Abstract
Aim
To synthesize international findings on the alcohol–dementia relationship, including representation from low‐ and middle‐income countries.
Methods
Individual participant data meta‐analysis of 15 prospective epidemiological cohort studies from countries situated in six continents. Cox regression investigated the dementia risk associated with alcohol use in older adults aged over 60 years. Additional analyses assessed the alcohol–dementia relationship in the sample stratified by sex and by continent. Participants included 24 478 community dwelling individuals without a history of dementia at baseline and at least one follow‐up dementia assessment. The main outcome measure was all‐cause dementia as determined by clinical interview.
Results
At baseline, the mean age across studies was 71.8 (standard deviation = 7.5, range = 60–102 years), 14 260 (58.3%) were female and 13 269 (54.2%) were current drinkers. During 151 636 person‐years of follow‐up, there were 2124 incident cases of dementia (14.0 per 1000 person‐years). When compared with abstainers, the risk for dementia was lower in occasional [hazard ratio (HR) = 0.78; 95% confidence interval (CI) = 0.68–0.89], light–moderate (HR = 0.78; 95% CI = 0.70–0.87) and moderate–heavy drinkers (HR = 0.62; 95% CI = 0.51–0.77). There was no evidence of differences between life‐time abstainers and former drinkers in terms of dementia risk (HR = 0.98; 95% CI = 0.81–1.18). In dose–response analyses, moderate drinking up to 40 g/day was associated with a lower risk of dementia when compared with lif‐time abstaining. Among current drinkers, there was no consistent evidence for differences in terms of dementia risk. Results were similar when the sample was stratified by sex. When analysed at the continent level, there was considerable heterogeneity in the alcohol–dementia relationship.
Conclusions
Abstinence from alcohol appears to be associated with an increased risk for all‐cause dementia. Among current drinkers, there appears to be no consistent evidence to suggest that the amount of alcohol consumed in later life is associated with dementia risk.
Keywords: Alcohol, cross‐national comparison, dementia, epidemiology, individual participant data meta‐analysis, older adults
INTRODUCTION
In recent decades, the estimated global prevalence of dementia has nearly tripled, from 20.2 million in 1990 to 57.4 million in 2019 [1]. By 2050, the number of individuals living with dementia globally is projected to increase to 152 million [2]. Due to increases in life expectancy and greater risk factor exposure, the largest increase in dementia prevalence is expected among those living in low‐ and middle‐income countries [2]. In the absence of disease‐modifying treatments for dementia, risk factor reduction is a fundamental strategy for preventing dementia onset [3]. To this end, in the 2020 report from the Lancet Commission for Dementia Prevention, Intervention and Care it was estimated that 40% of global dementia cases could be prevented or delayed if 12 key modifiable risk factors for dementia were eliminated [3].
Excessive or harmful alcohol use in mid‐life was newly included in the 2020 report from the Lancet Commission as one of the key modifiable risk factors for dementia [3]. This was supported by considerable evidence for the neurotoxic effects of ethanol on the brain [4, 5, 6], and by a recent study of hospital‐based records that identified alcohol use disorders as one of the strongest modifiable risk factors for dementia when compared with other established risk factors, including high blood pressure and diabetes [7]. In population‐based observational studies, often based on samples of older adults, heavy alcohol use has sometimes been found to increase the risk for dementia, although some studies have found heavy alcohol use to be unrelated to dementia risk [8]. In contrast to heavy use, population‐based studies have often found that light‐to‐moderate alcohol use appears to reduce dementia risk when compared with abstinence [8]. Overall, reviews of population‐based observational studies suggest that the alcohol–dementia relationship is likely to be J‐shaped, with low levels of alcohol use conferring some benefit when compared with abstinence from alcohol and progressively higher levels of alcohol use associated with a steadily increasing dementia risk in a dose–response trend [8, 9, 10].
While the evidence base for the alcohol–dementia relationship is large, prior meta‐analyses of published results have several limitations. There is a lack of standardization throughout studies in terms of alcohol categorization, with definitions of ‘light’, ‘moderate’ and ‘heavy’ alcohol use varying widely across studies and impeding cross‐study comparison. The abstaining group is often comprised of both former drinkers and life‐time abstainers, with former drinkers (or ‘sick quitters’) potentially driving the relationship between abstention and poorer health outcomes (i.e. reverse causation) [11]. Importantly, studies of the alcohol–dementia relationship are largely based on samples from high‐income countries [9, 10]. Evidence for the relationship between alcohol use and dementia is sparse in low‐ to middle‐income countries, where the future burden of dementia is likely to be concentrated [3], and where alcohol use is increasing [12].
The current study addresses these limitations by harmonizing individual participant‐level data from 15 prospective epidemiological cohort studies, including representation from countries situated throughout six continents, and examining the alcohol–dementia relationship. The overall aim of this study is to synthesize international findings on the alcohol–dementia relationship, including representation from low‐ and middle‐income countries.
METHODS
Contributing cohorts
All 15 contributing cohort studies are members of the Cohort Studies of Memory in an International Consortium (COSMIC) collaboration [13], and are detailed in Table 1. None of the cohorts reported participant exclusion criteria on the basis of alcohol use. Individuals were excluded from the current study if they were diagnosed with dementia at baseline, if they were missing baseline dementia status data, if they did not have any follow‐up dementia status assessment or if they were missing baseline alcohol use, age or sex data. For the current study, baseline year of data collection for each cohort was the first assessment occasion where both alcohol use and dementia status were assessed, and ranged from 1975 to 2011. The cohorts had various assessment schedules (two to 19 waves), follow‐up durations (4–40 years) and methods for establishing consensus diagnosis of dementia (Supporting information, Table S1). While the majority of the cohorts were based in high‐income countries, this study also includes representation from cohorts based in Brazil and the Republic of Congo. This project was approved by the University of New South Wales Human Research Ethics Committee (HC12446 and HC17292). The contributing cohort studies also had ethics approval. This study is reported as per the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines. The analysis was not pre‐registered and should therefore be considered exploratory.
TABLE 1.
Details of contributing studies.
| Study | Abbreviation | Location | Assessment years | Number of assessment waves | n | Criteria for dementia diagnosis |
|---|---|---|---|---|---|---|
| Einstein Ageing Study [14] | EAS | New York, USA | 1993–2017 | 19 | 1284 | DSM‐IV |
| Epidemiology of Dementia in Central Africa [15] | EPIDEMCA | Republic of Congo | 2011–14 | 2 | 721 | DSM‐IV |
| Etude Sante Psychologique Prevalence Risques et Traitement [16] | ESPRIT | Montpellier, France | 1999–2016 | 7 | 1917 | DSM‐IV |
| Framingham Heart Study (original cohort) [17] | FHS | Framingham, MA, USA | 1975–2015 | 15 | 1658 | DSM‐IV |
| Gothenburg H70 Birth Cohort Studies [18] | H70 | Gothenburg, Sweden | 2000–09 | 3 | 593 | DSM‐III‐R |
| Hellenic Longitudinal Investigation of Ageing and Diet [19] | HELIAD | Larissa and Marousi, Greece | 2009–18 | 2 | 972 | DSM‐IV |
| Korean Longitudinal Study on Cognitive Ageing and Dementia [20] | KLOSCAD | South Korea | 2009–18 | 4 | 5098 | DSM‐IV |
| Leipzig Longitudinal Study of the Aged [21] | LEILA75+ | Leipzig, Germany | 1997–2014 | 7 | 851 | DSM‐IV |
| Maastricht Ageing Study [22] | MAAS | South Limburg, the Netherlands | 1993–2018 | 3 | 433 | DSM‐III = R/DSM‐IV |
| Monongahela–Youghiogheny Healthy Ageing Team [23] | MYHAT | Small‐town region of PA, USA | 2006–16 | 11 | 1652 | CDR > 1 |
| Personality and Total Health Through Life Project [24] | PATH | Canberra, Australia | 2001–15 | 4 | 2238 | DSM‐IV |
| Sacramento Area Latino Study on Ageing [25] | SALSA | Latinos living in the Sacramento area, CA, USA | 1998–2008 | 7 | 1456 | DSM‐IV |
| São Paulo Ageing and Health Study [26] | SPAH | São Paulo, Brazil | 2003–08 | 2 | 1595 | DSM‐IV |
| Sydney Memory and Ageing Study [27] | MAS | Sydney, Australia | 2005–14 | 4 | 905 | DSM‐IV |
| Zaragoza Dementia Depression Project [28] | ZARADEMP | Zaragoza, Spain | 1994–2002 | 3 | 3099 | DSM‐IV |
CDR = Clinical Dementia Rating; DSM‐III = Diagnostic and Statistical Manual of Mental Disorders, 3rd edn; DSM‐IV = Diagnostic and Statistical Manual of Mental Disorders, 4th edn.
Measures
Criteria for dementia diagnoses are listed in Table 1. Given variability across the contributing cohorts in terms of data collected on dementia subtypes, as well as the low population incidence of dementia, the main outcome variable for the current study was all‐cause dementia. Date of death data were also provided for 13 of the 15 cohorts included in this study, allowing the implementation of competing risks models in these datasets (date of death data were not available for the PATH and SPAH cohorts).
For each cohort, alcohol use was converted into average grams of pure ethanol per day (g/day), taking into account the type of alcoholic beverage reported (in studies where beverage type was differentiated) and the definition of a standard drink in the different national contexts. This g/day variable was used to model the dose–response relationship between alcohol use and dementia. Using data from all cohorts, a five‐level alcohol use variable was calculated that included no current alcohol use (current abstaining), occasional alcohol use (< 1.3 g/day), light–moderate alcohol use (1.3–24.9 g/day), moderate–heavy alcohol use (25–44.9 g/day) and heavy alcohol use (> 45 g/day). In 11 of the 15 cohorts, data on historical alcohol use were also available (i.e. ever consumed alcohol over the life‐time), allowing the separation of the current abstaining group into former drinkers and life‐time abstainers in these 11 cohorts. Supporting information, Table S2 includes details on assessment of alcohol use within each cohort and detailed code for processing the alcohol use data within each cohort is included in the Supporting information (pp. 31–48). Four of the 15 cohorts also included information on frequency of alcohol use among current drinkers that could be harmonized so that daily drinkers could be compared with those drinking less than daily (see Supporting information, Table S2 for details on harmonization of frequency data).
All cohorts included data on age, sex and smoking status (categorized as current, former and never smoker). Additional demographic covariates included years of education at baseline (continuous variable; data available from 14 cohorts) and body mass index at baseline (BMI; continuous variable; data available from 14 cohorts). Clinical covariates included baseline depression status (absent/present; data available from all cohorts), a history of stroke at baseline (absent/present; data available from 14 cohorts), a history of diabetes at baseline (absent/present; data available from all cohorts), a history of myocardial infarction at baseline (absent/present; data available from 13 cohorts), hypertension at baseline (absent/present; data available from all cohorts) and high cholesterol at baseline (absent/present; data available from 14 cohorts). Supporting information, Tables S3–S8 include detail on the assessment, harmonization and distribution of all demographic and clinical covariates.
Statistical analysis
The proportion of missing data was generally less than 5% for any given covariate within a cohort, although extensive missing data were present for some covariates in some cohorts (see Supporting information, Tables S7 and S8 for details on missing data on baseline covariates). Prior to analysis, multiple imputation was used to account for missing data on baseline covariates within each cohort. For each cohort, 20 imputed data sets were created using the mice package in R [29]. To correct for the presence of dependent censoring, inverse probability of censoring weights were calculated using the WeightIt package in R [30]. See Supporting information for further details on multiple imputation and weight generation, as well as the R code. Data from individual cohorts were combined and analysed using a one‐stage individual participant data meta‐analytical approach. Event times were censored at the end of follow‐up/participant drop‐out, date of dementia diagnosis or date of death. A P‐value of 0.05 was considered statistically significant, and 95% confidence intervals (CI) are reported.
Alcohol use categories
Analyses first focused on the categorical alcohol use variable and were conducted in the full sample with current abstainers as the reference category. All analyses were then repeated in the sample of 11 cohorts where life‐time abstainers and former drinkers could be separated, with life‐time abstainers as the reference category. These analyses included inverse probability of censoring weights and were adjusted for age, sex and smoking status, as well as a random effect for cohort using the coxme package in R [31]. To identify sex‐specific relationships between alcohol use and dementia, these analyses were repeated in males and females.
Next, analyses were repeated in the subsample of cohorts that allowed adjustment for all additional demographic and clinical covariates considered (i.e. education, BMI, depression, stroke, diabetes, myocardial infarction, hypertension and high cholesterol). These ‘fully adjusted’ analyses were conducted to determine whether the relationship between dementia and alcohol use was robust to potential confounders.
Analyses were then conducted that accounted for competing risks of mortality in the cohorts that provided date of death data. This analysis accounted for the possibility that those who died may have developed dementia in the future. Competing risks models were conducted using the survival package in R [32], adjusting for age, sex and smoking status, as well as cohort as a clustering variable (as opposed to a random effect). All subgroup analyses based on sex, covariate adjustment and competing risks were planned a priori.
Dose–response curves
Dose–response analyses were first conducted with 0 g/day as the reference value. Former drinkers were excluded from these analyses and were therefore conducted in 11 of the 15 cohorts where life‐time abstainers could be separated from former drinkers. To allow health guidance among drinkers, these analyses were repeated using current drinkers only from each of the 15 cohorts, with the lowest volume of alcohol consumed per day set as the reference value (0.3 g/day). The rms package [33] in R was used to calculate hazard ratios for alcohol use modelled using restricted cubic splines (three knots at the 10th, 50th and 90th percentiles). These models included inverse probability of censoring weights and adjusted for the fixed effects of age, sex and smoking status, as well as cohort as a cluster variable. These analyses were also repeated in subsamples of males and females and in the subsample of cohorts that allowed for adjustment of all demographic and clinical covariates considered.
Post‐hoc sensitivity analyses
To correct for measurement error and within‐person variability in alcohol use over time, multi‐level regression calibration was implemented using information from 66 898 follow‐up assessments in 15 433 participants from 12 cohorts. A regression dilution ratio was estimated from a calibration model that regressed follow‐up alcohol consumption measurements on baseline alcohol consumption, adjusted for duration of follow‐up and baseline age, sex, smoking status, education, BMI, depression, stroke, diabetes, hypertension and high cholesterol. Nested random effects for follow‐up wave and study were also included in the calibration model. The resulting regression dilution ratio of 0.46 was extracted from this calibration model and hazard ratios (HRs) and confidence intervals were divided by this estimate to derive dose–response curves that accounted for measurement error and within‐person variability in alcohol use over time [34, 35, 36].
Post‐hoc sensitivity analyses were also conducted to determine whether the results replicated within cohorts grouped by continent where there were sufficient data, as well as to determine whether the results replicated after those reporting stroke at baseline were excluded from the analysis.
The relationship between daily drinking and dementia risk was also examined in four cohorts that included consistent information on frequency of alcohol use. These analyses were conducted with the coxme package in R and included the binary drinking frequency variable (daily drinking/not daily drinking) while adjusting for age, sex, smoking status and baseline alcohol consumption (g/day), as well as cohort as a random effect.
RESULTS
The combined sample of 15 cohorts included 33 532 individuals. Of these, 1522 individuals were excluded from the current study due to a dementia diagnosis at baseline, 270 had missing baseline dementia status data, 832 did not have baseline alcohol use data, six did not have data on sex and 6424 did not have any follow‐up dementia status assessment. The final analytical sample consisted of 24 478 individuals. Those included and excluded from the analyses differed in terms of alcohol use, as well as demographic and clinical characteristics (see Supporting information, Tables S9 and S10).
At baseline, the mean age across studies was 71.8 [standard deviation (SD) = 7.5, range = 60–102 years], 14 260 (58.3%) were female and 13 269 (54.2%) were current drinkers (Table 2). During 151 636 person‐years of follow‐up, there were 2124 incident cases of dementia (14.0 per 1000 person‐years). Baseline drinking patterns varied considerably across cohorts, particularly with respect to the number of abstainers (Table 2), as did demographic and clinical characteristics (Table 2, Supporting information, Tables S7 and S8). The relationships between each of the demographic and clinical characteristics and dementia risk in the combined sample are reported in Supporting information, Table S11.
TABLE 2.
Alcohol use and basic demographic characteristics of contributing cohorts.
| Current drinker categories for individual participant data meta‐analysis | Age | Sex (female) | Smoking status | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Abstainera % (n) | Former drinker % (n) | < 1.3 g/day % (n) | 1.3–24.9 g/day % (n) | 25–44.9 g/day % (n) | ≥ 45 g/day % (n) | Mean (SD) | % (n) | Current % (n) | Former % (n) | Never % (n) | |
| EAS (1284) | 28.4 (365) | 18.2 (234) | 29.4 (378) | 36.3 (466) | 5.3 (68) | 0.5 (7) | 78.0 (5.4) | 61.0 (783) | 6.8 (87) | 46.8 (601) | 45.2 (581) |
| EPIDEMCA (721) | 68.7 (495) | – | 10.7 (77) | 16.8 (121) | 2.5 (18) | 1.4 (10) | 73.6 (6.6) | 58.4 (421) | 13.3 (96) | 5.8 (42) | 80.6 (581) |
| ESPRIT (1917) | 16.6 (318) | 3.2 (61) | 8.4 (161) | 56.2 (1078) | 13.1 (251) | 5.7 (109) | 72.9 (5.4) | 59.0 (1130) | 6.4 (123) | 35.6 (681) | 58.0 (1112) |
| FHS (1658) | 36.9 (612) | – | 11.3 (187) | 34.4 (571) | 11.9 (198) | 5.4 (90) | 71.0 (6.5) | 60.6 (1005) | 16.3 (271) | 31.8 (527) | 50.1 (831) |
| H70 (576) | 12.7 (73) | 4.5 (26) | – | 81.9 (472) | 3.6 (21) | 1.7 (10) | 73.4 (4.9) | 73.3 (422) | 10.4 (60) | 28.8 (166) | 40.8 (235) |
| HELIAD (954) | 58.6 (559) | 16.0 (148) | 6.1 (59) | 29.4 (280) | 3.8 (36) | 2.1 (20) | 72.4 (5.3) | 60.7 (579) | 10.2 (97) | 26.8 (256) | 62.8 (599) |
| KLOSCAD (5139) | 67.6 (3475) | 7.8 (398) | 4.8 (246) | 18.9 (972) | 4.3 (220) | 4.4 (226) | 69.7 (6.4) | 56.7 (2915) | 8.2 (422) | 21.7 (1113) | 69.8 (3589) |
| LEILA75+ (851) | 12.0 (102) | 9.4 (80) | 41.4 (352) | 39.1 (333) | 6.0 (51) | 1.5 (13) | 81.2 (4.8) | 74.2 (632) | 1.8 (15) | 24.7 (210) | 68.3 (581) |
| MAAS (433) | 23.8 (103) | – | 19.9 (86) | 51.3 (222) | 0.5 (2) | 4.6 (20) | 68.4 (5.7) | 50.6 (219) | 19.6 (85) | 43.6 (189) | 36.7 (159) |
| MYHAT (1652) | 33.5 (554) | 19.5 (322) | 36.2 (598) | 25.9 (428) | 3.8 (62) | 0.6 (10) | 77.4 (7.3) | 62.2 (1028) | 6.9 (114) | 45.2 (746) | 47.7 (788) |
| PATH (2238) | 13.3 (299) | 8.4 (189) | 15.5 (348) | 56.9 (1274) | 13.3 (297) | 0.9 (20) | 62.5 (1.5) | 48.4 (1084) | 9.7 (218) | 37.2 (833) | 53.0 (1187) |
| SALSA (1456) | 43.6 (635) | – | 31.9 (465) | 17.5 (255) | 4.1 (59) | 2.9 (42) | 70.2 (6.6) | 58.3 (849) | 11.0 (160) | 43.3 (630) | 45.7 (666) |
| SPAH (1595) | 80.7 (1287) | 23.9 (381) | – | 12.2 (194) | 1.1 (18) | 6.0 (96) | 71.6 (5.7) | 61.6 (983) | 13.2 (211) | 40.9 (653) | 45.8 (731) |
| Sydney MAS (905) | 12.3 (111) | 6.2 (56) | 25.3 (229) | 37.8 (342) | 16.0 (145) | 8.6 (78) | 78.6 (4.8) | 54.4 (492) | 3.0 (27) | 50.6 (458) | 46.2 (418) |
| Zarademp (3099) | 71.7 (2221) | 11.2 (347) | 2.2 (69) | 19.9 (618) | 4.4 (135) | 1.8 (56) | 72.0 (8.5) | 55.4 (1718) | 13.6 (420) | 22.0 (683) | 64.4 (1996) |
| Total (24478) | 45.8 (11209) | 16.7 (4091) | 13.3 (3255) | 31.2 (7626) | 6.5 (1581) | 3.3 (807) | 71.8 (7.5) | 58.3 (14260) | 10.0 (2452) | 31.8 (7788) | 57.2 (14008) |
EAS = Einstein Aging Study; EPIDEMCA = Epidemiology of Dementia in Central Africa; ESPRIT = Etude Santé Psychologique Prévalence Risques et Traitement; FHS = Framingham Heart Study; H70 : Gothenburg H70 Birth Cohort Studies; HELIAD = Hellenic Longitudinal Investigation of Aging and Diet; KLOSCAD = Korean Longitudinal Study on Cognitive Aging and Dementia; LEILA75+ = Leipzig Longitudinal Study of the Aged; MAAS = Maastricht Ageing Study; MYHAT = Monongahela–Youghiogheny Healthy Aging Team; PATH = Personality and Total Health Through Life Project; SALSA = Sacramento Area Latino Study on Aging; SPAH = São Paulo Ageing and Health Study; Sydney MAS = Sydney Memory and Ageing Study; ZARADEMP = Zaragoza Dementia Depression Project; SD = standard deviation.
Abstainer group includes both life‐time abstainers and former drinkers.
Alcohol use categories
When compared with abstainers, the risk for dementia was lower in occasional, light–moderate and moderate–heavy drinkers (Table 3). Similar relationships were found in the fully adjusted model and competing risk model, as well as in the subsample of males. For women, the unadjusted model showed that, when compared with abstainers, the risk for dementia was lower in occasional, light–moderate and moderate–heavy drinkers. There was no evidence of a relationship between alcohol use and dementia in females when the models were fully adjusted and when the model adjusted for competing risk of death.
TABLE 3.
Combined sample hazard ratios.
| Main model a | Fully adjusted model b | Competing risk model c | |
|---|---|---|---|
| Combined sample | n = 24 478 | n = 20 878 | n = 20 645 |
| Abstainer | Ref | Ref | Ref |
| ≤ 1.3 g/day | 0.78 (0.68, 0.89) | 0.82 (0.71, 0.96) | 0.73 (0.54, 0.97) |
| 1.3–24.9 g/day | 0.78 (0.70, 0.87) | 0.85 (0.76, 0.96) | 0.78 (0.64, 0.95) |
| 25–44.9 g/day | 0.62 (0.51, 0.75) | 0.74 (0.60, 0.90) | 0.65 (0.45, 0.93) |
| ≥ 45 g/day | 0.81 (0.61, 1.08) | 0.78 (0.57, 1.05) | 0.79 (0.58, 1.06) |
| Male sample | n = 10 218 | n = 8873 | n = 8452 |
| Abstainer | Ref | Ref | Ref |
| ≤ 1.3 g/day | 0.69 (0.53, 0.91) | 0.71 (0.53, 0.95) | 0.62 (0.46, 0.82) |
| 1.3–24.9 g/day | 0.74 (0.62, 0.88) | 0.84 (0.69, 1.02) | 0.70 (0.55, 0.89) |
| 25–44.9 g/day | 0.59 (0.46, 0.77) | 0.70 (0.54, 0.92) | 0.61 (0.44, 0.84) |
| ≥ 45 g/day | 0.82 (0.60, 1.13) | 0.80 (0.57, 1.13) | 0.78 (0.58, 1.04) |
| Female sample | n = 14 260 | n = 12 005 | n = 12 193 |
| Abstainer | Ref | Ref | Ref |
| ≤ 1.3 g/day | 0.80 (0.68, 0.94) | 0.88 (0.73, 1.05) | 0.76 (0.53, 1.09) |
| 1.3–24.9 g/day | 0.82 (0.71, 0.94) | 0.88 (0.76, 1.02) | 0.82 (0.67, 1.01) |
| 25–44.9 g/day | 0.63 (0.46, 0.88) | 0.76 (0.55, 1.06) | 0.66 (0.40, 1.10) |
| ≥ 45 g/day | 0.37 (0.12, 1.11) | 0.36 (0.12, 1.10) | 0.40 (0.12, 1.35) |
Note: Bold = statistically significant at p < .05.
Model included all 15 cohorts and adjusted for age, sex, smoking status and random effect of study;
Model included 11 cohorts and adjusted for age, sex, smoking status, education, body mass index (BMI), depression, stroke, diabetes, myocardial infarction, hypertension, high cholesterol and random effect for study;
Model included 13 cohorts, adjusted for age, sex, smoking status and study as a cluster variable and accounted for competing risk of death.
In the 11 cohorts where life‐time abstainers could be separated from former drinkers (n = 20 187; Supporting information, Table S12), there was no evidence for statistically significant differences between life‐time abstainers (reference group) and former drinkers in terms of dementia risk. There was similarly no evidence for statistically significant differences between life‐time abstainers and former drinkers in the subsample of males, the subsample of females, when the analyses were fully adjusted for all demographic and clinical characteristics and when the competing risk of death was taken into account.
Dose–response curves
Among the 11 cohorts with data on former drinkers, 2223 participants were classified as former drinkers and excluded from the dose–response analysis with 0 g/day as the reference value (n = 17 964). In this analysis (Fig. 1), moderate drinking up to 40 g/day was associated with a lower risk of incident dementia when compared with life‐time abstaining (p‐value for non‐linearity = 0.0004). A similar relationship was identified in males (n = 7216; p‐value for non‐linearity = 0.0004) and females (n = 10 748; p‐value for non‐linearity = 0.0004), as well as in analyses which fully adjusted for demographic and clinical characteristics (n = 15 979; p‐value for non‐linearity = 0.043).
FIGURE 1.
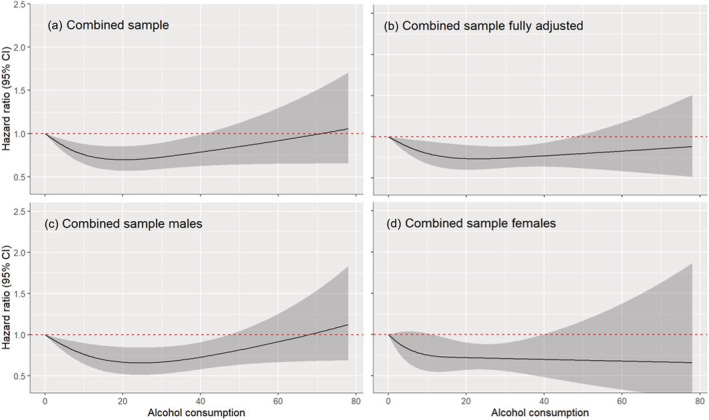
Dose–response relationship between alcohol use (g/day) and dementia including life‐time abstainers (reference group) and current drinkers
Meanwhile, a total of 13 335 participants (from 15 cohorts) were classified as current drinkers and included in the drinker‐only dose–response analysis. Among drinkers, there was no evidence for differences in terms of dementia risk (Fig. 2). There was also no evidence for a relationship between alcohol use and dementia risk identified in males (n = 7063) or females (n = 6272), as well as in analyses which adjusted for demographic and clinical characteristics (n = 11 722).
FIGURE 2.
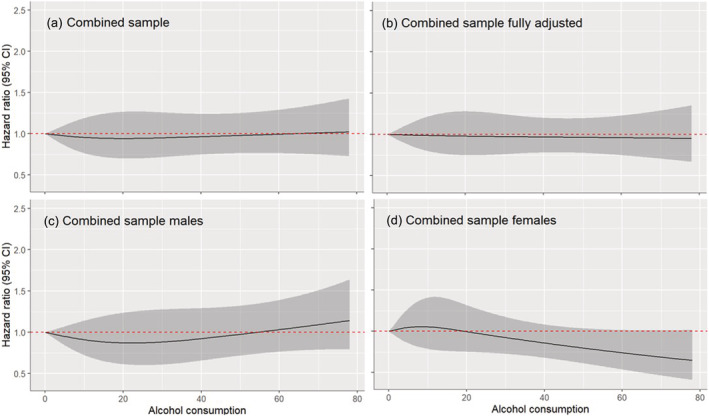
Dose–response relationship between alcohol use (g/day) and dementia among current drinkers
Sensitivity analyses
Results remained similar when baseline drinking status was adjusted for using the regression dilution ratio (Supporting information, Figs S1 and S2).
In the analysis comparing daily drinkers with those who were not daily drinkers (n = 4581), there was no evidence for statistically significant differences in terms of dementia risk (HR = 0.64; 95% CI = 0.41–1.0; P = 0.052).
When those who reported stroke at baseline were excluded from the analyses, a similar relationship was identified between alcohol use and dementia risk in the samples including life‐time abstainers (n = 17 016) and drinkers only (n = 12 343; see Supporting information, Fig. S3).
Subgroup analyses by continent were possible for North America, Europe, Oceania (Australia) and Asia (Korea). For the abstainer analysis (Supporting information, Fig. S4), there were non‐linear relationships between alcohol use and dementia risk for North America (n = 2380), Europe (n = 6735) and Asia (n = 4737), although there was no evidence for any statistically significant differences within these reduced sample sizes. In Oceania (n = 2898), there was evidence of a protective effect of alcohol use across the full spectrum of consumption when compared with life‐time abstainers. For the analysis including current drinkers only, results differed across continents (Supporting information, Fig. S5). When compared to those who drank minimally (0.3 g/day), there was evidence of lower dementia risk among light–moderate drinkers in Europe (n = 4527) and across the full spectrum of alcohol consumption in Oceania (n = 2733). Conversely, in North America (n = 3877), there was a higher dementia risk among light–moderate drinkers when compared to those who drank minimally. Meanwhile, there was no evidence of a relationship between alcohol use and dementia risk among current drinkers in Asia (n = 1664).
DISCUSSION
In a large international sample of older adults aged more than 60 years, the current study found that abstinence from alcohol is associated with an increased risk for all‐cause dementia. The increased risk associated with abstaining was evident in subsamples of both males and females, as well as in both former drinkers and life‐time abstainers. Among current drinkers in the general population, there was no consistent evidence to suggest that the amount of alcohol consumed in later life was significantly associated with dementia risk. While the current findings are relevant to the majority of older drinkers in the general population, the current study does not provide evidence on the relationships between dementia risk and heavier drinking or alcohol use disorder which are relatively rare in the general population.
Strengths and limitations
Through the use of data harmonization and individual participant data analysis, the current study overcomes many limitations of previous research. Among the 15 cohorts, alcohol use categories were harmonized so that comparisons were consistent across cohorts. The majority of cohorts allowed the separation of current abstainers into former drinkers and life‐time abstainers, allowing the exclusion of former drinkers from the abstainer category. Within each cohort, clinical consensus diagnosis of all‐cause dementia was used as the outcome variable. Importantly, this study included cohorts from high‐income countries and low‐ and middle‐income countries (i.e. Brazil and the Republic of Congo), providing evidence of the alcohol–dementia relationship in an international context.
Balanced against these strengths, the current findings also need to be considered within the context of some limitations. Alcohol use was assessed by self‐report, which is prone to under‐reporting. Beverage type was not consistently assessed across the cohorts and therefore could not be considered in the current study. Some studies have found that some beverage types (i.e. wine) are more protective against dementia when compared with other beverage types (i.e. spirits) [37]. However, predominant beverage type is highly confounded with other socio‐demographic characteristics and some reviews have suggested that ethanol itself should be the focus of study, rather than any particular beverage type [38]. While the current study was able to account for many demographic and clinical characteristics which were harmonized across cohorts, uncontrolled confounding may still impact this study’s results. Frequency of alcohol use is likely to be an important factor in dementia risk, but the current study was limited in the way it could examine alcohol frequency across cohorts. Healthy survivor bias may also impact the current findings, particularly given the older average age of the cohorts, and possibly reflected in the small numbers of participants in the more extreme drinking categories. While data were able to be stratified to investigate the alcohol–dementia relationship in four of the six continents represented in the current study, there was insufficient power to examine this relationship in the single cohorts representing South America (Brazil) and Africa (Republic of Congo). Future work is needed to better understand the alcohol–dementia relationship in low‐ and middle‐income countries.
Abstaining and increased dementia risk
While abstinence from alcohol has often been associated with a higher risk for dementia [37], this relationship is the subject of considerable debate. When compared with abstainers, light‐to‐moderate alcohol use has been found to be protective for vascular dementia (RR = 0.75; 95% CI: 0.57–0.98), Alzheimer's disease (RR = 0.61; 95% CI: 0.54–0.68; RR: 0.72; 95% CI: 0.61–0.86) and all‐cause dementia (RR = 0.74; 95% CI: 0.61–0.91) [10] in a previous systematic review of meta‐analyses. In a scoping review of systematic reviews, most reviews similarly found that light‐to‐moderate consumption was protective against a diagnosis of dementia, as well as death from dementia, when compared with abstinence [9]. Experimental evidence in animal models is consistent with this observational research, confirming the neurotoxicity of heavy alcohol use and the protective effects of alcohol at low doses [39, 40, 41].
It has been suggested that the increased risk of dementia associated with abstinence may be the result of including former drinkers who have ceased drinking due to other health conditions or the onset of cognitive problems [11]. Consistent with this hypothesis, previous studies have indicated that the increased risk of dementia associated with abstinence is not robust to careful control for confounding factors, particularly physical and mental health factors associated with dementia risk [42]. In the current study, however, the increased dementia risk associated with abstaining was evident after controlling for relevant demographic and clinical characteristics. In the analyses focused on categorical alcohol use, there was also no consistent difference in dementia risk for those designated as either former drinkers or life‐time abstainers. In the dose–response analysis, there was a higher dementia risk for abstainers after the exclusion of former drinkers.
While rates of abstinence varied considerably across the cohorts included in this study, the rates were high overall, and there may have been more power to identify statistically significant effects in this group when compared with current drinkers. The particularly high rates of life‐time abstaining across cohorts in the current study suggests that these data may be subject to recall and/or social desirability biases. The misidentification of former drinkers as life‐time abstainers may therefore explain some of the increased dementia risk in the abstainer group. Overall, however, there was consistent evidence from the current study to suggest that abstaining from alcohol is related to an increased dementia risk when compared to light‐moderate alcohol consumption.
Mechanisms underpinning the protective effect of light to moderate alcohol use are contested, but include indirect effects through reduced cardiometabolic disease [37] and the possible modulation of amyloid beta deposition and glymphatic function [39, 43]. While light to moderate alcohol use may reduce dementia risk, even low levels of alcohol use have been associated with reduced brain volume, grey matter atrophy and increased white matter hyperintensities [5, 44, 45], indicating that alcohol use is unlikely to be directly neuroprotective. In addition, light‐to‐moderate alcohol use has been associated with other health conditions, including some cancers [46], cautioning against recommending the commencement of alcohol use in those who abstain.
Current drinking and dementia risk
In the combined sample, dose–response analyses focused only on current drinkers found no evidence of differences in terms of dementia risk across the spectrum of consumption that could be investigated in the current study. It should be noted that the current study does not provide evidence on heavier drinking and alcohol use disorder, which are relatively rare in population‐based observational studies of older adults. There is evidence from other sources, such as hospital‐based studies, which indicate that heavy alcohol use and alcohol use disorders are strongly and causally associated with dementia (particularly young onset dementia) [7], as well as neurocognitive diseases where alcohol use is a contributing or necessary factor (i.e. alcohol‐related dementia and Wernicke–Korsakoff syndrome) [8].
CONCLUSION
The current study found consistent evidence to suggest that abstinence from alcohol in later life is associated with increased dementia risk internationally. Such findings need to be balanced against neuroimaging evidence suggesting that even low levels of alcohol use are associated with poorer brain health, as well as dose–response relationships between alcohol use and other health outcomes, including some cancers. For these reasons, advising those who currently abstain to initiate drinking is not recommended. Meanwhile, among current drinkers, alcohol use did not appear to be a consistent risk factor for dementia, although this relationship varied across continents and could not be examined among heavier drinkers. There is wide variability in alcohol guidelines across countries internationally, and findings from the current study support a more national‐level approach to the development of alcohol guidelines where local context can be taken into account. While other studies have demonstrated that heavy alcohol use and alcohol use disorders are strongly associated with neurocognitive disease and are key targets for preventions, the current study questions whether reducing less than heavy alcohol use in older adults aged over 60 years is an effective prevention strategy for dementia from a population‐level, or public health, perspective.
DECLARATION OF INTERESTS
None.
AUTHOR CONTRIBUTIONS
Louise Rua Mewton: Conceptualization; data curation; formal analysis; methodology; project administration. Rachel Visontay: Data curation; formal analysis; methodology; project administration. Nicholas Geoffrey Hoy: Data curation; methodology; project administration. Darren Lipnicki: Data curation; methodology. Matthew Sunderland: Methodology. Richard Lipton: Data curation; methodology. Maelenn Guerchet: Data curation; methodology. Karen Ritchie: Data curation; methodology. Jenna Najar: Data curation; methodology. Nikolaos Scarmeas: Data curation; methodology. Ki Woong Kim: Data curation; methodology. Steffi Riedel Heller: Data curation; methodology. Martin van Boxtel: Data curation; methodology. Erin Jacobsen: Data curation. Henry Brodaty: Data curation; funding acquisition; methodology; resources. Kaarin Anstey: Data curation; methodology. Mary Haan: Data curation; methodology. Marcia Scazufca: Data curation; methodology. Elena Lobo: Data curation; methodology. Perminder Sachdev: Data curation; methodology.
Supporting information
Table S1. Details of dementia assessment in each of the included cohorts
Table S2. Alcohol use: study‐specific details and harmonisation protocols
Table S3. Smoking: Study‐specific details and harmonisation protocols
Table S4. Depression: Study‐specific details and harmonisation protocols
Table S5. Hypertension: Study‐specific details and harmonisation protocols
Table S6. Cholesterol: Study‐specific details and harmonisation protocols
Table S7: Demographic characteristics of contributing cohorts
Table S8: Clinical characteristics of contributing cohorts
Table S9. Comparison of baseline demographic and clinical characteristics of the participants included and excluded from the analyses
Table S10. Comparison of baseline alcohol use characteristics of participants with and without baseline dementia diagnosis
Table S11. Relationships between demographic and clinical covariates and dementia risk in the combined sample (n = 24 478) a
Table S12. Combined sample hazard ratios with lifetime abstainers and former drinkers separated
Figure S1. Dose response relationship between usual alcohol use (grams/day; implemented using the regression dilution ratio) and dementia including lifetime abstainers (reference group) and current drinkers
Figure S2. Dose response relationship between usual alcohol use (grams/day; implemented using the regression dilution ratio) and dementia among current drinkers
Figure S3. Sensitivity analysis excluding participants reporting stroke at baseline
Figure S4. Sub‐group analyses by continent including lifetime abstainers (reference group) and current drinkers
Figure S5. Sub‐group analyses by continent including current drinkers
Figure S1. Love plots of covariate balance for each cohort.
ACKNOWLEDGEMENTS
Funding for COSMIC comes from the National Institute on Aging of the National Institutes of Health under Award Number RF1AG057531. HELIAD funding: IIRG‐09‐133014 from the Alzheimer’s Association, 189 10276/8/9/2011 from the NSRF‐EU program Excellence Grant (ARISTEIA) (which is co‐funded by the European Social Fund and Greek National resources) and ΔΥ2β/οικ.51657/14.4.2009 from the Ministry for Health and Social Solidarity (Greece). KLOSCAD funding: a grant from the Korean Health Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (grant no. HI09C1379). H70 funding: the study was financed by grants from the Swedish state under the agreement between the Swedish government and the county councils (the ALF‐agreement; ALF 716681), Stena Foundation, Swedish Research Council (11267, 2005‐8460, 2007‐7462, 2012‐5041, 2015‐02830, 2019‐01096, 2013‐8717, NEAR 2017‐00639), Swedish Research Council for Health, Working Life and Welfare (2004‐0145, 2006‐0596, 2008‐1111, 2010‐0870, 2013‐1202, 2018‐00471, 2001‐2646, 2003‐0234, 2004‐0150, 2006‐0020, 2008‐1229, 2012‐1138, AGECAP 2013‐2300, 2013‐2496), Konung Gustaf V’s och Drottning Victorias Frimurarestiftelse, Hjärnfonden (FO2014‐0207, FO2016‐0214, FO2018‐0214, FO2019‐0163) Alzheimerfonden, Eivind och Elsa K:son Sylvans stiftelse, The Alzheimer’s Association Zenith Award (ZEN‐01‐3151), The Alzheime’s Association Stephanie B. Overstreet Scholars (IIRG‐00‐2159), The Bank of Sweden Tercentenary Foundation, Stiftelsen Söderström‐Königska Sjukhemmet, Stiftelsen för Gamla Tjänarinnor, Handlanden Hjalmar Svenssons Forskningsfond, Systembolagets alkoholforskningsråd (CAN), Swedish Research Council for Health, Working Life and Welfare (2017‐1604). LEILA75+ funding: the Interdisciplinary Centre for Clinical Research at the University of Leipzig (Interdisziplinäres Zentrum für Klinische Forschung/IZKF; grant 01KS9504). The SPAH study was funded by Wellcome Trust, UK (GR066133MA); Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil (CNPQ) partially supported MS (307579/2019‐0). ZARADEMP funding: supported by grants from the Fondo de Investigación Sanitaria, Instituto de Salud Carlos III, Spanish Ministry of Economy and Competitiveness, Madrid, Spain (grants 94/1562, 97/1321E, 98/0103, 01/0255, 03/0815, 06/0617, G03/128) and the Fondo Europeo de Desarrollo Regional (FEDER) of the European Union and Gobierno de Aragón, Group #19. COSMIC management: the head of COSMIC is Perminder S. Sachdev, and the Study Co‐Ordinator is Darren M. Lipnicki. The Research Scientific Committee leads the scientific agenda of COSMIC and provides ongoing support and governance; it is composed of member study leaders (in alphabetical order): Kaarin Anstey, Carol Brayne, Henry Brodaty, Liang‐Kung Chen, Erico Costa, Michael Crowe, Oscar Del Brutto, Ding Ding, Jacqueline Dominguez, Mary Ganguli, Antonio Guaita, Maëlenn Guerchet, Oye Gureje, Jacobijn Gussekloo, Mary Haan, Hugh Hendrie, Ann Hever, Ki‐Woong Kim, Seb Koehler, Murali Krishna, Linda Lam, Bagher Larijani, Richard Lipton, Juan Llibre‐Rodriguez, Antonio Lobo, Richard Mayeux, Kenichi Meguro, Vincent Mubangizi, Toshiharu Ninimiya, Stella‐Maria Paddick, Maria Skaalum Petersen, Ng Tze Pin, Steffi Riedel‐Heller, Karen Ritchie, Kenneth Rockwood, Nikolaos Scarmeas, Marcia Scazufca, Suzana Shahar, Xiao Shifu, Kumagai Shuzo, Ingmar Skoog and Yuda Turana. Additional member study leaders: Marie‐Laure Ancelin, Mindy Katz, Martin van Boxtel, Iraj Nabipour, Pierre‐Marie Preux, Perminder Sachdev, Nicole Schupf, Richard Walker. COSMIC NIH grant investigators: Perminder Sachdev: Scientia Professor of Neuropsychiatry; Co‐Director, Centre for Healthy Brain Ageing (CHeBA), UNSW Sydney; Director, Neuropsychiatric Institute, Prince of Wales Hospital, Sydney, Australia; Mary Ganguli: Professor of Psychiatry, Neurology, and Epidemiology, University of Pittsburgh; Ronald Petersen: Professor of Neurology, Director, Mayo Clinic Alzheimer’s Disease Research Center and the Mayo Clinic Study of Aging; Richard Lipton: Edwin S. Lowe Professor and Vice Chair of Neurology, Albert Einstein College of Medicine; Karen Ritchie: Professor and Director of the Neuropsychiatry Research Unit of the French National Institute of Research (INSERM U1061); Ki‐Woong Kim: Professor of Brain and Cognitive Sciences, Director of National Institute of Dementia of Korea; Louisa Jorm: Director, Centre for Big Data Research in Health and Professor, Faculty of Medicine, UNSW Sydney, Australia; Henry Brodaty: Scientia Professor of Ageing and Mental Health; Co‐Director, Centre for Healthy Brain Ageing (CHeBA), UNSW Sydney; Director, Dementia Collaborative Research Centre (DCRC); Senior Consultant, Old Age Psychiatry, Prince of Wales Hospital. Open access publishing facilitated by University of New South Wales, as part of the Wiley ‐ University of New South Wales agreement via the Council of Australian University Librarians; EPIDEMCA: French National Agency (ANR) grant (ANR‐ 09‐MNPS‐009‐01 grant); AXA Research Fund (grant 2012 – Project – Public Health Institute (Inserm) – PREUX Pierre‐Marie); and the Limoges University Hospital through its APREL scheme. Dr Mewton was funded by a Dementia Centre for Research Collaboration Research Grant (RG180842‐A). Open access publishing facilitated by University of New South Wales, as part of the Wiley ‐ University of New South Wales agreement via the Council of Australian University Librarians.
Mewton L, Visontay R, Hoy N, Lipnicki DM, Sunderland M, Lipton RB, et al. The relationship between alcohol use and dementia in adults aged more than 60 years: a combined analysis of prospective, individual‐participant data from 15 international studies. Addiction. 2023;118(3):412–424. 10.1111/add.16035
Funding information Gobierno de Aragón; Fondo Europeo de Desarrollo Regional; Spanish Ministry of Economy and Competitiveness, Grant/Award Numbers: G03/128, 06/0617, 03/0815, 01/0255, 98/0103, 97/1321E, 94/1562; Instituto de Salud Carlos III; Fondo de Investigación Sanitaria; Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil, Grant/Award Number: 307579/2019‐0; Wellcome Trust, UK, Grant/Award Number: GR066133MA; Interdisciplinary Centre for Clinical Research at the University of Leipzig, Grant/Award Number: 01KS9504; Systembolagets alkoholforskningsråd (CAN); Handlanden Hjalmar Svenssons Forskningsfond; Stiftelsen för Gamla Tjänarinnor; Stiftelsen Söderström‐Königska Sjukhemmet; Bank of Sweden Tercentenary Foundation; Eivind och Elsa K:son Sylvans stiftelse; Alzheimerfonden; Konung Gustaf V’s och Drottning Victorias Frimurarestiftelse, Hjärnfonden, Grant/Award Numbers: FO2019‐0163, FO2018‐0214, FO2016‐0214, FO2014‐0207; Swedish Research Council for Health, Working Life and Welfare, Grant/Award Numbers: 2017‐1604, 2013‐2496, AGECAP 2013‐2300, 2012‐1138, 2008‐1229, 2006‐0020, 2004‐0150, 2003‐0234, 2001‐2646, 2018‐00471, 2013‐1202, 2010‐0870, 2008‐1111, 2006‐0596, 2004‐0145; Swedish Research Council, Grant/Award Numbers: NEAR 2017‐00639, 2013‐8717, 2019‐01096, 2015‐02830, 2012‐5041, 2007‐7462, 2005‐8460, 11267; Stena Foundation; ALF‐agreement;; Ministry of Health and Welfare, Republic of Korea, Grant/Award Number: HI09C1379; Ministry for Health and Social Solidarity, Grant/Award Number: ΔΥ2β/οικ.51657/14.4.2009; NSRF‐EU, Grant/Award Number: 189 10276/8/9/2011; Alzheimer’s Association, Grant/Award Numbers: IIRG‐00‐2159, ZEN‐01‐3151, IIRG‐09‐133014; National Institutes of Health, Grant/Award Number: RF1AG057531; French National Agency (ANR), Grant/Award Number: ANR‐09‐MNPS‐009‐01; AXA Research Fund, Grant/Award Number: 2012 – Project – Public Health Institute (Inserm) – PREUX Pierre‐Marie; Dementia Centre for Research Collaboration Research Grant, Grant/Award Number: RG180842‐A
REFERENCES
- 1. Global Burden of Disease (GBD) Collaborators . Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019. Lancet Public Health. 2022;7:e105–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Patterson C. World Alzheimer’s Report. The State of the Art of Dementia Research: New Frontiers. An Analysis of Prevalence, Incidence, Cost and Trends. London, UK: Alzheimer’s Disease International; 2018.
- 3. Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396:413–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rao R, Topiwala A. Alcohol use disorders and the brain. Addiction. 2020;115:1580–9. [DOI] [PubMed] [Google Scholar]
- 5. Topiwala A, Ebmeier KP. Effects of drinking on late‐life brain and cognition. Evid Based Ment Health. 2018;21:12–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Scarmeas N, Anastasiou CA, Yannakoulia M. Nutrition and prevention of cognitive impairment. Lancet Neurol. 2018;17:1006–15. [DOI] [PubMed] [Google Scholar]
- 7. Schwarzinger M, Pollock BG, Hasan OS, Dufoil C, Rehm J. Contribution of alcohol use disorders to the burden of dementia in France 2008–13: a nationwide retrospective cohort study. Lancet Public Health. 2018;3:e124–32. [DOI] [PubMed] [Google Scholar]
- 8. Visontay R, Rao RT, Mewton L. Alcohol use and dementia: new research directions. Curr Opin Psychiatry. 2021;34:165–70. [DOI] [PubMed] [Google Scholar]
- 9. Rehm J, Hasan OSM, Black SE, Shield KD, Schwarzinger M. Alcohol use and dementia: a systematic scoping review. Alzheimers Res Ther. 2019;11:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Anstey KJ, Ee N, Eramudugolla R, Jagger C, Peters R. A systematic review of meta‐analyses that evaluate risk factors for dementia to evaluate the quantity, quality, and global representativeness of evidence. J Alzheimers Dis. 2019;70:S165–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stockwell T, Zhao J, Panwar S, Roemer A, Naimi T, Chikritzhs T. Do ‘moderate’ drinkers have reduced mortality risk? A systematic review and meta‐analysis of alcohol consumption and all‐cause mortality. J Stud Alcohol Drugs. 2016;77:185–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Walls H, Cook S, Matzopoulos R, London L. Advancing alcohol research in low‐income and middle‐income countries: A global alcohol environment framework. BMJ Glob Health. 2020;5:e001958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sachdev PS, Lipnicki DM, Kochan NA, Crawford JD, Rockwood K, Xiao S, et al. COSMIC (Cohort Studies of Memory in an International Consortium): an international consortium to identify risk and protective factors and biomarkers of cognitive ageing and dementia in diverse ethnic and sociocultural groups. BMC Neurol. 2013;13:335–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Katz MJ, Lipton RB, Hall CB, Zimmerman ME, Sanders AE, Verghese J, et al. Age and sex specific prevalence and incidence of mild cognitive impairment, dementia and Alzheimer’s dementia in blacks and whites: a report from the Einstein Aging Study. Alzheimer Dis Assoc Disord. 2012;26:335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Guerchet M, Mbelesso P, Ndamba‐Bandzouzi B, Pilleron S, Desormais I, Lacroix P, et al. Epidemiology of Dementia in Central Africa (EPIDEMCA): Protocol for a multicentre population‐based study in rural and urban areas of the Central African Republic and the Republic of Congo. Springerplus. 2014;3:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ritchie K, Carrière I, Ritchie C, Berr C, Artero S. Designing prevention programmes to reduce incidence of dementia: prospective cohort study of modifiable risk factors. BMJ. 2010;341:c3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Satizabal CL, Beiser AS, Chouraki V, Chene G, Dufouil C, Seshadri S. Incidence of dementia over three decades in the Framingham Heart Study. N Engl J Med. 2016;374:523–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sterner TR, Ahlner F, Blennow K, Dahlin‐Ivanoff S, Falk H, Johansson LH, et al. The Gothenburg H70 Birth cohort study 2014–16: design, methods and study population. Eur J Epidemiol. 2019;34:191–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dardiotis E, Kosmidis MH, Yannakoulia M, Hadjigeogiou GM, Scarmeas N. The Hellenic Longitudinal Investigation of Aging and Diet (HELIAD): rationale, study design, and cohort description. Neuroepidemiology. 2014;43:9–14. [DOI] [PubMed] [Google Scholar]
- 20. Han JW, Kim TH, Kwak KP, Kim K, Kim BJ, Kim SJ, et al. Overview of the Korean longitudinal study on cognitive aging and dementia. Psychiatry Invest. 2018;15:767–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Riedel‐Heller SG, Busse A, Aurich C, Matschinger H, Angermeyer MC. Prevalence of dementia according to DSM‐III‐R and ICD‐10: results of the Leipzig Longitudinal Study of the Aged (LEILA75+), part 1. Br J Psychiatry. 2001;179:250–4. [DOI] [PubMed] [Google Scholar]
- 22. Jolles J, Houx P, Van Boxtel M, Ponds RW, Metsemakers JF, Houx PJ. The Maastricht Aging Study. Determinants of Cognitive Aging Maastricht: Neuropsych Publishers; 1995. p. 192. [Google Scholar]
- 23. Ganguli M, Snitz B, Vander Bilt J, Chang CC. How much do depressive symptoms affect cognition at the population level? The Monongahela–Youghiogheny Healthy Aging Team (MYHAT) study. Int J Geriatr Psychiatry. 2009;24:1277–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Anstey KJ, Christensen H, Butterworth P, Easteal S, Mackinnon A, Jacomb T, et al. Cohort profile: the PATH through life project. Int J Epidemiol. 2012;41:951–60. [DOI] [PubMed] [Google Scholar]
- 25. Haan MN, Mungas DM, Gonzalez HM, Ortiz TA, Acharya A, Jagust WJ. Prevalence of dementia in older Latinos: the influence of type 2 diabetes mellitus, stroke and genetic factors. J Am Geriatr Soc. 2003;51:169–77. [DOI] [PubMed] [Google Scholar]
- 26. Scazufca M, Menezes PR, Vallada HP, Crepaldi AL, Pastor‐Valero M, Coutinho LM, et al. High prevalence of dementia among older adults from poor socioeconomic backgrounds in São Paulo, Brazil. Int Psychogeriatr. 2008;20:394–405. [DOI] [PubMed] [Google Scholar]
- 27. Sachdev PS, Brodaty H, Reppermund S, Kochan NA, Trollor JN, Draper B, et al. The Sydney Memory and Ageing Study (MAS): methodology and baseline medical and neuropsychiatric characteristics of an elderly epidemiological non‐demented cohort of Australians aged 70–90 years. Int Psychogeriatr. 2010;22:1248–64. [DOI] [PubMed] [Google Scholar]
- 28. Lobo A, Saz P, Marcos G, Dia J‐L, De‐la‐Camara C, Ventura T, et al. The ZARADEMP Project on the incidence, prevalence and risk factors of dementia (and depression) in the elderly community: II. Methods and first results. Eur J Psychiatry. 2005;19:40–54. [Google Scholar]
- 29. van Buuren S, Groothuis‐Oudshoorn K. Mice: Multivariate Imputation by Chained Equations in R. J Stat Softw. 2011;45:1–67. [Google Scholar]
- 30. Greifer N. WeightIt: Weighting for Covariate Balance in Observational Studies. R package version 0.12.0. 2021. Available at: https://CRAN.R‐project.org/package=WeightIt (accessed 29 August 2022). [Google Scholar]
- 31. Therneau TM. coxme: Mixed Effects Cox Models. R package version 2.2‐16. 2020. Available at: https://CRAN.R‐project.org/package=coxme (accessed 29 August 2022).
- 32. Therneau T. A Package for Survival Analysis in R. R package version 3.3‐1. 2022. Available at: https://CRAN.R‐project.org/package=survival (accessed 29 August 2022).
- 33. Harrell FE Jr. rms: Regression Modeling Strategies. R package version 6.3‐0. 2022. Available at: https://CRAN.R‐project.org/package=rms (accessed 29 August 2022).
- 34. Wadström BN, Wulff AB, Pedersen KM, Jensen GB, Nordestgaard BG. Elevated remnant cholesterol increases the risk of peripheral artery disease, myocardial infarction, and ischaemic stroke: a cohort‐based study. Eur Heart J. 2021. 10.1093/eurheartj/ehab705 [DOI] [PubMed] [Google Scholar]
- 35. Fibrinogen Studies Collaboration . Correcting for multivariate measurement error by regression calibration in meta‐analyses of epidemiological studies. Stat Med. 2009;28:1067–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fibrinogen Studies Collaboration . Regression dilution methods for meta‐analysis: assessing long‐term variability in plasma fibrinogen among 27 247 adults in 15 prospective studies. Int J Epidemiol. 2006;35:1570–8. [DOI] [PubMed] [Google Scholar]
- 37. Sabia S, Fayosse A, Dumurgier J, Dugravot A, Akbaraly T, Britton A, et al. Alcohol consumption and risk of dementia: 23 year follow‐up of Whitehall II cohort study. BMJ. 2018;362:k2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Krenz M, Korthuis RJ. Moderate ethanol ingestion and cardiovascular protection: from epidemiologic associations to cellular mechanisms. J Mol Cell Cardiol. 2012;52:93–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lundgaard I, Wang W, Eberhardt A, Vinitsky HS, Reeves BC, Peng S, et al. Beneficial effects of low alcohol exposure, but adverse effects of high alcohol intake on glymphatic function. Sci Rep. 2018;8:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Charlton AJ, May C, Luikinga SJ, Burrows EL, Kim JH, Lawrence AJ, et al. Chronic voluntary alcohol consumption causes persistent cognitive deficits and cortical cell loss in a rodent model. Sci Rep. 2019;9:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kimoto A, Izu H, Fu C, Suidasari S, Kato N. Effects of low dose of ethanol on the senescence score, brain function and gene expression in senescence‐accelerated mice 8 (SAMP8). Exp Ther Med. 2017;14:1433–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Langballe EM, Ask H, Holmen J, Stordal E, Saltvedt I, Selbaek G, et al. Alcohol consumption and risk of dementia up to 27 years later in a large, population‐based sample: the HUNT study, Norway. Eur J Epidemiol. 2015;30:1049–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kim JW, Byun MS, Yi D, Lee JH, Ko K, Jeon SY, et al. Association of moderate alcohol intake with in vivo amyloid‐beta deposition in human brain: a cross‐sectional study. PLOS Med. 2020;17:e1003022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nixon SJ, Lewis B. Clarifying the neurobehavioral sequelae of moderate drinking lifestyles and acute alcohol effects with aging. Int Rev Neurobiol. 2019;148:39–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Topiwala A, Allan CL, Valkanova V, Zsoldos E, Filippini N, Sexton C, et al. Moderate alcohol consumption as risk factor for adverse brain outcomes and cognitive decline: longitudinal cohort study. BMJ. 2017;357:j2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rehm J, Soerjomataram I, Ferreira‐Borges C, Shield KD. Does alcohol use affect cancer risk? Curr Nutr Rep. 2019;8:222–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Details of dementia assessment in each of the included cohorts
Table S2. Alcohol use: study‐specific details and harmonisation protocols
Table S3. Smoking: Study‐specific details and harmonisation protocols
Table S4. Depression: Study‐specific details and harmonisation protocols
Table S5. Hypertension: Study‐specific details and harmonisation protocols
Table S6. Cholesterol: Study‐specific details and harmonisation protocols
Table S7: Demographic characteristics of contributing cohorts
Table S8: Clinical characteristics of contributing cohorts
Table S9. Comparison of baseline demographic and clinical characteristics of the participants included and excluded from the analyses
Table S10. Comparison of baseline alcohol use characteristics of participants with and without baseline dementia diagnosis
Table S11. Relationships between demographic and clinical covariates and dementia risk in the combined sample (n = 24 478) a
Table S12. Combined sample hazard ratios with lifetime abstainers and former drinkers separated
Figure S1. Dose response relationship between usual alcohol use (grams/day; implemented using the regression dilution ratio) and dementia including lifetime abstainers (reference group) and current drinkers
Figure S2. Dose response relationship between usual alcohol use (grams/day; implemented using the regression dilution ratio) and dementia among current drinkers
Figure S3. Sensitivity analysis excluding participants reporting stroke at baseline
Figure S4. Sub‐group analyses by continent including lifetime abstainers (reference group) and current drinkers
Figure S5. Sub‐group analyses by continent including current drinkers
Figure S1. Love plots of covariate balance for each cohort.


