Abstract
We propose a temperature-dependent scaling factor for the ‘one-point method’ to mitigate the overestimation of the maximum carboxylation capacity of photosynthesis at high temperatures.
Keywords: Global vegetation models, global warming, photosynthesis, temperature response, V cmax
The ‘one-point method’ (OPM) is a quick approach to estimate the maximum apparent carboxylation rate of Rubisco (Vʹ cmax ) based on a single measurement of leaf carbon assimilation rate taken under saturating light and ambient CO 2 , lately equal to 400 ppm ( De Kauwe et al., 2016 ). However, the OPM overestimates Vʹ cmax at high temperatures ( Burnett et al., 2019 ). This overestimation results from the reliance of the method on a linear relationship between Vʹ cmax and leaf respiration rates under light (R day ), as a fixed R day :V cmax ratio equal to 1.5%, while these parameters have different temperature dependencies. Here, we highlight the importance of the adoption of a temperature-dependent scaling factor for the R day :V cmax ratio as a way to prevent the overestimation of the photosynthetic capacity at temperatures >35 °C.
The maximum carboxylation rate of Rubisco (Vcmax) is a key photosynthetic enzyme characteristic that reflects plant individual fitness. It is also a prescribed variable of global vegetation models (GVMs), which calculate primary productivity of terrestrial vegetation. This parameter is usually estimated from CO2 response curves (A–Ci curves, where Ci is intercellular [CO2]) (e.g. Farquhar et al., 1980—the FvCB model). Recently, the OPM was proposed as a fast alternative to standard full biochemical curve-fitting methods (De Kauwe et al., 2016). Against the gold-standard A–Ci curves, the OPM has proven to be very useful as it allows for the characterization of a large number of species and individual leaves in a short period of time (5–10 min per individual), enabling a better parameterization of highly diverse tropical vegetation communities within GVMs. Another alternative available is the rapid A–Ci technique (RACiR) (Stinziano et al., 2017), which upon any change in conditions, such as leaf temperature, requires some data processing after the measurements are taken. Apart from being fast, another advantage of the OPM is the minimization of negative effects of performing multiple full A–Ci curves on the same leaf in order to obtain the temperature dependency of photosynthetic parameters. Therefore, providing measurements are taken with the proper care and allowing enough time for stabilization of gas exchange fluxes, the OPM has the potential to significantly improve our ability to describe the carbon uptake strategies of highly diverse plant communities.
According to De Kauwe et al. (2016), Vʹcmax can be calculated as:
| (1) |
Where Ci is the intercellular CO2 concentration, Γ* is the CO2 compensation point in the absence of mitochondrial respiration, Km is the Michaelis–Menten constant of Rubisco, and Rday is the leaf mitochondrial respiration in the light. When Rday is not determined experimentally, the authors suggest the use of an estimated Rday ratio equal to 1.5% of Vʹcmax (0.015 in Equation 2).
| (2) |
However, the OPM provides an overestimation of Vʹcmax, especially at high temperatures above 30 °C (De Kauwe et al. 2016; Slot and Winter, 2017; Burnett et al. 2019). As the temperature dependencies of Rday and Vcmax differ considerably from each other (Wang et al. 2020), assuming a linear relationship between these parameters as a constant ratio results in the overestimation of Vʹcmax, as Rday peaks at a much higher leaf temperature. A better accuracy of the OPM is needed to improve its performance at higher leaf temperatures. In this sense, we propose here the adoption of a temperature-dependent scaling factor for the Rday:Vʹcmax ratio in order to improve predictions of Vʹcmax.
The temperature dependencies of both Rday and Vcmax may be defined by standard Arrhenius functions, following Kumarathunge et al. (2019) as:
| (3) |
and
| (4) |
where RdayT and VcmaxT are respiration and carboxylation rates at a given leaf temperature (Tk in Kelvin), RdayR and VcmaxR are the rates at a reference leaf temperature of 25 °C, R is the universal gas constant (8.314 J mol−1 K−1), EaR (kJ mol−1) is the activation energy for respiration, and EaV (kJ mol−1), ΔSV (J mol−1 K−1), and HdV (kJ mol−1) are respectively the activation energy, entropy, and deactivation energy of Vcmax. The adopted values for model constants are available in Supplementary Table S1.
Our new suggested model modifies Equation 2 for:
| (5) |
where ρ is the temperature-dependent scaling on the Rday ratio (1.5% of Vʹcmax), which is calculated by dividing Equation 3 by Equation 4.
The robustness of this approach was tested using 278 unpublished A–Ci curves (12 CO2 concentration steps) under saturating light (2000 μmol m–2 s–1), collected from 31 tropical species naturally occurring in savannas and Amazon rainforest in Brazil (Supplementary Table S2) using two portable gas exchange systems (LI-6800, Li-Cor Inc., Lincoln, NE, USA). Leaf temperatures were kept constant during each curve, but ranged from 25 °C to 45 °C among curves, and the leaf chamber relative humidity was not controlled. Over the whole dataset, the leaf-to-air vapor pressure deficit ranged from 1.08 kPa to 7.71 kPa (3.84 ± 1.65), while stomatal conductance ranged from 0.05 mol m−2 s−1 to 0.34 mol m−2 s−1 (0.134 ± 0.06). To estimate Vʹcmax and Vʹcmax–ρ values, we selected a single point measurement from each A–Ci curve, corresponding to ambient CO2 concentrations (Ca) between 390 μmol mol–1 and 410 μmol mol–1. The Kc, Ko, and Γ* values used in Equations 2 and 3 were calculated according to the temperature dependencies listed in Bernacchi et al. (2002) and De Kauwe et al. (2016). Linear regressions were used to compare both Vʹcmax and Vʹcmax–ρ with Vcmax from full A–Ci curves (Duursma, 2015). The slopes between the two linear regressions were compared using ANOVA. Moreover, the distribution of the residuals of the regression models as a function of leaf temperature were used for method accuracy comparisons. Additionally, a multivariate sensitivity analysis was performed to investigate the role of temperature over the range values of the Arrhenius constants regarding the model outputs. All analyses we performed on R environment (R Core Team, 2020).
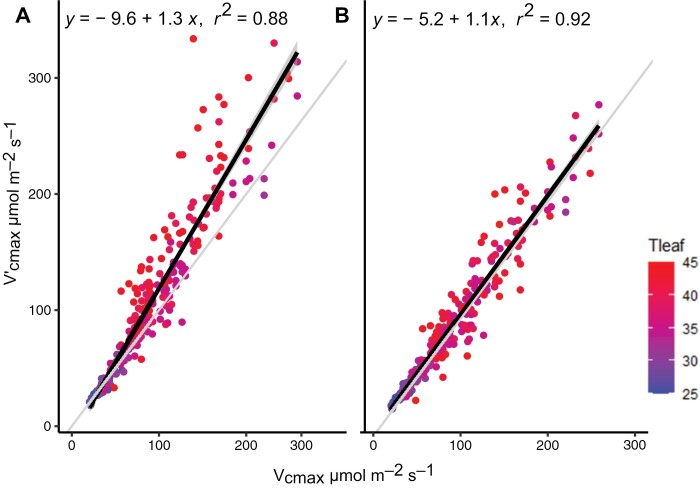
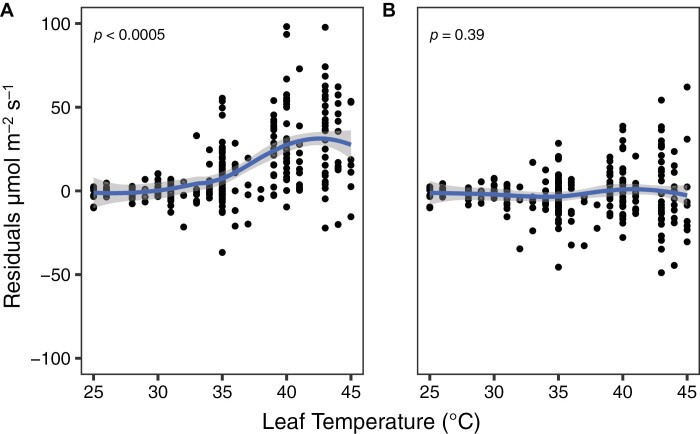
The temperature-dependent scaling factor provided a substantially better correspondence between Vcmax values from FvCB fits against the Vʹcmax–ρ values, when compared with Vʹcmax values from the original OPM (Fig. 1) (slope=1.10; r2=0.93). The ANOVA test revealed that the outputs from the two models were statistically different (P<0.005). The evaluation of the distribution of the residuals from the two regression models highlights how the overestimation of Vʹcmax by the original version of the OPM increases with leaf temperature (Fig. 2A), where overestimations of ~25% were observed above 35 °C. In contrast, no trend was observed for the residuals from the new model in relation to leaf temperature (Fig. 2B). The adoption of the Arrhenius temperature dependency for Rday (Equation 3) was preferred over a Q10 temperature coefficient (Atkin et al., 2015), the latter resulting in an overestimation of Vʹcmax–ρ values of ~25% (slope=1.29, r2=0.85) (Supplementary Fig. S1), with a non-significant difference between its slope against the model using the the original OPM approach (P=0.69). The sensitivity analysis revealed that the temperature-dependent scaling factor is strongly influenced by both EaR and EaV above 25°C (Supplementary Fig. S2). As temperature increases, their relative importance decreases, while ΔSV and the interaction among parameters become the main factors determining Rday:Vcmax.
Fig. 1.
Comparison of linear regression models between Vcmax estimated from full A–Ci curves against apparent photosynthetic capacity estimated by the ‘one-point method’ (Vʹcmax; Equation 2) (A), and the modified version including the temperature dependency (Vʹcmax–ρ; Equation 5) (B). The light gray line is the 1:1 relationship.
Fig. 2.
Residuals of maximum carboxylation rate, Vcmax, estimated from A–Ci curves estimated from apparent maximum carboxylation capacity, Vʹcmax (Equation 2) using the estimated Rday:Vcmax ratio (A), and using the temperature-dependent scaling factor (B) as a function of leaf temperature.
The adoption of a temperature-dependent scaling is indeed an important adjustment of the OPM, producing accurate estimations of Vʹcmax at high temperatures where there is proportionality of Vʹcmax and Rday responses to temperature (Wang et al., 2020), as demonstrated empirically in Supplementary Fig. S3. Therefore, the temperature-dependent scaling factor proposed here should allow for better determinations of the temperature response of Vʹcmax. However, providing reliable data for derivations of the Arrhenius constants is needed, as these constants vary among functional types of plants and species (Kumarathunge et al., 2019). Apart from the difficult task of adopting appropriate values for the Arrhenius constants for Rday and Vcmax, other aspects might be important as well. The application of biochemical models of photosynthesis often disregards the influence of gm over estimations of Vʹcmax, assuming it to be large enough to cause Ci to be equal to chloroplastic CO2 concentration (Cc). However, gm varies with leaf temperature as a result of both enzymatic dynamics and CO2 diffusion, resulting in an overestimation of Vcmax when assuming an infinite gm (von Caemmerer and Evans, 2015), therefore directly impacting the Rday:Vcmax ratio. Similarly, the temperature dependency of the Γ* is assumed to be invariant among plant species, and a single function is often used to scale this parameter to a specific leaf temperature (Bernacchi et al., 2002). However, depending on the Γ* assumption, the relationship between temperature and the Rday:Vcmax ratio may change (De Kauwe et al., 2016); this is because the stoichiometry of CO2 release by Rubisco oxygenation depends on how Γ* responds to temperature (Bernacchi et al., 2002). Therefore, a better understanding of possible variations of the temperature dependencies of both gm and Γ* is still needed in order to improve the understanding of temperature influence on the estimations of Vʹcmax, irrespective of the estimation method used.
In conclusion, the inclusion of a temperature-dependent scaling factor in the ‘one-point method’ extends its applicability to leaf temperatures >35 °C. Therefore, this approach should contribute to the characterization of vegetation communities and provide data for the estimation of Arrhenius parameters without stressing leaves by performing repeated CO2 response curves. Efforts should be directed to the understanding of the variation of temperature dependencies of the photosynthetic parameters, specifically the entropy and the activation and deactivation energies of Vcmax, Rday, and also the maximum electron transport rate (Jmax).
Supplementary data
The following supplementary data are available at JXB online.
Table S1. Dataset of primary parameters and their temperature dependency used to estimate Rday and Vʹcmax temperature response in Equations 3 and 4.
Table S2. Species studied with the biome, their family, number of individuals (N individuals) and curves (N curves), and temperature range curves.
Fig. S1. Comparison of linear regression models between Vcmax estimated from full A–Ci curves against apparent photosynthetic capacity estimated by the ‘one-point method’.
Fig. S2. Normalized partitioning of the variation of the influence of individual coefficients over model output at a broad leaf temperature range (sensitivity analysis).
Fig. S3. Estimated Rday (Rday:Vcmax ratio) as a function of leaf temperature using the De Kauwe et al. (2016) model.
Acknowledgements
We thank the University of São Paulo (USP), Wageningen University and Research (WUR), and National Institute for Amazonian Research (INPA) and the São Paulo Research Foundation—FAPESP for financial and logistical support for fieldwork, and the Coordination for Improvement of Higher-Level Personnel (CAPES) for the scholarships. We acknowledge the valuable inputs from Professor Jon Lloyd, Professor Keith Bloomfield, Vinicius Dorea, and Luiza Gallo.
Contributor Information
Tony César de Sousa Oliveira, FFCLRP, Department of Biology, University of São Paulo (USP), Ribeirão Preto, Brazil; Plant Ecology and Nature Conservation Group, Wageningen University and Research (WUR), Wageningen, The Netherlands.
Maquelle Neves Garcia, National Institute for Amazonian Research (INPA), Manaus, Brazil.
Elmar Veenendaal, Plant Ecology and Nature Conservation Group, Wageningen University and Research (WUR), Wageningen, The Netherlands.
Tomas Ferreira Domingues, FFCLRP, Department of Biology, University of São Paulo (USP), Ribeirão Preto, Brazil.
Johannes Kromdijk, University of Cambridge, UK.
Author contributions
TCSO, TFD, and MNG: design; TCSO and MNG: data collection. TCSO: data analysis; TCSO, TFD, EV, and MNG: interpreting the analysis and results; TCSO: writing—first draft; TFD, EV, and MNG: contributing to writing.
Conflict of interest
The authors have no conflicts to declare.
Funding
This study was supported by Wageningen University and Research (WUR) to TCSO (project number 5160957987); the Partnership for Enhanced Engagement in Research program to TFD (PEER grant agreement AID-OAA-A-11-00012); the National Council for Scientific and Technological Development (CNPq) to Flávia R.C. Costa (LTER/PELD-grants #441282/2016-4, 403764/2012-2, and 558244/2009-2); and the São Paulo Research Foundation (FAPESP) (Nordeste project thematic grant NE/N012488) to J. Lloyd.
Data availability
The data supporting the findings of this study are available from the corresponding author, Tony de Oliveira, upon request.
References
- Atkin OK, Bloomfield KJ, Reich PB, et al. 2015. Global variability in leaf respiration in relation to climate, plant functional types and leaf traits. New Phytologist 206, 614–636. [DOI] [PubMed] [Google Scholar]
- Bernacchi, CJ, Portis AR, Nakano H, Caemmerer S, Long SP.. 2002. Temperature response of mesophyll conductance. implications for the determination of rubisco enzyme kinetics and for limitations to photosynthesis in vivo. Plant Physiology 130, 1992–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett AC, Davidson KJ, Serbin SP, Rogers A.. 2019. The ‘one-point method’ for estimating maximum carboxylation capacity of photosynthesis: a cautionary tale. Plant, Cell & Environment 42, 2472–2481. [DOI] [PubMed] [Google Scholar]
- De Kauwe MG, Lin YS, Wright IJ, et al. 2016. A test of the ‘one-point method’ for estimating maximum carboxylation capacity from field-measured, light-saturated photosynthesis. New Phytologist 210, 1130–1144. [DOI] [PubMed] [Google Scholar]
- Duursma RA. 2015. Plantecophys—an R package for analysing and modelling leaf gas exchange data. PLoS One 10, e01433461–e01433413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar GD, Von Caemmerer S, Berry JA.. 1980. A biochemical-model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149, 78–90. [DOI] [PubMed] [Google Scholar]
- Kumarathunge DP, Medlyn BE, Drake JE, et al. 2019. Acclimation and adaptation components of the temperature dependence of plant photosynthesis at the global scale. New Phytologist 22, 768–784. [DOI] [PubMed] [Google Scholar]
- R Core Team. 2020. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. https://www.R-project.org/. [Google Scholar]
- Slot M, Winter K.. 2017. In situ temperature relationships of biochemical and stomatal controls of photosynthesis in four lowland tropical tree species. Plant, Cell & Environment 40, 3055–3068. [DOI] [PubMed] [Google Scholar]
- Stinziano JR, Morgan PB, Lynch DJ, et al. 2017. The rapid A–Ci response: photosynthesis in the phenomic era. Plant, Cell & Environment 40, 1256–1262. [DOI] [PubMed] [Google Scholar]
- Wang H, Atkin OK, Keenan TF, Smith NG, Wright IJ, Bloomfield KJ, Kattge J, Reich PB, Prentice IC.. 2020. Acclimation of leaf respiration consistent with optimal photosynthetic capacity. Global Change Biology 26, 2573–2583. [DOI] [PubMed] [Google Scholar]
- von Caemmerer S, Evans JR.. 2015. Temperature responses of mesophyll conductance. Plant, Cell & Environment 38, 629–637. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author, Tony de Oliveira, upon request.