Abstract
Bacteria can survive dramatic osmotic shifts. Osmoregulatory responses mitigate the passive adjustments in cell structure and the growth inhibition that may ensue. The levels of certain cytoplasmic solutes rise and fall in response to increases and decreases, respectively, in extracellular osmolality. Certain organic compounds are favored over ions as osmoregulatory solutes, although K+ fluxes are intrinsic to the osmoregulatory response for at least some organisms. Osmosensors must undergo transitions between “off” and “on” conformations in response to changes in extracellular water activity (direct osmosensing) or resulting changes in cell structure (indirect osmosensing). Those located in the cytoplasmic membranes and nucleoids of bacteria are positioned for indirect osmosensing. Cytoplasmic membrane-based osmosensors may detect changes in the periplasmic and/or cytoplasmic solvent by experiencing changes in preferential interactions with particular solvent constituents, cosolvent-induced hydration changes, and/or macromolecular crowding. Alternatively, the membrane may act as an antenna and osmosensors may detect changes in membrane structure. Cosolvents may modulate intrinsic biomembrane strain and/or topologically closed membrane systems may experience changes in mechanical strain in response to imposed osmotic shifts. The osmosensory mechanisms controlling membrane-based K+ transporters, transcriptional regulators, osmoprotectant transporters, and mechanosensitive channels intrinsic to the cytoplasmic membrane of Escherichia coli are under intensive investigation. The osmoprotectant transporter ProP and channel MscL act as osmosensors after purification and reconstitution in proteoliposomes. Evidence that sensor kinase KdpD receives multiple sensory inputs is consistent with the effects of K+ fluxes on nucleoid structure, cellular energetics, cytoplasmic ionic strength, and ion composition as well as on cytoplasmic osmolality. Thus, osmoregulatory responses accommodate and exploit the effects of individual cosolvents on cell structure and function as well as the collective contribution of cosolvents to intracellular osmolality.
OSMOSENSING
As inhabitants of natural and artificial aqueous environments, bacteria survive dramatic changes in extracellular osmolality (for definitions of terms used in this review, see the glossary in Table 1). For example, soil bacteria survive periods of low and high rainfall, uropathogens survive urine concentration and dilution, and industrial organisms tolerate concentrated nutrient solutions as well as the extracellular accumulation of metabolic products. Bacteria respond both passively and actively to changes in the osmolality of their environment (Fig. 1 provides a summary of this phenomenon as it occurs in Escherichia coli).
TABLE 1.
Glossary
| Term | Definition |
|---|---|
| Chaotrope | Cosolvents that decrease water structure are called chaotropes. Urea and other protein denaturants are chaotropes. |
| Chemosensor | Chemosensors are molecules that detect specific ligands. Many chemosensors act by binding a specific ligand at a structure-specific receptor site. |
| Compatible solute | A compatible solute is a cytoplasmic cosolvent whose level can be modulated over a broad range without disrupting cellular functions. |
| Cosolvent | A cosolvent is a solute that significantly affects the properties of water as a solvent, rendering the resulting solution nonideal. |
| Dehydration | Dehydration is water loss. (Desiccation is complete water removal.) |
| Hofmeister effect | The Hofmeister effect is the systematic effect of a series of inorganic salts on the solubility of proteins and was first reported by Franz Hofmeister in 1888. Many other solute effects have since been correlated with the Hofmeister effect, and it has been generalized to include both ionic and nonionic solutes. Solutes (or cosolvents) are categorized, according to the Hofmeister effect, as chaotropes or kosmotropes. |
| Kosmotrope | Cosolvents that increase water structure are called kosmotropes. Glycerol, glycine betaine, and other protein stabilizers are kosmotropes. |
| Osmolality | Osmolality is the osmotic pressure of a solution at a particular temperature, expressed as moles of solute per kilogram of solvent (osmolal) (see Appendix). Osmolality can be measured but not calculated. |
| Osmolarity | Osmolarity is an approximation for osmolality, expressed as moles of solute per liter of solution (osmolar) (see Appendix). Osmolarity is calculated as the sum of the concentrations of osmotically active solutes in a solution. |
| Osmoprotectant | Osmoprotectants are compounds that stimulate bacterial growth in high-osmolality media. |
| Osmoregulatory response | An osmoregulatory response is a physiological process that mitigates passive adjustments in cell structure caused by changes in the extracellular osmolality. |
| Osmosensor | An osmosensor is a device that detects changes in extracellular water activity (direct osmosensing) or resulting changes in cell structure or composition (indirect osmosensing). |
| Osmotolerance | The osmolality range for the media that support bacterial growth. |
| Osmotic downshift | An osmotic downshift is a decrease (over time) in the osmolality of the extracellular environment. |
| Osmotic upshift | An osmotic upshift is an increase (over time) in the osmolality of the extracellular environment. |
| Turgor pressure | Turgor pressure (ΔP) is the hydrostatic pressure difference which balances the osmotic pressure (or osmolality) difference between cell interior and exterior. Turgor pressure renders the chemical potentials of intracellular and extracellular water equal at equilibrium. |
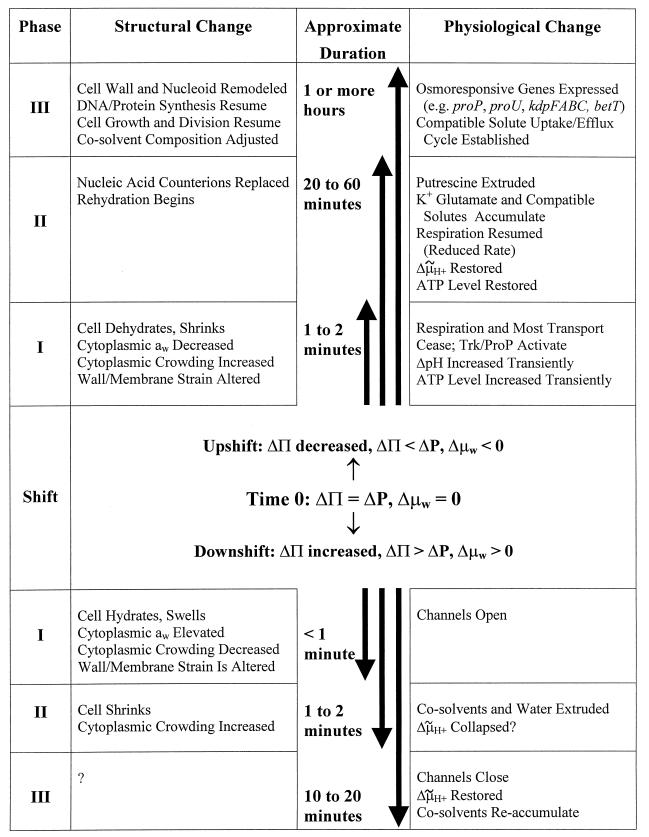
FIG. 1.
Phases of the osmotic stress response for E. coli K-12. Structural and physiological responses triggered by osmotic shifts (up or down) imposed at time zero proceed in parallel along the indicated, approximate timescales. The evidence supporting this scheme is discussed in the text.
Since the water permeability of the cytoplasmic membrane is high, imposed imbalances between turgor pressure and the osmolality gradient across the bacterial cell wall are short in duration. Changes in cell structure, organization, and composition that result from transmembrane water flux (Fig. 1, left column) trigger and are modulated by physiological responses (Fig. 1, right column). Bacteria respond to osmotic upshifts in three overlapping phases: dehydration (loss of some cell water) (phase I), adjustment of cytoplasmic solvent composition and rehydration (phase II), and cellular remodeling (phase III). Responses to osmotic downshifts are not yet well characterized, but they are also likely to proceed in three phases: water uptake (phase I), extrusion of water and cosolvents (phase II), and cytoplasmic cosolvent reaccumulation and cellular remodeling (phase III). Many of the cellular responses triggered by osmotic stimuli occur in parallel. Limited experimental evidence for sequential osmoregulatory processes (discussed below) offers insights into osmosensory mechanisms.
Modulation of cytoplasmic solvent composition is the only response that is known to reverse the growth-inhibitory effects of osmotic shifts on bacteria. The following principles govern this process as it occurs in diverse bacteria, both gram positive and gram negative. (i) The concentrations of certain cytoplasmic solutes increase and decrease in response to osmotic up- and downshifts, respectively. (ii) There is a hierarchy of preference among the solutes that accumulate in response to an osmotic upshift. Particular zwitterionic organic cosolvents such as ectoine and glycine betaine are selected for this role over inorganic solutes such as K+. (iii) Osmoregulation of uptake, efflux, biosynthesis, and/or catabolism is required to modulate the cytoplasmic levels of these osmoregulatory solutes.
The genes and enzymes responsible for modulation of osmoregulatory solute levels have been identified in diverse bacteria. The mechanisms by which bacteria sense osmotic shifts (osmosensory mechanisms) and the basis for osmoregulatory solute selection are still poorly defined, however.
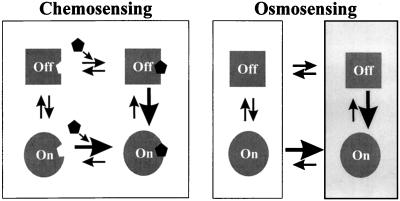
Chemosensors exploit the specificity of ligand-receptor interactions to detect the biochemistry of cellular environments, including both changes in nutrient supplies and signals with biological origins (Fig. 2). An osmosensor is a device that detects changes in extracellular water activity (direct osmosensing) or the resulting changes in cell structure (indirect osmosensing). In contrast to chemosensory mechanisms, osmosensory mechanisms cannot be based on stereospecific ligand-receptor interactions that occur at specific sites in receptor molecules. Instead, osmosensors are likely to be macromolecules that undergo changes in conformation and/or oligomerization in response to solvent changes or ensuing mechanical stimuli (Fig. 2). Our current knowledge suggests that osmosensors are located in the cytoplasmic membranes and nucleoids of bacteria. This review focuses on membrane-based osmosensors since they elicit primary osmoregulatory responses. In addition, current knowledge of the osmoregulation of transcription in bacteria has been reviewed whereas the basis for membrane-based osmosensing has not. The goals of this review are to define the properties of solvent-macromolecule and solvent-membrane interactions pertinent to osmosensing, to identify putative osmosensors and the changes that they detect, and to review our current understanding of cytoplasmic membrane-based osmosensory mechanisms in bacteria. This review is intended to stimulate broadly based research on osmosensing. Efforts have therefore been made to express microbiological, biochemical, and biophysical concepts in terms accessible to both specialists and nonspecialists. Readers who prefer textual explanations are therefore asked to accommodate the mathematical expressions preferred by those interested in the biophysical basis for osmosensing. This review also builds upon concepts developed by many other authors (2, 7, 25, 47, 74, 220, 228, 229, 256). Physiological responses to osmolality changes, to cooling-freezing, and to desiccation are related (44, 55, 124, 156). Only responses to osmolality changes are considered here, however. In addition, this review does not encompass the modifications to macromolecular structure that render organisms of the salt-in-cytoplasm type obligately halophilic (74).
FIG. 2.
Chemosensing versus osmosensing. Many biosensors are molecules whose conformations change between an “off” and an “on” conformation in response to a change in the biosensor environment. Chemosensors detect the biochemistry of cellular environments, including changes in nutrient supplies and signals with biological origins. The proportion of chemosensor molecules in the “on” conformation increases when the appropriate ligand binds to a specific site on the chemosensor surface. Osmosensors detect changes in extracellular water activity (direct osmosensing) or resulting changes in cell composition or structure (indirect osmosensing). At least some osmosensors are expected to change their conformations in response to solvent changes. The proportion of osmosensor molecules in the “on” conformation would then increase when the sensor surface was exposed to a suitably altered solvent (depicted here by a change from a white to a shaded background). Alternatively, sensing could involve a change in oligomeric state (for example, ligand- or solvent-induced dimerization).
SOLVENTS AND THEIR INTERACTIONS WITH BIOMOLECULES
Solvent Properties Pertinent to Osmosensing
For the purpose of this review, the chemical potential of water can be expressed as
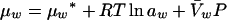 |
1 |
where μw* is the chemical potential of water under standard conditions, R is the gas constant, T is the temperature (Kelvin), aw is the activity of water, V̄w is the partial molar volume of water, and P is the pressure.
Water and its solutes can be characterized by their activities (ai), where
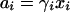 |
2 |
γi and xi are the activity coefficient and the mole fraction of the ith constituent, respectively, and O < ai < 1. In a physicochemically ideal system, the solvent activity approaches 1 and the solute concentrations (expressed as mole fractions or as the more familiar molar units), which approach zero, can be used as effective proxies for their activities. Experimental systems are often designed to approximate ideality, so that simplifying assumptions can be made about the resulting chemical and physical phenomena. Although some laboratory media and some natural microbial environments can be described as ideal solutions, many are nonideal. The aqueous compartments within microorganisms are certainly nonideal. Nonideality has important consequences for osmosensing (see “Solvent-macromolecule interactions”).
The ionic strength and osmotic pressure of the solvent arise through collective contributions of ionic solutes and of all solutes, respectively. Solutes that are present at high concentrations have the greatest effects on osmolality and (if they are ionic) ionic strength. The osmotic pressure of an aqueous solution (Π) is defined as
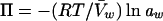 |
3 |
Note that definitions of osmotic pressure vary. This definition differs from those used in some previous reviews on bacterial osmoregulation (47, 178) and desiccation tolerance (221). It is used here for reasons discussed by Nobel (200). Osmotic pressure can also be expressed in terms of the osmolality (units of osmoles/kg of solvent or osmolal:
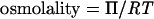 |
4 |
Osmolarity, the sum of the concentrations of osmotically active solutes in solution, provides a convenient approximation for osmolality:
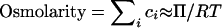 |
5 |
where the ci are the molar concentrations of the osmotically active solutes. Osmolarity and osmolality are not equal, and osmolality can be measured but not calculated (272).
Solvent-Macromolecule Interactions
Biologically relevant solvents, whether extracellular or intracellular, include both water and cosolvents. Cosolvents are solutes that significantly influence the behavior of water as a solvent (see “Solvent properties pertinent to osmosensing”). The term “cosolvent” is introduced here to emphasize that every solute molecule makes some contribution to solvent behavior while, more or less independently, playing a specific physiological role. Important solvent characteristics include cosolvent composition, ionic strength, osmotic pressure (or osmolality), and pH (see “Solvent properties pertinent to osmosensing”). Each cosolvent contributes, as part of the collective of cosolvents, to the osmolality of the solution. In addition, each cosolvent has particular effects on water, on its interactions with biomolecules, and hence on cellular functions. Both the osmolality and individual cosolvent effects are relevant to osmoregulatory mechanisms and their experimental study.
Diverse cosolvents are present outside and inside bacteria. NaCl and sugars (or other polyols) are the most prevalent and abundant extracellular cosolvents in natural environments. K+, organic anions, compatible solutes, proteins, and nucleic acids are the predominant intracellular cosolvents (28, 29, 74, 257, 304). The intracellular solvent of most bacteria is thus differentiated from the extracellular solvent by including a restricted array of low-molecular-weight cosolvents and a high concentration of macromolecular cosolvents. To understand osmosensing, it is necessary to deduce both the solvent changes to which osmosensors are actually exposed in vivo and the mechanisms by which those solvent changes can trigger changes in osmosensor conformation. Osmosensors located in the cytoplasmic membrane or nucleoid are exposed to the constrained solvent environments of the cytoplasm, membrane, and/or periplasm, not to the extracellular aqueous medium.
Effects of cosolvents on the behavior of water at interfaces (and their effects on proteins) have been described, systematically but empirically, in terms of the Hofmeister effect (41). Both ionic and nonionic cosolvents can be characterized in this way as kosmotropic (water structure making, e.g. phosphate, ammonium, sucrose, and glycerol), close to neutral (e.g., Na+ and Cl−), or chaotropic (water structure breaking, e.g., SCN− and urea). For example, kosmotropes and chaotropes tend to stabilize and destabilize native protein conformations, respectively (for a further discussion, see below). Effects of kosmotropes and chaotropes on protein function may be additive or mutually compensatory (see, e.g., references 292 and 308).
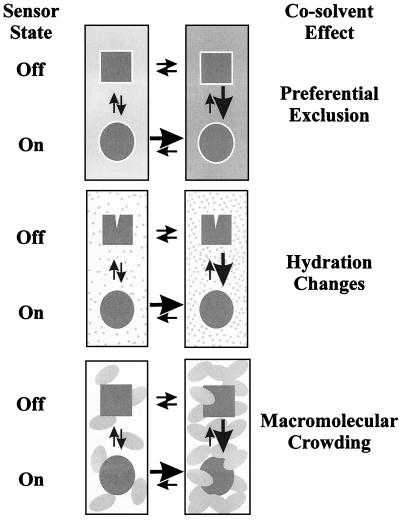
Efforts by biochemists and biophysicists to systematically describe and explain water–cosolvent–macromolecule–solid-matrix interactions now offer additional perspectives and tools to students of osmoregulation (Fig. 3). Included are studies of (i) very low affinity ligand-receptor interactions and the impacts of diverse cosolvents on macromolecular stability, solubility, and assembly explained in terms of preferential exclusion of certain solvent constituents from macromolecular surfaces (275); (ii) the significance of hydration changes, probed by varying the osmotic pressure, for macromolecular interactions and functions (209); and (iii) the impacts of crowding and confinement on the structures and interactions of macromolecules (182, 297). Each rests on the concept that “the local environment will influence reactions taking place in a biological medium when reactants, transition state complexes, and products interact unequally with background species” (311). In the context of osmosensing, the reactants and products would be the forms (“off” and “on”) of an osmosensor and the background species constituting the local environment would include water, cosolvents, and structural elements.
FIG. 3.
Solvent effects on macromolecular conformation. If different forms (conformations) of a macromolecule (or osmosensor) interact differently with their solvent, solvent changes will alter the distribution of the macromolecule (or sensor) population among those forms. Such effects could constitute a basis for osmosensing, as illustrated in Fig. 2. Simultaneous exposure of membrane-based osmosensors to multiple solvent environments would further enhance the scope for modulation of their structure by solvent changes. Three aspects of solvent-macromolecule interactions that have been demonstrated to influence macromolecular conformation are illustrated in this figure. For preferential exclusion, if water and cosolvent interact differently with the macromolecular surface, a change in cosolvent composition can trigger a change in macromolecular conformation and/or oligomerization. For example, an increased concentration of one or more cosolvents (indicated by a darker grey background) that are preferentially excluded from the sensor surface (indicated by a halo) favors a conformational change that results in a decreased sensor surface area (indicated by a change from square to round). For hydration changes, if entry of cosolvent molecules to macromolecule-associated solvent pools is restricted (as indicated by the relative sizes of the grey cosolvent molecules and the water-filled cleft on the macromolecular surface), an increase in cosolvent concentration will cause macromolecule-associated solvent to be extracted and macromolecular conformation to be altered (closing the water-filled cleft and possibly also causing a more generalized conformational change, indicated by a change from square to round). For macromolecular crowding or confinement within matrices, in crowded solutions (those containing high concentrations or networks of macromolecules) compact or globular conformations of a test macromolecule (or osmosensor) are favored. Changes in crowding of the bacterial cytoplasm, where macromolecules occupy as much as 50% of the available volume (indicated as a change in the concentration of oblong, grey molecules comparable in size to the test macromolecule), may therefore favor changes in conformation of osmosensor molecules (again indicated as a change from square to round).
Preferential interaction.
Some interactions among water, cosolvents, and osmosensors may be more appropriately considered in terms of preferential binding or exchange than in terms of classical binding theory. The latter describes the fractional occupancy of a specific receptor site(s) on a macromolecule by one or more ligands. This occupancy may vary from 0 (no binding) to the number of sites, which is usually small. In terms of preferential binding or exchange, the reference state is a macromolecule in pure water with all exposed surface sites hydrated (no sites occupied by cosolvent). A gradual exchange of water for cosolvent occurs as the cosolvent is added to the system. At any cosolvent concentration, the fractional occupancy of surface sites by cosolvent exceeds, matches, or falls short of the proportion (mole fraction) of cosolvent molecules in bulk solution if the surface sites prefer cosolvent over water, show no preference, or prefer water over cosolvent, respectively. This situation differs substantially from that treated by classical binding theory. Each solvent-macromolecule interaction is weak, but the number of sites involved is large and hence the potential impact of solvent-cosolvent exchange on macromolecular structure and function is also large. For example, it has been estimated that lysozyme offers a total of 266 surface sites for solvent and/or cosolvent occupancy (276). In addition, the global behavior of the macromolecule-solvent system is an average over weak interactions among macromolecule, cosolvent, and water at a large number of nonidentical surface sites.
The effects of various organic cosolvents on macromolecular solubility, conformation, and assembly can be described in terms of such preferential interactions (275, 276). A correlation is observed between cosolvent exclusion from protein surfaces and stabilization of native protein conformations and assemblies (which often have smaller surface areas exposed to solvent than denatured forms do) (Fig. 3). Steric restrictions on approach to the macromolecular surface by cosolvent molecules (as opposed to the smaller water molecules) and a thermodynamic preference of some cosolvent molecules for the bulk solvent over the solvent-protein interface are both believed to contribute to this cosolvent behavior. The protein-stabilizing effects of some compatible solutes have been described in these terms (248, 292, 308). It has been emphasized, however, that stabilization does not require preferential exclusion of the cosolvent from macromolecular surfaces. Stabilization can also be achieved if there is less preferential binding of the cosolvent to the denatured macromolecule than to the native macromolecule (as was shown for trehalose by Xie and Timasheff [301]). These observations indicate that the conformations and associations of macromolecules may be sensitive to the concentrations of cosolvents, but they also underscore the complex dependence of such phenomena on the chemical nature of cosolvent-macromolecule interactions, not on solution osmolality per se.
Hydration forces.
Osmotic pressure has been used as a tool to probe the biological significance of macromolecular hydration, i.e., of “hydration forces.” For example, the addition of osmotically active, polymeric cosolvent (e.g., polyethylene glycol [PEG]) to the surrounding medium can suppress ion channel opening and alter both KD and KM for the interaction of glucose with hexokinase (209). These data are interpreted as showing that the entry of cosolvent molecules to macromolecule-associated solvent pools is restricted and that solvent compartments which differ in osmolality are thus created. Thus, macromolecules may adjust to their solvent environments by assuming states that balance the (unfavorable) exclusion of cosolvent from inaccessible solvent pools against the (unfavorable) juxtaposition of macromolecular constituents. For example, withdrawal of solvent from intramolecular pools of low osmolality may force macromolecules to adopt different (and less energetically favorable) conformations than are assumed to be present in the absence of cosolvent (Fig. 3).
For hexokinase, this adjustment appears to be related to closure of a solvent-filled protein cleft containing the active site, although more generalized dehydration of the enzyme may also be involved. As would be anticipated for a purely osmotic effect, the number of water molecules implicated in this transition depends on the size of the cosolvent molecules used to impose osmotic stress. The difference in the number of PEG-accessible water molecules between the glucose-associated and glucose-free hexokinase conformations varies from 50 to 326 as the molecular weight of PEG increases from 300 to 1,000. It then remains constant as the molecular weight of PEG increases further to 10,000 (232). Thus, a larger quantity of hexokinase-associated water is inaccessible to high-molecular-weight than to low-molecular-weight PEG. These observations suggest that protein form and function can respond on an osmotic basis to certain cosolvents, including some that are similar in molecular weight and concentration to those which impose osmotic stress or serve as compatible solutes in nature.
Pressure effects on the specificity of ligand-receptor interactions.
Both osmotic and hydrostatic pressures have been used to perturb DNA-protein interactions (235). Stresses imposed with osmotic and hydrostatic pressures are fundamentally different; osmotic stress causes water molecules to be transferred from cosolvent-inaccessible to cosolvent-accessible regions around or within macromolecules, whereas hydrostatic pressure changes the weight density of the entire system. For some restriction endonucleases, DNA recognition sequence specificity changed as osmotic pressure was increased by the addition of diverse, low-molecular-weight cosolvents including sucrose, glycerol, 2-propanol, and N-methylformamide (0 to 3 osmolal, but with significant effect at 1 osmolal) (see, e.g., reference 234). These effects were reversed by elevated hydrostatic pressure (up to 500 atm). They were interpreted as indicating differential involvement of water at the enzyme-DNA interface for different DNA sequences. Hydration has also been recognized as playing a critical role in carbohydrate-protein interactions (152). Such behavior differs fundamentally from that described by preferential binding or exchange theory, however. It involves small numbers of water molecules that mediate high-affinity enzyme-substrate recognition at specific enzyme and substrate sites.
Crowding and confinement.
Considerations of macromolecular crowding and confinement may also be used to explain “background” effects on macromolecular structures and interactions (182, 311). They define the collective impact of macromolecules, whether soluble or present as structural elements, on cellular processes. Garner and Burg (77) have reviewed these concepts from a physiological perspective. The term “crowding” refers to effects of high collective volume occupancy by macromolecules on the structures and functions of individual macromolecular species with which they interact only weakly and nonspecifically. Such effects may be biologically significant since macromolecules occupy as much as 50% of the cytoplasmic volume. The term “confinement” refers to effects of entrapment within a subcellular matrix on macromolecular function.
Consideration of crowding and confinement effects on biological processes in terms of solution nonideality yields predictions that are supported by experimental evidence. (i) Under conditions of crowding comparable to those found in vivo, the values assumed by macromolecular association constants may be dominated by crowding effects and may exceed the corresponding values for dilute solution by up to several orders of magnitude. (ii) Compact or globular macromolecular conformations or assemblies are favored more in crowded than in dilute solutions (Fig. 3). (iii) For a given degree of crowding, effects on associations between macromolecules are expected to be much greater than effects on association of small molecules with macromolecules. (iv) Both crowding and confinement tend to enhance macromolecular associations—unlike crowding, confinement may favor the formation of extended (linear or discoid) rather than globular aggregates. Such considerations have led to the proposal that (some) animal cells may regulate macromolecular crowding rather than cell volume (183). This proposal rests on the concept that physiologically relevant changes in extracellular water activity, effected by modulating the extracellular concentrations of diverse cosolvents, may be translated into changes in cytoplasmic crowding or confinement, thereby avoiding the complication of specific cosolvent-osmosensor interactions.
These background effects on macromolecular structures and interactions are not mutually exclusive, and this list is not necessarily complete. For example, phenomena other than hydration have been proposed as origins for the mutual repulsion of macromolecular surfaces in aqueous solution (106). It has been argued that small cosolvent molecules may exert their effects on protein solubility, stability, and function through excluded volume rather than through osmotic or preferential binding effects (297). It has been proposed that water within enzyme active sites, ligand binding sites of receptors, and ion channels, like that within polymeric matrices, differs from bulk water in density and hence in its behavior as a solvent (295, 296). Resolution of these overlapping and/or conflicting interpretations is not the objective of this review. Rather, these concepts are useful to students of osmoregulation because they contribute to our understanding of solvent effects on cell structure and function and because they suggest potential osmosensory mechanisms (Table 2).
TABLE 2.
Potential stimuli for membrane- or nucleoid-based osmosensorsa
| Compartment sampled | Stimulus detected, change inb: |
|---|---|
| Periplasm | Thickness |
| Turgor pressure | |
| Concn of a specific cosolvent (e.g., glucan) | |
| Macromolecular crowding | |
| Osmolality | |
| Ionic strength | |
| Cytoplasmic membrane | Osmolality gradient |
| Lateral pressure | |
| Bilayer curvature | |
| Head group charge density | |
| Head group hydrogen bonding | |
| Head group hydration | |
| Thickness | |
| Lateral phospholipid distribution | |
| Intermonolayer phospholipid distribution | |
| Cytoplasm | Osmolality |
| Ionic strength | |
| Concn of kosmotropes | |
| Concn of a specific cosolvent (e.g., K glutamate) | |
| Macromolecular crowding or confinement | |
| Nucleoid | Turgor pressure |
| Counterion composition | |
| Protein composition | |
| Macromolecular crowding | |
| DNA topology |
The entries in this table were deduced by considering the ways in which solvent changes could affect the conformations of osmosensors placed in the cytoplasmic membrane or the nucleoid. A membrane-based osmosensor could sample properties of the periplasmic and/or cytoplasmic solvent as well as characteristics of the membrane itself.
Bold entries are relevant to membrane-based osmosensors that retain their effectiveness in cell, vesicle, and proteoliposome systems (in the absence of exogenous macromolecular crowding agents) (e.g., osmoprotectant transporter ProP, mechanosensitive channel MscL).
Solvent-Membrane Interactions
The principles outlined above apply to biomembrane constituents, proteins, phospholipids, and polysaccharides, as well as to other macromolecules. Solvent effects on the assembled membrane warrant particular attention in the context of osmosensing because biomembranes constitute the semipermeable barriers which define biologically relevant osmotic compartments and some osmosensors are located within biomembranes. When an osmotic shift is imposed on a vesicular membrane system (a cell, a biomembrane vesicle, or a liposome), membrane perturbations with multiple origins occur. The following discussion is designed to enumerate these effects, indicate which may be detected by osmosensors, and provide references to pertinent experimental approaches.
Solvent effects on membrane structure.
Biophysicists and biochemists have long been fascinated and puzzled by the diversity and complex phase behavior of membrane phospholipids. The net charge, hydrogen-bonding capacity, and hydration of each head group, the length and unsaturation of each acyl chain, and the resulting overall shape of each phospholipid molecule all contribute to the phospholipid-solvent interactions which determine phase behavior in aqueous phospholipid dispersions (57, 60, 78, 85, 155). Each phospholipid (and each phospholipid mixture) has a particular propensity to form the lamellar (L) phase characteristic of biological membranes versus alternative arrangements, including the nonlamellar hexagonal (HI or HII) or cubic phases. Phospholipid phase transitions, for example, the transitions from gel (Lβ) to liquid crystalline (Lα) lamellar phases and from the lamellar phase to the hexagonal phase, are characterized by phase transition temperatures (for these examples, TM and TH, respectively). Phospholipid phase behavior (often indicated by phase transition temperatures) is also influenced by amphiphiles which insert among phospholipid molecules (e.g., alcohols, fatty acids, detergents, anaesthetics, and peptides) and by such solvent properties as pH, ionic strength, and cosolvent composition.
Although physiological roles have been proposed for nonlamellar phospholipid, most biomembrane phospholipid, under most circumstances, is in the liquid crystalline lamellar phase. However, most biomembranes are enriched in phospholipids which, in isolation, have little propensity to form the lamellar phase (e.g., monoglucosyl diacylglycerol in Acholeplasma laidlawii and phosphatidylethanolamine in Clostridium butyricum and Escherichia coli) (57, 155). Some implications of the presence of “nonbilayer” lipid in biological membranes can be understood by comparing the behaviors of phospholipid monolayers and bilayers (60, 85, 161).
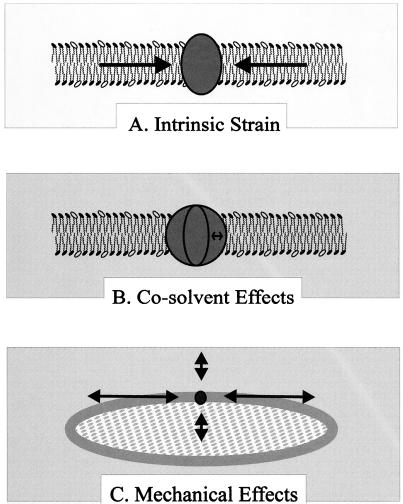
A planar phospholipid bilayer represents a balance between the intrinsic tendency of each monolayer to be curved (intrinsic curvature) and the requirement that the fatty acyl chains of each monolayer be sequestered from water (the hydrophobic effect) (Fig. 4A). The phospholipid bilayer is thus seen as an aggregate of “frustrated” monolayers, structures in which the frustration of intrinsic curvature creates a lateral pressure within the membrane (35, 85, 161). Electrostatic and van der Waals interactions, steric factors, and head group hydration are all predicted to contribute to lateral pressure within bilayers. The relative contributions of these factors are only now being defined as techniques are devised to detect the predicted lateral pressure (63, 132, 161). Epand and Epand demonstrated the thermodynamic significance of curvature strain by measuring an enthalpy associated with the diminution of intrinsic monolayer curvature upon introduction of amphipaths to frustrated lipid bilayers (63).
FIG. 4.
Solvent effects on membranes. Membrane strain is the relative displacement of membrane constituents in response to an imposed stress. Such strain may be communicated to osmosensors by the phospholipid bilayer. (A) Intrinsic membrane strain arises because phospholipid monolayers that have an intrinsic tendency to be curved must flatten in order to associate and form phospholipid bilayers in aqueous solution (satisfying the requirements of the hydrophobic effect). This phenomenon has a number of potential consequences including the possible existence of a lateral pressure, exerted in the bilayer plain, that is higher in the membrane core than at the membrane surface (see the text for a discussion of this phenomenon). (B) Changes in cosolvent composition (indicated in grey) may modulate intrinsic membrane strain by acting in the ways illustrated in Fig. 3. Such changes could be transmitted to (and modify the conformations of) osmosensors that are integral membrane proteins. (C) Since biomembranes are fluid, topologically closed, and differentially permeable to water and cosolvents, changes of intra- or extracellular water activity cause changes in membrane shape. Such changes may alter mechanically imposed membrane strain in ways that change the conformations of osmosensors that are integral membrane proteins.
Transitions from lamellar to nonlamellar lipid phases may occur when thermal or other changes shift the balance outlined above in favor of monolayer curvature. Intrinsic curvature strain (and hence lateral pressure within the membrane) is predicted to act in a more subtle but physiologically significant fashion on proteins associated with or embedded in lamellar phase lipid. Particularly relevant to osmosensing are arguments that lateral pressure or stress is a function of depth normal to the membrane plane and that variations in this stress may be coupled to conformational changes which alter the cross-sectional area of the protein in the membrane plane (35, 85, 161). Lipids that do not readily form bilayers have been implicated in the function of some peripheral membrane proteins and channels (61, 122), and E. coli cells deficient in phosphatidylethanolamine biosynthesis show complex phenotypes, some of which suggest signaling defects (103, 177).
How do cosolvents influence the phase behavior and lateral pressure of biomembranes? Although they have received much less attention than temperature effects, cosolvent effects on phospholipid phase behavior have been reported (13, 62, 97, 123, 240, 247, 270, 271). Effects of ionic and nonionic cosolvents on TM and TH for frustrated lipid systems have been correlated with cosolvent position within the Hofmeister series. These effects have been explained in terms of preferential-interaction theory as well as other factors (62, 247). For some phospholipid systems, kosmotropes (e.g., NaCl and compatible solutes) lowered TH, increasing the tendency of the lipid to form a nonbilayer phase at a given temperature. (Chaotropes had the opposite effects.) Membrane dehydration caused by exposure of the membrane to cosolvents which cannot penetrate membrane water pools also influences phase behavior in a manner that depends upon phospholipid structure and can be related to membrane curvature (166, 227). It is tempting to speculate that kosmotropes and molecules that are sterically excluded from the membrane increase lateral pressure within lamellar-phase biomembranes. An osmosensor might be designed to signal such a change (Fig. 4B; Table 2).
In addition to global phase changes, phospholipid bilayers may undergo local phase changes which create lateral phase separations. For certain mixed-lipid systems, some cosolvents (including ethanol, PEG, glucose, and cations) can cause lipid “demixing” (122, 148, 149, 189). Lipid characteristics that influence lipid miscibility include head group size, charge, and hydrogen-bonding capacity, as well as acyl chain length and unsaturation. For example, demixing can cluster phospholipids with similar head groups or acyl chain lengths, with the latter phenomenon resulting in localized membrane thinning or thickening. Lipid specificity has been predicted and in some cases demonstrated for membrane association of peripheral proteins (or protein domains) (60, 122), protein integration into membranes (24, 188), and the activities of peripheral and integral membrane proteins (references 23, 104, 105, and 122 and references cited in reference 150). An osmosensor could, in principle, be designed to detect a lipid phase formed by solvent- or curvature-induced lipid demixing.
Transverse asymmetry of phospholipid composition is well established for the outer membranes of gram-negative bacteria, for the cytoplasmic membranes of erythrocytes, and for very small unilamellar liposomes, but little is known about transverse asymmetry of lipids in the cytoplasmic membranes of bacteria (78). Nevertheless, transverse asymmetry may be a powerful determinant of the physical properties of biomembranes and the activities of membrane proteins.
Biomembrane permeability.
For small deviations from equilibrium, the net rate of volume flux across a membrane in response to a hydrostatic or osmotic driving force can be described in terms of an osmotic permeability coefficient (Pf [centimeters per second]) characteristic of the membrane and a reflection coefficient (ς [dimensionless]) characteristic of the membrane and the cosolvent used to impose the osmotic gradient (71, 200). The flow of volume across a membrane separating compartment 1 from compartment 2 can be described as
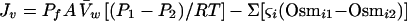 |
6 |
where Jv is the water flux (cubic centimeters per second), A is the membrane surface area (square centimeters), V̄w is the partial molar volume of water, R is the gas constant, T is the temperature (Kelvin), P1 and P2 are the hydrostatic pressures (atmospheres) in compartments 1 and 2, respectively, ςi (0 < ςi < 1) describes the relative rates at which the ith cosolvent and water cross the membrane (via all available pathways) and Osmi1 and Osmi2 are the osmolalities due to the ith cosolvent in compartments 1 and 2, respectively. The reflection coefficient (ςi) varies from close to 0 for a highly permeant cosolvent to 1 for a cosolvent which does not cross the cell surface (e.g., glycerol has a low ς and sucrose has a high ς with respect to the surface of E. coli [see below]). The significance of ς can be grasped by recognizing that a cosolvent flux sufficiently rapid (with respect to solvent flux) to collapse the osmolality gradient will attenuate the solvent flux (Jv). In terms which are important for osmosensing, Pf and ςi contribute to the rate and extent of solvent flux and hence to the duration of imposed osmotic gradients (ΔOsm), the rate of change of hydrostatic pressure (ΔP), and the rate at and degree to which a plastic, membrane-bounded osmotic compartment will be deformed in response to an osmotic shift.
Water crosses biomembranes via three pathways: the phospholipid bilayer, aquaporins (water-selective channels), and integral membrane proteins with other functions (e.g., transporters and channels designed to translocate substrates other than water) (52, 284, 307). The relative contributions of these pathways depend directly on the numbers of the relevant proteins per unit membrane area (and, in the case of the last pathway, the availability of substrates). The osmotic water permeabilities of phospholipid bilayers (Pf in the range 10−3 to 10−2 cm/s) are usually sufficient to permit equilibration of water across the membrane on a millisecond timescale. Aquaporins raise Pf to values in excess of 10−2 cm/s and hasten equilibration accordingly (284). The physiological roles of aquaporins have not been defined. However, their presence suggests that under at least some physiological conditions, transmembrane water flux via the phospholipid bilayer, alone, is unacceptably slow. Although water does cross biomembranes via transporters and channels other than aquaporins, the contributions of transporters and channels to total water flux may be relatively small (284, 307). Our understanding of the influence of cosolvents on the water permeabilities of phospholipid bilayers is currently limited (see, e.g., reference 17).
Like that of water, transmembrane cosolvent flux may in principle be either passive (occurring via the phospholipid bilayer or nonspecific pathways involving membrane proteins) or mediated (occurring via cosolvent-specific integral membrane proteins). Since the passive permeabilities of biomembranes for many biologically relevant solutes are orders of magnitude lower than that of water, solute-specific channels or transporters are required if high solute flux rates are to be attained (78) and the passive permeabilities for solutes are often ignored. In fact, passive permeabilities are important for certain biologically relevant cosolvents (e.g., glycerol, urea, and NH3), and they may become important when cosolvents, even those of low passive permeability, are used to impose large osmotic gradients (133, 180). The passive permeabilities of phospholipid bilayers and biomembranes for solutes are known to increase dramatically near both TM and TH (52, 60, 165). Although the temperature dependence of passive membrane permeability has been extensively explored, the degree to which absolute cosolvent levels (as opposed to cosolvent gradients) influence cosolvent permeabilities is not well characterized.
Mechanical properties of membranes.
A membrane can be defined, mechanically, as “a material with a very small thickness in comparison with its radii of curvature which separates two adjacent, liquid-like domains and supports the stresses created by the embedding medium” (19). To understand membrane-associated osmosensors, it may become necessary to link our understanding of membrane molecular structure and dynamics (with a length scale up to a few nanometers) with our understanding of the membrane as a continuum of condensed matter (with a length scale greater than 500 nm). Barriers to the attainment of that goal include both difficulties of communication among investigators and technical limits to the study of phenomena which occur on the relevant length scale and timescale (19).
To define the mechanical properties of a biological membrane, it would be necessary to fully describe the rates and extents of shape changes that occur upon application of defined forces (stresses) in vivo. For an elastic membrane, each applied stress will result in some strain, i.e., some reversible shape change. Stress and strain are related by proportionality constants (elastic moduli) determined by material properties of the membrane:
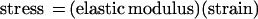 |
7 |
so that a membrane with a relatively large elastic modulus would undergo a relatively small shape change in response to a given stress. To define the rates of structural change, viscosity coefficients are also required. No membrane system has been fully described in these terms, but studies of eukaryotic cell membranes (particularly those of erythrocytes and sea urchin eggs), biomembrane vesicles, and liposomes (phospholipid vesicles) offer useful insights (see, e.g., references 19, 67, and 185). The following description refers specifically to unilamellar liposomes, since they can be described in relatively simple terms and hence serve as useful biochemical model systems. It is important to remember, however, that the mechanical properties of biomembranes in vivo may be strongly influenced by interactions with adjacent cellular material (19).
For a membrane segment composed of a constant quantity of lipid, the extent and rate of deformation can be expressed in terms of three independent, local shape changes (19, 67): area dilation or condensation, in-plane extension at constant area (surface shear), and bending (curvature change) at constant shape. Phospholipid membranes are remarkable because they are fluid materials (low in viscosity or stiffness) with mechanical properties (elastic moduli) that are isotropic in the membrane plane and highly anisotropic in the thickness dimension. (In this context, surface isotropy means that the material properties do not depend on orientation with respect to a chosen position. Properties may nevertheless be nonuniform, depending upon the chosen position.)
Imposition of an osmotic gradient on a topologically closed membrane system (e.g., a liposome) with a cosolvent of high reflection coefficient may invoke changes of all three types (Fig. 4). For example, upon imposition of an osmotic upshift, the surface of a spherical liposome may become nonspherical (requiring in-plane extension and curvature change) and condense (requiring a decrease in area per lipid molecule). Upon imposition of an osmotic downshift, the surface of a nonspherical liposome may become more spherical (requiring in-plane extension and curvature change) and dilate (requiring an increase in area per lipid molecule).
To analyze the mechanical properties of membranes, experimenters have applied mechanical stress either by performing direct physical manipulation (micropipette aspiration [19, 185] or the use of optical tweezers [see, e.g., reference 51]) or by imposing osmotic gradients (see, e.g., references 66, 94, and 190). Physical manipulation is applied to cells, individual giant vesicles, or liposomes and avoids chemical perturbation, but it is not applicable to all systems, and the resulting data cannot readily be correlated with population-based biochemical outcomes. In contrast, measurements based on osmotic perturbation require vesicle or liposome preparations that are monodisperse (uniform in size), and they are always complicated by chemical effects. Such measurements can be correlated with biochemical data, however.
Liposome studies have shown the area elastic modulus of the phospholipid bilayer to be high in comparison to the shear and curvature elastic moduli (19). Micropipette aspiration and osmotic perturbation yield similar estimates of the area elastic moduli of phospholipid membranes (reviewed by Nebel et al. [199]). Osmotic swelling causes changes in liposome radius no larger than approximately 5% (66, 94, 244). This limit is imposed by the high area elastic modulus of the membrane and by its limited ability to withstand strain. Once a yield point has been reached, the liposome membrane remains strained as a constant cosolvent gradient is maintained by cosolvent leakage (94). Since the shear and curvature elastic moduli are relatively low, osmotic shrinkage is associated with dramatic changes in liposome shape (245).
What are the consequences of mechanical stress and strain for the structure and organization of membranes? Although there are few clear answers to this question, it is a subject of active research. By altering the volume occupied by each phospholipid molecule, membrane area dilation and condensation will alter each of the chemical interactions which contribute to membrane lateral pressure as well as the membrane surface recognized by peripheral membrane proteins (or membrane protein domains). Microcalorimetry is now being used to define molecular processes associated with osmotically induced dilation and condensation of the membrane (see, e.g., reference 199). Mechanically and osmotically imposed alterations in membrane bilayer curvature, superimposed on intrinsic monolayer curvature, are known to influence lipid phase behavior and cause lateral phase separation (245). Each of these changes could, in principle, be detected by an osmosensor (Fig. 4C; Table 2).
TIMELINE OF OSMOSENSING
Osmoregulators are devices that implement the response of an organism to changing environmental osmolality. An individual device may both detect and respond to solvent changes (i.e., osmosensors may also be osmoregulators). Alternatively, osmosensors and osmoregulators may be separate and communication between or among them may require additional signal transduction machinery. The high water permeability and solute selectivity of biomembranes ensure that changes in extracellular osmolality imposed with membrane-impermeant cosolvents trigger extensive changes in cell structure and chemistry. Thus, in principle osmosensors may detect changes in extracellular water activity (direct osmosensing) or they may detect and elicit responses tailored to address secondary consequences of osmotic shifts (indirect osmosensing). It is therefore expected that an array of osmosensors may detect and control a temporal cascade of cellular changes and osmoregulatory responses. As a result, the “osmotic history” of each cell will determine its response to new osmotic stimuli (76, 261).
The following discussion is designed to place putative bacterial osmosensors within the cascade of osmotically induced changes to cell structure and physiology, thereby identifying the stimuli to which each osmosensor may (or may not) respond. This process of correlation and elimination is important, since the list of stimuli which could, in principle, be detected by each osmosensor is long (Table 2).
Phases of the Osmotic Stress Response
When subjected to an increase or decrease in the osmolality of the suspending medium (an osmotic upshift or downshift, respectively), E. coli cells change as illustrated in Fig. 1. This review focuses primarily on membrane-based sensory processes that occur during phase I of this response. Phases II and III are considered only to the extent that they illustrate the physiological objectives met by osmoregulatory processes and the relevant changes in cell structure associated with steady-state exposure to media of various osmolalities. The timescales of these events have not yet been fully defined, and the interpretation of existing evidence concerning the timescales of phases I and II is subject to ambiguities (see Appendix). Nevertheless, the events listed as phase I of the response to an osmotic upshift can be considered to occur within milliseconds to minutes of an osmotic shift. Phase II (which also begins on imposition of an osmotic shift) extends until approximately 40 min or 10 to 20 min after the shift for bacteria cultivated in the absence and presence of osmoprotectants, respectively. Phase III is highly condition dependent and can extend for 1 h or more after the shift. The timescales for water uptake (phase I) and release of cytoplasmic cosolvents (phase II) after an osmotic downshift are at or below the limits of the experimental techniques that have been applied to date. These phases are complete within 1 to 2 min, however, and the ensuing cosolvent reaccumulation (phase III) is complete within 10 to 20 min after the shift. The evidence on which these estimates are based is discussed further below.
Most studies have focused on effects of osmotic upshifts or of osmotic downshifts or of steady-state adaptation to either of these conditions. Osmotic upshifts and downshifts have usually been applied to bacteria cultivated in low- and high-osmolality media, respectively. Additional insight regarding osmosensing will be gained by considering, as a continuum, cellular responses to both increases and decreases in medium osmolality (see, for example, Parker’s assessment of changes in cell volume versus macromolecular crowding as triggers of regulatory volume changes in canine erythrocytes [207]). Such studies will be facilitated by examining organisms with sufficient osmotolerance to permit the application of significant osmotic shifts, both up and down, after cosolvent loading (e.g., Lactobacillus plantarum [80, 81] and Halomonas elongata).
Bacterial responses have been examined at different growth phases (usually either mid-exponential or late exponential phase) and with different growth status during the experiment (growing or partially or fully nutrient deprived). Significant cellular remodeling accompanies both long-term (minutes to hours) osmoadaptation and the transition to stationary-phase growth. Thus, the patterns outlined in Fig. 1 will be refined as the short- and long-term effects of osmolality, growth phase, and nutrient status are disentangled.
Cell Structure
Changes in extracellular osmolality may elicit changes in cell structure and/or water fluxes across the cell surface. The following discussion is designed to identify (i) osmotically induced structural changes which could be detected by membrane-based osmosensors, (ii) osmotically induced structural changes which may influence subsequent osmoregulatory responses, and (iii) experimental tools which may assist researchers in identifying osmosensors and the signals to which they respond.
Turgor pressure (ΔP) is defined as the hydrostatic pressure difference which balances the osmotic pressure (or osmolality) difference between cell interior (i) and exterior (o), rendering the chemical potentials (μw) of intracellular and extracellular water (see equation 1) equal at equilibrium. For cells which can be treated as two-compartment systems:
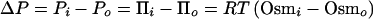 |
8 |
where P is hydrostatic pressure. Imposed changes in ΔP, Osmi or Osmo cause solvent flux across the cell surface to reestablish the equilibrium described by equation 8. On the basis of measured osmotic water permeability coefficients for phospholipid bilayers and membrane vesicles, equilibration is expected to occur within seconds of an osmotic shift (305). Since large cells (e.g., those of plants and some eukaryotic microorganisms) can be impaled, ΔP and Osmi can be manipulated directly (89, 102, 210). Such techniques may also be applicable to giant bacterial cells created by mutation and/or antibiotic treatment. The rate of solvent flux and the participation of particular cosolvents in passive cellular adjustment to osmotic shifts are illustrated (for fluxes of small magnitude) by equation 6.
Turgor pressure imposes stress on the cell surface. For a spherical cell with a very thin surface layer against which turgor pressure is exerted, the relationship between turgor pressure and the imposed stress (S) would be
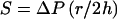 |
9 |
where r is the cell radius and h is the thickness of the surface layer. The surface layers of many bacteria are quite thick in relation to their radii of curvature, however, and for nonspherical cells, surface stress varies as a function of both location and direction along the cell surface in a manner which cannot be simply modeled (274).
Like those of phospholipid membranes, the mechanical properties of cell surfaces may in principle be described in terms of both their stiffness (or viscosity) and their elastic moduli (their ability to undergo various reversible deformations). For example, bacterial murein layers are both stiffer and more elastic than cytoplasmic membranes (as discussed by Thwaites and Mendelson [274]). For a spherical cell with a single, thin, elastic surface layer, the relationship between surface stress and strain would be
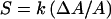 |
10 |
where k is a modulus describing the elasticity of the cell surface material and ΔA/A is the strain, i.e., the proportional change in the cell surface area in response to the imposed stress. In the simplest case, k would be constant regardless of the position on the cell surface, orientation on the cell surface, or magnitude of the applied stress.
Equations 9 and 10 can be combined to relate cell surface strain (ΔA/A) and the stress imposed by turgor pressure (ΔP) for a spherical cell:
 |
11 |
The following points emerge from this relationship. (i) The surfaces of larger cells experience greater strain than do those of smaller cells for a given stress (turgor pressure [ΔP]), elastic modulus (k), and surface layer thickness (h). (ii) Thicker cell surfaces expand and contract less (have lower strain) under a given stress (turgor pressure [ΔP]) than do thinner surfaces for a given elastic modulus (k) and cell size (r). (iii) In cells with elastic surfaces (small k), changes in extracellular osmolality may be accommodated by both water fluxes and adjustments in cell surface area. The time courses of solvent flux and of structural change after an osmotic perturbation are then determined by Pf, ςi, k, and viscosity coefficients which determine the rates at which structural changes can occur.
By using a microscopic pressure probe to measure and manipulate turgor pressure, osmotic responses and stress-strain relationships have been examined for large cells of plants and eukaryotic microbes. Such analyses suggest that for those systems at least, ςi, Pf, and k can vary with turgor pressure (89, 200, 210). Cellular properties such as surface stress and elastic modulus cannot readily be deduced from measurements of turgor pressure, osmotic gradients, and cell dimensions for nonspherical cells or for cells in which the elastic modulus varies with position on the cell surface, orientation on the cell surface, or magnitude of the applied stress. Since bacterial turgor pressure cannot readily be manipulated or measured (see Appendix), current prospects for quantifying surface stress and strain in whole bacteria are poor. However, pertinent information can be obtained by examining the mechanical properties of cellular constituents (see, e.g., reference 274).
The relationships outlined above suggest that the kinetics of osmotically induced solvent flux, of associated structural and biochemical changes, and hence of the time constants required of osmosensory and osmoregulatory processes can be adjusted by remodeling the cell surface to alter its reflection coefficient (ςi) and osmotic permeability coefficient (Pf) or its mechanical properties (its stiffness and elastic moduli). For example, reducing the reflection coefficient for a cosolvent would facilitate its rapid equilibration across the cytoplasmic membrane, reducing or eliminating osmotically induced water flux. For a benign cosolvent (e.g., glycerol), such equilibration may be more favorable to cellular survival and growth than cosolvent exclusion. Reduction of the osmotic permeability coefficient (Pf) would slow cellular dehydration in response to osmotic upshifts. Adjustment of surface stiffness and elasticity, either in general or within local cell surface regions, would modulate the degree to which swelling, shrinkage, and shape changes occur in response to extracellular osmolality changes.
Passive structural responses of bacteria to osmolality changes.
How are the structures of bacterial cells influenced by extracellular osmolality changes? Measurements of many cellular properties are pertinent to this topic. They include turgor pressure; cellular, cytoplasmic, and periplasmic volume (cell partitioning); cell density (not population density); cell size, shape and ultrastructure; and the mechanical properties of cell surface layers. Relevant techniques are discussed in the Appendix. Some bacteria have been shown to maintain turgor pressure (references 45 and 294 and references cited therein). For example, the turgor pressure for E. coli cells cultivated in standard, defined media is believed to be 3 to 5 atm. Although the mechanical properties of bacteria have not been fully analyzed (for reasons discussed above), studies of osmotic effects on cell size and hydration offer some insight into their osmotic relations.
Building upon earlier experiments performed with a variety of bacteria, Alemohammad and Knowles (1) used turbidimetry and solute distribution measurements in parallel to assess the immediate effects of salts, sucrose, and glycerol (osmotic shifts of up to 0.55 osmolal) on resting E. coli cells. The turbidity increased over 2 min to reach a new value that was stable for 1 h after treatment with NaCl, MgCl2, or sucrose. Decreases in both cell and cytoplasmic volume were observed by measuring solute exclusion 30 min after the shift. Stopped-flow spectrophotometry was used to establish that glycerol caused only a transient (maximal at approximately 0.5 s and reversed within 8 s) increase in the turbidity of such cell suspensions. Subsequent research has confirmed that the cytoplasmic membrane has a low reflection coefficient for glycerol (ςGly), which can be further reduced by the expression of facilitator GlpF (164).
These experiments established the time frame for the response of E. coli cells to osmotic upshifts and validated the use of glycerol as a membrane-permeant cosolvent during studies of osmoadaptation by E. coli. The fact that elevation of extracellular osmolality with glycerol fails to activate an osmoregulatory response is often taken as evidence that a change in transmembrane osmolality gradient (or its consequence) is sensed. However, dehydration of an osmosensor as a result of cosolvent exclusion from intramolecular water pools could also act as a stimulus (see “Hydration forces” above). Just as its lack of charge and its small size allow glycerol to permeate cell membranes, they may also allow it to penetrate intramolecular water pools and hence fail to alter macromolecular (osmosensor) conformation.
The impact of osmotic upshifts on the cellular, cytoplasmic, and periplasmic water content (microliters per milligram of protein or dry weight) of nutrient-deprived bacteria (E. coli and Salmonella only) has been assessed by applying the solute distribution technique (1, 38, 39, 260) (see Appendix). Decreases in both cell and cytoplasmic water contents were observed when osmotic upshifts were imposed with solutes expected to be excluded by the cell wall (polyglutamate) or the cytoplasmic membrane (sucrose, MgCl2, or NaCl) but not with cytoplasmic membrane-permeant solutes (glycerol or ethanol). Osmotic shifts imposed on Salmonella with sucrose or on E. coli with sucrose or NaCl were found to increase the fraction of cell water that was periplasmic. However, the degrees and morphological consequences of plasmolysis differed when osmotic upshifts of the same magnitude (0.365 osmolal) were imposed on E. coli cells with NaCl or sucrose (1).
Koch (125) used turbidimetry to analyze cellular changes 5 s and 1 min after small osmotic upshifts (no larger than 0.25 osmolal) were imposed by adding arabinose or xylose to growing E. coli cells. Measurements were performed by stopped-flow spectrophotometry and interpreted by fitting the data to a light-scattering model in which the total intensity of the scattered light (presumed to cover 0° through 180°) was related to cell size. Cells were treated as solid rods (shapes obtained by averaging that of a cylinder and that of an ellipse) of uniform and invariant refractive index (corrections for changes to the refractive index of the medium were applied). Turbidimetric changes were reported as cell surface area and volume changes. Arguing that the osmotic upshifts were too small to cause plasmolysis and that the measurement times were too short to accommodate metabolic changes (sugar uptake and/or osmoregulatory activity), Koch reported that increasing the osmotic upshifts elicited continuous cell surface area and volume decreases (without abrupt changes) to approximately 40 and 50%, respectively.
Baldwin et al. (12) measured changes in the buoyant density (density gradient centrifugation) and size (Coulter counter and light microscopy) of nutrient-deprived E. coli cells that were subjected to small (0.15- to 0.45-osmolar) osmotic up- and downshifts with NaCl or sucrose. Measurements performed within 10 min of the osmotic shift indicated that buoyant density increased as cell size decreased in response to osmotic upshifts and that buoyant density decreased as cell size increased in response to osmotic downshifts. However, the volume changes were smaller than those reported by Koch (125) (the surface area decreased 33% [microscopy] after a 0.15-osmolar upshift, and the volume decreased 21.5% [Coulter counter] after a 0.35-osmolar upshift). Since increased cellular buoyant density would certainly be accompanied by an increased cellular refractive index, Koch’s assumption that all turbidity changes would reflect changes in cell size (125) was not justified and may have contributed to overestimation of the reported area and volume changes.
By applying phase-contrast microscopy to filamentous (ftsA and ftsI) E. coli mutants, Koch et al. (127) observed decreases in cell length (on average, 17%) in response to detergent (sodium dodecyl sulfate) disruption of the cytoplasmic membrane. The observed shrinkage was attributed to elasticity of the murein layer. This elasticity was tested further by measuring the angular dependence (4° through 12°) of the intensity of unpolarized light scattered by sacculus suspensions as their pH was titrated over a range designed to adjust the net sacculus charge from positive through negative values (129). The data were fit to a model of the sacculi as hollow prolate ellipsoids, assuming a constant thickness and refractive index of the murein shell and a constant unique axial ratio of the ellipsoids, in order to extract changes in sacculus width, reported as sacculus area. Although systematic changes in light scattering by the sacculi were elicited by pH changes, their interpretation in terms of sacculus area may not be justified. Others have also reported murein elasticity, however (summarized in reference 58). Taken together, these results suggest that bacterial cell surfaces are elastic, with their elastic moduli varying with cell volume (perhaps turgor pressure) and ionic strength.
The studies summarized above suggest that the cytoplasmic membrane and cell wall of E. coli act as a unit structure with a sufficiently small elastic modulus (k) to permit overall cell shrinkage in response to cellular dehydration. The available measurements do not indicate whether turgor pressure is maintained at a constant level as cells shrink in this way or whether it declines steadily (see the discussion by Csonka and Hanson [47]). It is also difficult to assess whether these adjustments are accompanied by changes in periplasmic thickness that might be detected by an osmosensor. The cytoplasmic membrane partitions the cell interior into periplasmic and cytoplasmic compartments. The osmolalities of the cytoplasm and periplasm are sometimes assumed to be equal (228, 260), but the experimental evidence supporting that assumption is extremely limited. If periplasmic and cytoplasmic osmolalities were always equal, the cytoplasmic membrane would be subject to area dilation or condensation only as a result of mechanical stress transmitted by its linkage to the cell wall and/or the nucleoid (see below). Assessment of the relative osmolalities of the cytoplasm and periplasm for bacteria growing in diverse solvent environments should be a high experimental priority.
The outer membrane is covalently linked to the murein sacculus via lipoproteins (reference 147 and references therein) and some porins. Although structural links between the outer membrane and the murein layer may be stronger and/or more densely spaced than those between the cell wall and the cytoplasmic membrane, the latter links certainly exist (147). The potential involvement of Bayer’s bridges in protein secretion and of periseptal annuli in cell division has motivated extensive electron microscopic analyses of plasmolyzed E. coli cells. These observations clearly indicate that membranous links between the cytoplasmic membrane and the cell wall (Hechtian strands) are retained despite extensive plasmolysis (118, 126, 251, 298). Limitations on fluorescence recovery after photobleaching of periplasmic probes indicate that the periplasm is partitioned, presumably by links between the outer and cytoplasmic membranes (72). Woldringh et al. (299) and Nanninga (198) emphasize the extensive linkage of cell wall and cytoplasmic membrane effected by murein biosynthetic enzyme complexes as well as the linkage of cytoplasmic membrane and nucleoid effected by cotranslational protein secretion. Thus, the cytoplasmic membrane acts as a structural link between the cytoplasm (including the nucleoid) and the cell wall, suggesting that it may experience local variations in mechanical stress in response to both metabolic activity and osmotic shifts.
Plant cell plasmolysis has been examined to assess the molecular bases for freezing and salinity tolerance (203, 219). These studies, too, reveal the presence of Hechtian strands and plasma membrane vesiculation in plasmolyzed cells. It has been suggested that such structures may maintain the cytoplasmic membrane surface area, cell integrity, and polarity and focus mechanical stress from the wall to the membrane during the protoplast shrinkage that accompanies plasmolysis. In addition, vitronectin- and fibronectin-like proteins, present in enhanced numbers in NaCl-adapted tobacco cells, are proposed to mediate enhanced membrane-wall adhesion (309). It would be interesting to learn whether the incidence of wall-membrane adhesions also varies as a function of bacterial growth medium osmolality and/or salinity.
Cell surface modifications and osmosensing.
Does structural remodeling of the cell surface influence the osmotic stress response? Genetic screening has revealed genes encoding E. coli cell surface molecules whose expression is modulated by extracellular osmolality (Table 3). In most cases, steady-state levels of these elements have been reported but their influence on the kinetics of the osmotic stress response has not.
TABLE 3.
Osmoresponsive genes and operons in E. coli
| Gene or operon | Encoded function | Locus-specific transcriptional regulator(s) | Reference(s) |
|---|---|---|---|
| aqpZ | Aquaporin | 32 | |
| betTIBA | Choline uptake and oxidation | BetTa | 142 |
| kdpFABCDE | Potassium transport and transcriptional regulation | KdpD/KdpE | 2 |
| mdo | MDO synthesis | 140 | |
| ompF | Porin | EnvZ/OmpR | 255 |
| ompC | Porin | EnvZ/OmpR | 255 |
| osmB | Putative lipoprotein | 109 | |
| osmC | Putative lipoprotein | 88 | |
| osmE | Unknown | 88 | |
| osmY | Unknown | 145, 306 | |
| otsAB | Cytoplasmic trehalose synthesis | 100 | |
| proP | Compatible-solute transport | 302 | |
| proU | Compatible-solute transport | 159 (see text) | |
| stpA | Nucleoid protein | 73 | |
| treA | Periplasmic trehalose hydrolysis | 100 |
BetI is a repressor that modulates the transcription of betT and betIBA from divergent promoters in response to choline supply, and ArcA represses their transcription under anaerobic conditions. Osmotic induction of these loci is independent of BetI, H-NS and RpoS, however (142).
Reciprocal variations in the levels of porins OmpF and OmpC of E. coli are among the earliest discovered and the most intensively studied examples of osmoadaptation (184, 255). Despite detailed studies of the controls exerted by the two-component regulatory system EnvZ/OmpR over the transcription of ompF and ompC, no change in osmotolerance (in the steady state) has been associated with defects in the porins or their regulation. Recent analyses suggest that porin closure can be promoted by membrane-derived oligosaccharides (MDOs) and by polyamines (53, 54, 107). Polyamines reduced outer membrane aspartate permeability enough to influence the chemotactic response (53). E. coli cells cultivated in media of low osmolality and low ionic strength synthesize MDOs (121). Since these anionic oligosaccharides are unable to cross either the outer or the cytoplasmic membrane, they raise the osmolality and ionic strength of the periplasm. Reduced outer membrane permeability (limiting efflux of metabolic products) and/or increased MDO levels might increase periplasmic osmolality, increasing both the fraction of cell volume occupied by the periplasm and the crowding of macromolecules in the cytoplasm.
Modulation of porin levels and/or of their linkage to the murein sacculus may also influence the stiffness and elasticity of the cell surface. Since murein is anionic, elevation of the ionic strength of the periplasm with MDOs and their counterions may also influence the mechanical properties of the cell wall (274). Transcription of the genes encoding other cell wall proteins, including OsmB, OsmC, OsmE, and OsmY, is also induced at high osmolality (Table 3). OsmB and OsmE are believed to be lipoproteins. These proteins may be involved in osmoregulatory cell wall remodeling as structural elements and/or as enzymes. Each of these effects could influence the responsiveness of cell structure to osmotic changes and the kinetics of the osmotic stress response.
Since phospholipid composition profoundly influences the structure and permeability of membranes (see “Solvent-membrane interactions” above), it is also important to ascertain whether environmental osmolality influences membrane phospholipid composition. Temperature is a strong determinant of biomembrane lipid composition; effects of environmental osmolality on bacterial membrane lipids have been less extensively documented (98, 155, 242, 243, 258). For a variety of halotolerant and halophilic bacteria, both gram positive and gram negative, growth media of increasing salinity elicit a decrease in the proportion of zwitterionic phospholipids (e.g., phosphatidylethanolamine) and a corresponding increase in the proportion of anionic phospholipids (e.g., phosphatidylglycerol, cardiolipin) (242, 243). Diverse changes in fatty acid composition are observed, the strongest trend being an increase in the cyclopropane fatty acid content among the phospholipids of halophilic and halotolerant gram-negative bacteria. Since NaCl has most often been used as cosolvent for these studies, it is often not clear whether cells are responding to changes in osmolality, ionic strength, or NaCl concentration per se. In a few cases, however, NaCl and nonionic cosolvents elicit similar changes (242). Limited data suggest that the head group and acyl chain compositions of the phospholipids of E. coli are relatively insensitive to medium salinity (1a, 57).
High levels of NaCl (3 M) lowered the Th for membrane phospholipids from Vibrio costicola cells cultivated in media of low salinity (1 M NaCl), so that the lipids were not lamellar at 30°C (270). The shifts in head group and acyl chain composition that occurred during growth of this organism in high-salinity media (3 M NaCl) yielded membrane lipids which remained lamellar in media of low and high salinity (271). Changes in head group composition in response to osmotic shifts resulted from an immediate cessation of phospholipid biosynthesis followed, within a few minutes, by its resumption at relative rates designed to effect the ultimate compositional change (138, 242). Since these changes occurred rapidly and without protein synthesis, the phospholipid biosynthetic enzymes were believed to sense and respond to the osmotic shifts.
The recent discovery of aquaporin AqpZ in E. coli and of immunologically cross-reactive material or of aqpZ homologues in other bacteria challenges the view that water enters and leaves bacteria primarily via the phospholipid bilayer (32–34). The aqpZ mRNA is monocistronic. While disruption of aqpZ was not lethal for E. coli, aqpZ-deficient bacteria formed smaller colonies on Luria-Bertani medium and survived less well in liquid medium at 39°C or at low osmolality than did aqpZ+ bacteria. Expression of aqpZ, tested by using an aqpZ::lacZ fusion, peaked in mid-logarithmic phase and was suppressed up to 30-fold when the bacteria were cultivated in high-osmolality media (NaCl or KCl supplemented) (33). Further experimentation is required to assess whether modulation of Pf through aquaporin regulation alters the cellular response to osmotic shifts.
Osmotic shifts, cytoplasmic composition, and nucleoid organization.
For E. coli cells (exponential or stationary phase) cultivated in low-osmolality (Luria-Bertani) medium, the macromolecule concentration of the cytoplasm was estimated at 0.3 to 0.4 g/ml. Macromolecules were estimated to occupy 34 to 44% of the cytoplasmic volume (313). This volume exclusion (or macromolecular crowding) is a consequence of the collective presence of diverse macromolecules. A similar effect would be achieved in a homogeneous, 0.3- to 0.4-g/ml solution of a globular protein with a molecular mass in the range 72 to 79 kDa if it did not aggregate or undergo specific interactions with any other test molecule.
Phase separation—the formation of distinct liquid, aqueous phases—is a well-known correlate of macromolecular crowding (291). It can result in the formation of macromolecule-rich and -poor phases or in the formation of phases enriched for different, incompatible macromolecules. The phase separation between nucleoid and cytoplasm within bacteria (281) can be considered in these terms. Polyamines (e.g., putrescine [tetramethylene diamine]), K+, and proteins may all be expected to participate in the crowding-induced phase separation of the nucleoid from other cytoplasmic constituents.
Zimmerman and Murphy argue that bacterial DNA is always in a condensed state as a result of macromolecular crowding (312). The bacterial nucleoid is estimated to have a DNA concentration of 50 to 100 mg/ml and to occupy one-eighth to one-fifth of the volume within the cell envelope. Hildebrandt and Cozzarelli suggest that the balance among DNA condensation due to macromolecular crowding (expected to enhance associations), the connectivities of particular DNA sequences, and the masking or spatial sequestration of those sequences by proteins ensure their appropriate reactivity for processes such as transcription and replication (99). Stabilization by macromolecular crowding has now been reproduced in vitro for low-salt, spermidine nucleoids from E. coli, which retain nucleoid proteins (193, 194). Analysis of the resulting structures suggested that crowding might contribute to nucleoid condensation both directly, by condensing DNA, and indirectly, by enhancing partition of particular proteins into the nucleoid phase.
Specific cations and anions (and not ionic strength per se) are known to influence nucleoid structure and modulate transcription (36, 151). Cations accumulate in the bacterial cytoplasm and nucleoid because DNA and RNA, both present at high levels, are polyanions (3). The physiologically significant nucleic acid counterions in E. coli include K+, polyamines (e.g., putrescine), and proteins. Thus, nucleic acids act as ion-exchange matrices and cation exchange is required for gene expression (36, 91, 151).
Decreased cellular hydration and alterations in cytoplasmic ion content are immediate and sustained consequences of exposure of bacteria to high-osmolality media. It is therefore possible that osmosensors located in the cytoplasm (or on the surface of the cytoplasmic membrane) detect significant changes in macromolecular crowding under these circumstances. Osmotic upshifts also transiently inhibit DNA replication and cause transient or sustained induction of the transcription of certain genes (see below). To understand how changes in hydration influence replication or transcription, it is necessary to consider how macromolecules and inorganic ions, acting as cosolvents, influence nucleoid architecture. Further consideration of this topic is beyond the scope of this review. It is important to bear such effects in mind, however, when considering the sensory inputs to which osmoregulatory K+ transporters may respond (see below).
Energy Metabolism
Osmotic upshifts.
Avi-Dor and his colleagues first identified effects of osmotic upshifts on energy-transducing enzymes integral to bacterial cytoplasmic membranes through their studies of osmoadaptation by Ba1, a moderately halophilic, obligately aerobic gram-negative bacterium (summarized by Ken-Dror et al. [120]). In E. coli, respiration (both NADH and succinate dependent) (101, 172), facilitated diffusion of glycerol or xylitol (101), and solute uptake via sugar and amino acid transporters of the ATP binding cassette (ABC), phosphotransferase, and ion-linked categories (84, 101, 236, 237) are all inhibited within seconds when an osmotic upshift is imposed with salts or sucrose but not with glycerol. This attenuation of energy metabolism defines phase I of the physiological response to an osmotic upshift (Fig. 1). Activation of transporters implicated in osmoregulation occurs in strong contrast to these effects (see below).
Meury described strong (75%), transient inhibition of respiration by an osmotic upshift imposed with 0.6 M NaCl (172). This effect was partially reversed and a new, lower respiration rate was established approximately 30 min after the shift. Houssin et al. found respiration (20 min after the shift) to be systematically reduced as the osmolality increased (101). These effects were accompanied by a large and transient (reversed within less than 10 min) increase in cellular ATP concentration, but it could be attributed to cell volume changes (101, 201). Effects of osmotic shifts on the proton permeability of the membrane or F0F1-ATPase activity have not been reported.
Dinnbier et al. reported small, transient (reversed within 20 min) increases in cytoplasmic pH and ΔpH in response to an osmotic upshift imposed with NaCl (0.5 M) (56). E. coli possesses two Na+/H+ antiporters (NhaA and NhaB), both of which are implicated in the maintenance of ΔpNa+, pH homeostasis, and salinity tolerance (205). Wild-type bacteria can grow in minimal medium supplemented with up to 0.7 M NaCl or KCl. Bacteria lacking nhaA tolerate less than 0.4 M NaCl and remain unaffected by 0.7 M KCl (204). Deletion of nhaB, alone, is without effect, but deletion of both loci produces sensitivity to as little as 0.03 M NaCl (217). The Na+ sensitivity of nhaA and nhaA nhaB mutants is exacerbated as the medium pH increases, and an additional system, ChaA, is implicated in Na+ and Ca2+ extrusion at high pH (202). These systems, along with other K+/H+ and Ca2+/H+ antiporters, are expected to mediate alkalinization of the cytoplasm when osmotic upshifts are imposed with the corresponding salts. They are tightly controlled (205). Thus, although transient changes are likely, no steady-state alteration in either component of the proton motive force (neither ΔpH nor the membrane potential, Δψ) was detected during the period from 20 to 40 min after osmotic upshifts (0.28 to 1.88 osmolal) were imposed on E. coli with NaCl or sucrose at pH 7.6 (101). It would be interesting to know whether ionic and nonionic osmolytes exert differential effects in a more alkaline environment.
How do osmotic upshifts inhibit respiration? The rate and level at which respiration resumed after an osmotic upshift were dramatically attenuated for E. coli cells deficient in K+ uptake (kdpABC5 trkA405 trkD1) (172). Ken-Dror et al. (120) suggested that glycine betaine stimulated respiration in salt-stressed bacterium Ba1 because Na+/glycine betaine symport satisfied an Na+ requirement for respiration. These observations suggest that cation accumulation during osmoadaptation is required to restore membrane-linked energy metabolism. For E. coli, this requirement may be K+ specific (not merely a function of cytoplasmic rehydration) since glycine betaine accumulation did not fully replace K+ accumulation in this respect (172). A specific K+ requirement for respiration by EDTA-treated E. coli cells has been reported (206), but its mechanistic basis is unknown.
It may appear contradictory that ATP pools and the proton motive force remain stable while respiration is reduced in response to osmotic upshifts. These effects may be at least partially explained by respiratory control. As expected on that basis, the proton ionophore carbonyl cyanide m-chlorophenylhydrazone (CCCP) stimulated respiration by NaCl- or glucose-stressed E. coli cells (168). Direct effects of osmolality on membrane lipid and/or protein structure and organization are postulated to be responsible for the generalized inhibition of membrane-based functions (101). The operation of specific regulatory mechanisms has not been ruled out, however. The observations summarized above suggest that only small, transient perturbations to the proton motive force and ATP levels are associated with osmotic upshifts in E. coli. While such transient changes could conceivably trigger osmosensory responses (e.g., phosphorylation cascades), they cannot account for more sustained phenomena.
Osmotic downshifts.
Osmotic downshifts have long been known to elicit rapid and extensive solute efflux from bacteria, including E. coli (279), the cyanobacteria Synechococcus and Synechocystis (231), and the halophile Ectothiorhodospira halochloris (280). It is also evident that the specificity and extent of solute release depend upon the history of the bacteria, the magnitude of the osmotic gradient, and the speed with which it is imposed (see, e.g., references 16 and 176), as well as the temperature (see the discussion by Schleyer et al. [249]). For example, compatible-solute efflux (see below) can occur without (241, 249) or with (16) ATP efflux, and this difference is correlated with the magnitude and conditions of the imposed shifts (249). These observations are consistent with known effects of temperature and imposed osmotic gradients on phospholipid bilayer permeability.
Milner et al. observed decreases in the initial rates of glutamine uptake (via an ABC transporter [more than sixfold]), serine uptake (via an Na+/serine symporter [approximately twofold]), and proline uptake (via ProP, an osmoregulatory H+/proline symporter [sevenfold]) when E. coli cells cultivated in 0.3 M NaCl-supplemented minimal medium were introduced to a transport assay medium lacking that supplement (180). Cairney et al. showed strong reductions in the activities of the osmoregulatory transporters ProP and ProU (an ABC transporter) of Salmonella typhimurium under analogous conditions (31). No bioenergetic parameters (e.g., respiration rates, ATP levels, Δψ, ΔpH, and other ion gradients) were reported, but comparisons with other data suggest that the reduced transport activities may have reflected both ATP efflux and dissipation of the proton motive force. Ruffert et al. found that a transient decrease in ATP levels and a sustained and profound decrease in the membrane potential accompanied the specific efflux of glycine betaine elicited by continuous dilution of a Corynebacterium glutamicum suspension (241). The capacity for active solute uptake is rapidly regained after osmotic downshifts (see below), suggesting that energy metabolism resumes rapidly.
Adjustment of Cytoplasmic Solvent Composition
As noted above, the modulation of cytoplasmic solvent composition reverses the growth-inhibitory effects of environmental osmolality changes on bacteria. To identify putative osmosensors and the signals that they detect, the following discussion focuses on cytoplasmic cosolvent selection as well as the temporal sequence and consequences of cosolvent accumulation and release. More detailed information about osmoregulatory genes and enzymes in diverse organisms can be found in references 6, 45–47, 74, 87, 119, 178, 220, and 263.
Extensive studies of E. coli indicate that the pattern of response to an osmotic upshift is contingent upon the availability of osmoprotectants. The availability of K+, the magnitude of the imposed shift, the medium pH, and the supplies of carbon and nitrogen for growth are also important in tuning the osmoregulatory response to suit particular conditions. The relative importance of K+ for osmoregulation by other organisms is not yet clear, however (220). For E. coli cells in the absence of osmoprotectants, K+ influx and associated adjustments in levels of other ionic and nonionic cosolvents occur during phases II and III of the response, extending for as long as 1 h after the shift (Fig. 1). Phase II is compressed and altered in the presence of osmoprotectants, extending for as little as 10 to 20 min after the shift.
Cosolvent accumulation: absence of osmoprotectants.
K+ uptake and putrescine efflux are triggered as other energy-linked functions cease within seconds after an osmotic upshift. Transporters Trk and Kdp mediate K+ accumulation by E. coli in response to osmotic upshifts. Osmotic control over the synthesis and activity of these transporters, exerted at the level of the cytoplasmic membrane, is discussed below.
Putrescine efflux accompanies K+ uptake in response to osmotic upshifts (up to 1.6 osmolar) imposed with NaCl, MgCl2, KCl, or sucrose (but not glycerol), and putrescine is rapidly reaccumulated when cells are transferred to a low-osmolality medium (192). In experiments with whole bacteria, putrescine efflux in response to an osmotic upshift was contingent on the availability of exogenous K+, but K+ uptake was not reduced by prior depletion of endogenous putrescine. The PotE protein of E. coli, predicted to be an integral membrane protein with 12 transmembrane α-helices, possesses weak putrescine uptake and putrescine/ornithine antiport activities (5 and 1 nmol/min/mg of protein, respectively, in bacteria expressing recombinant plasmid-encoded potE) (114–116). The former activity is dependent upon the proton motive force. Neither the ion-coupling specificity and stoichiometry nor the dependence of this activity on K+ or osmolality has been reported, nor has the impact of a potE mutation on the initial phase of osmoadaptation. These experiments are necessary to assess whether this system may mediate the osmoresponsive putrescine efflux first described by Munro et al. (192).
K+ accumulation, particularly in cells exposed to very-high-osmolality media, exceeds the capacity for charge balance afforded by proton and putrescine efflux (6, 7, 56). Anions must therefore accumulate. Glutamate begins to accumulate within approximately 1 min of an osmotic upshift in E. coli (56, 168). Indeed, glutamate fulfills this role under most conditions and in most organisms examined to date, although other species may contribute (e.g., morpholinepropanesulfonic acid [MOPS] [40] and unknown species in nitrogen-limited E. coli cells [293]). In view of the high flux via the glutamate biosynthetic pathways even at low osmolality, reduced utilization of glutamate for the synthesis of other nitrogen-containing compounds (including proteins) may be sufficient to ensure glutamate accumulation in response to an osmotic upshift (168). However, K+ accumulation is not contingent upon glutamate accumulation in E. coli (168). The coincident glutamate and K+ efflux which occurred when ammonium was added to an S. typhimurium glnE mutant may have resulted from the activation of efflux mechanisms in response to massive glutamine accumulation, not to a specific glutamate requirement per se (303). Observed changes in K+, putrescine, and glutamate levels have been estimated to be sufficient to maintain electroneutrality (36, 168).
K+ replaces putrescine as a nucleic acid counterion during phase II of adaptation to an osmotic upshift. McLaggan et al. (168) estimated the quantity of K+ in E. coli not constrained by interactions with fixed anions (the K+ released by cold osmotic downshock). K+ release increased from 0.22 to 0.67 μmol/mg (dry weight) and the quantity of residual K+ increased from 0.36 to 0.64 μmol/mg (dry weight) for E. coli cells cultivated in medium of 0.17 to 1.24 osmolal. Richey et al. arrived at similar estimates on the basis of nuclear magnetic resonance spectroscopy (233). Guttman et al. concluded that interactions with rRNA (not DNA) influenced the nuclear magnetic resonance spectrum of approximately 60% of the K+ in the cytoplasm of E. coli (cultivated at low and high osmolality) (90). It has been suggested that the exchange of putrescine for K+ elevates cytoplasmic osmolality while reducing the impact of K+ uptake and glutamate accumulation on cytoplasmic ionic strength (36, 192). An increase in ionic strength would inevitably accompany the accumulation of osmotically active K+ and counterions in response to high-osmolality media, however. It is unfortunate that although two physicochemically different K+ pools have been identified, it has not been possible to estimate their osmotic activities.
For E. coli cells growing in the absence of osmoprotectants, biosynthetic accumulation of compatible solute trehalose follows glutamate accumulation and overlaps phases II and III of the response to an osmotic upshift (Fig. 1) (56). Trehalose accumulation is enhanced in bacteria growing in nitrogen-limited media and diminished in bacteria growing in carbon-limited media (293). It is effected through transcriptional control of the treA, treBC otsA, and otsB loci and perhaps also through the activation of the trehalose biosynthetic enzyme OtsA by K+ glutamate (see discussions by Lucht and Bremer [159] and Horlacher and Boos [100]). The mechanism of osmoregulatory trehalose accumulation is not discussed further here because it does not appear to involve membrane-based osmosensing.
Phases II and III of the response to an osmotic upshift are further defined by increased transcription of other osmoresponsive genes and operons (and by reduced transcription of the ompF locus) (Table 3). Expression of the genetic loci proP and proU by E. coli is induced in the absence of the substrates of their encoded transporters. Experiments performed with lacZ fusions indicate that transcription of the kdp, proP, and proU loci begins within minutes and proceeds with a half time of approximately 20 to 30 min. In bacteria proficient for glycine betaine transport, transcription of proP and proU (but not the kdp operon) is attenuated in the presence of glycine betaine, indicating that osmoprotectant accumulation attenuates the activating signal for the former systems (59, 108, 141, 169, 170, 269).
These osmoresponsive loci can be divided into two categories. For the first group, represented by proU and proP, no locus-specific, trans-acting regulatory factor appears to participate in osmotic induction. For the second group, represented by ompF-ompC and the kdp operon, transcription is controlled by a locus-specific two-component regulatory system (EnvZ-OmpR and KdpD-KdpE, respectively). Cytoplasmic membrane-associated sensor kinases EnvZ and KdpD alter transcription in response to osmotic changes and other signals. The mechanism by which KdpD detects osmotic shifts is discussed in more detail below (see “K+ transporters of E. coli”).
Cosolvent accumulation: presence of osmoprotectants.
Osmoprotectants are compounds that enter the cytoplasm through the action of osmoregulatory transporters and act as or are converted to compatible solutes. In all organisms examined to date, multiple transporters, falling into the ion symporter and ABC transporter classes and with overlapping substrate specificities, mediate osmoprotectant uptake.
ProP, a broad-specificity osmoprotectant/H+ symporter, is activated when an osmotic upshift is imposed on cells (E. coli or S. typhimurium) cultivated in low-osmolality media (59, 84, 179). It is now evident that secondary transporters which mediate osmoprotectant uptake fall into distinct phylogenetic groups (246). For example, members of the major facilitator superfamily include the ProP system of E. coli (50) and OusA of Erwinia chrysanthemi (82). A second group includes the choline transporter BetT of E. coli (264) and the glycine betaine transporters OpuD of Bacillus subtilis (113) and BetP of Corynebacterium glutamicum (213). Proline transporter OpuE of B. subtilis (287) is not linked to either of these groups. Analogous activities have been identified in a variety of other organisms but have not yet been defined at a structural level (220).
ProU (ProVWX), a broad-specificity osmoprotectant transporter of the ABC transporter class, is also activated when an osmotic upshift is imposed on E. coli cells cultivated in low-osmolality media (68). Systems OpuA and OpuC of B. subtilis (92, 113) are sequence homologues of E. coli ProU. Structurally undefined osmoprotectant transporters of the ABC class are present in other organisms including Rhizobium meliloti (178) and Listeria monocytogenes (283).
Although some osmoprotectant transporters are similar to those of E. coli in being both activated and induced by osmotic upshifts, others are reported to respond only at the genetic or biochemical level. Technical issues may have impeded the detection of transporter regulation in some cases, however (220). Like the K+ transporters Trk and Kdp, at least some osmoprotectant transporters are not merely resistant to inhibition by osmotic upshifts. Their activities increase, in the absence of protein synthesis, if the osmolality of the external medium is raised through the addition of salts or organic osmolytes (Table 4). It is now clear that the ProP transporter can both sense and respond to an osmotic upshift. The mechanism by which this occurs is discussed further below.
TABLE 4.
Osmotically activated osmoprotectant transportersa
| Transporter class | Transporter name | Organism | Activatorb
|
Sequence known | Reference | |
|---|---|---|---|---|---|---|
| I | NI | |||||
| ABC transporter | ProU | Escherichia coli | Yes | Yes | Yes | 68 |
| Not assigned | Listeria monocytogenes | Yes | NT | No | 283 | |
| Ion symporter | ProP | Escherichia coli | Yes | Yes | Yes | 84 |
| Salmonella typhimurium | Yes | NT | No | 31 | ||
| BetT | Escherichia coli | Yes | NT | Yes | 264 | |
| Not assigned | Staphylococcus aureus | Yes | Yes | No | 222 | |
| OpuD | Bacillus subtilis | Yes | NT | Yes | 113 | |
| BetP | Corynebacterium glutamicum | Yes | Yes | Yes | 213 | |
| Not assigned | Listeria monocytogenes | Yes | Yes | No | 79 | |
The listed transporters were activated by an osmotic upshift imposed with at least one ionic or nonionic cosolvent in the absence of protein synthesis.
I, ionic; NI, nonionic; NT, not tested.
Phase II of the response to an osmotic upshift is altered and truncated when it includes uptake of both K+ and compatible solutes. Respiration was inhibited to a lesser degree and the organism was resistant to inhibition over a wider osmolality range when glycine betaine was provided to bacterium Ba1 (120) or to E. coli (101, 172) than when it was absent. Glycine betaine also afforded some protection against osmotic inhibition of sugar transporters of the ABC, ion-linked, and phosphotransferase classes in E. coli (101). Compatible solutes are more effective than K+ and its counterions in effecting cytoplasmic rehydration, and their accumulation allows cells to reduce or avoid the pleiotropic effects of K+/counterion accumulation on cell structure and physiology. Indeed, proline or glycine betaine uptake reduced the rate and extent of K+ uptake and eliminated biosynthetic trehalose accumulation by E. coli cells subjected to osmotic upshifts (56, 172). Steady-state compatible-solute accumulation has been repeatedly observed to attenuate K+ accumulation by diverse bacteria cultivated in high-osmolality media. Thus bacteria clearly express a preference for compatible solutes over K+ as osmoregulators.
Since E. coli accumulates some K+ when an osmotic upshift is imposed in the presence of a compatible solute (56), K+ appears to meet needs not satisfied by compatible-solute accumulation. K+ uptake is generally believed to precede compatible-solute uptake in response to an osmotic upshift, and exogenous K+ is required for full activation of the ProP transporter in E. coli and S. typhimurium (131, 162). The timescales for activation of these transporters are near or even beyond those of most uptake assays, however, and no direct comparison of the activation kinetics of the transporters has been reported. It is therefore not clear whether K+ is required only for respiration (as discussed above) or whether it is integral to the osmoregulatory mechanism of ProP.
Cosolvent efflux.
Systematic analysis of solute efflux and exchange by bacteria is a recent phenomenon, motivated in part by the needs of the biotechnology industry (133). It is now clear that bacteria possess distinct uptake, exchange, and efflux mechanisms and that each of those processes is integral to metabolism. However, particular technical challenges face those who wish to differentiate efflux from exchange and “passive” efflux from efflux mediated by channels or by transporters (133). In particular, data must now be interpreted with the knowledge that multiple, poorly selective mechanosensitive channels mediate metabolite efflux across the cytoplasmic membranes of bacteria (16, 21, 49, 241).
Cosolvent efflux mechanisms may be required for the survival of bacteria that adapt to a high-osmolality medium and then experience an osmotic downshift, to offer an alternative to feedback mechanisms limiting cosolvent uptake and to provide opportunities for selective cosolvent retention (131). Rapid (1- to 2-min) release of K+, glutamate, and/or compatible solutes, without wholesale loss of metabolite pools, has been reported for Synechocystis (231), E. coli (249), B. subtilis (300), L. plantarum (80), C. glutamicum (144, 241), and L. monocytogenes (283). Thus, rapid but specific solute efflux defines phase II of bacterial responses to osmotic downshifts.
Gadolinium ions (Gd3+) inhibited both mechanosensitive channel activity and the release of ATP, glutamate, and lactose but not K+ or Rb+ from E. coli. Gd3+ also reduced osmotically induced cosolvent efflux from other organisms (16, 80, 144, 241). Since Gd3+ is an inhibitor of mechanosensitive channels, these studies suggested that channels play a role in adaptation to osmotic downshifts. Gd3+ may act directly on mechanosensitive channels, or its effects may be exerted via membrane lipids (64, 65).
In E. coli, K+ release, elicited by either an osmotic downshift or glycine betaine uptake, was independent of known K+ uptake and efflux mechanisms (Trk, Kdp, Kup, KefB, and KefC) (8, 249). Rapid K+ reaccumulation established a reduced K+ pool after the shift, and that process was compromised in bacteria lacking transporter Trk (249). Using osmotic upshifts to elicit K+ uptake and continuous slow dilution to elicit K+ release, Meury et al. demonstrated that these two processes followed distinct pathways (176). Unlike the former, the latter pathway excluded Rb+ and was inhibited by the eukaryotic K+ channel blockers tetraethylammonium and phencyclidine. Schleyer et al. reported nonspecific effects of Gd3+, quinine, and phencyclidine on K+ efflux and reuptake, however (249).
Glycine betaine was released from S. typhimurium in the absence of the osmoregulatory transporters ProP and ProU, but serine was also released under the conditions used in this experiment. Rapid, specific loss of glycine betaine, independent of ProP and ProU, was elicited by the thiol reagents N-ethylmaleimide and p-chloromercuribenzenesulfonic acid in the absence of osmotic shifts (131). A small amount of glycine betaine was released to the growth medium when bet+ E. coli cells were cultivated in a high-osmolality medium in the presence of choline. That release was dramatically enhanced by lesions inactivating the glycine betaine-specific transporters ProP and ProU (143). By analogy, a defect in the periplasmic trehalase, TreA, required to facilitate the uptake of exogenous trehalose as glucose, stimulated the excretion of trehalose during cultivation of E. coli in a high-osmolality medium (265). Schleyer et al. observed rapid reuptake of glutamate, but not trehalose, after both were released from E. coli by an osmotic downshift (249). Thus, osmotically stimulated cosolvent efflux appears to occur via a mechanism(s) distinct from that of cosolvent uptake. Furthermore, the cytoplasmic compatible-solute levels maintained during steady-state adaptation to high-osmolality media may be set by a balance between distinct active uptake and efflux mechanisms. The high rate and limited specificity of metabolite efflux have led some authors to propose that it is channel mediated (16).
Lively controversy surrounded the discovery of mechanosensitive channels in the cytoplasmic membranes of bacteria, including E. coli, B. subtilis, and Streptococcus faecalis (summarized by Berrier et al. [14]). Channels have been enumerated in patches excised from giant proteoliposomes reconstituted from an E. coli cytoplasmic membrane fraction and from giant round E. coli cells with disrupted walls. These studies revealed arrays of poorly selective stretch-activated conductances (0.1 to 2.3 nS in 0.1 M KCl) (14, 15). Whereas complex conductance patterns were observed during patch clamping of native membranes, reconstituted systems showed clusters of related conductances which differed from patch to patch. In both preparations, higher negative pressures were required to activate channels with higher conductance.
Two laboratories have added evidence that mechanosensitive channels contribute to osmoadaptation. Using whole-cell patch clamp recording, Cui et al. demonstrated that 0.35- and 1.1-nS channels (400 mM symmetric KCl) could be activated by applying positive pressure to the cell interior or by decreasing the extracellular osmolality (49). These channels remained in bacteria defective for K+ efflux systems KefB and KefC (not implicated in osmoregulation) and in those deficient for mscL. A kefA defect, previously shown to enhance the K+ sensitivity of E. coli, prolonged the channel open time and rendered the conductance more pressure sensitive without changing the magnitude of the channel conductance (48). The pressure sensitivity of these whole-cell preparations may have precluded the detection of channels that open only at higher pressures. MscL, present in the cytoplasmic membrane of E. coli, is the first mechanosensitive channel to be characterized at the molecular level (268). The osmosensory mechanism of MscL and evidence for its involvement in osmotic adaptation (20) are discussed below.
Osmoregulation, Turgor Pressure, Cell Growth, and Cell Division
The concept that turgor pressure is required for cell growth originated and is effectively described in the plant physiology literature (27, 200, 210). Cell growth requires plastic (irreversible) cell expansion, which can occur with or without the addition of new cell wall material. Breakage and reformation of chemical bonds can alter the shape of the cell wall (for example, making it larger in surface area and thinner) without changing its mass, but of course continuing cell growth and division require the addition of new material. In considering plant cell expansion, Lockhart (157, 158) noted two possibilities. (i) The cell wall area expands plastically by incorporating new material. Water uptake is a secondary response to the resulting decrease in turgor pressure and hence in the chemical potential of intracellular water. (ii) The cell wall area expands plastically, and without incorporating new wall material, by yielding to turgor pressure. Water uptake and the incorporation of new wall material are secondary responses to cell wall expansion.
The former theory predicts that all cell wall expansion depends quantitatively on the incorporation of new cell wall material. The latter predicts that cell wall expansion requires maintenance of turgor pressure above some threshold value, as articulated in the Lockhart equation:
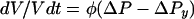 |
12 |
where V is cell volume, t is time, ϕ is a “yielding compliance,” describing the ability of the cell surface to expand plastically (irreversibly) in response to turgor pressure, and ΔPy is a critical turgor pressure or yield threshold. The equation states that the cell will grow, if turgor pressure exceeds ΔPy, at a rate determined by the yielding compliance (ϕ) and the degree to which ΔP exceeds ΔPy. The yielding compliance, defined in this way, incorporates plastic cell wall expansion with and without the addition of new cell wall material. During steady-state cell growth, the rate of increase of the cell volume (through uptake of water and other materials) must match that of wall yielding; no variation in turgor pressure is required.
Lockhart (157) concluded that the second of the above theories best described plant cell growth; the growth of walled cells has since been discussed in terms of the Lockhart equation (89, 200, 210). Nevertheless, a number of observations cast doubt on the generality of Lockhart’s conclusion for all walled cells and on its extension to encompass regulation of cell growth by turgor pressure (18, 27, 43, 89, 191, 273). For example, Harold and his colleagues have shown that the oomycete Saprolegnia ferax can extend and generate normal hyphal morphology without experiencing measurable turgor pressure (95, 96, 186).
Evaluation of the common assumption that bacteria osmoregulate to maintain turgor pressure and that they maintain turgor pressure to grow is central to any discussion of osmosensing. Do bacteria maintain turgor pressure at a set point or above a critical threshold? Is there a correlation between turgor pressure maintenance and cell growth or division? If these relationships exist, are they causally linked (42)? The answers will help determine whether turgor pressure sensors should or should not be sought by students of osmoregulation.
Difficulties associated with measurement of bacterial turgor pressure and cell volume, size, shape, and compartmentation are discussed in the Appendix. Although each of the applicable techniques has been used to estimate turgor pressure in bacteria, examinations of the turgor pressure of osmoregulating bacteria as a function of extracellular osmolality are rare. Studies of gas vesicle collapse in response to externally applied pressure suggested that the turgor pressures maintained by Microcystis sp. (230) and Ancylobacter aquaticus (215) cells decreased as a function of growth medium osmolality. Thus, turgor pressure might have been maintained above a minimum value, but it was not maintained at a unique “set point.” Whatmore and Reed (294) used plasmolysis titrations to examine the recovery of turgor pressure after osmotic upshifts were imposed on growing B. subtilis cells. Cell volumes were estimated with the Coulter counter, and all observed volume change was attributed to the cytoplasm. Turgor pressure varied from 0.753 osmolal before the shift to 0.325, 0.489, and 0.757 osmolal at intervals immediately, 2 h, and 5 h after an osmotic upshift was imposed with 0.4 M NaCl. These results suggested that B. subtilis cells growing under these conditions do osmoregulate to achieve a turgor pressure set point. However, they do not support the generalization that B. subtilis cells behave in this way under all conditions or that all bacteria osmoregulate to maintain turgor pressure. Effects of growth medium osmolality on the size, compartmentation, and hydration of metabolically active bacteria offer some additional insight.
Structural responses of metabolically active bacteria to osmotic shifts.
By applying the solute distribution technique to E. coli cells growing in the absence of osmoprotectants, Dinnbier et al. demonstrated transient decreases in cell and cytoplasmic water content (milliliters per gram of protein) in response to a 1-osmolal osmotic upshift imposed with NaCl (56). The cells recovered partially, reaching reduced steady-state water contents within approximately 10 min of the osmotic shift. Meury reported similar variations in cytoplasmic water content for E. coli (172). Analogous results were obtained when Skjerdal et al. applied inulin and sorbitol as probes to determine cellular and cytoplasmic hydration changes in Brevibacterium lactofermentum and C. glutamicum (the osmotic shift was imposed with 0.6 M NaCl), but no evidence was offered to validate this use of sorbitol (254). These observations suggest that if nutrients are provided, cells adapt within minutes to at least some effects of increased medium salinity on cell structure.
Meury et al. monitored culture turbidity, K+ uptake, DNA content, protein content, cell size, and cell concentration (Coulter counter) after E. coli cells (strains AB1157 [proA] and C600 [pro+]) were subjected to osmotic upshifts in minimal salts medium (171–174). They observed a transient increase in turbidity, rising within 2 to 3 min and reversing over approximately 30 min, for suspensions of growing E. coli cells subjected to large osmotic upshifts (up to 1.2 osmolar) imposed with NaCl or KCl in the absence of osmoprotectants (strain C600) or in the presence of osmoprotectant proline (strain AB1157). This transient was then followed by a sustained increase. The initial turbidity increase was probably related to cellular dehydration. Subsequent changes in turbidity were also related to changes in cell volume, shape, and concentration.
Following an osmotic upshift (1.2 osmolar, applied with NaCl), K+ uptake began immediately as most DNA and protein synthesis ceased. After 40 min, K+ had reached an elevated, steady-state level as both DNA and protein synthesis resumed. The observed K+ uptake was mediated by the Trk transporter (175). Cell division was arrested and the mean cell volume increased steadily following the osmotic shift. Phase-contrast microscopy revealed that cell length doubled at constant cell diameter, so that the observed volume change was not due to a change in cell shape at constant surface area (171). Cell division resumed, at a reduced rate, only after the mean cell volume had doubled. The size of Staphylococcus aureus cells cultivated in a defined medium (0.2 osmolal) also increased as the osmolality was elevated 10-fold with NaCl, NaNO3, or KCl but not with sucrose, sorbitol, or glycerol (285).
Increasing medium salinity was correlated with a decreased steady-state cell water content when Halomonas elongata and E. coli were cultivated in minimal salts media without osmoprotectants (for H. elongata, the media were supplemented with 0.05 to 3.4 M NaCl and data were expressed as microliters per milligram [dry weight] [288]; for E. coli, the media were supplemented with 0 to 0.65 M NaCl and the data were expressed as microliters per milligram of protein [38, 146, 233]). This was not true for L. monocytogenes, although high levels of errors may have obscured real reductions in that case (211).
For E. coli, increased medium salinity was correlated with reduced cytoplasmic water content (38, 101, 146, 233) and increased periplasmic water content (microliters per milligram of protein) (38, 233). The hydration of E. coli ML cells as a function of medium osmolality was also investigated by density gradient ultracentrifugation. Cellular buoyant density increased as medium salinity increased for E. coli (0.005 to 2.0 osmolal) (10, 11) and Salmonella (0.3 to 2.0 osmolal) (9), indicating decreasing hydration. However, neither cell age (as indicated by cell size at constant osmolality and nutrient supply) nor growth rate affected buoyant density for E. coli (136). At constant diameter and periplasmic thickness, an increase in E. coli cell volume (or length, as discussed above) would reduce the fraction of the cell volume occupied by the periplasm by less than 1%. Cayley et al. (38) reported an increase in the periplasmic fraction of cell water from 15% after growth in 0.28-osmolal growth medium to 37% after growth in 1.02-osmolal growth medium. Such results would indicate a sustained, two- to threefold increase in periplasmic thickness for cells with the dimensions reported by Meury (171).
Glycine betaine triggers further remodeling of bacterial cells under high-osmolality conditions. Meury et al. monitored the response to 1 mM glycine betaine of E. coli cells that had adapted to a high-osmolality medium (1.5 osmolar) (171–174). Following glycine betaine addition (and immediate uptake), the culture turbidity decreased and DNA synthesis accelerated as protein synthesis and cell division were attenuated. Glycine betaine attained a steady-state cytoplasmic level after 15 min. DNA synthesis accelerated further, and both protein synthesis and cell division resumed at elevated rates after 80 min. The mean cell volume fell steadily throughout this period, attaining a level near that in low-osmolality (0.3 osmolar) medium after 90 min. For E. coli cells cultivated in a minimal medium supplemented with 0.5 M NaCl (1.02 osmolar), the osmoprotectants glycine betaine and proline (1 mM) increased cytoplasmic hydration to approximately the levels observed for cells cultivated in the same medium supplemented with 0.2 M NaCl (0.47 osmolar) and 0.4 M NaCl (0.83 osmolar), respectively (39). The increase in size of S. aureus cells cultivated in a defined medium of high salinity was also reversed if the bacteria were provided with glycine betaine as osmoprotectant (285). These responses of S. aureus were related to salinity, not osmolality, and effects of salinity and osmoprotection on cell density were not reported.
These observations can be summarized as follows. (i) The rate of macromolecular synthesis correlates with cytoplasmic hydration and K+ content, at least for E. coli. After an osmotic upshift, attainment of an elevated steady-state K+ and/or compatible solute level precedes the resumption of DNA and (most) protein synthesis.
(ii) Steady-state cellular and cytoplasmic hydration vary inversely, whereas periplasmic (or cell wall) thickness or hydration and cell volume vary directly with growth medium osmolality, at least for some bacteria.
(iii) The growth rate of E. coli is inversely correlated with growth medium osmolality (38). Since chromosome copy number is inversely proportional to growth rate for E. coli, both reduced copy number and increased cell volume would counter the effect of sustained cytoplasmic dehydration on DNA concentration in the cytoplasm of cells grown in high-osmolality media.
These interpretations are consistent with the view that K+ and compatible-solute accumulation by osmoregulating bacteria are required to modulate nucleoid structure and facilitate macromolecular synthesis (228, 229). They also indicate striking effects of osmoadaptation on bacterial morphology and ultrastructure that warrant further investigation. Substantial changes in cell surface stress and strain are clearly associated with both transient and steady-state osmoadaptation, but these changes are not easy to define. The degree to which osmoregulating bacteria regain turgor pressure in the steady state is unclear.
CYTOPLASMIC MEMBRANE-BASED OSMOSENSORS
Cytoplasmic membrane-based proteins are implicated in the earliest bacterial responses to osmotic up- and downshifts (Table 5). Most mediate osmoregulatory transmembrane solute flux; two are sensor constituents of transcriptional regulatory systems. Two proteins, MscL and ProP, have been shown to function alone, in proteoliposomes, as both osmosensors and respondents to osmotic shifts. The stage is therefore set for the further elucidation of membrane-based osmosensory mechanisms.
TABLE 5.
Putative membrane-based osmosensorsa
| Osmoregulatory role | Function | Name | Organism | Purified/osmosensing shown in vitro |
|---|---|---|---|---|
| Unclear | Sensor kinase | EnvZ | E. coli | Yes/No |
| K+ accumulation | Transporter | TrkEFG(H) | E. coli | No/No |
| Sensor kinase | KdpD | E. colib | Yes/No | |
| P-type ATPase | KdpFABC | E. colib | Yes/No | |
| Compatible-solute accumulation | Ion-linked transporter | ProP | E. coli | Yes/Yes |
| S. typhimurium | No/No | |||
| BetT | E. coli | No/No | ||
| BetP | C. glutamicum | No/No | ||
| OpuE | B. subtilis | No/No | ||
| ABC transporter | ProU | E. coli | No/No | |
| OpuA | B. subtilis | No/No | ||
| OpuC | B. subtilis | No/No | ||
| Cosolvent efflux | Channel | MscL | E. coli | Yes/Yes |
The listed proteins have been shown to function as osmosensors (EnvZ, KdpD, and ProP) and/or as osmoregulators (all but EnvZ and KdpD) in vivo and/or in vitro, and their amino acid sequences are known. Related systems in other organisms have also been characterized (see text).
Cytoplasmic membrane localization of an osmosensor focuses the search for the stimulus that it detects. The array of possibilities is still large, however, particularly if sensory domains associated with the periplasmic and cytoplasmic faces of the membrane are included (Table 2). For systems that are functional, alone, in proteoliposomes, dependence on other cellular constituents (or their activities) can be ruled out. It will now be important to answer the following, overlapping questions. (i) Does each sensor protein detect changes in transmembrane solvent gradients or in absolute solvent osmolality? (ii) Does the sensor detect changes in solvent osmolality directly, or does it detect solvent-induced changes in the phospholipid membrane? Does the membrane act as an antenna? (iii) Does the sensor protein detect membrane changes that are mechanical and/or chemical in origin?
Since membrane enzymes, in general, are sensitive to membrane perturbation, the experiments that resolve these possibilities must be designed with particular care. For example, some membrane-based osmoregulatory systems are perturbed by amphipaths (e.g., procaine and tetracaine [see the examples below]). However, such compounds are pleiotropic in their impact on membrane structure and protein activity (see the reviews cited by Cantor [35]). Interpretation of amphipath effects in mechanistic terms will therefore require rigorous and painstaking dissection of coincident outcomes.
K+ Transporters of E. coli
The Trk and Kdp transporters can both be activated to mediate osmoregulatory K+ uptake by E. coli (2, 6, 7). Trk, which has a high KM for K+ (estimates of 0.3 to 3 mM), mediates K+ uptake by E. coli cells cultivated in media with K+ concentrations above approximately 1 mM. It is encoded in constitutively expressed genes that are dispersed on the chromosome, and it is unlike other transporters in structure. The KdpFABC transporter, a P-type ATPase with a low KM for K+ (2 μM), is responsible for osmoregulatory K+ uptake only by severely K+-depleted bacteria (2). Both Trk and Kdp (when present) are activated within seconds of an osmotic upshift.
K+ fluxes are primary and essential responses of E. coli cells to osmotic shifts. Since cytoplasmic K+ levels are directly correlated with extracellular osmolality (see the summary by Bakker [6]), cytoplasmic rehydration is usually assumed to be the objective of K+ accumulation. As outlined above, both osmotic upshifts and ensuing adjustments in the concentration of cytoplasmic K+ (and associated ions) influence nucleoid structure, cellular energetics, cytoplasmic ionic strength and ion composition, as well as cytoplasmic osmolality. K+ glutamate accumulation may specifically counter the effects of increased macromolecular crowding on nucleoid organization (229), and K+ is also implicated in energy metabolism. Some K+ accumulation occurs even in the presence of osmoprotectants, such as glycine betaine, which are more effective than K+ in restoring cellular hydration after an osmotic upshift. All these observations suggest that the adjustments in cytoplasmic ion composition (particularly the K+ level) which are triggered by osmotic shifts meet multiple objectives. If so, the enzymes that mediate these adjustments are likely to respond to multiple signals. Regardless of the validity of that prediction, the central role of K+ in cell physiology has rendered elucidation of the osmosensory mechanisms associated with K+ uptake particularly challenging.
Transporter Trk.
The homologous proteins TrkG (485 amino acids, encoded in the prophage rac region of the chromosome) and TrkH (483 amino acids) are believed to serve as alternative integral membrane subunits responsible for K+ translocation in E. coli. Although these proteins are predicted to span the membrane 12 times, they are not related in sequence to other transporters that share this topology. Both Δμ̃H+ and ATP are required for K+ uptake via the Trk system, but ATP is believed to regulate rather than to drive K+ uptake. Subunit TrkA (458 amino acids, corresponding to the SapG protein of S. typhimurium [208]) exists as both cytoplasmic and membrane-associated forms (26). Its association with membrane-integral subunits TrkG and TrkH is required for K+ uptake. Each half of TrkA includes a Rossman fold as well as having sequence similarity to the NADH binding domain of an NAD+-dependent dehydrogenase. TrkA has been demonstrated to bind NAD+ and NADH, but ATP binding could not be detected (250). These observations and the involvement of TrkA and SapG in both K+ uptake and resistance to antimicrobial peptides (208) imply that they play a regulatory role.
Mutations in the trkE locus eliminate and impair K+ transport via TrkH and TrkG, respectively. The trkE locus of E. coli is now known to correspond to the sapABCDF operon of S. typhimurium. Sequence similarities and the resistance of sap mutants to protamine, a cationic antimicrobial peptide, suggest that sapABCDF encodes a peptide transporter of the ABC transporter class (208). Various lines of evidence indicate that protamine disrupts energy transduction, that K+ uptake is required to counter those effects, and that K+ uptake and peptide resistance are distinct phenomena (5, 262).
The complexities summarized above have, until now, precluded further analysis of osmosensory mechanisms associated with the Trk systems. Since they are believed to be the earliest sensors of and the first respondents to osmotic upshifts in E. coli, such analysis should remain a particularly high priority for students of osmosensing.
Transporter Kdp.
Although it is best characterized in E. coli, the Kdp system is ubiquitous (reference 278 and references therein). The transport function of the Kdp-ATPase from E. coli is becoming well defined (2). Subunit KdpA is believed to span the cytoplasmic membrane 10 times and to translocate K+ in a process involving two sequentially occupied K+ binding sites (30). The catalytic subunit KdpB, also membrane associated, accepts phosphate from ATP during the K+ transport cycle (224, 253). The functions of essential subunit KdpC and hydrophobic peptide KdpF are unknown. K+ transport via the Kdp-ATPase has been demonstrated in cytoplasmic membrane vesicles provided with an ATP-regenerating system (130) and was shown to be electrogenic by an electrophysiological analysis involving black lipid membrane-associated proteoliposomes reconstituted with the purified enzyme (70). The mechanism by which osmotic upshifts activate the Kdp-ATPase is unknown, however.
Sensor kinase KdpD.
The kdpFABCDE operon encodes the Kdp-ATPase (KdpFABC) and two-component regulatory system KdpDE. An internal promoter mediates low-level kdpDE transcription, but readthrough of kdpDE from the upstream kdp promoter has also been observed (218, 286). Altendorf and Epstein summarized the evidence that KdpD acts via the phosphorelay mechanism characteristic of two-component systems (2). Much effort has been expended to elucidate the basis for transcriptional regulation of kdp operon expression from the upstream promoter. Laimins et al. (141) first used expression of β-galactosidase by bacteria containing a kdpA::lacZ fusion to demonstrate that transcription of kdpFABC responded to both low K+ concentrations and increased osmolarity. It is now well established that the K+ transport phenotype of the bacteria determines the threshold K+ concentration below which kdp transcription is observed. That threshold is approximately 5 mM for bacteria that cannot take up K+ via the KdpFABC transporter (Trk+ Kdp−) and 50 mM for bacteria unable to take up K+ via either transporter (Trk− Kdp−) (141). Frequently, kdp transcription is measured by using a kdp::lacZ fusion in bacteria that are phenotypically Trk− and/or Kdp−.
Transcription of a kdp::lacZ fusion was induced transiently (between approximately 10 and 30 min after the shift) when diverse salts, sugars, and hexitols were used to elevate medium osmolarity. Glycerol was not effective, however. The growth medium salinity (due to NaCl plus KCl) determined the maximum extracellular K+ concentration at which kdp transcription occurred. Since the cytoplasmic K+ concentration is a direct function of extracellular osmolarity (see the data summarized by Bakker [6]), Laimins et al. (141) suggested that neither the extracellular nor the intracellular K+ concentration per se controls kdp transcription. Rather, K+ was believed to influence kdp transcription indirectly, via its effect on turgor pressure.
The following observations challenge the view that turgor pressure alone controls kdp transcription. First, kdp transcription responds differently to osmotic upshifts of similar magnitude imposed with salts and nonionic cosolvents. The latter induce only transient kdp transcription in both Kdp− Trk+ and Kdp− Trk− bacteria (4, 83, 141, 267, 269). This characteristic distinguishes KdpD from other osmosensory systems. It implies that an aspect of the response to an osmotic upshift imposed with nonionic cosolvents quickly reverses or overrides the initial stimulus due to elevated extracellular osmolality whereas that stimulus is maintained in the steady state when osmotic stress is applied by adding ionic cosolvents. Studies of osmoregulation are usually, appropriately, focused on cellular responses that are neither electrolyte nor non-electrolyte specific. The different responses of the kdp system to ionic and nonionic cosolvents suggest that elucidation of the sensory mechanism for KdpD requires the opposite focus.
The fact that organic osmoprotectant uptake attenuates the transcription of the osmoresponsive loci proP and proU, but not that of the kdp locus, also contradicts a model in which turgor pressure alone controls kdp transcription. Like K+ uptake, osmoprotectant uptake is usually assumed to restore turgor pressure. Finally, Sugiura et al. (266) isolated kdpD mutations that caused K+-insensitive kdp transcription. Diverse ionic and nonionic cosolvents induced steady-state kdp transcription by the mutant bacteria. This result suggested that K+ and osmotic changes exert their opposing effects on kdp transcription, through KdpD, via distinct mechanisms.
The role of turgor pressure in osmosensing by kdp has been debated at length. Nevertheless, bacterial turgor pressure is difficult to measure and hence ill defined. Perhaps most open to question is the assumption that osmoregulatory K+ uptake, with or without compatible-solute accumulation, restores turgor pressure (to levels at or below that prevailing before the stress). Osmosensors are unlikely to detect turgor pressure (intracellular hydrostatic pressure) directly, not least because bacterial turgor pressures are much lower than pressure changes that are known to influence protein conformation and enzyme activity in vitro (239). Indeed, structural correlates of turgor pressure changes that could be sensed by KdpD have been proposed (47). Purification, reconstitution, and mutagenesis are now providing experimental systems that will support the evaluation of these alternatives (Table 6).
TABLE 6.
KdpD mutant phenotypes
| Mutationa | KdpD domainc | Induction of steady-state kdp expression byc:
|
Catalytic activityc
|
||||
|---|---|---|---|---|---|---|---|
| Low K+ | High osmolarity
|
KdpD kinase | KdpD:KdpE phosphotransferase | KdpE phosphatase | |||
| NaCl | Sucrose | ||||||
| Wild type | + | + | —b | + | + | ATP dep | |
| Δ(12–228) | N terminal | Weak | − | − | + | + | ATP indep |
| Δ(12–395) | N terminal | Weak | − | − | + | + | ATP indep |
| Δ(128–391) | N terminal | + | + | − | + | + | ATP dep |
| C32A | N terminal | + | + | − | + | + | + |
| G37A, K38A, T39C | N terminal | Weak | − | − | NT | NT | ATP dep |
| C256A | N terminal | + | + | − | + | + | + |
| Δ(392–530) | N terminal/TM1 | − | − | NT | − | NT | NT |
| C402A | TM1 | + | + | − | + | + | + |
| C409A | TM1 | Constit | Constit | Constit | + | + | + |
| C409V, C409Y | TM1 | Weak | − | − | + | + | + |
| C409S | TM1 | + | + | − | + | + | + |
| D460V | TM3 | K+ indep | + | + | NT | NT | NT |
| G492E | TM4 | K+ indep | + | + | NT | NT | NT |
| E509G | C terminal | K+ indep | + | + | NT | NT | NT |
| H673Q | C terminal | NT | NT | NT | − | NT | NT |
| C852A or C852S | C terminal | + | + | − | Low | + | + |
| C874A or C874S | C terminal | + | + | − | Low | + | + |
See discussion in text. Puppe et al. (225) observed stimulation of kdp transcription by sucrose (0.2 to 0.4 M) in bacteria overexpressing the wild-type KdpD protein in a Trk− Kdp+ background.
+, equivalent to wild type; −, no activity; indep, independent; dep, dependent; Constit, constitutive; NT, not tested; TM, transmembrane.
The KdpD protein (894 amino acids) includes a central domain with four cytoplasmic membrane-spanning segments and little periplasmic exposure (residues 401 to 498) flanked by extensive cytoplasmic N- and C-terminal domains (310). A KdpD derivative lacking the membrane domain (Kdp*, lacking residues 392 through 530) is also associated with the cytoplasmic membrane (225). KdpE, a 225-amino-acid cytoplasmic protein, is a member of the OmpR family of response regulators.
Autophosphorylation of KdpD, KdpD:KdpE phosphotransfer, and dephosphorylation of KdpE phosphate have been observed in vitro by using purified KdpE and membrane vesicle-associated or purified, proteoliposomal KdpD (112, 195–197, 286). The balance among these activities, set in response to environmental stimuli, is expected to determine the proportion of phosphorylated KdpE and hence the transcription of kdp. Although detergent-solubilized KdpD could bind ATP (112), autophosphorylation was not observed with either detergent-solubilized KdpD (112, 195, 196) or deletion mutant Kdp* (Δ392–530) (225), in contrast to earlier results (195, 196). Deletion analysis indicated that at least the first 128 residues of the N-terminal domain and the membrane domain of KdpD are implicated in its sensory capability.
The KdpD-KdpE phosphorelay detected in vitro requires transfer of phosphate from ATP to a KdpD protein domain that is cytoplasmic in vivo and then ultimately to KdpE. The expectation that the cytoplasmic domains of KdpD must be exposed on the external surface of both membrane vesicles and proteoliposomes (to allow access to exogenous ATP and KdpE) was confirmed by protease susceptibility (112). Current in vitro analyses of the phosphorelay therefore are performed with systems that are topologically inverted in comparison with the intact cell. Some efforts have been made to determine the dependence of the KdpD-KdpE phosphorelay on K+ and cosolvents. These experiments, described below, have involved (often simultaneous) variation of luminal and exogenous K+ concentration, transmembrane K+ and Na+ gradients, intra- and extraliposomal ionic strength, and intra- and extraliposomal osmolality.
Using membrane vesicles, Voelkner et al. (286) found KdpD phosphorylation to be absolutely dependent upon the presence of both divalent (Mg2+ or Ca2+) and monovalent (Na+ or K+) cations. At 0.05 M salt, the phosphorylation level attained with KCl exceeded that attained with NaCl. In contrast, 0.5 M NaCl elicited a higher KdpD phosphorylation level than did 0.5 M KCl. Sucrose did not substitute for NaCl or KCl in this reaction. The levels of KdpE phosphate attained upon addition of a cytoplasmic extract containing KdpE corresponded to the available KdpD phosphate. Solvent effects on the phosphotransfer and phosphatase reactions were not differentiated from those on KdpD autophosphorylation.
Nakashima et al. (195, 196) measured KdpD autophosphorylation and KdpD-KdpE phosphotransfer in the presence of added KCl or NaCl (0 to 400 mM) with proteoliposomes prepared in the presence of 0, 100, or 200 mM KCl and with partially purified KdpD (25 mM Tris HCl as the base buffer). Autophosphorylation and phosphotransfer appeared to be independent of the luminal (the equivalent of periplasmic) K+ concentration (and the resulting ionic and osmotic gradients) in this range (exogenous K+ at 100 mM). Phosphorylation of KdpE was a function of exogenous salt concentration (NaCl or KCl; no salt was added during reconstitution), increasing steadily in the range from 50 to 400 mM. Only net KdpE phosphorylation, the outcome of the combined in vitro kinase, transferase, and phosphatase reactions, was measured during these experiments. Salts also stimulate the phosphorylation of the response regulator OmpR by the sensor kinase EnvZ, a system that also responds to osmolality and other stimuli.
Procaine, an amphipathic local anaesthetic, enhanced kdp transcription in E. coli, but this effect was observed only in strains expressing K+-insensitive KdpD variants with altered membrane domains (266). Procaine and chlorpromazine were also observed to stimulate the phosphorylation of the response regulator OmpR in a process that required the membrane domain of the sensor kinase EnvZ. Like the effects of salts, the effect of procaine on EnvZ was attributed to inhibition of phospho-OmpR phosphatase (277).
Much remains to be learned about the signal(s) sensed by KdpD and its sensory mechanism(s). Unlike the ProP transporter (see below), KdpD does not respond clearly to medium osmolality once it is purified and reconstituted in proteoliposomes. Alternative interpretations of the above observations include the following. (i) The osmotic response of KdpD has not been reproduced in vitro because KdpD senses turgor pressure, which has not yet been imposed in vitro. (ii) For wild-type KdpD, the response to osmotic shifts is often obscured by the operation of other regulatory mechanisms related to K+ levels, energy metabolism, or nucleoid structure. The isolation of KdpD derivatives insensitive to those inputs will allow further elucidation of the osmotic response (Table 6). (iii) KdpD senses changes in cytoplasmic ionic strength as a manifestation of extracellular osmotic shifts. The salt dependence observed in vitro represents at least one of the regulatory mechanisms operative in vivo. (iv) KdpD, in vitro, does not show the osmotic response characteristic of whole bacteria, because protein or other macromolecular constituents of the periplasm or cytoplasm are absent from the in vitro systems. Such constituents might act on KdpD specifically or collectively (through macromolecular crowding). (v) Finally, KdpD does not respond to osmotic changes in vitro because the topology of the in vitro systems is opposite to that of the intact cell. As a result, the in vitro systems fail to reproduce mechanically induced changes in membrane strain that are sensed in vivo.
Osmoprotectant Transporters
Some bacterial osmoprotectant transporters, including both ABC transporters and ion symporters, are activated by an increase in salinity in the absence of protein synthesis. In some cases the osmotic origin of this phenomenon has been confirmed by demonstrating analogous responses to structurally dissimilar cosolvents, both ionic and nonionic (Table 4). The purified E. coli transporter ProP has now been shown to function as both an osmosensor and an osmoregulator (226). The rationale for the accumulation of organic cosolvents (osmoprotectants) in response to osmotic upshifts appears simple: these cosolvents are preferentially excluded from macromolecular surfaces. Recent research suggests that the related sensory mechanisms are also relatively simple. Analysis of the osmotic activation mechanism is most advanced for ion symporters from Listeria monocytogenes, Corynebacterium glutamicum (BetP), and E. coli (ProP).
Glycine betaine uptake by L. monocytogenes.
Glycine betaine uptake by L. monocytogenes cells was activated by both osmotic upshifts (124, 283) and reduced temperature (optimum, 7°C) (124). Although the dependence of uptake activity on the magnitude of the osmotic shift and on cosolvent chemistry was not defined, it increased within 2 min after an osmotic upshift and was maximal within 4 min (124). Osmotically activated glycine betaine uptake was also observed with cytoplasmic membrane vesicles. Glycine betaine uptake was absolutely Na+ dependent, and the initial rate was optimal when an osmotic upshift was imposed with 0.4 M NaCl (79). Both the fold stimulation of the uptake rate and the osmotic upshift yielding that rate were cosolvent dependent. KCl and sucrose stimulated the initial rate of glycine betaine uptake three- and sixfold when optimal osmotic upshifts of 0.64 and 0.28 osmolal, respectively, were imposed on vesicles in a medium containing 50 mM Na+ to meet the requirement for Na+ as a coupling ion. (In the presence of sucrose, the KM for Na+ was 75 mM.) Although the kinetics of osmotic activation were not defined, transport proceeded after a lag of approximately 4 min following exposure of the vesicles to a respiratory electron donor and an osmotic upshift. More recent data indicate that the cold-activated glycine betaine uptake activity observed in whole cells was attributable to a second glycine betaine transport system that is also osmotically activated (124a, 256).
Transporter BetP of C. glutamicum.
An absolute dependence on NaCl (optimum concentration, 0.625 M) was observed for glycine betaine uptake by cells of C. glutamicum cultivated in a complex, high-osmolality medium (brain heart infusion supplemented with 0.5 M NaCl) and washed with a cold, low-osmolality buffer (69). Most of this uptake activity (approximately 90%) was attributable to a Na+/glycine betaine symporter (KM for Na+, 4 mM) encoded in the betP locus (213). Upon expression in an E. coli strain lacking endogenous glycine betaine transporters, BetP activity was optimal when an osmotic upshift was imposed with 0.2 M NaCl. However, these bacteria were cultivated in minimal medium at low osmolality and not subjected to a cold, low-osmolality wash prior to the uptake assay. BetP was activated without a measurable lag when it was expressed in either C. glutamicum or E. coli (213).
BetP (595 amino acids), predicted to span the cytoplasmic membrane of C. glutamicum 12 times, is similar in sequence to the choline transporter BetT and the putative carnitine transporter CaiT of E. coli and to the glycine betaine transporter OpuD of B. subtilis (Table 4). Like BetP, BetT and OpuD catalyze osmoprotectant uptake and are activated by an osmotic upshift. CaiT is implicated in anaerobic carnitine catabolism and has no known relationship to osmoadaptation. (Osmoprotection of E. coli by l-carnitine [212] is eliminated by a proP defect [160].) The hydrophilic N-terminal sequence of BetP is predicted to be anionic and longer than those of BetT and OpuD; the C-terminal sequence is predicted to be cationic.
Peter et al. (214) examined BetP activity by expressing the transporter from a plasmid in a betP derivative of C. glutamicum. Uptake measurements were performed in the presence of ectoine to suppress a residual glycine betaine uptake activity that, unlike BetP, mediates ectoine uptake. The measured Vmax was approximately one-third of that attributable to BetP in wild-type bacteria. The amphipathic local anesthetic tetracaine stimulated BetP activity in a manner dependent upon both medium salinity (NaCl added to attain osmolalities of 400, 600, and 1000) and tetracaine concentration, with the optimal tetracaine concentration being in the range 0.6 to 0.8 mM.
Peter et al. (214) also examined the effects of N- and C-terminal deletions on BetP activity. Since the effects of these deletions on protein expression were not assessed, the impact of the mutations on absolute transporter activity could not be analyzed. For variant transporters with sufficiently high activity to support reliable determinations (all but two), the KM for glycine betaine did not differ from that of the wild-type. Deletion of 20, 32, 49, or 60 N-terminal residues yielded bacteria that retained glycine betaine uptake activity but required a larger osmotic upshift than did the wild type for maximal activation (1.4 M and 0.6 M NaCl, respectively). Deletion of 12, 23, or 32 C-terminal residues yielded glycine betaine uptake activity that was insensitive to osmotic shifts. Transport activity was stimulated by NaCl (up to 0.25 M), reflecting the requirement for Na+ as a coupling ion, but it was not stimulated by sorbitol. Deletion of 23 or 32 C-terminal residues also reduced the affinity of the transporter for Na+. Deletion of 52 C-terminal residues inactivated the transporter. Thus, both N and C termini are implicated in the sensory mechanism of BetP.
Recent evidence suggests that some transporters are subject to trans inhibition, i.e., inhibition by substrates previously accumulated within the cytoplasm (220, 223, 259, 283). trans inhibition of transport may act physiologically to limit compatible-solute accumulation. Since an osmotic upshift can override this inhibition for some systems, Poolman and Glaasker (220) suggest that modulation of the transporter-substrate interaction at the cytoplasmic membrane surface may also constitute an osmoregulatory mechanism. trans inhibition of glycine betaine uptake (by glycine betaine) was observed in C. glutamicum cells expressing wild-type BetP but not in those expressing the variant lacking 23 C-terminal residues (214). Thus, both the cytoplasmic glycine betaine level and extracellular osmolality may regulate BetP.
Transporter ProP of E. coli.
The ProP transporter of E. coli catalyzes H+/osmoprotectant symport (160). ProP was activated with a half time of approximately 1 min when E. coli cells or cytoplasmic membrane vesicles were subjected to osmotic upshifts imposed with NaCl or sucrose but not with glycerol (84, 179). A much larger osmotic upshift was required to elicit maximal activity in vesicles (0.8 osmolal) than in cells (0.2 osmolal), however (162). Exogenous K+ is required for full expression of ProP activity in intact cells of both S. typhimurium (131) and E. coli (162).
Overexpression of ProP and the addition of six C-terminal histidine residues (a His tag) facilitated the purification of the transporter and its reconstitution in proteoliposomes (226). Neither the KM of the transporter for proline nor its osmotic activation (in cells) was altered by this addition. ProP activity in proteoliposomes was absolutely dependent upon the imposition of a membrane potential (lumen negative) and an osmotic upshift. NaCl or sucrose (but not glycerol) was effective as a cosolvent. A further stimulation of uptake was observed when a pH gradient (lumen alkaline) was imposed. In contrast, an osmotic upshift imposed with sucrose inhibited the activity of the Na+/proline symporter PutP, which was purified and reconstituted under the same conditions. (PutP is implicated in proline catabolism by E. coli but not in the osmotic stress response.) Thus, proteoliposomal ProP alone acts as both an osmosensor and an osmoregulator. Since the imposition of a membrane potential, in this system, required luminal K+, it has not yet proven possible to further evaluate the role of K+ in ProP activity in vitro.
ProP (500 amino acids) is a member of the major facilitator superfamily and is most closely related in sequence to OusA of Erwinia chrysanthemi (498 amino acids, 93.6% similarity to ProP). Plasmid-borne ousA restored osmoprotection by glycine betaine, proline, and ectoine to an E. coli strain deficient in the ProP and ProU transporters. Although the corresponding uptake activities were observed, they were not stimulated when bacteria cultivated in low-osmolality medium were exposed to an osmotic upshift (0.5 M NaCl). This failure to observe OusA activation may have resulted from expression in a heterologous host or from the magnitude of the imposed osmotic shift. For ProP in E. coli cells, activity is optimal when an osmotic upshift is imposed with 0.2 M NaCl and fully inhibited at 0.5 M NaCl.
Citrate and α-ketoglutarate transporters from E. coli and other organisms, none of which are implicated in the osmotic stress response, show significant but lower similarity to ProP and OusA (approximately 30%). Given the absence of structural detail for this class of proteins, no structural basis for osmosensing by ProP is obvious from comparisons of their (putative) membrane integral domains. However, the central hydrophilic loops of ProP and OusA (between putative transmembrane domains 6 and 7) are predicted to be longer than those of the ProP homologues Cit, Ciu1, and KgtP (formerly WitA) (50, 82).
PhoA fusion analysis has placed the hydrophilic loop between transmembrane domains 11 and 12 of the α-ketoglutarate transporter KgtP in the periplasm of E. coli (252). Immunochemical analysis of membrane vesicles by using antibodies raised against a C-terminal peptide suggests that the C terminus of ProP is cytoplasmic, as is observed for other members of this transporter superfamily (162). Comparison of the topologies of ProP in cells and proteoliposomes is an immediate priority. ProP and OusA are distinguished from the related anion transporters by possessing a carboxyl-terminal extension comprising six to seven of the heptad repeats characteristic of α-helical coiled-coil-forming proteins (50, 82). Culham et al. (50) therefore proposed that the C-terminal extension of ProP might be involved in its osmotic activation. This hypothesis is attractive since solvent composition might be expected to influence the stability and/or conformation of an α-helical coiled-coil and/or its interaction with the membrane surface (see “Solvent-membrane interactions” above). No general coiled-coil-based mechanism can exist, however, since other osmotically activated transporters (e.g., BetP) are devoid of this structural motif.
A peptide replica of the ProP C terminus can form a homodimeric coiled-coil in vitro (278a). A ProP variant lacking 26 C-terminal amino acids is expressed at wild-type levels but is inactive, regardless of the growth medium or assay medium osmolality (50a). Thus, the C terminus of ProP has the potential to mediate homodimer formation and is critical for ProP activity. Further experimentation is required to determine whether coiled-coil stability is correlated with osmotic activation of ProP and whether the C terminus of ProP or OusA participates in homo- or heteromeric coiled-coil formation in vivo.
A Tn5 insertion in the distal locus proQ impairs ProP activity but does not reduce ProP expression (139, 181). The proQ defect eliminates ProP activity in bacteria cultivated in low-osmolality media, reduces by 5-fold the magnitude of the ProP activity attained after an osmotic upshift, and decreases the rate of ProP activation (the activation half time is increased 2.6-fold). ProQ (232 amino acids) is a cytoplasmic, hydrophilic protein (139). Since ProP alone can act as an osmosensor, ProQ must function to fine-tune that response and/or link ProP activation to other cellular processes. The magnitudes of the osmotic shifts eliciting maximal ProP activity in cells and membrane vesicles differ. While ProP is active without an osmotic shift in cells cultivated in low-osmolality media, proteoliposomes and membrane vesicles are devoid of ProP activity unless an osmotic upshift is imposed. These observations indicate that ProP can exist in fully inactive and active states and that the status of the signal sensed by ProP differs in intact bacteria and isolated membranes. This may reflect the absence, in the vesicle systems, of structural linkages present in whole bacteria.
Complex signal transduction cascades have now been shown to mediate the responses of many cells, both prokaryotic and eukaryotic, to environmental stimuli. On that basis, it may seem surprising that a molecule with the apparent structural simplicity of ProP can both sense and respond to osmotic shifts. Halophiles possess macromolecules that are designed to function in high-salinity environments. Perhaps ProP (like some E. coli promoters) represents a class of E. coli molecules that are designed to function when the cells face environments with low water activity.
Mechanosensitive Channel MscL of E. coli
Although mechanosensitive channels have been implicated in the cosolvent efflux that occurs when bacteria are subjected to osmotic downshifts, the evidence linking specific channels to specific efflux processes remains limited (see “Cosolvent efflux” above). Recent evidence suggests that MscL, a mechanosensitive channel with low ion selectivity and high conductance, opens in response to mechanically imposed membrane stress or osmotic shifts and mediates K+ efflux from E. coli cells. Only references of particular significance for osmosensing are cited below since MscL has been the subject of recent reviews (75, 268).
Although mscL null mutants are without phenotype under conditions examined to date, Blount et al. (20) showed K+ leakiness and slow growth in bacteria harbouring mscL mutations K31D and K31E. Both phenotypes were reversed during cultivation of the bacteria in a high-osmolality medium. In addition, an osmotic downshift elicited greater K+ loss from bacteria expressing the variant channels than from those expressing the wild-type channels, and patch clamp analysis revealed that the variant channels shared increased sensitivity to mechanical stress. This evidence implicates MscL in osmoadaptive K+ efflux but does not indicate whether it also mediates the efflux of other ions or nonionic cosolvents. Multiple mechanosensitive channel conductances have been detected in E. coli, but the E. coli chromosome encodes no MscL paralogues. (MscL homologues have been predicted in other organisms, however.)
MscL appears to detect osmolality changes indirectly as changes in mechanically imposed membrane stress. The presence of MscL in the cytoplasmic membrane of E. coli and in proteoliposomes reconstituted with the purified protein is correlated with the presence of a mechanosensitive conductance detected by patch clamping. The channel is not ion selective, and its conductance is high (2.5 nS in the presence of 0.2 M KCl and 0.04 M MgCl2). Voltage-dependent subconducting states are observed. The relationship between the channel-open probability and the applied pressure follows the Boltzmann distribution.
If it is determined by lateral membrane stress, the channel-open probability will be more sensitive to an imposed pressure for patches with large radii of curvature (see equation 11) (86). According to a simple two-state (open/closed) channel model, the following relationship would be expected:
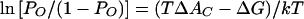 |
13 |
where PO is the channel-open probability, ΔAC is the stress-induced increase in channel area in the plane of the membrane (the channel strain), TΔAC is the work done to effect that expansion, ΔG is the free energy of the channel transition from the closed to the open state in the unperturbed membrane, and k is the Boltzmann constant. This relationship is now being applied to data correlating channel-open probability with patch curvature and applied pressure to yield estimates for the parameters ΔAC and ΔG. This effort is required to further test the assertion that mechanically imposed lateral stress gates MscL and to define stress-strain relationships for the channel in comparison to its host membrane.
The pressure required to activate channel conductance was reduced when various amphipaths (either cationic [e.g., procaine and chlorpromazine] or anionic [e.g., trinitrophenol]) were added to the solution bathing the cytoplasmic surface of excised E. coli membrane patches (163). The effects of chlorpromazine and trinitrophenol on patch conductance were compensatory. The authors assumed that the detected channels were located in the outer membrane and that the amphipaths partitioned into different membrane leaflets because of membrane lipid asymmetry. On that basis, they suggested that amphipaths potentiate channel opening by selectively expanding one membrane monolayer and imposing membrane bilayer curvature.
It is now clear that mechanosensitive channels are located in the cytoplasmic membrane of E. coli, which has unknown lipid asymmetry, and that channel MscL, at least, can function in the absence of bulk-phase, transverse lipid asymmetry. Recent evidence suggests that cosolvents and amphiphiles may exacerbate or compensate for the lateral pressure that creates intrinsic strain in membrane bilayers (61, 153, 161) (see “Solvent effects on membrane structure” above). The degree to which they exert these effects may depend on their molecular size, shape, and charge and hydrogen-bonding capacity as well as on membrane properties. The response of proteoliposomal MscL to amphipaths has not yet been reported. Their use may offer a means of testing whether both mechanically and chemically imposed membrane perturbation can activate this channel.
MscL is a homohexamer composed of a 136-amino-acid, largely α-helical protein that crosses the cytoplasmic membrane twice, with a small periplasmc loop (approximately residues 50 to 69) and cytoplasmic N and C termini (approximately residues 1 to 15 and 111 to 136, respectively). Although the MscL carboxyl-terminal sequence (residues 104 to 136) is conserved among MscL homologues, its deletion failed to alter the mechanosensitivity, unit conductance, or kinetics of the channel. These characteristics were very sensitive to N-terminal modifications, however. Point mutations that alter channel properties are now being described. Changes in the channel properties of variants with mutations at Q56 were correlated with changes in patch-clamping bath composition in a manner that substantiated its periplasmic location. Mutations K31D and K31E, predicted to fall within the N-terminal transmembrane domain of MscL, yielded channels with enhanced sensitivity to mechanical stress (20).
The following questions regarding osmosensing and osmoregulation by MscL can now be addressed: (i) What role does MscL play in the osmotic stress response of E. coli? Does it mediate fluxes of both ionic and nonionic cosolvents? If so, is it selective? (ii) How do voltage, membrane stress, and solvent composition influence the open probability of MscL? Can it respond to both mechanically and cosolvent-induced membrane stresses? (iii) Can MscL substates (defined electrophysiologically) be correlated with structural perturbations of MscL to map the process of channel gating? (iv) What is the nature of the protein-protein and protein-lipid interactions that allow MscL channels to open at a membrane stress insufficient to cause (presumably less specific) solute leakage via the membrane lipid phase (see, e.g., references 66 and 94)? How does channel opening depend upon changes in MscL secondary, tertiary, and quaternary structures?
CONCLUSION
Analyses of solvent-macromolecule and solvent-membrane interactions have revealed mechanisms by which osmosensors might detect changes in solvent osmolality, either directly or through their impact on cell structure. If different forms of a sensor (“off” and “on”) interact differently with solvent, a change in solvent composition can trigger a change in sensor form (Fig. 2 to 4). In principle, an osmosensor could detect changes in water activity and hence lack cosolvent specificity. In practice, however, bacterial osmosensors may be designed to detect collective changes in the intracellular concentration of individual or chemically similar cosolvents that result from changes in extracellular osmolality. Examples of such cosolvents (or cosolvent groups) might include (i) cosolvents that elevate cytoplasmic ionic strength, (ii) cosolvent molecules that interact differently with the surfaces of the “off” and “on” conformations of the osmosensor, (iii) cosolvent molecules large enough to be sterically excluded from the osmosensor surface or from solvent-filled cavities within the osmosensor, and (iv) cosolvent molecules large enough to effect macromolecular crowding. One or more of these principles may also underlie osmotic induction of genes or operons which occurs without the participation of locus-specific trans-acting regulatory elements (e.g., proP and proU [Table 3]).
Osmotic shifts elicit dramatic changes in bacterial cell structure. It is therefore reasonable to suppose that some osmosensors may be designed to detect these structural changes. Since it is difficult to precisely describe the effects of osmotic shifts on turgor pressure and on structural elements within intact cells, it is not currently possible to deduce osmosensory mechanisms by whole-cell experimentation. The cytoplasmic membrane is clearly a primary osmosensory locus (Table 5). Purified proteins ProP and MscL of E. coli, both cytoplasmic membrane constituents, respond to osmotic shifts and mechanical stimuli, respectively, when incorporated in proteoliposome systems in vitro. These experimental systems can now be exploited to further define the signal detected by each osmosensor, the resulting structural change in the osmosensor, and the role of the membrane. Further analysis of these sensory mechanisms may also offer new insights into the disposition of the cytoplasmic membrane, in vivo, and its perturbation by osmotic shifts. For example, indications that cosolvent accumulation by intact cells is a net consequence of transporter-mediated uptake and mechanosensitive channel-mediated leakage suggest that at least some regions of the cytoplasmic membrane in intact cells experience mechanically imposed membrane strain.
Students of membrane biophysics have predicted that variations in membrane strain modulate the activities of membrane enzymes (85). Membrane-based osmosensors (e.g., ProP and MscL) will now be used as probes to test that prediction. Just as the corpus of research on membrane physical properties will now serve as a resource to students of osmosensing, studies of osmosensory mechanisms may reveal which membrane properties make physiologically significant contributions to membrane strain.
ACKNOWLEDGMENTS
Thanks for assistance, encouragement, patience, comments on drafts of this review, and stimulating discussions are due to members of my laboratory; to Ross Hallett and Bernard Nickel and members of their laboratories (Department of Physics, University of Guelph); to Karlheinz Altendorf, Evert Bakker, and Kerstin Jung and their colleagues at the Universität Osnabrück; and to Kathryn Boys, Laszlo Csonka, Ursula Franklin, Arthur Koch, Reinhard Krämer, Ann Oaks, Jörg Kunte, Peter Rand, Sharon Taylor, and Henry Wiseman.
Research on osmosensing conducted in my laboratory and that of my Guelph collaborators (Ross Hallett and Bernard Nickel) is supported by the Natural Sciences and Engineering Research Council of Canada.
Appendix
Correlation of osmoregulatory responses with changes in turgor pressure, volume, and compartmentation for bacterial cells is a primary goal of research on osmosensing. This Appendix summarizes techniques that may be applicable to this problem and offers comments on their limitations.
Measurement of Turgor Pressure
The available techniques for the detection and monitoring of turgor pressure include (i) direct monitoring of individual cells with a microscopic pressure probe (102), (ii) cytoplasmic freezing-point analysis of whole cells (187), (iii) analysis of the relationship between extracellular osmolality and the pressure-induced collapse of cytoplasmic gas vesicles (154, 215, 216, 290), (iv) comparison of the osmolalities of extracellular media and corresponding cell extracts (260), and (v) analysis of the relationship between cell volume and extracellular osmolality (the plasmolysis titration) (200, 294).
Techniques i and ii have so far been applied only to the large cells of plants, algae, and fungi. It remains to be determined whether bacterial cells sufficiently large to admit pressure probes (for technique i) or to accommodate the necessary microscopic analysis (for technique ii) can be created through genetic manipulation and/or antibiotic treatment. Technique iii is applicable only to the limited array of organisms (Bacteria and Archaea) that construct gas vesicles. The recent expression of Bacillus megaterium gas vesicles in E. coli (154) suggests that it may be used more widely. Pinette and Koch (215, 216) applied the gas vesicle technique to Ancylobacter aquaticus cells to test the hypothesis that turgor pressure would vary during the cell cycle for these gram-negative rods. Their results suggested that the cell wall was elastic and that cells varied quite widely in turgor pressure but revealed no systematic correlation between turgor pressure and cell size (age). It remains to be determined whether this technique will permit the evaluation of osmoregulatory turgor pressure modulation. Difficulties to be overcome include the variability of collapse pressure among gas vesicles and among cells, as well as the sensitivity of collapse pressure to postharvesting changes in the bacteria. Techniques iv and v can be applied to all cells. Turgor pressure estimates obtained by techniques i to iii are independent of cell volume estimation and assumptions regarding cell surface properties (discussed above), whereas those obtained by techniques iv and v are not. As a result, the estimation of cell volume and subcellular compartmentation assumes particular importance for studies of osmosensing.
The cell volume within which turgor pressure exists is, of course, that bounded by the cell surface layer upon which turgor pressure acts. If the cell surface can be assumed to constitute a single semipermeable layer (as is usually assumed for gram-positive bacteria), the relevant volume is that of the cytoplasm. For certain euryhaline algae and plant cells, the vacuole has been considered the relevant compartment since the cytoplasm constitutes a thin layer of relatively constant volume underlying the cell wall (89). Cells that include multiple semipermeable surface layers (e.g., gram-negative bacteria) are composed of concentric compartments which may differ in hydrostatic pressure. It has been assumed that the periplasm and cytoplasm have the same osmolality. This interpretation was consistent with one set of measurements performed on S. typhimurium cells cultivated in a low-osmolality medium (260). It has not been widely tested, however. On the basis of this interpretation, the cytoplasm and periplasm would experience equal turgor pressure, and this turgor pressure would be exerted on the outer membrane!
Application of technique v (the plasmolysis titration) is based on the assumption that as the extracellular osmolality is elevated, the turgor pressure is reduced to zero. Beyond this point, the cell (cytoplasmic) volume adjusts according to equation 2, with the cytoplasmic membrane acting as a fluid surface with high elastic modulus (k) and constant surface area. Thus
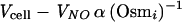 |
14 |
where Vcell is the total cell volume and VNO is the nonosmotic volume, i.e., the portion of the cell volume not responsive to extracellular osmolality changes (see, e.g., references 38, 260, and 294). A linear dependence of Vcell − VNO on the reciprocal of extracellular osmolality is taken as indicative of this behavior. Since intra- and extracellular osmolalities must be equal when turgor pressure is absent, intracellular osmolality is read off such plots and corrected for the difference between the corresponding osmotic volume (Vcell − VNO) and the estimated osmotic volume of unplasmolyzed cells. Cells are usually deprived of nutrients to prevent metabolic activity from altering intracellular osmolality or other cellular properties during the plasmolysis titration.
Measurement of Cell Volume and Compartmentation
Available techniques for the measurement of cell volume and compartmentation include (i) light and electron microscopy, (ii) determinations of the conductance of cell suspensions (with the Coulter counter or analogous instrumentation) (134, 137), (iii) (comparisons of the distributions of H2O and solutes within cell suspensions (260), and (iv) examinations of light scattering by cells and subcellular fractions (93, 128, 282). Technical limitations associated with each technique are discussed in the cited references, and issues of particular importance for studies of osmosensing are covered below.
Estimation of cell volume with the Coulter counter, a process developed by Kubitschek and colleagues (134, 135), depends upon comparison of electrical current interruption by cells and by particles of known size, suspended in a liquid electrolyte, as they pass through a small orifice. It is based on the assumption that current does not pass through the periplasm or cytoplasm. Kubitschek and Friske (137) demonstrated that for E. coli cells from exponential- and stationary-phase cultures, mean cell volumes estimated with the Coulter counter and by [14C]inulin exclusion (see below) were the same. This technique is relatively noninvasive and can provide rapid estimates of total cell volume. It is important to note that such cell volume estimates may be cell shape dependent, an issue of particular importance if cells compared in this way are likely to differ in shape as well as size.
Measurements of the relative distributions of water and solutes within cell suspensions yield estimates of total cell water (Wcell), periplasmic water (Wperi), cytoplasmic water (Wcyto), and hence the distribution of cell water between the cytoplasm and periplasm (subcellular compartmentation). Such measurements are based on the assumption that tritiated water equilibrates with all cellular water whereas other (usually 14C-labelled) hydrophilic solutes are physiologically inert and are excluded from particular cellular compartments only on the basis of their molecular size. Large, polymeric solutes (e.g., dextran, inulin, or PEG) are assumed to be excluded by the cell wall, and certain small, hydrophilic isolates (e.g., stachyose, sucrose, taurine, sorbitol, or mannitol) are assumed to penetrate the periplasm but to be excluded from the cytoplasm. Clearly, these assumptions must be justified experimentally for each solute-organism pair. Once water and solutes have equilibrated with a bacterial suspension, it is centrifuged, and the recovery of each solute in the pellet and supernatant is then compared to calculate, by difference, the volume of water associated with each cell compartment. Rottenberg (238) and Kashket (117) discussed this approach to the estimation of cytoplasmic water in a bioenergetic context.
Cayley et al. (38) extended this technique to determine the proportions of cell volume occupied by molecules other than water. Recognition of this issue is important since, given the large contributions of macromolecules to the volumes of cellular compartments (discussed above), estimates of water content cannot be taken as volume estimates for the corresponding compartments. By using cytoplasmic water as an estimator of cytoplasmic volume during plasmolysis titrations, researchers assume that all cytoplasmic volume changes result only from osmotically induced water flux across the cytoplasmic membrane. They thereby neglect possible changes in hydration of cytoplasmic molecules and in the accessibility of water to the probe solute.
The water contents of cells and their compartments, estimated by solute exclusion, are usually reported per unit cell mass or cell protein, not per cell. They thus provide population estimates, not “cell” estimates. Important information may be lost and erroneous conclusions may be drawn when populations which differ in cell shape and size (or shape and size distribution) are compared. It would therefore be helpful if these measurements were routinely combined with enumeration of cells and/or estimation of cell size and shape distributions (see “Osmoregulation, turgor pressure, cell growth, and cell division” above).
Two additional issues must be addressed if the solute exclusion technique is to be used effectively during studies of osmoadaptation. First, concentrated cell suspensions (cell slurries) are often used and cells are recovered as pellets to ensure that the errors in the required difference measurements are acceptably low. Slurries and pellets composed of respiration-competent bacteria are inevitably anaerobic and often nutrient limited. This may be desirable if the goal is to estimate the properties of “resting” cells, but efforts must be made to demonstrate whether changes in cell structure and physiology pertinent to osmoadaptation have occurred during such sample preparation. (For example, using taurine as probe, Castle et al. [37] measured cytoplasmic volumes of 1.25 μl · mg [dry weight]−1 and 1.8 μl · mg [dry weight]−1 for respiring and glycolyzing E. coli cells, respectively.) On the other hand, measurements may be made on metabolically active cells from dilute cell suspensions if they are recovered by centrifugation through silicone oil (see, e.g., reference 56).
Second, difficulties arise if assumptions regarding solute distribution are not justified. In addition to its size, the chemical nature of the probe solute may be important. For example, if solutes are accumulated in or excluded from macromolecule-associated water (see below), the volume associated with that water will be incorrectly attributed. If a solute (or a contaminant) is not metabolically inert but, rather, is accumulated in or excluded from the cytoplasm, the water content of the cytoplasm will be under- or overestimated and the water content of the periplasm will be over- or underestimated, respectively. For example, contrary to early assumptions, taurine is a low-affinity substrate for the osmoregulatory transporter ProP of E. coli (167). ProP is constitutive, it is activated at a biochemical level by osmotic upshifts, and expression of proP is also induced when the bacteria are cultivated in high-osmolality media. Although ProP will not mediate active solute uptake, it may facilitate solute entry into the cytoplasm in deenergized bacteria. Clearly, efforts must be made to validate the assumption of passive solute distribution into only the expected cellular compartment(s) when this technique is applied for the first time to new organisms and when its application is extended to organisms cultivated under new conditions.
Applications of Light-Scattering Spectroscopy
Turbidimetric techniques provide an attractive alternative to microscopy and solute distribution measurements since, in principle at least, they can be applied rapidly and continuously to dilute cell suspensions without perturbing cell structure and physiology. Sophisticated instrumentation may be required to obtain interpretable results, effects of cellular polydispersity may be difficult to assess, and, as a result, long sample acquisition times may compromise the fundamentally noninvasive nature of this technique. The intensity of light scattered by a suspension of cells in a liquid medium depends upon instrumental parameters as well as on the properties of the cells and their suspending medium. In terms of instrumentation, the critical factors are the wavelength of the incident light in the experimental medium, its polarization, and the angle(s) to the incident beam at which scattered light is detected.
When turbidimetric measurements are made with a conventional spectrophotometer, the incident light is unpolarized and usually has a wavelength (in the range 0.4 to 0.7 μm) comparable to cellular dimensions (0.3 to 3.0 μm). The detector captures light transmitted over a wide range of angles from 0° through approximately 20° to the incident beam. The total intensity of all light scattered at wider angles to the incident beam, which is the integral of intensity over those wider angles, is determined as the difference between the intensity of the incident light and that of the transmitted light. When analogous measurements are made with a modern light-scattering spectrometer, on the other hand, the incident light (usually originating from a helium-neon laser and with a wavelength of 632.8 nm) may be polarized. The intensity of the scattered light is measured directly and (either sequentially or simultaneously) at a series of angles to the incident beam (potentially from approximately 5° [forward scatter] through 180° [back scatter]).
The intensity of light scattered by cells in suspension depends on their concentration and the distributions of their sizes, their shapes, and the differences between their refractive indices and the refractive index of their suspending medium. Equations relating the intensity of scattered light to the listed instrument parameters and to particle size, shape, and refractive index are well known for certain particle populations. The applicability of each model depends on the relationship between particle size and the wavelength and polarization of the incident light (128, 282). Monodisperse populations of spheres, cylinders, and ellipses (and combinations of these shapes) have been considered in this way. The derived relationships usually apply specifically to solid particles or to thin-shelled particles, where the shell thickness and the refractive indices of the particle or of the particle shell and the particle lumen are critical. In some cases, scattering by polydisperse particle populations has also been predicted. These derivations and their experimental application to simple particle systems reveal that detailed information about particle size, shape, refractive index, and even polydispersity can be obtained if the incident light is polarized and the scattered-light intensity is measured as a function of scattering angle.
Light-scattering spectroscopy has potential for the study of bacterial cells, but they must be recognized as complex particles with internal structures that may change as a consequence of solvent perturbations. Turbidimetric measurements performed with a conventional spectrophotometer cannot be interpreted, a priori, in terms of any single particle characteristic such as size, shape, or refractive index. In the flow cytometer, light absorption, fluorescence, and/or scattering by individual particles is measured as they pass appropriate detectors in single file (22, 128). The issue of polydispersity is therefore addressed directly through individual examination of large numbers of particles, but other issues outlined above also apply to these measurements. Each of these techniques can be effectively applied to detect rapid cellular changes due to solvent perturbation, but the origins of those changes may be difficult to assign unequivocally. Indeed, in dynamic systems, structural changes may go undetected if simultaneous changes to multiple particle characteristics have opposing effects on light-scattering intensity and/or if only total scattered light intensity is measured.
REFERENCES
- 1.Alemohammad M M, Knowles C J. Osmotically induced volume and turbidity changes of Escherichia coli due to salts, sucrose and glycerol, with particular reference to the rapid permeation of glycerol into the cell. J Gen Microbiol. 1974;82:125–142. doi: 10.1099/00221287-82-1-125. [DOI] [PubMed] [Google Scholar]
- 1a.Alexander, D., A. Lipski, and J. M. Wood. Unpublished data.
- 2.Altendorf K, Epstein W. Kdp-ATPase of Escherichia coli. Cell Physiol Biochem. 1993;4:160–168. [Google Scholar]
- 3.Anderson C F, Record M T., Jr Salt-nucleic acid interactions. Annu Rev Phys Chem. 1995;46:657–700. doi: 10.1146/annurev.pc.46.100195.003301. [DOI] [PubMed] [Google Scholar]
- 4.Asha H, Gowrishankar J. Regulation of kdp operon expression in Escherichia coli: evidence against turgor as signal for transcriptional control. J Bacteriol. 1993;175:4528–4537. doi: 10.1128/jb.175.14.4528-4537.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aspedon A, Groisman E A. The antibacterial action of protamine: evidence for disruption of cytoplasmic membrane energization in Salmonella typhimurium. Microbiology. 1996;142:3389–3397. doi: 10.1099/13500872-142-12-3389. [DOI] [PubMed] [Google Scholar]
- 6.Bakker E P. Cell K+ and K+ transport systems in prokaryotes. In: Bakker E P, editor. Alkali cation transport systems in prokaryotes. Boca Raton, Fla: CRC Press, Inc.; 1993. pp. 205–224. [Google Scholar]
- 7.Bakker E P. Low-affinity K+ uptake systems. In: Bakker E P, editor. Alkali cation transport systems in prokaryotes. Boca Raton, Fla: CRC Press, Inc.; 1993. pp. 253–275. [Google Scholar]
- 8.Bakker E P, Booth I R, Dinnbier U, Epstein W, Gajewska A. Evidence for multiple K+ export systems in Escherichia coli. J Bacteriol. 1987;169:3743–3749. doi: 10.1128/jb.169.8.3743-3749.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baldwin W W, Kirkish M A, Koch A L. A change in a single gene of Salmonella typhimurium can dramatically change its buoyant density. J Bacteriol. 1994;176:5001–5004. doi: 10.1128/jb.176.16.5001-5004.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baldwin W W, Kubitschek H. Evidence for osmoregulation of cell growth and buoyant density in Escherichia coli. J Bacteriol. 1984;159:393–394. doi: 10.1128/jb.159.1.393-394.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baldwin W W, Myer R, Powell N, Anderson E, Koch A L. Buoyant density of Escherichia coli is determined solely by the osmolarity of the culture medium. Arch Microbiol. 1995;164:155–157. doi: 10.1007/s002030050248. [DOI] [PubMed] [Google Scholar]
- 12.Baldwin W W, Sheu M J T, Bankston P W, Woldringh C. Changes in buoyant density and cell size of Escherichia coli in response to osmotic shocks. J Bacteriol. 1988;170:452–455. doi: 10.1128/jb.170.1.452-455.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bartucci R, Belsito S, Sportelli L. Neutral lipid bilayers interacting with chaotropic ions. Chem Phys Lipids. 1996;79:171–180. [Google Scholar]
- 14.Berrier C, Besnard M, Ajouz B, Coulombe A, Ghazi A. Multiple mechanosensitive ion channels from Escherichia coli, activated at different thresholds of applied pressure. J Membr Biol. 1996;151:175–187. doi: 10.1007/s002329900068. [DOI] [PubMed] [Google Scholar]
- 15.Berrier C, Coulombe A, Houssin C, Ghazi A. A patch-clamp study of ion channels of inner and outer membranes and of contact zones of E. coli fused into giant liposomes. FEBS Lett. 1989;259:27–32. doi: 10.1016/0014-5793(89)81486-3. [DOI] [PubMed] [Google Scholar]
- 16.Berrier C, Coulombe A, Szabo I, Zoratti M, Ghazi A. Gadolinium ion inhibits loss of metabolites induced by osmotic shock and large stretch-activated channels in bacteria. Eur J Biochem. 1992;206:559–565. doi: 10.1111/j.1432-1033.1992.tb16960.x. [DOI] [PubMed] [Google Scholar]
- 17.Biondi A C, Féliz M R, Disalvo E A. Surface changes induced by osmotic stress and its influence on the glycerol permeability in lipid bilayers. Biochim Biophys Acta. 1991;1069:5–13. doi: 10.1016/0005-2736(91)90097-r. [DOI] [PubMed] [Google Scholar]
- 18.Bisson M A, Kirst G O. Osmotic acclimation and turgor pressure regulation in algae. Naturwissenschaften. 1995;82:461–471. [Google Scholar]
- 19.Bloom M, Evans E, Mouritsen O G. Physical properties of the fluid lipid-bilayer component of cell membranes: a perspective. Q Rev Biophys. 1991;24:293–397. doi: 10.1017/s0033583500003735. [DOI] [PubMed] [Google Scholar]
- 20.Blount P, Schroeder M J, Kung C. Mutations in a bacterial mechanosensitive channel change the cellular response to osmotic stress. J Biol Chem. 1997;272:32150–32157. doi: 10.1074/jbc.272.51.32150. [DOI] [PubMed] [Google Scholar]
- 21.Blount P, Sukharev S I, Moe P, Kung C. Mechanosensitive channels of E. coli: a genetic and molecular dissection. Biol Bull. 1997;192:126–127. doi: 10.2307/1542585. [DOI] [PubMed] [Google Scholar]
- 22.Blum J J, Balber A E. Osmotic and metabolic-induced changes in light scattering of Leishmania donovani as measured by flow cytometry. J Eukaryot Microbiol. 1996;43:213–217. [Google Scholar]
- 23.Bogdanov M, Dowhan W. Phosphatidylethanolamine is required for the in vivo function of the membrane-associated lactose permease of Escherichia coli. J Biol Chem. 1995;270:732–739. doi: 10.1074/jbc.270.2.732. [DOI] [PubMed] [Google Scholar]
- 24.Bogdanov M, Sun J, Kaback H R, Dowhan W. A phospholipid acts as a chaperone in assembly of a membrane transport protein. J Biol Chem. 1996;271:11615–11618. doi: 10.1074/jbc.271.20.11615. [DOI] [PubMed] [Google Scholar]
- 25.Booth I R, Higgins C F. Enteric bacteria and osmotic stress: intracellular potassium glutamate as a secondary signal of osmotic stress? FEMS Microbiol Rev. 1990;75:239–246. doi: 10.1111/j.1574-6968.1990.tb04097.x. [DOI] [PubMed] [Google Scholar]
- 26.Bossemeyer D, Borchard A, Dosch D C, Helmer G L, Epstein W, Booth I R, Bakker E P. K+-transport protein TrkA of Escherichia coli is a peripheral membrane protein that requires other trk gene products for attachment to the cytoplasmic membrane. J Biol Chem. 1989;264:16403–16410. [PubMed] [Google Scholar]
- 27.Boyer J S. Cell enlargement and growth-induced water potentials. Physiol Plant. 1988;73:311–316. [Google Scholar]
- 28.Burg M B. Molecular basis for accumulation of compatible osmolytes in mammalian cells. In: Somero G N, Osmond C B, Bolis C L, editors. Water and life: comparative analysis of water relationships at the organismic, cellular and molecular levels. Berlin, Germany: Springer-Verlag KG; 1992. pp. 33–51. [Google Scholar]
- 29.Burg M B. Coordinate regulation of organic osmolytes in renal cells. Kidney Int. 1996;49:1684–1685. doi: 10.1038/ki.1996.247. [DOI] [PubMed] [Google Scholar]
- 30.Buurman E T, Kim K-T, Epstein W. Genetic evidence for two sequentially occupied K+ binding sites in the Kdp transport ATPase. J Biol Chem. 1995;270:6678–6685. doi: 10.1074/jbc.270.12.6678. [DOI] [PubMed] [Google Scholar]
- 31.Cairney J, Booth I R, Higgins C F. Salmonella typhimurium proP gene encodes a transport system for the osmoprotectant betaine. J Bacteriol. 1985;164:1218–1223. doi: 10.1128/jb.164.3.1218-1223.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Calamita G, Bishai W R, Preston G M, Guggino W B, Agre P. Molecular cloning and characterization of AqpZ, a water channel from Escherichia coli. J Biol Chem. 1995;270:29063–29066. doi: 10.1074/jbc.270.49.29063. [DOI] [PubMed] [Google Scholar]
- 33.Calamita G, Kempf B, Bonhivers M, Bishai W R, Bremer E, Agre P. Regulation of the Escherichia coli water channel gene aqpZ. Proc Natl Acad Sci USA. 1998;95:3627–3631. doi: 10.1073/pnas.95.7.3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Calamita G, Kempf B, Rudd K E, Bonhivers M, Kneip S, Bishai W R, Bremer E, Agre P. The aquaporin-Z water channel gene of Escherichia coli: structure, organization and phylogeny. Biol Cell. 1997;89:321–329. [PubMed] [Google Scholar]
- 35.Cantor R S. The lateral pressure profile in membranes: a physical mechanism of general anesthesia. Biochemistry. 1997;36:2339–2344. doi: 10.1021/bi9627323. [DOI] [PubMed] [Google Scholar]
- 36.Capp M W, Cayley D S, Zhang W, Guttman H J, Melcher S E, Saecker R M, Anderson C F, Record M T., Jr Compensating effects of opposing changes in putrescine (2+) and K+ concentrations on lac repressor-lac operator binding: in vitro thermodynamic analysis and in vivo relevance. J Mol Biol. 1996;258:25–36. doi: 10.1006/jmbi.1996.0231. [DOI] [PubMed] [Google Scholar]
- 37.Castle A M, MacNab R M, Shulman R G. Measurement of intracellular sodium concentration and sodium transport in Escherichia coli by 23Na nuclear magnetic resonance. J Biol Chem. 1986;261:3288–3294. [PubMed] [Google Scholar]
- 38.Cayley S, Lewis B A, Guttman H J, Record M T., Jr Characterization of the cytoplasm of Escherichia coli K-12 as a function of external osmolarity: implications for protein-DNA interactions in vivo. J Mol Biol. 1991;222:281–300. doi: 10.1016/0022-2836(91)90212-o. [DOI] [PubMed] [Google Scholar]
- 39.Cayley S, Lewis B A, Record M T., Jr Origins of the osmoprotective properties of betaine and proline in Escherichia coli K-12. J Bacteriol. 1992;174:1586–1595. doi: 10.1128/jb.174.5.1586-1595.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cayley S, Record M T, Jr, Lewis B A. Accumulation of 3-(N-morpholino)propanesulfonate by osmotically stressed Escherichia coli K-12. J Bacteriol. 1989;171:3597–3602. doi: 10.1128/jb.171.7.3597-3602.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Collins K D, Washabaugh M W. The Hofmeister effect and the behaviour of water at interfaces. Q Rev Biophys. 1985;18:323–422. doi: 10.1017/s0033583500005369. [DOI] [PubMed] [Google Scholar]
- 42.Cooper S. Bacterial growth and division: biochemistry and regulation of prokaryotic and eukaryotic division cycles. San Diego, Calif: Academic Press, Inc.; 1991. pp. 247–252. [Google Scholar]
- 43.Cosgrove D. Biophysical control of plant growth. Annu Rev Plant Physiol. 1986;37:377–405. doi: 10.1146/annurev.pp.37.060186.002113. [DOI] [PubMed] [Google Scholar]
- 44.Crowe J H, Crowe L M. Membrane integrity in anhydrobiotic organisms: toward a mechanism for stabilizing dry cells. In: Somero G N, Osmond C B, Bolis C L, editors. Water and life: comparative analysis of water relationships at the organismic, cellular and molecular levels. Berlin, Germany: Springer-Verlag KG; 1992. pp. 87–103. [Google Scholar]
- 45.Csonka L N. Physiological and genetic responses of bacteria to osmotic stress. Microbiol Rev. 1989;53:121–147. doi: 10.1128/mr.53.1.121-147.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Csonka L N, Epstein W. Osmoregulation. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C: ASM Press; 1996. pp. 1210–1223. [Google Scholar]
- 47.Csonka L N, Hanson A D. Prokaryotic osmoregulation: genetics and physiology. Annu Rev Microbiol. 1991;45:569–606. doi: 10.1146/annurev.mi.45.100191.003033. [DOI] [PubMed] [Google Scholar]
- 48.Cui C, Adler J. Effect of mutation of potassium-efflux system, KefA, on mechanosensitive channels in the cytoplasmic membrane of Escherichia coli. J Membr Biol. 1996;150:143–152. doi: 10.1007/s002329900039. [DOI] [PubMed] [Google Scholar]
- 49.Cui C, Smith D O, Adler J. Characterization of mechanosensitive channels in Escherichia coli cytoplasmic membrane by whole-cell patch clamp recording. J Membr Biol. 1995;144:31–42. doi: 10.1007/BF00238414. [DOI] [PubMed] [Google Scholar]
- 50.Culham D E, Lasby B, Marangoni A G, Milner J L, Steer B A, van Nues R W, Wood J M. Isolation and sequencing of Escherichia coli gene proP reveals unusual structural features of the osmoregulatory proline/betaine transporter, ProP. J Mol Biol. 1993;229:268–276. doi: 10.1006/jmbi.1993.1030. [DOI] [PubMed] [Google Scholar]
- 50a.Culham, D. E., R. T. Voegele, and J. M. Wood. Unpublished data.
- 51.Dai J, Sheetz M P. Mechanical properties of neuronal growth cone membranes studied by tether formation with laser optical tweezers. Biophys J. 1995;68:988–996. doi: 10.1016/S0006-3495(95)80274-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.de Gier J. Osmotic behaviour and permeability properties of liposomes. Chem Phys Lipids. 1998;64:187–196. doi: 10.1016/0009-3084(93)90065-b. [DOI] [PubMed] [Google Scholar]
- 53.de la Vega A, Delcour A H. Cadaverine induces closing of E. coli porins. EMBO J. 1995;14:6058–6065. doi: 10.1002/j.1460-2075.1995.tb00294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Delcour A H, Adler J, Kung C, Martinac B. Membrane-derived oligosaccharides (MDO’s) promote closing of an E. coli porin channel. FEBS Lett. 1992;304:216–220. doi: 10.1016/0014-5793(92)80622-n. [DOI] [PubMed] [Google Scholar]
- 55.Deshnium P, Gombos Z, Nishiyama Y, Murata N. The action in vivo of glycine betaine in enhancement of tolerance of Synechococcus sp strain PCC 7942 to low temperature. J Bacteriol. 1997;179:339–344. doi: 10.1128/jb.179.2.339-344.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dinnbier U, Limpinsel E, Schmid R, Bakker E P. Transient accumulation of potassium glutamate and its replacement by trehalose during adaptation of growing cells of Escherichia coli K-12 to elevated sodium chloride concentrations. Arch Microbiol. 1988;150:348–357. doi: 10.1007/BF00408306. [DOI] [PubMed] [Google Scholar]
- 57.Dowhan W. Molecular basis for membrane phospholipid diversity: why are there so many lipids? Annu Rev Biochem. 1997;66:199–232. doi: 10.1146/annurev.biochem.66.1.199. [DOI] [PubMed] [Google Scholar]
- 58.Doyle R J, Marquis R E. Elastic, flexible peptidoglycan and bacterial cell wall properties. Trends Microbiol. 1998;2:57–60. doi: 10.1016/0966-842x(94)90127-9. [DOI] [PubMed] [Google Scholar]
- 59.Dunlap V J, Csonka L N. Osmotic regulation of l-proline transport in Salmonella typhimurium. J Bacteriol. 1985;163:296–304. doi: 10.1128/jb.163.1.296-304.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Epand R F, Polozov I. Liposomes and membrane stability. In: Barenholz Y, Lasic D D, editors. Handbook of nonmedical applications of liposomes. Boca Raton, Fla: CRC Press, Inc.; 1996. pp. 105–111. [Google Scholar]
- 61.Epand R M. Studies of membrane physical properties and their role in biological function. Biochem Soc Trans. 1997;25:1073–1079. doi: 10.1042/bst0251073a. [DOI] [PubMed] [Google Scholar]
- 62.Epand R M, Bryszewska M. Modulation of the bilayer to hexagonal phase transition and solvation of phosphatidylethanolamines in aqueous salt solutions. Biochemistry. 1988;27:8776–8779. doi: 10.1021/bi00424a013. [DOI] [PubMed] [Google Scholar]
- 63.Epand R M, Epand R F. Calorimetric detection of curvature strain in phospholipid bilayers. Biophys J. 1994;66:1450–1456. doi: 10.1016/S0006-3495(94)80935-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ermakov Y A, Averbakh A Z, Lobyshev V I, Sukharev S I. Effects of gadolinium on electrostatic and thermodynamic properties of lipid membranes. Biophys J. 1996;87:7574–7578. [Google Scholar]
- 65.Ermakov Y A, Averbakh A Z, Sukharev S I. Lipid and cell membranes in the presence of gadolinium and other ions with high affinity to lipids. I. Dipole and diffuse components of the boundary potential. Membr Cell Biol. 1997;11:539–554. [PubMed] [Google Scholar]
- 66.Ertel A, Marangoni A G, Marsh J, Hallett F R, Wood J M. Mechanical properties of vesicles. I. Coordinated analysis of swelling and lysis. Biophys J. 1993;64:426–434. doi: 10.1016/S0006-3495(93)81383-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Evans E A, Skalak R. Mechanics and thermodynamics of biomembranes. Boca Raton, Fla: CRC Press, Inc.; 1980. [Google Scholar]
- 68.Faatz E, Middendorf A, Bremer E. Cloned structural genes for the osmotically regulated binding-protein-dependent glycine betaine transport system (ProU) of Escherichia coli K-12. Mol Microbiol. 1988;2:265–279. doi: 10.1111/j.1365-2958.1988.tb00028.x. [DOI] [PubMed] [Google Scholar]
- 69.Farwick M, Siewe R M, Krämer R. Glycine betaine uptake after hyperosmotic shift in Corynebacterium glutamicum. J Bacteriol. 1995;177:4690–4695. doi: 10.1128/jb.177.16.4690-4695.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fendler K, Drose S, Altendorf K, Bamberg E. Electrogenic K+ transport by the Kdp-ATPase of Escherichia coli. Biochemistry. 1996;35:8009–8017. doi: 10.1021/bi960175e. [DOI] [PubMed] [Google Scholar]
- 71.Finkelstein A. Water movement through lipid bilayers, pores and plasma membranes: theory and reality. New York, N.Y: John Wiley & Sons, Inc.; 1987. [Google Scholar]
- 72.Foley M, Brass J M, Birmingham J, Cook W R, Garland P B, Higgins C F, Rothfield L I. Compartmentalization of the periplasm at cell division sites in Escherichia coli as shown by fluorescence photobleaching experiments. Mol Microbiol. 1989;3:1329–1336. doi: 10.1111/j.1365-2958.1989.tb00114.x. [DOI] [PubMed] [Google Scholar]
- 73.Free A, Dorman C J. The Escherichia coli stpA gene is transiently expressed during growth in rich medium and is induced in minimal medium and by stress conditions. J Bacteriol. 1997;179:909–918. doi: 10.1128/jb.179.3.909-918.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Galinski E A. Osmoadaptation in bacteria. Adv Microb Physiol. 1995;37:273–328. [PubMed] [Google Scholar]
- 75.García-Añoveros J, Corey D P. The molecules of mechanosensation. Annu Rev Neurosci. 1997;20:567–594. doi: 10.1146/annurev.neuro.20.1.567. [DOI] [PubMed] [Google Scholar]
- 76.Garcia-Romeu F, Borgese F, Guizouarn H, Fievet B, Motais R. A role for the anion exchanger AE1 (band 3 protein) in cell volume regulation. Cell Mol Biol. 1996;42:985–994. [PubMed] [Google Scholar]
- 77.Garner M M, Burg M B. Macromolecular crowding and confinement in cells exposed to hypertonicity. Am J Physiol. 1994;266:C877–C892. doi: 10.1152/ajpcell.1994.266.4.C877. [DOI] [PubMed] [Google Scholar]
- 78.Gennis R B. Biomembranes: molecular structure and function. New York, N.Y: Springer-Verlag; 1989. [Google Scholar]
- 79.Gerhardt P N M, Smith L T, Smith G M. Sodium-driven, osmotically activated glycine betaine transport in Listeria monocytogenes membrane vesicles. J Bacteriol. 1996;178:6105–6109. doi: 10.1128/jb.178.21.6105-6109.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Glaasker E, Konings W N, Poolman B. Glycine betaine fluxes in Lactobacillus plantarum during osmostasis and hyper- and hypo-osmotic shock. J Biol Chem. 1996;271:10060–10065. doi: 10.1074/jbc.271.17.10060. [DOI] [PubMed] [Google Scholar]
- 81.Glaasker E, Konings W N, Poolman B. Osmotic regulation of intracellular solute pools in Lactobacillus plantarum. J Bacteriol. 1996;178:575–582. doi: 10.1128/jb.178.3.575-582.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gouesbet G, Trautwetter A, Bonnassie S, Wu L F, Blanco C. Characterization of the Erwinia chrysanthemi osmoprotectant transporter gene ousA. J Bacteriol. 1996;178:447–455. doi: 10.1128/jb.178.2.447-455.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gowrishankar J. Identification of osmoresponsive genes in Escherichia coli: evidence for participation of potassium and proline transport systems in osmoregulation. J Bacteriol. 1985;164:434–445. doi: 10.1128/jb.164.1.434-445.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Grothe S, Krogsrud R L, McClellan D J, Milner J L, Wood J M. Proline transport and osmotic stress response in Escherichia coli K-12. J Bacteriol. 1986;166:253–259. doi: 10.1128/jb.166.1.253-259.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gruner S M. Coupling between bilayer curvature elasticity and membrane protein activity. In: Blank M, Vodyanoy I, editors. Biomembrane electrochemistry. Washington, D.C: American Chemical Society; 1994. pp. 129–149. [Google Scholar]
- 86.Gustin M C, Zhou X-L, Martinac B, Kung C. A mechanosensitive channel in the yeast plasma membrane. Science. 1988;242:762–765. doi: 10.1126/science.2460920. [DOI] [PubMed] [Google Scholar]
- 87.Gutierrez C, Abee T, Booth I R. Physiology of the osmotic stress response in microorganisms. Int J Food Microbiol. 1995;28:233–244. doi: 10.1016/0168-1605(95)00059-3. [DOI] [PubMed] [Google Scholar]
- 88.Gutierrez C, Gordia S, Bonnassie S. Characterization of the osmotically inducible gene osmE of Escherichia coli. Mol Microbiol. 1995;16:553–563. doi: 10.1111/j.1365-2958.1995.tb02418.x. [DOI] [PubMed] [Google Scholar]
- 89.Gutknecht J, Hastings D F, Bisson M A. Ion transport and turgor pressure regulation in giant algal cells. In: Giebisch G, editor. Transport across multi-membrane systems. Berlin, Germany: Springer-Verlag KG; 1978. pp. 125–174. [Google Scholar]
- 90.Guttman H J, Cayley S, Li M, Anderson C F, Record M T., Jr K+-ribosome interactions determine the large enhancements of 39K NMR transverse relaxation rates in the cytoplasm of Escherichia coli K-12. Biochemistry. 1995;34:1393–1404. doi: 10.1021/bi00004a034. [DOI] [PubMed] [Google Scholar]
- 91.Ha J-H, Capp M W, Hohenwalter M D, Baskerville M, Record M T., Jr Thermodynamic stoichiometries of participation of water, cations and anions in specific and non-specific binding of lac repressor to DNA: possible origins of the “glutamate effect” on protein-DNA interactions. J Mol Biol. 1992;228:252–264. doi: 10.1016/0022-2836(92)90504-d. [DOI] [PubMed] [Google Scholar]
- 92.Haardt M, Kempf B, Faatz E, Bremer E. The osmoprotectant proline betaine is a major substrate for the binding-protein-dependent transport system ProU of Escherichia coli K-12. Mol Gen Genet. 1995;246:783–786. doi: 10.1007/BF00290728. [DOI] [PubMed] [Google Scholar]
- 93.Hallett F R. Size distributions from static light scattering. In: Brown W, editor. Light scattering: principles and development. Oxford, United Kingdom: Clarendon Press; 1996. pp. 477–493. [Google Scholar]
- 94.Hallett F R, Marsh J, Nickel B G, Wood J M. Mechanical properties of vesicles. II. A model for osmotic swelling and lysis. Biophys J. 1993;64:435–442. doi: 10.1016/S0006-3495(93)81384-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Harold F M, Money N P. What forces drive cell wall expansion? Can J Bot. 1995;73(Suppl. 1):S379–S383. [Google Scholar]
- 96.Harold R L, Harold F M, Money N P. Growth and morphogenesis in Saprolegnia ferax: is turgor required? Protoplasma. 1996;191:105–114. [Google Scholar]
- 97.Hatanaka Y, Kinoshita K, Yamazaki M. Osmotic stress induces a phase transition from interdigitated gel phase to bilayer gel phase in multilamellar vesicles of dihexadecylphosphatidylcholine. Biophys Chem. 1997;65:229–233. doi: 10.1016/s0301-4622(97)00004-5. [DOI] [PubMed] [Google Scholar]
- 98.Hazel J R, Williams E E. The role of alterations in membrane lipid composition in enabling physiological adaptation of organisms to their physical environment. Prog Lipid Res. 1990;29:167–227. doi: 10.1016/0163-7827(90)90002-3. [DOI] [PubMed] [Google Scholar]
- 99.Hildebrandt E R, Cozzarelli N R. Comparison of recombination in vitro and in E. coli cells: measure of the effective concentration of DNA in vivo. Cell. 1995;81:331–340. doi: 10.1016/0092-8674(95)90386-0. [DOI] [PubMed] [Google Scholar]
- 100.Horlacher R, Boos W. Characterization of TreR, the major regulator of the Escherichia coli trehalose system. J Biol Chem. 1997;272:13026–13032. doi: 10.1074/jbc.272.20.13026. [DOI] [PubMed] [Google Scholar]
- 101.Houssin C, Eynard N, Shechter E, Ghazi A. Effect of osmotic pressure on membrane energy-linked functions in Escherichia coli. Biochim Biophys Acta. 1991;1056:76–84. doi: 10.1016/s0005-2728(05)80075-1. [DOI] [PubMed] [Google Scholar]
- 102.Hüsken D, Steudle E, Zimmerman U. Pressure probe technique for measuring water relations of cells in higher plants. Plant Physiol. 1978;61:158–163. doi: 10.1104/pp.61.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Inoue K, Matsuzaki H, Matsumoto K, Shibuya I. Unbalanced membrane phospholipid compositions affect transcriptional expression of certain regulatory genes in Escherichia coli. J Bacteriol. 1997;179:2872–2878. doi: 10.1128/jb.179.9.2872-2878.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.In’t Veld G, Driessen A J M, Konings W N. Bacterial solute transport proteins in their lipid environment. FEMS Microbiol Rev. 1991;12:293–314. doi: 10.1111/j.1574-6976.1993.tb00024.x. [DOI] [PubMed] [Google Scholar]
- 105.In’t Veld G, Driessen A J M, Op den Kamp J A F, Konings W N. Hydrophobic membrane thickness and lipid-protein interactions of the leucine transport system of Lactococcus lactis. Biochim Biophys Acta. 1991;1065:203–212. doi: 10.1016/0005-2736(91)90231-v. [DOI] [PubMed] [Google Scholar]
- 106.Israelachvili J, Wennerstroem H. Role of hydration and water structure in biological and colloidal interactions. Nature. 1996;379:219–225. doi: 10.1038/379219a0. [DOI] [PubMed] [Google Scholar]
- 107.Iyer R, Delcour A H. Complex inhibition of OmpF and OmpC bacterial porins by polyamines. J Biol Chem. 1997;272:18595–18601. doi: 10.1074/jbc.272.30.18595. [DOI] [PubMed] [Google Scholar]
- 108.Jovanovich S B, Martinell M, Record M T, Jr, Burgess R B. Rapid response to osmotic upshift by osmoregulated genes in Escherichia coli and Salmonella typhimurium. J Bacteriol. 1988;170:534–539. doi: 10.1128/jb.170.2.534-539.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jung J U, Gutierrez C, Martin F, Ardourel M, Villarejo M. Transcription of osmB, a gene encoding an Escherichia coli lipoprotein, is regulated by dual signals. J Biol Chem. 1990;265:10574–10581. [PubMed] [Google Scholar]
- 110.Jung K, Altendorf K. Truncation of amino acids 12–128 causes deregulation of the phosphatase activity of the sensor kinase KdpD of Escherichia coli. J Biol Chem. 1998;273:17406–17410. doi: 10.1074/jbc.273.28.17406. [DOI] [PubMed] [Google Scholar]
- 111.Jung K, Heermann R, Meyer M, Altendorf K. Effects of cysteine replacements on the properties of the turgor sensor KdpD of Escherichia coli. Biochim Biophys Acta. 1998;1372:311–322. doi: 10.1016/s0005-2736(98)00070-4. [DOI] [PubMed] [Google Scholar]
- 112.Jung K, Tjaden B, Altendorf K. Purification, reconstitution, and characterization of KdpD, the turgor sensor of Escherichia coli. J Biol Chem. 1997;272:10847–10852. doi: 10.1074/jbc.272.16.10847. [DOI] [PubMed] [Google Scholar]
- 113.Kappes R M, Kempf B, Bremer E. Three transport systems for the osmoprotectant glycine betaine operate in Bacillus subtilis: characterization of OpuD. J Bacteriol. 1996;178:5071–5079. doi: 10.1128/jb.178.17.5071-5079.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kashiwagi K, Miyamoto S, Suzuki F, Kobayashi H, Igarashi K. Excretion of putrescine by the putrescine-ornithine antiporter encoded by the potE gene of Escherichia coli. Proc Natl Acad Sci USA. 1992;89:4529–4533. doi: 10.1073/pnas.89.10.4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kashiwagi K, Shibuya S, Tomitori H, Kuraishi A, Igarishi K. Excretion and uptake of putrescine by the PotE protein in Escherichia coli. J Biol Chem. 1997;272:6318–6323. doi: 10.1074/jbc.272.10.6318. [DOI] [PubMed] [Google Scholar]
- 116.Kashiwagi K, Suzuki T, Suzuki F, Furuchi T, Kobayashi H, Igarashi K. Coexistence of the genes for putrescine transport protein and ornithine decarboxylase at 16 min on the Escherichia coli chromosome. J Biol Chem. 1991;266:20922–20927. [PubMed] [Google Scholar]
- 117.Kashket E R. The proton motive force in bacteria: a critical assessment of methods. Annu Rev Microbiol. 1985;39:219–242. doi: 10.1146/annurev.mi.39.100185.001251. [DOI] [PubMed] [Google Scholar]
- 118.Kellenberger E. The ‘Bayer bridges’ confronted with results from improved electron microscopy methods. Mol Microbiol. 1990;4:697–705. doi: 10.1111/j.1365-2958.1990.tb00640.x. [DOI] [PubMed] [Google Scholar]
- 119.Kempf B, Bremer E. Uptake and synthesis of compatible solutes as microbial stress responses to high osmolality environments. Arch Microbiol. 1998;170:319–330. doi: 10.1007/s002030050649. [DOI] [PubMed] [Google Scholar]
- 120.Ken-Dror S, Preger R, Avi-Dor Y. Role of betaine in the control of respiration and osmoregulation of a halotolerant bacterium. FEMS Microbiol Rev. 1986;39:115–120. [Google Scholar]
- 121.Kennedy E P. Membrane-derived oligosaccharides (periplasmic beta-d-glucans) of Escherichia coli. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C: ASM Press; 1996. pp. 1064–1072. [Google Scholar]
- 122.Kinnunen P K J, Lehtonen J Y A, Rytömaa M, Mustonen P. Lipid dynamics and peripheral interactions of proteins with membrane surfaces. Chem Phys Lipids. 1994;73:181–207. doi: 10.1016/0009-3084(94)90181-3. [DOI] [PubMed] [Google Scholar]
- 123.Kinoshita K, Yamazaki M. Phase transition between hexagonal II (HII) and liquid-crystalline phase induced by interaction between solvents and segments of the membrane surface of dioleoylphosphatidylethanolamine. Biochim Biophys Acta. 1997;1330:199–206. doi: 10.1016/s0005-2736(97)00161-2. [DOI] [PubMed] [Google Scholar]
- 124.Ko R, Smith L T, Smith G M. Glycine betaine confers enhanced osmotolerance and cryotolerance on Listeria monocytogenes. J Bacteriol. 1994;176:426–431. doi: 10.1128/jb.176.2.426-431.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124a.Ko, R., and L. T. Smith. Personal communication.
- 125.Koch A L. Shrinkage of growing Escherichia coli cells by osmotic challenge. J Bacteriol. 1984;159:919–924. doi: 10.1128/jb.159.3.919-924.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Koch A L. The geometry and osmotic relations of plasmolysis spaces in bacteria and the role of endocytosis, tubular structures and Scheie structures in their formation. J Theor Biol. 1995;176:471–492. [Google Scholar]
- 127.Koch A L, Lane S L, Miller J A, Nickens D G. Contraction of filaments of Escherichia coli after disruption of cell membrane by detergent. J Bacteriol. 1987;169:1979–1984. doi: 10.1128/jb.169.5.1979-1984.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Koch A L, Robertson B R, Button D K. Deduction of the cell volume and mass from forward scatter intensity of bacteria analyzed by flow cytometry. J Microbiol Methods. 1996;27:49–61. [Google Scholar]
- 129.Koch A L, Woeste S. Elasticity of the sacculus of Escherichia coli. J Bacteriol. 1992;174:4811–4819. doi: 10.1128/jb.174.14.4811-4819.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kollman R, Altendorf K. ATP-driven potassium transport in right-side-out membrane vesicles via the Kdp system of Escherichia coli. Biochim Biophys Acta. 1993;1143:62–66. doi: 10.1016/0005-2728(93)90216-3. [DOI] [PubMed] [Google Scholar]
- 131.Koo S-P, Higgins C F, Booth I R. Regulation of compatible solute accumulation in Salmonella typhimurium: evidence for a glycine betaine efflux system. J Gen Microbiol. 1991;137:2617–2625. doi: 10.1099/00221287-137-11-2617. [DOI] [PubMed] [Google Scholar]
- 132.Kraayenhof R, Sterk G J, Wong Fong Sang H W, Krab K, Epand R M. Monovalent cations differentially affect membrane surface properties and membrane curvature, as revealed by fluorescent probes and dynamic light scattering. Biochim Biophys Acta. 1996;1282:293–302. doi: 10.1016/0005-2736(96)00069-7. [DOI] [PubMed] [Google Scholar]
- 133.Krämer R. Secretion of amino acids by bacteria: physiology and mechanism. FEMS Microbiol Rev. 1994;13:75–94. [Google Scholar]
- 134.Kubitschek H E. Counting and sizing micro-organisms with the Coulter counter. Meth Microbiol. 1969;1:593–610. [Google Scholar]
- 135.Kubitschek H E. Buoyant density variation during the cell cycle in microorganisms. Crit Rev Microbiol. 1987;14:7397. doi: 10.3109/10408418709104436. [DOI] [PubMed] [Google Scholar]
- 136.Kubitschek H E, Baldwin W W, Schroeter S J, Graetzer R. Independence of buoyant cell density and growth rate in Escherichia coli. J Bacteriol. 1984;158:296–299. doi: 10.1128/jb.158.1.296-299.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kubitschek H E, Friske J A. Determination of bacterial cell volume with the Coulter counter. J Bacteriol. 1986;168:1466–1467. doi: 10.1128/jb.168.3.1466-1467.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kuchta T, Russell N J. Glycine betaine stimulates, but NaCl inhibits, fatty acid biosynthesis in the moderately halophilic eubacterium HX. Arch Microbiol. 1994;161:234–238. [Google Scholar]
- 139.Kunte, H. J., R. A. Crane, D. E. Culham, D. Richmond, and J. M. Wood. Protein ProQ influences osmotic activation of compatible solute transporter ProP in Escherichia coli K-12. J. Bacteriol., in press. [DOI] [PMC free article] [PubMed]
- 140.Lacroix J-M, Loubens I, Tempête M, Menichi B, Bohin J-P. The mdoA locus of Escherichia coli consists of an operon under osmotic control. Mol Microbiol. 1991;5:1745–1753. doi: 10.1111/j.1365-2958.1991.tb01924.x. [DOI] [PubMed] [Google Scholar]
- 141.Laimins L A, Rhoads D B, Epstein W. Osmotic control of kdp operon expression in Escherichia coli. Proc Natl Acad Sci USA. 1981;78:464–468. doi: 10.1073/pnas.78.1.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lamark T, Rokenes T P, McDougall J, Strom A R. The complex bet promoters of Escherichia coli: regulation by oxygen (ArcA), choline (BetI), and osmotic stress. J Bacteriol. 1996;178:1655–1662. doi: 10.1128/jb.178.6.1655-1662.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lamark T, Styrvold O B, Strom A R. Efflux of choline and glycine betaine from osmoregulating cells of Escherichia coli. FEMS Microbiol Lett. 1992;96:149–154. doi: 10.1016/0378-1097(92)90395-5. [DOI] [PubMed] [Google Scholar]
- 144.Lambert C, Erdmann A, Eikmanns M, Krämer R. Triggering glutamate excretion in Corynebacterium glutamicum by modulating the membrane state with local anesthetics and osmotic gradients. Appl Environ Microbiol. 1995;61:4334–4342. doi: 10.1128/aem.61.12.4334-4342.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lange R, Barth M, Hengge-Aronis R. Complex transcriptional control of the ςS-dependent stationary-phase-induced and osmotically regulated osmY (csi-5) gene suggests novel roles for Lrp, cyclic AMP (cAMP) receptor protein-cAMP complex, and integration host factor in the stationary phase response of Escherichia coli. J Bacteriol. 1993;175:7910–7917. doi: 10.1128/jb.175.24.7910-7917.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Larsen P I, Syndes L K, Landfald B, Strom A R. Osmoregulation in Escherichia coli by accumulation of organic osmolytes: betaines, glutamic acid and trehalose. Arch Microbiol. 1987;147:1–7. doi: 10.1007/BF00492896. [DOI] [PubMed] [Google Scholar]
- 147.Leduc M, Ishidate K, Shakibai N, Rothfield L I. Interactions of Escherichia coli membrane lipoproteins with the murein sacculus. J Bacteriol. 1992;174:7982–7988. doi: 10.1128/jb.174.24.7982-7988.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lehtonen J Y A, Kinnunen P K J. Changes in the lipid dynamics of liposomal membranes induced by poly(ethylene glycol): free volume alterations revealed by inter- and intramolecular excimer-forming phospholipid analogues. Biophys J. 1994;66:1981–1990. doi: 10.1016/S0006-3495(94)80991-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lehtonen J Y A, Kinnunen P K J. Poly(ethylene glycol)-induced and temperature-dependent phase separation in fluid binary phospholipid membranes. Biophys J. 1995;68:525–535. doi: 10.1016/S0006-3495(95)80214-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lehtonen J Y A, Kinnunen P K J. Evidence for phospholipid microdomain formation in liquid crystalline liposomes reconstituted with Escherichia coli lactose permease. Biophys J. 1997;72:1247–1257. doi: 10.1016/S0006-3495(97)78771-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Leirmo S, Harrison C, Cayley D S, Burgess R R, Record M T., Jr Replacement of potassium chloride by potassium glutamate dramatically enhances protein-DNA interactions in vitro. Biochemistry. 1987;26:2095–2101. doi: 10.1021/bi00382a006. [DOI] [PubMed] [Google Scholar]
- 152.Lemieux R U. How water provides the impetus for molecular recognition in aqueous solution. Acc Chem Res. 1996;29:373–380. [Google Scholar]
- 153.Lewis R N A H, Mannock D A, McElhaney R N. Membrane lipid molecular structure and polymorphism. In: Epand R, editor. Lipid polymorphism and membrane properties. San Diego, Calif: Academic Press, Inc.; 1997. pp. 25–102. [Google Scholar]
- 154.Li N, Cannon M C. Gas vesicle genes identified in Bacillus megaterium and functional expression in Escherichia coli. J Bacteriol. 1998;180:2450–2458. doi: 10.1128/jb.180.9.2450-2458.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lindblom G, Rilfors L. Nonlamellar phases formed by membrane lipids. Adv Colloid Interface Sci. 1992;41:101–125. doi: 10.1016/0001-8686(92)80009-m. [DOI] [PubMed] [Google Scholar]
- 156.Lippert K, Galinski E A. Enzyme stabilization by ectoine-type compatible solutes: protection against heating, freezing and drying. Appl Microbiol Biotechnol. 1992;37:61–65. [Google Scholar]
- 157.Lockhart J A. An analysis of irreversible plant cell elongation. J Theor Biol. 1965;8:264–275. doi: 10.1016/0022-5193(65)90077-9. [DOI] [PubMed] [Google Scholar]
- 158.Lockhart J A. Cell extension. In: Bonner J, Varner J E, editors. Plant biochemistry. New York, N.Y: Academic Press, Inc.; 1965. pp. 826–849. [Google Scholar]
- 159.Lucht J M, Bremer E. Adaptation of Escherichia coli to high osmolarity environments: osmoregulation of the high-affinity glycine betaine transport system ProU. FEMS Microbiol Rev. 1994;14:3–20. doi: 10.1111/j.1574-6976.1994.tb00067.x. [DOI] [PubMed] [Google Scholar]
- 160.MacMillan S V. The substrate and inhibitor specificity of the osmoregulatory transporter ProP. M.Sc. thesis. Guelph, Canada: University of Guelph; 1998. [Google Scholar]
- 161.Marsh D. Lateral pressure in membranes. Biochim Biophys Acta. 1996;1286:183–223. doi: 10.1016/s0304-4157(96)00009-3. [DOI] [PubMed] [Google Scholar]
- 162.Marshall E V. Osmoregulation of ProP: elucidating the roles of the ion coupling. Ph.D. thesis. Guelph, Canada: University of Guelph; 1996. [Google Scholar]
- 163.Martinac B, Adler J, Kung C. Mechanosensitive ion channels of E. coli activated by amphipaths. Nature. 1990;348:261–263. doi: 10.1038/348261a0. [DOI] [PubMed] [Google Scholar]
- 164.Maurel C, Reizer J, Schroeder J I, Chrispeels M J, Saier M H., Jr Functional characterization of the Escherichia coli glycerol facilitator, GlpF, in Xenopus oocytes. J Biol Chem. 1994;269:11869–11872. [PubMed] [Google Scholar]
- 165.McElhaney R N. The structure and function of the Acholeplasma laidlawii plasma membrane. Biochim Biophys Acta. 1984;779:1–42. doi: 10.1016/0304-4157(84)90002-9. [DOI] [PubMed] [Google Scholar]
- 166.McIntosh T J. Hydration properties of lamellar and non-lamellar phases of phosphatidylcholine and phosphatidylethanolamine. Chem Phys Lipids. 1996;81:117–131. doi: 10.1016/0009-3084(96)02577-7. [DOI] [PubMed] [Google Scholar]
- 167.McLaggan D, Epstein W. Escherichia coli accumulates the eukaryotic osmolyte taurine at high osmolarity. FEMS Microbiol Lett. 1991;81:209–214. doi: 10.1016/0378-1097(91)90304-s. [DOI] [PubMed] [Google Scholar]
- 168.McLaggan D, Naprstek J, Buurman E T, Epstein W. Interdependence of K+ and glutamate accumulation during osmotic adaptation of Escherichia coli. J Biol Chem. 1994;269:1911–1917. [PubMed] [Google Scholar]
- 169.Mellies J, Brems R, Villarejo M. The Escherichia coli proU promoter element and its contribution to osmotically signaled transcription activation. J Bacteriol. 1994;176:3638–3645. doi: 10.1128/jb.176.12.3638-3645.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Mellies J, Wise A, Villarejo M. Two different Escherichia coli proP promoters respond to osmotic and growth phase signals. J Bacteriol. 1995;177:144–151. doi: 10.1128/jb.177.1.144-151.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Meury J. Glycine betaine reverses the effects of osmotic stress on DNA replication and cellular division in Escherichia coli. Arch Microbiol. 1988;149:232–239. doi: 10.1007/BF00422010. [DOI] [PubMed] [Google Scholar]
- 172.Meury J. Immediate and transient inhibition of the respiration of Escherichia coli under hyperosmotic shock. FEMS Microbiol Lett. 1994;121:281–286. doi: 10.1111/j.1574-6968.1994.tb07113.x. [DOI] [PubMed] [Google Scholar]
- 173.Meury J, Bahloul A, Kohiyama M. Impairment of nucleoid segregation and cell division at high osmolarity in a strain of Escherichia coli overproducing the chaperone DnaK. FEMS Microbiol Lett. 1993;113:93–100. doi: 10.1111/j.1574-6968.1993.tb06494.x. [DOI] [PubMed] [Google Scholar]
- 174.Meury J, Bahloul A, Kohiyama M. Importance of the replication origin sequestration in cell division of Escherichia coli. Biochimie. 1995;77:875–879. doi: 10.1016/0300-9084(95)90006-3. [DOI] [PubMed] [Google Scholar]
- 175.Meury J, Kohiyama M. Potassium ions and changes in bacterial DNA supercoiling under osmotic stress. FEMS Microbiol Lett. 1992;99:159–164. doi: 10.1016/0378-1097(92)90018-j. [DOI] [PubMed] [Google Scholar]
- 176.Meury J, Robin A, Monnier-Champeix P. Turgor-controlled K+ fluxes and their pathways in Escherichia coli. Eur J Biochem. 1985;151:613–619. doi: 10.1111/j.1432-1033.1985.tb09148.x. [DOI] [PubMed] [Google Scholar]
- 177.Mileykovskaya E, Dowhan W. The Cpx two-component signal transduction pathway is activated in Escherichia coli mutant strains lacking phosphatidylethanolamine. J Bacteriol. 1997;179:1029–1034. doi: 10.1128/jb.179.4.1029-1034.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Miller K J, Wood J M. Osmoadaptation by rhizosphere bacteria. Annu Rev Microbiol. 1996;50:101–136. doi: 10.1146/annurev.micro.50.1.101. [DOI] [PubMed] [Google Scholar]
- 179.Milner J L, Grothe S, Wood J M. Proline porter II is activated by a hyperosmotic shift in both whole cells and membrane vesicles of Escherichia coli K12. J Biol Chem. 1988;263:14900–14905. [PubMed] [Google Scholar]
- 180.Milner J L, McClellan D J, Wood J M. Factors reducing and promoting the effectiveness of proline as an osmoprotectant in Escherichia coli K12. J Gen Microbiol. 1987;133:1851–1860. doi: 10.1099/00221287-133-7-1851. [DOI] [PubMed] [Google Scholar]
- 181.Milner J L, Wood J M. Insertion proQ220::Tn5 alters regulation of proline porter II, a transporter of proline and glycine betaine in Escherichia coli. J Bacteriol. 1989;171:947–951. doi: 10.1128/jb.171.2.947-951.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Minton A P. Influence of excluded volume upon macromolecular structure and associations in ‘crowded’ media. Curr Opin Biotechnol. 1997;8:65–69. doi: 10.1016/s0958-1669(97)80159-0. [DOI] [PubMed] [Google Scholar]
- 183.Minton A P, Coclasure G G, Parker J C. Model for the role of macromolecular crowding in regulation of cellular volume. Proc Natl Acad Sci USA. 1992;89:10504–10506. doi: 10.1073/pnas.89.21.10504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Mizuno T. His-Asp phosphotransfer signal transduction. J Biochem. 1998;123:555–563. doi: 10.1093/oxfordjournals.jbchem.a021972. [DOI] [PubMed] [Google Scholar]
- 185.Mohandas N, Evans E. Mechanical properties of the red cell membrane in relation to molecular structure and genetic defects. Annu Rev Biophys Biomol Struct. 1994;23:787–818. doi: 10.1146/annurev.bb.23.060194.004035. [DOI] [PubMed] [Google Scholar]
- 186.Money N P, Harold F M. Two water molds can grow without measurable turgor pressure. Planta. 1993;190:426–430. [Google Scholar]
- 187.Money N P, Howard R J. Confirmation of a link between fungal pigmentation, turgor pressure, and pathogenicity using a new method of turgor measurement. Fungal Genet Biol. 1996;20:217–227. [Google Scholar]
- 188.Mouritsen O G, Bloom M. Models of lipid-protein interactions in membranes. Annu Rev Biophys Biomol Struct. 1993;22:145–171. doi: 10.1146/annurev.bb.22.060193.001045. [DOI] [PubMed] [Google Scholar]
- 189.Mouritsen O G, Jorgensen K. Dynamic lipid-bilayer heterogeneity: a mesoscopic vehicle for membrane function? Bioessays. 1992;14:129–136. doi: 10.1002/bies.950140211. [DOI] [PubMed] [Google Scholar]
- 190.Mui B L S, Cullis P R, Evans E A, Madden T D. Osmotic properties of large unilamellar vesicles prepared by extrusion. Biophys J. 1993;64:443–453. doi: 10.1016/S0006-3495(93)81385-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Munns R. Physiological processes limiting plant growth in saline soils: some dogmas and hypotheses. Plant Cell Environ. 1993;16:15–24. [Google Scholar]
- 192.Munro G F, Hercules K, Morgan J, Sauerbier W. Dependence of the putrescine content of Escherichia coli on the osmotic strength of the medium. J Biol Chem. 1972;247:1272–1280. [PubMed] [Google Scholar]
- 193.Murphy L D, Zimmerman S B. Isolation and characterization of spermidine nucleoids from Escherichia coli. J Struct Biol. 1997;119:321–335. doi: 10.1006/jsbi.1997.3883. [DOI] [PubMed] [Google Scholar]
- 194.Murphy L D, Zimmerman S B. Stabilization of compact spermidine nucleoids from Escherichia coli under crowded conditions: implications for in vivo nucleoid structure. J Struct Biol. 1997;119:346. doi: 10.1006/jsbi.1997.3884. [DOI] [PubMed] [Google Scholar]
- 195.Nakashima K, Sugiura A, Kanamaru K, Mizuno T. Signal transduction between the two regulatory components involved in the regulation of the kdpABC operon in Escherichia coli: phosphorylation-dependent functioning of the positive regulator, KdpE. Mol Microbiol. 1993;7:109–116. doi: 10.1111/j.1365-2958.1993.tb01102.x. [DOI] [PubMed] [Google Scholar]
- 196.Nakashima K, Sugiura A, Mizuno T. Functional reconstitution of the putative Escherichia coli osmosensor, KdpD, in liposomes. J Biochem. 1993;114:615–621. doi: 10.1093/oxfordjournals.jbchem.a124226. [DOI] [PubMed] [Google Scholar]
- 197.Nakashima K, Sugiura H, Momoi H, Mizuno T. Phosphotransfer signal transduction between two regulatory factors involved in the osmoregulated kdp operon in Escherichia coli. Mol Microbiol. 1992;6:1777–1784. doi: 10.1111/j.1365-2958.1992.tb01350.x. [DOI] [PubMed] [Google Scholar]
- 198.Nanninga N. Morphogenesis of Escherichia coli. Microbiol Mol Biol Rev. 1998;62:110–129. doi: 10.1128/mmbr.62.1.110-129.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Nebel S, Ganz P, Seelig J. Heat changes in lipid membranes under sudden osmotic stress. Biochemistry. 1997;36:2853–2859. doi: 10.1021/bi961839n. [DOI] [PubMed] [Google Scholar]
- 200.Nobel P S. Physicochemical and environmental plant physiology. San Diego, Calif: Academic Press, Inc.; 1991. pp. 47–189. [Google Scholar]
- 201.Ohwada T, Sagisaka S. An immediate and steep increase in ATP concentration in response to reduced turgor pressure in Escherichia coli B. Arch Biochem Biophys. 1987;259:157–163. doi: 10.1016/0003-9861(87)90481-4. [DOI] [PubMed] [Google Scholar]
- 202.Ohyama T, Igarashi K, Kobayashi H. Physiological role of the chaA gene in sodium and calcium circulations at a high pH in Escherichia coli. J Bacteriol. 1994;176:4311–4315. doi: 10.1128/jb.176.14.4311-4315.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Oparka K J. Plasmolysis: new insights into an old process. New Phytol. 1994;126:571–591. [Google Scholar]
- 204.Padan E, Maisler N, Taglicht D, Karpel R, Schuldiner S. Deletion of ant in Escherichia coli reveals its function in adaptation to high salinity and an alternative Na+/H+ antiporter system. J Biol Chem. 1989;264:20297–20302. [PubMed] [Google Scholar]
- 205.Padan E, Schuldiner S. Molecular physiology of the Na+/H+ antiporter in Escherichia coli. J Exp Biol. 1994;196:443–456. doi: 10.1242/jeb.196.1.443. [DOI] [PubMed] [Google Scholar]
- 206.Padan E, Zilberstein D, Rottenberg H. The proton electrochemical gradient in Escherichia coli cells. Eur J Biochem. 1976;63:533–541. doi: 10.1111/j.1432-1033.1976.tb10257.x. [DOI] [PubMed] [Google Scholar]
- 207.Parker J C. In defense of cell volume? Am J Physiol. 1993;265:C1191–C1200. doi: 10.1152/ajpcell.1993.265.5.C1191. [DOI] [PubMed] [Google Scholar]
- 208.Parra-Lopez C, Lin R, Aspedon A, Groisman E A. A Salmonella protein that is required for resistance to antimicrobial peptides and transport of potassium. EMBO J. 1994;13:3964–3972. doi: 10.1002/j.1460-2075.1994.tb06712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Parsegian V A, Rand R P, Rau D C. Macromolecules and water: probing with osmotic stress. Methods Enzymol. 1995;259:43–95. doi: 10.1016/0076-6879(95)59039-0. [DOI] [PubMed] [Google Scholar]
- 210.Passioura J B, Fry S C. Turgor and cell expansion. Aust J Plant Physiol. 1992;19:565–576. [Google Scholar]
- 211.Patchett R A, Kelly A F, Kroll R G. Effect of sodium chloride on the intracellular solute pools of Listeria monocytogenes. Appl Environ Microbiol. 1992;58:3959–3963. doi: 10.1128/aem.58.12.3959-3963.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Peddie B A, Lever M, Hayman C M, Randall K, Chambers S T. Relationship between osmoprotection and the structure and intracellular accumulation of betaines by Escherichia coli. FEMS Microbiol Lett. 1994;120:125–132. doi: 10.1111/j.1574-6968.1994.tb07018.x. [DOI] [PubMed] [Google Scholar]
- 213.Peter H, Burkovski A, Kramer R. Isolation, characterization, and expression of the Corynebacterium glutamicum betP gene, encoding the transport system for the compatible solute glycine betaine. J Bacteriol. 1996;178:5229–5234. doi: 10.1128/jb.178.17.5229-5234.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Peter H, Burkovski A, Kramer R. Osmo-sensing by N- and C-terminal extensions of the glycine betaine uptake system BetP of Corynebacterium glutamicum. J Biol Chem. 1998;273:2567–2574. doi: 10.1074/jbc.273.5.2567. [DOI] [PubMed] [Google Scholar]
- 215.Pinette M F S, Koch A L. Variability of the turgor pressure of individual cells of the gram-negative heterotroph Ancylobacter aquaticus. J Bacteriol. 1987;169:4737–4742. doi: 10.1128/jb.169.10.4737-4742.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Pinette M F S, Koch A L. Turgor pressure responses of a gram-negative bacterium to antibiotic treatment, measured by collapse of gas vesicles. J Bacteriol. 1988;170:1129–1136. doi: 10.1128/jb.170.3.1129-1136.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Pinner E, Kotler Y, Padan E, Schuldiner S. Physiological role of NhaB, a specific Na+/H+ antiporter in Escherichia coli. J Biol Chem. 1993;268:1729–1734. [PubMed] [Google Scholar]
- 218.Polarek J W, Williams G, Epstein W. The products of the kdpDE operon are required for expression of the Kdp ATPase of Escherichia coli. J Bacteriol. 1992;174:2145–2151. doi: 10.1128/jb.174.7.2145-2151.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Pont-Lezica R F, McNally J G, Pickard B G. Wall-to-membrane linkers in onion epidermis: some hypotheses. Plant Cell Environ. 1993;16:111–123. [Google Scholar]
- 220.Poolman B, Glaasker E. Regulation of compatible solute accumulation in bacteria. Mol Microbiol. 1998;29:397–407. doi: 10.1046/j.1365-2958.1998.00875.x. [DOI] [PubMed] [Google Scholar]
- 221.Potts M. Desiccation tolerance of prokaryotes. Microbiol Rev. 1994;58:755–805. doi: 10.1128/mr.58.4.755-805.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Pourkomailian B, Booth I R. Glycine betaine transport by Staphylococcus aureus: evidence for two transport systems and for their possible roles in osmoregulation. J Gen Microbiol. 1992;138:2515–2518. doi: 10.1099/00221287-138-12-2515. [DOI] [PubMed] [Google Scholar]
- 223.Pourkomailian B, Booth I R. Glycine betaine transport by Staphylococcus aureus: evidence for feedback regulation of the activity of the two transport systems. Microbiology. 1994;140:3131–3138. doi: 10.1099/13500872-140-11-3131. [DOI] [PubMed] [Google Scholar]
- 224.Puppe W, Siebers A, Altendorf K. The phosphorylation site of the Kdp-ATPase of Escherichia coli: site directed mutagenesis of the aspartic acid residues 300 and 307 of the KdpB subunit. Mol Microbiol. 1992;6:3511–3520. doi: 10.1111/j.1365-2958.1992.tb01786.x. [DOI] [PubMed] [Google Scholar]
- 225.Puppe W, Zimmann P, Jung K, Lucassen M, Altendorf K. Characterization of truncated forms of the KdpD protein, the sensor kinase of the K+-translocating Kdp system of Escherichia coli. J Biol Chem. 1996;271:25027–25034. doi: 10.1074/jbc.271.40.25027. [DOI] [PubMed] [Google Scholar]
- 226.Racher, K. I., R. T. Voegele, E. V. Marshall, D. E. Culham, J. M. Wood, H. Jung, M. Bacon, M. T. Cairns, S. M. Ferguson, W.-J. Liang, P. J. F. Henderson, G. White, and F. R. Hallett. Purification and reconstitution of an osmosensor: transporter ProP of Escherichia coli senses and responds to osmotic shifts. Biochemistry, in press. [DOI] [PubMed]
- 227.Rand R P, Fuller N L, Gruner S M, Parsegian V A. Measured curvature, lipid segregation, and structural transitions for phospholipids under dual-solvent stress. Biochemistry. 1990;29:76–87. doi: 10.1021/bi00453a010. [DOI] [PubMed] [Google Scholar]
- 228.Record M T, Jr, Courtenay E S, Cayley D S, Guttman H J. Responses of E. coli to osmotic stress: large changes in amounts of cytoplasmic solutes and water. Trends Biochem Sci. 1998;23:143–148. doi: 10.1016/s0968-0004(98)01196-7. [DOI] [PubMed] [Google Scholar]
- 229.Record M T, Jr, Courtenay E S, Cayley S, Guttman H J. Biophysical compensation mechanisms buffering E. coli protein-nucleic acid interactions against changing environments. Trends Biochem Sci. 1998;23:190–194. doi: 10.1016/s0968-0004(98)01207-9. [DOI] [PubMed] [Google Scholar]
- 230.Reed R H, Walsby A E. Changes in cell turgor pressure in response to increases in external NaCl concentration in the gas-vacuolate cyanobacterium Microcystis sp. Arch Microbiol. 1985;143:290. [Google Scholar]
- 231.Reed R H, Warr S R C, Kerby N W, Stewart W D P. Osmotic shock-induced release of low molecular weight metabolites from free-living and immobilized cyanobacteria. Enzyme Microb Technol. 1986;8:101–104. [Google Scholar]
- 232.Reid C, Rand R P. Probing protein hydration and conformational states in solution. Biophys J. 1997;72:1022–1030. doi: 10.1016/S0006-3495(97)78754-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Richey B, Cayley D S, Mossing M C, Kolka C, Anderson C F, Farrar T C, Record M T., Jr Variability of the intracellular ionic environment of Escherichia coli. J Biol Chem. 1987;262:7157–7164. [PubMed] [Google Scholar]
- 234.Robinson C R, Sligar S G. Hydrostatic pressure reverses osmotic pressure effects on the specificity of EcoRI-DNA interactions. Biochemistry. 1994;33:3787–3793. doi: 10.1021/bi00179a001. [DOI] [PubMed] [Google Scholar]
- 235.Robinson C R, Sligar S G. Hydrostatic and osmotic pressure as tools to study macromolecular recognition. Methods Enzymol. 1995;259:395–426. doi: 10.1016/0076-6879(95)59054-4. [DOI] [PubMed] [Google Scholar]
- 236.Roth W G, Leckie M P, Dietzler D N. Osmotic stress drastically inhibits active transport of carbohydrates by Escherichia coli. Biochem Biophys Res Commun. 1985;126:434–441. doi: 10.1016/0006-291x(85)90624-2. [DOI] [PubMed] [Google Scholar]
- 237.Roth W G, Porter S E, Leckie M P, Porter B E, Dietzler D N. Restoration of cell volume and the reversal of carbohydrate transport and growth inhibition of osmotically upshocked Escherichia coli. Biochem Biophys Res Commun. 1985;126:442–449. doi: 10.1016/0006-291x(85)90625-4. [DOI] [PubMed] [Google Scholar]
- 238.Rottenberg H. The measurement of membrane potential and pH in cells, organelles, and vesicles. Methods Enzymol. 1979;55:547–569. doi: 10.1016/0076-6879(79)55066-6. [DOI] [PubMed] [Google Scholar]
- 239.Royer C A. Application of pressure to biochemical equilibria: the other thermodynamic variable. Methods Enzymol. 1995;259:357–377. doi: 10.1016/0076-6879(95)59052-8. [DOI] [PubMed] [Google Scholar]
- 240.Rudolph A S, Crowe J H, Crowe L M. Effects of three stabilizing agents—proline, betaine, and trehalose—on membrane phospholipids. Arch Biochem Biophys. 1986;245:134–143. doi: 10.1016/0003-9861(86)90197-9. [DOI] [PubMed] [Google Scholar]
- 241.Ruffert S, Lambert C, Peter H, Wendisch V F, Kramer R. Efflux of compatible solutes in Corynebacterium glutamicum mediated by osmoregulated channel activity. Eur J Biochem. 1997;247:572–580. doi: 10.1111/j.1432-1033.1997.00572.x. [DOI] [PubMed] [Google Scholar]
- 242.Russell N J. Lipids of halophilic and halotolerant microorganisms. In: Vreeland R H, Hochstein L I, editors. The biology of halophilic bacteria. Boca Raton, Fla: CRC Press, Inc.; 1993. pp. 163–210. [Google Scholar]
- 243.Russell N J. Membranes as a target for stress adaptation. Int J Food Microbiol. 1995;28:255–261. doi: 10.1016/0168-1605(95)00061-5. [DOI] [PubMed] [Google Scholar]
- 244.Rutkowski C A, Williams L M, Haines T H, Cummins H A. The elasticity of synthetic phospholipid vesicles obtained by photon correlation spectroscopy. Biochemistry. 1991;30:5688–5696. doi: 10.1021/bi00237a008. [DOI] [PubMed] [Google Scholar]
- 245.Sackmann E. Membrane bending energy concept of vesicle- and cell-shapes and shape-transitions. FEBS Lett. 1994;346:3–16. doi: 10.1016/0014-5793(94)00484-6. [DOI] [PubMed] [Google Scholar]
- 246.Saier M H., Jr Computer-aided analyses of transport protein sequences: gleaning evidence concerning function, structure, biogenesis, and evolution. Microbiol Rev. 1994;58:71–93. doi: 10.1128/mr.58.1.71-93.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Sanderson P W, Lis L J, Quinn P J, Williams W P. The Hofmeister effect in relation to membrane lipid phase stability. Biochim Biophys Acta. 1991;1067:43–50. doi: 10.1016/0005-2736(91)90024-3. [DOI] [PubMed] [Google Scholar]
- 248.Santoro M M, Liu Y, Khan S M A, Hou L-X, Bolen D W. Increased thermal stability of proteins in the presence of naturally occurring osmolytes. Biochemistry. 1992;31:5278–5283. doi: 10.1021/bi00138a006. [DOI] [PubMed] [Google Scholar]
- 249.Schleyer M, Schmid R, Bakker E P. Transient, specific and extremely rapid release of osmolytes from growing cells of Escherichia coli K-12 exposed to hypoosmotic shock. Arch Microbiol. 1993;160:424–431. doi: 10.1007/BF00245302. [DOI] [PubMed] [Google Scholar]
- 250.Schlösser A, Hamann A, Bossemeyer D, Schneider E, Bakker E P. NAD+ binding to the Escherichia coli K+-uptake protein TrkA and sequence similarity between TrkA and domains of a family of dehydrogenases suggest a role for NAD+ in bacterial transport. Mol Microbiol. 1993;9:533–543. doi: 10.1111/j.1365-2958.1993.tb01714.x. [DOI] [PubMed] [Google Scholar]
- 251.Schwarz H, Koch A L. Phase and electron microscopic observations of osmotically induced wrinkling and the role of endocytic vesicles in the plasmolysis of the Gram-negative cell wall. Microbiology. 1995;141:3161–3170. doi: 10.1099/13500872-141-12-3161. [DOI] [PubMed] [Google Scholar]
- 252.Seol W, Shatkin A J. Membrane topology model of Escherichia coli α-ketoglutarate permease by PhoA fusion analysis. J Bacteriol. 1993;175:565–567. doi: 10.1128/jb.175.2.565-567.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Siebers A, Altendorf K. Characterization of the phosphorylated intermediate of the K+-translocating Kdp-ATPase from Escherichia coli. J Biol Chem. 1989;264:5831–5838. [PubMed] [Google Scholar]
- 254.Skjerdal O T, Sletta H, Flenstad S G, Josefsen K D, Levine D W, Ellingsen T E. Changes in cell volume, growth and respiration rate in response to hyperosmotic stress of NaCl, sucrose and glutamic acid in Brevibacterium lactofermentum and Corynebacterium glutamicum. Appl Microbiol Biotechnol. 1995;43:1099–1106. [Google Scholar]
- 255.Slauch J M, Silhavy T J. The porin regulon: a paradigm for the two-component regulatory systems. In: Lin E C C, Lynch A S, editors. Regulation of gene expression in Escherichia coli. New York, N.Y: Chapman & Hall; 1996. pp. 383–417. [Google Scholar]
- 256.Smith G M, Smith L T, Gerhardt P N M, Ko R. Solute transport enzymes related to stress tolerance in Listeria monocytogenes: a review. J Food Biochem. 1998;22:269–285. [Google Scholar]
- 257.Somero G N. Adapting to water stress: convergence on common solutions. In: Somero G N, Osmond C B, Bolis C L, editors. Water and life: comparative analysis of water relationships at the organismic, cellular and molecular levels. Berlin, Germany: Springer-Verlag KG; 1992. pp. 3–18. [Google Scholar]
- 258.Steponkus P L, Lynch D V. Freeze-thaw-induced destabilization of the plasma membrane and the effects of cold acclimation. J Bioenerg Biomembr. 1989;21:21–41. doi: 10.1007/BF00762210. [DOI] [PubMed] [Google Scholar]
- 259.Stimmeling K W, Graham J E, Kaenjak A, Wilkinson B J. Evidence for feedback (trans) regulation of, and two systems for, glycine betaine transport by Staphylococcus aureus. Microbiology. 1994;140:3139–3144. doi: 10.1099/13500872-140-11-3139. [DOI] [PubMed] [Google Scholar]
- 260.Stock J B, Rauch B, Roseman S. Periplasmic space in Salmonella typhimurium and Escherichia coli. J Biol Chem. 1977;252:7850–7861. [PubMed] [Google Scholar]
- 261.Strange K. Are all cell volume changes the same? News Physiol Sci. 1994;9:223–228. [Google Scholar]
- 262.Stumpe S, Bakker E P. Requirement of a large K+-uptake capacity and of extracytoplasmic protease activity for protamine resistance of Escherichia coli. Arch Microbiol. 1997;167:126–136. [PubMed] [Google Scholar]
- 263.Stumpe S, Schlösser A, Schleyer M, Bakker E P. K+ circulation across the prokaryotic cell membrane: K+-uptake systems. In: Konings W N, Kaback H R, Lolkema J S, editors. Transport processes in eukaryotic and prokaryotic organisms. Amsterdam, The Netherlands: Elsevier Science B.V.; 1996. pp. 473–499. [Google Scholar]
- 264.Styrvold O B, Falkenberg P, Landfald B, Eshoo M W, Bjornsen T, Strom A R. Selection, mapping, and characterization of osmoregulatory mutants of Escherichia coli blocked in the choline-glycine betaine pathway. J Bacteriol. 1986;165:856–863. doi: 10.1128/jb.165.3.856-863.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Styrvold O B, Strom A R. Synthesis, accumulation, and excretion of trehalose in osmotically stressed Escherichia coli K-12 strains: influence of amber suppressors and function of the periplasmic trehalase. J Bacteriol. 1991;173:1187–1192. doi: 10.1128/jb.173.3.1187-1192.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Sugiura A, Hirokawa K, Nakashima K, Mizuno T. Signal-sensing mechanisms of the putative osmosensor KdpD in Escherichia coli. Mol Microbiol. 1994;14:929–938. doi: 10.1111/j.1365-2958.1994.tb01328.x. [DOI] [PubMed] [Google Scholar]
- 267.Sugiura A, Nakashima K, Tanaka K, Mizuno T. Clarification of the structural and functional features of the osmoregulated kdp operon of Escherichia coli. Mol Microbiol. 1992;6:1769–1776. doi: 10.1111/j.1365-2958.1992.tb01349.x. [DOI] [PubMed] [Google Scholar]
- 268.Sukharev S I, Blount P, Martinac B, Kung C. Mechanosensitive channels of Escherichia coli: the MscL gene, protein, and activities. Annu Rev Physiol. 1997;59:633–657. doi: 10.1146/annurev.physiol.59.1.633. [DOI] [PubMed] [Google Scholar]
- 269.Sutherland L, Cairney J, Elmore M J, Booth I R, Higgins C F. Osmotic regulation of transcription: induction of the proU betaine transport gene is dependent on accumulation of intracellular potassium. J Bacteriol. 1986;168:805–814. doi: 10.1128/jb.168.2.805-814.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Sutton G C, Quinn P J, Russell N J. Changes in phospholipid composition of bacterial membranes prevent formation of non-bilayer phases in vitro and in vivo by high solute concentrations. Biochem Soc Trans. 1990;18:950. doi: 10.1042/bst0180950. [DOI] [PubMed] [Google Scholar]
- 271.Sutton G C, Russell N J, Quinn P J. The effect of salinity on the phase behaviour of total lipid extracts and binary mixtures of the major phospholipids isolated from a moderately halophilic eubacterium. Biochim Biophys Acta. 1991;1061:235–246. doi: 10.1016/0005-2736(91)90289-k. [DOI] [PubMed] [Google Scholar]
- 272.Sweeney T E, Beuchat C A. Limitations of methods of osmometry: measuring the osmolality of biological fluids. Am J Physiol. 1993;264:R469–R480. doi: 10.1152/ajpregu.1993.264.3.R469. . (Editorial.) [DOI] [PubMed] [Google Scholar]
- 273.Taiz L. Plant cell expansion: regulation of cell wall mechanical properties. Annu Rev Plant Physiol. 1984;35:585–657. [Google Scholar]
- 274.Thwaites J J, Mendelson N H. Mechanical behaviour of bacterial cell walls. Adv Microb Physiol. 1991;32:174–221. doi: 10.1016/s0065-2911(08)60008-9. [DOI] [PubMed] [Google Scholar]
- 275.Timasheff S N. The control of protein stability and association by weak interactions with water: how do solvents affect these processes? Annu Rev Biophys Biomol Struct. 1993;22:67–97. doi: 10.1146/annurev.bb.22.060193.000435. [DOI] [PubMed] [Google Scholar]
- 276.Timasheff S N. Preferential interactions of water and cosolvents with proteins. In: Gregory R B, editor. Protein-solvent interactions. New York, N.Y: Marcel Dekker, Inc.; 1994. pp. 445–482. [Google Scholar]
- 277.Tokishita S, Yamada H, Aiba H, Mizuno T. Transmembrane signal transduction and osmoregulation in Escherichia coli. II. The osmotic sensor, EnvZ, located in the isolated cytoplasmic membrane displays its phosphorylation and dephosphorylation abilities as to the activator protein, OmpR. J Biochem. 1990;108:488–493. doi: 10.1093/oxfordjournals.jbchem.a123226. [DOI] [PubMed] [Google Scholar]
- 278.Treuner-Lange A, Kuhn A, Dürre P. The kdp system of Clostridium acetobutylicum: cloning, sequencing, and transcriptional regulation in response to potassium concentration. J Bacteriol. 1997;179:4501–4512. doi: 10.1128/jb.179.14.4501-4512.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278a.Tripet, B., and R. S. Hodges. Personal communication.
- 279.Tsapis A, Kepes A. Transient breakdown of the permeability barrier of the membrane of Escherichia coli upon hypoosmotic shock. Biochim Biophys Acta. 1977;469:1–12. doi: 10.1016/0005-2736(77)90320-0. [DOI] [PubMed] [Google Scholar]
- 280.Tschichholz I, Trüper H G. Fate of compatible solutes during dilution stress in Ectothiorhodospira halochloris. FEMS Microbiol Ecol. 1990;73:181–186. [Google Scholar]
- 281.Valkenburg J A C, Woldringh C. Phase separation between nucleoid and cytoplasm in Escherichia coli as defined by immersive refractometry. J Bacteriol. 1984;160:1151–1157. doi: 10.1128/jb.160.3.1151-1157.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Van Zanten J H. Characterization of vesicles and vesicular dispersions via scattering techniques. In: Rosoff M, editor. Vesicles. New York, N.Y: Marcel Dekker, Inc.; 1996. pp. 239–294. [Google Scholar]
- 283.Verheul A, Glaasker E, Poolman B, Abee T. Betaine and l-carnitine transport by Listeria monocytogenes Scott A in response to osmotic signals. J Bacteriol. 1997;179:6979–6985. doi: 10.1128/jb.179.22.6979-6985.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Verkman A S, van Hoek A N, Ma T, Frigeri A, Skach W R, Mitra A, Tamarappoo B K, Farinas J. Water transport across mammalian cell membranes. Am J Physiol. 1996;270:C12–C30. doi: 10.1152/ajpcell.1996.270.1.C12. [DOI] [PubMed] [Google Scholar]
- 285.Vijaranakul U, Nadakavukaren M J, Bayles D O, Wilkinson B J, Jayaswal R K. Characterization of an NaCl-sensitive Staphylococcus aureus mutant and rescue of the NaCl-sensitive phenotype by glycine betaine but not by other compatible solutes. Appl Environ Microbiol. 1997;63:1889–1897. doi: 10.1128/aem.63.5.1889-1897.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Voelkner P, Puppe W, Altendorf K. Characterization of KdpD protein, the sensor kinase of the K+-translocating Kdp system of Escherichia coli. Eur J Biochem. 1993;217:1019–1026. doi: 10.1111/j.1432-1033.1993.tb18333.x. [DOI] [PubMed] [Google Scholar]
- 287.von Blohn C, Kempf B, Kappes R M, Bremer E. Osmostress response in Bacillus subtilis: characterization of a proline uptake system (OpuE) regulated by high osmolarity and the alternative transcription factor sigma B. Mol Microbiol. 1997;25:175–187. doi: 10.1046/j.1365-2958.1997.4441809.x. [DOI] [PubMed] [Google Scholar]
- 288.Vreeland R H, Mierau B D, Litchfield C D, Martin E L. Relationship of the internal solute composition to the salt tolerance of Halomonas elongata. Can J Microbiol. 1983;29:407–414. [Google Scholar]
- 289.Walderhaug M O, Litwack E D, Epstein W. Wide distribution of homologs of Escherichia coli Kdp K+-ATPase among gram-negative bacteria. J Bacteriol. 1989;171:1192–1195. doi: 10.1128/jb.171.2.1192-1195.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Walsby A E. The pressure relationships of halophilic and non-halophilic prokaryotic cells determined by using gas vesicles as pressure probes. FEMS Microbiol Rev. 1986;39:45–49. [Google Scholar]
- 291.Walter H, Brooks D E. Phase separation in cytoplasm, due to macromolecular crowding, is the basis for microcompartmentation. FEBS Lett. 1995;361:135–139. doi: 10.1016/0014-5793(95)00159-7. [DOI] [PubMed] [Google Scholar]
- 292.Wang A J, Bolen D W. A naturally occurring protective system in urea-rich cells: mechanism of osmolyte protection of proteins against urea denaturation. Biochemistry. 1997;36:9101–9108. doi: 10.1021/bi970247h. [DOI] [PubMed] [Google Scholar]
- 293.Welsh D T, Reed R H, Herbert R A. The role of trehalose in the osmoadaptation of Escherichia coli NCIB 9484: interaction of trehalose, K+ and glutamate during osmoadaptation in continuous culture. J Gen Microbiol. 1991;137:745–750. doi: 10.1099/00221287-137-4-745. [DOI] [PubMed] [Google Scholar]
- 294.Whatmore A M, Reed R H. Determination of turgor pressure in Bacillus subtilis: a possible role for K+ in turgor regulation. J Gen Microbiol. 1990;136:2521–2526. doi: 10.1099/00221287-136-12-2521. [DOI] [PubMed] [Google Scholar]
- 295.Wiggins P M. Role of water in some biological processes. Microbiol Rev. 1990;54:432–449. doi: 10.1128/mr.54.4.432-449.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Wiggins P M. High and low density water in gels. Prog Polym Sci. 1995;20:1121–1163. [Google Scholar]
- 297.Winzor D J, Wills P R. Thermodynamic nonideality and protein solvation. In: Gregory R B, editor. Protein-solvent interactions. New York, N.Y: Marcel Dekker, Inc.; 1994. pp. 483–520. [Google Scholar]
- 298.Woldringh C. Significance of plasmolysis spaces as markers for periseptal annuli and adhesion sites. Mol Microbiol. 1994;14:597–607. doi: 10.1111/j.1365-2958.1994.tb01299.x. [DOI] [PubMed] [Google Scholar]
- 299.Woldringh C, Jensen P R, Westerhoff H V. Structure and partitioning of bacterial DNA: determined by a balance of compaction and expansion forces? FEMS Microbiol Lett. 1995;131:235–242. doi: 10.1111/j.1574-6968.1995.tb07782.x. [DOI] [PubMed] [Google Scholar]
- 300.Wong L S, Johnson M S, Sandberg L B, Taylor B L. Amino acid efflux in response to chemotactic and osmotic signals in Bacillus subtilis. J Bacteriol. 1995;177:4342–4349. doi: 10.1128/jb.177.15.4342-4349.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Xie G F, Timasheff S N. The thermodynamic mechanism of protein stabilization by trehalose. Biophys Chem. 1997;64:25–43. doi: 10.1016/s0301-4622(96)02222-3. [DOI] [PubMed] [Google Scholar]
- 302.Xu J, Johnson R C. Cyclic AMP receptor protein functions as a repressor of the osmotically inducible promoter proP P1 in Escherichia coli. J Bacteriol. 1997;179:2410–2417. doi: 10.1128/jb.179.7.2410-2417.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Yan D L, Ikeda T P, Shauger A E, Kustu S. Glutamate is required to maintain the steady-state potassium pool in Salmonella typhimurium. Proc Natl Acad Sci USA. 1996;93:6527–6531. doi: 10.1073/pnas.93.13.6527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Yancey P H. Compatible and counteracting aspects of organic osmolytes in mammalian kidney cells in vivo and in vitro. In: Somero G N, Osmond C B, Bolis C L, editors. Water and life: comparative analysis of water relationships at the organismic, cellular and molecular levels. Berlin, Germany: Springer-Verlag KG; 1992. pp. 19–32. [Google Scholar]
- 305.Ye R, Verkman A S. Simultaneous optical measurement of osmotic and diffusional water permeability in cells and liposomes. Biochemistry. 1989;28:824–829. doi: 10.1021/bi00428a062. [DOI] [PubMed] [Google Scholar]
- 306.Yim H, Brems R, Villarejo M. Molecular characterization of the promoter of osmY, an rpoS-dependent gene. J Bacteriol. 1994;176:100–107. doi: 10.1128/jb.176.1.100-107.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Zeuthen T. Molecular mechanisms for passive and active transport of water. Int Rev Cytol. 1995;160:99–160. doi: 10.1016/s0074-7696(08)61554-5. [DOI] [PubMed] [Google Scholar]
- 308.Zhang W, Capp M W, Bond J P, Anderson C F, Record M T., Jr Thermodynamic characterization of interactions of native bovine serum albumin with highly excluded (glycine betaine) and moderately accumulated (urea) solutes by a novel application of vapour pressure osmometry. Biochemistry. 1996;35:10506–10516. doi: 10.1021/bi960795f. [DOI] [PubMed] [Google Scholar]
- 309.Zhu J-K, Shi J, Singh U, Wyatt S E, Bressan R A, Hasegawa P M, Carpita N C. Enrichment of vitronectin- and fibronectin-like proteins in NaCl-adapted plant cells and evidence for their involvement in plasma membrane-cell wall adhesion. Plant J. 1993;3:637–646. [PubMed] [Google Scholar]
- 310.Zimmann P, Puppe W, Altendorf K. Membrane topology analysis of the sensor kinase KdpD of Escherichia coli. J Biol Chem. 1995;270:28282–28288. doi: 10.1074/jbc.270.47.28282. [DOI] [PubMed] [Google Scholar]
- 311.Zimmerman S B, Minton A P. Macromolecular crowding: biochemical, biophysical and physiological consequences. Annu Rev Biophys Biomol Struct. 1993;22:27–65. doi: 10.1146/annurev.bb.22.060193.000331. [DOI] [PubMed] [Google Scholar]
- 312.Zimmerman S B, Murphy L D. Macromolecular crowding and the mandatory condensation of DNA in bacteria. FEBS Lett. 1996;390:245–248. doi: 10.1016/0014-5793(96)00725-9. [DOI] [PubMed] [Google Scholar]
- 313.Zimmerman S B, Trach S O. Estimation of macromolecule concentrations and excluded volume effects for the cytoplasm of Escherichia coli. J Mol Biol. 1991;222:599–620. doi: 10.1016/0022-2836(91)90499-v. [DOI] [PubMed] [Google Scholar]