Abstract
INTRODUCTION:
Screening potential participants in Alzheimer disease (AD) clinical trials with amyloid PET is often time-consuming and expensive.
METHODS:
A web-based application was developed to model the time and financial cost of screening for AD clinical trials. Four screening approaches were compared; three approaches included an AD blood test at different stages of the screening process.
RESULTS:
The traditional screening approach using only amyloid PET was the most time-consuming and expensive. Incorporating an AD blood test at any point in the screening process decreased both the time and financial cost of trial enrollment. Improvements in AD blood test accuracy over currently available tests only marginally increased savings. Use of a high specificity cut-off may improve the feasibility of screening with only an AD blood test.
DISCUSSION:
Incorporating AD blood tests into screening for AD clinical trials may reduce the time and financial cost of enrollment.
Keywords: Modeling, web-based application, clinical trial enrollment, financial cost, time of enrollment, modeling, amyloid PET, blood test, Alzheimer disease, blood-based biomarkers, economics
1. Introduction
Alzheimer disease (AD) is one of the most expensive and devastating diseases of modern times and effective treatments are urgently needed [1–3]. Biomarkers that reflect the amyloid plaques and tau tangles characteristic of AD have provided drug development efforts with critically needed tools [4, 5]. Before AD biomarkers were widely available, ~25% of participants in AD clinical trials did not even have AD brain pathology, but rather had cognitive impairment from non-AD causes [6]. AD clinical trials now routinely use biomarkers to screen potential participants for AD brain pathology. Further, AD biomarkers are being used to assess the effects of treatments [7, 8].
Biomarkers have become central to AD clinical trials and their use has a major impact on the time and financial cost of trials. For example, while amyloid PET accurately detects brain amyloidosis and is well tolerated [4], screening potential participants with amyloid PET is often time-consuming and expensive, especially for prevention trials enrolling cognitively normal older individuals with a low frequency of brain amyloidosis. One major prevention trial, the Anti-Amyloid Treatment in Asymptomatic Alzheimer’s disease (A4) trial, performed 4,486 amyloid PET scans across 67 sites over the course of 3.8 years in order to identify 1,323 amyloid PET positive participants [9, 10]. Cerebrospinal fluid (CSF) levels of amyloid-β peptide 42, total tau, and phosphorylated tau can also be used to detect AD brain pathology and are strongly correlated with amyloid PET, but CSF biomarkers are not often used for screening potential participants because some individuals perceive lumbar punctures as overly invasive [5, 11]. While both amyloid PET and CSF biomarkers provide highly accurate information on AD brain pathology, their practical limitations can make clinical trials slower and more expensive.
There has recently been extremely rapid development of blood-based AD biomarkers, which have unique advantages that may transform and accelerate AD clinical trials [12–19]. Blood collection is minimally invasive and well tolerated by research participants, including individuals from groups that have been under-represented in AD research [11]. Venipuncture does not require expensive equipment or highly trained personnel, and can be performed at sites far from research centers. The potential throughput of AD blood tests is magnitudes higher than would be possible for tests requiring brain imaging or lumbar punctures. Further, AD blood tests could be relatively inexpensive compared to amyloid PET or CSF biomarkers. Overall, these advantages mean that AD blood tests provide an opportunity to rapidly test many individuals with relatively low burden and financial cost. Several analyses have concluded that screening potential clinical trial participants with currently available AD blood tests would reduce the number of amyloid PET scans required to enroll amyloid positive participants, decreasing the time and financial cost of enrollment [17, 20–24].
While some clinical trials have already started screening potential participants with AD blood tests (NCT04468659 and NCT05026866) [25, 26], the most effective approach to using AD blood tests is unclear. There has not been an analysis of when to perform an AD blood test, e.g., immediately after the initial phone screening for the study, or just before scheduling the amyloid PET scan. Additionally, the exact savings associated with an AD blood test is complex to estimate, especially given differences between study designs and sites. Therefore, we created a model to estimate the time and financial cost of four potential screening approaches, three of which include an AD blood test. Further, we present a novel web-based modeling tool that allows users to input time and cost parameters that are relevant to their own study. Finally, we examine how the characteristics of the AD blood test would affect clinical trial screening.
2. Methods
2.1. Selection of screening approaches
Approaches for screening potential participants for enrollment in AD clinical trials were selected by neurologists experienced in AD clinical trials (R.J.B., K.W., S.E.S) (Appendix A). The traditional approach uses only amyloid PET for screening. Three approaches that incorporate an AD blood test at different points were selected for comparison. Amyloid PET was assumed to be the gold standard for brain amyloidosis. The categorization of “meeting study criteria” was designed to be flexible given the significant differences between clinical trials, and could include considerations related to cognitive status (cognitively normal, mild cognitive impairment or AD dementia), performance within a specified range on cognitive tests, and/or the absence of exclusionary conditions.
2.2. Estimation of time and cost parameters
Factors that affect the time (Appendix B) and financial cost (Appendix C) to enroll clinical trial participants were examined using data from the Knight Alzheimer Disease Research Center Trials Unit, the Alzheimer’s Therapeutic Research Institute (ATRI), and an ongoing clinical study to validate an amyloid blood test in a general population (SEABIRD: Study to Evaluate Amyloid in Blood and Imaging Related to Dementia). The accuracy of the AD blood test was set at 0.85% sensitivity and 0.80% specificity for brain amyloidosis (Appendix D). Estimates for the cohort composition were made based on published data and institutional experience with screening for the A4 trial [9, 27, 28].
2.3. Modeling approach
The total time to enroll the target number of amyloid positive individuals who met study criteria was estimated for each screening approach by accounting for events occurring in parallel, such as multiple participants being contacted and moving through the screening process simultaneously, as well as rate-limiting steps (see Appendix E). The total financial cost of screening via each screening approach was estimated by adding the costs associated with each step (see Appendix F). Notably, the estimates for the time and financial cost parameters were derived from Washington University, which may have a higher capacity than many other sites. Because key parameters may vary considerably between trials and sites, a web-based application was created that enables users to input values for key factors that are appropriate for their study (https://amyloid.shinyapps.io/Optimizing_Screening/)[29]. Code for this application is provided in Appendix G. All modeling was performed in R version 4.0.5. Plots were created with GraphPad Prism version 9.2.0 (GraphPad Software, La Jolla, CA, USA).
3. Results
3.1. Estimated time and financial cost of screening approaches
Four approaches for screening potential participants for enrollment in AD clinical trials were considered (Figure 1). Approach A screens potential participants for brain amyloidosis with amyloid PET and does not include an AD blood test. Potential participants are called to determine eligibility based on easily ascertainable factors such as age and medical history (Step 1: Intake). Individuals remaining eligible after the initial phone call are scheduled for a screening visit (Step 2: Screening) that includes cognitive testing and a physical examination. Individuals who perform within the specified range on cognitive tests and who have no exclusionary conditions found on examination undergo routine laboratory tests to rule-out additional exclusionary conditions. Those who meet study criteria are evaluated for brain amyloidosis with an amyloid PET scan (Step 3: Amyloid PET), and individuals who are amyloid PET positive are enrolled in the trial.
Figure 1. Estimated time and financial cost of four different screening approaches (A-D).
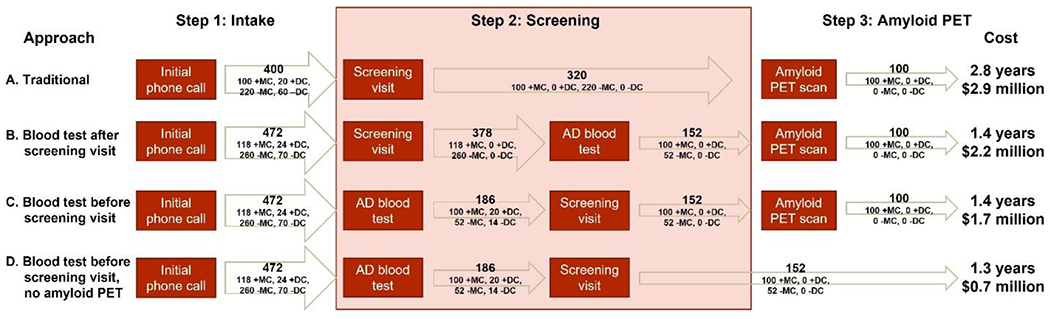
The time and financial cost of enrolling 100 amyloid PET positive participants who meet study criteria was estimated for each approach. Estimates are presented for an AD blood test with an 85% sensitivity and 80% specificity for brain amyloidosis. The number and type of individuals at the end of each step is shown (+MC: amyloid PET positive, meets study criteria; +DC: amyloid PET positive, does not meet study criteria; −MC: amyloid PET negative, meets study criteria; −DC: amyloid PET negative, does not meet study criteria.)
Three screening approaches that include an AD blood test were also considered. Approach B is identical to approach A until the conclusion of the screening visit, when individuals have samples collected not only for routine laboratory testing but also for an AD blood test. Only individuals with a positive AD blood test undergo an amyloid PET scan; individuals with a negative AD blood test are informed they are not eligible for further participation in the study. In approach C, an AD blood test is performed on individuals remaining eligible after the intake (initial phone call). Individuals with a negative AD blood test are informed they are not eligible for further participation, and only individuals with a positive AD blood test undergo the screening visit and potentially an amyloid PET scan. Approach D is identical to screening approach C, except that the AD blood test is used as the sole measure of brain amyloidosis and individuals do not undergo an amyloid PET scan.
To illustrate this modeling approach, the time and financial cost of enrolling participants via the four screening approaches was examined for a target enrollment of 100 amyloid PET positive individuals who meet study criteria. Importantly, the proportion of amyloid PET positive individuals who meet study criteria may vary markedly in screening populations and will have a major impact on the time and financial cost of enrolling participants. Estimates for key parameters were derived from AD clinical trials at Washington University and are summarized in Table 1. Based on these estimates, the traditional screening approach (approach A) would take 2.8 years and cost $2.9 million. In contrast, performing an AD blood test prior to the amyloid PET scan (approach B) would reduce the time for enrollment by 49% to 1.4 years and reduce the financial cost by 25% to $2.2 million. If the AD blood test were instead performed before the screening visit (approach C), the time of enrollment would remain at 1.4 years, but the cost of enrollment would be reduced to $1.7 million. Finally, if the AD blood test were used as the sole measure of brain amyloidosis and individuals did not undergo an amyloid PET scan (approach D), enrollment would take 1.3 years and cost $0.7 million. The savings of approaches B-D were primarily related to the reduced numbers of individuals who must undergo the most time-consuming and expensive aspects of screening, which are amyloid PET scans and screening visits.
Table 1. Summary of key parameters used in modeling the time and financial cost of enrollment.
For detailed descriptions of parameter estimates, see Appendix B for parameters that affect time and Appendix C for parameters that affect financial cost. A web-based application was developed that allows investigators to input their own values for these parameters into the model.
| Parameter type | Parameter | Value |
|---|---|---|
| Target enrollment | Number of amyloid PET+ individuals who meet study criteria | 100 |
| Characteristics of potentially eligible cohort (after initial phone call) | Amyloid PET positive, meets study criteria Amyloid PET positive, does not meet study criteria Amyloid PET negative, meets study criteria Amyloid PET negative, does not meet study criteria |
25% 5% 55% 15% |
| AD blood test accuracy | Sensitivity of AD blood test for brain amyloidosis Specificity of the AD blood test for brain amyloidosis |
85% 80% |
| Step 1: Intake | Percent of individuals called who meet eligibility criteria and follow-up for a screening visit | 75% |
| Initial phone calls per day | 1.3 | |
| Cost of initial phone call without AD blood test scheduling | $80 | |
| Cost of initial phone call with AD blood test scheduling | $175 | |
| Step 2: Screening | Waiting time between initial phone call and screening visit | 37 days |
| Waiting time between initial phone call/AD blood test and screening visit | 47 days | |
| Screening visits per day | 1.2 | |
| Cost of screening visit without routine laboratory tests | $1,326 | |
| Cost of screening visit with routine laboratory tests | $2,209 | |
| Cost of AD blood test | $540 | |
| Step 3: Amyloid PET | Waiting time between screening visit and amyloid PET scan | 30 days |
| Amyloid PET scans per day | 0.34 | |
| Cost of amyloid PET scan | $6,487 |
3.2. Reducing the time and financial cost of enrollment
Strategies to reduce the time of enrollment were explored for each screening approach by varying the values of key time parameters to evaluate for rate-limiting steps (Figure 2A–C). Based on the capacity to perform amyloid PET scans at Washington University, the major rate-limiting step for approaches A-C was the number of amyloid PET scans per day. For approach D, which does not include an amyloid PET scan, the major rate-limiting step was initial phone calls per day. Simultaneously increasing the number of initial phone calls per day and visits per day greatly reduced the time of enrollment for approach D, such that enrollment could be completed in just 94 days (0.3 years) (Figure 3).
Figure 2. Effects of modifying a single parameter on the time and financial cost of enrollment.
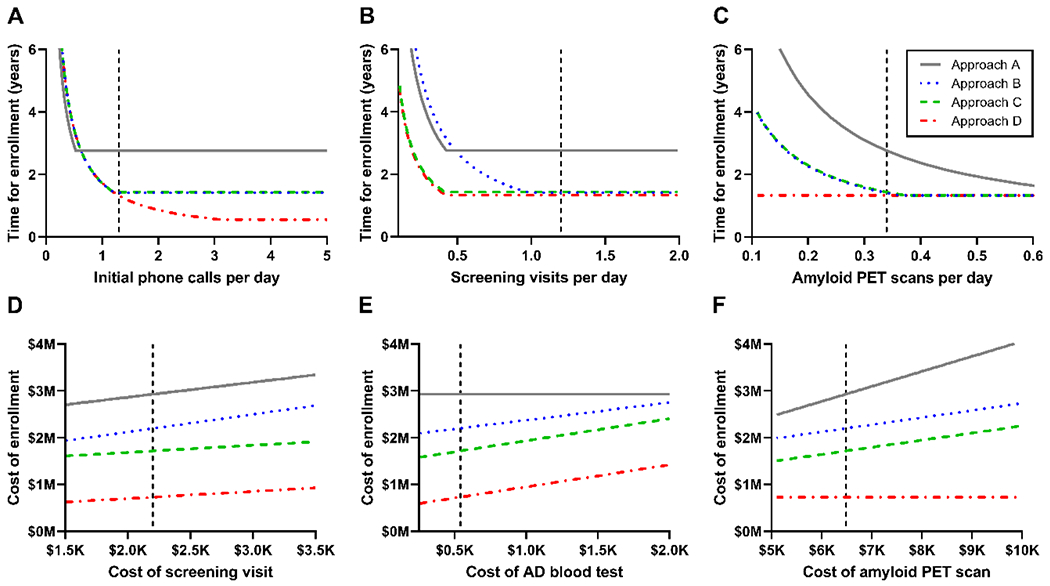
The time (A-C) or financial cost (D-F) of enrolling 100 amyloid PET positive participants who meet study criteria was estimated for each approach as one key parameter was varied. The dashed vertical line represents the estimated value for AD clinical trials at Washington University. The cost of the AD blood test did not affect approach A, which does not include an AD blood test, and the cost of an amyloid PET scan did not affect approach D, which does not include an amyloid PET scan.
Figure 3. Reducing the time of enrollment for approach D.

The estimated time of enrollment for approach D as a function of initial calls per day and screening visits per day.
The relative financial cost of enrollment via each of the screening approaches were comparatively stable for the key financial cost parameters because the financial costs were driven by the number of individuals rather than dependent on rate-limiting steps. Across a range of reasonable values for key financial cost parameters, approach A consistently cost the most ($2.5-4.1 million), followed by approach B ($1.9-2.8 million), then approach C ($1.5-2.4 million), and approach D cost the least ($0.6-1.4 million) (Figure 2D–F).
3.3. Effects of AD blood test accuracy on the time and financial cost of enrollment
The time and financial cost of enrollment were examined as a function of the sensitivity and specificity of the AD blood test for brain amyloidosis, using amyloid PET status as the reference standard. As previously described, integrating a highly accurate AD blood test (sensitivity 0.85/specificity 0.80) into screening (approach B, C or D) markedly decreased the time and financial cost of enrollment compared to the traditional approach based solely on amyloid PET (approach A) (Figure 4). Interestingly, using a nearly perfect AD blood test (sensitivity 0.95/specificity 0.90) only marginally reduced the time (~11-16%) and cost (~15-18%) of enrollment compared to using a highly accurate AD blood test. This suggests that significant savings can be realized by using currently available AD blood tests, and that improvements in blood test accuracy will only marginally increase these savings.
Figure 4. Time and cost of enrollment by AD blood test accuracy.
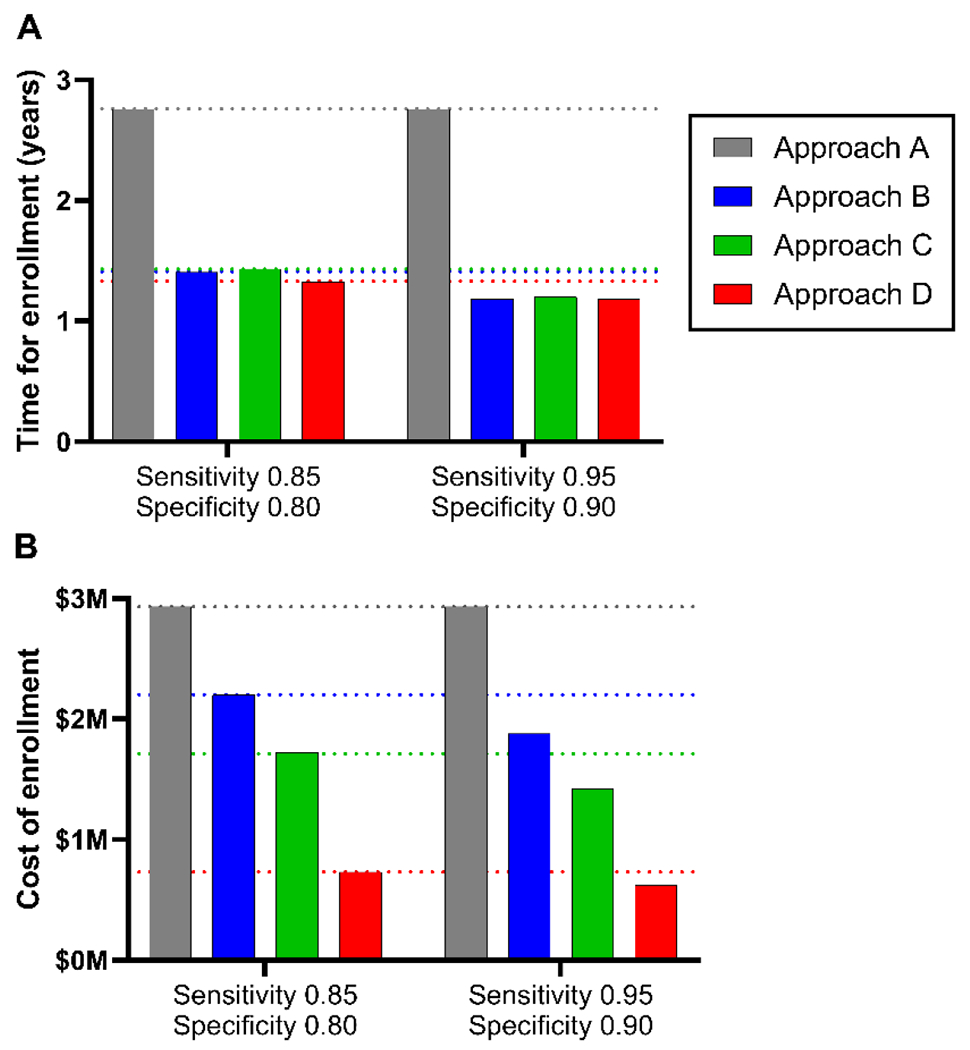
The time and financial cost of enrollment for each approach were examined as a function of the sensitivity and specificity of the AD blood test for brain amyloidosis.
3.4. Reducing individuals with false positive AD blood tests
A concern about approach D, which uses an AD blood test as the sole measure of brain amyloidosis, is the enrollment of some individuals with a false positive AD blood test (i.e., individuals with a positive AD blood test who would have a negative amyloid PET scan if one were performed). The number of false positive individuals enrolled via approach D is largely determined by the specificity of the AD blood test for brain amyloidosis (Figure 5A). For an AD blood test with a sensitivity of 0.85 and specificity of 0.80, 34% of participants enrolled via approach D would be expected to have a negative amyloid PET scan if one were performed. Reducing the number of false positive individuals can be achieved by selecting a cut-off that corresponds to a high specificity. For example, selecting an AD blood test cut-off with a specificity of 0.95, even with a lower sensitivity of 0.70, would reduce the proportion of enrolled participants having a negative amyloid PET scan to 14% (Figure 5B). The trade-off of selecting an AD blood test cut-off with a high specificity would be an increased proportion of false negatives, i.e., a higher proportion of amyloid PET positive participants would have a negative AD blood test result. Clinical trials can compensate for a higher false negative rate by increasing the number of individuals screened (Figure 5C). For example, an AD blood test with a sensitivity of 0.70 and a specificity of 0.95 would require screening 763 individuals (21% more) compared to an AD blood test with a sensitivity of 0.85 and a specificity of 0.80, which would require screening 629 individuals. Increasing the frequency of brain amyloidosis in the screening population by requiring an older age for study inclusion would also decrease the number of false positive AD blood tests [27, 28, 30].
Figure 5. False positive participants enrolled via approach D.
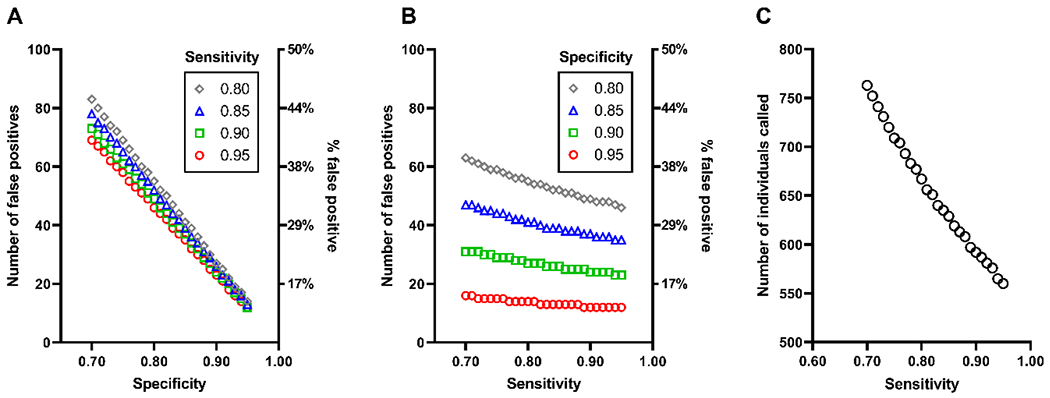
The number and proportion of individuals enrolled via approach D with a positive AD blood test who would be expected to have a negative amyloid PET scan (“false positives”) as a function of the specificity of the AD blood test for brain amyloidosis, stratified by the sensitivity (A). The number and percent of false positives as a function of the sensitivity stratified by the specificity (B). The number of individuals who must be called to yield 100 amyloid PET positive individuals who meet study criteria as a function of the AD blood test sensitivity (C), assuming that 75% of individuals called are eligible for the study.
4. Discussion
In this study, the time and financial cost of screening potential participants for enrollment in AD clinical trials was modeled using time and cost parameters derived from studies at Washington University, a large research institution with an active AD clinical trials unit. The traditional screening approach using only amyloid PET was the most expensive and time-consuming. Incorporating an AD blood test reduced the number of scans required, decreasing the time and financial cost of trial enrollment. The fastest and least expensive screening approach used an AD blood test as the sole measure of brain amyloidosis.
One major concern about using an AD blood test for screening is that some potential participants would have false positive or false negative results. If it is unacceptable for a trial to enroll individuals with false positive AD blood tests, screening approaches that use an AD blood test followed by an amyloid PET scan could be used and still have reduced time and financial costs of enrollment compared to the traditional approach using only amyloid PET. However, all screening approaches that use an AD blood test would not enroll individuals with a false negative AD blood test. Consequently, all screening approaches including an AD blood test would require recruiting a larger number of individuals (e.g., 18% more) to be screened for the trial. Recruiting more individuals may present a significant obstacle for some trials, especially those enrolling minoritized populations, and each trial would need to assess whether the efficiencies afforded by AD blood tests outweighed the additional recruitment burden.
Although the fastest and least expensive screening approach used an AD blood test as the sole measure of brain amyloidosis, this approach would lead to the enrollment of some individuals with false positive AD blood tests. Because AD blood tests may detect brain amyloidosis before amyloid PET [17], some individuals with false positive AD blood tests may have very early brain amyloidosis and be less likely to experience cognitive decline over the course of a clinical trial. Therefore, inclusion of individuals with false positive AD blood tests might reduce the power of a trial to discern the effect of a treatment on cognitive decline. Furthermore, the effect of a treatment on amyloid burden as measured by PET is a key endpoint in some trials and this effect may be difficult to detect in individuals who are below the threshold for amyloid PET positivity. For these reasons, it would be important to limit the number of participants with false positive AD blood tests, which could be achieved by selecting an AD blood test cut-off with a high specificity. A trade-off of using a high specificity cut-off is lower sensitivity, which would increase the number of false negative individuals and require screening more individuals.
Although biomarker cut-offs that minimize the combined number of false positive and false negative individuals are typically used diagnostically, strategic selection of cut-offs may increase the efficiency of clinical trial enrollment. The goal of clinical diagnosis is providing the most accurate diagnosis to each patient, and both false positive and false negative results should be minimized. In contrast, the goal of a clinical trial is to test the efficacy of a treatment as efficiently as possible. If a cut-off is applied that increases the frequency of inaccurate results, biomarker test results can be returned to the individual as showing they are “eligible” or “not eligible” for the trial, rather than being returned as providing a definitive diagnosis regarding brain amyloidosis. Some AD research studies disclose results to participants that are associated with dementia risk to varying degrees, and research participants generally cope well even with information that strongly affects dementia risk, such as apolipoprotein E (APOE) genotype or amyloid PET status [31, 32]. Especially since most observational research studies do not provide any results of biomarker testing to participants [33], it may be acceptable for a clinical trial not to provide a definitive diagnosis regarding brain amyloidosis to participants.
Some have argued that AD blood tests must be even more accurate and better validated before they are implemented. AD blood tests may not perform consistently across racial groups [34] and may give different results if individuals have certain medical conditions such as chronic kidney disease [35, 36]. However, there are also concerns about whether amyloid PET is a consistent AD biomarker across racial groups [37]. Much more work needs to be done to understand the relationships of biomarkers with race, medical conditions, AD brain pathology and cognitive impairment. The large studies necessary to understand these deeper questions will likely take several years to reach conclusions. In the meantime, assays for plasma Aβ42/Aβ40, p-tau181 and p-tau217 already exist that have high concordance with amyloid PET [14, 16, 21, 38, 39]. In this study, an AD blood test with the accuracy of currently available tests markedly reduced the time and financial costs of enrollment; a nearly perfect test only resulted in marginal added reductions. AD blood tests and our understanding of them will continue to improve [40], but waiting to implement AD blood tests in clinical trial screening may delay the realization of the benefits of these tools, slowing development of effective AD treatments.
Given the urgent need for effective AD treatments, accelerating clinical trial enrollment is critically important. Reducing the time of enrollment would also decrease the overall financial costs of running a clinical trial, which would reduce losses if a trial were unsuccessful and increase returns on investment if a trial were successful [41]. The time required for clinical trial enrollment is often dictated by a single rate-limiting step. At Washington University, this rate-limiting step was the number of amyloid PET scans that can be performed in a day, which is often constrained by competing demands for PET scanners and personnel. Increasing the number of amyloid PET scans per day requires a major investment in PET scanners, highly skilled technicians to operate the scanners, and clinicians to visually interpret the scans. For a screening approach in which the AD blood test was used as the sole measure of brain amyloidosis and individuals did not undergo an amyloid PET scan, the rate-limiting step was the numbers of screening phone calls, and increasing both the rate of screening phone calls and screening visits dramatically reduced the time of enrollment. Notably, hiring more research coordinators to call potential participants may be more achievable than building new PET facilities. Therefore, incorporating AD blood tests into screening may be a more practical approach to accelerating clinical trial enrollment than increasing amyloid PET capacity.
The modeling approach used in this study has significant limitations. The four screening approaches that are described do not encompass all potential approaches. Amyloid PET is used as the reference standard for brain amyloidosis, and although it has demonstrated biological relevance and clinical utility [42, 43], it is not a perfect test [42]. While the model is agnostic to the analyte(s) considered and therefore can be used to consider any blood test, it requires that a blood test result can be dichotomized as either positive or negative, and cut-offs are not yet established for many AD blood tests. The model assumes that the pool of potential clinical trial participants is not limited, but interested participants may be a limiting factor for some clinical trials, especially trials that aim to recruit a diverse cohort [44]. The model is also based on evaluating the financial costs and time of enrollment for a certain population at a single site, rather than a multi-center study that is typical of AD clinical trials. However, the model could be applied to each site individually, and then additional analyses could be performed to estimate the time and financial cost of the entire multi-center study. Estimating the time for enrollment of a multi-center study would require accounting for events occurring in parallel (e.g., enrollment at different sites), and the financial cost of screening would be a sum of the costs at each site plus the costs for coordinating the overall study. Notably, the code for the model is provided to enable interested investigators to modify the modeling approach to be more appropriate for their specific trial and/or screening strategy, and to serve as a starting point for more sophisticated models.
Because target populations and time and financial cost parameters vary markedly by clinical trial design and site, it was essential to develop a tool that enables investigators to examine parameters relevant to their own study. The estimates used for the illustrative models of time and financial cost of enrollment are based on AD clinical trials at Washington University, which is a large academic research center, and may vary considerably from other centers. Therefore, the conclusions drawn from the Washington University-derived estimates may not be generalizable to all sites, especially those with either a much higher or much lower availability of amyloid PET, which has a major influence on the time of enrollment for screening approaches that include amyloid PET. We created a web-based application that allows investigators to input participant characteristics, time parameters and financial cost parameters that will allow investigators to determine whether the conclusions drawn from Washington University estimates are relevant to their own study. Despite the inherent limitations, models that facilitate the design of more efficient clinical trials could save millions of dollars and accelerate the development of urgently needed treatments for AD.
Supplementary Material
Acknowledgements
We would like to express our gratitude to the research volunteers who participate in AD clinical trials and their supportive families. We would like to thank Dr. B. Joy Snider and Sonia Simons for assistance in gathering information on clinical trials at Washington University.
Funding
This study was supported by National Institute of Health (NIH) grants R01AG070941 (S.E. Schindler), RF1AG061900 (R.J. Bateman, PI), R56AG061900 (R.J. Bateman, PI), P30AG066444 (J.C. Morris, PI), P01AG003991 (J.C. Morris, PI), P01AG026276 (J.C. Morris, PI), U19AG032438 (J.C. Morris, PI), and U19AG024904 (J.C. Morris, PI).
Conflicts of interest
SES has analyzed data provided by C2N Diagnostics to Washington University, but she has not received any research funding or personal compensation from C2N Diagnostics or any other for-profit organizations. C2N Diagnostics had no role in this study, interpretation, or manuscript. YL, ML, AD, EP, LV, BHH, KW, BS, and MG report no disclosures. RJB and DMH co-founded C2N Diagnostics, which offers the PrecivityAD™ blood test. Washington University, RJB, and DMH have equity ownership interest in C2N Diagnostics and receive royalty income based on technology (stable isotope labeling kinetics and blood plasma assay) licensed by Washington University to C2N Diagnostics. RJB and DMH receive income from C2N Diagnostics for serving on the scientific advisory board. RJB has received honoraria as a speaker, consultant, or advisory board member from Amgen and Roche. DMH is on the scientific advisory board of Denali, Genentech, and Cajal Neuroscience and consults for Alector. Neither Dr. Morris nor his family owns stock or has equity interest (outside of mutual funds or other externally directed accounts) in any pharmaceutical or biotechnology company.
References
- [1].Winblad B, Amouyel P, Andrieu S, Ballard C, Brayne C, Brodaty H, et al. Defeating Alzheimer’s disease and other dementias: a priority for European science and society. The Lancet Neurology. 2016;15:455–532. [DOI] [PubMed] [Google Scholar]
- [2].Prados MJ, Liu Y, Jun H, Lam J, Mattke S. Projecting the long-term societal value of a disease-modifying treatment for Alzheimer’s disease in the United States. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2022;18:142–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].El-Hayek YH, Wiley RE, Khoury CP, Daya RP, Ballard C, Evans AR, et al. Tip of the Iceberg: Assessing the Global Socioeconomic Costs of Alzheimer’s Disease and Related Dementias and Strategic Implications for Stakeholders. Journal of Alzheimer’s disease : JAD. 2019;70:323–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Johnson KA, Minoshima S, Bohnen NI, Donohoe KJ, Foster NL, Herscovitch P, et al. Appropriate use criteria for amyloid PET: a report of the Amyloid Imaging Task Force, the Society of Nuclear Medicine and Molecular Imaging, and the Alzheimer’s Association. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2013;9:e-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Shaw LM, Arias J, Blennow K, Galasko D, Molinuevo JL, Salloway S, et al. Appropriate use criteria for lumbar puncture and cerebrospinal fluid testing in the diagnosis of Alzheimer’s disease. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2018;14:1505–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Karran E, Hardy J. Antiamyloid therapy for Alzheimer’s disease--are we on the right road? N Engl J Med. 2014;370:377–8. [DOI] [PubMed] [Google Scholar]
- [7].Salloway S, Farlow M, McDade E, Clifford DB, Wang G, Llibre-Guerra JJ, et al. A trial of gantenerumab or solanezumab in dominantly inherited Alzheimer’s disease. Nat Med. 2021;27:1187–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Sevigny J, Chiao P, Bussiere T, Weinreb PH, Williams L, Maier M, et al. The antibody aducanumab reduces Abeta plaques in Alzheimer’s disease. Nature. 2016;537:50–6. [DOI] [PubMed] [Google Scholar]
- [9].Sperling RA, Donohue M, Raman R, Sun CK, Yaari R, Siemers E, et al. The Anti-Amyloid Treatment in Asymptomatic Alzheimer’s Diseae (A4) Study: Report of Screening Data Results. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2018;14:P215–P6. [Google Scholar]
- [10].Clinical Trial of Solanezumab for Older Individuals Who May be at Risk for Memory Loss (A4).
- [11].Howell JC, Parker MW, Watts KD, Kollhoff A, Tsvetkova DZ, Hu WT. Research Lumbar Punctures among African Americans and Caucasians: Perception Predicts Experience. Front Aging Neurosci. 2016;8:296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Teunissen CE, Verberk IMW, Thijssen EH, Vermunt L, Hansson O, Zetterberg H, et al. Blood-based biomarkers for Alzheimer’s disease: towards clinical implementation. The Lancet Neurology. 2021. [DOI] [PubMed] [Google Scholar]
- [13].Ashton NJ, Leuzy A, Karikari TK, Mattsson-Carlgren N, Dodich A, Boccardi M, et al. The validation status of blood biomarkers of amyloid and phospho-tau assessed with the 5-phase development framework for AD biomarkers. Eur J Nucl Med Mol Imaging. 2021;48:2140–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Nakamura A, Kaneko N, Villemagne VL, Kato T, Doecke J, Dore V, et al. High performance plasma amyloid-beta biomarkers for Alzheimer’s disease. Nature. 2018;554:249–54. [DOI] [PubMed] [Google Scholar]
- [15].Ovod V, Ramsey KN, Mawuenyega KG, Bollinger JG, Hicks T, Schneider T, et al. Amyloid beta concentrations and stable isotope labeling kinetics of human plasma specific to central nervous system amyloidosis. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2017;13:841–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Palmqvist S, Janelidze S, Quiroz YT, Zetterberg H, Lopera F, Stomrud E, et al. Discriminative Accuracy of Plasma Phospho-tau217 for Alzheimer Disease vs Other Neurodegenerative Disorders. JAMA. 2020;324:772–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Schindler SE, Bollinger JG, Ovod V, Mawuenyega KG, Li Y, Gordon BA, et al. High-precision plasma beta-amyloid 42/40 predicts current and future brain amyloidosis. Neurology. 2019;93:e1647–e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Karikari TK. Blood Tests for Alzheimer’s Disease: Increasing Efforts to Expand and Diversify Research Participation Is Critical for Widespread Validation and Acceptance. Journal of Alzheimer’s disease : JAD. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Karikari TK, Ashton NJ, Brinkmalm G, Brum WS, Benedet AL, Montoliu-Gaya L, et al. Blood phospho-tau in Alzheimer disease: analysis, interpretation, and clinical utility. Nature reviews Neurology. 2022. [DOI] [PubMed] [Google Scholar]
- [20].Verberk IMW, Slot RE, Verfaillie SCJ, Heijst H, Prins ND, van Berckel BNM, et al. Plasma Amyloid as Prescreener for the Earliest Alzheimer Pathological Changes. Annals of neurology. 2018;84:648–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Li Y, Schindler SE, Bollinger JG, Ovod V, Mawuenyega KG, Weiner MW, et al. Validation of Plasma Amyloid-beta 42/40 for Detecting Alzheimer Disease Amyloid Plaques. Neurology. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Keshavan A, Pannee J, Karikari TK, Rodriguez JL, Ashton NJ, Nicholas JM, et al. Population-based blood screening for preclinical Alzheimer’s disease in a British birth cohort at age 70. Brain. 2021;144:434–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Mattke S, Cho SK, Bittner T, Hlavka J, Hanson M. Blood-based biomarkers for Alzheimer’s pathology and the diagnostic process for a disease-modifying treatment: Projecting the impact on the cost and wait times. Alzheimer’s & dementia. 2020;12:e12081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Karikari TK, Benedet AL, Ashton NJ, Lantero Rodriguez J, Snellman A, Suarez-Calvet M, et al. Diagnostic performance and prediction of clinical progression of plasma phospho-tau181 in the Alzheimer’s Disease Neuroimaging Initiative. Mol Psychiatry. 2021;26:429–42. [DOI] [PubMed] [Google Scholar]
- [25].Sperling RA, Johnson K, Zhou J, Irizarry MC, Dhadda S, Kramer LD, et al. Introduction of plasma biomarker screening for the AHEAD 3-45 study. The Journal of Prevention of Alzheimer’s Disease. 2021;8:S56. [Google Scholar]
- [26].Tariot PN, Reiman EM, Alexander RC, Langbaum JB, Holdridge K, Ferguson MB, et al. TRAILBLAZER-ALZ 3 trial design and rationale. The Journal of Prevention of Alzheimer’s Disease. 2021;8:S3–4. [Google Scholar]
- [27].Jansen WJ, Ossenkoppele R, Knol DL, Tijms BM, Scheltens P, Verhey FR, et al. Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. JAMA. 2015;313:1924–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Roberts RO, Aakre JA, Kremers WK, Vassilaki M, Knopman DS, Mielke MM, et al. Prevalence and Outcomes of Amyloid Positivity Among Persons Without Dementia in a Longitudinal, Population-Based Setting. JAMA Neurol. 2018;75:970–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Schindler SE, Li Y, Li M, Despotis A, Park E, Vittert L, et al. Time and financial cost of enrollment for Alzheimer disease clinical trials. 2022.
- [30].Ossenkoppele R, Jansen WJ, Rabinovici GD, Knol DL, van der Flier WM, van Berckel BN, et al. Prevalence of amyloid PET positivity in dementia syndromes: a meta-analysis. JAMA. 2015;313:1939–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Green RC, Roberts JS, Cupples LA, Relkin NR, Whitehouse PJ, Brown T, et al. Disclosure of APOE genotype for risk of Alzheimer’s disease. N Engl J Med. 2009;361:245–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Mozersky J, Sankar P, Harkins K, Hachey S, Karlawish J. Comprehension of an Elevated Amyloid Positron Emission Tomography Biomarker Result by Cognitively Normal Older Adults. JAMA Neurol. 2018;75:44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Gooblar J, Roe CM, Selsor NJ, Gabel MJ, Morris JC. Attitudes of Research Participants and the General Public Regarding Disclosure of Alzheimer Disease Research Results. JAMA Neurol. 2015;72:1484–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Schindler SE, Karikari TK, Ashton NJ, Henson RL, Yarasheski KE, West T, et al. Effect of Race on Prediction of Brain Amyloidosis by Plasma Abeta42/Abeta40, Phosphorylated Tau, and Neurofilament Light. Neurology. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Syrjanen JA, Campbell MR, Algeciras-Schimnich A, Vemuri P, Graff-Radford J, Machulda MM, et al. Associations of amyloid and neurodegeneration plasma biomarkers with comorbidities. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Mielke MM, Dage JL, Frank RD, Algeciras-Schimnich A, Knopman DS, Lowe VJ, et al. Performance of plasma phosphorylated tau 181 and 217 in the community. Nat Med. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Deters KD, Napolioni V, Sperling RA, Greicius MD, Mayeux R, Hohman T, et al. Amyloid PET Imaging in Self-Identified Non-Hispanic Black Participants of the Anti-Amyloid in Asymptomatic Alzheimer’s Disease (A4) Study. Neurology. 2021;96:e1491–e500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Thijssen EH, La Joie R, Wolf A, Strom A, Wang P, Iaccarino L, et al. Diagnostic value of plasma phosphorylated tau181 in Alzheimer’s disease and frontotemporal lobar degeneration. Nat Med. 2020;26:387–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Barthelemy NR, Horie K, Sato C, Bateman RJ. Blood plasma phosphorylated-tau isoforms track CNS change in Alzheimer’s disease. J Exp Med. 2020;217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Thijssen EH, Rabinovici GD. Rapid Progress Toward Reliable Blood Tests for Alzheimer Disease. JAMA Neurol. 2021;78:143–5. [DOI] [PubMed] [Google Scholar]
- [41].Cummings J, Reiber C, Kumar P. The price of progress: Funding and financing Alzheimer’s disease drug development. Alzheimers Dement (N Y). 2018;4:330–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Clark CM, Pontecorvo MJ, Beach TG, Bedell BJ, Coleman RE, Doraiswamy PM, et al. Cerebral PET with florbetapir compared with neuropathology at autopsy for detection of neuritic amyloid-beta plaques: a prospective cohort study. The Lancet Neurology. 2012;11:669–78. [DOI] [PubMed] [Google Scholar]
- [43].Rabinovici GD, Gatsonis C, Apgar C, Chaudhary K, Gareen I, Hanna L, et al. Association of Amyloid Positron Emission Tomography With Subsequent Change in Clinical Management Among Medicare Beneficiaries With Mild Cognitive Impairment or Dementia. JAMA. 2019;321:1286–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Salazar CR, Hoang D, Gillen DL, Grill JD. Racial and ethnic differences in older adults’ willingness to be contacted about Alzheimer’s disease research participation. Alzheimers Dement (N Y). 2020;6:e12023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


