Abstract
Background and Aims
Hunger, satiation, postprandial satiety, and hedonic eating constitute key food intake parameters. We aim to study whether these symptoms are associated with gastrointestinal symptoms (GIS) in patients with obesity.
Methods
This is a cross-sectional study of patients with obesity. Patients completed the following validated biomarkers and questionnaires: hunger was measured via visual analog scale (100 mm) following a standard meal, satiation was measured via ad libitum meal (calories to fullness; kcal), postprandial satiety was measured via gastric emptying scintigraphy (T1/2; mins), and hedonic eating was measured via the Hospital Anxiety and Depression Scale questionnaire. Participants completed the abridged Bowel Disease Questionnaire to evaluate their GIS. We calculated the odds ratios (ORs) adjusted for sex, weight, and age between food intake parameters <25th or >75th percentile observed in a prior cohort of 450 participants with obesity and GIS.
Results
A total of 274 participants (41 ± 10 [SD] years, 75% females, body mass index 39 ± 8 kg/m2) were included in the analysis. Increased hunger was associated with a lower prevalence of lumpy stools (OR = 0.18, P = .02). Satiation was associated with abdominal pain/discomfort (relieved by defecation [OR = 2.4, P = .02] or associated with change in stool consistency [OR = 2.92, P < .01]), loose/watery stools (OR = 2.09, P = .02), and bloating (OR = 2.49, P < .01). Abnormal postprandial satiety was associated with bloating (OR = 2.26, P < .01) and loose/watery stools (OR = 1.84, P = .04). Hedonic eating was associated with abdominal pain/discomfort with stool frequency change (OR = 2.4, P = .02), >3 bowel movements per day (OR = 1.93, P = .048), bloating (OR = 2.49, P = .01), abdominal pain after meals >1 per month (OR = 4.24, P < .01), and nausea >1 per week (OR = 4.51, P < .01).
Conclusion
Alterations in hunger, satiation, postprandial satiety, and hedonic eating are associated with GIS in patients with obesity.
Keywords: Obesity, Hunger, Satiation, Postprandial Satiety, Hedonic Eating
Introduction
Obesity is a chronic multifactorial disease that results from increased energy intake and/or decreased energy expenditure. Energy balance is governed by food intake and energy expenditure. An imbalance between these 2 key factors can lead to weight gain. Food intake is regulated by homeostatic and hedonic factors; homeostatic factors can be further divided into 3 stages: hunger, satiation, and postprandial satiety.1 Hunger is an internal motivational state elicited by a lack of nutrients in the body, which drives eating and food-seeking behavior.2 Satiation is the process that brings an eating episode to an end,3 whereas postprandial satiety is the constellation of sensations that inhibits eating in the postprandial period,1,4 and it is reflected objectively by gastric emptying (GE) time.5 In addition, hedonic eating is the desire to eat solely to elicit pleasurable feelings regardless of the individual’s nutritional status.6 Alterations in hunger, satiation, postprandial satiety, and hedonic eating contribute to food intake symptoms. These symptoms led to the development of a phenotype-guided method, which differentiates the causes of obesity based on the pathogenesis: satiation, postprandial satiety, hedonic eating, and resting energy expenditure.7 Importantly, these alterations have been only studied in patients with obesity or overweight. Such classification contributes to a better understanding5,8 and treatment of obesity.7
Obesity affects almost every system in the body, raising the risk of a variety of illnesses.9 It can either be the primary cause, as seen in nonalcoholic fatty liver disease,10,11 or a substantial risk factor for numerous gastrointestinal (GI) and hepatic diseases such as reflux esophagitis caused by gastroesophageal reflux disease.11, 12, 13 Low-grade chronic inflammation, fluctuations in GI hormones, and adipose tissue redistribution in the abdominal cavity contribute to GI morbidity in obesity.11 Moreover, several studies show that food intake results in a significant colonic response change (eg, fat composition).14, 15, 16 Obesity was also shown to be associated with chronic symptoms, including dyspepsia, upper abdominal pain, diarrhea, heartburn, vomiting, and retching.17 Furthermore, weight loss can play a possible role in the improvement of common GI symptoms in patients with obesity, such as gastroesophageal reflux disease,18 abdominal distention, diarrhea, and constipation.19
Previously, satiation and satiety tests have been used to explore the prevalence of dyspepsia in the community.20,21 Postprandial fullness and early satiation are typical dyspeptic symptoms that have been investigated22,23 using physiological GI tests such as GE of solids and liquids, gastric volumes, and liquid nutrient meals. However, little is known about the association between altered hunger, satiation, postprandial satiety, and hedonic eating with functional GI symptoms in patients with obesity. Although several studies measure the correlation between obesity and functional GI symptoms,17,24, 25, 26, 27 none explain their association with food intake symptoms. Moreover, these studies are limited by inconsistent conclusions,28,29 selection bias,29 young-age participants,26 small sample size,30 and using diverse tools to measure the same objective parameters (ie, GE).28 In addition, none of the studies in the literature simultaneously examined food intake symptoms and their relationship with common GI symptoms in obesity. We hypothesized that food intake symptoms (eg, altered hunger, satiation, postprandial satiety, and hedonic eating) are associated with diverse functional GI symptoms in obesity.
Methods
Study Design and Participants
We performed a cross-sectional study analyzing baseline characteristics of adult participants aged between 18 and 65 years with obesity (body mass index >30 kg/m2) with no evidence of any chronic gastrointestinal diseases, use of medications that may alter gastrointestinal motility, appetite or absorption, active psychiatric symptoms, eating disorders (eg, bulimia, binge eating disorder), or alcohol use disorder. This study was approved by the institutional human research review committee at Mayo Clinic. The participants were recruited from the community using standard advertisement, and here, we report the baseline characteristic of participants enrolled in the ClinicalTrials.gov NCT03374956 trial. In this study, all the physiological studies (ie, GE and ad libitum meal) and questionnaires were completed to assess the baseline characteristics of our patients before starting the clinical trial. All authors had access to the study data and reviewed and approved the final manuscript.
Measurements
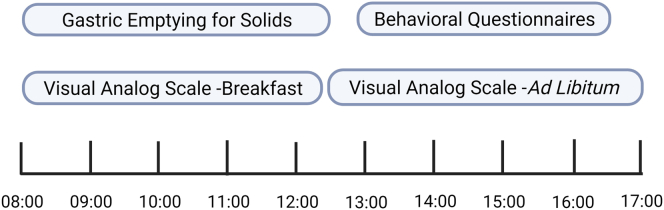
All tests were performed at the Mayo Clinic Clinical Research and Trial Unit after an 8-hour fasting period (Figures 1 and 2):
-
A.
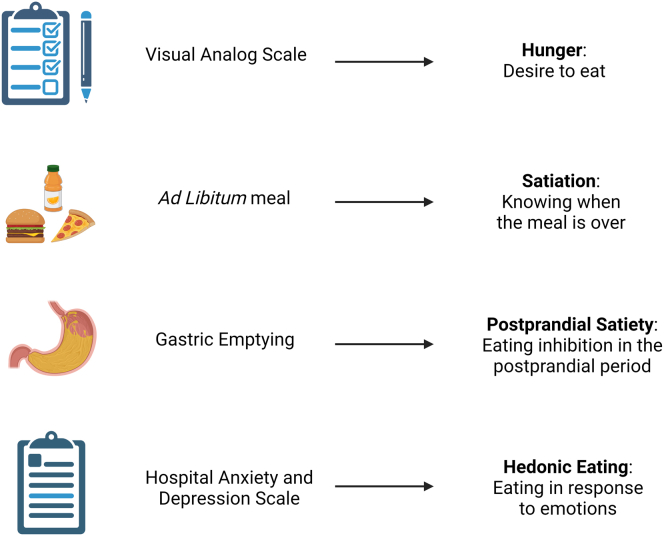
Hunger was evaluated using 100 mm visual analog scale after 240 minutes of a 320-kcal standard breakfast meal.5
-
B.
Satiation was assessed by measuring calories to fullness during an ad libitum meal.7
-
C.
Postprandial satiety was studied via measuring the gastric emptying (GE T1/2) by scintigraphy for a total of 240 minutes after radiolabeled solid (320 kcal, 30% fat) standard breakfast. Postprandial satiety has been previously associated with rapid GE by Gonzalez-Izundegui et al.5
-
D.
Hedonic eating was evaluated using the Hospital Anxiety and Depression Scale (HADS) questionnaire.7 A correlation between HADS anxiety score and the 3-factor eating questionnaire (emotional eating factor; r = .36) has been recently established. In addition, higher HADS anxiety scores were associated with emotional and uncontrolled eating (P < .001 for both) and lower levels of cognitive restraint (P = .04).31
Figure 1.
The flowchart of the testing day.
Figure 2.
The food intake parameters and their assessment methods.
Participants completed the abridged Bowel Disease Questionnaire, a 16-item form that has been used to evaluate various functional GI symptoms (Supplementary Material).32
Statistical Analysis
Using standard quantile regression approach to identify normal range33 and based on the fact that these variables are different in obesity when compared with healthy controls,34 the abnormal traits in the key components of food intake were determined based on quartiles—25th or 75th percentile as observed from our previous study.7 Thus, the cutoffs were increased hunger was defined as visual analog scale hunger: >80 mm for females and >87 mm for males; abnormal satiation was defined as ad libitum meal test >970 kcal for females and >1359 kcal for males; accelerated GE, which is a biomarker of abnormal postprandial satiety, was defined as <25th percentile of GE T1/2: <106 minutes for females and <87 minutes for males; and hedonic eating was defined with a score >7 for HADS-Anxiety for both sexes (Table 1). We used a multivariate logistic regression model to calculate the odds ratios (ORs) and 95% confidence intervals (CIs) associating hunger, satiation, postprandial satiety, and hedonic eating with the abridged Bowel Disease Questionnaire results while adjusting for sex, weight, and age. Statistical significance was set at 2-sided P < .05. We used JMP, Version 14.3.0 (SAS Institute Inc., Cary, NC, 1989–2019) to perform the statistical analysis. Data are summarized as mean (standard deviation).
Table 1.
Cohort Distribution of Participants Adjusted by Sex Upon the Food Intake Tests
| Food intake tests | Abnormal value |
Cutoff | |
|---|---|---|---|
| Female | Male | ||
| Hunger (VAS, mm) | >80 | >87 | 75% |
| Satiation (ad Libitum meal, kcal) | >970 | >1359 | 75% |
| Postprandial satiety (gastric emptying T½, min) | <106 | <87 | 25% |
| Hedonic eating behavior (HADS, score) | >7 | >7 | |
HADS, Hospital Anxiety and Depression Scale; VAS, visual analog scale.
Results
Participants Demographics
A total of 274 participants with obesity were recruited for this study. Our participants were predominantly females (75%), mean age 40.7 (10.3) years, and body mass index 39.2 (7.5) kg/m2. The distribution of our participants among the hunger, satiation, postprandial satiety, and hedonic eating groups is shown in Table 2.
Table 2.
Demographic Distribution of the Hunger, Satiation, Postprandial Satiety, and Hedonic Eating Groups of Participants
| Demographics | Hunger |
Satiation |
Postprandial satiety |
Hedonic eating |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Normal | Abnormal | P value | Normal | Abnormal | P value | Normal | Abnormal | P value | Normal | Abnormal | P value | |
| Participants, n | 215 | 51 | 205 | 68 | 205 | 69 | 222 | 51 | ||||
| Age, y | 41 ± 10 | 40 ± 10 | .76 | 41 ± 10 | 40 ± 10 | .24 | 41 ± 10 | 41 ± 11 | .65 | 41 ± 10 | 39 ± 11 | .1 |
| Sex, female (%) | 155 (72) | 45 (88) | .02 | 154 (75) | 51 (75) | 1 | 154 (75) | 52 (75) | 1 | 167 (75) | 38 (75) | 1 |
| Weight, kg | 114 ± 26 | 108 ± 21 | .09 | 110 ± 25 | 119 ± 25 | .02 | 112 ± 24 | 113 ± 27 | .72 | 113 ± 26 | 113 ± 20 | .94 |
| BMI, kg/m2 | 40 ± 7.6 | 38.5 ± 7.3 | .70 | 41 ± 8.3 | 38.7 ± 7.2 | .05 | 39 ± 7.4 | 40 ± 8 | .70 | 39 ± 7.8 | 39 ± 6.5 | .8 |
Bold value refers to statistical significance with a P-value <.05.
BMI, body mass index.
Association Between Food Intake and GI Symptoms
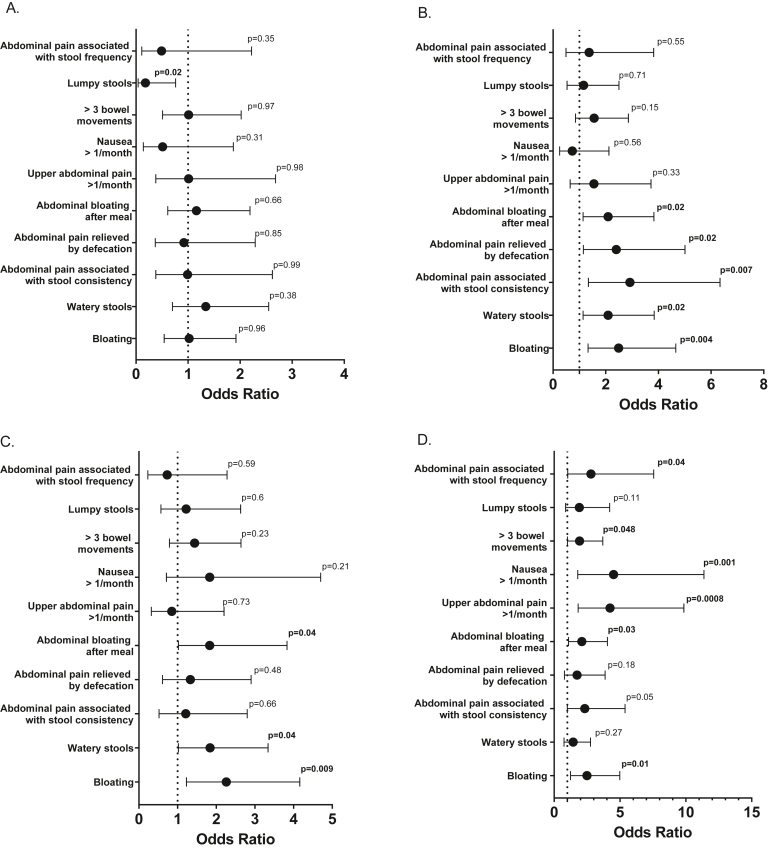
Increased hunger was associated with a lower prevalence of lumpy stools (OR 0.18, 95% CI 0.04–0.76; P = .02; Figure 3A).
Figure 3.
The odds ratio of gastrointestinal symptoms based in the Bowel Disease Questionnaire in patients with obesity and abnormal hunger (A), satiation (B), postprandial satiety (C), or hedonic eating (D).
Abnormal satiation was associated with a higher frequency for ≥3 months of continuous or recurrent symptoms of abdominal pain or discomfort that is relieved by defecation (OR 2.4, 95% CI 1.15–5.01; P = .02) or associated with change in stool consistency (OR 2.92, 95% CI 1.34–6.34 P < .01), bloating (OR 2.49, 95% CI 1.33–4.66; P < .01), bloating after meals (OR 2.09, 95% CI 1.14–3.83; P = .02), and loose/watery stools (OR 2.09, 95% CI 1.14–3.84; P = .02; Figure 3B).
Abnormal postprandial satiety was associated with a higher prevalence of bloating (OR 2.26, 95% CI 1.23–4.2; P < .01), bloating after meals (OR 1.83, 95% CI 1.02–3.29; P = .04), and loose/watery stools (OR 1.84, 95%CI 1.02–3.34; P = .04; Figure 3C).
Hedonic eating was associated with a higher frequency for ≥3 months of continuous or recurrent symptoms of abdominal pain or discomfort associated with a change in stool frequency (OR 2.4, 95% CI 1.15–5.01; P = .02), >3 bowel movements per day (OR 1.93, 95% CI 1.005–3.692; P = .048), bloating (OR 2.49, 95% CI 1.24–5; P = .01), upper abdominal pain after meals more than once a month (OR 4.24, 95% CI 1.82–9.85; P < .01), bloating after meals (OR 2.1, 95%CI 1.1–4.04; P = .03), and nausea regularly more than once a week (OR 4.51, 95% CI 1.79–11.37; P < .01; Figure 3D).
Discussion
Our present study shows an association between symptoms associated with food intake and chronic functional GI symptoms in patients with obesity. These GI symptoms, which are prevalent in obesity,17 seem to be linked to specific alterations in parameters of food intake. In fact, these symptoms associated with food intake are found to be coexisting with a wide range of upper (bloating, abdominal pain, nausea) and lower (diarrhea) GI symptoms.
Functional GI symptoms and obesity are highly prevalent in adults.17,24 Several studies show a high prevalence of various symptoms (ie, bloating and diarrhea) in patients with obesity.35 Here, we showed the association of each component of food intake with GI symptoms. These components were labeled as increased or decreased based on the data in an independent cohort of 450 adults with obesity, from which we proposed a pathophysiological and behavioral phenotype-based classification of obesity.7 The correlation with GI symptoms is of potential clinical importance. First, it might provide the rationale to propose treatments to address both the obesity phenotype as well as postprandial symptoms. For example, it would be advantageous to treat abnormal postprandial satiety (associated with accelerated GE) and watery diarrhea with an agent that delays gastrointestinal motility and GE such as a glucagon-like peptide 1 agonist.36,37 A second rationale is that both abnormal food intake and GI symptoms are highly prevalent and may coexist in the same person; thus, abnormal satiation is present in 32% of patients with obesity,7 and bloating is reported in 31% of the general population.38
Hunger and Lumpy Stools
The association between increased hunger and lower prevalence of lumpy stools may be explained by diverse mechanisms. Hunger is one of the driving factors of food intake39 and perhaps craving for high fat intake, which can stimulate a colonic motor response40 and increased colonic phasic contractile activity41 and therefore avoidance of constipation42 or the associated lumpy stools (type 1 or 2 on the Bristol Stool Form Scale43). Increased hunger may also reflect a higher level of ghrelin in the body.44 Ghrelin and its analogs (eg, relamorelin) regulate GI motility by accelerating gastric and intestinal motility45,46 and may play a potential role in treating constipation by stimulating gastrointestinal motility47 and significantly changing stool consistency as previously shown in a placebo-controlled trial of relamorelin.48
Satiation and GI Symptoms
Abnormal satiation in this study is reflected in increased intake of calories at an ad libitum meal; the sensation of bloating in patients with abnormal satiation is poorly understood, but it is conceivable that it reflects increased visceral afferent activation as occurs with dyspeptic symptoms49 and may result in changes in eating habits.50
Continuous or recurrent abdominal pain or discomfort for at least 6 months associated with a change in stool frequency, change in stool form, and related to defecation are the 3 Rome IV criteria for irritable bowel syndrome (IBS) classification. Any patient with ≥2 of these bowel function symptoms is sufficient for IBS diagnosis.51 Our study showed that participants with abnormally higher satiation level (ie, increased kcal intake) are more likely to have abdominal pain associated with change in stool frequency and/or relieved by defecation. However, other studies show that patients with IBS have a similar satiation level compared with healthy individuals.52 Similarly, ingesting certain types of food in high quantities can promote osmotic diarrhea, which can partially explain the high prevalence of diarrhea in patients with obesity. Patients with obesity and abnormal satiation are more likely to ingest a greater quantity of food27 containing poorly absorbed sugars (ie, fructose corn syrup) that can contribute to diarrhea.
Postprandial Satiety and GI Symptoms
Patients with rapid GE may present with dyspepsia symptoms (eg, bloating),13,53 possibly as a result of rapid transit of hyperosmolar food into the duodenum, as occurs in dumping syndrome,54 which is associated with bloating especially after meals.55
In a study on patients with chronic diarrhea, Charles et al showed a higher prevalence of rapid GE, which may be a possible mechanism of diarrhea in patients with functional bowel disorders.56 Similarly, in our study, patients with accelerated GE were more likely to report watery, loose stools.
Hedonic Eating and GI Symptoms
Anxiety has been previously linked to increased food intake in a subset of patients with obesity,7 and it has been associated with bloating,57 functional abdominal pain,58 and nausea.59 In addition, previous studies show an increased susceptibility of hedonic eating in female patients, which account for 75% of our cohort.60
Strength and Limitations
The strengths of our study include the adequate sample size of participants who completed simultaneously during the same day the required tests and questionnaires, exclusion of patients with any GI disease and eating disorder, and the nature of the cross-sectional study, which limits the attrition bias.
This study also has several potential limitations. Considering the nature of our study, no casual inference can be made for any of the results. In fact, we cannot study the temporal relation between the food intake and the chronic GI symptoms. For example, it cannot be concluded whether higher levels of anxiety were due to GI symptoms in obesity or vice versa. In addition, our participants were required to fill in questionnaires, which makes the study more susceptible to recall bias. This study also included mostly White Americans and female patients, which limits the generalizability of our results to other populations.
Conclusion
In patients with obesity, homeostatic (hunger, satiation, and postprandial satiety) and hedonic components of food intake are associated with various chronic GI symptoms. These symptoms are known to be observed with a significantly higher prevalence in patients with obesity.28 Our study further shows an association between food intake and functional GI symptoms. This linkage requires more study to better understand, treat, and prevent the occurrence of such GI symptoms in obesity.
Footnotes
Authors' Contributions: Wissam Ghusn contributed to writing, reviewing, and editing the article, software, and methodology. Lizeth Cifuentes, Alejandro Campos, Daniel Sacoto, Alan De La Rosa, and Fauzi Feris contributed to reviewing and editing the article. Gerardo Calderon, Daniel Gonzalez-Izundegui, and Jessica Stutzman contributed to investigation and resources. Maria Daniela Hurtado and Michael Camilleri contributed to supervision. Andres Acosta contributed to funding acquisition and supervision.
Conflicts of Interest: These authors disclose the following: A.A. is a stockholder in Gila Therapeutics and Phenomix Sciences; he served as a consultant for Rhythm Pharmaceuticals, General Mills, and Amgen Pharmaceuticals. M.C. is a stockholder in Phenomix Sciences and a consultant Q6 to Kallyope (with compensation to Mayo Clinic). The remaining authors disclose no conflicts.
Funding: A.A. is supported by NIH (NIDDK K23-DK114460). M.C. is supported by NIH (NIDDKRO1 DK67071).
Ethical Statement: The corresponding author, on behalf of all authors, jointly and severally, certifies that their institution has approved the protocol for any investigation involving humans or animals and that all experimentation was conducted in conformity with ethical and humane principles of research.
Data Transparency Statement: Deidentified individual participant data can be shared upon request: 1) Data on participants’ tests and questionnaire results, Bowel Disease Questionnaire, 2) Data can be sent to the journal if needed, and 3) No limited timeframe.
Material associated with this article can be found in the online version at https://doi.org/10.1016/j.gastha.2022.07.019.
Supplementary Materials
References
- 1.Cifuentes L., Acosta A. Homeostatic regulation of food intake. Clin Res Hepatol Gastroenterol. 2021;46:101794. doi: 10.1016/j.clinre.2021.101794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin S., Senapati B., Tsao C.-H. Neural basis of hunger-driven behaviour in Drosophila. Open Biology. 2019;9:180259. doi: 10.1098/rsob.180259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellisle F., Drewnowski A., Anderson G.H., et al. Sweetness, satiation, and satiety. J Nutr. 2012;142:1149S–1154S. doi: 10.3945/jn.111.149583. [DOI] [PubMed] [Google Scholar]
- 4.Blundell J.E. Perspective on the central control of appetite. Obesity. 2006;14:160S–163S. doi: 10.1038/oby.2006.298. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez-Izundegui D., Campos A., Calderon G., et al. Association of gastric emptying with postprandial appetite and satiety sensations in obesity. Obesity. 2021;29:1497–1507. doi: 10.1002/oby.23204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monteleone P., Piscitelli F., Scognamiglio P., et al. Hedonic eating is associated with increased Peripheral levels of ghrelin and the Endocannabinoid 2-Arachidonoyl-Glycerol in healthy humans: a pilot study. J Clin Endocrinol Metab. 2012;97:E917–E924. doi: 10.1210/jc.2011-3018. [DOI] [PubMed] [Google Scholar]
- 7.Acosta A., Camilleri M., Dayyeh B.A., et al. Selection of antiobesity medications based on phenotypes enhances weight loss: a pragmatic trial in an obesity clinic. Obesity (Silver Spring) 2021;29:662–671. doi: 10.1002/oby.23120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pajot G., Camilleri M., Calderon G., et al. Association between gastrointestinal phenotypes and weight gain in younger adults: a prospective 4-year cohort study. Int J Obes. 2020;44:2472–2478. doi: 10.1038/s41366-020-0593-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Apovian C.M. Obesity: definition, comorbidities, causes, and burden. Am J Manag Care. 2016;22:S176–S185. [PubMed] [Google Scholar]
- 10.Polyzos S.A., Kountouras J., Mantzoros C.S. Obesity and nonalcoholic fatty liver disease: from pathophysiology to therapeutics. Metabolism. 2019;92:82–97. doi: 10.1016/j.metabol.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 11.Camilleri M., Malhi H., Acosta A. Gastrointestinal complications of obesity. Gastroenterology. 2017;152:1656–1670. doi: 10.1053/j.gastro.2016.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anand G., Katz P.O. Gastroesophageal reflux disease and obesity. Gastroenterol Clin North Am. 2010;39:39–46. doi: 10.1016/j.gtc.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Delgado-Aros S., Camilleri M., Cremonini F., et al. Contributions of gastric volumes and gastric emptying to meal size and postmeal symptoms in functional dyspepsia. Gastroenterology. 2004;127:1685–1694. doi: 10.1053/j.gastro.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 14.Steed K.P., Bohemen E.K., Lamont G.M., et al. Proximal colonic response and gastrointestinal transit after high and low fat meals. Dig Dis Sci. 1993;38:1793–1800. doi: 10.1007/BF01296101. [DOI] [PubMed] [Google Scholar]
- 15.Deiteren A., Camilleri M., Burton D., et al. Effect of meal ingestion on ileocolonic and colonic transit in health and irritable bowel syndrome. Dig Dis Sci. 2010;55:384–391. doi: 10.1007/s10620-009-1041-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ragnarsson G., Bodemar G. Pain is temporally related to eating but not to defaecation in the irritable bowel syndrome (IBS). Patients' description of diarrhea, constipation and symptom variation during a prospective 6-week study. Eur J Gastroenterol Hepatol. 1998;10:415–421. doi: 10.1097/00042737-199805000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Eslick G. Gastrointestinal symptoms and obesity: a meta-analysis. Obes Rev. 2012;13:469–479. doi: 10.1111/j.1467-789X.2011.00969.x. [DOI] [PubMed] [Google Scholar]
- 18.Singh M., Lee J., Gupta N., et al. Weight loss can lead to resolution of gastroesophageal reflux disease symptoms: a prospective intervention trial. Obesity. 2013;21:284–290. doi: 10.1002/oby.20279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foster A., Laws H.L., Gonzalez Q.H., et al. Gastrointestinal symptomatic outcome after laparoscopic Roux-en-Y gastric bypass. J Gastrointest Surg. 2003;7:750–753. doi: 10.1016/s1091-255x(03)00092-1. [DOI] [PubMed] [Google Scholar]
- 20.Castillo E.J., Camilleri M., Locke G.R., et al. A community-based, controlled study of the epidemiology and pathophysiology of dyspepsia. Clin Gastroenterol Hepatol. 2004;2:985–996. doi: 10.1016/s1542-3565(04)00454-9. [DOI] [PubMed] [Google Scholar]
- 21.Jones K.L., Doran S.M., Hveem K., et al. Relation between postprandial satiation and antral area in normal subjects. Am J Clin Nutr. 1997;66:127–132. doi: 10.1093/ajcn/66.1.127. [DOI] [PubMed] [Google Scholar]
- 22.Fischler B., Tack J., De Gucht V., et al. Heterogeneity of symptom pattern, psychosocial factors, and pathophysiological mechanisms in severe functional dyspepsia. Gastroenterology. 2003;124:903–910. doi: 10.1053/gast.2003.50155. [DOI] [PubMed] [Google Scholar]
- 23.Parkman H.P., Hallinan E.K., Hasler W.L., et al. Early satiety and postprandial fullness in gastroparesis correlate with gastroparesis severity, gastric emptying, and water load testing. Neurogastroenterol Motil. 2017;29:3–9. doi: 10.1111/nmo.12981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Delgado-Aros S., Locke G.R., III, Camilleri M., et al. Obesity is associated with increased risk of gastrointestinal symptoms: a population-based study. Am J Gastroenterol. 2004;99:1801–1806. doi: 10.1111/j.1572-0241.2004.30887.x. [DOI] [PubMed] [Google Scholar]
- 25.Talley N.J., Quan C., Jones M.P., et al. Association of upper and lower gastrointestinal tract symptoms with body mass index in an Australian cohort. Neurogastroenterol Motil. 2004;16:413–419. doi: 10.1111/j.1365-2982.2004.00530.x. [DOI] [PubMed] [Google Scholar]
- 26.Talley N.J., Howell S., Poulton R. Obesity and chronic gastrointestinal tract symptoms in young adults: a birth cohort study. Am J Gastroenterol. 2004;99:1807–1814. doi: 10.1111/j.1572-0241.2004.30388.x. [DOI] [PubMed] [Google Scholar]
- 27.Aro P., Ronkainen J., Talley N.J., et al. Body mass index and chronic unexplained gastrointestinal symptoms: an adult endoscopic population based study. Gut. 2005;54:1377–1383. doi: 10.1136/gut.2004.057497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ho W., Spiegel B.M.R. The relationship between obesity and functional gastrointestinal disorders: causation, association, or neither? Gastroenterol Hepatol. 2008;4:572–578. [PMC free article] [PubMed] [Google Scholar]
- 29.van Oijen M.G., Josemanders D.F., Laheij R.J., et al. Gastrointestinal disorders and symptoms: does body mass index matter? Neth J Med. 2006;64:45–49. [PubMed] [Google Scholar]
- 30.Bluemel S., Menne D., Milos G., et al. Relationship of body weight with gastrointestinal motor and sensory function: studies in anorexia nervosa and obesity. BMC Gastroenterol. 2017;17:4. doi: 10.1186/s12876-016-0560-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cifuentes L., Campos A., Silgado M.L.R., et al. Association between anxiety and eating behaviors in patients with obesity. Obes Pillars. 2022;3:100021. doi: 10.1016/j.obpill.2022.100021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brandler J., Miller L.J., Wang X.J., et al. Secretin effects on gastric functions, hormones and symptoms in functional dyspepsia and health: randomized crossover trial. Am J Physiol Gastrointest Liver Physiol. 2020;318:G635–G645. doi: 10.1152/ajpgi.00371.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koenker R. Cambridge University Press; Cambridge, New York: 2005. Quantile regression. [Google Scholar]
- 34.Acosta A., Camilleri M., Shin A., et al. Quantitative gastrointestinal and psychological traits associated with obesity and response to weight-loss therapy. Gastroenterology. 2015;148:537–546.e4. doi: 10.1053/j.gastro.2014.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alkhowaiter S., Alotaibi R.M., Alwehaibi K.K., et al. The effect of body mass index on the prevalence of gastrointestinal symptoms among a Saudi population. Cureus. 2021;13:e17751. doi: 10.7759/cureus.17751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Acosta A., Camilleri M., Burton D., et al. Exenatide in obesity with accelerated gastric emptying: a randomized, pharmacodynamics study. Physiol Rep. 2015;3:e12610. doi: 10.14814/phy2.12610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Halawi H., Khemani D., Eckert D., et al. Effects of liraglutide on weight, satiation, and gastric functions in obesity: a randomised, placebo-controlled pilot trial. Lancet. Gastroenterol Hepatology. 2017;2:890–899. doi: 10.1016/S2468-1253(17)30285-6. [DOI] [PubMed] [Google Scholar]
- 38.Lacy B.E., Cangemi D., Vazquez-Roque M. Management of chronic abdominal distension and bloating. Clin Gastroenterol Hepatol. 2021;19:219–231.e1. doi: 10.1016/j.cgh.2020.03.056. [DOI] [PubMed] [Google Scholar]
- 39.Hunger Davis J. Ghrelin and the gut. Brain Res. 2018;1693:154–158. doi: 10.1016/j.brainres.2018.01.024. [DOI] [PubMed] [Google Scholar]
- 40.Bouchoucha M., Odinot J.M., Devroede G., et al. Simple clinical assessment of colonic response to food. Int J Colorectal Dis. 1998;13:217–222. doi: 10.1007/s003840050164. [DOI] [PubMed] [Google Scholar]
- 41.Ford M.J., Camilleri M., Wiste J.A., et al. Differences in colonic tone and phasic response to a meal in the transverse and sigmoid human colon. Gut. 1995;37:264–269. doi: 10.1136/gut.37.2.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sarna S.K., Integrated Systems Physiology: From Molecule to Function to Disease . Morgan & Claypool Life Sciences; San Rafael (CA): 2010. Colonic motility: from bench side to bedside. [PubMed] [Google Scholar]
- 43.Chumpitazi B.P., Self M.M., Czyzewski D.I., et al. Bristol Stool Form Scale reliability and agreement decreases when determining Rome III stool form designations. Neurogastroenterol Motil. 2016;28:443–448. doi: 10.1111/nmo.12738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Müller T.D., Nogueiras R., Andermann M.L., et al. Ghrelin Mol Metab. 2015;4:437–460. doi: 10.1016/j.molmet.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.El-Salhy M., Lillebø E., Reinemo A., et al. Ghrelin in patients with irritable bowel syndrome. Int J Mol Med. 2009;23:703–707. doi: 10.3892/ijmm_00000183. [DOI] [PubMed] [Google Scholar]
- 46.Tack J., Depoortere I., Bisschops R., et al. Influence of ghrelin on interdigestive gastrointestinal motility in humans. Gut. 2006;55:327–333. doi: 10.1136/gut.2004.060426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gürkan O.E., Dalgıç B., Bideci A. Relation between ghrelin level and treatment response in functional constipation. Turk J Gastroenterol. 2013;24:515–520. [PubMed] [Google Scholar]
- 48.Acosta A., Kolar G., Iturrino J., et al. Sa2051 A phase II, randomized, double-blind, placebo-controlled, multiple-dose, Parallel-Group Study to evaluate the efficacy, safety, and pharmacodynamics of RM-131 in patients with chronic constipation. Gastroenterology. 2014;146:S-364. [Google Scholar]
- 49.Giurcan R., Voiosu T.A. Functional dyspepsia: a pragmatic approach. Rom J Intern Med. 2010;48:9–15. [PubMed] [Google Scholar]
- 50.Levine M.E. Sickness and satiety: physiological mechanisms underlying perceptions of nausea and stomach fullness. Curr Gastroenterol Rep. 2005;7:280–288. doi: 10.1007/s11894-005-0020-2. [DOI] [PubMed] [Google Scholar]
- 51.Patel N., Shackelford K.S. StatPearls [Internet] StatPearls Publishing; Treasure Island, FL: 2019. Irritable syndrome syndrome.https://www.ncbi.nlm.nih.gov/books/NBK534810/ [Google Scholar]
- 52.Camilleri M., McKinzie S., Busciglio I., et al. Prospective study of motor, sensory, psychologic, and autonomic functions in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2008;6:772–781. doi: 10.1016/j.cgh.2008.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bharucha A.E., Manduca A., Lake D.S., et al. Gastric motor disturbances in patients with idiopathic rapid gastric emptying. Neurogastroenterol Motil. 2011;23 doi: 10.1111/j.1365-2982.2011.01710.x. 617-e252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hui C., Dhakal A., Bauza G.J. StatPearls Publishing; Treasure Island, FL: 2020. Dumping syndrome.https://www.ncbi.nlm.nih.gov/books/NBK470542/ (StatPearls [Internet]). [Google Scholar]
- 55.Scarpellini E., Arts J., Karamanolis G., et al. International consensus on the diagnosis and management of dumping syndrome. Nat Rev Endocrinol. 2020;16:448–466. doi: 10.1038/s41574-020-0357-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Charles F., Phillips S.F., Camilleri M., et al. Rapid gastric emptying in patients with functional diarrhea. Mayo Clin Proc. 1997;72:323–328. doi: 10.4065/72.4.323. [DOI] [PubMed] [Google Scholar]
- 57.Seo A.Y., Kim N., Oh D.H. Abdominal bloating: pathophysiology and treatment. J Neurogastroenterol Motil. 2013;19:433–453. doi: 10.5056/jnm.2013.19.4.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Walter S.A., Jones M.P., Talley N.J., et al. Abdominal pain is associated with anxiety and depression scores in a sample of the general adult population with no signs of organic gastrointestinal disease. Neurogastroenterol Motil. 2013;25 doi: 10.1111/nmo.12155. 741-e576. [DOI] [PubMed] [Google Scholar]
- 59.Haug T.T., Mykletun A., Dahl A.A. The prevalence of nausea in the community: psychological, social and somatic factors. Gen Hosp Psychiatry. 2002;24:81–86. doi: 10.1016/s0163-8343(01)00184-0. [DOI] [PubMed] [Google Scholar]
- 60.Buczek L., Migliaccio J., Petrovich G.D. Hedonic eating: sex differences and characterization of orexin activation and signaling. Neuroscience. 2020;436:34–45. doi: 10.1016/j.neuroscience.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.