Significance
Memory updating is an adaptive mechanism that allows modifying stored memories. Consolidated memories can be enhanced or weakened by drug treatments, amnestic agents, or new information associated with previous memory through memory updating. The hippocampus is essential for spatial contextual detection underlying the recognition memory. Here, we show that modulation of catecholamine release in the dorsal hippocampal CA1 region from the locus coeruleus is required to update spatial contextual recognition memory. Modulating the extracellular concentration of catecholamines in the hippocampus could modify contextual maladaptive memories, such as aversive memories, drug addiction, and phobias, through memory updating.
Keywords: memory updating, locus coeruleus, hippocampus, optogenetics, catecholamines
Abstract
Detecting novelty is critical to consolidate declarative memories, such as spatial contextual recognition memory. It has been shown that stored memories, when retrieved, are susceptible to modification, incorporating new information through an updating process. Catecholamine release in the hippocampal CA1 region consolidates an object location memory (OLM). This work hypothesized that spatial contextual memory updating could be changed by decreasing catecholamine release in the hippocampal CA1 terminals from the locus coeruleus (LC). In a mouse model expressing Cre-recombinase under the control of the tyrosine hydroxylase (TH) promoter, memory updating was impaired by photoinhibition of the CA1 catecholaminergic terminals from the LC (LC-CA1) but not from the ventral tegmental area (VTA-CA1). In vivo microdialysis confirmed that the extracellular concentration of both dopamine (DA) and noradrenaline (NA) decreased after photoinhibition of the LC-CA1 terminals (but not VTA-CA1) during the OLM update session. Furthermore, DA D1/D5 and beta-adrenergic receptor antagonists disrupted behavior, but only the former impaired memory updating. Finally, photoinhibition of LC-CA1 terminals suppressed long-term potentiation (LTP) induction in Schaffer’s collaterals as a plausible mechanism for memory updating. These data will help understand the underpinning mechanisms of DA in spatial contextual memory updating.
Long-term memory encoding and storage are achieved through a consolidation process where structural and molecular changes transform new information into a stable trace (1). However, retrieving memories allows behavioral expression and could initiate a dynamic process by which memory can integrate novel information. This process is called memory updating—that is, novel information is compared with the stored memories during retrieval, destabilizing the stored memory (2–6). Subsequently, the destabilized memory and the incoming information are restabilized, and the updating consolidation period results in the memory returning to a steady state (7–9).
Memory updating is an adaptive mechanism present in many species (10). Consequently, established memories may be modified and modulated by events during the retrieval process, implying the occurrence of plastic changes. Hence, memory retrieval could trigger the incorporation of novel information that results in memory updating (3, 9, 11). After reactivation, memory can be weakened or strengthened by pharmacological treatments, amnesic agents, or new competitive learning (11–13). In a clinical setting, these approaches could modify maladaptive memories such as aversive memories, phobias, and drug addictions (2, 6, 14–16). Human studies have shown that it is possible to reduce cigarette consumption by updating the memories associated with smoking cues (17). In addition, oral administration of beta-adrenoreceptor blockers after exposure to smoking cues reduces craving (18, 19).
The hippocampus has long been proposed to play a critical role in the novelty detection of spatial contextual memory (20–22). In particular, the hippocampal CA1 region detects familiar/novel spatial contextual stimuli during episodic memory updating (1, 23–26). Hippocampal lesions and inhibition of hippocampal protein synthesis disrupt spatial contextual memory updating (27, 28). Recent studies have shown that spatial contextual object recognition memory updating depends on memory reactivation and hippocampal protein synthesis (24). Researchers have proposed that dopamine (DA) is involved in novel stimulus processing (29–31). Blockade of hippocampal DA D1/D5 receptors impairs memory updating of object recognition memory (32). Similarly, spatial contextual memory depends on catecholaminergic hippocampal activity (22, 33, 34).
The ventral tegmental area (VTA) is a structure that releases DA to the hippocampus (18), and the locus coeruleus (LC) is a source of noradrenaline (NA) to the hippocampus (35, 36). It had been thought that the VTA is the primary DA source in the hippocampus (37, 38). However, recent investigations have demonstrated that the LC releases both DA and NA to the hippocampus (39, 40). Moreover, acquisition of spatial contextual recognition memory is modulated by DA and NA release in the hippocampus (25, 39). However, the precise relationship between these neurotransmitters and recognition memory updating remains to be elucidated. Specifically, DA and NA seem to be involved in the detection of familiar/novel information (41, 42), spatial memory (39, 43, 44), and contextually motivated behaviors (25, 45, 46). Recent studies have shown the relationship between synaptic plasticity and memory through DA and NA release. Specifically, an inhibitory avoidance paradigm induced in vivo long-term potentiation (LTP) dependent on the hippocampal CA1 DA receptor activation (45). Another study showed that novel experience facilitates hippocampal LTP induction requiring beta-adrenoreceptor activation (47). Therefore, hippocampal catecholamine modulation may be a potential target to modify the stored spatial contextual memories through memory updating and its underlying plastic modifications.
We assessed the influence of catecholaminergic fibers from the LC and VTA on the hippocampal CA1 region during spatial contextual recognition memory updating. Furthermore, we analyzed the specific involvement of DA and NA during retrieval and updating of spatial contextual memories. We performed optogenetic manipulations using transgenic tyrosine hydroxylase (TH) Cre-recombinase-dependent (TH-cre) mice, pharmacological procedures, and in vivo microdialysis techniques during a slightly modified object location memory (OLM) task (24). Optogenetic inhibition of hippocampal axons from the LC-CA1, but not from the VTA-CA1, impaired OLM updating by decreasing DA and NA extracellular levels in the dorsal hippocampal CA1 region. Pharmacological manipulations allowed us to identify how DA and NA participate in different phases of memory. DA and NA modulate behavioral expression, whereas only DA is involved in destabilization and re-stabilization for memory updating in the dorsal hippocampal CA1 region. Our behavioral data are consistent with synaptic plastic modifications. Optogenetic inhibition of catecholaminergic axons from the LC-CA1 during high-frequency stimulation (HFS) in the Schaffer collaterals produced an LTP to long-term depression (LTD) shift. We obtained similar results when we concomitantly administered NA and DA receptor antagonists before performing the HFS. These data show that the LC-CA1 pathway is highly involved in synaptic plasticity that may underlie spatial contextual memory updating.
Results
Catecholaminergic Modulation from LC-CA1 but not from VTA-CA1 Terminals Is Required to Update Spatial Contextual Recognition Memory.
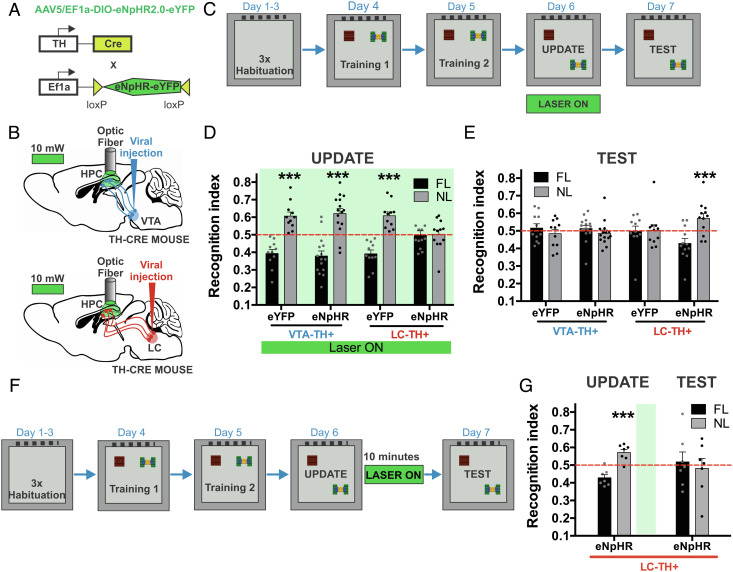
We first investigated how catecholamine release in the hippocampal CA1 region from the LC and VTA modulates spatial contextual recognition memory updating. TH-cre mice were infused with a viral vector to express eNpHR or eYFP into the VTA or LC and its projecting axons (Fig. 1 A and B). We tested an OLM hippocampus-dependent spatial and contextual paradigm (24, 25) (Fig. 1C). This protocol allowed us to manipulate memory updating. First, the mice acquired spatial and contextual information by exploring two objects for 10 min for 2 d during training sessions (SI Appendix, Fig. S1). The update session was performed 24 h after the last training session. In the update session, one object remained in the original, familiar location. The other object was moved to a novel location, thus representing relevant information. In this session, we observed behavioral expression and, at the same time, memory updating begins. During this session, we optically inhibited the catecholaminergic LC-CA1 and VTA-CA1 axons with green light through bilaterally implanted optic fibers into the dorsal hippocampal CA1 region. A correct update session performance would increase exploration of the object with the novel location. The memory updating was measured in a test session that occurred 24 h after the update session. Correct memory updating did not show changes in the recognition index, indicating that this new configuration was updated and both objects are detected in a familiar position.
Fig. 1.
Catecholaminergic modulation from the LC to the hippocampal CA1 region in spatial contextual recognition memory updating. A. Viral Cre-inducible vectors were injected in TH-cre mice. B. Schematic bilateral viral infection eNpHR or control eYFP in the VTA (blue) or LC (red) and bilateral optic fiber implantation into the dorsal hippocampal CA1 region. C. OLM protocol. D. Recognition index in update session with objects in a familiar location (FL) and a novel location (NL) for the VTA-eYFP (n = 11), VTA-eNpHR (n = 15), LC-eYFP (n = 11), and LC-eNpHR (n = 12) groups. The green bar represents continuous delivery of 10–15 mW green light during the update session. E. Memory test session. F. Photoinhibition of catecholaminergic hippocampal projection after the update session. G. Recognition index in the update and test sessions (n = 7) of TH-cre mice with viral infection in the LC. The green bar represents 10 min of laser (10–15 mW) from the fiber optic tip to continuous pulse after the update session. All results show the mean recognition index ± SEM. ***P < 0.001.
Photoinhibition in the hippocampal CA1 led to significant group differences in the recognition indexes during the update session of the OLM task (group × object interaction, F(3,90) = 9.235, P < 0.0001). Photoinhibition of the hippocampal axons from the VTA-eNpHR and VTA-eYFP groups produced adequate behavioral expression: Both groups exhibited increased recognition indexes for the object placed in the novel location compared with the familiar location (Holm–Sidak, VTA-eNpHR: t(28) = 6.015, P < 0.0001, VTA-eYFP: t(20) = 6.503, P < 0.0001) (Fig. 1D). Similarly, the control LC-eYFP group showed a significantly increased object recognition index to the object with the novel location under the light (Holm–Sidak LC-eYFP: t(20) = 6.988, P < 0.0001). However, photoinhibition of hippocampal CA1 axons in the LC-NpHR group showed a similar recognition index for the familiar and novel locations (LC-eNpHR: Holm–Sidak, t(22) = 0.141, P = 0.889). Photoinhibition of LC-CA1 axons impaired behavioral expression (Fig. 1D).
Memory updating was evidenced during the test session. There were significant differences among the groups regarding spatial contextual recognition memory indexes (group × object interaction, F(3,90) = 5.392, P = 0.002). The LC-eYFP, VTA-eNpHR, and VTA-eYFP groups showed correct memory updating with similar recognition indexes for both objects during the test session (Holm–Sidak, LC-eNpHR: t(20) = 0.166, P = 0.869, VTA-eNpHR: t(28) = 1.041, P = 0.306, VTA-eYFP: t(20) = 0.912, P = 0.372) (Fig. 1E). However, photoinhibition of hippocampal projections from the LC-TH during the update session impaired memory updating of the moving object because the recognition time to this object was significantly higher during the test session (Holm–Sidak, LC-eNpHR: t(22) = 3.655, P = 0.001). This result indicates that the LC-eNpHR group did not incorporate the novel information of the object during and after the update session.
Our findings prove that photoinhibition of LC-CA1 axons, but not VTA-CA1 axons, modulates spatial contextual recognition memory updating. The first step for memory updating is reactivation through retrieval. Thus, we showed that photoinhibition of LC-CA1 terminals just after the update session did not block memory updating because animals showed similar recognition memory indexes to both objects during the test session (session × object interaction, F(1,24) = 4.687, P < 0.0041; Holm–Sidak, update session: t(12) = 5.722, P < 0.0001, test session: t(12) = 0.468, P = 0.648) (Fig. 1 F and G). Memory updating requires memory reactivation and a temporal window to incorporate the updated information initiated during the update session. In this case, temporal inhibition of catecholamine release after the update session was not sufficient to block memory updating. Additionally, all groups showed a similar total time spent in the exploration of objects during all sessions of the OLM task (SI Appendix, Table S1). Importantly, we quantified the density of nuclei in the hippocampal CA1 region and determined that photoinhibition performed during the OLM protocol did not reduce hippocampal CA1 nuclei compared with mice without photoinhibition (t(5) = 0.240, P = 0.820) (SI Appendix, Fig. S2).
Hippocampal CA1 TH Terminals Are More Abundant from the LC than the VTA.
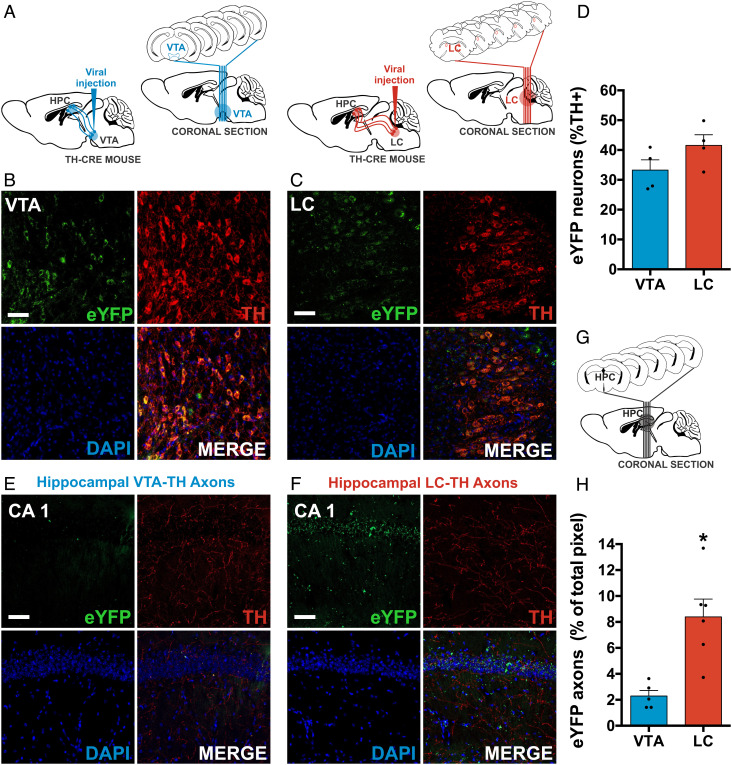
Hippocampal catecholaminergic modulation from the LC is required for spatial contextual memory updating (33, 39, 40, 43). To identify the distribution of hippocampal CA1 catecholaminergic fibers from the LC and VTA, we performed immunofluorescence detection of TH and observed colocalization of YFP with TH labeling (Fig. 2). We infused TH-cre mice with a viral vector eNpHR into the VTA or LC (Fig. 2A). Then, we quantified the eYFP+ and TH+ cells in the LC and VTA (Fig. 2 B and C); there was a similar percent of eYFP+/TH+ neurons in both the VTA and LC (t(6) = 1.677, P = 0.145) (Fig. 2D and SI Appendix, Fig. S3). We compared the distribution of catecholaminergic fibers from the VTA and LC with the dorsal hippocampal CA1 region (Fig. 2 E and F). To quantify the projection from the VTA and LC to the dorsal hippocampal CA1 region (Fig. 2G), we measured the pixels with eYFP axonal projections coming from VTA and LC. Projection from the LC-CA1 was more abundant than the VTA-CA1 (t(9) = 3.912, P = 0.004) (Fig. 2H and SI Appendix, Fig. S3). Like previous reports, we confirmed that the LC has a higher axonal density in the dorsal CA1 than the VTA (39, 40).
Fig. 2.
Hippocampal TH terminals are more abundant from the LC than the VTA. A. Schematic bilateral viral injection and representative coronal sections of eNpHR in the VTA (blue) or LC (red). B, and C. Representative images of coronal immunofluorescence section of bilateral viral injection of AAV5-eNpHR in the VTA (B) or LC (C); eYFP (green), TH (red), DAPI (blue), and MERGE (colocalization). D. Quantification of eYFP+ and TH+neurons of the VTA (n = 4 mice with four or five coronal sections) or the LC (n= 4 mice with four or five coronal sections). The graph shows the eYFP area related to the TH area. E, and F. Representative images of coronal immunofluorescence section of dorsal hippocampal CA1 axons from the VTA (E) or LC (F) with a bilateral viral injection of AAV5-eNpHR in the VTA or LC, respectively. G. Representative coronal sections of dorsal hippocampal CA1analysis. H. Quantifying hippocampal axons from the VTA (n = 5 mice with six coronal sections) or the LC (n = 6 mice with six coronal sections). The graph shows the eYFP area. All graphs show mean ± SEM. (Scale bar: 50 µm.) *P < 0.01.
Behavioral Expression and Memory Updating of Spatial Contextual Recognition Memory Are Modulated by the Release of DA and NA from the LC to the Hippocampal CA1 Region.
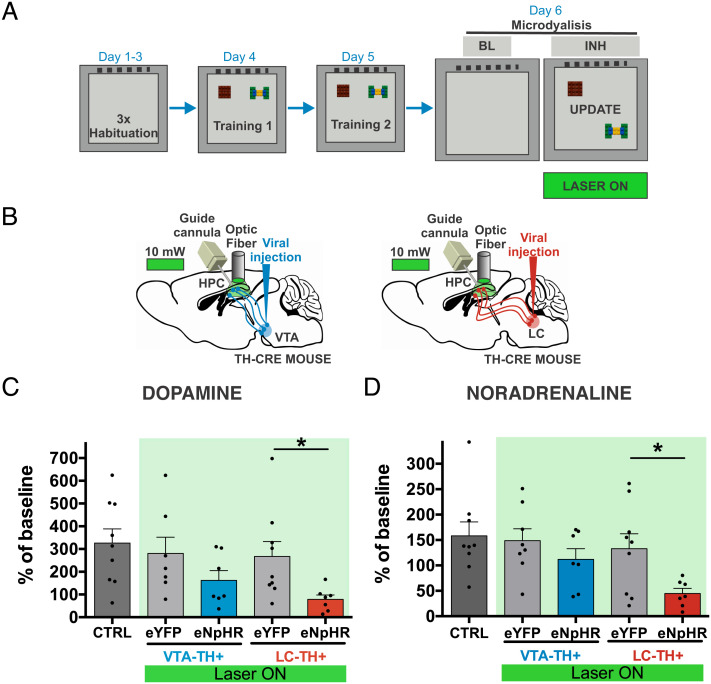
Our results showed that dorsal LC-CA1 fibers modulate memory updating through DA and NA release. We used in vivo microdialysis with electrophoretic detection during the update session of the OLM paradigm to quantify the extracellular DA and NA levels in the hippocampus (Fig. 3 A and B). A one-way ANOVA yielded significant differences among the groups in DA (F(4,34) = 2.892, P = 0.037) and NA (F(4,35) = 3.169, P = 0.025). As expected, biochemical analysis of intact wild-type mice (CTRL) revealed that during the update session, there was release of NA (158.0% ± 27.2%) and DA (326.2% ± 62.4%) in the hippocampal CA1 region. Photoinhibition of VTA-CA1 axons did not affect the extracellular DA (162.1% ± 42.6%) and NA (111.9% ± 21.2%) release compared with the VTA-eYFP extracellular concentration of DA (280.4% ± 72.6%, t(12) = 1.417, P = 0.182) and NA (148.7% ± 23.5%, t(13) = 1.148, P = 0.272). Photoinhibition of LC-CA1 axons decreased the extracellular DA (78.4% ± 19.5%) and NA (44.7% ± 9.9%) during the update session compared with the LC-eYFP group for DA (267.0% ± 65.8%, t(14) = 2.444, P = 0.028) and NA (133.0% ± 29.2%, t(14) = 2.565, P = 0.022, Fig. 3 C and D). Therefore, LC modulates the release of DA and NA during the update session, underlying behavioral expression followed by recognition memory updating in the hippocampal CA1 region.
Fig. 3.
DA and NA release from the LC to the hippocampal CA1 region modulates behavioral expression and memory updating. A. OLM protocol. Microdialysis samples were collected at baseline (BL) and during the update session with photoinhibition (INH). B. Schematic bilateral viral injection of eNpHR or control eYFP in the VTA (blue) or LC (red), bilateral optic fiber implantation into the dorsal hippocampal CA1 region, and unilateral guide cannula implantation into the dorsal hippocampal CA1 region. C, and D. Hippocampal CA1 release of DA (C) and NA (D) during update session in the CTRL (DA and NA: n = 9), VTA-eYFP (DA: n = 7, NA: n = 8), VTA-eNpHR (DA and NA: n = 7), LC-eYFP (DA and NA: n = 9), and LC-eNpHR (DA and NA: n = 7) groups. The green bar represents the time with a continuous laser pulse (10–15 mW) from the fiber optic tip. All results show the mean percent of baseline ± SEM. *P < 0.05.
DA and NA Modulate OLM Behavioral Expression, but Only DA Modulates Spatial Contextual Recognition Memory Updating.
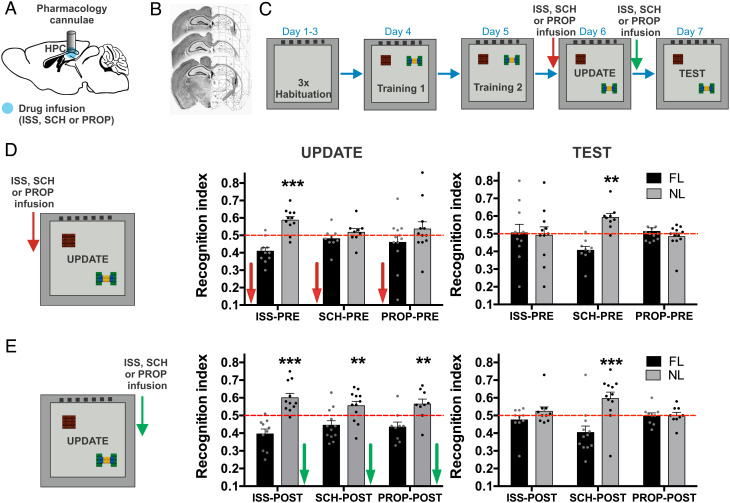
We tested whether DA or NA receptor antagonists could pharmacologically inhibit memory updating. Our previous results showed that hippocampal CA1 extracellular DA and NA concentration reduction during the update session impairs behavioral expression and memory updating. Hence, we hypothesized that DA and NA receptor antagonists have a dissociable function in retrieving and memory updating.
To test this hypothesis, before the update session we infused the CA1 hippocampal region with either SCH23390 (SCH), a DA D1/D5 receptor antagonist, or propranolol (PROP), a beta-adrenergic receptor antagonist (Fig. 4D). First, the mice explored two objects for 10 min for 2 d during training sessions (SI Appendix, Fig. S1). In the update session, the control mice that received infusion of isotonic saline solution (ISS) preferred the novel moved object compared with the familiar object (group × object interaction, F(2,58) = 2.636, P = 0.080; object effect F(1,58) = 14.29, P = 0.0004; Holm–Sidak, ISS: t(20) = 6.274, P < 0.0001). However, the SCH and PROP groups demonstrated disrupted memory expression (Holm–Sidak, SCH: t(16) = 1.261, P = 0.225, PROP: t(22) = 1.306, P = 0.205). Twenty-four hours after the update session, we proved memory updating occurred during the test session. There was a significant difference among the groups (group × object interaction, F(2,58) = 5.966, P = 0.004). The PROP and ISS groups showed memory updating, with a similar exploration time during the test session (Holm–Sidak, ISS: t(20) = 0.202, P = 0.842, PROP: t(22) = 0.205, P = 1.307). However, memory updating was only disrupted in the SCH group: The mice showed a higher preference index for the moving object during the test session (Holm–Sidak, SCH: t(16) = 5.813, P < 0.0001) (Fig. 4D). These findings indicate that beta-adrenergic receptors modulate memory expression without affecting reactivation and, subsequently, memory updating. Our results show that memory updating depends on DA D1/D5 receptor activation.
Fig. 4.
OLM behavioral expression is dependent on catecholaminergic receptor activation; however, DA D1/D5 receptor activation modulates OLM memory updating. A. Cannula implantation in the hippocampal CA1 for pharmacological dosing. B. Histology of coronal brain sections of the hippocampal CA1 region with a cannula. C. OLM protocol. D. Recognition index in the update and test sessions with an object in the familiar location (FL) and the novel location (NL) in mice infused with ISS (n = 11), SCH (n = 9), or PROP (n = 12) before the update session. E. Recognition index in the update and test sessions in mice infused with ISS (n = 10), SCH (n = 12), or PROP (n = 9) after the update session. All results show the mean recognition index ± SEM. ***P < 0.001; **P < 0.01.
To further investigate the role of both catecholamines for memory updating, we infused either DA or NA receptor antagonists immediately after the update session concluded (Fig. 4E). The mice explored two objects for 2 d during training sessions (SI Appendix, Fig. S1). As expected, all groups recognized the novel object during the update session prior to the pharmacological intervention (group × object interaction, F(2,56) = 1.825, P = 0.172; object effect, F(1,56) = 45.020, P < 0.0001). All groups showed more time spent with the novel moved object than the familiar object (Holm–Sidak, ISS: t(18) = 5.465, P < 0.0001, SCH: t(22) = 3.070, P = 0.005, PROP: t(16) = 3.253, P = 0.005). Nonetheless, during the test session to evaluate memory updating (group × object interaction, F(2,56) = 5.915, P = 0.005), only the SCH group showed memory updating impairment: This group spent more time with the moved object (Holm–Sidak, ISS: t(18) = 1.411, P = 0.175, SCH: t(22) = 3.760, P = 0.001, PROP: t(16) = 0.138, P = 0.892) (Fig. 4E). Notably, pharmacological manipulation did not affect the total object exploration time during any OLM session (SI Appendix, Table S1). Taken together, these results indicate that hippocampal catecholamines play an essential role during retrieval in behavioral expression, trace reactivation, and memory updating. We conclude that NA is only essential in behavioral expression but not in memory updating. On the contrary, DA is needed for behavioral expression and memory updating.
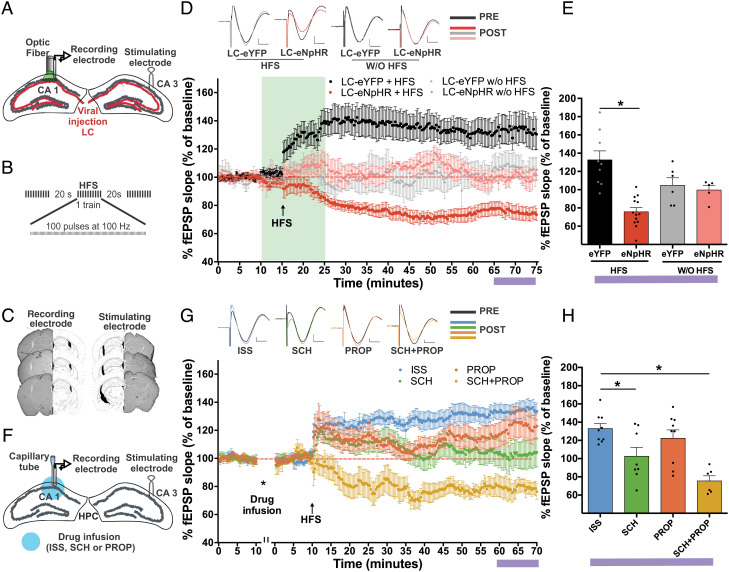
LC Catecholaminergic Fibers Modulate the LTP/LTD Threshold in the Hippocampal CA1 Region.
Previous reports have demonstrated the effects of DA and NA on synaptic plasticity (47, 48). To determine whether the catecholaminergic LC-CA1 fibers modulate synaptic plasticity, we photoinhibited the LC-CA1 fibers during an LTP protocol in anesthetized mice. After 3 wk, preinjecting animals with eNpHR in the LC, we induced LTP in the Shaffer collateral pathway by applying HFS and measuring the corresponding electrical responses in the hippocampal CA1 region (Fig. 5 A–C). For the LC-eYFP group, we applied green light for 5 min during baseline and 10 min after HFS. After 1 h, the LC-eYFP group had induced and maintained strong LTP (132.6% ± 9.8% of the field excitatory postsynaptic potential [fEPSP] slope at 1 h). In contrast, HFS stimulation and photoinhibition in the LC-eNpHR group switched LTP to LTD (75.7% ± 4.5% of the fEPSP slope at 1 h). These results showed that inhibition of hippocampal CA1 release of DA and NA changes the LTP/LTD threshold compared with the control LC-eYFP group (F(3,29) = 13.25, P < 0.0001, Tukey, LC-eYFP HFS vs. LC-eNpHR HFS, P < 0.0001). Furthermore, when we applied light without HFS, neither the LC-NpHR group (104.8% ± 8.3% of fEPSP slope at 1 h) nor the LC-eYFP group (99.6% ± 5.1% of fEPSP slope at 1 h) showed induced and maintained LTP (104.8% ± 8.3% of fEPSP slope at 1 h), and the groups had similar fEPSP (F(3,29) = 13.25, P < 0.0001, Tukey, LC-eYFP W/O HFS vs. LC-eNpHR W/O HFS, P = 0.976) (Fig. 5 D and E).
Fig. 5.
Associated modulation of DA and NA regulates the threshold of LTD/LTP after HFS. A. Schematic infusion of eNpHR or eYFP in the LC, and implantation of a fiber optic and recording electrode in the hippocampal CA1 region and a stimulating electrode in the hippocampal CA3 region. B. HFS protocol (three trains of 100 pulses at 100 Hz). C. Histology of coronal brain sections of the hippocampal CA1 (recording electrode) and CA3 (stimulating electrode) regions. D. Response as the percent slope of fEPSP in the LC-eNpHR+HFS (n = 13), LC-eYFP+HFS (n = 9), LC-eNpHR+W/OHFS (n = 5), and LC-eYFP+W/O HFS (n = 6) groups. Representative traces of EPSP before (PRE) and after exposure to the laser (POST) (green bar, 573 nm at 10–15 mW from the fiber optic tip to continuous pulse for 15 min). E. Representative graph of the last 10 min (purple bar) of the % EPSP slope. F. Schematic of drug infusion and the recording electrode in the hippocampal CA1 region and the stimulating electrode in the hippocampal CA3 region. G. Response in % fEPSP slope after HFS administrated with ISS (n = 9), SCH23390 (SCH, n = 8), PROP (n = 9), or SCH with PROP (SCH+PROP, n = 6). Representative traces of EPSP before (PRE) and after HFS (POST) (horizontal bar: 5 ms, vertical bar: 200 mV). H. Representative graph of the last 10 min (purple bar) of the % fEPSP slope. All results show the mean % fEPSP slope of baseline ± SEM. ns: not significant; *P < 0.05.
Finally, we studied the relevance of the DA and NA receptors during LTP induction. We infused DA and NA receptor antagonists before HFS in the hippocampal CA1 region and followed the same protocol described above (Fig. 5F). Administration of the beta-adrenergic receptor antagonist PROP and ISS did not affect the induction and maintenance of LTP after HFS. However, LTP induction after administration of DA D1/D5 antagonist SCH was significantly lower than the saline group. Coadministration of PROP and SCH switched the LTP/LTD threshold after HFS (F(3,28) = 8.773, P = 0.0003, Tukey, ISS vs. SCH P = 0.0478, ISS vs. PROP P = 0.7578, ISS vs. SCH+PROP P = 0.0003) (Fig. 5 G and H). These results suggest that catecholaminergic modulation from LC-TH terminals in the hippocampal CA1 region is required to modulate the LTP/LTD threshold.
Discussion
This work evidences that DA release in the dorsal hippocampal CA1 region from the LC is required during spatial contextual memory updating, while both DA and NA support behavioral expression. The LC and VTA are presumably the primary catecholaminergic providers. In this work, we investigated the terminal pathways from the LC and VTA by which the dorsal hippocampus is supplied. We determined how these pathways are involved by optogenetically suppressing them during spatial contextual memory updating. Our results showed that photoinhibition of LC-CA1 but not VTA-CA1 terminals impairs spatial contextual recognition memory updating. Previous reports have shown that acquisition and consolidation of spatial contextual memory are modulated by DA release in the dorsal hippocampal CA1 region from the LC (39, 40). However, NA modulation seems less necessary for acquiring and consolidating spatial memory in the same area (39, 40). As we will see, both DA and NA receptor antagonists impair behavioral expression, but only DA receptor antagonists block spatial memory updating. These studies suggest that LC releases DA in the dorsal hippocampus and is involved in spatial contextual memory updating.
Our behavioral results showed that catecholamine release from LC-CA1 terminals but not VTA terminals modulates spatial contextual memory updating. Optogenetic inhibition of the catecholaminergic LC-CA1 terminals during the update phase impaired recognition of the novel position during the OLM protocol, associated with decreased DA and NA release in the hippocampal CA1 region and diminished spatial contextual memory updating. Previous studies with pharmacological manipulation have shown that catecholamines in the dorsal hippocampus modulate the acquisition of contextual memory. DA has been associated with familiarity/novelty detection, and NA is linked to attention and the identification of contextual cues (25, 40). In addition, studies have shown the role of hippocampal DA during memory consolidation and reconsolidation of recognition memory (25, 41, 49, 50). However, the hippocampal pathway of DA and NA release during spatial contextual memory updating had been unknown. In a previous study, we demonstrated that DA and NA release from VTA in the insular cortex is required during retrieval for updating a contextual reward memory (51). Recent work has shown a functional loop between the hippocampus and dopaminergic neurons of the VTA, resulting in DA release in the hippocampus and enhanced reward novelty memory (38). These data and other findings suggest that catecholamine modulation from the VTA or LC could depend on the type and stage of memory and the anatomical structure (38, 40, 51).
Our histological results confirm that the LC is the primary source of catecholaminergic projection to the dorsal hippocampal CA1 region. These results are consistent with recent reports that have shown the LC sends primary projections to the dorsal hippocampus. Meanwhile, TH fibers from the VTA are more abundant in the ventral than the dorsal hippocampus (39, 40). The number of catecholaminergic fibers from the VTA and LC in the hippocampus could be associated with modulation of spatial contextual memory updating. Several studies have determined that these neurotransmitters are needed during object recognition (25, 39, 40, 52, 53). DA and NA are extracellularly enhanced in the hippocampal CA1 region during retrieval on the OLM task (25). In contrast, object recognition memory retrieval does not increase cortical extracellular DA and NA levels (25, 54). Similarly, hippocampal lesions with 6-hydroxydopamine impair CA1 release of both DA and NA and do not affect object recognition memory acquisition and retrieval (25). These results indicate that DA and NA release in the hippocampus is not necessary for recognizing the identity of objects but only for detecting a spatial contextual novelty associated with an object in a novel position (25, 54). Taken together, these results suggest that two catecholaminergic circuits could be involved in recognition memory, one for the object’s novelty/familiarity and the other in the spatial contextual novel/familiar configuration where the objects are ubicated. In this way, to determine whether the extracellular concentration of DA and NA is modified by photoinhibition of LC-CA1 or VTA-CA1 fibers, we used in vivo microdialysis during the update session of the OLM task. We found that dorsal hippocampal DA and NA extracellular concentrations are significantly decreased after inhibiting the terminals originating from the LC but not from the VTA during the update session. Accordingly, our data obtained through photoinhibition support the idea that the LC contributes to the corelease of NA and DA in the dorsal hippocampus (39, 40, 55, 56).
As mentioned, memory updating is initiated when novel information destabilizes the original memory during retrieval (7–9). Then, the restabilization processes induce the incorporation of new information in the original memory, promoting memory updating (3, 7, 32, 57). Although amnesic agents do not affect the retrieval of familiar memories, they impair the updating processes (24, 32). Interestingly, recent work using cellular analysis of the temporal activity of in situ hybridization fluorescence Arc activity has shown that the original memory coexists with the updated memory (24). The authors demonstrated an overlap between neurons related to the original memory and neurons activated to update the OLM (24). In this way, the update session represents an opportunity to incorporate a novel spatial configuration into the original memory for the objects. Based on optogenetic manipulation, we could not determine whether DA and NA have differential effects on memory retrieval and updating. However, our data highlight an essential role of LC-CA1 axons in DA and NA release during spatial contextual recognition memory. In this regard, we performed pharmacological manipulation to determine the role of DA and NA receptors during memory retrieval and updating. We found that injection of DA D1/D5 receptors or beta-adrenergic receptor antagonists before the update session in the hippocampal CA1 impair OLM expression. However, only the DA D1/D5 receptor antagonists impair OLM updating. Furthermore, our pharmacological posttrial injections confirmed that updating processes continue even after presenting novel information and are modulated by DA but not by NA receptors. These data show a dissociable function of catecholaminergic receptors in memory retrieval and updating. In this way, we have shown that beta-adrenergic receptors are essential to behavioral expression, whereas DA receptors modulate behavioral expression and memory updating. Consistent with our results, relevant stimuli during retrieval elicit NA release in the forebrain (58). Our data are in line with other studies showing that behavioral expression is an independent process of memory updating (3, 5, 6, 59–61).
Our data showed that beta-adrenergic antagonists in the CA1 only block behavioral expression. Hence, we propose that hippocampal NA release is processed via beta-adrenergic receptors and modulates memory retrieval. Consistently, Murchison and colleagues (62) proposed that hippocampal NA release is processed via beta-adrenergic receptors and modulates memory retrieval. Accordingly, it has been demonstrated that salient cues activate the LC (63), and beta-adrenergic receptor antagonists in the hippocampus impair the expression of different behaviors (62, 64, 65). In addition, NA is also involved in attention, helping optimize task performance (66–68). These data could explain the effects of beta-adrenergic receptor antagonists on memory expression and not memory updating. Then, the trace activation is susceptible to memory updating via DA activity in the hippocampal CA1 region.
In the process, trace reactivation only triggers memory updating when the information is relevant and susceptible to amnesic agents (24, 32, 57, 69). Our data showed that DA D1/D5 receptors are involved in memory expression and updating. Recent studies have confirmed that DA D1/D5 receptor antagonist hippocampal CA1 infusion during object recognition retrieval impairs memory destabilization, preventing the incorporation of new information into the original memory (50). Another study showed that DA signals the novelty during recall, inducing memory destabilization and determining whether a new trace will link to previously stored memories (32). When a previous memory is reactivated and associated with new information, this can be updated and the new information can be linked with preexisting memories. Blocking hippocampal DA receptors prevents new information from being linked with previous storage memory (32, 50). These results suggest that when a previous memory is reactivated, the DA modulation is involved in associating new information with storage memories (32, 50).
Additionally, our results showed that photoinhibition after the update session does not impair memory updating. In contrast, pharmacological DA blockade impairs memory updating. However, the similarities of the cellular mechanisms involved in the photoinhibition of catecholaminergic terminals and the DA receptor antagonists remain to be determined. Perhaps optogenetic inhibition of LC-CA1 terminals in the hippocampus during updating induces similar molecular events as blockade of the DA receptor but in different time profiles. Many researchers have shown that pharmacological treatments only have effects during vulnerability windows that modify the stabilization process (51, 69, 70). Indeed, SCH has effects even 3 h after administration (71). Our optogenetic experiments immediately after the update test were ineffective because temporal inhibition was only for 10 min compared with the activity windows of the SCH administered after the update test. This result showed that memory updating needs a longer temporal window to incorporate and stabilize the updated information.
The precise mechanism for memory updating is unclear, but researchers have shown that modulation of some neurotransmitters would impact the different stages of memory updating (10, 50, 72, 73). In this regard, AMPA receptors are involved in destabilizing the memory trace. The balance between calcium-permeable and calcium-impermeable AMPA receptors allows synaptic malleability. When new information is present, the memory trace is destabilized, and the calcium-impermeable AMPA receptors are replaced with calcium-permeable AMPA receptors (74). The role of DA in this process is through phosphorylation of the GluA1 subunit via PKA. GluA1 subunit phosphorylation involves insertion of the calcium-permeable AMPA receptor (75, 76). In addition, after spatial contextual memory retrieval, the membrane excitability of hippocampal CA1 neurons increases and becomes dependent on DA receptors (73). The next step for memory updating is restabilization. This process is dependent on protein synthesis and transcription factors. Studies have shown that the transcription factor Zif268 is necessary for memory updating (1, 10, 77). The administration of Zif268 antisense oligonucleotides in the hippocampus revealed that this molecule is necessary during recognition memory updating. Therefore, DA modulation is required for Zif268 expression through DA D1 receptor activation (48, 77, 78).
Several studies have demonstrated that the plastic synaptic changes induced by DA are related to the molecular changes underlying memory (45, 47, 79). The induction and maintenance of LTP share mechanisms of memory updating (80), such as the expression of Zif268 in the hippocampus (48). Recent investigations have shown that LC modulation can induce modest LTP in the hippocampus after HFS or optogenetic stimulation of LC somata (81, 82). Additionally, DA and NA levels in the CA1 region could modify LTP induced by HFS (83, 84). Similarly, administration of catecholaminergic agonists or antagonists increases or decreases LTP, respectively (45, 85–87). Accordingly, enhancement of catecholamines by blockers of monoamine reuptake transporters, like cocaine, induces LTP in the VTA (88). Our electrophysiological results demonstrated that optogenetic inhibition of the hippocampal projections from LC can modify the catecholaminergic concentrations in the hippocampus. Modification of catecholamine release could transform LTP into LTD after HFS. This phenomenon has been observed in the prefrontal cortex by reducing tonic DA levels, transforming LTP induction into LTD (89, 90). This model demonstrated that the DA background levels could modify the threshold to induce LTD instead of LTP.
In addition, our experiments with concomitant infusion of DA D1/D5 and beta-adrenergic receptor antagonists showed a similar result as inhibition of hippocampal CA1 projections from the LC, namely modulation of the threshold of LTP/LTD. Accordingly, other authors have shown that NA enhances the excitatory effects of DA in the prefrontal cortex (91). The synergic effect has been associated with the corelease of DA and NA from the LC, convergent innervation in the hippocampus, and shared intracellular signaling pathways (92). Our result showed that hippocampal DA extracellular concentrations modulate the LTP/LTD threshold associated with impaired spatial contextual memory updating.
This study provides information about the mechanisms underlying memory updating. This study’s clinical implications are related to treating problems associated with spatial contextual emotional memories. Specifically, some authors have observed that the administration of beta-adrenergic antagonists blocks the reconsolidation of drug addictions (12–14, 16, 28, 42). A meta-analysis showed clinical efficacy for intervention in memory disorders through reconsolidation impairment using PROP (93). Recent human studies have shown that after exposure to emotional pictures and associated context, administration of PROP before the reactivation session reduces the subsequent emotional effects (94). Additionally, functional magnetic resonance imaging showed increased hippocampal and amygdalar activity during recognition of cues related to emotional memory reactivation (94). Blocking beta-adrenergic receptors after memory reactivation by a familiar cue decreases the nicotine craving of smokers (15). Our study sheds light on understanding the underpinning of hippocampal catecholaminergic modulation in spatial contextual memory updating. Furthermore, in the future it would be interesting to evaluate the role of DA in modulating maladaptive memories in humans.
In conclusion, optogenetic inhibition of LC-CA1 fibers impairs spatial contextual memory updating. This photoinhibition modifies DA and NA concentrations in the hippocampus during behavioral and electrophysiological studies. These DA and NA changes are associated with receptor activation that unleashes several cellular mechanisms involved in memory updating. We demonstrated that beta-adrenoreceptors and DA D1/D5 receptors are required during behavioral expression, while memory updating relies only on DA D1/D5 receptors. In addition, by reducing DA and NA levels, we observed a threshold modification of LTP to LTD after performing HFS. Hence, we have shown that hippocampal catecholaminergic projections from the LC modulate spatial contextual recognition memory updating through DA release.
Materials and Methods
Animals were habituated to the experimental arena for three consecutive days, allowing them to explore the arena without stimulus objects (Habituation). In two training sessions, mice were introduced into the arena containing the same object configuration (Training). Twenty-four hours after training, one update session was conducted in which animals were reintroduced into the arena with one object placed in the original position and the other in a novel position (UPDATE). The following day, the last test session was completed using the same object configuration of the update session to measure memory updating (TEST) (24). Details on mice and procedures regarding behavioral protocols (24, 51, 95), optogenetics (51, 96–98), pharmacology (39, 44, 46), microdialysis (99), histology, electrophysiology and statistics are detailed in SI Appendix, Material and Methods.
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
We would like to thank Dr. Daniel Osorio Gómez for their comments on the manuscript and technical support. We are grateful to Dr. Rui M. Costa for the TH-Cre mice. We also thank the Unidad de Imagenología of the Instituto de Fisiología Celular, UNAM, especially Dr. Ruth Rincón Heredia and Dr. Abraham Rosas Arellano for technical support. D.K.G.-M. is a doctoral student from Programa de Doctorado en Ciencias Bioquímicas, UNAM and received the fellowship 573989 from Consejo Nacional de Ciencia y Tecnología (CONACYT), México. This project was supported by the CONACyT grants FOINS 474, CF-2023-I-189, and DGAPA-PAPIIT-UNAM grant IN212919 for F.B.-R.
Author contributions
D.K.G.-M., M.S.-M., P.M.-C., L.R.-D., M.L.E., F.T., and F.B.-R. designed research; D.K.G.-M. and M.S.-M. performed research; D.K.G.-M., L.R.-D., M.L.E., and F.B.-R. analyzed data; F.T. contributed new reagents/analytic tools; and D.K.G.-M. and F.B.-R. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission.
Data, Materials, and Software Availability
All study data are included in the article and/or SI Appendix.
Supporting Information
References
- 1.Lee J. L. C., Independent cellular processes for hippocampal memory consolidation and reconsolidation. Science 304, 839–843 (2004). [DOI] [PubMed] [Google Scholar]
- 2.Lee J. L. C., Nader K., Schiller D., An update on memory reconsolidation updating. Trends Cogn. Sci. 21, 531–545 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodriguez-Ortiz C. J., Bermúdez-Rattoni F., Determinants to trigger memory reconsolidation: The role of retrieval and updating information. Neurobiol. Learn. Mem. 142, 4–12 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Trent S., Barnes P., Hall J., Thomas K. L., Rescue of long-term memory after reconsolidation blockade. Nat. Commun. 6, 1–7 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balderas I., Rodriguez-Ortiz C. J., Bermudez-Rattoni F., Retrieval and reconsolidation of object recognition memory are independent processes in the perirhinal cortex. Neuroscience 253, 398–405 (2013). [DOI] [PubMed] [Google Scholar]
- 6.Flavell C. R., Barber D. J., Lee J. L. C., Behavioural memory reconsolidation of food and fear memories. Nat. Commun. 2, 504 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bermúdez-Rattoni F., McGaugh J. L., Memory reconsolidation and memory updating: Two sides of the same coin? Neurobiol. Learn. Mem. 142, 1–3 (2017). [DOI] [PubMed] [Google Scholar]
- 8.Nader K., Einarsson E. O., Memory reconsolidation: An update. Ann. N. Y. Acad. Sci. 1191, 27–41 (2010). [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez-Ortiz C. J., De la Cruz V., Gutiérrez R., Bermudez-Rattoni F., Protein synthesis underlies post-retrieval memory consolidation to a restricted degree only when updated information is obtained. Learn. Mem. Cold Spring Harb. N. 12, 533–537 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tronson N. C., Taylor J. R., Molecular mechanisms of memory reconsolidation. Nat. Rev. Neurosci. 8, 262–275 (2007). [DOI] [PubMed] [Google Scholar]
- 11.Lee J. L. C., Memory reconsolidation mediates the strengthening of memories by additional learning. Nat. Neurosci. 11, 1264–1266 (2008). [DOI] [PubMed] [Google Scholar]
- 12.Goltseker K., Bolotin L., Barak S., Counterconditioning during reconsolidation prevents relapse of cocaine memories. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 42, 716–726 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olshavsky M., et al. , Updating appetitive memory during reconsolidation window: Critical role of cue-directed behavior and amygdala central nucleus. Front. Behav. Neurosci. 7, 186 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elsey J. W. B., et al. , Reconsolidation-based treatment for fear of public speaking: A systematic pilot study using propranolol. Transl. Psychiatry 10, 1–12 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin X., et al. , Neural substrates of propranolol-induced impairments in the reconsolidation of nicotine-associated memories in smokers. Transl. Psychiatry 11, 1–10 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roullet P., et al. , Traumatic memory reactivation with or without propranolol for PTSD and comorbid MD symptoms: A randomised clinical trial. Neuropsychopharmacology 46, 1643–1649 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Germeroth L. J., et al. , Effect of a brief memory updating intervention on smoking behavior. JAMA Psychiatry 74, 214–223 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xue Y.-X., et al. , Effect of selective inhibition of reactivated nicotine-associated memories with propranolol on nicotine craving. JAMA Psychiatry 74, 224–232 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lonergan M. H., Olivera-Figueroa L. A., Pitman R. K., Brunet A., Propranolol’s effects on the consolidation and reconsolidation of long-term emotional memory in healthy participants: A meta-analysis. J. Psychiatry Neurosci. JPN 38, 222–231 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown M. W., Aggleton J. P., Recognition memory: What are the roles of the perirhinal cortex and hippocampus?. Nat. Rev. Neurosci. 2, 51–61 (2001). [DOI] [PubMed] [Google Scholar]
- 21.Yonelinas A. P., Otten L. J., Shaw K. N., Rugg M. D., Separating the brain regions involved in recollection and familiarity in recognition memory. J. Neurosci. Off. J. Soc. Neurosci. 25, 3002–3008 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moreno-Castilla P., Guzman-Ramos K., Bermudez-Rattoni F., “Object recognition and object location recognition memory – the role of dopamine and noradrenaline” in Handbook of Behavioral Neuroscience, Handbook of Object Novelty Recognition, Ennaceur A., de Souza Silva M. A., Eds. (Elsevier, 2018), chap. 28, pp. 403–413. [Google Scholar]
- 23.Lee J. L. C., Memory reconsolidation mediates the updating of hippocampal memory content. Front. Behav. Neurosci. 4, (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwapis J. L., et al. , Aging mice show impaired memory updating in the novel OUL updating paradigm. Neuropsychopharmacology 45, 337–346 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moreno-Castilla P., Pérez-Ortega R., Violante-Soria V., Balderas I., Bermúdez-Rattoni F., Hippocampal release of dopamine and norepinephrine encodes novel contextual information. Hippocampus 27, 547–557 (2017). [DOI] [PubMed] [Google Scholar]
- 26.Rossato J. I., Bevilaqua L. R. M., Medina J. H., Izquierdo I., Cammarota M., Retrieval induces hippocampal-dependent reconsolidation of spatial memory. Learn. Mem. 13, 431–440 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morris R. G. M., et al. , Memory reconsolidation: Sensitivity of spatial memory to inhibition of protein synthesis in dorsal hippocampus during encoding and retrieval. Neuron 50, 479–489 (2006). [DOI] [PubMed] [Google Scholar]
- 28.Winocur G., Frankland P. W., Sekeres M., Fogel S., Moscovitch M., Changes in context-specificity during memory reconsolidation: Selective effects of hippocampal lesions. Learn. Mem. 16, 722–729 (2009). [DOI] [PubMed] [Google Scholar]
- 29.Ljungberg T., Apicella P., Schultz W., Responses of monkey dopamine neurons during learning of behavioral reactions. J. Neurophysiol. 67, 145–163 (1992). [DOI] [PubMed] [Google Scholar]
- 30.Lisman J., Grace A. A., Duzel E., A neoHebbian framework for episodic memory; role of dopamine-dependent late LTP. Trends Neurosci. 34, 536–547 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schultz W., Multiple dopamine functions at different time courses. Annu. Rev. Neurosci. 30, 259–288 (2007). [DOI] [PubMed] [Google Scholar]
- 32.Gonzalez M. C., Rossato J. I., Radiske A., Bevilaqua L. R. M., Cammarota M., Dopamine controls whether new declarative information updates reactivated memories through reconsolidation. Proc. Natl. Acad. Sci. U.S.A. 118, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Assini F. L., Duzzioni M., Takahashi R. N., Object location memory in mice: Pharmacological validation and further evidence of hippocampal CA1 participation. Behav. Brain Res. 204, 206–211 (2009). [DOI] [PubMed] [Google Scholar]
- 34.Murai T., Okuda S., Tanaka T., Ohta H., Characteristics of object location memory in mice: Behavioral and pharmacological studies. Physiol. Behav. 90, 116–124 (2007). [DOI] [PubMed] [Google Scholar]
- 35.Berridge C. W., Abercrombie E. D., Relationship between locus coeruleus discharge rates and rates of norepinephrine release within neocortex as assessed by in vivo microdialysis. Neuroscience 93, 1263–1270 (1999). [DOI] [PubMed] [Google Scholar]
- 36.Hansen N., The longevity of hippocampus-dependent memory is orchestrated by the locus coeruleus-noradrenergic system. Neural Plast. 2017, e2727602. (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gasbarri A., Verney C., Innocenzi R., Campana E., Pacitti C., Mesolimbic dopaminergic neurons innervating the hippocampal formation in the rat: A combined retrograde tracing and immunohistochemical study. Brain Res. 668, 71–79 (1994). [DOI] [PubMed] [Google Scholar]
- 38.Lisman J. E., Grace A. A., The hippocampal-VTA loop: Controlling the entry of information into long-term memory. Neuron 46, 703–713 (2005). [DOI] [PubMed] [Google Scholar]
- 39.Kempadoo K. A., Mosharov E. V., Choi S. J., Sulzer D., Kandel E. R., Dopamine release from the locus coeruleus to the dorsal hippocampus promotes spatial learning and memory. Proc. Natl. Acad. Sci. U.S.A. 113, 14835–14840 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takeuchi T., et al. , Locus coeruleus and dopaminergic consolidation of everyday memory. Nature 537, 357–362 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moncada D., Viola H., Induction of long-term memory by exposure to novelty requires protein synthesis: evidence for a behavioral tagging. J. Neurosci. 27, 7476–7481 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang S.-H., Novelty enhances memory persistence and remediates propranolol-induced deficit via reconsolidation. Neuropharmacology 141, 42–54 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun H., Mao Y., Wang J., Ma Y., Effects of beta-adrenergic antagonist, propranolol on spatial memory and exploratory behavior in mice. Neurosci. Lett. 498, 133–137 (2011). [DOI] [PubMed] [Google Scholar]
- 44.Wang F., et al. , Dopamine in the hippocampal dentate gyrus modulates spatial learning via D1-like receptors. Brain Res. Bull. 144, 101–107 (2019). [DOI] [PubMed] [Google Scholar]
- 45.Broussard J. I., et al. , Dopamine regulates aversive contextual learning and associated in vivo synaptic plasticity in the hippocampus. Cell Rep. 14, 1930–1939 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murchison C. F., Schutsky K., Jin S.-H., Thomas S. A., Norepinephrine and ß1adrenergic signaling facilitate activation of hippocampal CA1 pyramidal neurons during contextual memory retrieval. Neuroscience 181, 109–116 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hansen N., Manahan-Vaughan D., Hippocampal long-term potentiation that is elicited by perforant path stimulation or that occurs in conjunction with spatial learning is tightly controlled by beta-adrenoreceptors and the locus coeruleus. Hippocampus 25, 1285–1298 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Granado N., et al. , D1 but not D5 dopamine receptors are critical for LTP, spatial learning, and LTP-Induced arc and zif268 expression in the hippocampus. Cereb. Cortex N. Y. N. 1991 18, 1–12 (2008). [DOI] [PubMed] [Google Scholar]
- 49.Balderas I., Rodriguez-Ortiz C. J., Bermudez-Rattoni F., Consolidation and reconsolidation of object recognition memory. Behav. Brain Res. 285, 213–222 (2015). [DOI] [PubMed] [Google Scholar]
- 50.Rossato J. I., et al. , State-dependent effect of dopamine D1/D5 receptors inactivation on memory destabilization and reconsolidation. Behav. Brain Res. 285, 194–199 (2015). [DOI] [PubMed] [Google Scholar]
- 51.Gil-Lievana E., et al. , Glutamatergic basolateral amygdala to anterior insular cortex circuitry maintains rewarding contextual memory. Commun. Biol. 3, 139 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Devoto P., Flore G., Saba P., Fà M., Gessa G. L., Co-release of noradrenaline and dopamine in the cerebral cortex elicited by single train and repeated train stimulation of the locus coeruleus. BMC Neurosci. 6, 31 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smith C. C., Greene R. W., CNS dopamine transmission mediated by noradrenergic innervation. J. Neurosci. Off. J. Soc. Neurosci. 32, 6072–6080 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guzmán-Ramos K., et al. , Restoration of dopamine release deficits during object recognition memory acquisition attenuates cognitive impairment in a triple transgenic mice model of Alzheimer’s disease. Learn. Mem. Cold Spring Harb. N. 19, 453–460 (2012). [DOI] [PubMed] [Google Scholar]
- 55.Fog J. U., et al. , Calmodulin kinase II Interacts with the dopamine transporter C terminus to regulate amphetamine-induced reverse transport. Neuron 51, 417–429 (2006). [DOI] [PubMed] [Google Scholar]
- 56.Sonneborn A.,Greene R. W.,Norepinephrine transporter antagonism prevents dopamine-dependent synaptic plasticity in the mouse dorsal hippocampus. Neurosci. Lett. 740, 135450 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rodriguez-Ortiz C. J., Balderas I., Garcia-DeLaTorre P., Bermudez-Rattoni F., Taste aversion memory reconsolidation is independent of its retrieval. Neurobiol. Learn. Mem. 98, 215–219 (2012). [DOI] [PubMed] [Google Scholar]
- 58.Morilak D. A., et al. , Role of brain norepinephrine in the behavioral response to stress. Prog. Neuropsychopharmacol. Biol. Psychiatry 29, 1214–1224 (2005). [DOI] [PubMed] [Google Scholar]
- 59.Ben Mamou C., Gamache K., Nader K., NMDA receptors are critical for unleashing consolidated auditory fear memories. Nat. Neurosci. 9, 1237–1239 (2006). [DOI] [PubMed] [Google Scholar]
- 60.Delorenzi A., et al. , Memory beyond expression. J. Physiol. Paris 108, 307–322 (2014). [DOI] [PubMed] [Google Scholar]
- 61.Osorio-Gómez D., Guzmán-Ramos K., Bermúdez-Rattoni F., Differential involvement of glutamatergic and catecholaminergic activity within the amygdala during taste aversion retrieval on memory expression and updating. Behav. Brain Res. 307, 120–125 (2016). [DOI] [PubMed] [Google Scholar]
- 62.Murchison C. F., et al. , A distinct role for norepinephrine in memory retrieval. Cell 117, 131–143 (2004). [DOI] [PubMed] [Google Scholar]
- 63.Aston-Jones G., Rajkowski J., Kubiak P., Conditioned responses of monkey locus coeruleus neurons anticipate acquisition of discriminative behavior in a vigilance task. Neuroscience 80, 697–715 (1997). [DOI] [PubMed] [Google Scholar]
- 64.Kroes M. C. W., Strange B. A., Dolan R. J., Beta-adrenergic blockade during memory retrieval in humans evokes a sustained reduction of declarative emotional memory enhancement. J. Neurosci. Off. J. Soc. Neurosci. 30, 3959–3963 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Otis J. M., Fitzgerald M. K., Mueller D., Inhibition of hippocampal β-adrenergic receptors impairs retrieval but not reconsolidation of cocaine-associated memory and prevents subsequent reinstatement. Neuropsychopharmacology 39, 303–310 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Aston-Jones G., Cohen J. D., Adaptive gain and the role of the locus coeruleus-norepinephrine system in optimal performance. J. Comp. Neurol. 493, 99–110 (2005). [DOI] [PubMed] [Google Scholar]
- 67.Berridge C. W., Waterhouse B. D., The locus coeruleus-noradrenergic system: Modulation of behavioral state and state-dependent cognitive processes. Brain Res. Brain Res. Rev. 42, 33–84 (2003). [DOI] [PubMed] [Google Scholar]
- 68.Tsukahara J. S., Engle R. W., Fluid intelligence and the locus coeruleus-norepinephrine system. Proc. Natl. Acad. Sci. U.S.A. 118, e2110630118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dudai Y., The restless engram: Consolidations never end. Annu. Rev. Neurosci. 35, 227–247 (2012). [DOI] [PubMed] [Google Scholar]
- 70.McGaugh J. L., Time-dependent processes in memory storage. Science 153, 1351–1358 (1966). [DOI] [PubMed] [Google Scholar]
- 71.Epping-Jordan M. P., Markou A., Koob G. F., The dopamine D-1 receptor antagonist SCH 23390 injected into the dorsolateral bed nucleus of the stria terminalis decreased cocaine reinforcement in the rat. Brain Res. 784, 105–115 (1998). [DOI] [PubMed] [Google Scholar]
- 72.Bellfy L., Kwapis J. L., Molecular mechanisms of reconsolidation-dependent memory updating. Int. J. Mol. Sci. 21, E6580 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wideman C. E., Jardine K. H., Winters B. D., Involvement of classical neurotransmitter systems in memory reconsolidation: Focus on destabilization. Neurobiol. Learn. Mem. 156, 68–79 (2018). [DOI] [PubMed] [Google Scholar]
- 74.Hong I., et al. , AMPA receptor exchange underlies transient memory destabilization on retrieval. Proc. Natl. Acad. Sci. U.S.A. 110, 8218–8223 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ferrara N. C., et al. , GluR2 endocytosis-dependent protein degradation in the amygdala mediates memory updating. Sci. Rep. 9, 5180 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hasegawa S., et al. , Hippocampal clock regulates memory retrieval via Dopamine and PKA-induced GluA1 phosphorylation. Nat. Commun. 10, 5766 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gonzalez M. C., Rossato J. I., Radiske A., Pádua Reis M., Cammarota M., Recognition memory reconsolidation requires hippocampal Zif268. Sci. Rep. 9, 16620 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bhat R. V., Cole A. J., Baraban J. M., Role of monoamine systems in activation of zif268 by cocaine. J. Psychiatry Neurosci. JPN 17, 94–102 (1992). [PMC free article] [PubMed] [Google Scholar]
- 79.Baltaci S. B., Mogulkoc R., Baltaci A. K., Molecular mechanisms of early and late LTP. Neurochem. Res. 44, 281–296 (2019). [DOI] [PubMed] [Google Scholar]
- 80.Stuchlik A., Dynamic learning and memory, synaptic plasticity and neurogenesis: An update. Front. Behav. Neurosci. 8, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Reid A. T., Harley C. W., An associativity requirement for locus coeruleus-induced long-term potentiation in the dentate gyrus of the urethane-anesthetized rat. Exp. Brain Res. 200, 151–159 (2010). [DOI] [PubMed] [Google Scholar]
- 82.Quinlan M. A. L., et al. , Locus coeruleus optogenetic light activation induces long-term potentiation of perforant path population spike amplitude in rat dentate gyrus. Front. Syst. Neurosci. 12, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li S., Cullen W. K., Anwyl R., Rowan M. J., Dopamine-dependent facilitation of LTP induction in hippocampal CA1 by exposure to spatial novelty. Nat. Neurosci. 6, 526–531 (2003). [DOI] [PubMed] [Google Scholar]
- 84.Maity S., Rah S., Sonenberg N., Gkogkas C. G., Nguyen P. V., Norepinephrine triggers metaplasticity of LTP by increasing translation of specific mRNAs. Learn. Mem. 22, 499–508 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Huang Y. Y., Kandel E. R., Modulation of both the early and the late phase of mossy fiber LTP by the activation of beta-adrenergic receptors. Neuron 16, 611–617 (1996). [DOI] [PubMed] [Google Scholar]
- 86.Kemp A., Manahan-Vaughan D., Beta-adrenoreceptors comprise a critical element in learning-facilitated long-term plasticity. Cereb. Cortex N. Y. N. 1991 18, 1326–1334 (2008). [DOI] [PubMed] [Google Scholar]
- 87.Papaleonidopoulos V., Kouvaros S., Papatheodoropoulos C., Effects of endogenous and exogenous D1/D5 dopamine receptor activation on LTP in ventral and dorsal CA1 hippocampal synapses. Synapse 72, e22033 (2018). [DOI] [PubMed] [Google Scholar]
- 88.Argilli E., Sibley D. R., Malenka R. C., England P. M., Bonci A., Mechanism and time course of cocaine-induced long-term potentiation in the ventral tegmental area. J. Neurosci. 28, 9092–9100 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kolomiets B., Marzo A., Caboche J., Vanhoutte P., Otani S., Background dopamine concentration dependently facilitates long-term potentiation in rat prefrontal cortex through postsynaptic activation of extracellular signal-regulated kinases. Cereb. Cortex N. Y. N. 1991 19, 2708–2718 (2009). [DOI] [PubMed] [Google Scholar]
- 90.Otani S., Bai J., Blot K., Dopaminergic modulation of synaptic plasticity in rat prefrontal neurons. Neurosci. Bull. 31, 183–190 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ranjbar-Slamloo Y., Fazlali Z., Dopamine and noradrenaline in the brain; overlapping or dissociate functions? Front. Mol. Neurosci. 12, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shinohara F., Arakaki S., Amano T., Minami M., Kaneda K., Noradrenaline enhances the excitatory effects of dopamine on medial prefrontal cortex pyramidal neurons in rats. Neuropsychopharmacol. Rep. 40, 348–354 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pigeon S., Lonergan M., Rotondo O., Pitman R. K., Brunet A., Impairing memory reconsolidation with propranolol in healthy and clinical samples: A meta-analysis. J. Psychiatry Neurosci. JPN 47, E109–E122 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schwabe L., Nader K., Wolf O. T., Beaudry T., Pruessner J. C., Neural signature of reconsolidation impairments by propranolol in humans. Biol. Psychiatry 71, 380–386 (2012). [DOI] [PubMed] [Google Scholar]
- 95.Dong H. W., The Allen reference atlas: A digital color brain atlas of the C57Bl/6J male mouse (John Wiley & Sons Inc, 2008). [Google Scholar]
- 96.Chen B. T., et al. , Rescuing cocaine-induced prefrontal cortex hypoactivity prevents compulsive cocaine seeking. Nature 496, 359–362 (2013). [DOI] [PubMed] [Google Scholar]
- 97.Clarkson J., et al. , Definition of the hypothalamic GnRH pulse generator in mice. Proc. Natl. Acad. Sci. U.S.A. 114, E10216–E10223 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kang M. S., Han J.-H., Optogenetic inhibition of medial entorhinal cortex inputs to the hippocampus during a short period of time right after learning disrupts contextual fear memory formation. Mol. Brain 14, 2 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Guzmán-Ramos K., Osorio-Gómez D., Moreno-Castilla P., Bermúdez-Rattoni F., Off-line concomitant release of dopamine and glutamate involvement in taste memory consolidation. J. Neurochem. 114, 226–236 (2010). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
All study data are included in the article and/or SI Appendix.