Significance
Ambient fine particulate matter (PM2.5) is the world’s leading environmental health hazard. Understanding the health benefits that result from interventions that reduce source contributions to PM2.5is fundamental to public health decision-making. In Canada, had PM2.5contributions from major anthropogenic sources been reduced over 2007–2016, there would have been tangible public health benefits. The magnitudes of these benefits varied greatly by source, intervention strategy, and time from intervention, underscoring the critical importance of clearly specifying air quality interventions to understand the ensuing benefits. The protective effects of reducing PM2.5were larger for men, older adults, and people earning lower incomes, highlighting the potential equitable benefits of continuing improving air quality, even at the relatively low levels seen in Canada.
Keywords: fine particulate matter, emission source, survival, g-formula, Canada
Abstract
Emissions of fine particulate matter (PM2.5) from human activities have been linked to substantial disease burdens, but evidence regarding how reducing PM2.5 at its sources would improve public health is sparse. We followed a population-based cohort of 2.7 million adults across Canada from 2007 through 2016. For each participant, we estimated annual mean concentrations of PM2.5 and the fractional contributions to PM2.5 from the five leading anthropogenic sources at their residential address using satellite observations in combination with a global atmospheric chemistry transport model. For each source, we estimated the causal effects of six hypothetical interventions on 10-y nonaccidental mortality risk using the parametric g-formula, a structural causal model. We conducted stratified analyses by age, sex, and income. This cohort would have experienced tangible health gains had contributions to PM2.5 from any of the five sources been reduced. Compared with no intervention, a 10% annual reduction in PM2.5 contributions from transportation and power generation, Canada’s largest and fifth-largest anthropogenic sources, would have prevented approximately 175 (95%CI: 123–226) and 90 (95%CI: 63–117) deaths per million by 2016, respectively. A more intensive 50% reduction per year in PM2.5 contributions from the two sources would have averted 360 and 185 deaths per million, respectively, by 2016. The potential health benefits were greater among men, older adults, and low-income earners. In Canada, where PM2.5 levels are among the lowest worldwide, reducing PM2.5 contributions from anthropogenic sources by as little as 10% annually would yield meaningful health gains.
Ambient fine particulate matter (PM2.5) is the world’s leading environmental health hazard, responsible for an estimated 4.1 million premature deaths globally in 2019 (1). Constituting a spectrum of organic compounds, metals, carbon, sulfates, and nitrates, PM2.5 can easily enter the bloodstream via alveoli and have widespread systemic effects, resulting in various morbidity and mortality outcomes (2, 3). Despite substantial efforts to lower emissions (4, 5), PM2.5 levels still exceed the current World Health Organization air quality guideline of 5 µg/m3 per annum in most of the world (6).
Formed by direct emissions and atmospheric chemical reactions involving precursors, PM2.5 originates from multiple anthropogenic (e.g., industry, transportation) and natural (e.g., wildfires, dust) sources (7–9). Using zero-out approaches with previously published concentration-response functions (CRF) and region-level population data, increasing numbers of studies have conducted health impact assessments to quantify the health burden of specific emissions sources at the national or global scales (7, 8, 10–16). For example, McDuffie et al. have estimated that fossil fuel combustion is responsible for one million annual deaths globally (8). Transportation is the largest contributor to PM2.5-related deaths in North America (8, 11, 13), whereas it is agriculture sector in western Europe (7, 8, 13). In many middle- and low-income countries (e.g., India, China), commercial and residential energy use is the dominant contributor to PM2.5-related deaths (7, 13).
While previous studies have estimated the mortality burden from major sources of PM2.5, there remain important knowledge gaps in our understanding of how interventions on emission sources may improve public health. First, idealistic zero-out scenarios assume instantaneous removal of pollution sources. Such drastic changes are unlikely to be realized in the real world for a variety of technical, political, and societal reasons; incremental reductions in source contributions over multiple years (or decades) are more realistic. Second, most previous studies have attributed health benefits to changes in source contributions to PM2.5 within the same year, but outcomes (e.g., deaths) may be lagged. Understanding how interventions influence health in both the short and long terms is critical. Finally, existing studies indirectly estimated the hypothetical benefits because they relied on published CRFs derived from different populations, often under the assumption that CRFs are time-invariant, despite the dynamic nature of populations and ambient particles.
To close the gap between what is expected and what may be realistically achievable, we conducted a population-based cohort study to evaluate the potential health benefits of lowering PM2.5 source contributions in Canada using the 2006 wave of the Canadian Census Health and Environment Cohort (CanCHEC). This cohort collected person-level information on the health and socioeconomic characteristics of approximately three million adults across Canada, along with high-resolution data on source contributions to PM2.5 and related mass concentrations from satellite-based observations and chemical transport modeling (17). Through applying the g-formula, a causal technique uniquely suited to evaluate interventions using observational data with time-varying air pollution exposures, confounders, and health outcomes, we aimed to provide nationally representative estimates of the health benefits resulting from PM2.5 reductions across multiple mitigation strategies, emission sources, and time periods over the course of a decade. This improves upon a small number of past studies that examined the health impacts of emission controls focusing on a single action (or source), a confined study region, or a short time period (18–22). Another strength of this research is that under less restrictive conditions than typical regression-based methods, the g-formula approach allows us to evaluate sustained and dynamic interventions and estimate marginal causal effects on both relative and additive scales, thus enhancing policy relevance (23, 24).
Methods
Study Design and Population.
We conducted a population-based cohort study involving CanCHEC (17), which comprises respondents to the Canadian long-form censuses that are conducted every 5 y. Sampling 20% households, long-form censuses collect personal information on socioeconomic and ethnocultural characteristics. To construct CanCHEC, Statistics Canada used standard deterministic and probabilistic record linkage techniques to link long-form census responses to nationwide health administrative data and family tax files, thereby obtaining detailed health status, annual income, and residential history for all respondents (17). CanCHEC has been widely used to evaluate the health impacts of PM2.5 (25–31).
To better represent contemporary exposures to PM2.5, we included participants in the 2006 wave of CanCHEC. We restricted the cohort to adults aged 30–79 y who had lived in Canada for >5 y. We excluded individuals with missing information on exposure and covariates and those who died before January 1, 2007 (baseline). The outcome of interest was death from natural causes (International Classification of Diseases, Ninth Revision ICD-9 code: <800 and Tenth Revision ICD-10 code: A00-R99). We obtained the cause and date of death from the Canadian Vital Statistics Death Database.
The mortality follow-up extended through December 31, 2016 (10 y after cohort inception). The Health Canada-Public Health Agency of Canada Research Ethics Board approved the study.
Annual Mean Concentrations and Source Contributions of PM2.5.
We obtained annual mean estimates of PM2.5 concentrations for all participants based on postal code addresses between 2001 (6 y before baseline) and 2016 (end of follow-up) using version V4.NA.02.MAPLE of PM2.5 surfaces provided by the Washington University Atmospheric Compositional Analysis Group (32). This dataset combined satellite retrievals of aerosol optical depth with outputs from a global atmospheric chemistry transport model (GEOS-Chem CTM) and ground-based PM2.5 observations (32). Ground-based observations were incorporated using a geographically weighted regression that included information on land cover, elevation, and aerosol composition. These estimates were validated against PM2.5 concentrations measured at fixed-site monitors across North America (n = 2,312) and showed excellent long-term mean cross-validated performance between 2000 and 2016 at 1 × 1-km resolution (R2 = 0.73) (32).
We also obtained fractional contributions to PM2.5 from emission sources in Canada by conducting a series of baseline and sensitivity simulations using the GEOS-Chem CTM. The technical details, including information on model validation, have been previously published (9). Briefly, we conducted baseline global simulations to achieve boundary conditions, followed by regional simulations across North America using the nested-grid capability of the GEOS-Chem CTM. All simulations were driven by assimilated meteorological data. Regional emission inventories were scaled to the simulation year in 2013 (roughly the midpoint of the study period) using annual scale factors from Canada’s Air Pollutant Emission Inventory (9). The baseline simulations were then downscaled to 1 × 1-km resolution based on annual mean satellite-derived PM2.5 to better represent the spatial variation of population density. Next, we conducted simulations by individually excluding each emission source from the baseline simulations, yielding the relative contributions of individual sources of PM2.5 across Canada. This method has been extensively used to evaluate the contributions of different sources to PM2.5 exposures or health impacts (7, 8, 10, 13). We a priori considered five leading anthropogenic sources in Canada: agriculture; industry; power generation; residential combustion; and transportation (9).
Covariates.
We obtained the following individual-level data from the long-form census questionnaire: age; sex; race/ethnicity (i.e., visible minority status and Indigenous identity); nativity; marital status; educational attainment; occupational class; and employment status. From family income tax files, we also derived annual household income adequacy (in deciles) which accounted for household income, family size, region, and year.
Using 2006 and 2011 Canadian Census data, we derived four neighborhood-level deprivation measures based on the Canadian Marginalization Index: (33) residential instability; material deprivation; dependency; and ethnic concentration (26, 28). Additionally, we created an urban form variable to characterize active commuting and transit-use using census tract data (34). Furthermore, to account for regional differences in mortality that might be caused by factors other than pollution, we created a variable representing the population size of participants’ home communities and another variable representing airsheds. All area-level variables were assigned to annual residential postal codes and the nearest census year.
Hypothetical Interventions on Source Contributions.
For each emission source, we considered six hypothetical interventions: a zero-out strategy; three incremental strategies; and two phased strategies. The zero-out strategy would instantaneously eliminate source contributions to PM2.5 in 2007. The incremental strategies would annually reduce PM2.5 contributions by 10%, 25%, or 50% of the observed fractional contributions over the 10 y (SI Appendix, Table S1). The phased strategies would reduce PM2.5 contribution by 25% per period over four periods (referred to as 25% phased) or by 50% per period over two periods (50% phased). Given residential mobility and space–time variations in PM2.5, each participant would experience varied reductions in PM2.5 exposure between 2007 and 2016. We considered the observed PM2.5 exposure values as the reference scenario (i.e., natural course).
Statistical Analysis.
To estimate the causal effects of the above-mentioned source reduction interventions on 10-y mortality risk, we implemented the parametric g-formula. Under standard identifiability assumptions (exchangeability, positivity, and consistency) (see SI Appendix for detail), the parametric g-formula simulates the counterfactual outcome and covariate history that would have been observed if everybody in the cohort had followed a specified intervention (23, 24). This approach has been used to evaluate public various health interventions, including lifestyle interventions on the risk of coronary heart disease (23), weight gain (35), and mortality (36), and recently the potential benefit of improving ambient air quality on reducing morbidity and mortality (37, 38).
The g-formula identifies the counterfactual outcome probability conditional on time-varying exposure and covariates and weighted by the distribution of covariate histories compatible with the conditional distribution of covariates under a specified intervention (23, 24). Following previous studies (23, 24), we first estimated the joint distributions of PM2.5 and all covariates as well as the probability of nonaccidental mortality at each time interval given covariate histories by fitting separate parametric models (See SI Appendix for detail). Then, we conducted Monte Carlo simulation, based on these parametric models, to estimate the predicted probability of nonaccidental mortality using random draws with replacement (n = 10,000) from the original study population under each of the above-mentioned intervention scenarios. To estimate time-to-mortality probability, we fitted a pooled logistic regression model, whereas to estimate the distributions of PM2.5 and time-varying covariates, we used linear regression models. Accidental deaths were treated as censoring events because they accounted for a small fraction of total deaths (~6%).
In all models, we included baseline age (using a restricted cubic spline with five knots), sex, race/ethnicity, nativity, marital status, education, occupation, employment, and a quadratic function of years since baseline (SI Appendix, Table S2). In the mortality model, we additionally included the 3-y history of annual PM2.5 and all other time-varying covariates (income, urban form, community size, airshed, and neighborhood-level material deprivation, dependency, instability, and ethnic concentration). Thus, an individual’s moving window of exposure for 2010, for example, would be estimated as the mean of exposures over 2007–2009 according to the postal code residence of the individual. In addition, we introduced an interaction between PM2.5 and categorical years in follow-up to allow the PM2.5-mortality association to vary temporally. Using the Shape Constrained Health Impact Function approach (39), we examined the shape of the PM2.5-mortality association with pooled logistic regression and found that the log–log shape was optimal (SI Appendix, Table S3). Thus, we specified this shape in the mortality model. In each covariate model, we similarly included the 3-y histories of PM2.5 and all other time-varying covariates, as well as concurrent covariates that preceded the time-varying covariate of interest based on a directed acyclic graph (SI Appendix, Fig. S1).
Due to its large size, we split our study population into 10 random subcohorts and performed all the analyses for each subcohort. For each emission source, we computed the absolute difference in the average mortality risk under each hypothetical intervention relative to the natural course for all years between 2007 and 2016, expressed as a risk difference in mortality (per million population). As a complementary effect measure, we calculated the ratio of mortality risks, expressed as the percentage change in mortality ((1−risk ratio)* 100%). The 95% CI were constructed using 200 nonparametric bootstrap resamples. The subcohort-specific estimates were pooled using fixed-effects meta-analysis to obtain summary estimates for the entire cohort. To contextualize our findings, we calculated the number of premature deaths in the study population that would have been averted over the 10-y period. Using the restricted mean survival time between each intervention and the natural course, we also derived the number of years of life that would have been gained over the 10-y period. Furthermore, we computed the economic benefits from these interventions using willingness-to-pay metrics that account for both direct and indirect costs (40). This was achieved using the value of statistical life of $6.5 million CAD and the currency year in 2016 (the end of follow-up) (41). The analyses were repeated for each combination of intervention and emission source.
Secondary Analysis.
To assess the robustness of the study findings, we conducted sensitivity analyses that evaluated the influence of the causal ordering of time-varying covariates by considering four alternative orderings (and thus specifications) of the time-varying covariates, considered 6-y histories of PM2.5 and time-varying covariates, and specified a log-linear association between PM2.5 and mortality. To facilitate comparison with the literature of the PM2.5-mortality relationship, we also used Cox proportional hazards regression to examine this relationship according to three alternative exposure time windows (3 y prior, 6 y prior, and same year exposure), all under the assumption of a log-linear CRF (see SI Appendix, Table S4 about main differences between Cox regression and the g-formula) (3, 42). Furthermore, we performed stratified analyses by age (<50 vs. ≥50 y), sex, and income (<median income vs. ≥median income of the entire cohort) at baseline. The presence of interactions was assessed on the difference scale because it is the most relevant for evaluating the public health significance of interventions (43).
All analyses were conducted using SAS Enterprise Guide (version 7.11) and R software (version 4.0.5) with the gfoRmula package (R code to carry out the g-formula is provided in SI Appendix) (44).
Results
Among 3,343,370 potential study participants, the following exclusions were made: 432,580 (12.9%) because they did not meet the age criteria; 83,630 (2.5%) because they had lived in Canada for ≤5 y; 31,835 (1%) due to missing PM2.5 exposure; 126,805 (3.8%) due to missing covariates; and 4,880 (0.1%) due to prebaseline (January 1, 2007) death. The analytical cohort comprised 2,663,645 participants (SI Appendix, Fig. S2) and 25.7 million person-years of observations. The mean age at baseline was 50.9 y, 48.6% were men, 10.5% were visible minorities, 20.4% had less than high school education, and 65.5% were employed (SI Appendix, Table S5). The mean follow-up time was 6.4 y. The annual mortality rate was 0.8%.
The annual mean of PM2.5 exposure in the cohort varied between 5.5 and 7.2 µg/m3, with standard deviations ranging from 1.9 to 2.4 µg/m3 (or interquartile ranges varying from to 2.7 to 3.7 µg/m3), depending on year (SI Appendix, Fig. S3). Specifically, at baseline mean annual concentration of PM2.5 at participants’ residences was 7.1 μg/m3 (range, 0.5–15.7), of which 1.1 μg/m3 (14.3%) originated from transportation, 1.0 μg/m3 (13.4%) from residential combustion, 1.0 μg/m3 (13.4%) from industry, 0.7 μg/m3 (9.5%) from agriculture, and 0.6 μg/m3 (7.3%) from power generation. In 2015, the observed fractional contributions to PM2.5 by these emission sources ranged between 0.5 μg/m3 and 1.0 μg/m3. Across all sources examined, reducing PM2.5 contributions from transportation and power generation would have resulted in the largest and smallest declines in annual exposure to PM2.5, respectively (SI Appendix, Fig. S4).
Estimated Health and Economic Impacts of Interventions.
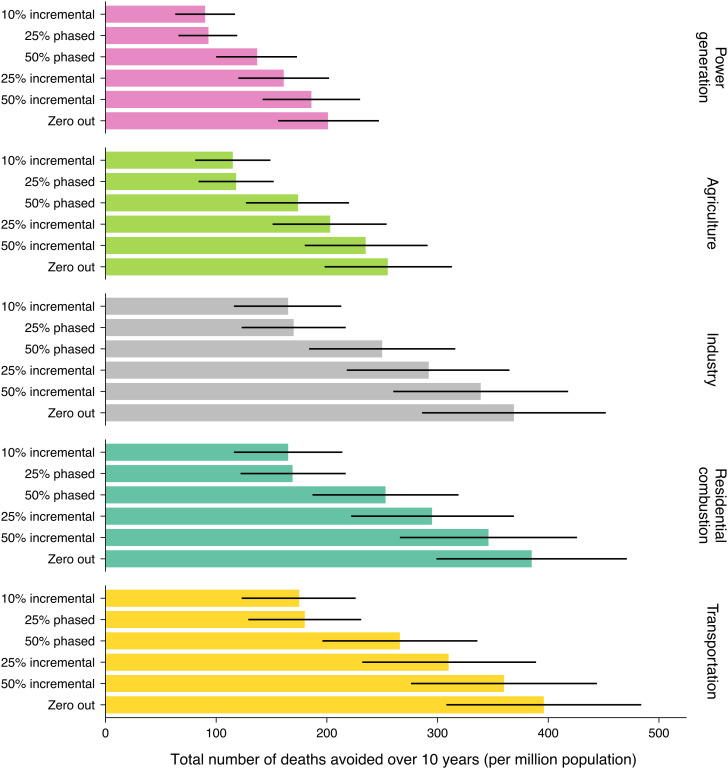
Among the 30 possible intervention scenarios, even modest interventions would have resulted in tangible reductions in mortality over 2007–2016: 90 fewer deaths (95%CI: 63–117) per million would have occurred by 2016 if PM2.5 from power generation was reduced annually by 10% compared with the natural course (Fig. 1). This corresponds to a −0.1% change in mortality risk (SI Appendix, Tables S6 and S7). In comparison, had the same 10% incremental strategy been applied to PM2.5 contributions from agriculture, industry, residential combustion, and transportation, there would have been 115, 165, 165, and 175 fewer deaths per million or −0.2%, −0.2%, −0.2% and −0.3% change in mortality, respectively, compared with the natural course. If PM2.5 contributions from all five sources had been reduced annually by 10%, 710 deaths per million would have been averted over 2007–2016.
Fig. 1.
Cumulative number of premature deaths that would have been avoided (per million population) if source contributions to PM2.5 exposure had been reduced in Canada over the period 2007–2016, by selected emission sources and intervention strategies relative to the natural course of observed PM2.5 exposures ("no intervention" scenario). Error bars represent 95% CIs from bootstrap replications.
Compared with the 10% incremental reduction, had PM2.5 contributions from the five sources been instantaneously zeroed out at baseline (in 2007), twice as many PM2.5-related deaths would have been averted by 2016 (1,607 fewer deaths per million) (Fig. 1). However, such drastic interventions are unlikely to occur. Of the more realistic incremental strategies, a 25% incremental reduction was 80% more effective in averting PM2.5-related mortality than the 10% incremental reduction for the same sources (e.g., avoiding 311 vs. 175 deaths per million if intervening on transportation PM2.5 contributions). This strategy would eliminate source contributions in year four as opposed to in year 10 under the 10% incremental reduction. Deploying other intensive strategies to reduce transportation PM2.5 contributions would prevent between 266 (under 50% phased reduction) and 360 (under 50% incremental reduction) deaths per million by 2016. Conversely, had a 25% phased strategy been used for transportation, it would have yielded a similar benefit as the 10% incremental strategy.
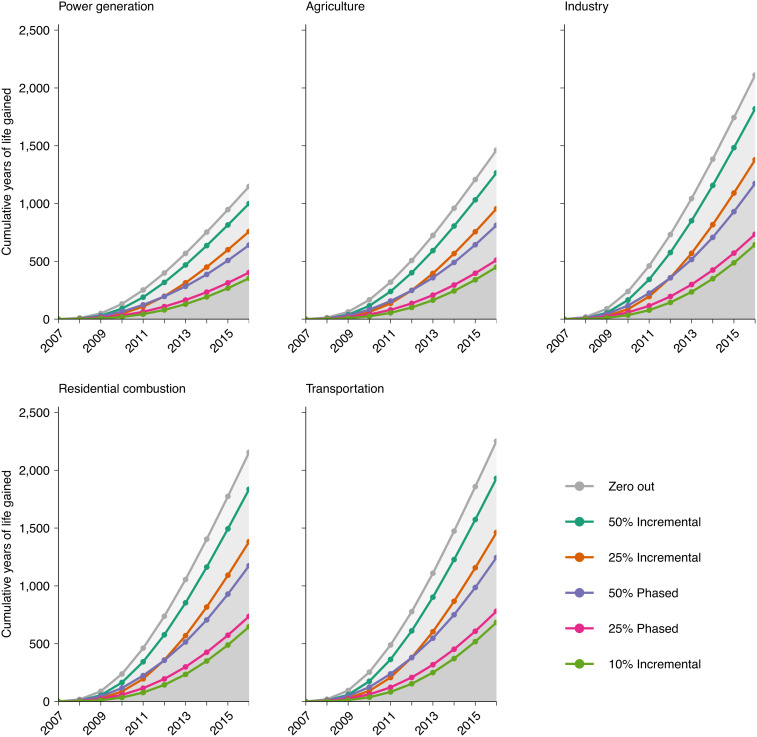
Large variations were also observed in terms of years of life saved (Fig. 2). Had PM2.5 from power generation been incrementally reduced by 10%, 25%, or 50% annually over 10 y, this cohort would have gained approximately 354, 760, and 1,001 life-years per million by 2016, respectively. In comparison, had transportation PM2.5 contributions been reduced by the three above-mentioned strategies, 685, 1,465, and 1,934 life-years per million would have been saved by 2016. Combined, reducing all five sources under these three strategies would have saved 2,783, 5,947, and 7,865 life-years per million, respectively. Fig. 2 also illustrates that the vast majority of life-years saved would have been experienced in the longer term. For example, had transportation PM2.5 contributions been reduced annually by 10%, per million population, 11 life-years would have been saved between 2007 and 2009 vs. 435 life-years saved between 2014 and 2016 (2% and 63% of all life-years saved by 2016 under this scenario). Likewise, had transportation PM2.5 contributions been zeroed out at baseline, per million population, 95 life-years would have been saved in the first three years vs. 1,144 life-years saved during the final 3 y (4% and 51% of all life-years saved by 2016 under this scenario).
Fig. 2.
Additional years of life that would have been gained (per million population) if source contributions to PM2.5 exposure had been reduced in Canada over the period 2007–2016, by selected emission sources and intervention strategies relative to the natural course of observed PM2.5 exposures ("no intervention" scenario)
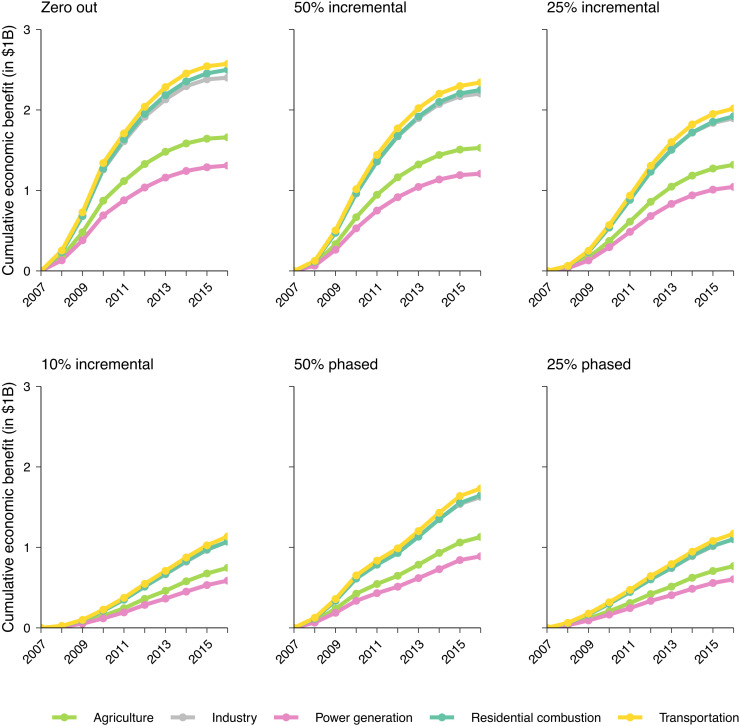
If the zero-out strategy had been deployed at baseline, per million population, the related health impacts by 2016 would have the economic valuation of $1.3B (95% CI: 1.0–1.6) from power generation, $1.7B (95% CI: 1.3–2.0) from agriculture, $2.4B (95% CI: 1.9–2.9) from industry, $2.5B (95% CI: 1.9–3.3) from residential combustion, and $2.6B (95% CI: 2.0–3.0) from transportation, with a combined economic valuation of $10.4B (95% CI: 8.1–12.7) (Fig. 3). If these five source contributions had been more realistically reduced under an incremental strategy, for example, 25% incremental reduction, the economic valuation of the related health impacts would have been $1.0B, $1.3B, $1.9B, $1.9B, and $2.0B per million, respectively, with a combined economic valuation of $8.2B/million. Under a more modest 10% reduction per year, the corresponding health impacts would have an economic valuation of $4.6B/million for all five sources combined. Like the results with life-years saved, the vast majority of economic benefits from these interventions occurred over the long term, indicating that rather than arising instantaneously, it would take multiple years to accrue public health gains after intervention initiation.
Fig. 3.
Economic benefits that would have been accrued (per million population) if source contributions to PM2.5 exposure had been reduced in Canada over the period 2007–2016, by selected emission sources and intervention strategies relative to the natural course of observed PM2.5 exposures ("no intervention" scenario)
Secondary Analyses.
Our findings did not materially change when we varied the sequence of models for time-varying covariates and we considered earlier histories of PM2.5 and other time-varying covariates over the 6 y prior (SI Appendix, Figs. S5 and S6). Similar estimates (albeit somewhat attenuated) were observed when specifying a log-linear shape for the PM2.5-mortality association. All models performed very well in estimating the mortality risk and the distributions of risk factors under the natural course (SI Appendix, Fig. S7). Our additional sensitivity analyses based on Cox models further yielded hazard ratios of 1.05 (95% CI: 1.04–1.06) for average exposure over 3 y prior, 1.07 (95% CI: 1.06–1.08) over 6 y prior, and 1.01 (95% CI: 1.00–1.02) in the same year of PM2.5 per interquartile range change in PM2.5 exposure (3.26 μg/m3).
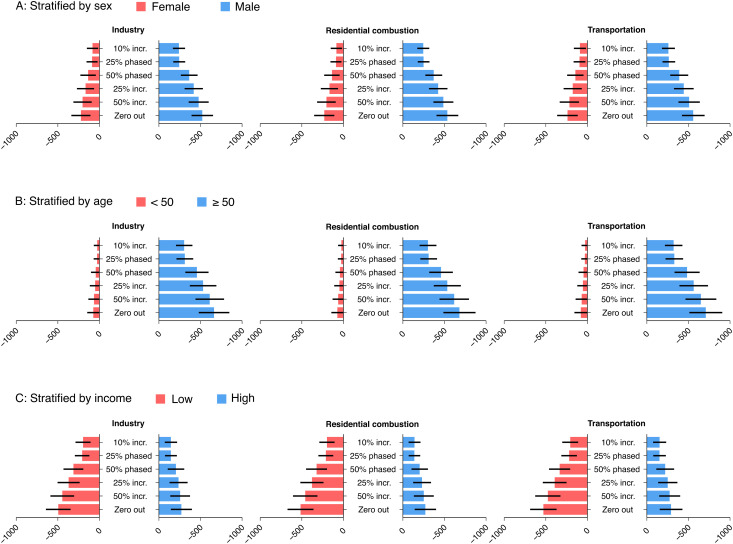
In stratified analyses, we found that the effects of PM2.5 source reductions on mortality were higher for men than women, for older adults than younger adults, and to a lesser degree, for low-income earners than high-income earners (Fig. 4 and SI Appendix, Fig. S8). For example, had industry PM2.5 contributions been reduced yearly by 25%, 419 fewer deaths (95% CI: 309–529) would have been observed per million men vs. 167 fewer deaths (95% CI: 64–270) per million women by 2016 (P-interaction: <0.01). The protective effect of this intervention strategy against mortality was 10 times larger in older adults than younger adults (P-interaction: <0.01). Additionally, this intervention strategy would lead to 370 fewer deaths (95% CI: 238–503) per million low-income earners vs. 236 fewer deaths (95% CI: 128–344) per million high-income earners (P-interaction: 0.12). All subgroups experienced similar reductions in PM2.5 each year (e.g., in 2010, reductions ranging from 0.95 to 0.96 μg/m3, depending on the subgroup). The observed findings were consistent across all 30 intervention scenarios.
Fig. 4.
Absolute change in mortality risk and 95% CI per million persons for the associations of reductions in source contributions to PM2.5 with premature mortality in the 2006 CanCHEC cohort over the period 2007–2016, by the three largest anthropogenic emission sources, intervention strategies, and selected personal characteristics at baseline (A: by sex, B: by age, C: by income).
Discussion
In this causal analysis of 2.7 million adults in Canada, we estimated that had PM2.5 contributions from any of five major anthropogenic sources been eliminated between 2007 and 2016, this cohort would have experienced tangible health gains. Even a modest 10% annual reduction in PM2.5 from power generation–Canada’s fifth-largest anthropogenic source of PM2.5–would have prevented approximately 90 deaths per million in this cohort by 2016 compared with no intervention. The avoidance of premature deaths during this period was consistently noted in all scenarios, but their impacts varied greatly, with the averted deaths being up to four times higher under certain incremental strategies and sources. Our results were robust to sensitivity analyses and proved stronger in men, older adults, and low-income individuals. Lastly, most public health gains from reducing source contributions to PM2.5 unfolded gradually, suggesting that PM2.5 source reductions provide health benefits inherently at the decadal (or centennial) timescale.
Increasing numbers of studies have quantified mortality burden attributable to human-caused emissions of PM2.5 (7, 8, 11, 13, 15, 16). This was routinely achieved indirectly by combining published CRF with region-level population and PM2.5 data. Using this approach, Silva et al. showed that in 2005, PM2.5 exposures emanating from residential and commercial combustion had the greatest impact globally – 675,000 deaths/year – and in North America, transportation was the largest contributor, responsible for 32% of total anthropogenic PM2.5 mortality (13). McDuffie et al. also estimated that in 2017, residential combustion remained the world’s dominant contributor to PM2.5, accounting for 740,000 deaths/year, whereas in North America, the largest three sources of PM2.5 (transportation, industry, and residential combustion) collectively accounted for half of the total anthropogenic PM2.5 mortality burden (8). The differential impacts of individual emission sources on PM2.5 disease burden were also reported in several other health impact assessment studies, all of which demonstrated the enormous health impacts of fossil-fuel combustion (e.g., emitted from transportation) (7, 11). Although not directly comparable with our study, our results support these previous findings. Our study further demonstrates that the impact of PM2.5 source reductions is highly intervention-dependent. Compared with the zero-out strategy, for example, deploying the 25% phased strategy to the same emission sources prevented ~25% by year three and 50% by year 10 of PM2.5-related deaths that would be averted under the idealized instantaneous elimination. If a more intensive strategy (e.g., 50% phased strategy) were to be used, the gap in effectiveness that results from the idealized zero-out strategy would be reduced to 35% over the 10-y period. Given large variations in the effectiveness across the different intervention strategies and that the idealistic zero-out strategy is likely infeasible in practice, specifying more realistic interventions allows for meaningful interpretations of the health effects of reducing source contributions to PM2.5.
Under the assumption of instantly eliminating source contributions to PM2.5, previous studies have often predicted sizable near-term reductions in the mortality burden of air pollution. However, our study shows that the vast majority of public health gains from eliminating PM2.5 source contributions occurred in the long term. This is unsurprising since removal of PM2.5 would not alter health outcomes arising from past exposures, but rather would only influence future years. This aligns with a large and growing body of literature linking the dominant health impacts of air pollution to long-term cumulative exposure spanning over multiple years (26, 45–47). This was also supported by our observation of the lack of strong evidence associating same-year exposure to PM2.5 with mortality in the sensitivity analyses. Consistent with previous studies of air health effects (25, 26, 48), we a priori considered mean PM2.5 exposure over 3 y preceding the follow-up year to capture the cumulative and delayed effects. While the average exposures over multiple years have been regularly used in air health research, the temporal relationships between past PM2.5 exposure and health outcomes remain incompletely understood. Emerging evidence suggests that longer exposure windows might be more important (27, 47, 49, 50). In this study, we considered cumulative PM2.5 exposure over 6 y prior and found that the estimated health benefits from removing PM2.5 source contributions remain similar.
Previous epidemiological studies of air pollution suggested stronger associations of long-term PM2.5 exposure with mortality in younger adults than older adults (42, 46, 51, 52), however, we found the health gains due to PM2.5 source reductions to be 10 times larger among older adults (≥50 y) compared with younger adults (<50 y). This inconsistency might be attributable to differences in the inferential goals, study methodologies, and underlying population characteristics, but the different scales on which the interactions were assessed are probably important. There is a growing recognition that additive interaction is more appropriate than multiplicative interaction for identifying groups that will benefit most from intervention–something of particular interest to this study (43). We also found larger health benefits from reducing PM2.5 sources in men, a pattern that has emerged in the recent literature (28, 46, 52–54) Furthermore, several cohort studies have tied greater mortality risk from PM2.5 to low socioeconomic status (SES) (52, 53, 55). For example, in a large U.S. cohort study comprising 61 million Medicare recipients, living with lower SES consistently heightened the association of premature death with long-term exposure to PM2.5 (53). In this study, we observed a tendency for individuals earning low incomes to experience larger health benefits than individuals earning high incomes. The fact that both income groups underwent similar reductions in PM2.5 exposure reinforces the notion that socially disadvantaged persons are more vulnerable to the effects of air pollution, in part due to existing susceptibility to poor health. Our observations provide key insights that improving air quality would reduce health disparities, even at the relatively low levels of PM2.5 seen in Canada (56). It is noteworthy that in 2018, annual mean concentration of PM2.5 was 11.4 µg/m3 in Los Angeles, the United States, 14.1 µg/m3 in Rome, Italy, and 50.6 µg/m3 in Beijing, China, whereas in Toronto, the largest city in Canada, it was 7.6 µg/m3 (56).
Several limitations merit mention. First, we assumed equal toxicity by emission sources in this study. While there is emerging evidence that PM2.5 emitted from fossil-fuel combustion might be more toxic than from other sources (e.g., soil or biomass) (57, 58), the current evidence about differential toxicity by PM2.5 sources remains inconclusive (59, 60). Given the uncertainty about the relative toxicity of PM2.5 sources, we cannot completely eliminate the imprecision in our specifications of intervention strategies which might lead to over- or under-estimation of the benefits of intervening on some PM2.5 sources (e.g., transportation). Second, we did not have information on individual behavioral factors such as smoking and physical activity. To control for potential confounding by these lifestyle variables, we included various personal and socioeconomic characteristics and neighborhood deprivation. Since socioeconomic characteristics are strongly associated with lifestyle variables (61), adjusting for these variables should reduce the influence of these unmeasured variables on our effect estimates. Additionally, in a sensitivity analysis using the Cox model, our estimated PM2.5-mortality association was found consistent with those reported elsewhere (50, 53, 54, 62–65). For example, in a recent large multiple-country cohort study, Strak et al. (54) reported that each μg/m3 increase in PM2.5 exposure was associated with a hazard ratio of 1.02 (95% CI: 1.02–1.03) with nonaccidental mortality (54). Similarly, in a large cohort study of 61 million adults in the continental U.S., Di et al. estimated that every μg/m3 increase in PM2.5 exposure was associated with a hazard ratio of 1.01 (95% CI: 1.01–1.01) with nonaccidental mortality (53). Despite our efforts, we acknowledge that, due to the nature of observational data, the possibility of residual confounding cannot be eliminated. As well, we cannot completely rule out the possibility of model misspecification. However, because the modeled natural course values were similar to the observed values and because the results were similar across many covariate model specifications, gross model misspecification under the natural course is highly unlikely. Third, our exposure assessments were based on modeled annual mean concentrations of PM2.5 and source contributions that were assigned to annual postal code addresses, which do not completely reflect personal exposure. As well, quantitative estimation of uncertainty in PM2.5 sources remains elusive given the roles of multiple sources and processes affecting the relation of specific sources with specific monitors. Furthermore, the data for PM2.5 source contributions were available at the midpoint of our study period only. Given the inherent imprecision of PM2.5 exposure, our exposure assessment was probably subject to nondifferential misclassification. Lastly, our estimated marginal causal effects are closely tied to our study population and correspond directly to our inferential goal. As such, they may not be generalizable to other populations with a different mix of effect modifiers such as age and SES.
This is a national study to quantify potential public health impacts of reducing contributions to PM2.5 across multiple emission sources, intervention strategies, and time periods. Strengths of this study include its large size and population-based representation of adults across Canada. Another novel aspect of our study is the application of the g-formula, a powerful technique for causal inference that is uniquely suited to evaluate sustained and dynamic interventions. In tandem with the well-characterized CanCHEC cohort, it overcomes the limitations of past health impact assessments that have relied on region-level population data and published CRF estimates from different populations. This approach also allowed us to make causal inferences about more realistic interventions than the idealistic zero-out scenario that further strengthen future efforts to reduce source-specific contributions to PM2.5. In addition, we obtained extensive individual-level information on annual income, education, race or ethnicity, and many other characteristics, which allowed for good control for known risk factors. Furthermore, the use of high-resolution data measuring PM2.5 concentrations along with personal residential histories offered a unique opportunity to construct detailed exposures for the cohort across Canada over a decade. Large-scale air quality interventions have continued to play a major role in protecting the public worldwide. To complement them, interest has grown in community-level interventions (66). Future research is warranted to further evaluate the health impacts of potential interventions to reduce exposure to PM2.5 (and other pollutants) among population subgroups or regions (e.g., urban/rural).
Conclusion
This nationwide causal analysis showed that reducing PM2.5 at anthropogenic emission sources would yield meaningful public health benefits in Canada. Given that PM2.5 levels in Canada are among the lowest worldwide, these findings can have important public health implications globally.
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
© His Majesty the King in Right of Canada, as represented by the Minister of Health, 2022. Funding: Funding for this study was provided by Health Canada (#810630) under the Addressing Air Pollution Horizontal Initiative (AAPHI) of the Government of Canada.
Author contributions
H.C. conceived the study; H.C. and R.T.B. designed research; H.C., M.Q., J.S.K., C.C., J.C.K., A.v.D., J.M., R.V.M., J.K., E.L., L.B., Y.L., M.T., T.B., and R.T.B. performed research; H.C. and M.Q. analyzed data; H.C., M.Q., J.K. and T.B. contributed to statistical methodology; H.C., M.Q., J.S.K., C.C., J.C.K., A.v.D., J.M., R.V.M., J.K., E.L., L.B., Y.L., M.T., T.B., and R.T.B. contributed to the interpretation of data, provided critical revisions to the manuscript, and approved the final draft; A.v.D., J.M., and R.V.M. contributed to exposure assessment; and H.C. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission.
Data, Materials, and Software Availability
Statistics Canada’s policy on data privacy and confidentiality prohibits the analytical cohort used to be freely available in the manuscript or in a public repository. However, access can be granted through Statistics Canada’s Research Data Centre program. Environmental exposures are available upon request to the original authors of the data. The analytical code used was all standard R and SAS code (e.g., gfoRmula, data steps).
Supporting Information
References
- 1.GBD 2019 Risk Factors Collaborators, Global burden of 87 risk factors in 204 countries and territories, 1990-2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 396, 1223–1249 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brook R. D., et al. , Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation 121, 2331–2378 (2010). [DOI] [PubMed] [Google Scholar]
- 3.Hoek G., et al. , Long-term air pollution exposure and cardio- respiratory mortality: A review. Environ. Health 12, 43 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greenbaum D. S., The clean air act: Substantial success and the challenges ahead. Ann. Am. Thorac. Soc. 15, 296–297 (2018). [DOI] [PubMed] [Google Scholar]
- 5.Zhang Q., et al. , Drivers of improved PM(2.5) air quality in China from 2013 to 2017. Proc. Natl. Acad. Sci. U.S.A. 116, 24463–24469 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization, WHO Global Air Quality Guidelines: Particulate Matter (PM2.5 and PM10), Ozone, Nitrogen Dioxide, Sulfur Dioxide and Carbon Monoxide (World Health Organization, Geneva, Switzerland, 2021). [PubMed] [Google Scholar]
- 7.Lelieveld J., Evans J. S., Fnais M., Giannadaki D., Pozzer A., The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature 525, 367–371 (2015). [DOI] [PubMed] [Google Scholar]
- 8.McDuffie E. E., et al. , Source sector and fuel contributions to ambient PM(2.5) and attributable mortality across multiple spatial scales. Nat. Commun. 12, 3594 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meng J., et al. , Source contributions to ambient fine particulate matter for Canada. Environ. Sci. Technol. 53, 10269–10278 (2019). [DOI] [PubMed] [Google Scholar]
- 10.GBD MAPS Working Group, Burden of Disease Attributable to Coal-Burning and Other Major Sources of Air Pollution in China, Special Report 20. Health Effects Institute. https://www.healtheffects.org/publication/burden-disease-attributable-coal-burning-and-other-air-pollutionsources-china (2016).
- 11.Thakrar S. K., et al. , Reducing mortality from air pollution in the United States by targeting specific emission sources. Environ. Sci. Technol. Lett. 7, 639–645 (2020). [Google Scholar]
- 12.Lelieveld J., et al. , Effects of fossil fuel and total anthropogenic emission removal on public health and climate. Proc. Natl. Acad. Sci. U.S.A. 116, 7192–7197 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silva R. A., Adelman Z., Fry M. M., West J. J., The impact of individual anthropogenic emissions sectors on the global burden of human mortality due to ambient air pollution. Environ. Health Perspect. 124, 1776–1784 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conibear L., Butt E. W., Knote C., Arnold S. R., Spracklen D. V., Residential energy use emissions dominate health impacts from exposure to ambient particulate matter in India. Nat. Commun. 9, 617 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yun X., et al. , Residential solid fuel emissions contribute significantly to air pollution and associated health impacts in China. Sci. Adv. 6, eaba7621 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao M., et al. , The impact of power generation emissions on ambient PM(2.5) pollution and human health in China and India. Environ. Int. 121, 250–259 (2018). [DOI] [PubMed] [Google Scholar]
- 17.Tjepkema M., Christidis T., Bushnik T., Pinault L., Cohort profile: The Canadian census health and environment cohorts (CanCHECs). Health Rep. 30, 18–26 (2019). [DOI] [PubMed] [Google Scholar]
- 18.Dockery D. W., et al. , Effect of air pollution control on mortality and hospital admissions in Ireland. Res. Rep. Health Eff. Inst. 176, 3–109 (2013). [PubMed] [Google Scholar]
- 19.Casey J. A., et al. , Improved asthma outcomes observed in the vicinity of coal power plant retirement, retrofit, and conversion to natural gas. Nat. Energy 5, 398–408 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelly F., et al. , The london low emission zone baseline study. Res. Rep. Health Eff. Inst. 163, 3–79 (2011). [PubMed] [Google Scholar]
- 21.Hedley A. J., et al. , Cardiorespiratory and all-cause mortality after restrictions on sulphur content of fuel in Hong Kong: An intervention study. Lancet 360, 1646–1652 (2002). [DOI] [PubMed] [Google Scholar]
- 22.Meng Y., et al. , Improvements in air quality and health outcomes among california medicaid enrollees due to goods movement actions. Res. Rep. Health Eff. Inst. 205, 1–61 (2021). [PMC free article] [PubMed] [Google Scholar]
- 23.Taubman S. L., Robins J. M., Mittleman M. A., Hernán M. A., Intervening on risk factors for coronary heart disease: An application of the parametric g-formula. Int. J. Epidemiol. 38, 1599–1611 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Westreich D., et al. , The parametric g-formula to estimate the effect of highly active antiretroviral therapy on incident AIDS or death. Stat. Med. 31, 2000–2009 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pinault L. L., et al. , Associations between fine particulate matter and mortality in the 2001 canadian census health and environment cohort. Environ. Res. 159, 406–415 (2017). [DOI] [PubMed] [Google Scholar]
- 26.Brauer M., et al. , Mortality-air pollution associations in low-exposure environments (MAPLE): Phase 1. Res. Rep. Health Eff. Inst. 203, 1–87 (2019). [PMC free article] [PubMed] [Google Scholar]
- 27.Crouse D. L., et al. , Evaluating the sensitivity of PM2.5-mortality associations to the spatial and temporal scale of exposure assessment. Epidemiology 31, 168–176 (2020). [DOI] [PubMed] [Google Scholar]
- 28.Pappin A. J., et al. , Examining the shape of the association between low levels of fine particulate matter and mortality across three cycles of the canadian census health and environment cohort. Environ. Health Perspect. 127, 107008 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen H., et al. , Changes in exposure to ambient fine particulate matter after relocating and long term survival in Canada: quasi-experimental study. BMJ 375, n2368 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crouse D. L., et al. , Risk of nonaccidental and cardiovascular mortality in relation to long-term exposure to low concentrations of fine particulate matter: A canadian national-level cohort study. Environ. Health Perspect. 120, 708–714 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olaniyan T., et al. , Ambient air pollution and the risk of acute myocardial infarction and stroke: A national cohort study. Environ. Res. 204, 111975 (2021). [DOI] [PubMed] [Google Scholar]
- 32.van Donkelaar A., Martin R. V., Li C., Burnett R. T., Regional estimates of chemical composition of fine particulate matter using a combined geoscience-statistical method with information from satellites, models, and monitors. Environ. Sci. Technol. 53, 2595–2611 (2019). [DOI] [PubMed] [Google Scholar]
- 33.Matheson F. I., Dunn J. R., Smith K. L., Moineddin R., Glazier R. H., Development of the canadian marginalization index: A new tool for the study of inequality. Can. J. Public Health 103, S12–S6 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Celis-Morales C. A., et al. , Association between active commuting and incident cardiovascular disease, cancer, and mortality: Prospective cohort study. BMJ 357, j1456 (2017). [DOI] [PubMed] [Google Scholar]
- 35.Jain P., Danaei G., Manson J. E., Robins J. M., Hernán M. A., Weight gain after smoking cessation and lifestyle strategies to reduce it. Epidemiology 31, 7–14 (2020). [DOI] [PubMed] [Google Scholar]
- 36.Chiu Y. H., et al. , Estimating the effect of nutritional interventions using observational data: The American heart association’s 2020 dietary goals and mortality. Am. J. Clin. Nutr. 114, 690–703 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Urman R., et al. , The potential effects of policy-driven air pollution interventions on childhood lung development. Am. J. Respir. Crit. Care Med. 201, 438–444 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garcia-Aymerich J., Varraso R., Danaei G., Camargo C. A. Jr., Hernán M. A., Incidence of adult-onset asthma after hypothetical interventions on body mass index and physical activity: An application of the parametric g-formula. Am. J. Epidemiol. 179, 20–26 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nasari M. M., et al. , A class of non-linear exposure-response models suitable for health impact assessment applicable to large cohort studies of ambient air pollution. Air Qual. Atmos. Health 9, 961–972 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mishan E. J., Evaluation of life and limb: A theoretical approach. J. Political Econ. 79, 687–705 (1971). [Google Scholar]
- 41.Health Canada, Health Impacts of Air Pollution in Canada: Estimates of premature deaths and nonfatal outcomes (2021 Report), https://www.canada.ca/en/health-canada/services/publications/healthy-living/2021-health-effects-indoor-air-pollution.html (Accessed 9 September 2021).
- 42.Chen J., Hoek G., Long-term exposure to PM and all-cause and cause-specific mortality: A systematic review and meta-analysis. Environ. Int. 143, 105974 (2020). [DOI] [PubMed] [Google Scholar]
- 43.VanderWeele T. J., Knol M. J., A tutorial on interaction. Epidemiol. Met. 3, 33–72 (2014). [Google Scholar]
- 44.McGrath S., Lin V., Zhang Z.et al. gfoRmula: An R package for estimating the effects of sustained treatment strategies via the parametric g-formula. Patterns (N. Y.) 1, 100008 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thurston G. D., et al. , Ambient particulate matter air pollution exposure and mortality in the NIH-AARP diet and health cohort. Environ. Health Perspect. 124, 484–490 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cesaroni G., et al. , Long-term exposure to urban air pollution and mortality in a cohort of more than a million adults in Rome. Environ. Health Perspect. 121, 324–331 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Puett R. C., et al. , Chronic fine and coarse particulate exposure, mortality, and coronary heart disease in the Nurses’ health study. Environ. Health Perspect. 117, 1697–1701 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pinault L., et al. , Risk estimates of mortality attributed to low concentrations of ambient fine particulate matter in the Canadian community health survey cohort. Environ. Health 15, 18 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arden Pope C., Mortality effects of longer term exposures to fine particulate air pollution: Review of recent epidemiological evidence. Inhal. Toxicol. 19 Suppl 1, 33-38 (2007). [DOI] [PubMed] [Google Scholar]
- 50.Lepeule J., Laden F., Dockery D., Schwartz J., Chronic exposure to fine particles and mortality: An extended follow-up of the Harvard six cities study from 1974 to 2009. Environ. Health Perspect. 120, 965–970 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bauwelinck M., et al. , Variability in the association between long-term exposure to ambient air pollution and mortality by exposure assessment method and covariate adjustment: A census-based country-wide cohort study. Sci. Total Environ. 804, 150091 (2022). [DOI] [PubMed] [Google Scholar]
- 52.So R., et al. , Long-term exposure to air pollution and mortality in a Danish nationwide administrative cohort study: Beyond mortality from cardiopulmonary disease and lung cancer. Environ. Int. 164, 107241 (2022). [DOI] [PubMed] [Google Scholar]
- 53.Di Q., et al. , Air pollution and mortality in the medicare population. N. Engl. J. Med. 376, 2513–2522 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Strak M., et al. , Long term exposure to low level air pollution and mortality in eight European cohorts within the ELAPSE project: Pooled analysis. BMJ 374, n1904 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang Y., et al. , Long-term exposure to PM2.5 and mortality among older adults in the southeastern US. Epidemiology 28, 207–214 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.World Health Organization, WHO Global Urban Ambient Air Pollution Database (update 2016) (World Health Organization, Switzerland, Geneva, 2017). [Google Scholar]
- 57.Park M., et al. , Differential toxicities of fine particulate matters from various sources. Sci. Rep. 8, 17007 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thurston G., Awe Y., Ostro B., Sanchez-Triana E., Are All Air Pollution Particles Equal? How Constituents and Sources of Fine Air Pollution Particles (PM2. 5) Affect Health (World Bank, Washington, DC, 2021). [Google Scholar]
- 59.U.S. EPA, Integrated science assessment (ISA) for particulate matter (Final report, Dec 2019) (U.S. Environmental Protection Agency, Washington, DC, 2019). [PubMed] [Google Scholar]
- 60.World Health Organization, Regional Office for Europe (2021). Review of evidence on health aspects of air pollution: REVIHAAP project: technical report. World Health Organization. Regional Office for Europe. https://apps.who.int/iris/handle/10665/341712. [PubMed]
- 61.Janssen I., Boyce W. F., Simpson K., Pickett W., Influence of individual- and area-level measures of socioeconomic status on obesity, unhealthy eating, and physical inactivity in Canadian adolescents. Am. J. Clin. Nutr. 83, 139–145 (2006). [DOI] [PubMed] [Google Scholar]
- 62.Bowe B., The 2016 global and national burden of diabetes mellitus attributable to PM2.5 air pollution. Lancet Planet. Health 2, e301–e312 (2018). [DOI] [PubMed] [Google Scholar]
- 63.Carey I. M., et al. , Mortality associations with long-term exposure to outdoor air pollution in a national english cohort. Am. J. Respir. Crit. Care Med. 187, 1226–1233 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang Y., et al. , Long term exposure to air pollution and mortality in an elderly cohort in Hong Kong. Environ. Int. 117, 99–106 (2018). [DOI] [PubMed] [Google Scholar]
- 65.Yin P., et al. , Long-term fine particulate matter exposure and nonaccidental and cause-specific mortality in a large national cohort of Chinese Men. Environ. Health Perspect. 125, 117002 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Boogaard H., van Erp A. M., Walker K. D., Shaikh R., Accountability studies on air pollution and health: The HEI experience. Curr. Environ. Health Rep. 4, 514–522 (2017). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
Statistics Canada’s policy on data privacy and confidentiality prohibits the analytical cohort used to be freely available in the manuscript or in a public repository. However, access can be granted through Statistics Canada’s Research Data Centre program. Environmental exposures are available upon request to the original authors of the data. The analytical code used was all standard R and SAS code (e.g., gfoRmula, data steps).