Abstract
The estrous cycle is a potent modulator of neuron physiology. In rodents, in vivo ventral tegmental area (VTA) dopamine (DA) activity has been shown to fluctuate across the estrous cycle. Although the behavioral effect of fluctuating sex steroids on the reward circuit is well studied in response to drugs of abuse, few studies have focused on the molecular adaptations in the context of stress and motivated social behaviors. We hypothesized that estradiol fluctuations across the estrous cycle acts on the dopaminergic activity of the VTA to alter excitability and stress response. We used whole-cell slice electrophysiology of VTA DA neurons in naturally cycling, adult female C57BL/6J mice to characterize the effects of the estrous cycle and the role of 17β-estradiol on neuronal activity. We show that the estrous phase alters the effect of 17β-estradiol on excitability in the VTA. Behaviorally, the estrous phase during a series of acute variable social stressors modulates subsequent reward-related behaviors. Pharmacological inhibition of estrogen receptors in the VTA before stress during diestrus mimics the stress susceptibility found during estrus, whereas increased potassium channel activity in the VTA before stress reverses stress susceptibility found during estrus as assessed by social interaction behavior. This study identifies one possible potassium channel mechanism underlying the increased DA activity during estrus and reveals estrogen-dependent changes in neuronal function. Our findings demonstrate that the estrous cycle and estrogen signaling changes the physiology of DA neurons resulting in behavioral differences when the reward circuit is challenged with stress.
SIGNIFICANCE STATEMENT The activity of the ventral tegmental area encodes signals of stress and reward. Dopaminergic activity has been found to be regulated by both local synaptic inputs as well as inputs from other brain regions. Here, we provide evidence that cycling sex steroids also play a role in modulating stress sensitivity of dopaminergic reward behavior. Specifically, we reveal a correlation of ionic activity with estrous phase, which influences the behavioral response to stress. These findings shed new light on how estrous cycle may influence dopaminergic activity primarily during times of stress perturbation.
Keywords: dopamine, estrogen, estrous cycle, potassium channel, stress, ventral tegmental area
Introduction
With the onset of puberty and fluctuating sex steroids, sex differences in the prevalence of dopamine (DA)-related disorders emerge (Weissman et al., 1996; Salk et al., 2017; Eid et al., 2019). Mood disorders like major depressive disorder (MDD) are diagnosed twice as frequently in females starting in adolescence (Kessler et al., 1994). Further, 3–8% of reproductive cycling females meet the criteria for premenstrual dysphoric disorder (PMDD), which is typified by the shared symptomology of depression and irritability (Yonkers et al., 2008). Depression research has revealed biological neuronal adaptations in the mesolimbic circuit (Nestler et al., 2002; Russo et al., 2012; Russo and Nestler, 2013). Despite the sex difference in the prevalence of MDD and the occurrence of PMDD, rodent models of depressive behaviors have historically used male subjects to determine the neuronal substrates underlying stress susceptibility and depression (Shansky and Woolley, 2016). These studies show that alterations in the firing patterns of DA neurons in the ventral tegmental area (VTA) occur after chronic stress through changes in excitability and potassium (K+) channel activity (Krishnan et al., 2007; Cao et al., 2010; Chaudhury et al., 2013; Friedman et al., 2014). These adaptations are crucial in regulating a healthy stress response and determining a behavioral response to stress (Friedman et al., 2016). As the frequency differences in MDD diagnosis and PMDD diagnosis emerge with the onset of puberty and end after menopause (Salk et al., 2017), circulating sex steroids, such as estrogens and progestogens, are currently under investigation for their role in regulating the behavioral response to stress (Bangasser and Valentino, 2014; Newhouse and Albert, 2015).
Given the research on the interaction between sex steroids and the reward system, understanding the functional and ionic differences across the phases of estrous in naturally cycling female rodents is translationally valuable. Sex steroids can be potent modulators of neuronal excitability throughout the brain and can have rapid and long-lasting effects on intrinsic and synaptic mechanisms of neuronal activity (McEwen and Milner, 2017). Interestingly, many ion channels that are involved in the stress adaptation response in the male VTA, such as HCN (hyperpolarization-activated cyclic nucleotide-gated channels) and potassium channels, are well known to be targets of estrogen signaling throughout the brain (Kow and Pfaff, 2016). Moreover, a large population of VTA DA neurons also express estrogen receptors (ERs; Kritzer, 1997; Milner et al., 2010; Vandegrift et al., 2020), although the effects of estrogen signaling on the ionic mechanisms underlying intrinsic excitability have yet to be characterized in the VTA.
More broadly, the impact of estrogen signaling on neuronal excitability is diverse and region specific, even having opposing effects on different cell types within the hypothalamus (Kelly and Rønnekleiv, 2015; Kow and Pfaff, 2016). Previous research has shown that the magnitude of DA release in the striatum and prefrontal cortex is influenced by estrous cycle and estrogen treatment (McDermott et al., 1994; Dazzi et al., 2007; Almey et al., 2015; Calipari et al., 2017). This suggests that the estrous cycle, and estrogen signaling specifically, are important modulators of DA neuron activity and the reward system as a whole. Using a 1 d acute variable stress model, which consists of five mild stressors, we explored estrous cycle differences in the stress response. We propose that the ionic differences recorded in the naturally cycling females are associated with the differences in the stress response. In this article, we demonstrate changes in DA neuron activity across the estrous cycle, establish the role of estrogen signaling in modulating the ionic mechanisms of excitability, and behaviorally assess the interaction between stress acquisition and estrogen signaling in the VTA.
Materials and Methods
Animals
Female C57BL6/J mice were purchased from The Jackson Laboratory at 7 weeks of age and acclimated for at 1 week in the animal facility before use in behavioral experiments or surgeries. Mice were kept in a 12 h light/dark cycle (lights on at 08:00) in corncob bedding and fed regular mouse chow ad libitum. Animals used for electrophysiology experiments were housed in groups of 2–4 individuals to reduce the effect of single house stress. Animals used for stress were weighed and singly housed the day before the beginning of stress. Animals were killed for electrophysiology experiments between 09:00 and 10:00. Stress acquisition and behavior experiments began between 09:00 and 10:00, and continued until completion at around 13:00. All females were monitored for estrous cycle on testing day after all behavior was completed and on the following 2 d, or on the morning (9:00–10:00 A.M.) of slice electrophysiology. Ten to 20 microliters of PBS was gently washed into the opening of the vagina and collected using a P200 pipettor. Samples were immediately visualized under 10× to 40× magnification, and photographs were captured using an iPhone camera for future reference (Pantier et al., 2019). Estrous stage was determined based on presence, absence and relative quantities of cornified epithelial cells, nucleated epithelial cells and leucocytes, and estrous stage on prior day or days (Fig. 1). Briefly, diestrus is characterized by the abundance of leucocytes (more than 80%), proestrus is characterized by an abundance of nucleated epithelial cells (more than 80%), metestrus is characterized by ∼50% cornified epithelial cells and 50% leukocytes, and estrus is characterized by having primarily cornified epithelial cells, with no visible nucleus (more than 80%). Pseudopregnancy induced by repeated stimulation of the vagina and cervix from repeated vaginal lavage, defined by extended presence of leucocytes, was not detected. Cell cytology was the primary method of determining estrous cycle stage and is based on the proportion of the cell types. For terminal slice electrophysiology experiments, estrous cycle stage was also determined by estrogen and progesterone levels determined by enzyme-linked immunoassay (ELISA) and uterine weight. Uterine weight and estrogen and progesterone levels are used to confirm estrous phase determined from cell cytology. If uterine weight and/or hormone levels did not align with vaginal smears and published data on hormone levels, these additional measurements were used to exclude animals from electrophysiology analysis (n = 3 mice excluded from analysis; Nelson et al., 1981; Nilsson et al., 2015). All animal studies were approved by the Hunter College Institutional Animal Care and Use Committee and were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Figure 1.
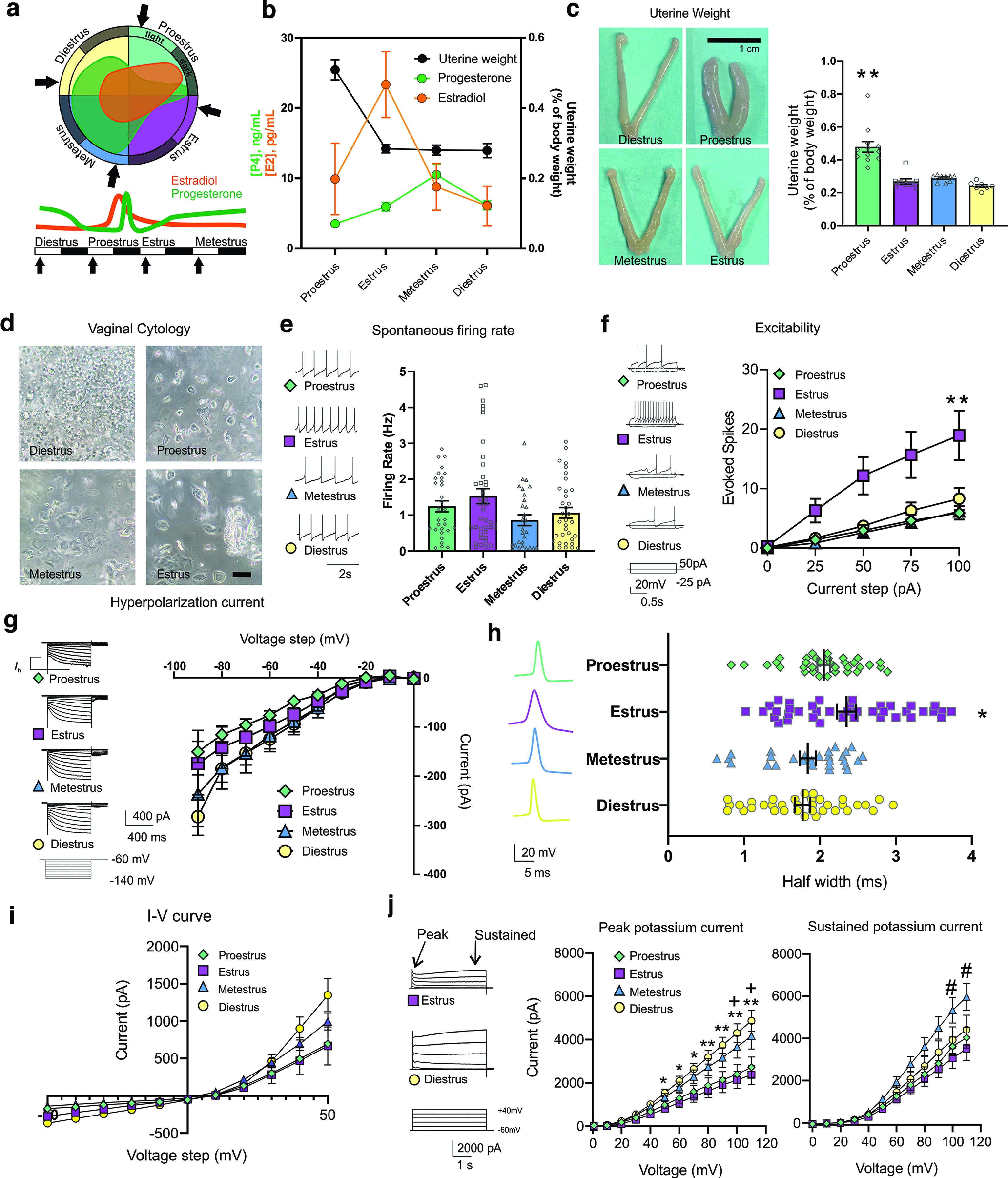
The estrous cycle modulates electrophysiological properties of VTA DA neurons. A three-pronged approach to determining estrous cycle stage was used in terminal experiments. a, A representation of the estrous cycle and relative estrogen and progesterone levels at each stage of estrous. b, Measured serum concentrations of estrogen (E2), progesterone (P4), and uterine weights at each estrous cycle stage determined by vaginal cell cytology. c, Uterine weight was significantly higher in animals during proestrus (F(3,32) = 23.52, p < 0.0001, n = 7–12 mice per group). Scale bar, 1 cm. d, Vaginal cytology showing leucocytes, nucleated epithelial cells, and cornified epithelial cells at each stage of the estrous cycle. Scale bars: 100 μm. Proestrus is typified by the presence of small, round, nucleated epithelial cells, which are often observed in clusters. Estrus was typified by the presence of anucleated keratinized epithelial cells and the absence of neutrophils. Metestrus is typified by the presence of anucleated keratinized epithelial cells and neutrophils. Diestrus is typified by the presence of primarily neutrophils. Arrows indicate time points used for electrophysiology studies. e, Spontaneous firing rate does not change across the estrous cycle (1-way ANOVA, F(3,128) = 1.88, p = 0.14, n = 28–39 cells per estrous stage, 7–12 mice per group; nested ANOVA, F(3,33) = 1.23, p = 0.32, 2–7 cells per mouse, 7–12 mice per group). f, Excitability is increased during estrus compared with proestrus and metestrus (2-way ANOVA, current injection × estrous cycle stage interaction, p < 0.05, F(12,360) = 3.28, p = 0.0002, n = 10–33 = cells per group, 7–12 mice per group; nested ANOVA, +100 pA, F(3,32) = 4.44, p = 0.006; +75 pA, F(3,32) = 4.37, p = 0.01, 2–7 cells per mouse, 7–12 mice per group). g, Ih across the estrous cycle does not change (2-way ANOVA, voltage step × cycle interaction, F(24,719) = 0.368, p = 0.99, n = 15–33 cells, 7–12 mice per group). h, Action potential width across the estrous cycle (2-way ANOVA, estrous stage vs AP width, F(3,94) = 5.044, p = 0.002, n = 28–39 cells per estrous stage, 7–12 mice per group; nested ANOVA, F(3,133)= 6.17, p = 0.0006, 2–7 cells per mouse, 7–12 mice per group). i, I–V curves obtained from neurons across the estrous cycle (2-way ANOVA, +50 pA current injection × estrous cycle stage interaction, F(3,16) = 1.427, p = 0.271, n = 5–12 = cells per group, 3 mice per group; nested ANOVA, +50 pA, F(3,8) = 1.29, p = 0.34, n = 2–4 cells per mouse, 3 mice per group). j, Peak potassium current across the estrous cycle is increased in diestrus (2-way ANOVA, voltage step × cycle interaction, F(33,594) = 4.835, p < 0.0001; multiple comparisons, *p < 0.05 **p < 0.01 between estrus and diestrus; +p < 0.05 between proestrus and diestrus; nested two-way ANOVA, significant voltage × cycle interaction, F(33,429) = 4.88, p < 0.001, 2–3 cells per mouse, 7–12 mice per group). Sustained potassium current is increased during metestrus (2-way ANOVA significant voltage × cycle interaction, F(33,550) = 2.913, p < 0.0001; multiple comparisons, #p < 0.01 between estrus and metestrus, n = 8–22 cells per phase, 7–12 mice per group; nested 2-way ANOVA, significant voltage × cycle interaction, F(33,429) = 3.35, p < 0.001, 2–3 cells per mouse, 7–12 mice per group). Error bars indicate mean ± SEM.
Slice electrophysiology
For slice preparation, coronal brain slices of VTA were prepared according to previously published protocols between 09:00 and 10:00 (Cao et al., 2010; Chaudhury et al., 2013; Friedman et al., 2016; Papouin and Haydon, 2018). Briefly, animals were anesthetized with isoflurane and transcardially perfused with ice-cold, oxygenated artificial Cerebrospinal fluid (aCSF) containing the following (in mm): 128 NaCl, 10 d-glucose, 1.25 sodium phosphate monobasic, 25 sodium bicarbonate, 2 MgCl, 3 KCl, and 2 CaCl2. The brain was extracted and placed in ice-cold oxygenated sucrose aCSF containing the following (in mm): 227 sucrose, 10 d-glucose, 1.25 sodium phosphate monobasic, 24 sodium bicarbonate, 2 MgCl, 3 KCl, and 2 CaCl2 for 1 min. Brain tissue was blocked for the region of interest using a sharp razor blade, then sliced using a DSK microslicer (Ted Pella) in ice-cold sucrose aCSF solution under constant oxygenation. Two-hundred-fifty micron slices containing the region of interest were collected and allowed to recover for 1 h at 37°C in aCSF with constant oxygenation. The slice recovery chamber was removed from the water bath after 1 h and set aside at room temperature until collection for recording. Brain slices were transferred into the recording chamber with a constant flow rate of 3 ml per minute of oxygenated (95% O2 and 5% CO2) aCSF at 35°C.
All recordings were conducted blind to the estrous stage. The timing and sequence of recordings were consistent throughout recording, with all recordings occurring between 9:00 and 10:00 A.M. to ensure consistency of the estrous phase. Putative DA neurons were identified by their location, infrared differential interference contrast microscopy, electrophysiological criteria including waveform, and the presence of hyperpolarization-activated current (Ih), and regular spontaneous firing as previously described (Ungless et al., 2003; Cao et al., 2010; Iñiguez et al., 2010). Recordings were primarily done in the lateral VTA, where the highest population of tyrosine hydroxylase positive neurons are located (Lammel et al., 2011; Yamaguchi et al., 2011). The presence of an Ih above 80 pA and triphasic action potential, with a long duration (>2.0 ms) was required for inclusion. Further, neurons were selected for an action potential width from start to negative trough ≥1.1 and firing rate below 10 Hz. Neurons that did not fit this criteria were not included in the analysis, thus eliminating GABA neurons and restricting the data to a subset of dopaminergic neurons. Previous research has indicated that although a majority of dopamine neurons express an Ih, there is a subpopulation that does not, and they are not included in this study (Margolis et al., 2008). Additionally, a small percentage of neurons has been identified to express Ih, but not DA, allowing for the possibility that some portion of neurons identified as dopaminergic is not correct. To overcome this, we have ensured having a large enough sample size.
Whole-cell recordings
A gigaohm seal was obtained using a 3–6 MΩ pipette filled with standard internal solution containing the following (in mm): 115 potassium gluconate, 20 potassium chloride, 1.5 magnesium chloride, 10 phosphocreatine-tris, 2 ATP-Mg, 0.5 GTP-Na, and 10 HEPES, pH 7.21, 825 mOsm. The membrane was ruptured using pulses of fast negative suction. Membrane potential and baseline firing rate were recorded immediately after breaking through the membrane in I = 0 current-clamp configuration. Cell health and seal quality were regularly monitored using the membrane test feature of Clampex. Spontaneous action potentials (APs) were extracted from >3 min traces using the template search feature of Clampfit 9.2 software. Ih was recorded in whole-cell voltage clamp with a series of 800 ms pulses with 10 mV command voltage steps from –120 to –60 mV with a holding potential of –60 mV at the start of each recording. Once a stable baseline is achieved for 8 min, excitability was measured. Excitability measurements were recorded with a series of 2 s pulses with 25 pA command current steps from −25 to 100 pA, or 10 pA command current steps from −20 to 70 pA. Responses to intracellular current injection were averaged and used to determine the mean number of action potentials elicited per current step. I–V curves were generated from 50 ms pulses with command voltage steps from −50 to +50 mV from a holding potential of −60 mV. To isolate K+ currents, recordings were performed in a solution containing the following: 1× aCSF solution, 1 um TTX, 200 um CdCl2, 1 mm kynurenic acid, and 100 um picrotoxin perfused onto the slice for 5 min before recording. Potassium currents were recorded in whole-cell voltage clamp using a series of 4 s long depolarizing steps from −60 mV holding potential from 10 mV to +110 mV. Stock solutions of 17 β-estradiol (E2; catalog #2842, Tocris Bioscience) were first dissolved in DMSO, then diluted to a final concentration of 1 um (in 0.1% DMSO) in oxygenated aCSF. DMSO was added to control aCSF solutions at the same concentration. These solutions were used for slice recovery and were continuously perfused through the slice chamber during recordings.
Acute variable social stress
Experimental mice were exposed to each of the following stressors for 20 min each, with a 10 min break between each stressor. The following stressors specifically disturb the natural drive for female mice to safely nest and have social stability. For predator odor stress, the cotton tip of a sterile swab applicator was removed, and 2 µl of 2,5-dihydro-2,4,5-trimethylthiazoline (catalog #W332518, Sigma-Aldrich) was added to the tip. Tips were placed in the home cage of the mouse for 20 min. Control animals were kept in an adjacent room to avoid exposure to the odor (Janitzky et al., 2014). In restraint and witness to restraint stress, animals were split into two groups (group A and group B). Group A was restrained in a 50 ml conical tube with air holes drilled into the side and caps. Group A animals were placed into the home cage of group B for 20 min. On the next set of witness/restraint stress, group B was restrained and placed into the home cage of group A (Koehl et al., 2006; Sial et al., 2016). For overcrowding stress, all mice were tail marked with unique identifiers using a colored permanent marker for later identification and returned to their established home cage. Eight to ten animals were placed in a standard size cage for 20 min (Reber et al., 2006; Lin et al., 2015). In home cage instability stress, cages were placed on a 5° incline, and 800 ml of room-temperature tap water was added to the bedding (Saavedra-Rodríguez and Feig, 2013). Animals were closely monitored for signs of hypothermia. If signs of hypothermia were observed, animals were removed and placed on a heating pad to recover. After 20 min, animals were placed in a new cage with fresh bedding. Control animals were transported and handled but were placed in an adjacent room for the duration of the stressors to avoid exposure to stress pheromones and vocalizations from the stress group. Stressed animals recovered for at least 30 min before returning to the animal facility.
Social interaction test
As a measure of the rewarding nature of a social interaction, the time spent interacting with a novel social target was assessed with a social interaction test. Animals were acclimated to the testing room for 1 h in the dark before the beginning of testing. Testing was performed in the dark with a red light (<15 lux). Animals were allowed to explore a square arena for 2.5 min in the presence of a small cylindrical empty cage. Animals were removed, the arena and empty cage were cleaned, and a sex- and age-matched novel mouse was placed in the empty cage. Animals were allowed to interact with the novel mouse for 2.5 min. Velocity and location were tracked using Noldus EthoVision software.
Splash test
As a measure of self-care and motivation (Isingrini et al., 2010), the duration of time spent grooming following a spray of sucrose solution on the dorsal coat was assessed with a splash test. Animals were acclimated in red light for 1 h before testing. A 10% sucrose solution was sprayed on the dorsal coat of the mouse, and the time spent grooming and the latency to groom were recorded using Noldus EthoVision. The duration of grooming behaviors, including manual, oral, and scratching were assessed.
Stereotaxic surgeries and cannula infusions
Animals were anesthetized using a cocktail of ketamine/xylazine (2/0.2 mg/kg). Animals were head fixed in a stereotaxic apparatus (Kopf). Bilateral cannulas 4 mm long were implanted into the VTA (AP, −3.3 mm; DV, −4.4 mm; LM, ±1.05 mm from bregma), targeting the area slightly dorsal to the VTA (scaled to bregma–lambda distance; Moore and Boehm, 2009). Cannula were fixed to the scull using MetaBond dental cement (Parkell). Animals recovered for 1 week before drug infusion and were monitored for their recovery postsurgery. Pharmacological agents were first dissolved in stock solutions of DMSO, then further diluted into sterile 1× PBS solution for a final DMSO concentration of 0.1%. Vehicle solutions were 1× PBS with 0.1% DMSO. ICI 182,780 or retigabine [D-23129; N-(2-amino-4-(4-fluorobenzylamino)-phenyl) carbamic acid ethyl ester] was infused bilaterally over 5 min using a programmable syringe pump (Harvard Apparatus) at 0.1 μl/min. The following concentrations were based on previous studies: ICI 182,780, a nonspecific ER antagonist (10 µg/µl; catalog #1047, Tocris Bioscience; Fernandez et al., 2008) and retigabine, a positive allosteric modulator of noninactivating neuronal Kv7.2/Kv7.3 M-currents (5 µg/µl; Alomone Labs; Main et al., 2000; Wickenden et al., 2000). The drugs were allowed to diffuse for 3 additional minutes after infusion before the infusion cannula was removed.
Enzyme-linked immunoassay
Trunk blood was collected from eight control and twenty stressed animals [four animals per stressor, 10 min after each acute variable social stress (AVSS) stressor]. Approximately 500 μl of whole-blood samples were collected transcardially from anesthetized mice and allowed to coagulate on ice for 30 min. Samples were centrifuged at 2500 rpm for 5 min to isolate the serum. Serum samples were stored at –20°C until use in the ELISA kit (catalog #ADI-900-097, Enzo Life Sciences). The ELISA was performed following the instructions of the manufacturer for a small volume. Further, molecular measurements of estrogen (E2; Estradiol ELISA Kit, catalog #3582251, Cayman Chemical) and progesterone (Progesterone Mouse/Rat ELISA Kit, catalog #IB79183, IBL) levels were performed with commercially available low-volume ELISA kits from serum samples. Given the detection limitation of commercially available ELISA kits, we also used uterine weight, which can be precisely quantified and is directly affected by estrogen and progesterone produced by the ovaries. We assessed vaginal epithelial cells by cytological analysis over 3 consecutive days including the morning of recording and the blood serum levels and uterine weight on the day of recording. For whole-cell electrophysiology terminal experiments, we collected blood serum and the uterine weight at the time of slicing. Brain slices and trunk blood were collected in the morning.
Experimental design and statistical analyses
Analysis was performed blind to the experimental conditions, and all mice with off-target cannula implantation were removed from the study. All behaviors were scored using automated and unbiased EthoVision software. Electrophysiology data were analyzed in Clampfit 10.7 software (Molecular Devices). GraphPad Prism 9 was used for statistical analysis. Comparisons of electrophysiological characteristics between estrous cycle groups were analyzed using a Student's t test, or one- or two-way ANOVA or mixed model analysis where appropriate. Results are presented as mean ± SEM.
Results
The estrous cycle modulates VTA dopamine neuron physiology
To examine the physiology of VTA neurons across the estrous cycle we performed in vitro brain slice recordings from 36 gonadally intact, naturally cycling C57BL/6 female mice (Fig. 1a). We used vaginal cell cytology, uterine weight, and serum steroid hormone levels in a three-pronged approach to confirm estrous cycle stage (Proestrus, n = 12; Estrus, n = 8; Metestrus, n = 9; Diestrus, n = 7; Fig. 1b–d). In each animal, we performed whole-cell recordings in acute brain slices containing the VTA, selecting for neurons that exhibited an Ih as a marker for a subgroup of DA neurons. We found that estrous cycle phase had no significant effect on the average in vitro spontaneous firing rate (Fig. 1e). Despite the similar firing activity across all stages of the estrous cycle during an unperturbed state, we found a significantly higher number of action potentials are elicited from the same size current injection during estrus (Fig. 1f). Our finding of increased excitability during estrus is consistent with previous findings of increased DA bursting activity during this phase and indicates differential underlying ion channel activity across the estrous cycle (Zhang et al., 2008; Calipari et al., 2017). As excitability is dynamically governed by a combination of intrinsic ion function, synaptic efficacy, and changing behavioral states introducing a lot of variability, we next measured individual ion currents.
To gain further insight into the ionic mechanisms underlying excitability across the estrous cycle, we next performed whole-cell recordings isolating Ih. Ih is an established excitatory driving force in VTA DA neurons and drives changes in excitability in response to exposure to stress and ethanol (Neuhoff et al., 2002; Okamoto et al., 2006; Zhong et al., 2018). Despite significant differences in excitability across the estrous cycle, we found no difference in the size of Ih (Fig. 1g). The VTA appears more excitable during estrus without an increase in Ih. We next examined the action potential width and found that there are significant differences across the estrous cycle, with estrus exhibiting a wider spike shape on average compared with diestrus and metestrus (Fig. 1h). A further indication of changes in conductance across estrous was revealed with an analysis of the current-voltage (I-V) relationship (Fig. 1i; n = 5–12 cells per group, 3 mice per group).
An important modulator of DA neuron responsivity, spike shape and excitability is K+ current function, which has been shown to be rapidly modulated by estrogen signaling throughout the brain (Rønnekleiv et al., 2015; Tarfa et al., 2017). Therefore, using a series of specific channel blockers, TTX, CdCl2, kynurenic acid, and picrotoxin, we isolated and recorded potassium currents. In diestrus, when circulating steroid hormones are relatively low, the rapidly inactivating and sustained K+ currents are higher compared with estrus, a time of rapidly dropping estrogen and relatively low progesterone (Fig. 1j). The increased spike width during estrus and the relatively lower K+ channel function during estrus provides one possible ion channel mechanism for the increased excitability observed in vitro and the increased bursting previously found in vivo. During proestrus the peak and sustained K+ currents are also relatively lower, with effects on excitability likely counteracted by the changing hormonal state. Because a subset of VTA neurons express ERs, and estrogen signaling has been shown to modulate potassium conductance in other brain regions, we next examined whether the changes in excitability and K+ channel currents may be due to changes in estrogen signaling within the VTA.
Estrogen signaling contributes to estrous cycle changes in the VTA
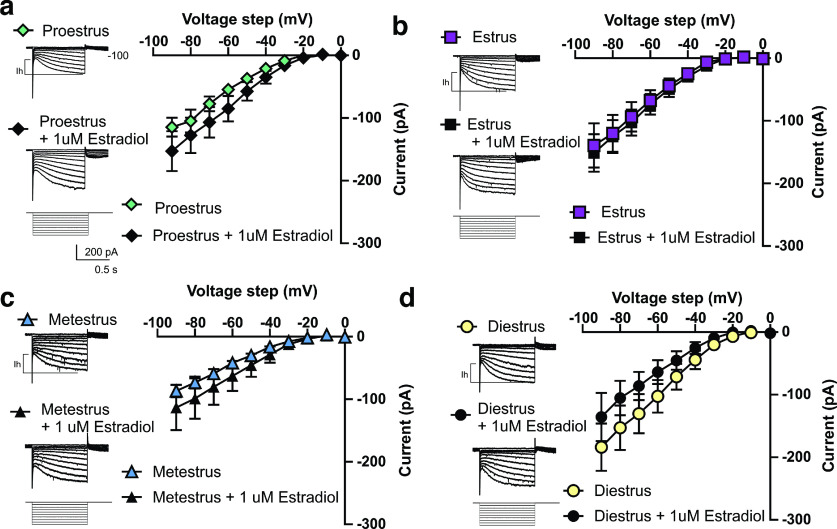
To determine the contribution of estrogen in modulating excitability, we performed a series of in vitro within-animal recordings of excitability in the presence of E2, the most prevalent naturally produced estrogen. Given that the relative ratio of fluctuating hormones is known to regulate neuronal activity, we used a within-animal experimental design to isolate the role of estrogen across the estrous cycle. To do this, we bisected each brain slice, so each half contains a hemisphere of VTA cells with the same hormonal profile, then incubated one half in aCSF with 1 μm E2 in 0.01% DMSO, and half in aCSF with 0.01% DMSO for 1 h (n = 6 mice per estrous phase). Consistent with our electrophysiological findings across the estrous cycle, we found no difference in spontaneous firing rate or Ih at any stage of the estrous cycle following estrogen incubation (Fig. 2; cells during proestrus, n = 27–34; estrus, n = 13–19; metestrus, n = 13–15; diestrus, n = 14–15; 6 mice per cycle phase). However, we revealed that the effect of E2 incubation on VTA DA neuron excitability was dependent on the phase of estrous, which is known to regulate estrogen and progesterone receptor levels (Quadros and Wagner, 2008; Mitterling et al., 2010; Liu and Shi, 2015; Vastagh and Liposits, 2017). During estrus and early proestrus, a time of relatively low progesterone and high ER activity, E2 incubation decreases excitability (Fig. 3a,b). Conversely, during metestrus, a time of relatively low estrogen and rising progesterone that downregulates ER activity, E2 incubation increased excitability (Fig. 3c). Further, during diestrus, a time of stable estrogen and progesterone, E2 incubation has no effect on excitability (Fig. 3d; cells during proestrus, n = 18–24; estrus, n = 10–16; metestrus, n =13–15; diestrus, n = 18–25; 6 mice per cycle phase). We identified an increase in excitability during estrus suggesting that DA neurons are more sensitive to perturbation during this phase, as well as a possible role of estrogen in mediating these differences. The variability in the modulation makes it clear that the changes in excitability are not solely dependent on changes in potassium channel activity and are likely counteracted by the action of progesterone and its metabolites. Together, these changes in electrophysiological responses across the phases of estrous indicate that the VTA may respond differently to incoming signals depending on the phase of estrous.
Figure 2.
Effect of estradiol incubation on Ih across the estrous cycle. a, During proestrus there was no change in Ih during estradiol incubation (2-way ANOVA, voltage step × treatment interaction F(9,531) = 0.7959, p = 0.6202, n = 27–34 cells, n = 6 mice). b, During estrus there was no change in Ih during estradiol incubation (2-way ANOVA, voltage step × treatment interaction, F(9,261) = 0.460, p > 0.9999, n = 13–19 cells, n = 6 mice). c, During metestrus there was no change in Ih during estradiol incubation (2-way ANOVA, voltage step × treatment interaction, F(9,234) = 0.50, p = 0.86, 13–15 cells, n = 6 mice). d, During diestrus there was no change in Ih during estradiol incubation (voltage step × treatment, F(9,385) = 1.021, p = 0.422, n = 14-15 cells, n = 6 mice).
Figure 3.
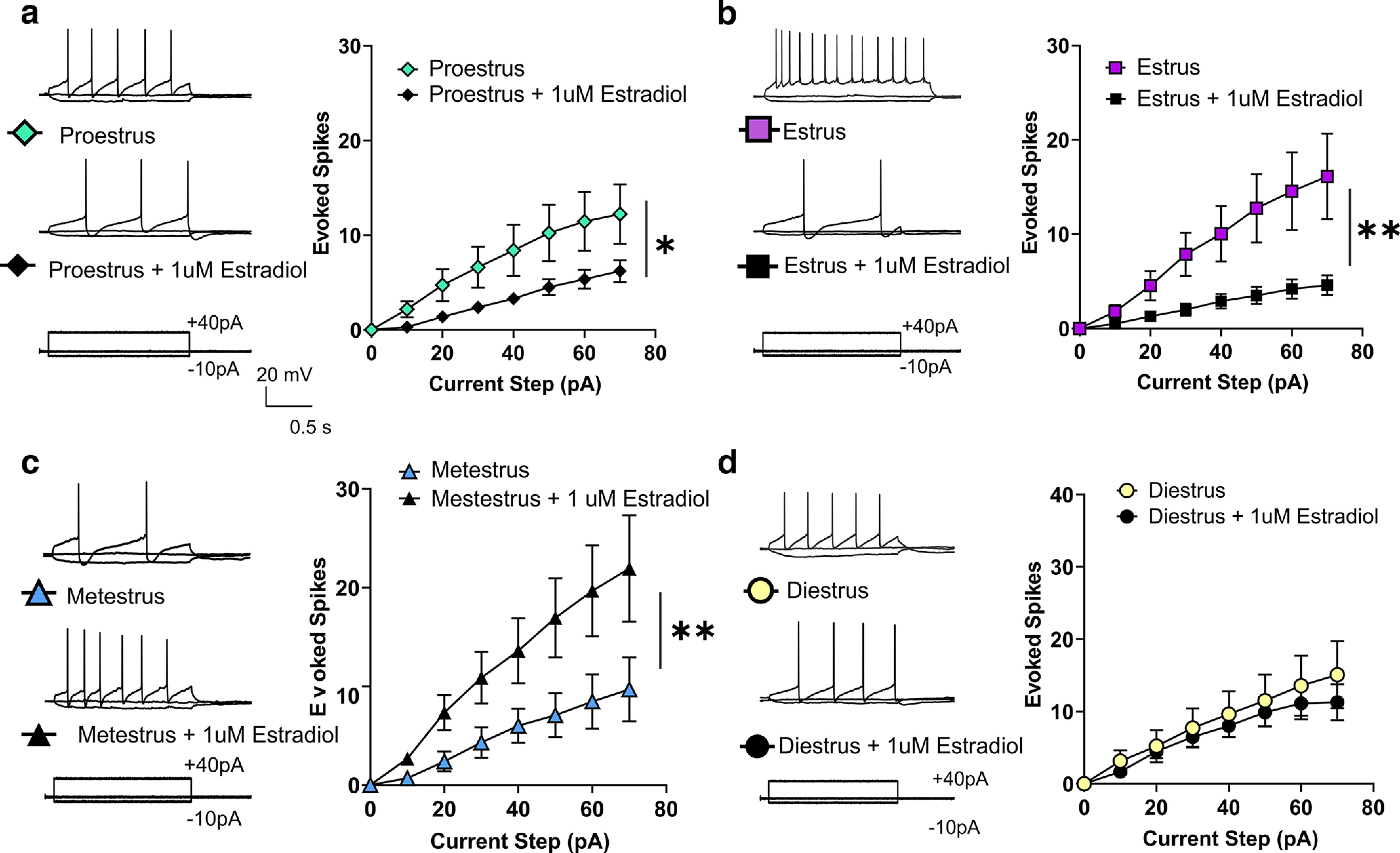
Effects of estradiol incubation and constant perfusion on VTA DA neuron excitability across the estrous cycle. a, Proestrus, estradiol incubation significantly reduces the excitability of DA neurons during proestrus (2-way ANOVA, current step × treatment interaction, F(7,280) = 3.49, p = 0.001, n = 18–24 cells, n = 6 mice). b, Estrus, estradiol incubation significantly reduces the excitability of DA neurons during estrus (2-way ANOVA, current step × treatment interaction, F(7,168) = 3.602, p = 0.001, n = 10–16 cells, n = 6 mice). c, Metestrus, estradiol treatment significantly increases excitability during metestrus (2-way ANOVA current step × treatment interaction, F(7,182) = 3.30, p = 0.003, n = 13–15 cells, n = 6 mice). d, Diestrus, estradiol incubation does not affect excitability during diestrus (2-way ANOVA, current step × treatment interaction F(7,189) = 0.363, p = 0.923, n = 18–25 cells, n = 6 mice). (*p < 0.05, **p < 0.005) Error bars indicate mean ± SEM.
Interaction of stress and estrogen signaling on social behavior
To determine whether steroid hormone signaling interacts with stress acquisition to alter social behavior in an estrous cycle-dependent manner, we examined reward-related behaviors following an acute series of stressors at different hormonal states. Stress has been previously found to alter the dopaminergic activity of the VTA (Lowes and Harris, 2022). To probe the behavioral effect of the differential ion channel function of the VTA at each individual stage of estrous we developed a subthreshold one-day stress paradigm consisting of five sex-independent stressors, called acute variable social stress (AVSS; Fig. 4a). This 1 d series of mild stressors results in significantly elevated levels of serum corticosterone (Fig. 4b) but does not result in long-term changes in social interaction behaviors. As a readout of the behavioral effect of the stress at different hormone profiles, we measured social interaction behaviors and self-grooming behavior during a splash test. The social interaction test, in which the time spent investigating an age- and sex-matched novel social target, has been reliably used as a measure of the salience of a social reward. Further social interaction behaviors have been closely linked with VTA dopaminergic activity, as well as activity in other brain regions (Krishnan et al., 2007; Chaudhury et al., 2013; Gunaydin et al., 2014). We found that female mice that undergo the subthreshold AVSS paradigm show no difference in their average social interaction time, but it does increase their time spent in the corner compared with unstressed control female mice 24 h later (Fig. 4c,d; n = 34–38 mice per group). There are no significant differences in velocity (Fig. 4e) or distance traveled (Fig. 4f). Given the behavioral variability following AVSS and our finding that DA neuron excitability is altered across the estrous cycle, we examined whether cycle phase alters social interaction behaviors. We first analyzed whether the estrous cycle phase on the day of social interaction altered the time interacting with a social target and found no significant interaction (two-way ANOVA, F(3,60) = 0.9704, p = 0.4127; data not shown). We next examined whether estrous cycle phase on the day of stress acquisition influences the resulting social interaction behaviors 24 h later. We found that females that undergo AVSS during estrus, a time of higher VTA excitability, exhibit a reduction in social interaction time with a novel social target (Fig. 4g) and an increase in the time spent in the corner (Fig. 4h) compared with estrus control (n = 7–12 mice per cycle phase). This is consistent with our electrophysiological findings in which the excitability is only increased during estrus, whereas spontaneous firing activity across the estrous cycle on average is unchanged. Further, we did not observe any significant interaction between AVSS and estrous cycle in the average velocity (Fig. 4i). In a separate cohort of mice during a splash test, mice were sprayed with a 10% sucrose solution, and time spent grooming was recorded (Fig. 5a). We found no significant difference in time spent grooming between stressed and nonstressed mice (Fig. 5b), but we did find an overall increase in the latency to groom (Fig. 5c; n = 21–22 mice per group). Although we observed no significant differences in time spent grooming across the estrous cycle in nonstressed mice, mice that underwent AVSS during estrus spent significantly less time grooming compared with the other phases of estrous (Fig. 5d; n = 5–6 mice per cycle phase).
Figure 4.
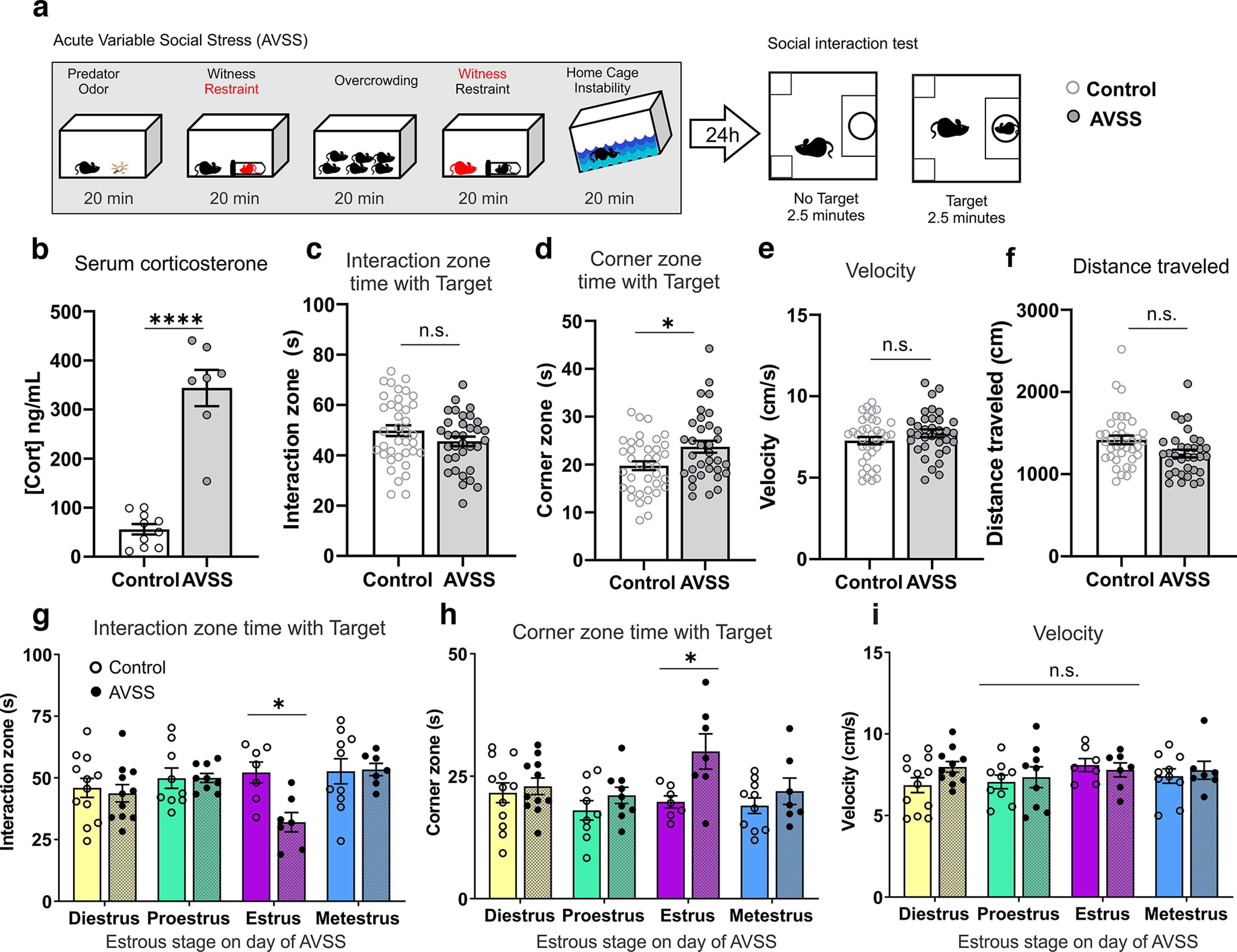
Estrous cycle stage during AVSS influences social interaction behaviors. a, Timeline of acute variable social stress and behavioral testing. b, AVSS increases serum corticosterone (Cort) levels in female mice (t(15) = 8.74, p< 0.0001). c, Social interaction time 24 h after AVSS (t(70) = 1.48, p = 0.14, n = 34–38 mice per group). d, Corner zone time is increased 24 h after AVSS (t(70) = 2.61, p = 0.01, n = 34–38). e, There is no significant effect of AVSS on average velocity with no target present (t(70) = 1.37, p = 0.17, n = 34–38). f, Distance traveled with no target present (t(70) = 2.37, p = 0.02, n = 34–38). g, Reduced social interaction time is driven by females in estrus on the day of AVSS stress acquisition (2-way ANOVA, cycle × stress interaction, F(3,64) = 2.87, p = 0.04, n = 7–12 mice per group). h, Corner zone time is significantly higher in mice that undergo AVSS during estrus compared with unstressed control (2-way ANOVA, cycle × stress interaction, F(3,64) = 3.47, p = 0.02). i, There is no significant effect of estrous phase on velocity following AVSS (2-way ANOVA, cycle × stress interaction, F(3,64) = 0.79, p = 0.51). (****p < 0.0001, *p < 0.05, n.s. = not significant) Error bars indicate mean ± SEM.
Figure 5.
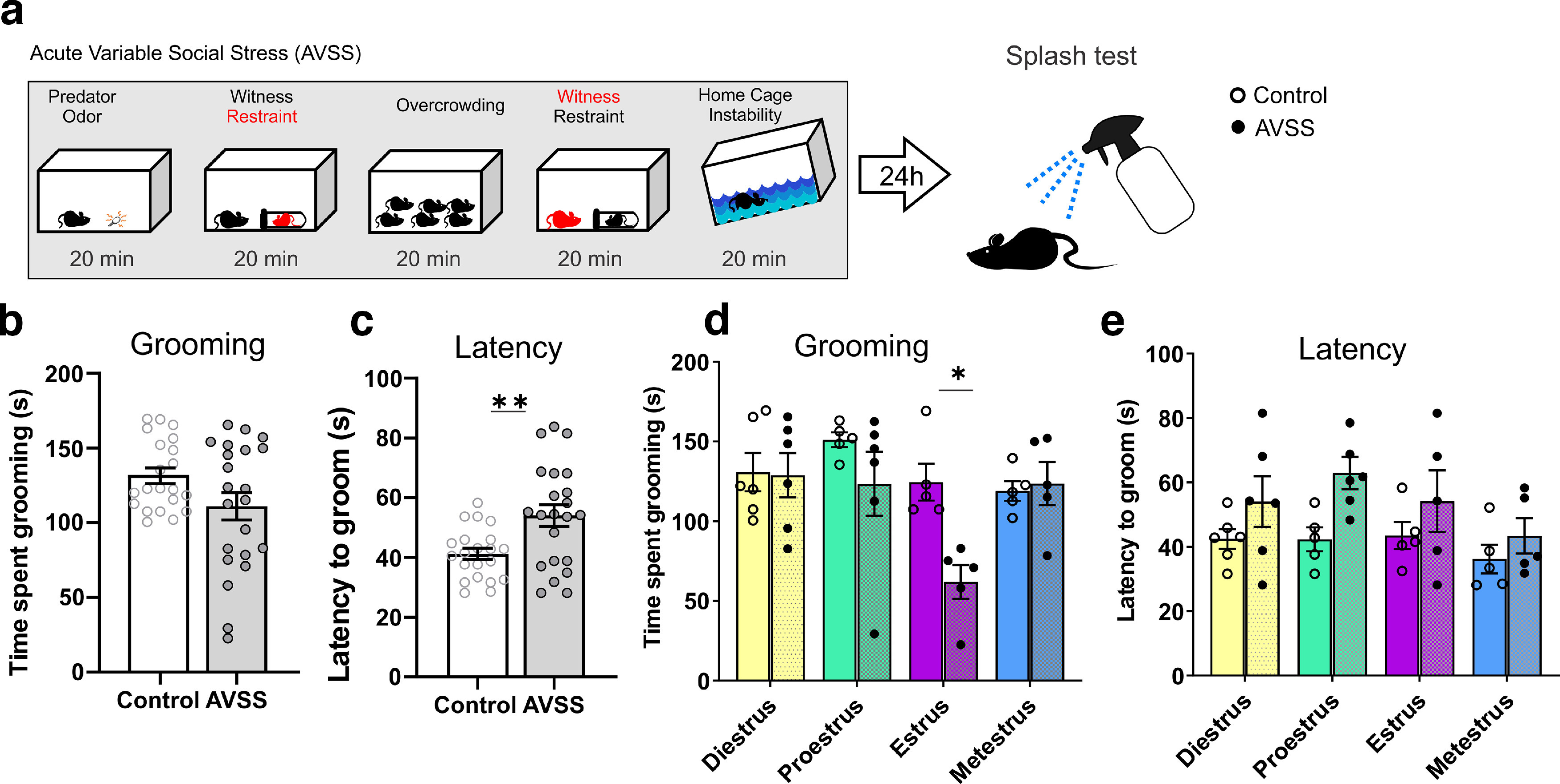
Estrous cycle stage during AVSS influences time spent grooming in the the splash test. a, Timeline. b, AVSS does not significantly alter time spent grooming (t(41) = 1.9, p = 0.06, n =21–22). c, AVSS increases the latency to groom (t(41) = 3.1, p = 0.003, n = 21–22). d, Estrous phase has no significant effect on time spent grooming during the splash test in nonstressed mice (open circle) (F(3,17) = 2.04, p = 0.15, n = 5–6 per cycle phase). AVSS during the estrus phase decreases the time spent grooming (black circles) (2-way ANOVA, cycle × stress interaction, F(3,35) = 4.20, p = 0.01, n = 5–6 mice per cycle phase). e, There is no significant interaction between cycle and stress in latency to groom (2-way ANOVA, cycle × stress interaction, F(3,35) = 1.65, p = 0.20, n = 5–6 mice per cycle phase). (*p < 0.05, **p < 0.01) Error bars indicate mean ± SEM.
To test ER involvement in altering stress acquisition we used ICI 182,780, a nonspecific ER antagonist, to mimic the rapid decline of estrogen observed during estrus. We first bath applied in vitro ICI 182,780 to VTA slices from mice in diestrus and found an increase in excitability (Fig. 6a; n = 14 cells, 5 mice). Next, to test whether the behavioral change is because of estrogen signaling in the VTA, we implanted a chronic bilateral cannula into the VTA to pharmacologically target ERs during AVSS stress acquisition (Fig. 6b). Using females in diestrus only, we infused ICI 182,780 before AVSS or control conditions (n = 8–12 mice per group). The following day we performed a social interaction test. We found no interaction between time spent in the interaction zone with no target present (Fig. 6c,d), time spent in the corner zone (Fig. 6e) or velocity (Fig. 6f) among AVSS, control, or ICI 182,780 or vehicle DMSO infusion. With the social target present we found a significant reduction in time spent in the social interaction zone in the presence of a social target similar to what we found during estrus phase (Fig. 6g,h). We also found mice that were infused in ICI 182,780 and underwent AVSS spent significantly more time in the corner zone (Fig. 6i) without any changes velocity (Fig. 6j). These results indicate ERs are involved in maintaining healthy social behaviors when faced with stress.
Figure 6.
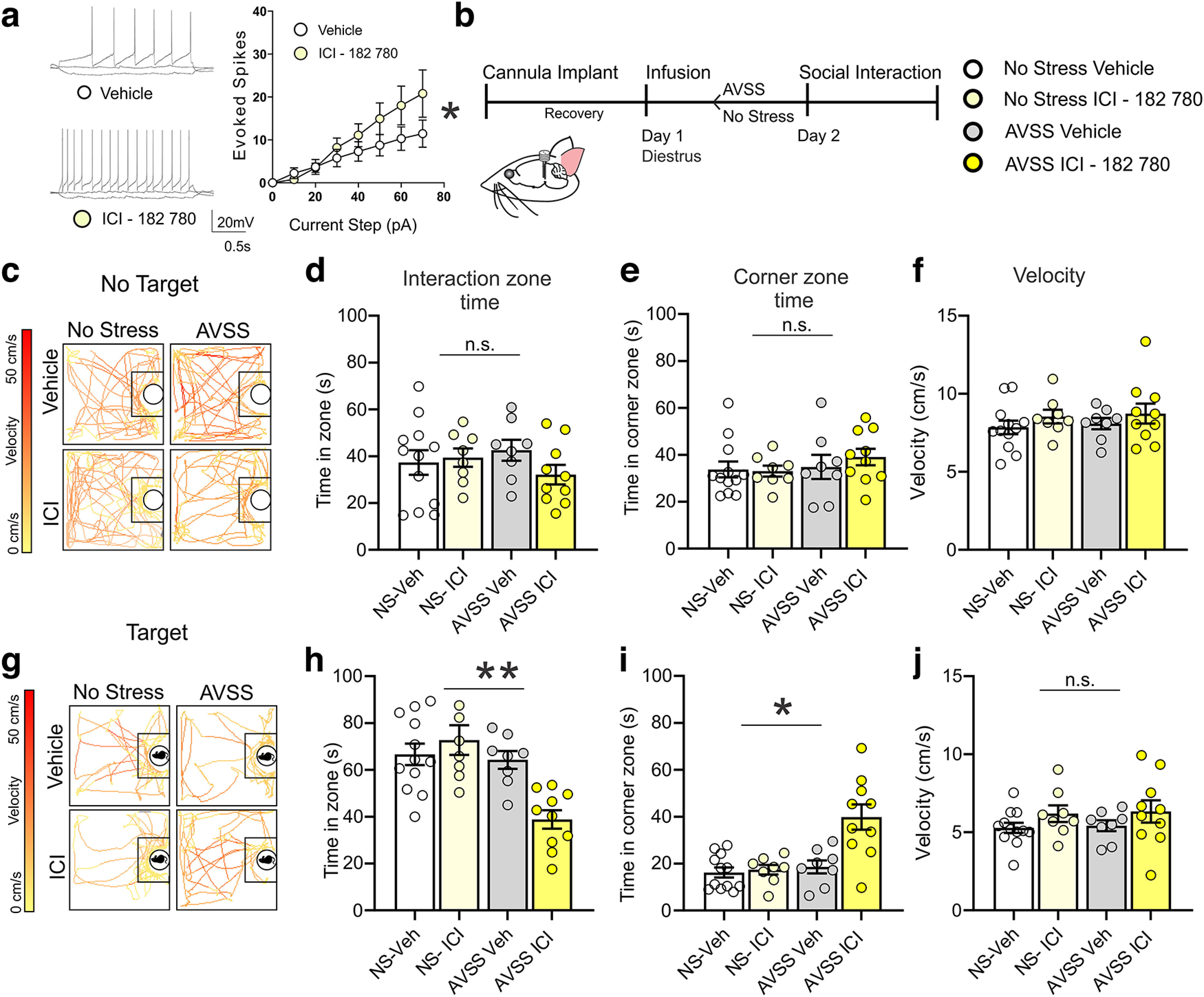
VTA infusion of ICI 182,780 before AVSS in female mice during diestrus increases behavioral stress susceptibility. a, In vitro ICI 182,780 bath perfusion increases excitability in VTA brain slices of diestrus mice (2-way ANOVA, F(7,104) = 2.30, p = 0.032, n = 14 cells, 5 mice). b, Timeline of behavioral assessment following infusion and AVSS in diestrus mice. c, Sample traces of animal location and velocity in the absence of a social target. d, ICI 182,780 in vivo infusion and stress has no significant interaction on time spent in social interaction zone (2-way ANOVA, F(3,23) = 0.96, p = 0.42, n = 8–12 mice per group). e, Time spent in corner zone (F(3,23) = 0.43, p = 0.73). f, velocity with no target present (F(3,23) = 0.53, p = 0.67). g, Sample traces of animal location and velocity with novel female social target. h, ICI 182,780 infusion before AVSS significantly decreases time spent in the interaction zone in the presence of a social target (ICI × stress interaction (F(3,23) = 6.68, p = 0.002). i, ICI 182,780 infusion before AVSS significantly increases time spent in the corner zone in the presence of a social target (F(3,23) = 12.2, p <0.0001). j, There is no significant effect of ICI or AVSS on average velocity F(3,23) = 1.184, p = 0.34). (*p < 0.05, **p < 0.01, n.s. = not significant) Error bars indicate mean ± SEM.
To more closely link the changes in social behavior following stress that occur during estrus to the observed changes in VTA excitability, we increased potassium channel function in the VTA before AVSS in mice during the estrus phase. To reduce the increased excitability observed during estrus in the VTA and test its involvement in modulating stress acquisition, we used retigabine, a potassium channel opener. We first bath applied retigabine in vitro to VTA slices from mice in estrus and found a significant decrease in excitability (Fig. 7a; n = 12 cells, 6 mice). To determine whether the increased susceptibility during estrus is linked to the reduced VTA potassium currents, we implanted a chronic bilateral cannula into the VTA to pharmacologically enhance potassium currents immediately prior to stress acquisition (Fig. 7b). Using females in estrus only, we infused retigabine or vehicle before AVSS (n = 8 mice per group). The following day we performed a social interaction test. We found that the mice in estrus that received retigabine infusion before stress spent more time in the interaction zone with a novel social target compared with mice in estrus that received a vehicle infusion (Fig. 7d). We also found mice that were infused with retigabine and underwent AVSS spent significantly less time in the corner zone with the target present (Fig. 7e) without any changes in velocity compared with vehicle (Fig. 7f). These results indicate that the reduced potassium current in the VTA during estrus is involved in increased susceptibility during estrus.
Figure 7.
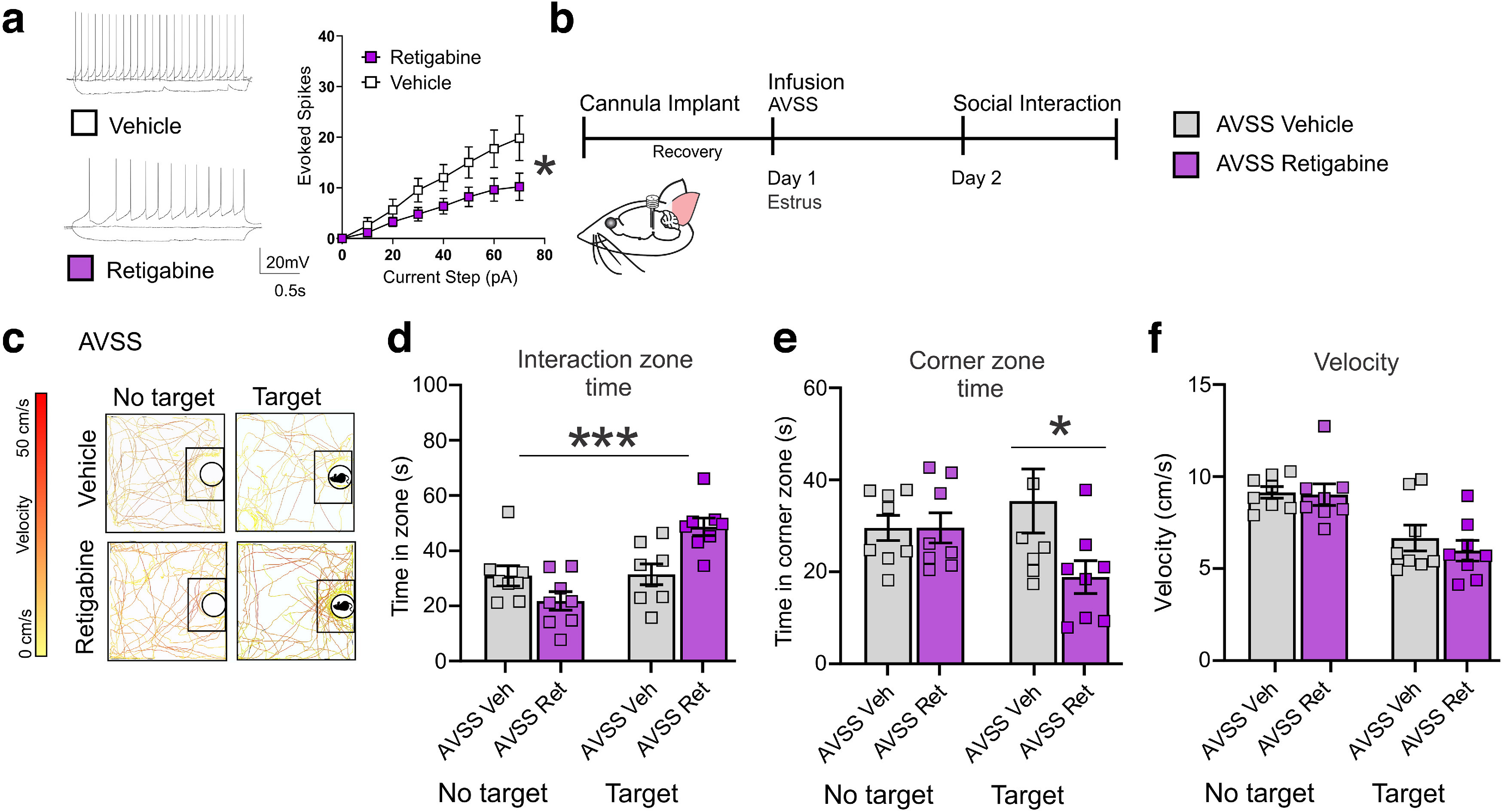
Retigabine infusion to the VTA decreases behavioral susceptibility to AVSS in mice during estrus. a, In vitro retigabine bath perfusion decreases excitability in VTA brain slices of estrus mice (paired t test, 60 pA step, t(11) = 2.65, *p < 0.05 12 cells, 6 mice). b, Timeline of behavioral assessment following infusion and AVSS in estrus mice. c, Sample traces of animal location and velocity during social interaction test. d, In vivo infusion of retigabine to the VTA before stress increases the time spent in social interaction zone with novel social target (ANOVA, F(3,28) = 10.32, p < 0.0001, n = 8 mice per group) and e, reduces time spent in corner zone (F(3,28) = 2.40, p = 0.08). f, There is no significant effect of retigabine on average velocity with the target present (paired t tests, t(14) = 0.763, p = 0.46). Error bars indicate mean ± SEM.
Discussion
A large body of research has been dedicated to revealing the broad role of DA in encoding different behaviors. Beyond reproduction, the complex role of sex steroid modulation has the potential to modulate many of these different DA-regulated behaviors. This interaction has previously been demonstrated in the drug reward field, as well as in more natural behaviors such as food reward (Fattore et al., 2008; Anker and Carroll, 2011; Richard et al., 2017). In particular, increased bursting behavior of VTA DA neurons, as well as an increase in striatal release of DA, is consistently observed during estrus (Becker, 1990). During estrus, DA neurons in the VTA also show increased sensitivity to ethanol and DA (Vandegrift et al., 2017). Further, differences in electrophysiological properties of medium spiny neurons in the nucleus accumbens (NAc) emerge across the estrous cycle from both direct action of estradiol ER activation (Almey et al., 2022) and possible changes in DA signaling (Proaño et al., 2018, 2020). Here, using whole-cell electrophysiological recordings in freely cycling female mice, we demonstrate ionic differences in DA neuron activity associated with increased sensitivity to a stimulating current input. Our pharmacological manipulation of estrogen signaling during a behaviorally salient event indicates the complex interaction between sex steroids and stress in the reward system.
We demonstrate that the average spontaneous in vitro firing activity of VTA DA neurons is not significantly different across the estrous cycle. This is highly consistent with the range of female mice behavior that does not show significant differences across the estrous cycle (Meziane et al., 2007). However, our study demonstrates that the excitability, which determines the functional response of the VTA, is fundamentally modulated across the estrous cycle, with excitability significantly higher during estrus. We further found that Ih, a primary driver of excitability that has previously been found to be modulated by stress, is not sensitive to fluctuating sex steroids, indicating that other mechanisms are involved. This upregulation of excitability during estrus is likely in part driven by the concomitant downregulation of K+ channel function, a known regulator of DA neuron activity. We found that estrogen incubation significantly changes the excitability response in a cycle-dependent pattern, indicating that estrogen signaling is partially involved in modulating excitability across the cycle. Estrogen incubation decreases excitability in both proestrus and estrus phases and conversely increases excitability during metestrus. These phase dependent responses to estrogen highlight the functional changes in the VTA across the estrous cycle. Importantly, our study provides a possible ionic mechanism for differences previously recorded during in vivo drug studies and at the same time revealing why some behavioral and physiological differences across the estrous cycle only emerge in the presence of a drug, stress, or other perturbance (Zhang et al., 2008; Zhao et al., 2021).
As previously established, the estrous cycle alone does not influence the outcome of widely accepted behavioral tests commonly used to assess behavioral changes in rodents, including the social interaction assay (Zhao et al., 2021). We similarly found that social interaction behavior is not altered across the estrous cycle. However, when a salient stimulus such as AVSS is experienced, the estrous cycle stage at the time of the stimulus becomes an important factor for the social interaction behavioral output. Similar to the findings that the physiological effects of drugs of reward are modified across the estrous cycle, our results support the role of steroid hormones in modulating the acquisition of stress and the resulting behavioral output.
Two ER types, ERα and ERβ, are expressed on subpopulations of VTA DA neurons (Milner et al., 2010; Vandegrift et al., 2020). These receptors have opposing effects in some brain regions and may account for some of the complicated effects we have found at different estrous cycle stages. Alternatively, (or concomitantly) the expression of ERs in the VTA may change across the estrous cycle as estrous stage modulates ER expression throughout the brain. The relative expression levels of these receptors may play a role in the behavioral outcome and physiological activity of VTA DA neurons. A third G-protein-coupled estrogen receptor (GPER-1) has been identified, which along with ERα, is expressed on neurons in the medial preoptic area that project to dopamine neurons in the VTA but not on the dopamine neurons themselves (Tobiansky et al., 2016). GPER rapidly effects neuronal excitability on binding with estrogen (Revankar et al., 2005). This transmembrane receptor is localized to the endoplasmic reticulum and on activation increases the release of internal calcium stores from the endoplasmic reticulum, activates IP3, and modulates other calcium signaling pathways (Hazell et al., 2009). Future experiments will explore the role of each receptor type in modulating the activity of VTA DA neurons and the behavioral response to stress across the estrous cycle.
We found strikingly different effects of estrogen incubation on VTA DA neuron activity across the estrous cycle, indicating an important role of cycling sex steroids, including progesterone signaling in modulating the physiology of DA neurons. Although our experiments did not directly manipulate progesterone levels, the synergistic balance between progesterone and estrogen signaling likely plays a role in differences observed across estrous. Progesterone signaling is well known to play a role in facilitating sexual receptivity through direct, rapid action in the VTA (Frye, 2001). Progesterone receptors have been detected in the VTA, although their cell-type distribution has not been thoroughly described (Quadros et al., 2008; Frye et al., 2013). In addition to modulating ER expression, activation of progesterone receptors has immediate and long-lasting effects on neuronal physiology, possibly contributing to the differences recorded (Kapur and Joshi, 2021).
In addition to the effects on the DA neurons in the VTA, steroid hormones likely play a role in modulating the local circuitry in the VTA, including locally projecting GABAergic interneurons. These neurons respond to stress and aversive stimuli and are acutely integrated into the circuitry involved in motivation and reward (Bouarab et al., 2019). Progesterone and allopregnanolone, a progesterone metabolite, are allosteric modulators of GABAA receptors (Callachan et al., 1987) that induce plasticity in local VTA circuits (Vashchinkina et al., 2014), reduce the amount of DA released in the NAc (Dornellas et al., 2020), and can have an anxiolytic effect on behavior (Reddy et al., 2005). Working together with estrogen signaling, progesterone and its metabolites may balance changes in VTA excitability. This further highlights the role of the VTA as an integration site for internal state (i.e., hormonal state) and external state (i.e., stress).
A reductionist method of using gonadectomy to control the levels of hormones has been performed to examine this complex system. However, the removal of all steroid hormone production can cause unintended widespread changes in response to the deprivation of natural levels of peripheral steroid hormones, such as changes in metabolism and changes in the behavioral response to stress, as well as a possible increase in extragonadal aromatization and synthesis of estrogen (Zhao et al., 2005; Levin, 2009; Lagunas et al., 2010). Removing this natural phenomenon of estrous cycle may therefore result in an incomplete analysis of its modulatory role on the reward pathways. Further experimental approaches of controlled hormone replacement have provided insight but are still fraught with questions of hormone interactions, temporal effects, and withdrawal states.
Our study establishes a correlation between VTA electrophysiological signatures across estrous with susceptibility to changes in DA-related social interaction behavior during stress acquisition. These findings are consistent with clinical observations of a subset of females being more susceptible to disorders that have been linked to disturbances in DA signaling, such as depression, anxiety, and substance use disorders (Weissman et al., 1996; Fattore et al., 2008). Many symptoms of these disorders emerge at puberty, when sex steroids begin to fluctuate, and increase with changing hormone levels across the menstrual cycle (Salk et al., 2017; Eid et al., 2019). Moreover, during menopause, another period of intense hormonal fluctuations, symptoms of depression are worsened or even emerge in females who have never experienced depression (Noble, 2005; Freeman et al., 2006). Although stress is a known precipitating factor in MDD, its interaction with sex steroids is theorized to contribute to the sex difference in MDD prevalence (Bloch et al., 2000; Balzer et al., 2015). Our study provides a neural substrate for this interaction, which may lead to improved treatment patterns for females (Lebrón-Milad et al., 2013). In light of the modulatory role of estrous cycle on the VTA ion channel activity, our study shows the importance of considering both hormonal state and sex in future investigations of reward circuits, as well as for translational research aiming for therapeutic interventions targeting the DA system.
Footnotes
This work was supported by National Science Foundation Grant 201825283 (M.R.S.); National Institutes of Health (NIH)—National Institute of Neurological Disorders and Stroke Grant R25NS080686 (R.K. and A.S.); NIH–National Heart, Lung, and Blood Institute Grants HL098351 and HL136520 (T.A.M.); and National Institute of General Medical Sciences Grant GM122646 (A.K.F.).
The authors declare no competing financial interests.
References
- Almey A, Milner TA, Brake WG (2015) Estrogen receptors in the central nervous system and their implication for dopamine-dependent cognition in females. Horm Behav 74:125–138. 10.1016/j.yhbeh.2015.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almey A, Milner TA, Brake WG (2022) Estrogen receptors observed at extranuclear neuronal sites and in glia in the nucleus accumbens core and shell of the female rat: evidence for localization to catecholaminergic and GABAergic neurons. J Comp Neurol 530:2056–2072. 10.1002/cne.25320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker JJ, Carroll ME (2011) Females are more vulnerable to drug abuse than males: evidence from preclinical studies and the role of ovarian hormones. Curr Top Behav Neurosci 8:73–96. 10.1007/7854_2010_93 [DOI] [PubMed] [Google Scholar]
- Balzer BW, Duke SA, Hawke CI, Steinbeck KS (2015) The effects of estradiol on mood and behavior in human female adolescents: a systematic review. Eur J Pediatr 174:289–298. 10.1007/s00431-014-2475-3 [DOI] [PubMed] [Google Scholar]
- Bangasser DA, Valentino RJ (2014) Sex differences in stress-related psychiatric disorders: neurobiological perspectives. Front Neuroendocrinol 35:303–319. 10.1016/j.yfrne.2014.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB (1990) Direct effect of 17 beta-estradiol on striatum: sex differences in dopamine release. Synapse 5:157–164. 10.1002/syn.890050211 [DOI] [PubMed] [Google Scholar]
- Bloch M, Schmidt PJ, Danaceau M, Murphy J, Nieman L, Rubinow DR (2000) Effects of gonadal steroids in women with a history of postpartum depression. Am J Psychiatry 157:924–930. 10.1176/appi.ajp.157.6.924 [DOI] [PubMed] [Google Scholar]
- Bouarab C, Thompson B, Polter AM (2019) VTA GABA neurons at the interface of stress and reward. Front Neural Circuits 13:78. 10.3389/fncir.2019.00078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari ES, Juarez B, Morel C, Walker DM, Cahill ME, Ribeiro E, Roman-Ortiz C, Ramakrishnan C, Deisseroth K, Han M-H, Nestler EJ (2017) Dopaminergic dynamics underlying sex-specific cocaine reward. Nat Commun 8:13877. 10.1038/ncomms13877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callachan H, Cottrell GA, Hather NY, Lambert JJ, Nooney JM, Peters JA (1987) Modulation of the GABAA receptor by progesterone metabolites. Proc R Soc Lond B Biol Sci 231:359–369. 10.1098/rspb.1987.0049 [DOI] [PubMed] [Google Scholar]
- Cao J-L, Covington HE, Friedman AK, Wilkinson MB, Walsh JJ, Cooper DC, Nestler EJ, Han M-H (2010) Mesolimbic dopamine neurons in the brain reward circuit mediate susceptibility to social defeat and antidepressant action. J Neurosci 30:16453–16458. 10.1523/JNEUROSCI.3177-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhury D, et al. (2013) Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature 493:532–536. 10.1038/nature11713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dazzi L, Seu E, Cherchi G, Barbieri PP, Matzeu A, Biggio G (2007) Estrous cycle-dependent changes in basal and ethanol-induced activity of cortical dopaminergic neurons in the rat. Neuropsychopharmacology 32:892–901. 10.1038/sj.npp.1301150 [DOI] [PubMed] [Google Scholar]
- Dornellas APS, Macedo GC, McFarland MH, Gomez AA, O'Buckley TK, Da Cunha C, Morrow AL, Robinson DL (2020) Allopregnanolone decreases evoked dopamine release differently in rats by sex and estrous stage. Front Pharmacol 11:608887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eid RS, Gobinath AR, Galea LAM (2019) Sex differences in depression: insights from clinical and preclinical studies. Prog Neurobiol 176:86–102. 10.1016/j.pneurobio.2019.01.006 [DOI] [PubMed] [Google Scholar]
- Fattore L, Altea S, Fratta W (2008) Sex differences in drug addiction: a review of animal and human studies. Womens Health (Lond) 4:51–65. 10.2217/17455057.4.1.51 [DOI] [PubMed] [Google Scholar]
- Fernandez SM, Lewis MC, Pechenino AS, Harburger LL, Orr PT, Gresack JE, Schafe GE, Frick KM (2008) Estradiol-induced enhancement of object memory consolidation involves hippocampal extracellular signal-regulated kinase activation and membrane-bound estrogen receptors. J Neurosci 28:8660–8667. 10.1523/JNEUROSCI.1968-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman EW, Sammel MD, Lin H, Nelson DB (2006) Associations of hormones and menopausal status with depressed mood in women with no history of depression. Arch Gen Psychiatry 63:375–382. 10.1001/archpsyc.63.4.375 [DOI] [PubMed] [Google Scholar]
- Friedman AK, Walsh JJ, Juarez B, Ku SM, Chaudhury D, Wang J, Li X, Dietz DM, Pan N, Vialou VF, Neve RL, Yue Z, Han M-H (2014) Enhancing depression mechanisms in midbrain dopamine neurons achieves homeostatic resilience. Science 344:313–319. 10.1126/science.1249240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman AK, Juarez B, Ku SM, Zhang H, Calizo RC, Walsh JJ, Chaudhury D, Zhang S, Hawkins A, Dietz DM, Murrough JW, Ribadeneira M, Wong EH, Neve RL, Han M-H (2016) KCNQ channel openers reverse depressive symptoms via an active resilience mechanism. Nat Commun 7:11671. 10.1038/ncomms11671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA (2001) The role of neurosteroids and non-genomic effects of progestins and androgens in mediating sexual receptivity of rodents. Brain Res Brain Res Rev 37:201–222. 10.1016/s0165-0173(01)00119-9 [DOI] [PubMed] [Google Scholar]
- Frye CA, Walf AA, Kohtz AS, Zhu Y (2013) Membrane progestin receptors in the midbrain ventral tegmental area are required for progesterone-facilitated lordosis of rats. Horm Behav 64:539–545. 10.1016/j.yhbeh.2013.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunaydin LA, Grosenick L, Finkelstein JC, Kauvar IV, Fenno LE, Adhikari A, Lammel S, Mirzabekov JJ, Airan RD, Zalocusky KA, Tye KM, Anikeeva P, Malenka RC, Deisseroth K (2014) Natural neural projection dynamics underlying social behavior. Cell 157:1535–1551. 10.1016/j.cell.2014.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazell GG, Yao ST, Roper JA, Prossnitz ER, O'Carroll AM, Lolait SJ (2009) Localisation of GPR30, a novel G protein-coupled oestrogen receptor, suggests multiple functions in rodent brain and peripheral tissues. J Endocrinol 202:223–236. 10.1677/JOE-09-0066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iñiguez SD, Vialou V, Warren BL, Cao JL, Alcantara LF, Davis LC, Manojlovic Z, Neve RL, Russo SJ, Han MH, Nestler EJ, Bolaños-Guzmán CA (2010) Extracellular signal-regulated kinase-2 within the ventral tegmental area regulates responses to stress. J Neurosci 30:7652–7663. 10.1523/JNEUROSCI.0951-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isingrini E, Camus V, Le Guisquet AM, Pingaud M, Devers S, Belzung C (2010) Association between repeated unpredictable chronic mild stress (UCMS) procedures with a high fat diet: a model of fluoxetine resistance in mice. PLoS One 5:e10404. 10.1371/journal.pone.0010404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janitzky K, Peine A, Kröber A, Yanagawa Y, Schwegler H, Roskoden T (2014) Increased CRF mRNA expression in the sexually dimorphic BNST of male but not female GAD67 mice and TMT predator odor stress effects upon spatial memory retrieval. Behav Brain Res 272:141–149. 10.1016/j.bbr.2014.06.020 [DOI] [PubMed] [Google Scholar]
- Kapur J, Joshi S (2021) Progesterone modulates neuronal excitability bidirectionally. Neurosci Lett 744:135619. 10.1016/j.neulet.2020.135619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly MJ, Rønnekleiv OK (2015) Minireview: neural signaling of estradiol in the hypothalamus. Mol Endocrinol 29:645–657. 10.1210/me.2014-1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Wittchen HU, Kendler KS (1994) Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry 51:8–19. 10.1001/archpsyc.1994.03950010008002 [DOI] [PubMed] [Google Scholar]
- Koehl M, Battle S, Meerlo P (2006) Sex differences in sleep: the response to sleep deprivation and restraint stress in mice. Sleep 29:1224–1231. 10.1093/sleep/29.9.1224 [DOI] [PubMed] [Google Scholar]
- Kow LM, Pfaff DW (2016) Rapid estrogen actions on ion channels: a survey in search for mechanisms. Steroids 111:46–53. 10.1016/j.steroids.2016.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, et al. (2007) Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell 131:391–404. 10.1016/j.cell.2007.09.018 [DOI] [PubMed] [Google Scholar]
- Kritzer MF (1997) Selective colocalization of immunoreactivity for intracellular gonadal hormone receptors and tyrosine hydroxylase in the ventral tegmental area, substantia nigra, and retrorubral fields in the rat. J Comp Neurol 379:247–260. [DOI] [PubMed] [Google Scholar]
- Lagunas N, Calmarza-Font I, Diz-Chaves Y, Garcia-Segura LM (2010) Long-term ovariectomy enhances anxiety and depressive-like behaviors in mice submitted to chronic unpredictable stress. Horm Behav 58:786–791. 10.1016/j.yhbeh.2010.07.014 [DOI] [PubMed] [Google Scholar]
- Lammel S, Ion DI, Roeper J, Malenka RC (2011) Projection-specific modulation of dopamine neuron synapses by aversive and rewarding stimuli. Neuron 70:855–862. 10.1016/j.neuron.2011.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebrón-Milad K, Tsareva A, Ahmed N, Milad MR (2013) Sex differences and estrous cycle in female rats interact with the effects of fluoxetine treatment on fear extinction. Behav Brain Res 253:217–222. 10.1016/j.bbr.2013.07.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ER (2009) Plasma membrane estrogen receptors. Trends Endocrinol Metab 20:477–482. 10.1016/j.tem.2009.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin EJ, Sun M, Choi EY, Magee D, Stets CW, During MJ (2015) Social overcrowding as a chronic stress model that increases adiposity in mice. Psychoneuroendocrinology 51:318–330. 10.1016/j.psyneuen.2014.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Shi H (2015) Regulation of estrogen receptor α expression in the hypothalamus by sex steroids: implication in the regulation of energy homeostasis. Int J Endocrinol 2015:949085. 10.1155/2015/949085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowes DC, Harris AZ (2022) Stressed and wired: the effects of stress on the VTA circuits underlying motivated behavior. Curr Opin Endocr Metab Res 26:100388. 10.1016/j.coemr.2022.100388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Main MJ, Cryan JE, Dupere JR, Cox B, Clare JJ, Burbidge SA (2000) Modulation of KCNQ2/3 potassium channels by the novel anticonvulsant retigabine. Mol Pharmacol 58:253–262. 10.1124/mol.58.2.253 [DOI] [PubMed] [Google Scholar]
- Margolis EB, Mitchell JM, Ishikawa J, Hjelmstad GO, Fields HL (2008) Midbrain dopamine neurons: projection target determines action potential duration and dopamine D(2) receptor inhibition. J Neurosci 28:8908–8913. 10.1523/JNEUROSCI.1526-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott JL, Liu B, Dluzen DE (1994) Sex differences and effects of estrogen on dopamine and DOPAC release from the striatum of male and female CD-1 mice. Exp Neurol 125:306–311. 10.1006/exnr.1994.1034 [DOI] [PubMed] [Google Scholar]
- McEwen BS, Milner TA (2017) Understanding the broad influence of sex hormones and sex differences in the brain. J Neurosci Res 95:24–39. 10.1002/jnr.23809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meziane H, Ouagazzal AM, Aubert L, Wietrzych M, Krezel W (2007) Estrous cycle effects on behavior of C57BL/6J and BALB/cByJ female mice: implications for phenotyping strategies. Genes Brain Behav 6:192–200. 10.1111/j.1601-183X.2006.00249.x [DOI] [PubMed] [Google Scholar]
- Milner TA, Thompson LI, Wang G, Kievits JA, Martin E, Zhou P, McEwen BS, Pfaff DW, Waters EM (2010) Distribution of estrogen receptor β containing cells in the brains of bacterial artificial chromosome transgenic mice. Brain Res 1351:74–96. 10.1016/j.brainres.2010.06.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitterling KL, Spencer JL, Dziedzic N, Shenoy S, McCarthy K, Waters EM, McEwen BS, Milner TA (2010) Cellular and subcellular localization of estrogen and progestin receptor immunoreactivities in the mouse hippocampus. J Comp Neurol 518:2729–2743. 10.1002/cne.22361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore EM, Boehm SL II (2009) Site-specific microinjection of baclofen into the anterior ventral tegmental area reduces binge-like ethanol intake in male C57BL/6J mice. Behav Neurosci 123:555–563. 10.1037/a0015345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson JF, Felicio LS, Osterburg HH, Finch CE (1981) Altered profiles of estradiol and progesterone associated with prolonged estrous cycles and persistent vaginal cornification in aging C57BL/6J mice. Biol Reprod 24:784–794. 10.1095/biolreprod24.4.784 [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM (2002) Neurobiology of depression. Neuron 34:13–25. 10.1016/s0896-6273(02)00653-0 [DOI] [PubMed] [Google Scholar]
- Neuhoff H, Neu A, Liss B, Roeper J (2002) I(h) channels contribute to the different functional properties of identified dopaminergic subpopulations in the midbrain. J Neurosci 22:1290–1302. 10.1523/JNEUROSCI.22-04-01290.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newhouse P, Albert K (2015) Estrogen, stress, and depression: a neurocognitive model. JAMA Psychiatry 72:727–729. 10.1001/jamapsychiatry.2015.0487 [DOI] [PubMed] [Google Scholar]
- Nilsson ME, Vandenput L, Tivesten Å, Norlén AK, Lagerquist MK, Windahl SH, Börjesson AE, Farman HH, Poutanen M, Benrick A, Maliqueo M, Stener-Victorin E, Ryberg H, Ohlsson C (2015) Measurement of a comprehensive sex steroid profile in rodent serum by high-sensitive gas chromatography-tandem mass spectrometry. Endocrinology 156:2492–2502. 10.1210/en.2014-1890 [DOI] [PubMed] [Google Scholar]
- Noble RE (2005) Depression in women. Metabolism 54:49–52. 10.1016/j.metabol.2005.01.014 [DOI] [PubMed] [Google Scholar]
- Okamoto T, Harnett MT, Morikawa H (2006) Hyperpolarization-activated cation current (Ih) is an ethanol target in midbrain dopamine neurons of mice. J Neurophysiol 95:619–626. 10.1152/jn.00682.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantier LK, Li J, Christian CA (2019) Estrous cycle monitoring in mice with rapid data visualization and analysis. Bio Protoc 9:e3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papouin T, Haydon PG (2018) Obtaining acute brain slices. Bio Protoc 8:e2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proaño SB, Morris HJ, Kunz LM, Dorris DM, Meitzen J (2018) Estrous cycle-induced sex differences in medium spiny neuron excitatory synaptic transmission and intrinsic excitability in adult rat nucleus accumbens core. J Neurophysiol 120:1356–1373. 10.1152/jn.00263.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proaño SB, Krentzel AA, Meitzen J (2020) Differential and synergistic roles of 17β-estradiol and progesterone in modulating adult female rat nucleus accumbens core medium spiny neuron electrophysiology. J Neurophysiol 123:2390–2405. 10.1152/jn.00157.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadros PS, Wagner CK (2008) Regulation of progesterone receptor expression by estradiol is dependent on age, sex and region in the rat brain. Endocrinology 149:3054–3061. 10.1210/en.2007-1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadros PS, Schlueter LJ, Wagner CK (2008) Distribution of progesterone receptor immunoreactivity in the midbrain and hindbrain of postnatal rats. Dev Neurobiol 68:1378–1390. 10.1002/dneu.20664 [DOI] [PubMed] [Google Scholar]
- Reber SO, Obermeier F, Straub RH, Falk W, Neumann ID (2006) Chronic intermittent psychosocial stress (social defeat/overcrowding) in mice increases the severity of an acute DSS-induced colitis and impairs regeneration. Endocrinology 147:4968–4976. 10.1210/en.2006-0347 [DOI] [PubMed] [Google Scholar]
- Reddy DS, O'Malley BW, Rogawski MA (2005) Anxiolytic activity of progesterone in progesterone receptor knockout mice. Neuropharmacology 48:14–24. 10.1016/j.neuropharm.2004.09.002 [DOI] [PubMed] [Google Scholar]
- Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER (2005) A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science 307:1625–1630. 10.1126/science.1106943 [DOI] [PubMed] [Google Scholar]
- Richard JE, López-Ferreras L, Anderberg RH, Olandersson K, Skibicka KP (2017) Estradiol is a critical regulator of food-reward behavior. Psychoneuroendocrinology 78:193–202. 10.1016/j.psyneuen.2017.01.014 [DOI] [PubMed] [Google Scholar]
- Rønnekleiv OK, Zhang C, Bosch MA, Kelly MJ (2015) Kisspeptin and gonadotropin-releasing hormone neuronal excitability: molecular mechanisms driven by 17β-estradiol. Neuroendocrinology 102:184–193. 10.1159/000370311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SJ, Nestler EJ (2013) The brain reward circuitry in mood disorders. Nat Rev Neurosci 14:609–625. 10.1038/nrn3381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SJ, Murrough JW, Han MH, Charney DS, Nestler EJ (2012) Neurobiology of resilience. Nat Neurosci 15:1475–1484. 10.1038/nn.3234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saavedra-Rodríguez L, Feig LA (2013) Chronic social instability induces anxiety and defective social interactions across generations. Biol Psychiatry 73:44–53. 10.1016/j.biopsych.2012.06.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salk RH, Hyde JS, Abramson LY (2017) Gender differences in depression in representative national samples: meta-analyses of diagnoses and symptoms. Psychol Bull 143:783–822. 10.1037/bul0000102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shansky RM, Woolley CS (2016) Considering sex as a biological variable will be valuable for neuroscience research. J Neurosci 36:11817–11822. 10.1523/JNEUROSCI.1390-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sial OK, Warren BL, Alcantara LF, Parise EM, Bolaños-Guzmán CA (2016) Vicarious social defeat stress: bridging the gap between physical and emotional stress. J Neurosci Methods 258:94–103. 10.1016/j.jneumeth.2015.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarfa RA, Evans RC, Khaliq ZM (2017) Enhanced sensitivity to hyperpolarizing inhibition in mesoaccumbal relative to nigrostriatal dopamine neuron subpopulations. J Neurosci 37:3311–3330. 10.1523/JNEUROSCI.2969-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobiansky DJ, Will RG, Lominac KD, Turner JM, Hattori T, Krishnan K, Martz JR, Nutsch VL, Dominguez JM (2016) Estradiol in the preoptic area regulates the dopaminergic response to cocaine in the nucleus accumbens. Neuropsychopharmacology 41:1897–1906. 10.1038/npp.2015.360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless MA, Singh V, Crowder TL, Yaka R, Ron D, Bonci A (2003) Corticotropin-releasing factor requires CRF binding protein to potentiate NMDA receptors via CRF receptor 2 in dopamine neurons. Neuron 39:401–407. 10.1016/s0896-6273(03)00461-6 [DOI] [PubMed] [Google Scholar]
- Vandegrift BJ, You C, Satta R, Brodie MS, Lasek AW (2017) Estradiol increases the sensitivity of ventral tegmental area dopamine neurons to dopamine and ethanol. PLOS ONE 12:e0187698. 10.1371/journal.pone.0187698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandegrift BJ, Hilderbrand ER, Satta R, Tai R, He D, You C, Chen H, Xu P, Coles C, Brodie MS, Lasek AW (2020) Estrogen receptor α regulates ethanol excitation of ventral tegmental area neurons and binge drinking in female mice. J Neurosci 40:5196–5207. 10.1523/JNEUROSCI.2364-19.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vashchinkina E, Manner AK, Vekovischeva O, den Hollander B, Uusi-Oukari M, Aitta-Aho T, Korpi ER (2014) Neurosteroid agonist at GABAA receptor induces persistent neuroplasticity in VTA dopamine neurons. Neuropsychopharmacology 39:727–737. 10.1038/npp.2013.258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vastagh C, Liposits Z (2017) Impact of proestrus on gene expression in the medial preoptic area of mice. Front Cell Neurosci 11:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman MM, Bland RC, Canino GJ, Faravelli C, Greenwald S, Hwu HG, Joyce PR, Karam EG, Lee CK, Lellouch J, Lepine JP, Newman SC, Rubio-Stipec M, Wells JE, Wickramaratne PJ, Wittchen H, Yeh EK (1996) Cross-national epidemiology of major depression and bipolar disorder. JAMA 276:293. 10.1001/jama.1996.03540040037030 [DOI] [PubMed] [Google Scholar]
- Wickenden AD, Yu W, Zou A, Jegla T, Wagoner PK (2000) Retigabine, a novel anti-convulsant, enhances activation of KCNQ2/Q3 potassium channels. Mol Pharmacol 58:591–600. 10.1124/mol.58.3.591 [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Wang H-L, Li X, Ng TH, Morales M (2011) Mesocorticolimbic glutamatergic pathway. J Neurosci 31:8476–8490. 10.1523/JNEUROSCI.1598-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonkers KA, O'Brien PM, Eriksson E (2008) Premenstrual syndrome. Lancet 371:1200–1210. 10.1016/S0140-6736(08)60527-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Yang S, Yang C, Jin G, Zhen X (2008) Estrogen regulates responses of dopamine neurons in the ventral tegmental area to cocaine. Psychopharmacology (Berl)) 199:625–635. 10.1007/s00213-008-1188-6 [DOI] [PubMed] [Google Scholar]
- Zhao H, Tian Z, Hao J, Chen B (2005) Extragonadal aromatization increases with time after ovariectomy in rats. Reprod Biol Endocrinol 3:6. 10.1186/1477-7827-3-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, et al. (2021) Behaviors related to psychiatric disorders and pain perception in C57BL/6J mice during different phases of estrous cycle. Front Neurosci 15:650793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong P, Vickstrom CR, Liu X, Hu Y, Yu L, Yu HG, Liu QS (2018) HCN2 channels in the ventral tegmental area regulate behavioral responses to chronic stress. Elife 7:e32420. 10.7554/eLife.32420 [DOI] [PMC free article] [PubMed] [Google Scholar]