Abstract
Rationale
High circulating galectin-3 is associated with poor outcomes in patients with coronavirus disease (COVID-19). We hypothesized that GB0139, a potent inhaled thiodigalactoside galectin-3 inhibitor with antiinflammatory and antifibrotic actions, would be safely and effectively delivered in COVID-19 pneumonitis.
Objectives
Primary outcomes were safety and tolerability of inhaled GB0139 as an add-on therapy for patients hospitalized with COVID-19 pneumonitis.
Methods
We present the findings of two arms of a phase Ib/IIa randomized controlled platform trial in hospitalized patients with confirmed COVID-19 pneumonitis. Patients received standard of care (SoC) or SoC plus 10 mg inhaled GB0139 twice daily for 48 hours, then once daily for up to 14 days or discharge.
Measurements and Main Results
Data are reported from 41 patients, 20 of which were assigned randomly to receive GB0139. Primary outcomes: the GB0139 group experienced no treatment-related serious adverse events. Incidences of adverse events were similar between treatment arms (40 with GB0139 + SoC vs. 35 with SoC). Secondary outcomes: plasma GB0139 was measurable in all patients after inhaled exposure and demonstrated target engagement with decreased circulating galectin (overall treatment effect post-hoc analysis of covariance [ANCOVA] over days 2–7; P = 0.0099 vs. SoC). Plasma biomarkers associated with inflammation, fibrosis, coagulopathy, and major organ function were evaluated.
Conclusions
In COVID-19 pneumonitis, inhaled GB0139 was well-tolerated and achieved clinically relevant plasma concentrations with target engagement. The data support larger clinical trials to determine clinical efficacy.
Clinical trial registered with ClinicalTrials.gov (NCT04473053) and EudraCT (2020–002230–32).
Keywords: COVID-19, galectin-3, GB0139
At a Glance Commentary
Scientific Knowledge on the Subject
High circulating galectin-3 is associated with poor outcomes in patients with coronavirus disease (COVID-19). We hypothesized that GB0139, a potent inhaled thiodigalactoside galectin-3 inhibitor with antiinflammatory and antifibrotic actions, would be safely and effectively delivered in COVID-19 pneumonitis.
What This Study Adds to the Field
In COVID-19 pneumonitis, inhaled GB0139 was well-tolerated and achieved clinically relevant plasma concentrations with target engagement. The data support larger clinical trials to determine clinical efficacy.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the disease it causes (coronavirus disease [COVID-19]) has put major stress on the world’s healthcare services and, despite vaccination, is likely to remain a problem for the foreseeable future (1). Severe morbidity and mortality from COVID-19 are predominantly a consequence of immunopathology in response to viral infection (2), characterized by aberrant monocyte and macrophage-driven lung injury, T-cell depletion (3), cytokine excess, inflammation-driven thrombosis, and fibrotic lung damage (4–7). Among the many interventions tested (8), treatments that target this immunopathology, such as corticosteroids (9) and IL-6 antagonists (10), have been shown to improve outcomes in hospitalized patients with COVID-19, with the greatest effect observed in those requiring respiratory support. Despite the approval of these agents as the standard of care (SoC) for COVID-19, there is still an urgent need to identify other effective and tolerable therapies, particularly those that target COVID-19 immunopathology (11), alongside antiviral effects.
Galectin-3, a mammalian β-galactoside– binding lectin, is an important regulator of immune homeostasis highly upregulated after lung injury (i.e., a biomarker for lung damage) (12–15). There is an illness severity-dependent increase in galectin-3 concentration in COVID-19 (16, 17), and given the immunopathology of COVID-19, galectin-3 blockade is considered a plausible therapeutic target (7, 18). Galectin-3 drives a systemic and pulmonary proinflammatory cytokine profile after coinfection of pneumococcus and H1N1 avian influenza virus (19) via upregulation of macrophage IL-1β production (20) and NLRP3 inflammasome activation (19, 20). Therefore, anti–galectin-3 therapy can reduce macrophage and dendritic cell secretion of cytokines IL-1, IL-6, and TNF-α and attenuate other inflammatory COVID-19–associated consequences (16, 18, 21), including inflammation-driven thrombosis (22). LGAL3SBP (galectin-3–binding protein) (23) is an interaction partner of SARS-CoV-2 spike glycoprotein, enhancing syncytia formation. Furthermore, inhaled GB0139, a potent thiodigalactoside galectin-3 inhibitor, in a phase Ib/IIa trial, suppressed galectin-3 expression and decreased key plasma biomarkers associated with idiopathic pulmonary fibrosis (IPF) disease progression (24). These initial findings support the hypothesis that inhaled GB0139 may counteract the SARS-CoV-2–associated pathobiology enhanced by galectin-3, thereby reducing the severity of COVID-19 and potentially the development of postviral pulmonary fibrosis.
Therefore, we sought to investigate the safety, pharmacokinetics, and pharmacodynamics of inhaled GB0139 in hospitalized patients with COVID-19 pneumonitis before evaluating clinical efficacy in larger trials. Biological and clinical indices of efficacy were also explored.
Some of the results of these studies have been previously reported in the form of an abstract (25) and preprint (medRxiv 10 January 2022 https://www.medrxiv.org/content/10.1101/2021.12.21.21267983v2).
Methods
Study Design
DEFINE (University of Edinburgh COVID-19 Define trial, 2020; ClinicalTrials.gov identifier: NCT04473053, EurdraCT number: 2020–002230–32) was a randomized, controlled, open-label, parallel-group, experimental medicine platform study evaluating the safety and pharmacokinetics and pharmacodynamics of GB0139 and nafamostat (data presented separately [26]) in patients with COVID-19.
After eligibility screening, patients were randomly assigned (1:1:1) to receive GB0139 plus SoC, nafamostat plus SoC, or SoC alone. Separate statistical analysis plans were used for each active treatment versus SoC comparison. Treatment arms have been reported separately. Additional information regarding randomization and SoC treatment is included in the online supplement. For this analysis, we report data from the GB0139 + SoC and SoC cohorts.
GB0139 was administered as an inhaled formulation of 10 mg twice daily via a dry powder inhaler (Plastiape; Berry Bramlage) for the first 48 hours, followed by 10 mg once daily for up to 14 days or until discharge from the hospital or withdrawal from the trial.
The dose of GB0139 is on the basis of previous experience in patients with IPF receiving 10 mg of GB0139 daily for 14 days. In patients with IPF, a near-total attenuation of Gal-3 concentrations in lung macrophages was observed, showing near-total target engagement in the target organ (24). A loading dose strategy was implemented in DEFINE for the first 2 days of treatment (10 mg twice daily) to reach the desired lung concentrations of GB0139 faster.
Independent ethics committee approval (Scotland A Research Ethics Committee: 20/SS/0066) was obtained before the initiation of the study. The independent Data Monitoring Committee scrutinized accumulating data.
Study Participants
The trial protocol has been previously reported (27). Eligible patients were aged 16 years or older, had polymerase chain reaction-confirmed COVID-19 infection, and required oxygen and had evidence of pneumonitis on X-ray. Inclusion and exclusion criteria are provided in Table 1.
Table 1.
Inclusion and Exclusion Criteria for Patient Eligibility for Study Participation
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| - COVID-19 positive test result within the last 14 d - Hospitalized with breathlessness requiring oxygen and evidence of pneumonitis on X-ray - Provision of informed consent from the patient or representative - Aged at least 16 yr - If the patient is of child-bearing potential, the patient, and their partner(s), agree to use medically accepted double-barrier methods of contraception during the study and for at least 90 d after termination of study therapy |
- Current or recent history, as determined by the Investigator, of severe, progressive, and/or uncontrolled cardiac disease(NYHA class IV), uncontrolled renal disease (eGFR < 30 ml/min/1.73 m2), severe liver dysfunction (ALT >5 × ULN) or anemia (Hb < 80 g/L) - Women who are pregnant or breastfeeding - Participation in another clinical trial of an investigational medicinal product - Known hypersensitivity to the IMP or excipients (e.g., lactose) - Concomitant use of treatments for COVID-19 that are not recognized as locally approved standard care - Significant electrolyte disturbance (hyperkalemia K+ >5.0 mmol/L or hyponatremia Na+ <120 mmol/L) - Currently receiving potassium-sparing diuretics that cannot be reasonably withheld - Currently receiving anticoagulation or antiplatelet agents that cannot be reasonably withheld if randomized to receive nafamostat - Patients (or their partners) planning on donating sperm and/or eggs during the trial period - Ongoing dialysis - History of serious liver disease (Child Pugh score >10) - Severe uncontrolled diabetes mellitus - In the Investigator’s opinion, patient is unwilling or unable to comply with drug administration plan, laboratory tests, or other study procedures |
Definition of abbreviations: ALT = alanine aminotransferase; AST = aspartate transaminase; COVID-19 = coronavirus disease; eGFR = estimated glomerular filtration rate; Hb = hemoglobin; IMP = investigational medicinal product; NYHA = New York Heart Association; ULN = upper limit of normal.
Objectives
The primary objective was to evaluate the safety of inhaled GB0139 as add-on therapy in patients with COVID-19 pneumonitis. Secondary objectives included assessments of the pharmacokinetic and pharmacodynamic properties of GB0139. Secondary exploratory biomarkers were assessed during treatment, as was the potential for clinical efficacy.
Safety was assessed daily using observations and blood parameters (Table E1 in the online supplement). Adverse events (AEs) and concomitant medications were recorded from the screening visit until discharge, withdrawal, death, or 90-day follow-up.
Pharmacokinetics
Blood plasma samples for pharmacokinetic assessments were drawn before the dose on Days 1, 3, 5, 8, and 11 (±1 d) for the determination of trough plasma GB0139 concentrations. Sample analysis is detailed in the online supplement.
Flow cytometry
Inflammatory cell phenotypes were analyzed by fluorescence-activated cell sorting of peripheral blood. Methods are described in the Appendix, and gating strategies and a list of antibodies used are available in Figure E1 and Table E2, respectively.
Cytokine analysis
Blood samples were collected daily for biomarker and cytokine measurement, subject to patient tolerance. Details of biomarkers selected and analysis can be found in the online supplement.
Viral load
Viral load was determined as previously described (26).
Statistical Analysis
Note that this study is an early phase clinical trial with a small sample size, and, therefore, we only have sufficient power to detect large differences between groups.
An indicative sample size calculation suggested that 20 patients per group provides 80% power to detect an effect size of 0.7 using a two-group t test with a 10% one-sided degree of significance (equivalent to a two-sided 20% degree) and assuming 5% missing data, for the difference of means in a biomarker between GB0139 and control groups. For ease of presentation and to reduce confusion for the reader, we chose to present the usual two-sided 95% credible intervals.
Nonsignificant results should be interpreted cautiously.
Bayesian generalized linear mixed effects models were fitted to continuous safety outcomes, with baseline and trial arm as explanatory variables and a random effect for the patient. Day of measurement after randomization was also included as a categorical factor variable. Noninformative flat priors were used. Results were reported as posterior mean differences and the highest posterior density 95% credible intervals. Further details are provided in the online supplement. Note that whenever we use the phrase “statistically significant” in the post hoc analysis of trends, this means that the credible intervals do not contain zero, so the posterior probability of a treatment benefit (or harm) will be at least 97.5%.
Results
Patient Disposition and Baseline Characteristics
The study population, recruited between September 2020 and February 2021, comprised 43 patients aged 27–87 years admitted to the Royal Infirmary of Edinburgh and Western General Hospital, Edinburgh. Twenty-two patients were randomized to receive GB0139 + SoC and 21 to receive SoC (Figure 1). Two patients assigned to the GB0139 + SoC arm did not receive any study drug because of clinical deterioration after randomization but before administration and were excluded from the analyses as per the predefined statistical analysis plan. One patient missed a loading dose. Full baseline demographics and disease characteristics are provided in Table 2, with a patient-degree breakdown of comorbidities in Table E3. Details of SoC treatment on an individual basis are provided in Table E4.
Figure 1.
Consort diagram of patient disposition.
Table 2.
Patient Demographics and Disease Characteristics at Study Entry
| Overall Population |
Subgroup with Baseline NEWS⩾ 4 |
|||
|---|---|---|---|---|
| Characteristic | GB0139 + SoC | SoC | GB0139 + SoC | SoC |
| (n = 20) | (n = 21) | (n = 14) | (n = 10) | |
| Male sex, n (%) | 11 (55.0) | 12 (57.1) | 10 (71.4) | 5 (50.0) |
| Age, yr | ||||
| Mean (SD) | 65.2 (13.9) | 65.0 (16.4) | 65.1 (14.8) | 64.5 (19.0) |
| Median (range) | 66.5 (29.0–87.0) | 65.0 (27.0–29.0) | 66.5 (29.0–86.0) | 67.5 (27.0–87.0) |
| BMI (kg/m2), mean (SD) | 32.1 (5.5) | 32.4 (6.3) | 32.8 (5.4) | 35.9 (5.4) |
| Ethnicity, n (%) | ||||
| White | 19 (95.0) | 19 (90.5) | 13 (92.9) | 10 (100) |
| Asian | 1 (5.0) | 1 (4.8) | 1 (7.1) | 0 |
| Black | 0 | 1 (4.8) | 0 | 0 |
| Smoking status, n (%) | ||||
| Nonsmoker | 12 (60.0) | 11 (52.4) | 9 (64.3) | 5 (50.0) |
| Ex-smoker | 8 (40.0) | 10 (47.6) | 5 (35.7) | 5 (50.0) |
| D since first symptoms (n), mean (SD) | 8.5 (3.8) | 8.6 (3.7) | 9.8 (4.4) | 9.4 (3.7) |
| Any comorbidities | 12 (85.7) | 9 (90.0) | ||
| Chronic cardiac disease | 5 (26.3) | 5 (23.8) | 3 (28.6) | 3 (30.0) |
| Hypertension | 8 (42.1) | 8 (38.1) | 7 (50.0) | 4 (40.0) |
| Chronic pulmonary disease | 6 (31.6) | 5 (23.8) | 5 (35.7) | 3 (30.0) |
| Asthma | 3 (15.8) | 3 (14.3) | 2 (14.3) | 3 (30.0) |
| Chronic kidney disease | 2 (10.5) | 1 (4.8) | 1 (7.1) | 1 (10.0) |
| Chronic liver disease | 1 (5.3) | 0 | 0 | 0 |
| Chronic neurological disorder | 2 (10.5) | 4 (19.0) | 2 (14.3) | 2 (10.0) |
| Diabetes | 4 (21.1) | 3 (14.3) | 4 (28.6) | 3 (30.0) |
| Malignancy | 1 (5.3) | 3 (14.3) | 1 (7.1) | 2 (20.0) |
| Immunocompromised | 0 | 2 (9.5) | 0 | 1 (10.0) |
| Other | 8 (42.1) | 11 (52.4) | 5 (35.7) | 4 (40.0) |
| NEWS2 | ||||
| Mean (SD) | 4 (1.8) | 4 (1.7) | 5 (1.5) | 5 (1.6) |
Definition of abbreviations: BMI = body mass index; D = days; NEWS2 = National Early Warning Score 2; SD = standard deviation; SoC = standard of care.
Mean NEWS2 rounded to whole integers to represent the scale. For a full breakdown of comorbidities at an individual participant degree, please see Table E3.
It was noted that in the GB0139 + SoC arm, 30% of patients had a National Early Warning Score 2 (NEWS2) (28) of three or less at trial entry, and 28.6% of patients had a NEWS2 of six or more at baseline. In the SoC arm, 50% of patients had a baseline NEWS2 of three or less, and 9% of patients had a NEWS2 of six or more. A post hoc subgroup analysis of patients with a baseline NEWS2 of four or more was therefore undertaken. Please see the online supplement for these additional results.
Safety and Tolerability of GB0139
There were no safety or tolerability concerns associated with inhaled administration of GB0139, and no statistically significant differences were observed in hematological and biochemical safety laboratory outcomes or vital signs (Table 3). The GB0139 + SoC group experienced 40 AEs compared with 35 in the SoC group. The total number of patients who experienced at least 1 AE was 14 (70.0%) in the GB0139 + SoC group versus 12 (57.1%) in the SoC group (Table 4). The odds ratio (95% credible interval [CI]) of 1 or more AE occurring between trial arms was 1.83 (95% CI, 0.48–6.81), and the rate ratio (95% CI) between arms for the number of AEs per patient was 1.20 (95% CI, 0.75–1.89). Three serious AEs were recorded from one patient in the GB0139 + SoC group after hospital discharge (small bowel obstruction and paracetamol overdose, both requiring hospital admission, and worsening of hyponatremia). These were considered unrelated to the study treatment.
Table 3.
Primary Outcome of Safety Assessments. Continuous Outcome Variable Results: Hematological and Biochemical Safety Laboratory Outcomes and Vital Signs
| 95% HPD Interval |
|||
|---|---|---|---|
| Variable | Mean | Lower Limit | Upper Limit |
| Hemoglobin (g/L) | −0.495 | −7.21 | 5.963 |
| Hematocrit (ratio) | −0.206 | −5.04 | 4.996 |
| Red cell count (109/L) | −0.128 | −4.87 | 4.641 |
| Mean cell volume (fl) | −0.136 | −5.73 | 5.546 |
| Mean cell Hb (pg) | −0.344 | −5.36 | 4.608 |
| Mean cell Hb concentration (g/L) | 0.050 | −6.94 | 7.375 |
| White cell count (109/L) | −0.489 | −6.04 | 5.255 |
| Neutrophil count (109/L) | 0.031 | −5.80 | 6.003 |
| Lymphocytes (109/L) | −0.030 | −5.64 | 5.422 |
| Monocyte count (109/L) | 0.112 | −5.93 | 5.751 |
| Basophil count (109/L) | −0.640 | −5.88 | 4.626 |
| Eosinophil count (109/L) | 0.691 | −10.8 | 12.99 |
| Platelet count (109/L) | 17.76 | −25.5 | 59.05 |
| Random glucose (micromole/L) | −0.536 | −6.45 | 4.911 |
| Urea (mmol/L) | 0.533 | −5.08 | 6.125 |
| Sodium (mmol/L) | −0.203 | −5.25 | 5.327 |
| Potassium (mmol/L) | 0.144 | −4.96 | 5.791 |
| Chloride (mmol/L) | −1.05 | −7.05 | 4.631 |
| Magnesium (mmol/L) | −0.090 | −5.95 | 6.038 |
| Bicarbonate (mmol/L) | 0.856 | −4.65 | 6.472 |
| Creatinine (micromole/L) | −1.77 | −8.39 | 4.721 |
| Total protein (g/L) | −0.896 | −6.93 | 5.036 |
| Albumin (g/L) | −1.05 | −6.37 | 4.081 |
| AST (U/L) | −10.3 | −28.4 | 7.814 |
| Total bilirubin (micromole/L) | 0.741 | −4.83 | 6.798 |
| GGT (U/L) | −15.2 | −44.2 | 15.82 |
| ALT (U/L) | −2.97 | −26.2 | 20.77 |
| Alkaline phosphatase (U/L) | −1.37 | −9.75 | 6.990 |
| LDH (U/L) | −18.8 | −62.7 | 25.53 |
| Ferritin (micromole/L) | 63.66 | −96.9 | 220.1 |
| CRP (mg/L) | 0.168 | −27.1 | 27.82 |
| Troponin (ng/L) | −0.469 | −10.5 | 10.06 |
| Triglycerides (mmol/L) | −0.696 | −6.69 | 4.959 |
| D-dimer (ng/ml) | 5.609 | −208 | 209.1 |
| Creatinine kinase (U/L) | −23.1 | −76.8 | 25.12 |
| INR (ratio) | −0.444 | −5.43 | 4.268 |
| Prothrombin time (seconds) | −0.484 | −5.42 | 4.740 |
| Fibrinogen (g/L) | −0.071 | −6.14 | 5.822 |
| aPTT (seconds) | 0.226 | −6.06 | 6.674 |
| Protein C (IU/ml) | −0.134 | −5.78 | 5.671 |
| Antithrombin (IU/ml) | −0.131 | −6.16 | 5.718 |
| O2 saturation (%) | 0.616 | −3.49 | 4.848 |
| Respiratory rate (bpm) | 0.774 | −5.06 | 6.715 |
| Systolic blood pressure (mm Hg) | −3.62 | −13.6 | 6.819 |
| Diastolic blood pressure (mm Hg) | −0.773 | −7.81 | 6.425 |
| Temperature (degrees C) | 0.196 | −4.78 | 5.035 |
| Pulse (bpm) | 5.242 | −4.52 | 14.52 |
| ECG rate | 2.143 | −8.11 | 12.86 |
| ECG Q̇tc | −4.37 | −22.5 | 13.09 |
Definition of abbreviations: ALT = alanine aminotransferase; aPTT = activated partial thromboplastin time; AST = aspartate aminotransferase; CRP = C-reactive protein; GGT = γ-glutamyltransferase; Hb = hemoglobin; HPD = highest posterior density; INR = international normalized ratio; LDH = lactate dehydrogenase; Q̇tc = corrected Q̇t interval.
All of the 95% HPD-credible intervals include zero, and therefore none are statistically significant.
Table 4.
Adverse Events in the Safety Population
| Total Population |
Subgroup with Baseline NEWS2⩾ 4 |
|||
|---|---|---|---|---|
| Study Group, n (%) | GB0139 + SoC | SoC | GB0139 + SoC | SoC |
| (n = 20) | (n = 21) | (n = 14) | (n = 10) | |
| Any AE | 14 (70.0) | 12 (57.1) | 10 (71.4) | 4 (40.0) |
| Any serious AE | 1* (5.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Definition of abbreviations: AE = adverse event; NEWS2 = National Early Warning Score 2; SoC = standard of care.
Three serious AEs were reported for one patient in the GB0139 study group: small bowel obstruction, paracetamol overdose, and worsening of hyponatremia.
Among patients who received GB0139 + SoC, five reported an AE considered by the clinical study team to be possibly related to treatment. These comprised an isolated instance of prolonged corrected Q̇t interval on ECG with no arrhythmia that spontaneously resolved, nausea, sore throat, oral thrush, and hair loss (all mild severity). Over 400 patients with IPF have been treated with GB0139 (ClinicalTrials.gov Identifiers: NCT02257177 and NCT03832946), and none of these AEs have previously been reported with treatment. Table E5 details all AEs.
Plasma Concentrations of GB0139
After an initial peak of 19.94 ng/ml on Day 3, consistent with the higher 10 mg twice daily dosing for the first 2 days, geometric mean trough plasma concentrations of GB0139 decreased to 7.81 ng/ml on Day 5 and had reached steady-state by Day 8 (6.01 ng/ml). Similar trough concentrations were measured at the end of the pharmacokinetics assessment period on Day 11 (5.50 ng/ml). The apparent decrease of plasma concentrations between Days 3 and 5 was because of the twofold lower daily dose administered from Day 3 onwards (10 mg every day from Day 3 vs. 10 mg twice daily for the first 48 h). Ranges of individual values overlapped between Days 5, 8, and 11, and interindividual variability on trough plasma concentrations was moderate, with coefficients of variation ranging across days from 39% to 57%, indicating consistent exposure between patients and across days (Table 5). Inhaled GB0139 achieved plasma concentrations in patients with COVID-19 were comparable to that previously observed in patients with IPF (14, 24), with individual ranges overlapping between the two populations (Figure E2).
Table 5.
Trough (Before Dose) Plasma GB0139 Concentrations (ng/ml) in Patients with COVID-19
| Baseline | Day 3 | Day 5 | Day 8 | Day 11 | |
|---|---|---|---|---|---|
| N | 18 | 20 | 12 | 9 | 4 |
| Arithmetic mean (SD) | BLQ | 22.91 (12.03) | 8.58 (4.60) | 6.66 (3.80) | 5.85 (2.30) |
| CV, % | — | 53 | 54 | 57 | 39 |
| Geometric mean | — | 19.94 | 7.81 | 6.01 | 5.50 |
| Median (min–max) | BLQ | 18.28 | 7.32 | 5.27 | 5.70 |
| (BLQ–BLQ) | (7.45–51.21) | (3.94–21.62) | (3.87–15.99) | (3.22–8.81) |
Definition of abbreviations: BLQ = below the limit of quantitation (0.5 ng/ml); CV = coefficient of variation; SD = standard deviation.
GB0139 Effect on Biomarkers
There was a decrease in the mean serum concentration of galectin-3 over time with GB0139 + SoC treatment (overall treatment effect post hoc analysis of covariance (ANCOVA) vs. SoC over Days 2–7: P = 0.0099), indicating target engagement activity (Figure 2). Further post hoc analysis, using Bayesian generalized linear mixed effects models, showed a significantly greater rate of decline of galectin-3 concentrations between patients receiving GB0139 + SoC compared with those receiving SoC (Table E6.1 and E6.2).
Figure 2.
Individual patient serum galectin-3 concentrations from all patients receiving GB0139 + SoC (standard of care) (red) or SoC (blue). Linear regression analysis showing the line of best fit with a 95% confidence interval (shaded area). Of note, overall treatment effect, using all available data to Day 16 was also significant for GB0139 + SoC versus SoC (post hoc analysis of covariance [ANCOVA] over days 2–16: P = 0.0001); however, the last day for data availability in the SoC arm was Day 14.
Biomarkers of inflammation
All biomarkers were assayed as planned. Although GB0139 + SoC-treated patients had higher baseline C-reactive protein (CRP) concentrations than those receiving SoC (115 vs. 45 mg/L, respectively), a post hoc analysis showed that the rate of decline of CRP was significantly greater in patients receiving GB0139 + SoC compared with those receiving SoC (Figure 3A and Table E6.1). Similarly, post hoc analysis showed the rates of decline of lactate dehydrogenase and neutrophil to lymphocyte ratio (NLR) were also significantly greater in patients receiving GB0139 + SoC compared with those receiving SoC (Figures 3B and 3C and Table E6.1).
Figure 3.

Absolute change from baseline in markers of inflammation in the overall population: (A) C-reactive protein, (B) lactate dehydrogenase, (C) neutrophil/lymphocyte ratio, and (D) CXCL10. Individual patient-degree data from all those who received GB0139 + SoC (standard of care) (red) or SoC (blue): linear regression analysis showing the line of best fit with 95% confidence interval (shaded area). CXCL10 = C-X-C motif chemokine ligand 10.
In post hoc analysis, the rate of decline of CXCL10 was significantly greater in those receiving GB0139 + SoC rather than SoC (Figure 3D and Table E6.1). Cytokine IL-1b and granulocyte–macrophage colony stimulating factor are not reported because of low or undetectable concentrations (below the lower limit of quantification). IL-1rα, IL-8, IL-17, and amphiregulin were measured, but no differences were noted between treatment arms.
Biomarkers of coagulopathy
Patients receiving GB0139 + SoC compared with patients receiving SoC alone had consistently low concentrations of D-dimer (Figure 4A). In post hoc analysis, the rate of decline of plasma concentrations of fibrinogen/platelet ratio was significantly greater in patients receiving GB0139 + SoC compared with those receiving SoC (Figure 4B and Table E6.1). We observed an increase in platelet count and activated partial thromboplastin time in GB0139 + SoC-treated patients compared with patients receiving SoC alone (Figures 4C and 4D).
Figure 4.
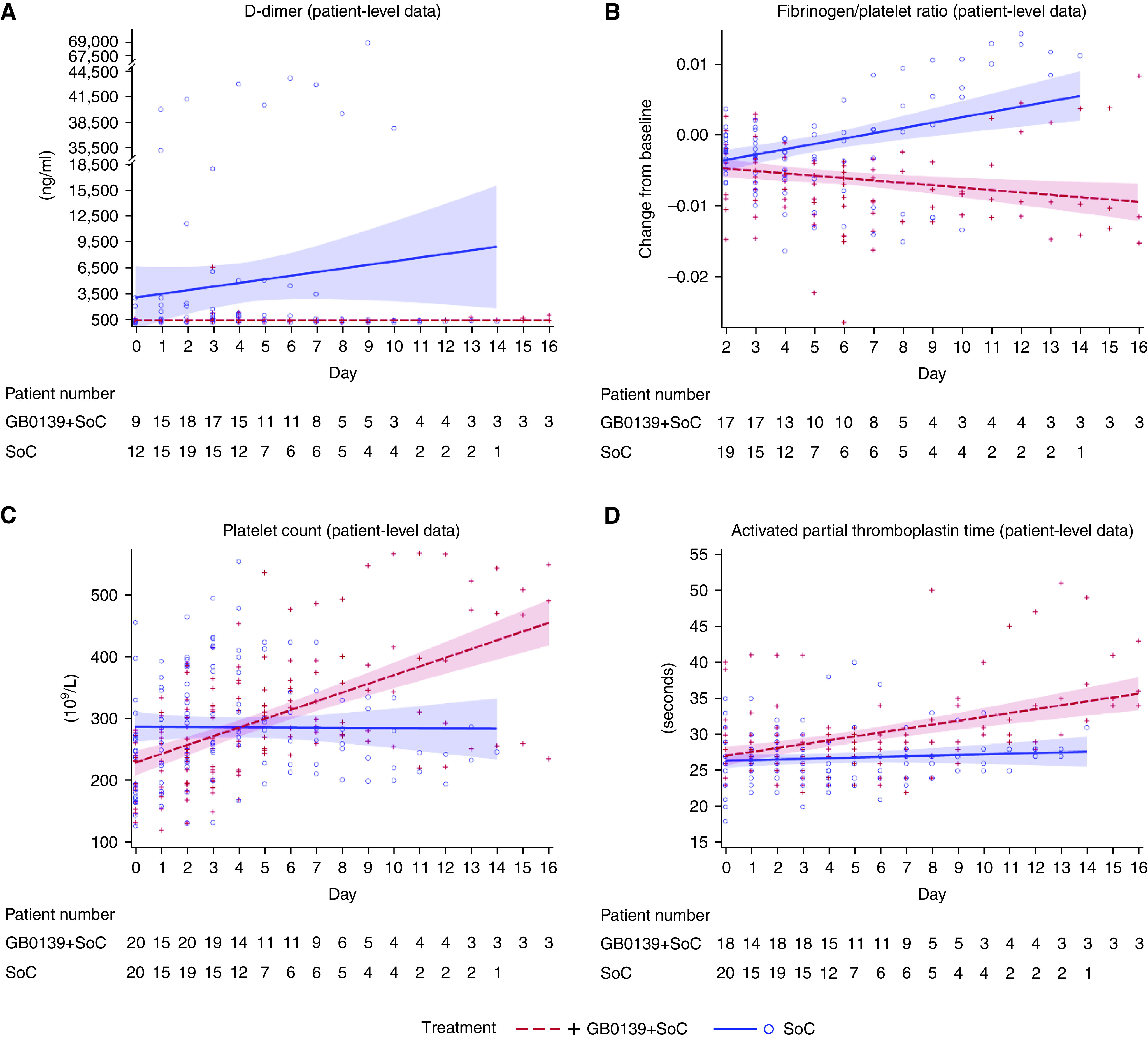
Effect of GB0139 + SoC (standard of care) (blue) and SoC (red) on coagulopathy: individual patient-degree data for (A) D-dimer concentrations, (B) fibrinogen/platelet ratio, (C) platelets, and (D) activated partial thromboplastin time. (A) and (B) display linear regression analysis showing the line of best fit with a 95% confidence interval (shaded area).
Biomarkers of fibrosis
GB0139 + SoC-treated patients had lower concentrations (from Days 4–7) of YKL-40 from baseline compared with SoC (Figure E4A). Furthermore, flow analyses of peripheral blood in the patient subgroup with a NEWS2 of four or more suggest that GB0139 may change monocyte phenotype from profibrotic transitional to antifibrotic classical and decrease transitional monocytes (Figure E4C).
Patient Outcomes and Mortality Rates
Seven deaths occurred in the overall population of 41 randomized patients: four deaths in 20 patients in the GB0139 + SoC arm (in which patients received a median of two GB0139 doses) and three deaths in 21 patients in the SoC group. In the NEWS2 of four or more group, 6 deaths occurred, 3/11 deaths in SOC compared with 3/14 deaths in GB0139 + SOC (full details are available in Table E7). No deaths were attributed to GB0139. There were no patients who received invasive ventilation or renal replacement therapy while taking part in the trial. The median duration of hospital stay for GB0139 + SoC patients was 3 days longer on average compared with the SoC arm (SoC: 3 d, minimum 1 d, maximum 21 d; GB0139 + SoC: 6 d, minimum 2 d, maximum 35 d). This was nonsignificant.
The GB0139 arm had a significantly lower number of oxygen-free days compared with standard care (19/123 vs. 29/86; rate ratio 0.45, 95% highest posterior density credible interval 0.25–0.82), after taking into account the number of times oxygen status was recorded in each arm. There was a significantly greater reduction in FiO2 observed in patients treated with GB0139 + SoC compared with SoC (Figure E4).
Viral Data
Nasopharyngeal and saliva samples were taken at baseline, Day 3, and Day 5 for RT-PCR analysis. Viral load decreased over time in both groups (Figure E5). During the course of the study, the virus strain dominant in the community was initially α for the first half and then β.
Discussion
DEFINE is the first reported clinical trial of an inhaled galectin-3 inhibitor for the treatment of COVID-19. GB0139 had an acceptable safety and tolerability profile in hospitalized patients with COVID-19 pneumonitis. Despite being breathless and requiring oxygen, patients were able to inhale and achieve consistent exposure of GB0139. The 10 mg twice-daily dose used for the first 2 days is higher than the top dose used in an ongoing study in IPF (NCT03832946) and was well tolerated. Inhaled GB0139 caused a significant reduction of galectin-3 concentrations in patients with COVID-19 and may reduce plasma concentrations of other key prognostic biomarkers associated with severe disease.
High galectin-3 concentrations correlate with the severity of COVID-19, and it performs as a biomarker for the severity of acute respiratory distress syndrome in COVID-19 (29). Patients with high galectin-3 serum concentrations also have a markedly higher risk of ICU admission or death (30).
Despite a small sample size, this study adds further weight to the therapeutic potential of galectin-3 inhibition in the treatment of patients with COVID-19. Although the trial was not powered to assess efficacy, GB0139 treatment showed a greater decrease in the rate of inflammatory and physiological biomarkers associated with COVID-19 severity in patients on GB0139 + SOC compared with SOC. CRP and NLR are measures of systemic inflammation, with increased concentrations indicative of severe COVID-19 infection. CXCL10 has been identified as a cardinal chemokine driving COVID-19 immunopathogenesis (31, 32). Raised CXCL10 concentrations are often observed in both plasma and BAL of patients who are critically ill with the disease (33). We have shown a greater rate of decline of CRP, NLR, and CXCL10 in patients treated with GB0139 + SoC compared with SoC. This suggests that GB0139, as an add-on to SoC, may have the potential to reduce the severity of systemic inflammation and the cytokine excess in patients with COVID-19. Lymphocyte exhaustion is also a feature of both infection and progression of severe COVID-19 (34). In the NEWS2 of four or more subgroup, we observed that whereas the concentration of B cells and T cells may have been higher in the GB0139 + SoC-treated group compared with SoC, exhaustion markers for CD4 and CD8 T cells still had a greater decrease with GB0139 + SoC treatment. These observations suggest that GB0139 therapy may possess a role in enhanced immunological responses to COVID-19 infection and improve recovery compared with SoC.
A high incidence of micro/intravascular thrombosis and thromboembolic events are notable features of severe COVID-19 infection (35). D-dimer is a fibrin degradation product, and elevated concentrations (>1,000 ng/ml−1) are strongly linked to the rate of disease, poor outcomes, and death (36–40). In the overall population, low D-dimer concentrations were maintained with GB0139 + SoC treatment, in contrast to SoC, in which a steady increase was observed. The observations in additional markers of coagulopathy (fibrinogen/platelet ratio, platelets, and activated partial thromboplastin time) also suggest that inhibition of galectin-3 by GB0139 may impact the COVID-19–induced coagulopathy.
Lung injury with subsequent respiratory failure is a key feature of COVID-19 (41, 42). The reduction in FiO2 observed in patients treated with GB0139 + SoC compared with SoC requires further investigation.
Recovery from COVID is an emerging healthcare burden (6), with studies demonstrating persistent pulmonary dysfunction (43). We showed that inhaled GB0139 leads to systemic exposure in patients with COVID-19 comparable to that previously observed in patients with IPF who subsequently had reduced concentrations of biomarkers associated with fibrotic disease progression (24). YKL-40 is secreted by activated macrophages and neutrophils in response to IL-1 and IL-6 and is associated with extracellular tissue remodeling and fibrosis. PAI-1 is a positive regulator of inflammation, clotting, and fibrosis (44–46). These drivers of lung fibrosis were both consistently lower after GB0139 + SoC treatment versus SoC in the current analysis, particularly in patients with baseline NEWS2 of four or more. Transitional monocytes are associated with fibrosis, and classical monocytes are associated with antimicrobial effects (47, 48). In the NEWS2 of four or more subgroup, we observed low concentrations of transitional monocytes with GB0139 + SoC versus SoC (<100% vs. 100–300%) but higher overall concentrations of classical monocytes in the former group. These potentially beneficial effects on monocyte subsets may augment the immune response to infection and decrease fibrotic tendency in patients with moderate-to-severe COVID-19.
In the post hoc analysis of the NEWS2 of four or more subpopulation, there was an imbalance in clinical severity between the 2 arms (NEWS2 of six or more, 9% in SOC and 28.6% on GB0139). In this NEWS2 of four or more subgroup, there were 3/14 (21.4%) deaths among patients who received GB0139 + SOC versus 3/11 (27.3%) in patients receiving SOC. These numbers are too small to draw any definitive conclusion, and this warrants further investigation in a larger clinical study.
Limitations
This study aimed to evaluate the safety and tolerability of inhaled GB0139 in hospitalized patients with COVID-19 requiring oxygen and was limited by a small sample size, particularly as patients left the trial toward the end of the 16-day trial period. Given the small number of patients, differences in secondary outcomes such as length of hospital stay become difficult to interpret, as they are liable to skewing by individual patients. The numbers in the study are too small to evaluate if biomarker changes translate into improved clinical outcomes. The number of patients in the post hoc analysis of NEWS2 of 4 or more is, therefore, smaller again, and while the observations are of interest, they should be interpreted with caution. When analyzing results, the duration of symptoms or treatment was not taken into account. Investigator knowledge of treatment allocation in this open-label trial may have biased the provision of inspired oxygen; however, the investigators were not part of the clinical team caring for the patient. The use of the NEWS2 score in the post hoc analysis may have been skewed because of underlying comorbidities.
Conclusions
This is the first trial of an inhaled galectin-3 inhibitor in COVID-19. It has demonstrated that hospitalized patients with COVID-19 can safely and effectively inhale GB0139 and achieve plasma concentrations known to induce biomarker changes. Our data indicate that inhaled GB0139 decreases concentrations of circulating galectin-3 and may reduce inflammation. Our early phase trial data support the need for further investigation in larger clinical trials for GB0139 in treating hospitalized patients with COVID-19.
Acknowledgments
Acknowledgment
The authors thank the participants and their families and all GB0139 study team members and investigators. The authors also thank the Clinical Research Facility staff in the Royal Infirmary of Edinburgh; the Emergency Medicine Research Group Edinburgh (EMERGE) research team; the Academic and Clinical Central Office for Research and Development (ACCORD) Facilitation; and the quality assurance and monitoring teams at the University of Edinburgh/NHS Lothian (Sponsor). Flow cytometry data were generated with support from the Queen’s Medical Research Institute (QMRI) Flow Cytometry and cell sorting facility, University of Edinburgh. GB0139 is being developed by Galecto Biotech. Galecto Biotech participated in the design, study conduct, analysis, and interpretation of data, as well as the writing, review, and approval of the manuscript. The authors thank the data monitoring committee and steering committee members. Third-party medical writing assistance under the direction of the authors was provided by Sinéad Holland, Ph.D., of Ashfield MedComms, an Ashfield Health company, and was funded by Galecto Biotech.
Footnotes
Supported by LifeArc and funding was provided to the University of Edinburgh under the STOPCOVID award. Support also received as part of Baillie Gifford Pandemic Science Hub.
Author Contributions: K.D. was the chief investigator of the study. T.M.Q. and E.E.G. were the clinical delivery team. A.M.B. and J.A. were the clinical management team. D.H., R.O’C., F.L., C.B., A.V., M.B., P.E., B.M., G.R., G.H., E.G.F, R.M., and E.S. were the laboratory team. T.S., B.L., A.M.B., J.A., D.H.D., T.S.W., J.N., R.A.P., J.W.D., O.K., K.T., M.S.-H., A.R.A., N.H., and K.D. contributed to trial protocol development. T.M.Q., E.E.G., R.O’C., F.L., A.M., V.A., J.W.-J., R.J.S., B.L., H.S., S.P., and T.S. contributed to the data analysis. L.G. provided drug supply logistics and design. J.N., R.A.P., D.P., and A.C.M. provided statistical input. All authors contributed to the manuscript preparation. T.M.Q. and E.E.G. have verified the underlying data.
Data sharing: Ownership of the data arising from this study resides with the study team. Scientific publications and the sharing of clinical data generated as part of this trial are crucial to better understanding COVID-19 and developing new treatments. Data will be shared, with appropriate data sharing agreements, on request.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202203-0477OC on August 16, 2022
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.WHO. Coronavirus disease (COVID-19) pandemic; 2021. [accessed 15 June 2021]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019
- 2. Dorward DA, Russell CD, Um IH, Elshani M, Armstrong SD, Penrice-Randal R, et al. Tissue-specific immunopathology in fatal COVID-19. Am J Respir Crit Care Med . 2021;203:192–201. doi: 10.1164/rccm.202008-3265OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Laing AG, Lorenc A, Del Molino Del Barrio I, Das A, Fish M, Monin L, et al. A dynamic COVID-19 immune signature includes associations with poor prognosis. Nat Med . 2020;26:1623–1635. doi: 10.1038/s41591-020-1038-6. [DOI] [PubMed] [Google Scholar]
- 4. Zhang C, Wu Z, Li JW, Zhao H, Wang GQ. Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int J Antimicrob Agents . 2020;55:105954. doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Merad M, Martin JC. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol . 2020;20:355–362. doi: 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. John AE, Joseph C, Jenkins G, Tatler AL. COVID-19 and pulmonary fibrosis: a potential role for lung epithelial cells and fibroblasts. Immunol Rev . 2021;302:228–240. doi: 10.1111/imr.12977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Caniglia JLA, Asuthkar S, Tsung AJ, Guda MR, Velpula KK. Immunopathology of galectin-3: an increasingly promising target in COVID-19. F1000 Res . 2020;9:1078. doi: 10.12688/f1000research.25979.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Update to living WHO guideline on drugs for covid-19. BMJ . 2022;376:o80. doi: 10.1136/bmj.o80. [DOI] [PubMed] [Google Scholar]
- 9. Sterne JAC, Murthy S, Diaz JV, Slutsky AS, Villar J, Angus DC, et al. WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA . 2020;324:1330–1341. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shankar-Hari M, Vale CL, Godolphin PJ, Fisher D, Higgins JPT, Spiga F, et al. WHO rapid evidence appraisal for COVID-19 therapies (REACT) working group. JAMA . 2021;326:499–518. doi: 10.1001/jama.2021.11330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van de Veerdonk FL, Giamarellos-Bourboulis E, Pickkers P, Derde L, Leavis H, van Crevel R, et al. A guide to immunotherapy for COVID-19. Nat Med . 2022;28:39–50. doi: 10.1038/s41591-021-01643-9. [DOI] [PubMed] [Google Scholar]
- 12. Díaz-Alvarez L, Ortega E. The many roles of galectin-3, a multifaceted molecule, in innate immune responses against pathogens. Mediators Inflamm . 2017;2017:9247574. doi: 10.1155/2017/9247574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Slack RJ, Mills R, Mackinnon AC. The therapeutic potential of galectin-3 inhibition in fibrotic disease. Int J Biochem Cell Biol . 2021;130:105881. doi: 10.1016/j.biocel.2020.105881. [DOI] [PubMed] [Google Scholar]
- 14. Mackinnon AC, Gibbons MA, Farnworth SL, Leffler H, Nilsson UJ, Delaine T, et al. Regulation of transforming growth factor-β1-driven lung fibrosis by galectin-3. Am J Respir Crit Care Med . 2012;185:537–546. doi: 10.1164/rccm.201106-0965OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nishi Y, Sano H, Kawashima T, Okada T, Kuroda T, Kikkawa K, et al. Role of galectin-3 in human pulmonary fibrosis. Allergol Int . 2007;56:57–65. doi: 10.2332/allergolint.O-06-449. [DOI] [PubMed] [Google Scholar]
- 16. De Biasi S, Meschiari M, Gibellini L, Bellinazzi C, Borella R, Fidanza L, et al. Marked T cell activation, senescence, exhaustion and skewing towards TH17 in patients with COVID-19 pneumonia. Nat Commun . 2020;11:3434. doi: 10.1038/s41467-020-17292-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang J, Jiang M, Chen X, Montaner LJ. Cytokine storm and leukocyte changes in mild versus severe SARS-CoV-2 infection: Review of 3939 COVID-19 patients in China and emerging pathogenesis and therapy concepts. J Leukoc Biol . 2020;108:17–41. doi: 10.1002/JLB.3COVR0520-272R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Caniglia JL, Guda MR, Asuthkar S, Tsung AJ, Velpula KK. A potential role for galectin-3 inhibitors in the treatment of COVID-19. PeerJ . 2020;8:e9392. doi: 10.7717/peerj.9392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nita-Lazar M, Banerjee A, Feng C, Vasta GR. Galectins regulate the inflammatory response in airway epithelial cells exposed to microbial neuraminidase by modulating the expression of SOCS1 and RIG1. Mol Immunol . 2015;68:194–202. doi: 10.1016/j.molimm.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen YJ, Wang SF, Weng IC, Hong MH, Lo TH, Jan JT, et al. Galectin-3 enhances avian H5N1 influenza A virus-induced pulmonary inflammation by promoting NLRP3 inflammasome activation. Am J Pathol . 2018;188:1031–1042. doi: 10.1016/j.ajpath.2017.12.014. [DOI] [PubMed] [Google Scholar]
- 21. Kalfaoglu B, Almeida-Santos J, Tye CA, Satou Y, Ono M. T-cell hyperactivation and paralysis in severe COVID-19 infection revealed by single-cell analysis. Front Immunol . 2020;11:589380. doi: 10.3389/fimmu.2020.589380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Diaz JA, Ramacciotti E, Wakefield TW. Do galectins play a role in venous thrombosis? A review. Thromb Res . 2010;125:373–376. doi: 10.1016/j.thromres.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gutmann C, Takov K, Burnap SA, Singh B, Ali H, Theofilatos K, et al. SARS-CoV-2 RNAemia and proteomic trajectories inform prognostication in COVID-19 patients admitted to intensive care. Nat Commun . 2021;12:3406. doi: 10.1038/s41467-021-23494-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hirani N, MacKinnon AC, Nicol L, Ford P, Schambye H, Pedersen A, et al. Target inhibition of galectin-3 by inhaled TD139 in patients with idiopathic pulmonary fibrosis. Eur Respir J . 2021;57:2002559. doi: 10.1183/13993003.02559-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gaughan E, Quinn T, Hirani N, Mills A, Bruce AM, MacKinnon A, et al. DEFINE – a randomized, open-label trial of the inhaled galectin-3 inhibitor GB0139 in hospitalized patients with moderate-to-severe COVID-19. Am J Respir Crit Care Med . 2022;205:A3127. [Google Scholar]
- 26. Quinn TM, Gaughan EE, Bruce A, Antonelli J, O’Connor R, Li F, et al. Randomised controlled trial of intravenous nafamostat mesylate in COVID pneumonitis: Phase 1b/2a experimental study to investigate safety, Pharmacokinetics and Pharmacodynamics. EBioMedicine . 2022;76:103856. doi: 10.1016/j.ebiom.2022.103856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gaughan E, Quinn T, Bruce A, Antonelli J, Young V, Mair J, et al. Evaluation of new or repurposed treatments for COVID-19: protocol for the phase Ib/IIa DEFINE trial platform. BMJ Open . 2021;11:e054442. doi: 10.1136/bmjopen-2021-054442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kostakis I, Smith GB, Prytherch D, Meredith P, Price C, Chauhan A, Portsmouth Academic ConsortIum For Investigating COVID-19 (PACIFIC-19) The performance of the National Early Warning Score and National Early Warning Score 2 in hospitalised patients infected by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Resuscitation . 2021;159:150–157. doi: 10.1016/j.resuscitation.2020.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Portacci A, Diaferia F, Santomasi C, Dragonieri S, Boniello E, Di Serio F, et al. Galectin-3 as prognostic biomarker in patients with COVID-19 acute respiratory failure. Respir Med . 2021;187:106556. doi: 10.1016/j.rmed.2021.106556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Xu Z, Li X, Huang Y, Mao P, Wu S, Yang B, et al. The predictive value of plasma galectin-3 for ards severity and clinical outcome. Shock . 2017;47:331–336. doi: 10.1097/SHK.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 31. Mahat RK, Panda S, Rathore V, Swain S, Yadav L, Sah SP. The dynamics of inflammatory markers in coronavirus disease-2019 (COVID-19) patients: a systematic review and meta-analysis. Clin Epidemiol Glob Health . 2021;11:100727. doi: 10.1016/j.cegh.2021.100727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Coperchini F, Chiovato L, Ricci G, Croce L, Magri F, Rotondi M. The cytokine storm in COVID-19: further advances in our understanding the role of specific chemokines involved. Cytokine Growth Factor Rev . 2021;58:82–91. doi: 10.1016/j.cytogfr.2020.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Saris A, Reijnders TDY, Nossent EJ, Schuurman AR, Verhoeff J, van Asten S, et al. Distinct cellular immune profiles in the airways and blood of critically ill patients with COVID-19. Thorax . 2021;76:1010–1019. doi: 10.1136/thoraxjnl-2020-216256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zheng M, Gao Y, Wang G, Song G, Liu S, Sun D, et al. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol Immunol . 2020;17:533–535. doi: 10.1038/s41423-020-0402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Loo J, Spittle DA, Newnham M. COVID-19, immunothrombosis and venous thromboembolism: biological mechanisms. Thorax . 2021;76:412–420. doi: 10.1136/thoraxjnl-2020-216243. [DOI] [PubMed] [Google Scholar]
- 36. Moresco RN, Vargas LC, Voegeli CF, Santos RC. D-dimer and its relationship to fibrinogen/fibrin degradation products (FDPs) in disorders associated with activation of coagulation or fibrinolytic systems. J Clin Lab Anal . 2003;17:77–79. doi: 10.1002/jcla.10072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Price LC, McCabe C, Garfield B, Wort SJ. Thrombosis and COVID-19 pneumonia: the clot thickens! Eur Respir J . 2020;56:2001608. doi: 10.1183/13993003.01608-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Vidali S, Morosetti D, Cossu E, Luisi MLE, Pancani S, Semeraro V, et al. D-dimer as an indicator of prognosis in SARS-CoV-2 infection: a systematic review. ERJ Open Res . 2020;6:00260–02020. doi: 10.1183/23120541.00260-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet . 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E, et al. Global COVID-19 Thrombosis Collaborative Group, Endorsed by the ISTH, NATF, ESVM, and the IUA, Supported by the ESC Working Group on Pulmonary Circulation and Right Ventricular Function COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am Coll Cardiol . 2020;75:2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gu Y, Wang D, Chen C, Lu W, Liu H, Lv T, et al. PaO2/FiO2 and IL-6 are risk factors of mortality for intensive care COVID-19 patients. Sci Rep . 2021;11:7334. doi: 10.1038/s41598-021-86676-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Santus P, Radovanovic D, Saderi L, Marino P, Cogliati C, De Filippis G, et al. Severity of respiratory failure at admission and in-hospital mortality in patients with COVID-19: a prospective observational multicentre study. BMJ Open . 2020;10:e043651. doi: 10.1136/bmjopen-2020-043651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Siddiqui S, Brightling CE. Pathological disease in the lung periphery after acute COVID-19. Lancet Respir Med . 2021;9:1089–1090. doi: 10.1016/S2213-2600(21)00378-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ghosh AK, Vaughan DE. PAI-1 in tissue fibrosis. J Cell Physiol . 2012;227:493–507. doi: 10.1002/jcp.22783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zuo Y, Warnock M, Harbaugh A, Yalavarthi S, Gockman K, Zuo M, et al. Plasma tissue plasminogen activator and plasminogen activator inhibitor-1 in hospitalized COVID-19 patients. Sci Rep . 2021;11:1580. doi: 10.1038/s41598-020-80010-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhao T, Su Z, Li Y, Zhang X, You Q. Chitinase-3 like-protein-1 function and its role in diseases. Signal Transduct Target Ther . 2020;5:201. doi: 10.1038/s41392-020-00303-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fraser E, Denney L, Antanaviciute A, Blirando K, Vuppusetty C, Zheng Y, et al. Multi-modal characterization of monocytes in idiopathic pulmonary fibrosis reveals a primed type I interferon immune phenotype. Front Immunol . 2021;12:623430. doi: 10.3389/fimmu.2021.623430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol . 2006;7:311–317. doi: 10.1038/ni1309. [DOI] [PubMed] [Google Scholar]