Abstract
The overall goal for this review is to summarize the current body of knowledge about the structure and function of major known antigens of Streptococcus pneumoniae, a major gram-positive bacterial pathogen of humans. This information is then related to the role of these proteins in pneumococcal pathogenesis and in the development of new vaccines and/or other antimicrobial agents. S. pneumoniae is the most common cause of fatal community-acquired pneumonia in the elderly and is also one of the most common causes of middle ear infections and meningitis in children. The present vaccine for the pneumococcus consists of a mixture of 23 different capsular polysaccharides. While this vaccine is very effective in young adults, who are normally at low risk of serious disease, it is only about 60% effective in the elderly. In children younger than 2 years the vaccine is ineffective and is not recommended due to the inability of this age group to mount an antibody response to the pneumococcal polysaccharides. Antimicrobial drugs such as penicillin have diminished the risk from pneumococcal disease. Several pneumococcal proteins including pneumococcal surface proteins A and C, hyaluronate lyase, pneumolysin, autolysin, pneumococcal surface antigen A, choline binding protein A, and two neuraminidase enzymes are being investigated as potential vaccine or drug targets. Essentially all of these antigens have been or are being investigated on a structural level in addition to being characterized biochemically. Recently, three-dimensional structures for hyaluronate lyase and pneumococcal surface antigen A became available from X-ray crystallography determinations. Also, modeling studies based on biophysical measurements provided more information about the structures of pneumolysin and pneumococcal surface protein A. Structural and biochemical studies of these pneumococcal virulence factors have facilitated the development of novel antibiotics or protein antigen-based vaccines as an alternative to polysaccharide-based vaccines for the treatment of pneumococcal disease.
Human infections caused by gram-positive bacterial pathogens are increasingly difficult to treat, predominantly due to the emergence of antibiotic-resistant strains not only against penicillin and penicillin-like antibiotics but even against novel antibiotics such as vancomycin (108). One such gram-positive bacterial organism is Streptococcus pneumoniae, a human pathogen that colonizes the upper respiratory tract and causes life-threatening diseases such as pneumonia, bacteremia, and meningitis throughout the world (104). Disease rates are particularly high in young children, the elderly, and patients with predisposing conditions such as asplenia, chronic medical conditions, or immunosuppressive illnesses, particularly AIDS (57, 77, 105). Around 5 million children worldwide younger than 5 years die each year from pneumonia, with S. pneumoniae being the main causative agent (85). In the United States alone, more than half a million cases of pneumococcal pneumonia are reported each year, with 5 to 7% of them being fatal (5, 27, 75, 146, 163). Most of these infections are found in the elderly. S. pneumoniae also causes less serious but very prevalent diseases like otitis media and sinusitis. Each year there are approximately 7 million cases of otitis media in the United States alone, accounting for up to 12 million office visits to pediatricians (146). It also causes significant morbidity and leads to high medical costs. There are an estimated 3,000 cases of meningitis and 50,000 cases of bacteremia per year in the United States (5, 24, 146, 163). In adults, 60 to 87% of pneumococcal bacteremia is associated with pneumonia (1, 20, 83). The mortality rate of 40,000 per year caused by this pathogen in the United States is larger than the mortality rate caused by any other bacterial pathogen (2, 46, 85). An improved treatment and/or vaccine against S. pneumoniae is one of the top vaccine priorities in the world (31).
Treatment of Pneumococcal Infections
The currently licensed 23-valent polysaccharide pneumococcal vaccine is only moderately effective, and it is not prescribed for children younger than 2 years due to poor antibody responses to the polysaccharides. The vaccine contains 23 purified capsular polysaccharide antigens of S. pneumoniae (serotypes 1 to 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19A, 19F, 20, 22F, 23F, and 33F) that represent at least 85 to 90% of the serotypes that cause invasive infections. This vaccine, which is manufactured by Merck & Co., Inc., and Lederle Laboratories, replaced an earlier 14-valent vaccine in 1983. The serotypes 6B, 9V, 14, 19A, 19F, and 23F cause most drug-resistant infections in the United States (23, 24, 52, 64), and although antimicrobial drugs such as penicillin have diminished the risk from pneumococcal disease, the proportion of strains that are resistant to antibiotics in the United States (142) and other parts of the world (7) is steadily increasing. In some areas, 35% of pneumococcal isolates are resistant to penicillin (3, 42, 64). Many of these isolates are also resistant to other antimicrobial drugs, with some isolates susceptible only to vancomycin, a last resort in many hospitals. Recently, however, vancomycin tolerance emerged in pneumococci (108). This reinforces the need for an effective improved vaccine and/or effective new drugs against pneumococcal infections.
Pneumococcal Virulence Factors
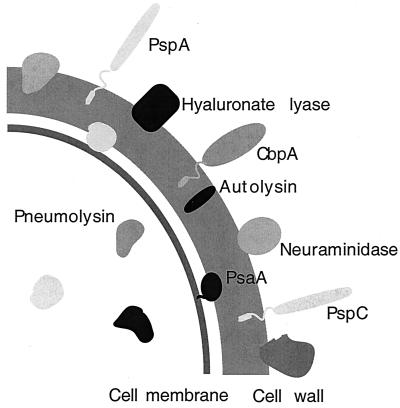
Certain proteins or enzymes displayed on the surface of gram-positive organisms significantly contribute to pathogenesis and might be involved in the disease process caused by these pathogens. Often, these proteins are involved in direct interactions with host tissues or in concealing the bacterial surface from the host defense mechanisms. S. pneumoniae is not an exception in this regard. In the past, the polysaccharide capsule was considered the primary virulence factor of S. pneumoniae because nonencapsulated bacteria are almost completely harmless compared with the same encapsulated strain. Recent studies, however, have suggested that certain pneumococcal proteins, including hyaluronate lyase (Hyl) (90), pneumolysin (Ply) (48), two neuraminidases (NanA and NanB) (90), major autolysin (LytA) (90), choline binding protein A (CbpA) (129), pneumococcal surface antigen A (PsaA) (134), and pneumococcal surface protein A (PspA) (96), could be used as potential vaccine candidates (Fig. 1). If antibodies to these proteins could offer better protection to humans, they could provide the source of a pneumococcal vaccine to be used in conjunction with or in place of the more traditional capsular polysaccharide vaccine or the conjugate vaccine that is under development. Some of these proteins, such as PspA or Ply, have already shown significant promise for use in an alternative vaccine approach. PspA, for example, can elicit antibodies in mice that protect against inocula more than 100 times the 50% lethal dose (97).
FIG. 1.
Schematic diagram of the virulence factors of S. pneumoniae.
Pneumococcal Vaccine Development
Individual contributions of known virulence factors of S. pneumoniae to the pathogenesis of this organism and the likely development of a novel pneumococcal vaccine have recently been investigated (8). The antigens that reduced the virulence of the organism and are probably the best candidates for a vaccine developments appear to be Ply, LytA, or PspA. The studies of D39 pneumococci based on deleting selected genes from the chromosome showed that in an intraperitoneal challenge experiment, nanA, hyl, and cbpA deletions did not have a significant impact on virulence. However, when two genes were compromised, a significant attenuation in the virulence has been achieved for ply hyl, ply pspA, and ply cbpA double mutants. Mutagenesis of nanA or lytA in addition to ply did not yield additionally attenuated virulence. The analysis of these studies suggested that the major role of LytA in pathogenesis of pneumococci is probably the release of cytoplasmic Ply (8, 89). Individually, Ply and LytA contribute significantly to pneumococcal virulence, but when Ply is not expressed in the mutated cells, LytA does not provide additional protection since there are no Ply molecules to release from the cytoplasm of these cells.
PNEUMOCOCCAL SURFACE PROTEIN A
Antibody studies have shown that PspA is located on the cell wall of pneumococci (98) and that it is found on every S. pneumoniae strain discovered to date (34). PspA is a surface protein with variable molecular size ranging from 67 to 99 kDa (161). Based on sequence analysis, the protein has four distinct domains: an N-terminal highly charged α-helical region (288 amino acids in strain Rx1), a proline-rich domain (83 amino acids), a stretch of 10 highly conserved 20-amino-acid repeats, and a tail of 17 slightly hydrophobic residues at the C terminus (166). The N-terminal end extends from the cell wall and possibly protrudes outside the capsule (166). Secondary-structure predictions have shown that the N-terminal domain is highly helical and most probably a coiled-coil structure (166).
The function of PspA, a protective antigen for pneumococci, appears to be protection against host complement system (38, 166, 167). Structural studies showed that PspA has a highly polar electrostatic charge, which results in capsular charge stabilization through the electropositive end of the molecule and in prevention of complement activation through the predominant electronegative part of PspA (70). Biological evidence of the anticomplementary properties of PspA has been observed and has shown that PspA reduced the complement-mediated clearance and phagocytosis of S. pneumoniae (19). All protective monoclonal antibodies reactive to PspA on the pneumococcal cell surface bind to the N-terminal half of the molecule, suggesting that this part of the molecule is surface exposed (94). This α-helical domain also exhibits more variability due to accumulation of mutations, reinforcing the surface exposed character of PspA.
Coiled-Coil Structure
A number of other surface molecules of gram-positive organisms discovered to date have been cloned and sequenced (51). Conformational analysis of their sequences showed that many of these molecules have a very highly α-helical component with repeating seven-residue blocks that are characteristic only to proteins exhibiting a coiled-coil structure. Other known surface molecules of bacterial organisms usually exhibit predominant β-sheet, β-turn, and random-coil structures with only a small number of helical conformations (51).
The seven-residue repeat or the heptad pattern has been clearly identified in the N-terminal part of the PspA molecule (166). In this respect, the N-terminal PspA sequence is similar to other coiled-coil α-helical proteins that show this characteristic motif (residues at positions a, b, c, d, e, f, and g in a coiled coil), with hydrophobic residues at positions a and d and hydrophilic residues at positions b, c, e, f, and g (37, 99) (Fig. 2). This conformational analysis suggests the coiled-coil structure of the N-terminal module of PspA. Outside of bacterial proteins, the heptad repeats are found in eukaryotic coiled-coil proteins like myosin or tropomyosin (84, 119). In addition, the structural properties of PspA were analyzed using biophysical methods such as circular dichroism spectroscopy and sedimentation velocity and equilibrium studies, which provided evidence for an elongated, rod-like, coiled-coil structure for the molecule (Fig. 3A and B). The biophysical studies, together with computer modeling, led to the formulation of a three-dimensional model of the molecule and the subsequent elucidation of its likely role and function in pneumococcal infection. The molecule's N-terminal functional part is highly charged and polar. The C-terminal attachment module anchors PspA to the pneumococcal surface. The proline-rich region acts as a tether and allows for greater flexibility and movement of the N-terminal functional module. The electropositive end of the functional module of the molecule stabilizes the electronegative capsule (capsular polysaccharides of essentially all pathogenic organisms, especially pneumococci, are highly electronegative) (Fig. 3B and C). The other end of the functional module of PspA is electronegative and points away from pneumococci due to repulsive interactions with the electronegative capsule. This electronegative part of the PspA molecule on the pneumococcal surface probably prevents C3-mediated binding of the host complement to pneumococci, which ultimately would lead to the lysis of S. pneumoniae cells. Biological studies described below confirmed that PspA reduces the complement-mediated clearance and phagocytosis of S. pneumoniae (19). As reported by Briles et al. (19), these studies involved a bystander complement assay with an encapsulated mutant S. pneumoniae strain lacking PspA as well as the isogenic pneumococcal strain expressing PspA. The PspA− mutant was found to fix more complement. The same assay with a PspA+ strain detected activated C3 but not C3 deposited on pneumococci. Similarly, infections of nonimmune mice with PspA− type 3 capsular S. pneumoniae lead to greater early activation serum complement (as evidenced from the increased disappearance of antigenic C3 from the circulation) than do infections with the PspA+ isogenic parent (19). All these results suggest that PspΔ can reduce complement consumption by pneumococci and lead to their reduced complement-mediated clearance and phagocytosis.
FIG. 2.
The heptad repeat pattern of the N-terminal helical part of the deduced amino acid sequence of the Rx1 PspA. The sequence of the first 288 amino acids of the Rx1 PspA is arranged in heptad repeat blocks characteristic of coiled-coil proteins. The residues in the right part of the figure (residues 120 to 123, 173 to 175, and 208 to 212) do not satisfy the requirements for the coiled-coil structure.
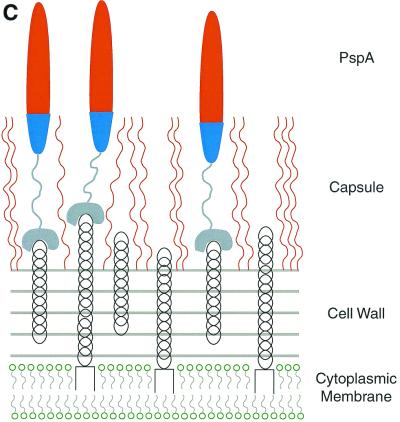
FIG. 3.
Structural model of PspA (A), the electrostatic potential distribution on its surface (B), and the schematic arrangement of PspA molecules on the surface of S. pneumoniae (C). (A) The N-terminal part of PspA has an elongated rod-like shape built from antiparallel coiled-coil α-helices. The drawing is based on the model of a PspA molecule containing amino acids 1 to 288 (70). (B) The color coding of the PspA surface corresponds to the magnitude of the electrostatic potential: blue, electropositive; red, electronegative. The model also depicts the highly charged and polar character of PspA. (C) The character of the surface interactions of PspA with teichoic acids and the capsule exposes the highly electronegative end of the molecule outside of the bacterial cell.
Attachment to the Surface of S. pneumoniae
Pneumococci display an unusual surface molecule, phosphocholine, on the cell wall teichoic acid and the membrane-bound lipoteichoic acid (154). Studies have shown that PspA attaches itself to S. pneumoniae by noncovalent binding to the choline of both lipoteichoic and teichoic acids via its C-terminal end, consisting of the repeat region, also called the choline binding region (CBR) (Fig. 3C) (167). PspA is not the only choline binding protein (CBP) present on the surface of this pathogen. Examples of other pneumococcal CBPs are the major cell wall hydrolase LytA (54) and an adhesin choline binding protein A (CbpA) (129). LytA is an amidase functioning in the separation of daughter cells during cell division (127) and is required for cell lysis (155), whereas CbpA appears to be the first known protein adhesin on the pneumococcal surface (129). The CBR motif has also been found among the surface proteins of other bacteria (164) like Clostridium acetobutylicum, Clostridium difficile, Streptococcus mutans, and Streptococcus downei. All these CBRs have the characteristic feature of the repeat sequences at the C-terminal region. This family of such ligand binding proteins seems to have a modular structure, with the CBR being responsible for the attachment to the pathogen and the other module being responsible for the function of the molecule. This analysis reinforces the notion that the N-terminal part of PspA, having such modular design, is responsible for its function.
Expression by Other Bacteria
It would be unlikely if PspA or PspA-like molecules were expressed only by S. pneumoniae bacteria. Analysis of the genomic sequences to identify similar proteins to the functional part of PspA yielded PspC, a PspA-like molecule with similar structure and function and the only homologous molecule which is closely related in its properties to PspA (19). Low homology was also identified to other highly α-helical molecules like myosin and tropomyosin (70). However, analysis of yet unfinished microbial genomes resulted in multiple homology hits, suggesting that PspA or PspA-like molecules might be present in other bacterial organisms (Fig. 4). The predominant coiled-coil pattern has been detected among all of these homologous proteins (M. J. Jedrzejas and R. S. Becker, submitted for publication). The coiled-coil pattern of these novel proteins was seldom disrupted, as was observed in PspA molecules examined earlier.
FIG. 4.
Multiple alignment of the N-terminal part of Rx1 PspA with sequences from selected microbial genomes. The sequences were aligned using Multalin program (32) and displayed using Multiple Protein Sequence Alignment (MPSA) software (14). The sequence data represented correspond to unfinished genome sequences obtained from www.ncbi.nlm.nih.gov/BLAST/unfinishedgenome.html: S. pyogenes, Streptococcus pyogenes Contig1; E.faecalis, Enterococcus faecalis gef_6217; S.aureus, Staphylococcus aureus 4433; P.falciparum, Plasmodium falciparum Contig34. The color coding for the alignment is as follows: red, single fully conserved residue; green, fully conserved strong or weaker groups; blue, no particularity.
It is likely that other bacterial organisms utilize a PspA-like-mediated anticomplementary defense mechanism, as does S. pneumoniae. The sequence similarities suggest that other bacterial organisms in the genus Streptococcus, such as Streptococcus pyogenes, as well as other bacteria, such as Plasmodium falciparum, Enterococcus faecalis, and Staphylococcus aureus, might use similar defense elements (Fig. 4).
HYALURONATE LYASE
Hyaluronate lyase (Hyl) is another major surface protein of S. pneumoniae with potential antigenetically variable properties that might be essential for full pneumococcal virulence (15). Thus, it might represent another alternative for a pneumococcal vaccine or drug target, especially when combined with other pneumococcal virulence factors such as PspA or pneumolysin (8).
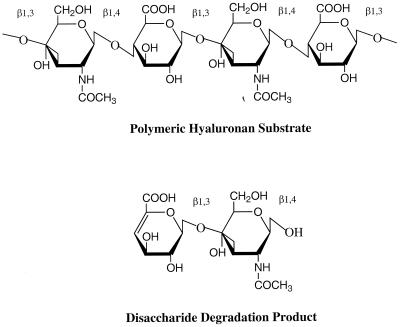
Hyaluronate lyase is part of a broader group of enzymes called hyaluronidases. The hyaluronidase enzyme-mediated facilitation of tissue invasion by breaking down the extracellular matrix (ECM) components was first suggested in a report by Duran-Reynals (43) describing bacterial “spreading factors” (101). Increased tissue permeability caused by the action of hyaluronidase on the ECM appears to play a role in wound infections, pneumonia, and other sepses such as bacteremia and meningitis. In the past few years, the first sequences of prokaryotic and animal hyaluronidases have been determined. There are at least three types of hyaluronidases which degrade hyaluronan by different mechanisms (81): (i) endo-β-N-acetyl-d-hexosaminidases degrade the high-molecular-weight substrate by hydrolysis to smaller end products, mainly tetrasaccharides, although other saccharides can also be present; (ii) β-endoglucuronidase degrades HA, also using hydrolysis, and produces similar-size degradation end products to those produced by the enzyme above, but they degrade the substrate at a different place, yielding mainly tetrasaccharides but of different composition from those above; and (iii) bacterial hyaluronate lyases use β-elimination to degrade hyaluronan and often yield disaccharides as the main end product; however, saccharides of different size are often present. S. pneumoniae hyaluronate lyase cleaves the 1,4-glycosidic linkage between N-acetyl-β-d-glucosamine and d-glucuronic acid residues in hyaluronan and catalyzes the release of unsaturated polysaccharides, with the disaccharide unit 2-acetamido-2-deoxy-3-O-(β-d-gluco-4-enepyranosyluronic acid)-d-glucose being the main end product (123) (Fig. 5).
FIG. 5.
Structure of hyaluronan. Alternating units of glucuronic acid (GlcUA) and N-acetylglucosamine (GlcNAc) are the building blocks of the hyaluronan polymer. The main digestive product of the action of bacterial hyaluronate lyase on hyaluronan is the disaccharide unit. The glycosidic linkages are also marked.
Hyaluronan
The primary reported substrate of hyaluronan lyases in hyaluronan, a ubiquitous and important component of the ECM of vertebrates. Hyaluronan is composed of repeating units of d-glucuronic acid(1-β-3)N-acetyl-d-glucosamine(1-β-4) (124) (Fig. 5). It is detectable in every studied tissue and fluid in higher animals and humans (81) (Table 1). In addition to its role as a structural component, there is growing data that hyaluronan and the molecules with which it specifically interacts have many other functions in the host defense mechanisms. It has been implicated in many biological processes including fertilization, embryonic development, cell migration and differentiation, wound healing, inflammation, and growth and metastasis of tumor cells (150, 162). This polymer interacts with receptors and binding proteins on cell surfaces like CD44 and RHAMM (4, 58, 165). The cell surface receptor molecule for hyaluronan is CD44, which is present on many different cell types and seems to be important in various different steps of the normal immune response (59). CD44 receptor, a member of a family of cell surface adhesion molecules (103), is known to be present on the surfaces of macrophages (63), neutrophils, T cells, B cells, and various epithelial cells. There is also mounting evidence that cytokines are involved in S. pneumoniae sepsis and in hyaluronan metabolism (45). It seems that hyaluronan levels on endothelial cells, lung fibroblasts, and other cell surfaces are finely controlled by various cytokines, which seem to regulate the rates of hyaluronan biosynthesis and degradation (52, 92, 135). Additionally, it has been recently discovered that the secondary substrate for hyaluronate lyases are chondroitin sulfates. Chondroitin sulfate proteoglycans bind significant amounts of water, which allows protection and cushioning of surrounding structures, and limit the freedom of diffusion of other macromolecules. Chondroitin sulfate is cleaved by hyaluronate lyase only at regions with certain sulfation patterns (78, 93, 123). Sulfation patterns of glycosaminoglycans (GAGs) appear to be critical for their biological function.
TABLE 1.
Concentration of hyaluronan in human tissues and tissue fluids
| Tissue or fluid | Hyaluronan concn (mg/liter) |
|---|---|
| Umbilical cord | 14,100 |
| Synovial fluid | 1,420–3,600 |
| Vitreous body | 140–338 |
| Dermis | 200 |
| Thoracic lymph | 8.5–18 |
| Urine | 0.1–0.5 |
| Serum | 0.01–0.1 |
Production by S. pneumoniae
Most strains of S. pneumoniae, as well as other gram-positive bacterial pathogens, produce hyaluronate lyase (11, 65–66, 122). The pneumococcal hyaluronate lyase enzyme, by breaking down hyaluronan, a ubiquitous and important constituent of connective tissues, is directly involved in host invasion by S. pneumoniae. The exact mechanism of how the enzyme facilitates bacterial penetration of the physical defenses of the host and the subsequent spread to its tissues was poorly understood (16, 22, 77) until recent structural studies were reported (see below) (88, 121). In S. pneumoniae cultures, the enzyme is found in both the culture and the cell-associated fractions. This may suggest that at least part of the enzyme is released by the pathogen to surrounding host tissues during infection to facilitate the bacterial invasion (11).
Mechanism of Attachment to Pneumococci
The full-length Hyl has a molecular mass of 107 kDa when expressed in E. coli (11). The carboxy terminus of the mature enzyme contains a hydrophobic tail preceded by a signature sequence from Leu-919 to Gly-923 of LPQTG and a group of charged residues found in enzymes binding covalently to peptidoglycan structures to form a cell wall anchor (137). The general mechanism of such attachment comprises a group of signature sequence properties. It requires a sorting signal located at the carboxy terminus of proteins, which consists of an LPXTGX (X represents any amino acid) motif followed by a carboxy-terminal hydrophobic domain and preceded by a tail of mostly positively charged residues (50, 137).
Structure and Mechanism of Action
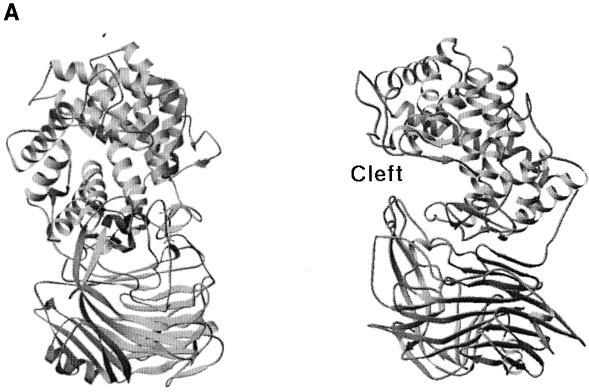
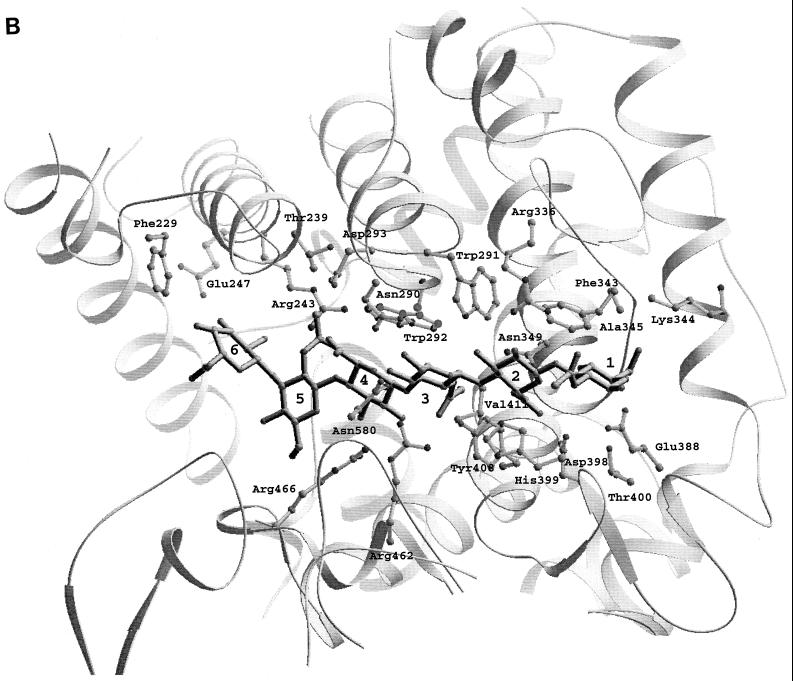
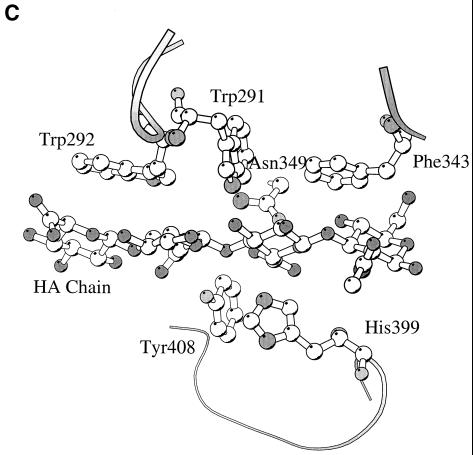
The truncated but functional form of the Hyl enzyme containing amino acids Ala-168 to Glu-891 was recently crystallized (72, 73), and the three-dimensional structure was determined based on X-ray diffraction (88). This form of the enzyme has molecular mass of 89 kDa and lacks the first 167 N-terminal residues as well as the anchoring part to pneumococcal peptidoglycan (137). The enzyme structure contains two domains, with the catalytic domain built largely from helices resembling a horseshoe with an α5/α5-barrel structure (Fig. 6A). The C-terminal domain consists almost entirely of β-sheets arranged in a four-layered sandwich. The helical domain comprises a large, deep, and elongated cleft located at the wider opening of the barrel structure, which is responsible for substrate binding and degradation (88, 121). The central part of such cleft contains three catalytic residues, Asn-349, His-399, and Tyr-408, which degrade the substrate through a proton acceptance and donation mechanism (Fig. 6B to D) (69, 88). In addition to these three catalytic residues, the central part of the cleft has a patch of aromatic residues, Trp-291, Trp-292, and Phe-343, that were implicated in the selection process of the final product of hyaluronan degradation (Fig. 6C). The aromatic patch interacts exactly with two disaccharide units of HA, positioning the disaccharides precisely for catalysis, which results in disaccharides being the smallest degradation product of HA. The structure of the enzyme complex with the product of degradation, the disaccharide unit of HA, revealed that the enzyme degrades the substrate from the reducing end. Also, the degradation process is endolytic (progressive); once the enzyme binds the substrate and degrades it, it progressively moves towards the nonreducing end until the enzyme finishes degrading the HA chain (69, 110).
FIG. 6.
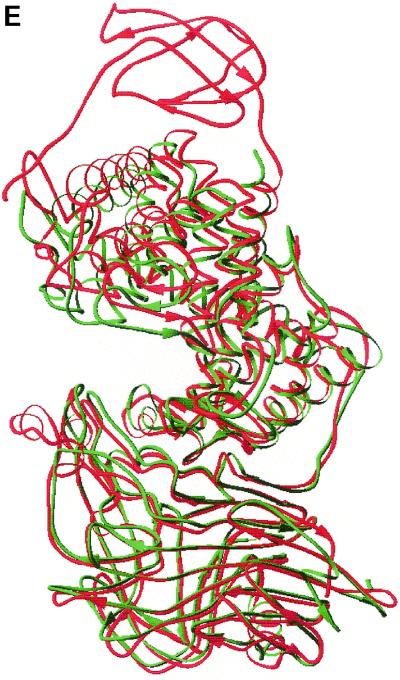
(A) Overall structure of S. pneumoniae hyaluronate lyase (pdb: 1egu). (B) Structure of the modeled hexasaccharide substrate in the binding/catalytic cleft. (C) Catalytic residues of the enzyme. (D) Proposed mechanism of catalysis. (E) Alignment with the structure of S. agalactiae hyaluronate lyase (pdb: 1f1s). (A) The enzyme is built from an α-helical catalytic domain and a supportive β-sheet domain. The two domains are connected by one flexible peptide linker. Two perpendicular views of the enzyme are shown. (B) The cleft present at one end of the α-domain is where the substrate binds and is being degraded. (C) The residues directly involved in catalysis are Asn-349, His-399, and Tyr-408. (D) These residues degrade hyaluronan through a five-step proton acceptance and donation mechanism. (E) The S. agalactiae enzyme structure shows the presence of an additional supportive β-sheet domain at its N terminus.
The proton acceptance and donation HA degradation mechanism was proposed with the help of the structural and mutation analysis and involves five steps: (i) substrate binding in the catalytic cleft; (ii) Asn-349 neutralization of the carboxyl group of glucuronic moiety of HA on the C-5 carbon atom; (iii) His-399 extraction of the C-5 proton to form a double bond between carbon atoms C-4 and C-5; (iv) Tyr-408 donation of a proton to the glycosidic bond, causing breakage of the β1,4 glycosidic bond; and (v) cleavage of the disaccharide substrate, leaving the active site and catalytic residues His-399 and Tyr-408 to balance their protons via exchange with water, making the enzyme ready for the next round of the catalytic process (Fig. 6B to D).
The role of the β-sheet domain is probably only supportive in maintaining the structure of the catalytic cleft as well as modulating access to the cleft. These two goals might be accomplished through a Ca2+-based influence on the structure of the loops at the edge of the cleft, where Ca2+ is thought to bind. It has been shown that Ca2+ ions are activators of the enzyme and are necessary for its activity (72). However, the precise role of the β-sheet domain and the calcium ions is still largely unknown (for more details, see references 69 and 121).
Hyaluronate Lyases in Other Bacteria
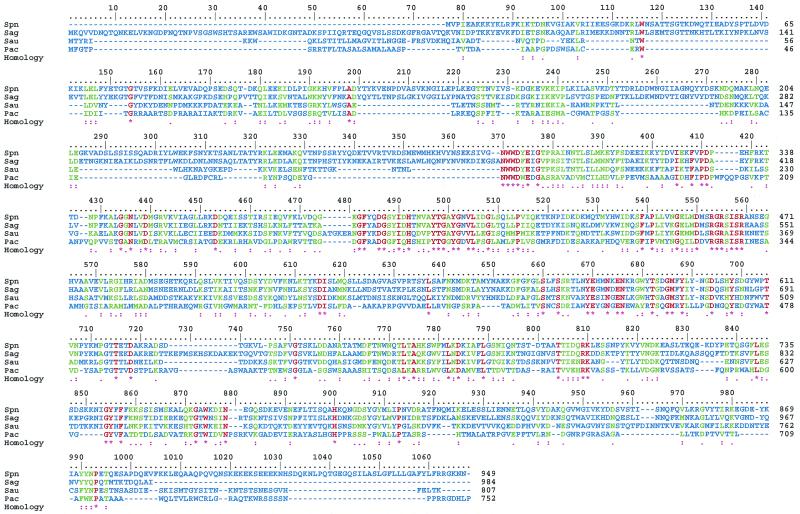
Another member of the Streptococcus genus, S. agalactiae (group B streptococcus), also produces hyaluronate lyase (121). S. agalactiae, although less lethal than to the pneumococci, can cause serious disease, especially in neonates (6, 28, 138). The amino acid sequences for the two streptococcal hyaluronate lyase enzymes from S. pneumoniae and S. agalactiae are 68% homologous, suggesting possible structural similarities (71). Also, recently reported sequences for hyaluronate lyase from Staphylococcus aureus (67, 144) and Propionibacterium acnes (144) (GenBank accession no. U27583) show high amino acid similarity to the pneumococcal and GBS enzymes, implying their structural similarity (Fig. 7).
FIG. 7.
Sequence alignment of selected bacterial hyaluronate lyases. The sequence data correspond to the following enzymes: Spn, S. pneumoniae; Sag, S. agalactiae; Sau, Staphylococcus aureus; Pac, Propionibacterium acnes. The sequence data were obtained from GenBank. The method used to produce this figure and the color coding used are the same as in Fig. 4.
The three-dimensional structure of the S. agalactiae hyaluronate lyase has recently been elucidated by X-ray crystallography (Fig. 6E) (69, 71). This structure is very similar to that of the pneumococcal enzyme. Similarly, the S. agalactiae structure also represents a truncated enzyme but contains an additional part of the N-terminal domain, comprising 74 amino acids, that was not present in the truncated form of the pneumococcal enzyme (Fig. 6E). The additional domain probably plays a similar role to the C-terminal β-sheet domain, and its function is based on the modulation of substrate access to the catalytic cleft. Furthermore, the structure of S. agalactiae Hyl suggests that the enzymes might be a novel group of allosteric enzymes with the substrate modulating the shape of the catalytic cleft. All domains are connected by single flexible linkers that might easily accommodate some substrate-modulated interdomain movements. However, more studies are needed to elucidate fully this property of bacterial hyaluronate lyase enzymes.
PNEUMOLYSIN
Pneumolysin (Ply) is yet another virulence factor of pneumococci that penetrates the physical defenses of the host. Pneumolysin is a 53-kDa protein produced by all clinical isolates of the pathogen (115–117). Unlike other pneumococcal antigens, this molecule is not surface exposed. It is a cytoplasmic enzyme that is released due to the action of surface pneumococcal autolysin (described below). The virulence properties of Ply are therefore directly dependent on the action of autolysin. Ply has several distinct functions, especially in the early pathogenesis of pneumococcal infection. The enzyme is cytotoxic to ciliated bronchial epithelial cells, slows ciliary beating in organ culture, and disrupts tight junctions and the integrity of the bronchial epithelial monolayer (125, 145). Due to Ply function, the ability of ciliated bronchial cells to clear mucus from the lower respiratory tract is reduced, which facilitates the spread of pneumococcal infection. In addition, Ply interactions with alveolar epithelial cells and pulmonary endothelial cells probably cause alveolar edema and hemorrhage during pneumococcal pneumonia. Ply action during pneumococcal infection disrupts the alveolar-capillary boundary, which produces an alveolar flooding providing nutrients for bacterial growth and facilitates penetration through the epithelium into the pulmonary interstitium and ultimately into the bloodstream (132). The cytotoxic effects of pneumolysin can directly inhibit phagocyte and immune cell function, which leads to suppression of the host inflammatory and immune responses. Low concentrations of Ply are able to inhibit human neutrophil and monocyte respiratory bursts, chemotaxis, bactericidal activity, and production of lymphokines and immunoglobulins (132). The virulence and multiple function of pneumolysin, especially in early stages of infection by pneumococci, are crucial to the pneumococcal colonization of a host.
Cholesterol-Dependent Cytolysins
Pneumolysin is part of a larger group of proteins of pathogenic gram-positive bacteria known as cholesterol-dependent cytolysins (CDCs) (Fig. 8). All of them are virulence factors for their organisms. Their mode of action is based on binding to the host cell cytoplasmic membrane cholesterol, a process that is followed by insertion into the targeted membranes and formation of relatively large pores. Once the pores are formed, the targeted cell undergoes lysis. The exception to this is found in the Listeria monocytogenes CDC, listerolysin O, which lyses phagosomal membranes but not the cell membrane due to its low pH activity optimum at around pH 5.5 (55). All these proteins have some similarities in their primary structures as well as in their modes of action. Their structural properties seem to vary depending on their environment. CDCs have a water-soluble form, a cholesterol-bound form, and a cholesterol- and membrane-bound form. The forms differ in structure to various degrees. The water-soluble CDCs usually exist in aqueous solution as monomers and dimers, probably becoming dimers after association with cholesterol (80), and then form large oligomers while embedded in the lipid bilayer of a target cell membrane. For pneumolysin, its membrane pore assembly consists of 30 to 50 monomeric Ply molecules with the assembly diameter of 35 to 45 nm (132). The presence of cholesterol in the target membrane is absolutely necessary for the cytotoxicity of CDCs, and Ply is no exception.
FIG. 8.
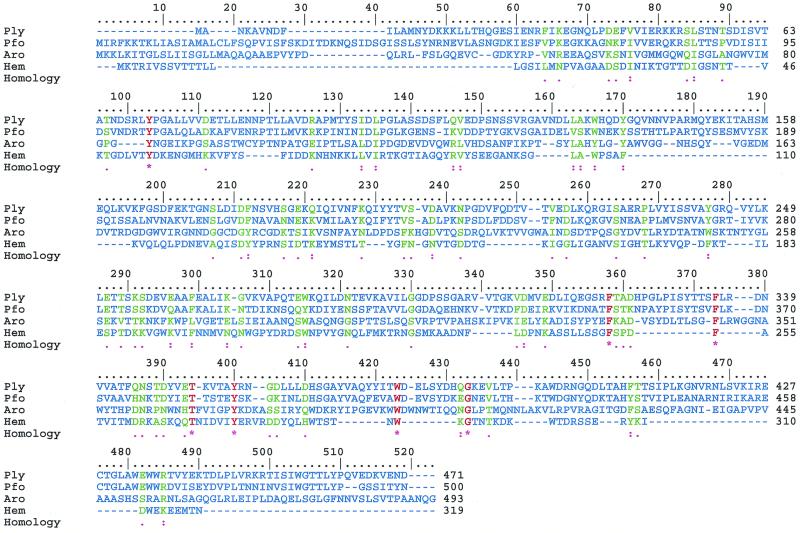
Sequence alignment of CDCs of known three-dimensional structures. The origin of the proteins is as follows: Ply, S. pneumoniae; Pfo, Clostridium perfringens; Aro, Aeromonas hydrophilia; Hem, Staphylococcus aureus. Overall, the molecules have sequences that are only 14% homologous, but the three-dimensional structures have a similar fold and similar general structural arrangement. The sequence data were from GenBank. The method used to construct this figure and the color coding used are the same as in Fig. 4.
Surprisingly, Ply is not actively secreted during bacterial growth due to the lack of the N-terminal secretion signal sequence. However, its function is necessarily coupled to being released from the cell. This release of Ply from the cytoplasm is dependent on another S. pneumoniae enzyme, autolysin (LytA). Autolysin naturally degrades the bacterial cell wall during cell division, but under certain conditions such as in the stationary phase of growth in vitro or on antibiotic or detergent treatment it induces cell lysis (132). The details of the process of autolysis during infection are still under investigation and are not totally clear.
Homology-Based Structural Properties
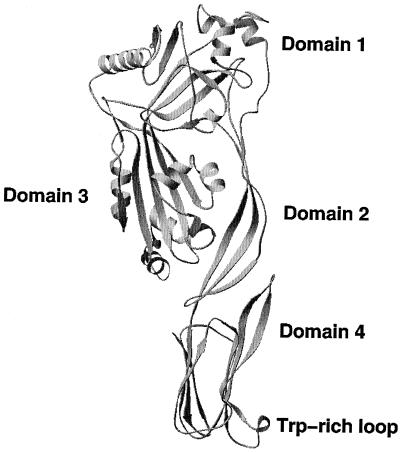
Although the structure of Ply is not known, it is expected to be similar to the structures of CDC molecules from other bacterial organisms that have already been elucidated structurally by X-ray crystallography. Of these CDC molecules, the structure of perfringolysin (Pfo) from Clostridium perfringens probably is the most similar to that of Ply, based on the high sequence similarity of 60% (47, 130). Other CDC molecules studied by structural X-ray crystallographic methods include aerolysin from Aeromonas hydrophilia (111), α-hemolysin from Staphylococcus aureus (141), and protective antigen from Bacillus anthracis (118) (Fig. 8). All of these proteins have a similar three-dimensional fold with a characteristic domain-based structure, elongated shape, and high percentage of β-sheet structure (Fig. 8 and 9). Detailed comparison of these macromolecules shows significant differences, mainly based on different arrangements of the domains. Based on the comparison of Ply to Pfo and modeling studies, a structural model of Ply has been proposed (131). The modeling studies were followed by mutation analysis, and together these studies provided some insight into the structural and functional properties of Ply. Pfo-based modeling shows that the Ply molecule is composed of four domains (1 through 4), with domain 4 being involved in cholesterol binding on membranes of target cells. In general, the Ply model is very similar to the crystal structure of Pfo (root mean square difference of 0.6 Å for Cα carbon atoms). The only sequence insertion in Ply that is not found in Pfo is in domain 1 and is located in a surface-exposed loop. The most conserved region at the sequence and structural levels seems to be the Trp-rich segment in domain 4, which is thought to be responsible for cholesterol binding (82, 131). Only detailed structural information obtained from X-ray crystallography or nuclear magnetic resonance spectroscopy will facilitate the elucidation of the differences and similarities between Ply and Pfo in significant detail.
FIG. 9.
Structure of perfringolysin, a model structure for pneumolysin. All domains are labeled from 1 to 4, along with the Trp-rich loop in domain 4 (pdb: 1pfo). Domains 3 and 4 were implicated in membrane insertion accompanied by conformational changes leading to an α-helix–to–β-strand secondary structure shift.
Model of Cell Membrane Insertion
The region of domain 4 of Ply that is directly implicated in cholesterol binding is a Trp-rich loop (Fig. 9). This loop is the most highly conserved region of the CDC family of enzymes and contains an 11-amino-acid segment (61). In pneumolysin, this region contains the only cysteine residue in the whole molecule, in addition to three tryptophan residues all of which are implicated in the mechanism of membrane binding, complement activation, and pore formation (131, 133). The mutations of this Cys residue that most probably do not change the conformation of the Trp-rich loop do not have any detectable effect on the hemolytic activity and on pneumococcal virulence. However, mutations changing the loop conformation result in decreased hemolytic activity, but the mutants still could be inhibited by cholesterol binding. Recent studies have shown that certain regions of the Ply monomer change their structural conformation, first decreasing the α-helical conformation along with a decrease in β-sheets upon binding to cholesterol (82, 107, 112, 130), leading to a shift in the Trp-rich loop of Ply and an increase in the β-sheet content, probably in domain 3, during the membrane insertion associated with the transition to the oligomeric form of the toxin (56, 80, 139, 140).
The conformational change associated with cholesterol binding exposes a hydrophobic part, probably on domain 4, that can then undergo the insertion process into the host cell membrane. As the protein shifts, Ply molecules aggregate into large ring structures and form pores. Such pores within the target cell membrane upset the delicate osmotic balance between the cell and its environment, allowing material to leak in and out freely, quickly leading to lysis of the target cells.
AUTOLYSIN
Autolysins are members of a widely distributed group of enzymes that degrade the peptidoglycan backbone of bacterial organisms. The action of these cell wall-degrading enzymes ultimately leads to cell lysis (126). These enzymes are located in the cell envelope and are also postulated to play roles in a variety of physiological cell functions associated with cell wall growth, its turnover, and cell separation in microorganisms (152). The major function of this group of enzymes, cell wall degradation, has significant physiological consequences, such as cell lysis which leads directly to cell death (152, 156). An example of one such enzyme is the S. pneumoniae N-acetylmuramoyl-l-alanine amidase, also known as LytA amidase (91, 127). It probably is the best characterized autolysin of this group of enzymes, and it has been implicated in the pathogenicity of pneumococci. One of the direct implications is the release of the components of cell wall shown to be highly inflammatory in some animals (158). The indirect implication involves the release of cytoplasmic bacterial proteins including bacterial virulence factors such as pneumolysin (76, 102). The precise role of LytA in virulence is still under debate (151). The mutations of lytA gene in the S. pneumoniae chromosome lead to significantly decreased virulence of this organism compared to wild-type strain in mouse intraperitoneal challenge (8). Such behavior clearly indicates that the lytA gene is important to pneumococcal pathogenesis. Other studies of LytA also have shown that this amidase induces a protective response in mice to streptococci when inoculated in the lungs (11, 12, 26). This protective property of LytA that contributed to a significantly longer survival for mice challenged intranasally with autolysin makes it a potential component of novel antipneumococcal vaccines (13, 89). The degrees of protection in mice immunized with autolysin and pneumolysin were shown to be similar, and no additional protection was observed in animals immunized with both proteins. In addition, no increase in survival time was observed in mice challenged with a pneumolysin-negative strain. These data are consistent with the idea that, at least in a mouse model, the antiautolysin antibodies exert their effects primarily by preventing the release of pneumolysin. On the other hand, autolysin played a major effect in middle ear infection in a chinchilla otitis media model, where the role of Ply was more limited (136). Yet other reports show a lack of influence of autolysin on the virulence of pneumococci (151). There clearly is still some controversy regarding the role of autolysin in pathogenesis.
The contribution of LytA and other virulence factors appears to vary among different disease states and different animal models (114). The gene for pneumococcal LytA amidase, lytA, has been cloned, and the protein was produced from Escherichia coli, allowing its characterization (53, 54). Pneumococcal LytA amidase has a molecular mass of ∼36 kDa and has a modular organization; it is composed of two distinct domains. One domain is composed of six 20 to 21-amino-acid repeats located at the C-terminal part (similar to that of the PspA repeat domain or CBR) and is responsible for the attachment to teichoic or lipoteichoic acid residues on the surface of pneumococci. The other domain, located at the N terminus, is probably directly responsible for the lytic activity against pneumococcal peptidoglycan structures. Attachment of the enzyme to the choline of the S. pneumoniae cell wall teichoic acid is essential for the lytic activity of the enzyme (155). The carboxy-terminal attachment module probably influences the activity of the enzyme by stabilizing or inducing its active conformation (17). Also, it recently became clear that all bacterial cell lytic enzymes appear to have a two-domain architecture.
Structural Properties of LytA
Although the three-dimensional structure of LytA is unknown, the enzyme was characterized to a significant degree using other biophysical methods such as analytical sedimentation and spectroscopy. The LytA enzyme forms dimers in solution, with an equilibrium constant between dimers and monomers of ∼105 M−1. The monomer-dimer equilibrium shifts toward more dimer formation on an increase in choline, with an equilibrium constant of ∼108 M−1. No higher-order aggregates were reported (159). Even in the absence of choline, as much as 80% of the enzyme is in the dimeric population. The shape of the molecule based on sedimentation velocity analysis is an elongated, rod-like molecule with a length/width ratio of ∼15/1 and dimensions of 190 by 13 Å for the LytA functional dimeric form. The catalytic sites are probably located at both ends of the elongated ellipsoidal molecule. The elongated shape might facilitate the diffusion of the enzyme in the cross-linked peptidoglycan structures, allowing greater access to peptidoglycan substrates that are hydrolyzed. The secondary structure of this autolysin consists of 47% β-sheets, 19% α-helices, and 23% turns, as well as 11% irregular structures (100). The secondary structure of the C-terminal module is very similar to that of the whole LytA molecule, with a characteristic high β-sheet content. The composition of the N-terminal part, therefore, was concluded to also have similar secondary structure to the whole LytA molecule or its C-terminal module. The binding of LytA to choline residues does not cause any significant change in the secondary-structure composition, which is indicative of the lack of major structural changes for the molecule during this event (100). The secondary structure is essentially unaffected by the increased amounts of choline present. However, two modes of choline binding to the C-terminal module have been detected; one is low affinity and another is high affinity. Saturation of choline binding to the high-affinity sites induces dimerization and subsequent increase of the affinity for the substrate (100, 159). The dimerization involves primarily the C-terminal part of the molecule, with preferential binding of two choline molecules to the dimer. On the other hand, saturation of the low-affinity sites requires a choline concentration similar to those necessary for inhibition of this amidase in in vivo assays (100). The presence of these two choline binding sites, together with inducible dimerization, might play an essential role in LytA cellular targeting by causing a preferential location of the enzyme at the sites of its action on the cell wall. It is common knowledge that other bacterial organisms have similar lytic enzymes with the ability to degrade their peptidoglycans. LytA is therefore a member of a large group of bacterial lytic enzymes.
PNEUMOCOCCAL SURFACE ANTIGEN A
Pneumococcal surface antigen A (PsaA) is yet another virulence factor of pneumococci; it has a molecular weight of 34,539 and is composed of 309 residues. It elicits protective properties in mice against S. pneumoniae (148), and the PsaA− mutants of pneumococci were also avirulent in a mouse model (9). The likely function of PsaA is the transport of Mn2+ and Zn2+ into the cytoplasm of the bacteria (41). The protein is thought to be anchored to S. pneumoniae via the bacterial cell membrane and a lipid component that is covalently attached to the protein. Such attachment has a characteristic signature in the C-terminal part of the protein signal sequence, i.e., LX1X2C, where X1 usually is A, S, V, Q, or T and X2 is G or A (149). In this attachment mechanism the protein forms a covalent linkage with diacylglyceryl to the Cys residue followed by proteolytic cleavage of the signal sequence. This way, the modified Cys is present at the amino-terminal part of the protein. Such a signature sequence for covalent attachment of lipid is present in the PsaA sequence, and this mechanism probably keeps this protein attached to the bacterium.
Structure
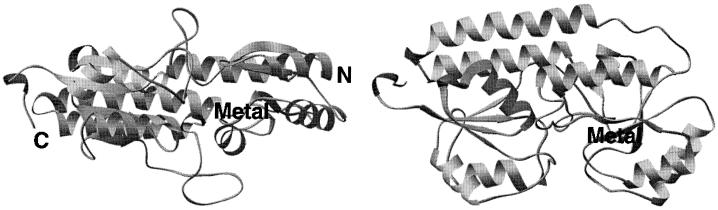
The three-dimensional structure of PsaA has recently been elucidated by X-ray crystallographic techniques (87). The structure includes 290 amino acids, Ala-19 to Lys-309, out of 309 amino acids of the full-length mature protein. A few additional residues were added to implement an N-terminal fusion with a polyhistidine tag, which is convenient for purification (120). The structure of PsaA consists of two twofold-pseudosymmetrical (β/α)4 sandwich domains and amino- and carboxy-terminal domains (Fig. 10). The β-strands of each domain form parallel β-sheets (87). The N-terminal part of the structure, including the LX1X2C (X1 and X2 are defined above) signature sequence, is not visible in the structure. However, the whole N-terminal part of the amino-terminal domain structure protrudes somewhat outside the PsaA molecule and does not form any significant contacts outside of the expected crystal-packing interactions. It is likely that these residues of the N-terminal part of PsaA are somewhat flexible, accommodating protein binding to lipids of the S. pneumoniae cytoplasmic lipid bilayer and facilitating the attachment to bacteria. A sequence comparison of different strains of PsaA shows that this region of the molecule is the least highly conserved and therefore is likely to assume a variety of structures, including highly flexible and/or disordered structures, accommodating the flexible attachment to the lipid bilayer (9). In gram-negative bacteria these proteins lack a membrane anchor and are found free in the periplasm.
FIG. 10.
Three-dimensional structure of PsaA based on the X-ray diffraction data (pdb: 1psz). The metal bound in its binding site is labeled. Two perpendicular views of the protein are shown.
Metal Binding and Function
The metal binding site is present at the interface between the amino- and carboxy-terminal domains. The metal-coordinating ligands include His-67, His-139, Glu-205, and Asp-280 in a tetrahedral geometry. The atoms coordinating to metal are NE2 from both histidines, OE1 from Glu-205, and OD2 from Asp-280 (87). Crystallographic refinements suggest the presence of Zn2+ in the metal binding site, which is consistent with the tetrahedral coordination geometry of this metal. In nature, zinc usually has a tetrahedral or distorted tetrahedral coordination of its ligands when bound to proteins (29). It is also less discriminating in the type of its ligands than is Mn2+, for example. The coordination geometry of Mn2+ bound to proteins is usually square pyramidal, trigonal bipyramidal, or octahedral (30). The coordination sphere for Mn2+ is usually filled with the carboxyl groups of Glu and Asp, the carboxyamide of Asn or Gln, or a nitrogen atom of His. The sizes of the two metals are similar: 0.80 Å for Mn2+ and 0.74 Å for Zn2+ (33). Therefore, the coordination geometry suggests that PsaA interacts with Zn2+, but the type of the metal ligands could easily accommodate Mn2+. The tetrahedral geometry is similar to the square pyramidal geometry typical of how Mn2+ binds to proteins. The absolute determination of PsaA specificity for Mn2+ and/or Zn2+ still remains to be done. However, the growth requirements of PsaA− mutants suggest that PsaA plays an essential role in the transport of Mn2+ into the cytoplasm of pneumococci (41).
The investigations of PsaA clearly suggest that this molecule is not an adhesin, as was initially thought (9, 134). The lipid-linked PsaA protein, with dimensions of 40 by 40 by 70 Å, is probably present beneath the peptidoglycan and capsule structures of pneumococci. The total thickness of the pneumococcal cell wall is approximately ∼0.36 μm (153). PsaA therefore has no possibility of protruding outside the cell wall. The initial adhesin features of PsaA deduced based on analysis of PsaA− pneumococcal cells may have been due to a secondary effect causing the absence of another adhesin molecule like choline binding protein A (CbpA) modulated by the presence or absence (in PsaA− mutant pneumococci) of Mn2+ or Zn2+.
Sequence Analysis
Genomic sequence analysis shows that PsaA belongs to an ATP binding cassette (ABC)-type transport system (9) and constitutes the extracellular component responsible for solute (metal) binding. The ABC-type transport system is characteristic of prokaryotic and eukaryotic cells (60) and has up to three components, an extracytoplasmic protein responsible for solute binding (such as PsaA), an integral membrane part responsible for transport of the solute through the cell membrane, and a cytoplasmic protein binding ATP. ATP hydrolysis is coupled with solute transport since it provides energy necessary for transport. In S. pneumoniae and other gram-positive bacteria, the solute binding proteins have only an extracellular component and are anchored to bacteria by a lipid attached to the protein. These proteins also have a characteristic sequence motif, LX1X2C, which is found in PsaA and was described above, which is responsible for lipid attachment. ABC-type proteins are often grouped into clusters based on sequence and ligand identity. Another member of the ABC-type proteins in S. pneumoniae is AdcA, which, together with PsaA, belongs to cluster 9 of metal transporters (41).
CHOLINE BINDING PROTEIN A
Pneumococci have a series of surface-exposed proteins that attach to bacteria through a specific choline binding motif. Two antigens already mentioned, PspA and LytA, are examples of such proteins. These proteins, called CBPs, have modular structures consisting of a C-terminal choline binding module that is usually followed by a flexible linker peptide built from a proline-rich segment and finally by a functional N-terminal module. The choline binding module consists of several (usually around 10) repeat regions of usually ∼20 amino acids. This module has been identified to bind to terminal choline residues of teichoic or lipoteichoic acid structures present on the surface of S. pneumoniae and serves as an anchoring device to pneumococci for these proteins. The CBP motif has also been found among the surface proteins of other bacteria like Clostridium acetobutylicum, Clostridium difficile, Streptococcus mutans, and Streptococcus downei (164).
Structural Properties
Choline binding protein A (CbpA) was specifically identified and characterized as a major CBP. It is a predominant protein in the mixtures of CBPs isolated from pneumococci (129). It is surface exposed and has a strong ability to react with both human convalescent-phase antibody and the mouse protective anti-CBP serum. The cloning of this protein allowed the determination of its sequence. It consists of 663 amino acids and has a molecular mass of 75 kDa. However, it apparently migrates in sodium dodecyl sulfate-polyacrylamide gel electrophoresis at a higher mass (approximately 112 kDa). Like other CBPs, CbpA has an N-terminal repeat region from residues 404 to 663 responsible for attachment to teichoic acid choline residues, a proline linker from amino acids 374 to 403, and the functional N-terminal module consisting of amino acids 1 to 373 (129). The repeat region consists of 10 20-amino-acid repeats. However, the primary sequence of the N-terminal module of CbpA is clearly different from that of the PspA N-terminal domain. Sequence analysis of this N-terminal CbpA region has shown the existence of common structural features with PspA. CbpA comprises structural motifs such as a large α-helical region (six α-helices) and a coiled-coil region (five coiled-coils). Such secondary structure is consistent with a fibrous molecule, which suggests a high structural similarly of highly elongated, fibrous PspA (70). The structural features of PspA have been investigated in greater detail than those of CbpA, and, at least for the present, the structural model of PspA can serve as a general structural model for CbpA, even though many structural details of these two proteins are different.
Function
The function of CbpA in adherence to host tissues and in colonization was confirmed by studies of CbpA− mutant pneumococcal cells. The cbpA-deficient mutant cells have lost the ability to interact with cytokine-activated host cells, with immobilized 6′-siallylactose–human serum albumin (HSA) (6′SL), and with lacto-N-neotetraose-HSA (LnNT). Therefore, CbpA seems to be the first known protein adhesin identified on the pneumococcal surface. The molecule appears to act as a bridging element between the choline of teichoic acid or lipoteichoic acid and human cell glycoconjugates by utilizing the repeat region binding to choline and the N-terminal region binding to cells, respectively. This interaction is, however, restricted to cytokine-activated human cells. This process might be involved in advancing the pneumococcal disease from colonization to invasion.
It has been suggested that CbpA as well as other CBPs might simply block the cell wall choline residues from interacting with the host cells. It is likely that cytokine-activated human cells express platelet-activating factor (PAF) receptor, which has the ability to bind phosphocholine of the pneumococcal cell wall (39). In this case, the role of CbpA and other CBPs would only be to block such attachment. Both of these possibilities, pneumococcal adhesion and pneumococcal surface blocking, are likely.
NEURAMINIDASE
Neuraminidase is yet another virulence factor of S. pneumoniae that is present on all strains of pneumococci examined (10, 79, 109). The enzyme cleaves terminal sialic acid from cell surface glycans such as mucin, glycolypids, and glycoproteins, which probably causes significant damage to host cell glycans as well as to the host. This action changes the glycosylation patterns of the host and probably exposes more of the host cell surface, which may reveal surface receptors for possible interaction with pneumococci, contributing to increased adhesion and other processes (82). The precise role of S. pneumoniae neuraminidase in pathogenesis has not been clearly established, but it probably enhances colonization due to its action on glycans (128).
Types of Neuraminidases in Pneumococci
There appear to be at least two forms of the pneumococcal neuraminidase enzymes, NanA and NanB, with NanA having a molecular mass of ∼108 kDa (25) and NanB having a mass of ∼75 kDa (10). The structural genes for NanA and NanB have been cloned and sequenced (Fig. 11) (10, 25). Their sequences exhibit negligible homology. Both enzymes seem to have a propensity for degradation to smaller fragments during in vitro growth and protein purification, and some of these fragments preserve neuraminidase activity. For NanA, active fragments as small as 85 kDa were isolated (90). NanA has been located on the surface of pneumococci through antibody studies (25). The activity of NanB is approximately 100 times lower than that of NanA (10, 90). NanA but not NanB contains a C-terminal signature sequence containing an LPXTGX motif (discussed above for hyaluronate lyase), which probably reflects the covalent binding of NanA to peptidoglycan structures of S. pneumoniae (Fig. 11) (25, 49, 137).
FIG. 11.
Sequence alignment of selected microbial neuraminidase enzymes. The abbreviations are as follows: NanA and NanB, two neuraminidases of Streptococcus pneumoniae; Tcru, Trypanosoma cruzi; Cper, Clostridium perfringens; Styp = Salmonella enterica serovar Typhimurium. The sequence data were from GenBank. The method used to construct this figure and the color coding used are the same as in Fig. 4.
It is peculiar that an organism would have two different neuraminidases. It is still uncertain why S. pneumoniae produces at least two of them. It is likely that they specialize to be most efficient in different environments for pneumococci that are important during infection or invasion of the host. Such a possibility is supported by their different molecular sizes as well as by different activities of both enzymes at different pHs. NanA has maximum activity at ∼pH 5, whereas NanB is most active at pH ∼7 (10).
Structural Homology of Neuraminidases
No three-dimensional structural data are available for NanA or NanB neuraminidases from S. pneumoniae. However, the structures of various forms of neuraminidase of viral origin (21, 74, 147, 157, 160) and of bacterial origin from Salmonella enterica serovar Typhimurium are available (Fig. 11 and 12) (35, 36). The monomer of the S. enterica serovar Typhimurium neuraminidase is composed of only 379 residues, whereas NanA has 1,035 amino acids. The sequence similarity between the two enzymes is only 20% (Fig. 11). However, due to their similar function, there is probably some structural similarity. Both enzymes are monomers, whereas the viral enzyme is a tetramer. Only the three-dimensional structural information of both NanA and NanB will show the details of their function and mechanism of action. However, currently the S. enterica serovar Typhimurium structure may serve as a model to provide insights into bacterial neuraminidase structure and function.
FIG. 12.
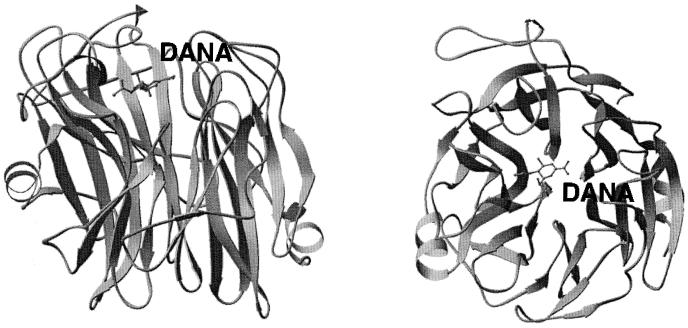
Three-dimensional structure of Salmonella enterica serovar Typhimurium LT2 neuraminidase with 2-deoxy-2,3-dehydro-N-acetylneuraminic acid (DANA) bound in its active site (pdb: 3sim). Two perpendicular views of the enzyme are shown.
The enzyme from S. enterica serovar Typhimurium consists of six four-stranded antiparallel β-sheets arranged in a propeller-like fashion (Fig. 12). The active site is located on the surface in a shallow crevice in the middle of the enzyme. The catalytic site includes three arginine residues responsible for stabilizing the carboxyl group of the substrate, sialic acid. At the bottom of the crevice is a tyrosine residue interacting with the substrate ring structure. A hydrophobic pocket accommodates the N-acetyl group. Glu-231 is involved in the catalysis by proton donation (36).
CONCLUSIONS
Pneumococcal Adherence and Interactions with Host Cells
Pneumococcal adherence to host cells has been suggested to be a two-step process (38). The first step involves targeting an anatomic niche of the host like the nasopharynx to bind to the host surface glycoconjugates on respiratory epithelial and endothelial cells. Following this step, cytokine activation is induced, which results in expression of novel glycans on the surface of activated cells and increased pneumococcal adherence. An example of such cytokine activation is the expression of platelet-activating factor receptors on some host cell surfaces (39). These two steps lead to pneumococcal invasion of the host and to pneumococcal disease. S. pneumoniae adherence to the host probably involves an array of adhesin molecules expressed on its surface. Examples of such molecules that take part or modulate adhesion to host cells are discussed above for CbpA and both neuraminidases.
In addition to adherence to the host cells, pneumococci have developed other ways to interact with the host and its tissues. At least in part, such interactions involve a variety of host cells, tissues, or tissue components and the pathogen utilizes an array of its predominantly surface macromolecules. Surface molecules not predominantly involved in host adherence have been discussed above and include PspA/PspC, Hyl, Ply, LytA, and PsaA (Fig. 1). These protein are involved in interactions with the host complement system (PspA), degradation of hyaluronan of the ECM, lysis of cholesterol containing membranes (Ply), degradation of peptidoglycan layers of pneumococci most likely to release cytoplasmic Ply and inflammatory degradation products of cell wall (LytA), and binding of metals (divalent cations) such as Mn2+ or Zn2+ (PsaA) followed by their transport inside cytoplasm of pneumococci (ABC-type transporters).
The functions of all the above proteins facilitate significant aspects of pneumococcal colonization and/or invasion; compromising these function leads to compromised pathogenicity of S. pneumoniae. Therefore; these proteins can serve as targets for the development of novel therapies to treat pneumococcal disease. On one hand, the antibodies against the majority of these surface antigens are protective against the disease, and therefore these antigens can be used as protein-based vaccine candidates. One of these antigens, PspA, is already being used to develop a novel vaccine, and the initial evaluation of this vaccine candidate is very promising. On the other hand, some of these proteins can have their function compromised or totally abolished by the use of small molecules that most probably bind in their active sites; these small molecules can be used to develop potent drugs.
The availability of three-dimensional structural information about pneumococcal proteins will most probably facilitate the elucidation of the detailed function and detailed mechanism involved in such function. Such knowledge will aid the development of treatment strategies for pneumococcal disease as well as aiding the progression of science in general. However, structural information must be accompanied by an increased understanding of the role of such proteins during various stages of pathogenesis in animals and finally in humans.
Pneumococcal Vaccines
Polyvalent vaccines based on purified capsular polysaccharides are limited in their potency because of their poor immunogenicity, especially in susceptible groups of patients like young children and the elderly (143). The poor immunogenicity of polysaccharide vaccines is due to a poor antibody response elicited by these vaccines and to the fact that the T-cell independence of the response fails to induce memory. In addition, the available vaccines make up only a limited number of serotypes out of 90 known. The development of conjugated vaccines by coupling the polysaccharides with protein carriers should increase the potency of the vaccine, but it also will limit the serotypes included in such conjugate mixtures. The combination of polysaccharides with a protein significantly increases immunogenicity and memory to polysaccharide antigens. If the protein carrier(s) has the ability to induce additional protection (e.g., the antigens discussed in this review), the resultant vaccine would be improved by the induction of anti-protein antigen antibodies. Such additional protection might also be independent of the serotypes. Therefore, the development of a two-component vaccine comprising a polysaccharide and a nonpolysaccharide part, such as a protein (discussed above), might be the best approach (2, 18, 106, 113, 158). Vaccines composed of mixtures of polysaccharides and protein antigens are likely to provide better protection against S. pneumoniae than are vaccines based only on one or limited mixtures of the possible single-type (polysaccharide or protein) protective components. Mixtures of some possible vaccine components can provide an additive attenuation of virulence. The possible protein candidates for such vaccines have been discussed above. However, more studies are needed to assess the usefulness of various antigens or their mixtures in various modes of pneumococcal challenge.
Pneumococci and Patients with Other Predisposed Conditions
Patients with a predisposing condition of weakened immune system (e.g., AIDS patients or cancer patients) are susceptible to serious infections from encapsulated bacteria, particularly pneumococci (44, 62). Specific examples of cancer patients include, but are not limited to, patients with splenectomy (44) and those undergoing radiotherapy of the head and neck region (62). A rapid onset of severe pneumococcal infections has been observed in asplenic patients. In addition, it is well known that radiotherapy of the head and neck region increases the frequency of upper respiratory infections. Therefore, vaccination against pneumococci is strongly recommended for all patients undergoing splenectomy or radiotherapy. The radiation-induced changes in the oral microflora increase the numbers of virulent streptococci and other exogenous pathogens. Presumably the change in the microflora results from a compromised immune system as a result of radiation therapy, and not from irradiation of the microflora. By increasing our knowledge about pneumococcal virulence factors, we will aid the development of a better vaccine or drug and contribute to the increase in the survival rate of infected patients.
ACKNOWLEDGMENTS
I acknowledge grant support from the National Institutes of Health (AI 44079) and from Aventis Pasteur.
REFERENCES
- 1.Afessa B, Greaves W L, Frederick W R. Pneumococcal bacteremia in adults: a 14-year experience in an inner-city university hospital. Clin Infect Dis. 1995;21:345–351. doi: 10.1093/clinids/21.2.345. [DOI] [PubMed] [Google Scholar]
- 2.Alexander J E, Lock R A, Peeters C C, Poolman J T, Andrew P W, Mitchell T J, Hansman D, Paton J C. Immunization of mice with pneumolysin toxoid confers a significant degree of protection against at least nine serotypes of Streptococcus pneumoniae. Infect Immun. 1994;62:5683–5688. doi: 10.1128/iai.62.12.5683-5688.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnold K E, Leggiadro R J, Breiman R F, et al. Risk factors for carriage of drug-resistant Streptococcus pneumoniae among children in Memphis, Tennessee. J Pediatr. 1996;128:757–764. doi: 10.1016/s0022-3476(96)70326-8. [DOI] [PubMed] [Google Scholar]
- 4.Aruffo A, Stamenkovic I, Melnick M, Underhill C B, Seed B. CD44 is the principal cell surface receptor for hyaluronan. Cell. 1990;61:1303–1313. doi: 10.1016/0092-8674(90)90694-a. [DOI] [PubMed] [Google Scholar]
- 5.Austrian R. Some observations on the pneumococcus and on the current status of pneumococcal disease and its prevention. In: Quie P G, Kass E H, editors. The pneumococcus and the pneumococcal vaccine. Chicago, Ill: University of Chicago Press; 1982. pp. 191–207. [Google Scholar]
- 6.Baker C J, Edwards M S. Group B streptococcal infections. In: Remington J, Klein J O, editors. Infectious diseases of the fetus and newborn infant. 4th ed. Philadelphia, Pa: The W. B. Saunders Co.; 1995. pp. 980–1054. [Google Scholar]
- 7.Baquero F, Martinez-Beltran J, Loza E. A review of antibiotic resistance patterns of Streptococcus pneumoniae in Europe. J Antimicrob Clemother. 1991;28(Suppl. C):31–38. doi: 10.1093/jac/28.suppl_c.31. [DOI] [PubMed] [Google Scholar]
- 8.Berry A M, Paton J C. Additive attenuation of virulence of Streptococcus pneumoniae by mutation of the genes encoding pneumolysin and other putative pneumococcal virulence proteins. Infect Immun. 2000;68:133–140. doi: 10.1128/iai.68.1.133-140.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berry A M, Paton J C. Sequence heterogeneity of PsaA, a 37-kilodalton putative adhesin essential for virulence of Streptococcus pneumoniae. Infect Immun. 1996;64:5255–5262. doi: 10.1128/iai.64.12.5255-5262.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berry A M, Lock R A, Paton J C. Cloning and characterization of nanB, a second Streptococcus pneumoniae neuraminidase gene, and purification of the NanB enzyme from recombinant Escherichia coli. J Bacteriol. 1996;178:4854–4860. doi: 10.1128/jb.178.16.4854-4860.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berry A M, Lock R A, Thomas S M, Rajan D P, Hansman D, Paton J C. Cloning and nucleotide sequence of the Streptococcus pneumoniae hyaluronidase gene and purification of the enzyme from recombinant Escherichia coli. Infect Immun. 1994;62:1101–1108. doi: 10.1128/iai.62.3.1101-1108.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berry A M, Paton J C, Hansman D. Effect of insertional inactivation of the genes encoding pneumolysin and autolysin on the virulence of Streptococcus pneumoniae type 3. Microb Pathog. 1992;12:87–93. doi: 10.1016/0882-4010(92)90111-z. [DOI] [PubMed] [Google Scholar]
- 13.Berry A M, Lock R A, Hansman D, Paton J C. Contribution of autolysin to virulence of Streptococcus pneumoniae. Infect Immun. 1989;57:2324–2330. doi: 10.1128/iai.57.8.2324-2330.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blanchet C, Geourjon C, Deleage G. Multiple protein sequence analysis. Lyon, France: IBCP; 1997. [Google Scholar]
- 15.Boulnois G J, Mitchel T J, Saunders K, Owen R, Canvin J, Shepherd A, Camara M, Wilson R, Feldman C, Steinford C, Bashford L, Pasternak C, Andrew P W. Analysis of some putative protein virulence factors of Streptococcus pneumoniae. In: Dunny G M, Cleary P P, McKay L L, editors. Genetics and molecular biology of streptococci, latococci, and enterococci. Washington, D.C.: American Society for Microbiology; 1991. pp. 83–87. [Google Scholar]
- 16.Boulnois G J. Pneumococcal proteins and the pathogenesis of disease caused by Streptococcus pneumoniae. J Gen Microbiol. 1992;138:249–259. doi: 10.1099/00221287-138-2-249. [DOI] [PubMed] [Google Scholar]
- 17.Briese T, Hakenbeck R. Interaction of the pneumococcal amidase with lipoteichoic acid and choline. Eur J Biochem. 1985;146:417–427. doi: 10.1111/j.1432-1033.1985.tb08668.x. [DOI] [PubMed] [Google Scholar]
- 18.Briles D E, Tart R C, Swiatlo E, Dillard J P, Smith P, Benton K A, Ralph B A, Brooks-Walter A, Crain M J, Hollingshead S K, McDaniel L S. Pneumococcal diversity: considerations for new vaccine strategies with emphasis on pneumococcal surface protein A (PspA) Clin Microbiol Rev. 1998;11:645–657. doi: 10.1128/cmr.11.4.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Briles D E, Hollingshead S K, Swiatlo E, Brooks-Walter A, Szalai A, Virolainen A, McDaniel L S, Benton K A, White P, Prellner K, Hermansson A, Aerts P C, Van Dijk H, Crain M J. PspA and PspC: their potential for use as pneumococcal vaccines. Microb Drug Resist. 1997;3:401–408. doi: 10.1089/mdr.1997.3.401. [DOI] [PubMed] [Google Scholar]
- 20.Burman L A, Norrby R, Trollfors B. Invasive pneumococcal infections: incidence, predisposing factors, and prognosis. Rev Infect Dis. 1985;7:133–142. doi: 10.1093/clinids/7.2.133. [DOI] [PubMed] [Google Scholar]
- 21.Burmeister W P, Ruigrok R W, Cusack S. The 2.2 Å resolution crystal structure of influenza B neuraminidase and its complex with sialic acid. EMBO J. 1992;11:49–56. doi: 10.1002/j.1460-2075.1992.tb05026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Busse W. Pathogenesis and sequelae of respiratory infections. Rev Infect Dis. 1991;13:S477–S485. doi: 10.1093/clinids/13.supplement_6.s477. [DOI] [PubMed] [Google Scholar]
- 23.Butler J C, Hofmann J, Cetron M S, Elliott J A, Facklam R R, Breiman R F. The continued emergence of drug-resistant Streptococcus pneumoniae in the United States: an update from the Centers for Disease Control and Prevention's Pneumococcal Sentinel Surveillance System. J Infect Dis. 1996;174:986–993. doi: 10.1093/infdis/174.5.986. [DOI] [PubMed] [Google Scholar]
- 24.Butler J C, Shapiro E D, Carlone G M. Pneumococcal vaccines: history, current status, and future directions. Am J Med. 1999;107:69S–76S. doi: 10.1016/s0002-9343(99)00105-9. [DOI] [PubMed] [Google Scholar]
- 25.Camara M, Boulnois G J, Andrew P W, Mitchell T J. A neuraminidase from Streptococcus pneumoniae has the features of a surface protein. Infect Immun. 1994;62:3688–3695. doi: 10.1128/iai.62.9.3688-3695.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Canvin J R, Marvin A P, Sivakumaran M, Paton J C, Boulnois G J, Andrew P W, Mitchell T J. The role of pneumolysin and autolysin in the pathology of pneumonia and septicemia in mice infected with a type 2 pneumococcus. J Infect Dis. 1995;172:119–123. doi: 10.1093/infdis/172.1.119. [DOI] [PubMed] [Google Scholar]
- 27.Center for Disease Control and Prevention. Pneumococcal polysaccharide vaccine usage, United States. Morb Mortal Wkly Rep. 1984;33:273–281. [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention. Prevention of perinatal group B streptococcal disease: a public health perspective, United States. Morb Mortal Wkly Rep. 1996;45:1–24. [PubMed] [Google Scholar]
- 29.Christianson D W, Cox J D. Catalysis by metal-activated hydroxide in zinc and manganese metalloenzymes. Annu Rev Biochem. 1999;68:35–57. doi: 10.1146/annurev.biochem.68.1.33. [DOI] [PubMed] [Google Scholar]
- 30.Christianson D W. Structural chemistry and biology of manganese metalloenzymes. Prog Biophys Mol Biol. 1997;67:217–252. doi: 10.1016/s0079-6107(97)88477-5. [DOI] [PubMed] [Google Scholar]
- 31.Cohen J. Bumps on the vaccine road. Science. 1994;265:1371–1373. doi: 10.1126/science.8073271. [DOI] [PubMed] [Google Scholar]
- 32.Corpet F. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res. 1988;16:10881–10890. doi: 10.1093/nar/16.22.10881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cotton F A, Wilkinson G. Advanced inorganic chemistry. 4th ed. New York, N.Y: John Wiley & Sons, Inc.; 1980. [Google Scholar]
- 34.Crain M J, Waltman W D II, Turner J S, Yother J, Talkington D F, McDaniel L S, Gray B M, Briles D E. Pneumococcal surface protein A (PspA) is serologically highly variable and is expressed by all clinically important capsular serotypes of Streptococcus pneumoniae. Infect Immun. 1990;58:3293–3299. doi: 10.1128/iai.58.10.3293-3299.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crennell S J, Garman E F, Philippon C, Vasella A, Laver W G, Vimr E R, Taylor G L. The structures of Salmonella typhimurium LT2 neuraminidase and its complexes with three inhibitors at high resolution. J Mol Biol. 1996;259:264–280. doi: 10.1006/jmbi.1996.0318. [DOI] [PubMed] [Google Scholar]
- 36.Crennell S J, Garman E F, Laver W G, Vimr E R, Taylor G L. Crystal structure of a bacterial sialidase (from Salmonella typhimurium LT2) shows the same fold as an influenza virus neuraminidase. Proc Natl Acad Sci USA. 1993;90:9852–9856. doi: 10.1073/pnas.90.21.9852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crick F H C. The packing of α-helices: simple coiled-coils. Acta Crystallogr. 1953;6:689–697. [Google Scholar]
- 38.Cundell D R, Pearce B J, Sandros J, Naughton A M, Masure H R. Peptide permeases from Streptococcus pneumoniae affect adherence to eukaryotic cells. Infect Immun. 1995;63:2493–2498. doi: 10.1128/iai.63.7.2493-2498.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cundell D R, Gerard N P, Gerard C, Idanpaan-Heikkila I, Toumanen E. Streptococcus pneumoniae anchor to human cells by the receptor for platelet-activating factor. Nature. 1995;377:435–438. doi: 10.1038/377435a0. [DOI] [PubMed] [Google Scholar]
- 40.Diaz E, Lopez R, Garcia J L. Chimeric phage-bacterial enzymes: a clue to the modular evolution of genes. Proc Natl Acad Sci USA. 1990;87:8125–8129. doi: 10.1073/pnas.87.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dintilhac A, Alloing G, Granadel C, Claverys J P. Competence and virulence of Streptococcus pneumoniae: Adc and PsaA mutants exhibit a requirement for Zn and Mn resulting from inactivation of putative ABC metal permeases. Mol Microbiol. 1997;25:727–739. doi: 10.1046/j.1365-2958.1997.5111879.x. [DOI] [PubMed] [Google Scholar]
- 42.Duchin J S, Breiman R F, Diamond A, et al. High prevalence of multidrug-resistant Streptococcus pneumoniae among children in a rural Kentucky community. Pediatr Infect Dis J. 1995;14:745–750. doi: 10.1097/00006454-199509000-00004. [DOI] [PubMed] [Google Scholar]
- 43.Duran-Reynals F. Studies on a certain spreading factor existing in bacteria and its significance for bacterial invasivness. J Exp Med. 1933;58:161–181. doi: 10.1084/jem.58.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ejstrud P, Hansen J B, Andreasen D A. Prophylaxis against pneumococcal infection after splenectomy: a challenge for hospitals and primary care. Eur J Surg. 1997;163:733–738. [PubMed] [Google Scholar]
- 45.Elias J A, Krol R C, Freundlich B, Sampson P M. Regulation of human lung fibroblast glycosaminoglycan by recombinant interferons, tumor necrosis factor, and lymphotoxin. J Clin Investig. 1988;81:325–333. doi: 10.1172/JCI113324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fedson D S, Musher D M. Pneumococcal vaccine. In: Plotkin S A, Mortimer E A Jr, editors. Vaccines. 2nd ed. Philadelphia, Pa: The W. B. Saunders Co.; 1994. pp. 517–563. [Google Scholar]
- 47.Feil S C, Rossjohn J, Rohde K, Tweten R K, Parker M W. Crystallization and preliminary X-ray analysis of a thiol-activated cytolysin. FEBS Lett. 1996;397:290–292. doi: 10.1016/s0014-5793(96)01200-8. [DOI] [PubMed] [Google Scholar]
- 48.Feldman C, Munro N C, Jeffery P K, et al. Pneumolysin induces the salient histologic features of pneumococcal infection in the rat lung in vivo. Am J Repir Cell Mol Biol. 1992;5:416–423. doi: 10.1165/ajrcmb/5.5.416. [DOI] [PubMed] [Google Scholar]
- 49.Fischetti V A, Landau G M, Sellers P H, Schmidt J P. Identifying periodic occurrences of a template with applications to protein structure. Inf Process Lett. 1993;45:11–18. [Google Scholar]
- 50.Fischetti V A, Pancholi V, Schneewind O. Conservation of a hexapeptide sequence in the anchor region of surface proteins from gram-positive cocci. Mol Microbiol. 1990;4:1603–1605. doi: 10.1111/j.1365-2958.1990.tb02072.x. [DOI] [PubMed] [Google Scholar]
- 51.Fischetti V A, Pancholi V, Sellers P, Schmidt J, Landau G, Xu X, Schneewind O. Streptococcal M protein: a common structural motif used by gram-positive bacteria for biological active surface molecules. In: Korhonen T K, et al., editors. Molecular recognition in host-parasite interactions. New York, N.Y: Plenum Press; 1992. pp. 112–119. [Google Scholar]
- 52.Fraser J R E, Laurant T C. Turnover and metabolism of hyaluronan. Ciba Found Symp. 1989;143:42–59. [PubMed] [Google Scholar]
- 53.Garcia P, Garcia J L, Garcia E, Sanchez-Puelles J M, Lopez R. Modular organization of the lytic enzymes of Streptococcus pneumoniae and its bacteriophages. Gene. 1990;86:81–88. doi: 10.1016/0378-1119(90)90116-9. [DOI] [PubMed] [Google Scholar]
- 54.Garcia P, Garcia J L, Garcia E, Lopez R. Nucleotide sequence and expression of the pneumococcal autolysin gene from its own promoter in Escherichia coli. Gene. 1986;43:265–272. doi: 10.1016/0378-1119(86)90215-5. [DOI] [PubMed] [Google Scholar]
- 55.Geoffroy C, Gaillard J L, Alouf J E, Berche P. Purification, characterization, and toxicity of the sulfhydryl-activated hemolysin listeriolysin O from Listeria monocytogenes. Infect Immun. 1987;55:1641–1646. doi: 10.1128/iai.55.7.1641-1646.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gilbert R J, Jimenez J L, Chen S, Tickle I J, Rossjohn J, Parker M, Andrew P W, Saibil H R. Self-interaction of pneumolysin, the pore-forming protein toxin of Streptococcus pneumoniae. Cell. 1999;97:647–655. doi: 10.1016/s0092-8674(00)80775-8. [DOI] [PubMed] [Google Scholar]
- 57.Gray B M, Converse III G M, Dillon H C., Jr Epidemiologic studies of Streptococcus pneumoniae in infants: acquisition, carriage, and infection during the first 24 months of life. J Infect Dis. 1980;142:923–933. doi: 10.1093/infdis/142.6.923. [DOI] [PubMed] [Google Scholar]
- 58.Hardwick C, Hoare K, Owens R, Hohn H P, Hook M, Moore D, Cripps V, Austen L, Nance D M, Turley E A. Molecular cloning of a novel hyaluronan receptor that mediates tumor cell motility. J Cell Biol. 1992;117:1343–1350. doi: 10.1083/jcb.117.6.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Haynes B F, Telen M J, Hale L P, Denning S M. CD44—a molecule involved in leukocyte adherence and T-cell activation. Immunol Today. 1989;10:423–428. doi: 10.1016/0167-5699(89)90040-6. [DOI] [PubMed] [Google Scholar]
- 60.Higgins C F. ABC transporters: from microorganisms to man. Annu Rev Cell Biol. 1992;8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- 61.Hill J, Andrew P W, Mitchell T J. Amino acids in pneumolysin important for hemolytic activity identified by random mutagenesis. Infect Immun. 1994;62:757–758. doi: 10.1128/iai.62.2.757-758.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Himi T, Kukuminato Y, Kita H, Yoshioka I, Kataura A. Effect of radiotherapy on the levels of secretory immunoglobulin A against indigenous and virulent streptococci. Otolaryngol Head Neck Surg. 1997;117:433–437. doi: 10.1016/S0194-59989770010-X. [DOI] [PubMed] [Google Scholar]
- 63.Hiro D A, Ito K, Matsuta K, Mori Y. Hyaluronic acid is an endogenous inducer of interleukin-1 production by human monocytes and rabbit macrophages. Biochem Biophys Res Commun. 1986;140:715–722. doi: 10.1016/0006-291x(86)90790-4. [DOI] [PubMed] [Google Scholar]
- 64.Hofmann J, Cetron M S, Farley M M, et al. The prevalence of drug-resistant Streptococcus pneumoniae in Atlanta. N Engl J Med. 1995;333:481–486. doi: 10.1056/NEJM199508243330803. [DOI] [PubMed] [Google Scholar]
- 65.Humphrey J H. Hyaluronidase production by pneumococci. J Pathol. 1948;55:273–275. [Google Scholar]
- 66.Hynes W L, Ferretti J J. Sequence analysis and expression in Escherichia coli of the hyaluronidase gene of Streptococcus pyogenes bacteriophage. Infect Immun. 1989;57:533–539. doi: 10.1128/iai.57.2.533-539.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hynes W L, Hancock L. Analysis of a second bacteriophage hyaluronidase gene from Streptococcus pyogenes: evidence for a third hyaluronidase involved in extracellular enzymatic activity. Infect Immun. 1995;63:3015–3020. doi: 10.1128/iai.63.8.3015-3020.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Reference deleted.
- 69.Jedrzejas M J. Structural and functional comparison of polysaccharide-degrading enzymes. Crit Rev Biochem Mol Biol. 2000;35:221–251. doi: 10.1080/10409230091169195. [DOI] [PubMed] [Google Scholar]
- 70.Jedrzejas M J, Hollingshead S K, Lebowitz J, Chantalat L, Briles D E, Lamani E. Production and characterization of functional fragment of pneumococcal surface protein A. Arch Biochem Biophys. 2000;373:116–125. doi: 10.1006/abbi.1999.1544. [DOI] [PubMed] [Google Scholar]
- 71.Jedrzejas M J, Chantalat L. Structural studies of Streptococcus agalactiae hyaluronate lyase. Acta Crystallogr. 2000;D56:460–463. doi: 10.1107/s0907444900000706. [DOI] [PubMed] [Google Scholar]
- 72.Jedrzejas M J, Mewbourne R B, Chantalat L, McPherson D. Expression and purification of Streptococcus pneumoniae hyaluronate lyase from Escherichia coli. Protein Expression Purif. 1998;13:83–89. doi: 10.1006/prep.1997.0864. [DOI] [PubMed] [Google Scholar]
- 73.Jedrzejas M J, Chantalat L, Mewbourne R B. Crystallization and preliminary X-ray analysis of Streptococcus pneumoniae hyaluronate lyase. J Struct Biol. 1998;121:71–73. doi: 10.1006/jsbi.1998.3963. [DOI] [PubMed] [Google Scholar]
- 74.Jedrzejas M J, Singh S, Brouillette W J, Laver W G, Air G M, Luo M. Structures of aromatic inhibitors of influenza virus neuraminidase. Biochemistry. 1995;34:3144–3151. doi: 10.1021/bi00010a003. [DOI] [PubMed] [Google Scholar]
- 75.Jernigan D B, Cetron M S, Breiman R F. Minimizing the impact of drug-resistant Streptococcus pneumoniae (DRSP): a strategy from the DRSP working group. JAMA. 1996;275:206–209. [PubMed] [Google Scholar]
- 76.Johnson M K. Cellular location of pneumolysin. FEMS Microbiol Lett. 1977;2:243–245. [Google Scholar]
- 77.Johnston R B., Jr Pathogenesis of pneumococcal pneumonia. Rev Infect Dis. 1991;13:S509–S517. doi: 10.1093/clinids/13.supplement_6.s509. [DOI] [PubMed] [Google Scholar]
- 78.Karamanos N K, Syrokou A, Vanky P, Nurminen M, Hjerpe A. Determination of 24 variously sulfated galactosaminoglycan and hyaluronan-derived disaccharides by high-performance liquid chromatography. Ann Biochem. 1994;221:189–199. doi: 10.1006/abio.1994.1396. [DOI] [PubMed] [Google Scholar]
- 79.Kelly R, Greiff D. Toxicity of pneumococcal neuraminidase. Infect Immun. 1970;2:115–117. doi: 10.1128/iai.2.1.115-117.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kelly S J, Jedrzejas M J. Structure and molecular mechanism of a functional form of pneumolysin: a cholesterol-dependent cytolysin from Streptococcus pneumoniae. J Struct Biol. 2000;132:72–81. doi: 10.1006/jsbi.2000.4308. [DOI] [PubMed] [Google Scholar]
- 81.Kreil G. Hyaluronidases—a group of neglected enzymes. Protein Sci. 1995;4:1666–1669. doi: 10.1002/pro.5560040902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Krivan H C, Roberts D D, Ginsburg V. Many pulmonary pathogenic bacteria bind specifically to the carbohydrate sequence GalNAc beta 1-4Gal found in some glycolipids. Proc Natl Acad Sci USA. 1988;85:6157–6161. doi: 10.1073/pnas.85.16.6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kuikka A, Syrjanen J, Renkonen O-V, Valtonen V. Pneumococcal bacteremia during a recent decade. J Infect. 1992;24:157–168. doi: 10.1016/0163-4453(92)92850-i. [DOI] [PubMed] [Google Scholar]
- 84.Lamani E, McPherson D T, Hollingshead S K, Jedrzejas M J. Production, characterization, and crystallization of truncated forms of pneumococcal surface protein A from Escherichia coli. Protein Expression Purif. 2000;20:379–388. doi: 10.1006/prep.2000.1320. [DOI] [PubMed] [Google Scholar]
- 85.Lancet. Acute respiratory infections in under-fives: 15 million deaths a year. Lancet. 1985;ii:699–701. [PubMed] [Google Scholar]
- 86.Lannering B, Larsson L E, Rojas J, Stahlman M T. Early onset group B streptococcal disease. Seven year experience and clinical scoring system. Acta Paediatr Scand. 1983;72:597–602. doi: 10.1111/j.1651-2227.1983.tb09777.x. [DOI] [PubMed] [Google Scholar]
- 87.Lawrence M C, Pilling P A, Epa V C, Berry A M, Ogunniyi A D, Paton J C. The crystal structure of pneumococcal surface antigen PsaA reveals a metal-binding site and a novel structure for a putative ABC-type binding protein. Structure. 1998;6:1553–1561. doi: 10.1016/s0969-2126(98)00153-1. [DOI] [PubMed] [Google Scholar]
- 88.Li S, Kelly S J, Lamani E, Ferraroni M, Jedrzejas M J. Streptococcus pneumoniae hyaluronate lyase crystal structure at 1.56 Å resolution: mechanism of hyaluronan binding and degradation. EMBO J. 2000;19:1228–1240. doi: 10.1093/emboj/19.6.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lock R A, Hansman D, Paton J C. Comparative efficacy of autolysin and pneumolysin as immunogens protecting mice against infection by Streptococcus pneumoniae. Microb Pathog. 1992;12:137–143. doi: 10.1016/0882-4010(92)90116-6. [DOI] [PubMed] [Google Scholar]
- 90.Lock R A, Paton J C, Hansman D. Purification and immunological characterization of neuraminidase produced by Streptococcus pneumoniae. Microb Pathog. 1988;5:461–467. doi: 10.1016/0882-4010(88)90046-0. [DOI] [PubMed] [Google Scholar]
- 91.Lopez R, Garcia J L, Garcia E, Ronda C, Garcia P. Structural analysis and biological significance of the cell wall lytic enzymes of Streptococcus pneumoniae and its bacteriophage. FEMS Microbiol Lett. 1992;79:439–447. doi: 10.1111/j.1574-6968.1992.tb14074.x. [DOI] [PubMed] [Google Scholar]
- 92.Lucas M, Mazzone T. Cell surface proteoglycans modulate net synthesis and secretion of macrophage apoliprotein. Eur J Biochem. 1996;271:14454–14460. doi: 10.1074/jbc.271.23.13454. [DOI] [PubMed] [Google Scholar]
- 93.Mark M P, Baker J R, Morrison K, Ruch J. Chondroitin sulfates in developing mouse tooth germs: an immunohistochemical study with monoclonal antibodies against chondroitin-4 and chondroitin-6 sulfates. Differentiation. 1990;43:37–50. doi: 10.1111/j.1432-0436.1990.tb00428.x. [DOI] [PubMed] [Google Scholar]
- 94.McDaniel L S, Ralph B A, McDaniel D O, Briles D E. Localization of protection-eliciting epitopes on PspA of Streptococcus pneumoniae between amino acid residues 192 and 260. Microb Pathog. 1994;17:323–337. doi: 10.1006/mpat.1994.1078. [DOI] [PubMed] [Google Scholar]
- 95.McDaniel L S, Sheffield J S, Swiatlo E, Yother J, Crain M J, Briles D E. Molecular localization of variable and conserved regions of PspA and identification of additional PspA homologous sequences in Streptococcus pneumoniae. Microb Pathog. 1992;13:261–269. doi: 10.1016/0882-4010(92)90036-n. [DOI] [PubMed] [Google Scholar]
- 96.McDaniel L S, Sheffield J S, DeLucchi P, Briles D E. PspA, a surface protein of Streptococcus pneumoniae, is capable of eliciting protection against pneumococci of more than one capsular type. Infect Immun. 1991;59:222–228. doi: 10.1128/iai.59.1.222-228.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.McDaniel L S, Yother J, Vijayakumar M, Guild W R, Briles D E. Use of insertional inactivation to facilitate studies of biological properties of pneumococcal surface protein A, PspA. J Exp Med. 1987;165:381–394. doi: 10.1084/jem.165.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.McDaniel L S, Scott G, Kearney J F, Briles D E. Monoclonal antibodies against protease-sensitive pneumococcal antigens can protect mice from fatal infection with Streptococcus pneumoniae. J Exp Med. 1984;160:386–397. doi: 10.1084/jem.160.2.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.McLachlan A D, Stewart M, Smillie L B. Sequence repeats in alpha-tropomyosin. J Mol Biol. 1975;98:281–291. doi: 10.1016/s0022-2836(75)80118-5. [DOI] [PubMed] [Google Scholar]
- 100.Medrano F J, Gasset M, Lopez-Zumel C, Usobiaga P, Garcia J L, Menendez M. Structural characterization of the unligated and choline-bound forms of the major pneumococcal autolysin LytA amidase. Conformational transitions induced by temperature. J Biol Chem. 1996;271:29152–29161. doi: 10.1074/jbc.271.46.29152. [DOI] [PubMed] [Google Scholar]
- 101.Meyer K, Chaffee E, Hobby G L, Dawson M H. Hyaluronidases of bacterial and animal origin. J Exp Med. 1941;73:309–326. doi: 10.1084/jem.73.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mitchell T J, Alexander J E, Morgan P J, Andrew P W. Molecular analysis of virulence factors of Streptococcus pneumoniae. Soc Appl Bacteriol Symp Ser. 1997;26:62S–71S. [PubMed] [Google Scholar]
- 103.Miyake K C, Underhill C B, Lesley J, Kincade P W. Hyaluronate can function as a cell adhesion molecule and CD44 participates in hyaluronate recognition. J Exp Med. 1990;172:69–75. doi: 10.1084/jem.172.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mufson M A. Streptococcus pneumoniae. In: Mandell G L, Douglas R G Jr, Bennett J E, editors. Principles and practice of infectious diseases. New York, N.Y: Churchill Livingstone, Inc.; 1990. pp. 1539–1550. [Google Scholar]
- 105.Musher D M. Infections caused by Streptococcus pneumoniae: clinical spectrum, pathogenesis, immunity, and treatment. Clin Infect Dis. 1991;14:801–807. doi: 10.1093/clinids/14.4.801. [DOI] [PubMed] [Google Scholar]
- 106.Nabors G S, Braun P A, Herrmann D J, Heise M L, Pyle D J, Gravenstein S, Schilling M, Ferguson L M, Hollingshead S K, Briles D E, Becker R S. Immunization of healthy adults with a single recombinant pneumococcal surface protein A (PspA) variant stimulates broadly cross-reactive antibodies to heterologous PspA molecules. Vaccine. 2000;18:1743–1754. doi: 10.1016/s0264-410x(99)00530-7. [DOI] [PubMed] [Google Scholar]
- 107.Nakamura M, Sekino N, Iwamoto M, Ohno-Iwashita Y. Interaction of theta-toxin (perfringolysin O), a cholesterol-binding cytolysin, with liposomal membranes: change in the aromatic side chains upon binding and insertion. Biochemistry. 1995;34:6513–6520. doi: 10.1021/bi00019a032. [DOI] [PubMed] [Google Scholar]
- 108.Novak R, Henriques B, Charpentier E, Normark S, Tuomanen E. Emergence of vancomycin tolerance in Streptococcus pneumoniae. Nature. 1999;399:590–593. doi: 10.1038/21202. [DOI] [PubMed] [Google Scholar]
- 109.O'Toole R D, Goode L, Howe C. Neuraminidase activity in bacterial meningitis. J Clin Investig. 1971;50:979–985. doi: 10.1172/JCI106591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Park Y, Cho S, Lindhardt R J. Exploration of the action pattern of Streptomyces hyaluronate lyase using high-resolution capilary electrophoresis. Biochim Biophys Acta. 1997;1337:217–226. doi: 10.1016/s0167-4838(96)00167-7. [DOI] [PubMed] [Google Scholar]
- 111.Parker M W, Buckley J T, Postma J P, Tucker A D, Leonard K, Pattus F, Tsernoglou D. Structure of the Aeromonas toxin proaerolysin in its water-soluble and membrane-channel states. Nature. 1994;367:292–295. doi: 10.1038/367292a0. [DOI] [PubMed] [Google Scholar]
- 112.Parker M W, Tucker A D, Tsernoglou D, Pattus F. Insights into membrane insertion based on studies of colicins. Trends Biochem Sci. 1990;15:126–129. doi: 10.1016/0968-0004(90)90205-p. [DOI] [PubMed] [Google Scholar]
- 113.Paton J C. Novel pneumococcal surface proteins: role in virulence and vaccine potential. Trends Microbiol. 1998;6:85–87. doi: 10.1016/s0966-842x(98)01220-7. [DOI] [PubMed] [Google Scholar]
- 114.Paton J C, Berry A M, Lock R A. Molecular analysis of putative pneumococcal virulence proteins. Microb Drug Resist. 1997;3:1–10. doi: 10.1089/mdr.1997.3.1. [DOI] [PubMed] [Google Scholar]
- 115.Paton J C, Andrew P W, Boulnois G J, Mitchell T J. Molecular analysis of the pathogenicity of Streptococcus pneumoniae: the role of pneumococcal proteins. Annu Rev Microbiol. 1993;47:89–115. doi: 10.1146/annurev.mi.47.100193.000513. [DOI] [PubMed] [Google Scholar]
- 116.Paton J C, Berry A M, Lock R A, Hansman D, Manning P A. Cloning and expression in Escherichia coli of the Streptococcus pneumoniae gene encoding pneumolysin. Infect Immun. 1986;54:50–55. doi: 10.1128/iai.54.1.50-55.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Paton J C, Lock R A, Hansman D J. Effect of immunization with pneumolysin on survival time of mice challenged with Streptococcus pneumoniae. Infect Immun. 1983;40:548–552. doi: 10.1128/iai.40.2.548-552.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Petosa C, Collier R J, Klimpel K R, Leppla S H, Liddington R C. Crystal structure of the anthrax toxin protective antigen. Nature. 1997;385:833–838. doi: 10.1038/385833a0. [DOI] [PubMed] [Google Scholar]
- 119.Phillips G N, Jr, Fillers J P, Cohen C. Tropomyosin crystal structure and muscle regulation. J Mol Biol. 1986;192:111–131. doi: 10.1016/0022-2836(86)90468-7. [DOI] [PubMed] [Google Scholar]
- 120.Pilling P A, Lawrence M C, Berry A M, Ogunniyi A D, Lock R A, Paton J C. Expression, purification and preliminary X-ray crystallographic analysis of PsaA, a putative metal-transporter protein of Streptococcus pneumoniae. Acta Crystallogr. 1998;D54:1464–1466. doi: 10.1107/s0907444998005812. [DOI] [PubMed] [Google Scholar]
- 121.Ponnuraj K, Jedrzejas M J. Hyaluronan binding and degradation: a complex structure of pneumococcal hyaluronate lyase with hyaluronan substrate. J Mol Biol. 2000;299:885–895. doi: 10.1006/jmbi.2000.3817. [DOI] [PubMed] [Google Scholar]
- 122.Pritchard D G, Lin B. Group B streptococcal neuraminidase is actually a hyaluronidase. Infect Immun. 1993;61:3234–3239. doi: 10.1128/iai.61.8.3234-3239.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pritchard D G, Lin B, Willingham T R, Baker J R. Characterization of the group B streptococcal hyaluronate lyase. Arch Biochem Biophys. 1994;315:431–437. doi: 10.1006/abbi.1994.1521. [DOI] [PubMed] [Google Scholar]
- 124.Qui G N, Toyoda H, Toida T, Koshiishi I, Imanari T. Compositional analysis of hyaluronan, chondroitin sulfate and dermatan sulfate: HPLC of disaccharides produced from the glycosaminoglycans by solvolysis. Chem Pharm Bull (Tokyo) 1996;44:1017–1020. doi: 10.1248/cpb.44.1017. [DOI] [PubMed] [Google Scholar]
- 125.Rayner C F J, Jackson A D, Rutman A. Interaction of pneumolysin-sufficient and -deficient isogenic variants of Streptococcus pneumoniae with human respiratory mucosa. Infect Immun. 1995;63:422–427. doi: 10.1128/iai.63.2.442-447.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rogers H J, Perkins H R, Ward J B. Formation of cell wall polymers. In: Nombela C, editor. Microbial cell wall and membranes. London, United Kingdom: Chapman & Hall, Ltd.; 1980. pp. 437–460. [Google Scholar]
- 127.Ronda C, Garcia Garcia J L, Sanchez-Puelles E J M, Lopez R. Biological role of the pneumococcal amidase. Cloning of the lytA gene in Streptococcus pneumoniae. Eur J Biochem. 1987;164:621–624. doi: 10.1111/j.1432-1033.1987.tb11172.x. [DOI] [PubMed] [Google Scholar]
- 128.Rosenfeld R M, Doyle W J, Swarts J D, Seroky J, Pinero B P. Third-generation cephalosporins in the treatment of acute pneumococcal otitis media. An animal study. Arch Otolaryngol Head Neck Surg. 1992;118:49–52. doi: 10.1001/archotol.1992.01880010053015. [DOI] [PubMed] [Google Scholar]
- 129.Rosenow C, Ryan P, Weiser J N, Johnson S, Fontan P, Ortqvist A, Masure H R. Contribution of novel choline-binding proteins to adherence, colonization and immunogenicity of Streptococcus pneumoniae. Mol Microbiol. 1997;25:819–829. doi: 10.1111/j.1365-2958.1997.mmi494.x. [DOI] [PubMed] [Google Scholar]
- 130.Rossjohn J, Feil S C, McKinstry W J, Tweten R K, Parker M W. Structure of a cholesterol-binding thiol-activated cytolysin and a model of its membrane form. Cell. 1997;89:685–692. doi: 10.1016/s0092-8674(00)80251-2. [DOI] [PubMed] [Google Scholar]
- 131.Rossjohn J, Gilbert R J, Crane D, Morgan P J, Mitchell T J, Rowe A J, Andrew P W, Paton J C, Tweten R K, Parker M W. The molecular mechanism of pneumolysin, a virulence factor from Streptococcus pneumoniae. J Mol Biol. 1998;284:449–461. doi: 10.1006/jmbi.1998.2167. [DOI] [PubMed] [Google Scholar]
- 132.Rubins J B, Janoff E N. Pneumolysin: a multifunctional pneumococcal virulence factor. J Lab Clin Med. 1998;131:21–27. doi: 10.1016/s0022-2143(98)90073-7. [DOI] [PubMed] [Google Scholar]
- 133.Sambandam T J, Baker J R, Christner J E, Ekborg S L. Specificity of the low density lipoprotein-glycosaminoglycan interactions. Arteriosclerosis. 1991;11:561–568. doi: 10.1161/01.atv.11.3.561. [DOI] [PubMed] [Google Scholar]
- 134.Sampson J S, O'Connor S P, Stinson A R, Tharpe J A, Russel H. Cloning and nucleotide sequence analysis of psaA, the Streptococcus pneumoniae gene encoding a 37-kilodalton protein homologous to previously reported Streptococcus sp. adhesins. Infect Imunn. 1994;62:319–324. doi: 10.1128/iai.62.1.319-324.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sampson P M, Rochester C L, Freundlich B, Elias J A. Cytokine regulation of human lung fibroblast hyaluronan (hyaluronic acid) production. J Clin Investig. 1992;90:1492–1503. doi: 10.1172/JCI116017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sato K, Quartey M K, Liebeler C L, Le C T, Giebink G S. Roles of autolysin and pneumolysin in middle ear inflammation caused by a type 3 Streptococcus pneumoniae strain in the chinchilla otitis media model. Infect Immun. 1996;64:1140–1145. doi: 10.1128/iai.64.4.1140-1145.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Schneewind O, Fowler A, Faull K F. Structure of the cell wall anchor of surface proteins in Staphylococcus aureus. Science. 1995;268:103–106. doi: 10.1126/science.7701329. [DOI] [PubMed] [Google Scholar]
- 138.Schwartz B, Schuchat A, Oxtoby M J, Cochi S L, Hightower A, Broome C V. Invasive group B streptococcal disease in adults: a population-based study in metropolitan Atlanta. JAMA. 1991;266:1112–1114. [PubMed] [Google Scholar]
- 139.Shatursky O, Heuck A P, Shepard L A, Rossjohn J, Parker M W, Johnson A E, Tweten R K. The mechanism of membrane insertion for a cholesterol-dependent cytolysin: a novel paradigm for pore-forming toxins. Cell. 1999;99:293–299. doi: 10.1016/s0092-8674(00)81660-8. [DOI] [PubMed] [Google Scholar]
- 140.Shepard L A, Heuck A P, Hamman B D, Rossjohn J, Parker M W, Ryan K R, Johnson A E, Tweten R K. Identification of a membrane-spanning domain of the thiol-activated pore-forming toxin Clostridium perfringens perfringolysin O: an alpha-helical to beta-sheet transition identified by fluorescence spectroscopy. Biochemistry. 1998;37:14563–14574. doi: 10.1021/bi981452f. [DOI] [PubMed] [Google Scholar]
- 141.Song L, Hobaugh M R, Shustak C, Cheley S, Bayley H, Gouaux J E. Structure of staphylococcal alpha-hemolysin, a heptameric transmembrane pore. Science. 1996;274:1859–1866. doi: 10.1126/science.274.5294.1859. [DOI] [PubMed] [Google Scholar]
- 142.Spika J S, Facklam R R, Plikaytis B D, Oxtoby M J. Antimicrobial resistance of Streptococcus pneumoniae in the United States, The Pneumococcal Surveillance Working Group. J Infect Dis. 1991;163:1273–1278. doi: 10.1093/infdis/163.6.1273. [DOI] [PubMed] [Google Scholar]
- 143.Stansfield S K. Acute respiratory infections in the developing world: strategies for prevention, treatment and control. Pediatr Infect Dis J. 1987;6:622–629. doi: 10.1097/00006454-198707000-00002. [DOI] [PubMed] [Google Scholar]
- 144.Steiner B, Cruce D. A zymographic assay for detection of hyaluronidase activity on polyacrylamide gels and its application to enzymatic activity found in bacteria. Anal Biochem. 1992;200:405–410. doi: 10.1016/0003-2697(92)90487-r. [DOI] [PubMed] [Google Scholar]
- 145.Steinfort C, Wilson R, Mitchell T. Effects of Streptococcus pneumoniae on human respiratory epithelium in vitro. Infect Immun. 1989;57:2006–2013. doi: 10.1128/iai.57.7.2006-2013.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Stool S E, Field M J. The impact of otitis media. Pediatr Infect Dis J. 1989;8:S11–S14. [PubMed] [Google Scholar]
- 147.Sudbeck E A, Jedrzejas M J, Singh S, Brouillette W J, Air G M, Laver W G, Babu Y S, Bantia S, Chand P, Chu N, Montgomery J A, Walsh D A, Luo M. Guanidinobenzoic acid inhibitors of influenza virus neuraminidase. J Mol Biol. 1997;267:584–594. doi: 10.1006/jmbi.1996.0885. [DOI] [PubMed] [Google Scholar]
- 148.Talkington D F, Brown B G, Tharpe J A, Koenig A, Russell H. Protection of mice against fatal pneumococcal challenge by immunization with pneumococcal surface adhesin A (PsaA) Microb Pathog. 1996;21:17–22. doi: 10.1006/mpat.1996.0038. [DOI] [PubMed] [Google Scholar]
- 149.Tam R, Saier M H., Jr Structural, functional, and evolutionary relationships among extracellular solute-binding receptors of bacteria. Microbiol Rev. 1993;57:320–346. doi: 10.1128/mr.57.2.320-346.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Teti G, Mancuso G, Tomasello F. Cytokine appearance and effects of anti-tumor necrosis factor alpha antibodies in a neonatal rat model of group B streptococcal infection. Infect Immun. 1993;61:227–235. doi: 10.1128/iai.61.1.227-235.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tomasz A, Moreillon P, Pozzi G. Insertional inactivation of the major autolysin gene of Streptococcus pneumoniae. J Bacteriol. 1988;170:5931–5934. doi: 10.1128/jb.170.12.5931-5934.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tomasz A. Building and breaking of bonds in the cell wall of bacteria—the role for autolysin. In: Nombela C, editor. Microbial cell wall and autolysins. Amsterdam, The Netherlands: Elsvier Science Publishers; 1984. pp. 3–12. [Google Scholar]
- 153.Tomasz A. Surface components of Streptococcus pneumoniae. Rev Infect Dis. 1981;3:190–211. doi: 10.1093/clinids/3.2.190. [DOI] [PubMed] [Google Scholar]
- 154.Tomasz A, Westphal M, Briles E B, Fletcher P. On the physiological functions of teichoic acids. J Supramol Struct. 1975;3:1–16. doi: 10.1002/jss.400030102. [DOI] [PubMed] [Google Scholar]
- 155.Tomasz A, Zanati E, Ziegler R. DNA uptake during genetic transformation and the growing zone of the cell envelope. Proc Natl Acad Sci USA. 1971;68:1848–1852. doi: 10.1073/pnas.68.8.1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tomasz A, Albino A, Zanati E. Multiple antibiotic resistance in a bacterium with suppressed autolytic system. Nature. 1970;227:138–140. doi: 10.1038/227138a0. [DOI] [PubMed] [Google Scholar]
- 157.Tulip W R, Varghese J N, Baker A T, van Donkelaar A, Laver W G, Webster R G, Colman P M. Refined atomic structures of N9 subtype influenza virus neuraminidase and escape mutants. J Mol Biol. 1991;221:487–497. doi: 10.1016/0022-2836(91)80069-7. [DOI] [PubMed] [Google Scholar]
- 158.Tuomanen E. Molecular and cellular biology of pneumococcal infection. Curr Opin Microbiol. 1999;2:35–39. doi: 10.1016/s1369-5274(99)80006-x. [DOI] [PubMed] [Google Scholar]
- 159.Usobiaga P, Medrano F J, Gasset M, Garcia J L, Saiz J L, Rivas G, Laynez J, Menendez M. Structural organization of the major autolysin from Streptococcus pneumoniae. J Biol Chem. 1996;271:6832–6838. doi: 10.1074/jbc.271.12.6832. [DOI] [PubMed] [Google Scholar]
- 160.Varghese J N, Colman P M. Three-dimensional structure of the neuraminidase of influenza virus A/Tokyo/3/67 at 2.2 Å resolution. J Mol Biol. 1991;221:473–486. doi: 10.1016/0022-2836(91)80068-6. [DOI] [PubMed] [Google Scholar]
- 161.Waltman W D, II, McDaniel L S, Gray B M, Briles D E. Variation in the molecular weight of PspA (pneumococcal surface protein A) among Streptococcus pneumoniae. Microb Pathog. 1990;8:61–69. doi: 10.1016/0882-4010(90)90008-e. [DOI] [PubMed] [Google Scholar]
- 162.Williams P A, Bohnsack J F, Augustine N H, Rubens C E, Hill H R. Production of tumor necrosis factor by human cells in vitro and in vivo, induced by group B streptococci. J Pediatr. 1993;123:292–300. doi: 10.1016/s0022-3476(05)81706-8. [DOI] [PubMed] [Google Scholar]
- 163.Williams W W, Hickson M A, Kane M A, Kendal A P, Spika J S, Hinman A R. Immunization policies and vaccine coverage among adults: the risk for missed opportunities. Ann Intern Med. 1988;108:616–625. doi: 10.7326/0003-4819-108-4-616. [DOI] [PubMed] [Google Scholar]
- 164.Wren B W. A family of clostridial and streptococcal ligand-binding proteins with conserved C-terminal repeat sequences. Mol Microb. 1991;5:797–803. doi: 10.1111/j.1365-2958.1991.tb00752.x. [DOI] [PubMed] [Google Scholar]
- 165.Yang B, Yang B L, Savani R C, Turley E A. Identification of a common hyaluronan binding motif in the hyaluronan binding proteins RHAMM, CD44 and link protein. EMBO J. 1994;13:286–296. doi: 10.1002/j.1460-2075.1994.tb06261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Yother J, Briles D E. Structural properties and evolutionary relationships of PspA, a surface protein of Streptococcus pneumoniae, as revealed by sequence analysis. J Bacteriol. 1992;174:601–609. doi: 10.1128/jb.174.2.601-609.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Yother J, White J M. Novel surface attachment mechanism of the Streptococcus pneumoniae protein PspA. J Bacteriol. 1994;176:2976–2985. doi: 10.1128/jb.176.10.2976-2985.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]