Abstract
Cuticular hydrocarbons (CHCs) cover the cuticle of insects and serve as desiccation barrier and as semiochemicals. While the main enzymatic steps of CHC biosynthesis are well understood, few of the underlying genes have been identified. Here we show how exploitation of intrasexual CHC dimorphism in a mason wasp, Odynerus spinipes, in combination with whole-genome sequencing and comparative transcriptomics facilitated identification of such genes. RNAi-mediated knockdown of twelve candidate gene orthologs in the honey bee, Apis mellifera, confirmed nine genes impacting CHC profile composition. Most of them have predicted functions consistent with current knowledge of CHC metabolism. However, we found first-time evidence for a fatty acid amide hydrolase also influencing CHC profile composition. In situ hybridization experiments furthermore suggest trophocytes participating in CHC biosynthesis. Our results set the base for experimental CHC profile manipulation in Hymenoptera and imply that the evolutionary origin of CHC biosynthesis predates the arthropods’ colonization of land.
Subject terms: RNAi, Gene expression, Phylogenetics
Transcriptomic and molecular analyses on Hymenoptera facilitated identification of cuticular hydrocarbon (CHC) pathway genes, whose phylogenetic distribution implies that the origin of CHC biosynthesis predates the arthropods’ colonization of land.
Introduction
Cuticular hydrocarbons (CHCs) are long-chained molecules that cover the cuticle of most, if not all, insects1. They function as important desiccation barrier and are thought to have fostered the transition from marine to terrestrial habitats in the lineage leading to Hexapoda2. CHCs additionally serve as semiochemicals for inter- and intraspecific communication2. In Hymenoptera, they play a particularly important role in establishing eusociality by enabling kin, nest-mate, and caste recognition2. CHCs have also been exploited for chemical deception and chemical mimicry2. Identifying genes involved in CHC biosynthesis of Hymenoptera is of major interest, as it would open the door for CHC profile manipulation (e.g., via CRISPR-Cas9 or RNA interference) in behavioral, ecological, and socio-biological studies on Hymenoptera.
Most insects synthesize CHCs de novo from fatty acid precursors3 in oenocytes4. These secretory cells of ectodermal origin are found in the fat body of all major insect lineages4. The main enzymatic steps of CHC biosynthesis are well understood and involve fatty acid synthases (FAS), fatty acid elongases (ELO), fatty acid desaturases (Desat), fatty acyl-CoA reductases (FAR), and cytochrome p450 decarbonylases (CYP4G subfamily)5–10 (Fig. 1). However, the details of CHC biosynthesis and which genes encode the participating enzymes are not yet fully elucidated, particularly in the species-rich insect order Hymenoptera, in which only a single gene (CYP4G) has so far been shown to be involved in CHC biosynthesis10,11. Furthermore, insights obtained from studying species of one insect lineage (e.g., Diptera, in which three genes coding for fatty acid desaturases have been shown to be involved in alkene synthesis based on research on Drosophila10) are not necessarily representative for species of a distantly-related lineage (e.g., Hymenoptera). Most genes associated with CHC biosynthesis belong to multi-gene families, and some of these exhibit remarkably high turnover rates (e.g., fatty acid desaturases12). Therefore, it is highly desirable to identify CHC biosynthesis-related genes in multiple insect lineages. A promising approach to this end is transcriptomic analysis comparing gene expression between samples of the same species that differ in their CHC composition from each other.
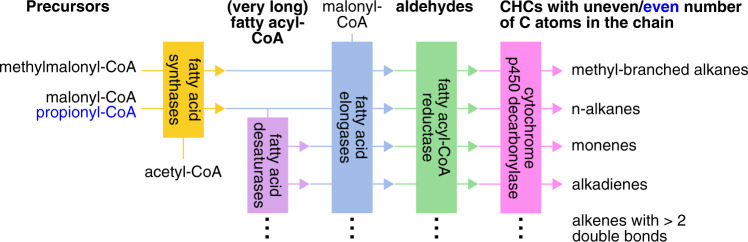
Fig. 1. Schematic overview of the main steps of cuticular hydrocarbon biosynthesis.
The pathway starts with a fatty acid synthase (FAS) that binds acetyl-coenzyme A (CoA) with malonyl-CoA units. The resulting acyl-CoA (with an even number of carbon atoms) is repeatedly elongated by a FAS which binds acyl-CoA with malonyl-CoA. During each elongation step, the chain length of the fatty acyl-CoA increases by two carbon atoms. Fatty acyl-CoA chains with an uneven number of carbon atoms are thought to arise from substituting malonyl-CoA with propionyl-CoA during the elongation step. The consecutive elongation of the fatty acyl-CoA chain by FAS results in long chain fatty acyl-CoAs. Additional elongation of these fatty acyl-CoA chains to very-long fatty acyl-CoA requires the action of fatty acid elongases that add additional malonyl-CoA molecules to the long chain fatty acyl-CoA. These are reduced into aldehydes by fatty acyl-CoA reductases. Before the latter step, double bonds can be inserted in the (very-)long chain fatty acyl-CoAs by fatty acid desaturases, resulting in the biosynthesis of unsaturated hydrocarbons with a different number of double bonds (alkenes, alkadienes). The biosynthesis of methyl-branched alkanes is thought to depend on the action of a specialized fatty acid synthase that incorporates methylmalonyl-CoA instead of malony-CoA into the chain at specific positions during the chain elongation. The last step of CHC biosynthesis is the oxidative decarbonylation of aldehydes leading to hydrocarbons (with the loss of one carbon atom) by a cytochrome p450. Based on2,5–9.
In most species, individuals of the same sex share the same set of CHCs, which is referred to as CHC profile type13. Intriguingly, previous research found that females of the mason wasp Odynerus spinipes are able to express two CHC profile types14. The two chemotypes (c1 and c2) qualitatively differ in 77 CHCs from each other, with most of the differing CHCs being alkenes14. Specifically, chemotype 1 is characterized by alkenes with double bonds at uneven positions, whereas chemotype 2 is characterized by alkenes with double bonds at even positions14,15. Furthermore, wasps expressing chemotype 2 feature a higher relative abundance of alkenes with chain lengths greater than 27 carbon atoms than wasps expressing chemotype 114,15 (Supplementary Fig. 1). The CHC dimorphism of the spiny mason wasps provides an exceptional opportunity to identify genes involved in CHC biosynthesis, as O. spinipes females with different chemotypes do not appear to differ in any other trait from each other; hence, they should systematically differ only in the expression of CHC biosynthesis-related genes from each other14,15. O. spinipes females additionally show age-related quantitative changes in their CHC profile composition, which can also be used to identify candidate genes.
In this study, we report results from exploiting the CHC dimorphism in the spiny mason wasp for identifying CHC biosynthesis-related candidate genes in Hymenoptera and testing the functional involvement of the candidate genes in CHC biosynthesis in the honey bee — a more trackable species of Hymenoptera for reverse genetic analyses than the mason wasp. We first tested whether O. spinipes females retain a given chemotype throughout their adult life. We concurrently sequenced, assembled, and annotated the genome of O. spinipes to aid accurate analysis of gene expression levels in this species. We next identified candidate genes differentially expressed between O. spinipes females with different CHC profile composition. We then identified candidate gene orthologs in the Apis mellifera genome to facilitate reverse genetic analyses in species that allows us to study a larger number of replicates per knockdown experiment than O. spinipes. Specifically, we investigated which cell types of the worker honey bee fat body express the candidate genes and tested whether knockdown of selected candidate genes in worker honey bees via RNA interference (RNAi) impacts their CHC profile composition. These experiments allowed us to identify an array of genes impacting the honey bee’s CHC profile composition and provided hints what functions the encoded enzymes might have in the CHC biosynthesis of this species and of Hymenoptera in general.
Results
Odynerus spinipes chemotype persistency
None of the O. spinipes females whose CHC profile we sampled multiple times during their adult life changed their chemotype (c1 and c2): neither those kept under laboratory conditions (Nc1 = 10; Nc2 = 17; sampled on average three times during the first 16 days after their eclosion; Supplementary Table 1), nor those studied in field populations (Nc1 = 8; Nc2 = 10; sampled on average two times and with a time difference of on average five days; Supplementary Table 2). These results are consistent with the hypothesis that adult O. spinipes females cannot change their chemotype and justify associating CHC chemotype differences between females with gene expression differences between these females.
Odynerus spinipes draft genome
To foster comparative transcriptomic analyses on O. spinipes, we sequenced the genome of this species using short read sequencing technology. The assembly of the O. spinipes genome resulted in 21,922 scaffolds spanning a total of 171 Mbp, 89.5% of the total genome, which we estimated via flow cytometry to be ca. 191 Mbp. The N50 value and L50 value of the draft genome assembly are 3.6 Mbp and twelve scaffolds, respectively. Exploiting whole body transcript libraries of one adult male and of two adult females (representing each chemotype) to foster annotation of nuclear-encoded protein-coding genes (Supplementary Table 3), we inferred a total of 16,677 gene models. The nucleotide sequence data of the genome shotgun project are deposited under the NCBI Bioproject PRJNA735081. The draft genome assembly and the corresponding gene annotations are available under the NCBI accession JAIFRP000000000 and from Zenodo (10.5281/zenodo.7334422).
Candidate genes in Odynerus spinipes
We first identified statistically significant differentially expressed genes in transcriptomes of females with different chemotype in two batches of adult females that differed in sampling time: 12–38 h (batch 1) and 48–62 h (batch 2) after the wasps’ eclosion (Supplementary Tables 4–6). Considering only genes that were consistently differentially expressed between chemotypes in the two batches, we retained five candidate genes (Table 1; Supplementary Tables 7 and 8). We next searched for genes that were significantly differentially expressed between the two above batches of adult female wasps that differed in their age from each other, ignoring the wasps’ chemotype. However, we only considered genes belonging to four gene families known to include genes involved in CHC biosynthesis (i.e., FAS, ELO, Desat, FAR) based on prior research10. This procedure resulted in the identification of eleven candidate genes (Table 1; Supplementary Table 9).
Table 1.
Cuticular hydrocarbon biosynthesis candidate genes in the mason wasp Odynerus spinipes.
| Wasp gene ID | DGE between | Honey bee gene ID | %ID | Predicted function | Cell type |
|---|---|---|---|---|---|
| g3158 | age classes | GB52590 | 54.7 | fatty acid synthase | oenocytes (weak) |
| g6537 | age classes | GB46038 | 85.9 | fatty acid elongase | oenocytes + trophocytes |
| g7609 | age classes | GB54399 | 58.7 | fatty acid elongase | oenocytes (weak) |
| g7610 | age classes | GB54397 | 55.2 | fatty acid elongase | oenocytes |
| g7616 | age classes / chemotypes | GB51247 | 82.9 | fatty acid elongase | oenocytes |
| g7620 | age classes | GB54401 | 84.0 | fatty acid elongase | trophocytes |
| g7621 | age classes | GB54302 | 67.7 | fatty acid elongase | NA |
| g14708 | age classes | NAa | NAa | fatty acid desaturase | oenocytes |
| g14712 | chemotypes | GB42218 | 54.2 | fatty acid desaturase | oenocytes |
| GB51236 | 64.1 | oenocytes | |||
| GB51238 | 55.2 | trophocytes | |||
| g2413 | age classes | GB50627 | 64.2 | fatty acyl-CoA reductase | oenocytes |
| g1571 | age classes | GB52087 | 48.1 | fatty acyl-CoA reductase | oenocytes + trophocytes |
| g7842 | age classes | GB49380 | 72.3 | fatty acyl-CoA reductase | oenocytes |
| g283 | chemotypes | GB44756 | 36.9 | rhythmically expressed gene 5 protein | NA |
| g2290 | chemotypes | GB53695b | 34.3 | fatty acid amide hydrolase | oenocytes + trophocytes (weak) |
| g3059 | chemotypes | GB52820 | 62.4 | parathyroid hormone-related peptide receptor-like protein | oenocytes (weak) + trophocytes (weak) + hexagonal cells (strong) |
The table informs about 1) the mason wasp gene ID, 2) between which groups differential expression differences (DGE) were detected, 3) orthologous/homologous genes in the honey bee (Apis mellifera) genome, 4) the percentage of identical amino acids between the encoded sequences of the mason wasp and those of the honey bee, 5) the genes’ predicted functions, and 6) the expression of the candidate genes in honey bee fat body cells.
aSince we found no 1:1 ortholog in the honey bee genome, we assessed the phylogenetically most closely related homolog, GB40659 (39.6%), for being involved in CHC biosynthesis.
bOne of three co-orthologs in the honey bee, the other two being GB48850 (36.4%) and GB47832 (34.3%) (not studied by us).
Combining the results from both approaches and accounting for redundancy (g7616 was identified with both search strategies), we identified a total of 15 candidate genes affecting CHC biosynthesis in O. spinipes (Table 1). Based on amino acid sequence homology to proteins whose functions have been characterized in other species before, the candidate genes encode one fatty acid synthase, six fatty acid elongases, two fatty acid desaturases, three fatty acyl-CoA reductases, one fatty acid amide hydrolase, one parathyroid hormone-related peptide receptor-like protein, and one rhythmically expressed gene 5 protein.
Honey bee candidate gene orthologs
We applied a phylogenetic approach to identify orthologs of twelve candidate genes belonging to multi-gene families (i.e., FAS, ELO, Desat, FAR) in the honey bee genome. The majority (ten) of the O. spinipes candidate genes share a 1:1 ortholog relationship to honey bee genes (Figs. 2 and 3; Supplementary Figs. 2 and 3; Table 1). However, the fatty acid desaturase candidate gene (g14712) of O. spinipes was found to have three co-orthologs in A. mellifera. We decided to consider all of them for further analyses (Table 1; Fig. 3). We found one fatty acid desaturase candidate gene (g14708) to not have an ortholog in A. mellifera. We decided to include the phylogenetically most closely related A. mellifera gene for further experimental assessment (Table 1; Fig. 3).
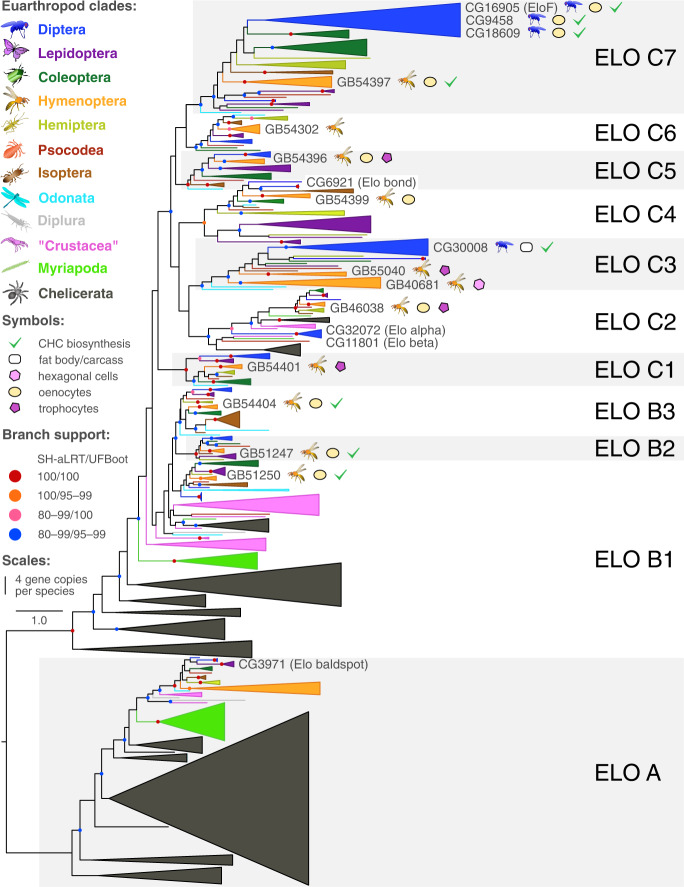
Fig. 2. Gene tree of fatty acid elongases from 37 species of Euarthropoda showing copy numbers and involvement of genes in CHC biosynthesis.
Branch support was calculated from ultrafast bootstrap (UFBoot2) replicates (in %) and Shimodaira-Hasegawa approximate likelihood ratio tests (SH-aLRT in %). Supported branches are represented by a color circle, whereas those with SH-aLRT<80% and UFBoot<95% do not have a circle. The width of each clade represents the number of gene copies per species (single copies represented by a line).
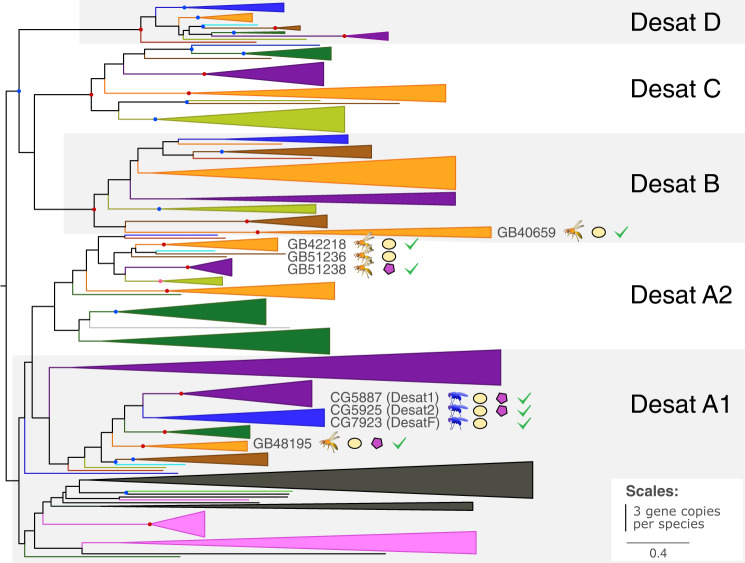
Fig. 3. Gene tree of fatty acid desaturases from 37 species Euarthropoda showing copy numbers and involvement of genes in CHC biosynthesis.
Color coding and symbols are as in the legend of Fig. 2.
We applied the best reciprocal hit criterion to identify orthologs of the remaining three candidate genes (g283, g2290, g3059) in the honey bee genome (Table 1). In all instances except one did we find 1:1 orthologs. The exception was a gene predicted to encode a fatty acid amide hydrolase (FAAH) (g2290) that we found to be represented by three co-orthologs in the honey bee genome. Because this gene family had previously not been implicated with CHC biosynthesis, we considered only one ortholog (GB53695), that with the best reciprocal hit to g2290 (i.e. with the smallest e-value), in subsequent experiments.
We extended our list of candidate genes by seven genes (Table 2). Two of them are A. mellifera orthologs of genes previously shown to be involved in the CHC biosynthesis in the fruit fly Drosophila melanogaster (i.e., the honey bee ortholog of the two fatty acid synthases FASN116/FASN29, Supplementary Fig. 2, and the honey bee ortholog of the three desaturases Desat117, Desat217, and DesatF18 (Fig. 3). The remaining five genes encode fatty acid elongases that belong to clades phylogenetically closely related to genes shown in previous studies (CG3000819, EloF20) and in this study to be involved in CHC biosynthesis (Fig. 2). The total number of candidate genes selected for further investigation in A. mellifera was thus 24 (Tables 1 and 2).
Table 2.
Additional cuticular hydrocarbon biosynthesis candidate genes studied in the honey bee (Apis mellifera).
| Fly gene ID | Honey bee gene ID | Predicted function | Cell type |
|---|---|---|---|
| FASN1/2 | GB53412 | fatty acid synthase | hexagonal cells |
| CG30008 | GB55040 | fatty acid elongase | oenocytes + trophocytes |
| CG30008 | GB40681 | fatty acid elongase | hexagonal cells |
| CG5326 | GB54396 | fatty acid elongase | oenocytes + trophocytes |
| CG31522 | GB51250 | fatty acid elongase | oenocytes |
| CG2781 | GB54404 | fatty acid elongase | oenocytes |
| Desat1, 2, and F | GB48195 | fatty acid desaturase | oenocytes + trophocytes |
Given are 1) the fly and 2) the honey bee gene IDs, 3) the genes’ predicted functions, and 4) the expression of the candidate genes in honey bee fat body cells.
Candidate gene expression sites
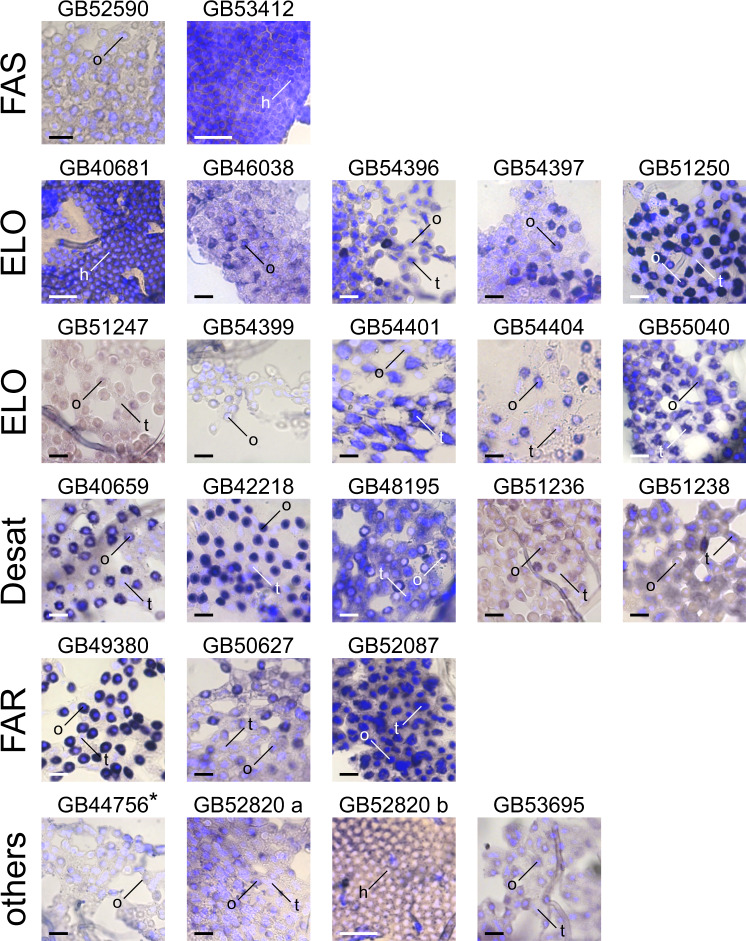
In situ hybridization (ISH) experiments on fat body tissues from honey bee metasomas revealed 18 of the 24 candidate genes to be expressed in oenocytes: a fatty acid synthase (GB52590), eight fatty acid elongases (GB46038, GB54396, GB54397, GB51250, GB51247, GB54399, GB54404, GB55040), four fatty acid desaturases (GB40659, GB42218, GB48195, GB51236), three fatty acyl-CoA reductases (GB49380, GB50627, GB52087), one fatty acid amide hydrolase (GB53695), and one parathyroid hormone-related peptide receptor-like protein (GB52820) (Fig. 4; Tables 1 and 2; negative controls performed with sense probes are shown in Supplementary Fig. 4). Six of them (three fatty acid elongases, one fatty acid desaturase, one fatty acyl-CoA reductase, one fatty acid amide hydrolase) were found to be co-expressed in trophocytes, a fat body cell type different from oenocytes (Fig. 4; Tables 1 and 2). We found two candidate genes, one fatty acid desaturase (GB51238) and one fatty acid elongase (GB54401), to be expressed in trophocytes only (Fig. 4; Table 1). Three candidate genes, predicted to encode a fatty acid synthase (GB53412), a fatty acid elongase (GB40681), and a parathyroid hormone-related peptide receptor-like protein (GB52820), respectively, were found to be expressed in cells located between the cuticle and the fat body layer (GB52820 was found also slightly expressed in oenocytes and trophocytes). We refer to these cells as hexagonal cells (Fig. 4; Tables 1 and 2). In situ hybridization experiments on one candidate gene, predicted to encode a rhythmically expressed protein (GB44756), were inconclusive (marked by an asterisk in Fig. 4), because the staining intensity of the treatment group did not differ from that of the negative controls (Supplementary Fig. 4). We were unable to study cell-specific expression of one candidate gene (GB54302) encoding a fatty acid elongase, as its probe synthesis failed.
Fig. 4. Cell-specific expression of candidate genes in the fat body of worker honey bee (Apis mellifera).
The photomicrographs visualize the expression (dark blue/purple) of fatty acid synthases (FAS), fatty acid elongases (ELO), fatty acid desaturases (Desat), fatty acid reductases (FAR), and additional candidate gene transcripts (others) in fat body cells. Three fat body cell types are indicated: hexagonal cells (h), oenocytes (o), and trophocytes (t). Two separate photomicrographs visualize the expression of GB52820. DAPI (in light blue) was used to counterstain nuclei. Staining in the photomicrograph for GB44756 (marked with an asterisk) did not significantly differ from that of the corresponding control (Supplementary Fig. 4). Scale bar: 50 μm.
Candidate gene knockdown experiments
We assessed twelve candidate genes (selection criteria given in Supplementary Methods) for influencing CHC profile composition by conducting RNAi-mediated knockdown experiments on worker honey bees (Figs. 5 and 6). Knockdown of nine candidate genes resulted in statistically significant changes of the CHC profile composition of the treated bees relative to control experiments in which we injected double-stranded RNA (dsRNA) of green florescent protein (GFP) (powered partial least squares discriminant analysis [PPLS-DA]: p ≤ 0.05 after Benjamini-Hochberg correction for multiple testing). The nine genes were: four fatty acid elongases (GB51250, GB51274, GB54397, GB54404) (Fig. 5), four fatty acid desaturases (GB40659, GB42218, GB48195, GB51238) (Fig. 6), and one fatty acid amide hydrolase (GB53695) (Fig. 5). CHC profile compositional changes associated with the knockdown of fatty acid desaturase GB51236 were statistically insignificant after Benjamini-Hochberg correction for multiple testing (PPLS-DA, p = 0.087; Supplementary Fig. 5). RNAi-mediated knockdown of the remaining two genes, a fatty-acid synthase (GB52590) and a fatty-acyl-CoA reductase (GB52087), caused a premature death of the treated bees within the first two days after dsRNA injection.
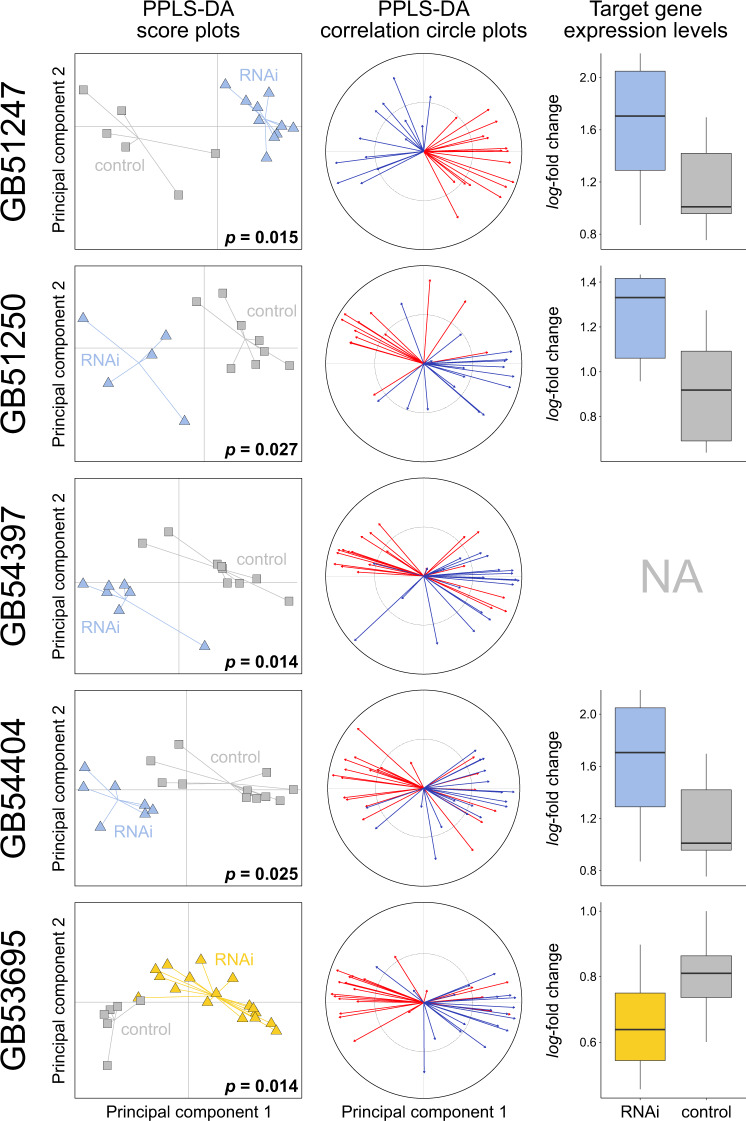
Fig. 5. Effects of RNAi-mediated knockdown of four fatty acid elongases and of one fatty acid amide hydrolase (FAAH) in worker honey bees (Apis mellifera) 2–5 days after dsRNA treatment.
The first two columns show score plots and correlation circle plots from a powered partial least squares discriminant analysis (PPLS-DA) of the cuticular hydrocarbon (CHC) profile data of target gene-treated (in blue for fatty acid elongases RNAi: GB51247, GB51250, GB54397, GB54404, in yellow for FAAH RNAi: GB53695) and of control bees (in grey, injected with dsRNA targeting GFP). P-values indicate the statistical probability of group differences representing random variation after Benjamini-Hochberg correction for multiple (N = 10) testing. The correlation circle plots indicate how many CHCs of a given chain length class (i.e., ≤ 27 [red] or > 27 [blue] carbon atoms) correlate with the first two principal components. Box plots in the third column show gene expression levels (log2-fold change) of the target genes in target gene-treated (in blue for elongase RNAi, in yellow for FAAH RNAi) and in control bees (in grey). None of the expression levels differed statistically significantly between target gene-treated and control bees after applying Benjamini-Hochberg correction for multiple testing (N = 9; Welch’s t-test and Wilcoxon signed-rank test). Additional information is shown in Supplementary Fig. 6.
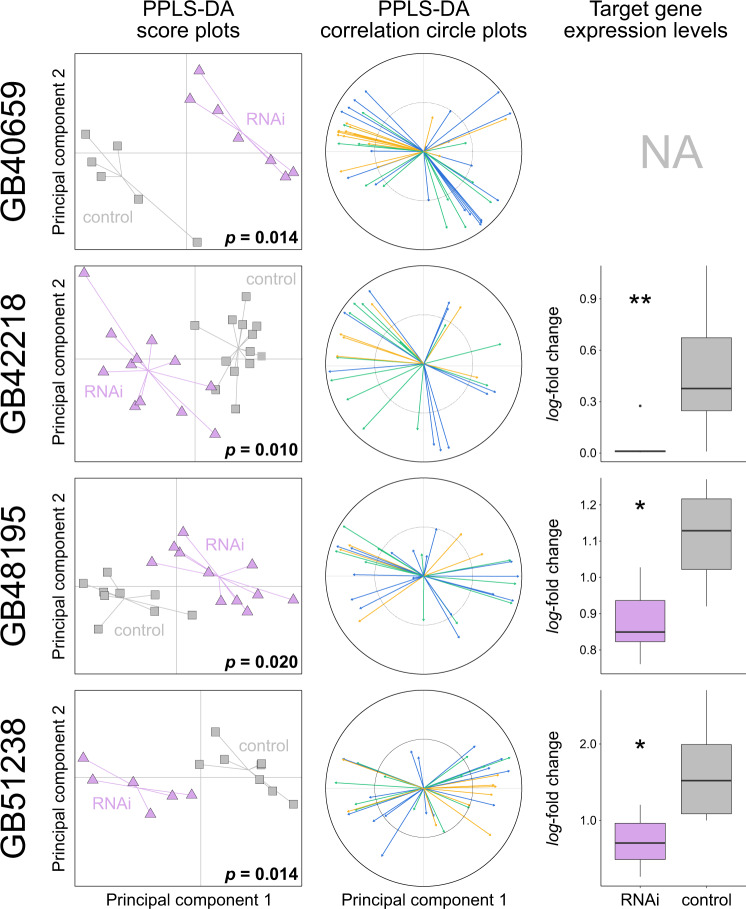
Fig. 6. Effects of RNAi-mediated knockdown of four fatty acid desaturases in worker honey bees (Apis mellifera) 2–5 days after dsRNA treatment.
The first two columns show score plots and correlation circle plots from a powered partial least squares discriminant analysis (PPLS-DA) of cuticular hydrocarbon (CHC) profile data of target gene-treated (in purple: GB40659, GB42218, GB48195, GB51238) and of control bees (in grey, injected with dsRNA targeting GFP). P-values indicate the statistical probability of group differences representing random variation after Benjamini-Hochberg correction for multiple (N = 10) testing. The correlation circle plots indicate how many CHCs of a given compound class (i.e., alkanes [green], alkenes/alkadienes [blue], methyl-branched alkanes [yellow]) correlate with the first two principal components. Box plots in the third column show gene expression levels (log2-fold change) of the target genes in target gene-treated (in purple) and in control bees (in grey). Asterisks indicate the statistical significance of expression differences between target gene-treated and control bees after applying Benjamini-Hochberg correction for multiple testing (N = 9; Welch’s t-test/Wilcoxon signed-rank test; p ≤ 0.05 [*], p ≤ 0.01 [**]). Additional information is shown in Supplementary Fig. 7.
While we found RNAi of the nine genes outlined above to generally result in a strong phenotypic effect 2–5 days after dsRNA injection, we did not detect statistically significant differences in expression levels of most target genes at the same point in time (p = 0.126–0.607; Welch’s t-tests; Figs. 5 and 6). The exceptions are three fatty acid desaturases (GB42218, GB48195, GB51238) that showed significantly lower expression levels in treated bees relative to control bees after Benjamini-Hochberg correction for multiple testing (GB42218: Wilcoxon signed-rank test, W = 122, p = 0.002; GB48195: Welch’s t-test, t(10.85) = 4.01, p = 0.010; GB51238: Welch’s t-test, t(9.67) = 3.21, p = 0.033; Fig. 6). Note that we were unable to measure the expression levels of GB54397 (encoding a fatty acid elongase) and of GB40659 (encoding a fatty acid desaturase), because the qRT-PCR oligonucleotide primers did not efficiently amplify a unique stretch of the genes’ cDNA.
PPLS-DA of the CHC profiles provided information on the compounds that dominantly discriminate the RNAi treatment group and the control group. Knockdown of fatty acid elongases caused major CHC profile perturbations in several compound classes (Supplementary Fig. 6), but it most notably impacted CHC chain lengths (Fig. 5). Specifically, we found the chain lengths of CHCs positively correlating more than 50% along the first principal component of the PPLS-DA (Fig. 5) with a fatty acid elongase dsRNA treatment (GB51247, GB51250, GB54397, GB54404) to be significantly lower than CHC chain lengths positively correlating with a control dsRNA treatment after correcting for multiple testing (Holm-Bonferroni) (GB51247: Welch’s t-test, t(12.04) = 6.67, p < 0.001; GB51250: Welch’s t-test, t(15.98) = 6.18, p < 0.001; GB54397: Welch’s t-test, t(27.76) = 3.54, p = 0.003; GB54404: Welch’s t-test, t(24.93) = 2.58, p = 0.016) (Fig. 7). We found the opposite pattern when interfering with the expression of GB53695 (encoding a fatty acid amide hydrolase): the CHC profiles of the treatment bees contained CHCs with significantly longer chain lengths than the CHC profiles of the control bees (Welch’s t-test, t(27.71) = −5.92, p < 0.001; Figs. 5and 7; Supplementary Fig. 6).
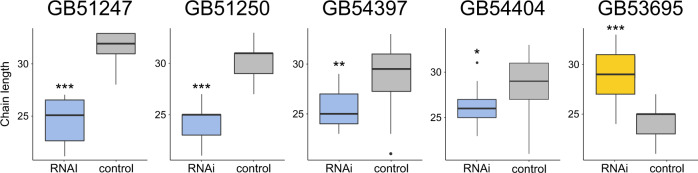
Fig. 7. Effects on chain lengths of cuticular hydrocarbons (CHCs) of RNAi-mediated knockdown of four fatty acid elongases and of one fatty acid amide hydrolase (FAAH) in worker honey bees (Apis mellifera) 2–5 days after dsRNA treatment.
Box plots of the chain lengths of CHCs positively associated in a powered partial least squares discriminant analysis along the first principal component with bees treated with dsRNA targeting fatty acid elongases (in bue: GB51247, GB51250, GB54397, GB54404) or fatty acid amide hydrolase (FAAH, in yellow: GB53695) and with control bees (in grey, injected with dsRNA targeting GFP). Asterisks indicate statistical significance of abundance differences between the two treatment groups after Holm-Bonferroni correction for multiple (N = 5) testing (Welch’s t-test: p ≤ 0.05 [*], p ≤ 0.01 [**], p ≤ 0.001 [***]).
Knockdown of fatty acid desaturases also caused major CHC profile perturbations and impacted the relative abundance of several compound classes (i.e., alkanes, alkenes, and methyl-branched alkanes) (Fig. 6; Supplementary Fig. 7). Depending on what gene we targeted with dsRNA, we found statistically significant differences in the relative abundance of alkenes with specific double bond positions between treated bees and control bees. Specifically, we found the relative abundance of alkenes with a double bond at even positions (8,10) to be significantly smaller in bees treated with dsRNA of GB48195 (Wilcoxon signed-rank test, W = 87, p = 0.022) and of GB51238 (Welch’s t-test, t(11.94) = 5.31, p = 0.002) than in control bees after correcting for multiple testing (Holm-Bonferroni) (Fig. 8). We did not study the impact of target gene knockdown on alkadienes (hydrocarbons with two double bonds), since the relative abundance of alkadienes was close to the detection limit of the used GC-MS instruments.
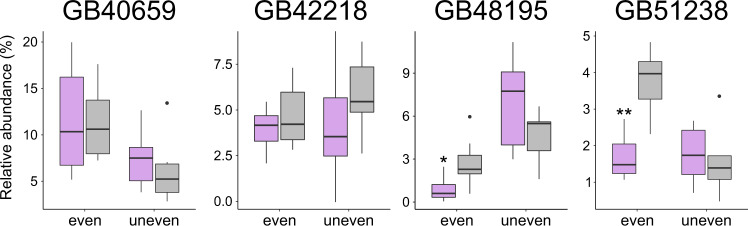
Fig. 8. Relative abundance of alkenes with double bonds at even and at uneven positions in fatty acid desaturase RNAi-treated bees and control bees.
Box plots of the relative abundances of alkenes in bees treated with dsRNA of fatty acid desaturases (GB40659, GB42218, GB48195, GB51238; purple) or dsRNA of GFP (control; grey). Asterisks indicate statistically significant abundance differences between the two groups (RNAi treated vs. control) after Holm-Bonferroni correction for multiple (N = 10) testing (Welch’s t-test/Wilcoxon signed-rank test: p ≤ 0.05 [*], p ≤ 0.01 [**]).
Discussion
Studying intrasexual CHC profile differences in the mason wasp O. spinipes provided promising candidate genes that modulate CHC profile composition in Hymenoptera. Knockdown experiments on twelve selected candidate genes in A. mellifera resulted in statistically significant CHC profile changes in nine of the experiments. In the light of these results, the remaining candidate genes not further experimentally assessed in the present investigation also appear highly promising targets for future reverse genetic and heterologous expression experiments, as done, for example, by Tupec et al.21 on fatty acyl-CoA reductases.
Our study provided strong evidence for four fatty acid elongases (GB51247, GB51250, GB54397, GB54404) modulating CHC profile composition in Hymenoptera (Figs. 5 and 7). Fatty acid elongases previously identified in Diptera to be involved in CHC biosynthesis belong to clade ELO C3 (CG3000819) and clade ELO C7 (i.e., EloF20, CG945819, CG1860919) (Fig. 2). Our RNAi experiments on honey bees confirmed that genes of clade ELO C7 (GB54397) are also involved in CHC biosynthesis of Hymenoptera. They further revealed that genes of three additional major clades of this gene family, that is ELO B1 (GB51250), ELO B2 (GB51247), and ELO B3 (GB54404), are involved in shaping CHC profiles in this mega-diverse insect order (Fig. 2). The numerous clades with known CHC biosynthesis-related genes in the gene family tree and of candidate genes shown in this study to be expressed in oenocytes or trophocytes (see below) (Fig. 2) within this gene family makes genes belonging to clades ELO C1–7 promising targets for future knockdown experiments. In contrast, the involvement of genes of clade ELO A in CHC biosynthesis currently appears unlikely, as the only gene of this clade functionally studied so far (i.e., baldspot of D. melanogaster) participates in spermatogenesis22.
We did not measure a statistically significant decrease in the expression levels of RNAi-targeted elongates at the point in time when we sampled the honey bees’ CHC profiles (Fig. 5). This at first glance surprising result could be due to a combination of two factors: 1) lag-time between gene expression level changes and CHC profile changes, and 2) short-term duration of the knockdown effect on the target genes’ expression levels23. Because the time points for sampling dsRNA-treated bees had been chosen based on when phenotypic changes became visible in a subset of samples (typically 2–5 days after dsRNA injection; Supplementary Table 10), the time points were possibly too late to still detect transient gene expression level changes. We consider the alternative explanation, namely that RNAi treatment did not have an effect on the target genes’ expression levels, as unlikely, because the observed phenotypic changes were in most instances consistent with the predicted function of the target genes. Moreover, the observed increase of the expression levels of three elongase genes after RNAi treatment could be explained by a temporary overexpression of these genes after their successful prior knockdown23.
Fatty acid elongases have been shown to catalyze the elongation of long fatty acyl-CoAs2,10,24. Consistent with this idea, we found knockdown of fatty acid elongase genes to result in a decrease in the relative abundance of CHCs with chain lengths > 27 carbon atoms (Figs. 5 and 7). Yet, we did not find any additional differences between the knockdown phenotypes. Lack of distinct phenotypes could be due to off-target effects (i.e., knockdown of a gene coding for one fatty acid elongase simultaneously decreases the expression of another gene coding for a fatty acid elongase). However, except for two target genes, this scenario seems unlikely, as the nucleotide sequences of the synthesized dsRNAs (provided on Zenodo: 10.5281/zenodo.7334422) differed substantially from each other. The exceptions are GB51250 and GB54404, which differed only in 43% of their nucleotides from each other. An alternative explanation for the lack of distinct phenotypic effects could be that the targeted fatty acid elongases exhibit, in stark contrast to those of mammals24, little substrate specificity. Finally, manipulation of the elongation steps could cause a shift of substrate concentrations along the entire CHC biosynthetic pathway, making it difficult to detect substrate specificity. Additional tools have to be used in future investigations to further interrogate the specific function and the substrate specificity of the enzymes encoded by the here-identified genes (e.g., heterologous expression, as done by Pei et al.25, or in vivo synthesis using labeled precursors). The results of our study provide the foundation for such investigations.
We identified four fatty acid desaturase genes (GB40659, GB42218, GB48195, GB51238) that modulate CHC composition in worker honey bees. Previous research on D. melanogaster had led to the identification of three closely related fatty acid desaturases (Desat1, Desat2, and DesatF) for being involved in CHC biosynthesis and introducing double bonds in fatty acyl-CoA17,18,26. All three desaturases belong to clade Desat A1 (Fig. 3). In the honey bee, clade Desat A1 is represented by a single gene (GB48195), which our knockdown experiments confirmed to be also involved in CHC biosynthesis in Hymenoptera. Intriguingly, the three additional fatty acid desaturases identified in our study to influence CHC profile composition in worker honey bees belong to two clades previously not associated with CHC biosynthesis (Fig. 3): Desat A2 (GB42218, GB51238) and Desat B (GB40659). This demonstrates that fatty acid desaturases from very distantly related clades participate in CHC metabolism (i.e., Desat A1, Desat A2, Desat B; possibly also Desat D, which contains the O. spinipes candidate gene g14708, which lacks an ortholog in A. mellifera).
Knockdown of the above-outlined four fatty acid desaturases had a strong effect in worker honey bees on both target gene expression (except GB40659) and on CHC profile composition (Fig. 6). Moreover, we showed that knockdown of GB48195 and of GB51238 resulted in a decrease of alkenes, consistent with the idea of the encoded proteins introducing double bonds in fatty acyl-CoA. While all desaturases in D. melanogaster known to be involved in CHC biosynthesis (Desat1, Desat2, and DesatF) show Δ9-specificity and thus facilitate synthesis of alkenes with double bonds at uneven positions17,18, GB48195 and GB51238 are the first fatty acid desaturases identified to be involved in the production of alkenes with double bonds at even positions (8 and 10) (Fig. 8). Knockdown of the remaining two fatty acid desaturases (GB40659, GB42218) led to broad and complex compositional changes involving multiple CHCs. As discussed above, lack of distinct phenotypes could be due to off-target effects, which in our case seems unlikely, however, as the nucleotide sequences of the synthesized dsRNAs (provided on Zenodo: 10.5281/zenodo.7334422) differed between all genes except two substantially from each other. The exceptions are GB42218 and GB51238, whose dsRNAs differed only in 29% of their nucleotides from each other. We consider off-target effects in this specific instance to unlikely have significantly impacted the results, as the nucleotide sequence used for antisense riboprobe (ISH) and the dsRNA probe (RNAi) caused different staining in the ISH experiments and different phenotypes in the knockdown experiments, respectively. The lack of distinct phenotypes and the difficulty to detect substrate specificity could be for the similar reasons as discussed above for fatty acid elongases.
We identified a fatty-acid amide hydrolase (FAAH, GB53695) influencing CHC profile composition in worker honey bees. This result is remarkable, as fatty-acid amide hydrolases had never been associated with CHC biosynthesis before. Knockdown of GB53695, which we found to be expressed in oenocytes, resulted in a significant increase of the relative abundance of CHCs with chain lengths > 27 carbon atoms (Fig. 7). However, we did not detect statistically significant gene expression changes (Fig. 5). Fatty-acid amide hydrolases are characterized by their amidase signature and are — in mammals — known to degrade fatty acid amides of endogenous lipids into fatty acids by removing the amide group27. How the functions of fatty-acid amide hydrolases relate to the modulation of CHC profiles in Hymenoptera remains unclear at this point. We deem it possible that the studied fatty-acid amide hydrolase impacts the availability of CHC precursors, by, for instance, releasing short-chain fatty acids that are then possibly reduced to aldehydes, which could be transformed to CHCs by a cytochrome p450 decarbonylase (Fig. 1). It is likely that another honey bee gene phylogenetically closer to the candidate gene g2290 (GB48850) is also involved in CHC biosynthesis (Supplementary Fig. 8). Future experiments (e.g., heterologous expression) should shed light on the role of this small gene family in CHC biosynthesis.
Knockdown of candidate genes encoding a fatty acid synthase (GB52590) and a fatty acyl-CoA reductase (GB52087) resulted in a premature death of the treated bees. A similar observation was made by other researchers when targeting genes of these two families in various insects (e.g.,16,28–31). We hypothesize that interfering with the expression of genes of the two gene families reduced — due to their central role in CHC biosynthesis — the overall availability of CHCs to an extend that it caused desiccation-related death of the treated bees16,29,31. Knockdown of fatty acid synthase could additionally have reduced the synthesis of other molecules fundamentally important for survival (e.g., cuticular lipids29). Despite the fact that our RNAi experiments on GB52590 and GB52087 remained inconclusive, alternative approaches should consider them highly promising candidate genes, as multiple lines of evidence strongly suggest both genes to be involved in CHC biosynthesis: a) ortholog relationship to D. melanogaster genes known to be involved in CHC biosynthesis (i.e., GB52590, ortholog of FASN316 fatty acid synthase which belongs to clade FAS B2; Supplementary Fig. 2; GB52087, ortholog of the three fatty acyl-CoA reductases CG10097, CG13091, and CG1756019, all of which belong to clade FAR F; Supplementary Fig. 3), b) expression in honey bee oenocytes (Fig. 4), and c) statistically significant expression differences of their O. spinipes orthologs (i.e, g3158, ortholog of GB52590; g1571, ortholog of GB52087) between 12 and 38 h-old O. spinipes females with different chemotype (Supplementary Tables 5 and 7) and between females differing in age from each other (Table 1; Supplementary Table 9). Two other FAR-encoding candidate genes, GB50627 (clade FAR F) and GB49380 (clade FAR B) also seem promising candidate genes, as they are both expressed in honey bee oenocytes (Fig. 4) and their O. spinipes orthologs (g2413 and g7842) were found to be differentially expressed between females differing in age from each other (Table 1; Supplementary Table 9).
The phylogenetic distribution of genes involved in CHC biosynthesis across Euarthropoda (i.e., Chelicerata, Myriapoda, “Crustacea” (paraphyletic), and Hexapoda) has implications for our understanding of the evolution of CHC biosynthesis. Based on the four inferred gene trees and the ISH and knockdown results on A. mellifera, our study shows that all major gene families (Fig. 1) and most subclades within them harboring genes known to be involved in CHC biosynthesis (i.e., FAS A, ELO C, ELO B, Desat A1, and potentially FAR B) are present in the extant major lineages of Euarthropoda. The phylogenetic relationships in the gene trees of fatty acid synthases and fatty acid elongases inferred by Finck et al.32 are consistent with this interpretation. CHCs and oenocytes have also been reported from all major Euarthropoda lineages33–40. We therefore consider it reasonable to hypothesize that the capacity of CHC biosynthesis evolved before the divergence of the major lineages of Euarthropoda and, consequently, before the colonization of land. An alternative hypothesis, namely convergent exaptation of the same genes for CHC biosynthesis in different lineages of Euarthropoda, is far less parsimonious. The only gene whose phylogenetic distribution is currently not fully consistent with a CHC origin in a species inhabiting an aquatic environment encodes a cytochrome p450 decarbonylase (CYP4G gene family) (Fig. 1) that catalyzes the final step of CHC biosynthesis8,41 and that has — so far — only been found in Hexapoda41,42. As the cytochrome p450 gene family is known for its high turnover rate38, we do not put too much weight in this single exception.
Although CHCs serve social insects as important semiochemicals (e.g., cast and nest-mate recognition), social species do not possess more complex CHC profiles than solitary ones43. Indeed, CHCs also serve fundamental functions in solitary species (e.g., cues and signals for species and sex recognition2). We found the absence of systematic differences between social and solitary species in their CHC profiles to be reflected at the genetic level: none of the taxonomic lineages represented in the gene trees stands out by a particularly rich or poor set of genes, irrespective of whether or not the lineage contains solitary or social species (Figs. 2 and 3; Supplementary Figs. 2 and 3). What we found are species-specific gene duplications in Amyelois transitella, Manduca sexta, Nasonia vitripennis, and Tribolium castaneum (Supplementary Fig. 9), possibly linked to species-specific ecology and behaviors other than sociality.
We found almost all genes whose knockdown resulted in a CHC profile change to be expressed in oenocytes — consistent with the common view that CHC metabolism occurs in oenocytes (e.g.,8,44–46). A major exception was GB51238, encoding a fatty acid desaturase. We found this gene exclusively and highly expressed in trophocytes (Fig. 4), another cell type of the insects’ fat body. While we cannot exclude the possibility that the gene is expressed at a very low level in oenocytes, we deem the result noteworthy, as other researchers previously already suggested fat body cells other than oenocytes to be possibly involved in CHC biosynthesis16,47. Given the possible implications of CHC biosynthesis involving additional types of fat body cells, we recommend future studies to not exclusively focus on oenocytes when studying CHC metabolism.
RNAi experiments are difficult to conduct on O. spinipes, because these wasps cannot be cultured (at least so far), occurs at most known field sites in comparatively small numbers, and because rearing age-controlled specimens of this species is extremely time consuming (i.e., can only be done once per year). We therefore decided to assess a posteriori whether the possible functions of genes — inferred from our knockdown experiments on honey bees — that are differentially expressed in O. spinipes females with different chemotypes, can explain the specific phenotypic differences between the two chemotypes. This approach rests on the ortholog conjecture, which states that orthologous genes in different species tend to retain the same function48. Intriguingly, two of the five genes that we found differentially expressed between O. spinipes females with different chemotypes (i.e., g14712, g7616) are likely sufficient to explain the majority of the phenotypic differences between the two chemotypes. Knockdown of GB51238, a co-ortholog of the O. spinipes fatty acid desaturase g14712, caused a decrease in the relative abundance of alkenes with double bonds at even carbon chain positions in worker honey bees. Consistent with this result, we found g14712 to be higher expressed in O. spinipes females expressing chemotype 2, which is characterized by alkenes with double bonds at even carbon chain positions (i.e., 8, 10, 12, and 14) than in O. spinipes females expressing chemotype 1, which is characterized by alkenes with double bonds at uneven carbon chain positions (i.e., 7 and 9). Likewise, the knockdown of GB51247, an ortholog of the O. spinipes fatty acid elongase g7616, caused a decrease of the relative abundance of CHCs with chain lengths larger than 27 carbon atoms in worker honey bees. Consistent with this result, we found g7616 to be higher expressed in O. spinipes females expressing chemotype 2, which is characterized by a high relative abundance of alkenes with chain lengths > 27 carbon atoms, than in O. spinipes females expressing chemotype 1, which is characterized by low relative abundance of alkenes with chain lengths > 27 carbon atoms (Supplementary Fig. 1). Heterologous expression experiments21 could provide the means to test our above hypotheses and to shed light on the specific enzymatic reactions catalyzed by the remaining identified candidate genes in O. spinipes.
Our study demonstrates how comparative transcriptomic analyses on a non-model organism featuring unique characteristics can deliver valuable information that research on established model organisms may not be able to provide. We presented an array of genes that impact CHC profile composition in Hymenoptera. These enable future experimental manipulation of CHC profiles in this mega-diverse insect order and functional investigations on the activity and substrate specificity of the encoded proteins.
Methods and material
Odynerus spinipes chemotype persistency analysis
We collected prepupae of O. spinipes females and males from trap nests (details in Supplementary Methods). Each female was placed in a separate observation cage (30 cm×30 cm x 30 cm, Bioform, Nürnberg, Germany) and provided with a conspecific male and absorbent paper soaked with honey and water. The presence of males was meant to simulate field conditions for the females. The cages were kept in a climate chamber (70% humidity, 23 °C during day, 18 °C during night, and with a 12/12 h day/night cycle). We sampled the cuticular hydrocarbons (CHCs) of 23 females with the aid of Solid Phase Micro-Extraction (SPME) fibers (see below) at two days, seven/eight days, and 14 days (or immediately after they died) after they had eclosed (Supplementary Table 1).
We sampled the CHCs of 18 females two to four times during their adult life in the field (Supplementary Table 2). Females were caught at their nest entrance, color-coded with water-resistant paint markers (Edding 780, 0.8 mm, Ahrensburg, Germany), CHC-sampled with the aid of SPME fibers, and released the next morning at their nest site. The sampling time coincided with the start of the nest building activity.
CHCs from living wasps were sampled by stroking a SPME fiber (Supelco, coating: polydimethylsiloxane, 100 µm, Sigma-Aldrich, Bellefonte, PA, USA) for 2 min. (field populations) or 4 min. (lab population) over the cuticle of a female’s metasoma. Wasps processed in 2016 were anesthetized by exposing them for 1 min. to CO2, those processed in 2017 by cooling them down for 3 min. at −20 °C (because we suspected CO2 treatment to cause amnesia in the treated wasp, as wasps treated this way in 2016 rarely returned to their nest; see49).
All CHC extracts were either analyzed with a gas chromatograph coupled to a mass spectrometer (GC-MS) or with a gas chromatograph-flame ionization detector equipped with a SLB-5 MS non-polar capillary (GC-FID) applying the same temperature profile (details in Supplementary Methods). The GC-MS data were recorded and quantified with the software HP Enhanced ChemStation G1701AA (version A.03.00; Hewlett Packard, Palo Alto, California, USA). The GC-FID data were recorded and quantified with the software GCSolution (Shimadzu, Wemmel, Belgium). The chemotype of the female wasps was inferred from the presence/absence of chemotype-specific alkenes14,15 with diagnostic Kovats indices50.
Odynerus spinipes draft genome
We sequenced the nuclear genome at a base-coverage depth of ca. 86x using Illumina HiSeq2000 sequencing technology and three different types of NGS libraries: 250 bp paired-end [10.58 Gbp] (55x), 800 bp paired-end [2.45 Gbp] (13x), and 3 kbp mate-pair [3.4 Gbp] (18x), generated from a single pool of DNA extracted from males and females (details in Supplementary Methods). To foster gene annotation, we sequenced whole-body transcriptomes of one adult male and of two adult females (one of each chemotype). DNA and RNA extraction, library preparation, and Illumina DNA sequencing were done by BGI Tech Solutions (Hong Kong, PRC).
All raw reads of genomic DNA were processed with trimmomatic51 version 0.33 (details in Supplementary Methods). The processed reads were then used to assemble the genome with Platanus52 version 1.2.4 using all three libraries in each of the three assembly steps (contig construction, scaffolding, and gap closing) and applying the software’s default parameters. The protein-coding gene repertoire was annotated with the BRAKER ab initio gene prediction pipeline53 version 2.1 and providing the RNA-seq data of the three above-mentioned adult wasps as intrinsic evidence (details in Supplementary Methods).
Comparative gene expression analysis
We sequenced metasoma transcriptomes of twelve O. spinipes females raised under laboratory conditions from field-collected prepupae (Supplementary Table 4): six of them (three of each chemotype) were sampled 12–38 h after they had eclosed, the other six (three of each chemotype) were sampled 48–62 h after they had eclosed. The prepupae were collected from three field sites (Supplementary Table 4).
Prior to RNA fixation, the eclosed females were kept separate from each other at 21 °C in polystyrene tubes (53 mm×100 mm, Bioform, Nuremberg, Germany) containing moistened cotton and experienced a 12/12 h day/night cycle. All samples were euthanized at the same time of the day (1 pm) to minimize a potential impact of expression differences by rhythmically expressed genes (note that expression of Desat1 follows such a diurnal rhythm in D. melanogaster54). Wasps were euthanized by submersing them for 60–120 sec. in hexane (n-hexane for gas chromatography, SupraSolv, Merck, Germany). The CHC extracts were used to infer the wasps’ chemotypes as described above. Each wasp’s metasoma was ground with a sterile pestle in 1 mL RNAlater (QIAGEN, Hilden, Germany) and kept at −80 °C before further processing it.
RNA extraction, NGS library preparation, and 150 bp paired-end nucleotide sequencing with an Illumina NextSeq 500 (Illumina, San Diego, USA) were done by StarSEQ (Mainz, Germany) using standard protocols. We collected on average 20–44 million reads per sample. All raw reads are available from GenBank in Bioproject PRJNA609595 (Supplementary Table 4).
The RNA-seq data were analyzed with software integrated in the Galaxy Europe platform (https://usegalaxy.eu). We quality-trimmed the raw reads and removed NGS library adapters with Trim Galore version 0.4.3.0 (http://www.bioinformatics.babraham.ac.uk/projects/trim_galore/). The quality of the raw reads was assessed with FastQC version 0.67 (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/). The gene model file (GTF) of the O. spinipes genome was used to model splice junctions with the software STAR55 version 2.5.2b0 via mapping the transcript raw reads onto the genome assembly. We inferred a matrix of normalized read counts per gene, considering the genes in the above GTF file, with featureCounts56 version 1.5.3.
Because our initial experimental design included only one batch of six transcriptomes (three of each chemotype) (Supplementary Table 4), we applied a two-step procedure for identifying genes differentially expressed between females with different chemotypes: we first searched with DESeq257 version 2.11.39 for differentially expressed genes in each of the two batches, using a False discovery rate (FDR) of 0.05 as cutoff. We then reciprocally assessed genes that this analysis indicated to be differentially expressed between the two chemotypes in the other batch, this time applying a more conservative Holm-Bonferroni correction for multiple testing. Only genes that were significantly differentially expressed in both analyses were kept as candidate genes. We assessed with edgeR58 version 3.28.0 in the R statistical software version 3.4.1 (http://www.R-project.org) whether the five genes identified with the above-described procedure were also found differentially expressed in the two batches (p-value< 0.05 after Holm-Bonferroni correction for five tests). We used DESeq2 as well as edgeR to assess the statistical significance of gene expression differences between O. spinipes females of different age classes, ignoring the wasps’ chemotype. We considered genes, judged to differ in their expression by either of the two programs, as candidates if they encoded fatty acid synthases (FAS), fatty acid elongases (ELO), fatty acid desaturases (DESAT), or fatty acyl-CoA reductases (FAR).
Identification of candidate gene orthologs in the honey bee genome
Honey bee orthologs of O. spinipes candidate genes belonging to the gene families Desat, ELO, FAR, and FAS were identified by their phylogenetic position in the corresponding gene trees. To this end, we identified the proteins of the four gene families by searching for proteins with the protein family-characteristic protein domains (see below) available as HMM models from PFAM database59 (PFAM-A, version 28). The HMM models were searched with the aid of hmmer60 version 3.1b1 against the protein sets of 37 Euarthropoda, including O. spinipes, with sequenced genomes (details in Supplementary Methods). To identify fatty acid synthases, we searched for proteins containing the PFAM domains ketoacyl-synt KS (Pfam family accession PF00109), acyl-transferase-1 (PF00698), and PS-DH (PF14765) (e-value < 1e-5). To identify fatty acid elongases, we searched for proteins containing the ELO PFAM domain PF01151 (e-value < 1e-30), and consisting at least of 200 amino acids. To identify fatty acid desaturases, we searched for proteins containing the PFAM domain PF00487 (e-value < 1e-20). Note that we did not consider sphingolipid desaturases, as they show little amino acid sequence similarity to other desaturases (see12). To identify fatty acyl-CoA reductases, we searched for the simultaneous presence of the two PFAM domains PF07993 (e-value < 1e-57) and PF03015 (e-value < 1e-15). To identify fatty acid amide hydrolases, we searched for proteins containing the amidase PFAM domain PF01425 (e-value < 1e-65). The amino acid sequences of a given gene family were aligned with MAFFT61 version 7.123 and phylogenetically analyzed with the software IQ-TREE62 version 1.6 (details in Supplementary Methods).
Honey bee orthologs of the remaining O. spinipes candidate genes and which did not belong to the above gene families were identified by applying the best reciprocal hit criterion and searching the protein repertoires of the two species with the blastp program of the BLAST + software suite63,64.
In situ hybridization
RNA probes were synthesized according to standard protocols65 from cloned partial mRNA sequences of all candidate genes (Tables 1 and 2) using DIG and FLU RNA Labeling Kits (Roche, Basel, Switzerland). Strand cDNA was synthesized from isolated total RNA (1 µg, DNase-treated) of fat body tissues isolated from A. mellifera using the Quantitect kit (QIAGEN GmbH, Hilden, Germany). The resulting cDNA library was used to amplify target regions via polymerase chain reactions (PCRs) (Mastercycle DNA Engine Thermal cycler PCR, Eppendorf AG, Hamburg, Germany) using the Phusion high fidelity kit (New England Biolabs, Ipswich, MA, USA). Amplicons were cloned with the NEB PCR Cloning kit (New England Biolabs GmbH, Ipswich, MA, USA), and the plasmids were extracted using the Gen Elute Plasmid Miniprep kit (Sigma-Aldrich, Steinheim, Germany) and were sent to Genewiz (Genewiz, Leipzig, Germany) for nucleotide sequencing. The cloned gene fragments (387–774 bp long) were used to synthesize anti-sense and sense RNA probes using T7 (Roche, Mannheim, Germany) or SP6 (Roche, Mannheim, Germany) RNA polymerases. Sense probes served as negative control to each anti-sense probe. Oligonucleotide primers used for probe cloning were designed with the online software Primer-BLAST66 and synthesized by Metabion (Planegg, Germany) or Sigma-Aldrich (Steinheim am Albuch, Germany) (Supplementary Table 11).
In situ hybridization was done on worker honey bees collected from a single hive at Würzburg University. All bees eclosed 4–8 days prior to their processing, were reared from prepupae in climate chambers at 28 °C, and were collectively kept in observation cages at 32 °C with 30% sugar solution. Metasoma tergites and sternites were dissected from living bees (placed on ice for 5 min) in 4% paraformaldehyde (PFA) (Sigma-Aldrich, Steinheim am Albuch, Germany; dilution in Phosphate buffered saline [PBS]). The dissected segments were fixed overnight in a solution consisting of 2 mL heptane (Carl Roth, Karlsruhe, Germany) and 2 mL of 4% PFA on a rotator at room temperature. The fixation was finalized following the protocol given by Dearden et al.67. Whole-mount RNA in situ hybridization was done as described by Dearden et al.68, with minor modifications outlined in Supplementary Methods. All mounting media included DAPI (0.5 µg/mL; Thermofisher Scientific, Waltham, Massachusetts, USA) to achieve nuclear counterstaining. High-resolution images of stained tissues mounted on glass slides were obtained with a Zeiss Axio Imager A1 fluorescence microscope, a Zeiss AxioCam MRc, DAPI fluorescence filters, a Zeiss HXP120 UV light unit, and the Zeiss Axiovision software (Carl Zeiss Jena GmbH, Jena, Germany). Overlay of brightfield images and DAPI fluorescence was done with ImageJ/Fiji (https://fiji.sc/). Trophocytes were distinguished from oenocytes by their polyploid or polygonal cells (oenocytes are round) with a large irregular nucleus (the nucleus of oenocytes is round or oval)69.
RNAi-mediated knockdown experiments
Double-stranded RNA (dsRNA) was synthesized in vitro from 387–774-bp-long inserts — previously generated for RNA probe synthesis — using MEGAscript RNAi kit (Ambion, Texas, USA) (details in Supplementary Methods). T7 promotor was added to the oligonucleotide primers (listed in Supplementary Table 11) used to amplify the plasmids. A plasmid with a DNA fragment encoding green florescent protein (GFP), kindly provided by Anne-Kathrin Rohlfing (nucleotide sequence on Zenodo: 10.5281/zenodo.7334422), was used to synthesize dsRNA that served as negative control. We verified that the GFP nucleotide sequence had no significant match in the honey bee genome70 by searching it with tools of the BLAST + software suite63,64 against the honey bee genome (GCF_000002195.4_Amel_4.5). The identity of the amplicons was verified by nucleotide-sequencing them. All dsRNA probes were diluted in MOPS Ringer solution (270 mM NaCl, 3.2 mM KCl, 1.2 mM CaCl2, 10 mM MgCl2, 10 mM γ-(N-Morpholino) propane sulfonic acid (MOPS), ph 7.4) to reach a final concentration of 5 µg/µL.
Knockdown experiments were done on worker honey bees reared as described above and chilled prior to the dsRNA injection. All bees of a given experiment came from the same hive, eclosed 6–7 days prior to dsRNA treatment and belonged to the same caste (workers, nurse bee). We injected between the 2nd and 3rd tergite of each bee’s the metasoma 1 µL dsRNA (5 µg) with a Hamilton microliter syringe (MICROLITER Series 7000 Tip type 2, 7105 KH, Carl Roth, Karlsruhe, Germany). Per the experiment, we treated 6–12 bees with dsRNA of the target gene of interest and 6–13 bees with dsRNA of GFP, which served as control (Supplementary Table S11). Bees of different experiments were kept in separated observation cages and were fed with fed 30% sugar water. Note that we were forced to use the same control group when assessing knockdown of GB51250 and GB48195 (which have been carried out at the same time and with bees from the same hive), as we lacked bees to make an extra control group at that specific time.
After dsRNA injection, we daily sampled the CHCs of six bees (three of the treatment group and three of the control group) from a given RNAi experiment with SPME fibers, probing each bee’s metasoma. As soon as notable CHC profile differences between treatment bees and control bees were detected (sampling times given in Supplementary Table 10), all samples from the corresponding RNAi experiment were euthanized by immersing them for 2 min. in hexane (n-hexane for gas chromatography, SupraSolv, Merck, Germany) and were subsequently frozen in liquid nitrogen. The bees’ metasoma were subsequently stored at −80 °C before further processing them.
CHC extracts were analyzed with a GC-MS (details in Supplementary Methods). CHC abundance values were normalized by dividing them by the total abundance of CHCs identified in a given CHC extract (available on Zenodo: 10.5281/zenodo.7334422). The normalized data were analyzed in Rstudio (R version 4.0.3), using functionalities from the following packages: RVAideMemoire version 0.9–80 (https://CRAN.R-project.org/package=RVAideMemoire), vegan version 2.5–7, Hotelling version 1.0–7, pls version 2.7–3, FactoMineR71 version 2.4, and Factoextra version 1.0.6 (https://CRAN.R-project.org/package=factoextra). The statistical significance of CHC profile differences between groups was assessed with a powered partial least squares discriminant analysis (PPLS-DA) and applying pairwise permutation tests72,73. In order to test the effect of RNAi of elongases on CHC profiles, we plotted the chain lengths of cuticular hydrocarbons (CHCs) positively associated (threshold = 0.5, CHC plots available on Zenodo: 10.5281/zenodo.7334422) in a PPLS-DA with the first principal component (Fig. 5: correlation circle plots) separating bees treated with dsRNA targeting fatty acid elongases (GB51247, GB51250, GB54397, GB54404) or a fatty acid hydrolase (GB53695) and with bees treated with dsRNA targeting GFP (control).
Differences in chain lengths and the relative abundance of specific alkenes were assessed with Welch’s t-test or with the Wilcoxon signed-rank test, depending on whether or not the data appeared normally distributed (assessed with the Shapiro-Wilk test), using the stats package version 3.6.2 in Rstudio. P-values were corrected with the Holm-Bonferroni method for multiple testing. Boxplots were drawn with the ggplot2 package (https://ggplot2.tidyverse.org) in Rstudio.
We extracted the total RNA from the metasoma of each bee treated with dsRNA using RNeasy Plus Mini kit (QIAGEN, Hilden, Germany) and quantified the relative expression of the target genes via real-time quantitative PCR (RT-qPCR) using the QuantiTect SYBR Green RT-PCR kit (QIAGEN, Hilden, Germany) and a StepOnePlus Real-Time PCR System (Applied Biosystems-Life technologies, ThermoFisher Scientific, Waltham, Massachusetts, USA). We quantified expression levels in at least five samples per group (i.e., treatment and control) and in three technical replicates per sample. RT-qPCR oligonucleotide primers used to assess the target genes’ expression levels are listed in Supplementary Table 12 and were designed with Primer-BLAST66. If possible, oligonucleotides were designed so that the resulting amplicon was 150–250 bp in length, that the oligonucleotides’ melting temperature was between 57 and 63 °C, that the oligonucleotides would span exon-exon junctions, that the amplified region was localized at the 5’ to the dsRNA target region74, and that the amplified region was not overlapping with the dsRNA target region. We used GB49975 (predicted ribosomal protein L1) as reference gene (Supplementary Table 11), because its O. spinipes ortholog was one of the genes that exhibited the least expression differences between females with different chemotype. All oligonucleotide primer pairs exhibited an amplification efficiency of more than 95%, and each amplicon showed a single melting temperature maximum. The expression data were analyzed following the protocol given by Pfaffl, 200175. Gene expression levels were statistically assessed with the Welch’s t-test and with the Wilcoxon signed-rank test, as described above. P-values were corrected with the Benjamini-Hochberg method for multiple testing.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
We are indebted to Karl-Heinz Schmalz and Wolf-Harald Liebig for providing us O. spinipes samples and to Jean-Yves Baugnée for providing information where to find O. spinipes in Belgium. We thank Michael Schnell, Harald Noeske, and Rainer Blum for help with building trap nests. We are thankful to the following people for fruitful discussions, help in the lab, and/or with handling bees and wasps: Rabea Blümel, Dr. Nicolas Braseroa, Dr. Ruth Castillo, Dr. Laura Degirmenci, Janina Diehl, Claudia Etzbauer, Dr. Hermes Escalona, Martin Gabel, Dr. Ewald Grosse-Wilde, Wolf Haberer, Sinan Kaya-Zeeb, Sabine Knaup, Sandra Kukowka, Ruth Lieberth, Dr. Jana Maehner, Dr. Baptiste Martinet, Dr. Christine Missbach, Karin Möller, Maya Nakajima, Dr. Fabian Rüdenauer, Florentine Schaub, Felix Schilcher, Doris Waffler, Katharina Wagler. We are thankful to Mareen Geyer for help with analyzing CHC profile data. We are grateful to Dr. Daniel Elsner, Dr. Jose Manuel Monroy Kuhn, and Dr. Karen Meusemann for their support regarding differential gene expression analyses. We thank Prof. Dr. Judith Korb and PD Dr. Volker Nehring for advice regarding statistical analyses, and we thank Dr. Thomas Pauli for bioinformatic support. We acknowledge support by the Freiburg Galaxy Team: (i.e., Prof. Rolf Backofen, Bérénice Batut, Bjoern Gruening, Torsten Houwaart), funded by the Collaborative Research Centre 992 Medical Epigenetics (DFG grant SFB 992/1 2012) and German Federal Ministry of Education and Research (BMBF grant 031 A538A RBC). We acknowledge the Struktur- und Genehmigungsbehörde Süd and the Struktur- und Genehmigungsdirektion Nord (both Rhineland Palatinate, Germany) for granting permission to collect samples and Gaby Schöning for granting permission to place trap nests at a bee hotel in Büchelberg. Major parts of the present study were funded by the German Research Foundation (DFG; NI1387/2-1, SCHM 2645/6-1).
Author contributions
O.N., T.S., and V.C.M. conceived and designed the project. A.D., B.M., J.W., M.P., O.N., and S.M. sequenced, assembled, and annotated the Odynerus spinipes genome. J.P.O. and V.C.M. performed the comparative transcriptomic analyses. L.P. and V.C.M. inferred the gene trees. D.L. and V.C.M. designed the in situ hybridization experiments. M.T., O.N., R.S., T.S., and V.C.M. contributed to the design of the knockdown experiments. V.C.M. conducted the chemotype persistency, the in situ hybridization, the knockdown experiments, and all chemical and statistical analyses. D.L., O.N., R.S., T.S. provided instruments and reagents. O.N. and V.C.M. wrote the manuscript, with contributions from T.S. All authors provided feedback on the manuscript.
Peer review
Peer review information
Communications Biology thanks Henry Chung and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Luciano Matzkin and Luke R. Grinham. Peer reviewer reports are available.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Data availability
The nucleotide sequence data of the genome shotgun project are deposited under the NCBI Bioproject PRJNA735081. The draft genome assembly and the corresponding gene annotations are available under the NCBI accession JAIFRP000000000 and from Zenodo (10.5281/zenodo.7334422). The transcriptomic raw reads are available from GenBank in Bioproject PRJNA609595. Additional data that support the findings of this study have been deposited on Zenodo (10.5281/zenodo.7334422).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Victoria C. Moris, Email: victoria.carla.moris@gmail.com
Oliver Niehuis, Email: oliver.niehuis@biologie.uni-freiburg.de.
Supplementary information
The online version contains supplementary material available at 10.1038/s42003-022-04370-0.
References
- 1.Lockey KH. Lipids of the insect cuticle: origin, composition and function. Comp. Biochem. Physiol. B: Comp. Biochem. 1988;89:595–645. [Google Scholar]
- 2.Blomquist, G. J. & Bagnères, A. G. Insect hydrocarbons: biology, biochemistry, and chemical ecology. (Cambridge University Press, 2010).
- 3.Nelson, D. R. & Blomquist, G. J. Insect waxes. in Waxes: Chemistry, Molecular Biology and Functions (Hamilton, R. J. ed.). 1-90 (Oily Press, 1995).
- 4.Makki R, Cinnamon E, Gould AP. The development and functions of oenocytes. Annu. Rev. Entomol. 2014;59:405–425. doi: 10.1146/annurev-ento-011613-162056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Juarez P, Chase J, Blomquist GJ. A microsomal fatty acid synthetase from the integument of Blattella germanica synthesizes methyl-branched fatty acids, precursors to hydrocarbon and contact sex pheromone. Arch. Biochem. Biophys. 1992;293:333–341. doi: 10.1016/0003-9861(92)90403-j. [DOI] [PubMed] [Google Scholar]
- 6.Reed JR, Quilici DR, Blomquist GJ, Reitz RC. Proposed mechanism for the cytochrome P 450-catalyzed conversion of aldehydes to hydrocarbons in the house fly, Musca domestica. Biochemistry. 1995;34:16221–16227. doi: 10.1021/bi00049a038. [DOI] [PubMed] [Google Scholar]
- 7.Gu P, Welch WH, Guo L, Schegg KM, Blomquist GJ. Characterization of a novel microsomal fatty acid synthetase (FAS) compared to a cytosolic FAS in the housefly, Musca domestica. Comp. Biochem. Physiol. B: Biochem. Mol. Biol. 1997;118:447–456. doi: 10.1016/s0305-0491(97)00112-0. [DOI] [PubMed] [Google Scholar]
- 8.Qiu Y, et al. An insect-specific P450 oxidative decarbonylase for cuticular hydrocarbon biosynthesis. Proc. Natl Acad. Sci. USA. 2012;109:14858–14863. doi: 10.1073/pnas.1208650109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chung H, Carroll SB. Wax, sex and the origin of species: dual roles of insect cuticular hydrocarbons in adaptation and mating. BioEssays. 2015;37:822–830. doi: 10.1002/bies.201500014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holze H, Schrader L, Büllesbach J. Advances in deciphering the genetic basis of insect cuticular hydrocarbon biosynthesis and variation. Heredity. 2021;126:219–234. doi: 10.1038/s41437-020-00380-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calla B, et al. Functional characterization of CYP4G11—a highly conserved enzyme in the western honey bee Apis mellifera. Insect Mol. Biol. 2018;27:661–674. doi: 10.1111/imb.12516. [DOI] [PubMed] [Google Scholar]
- 12.Helmkampf M, Cash E, Gadau J. Evolution of the insect desaturase gene family with an emphasis on social Hymenoptera. Mol. Biol. Ecol. 2015;32:456–471. doi: 10.1093/molbev/msu315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomas ML, Simmons LW. Cuticular hydrocarbons are heritable in the cricket Teleogryllus oceanicus. J. Evol. Biol. 2008;21:801–806. doi: 10.1111/j.1420-9101.2008.01514.x. [DOI] [PubMed] [Google Scholar]
- 14.Wurdack M, et al. Striking cuticular hydrocarbon dimorphism in the mason wasp Odynerus spinipes and its possible evolutionary cause (Hymenoptera: Chrysididae, Vespidae) Proc. R. Soc. B: Biol. Sci. 2015;282:20151777. doi: 10.1098/rspb.2015.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moris VC, et al. Cuticular hydrocarbons on old museum specimens of the spiny mason wasp, Odynerus spinipes (Hymenoptera: Vespidae: Eumeninae), shed light on the distribution and on regional frequencies of distinct chemotypes. Chemoecology. 2021;31:311–322. [Google Scholar]
- 16.Wicker-Thomas C, et al. Flexible origin of hydrocarbon/pheromone precursors in Drosophila melanogaster. J. Lipid Res. 2015;56:2094–2101. doi: 10.1194/jlr.M060368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dallerac R, et al. A delta 9 desaturase gene with a different substrate specificity is responsible for the cuticular diene hydrocarbon polymorphism in Drosophila melanogaster. Proc. Natl Acad. Sci. USA. 2000;97:9449–9454. doi: 10.1073/pnas.150243997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chertemps T, Duportets L, Labeur C, Ueyama M, Wicker‐Thomas C. A female‐specific desaturase gene responsible for diene hydrocarbon biosynthesis and courtship behaviour in Drosophila melanogaster. Insect Mol. Biol. 2006;15:465–473. doi: 10.1111/j.1365-2583.2006.00658.x. [DOI] [PubMed] [Google Scholar]
- 19.Dembeck LM, et al. Genetic architecture of natural variation in cuticular hydrocarbon composition in Drosophila melanogaster. eLife. 2015;4:e09861. doi: 10.7554/eLife.09861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chertemps T, et al. A female-biased expressed elongase involved in long-chain hydrocarbon biosynthesis and courtship behavior in Drosophila melanogaster. Proc. Natl Acad. Sci. USA. 2007;104:4273–4278. doi: 10.1073/pnas.0608142104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tupec M, et al. Expansion of the fatty acyl reductase gene family shaped pheromone communication in Hymenoptera. eLife. 2019;8:e39231. doi: 10.7554/eLife.39231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Szafer-Glusman E, et al. A role for very-long-chain fatty acids in furrow ingression during cytokinesis in Drosophila spermatocytes. Curr. Biol. 2008;18:14261431. doi: 10.1016/j.cub.2008.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sapountzis P, et al. New insight into the RNA interference response against cathepsin-L gene in the pea aphid, Acyrthosiphon pisum: molting or gut phenotypes specifically induced by injection or feeding treatments. Insect Biochem. Mol. 2014;51:20–32. doi: 10.1016/j.ibmb.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 24.Jakobsson A, Westerberg R, Jacobsson A. Fatty acid elongases in mammals: their regulation and roles in metabolism. Prog. Lipid Res. 2006;45:237–249. doi: 10.1016/j.plipres.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 25.Pei XJ, et al. Modulation of fatty acid elongation in cockroaches sustains sexually dimorphic hydrocarbons and female attractiveness. PLoS Biol. 2021;19:e3001330. doi: 10.1371/journal.pbio.3001330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shirangi TR, Dufour HD, Williams TM, Carroll SB. Rapid evolution of sex pheromone-producing enzyme expression in Drosophila. PLoS Biol. 2009;7:e1000168. doi: 10.1371/journal.pbio.1000168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McKinney MK, Cravatt BF. Structure and function of fatty acid amide hydrolase. Annu. Rev. Biochem. 2005;74:411–432. doi: 10.1146/annurev.biochem.74.082803.133450. [DOI] [PubMed] [Google Scholar]
- 28.Finet C, Slavik K, Pu J, Carroll SB, Chung H. Birth-and-death evolution of the fatty acyl-CoA reductase (FAR) gene family and diversification of cuticular hydrocarbon synthesis in Drosophila. Genome Biol. Evol. 2019;11:1541–1551. doi: 10.1093/gbe/evz094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pei XJ, et al. BgFas1: A fatty acid synthase gene required for both hydrocarbon and cuticular fatty acid biosynthesis in the German cockroach, Blattella germanica (L.) Insect Biochem. Mol. Biol. 2019;112:103203. doi: 10.1016/j.ibmb.2019.103203. [DOI] [PubMed] [Google Scholar]
- 30.Grigoraki L, Grau-Bové X, Yates HC, Lycett GJ, Ranson H. Isolation and transcriptomic analysis of Anopheles gambiae oenocytes enables the delineation of hydrocarbon biosynthesis. eLife. 2020;9:e58019. doi: 10.7554/eLife.58019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moriconi DE, Dulbecco AB, Juárez MP, Calderón‐Fernández GM. A fatty acid synthase gene (FASN3) from the integument tissue of Rhodnius prolixus contributes to cuticle water loss regulation. Insect Mol. Biol. 2019;28:850–861. doi: 10.1111/imb.12600. [DOI] [PubMed] [Google Scholar]
- 32.Finck J, Berdan EL, Mayer F, Ronacher B, Geiselhardt S. Divergence of cuticular hydrocarbons in two sympatric grasshopper species and the evolution of fatty acid synthases and elongases across insects. Sci. Rep. 2016;6:1–13. doi: 10.1038/srep33695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grinsted L, Bilde T, d’Ettorre P. Cuticular hydrocarbons as potential kin recognition cues in a subsocial spider. Behav. Ecol. 2011;22:1187–1194. [Google Scholar]
- 34.de Carvalho Guimarães I, Cardoso CAL, Caramão EB, Schneider JK. & Junior, W. F. A. Analysis of cuticular chemical profiles of Latrodectus geometricus (Araneae: Theridiidae) females and juveniles using GC× GC/qMS. Ciência e. Natura. 2018;40:1. [Google Scholar]
- 35.Oudejans RCHM, Zandee DI. The biosynthesis of the hydrocarbons in males and females of the millipede Graphidostreptus tumuliporus. J. Insect Physiol. 1973;19:2245–2253. [Google Scholar]
- 36.Zhang D, Terschak JA, Harley MA, Lin J, Hardege JD. Simultaneously hermaphroditic shrimp use lipophilic cuticular hydrocarbons as contact sex pheromones. PLoS One. 2011;6:e17720. doi: 10.1371/journal.pone.0017720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Copeman LA, et al. Total lipids, lipid classes, and fatty acids of newly settled red king crab (Paralithodes camtschaticus): comparison of hatchery-cultured and wild crabs. J. Shellfish Res. 2012;31:153–165. [Google Scholar]
- 38.Romer F, Gnatzy W. Arachnid oenocytes: ecdysone synthesis in the legs of harvestmen (Opilionidae) Cell Tissue Res. 1981;216:449–453. doi: 10.1007/BF00233631. [DOI] [PubMed] [Google Scholar]
- 39.Fontanetti CS, Camargo-Mathias MI, Tiritan B. The fat body in Rhinocricus padbergi (Diplopoda, Spirobolida. Iheringia. Série Zoologia. 2004;94:351–355. [Google Scholar]
- 40.Symonová R, Smrž J. First record of hemocytes and oenocytes in freshwater ostracodes. J. Crustac. 2009;29:18–25. [Google Scholar]
- 41.Feyereisen R. Origin and evolution of the CYP4G subfamily in insects, cytochrome P450 enzymes involved in cuticular hydrocarbon synthesis. Mol. Phylogenet. Evol. 2020;143:106695. doi: 10.1016/j.ympev.2019.106695. [DOI] [PubMed] [Google Scholar]
- 42.Nelson DR. Cytochrome P450 diversity in the tree of life. Biochim. Biophys. Acta. 2018;1866:141–154. doi: 10.1016/j.bbapap.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kather R, Martin SJ. Evolution of cuticular hydrocarbons in the Hymenoptera: a meta-analysis. J. Chem. Ecol. 2015;41:871–883. doi: 10.1007/s10886-015-0631-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Piek T. Synthesis of wax in the honeybee (Apis mellifera L.) J. Insect Physiol. 1964;10:563–572. [Google Scholar]
- 45.Katase H, Chino H. Transport of hydrocarbons by haemolymph lipophorin in Locusta migratoria. Insect Biochem. 1984;14:1–6. [Google Scholar]
- 46.Billeter JC, Atallah J, Krupp JJ, Millar JG, Levine JD. Specialized cells tag sexual and species identity in Drosophila melanogaster. Nature. 2009;461:987–991. doi: 10.1038/nature08495. [DOI] [PubMed] [Google Scholar]
- 47.Savarit F, Ferveur JF. Genetic study of the production of sexually dimorphic cuticular hydrocarbons in relation with the sex-determination gene transformer in Drosophila melanogaster. Genet. Res. 2002;79:23–40. doi: 10.1017/s0016672301005481. [DOI] [PubMed] [Google Scholar]
- 48.Altenhoff AM, Studer RA, Robinson-Rechavi M, Dessimoz C. Resolving the ortholog conjecture: orthologs tend to be weakly, but significantly, more similar in function than paralogs. PLoS Comput. Biol. 2012;8:e1002514. doi: 10.1371/journal.pcbi.1002514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beckmann HE. Beeinflussung des Gedächtnisses der Honigbiene durch Narkose, Kühlung und Streß. J. Comp. Physiol. 1974;94:249–266. [Google Scholar]
- 50.Kovats VE. Gas‐chromatographische charakterisierung organischer Verbindungen. Teil 1: Retentionsindices aliphatischer Halogenide, Alkohole, Aldehyde und Ketone. Helv. Chim. Acta. 1958;41:1915–1932. [Google Scholar]
- 51.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kajitani R, et al. Efficient de novo assembly of highly heterozygous genomes from whole-genome shotgun short reads. Genome Res. 2014;24:1384–1395. doi: 10.1101/gr.170720.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hoff KJ, Lange S, Lomsadze A, Borodovsky M, Stanke M. BRAKER1: unsupervised RNA-Seq-based genome annotation with GeneMark-ET and AUGUSTUS. Bioinformatics. 2016;32:767–769. doi: 10.1093/bioinformatics/btv661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Krupp JJ, et al. Social experience modifies pheromone expression and mating behavior in male Drosophila melanogaster. Curr. Biol. 2008;18:1373–1383. doi: 10.1016/j.cub.2008.07.089. [DOI] [PubMed] [Google Scholar]
- 55.Dobin A, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liao Y, Smyth GK, Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014;30:923–930. doi: 10.1093/bioinformatics/btt656. [DOI] [PubMed] [Google Scholar]
- 57.Love MI, Anders S, Huber W. Differential analysis of count data – the DESeq2 package. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Finn RD, et al. The Pfam protein families database: towards a more sustainable future. Nucleic Acids Res. 2016;44:D279–D285. doi: 10.1093/nar/gkv1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Eddy SR. Accelerated Profile HMM Searches. PloS Comput. Biol. 2011;7:e1002195. doi: 10.1371/journal.pcbi.1002195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nguyen LT, Schmidt HA, Von Haeseler A, Minh BQ. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015;32:268–274. doi: 10.1093/molbev/msu300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Camacho C, et al. “BLAST+: architecture and applications.”. BMC Bioinforma. 2008;10:421. doi: 10.1186/1471-2105-10-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Johnson M, et al. NCBI BLAST: a better web interface. Nucleic Acids Res. 2008;36:W5–W9. doi: 10.1093/nar/gkn201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thisse C, Thisse B. High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat. Protoc. 2008;3:59–69. doi: 10.1038/nprot.2007.514. [DOI] [PubMed] [Google Scholar]
- 66.Ye J, et al. Primer-BLAST: a tool to design target-specific primers for polymerase chain reaction. BMC Bioinforma. 2012;13:134. doi: 10.1186/1471-2105-13-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dearden PK, Duncan EJ, Wilson MJ. Fixation and storage of honeybee (Apis mellifera) tissues. Cold Spring Harb. Protoc. 2009;2009:pdb-prot5224. doi: 10.1101/pdb.prot5224. [DOI] [PubMed] [Google Scholar]
- 68.Dearden PK, Duncan EJ, Wilson MJ. Whole-mount in situ hybridization of honeybee (Apis mellifera) tissues. Cold Spring Harb. Protoc. 2009;2009:pdb-prot5225. doi: 10.1101/pdb.prot5225. [DOI] [PubMed] [Google Scholar]
- 69.Roma GC, Bueno OC, Camargo-Mathias MI. Morpho-physiological analysis of the insect fat body: a review. Micron. 2010;41:395–401. doi: 10.1016/j.micron.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 70.Weinstock GM, et al. Insights into social insects from the genome of the honeybee Apis mellifera. Nature. 2006;443:931–949. doi: 10.1038/nature05260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lê S, Josse J, Husson F. FactoMineR: an R package for multivariate analysis. J. Stat. Softw. 2008;25:1–18. [Google Scholar]
- 72.Brereton RG, Lloyd GR. Partial least squares discriminant analysis: taking the magic away. J. Chemom. 2014;28:213–225. [Google Scholar]
- 73.Liland KH, Indahl UG. Powered partial least squares discriminant analysis. J. Chemom. 2009;23:7–18. [Google Scholar]
- 74.Mainland RL, Lyons TA, Ruth MM, Kramer JM. Optimal RNA isolation method and primer design to detect gene knockdown by qPCR when validating Drosophila transgenic RNAi lines. BMC Res. Notes. 2017;10:647. doi: 10.1186/s13104-017-2959-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pfaffl MW. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001;29:E45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The nucleotide sequence data of the genome shotgun project are deposited under the NCBI Bioproject PRJNA735081. The draft genome assembly and the corresponding gene annotations are available under the NCBI accession JAIFRP000000000 and from Zenodo (10.5281/zenodo.7334422). The transcriptomic raw reads are available from GenBank in Bioproject PRJNA609595. Additional data that support the findings of this study have been deposited on Zenodo (10.5281/zenodo.7334422).