Abstract
Persistence or recurrence of large B-cell lymphoma after CD19-CAR-T is common, yet data guiding management are limited. We describe outcomes and features following CAR-T treatment failure. Of 305 adults who received CD19-CAR-T, 182 experienced disease recurrence or progression (1-year cumulative incidence 63% [95%CI: 57–69]). Of 52 post-CAR-T biopsies evaluated by flow cytometry, 49 (94%) expressed CD19. Subsequent anti-cancer treatment was administered in 135/182 (74%) patients with CAR-T treatment failure. Median OS from the first post-CAR-T treatment was 8 months (95%CI 5.6–11.0). Polatuzumab-, standard chemotherapy-, and lenalidomide-based treatments were the most common approaches after CAR-T. No complete responses (CRs) were observed with conventional chemotherapy, while CR rates exceeding 30% were seen following polatuzumab- or lenalidomide-based therapies. Factors associated with poor OS among patients treated post-CAR-T were pre-CAR-T bulky disease (HR 2.27 [1.10–4.72]), lack of response to CAR-T (2.33 [1.02–5.29]), age >65 years (HR 2.65 [1.49–4.73]) and elevated LDH at post-CAR-T treatment (HR 2.95 [1.61–5.38]). The presence of ≥2 of these factors was associated with inferior OS compared to ≤1 (56% vs. 19%). In this largest analysis to date of patients who progressed or relapsed after CD19-CAR-T, survival is poor, though novel agents such as polatuzumab and lenalidomide may have hold promise.
INTRODUCTION
CD19-directed chimeric antigen receptor T-cell (CD19-CAR-T) therapy has transformed the care of relapsed or refractory (r/r) large B-cell lymphoma (LBCL). Both FDA-approved and Point-of-Care CD19-CAR-T cell (POC) products have resulted in unprecedented response rates of approximately 70% in this population.1–7 Unfortunately, over 60% of patients will ultimately progress or relapse following CD19-CAR-T.8–13
The treatment landscape for r/r LBCL is expanding. Polatuzumab, tafasitamab, selinexor, and loncastuximab are FDA-approved in this setting.14–17 Immune checkpoint inhibitors, lenalidomide, bi-specific antibodies, investigational CAR-T products, and allogeneic hematopoietic cell transplantation, as well as salvage chemotherapy, represent additional options. Nevertheless, it is unclear how they should be utilized after exposure to CAR-T.18, 19 Several groups have reported their experience treating relapses after CAR-T cell therapy, albeit with limited sample sizes and heterogeneous treatment strategies.11, 13, 19–22,
In this retrospective observational research study, our aims were: 1. Report characteristics and outcomes of LBCL patients whose disease relapsed or progressed after CD19-CAR-T therapy; 2. Characterize response and overall survival of first-line interventions after CAR-T therapy; 3. Identify risk factors for poor outcomes and develop a model for stratifying the mortality risk in patients receiving therapeutic interventions after CAR-T treatment.
MATERIAL AND METHODS
Study Population
This retrospective analysis included patients with r/r LBCL treated at Memorial Sloan Kettering Cancer Center (MSKCC, New York) and Sheba Medical Center (Tel Hashomer, Israel) with CD19-directed CAR-T cell therapy between April 2016 to May 2021 (Figure 1A). We included adults (age ≥ 18 years) treated with one of the following CD19-CAR-T products: axicabtagene ciloleucel (axi-cel), tisagenlecleucel (tisa-cel), lisocabtagene maraleucel (liso-cel), or a POC CD28-based product (NCT02772198).6, 7 Axi-cel and tisa-cel were given as standard-of-care therapy, and liso-cel was administered under the TRANSCEND NHL 001 study (NCT02631044). Patients who received additional anti-cancer treatment concomitantly with CAR-T administration were excluded. Patient data were captured in REDCap databases.23 The Institutional Review Boards of the participating institutions approved the study in accordance with the Declaration of Helsinki.
Figure 1. Outcomes following CD19-CAR-T in LBCL.
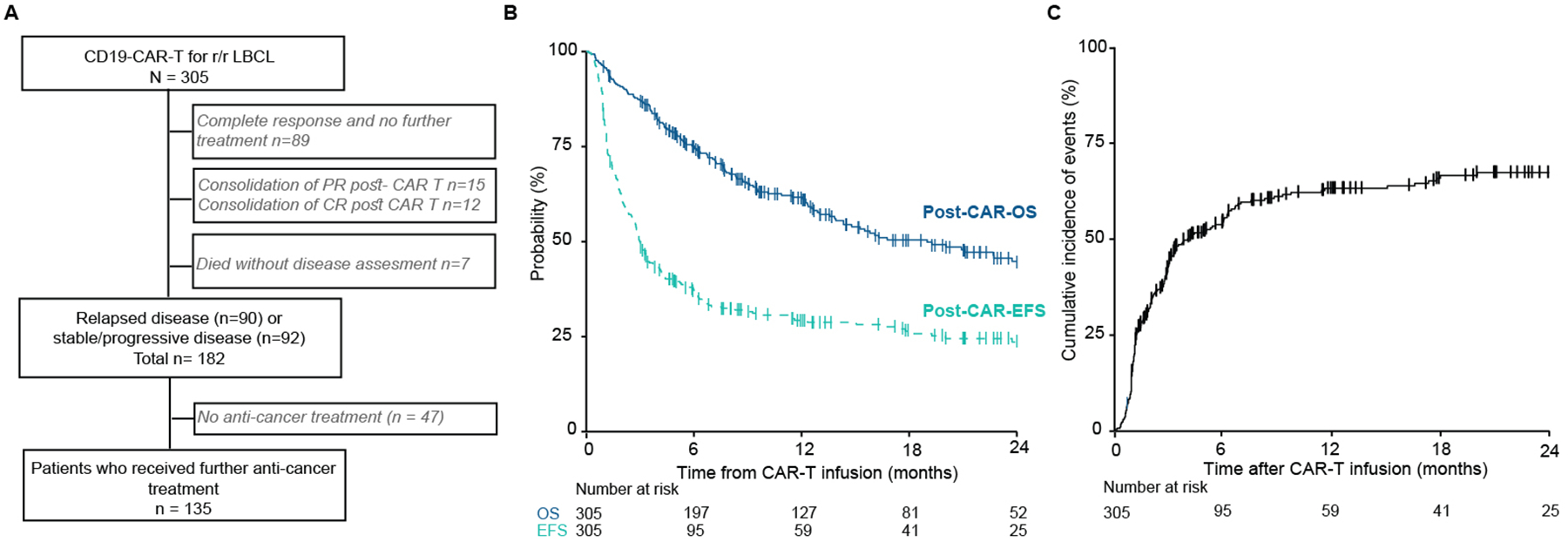
(A) A study flow diagram describing the population. Three hundred five patients were included; 165 patients were treated at MSKCC and 140 at Sheba Medical Center. One hundred thirty-five patients received subsequent anti-cancer treatment for relapsed or progressive or stable disease post-CAR-T therapy. (B) Post-CAR-overall survival (OS) and post-CAR-event-free survival (EFS) from the day of CAR-T infusion across the entire cohort. (C) Cumulative incidence of relapse or progression after CD19-CAR-T therapy.
MSKCC – Memorial Sloan Kettering Cancer Center; r/r – relapsed/refractory; LBCL – large B-cell lymphoma; PR – partial response; CR – complete response.
Definitions
Bulky disease was defined as the presence of any mass with a single diameter > 10 cm. Response assessment was performed locally according to the Lugano criteria.24 Best response denotes the best response achieved up to 100 days following cell infusion. Disease status before CAR-T infusion was determined according to the most recent disease assessment before infusion. Cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS) were graded using the American Society of Transplant and Cellular Therapy grading criteria.25 Event-free survival (EFS) was defined as the time from (1) CAR-T infusion (“post-CAR-EFS”) and (2) the initiation of first-line treatment after CAR-T (“post-treatment-EFS”) to the date of first documented progression, death due to any cause, or the initiation of the subsequent line of treatment. Similarly, post-CAR-overall survival (OS) and post-treatment-OS corresponded to time from CAR-T infusion and first-line treatment after CAR-T to vital status at last-follow up. Time to progression or relapse was measured from time of CAR-T infusion. Death without documented relapse or progression was considered a competing event. Table S1 lists additional definitions.
Flow Cytometry
Flow cytometry was performed as previously described26. Panels used for B cell analysis and methodology are presented in Table S2.
Statistical Analysis
Descriptive statistics, including median and interquartile range (IQR) for continuous variables and percentages for categorical variables, are provided. OS and EFS were estimated using the Kaplan-Meier method. Fisher’s exact or χ2 tests evaluated the association between categorical variables. The Wilcoxon rank-sum test or Kruskal-Wallis test was used to assess the difference in a continuous variable between/among patient groups. Clinically relevant variables were included in univariable Cox regression models for post-CAR-EFS and post-treatment-overall survival (OS). Variables meeting a P ≤ .1 in univariable models were introduced into multivariable Cox regression models stratified by center. Characteristics associated with adverse post-treatment-OS in the multivariable model were aggregated as a score into a risk-stratification system. An exploratory multivariable Cox regression analysis stratified by center and adjusted for age, response to CAR-T, and pre-treatment LDH was used to compare post-treatment-OS between different post-CAR-T treatment strategies. Data were analyzed using R (version 4.1.2).
RESULTS
Patient characteristics and outcomes
A total of 305 patients (MSKCC n=165 [54%], Sheba n=140 [46%]; Table 1) with a median age of 63 years (range: 20–86) received CD19-CAR-T therapy (axi-cel [n=116, 38%]; tisa-cel [n=83, 27%]; lisocel [n=28, 9%]; POC-CAR-T [n=78, 26%]). The primary histological subtype of LBCL was diffuse large B cell lymphoma not otherwise specified (n=236, 77%). A majority (211, 69%) had received 3 or more previous lines of therapy, 125 patients (41%) were generally heavily pretreated (>3 lines; 125, 41%), most had stage III-IV disease (216, 71%), and 134 (44%) and 43 (14%) had primary refractory disease and bulky disease, respectively.
Table 1.
Population characteristics
| Characteristic | Overall, N = 3051 |
|---|---|
| Age (years) at infusion | 63 (IQR: 51 – 71) |
| ≤ 65 | 176 (58%) |
| > 65 | 129 (42%) |
| Patient sex | |
| Female | 112 (37%) |
| Male | 193 (63%) |
| LBCL origin | |
| De novo | 191 (63%) |
| Transformed low-grade | 111 (37%) |
| Unknown | 3 |
| Primary diagnosis | |
| DLBCL NOS | 236 (77%) |
| High-grade b-cell lymphoma2 | 42 (14%) |
| Primary mediastinal B-cell lymphoma | 21 (7%) |
| Other3 | 6 (2%) |
| Cell of origin | |
| Germinal Center B cells | 125 (47%) |
| non- Germinal Center B cells | 139 (53%) |
| Unknown | 41 |
| Double/triple hit | 32 (15%) |
| Unknown | 92 |
| Number of prior lines of therapy | |
| 2 lines | 94 (31%) |
| 3 lines | 86 (28%) |
| 4–5 lines | 94 (31%) |
| 6 or more lines | 31 (10%) |
| Prior autologous transplantation | 76 (25%) |
| Prior allogeneic transplantation | 14 (5%) |
| Primary refractory disease up to apheresis | 134 (44%) |
| Unknown | 1 |
| Bulky disease at apheresis | 43 (14%) |
| Stage at apheresis | |
| ≤ II | 87 (29%) |
| III-IV | 216 (71%) |
| Unknown | 2 |
| Bridging therapy | 172 (56%) |
| Pre-CAR-T LDH | |
| Normal range | 138 (46%) |
| > ULN | 160 (54%) |
| Unknown | 7 |
| Disease status at the time of CAR-T infusion | |
| Complete response | 13 (4%) |
| Partial response | 60 (20%) |
| Stable/Progressive disease | 229 (76%) |
| Unknown | 3 |
| Days from diagnosis to CAR-T | 456 (286 – 810) |
| Unknown | 2 |
| CAR-T product | |
| Axicabtagene ciloleucel | 116 (38%) |
| Lisocabtagene maraleucel | 28 (9%) |
| POC-CAR-T | 78 (25%) |
| Tisagenlecleucel | 83 (27%) |
Median (IQR); n (%)
High-grade b-cell lymphomas with MYC and BCL2 and/or BCL6 rearrangement (n =30, 10%), High-grade B-cell lymphoma, NOS (n=12, 4%)
EBV-positive DLBCL, T-cell rich DLBCL, Intravascular large B-cell lymphoma
Abbreviations: Large B cell lymphoma (LBCL), Diffuse large B cell lymphoma Not otherwise specified (DLBCL NOS) Germinal Center B cells (GCB), Lactate dehydrogenase (LDH), Upper Limit of Normal (ULN), Point-of-Care CD19-CAR-T cell (POC)
CRS and ICANS of any grade were observed in 76% and 32% of patients, respectively, with severe (grade ≥2) CRS and ICANS developing in 33% and 23%. Most patients responded to CAR-T treatment (best overall response was 67%, 48% complete response [CR], 19% partial response [PR]). CAR-T product specific toxicity and response are presented in Table S3. With a median follow-up of 20 months (IQR: 10–30), the probability of 1-year post-CAR-OS and post-CAR-EFS was 62% (95% confidence interval [CI] 56–68) and 29% (95% CI 24–35), respectively (Figure 1B). At one year, the cumulative relapse or progression incidence rate was 63% ([95% CI 57–69], Figure 1C).
Predictors of post-CAR-EFS
Post-CAR-EFS reflects cases with CAR-T treatment failure due to relapse, progression, or death. Therefore, it is imperative to understand its determinants. We screened for candidate predictors in univariable analysis. Those meeting a significance criteria of P < .1 were introduced to a multivariable Cox regression model (Table 2). Non-germinal center lymphomas (Hazard Ratio [HR], 1.43; 95% CI, 1.04–1.97); P= .026), pre-apheresis primary refractory disease (HR, 1.64; 95% CI, 1.18–2.28); P = .003), elevated pre-CAR-T LDH (HR, 1.89; 95% CI, 1.37–2.60); P < .001), and presence of active disease at time of CAR-T infusion relative to complete metabolic remission (PR [HR, 1.90; 95% CI] and SD/PD [HR, 3.32; 95% CI, 0.80–13.8]; global P = .012). Apart from these traditional risk factors, Tisa-cel was also associated higher risk of treatment failure compared to axi-cel (HR, 2.03; 95% CI, 1.37–3.00, P = .005).
Table 2.
Univariable and multivariable Cox regression model for predictors of post-CAR-EFS
| Univariable analysis | Multivariable analysis* | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | N | HR1 | 95% CI1 | p-value | N | HR1 | 95% CI1 | p-value |
| Age at infusion (binary) | 305 | 0.36 | ||||||
| ≤ 65 | — | — | ||||||
| > 65 | 0.88 | 0.67, 1.15 | ||||||
| LBCL type | 302 | 0.061 | ||||||
| De novo | — | — | ||||||
| Transformed low-grade | 0.77 | 0.58, 1.02 | ||||||
| Cell of origin | 264 | 0.052 | 255 | 0.026 | ||||
| Germinal Center B cells | — | — | — | — | ||||
| non- Germinal Center B cells | 1.33 | 1.00, 1.77 | 1.43 | 1.04, 1.97 | ||||
| Double/triple hit 2 | 213 | 0.62 | ||||||
| No | — | — | ||||||
| Yes | 1.12 | 0.72, 1.75 | ||||||
| Number of prior lines of therapy | 305 | 0.36 | ||||||
| 2 lines | — | — | ||||||
| 3 lines | 1.19 | 0.84, 1.68 | 1.19 | |||||
| 4–5 lines | 0.88 | 0.62, 1.25 | 0.88 | |||||
| 6+ lines | 1.15 | 0.71, 1.85 | 1.15 | |||||
| Prior autologous transplantation | 305 | 0.14 | ||||||
| No | — | — | ||||||
| Yes | 0.79 | 0.58, 1.09 | ||||||
| Prior allogeneic transplantation | 305 | 0.77 | ||||||
| No | — | — | ||||||
| Yes | 1.10 | 0.60, 2.01 | ||||||
| Primary refractory disease up to apheresis | 304 | <0.001 | 255 | 0.003 | ||||
| No | — | — | — | — | ||||
| Yes | 1.70 | 1.30, 2.22 | 1.64 | 1.18, 2.28 | ||||
| Bulky disease at apheresis | 305 | 0.003 | 255 | 0.007 | ||||
| No | — | — | — | — | ||||
| Yes | 1.81 | 1.26, 2.59 | 1.82 | 1.18, 2.82 | ||||
| Stage at apheresis | 303 | 0.035 | 255 | 0.31 | ||||
| ≤ II | — | — | — | — | ||||
| III-IV | 1.38 | 1.01, 1.88 | 1.21 | 0.84, 1.73 | ||||
| Bridging therapy | 305 | 0.50 | ||||||
| No | — | — | ||||||
| Yes | 1.10 | 0.84, 1.44 | ||||||
| Pre-CAR-T LDH | 298 | <0.001 | 255 | <0.001 | ||||
| Normal range | — | — | — | — | ||||
| > ULN | 2.03 | 1.53, 2.69 | 1.89 | 1.37, 2.60 | ||||
| Disease status at the time of CAR-T infusion | 302 | <0.001 | 255 | 0.012 | ||||
| Complete response | — | — | — | — | ||||
| Partial response | 2.46 | 0.88, 6.90 | 1.90 | 0.44, 8.18 | ||||
| Stable/Progressive disease | 3.72 | 1.38, 10.0 | 3.32 | 0.80, 13.8 | ||||
| CART product | 305 | 0.002 | 255 | 0.005 | ||||
| Axicabtagene ciloleucel | — | — | — | — | ||||
| Lisocabtagene maraleucel | 1.15 | 0.71, 1.88 | 1.32 | 0.79, 2.23 | ||||
| POC-CAR-T | 1.88 | 1.33, 2.65 | 1.64 | 0.98, 2.74 | ||||
| Tisagenlecleucel | 1.56 | 1.11, 2.20 | 2.03 | 1.37, 3.00 | ||||
Multivariable cox regression analysis were stratified by center
HR = Hazard Ratio, CI = Confidence Interval
Double/triple hit is defined by two or three recurrent chromosome translocations; MYC/8q24 loci in combination with the t (14; 18) (q32; q21) bcl-2 gene or/and BCL6/3q27 chromosomal translocation.
Abbreviations: Large B cell lymphoma (LBCL), Lactate dehydrogenase (LDH), Upper Limit of Normal (ULN), Point-of-Care CD19-CAR-T cell (POC).
Disease features at CAR-T treatment failure
Of 182 patients with disease recurrence or persistence after CAR-T, 51% had CAR-T refractory disease, and 49% initially responded to CAR-T but then relapsed. Tissue biopsies to confirm disease persistence or recurrence were performed in 77 patients. Since antigen escape is a potential mechanism of CAR-T resistance,27–31 we assessed CD19 expression in 52 tumor biopsies. Interestingly, CD19 expression (Figure 2A) was absent in only 3/52 (6%), while the remaining samples had either dim (14/52, 27%) or normal (35/52, 67%) expression.
Figure 2. Characteristics at time of CAR-T treatment failure.
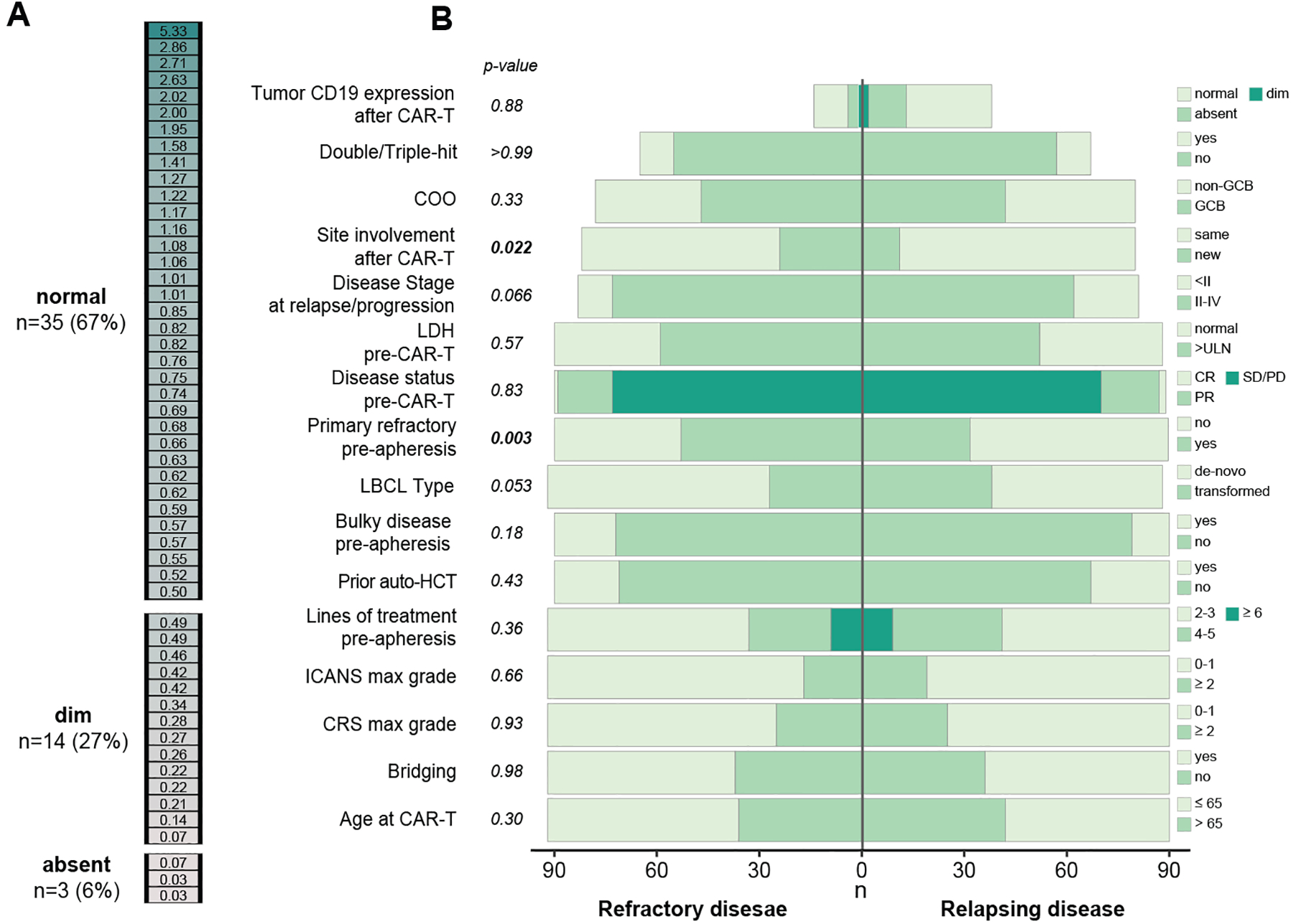
(A) CD19 expression was assessed by flow cytometry in 52 tumor biopsies that were performed after CD19-CAR-T therapy. The color intensity represent CD19 expression intensity as measured by the MFI ratio (see methods). Expression was categorized into dim and normal based on the MFI ratio level. Absent (B) A divergent bar plot representing differences between pre- and post-CAR-T features of LBCL refractory to CAR-T vs. LBCL that initially responded to CAR-T and then relapsed (Table S2). P-values represent Pearson’s Chi-squared test or Fisher’s exact test.
COO– Cell of origin; LDH – lactate dehydrogenase; LBCL – large B-cell lymphoma; auto-HCT – autologous hematopoietic stem cell transplantation; PR – partial response; CR – complete response; CRS–Cytokine release syndrome (CRS); ICANS– Central Nervous System (CNS); GCB– Germinal center B cells; ULN–Upper Limit of Normal; PR– Partial response; CR– Complete response; PD– Progressive disease; SD– Stable disease.
We compared baseline characteristics between refractory patients versus those relapsing after CAR-T to identify those potentially related to the type of CAR-T treatment failure (Figure 2B, Table S4). Primary refractory disease before apheresis was the only pre-CAR-T that varied between the groups, with a greater percentage in the CAR-T-refractory population (58% vs. 36%, P = .003).
To further understand the differences between these two patterns of disease response to CAR-T, we studied additional variables measured after CAR-T infusions. Patients in the refractory group were more likely to have new sites of disease involvement at time of disease assessment compared to the relapse group (30% vs. 14%, P = .017). Patients with CAR-T resistance had similar CD19 expression at disease progression to those with relapsed disease, both when considering expression categories (absent/dim/normal; P = .88) and MFI ratio (relative to a reference value) as a continuous covariate (P = .60). Collectively, our findings suggest that disease resistance and relapse are both common modes of CAR-T treatment failure and that better biomarkers are required to describe the differences in disease biology between them.
Therapy following CAR-T treatment failure
Of 182 patients with relapsed or stable/progressive disease post-CAR-T, 135 patients (74%; Figure 1A) received subsequent anti-cancer therapy at a median of 83 days (IQR: 53–130) after CAR-T infusion. Most patients (75/135, 56%) were treated for disease relapsing after response to CAR-T (CR 32%, PR 24%); the remaining patients (60/135, 44%) were treated for CAR-T-unresponsive disease.
To provide an indirect assessment of considerations underlying the decision to treat after CAR-T treatment failure, we compared patients receiving and not receiving anti-cancer treatment following CAR-T (Table S5). Untreated patients were more likely to be older at the time of CAR-T infusion (median age 65 [IQR: 57–72] vs. 60 [IQR: 49–69], P = .029) and have bulky disease before apheresis (28% vs. 12%).
Among patients who received further therapy after CAR-T treatment failure, the overall response rate was 39% (CR 20%; PR 19%). As expected, the median time from CAR-T to next line treatment for patients who had progressive or stable disease after CAR-T was shorter than those who initially responded and then relapsed (50 days [IQR 37–66] vs. 123 days [IQR 92–236], p<0.001). Median follow-up from time of first-line therapy after CAR-T was 15 months (IQR: 8–33). Median post-treatment-OS and post-treatment-EFS (Figure 3A) were 8.5 months (95% CI 5.6–11) and 1.9 months (95% CI 1.4–2.3), respectively. These poor outcomes highlight the urgent need for interventions to improve the care of patients requiring anti-cancer treatment after CAR-T.
Figure 3. Outcomes among patients receiving first treatment after CD19-CAR-T.
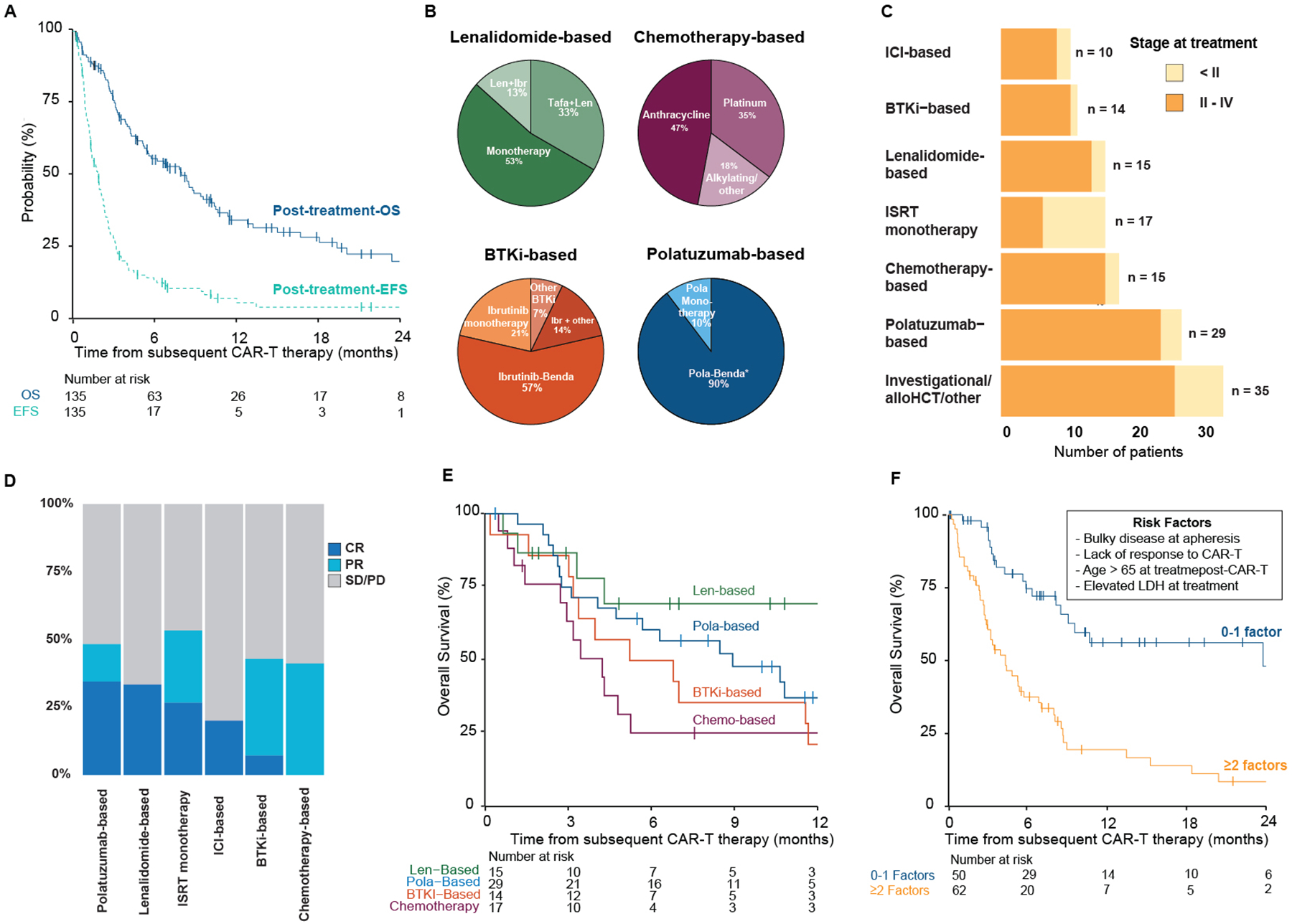
(A) Post-treatment-overall survival (OS) and post-treatment-event-free survival (EFS) from initiation of first-line treatment post-CD19-CAR-T. (B) Treatment components among the most common treatments groups. Treatment strategies of the remaining treatment groups are listed in Table S3. (C) First-line treatment strategies, by disease stage at time of relapse or progression, after CD19-CAR-T treatment failure. (D) Response rates by treatment group. (E) Post-treatment-OS by leading treatment strategies. (F) Overall survival by prognostic strata.
AutoSCT – autologous stem cell transplantation; GCB – germinal center B-cell; CRS – cytokine release storm; ICANS – immune effector cell-associated neurotoxicity syndrome; LDH – lactate dehydrogenase; ICI – immune checkpoint inhibitor; BTKi – Bruton’s tyrosine kinase inhibitor; ISRT – involved site radiation therapy; alloHCT – allogeneic hematopoietic stem cell transplantation; Ibr - Ibrutinib; Tafa – tafasitimab; Benda - bendamustine; CR – complete response; PR – partial response; SD/PD – stable disease/progressive disease
More than 30 distinct treatment strategies were employed for first-line therapy after CAR-T. Treatments were grouped by major drug classes or agents (Table S6). The predominant treatment categories (Figure 3B) included polatuzumab-based (n=29), chemotherapy approaches (n=17; anthracycline or platinum-based), lenalidomide-based (n=15), involved site radiation therapy (ISRT) monotherapy (n=15), and Bruton’s tyrosine kinase inhibitor-based (BTKi; n=14). Treatment assignments were made at the treating physician’s discretion and varied by center Monotherapy ISRT was primarily administered for localized diseases (Figure 3C).
Patient characteristics across the various treatment strategies differed meaningfully. Aggressive disease features such as bulky disease, primary refractory disease before apheresis, and lower response rates to CAR-T were more frequently observed in the chemotherapy group. Patients receiving lenalidomide were older and more heavily pretreated than other treatment groups while also having favorable CAR-T responses and lower pre-treatment LDH (i.e., before administration of post-CAR-T treatment). Patients treated with polatuzumab and BTKi-based strategies were typically younger than 60 years old, and over 70% of these patients had elevated pre-treatment LDH (Table S7). CR rates of systemic therapies ranged from 0% with chemotherapy to over 30% with lenalidomide and polatuzumab-based strategies (Figure 3D; Table S8). Patient-level outcome to the most common systemic treatment groups (polatuzumab-based (n=29), chemotherapy approaches (n=17), lenalidomide-based (n=15, and BTKi n=14) are depicted in Figure S1.
One-year survival following the most common systemic treatment strategies (1-year post-treatment-OS) ranged from 21% (95% CI 8–58) with BTKi and 25% (95% CI 11–59) with chemotherapy-based approaches to 69% (95% CI 48–100) with lenalidomide-based treatment (Figure 3E; Table S9). Polatuzumab-based strategies induced complete or partial responses in 48% of patients (CR n=10/29 [34%], PR n=4/29 [14%]), though these responses did not translate into prolonged 1-year post-treatment-OS (37% [95% CI 22–63]). Given the differences in post-treatment-OS rates, we performed an exploratory analysis to compare the leading treatment strategies. In a multivariable Cox regression model stratified by center and adjusted for potential drivers of treatment selection, including age, LDH at treatment, and previous response to CAR-T (Table S10), lenalidomide-based treatment was associated with improved post-treatment-OS compared to standard chemotherapy (HR 0.25 [0.07–0.85], P = .027). Our findings suggest that novel agents may induce remission after CAR-T treatment failure.
Post-CAR-T risk stratification system
Given poor outcomes following first-line treatment after CAR-T treatment failure, we sought to identify key predictors of mortality in this setting. We investigated associations between disease, patient, and therapy-related features with post-treatment-OS in univariable and multivariable Cox regression models. Older age (>65 years), bulky disease at apheresis, elevated LDH pre-treatment, shorter period between CAR-T to treatment (<100 days), advanced disease stage at treatment, and CAR-T refractoriness were candidate predictors of shorter post-treatment-OS in univariable analysis (Table 3). A multivariable Cox model for post-treatment OS, including candidate predictors, confirmed an independent association of bulky disease (HR 2.27 [95% CI: 1.10–4.72]), older age (HR 2.65 [95% CI 1.49–4.73]), elevated LDH, (HR 2.95 [95% CI 1.61–5.38]) and CAR-T refractoriness (95% CI 2.33 [1.02–5.29]). To stratify the risk of mortality for these patients, we propose a prognostic system including these four factors (pre-apheresis bulky disease, CAR-T refractoriness, age > 65, and pre-treatment LDH). Grouped by the presence of 0–1 versus ≥ 2 of these factors, the corresponding probability of 1-year survival was 56% (95% CI 42–75) vs. 19% (95% CI 11–35, Figure 3F), respectively.
Table 3.
Univariable and multivariable Cox regression models for post-treatment-OS in patients receiving anti-cancer treatment after CAR-T
| Univariable analysis | Multivariable analysis* | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | N | HR1 | 95% CI1 | p-value | N | HR1 | 95% CI1 | p-value |
| Age at infusion (years) | 135 | 0.035 | 112 | <0.001 | ||||
| ≤ 65 | — | — | — | — | ||||
| > 65 | 1.60 | 1.04, 2.47 | 2.65 | 1.49, 4.73 | ||||
| LBCL type | 133 | 0.64 | ||||||
| De novo | — | — | ||||||
| Transformed low-grade | 0.90 | 0.56, 1.43 | ||||||
| Cell of origin | 119 | 0.88 | ||||||
| Germinal Center B cells | — | — | ||||||
| non- Germinal Center B cells | 1.04 | 0.66, 1.63 | ||||||
| Double/triple hit cytogenetics translocations | 98 | 0.15 | ||||||
| No | — | — | ||||||
| Yes | 1.62 | 0.87, 3.00 | ||||||
| Prior autologous transplantation | 135 | 0.39 | ||||||
| No | — | — | ||||||
| Yes | 0.81 | 0.49, 1.33 | ||||||
| Primary refractory disease up to apheresis | 135 | 0.15 | ||||||
| No | — | — | ||||||
| Yes | 1.37 | 0.89, 2.10 | ||||||
| Bulky disease at apheresis | 135 | 0.090 | 112 | 0.027 | ||||
| no | — | — | — | — | ||||
| yes | 1.73 | 0.96, 3.12 | 2.27 | 1.10, 4.72 | ||||
| Bridging therapy | 135 | 0.31 | ||||||
| No | — | — | ||||||
| Yes | 1.25 | 0.81, 1.94 | ||||||
| CART costimulatory domain | 135 | 0.85 | ||||||
| 41bb | — | — | ||||||
| CD28 | 0.96 | 0.63, 1.47 | ||||||
| Maximal CRS grade | 135 | 0.42 | ||||||
| 0–1 | — | — | ||||||
| ≥ 2 | 1.23 | 0.75, 2.03 | ||||||
| Maximal ICANS grade | 135 | 0.30 | ||||||
| 0–1 | — | — | ||||||
| ≥ 2 | 1.37 | 0.77, 2.44 | ||||||
| Overall response to CAR-T | 135 | 0.058 | 112 | 0.044 | ||||
| Responder | — | — | — | — | ||||
| Nonresponder | 1.51 | 0.99, 2.32 | 2.33 | 1.02, 5.29 | ||||
| Pre-treatment LDH | 116 | <0.001 | 112 | <0.001 | ||||
| Normal range | — | — | — | — | ||||
| > ULN | 2.94 | 1.72, 5.02 | 2.95 | 1.61, 5.38 | ||||
| Disease stage at relapse or progression | 126 | 0.013 | 112 | 0.52 | ||||
| Stage I | — | — | — | — | ||||
| Stages II-IV | 2.07 | 1.11, 3.83 | 1.32 | 0.56, 3.08 | ||||
| Time from CAR-T to next treatment- 100 (days) | 135 | 0.058 | 112 | 0.73 | ||||
| >100 | — | — | — | — | ||||
| 0–100 | 1.54 | 0.98, 2.42 | 0.88 | 0.42, 1.83 | ||||
Multivariable cox regression model stratified by center
HR = Hazard Ratio, CI = Confidence Interval
Abbreviations: Large B cell lymphoma (LBCL), Lactate dehydrogenase (LDH), Upper Limit of Normal (ULN), Point-of-Care CD19-CAR-T cell (POC), Immune effector cell-associated neurotoxicity syndrome (ICANS), Cytokine release syndrome (CRS), Central Nervous System (CNS) Immunohistochemistry (IHC).
DISCUSSION
We sought to address knowledge gaps related to outcomes and management of patients who were failed by CAR-T therapy. Our results reflect the dismal consequences of lymphoma persistence after CAR-T and highlight the unmet need for therapies with long-lasting effects. Nonetheless, newer agents show activity after CAR-T exposure and should be further explored in this context. Finally, we propose a prognostic tool for identifying patients at the highest risk for early mortality after CAR-T, which may help stratify patients for future investigations in the post-CAR-T setting.
This observational study illustrates the immense contribution of CAR-T cells to our field. However, and in line with the pivotal trials and real-world data,1–3, 8, 9, 12, 32 60% of the studied population did not achieve long-term disease control, confirming that disease recurrence or progression represents the most significant challenge in r/r LBCL treated with CAR-T. We identified several risk factors for CAR-T treatment failure concordant with the literature and included treatment-refractory disease, bulky disease before apheresis, and elevated LDH before CAR-T infusion.1, 11, 12, 33 Notably, non-GCB cell-of-origin, was likewise associated with shorter post-CAR-EFS. To our knowledge, this represents the first report associating COO and adverse outcome after CAR-T. Previous studies have not identified the same effect but may have had less power to detect it or looked at endpoints other than post-CAR-EFS.10–13 The choice of CAR-T product was also notable. In a multivariable analysis, patients treated with tisa-cel were at increased risk for an EFS event compared to axi-cel. This finding should be interpreted with caution as latent confounders such as performance status may have guided product selection and could underlie worse outcomes. While many of the identified risk factors for treatment failure are fixed, they can be used to identify high-risk populations who could potentially benefit from further interventions such as post-CAR-T maintenance.
Antigen loss is a predominant immune escape mechanism in B-cell acute lymphoblastic leukemia.27–30 In our cohort, which we believe is the most extensive collection of LBCL post-CAR-T tumor samples reported to date, loss of CD19 was infrequent (only 6%). Furthermore, CD19 expression at CAR-T treatment failure showed no correlation with CAR-T refractoriness versus later relapse. Our findings echo those reported by Plaks et al. in suggesting that antigen loss is a minor resistance mechanism in LBCL.31 The persistence of CD19 may render tumors susceptible to further CD19-directed interventions such as tafasitamab or loncastuximab,14, 17 Disappointingly, Gauthier et al. have reported low response rates for a second CD19-directed CAR-T infusion.34 However, bispecific constructs simultaneously targeting CD19 and CD20 appear to have promising activity among this patient population.35
CAR-T use is rapidly growing and is being introduced as an earlier line of treatment,36–38 leading to greater opportunity for therapeutic decisions in the CAR-T treatment failure setting. Several small patient series have reported outcomes of post-CAR-T interventions.13, 19–22, 34 While treatment assignments following CAR-T varied, we believe our study provides the most comprehensive experience reported to date.
Overall survival and complete response rates with lenalidomide as first-line therapy after CAR-T treatment failure were encoring (69% and 33%, respectively). Lenalidomide was primarily given as monotherapy (8/15). These results could be driven by the relatively favorable initial responses to CAR-T, along with generally lower pre-treatment LDH, in this group. However, these promising results could also be linked to distinct immunomodulatory activity of lenalidomide in the presence of CAR-T cells. In preclinical models, lenalidomide appears to mitigate T-cell exhaustion and induce CAR-T cell activation.39–41 Preliminary clinical data suggest that lenalidomide can induce high response rates in LBCL rapidly progressing after CAR-T cell therapy.42 Polatuzumab treatment, typically administered with bendamustine (n=26), also resulted in promising overall response rates (48%), albeit lower 1-year overall survival in our cohort. Similar results were reported by Liebers et al.21 Others showed a similar response to polatuzumab but with limited durability.43 Therefore, polatuzumab may serve as a valuable agent for salvage therapy post-CAR-T. A follow-up study on the CORAL trial demonstrated that salvage chemotherapy, administered as third-line treatment in r/r LBCL, induced CR in approximately 30% of cases.44 However, chemotherapy-based approaches did not result in CRs in our cohort and OS was poor. In an exploratory comparison of survival between chemotherapy-based treatment and novel agents, the disadvantage of post-CAR-T salvage chemotherapy persisted when adjusting for features that may contribute to treatment selection. Nevertheless, aggressive disease features more prevalent in this subgroup (Table S6) may have driven results in this group. Our results suggest that chemotherapy should be deprioritized in sequencing therapies post-CAR-T in contemporary practice. The proliferation of new treatment options for r/r LBCL complicates the identification of the best sequence of treatments post-CAR-T therapies and warrants the development of prospective trials.
Tools for risk stratification in a population with CAR-T-resistant LBCL could inform clinical trial design and goals of care discussion. We identified four key predictors of OS in patients receiving treatment for relapse or progressive disease after CAR-T therapy: (1) bulky disease at apheresis, (2) refractoriness to CAR-T, (3–4) age, and elevated LDH at the time of subsequent therapy. Notably, bulky disease was the only factor preceding CAR-T, suggesting it is a biomarker of disease biology. We propose a simple prognostic tool incorporating these factors for identifying patients at the highest risk of early adverse outcomes after post-CAR-T therapy and should be used for stratifying or selecting clinical trial participants in the post-CAR-T setting. The proposed system is intended to be a research instrument, not a clinical decision-making tool.
Several limitations of the analysis should be noted. First, treatment decisions post-CAR-T varied considerably and were not protocol-driven. Therefore, selection bias and latent confounders such as pre-existing hematological toxicities, performance status, comorbidities, and health insurance complicate comparisons between the treatments. However, we have extensively characterized the differences among the groups and restricted comparisons to only the most common treatments while adjusting for key known confounders to mitigate this bias. Second, despite describing what we believe is the largest cohort of post-CAR-T relapse and progression to date, the sample size remains limited and spread across varied treatment groups. Third, the intent of treatments administered after CAR-T was not captured. Treatment goals are essential for interpreting results in interventions such as monotherapy ISRT. Imber et al. recently reported ISRT as an effective bridging treatment for patients relapsing with localized disease after CAR-T.20 However, a portion of patients in our cohort likely received monotherapy ISRT for palliation. Therefore, we only describe outcomes with radiation therapy and avoid comparisons. Finally, biological biomarkers such as the continued presence of CAR-T cells at relapse or progression are lacking.
In conclusion, the poor outcomes following CAR-T treatment failure suggest that the community should focus first and foremost on relapse prevention. In patients treated with curative intent after CAR-T therapy failure, our findings indicate that definitive approaches are needed as the vast majority progress. Future trials should aim to tailor post-CAR-T management strategies based on patient and disease features as well as exposure to previous therapies.
Supplementary Material
Acknowledgment:
This research was supported in part by the Memorial Sloan Kettering Cancer Center Core grant (P30 CA008748) from the National Institutes of Health/National Cancer Institute. RS was supported by the American Society of Transplantation and Cellular Therapy New Investigator Award, the American Society of Hematology Fellow Scholar Award, a grant from the Long Island Sound Chapter, Swim Across America, the Robert Hirschhorn Award, and the Memorial Sloan Kettering Steven Greenberg Lymphoma Research Award. A.A.T was supported by a grant from the Alfonso Martín Escudero. JAF was supported by an HONORS Award from the American Society of Hematology.
Footnotes
Previous Publication: This work was presented in part as an oral presentation at the 63rd American Society of Hematology Annual Meeting (Atlanta, GA).
| Author | Name of organization | Type of relationship |
|---|---|---|
| Roni Shouval | Medexus | Consultancy |
| Parastoo B. Dahi | Kite/Gilead. | Advisory board |
| Sergio A. Giralt | Actinnum | Membership on an entity’s Board of Directors or advisory committees |
| CELGENE | Membership on an entity’s Board of Directors or advisory committees | |
| BMS. | Membership on an entity’s Board of Directors or advisory committees | |
| SANOFI | Membership on an entity’s Board of Directors or advisory committees | |
| AMGEN | Membership on an entity’s Board of Directors or advisory committees | |
| PFIZER | Membership on an entity’s Board of Directors or advisory committees | |
| JENSENN | Membership on an entity’s Board of Directors or advisory committees | |
| GSK. | Membership on an entity’s Board of Directors or advisory committees | |
| JAZZ | Membership on an entity’s Board of Directors or advisory committees | |
| Gilles Salles | AbbVie Inc, Allogene Therapeutics, Autolus Therapeutics, BeiGene Ltd, Bristol-Myers Squibb Company, Celgene Corporation, Debiopharm Group, Genmab, Kite, A Gilead Company, Incyte Corporation, Janssen Biotech Inc, Miltenyi Biotec, MorphoSys, Novartis, Roche | Advisory Committee |
| Bristol-Myers Squibb Company, Celgene Corporation, Debiopharm Group, Genmab, Kite, A Gilead Company, Incyte Corporation, Miltenyi Biotec, MorphoSys, Novartis, Roche Laboratories Inc | Consultancy | |
| Craig S. Sauter | Juno Therapeutics | Consultancy and Research Funding |
| Sanofi-Genzyme | Consultancy and Research Funding | |
| Spectrum Pharmaceuticals | Consultancy | |
| Novartis | Consultancy | |
| Genmab | Consultancy | |
| Precision Biosciences | Consultancy | |
| Kite/Gilead | Consultancy | |
| Celgene | Consultancy and Research Funding | |
| Gamida Cell | Consultancy | |
| GSK. | Consultancy | |
| Bristol-Myers Squibb | Research Funding | |
| Michael Scordo | McKinsey & Company | Consultancy |
| Angiocrine Bioscience | Consultancy and Research Funding | |
| Omeros Corporation | Consultancy and research funding | |
| Amgen, Inc. | Research funding | |
| Kite - A Gilead Company | Advisory Board | |
| i3 Health | Other: Honorarium, CME activity | |
| Medscape, LLC | Other: Honorarium, CME activity | |
| Gunjan Shah | Amgen | Research Funding |
| Janssen Pharmaceutica | Research Funding | |
| Abraham Avigdor | Takeda | Consultancy and honoraria |
| Janseen | Research Funding | |
| BMS | Research Funding | |
| Gilead | Consultancy and honoraria | |
| Pfizer | Consultancy and honoraria | |
| Miguel-Angel Perales | Bristol-Myers Squibb | Honoraria |
| Celgene | Honoraria | |
| Equilium | Honoraria | |
| Incyte | Honoraria and Other: Clinical trial support to institution | |
| Karyopharm | Honoraria | |
| Kite/Gilead | Honoraria and Other: Clinical trial support to institution | |
| Merck | Honoraria | |
| Miltenyi Biotec | Honoraria and Other | |
| MorphoSys | Honoraria | |
| Novartis | Honoraria and Other: Clinical trial support to institution | |
| Nektar Therapeutics | Honoraria and Other | |
| Omeros | Honoraria | |
| Takeda | Honoraria | |
| Cidara Therapeutics | Honoraria | |
| Medigene | Honoraria | |
| Sellas Life Sciences | Honoraria | |
| Servier | Honoraria | |
| NexImmune | Honoraria | |
| Elad Jacoby | Novartis | Advisory board, Honoraria |
| Joachim Yahalom | Convergent R.N.R Ltd. | Advisory board |
| Mikhail Roshal | Celgene | Provision of Services |
| Auron Therapeutics, Inc. | Ownership / Equity Interests; Provision of Services | |
| Physicians’ Education Resource | Provision of Services |
Data availability statement
The datasets generated during and/or analyzed during the current study are not publicly available due to protect study participant privacy but are available from the corresponding author upon reasonable request.
References
- 1.Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N Engl J Med 2017. Dec 28; 377(26): 2531–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abramson JS, Palomba ML, Gordon LI, Lunning MA, Wang M, Arnason J, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet 2020. Sep 19; 396(10254): 839–852. [DOI] [PubMed] [Google Scholar]
- 3.Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N Engl J Med 2019. Jan 3; 380(1): 45–56. [DOI] [PubMed] [Google Scholar]
- 4.Sauter CS, Senechal B, Riviere I, Ni A, Bernal Y, Wang X, et al. CD19 CAR T cells following autologous transplantation in poor-risk relapsed and refractory B-cell non-Hodgkin lymphoma. Blood 2019. Aug 15; 134(7): 626–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ortíz-Maldonado V, Rives S, Castellà M, Alonso-Saladrigues A, Benítez-Ribas D, Caballero-Baños M, et al. CART19-BE-01: a multicenter Trial of ARI-0001 Cell Therapy in Patients with CD19+ Relapsed/Refractory malignancies. Molecular Therapy 2021; 29(2): 636–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kedmi M, Shouval R, Fried S, Bomze D, Fein J, Cohen Z, et al. Point-of-care anti-CD19 CAR T-cells for treatment of relapsed and refractory aggressive B-cell lymphoma. Transplantation and cellular therapy 2022. Feb 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Itzhaki O, Jacoby E, Nissani A, Levi M, Nagler A, Kubi A, et al. Head-to-head comparison of in-house produced CD19 CAR-T cell in ALL and NHL patients. J Immunother Cancer 2020. Mar; 8(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cappell KM, Sherry RM, Yang JC, Goff SL, Vanasse DA, McIntyre L, et al. Long-Term Follow-Up of Anti-CD19 Chimeric Antigen Receptor T-Cell Therapy. J Clin Oncol 2020. Nov 10; 38(32): 3805–3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Locke FL, Ghobadi A, Jacobson CA, Miklos DB, Lekakis LJ, Oluwole OO, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1–2 trial. The Lancet Oncology 2019. Jan; 20(1): 31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vercellino L, Di Blasi R, Kanoun S, Tessoulin B, Rossi C, D’Aveni-Piney M, et al. Predictive factors of early progression after CAR T-cell therapy in relapsed/refractory diffuse large B-cell lymphoma. Blood Adv 2020. Nov 24; 4(22): 5607–5615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chow VA, Gopal AK, Maloney DG, Turtle CJ, Smith SD, Ujjani CS, et al. Outcomes of patients with large B-cell lymphomas and progressive disease following CD19-specific CAR T-cell therapy. Am J Hematol 2019. Aug; 94(8): E209–E213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nastoupil LJ, Jain MD, Feng L, Spiegel JY, Ghobadi A, Lin Y, et al. Standard-of-Care Axicabtagene Ciloleucel for Relapsed or Refractory Large B-Cell Lymphoma: Results From the US Lymphoma CAR T Consortium. J Clin Oncol 2020. Sep 20; 38(27): 3119–3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spiegel JY, Dahiya S, Jain MD, Tamaresis J, Nastoupil LJ, Jacobs MT, et al. Outcomes of patients with large B-cell lymphoma progressing after axicabtagene ciloleucel therapy. Blood, The Journal of the American Society of Hematology 2021; 137(13): 1832–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salles G, Duell J, Gonzalez Barca E, Tournilhac O, Jurczak W, Liberati AM, et al. Tafasitamab plus lenalidomide in relapsed or refractory diffuse large B-cell lymphoma (L-MIND): a multicentre, prospective, single-arm, phase 2 study. The Lancet Oncology 2020. Jul; 21(7): 978–988. [DOI] [PubMed] [Google Scholar]
- 15.Tilly H, Morschhauser F, Bartlett NL, Mehta A, Salles G, Haioun C, et al. Polatuzumab vedotin in combination with immunochemotherapy in patients with previously untreated diffuse large B-cell lymphoma: an open-label, non-randomised, phase 1b-2 study. The Lancet Oncology 2019. Jul; 20(7): 998–1010. [DOI] [PubMed] [Google Scholar]
- 16.Kalakonda N, Maerevoet M, Cavallo F, Follows G, Goy A, Vermaat JSP, et al. Selinexor in patients with relapsed or refractory diffuse large B-cell lymphoma (SADAL): a single-arm, multinational, multicentre, open-label, phase 2 trial. Lancet Haematol 2020. Jul; 7(7): e511–e522. [DOI] [PubMed] [Google Scholar]
- 17.Hamadani M, Radford J, Carlo-Stella C, Caimi PF, Reid E, O’Connor OA, et al. Final results of a phase 1 study of loncastuximab tesirine in relapsed/refractory B-cell non-Hodgkin lymphoma. Blood 2021. May 13; 137(19): 2634–2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Logue JM, Chavez JC. How to Sequence Therapies in Diffuse Large B-Cell Lymphoma Post-CAR-T Cell Failure. Current Treatment Options in Oncology 2021. 2021/10/26; 22(12): 112. [DOI] [PubMed] [Google Scholar]
- 19.Byrne M, Oluwole OO, Savani B, Majhail NS, Hill BT, Locke FL. Understanding and Managing Large B Cell Lymphoma Relapses after Chimeric Antigen Receptor T Cell Therapy. Biol Blood Marrow Transplant 2019. Nov; 25(11): e344–e351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Imber BS, Sadelain M, DeSelm C, Batlevi C, Brentjens RJ, Dahi PB, et al. Early experience using salvage radiotherapy for relapsed/refractory non-Hodgkin lymphomas after CD19 chimeric antigen receptor (CAR) T cell therapy. British journal of haematology 2020; 190(1): 45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liebers N, Duell J, Fitzgerald D, Kerkhoff A, Noerenberg D, Kaebisch E, et al. Polatuzumab vedotin as a salvage and bridging treatment in relapsed or refractory large B-cell lymphomas. Blood Advances 2021; 5(13): 2707–2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gouni S, Rosenthal AC, Crombie JL, Ip A, Kamdar M, Hess B, et al. A Multicenter Retrospective Study of Polatuzumab Vedotin in Patients with Large B-cell Lymphoma After CAR T-cell Therapy. Blood Adv 2022. Mar 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform 2019. Jul; 95: 103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol 2014. Sep 20; 32(27): 3059–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee DW, Santomasso BD, Locke FL, Ghobadi A, Turtle CJ, Brudno JN, et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biol Blood Marrow Transplant 2019. Apr; 25(4): 625–638. [DOI] [PubMed] [Google Scholar]
- 26.Palomba ML, Qualls D, Monette S, Sethi S, Dogan A, Roshal M, et al. CD19-directed chimeric antigen receptor T cell therapy in Waldenstrom macroglobulinemia: a preclinical model and initial clinical experience. J Immunother Cancer 2022. Feb; 10(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Orlando EJ, Han X, Tribouley C, Wood PA, Leary RJ, Riester M, et al. Genetic mechanisms of target antigen loss in CAR19 therapy of acute lymphoblastic leukemia. Nature medicine 2018; 24(10): 1504–1506. [DOI] [PubMed] [Google Scholar]
- 28.Dourthe M-E, Rabian F, Yakouben K, Chevillon F, Cabannes-Hamy A, Méchinaud F, et al. Determinants of CD19-positive vs CD19-negative relapse after tisagenlecleucel for B-cell acute lymphoblastic leukemia. Leukemia 2021. 2021/12/01; 35(12): 3383–3393. [DOI] [PubMed] [Google Scholar]
- 29.Larson RC, Maus MV. Recent advances and discoveries in the mechanisms and functions of CAR T cells. Nature Reviews Cancer 2021: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hay KA, Gauthier J, Hirayama AV, Voutsinas JM, Wu Q, Li D, et al. Factors associated with durable EFS in adult B-cell ALL patients achieving MRD-negative CR after CD19 CAR T-cell therapy. Blood 2019. Apr 11; 133(15): 1652–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Plaks V, Rossi JM, Chou J, Wang LH, Poddar S, Han GC, et al. CD19 target evasion as a mechanism of relapse in large B-cell lymphoma treated with axicabtagene ciloleucel. Blood 2021. Sep 23; 138(12): 1081–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Reagan PM, Miklos DB, et al. Comparison of 2-year outcomes with CAR T cells (ZUMA-1) vs salvage chemotherapy in refractory large B-cell lymphoma. Blood Advances 2021; 5(20): 4149–4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garcia-Recio M, Wudhikarn K, Pennisi M, Alonso-Trillo R, Flynn J, Shouval R, et al. The International Prognostic Index Is Associated with Outcomes in Diffuse Large B Cell Lymphoma after Chimeric Antigen Receptor T Cell Therapy. Transpl Cell Ther 2021. Mar; 27(3): 233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gauthier J, Bezerra ED, Hirayama AV, Fiorenza S, Sheih A, Chou CK, et al. Factors associated with outcomes after a second CD19-targeted CAR T-cell infusion for refractory B-cell malignancies. Blood 2021; 137(3): 323–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Y, Wang Y, Liu Y, Tong C, Wang C, Guo Y, et al. Long-term activity of tandem CD19/CD20 CAR therapy in refractory/relapsed B-cell lymphoma: a single-arm, phase 1–2 trial. Leukemia 2021. Jul 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Locke FL, Miklos DB, Jacobson CA, Perales M-A, Kersten M-J, Oluwole OO, et al. Axicabtagene Ciloleucel as Second-Line Therapy for Large B-Cell Lymphoma. New England Journal of Medicine 2021. [DOI] [PubMed] [Google Scholar]
- 37.Bishop MR, Dickinson M, Purtill D, Barba P, Santoro A, Hamad N, et al. Second-Line Tisagenlecleucel or Standard Care in Aggressive B-Cell Lymphoma. N Engl J Med 2021. Dec 14. [DOI] [PubMed] [Google Scholar]
- 38.Neelapu SS, Dickinson M, Munoz J, Ulrickson ML, Thieblemont C, Oluwole OO, et al. Axicabtagene ciloleucel as first-line therapy in high-risk large B-cell lymphoma: the phase 2 ZUMA-12 trial. Nature Medicine 2022. 2022/03/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Works M, Soni N, Hauskins C, Sierra C, Baturevych A, Jones JC, et al. Anti–B-cell maturation antigen chimeric antigen receptor T cell function against multiple myeloma is enhanced in the presence of lenalidomide. Molecular cancer therapeutics 2019; 18(12): 2246–2257. [DOI] [PubMed] [Google Scholar]
- 40.Otáhal P, Průková D, Král V, Fabry M, Vočková P, Latečková L, et al. Lenalidomide enhances antitumor functions of chimeric antigen receptor modified T cells. Oncoimmunology 2016; 5(4): e1115940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuramitsu S, Ohno M, Ohka F, Shiina S, Yamamichi A, Kato A, et al. Lenalidomide enhances the function of chimeric antigen receptor T cells against the epidermal growth factor receptor variant III by enhancing immune synapses. Cancer gene therapy 2015; 22(10): 487–495. [DOI] [PubMed] [Google Scholar]
- 42.Thieblemont C, Chevret S, Allain V, Di Blasi R, Morin F, Vercellino L, et al. Lenalidomide Enhance CAR T-Cells Response in Patients with Refractory/Relapsed Large B Cell Lymphoma Experiencing Progression after Infusion. Blood 2020. Nov 5; 136. [Google Scholar]
- 43.Gouni S, Rosenthal AC, Crombie JL, Ip A, Kamdar MK, Hess B, et al. A multicenter retrospective study of polatuzumab vedotin in patients with large B-cell lymphoma after CAR T-cell therapy. Blood Advances 2022; 6(9): 2757–2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Den Neste E, Schmitz N, Mounier N, Gill D, Linch D, Trneny M, et al. Outcome of patients with relapsed diffuse large B-cell lymphoma who fail second-line salvage regimens in the International CORAL study. Bone Marrow Transplant 2016. Jan; 51(1): 51–57. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available due to protect study participant privacy but are available from the corresponding author upon reasonable request.


