Abstract
Women with BRCA1 germline mutations have approximately an 80% lifetime chance of developing breast cancer. While the tumor suppressor function of BRCA1 in breast epithelium has been studied extensively, it is not clear whether BRCA1 deficiency in non-breast somatic cells also contribute to tumorigenesis. Here, we report that mouse Brca1 knockout (KO) in mature T lymphocytes compromises host antitumor immune response to transplanted syngeneic mouse mammary tumors. T cell adoptive transfer further corroborates CD8+ T cell-intrinsic impact of Brca1 KO on antitumor adaptive immunity. T cell-specific Brca1 KO mice exhibit fewer total CD8+, more exhausted, reduced cytotoxic, and reduced memory tumor-infiltrating T cell populations. Consistent with the preclinical data, cancer-free BRCA1 mutation-carrying women display lower abundance of circulating CD8+ lymphocytes than the age-matched control group. Thus, our findings support the notion that BRCA1 deficiency in adaptive immunity could contribute to BRCA1-related tumorigenesis. We also suggest that prophylactic boosting of adaptive immunity may reduce cancer incidence among at-risk women.
Keywords: Breast Neoplasms, CD8-Positive T-Lymphocytes, Adaptive Immunity
Background
Germ-line mutations in the breast cancer susceptibility gene BRCA1 are associated with a significantly increased incidence of breast cancer in women. Extensive investigations have shed light on the molecular functions of BRCA1 in tumor suppression. The most prominent BRCA1 functions in the context of genomic stability are promotion of homologous recombination-based double-strand break (DSB) DNA repair and stabilization of DNA replication forks during replication stress.1 Within the breast epithelium, luminal progenitor cells are considered the cell of origin of BRCA1-associated breast tumors.2–4 In further support, it has been shown that the RANK-RANKL axis, which drives paracrine actions in luminal homeostasis, is aberrantly activated in BRCA1 mutation carriers prior to any clinical evidence of breast cancer.5 6 While findings of breast epithelium-focused research have added significantly to the current understanding of BRCA1-related tumor etiology, it remains unclear whether BRCA1 deficiency in somatic cells beyond breast epithelium could contribute to tumor development and progression.
CD8+ T lymphocytes are a critical component of host antitumor immunity. On encountering specific tumor-associated antigens, naïve CD8+ T cells undergo rapid proliferation and differentiation to acquire the cytotoxic effector function.7 A subset of short-lived effector cells develops into memory T cells. Both effector and memory T cells play important roles in deterring tumor growth. Persistent and excessive presence of tumor antigens often induces an exhaustion phenotype of tumor-reactive T cells, which is associated with upregulation of multiple inhibitory receptors and loss of T cell polyfunctionality.8 Anticancer immunotherapies aimed at reinvigorating antitumor T cells have transformed cancer treatment. Here, we provide functional evidence for a potential T cell-intrinsic BRCA1 function in promoting antitumor immunity. Our findings could inform novel preventive measures to reduce cancer risk reduction among BRCA1 mutation carriers.
Results
By mining a recent single-cell transcriptomic dataset from human breast cancer-associated T cells,9 we found that human BRCA1 and BRCA2, another breast cancer susceptibility gene, were both highly expressed in tumor infiltrating, CD8+ mitotic tissue-resident memory T cells (online supplemental figure 1A). In addition, using a separate dataset from a more recent study of human germline variants and cancer-related immune traits,10 we observed an association of BRCA1 germline mutations with fewer CD8+ tumor-infiltrating lymphocytes (TILs) in human breast cancer (online supplemental figure 1B). Based on publicly available mouse transcriptomic data,11 we also noted that mouse Brca1 mRNA levels were significantly elevated in activated CD8+ T cells versus their naïve counterparts (online supplemental figure 2A). Consistent with the mRNA data, BRCA1 protein was undetectable in mouse naïve CD8+ T cells, but its abundance was substantially increased on T cell receptor (TCR)-mediated activation in vitro (online supplemental figure 2B). Thus, these data concerning the BRCA1 gene status and its expression pattern are consistent with a possible T cell-intrinsic role of BRCA1 in tumorigenesis.
jitc-2022-005852supp001.pdf (284.5KB, pdf)
jitc-2022-005852supp002.pdf (158.4KB, pdf)
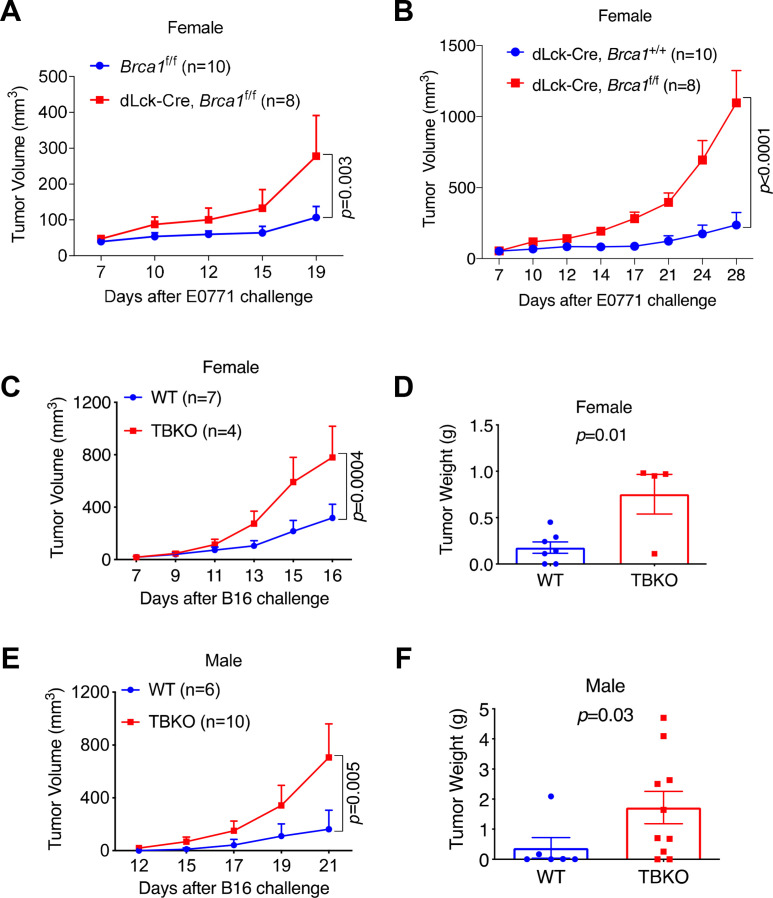
To interrogate the functionality of mouse Brca1 in CD8+ T cells, we generated a conditional mouse Brca1 mutant strain in which exon 11 of both Brca1 alleles was deleted specifically in mature T cells (dLck-Cre, Brca1f/f, referred to as TBKO thereafter). Mature T cell-specific action of the dLck-Cre strain was demonstrated previously.12 13 Immunoblotting confirmed effective depletion of BRCA1 protein in the CD8+ T cell compartment (online supplemental figure 2B). To assess the consequence of T cell-specific Brca1 KO on antitumor immunity, we transplanted syngeneic mouse mammary tumor cells E0771 into female TBKO mice and their controls, Brca1f/f or dLck-Cre. TBKO mice gave rise to more aggressive tumor growth than both control groups (figure 1A, B and online supplemental figure 2C). We, therefore, used Brca1f/f as the wild-type (WT) control in the following experiments. More aggressive tumor growth in TBKO hosts was also observed in a melanoma tumor model B16 (figure 1C, D), and in both female and male TBKO hosts (figure 1E, F). These results indicate that Brca1 KO in mature T cells of both sexes impairs host adaptive immune response to the growth of multiple tumor types.
Figure 1.
TBKO mice have defective antitumor immunity. (A) E0771 tumor growth curves in Brca1f/f and TBKO mice. (B) E0771 tumor growth curves in dLck-Cre, Brca1+/+ and TBKO mice. (C, D) B16 melanoma tumor growth curves (C) and weights (D) in female WT and TBKO mice. (E, F) B16 melanoma tumor growth curves (E) and weights (F) in male WT and TBKO mice. WT, wild type; TBKO, dLck-Cre, Brca1f/f.
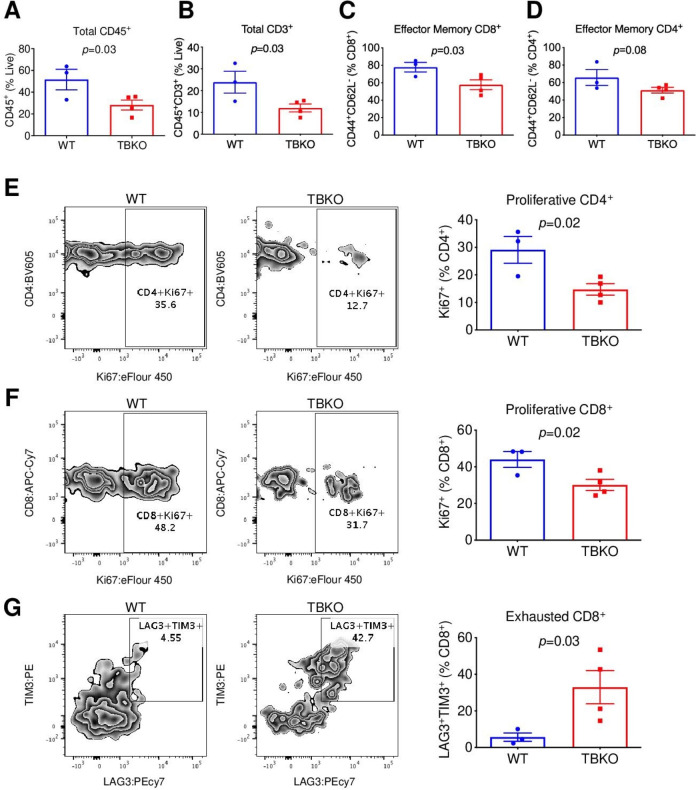
Flow cytometry analysis of splenocytes from naïve mice prior to tumor challenge showed that TBKO mice had lower abundance of total CD45+ and CD8+ T cells than their WT counterparts (online supplemental figure 3A, B). In addition, central memory, but not naïve or effector memory, CD8+ T cells were less abundant in tumor-free TBKO mice versus control (online supplemental figure 3C). By analyzing TILs from tumor-bearing mice, we found substantially fewer total CD45+ (figure 2A), CD3+ (figure 2B) and effector memory CD8+ (figure 2C, online supplemental figure 3D), but not effector memory CD4+ (figure 2D) T cells in TBKO mice versus WT control. Compared with WT, TBKO mice displayed lower proliferative CD4+ (figure 2E) and CD8+ T cells (figure 2F), but markedly more abundant CD8 T cells with the exhaustion markers LAG3 and TIM3 (figure 2G). Taken together, these results support the notion that Brca1 KO impedes the proliferative and cytotoxic functions of antitumor T cells.
Figure 2.
TBKO mice have impaired T cell functions. (A–F) TIL analysis by flow cytometry for total CD45+ leucocytes (A), CD3+ T cells (B), effector memory CD44+CD62L− of CD8+ (C), effector memory CD44+CD62L− of CD4+ (D), proliferative Ki67+ of CD4+ (E), proliferative Ki67+ of CD8+ (F), and exhausted TIM3+LAG3+ of CD8+ (G). TIL, tumor-infiltrating lymphocyte; WT, wild type; TBKO, dLck-Cre, Brca1f/f.
jitc-2022-005852supp003.pdf (1.4MB, pdf)
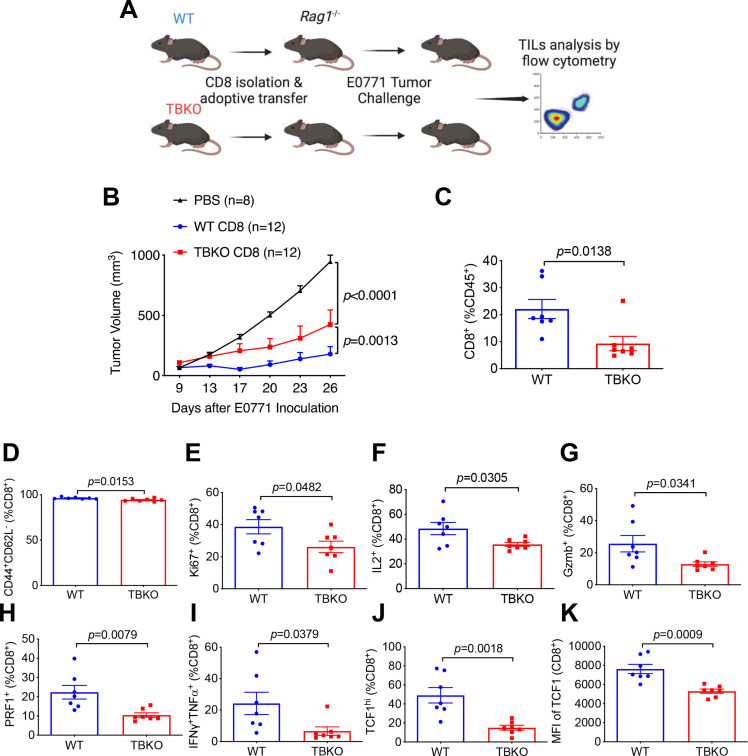
To corroborate a T cell-autonomous effect of Brca1 on antitumor immunity, we adoptively transferred CD8+ T cells from WT or TBKO mice to immunodeficient Rag1-/- recipient mice. The resulting chimeric mice were subsequently grafted with E0771 tumor cells (figure 3A). Compared with chimeric mice with WT CD8+ cells, those adoptively transferred with TBKO CD8+ cells exhibited more robust tumor growth (figure 3B). Furthermore, TILs from TBKO chimeric hosts had substantially lower amounts of total (figure 3C), effector memory (figure 3D), and proliferative (figure 3E) CD8+ T cells versus WT chimeras. Similar differences were also present in CD8+ cells from TBKO and WT chimeras that expressed known activation-related or cytotoxicity-related markers including IL2 (figure 3F), Gzmb (figure 3G), PRF1 (figure 3H), and those that were IFNγ+/TNFα+ polyfunctional (figure 3I). In addition, TCF1, a master transcriptional regulator of memory and stem-like CD8+ T cells,14 was expressed at lower levels in TBKO chimeras than their WT counterparts (figure 3J, K). Collectively, these data strongly suggest an intrinsic role of BRCA1 in supporting CD8+ T cell functions.
Figure 3.
CD8-intrinsic BRCA1 is important for antitumor immunity. (A) scheme of CD8+ adoptive transfer to Rag1-/- mice followed by E0771 tumor challenge. (B) E0771 tumor growth curves in Rag1-/- mice receiving PBS, WT, or TBKO CD8+ cells. (C–K) TIL analysis by flow cytometry for total CD8+ T cell percentage of CD45+ (C), effector memory CD44+CD62L− percentage of CD8+ cells (D), proliferative Ki67+ percentage of CD8+ cells (E), IL2+ percentage of CD8+ cells (F), cytotoxic Gzmb+ percentage of CD8+ cells (G), cytotoxic PRF1+ percentage of CD8+ cells (H), polyfunctional IFNγ+TNFα+ percentage of CD8+ cells (I), TCF1hi percentage of CD8+ cells (J), and TCF1 MFI in CD8+ cells (K) in Rag1-/- mice with adoptively transferred WT or TBKO CD8+ T cells. TIL, tumor-infiltrating lymphocyte; WT, wild type; TBKO, dLck-Cre, Brca1f/f; PBS, Phosphate-Buffered Saline.
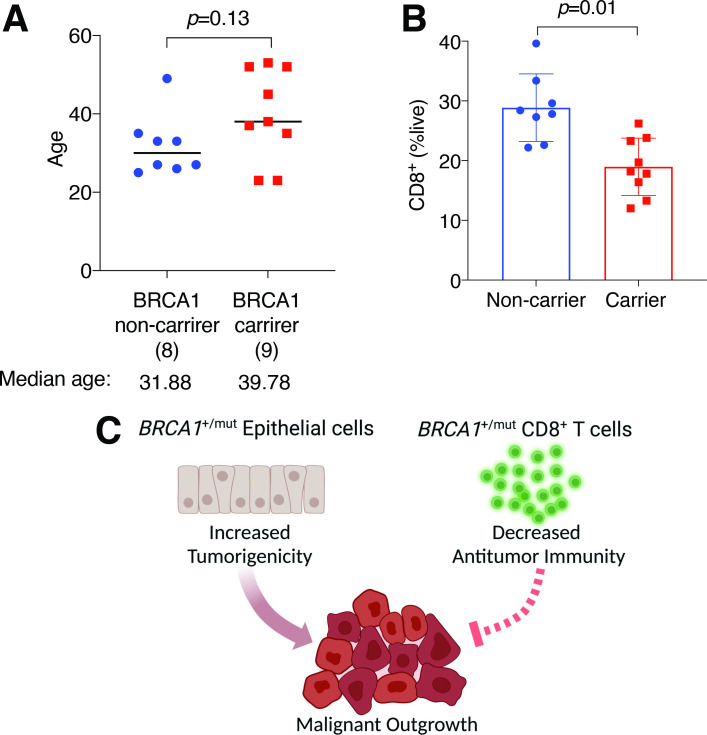
To seek the clinical relevance of our preclinical findings, we procured whole-blood samples from aged-matched, cancer-free control and BRCA1 mutation-carrying women (figure 4A, online supplemental table 1). None of the donors in either group has ever had cancers of any type. Consistent with findings from TBKO mice (online supplemental figure 3A, B), flow cytometry showed that circulating CD8+ T cell abundance was significantly lower in BRCA1 mutation carriers than those in the control group (figure 4B). While the clinical data do not necessarily imply a CD8+ T cell-intrinsic effect of germline BRCA1 mutations, they are in line with the notion that the BRCA1 germline mutation status is associated with altered abundance and/or activity of immune cells in cancer-free BRCA1 mutation carriers.
Figure 4.
Human BRCA1 mutation carriers are associated with lower abundance of CD8+ T cells. (A) Donor ages of non-carriers and carriers of BRCA1 mutation. (B) CD8+ percentages of total live cells in human blood. (C) Proposed dual impact of BRCA1 heterozygosity on breast epithelium and CD8+ T cells.
jitc-2022-005852supp005.pdf (64.1KB, pdf)
Discussion
In this study, we provide evidence for a CD8+ T cell-intrinsic function of BRCA1 in antitumor immunity. The reduced number of total CD8+ in TBKO versus WT tumor-free mice likely explains the lower total leucocytes counts in the spleens. Our detailed immunophenotyping data further indicate that this BRCA1 function is likely due to its role in supporting T cell proliferation, cytotoxic polyfunctionality, and sustaining memory stem cells pool. A previous study showed that deletion of both Brca1 alleles during early T cell development resulted in impaired thymocyte development.15 In contrast to the proximal Lck-Cre system used in the previous work, the distal Lck-Cre (dLck-Cre) system used in our current work was designed specifically to achieve gene ablation without altering T cell development.13 Indeed, it was experimentally confirmed that Cre recombinase in the dLck-Cre strain was only activated after thymocyte positive selection and, therefore, has minimal impact on thymocyte development.12 13 Therefore, the TBKO phenotype observed in the current work strongly suggests a functional role of BRCA1 in mature peripheral T cells, distinct from its reported role in early thymocyte development. Furthermore, because immunophenotyping of both splenocytes from tumor-free mice and TILs from tumor-bearing mice showed reduction of subsets of memory CD8+ cells in TBKO versus WT, these data suggest a role of BRCA1 in promoting the memory population of CD8+ T cells.
Approximately 90% of BRCA1 mutations found clinically result in protein truncation or large-scale gene disruption through various mechanisms, whereas the remaining 10% mutations lead to single amino acid substitutions. Therefore, our preclinical model with the exon 11 deletion recapitulates to a large degree what is found in the clinic and in patients’ CD8+ T cells. The mechanistic basis of the biological role of BRCA1 in supporting T cell functions warrants further investigation. Given the well-established function of BRCA1 in DSB DNA repair and resolution of DNA replication stress in tumor epithelial cells, it is reasonable to speculate that compromised DNA repair or resolution of DNA replication stress likely contribute to impaired proliferation of T cells detected in TBKO mice. In further support, using published transcriptomic datasets from human melanoma TILs,16 we found that BRCA1 mRNA abundance was correlated with genes involved in cell cycle- and DNA repair-related pathways (online supplemental figure 4A). In keeping with this, we observed more apoptosis in TBKO versus WT CD8+ cells post in vitro activation (online supplemental figure 4B). In addition, BRCA1 functions other than maintenance of genetic integrity, such as chromatin remodeling and transcriptional regulation, could also account for the aberrant antitumor activity in TBKO mice and reduced circulating CD8+ T cells in human BRCA1 mutation carriers. In this regard, we recently showed that breast epithelial cells from BRCA1 mutation carriers displayed significant loss of transcriptional super-enhancer functions.17 It is conceivable that impaired BRCA1 expression in T cells could lead to aberrant transcription related to antitumor immunity, which in turn enables immune evasion of BRCA1 mutation-associated breast tumor cells and contributes to the elevated breast cancer risk among BRCA1 mutation-carrying women (figure 4C).
jitc-2022-005852supp004.pdf (1.9MB, pdf)
TCF1, encoded by TCF7, plays a key role in maintaining memory stem cell pool of antitumor CD8+ T cells and dictating response to various immunotherapies including immune checkpoint inhibitors.14 From our TILs analysis (figure 3J, K) we observed significantly lower expression of TCF1 in CD8+ T cells from TBKO versus WT mice, suggesting a potential role of BRCA1 in promoting TCF1-related transcriptional program. In keeping with this model, a recent study demonstrated that BRCA1 mutation carriers had significant lower frequency of p63+TCF7+ myoepithelial cells.18 Future work is needed to determine how BRCA1 affects TCF1 expression and/or activity.19
A previous study reported a higher percentage of stromal TILs in BRCA1-mutated versus WT primary triple negative breast cancer samples.20 Due to deficiency in DNA repair and elevated genomic instability, BRCA1-mutated tumor cells are expected to have increased tumor mutation load and a greater number of neoantigens, which can in turn attract more TILs. Therefore, in BRCA1 mutation carrying patients with breast cancer, the effect of tumor-intrinsic BRCA1 deficiency on TIL abundance could offset compromised antitumor immunity due to T cell-intrinsic loss of BRCA1. In our preclinical tumor models, we bypassed the influence of tumor BRCA1 deficiency by using the same tumor cell line in both WT and TBKO hosts, which enabled us to assess the specific consequences of BRCA1 deficiency in antitumor T cells.
Prophylactic bilateral mastectomy and prophylactic bilateral salpingo-oophorectomy are currently the primary risk-reducing measures for germline BRCA1 mutation carriers.21 Despite their proven clinical efficacy, these prophylactic surgeries are associated with various side effects including pain and postoperative complications, undesired cosmetic outcomes as well as psychosocial morbidity,22 which has prompted exploration of nonsurgical risk-reducing tools for BRCA1 mutation carriers.23 Furthermore, the current prophylactic considerations are aimed at the cell of origin for BRCA1-associated tumors rather than non-breast epithelial cells. On further validation of the concept of T cell-specific BRCA1 function, it may be clinically relevant to search for therapeutic agents that can boost functions of antitumor immunity of CD8+ T cells with BRCA1 deficiency, which could reduce life-time cancer risk for BRCA1 mutation-carrying women.
Methods
Donor age distributions were shown in figure 4A, and the mutation information for individual donors were detailed in online supplemental table 1.
Mice and in vivo tumor study
Brca1f/f (backcrossed to pure C57BL/6J background for 10 generations) and distal Lck-cre (dLck-cre) mice were established as previously described.12 24 Brca1f/f mice were used as the WT control in most of our experiments. Rag1-/- (stock no. 002216) mice were purchased from The Jackson Laboratory. Mice older than 8 weeks were considered adults and used for all the experiments.
For tumor challenge, syngeneic mammary tumor E0771 cells (1×106 cells) (CH3 Biosystems, 940001) or B16 (1×105 cells) (a generous gift from Tyler Curiel25) were injected into the fourth mammary fat pad or back flank of C57BL/6 mice, respectively. For adoptive CD8+ T cell transfer experiments, CD8+ cells were isolated from mouse spleen using an EasySep Mouse CD8+ T Cell Isolation Kit (STEMCELL Technologies, 19853). WT or TBKO (2.5×106) CD8+ were transferred by tail vein injection into Rag1-/- recipients. E0771 (1×106 cells) mammary tumor cells were then inoculated into the fourth mammary fat pad 1 day after CD8+ adoptive transfer.
Flow cytometry for mouse samples
Cells were stained with Viability Ghost Dye 510 (Tonbo Biosciences, 13-0870) and blocked by anti-CD16/32 (Tonbo Bioscience, 70-0161). Cells were further stained with anti-CD45 (Invitrogen, 11-0451-82), anti-CD3 (Tonbo Biosciences, 65-0031 U100), anti-CD8 (BD Pharmingen, 557654), anti-CD44 (BioLegend, 103041), anti-CD62L (BioLegend, 104424), anti-TIM3 (Biolegend, 119704), and anti-LAG3 (Invitrogen, 25223182). For nuclear transcription factor staining, cells were permeabilized using a FoxP3/transcription factor staining kit (eBioscience, 00-5523-00) and stained with anti-Ki67 (Invitrogen, 48-5698-82) and anti-TCF1 (Biolegend, 655203). For cytokine staining, cells were stimulated by anti-CD3/CD28 (ThermoFisher, 11 452D) and then treated with BD GolgiPlug (BD Biosciences, 550583). Cells were permeabilized using a BD Cytofix/Cytoperm kit (BD Biosciences, 554714) and stained with anti-IFNγ (BioLegend, 505826), anti-TNFα (BioLegend, 506314), anti-IL2 (Invitrogen, 45-7021-82), anti-Granzyme B (Invitrogen, 12-8898-82), anti-Perforin (Invitrogen, 11-9392-82). Apoptosis was assessed as previously mentioned.19 Data were analyzed using BD FACSDiva and FlowJo software.
In vitro T cell activation and immunoblotting
Naive CD8+ cells were isolated from mouse spleen using an EasySep Mouse CD8+ T Cell Isolation Kit (STEMCELL Technologies, 19858) and then activated by anti-CD3/CD28 (Thermo Fisher, 11 452D). After 48 hours of activation, cells were lysed for immunoblotting. Anti-BRCA1 antibody (Santa Cruz, sc-135732) and corresponding secondary antibody were used.
Flow cytometry analysis for human blood samples
Whole blood samples of BRCA1 non-carriers and mutation carriers were collected in lithium-heparin-coated tubes (BD, 367880). Samples were incubated with anti-human Fc receptor binding inhibitor antibody (Invitrogen, 14-9161-73) for 15 min in 4°C. Red blood cells were lysed with RBC lysis buffer (Invitrogen, 00-4300-54). Cells were then incubated with Viability Ghost Dye 510 (Tonbo Biosciences, 13-0870) and blocking buffer (Invitrogen, 14-9161-73), followed by anti-CD3 (Invitrogen, 63-0037-42), anti-CD4 (Biolegend, 344710), anti-CD8 (Biolegend, 344710) staining. Flow cytometry data were acquired by a BD FACSCelesta and analyzed using BD FACSDiva and FlowJo software.
Statistics
Mean differences between two groups were tested using Student’s t-test. Mean differences between three or more groups were tested using one-way analysis of variance (ANOVA). Tumor curves were compared using two-way ANOVA, followed by multiple comparisons. Statistics were performed using GraphPad Prism software. A p<0.05 was considered significant.
Acknowledgments
We thank Dr. Tyler Curiel for providing B16 tumor cell line.
Footnotes
Twitter: @bogang_wu
BW and LQ contributed equally.
Contributors: RL and YH managed and oversaw the overall project. RL, BW and LQ designed the experiments and wrote the manuscript. BW, LQ, and H-CC carried out the experiments. BW, LQ, HP, XZ, L-JW, YC, AH, YH, and RL analyzed the data. AG, ES, BH, DC, CI and RE were involved in clinical sample procurement, analysis, and data interpretation.
Funding: The work was supported by grants to RL from NIH (CA220578 and CA246707), to YH from NIH (CA212674) and the Congressionally Directed Medical Research Program (W81XWH-17-1-0008).
Competing interests: Claudine Isaacs: Consultancies: Genentech, PUMA, Seagen, AstraZeneca, Novartis, Pfizer, ION; Gilead; Royalties: Wolters Kluwer (UptoDate); McGraw Hill (Goodman and Gillman); Research support (to institution): Tesaro/GSK; Seattle Genetics; Pfizer; AZ; BMS; Genentech; Novartis.
Rong Li: Scientific advisory board of Parthenon Therapeutics.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as online supplemental information.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Blood samples were procured from cancer-free BRCA1 mutation carriers and non-carriers, following a protocol approved by the Institutional Review Board (NCR191157) at the George Washington University. All donors signed written consent forms authorizing the use of the specimens for breast cancer-related laboratory investigations.
References
- 1.Venkitaraman AR. Cancer suppression by the chromosome custodians, BRCA1 and BRCA2. Science 2014;343:1470–5. 10.1126/science.1252230 [DOI] [PubMed] [Google Scholar]
- 2.Lim E, Vaillant F, Wu D, et al. Aberrant luminal progenitors as the candidate target population for basal tumor development in BRCA1 mutation carriers. Nat Med 2009;15:907–13. 10.1038/nm.2000 [DOI] [PubMed] [Google Scholar]
- 3.Molyneux G, Geyer FC, Magnay F-A, et al. BRCA1 basal-like breast cancers originate from luminal epithelial progenitors and not from basal stem cells. Cell Stem Cell 2010;7:403–17. 10.1016/j.stem.2010.07.010 [DOI] [PubMed] [Google Scholar]
- 4.Proia TA, Keller PJ, Gupta PB, et al. Genetic predisposition directs breast cancer phenotype by dictating progenitor cell fate. Cell Stem Cell 2011;8:149–63. 10.1016/j.stem.2010.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nolan E, Vaillant F, Branstetter D, et al. RANK ligand as a potential target for breast cancer prevention in BRCA1-mutation carriers. Nat Med 2016;22:933–9. 10.1038/nm.4118 [DOI] [PubMed] [Google Scholar]
- 6.Sigl V, Owusu-Boaitey K, Joshi PA, et al. RANKL/RANK control BRCA1 mutation-driven mammary tumors. Cell Res 2016;26:761–74. 10.1038/cr.2016.69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Philip M, Schietinger A. CD8+ T cell differentiation and dysfunction in cancer. Nat Rev Immunol 2022;22:209–23. 10.1038/s41577-021-00574-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ando M, Ito M, Srirat T, et al. Memory T cell, exhaustion, and tumor immunity. Immunol Med 2020;43:1–9. 10.1080/25785826.2019.1698261 [DOI] [PubMed] [Google Scholar]
- 9.Savas P, Virassamy B, Ye C, et al. Single-cell profiling of breast cancer T cells reveals a tissue-resident memory subset associated with improved prognosis. Nat Med 2018;24:986–93. 10.1038/s41591-018-0078-7 [DOI] [PubMed] [Google Scholar]
- 10.Sayaman RW, Saad M, Thorsson V, et al. Germline genetic contribution to the immune landscape of cancer. Immunity 2021;54:367–86. 10.1016/j.immuni.2021.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meng X, Liu X, Guo X, et al. FBXO38 mediates PD-1 ubiquitination and regulates anti-tumour immunity of T cells. Nature 2018;564:130–5. 10.1038/s41586-018-0756-0 [DOI] [PubMed] [Google Scholar]
- 12.Zhang N, Bevan MJ. TGF-β signaling to T cells inhibits autoimmunity during lymphopenia-driven proliferation. Nat Immunol 2012;13:667–73. 10.1038/ni.2319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang DJ, Wang Q, Wei J, et al. Selective expression of the cre recombinase in late-stage thymocytes using the distal promoter of the Lck gene. J Immunol 2005;174:6725–31. 10.4049/jimmunol.174.11.6725 [DOI] [PubMed] [Google Scholar]
- 14.Siddiqui I, Schaeuble K, Chennupati V, et al. Intratumoral TCF1+PD-1+CD8+ T cells with stem-like properties promote tumor control in response to vaccination and checkpoint blockade immunotherapy. Immunity 2019;50:195–211. 10.1016/j.immuni.2018.12.021 [DOI] [PubMed] [Google Scholar]
- 15.Mak TW, Hakem A, McPherson JP, et al. BRCA1 required for T cell lineage development but not TCR loci rearrangement. Nat Immunol 2000;1:77–82. 10.1038/76950 [DOI] [PubMed] [Google Scholar]
- 16.Tirosh I, Izar B, Prakadan SM, et al. Dissecting the multicellular ecosystem of metastatic melanoma by single-cell RNA-seq. Science 2016;352:189–96. 10.1126/science.aad0501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang X, Wang Y, Chiang H-C, et al. BRCA1 mutations attenuate super-enhancer function and chromatin looping in haploinsufficient human breast epithelial cells. Breast Cancer Res 2019;21:51. 10.1186/s13058-019-1132-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ding L, Su Y, Fassl A, et al. Perturbed myoepithelial cell differentiation in BRCA mutation carriers and in ductal carcinoma in situ. Nat Commun 2019;10:4182. 10.1038/s41467-019-12125-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu B, Zhang X, Chiang H-C, et al. RNA polymerase II pausing factor NELF in CD8+ T cells promotes antitumor immunity. Nat Commun 2022;13:2155. 10.1038/s41467-022-29869-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nolan E, Savas P, Policheni AN, et al. Combined immune checkpoint blockade as a therapeutic strategy for BRCA1-mutated breast cancer. Sci Transl Med 2017;9. doi: 10.1126/scitranslmed.aal4922. [Epub ahead of print: 07 Jun 2017]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ludwig KK, Neuner J, Butler A, et al. Risk reduction and survival benefit of prophylactic surgery in BRCA mutation carriers, a systematic review. Am J Surg 2016;212:660–9. 10.1016/j.amjsurg.2016.06.010 [DOI] [PubMed] [Google Scholar]
- 22.Galimberti V, Vicini E, Corso G, et al. Nipple-sparing and skin-sparing mastectomy: review of aims, oncological safety and contraindications. Breast 2017;34 Suppl 1:S82–4. 10.1016/j.breast.2017.06.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singer CF. Nonsurgical prevention strategies in BRCA1 and BRCA2 mutation carriers. Breast Care 2021;16:144–8. 10.1159/000507503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang X, Chiang H-C, Wang Y, et al. Attenuation of RNA polymerase II pausing mitigates BRCA1-associated R-loop accumulation and tumorigenesis. Nat Commun 2017;8:15908. 10.1038/ncomms15908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clark CA, Gupta HB, Sareddy G, et al. Tumor-intrinsic PD-L1 signals regulate cell growth, pathogenesis, and autophagy in ovarian cancer and melanoma. Cancer Res 2016;76:6964–74. 10.1158/0008-5472.CAN-16-0258 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2022-005852supp001.pdf (284.5KB, pdf)
jitc-2022-005852supp002.pdf (158.4KB, pdf)
jitc-2022-005852supp003.pdf (1.4MB, pdf)
jitc-2022-005852supp005.pdf (64.1KB, pdf)
jitc-2022-005852supp004.pdf (1.9MB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as online supplemental information.