Abstract
Study Objectives:
The relationship between open-angle glaucoma (OAG) and obstructive sleep apnea (OSA) is unclear. The long-term risk for OAG after OSA diagnosis has not been investigated. Therefore, we assessed the risk for OAG among patients with OSA over a 12-year follow-up period using nationwide, population-based data.
Methods:
The OSA group was randomly selected from among 3.5 million individuals registered with the National Health Insurance Service. The non-OSA group was obtained through propensity score matching considering several variables. The primary endpoint was glaucoma diagnosis.
Results:
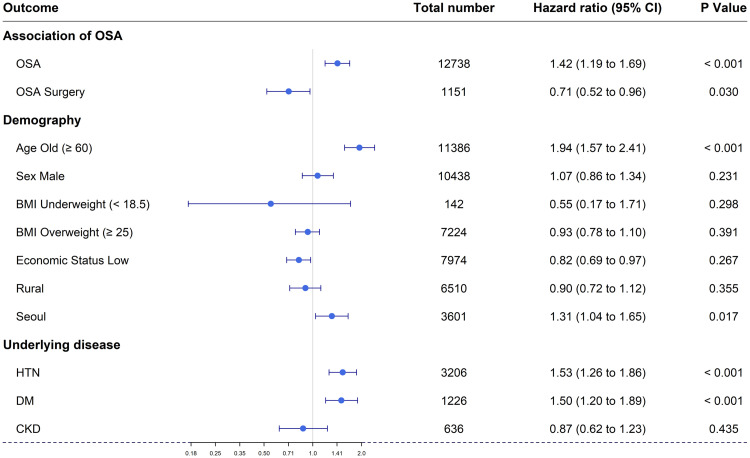
The OSA and non-OSA groups both included 6,369 individuals. The overall hazard ratio for OAG in the OSA group was 1.42 (95% confidence interval [CI]: 1.19–1.69). In subgroup analysis, the hazard ratio for OAG was 1.94 (95% CI: 1.57–2.41) for those aged > 60 years, 1.50 (95% CI: 1.20–1.89) for those with diabetes mellitus, 1.53 (95% CI: 1.26–1.86) for those with hypertension, and 0.71 (95% CI: 0.52–0.96) for those with a history of OSA surgery.
Conclusions:
Over the 12-year follow-up, the risk for OAG increased after OSA diagnosis. Further research will be necessary to determine if treating OSA can mitigate this association.
Citation:
Lee T-E, Kim JS, Yeom SW, Lee MG, Lee JH, Lee H-J. Long-term effects of obstructive sleep apnea and its treatment on open-angle glaucoma: a big-data cohort study. J Clin Sleep Med. 2023;19(2):339–346.
Keywords: obstructive sleep apnea, open-angle glaucoma
BRIEF SUMMARY
Current Knowledge/Study Rationale: The relationship between open-angle glaucoma (OAG) and obstructive sleep apnea (OSA) is unclear. The long-term risk for OAG after OSA diagnosis has not been investigated.
Study Impact: The hazard ratio for OAG in the OSA group was 1.42 (95% confidence interval: 1.19–1.69). In the subgroup analysis, the hazard ratio for OAG was 1.94 (95% confidence interval: 1.57–2.41) among patients aged > 60 years, 0.71 (95% confidence interval: 0.52–0.96) for those with a history of OSA surgery, and 0.06 (95% confidence interval: 0.05–0.08) for those receiving continuous positive airway pressure treatment. The OAG risk was increased at 12 years after OSA diagnosis. OSA treatment was associated with a lower likelihood of OAG.
INTRODUCTION
Glaucoma is a progressive optic neuropathy that causes structural changes in the optic disc, as well as visual field loss.1 Vision loss in glaucoma is caused by retinal ganglion cell death. Increased intraocular pressure (IOP) is the best-known risk factor for the development and progression of glaucoma.2–5 Other causes of retinal ganglion cell death include vascular dysregulation, autonomic dysfunction, ischemia, inflammation, and oxidative stress.6
Sleep-disordered breathing, characterized by repeated episodes of apnea during sleep, causes sleep deprivation and decreases oxyhemoglobin saturation.7 Obstructive sleep apnea (OSA), the most common form of sleep-disordered breathing, is characterized by repeated partial or complete obstruction of the upper airway during sleep. Complete upper airway obstruction causes apnea, while partial obstruction causes hypopnea. OSA is associated with several metabolic conditions, such as diabetes mellitus (DM), insulin resistance, hypertension (HTN), and cardiovascular diseases,8–10 in addition to ophthalmic diseases such as glaucoma, nonarteritic ischemic optic neuropathy, droopy eyelid syndrome, blepharitis, ptosis, papillary conjunctivitis, retinal vessel torsion, and central serous chorioretinopathy.11
The correlation between glaucoma and OSA is controversial, but several studies have demonstrated a high prevalence of glaucoma in OSA patients. Some studies have also demonstrated structural optic nerve changes in OSA patients, even prior to diagnosis of glaucoma.12–14 However, the long-term risk for glaucoma after OSA diagnosis has not been investigated. Therefore, we investigated the risk for open-angle glaucoma (OAG) over a 12-year follow-up period after OSA diagnosis by analyzing a nationwide Korean dataset. Furthermore, we investigated whether surgical correction of OSA affects the incidence of OAG in OSA patients.
METHODS
Ethical consideration, database, and study design
This study was approved by the Institutional Review Board of Jeonbuk National University Hospital (Institutional Review Board number 2021-09-026). The requirement for informed consent was waived because no personal information was included in this study. The study was conducted and designed in accordance with the Declaration of Helsinki. We accessed the National Health Insurance Service Information Database, which contains information on 3.5 million randomly selected individuals (National Health Insurance Service 2021-1-689). Since 1989, almost all people in Korea have been enrolled in the National Health Insurance Service. Patient data such as hospital visit day, prescriptions, diagnostic codes, medications, treatment history, insurance claim data, demographics, and mortality were recorded.
Study population
The experimental cohort consisted of patients with OSA (International Classification of Diseases, 10th Revision diagnostic code = G473) diagnosed between January 1, 2008 and December 31, 2010. The International Classification of Diseases, 10th Revision code for OAG is H401. The exclusion criteria were a diagnosis of OSA during the period 2011–2019 and a diagnosis of glaucoma earlier (or at the same time) than that for OSA. In our statistical model, the starting point was the date of OSA diagnosis, and the endpoint was the date of glaucoma diagnosis or December 31, 2019 (in cases without this diagnosis).
Control (non-OSA) group
The control group was obtained by 1:1 propensity score (PS) matching using a greedy nearest-neighbor algorithm. Eight independent variables (sex, residential area, economic status, HTN, DM, chronic kidney disease, and age combined with the body mass index [all continuous variables]) were used during PS matching. The success of PS matching was confirmed by the lack of large differences in the standardized mean differences between the groups15 (Table 1).
Table 1.
Characteristics of the control (non-OSA) and study (OSA) groups.
| Variable | Control (n = 6,369) | Study (n = 6,369) | SMD |
|---|---|---|---|
| Sex | 0.034 | ||
| Female (%) | 1,098 (17.2) | 1,181 (18.5) | |
| Male (%) | 5,271 (82.8) | 5,188 (81.5) | |
| Age, y | 0.098 | ||
| Mean (SD) | 42.14 (12.8) | 41.67 (14.6) | |
| BMI | 0.016 | ||
| Mean (SD) | 25.90 (3.6) | 25.83 (3.7) | |
| Economic status | 0.031 | ||
| High (%) | 2,428 (38.1) | 2,331 (36.6) | |
| Low (%) | 3,941 (61.9) | 4,038 (63.4) | |
| Region | 0.037 | ||
| Metropolitan (%) | 1,306 (20.5) | 1,356 (21.3) | |
| Rural (%) | 3,331 (52.3) | 3,212 (50.4) | |
| Seoul (%) | 1,732 (27.2) | 1,801 (28.3) | |
| HTN | 0.075 | ||
| No (%) | 4,854 (76.2) | 4,646 (72.9) | |
| Yes (%) | 1,515 (23.8) | 1,723 (27.1) | |
| DM | 0.098 | ||
| No (%) | 5,844 (91.8) | 5,660 (88.9) | |
| Yes (%) | 525 (8.2) | 709 (11.1) | |
| CKD | 0.071 | ||
| No (%) | 6,110 (95.9) | 6,013 (94.4) | |
| Yes (%) | 259 (4.1) | 356 (5.6) | |
| OSA surgery | 0.80 | ||
| No (%) | 6,369 (100) | 4,818 (75.7) | |
| Yes (%) | 0 (0) | 1,551 (24.3) |
BMI = body mass index, CKD = chronic kidney disease, DM = diabetes mellitus, HTN = hypertension, OSA = obstructive sleep apnea, SD = standard deviation, SMD = standardized mean difference.
We analyzed 2 continuous independent variables and 7 categorical variables. The former were the normalized age and body mass index; the latter were sex (male or female), economic status (high [upper 30%] or low [bottom 70%]), residential area (metropolitan city, rural area, or Seoul), and prior/current HTN, DM, chronic kidney disease, and OSA surgery (yes or no).16 The underlying diseases are listed in Table S1 (79.2KB, pdf) in the supplemental material.
The starting point for the non-OSA group was the day of the first hospital visit for any reason between 2008 and 2010, and the endpoint was the date of first diagnosis of glaucoma or December 31, 2019 (in cases without this diagnosis).
Outcome variables and statistical analysis
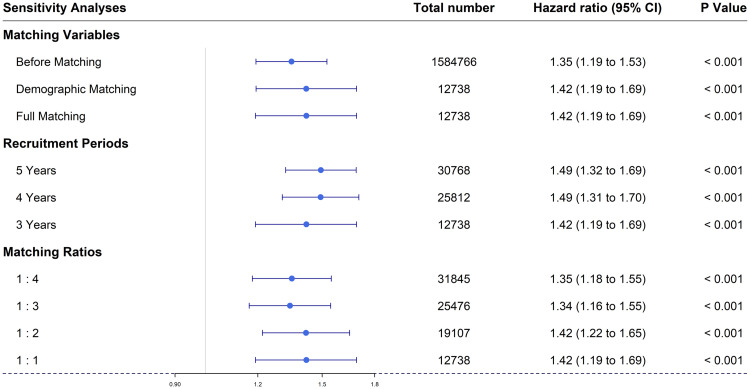
Data analysis was performed independently by 2 data analysts (J.S.K. and S.W.Y.) between March and December 2021. The hazard ratio (HR) in the Cox proportional hazards model was calculated based on the time between the endpoint and start point. When considering the relationship between an independent variable and the dependent variable, the adjusted HR was obtained considering age, sex, residential area, economic status, and underlying diseases (HTN, DM, and chronic kidney disease), while the unadjusted HR was calculated without considering the other variables. The cumulative HR was obtained by Kaplan-Meier survival analysis. To render the study robust and to show that the results were not affected by the recruitment period, matching ratio, or other variables, we performed sensitivity tests on the variables used for PS matching (before matching: age, sex, residence, and economic status), the recruitment period (5, 4, and 3 years), and the matching ratios (1:4, 1:3, 1:2, and 1:1). All analyses were performed using R 4.0.3 software (R Foundation for Statistical Computing, Vienna, Austria).
Subgroup analysis
In subgroup analysis of the OSA group, we examined sex, residential area, economic status, age, and underlying diseases. We also evaluated the effect of OSA surgery (uvulopalatopharyngoplasty, uvulopalatal flap placement, uvulectomy, septoplasty, tonsillectomy, and adenoidectomy) and continuous positive airway pressure (CPAP) treatment on the incidence of OAG. CPAP is the most effective treatment for OSA and has been covered by insurance in South Korea since July 2018. Therefore, in our 12-year database, data regarding CPAP treatment and OSA surgery were available for only 2 years (2018–2019).
RESULTS
Of the approximately 3.5 million samples, 12,738 individuals (6,369 in the OSA group and 6,369 in the non-OSA group) were followed up for 12 years, from January 2008 to December 2019 (Table 1).
Validation of PS matching
The control group was generated by 1:1 PS matching based on 8 independent variables. The standardized mean differences did not exceed 0.1, so there were no significant differences between the OSA and non-OSA groups (Table 1).
Incidence rate and HR for glaucoma
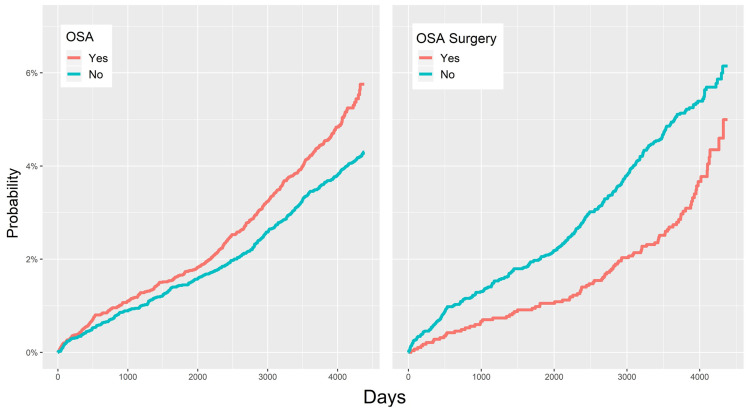
Table 2 shows the relationships between the 10 independent variables and OAG. The incidence per 10,000 person years is a measure of the frequency rate of diseases.16 Figure 1 and Table 2 show that the HR for glaucoma in the OSA group was 1.42 (95% confidence interval [CI]: 1.19–1.69), ie, the prevalence of OAG was 1.42 times higher in the OSA than in the control group.
Table 2.
Incidence per 10,000 person-years and hazard ratios for glaucoma during the 12-year follow-up period.
| Variable | Study Participants | Number of Cases | Incidence per 10,000 person-years | Hazard Ratio | P (adjusted hazard ratio) | |
|---|---|---|---|---|---|---|
| Adjusted | Unadjusted | |||||
| Total | 12,738 | 577 | ||||
| OSA | ||||||
| No | 6,369 | 272 | 37.31 | 1 | 1 | |
| Yes | 6,369 | 305 | 46.24 | 1.42 (1.19–1.69) | 1.31 (1.11–1.55) | <.001 |
| Sex | ||||||
| Female | 2,279 | 103 | 41.38 | 1 | 1 | |
| Male | 10,459 | 474 | 41.59 | 1.07 (0.86–1.34) | 1.01 (0.81–1.25) | .231 |
| Age, y | ||||||
| < 60 (young) | 11,386 | 438 | 35.25 | 1 | 1 | |
| ≥ 60 (old) | 1,352 | 139 | 95.20 | 1.94 (1.57–2.41) | 2.67 (2.21–3.24) | <.001 |
| BMI | ||||||
| 18.5–25 (normal) | 5,372 | 250 | 42.75 | 1 | 1 | |
| < 18.5 (underweight) | 142 | 3 | 19.51 | 0.55 (0.17–1.71) | 0.89 (0.72–1.11) | .298 |
| ≥ 25 (overweight) | 7,224 | 324 | 41.10 | 0.93 (0.78–1.10) | 1.35 (1.08–1.70) | .391 |
| Economic status | ||||||
| High | 4,759 | 257 | 49.56 | 1 | 1 | |
| Low | 7,979 | 320 | 36.78 | 0.82 (0.69–0.97) | 0.74 (0.63–0.88) | .267 |
| Region | ||||||
| Metropolitan | 2,662 | 116 | 39.91 | 1 | 1 | |
| Rural | 6,543 | 255 | 35.62 | 0.90 (0.72–1.12) | 0.89 (0.72–1.11) | .355 |
| Seoul | 3,533 | 206 | 53.93 | 1.31 (1.04–1.65) | 1.35 (1.08–1.70) | .017 |
| HTN | ||||||
| No | 9,500 | 334 | 32.20 | 1 | 1 | |
| Yes | 3,238 | 243 | 69.20 | 1.53 (1.26–1.86) | 2.14 (1.81–2.52) | .259 |
| DM | ||||||
| No | 11,504 | 463 | 36.85 | 1 | 1 | |
| Yes | 1,234 | 114 | 86.18 | 1.50 (1.20–1.89) | 2.34 (1.90–2.87) | .009 |
| CKD | ||||||
| No | 12,123 | 540 | 40.85 | 1 | 1 | |
| Yes | 615 | 37 | 55.52 | 0.87 (0.62–1.23) | 1.36 (0.97–1.89) | .216 |
| OSA surgery | ||||||
| No | 11,187 | 527 | 42.94 | 1 | 1 | |
| Yes | 1,551 | 50 | 30.98 | 0.71 (0.52–0.96) | 0.74 (0.56–0.99) | .03 |
BMI = body mass index, CKD = chronic kidney disease, DM = diabetic mellitus, HTN = hypertension, OSA = obstructive sleep apnea.
Figure 1. Probability of open-angle glaucoma.
(Left) Cumulative hazard ratios for open-angle glaucoma in OSA and control (non-OSA) groups. (Right) Cumulative incidence rates for open-angle glaucoma in OSA patients with and without surgery. OSA = obstructive sleep apnea.
Subgroup analysis
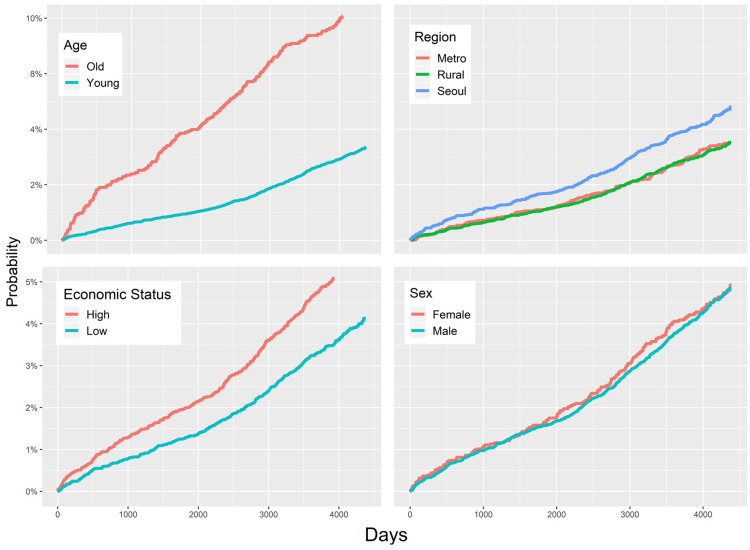
The prevalence of OAG was lower in patients who underwent OSA surgery compared to those who did not (adjusted HR = 0.71; 95% CI: 0.52–0.96) (Figure 1, Figure 3, and Table 2). In subgroup analyses according to demographic characteristics, the adjusted HRs for OAG were as follows: males vs females, 1.07 (95% CI: 0.86–1.34); low vs high economic status, 0.82 (95% CI: 0.69–0.97); aged ≥ 60 vs < 60 years, 1.94 (95% CI: 1.57–2.41); residence in Seoul vs metropolitan city, 1.31 (95% CI: 1.04–1.65); and residence in a rural area vs metropolitan city, 0.90 (95% CI: 0.72–1.12). Unadjusted HRs and plots are shown in Figure 2 and Table 2. Finally, the adjusted HRs were 1.53 (95% CI: 1.26–1.86) for the HTN subgroup, 1.50 (95% CI: 1.20–1.89) for the DM subgroup, and 0.87 (95% CI: 0.62–1.23) for the chronic kidney disease subgroup (Figure 3 and Table 2).
Figure 3. Forest plot of the cumulative hazard ratio for each factor: OSA, OSA surgery, age, sex, economic status, residential area, and underlying disease (HTN, DM, and CKD).
BMI = body mass index, CKD = chronic kidney disease, DM = diabetes mellitus, HTN = hypertension, OSA = obstructive sleep apnea.
Figure 2. Cumulative hazard plot for the subgroup analysis (age, residential region, economic status, and sex).
Data from the final 2 years of the study period showed that CPAP treatment significantly lowered the risk for OAG compared to OSA surgery (HR = 0.06 [95% CI: 0.04–0.08] and 0.57 [95% CI: 0.35–0.93], respectively; Figure S1 (79.2KB, pdf) ).
Sensitivity analyses
Figure 4 shows the results of sensitivity analysis; our study was robust. The risks for OAG revealed by all 8 models that varied 3 parameters were statistically significant.
Figure 4. Forest plot of three sensitivity analyses: (1) variables used for propensity score matching (before matching: age, sex, residence, and economic status), (2) recruitment period (5, 4, and 3 years), and (3) matching ratios (1:4, 1:3, 1:2, and 1:1). CI = confidence interval.
DISCUSSION
This nationwide longitudinal study was performed to compare the risk for OAG between OSA patients and age- and sex-matched controls. The OSA patients had a 1.42-fold greater risk of developing OAG than controls after adjusting for confounding factors. In addition, OSA surgery and CPAP treatment reduced the incidence of OAG in OSA patients.
The association between OSA and OAG has been attributed to various factors, including vascular changes, hypoxia, and mechanical factors. Several studies indicated roles of vascular factors in the development and progression of OAG in the absence of increased IOP. Apnea-related vascular dysregulation can compromise retinal blood supply.6 Several studies reported negative effects of OSA on the endothelial regulation of peripheral vasomotor tone, which increases the risk for cardiovascular diseases in OSA patients.17 Vascular endothelial dysfunction in the eyes can lead to unstable ocular perfusion pressure because of the reduced capacity for blood flow autoregulation.18 Several large epidemiological studies have demonstrated a role of reduced ocular perfusion pressure in OAG.19–22
OSA patients experience repeated episodes of apnea-hypopnea due to partial or complete obstruction of the upper airway during sleep. The retina is composed of nervous tissue that requires a large amount of oxygen, and apnea-hypopnea can directly damage the optic nerve.23 Repetitive hypoxia and reoxygenation also increase cellular oxidative stress and inflammation, which are known to be associated with OAG.24
Although elevated IOP is the most important factor in the development and progression of glaucoma, the effect of IOP on optic nerve damage in OSA patients is not yet fully understood.25 Ischemia and the abnormal perfusion associated with vascular dysregulation can potentially make the optic nerve vulnerable to slight IOP changes (ie, changes within the normal range). Posture changes during sleep can also affect IOP.26–28 Although obesity is a risk factor for OSA and IOP elevation in the supine position,29 body mass index did not affect the incidence of OAG in the present study.
Despite plausible mechanisms for an association between OSA and OAG, conflicting results have been reported. Several cross-sectional studies demonstrated a significantly higher prevalence of OAG in OSA patients,23,30–36 while other studies found no such association.37,38 Studies analyzing large databases to determine the risk of OAG development in patients with OSA also yielded conflicting results. Three studies, conducted in the United States and France, reported no increase in risk for OAG in patients with OSA than in controls,39–41 whereas a Taiwanese longitudinal study demonstrated an increased incidence of OAG after OSA diagnosis.42 Lin et al42 used a design similar to our study and a 5-year follow-up period and yielded results comparable to ours, with an HR of 1.67 for the development of OAG after OSA diagnosis. The results of these Asian studies may be attributed to the higher prevalence of normal-tension glaucoma in Asian populations.43
In the present study, individuals with higher economic status residing in Seoul had a higher prevalence of OAG. Our results are consistent with those of previous studies.44,45 Because glaucoma, particularly OAG, is a chronic disease that may be asymptomatic in the early stage, access to medical services affects the likelihood of detection of OAG. Although South Korea provides medical insurance to most citizens, the accessibility of medical services is affected by economic status.46,47 In addition, in large cities, such as Seoul, access to eye care is better than in rural areas because of the greater availability of medical services, such as eye clinics and ophthalmologists.
Interestingly, our results showed a reduction in the incidence of OAG in patients who underwent OSA corrective surgery. OSA has multifactorial and heterogeneous etiologies, and surgery plays an important role in treatment. There have been reports that OSA surgery can reduce OSA-related mortality and morbidity.48 Qian et al49 reported improvements of metabolic biomarkers and reduction in cardiovascular risk after OSA surgery. Increases in visual sensitivity have been reported after CPAP treatment and OSA surgery.50,51 These results are consistent with those of the present study, in which OSA increased the risk for OAG 1.42-fold and OSA surgery lowered the incidence of OAG 0.71-fold. We also evaluated the effects of CPAP treatment on the incidence of OAG. CPAP treatment has been covered by the medical insurance in South Korea since July 2018; therefore, data related to CPAP treatment were only analyzed for the period 2018–2019. Despite the short recruitment (2008–2010) and follow-up period (2018–2019), the HRs of OSA surgery did not differ according to the follow-up period (2008–2019, HR = 0.71 [95% CI: 0.52–0.96]; 2018–2019, HR = 0.57 [95% CI: 0.35–0.93]). In the final 2 years of the study period (2018–2019), the adjusted HR was lower for CPAP treatment than for OSA surgery (HR = 0.06 and 0.57, respectively). However, our results regarding the effect of CPAP treatment on the incidence of OAG in OSA patients should be interpreted cautiously. Because OAG is a chronic disease, a study duration of 2 years is insufficient to determine the effect of CPAP treatment. In addition, previous studies have reported that CPAP affects the IOP,52,53 and the effects of long-term CPAP use on the eyes are still unknown. Therefore, a long-term study of OAG in OSA patients treated with CPAP is needed.
This study had several limitations. Detailed clinical information about disease severity, such as the apnea-hypopnea index and visual field results, were not available, so quantitative analysis of OSA severity (mild, moderate, and severe) could not be performed. However, our study had the advantages of a large sample size and a 12-year longitudinal, rather than cross-sectional, design. To the best of our knowledge, this is the first study to report that OSA treatment may affect the development of OAG.
In this study using the Korean National Health Insurance Service–National Sample Cohort database, the risk of developing OAG was found to be 1.42 times greater after OSA diagnosis and OSA treatment was associated with a reduced incidence of OAG. In light of these findings, clinicians should be aware of the risk of glaucoma development in OSA patients and implement regular screening. Further research is necessary to clarify the relationship between OSA and OAG, and hospital-based studies will be required to confirm the relationship between OSA treatment and OAG development.
DISCLOSURE STATEMENT
This study was funded by project for Industry-Academic Cooperation-based R&D Platform funded by the Korean Ministry of Small and Medium Enterprises (SMEs) and Startups (2021) (no. S3017921). The authors report no conflicts of interest.
ACKNOWLEDGMENTS
Author contributions: T.E.L., J.S.K., and H.J.L. contributed to study design and protocol and prepared materials. S.W.Y. and M.G.L. collected data. J.S.K., S.W.Y., M.G.L., and J.H.L. planned the statistical evaluation; S.W.Y. and M.G.L. performed all analyses. T.E.L., J.S.K., and S.W.Y. wrote the first draft of the manuscript. All authors contributed to data interpretation data and manuscript revision.This study was supported by Fund of Biomedical Research Institute, Jeonbuk National University Hospital.
ABBREVIATIONS
- CI
confidence interval
- CPAP
continuous positive airway pressure
- DM
diabetes mellitus
- HR
hazard ratio
- HTN
hypertension
- IOP
intraocular pressure
- OAG
open-angle glaucoma
- OSA
obstructive sleep apnea
- PS
propensity score
REFERENCES
- 1. Weinreb RN , Khaw PT . Primary open-angle glaucoma . Lancet. 2004. ; 363 ( 9422 ): 1711 – 1720 . [DOI] [PubMed] [Google Scholar]
- 2. The AGIS Investigators . The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration . Am J Ophthalmol. 2000. ; 130 ( 4 ): 429 – 440 . [DOI] [PubMed] [Google Scholar]
- 3. Leske MC , Heijl A , Hussein M , Bengtsson B , Hyman L , Komaroff E ; Early Manifest Glaucoma Trial Group . Factors for glaucoma progression and the effect of treatment: the early manifest glaucoma trial . Arch Ophthalmol. 2003. ; 121 ( 1 ): 48 – 56 . [DOI] [PubMed] [Google Scholar]
- 4. Bengtsson B , Leske MC , Hyman L , Heijl A ; Early Manifest Glaucoma Trial Group . Fluctuation of intraocular pressure and glaucoma progression in the early manifest glaucoma trial . Ophthalmology. 2007. ; 114 ( 2 ): 205 – 209 . [DOI] [PubMed] [Google Scholar]
- 5. De Moraes CG , Juthani VJ , Liebmann JM , et al . Risk factors for visual field progression in treated glaucoma . Arch Ophthalmol. 2011. ; 129 ( 5 ): 562 – 568 . [DOI] [PubMed] [Google Scholar]
- 6. Agarwal R , Gupta SK , Agarwal P , Saxena R , Agrawal SS . Current concepts in the pathophysiology of glaucoma . Indian J Ophthalmol. 2009. ; 57 ( 4 ): 257 – 266 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Guilleminault C . Clinical Features and Evaluation of Obstructive Sleep Apnea-Hypopnea Syndrome . In: Kryger MH , Roth T , Dement WC , eds. Principles and Practice of Sleep Medicine. Philadelphia: : Saunders; ; 2005. : 1043 – 1052 . [Google Scholar]
- 8. Peppard PE , Young T , Palta M , Skatrud J . Prospective study of the association between sleep-disordered breathing and hypertension . N Engl J Med. 2000. ; 342 ( 19 ): 1378 – 1384 . [DOI] [PubMed] [Google Scholar]
- 9. Peker Y , Carlson J , Hedner J . Increased incidence of coronary artery disease in sleep apnoea: a long-term follow-up . Eur Respir J. 2006. ; 28 ( 3 ): 596 – 602 . [DOI] [PubMed] [Google Scholar]
- 10. Punjabi NM , Polotsky VY . Disorders of glucose metabolism in sleep apnea . J Appl Physiol. 2005. ; 99 ( 5 ): 1998 – 2007 . [DOI] [PubMed] [Google Scholar]
- 11. Waller EA , Bendel RE , Kaplan J . Sleep disorders and the eye . Mayo Clin Proc. 2008. ; 83 ( 11 ): 1251 – 1261 . [DOI] [PubMed] [Google Scholar]
- 12. Casas P , Ascaso FJ , Vicente E , Tejero-Garcés G , Adiego MI , Cristóbal JA . Retinal and optic nerve evaluation by optical coherence tomography in adults with obstructive sleep apnea-hypopnea syndrome (OSAHS) . Graefes Arch Clin Exp Ophthalmol. 2013. ; 251 ( 6 ): 1625 – 1634 . [DOI] [PubMed] [Google Scholar]
- 13. Lin PW , Friedman M , Lin HC , Chang HW , Pulver TM , Chin CH . Decreased retinal nerve fiber layer thickness in patients with obstructive sleep apnea/hypopnea syndrome . Graefes Arch Clin Exp Ophthalmol. 2011. ; 249 ( 4 ): 585 – 593 . [DOI] [PubMed] [Google Scholar]
- 14. Yu JG , Mei ZM , Ye T , et al . Changes in retinal nerve fiber layer thickness in obstructive sleep apnea/hypopnea syndrome: a meta-analysis . Ophthalmic Res. 2016. ; 56 ( 2 ): 57 – 67 . [DOI] [PubMed] [Google Scholar]
- 15. You YS , Kim JS , Jeong JS , et al . Septal deviation could be associated with the development of bronchial asthma: a nationwide cohort study . J Allergy Clin Immunol Pract. 2021. ; 10 ( 4 ): 1099 – 1101.e1 . [DOI] [PubMed] [Google Scholar]
- 16. Yeom SW , Kim MG , Lee EJ , et al . Association between septal deviation and OSA diagnoses: a nationwide 9-year follow-up cohort study . J Clin Sleep Med. 2021. ; 17 ( 10 ): 2099 – 2106 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Atkeson A , Yeh SY , Malhotra A , Jelic S . Endothelial function in obstructive sleep apnea . Prog Cardiovasc Dis. 2009. ; 51 ( 5 ): 351 – 362 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Flammer J , Orgül S , Costa VP , et al . The impact of ocular blood flow in glaucoma . Prog Retin Eye Res. 2002. ; 21 ( 4 ): 359 – 393 . [DOI] [PubMed] [Google Scholar]
- 19. Tielsch JM , Katz J , Sommer A , Quigley HA , Javitt JC . Hypertension, perfusion pressure, and primary open-angle glaucoma. A population-based assessment . Arch Ophthalmol. 1995. ; 113 ( 2 ): 216 – 221 . [DOI] [PubMed] [Google Scholar]
- 20. Bonomi L , Marchini G , Marraffa M , Bernardi P , Morbio R , Varotto A . Vascular risk factors for primary open angle glaucoma: the Egna-Neumarkt Study . Ophthalmology. 2000. ; 107 ( 7 ): 1287 – 1293 . [DOI] [PubMed] [Google Scholar]
- 21. Memarzadeh F , Ying-Lai M , Azen SP , Varma R ; Los Angeles Latino Eye Study Group . Associations with intraocular pressure in Latinos: the Los Angeles Latino Eye Study . Am J Ophthalmol. 2008. ; 146 ( 1 ): 69 – 76 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Leske MC , Wu SY , Hennis A , Honkanen R , Nemesure B ; BESs Study Group . Risk factors for incident open-angle glaucoma: the Barbados Eye Studies . Ophthalmology. 2008. ; 115 ( 1 ): 85 – 93 . [DOI] [PubMed] [Google Scholar]
- 23. Tsang CS , Chong SL , Ho CK , Li MF . Moderate to severe obstructive sleep apnoea patients is associated with a higher incidence of visual field defect . Eye (Lond). 2006. ; 20 ( 1 ): 38 – 42 . [DOI] [PubMed] [Google Scholar]
- 24. Kimura A , Namekata K , Guo X , Noro T , Harada C , Harada T . Targeting oxidative stress for treatment of glaucoma and optic neuritis . Oxid Med Cell Longev. 2017. ; 2017 : 2817252 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Faridi O , Park SC , Liebmann JM , Ritch R . Glaucoma and obstructive sleep apnoea syndrome . Clin Exp Ophthalmol. 2012. ; 40 ( 4 ): 408 – 419 . [DOI] [PubMed] [Google Scholar]
- 26. Krieglstein G , Langham ME . Influence of body position on the intraocular pressure of normal and glaucomatous eyes . Ophthalmologica. 1975. ; 171 ( 2 ): 132 – 145 . [DOI] [PubMed] [Google Scholar]
- 27. Lee TE , Yoo C , Kim YY . Effects of different sleeping postures on intraocular pressure and ocular perfusion pressure in healthy young subjects . Ophthalmology. 2013. ; 120 ( 8 ): 1565 – 1570 . [DOI] [PubMed] [Google Scholar]
- 28. Lee JY , Yoo C , Jung JH , Hwang YH , Kim YY . The effect of lateral decubitus position on intraocular pressure in healthy young subjects . Acta Ophthalmol. 2012. ; 90 ( 1 ): e68 – e72 . [DOI] [PubMed] [Google Scholar]
- 29. Cheung N , Wong TY . Obesity and eye diseases . Surv Ophthalmol. 2007. ; 52 ( 2 ): 180 – 195 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mojon DS , Hess CW , Goldblum D , et al . High prevalence of glaucoma in patients with sleep apnea syndrome . Ophthalmology. 1999. ; 106 ( 5 ): 1009 – 1012 . [DOI] [PubMed] [Google Scholar]
- 31. Mojon DS , Hess CW , Goldblum D , Böhnke M , Körner F , Mathis J . Primary open-angle glaucoma is associated with sleep apnea syndrome . Ophthalmologica. 2000. ; 214 ( 2 ): 115 – 118 . [DOI] [PubMed] [Google Scholar]
- 32. Onen SH , Mouriaux F , Berramdane L , Dascotte JC , Kulik JF , Rouland JF . High prevalence of sleep-disordered breathing in patients with primary open-angle glaucoma . Acta Ophthalmol Scand. 2000. ; 78 ( 6 ): 638 – 641 . [DOI] [PubMed] [Google Scholar]
- 33. Marcus DM , Costarides AP , Gokhale P , et al . Sleep disorders: a risk factor for normal-tension glaucoma? J Glaucoma. 2001. ; 10 ( 3 ): 177 – 183 . [DOI] [PubMed] [Google Scholar]
- 34. Sergi M , Salerno DE , Rizzi M , et al . Prevalence of normal tension glaucoma in obstructive sleep apnea syndrome patients . J Glaucoma. 2007. ; 16 ( 1 ): 42 – 46 . [DOI] [PubMed] [Google Scholar]
- 35. Bendel RE , Kaplan J , Heckman M , Fredrickson PA , Lin SC . Prevalence of glaucoma in patients with obstructive sleep apnoea–a cross-sectional case-series . Eye (Lond). 2008. ; 22 ( 9 ): 1105 – 1109 . [DOI] [PubMed] [Google Scholar]
- 36. Lin PW , Friedman M , Lin HC , Chang HW , Wilson M , Lin MC . Normal tension glaucoma in patients with obstructive sleep apnea/hypopnea syndrome . J Glaucoma. 2011. ; 20 ( 9 ): 553 – 558 . [DOI] [PubMed] [Google Scholar]
- 37. Geyer O , Cohen N , Segev E , et al . The prevalence of glaucoma in patients with sleep apnea syndrome: same as in the general population . Am J Ophthalmol. 2003. ; 136 ( 6 ): 1093 – 1096 . [DOI] [PubMed] [Google Scholar]
- 38. Kadyan A , Asghar J , Dowson L , Sandramouli S . Ocular findings in sleep apnoea patients using continuous positive airway pressure . Eye (Lond). 2010. ; 24 ( 5 ): 843 – 850 . [DOI] [PubMed] [Google Scholar]
- 39. Girkin CA , McGwin G Jr , McNeal SF , Owsley C . Is there an association between pre-existing sleep apnoea and the development of glaucoma? Br J Ophthalmol. 2006. ; 90 ( 6 ): 679 – 681 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stein JD , Kim DS , Mundy KM , et al . The association between glaucomatous and other causes of optic neuropathy and sleep apnea . Am J Ophthalmol. 2011. ; 152 ( 6 ): 989 – 998.e3 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Aptel F , Chiquet C , Tamisier R , et al. Sleep Registry of the French Federation of Pneumology Paris, France . Association between glaucoma and sleep apnea in a large French multicenter prospective cohort . Sleep Med. 2014. ; 15 ( 5 ): 576 – 581 . [DOI] [PubMed] [Google Scholar]
- 42. Lin CC , Hu CC , Ho JD , Chiu HW , Lin HC . Obstructive sleep apnea and increased risk of glaucoma: a population-based matched-cohort study . Ophthalmology. 2013. ; 120 ( 8 ): 1559 – 1564 . [DOI] [PubMed] [Google Scholar]
- 43. Chen MJ . Normal tension glaucoma in Asia: epidemiology, pathogenesis, diagnosis, and management . Taiwan J Ophthalmol. 2020. ; 10 ( 4 ): 250 – 254 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ko YC , Hwang DK , Chen WT , Lee CC , Liu CJ . Impact of socioeconomic status on the diagnosis of primary open-angle glaucoma and primary angle closure glaucoma: a nationwide population-based study in Taiwan . PLoS One. 2016. ; 11 ( 2 ): e0149698 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Vijaya L , George R , Baskaran M , et al . Prevalence of primary open-angle glaucoma in an urban south Indian population and comparison with a rural population. The Chennai Glaucoma Study . Ophthalmology. 2008. ; 115 ( 4 ): 648 – 654.e1 . [DOI] [PubMed] [Google Scholar]
- 46. Zhang X , Beckles GL , Chou CF , et al . Socioeconomic disparity in use of eye care services among US adults with age-related eye diseases: National Health Interview Survey, 2002 and 2008 . JAMA Ophthalmol. 2013. ; 131 ( 9 ): 1198 – 1206 . [DOI] [PubMed] [Google Scholar]
- 47. Zhang X , Cotch MF , Ryskulova A , et al . Vision health disparities in the United States by race/ethnicity, education, and economic status: findings from two nationally representative surveys . Am J Ophthalmol. 2012. ; 154 ( 6 Suppl ): S53 – 62.e1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Carvalho B , Hsia J , Capasso R . Surgical therapy of obstructive sleep apnea: a review . Neurotherapeutics. 2012. ; 9 ( 4 ): 710 – 716 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Qian Y , Zou J , Xu H , et al . Association of upper airway surgery and improved cardiovascular biomarkers and risk in OSA . Laryngoscope. 2020. ; 130 ( 3 ): 818 – 824 . [DOI] [PubMed] [Google Scholar]
- 50. Lin PW , Lin HC , Friedman M , et al . Effects of CPAP for patients with OSA on visual sensitivity and retinal thickness . Sleep Med. 2020. ; 67 : 156 – 163 . [DOI] [PubMed] [Google Scholar]
- 51. Lin PW , Lin HC , Friedman M , et al . Effects of OSA surgery on ophthalmological microstructures . Ann Otol Rhinol Laryngol. 2019. ; 128 ( 10 ): 938 – 948 . [DOI] [PubMed] [Google Scholar]
- 52. Kiekens S , De Groot V , Coeckelbergh T , et al . Continuous positive airway pressure therapy is associated with an increase in intraocular pressure in obstructive sleep apnea . Invest Ophthalmol Vis Sci. 2008. ; 49 ( 3 ): 934 – 940 . [DOI] [PubMed] [Google Scholar]
- 53. Wozniak DJ , Schneiders M , Bourne RR , Smith IC . CPAP related intraocular pressure increase at night in people with and without glaucoma . Eur Respir J. 2018. ; 52 : PA430 . [Google Scholar]