Abstract
A model is proposed in which bending and wrapping of DNA around RNA polymerase causes untwisting of the DNA helix at the RNA polymerase catalytic center to stimulate strand separation prior to initiation. During elongation, DNA bending through the RNA polymerase active site is proposed to lower the energetic barrier to the advance of the transcription bubble. Recent experiments with mammalian RNA polymerase II along with accumulating evidence from studies of Escherichia coli RNA polymerase indicate the importance of DNA bending and wrapping in transcriptional mechanisms. The DNA-wrapping model describes specific roles for general RNA polymerase II transcription factors (TATA-binding protein [TBP], TFIIB, TFIIF, TFIIE, and TFIIH), provides a plausible explanation for preinitiation complex isomerization, suggests mechanisms underlying the synergy between transcriptional activators, and suggests an unforseen role for TBP-associating factors in transcription.
HOMOLOGY AMONG MULTISUBUNIT RNA POLYMERASES, THEIR MECHANISM, AND THEIR TRANSCRIPTION FACTORS
Multisubunit DNA-dependent RNA polymerases are evolutionarily conserved among the three phylogenetic domains of Eubacteria, Archaea, and Eucarya and are found in plant chloroplasts and some viral species. This review concentrates on the evidence for DNA wrapping around RNA polymerases in the eubacterial Escherichia coli system and the eukaryotic human RNA polymerase II system. In Table 1, multisubunit RNA polymerases are compared to RNA polymerase II of brewer’s yeast, Saccharomyces cerevisiae. Human RNA polymerase II is homologous to the yeast enzyme. RNA polymerase II subunit homologues are aligned horizontally in Table 1. Table 2 shows a comparison between the general transcription factors for human RNA polymerase II and similar factors for other multisubunit RNA polymerases.
TABLE 1.
Evolution and peptide functions of multisubunit DNA-dependent RNA polymerasesa
| Pol II (S. cerevisiae) | Start site | DNA | RNA | NTPs | Assembly | Eubacteria (E. coli) | Archaea (Sulfolobus) | Pol I (S. cerevisiae) | Shared Pol I, II, and III | Pol III (S. cerevisiae) | Vaccinia virus |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Catalytic subunits | |||||||||||
| Rpb1/B190/220 (Zn, P) | + | + | + | β′ (Zn) | A′(101) (Zn) | C160 (Zn, P) | rpo147 (Zn) | ||||
| A" (44) | |||||||||||
| Rpb2/B140/150 (Zn) | + | + | + | + | β | B(122) (Zn) | A135 (Zn) | C128 (Zn) | rpo132 (Zn) | ||
| Assembly subunits | |||||||||||
| Rpb3/B35/44 | + | α | D(30) | AC40 | |||||||
| Rpb11/B14/12.5 | + | α | L(10) | AC19 | |||||||
| Shared subunits between Pol I, II, and III | |||||||||||
| Rpb5/B25/27 | H(11.8) | ABC27 | |||||||||
| Rpb6/B18/23 (P) | K(9.7) | ABC23 | |||||||||
| Rpb8/B17/14.5 | ABC14.5 | ||||||||||
| Rpb10/B8.3/10β (Zn) | N(7.5) (Zn) | ABC10β (Zn) | rpo7 (Zn) | ||||||||
| Rpb12/B7.7/10α (Zn) | ABC10α (Zn) | ||||||||||
| Other Pol II subunits: | |||||||||||
| Rpb4/B25/32* | |||||||||||
| Rpb7/B19/16 | E(27) | C25 | |||||||||
| Rpb9/B14/12.6 (Zn)* | + | (SII-like) (Zn) | A12.5 (Zn) | C11 (Zn) | rpo30 (Zn) | ||||||
| Subunits specific to other RNA polymerases | ς | F(12) | A49* | C82 | rpo35 | ||||||
| ω* | G(13.8) | A43 (P) | C53 (P) | rpo22 | |||||||
| I(9.7) | A34.5 (P)* | C34 | rpo19 | ||||||||
| A14 | C31 | rpo18 |
The table is organized around the structure of RNA polymerase II (Rpb/B; Pol II) from S. cerevisiae. The two numbers given (e.g., B190/220) are the sequence- and electrophoresis-derived molecular weights. Subunits are categorized as catalytic subunits, assembly subunits, shared subunits, and RNA polymerase-specific subunits. Homologous subunits from E. coli RNA polymerase, Sulfolobus RNA polymerase, S. cerevisiae RNA polymerase I (A; Pol I), S. cerevisiae RNA polymerase III (C; Pol III), and vaccinia virus RNA polymerase (rpo) are aligned with their RNA polymerase II counterparts. The asterisk indicates that a subunit is dispensable for cell viability under some growth conditions. NTP, nucleoside triphosphate.
TABLE 2.
Comparison of human general transcription factors for RNA polymerase II with factors for other multisubunit RNA polymerases
| Pol II GTFa | Subunits | Descriptiona | Archaeal GTF | Pol I GTF | Pol III GTF |
|---|---|---|---|---|---|
| TBP | TBP | TATA-binding protein, promoter bending (isomerization of the PIC), transcription factor for Pol I, II, and III | TFA/TBP | TBP | TBP |
| TFIIA | TFIIAα | Stabilizes TFIID or TBP binding, coactivator, antirepressor | |||
| TFIIAβ | |||||
| TFIIAγ | |||||
| TFIIB | TFIIB | Promoter binding, promoter bending (isomerization of the PIC), Pol II-TFIIF recruitment | TFB | Brf1/Tds4/Pcf4 (subunit of TFIIIB) | |
| TFIIF | RAP30 | Pol II recruitment, isomerization of the PIC and EC | |||
| RAP74 | |||||
| TFIIE | TFIIE34 | Isomerization of the PIC, TFIIH recruitment | |||
| TFIIE56 | |||||
| TFIIH | Isomerization of the PIC, open-complex formation, CTD kinase | ||||
| XPB | DNA helicase, DNA repair | ||||
| XPD | DNA helicase, DNA repair | ||||
| p62 | |||||
| p52 | |||||
| p44 | |||||
| p34 | |||||
| p36/MAT1 | Interacts with cyclin H/Cdk7 | ||||
| Cyclin H | Cyclin for Cdk7 | ||||
| Cdk7 | CTD kinase |
GTF, general transcription factor; PIC, preinitiation complex; EC, elongation complex; Pol, polymerase.
In eubacteria and archaea, a single multisubunit RNA polymerase is responsible for transcription of the major classes of genes including rRNA, mRNA, and tRNA. In eukaryotic species, three distinct multisubunit RNA polymerases are found within the cell nucleus. RNA polymerase I synthesizes rRNA, RNA polymerase II synthesizes mRNA and some small nuclear RNAs, and RNA polymerase III synthesizes 5S rRNA, tRNAs, and some small nuclear RNAs.
In E. coli, the “core” RNA polymerase is made up of two large subunits, named β and β′, and two smaller α subunits. An ω subunit is also identified in the enzyme, but ω is not required for cell viability or transcription. Core α2ββ′ RNA polymerase supports RNA elongation but is incapable of accurate initiation. Promoter recognition is conferred on core RNA polymerase by binding of a ς factor, and the initiating α2ββ′ς RNA polymerase is referred to as holoenzyme (48, 53). In E. coli, most promoters are recognized by holoenzyme containing the ς70 subunit. These promoters are characterized by −35 (TTGACA) and −10 (TATAAT) consensus sequences separated by a defined spacing (usually 17 bp). ς70 has subdomains that interact directly and simultaneously with the −35 and −10 DNA sequences. A helix-turn-helix motif within ς70 contacts the −35 region.
Structure-function and genetic studies (Fig. 1) of multisubunit RNA polymerases have begun to provide insight into the basic mechanism of RNA synthesis. The two largest subunits of RNA polymerases comprise the catalytic apparatus. The active site includes subdomains of both catalytic subunits. The β/Rpb2 homologue appears to be arranged like a donut, with the N and C termini of this large polypeptide located close to one another. There are two or three metal centers within the catalytic subunits: an Mg2+ ion located at the active site that participates directly in phosphodiester bond formation and, depending on the species, one or two Zn2+ ions located near the closing site of a deep channel. Closing the channel is thought to engulf template DNA in a sliding processivity clamp for elongation, and termination might require breaking open the clamp. The active center of RNA polymerase appears to be buried just behind the clamp, effectively insulating the catalytic apparatus from most accessory transcription factors. Therefore, regulators of initiation and elongation may have to alter the conformation of RNA polymerase and/or template DNA to modulate transcription. Interestingly, during elongation, DNA appears to enter the sliding-clamp channel very close to the channel by which RNA exits from the enzyme.
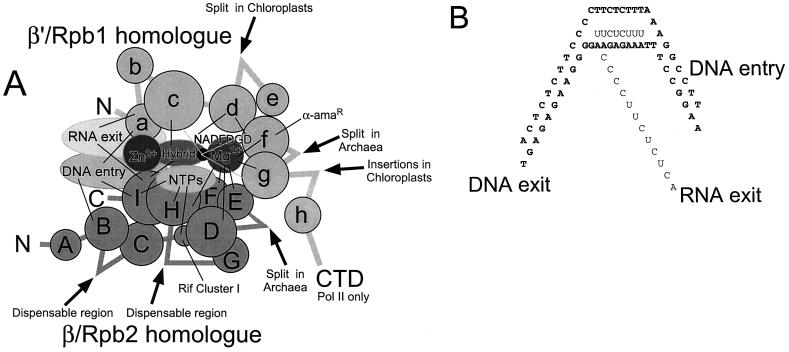
FIG. 1.
Functional domains of the catalytic subunits of multisubunit RNA polymerases, based primarily on experiments with E. coli RNA polymerase. (A) Homology regions within the catalytic subunits that make specific functional interactions. The catalytic center is a modular structure of β and β′ domains. The active-site Mg2+ is held by three clustered aspartic acid residues in region d. The a homology region of β′ holds an atom of Zn2+. The a and I regions are thought to make up the processivity sliding clamp, because the protein channels for DNA entry and RNA exit are within the a and I regions. The J region (which also binds Zn2+), found in most homologues of Rpb2 but not in β, is not shown. “Hybrid” is the RNA-DNA hybrid. The 3′ end of the RNA chain is indicated by a black dot. (B) Bending of template DNA through the RNA polymerase active site. The open complex appears to be about 10 to 12 single-stranded bases. The RNA-DNA hybrid appears to be 8 to 9 bp. See the text for references.
Despite the similarity in their size and names, β and β′ bear no sequence similarity to one another. The β and β′ subunits form the catalytic core of the enzyme. Comparing the E. coli enzyme with multisubunit RNA polymerases from members of the Archaea and Eucarya, β and its homologues contain similar regions A to I (or J) and β′ and its homologues contain similar regions a to h (Fig. 1) (138). The active site of E. coli RNA polymerase is a modular structure that includes portions of the β and β′ subunits (104). An Mg2+ ion is located at the catalytic center, held by a cluster of three aspartic acid residues (underlined) within the d region of β′ (NADFDGD) (166). The active site Mg2+ can be replaced by Fe2+, and hydroxyl radical cleavage can be induced in nearby protein domains. β′ regions d, f, and g and β regions H, F, D, and E are sensitive to Fe2+-induced cleavage, indicating the proximity of these protein sequences to the catalytic center (104, 166). Another approach to mapping protein domains surrounding the active site is to develop cross-links between protein and the ribonucleotide base at the 3′ end of the RNA chain. By using this method, β′ c, f, and g are identified near the active site (105, 141). Homology region c makes additional contacts with the RNA-DNA hybrid, strengthening the argument that c is a part of the enzyme catalytic core (91, 109).
Genetic and biochemical studies with the antibiotic rifampin have led to significant insight into the structure and function of the E. coli RNA polymerase active site. When initiation is from a natural promoter, rifampin appears to allow the formation of the first phosphodiester bond by RNA polymerase but not subsequent bonds (94). If a trinucleotide or longer RNA chain is synthesized before the addition of rifampin, RNA polymerase becomes resistant to inhibition, as if nascent RNA occupies the drug binding site. The binding site for rifampin has been mapped genetically (69, 80) and by cross-linking studies (141) within the D homology region, extending toward the C homology region of the β subunit. Cross-linking studies with hybrid rifampin-nucleotide probes (105) and analogues of the initiating nucleoside triphosphate (141) show that the rifampin binding site must be within about 15 Å of the gamma phosphate of the initiating nucleoside triphosphate. Synthetic rifampin-nucleotide hybrid molecules can be extended by RNA polymerase to up to 8 nucleotides, but these products probably do not maintain normal register with the DNA template (105). Because the antibiotic inhibits the first translocation, rifampin appears to block the 5′ base of an RNA trinucleotide from base-pairing with DNA. Consistent with this idea, rifampin-nucleotide probes cross-link to the −2 and −3 positions of the DNA template (105).
A complementary view of the RNA polymerase active site is obtained by cross-linking studies with analogues of the initiating nucleoside triphosphate as probes. As noted above, the 5′ face of the initiating base is located about 15 Å from bound rifampin (105). The initiating base can also be cross-linked to the β subunit between homology regions C and D (141), a region identified as a hot spot for rifampin-resistant mutants (termed Rifr cluster I) (69, 80). The initiating base can also be cross-linked to the H and I homology regions of β (45). Because of the short range of the cross-linking reagents used, H and I must contribute to a single functional domain located at the active center (103), very close to Rifr cluster I, which is the presumed binding site for rifampin between homology regions C and D (141). The initiating nucleoside triphosphate also cross-links to the ς70 promoter specificity subunit of RNA polymerase (139). The region of ς70 that contacts the RNA polymerase catalytic center is referred to as ς homology region 3, located between ς homology region 2, which contacts the −10 promoter region, and ς homology region 4, which contacts the −35 region. This is an interesting result, because it indicates that ς70 must have an elongated conformation on core RNA polymerase, extending simultaneously over the −35 region and the +1 region and bending back over the −10 region of the template DNA. Only about 150 amino acids (encompassing ς homology regions 3 and 4) (88) are available to make these DNA contacts, which span about 40 bp (140 Å of B-form DNA) (139). Many mutants with mutations in Rifr cluster I and neighboring homology region D have altered transcriptional properties including elongation rate, termination efficiency, and tendency to pause (70, 80). Some mutants have low elongation rates. Some have increased and some have decreased pausing and termination efficiencies. A number of mutants with mutations in the I homology region of β have similar properties (80). Mutations have also been identified in the H and I homology regions that affect the transition from initiation to elongation (103), in a manner reminiscent of rifampin inhibition. These genetic studies strengthen the argument that the D, H, and I homology regions cluster around the RNA-DNA hybrid and the catalytic center, and, as expected, these regions are important for RNA synthesis.
Cysteine-rich ribbons that bind Zn2+ are found within the a region of the β′/Rpb1 homologue (consensus CX2CX6–12CXGHXGX24–37CX2C) and the J region of the Rpb2 homologue (consensus CX2CX8–25CX2C) (β itself lacks this Zn2+ binding sequence) (138). The a-region Zn2+ interaction region and the I homology region are likely to correspond to a “sliding clamp” of RNA polymerase that holds the DNA template during elongation (108). Consistent with this idea, regions a and I contact DNA downstream of the transcription bubble (the DNA entry site), along with region B (109). This tight DNA-protein contact at the DNA entry site renders the transcription elongation complex highly resistant to disruption with salt (108). That β homology regions B and I both cross-link to DNA at the DNA entry site indicates that the N and C termini of β are located in close proximity.
At the DNA entry site, about 9 bp of double-stranded DNA is held tightly by E. coli RNA polymerase (108). Prior to initiation, the open complex has been measured as 11 to 12 single-stranded bases (146, 147). During elongation, the RNA-DNA hybrid is 8 to 9 bp (110, 145). Within the transcription bubble, the I region of β makes tight contacts to the template DNA strand extending from 1 base downstream of the 3′ end of the RNA to about 6 bases upstream (108). RNA from 10 to 23 bases upstream of the 3′ end fills the RNA exit channel. RNA more than 10 bases upstream of the catalytic center is not base-paired to DNA and makes protein contacts with the a and c regions of β′ and the I region of β (109). Because homology regions a and I make up both the DNA entry channel and the RNA exit channel, DNA must enter the enzyme very close to the channel for RNA exit (109). This arrangement cannot be maintained without significant bending of the DNA template through the RNA polymerase catalytic center (Fig. 1).
An α2 dimer nucleates assembly of the α2ββ′ core enzyme by the pathway 2α→α2→α2β→α2ββ′ (54, 61, 62). High-resolution structures are available for the α dimer (68, 167). More detailed images of the catalytic subunits are eagerly awaited.
Many ς factors have been identified and characterized. Different ς factors recognize distinct promoters, so that regulation of binding of a ς factor to the core is a mechanism for altering the pattern of gene expression (48, 53). Regulation of ς-factor activity is a primary control for cell adaptation and for progression of developmental programs, such as the sporulation pathway in Bacillus subtilis. Heat shock-regulated genes are controlled by ς32, which, like many bacterial ς factors, is related by sequence to ς70 but which recognizes a distinct promoter consensus sequence. Another class of ς factor is represented by ς54, which bears no homology to ς70 or ς32 and which functions through a distinct initiation mechanism involving stimulation by bacterial enhancer binding proteins and ATP hydrolysis.
The single RNA polymerase of the domain Archaea resembles the enzyme of eukaryotes in subunit complexity (81). Interestingly, the genes encoding the catalytic subunits of archaeal RNA polymerases have been split into two genes in some species. For instance, Sulfolobus has two β′ homologues, one corresponding to the N-terminal two-thirds of β′ and one corresponding to the C-terminal one-third of β′. The split is between the f and g regions (Fig. 1). Chloroplast RNA polymerase has a different split-gene arrangement, in which its β′ homologue is split into two genes corresponding to the N- and C-terminal halves of β′. The chloroplast split site is found between the d and e regions. In halophilic bacteria and methanobacteria, both the β′ and β homologues are found as split genes. The two genes homologous to β′ resemble the gene organization in Sulfolobus. The two genes homologous to β represent the N- and C-terminal halves, split between the D and E regions. Introducing several of these split sites into E. coli RNA polymerase does not inactivate the enzyme (140). There are also two dispensable regions within β between regions B and C and between regions G and H. Deletion of these regions does not inactivate the E. coli enzyme (6). Split sites, dispensable regions, and insertions may indicate looped structures or boundaries between domains, although regions D and E and regions f and g, which are split in some species of the Archaea, all appear to interact with the enzyme catalytic center.
Archaeal RNA polymerase recognizes promoters by a similar mechanism to eukaryotic RNA polymerase II (Table 2) (81, 120–123). TFA/TBP binds to a TATA recognition element in the upstream promoter region. TFB participates in promoter recognition and RNA polymerase binding. TFA/TBP is homologous to eukaryotic TATA-binding protein (TBP), and TFB is homologous to eukaryotic transcription factor IIB (TFIIB). No correlates to other eukaryotic general transcription factors have been identified in the Archaea.
Eukaryotic RNA polymerases I, II, and III are homologous to one another, to archaeal RNA polymerase, and to eubacterial RNA polymerase (Table 1) (138, 164). Like archaeal RNA polymerase, these enzymes have a complex subunit structure. Yeast RNA polymerase II is used here as an example. This enzyme has 12 subunits termed Rpb1 through Rpb12. Rpb1 is structurally and functionally homologous to β′, Rpb2 is homologous to β, and these subunits make up the catalytic core of the enzyme. Rpb1 binds DNA, as does β′, and Rpb2 binds nucleoside triphosphates, as does β. In metazoan RNA polymerase II, the potent and specific transcriptional inhibitor α-amanitin interacts primarily with the RNA polymerase II Rpb1 subunit and the RNA polymerase III C160 subunit within the f homology region (155). Rpb3 and Rpb11 appear to be distant homologs of E. coli α and are similar to the AC40 and AC19 subunits of RNA polymerases I and III (152, 164). AC40 and AC19 form a heterodimeric unit, but this has not yet been demonstrated for Rpb3 and Rpb11. By analogy to α, these proteins are likely to nucleate the assembly of their respective RNA polymerases, and they are likely to be major structural components of the core RNA polymerase structure. Rpb5, Rpb6, Rpb8, Rpb10, and Rpb12 are also found as subunits of RNA polymerases I and III and are likely to be central components of the RNA polymerase structural core. Eubacteria have apparently evolved to lose the requirement for these core RNA polymerase subunits that are shared by RNA polymerases I, II, and III. Rpb4 and Rpb7 can be removed together from RNA polymerase II. RPB4 is not an essential gene in yeast, although RPB7 is essential. RNA polymerase II isolated from rpb4 deletion mutants lacks Rpb7, but presumably Rpb7 fulfills some essential role in both wild-type and rpb4 mutant strains. Perhaps Rpb7 can associate with RNA polymerase II in the absence of Rpb4, albeit too weakly to copurify with RNA polymerase II (138, 164).
Rpb9 is a nonessential function in yeast; it has a genetic connection to start site selection (50) and an interesting similarity to the RNA cleavage factor TFIIS/SII, within a Zn2+ binding ribbon. TFIIS/SII allows RNA polymerase II to overcome irreversible transcriptional arrest by stimulating the polymerase to cleave the nascent RNA chain within the RNA-DNA hybrid and resume elongation (126). Whether Rpb9 or similar factors, including a Zn2+ ribbon-containing factor from Sulfolobus, the A12.5 subunit of RNA polymerase I, the C11 subunit of RNA polymerase III, and rpo30 of vaccinia virus, play roles in transcript cleavage is not known. Similarities between these factors are limited to the Zn2+ ribbon, and so these proteins cannot clearly be categorized as Rpb9-like or TFIIS/SII-like, and perhaps they are related to both in structure and function.
The C82, C53, C34, and C31 subunits of RNA polymerase III do not have known counterparts in other multisubunit RNA polymerases, but these functions are essential for cell viability. The C82, C34, and C31 subunits can be removed together from RNA polymerase III. C34 binds to the 70-kDa subunit of TFIIIB (162). TFIIIB is made up of three subunits. In addition to TFIIIB-70, TFIIIB contains TBP and the TFIIB homologue Brf1/Tds4/Pcf4 (Table 2). C34, therefore, is thought to be important for specifying that RNA polymerase III will be recruited to appropriate 5S rRNA, tRNA, and small nuclear RNA promoters.
In this review, core promoter elements are considered to be those whose spacing must be maintained relative to the transcriptional start site, because factors interacting with these sites direct the assembly and topology of the RNA polymerase preinitiation complex. Core promoters recognized by Drosophila and vertebrate RNA polymerase II are characterized by multiple elements including a TATA box (around −30), an initiator element (around +1), and a downstream promoter element (DPE) (around +30) (10, 11, 148). Not all promoters have each of these elements, and in some cases a single recognizable element is sufficient for core promoter function with the aid of appropriate upstream or downstream regulatory sites and binding factors. Small nuclear RNA promoters recognized by RNA polymerase II have a proximal sequence element (extending to around −60), which is also a core promoter element (87). It is interesting that core promoter elements for RNA polymerase II can extend for about 90 bp or about 300 Å of linear B-form DNA surrounding the transcriptional start. E. coli core promoters that interact with the α2ββ′ς RNA polymerase holoenzyme similarly extend for about 90 bp of DNA, well beyond the consensus −35 and −10 regions (see below).
In eukaryotes, TBP binds to the TATA box of the promoter (12). TBP is required for accurate transcription by nuclear RNA polymerases I, II, and III and from promoters that contain and those that do not contain a TATA box (Table 2). TBP is found in cell extracts in association with alternate collections of transcription factors that direct it to the appropriate promoter and help to specify the RNA polymerase and general factors for transcription of the downstream gene. For mRNA synthesis, TBP is found in a complex with the TBP-associated factors (TAFIIs). In the human system, for small nuclear RNA synthesis by both RNA polymerases II and III, TBP associates with the small nuclear RNA-activating promoter complex (SNAP-c) (83). SNAP-c can be viewed as an alternate TBP-TAFII/III complex that interacts with the far-upstream core promoter element, termed the proximal sequence element. It is not yet clear how SNAP-c specifically directs RNA polymerase II and RNA polymerase III to distinct snRNA promoters.
At metazoan mRNA promoters, TFIIA and TFIIB can bind to and stabilize the complex between TATA box-containing promoter DNA and TBP (12, 50, 114). TFIIA is generally a requirement for transcription reactions that require TFIID but not for reactions in which the TBP subunit replaces TFIID. TFIIA appears to function as an antirepressor and/or coactivator to counteract negative transcriptional activities of the TAFII subunits of TFIID. The TFIIA-TBP-TFIIB-promoter assembly can bind a preformed complex of RNA polymerase II and TFIIF. One of the major roles of TFIIF is to escort RNA polymerase II to the promoter. TFIIE and TFIIH can then assemble into the complex. TFIIH contains two helicase subunits and a kinase-cyclin complex. At least one of the TFIIH helicases is thought to participate in forming the open complex for initiation. Both TFIIH helicases are additionally important in DNA repair. The TFIIH carboxy-terminal domain (CTD) kinase phosphorylates a repeating heptapeptide repeat (consensus YSPTSPS) found uniquely at the C terminus of the Rpb1 subunit of RNA polymerase II (Fig. 1). This stepwise-assembly model for an RNA polymerase II initiation complex represents the minimal factor-promoter interactions that may be necessary for accurate transcription. In cell extract preparations, however, RNA polymerase II is found in high-molecular-weight holoenzyme forms that contain most of the transcription factors that are necessary for accurate initiation. In many cases, RNA polymerase II holoenzyme preparations will respond to transcriptional regulators in vitro (46).
There does not appear to be a close eukaryotic counterpart to the bacterial sigma factor. ς70 functions as a monomer that must form an extended structure on RNA polymerase to reach along 30 to 40 bp of DNA with which it interacts (48, 53, 139). A helix-turn-helix domain binds the −35 region of the promoter, making contacts within the DNA major groove. A distinct subdomain of ς70 contacts the −10 promoter region. −35 and −10 contacts must be maintained simultaneously by ς70 during initiation. Although −10 regions and eukaryotic TATA boxes are both AT rich, these sequences are contacted by their recognition proteins in different ways. Contacts between ς homology region 2.4 and the −10 (TATAAT) sequence appear to be through the DNA major groove. TBP contacts TATAAA through the minor groove (12, 73, 75), and TBP bears no sequence or structural resemblance to ς70.
In solution (20, 38) and in the preinitiation complex (129), TFIIF is a heterotetramer of two RAP30 and two RAP74 subunits. The RAP30 subunit has some potential sequence similarity to bacterial sigma factors both within a central region that is thought to bind to RNA polymerase II and within a C-terminal region that interacts with DNA (42, 47, 149, 153). Consistent with these sequence alignments, RAP30 binds to E. coli RNA polymerase and ς70 binds to mammalian RNA polymerase II (95). TFIIF suppresses nonspecific DNA binding by RNA polymerase II just as ς70 suppresses nonspecific DNA binding by E. coli RNA polymerase (21, 72). It is possible that there is a very distant evolutionary relationship between RAP30 and ς70, but TFIIF appears, by its α2β2 stoichiometry and its lack of obvious promoter recognition capability, to play distinct roles from ς70 in its initiation pathway. Because of its monomeric structure, ς70 is expected to have a single extended binding surface on RNA polymerase. Both RAP30 and RAP74 subunits have RNA polymerase II interaction regions, and because TFIIF is a heterotetramer, RNA polymerase II may have two distinct interaction sites for RAP30 and two interaction sites for RAP74. Because ς70 functions as a monomer, only one of the RNA polymerase II interaction sites for RAP30 can be conserved with the RNA polymerase interaction site for ς70.
The initiator element of the core promoter appears to be a compound element recognized by several proteins (148). RNA polymerase II must recognize this site directly. Start site selection in yeast is altered by mutations in the Rpb1, Rpb2, and Rpb9 subunits of RNA polymerase II (50). Mutations in the general factor TFIIB also alter start site selection, and so TFIIB is thought to be involved either directly or indirectly in initiator identification. TAFIIs interact with the initiator and surrounding sequences. The initiator can also interact with some sequence-specific DNA binding transcriptional regulators including YY1, USF, and TFII-I (132, 148). The highly variable consensus sequence for the initiator may reflect this multiplicity of interactions. The DPE, initially identified in Drosophila, interacts with TAFIIs (10).
Multisubunit RNA polymerases have related structures and conserved functions. The buried active site of RNA polymerase and the coincidence of the DNA entry and RNA exit channels imply that template DNA must bend as it passes through the enzyme catalytic center. The factors that aid in promoter recognition and accurate initiation appear to be divergent between eubacterial and eukaryotic RNA polymerases. The factors required for archaeal RNA polymerase, on the other hand, appear to be a subset of those required for eukaryotic RNA polymerase II transcription. In this review, we advance the argument that the general mechanisms of transcription initiation and elongation are conserved between eubacterial and eukaryotic multisubunit RNA polymerases.
DNA IS WRAPPED AROUND E. COLI RNA POLYMERASE IN THE PREINITIATION COMPLEX
The α2ββ′ς E. coli RNA polymerase holoenzyme is capable of promoter binding, accurate initiation, and response to diverse transcriptional regulators, some of which alter the conformation of DNA in the upstream promoter region. Architectural changes in DNA may be important to initiate the formation of a DNA loop around RNA polymerase. Wrapping of DNA around E. coli RNA polymerase in the preinitiation complex has been proposed by several laboratories (24, 117, 136, 156), but this important literature has not been prominently reviewed. The primary motivation for a wrapping model is to resolve the paradox shown in Fig. 2. The largest dimension of E. coli RNA polymerase holoenzyme is 160 Å (160 by 95 by 90 Å) (27), but footprints of holoenzyme on promoter DNA in both closed and open complexes extend to 310 Å or more (15, 23, 24, 32, 77, 96, 97, 136, 150). These extensive footprints are best explained by wrapping the DNA around RNA polymerase. Additionally, DNA wrapping can account for several observations from a number of laboratories, including promoter DNA bending (99, 124) and hypersensitivity to enzymatic and chemical cleavage reagents (23, 24, 32, 96).
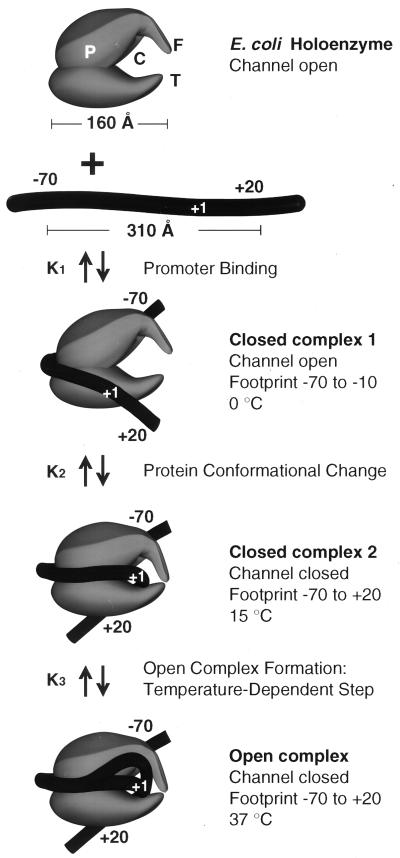
FIG. 2.
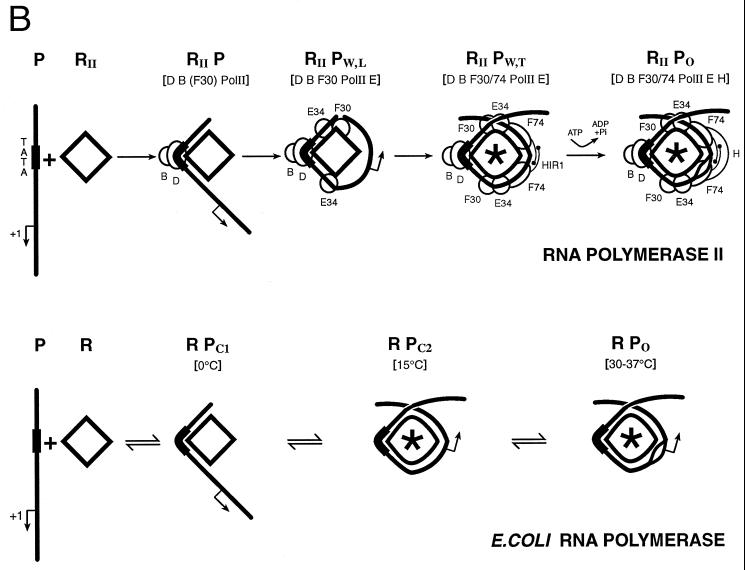
A three-step model for initiation by E. coli RNA polymerase. The holoenzyme measures 160 Å in its longest dimension. The footprint of holoenzyme on DNA, however, measures over 300 Å, indicating that promoter DNA must wrap around RNA polymerase. Isomerization is a progression of conformational changes in DNA and protein accompanying each step in the reaction pathway. A major change in protein conformation, the closing of the sliding clamp, is thought to occur during formation of closed complex II. This step results in substantial DNA unwinding but not DNA strand separation. Features of the holoenzyme structure include a palm (P), a channel (C), fingers (F), and a thumb (T). Closing of the fingers and thumb forms the sliding clamp for elongation.
Initiation of transcription by holoenzyme appears to occur by at least a three-step mechanism (Fig. 2) described as follows:
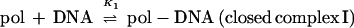 |
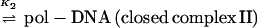 |
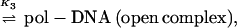 |
where K1 = k1/k−1 (the ratio of the forward and reverse rate constants), K2 = k2/k−2, and K3 = k3/k−3 (9, 131, 150). Rapid equilibrium promoter binding by RNA polymerase (pol), represented by K1, results in the formation of closed complex I. At 0°C, holoenzyme forms a complex on the promoter that is inferred to be similar to the closed complex I kinetic intermediate. The 0°C complex has a shorter footprint than that observed for closed complex II and the open complex (23, 77, 96, 97, 150). The footprint of closed complex I extends far upstream of the transcriptional start site, between about −70 or −50 to −10, depending on the promoter. Because protection of the DNA is not observed in the region near the transcriptional start site in closed complex I, initial promoter binding (K1) appears to require only contacts with DNA upstream of +1. Interestingly, DNA appears to be bent in these initial complexes, because footprints reveal regions of DNase I hypersensitivity in the vicinity of the −35 region (between −50 and −20, depending on the promoter and the ς factor) (23, 32, 96, 150). At about 15°C, closed complex II predominates (23, 96, 97, 150). Conversion of closed complex I to closed complex II (k2) is characterized by extension of the footprint from −70 or −50 to +20 (23, 24, 96, 97, 150) and by a significant protein conformational change (131). Record and coworkers postulate this conformational change in holoenzyme based on the temperature dependence of k2 and the inferred heat capacity change of this transition. The observed transition is most consistent with a change in holoenzyme conformation that reduces the exposed hydrophobic surface of the protein (131) and could be attributable in part to the closing of the RNA polymerase sliding clamp (108). At higher temperatures such as 30 and 37°C, the DNA strands separate to form the open complex (23, 96, 97, 150). The footprint of the open complex is very similar to that of closed complex II, indicating that the DNA wrap and both upstream and downstream DNA contacts are largely maintained (23, 96, 97, 150). One notable characteristic of the footprints of both closed complex II and the open complex is that the region of helix surrounding the transcriptional start site is strongly protected, as if this region of the DNA were buried in protein, perhaps the sliding clamp (positions −5 to +15) (23, 24, 96, 97, 136, 150). At specific positions within the regions of protein-DNA contact, however, subtle differences in DNase I protection and hypersensitivity accompany each step in complex assembly and isomerization. These changes in DNA reactivity have been used to monitor progression between intermediates as a function of temperature. Formation of the open complex (k3), for instance, is a highly temperature-dependent process, such that if the extent of DNA melting is plotted as a function of temperature, the resulting plot resembles a DNA-melting curve with the midpoint of the transition around 20 to 25°C (9, 23, 96, 131, 150).
The protein envelope structure of various RNA polymerases has been determined by electron crystallography of two-dimensional microcrystalline arrays formed on mica sheets and stained with heavy metals (4, 26, 27, 117). Prominent common features of these structures are finger-like projections in RNA polymerase that close to form a channel large enough to accommodate DNA. For E. coli RNA polymerase, the channel is open in the initiating holoenzyme and closed in the elongating core enzyme, leading to the suggestion that DNA penetrates the channel, which closes around the template during elongation to prevent termination (27, 117). In this review, the structure of RNA polymerase is described as a “hand” with a “palm,” “thumb,” and “finger” (Fig. 2). Single-subunit polymerases, including E. coli DNA polymerase I, human immunodeficiency virus type 1 reverse transcriptase, T7 RNA polymerase, and rat DNA polymerase β, have a palm, thumb, and finger arrangement (76, 151), but these enzymes are not homologous to multisubunit RNA polymerases in which the active site is made up of a modular arrangement of the two largest subunits rather than a single subunit (Fig. 1) (104). On E. coli RNA polymerase, the DNA template is suggested to run across the palm and through the channel, grasped by the thumb and finger, thought to comprise the sliding clamp (Fig. 2). The direction of transcription points along the palm toward the channel. The active site for RNA synthesis may be close to the channel between the thumb and finger (Fig. 1 and 2). This molecular structure is similar in yeast RNA polymerase II, which can display the thumb and finger either apart or together, depending on the presence of RNA polymerase II subunits Rpb4 and Rpb7 and the general transcription factor TFIIE in the complex (4, 26).
According to Darst and coworkers (117), in closed complex I the thumb and finger are apart and the DNA does not fill the active-site channel. This explains why the DNA is exposed to modification and cleavage in the region of closed complex I from −5 to +20. In closed complex II and the open complex, the thumb and finger grasp the DNA, protecting the helix from cleavage agents, particularly in the region from −5 to +15. As downstream DNA contacts develop on the pathway to initiation, the upstream contacts are maintained, so that in closed complex II and the open complex, RNA polymerase may contact as many as 90 bp of DNA (−70 to +20) (15, 23, 24, 32, 53, 77, 96, 97, 136, 150). Protection is even more extensive on some promoters (24).
In both the closed complex and the open complex, the topology of the promoter is altered to introduce 1.7 negative supercoils (1). The extent of the topological change in formation of closed complex I was not determined in this study, but this result shows that significant helix unwinding is present in closed complex II, prior to open-complex formation. Upstream promoter DNA may be partially wrapped around RNA polymerase in closed complex I, with a significant DNA bend near the −35 region of the promoter. In closed complex II, the DNA wrapping must become more extensive. In particular, there is a conformational change in the holoenzyme that closes the hand around the DNA and introduces another major DNA bend near the transcriptional start site. This conformational change has the effect of developing significant strain on the DNA helix around +1. Helix unwinding developed in closed complex II is rapidly converted to the open complex if the temperature is high enough to support k3.
DNA WRAPPING AS A MECHANISM OF TRANSCRIPTIONAL CONTROL IN PROKARYOTES
The α subunits of RNA polymerase are important for interactions with upstream promoter DNA and regulators. The α structure comprises an amino-terminal domain (α-NTD) and a CTD (α-CTD) domain separated by a flexible central region. Some very strong promoters have a DNA sequence called an “up” element, upstream of the −35 region, that binds the α-CTD (40).
A DNA-wrapping model has been proposed to describe catabolite gene activator protein (CAP)-cyclic AMP (cAMP)-mediated activation from the lac and gal promoters (25). CAP binding sites can be located at variable positions relative to the transcriptional start site, but the face of the helix on which CAP lies relative to RNA polymerase must be maintained for activation. Maintaining the orientation of CAP relative to RNA polymerase is thought to serve two purposes: (i) to allow for specific CAP-RNA polymerase interactions and (ii) to maintain the directionality of the CAP-induced bend in DNA toward RNA polymerase. In the lactose promoter, the CAP binding site is centered at position −61.5. On the galactose P1 promoter, the CAP binding site is centered at position −41.5. Moving the CAP binding site to position −51.5 allows for activation, but moving it to position −46.5, on the opposite face of the DNA helix, does not. On the malT promoter, the CAP binding site is at position −70.5 (35), and on the uhpT promoter, the site is centered at −103.5 (98). CAP-cAMP bends DNA by 90° (137), bending promoter DNA back toward RNA polymerase. On the lac promoter, CAP-cAMP (centered at position −61.5) makes specific contacts with activation regions on the α-CTD. On gal P1 and related synthetic promoters, CAP-cAMP (centered at position −41.5) contacts the same region of the α-CTD and a distinct region on the α-NTD (13, 107, 134, 169).
DNA bending and α-binding by CAP-cAMP might contribute to promoter strength in two ways: (i) by enhancing RNA polymerase binding to the promoter (K1) and (ii) by promoting isomerization (k2). Because initial binding of holoenzyme induces DNA bending in the upstream promoter region, DNA bending by CAP-cAMP could stimulate binding (K1). RNA polymerase binding is also expected to be stimulated by interactions between CAP-cAMP and the α subunit of RNA polymerase. According to the DNA-wrapping model, however, appropriately phased bends in upstream DNA initiate DNA wrapping around RNA polymerase, so that DNA bending in the upstream promoter region can also contribute to isomerization (k2). At class II CAP-dependent promoters such as gal P1, CAP-cAMP is observed to stimulate both K1 and k2 (25, 82, 107). This observation is consistent with predictions of the DNA-wrapping model, because bending and wrapping of upstream promoter DNA is expected to affect isomerization of the preinitiation complex. Sequence-induced bends in gal P1 promoter DNA upstream of the CAP site (around positions −84.5, −74, and −63) also accelerate k2 (82). At class I CAP-dependent promoters such as wild-type lac, CAP-cAMP is observed only to increase K1 (25), as if RNA polymerase recruitment to the promoter is the only step affected by CAP-cAMP binding and DNA bending at this promoter. This result suggests that DNA bending alone is not sufficient to promote isomerization. To accelerate isomerization, bends in the upstream promoter region may require the correct directionality and spacing to alter DNA conformation in the region surrounding +1. Formation of the appropriate DNA loop around holoenzyme requires bending and wrapping of the DNA, facilitated by a multitude of protein-protein and protein-DNA contacts. In the DNA-wrapping model, RNA polymerase binding and isomerization of the DNA are linked processes, explaining why regulators can affect isomerization even though they bind to and restructure DNA far upstream of the transcriptional start.
The relationship between DNA wrapping and CAP-cAMP stimulation of transcription is perhaps most clearly demonstrated by experiments with the malT promoter, at which CAP-cAMP binds to a DNA region centered around position −70.5 (35). In that study, a UV laser was used to monitor DNA-protein contacts during formation of transcription intermediates. At the malT promoter, a CAP-cAMP-dependent cross-link between upstream DNA at position −94 and RNA polymerase was strongly correlated with the promoter escape step of the initiation process. DNA bending and straining by CAP-cAMP in the upstream promoter region therefore appear to cause DNA to wrap around RNA polymerase. Surprisingly, isomerization of the DNA in the upstream promoter region affects a very late stage of the initiation process, by exerting an effect on the early progression of the open complex. In this case, a contact between far upstream DNA and RNA polymerase affects events downstream of the transcriptional start site.
The bacteriophage λ cI repressor binds to the rightward λ operators oR1 and oR2 and activates transcription from neighboring pRM (promoter for repressor maintenance). The λ repressor interacts with the ς70 subunit of RNA polymerase and activates transcription from pRM by stimulating k2 (52). Protein-protein interactions between the C-terminal domains of λ repressor molecules bound at the two operator sites are likely to induce conformational changes in DNA just upstream of pRM, and these changes in DNA topology may induce DNA wrapping around RNA polymerase to stimulate isomerization. Other factors that bend or wrap DNA in the upstream promoter region include integration host factor (44) and FIS (37, 102). These factors may stimulate transcription from target promoters by inducing or stabilizing a DNA wrap around holoenzyme, as suggested for CAP and λ repressor.
The suggestion that DNA wrapping is a key feature of the initiation mechanism for E. coli RNA polymerase has been made by several investigators (24, 117, 136, 156). Based on recent experimental findings in the mammalian RNA polymerase II system, this dynamic mechanism in which RNA polymerase causes DNA to bend sharply in the upstream promoter region and again downstream of +1 to isomerize and then open the helix appears to be a general feature of RNA polymerase mechanisms.
DNA IS WRAPPED AROUND MAMMALIAN RNA POLYMERASE II IN THE PREINITIATION COMPLEX
As discussed above, binding of TBP within the DNA minor groove at the TATA sequence induces a bend of about 80° in promoter DNA (73, 75). This bend is stabilized by the binding of TFIIB (79, 106). To bring RNA polymerase II into the TBP-TFIIB-promoter complex generally requires TFIIF. TFIIE cooperates with TFIIF in the assembly process and contributes to complex stability. Both TFIIF and TFIIE are α2β2 heterotetramers in solution and appear to assemble in this form in the preinitiation complex (129). As discussed below, there are potential parallels between TFIIF and TFIIE functions in DNA wrapping. In addition to this postulated role for TFIIF and TFIIE in preinitiation complex assembly and isomerization, TFIIE helps to bring TFIIH into the preinitiation complex.
Evidence for wrapping of DNA around RNA polymerase II in the preinitiation complex is emerging from site-specific DNA-protein photo-cross-linking studies by the groups of Ebright and Reinberg (74, 79), and Coulombe, Greenblatt, and Burton (22, 39, 129, 130) (Fig. 3). Both groups identified an extensive cross-linking “footprint” of the RNA polymerase II preinitiation complex on DNA. Both groups have also analyzed distinct intermediate complexes by using cross-linking reagents located at different positions on the DNA, and their results differ in some particulars. However, they both propose that extensive bending of DNA occurs in the preinitiation complex.
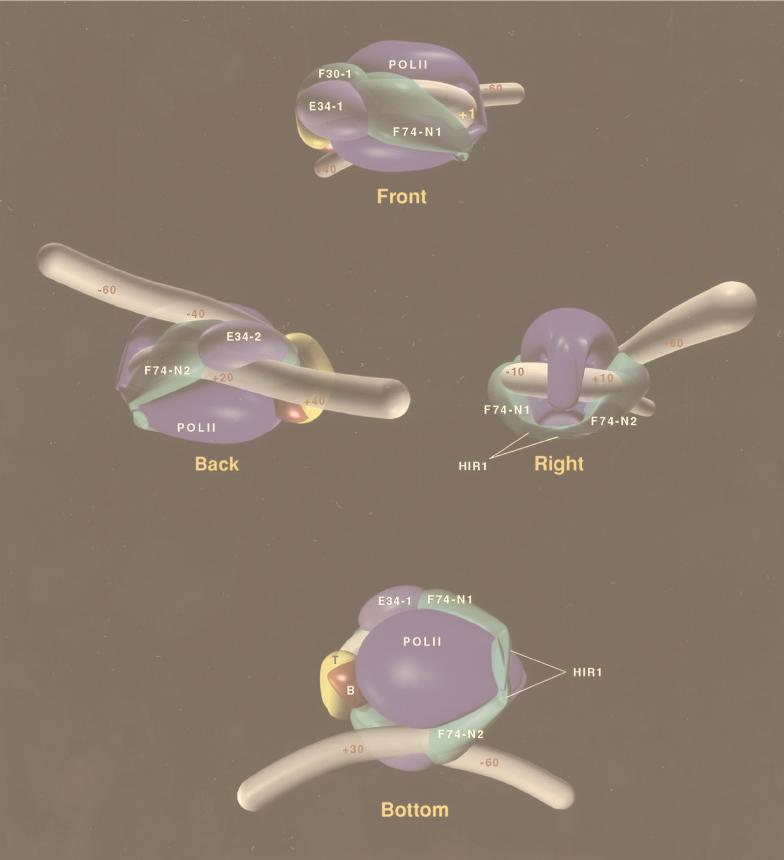
FIG. 3.
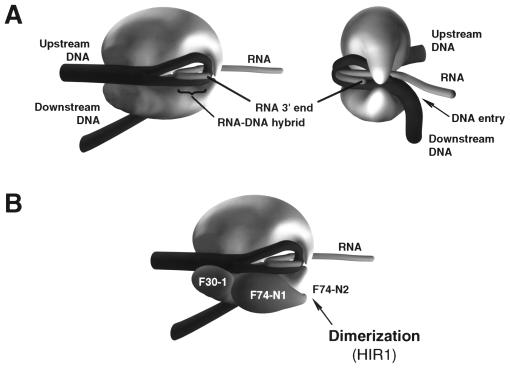
DNA wrapping in the RNA polymerase II preinitiation complex. TBP is in yellow; B, TFIIB (red); F30, TFIIF RAP30 subunit (green); F74-N, TFIIF RAP74 subunit (amino acids 1 to 205) (green); E34, TFIIE 34-kDa subunit (clear blue); Pol II, RNA polymerase II (opaque blue); HIR1, RAP74 dimerization region (amino acids 172 to 205) (129). Models of transcriptional mechanisms can be viewed at http://labcoulombe.usherb.ca.
Ebright and colleagues performed cross-linking studies of the TBP-TFIIB-RNA polymerase II-TFIIF complex (74). In these studies, TBP was used instead of TFIID to eliminate problems in interpretation that would result from cross-linking of the TAFIIs and from architectural changes in DNA induced by the TAFIIs (111). To place site-specific cross-linking reagents into the DNA, Ebright and coworkers developed a novel method in which a thiophosphate within a DNA primer is used to attach a 9.7-Å arm linked to an azido group. UV light causes covalent attachment between the azido group and nearby proteins. Using an extensive set of primers, these investigators positioned cross-linking arms at every other phosphate group on the two DNA strands of the adenovirus major late promoter from positions −55 to +25. They then combined their cross-linking results with structural data for the protein envelope of yeast RNA polymerase II and the crystal structures of TBP and TFIIB bound to promoter DNA. As a result, they postulated significant DNA wrapping within the complex with two major DNA bends: one at the TATA box and one centered around +1. Their model accounts for architectural changes introduced into the DNA by binding of TBP and TFIIB at the TATA box, for envelopment of the DNA around the transcriptional start site (as also observed in E. coli RNA polymerase-promoter complexes), and for unwinding of the DNA helix near the site of strand opening.
Coulombe and colleagues have studied the distribution of protein factors within the TBP-TFIIB-RNA polymerase II-TFIIF-TFIIE complex, using TBP in place of TFIID and including TFIIE (in contrast to Ebright and coworkers) (129). They used an azido-substituted deoxyuridine photo-cross-linking probe that replaces thymidine residues at specific DNA positions (8). By using this method, the photo-cross-linking agent protrudes from specific thymidine base positions into the DNA major groove. This technique has the limitation that cross-linking can be done only from certain DNA positions without modifying the nucleotide sequence and so fewer positions are analyzed. Where multiple thymidines occur in a sequence, multiple cross-linkers are inserted, limiting resolution but potentially increasing the strength of the cross-linking signal. Whether there is an advantage to having the cross-linking reagent located on the phosphate backbone or at a thymidine base will depend on the precise disposition of protein targets surrounding a particular probe.
The first indication of a wrapped DNA structure within the RNA polymerase II initiation complex (Fig. 3) arose from the length of the photo-cross-linking “footprint” on promoter DNA. RNA polymerase II contacts the adenovirus major late promoter between positions −40 and +13 (74, 129), representing contact along 180 Å of B-form DNA. The longest dimension of RNA polymerase II is 140 Å (140 by 140 by 110 Å) (26), and DNA bending is necessary to accommodate such extensive contact. TFIIF subunits interact with DNA between positions −56 and +32, representing about 300 Å of B-form DNA. This extensive contact cannot reasonably be explained except by DNA wrapping. Also, electron micrographs of preinitiation complexes allow direct visualization of DNA wrapping in the complex (39, 74). These studies indicate that 50 ± 21 bp (160 Å) of DNA is consumed within the structure (39, 74), fully supporting the notion of tight DNA bending and wrapping.
If promoter DNA must be bent into a looped configuration for initiation, upstream and downstream promoter DNA segments will be found to closely approach one another near the approximate cross-point of the loop. Close approach of upstream and downstream DNA segments on the adenovirus major late promoter is strongly supported by the pattern of photo-cross-linking of RAP30, RAP74, and TFIIE-34 (129). Because both TFIIF and TFIIE assemble in the preinitiation complex as heterotetramers (129), two molecules of each subunit are present in the preinitiation complex and one molecule each of RAP30, RAP74, and TFIIE-34 appears to be located very near to the approximate cross-point of the looped DNA structure (Fig. 3). The second subunit of the RAP30, RAP74, and TFIIE-34 dimers is located close to DNA between the TATA element and the +1 position (39, 74, 129, 130). The evidence for location of general factors near to the DNA cross-point comes from the development of DNA contacts between a single molecule each of RAP74 and RAP30 upstream of TATA and simultaneously downstream of +1 (between positions +13 and +34). A single molecule of TFIIE-34 may also be found near the DNA cross-point.
In studies of the TBP-TFIIB-RNA polymerase II-TFIIF-TFIIE complex, RAP74 was found to be essential to tighten a DNA loop around RNA polymerase II (129). The deletion mutant RAP74(1–172) enters into and supports the formation of a stable preinitiation complex but has very low activity in transcription (84). In photo-cross-linking studies, RAP74(1–172) is found to occupy the core promoter region (positions −15 and −5), but this protein is not found near the DNA cross-point (upstream positions −56/−61, −45/−48, and −39/−40; downstream positions +13, +16/+18, and +26), at positions occupied by RAP74(1–205) and RAP74(1–517, wt) (129). This result indicates that in a complex containing wild-type TFIIF, a single molecule of RAP74 occupies the core promoter region and a second is located near the DNA cross-point. In the absence of RAP74, RAP30 is found near the DNA cross-point (upstream positions −56/−61 and −39/−40 and downstream positions +13, +16/+18, +26, and +32/+34) but not within the core promoter (position −19). In the presence of RAP74(1–172) and RAP74(1–517, wt), however, both molecules of RAP30 are detected. Photo-cross-linking of RAP30 therefore indicates that the promoter is in a “loose” looped conformation, even in the absence of RAP74, and that distinct molecules of RAP30 occupy the core promoter region and the DNA cross-point. The RAP74(1–172) mutant supports a complex in which TFIIE-34 does not form appropriate downstream promoter contacts (positions +13 and +26), although in this context, TFIIE-34 does contact DNA in the upstream promoter region (position −39/−40) and in the core promoter region (position −2/−13). The transcriptional defect of RAP74(1–172) may therefore be attributable to its inability to tightly wrap DNA around the preinitiation complex, as indicated by its failure to appropriately locate TFIIE-34 near to the DNA cross-point. In the complex containing wild-type TFIIF, the TFIIE-34 contacts made with DNA at upstream (position −39/−40) and downstream (positions +13 and +26) positions are thought to be attributable to a TFIIE-34 molecule located near the DNA cross-point (129). In addition, RNA polymerase II subunits Rpb1 and Rpb2 make inappropriate contacts with the promoter in the absence of RAP74 or in the presence of the defective mutant RAP74(1–172). Rpb1 fails to make specific contacts in the core promoter region (positions −19 and +1), and Rpb2 fails to make contacts in the upstream promoter region (position −39/−40) and in the downstream promoter region (position +13), indications that DNA is not tightly wrapped around RNA polymerase II unless RAP74 is present in the complex.
Tight wrapping of DNA in the preinitiation complex also appears to depend on the presence of TFIIE. In studies of the TBP-TFIIB-TFIIF-RNA polymerase II-promoter complex lacking TFIIE, Kim et al. (74) did not observe extensive contact of general factors downstream of +1. Only inclusion of TFIIE in the complex revealed these downstream contacts (129), as if the DNA is not tightly wrapped in the absence of TFIIE. As with RAP74, the effect of TFIIE on isomerization is indicated by enhancement and induction of specific TFIIF-DNA contacts within the core promoter region (39, 130). From photo-cross-linking studies, therefore, TFIIF and TFIIE appear to have cooperative functions in DNA wrapping and complex isomerization.
In transcription systems with supercoiled or premelted templates, the TBP-TFIIB-RNA polymerase II-RAP30 complex is functional in accurate initiation (59, 115, 116). In this minimal system, addition of RAP74 and further addition of TFIIE stimulate open-complex formation in the absence of TFIIH and an ATP cofactor. Photo-cross-linking studies indicate that stimulation occurs because RAP74 and TFIIE promote complex isomerization and helix unwinding. On a linear DNA template, assembly of TFIIH into the complex enhances and induces specific RNA polymerase II-DNA contacts within the core promoter (129), suggesting that TFIIH further helps to tighten the DNA wrap around RNA polymerase II.
ISOMERIZATION OF RNA POLYMERASE AND PROMOTER DNA AS A GENERAL TRANSCRIPTIONAL MECHANISM
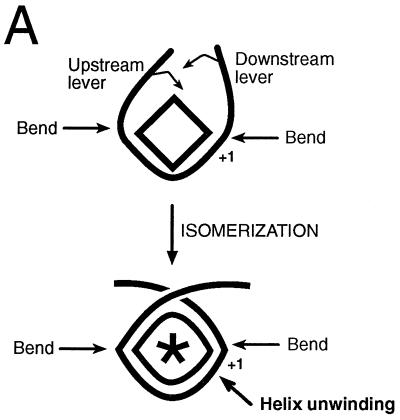
Isomerization is a progression of specific conformational changes in protein and DNA that accompanies topological untwisting of the helix and, finally, separation of promoter DNA strands for initiation. In this review, we argue that isomerization involves bending of promoter DNA in the upstream promoter region, bending of DNA through the RNA polymerase active site, and a protein conformational change. The change in RNA polymerase conformation involves closing of a channel, the sliding clamp, to surround the template within the polymerase active site. Tightening of the DNA wrap between two DNA bends results in helix unwinding (Fig. 4A).
FIG. 4.
Isomerization of transcription complexes. (A) DNA wrapping around RNA polymerase in the preinitiation complex. The diamond shape represents RNA polymerase prior to isomerization. The football shape containing an asterisk represents the isomerized holoenzyme. A DNA bend in the upstream promoter region and another bend around +1 coupled with a tight DNA wrap around RNA polymerase leads to helix unwinding. (B) Comparison of the E. coli RNA polymerase three-step mechanism and the mammalian RNA polymerase II stepwise-assembly model. Proposed similarities between these mechanisms include DNA bending in the upstream promoter region, DNA bending just downstream of +1, wrapping of DNA around RNA polymerase, and isomerization of RNA polymerase and DNA on the pathway to formation of the open complex. RII, RNA polymerase II (Pol II); R, RNA polymerase; P, promoter DNA; D, TBP; W, wrapped; L, loose; T, taut; O, open; C1, closed complex I; C2, closed complex II; HIR1, a RAP74 dimerization region (amino acids 172 to 205); ∗, conformational change in RNA polymerase. (C) The TBP-TAFII complex and the TBP-TFIIB-RNA polymerase II-TFIIF-TFIIE complex (lacking TAFIIs) (RIIPW,T in panel B) appear to isomerize promoter DNA into a similar conformation.
How does DNA wrapping promote DNA unwinding, and what is the relationship between the DNA bend in the upstream promoter region and the DNA bend just downstream of +1? We propose that the two DNA bends are spaced in such a manner that as the DNA wrap is tightened, the short stretch of helix between the two bends is forced to unwind. This process of helix unwinding prior to the separation of the DNA strands appears to be a critical step in isomerization (Fig. 2 and 4A). In effect, DNA upstream and downstream of the two DNA bends acts as two levers to pry the helix into the unwound conformation that precedes open-complex formation. Transcriptional regulators can affect this mechanism by pulling the upstream DNA lever, the downstream lever, or both.
In transcription by mammalian RNA polymerase II, TBP and TFIIB initiate wrapping in the upstream promoter region by bending the DNA by 80° at the TATA box. To isomerize the complex, however, the downstream bend must be formed and the DNA wrap must be tightened both upstream of the TATA box and downstream of +1. The TFIIE and TFIIF heterotetramers appear to play key roles in pulling the upstream DNA lever, forming the DNA bend around +1, and pulling the downstream DNA lever (39, 129). DNA wrapping may create just enough strand separation to allow the DNA helicase of TFIIH access to single-stranded DNA to catalyze open-complex formation in the presence of ATP.
In E. coli transcription, the precise position of the upstream DNA bend appears to vary between promoters and with different ς factors. Regulators λ cI and CAP-cAMP, rRNA promoter up elements, the α-CTD, and architectural factors FIS and IHF appear to be involved in forming DNA bends upstream of the promoter and pulling the upstream transcriptional lever to wrap DNA around RNA polymerase.
Isomerization appears to proceed by very similar pathways for mammalian RNA polymerase II and E. coli RNA polymerase (Fig. 4B). Upstream of the TATA element, RNA polymerase II and TFIIF approach DNA at positions −39/−40 and −56/−61 (129). These upstream promoter contacts are analogous to contacts established by E. coli RNA polymerase in closed complex I and maintained in closed complex II and the open complex (Fig. 2). The bend induced in promoter DNA by binding of TBP and TFIIB is reminiscent of the DNase I hypersensitivity sites induced in bacterial promoters in the formation of closed complex I. DNA bending by E. coli CAP-cAMP, particularly at promoters such as gal P1, where CAP binds centered around position −41.5, may also be analogous to TBP-TFIIB-promoter bending. The contacts of RNA polymerase II and TFIIF downstream of +1 resemble those made by E. coli RNA polymerase after isomerization in closed complex II and the open complex. By further analogy to E. coli, the TBP-TFIIB-RNA polymerase II-RAP30-TFIIE-promoter complex (lacking RAP74) resembles an incompletely isomerized intermediate between closed complexes I and II. The complex containing both RAP30 and RAP74 more closely resembles a fully isomerized closed complex II (Fig. 4B). Addition of TFIIH to the complex tightens the DNA wrap (129). TBP and TFIIB induce wrapping by bending the template in the upstream promoter region. TFIIF, TFIIE, and TFIIH help to complete and tighten the wrapped DNA structure. All of the general factors therefore contribute to isomerization of the preinitiation complex.
Similar to E. coli RNA polymerase (26, 117), the fingers and thumb of RNA polymerase II appear to be closed around promoter DNA in the preinitiation complex, as indicated by full protection of both DNA strands in the region surrounding +1 from digestion with DNase I (74). The notion of the closing channel is also supported because the finger and thumb structures of yeast RNA polymerase II can be imaged in both the open and closed configurations (4, 26). As noted by Darst and coworkers (117), the closing of the RNA polymerase channel around DNA is likely to represent part of the protein conformational change that accompanies isomerization (39, 129–131).
E. coli core RNA polymerase forms a holoenzyme by binding a ς factor. Mammalian RNA polymerase II is found in complex holoenzyme forms containing core RNA polymerase II, most of the known general initiation factors, and additional polypeptides (46). These holoenzyme preparations support accurate initiation from promoters in vitro with minimal supplementation and respond appropriately to some transcriptional regulators. Uncharacterized protein components of the holoenzyme are likely to play important roles in modifying DNA wrapping and bending around RNA polymerase II to regulate initiation and elongation.
FUNCTIONS OF TAFIIS IN TRANSCRIPTION BY RNA POLYMERASE II
TFIID is a large protein complex of TBP and 8 to 11 TAFIIs. Several excellent reviews and many manuscripts recently have been published describing the potential roles of the TAFIIs, and some of this large body of literature is summarized in Table 3 (12, 51, 58, 83, 154, 158). TAFIIs interact with transcriptional regulators as both coactivators and corepressors. In addition to these regulatory functions, TAFIIs interact directly with promoter DNA upstream of the TATA box, within the initiator region, and at the DPE, which is found in some promoters. Consistent with their role in binding core promoter DNA sequences, TAFIIs play important roles in promoter selection by RNA polymerase II. TAFIIs are particularly important for recognition of several genes that encode cyclins and other genes that are important for cell cycle progression, and this requirement may be the basis for the cell cycle arrest phenotypes associated with particular TAFII mutants. A subset of the TAFIIs have significant sequence similarity to core histones H4, H3, and H2B and may comprise a structure similar to a core histone octamer within the TFIID complex (58). The TAFIIs make many contacts to the general transcription factors. Additionally, human hTAFII250/230 and its homologue from Drosophila, dTAFII250, have catalytic activities including histone acetyltransferase activity and protein kinase activity.
TABLE 3.
Comparison of yeast, Drosophila, and human TAFIIsa
| Yeast | Drosophila | Human | Comments |
|---|---|---|---|
| yTBP (27) | dTBP (38) | hTBP (43) | TBP, contacts NC2 (Dr1-DRAP1), contacts TFIIA and TFIIB |
| TAFs | |||
| yTAF145/130 (121) | dTAF250 | hTAF250/230 | Cell cycle progression (G1/S), HMG box, bromodomains, HAT activity, contacts E1A, TFIIF kinase activity, contacts TBP |
| yTAF150 (161) | dTAF150 | hTAF150/CIF150 | Cell cycle progression, contacts NTF-1, contacts TBP, contacts Inr |
| dTAF110 | hTAF135/130 | Contacts Sp1, contains Sp1-like Q-rich regions, contacts E1A and NF-E2, contacts TFIIA, contacts Inr | |
| hTAF105 | Cell-specific hTAF135/130 variant | ||
| yTAF90 (89) | dTAF85/80 | hTAF100/95 | WD-40 repeats, cell cycle progression (G2/M), contacts TFIIF (RAP30) and TBP |
| yTAF67 (67) | hTAF55 | Contacts Sp1, USF, CTF, HIV-1 Tat, E1A, and YY1 | |
| hTAF43 | |||
| yTAF25/23 (23) | hTAF30 | Contacts estrogen receptor, contacts TBP | |
| yTAF47 (40) | |||
| yTAF30 (27) | (AF-9, ENL) | Contacts TFIIF, contacts SWI/SNF, nonessential, human leukemia breakpoint proteins | |
| TAFs with histone similarities | |||
| yTAF60 (58) | dTAF62/60 | hTAF80/70 | Histone H4 similarity, contacts p53, NF-κB/p65, and NTF-1, contacts TFIIF (RAP74), TFIIE56, and TBP, contacts DPE |
| yTAF20/17 (17) | dTAF42/40 | hTAF32/31 | Histone H3 similarity, contacts VP16, p53, and NF-κB/p65, contacts N-Cor, contacts TFIIB, contacts DPE |
| yTAF68/61 (61) | dTAF30α/28/22 | hTAF20/15 | Histone H2B similarity, contacts TBP |
| yTAF40 (41) | dTAF30β | hTAF28 | Histone fold, SPT3 (SAGA) similarity |
| yTAF19 (19) | hTAF18 | Histone fold, SPT3 (SAGA) similarity |
Numbers in parentheses are molecular weights determined from the gene sequence. Homologous TAFIIs are aligned horizontally. E1a, NTF-1, Sp1, NF-E2, USF, CTF, human immunodeficiency virus (HIV-1) Tat, YY1, estrogen receptor, p53, and NF-κB/p65 are transcriptional activators or coactivators. NC2 (Dr1-DRAP1) and N-Cor are repressors or corepressors. HAT is histone acetyltransferase activity. SWI/SNF is a large protein complex that hydrolyzes ATP and remodels chromatin structure. SAGA is a large protein complex that bears some similarity to TFIID. For instance, SAGA contains some TAFII components, including histone-like TAFIIs, and a subunit with histone acetyltransferase activity. Contacts to the initiator (Inr) and DPE core promoter sequences are indicated.
The TFIID complex makes an extended footprint on DNA that mimics the footprint of the preinitiation complex formed with TBP, RNA polymerase II, and the general transcription factors in the absence of TAFIIs. TBP appears to make DNA contacts limited to the minor groove at the TATA sequence, but the TFIID complex additionally contacts DNA upstream of TATA, between TATA and +1, and downstream of TATA to almost +40 (111). In the absence of TBP and other TAFIIs, dTAFII150 makes footprints on DNA in the downstream promoter region between +1 and +38 (159). In the presence of TBP, dTAFII150 also occupies the core promoter between the TATA box and +1, so that dTAFII150 by itself appears to make contact both between the TATA box and +1 and between +1 and +38. dTAFII150 also induces DNase I hypersensitivity around the region of the transcriptional start site, indicative of DNA bending. In the presence of TBP, therefore, dTAFII150 appears to bend the DNA near +1 and wrap it around so that the promoter is in a similar configuration to that which forms around RNA polymerase II in the presence of TBP, TFIIB, TFIIF, and TFIIE (129). On several promoters, dTFIID induces footprint patterns characterized by DNase I hypersensitivity sites in the region of the TATA box and surrounding +1 and by protection of the DNA upstream of the TATA box and downstream to about +40 (10, 11). On promoters lacking a TATA box, DNA protection between about −30 and +40 is maintained and the DNA, in some cases, appears to be bent in the region where a TATA box would be located. The human homologue of dTAFII150 is named hTAFII150 or CIF150 (for “cofactor for initiator function”) (71). Recently, hTAFII150/CIF150 was reported to be a component of hTFIID, although its presence in the complex was previously obscured by hTAFII135/130, which has a similar mobility in gels (92).
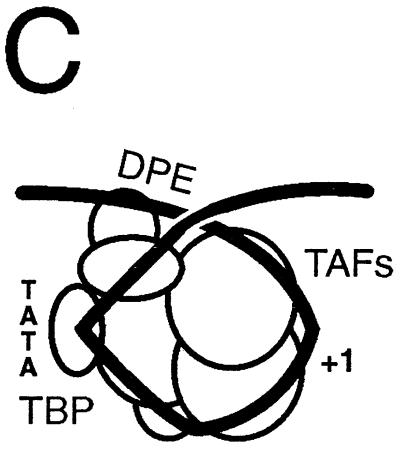
In Drosophila, a DPE has been identified around positions +28 to +34 in many promoters lacking a TATA sequence (10, 11). The DPE is very important for interactions between dTAFIIs and DNA in the downstream promoter region, and photo-cross-linking studies show that the DPE selectively interacts with dTAFII60 and dTAFII40, which are similar in sequence and structure to histones H4 and H3. Simultaneous binding of TBP to the TATA sequence and dTAFII60 and dTAFII40 to the DPE should bring these sequences into approximate juxtaposition, as expected in a looped DNA structure (Fig. 4C).
Photo-cross-linking studies show that a hTAFII of about 150 kDa contacts DNA upstream of TATA, between TATA and +1, and in the downstream promoter region between +5 and +22 (111). It is not clear whether these cross-links are to hTAFII150/CIF150 or hTAFII135/130, because hTAFII150 was not known to be present in hTFIID when this study was done (92). Combining the photo-cross-linking studies with antibody analysis of the products would be useful, because if all of these cross-links can be shown to be to the same protein, this would strongly support the wrapping model shown in Fig. 5. General transcription factor TFIIF makes DNA contacts upstream of TATA, within the core promoter, and downstream of +1 in the preinitiation complex. If hTAFII150 (or hTAFII135) makes similar contacts, the possibility must be considered that DNA is wrapped around TFIID in a similar manner to the way DNA is wrapped around TFIIF, TFIIE, and RNA polymerase II in the preinitiation complex (Fig. 3, 4B and C, and 5). The H4-related hTAFII80 interacts with TFIIE-56 and RAP74 (55), consistent with a common DNA-wrapping pathway in the formation of the TFIID-promoter complex and the preinitiation complex lacking TAFIIs. Of potential interest in this regard is the recent observation that the DNA binding domain of the RAP30 subunit of TFIIF has structural homology to the linker histone H5 (47).
FIG. 5.
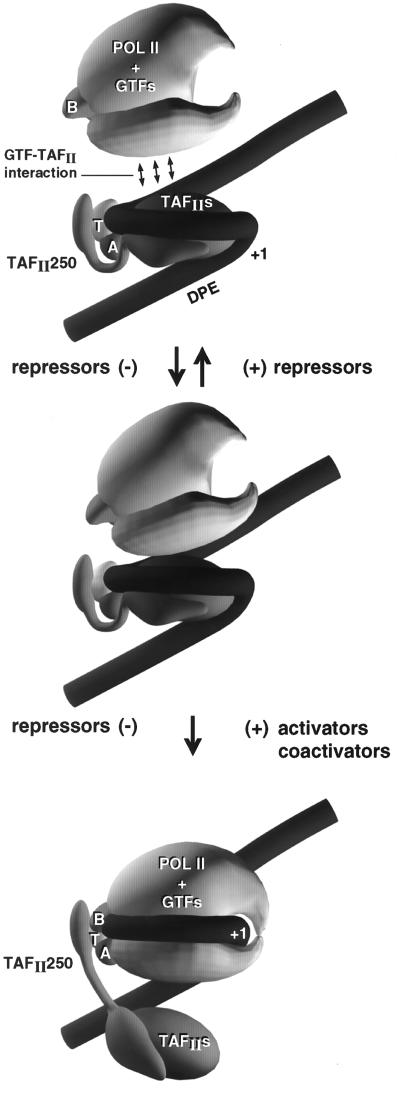
A proposed additional step in preinitiation complex assembly involving movement of TAFIIs from the core promoter region, in order to bind RNA polymerase II and the general transcription factors (GTFs). The promoter is depicted as an isomerized DNA structure tightly wrapped around a TAFII core, part of which may resemble a core histone octamer (hTAFII80/70-hTAFII32/31 heterotetramer-hTAFII20/15 tetramer). RNA polymerase II and associated general transcription factors dock first in the upstream promoter region through interactions with TBP, TFIIA, promoter DNA, and TAFIIs. TAFIIs must be displaced from the core promoter for RNA polymerase II to bend the initiator sequence through the enzyme active site. This step requires some movement of the DNA. Notice the similarities between this mechanism and that proposed for E. coli RNA polymerase (Fig. 2). hTAFII250 is indicated as an extended structure linking the TAFII core with TBP. This connection between hTAFII250 and TBP appears to be missing in yTAFII145/130.
In the human system, histone homologues hTAFII80, hTAFII31, and hTAFII20/15 (57) were not observed to cross-link to DNA (111); however, the precise location of cross-linking reagents may not have been appropriate to detect these interactions. Histone cores primarily contact DNA through the minor groove (89), but the photo-cross-linking reagent used in TFIID-promoter binding studies protrudes from the major groove. It is not known whether the histone-like TAFIIs play a similar role to histones in the formation of the TFIID complex, but promoter DNA appears to be wrapped around TFIID much as DNA is wrapped around a nucleosome, except that the DNA coil around TFIID appears to be more sharply bent, particularly around the transcriptional start site. In the case of the TFIID complex, DNA is bent at about 80° at the TATA box through binding of TBP, DNA appears to be bent again in the vicinity of +1, and TAFIIs make extensive contacts with DNA upstream of the TATA box, between TATA and +1 and from +1 to +40. dTAFII150 appears to play a role in forming the DNA bends around +1 (10, 11, 159). Similarly to histones, TAFIIs induce negative supercoiling in promoter DNA (111). TAFIIs appear to isomerize the DNA into a looped structure that is appropriate to accept binding by the RNA polymerase II holoenzyme (compare Fig. 3 and 5). Because the promoter DNA contacts made by TAFIIs and the general transcription factors are so similar, it is very doubtful that these contacts can be maintained simultaneously. The TAFIIs must release contacts with the core promoter for assembly of the RNA polymerase II preinitiation complex.
Transcriptional activators influence TFIID binding to promoters by extending the TFIID footprint in the downstream promoter region and by inducing DNase I-accessible or -hypersensitive sites in the vicinity of the transcriptional start site (17, 60, 85, 135, 168). According to the DNA-wrapping model, these observations might indicate that activators stimulate transcription by enhancing TAFII binding and TAFII-mediated isomerization of promoter DNA. As in bacterial systems, mammalian activators may enhance transcription by stimulating DNA wrapping around RNA polymerase II. Mammalian transcriptional activators appear to stimulate the recruitment of the general transcriptional machinery and isomerization of the preinitiation complex in the same way that bacterial transcriptional activators affect K1 and k2, although those that interact with TAFIIs may function at an intermediate stage in complex assembly.
The RNA polymerase II holoenzyme is suggested to interact with both promoter DNA and the TAFIIs for initial promoter recognition. TAFIIs interact with a number of the basal transcription factors (Table 3). hTAFII250 is a kinase for the RAP74 subunit of TFIIF (28). dTAFII110 interacts with TFIIA, and its human counterpart, hTAFII135/130, may contact the initiator sequence (111), possibly indicating DNA wrapping around hTFIID. hTAFII100/95 interacts with the RAP30 subunit of TFIIF (31). Histone-like TAFIIs also make contacts to general factors. The H4-like hTAFII80/70 contacts the RAP74 subunit of TFIIF and TFIIE56 (55). The H3-like dTAFII42/40 contacts TFIIB (43). The H3- and H4-like TAFIIs from Drosophila interact with the DPE (10), which may be juxtaposed to DNA upstream of TATA, where RAP30, RAP74, and TFIIB interact (78, 129). Contacts between the general transcription factors and TAFIIs could therefore be important for recruitment of holoenzyme to the promoter.
The current state of knowledge of TAFIIs and the concept of DNA wrapping in the preinitiation complex have been combined to develop a model for TAFII function. This model indicates that TAFIIs are involved in an intermediate step in the assembly of a preinitiation complex (Fig. 4C and 5). Similar DNA-wrapping models for TFIID interaction with promoter DNA have been suggested by others (111, 154). The difference between those models and ours is that we strongly suggest that the TFIID-promoter complex and the TBP-TFIIB-RNA polymerase II-TFIIF-TFIIE-promoter complex are incompatible. Because these structures cannot coexist, assembly of the preinitiation complex requires that the TAFIIs release their DNA contacts within the core promoter. Our model supports the following ideas: (i) TFIID bound to DNA is a form of modified nucleosome core surrounded by a single left-handed loop of DNA; (ii) in many cases, a promoter may be a nucleoprotein complex of TBP, TAFIIs, and DNA, rather than a linear DNA sequence, and RNA polymerase II and the GTFs may recognize both DNA and TFIID in promoter binding; (iii) TAFIIs have repressive transcriptional functions; (iv) some activators and coactivators function by antirepression mechanisms involving the release of TAFIIs from promoter DNA; (v) some repressors function by locking the TFIID-promoter structure; and (vi) TAFIIs must vacate the core promoter to assemble the preinitiation complex.
In our model, DNA is wrapped around the TAFII core in much the same fashion that DNA is wrapped around the nucleosome, except that the DNA is looped once rather than twice. The DNA is isomerized into a very similar conformation to that found in the fully assembled preinitiation complex, as if the promoter is restructured into a form ready to accept the binding of RNA polymerase II holoenzyme. TBP occupies the TATA box and bends DNA as expected. hTAFII250 is depicted as a hinged connector to TBP, which is located on the outside of the loop, bending the DNA back toward the hTAFII core. hTAFII250 also contacts the hTAFIIs as they interact with the core promoter. As holoenzyme docks with hTAFIIs, hTAFII250 is available to interact with the general transcription factors, such as TFIIF, which is a substrate for the hTAFII250 kinase activity. The hTAFII histone-like core is drawn in contact with the core promoter. Placement of TBP outside the loop is a likely arrangement, because hTAFIIs interact with DNA upstream of TATA. These upstream contacts are difficult to imagine if TBP is brought inside the structure and the DNA is bent away from the loop and the hTAFIIs. A looped structure is also more stable than one with two independent DNA bends. Inspection of the amino acid sequence indicates that hTAFII250 and dTAFII250 could easily be hinged just C-terminal to their TBP binding domains found near their N termini (86). For instance, these regions include polyproline tracts and highly charged sequences. The yeast homologue yTAFII145/130 appears to be missing this TBP binding region, which may explain why yTBP is found free of the yTAFIIs during isolation. A form of hTFIID lacking TBP has also recently been reported (163). A potential criticism of our model is that TBP has been shown in vitro to contact several TAFIIs. Some of these contacts, however, may be more important for transport of the TFIID complex in solution than they are within the complex assembled on the promoter (86).
For assembly of the preinitiation complex, the TAFIIs must release their contacts to the core promoter. This extra step in formation of an active transcription complex provides unforeseen roles for regulators. In this model, TAFIIs have an important function in repression, because they lock up the promoter. Transcriptional repressors could block transcription by holding this structure in place. The corepressor N-Cor and its homologues interact with the H3-like hTAFII32/31 and may have this locking function (101). The mammalian repressor RBP/CBF1 can interact with dTAFII110, blocking the coactivator site for TFIIA (113). In vitro transcription studies have also indicated repressive activities of TAFIIs (90, 112). We expect that the TAFIIs will be found to have many additional corepressor functions when the mechanisms for transcriptional repression are better understood. Many activators and coactivators are expected to function as derepressors in this model, to unlock the repressive TAFII structure. The coactivator PC4 appears to function in this manner (90).
The DNA-wrapping model can also address some confusing observations about the role of TAFIIs in transcription. In some cases, TAFIIs appear to function as coactivators (2, 16, 18, 19, 43, 67, 158) or as corepressors (101, 113). In other cases, TAFIIs appear to function as promoter selectivity factors (157) or may be dispensable for transcription and transcriptional activation altogether (100, 160). If TAFIIs wrap promoter DNA into a structure that is similar to the loop formed around RNA polymerase II and the general transcription factors, the function of TAFIIs is partially redundant with respect to the general transcriptional mechanism. In the DNA-wrapping model, therefore, TAFIIs can be viewed as targets of transcriptional activators (coactivators) or repressors (corepressors), as basal factors (promoter selectivity factors), and as potentially redundant transcriptional functions.
TRANSCRIPTIONAL ACTIVATION: SYNERGY, TETHERING, AND WRAPPING
The DNA-wrapping model may help to explain synergy by transcriptional regulators (14). Transcriptional synergy may result from activators making multiple, relatively weak contacts with the RNA polymerase II holoenzyme and associated factors. Formation of the DNA wrap is an inherently cooperative process, because as DNA is docked into grooves on the RNA polymerase holoenzyme surface, many favorable binding contacts are developed simultaneously along an extended protein-DNA interface. Transcriptional activators can affect this process cooperatively through multiple distinct protein-protein and/or protein-DNA contacts. Cooperativity between transcriptional activators and the natural DNA-wrapping process is expected to stimulate the recruitment of RNA polymerase II and isomerization of the holoenzyme and promoter DNA, in much the same way that CAP-cAMP and λ repressor are suggested to activate transcription in E. coli.
A popular technique to study the mechanism of action of eukaryotic transcriptional activators is to tether a DNA binding domain such as the E. coli lex repressor or the yeast Gal4 N-terminal DNA binding domain to a polypeptide that is a component of the general transcriptional machinery (119). In many cases, this strategy bypasses the requirement for a transcriptional activation domain. Tethered factors are then tested for the ability to activate transcription from promoters containing an appropriate DNA binding site. When general factors such as TBP have been tethered and activation results, these experiments have been interpreted to mean that binding of TBP and therefore recruitment of RNA polymerase II to the promoter have been enhanced. Recently, the tethering technology has been extended to bacterial systems (29, 30, 56). In the E. coli system, the α and ω subunits of RNA polymerase have been tethered to the λ repressor by using various strategies.
According to the DNA-wrapping model, however, another interpretation of tethering experiments is possible. Activation may result not only from enhanced binding of general factors to the promoter (termed “recruitment”) but also from stimulation of open-complex formation resulting from the DNA wrap that must result from looping the DNA between the upstream DNA element and the promoter. In this way, tethered transcription factors can stimulate not only binding (K1) but also isomerization (k2), as seen with CAP and λ repressor. In their review on activation by recruitment, Ptashne and Gann (119) acknowledged that tethering might influence both binding and isomerization, as we suggest based on the wrapping model.
It is perhaps somewhat surprising that there are so many different contacts between transcriptional regulators and the general factors that can stimulate initiation and that so many combinations of interactions can produce synergistic activation. We attribute much of this plasticity to the cooperative nature of the wrapped DNA structure. As the upstream and downstream DNA levers are pulled, many favorable DNA-protein contacts are developed simultaneously. Weak protein-protein or protein-DNA interactions can therefore contribute in multiple ways to stimulation of the wrapping mechanism.
DNA BENDING THROUGH THE RNA POLYMERASE ACTIVE SITE DURING ELONGATION
Multisubunit RNA polymerases elongate RNA chains according to a branched kinetic pathway, in which the enzyme alternates between a rapid and a slow (or inactive) conformation (Fig. 6). One line of evidence for a branched kinetic pathway comes from studies of E. coli RNA polymerase (36). The experimental approach was to initiate transcription with three of four ribonucleoside triphosphates so that RNA polymerase was blocked for elongation at a particular base position. In this case, no CTP was added to the reaction, and at the position for insertion of the first CMP, RNA polymerase was forced to either stop elongation or misincorporate a UMP in place of CMP. At the base position just prior to the misincorporation site, RNA polymerase was observed to fall into a very slow or inactive elongation state. An arrested elongation complex is one in which the RNA polymerase active site has backtracked irreversibly from the 3′-hydroxyl of the RNA chain to a position over the RNA-DNA hybrid. A paused complex may also backtrack, but paused complexes are capable of resuming elongation. Therefore, if backtracking occurs at pause positions, it is reversible. Just prior to the misincorporation site, a small fraction of complexes arrest, but most are paused, because these complexes are extended when they are supplied with CTP, which is the natural substrate for addition of the next base. That a fraction of complexes can misincorporate UMP for CMP but a separate fraction at the same base position becomes incapable of misincorporation indicates a branched kinetic pathway. The authors argue that three different conformational states must be possible at the stall position: (i) a short-lived activated elongation state that can misincorporate UMP in place of CMP; (ii) an inactive state that requires the addition of CTP, the next natural substrate, to reenter the activated elongation state; and (iii) an arrested state. Based on similar arguments, multiple conformational modes are proposed for elongation complexes at the next base position that have misincorporated UMP for CMP. Initially, a fraction of these complexes are extended, indicating that these complexes added the next base before exiting from the activated elongation conformation. Other complexes arrest at this position. Apparently, once the elongation complex containing the misincorporated UMP leaves the activated elongation state, it cannot reenter the activated elongation mode. Perhaps the base mismatch promotes irreversible backtracking of the complex. Again, these observations indicate a branched kinetic pathway.
FIG. 6.
Isomerization of the transcription elongation complex. (A) Proposed kinetic pathways for isomerization, phosphodiester bond formation, backtracking, arrest, and editing by RNA polymerases. EC is the unisomerized elongation complex, and EC* is the isomerized elongation complex. TFIIF stabilizes EC* by stimulating ki(n) and/or inhibiting k−i(n). TFIIF is not expected to affect k*(n + 1), the rate constant for phosphodiester bond synthesis. Rates for RNA polymerase active-site translocation are not shown in this model. Translocation rates are expected to be rapid relative to the rates shown. TFIIS, GreA, and GreB are expected to act on the EC, helping to resolve backtracked complexes by stimulating an endonuclease activity of their respective RNA polymerase, releasing oligonucleotides such as dinucleotides (N2). (B) EC* is thought to differ from EC by sharp DNA bending through the RNA polymerase active site and more extensive helix unwinding.
In another approach, a detailed analysis was done of elongation by yeast RNA polymerase III on the SUP4 tRNATyr gene (93). In that study, 1-s time points were recorded to measure the rates of entry and exit of RNA polymerase III at each base position. As is the case with RNA polymerase II and E. coli RNA polymerase, RNA polymerase III pauses at specific nucleotide addition sites. Moreover, even with a highly synchronous start, RNA polymerase III elongates the RNA chain asynchronously, as if the enzyme is converting between two forms: a rapid and a slow elongation form.
In the kinetic model shown in Fig. 6A, the elongation complex is suggested to convert between an isomerized EC* form and a low-activity EC form. Interconversion of the slow or inactive EC form and the rapid, isomerized EC* form is described by the equilibrium constant Ki(n) = ki(n)/k−i(n) (where i indicates isomerization and n indicates a defined nucleotide position in the RNA chain). In this scheme, the rate of nucleotide addition is described by k*(n + 1). This rate is a composite of the rate of translocation of the RNA polymerase active site and of the rate of phosphodiester bond formation. If k*(n + 1) is slow relative to k*(average), EC*(n) is a pause site. Pausing also results if k−i(n) > k*(n + 1). As indicated in the figure, pausing can also involve reversible backtracking of the RNA polymerase active site. Asynchrony of elongation results from continual partitioning between the EC* and EC forms and is further complicated by backtracking.
TFIIF stimulates the elongation rate by RNA polymerase II by decreasing the dwell time at pause sites or possibly at all nucleotide addition sites (3, 5, 63, 126). According to the model in Fig. 6A, TFIIF can stimulate the elongation rate by stimulating ki, inhibiting k−i, or stimulating k*(n + 1). TFIIF may have a somewhat low affinity for elongation complexes, or stalled elongation complexes (EC form), because dilution of reactions inhibits TFIIF stimulation of elongation (118). If TFIIF has a reduced affinity for the EC, this could explain the tendency of TFIIF to cycle off of the elongation complex. If TFIIF has a higher affinity for EC* than for EC, then TFIIF will stabilize EC* and inhibit k−i(n). Because the RNA polymerase II active site appears to be buried behind the sliding-clamp structure (Fig. 1 and 3), the catalytic mechanism of polymerization may be insulated from TFIIF, and TFIIF may not affect k*(n + 1). TFIIF may stimulate elongation by stimulating ki and inhibiting k−i. In this model, TFIIF helps to support an activated conformation of RNA polymerase II during elongation. Experiments to test these hypotheses are in progress.
Assuming that TFIIF accelerates elongation by maintaining RNA polymerase II in the EC* state, what does this activated conformation look like? We propose that during rapid elongation, template DNA may be bent sharply through the RNA polymerase II active site (Fig. 6B and 7). In the unisomerized EC state, template DNA is proposed to be less sharply bent. In this model, the rapid elongation complex partially resembles the isomerized preinitiation complex (compare Fig. 7 with Fig. 3). One reason why we favor this model is that mutants within the critical HIR-1 segment of the RAP74 subunit of TFIIF (Fig. 3 and 4B) have very similar effects on both initiation and elongation (84). Because these mutations affect DNA wrapping in the preinitiation complex (129), we propose that these mutants may also affect the conformational state of elongating RNA polymerase II. Based on our mutagenic studies, TFIIF appears to play very similar roles during distinct phases of the transcription cycle. Perhaps TFIIF stimulates elongation by helping to stabilize the DNA bend through the RNA polymerase II active site, resulting in progressive untwisting of the DNA helix during elongation. In this way, TFIIF could have a similar function in initiation and elongation.
FIG. 7.
Models for isomerized RNA polymerase II elongation complexes in the absence (A) and presence (B) of TFIIF. The RAP74 dimerization region HIR1 (amino acids 172 to 205) is critical for accurate initiation (84), elongation stimulation (84), and DNA wrapping in the preinitiation complex (129). The RNA exit channel and DNA entry channel are drawn so that they contact the RNA polymerase sliding clamp (109).
In the preinitiation complex, the adenovirus major late promoter appears to be wrapped almost a full turn around RNA polymerase II (Fig. 3) (129). According to the DNA-wrapping model, however, the minimal requirement for initiation might be simply a sharp DNA bend through the RNA polymerase II active site. In the initiation mechanism, the DNA wrap in the upstream promoter region could be viewed as a means to achieve this end. DNA bending and wrapping in the upstream promoter region is proposed to combine with the sharp DNA bend around the initiation site to unwind the DNA helix for initiation. If an alternate mechanism were used to induce helix unwinding by sharp DNA bending through the catalytic site, DNA bending and wrapping in the upstream promoter region would not be necessary for initiation. During elongation, untwisting of the helix is supported in part by the RNA-DNA hybrid and the transcription bubble. Wrapping of DNA upstream of elongating RNA polymerase may therefore not be necessary to untwist the DNA helix for rapid elongation. We argue, however, that maintenance of a bend through the RNA polymerase catalytic center is very probably a requirement for rapid elongation and that the elongation factor TFIIF supports this bend in DNA.
Other elongation factors, including SIII/elongin, ELL1, and ELL2, appear to stimulate elongation by a similar mechanism to TFIIF (7, 126, 142–144). We suggest that these factors also stabilize the EC* form of the elongation complex. SII/TFIIS resolves paused and irreversibly arrested transcription complexes by stimulating RNA polymerase II to cleave the RNA chain within the RNA-DNA hybrid to reset the RNA polymerase active site at the newly generated 3′ end of the RNA (49, 64–66, 125, 126, 133, 161). E. coli GreA and GreB proteins stimulate transcript cleavage by bacterial RNA polymerase in a manner similar to that of eukaryotic SII. As indicated in Fig. 6, SII, GreA, and GreB may work on the EC form of the elongation complex to reset the RNA polymerase catalytic center. Our model suggests that stalled elongation complexes are in the EC form, a cautionary note for researchers who study stalled complexes. When stalled, RNA polymerase can also backtrack from the RNA 3′ end to a position on the RNA-DNA hybrid. In some cases, sliding is reversible and the position is a long-duration pause site. In other cases, sliding cannot be reversed to reset RNA polymerase at the 3′ end of the RNA to resume elongation. In this case, the complex is irreversibly arrested and can be resolved only through SII-, GreA- or GreB-stimulated RNA cleavage.
In the elongation complex, the DNA entry channel and the RNA exit channel can photo-cross-link to the same regions of E. coli RNA polymerase that comprise the processivity sliding clamp (Fig. 1). This arrangement requires that the DNA template be bent through the RNA polymerase catalytic center during elongation (Fig. 7).
DNA bending in the initiation and elongation complexes may also be indicated from studies of promoter escape by RNA polymerase II. Conaway and coworkers (34) have shown that template DNA 40 to 50 bp downstream of the transcriptional start site of the adenovirus major late promoter is necessary for RNA polymerase II to transcribe through a strong transcriptional pause site located around positions +9 to +13. It is difficult to account for this effect of downstream DNA unless this region contacts RNA polymerase II and/or a general transcription factor(s). Promoter escape at this position is dependent on TFIIE, TFIIH, and ATP hydrolysis (33), so that TFIIH may account for some of these far-downstream DNA contacts, consistent with the ability of this factor to conformationally tighten the preinitiation complex (129).
DNA TOPOLOGY, THE OPEN COMPLEX, THE RNA-DNA HYBRID, HELIX UNWINDING, AND DNA WRAPPING
The DNA-wrapping models for E. coli RNA polymerase and human RNA polymerase II require a change in DNA topology of almost −2 turns within the preinitiation complex. Wrapping DNA 360° around RNA polymerase in a left-handed helical loop would induce a single negative supercoil, and untwisting DNA through a single turn (10.5 bp) to form the open complex induces a single negative supercoil. Therefore, accurate measurements of the change in DNA topology, the number of times DNA wraps around the transcription complex, and the extent of DNA unwinding must be considered in evaluating the DNA-wrapping model.
For E. coli RNA polymerase, the topological change in DNA has been carefully determined for an unstimulated wild-type lac promoter, the much stronger mutant lacUV5 promoter, and a fortuitous, near-consensus promoter that is found within simian virus 40 DNA but is recognized by E. coli RNA polymerase (1, 41). From each of these promoters, the topological change in DNA was determined to be −1.7 turns. RNA polymerase bound to the wild-type lac promoter does not melt in or initiate efficiently, unless stimulated by CAP-cAMP. Therefore, an unstimulated wild-type lac promoter is expected to be in a form similar to the closed complex II kinetic intermediate (Fig. 2). Nevertheless, RNA polymerase binds the wild-type lac promoter and reduces the linking number of the DNA just as extensively as does RNA polymerase bound to the stronger lacUV5 and simian virus 40 promoters at which polymerase is able to melt in. Negative supercoiling can be induced either by changes in DNA twist (unwinding) or writhe (wrapping); therefore, determining the number of base pairs that are unwound at a promoter is significant. By using topology measurements, twist and writhe terms cannot be separately evaluated, but the twist term can be estimated from the extent of the open complex. The extent of the transcription bubble prior to initiation has been determined for the mutant lacUV5 promoter and the bacteriophage T7 A3 promoter as 11 to 12 single-stranded bases (positions −9 to +2 or +3) (146, 147). This estimation was based on dimethyl sulfate sensitivity of single-stranded cytosines (N-3) and adenines (N-1). Modification at these positions prevents the formation of Watson-Crick base pairs, so that methylation was revealed by S1 nuclease digestion after dissociation of RNA polymerase from the DNA. On the other hand, using a direct measurement of cytosine sensitivity to dimethyl sulfate, the cytosine at position −9 of the template strand of the lacUV5 promoter was not found to be reactive (in contrast to cytosines at positions −6, −4, −2, and −1), indicating that the open complex may not extend to position −9 (1). This observation appears to reduce the size of the open complex to fewer than 11 single-stranded bases. The rest of the topological change in DNA linking number may be due to DNA wrapping and could account for almost a full turn of DNA around RNA polymerase. Assuming that the transcription bubble is 10 to 12 single-stranded bases, that the topological change in the DNA is −1.7 turns, and that there are 10.5 bp per turn of the helix, the DNA wrap in the preinitiation complex is between 270 and 200°. This range represents substantial DNA bending but slightly less extensive wrapping than we would estimate based on photo-cross-linking studies of the mammalian preinitiation complex (129). More careful determination of the extent of the transcription bubble, the change in DNA topology, and the pitch of the helix around +1 will be necessary to estimate the DNA wrap with accuracy.
The extent of the transcription bubble has also been measured during elongation. The length of the open complex appears to vary between 12 and 19 single-stranded bases (165). This determination, however, may be overestimated at some positions because stalled elongation complexes are not static. If translocation is reversible, stalled elongation complexes will move back and forth by at least one base as the active site of RNA polymerase moves between the RNA 3′-hydroxyl group and the last phosphodiester bond that was formed. More extensive backtracking of complexes and cleavage of RNA within the RNA-DNA hybrid indicates that the RNA polymerase active site can move much more extensively over the RNA-DNA hybrid, so that exposure of bases will reflect time-averaged movement of the transcription bubble. Another consideration is that the stalled elongation complex may have to advance to isomerize (Fig. 6A). The topology of the stalled elongation complex is indistinguishable from that of the initiation complex (41), and so if the open complex is 12 single-stranded bases or fewer, there may be significant DNA bending even within the stalled elongation complex. The length of the RNA-DNA hybrid is 8 to 9 nucleotides during elongation (110, 145), and so this may be the minimum size of the transcription bubble.
No topology, elongation open-complex, or RNA-DNA hybrid measurements are yet available for RNA polymerase II transcription complexes. No topology studies have been reported for E. coli promoters in the presence of architectural activators such as CAP-cAMP, λ repressor, integration host factor, or FIS, but binding of these factors in the upstream promoter region is expected to enhance DNA wrapping.
As noted above, DNA wrapping around RNA polymerases in initiation mechanisms could be viewed as a means to an end. Sharp DNA bending through the RNA polymerase active site is expected to be sufficient for isomerization and initiation. DNA wrapping in the upstream promoter region appears to be a major and general strategy to help bend DNA through the active site and to untwist the helix. At some promoters, extensive DNA wrapping may not be necessary to cause DNA bending around +1, and for these promoters, upstream DNA wrapping may not be as extensive or important for initiation.
FUTURE DIRECTIONS
The DNA-wrapping model described here provides explanations for an important number of observations made in many laboratories studying prokaryotic and eukaryotic transcription. Future work must better define the precise role of DNA bending and wrapping in isomerization of the preinitiation complex, open-complex formation, promoter escape, and elongation. Studies of multiple promoters will challenge the generality of the DNA-wrapping mechanism, although the similarities between prokaryotic and eukaryotic transcription systems indicate that this mechanism may have significant generality for multisubunit RNA polymerases.
Techniques for direct visualization of DNA wrapping, such as electron or atomic force microscopy, may not have the discrimination or resolution to map the trajectory of DNA around the protein core. One very direct experiment, however, is to demonstrate the juxtaposition of upstream and downstream DNA sequences by using protein-DNA photo-cross-linking. By detection of a single molecule of RAP30, RAP74, and TFIIE-34 in close approach to DNA both upstream of TATA (position −39 to −40) and downstream of +1 (positions +13 to +34) Robert et al. (129) have demonstrated the approximate crossing of helix segments separated by 53 to 74 bp (180 to 250 Å of B-form DNA). This observation strongly supports the DNA-wrapping model for the RNA polymerase II initiation system. Using photo-cross-linking technology to search for other factors (e.g., TFIIA or TFIIB) located near the cross-point of looped DNA structures should be a useful approach to demonstrate wrapping in preinitiation complexes and TBP-TAFII complexes. Fluorescence transfer or DNA ligation studies may provide another method of demonstrating the close approach of upstream and downstream promoter DNA sequences.
Footprinting experiments demonstrating stepwise preinitiation complex assembly, using highly purified RNA polymerase II, TFIIH, and TFIID (or recombinant TBP) and recombinant general factors, would be very useful to supplement photo-cross-linking studies. Comprehensive footprinting experiments have been done in the E. coli system and strongly support a DNA wrapping model. Similarly, photo-cross-linking studies with E. coli transcription complexes will help to support the generality of the wrapping model. A systematic determination of the DNA topology changes that are induced by transcription complexes at various stages of assembly would be of great interest. Some of these experiments have been reported, but many remain to be done. Another item on our wish list is new technology to visualize DNA bending and DNA conformation at the transcriptional start site within complexes in which this DNA is buried within the RNA polymerase active site.
In prokaryotic systems, transcriptional control works by stimulation of the basal transcriptional mechanism, by stimulating recruitment of RNA polymerase and isomerization of the polymerase-promoter complex. Because DNA binding by RNA polymerase involves significant bending and restructuring of the promoter, transcription factor recruitment and preinitiation complex isomerization are linked processes, as indicated by kinetic and footprinting studies. We argue here that many bacterial transcriptional activators function by enhancing the wrapping of DNA around RNA polymerase, particularly by inducing DNA bending and protein-DNA contacts in the upstream promoter region (described above as pulling the upstream transcriptional “lever” [Fig. 4A]). Many activators and repressors are similarly expected to work through the basal mechanism in eukaryotic systems. We confidently predict that many eukaryotic transcriptional regulators will be shown to function by stimulating or inhibiting DNA bending and wrapping.
ACKNOWLEDGMENTS
We thank Jack Greenblatt, David Arnosti, Steve Triezenberg, Bill Henry, James Geiger, Laurie Kaguni, and Lee Kroos for fruitful discussions and critical reading of the manuscript. We thank Konstantin Severinov for his incisive questions about DNA topology and the DNA-wrapping model. We thank Pierre Vandenberghe for the excellent graphics, prepared in Cinema 4D for MacIntosh.
B.C. is a Junior Research Scholar of the Fonds de la recherche en santé du Québec and is supported by grants from the Medical Research Council of Canada and the Cancer Research Society Inc. Z.F.B. is supported by a grant from the American Cancer Society, Inc.; Michigan State University Research Excellence Funds; and the Michigan State University Agricultural Experiment Station.
REFERENCES
- 1.Amouyal M, Buc H. Topological unwinding of strong and weak promoters by RNA polymerase. A comparison between the lac wild-type and the UV5 sites of E. coli. J Mol Biol. 1987;195:795–808. doi: 10.1016/0022-2836(87)90485-2. [DOI] [PubMed] [Google Scholar]
- 2.Amrolia P J, Ramamurthy L, Saluja D, Tanese N, Jane S M, Cunningham J M. The activation domain of the enhancer binding protein p45NF-E2 interacts with TAFII130 and mediates long-range activation of the α- and β-globin gene loci in an erythroid cell line. Proc Natl Acad Sci USA. 1997;94:10051–10056. doi: 10.1073/pnas.94.19.10051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aso T, Conaway J W, Conaway R C. The RNA polymerase II elongation complex. FASEB J. 1995;9:1419–1428. doi: 10.1096/fasebj.9.14.7589983. [DOI] [PubMed] [Google Scholar]
- 4.Asturias F J, Meredith G D, Poglitsch C L, Kornberg R D. Two conformations of RNA polymerase II revealed by electron crystallography. J Mol Biol. 1997;272:536–540. doi: 10.1006/jmbi.1997.1273. [DOI] [PubMed] [Google Scholar]
- 5.Bengal E, Flores O, Krauskopf A, Reinberg D, Aloni Y. Role of the mammalian transcription factors IIF, IIS, and IIX during elongation by RNA polymerase II. Mol Cell Biol. 1991;11:1195–1206. doi: 10.1128/mcb.11.3.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borukhov S, Severinov K, Kashlev M, Lebedev A, Bass I, Rowland G C, Lim P P, Glass R E, Nikiforov V, Goldfarb A. Mapping of trypsin cleavage and antibody-binding sites and delineation of a dispensable domain in the β subunit of E. coli RNA polymerase. J Biol Chem. 1991;266:23921–23926. [PubMed] [Google Scholar]
- 7.Bradsher J N, Jackson K W, Conaway R C, Conaway J W. RNA polymerase II transcription factor SIII. I. Identification, purification, and properties. J Biol Chem. 1993;268:25587–25593. [PubMed] [Google Scholar]
- 8.Braun B R, Bartholomew B, Kassavetis G A, Geiduschek E P. Topography of transcription factor complexes on the S. cerevisiae 5 S RNA gene. J Mol Biol. 1992;228:1063–1077. doi: 10.1016/0022-2836(92)90315-b. [DOI] [PubMed] [Google Scholar]
- 9.Buc H, McClure W R. Kinetics of open complex formation between E. coli RNA polymerase and the lac UV5 promoter. Evidence for a sequential mechanism involving three steps. Biochemistry. 1985;24:2712–2723. doi: 10.1021/bi00332a018. [DOI] [PubMed] [Google Scholar]
- 10.Burke T W, Kadonaga J T. The downstream core promoter element, DPE, is conserved from Drosophila to humans and is recognized by TAFII60 of Drosophila. Genes Dev. 1997;11:3020–3031. doi: 10.1101/gad.11.22.3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burke T W, Kadonaga J T. Drosophila TFIID binds to a conserved downstream basal promoter element that is present in many TATA-box-deficient promoters. Genes Dev. 1996;10:711–724. doi: 10.1101/gad.10.6.711. [DOI] [PubMed] [Google Scholar]
- 12.Burley S K, Roeder R G. Biochemistry and structural biology of transcription factor IID (TFIID) Annu Rev Biochem. 1996;65:769–799. doi: 10.1146/annurev.bi.65.070196.004005. [DOI] [PubMed] [Google Scholar]
- 13.Busby S, Ebright R H. Transcription activation at class II CAP-dependent promoters. Mol Microbiol. 1997;23:853–859. doi: 10.1046/j.1365-2958.1997.2771641.x. [DOI] [PubMed] [Google Scholar]
- 14.Carey M. The enhanceosome and transcriptional synergy. Cell. 1998;92:5–8. doi: 10.1016/s0092-8674(00)80893-4. [DOI] [PubMed] [Google Scholar]
- 15.Carpousis A J, Gralla J D. Interaction of RNA polymerase with lacUV5 promoter DNA during mRNA initiation and elongation. Footprinting, methylation, and rifampicin-sensitivity changes accompanying transcription initiation. J Mol Biol. 1985;183:165–177. doi: 10.1016/0022-2836(85)90210-4. [DOI] [PubMed] [Google Scholar]
- 16.Chen J L, Attardi L D, Verrijzer C P, Yokomori K, Tjian R. Assembly of recombinant TFIID reveals differential coactivator requirements for distinct transcriptional activators. Cell. 1994;79:93–105. doi: 10.1016/0092-8674(94)90403-0. [DOI] [PubMed] [Google Scholar]
- 17.Chi T, Lieberman P, Ellwood K, Carey M. A general mechanism for transcriptional synergy by eukaryotic activators. Nature. 1995;377:254–257. doi: 10.1038/377254a0. [DOI] [PubMed] [Google Scholar]
- 18.Chiang C M, Ge H, Wang Z, Hoffmann A, Roeder R G. Unique TATA-binding protein-containing complexes and cofactors involved in transcription by RNA polymerases II and III. EMBO J. 1993;12:2749–2762. doi: 10.1002/j.1460-2075.1993.tb05936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chiang C M, Roeder R G. Cloning of an intrinsic human TFIID subunit that interacts with multiple transcriptional activators. Science. 1995;267:531–536. doi: 10.1126/science.7824954. [DOI] [PubMed] [Google Scholar]
- 20.Conaway J W, Conaway R C. A multisubunit transcription factor essential for accurate initiation by RNA polymerase II. J Biol Chem. 1989;264:2357–2362. [PubMed] [Google Scholar]
- 21.Conaway J W, Conaway R C. An RNA polymerase II transcription factor shares functional properties with E. coli ς70. Science. 1990;248:1550–1553. doi: 10.1126/science.2193400. [DOI] [PubMed] [Google Scholar]
- 22.Coulombe B, Li J, Greenblatt J. Topological localization of the human transcription factors IIA, IIB, TATA box-binding protein, and RNA polymerase II-associated protein 30 on a class II promoter. J Biol Chem. 1994;269:19962–19967. [PubMed] [Google Scholar]
- 23.Cowing D W, Mecsas J, Record M T, Jr, Gross C A. Intermediates in the formation of the open complex by RNA polymerase holoenzyme containing the ς factor ς32 at the groE promoter. J Mol Biol. 1989;210:521–530. doi: 10.1016/0022-2836(89)90128-9. [DOI] [PubMed] [Google Scholar]
- 24.Craig M L, Suh W C, Record M T., Jr HO. and DNase I probing of E ς70 RNA polymerase--λ PR promoter open complexes: Mg2+ binding and its structural consequences at the transcription start site. Biochemistry. 1995;34:15624–15632. doi: 10.1021/bi00048a004. [DOI] [PubMed] [Google Scholar]
- 25.Crothers D M, Steitz T A. Transcriptional activation by E. coli CAP protein. In: McKnight S L, Yamamoto K R, editors. Transcriptional regulation. Vol. 1. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1992. pp. 501–534. [Google Scholar]
- 26.Darst S A, Edwards A M, Kubalek E W, Kornberg R D. Three-dimensional structure of yeast RNA polymerase II at 16 angstrom resolution. Cell. 1991;66:121–129. doi: 10.1016/0092-8674(91)90144-n. [DOI] [PubMed] [Google Scholar]
- 27.Darst S A, Kubalek E W, Kornberg R D. Three-dimensional structure of E. coli RNA polymerase holoenzyme determined by electron crystallography. Nature. 1989;340:730–732. doi: 10.1038/340730a0. [DOI] [PubMed] [Google Scholar]
- 28.Dikstein R, Ruppert S, Tjian R. TAFII250 is a bipartite protein kinase that phosphorylates the base transcription factor RAP74. Cell. 1996;84:781–790. doi: 10.1016/s0092-8674(00)81055-7. [DOI] [PubMed] [Google Scholar]
- 29.Dove S L, Hochschild A. Conversion of the ω subunit of E. coli RNA polymerase into a transcriptional activator or an activation target. Genes Dev. 1998;12:745–754. doi: 10.1101/gad.12.5.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dove S L, Joung J K, Hochschild A. Activation of prokaryotic transcription through arbitrary protein- protein contacts. Nature. 1997;386:627–630. doi: 10.1038/386627a0. [DOI] [PubMed] [Google Scholar]
- 31.Dubrovskaya V, Lavigne A C, Davidson I, Acker J, Staub A, Tora L. Distinct domains of hTAFII100 are required for functional interaction with transcription factor TFIIF β (RAP30) and incorporation into the TFIID complex. EMBO J. 1996;15:3702–3712. [PMC free article] [PubMed] [Google Scholar]
- 32.Duval-Valentin G, Ehrlich R. Interaction between E. coli RNA polymerase and the tetR promoter from pSC101: homologies and differences with other E. coli promoter systems from close contact point studies. Nucleic Acids Res. 1986;14:1967–1983. doi: 10.1093/nar/14.5.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dvir A, Conaway R C, Conaway J W. A role for TFIIH in controlling the activity of early RNA polymerase II elongation complexes. Proc Natl Acad Sci USA. 1997;94:9006–9010. doi: 10.1073/pnas.94.17.9006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dvir A, Tan S, Conaway J W, Conaway R C. Promoter escape by RNA polymerase II. Formation of an escape-competent transcriptional intermediate is a prerequisite for exit of polymerase from the promoter. J Biol Chem. 1997;272:28175–28178. doi: 10.1074/jbc.272.45.28175. [DOI] [PubMed] [Google Scholar]
- 35.Eichenberger P, Dethiollaz S, Buc H, Geiselmann J. Structural kinetics of transcription activation at the malT promoter of E. coli by UV laser footprinting. Proc Natl Acad Sci USA. 1997;94:9022–9027. doi: 10.1073/pnas.94.17.9022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Erie D A, Hajiseyedjavadi O, Young M C, von Hippel P H. Multiple RNA polymerase conformations and GreA: control of the fidelity of transcription. Science. 1993;262:867–873. doi: 10.1126/science.8235608. [DOI] [PubMed] [Google Scholar]
- 37.Finkel S E, Johnson R C. The Fis protein: it’s not just for DNA inversion anymore. Mol Microbiol. 1992;6:3257–3265. doi: 10.1111/j.1365-2958.1992.tb02193.x. [DOI] [PubMed] [Google Scholar]
- 38.Flores O, Ha I, Reinberg D. Factors involved in specific transcription by mammalian RNA polymerase II. Purification and subunit composition of transcription factor IIF. J Biol Chem. 1990;265:5629–5634. [PubMed] [Google Scholar]
- 39.Forget D, Robert F, Grondin G, Burton Z F, Greenblatt J, Coulombe B. RAP74 induces promoter contacts by RNA polymerase II upstream and downstream of a DNA bend centered on the TATA box. Proc Natl Acad Sci USA. 1997;94:7150–7155. doi: 10.1073/pnas.94.14.7150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gaal T, Ross W, Blatter E E, Tang H, Jia X, Krishnan V V, Assa-Munt N, Ebright R H, Gourse R L. DNA-binding determinants of the α subunit of RNA polymerase: novel DNA-binding domain architecture. Genes Dev. 1996;10:16–26. doi: 10.1101/gad.10.1.16. [DOI] [PubMed] [Google Scholar]
- 41.Gamper H B, Hearst J E. A topological model for transcription based on unwinding angle analysis of E. coli RNA polymerase binary, initiation and ternary complexes. Cell. 1982;29:81–90. doi: 10.1016/0092-8674(82)90092-7. [DOI] [PubMed] [Google Scholar]
- 42.Garrett K P, Serizawa H, Hanley J P, Bradsher J N, Tsuboi A, Arai N, Yokota T, Arai K, Conaway R C, Conaway J W. The carboxyl terminus of RAP30 is similar in sequence to region 4 of bacterial ς factors and is required for function. J Biol Chem. 1992;267:23942–23949. [PubMed] [Google Scholar]
- 43.Goodrich J A, Tjian R. TBP-TAF complexes: selectivity factors for eukaryotic transcription. Curr Opin Cell Biol. 1994;6:403–409. doi: 10.1016/0955-0674(94)90033-7. [DOI] [PubMed] [Google Scholar]
- 44.Goosen N, van de Putte P. The regulation of transcription initiation by integration host factor. Mol Microbiol. 1995;16:1–7. doi: 10.1111/j.1365-2958.1995.tb02386.x. [DOI] [PubMed] [Google Scholar]
- 45.Grachev M A, Lukhtanov E A, Mustaev A A, Zaychikov E F, Abdukayumov M N, Rabinov I V, Richter V I, Skoblov Y S, Chistyakov P G. Studies of the functional topography of E. coli RNA polymerase. A method for localization of the sites of affinity labelling. Eur J Biochem. 1989;180:577–585. doi: 10.1111/j.1432-1033.1989.tb14684.x. [DOI] [PubMed] [Google Scholar]
- 46.Greenblatt J. RNA polymerase II holoenzyme and transcriptional regulation. Curr Opin Cell Biol. 1997;9:310–319. doi: 10.1016/s0955-0674(97)80002-6. [DOI] [PubMed] [Google Scholar]
- 47.Groft C M, Uljon S N, Wang R, Werner M H. Structural homology between the RAP30 DNA-binding domain and linker histone H5: implications for preinitiation complex assembly. Proc Natl Acad Sci USA. 1998;95:9117–9122. doi: 10.1073/pnas.95.16.9117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gross C A, Lonetto M, Losick R. Bacterial ς factors. In: McKnight S L, Yamamoto K R, editors. Transcriptional regulation. Vol. 1. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1992. pp. 129–176. [Google Scholar]
- 49.Gu W, Reines D. Variation in the size of nascent RNA cleavage products as a function of transcript length and elongation competence. J Biol Chem. 1995;270:30441–30447. doi: 10.1074/jbc.270.51.30441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hampsey M. Molecular genetics of the RNA polymerase II general transcriptional machinery. Microbiol Mol Biol Rev. 1998;62:465–503. doi: 10.1128/mmbr.62.2.465-503.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hampsey M, Reinberg D. Transcription: why are TAFs essential? Curr Biol. 1997;7:R44–R46. doi: 10.1016/s0960-9822(06)00018-2. [DOI] [PubMed] [Google Scholar]
- 52.Hawley D K, McClure W R. Mechanism of activation of transcription initiation from the λ PRM promoter. J Mol Biol. 1982;157:493–525. doi: 10.1016/0022-2836(82)90473-9. [DOI] [PubMed] [Google Scholar]
- 53.Helmann J D. Bacterial ς factors. In: Conaway R C, Conaway J W, editors. Transcription: mechanisms and regulation. New York, N.Y: Raven Press; 1994. pp. 1–18. [Google Scholar]
- 54.Heyduk T, Heyduk E, Severinov K, Tang H, Ebright R H. Determinants of RNA polymerase α subunit for interaction with β, β′, and ς subunits: hydroxyl-radical protein footprinting. Proc Natl Acad Sci USA. 1996;93:10162–10166. doi: 10.1073/pnas.93.19.10162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hisatake K, Ohta T, Takada R, Guermah M, Horikoshi M, Nakatani Y, Roeder R G. Evolutionary conservation of human TATA-binding-polypeptide-associated factors TAFII31 and TAFII80 and interactions of TAFII80 with other TAFIIs and with general transcription factors. Proc Natl Acad Sci USA. 1995;92:8195–8199. doi: 10.1073/pnas.92.18.8195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hochschild A, Dove S L. Protein-protein contacts that activate and repress prokaryotic transcription. Cell. 1998;92:597–600. doi: 10.1016/s0092-8674(00)81126-5. [DOI] [PubMed] [Google Scholar]
- 57.Hoffmann A, Chiang C M, Oelgeschlager T, Xie X, Burley S K, Nakatani Y, Roeder R G. A histone octamer-like structure within TFIID. Nature. 1996;380:356–359. doi: 10.1038/380356a0. [DOI] [PubMed] [Google Scholar]
- 58.Hoffmann A, Oelgeschlager T, Roeder R G. Considerations of transcriptional control mechanisms: do TFIID-core promoter complexes recapitulate nucleosome-like functions? Proc Natl Acad Sci USA. 1997;94:8928–8935. doi: 10.1073/pnas.94.17.8928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Holstege F C, Tantin D, Carey M, van der Vliet P C, Timmers H T. The requirement for the basal transcription factor IIE is determined by the helical stability of promoter DNA. EMBO J. 1995;14:810–819. doi: 10.1002/j.1460-2075.1995.tb07059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Horikoshi M, Hai T, Lin Y S, Green M R, Roeder R G. Transcription factor ATF interacts with the TATA factor to facilitate establishment of a preinitiation complex. Cell. 1988;54:1033–1042. doi: 10.1016/0092-8674(88)90118-3. [DOI] [PubMed] [Google Scholar]
- 61.Ishihama A. Subunit of assembly of E. coli RNA polymerase. Adv Biophys. 1981;14:1–35. [PubMed] [Google Scholar]
- 62.Ishihama A, Ito K. Subunits of RNA polymerase in function and structure. II. Reconstitution of E. coli RNA polymerase from isolated subunits. J Mol Biol. 1972;72:111–123. doi: 10.1016/0022-2836(72)90073-3. [DOI] [PubMed] [Google Scholar]
- 63.Izban M G, Luse D S. Factor-stimulated RNA polymerase II transcribes at physiological elongation rates on naked DNA but very poorly on chromatin templates. J Biol Chem. 1992;267:13647–13655. [PubMed] [Google Scholar]
- 64.Izban M G, Luse D S. The increment of SII-facilitated transcript cleavage varies dramatically between elongation competent and incompetent RNA polymerase II ternary complexes. J Biol Chem. 1993;268:12974–12985. [PubMed] [Google Scholar]
- 65.Izban M G, Luse D S. The RNA polymerase II ternary complex cleaves the nascent transcript in a 3′→5′ direction in the presence of elongation factor SII. Genes Dev. 1992;6:1342–1356. doi: 10.1101/gad.6.7.1342. [DOI] [PubMed] [Google Scholar]
- 66.Izban M G, Luse D S. SII-facilitated transcript cleavage in RNA polymerase II complexes stalled early after initiation occurs in primarily dinucleotide increments. J Biol Chem. 1993;268:12964–12973. [PubMed] [Google Scholar]
- 67.Jacq X, Brou C, Lutz Y, Davidson I, Chambon P, Tora L. Human TAFII30 is present in a distinct TFIID complex and is required for transcriptional activation by the estrogen receptor. Cell. 1994;79:107–117. doi: 10.1016/0092-8674(94)90404-9. [DOI] [PubMed] [Google Scholar]
- 68.Jeon Y H, Negishi T, Shirakawa M, Yamazaki T, Fujita N, Ishihama A, Kyogoku Y. Solution structure of the activator contact domain of the RNA polymerase α subunit. Science. 1995;270:1495–1497. doi: 10.1126/science.270.5241.1495. [DOI] [PubMed] [Google Scholar]
- 69.Jin D J, Gross C A. Mapping and sequencing of mutations in the E. coli rpoB gene that lead to rifampicin resistance. J Mol Biol. 1988;202:45–58. doi: 10.1016/0022-2836(88)90517-7. [DOI] [PubMed] [Google Scholar]
- 70.Jin D J, Gross C A. RpoB8, a rifampicin-resistant termination-proficient RNA polymerase, has an increased Km for purine nucleotides during transcription elongation. J Biol Chem. 1991;266:14478–14785. [PubMed] [Google Scholar]
- 71.Kaufmann J, Verrijzer C P, Shao J, Smale S T. CIF, an essential cofactor for TFIID-dependent initiator function. Genes Dev. 1996;10:873–886. doi: 10.1101/gad.10.7.873. [DOI] [PubMed] [Google Scholar]
- 72.Killeen M T, Greenblatt J F. The general transcription factor RAP30 binds to RNA polymerase II and prevents it from binding nonspecifically to DNA. Mol Cell Biol. 1992;12:30–37. doi: 10.1128/mcb.12.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim J L, Nikolov D B, Burley S K. Co-crystal structure of TBP recognizing the minor groove of a TATA element. Nature. 1993;365:520–527. doi: 10.1038/365520a0. [DOI] [PubMed] [Google Scholar]
- 74.Kim T K, Lagrange T, Wang Y H, Griffith J D, Reinberg D, Ebright R H. Trajectory of DNA in the RNA polymerase II transcription preinitiation complex. Proc Natl Acad Sci USA. 1997;94:12268–12273. doi: 10.1073/pnas.94.23.12268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim Y, Geiger J H, Hahn S, Sigler P B. Crystal structure of a yeast TBP/TATA-box complex. Nature. 1993;365:512–520. doi: 10.1038/365512a0. [DOI] [PubMed] [Google Scholar]
- 76.Kohlstaedt L A, Wang J, Friedman J M, Rice P A, Steitz T A. Crystal structure at 3.5 A resolution of HIV-1 reverse transcriptase complexed with an inhibitor. Science. 1992;256:1783–1790. doi: 10.1126/science.1377403. [DOI] [PubMed] [Google Scholar]
- 77.Kovacic R T. The 0°C closed complexes between E. coli RNA polymerase and two promoters, T7-A3 and lacUV5. J Biol Chem. 1987;262:13654–13661. [PubMed] [Google Scholar]
- 78.Lagrange T, Kapanidis A N, Tang H, Reinberg D, Ebright R H. New core promoter element in RNA polymerase II-dependent transcription: sequence-specific DNA binding by transcription factor IIB. Genes Dev. 1998;12:34–44. doi: 10.1101/gad.12.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lagrange T, Kim T K, Orphanides G, Ebright Y W, Ebright R H, Reinberg D. High-resolution mapping of nucleoprotein complexes by site-specific protein-DNA photocrosslinking: organization of the human TBP-TFIIA-TFIIB-DNA quaternary complex. Proc Natl Acad Sci USA. 1996;93:10620–10625. doi: 10.1073/pnas.93.20.10620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Landick R, Stewart J, Lee D N. Amino acid changes in conserved regions of the β-subunit of E. coli RNA polymerase alter transcription pausing and termination. Genes Dev. 1990;4:1623–1636. doi: 10.1101/gad.4.9.1623. [DOI] [PubMed] [Google Scholar]
- 81.Langer D, Hain J, Thuriaux P, Zillig W. Transcription in archaea: similarity to that in eucarya. Proc Natl Acad Sci USA. 1995;92:5768–5772. doi: 10.1073/pnas.92.13.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lavigne M, Herbert M, Kolb A, Buc H. Upstream curved sequences influence the initiation of transcription at the E. coli galactose operon. J Mol Biol. 1992;224:293–306. doi: 10.1016/0022-2836(92)90995-v. [DOI] [PubMed] [Google Scholar]
- 83.Lee T I, Young R A. Regulation of gene expression by TBP-associated proteins. Genes Dev. 1998;12:1398–1408. doi: 10.1101/gad.12.10.1398. [DOI] [PubMed] [Google Scholar]
- 84.Lei L, Ren D, Finkelstein A, Burton Z F. Functions of the N- and C-terminal domains of human RAP74 in transcriptional initiation, elongation, and recycling of RNA polymerase II. Mol Cell Biol. 1998;18:2130–2142. doi: 10.1128/mcb.18.4.2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lieberman P M, Berk A J. A mechanism for TAFs in transcriptional activation: activation domain enhancement of TFIID-TFIIA-promoter DNA complex formation. Genes Dev. 1994;8:995–1006. doi: 10.1101/gad.8.9.995. [DOI] [PubMed] [Google Scholar]
- 86.Liu D, Ishima R, Tong K I, Bagby S, Kokubo T, Muhandiram D R, Kay L E, Nakatani Y, Ikura M. Solution structure of a TBP-TAFII230 complex: protein mimicry of the minor groove surface of the TATA box unwound by TBP. Cell. 1998;94:573–583. doi: 10.1016/s0092-8674(00)81599-8. [DOI] [PubMed] [Google Scholar]
- 87.Lobo S M, Hernandez N T. Transcription of snRNA genes by RNA polymerases II and III. In: Conaway R C, Conaway J W, editors. Transcription: mechanisms and regulation. New York, N.Y: Raven Press; 1994. pp. 127–159. [Google Scholar]
- 88.Lonetto M, Gribskov M, Gross C A. The ς70 family: sequence conservation and evolutionary relationships. J Bacteriol. 1992;174:3843–3849. doi: 10.1128/jb.174.12.3843-3849.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Luger K, Mader A W, Richmond R K, Sargent D F, Richmond T J. Crystal structure of the nucleosome core particle at 2.8 angstrom resolution. Nature. 1997;389:251–260. doi: 10.1038/38444. [DOI] [PubMed] [Google Scholar]
- 90.Malik S, Guermah M, Roeder R G. A dynamic model for PC4 coactivator function in RNA polymerase II transcription. Proc Natl Acad Sci USA. 1998;95:2192–2197. doi: 10.1073/pnas.95.5.2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Markovtsov V, Mustaev A, Goldfarb A. Protein-RNA interactions in the active center of transcription elongation complex. Proc Natl Acad Sci USA. 1996;93:3221–3226. doi: 10.1073/pnas.93.8.3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Martinez E, Ge H, Tao Y, Yuan C X, Palhan V, Roeder R G. Novel cofactors and TFIIA mediate functional core promoter selectivity by the human TAFII150-containing TFIID complex. Mol Cell Biol. 1998;18:6571–6583. doi: 10.1128/mcb.18.11.6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Matsuzaki H, Kassavetis G A, Geiduschek E P. Analysis of RNA chain elongation and termination by S. cerevisiae RNA polymerase III. J Mol Biol. 1994;235:1173–1192. doi: 10.1006/jmbi.1994.1072. [DOI] [PubMed] [Google Scholar]
- 94.McClure W R, Cech C L. On the mechanism of rifampicin inhibition of RNA synthesis. J Biol Chem. 1978;253:8949–8956. [PubMed] [Google Scholar]
- 95.McCracken S, Greenblatt J. Related RNA polymerase-binding regions in human RAP30/74 and E. coli ς70. Science. 1991;253:900–902. doi: 10.1126/science.1652156. [DOI] [PubMed] [Google Scholar]
- 96.Mecsas J, Cowing D W, Gross C A. Development of RNA polymerase-promoter contacts during open complex formation. J Mol Biol. 1991;220:585–597. doi: 10.1016/0022-2836(91)90102-c. [DOI] [PubMed] [Google Scholar]
- 97.Meier T, Schickor P, Wedel A, Cellai L, Heumann H. In vitro transcription close to the melting point of DNA: analysis of Thermotoga maritima RNA polymerase-promoter complexes at 75°C using chemical probes. Nucleic Acids Res. 1995;23:988–994. doi: 10.1093/nar/23.6.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Merkel T J, Dahl J L, Ebright R H, Kadner R J. Transcription activation at the E. coli uhpT promoter by the catabolite gene activator protein. J Bacteriol. 1995;177:1712–1718. doi: 10.1128/jb.177.7.1712-1718.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Meyer-Almes F J, Heumann H, Porschke D. The structure of the RNA polymerase-promoter complex. DNA-bending-angle by quantitative electrooptics. J Mol Biol. 1994;236:1–6. doi: 10.1006/jmbi.1994.1112. [DOI] [PubMed] [Google Scholar]
- 100.Moqtaderi Z, Bai Y, Poon D, Weil P A, Struhl K. TBP-associated factors are not generally required for transcriptional activation in yeast. Nature. 1996;383:188–191. doi: 10.1038/383188a0. [DOI] [PubMed] [Google Scholar]
- 101.Muscat G E, Burke L J, Downes M. The corepressor N-CoR and its variants RIP13a and RIP13Δ1 directly interact with the basal transcription factors TFIIB, TAFII32 and TAFII70. Nucleic Acids Res. 1998;26:2899–2907. doi: 10.1093/nar/26.12.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Muskhelishvili G, Travers A A, Heumann H, Kahmann R. FIS and RNA polymerase holoenzyme form a specific nucleoprotein complex at a stable RNA promoter. EMBO J. 1995;14:1446–1452. doi: 10.1002/j.1460-2075.1995.tb07131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mustaev A, Kashlev M, Lee J Y, Polyakov A, Lebedev A, Zalenskaya K, Grachev M, Goldfarb A, Nikiforov V. Mapping of the priming substrate contacts in the active center of E. coli RNA polymerase. J Biol Chem. 1991;266:23927–23931. [PubMed] [Google Scholar]
- 104.Mustaev A, Kozlov M, Markovtsov V, Zaychikov E, Denissova L, Goldfarb A. Modular organization of the catalytic center of RNA polymerase. Proc Natl Acad Sci USA. 1997;94:6641–6645. doi: 10.1073/pnas.94.13.6641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mustaev A, Zaychikov E, Severinov K, Kashlev M, Polyakov A, Nikiforov V, Goldfarb A. Topology of the RNA polymerase active center probed by chimeric rifampicin-nucleotide compounds. Proc Natl Acad Sci USA. 1994;91:12036–12040. doi: 10.1073/pnas.91.25.12036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nikolov D B, Chen H, Halay E D, Usheva A A, Hisatake K, Lee D K, Roeder R G, Burley S K. Crystal structure of a TFIIB-TBP-TATA-element ternary complex. Nature. 1995;377:119–129. doi: 10.1038/377119a0. [DOI] [PubMed] [Google Scholar]
- 107.Niu W, Kim Y, Tau G, Heyduk T, Ebright R H. Transcription activation at class II CAP-dependent promoters: two interactions between CAP and RNA polymerase. Cell. 1996;87:1123–1134. doi: 10.1016/s0092-8674(00)81806-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nudler E, Avetissova E, Markovtsov V, Goldfarb A. Transcription processivity: protein-DNA interactions holding together the elongation complex. Science. 1996;273:211–217. doi: 10.1126/science.273.5272.211. [DOI] [PubMed] [Google Scholar]
- 109.Nudler E, Gusarov I, Avetissova E, Kozlov M, Goldfarb A. Spatial organization of transcription elongation complex in E. coli. Science. 1998;281:424–428. doi: 10.1126/science.281.5375.424. [DOI] [PubMed] [Google Scholar]
- 110.Nudler E, Mustaev A, Lukhtanov E, Goldfarb A. The RNA-DNA hybrid maintains the register of transcription by preventing backtracking of RNA polymerase. Cell. 1997;89:33–41. doi: 10.1016/s0092-8674(00)80180-4. [DOI] [PubMed] [Google Scholar]
- 111.Oelgeschlager T, Chiang C M, Roeder R G. Topology and reorganization of a human TFIID-promoter complex. Nature. 1996;382:735–738. doi: 10.1038/382735a0. [DOI] [PubMed] [Google Scholar]
- 112.Oelgeschlager T, Tao Y, Kang Y K, Roeder R G. Transcription activation via enhanced preinitiation complex assembly in a human cell-free system lacking TAFIIs. Mol Cell. 1998;1:925–931. doi: 10.1016/s1097-2765(00)80092-1. [DOI] [PubMed] [Google Scholar]
- 113.Olave I, Reinberg D, Vales L D. The mammalian transcriptional repressor RBP (CBF1) targets TFIID and TFIIA to prevent activated transcription. Genes Dev. 1998;12:1621–1637. doi: 10.1101/gad.12.11.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Orphanides G, Lagrange T, Reinberg D. The general transcription factors of RNA polymerase II. Genes Dev. 1996;10:2657–2683. doi: 10.1101/gad.10.21.2657. [DOI] [PubMed] [Google Scholar]
- 115.Pan G, Greenblatt J. Initiation of transcription by RNA polymerase II is limited by melting of the promoter DNA in the region immediately upstream of the initiation site. J Biol Chem. 1994;269:30101–30104. [PubMed] [Google Scholar]
- 116.Parvin J D, Shykind B M, Meyers R E, Kim J, Sharp P A. Multiple sets of basal factors initiate transcription by RNA polymerase II. J Biol Chem. 1994;269:18414–18421. [PubMed] [Google Scholar]
- 117.Polyakov A, Severinova E, Darst S A. Three-dimensional structure of E. coli core RNA polymerase: promoter binding and elongation conformations of the enzyme. Cell. 1995;83:365–373. doi: 10.1016/0092-8674(95)90114-0. [DOI] [PubMed] [Google Scholar]
- 118.Price D H, Sluder A E, Greenleaf A L. Dynamic interaction between a Drosophila transcription factor and RNA polymerase II. Mol Cell Biol. 1989;9:1465–1475. doi: 10.1128/mcb.9.4.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ptashne M, Gann A. Transcriptional activation by recruitment. Nature. 1997;386:569–577. doi: 10.1038/386569a0. [DOI] [PubMed] [Google Scholar]
- 120.Qureshi S A, Baumann P, Rowlands T, Khoo B, Jackson S P. Cloning and functional analysis of the TATA binding protein from Sulfolobus shibatae. Nucleic Acids Res. 1995;23:1775–1781. doi: 10.1093/nar/23.10.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Qureshi S A, Bell S D, Jackson S P. Factor requirements for transcription in the Archaeon Sulfolobus shibatae. EMBO J. 1997;16:2927–2936. doi: 10.1093/emboj/16.10.2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Qureshi S A, Jackson S P. Sequence-specific DNA binding by the S. shibatae TFIIB homolog, TFB, and its effect on promoter strength. Mol Cell. 1998;1:389–400. doi: 10.1016/s1097-2765(00)80039-8. [DOI] [PubMed] [Google Scholar]
- 123.Qureshi S A, Khoo B, Baumann P, Jackson S P. Molecular cloning of the transcription factor TFIIB homolog from Sulfolobus shibatae. Proc Natl Acad Sci USA. 1995;92:6077–6081. doi: 10.1073/pnas.92.13.6077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rees W A, Keller R W, Vesenka J P, Yang G, Bustamante C. Evidence of DNA bending in transcription complexes imaged by scanning force microscopy. Science. 1993;260:1646–1649. doi: 10.1126/science.8503010. [DOI] [PubMed] [Google Scholar]
- 125.Reines D. Elongation factor-dependent transcript shortening by template-engaged RNA polymerase II. J Biol Chem. 1992;267:3795–3800. [PMC free article] [PubMed] [Google Scholar]
- 126.Reines D, Conaway J W, Conaway R C. The RNA polymerase II general elongation factors. Trends Biochem Sci. 1996;21:351–355. [PMC free article] [PubMed] [Google Scholar]
- 127.Reference deleted.
- 128.Reference deleted.
- 129.Robert F, Douziech M, Forget D, Egly J-M, Greenblatt J, Burton Z F, Coulombe B. Wrapping of promoter DNA around the RNA polymerase II initiation complex induced by TFIIF. Mol Cell. 1998;2:341–351. doi: 10.1016/s1097-2765(00)80278-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Robert F, Forget D, Li J, Greenblatt J, Coulombe B. Localization of subunits of transcription factors IIE and IIF immediately upstream of the transcriptional initiation site of the adenovirus major late promoter. J Biol Chem. 1996;271:8517–8520. doi: 10.1074/jbc.271.15.8517. [DOI] [PubMed] [Google Scholar]
- 131.Roe J H, Burgess R R, Record M T., Jr Temperature dependence of the rate constants of the E. coli RNA polymerase-lambda PR promoter interaction. Assignment of the kinetic steps corresponding to protein conformational change and DNA opening. J Mol Biol. 1985;184:441–453. doi: 10.1016/0022-2836(85)90293-1. [DOI] [PubMed] [Google Scholar]
- 132.Roy A L, Du H, Gregor P D, Novina C D, Martinez E, Roeder R G. Cloning of an inr- and E-box-binding protein, TFII-I, that interacts physically and functionally with USF1. EMBO J. 1997;16:7091–7104. doi: 10.1093/emboj/16.23.7091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rudd M D, Izban M G, Luse D S. The active site of RNA polymerase II participates in transcript cleavage within arrested ternary complexes. Proc Natl Acad Sci USA. 1994;91:8057–8061. doi: 10.1073/pnas.91.17.8057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Savery N J, Lloyd G S, Kainz M, Gaal T, Ross W, Ebright R H, Gourse R L, Busby S J. Transcription activation at class II CRP-dependent promoters: identification of determinants in the C-terminal domain of the RNA polymerase α subunit. EMBO J. 1998;17:3439–3447. doi: 10.1093/emboj/17.12.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sawadogo M, Roeder R G. Interaction of a gene-specific transcription factor with the adenovirus major late promoter upstream of the TATA box region. Cell. 1985;43:165–175. doi: 10.1016/0092-8674(85)90021-2. [DOI] [PubMed] [Google Scholar]
- 136.Schickor P, Metzger W, Werel W, Lederer H, Heumann H. Topography of intermediates in transcription initiation of E. coli. EMBO J. 1990;9:2215–2220. doi: 10.1002/j.1460-2075.1990.tb07391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Schultz S C, Shields G C, Steitz T A. Crystal structure of a CAP-DNA complex: the DNA is bent by 90 degrees. Science. 1991;253:1001–1007. doi: 10.1126/science.1653449. [DOI] [PubMed] [Google Scholar]
- 138.Sentenac A, Riva M, Thuriaux P, Buhler J-M, Treich I, Carles C, Werner M, Ruet A, Huet J, Mann C, Chiannilkulchai N, Stettler S, Mariotte S. Yeast RNA polymerase subunits and genes. In: McKnight S L, Yamamoto K R, editors. Transcriptional regulation. Vol. 1. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1992. pp. 27–54. [Google Scholar]
- 139.Severinov K, Fenyo D, Severinova E, Mustaev A, Chait B T, Goldfarb A, Darst S A. The ς subunit conserved region 3 is part of “5′-face” of active center of E. coli RNA polymerase. J Biol Chem. 1994;269:20826–20828. [PubMed] [Google Scholar]
- 140.Severinov K, Mustaev A, Kukarin A, Muzzin O, Bass I, Darst S A, Goldfarb A. Structural modules of the large subunits of RNA polymerase. Introducing archaebacterial and chloroplast split sites in the β and β′ subunits of E. coli RNA polymerase. J Biol Chem. 1996;271:27969–27974. doi: 10.1074/jbc.271.44.27969. [DOI] [PubMed] [Google Scholar]
- 141.Severinov K, Mustaev A, Severinova E, Kozlov M, Darst S A, Goldfarb A. The β subunit Rif-cluster I is only angstroms away from the active center of E. coli RNA polymerase. J Biol Chem. 1995;270(49):29428–29432. doi: 10.1074/jbc.270.49.29428. [DOI] [PubMed] [Google Scholar]
- 142.Shilatifard A, Duan D R, Haque D, Florence C, Schubach W H, Conaway J W, Conaway R C. ELL2, a new member of an ELL family of RNA polymerase II elongation factors. Proc Natl Acad Sci USA. 1997;94:3639–3643. doi: 10.1073/pnas.94.8.3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Shilatifard A, Haque D, Conaway R C, Conaway J W. Structure and function of RNA polymerase II elongation factor ELL. Identification of two overlapping ELL functional domains that govern its interaction with polymerase and the ternary elongation complex. J Biol Chem. 1997;272:22355–22363. doi: 10.1074/jbc.272.35.22355. [DOI] [PubMed] [Google Scholar]
- 144.Shilatifard A, Lane W S, Jackson K W, Conaway R C, Conaway J W. An RNA polymerase II elongation factor encoded by the human ELL gene. Science. 1996;271:1873–1876. doi: 10.1126/science.271.5257.1873. [DOI] [PubMed] [Google Scholar]
- 145.Sidorenkov I, Komissarova N, Kashlev M. Crucial role of the RNA:DNA hybrid in the processivity of transcription. Mol Cell. 1998;2:55–64. doi: 10.1016/s1097-2765(00)80113-6. [DOI] [PubMed] [Google Scholar]
- 146.Siebenlist U. RNA polymerase unwinds an 11-base pair segment of a phage T7 promoter. Nature. 1979;279:651–652. doi: 10.1038/279651a0. [DOI] [PubMed] [Google Scholar]
- 147.Siebenlist U, Simpson R B, Gilbert W. E. coli RNA polymerase interacts homologously with two different promoters. Cell. 1980;20:269–281. doi: 10.1016/0092-8674(80)90613-3. [DOI] [PubMed] [Google Scholar]
- 148.Smale S T. Core promoter architecture for eukaryotic protein-coding genes. In: Conaway R C, Conaway J W, editors. Transcription: mechanisms and regulation. New York, N.Y: Raven Press; 1994. pp. 63–81. [Google Scholar]
- 149.Sopta M, Burton Z F, Greenblatt J. Structure and associated DNA-helicase activity of a general transcription initiation factor that binds to RNA polymerase II. Nature. 1989;341:410–414. doi: 10.1038/341410a0. [DOI] [PubMed] [Google Scholar]
- 150.Spassky A, Kirkegaard K, Buc H. Changes in the DNA structure of the lacUV5 promoter during formation of an open complex with E. coli RNA polymerase. Biochemistry. 1985;24:2723–2731. doi: 10.1021/bi00332a019. [DOI] [PubMed] [Google Scholar]
- 151.Steitz T A, Smerdon S J, Jager J, Joyce C M. A unified polymerase mechanism for nonhomologous DNA and RNA polymerases. Science. 1994;266:2022–2025. doi: 10.1126/science.7528445. [DOI] [PubMed] [Google Scholar]
- 152.Svetlov V, Nolan K, Burgess R R. Rpb3, stoichiometry and sequence determinants of the assembly into yeast RNA polymerase II in vivo. J Biol Chem. 1998;273:10827–10830. doi: 10.1074/jbc.273.18.10827. [DOI] [PubMed] [Google Scholar]
- 153.Tan S, Garrett K P, Conaway R C, Conaway J W. Cryptic DNA-binding domain in the C terminus of RNA polymerase II general transcription factor RAP30. Proc Natl Acad Sci USA. 1994;91:9808–9812. doi: 10.1073/pnas.91.21.9808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Tansey W P, Herr W. TAFs: guilt by association? Cell. 1997;88:729–732. doi: 10.1016/s0092-8674(00)81916-9. [DOI] [PubMed] [Google Scholar]
- 155.Thuillier V, Brun I, Sentenac A, Werner M. Mutations in the α-amanitin conserved domain of the largest subunit of yeast RNA polymerase III affect pausing, RNA cleavage and transcriptional transitions. EMBO J. 1996;15:618–629. [PMC free article] [PubMed] [Google Scholar]
- 156.Travers A A. Why bend DNA? Cell. 1990;60:177–180. doi: 10.1016/0092-8674(90)90729-x. [DOI] [PubMed] [Google Scholar]
- 157.Verrijzer C P, Chen J L, Yokomori K, Tjian R. Binding of TAFs to core elements directs promoter selectivity by RNA polymerase II. Cell. 1995;81:1115–1125. doi: 10.1016/s0092-8674(05)80016-9. [DOI] [PubMed] [Google Scholar]
- 158.Verrijzer C P, Tjian R. TAFs mediate transcriptional activation and promoter selectivity. Trends Biochem Sci. 1996;21:338–342. [PubMed] [Google Scholar]
- 159.Verrijzer C P, Yokomori K, Chen J L, Tjian R. Drosophila TAFII150: similarity to yeast gene TSM-1 and specific binding to core promoter DNA. Science. 1994;264:933–941. doi: 10.1126/science.8178153. [DOI] [PubMed] [Google Scholar]
- 160.Walker S S, Reese J C, Apone L M, Green M R. Transcription activation in cells lacking TAFIIS. Nature. 1996;383:185–188. doi: 10.1038/383185a0. [DOI] [PubMed] [Google Scholar]
- 161.Wang D, Hawley D K. Identification of a 3′⇒5′ exonuclease activity associated with human RNA polymerase II. Proc Natl Acad Sci USA. 1993;90:843–847. doi: 10.1073/pnas.90.3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Werner M, Chaussivert N, Willis I M, Sentenac A. Interaction between a complex of RNA polymerase III subunits and the 70- kDa component of transcription factor IIIB. J Biol Chem. 1993;268:20721–20724. [PubMed] [Google Scholar]
- 163.Wieczorek E, Brand M, Jacq X, Tora L. Function of TAFII-containing complex without TBP in transcription by RNA polymerase II. Nature. 1998;393:187–191. doi: 10.1038/30283. [DOI] [PubMed] [Google Scholar]
- 164.Woychik N A, Young R A. Exploring RNA polymerase II structure and function. In: Conaway R C, Conaway J W, editors. Transcription: mechanisms and regulation. Vol. 3. New York, N.Y: Raven Press; 1994. pp. 227–242. [Google Scholar]
- 165.Zaychikov E, Denissova L, Heumann H. Translocation of the E. coli transcription complex observed in the registers 11 to 20: “jumping” of RNA polymerase and asymmetric expansion and contraction of the “transcription bubble.”. Proc Natl Acad Sci USA. 1995;92:1739–1743. doi: 10.1073/pnas.92.5.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zaychikov E, Martin E, Denissova L, Kozlov M, Markovtsov V, Kashlev M, Heumann H, Nikiforov V, Goldfarb A, Mustaev A. Mapping of catalytic residues in the RNA polymerase active center. Science. 1996;273:107–109. doi: 10.1126/science.273.5271.107. [DOI] [PubMed] [Google Scholar]
- 167.Zhang G, Darst S A. Structure of the E. coli RNA polymerase α subunit amino-terminal domain. Science. 1998;281:262–266. doi: 10.1126/science.281.5374.262. [DOI] [PubMed] [Google Scholar]
- 168.Zhou Q, Lieberman P M, Boyer T G, Berk A J. Holo-TFIID supports transcriptional stimulation by diverse activators and from a TATA-less promoter. Genes Dev. 1992;6:1964–1974. doi: 10.1101/gad.6.10.1964. [DOI] [PubMed] [Google Scholar]
- 169.Zhou Y, Merkel T J, Ebright R H. Characterization of the activating region of E. coli catabolite gene activator protein (CAP). II. Role at class I and class II CAP-dependent promoters. J Mol Biol. 1994;243:603–610. doi: 10.1016/0022-2836(94)90035-3. [DOI] [PubMed] [Google Scholar]