Abstract
Background
International COVID-19 guidelines recommend thromboprophylaxis for non-critically ill inpatients to prevent thrombotic complications. It is still debated whether full-dose thromboprophylaxis reduces all-cause mortality. The main aim of this updated systematic review and meta-analysis is to evaluate the effect of full-dose heparin-based thromboprophylaxis on survival in hospitalized non-critically ill COVID-19 patients.
Methods
A systematic review was performed across Pubmed/Medline, EMBASE, Cochrane Central Register of clinical trials, Clinicaltrials.gov, and medRxiv.org from inception to November 2022. We conducted a meta-analysis of randomized clinical trials (RCTs) comparing full-dose heparin-based anticoagulation to prophylactic or intermediate dose anticoagulation or standard treatment in hospitalized non-critically ill COVID-19 patients. The risk of bias was assessed using the Cochrane risk-of-bias tool for randomized trials and Grading of Recommendations Assessment, Development and Evaluation was applied. The primary outcome was all-cause mortality at the longest follow-up available.
Results
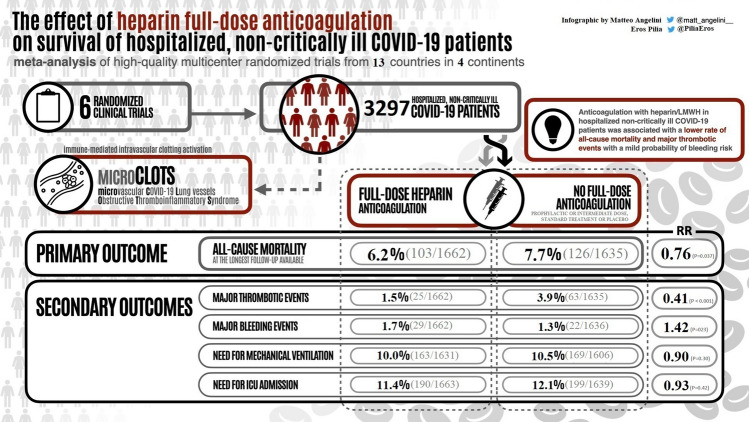
We identified 6 multicenter RCTs involving 3297 patients from 13 countries across 4 continents. The rate of all-cause mortality was 6.2% (103/1662) in the full-dose group vs 7.7% (126/1635) in the prophylactic or intermediate dose group (Risk Ratio [RR] = 0.76; 95% confidence interval [CI] = 0.59–0.98; P = 0.037). The probabilities of any mortality difference and of NNT ≤ 100 were estimated at 98.2% and 84.5%, respectively. The risk of bias was low for all included RCTs and the strength of the evidence was “moderate.”
Conclusion
Our meta-analysis of high-quality multicenter RCTs suggests that full-dose anticoagulation with heparin or low molecular weight heparin reduces all-cause mortality in hospitalized non-critically ill COVID-19 patients.
Study registration: PROSPERO, review no. CRD42022348993.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s00408-023-00599-6.
Keywords: Hospital mortality, Heparin, LMWH, Anticoagulant, SARS-CoV-2, COVID-19
Introduction
Globally, according to the WHO’s weekly epidemiological updates until January 9, 2023, there have been 659,108,952 confirmed cases of COVID-19 that caused 6,684,756 deaths [1]. A total of 13,073,712,554 vaccine doses have been administered worldwide so far [1, 2] and most cases of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infections are frequently asymptomatic or associated with mild symptoms.
Nowadays, severe COVID-19 cases primarily affect unvaccinated patients or those with comorbidities, and are associated with progressive respiratory failure with a high rate of mortality [3]. In the most severe COVID-19 cases, mortality is related to a particular form of acute respiratory distress syndrome involving vascular inflammation and endothelial injury. The evidence supporting microvascular COVID-19 lung vessels obstructive thromboinflammatory syndrome (MicroCLOTS), mediated by an immune-mediated micro-thrombosis, is growing [4]. Thromboprophylaxis has been proposed to prevent MicroCLOTS, although the best timing, method, and dosage of anticoagulation remains to be clarified [5, 6].
International guidelines recommend anticoagulation thromboprophylaxis in hospitalized COVID-19 patients [7–12]. Whereas full-dose anticoagulation reduces major arterial and venous thrombotic events in COVID-19 patients, survival benefits were not documented in non-critically ill patients [13] and in the overall population of COVID-19 hospitalized patients [14].
Consequently, we conducted an updated systematic review and meta-analysis of all available randomized controlled trials (mRCTs) evaluating therapeutic-dose heparin-based anticoagulation in non-critically ill patients and evaluated the impact of thromboprophylaxis on mortality.
Methods
The present systematic review and meta-analysis was conducted following the Preferred Reporting for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [15] and Cochrane methodology [16]. The original protocol for this study was registered in the International Prospective Register of Systematic Reviews (PROSPERO). The registration number is CRD42022348993.
We designed the review following the PICOS framework: (1) Population: hospitalized non-critically ill COVID-19 patients, (2) Intervention: therapeutic-dose anticoagulation with heparin/LMWH, (3) Comparison: no therapeutic-dose anticoagulation (including standard treatment, placebo, or prophylactic or intermediate dose anticoagulation), (4) Outcome: the primary and secondary outcomes as listed below, (5) Study design: multicenter randomized controlled trials.
Search Strategy and Study Selection
Two reviewers independently searched PubMed/Medline, Embase, the Cochrane Central Register of clinical trials, medRxiv.org and Clinicaltrials.gov (from inception to November 2022) with no language restrictions. Studies were included if there was agreement between the two reviewers, with disagreements resolved by discussion including a third author. The search strategy (Supplementary material) sought to identify RCTs that compared full-dose heparin-based anticoagulation to prophylactic-dose or intermediate-dose heparin-based anticoagulation in hospitalized non-critically ill COVID-19 patients. We included (1) randomized trials (2) enrolling hospitalized non-critically ill adults (age ≥ 18 years) with a positive RT-PCR or antigen test for SARS-CoV-2 viral infection regardless of gender or ethnicity (3) comparing therapeutic-dose anticoagulation with a heparin product (unfractionated or low-molecular weight heparin) versus no therapeutic-dose anticoagulation. We excluded: trials enrolling critically ill patients (defined according to Authors of each individual study); non-hospitalized patients; pediatric patients; and those involving a non-parallel, non-randomized, or quasi-randomized trial design.
Study Outcomes
The primary outcome of our study was all-cause mortality at the longest follow-up available. Secondary outcomes included: the rate of major thrombotic events (as defined by each individual trial); the rate of major bleeding events (as defined by the International Society on Thrombosis and Haemostasis ISTH); the need for mechanical ventilation; and the need for intensive care unit (ICU) admission [17].
Data Abstraction and Risk of Bias Assessment
For each identified trial, two reviewers independently abstracted data about the overall sample size, treatment type and dose, control therapy and the specified outcomes. Trial investigators were contacted by e-mail for additional data if it was not available in the trial manuscript (Supplementary Material Table 1).
Two independent reviewers evaluated the risk of bias with the Cochrane risk-of-bias tool for randomized trials version 2 (RoB 2) [18]. The overall risk of bias was classified as “Low,” “Some concerns,” or “High” for each study. Small study effect and publication bias were investigated for primary outcome by visual inspection funnel plot and formally tested using the Egger’s test with STATA v.17 (STATACorp, College Station, USA).
Data Analysis
Analyses were conducted using STATA v.17 (STATACorp, College Station, USA) and RevMan 5.4.1 (Review Manager, The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark). Pooled risk ratios (RRs) were calculated for dichotomous outcomes using the fixed-effects Mantel–Haenszel method. The intention-to-treat principle was followed for all analyses.
To contextualize and visualize the findings, we used the RR and 95% CI for each outcome and simulated 100,000 trials on the log scale, generated a representative probability density function on the RR scale using kernel density estimation, and estimated the probability of any benefit (RR < 1; bleeding: any harm RR > 1) and of a number needed to treat ≤ 100 based on a 1% reduction vs. the overall control event rate on the RR scale (bleeding: number needed to harm based on a 1% increased risk) using STATA v.17 (STATACorp, College Station, USA). Sensitivity analyses were performed by sequentially removing each study and re-assessing the pooled estimates and by changing of summary statistics (RR to odds ratio [OR] or risk difference [RD]).
Heterogeneity Analysis
At first, heterogeneity was evaluated by visual inspection of the forest plots, and then formally assessed with the I2 statistic. We used STATA v.17 (STATACorp, College Station, USA) and RevMan 5.4.1 (Review Manager, The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark) to test the heterogeneity hypothesis. We considered statistical heterogeneity for an I2 ˂ 25% as low, 26–50% as moderate, and > 50% as high. Fixed-effects models were used in case of low-to-moderate heterogeneity, while random-effects models were to be used in case of high heterogeneity.
Strength of the Evidence Assessment
The GRADE (Grading of Recommendations Assessment, Development and Evaluation) was applied to rate the strength of evidence. The primary and secondary outcomes were evaluated taking into account the following items: study limitations (risk of bias), inconsistency, indirectness, and imprecision [19]. These items were scored as “not serious concerns,” “serious concerns,” and “very serious concerns.” Publication bias, the number of individuals in each group, and the effect measure of the meta-analysis were also evaluated. At the end, the strength of the evidence was evaluated as very low, low, moderate, or high.
Results
Study Characteristics
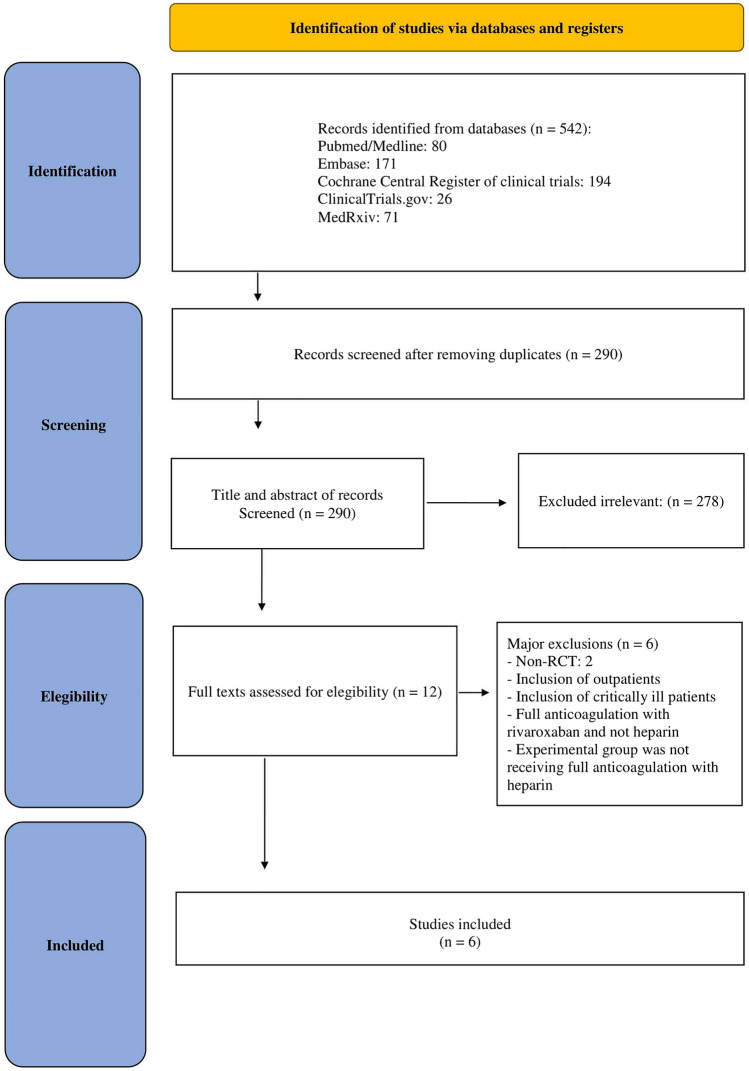
The search strategy in online databases identified potential studies in Pubmed/Medline (N = 80), Embase (N = 171), Cochrane Central Register of clinical trials (N = 194), ClinicalTrials.gov (N = 26), and MedRxiv (N = 71). After removing duplicates and applying our eligibility criteria, we identified 6 studies for inclusion in the analysis [20–25] (Fig. 1).
Fig. 1.
PRISMA diagram
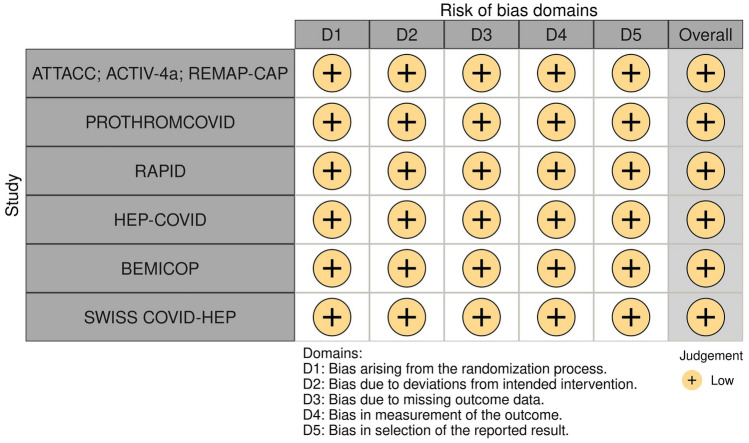
The 6 trials included were multicenter and all included prophylactic or intermediate dose anticoagulation (administered according to local clinical practice guidelines) as control treatment. The studies included a total of 3297 patients (1662 therapeutic-dose heparin-based anticoagulation vs 1635 with prophylactic or intermediate dose anticoagulation) from 13 different countries across 4 continents. (Table 1). The included 6 trials were considered at overall low risk of bias [26] (Fig. 2).
Table 1.
Main characteristics of the included studies
| mRCT | Full-dose anticoagulation (drug and dosage) | Prophylactic or intermediate dose anticoagulation (drug and dosage) | N patients treatment group | N patients control group | Country | Enrollment period |
|---|---|---|---|---|---|---|
| ATTACC; ACTIV-4a; REMAP-CAP20 | LMWH dosed according to patient weight and CrCl according to local practice; enoxaparin starting at 1 mg/kg twice daily or enoxaparin starting at 1.5 mg/kg once daily; dalteparin: 100 U/kg twice daily or starting at 200 U/kg once daily; tinzaparin 175 anti-Xa units/kg once daily. UFH continuous iv administration per local protocol | LMWH dosed according to patient weight and CrCl according to local practice enoxaparin 40 mg once daily, up to and including (a) 0.5 mg/kg twice daily or (b) 40 mg twice daily; dalteparin 5000 units once daily or 5,000 units twice daily; tinzaparin up to and including (a) 75 anti-Xa units/kg once daily or (b) 4500 units once daily; tinzaparin 4,500 units twice daily; UFH 5,000 units twice or three times daily or 7,500 units three times daily or 10,000 units twice daily | 1180 | 1046 | USA, Canada, UK, Brazil, Mexico, Nepal, Australia,the Netherlands, Spain | April 2020–January 2021 |
| PROTHROMCOVID21 | Tinzaparin 175 IU/kg/day | Tinzaparin sc 4500 IU/day or 100 IU/kg/day | 103 | 197 | Spain | February 2021–September 2021 |
| BEMICOP22 | Bemiparin 7500 IU qd if bwt between 50 and 70 kg; bemiparin 10,000 IU qd if bwt between 70–100 kg; bemiparin 12,500 IU qd if bwt > 100 kg | Bemiparin 3500 IU sc qd | 32 | 33 | Spain | October 2020–May 2021 |
| HEP23 | Enoxaparin 1 mg/kg sc bid if CrCl ≥ 30 mL/min/1.73 m2; enoxaparin 0.5 mg/kg sc bid if CrCl 15–29 mL/min/1.73 m2 | UFH up to 22,500 IU sc (divided twice or thrice daily); enoxaparin 30–40 mg sc qd/bid; dalteparin 2500–5000 IU sc qd. If CrCl ˂ 15 mL/min/1.73 m2, enoxaparin was converted to iv UFH until kidney function improved to CrCl ≥ 15 mL/min/1.73 m2 | 84 | 86 | USA | May 2020–May 2021 |
| RAPID24 | Enoxaparin 1 mg/kg sc bid or enoxaparin 1.5 mg/kg sc qd or dalteparin 100 IU/kg sc bid or dalteparin 200 IU/kg sc qd or tinzaparin 175 IU/kg sc qd or UFH iv (titrate to institution specific anti-Xa or aPTT values) if CrCl ≥ 30 mL/min/1.73 m2 and bmi ˂ 40; enoxaparin 1 mg/kg sc bid or dalteparin 100 IU/kg sc bid or tinzaparin 175 IU/kg sc qd or UFH iv titrate to institution specific anti-Xa or aPTT values if CrCl ≥ 30 mL/min/1.73 m2 and bmi ≥ 40; UFH iv titrate to institution specific anti-Xa or aPTT values or LMWH per hospital protocol taking BMI into consideration if CrCl ˂30 mL/min/1.73 m2 | Enoxaparin 40 mg sc qd or or dalteparin 5000 IU sc qd or tinzaparin 4500 IU sc qd or fondaparinux 2.5 mg sc qd or UFH 5000 UI iv bid/tid if CrCl ≥ 30 mL/min/1.73 m2 and bmi ˂ 40; enoxaparin 40 mg sc bid or dalteparin 5000 IU sc bid or tinzaparin 9000 (± 1000) UI sc qd or UFH 7500 UI sc tid if CrCl ≥ 30 mL/min/1.73 m2 and bmi ≥ 40; UFH 5000 UI iv bid/tid if CrCl ˂30 mL/min/1.73 m2 and bmi ˂ 40 or LMWH per hospital protocol taking BMI into consideration as above; UFH 7500 UI sc tid if CrCl ˂30 mL/min/1.73 m2 and bmi ˃40 or LMWH per hospital protocol taking BMI into consideration as above | 228 | 237 |
USA, Canada, Ireland, Brazil, Saudi Arabia, United Arab Emirates |
May 2020–April 2021 |
| SWISS COVID-HEP25 | Enoxaparin 1 mg/kg bid or or therapeutic intravenous or UFH iv if CrCl < 30 mL/min titrate to institution specific anti-Xa values | Enoxaparin once-daily weight-based (20 mg if 40–49.9 kg, 40 mg if 50–99.9 kg, 60 mg if ≥ 100 kg), or UFH sc (5000 IU twice daily if < 100 kg, three times daily if ≥ 100 kg) if CrCl < 30 mL/min | 35 | 36 | Switzerland | April 2020–June 2021 |
No number, QD quaque die, BID bis in die, TID ter in die, iv intravenous, bwt body weight, UFH unfractionated heparin, CrCl creatinine clearance, BMI body mass index, LMWH low molecular weight heparin, mRCT multicenter randomized controlled trial
Fig. 2.
Traffic plot of mRCTs included in the meta-analysis
Quantitative Data Synthesis
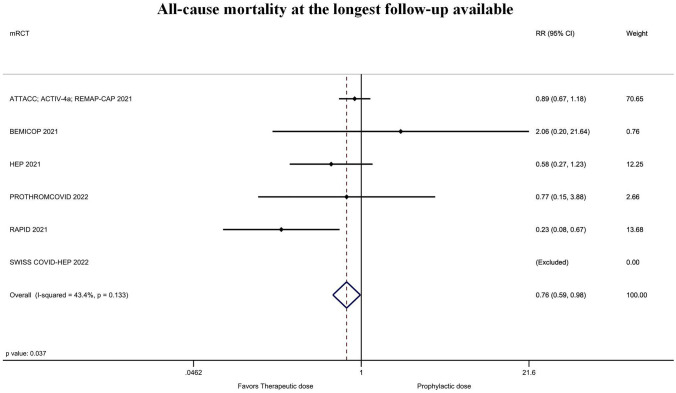
Figure 3 shows the forest plot of the rate of all-cause mortality at the longest follow-up available according to the six included randomized studies. All-cause mortality of patients treated with heparin full-dose was 6.2% (103/1662) vs 7.7% (126/1635) in those who received prophylactic or intermediate dose (risk ratio [RR] = 0.76; 95% confidence interval [CI] = 0.59–0.98; P = 0.037; I2 = 43%; Egger’s test P = 0.52). This corresponded to a 98.2% probability of any benefit and to an 84.5% probability that the number needed to treat was ≤ 100 (Supplementary material Fig. 3). Sensitivity analyses showed that the magnitude and direction of the effect size was not modified by sequentially removing each study and re-assessing the pooled estimates (lowest RR = 0.47; 95% CI = 0.27–0.81; P ˂ 0.01; I2 = 22%; removing ATTACC; ACTIV-4a; REMAP-CAP and highest RR = 0.85; 95% CI = 0.65–1.10; P = 0.22; I2 = 0%; removing RAPID). Sensitivity analyses by changing RR to odds ratio (OR = 0.75; CI = 0.57–0.98; P = 0.04; I2 = 44%) or risk difference (RD = − 0.02; CI − 0.04, − 0.00; P = 0.04; I2 = 38%) did not result in a change in significance of study findings.
Fig. 3.
Forest plot of the rate of all-cause mortality at the longest follow-up available
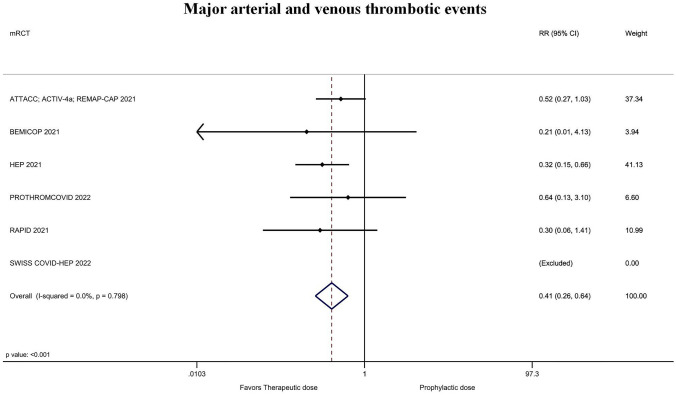
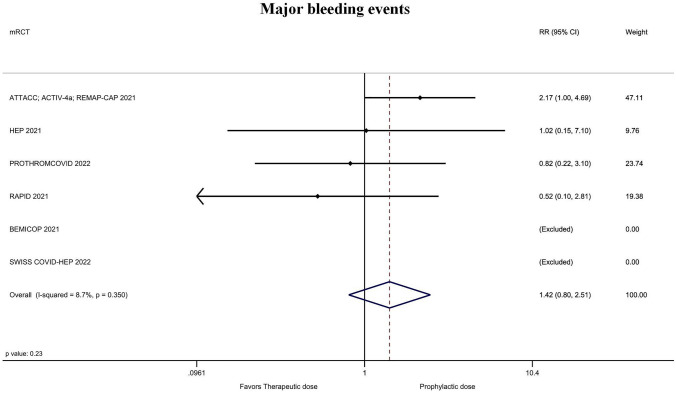
The rate of major arterial and venous thrombotic events was significantly lower for treatment-dose anticoagulation 1.5% (25/1662) vs 3.9% (63/1635) with prophylactic or intermediate dose (RR = 0.41; 95% CI = 0.26–0.64; P ˂ 0.001; I2 = 0%; Fig. 4; Supplementary material Fig. 4). The magnitude and direction of findings did not change with the sequential removal of each trial (lowest RR = 0.29; 95% CI = 0.15–0.57; P ˂ 0.001; I2 = 0%; removing ATTACC; ACTIV-4a; REMAP-CAP and highest RR = 0.47; 95% CI = 0.26–0.83; P ˂ 0.01; I2 = 0%; removing HEP). The rate of major bleeding with treatment-dose anticoagulation was 1.7% (29/1662) vs. 1.3% (22/1636) in those receiving prophylactic or intermediate dose (RR = 1.42; 95% CI = 0.80–2.51; P = 0.23; I2 = 9%; Fig. 5; Supplementary material Fig. 5). This corresponded to an 88.4% probability of increased bleeding with only a 24.4% probability that the number needed to harm would be ≤ 100. The other secondary outcomes are presented in (Table 2 and Supplementary Material Figs. 1, 2, 6, 7).
Fig. 4.
Forest plot of the rate of major arterial and venous thrombotic events
Fig. 5.
Forest plot of the rate of major bleeding events
Table 2.
Pooled analysis of studies comparing full-dose heparin anticoagulation to prophylactic or intermediate dose anticoagulation
| Outcomes | Events/patients full-dose anticoagulation (%) | Events/patients prophylactic or intermediate dose anticoagulation (%) | Risk ratio (95% CI) | P-value | I2 (%) | Probability RR < 1* (%) | Probability NNT* ≤ 100*(%) |
|---|---|---|---|---|---|---|---|
| Primary outcome | |||||||
| All-cause mortality at the longest follow-up available | 103/1662 (6.2%) | 126/1635 (7.7%) | 0.76 (0.59–0.98) | 0.037 | 43% | 98.2 | 84.5 |
| Secondary outcomes | |||||||
| Major thrombotic events (arterial and venous) | 25/1662 (1.5%) | 63/1635 (3.9%) | 0.41 (0.26–0.64) | ˂ 0.001 | 0% | 99.9 | 99.5 |
| Major bleeding events | 29/1662 (1.7%) | 22/1636 (1.3%) | 1.42 (0.80–2.51) | 0.23 | 9% | 88.4 | 24.4 |
| Need for mechanical ventilation | 163/1631 (10.0%) | 169/1606 (10.5%) | 0.90 (0.73–1.10) | 0.30 | 2% | 84.4 | 52.0 |
| Need for ICU admission | 190/1663 (11.4%) | 199/1639 (12.1%) | 0.93 (0.77–1.12) | 0.42 | 0% | 77.6 | 44.4 |
ICU intensive care unit, CI confidence interval
*RR > 1 and NNH for bleeding
Strength of the Evidence Assessment
According to the GRADE approach (Supplementary Material Table 2) there were not “very serious concerns” about the risk of bias, inconsistency, indirectness, and imprecision [19]. The strength of evidence was moderate for the primary outcome (all-cause mortality), for major bleeding events and for need for mechanical ventilation, while it was high for major arterial and venous thrombotic events and for need for ICU admission.
Discussion
Key Findings
Our systematic review and meta-analysis shows that full-dose thromboprophylaxis (predominantly with LMWH such as enoxaparin, tinzaparin, bemiparin, or dalteparin) might improve survival in hospitalized non-critically ill patients with COVID-19.
Relationship to Previous Studies
There have been a few prior meta-analyses which studied the effect of anticoagulants in hospitalized patients with Covid-19. Important limitations of these previous studies include the following: (1) the combination of direct oral anticoagulants (DOACs) together with heparin-based anticoagulation, (2) the inclusion of patients from different settings (e.g., the inclusion of critically ill patients or outpatients), (3) the inclusion of intermediate doses of anticoagulation in the treatment group, and (4) the inclusion of observational studies which are subject to both have selection and immortal time biases [27–29]. By contrast, our meta-analysis focused only on full-dose heparin-based thromboprophylaxis involving non-critically ill hospitalized COVID-19 patients.
In 2021, Giossi et al. did not find a difference in overall all-cause mortality between prophylactic-dose and full-dose of anticoagulation. However, they included trials with heterogeneous treatment (e.g., apixaban or heparin) and high risk of bias studies (e.g., observational) in their analysis [30].
In 2022, Jorda A. et al. included patients receiving heterogeneous treatment (i.e., rivaroxaban or heparin) in different settings of care (critically ill and non-critically ill patients or outpatients) [31]. In addition, the intervention group of their meta-analysis included patients who were not subjected only to full-dose thromboprophylaxis due to the inclusion of the INSPIRATION trial and the X-COVID trial [32, 33].
Significance of Study Findings and What this Study Adds to Our Knowledge
SARS-CoV-2 induced infection, compared with other respiratory infections, seems to be associated with higher rates and severity of thrombotic complications which are associated with worse patient outcomes [10]. Consequently, several international guidelines recommend heparin-based thromboprophylaxis in all COVID-19 hospitalized patients [9, 11, 12]. The suitable dosage (full-dose or intermediate or prophylactic dose) of thromboprophylaxis is still debated [5, 12]. However, practical position statements already suggest that in high thrombotic risk, non-critically ill patients, a full-dose thromboprophylaxis is recommended, taking into consideration the individual patient’s bleeding risk [34].
Severe forms of COVID-19 seem to be linked to the formation in situ of micro-clots caused by an abnormal activation of the immune system in the lungs [4]. It has been hypothesized that anticoagulant therapy may exert a prophylactic role against these immune-thrombotic events by preventing thrombi formation and progression and, furthermore, that this benefit may best be demonstrated early [7] because anticoagulants may have a lesser ability to act on already formed thrombi [8]. In our updated systematic review and meta-analysis of randomized controlled trials involving non-critically ill hospitalized patients, we demonstrated that thromboprophylaxis with therapeutic-dose heparins (primarily LMWH) was associated with a statistically significant reduction of all-cause mortality and thrombotic events balanced with a modest probability of increased major bleeding. We suggest that the overall benefits outweigh the disadvantages and that for most non-critically ill patients at low risk of bleeding, thromboprophylaxis with LMWH should be recommended. Whether or not patients receiving other forms of thromboprophylaxis at admission should be switched to therapeutic-dose heparin-based thromboprophylaxis is an important question for future research.
The reason of the differences in results of previous meta-analyses grouping critically ill and non-critically ill patients is likely related to this aspect. Final results of our meta-analysis show overall benefits of full-dose anticoagulation in hospitalized non-critically ill COVID-19 patients rather than disadvantages. In addition, because the most favorable thromboprophylactic approach during hospitalization is still not defined, our results may help physicians in the decision-making process when treating non-critically ill COVID-19 patients. Our results can help in reducing COVID-19 all-cause in hospital mortality over time and the consequences of venous thromboembolism (e.g., deep vein thrombosis, pulmonary embolism) and arterial thromboembolism (e.g., myocardial infarction and ischemic stroke) in hospitalized non-critically ill COVID-19 patients [35–38].
Furthermore, there are numerous plausible biological explanations for ancillary beneficial properties of heparin in terms of antiviral effects [39, 40]. Several mechanisms were suggested and are still a research object: (1) the capability of LMWH to bind with high affinity IFNγ, (2) the capability of heparin to influence the biological activity of IL-6 by binding either IL-6 or IL-6/IL-6Rα, (3) the heparin capability to inhibit multiple coagulation proteases, some of which might be specific to COVID-19, determining an overall anti-inflammatory action [41]. Indoubitably more clinical evidence is required to clarify heparin antiviral mechanisms of action and its capability to influence infectious conditions such as COVID-19.
Strengths and Limitations of the Study
The main strength of this meta-analysis is the high quality of the low risk of bias multicenter RCTs included [20–25]. Generalizability seems likely as the included patients were not considered critically ill and were recruited from 13 different countries across 4 continents. Disease progression may be too advanced or bleeding complications too high-risk in critically ill patients for them to benefit from full-dose anticoagulation. Another aspect to note is that the use of heparins was heterogeneous across the 6 trials and it is not possible to attribute clinical benefits to a specific molecule. Importantly, few patients were treated with therapeutic dose unfractionated heparin and so the implications for patients with advanced renal dysfunction, for whom LMWH are relative contraindicated, are less clear. Anyway, following Cochrane indications, meta-regression analysis was not included in our investigation because the pooled analysis included fewer than ten studies [42]. To note, meta-analyses should be considered hypothesis-generating, and hence our results remain speculative. Studies included in our analysis included data from the earlier phases of the pandemic when multiple reinfections were uncommon and vaccination was not widely implemented. Our findings may not apply to current COVID-19 epidemiology. However, coagulopathy in COVID-19 patients is still a field of research and there is a need for more structured clinical trials to make physicians able to take precise decisions regarding thromboprophylaxis in COVID-19 patients [43, 44]. In addition, the role of intermediate dose heparin thromboprophylaxis in non-critically ill patients has not been adequately studied in the available RCTs [34]. We searched also for preprint studies, which may be at high risk of bias [45]. However, all of the identified and included trials were published on peer-reviewed journals.
Conclusion
Pooled data from high-quality multicenter randomized trials suggest a reduction in all-cause mortality at the longest follow-up available in hospitalized non-critically ill COVID-19 patients receiving full-dose heparin-based thromboprophylaxis. This is associated with a significant reduction in thrombotic events with the benefits being partly offset by a modest probability of increased bleeding. Overall, the benefits suggest that therapeutic-dose heparin-based thromboprophylaxis should be recommended in most non-critically ill patients who are not at high risk of bleeding.
Supplementary Information
Below is the link to the electronic supplementary material.
Author Contributions
EP: conceptualization, visualization, data curation, writing—review & editing, formal analysis, writing—original draft. AB: conceptualization, visualization, data curation, writing—review & editing, writing—original draft. SF: conceptualization, visualization, data curation, writing—original draft. TCL: visualization, formal analysis, writing—review & editing. AZ: conceptualization, project administration, data curation, visualization writing—original draft, writing—review & editing, supervision. GF: conceptualization, resources, writing—original draft, supervision. GL: conceptualization, project administration, data curation, visualization writing—original draft, writing—review & editing, supervision.
Funding
Todd C. Lee receives research salary support from the Fonds de Recherche du Québec—Santé, unrelated to this project.
Declarations
Conflict of interest
The authors declare they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Giovanni Landoni, Email: landoni.giovanni@hsr.it.
full anticoagulation:
Matteo Angelini, Rosaria Sofia, Iliyan Vlasakov, and Alessandro Pruna
References
- 1.World Health Organization (WHO) Coronavirus (COVID-19) dashboard: weekly epidemiological updates until January 9, 2023. https://covid19.who.int/. Accessed 09 Jan 2023
- 2.Flacco ME, Acuti Martellucci C, Baccolini V, De Vito C, Renzi E, Villari P, Manzoli L. COVID-19 vaccines reduce the risk of SARS-CoV-2 reinfection and hospitalization: meta-analysis. Front Med (Lausanne) 2022;9(9):1023507. doi: 10.3389/fmed.2022.1023507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wieteska-Miłek M, Kuśmierczyk-Droszcz B, Ryczek R, Szmit S, Florczyk M, Mańczak R, Betkier-Lipińska K, Hoffman P, Krzesiński P, Torbicki A, Kurzyna M. Outcomes of COVID-19 in patients vaccinated and unvaccinated against SARS-CoV-2 and suffering from pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension. Pol Arch Intern Med. 2023;5:16406. doi: 10.20452/pamw.16406. [DOI] [PubMed] [Google Scholar]
- 4.Ciceri F, Beretta L, Scandroglio AM, Colombo S, Landoni G, Ruggeri A, et al. Microvascular COVID 19 lung vessels obstructive thromboinflammatory syndrome (MicroCLOTS): an atypical acute respiratory distress syndrome working hypothesis. Crit Care Resusc. 2020;22:95–97. doi: 10.51893/2020.2.pov2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bikdeli B, Madhavan MV, Jimenez D, et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J Am Coll Cardiol. 2020;75(23):2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Information on COVID-19 treatment, prevention and research. COVID-19 treatment guidelines. https://www.covid19treatmentguidelines.nih.gov/. Accessed 5 Oct 5 2021
- 7.Miesbach W, Makris M. COVID-19: coagulopathy, risk of thrombosis, and the rationale for anticoagulation. Clin Appl Thromb Hemost. 2020;26:1076029620938149. doi: 10.1177/1076029620938149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sofia R, Carbone M, Landoni G, Zangrillo A, Dagna L. Anticoagulation as secondary prevention of massive lung thromboses in hospitalized patients with COVID-19. Eur J Internal Med. 2022 doi: 10.1016/j.ejim.2022.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cuker A, Tseng EK, Nieuwlaat R, et al. American Society of Hematology living guidelines on the use of anticoagulation for thromboprophylaxis in patients with COVID-19: May 2021 update on the use of intermediate intensity anticoagulation in critically ill patients. Blood Adv. 2021 doi: 10.1182/bloodadvances.2021005493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spyropoulos AC, Levy JH, Ageno W, et al. Scientific and Standardization Committee communication: clinical guidance on the diagnosis, prevention, and treatment of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18(8):1859–1865. doi: 10.1111/jth.14929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barnes GD, Burnett A, Allen A, et al. Thromboembolism and anticoagulant therapy during the COVID-19 pandemic: interim clinical guidance from the anticoagulation forum. J Thromb Thrombolysis. 2020;50(1):72–81. doi: 10.1007/s11239-020-02138-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moores LK, Tritschler T, Brosnahan S, et al. Prevention, diagnosis, and treatment of VTE in patients with coronavirus disease 2019. Chest. 2020;158(3):1143–1163. doi: 10.1016/j.chest.2020.05.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pilia E, Belletti A, Fresilli S, Finco G, Landoni G. Efficacy and safety of heparin full-dose anticoagulation in hospitalized non-critically ill COVID-19 patients: a meta-analysis of multicenter randomized controlled trials. J Thromb Thrombolysis. 2022;54(3):420–430. doi: 10.1007/s11239-022-02681-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ortega-Paz L, Galli M, Capodanno D, Franchi F, Rollini F, Bikdeli B, Mehran R, Montalescot G, Gibson CM, Lopes RD, Andreotti F, Angiolillo DJ. Safety and efficacy of different prophylactic anticoagulation dosing regimens in critically and non-critically ill patients with COVID-19: a systematic review and meta-analysis of randomized controlled trials. Eur Heart J Cardiovasc Pharmacother. 2021 doi: 10.1093/ehjcvp/pvab070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cochrane Handbook for Systematic Reviews of Interventions. http://handbook-5-1.cochrane.org/. Accessed 22 Mar 2019
- 17.Schulman S, Kearon C, the Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3:692–694. doi: 10.1111/j.1538-7836.2005.01204.x. [DOI] [PubMed] [Google Scholar]
- 18.Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;28(366):l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 19.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann HJ, GRADE Working Group GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.ATTACC Investigators, ACTIV-4a Investigators, REMAP-CAP Investigators, Lawler PR, Goligher EC, Berger JS, Neal MD, McVerry BJ, Nicolau JC, Gong MN, Carrier M, Rosenson RS, Reynolds HR, Turgeon AF, Escobedo J, Huang DT, Bradbury CA, Houston BL, Kornblith LZ, Kumar A, Kahn SR, Cushman M, McQuilten Z, Slutsky AS, Kim KS, Gordon AC, Kirwan BA, Brooks MM, Higgins AM, Lewis RJ, Lorenzi E, Berry SM, Berry LR, Aday AW, Al-Beidh F, Annane D, Arabi YM, Aryal D, Baumann Kreuziger L, Beane A, Bhimani Z, Bihari S, Billett HH, Bond L, Bonten M, Brunkhorst F, Buxton M, Buzgau A, Castellucci LA, Chekuri S, Chen JT, Cheng AC, Chkhikvadze T, Coiffard B, Costantini TW, de Brouwer S, Derde LPG, Detry MA, Duggal A, Džavík V, Effron MB, Estcourt LJ, Everett BM, Fergusson DA, Fitzgerald M, Fowler RA, Galanaud JP, Galen BT, Gandotra S, García-Madrona S, Girard TD, Godoy LC, Goodman AL, Goossens H, Green C, Greenstein YY, Gross PL, Hamburg NM, Haniffa R, Hanna G, Hanna N, Hegde SM, Hendrickson CM, Hite RD, Hindenburg AA, Hope AA, Horowitz JM, Horvat CM, Hudock K, Hunt BJ, Husain M, Hyzy RC, Iyer VN, Jacobson JR, Jayakumar D, Keller NM, Khan A, Kim Y, Kindzelski AL, King AJ, Knudson MM, Kornblith AE, Krishnan V, Kutcher ME, Laffan MA, Lamontagne F, Le Gal G, Leeper CM, Leifer ES, Lim G, Lima FG, Linstrum K, Litton E, Lopez-Sendon J, Lopez-Sendon Moreno JL, Lother SA, Malhotra S, Marcos M, Saud Marinez A, Marshall JC, Marten N, Matthay MA, McAuley DF, McDonald EG, McGlothlin A, McGuinness SP, Middeldorp S, Montgomery SK, Moore SC, Morillo Guerrero R, Mouncey PR, Murthy S, Nair GB, Nair R, Nichol AD, Nunez-Garcia B, Pandey A, Park PK, Parke RL, Parker JC, Parnia S, Paul JD, Pérez González YS, Pompilio M, Prekker ME, Quigley JG, Rost NS, Rowan K, Santos FO, Santos M, Olombrada Santos M, Satterwhite L, Saunders CT, Schutgens REG, Seymour CW, Siegal DM, Silva DG Jr, Shankar-Hari M, Sheehan JP, Singhal AB, Solvason D, Stanworth SJ, Tritschler T, Turner AM, van Bentum-Puijk W, van de Veerdonk FL, van Diepen S, Vazquez-Grande G, Wahid L, Wareham V, Wells BJ, Widmer RJ, Wilson JG, Yuriditsky E, Zampieri FG, Angus DC, McArthur CJ, Webb SA, Farkouh ME, Hochman JS, Zarychanski R (2021) Therapeutic anticoagulation with heparin in noncritically ill patients with covid-19. N Engl J Med 385(9):790–802. 10.1056/NEJMoa2105911. [DOI] [PMC free article] [PubMed]
- 21.Muñoz-Rivas N, Aibar J, Gabara-Xancó C, Trueba-Vicente Á, Urbelz-Pérez A, Gómez-Del Olmo V, Demelo-Rodríguez P, Rivera-Gallego A, Bosch-Nicolau P, Perez-Pinar M, Rios-Prego M, Madridano-Cobo O, Ramos-Alonso L, Alonso-Carrillo J, Francisco-Albelsa I, Martí-Saez E, Maestre-Peiró A, Méndez-Bailón M, Hernández-Rivas JÁ, Torres-Macho J, PROTHROMCOVID Trial Investigators Efficacy and safety of tinzaparin in prophylactic, intermediate and therapeutic doses in non-critically ill patients hospitalized with COVID-19: The PROTHROMCOVID randomized controlled trial. J Clin Med. 2022;11(19):5632. doi: 10.3390/jcm11195632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marcos-Jubilar M, Carmona-Torre F, Vidal R, Ruiz-Artacho P, Filella D, Carbonell C, Jiménez-Yuste V, Schwartz J, Llamas P, Alegre F, Sádaba B, Núñez-Córdoba J, Yuste JR, Fernández-García J, Lecumberri R, BEMICOP Investigators Therapeutic versus prophylactic bemiparin in hospitalized patients with nonsevere COVID-19 pneumonia (BEMICOP Study): an open-label, multicenter, randomized, controlled trial. Thromb Haemost. 2022;122(2):295–299. doi: 10.1055/a-1667-7534. [DOI] [PubMed] [Google Scholar]
- 23.Spyropoulos AC, Goldin M, Giannis D, Diab W, Wang J, Khanijo S, Mignatti A, Gianos E, Cohen M, Sharifova G, Lund JM, Tafur A, Lewis PA, Cohoon KP, Rahman H, Sison CP, Lesser ML, Ochani K, Agrawal N, Hsia J, Anderson VE, Bonaca M, Halperin JL, Weitz JI, HEP-COVID Investigators (2021) Efficacy and safety of therapeutic-dose heparin vs standard prophylactic or intermediate-dose heparins for thromboprophylaxis in high-risk hospitalized patients with COVID-19: the HEP-COVID randomized clinical trial. JAMA Intern Med 181(12):1612–1620. 10.1001/jamainternmed.2021.6203. Erratum in 2022, JAMA Intern Med. 182(2):239 [DOI] [PMC free article] [PubMed]
- 24.Sholzberg M, Tang GH, Rahhal H, AlHamzah M, Kreuziger LB, Áinle FN, Alomran F, Alayed K, Alsheef M, AlSumait F, Pompilio CE, Sperlich C, Tangri S, Tang T, Jaksa P, Suryanarayan D, Almarshoodi M, Castellucci LA, James PD, Lillicrap D, Carrier M, Beckett A, Colovos C, Jayakar J, Arsenault MP, Wu C, Doyon K, Andreou ER, Dounaevskaia V, Tseng EK, Lim G, Fralick M, Middeldorp S, Lee AYY, Zuo F, da Costa BR, Thorpe KE, Negri EM, Cushman M, Jüni P, RAPID trial investigators Effectiveness of therapeutic heparin versus prophylactic heparin on death, mechanical ventilation, or intensive care unit admission in moderately ill patients with covid-19 admitted to hospital: RAPID randomised clinical trial. BMJ. 2021;375:n2400. doi: 10.1136/bmj.n2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blondon M, Cereghetti S, Pugin J, Marti C, Darbellay Farhoumand P, Reny JL, Calmy A, Combescure C, Mazzolai L, Pantet O, Ltaief Z, Méan M, Manzocchi Besson S, Jeanneret S, Stricker H, Robert-Ebadi H, Fontana P, Righini M, Casini A. Therapeutic anticoagulation to prevent thrombosis, coagulopathy, and mortality in severe COVID-19: The Swiss COVID-HEP randomized clinical trial. Res Pract Thromb Haemost. 2022;6(4):e12712. doi: 10.1002/rth2.12712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGuinness LA, Higgins JPT. Risk-of-bias VISualization (robvis): an R package and Shiny web app for visualizing risk-of-bias assessments. Res Syn Methods. 2020 doi: 10.1002/jrsm.1411. [DOI] [PubMed] [Google Scholar]
- 27.Kow CS, Ramachandram DS, Hasan SS. The effect of higherintensity dosing of anticoagulation on the clinical outcomes in hospitalized patients with COVID-19: a meta-analysis of randomized controlled trials. J Infect Chemother. 2022;28(2):257–265. doi: 10.1016/j.jiac.2021.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sholzberg M, da Costa BR, Tang GH, Rahhal H, AlHamzah M, Baumann KL. Randomized trials of therapeutic heparin for COVID-19: a meta-analysis. Res Pract Thromb Haemost. 2021;5(8):e12638. doi: 10.1002/rth2.12638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tleyjeh IM, Kashour T, Mandrekar J, Petitti DB. Overlooked shortcomings of observational studies of interventions in coronavirus disease 2019: an illustrated review for the clinician. Open Forum Infect Dis. 2021;8(8):ofab317. doi: 10.1093/ofid/ofab317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giossi R, Menichelli D, Pani A, Tratta E, Romanini A, Roncato R, Nani A, et al. A systematic review and a meta-analysis comparing prophylactic and therapeutic low molecular weight heparins for mortality reduction in 32,688 COVID-19 patients. Front Pharmacol. 2021;2(12):698008. doi: 10.3389/fphar.2021.698008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jorda A, Siller-Matula JM, Zeitlinger M, Jilma B, Gelbenegger G. Anticoagulant treatment regimens in patients with covid-19: a meta-analysis. Clin Pharmacol Ther. 2022;111(3):614–623. doi: 10.1002/cpt.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Investigators I, et al. Effect of intermediate-dose vs standard-dose prophylactic anticoagulation on thrombotic events, extracorporeal membrane oxygenation treatment, or mortality among patients with COVID-19 admitted to the intensive care unit: the INSPIRATION randomized clinical trial. JAMA. 2021;325:1620–1630. doi: 10.1001/jama.2021.4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morici N, Podda G, Birocchi S, Bonacchini L, Merli M, Trezzi M, Massaini G, Agostinis M, Carioti G, Saverio Serino F, Gazzaniga G, Barberis D, Antolini L, Grazia Valsecchi M, Cattaneo M. Enoxaparin for thromboprophylaxis in hospitalized COVID-19 patients: the X-COVID-19 randomized trial. Eur J Clin Invest. 2022;52(5):e13735. doi: 10.1111/eci.13735. [DOI] [PubMed] [Google Scholar]
- 34.Kyriakoulis KG, Dimakakos E, Kyriakoulis IG, Catalano M, Spyropoulos AC, Schulman S, Douketis J, Falanga A, Maraveyas A, Olinic DM, Belch J, Gerotziafas G, Syrigos K, Kollias A, COVID-19 Thrombosis Collaborative Group, Endorsed by VAS-European Independent Foundation in Angiology/Vascular Medicine, UEMS Division of Angiology/Vascular Medicine/and ESVM-European Society of Vascular Medicine and Supported by the Balkan Working Group (2022) Practical recommendations for optimal thromboprophylaxis in patients with COVID-19: A consensus statement based on available clinical trials. J Clin Med 11(20):5997. 10.3390/jcm11205997 [DOI] [PMC free article] [PubMed]
- 35.De Cobelli F, Palumbo D, Ciceri F, Landoni G, Ruggeri A, Rovere-Querini P, D'Angelo A, Steidler S, Galli L, Poli A, Fominskiy E, Calabrò MG, Colombo S, Monti G, Nicoletti R, Esposito A, Conte C, Dagna L, Ambrosio A, Scarpellini P, Ripa M, Spessot M, Carlucci M, Montorfano M, Agricola E, Baccellieri D, Bosi E, Tresoldi M, Castagna A, Martino G, Zangrillo A (2021) Pulmonary vascular thrombosis in COVID-19 pneumonia. J Cardiothorac Vasc Anesth 35(12):3631–3641. 10.1053/j.jvca.2021.01.011 [DOI] [PMC free article] [PubMed]
- 36.Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers D, Kant KM, Kaptein FHJ, van Paassen J, Stals MAM, Huisman MV, Endeman H. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb Res. 2020;191:148–150. doi: 10.1016/j.thromres.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nopp S, Moik F, Jilma B, Pabinger I, Ay C. Risk of venous thromboembolism in patients with COVID-19: a systematic review and meta-analysis. Res Pract Thromb Haemost. 2020;4(7):1178–1191. doi: 10.1002/rth2.12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roncon L, Zuin M, Barco S, et al. Incidence of acute pulmonary embolism in COVID-19 patients: Systematic review and meta-analysis. Eur J Intern Med. 2020;82:29–37. doi: 10.1016/j.ejim.2020.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buijsers B, Yanginlar C, Maciej-Hulme ML, de Mast Q, van der Vlag J. Beneficial non-anticoagulant mechanisms underlying heparin treatment of COVID-19 patients. EBioMedicine. 2020 doi: 10.1016/j.ebiom.2020.102969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mycroft-West CJ, Su D, Pagani I, Rudd TR, Elli S, Gandhi NS, Guimond SE, Miller GJ, Meneghetti MCZ, Nader HB, Li Y, Nunes QM, Procter P, Mancini N, Clementi M, Bisio A, Forsyth NR, Ferro V, Turnbull JE, Guerrini M, Fernig DG, Vicenzi E, Yates EA, Lima MA, Skidmore MA. Heparin inhibits cellular invasion by SARS-CoV-2: structural dependence of the interaction of the spike S1 receptor-binding domain with heparin. Thromb Haemost. 2020;120(12):1700–1715. doi: 10.1055/s-0040-1721319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Litov L, Petkov P, Rangelov M, Ilieva N, Lilkova E, Todorova N, Krachmarova E, Malinova K, Gospodinov A, Hristova R, Ivanov I, Nacheva G. Molecular mechanism of the anti-inflammatory action of heparin. Int J Mol Sci. 2021;22(19):10730. doi: 10.3390/ijms221910730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deeks JJ, Higgins JPT, Altman DG (2022). Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (ed) Cochrane handbook for systematic reviews of interventions version 6.3 (updated February 2022), Cochrane. www.training.cochrane.org/handbook. Accessed 09 Jan 2023
- 43.Mehrabi F, Farshbafnadi M, Rezaei N. Post-discharge thromboembolic events in COVID-19 patients: a review on the necessity for prophylaxis. Clin Appl Thromb Hemost. 2023;29:10760296221148477. doi: 10.1177/10760296221148477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang H, Lao Q, Zhang J, Zhu J. Coagulopathy in COVID-19 and anticoagulation clinical trials. Best Pract Res Clin Haematol. 2022;35(3):101377. doi: 10.1016/j.beha.2022.101377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ravinetto R, Caillet C, Zaman MH, Singh JA, Guerin PJ, Ahmad A, Durán CE, Jesani A, Palmero A, Merson L, Horby PW, Bottieau E, Hoffmann T, Newton PN. Preprints in times of COVID19: the time is ripe for agreeing on terminology and good practices. BMC Med Ethics. 2021;22(1):106. doi: 10.1186/s12910-021-00667-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.