Abstract
With developments in synthetic biology, “engineering biology” has emerged through standardization and platformization based on hierarchical, orthogonal, and modularized biological systems. Genome engineering is necessary to manufacture and design synthetic cells with desired functions by using bioparts obtained from sequence databases. Among various tools, the CRISPR-Cas system is modularly composed of guide RNA and Cas nuclease; therefore, it is convenient for editing the genome freely. Recently, various strategies have been developed to accurately edit the genome at a single nucleotide level. Furthermore, CRISPR-Cas technology has been extended to molecular diagnostics for nucleic acids and detection of pathogens, including disease-causing viruses. Moreover, CRISPR technology, which can precisely control the expression of specific genes in cells, is evolving to find the target of metabolic biotechnology. In this review, we summarize the status of various CRISPR technologies that can be applied to synthetic biology and discuss the development of synthetic biology combined with CRISPR technology in microbiology.
Keywords: CRISPR-Cas technologies, Synthetic biology, Microbiology
Introduction
Advances in biotechnology are essential for sustainable human life to solve various problems caused by population growth in the fields of environment, food, energy, and health care. Synthetic biology has developed remarkably to maximize the utilization of biological systems through standardization and platformization (Purnick & Weiss, 2009). Life systems replicate and grow as programmed in the genome; they can also provide the intended values. Therefore, a new genome should be efficiently constructed for a desired purpose. With the development of sequencing technology, the nucleotide sequences that make up and program living organisms have been revealed tremendously, and new systems can be designed by extracting necessary parts, circuits, and pathways from the database (Chen et al., 2012; Quince et al., 2017).
Since the genetic code is universal from microorganisms to higher organisms, genes obtained from living organisms can be recombined in new cells (Ostrov et al., 2020). Based on the rapid in vitro chemical synthesis of oligonucleotides, several DNA assembly methods have been developed (Hughes & Ellington, 2017). Among them, sequence homology-based methods such as Gateway, circular polymerase extension cloning (CPEC), and Gibson assembly have been applied to develop a larger DNA assembly (Chao et al., 2015; Gibson et al., 2010; Wang et al., 2018a). A megabase-sized genome can be synthesized in cells, and a synthetic cell can be built for microbial chassis cells (Hutchison et al., 2016).
Various functions, including environmental signal sensing, intracellular signal transduction, and biochemical production, can be implemented in synthetic cells (Brophy & Voigt, 2014). Synthetic genetic circuits, such as a toggle switch (Gardner et al., 2000), an oscillator (Stricker et al., 2008), feedback loops (Dahl et al., 2013), and a Boolean logic gate (Green et al., 2017), can control cell performance and behavior. Moreover, microorganisms are reprogrammed to diagnose and treat diseases (Riglar & Silver, 2018) and can be utilized as biosensors capable of detecting metabolites, enzyme products, and harmful substances (Kim et al., 2016a). In addition, reprogrammed microorganisms are used to produce desired substances from synthetic cells in the fields of bioenergy, chemistry, and medicine (Nielsen & Keasling, 2016). For example, the biosynthetic pathway from Artemisia annua L. (sweet wormwood) can be transplanted to Saccharomyces cerevisiae to produce artemisinic acid, an antimalarial drug precursor in microbial cell factories (Ro et al., 2006).
Synthetic biology tools have been developed to design and optimize living systems (Leon-Buitimea et al., 2022). Among them, CRISPR-Cas, an adaptive immune system of microorganisms, has been developed as a genome editing technology (Jinek et al., 2012). CRISPR gene scissors are divided into two modules: nucleolytic protein and target recognition RNA; therefore, they can be used and applied more synthetic-biologically than any other tool (Clarke et al., 2021). Unlike restriction enzymes, any target sequence can be easily and freely designed, and CRISPR-Cas can operate from bacteria to higher organisms.
In microbiology, CRISPR technology has advanced in the following directions. CRISPR genome editing tools have been developed in various microorganisms (Lee & Lee, 2021). The function of recognizing specific sequences in CRISPR-Cas has been expanded and applied to molecular diagnosis (Kim et al., 2021). In addition, with nucleolytic activity-free Cas proteins, gene expression and biosynthetic circuits in synthetic cells can be precisely regulated (Santos-Moreno & Schaerli, 2020). Here, we review the latest trends in CRISPR technology, an essential tool for the development of synthetic biology, and summarize how it is applied to microbiology. We also discuss the prospects for the development of CRISPR-Cas technologies in microbiology.
CRISPR-Mediated Genome Editing
The CRISPR-Cas system, which exhibits adaptive immunity in microorganisms, is modularized with single guide RNA (sgRNA) that recognizes nucleic acid targets and Cas protein that causes cleavage (Mali et al., 2013). The target nucleotide sequence can be freely modified on the basis of the change in the RNA sequence; it is also used as a genome editing tool in many organisms, including bacteria and yeasts. Studies on genome editing with CRISPR-Cas in various microorganisms, such as archaea, bacteria, and yeasts, are summarized in Table 1.
Table 1.
CRISPR-mediated genome editing in microorganisms
| Organism | Cas protein | Species | Donor DNA | Types of edits (bp) | Feature | References |
|---|---|---|---|---|---|---|
| Archaea | Cas9 | Methanosarcina acetivorans |
Plasmid None* |
D (34–2624) I (2526 and 3045) |
Multiple two different gene deletion using single plasmid harboring four sgRNAs | Nayak and Metcalf (2017) |
| Sulfolobus islandicus | Plasmid |
D (442) I (18) S (9) |
X-Gal blue-white colony screening | Li et al. (2016b) | ||
| Bacteria | Cas9 | Bacillus subtilis | Plasmid |
D (4100–25,000) S (3) |
PAM substitution | Altenbuchner (2016) |
| Clostridium acetobutylicum | Plasmid |
D (306) I (2985) S (2) |
Two-plasmid system (Cas9 plasmid, sgRNA + donor DNA plasmid) | Wasels et al. (2017) | ||
| Escherichia coli | Oligo | S (1) | Target-mismatched sgRNA | Lee et al. (2020a) | ||
| E. coli | Oligo |
D (1) I (1) S (1) |
5′-truncated sgRNA | Lee et al. (2021) | ||
| nCas9 | B. licheniformis | Plasmid |
D (997–40,909) I (1335) |
Simultaneous multiple-gene disruption | Li et al. (2018a) | |
| B. subtilis | Plasmid |
D (1000–20,500) I (1000–2000) S (1) |
Improving editing efficiency by using a ligD-deficient strain | Liu et al. (2019a) | ||
| C. beijerinckii | Plasmid | D (20–1149) | All-in-one plasmid (nCas9, sgRNA, and donor DNA) | Li et al. (2016a) | ||
| Cas12a | Corynebacterium glutamicum | Oligo | S (1) | Target-mismatched crRNA | Kim et al. (2020c) | |
| E. coli | Oligo |
D (1) I (1) S (1) |
3′-truncated crRNA | Lee et al. (2022b) | ||
| Mycobacterium smegmatis | Oligo |
D (1) I (1) S (2) |
gp60, gp61-mediated ssDNA recombineering, X-Gal blue-white colony screening | Yan et al. (2017) | ||
| PCR product |
D (2–4000) I (642–1000) |
|||||
| Cas12f1 | B. anthracis | Plasmid | D (1242–14,600) | Co-expressing AsCas12f1 and I-SceI nuclease | Wang et al. (2022c) | |
| E. coli | Plasmid | D (891–2145) | Okano et al. (2021) | |||
| Fungi | Cas9 | Aspergillus fumigatus | None |
D (1) I (219) |
Verification of editing efficiency with albino phenotype | Fuller et al. (2015) |
| Penicillium chrysogenum | PCR product |
D (1275–23,439) I (1449–3170) |
Genome editing using preassembled sgRNA/Cas9 ribonucleoprotein | Pohl et al. (2016) | ||
| Trichoderma reesei | PCR product | D (2,244) | Multiple gene deletion using separate gRNA and donor DNA plasmids | Liu et al. (2015) | ||
| Cas12a | A. aculeatus | None |
D (7) I (2) |
crRNA-flanking tRNA fusion transcript with U3 Pol III promoter |
Abdulrachman et al. (2021) | |
| A. nidulans |
Oligo PCR product |
I (6) I (678) |
Marker-free genome editing with NHEJ-deficient strain | Vanegas et al. (2019) | ||
| Yeast | Cas9 | Kluyveromyces lactis | PCR | I (3574) | Multiplexed gene integration by single plasmid harboring multiple sgRNA | Horwitz et al. (2015) |
| Pichia pastoris | PCR | D (100 ~ 1800) | ribozyme strategy for gRNA expression with self-splicing RNA element | Weninger et al. (2016) | ||
| Scheffersomyces stipitis | PCR | D (1,463) | Elimination of the NHEJ mechanism by ku70/ku80 genes to improve homologous recombination | Cao et al. (2018) | ||
| Yarrowia lipolytica | PCR | I (1026) | Expression of sgRNA with tRNA-based synthetic promoters | Schwartz et al. (2016) | ||
| Cas12a | Saccharomyces cerevisiae | PCR | I (8429) | Evaluation of editing efficiency using three Cpf1 orthologues | Verwaal et al. (2018) | |
| Schizosaccharomyces pombe | PCR | D (1659) | Multiple crRNA array using a strong constitutive pol II promoter | Zhao and Boeke (2020) | ||
| Y. lipolytica | None | D (2) | Improving editing efficiency by addition of poly-thymidine to the 3ʹ-end of crRNA | Yang et al. (2020) |
*NHEJ-mediated genome editing
CRISPR-Cas9-mediated gene editing was first reported in Escherichia coli among microorganisms (Jiang et al., 2013). In Staphylococcus aureus, gene deletion, insertion, and base substitution are performed using a single plasmid containing Cas9, sgRNA, λ-Red recombinase, and donor DNA (Chen et al., 2017). The CRISPR-Cas9 system is also used in Trichoderma reesei, a filamentous fungus, and a relatively high homologous recombination efficiency (> 93%) is achieved when the length of the donor DNA homology arm is 200 bp (Liu et al., 2015). Genes are edited with > 70% efficiency in Streptomyces by using a single plasmid containing Cas9, sgRNAs, and donor templates (Cobb et al., 2015). Up to three heterologous genes are simultaneously inserted into various regions of the E. coli genome by using CRISPR-Cpf1 and λ-Red recombinase (Ao et al., 2018).
Recently, Cas12f1, which has a relatively smaller gene than Cas9 and Cpf1, was discovered from metagenomic data (Harrington et al., 2018); studies have reported gene deletion in E. coli and Bacillus anthracis by using CRISPR-Cas12f1 (Okano et al., 2021; Wang et al., 2022c). In addition, new class of RNA-guided nucleases such as IscB and TnpB were discovered by exploring the evolutionary origin of Cas9 and Cpf1 nucleases (Karvelis et al., 2021; Schuler et al., 2022). IscB and TnpB, which are considered ancestors of Cas9 and Cas12 nucleases, have lower editing efficiency. However, since the protein size is small, it has the advantage of being used for gene therapy.
Target Sequence Identification
As CRISPR-mediated genome editing was studied, an off-target effect of cutting similar sequences outside the target was observed in the eukaryotic system with high genome complexity. In some cases, cleavage occurs at undesired locations in the genome, and this process has been recognized as an obstacle to editing (Lin et al., 2014). In order to solve this problem in eukaryotic cells, studies have been conducted to increase target specificity and editing efficiency by engineering guide RNA or Cas nuclease.
The chemical modification of the crRNA terminus of Cpf1, including methylation and fluorination, improves crRNA stability and editing efficiency (McMahon et al., 2018). 2′-O-methyl-3′-phosphonoacetate modification of ribose in crRNA improves Cas9 function and target specificity in some cases (Ryan et al., 2018). The extension of the 5ʹ end of crRNA in Cpf1 enhances the efficiency of genome editing such as gene knockout and homology-directed repair (Park et al., 2018a), and uridylation of the 3ʹ end improves the efficiency of indel editing by using Cpf1 (Moon et al., 2018). When a part of the spacer in crRNA is replaced with DNA, the editing efficiency is improved and the off-target effect is reduced by changing the binding energy to the target (Kim et al., 2020b).
Cas protein engineering has been reported as another strategy to improve target specificity. For example, on-target activity can be improved by fusing a chromatin-modulating peptide with Cas9 (Ding et al., 2019). In another study, FokI nuclease is fused with deactivated Cas9 (dCas9) to form nicks at different positions and strands, thereby reducing the off-target effect (Ding et al., 2019; Guilinger et al., 2014).
Cas9 has a PAM strand-specific RuvC nuclease domain and a target strand-specific HNH nuclease domain (Jinek et al., 2012). When Asp10 or His840 (the catalytic residues of RuvC and HNH domains, respectively) is substituted with Ala, Cas9 nickase (nCas9), which cleaves a single strand of target DNA, can be generated (Cong et al., 2013; Gasiunas et al., 2012). The CRISPR-nCas9 (D10A) system is used to insert or delete gene cassettes of 1 kb or less with an editing efficiency of up to 100% in Lactobacillus casei (Song et al., 2017). Since nCas9 was designed to target two adjacent sites on different strands, it has been used as a genome editing method with improved target specificity by targeting more nucleotide sequences and resulting in double-strand break (DSB) (Cho et al., 2014; Ran et al., 2013). Multiple nicks are formed at different positions and strands in E. coli to perform a final 133 kb deletion (Standage-Beier et al., 2015).
In mammalian cells, the development of technologies for detecting off-target effects remains a key challenge. Recently, strategies for detecting off-target effects have been developed. Various methods for detecting off-target effects including Web-based prediction tools, CHIP-seq, GUIDE-seq, and HTGTS have been developed and applied (Zhang et al., 2015). Recently, unwanted mutations can be avoided by profiling the off-target effect of nucleases including Cas9 through Digenome-seq (Kim et al., 2015).
The protospacer adjacent motif (PAM) sequence is located near the target DNA and helps the bacterial adaptive immune CRISPR-Cas system to discriminate between self- and non-self-target sequences (Marraffini & Sontheimer, 2010). However, PAM sequences limit the range of target sequences that Cas proteins can recognize in genome editing. Therefore, studies have been performed to relieve the restriction of the PAM sequence by engineering Cas nucleases and solve this problem. For example, a Cas9 variant (xCas9) that can recognize PAMs of different sequences, such as NG, GAA, and GAT, has been developed through phage-assisted continuous evolution [PACE; (Hu et al., 2018)]. Cas9-NG that recognizes 5ʹ-NG as a PAM sequence has also been designed by eliminating the dependence of the Cas9 protein on the third guanine of 5ʹ-NGG, thereby expanding the range of target selection (Nishimasu et al., 2018). Moreover, chimeric Cas9 produced through ortholog analysis and Cas12a (Cpf1) variant produced through structure-guided mutagenesis show the effect of PAM sequence expansion and off-target effect reduction (Kleinstiver et al., 2019; Ma et al., 2019).
Recently, RNA-guided large DNA insertion tools were developed by combining the DNA integration capability of transposases and the function of target recognition of CRISPR-Cas. Insertion of Transposable Elements by Guide RNA-Assisted Targeting (INTEGRATE) and CRISPR-associated transposase from cyanobacteria Scytonema hofmanni (ShCAST) can efficiently integrate DNA segments into the genome of E. coli (Klompe et al., 2019; Strecker et al., 2019). These technologies can insert desired genes and pathways into the genome, and thus can be used to synthesize biological systems with intended functions.
Single Nucleotide Editing
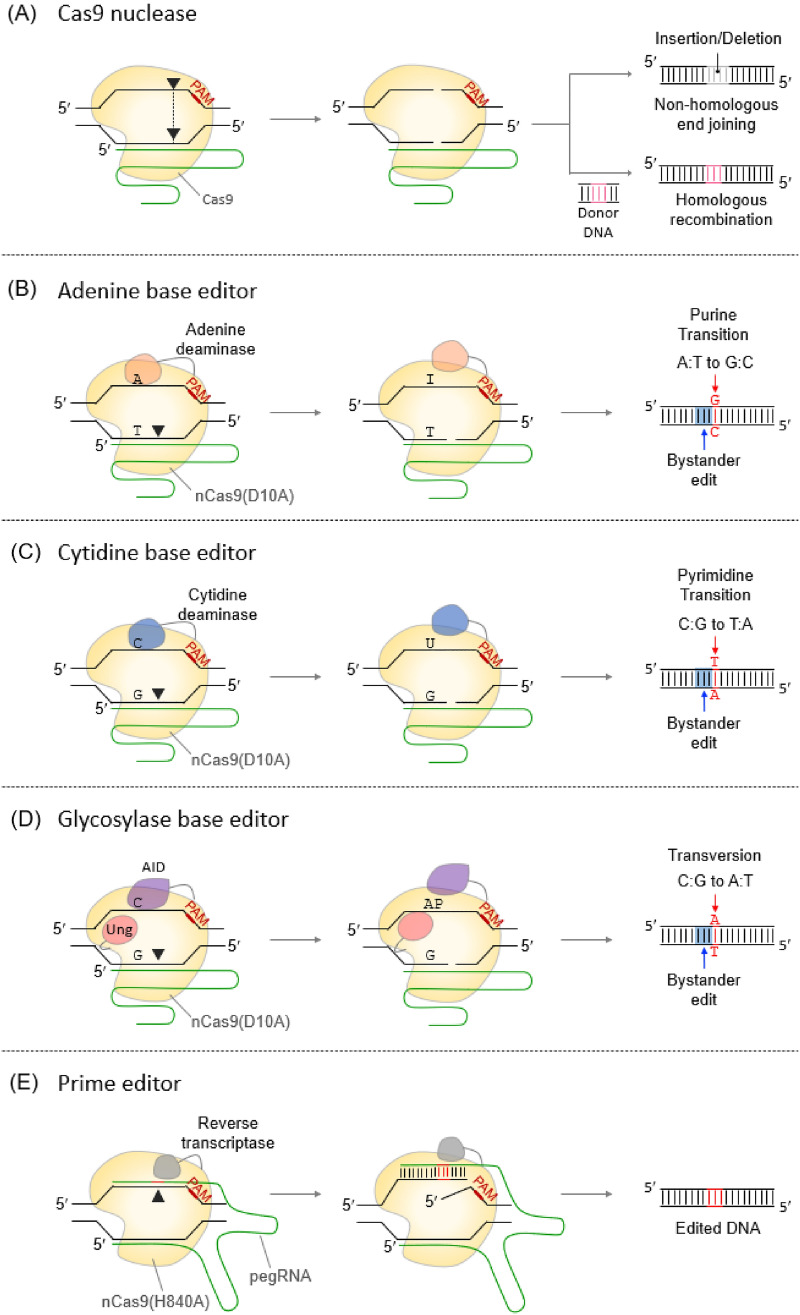
Genome editing mediated by the CRISPR-Cas system requires cleavage of two strands of a target DNA and recombination (Hsu et al., 2014; Knott & Doudna, 2018). Since double-strand breaks of a target DNA can occur even if a mismatch exists between the edited target DNA and the guide RNA, genome editing at the single nucleotide level is hardly achieved even in microbial cells with low genome complexity (Lee et al., 2020a). Several CRISPR-Cas genome editing technologies, such as base editor and prime editor, have been developed to bypass the off-target effect without double-strand breaks (Fig. 1).
Fig. 1.
CRISPR-Cas-based genome editing tools. A Cas9 nucleases create double-strand breaks in a target DNA. Indel and subsequent nonhomologous end joining inactivate the target gene. If a donor DNA is added, the target gene can be edited to the desired sequence. Filled triangles indicate cleavage sites. B Adenine base editors (ABEs), composed of an nCas9 fused with an adenine deaminase, mediate A-to-G transition. Adenine deaminase catalyzes the deamination of A to hypoxanthine (I), which is recognized as G, forming a G:C base pair. C Cytosine base editors (CBEs) convert C:G into T:A base pairs by using cytidine deaminase. D Glycosylase base editors (GBEs) mediate C-to-A transversion by using an nCas9 (D10A) fused with activation-induced cytidine deaminase (AID) and uracil DNA glycosylase (Ung). AID-nCas9-Ung binds to the target DNA and creates a nick. AID cleaves the amine group at C to form U. Ung excises the U base, forming an apurinic/apyrimidinic (AP) site that initiates DNA repair. E Prime editors are composed of nCas9, reverse transcriptase (RT), and prime editing guide RNA (pegRNA). nCas9 (H840A) cleaves the target DNA. The RT polymerizes a new DNA strand complementary to the pegRNA sequence on the nicked strand
A base editor (BE), made through the fusion of dCpf1, dCas9, and nCas9 with a base deaminase, introduces point mutations into the target DNA without DSB (Gaudelli et al., 2017; Grunewald et al., 2019; Li et al., 2018d). An adenine base editor (ABE) facilitates the conversion of A:T to G:C, and cytidine base editor (CBE) converts C:G to T:A base pairs (Gaudelli et al., 2017; Komor et al., 2016). For example, in S. aureus, ABE converts A to G in 4–8 editing windows with > 50% editing efficiency (Zhang et al., 2020). In galK of E. coli, CBE achieves point mutagenesis with an efficiency of 61%–95%, and C to T substitution is mainly induced in 17–20 bases in the upstream region of the PAM sequence (Banno et al., 2018). ABE and CBE have been applied to various organisms, including eukaryotes and some bacteria, to introduce transition point mutations (Chen et al., 2018b; Luo et al., 2020; Wang et al., 2018d). A recently developed glycosylase base editor (GBE) can mediate base transversion such as C to A and C to G. It is composed of nCas9, a cytidine deaminase, and an uracil-DNA glycosylase (Ung). It converts C to A with an average editing specificity of 93.8% in E. coli (Zhao et al., 2021). However, if these BEs are adjacent to the same base, an unwanted bystander editing effect may occur (Lee et al., 2020b).
CasMINI, half the size of Cas9 and Cas12, was engineered from natural type V-F Cas12f (Cas14) system by gRNA and protein engineering (Xu et al., 2021). Deactivated CasMINI-mediated adenine base editor (dCasMINI-ABE) showed the most efficient A to G conversion in a narrow window (3–4 bp downstream region from the PAM). TnpB-based ABE, made through the fusion of the C-terminus of dTnpB with a modified dimer of TadA adenosine deaminase, facilitates A to G conversion in the PAM-proximal region (Kim et al., 2022). The conversion efficiency of TnpB-based ABE was higher than that of Cas12f-based ABE, but still lower than that of SpCas9-based ABE.
A prime editor (PE) uses Cas9 nickase fused with reverse transcriptase and prime editing sgRNA (pegRNA). It can edit the genome through various processes, such as insertion, deletion, and point mutation, as programmed in the pegRNA sequence (Anzalone et al., 2019). PE performs 2–3 bp substitution, insertion, and deletion in the chromosomal DNA of E. coli with an efficiency of 26% (Tong et al., 2021). However, loading a PE system is slightly difficult in a size-limited vector because of the large size of the construct consisting of an nCas protein, a reverse transcriptase, and pegRNA (Arroyo-Olarte et al., 2021).
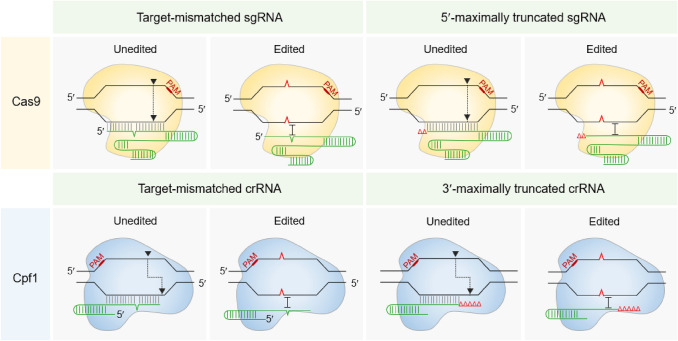
Accurate genome editing with Cas dsDNA nuclease and target-mismatched and truncated guide RNAs has also been developed (Lee & Lee, 2021) (Fig. 2). Target-mismatched guide RNAs of Cas9 and Cpf1 can discriminate between a single-nucleotide-edited target and an unedited target and efficiently edit a single nucleotide in the genomes of E. coli and Corynebacterium glutamicum, respectively (Kim et al., 2020c; Lee et al., 2020a). Furthermore, a single-nucleotide in the cI857 repressor gene of the bacteriophage λ genome was accurately corrected by the aid of target-mismatched sgRNA and Cas9 complex, which restored thermostable λ lysogenic E. coli cells (Lee et al., 2022a). Cas9 with 5′-truncated sgRNA and Cpf1 with 3′-truncated crRNA improve on-target specificity and reduce off-target effect (Fu et al., 2014; Kim et al., 2017). Besides, 5ʹ-truncation of sgRNA in Cas9 and 3ʹ-truncation of crRNA in Cpf1 greatly enhance the efficiency of oligonucleotide-directed single nucleotide editing in the microbial genome (Lee et al., 2021, 2022b).
Fig. 2.
Accurate genome editing with target-mismatched or maximally truncated guide RNAs in CRISPR-Cas systems. Unedited target DNAs are cleaved by Cas nucleases (Cas9 or Cpf1) with target-mismatched or maximally truncated guide RNAs (sgRNA for Cas9, and crRNA for Cpf1). Single base/nucleotide edited targets are not cleaved by Cas nucleases with target-mismatched or maximally truncated guide RNAs. The intolerance of single-base/nucleotide mismatch between the edited target DNA and target-mismatched or maximally truncated guide RNAs enables single base/nucleotide genome editing
Nucleic Acid Diagnostics
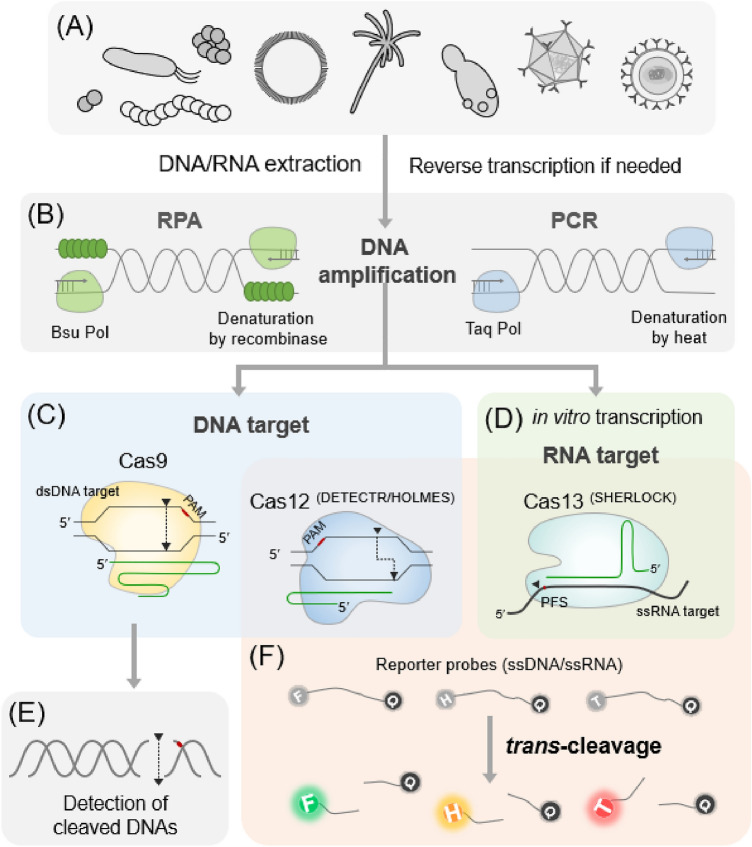
The sequence of nucleic acids characteristic of each disease-causing virus or microbial pathogen can be used as a diagnostic biomarker. The CRISPR-Cas complex can recognize and cut the target nucleic acid, thereby indicating the presence of a nucleic acid of a specific sequence in the sample (Fig. 3). Among various Cas nucleases, Cas9, Cas12, and Cas13, which belong to Class 2 whose effector complex is a single polypeptide, are mainly used to develop nucleic acid sensors (Liu et al., 2022c).
Fig. 3.
Nucleic acid diagnostics via CRISPR-Cas systems. Schematic of the operating principle of a CRISPR-based diagnostic system is shown. A Depending on the target gene to be detected, DNA or RNA is extracted from pathogens such as viruses, bacteria, fungi, and yeasts. DNA is amplified via recombinase polymerase amplification (RPA) or PCR and used for nucleic acid detection. RNA can be reversely transcribed to cDNA, and subsequent RPA or PCR can amplify the cDNA. B In RPA, double-stranded DNA denatured by recombinase is isothermally amplified through the extension of the template DNA with Bsu DNA polymerase with a strand displacement activity. In PCR, double-stranded DNA is denatured by heat, and Taq DNA polymerase with high thermal stability is used for DNA amplification. C Cas9 or Cas12 and the corresponding guide RNA complex recognizes and cleaves the amplified DNA. D For RNA-targeting CRISPR enzymes, including Cas13, the amplified DNA is transcribed into RNA via in vitro transcription. E Cas9-based diagnosis is performed by detecting the cleaved target DNA itself. F Cas12- and Cas13-based diagnoses are conducted by detecting signals released when a single-stranded DNA or RNA reporter probe is cleaved by trans-cleavage. Each letter in a circle indicates the following: F, carboxyfluorescein (green); H, hexachlorofluorescein (orange); T, Texas Red; and Q, Quencher
CRISPR-based diagnosis has the advantage of not requiring a sequencing step of target nucleic acids. However, since a sufficient amount of nucleic acids is necessary to detect a signal, DNA or RNA should be amplified. For rapid diagnosis in a single tube without equipment, DNA is amplified using isothermal reactions such as recombinase polymerase amplification (RPA) and loop-mediated isothermal amplification (LAMP).
Target Cleavage and Detection
When a specific DNA sequence is recognized as a target by the Cas9-sgRNA complex, a cleaved double-stranded target DNA is retained and can be detected in various ways (Strich & Chertow, 2019). For example, a toehold switch method has been proposed to identify Zika virus strains with CRISPR-Cas9 by designing strain variant sequences with PAM sequences. If variation exists in the viral DNA sequence, the Cas9/sgRNA complex is unable to cleave the target DNA; subsequently, the full-length trigger mRNA is transcribed to activate the toehold switch (Pardee et al., 2016). Bacterial antibiotic resistance genes are detected by designing a fluorescent probe to bind to the strand cut via the Cas9-sgRNA complex (Muller et al., 2016). Moreover, a sensitive DNA detection method is developed to detect isothermally amplified DNA by using the DNA cut by Cas9-sgRNA as a primer (Huang et al., 2018). Similarly, DNA sequence variation can be detected by inducing the target cleavage by Cas9/sgRNA in two different positions, thereby producing universal primer binding sequences at the 5ʹ-ends of the cleaved strands; this variation can also be observed by performing qPCR (Gao et al., 2021). In addition, FnCas9 Editor Linked Uniform Detection Assay (FELUDA) was developed to detect single nucleotide variants of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (Azhar et al., 2021).
With the development of deactivated Cas9 (dCas9) in which the nucleolytic activity of the RuvC and HNH nuclease domains of Cas9 has been removed (Qi et al., 2013), a diagnostic method for detecting the binding of the dCas9/sgRNA complex to the target DNA has been studied. When the dCas9/sgRNA complex binds to two different targets, the association of the partial luciferase fragments fused with each dCas9 can form an active full-length luciferase, which produces the luminescence (Zhang et al., 2017b). For miRNA detection, rolling circle amplification and dCas9-fused split-horseradish peroxidase techniques can be used (Qiu et al., 2018). Methicillin-resistant Staphylococcus aureus (MRSA) can be detected through fluorescence in situ hybridization (FISH) by connecting a magnetic bead to the dCas9 protein (Guk et al., 2017). In addition, a diagnostic method for detecting the change in the ionic current rectification caused by the binding of dCas9/sgRNA to a target in an aluminum-based sensing chip has been reported (Sun et al., 2022).
Trans-Cleavage Activity
The nuclease activity of Cas protein is activated through the binding of the gRNA/Cas complex to the target nucleic acid (Chen et al., 2018a; Lim et al., 2016). Unlike Type II Cas9, whose nuclease activity is lost upon target cleavage, Type V and Type VI Cas nucleases form a complex with a guide RNA and exhibit a nonspecific cleavage activity on ssDNA present in the vicinity even after the target DNA/RNA is cleaved, which is called trans-cleavage (Yuan et al., 2020). Based on this phenomenon, a diagnostic method has been developed to indirectly measure the trans-cleavage activity of Cas12 or Cas13 by fluorescence (Wang et al., 2020b), color development (Wang et al., 2022a), potential difference (Hajian et al., 2019), or other techniques.
Unlike Cas9, whose PAM sequence is located at the 3ʹ end of the target, Cas12 mainly has a T-rich PAM located at the 5ʹ end of the target and recognizes dsDNA and ssDNA as targets (Zetsche et al., 2015). The one-HOur Low-cost Multipurpose highly Efficient System (HOLMES) was developed as a diagnostic method that can detect single nucleotide variation by amplifying DNA/RNA in a sample via (RT-)PCR and optimizing crRNA that forms a complex with LbCas12a (Li et al., 2018c). In HOLMESv2 with AacCas12b, a diagnostic method has been constructed for detecting dsDNA, and ssDNA through (RT-)LAMP and asymmetric PCR (Li et al., 2019a). It can also distinguish a single nucleotide polymorphism (SNP) locus in the target DNA of the human genome. Through this method, SARS‑CoV‑2 variant (Najjar et al., 2022; Rossetti et al., 2022; Wu et al., 2022) and African swine fever virus (Qin et al., 2022) are diagnosed.
Unlike HOLMES, DNA Endonuclease-Targeted CRISPR Trans Reporter (DETECTR) is a diagnostic method to distinguish between different HPV strains by isothermally amplifying target DNA in the sample with RPA and then detecting the fluorescent signal of the ssDNA-FQ reporter generated by trans-cleavage following the cleavage of the dsDNA target of Cas12a (Chen et al., 2018a).
With this method, SNPs between Bacillus anthracis and B. cereus, have been distinguished, and specific species have been identified (Wang et al., 2022a). In addition, various types of pathogens, including MRSA (Wang et al., 2022b), Mycoplasma pneumoniae (Deng et al., 2022), and SARS-CoV-2 (Sun et al., 2021), have been diagnosed. All-In-One-Dual CRISPR-Cas12a (AIOD-CRISPR) is a method that can detect nucleic acids in a single reaction system without a separate pre-amplification step (Ding et al., 2020). It can sensitively detect the nucleic acids of SARS-CoV-2 and human immunodeficiency virus (HIV) by utilizing dual crRNAs.
Unlike Type II Cas9 and Type V Cas12 that cut DNA targets, Type VI Cas13 nucleases recognize and cut RNA as a target (Abudayyeh et al., 2016). Because of a higher turnover, trans-cleavage of Cas13a occurs relatively faster than that of Cas12a (Nalefski et al., 2021). Therefore, with the advantage of the fast detection of fluorescence signals, after the DNA target is converted to RNA, Cas13 is used for diagnosis. Specific high-sensitivity enzymatic reporter unlocking (SHERLOCK) amplifies DNA from a target DNA/RNA via (RT-)RPA and produces target RNA through in vitro transcription. Then, the RNA probe is cleaved via Cas13a trans-cleavage, which occurs in the presence of the target RNA, to generate a fluorescence signal (Gootenberg et al., 2017).
SHERLOCK diagnosis is mainly used to detect a pseudovirus in swab or food (Wang et al., 2021) or to detect RNA viruses such as feline calicivirus (Huang et al., 2022) or SARS-CoV-2 (Casati et al., 2022). SHERLOCK can also detect cancer mutations in cell-free DNA, and SNPs from human saliva (Gootenberg et al., 2017). Heating unextracted diagnostic samples to obliterate nucleases (HUDSON), a thermal/chemical processing method of collected samples, has been combined with SHERLOCK to enable faster and more accurate diagnosis and analysis (Myhrvold et al., 2018). This platform can distinguish four dengue virus (DENV) serotypes, and detect region-specific SNPs in zika virus (ZIKV) samples (Chertow, 2018). SHERLOCKv2 has also been developed to detect DNA at a concentration of 8 zM through the amplification of a fluorescence signal by using synergistically activated Csm and Cas13a (Gootenberg et al., 2018).
Cas13a-Based, Rugged, Equitable, and Scalable Testing (CREST) was developed to address major hurdles in limiting scalability of RT-qPCR method detecting SARS-CoV-2 using widely available enzymes, fluorescent visualizers, and portable thermocyclers. CREST has been shown to have sensitivity comparable to that of RT-qPCR in COVID-19 test (Rauch et al., 2021). Microfluidic Combinatorial Arrayed Reactions for Multiplexed Evaluation of Nucleic acids (mCARMEN) is a method that can detect multiple viruses and mutants simultaneously. Six SARS-CoV-2 variant lineages, including Delta and Omicron, can be identified by using 26 crRNA pairs, individually or in combination (Welch et al., 2022). Multiple samples can be examined and detected using four Cas nucleases with different types of single-stranded nucleic acid probes. Other representative cases of Cas nuclease diagnosis are summarized in Table 2.
Table 2.
CRISPR-based diagnosis in microbial pathogens
| Cas effector | Target amplification | Target pathogen | Sample type | Limit of Detection | Chromophores/fluorophores | Time | Description | References |
|---|---|---|---|---|---|---|---|---|
| Cas9 | Reverse transcription, NASBA, in vitro transcription (T7) | ZIKV | Synthetic viral vector | 1 fM | Colorimetry (Toehold switch) | 3 h | Toehold inactivation by truncated RNA result from Cas9 cleavage | Pardee et al. (2016) |
| Reverse transcription, EXPAR | Listeria monocytogenes | Total RNA | 0.82 amole | Fluorescence (SYBR Green I) | 1 h | ssDNA primer generation by Cas9 cleavage | Huang et al. (2018) | |
| PCR, RPA | L. monocytogenes | gDNA | 150 copies | Colorimetry (AuNP) | 1 h | Probe hybridization by Cas9 cleavage | Wang et al. (2020a) | |
| LAMP | Salmonella, Neisseria meningitidis | gDNA | 80 copies | Fluorescence (SYBR Green I) | 1 h | Removal of contaminant by Cas9 cleavage | Bao et al. (2020) | |
| Reverse transcription, PCR, RPA | SARS-CoV-2 | gDNA | 10 copies | Fluorescence (FAM) | 1 h | FELUDA | Azhar et al. (2021) | |
| dCas9 | PCR | M. tuberculosis | gDNA | 50 pM* | Luminescence (Firefly luciferase) | 2 h | Partial protein hybridization by dCas9 binding | Zhang et al. (2017b) |
| - | Methicillin-resistant Staphylococcus aureus (MRSA) | Bacterial cell lysate | 10 CFU/ml | Fluorescence (SYBR Green I) | 3 h | Captured with His-tagged dCas9, Ni–NTA column | Guk et al. (2017) | |
| RCA | – | Synthetic miRNA | 35.4 aM | Colorimetry (TMB) | 4 h | TMB oxidation and color development by full-length horseradish peroxidase | Qiu et al. (2018) | |
| Cas12a | PCR | Pseudorabies virus (PRV), Japanese encephalitis virus (JEV) | gDNA | 0.5 aM | Fluorescence (HEX) | 1 h | HOLMES (truncated crRNA) | Li et al. (2018c) |
| RPA | Human papillomavirus (HPV) | Synthetic viral vector | 1 aM | Fluorescence (FAM) | 2 h | DETECTR | Chen et al. (2018a) | |
| RPA | Mycoplasma | Synthetic vector | 10 aM | Fluorescence (FAM) | 0.5 h | One-pot reaction | Wang et al. (2019) | |
| RPA | E. coli O157:H7, S. aureus | gDNA | 1 CFU/ml | Fluorescence (HEX) | 1 h | One-pot reaction | Wang et al. (2020b) | |
| RPA | B. anthracis | Bacterial Plasmid | 1 copy/rxn | Colorimetry (ABTS) | 1.5 h | Visible to the unaided eye | Wang et al. (2022a) | |
| RAA (similar to the RPA) | P. aeruginosa | Bacterial cell culture | 1 aM, 103 CFU/ml | Fluorescence (FAM) | 4 h | Centrifugal microfluidic chip | Chen et al. (2020) | |
| PCR | Shigella dysenteriae | Bacterial cell culture | 10 aM | Fluorescence (FAM) | 2 h | Biotinylated ssDNA reporter + gold nanoparticle | Sun et al. (2020) | |
| RT-RPA | SARS-CoV-2 | Pseudoviruses | 1 copy/μl | Fluorescence (FAM) | 1 h | One-pot reaction | Sun et al. (2021) | |
| RPA | SARS-CoV-2, HIV-1 | Synthetic viral vector | 1.3 copies, 1.2 copies | Fluorescence (FAM) | 40 min | AIOD-CRISPR (One-pot reaction) | Ding et al. (2020) | |
| Cas12b | LAMP, Asymmetric PCR | JEV | Virus | 10 aM | Fluorescence (FAM) | 1 h | HOLMESv2 (Cas12b) | Li et al. (2019a) |
| Cas13a | RPA, in vitro transcription (T7) | P. aeruginosa, ZIKV, Dengue virus (DENV) | gDNA, Synthetic viral vector | 2 aM | Fluorescence (FAM) | 2 h | SHERLOCK | Gootenberg et al. (2017) |
| RPA, in vitro transcription (T7) | ZIKV, DENV | Clinical sample | 20 aM | Fluorescence (FAM) | 2 h | HUDSON (sample treatment) | Myhrvold et al. (2018) | |
| PCR, in vitro transcription (T7) | S. aureus | Food sample | 1 CFU/mL | Fluorescence (FAM) | 4 h | Cas13a has stronger trans-cleavage activity than Cas12 | Zhou et al. (2020) | |
| in vitro transcription (T7) | SARS-CoV-2 | Synthetic viral vector | 82 copies | Fluorescence (Broccoli) | 1 h | RNA fluorescence using light-up aptamers | Wang et al. (2021) | |
| PCR, in vitro transcription (T7) | 113 pathogenic strains | gDNA | 1 copy | Fluorescence | 3 h | One-pot amplification of over 50 targets | Thakku et al. (2022) | |
| RPA, in vitro transcription (T7) | SARS-CoV-2 variants | Clinical sample | 2.5 copies/μl | Fluorescence (TEX 615) | 1 h | Sensitive detection of Delta and Omicron variants | Casati et al. (2022) | |
| Isothermal amplification, in vitro transcription (T7) | S. enterica Enteritidis | gDNA | 1 copy | Fluorescence (FAM) | 2.5 h | Probe contains aptamer domain as identification element | Shen et al. (2020) | |
| RPA, in vitro transcription (T7) | P. aeruginosa, S. aureus, ZIKV, DENV | gDNA, Total RNA | 8 zM | Fluorescence (FAM) | 1 h | SHERLOCKv2 | Gootenberg et al. (2018) | |
| Reverse transcription, PCR, in vitro transcription | SARS-CoV-2 | Total RNA | 10 copies/μl | Fluorescence (FAM) | 3 h | CREST (SARS-CoV-2 RNA detection method) | Rauch et al. (2021) |
ABTS 2,2′-Azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt, AIOD-CRISPR All-in-One Dual CRISPR-Cas12a, AuNP Gold Nanoparticle, CREST Cas13-based, Rugged, Equitable, Scalable Testing, DENV Dengue virus, DETECTR DNA Endonuclease-Targeted CRISPR Trans Reporter, EXPAR Exponential Amplification Reaction, FAM Fluorescein amidite, FELUDA FnCas9 Editor-Limited Uniform Detection Assay, gDNA Genomic DNA, HEX Hexachloro-fluorescein, His-tagged Polyhistidine-tagged, HIV-1 Human immunodeficiency virus type 1, HOLMES One-hour Low-cost Multipurpose Highly Efficient System, HPV Human papillomavirus, HUDSON Heating Unextracted Diagnostic Samples to Obliterate Nucleases, JEV Japanese encephalitis virus, LAMP Loop-mediated isothermal amplification, miRNA microRNA, MRSA Methicillin-resistant Staphylococcus aureus, NASBA Nucleic Acid Sequenced Based Amplification, Ni–NTA Nickel-nitrilotriacetic acid, PCR Polymerase chain reaction, PRV Pseudorabies virus, RAA Recombinase-aided amplification, RCA Rolling circle amplification, RPA Recombinase polymerase amplification, RT Reverse transcription, SARS-CoV-2 Severe acute respiratory syndrome coronavirus 2, TEX Texas Red, TMB 3,3ʹ,5,5ʹ-Tetramethylbenzidine, ZIKV Zika virus
In vivo CRISPR Regulation
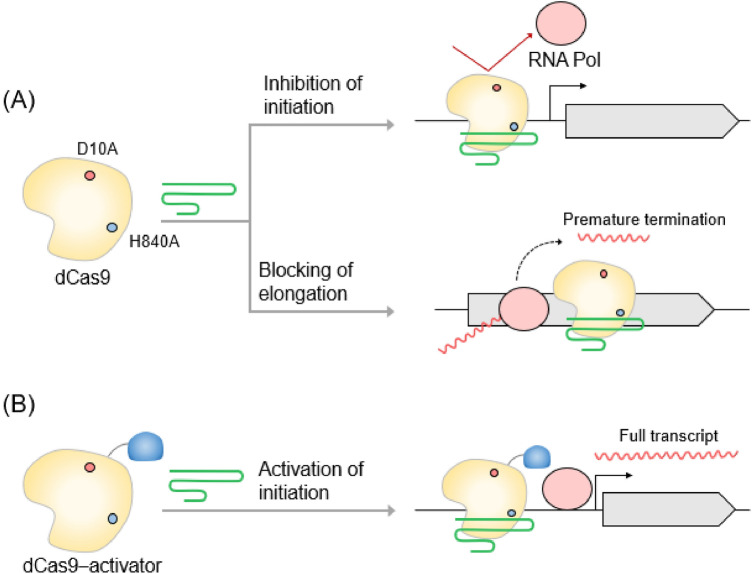
CRISPR-Cas evolved into deactivated Cas9 (dCas9) that can regulate gene transcription beyond genome editing. The CRISPR-dCas system has been expanded through fusion with various effector domains such as transcriptional repressors or activators (Farzadfard et al., 2013; Konermann et al., 2015). As shown in Fig. 4, the binding of the sgRNA-dCas protein complex to the target DNA can block the initiation and elongation of transcription by interfering with the binding of RNA polymerase or transcription factors (Qi et al., 2013) through a process called CRISPR interference (CRISPRi). The CRISPRi system can reversibly inhibit the transcription of multiple target genes (Qi et al., 2013; Zhang et al., 2021). The combination of a DNase-dead Cpf1 mutant (ddCpf1), and a crRNA array is used to simultaneously inhibit the transcription of four genes (Zhang et al., 2017a). In yeast, various transcriptional repressors are fused with dCas protein to regulate transcription more effectively (Schwartz et al., 2017; Wensing et al., 2019).
Fig. 4.
Transcriptional regulation by deactivated Cas proteins. A CRISPR interference (CRISPRi). Deactivated Cas9 (dCas9) enables the regulation of gene expression by blocking the transcriptional initiation or elongation of RNA polymerase (RNA Pol). B CRISPR activation (CRISPRa). dCas9 fused with a transcription activator binds upstream of the target promoter to recruit RNA Pol and activates the transcription of the target gene
CRISPR activation (CRISPRa) that can activate gene expression by promoting the recruitment of transcriptional activators to target DNA sequences has been developed (Bikard et al., 2013). Various transcriptional activators, including the ω subunit of RNA polymerase and phage activator AsiA, have been fused with dCas9 in a bacterial system (Bikard et al., 2013; Ho et al., 2020). In S. cerevisiae, dCas9 fused with VP64 significantly enhances the activity of a reporter gene when sgRNA is located upstream of the TATA box (Farzadfard et al., 2013). CRISPRi/a has been used as a molecular tool for industrially important host gene expression regulation and gene function identification because of its easily programmable properties (Liu et al., 2017; Rousset et al., 2018). Representative studies on CRISPR-mediated microbial gene transcriptional regulation are summarized in Table 3.
Table 3.
CRISPR-mediated gene regulation
| Organism | dCas protein | Species | Effector domain | Target | Feature | References |
|---|---|---|---|---|---|---|
| Bacteria | dCas9 | Escherichia coli | None | mRFP, sfGFP | Characterization of factors that affect silencing efficiency | Qi et al. (2013) |
| PhlF | rfp | Construction of the nontoxic version of dCas9 by making R1335K mutation to dCas9 and fusing it to the PhlF repressor | Zhang and Voigt (2018) | |||
| None | gal promoter, galETK | Repression of DNA targets by CRISPRi with expanded PAM sequences | Kim et al. (2020a) | |||
| Corynebacterium glutamicum | None | pyc, gltA, idsA, glgC | Repression of single or double target genes by using two plasmid system (dCas9 plasmid, sgRNA plasmid) | Park et al. (2018b) | ||
| Cyanobacterium Anabaena | None | glnA | Fine-tuning the GlnA protein by strictly regulating dCas9 expression via the TetR induction system | Higo et al. (2018) | ||
| Mycobacterium tuberculosis | None | dnaE1, pptT | Achieved 20- to 100-fold knockdown of target genes using dCas9Sth1 with minimal proteotoxicity | Rock et al. (2017) | ||
| dCas9 − activator | Bacillus subtilis | ω subunit | prsA, nprB, bpr, vpr | 260-fold enhancement of BLA production via the promoter engineering strategy OAPS | Lu et al. (2019) | |
|
E. coli Salmonella enterica Klebsiella oxytoca |
AsiA | gfp | Improving the potency of dCas9-AsiA using random PCR mutagenesis | Ho et al. (2020) | ||
| Pseudomonas putida | MCP-SoxS | gtpch, ptps, sr, mvaES | Using a modified gRNA (scRNA) that recruits RNA-binding protein fused with an effector domain | Kiattisewee et al. (2021) | ||
| dCpf1 | C.glutamicum | None | pck, hom, pgi, gltA | Simultaneous repression of four target genes by a single crRNA array | Li et al. (2020) | |
| E. coli | None | malT, prop, degP, rseA | Multiplex gene regulation using ddCpf1 with the remaining RNase activity | Zhang et al. (2017a) | ||
| dCpf1 − activator | B. subtilis | RemA | bdhA, acoA, ldh, pta, alsR | Construction of CRISPR-assisted multiple genes editing and regulation system and crRNA array using SOMACA method | Wu et al. (2020) | |
| B. amyloliquefaciens | ω subunit | secE, secDF, prsA | Simultaneous activation of the expression of three genes by designing a crRNA array | Xin et al. (2022) | ||
| dxCas9 − activator | E. coli | PspF | sfGFP, mCherry, mTagBFP2, vioADC | Using scRNA, which contained BoxB aptamers that bind to λN22plus peptide fused with activation domain | Liu et al. (2019b) | |
| Fungi | dCas9 − activator | Aspergillus niger | p300 | breF, fuml, fwnA | Regulation of the expression of secondary metabolic genes via dCas9-based histone modification | Li et al. (2021) |
| Penicillium rubens | VPR | DsRed, macR | Using an AMA1 shuttle vector with a ribozyme-based sgRNA “plug-and-play” module | Mozsik et al. (2021) | ||
| A. nidulans | VPR | mdp cluster, AN8504 cluster | Optimizing sgRNA positioning by creating genome-wide nucleosome maps | Schuller et al. (2020) | ||
| Yeast | dCas9 | Yarrowia lipolytica | Mxi1 | ku70, ku80 | Achieving high HR rates by enhancing repression with Mxi1 fused with dCas9 | Schwartz et al. (2017) |
| Schizosaccharomyces pombe | None | his2+, his7+, ade6+, ura4+ | Determination of the directionality of targeting sequences of sgRNAs | Ishikawa et al. (2021) | ||
| Saccharomyces cerevisiae | Mxi1 | GFP | Optimizing gRNA-promoter combinations by testing 101 gRNA structures on 14 promoters | Jensen et al. (2017) | ||
| Candida albicans | Mxi1, Mig1 | ADE2, HSP90 | First CRISPRi system for use in C. albicans | Wensing et al. (2019) | ||
| dCas9 − activator | S. cerevisiae | VP64 | GFP | By targeting different sites, single crisprTF can be programmed to act as an activator and repressor | Farzadfard et al. (2013) | |
| Ogataea thermomethanolica | VP64 | Promoter sequence of SOD1, VPS1, YPT7 | Evaluation of the role of genes on heterologous protein secretion | Kruasuwan et al. (2021) | ||
| dCpf1 | Pichia pastoris | Mit1 | eGFP | Construction of SynPic-X, a synthetic expression platform with iTSAD | Liu et al. (2022b) | |
| Y. lipolytica | KRAB | gfp, vioABE | Using multiplex gRNA strategy based on one-step golden-brick assembly technology | Zhang et al. (2018) | ||
| dCpf1 − activator | S. cerevisiae |
VP64 VP64-p65AD VP64-VPR |
CYC1p, RNR2p | Designing a combinational metabolic engineering strategy based on an orthogonal tri-functional CRISPR system | Lian et al. (2017) |
OAPS Oligonucleotide Annealing-based Promoter Shuffling, scRNA scaffold RNA, ddCpf1 DNase-dead Cpf1, SOMACA Synthetic oligonucleotide-mediated assembly method, VPR VP64-p65-Rta, iTSAD improved transcriptional signal amplification device
Cellular System Optimization
Since the development of artificial gene circuits in 2000 (Elowitz & Leibler, 2000; Gardner et al., 2000), transcription factors (TFs) have been used to regulate gene expression in most synthetic circuits. TFs generally offer a high dynamic range, but their orthogonality, modularity, and programmability are limited; therefore, TFs are less ideal for synthetic biology (Zhang & Voigt, 2018). CRISPR-based gene circuits can be constructed to easily target and manipulate individual genes in complex regulatory networks within cells. In prokaryotes, CRISPR-based synthetic circuits mainly include logic circuits such as AND, NOR, and NIMPLY (Santos-Moreno & Schaerli, 2020). In S. cerevisiae, NOR gates are constructed via chromatin remodeler-combined CRISPRi, which allows minimal leak and digital responses (Gander et al., 2017).
The CRISPRi/a circuit can be linked to a cellular sensor system to control host metabolism in response to external stimuli (Mimee et al., 2015; Taketani et al., 2020). However, in the case of complex gene circuits, multiple gRNAs must share a limited intracellular pool of dCas9, which can reduce target gene repression (Li et al., 2018b). Therefore, a non-toxic version of dCas9R1335K with impaired ability to recognize PAM has been developed and fused with a PhlF inhibitor to solve this problem; in this way, the dCas9-based circuit design has been expanded in metabolic engineering and synthetic biology (Zhang & Voigt, 2018).
CRISPR-mediated gene regulation technology has been utilized to optimize the biosynthetic pathways of various metabolites in microorganisms and identify chemical–genetic interactions (Vanegas et al., 2017). CRISPRi has been mainly used to inhibit target genes, including essential genes, and direct carbon flux toward a desired product or bioactive compound (Kim et al., 2016b). In Corynebacterium glutamicum, the production of l-lysine and l-glutamate is improved by simultaneously inhibiting the expression of pgi, pck, and pyk via CRISPRi (Cleto et al., 2016). CRISPRa can be used to activate metabolic pathways related to the biosynthesis of a desired product. In Pseudomonas putida, mevalonate production was increased 40-fold through the activation of related genes via CRISPRa (Kiattisewee et al., 2021). In addition, CRISPR-mediated gene circuits or biosensors are used to alternately switch between cell growth and production phases and improve the production of desired metabolites (Shabestary et al., 2021). Furthermore, metabolite production can be further increased by combining CRISPRi/a technology with other metabolic engineering techniques, such as deletion, overexpression of specific genes, or optimization of growth media (Kozaeva et al., 2021; Lian et al., 2017).
Target Gene Screening
To adapt to various environments or conditions, cells are regulated by a complex network of numerous genes. CRISPR technology can be used for high-throughput genome-wide screening to achieve the desired cellular performance or obtain gene targets corresponding to phenotypes. Prior to CRISPR technology, RNAi-based screening has been widely used to identify genes involved in specific pathways, structures, or functions (Cronin et al., 2009). However, this approach has difficulties in finding the association between phenotype and gene knockdown because of the off-target effect on mRNA and incomplete gene suppression (So et al., 2019).
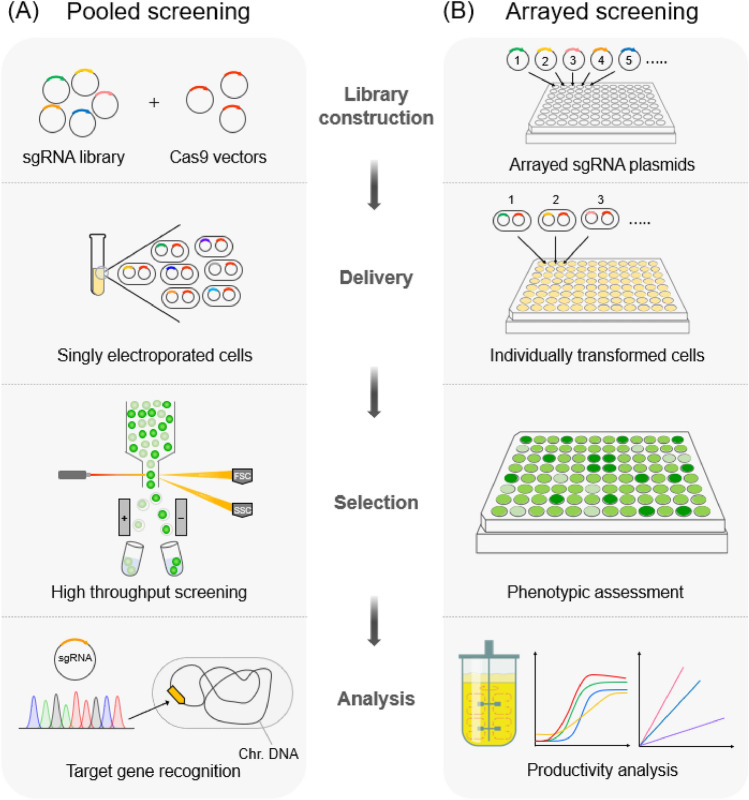
CRISPR-Cas screening via a gRNA library has been used to reveal genes, pathways, and mechanisms related to specific phenotypes or biological characteristics. CRISPR-Cas9 screening has also been used to identify genes associated with bacterial invasion (Pacheco et al., 2018) and resistance to antibiotics or chemicals (Garst et al., 2017). However, CRISPR knockout (CRISPR-KO) screening cannot reversibly regulate gene expression because it causes permanent gene disruption (So et al., 2019). CRISPRi/a, which can modulate gene expression levels and mediate reversible gene expression, has been developed and utilized for gene screening. CRISPRi screening is generally performed in two ways: pooled or arrayed (Bock et al., 2022).
In pooled CRISPR screening, a target gene is identified by sequencing the sgRNA extracted from cells showing a specific phenotypic change after a large amount of sgRNA library is introduced into them (Fig. 5). For example, essential auxotrophic and antibiotic resistance-related genes are screened using a sgRNA library (~ 60,000 sgRNAs) in E. coli (Wang et al., 2018b). CRISPRa in S. cerevisiae has confirmed that OLE1 is important for the heat resistance of yeast (Li et al., 2019b).
Fig. 5.
Pooled and arrayed CRISPR screening methods. A In pooled screening, sgRNA library and Cas9 vector are delivered into cells within a single tube. Then, cells with specific phenotypes can be screened using high throughput facilities. Target genes are identified by DNA sequencing of sgRNAs extracted from the selected cells. B In arrayed screening, Cas9 vector and each sgRNA vector are delivered into individual cells. Cells with the desired phenotype are selected, and the productivity of the selected cells is analyzed
In arrayed CRISPRi screening, one sgRNA targeting one gene per well is introduced into cells in a multi-well plate and the phenotype occurring in each cell is observed. For example, an L-proline exporter was discovered for L-proline hyperproduction by using a sgRNA library targeting the potential L-proline transporter genes in Corynebacterium glutamicum (Liu et al., 2022a). In addition, 28 phosphatase-encoding genes that increase terpenoid production have been identified in E. coli by using the CRISPRi system (Wang et al., 2018c). Thus, genome-wide CRISPRi/a screening is evolving into an effective synthetic biology tool that can help profile the relationship between phenotype and genotype and find targets for engineering in complex cellular networks.
Perspectives
CRISPR-Cas technology has been applied as a genome editing tool for various organisms, including prokaryotes and human cells (Jinek et al., 2012). Currently, highly specific and trans-cleaving nucleolytic activities of CRISPR-Cas are used for accurate genome editing (Lee & Lee, 2021) and diagnosis (Kaminski et al., 2021), respectively. Deactivated Cas protein is used not only to regulate the transcription of target genes but also to reveal the function of genes related to specific phenotypes through CRISPR screening.
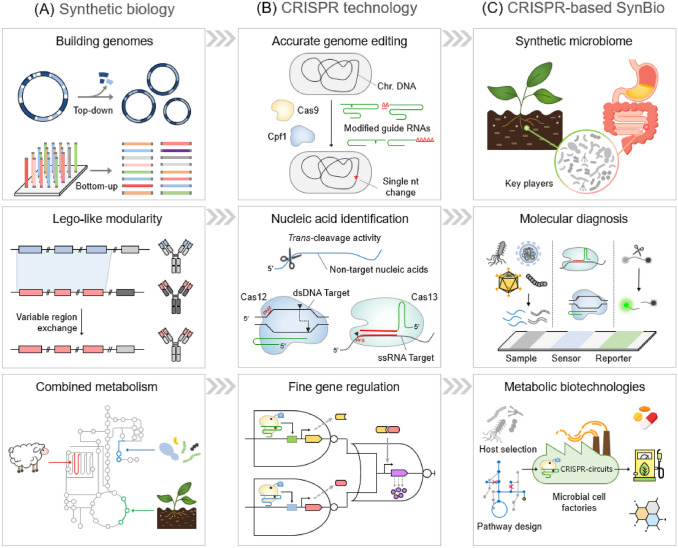
In the future, CRISPR-based genome editing tools will be applied to various cells, from bacteria to humans via codon optimization and established genetic vectors. Changing the gut and soil microbiome through the production of customized strains will enable individual health management and improve crop yield and quality. In addition, CRISPR-Cas will be developed to innovate quick and easy biosensors that can detect pathogenic microorganisms and genetic markers without using expensive equipment, which can greatly help in disease diagnosis and treatment. CRISPR-mediated gene regulation will improve the performance of industrial strains that produce useful biochemicals (Fig. 6).
Fig. 6.
CRISPR-Cas systems accelerate the development of synthetic biology. A Current synthetic biology can establish a new genome in top-down or bottom-up manners. Biomolecular engineering can be accelerated by Lego-like modularization. Combining the metabolic pathways of different origins can create pathways for the synthesis of new substances. B Development of various CRISPR technologies for accurate genome editing using modified guide RNAs, nucleic acid detection using trans-cleavage activity of Cas enzymes, and fine gene regulation by CRISPR-based artificial transcription factors. C Applications of microbial CRISPR technologies in synthetic biology: synthetic microbiome (e.g., regulation of plant/human-microbe interaction), molecular diagnosis (e.g., point-of-care testing to detect pathogens or genetic markers), and metabolic biotechnologies (e.g., production of pharmaceutical, bioenergy, and chemicals in microbial cell factories carrying CRISPR-based circuits)
Among various biotechnologies, CRISPR-Cas technology has become a key tool in the field of microbial metabolic engineering and synthetic biology by maximizing advantages such as ease of use and scalability of modular components. It has accelerated the design–build–test–learn cycle of synthetic biology to solve current problems such as healthcare, global epidemics, food shortages, and environmental pollution; thus, it will help achieve a sustainable future human life.
Acknowledgements
This research was supported by the National Research Foundation of Korea (2021R1A2C1013606), Republic of Korea. This study was also supported by Rural Development Administration (Project No. PJ015001032021) and the Chung-Ang University Graduate Research Scholarship in 2021.
Declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- Abdulrachman D, Eurwilaichitr L, Champreda V, Chantasingh D, Pootanakit K. Development of a CRISPR/Cpf1 system for targeted gene disruption in Aspergillus aculeatus TBRC 277. BMC Biotechnology. 2021;21:15. doi: 10.1186/s12896-021-00669-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abudayyeh OO, Gootenberg JS, Konermann S, Joung J, Slaymaker IM, Cox DBT, Shmakov S, Makarova KS, Semenova E, Minakhin L, Severinov K, Regev A, Lander ES, Koonin EV, Zhang F. C2c2 is a single-component programmable RNA-guided RNA-targeting CRISPR effector. Science. 2016;353:aaf5573. doi: 10.1126/science.aaf5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altenbuchner J. Editing of the Bacillus subtilis Genome by the CRISPR-Cas9 System. Applied and Environmental Microbiology. 2016;82:5421–5427. doi: 10.1128/AEM.01453-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anzalone AV, Randolph PB, Davis JR, Sousa AA, Koblan LW, Levy JM, Chen PJ, Wilson C, Newby GA, Raguram A, Liu DR. Search-and-replace genome editing without double-strand breaks or donor DNA. Nature. 2019;576:149–157. doi: 10.1038/s41586-019-1711-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ao X, Yao Y, Li T, Yang TT, Dong X, Zheng ZT, Chen GQ, Wu Q, Guo YY. A multiplex genome editing method for Escherichia coli based on CRISPR-Cas12a. Frontiers in Microbiology. 2018;9(10):3389. doi: 10.3389/fmicb.2018.02307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arroyo-Olarte RD, Rodriguez RB, Morales-Rios E. Genome editing in bacteria: CRISPR-Cas and beyond. Microorganisms. 2021;9:844. doi: 10.3390/microorganisms9040844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azhar M, Phutela R, Kumar M, Ansari AH, Rauthan R, Gulati S, Sharma N, Sinha D, Sharma S, Singh S, Acharya S, Sarkar S, Paul D, Kathpalia P, Aich M, Sehgal P, Ranjan G, Bhoyar RC, Singhal K, Lad H, Patra PK, Makharia G, Chandak GR, Pesala B, Chakraborty D, Maiti S, Epide ICGG. Rapid and accurate nucleobase detection using FnCas9 and its application in COVID-19 diagnosis. Biosensors & Bioelectronics. 2021;183:113207. doi: 10.1016/j.bios.2021.113207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banno S, Nishida K, Arazoe T, Mitsunobu H, Kondo A. Deaminase-mediated multiplex genome editing in Escherichia coli. Nature Microbiology. 2018;3:423–429. doi: 10.1038/s41564-017-0102-6. [DOI] [PubMed] [Google Scholar]
- Bao Y, Jiang Y, Xiong E, Tian T, Zhang Z, Lv J, Li Y, Zhou X. CUT-LAMP: contamination-free loop-mediated isothermal amplification based on the CRISPR/Cas9 cleavage. ACS Sensors. 2020;5:1082–1091. doi: 10.1021/acssensors.0c00034. [DOI] [PubMed] [Google Scholar]
- Bikard D, Jiang W, Samai P, Hochschild A, Zhang F, Marraffini LA. Programmable repression and activation of bacterial gene expression using an engineered CRISPR-Cas system. Nucleic Acids Research. 2013;41:7429–7437. doi: 10.1093/nar/gkt520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock, C., Datlinger, P., Chardon, F., et al. (2022). High-content CRISPR screening. Nature Reviews Methods Primers2. [DOI] [PMC free article] [PubMed]
- Brophy JAN, Voigt CA. Principles of genetic circuit design. Nature Methods. 2014;11:508–520. doi: 10.1038/nmeth.2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao M, Gao M, Ploessl D, Song C, Shao Z. CRISPR-mediated genome editing and gene repression in Scheffersomyces stipitis. Biotechnology Journal. 2018;13:e1700598. doi: 10.1002/biot.201700598. [DOI] [PubMed] [Google Scholar]
- Casati B, Verdi JP, Hempelmann A, Kittel M, Klaebisch AG, Meister B, Welker S, Asthana S, Di Giorgio S, Boskovic P, Man KH, Schopp M, Ginno PA, Radlwimmer B, Stebbins CE, Miethke T, Papavasiliou FN, Pecori R. Rapid, adaptable and sensitive Cas13-based COVID-19 diagnostics using ADESSO. Nature Communications. 2022;13:3308. doi: 10.1038/s41467-022-30862-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao R, Yuan Y, Zhao H. Recent advances in DNA assembly technologies. FEMS Yeast Research. 2015;15:1–9. doi: 10.1111/1567-1364.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YY, Galloway KE, Smolke CD. Synthetic biology: Advancing biological frontiers by building synthetic systems. Genome Biology. 2012;13:240. doi: 10.1186/gb-2012-13-2-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JS, Ma E, Harrington LB, Da Costa M, Tian X, Palefsky JM, Doudna JA. CRISPR-Cas12a target binding unleashes indiscriminate single-stranded DNase activity. Science. 2018;360:436–439. doi: 10.1126/science.aar6245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Mei Y, Zhao X, Jiang X. Reagents-loaded, automated assay that integrates recombinase-aided amplification and Cas12a Nucleic Acid detection for a point-of-care test. Analytical Chemistry. 2020;92:14846–14852. doi: 10.1021/acs.analchem.0c03883. [DOI] [PubMed] [Google Scholar]
- Chen WH, Zhang YF, Yeo WS, Bae T, Ji QJ. Rapid and efficient genome editing in Staphylococcus aureus by using an engineered CRISPR/Cas9 system. Journal of the American Chemical Society. 2017;139:3790–3795. doi: 10.1021/jacs.6b13317. [DOI] [PubMed] [Google Scholar]
- Chen W, Zhang Y, Zhang Y, Pi Y, Gu T, Song L, Wang Y, Ji Q. CRISPR/Cas9-based genome editing in Pseudomonas aeruginosa and cytidine deaminase-mediated base editing in Pseudomonas species. iScience. 2018;6:222–231. doi: 10.1016/j.isci.2018.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chertow DS. Next-generation diagnostics with CRISPR. Science. 2018;360:381–382. doi: 10.1126/science.aat4982. [DOI] [PubMed] [Google Scholar]
- Cho SW, Kim S, Kim Y, Kweon J, Kim HS, Bae S, Kim JS. Analysis of off-target effects of CRISPR/Cas-derived RNA-guided endonucleases and nickases. Genome Research. 2014;24:132–141. doi: 10.1101/gr.162339.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke R, Terry AR, Pennington H, Hasty C, MacDougall MS, Regan M, Merrill BJ. Sequential activation of guide RNAs to enable successive CRISPR-Cas9 activities. Molecular Cell. 2021;81:226–238.e225. doi: 10.1016/j.molcel.2020.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleto S, Jensen JV, Wendisch VF, Lu TK. Corynebacterium glutamicum metabolic engineering with CRISPR interference (CRISPRi) ACS Synthetic Biology. 2016;5:375–385. doi: 10.1021/acssynbio.5b00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb RE, Wang YJ, Zhao HM. High-efficiency multiplex genome editing of Streptomyces species using an engineered CRISPR/Cas system. ACS Synthetic Biology. 2015;4:723–728. doi: 10.1021/sb500351f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong L, Ran FA, Cox D, Lin SL, Barretto R, Habib N, Hsu PD, Wu XB, Jiang WY, Marraffini LA, Zhang F. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronin SJF, Nehme NT, Limmer S, Liegeois S, Pospisilik JA, Schramek D, Leibbrandt A, Simoes RD, Gruber S, Puc U, Ebersberger I, Zoranovic T, Neely GG, von Haeseler A, Ferrandon D, Penninger JM. Genome-wide RNAi screen identifies genes involved in intestinal pathogenic bacterial infection. Science. 2009;325:340–343. doi: 10.1126/science.1173164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl RH, Zhang F, Alonso-Gutierrez J, Baidoo E, Batth TS, Redding-Johanson AM, Petzold CJ, Mukhopadhyay A, Lee TS, Adams PD, Keasling JD. Engineering dynamic pathway regulation using stress-response promoters. Nature Biotechnology. 2013;31:1039–1046. doi: 10.1038/nbt.2689. [DOI] [PubMed] [Google Scholar]
- Deng Z, Hu H, Tang D, Liang J, Su X, Jiang T, Hu X, Ying W, Zhen D, Xiao X, He J. Ultrasensitive, specific, and rapid detection of Mycoplasma pneumoniae using the ERA/CRISPR-Cas12a dual system. Frontiers in Microbiology. 2022;13:811768. doi: 10.3389/fmicb.2022.811768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X, Seebeck T, Feng Y, Jiang Y, Davis GD, Chen F. Improving CRISPR-Cas9 genome editing efficiency by fusion with chromatin-modulating peptides. CRISPR J. 2019;2:51–63. doi: 10.1089/crispr.2018.0036. [DOI] [PubMed] [Google Scholar]
- Ding, X., Yin, K., Li, Z., and Liu, C. (2020). All-in-one dual CRISPR-Cas12a (AIOD-CRISPR) assay: A case for rapid, ultrasensitive and visual detection of novel coronavirus SARS-CoV-2 and HIV virus. bioRxiv, 998724.
- Elowitz MB, Leibler S. A synthetic oscillatory network of transcriptional regulators. Nature. 2000;403:335–338. doi: 10.1038/35002125. [DOI] [PubMed] [Google Scholar]
- Farzadfard F, Perli SD, Lu TK. Tunable and multifunctional eukaryotic transcription factors based on CRISPR/Cas. ACS Synthetic Biology. 2013;2:604–613. doi: 10.1021/sb400081r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu YF, Sander JD, Reyon D, Cascio VM, Joung JK. Improving CRISPR-Cas nuclease specificity using truncated guide RNAs. Nature Biotechnology. 2014;32:279–284. doi: 10.1038/nbt.2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller KK, Chen S, Loros JJ, Dunlap JC. Development of the CRISPR/Cas9 system for targeted gene disruption in Aspergillus fumigatus. Eukaryotic Cell. 2015;14:1073–1080. doi: 10.1128/EC.00107-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gander MW, Vrana JD, Voje WE, Carothers JM, Klavins E. Digital logic circuits in yeast with CRISPR-dCas9 NOR gates. Nature Communications. 2017;8:15459. doi: 10.1038/ncomms15459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Wu L, Yang D, Gong W, Wang J. A one-pot CRISPR/Cas9-typing PCR for DNA detection and genotyping. The Journal of Molecular Diagnostics. 2021;23:46–60. doi: 10.1016/j.jmoldx.2020.10.004. [DOI] [PubMed] [Google Scholar]
- Gardner TS, Cantor CR, Collins JJ. Construction of a genetic toggle switch in Escherichia coli. Nature. 2000;403:339–342. doi: 10.1038/35002131. [DOI] [PubMed] [Google Scholar]
- Garst AD, Bassalo MC, Pines G, Lynch SA, Halweg-Edwards AL, Liu RM, Liang LY, Wang ZW, Zeitoun R, Alexander WG, Gill RT. Genome-wide mapping of mutations at single-nucleotide resolution for protein, metabolic and genome engineering. Nature Biotechnology. 2017;35:48–55. doi: 10.1038/nbt.3718. [DOI] [PubMed] [Google Scholar]
- Gasiunas G, Barrangou R, Horvath P, Siksnys V. Cas9-crRNA ribonucleoprotein complex mediates specific DNA cleavage for adaptive immunity in bacteria. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:E2579–E2586. doi: 10.1073/pnas.1208507109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudelli NM, Komor AC, Rees HA, Packer MS, Badran AH, Bryson DI, Liu DR. Programmable base editing of A.T to G.C in genomic DNA without DNA cleavage. Nature. 2017;551:464–471. doi: 10.1038/nature24644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson DG, Glass JI, Lartigue C, Noskov VN, Chuang RY, Algire MA, Benders GA, Montague MG, Ma L, Moodie MM, Merryman C, Vashee S, Krishnakumar R, Assad-Garcia N, Andrews-Pfannkoch C, Denisova EA, Young L, Qi ZQ, Segall-Shapiro TH, Calvey CH, Parmar PP, Hutchison CA, 3rd, Smith HO, Venter JC. Creation of a bacterial cell controlled by a chemically synthesized genome. Science. 2010;329:52–56. doi: 10.1126/science.1190719. [DOI] [PubMed] [Google Scholar]
- Gootenberg JS, Abudayyeh OO, Kellner MJ, Joung J, Collins JJ, Zhang F. Multiplexed and portable nucleic acid detection platform with Cas13, Cas12a, and Csm6. Science. 2018;360:439–444. doi: 10.1126/science.aaq0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gootenberg JS, Abudayyeh OO, Lee JW, Essletzbichler P, Dy AJ, Joung J, Verdine V, Donghia N, Daringer NM, Freije CA, Myhrvold C, Bhattacharyya RP, Livny J, Regev A, Koonin EV, Hung DT, Sabeti PC, Collins JJ, Zhang F. Nucleic acid detection with CRISPR-Cas13a/C2c2. Science. 2017;356:438–442. doi: 10.1126/science.aam9321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AA, Kim JM, Ma D, Ilver PAS, Collins JJ, Yin P. Complex cellular logic computation using ribocomputing devices. Nature. 2017;548:117–121. doi: 10.1038/nature23271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunewald J, Zhou RH, Iyer S, Lareau CA, Garcia SP, Aryee MJ, Joung JK. CRISPR DNA base editors with reduced RNA off-target and self-editing activities. Nature Biotechnology. 2019;37:1041–1048. doi: 10.1038/s41587-019-0236-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilinger JP, Thompson DB, Liu DR. Fusion of catalytically inactive Cas9 to FokI nuclease improves the specificity of genome modification. Nature Biotechnology. 2014;32:577–582. doi: 10.1038/nbt.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guk K, Keem JO, Hwang SG, Kim H, Kang T, Lim EK, Jung J. A facile, rapid and sensitive detection of MRSA using a CRISPR-mediated DNA FISH method, antibody-like dCas9/sgRNA complex. Biosensors & Bioelectronics. 2017;95:67–71. doi: 10.1016/j.bios.2017.04.016. [DOI] [PubMed] [Google Scholar]
- Hajian R, Balderston S, Tran T, deBoer T, Etienne J, Sandhu M, Wauford NA, Chung JY, Nokes J, Athaiya M, Paredes J, Peytavi R, Goldsmith B, Murthy N, Conboy IM, Aran K. Detection of unamplified target genes via CRISPR-Cas9 immobilized on a graphene field-effect transistor. Nature Biomedical Engineering. 2019;3:427–437. doi: 10.1038/s41551-019-0371-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington LB, Burstein D, Chen JS, Paez-Espino D, Ma E, Witte IP, Cofsky JC, Kyrpides NC, Banfield JF, Doudna JA. Programmed DNA destruction by miniature CRISPR-Cas14 enzymes. Science. 2018;362:839–842. doi: 10.1126/science.aav4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higo A, Isu A, Fukaya Y, Ehira S, Hisabori T. Application of CRISPR interference for metabolic engineering of the heterocyst-forming multicellular Cyanobacterium Anabaena sp PCC 7120. Plant and Cell Physiology. 2018;59:119–127. doi: 10.1093/pcp/pcx166. [DOI] [PubMed] [Google Scholar]
- Ho HI, Fang JR, Cheung J, Wang HH. Programmable CRISPR-Cas transcriptional activation in bacteria. Molecular Systems Biology. 2020;16:e9427. doi: 10.15252/msb.20199427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz AA, Walter JM, Schubert MG, Kung SH, Hawkins K, Platt DM, Hernday AD, Mahatdejkul-Meadows T, Szeto W, Chandran SS, Newman JD. Efficient multiplexed integration of synergistic alleles and metabolic pathways in yeasts via CRISPR-Cas. Cell Systems. 2015;1:88–96. doi: 10.1016/j.cels.2015.02.001. [DOI] [PubMed] [Google Scholar]
- Hsu PD, Lander ES, Zhang F. Development and applications of CRISPR-Cas9 for genome engineering. Cell. 2014;157:1262–1278. doi: 10.1016/j.cell.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu JH, Miller SM, Geurts MH, Tang W, Chen L, Sun N, Zeina CM, Gao X, Rees HA, Lin Z, Liu DR. Evolved Cas9 variants with broad PAM compatibility and high DNA specificity. Nature. 2018;556:57–63. doi: 10.1038/nature26155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Liu Y, He Y, Yang X, Li Y. CRISPR-Cas13a based visual detection assays for feline calicivirus circulating in southwest China. Frontiers in Veterinary Science. 2022;9:913780. doi: 10.3389/fvets.2022.913780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang MQ, Zhou XM, Wang HY, Xing D. Clustered regularly interspaced short palindromic repeats/Cas9 triggered isothermal amplification for site-specific nucleic acid detection. Analytical Chemistry. 2018;90:2193–2200. doi: 10.1021/acs.analchem.7b04542. [DOI] [PubMed] [Google Scholar]
- Hughes RA, Ellington AD. Synthetic DNA synthesis and assembly: Putting the synthetic in synthetic biology. Cold Spring Harbor Perspectives in Biology. 2017;9:a023812. doi: 10.1101/cshperspect.a023812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison CA, Chuang RY, Noskov VN, Assad-Garcia N, Deerinck TJ, Ellisman MH, Gill J, Kannan K, Karas BJ, Ma L, Pelletier JF, Qi ZQ, Richter RA, Strychalski EA, Sun LJ, Suzuki Y, Tsvetanova B, Wise KS, Smith HO, Glass JI, Merryman C, Gibson DG, Venter JC. Design and synthesis of a minimal bacterial genome. Science. 2016;351:aad6253. doi: 10.1126/science.aad6253. [DOI] [PubMed] [Google Scholar]
- Ishikawa K, Soejima S, Masuda F, Saitoh S. Implementation of dCas9-mediated CRISPRi in the fission yeast Schizosaccharomyces pombe. G3 (bethesda) 2021;11:1051. doi: 10.1093/g3journal/jkab051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen ED, Ferreira R, Jakociunas T, Arsovska D, Zhang J, Ding L, Smith JD, David F, Nielsen J, Jensen MK, Keasling JD. Transcriptional reprogramming in yeast using dCas9 and combinatorial gRNA strategies. Microbial Cell Factories. 2017;16:46. doi: 10.1186/s12934-017-0664-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang WY, Bikard D, Cox D, Zhang F, Marraffini LA. RNA-guided editing of bacterial genomes using CRISPR-Cas systems. Nature Biotechnology. 2013;31:233–239. doi: 10.1038/nbt.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337:816–821. doi: 10.1126/science.1225829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski MM, Abudayyeh OO, Gootenberg JS, Zhang F, Collins JJ. CRISPR-based diagnostics. Nature Biomedical Engineering. 2021;5:643–656. doi: 10.1038/s41551-021-00760-7. [DOI] [PubMed] [Google Scholar]
- Karvelis T, Druteika G, Bigelyte G, Budre K, Zedaveinyte R, Silanskas A, Kazlauskas D, Venclovas C, Siksnys V. Transposon-associated TnpB is a programmable RNA-guided DNA endonuclease. Nature. 2021;599:692–696. doi: 10.1038/s41586-021-04058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiattisewee C, Dong C, Fontana J, Sugianto W, Peralta-Yahya P, Carothers JM, Zalatan JG. Portable bacterial CRISPR transcriptional activation enables metabolic engineering in Pseudomonas putida. Metabolic Engineering. 2021;66:283–295. doi: 10.1016/j.ymben.2021.04.002. [DOI] [PubMed] [Google Scholar]
- Kim D, Bae S, Park J, Kim E, Kim S, Yu HR, Hwang J, Kim JI, Kim JS. Digenome-seq: Genome-wide profiling of CRISPR-Cas9 off-target effects in human cells. Nature Methods. 2015;12:237–243. doi: 10.1038/nmeth.3284. [DOI] [PubMed] [Google Scholar]
- Kim DY, Chung Y, Lee YJ, Jeong D, Park KH, Chin HJ, Lee JM, Park S, Ko S, Ko JH, Kim YS. Hypercompact adenine base editors based on transposase B guided by engineered RNA. Nature Chemical Biology. 2022;18:1005–1013. doi: 10.1038/s41589-022-01077-5. [DOI] [PubMed] [Google Scholar]
- Kim SK, Han GH, Seong W, Kim H, Kim SW, Lee DH, Lee SG. CRISPR interference-guided balancing of a biosynthetic mevalonate pathway increases terpenoid production. Metabolic Engineering. 2016;38:228–240. doi: 10.1016/j.ymben.2016.08.006. [DOI] [PubMed] [Google Scholar]
- Kim S, Ji S, Koh HR. CRISPR as a diagnostic tool. Biomolecules. 2021;11:1162. doi: 10.3390/biom11081162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B, Kim HJ, Lee SJ. Effective blocking of microbial transcriptional initiation by dCas9-NG-mediated CRISPR interference. Journal of Microbiology and Biotechnology. 2020;30:1919–1926. doi: 10.4014/jmb.2008.08058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Lee WJ, Oh Y, Kang SH, Hur JK, Lee H, Song W, Lim KS, Park YH, Song BS, Jin Y, Jun BH, Jung C, Lee DS, Kim SU, Lee SH. Enhancement of target specificity of CRISPR-Cas12a by using a chimeric DNA-RNA guide. Nucleic Acids Research. 2020;48:8601–8616. doi: 10.1093/nar/gkaa605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Lim JW, Jeong H, Lee SJ, Lee DW, Kim T, Lee SJ. Development of a highly specific and sensitive cadmium and lead microbial biosensor using synthetic CadC-T7 genetic circuitry. Biosensors & Bioelectronics. 2016;79:701–708. doi: 10.1016/j.bios.2015.12.101. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Oh SY, Lee SJ. Single-base genome editing in Corynebacterium glutamicum with the help of negative selection by target-mismatched CRISPR/Cpf1. Journal of Microbiology and Biotechnology. 2020;30:1583–1591. doi: 10.4014/jmb.2006.06036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HK, Song M, Lee J, Menon AV, Jung S, Kang YM, Choi JW, Woo E, Koh HC, Nam JW, Kim H. In vivo high-throughput profiling of CRISPR-Cpf1 activity. Nature Methods. 2017;14:153–159. doi: 10.1038/nmeth.4104. [DOI] [PubMed] [Google Scholar]
- Kleinstiver BP, Sousa AA, Walton RT, Tak YE, Hsu JY, Clement K, Welch MM, Horng JE, Malagon-Lopez J, Scarfo I, Maus MV, Pinello L, Aryee MJ, Joung JK. Engineered CRISPR-Cas12a variants with increased activities and improved targeting ranges for gene, epigenetic and base editing. Nature Biotechnology. 2019;37:276–282. doi: 10.1038/s41587-018-0011-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klompe SE, Vo PLH, Halpin-Healy TS, Sternberg SH. Transposon-encoded CRISPR-Cas systems direct RNA-guided DNA integration. Nature. 2019;571:219–225. doi: 10.1038/s41586-019-1323-z. [DOI] [PubMed] [Google Scholar]
- Knott GJ, Doudna JA. CRISPR-Cas guides the future of genetic engineering. Science. 2018;361:866–869. doi: 10.1126/science.aat5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komor AC, Kim YB, Packer MS, Zuris JA, Liu DR. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature. 2016;533:420–424. doi: 10.1038/nature17946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konermann S, Brigham MD, Trevino AE, Joung J, Abudayyeh OO, Barcena C, Hsu PD, Habib N, Gootenberg JS, Nishimasu H, Nureki O, Zhang F. Genome-scale transcriptional activation by an engineered CRISPR-Cas9 complex. Nature. 2015;517:583–588. doi: 10.1038/nature14136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozaeva E, Volkova S, Matos MRA, Mezzina MP, Wulff T, Volke DC, Nielsen LK, Nikel PI. Model-guided dynamic control of essential metabolic nodes boosts acetyl-coenzyme A-dependent bioproduction in rewired Pseudomonas putida. Metabolic Engineering. 2021;67:373–386. doi: 10.1016/j.ymben.2021.07.014. [DOI] [PubMed] [Google Scholar]
- Kruasuwan W, Puseenam A, Phithakrotchanakoon C, Tanapongpipat S, Roongsawang N. Modulation of heterologous protein secretion in the thermotolerant methylotrophic yeast Ogataea thermomethanolica TBRC 656 by CRISPR-Cas9 system. PLoS One. 2021;16:e0258005. doi: 10.1371/journal.pone.0258005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SS, Ding N, Sun YD, Yuan TL, Li J, Yuan QC, Liu LZ, Yang J, Wang Q, Kolomeisky AB, Hilton IB, Zuo EW, Gao X. Single C-to-T substitution using engineered APOBEC3G-nCas9 base editors with minimum genome- and transcriptome-wide off-target effects. Science Advances. 2020;6:eaba1773. doi: 10.1126/sciadv.aba1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Kim HJ, Lee SJ. CRISPR-Cas9-mediated pinpoint microbial genome editing aided by target-mismatched sgRNAs. Genome Research. 2020;30:768–775. doi: 10.1101/gr.257493.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Kim HJ, Lee SJ. Mismatch intolerance of 5'-truncated sgRNAs in CRISPR/Cas9 enables efficient microbial single-base genome editing. International Journal of Molecular Sciences. 2021;22:6427. doi: 10.3390/ijms22126457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Kim HJ, Lee SJ. Control of λ lysogenic Escherichia coli cells by synthetic λ phage carrying cIantisense. ACS Synthetic Biology. 2022;11:3829–3835. doi: 10.1021/acssynbio.2c00409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Kim HJ, Park YJ, Lee SJ. Efficient single-nucleotide microbial genome editing achieved using CRISPR/Cpf1 with maximally 3'-end-truncated crRNAs. ACS Synthetic Biology. 2022;11:2134–2143. doi: 10.1021/acssynbio.2c00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Lee SJ. Advances in accurate microbial genome-editing CRISPR technologies. Journal of Microbiology and Biotechnology. 2021;31:903–911. doi: 10.4014/jmb.2106.06056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon-Buitimea A, Balderas-Cisneros FJ, Garza-Cardenas CR, Garza-Cervantes JA, Morones-Ramirez JR. Synthetic biology tools for engineering microbial cells to fight superbugs. Frontiers in Bioengineering and Biotechnology. 2022;10:869206. doi: 10.3389/fbioe.2022.869206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li KF, Cai DB, Wang ZQ, He ZL, Chen SW. Development of an efficient genome editing tool in Bacillus licheniformis using CRISPR-Cas9 Nickase. Applied and Environmental Microbiology. 2018;84:e02608–02617. doi: 10.1128/AEM.02608-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Chen J, Minton NP, Zhang Y, Wen Z, Liu J, Yang H, Zeng Z, Ren X, Yang J, Gu Y, Jiang W, Jiang Y, Yang S. CRISPR-based genome editing and expression control systems in Clostridium acetobutylicum and Clostridium beijerinckii. Biotechnology Journal. 2016;11:961–972. doi: 10.1002/biot.201600053. [DOI] [PubMed] [Google Scholar]
- Li MY, Chen JZ, Wang Y, Liu J, Huang JW, Chen N, Zheng P, Sun JB. Efficient multiplex gene repression by CRISPR-dCpf1 in Corynebacterium glutamicum. Frontiers in Bioengineering and Biotechnology. 2020;8:357. doi: 10.3389/fbioe.2020.00357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SY, Cheng QX, Wang JM, Li XY, Zhang ZL, Gao S, Cao RB, Zhao GP, Wang J. CRISPR-Cas12a-assisted nucleic acid detection. Cell Discov. 2018;4:20. doi: 10.1038/s41421-018-0028-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li PS, Fu XF, Zhang L, Li SZ. CRISPR/Cas-based screening of a gene activation library in Saccharomyces cerevisiae identifies a crucial role of OLE1 in thermotolerance. Microbial Biotechnology. 2019;12:1154–1163. doi: 10.1111/1751-7915.13333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XJ, Huang LG, Pan LJ, Wang B, Pan L. CRISPR/dCas9-mediated epigenetic modification reveals differential regulation of histone acetylation on Aspergillus niger secondary metabolite. Microbiological Research. 2021;245:126694. doi: 10.1016/j.micres.2020.126694. [DOI] [PubMed] [Google Scholar]
- Li L, Li S, Wu N, Wu J, Wang G, Zhao G, Wang J. HOLMESv2: A CRISPR-Cas12b-assisted platform for nucleic acid detection and DNA methylation quantitation. ACS Synthetic Biology. 2019;8:2228–2237. doi: 10.1021/acssynbio.9b00209. [DOI] [PubMed] [Google Scholar]
- Li YJ, Pan SF, Zhang Y, Ren M, Feng MX, Peng N, Chen LM, Liang YX, She QX. Harnessing Type I and Type III CRISPR-Cas systems for genome editing. Nucleic Acids Research. 2016;44:e34. doi: 10.1093/nar/gkv1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Wang Y, Liu Y, Yang B, Wang X, Wei J, Lu Z, Zhang Y, Wu J, Huang X, Yang L, Chen J. Base editing with a Cpf1-cytidine deaminase fusion. Nature Biotechnology. 2018;36:324–327. doi: 10.1038/nbt.4102. [DOI] [PubMed] [Google Scholar]
- Li L, Wei KK, Zheng GS, Liu XC, Chen SX, Jiang WH, Lu YH. CRISPR-Cpf1-assisted multiplex genome editing and transcriptional repression in Streptomyces. Applied and Environmental Microbiology. 2018;84:e00827–e1818. doi: 10.1128/AEM.00827-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian JZ, HamediRad M, Hu SM, Zhao HM. Combinatorial metabolic engineering using an orthogonal tri-functional CRISPR system. Nature Communications. 2017;8:1688. doi: 10.1038/s41467-017-01695-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim Y, Bak SY, Sung K, Jeong E, Lee SH, Kim JS, Bae S, Kim SK. Structural roles of guide RNAs in the nuclease activity of Cas9 endonuclease. Nature Communications. 2016;7:13350. doi: 10.1038/ncomms13350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YN, Cradick TJ, Brown MT, Deshmukh H, Ranjan P, Sarode N, Wile BM, Vertino PM, Stewart FJ, Bao G. CRISPR/Cas9 systems have off-target activity with insertions or deletions between target DNA and guide RNA sequences. Nucleic Acids Research. 2014;42:7473–7485. doi: 10.1093/nar/gku402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Chen L, Jiang YP, Zhou ZH, Zou G. Efficient genome editing in filamentous fungus Trichoderma reesei using the CRISPR/Cas9 system. Cell Discovery. 2015;1:15007. doi: 10.1038/celldisc.2015.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Gallay C, Kjos M, Domenech A, Slager J, van Kessel SP, Knoops K, Sorg RA, Zhang JR, Veening JW. High-throughput CRISPRi phenotyping identifies new essential genes in Streptococcus pneumoniae. Molecular Systems Biology. 2017;13:931. doi: 10.15252/msb.20167449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Huang C, Guo J, Zhang P, Chen T, Wang Z, Zhao X. Development and characterization of a CRISPR/Cas9n-based multiplex genome editing system for Bacillus subtilis. Biotechnology for Biofuels. 2019;12:197. doi: 10.1186/s13068-019-1537-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XL, Hussain M, Dai JG, Li YH, Zhang LJ, Yang J, Ali Z, He NY, Tang YJ. Programmable biosensors based on RNA-guided CRISPR/Cas endonuclease. Biological Procedures Online. 2022;24:2. doi: 10.1186/s12575-021-00163-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Liu MS, Shi T, Sun GN, Gao N, Zhao XJ, Guo X, Ni XM, Yuan QQ, Feng JH, Liu ZM, Guo YM, Chen JZ, Wang Y, Zheng P, Sun JB. CRISPR-assisted rational flux-tuning and arrayed CRISPRi screening of an l-proline exporter for l-proline hyperproduction. Nature Communications. 2022;13:891. doi: 10.1038/s41467-022-28501-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Song L, Peng Q, Zhu Q, Shi X, Xu M, Wang Q, Zhang Y, Cai M. A programmable high-expression yeast platform responsive to user-defined signals. Science Advances. 2022;8:eabl5166. doi: 10.1126/sciadv.abl5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wan XY, Wang BJ. Engineered CRISPRa enables programmable eukaryote-like gene activation in bacteria. Nature Communications. 2019;10:3693. doi: 10.1038/s41467-019-11479-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu ZH, Yang SH, Yuan X, Shi YY, Ouyang L, Jiang SJ, Yi L, Zhang GM. CRISPR-assisted multi-dimensional regulation for fine-tuning gene expression in Bacillus subtilis. Nucleic Acids Research. 2019;47:e40. doi: 10.1093/nar/gkz072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Ge M, Wang B, Sun C, Wang J, Dong Y, Xi JJ. CRISPR/Cas9-deaminase enables robust base editing in Rhodobacter sphaeroides 2.4.1. Microbial Cell Factories. 2020;19:93. doi: 10.1186/s12934-020-01345-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma D, Xu Z, Zhang Z, Chen X, Zeng X, Zhang Y, Deng T, Ren M, Sun Z, Jiang R, Xie Z. Engineer chimeric Cas9 to expand PAM recognition based on evolutionary information. Nature Communications. 2019;10:560. doi: 10.1038/s41467-019-08395-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mali P, Yang LH, Esvelt KM, Aach J, Guell M, DiCarlo JE, Norville JE, Church GM. RNA-guided human genome engineering via Cas9. Science. 2013;339:823–826. doi: 10.1126/science.1232033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marraffini LA, Sontheimer EJ. Self versus non-self discrimination during CRISPR RNA-directed immunity. Nature. 2010;463:568–571. doi: 10.1038/nature08703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon MA, Prakash TP, Cleveland DW, Bennett CF, Rahdar M. Chemically modified Cpf1-CRISPR RNAs mediate efficient genome editing in mammalian cells. Molecular Therapy. 2018;26:1228–1240. doi: 10.1016/j.ymthe.2018.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimee M, Tucker AC, Voigt CA, Lu TK. Programming a human commensal bacterium, Bacteroides thetaiotaomicron, to sense and respond to stimuli in the Murine Gut Microbiota. Cell Systems. 2015;1:62–71. doi: 10.1016/j.cels.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon SB, Lee JM, Kang JG, Lee NE, Ha DI, Kim DY, Kim SH, Yoo K, Kim D, Ko JH, Kim YS. Highly efficient genome editing by CRISPR-Cpf1 using CRISPR RNA with a uridinylate-rich 3'-overhang. Nature Communications. 2018;9:3651. doi: 10.1038/s41467-018-06129-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozsik L, Hoekzema M, de Kok NAW, Bovenberg RAL, Nygard Y, Driessen AJM. CRISPR-based transcriptional activation tool for silent genes in filamentous fungi. Scientific Reports. 2021;11:1118. doi: 10.1038/s41598-020-80864-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller V, Rajer F, Frykholm K, Nyberg LK, Quaderi S, Fritzsche J, Kristiansson E, Ambjornsson T, Sandegren L, Westerlund F. Direct identification of antibiotic resistance genes on single plasmid molecules using CRISPR/Cas9 in combination with optical DNA mapping. Scientific Reports. 2016;6:37938. doi: 10.1038/srep37938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myhrvold C, Freije CA, Gootenberg JS, Abudayyeh OO, Metsky HC, Durbin AF, Kellner MJ, Tan AL, Paul LM, Parham LA, Garcia KF, Barnes KG, Chak B, Mondini A, Nogueira ML, Isern S, Michael SF, Lorenzana I, Yozwiak NL, MacInnis BL, Bosch I, Gehrke L, Zhang F, Sabeti PC. Field-deployable viral diagnostics using CRISPR-Cas13. Science. 2018;360:444–448. doi: 10.1126/science.aas8836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najjar D, Rainbow J, Sharma Timilsina S, Jolly P, de Puig H, Yafia M, Durr N, Sallum H, Alter G, Li JZ, Yu XG, Walt DR, Paradiso JA, Estrela P, Collins JJ, Ingber DE. A lab-on-a-chip for the concurrent electrochemical detection of SARS-CoV-2 RNA and anti-SARS-CoV-2 antibodies in saliva and plasma. Nature Biomedical Engineering. 2022;6:968–978. doi: 10.1038/s41551-022-00919-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalefski EA, Patel N, Leung PJY, Islam Z, Kooistra RM, Parikh I, Marion E, Knott GJ, Doudna JA, Le Ny AM, Madan D. Kinetic analysis of Cas12a and Cas13a RNA-Guided nucleases for development of improved CRISPR-Based diagnostics. iScience. 2021;24:102996. doi: 10.1016/j.isci.2021.102996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak DD, Metcalf WW. Cas9-mediated genome editing in the methanogenic archaeon Methanosarcina acetivorans. Proceedings of the National Academy of Sciences of the United States of America. 2017;114:2976–2981. doi: 10.1073/pnas.1618596114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J, Keasling JD. Engineering cellular metabolism. Cell. 2016;164:1185–1197. doi: 10.1016/j.cell.2016.02.004. [DOI] [PubMed] [Google Scholar]
- Nishimasu H, Shi X, Ishiguro S, Gao L, Hirano S, Okazaki S, Noda T, Abudayyeh OO, Gootenberg JS, Mori H, Oura S, Holmes B, Tanaka M, Seki M, Hirano H, Aburatani H, Ishitani R, Ikawa M, Yachie N, Zhang F, Nureki O. Engineered CRISPR-Cas9 nuclease with expanded targeting space. Science. 2018;361:1259–1262. doi: 10.1126/science.aas9129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okano K, Sato Y, Hizume T, Honda K. Genome editing by miniature CRISPR/Cas12f1 enzyme in Escherichia coli. Journal of Bioscience and Bioengineering. 2021;132:120–124. doi: 10.1016/j.jbiosc.2021.04.009. [DOI] [PubMed] [Google Scholar]
- Ostrov N, Nyerges A, Chiappino-Pepe A, Rudolph A, Baas-Thomas M, Church GM. Synthetic genomes with altered genetic codes. Current Opinion in Systems Biology. 2020;24:32–40. doi: 10.1016/j.coisb.2020.09.007. [DOI] [Google Scholar]
- Pacheco AR, Lazarus JE, Sit B, Schmieder S, Lencer WI, Blondel CJ, Doench JG, Davis BM, Waldor MK. CRISPR screen reveals that EHEC's T3SS and Shiga Toxin Rely on shared host factors for infection. mBio. 2018;9:e01003–e01018. doi: 10.1128/mBio.01003-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardee K, Green AA, Takahashi MK, Braff D, Lambert G, Lee JW, Ferrante T, Ma D, Donghia N, Fan M, Daringer NM, Bosch I, Dudley DM, O'Connor DH, Gehrke L, Collins JJ. Rapid, low-cost detection of Zika virus using programmable biomolecular components. Cell. 2016;165:1255–1266. doi: 10.1016/j.cell.2016.04.059. [DOI] [PubMed] [Google Scholar]
- Park HM, Liu H, Wu J, Chong A, Mackley V, Fellmann C, Rao A, Jiang F, Chu H, Murthy N, Lee K. Extension of the crRNA enhances Cpf1 gene editing in vitro and in vivo. Nature Communications. 2018;9:3313. doi: 10.1038/s41467-018-05641-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Shin H, Lee SM, Um Y, Woo HM. RNA-guided single/double gene repressions in Corynebacterium glutamicum using an efficient CRISPR interference and its application to industrial strain. Microbial Cell Factories. 2018;17:4. doi: 10.1186/s12934-017-0843-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl C, Kiel JAKW, Driessen AJM, Bovenberg RAL, Nygard Y. CRISPR/Cas9 based genome editing of Penicillium chrysogenum. ACS Synthetic Biology. 2016;5:754–764. doi: 10.1021/acssynbio.6b00082. [DOI] [PubMed] [Google Scholar]
- Purnick PE, Weiss R. The second wave of synthetic biology: From modules to systems. Nature Reviews Molecular Cell Biology. 2009;10:410–422. doi: 10.1038/nrm2698. [DOI] [PubMed] [Google Scholar]
- Qi LS, Larson MH, Gilbert LA, Doudna JA, Weissman JS, Arkin AP, Lim WA. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell. 2013;152:1173–1183. doi: 10.1016/j.cell.2013.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin C, Liu J, Zhu W, Zeng M, Xu K, Ding J, Zhou H, Zhu J, Ke Y, Li LY, Sheng G, Li Z, Luo H, Jiang S, Chen K, Ding X, Meng H. One-pot visual detection of African swine fever virus using CRISPR-Cas12a. Front. Vet. Sci. 2022;9:962438. doi: 10.3389/fvets.2022.962438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu XY, Zhu LY, Zhu CS, Ma JX, Hou T, Wu XM, Xie SS, Min L, Tan DA, Zhang DY, Zhu L. Highly effective and low-cost microRNA detection with CRISPR-Cas9. ACS Synthetic Biology. 2018;7:807–813. doi: 10.1021/acssynbio.7b00446. [DOI] [PubMed] [Google Scholar]
- Quince C, Walker AW, Simpson JT, Loman NJ, Segata N. Shotgun metagenomics, from sampling to analysis. Nature Biotechnology. 2017;35:833–844. doi: 10.1038/nbt.3935. [DOI] [PubMed] [Google Scholar]
- Ran FA, Hsu PD, Lin CY, Gootenberg JS, Konermann S, Trevino AE, Scott DA, Inoue A, Matoba S, Zhang Y, Zhang F. Double nicking by RNA-guided CRISPR Cas9 for enhanced genome editing specificity. Cell. 2013;154:1380–1389. doi: 10.1016/j.cell.2013.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch JN, Valois E, Solley SC, Braig F, Lach RS, Audouard M, Ponce-Rojas JC, Costello MS, Baxter NJ, Kosik KS, Arias C, Acosta-Alvear D, Wilson MZ. A scalable, easy-to-deploy protocol for Cas13-based detection of SARS-CoV-2 genetic material. Journal of Clinical Microbiology. 2021;59:e02402–02420. doi: 10.1128/JCM.02402-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riglar DT, Silver PA. Engineering bacteria for diagnostic and therapeutic applications. Nature Reviews Microbiology. 2018;16:214–225. doi: 10.1038/nrmicro.2017.172. [DOI] [PubMed] [Google Scholar]
- Ro DK, Paradise EM, Ouellet M, Fisher KJ, Newman KL, Ndungu JM, Ho KA, Eachus RA, Ham TS, Kirby J, Chang MC, Withers ST, Shiba Y, Sarpong R, Keasling JD. Production of the antimalarial drug precursor artemisinic acid in engineered yeast. Nature. 2006;440:940–943. doi: 10.1038/nature04640. [DOI] [PubMed] [Google Scholar]
- Rock JM, Hopkins FF, Chavez A, Diallo M, Chase MR, Gerrick ER, Pritchard JR, Church GM, Rubin EJ, Sassetti CM, Schnappinger D, Fortune SM. Programmable transcriptional repression in Mycobacteria using an orthogonal CRISPR interference platform. Nature Microbiology. 2017;2:16274. doi: 10.1038/nmicrobiol.2016.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossetti M, Merlo R, Bagheri N, Moscone D, Valenti A, Saha A, Arantes PR, Ippodrino R, Ricci F, Treglia I, Delibato E, van der Oost J, Palermo G, Perugino G, Porchetta A. Enhancement of CRISPR/Cas12a trans-cleavage activity using hairpin DNA reporters. Nucleic Acids Research. 2022;50:8377–8391. doi: 10.1093/nar/gkac578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousset F, Cui L, Siouve E, Becavin C, Depardieu F, Bikard D. Genome-wide CRISPR-dCas9 screens in E. coli identify essential genes and phage host factors. PLoS Genetics. 2018;14:e1007749. doi: 10.1371/journal.pgen.1007749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan DE, Taussig D, Steinfeld I, Phadnis SM, Lunstad BD, Singh M, Vuong X, Okochi KD, McCaffrey R, Olesiak M, Roy S, Yung CW, Curry B, Sampson JR, Bruhn L, Dellinger DJ. Improving CRISPR-Cas specificity with chemical modifications in single-guide RNAs. Nucleic Acids Research. 2018;46:792–803. doi: 10.1093/nar/gkx1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Moreno J, Schaerli Y. CRISPR-based gene expression control for synthetic gene circuits. Biochemical Society Transactions. 2020;48:1979–1993. doi: 10.1042/BST20200020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuler G, Hu C, Ke A. Structural basis for RNA-guided DNA cleavage by IscB-omegaRNA and mechanistic comparison with Cas9. Science. 2022;376:1476–1481. doi: 10.1126/science.abq7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuller A, Wolansky L, Berger H, Studt L, Gacek-Matthews A, Sulyok M, Strauss J. A novel fungal gene regulation system based on inducible VPR-dCas9 and nucleosome map-guided sgRNA positioning. Applied Microbiology and Biotechnology. 2020;104:9801–9822. doi: 10.1007/s00253-020-10900-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz C, Frogue K, Ramesh A, Misa J, Wheeldon I. CRISPRi repression of nonhomologous end-joining for enhanced genome engineering via homologous recombination in Yarrowia lipolytica. Biotechnology and Bioengineering. 2017;114:2896–2906. doi: 10.1002/bit.26404. [DOI] [PubMed] [Google Scholar]
- Schwartz CM, Hussain MS, Blenner M, Wheeldon I. Synthetic RNA polymerase III promoters facilitate high-efficiency CRISPR-Cas9-mediated genome editing in Yarrowia lipolytica. ACS Synthetic Biology. 2016;5:356–359. doi: 10.1021/acssynbio.5b00162. [DOI] [PubMed] [Google Scholar]
- Shabestary K, Hernandez HP, Miao R, Ljungqvist E, Hallman O, Sporre E, dos Santos FB, Hudson EP. Cycling between growth and production phases increases cyanobacteria bioproduction of lactate. Metabolic Engineering. 2021;68:131–141. doi: 10.1016/j.ymben.2021.09.010. [DOI] [PubMed] [Google Scholar]
- Shen J, Zhou X, Shan Y, Yue H, Huang R, Hu J, Xing D. Sensitive detection of a bacterial pathogen using allosteric probe-initiated catalysis and CRISPR-Cas13a amplification reaction. Nature Communications. 2020;11:267. doi: 10.1038/s41467-019-14135-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So RWL, Chung SW, Lau HHC, Watts JJ, Gaudette E, Al-Azzawi ZAM, Bishay J, Lin LTW, Joung J, Wang XZ, Schmitt-Ulms G. Application of CRISPR genetic screens to investigate neurological diseases. Molecular Neurodegeneration. 2019;14:41. doi: 10.1186/s13024-019-0343-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Huang H, Xiong ZQ, Ai LZ, Yang S. CRISPR-Cas 9(D10A) nickase-assisted genome editing in Lactobacillus case i. Applied and Environmental Microbiology. 2017;83:e01259–e11217. doi: 10.1128/AEM.01259-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standage-Beier K, Zhang Q, Wang X. Targeted large-scale deletion of bacterial genomes using CRISPR-nickases. ACS Synthetic Biology. 2015;4:1217–1225. doi: 10.1021/acssynbio.5b00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strecker J, Ladha A, Gardner Z, Schmid-Burgk JL, Makarova KS, Koonin EV, Zhang F. RNA-guided DNA insertion with CRISPR-associated transposases. Science. 2019;365:48–53. doi: 10.1126/science.aax9181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strich JR, Chertow DS. CRISPR-Cas biology and its application to infectious diseases. Journal of Clinical Microbiology. 2019;57:e01307–01318. doi: 10.1128/JCM.01307-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stricker J, Cookson S, Bennett MR, Mather WH, Tsimring LS, Hasty J. A fast, robust and tunable synthetic gene oscillator. Nature. 2008;456:516–519. doi: 10.1038/nature07389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Guo W, Liu Z, Qiao S, Wang Z, Wang J, Qu L, Shan L, Sun F, Xu S, Bai O, Liang C. Direct MYD88(L265P) gene detection for diffuse large B-cell lymphoma (DLBCL) via a miniaturised CRISPR/dCas9-based sensing chip. Lab on a Chip. 2022;22:768–776. doi: 10.1039/D1LC01055G. [DOI] [PubMed] [Google Scholar]
- Sun Y, Liu H, Shen Y, Huang X, Song F, Ge X, Wang A, Zhang K, Li Y, Li C, Wan Y, Li J. Cas12a-activated universal field-deployable detectors for bacterial diagnostics. ACS Omega. 2020;5:14814–14821. doi: 10.1021/acsomega.0c01911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Yu L, Liu C, Ye S, Chen W, Li D, Huang W. One-tube SARS-CoV-2 detection platform based on RT-RPA and CRISPR/Cas12a. Journal of Translational Medicine. 2021;19:74. doi: 10.1186/s12967-021-02741-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taketani M, Zhang JB, Zhang SY, Triassi AJ, Huang YJ, Griffith LG, Voigt CA. Genetic circuit design automation for the gut resident species Bacteroides thetaiotaomicron. Nature Biotechnology. 2020;38:962–969. doi: 10.1038/s41587-020-0468-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakku SG, Ackerman CM, Myhrvold C, Bhattacharyya RP, Livny J, Ma P, Gomez GI, Sabeti PC, Blainey PC, Hung DT. Multiplexed detection of bacterial nucleic acids using Cas13 in droplet microarrays. PNAS Nexus. 2022;1:pgac021. doi: 10.1093/pnasnexus/pgac021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Y, Jorgensen TS, Whitford CM, Weber T, Lee SY. A versatile genetic engineering toolkit for E. coli based on CRISPR-prime editing. Nature Communications. 2021;12:5206. doi: 10.1038/s41467-021-25541-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanegas KG, Jarczynska ZD, Strucko T, Mortensen UH. Cpf1 enables fast and efficient genome editing in Aspergilli. Fungal Biology and Biotechnology. 2019;6:6. doi: 10.1186/s40694-019-0069-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanegas KG, Lehka BJ, Mortensen UH. SWITCH: A dynamic CRISPR tool for genome engineering and metabolic pathway control for cell factory construction in Saccharomyces cerevisiae. Microbial Cell Factories. 2017;16:25. doi: 10.1186/s12934-017-0632-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verwaal R, Buiting-Wiessenhaan N, Dalhuijsen S, Roubos JA. CRISPR/Cpf1 enables fast and simple genome editing of Saccharomyces cerevisiae. Yeast. 2018;35:201–211. doi: 10.1002/yea.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Chen G, Lyu Y, Feng E, Zhu L, Pan C, Zhang W, Liu X, Wang H. A CRISPR/Cas12a-based DNAzyme visualization system for rapid, non-electrically dependent detection of Bacillus anthracis. Emerging Microbes & Infections. 2022;11:428–437. doi: 10.1080/22221751.2021.2012091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Guan C, Guo J, Liu B, Wu Y, Xie Z, Zhang C, Xing XH. Pooled CRISPR interference screening enables genome-scale functional genomics study in bacteria with superior performance. Nature Communications. 2018;9:2475. doi: 10.1038/s41467-018-04899-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TM, Guo JH, Liu YY, Xue ZL, Zhang C, Xing XH. Genome-wide screening identifies promiscuous phosphatases impairing terpenoid biosynthesis in Escherichia coli. Applied Microbiology and Biotechnology. 2018;102:9771–9780. doi: 10.1007/s00253-018-9330-9. [DOI] [PubMed] [Google Scholar]
- Wang L, Jiang S, Chen C, He W, Wu X, Wang F, Tong T, Zou X, Li Z, Luo J, Deng Z, Chen S. Synthetic genomics: From DNA synthesis to genome design. Angewandte Chemie (international Ed. in English) 2018;57:1748–1756. doi: 10.1002/anie.201708741. [DOI] [PubMed] [Google Scholar]
- Wang Y, Ke Y, Liu W, Sun Y, Ding X. A one-pot toolbox based on cas12a/crRNA enables rapid foodborne pathogen detection at attomolar level. ACS Sensors. 2020;5:1427–1435. doi: 10.1021/acssensors.0c00320. [DOI] [PubMed] [Google Scholar]
- Wang Y, Liang X, Xu J, Nan L, Liu F, Duan G, Yang H. Rapid and ultrasensitive detection of methicillin-resistant Staphylococcus aureus based on CRISPR-Cas12a combined with recombinase-aided amplification. Frontiers in Microbiology. 2022;13:903298. doi: 10.3389/fmicb.2022.903298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YC, Sang SL, Zhang X, Tao HX, Guan Q, Liu CJ. Efficient genome editing by a miniature CRISPR-AsCas12f1 nuclease in Bacillus anthracis. Frontiers in Bioengineering and Biotechnology. 2022;9:825493. doi: 10.3389/fbioe.2021.825493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wang S, Chen W, Song L, Zhang Y, Shen Z, Yu F, Li M, Ji Q. CRISPR-Cas9 and CRISPR-assisted cytidine deaminase enable precise and efficient genome editing in Klebsiella pneumoniae. Applied and Environmental Microbiology. 2018;84:e01834–e11818. doi: 10.1128/AEM.01834-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Wang R, Wang D, Wu J, Li J, Wang J, Liu H, Wang Y. Cas12aVDet: a CRISPR/Cas12a-based platform for rapid and visual nucleic acid detection. Analytical Chemistry. 2019;91:12156–12161. doi: 10.1021/acs.analchem.9b01526. [DOI] [PubMed] [Google Scholar]
- Wang X, Xiong E, Tian T, Cheng M, Lin W, Wang H, Zhang G, Sun J, Zhou X. Clustered regularly interspaced short palindromic repeats/Cas9-mediated lateral flow nucleic acid assay. ACS Nano. 2020;14:2497–2508. doi: 10.1021/acsnano.0c00022. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhang Y, Chen J, Wang M, Zhang T, Luo W, Li Y, Wu Y, Zeng B, Zhang K, Deng R, Li W. Detection of SARS-CoV-2 and its mutated variants via CRISPR-Cas13-based transcription amplification. Analytical Chemistry. 2021;93:3393–3402. doi: 10.1021/acs.analchem.0c04303. [DOI] [PubMed] [Google Scholar]
- Wasels F, Jean-Marie J, Collas F, Lopez-Contreras AM, Lopes Ferreira N. A two-plasmid inducible CRISPR/Cas9 genome editing tool for Clostridium acetobutylicum. Journal of Microbiological Methods. 2017;140:5–11. doi: 10.1016/j.mimet.2017.06.010. [DOI] [PubMed] [Google Scholar]
- Welch NL, Zhu ML, Hua C, Weller J, Mirhashemi ME, Nguyen TG, Mantena S, Bauer MR, Shaw BM, Ackerman CM, Thakku SG, Tse MW, Kehe J, Uwera MM, Eversley JS, Bielwaski DA, McGrath G, Braidt J, Johnson J, Cerrato F, Moreno GK, Krasilnikova LA, Petros BA, Gionet GL, King E, Huard RC, Jalbert SK, Cleary ML, Fitzgerald NA, Gabriel SB, Gallagher GR, Smole SC, Madoff LC, Brown CM, Keller MW, Wilson MM, Kirby MK, Barnes JR, Park DJ, Siddle KJ, Happi CT, Hung DT, Springer M, MacInnis BL, Lemieux JE, Rosenberg E, Branda JA, Blainey PC, Sabeti PC, Myhrvold C. Multiplexed CRISPR-based microfluidic platform for clinical testing of respiratory viruses and identification of SARS-CoV-2 variants. Nature Medicine. 2022;28:1083–1094. doi: 10.1038/s41591-022-01734-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weninger A, Hatzl AM, Schmid C, Vogl T, Glieder A. Combinatorial optimization of CRISPR/Cas9 expression enables precision genome engineering in the methylotrophic yeast Pichia pastoris. Journal of Biotechnology. 2016;235:139–149. doi: 10.1016/j.jbiotec.2016.03.027. [DOI] [PubMed] [Google Scholar]
- Wensing L, Sharma J, Uthayakumar D, Proteau Y, Chavez A, Shapiro RS. A CRISPR interference platform for efficient genetic repression in Candida albicans. mSphere. 2019;4:e00002–e00019. doi: 10.1128/mSphere.00002-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Chen Z, Li C, Hao Y, Tang Y, Yuan Y, Chai L, Fan T, Yu J, Ma X, Al-Hartomy OA, Wageh S, Al-Sehemi AG, Luo Z, He Y, Li J, Xie Z, Zhang H. CRISPR-Cas12a-empowered electrochemical biosensor for rapid and ultrasensitive detection of SARS-CoV-2 delta variant. Nanomicro Letters. 2022;14:159. doi: 10.1007/s40820-022-00888-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YK, Liu YF, Lv XQ, Li JH, Du GC, Liu L. CAMERS-B: CRISPR/Cpf1 assisted multiple-genes editing and regulation system for Bacillus subtilis. Biotechnology and Bioengineering. 2020;117:1817–1825. doi: 10.1002/bit.27322. [DOI] [PubMed] [Google Scholar]
- Xin Q, Wang B, Pan L. Development and application of a CRISPR-dCpf1 assisted multiplex gene regulation system in Bacillus amyloliquefaciens LB1ba02. Microbiological Research. 2022;263:127131. doi: 10.1016/j.micres.2022.127131. [DOI] [PubMed] [Google Scholar]
- Xu XS, Chemparathy A, Zeng LP, Kempton HR, Shang S, Nakamura M, Qi LS. Engineered miniature CRISPR-Cas system for mammalian genome regulation and editing. Molecular Cell. 2021;81:4333–4345.e4334. doi: 10.1016/j.molcel.2021.08.008. [DOI] [PubMed] [Google Scholar]
- Yan MY, Yan HQ, Ren GX, Zhao JP, Guo XP, Sun YC. CRISPR-Cas12a-assisted recombineering in bacteria. Applied and Environmental Microbiology. 2017;83:e00947–e1917. doi: 10.1128/AEM.00947-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Edwards H, Xu P. CRISPR-Cas12a/Cpf1-assisted precise, efficient and multiplexed genome-editing in Yarrowia lipolytica. Metabolic Engineering Communications. 2020;10:e00112. doi: 10.1016/j.mec.2019.e00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan CQ, Tian T, Sun J, Hu ML, Wang XS, Xiong EH, Cheng M, Bao YJ, Lin W, Jiang JM, Yang CW, Chen Q, Zhang H, Wang H, Wang XR, Deng XB, Liao XP, Liu YH, Wang Z, Zhang GH, Zhou XM. Universal and naked-eye gene detection platform based on the clustered regularly interspaced short palindromic repeats/Cas12a/13a system. Analytical Chemistry. 2020;92:4029–4037. doi: 10.1021/acs.analchem.9b05597. [DOI] [PubMed] [Google Scholar]
- Zetsche B, Gootenberg JS, Abudayyeh OO, Slaymaker IM, Makarova KS, Essletzbichler P, Volz SE, Joung J, van der Oost J, Regev A, Koonin EV, Zhang F. Cpf1 is a single RNA-guided endonuclease of a class 2 CRISPR-Cas system. Cell. 2015;163:759–771. doi: 10.1016/j.cell.2015.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JL, Peng YZ, Liu D, Liu H, Cao YX, Li BZ, Li C, Yuan YJ. Gene repression via multiplex gRNA strategy in Y. lipolytica. Microbial Cell Factories. 2018;17:62. doi: 10.1186/s12934-018-0909-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Qian L, Wei W, Wang Y, Wang B, Lin P, Liu W, Xu L, Li X, Liu D, Cheng S, Li J, Ye Y, Li H, Zhang X, Dong Y, Zhao X, Liu C, Zhang HM, Ouyang Q, Lou C. Paired design of dCas9 as a systematic platform for the detection of featured nucleic acid sequences in pathogenic strains. ACS Synthetic Biology. 2017;6:211–216. doi: 10.1021/acssynbio.6b00215. [DOI] [PubMed] [Google Scholar]
- Zhang XH, Tee LY, Wang XG, Huang QS, Yang SH. Off-target effects in CRISPR/Cas9-mediated genome engineering. Molecular Therapy - Nucleic Acids. 2015;4:e264. doi: 10.1038/mtna.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Voigt CA. Engineered dCas9 with reduced toxicity in bacteria: Implications for genetic circuit design. Nucleic Acids Research. 2018;46:11115–11125. doi: 10.1093/nar/gky884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XC, Wang JM, Cheng QX, Zheng X, Zhao GP, Wang J. Multiplex gene regulation by CRISPR-ddCpf1. Cell Discovery. 2017;3:17018. doi: 10.1038/celldisc.2017.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang RY, Xu WS, Shao S, Wang QY. Gene silencing through CRISPR interference in bacteria: Current advances and future prospects. Frontiers in Microbiology. 2021;12:635227. doi: 10.3389/fmicb.2021.635227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhang HY, Wang ZP, Wu ZW, Wang Y, Tang N, Xu XX, Zhao SW, Chen WZ, Ji QJ. Programmable adenine deamination in bacteria using a Cas9-adenine-deaminase fusion. Chemical Science. 2020;11:1657–1664. doi: 10.1039/C9SC03784E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Boeke JD. CRISPR-Cas12a system in fission yeast for multiplex genomic editing and CRISPR interference. Nucleic Acids Research. 2020;48:5788–5798. doi: 10.1093/nar/gkaa329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao D, Li J, Li S, Xin X, Hu M, Price MA, Rosser SJ, Bi C, Zhang X. Glycosylase base editors enable C-to-A and C-to-G base changes. Nature Biotechnology. 2021;39:35–40. doi: 10.1038/s41587-020-0592-2. [DOI] [PubMed] [Google Scholar]
- Zhou J, Yin L, Dong Y, Peng L, Liu G, Man S, Ma L. CRISPR-Cas13a based bacterial detection platform: Sensing pathogen Staphylococcus aureus in food samples. Analytica Chimica Acta. 2020;1127:225–233. doi: 10.1016/j.aca.2020.06.041. [DOI] [PubMed] [Google Scholar]