Abstract
Antibiotic use is increasing worldwide. However, the use of antibiotics is clearly associated with changes in gut microbiome composition and function, and perturbations have been identified as potential environmental risk factors for chronic inflammatory disorders of the gastrointestinal tract. In this Review, we examine the association between the use of antibiotics and the onset and development of both type 1 and type 2 diabetes, inflammatory bowel disease, including ulcerative colitis and Crohn’s disease, as well as coeliac disease and eosinophilic oesophagitis. We discuss the key findings of epidemiological studies, provide mechanistic insights into the pathways by which the gut microbiota might contribute to these diseases, and assess clinical trials investigating the effects of antibiotics. Such studies indicate that antibiotic exposures, varying in type, timing and dosage, could explain differences in disease risk. There seems to be a critical window in early life in which perturbation of the microbiome has a substantial effect on disease development. Identifying the antibiotic-perturbed gut microbiota as a factor that contributes to the pathophysiology of these inflammatory disorders might stimulate new approaches to prevention, diagnosis and treatment.
The gastrointestinal tract is subject to important chronic inflammatory diseases. These include diseases that affect the wall of the gastrointestinal tract, such as inflammatory bowel disease (IBD), including ulcerative colitis and Crohn’s disease, as well as coeliac disease and eosinophilic oesophagitis (EoE) (BOX 1). In addition, the pancreas is subject to inflammatory processes that can lead to either type 1 or type 2 diabetes. In this Review, we consider the relationship between these diseases and the gut microbiome, especially with respect to how antibiotic treatment for other indications can perturb the microbiome and affect the risk and course of these illnesses.
Box 1 ∣. Eosinophilic oesophagitis.
Eosinophilic oesophagitis (EoE) is a chronic immune-mediated disease that is defined by the presence of symptoms consistent with oesophageal dysfunction, with an oesophageal biopsy sample showing ≥15 eosinophils per high power field, while excluding other causes of oesophageal eosinophilia244,245.
The immune response in EoE is mainly mediated by T helper 2 (TH2) interleukins244,246; overexpression of IL-13 selectively induces eotaxin 3 (also known as CCL26) expression in oesophageal epithelial cells, leading to eosinophilic infiltration and activation within oesophageal tissue246-249.
Epidemiology
As with other atopic diseases, the prevalence of EoE has increased in the past few decades250-254. EoE now accounts for approximately one-quarter of histologically proven oesophageal disease in children undergoing oesophagogastroduodenoscopy (EGD)250,251. EoE affects all age groups, is male-predominant, and is global, although it seems to be more common in temperate climates and in those of European descent244. Improved detection alone does not account for the increase in prevalence250,252,253,255,256. Although genetic variants might relate to EoE risk, a retrospective cross-sectional study utilizing two cohorts, including an international registry of EoE twin probands, showed that there was a stronger environmental contribution than genetic contribution to EoE255. In the twin cohort, genetic heritability was 14.5 ± 4%, and the common family environment contributed 81 ± 4% to risk255.
Inverse association with Helicobacter pylori
Helicobacter pylori has been inversely associated with atopic diseases such as asthma257 and oesophageal diseases including gastroesophageal reflux disease (GERD)258-261, Barrett oesophagus261 and oesophageal adenocarcinoma262. Six studies have shown an inverse relationship between the presence of H. pylori and either oesophageal eosinophilia and/or EoE263-268. Humans have been colonized by H. pylori for at least 100,000 years, and probably longer118. Loss of key microbial species, including H. pylori, as a result of antibiotic exposures could plausibly contribute to EoE risk269.
Clinical studies
In six clinical studies evaluating the relationship between antibiotic exposure and EoE risk, the odds ratios ranged from 1.3 to 6 (REFS.233-238) (TABLE 4). A 2021 meta-analysis of five of these studies showed significant associations between antibiotic exposure and EoE risk in four studies219,233-236. In one study, there were similar rates of early-life exposure to antibiotics between EoE and GERD cohorts (81% and 73%, respectively), which were higher than the exposure rate (42%) for a control cohort of asymptomatic children234. Although these findings provide evidence that antibiotic exposure in early life might increase the risk of developing EoE, they are limited by the small numbers of individuals studied.
Timing of EoE onset and antibiotic exposure.
A case–control study evaluated patients with EoE who did not develop symptoms until ≥18 years of age, nested within a prospective cohort study of adults undergoing EGD for evaluation of gastrointestinal symptoms237. Both the individuals and their mothers provided information about early-life antibiotic exposures; antibiotic exposure in the first year of life was associated with adult-onset EoE (OR 4.6)237 (TABLE 4).
EoE disease severity and antibiotic exposure.
In a retrospective review of Italian children and adults with EoE, antibiotic exposure in relation to EoE disease activity was studied270. The researchers defined refractory EoE as symptom ‘flare-ups’ and responsive EoE as asymptomatic, but histological data to confirm EoE disease activity was not reported. Antibiotic exposure was defined as repeated courses (three or more courses per year) in the first 3 years of life. Adults with refractory (symptomatic) EoE were significantly more likely to have repeated antibiotic exposure (as defined by the authors): 70% versus 33.3% in asymptomatic EoE (P = 0.03)270. Further studies are needed to assess the relationship between antibiotic exposure and EoE disease severity.
Humans, like other mammals, develop in a uterus that is routinely sterile or only occasionally visited by adventitious microbial pathogens1,2. The major exposure of the baby to the world of microorganisms occurs after rupture of the membranes and its descent through the birth canal and exposure to maternal vaginal and faecal microorganisms1,2. This is followed by successional colonization and blooms of taxa in the intestine that are highly conserved across all healthy infants3-5. The most dynamic period for the human microbiome is the first 3 years of life6, which is also the period in which immunity, metabolism and cognition become well established. Studies in animal models have shown that perturbing the early-life microbiome, even transiently, can have long-term effects on these crucial developmental steps7-12. This conserved biology and the effects of experimental perturbation have led to the theory that an altered microbiota underlies a number of the diseases that are currently epidemic globally13,14, including inflammatory conditions affecting the gastrointestinal tract.
Studies have shown that visceral, inflammatory and neuropathic pain can be influenced by the gut microbiome, which is particularly relevant in conditions in which pain can be a prominent symptom, including IBD and coeliac disease15,16. Inflammatory foot pad pain induced by carrageenan, lipopolysaccharide (LPS), tumour necrosis factor (TNF), IL-1β, and the chemokine CXCL1 in conventional mice was reduced in germ-free mice17. Similar experiments have demonstrated that mice develop visceral hypersensitivity following dextran sulfate sodium (DSS)-induced colitis, even after the intestinal inflammation has resolved18. This hypersensitivity was transferable by transplantation of a post-inflammatory microbiome to mice that had never been exposed to DSS but not by transplanting a control microbiome18. These findings provide evidence that the gut microbiota in mice contributes to the development of inflammatory hypernociception18,19. Specific data on the effect of the gut microbiome on pain in patients with IBD and coeliac disease are preliminary. In a pilot study of 21 children with coeliac disease, significant differences in relative abundance of specific bacterial taxa were associated with symptoms including abdominal pain20, but further studies are warranted.
Antibiotics entered the general practice of medicine in the late 1940s and have since become pillars of modern medicine. Consequently, their use has steadily grown, and health practitioners increasingly rely on them. Estimated use exceeds one course per year for every person worldwide21, and the numbers are growing. There is extensive variation in antibiotic use within localities, regions and countries, reflecting important differences in the culture of medicine and personal characteristics of both patients and practitioners22. Antibiotics also vary considerably in their antimicrobial spectrum of activity23. Antibiotics were developed to treat infections caused by bacterial pathogens, which has been the major thrust of both their development and their use. However, when an antibiotic is taken, it also has collateral effects on the resident microbiota: inhibiting some, and thus reciprocally selecting for others. In addition to selecting for potential pathogens within the microbiome, including Staphylococcus aureus and Clostridioides difficile, antibiotics considerably perturb the human gut microbiome, with effects lasting for months or longer24-26. In the past, it was widely assumed that after a course of antibiotics the ‘normal flora’ would bounce back to its pretreatment state. Unfortunately, using molecular tools that have greater precision than culture-based studies, it has become clear that the microbiome is perturbed for months, and might never resume to its pretreatment state24-26. This finding is especially important for young children, in whom the microbial succession is highly choreographed and perturbations, even if transient, can affect both microbiome and host development3-5,8,25.
In mixed microbial populations, such as the gut microbiome, fungal numbers usually increase after exposure to antibiotics27. Many gut commensal fungi, including Candida species, interact with host epithelial and immune cells28,29. The host adaptive immune response is targeted to hyphal cells29, and thus any shift in the balance between yeasts and hyphae will affect the immunological milieu. Such phenomena could contribute to antibiotic-induced exacerbations of both disease predisposition and the disease itself.
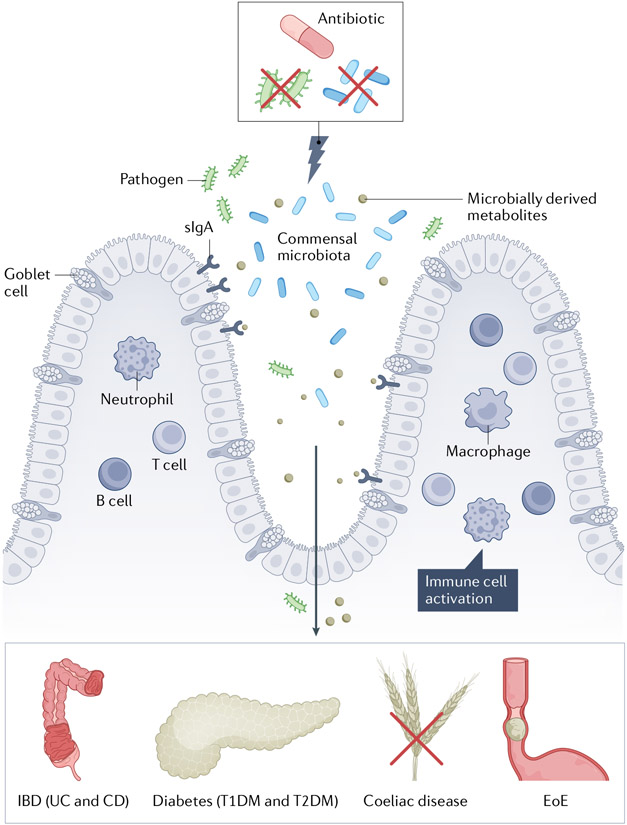
As such, there is growing interest in the hypothesis that owing to their effects on the gut microbiome, antibiotic use might have unintended collateral clinical consequences, especially when given to young children, whose developing microbiome is both plastic and interlinked with host development30. In this Review, we consider this hypothesis in the context of several chronic inflammatory diseases that affect the human gastrointestinal tract and that have increased in incidence during the antibiotic era (FIG. 1).
Fig. 1 ∣. The gut microbiota and antibiotics in the pathogenesis of inflammatory diseases of the gastrointestinal tract.
Schematic overview of the role of the microbiota in the pathogenesis of inflammatory diseases injuring organs in the gastrointestinal tract, leading to the onset and development of both type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM), inflammatory bowel disease (IBD; including ulcerative colitis (UC) and Crohn’s disease (CD)), coeliac disease and eosinophilic oesophagitis (EoE). Exposure to antibiotics leads to profound effects on both the composition and functionality of the gut microbiome, leading to decreased diversity. These changes might then lead to secondary effects involving the intestinal wall including altered epithelial cell signalling to adaptive immune effectors, and/or increased intestinal permeability, leading to translocation of microbial constituents and products into the systemic circulation, among other mechanisms. sIgA, secretory IgA.
Diabetes
Approximately 537 million people worldwide have either type 1 diabetes mellitus (T1DM) or type 2 diabetes mellitus (T2DM)31. Epidemiological studies of the incidence and prevalence of T1DM and T2DM in 212 countries/regions have been extensively reviewed by the International Diabetes Federation and published in the Diabetes Atlas31. Although hyperglycaemia is the common element in T1DM and T2DM, the diseases differ extensively in epidemiology and pathogenesis, and are therefore considered separately.
Type 1 diabetes
Epidemiology
An important factor in T1DM is the genetic predisposition provided by specific HLA haplotypes, mainly DR3-DQ2 and DR4-DQ8 (REF.32). However, as the age of onset is swiftly decreasing, these predisposing genes cannot solely explain the rapidly rising incidence of T1DM worldwide32-34. Altered gut microbiome composition (referred to as dysbiosis) has been identified as a potential environmental risk factor35. The gut microbiota of patients with T1DM harbour a lower ratio of Firmicutes to Bacteroidetes, have decreased Bifidobacterium spp. abundance, reduced bacterial richness and diversity, and lower production of short-chain fatty acids (SCFAs) compared with healthy individuals36-39. Although such changes are seen largely in children already affected by the disease, some were already present before clinical onset.
Changing epidemiology of T1DM in the antibiotic era.
In the past few decades, the worldwide incidence of T1DM has risen dramatically, particularly in children under 14 years old31,40. The estimated annual global number of newly diagnosed children rose by approximately 50% from 65,000 in 2003 (REF.41) to 98,300 in 2021 (REF.31), a 3% annual increase. In 2021, >1.2 million children and adolescents worldwide had T1DM31. However, there is striking geographic variation in the reported incidence of T1DM, with the highest annual incidence reported in children in Europe (~31,000 cases (5.26% of all children in Europe)) and the lowest in children in the Western Pacific (~11,600 cases (1.88% of all children in the Western Pacific))31. Discrepancies between regions must be interpreted with caution, as data sources on T1DM incidence in low-income regions are scarce.
Epidemiological linkages with the disease.
The exact causes of the steep increases in incidence of T1DM are not yet known42. Rapid changes within a short span of time are more likely a result of changes in environmental risk factors than changes in genetic risk32. Intriguingly, the rising incidence of T1DM in children began in many countries/regions in the middle of the twentieth century, coinciding with the start of the antibiotic era43. For example, the rise in Finland preceded the widespread introduction of antibiotics, which is consistent with changes in sanitation, such as the use of chlorinated drinking water. These improved hygiene conditions led to reduced exposure to infectious agents in early childhood. This ‘hygiene hypothesis’ is supported by the negative correlation between hygiene conditions and T1DM incidence44.
Two important changes in medical practice in the second half of the twentieth century were antibiotics and the increased frequency of caesarean section. Antibiotic use in early life clearly leads to changes in the intestinal microbiome3,45. Similarly, children born by caesarean section begin life with an altered microbiome3,5,46,47, and the changes, including reduced Bacteroides species, and altered community composition can persist throughout the first year of life3,46-48. Birth by caesarean section is usually a compounded insult to normal microbiome development, involving loss of the natural passage through the birth canal, and the administration of high doses of antibiotics to the mother in the peripartum period49. Children born by caesarean section also seem to be more likely to receive antibiotics in early life50,51 (TABLE 1). Antibiotics are widely administered to children on the basis of the clinical premise of important benefit and minimal risk; however, antibiotic overuse is well documented in children as well as in older people52,53, and prescribing rates vary widely with differences between countries/regions (both high-income and low-income to middle-income countries) as well as regional differences, even among children with similar clinical presentations22. Parallel statements can be made about caesarean section49. Currently, only limited data on the association between the risk of T1DM and the use of antibiotics are available, mostly provided by Scandinavian nationwide cohort studies50,51,54,55 (TABLE 1), where most children are exposed to antibiotics in early life.
Table 1 ∣.
Clinical studies evaluating antibiotic exposure and risk of diabetes and childhood obesity
| Study | Antibiotic exposure timing |
Date (location) |
Study design | Antibiotic data source |
Disease diagnosis | Key findingsa |
|---|---|---|---|---|---|---|
| Type 1 diabetes mellitus | ||||||
| Wernroth et al.50 | Prenatal to 12 months | 2020 (Sweden) | Cohort | Prescriber registry (ATC code) | Database diagnostic code for T1DM (ICD-10: E10) | Increased risk of T1DM before age of 10 years: ≤1 year: aHR 1.19 (1.05–1.36): 44.3/100,000 person-years among exposed children versus 39.0/100,000 person-years among non-exposed children: ≤6 months: aHR 1.26 (1.04 – 1.5) Modified by mode of delivery |
| Clausen et al.51 | Birth to 24 months | 2016 (Denmark) | Cohort | Prescriber registry (ATC code) | Database diagnostic code for T1DM | Increased risk after exposure to broad-spectrum antibiotics HR 1.13 (1.02–1.25) Modified by mode of delivery |
| Hviid and Svanström54 | 12 months to year 2005 | 2009 (Denmark) | Cohort | Prescriber registry (ATC code) | Database diagnostic code for T1DM (ICD-10: E10) | Differences not significant RR 1.16 (0.91–1.50) |
| Tapia et al.55 | Prenatal to 18 months | 2018 (Norway) | Cohort | Repeated questionnaires | Database diagnostic code for T1DM (ICD-10: E10) | Differences not significant Prenatal: aHR 1.09 (0.85–1.35) In early life: aHR 1.11 (0.81–1.50) |
| Childhood obesity | ||||||
| Trasande et al.90 | <6 months 6–14 months 14–23 months | 2013 (UK) | Longitudinal birth cohort | Repeated questionnaires | Measured during five study visits | Increased body mass at 10–38 months, after antibiotic exposure during first 6 months of life aOR 1.22 (P = 0.029) |
| Bailey et al.91 | 0–23 months 24–59 months | 2014 (Philadelphia, USA) | Cohort | Outpatient prescriptions and patient-reported medications | Measured during recurring study visits | Cumulative exposure to antibiotics was associated with development of obesity ≥4 courses: RR 1.11 (1.02–1.21) Stronger effect for broad-spectrum antibiotics: RR 1.16 (1.03–1.19) |
| Azad et al.92 | 0–5 years | 2014 (Canada) | Longitudinal birth cohort | Prescription records | Measured at 9 and 12 years of age | Increased risk of overweight and central adiposity in preadolescent boys, but not girls Age 9 years: aOR 2.19 (1.06–4.54) Age 12 years: aOR 5.35 (1.94–14.72) |
| Murphy et al.93 | 0–12 months | 2014 (18 countries/regions) | Cross-sectional | Repeated questionnaires | Self-reported or measured | With antibiotic exposure, increased childhood BMI in boys aged 5–8 years, but not girls: BMI + 0.107 kg/m2 (P < 0.0001) |
| Aversa et al.94 | 0–6 months 6–12 months 12–24 months | 2021 (Minnesota, USA) | Population-based cohort | Medical records linkage system | Medical records linkage system | Increased risk for overweight and obesity, depending on number, type and timing of antibiotic exposure Girls with overweight: HR 1.19 (1.09–1.30) Girls with obesity: HR 1.13 (0.99–1.29) Boys with overweight: HR 1.22 (1.12–1.34) Boys with obesity: HR 1.12 (1.08–1.39) |
| Mueller et al.48 | Prenatal | 2014 (New York, USA) | Cohort | Questionnaire | Measured at age 7 years | Exposure to antibiotics in the second or third trimester associated with higher risk of childhood obesity aRR 1.77 (1.25–2.51) |
| Mbakwa et al.95 | Birth to 10 years | 2016 (Netherlands) | Longitudinal cohort | Repeated questionnaires | Self-reported over 7 time points | Increased height and weight in children exposed to: One course during first 6 months of life; adjusted β 0.24 and 0.23 Two or more courses during second year of life; adjusted β 0.34 and 0.29 |
| Type 2 diabetes mellitus in adults | ||||||
| Mikkelsen et al.87 | Adulthood | 2015 (Denmark) | Case–control | Prescriber registry (ATC code) | First-ever prescription of a non-insulin glucose-lowering agent (ATC A10B) | Dose-dependent relationship with number of antibiotic courses: For 2–4, OR 1.21 (1.19–1.24) For ≥5, OR 1.53 (1.50–1.55) |
| Davis et al.88 | Adulthood | 2019 (USA) | Retrospective cohort | Outpatient antibiotic prescriptions >6 months prior to diabetes diagnosis | Two or more ICD-9 codes for diabetes or two or more prescriptions of diabetes medications, other than metformin | Increased risk after exposure to more than one prescription of antibiotics HR = 1.13 (1.01–1.25) |
| Boursi et al.89 | Adulthood | 2015 (UK) | Nested case–control | Antibiotic prescriptions >1 year before diabetes diagnosis | At least one Read code (general practitioners) | No significant difference after a single antibiotic course Dose-dependent relationship for number of antibiotic courses (two or more), with OR depending on antibiotic type |
aHR, adjusted hazard ratio; aOR, adjusted odds ratio; aRR, adjusted relative risk; ATC, Anatomical Therapeutic Chemical classification system; ICD, International Classification of Disease; RR, rate ratio; T1DM, type 1 diabetes mellitus.
Value ranges in parentheses are 95% confidence intervals; adjusted β are adjusted generalized estimating equation estimates in relation to z-scores.
Two longitudinal cohort studies from Sweden and Denmark found an increased risk of T1DM after antibiotic exposure in early life50,51, However, mode of delivery is a strong confounder, as a larger effect was observed in children delivered by caesarean section compared with those delivered vaginally. Although similar in the magnitude of the increased risk ratio, two other cohort studies from Denmark and Norway found no significant association between the use of antibiotics and T1DM onset, irrespective of antimicrobial spectrum or use in an age-specific period54,55 (TABLE 1). These results might reflect the differences between countries/regions, types of antibiotics used, and exposure to probiotics, among other factors. Such variation might lead to non-significant associations. More homogeneous cohorts, with varying ethnicities and geographical regions, will better assess whether perturbation of gut microbiome composition as a result of caesarean section and/or antibiotic exposure in early life influences the onset of T1DM.
Experimental studies
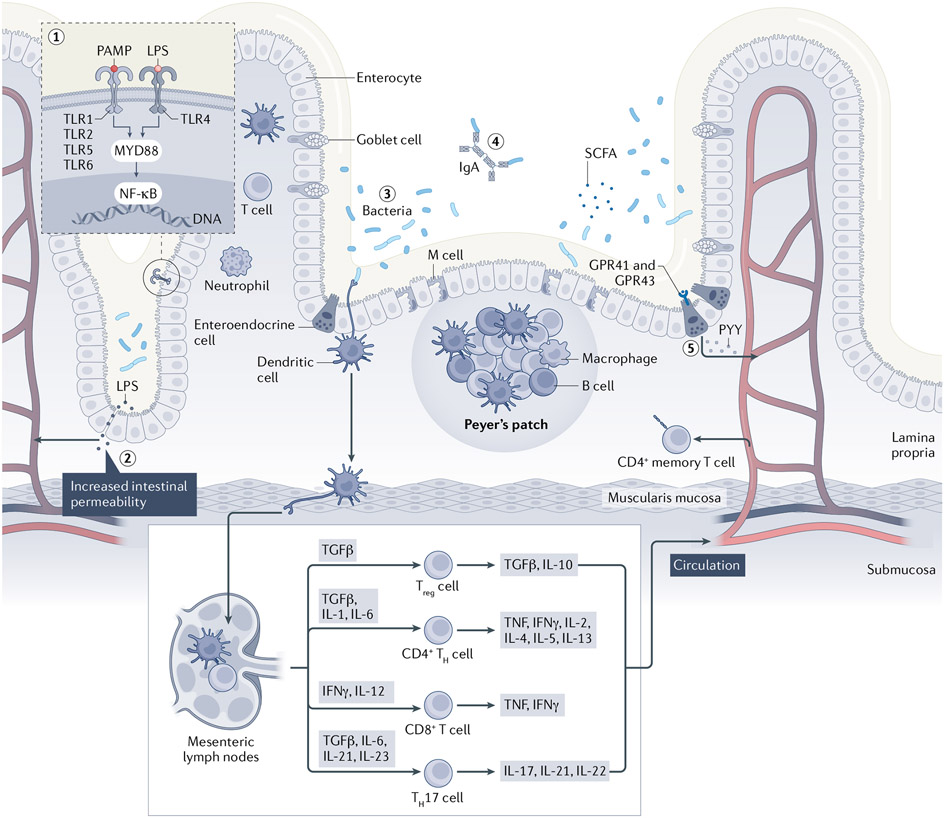
The composition of the intestinal microbiota in early life has a large effect on immunological development in both intestinal and systemic sites10,11,56-59. Therefore, perturbations of microbiome composition during this critical window might have a key role in T1DM onset, which is shown by studies using non-obese diabetic mice (NOD), an experimental model resembling T1DM in humans60. The variation in T1DM incidence in these NOD mice is dependent on the composition of the microbiome to which the mice are exposed61-63. A general rule of thumb is that ‘dirty protects’; NOD mice reared in ultra-clean facilities develop T1DM at higher rates than those in more standard facilities64. As such, germ-free mice are more prone to develop T1DM than NOD mice exposed to a single bacterium62,63. NOD mice with deficient innate immunity owing to a null mutation of the Toll-like receptor adapter signalling molecule MYD88 are protected from T1DM development under specific-pathogen-free conditions, but not under germ-free conditions65, indicating that the microbiota signal is transduced through MYD88. A particular taxon, the genus Candidatus Savagella (formerly known as segmented filamentous bacteria (SFB)), protected NOD mice against T1DM by inducing small intestinal TH17 cell populations63. These results indicate an important and complex interplay between the microbiota and immunological effectors in T1DM (FIG. 2).
Fig. 2 ∣. Complex interplay between the gut microbiota and the immune system in diabetes and IBD.
Microbially derived peptides, such as pathogen-associated molecular patterns (PAMPs) and lipopolysaccharide (LPS), bind to Toll-like receptors (TLRs) on the cell membrane of enterocytes (1). Activation of these TLR/MYD88-dependent signalling pathways leads to translocation of nuclear factor-κB (NF-κB) into the nucleus, promoting transcription of numerous cytokines63,65,239. Dysbiosis of the gut microbiota can lead to intestinal barrier dysfunction and increased intestinal permeability (2). This facilitates the translocation of PAMPs and LPS into the systemic circulation, leading to a persistent, low-grade inflammatory state of liver, muscles, and visceral and subcutaneous adipose tissue as observed in both diabetes (both type 1 and type 2 diabetes mellitus) and inflammatory bowel disease (IBD)109-114. Across the intestinal epithelium, antigen-presenting cells (APCs), including macrophages and dendritic cells, detect pathogenic bacteria and promote the antigens on the cell surface (3). Thereafter, the APCs migrate to mesenteric lymph nodes, mediating an alteration of T lymphocyte subsets66,71. Secretory IgA (sIgA) serves as the first line of defence in protecting the intestinal epithelium from enteric toxins and pathogenic microorganisms (4). Antibiotic exposure leads to lower levels of sIgA, potentially leading to an increased inflammatory environment9. Gut microbiota ferment diet-derived carbohydrates into short-chain fatty acids (SCFAs) (5). SCFAs are ligands of the G protein-coupled receptors GPR41 and GPR43, which are expressed by intestinal enteroendocrine cells and enhance production of peptide YY (PYY), a hormone that affects insulin utilization by increasing the intestinal transit time, and increases satiety and energy harvest from the diet105,106. TH cell, T helper cell; Treg cell, regulatory T cell. The original version of this figure was created with BioRender.com.
Timing and nature of antibiotic exposure.
As specific types of antibiotics exert differential effects on gut microbiota composition, exposure to particular antibiotic classes can differentially affect T1DM development66-68. Timing of antibiotic exposure is also a potential factor66,69. In NOD/Caj mice, a substrain of NOD mice used to understand the role of B cells as antigen-presenting cells, maternal (prenatal) exposure to neomycin or vancomycin both induced long-term changes in the gut microbiome composition of the offspring compared with the offspring of untreated control mice66. However, only vancomycin (which targets mainly Gram-positive bacteria and anaerobes) strongly accelerated T1DM development66. In mice prenatally treated with vancomycin, T cells from the spleen, pancreatic lymph nodes and Peyer’s patches showed significant decreases in naive T cell markers (CD44−CD62L+) and increased numbers of CD4+ memory T cells (CD44+CD62L−). Consistent with this more-activated T cell repertoire, these T cells expressed higher levels of the pro-inflammatory cytokines IL-17, interferon-γ (IFNγ) and TNF. By contrast, prenatal treatment with neomycin (which targets mainly aerobic microorganisms) significantly protected the progeny from T1DM compared with untreated counterparts, and was associated with increased Bacteroidetes abundance66. The protection was also associated with induced immunotolerogenic responses of antigen-presenting cells in both spleen and mesenteric lymph nodes66.
In another study, lifelong treatment of NOD mice with either vancomycin or neomycin, started prenatally until the onset of diabetes, accelerated T1DM onset and altered effector T cell populations, with increased IFNγ CD4+ T cells and, in contrast to the previously discussed study66, reduced IL-17+CD4+ T cells70. In male but not female NOD mice treated with a broad-spectrum high-dose antibiotic cocktail (streptomycin, colistin and ampicillin) or vancomycin only, T1DM incidence was significantly increased compared with the incidence in untreated control mice68 (P < 0.0001 for the antibiotic cocktail and P < 0.0004 for vancomycin) and showed a significant decrease in Il17a gene expression or IL-17-producing cells in colon, Peyer’s patches and mesenteric lymph nodes68. However, another study showed that T1DM was attenuated in NOD mice that received vancomycin from birth through weaning at age 4 weeks69. These conflicting results might reflect the nature of the microbiota in the mouse colony being studied, but nevertheless they provide experimental evidence that antibiotic perturbation of the microbiome affects T1DM development.
Most studies investigating the effects of antibiotics in murine models have used a continuous antibiotic regimen, often at super-therapeutic levels67,68. However, such interventions do not mimic paediatric antibiotic use, which consists of discrete courses that are modelled better by therapeutic-dose pulsed antibiotic treatment (PAT). A study in which NOD mice received PAT (with a macrolide) in early life showed accelerated development of T1DM and insulitis compared with mice continuously treated with subtherapeutic antibiotic treatment (STAT) and controls71. Male mice exposed to PAT showed reduced α-diversity and β-diversity in their microbial population structure and decreased proportions of small intestinal lamina propria TH17 and regulatory T (Treg) cells before T1DM onset. These immunological changes were accompanied by altered ileal gene expression (concomitant with upregulated cholesterol biosynthesis) and altered metabolomic profiles in the caecum, liver and serum71. Transfer of the antibiotic-altered microbiota to adult germ-free mice showed similar changes in intestinal T cell populations, confirming that the perturbed microbiome was responsible for the altered immunological signal. However, transfer of antibiotic-perturbed microbiota from 6-week-old mice to pregnant germ-free mice showed an unexpected protection of the offspring from T1DM71. One potential explanation for this observation is that the 6-week time point (P42) microbiota were highly selected for opportunistic microorganisms, and their transfer to the newborn mice led to tolerance, consistent with the adage in NOD mice that ‘dirty protects’. This complexity, shown in a single series of experiments by the same group9,72, illustrates that the relationship between the microbiota and host phenotypes depends on antibiotic type, dosage and timing. In independent experiments, even a single 5-day macrolide PAT course in early life was sufficient to accelerate and enhance T1DM onset in male mice, leading to profound changes in expression of genes encoding immunological effectors in the ileum72. A study published in 2021 explored whether the phenotype of mice that had been exposed to antibiotics that perturbed the microbiome, changed immunological phenotypes and accelerated and enhanced T1DM, can be rescued by attempting to restore their microbiota73. To investigate this, during a period between 3 and 7 days after antibiotic treatment ended, mouse pups were gavaged with caecal microbiota of mothers obtained on the day of their pups’ birth. This treatment largely restored the baseline T1DM phenotype, partially restored the intestinal microbiome composition, metagenome and metabolome, and restored ileal RNA and microRNA expression. These studies demonstrate the importance of the effect of antibiotic perturbation of the microbiome on T1DM development, and point towards the role of post-exposure restorative approaches. They also provide a path to discovery of relevant microorganisms, microbial genes, metabolites and host genes that influence the propensity for T1DM73.
Clinical trials
An intervention study that used the antimicrobial fusidic acid in 28 patients with newly diagnosed T1DM showed no significant differences in β-cell function, C-peptide values or quantitative insulin requirements compared with a control group who received placebo74. In addition, a clinical trial with an oral dose of the SCFA butyrate showed no effect on either innate or adaptive immunity markers in 30 patients with long-standing T1DM74,75. By contrast, in a pilot trial including 20 patients with recent T1DM onset, use of faecal microbiota transplantation (FMT) led to an increased abundance of both Desulfovibrio strains and microbiota-derived plasma metabolites of tryptophan origin, which were associated with the stabilization of residual β-cell function76. This result provides a proof-of-principle that even after T1DM commencement, interventions affecting gut microbiome composition and activity can have salutary effects, extending the findings observed in mice. Taken together, such findings warrant added caution in the use of antibiotics in pregnant women and newborns, and minimizing the practice of caesarean section.
Type 2 diabetes and childhood obesity
Epidemiology
Changing epidemiology of the disease in the antibiotic era.
In the USA, the prevalence of T2DM has increased from 0.93% in 1958 to 7.40% in 2015 (REF.77). This steep rise coincides with an increased prevalence of obesity, one of the hallmarks of T2DM, as well as with the cumulative and increasing use of antibiotics52,53,78. The worldwide prevalence of T2DM is estimated to increase to 12.2% by 2045 (REF.31).
Metagenomic analysis of the gut microbiome of patients with T2DM revealed distinct perturbations in composition and function, characterized by a decreased abundance of butyrate-producing bacteria and an enrichment of opportunistic pathogens, often mucin-degrading (Akkermansia muciniphila) and sulfate-reducing (Desulfovibrio sp.)79. Although some studies showed an increased Firmicutes to Bacteroidetes ratio in patients with T2DM80,81, this should not be considered a T2DM hallmark as the relative abundance of these phyla is highly variable between individuals with T2DM82,83. These compositional changes are partly explained by differences in ethnicity; lower α-diversity was observed in the gut microbiomes of populations of South Asian and African origin compared with those of European origin living in the same city84,85. Such differences correspond to a higher risk of T2DM in these ethnic minority populations86.
Epidemiological linkages with the disease.
As T2DM mostly affects adults, studies examining the association between antibiotic use and T2DM diagnosis have only been performed in adult cohorts87-89 (TABLE 1). However, multiple longitudinal cohort studies have shown that exposure to antibiotics in the first 3 years of life is associated with an increased risk of childhood obesity and central adiposity, which are well-known risk factors for T2DM48,90-95 (TABLE 1). Strikingly, these studies showed that most children (>70%) in high-income countries/regions are exposed to antibiotic therapy at least once by the age of 2 years90, whereas the average incidence of antibiotic use in low-income and middle-income countries/regions is even higher (4.9 courses per child per year)96. This enormous use is at a scale consistent with the extent of disease incidence; antibiotic use in developing countries/regions started later, but cumulatively they are catching up quite rapidly97.
Antibiotic use during pregnancy also has effects on the development of the infant microbiome98. Exposure to antibiotics in the second or third trimester has been associated with an 84% (95% CI 33–154%) higher risk of childhood obesity, including higher waist circumference (3.13 cm, 95% CI 0.68–5.59 cm) and increased body fat percentage (1.86%, 95% CI 0.33–3.39%)48. Although no direct information on T2DM was provided in these childhood studies, these findings provide consistent evidence that exposure to antibiotics during the critical window in early life, even if brief, could lead to long-term effects that increase the risk of developing T2DM.
Adult cohort studies consistently show that antibiotic exposure at least 6 months before the date of diabetes diagnosis is associated with increased risk of T2DM (TABLE 1). The risk increases with more frequent antibiotic exposure87-89 and varies depending on antibiotic type88,89. However, these studies vary in their methodological approaches. For example, not all studies adjusted the results for variables including obesity, dyslipidaemia, hypertension and other cardiovascular comorbidities88, conditions that are part of the metabolic syndrome and are associated with T2DM as well as gut dysbiosis99,100. That the gut microbiome is influenced by ethnicity84, as well as environmental factors such as diet, physical activity, smoking and other medications101, limits the interpretation of epidemiological studies. As such, the causal link between antibiotic exposure and its subsequent perturbation of the gut microbiome, with T2DM development, has not been established by the epidemiological studies.
Experimental studies
Murine models and faecal transplant experiments have provided mechanistic insights into how the gut microbiota might contribute to T2DM development. In particular, they have shown that the intestinal microbiota influences both host metabolism and immunological interactions. Early-life STAT exposure in C57BL/6J mice, either continuously or for a short time span only, led to significantly increased fat mass compared with control mice7,8,102,103, with increased incretin secretion and glucose intolerance compared with controls7,8,103. Antibiotic treatment also altered expression of hepatic and ileal genes involved in fatty acid metabolism and triglyceride uptake, as well as hepatic steatosis9,102,103. The gut microbiota of STAT mice showed a shift in taxonomic composition, with higher levels of Firmicutes and lower levels of Bacteroidetes7,8,102, similar to that observed in ob/ob (leptin-deficient, obese) mice99,104. Importantly, the onset of adiposity occurred after the alterations in microbiome composition and remained later in life (32 weeks)7,8, even after the perturbation of the microbiome recovered following STAT withdrawal8,102. Transferring caecal contents from STAT mice into germ-free mice by oral gavage replicated the obesity phenotype8. These data are consistent with the hypothesis that there is a critical early-life period in which later-in-life metabolic development is set, and that alterations to the gut microbiota in that period have long-term consequences. Caecal contents of STAT mice showed substantially higher levels of SCFAs (acetate, butyrate and propionate), which indicates an increased capacity to harvest energy by bacterial fermentation of complex dietary carbohydrates7. SCFAs are ligands of the G protein-coupled receptors GPR41 and GPR43, which are expressed by intestinal enteroendocrine cells that produce peptide YY105, a hormone that affects insulin utilization by increasing intestinal transit time and increases satiety and energy harvest from the diet. However, the role of GPR41 and GPR43 in obesity is inconsistent between studies. A study with GPR41-knockout mice showed that they have reduced body weight, less fat accumulation and less insulin resistance than their wild-type counterparts106. Another study showed that weight gain is suppressed in mice that overexpress GPR43 in white adipose tissue and that are fed a high-fat diet (HFD), whereas GPR43-knockout mice become obese, even on a normal diet107. These seemingly contradictory results might reflect factors such as differences in the disease models used, the inbred mouse strains used and their microbiota, or non-specific effects of the knockouts themselves108. Epithelial cell expression of GPR41 and GPR43 in human physiology needs more study. Nevertheless, these studies provide evidence that SCFAs, and therefore the gut microbiota, regulate host energy expenditure. As such, disruption of gut microbiome composition and functionality by antibiotic exposure affects host energy balance, at least in part.
High circulating levels of inflammatory effector molecules, including TNF, IL-6, IFNγ and bacterial LPS, have been consistently associated with both obesity and T2DM, providing a potential mechanism for the persistent, low-grade inflammatory state of liver, muscle and adipose tissue frequently observed in both obesity and T2DM109-114. These changes might reflect increased intestinal permeability, leading to translocation of microbial constituents and products into the systemic circulation110. Conventionally raised mice receiving continuous LPS infusions showed metabolic responses, including increased hepatic insulin resistance, similar to those in mice receiving a HFD109. In 6-week-old ob/ob mice characterized by high LPS levels, antibiotic treatment lowered plasma LPS levels and inflammatory markers in adipose tissue110. These changes occurred concomitantly with improved metabolic parameters (such as improved glucose tolerance, less weight gain and lower fat mass). Notably, similar beneficial effects on glucose metabolism, including improved glucose tolerance and reduced fasting glucose levels, were seen in lean, healthy male mice after different 4-week antibiotic regimens115. These improved metabolic processes were accompanied by changes in hepatic and ileal gene expression involving glucose regulation and bile metabolism. Thus, after perturbations that increase translocation of intestinal contents, antibiotic treatments reduce secondary effects, and are therefore useful tools for future experiments. However, early-life exposure to antibiotics also directly affects host immune phenotypes, as illustrated by altered CD4+ T cell subsets and reduced intestinal secretory IgA levels in mice exposed to antibiotics compared with conventionally raised mice9. Germ-free mice exposed to antibiotics did not exhibit any substantial immunological changes, indicating that the metabolic and immunological effects were not a direct result of the antibiotics but rather are consequences of the antibiotic-induced gut microbiome alterations9 (FIG. 2). Thus, perhaps paradoxically, although early-life antibiotic exposures can drive the altered pathophysiology, once the damage has been done (from whichever cause), antibiotics have the potential to improve outcomes.
Dietary intake is one of the key modifiable extrinsic factors that influences gut microbiome composition and contributes to the onset of both T2DM and obesity. Antibiotic exposure in early life aggravates the negative effects (which include dyslipidaemia, insulin resistance and increased visceral fat mass) that accompany a HFD in female BALB/c mice compared with mice similarly exposed to antibiotics but fed a normal diet, even when the dysbiosis progressively recovers116. In another study, mice fed an obesogenic diet showed increased weight and fat mass when receiving lifelong STAT compared with their unexposed HFD-fed counterparts103. Such an obesogenic diet also affects antibiotic susceptibility. HFD-fed mice had impaired efficacy of bactericidal antibiotics compared with normally fed mice, a difference that was not observed in microbiota-depleted animals117. These findings suggest that antibiotic exposure worsens the diet-induced adiposity phenotype, leading to increased T2DM risk, and that obesity also reduces antimicrobial susceptibility.
Clinical trials
Owing to concerns about antibiotic resistance, there are few clinical trials that have investigated the effects of antibiotic exposure on weight gain in children. The trials that have been performed show conflicting results, with early-life exposure to antibiotics either having pronounced effects on weight gain118 or no effect119. A meta-analysis of ten randomized controlled trials including 4,316 children showed that undernourished children (<12 years old) from low-income and middle-income countries/regions treated with antibiotics were significantly taller (0.04 cm/month, 95% CI 0.00–0.07 cm/month) and had increased weight gain (23.8 g/month, 95% CI 4.3–43.3 g/month) compared with their placebo (nine studies) or untreated (one study) control groups117. However, there was significant geographic variation in weight gain, with children from trials conducted in Africa gaining 35.6 g/month (95% CI 12.8–58.3 g/month) of body weight more than children from other regions, which possibly is a reflection of more severe malnutrition118. In a US trial, 302 children (<6 years old) taking long-term oral trimethoprim–sulfamethoxazole prophylaxis did not gain substantially more weight than 305 control individuals, although approximately 25% of children in both groups had overweight or obesity119.
Studies of antibiotic interventions to alter T2DM progression have been limited in number. Treatment with a broad-spectrum antibiotic mixture in healthy lean men led to a drastic reduction in the abundance of gut microbiota (in colony-forming units (CFU)), but without significant changes in fasting or postprandial glucose, insulin secretion or plasma lipid levels120. Similar effects on gut microbiota composition were seen after oral vancomycin therapy121,122. Bacterial diversity was significantly (P < 0.001) reduced, with lower abundance of Gram-positive bacteria and increased abundance of Gram-negative bacteria, concomitant with increased levels of circulating LPS121,122. These perturbations affected glucose metabolism by decreasing peripheral insulin sensitivity in both lean individuals and patients with metabolic syndrome. In another study, 48 patients with bacterial endocarditis treated with intravenous vancomycin and gentamicin showed substantially increased BMI (+2.3 ± 0.9 kg/m2), which persisted 1 year after treatment123. However, as the therapy improved clinical status, weight gain could have been a reflection of the overall health improvement.
Novel therapies that aim to restore the dysbiotic gut microbiota in individuals with obesity and T2DM have been investigated in humans using different prebiotic124, probiotic125 or synbiotic126 regimens, but beneficial effects have been elusive. FMT has been studied as a means of counteracting the dysbiotic gut microbiome to improve insulin sensitivity in patients with metabolic syndrome or T2DM. Transfer of faeces from healthy donors to patients with metabolic syndrome improved insulin sensitivity in some, but not in all, patients127,128. This dichotomy might reflect baseline intestinal bacterial differences, with responders having lower diversity before FMT128, or differences in donor FMT composition and its administration129.
Inflammatory bowel disease
Definitions and epidemiology of IBD
IBD is a chronic inflammatory condition of the intestines, with two main subtypes: ulcerative colitis and Crohn’s disease. Although the precise aetiology of IBD remains unknown, increasing data suggest that alterations in the intestinal microbiota are a major contributor to IBD risk. As such, antibiotic exposure might have a role in IBD development in the current era.
One feature of IBD that might help in understanding its underlying pathophysiology is its temporal variation. Not only in disease activity, extent or behaviour within a patient, but also in epidemiological features that have evolved globally over time. Although the disease initially seemed to mostly be limited to Northern and Western Europe, the USA and Canada, data from 1960–2017 show that IBD has become increasingly prevalent worldwide, including in Central and South America, Africa, and the Caribbean and Asia–Pacific regions130-132. Furthermore, incidence in newly identified hotspots might outpace that of regions with traditionally high prevalence130,131, which is consistent with the rising cumulative exposure to antibiotics in those areas.
The demographics of those with IBD have also evolved. Ulcerative colitis is the predominant IBD subtype in regions with newly-incident IBD, and Crohn’s disease incidence then increases over time130. Countries/regions with traditionally high prevalence tend to have similar distributions of Crohn’s disease and ulcerative colitis130. Among the 34 countries/regions in the Organisation for Economic Co-operation and Development (OECD), IBD hospitalizations (a proxy for disease severity) were highest in traditionally high-prevalence regions (North America, Europe and Oceania) and lowest, but also most rapidly increasing, in new-incidence regions (Asia, Latin America and the Caribbean)133. In the Asia–Pacific Crohn’s and Colitis Epidemiology Study (ACCESS) inception cohort of 413 patients (181 with Crohn’s disease, 222 with ulcerative colitis and 10 with unclassified IBD), ~20% of patients with Crohn’s disease who initially presented with a non-fistulizing, non-stricturing phenotype developed these complications after a median follow-up of 18 months134, which is similar to rates in Western populations135. However, patients with ulcerative colitis in the ACCESS cohort were less likely to need advanced medical therapies and also less likely to have colectomies compared with their counterparts with Crohn’s disease, as well as in comparison to patients with ulcerative colitis in Western populations134,136.
Although the time of IBD onset can span from infancy to older age, diagnoses occur most commonly around the third decade of life132,137. However, there has been an increase in paediatric-onset IBD incidence in the past few decades, particularly in children aged <10 years137-139, in countries/regions with a high prevalence of IBD. For example, a population cohort study in Ontario, Canada, found an annual increase of 9.7% from 1999 to 2008, although this might have reflected improved early diagnosis137. However, IBD incidence increases following immigration from a low-prevalence to a high-prevalence country/region, with stronger risk associated with younger age at time of immigration (similar to T1DM) and among subsequent generations born in the high-prevalence countries/regions140-142. Such studies provide direct evidence of the importance of environmental factors in IBD risk. Although genome-wide association studies143,144 and twin studies145,146 have clearly identified genetic contributors to IBD risk, the changes in global IBD patterns and the time frame in which they have occurred emphasize the critical role of environmental triggers. Notably, many of the environmental factors identified as being associated with IBD risk or protection (for example, migration, diet and breastfeeding)145,146 are related to changes in the intestinal microbiome147-149.
Antibiotic exposure and IBD risk in humans
Several large cohort studies have implicated the use of antibiotics in the risk of IBD. Two large national database studies, one from the UK150 (a registry of 1,072,426 children aged <18 years with 6.6 million person-years of follow-up from 1994 to 2009) and another from Denmark151 (a registry of 577,627 children with 3,173,117 person-years of follow-up from 1995 to 2003), both found that antibiotic exposure in childhood is associated with IBD risk in a dose-dependent manner. This relationship has been confirmed in both paediatric-onset and adult-onset IBD (with approximate odds ratios ranging from 1.3 to 3.4), although the risk is higher with childhood exposure, with the greatest quantity of evidence and strength of effect in children exposed to antibiotics before the age of 1 year150-157. Although some studies have found associations between antibiotic treatment and subsequent development of both Crohn’s disease and ulcerative colitis152,154, in other studies the relationship was stronger either for Crohn’s disease (that is, significant findings at lower exposure levels)150 or present for Crohn’s disease only151,155,158. However, many of these studies were conducted in children, and the distribution of Crohn’s disease compared with ulcerative colitis, as well as the primary drivers of disease aetiology, may differ from those in adults158. For example, although the increasing incidence of very early onset IBD in Canada (that is, onset before age 6 years)159 suggests that its aetiology contains an environmental component, it is more commonly associated with monogenic mutations than IBD that starts later in childhood or in adulthood160. A potentially important confounding factor is the nature and severity of the illnesses for which antibiotics are prescribed. One hypothesis is that antibiotic exposure is merely a proxy for the underlying acute infections, which themselves might be the primary factor for increased risk of IBD, or alternatively might be a protective factor against IBD (the ‘hygiene hypothesis’). Unsurprisingly, such issues are difficult to tease out from studies in children161, which is why models in experimentally exposed animals, in the absence of initiating infections, are useful.
Animal models of antibiotics in IBD
Given the lag between antibiotic exposure and IBD onset, as well as potential clinical confounders associated with antibiotic use, animal studies have been particularly helpful in establishing causal relationships between antibiotic treatment and changes in intestinal inflammation in IBD models. IL-10-deficient162 and SAMP1/YitFc163 mice spontaneously develop bowel inflammation, and disease activity is responsive to microbiome manipulations164,165. Studies in both models have shown that antibiotic treatment improves intestinal inflammation when used in either preventive or treatment strategies165,166. Studies in the IL-10-deficient model demonstrated that particular antibiotic classes improve colitis in different intestinal regions, suggesting that specific microbiome populations have roles in modulating intestinal inflammation166. In experiments that model how vertical transmission of the maternal human microbiome to infants might affect IBD development, Schulfer et al. gavaged gnotobiotic IL-10-deficient mouse dams with faecal microbiota from wild-type mice that had either been perturbed by antibiotics administered at weaning in their drinking water or had not (control microbiota)167. In the absence of any other intervention, pups born to dams gavaged with the antibiotic-perturbed microbiota developed substantially more severe colitis than those born to dams given the control microbiota. In this experiment, the fact that neither the pups nor their mothers were actually exposed to any antibiotic established that the antibiotic-perturbed microbiota passed down from the mothers was sufficient for the enhanced disease. Parenthetically, this experiment also provides evidence that in diseases with familial tendencies, the risk factors might not be only the inherited host genes, but also the intergenerational transfer of microorganisms. Similar experiments were conducted by Miyoshi et al. with similar results168. Taken together, these studies provide an important link between cumulative antibiotic use in populations and the increasing IBD disease risks observed over the past few decades. They are consistent with the notion that antibiotic effects are cumulative across generations, as previously postulated169. Studies in humans investigating the effect of inheriting a perturbed microbiome on IBD risk are ongoing170.
Another important experimental model of IBD is DSS-induced colitis171,172. Ozkul and colleagues173 investigated whether previous exposure to antibiotics worsened the course of DSS colitis. They observed that mice with early-life exposure to a macrolide develop more severe colitis than mice without the exposure. That the antibiotic exposure ended more than 2 weeks before the DSS challenge indicates potential latency in antibiotic effects, which is consistent with observations in children153. In a subsequent experiment, the investigators transferred antibiotic-perturbed microbiota obtained 30 days after the exposure ended to germ-free mice, who were subsequently challenged with DSS; the recipients of the antibiotic-perturbed microbiota developed more severe colitis than those given the normal microbiota. Taken together, these studies suggest that antibiotic perturbation of the microbiota, in the absence of any infection, worsens experimental models of colitis. In other experiments in which the antibiotic challenge preceded exposure to a colonic pathogen of mice (Citrobacter rodentium) by as much as 80 days, there was worsened inflammation compared with mice not exposed to antibiotics174; the antibiotic-perturbed microbiota also transmitted more severe disease to germ-free mice that had been conventionalized, indicating that the perturbed microbiota per se had pathogenic properties174.
Antibiotics in the treatment of IBD
A separate question is whether antibiotics can modulate disease activity in established IBD, as with diabetes. There has been a long-established, but under-studied, clinical practice of using antibiotics in treating complications of IBD, such as fistulae, abscesses and pouchitis175-178. A related question is whether antibiotics can be used more routinely to alter the natural history of IBD. However, randomized controlled trials (and related meta-analyses) have yielded conflicting results179,180 (TABLE 2). The literature is difficult to interpret given the diversity of antibiotics studied as well as the differing indications and timing of treatment. The end points for some of these trials were based on clinical symptoms, and were therefore subject to potential confounding by treatment of irritable bowel syndrome, a condition that frequently co-exists with IBD181 and for which antibiotic treatment can have some efficacy182. Nonetheless, one of the clearest examples of the utility of antibiotics in the management of IBD is the use of nitroimidazoles, specifically metronidazole and ornidazole, to prevent postoperative recurrences of Crohn’s disease. Compared with placebo, both medications reduced (by 25–30%) the proportion of patients with endoscopic recurrence 3 months after surgery183,184. In the setting of defined clinical interventions, such as surgery, antibiotics can clearly improve outcomes.
Table 2 ∣.
Clinical studies evaluating antibiotic exposure and inflammatory bowel disease
| Study | Antibiotic exposure timing |
Date (location) | Study design | Antibiotic data source |
Disease diagnosis |
Key findingsa |
|---|---|---|---|---|---|---|
| Kronman et al.150 | Childhood | 2012 (UK) | Retrospective cohort | Health records registry | Diagnosis (Read) codes | HR IBD 5.51 (1.66–18.28) — antibiotic exposure before 1 year of age; HR 2.62 (1.61–4.25) — by 5 years of age; HR 1.57 (1.35–1.84) — by 15 years of age Dose dependency |
| Hviid et al.151 | Childhood | 2011 (Denmark) | Retrospective cohort | National health registry | Diagnosis (ICD) codes | IBD RR 1.84 (1.08–3.15) Dose dependency |
| Nguyen et al.152 | All | 2020 (Sweden) | Case–control | National health registry | Diagnosis (ICD) codes | aOR IBD 1.94 (1.85–2.03) Dose dependency |
| Shaw et al.153 | Infants <1 year of age | 2010 (Manitoba, Canada) | Nested case–cohort | Provincial health registry | Diagnosis (ICD) codes | OR IBD 2.9 (1.2–7.0) Dose dependency |
| Shaw et al.154 | 2–5 years before diagnosis | 2011 (Manitoba, Canada) | Case–control | Provincial health registry | Diagnosis (ICD) codes | aOR IBD 1.27 (1.20–1.35) Dose dependency |
| Card et al.157 | 2–5 years before diagnosis | 2004 (UK) | Case–control | Research database | Diagnosis (Oxmis, Read) codes | aOR IBD (1.05–1.65) No dose dependency |
| Virta et al.158 | Childhood | 2012 (Finland) | Case–control | National health registry | Diagnosis (ICD) codes | aOR CD 1.87 (1.37–2.56) aOR UC 1.18 (0.92–1.52) |
| Ungaro et al.155 | Variable | 2014 (Europe, Canada, New Zealand) | Meta-analysis | Mostly health registries, some surveys | Varies | Pooled OR IBD 1.57 (1.27–1.94) |
| Zou et al.156 | Childhood | 2020 (North America, Eurasia, Oceania) | Meta-analysis | Varies | Varies | Pooled OR IBD 1.5 (1.22–1.85) |
aOR, adjusted odds ratio; CD, Crohn’s disease; IBD, inflammatory bowel disease; ICD, international Classification of Disease; RR, rate ratio; UC, ulcerative colitis.
Value ranges in parentheses are 95% confidence intervals.
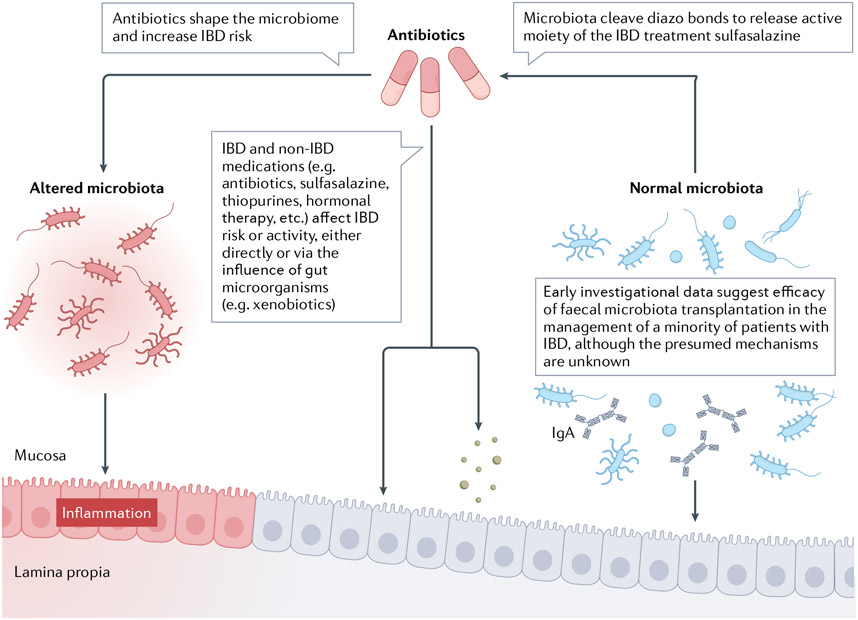
Beyond the direct effect of antibiotic-perturbed microbiota on IBD risk and activity, antibiotics might also influence disease by altering the metabolism of IBD medications by the gut microbiota185,186 (FIGS. 2,3). Sulfasalazine, which was among the earliest recognized IBD treatments, comprises an anti-inflammatory 5-aminosalicylate (such as mesalamine) joined to the antimicrobial sulfapyridine via a diazo bond. The cleavage of this bond, and the subsequent release of the active moiety, is mediated by diazo reductases, which are produced by many gut bacterial taxa but in the largest quantity by Clostridia186,187. Notably, antibiotic-treated germ-free rats do not excrete cleaved sulfasalazine186,187. The gut microbiota also has roles in metabolism of other IBD medications including glucocorticoids188, methotrexate189 and thioguanine190. Thus, antibiotic-induced microbiome manipulation might affect established IBD in addition to having effects on disease development.
Fig. 3 ∣. Influence of the gut microbiota on inflammatory bowel disease activity.
Antibiotics shape the microbiota and increase the risk of inflammatory bowel disease (IBD)150-158,161. Normal microbiota constituents cleave sulfa-diazo bonds to release the active moiety of the IBD treatment sulfasalazine186,187. IBD and non-IBD medications (for example, antibiotics, sulfasalazine, thiopurines, hormonal therapy, etc.) affect IBD risk or activity, either directly or with the influence of gut microorganisms (for example, xenobiotics)150-158,161,175-180,183,184,186-190,240-242. Early investigational data suggest efficacy for crude faecal microbiota transplantation in IBD management, although the precise mechanism is unknown191,193,243. The original version of this figure was created with BioRender.com.
FMT as an approach for treating IBD
The growing interest in FMT, coupled with high-throughput sequencing, permits a new understanding of the interactions between the intestinal microbiota and IBD activity. Despite differing delivery routes and treatment regimens, three of the four randomized clinical trials of FMT for the treatment of mildly to moderately active ulcerative colitis yielded remission rates of ~25–30% compared with 5–10% in controls191-194. In a pilot, randomized, controlled study of 12 patients with mild-to-moderate ulcerative colitis published in 2021, remission was numerically more common in the treatment group but was not significantly different between the treatment group and the control group (two of six versus none of six, respectively)195. Another negative study, published in 2015, evaluated 50 patients who were randomized to either two donor FMTs over 3 weeks by nasoduodenal tube or placebo (autologous FMT). The primary end point evaluation at 12 weeks was completed by 37 patients. In both the per-protocol and intention-to-treat analyses, there were no significant differences between groups, although some have attributed the negative result to the less-intensive dosing interval compared with the interval used in the positive FMT trials192. Across trials, consistent taxa or mechanisms underpinning the successful FMTs have not been identified. Nevertheless, the proof of principle underlying these trials is promising, and has spurred development of defined microbial consortia instead of stool. Although there have been a few open-label studies and case reports of FMT for Crohn’s disease that have had positive results196, the only two randomized clinical trials to date did not show significant effects197,198. However, data interpretation is limited by heterogeneity in disease location, behaviour and extent, which makes Crohn’s disease difficult to study.
Coeliac disease
Definition and epidemiology
Coeliac disease is a chronic immune-mediated inflammatory disease that affects the small intestine and is triggered by gluten exposure in a genetically susceptible host. Coeliac disease affects about 1% of the population worldwide and is associated with increased morbidity and mortality199-202. A meta-analysis published in 2019 revealed that the incidence of coeliac disease has been increasing by 8.4% (95% CI 6.0–10.8%) annually over the past few decades (since the 1990s), with female predominance203. Coeliac disease can occur at any age, and studies have demonstrated that a loss of gluten tolerance can occur during adulthood204. Although increased incidence and prevalence might reflect improved detection, disease development in adulthood and the dramatic increases observed suggest that environmental factors are contributing to the risk of coeliac disease.
Environmental factors
Coeliac disease has been strongly linked to HLA variants within the DQ2 and DQ8 heterodimers205. Although the presence of risk alleles is generally necessary for coeliac disease development, it is not sufficient, indicating the importance of environmental factors205. Earlier studies evaluating the microbial populations associated with coeliac disease found intestinal dysbiosis in patients, with an increased abundance of Escherichia coli and Bacteroides and a decreased abundance of Bifidobacterium206-211. Some differences in relative abundance resolve with a gluten-free diet, whereas others persist206-211. In 2015, Galipeau et al. showed that in mice with genetic susceptibility (that is, expressing the human HLA-DQ8 gene) for coeliac disease, antibiotic exposure influenced gluten-induced immunopathogenicity that depended on the specific bacterial taxa expanded by the antibiotic212. These findings are consistent with cohort studies showing that an increased risk of coeliac disease is associated with such microbiome perturbations as caesarean delivery and proton pump inhibitor exposure213,214, but studies now suggest that particular taxa are potentially implicated in pathogenesis.
Additional evidence is emerging that gut microbiota composition and function contribute to the development of coeliac disease in genetically susceptible hosts212,215-217. Opportunistic pathogens can induce immune activation of gluten-specific T cells relevant for coeliac disease, either through bacterial elastase modification (increasing immunogenicity and mucosal translocation)215,216 or via molecular mimicry217 in animal and preclinical studies. Caminero et al. colonized germ-free mice with opportunistic pathogens derived from small intestinal biopsy samples from patients with coeliac disease, including Pseudomonas aeruginosa, or with Lactobacillus spp. from healthy control individuals216. P. aeruginosa showed enhanced mucosal translocation in the mouse intestine216, and P. aeruginosa-modified gluten peptides which were then recognized by activated gluten-specific T cells from patients with coeliac disease, leading to increased immune recognition. By contrast, Lactobacillus spp. from healthy individuals degraded gluten peptides, resulting in decreased immunogenicity216. Taken together, multiple factors modulated by specific opportunistic pathogens associated with coeliac disease could reduce tolerance towards gluten in genetically susceptible individuals.
Clinical studies
Coeliac disease and antibiotic exposure.
To date, there have been ten studies evaluating antibiotic exposure and risk of developing coeliac disease (TABLE 3). In addition, systematic reviews and meta-analyses of the literature from 2018 to 2020 have demonstrated increased risk of coeliac disease and antibiotic exposure218-220. Jiang et al. found increased risk of coeliac disease after antibiotic exposure (pooled OR 1.2, 95% CI 1.04–1.39), and specifically for antibiotic exposure during childhood (OR 1.15, 95% CI 1.02–1.29)218. Kamphorst et al. also concluded that antibiotic exposure in the first 2 years of life is associated with coeliac disease risk219. Although the studies evaluated in this meta-analysis were of high quality, there were only four, and the odds ratios were modest, ranging from 1.13 to 1.4 (REFS.221-223). However, the clear dose–response relationship in three of the studies provides further evidence of a relationship221-223 (TABLE 3). In another population-based birth cohort study of >14,000 children born in Olmsted County, Minnesota, between January 2003 and December 2011, antibiotic exposure in the first 2 years of life was studied as a possible risk factor for the development of ten conditions with childhood onset, including coeliac disease. In the cohort, which included 45 children who were subsequently diagnosed with coeliac disease, there was a significant antibiotic dose-dependent relationship, which was stronger in girls than in boys.
Table 3 ∣.
Clinical studies evaluating antibiotic exposure and coeliac disease risk
| Study | Antibiotic exposure timing |
Date (location) | Study design | Antibiotic data source |
Coeliac disease diagnosis |
Key findingsa |
|---|---|---|---|---|---|---|
| Mårild et al.225 | Prenatal | 2014 (Sweden) | Cohort | Prospective questionnaire | Histology (Marsh 3) and either positive coeliac serologies or symptoms consistent with coeliac which resolved on GFDb | No significant difference HR 1.33 (0.69–2.56) |
| Mårild et al.226 | Prenatal | 2017 (Norway) | Cohort | Prospective questionnaire | Questionnaire or database diagnostic codes for coeliac diseasec | No significant difference aOR 1.16 (0.94–1.43) |
| Myléus et al.232 | Birth to 6 months | 2012 (Sweden) | Case–control | Parental questionnaire | Three consecutive duodenal biopsy samples (Marsh 3) | No significant difference between coeliac disease and controls OR 1.2 (0.87–1.6) |
| Canova et al.221 | Birth to 12 months | 2014 (Italy) | Cohort | Prescriber registry | Database diagnostic codes for coeliac disease | OR 1.3 (1.10–1.56) Dose–response relationship with five or more antibiotic courses OR 2.66 (1.79–3.95) |
| Dydensborg Sander et al.222 | Birth to 12 months | 2019 (Denmark and Norway) | Observational cohort | Prescriber registry | Database diagnostic codes for coeliac disease | OR 1.26 (1.16–1.36) Dose-dependent relationship for each additional antibiotic OR 1.08 (1.05–1.11) |
| Kemppainen et al.228 | Birth to 48 months | 2017 (Finland, Germany, Sweden and USA (TEDDY)) | Cohort with T1DM and permissive HLA for CD | Prospective questionnaire | Risk of coeliac disease defined as two consecutive positive serum TTG IgA at least 3 months apartd | No increased risk of positive TTG IgA and antibiotic exposure HR 1.00 (0.98–1.02) |
| Bittker and Bell223 | Birth to 48 months | 2019 (USA) | Case–control Internet-based survey | Parental report | Diagnosis from medical professional | aOR 1.13 (1.04–1.24) Dose-dependent relationship for number of antibiotic courses For four to seven, OR 1.62 (1.03–2.55) For eight or more, OR 2.48 (1.29–4.75) |
| Aversa et al.94 | Birth to 24 months | 2021 (USA) | Cohort | Prescriber registry | Database diagnostic codes for coeliac disease | Dose-dependent relationship Gender specific for girls: For one or two antibiotic prescriptions, HR 8.12 (1.03–64.10) For more than five antibiotic prescriptions, HR 12.32 (1.56–97.32) |
| Simre et al.227 | Birth to 60 months | 2016 (Estonia and Finland (DIABIMMUNE)) | Cohort with T1DM and permissive HLA for CD | Parental report of antibiotic exposure | Positive coeliac serology and duodenal biopsy sample (Marsh 3) | No significant difference (number of antibiotic courses 1.1 versus 1.0 Finland) |
| Mårild et al.224 | All ages | 2013 (Sweden) | Case–control | Prescriber registry | Histology database (Marsh 3) | OR 1.40 (1.27–1.53) Also found increased risk with inflammation (Marsh 1 and 2) and those with normal histology (Marsh 0) but positive coeliac serologies |
aOR, adjusted odds ratio; CD, coeliac disease; GFD, gluten-free diet; OR, odds ratio; T1DM, type 1 diabetes mellitus; TTG, tissue transglutaminase.
Value ranges in parentheses are 95% confidence intervals.
Data obtained from prior study published in 2004.
Validation study performed by authors.
Primary outcome was risk of coeliac disease.
Timing of antibiotic exposure.
Five studies showed an increased risk of coeliac disease associated with antibiotic exposure. In four of the studies, the exposure was within the first 2 years of life94,221-223, whereas the fifth study examined exposures at all ages in childhood224 (TABLE 3). It is biologically plausible to interpret these findings as supporting a causal relationship given that the gut microbiota becomes well-established by 3 years of age6. Both studies that evaluated maternal (prenatal) antibiotic exposure did not show a statistically significant difference in the risk of coeliac disease in the offspring between prenatal exposure to antibiotics and no exposure225,226 (TABLE 3). Taken together, these findings suggest that timing of exposure might be a significant factor for the risk of developing coeliac disease94,221-223,225,226. In addition, two large cohort studies evaluated the relationships between coeliac disease and antibiotic exposure in specific at-risk populations, specifically children with T1DM with coeliac disease-permissive HLA alleles227,228 (TABLE 3). These studies followed children from birth, based on parental reporting of antibiotic exposure, monitoring for development of positive coeliac serologies, including tissue transglutaminase IgA, and in one study for development of histologically proven coeliac disease227. In these restricted populations, neither study showed an association between antibiotic exposure and the development of either coeliac disease or positive serologies.
Pathogenesis
Several high-quality studies have shown associations between the development of coeliac disease and early-life antibiotic exposure with dose-dependent relationships94,221-223; however, we know little about the specific mechanisms, other than the evidence of major changes in immunological development seen in other studies10,68,94,166. There seems to be a critical window, perhaps within the first 2 years of life, in which antibiotic exposure, by perturbing the gut microbiome and consequently altering immunological maturation, affects coeliac disease development. However, other windows might exist in later life, given that coeliac disease can develop in adulthood. Certain HLA haplotypes affect disease onset. For example, having two copies of HLADQB1*02 has been associated with earlier disease onset, classic clinical presentation and more severe histological damage229. Future studies to help better understand the interplay between genetic susceptibility and environmental contributions, such as that from a perturbed microbiota, should also include HLA genotyping. Our understanding of the pathogenetic steps has been limited by the lack of a proper animal model. The development of a mouse model in 2020 that approximates coeliac disease through overexpression of IL-15 and expression of the predisposing HLA-DQ8 molecule, leading to development of villous atrophy after ingestion of gluten, has great promise for developing a greater mechanistic understanding230.
Discussion and conclusions
In this Review, we consider several distinct diseases. Yet, all are centred on the development of abnormal patterns of inflammation of the gastrointestinal tract, an organ system that hosts an enormous, complex and varied microbiota. Although our discussion of the aetiology of these diseases centres on perturbation of the hindgut microbiome, we also consider another disease that is increasing in incidence, EoE (TABLE 4), for which foregut microbiota perturbation might be important (BOX 1). In reality, the principles being considered are parallel for the foregut and hindgut.
Table 4 ∣.
Clinical studies evaluating antibiotic exposure and eosinophilic oesophagitis risk
| Study | Timing of antibiotic exposure |
Age of EoE onset |
Date (location) | Study design | Antibiotic data source |
EoE diagnosis | Key findingsa |
|---|---|---|---|---|---|---|---|
| Witmer et al.233 | Birth to 6 months | Paediatric | 2018 (USA) | Case–control | Pharmaceutical coding records | National military database | aOR 1.31 (1.10–1.56) |
| Jensen et al.234 | Birth to 12 months | Paediatric | 2013 (NC, USA) | Case–control | Retrospective survey | Databaseb | OR 6 (1.7–20.8) |
| Radano et al.235 | Birth to 12 months | Paediatric | 2014 (Boston, MA, USA) | Case–control | Retrospective questionnaire | Databaseb | OR 3.61 (1.11–11.7) |
| Jensen et al.236 | Birth to 12 months | Paediatric | 2018 (Cincinnati, OH, USA) | Case–control | Retrospective questionnaire | Databaseb | aOR 2.30 (1.21–4.38) |
| Dellon et al.237 | Birth to 12 months | Adult | 2021 (NC, USA) | Nested case–controlc | Retrospective questionnaire | Databaseb | OR 4.64 (1.63–13.2) |
| Slae et al.238 | Birth to 12 monthsd | Paediatric | 2015 (Canada) | Case–control | Retrospective questionnaire | Databaseb | No significant difference OR 1.00 |
aOR, adjusted odds ratio; EoE, eosinophilic oesophagitis; OR, odds ratio.
Value ranges in parentheses are 95% confidence intervals.
Database included histopathological data to confirm EoE diagnosis based on accepted definitions.
Case–control study nested within a previously conducted prospective cohort study of adults undergoing outpatient oesophagogastroduodenoscopy for evaluation of gastrointestinal symptoms.
This study included questions regarding recent antibiotic exposure, which was defined as ‘less than once a year’, ‘once a year’, ‘two to three times per year’ and ‘four or more times per year’.
For each of these diseases, there is a growing body of evidence that a perturbed microbiota is associated with onset and pathogenesis. There is a complex interplay between host genetics and environmental factors that influence gut microbiota composition and functionality, with multiple confounding factors. Epidemiological studies suggest that antibiotic treatments, especially in early life, are associated with increased risk of these diseases by altering the microbiota. However, as they are designed to test associations rather than causal roles, they can never be conclusive. However, experimental studies of several antibiotic treatments in mice have shown substantial early-life perturbations of the gut microbiota, with downstream metabolic and immunological effects. From such experiments, it is possible to reach conclusions about causality (in mice), which can be juxtaposed with the human epidemiological and clinical data to reach broader conclusions. Experiments further indicate that variation in antibiotic types, dosages and timing could explain differences in disease risk.
If this notion is correct, then it can help us to understand the pathogenesis of each of these diseases in new ways, beginning with how microbial populations in the lumen signal to host tissues in beneficial or pathogenic ways. Understanding the pathogenetic mechanisms might lead to new approaches to the diagnosis and treatment of these diseases. In addition, such investigations should lead us to a new appreciation of the biological costs of antibiotic treatments. Although antibiotic treatment can be life-saving, most patients receive antibiotics for treatment of mild infections, often without strong indications of utility22. Such wide use reflects a general sense by both practitioners and the public that antibiotics are very safe, and that benefits outweigh any risks. But if antibiotic exposure is indeed playing a part in any or all of these diseases, then consideration of their risks must grow, and their use must be tempered by a more transparent risk–benefit assessment.
Antibiotics have been pillars of medicine for the past 75 years, and have so much benefit and so little immediate cost that they are widely used even for the most marginal indications22. However, increasing evidence of long-term costs propels us to find alternative approaches to control bacterial infections. One important avenue is the development of narrow-spectrum agents, whether they be antibiotics, peptides, bacteriophages or other approaches, to reduce the unintended collateral consequences of broad-spectrum agents. Other approaches are ecological: to select for, or introduce, competitors of the pathogens; this could be done with single agents or mixtures of beneficial organisms, using probiotics, prebiotics or FMT. Retreating from antibiotic ‘carpet bombing’ of the intestinal microbiota will probably prevent much future disease.
However, as discussed throughout this Review, the data are incomplete, and limited by the complexity of testing hypotheses that involve exposures months, years or even decades before the development of a disease. Going forwards, these issues should be considered in light of the nine criteria for understanding causal relationships developed in 1965 by Austin Hill231. These criteria, which include the strength of the association (effect size), consistency (reproducibility), specificity, temporality, biological gradient (dose–response relationship), plausibility, coherence, experiment and analogy, are highly relevant to the questions raised here. For each of the illnesses, there is a partial match with the criteria, but the data are incomplete to fully assess the link and its magnitude. As such, data interpretation in humans is challenging, and many pathways remain to be discovered (BOX 2). Further knowledge will require more prospective epidemiological studies, clinical trials, and disease models in experimental animals that mimic the conditions of interest. Nevertheless, this interface involving some of the most common medicines used in the world represents an important frontier of medical science for diseases of global significance.
Box 2 ∣. Open research questions.
What is the relationship of the timing of antibiotic exposure to disease risk?
Do all antibiotics have similar metabolic and immunological effects mediated by microbiota disturbances?
Are there particularly bad combinations of antibiotic exposures that magnify risk?
What is the relationship between antibiotics and other exposures that perturb the early-life microbiome (for example, caesarean birth, formula feeding)?
What are the mechanisms by which a perturbed microbiota aberrantly signals to host tissues?
- After a damaging exposure, can there by restoration to baseline?
- What is the relevant time window?
- Is there a point of no return?
- What are the optimal ways to accomplish this (for example, prebiotic, probiotic or synbiotic supplementation, or faecal microbiota transplantation)?
Key points.
The widespread use of antibiotics worldwide is consistent with a rise in chronic inflammatory diseases of the gastrointestinal tract, including inflammatory bowel disease, coeliac disease, eosinophilic oesophagitis, and type 1 and type 2 diabetes.
Exposure to antibiotics leads to profound effects on both the composition and the functionality of the gut microbiota, leading to potential pathogenic mechanisms for disease onset.
Experimental studies have shown that antibiotic-induced perturbations of the microbiota are transferable and affect disease development.
Differential levels of antibiotic exposures, and their types and timing — particularly exposure in early childhood — could explain differences in disease risk.
A growing body of evidence indicates that an antibiotic-perturbed microbiota is associated with disease development, although current knowledge is limited by microbiota complexity. Research including prospective epidemiological studies, clinical trials and experimental studies is required.
Novel therapies aiming to remediate the perturbation of the gut microbiome are being researched, including prebiotics, probiotics, synbiotics and faecal microbiota transplantation; however, the strong application of antibiotic stewardship is most warranted to prevent perturbing the microbiome.
Lipopolysaccharide (LPS).
A microbiota-derived endotoxin found in the outer membranes of Gram-negative bacteria. Bacterial LPS has a key role as an elicitor of innate immune responses through binding to CD44, LBP and Toll-like receptor 4.
Germ-free mice.
Mice born and raised in sterile conditions and thus free of bacteria and fungi.
Dysbiosis.
Perturbation of the homeostasis of gut microbiota composition, potentially leading to changes in both functional and metabolic activities.
Short-chain fatty acids (SCFAs).
Predominantly butyrate, propionate and acetate. Metabolic products of complex carbohydrate fermentation, chiefly from anaerobic bacteria, that are both energy sources for hosts as well as signalling molecules to host tissues.
Probiotics.
Viable microorganisms that reach the intestine in an active state and might elicit a favourable effect on host metabolism or re-establishment of gut microbial composition.
Non-obese diabetic mice (NOD mice).
A mouse strain developing an autoimmune illness resembling type 1 diabetes mellitus in humans. The substrain NOD/Caj have membrane-bound immunoglobulins and do not secrete antibodies, so as to understand the role of B cells as antigen-presenting cells rather than production of auto-antibodies only.
Prebiotic.
Dietary components, mostly consisting of non-digestible fibres, that might have a potential beneficial effect on gut microbial composition and function.
Synbiotic.
Nutritional supplements that consist of a synergistic combination of both prebiotics and probiotics.
Acknowledgements
Supported in part by 1K23DK119544-01A1 (L.A.C.) and U01AI22285 (M.J.B.) from the National Institutes of Health, and Sergei S. Zlinkoff Fund (M.J.B.), a personal ZONMW VICI grant 2020 (09150182010020) (M.N.), and the TransAtlantic Networks of Excellence Program (33.17CVD01) from the Fondation Leducq (all authors).
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Ferretti P et al. Mother-to-infant microbial transmission from different body sites shapes the developing infant gut microbiome. Cell Host Microbe 24, 133–145.e5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Korpela K & de Vos WM Early life colonization of the human gut: microbes matter everywhere. Curr. Opin. Microbiol 44, 70–78 (2018). [DOI] [PubMed] [Google Scholar]
- 3. Bokulich N et al. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci. Transl Med 176, 139–148 (2016). This paper shows the profound effects of caesarean section and antibiotic exposure on how the early-life microbiome develops.
- 4.Yassour M et al. Natural history of the infant gut microbiome and impact of antibiotic treatment on bacterial strain diversity and stability. Sci. Transl Med 8, 343ra81 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bäckhed F et al. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe 17, 690–703 (2015). [DOI] [PubMed] [Google Scholar]
- 6.Yatsunenko T et al. Human gut microbiome viewed across age and geography. Nature 486, 222–227 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cho I et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature 488, 621–626 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cox LM et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell 158, 705–721 (2014). This paper shows that transient antibiotic-induced perturbation in early life can lead to late long-term metabolic changes in experimental models of obesity.
- 9.Ruiz VE et al. A single early-in-life macrolide course has lasting effects on murine microbial network topology and immunity. Nat. Commun 8, 518 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Macpherson AJ & Harris NL Interactions between commensal intestinal bacteria and the immune system. Nat. Rev. Immunol 4, 478–485 (2004). [DOI] [PubMed] [Google Scholar]
- 11.Honda K & Littman DR The microbiota in adaptive immune homeostasis and disease. Nature 535, 75–84 (2016). [DOI] [PubMed] [Google Scholar]
- 12. Hsiao EY et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 155, 1451–1463 (2013). This paper provides direct evidence of linkage of the gut microbiome with neurodevelopment.
- 13. Blaser MJ Who are we? Indigenous microbes and the ecology of human diseases. EMBO Rep. 7, 956–960 (2006). This paper introduces the concept that loss of ancestral commensals is leading to the modern epidemics of chronic diseases.
- 14.Blaser MJ The past and future biology of the human microbiome in an age of extinctions. Cell 172, 1173–1177 (2018). [DOI] [PubMed] [Google Scholar]
- 15.Guo R, Chen L-H, Xing C & Liu T Pain regulation by gut microbiota: molecular mechanisms and therapeutic potential. Br. J. Anaesth 123, 637–654 (2019). [DOI] [PubMed] [Google Scholar]
- 16.O’ Mahony SM, Dinan TG & Cryan JF The gut microbiota as a key regulator of visceral pain. Pain 158 (Suppl. 1), S19–S28 (2017). [DOI] [PubMed] [Google Scholar]
- 17.Ding W et al. Gut microbiota influences neuropathic pain through modulating proinflammatory and anti-inflammatory T cells. Anesth. Analg 132, 1146–1155 (2021). [DOI] [PubMed] [Google Scholar]
- 18.Esquerre N et al. Colitis-induced microbial perturbation promotes postinflammatory visceral hypersensitivity. Cell Mol. Gastroenterol. Hepatol 10, 225–244 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amaral FA et al. Commensal microbiota is fundamental for the development of inflammatory pain. Proc. Natl Acad. Sci. USA 105, 2193–2197 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.di Biase AR et al. Gut microbiota signatures and clinical manifestations in celiac disease children at onset: a pilot study. J. Gastroenterol. Hepatol 36, 446–454 (2021). [DOI] [PubMed] [Google Scholar]
- 21.van Boeckel TP et al. Global antibiotic consumption 2000 to 2010: an analysis of national pharmaceutical sales data. Lancet Infect. Dis 14, 742–750 (2014). [DOI] [PubMed] [Google Scholar]
- 22. Blaser MJ, Melby MK, Lock M & Nichter M Accounting for variation in and overuse of antibiotics among humans. Bioessays 43, e2000163 (2021). This paper indicates the extensive variation in antibiotic use and the means to rationalize therapeutic approaches.
- 23.Maier L et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature 555, 623–628 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dethlefsen L & Relman DA Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc. Natl Acad. Sci. USA 108, 4554–4561 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Korpela K et al. Intestinal microbiome is related to lifetime antibiotic use in Finnish pre-school children. Nat. Commun 7, 10410 (2016). This study shows long-term effects of early-life antibiotic exposures.
- 26.Abeles SR et al. Microbial diversity in individuals and their household contacts following typical antibiotic courses. Microbiome 4, 39 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ventin-Holmberg R et al. The effect of antibiotics on the infant gut fungal microbiota. J. Fungi 8, 328 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Basmaciyan L, Bon F, Paradis T, Lapaquette P & Dalle F Candida albicans interactions with the host: crossing the intestinal epithelial barrier. Tissue Barriers 7, 1612661 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ost KS et al. Adaptive immunity induces mutualism between commensal eukaryotes. Nature 596, 114–118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dominguez-Bello MG, Godoy-Vitorino F, Knight R & Blaser MJ Role of the microbiome in human development. Gut 68, 1108–1114 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.International Diabetes Federation. IDF Diabetes Atlas 10th edn (IDF, 2021). [Google Scholar]
- 32.Ilonen J, Lempainen J & Veijola R The heterogeneous pathogenesis of type 1 diabetes mellitus. Nat. Rev. Endocrinol 15, 635–650 (2019). [DOI] [PubMed] [Google Scholar]
- 33.Patterson CC et al. Incidence trends for childhood type 1 diabetes in Europe during 1989-2003 and predicted new cases 2005-20: a multicentre prospective registration study. Lancet 373, 2027–2033 (2009). [DOI] [PubMed] [Google Scholar]
- 34.Hussen HI, Persson M & Moradi T The trends and the risk of type 1 diabetes over the past 40 years: an analysis by birth cohorts and by parental migration background in Sweden. BMJ Open 3, e003418 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Akerblom HK, Vaarala O, Hyöty H, Ilonen J & Knip M Environmental factors in the etiology of type 1 diabetes. Am. J. Med. Genet 115, 18–29 (2002). [DOI] [PubMed] [Google Scholar]
- 36.Giongo A et al. Toward defining the autoimmune microbiome for type 1 diabetes. ISME J. 5, 82–91 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Goffau MC et al. Fecal microbiota composition differs between children with β-cell autoimmunity and those without. Diabetes 62, 1238–1244 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Groot PF et al. Distinct fecal and oral microbiota composition in human type 1 diabetes, an observational study. PLoS ONE 12, e0188475 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kostic AD et al. The dynamics of the human infant gut microbiome in development and in progression towards type 1 diabetes. Cell Host Microbe 17, 260–273 (2015). This study links changes in microbiome characteristics with type 1 diabetes risk.
- 40.Rogers MAM, Kim C, Banerjee T & Lee JM Fluctuations in the incidence of type 1 diabetes in the United States from 2001 to 2015: a longitudinal study. BMC Med. 15, 199 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.International Diabetes Federation. IDF Diabetes Atlas 2nd edn (IDF, 2003). [Google Scholar]
- 42.Abela AG & Fava S Why is the incidence of type 1 diabetes increasing? Curr Diabetes Rev. 17, e030521193110 (2021). [DOI] [PubMed] [Google Scholar]
- 43.Gale EAM The rise of childhood type 1 diabetes in the 20th century. Diabetes 51, 3353–3361 (2002). [DOI] [PubMed] [Google Scholar]
- 44.Chapman NM, Coppieters K, von Herrath M & Tracy S The microbiology of human hygiene and its impact on type 1 diabetes. Islets 4, 253–261 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Korpela K et al. Antibiotics in early life associate with specific gut microbiota signatures in a prospective longitudinal infant cohort. Pediatr. Res 88, 438–443 (2020). [DOI] [PubMed] [Google Scholar]
- 46.Dominguez-Bello MG et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl Acad. Sci. USA 107, 11971–11975 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Korpela K et al. Maternal fecal microbiota transplantation in cesarean-born infants rapidly restores normal gut microbial development: a proof-of-concept study. Cell 183, 324–334.e5 (2020). [DOI] [PubMed] [Google Scholar]
- 48.Mueller NT et al. Prenatal exposure to antibiotics, cesarean section and risk of childhood obesity. Int. J. Obes 39, 665–670 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Williams MJ, Ribeiro do Valle CC & Gyte GML Different classes of antibiotics given to women routinely for preventing infection at caesarean section. Cochrane Database Syst. Rev 3, CD008726 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wernroth M-L et al. Early childhood antibiotic treatment for otitis media and other respiratory tract infections is associated with risk of type 1 diabetes: a nationwide register-based study with sibling analysis. Diabetes Care 43, 991–999 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Clausen TD et al. Broad-spectrum antibiotic treatment and subsequent childhood type 1 diabetes: a Nationwide Danish Cohort Study. PLoS ONE 11, e0161654 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hicks LA, Taylor TH Jr & Hunkler RJ U.S. outpatient antibiotic prescribing, 2010. N. Engl. J. Med 368, 1461–1462 (2013). [DOI] [PubMed] [Google Scholar]
- 53.Cars O, Mölstad S & Melander A Variation in antibiotic use in the European Union. Lancet 357, 1851–1853 (2001). [DOI] [PubMed] [Google Scholar]
- 54.Hviid A & Svanström H Antibiotic use and type 1 diabetes in childhood. Am. J. Epidemiol 169, 1079–1084 (2009). [DOI] [PubMed] [Google Scholar]
- 55.Tapia G et al. Antibiotics, acetaminophen and infections during prenatal and early life in relation to type 1 diabetes. Int. J. Epidemiol 47, 1538–1548 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Suzuki K et al. Aberrant expansion of segmented filamentous bacteria in IgA-deficient gut. Proc. Natl Acad. Sci. USA 101, 1981–1986 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hooper LV, Littman DR & Macpherson AJ Interactions between the microbiota and the immune system. Science 336, 1268–1273 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Geuking MB et al. Intestinal bacterial colonization induces mutualistic regulatory T cell responses. Immunity 34, 794–806 (2011). [DOI] [PubMed] [Google Scholar]
- 59.El-Aidy S, Hooiveld G, Tremaroli V, Bäckhed F & Kleerebezem M The gut microbiota and mucosal homeostasis: colonized at birth or at adulthood, does it matter? Gut Microbes 4, 118–124 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Makino S et al. Breeding of a non-obese, diabetic strain of mice. Jikken Dobutsu 29, 1–13 (1980). [DOI] [PubMed] [Google Scholar]
- 61.Alam C et al. Effects of a germ-free environment on gut immune regulation and diabetes progression in non-obese diabetic (NOD) mice. Diabetologia 54, 1398–1406 (2011). [DOI] [PubMed] [Google Scholar]
- 62.King C & Sarvetnick N The incidence of type-1 diabetes in NOD mice is modulated by restricted flora not germ-free conditions. PLoS ONE 6, 6–8 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kriegel MA et al. Naturally transmitted segmented filamentous bacteria segregate with diabetes protection in nonobese diabetic mice. Proc. Natl Acad. Sci. USA 108, 11548–11553 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pozzilli P, Signore A, Williams AJ & Beales PE NOD mouse colonies around the world–recent facts and figures. Immunol. Today 14, 193–196 (1993). [DOI] [PubMed] [Google Scholar]
- 65.Wen L et al. Innate immunity and intestinal microbiota in the development of type 1 diabetes. Nature 455, 1109–1113 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hu Y et al. Different immunological responses to early-life antibiotic exposure affecting autoimmune diabetes development in NOD mice. J. Autoimmun 72, 47–56 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brugman S et al. Antibiotic treatment partially protects against type 1 diabetes in the Bio-Breeding diabetes-prone rat. Is the gut flora involved in the development of type 1 diabetes? Diabetologia 49, 2105–2108 (2006). [DOI] [PubMed] [Google Scholar]
- 68.Candon S et al. Antibiotics in early life alter the gut microbiome and increase disease incidence in a spontaneous mouse model of autoimmune insulin-dependent diabetes. PLoS ONE 10, e0125448 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hansen CHF et al. Early life treatment with vancomycin propagates Akkermansia muciniphila and reduces diabetes incidence in the NOD mouse. Diabetologia 55, 2285–2294 (2012). [DOI] [PubMed] [Google Scholar]
- 70.Brown K et al. Prolonged antibiotic treatment induces a diabetogenic intestinal microbiome that accelerates diabetes in NOD mice. ISME J. 10, 321–332 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Livanos AE et al. Antibiotic-mediated gut microbiome perturbation accelerates development of type 1 diabetes in mice. Nat. Microbiol 1, 16140 (2016). This paper discusses the use of an experimental model showing a causal role for an antibiotic-perturbed microbiota in type 1 diabetes.
- 72.Zhang X-S et al. Antibiotic-induced acceleration of type 1 diabetes alters maturation of innate intestinal immunity. eLife 7, e37816 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Zhang X et al. Maternal cecal microbiota transfer rescues early-life antibiotic-induced enhancement of type 1 diabetes in mice. Cell Host Microbe 29, 1249–1265.e9 (2021). This study demonstrated that after antibiotic-induced increases in experimental type 1 diabetes, microbiome transplant can return phenotype to the baseline.
- 74.Conget I et al. Lack of effect of intermittently administered sodium fusidate in patients with newly diagnosed type 1 diabetes mellitus: the FUSIDM trial. Diabetologia 48, 1464–1468 (2005). [DOI] [PubMed] [Google Scholar]
- 75.de Groot PF et al. Oral butyrate does not affect innate immunity and islet autoimmunity in individuals with longstanding type 1 diabetes: a randomised controlled trial. Diabetologia 63, 597–610 (2020). [DOI] [PubMed] [Google Scholar]
- 76.de Groot P et al. Faecal microbiota transplantation halts progression of human new-onset type 1 diabetes in a randomised controlled trial. Gut 70, 92–105 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.National Center for Chronic Disease Prevention and Health Promotion (U.S.). Division of Diabetes Translation. Long-term trends in diabetes. April 2017. CDC https://stacks.cdc.gov/view/cdc/46096 (2017). [Google Scholar]
- 78.Centers for Disease Control and Prevention. Diabetes Report Card 2019 (CDC, 2020). [Google Scholar]
- 79.Wang J et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 490, 55–60 (2012). [DOI] [PubMed] [Google Scholar]
- 80.Zhao L et al. Comprehensive relationships between gut microbiome and faecal metabolome in individuals with type 2 diabetes and its complications. Endocrine 66, 526–537 (2019). [DOI] [PubMed] [Google Scholar]
- 81.Ahmad A et al. Analysis of gut microbiota of obese individuals with type 2 diabetes and healthy individuals. PLoS ONE 14, e0226372 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Magne F et al. The firmicutes/bacteroidetes ratio: a relevant marker of gut dysbiosis in obese patients? Nutrients 12, 1474 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zmora N, Suez J & Elinav E You are what you eat: diet, health and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol 16, 35–56 (2019). [DOI] [PubMed] [Google Scholar]
- 84.Deschasaux M et al. Depicting the composition of gut microbiota in a population with varied ethnic origins but shared geography. Nat. Med 24, 1526–1531 (2018). [DOI] [PubMed] [Google Scholar]
- 85.Muilwijk M et al. The high risk for type 2 diabetes among ethnic minority populations is not explained by low-grade inflammation. Sci. Rep 9, 19871 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tillin T et al. Insulin resistance and truncal obesity as important determinants of the greater incidence of diabetes in Indian Asians and African Caribbeans compared with Europeans: the Southall and Brent Revisited (SABRE) cohort. Diabetes Care 36, 383–393 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Mikkelsen KH, Knop FK, Frost M, Hallas J & Pottegard A Use of antibiotics and risk of type 2 diabetes: a population-based case-control study. J. Clin. Endocrinol. Metab 100, 3633–3640 (2015). This was a large epidemiological study in Denmark linking prior antibiotic exposures, even years earlier, with the risk of developing T2DM.
- 88.Davis PJ et al. Prior antibiotic exposure and risk of type 2 diabetes among veterans. Prim. Care Diabetes 13, 49–56 (2019). [DOI] [PubMed] [Google Scholar]
- 89. Boursi B, Mamtani R, Haynes K & Yang Y-X The effect of past antibiotic exposure on diabetes risk. Eur. J. Endocrinol 172, 639–648 (2015). This was a large epidemiological study in the UK linking prior antibiotic exposures to risk of type 2 diabetes in adults.
- 90.Trasande L et al. Infant antibiotic exposures and early-life body mass. Int. J. Obes 37, 16–23 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bailey LC et al. Association of antibiotics in infancy with early childhood obesity. JAMA Pediatr. 168, 1063–1069 (2014). [DOI] [PubMed] [Google Scholar]
- 92.Azad MB, Bridgman SL, Becker AB & Kozyrskyj AL Infant antibiotic exposure and the development of childhood overweight and central adiposity. Int. J. Obes 38, 1290–1298 (2014). [DOI] [PubMed] [Google Scholar]
- 93.Murphy R et al. Antibiotic treatment during infancy and increased body mass index in boys: an international cross-sectional study. Int. J. Obes 38, 1115–1119 (2014). [DOI] [PubMed] [Google Scholar]
- 94. Aversa Z et al. Association of infant antibiotic exposure with childhood health outcomes. Mayo Clin. Proc 96, 66–77 (2021). This was an epidemiological study linking early-life antibiotic exposure to ten common childhood disorders.
- 95.Mbakwa CA et al. Early life antibiotic exposure and weight development in children. J. Pediatrics 176, 105–113.e2 (2016). [DOI] [PubMed] [Google Scholar]
- 96. Rogawski ET et al. Use of antibiotics in children younger than two years in eight countries: a prospective cohort study. Bull. World Health Organ 95, 49–61 (2017). This study indicates the extremely high use of antibiotics in young children in many developing countries/regions.
- 97.Klein EY et al. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc. Natl Acad. Sci. USA 115, E3463–E3470 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dierikx TH et al. The influence of prenatal and intrapartum antibiotics on intestinal microbiota colonisation in infants: a systematic review. J. Infect 81, 190–204 (2020). [DOI] [PubMed] [Google Scholar]
- 99.Ley RE et al. Obesity alters gut microbial ecology. Proc. Natl Acad. Sci. USA 102, 11070–11075 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Verhaar BJH et al. Associations between gut microbiota, faecal short-chain fatty acids, and blood pressure across ethnic groups: the HELIUS study. Eur. Heart J 41, 4259–4267 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rothschild D et al. Environment dominates over host genetics in shaping human gut microbiota. Nature 555, 210–215 (2018). [DOI] [PubMed] [Google Scholar]
- 102.Schulfer AF et al. The impact of early-life sub-therapeutic antibiotic treatment (STAT) on excessive weight is robust despite transfer of intestinal microbes. ISME J. 13, 1280–1292 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mahana D et al. Antibiotic perturbation of the murine gut microbiome enhances the adiposity, insulin resistance, and liver disease associated with high-fat diet. Genome Med. 8, 48 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Turnbaugh PJ, Bäckhed F, Fulton L & Gordon JI Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe 3, 213–223 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cherbut C et al. Short-chain fatty acids modify colonic motility through nerves and polypeptide YY release in the rat. Am. J. Physiol 275, G1415–G1422 (1998). [DOI] [PubMed] [Google Scholar]
- 106.Samuel BS et al. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc. Natl Acad. Sci. USA 105, 16767–16772 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kimura I et al. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat. Commun 4, 1829 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ang Z & Ding JL GPR41 and GPR43 in obesity and inflammation–protective or causative? Front. Immunol 7, 1–5 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cani PD et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56, 1761–1772 (2007). [DOI] [PubMed] [Google Scholar]
- 110.Cani PD, Bibiloni R, Knauf C, Neyrinck AM & Delzenne NM Changes in gut microbiota control metabolic diet-induced obesity and diabetes in mice. Diabetes 57, 1470–1481 (2008). [DOI] [PubMed] [Google Scholar]
- 111.Moreno-Navarrete JM et al. Circulating lipopolysaccharide-binding protein (LBP) as a marker of obesity-related insulin resistance. Int. J. Obes 36, 1442–1449 (2012). [DOI] [PubMed] [Google Scholar]
- 112.Gonzalez-Quintela A et al. Determinants of serum concentrations of lipopolysaccharide-binding protein (LBP) in the adult population: the role of obesity. PLoS ONE 8, e54600 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ortiz S et al. Bacterial DNA translocation holds increased insulin resistance and systemic inflammatory levels in morbid obese patients. J. Clin. Endocrinol. Metab 99, 2575–2583 (2014). [DOI] [PubMed] [Google Scholar]
- 114.Lassenius MI et al. Bacterial endotoxin activity in human serum is associated with dyslipidemia, insulin resistance, obesity, and chronic inflammation. Diabetes Care 34, 1809–1815 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rodrigues RR et al. Antibiotic-induced alterations in gut microbiota are associated with changes in glucose metabolism in healthy mice. Front. Microbiol 8, 2306 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Miao ZH et al. Dysbiosis of intestinal microbiota in early life aggravates high-fat diet induced dysmetabolism in adult mice. BMC Microbiol. 21, 209 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Liu Y et al. Gut microbiome alterations in high-fat-diet-fed mice are associated with antibiotic tolerance. Nat. Microbiol 6, 874–884 (2021). [DOI] [PubMed] [Google Scholar]
- 118.Gough EK et al. The impact of antibiotics on growth in children in low and middle income countries: systematic review and meta-analysis of randomised controlled trials. BMJ 348, g2267 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Edmonson MB & Eickhoff JC Weight gain and obesity in infants and young children exposed to prolonged antibiotic prophylaxis. JAMA Pediatr. 171, 150–156 (2017). [DOI] [PubMed] [Google Scholar]
- 120.Mikkelsen KH et al. Effect of antibiotics on gut microbiota, gut hormones and glucose metabolism. PLoS ONE 10, e0142352 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Vrieze A et al. Impact of oral vancomycin on gut microbiota, bile acid metabolism, and insulin sensitivity. J. Hepatol 60, 824–831 (2014). [DOI] [PubMed] [Google Scholar]
- 122.Bakker GJ et al. Oral vancomycin treatment does not alter markers of postprandial inflammation in lean and obese subjects. Physiol. Rep 7, e14199 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Thuny F et al. Vancomycin treatment of infective endocarditis is linked with recently acquired obesity. PLoS ONE 5, e9074 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Colantonio AG, Werner SL & Brown M The effects of prebiotics and substances with prebiotic properties on metabolic and inflammatory biomarkers in individuals with type 2 diabetes mellitus: a systematic review. J. Acad. Nutr. Diet 120, 587–607.e2 (2020). [DOI] [PubMed] [Google Scholar]
- 125.Hendijani F & Akbari V Probiotic supplementation for management of cardiovascular risk factors in adults with type II diabetes: a systematic review and meta-analysis. Clin. Nutr 37, 532–541 (2018). [DOI] [PubMed] [Google Scholar]
- 126.Tabrizi R et al. The effects of synbiotic supplementation on glucose metabolism and lipid profiles in patients with diabetes: a systematic review and meta-analysis of randomized controlled trials. Probiotics Antimicrob. Proteins 10, 329–342 (2018). [DOI] [PubMed] [Google Scholar]
- 127.Vrieze A et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 143, 913–916.e7 (2012). [DOI] [PubMed] [Google Scholar]
- 128.Kootte RS et al. Improvement of insulin sensitivity after lean donor feces in metabolic syndrome is driven by baseline intestinal microbiota composition. Cell Metab. 26, 611–619.e6 (2017). [DOI] [PubMed] [Google Scholar]
- 129.Hanssen NMJ, de Vos WM & Nieuwdorp M Fecal microbiota transplantation in human metabolic diseases: from a murky past to a bright future? Cell Metab. 33, 1098–1110 (2021). [DOI] [PubMed] [Google Scholar]
- 130.Ng SC et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 390, 2769–2778 (2017). [DOI] [PubMed] [Google Scholar]
- 131.Alatab S et al. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol 5, 17–30 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Molodecky NA et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 142, 46–54.e42; quiz e30 (2012). [DOI] [PubMed] [Google Scholar]
- 133.King JA et al. Trends in hospitalisation rates for inflammatory bowel disease in western versus newly industrialised countries: a population-based study of countries in the Organisation for Economic Co-operation and Development. Lancet Gastroenterol. Hepatol 4, 287–295 (2019). [DOI] [PubMed] [Google Scholar]
- 134.Ng SC et al. Early course of inflammatory bowel disease in a population-based inception cohort study from 8 countries in Asia and Australia. Gastroenterology 150, 84–86 (2016). [DOI] [PubMed] [Google Scholar]
- 135.Thia KT, Sandborn WJ, Harmsen WS, Zinsmeister AR & Loftus EVJ Risk factors associated with progression to intestinal complications of Crohn’s disease in a population-based cohort. Gastroenterology 139, 1147–1155 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Fumery M et al. Natural history of adult ulcerative colitis in population-based cohorts: a systematic review. Clin. Gastroenterol. Hepatol 16, 343–356.e3 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Benchimol EI et al. Changing age demographics of inflammatory bowel disease in Ontario, Canada: a population-based cohort study of epidemiology trends. Inflamm. Bowel Dis 20, 1761–1769 (2014). [DOI] [PubMed] [Google Scholar]
- 138.Benchimol EI et al. Incidence, outcomes, and health services burden of very early onset inflammatory bowel disease. Gastroenterology 147, 803–805 (2014). [DOI] [PubMed] [Google Scholar]
- 139.Ghione S et al. Dramatic increase in incidence of ulcerative colitis and Crohn’s disease (1988–2011): a population-based study of French adolescents. Am. J. Gastroenterol 113, 265–272 (2018). [DOI] [PubMed] [Google Scholar]
- 140.Benchimol EI et al. Inflammatory bowel disease in immigrants to Canada and their children: a population-based cohort study. Am. J. Gastroenterol 110, 553–563 (2015). [DOI] [PubMed] [Google Scholar]
- 141.Benchimol EI et al. Asthma, type 1 and type 2 diabetes mellitus, and inflammatory bowel disease amongst South Asian immigrants to Canada and their children: a population-based cohort study. PLoS ONE 10, e0123599 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Li X, Sundquist J, Hemminki K & Sundquist K Risk of inflammatory bowel disease in first- and second-generation immigrants in Sweden: a nationwide follow-up study. Inflamm. Bowel Dis 17, 1784–1791 (2011). [DOI] [PubMed] [Google Scholar]
- 143.Jostins L et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 491, 119–124 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Liu JZ et al. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat. Genet 47, 979–986 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ananthakrishnan AN Epidemiology and risk factors for IBD. Nat. Rev. Gastroenterol. Hepatol 12, 205–217 (2015). [DOI] [PubMed] [Google Scholar]
- 146.Xu L et al. Systematic review with meta-analysis: breastfeeding and the risk of Crohn’s disease and ulcerative colitis. Aliment. Pharmacol. Ther 46, 780–789 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Vangay P et al. US immigration westernizes the human gut microbiome. Cell 175, 962–972.e10 (2018). This study demonstrates the influence of the environment on microbiome characteristics, with the microbiome of immigrants becoming progressively more westernized.
- 148.Lewis JD & Abreu MT Diet as a trigger or therapy for inflammatory bowel diseases. Gastroenterology 152, 398–414.e6 (2017). [DOI] [PubMed] [Google Scholar]
- 149.Pannaraj PS et al. Association between breast milk bacterial communities and establishment and development of the infant gut microbiome. JAMA Pediatr. 171, 647–654 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kronman MP, Zaoutis TE, Haynes K, Feng R & Coffin SE Antibiotic exposure and IBD development among children: a population-based cohort study. Pediatrics 130, e794–e803 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Hviid A, Svanström H & Frisch M Antibiotic use and inflammatory bowel diseases in childhood. Gut 60, 49–54 (2011). This study shows a dose–response relationship between antibiotic exposure in the first year of life and subsequent IBD development.
- 152.Nguyen LH et al. Antibiotic use and the development of inflammatory bowel disease: a national case-control study in Sweden. Lancet Gastroenterol. Hepatol 5, 986–995 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Shaw SY, Blanchard JF & Bernstein CN Association between the use of antibiotics in the first year of life and pediatric inflammatory bowel disease. Am. J. Gastroenterol 105, 2687–2692 (2010). [DOI] [PubMed] [Google Scholar]
- 154.Shaw SY, Blanchard JF & Bernstein CN Association between the use of antibiotics and new diagnoses of Crohn’s disease and ulcerative colitis. Am. J. Gastroenterol 106, 2133–2142 (2011). [DOI] [PubMed] [Google Scholar]
- 155.Ungaro R et al. Antibiotics associated with increased risk of new-onset Crohn’s disease but not ulcerative colitis: a meta-analysis. Am. J. Gastroenterol 109, 1728–1738 (2014). [DOI] [PubMed] [Google Scholar]
- 156.Zou Y et al. Correlation between antibiotic use in childhood and subsequent inflammatory bowel disease: a systematic review and meta-analysis. Scand. J. Gastroenterol 55, 301–311 (2020). [DOI] [PubMed] [Google Scholar]
- 157.Card T, Logan RFA, Rodrigues LC & Wheeler JG Antibiotic use and the development of Crohn’s disease. Gut 53, 246–250 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Virta L, Auvinen A, Helenius H, Huovinen P & Kolho K-L Association of repeated exposure to antibiotics with the development of pediatric Crohn’s disease–a nationwide, register-based Finnish case-control study. Am. J. Epidemiol 175, 775–784 (2012). [DOI] [PubMed] [Google Scholar]
- 159.Benchimol EI et al. Trends in epidemiology of pediatric inflammatory bowel disease in Canada: distributed network analysis of multiple population-based provincial health administrative databases. Am. J. Gastroenterol 112, 1120–1134 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Uhlig HH et al. The diagnostic approach to monogenic very early onset inflammatory bowel disease. Gastroenterology 147, 990–1007.e3 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hildebrand H, Malmborg P, Askling J, Ekbom A & Montgomery SM Early-life exposures associated with antibiotic use and risk of subsequent Crohn’s disease. Scand. J. Gastroenterol 43, 961–966 (2008). [DOI] [PubMed] [Google Scholar]
- 162.Sellon RK et al. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect. Immun 66, 5224–5231 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Pizarro TT et al. SAMP1/YitFc mouse strain: a spontaneous model of Crohn’s disease-like ileitis. Inflamm. Bowel Dis 17, 2566–2584 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Oka A et al. Human-derived Clostridium VE202 strains reduce enterobacteriaceae and fusobacteria and reverse experimental colitis induced by human gut microbiota [abstract P074]. Inflamm. Bowel Dis 26, S36–S37 (2020). [Google Scholar]
- 165.Bamias G et al. Down-regulation of intestinal lymphocyte activation and Th1 cytokine production by antibiotic therapy in a murine model of Crohn’s disease. J. Immunol 169, 5308–5314 (2002). [DOI] [PubMed] [Google Scholar]
- 166.Hoentjen F et al. Antibiotics with a selective aerobic or anaerobic spectrum have different therapeutic activities in various regions of the colon in interleukin 10 gene deficient mice. Gut 52, 1721–1727 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Schulfer AF et al. Intergenerational transfer of antibiotic-perturbed microbiota enhances colitis in susceptible mice. Nat. Microbiol 3, 234–242 (2018). This study provides experimental evidence that an antibiotic-perturbed microbiota is sufficient to drive colitis in genetically susceptible individuals.
- 168. Miyoshi J et al. Peripartum antibiotics promote gut dysbiosis, loss of immune tolerance, and inflammatory bowel disease in genetically prone offspring. Cell Rep. 20, 491–504 (2017). This study complements the study of Schulfer et al. (2018) in showing the effects of an altered microbiota on IBD development.
- 169.Blaser MJ & Falkow S What are the consequences of the disappearing human microbiota? Nat. Rev. Microbiol 7, 887–894 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Torres J et al. Infants born to mothers with IBD present with altered gut microbiome that transfers abnormalities of the adaptive immune system to germ-free mice. Gut 69, 42–51 (2020). [DOI] [PubMed] [Google Scholar]
- 171.Okayasu I et al. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology 98, 694–702 (1990). [DOI] [PubMed] [Google Scholar]
- 172.Chassaing B, Aitken JD, Malleshappa M & Vijay-Kumar M Dextran sulfate sodium (DSS)-induced colitis in mice. Curr. Protoc. Immunol 104, 15.25.1–15.25.14 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ozkul C et al. A single early-in-life antibiotic course increases susceptibility to DSS-induced colitis. Genome Med. 12, 65 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Roubaud-Baudron C et al. Long-term effects of early-life antibiotic exposure on resistance to subsequent bacterial infection. mBio 10, e02820–19 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Bermejo F et al. Efficacy of different therapeutic options for spontaneous abdominal abscesses in Crohn’s disease: are antibiotics enough? Inflamm. Bowel Dis 18, 1509–1514 (2012). [DOI] [PubMed] [Google Scholar]
- 176.Nitzan O, Elias M, Peretz A & Saliba W Role of antibiotics for treatment of inflammatory bowel disease. World J. Gastroenterol 22, 1078–1087 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Panes J & Rimola J Perianal fistulizing Crohn’s disease: pathogenesis, diagnosis and therapy. Nat. Rev. Gastroenterol. Hepatol 14, 652–664 (2017). [DOI] [PubMed] [Google Scholar]
- 178.Akiyama S, Rai V & Rubin DT Pouchitis in inflammatory bowel disease: a review of diagnosis, prognosis, and treatment. Intest. Res 19, 1–11 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Khan KJ et al. Antibiotic therapy in inflammatory bowel disease: a systematic review and meta-analysis. Am. J. Gastroenterol 106, 661–673 (2011). [DOI] [PubMed] [Google Scholar]
- 180.Townsend CM et al. Antibiotics for induction and maintenance of remission in Crohn’s disease. Cochrane Database Syst. Rev 2, CD012730 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Halpin SJ & Ford AC Prevalence of symptoms meeting criteria for irritable bowel syndrome in inflammatory bowel disease: systematic review and meta-analysis. Am. J. Gastroenterol 107, 1474–1482 (2012). [DOI] [PubMed] [Google Scholar]
- 182.Pimentel M, Park S, Mirocha J, Kane SV & Kong Y The effect of a nonabsorbed oral antibiotic (rifaximin) on the symptoms of the irritable bowel syndrome: a randomized trial. Ann. Intern. Med 145, 557–563 (2006). [DOI] [PubMed] [Google Scholar]
- 183.Rutgeerts P et al. Controlled trial of metronidazole treatment for prevention of Crohn’s recurrence after ileal resection. Gastroenterology 108, 1617–1621 (1995). [DOI] [PubMed] [Google Scholar]
- 184.Rutgeerts P et al. Ornidazole for prophylaxis of postoperative Crohn’s disease recurrence: a randomized, double-blind, placebo-controlled trial. Gastroenterology 128, 856–861 (2005). [DOI] [PubMed] [Google Scholar]
- 185.Spanogiannopoulos P, Bess EN, Carmody RN & Turnbaugh PJ The microbial pharmacists within us: a metagenomic view of xenobiotic metabolism. Nat. Rev. Microbiol 14, 273–287 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Crouwel F, Buiter HJC & de Boer NK Gut microbiota-driven drug metabolism in inflammatory bowel disease. J. Crohns Colitis 15, 307–315 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Rafii F, Franklin W & Cerniglia CE Azoreductase activity of anaerobic bacteria isolated from human intestinal microflora. Appl. Env. Microbiol 56, 2146–2151 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Yadav V, Gaisford S, Merchant HA & Basit AW Colonic bacterial metabolism of corticosteroids. Int. J. Pharm 457, 268–274 (2013). [DOI] [PubMed] [Google Scholar]
- 189.Valerino DM, Johns DG, Zaharko DS & Oliverio VT Studies of the metabolism of methotrexate by intestinal flora. I. Identification and study of biological properties of the metabolite 4-amino-4-deoxy-N10-methylpteroic acid. Biochem. Pharmacol 21, 821–831 (1972). [DOI] [PubMed] [Google Scholar]
- 190.Oancea I et al. Colonic microbiota can promote rapid local improvement of murine colitis by thioguanine independently of T lymphocytes and host metabolism. Gut 66, 59–69 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Moayyedi P et al. Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial. Gastroenterology 149, 102–109.e6 (2015). [DOI] [PubMed] [Google Scholar]
- 192.Rossen NG et al. Findings from a randomized controlled trial of fecal transplantation for patients with ulcerative colitis. Gastroenterology 149, 110–118.e4 (2015). [DOI] [PubMed] [Google Scholar]
- 193.Paramsothy S et al. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: a randomised placebo-controlled trial. Lancet 389, 1218–1228 (2017). [DOI] [PubMed] [Google Scholar]
- 194.Costelloe C, Metcalfe C, Lovering A, Mant D & Hay AD Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ 340, c2096 (2010). [DOI] [PubMed] [Google Scholar]
- 195.Crothers JW et al. Daily, oral FMT for long-term maintenance therapy in ulcerative colitis: results of a single-center, prospective, randomized pilot study. BMC Gastroenterol. 21, 281 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Fehily SR, Basnayake C, Wright EK & Kamm MA Fecal microbiota transplantation therapy in Crohn’s disease: systematic review. J. Gastroenterol. Hepatol 36, 2672–2686 (2021). [DOI] [PubMed] [Google Scholar]
- 197.Sokol H et al. Fecal microbiota transplantation to maintain remission in Crohn’s disease: a pilot randomized controlled study. Microbiome 8, 12 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Yang Z et al. Fecal microbiota transplant via endoscopic delivering through small intestine and colon: no difference for Crohn’s disease. Dig. Dis. Sci 65, 150–157 (2020). [DOI] [PubMed] [Google Scholar]
- 199.Mustalahti K et al. The prevalence of celiac disease in Europe: results of a centralized, international mass screening project. Ann. Med 42, 587–595 (2010). [DOI] [PubMed] [Google Scholar]
- 200.Rubio-Tapia A, Ludvigsson JF, Brantner TL, Murray JA & Everhart JE The prevalence of celiac disease in the United States. Am. J. Gastroenterol 107, 1538–1544 quiz 1537–1545 (2012). [DOI] [PubMed] [Google Scholar]
- 201.Ludvigsson JF, Montgomery SM, Ekbom A, Brandt L & Granath F Small-intestinal histopathology and mortality risk in celiac disease. JAMA 302, 1171–1178 (2009). [DOI] [PubMed] [Google Scholar]
- 202.Rubio-Tapia A et al. Increased prevalence and mortality in undiagnosed celiac disease. Gastroenterology 137, 88–93 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.King JA et al. Incidence of celiac disease is increasing over time: a systematic review and meta-analysis. Am. J. Gastroenterol 115, 507–525 (2020). [DOI] [PubMed] [Google Scholar]
- 204.Catassi C et al. Natural history of celiac disease autoimmunity in a USA cohort followed since 1974. Ann. Med 42, 530–538 (2010). [DOI] [PubMed] [Google Scholar]
- 205.Sollid LM et al. Evidence for a primary association of celiac disease to a particular HLA-DQ alpha/beta heterodimer. J. Exp. Med 169, 345–350 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Collado MC, Calabuig M & Sanz Y Differences between the fecal microbiota of coeliac infants and healthy controls. Curr. Issues Intest. Microbiol 8, 9–14 (2007). [PubMed] [Google Scholar]
- 207.Collado MC, Donat E, Ribes-Koninckx C, Calabuig M & Sanz Y Specific duodenal and faecal bacterial groups associated with paediatric coeliac disease. J. Clin. Pathol 62, 264–269 (2009). [DOI] [PubMed] [Google Scholar]
- 208.Schippa S et al. A distinctive ‘microbial signature’ in celiac pediatric patients. BMC Microbiol. 10, 175 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Nadal I, Donant E, Ribes-Koninckx C, Calabuig M & Sanz Y Imbalance in the composition of the duodenal microbiota of children with coeliac disease. J. Med. Microbiol 56, 1669–1674 (2007). [DOI] [PubMed] [Google Scholar]
- 210.de Palma G et al. Intestinal dysbiosis and reduced immunoglobulin-coated bacteria associated with coeliac disease in children. BMC Microbiol. 10, 63 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.di Cagno R et al. Duodenal and faecal microbiota of celiac children: molecular, phenotype and metabolome characterization. BMC Microbiol. 11, 219 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Galipeau HJ et al. Intestinal microbiota modulates gluten-induced immunopathology in humanized mice. Am. J. Pathol 185, 2969–2982 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Mårild K, Stephansson O, Montgomery S, Murray JA & Ludvigsson JF Pregnancy outcome and risk of celiac disease in offspring: a nationwide case-control study. Gastroenterology 142, 39–45.e3 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Lebwohl B, Spechler SJ, Wang TC, Green PHR & Ludvigsson JF Use of proton pump inhibitors and subsequent risk of celiac disease. Dig. Liver Dis 46, 36–40 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Caminero A & Verdu EF Celiac disease: should we care about microbes? Am. J. Physiol. Gastrointest. Liver Physiol 317, G161–G170 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Caminero A et al. Duodenal bacteria from patients with celiac disease and healthy subjects distinctly affect gluten breakdown and immunogenicity. Gastroenterology 151, 670–683 (2016). [DOI] [PubMed] [Google Scholar]
- 217.Petersen J et al. T cell receptor cross-reactivity between gliadin and bacterial peptides in celiac disease. Nat. Struct. Mol. Biol 27, 49–61 (2020). [DOI] [PubMed] [Google Scholar]
- 218.Jiang H-Y, Zhang X, Zhou Y-Y, Jiang C-M & Shi Y-D Infection, antibiotic exposure, and risk of celiac disease: a systematic review and meta-analysis. J. Gastroenterol. Hepatol 35, 557–566 (2020). [DOI] [PubMed] [Google Scholar]
- 219.Kamphorst K et al. Early life antibiotics and childhood gastrointestinal disorders: a systematic review. BMJ Paediatr. Open 5, e001028 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Kołodziej M et al. Association between early life (prenatal and postnatal) antibiotic administration and coeliac disease: a systematic review. Arch. Dis. Child 104, 1083–1089 (2019). [DOI] [PubMed] [Google Scholar]
- 221.Canova C et al. Association of maternal education, early infections, and antibiotic use with celiac disease: a population-based birth cohort study in northeastern Italy. Am. J. Epidemiol 180, 76–85 (2014). [DOI] [PubMed] [Google Scholar]
- 222.Dydensborg Sander S et al. Association between antibiotics in the first year of life and celiac disease. Gastroenterology 156, 2217–2229 (2019). [DOI] [PubMed] [Google Scholar]
- 223.Bittker SS & Bell KR Potential risk factors for celiac disease in childhood: a case-control epidemiological survey. Clin. Exp. Gastroenterol 12, 303–319 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Mårild K et al. Antibiotic exposure and the development of coeliac disease: a nationwide case-control study. BMC Gastroenterol. 13, 109 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Mårild K, Ludvigsson J, Sanz Y & Ludvigsson JF Antibiotic exposure in pregnancy and risk of coeliac disease in offspring: a cohort study. BMC Gastroenterol. 14, 75 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Mårild K, Kahrs CR, Tapia G, Stene LC & Størdal K Maternal infections, antibiotics, and paracetamol in pregnancy and offspring celiac disease: a cohort study. J. Pediatr. Gastroenterol. Nutr 64, 730–736 (2017). [DOI] [PubMed] [Google Scholar]
- 227.Simre K et al. Exploring the risk factors for differences in the cumulative incidence of coeliac disease in two neighboring countries: the prospective DIABIMMUNE study. Dig. Liver Dis 48, 1296–1301 (2016). [DOI] [PubMed] [Google Scholar]
- 228.Kemppainen KM et al. Association between early-life antibiotic use and the risk of islet or celiac disease autoimmunity. JAMA Pediatr. 171, 1217–1225 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Martínez-Ojinaga E et al. Influence of HLA on clinical and analytical features of pediatric celiac disease. BMC Gastroenterol. 19, 91 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Abadie V et al. IL-15, gluten and HLA-DQ8 drive tissue destruction in coeliac disease. Nature 578, 600–604 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Hill AB The environment and disease: association or causation? Proc. R. Soc. Med 58, 295–300 (1965). This study is a classic: it established nine criteria to examine causal relationships.
- 232.Myléus A et al. Early infections are associated with increased risk for celiac disease: an incident case-referent study. BMC Pediatr. 12, 194 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Witmer CP, Susi A, Min SB & Nylund CM Early infant risk factors for pediatric eosinophilic esophagitis. J. Pediatr. Gastroenterol. Nutr 67, 610–615 (2018). [DOI] [PubMed] [Google Scholar]
- 234.Jensen ET, Kappelman MD, Kim HP, Ringel-Kulka T & Dellon ES Early life exposures as risk factors for pediatric eosinophilic esophagitis. J. Pediatr. Gastroenterol. Nutr 57, 67–71 (2013). [DOI] [PubMed] [Google Scholar]
- 235.Radano MC et al. Cesarean section and antibiotic use found to be associated with eosinophilic esophagitis. J. Allergy Clin. Immunol. Pract 2, 475–477.e1 (2014). [DOI] [PubMed] [Google Scholar]
- 236.Jensen ET, Kuhl JT, Martin LJ, Rothenberg ME & Dellon ES Prenatal, intrapartum, and postnatal factors are associated with pediatric eosinophilic esophagitis. J. Allergy Clin. Immunol 141, 214–222 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Dellon ES et al. Early life factors are associated with risk for eosinophilic esophagitis diagnosed in adulthood. Dis. Esophagus 34, doaa074 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Slae M et al. Role of environmental factors in the development of pediatric eosinophilic esophagitis. Dig. Dis. Sci 60, 3364–3372 (2015). [DOI] [PubMed] [Google Scholar]
- 239.Walsh D, McCarthy J, O’Driscoll C & Melgar S Pattern recognition receptors–molecular orchestrators of inflammation in inflammatory bowel disease. Cytokine Growth Factor Rev. 24, 91–104 (2013). [DOI] [PubMed] [Google Scholar]
- 240.Klotz U, Maier K, Fischer C & Heinkel K Therapeutic efficacy of sulfasalazine and its metabolites in patients with ulcerative colitis and Crohn’s disease. N. Engl. J. Med 303, 1499–1502 (1980). [DOI] [PubMed] [Google Scholar]
- 241.Pellock SJ & Redinbo MR Glucuronides in the gut: sugar-driven symbioses between microbe and host. J. Biol. Chem 292, 8569–8576 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Khalili H et al. Hormone therapy increases risk of ulcerative colitis but not Crohn’s disease. Gastroenterology 143, 1199–1206 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Costello SP et al. Effect of fecal microbiota transplantation on 8-week remission in patients with ulcerative colitis: a randomized clinical trial. JAMA 321, 156–164 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Reed CC & Dellon ES Eosinophilic esophagitis. Med. Clin. North. Am 103, 29–42 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Dellon ES et al. Updated international consensus diagnostic criteria for eosinophilic esophagitis: proceedings of the AGREE conference. Gastroenterology 155, 1022–1033.e10 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Blanchard C et al. IL-13 involvement in eosinophilic esophagitis: transcriptome analysis and reversibility with glucocorticoids. J. Allergy Clin. Immunol 120, 1292–1300 (2007). [DOI] [PubMed] [Google Scholar]
- 247.Zuo L et al. IL-13 induces esophageal remodeling and gene expression by an eosinophil-independent, IL-13Rα2-inhibited pathway. J. Immunol 185, 660–669 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Mishra A & Rothenberg ME Intratracheal IL-13 induces eosinophilic esophagitis by an IL-5, eotaxin-1, and STAT6-dependent mechanism. Gastroenterology 125, 1419–1427 (2003). [DOI] [PubMed] [Google Scholar]
- 249.Blanchard C et al. Inhibition of human interleukin-13-induced respiratory and oesophageal inflammation by anti-human-interleukin-13 antibody (CAT-354). Clin. Exp. Allergy 35, 1096–1103 (2005). [DOI] [PubMed] [Google Scholar]
- 250.Dellon ES Epidemiology of eosinophilic esophagitis. Gastroenterol. Clin. North. Am 43, 201–218 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Soon IS, Butzner JD, Kaplan GG & deBruyn JCC Incidence and prevalence of eosinophilic esophagitis in children. J. Pediatr. Gastroenterol. Nutr 57, 72–80 (2013). [DOI] [PubMed] [Google Scholar]
- 252.Dellon ES et al. The increasing incidence and prevalence of eosinophilic oesophagitis outpaces changes in endoscopic and biopsy practice: national population-based estimates from Denmark. Aliment. Pharmacol. Ther 41, 662–670 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Giriens B et al. Escalating incidence of eosinophilic esophagitis in Canton of Vaud, Switzerland, 1993-2013: a population-based study. Allergy 70, 1633–1639 (2015). [DOI] [PubMed] [Google Scholar]
- 254.Homan M, Blagus R, Jeverica AK & Orel R Pediatric eosinophilic esophagitis in Slovenia: data from a retrospective 2005-2012 epidemiological study. J. Pediatr. Gastroenterol. Nutr 61, 313–318 (2015). [DOI] [PubMed] [Google Scholar]
- 255.Alexander ES et al. Twin and family studies reveal strong environmental and weaker genetic cues explaining heritability of eosinophilic esophagitis. J. Allergy Clin. Immunol 134, 1084–1092.e1 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Dellon ES et al. Clinical, endoscopic, and histologic findings distinguish eosinophilic esophagitis from gastroesophageal reflux disease. Clin. Gastroenterol. Hepatol 7, 1305–1313 quiz 1261 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Kummeling I et al. Early life exposure to antibiotics and the subsequent development of eczema, wheeze, and allergic sensitization in the first 2 years of life: the KOALA birth cohort study. Pediatrics 119, e225–e231 (2007). [DOI] [PubMed] [Google Scholar]
- 258.Chen Y & Blaser MJ Helicobacter pylori colonization is inversely associated with childhood asthma. J. Infect. Dis 198, 553–560 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Mou W-L, Feng M-Y & Hu L-H Eradication of Helicobacter pylori infections and GERD: a systematic review and meta-analysis. Turk. J. Gastroenterol 31, 853–859 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Vicari JJ et al. The seroprevalence of cagA-positive Helicobacter pylori strains in the spectrum of gastroesophageal reflux disease. Gastroenterology 115, 50–57 (1998). [DOI] [PubMed] [Google Scholar]
- 261.Loffeld RJ et al. Colonization with cagA-positive Helicobacter pylori strains inversely associated with reflux esophagitis and Barrett’s esophagus. Digestion 62, 95–99 (2000). [DOI] [PubMed] [Google Scholar]
- 262.Chow WH et al. An inverse relation between cagA+ strains of Helicobacter pylori infection and risk of esophageal and gastric cardia adenocarcinoma. Cancer Res. 58, 588–590 (1998). [PubMed] [Google Scholar]
- 263.Ronkainen J et al. Prevalence of oesophageal eosinophils and eosinophilic oesophagitis in adults: the population-based Kalixanda study. Gut 56, 615–620 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Dellon ES et al. Inverse association of esophageal eosinophilia with Helicobacter pylori based on analysis of a US pathology database. Gastroenterology 141, 1586–1592 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Furuta K et al. Case-control study of association of eosinophilic gastrointestinal disorders with Helicobacter pylori infection in Japan. J. Clin. Biochem. Nutr 53, 60–62 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Elitsur Y, Alrazzak BA, Preston D & Demetieva Y Does Helicobacter pylori protect against eosinophilic esophagitis in children? Helicobacter 19, 367–371 (2014). [DOI] [PubMed] [Google Scholar]
- 267.Sonnenberg A, Dellon ES, Turner KO & Genta RM The influence of Helicobacter pylori on the ethnic distribution of esophageal eosinophilia. Helicobacter 22, e12370 (2017). [DOI] [PubMed] [Google Scholar]
- 268.von Arnim U et al. Helicobacter pylori infection is associated with a reduced risk of developing eosinophilic oesophagitis. Aliment. Pharmacol. Ther 43, 825–830 (2016). [DOI] [PubMed] [Google Scholar]
- 269.Atherton JC & Blaser MJ Coadaptation of Helicobacter pylori and humans: ancient history, modern implications. J. Clin. Invest 119, 2475–2487 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Ridolo E, Martignago I, Pellicelli I & Incorvaia C Assessing the risk factors for refractory eosinophilic esophagitis in children and adults. Gastroenterol. Res. Pract 2019, 1654543 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]