Relapse and graft-versus-host disease (GvHD) are the main impediments to the clinical success of allogeneic hematopoietic cell transplantation (HCT) in curing malignant blood disorders. Alloreactive donor T cells are important mediators of both relapse control by graft-versus-leukemia (GvL) effects, and of GvHD.1 In unrelated donor (UD)-HCT, frequent human leukocyte antigen (HLA)-DPB1 disparity is the target of T-cell alloreactivity, contributing to both leukemia control and GvHD.2 We have previously shown that two biological models of HLA-DPB1 mismatching, namely permissiveness according to T-cell epitope (TCE) groups and genetically determined high-expression levels, are individually associated with the risks of non-relapse mortality and GvHD/relapse, respectively.3,4 This led us to hypothesize that combined TCE-permissive and high-expression (TPHE) HLA-DPB1 mismatches might synergize for best outcomes. We tested this novel hypothesis in over 6,000 HCT from 8/8 HLA-matched UD reported to the European Group for Blood and Marrow Transplantation (EBMT), to demonstrate that HLA-DPB1 non-TPHE mismatches were associated with worse relapse-free survival and overall survival than TPHE mismatches, present in 21.7% of single HLA-DPB1 disparate pairs. Our work provides a synthesis of previous algorithms, mechanistically based on HLA-DPB1 immunopeptidome divergence5 and expression by residual leukemia cells,6,7 respectively, into a new and integrative model for intelligent mismatching in UD-HCT, to improve survival for future patients.
Alloreactive donor T cells recognizing patient-specific genetic polymorphisms, including mismatched HLA allotypes, play a major role in both beneficial GvL and severe GvHD after UD-HCT. One of the best examples of these two contrasting aspects of T-cell alloreactivity is donorrecipient HLA-DPB1 disparity, present in over 80% of transplants from UD.3 HLA-DPB1 disparity has been extensively explored for biological models apt to tease out clinically permissive mismatch combinations.2 These include sharing of alloreactive TCE groups between mismatched HLA-DPB1 alleles (TCE-permissiveness), mainly associated with non-relapse mortality,3,8 and high or low expression levels determined by a specific single nucleotide polymorphism in the HLA-DPB1 3’ untranslated region (expression-permissiveness), mainly associated with acute GvHD.4,9 Numerous studies have since investigated the clinical role of these two models in different national and international cohorts from the USA and Europe, both individually and in direct comparison.10-13 However, it is unclear whether the two models can be integrated, and whether their integration improves prediction of survival. These two questions were addressed in the present study. We investigated a previously unexplored cohort of 6,627 HLA-A, -B, -C, -DRB1-matched (HLA-8/8) first UD-HCT for adult patients with hematologic malignancies, reported by 160 EBMT centers between 2005 and 2017 (Table 1). Reflecting the increasing numbers of available six-loci HLA-typed UD in worldwide registries, and mounting recognition of the relevance of HLA-DPB1 matching for outcome, the percentage of HLA-DPB1 allele-matched pairs was higher in later years compared to earlier years (Table 1). Informed consent was obtained from all patients according to the Declaration of Helsinki, and protocols were approved by the institutional review boards of the participating institutions. Patients had received mainly peripheral blood stem cells under reduced intensity conditioning and cyclosporine-based GvHD prophylaxis with in vivo T-cell depletion by anti-thymocyte globulin; use of post-transplant cyclophosphamide was excluded. The cohort was stratified by HLA-DPB1 allele matches or mismatches, by TCE-permissiveness or TCE-nonpermissiveness according to the three-group model,3 or in the single HLA-DPB1 graft-versus-host (GvH) mismatched group by high expression or low expression according to the rs9277534 G/A single nucleotide polymorphism4 and/or TCE-permissiveness, for the discrimination between TPHE mismatches and others (Online Supplementary Figure S1A). The primary study endpoint was relapse-free survival; secondary endpoints included non-relapse mortality, relapse, acute GvHD, chronic GvHD, GvHD-free relapse-free survival (GRFS) and overall survival. Univariable and multivariable analyses by the log-rank test, cumulative-incidence functions and (cause-specific) Coxregression models were adjusted for non-HLA-DPB1 factors (Online Supplementary Table S1). For this hypothesis-testing analysis, P-values <0.05 were considered statistically significant.
HLA-DPB1 allele mismatches were associated with lower risks of relapse, and increased risks of acute GvHD and non-relapse mortality without improved relapse-free survival, compared to matches (Figure 1, Online Supplementary Table S2). When considered individually, TCE-nonpermissive and high-expression HLA-DPB1 single GvH-mismatches were each associated with lower relapse but higher acute
Table 1.
Patient, donor and transplant characteristics.
GvHD and non-relapse mortality, compared to allele matches (Online Supplementary Table S2). In contrast, the risk of non-relapse mortality was not higher in TCE-permissive or low-expression HLA-DPB1 single GvH-mismatches compared to allele matches. Importantly, TCE-permissive but not low-expression HLA-DPB1 single GvH-mismatches were associated with lower relapse risks compared to allele matches, albeit with higher GvHD. Neither permissive or nonpermissive TCE-mismatches nor high- or low-expression, were individually associated with relapse-free survival (Online Supplementary Table S2) or overall survival (data not shown).
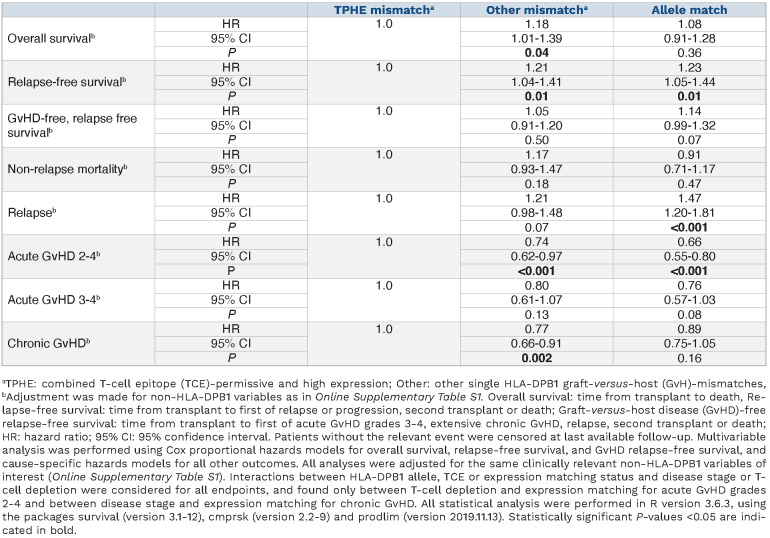
Reflecting the strong linkage disequilibrium between exon variation of HLA-DPB1 determining TCE groups and the rs9277534 single nucleotide polymorphism determining expression levels,14 there was some overlap between the TCE-model and the expression-model, with 65% of single HLA-DPB1 GvH-mismatches being TCE-nonpermissive and high expression (685/2,745, 25%) or TCE-permissive and low expression (1,100/2,745, 40%) (Online Supplementary Figure S1A). In contrast, the remaining 35% of single HLA-DPB1 GvH-mismatches were TCE-nonpermissive and low-expression (363/2,745, 13.3%) or TCE-permissive and high expression (TPHE; 597/2,745, 21.7%). We developed and tested the novel hypothesis that the latter group might identify pairs with best outcomes, given that both TCE-permissive and high-expression mismatches were individually associated with reduced relapse risks, but at the same time, TCE-permissive pairs did not have increased risks of non-relapse mortality (Online Supplementary Table S2). Univariable associations with relapse-free survival were better for TPHE-mismatches than for non-TPHE-mismatches or allele-matches, reflecting lower non-relapse mortality and lower relapse (Figure 1, Online Supplementary Figure S1B). Multivariable analysis adjusted for non-HLA-DPB1 factors (Online Supplementary Table S1) confirmed the association between non-TPHE mismatches or allele matches with lower relapse-free survival, compared to TPHE mismatches (hazard ratio [HR]=1.21, 95% confidence interval [95% CI]: 1.04-1.41, P=0.01) (Table 2). With TPHE mismatches as the reference, overall survival was worse for non-TPHE mismatches but not for allele matches (HR=1.18, 95% CI: 1.01-1.39, P=0.04), while there were no differences in GRFS (Table 2). To confirm the observation that the association of high-expression mismatches with acute GvHD and non-relapse mortality was dependent on the TCE status, we performed interaction analyses. We found a significant interaction between high expression and TCE permissiveness in opposing directions for acute GvHD (interaction HR=0.62; 95% CI: 0.45-0.85, P=0.003) and non-relapse mortality (interaction HR=1.64; 95% CI: 1.09-2.48, P=0.018), with lower mortality associated with TCE permissiveness in the high-expression group despite increased acute GvHD.
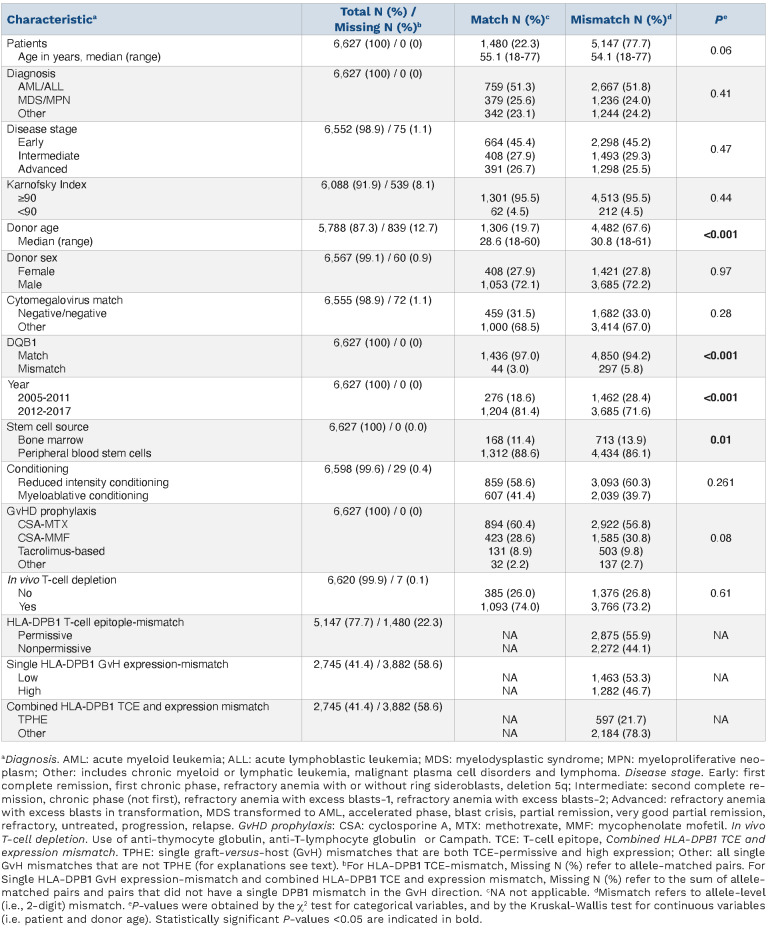
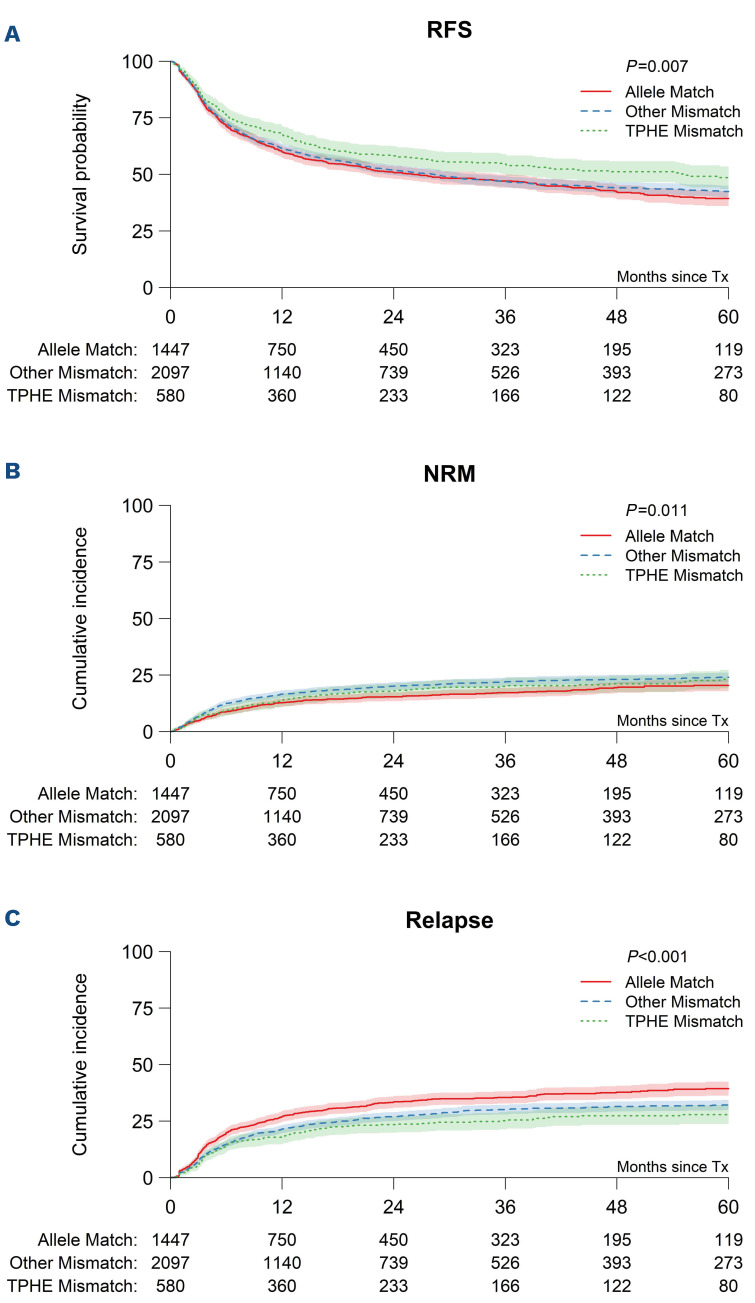
Figure 1.
Univariable associations between combined HLA-DPB1 T-cell epitope and expression matching and outcome afer 8/8 HLA-matched unrelated donor hematopoietic cell transplantation. Five-year outcome probabilities (A,B) or (C) 5-year cumulative incidence are shown for HLA-DPB1 T-cell epitope (TCE)-permissive and high expression (TPHE) mismatches, other (i.e. non-TPHE) mismatches or allele matches. (A) Relapse-free survival (RFS) (49% [95% CI: 44-53%], 42% [95% CI: 40-45%] or 39% [95% CI: 36-43%], P=0.007), (B) non-relapse mortality (NRM) (23% [95% CI: 19-27%], 24% [95% CI: 22-26%], 20% [95% CI: 18-23%], P=0.01), (C) relapse (28% [95% CI: 24-32%], 32% [95% CI: 30-34%], 39% [95% CI: 36-42%], P<0.001). Overall survival and relapse-free survival were assessed using the Kaplan-Meier (KM) method, with reverse KM for determination of median follow-up. Univariable comparisons were performed using the log-rank test. Outcomes with competing risks were assessed using cumulative incidence curves and univariable comparisons for these outcomes were performed using the Gray test. For each of relapse, acute GvHD, and chronic GvHD, death without the event of interest was a competing event, as was second transplantation.
Our study is the first to investigate the combined effects of the biological HLA-DPB1 TCE and expression models in HLA-8/8-matched transplantation for association with survival. The results provide a synthesis of both biological algorithms into a new and integrative model for intelligent mismatching in UD-HCT. Mechanistically, HLA-DPB1 TCE permissiveness reflects limited immunopeptidome diver gence between mismatched allotypes, constraining the number and diversity of the alloreactive T-cell receptor repertoire.5 HLA class II expression, on the other hand, is a hallmark of different hematologic malignancies, including acute myeloid leukemia, in which its downregulation is an established mechanism of immune escape after allogeneic HCT.6,7 The favorable associations between HLA-DPB1 TPHE mismatches and survival observed in this study suggest that the GvL effect mediated by limited T-cell alloreactivity in the TCE-permissive setting might be most effective when HLA-DP expression levels on residual malignant cells are high. Concordantly, we found that in the high-expression group, the mean (± standard deviation) immunopeptidome overlap of the informative mismatched HLA-DPB1 alleles was 11% (± 13%) for TCE-permissive pairs, and 1.9% (± 0.8%) for TCE-nonpermissive pairs (P<0.0001 in the 2-tailed Mann-Whitney test). It should be noted that due to the aforementioned linkage disequilibrium within the HLA-DPB1 locus, TPHE mismatches mostly involve certain HLA-DP allotypes carrying the DEAV motif in the patient but not in the donor, whose immunoepepti-domes have only partly been explored.5,15 Specific immunopeptidome characterization of the relevant TPHE mismatches will help to improve our understanding of the mechanisms underlying alloreactive T-cell-mediated GvL effects.
Table 2.
Multivariable analysis of associations between combined HLA-DPB1 T-cell epitope and expression status and clinical endpoints.
The present study confirmed previously described associations of HLA-DPB1 allele mismatches with relapse, and of the individual TCE and expression models with non-relapse mortality3,8,12,13 and acute GvHD grade 2-4,4,9,11 but also revealed some differences. Overall survival was not improved for TCE-permissive mismatches, and single HLA-DPB1 high-expression mismatches were not associated with severe acute GvHD grade 3-4 (data not shown). Patients in our study received more reduced-intensity/non-ablative conditioning regimens and peripheral blood stem cell products with T-cell depletion, compared to previous cohorts.3,4,11 These transplant characteristics might explain why the GvL advantage for TPHE-mismatches was not negated by GvHD toxicity, resulting in the observed survival benefits in the current study.
Our study has limitations related to its retrospective nature and its current applicability only to patients with single GvH mismatches, resulting in a relatively limited number of patients in the TPHE subset of interest. Further studies are needed to explore the possibility that our observations apply to the entire set of HLA-DPB1 mismatched patients. Moreover and importantly, confirmation of our findings in additional, independent cohorts is clearly warranted. Nevertheless, our data suggest that the combined consideration of two biological mechanisms of T-cell alloreactivity, TCE and expression, can teach us new lessons regarding the mechanisms underlying cellular immunotherapy of malignant blood disorders, and help to improve survival after UD-HCT.
Supplementary Material
Appendix: EBMT members contributing to this study
Austria: Johannes Clausen (Elisabethinen-Hospital, Linz); Wolfgang Holter (St. Anna Kinderspital, Vienna); Peter Kalhs (Medizinische Universität Wien, Vienna). Belgium: Yves Beguin (University of Liege, Liege); Dominique Bron (Institut Jules Bordet, Brussels); Dries Deeren (AZ Delta, Roeselare); Wu Ka Lung (ZNA, Antwerp); Tessa Kerre (Ghent University Hospital, Gent); Xavier Poiré (Cliniques Universitaires St. Luc, Brussels); Dominik Selleslag (A.Z. Sint-Jan, Brugge); Wilfried Schroyens (Antwerp University Hospital (UZA), Antwerp Edegem). Brazil: Vanderson Rocha (Hospital Sirio-Libanes, Sao Paulo). Czech Republic: Pavel Jindra (Charles University Hospital, Pilsen); Jiri Mayer (University Hospital Brno, Brno); Jan Vydra (Institute of Hematology and Blood Transfusion, Prague); Pavel Zák (Charles University Hospital, Hradec Kralove). Denmark: Bendt Nielsen (University Department of Hematology, Aarhus); Henrik Sengeloev (Bone Marrow Transplant Unit L 4043, Copenhagen). Estonia: Ain Kaare (Tartu University Hospital, Tartu). Finland: Maija Itäla-Remes (Turku University Hospital, Turku); Riitta Niittyvuopio (HUCH Comprehensive Cancer Center, Helsinki); Anu Partanen (Kuopio University Hospital, Kuopio); Juha Peräsaari (Clinical Laboratory Services, Histocompatibility Testing, Finnish Red Cross Blood Service, Helsinki). France: Ibrahim Yakoub-Agha (CHU de Lille, Lille); Jacques-Olivier Bay (CHU Estaing, Clermont Ferrand); Yves Bertrand (Institut d`Hematologie et d`Oncologie Pediatrique, Lyon); Didier Blaise (Programme de Transplantation&Therapie Cellulaire, Marseille); Jean Henri Bourhis (Gustave Roussy Cancer Campus, Villejuif); Claude-Eric Bulabois (CHU Grenoble Alpes -Université Grenoble Alpes, Grenoble); Christian Chabanon (Institut Paoli-Calmettes, Marseille); Patrice Chevallier (CHU Nantes, Nantes); Thomas Cluzeau (CHU Nice - Hôpital de L`Archet, Nice); Gandhi Damaj (CHU Caen, Caen); Eric Deconinck (Hopital Jean Minjoz, Besancon); Valérie Dubois (Histocompatibility Laboratory, EFS Lyon); Nathalie Fegueux (CHU Lapeyronie, Montpellier); Edouard Forcade (CHU Bordeaux, Pessac); Virginie Gandemer (Centre Hospitalier Universitaire de Rennes, Rennes); Denis Guyotat (Institut de Cancerologie Lucien Neuwirth, Saint Etienne); Mathilde HunaultBerger (CHRU, Angers); Anne Huynh (CHU - Institut Universitaire du Cancer Toulouse, Toulouse); Hélène Labussière-Wallet (Centre Hospitalier Lyon Sud, Lyon); Xavier Leleu (Hopital La Miletrie, Poitiers); Bruno Lioure (Techniciens d`Etude Clinique Suivi de Patients Greffes, Strasbourg); Sebastien Maury (Hôpital Henri Mondor, Creteil); Gérard Michel (Hopital d`Enfants de la Timone, CHU, Marseille); Mohamad Mohty (Hopital Saint Antoine, Paris); Marie Thérèse Rubio (CHRU Brabois, Nancy); Gerard Socié (Hôpital St. Louis, Paris); Herve Tilly (Centre Henri Becquerel, Rouen); Pascal Turlure (CHRU Limoges, Limoges); Germany: Wolfgang Bethge (Universität Tübingen, Tübingen); Jochen Casper (Klinikum Oldenburg, Oldenburg); Pietro Crivello (University Hospital Essen, Essen); Hermann Einsele (Universitätsklinikum Würzburg, Würzburg); Katharina Fleischhauer (University Hospital Essen, Essen); Arnold Ganser (Hannover Medical School, Hannover); Nicolaus Kröger (University Hospital Eppendorf, Hamburg); Sonja Martin (Robert Bosch Krankenhaus, Stuttgart); Carlheinz Müller (German Donor Registry, Ulm); Uwe Platzbecker (Medical Clinic and Policinic 1, Leipzig); Christian Reinhardt (University Hospital Essen, Essen); Kerstin Schäfer-Eckart (Klinikum Nürnberg, Nuünberg); Lorenz Thurner (University of Saarland, Homburg); Thomas Valerius (University Medical Center Schleswig-Holstein, Campus Kiel, Kiel); Gerald. G. Wulf (Universitätsklinikum Göttingen, Göttingen). Greece: Dimitrios Karakasis (Evangelismos Hospital, Athens); Alexandros Spyridonidis (University Hospital of Patras, Patras). Hungary: Peter Hauser (Borsod-Abaúj- Zemplén County Central Hospital, Miskolc); Péter Reményi (Dél-pesti Centrumkórház, Budapest). Iceland: Sigrun Reykdal (Landspitali University Hospital, Reykjavik). Iran: Ashrafsadat Mousavi (Shariati Hospital, Teheran). Italy: Emanuele Angelucci (Ospedale San Martino, Genova); William Arcese (Tor Vergata¨ University of Rome, Rome); Fabio Benedetti (Policlinico G.B. Rossi, Verona); Paolo Bernasconi (BMT Unit, Pavia); Andrea Biondi (Centro Trapianti di Midollo Osseo, Monza); Francesca Bonifazi (Bologna University, S.Orsola-Malpighi Hospital, Bologna); Chiara Bonini (Ospedale San Raffaele s.r.l., Milano); Angelo Michele Carella (IRCCS, Casa Sollievo della Sofferenza, S. Giovanni Rotondo); Paola Carluccio (U.O. Ematologia con Trapianto, Bari); Marco Casini (Hospital San Maurizio, Bolzano); Luigi Cavanna (Hospital Guglielmo da Saliceto, Piacenza); Fabio Ciceri (Ospedale San Raffaele s.r.l., Milano); Giuseppe Cimino (Ospedale Santa Maria Goretti, Latina); Paolo Corradini (University of Milano, Milano); Renato Fanin (Azienda Ospedaliero Universitaria di Udine, Udine); Piero Galieni (Mazzoni Hospital, Ascoli Piceno); Giovanni Grillo (ASST Grande Ospedale Metropolitano Niguarda, Milano); Anna Paola Iori (University La Sapienza, Rome); Giorgio La Nasa (Centro Trapianti Unico Di CSE Adulti e Pediatrico A. O Brotzu, Cagliari); Franco Locatelli (IRRCS Ospedale Pediatrico Bambino Gesù, Rome); Giuseppe Marotta (U.O.S.A Centro Trapianti e Terapia Cellulare, Siena); Massimo Martino (Grande Ospedale Metropolitano Bianchi Melacrino Morelli - Centro Unico Trapianti A. Neri, Reggio Calabria); Patrizio Mazza (Ospedale Nord, Taranto); Nicola Mordini (Az. Ospedaliera S. Croce e Carle, Cuneo); Maurizio Musso (Ospedale La Maddalena - Dpt. Oncologico, Palermo); Attilio Olivieri (Azienda Ospedali Riuniti di Ancona, Ancona); Vincenzo Pavone (Hospital C. Panico, Tricase Lecce); Fabrizio Pane (University of Napoli, Napoli); Mario Petrini (Azienda Ospedaliero Universitaria Pisana, Pisa); Pietro Pioltelli (Ospedale San Gerardo, Monza); Alessandro Rambaldi (ASST Papa Giovanni XXIII, Bergamo); Annalisa Ruggeri (Ospedale San Raffaele s.r.l., Milano); Marco Ruggeri (S. Bortolo Hospital, Vicenza); Riccardo Saccardi (Azienda Ospedaliera Universitaria Careggi, Firenze); Stella Santarone (Ospedale Civile, Pescara); Rosanna Scimè (U.O.D Trapianti di Midollo Osseo, Palermo); Simona Sica (Universita Cattolica S. Cuore, Rome); Corrado Tarella (European Institute of Oncology, Milano); Luca Vago (Ospedale San Raffaele s.r.l., Milano); Andrea Velardi (Sezione di Ematologia, Perugia); Giuseppe Visani (AORMN Hospital, Pesaro); Marco Zecca (Fondazione IRCCS Policlinico San Matteo, Pavia). Norway: Tobias Gedde-Dahl,Oslo University Hospital, Rikshospitalet, Oslo). Romania: Alina Tanase (Fundeni Clinical Institute, Bucharest). Russia: Aleksandr Kulagin (First State Pavlov Medical University of St. Petersburg, St. Petersburg); Valery Savchenko (National Research Center for Hematology, Moscow). Spain: Carmen Albo López (Hospital Álvaro Cunqueiro - Complejo Hospitalario Universitario de Vigo, Vigo); Adrián Alegre Amor (Hospital de la Princesa, Madrid); Jose Luis Bello López (Hospital Clinico Universitario, S. De Compostela); Dolores Caballero (Hospital Clínico, Salamanca); Rafael Duarte (Clinica Puerta de Hierro, Madrid); Maria Jesús Pascual Cascon (Hospital Regional de Málaga, Malaga); Rocio Parody Porras (ICO – Hospital Duran i Reynals, Barcelona); Jose Antonio Pérez-Simón (Hospital Universitario Virgen del Rocío, Sevilla); Montserrat Rovira (Hospital Clinic, Barcelona); Jaime Sanz (University Hospital La Fe, Valencia); Juan Pio Torres Carrete (Complejo Hospitalario de A Coruña, La Coruna). Sweden: Mats Bengtson (Department of Immunology, Genetics and Pathology, Uppsala University, Uppsala); Jörg Cammenga (University Hospital, Linkoeping); Cecilia Isaksson (Umea University Hospital, Umea); Jan-Erik Johansson (Sahlgrenska University Hospital, Goeteborg); Stig Lenhoff (Skanes University Hospital, Lund); Stephan Mielke (Karolinska University Hospital, Stockholm). Switzerland: Yves Chalandon (Département d`Oncologie, Service d`Hématologie, Geneva); Jakob Passweg (University Hospital, Basel); Urs Schanz (University Hospital, Zürich). The Netherlands: Edouard F. Bonneville (Leiden University Medical Center, Leiden); Goda Choi (University Medical Center Groningen, Groningen); Jan J. Cornelissen (Erasmus MC Cancer Institute, Rotterdam); Liesbeth C. de Wreede (Leiden University Medical Center, Leiden); Jorinde D. Hoogenboom (EBMT Leiden Study Unit, Leiden); Ellen Meijer (VU University Medical Center, Amsterdam); Jürgen Kuball (University Medical Centre, Utrecht); Gwendolyn van Gorkom (University Hospital Maastricht, Maastricht); Joan Hendrik Veelken (Leiden University Hospital, Leiden); Lotte Wieten (University Hospital Maastricht, Maastricht). United Kingdom: Jane Apperley (Imperial College, London); Adrian Bloor (Christie NHS Trust Hospital, Manchester); Jenny Byrne (Nottingham University, Nottingham); Ben Carpenter (University College London Hospital, London); Andrew Clark (Bone Marrow Transplant Unit, Glasgow); Matthew Collin (Adult HSCT Unit, Newcastle upon Tyne); Charles Craddock (University Hospital Birmingham NHS Trust, Birmingham); Charles R. Crawley (Addenbrookes Hospital, Cambridge); Brenda E. Gibson (Royal Hospital for Children, Glasgow); Anjum Khan (Yorkshire Blood & Marrow Transplant Programme, Leeds); Steven G.E. Marsh (Immunogenetics Research Laboratory, Anthony Nolan Research Institute, Royal Free Hospital, London); Murray Martin (Leicester Royal Infirmary, Leicester); Patrick Medd (University Hospitals Plymouth NHS Trust, Plymouth); Emma Nicholson (Royal Marsden Hospital, London); Kim Orchard (Southampton General Hospital, Southampton); Amit Patel (Clatterbridge Cancer Centre - Liverpool, Royal Liverpool University Hospital, Liverpool); Andy Peniket (Department of Haematology, Oxford); Victoria Potter (Kings College Hospital, London); John Snowden (Sheffield Teaching Hospitals NHS Trust, Sheffield); Eleni Tholouli (Manchester Royal Infirmary, Manchester); Keith M. O. Wilson, (Department of Haematology, Cardiff). USA: Effie W. Petersdorf (Fred Hutchinson Cancer Research Center, Seattle).
Funding Statement
Funding: This work was supported by grants from the Deutsche Forschungsgemeinschaft (DFG FL 843/1-1), the Deutsche José Carreras Leukämie Stiftung (DJCLS R 15-02; DJCLS 20R/2019), and the Joseph-Senker Stiftung to KF, and the DKMS (DKMS-SLS-MHG-2018-01) to PC.
References
- 1.Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354(17):1813-1826. [DOI] [PubMed] [Google Scholar]
- 2.Fleischhauer K, Shaw BE. HLA-DP in unrelated hematopoietic cell transplantation revisited: challenges and opportunities. Blood. 2017;130(9):1089-1096. [DOI] [PubMed] [Google Scholar]
- 3.Fleischhauer K, Shaw BE, Gooley T, et al. Effect of T-cell-epitope matching at HLA-DPB1 in recipients of unrelated-donor haemopoietic-cell transplantation: a retrospective study. Lancet Oncol. 2012;13(4):366-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petersdorf EW, Malkki M, O'Huigin C, et al. High HLA-DP expression and graft-versus-host disease. N Engl J Med. 2015;373(7):599-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meurer T, Crivello P, Metzing M, et al. Permissive HLA-DPB1 mismatches in HCT depend on immunopeptidome divergence and editing by HLA-DM. Blood. 2021;137(7):923-928. [DOI] [PubMed] [Google Scholar]
- 6.Toffalori C, Zito L, Gambacorta V, et al. Immune signature drives leukemia escape and relapse after hematopoietic cell transplantation. Nat Med. 2019;25(4):603-611. [DOI] [PubMed] [Google Scholar]
- 7.Christopher MJ, Petti AA, Rettig MP, et al. Immune escape of relapsed AML cells after allogeneic transplantation. N Engl J Med. 2018;379(24):2330-2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pidala J, Lee SJ, Ahn KW, et al. Nonpermissive HLA-DPB1 mismatch increases mortality after myeloablative unrelated allogeneic hematopoietic cell transplantation. Blood. 2014;124(16):2596-2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morishima S, Shiina T, Suzuki S, et al. Evolutionary basis of HLA-DPB1 alleles affects acute GVHD in unrelated donor stem cell transplantation. Blood. 2018;131(7):808-817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lorentino F, Sacchi N, Oldani E, et al. Comparative evaluation of biological HLA-DPB1 mismatch models for survival and graft versus host disease prediction after unrelated donor hematopoietic cell transplantation. Haematologica. 2020;105(4):e-186-e189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petersdorf EW, Bengtsson M, De Santis D, et al. Role of HLA-DP expression in graft-versus-host disease after unrelated donor transplantation. J Clin Oncol. 2020;38(24):2712-2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mytilineos D, Tsamadou C, Neuchel C, et al. The human leukocyte antigen-DPB1 degree of compatibility is determined by its expression level and mismatch permissiveness: a German multicenter analysis. Front Immunol. 2020;11:614976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buhler S, Baldomero H, Ferrari-Lacraz S, et al. Analysis of biological models to predict clinical outcomes based on HLA-DPB1 disparities in unrelated transplantation. Blood Adv. 2021;5(17):3377-3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fleischhauer K. Immunogenetics of HLA-DP--a new view of permissible mismatches. N Engl J Med. 2015;373(7):669-672. [DOI] [PubMed] [Google Scholar]
- 15.van Balen P, Kester MGD, de Klerk W, et al. Immunopeptidome analysis of HLA-DPB1 allelic variants reveals new functional hierarchies. J Immunol. 2020;204(12):3273-3282. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.