Abstract
Background
Recent studies suggest increased complications when surgery closely follows corticosteroid injection. The purpose of this study was to understand the occurrence of surgical site infection (SSI), wound complications, and reoperation rates after carpal tunnel release (CTR) when corticosteroid injections were given within 180 days preoperatively.
Methods
Data were collected from the Truven MarketScan database from 2009 to 2018. Cohorts were created based on preoperative timing of injection (<30, 30-60, 61-90, 91-180 days) and number of injections (0, 1, 2, 3+). Logistic regression was performed to assess the contribution of preoperative injections on 90-day surgical site complications and 1-year reoperation while controlling for demographics and comorbidities.
Results
Overall, 223 899 patients underwent CTR. Of these, 17 391 (7.76%) had a preoperative injection in the 180 days preceding surgery. Univariate analysis demonstrated a relationship between timing of injections and noninfectious wound complications (P = .006) and rate of 1-year reoperation (P = .045). Univariate analysis demonstrated a relationship between number of injections and 1-year reoperation (P < .001). On multivariate analysis, those receiving injections within 30 days preoperatively had increased rates of SSI (P = .034) and noninfectious wound complications (P = .006) compared with those with no injection or at other time points. Patients with 2 (P = .002) or 3 or more injections (P < .001) in the 180-day preoperative period had increased odds of 1-year reoperation.
Conclusion
Our study suggests increased risk of SSI, wound complications, and 1-year reoperation when corticosteroid injections are administered in the 30-day preoperative period. In addition, multiple steroid injections may increase the risk of 1-year reoperation.
Keywords: Carpal tunnel syndrome, carpal tunnel release, corticosteroid injections, surgical site infection
Introduction
Carpal tunnel syndrome (CTS) is a common and potentially debilitating condition.1-3 Management is dependent on the severity of a patient’s symptoms.1-3 Conservative approaches to management of CTS include anti-inflammatory drugs, immobilization, splinting, and hand therapy.2,4 Steroid injections are easily administered in the office and provide symptom relief by suppressing local inflammation.5,6 Therapeutic steroid injections have been repeatedly demonstrated as a cost-effective and safe choice to relieve CTS symptoms.7-10 For some, the symptom relief is not sufficient, eventually requiring surgical intervention. 11
The effects of steroid injections on postoperative outcomes have long been a focus of research in total joint arthroplasty12-15 and spine surgery,16-18 with prior publications revealing increased postoperative complications at follow-up intervals ranging from 1 to 12 months. Specific to the upper extremity, a potential association between preoperative injections and postoperative complications in carpometacarpal (CMC) arthroplasty and trigger finger release has been suggested.19,20
It has been demonstrated that preoperative injections may increase the rates of postoperative complications in numerous orthopedic surgical procedures. Despite this, few studies have explored the potential harm of the frequency of injections administered so often before surgery. The purpose of this study was to understand how these 2 factors influence optimal surgical timing as demonstrated by specific postoperative complications (ie, surgical site infection [SSI], noninfectious wound complications, and 1-year reoperation rates). We hypothesized there would be an increased risk of SSI, wound complication, and reoperation in patients receiving multiple injections or injections less than 30 days prior to surgery.
Methods
Data Source
Data for this study were identified and collected from the Truven MarketScan Commercial Claims and Encounters and Medicare Supplemental and Coordination of Benefit database which comprises billing data from patients with both private insurance and Medicare. The utilization of this database allows for following a large, geographically diverse cohort of patients longitudinally. This database provides a holistic health care summary for each patient because it collect data from pharmaceutical records and inpatient and outpatient visits. 21
Patient Selection
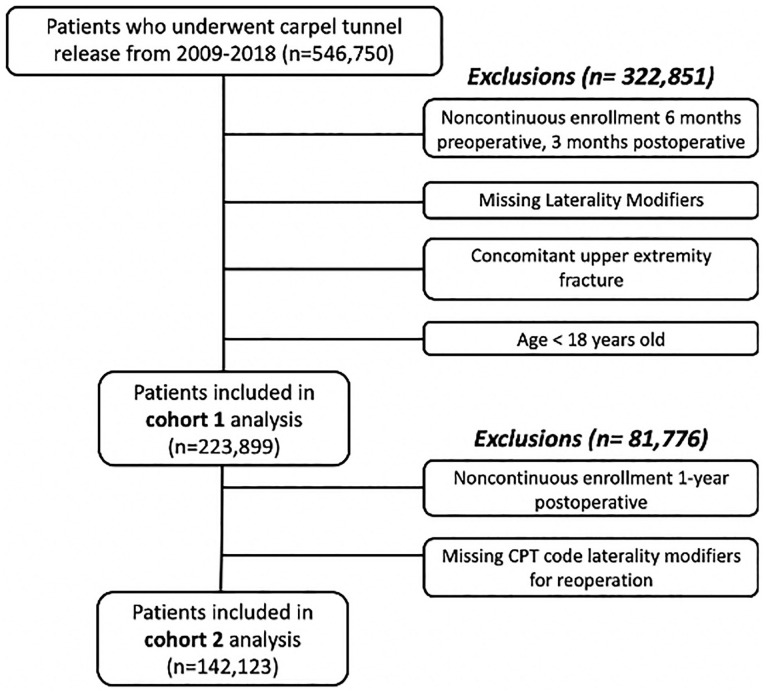
The database was queried from 2009 to 2018 for all patients undergoing carpal tunnel release (CTR) with Common Procedural Terminology (CPT) codes 29848 (endoscopic carpal tunnel release) and 64721 (neuroplasty and/or transposition; median nerve at carpal tunnel). We eventually separated the group into multiple cohorts for tracking 90-day (223 899) and 1-year (142 123) outcomes (Figure 1). General exclusion criteria were age less than 18 years, lack of CPT code laterality modifiers for the index CTR, concurrent distal upper extremity fracture, lack of enrollment in the database 6 months prior to surgery, and lack of enrollment in the database 3 months after surgery. Those who were analyzed for 1-year reoperation rates were excluded if they did not have 1 year of continuous postoperative enrollment or CPT code laterality modifiers for the reoperation.
Figure 1.
Schematic of database query and subjects included in analysis.
Preoperative Patient Data
In the 6 months prior to the CTR, injections were queried. To ensure that the majority of preoperative carpal tunnel injections were captured, the following CPT codes were used: 20526 (injection, carpal tunnel), 20600 (arthrocentesis, aspiration, and/or injection; small joint or bursa), and 20605 (injection, intermediate joint or bursa). These injection codes were queried for laterality (ie, left or right) with CPT code laterality modifiers and diagnosis via International Classification of Diseases, 9th Revision (ICD-9) and International Classification of Diseases, 10th Revision (ICD-10). This was done to ensure that the injection was performed for a diagnosis of CTS and that the injection was performed on the ipsilateral wrist as the CTR. Patients were then stratified into cohorts based on the timing of their most recent injection prior to surgery: ≤30, 31-60, 61-90, and 91-180 days. In addition, we stratified patients by the total number of injections received in the 180-day preoperative period, dividing into cohorts of 0, 1, 2, or 3+.
Demographic information was collected for each patient, which included age, sex, comorbidities, surgery type (endoscopic or open), and smoking status. Comorbidity information was collected using the Elixhauser comorbidity index. 22 The number of comorbidities were tallied and quantified as the total number of comorbidities present. These were grouped from 0 to 4+. Each comorbidity was weighted equally.
Outcomes
Postoperative complications were identified with the use of ICD-9 and ICD-10 diagnosis codes and CPT codes. For 90-day outcomes, we collected SSI and noninfectious wound complications, such as hematoma or seroma formation. Reoperation within 1 year was also queried and was defined as the patient undergoing an additional CTR on the ipsilateral wrist within 1 year of surgery. Patients without CPT code laterality modifiers present for revision procedures were excluded from the analysis, as we could not definitely determine whether this was a reoperation or an index operation on the contralateral wrist.
Statistical Analysis
Statistical analysis was conducted with SAS version 9.4 (SAS, Cary, North Carolina). A value of P < .05 was considered significant. After patients in this study were divided into their respective cohorts, baseline patient demographic and comorbidity data were calculated and compared using χ2 analysis. Thereafter, binomial logistic regression was performed controlling for the baseline demographic, surgical, and patient comorbidity data contained in Table 1 to assess the independent association between preoperative injection and postoperative complications.
Table 1.
Preoperative Injection Timing by Patient Demographics and Comorbidities.
| Characteristic | Injection timing | value | ||||
|---|---|---|---|---|---|---|
| None | <30 days | 30-60 days | 61-90 days | 91-180 days | ||
| Total | 206 508 (92.2%) | 2600 (1.16%) | 5034 (2.25%) | 3645 (1.62%) | 6112 (2.72%) | — |
| Age category, y | ||||||
| <45 | 37 240 (18.03%) | 577 (22.19%) | 1185 (23.54%) | 902 (24.75%) | 1453 (23.77%) | <.001 |
| 45-54 | 58 079 (28.12%) | 718 (27.62%) | 1428 (28.37%) | 1018 (27.93%) | 1781 (29.14%) | |
| 55-64 | 69 756 (33.78%) | 796 (30.62%) | 1492 (29.64%) | 1158 (31.77%) | 1909 (31.23%) | |
| >65 | 41 433 (20.06%) | 509 (19.58%) | 929 (18.45%) | 567 (15.56%) | 969 (15.85%) | |
| Sex | ||||||
| Male | 75 992 (36.80%) | 956 (36.77%) | 1707 (33.91%) | 1150 (31.55%) | 1757 (28.75%) | < .001 |
| Female | 130 516 (63.20%) | 1644 (63.23%) | 3327 (66.09%) | 2495 (68.45%) | 4355 (71.25%) | |
| Number of comorbidities | ||||||
| 0 | 85 693 (41.50%) | 1034 (39.77%) | 2039 (40.50%) | 1524 (41.81%) | 2637 (43.14%) | .111 |
| 1 | 55 851 (27.05%) | 727 (27.96%) | 1376 (27.33%) | 979 (26.86%) | 1655 (27.08%) | |
| 2 | 36 235 (17.55%) | 450 (17.31%) | 894 (17.76%) | 652 (17.89%) | 1017 (16.64%) | |
| 3 | 17 465 (8.46%) | 218 (8.38%) | 433 (8.60%) | 285 (7.82%) | 483 (7.90%) | |
| 4+ | 11 264 (5.45%) | 171 (6.58%) | 292 (5.80%) | 205 (5.62%) | 320 (5.24%) | |
| Surgery type | ||||||
| Open | 164 544 (79.68%) | 1991 (76.58%) | 3929 (78.05%) | 2790 (76.54%) | 4703 (76.95%) | .059 |
| Endoscopic | 41 964 (20.32%) | 609 (23.42%) | 1105 (21.95%) | 855 (23.46%) | 1409 (23.05%) | |
| Smoking status | ||||||
| No | 91 232 (92.60%) | 2379 (91.50%) | 4596 (91.30%) | 3359 (92.15%) | 5684 (93.00%) | < .001 |
| Yes | 15 276 (7.40%) | 221 (8.50%) | 438 (8.70%) | 286 (7.85%) | 428 (7.00%) | |
Results
Baseline Cohort Information
We identified 223 899 patients with 90-day follow-up who had undergone CTR during the time period of interest meeting our exclusion and inclusion criteria (Figure 1). Patients were separated into cohorts, as described above, with the distribution of preoperative patient variables described in Table 1. Of the 223 899 patients included, 2600 (1.16%) had injections at less than 30 days, 5034 (2.25%) at 30-60 days, 3645 (1.62%) at 61-90 days, and 6112 (2.72%) at 91-180 days before surgery. There were a number of demographic differences between the 2 cohorts; however, the differences were small and likely clinically insignificant. Cohorts receiving an injection tended to be younger, on average, than the cohort not receiving injections (P = .011). In addition, those receiving injections were more likely to be women (P < .001). Differences in smoking status were statistically significant but small with no apparent pattern between cohorts (Table 1). Complications were then collected and compared between cohorts, both by time (in days) between injection and surgery and by the total number of injections within the 180-day period.
Surgical Site Infections
Infections rates were 1.77%, 1.21%, 1.40%, and 1.19% for less than 30 days, 30-60 days, 61-90 days, and 91-180 days, respectively (Table 2). Those not receiving injections had an infection rate of 1.28%. On univariate analysis, this trend was not significant (P = .207) (Table 2). On multivariate analysis, there was a significant relationship between injections at <30 days and SSIs (P = .034) (Table 2). Those receiving injections within 30 days prior to surgery had a 1.37 (confidence Interval [CI]: 1.02-1.84, P = .034) times increased odds of SSIs compared with those who did not receive a preoperative injection.
Table 2.
Univariate Analysis of Complications.
| Characteristic | 90-day follow-up | 1-year follow-up | ||||||
|---|---|---|---|---|---|---|---|---|
| Infection | Noninfectious complication | Total | 1-year reoperation a | |||||
| Total | No. (%) | P value | No. (%) | P value | No. (%) | P value | ||
| Injection timing | ||||||||
| None | 206 508 | 2636 (1.28) | .207 | 1306 (0.63) | .006 | 131 425 | 1890 (1.44) | .045 |
| <30 d | 2600 | 46 (1.77) | 29 (1.12) | 1576 | 33 (2.09) | |||
| 30-60 d | 5034 | 61 (1.21) | 38 (0.75) | 3136 | 59 (1.88) | |||
| 61-90 d | 3645 | 51 (1.40) | 23 (0.63) | 2240 | 28 (1.25) | |||
| 91-180 d | 6112 | 73 (1.19) | 27 (0.44) | 3746 | 59 (1.58) | |||
| Number of injections | ||||||||
| 0 | 206 508 | 2615 (1.27) | .684 | 1306 (0.63) | .374 | 131 425 | 1890 (1.44) | <.001 |
| 1 | 8858 | 114 (1.29) | 62 (0.70) | 5851 | 67 (1.15) | |||
| 2 | 6228 | 81 (1.30) | 35 (0.56) | 3636 | 76 (2.09) | |||
| 3+ | 2305 | 36 (1.56) | 20 (0.87) | 1211 | 36 (2.97) | |||
Confirmed with Common Procedural Terminology Code Laterality Modifiers.
Noninfectious Wound Complications
Noninfectious wound complication rates were 1.12%, 0.75%, 0.63%, and 0.44% for less than 30 days, 30-60 days, 61-90 days, and 91-180 days, respectively (Table 2). On univariate analysis, those receiving injections had an increased rate of complications compared with those not receiving injections (P = .0006) (Table 2). On multivariate analysis, those receiving injections within 30 days prior to surgery had notably increased rates of complications (1.12%) compared with those receiving no injection (0.63%) (P = .006) (Table 3). Similar findings were seen with noninfectious wound complications, where a 1.73 (CI: 1.20-2.52, P = .003) times increased odds of a complication were seen in those receiving injections 30 days prior to surgery compared with those who did not receive injections. Corticosteroid injections received at other time points were not shown to significantly increase infection risk (P > .05; Table 3). In addition, there was no difference in the univariate or multivariate rates of these complications with increasing injection counts in the total 180-day preoperative period (Tables 2 and 3).
Table 3.
Multivariate Analysis of Complications.
| Characteristic | Infection | Non-infectious complication | 1-year reoperation a | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |
| Injection timing | ||||||
| None | Ref. | — | Ref. | — | Ref. | — |
| <30 d | 1.37 (1.02-1.84) | .034 | 1.73 (1.20-2.52) | .003 | 1.46 (1.03-2.07) | .033 |
| 30-60 d | 0.94 (0.73-1.22) | .656 | 1.18 (0.85-1.63) | .318 | 1.31 (1.01-1.70) | .044 |
| 61-90 d | 1.11 (0.83-1.46) | .476 | 1.00 (0.66-1.52) | .982 | 0.88 (0.60-1.28) | .503 |
| 91-180 d | 0.95 (0.75-1.21) | .711 | 0.72 (0.49-1.05) | .091 | 1.12 (0.86-1.46) | .390 |
| Number of injections | ||||||
| 0 | Ref. | — | Ref. | — | Ref. | — |
| 1 | 1.05 (0.87-1.25) | .602 | 1.12 (0.88-1.43) | .342 | 0.80 (0.63-1.03) | .081 |
| 2 | 1.03 (0.82-1.28) | .794 | 0.92 (0.66-1.27) | .619 | 1.47 (1.17-1.86) | .002 |
| 3+ | 1.23 (0.89-1.71) | .206 | 1.35 (0.86-2.10) | .183 | 2.09 (1.52-2.94) | <.001 |
Note. OR = odds ratio; CI = confidence interval.
Confirmed with Common Procedural Terminology Code Laterality Modifiers.
1-Year Reoperation
We identified 142 123 patients with 1-year follow-up who met the inclusion criteria (Figure 1). One-year reoperation rates were 2.09%, 1.88%, 1.25%, and 1.58 % for less than 30 days, 30-60 days, 61-90 days, and 91-180 days, respectively (Table 2). Patients who received no injections averaged a 1.44% rate of reoperation (Table 2). Univariate analysis showed significant differences in outcome with respect to timing of injection (P = .045) and number of injections (P < .001) (Table 2). Multivariate analysis showed increased odds of reoperation when an injection was received within 30 days prior to surgery (odds ratio [OR], 1.46; CI, 1.03-2.07; P = .033) and 31-60 days prior to surgery (OR, 1.31; CI, 1.01-1.70; P = .044). Those receiving 2 total injections (OR, 1.47; 95% CI, 1.17-1.86; P = .002) and 3 or more injections (OR, 2.09; CI, 1.52-2.94; P < .001) also had increased odds of reoperation compared with those not receiving any preoperative injections.
Discussion
Prior studies have investigated the association between steroid injections and postoperative complications in numerous orthopedic surgical procedures.12-17 Specific to patients undergoing CTR, preoperative steroid injections have been shown to be associated with greater postoperative complaints, specifically pain, paresthesia, and nocturnal awakening following CTR, but an association with reoperation and wound complication rates has yet to be investigated. 23 The purpose of this study was to understand how the timing and number of preoperative steroid injections impact surgical risk by examining 3 postoperative complications (SSIs, wound complications, and reoperation rates) to better inform the discussion between patients and surgeons when deliberating the treatment plan.
Our findings suggest that both days since most recent injection and number of therapeutic injections are potentially modifiable risk factors for early postoperative infections, wound complications, and 1-year reoperation following CTR. Injection within 30 days of surgery increased the rates of infection, noninfectious wound complications, and reoperation at 1 year. This trend was not statistically significant in time periods of 30-60, 61-90, or 91-180 days, suggesting that injections given outside of the 30-day preoperative window can more safely be given to patients. Rates of reoperation at 1 year increased in patients receiving 2 or more injections prior to surgery.
The results of this study expand upon those of prior investigations which have shown therapeutic corticosteroid injections to be a modifiable risk factor for prosthetic joint infection following total joint arthroplasty,13,24-26 infection and revision following rotator cuff repair,12,14,27 and infection following hip arthoscopy. 15 Specific to the hand investigations, preoperative corticosteroid injections have been associated with increased rates of infection following surgical treatment of CMC arthritis, where a dose-dependent relationship was observed between steroid injection and complications. 19 Similarly, in trigger finger release, preoperative injections seem to be associated with increased rates of infection in a time-dependent manner, whereas most deep infections occurred in those patients receiving injections within the immediate 90-day preoperative period. 20 Our findings strengthen the body of evidence that operative intervention within 30 days after the most recent steroid injection comes along with a risk significantly increased over that of baseline. 28
There are several mechanisms that may be driving the injection-outcome relationship present in this study. The increased rates of infection may be secondary to the local immunosuppressant effects at the injection site—in addition to breaking the skin barrier prior to surgery—which may potentially seed the area and predispose to infection following surgery.29-32 Similarly, corticosteroids have also been shown to lower tissue deposition of important wound healing growth factors, which may predispose to the wound complications present in this study and others. 32
Like any large database study, this article has limitations rooted in the use of the Truven database. 33 Our query was limited to commonly used CPT codes with concurrent ICD-9 and ICD-10 codes of CTS; thus, we could have potentially missed injections that were miscoded, such as surgeons using 20550 (injections, single tendon sheath), which does not adequately characterize the procedure performed but may still be used. Furthermore, this association is certainly not evidence of causation as those patients more likely to have postoperative complications may also be more likely to be recommended for injection preoperatively by their surgeon. This is certainly possible and should be established, but is unlikely based on multiple prior studies with similar findings. In contrast, there does not seem to be a logical physiologic explanation for causation of revision surgery after multiple injections. This, in the authors’ opinion, is likely related to an association between those patients chosen for multiple injections and those patients who may require revision surgery, potentially due to atypical presentations of this pathology or unclear diagnoses. Furthermore, the association noted between wound complication and injection is at its worst, within 30 days, an increased risk of 1%, which although is statistically significant may be a risk that some providers and patients would be willing to accept. As Truven only collects data for claims through insurance, the generalizability of our findings may be limited to only those with insurance and not representative of those underinsured and uninsured patients who historically have worse health outcomes, including after CTR.34-36 Nevertheless, researchers have used Truven MarketScan extensively in orthopedic clinical outcomes research, including several studies looking at the injection-outcome relationship, and the patient volumes that it affords represent a major strength of this study.37-40
Conclusion
This study comprises an important contribution to the understanding of risk in relation to timing between steroid injections and surgical release for the treatment of CTS. Our findings reveal an association with steroid injections and the occurrence of SSI, noninfectious wound complications, and reoperation at 1 year. There is a small, but statistically significant, rate of postoperative wound complications when an injection for symptomatic relief of CTS is performed within 30 days of surgery. Our hope is that the findings of this study will lead to shared decision-making between patients and surgeons to carefully select the timing of preoperative injections prior to surgery so as to minimize risks and maximize benefits.
Footnotes
Ethical Approval: Each author certifies that his or her institution approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
Statement of Human and Animal Rights: Given that the Truven MarketScan Commercial Claims and Encounters and Medicare Supplemental and Coordination of Benefit database was used for this study, researchers did not have any access to identifiable patient information. All procedures followed were in accordance with the ethical standards of the Helsinki Declaration of 1975, as revised in 2008, and conducted in conformity with ethical principles of research.
Statement of Informed Consent: Informed consent did not apply to this study as no identifiable patient information was used. By agreeing to be included in the Truven MarketScan database, patients are informed that deidentified information can be used for research purposes.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Emily L. DeMaio  https://orcid.org/0000-0001-6976-8761
https://orcid.org/0000-0001-6976-8761
References
- 1. Aroori S, Spence RA. Carpal tunnel syndrome. Ulster Med J. 2008;77(1):6-17. [PMC free article] [PubMed] [Google Scholar]
- 2. Bland JD. Treatment of carpal tunnel syndrome. Muscle Nerve. 2007;36(2):167-171. [DOI] [PubMed] [Google Scholar]
- 3. Middleton SD, Anakwe RE. Carpal tunnel syndrome. BMJ. 2014;349:g6437. [DOI] [PubMed] [Google Scholar]
- 4. Bland JD. Carpal tunnel syndrome. BMJ. 2007;335(7615):343-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gottlieb NL, Riskin WG. Complications of local corticosteroid injections. JAMA. 1980;243(15):1547-1548. [PubMed] [Google Scholar]
- 6. Karimzadeh A, Bagheri S, Raeissadat SA, et al. The comparison of the effectiveness between different doses of local methylprednisolone injection versus triamcinolone in Carpal Tunnel Syndrome: a double-blind clinical trial. J Pain Res. 2019;12:579-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Milone MT, Karim A, Klifto CS, et al. Analysis of expected costs of carpal tunnel syndrome treatment strategies. Hand (NY). 2019;14(3):317-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hameso A, Bland JD. Prevalence of decompression surgery in patients with carpal tunnel syndrome 8 years after initial treatment with a local corticosteroid injection. J Hand Surg Eur Vol. 2017;42(3):275-280. [DOI] [PubMed] [Google Scholar]
- 9. Huisstede BM, van den Brink J, Randsdorp MS, et al. Effectiveness of surgical and postsurgical interventions for carpal tunnel syndrome: a systematic review. Arch Phys Med Rehabil. 2018;99(8):1660-1680.e1621. [DOI] [PubMed] [Google Scholar]
- 10. Kaile E, Bland JDP. Safety of corticosteroid injection for carpal tunnel syndrome. J Hand Surg Eur Vol. 2018;43(3):296-302. [DOI] [PubMed] [Google Scholar]
- 11. DeStefano F, Nordstrom DL, Vierkant RA. Long-term symptom outcomes of carpal tunnel syndrome and its treatment. J Hand Surg Am. 1997;22(2):200-210. [DOI] [PubMed] [Google Scholar]
- 12. Agarwalla A, Puzzitiello RN, Mascarenhas R, et al. Preoperative injections may be an iatrogenic cause of reoperation after arthroscopic rotator cuff repair. Arthroscopy. 2019;35(2):325-331. [DOI] [PubMed] [Google Scholar]
- 13. Schairer WW, Nwachukwu BU, Mayman DJ, et al. Preoperative hip injections increase the rate of periprosthetic infection after total hip arthroplasty. J Arthroplasty. 2016;31(suppl 9):166-169.e161. [DOI] [PubMed] [Google Scholar]
- 14. Traven SA, Brinton D, Simpson KN, et al. Preoperative shoulder injections are associated with increased risk of revision rotator cuff repair. Arthroscopy. 2019;35(3):706-713. [DOI] [PubMed] [Google Scholar]
- 15. Wang D, Camp CL, Ranawat AS, et al. The timing of hip arthroscopy after intra-articular hip injection affects postoperative infection risk. Arthroscopy. 2017;33(11):1988-1994.e1981. [DOI] [PubMed] [Google Scholar]
- 16. Ranson WA, White SJW, Cheung ZB, et al. The effects of chronic preoperative steroid therapy on perioperative complications following elective posterior lumbar fusion. Global Spine J. 2018;8(8):834-841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. White SJW, Carrillo O, Cheung ZB, et al. The effects of preoperative steroid therapy on perioperative complications after elective anterior lumbar fusion. World Neurosurg. 2019;126:e314-e322. [DOI] [PubMed] [Google Scholar]
- 18. White SJW, Ranson WA, Cho B, et al. The effects of preoperative steroid therapy on perioperative morbidity and mortality after adult spinal deformity surgery. Spine Deform. 2019;7(5):779-787. [DOI] [PubMed] [Google Scholar]
- 19. Giladi AM, Rahgozar P, Zhong L, et al. Corticosteroid or hyaluronic acid injections to the carpometacarpal joint of the thumb joint are associated with early complications after subsequent surgery. J Hand Surg Eur Vol. 2018;43(10):1106-1110. [DOI] [PubMed] [Google Scholar]
- 20. Matzon JL, Lebowitz C, Graham JG, et al. Risk of infection in trigger finger release surgery following corticosteroid injection. J Hand Surg Am. 2020;45(4):310-316. [DOI] [PubMed] [Google Scholar]
- 21. Truven Health Analytics. The Truven Health MarketScan Databases for health services researchers: thought leadership white paper. Published 2019. [Google Scholar]
- 22. Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. [DOI] [PubMed] [Google Scholar]
- 23. Vahi PS, Kals M, Koiv L, et al. Preoperative corticosteroid injections are associated with worse long-term outcome of surgical carpal tunnel release. Acta Orthop. 2014;85(1):102-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Richardson SS, Schairer WW, Sculco TP, et al. Comparison of infection risk with corticosteroid or hyaluronic acid injection prior to total knee arthroplasty. J Bone Joint Surg Am. 2019;101(2):112-118. [DOI] [PubMed] [Google Scholar]
- 25. Werner BC, Cancienne JM, Browne JA. The timing of total hip arthroplasty after intraarticular hip injection affects postoperative infection Risk. J Arthroplasty. 2016;31(4):820-823. [DOI] [PubMed] [Google Scholar]
- 26. McIntosh AL, Hanssen AD, Wenger DE, et al. Recent intraarticular steroid injection may increase infection rates in primary THA. Clin Orthop Relat Res. 2006;451:50-54. [DOI] [PubMed] [Google Scholar]
- 27. Forsythe B, Agarwalla A, Puzzitiello RN, et al. The timing of injections prior to arthroscopic rotator cuff repair impacts the risk of surgical site infection. J Bone Joint Surg Am. 2019;101(8):682-687. [DOI] [PubMed] [Google Scholar]
- 28. Brown TS. Intra-articular injections should be avoided in the 3 months prior to total knee arthroplasty: commentary on an article by Shawn S. Richardson, MD, et al.: “comparison of infection risk with corticosteroid or hyaluronic acid injection prior to total knee arthroplasty.” J Bone Joint Surg Am. 2019;101(2):e8. [DOI] [PubMed] [Google Scholar]
- 29. Anstead GM. Steroids, retinoids, and wound healing. Adv Wound Care. 1998;11(6):277-285. [PubMed] [Google Scholar]
- 30. Asomugha EU, Miller JA, McLain RF. Surgical site infections in posterior lumbar surgery: a controlled-cohort study of epidural steroid paste. Spine (Phila Pa 1976). 2017;42(1):63-69. [DOI] [PubMed] [Google Scholar]
- 31. Valizadeh N, Murray AC, Suradkar K, et al. Impact of preoperative steroid or immunosuppressant use on short-term outcomes following colectomy in Crohn’s disease patients. Tech Coloproctol. 2017;21(3):217-223. [DOI] [PubMed] [Google Scholar]
- 32. Wicke C, Halliday B, Allen D, et al. Effects of steroids and retinoids on wound healing. Arch Surg. 2000;135(11):1265-1270. [DOI] [PubMed] [Google Scholar]
- 33. Kulaylat AS, Schaefer EW, Messaris E, et al. Truven health analytics MarketScan databases for clinical research in colon and rectal surgery. Clin Colon Rectal Surg. 2019;32(1):54-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chaise F, Bellemère P, Fril JP, et al. Return-to-work interval and surgery for Carpal Tunnel Syndrome. Results of a prospective series of 233 patients. J Hand Surg Br. 2004;29(6):568-570. [DOI] [PubMed] [Google Scholar]
- 35. Katz JN, Amick BC, III, Keller R, et al. Determinants of work absence following surgery for carpal tunnel syndrome. Am J Ind Med. 2005;47(2):120-130. [DOI] [PubMed] [Google Scholar]
- 36. Nathan PA, Meadows KD, Keniston RC. Rehabilitation of carpal tunnel surgery patients using a short surgical incision and an early program of physical therapy. J Hand Surg Am. 1993;18(6):1044-1050. [DOI] [PubMed] [Google Scholar]
- 37. Schwartz RH, Urits I, Viswanath O. Carpal Tunnel Injection. 2020. Apr 24. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan. [PubMed] [Google Scholar]
- 38. Wilson JM, Farley KX, Bradbury TL, et al. Preoperative opioid use is a risk factor for complication and increased healthcare utilization following revision total knee arthroplasty. Knee. 2020;27(4):1121-1127. [DOI] [PubMed] [Google Scholar]
- 39. Wilson JM, Farley KX, Bradbury TL, Erens GA, Guild GN. Preoperative opioid use is a risk factor for complication and increased healthcare utilization following revision total knee arthroplasty. Knee. 2020;27(4):1121-1127. [DOI] [PubMed] [Google Scholar]
- 40. Wilson JM, Farley KX, Erens GA, et al. Preoperative depression is associated with increased risk following revision total joint arthroplasty. J Arthroplasty. 2020;35(4):1048-1053. [DOI] [PMC free article] [PubMed] [Google Scholar]