Abstract
Findings from numerous laboratories and across neuroimaging modalities have consistently shown that exogenous administration of cytokines or inflammatory stimuli that induce cytokines disrupt circuits and networks involved in motivation and motor activity, threat detection, anxiety, interoceptive and emotional processing. While inflammatory effects on neural circuits and relevant behaviors may represent adaptive responses promoting conservation of energy and heightened vigilance during immune activation, chronically elevated inflammation may contribute to symptoms of psychiatric illnesses. Indeed, biomarkers of inflammation such as cytokines and acute phase reactants are reliably elevated in a subset of patients with unipolar or bipolar depression, anxiety-related disorders, and schizophrenia, and have been associated with differential treatment responses and poor clinical outcomes. A growing body of literature also describes higher levels of endogenous inflammatory markers and altered, typically lower functional or structural connectivity within these circuits in association with transdiagnostic symptoms like anhedonia and anxiety in psychiatric and at-risk populations. This review will present recent evidence that inflammation and its effects on the brain may serve as one molecular and cellular mechanism of dysconnectivity within anatomically and/or functionally connected cortical and subcortical regions in association with transdiagnostic symptoms. We also discuss the need to establish reproducible methods to assess inflammation-associated dysconnectivity in relation to behavior for use in translational studies or biomarker-driven clinical trials for novel pharmacological or behavioral interventions targeting inflammation or its effects on the brain.
Keywords: inflammation, functional connectivity, fMRI, depression, anxiety, anhedonia, interoception
1. Inflammation in psychiatric disorders: sources and symptoms
1a. Mechanisms and prevalence of increased inflammation in psychiatric patients
In otherwise medically-healthy psychiatric patients, genetic predisposition may interact with environmental/lifestyle factors that contribute to low-grade inflammation including pathogens (e.g., latent infections, gut dysbiosis) and “sterile” inflammatory signals that trigger innate immune responses in the absence of pathogens (Figure 1a)(1). Many of these factors increase risk for both psychiatric and medical illnesses, suggesting shared pathophysiologic mechanisms that explain high rates of comorbidity (2). Activated innate immune cells release inflammatory cytokines like interleukins (ILs), tumor necrosis factor-alpha (TNF), and interferons (IFNs), which then induce acute phase reactants like CRP from the liver. Numerous studies and meta-analyses report higher CRP and protein or gene expression markers of circulating inflammatory cytokines (e.g., IL-1, IL-6, TNF, IFNs) in depression as well as other psychiatric disorders sharing commons symptom domains of reduced motivation, psychomotor slowing and anxiety, including bipolar disorder, schizophrenia, anxiety disorders and post-traumatic stress disorder (PTSD)(3–9). High peripheral inflammation defined as CRP >3 mg/L (i.e., high risk for cardiometabolic disease) is observed for example in ~25-40% of depressed patients (10–13), and may reflect increased activity of some but not all inflammatory cytokines. Longitudinal studies found CRP to predict depressive symptoms (14–16) even beyond prior depression severity (17). Importantly, higher CRP and inflammatory cytokines have also been associated with resistance to conventional antidepressants in depression and worse clinical outcomes in schizophrenia (18–23).
Figure 1. Sources and mechanisms of inflammation and its effects on neurotransmitters and circuits that contribute to psychiatric symptoms.
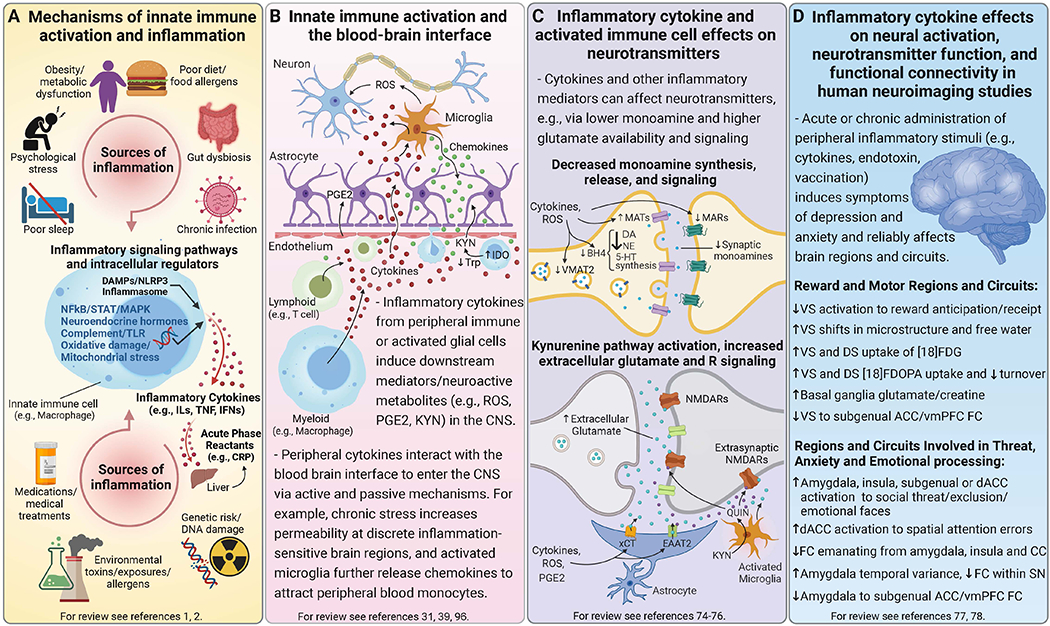
Mechanisms of innate immune activation and chronic low-grade inflammation. Panel A. Genetic predisposition may interact with multiple environmental and lifestyle factors that contribute to chronic low-grade inflammation, many of which are risk factors for both psychiatric disorders and major medical illnesses including psychological stress, disturbed sleep, poor diet, metabolic changes and gut dysbiosis, as well as chronic infections and environmental toxins. Innate immune cells are activated by pathogens or sterile inflammatory (e.g., DAMPs, metabolic, neuroendocrine, or oxidative stress) pathways to synthesize and release inflammatory mediators like cytokines (e.g., ILs, TNF, IFNs), which in turn induce acute phase reactants such as CRP from the liver. Acute inflammatory activity is typically resolved by homeostatic processes, but disruption of these mechanisms or prolonged immune activation can lead to chronic low-grade inflammation that impacts multiple systems including the brain. Bidirectional inflammatory processes at the blood-brain interface. Panel B. Activated innate immune cells interact with adaptive immune cells (e.g., lymphocytes), migrate into circulation, and traffic to organs and tissues including the brain. Circulating inflammatory cytokines and activated immune cells communicate with brain endothelial cells to induce other inflammatory mediators (e.g., PGE2). Inflammatory cytokines can enter the CNS via active transport or passively at circumventricular organs or openings in tight junctions of the BBB, while also signaling to the brain via vagal afferents (not shown). Microglia can be activated by inflammatory stimuli originating in the CNS or by these inflammatory signals from the periphery, and elaborate release of inflammatory mediators in the CNS like cytokines, ROS and nitrogen intermediates, as well as chemokines that further recruit peripheral inflammatory cells to perivascular regions or brain parenchyma. Inflammation also increases neuroactive metabolites from the catabolism of KYN, which is synthesized from Trp by IDO either locally in activated microglia or by macrophages followed by active transport into the brain. Inflammatory cytokines and associated oxidative molecules affect monoamine and glutamate neurotransmission. Panel C. Inflammatory cytokines and the associated release of ROS and nitrogen species can impact neuronal function through several ways including effects on neurotransmitters like monoamines and glutamate. For example, oxidation of BH4, a cofactor required for the synthesis of monoamine precursors, leads to decreased availability and release of monoamines - particularly DA, which requires BH4 for conversion of both Phe to Tyr and Tyr to L-DOPA. Evidence also exists that inflammatory cytokines can decrease expression or function of VMAT2, increase expression and activity of MATs especially the 5-HTT, and reduce expression of MARs like D2R. These effects together lead to a net decrease in synaptic monoamine availability and signaling. Inflammatory and oxidative factors also affect multiple aspects of glutamate transmission particularly by decreasing astrocytic buffering of glutamate by EAAT2, including reversing its efflux while promoting activity of the xCT to increase extracellular glutamate. Increased transport or local production of KYN in the brain and subsequent generation of neurotoxic metabolites like QUIN (a NMDAR agonist) further increase glutamate signaling including at extrasynaptic Rs, which lead to excitotoxicity and downstream generation of ROS (not shown). Peripherally administered acute and chronic inflammatory stimuli, which induce symptoms of depression and anxiety, reliably impact relevant brain regions and circuits in human neuroimaging studies. Panel D. Neuroimaging of patients chronically treated with inflammatory cytokines (e.g., IFN-α) or healthy participants administered stimuli that induce cytokines (e.g., endotoxin, vaccination) have shown that cytokines affect reward and motor-related regions and circuits, as well as those involved in threat, anxiety and emotional processing. For example, PET and MRS studies in IFN-α-treated patients reflect the impact of cytokines on neurotransmitters including reduced striatal DA availability and release as well as increased extracellular glutamate, both of which correlated with reduced motivation and low energy. Corresponding striatal microstructural as well as functional changes have included attenuated VS responses to reward anticipation/receipt and reduced FC between key regions of vmPFC and VS, after acute or chronic administration of IFN-α or other inflammatory stimuli. Inflammatory stimuli also increased neural activation of the amygdala, insula and dACC either independently or together during tasks designed to trigger emotional responses. Importantly, endotoxin also induced greater temporal variance in the amygdala at rest that correlated with lower FC within the SN as well as greater inflammation-induced anxiety. These findings on the impact of exogenous inflammatory stimuli on the brain have established a framework for the growing body of work assessing relationships between endogenous inflammatory markers and structure and function of these regions/circuits in psychiatric patients. Abbreviations: 5-HT - serotonin; 5-HTT - serotonin transporter; ACC - anterior cingulate cortex; BBB - blood-brain barrier; BH4 - tetrahydrobiopterin; CNS - central nervous system; CRP - C-reactive protein; D2R - dopamine 2 receptor; DA - dopamine; dACC - dorsal anterior cingulate cortex; DAMPs - danger-associated molecular patterns; DS - dorsal striatum; EAAT2 - excitatory amino-acid transporter 2; FC - functional connectivity; [18]FDG - fluorodeoxyglucose; [18]FDOPA - fluorodopa; IDO - indoleamine 2, 3-dioxygenase; IFN - interferon; ILs - interleukins; KYN - kynurenine; L-DOPA - levodopa; MAPK- mitogen-activated protein kinase; MARs - monoamine receptors; MATs - monoamine transporters; MRS - magnetic resonance spectroscopy; NE - norepinephrine; NFkB - nuclear factor kappa B; NLRP3 - NOD- LRR- and pyrin domain-containing protein-3; NMDAR - N-methyl-D-aspartate receptor; PET - positron emission tomography; PGE2 - prostaglandin E2; Phe - phenylalanine; QUIN - quinolinic acid; ROS - reactive oxygen species; SN - salience network; TLR - toll-like receptor; STAT - signal transducer and activator of transcription; TNF - tumor necrosis factor; Trp - tryptophan; Tyr - tyrosine; VMAT2 - vesicular monoamine transporter 2; vmPFC - ventromedial prefrontal cortex; VS - ventral striatum; xCT - cystine-glutamate exchanger
Peripheral-central immune crosstalk at the blood-brain interface.
Elevated CRP, innate/inflammatory cytokines, and peripheral white blood cells are found in CSF (11, 24), and postmortem studies show evidence of increased inflammatory cytokines and signaling pathways, activated microglia, and/or peripheral immune cell trafficking to brain parenchyma in depression, bipolar disorder and schizophrenia (25–29). However, lack of evidence of widespread BBB disruption (e.g., as indicated by CSF/circulating albumin, IgG ratios) observed in depression versus controls (n=106/group) or in relation to CRP (n=73)(11, 24) is consistent with evidence from animal models. For example, while monocyte trafficking and peripheral IL-6 are required for expression of anhedonic and depressive behaviors in chronic stress-induced depression models, the BBB remains relatively intact with region-specific decreases in integrity characterized by increased permeability to IL-6 in nucleus accumbens (NAc)(30, 31). Reduced BBB proteins (e.g., claudin 5) were also seen in NAc of susceptible mice, and postmortem NAc but not prefrontal cortex (PFC) or hippocampus of depressed patients (n=39)(30).
While peripheral immune activation is sufficient to cause depressive symptoms (see below), it is important to note that this involves bidirectional processes whereby peripheral cytokines and immune cells interact with endothelial cells, astrocytes, and microglia to elaborate production of cytokines and inflammatory mediators; microglia in turn release chemokines that recruit peripheral cells (Figure 1b). These bidirectional mechanisms may be particularly relevant in disorders like schizophrenia involving genetic, developmental, or autoimmune predispositions and inflammatory processes potentially initiating in the brain to engage the peripheral immune system (29). For example, and distinct from depression, in addition to CSF/serum albumin ratio and other markers of BBB disruption (n=104/group)(32), CSF evidence of complement activation and in some cases anti-neuronal antibodies are seen in psychotic disorders (32–34). Conversely, translocator protein (TSPO) positron emission tomography (PET) imaging thought to reflect microglial/macrophage activation is often reported to be increased in depression (despite not correlating with peripheral inflammatory markers) but lower in schizophrenia (35, 36), likely reflecting method limitations, e.g., off-target binding, competition from activated peripheral immune cells (37, 38), rather than a reduced inflammatory state. Nevertheless, similar patterns of innate/inflammatory cytokines and acute phase reactants in blood and CSF of patients in relation to symptom domains across disorders reflect potential common mechanisms of the effects of innate immune activation on the brain (see below), regardless of its source.
1b. Increased inflammation and transdiagnostic symptoms: cause-effect relationships
Inflammatory cytokines induce symptoms of reduced motivation, motor slowing and anxiety.
Consistent with an above-described role for inflammation in psychiatric disorders, a wealth of clinical and translational data demonstrate that administration of cytokines or inflammatory stimuli that induce cytokines affects neurotransmitters and circuits implicated in the pathophysiology of multiple disorders in association with symptoms of depression and anxiety (39–41)(Figure 1c,d). Some of the strongest clinical evidence of a potentially causal role for inflammation in psychiatric symptoms comes from patients chronically administered antiviral/antiproliferative cytokines like IFN-α for infectious diseases/cancer (42, 43). Up to 50% of IFN-α-treated patients reliably developed symptoms meeting criteria for major depression, and ~80% experienced fatigue, reduced energy, and/or psychomotor slowing (43–50). Anxiety and reduced motivation or anhedonia are also frequently reported post-IFN-α (42, 51). Indeed, instruments specifically probing anhedonia or reduced motivation yielded comparable effect sizes (r=0.47-0.49) as IFN-α-induced increase in total depression or fatigue scores (42, 52). Acute administration of inflammatory stimuli like low-dose endotoxin/vaccination, which potently induces cytokines, transiently increases depressive/anxiety symptoms (53, 54) in humans and laboratory animals and is used to study the impact of peripheral inflammation on the brain.
Endogenous inflammation, transdiagnostic symptoms, and reversal with anti-cytokine therapies.
While inflammatory effects on relevant circuits and behaviors described above may represent adaptive responses promoting conservation of energy (re. reduced motivation/anhedonia, psychomotor slowing), heightened vigilance (re. threat detection, anxiety), or social/emotional adaptations during immune activation, chronically elevated inflammation may contribute to psychiatric symptoms (40). Accordingly, relationships between biomarkers of low-grade inflammation and symptoms consistent with those induced by exogenous inflammatory stimuli and common to depression and other psychiatric disorders are frequently reported. For example, in medically-stable, unmedicated depressed patients, we found associations between anhedonia and both plasma CRP and clusters of inflammatory cytokines (IL-1, IL-6, TNF) and their soluble receptors in CSF (n=76)(11, 55). Results were extended by studies reporting correlations between both T and non-T cell cytokines with anhedonia (56), and longitudinal correlations between baseline TNF and 4-month anhedonia in depression (57). We also uncovered relationships between psychomotor slowing and inflammatory markers in depression (58, 59), and numerous studies report high inflammation in schizophrenia in association with negative symptoms including motivational deficits, blunted affect, and social withdrawal (60, 61). A growing literature further describes correlations between CRP/cytokines and anxiety (62–64), including longitudinally (65) and depression (66).
The TNF antagonist infliximab reduced overall depression severity only in treatment-resistant depressed (TRD) patients with higher plasma CRP (67), and anhedonia (work and activities) was the symptom most improved followed by motor slowing (retardation) and anxiety (psychic anxiety). Recent studies similarly found that infliximab or sirukumab (anti-IL-6) preferentially reduced anhedonia in unipolar or bipolar depressed patients with evidence of increased inflammation (68, 69). These cause-effect relationships indicating that transdiagnostic symptoms like anhedonia, motor slowing and anxiety can be both induced by inflammatory stimuli and reversed by cytokine antagonism, support specificity of inflammation effects on relevant brain regions/circuits that may serve as translational targets for development of treatments for patients with high inflammation (70, 71).
2. Impact of inflammatory stimuli on brain regions and circuits: from the clinic to the lab
As described above, chronically administered inflammatory cytokine therapies like IFN-α cause clinically-significant depressive symptoms at high rates, and this model was used in early work examining peripheral inflammation effects on the brain. Whole-brain analyses of fluorodeoxyglucose PET in patients undergoing IFN-α therapy revealed increased resting glucose metabolism in basal ganglia (consistent with low dopamine signaling in neurologic disorders) and decreased PFC metabolism (72, 73). These findings were subsequently linked to both low dopamine availability/release in caudate, putamen, and ventral striatum (VS: including NAc) by PET(42) and increased glutamate in basal ganglia and dorsal anterior cingulate cortex (dACC) by magnetic resonance spectroscopy (MRS)(74)(Figure 1d), all of which correlated with IFN-α-induced symptoms including reduced motivation and anergia. These clinical findings in patients during chronic IFN-α therapy indicating that peripheral inflammation impacts cortical and subcortical regions via effects on neurotransmitters like dopamine and glutamate have been confirmed and complemented by numerous laboratory human and animal studies using a variety of inflammatory stimuli, the neurobiological mechanisms of which are reviewed elsewhere (75–77)(see Figure 1c). Relevant to a larger body of work addressing the functional consequences of exogenous inflammatory stimuli on brain regions/circuits (78, 79), seminal fMRI studies are briefly summarized herein (Figure 1d) as they provided a foundation for a newer literature primarily using circuit and network-based approaches to understand relationships between endogenous low-grade inflammation and altered functional and structural connectivity in psychiatric patients (Section 3).
2a. Impact of inflammation on reward and motor regions and circuits
Complementary to the above-described effects of inflammation on dopamine availability, functional effects of peripheral inflammation on brain regions relevant to reduced motivation and psychomotor slowing have been consistently revealed by functional MRI (fMRI) in subjects administered inflammatory cytokines (e.g., IFN-α therapy) or inflammatory stimuli (e.g., endotoxin or vaccination given in the laboratory)(75). For example, chronic IFN-α treatment decreased VS neural activation to receipt of reward in association with reduced motivation (42). Similar effects on reward processing have been observed after acute endotoxin/vaccine in healthy volunteers (80, 81), and complemented by vaccine effects on task-based activity in substantia nigra that correlated with psychomotor slowing and IL-6 (82, 83). In addition to findings from region-focused task fMRI, whole-brain analysis revealed rapid (4-hour) IFN-α-induced microstructural changes in free water signal (consistent with edema) localized to left striatum that predicted subsequent fatigue (84). Importantly, evidence from acute IFN-α or vaccine suggests these neurotransmitter, structural and functional changes in discrete regions known to regulate motivation and motor activity contribute more broadly to inflammation effects on functional connectivity (FC) in key circuits including VS-ventromedial (vm)PFC, or as primary nodes within a global network that predicted depressive symptoms (85, 86).
2b. Impact of inflammation on regions and circuits for threat, anxiety, emotional and interoceptive processing
In addition to effects on reward and motor regions (per Section 2a/Figure 1d), peripheral inflammatory stimuli have been shown to increase neural activation of amygdala, dACC and insula (79), similar to findings reported in depression, anxiety disorders and PTSD. While analyses targeting amygdala showed higher right amygdala responses to emotional or socially threatening stimuli in relation to IFN-α or endotoxin-induced depressive or social disconnection symptoms (87, 88), whole-brain-analyses have revealed inflammation-by-task-related increases in dACC, mPFC and insula activation independently or in concert with each other or amygdala (89–92). For example, typhoid vaccine increased task activation of an interoceptive network including mid/posterior insula as well as amygdala and dACC (90). Given the role of insula in interoception, it is not surprising this region showed increased PET resting glucose metabolism (93) and lower seed-to-voxel resting-state (rs)FC with a number of cortical regions (94) after endotoxin. While task and seed-based analyses may bias or limit observed effects of inflammation to specific regions, a more agnostic network approach revealed reduced rsFC within a salience network (including amygdala, insula, dACC) in association with increased temporal variation of the rsFC signal only in amygdala, which in turn correlated with endotoxin-induced anxiety (95). These findings are consistent with animal studies showing rapid and behaviorally-relevant activation of amygdala by peripheral inflammatory stimuli in part via direct cytokine effects (96–98), and reinforce the importance of functionally connected regions involved in interoceptive/emotional processing, vigilance/threat detection, to contribute to relevant symptoms induced by inflammation including anxiety.
3. Structural and functional dysconnectivity in patients with high inflammation
As discussed in Sections 1 and 2, inflammation can influence neurotransmitters and key regions and circuits thought to underlie network dysfunction observed across psychiatric diagnoses (79, 99)(Figure 1d), and may contribute to disease pathophysiology and discrete symptomologies in a subset of patients. While inflammation-associated structural and free-water changes are reported in schizophrenia (23, 100–102), studies contributing to our evolving understanding of relationships between endogenous inflammation and brain activity or FC relevant to transdiagnostic symptoms in psychiatric disorders have focused primarily on depression or bipolar disorder and taken hypothesis-driven, symptom-focused approaches to examine relationships between inflammation and frontostriatal, amygdala-prefrontal, and interoceptive circuits/networks (Table 1). Accordingly, findings are presented with a circuit/symptom focus. As relationships between inflammatory markers in psychiatric patients and low rsFC have emerged as the most consistent findings for inflammation-associated dysconnectivity, with potential for translational use as a reliable brain biomarker of inflammation, these studies are highlighted in Figure 2.
Table 1.
Summary of studies assessing relationships between endogenous inflammation and functional or structural neuroimaging outcomes in the context of significant psychiatric symptoms or diagnoses.
| Study | Population | Inflammatory Markers | Outcome | Brain Region/White Matter Tract |
|---|---|---|---|---|
| Resting State Functional Connectivity and Supporting fMRI Studies | ||||
| Circuits and Regions Relevant to Reduced Motivation or Psychomotor Slowing | ||||
| Felger et al., 2016 (55) | MDD | CRP | ↓ seed-to-voxel and seed-to-ROI, resting-state FC | (1) Left VS-vmPFC (2) Dorsal striatum and vmPFC, presupplementary motor area |
| Yin et al., 2019 (110) | MDD | CRP | ↓ Voxel-wise GBC and PBA of 100 ROIs, resting-state FC | Network with central hubs in vmPFC followed by VS |
| Mehta et al., 2020 (112) | Trauma-exposed women with or without clinically significant PTSD symptoms | CRP, inflammatory cytokine composite score | ↓ seed-to-ROI, resting-state FC | VS-vmPFC |
| Tang et al., 2021 (114) | BD (current episode depressed) | IL-8 | ↓ ICA, resting-state FC | Right precentral gyrus (somatomotor network) |
| Tseng et al., 2021 (113) | BD (euthymic) | CRP | ↑ seed-to-voxel, resting-state FC | Right dorsal caudal putamen-middle orbitofrontal gyrus |
| Rengasamy et al, 2022 (111) | TRD | IL-6 | ↓ seed-to-ROI, resting-state FC | Left VS-vmPFC |
| Haroon et al., 2018 (120) | MDD | CRP | ↓ local and network, resting-state ReHo functional integrity | Left basal ganglia and network varying by levels of CRP and MRS glutamate |
| Bradley et al., 2019 (118) | Adolescents presenting with clinically significant psychiatric symptoms | Inflammatory composite factors | ↓ activation to reward attainment (1) and anticipation (2) | (1)Basal ganglia (ROI), angular gyrus (whole-brain) (2) Precuneus/PCC (whole-brain) |
| Liu et al., 2020 (117) | Adolescents presenting with clinically significant psychiatric symptoms | CRP | ↑activation to reward attainment (1) ↓ activation to reward anticipation (2) ↑ activation to positive prediction error (3) |
(1) Visual and dorsal attention networks (whole-brain) (2) dACC (ROI) (3) NAc (ROI) |
| Burrows et al., 2021 (115) | MDD | CRP | ↓ activation to anticipation of small rewards | Dorsal caudate, thalamus, left insula, left precuneus (whole-brain) |
| Costi et al., 2021 (116) | MDD and healthy adults | Stimulated blood immune markers | ↓ activation to reward anticipation | VS (ROI) |
| Circuits and Regions Relevant to Threat, Anxiety and Emotional Processing | ||||
| Mehta et al., 2018 (126) | MDD with or without comorbid anxiety disorders or PTSD | CRP, IL-6, IL-1ra | ↓ ROI-to-voxel and ROI-to-ROI, resting-state FC | (1) Right amygdala-vmPFC (2) Right amygdala-left precentral gyrus |
| Gong et al., 2022 (128) | BD (>75% depressive episode) | TNF | ↓ seed-to-voxel, resting-state FC | (1) Right amygdala-bilateral medial PFC (2) Left amygdala-left temporal pole |
| Mehta et al., 2022 (127) | Trauma-exposed women with or without clinically significant PTSD symptoms | CRP, inflammatory cytokine composite score | ↓ ROI-to-ROI, resting-state FC | Right amygdala-vmPFC |
| Savitz et al., 2013 (131) | MDD | Expression of immune-related PBMC genes | ↑ activation to sad vs happy faces | Amygdala, left hippocampus, and vmPFC (ROIs from whole-brain MDD > HC) |
| Mocking et al, 2017 (130) | MDD | CRP | No association with reactivity to negative faces | Bilateral amygdala (ROI) |
| Poletti et al., 2017 (133) | BD (current episode depressed) | T regulatory cells | ↓ activation to negative vs. positive stimuli | Right dorsolateral PFC/inferior frontal gyrus (whole-brain) |
| Conejero et al., 2019 (132) | Women with history of MDD and/or SA and healthy controls | IL-1β, IL-2 | ↓ activation related to IL-1β (1) ↑ activation related to IL-2 (2) during social exclusion |
(1) Right orbitofrontal cortex (2) Right orbitofrontal cortex, insula and ACC (ROI) |
| Boukezzi et al., 2022 (129) | MDD | Stimulated blood immune markers | ↑ activation to fear vs happy faces | Amygdala (ROI) |
| Interoceptive, Default Mode and other Large-Scale Networks | ||||
| Chen et al., 2020 (143) | BD (current episode depressed) | IL-6 | ↓ seed-to-voxel, resting-state FC | Right posterior insula-left postcentral gyrus |
| Aruldass et al., 2021 (140) | MDD | CRP, IL-6, neutrophils, CD4+ T-cells | ↓ NBS of 8 networks, resting-state FC | Within insular/frontal opercular cortex (VAN) and the posterior cingulate cortex (DMN) |
| Kitzbichler et al., 2021 (141) | MDD | CRP | ↓↑ PBA of 360 cortical and 16 subcortical ROIs, resting-state FC |
Lower within DMN, higher DMN-hippocampus, in association with PD free water edema signal |
| King et al., 2021 (144) | SZ | IL-6 | ↓ seed-to-voxel, resting-state FC | Left lateral parietal cortex-precuneus of DMN |
| Beckmann et al., 2022 (142) | MDD | CRP | ↑ seed-based analysis of 5 networks, resting-state FC | Internetwork DMN to AN |
| Structural Connectivity Studies | ||||
| Prasad et al., 2015 (100) | SZ | CRP, IL-6 | ↓ FA, ↑ RD | Inferior longitudinal fasciculus, inferior fronto-occipital fasciculus, forceps major (RD to IL-6 only) |
| Benedetti et al., 2016 (125) | BD (current episode depressed) | TNF, IL-8, IFN-γ | ↓ FA ↑ RD | Corpus callosum, cingulum, superior and inferior longitudinal fasciculi, inferior fronto-occipital fasciculi, uncinate, forceps, corona radiata, thalamic radiation, internal capsule |
| Sugimoto et al., 2018 (123) | MDD | IL-1β | ↓ FA | Inferior fronto-occipital fasciculi, left uncinate fasciculus |
| Wang et al., 2020 (102) | SZ | IL-6 | ↓ FA | Genu and body of corpus callosum, anterior/posterior limbs of internal capsule |
| Lim et al., 2021 (124) | MDD | TNF | ↓ FA | Genu of corpus callosum, left anterior and superior corona radiata |
| Thomas et al., 2021 (122) | MDD | CRP | ↓ QA | Corticostriatal tracts, thalamic radiations, inferior longitudinal fasciculi, corpus callosum, cingulum, and left superior longitudinal fasciculus |
AN - affective network; BD - bipolar disorder; CRP - C-reactive protein; dACC - dorsal anterior cingulate cortex; DMN - default mode network; FA - fractional anisotropy; FC - functional connectivity; GBC - global brain connectivity; HC - healthy controls; IFN - interferon; IL - interleukin; L-DOPA - levodopa; MDD - major depressive disorder; MRS - magnetic resonance spectroscopy; NAc - nucleus accumbens; NBS - network-based statistics; PBA - parcellation-based analysis; PBMC - peripheral blood mononuclear cells; PCC - posterior cingulate cortex; PD - proton density; PTSD - post-traumatic stress disorder; QA - quantitative anisotropy; ra - receptor antagonist; RD - radial diffusivity; ReHo - regional homogeneity; SA - suicide attempt; SZ - schizophrenia; TNF - tumor necrosis factor; TRD - treatment-resistant depression; VAN - ventral attention network; vmPFC - ventromedial prefrontal cortex; VS - ventral striatum
Figure 2. Inflammation-associated resting-state functional “dysconnectivity” in key circuits and networks that contribute to psychiatric disorders.
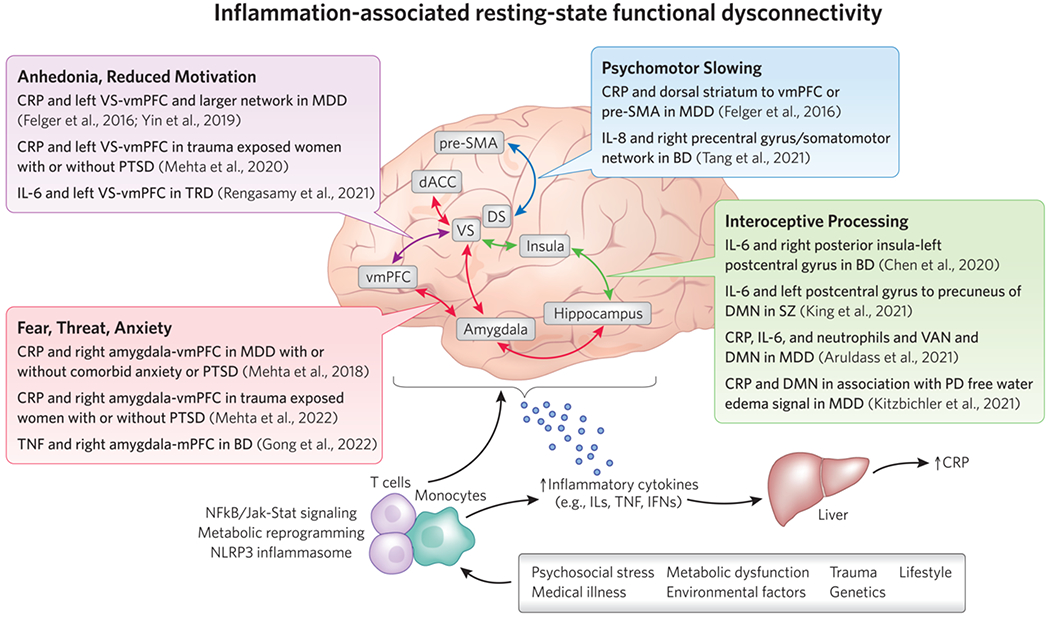
Summary of key studies from an emerging literature describing associations between circulating biomarkers of inflammation, such as inflammatory cytokines and the acute phase reactant CRP, in patients with depression or other psychiatric illnesses and low resting-state functional connectivity (rsFC) in frontostriatal circuits regulating motivation or motor activity, amygdala-prefrontal circuits involved in fear, threat and anxiety, and circuits/networks involved in interoceptive and emotional processing, all of which may contribute to transdiagnostic symptoms in patients with psychiatric disorders. A host of medical, environmental, and lifestyle factors contribute to innate immune activation in patients with depression and other psychiatric disorders. Peripheral immune cells like monocytes and T cells activate inflammatory signaling pathways and undergo a metabolic reprograming to facilitate the release of cytokines and cell trafficking to the brain. Inflammatory cytokines produced in the periphery and CNS can impact neural activation and functional connectivity within key brain regions, circuits, and networks relevant to psychiatric disorders through effects on neurotransmitters like dopamine and glutamate, or structural changes like reduced white matter integrity. Abbreviations: BD - bipolar disorder; CRP - C- reactive protein; DMN - default mode network; FA - fractional anisotropy; FC - functional connectivity; IFN - interferon; IL - interleukin; Jak-Stat - Janus kinase-Signal transducer and activator of transcription; MDD - major depressive disorder; MRS - magnetic resonance spectroscopy; NAc - nucleus accumbens; NFkB - nuclear factor kappa B; PCC - posterior cingulate cortex; PD - proton density; pSMA - pre-supplementary motor area; PTSD - post-traumatic stress disorder; TNF - tumor necrosis factor; TRD - treatment-resistant depression; VAN - ventral attention network; vmPFC - ventromedial prefrontal cortex; VS - ventral striatum
Inflammation and low rsFC in non-psychiatric populations: risk factors across the lifespan.
As our discussion focuses on endogenous inflammation and rsFC in the context of psychiatric disorders, it is worthy to mention representative studies from a similar body of work in non-psychiatric populations. Consistent with the above-described effects of peripheral inflammation on regions and circuits involved in emotion regulation (Section 2b), a composite index of CRP/inflammatory cytokines, or numbers of classical monocytes, associated with low rsFC in an emotion-regulation network in cohorts of at-risk African American (AA) youth (103). Associations between cytokines (TNF) and altered rsFC in adolescents also extended to other inflammation-sensitive regions including right amygdala and left VS (104). In adults, IL-6 partially mediated relationships between childhood abuse and lower amygdala-vmPFC rsFC (105), and an inflammatory cytokine composite related to low salience, default mode network (DMN), and central executive inter-network rsFC in association with sub-clinical PTSD symptoms in stress-exposed firefighters (106). Finally, an inflammatory cell index (neutrophil/lymphocyte ratio) in older adults negatively correlated with rsFC within regions of vmPFC in relation to geriatric depression symptoms (107). These findings, together with the impact of inflammatory stimuli on the brain (Section 2), suggest that inflammation-effects on FC within sensitive regions/circuits serve as potential brain mechanisms of the frequently reported associations between inflammatory markers and psychiatric symptoms in individuals exposed to risk factors like stress, early life adversity, and aging (2, 40), and support these pathways as mechanisms of inflammation-related dysconnectivity in psychiatric patients.
3a. Frontostriatal circuits and transdiagnostic symptoms of reduced motivation and psychomotor slowing
Inflammation and low rsFC in reward and motor circuits.
Similar to the effects of exogenous inflammatory stimuli on reward-relevant regions (Section 2a, Figure 1d), we and other have found relationships between endogenous inflammation in psychiatric patients and low rsFC in frontostriatal circuits including VS-vmPFC, a classic reward circuit found to be disrupted in depression and other psychiatric disorders (108, 109). For example, negative associations between plasma CRP and left VS-vmPFC rsFC were observed using both seed-to-voxel-wise and targeted seed-to-ROI approaches in medically-stable, unmedicated depressed patients (n=48), whereby lower VS-vmPFC rsFC in turn correlated with and mediated relationships between CRP and anhedonia (55). Frontostriatal rsFC also negatively associated with inflammatory cytokines and their soluble receptors (55). These relationships in depression were corroborated by parcellation-based network analyses whereby primary (vmPFC) and secondary (VS, as anterior caudate) hubs, along with multiple other edges of a 63-feature network of CRP-associated dysconnectivity, highly predicted anhedonia symptoms in support vector regression (110). Negative associations between inflammatory markers and left VS-vmPFC rsFC were replicated by Rengasamy et al. for IL-6 in TRD (111), and in our group for CRP in association with anhedonia in trauma-exposed inner-city AA women, while a composite of inflammatory cytokines and their receptors (11) only correlated with rsFC in women with significant PTSD symptoms (112). Relevant to risk factors for associations between high inflammation and low rsFC, early-life adversity modified these relationships whereby severity of childhood maltreatment predicted stronger negative cytokine-rsFC associations in both studies (111, 112).
Consistent with inflammatory stimuli effects on motor activity (75), endogenous inflammation also correlated with dysconnectivity within corticostriatal circuits involving cognitive and motor regions of dorsal striatum. For example, we found negative relationships between CRP and rsFC for dorsal caudate and dorsal caudal putamen with vmPFC and/or presupplementary motor area in association with psychomotor slowing in depression (55). These relationships along with additional rsFC features identified in network analyses highly predicted psychomotor slowing (110). Similar relationships have been seen in depressed, but not euthymic (113), bipolar disorder patients including negative correlations between IL-8 and rsFC of right precentral gyrus within a somatomotor network, as demonstrated by Tang et al. with independent component analysis (ICA)(114).
Complementary task fMRI, neurotransmitter, and structural studies.
Relationships between endogenous inflammation and low rsFC in frontostriatal reward and motor circuits in patients are complemented by a study from Burrows et al. showing decreased activation of dorsal caudate, thalamus, left insula, and left precuneus in anticipation of small wins in depression with higher CRP (>3 mg/L)(115). Costi et al. also found an endotoxin-stimulated inflammatory factor to negatively correlate with VS response to reward anticipation in association with anhedonia in depression (116). Relationships between either CRP or inflammatory cytokine factors and reward anticipation/attainment in striatal and prefrontal regions were similarly seen in adolescents with clinically-significant psychiatric symptoms (117, 118). These associations between inflammation and low activity or rsFC within frontostriatal circuits may involve its effects on neurotransmitters like dopamine and glutamate (75, 76)(Figure 1c,d). Plasma and CSF CRP in medically-stable, unmedicated depressed patients associated with left basal ganglia glutamate (using single-voxel MRS)(119), which jointly identified a larger network of low regional homogeneity (“ReHo,” concordance of oscillatory activity in neighboring MRS voxels, including a reward-related subnetwork)(120), and correlated with anhedonia and psychomotor slowing. Regarding links to dopamine, we recently reported that acute challenge with its precursor levodopa (which rapidly increases dopamine availability) improved left VS-vmPFC rsFC only in depressed patients with higher CRP (>2 mg/L) in association with higher task-FC during reward anticipation and levodopa-induced decreases in anhedonia (121). In addition to neurotransmitter influence on functional circuits/networks, inflammation may impact rsFC through effects on structural connectivity as relationships between CRP/cytokines and low white matter integrity (quantitative and fractional anisotropy) have been observed in depressed and bipolar patients in numerous important tracts including corticostriatal and thalamic radiations connecting cortical and subcortical structures (122–125).
3b. Amygdala-prefrontal circuits involved in threat detection, anxiety, and emotional processing
Inflammation and low amygdala-PFC rsFC.
In addition to effects on motivation and motor activity, inflammatory stimuli induce symptoms of anxiety in the context of heightened reactivity and low rsFC of amygdala and prefrontal regions (53, 88, 94, 95)(Section 2, Figure 1d), consistent with reports of associations between inflammation and low rsFC in this circuitry in psychiatric patients. Accordingly, we found associations between endogenous inflammatory markers, plasma CRP and inflammatory cytokines and their soluble receptors, and low right amygdala-vmPFC rsFC in medically-stable, unmedicated patients with a primary diagnosis of depression (126). Right amygdala-vmPFC rsFC in turn negatively correlated with and mediated relationships between CRP and symptoms of anxiety, and these findings were strongest in patients with co-morbid anxiety disorders or PTSD. Relationships between CRP or cytokines and rsFC between right amygdala and mPFC/vmPFC were also generalizable to trauma-exposed AA women with or without PTSD in association with anxiety (127), and to unmedicated bipolar patients (128).
Complementary task-fMRI studies.
Relationships between endogenous inflammatory markers and low rsFC with amygdala is supported by a recent report that an endotoxin-stimulated inflammatory factor associated with heighted amygdala activation to fear>happy faces, which in turn associated with symptoms of anxious arousal in depression (129). Importantly, while not all studies report similar associations between inflammation and amygdala reactivity (130), TNF-antagonism with infliximab in patients with inflammatory illness reduced depressive symptoms in association with decreased amygdala reactivity to emotional face-processing (87). Savitz and colleagues also extended findings of relationships between inflammation and amygdala reactivity to a broader network of regions activated by inflammatory stimuli in concert with amygdala (Section 3b, Figure 1d) by showing positive correlations between inflammatory genes in peripheral blood immune cells and activation of amygdala, vmPFC, and hippocampus to sad>happy faces (131). Emotional task fMRI also revealed relationships between inflammatory cytokines (IL-1beta, IL2) and PFC, insula and/or ACC activation in women with a history of suicidality or depression (132), and generally anti-inflammatory T regulatory cells inversely correlated with dorsolateral PFC activation in bipolar depression (133). Therefore, endogenous inflammation-associated increases in reactivity of amygdala, PFC and functionally-related regions like insula are consistent with effects of inflammatory stimuli on these regions, and may contribute to low amygdala-vmPFC rsFC seen in psychiatric patients.
3.c. Interoceptive, Default Mode, and other Large-Scale Networks
Similar to findings that inflammation effects on the brain extend beyond individual regions/circuits (Section 2b), network analyses have revealed endogenous inflammation/rsFC associations within largescale networks in psychiatric patients that overlap with inflammation-sensitive regions/circuits constructed by meta-analysis (134), e.g., DMN (135, 136), ventral attention network (VAN)(137), and interoceptive pathways involving insula (138, 139). In high CRP-depressed (>3mg/L, n=33/83) versus healthy controls (n=46), Aruldass et al. reported low rsFC within VAN (insular/frontal opercular cortex) and posterior cingulate cortex (PCC) of DMN, and many features of this predefined network negatively correlated with CRP, IL-6, and neutrophils in all patients (140). While Kitzbichler et al. reported a greater proportion of negatively weighted rsFC features within DMN in depressed, particularly high CRP, patients in this same cohort along with positive correlations between CRP and proton density (PD; tissue-free water/edema) within DMN (141), analysis of all 70,500 possible pair-wise correlations between individual edges and CRP in all patients also revealed positive associations primarily with hippocampus. However, positive correlations between CRP and PD in PCC subregions mediated negative relationships between CRP and PCC-mPFC rsFC, but not positive relationships between CRP and PCC-hippocampus rsFC. Thus, inflammation-related structural changes in key regions of high CRP patients may contribute to low within-network rsFC, subsequently influencing rsFC with outside networks/regions possibly not as directly impacted by inflammation. While exclusively negative CRP-rsFC associations found using a similar strategy with less parcellations (100 versus 376) in another depressed cohort (n=44)(110) suggest that fine-grained, agnostic approaches may be necessary to reveal positive correlations. While stronger internetwork DMN-VAN rsFC was also seen in association with CRP in a small depression cohort (n=27)(142), negative seed-to-voxel rsFC correlations for insula and DMN were reported for IL-6 in unmedicated bipolar depressed and schizophrenic patients (143, 144). Thus, peripheral inflammation in psychiatric patients primarily associated with low connectivity within large-scale networks, with some evidence of increased connectivity across networks or with other brain regions.
4. Conclusions and translational implications
Herein, we discuss key findings from an emerging literature describing associations between inflammatory markers and functional dysconnectivity in both discrete circuits and broad networks relevant to transdiagnostic symptoms in depression and other disorders (Figure 2). Results are consistent with and described in the context of a wealth of data demonstrating the causal impact of clinically or experimentally administered cytokines or inflammatory stimuli on neurotransmitters and functional activity and connectivity in the same regions and circuits in association with relevant symptoms (Section 2/Figure 1). Supporting evidence of inflammation-associated alterations in neurotransmitters, task activation, structural connectivity, and edema in patients (Section 3/Table 1) further serve as potential mechanisms of functional dysconnectivity.
Most studies reported relationships between low structural or functional connectivity and innate/inflammatory cytokines (ILs, TNF, IFNs assessed individually or as a composite) or CRP (thought to reflect activity of multiple cytokines). While a handful of studies measured more than one cytokine (but not CRP) and only reported on one marker (IL-1beta, IL-6, IL-8, TFN)(102, 111, 114, 123, 124, 128, 143), it is not clear whether this represents biologically significant cytokine-circuit associations within the context of chronic low-grade inflammation in patients, or rather inter-study/marker variability in methods/detection. As this area of research expands, relationships between connectivity and individual immune markers can be examined in large datasets, and longitudinal and experimental studies can confirm stability, neurobiological mechanisms, and causal associations/pathway specificity.
For example, in region/circuit analyses, relationships between inflammation and low rsFC in frontostriatal reward/motor-related circuits have as emerged as a consistent finding across laboratories and samples (Figure 2), and parallel findings on the impact of inflammatory stimuli on multimodal neuroimaging outcomes in these regions (Section 2)(145). Our recent report that levodopa increased VS-vmPFC rsFC only in depressed patients with higher CRP in association with improved anhedonia not only link inflammation-related reward circuit deficits to dopamine (121)(Section 3a), but also indicate rsFC as a modifiable imaging biomarker for the efficacy of interventions to reverse the impact of inflammation on the brain. Future research using this approach in patients with high inflammation will focus on other targetable substrates, e.g., glutamate or immune-modulating therapies (71, 145).
In sum, a growing field describes reliable associations between endogenous innateimmune/inflammatory markers and structural/functional dysconnectivity in regions, circuits and networks known to be sensitive to inflammatory stimuli in association with transdiagnostic symptoms in psychiatric patients. Future work will examine specificity/causality of these associations and their potential use as brain biomarkers to develop therapies targeted to patients with high inflammation (70, 71).
Acknowledgements:
This work was supported by funds from the National Institute of Mental Health grants R01MH109637, R61MH121625 (JCF), K23MH114037 (DRG), F32MH119750 (MB), and F31MH119745 (NDM). Figure 2 artwork is credited to Katie Vicari: KatieRisVicari@gmail.com; www.katierisvicari.com.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Dr. Felger recently consulted for Cello Health BioConsulting on a topic unrelated to this work. All other authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Berk M, Williams LJ, Jacka FN, O’Neil A, Pasco JA, Moylan S, et al. (2013): So depression is an inflammatory disease, but where does the inflammation come from? BMC Med. 11:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, Franceschi C, et al. (2019): Chronic inflammation in the etiology of disease across the life span. Nature medicine. 25:1822–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Costello H, Gould RL, Abrol E, Howard R (2019): Systematic review and meta-analysis of the association between peripheral inflammatory cytokines and generalised anxiety disorder. BMJ open. 9:e027925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Passos IC, Vasconcelos-Moreno MP, Costa LG, Kunz M, Brietzke E, Quevedo J, et al. (2015): Inflammatory markers in post-traumatic stress disorder: a systematic review, meta-analysis, and meta-regression. The Lancet Psychiatry. 2:1002–1012. [DOI] [PubMed] [Google Scholar]
- 5.Goldsmith DR, Rapaport MH, Miller BJ (2016): A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatry. 21:1696–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Munkholm K, Vinberg M, Vedel Kessing L (2013): Cytokines in bipolar disorder: a systematic review and meta-analysis. Journal of affective disorders. 144:16–27. [DOI] [PubMed] [Google Scholar]
- 7.Mostafavi S, Battle A, Zhu X, Potash JB, Weissman MM, Shi J, et al. (2014): Type I interferon signaling genes in recurrent major depression: increased expression detected by whole-blood RNA sequencing. Mol Psychiatry. 19:1267–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Howren MB, Lamkin DM, Suls J (2009): Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosomatic medicine. 71:171–186. [DOI] [PubMed] [Google Scholar]
- 9.Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, et al. (2010): A meta-analysis of cytokines in major depression. Biol Psychiatry. 67:446–457. [DOI] [PubMed] [Google Scholar]
- 10.Rapaport MH, Nierenberg AA, Schettler PJ, Kinkead B, Cardoos A, Walker R, et al. (2016): Inflammation as a predictive biomarker for response to omega-3 fatty acids in major depressive disorder: a proof-of-concept study. Mol Psychiatry. 21:71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Felger JC, Haroon E, Patel TA, Goldsmith DR, Wommack EC, Woolwine BJ, et al. (2020): What does plasma CRP tell us about peripheral and central inflammation in depression? Mol Psychiatry. 25:1301–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uher R, Tansey KE, Dew T, Maier W, Mors O, Hauser J, et al. (2014): An inflammatory biomarker as a differential predictor of outcome of depression treatment with escitalopram and nortriptyline. Am J Psychiatry. 171:1278–1286. [DOI] [PubMed] [Google Scholar]
- 13.Pitharouli MC, Hagenaars SP, Glanville KP, Coleman JRI, Hotopf M, Lewis CM, et al. (2021): Elevated C-Reactive Protein in Patients With Depression, Independent of Genetic, Health, and Psychosocial Factors: Results From the UK Biobank. Am J Psychiatry. 178:522–529. [DOI] [PubMed] [Google Scholar]
- 14.Wium-Andersen MK, Orsted DD, Nielsen SF, Nordestgaard BG (2013): Elevated C-reactive protein levels, psychological distress, and depression in 73, 131 individuals. JAMA psychiatry. 70:176–184. [DOI] [PubMed] [Google Scholar]
- 15.Gimeno D, Kivimaki M, Brunner EJ, Elovainio M, De Vogli R, Steptoe A, et al. (2009): Associations of C-reactive protein and interleukin-6 with cognitive symptoms of depression: 12-year follow-up of the Whitehall II study. Psychol Med. 39:413–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Au B, Smith KJ, Gariepy G, Schmitz N (2015): The longitudinal associations between C-reactive protein and depressive symptoms: evidence from the English Longitudinal Study of Ageing (ELSA). International journal of geriatric psychiatry. 30:976–984. [DOI] [PubMed] [Google Scholar]
- 17.Bondy E, Norton SA, Voss MD, Marks RB, Bourdreaux MJ, Treadway MT, et al. (2021): Inflammation is associated with future depressive symptoms among older adults. Brain, Behhavior, & Immunity - Health. 13:100226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haroon E, Daguanno AW, Woolwine BJ, Goldsmith DR, Baer WM, Wommack EC, et al. (2018): Antidepressant treatment resistance is associated with increased inflammatory markers in patients with major depressive disorder. Psychoneuroendocrinology. 95:43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chamberlain SR, Cavanagh J, de Boer P, Mondelli V, Jones DNC, Drevets WC, et al. (2018): Treatment-resistant depression and peripheral C-reactive protein. The British journal of psychiatry: the journal of mental science.1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uher R, Tansey KE, Dew T, Maier W, Mors O, Hauser J, et al. (2014): An Inflammatory Biomarker as a Differential Predictor of Outcome of Depression Treatment With Escitalopram and Nortriptyline. Am J Psychiatry. [DOI] [PubMed] [Google Scholar]
- 21.Jha MK, Minhajuddin A, Gadad BS, Greer T, Grannemann B, Soyombo A, et al. (2017): Can C-reactive protein inform antidepressant medication selection in depressed outpatients? Findings from the CO-MED trial. Psychoneuroendocrinology. 78:105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nettis MA, Pergola G, Kolliakou A, O’Connor J, Bonaccorso S, David A, et al. (2019): Metabolic-inflammatory status as predictor of clinical outcome at 1-year follow-up in patients with first episode psychosis. Psychoneuroendocrinology. 99:145–153. [DOI] [PubMed] [Google Scholar]
- 23.Kose M, Pariante CM, Dazzan P, Mondelli V (2021): The Role of Peripheral Inflammation in Clinical Outcome and Brain Imaging Abnormalities in Psychosis: A Systematic Review. Front Psychiatry. 12:612471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sorensen NV, Orlovska-Waast S, Jeppesen R, Klein-Petersen AW, Christensen RHB, Benros ME (2022): Neuroinflammatory Biomarkers in Cerebrospinal Fluid From 106 Patients With Recent-Onset Depression Compared With 106 Individually Matched Healthy Control Subjects. Biol Psychiatry. [DOI] [PubMed] [Google Scholar]
- 25.Torres-Platas SG, Cruceanu C, Chen GG, Turecki G, Mechawar N (2014): Evidence for increased microglial priming and macrophage recruitment in the dorsal anterior cingulate white matter of depressed suicides. Brain Behav Immun. 42:50–59. [DOI] [PubMed] [Google Scholar]
- 26.Pandey GN (2017): Inflammatory and Innate Immune Markers of Neuroprogression in Depressed and Teenage Suicide Brain. Modern trends in pharmacopsychiatry. 31:79–95. [DOI] [PubMed] [Google Scholar]
- 27.North HF, Weissleder C, Fullerton JM, Sager R, Webster MJ, Weickert CS (2021): A schizophrenia subgroup with elevated inflammation displays reduced microglia, increased peripheral immune cell and altered neurogenesis marker gene expression in the subependymal zone. Transl Psychiatry. 11:635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cai HQ, Catts VS, Webster MJ, Galletly C, Liu D, O’Donnell M, et al. (2020): Increased macrophages and changed brain endothelial cell gene expression in the frontal cortex of people with schizophrenia displaying inflammation. Mol Psychiatry. 25:761–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu Y, Webster MJ, Murphy CE, Middleton FA, Massa PT, Liu C, et al. (2022): Distinct Phenotypes of Inflammation Associated Macrophages and Microglia in the Prefrontal Cortex Schizophrenia Compared to Controls. Frontiers in neuroscience. 16:858989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Menard C, Pfau ML, Hodes GE, Kana V, Wang VX, Bouchard S, et al. (2017): Social stress induces neurovascular pathology promoting depression. Nat Neurosci. 20:1752–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dantzer R, Cohen S, Russo SJ, Dinan TG (2018): Resilience and immunity. Brain, behavior, and immunity. 74:28–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jeppesen R, Orlovska-Waast S, Sorensen NV, Christensen RHB, Benros ME (2022): Cerebrospinal Fluid and Blood Biomarkers of Neuroinflammation and Blood-Brain Barrier in Psychotic Disorders and Individually Matched Healthy Controls. Schizophr Bull. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mayilyan KR, Weinberger DR, Sim RB (2008): The complement system in schizophrenia. Drug News Perspect. 21:200–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Endres D, Meixensberger S, Dersch R, Feige B, Stich O, Venhoff N, et al. (2020): Cerebrospinal fluid, antineuronal autoantibody, EEG, and MRI findings from 992 patients with schizophreniform and affective psychosis. Transl Psychiatry. 10:279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Setiawan E, Wilson AA, Mizrahi R, Rusjan PM, Miler L, Rajkowska G, et al. (2015): Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA Psychiatry. 72:268–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Plaven-Sigray P, Matheson GJ, Collste K, Ashok AH, Coughlin JM, Howes OD, et al. (2018): Positron Emission Tomography Studies of the Glial Cell Marker Translocator Protein in Patients With Psychosis: A Meta-analysis Using Individual Participant Data. Biol Psychiatry. 84:433–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Notter T, Schalbetter SM, Clifton NE, Mattei D, Richetto J, Thomas K, et al. (2020): Neuronal activity increases translocator protein (TSPO) levels. Mol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nettis MA, Veronese M, Nikkheslat N, Mariani N, Lombardo G, Sforzini L, et al. (2020): PET imaging shows no changes in TSPO brain density after IFN-alpha immune challenge in healthy human volunteers. Transl Psychiatry. 10:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW (2008): From inflammation to sickness and depression: when the immune system subjugates the brain. Nature reviews Neuroscience. 9:46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller AH, Raison CL (2016): The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nature reviews Immunology. 16:22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Felger JC, Lotrich FE (2013): Inflammatory cytokines in depression: Neurobiological mechanisms and therapeutic implications. Neuroscience. 246:199–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Capuron L, Pagnoni G, Drake DF, Woolwine BJ, Spivey JR, Crowe RJ, et al. (2012): Dopaminergic mechanisms of reduced basal ganglia responses to hedonic reward during interferon alfa administration. Arch Gen Psychiatry. 69:1044–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Capuron L, Gumnick JF, Musselman DL, Lawson DH, Reemsnyder A, Nemeroff CB, et al. (2002): Neurobehavioral effects of interferon-alpha in cancer patients: phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 26:643–652. [DOI] [PubMed] [Google Scholar]
- 44.Donnelly S (1998): Patient management strategies for interferon alfa-2b as adjuvant therapy of high-risk melanoma. Oncol Nurs Forum. 25:921–927. [PubMed] [Google Scholar]
- 45.Capuron L, Neurauter G, Musselman DL, Lawson DH, Nemeroff CB, Fuchs D, et al. (2003): Interferon-alpha-induced changes in tryptophan metabolism. relationship to depression and paroxetine treatment. Biol Psychiatry. 54:906–914. [DOI] [PubMed] [Google Scholar]
- 46.Musselman DL, Lawson DH, Gumnick JF, Manatunga AK, Penna S, Goodkin RS, et al. (2001): Paroxetine for the prevention of depression induced by high-dose interferon alfa. N Engl J Med. 344:961–966. [DOI] [PubMed] [Google Scholar]
- 47.Raison CL, Borisov AS, Broadwell SD, Capuron L, Woolwine BJ, Jacobson IM, et al. (2005): Depression during pegylated interferon-alpha plus ribavirin therapy: prevalence and prediction. J Clin Psychiatry. 66:41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Raison CL, Rye DB, Woolwine BJ, Vogt GJ, Bautista BM, Spivey JR, et al. (2010): Chronic interferon-alpha administration disrupts sleep continuity and depth in patients with hepatitis C: association with fatigue, motor slowing, and increased evening cortisol. Biol Psychiatry. 68:942–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Raison CL, Borisov AS, Majer M, Drake DF, Pagnoni G, Woolwine BJ, et al. (2009): Activation of central nervous system inflammatory pathways by interferon-alpha: relationship to monoamines and depression. Biol Psychiatry. 65:296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Capuron L, Hauser P, Hinze-Selch D, Miller AH, Neveu PJ (2002): Treatment of cytokine-induced depression. Brain, behavior, and immunity. 16:575–580. [DOI] [PubMed] [Google Scholar]
- 51.Capuron L, Fornwalt FB, Knight BT, Harvey PD, Ninan PT, Miller AH (2009): Does cytokine-induced depression differ from idiopathic major depression in medically healthy individuals? Journal of affective disorders. 119:181–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Majer M, Welberg LA, Capuron L, Pagnoni G, Raison CL, Miller AH (2008): IFN-alpha-induced motor slowing is associated with increased depression and fatigue in patients with chronic hepatitis C. Brain Behav Immun. 22:870–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lasselin J, Elsenbruch S, Lekander M, Axelsson J, Karshikoff B, Grigoleit JS, et al. (2016): Mood disturbance during experimental endotoxemia: Predictors of state anxiety as a psychological component of sickness behavior. Brain Behav Immun. 57:30–37. [DOI] [PubMed] [Google Scholar]
- 54.Eisenberger NI, Inagaki TK, Mashal NM, Irwin MR (2010): Inflammation and social experience: an inflammatory challenge induces feelings of social disconnection in addition to depressed mood. Brain, behavior, and immunity. 24:558–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Felger JC, Li Z, Haroon E, Woolwine BJ, Jung MY, Hu X, et al. (2016): Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Mol Psychiatry. 21:1358–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jha MK, Miller AH, Minhajuddin A, Trivedi MH (2018): Association of T and non-T cell cytokines with anhedonia: Role of gender differences. Psychoneuroendocrinology. 95:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rengasamy M, Marsland A, McClain L, Kovats T, Walko T, Pan L, et al. (2021): Longitudinal relationships of cytokines, depression and anhedonia in depressed adolescents. Brain, behavior, and immunity. 91:74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bekhbat M, Goldsmith DR, Woolwine BJ, Haroon E, Miller AH, Felger JC (2021): Transcriptomic signatures of psychomotor slowing in peripheral blood of depressed patients: evidence for immunometabolic reprogramming. Mol Psychiatry. 26:7384–7392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goldsmith DR, Haroon E, Woolwine BJ, Jung MY, Wommack EC, Harvey PD, et al. (2016): Inflammatory markers are associated with decreased psychomotor speed in patients with major depressive disorder. Brain Behav Immun. 56:281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Goldsmith DR, Rapaport MH (2020): Inflammation and Negative Symptoms of Schizophrenia: Implications for Reward Processing and Motivational Deficits. Front Psychiatry. 11:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Goldsmith DR, Massa N, Pearce BD, Wommack EC, Alrohaibani A, Goel N, et al. (2020): Inflammatory markers are associated with psychomotor slowing in patients with schizophrenia compared to healthy controls. NPJ Schizophr. 6:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ye Z, Kappelmann N, Moser S, Davey Smith G, Burgess S, Jones PB, et al. (2021): Role of inflammation in depression and anxiety: Tests for disorder specificity, linearity and potential causality of association in the UK Biobank. EClinicalMedicine. 38:100992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Milaneschi Y, Kappelmann N, Ye Z, Lamers F, Moser S, Jones PB, et al. (2021): Association of inflammation with depression and anxiety: evidence for symptom-specificity and potential causality from UK Biobank and NESDA cohorts. Mol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liukkonen T, Räsänen P, Jokelainen J, Leinonen M, Järvelin MR, Meyer-Rochow VB, et al. (2011): The association between anxiety and C-reactive protein (CRP) levels: results from the Northern Finland 1966 birth cohort study. Eur Psychiatry. 26:363–369. [DOI] [PubMed] [Google Scholar]
- 65.van Eeden WA, El Filali E, van Hemert AM, Carlier IVE, Penninx B, Lamers F, et al. (2021): Basal and LPS-stimulated inflammatory markers and the course of anxiety symptoms. Brain Behav Immun. 98:378–387. [DOI] [PubMed] [Google Scholar]
- 66.Gaspersz R, Lamers F, Wittenberg G, Beekman ATF, van Hemert AM, Schoevers RA, et al. (2017): The role of anxious distress in immune dysregulation in patients with major depressive disorder. Transl Psychiatry. 7:1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, et al. (2013): A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry. 70:31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Salvadore G, Nash A, Bleys C, Hsu B, Saad Z, Gause A, et al. (2018): A Double-Blind, Placebo-Controlled, Multicenter Study of Sirukumab as Adjunctive Treatment to a Monoaminergic Antidepressant in Adults With Major Depressive Disorder, in ACNP 57th Annual Meeting: Poster Session II, Hollywood, FL. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 43:228–382. [Google Scholar]
- 69.Lee Y, Mansur RB, Brietzke E, Carmona NE, Subramaniapillai M, Pan Z, et al. (2020): Efficacy of adjunctive infliximab vs. placebo in the treatment of anhedonia in bipolar I/II depression. Brain Behav Immun. 88:631–639. [DOI] [PubMed] [Google Scholar]
- 70.Miller AH, Haroon E, Felger JC (2017): Therapeutic Implications of Brain-Immune Interactions: Treatment in Translation. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 42:334–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Drevets WC, Wittenberg GM, Bullmore ET, Manji HK (2022): Immune targets for therapeutic development in depression: towards precision medicine. Nat Rev Drug Discov. 21:224–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Capuron L, Pagnoni G, Demetrashvili MF, Lawson DH, Fornwalt FB, Woolwine B, et al. (2007): Basal ganglia hypermetabolism and symptoms of fatigue during interferon-alpha therapy. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 32:2384–2392. [DOI] [PubMed] [Google Scholar]
- 73.Juengling FD, Ebert D, Gut O, Engelbrecht MA, Rasenack J, Nitzsche EU, et al. (2000): Prefrontal cortical hypometabolism during low-dose interferon alpha treatment. Psychopharmacology. 152:383–389. [DOI] [PubMed] [Google Scholar]
- 74.Haroon E, Woolwine BJ, Chen X, Pace TW, Parekh S, Spivey JR, et al. (2014): IFN-alpha-induced cortical and subcortical glutamate changes assessed by magnetic resonance spectroscopy. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 39:1777–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Felger JC, Treadway MT (2017): Inflammation Effects on Motivation and Motor Activity: Role of Dopamine. Neuropsychopharmacology. 42:216–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Haroon E, Miller AH, Sanacora G (2017): Inflammation, Glutamate, and Glia: A Trio of Trouble in Mood Disorders. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 42:193–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hunt C, Macedo ECT, Suchting R, de Dios C, Cuellar Leal VA, Soares JC, et al. (2020): Effect of immune activation on the kynurenine pathway and depression symptoms - A systematic review and meta-analysis. Neuroscience and biobehavioral reviews. 118:514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Harrison NA (2017): Brain Structures Implicated in Inflammation-Associated Depression. Current topics in behavioral neurosciences. 31:221–248. [DOI] [PubMed] [Google Scholar]
- 79.Felger JC (2018): Imaging the Role of Inflammation in Mood and Anxiety-related Disorders. Curr Neuropharmacol. 16:533–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Eisenberger NI, Berkman ET, Inagaki TK, Rameson LT, Mashal NM, Irwin MR (2010): Inflammation-induced anhedonia: endotoxin reduces ventral striatum responses to reward. Biol Psychiatry. 68:748–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Harrison NA, Voon V, Cercignani M, Cooper EA, Pessiglione M, Critchley HD (2016): A Neurocomputational Account of How Inflammation Enhances Sensitivity to Punishments Versus Rewards. Biol Psychiatry. 80:73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Brydon L, Harrison NA, Walker C, Steptoe A, Critchley HD (2008): Peripheral inflammation is associated with altered substantia nigra activity and psychomotor slowing in humans. Biol Psychiatry. 63:1022–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Harrison NA, Cercignani M, Voon V, Critchley HD (2015): Effects of inflammation on hippocampus and substantia nigra responses to novelty in healthy human participants. Neuropsychopharmacology. 40:831–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dowell NG, Cooper EA, Tibble J, Voon V, Critchley HD, Cercignani M, et al. (2016): Acute Changes in Striatal Microstructure Predict the Development of Interferon-Alpha Induced Fatigue. Biol Psychiatry. 79:320–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dipasquale O, Cooper EA, Tibble J, Voon V, Baglio F, Baselli G, et al. (2016): Interferon-alpha acutely impairs whole-brain functional connectivity network architecture - A preliminary study. Brain, behavior, and immunity. 58:31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Critchley HD (2009): Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biol Psychiatry. 66:407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Davies KA, Cooper E, Voon V, Tibble J, Cercignani M, Harrison NA (2021): Interferon and anti-TNF therapies differentially modulate amygdala reactivity which predicts associated bidirectional changes in depressive symptoms. Mol Psychiatry. 26:5150–5160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Inagaki TK, Muscatell KA, Irwin MR, Cole SW, Eisenberger NI (2012): Inflammation selectively enhances amygdala activity to socially threatening images. NeuroImage. 59:3222–3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Capuron L, Pagnoni G, Demetrashvili M, Woolwine BJ, Nemeroff CB, Berns GS, et al. (2005): Anterior cingulate activation and error processing during interferon-alpha treatment. Biol Psychiatry. 58:190–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Dolan RJ, et al. (2009): Neural origins of human sickness in interoceptive responses to inflammation. Biol Psychiatry. 66:415–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Eisenberger NI, Inagaki TK, Rameson LT, Mashal NM, Irwin MR (2009): An fMRI study of cytokine-induced depressed mood and social pain: the role of sex differences. NeuroImage. 47:881–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kullmann JS, Grigoleit JS, Lichte P, Kobbe P, Rosenberger C, Banner C, et al. (2013): Neural response to emotional stimuli during experimental human endotoxemia. Human brain mapping. 34:2217–2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hannestad J, Subramanyam K, Dellagioia N, Planeta-Wilson B, Weinzimmer D, Pittman B, et al. (2012): Glucose metabolism in the insula and cingulate is affected by systemic inflammation in humans. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 53:601–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Labrenz F, Wrede K, Forsting M, Engler H, Schedlowski M, Elsenbruch S, et al. (2016): Alterations in functional connectivity of resting state networks during experimental endotoxemia - An exploratory study in healthy men. Brain, behavior, and immunity. 54:17–26. [DOI] [PubMed] [Google Scholar]
- 95.Labrenz F, Ferri F, Wrede K, Forsting M, Schedlowski M, Engler H, et al. (2019): Altered temporal variance and functional connectivity of BOLD signal is associated with state anxiety during acute systemic inflammation. NeuroImage. 184:916–924. [DOI] [PubMed] [Google Scholar]
- 96.Engler H, Doenlen R, Engler A, Riether C, Prager G, Niemi M-B, et al. (2011): Acute amygdaloid response to systemic inflammation. Brain, behavior, and immunity. 25:1384–1392. [DOI] [PubMed] [Google Scholar]
- 97.Munshi S, Rosenkranz JA (2018): Effects of Peripheral Immune Challenge on In Vivo Firing of Basolateral Amygdala Neurons in Adult Male Rats. Neuroscience. 390:174–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dantzer R, Konsman JP, Bluthé RM, Kelley KW (2000): Neural and humoral pathways of communication from the immune system to the brain: parallel or convergent? Auton Neurosci. 85:60–65. [DOI] [PubMed] [Google Scholar]
- 99.van den Heuvel MP, Sporns O (2019): A cross-disorder connectome landscape of brain dysconnectivity. Nature reviews Neuroscience. 20:435–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Prasad KM, Upton CH, Nimgaonkar VL, Keshavan MS (2015): Differential susceptibility of white matter tracts to inflammatory mediators in schizophrenia: an integrated DTI study. Schizophr Res. 161:119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Di Biase MA, Zalesky A, Cetin-Karayumak S, Rathi Y, Lv J, Boerrigter D, et al. (2021): Large-Scale Evidence for an Association Between Peripheral Inflammation and White Matter Free Water in Schizophrenia and Healthy Individuals. Schizophr Bull. 47:542–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang Y, Wei Y, Edmiston EK, Womer FY, Zhang X, Duan J, et al. (2020): Altered structural connectivity and cytokine levels in Schizophrenia and Genetic high-risk individuals: Associations with disease states and vulnerability. Schizophr Res. 223:158–165. [DOI] [PubMed] [Google Scholar]
- 103.Nusslock R, Brody GH, Armstrong CC, Carroll AL, Sweet LH, Yu T, et al. (2019): Higher Peripheral Inflammatory Signaling Associated With Lower Resting-State Functional Brain Connectivity in Emotion Regulation and Central Executive Networks. Biol Psychiatry. 86:153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Swartz JR, Carranza AF, Tully LM, Knodt AR, Jiang J, Irwin MR, et al. (2021): Associations between peripheral inflammation and resting state functional connectivity in adolescents. Brain Behav Immun. 95:96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kraynak TE, Marsland AL, Hanson JL, Gianaros PJ (2019): Retrospectively reported childhood physical abuse, systemic inflammation, and resting corticolimbic connectivity in midlife adults. Brain, behavior, and immunity. 82:203–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kim J, Yoon S, Lee S, Hong H, Ha E, Joo Y, et al. (2020): A double-hit of stress and low-grade inflammation on functional brain network mediates posttraumatic stress symptoms. Nat Commun. 11:1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.McIntosh RC, Lobo J, Paparozzi J, Goodman Z, Kornfeld S, Nomi J (2022): Neutrophil to lymphocyte ratio is a transdiagnostic biomarker of depression and structural and functional brain alterations in older adults. Journal of neuroimmunology. 365:577831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA (2015): Large-Scale Network Dysfunction in Major Depressive Disorder: A Meta-analysis of Resting-State Functional Connectivity. JAMA psychiatry. 72:603–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Whitton AE, Treadway MT, Pizzagalli DA (2015): Reward processing dysfunction in major depression, bipolar disorder and schizophrenia. Curr Opin Psychiatry. 28:7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yin L, Xu X, Chen G, Mehta ND, Haroon E, Miller AH, et al. (2019): Inflammation and decreased functional connectivity in a widely-distributed network in depression: Centralized effects in the ventral medial prefrontal cortex. Brain, behavior, and immunity. 80:657–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rengasamy M, Brundin L, Griffo A, Panny B, Capan C, Forton C, et al. (2022): Cytokine and Reward Circuitry Relationships in Treatment-Resistant Depression. Biol Psychiatry Glob Open Sci. 2:45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mehta ND, Stevens JS, Li Z, Gillespie CF, Fani N, Michopoulos V, et al. (2020): Inflammation, reward circuitry and symptoms of anhedonia and PTSD in trauma-exposed women. Soc Cogn Affect Neurosci. 15:1046–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tseng HH, Chang HH, Wei SY, Lu TH, Hsieh YT, Yang YK, et al. (2021): Peripheral inflammation is associated with dysfunctional corticostriatal circuitry and executive dysfunction in bipolar disorder patients. Brain, behavior, and immunity. 91:695–702. [DOI] [PubMed] [Google Scholar]
- 114.Tang G, Chen P, Chen G, Zhong S, Gong J, Zhong H, et al. (2021): Inflammation is correlated with abnormal functional connectivity in unmedicated bipolar depression: an independent component analysis study of resting-state fMRI. Psychol Med.1–11. [DOI] [PubMed] [Google Scholar]
- 115.Burrows K, Stewart JL, Kuplicki R, Figueroa-Hall L, Spechler PA, Zheng H, et al. (2021): Elevated peripheral inflammation is associated with attenuated striatal reward anticipation in major depressive disorder. Brain, behavior, and immunity. 93:214–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Costi S, Morris LS, Collins A, Fernandez NF, Patel M, Xie H, et al. (2021): Peripheral immune cell reactivity and neural response to reward in patients with depression and anhedonia. Translational psychiatry. 11:565–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Liu Q, Ely B, Simkovic S, Tao A, Wolchok R, Alonso CM, et al. (2020): Correlates of C-reactive protein with neural reward circuitry in adolescents with psychiatric symptoms. Brain Behav Immun Health. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bradley KA, Stern ER, Alonso CM, Xie H, Kim-Schulze S, Gabbay V (2019): Relationships between neural activation during a reward task and peripheral cytokine levels in youth with diverse psychiatric symptoms. Brain, behavior, and immunity. 80:374–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Haroon E, Fleischer CC, Felger JC, Chen X, Woolwine BJ, Patel T, et al. (2016): Conceptual convergence: increased inflammation is associated with increased basal ganglia glutamate in patients with major depression. Mol Psychiatry. 21(10):1351–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Haroon E, Chen X, Li Z, Patel T, Woolwine BJ, Hu XP, et al. (2018): Increased inflammation and brain glutamate define a subtype of depression with decreased regional homogeneity, impaired network integrity, and anhedonia. Transl Psychiatry. 8:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bekhbat M, Li Z, Mehta ND, Treadway MT, Lucido MJ, Woolwine BJ, et al. (2022): Functional connectivity in reward circuitry and symptoms of anhedonia as therapeutic targets in depression with high inflammation: evidence from a dopamine challenge study. Mol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Thomas M, Savitz J, Zhang Y, Burrows K, Smith R, Figueroa-Hall L, et al. (2021): Elevated Systemic Inflammation Is Associated with Reduced Corticolimbic White Matter Integrity in Depression. Life (Basel). 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sugimoto K, Kakeda S, Watanabe K, Katsuki A, Ueda I, Igata N, et al. (2018): Relationship between white matter integrity and serum inflammatory cytokine levels in drug-naive patients with major depressive disorder: diffusion tensor imaging study using tract-based spatial statistics. Transl Psychiatry. 8:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lim J, Sohn H, Kwon MS, Kim B (2021): White Matter Alterations Associated with Pro-inflammatory Cytokines in Patients with Major Depressive Disorder. Clin Psychopharmacol Neurosci. 19:449–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Benedetti F, Poletti S, Hoogenboezem TA, Mazza E, Ambrée O, de Wit H, et al. (2016): Inflammatory cytokines influence measures of white matter integrity in Bipolar Disorder. Journal of affective disorders. 202:1–9. [DOI] [PubMed] [Google Scholar]
- 126.Mehta ND, Haroon E, Xu X, Woolwine BJ, Li Z, Felger JC (2018): Inflammation negatively correlates with amygdala-ventromedial prefrontal functional connectivity in association with anxiety in patients with depression: Preliminary results. Brain, Behavior, and Immunity. 73:725–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mehta ND, Stevens JS, Li Z, Fani N, Gillespie CF, Ravi M, et al. (2022): Inflammation, amygdala-ventromedial prefrontal functional connectivity and symptoms of anxiety and PTSD in African American women recruited from an inner-city hospital: Preliminary results. Brain, behavior, and immunity. 105:122–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gong J, Chen G, Chen F, Zhong S, Chen P, Zhong H, et al. (2022): Association between resting-state functional connectivity of amygdala subregions and peripheral pro-inflammation cytokines levels in bipolar disorder. Brain Imaging Behav. [DOI] [PubMed] [Google Scholar]
- 129.Boukezzi S, Costi S, Shin LM, Kim-Schulze S, Cathomas F, Collins A, et al. (2022): Exaggerated amygdala response to threat and association with immune hyperactivity in depression. Brain, behavior, and immunity. 104:205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mocking RJT, Nap TS, Westerink AM, Assies J, Vaz FM, Koeter MWJ, et al. (2017): Biological profiling of prospective antidepressant response in major depressive disorder: Associations with (neuro)inflammation, fatty acid metabolism, and amygdala-reactivity. Psychoneuroendocrinology. 79:84–92. [DOI] [PubMed] [Google Scholar]
- 131.Savitz J, Frank MB, Victor T, Bebak M, Marino JH, Bellgowan PS, et al. (2013): Inflammation and neurological disease-related genes are differentially expressed in depressed patients with mood disorders and correlate with morphometric and functional imaging abnormalities. Brain, behavior, and immunity. 31:161–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Conejero I, Jaussent I, Cazals A, Thouvenot E, Mura T, Le Bars E, et al. (2019): Association between baseline pro-inflammatory cytokines and brain activation during social exclusion in patients with vulnerability to suicide and depressive disorder. Psychoneuroendocrinology. 99:236–242. [DOI] [PubMed] [Google Scholar]
- 133.Poletti S, de Wit H, Mazza E, Wijkhuijs AJM, Locatelli C, Aggio V, et al. (2017): Th17 cells correlate positively to the structural and functional integrity of the brain in bipolar depression and healthy controls. Brain, behavior, and immunity. 61:317–325. [DOI] [PubMed] [Google Scholar]
- 134.Kraynak TE, Marsland AL, Wager TD, Gianaros PJ (2018): Functional neuroanatomy of peripheral inflammatory physiology: A meta-analysis of human neuroimaging studies. Neuroscience and biobehavioral reviews. 94:76–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sheline YI, Barch DM, Price JL, Rundle MM, Vaishnavi SN, Snyder AZ, et al. (2009): The default mode network and self-referential processes in depression. Proceedings of the National Academy of Sciences of the United States of America. 106:1942–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hamilton JP, Farmer M, Fogelman P, Gotlib IH (2015): Depressive Rumination, the Default-Mode Network, and the Dark Matter of Clinical Neuroscience. Biol Psychiatry. 78:224–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Seeley WW (2019): The Salience Network: A Neural System for Perceiving and Responding to Homeostatic Demands. The Journal of neuroscience : the official journal of the Society for Neuroscience. 39:9878–9882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Savitz J, Harrison NA (2018): Interoception and Inflammation in Psychiatric Disorders. Biol Psychiatry Cogn Neurosci Neuroimaging. 3:514–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Barrett LF, Simmons WK (2015): Interoceptive predictions in the brain. Nature reviews Neuroscience. 16:419–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Aruldass AR, Kitzbichler MG, Morgan SE, Lim S, Lynall M-E, Turner L, et al. (2021): Dysconnectivity of a brain functional network was associated with blood inflammatory markers in depression. Brain, behavior, and immunity. 98:299–309. [DOI] [PubMed] [Google Scholar]
- 141.Kitzbichler MG, Aruldass AR, Barker GJ, Wood TC, Dowell NG, Hurley SA, et al. (2021): Peripheral inflammation is associated with micro-structural and functional connectivity changes in depression-related brain networks. Mol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Beckmann FE, Seidenbecher S, Metzger CD, Gescher DM, Carballedo A, Tozzi L, et al. (2022): C-reactive protein is related to a distinct set of alterations in resting-state functional connectivity contributing to a differential pathophysiology of major depressive disorder. Psychiatry Res Neuroimaging. 321:111440. [DOI] [PubMed] [Google Scholar]
- 143.Chen P, Chen F, Chen G, Zhong S, Gong J, Zhong H, et al. (2020): Inflammation is associated with decreased functional connectivity of insula in unmedicated bipolar disorder. Brain, behavior, and immunity. 89:615–622. [DOI] [PubMed] [Google Scholar]
- 144.King S, Holleran L, Mothersill D, Patlola S, Rokita K, McManus R, et al. (2021): Early life Adversity, functional connectivity and cognitive performance in Schizophrenia: The mediating role of IL-6. Brain, behavior, and immunity. 98:388–396. [DOI] [PubMed] [Google Scholar]
- 145.Bekhbat M, Treadway MT, Felger JC (2022): Inflammation as a Pathophysiologic Pathway to Anhedonia: Mechanisms and Therapeutic Implications. Current topics in behavioral neurosciences. [DOI] [PubMed] [Google Scholar]


