performed from day+14 to +21 after CAR T infusion,13 none of these patients developed CMV organ disease. Interpretation of these studies with regard to CMV reactivation incidence is limited by the unknown or low frequency of testing. Active monitoring with CMV DNA quantitative polymerase chain reaction (qPCR) testing was not described, and CMV reactivation is less likely to be detected if the frequency of testing is low.
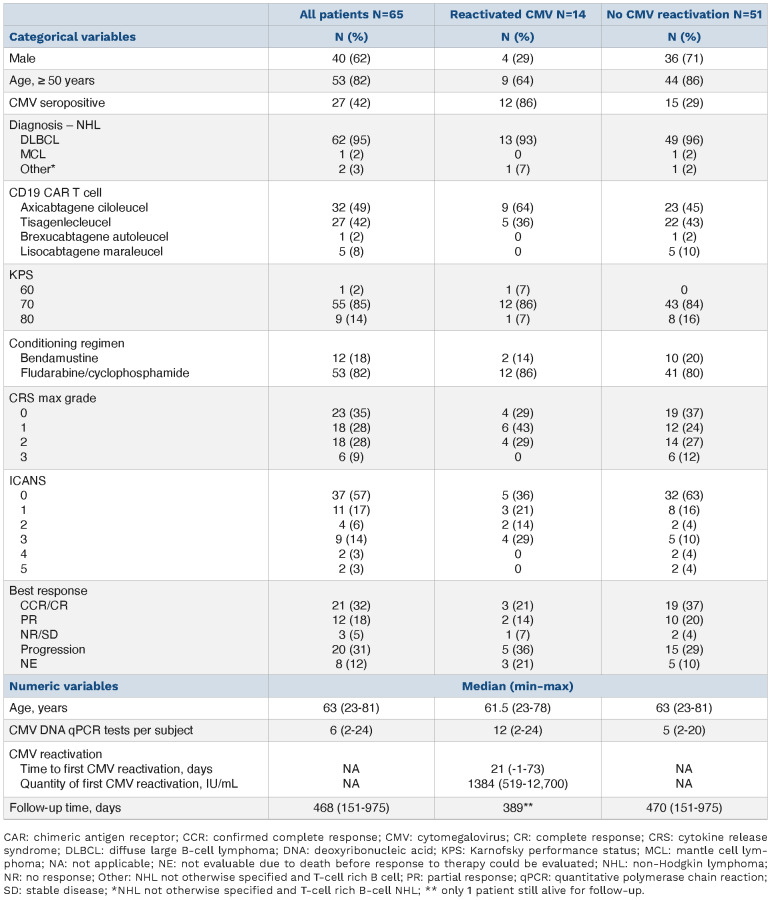
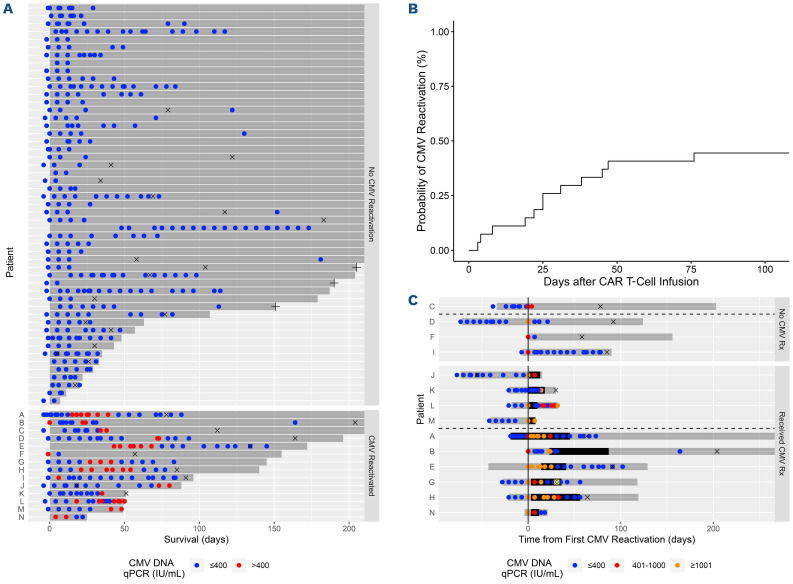
Therefore, we retrospectively analyzed the clinical outcomes of 65 consecutive patients treated with autologous CD19 targeted CAR T therapy for non-Hodgkin lymphoma from May 2018 to June 2021 who received at least two CMV DNA qPCR tests (Table 1) to determine the incidence of CMV reactivation and its clinical impact. All patients were treated at a single comprehensive cancer center with a dedicated transplant and cellular therapy unit and transfused with leukocyte-reduced blood products that were considered CMV safe. None of the patients received prophylaxis for CMV infection. Patients received prophylaxis against herpes viruses with acyclovir 400 mg by mouth twice a day (renally dosed as needed). CAR T-related clinical data were prospectively gathered as part of the Transplant and Cellular Therapy Program monitoring plan. American Society for Transplant and Cellular Therapy guidelines were used to define and grade cytokine release syndrome (CRS) and immune effector cell-mediated neurotoxicity syndrome (ICANS).14 Reactivation was defined as a CMV DNA qPCR result >400 IU/mL occurring from 4 days before CAR T infusion until the time of death or last follow-up. This CMV DNA qPCR threshold was chosen because it is the minimum level at which detection of CMV DNA by the qPCR assay is more than 95% reliable (the 95% limit of detection). The 95% limit of quantification of the qPCR assay was 1,000 IU/mL. The test was performed on plasma samples in all patients and at all time points. The CMV qPCR assay was developed and validated in the Roswell Park clinical laboratory and approved for clinical use by the New York State Clinical Laboratory Evaluation Program. CMV DNA qPCR testing was started upon the initial hospitalization for CAR T infusion and as clinically indicated in the outpatient setting. Five hundred and sixty CMV DNA qPCR measurements were included in this analysis (Figure 1A). CMV DNA qPCR testing was performed a median of every 8.4 (min-max 2.8-197.8) days. The median number of CMV DNA qPCR assays per patient was 6 (min-max 2–24).
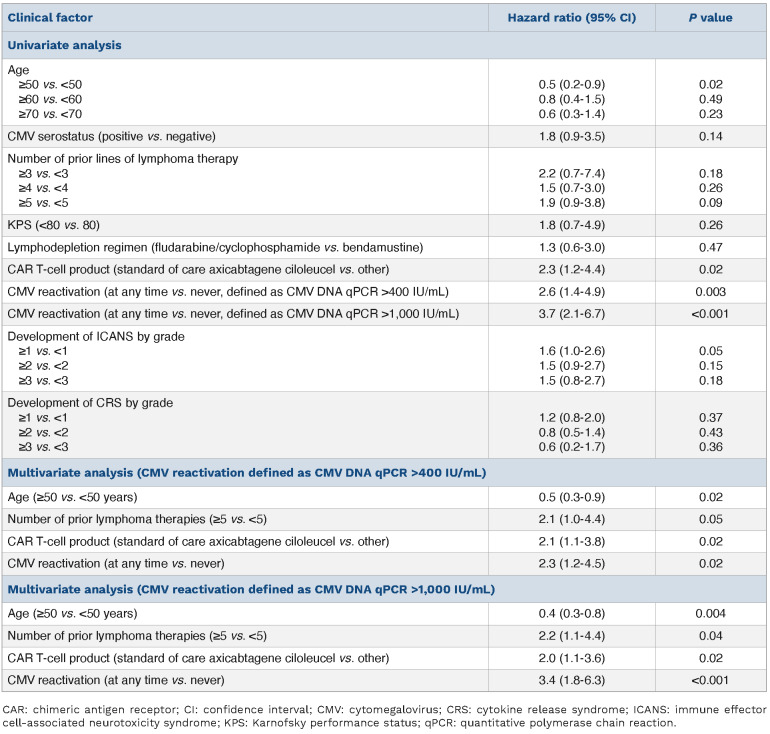
Estimated with the cumulative incidence function, 44% (95% confidence interval [CI]: 25-62, Figure 1B) of CMV-seropositive patients reactivated CMV by day+100. The cumulative incidence of CMV reactivation for all patients was 22% (95% CI: 13-32) at day+100 after CAR T infusion. Two of 38 (5.2%) CMV-seronegative patients had detectable CMV DNA >400 IU/mL indicative of new infection or reactivation in the setting of falsely negative prior CMV serology. For this analysis, these patients were classified as reactivating CMV. The median CMV DNA qPCR level of the first reactivation episode was 1,384 (min-max 519-12,700) IU/mL. Multivariate Cox proportional hazards modeling of clinical factors univariately associated with CMV reactivation at P<0.1 revealed that age ≥50 years (hazard ratio [HR] 0.2, 95% CI: 0.1–0.4, P<.001) was significantly associated with a decreased risk of reactivation, and positive CMV serostatus (HR 18.5, 95% CI: 4.5–76.6, P<0.001) was significantly associated with an increased risk of reactivation (Online Supplementary Table S1). Age ≥50 years was not significantly associated with non-Hodgkin lymphoma subtype or the number of lines of lymphoma therapy received.
Of the 14 (of a 65 total) patients who reactivated CMV, ten received CMV-specific therapy with foscarnet, ganciclovir, or valganciclovir. This therapy was initiated by the treating physician according to his/her clinical judgement. All ten of these patients had a CMV DNA qPCR >1,000 IU/mL. One patient developed CMV enteritis of the jejunum that was resolved with intravenous foscarnet but complicated by pancreatic necrosis, gastrointestinal perforation and bleeding requiring embolization, and mesenteric ischemia requiring wedge resection and repair. Prior to CAR T therapy the patient presented with a duodenal lesion that was diffuse large B-cell lymphoma. This patient died from his underlying lymphoma and not from CMV-related causes. Six of 10 (60%) patients receiving CMV therapy responded (Figure 1C). Of the four patients with CMV reactivation who did not receive therapy, three of four (75%) had resolution of reactivation without intervention.
Table 1.
Demographic characteristics (n=65).
The probability of surviving until day+365 for all patients studied was 54.7% (95% CI: 43.1-68.1). Multivariate Cox proportional hazards modeling of clinical factors univariately associated with survival at P<0.1 revealed that CMV reactivation (defined as CMV DNA qPCR >400 IU/mL, HR 2.3, 95% CI: 1.2–4.5, P=0.02) and treatment with standard of care axicabtagene ciloleucel (HR 2.1, 95% CI: 1.1–3.8, P=0.02) were significantly associated with an increased risk of death, while age ≥50 years (HR 0.5, 95% CI: 0.3-0.9, P=0.02) was significantly associated with a decreased risk of death (Table 2). Significant associations were observed between treatment with standard of care axicabtagene ciloleucel and the development of ICANS (P=0.005) or CRS (P=0.008), and between CMV serostatus and CMV reactivation (P=0.001). When CMV reactivation was redefined as CMV DNA qPCR >1,000 IU/mL, multivariate analysis resulted in similar conclusions with an increased risk for death associated with CMV reactivation (HR 3.4, 95% CI: 1.8–6.3, P<0.001, Table 2). Recursive partitioning analysis corroborated the significance of CMV reactivation to survival (Online Supplementary Figure S1).
Figure 1.
Cytomegalovirus reactivation occurs at moderate incidence afer CD19 CAR T-cell therapy and can be treated by cytomegalovirus-specific therapies. Cytomegalovirus (CMV) reactivation was defined as >400 IU/mL copies of CMV DNA. (A) Distribution of CMV DNA quantitative polymerase chain reaction (qPCR) testing after CD19 chimeric antigen receptor (CAR) T-cell therapy. CMV DNA qPCR testing occurred primarily during the first 180 days after CD19 CAR T-cell therapy. Testing occurred at the time points indicated by a dot. Red dots indicate CMV reactivation and blue dots indicate no CMV reactivation. (B) Cumulative incidence of CMV reactivation in CMV-seropositive patients. The cumulative incidence was 44% (95% confidence interval [CI]: 25-62) by day+100. (C) Clinical course of patients with CMV reactivation. Fourteen of 65 patients reactivated CMV. Four of these patients were not treated with CMV specific therapy (upper facet). Ten of these patients were treated with CMV-specific therapy (lower facet). Three of 4 patients who were not treated with CMV-specific therapy resolved the infection without intervention as defined by a subsequent CMV DNA qPCR test <400 IU/mL (blue dot, upper facet, below dashed line). The CMV status of the fourth untreated patient was indeterminate due to the lack of follow up CMV DNA qPCR testing. Six of the 10 patients treated with anti-CMV therapy responded to treatment as defined by a subsequent CMV DNA qPCR test ≤400 IU/mL (blue dot) after therapy (lower facet, below dashed line). Level of CMV reactivation is represented by the color of the circle as indicated in the legend with orange indicating CMV DNA qPCR ≥1,001, red indicating CMV DNA qPCR 401-1,000, and blue indicating CMV DNA qPCR ≤400. One patient (G) developed CMV enteritis which was diagnosed with a biopsy of the jejenum (yellow circled X) which demonstrated immunohistochemical staining for CMV in the context of xanthogranulomatous inflammation at day+31 after CAR T infusion. Time and duration of CMV treatment is indicated by the thicker horizontal black bar. Survival time of the patient is indicated by the narrower horizontal gray bar. The events for each patient have been recentered on the day of earliest CMV reactivation. ICANS: immune effector cell associated neurotoxicity syndrome; IU: international units; Rx: treatment; +: censored at last follow-up; x: disease relapse.
Among the 14 patients with CMV reactivation, the most frequent cause of death was lymphoma (n=9, Online Supplementary Table S2). The second most frequent cause of death was infection (n=2). The causes of infection were Candida krusei fungemia and Mucormycosis hepatitis.
In the 51 patients without CMV reactivation, the most frequent cause of death was lymphoma (n=11) and the second most frequent cause of death was ICANS (n=3). Infectious causes of death occurred in three patients. The etiology was blood infection (often polymicrobial) by Klebsiella pneumoniae, Enterococcus, Stenotrophomonas maltophillia, Staphylococcus hemolyticus, and Candida glabrata.
Table 2.
Clinical factors affecting overall survival.
Estimated with the cumulative incidence function, disease relapse occurred by day+180 in 42% (95% CI: 29-53). The cumulative incidence of non-relapse mortality was 14% (95% CI: 7-23) by day+180. Ten of the 31 (32%) patients who experienced relapse also reactivated CMV. In nine of these ten patients, CMV reactivation preceded relapse. The median time of CMV reactivation was 21 (min-max -1 to 73) days. The median time of relapse was 88 (min-max 18 to 204) days. Four of the 11 (36%) patients who experienced non-relapse mortality also reactivated CMV at 4, 18, 27, and 41 days.
These data indicate that CMV reactivation occurs at moderate frequency after CAR T therapy and may have clinically significant consequences. We report a higher incidence of CMV reactivation than previously recognized. This was likely due to a more accurate ascertainment of cases by frequent CMV DNA qPCR testing. Although CMV surveillance was not prospectively defined in this study, the frequency of the actual measurements suggests a monitoring level sufficient to reasonably detect CMV reactivation, at least during the first 100 days after CAR T infusion. The cumulative incidence is likely to be an underestimate due to patients who may have had an asymptomatic CMV infection but were not tested. As with CMV reactivation after alloHCT,2 CMV reactivation after CAR T therapy was associated with increased mortality not due to CMV disease. This was corroborated by the increased risk of death when a higher threshold for CMV reactivation was used (1,000 vs. 400 IU/mL). Paradoxically, age ≥50 was significantly associated with a decreased risk of death. In this patient cohort, younger age may be a surrogate for biologically worse lymphoma. The association between standard of care axicabtagene ciloleucel and increased mortality may have been through ICANS and CRS, both of which were closely associated with axicabtagene ciloleucel use.
The probability that CMV reactivation after CAR T therapy will progress to organ disease is still not known. CMV reactivation resolved without intervention in three cases in our study, suggesting that CMV reactivation may be self-limited in some cases, possibly dependent on the level of CMV reactivation. Future CMV surveillance studies may provide information about the rate of self-resolution of CMV reactivation and the level of CMV reactivation that is associated with adverse clinical outcomes. However, there will be a limitation to the level of CMV reactivation that will be tolerated (and subsequently analyzed) before initiating early therapy given the clinical experience with CMV reactivation in allogeneic HCT. Studies of prophylactic or early therapy may also provide an answer if an appropriate control arm is included. This study is limited by its retrospective design and the lower frequency of CMV DNA qPCR testing after day+30 and especially day+100. Patients who received CD19 CAR T therapy but did not have at least two CMV DNA qPCR tests performed (n=5) were excluded from this analysis. In conclusion, CMV reactivation after CAR T therapy is clinically significant. Therefore, prospectively defined surveillance plans to detect reactivation in high risk populations as well as clinical trials to evaluate the risk and benefit of CMV prophylaxis or early treatment after CAR T therapy are necessary.
Supplementary Material
Acknowledgements
We gratefully acknowledge the patients described in the study and the clinical team providing care to the patients; without their support, this study could not have been completed.
Funding Statement
Funding: This work was supported by Roswell Park Comprehensive Cancer Center and National Cancer Institute (NCI) grant P30CA016056.
References
- 1.Zuhair M, Smit GSA, Wallis G, et al. Estimation of the worldwide seroprevalence of cytomegalovirus: a systematic review and meta-analysis. Rev Med Virol. 2019;29(3):e2034. [DOI] [PubMed] [Google Scholar]
- 2.Green ML, Leisenring W, Xie H, et al. Cytomegalovirus viral load and mortality after haemopoietic stem cell transplantation in the era of pre-emptive therapy: a retrospective cohort study. Lancet Haematol. 2016;3(3):e119-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen GL, Wallace PK, Zhang Y, et al. Low-Level Cytomegalovirus antigenemia promotes protective cytomegalovirus antigen-specific T cells after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2020;26(11):2147-2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wittmann Dayagi T, Sherman G, Bielorai B, et al. Characteristics and risk factors of infections following CD28-based CD19 CAR-T cells. Leuk Lymphoma. 2021;62(7):1692-1701. [DOI] [PubMed] [Google Scholar]
- 5.Hill JA, Li D, Hay KA, et al. Infectious complications of CD19-targeted chimeric antigen receptor-modified T-cell immunotherapy. Blood. 2018;131(1):121-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wudhikarn K, Palomba ML, Pennisi M, et al. Infection during the first year in patients treated with CD19 CAR T cells for diffuse large B cell lymphoma. Blood Cancer J. 2020;10(8):79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baird JH, Epstein DJ, Tamaresis JS, et al. Immune reconstitution and infectious complications following axicabtagene ciloleucel therapy for large B-cell lymphoma. Blood Adv. 2021;5(1):143-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abramson JS, Palomba ML, Gordon LI, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396(10254):839-852. [DOI] [PubMed] [Google Scholar]
- 9.Logue JM, Zucchetti E, Bachmeier CA, et al. Immune reconstitution and associated infections following axicabtagene ciloleucel in relapsed or refractory large B-cell lymphoma. Haematologica. 2021;106(4):978-986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Locke FL, Ghobadi A, Jacobson CA, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. Lancet Oncol. 2019;20(1):31-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zu C, Xu Y, Wang Y, et al. Cytomegalovirus retinitis and retinal detachment following chimeric antigen receptor T cell therapy for relapsed/refractory multiple myeloma. Curr Oncol. 2022;29(2):490-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cousin E, Belicard F, Michel L, et al. Severe cytomegalovirus disease with encephalitis after CAR-T cell therapy: a rare but potentially fatal complication. J Med Virol. 2021;93(11):6398-6403. [DOI] [PubMed] [Google Scholar]
- 13.Beyar-Katz O, Kikozashvili N, Bar On Y, et al. Characteristics and recognition of early infections in patients treated with commercial anti-CD19 CAR-T cells. Eur J Haematol. 2022;108(1):52-60. [DOI] [PubMed] [Google Scholar]
- 14.Lee DW, Santomasso BD, Locke FL, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. 2019;25(4):625-638. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.