Abstract
The chemotherapeutic agent cisplatin accumulates in the kidney and induces acute kidney injury (AKI). Preclinical and clinical studies suggest that young female mice and women show greater recovery from cisplatin-AKI compared to young male mice and men. The endothelin (ET) and ET receptors are enriched in the kidney and may be dysfunctional in cisplatin-AKI; however, there is a gap in our knowledge about the putative effects of sex and cisplatin on the renal ET system. We hypothesized that cisplatin-AKI male and female mice will have increased expression of the renal ET system. As expected, all cisplatin-AKI mice had kidney damage and body weight loss greater than control mice. Cisplatin-AKI mice had greater cortical Edn1, Edn3, Ednra, and Ednrb, while outer medullary Ednra was significantly suppressed in both sexes. Of the ~25 000 genes sequenced from the inner medulla, only 91 genes (comparing saline mice) and 134 genes (comparing cisplatin-AKI mice) were differentially expressed and they were unrelated to the ET system. However, Edn1 was significantly greater in the inner medulla of male and female cisplatin-AKI mice. Thus, RNA profiles of the ET system were significantly affected by cisplatin-AKI throughout the kidney regardless of sex and this may help determine the therapeutic potential of targeting the ET receptors in cisplatin-AKI.
Keywords: endothelin, kidney, RNA, cisplatin, acute kidney injury, sex
Résumé
Le cisplatine, un agent chimiothérapeutique, s’accumule dans le rein, où il entraîne des lésions rénales aiguës (LRA). Les études précliniques et cliniques laissent entendre que les jeunes souris femelles et les jeunes femmes parviendraient mieux à se rétablir de la situation cisplatine-LRA que les jeunes mâles et les jeunes hommes. L’endothéline (ET) et les récepteurs de l’ET sont plus présents dans les reins et pourraient être dysfonctionnels en situation cisplatine-LRA; cependant nos connaissances quant aux effets éventuels du sexe et du cisplatine dans le système ET rénal sont lacunaires. Nous avons formulé une hypothèse selon laquelle l’expression du système ET rénal est accrue chez les souris mâles et femelles cisplatine-LRA. Comme prévu, les souris cisplatine-LRA présentaient toutes des lésions rénales et une perte de poids plus importantes que les souris témoin. Les souris cisplatine-LRA exprimaient Edn1, Edn3, Ednra, et Ednrb dans le cortex dans une plus grande mesure, tandis qu’Ednra était nettement inhibé chez les deux sexes. Parmi environ 25 000 gènes séquencés dans la médulla interne, uniquement 91 gènes (comparaison avec les souris solution saline) et 134 gènes (comparaison avec les souris cisplatine-LRA) s’exprimaient de manière différentielle, et ils n’étaient pas liés au système ET. Cependant, Edn1 s’exprimait nettement plus dans la médulla interne des souris cisplatine-LRA mâles et femelles. Par conséquent, les profils du système ET en ARN étaient affectés de façon marquée avec le couple cisplatine-LRA à l’échelle du rein entier, sans égard au sexe. Cette observation pourrait contribuer à établir le potentiel thérapeutique du ciblage des récepteurs de l’ET en situation cisplatine-LRA.
Mots-clés : endothéline, reins, ARN, cisplatine, lésions rénales aiguës, sexe
Introduction
The endothelin (ET) system is composed of three different peptides ET-1 (gene Edn1), ET-2 (Edn2), and ET-3 (Edn3) that undergo proteolytic cleavage by the ET converting enzymes (Ece1 and Ece2) to form the 21 amino acid active peptides (Davenport et al. 2016). The ETs bind to two different G-protein-coupled receptors, ETA (Ednra) and ETB (Ednrb) (Davenport et al. 2016). Although the endothelium produces ET-1 (Yanagisawa et al. 1988), the inner medulla (IM) of the kidney is a rich source of ET (Kitamura et al. 1989; Morita et al. 1991). Both ET-1 and ET-3 are produced in rodents by the IM (Kitamura et al. 1989), with greatest expression by the IM collecting duct (IMCD) (Kohan 1991). ET-1 is the predominant isoform produced by the IM in humans (Morita et al. 1991). The renal ET system plays a critical physiological role in fluid–electrolyte balance by promoting sodium excretion (Kohan 2011) and dysfunctional renal ET signaling can lead to vasoconstriction, fibrosis, and (or) inflammation (Dhaun et al. 2012). The ET system is therefore hypothesized to promote the pathogenesis of different renal diseases including acute kidney injury (AKI) (Lopez-Farre et al. 1991; Kohan 1994), chronic kidney disease (CKD) (Dhaun et al. 2012, 2013), diabetic kidney disease (Heerspink et al. 2019, 2021a, 2021b; Waijer et al. 2021), and sickle cell nephropathy (Heimlich et al. 2016; Kasztan et al. 2017).
AKI is an abrupt decrease in kidney function that is associated with many complications and can lead to CKD, end-stage kidney disease, other organ dysfunction, or death (Kellum et al. 2021). There are many factors that can lead to the onset of AKI including the use of nephrotoxic drugs such as cisplatin (cis-diamminedichloroplatinum(II)), a chemotherapeutic drug used to treat a large number of cancers (McSweeney et al. 2021). Cisplatin accumulates in the proximal tubules of the kidneys leading to acute tubular necrosis, alters hemodynamics, and can evoke an inflammatory response (McSweeney et al. 2021). Despite adequate hydration and dosing adjustments used in the clinic, nephrotoxicity is still a major concern (Perazella et al. 2022) especially in patients with preexisting conditions (Duan et al. 2020). Biological sex may be an important risk factor for cisplatin-AKI. Older women (Chen et al. 2017) and older female mice in preclinical studies (Boddu et al. 2017) have a higher risk of developing cisplatin-AKI than men or male mice. Thus, there is a need to understand sex-specific signaling and mechanisms, especially in the kidney, to help prevent cisplatin-AKI.
There is existing evidence suggesting that sex differences exist in the renal ET system (Gohar et al. 2016), but data are limited on whether these differences are maintained with cisplatin-AKI. Previous studies have determined that male mice had an increase in whole kidney Edn1 without a change in Ednra or Ednrb expression 3 days after a cisplatin-AKI (Lee and Ahn 2008). In male rats with cisplatin-AKI, co-treatment with the ETA antagonist, BQ-123, attenuated kidney damage (Helmy et al. 2014; Abdel Moneim et al. 2019); however, the dual ET receptor antagonist, bosentan, did not prevent cisplatin-AKI in either male or female rats (Jokar et al. 2015). It is still unclear whether there are sex-specific differences in the kidney ET system in response to cisplatin-AKI. The aim of this study was to determine the expression of the ET system in both male and female mice at baseline and following cisplatin-AKI. We hypothesized that male and female mice have an increase in the expression of the kidney ET system following cisplatin-AKI.
Materials and methods
Animals and tissue collection
All animal use and welfare adhered to the NIH Guide for the Care and Use of Laboratory Animals following a protocol reviewed and approved by the Institutional Laboratory Animal Care and Use Committee of the University of Alabama at Birmingham (UAB). Male and female C57bl/6 J mice at 8 weeks of age were purchased from Jackson Labs (Bar Harbor, ME). Mice were acclimated to the UAB animal facility in a room with a 12 h light:12 h dark cycle and fed an ad libitum standard chow diet (0.49% NaCl Teklad #96208) and autoclaved tap water. At 9 weeks of age, each cage of mice was randomly assigned to either saline (vehicle control) or cisplatin. Between 7 and 8 am, when the animal room lights switched on, each mouse received a single, intraperitoneal injection of 100 μL of saline or 15 mg/kg cisplatin (Sigma, St. Louis, MO) dissolved in saline. This dose of cisplatin was used because both male and female mice survive at least a week, while a 20 mg/kg dose led to significant mortality between 48 and 72 h in our lab. At 72 h after the 15 mg/kg injection, the mice were anesthetized with 2.5% vaporized isoflurane, and terminal tissues and blood were collected by a person blinded to the groups.
Blood was collected into a heparinized syringe via cardiac puncture, and plasma was isolated following centrifugation at 4 °C, 1000 g for 10 min. Plasma was used to measure creatinine at the UAB-UCSD O’Brien Bioanalytical core using liquid chromatography coupled to tandem mass spectrometry. Plasma urea concentration was measured using colorimetric assay (BioAssay Systems, QuantiChrom DIUR-100).
Kidneys were excised and decapsulated, and the right kidney was dissected into cortex, outer medulla (OM), and IM. These samples were immediately snap frozen with liquid nitrogen and stored at −80 °C. The left kidney was cut into cross-sections and placed in 10% neutral buffered formalin for 24 h at room temperature (25°C) and stored in 70% ethanol in water until embedded in paraffin for histological analyses.
Histology
The kidney cross-sections were processed and embedded in paraffin with the UAB Animal Resource Program histopathology core. These kidney blocks were then cut at 4 μm sections and placed on superfrost plus slides (Fisher Scientific). Slides were then stained with Gomori’s trichrome (Epredia, Richard Allen, Kalamazoo, MI), dehydrated, cleared with Xylenes, and mounted with Cytoseal 60 and a glass coverslip. A researcher blinded to the groups took images of each sample and assessed tubular injury by noting the presence of protein casts or dilated tubules. Representative images reported in this study were taken with an Olympus Bx53 and DP28 digital camera.
RNA isolation, cDNA, and real-time quantitative PCR
RNA was isolated from cortex and OM samples, using the Zymo Quick-RNA miniprep kit. Quantity was determined by the nanodrop and 1 μg of total RNA reversed transcribed with the VILO Superscript IV (Thermo Fisher Scientific). All cDNA was diluted 1/10 with sterile, RNAse-, DNase-free water. Relative transcript expression was determined with SYBR green-based real-time quantitative PCR (qPCR). Each sample was run in duplicate with SSoAdvanced Universal SYBR green master mix (Bio-Rad, Carlsbad, CA), 7.5 pmol of each primer (Table 1), and 1 μL of 1/10 cDNA. Cycle thresholds were determined using the CXF96 system (Bio-Rad).
Table 1.
Real-time quantitative PCR primers used in the study.
| Transcript | Forward | Reverse | Amplicon |
|---|---|---|---|
| Edn1 | GCACCGGAGCTGAGAATGG | GTGGCAGAAGTAGACACACTC | 119 |
| Edn2 | CACCTGCGTTTTCGTCGATG | CCAGTGTCTTCGATGGCAGAA | 219 |
| Edn3 | CCCTGGTGAGAGGATTGTGTC | CCTTGTCCTTGTAAGTGAAGCAC | 161 |
| Ece1 | TCTCCGAGGGCGATGTGTA | CTTCTCCACCGAGGTCCGA | 98 |
| Ece2 | GGTGTTGGGGAAGTGTACTGA | AAGCCAGCAGTCCTTCTCTTT | 114 |
| Ednra | ATGAGTATCTTTTGCCTTGCGG | GTCTTCCATGTGGCTGCTTAG | 108 |
| Ednrb | GTGGCTTCTTGGGGGTATGG | TCTTAGTGGGTGGCGTCATTA | 102 |
| Gapdh | AGGTCGGTGTGAACGGATTTG | TGTAGACCATGTAGTTGAGGTCA | 123 |
RNA sequencing of the inner medulla
Because the ET system is most highly expressed in the IMCD (Kitamura et al. 1989; Kohan 1991; Morita et al. 1991), we performed unbiased RNA sequencing of the IM. Total RNA was extracted as mentioned above and sent to GeneWiz (South Plainfield, NJ) where RNA quality was confirmed with Qubit assay and quantitated by qPCR. All samples had an RNA integrity number value >8 except for male cisplatin sample 3 that was 7.9. cDNA libraries were generated and included poly A selection (Illumina, San Diego, CA), and they were sequenced on the Illumina HiSeq3000/4000 in a 2 × 50 base pair configuration targeting 30 million reads per sample. Raw fastq files were analyzed at UAB and have been deposited into Gene Expression Omnibus (GSE195786). The fastq file quality was checked with FASTQC in the package Trim_Galore! and adapter sequences trimmed. Again, file quality was checked with FASTQC, and the files were then mapped to the mouse genome (mm10) with the program STAR. The BAM files generated were then used to determine raw counts with featureCounts in the package subread. Finally, these count matrixes were used in the R package, DESeq2, to determine differentially expressed genes (DEGs). To determine pathways that may be enriched in either sex, the DEG (adjusted p value <0.05) gene symbols were queried in the DAVID Bioinformatics Resource (v.6.8) and significant (Benjamini p value <0.05) Gene Ontology pathways were determined (Jiao et al. 2012).
Sample sizes, data reporting, and statistics
For sample size determination, we used an a priori alpha = 0.05, with a power of 80%, to detect a 20% difference in mean value and assuming a variation of 10% for a two-mean comparison of a continuous variable. A sample size of four per group was calculated. We collected samples from three cohorts of mice over a 1-year period that resulted in a final sample size reported in Table 2. For qPCR analyses, we extracted RNA from the cortex of n = 5–7 mice and RNA from the OM of n = 5/group. For qPCR data, all genes of interest were standardized to the housekeeping gene, Gapdh, and normalized to the male saline group using the 2ΔΔCT method. Statistical significance was determined by a two-factor analysis of variance (ANOVA) (sex and cisplatin) and Tukey’s post hoc analysis. p < 0.05 was considered statistically significant and group effects and post hoc test p values are reported in the figures. All data are reported as mean ± standard error of the mean. For the RNA sequencing, we extracted RNA from all mice in the study and pairwise comparisons were made between (i) male saline and female saline to test for sex differences; (ii) male saline and cisplatin; or (iii) female saline and cisplatin to test for the effects of cisplatin; or (iv) female cisplatin and male cisplatin to test for the effects of sex on cisplatin-related DEGs. Normalized counts from these pairwise comparisons are reported and defined as the raw counts divided by the median ratio of gene counts relative to the geometric mean per gene. Statistical significance was determined by the Wald test with Benjamini and Hochberg correction and adjusted p value <0.05 were considered significant. Volcano plots of the DEG between males and females represent the log2 fold change versus the −log adjusted p value.
Table 2.
Characteristics of the mice used in the study.
| Female | Male | Two-factor ANOVA | ||||||
|---|---|---|---|---|---|---|---|---|
| Saline | Cisplatin | Saline | Cisplatin | P sex | P cisplatin | P Interaction | ||
| Sample size | 7 | 5 | 6 | 6 | ||||
| Age | 9 | 9 | 9 | 9 | ||||
| Starting mass | g | 20.38 (0.32) | 20.77 (0.55) | 26.49 (0.69) * | 27.70 (0.41) * | <0.001 | 0.13 | 0.43 |
| Change in mass | % | −0.55 (1.49) | −13.2 (3.80)* | 0.56 (0.92) | −14.33 (2.78)* | 0.99 | <0.001 | 0.62 |
| Total kidney mass | mg | 0.24 (0.004) | 0.21 (0.004) | 0.30 (0.01) | 0.29 (0.006) | <0.001 | 0.072 | 0.38 |
| Kidney/starting body mass | mg/g | 11.56 (0.13) | 10.20 (0.21) * | 11.23 (0.36) | 10.43 (0.26) | 0.85 | 0.0006 | 0.30 |
| Kidney/dissection body mass | mg/g | 11.64 (0.12) | 12.03 (0.57) | 11.13 (0.33) | 12.12 (0.55) | 0.68 | 0.069 | 0.37 |
| Heart mass | mg/dL | 0.11 (0.008) | 0.092 (0.001) | 0.13 (0.007) | 0.12 (0.006) | 0.0045 | 0.073 | 0.76 |
| Heart/starting body mass | mg/g | 5.10 (0.32) | 4.48 (0.14) | 4.79 (0.19) | 4.20 (0.19) | 0.20 | 0.016 | 0.95 |
| Heart/dissection body mass | mg/g | 5.20 (0.34) | 5.26 (0.14) | 4.78 (0.16) | 4.96 (0.17) | 0.12 | 0.60 | 0.77 |
| Plasma creatinine | mg/dL | 0.14 (0.01) | 0.16 (0.03) | 0.13 (0.007) | 0.25 (0.06) * | 0.19 | 0.019 | 0.11 |
| Plasma urea | mg/dL | 53.2 (6.6) | 76.7 (15.6) | 66.9 (4.2) | 110.7 (20.5) | 0.072 | 0.014 | 0.43 |
Note: Mean (standard error of mean) values are reported. Bold represents statistical significance.
indicates significant difference from post hoc Tukey’s analysis.
Results
Cisplatin-induced kidney injury
Three days after cisplatin or vehicle control injection, cisplatin-treated male and female mice had a significantly higher plasma creatinine (Psex = 0.19, Pcisplatin = 0.019, and PInteraction = 0.11) and plasma urea (Psex = 0.072, and Pcisplatin = 0.014, and PInteraction = 0.43) compared to vehicle-injected mice (Table 2). Moreover, cisplatin-injected mice had a significant 13%–14% loss in body mass, while there was no significant change in body mass of control mice (Table 2). Kidney and heart masses were smaller in the female mice compared to male mice, regardless of cisplatin treatment; however, when corrected for starting body mass, cisplatin-treated mice had significantly lower kidney/body mass (Psex = 0.85, Pcisplatin = 0.006, and PInteraction = 0.3) and lower heart/body mass (Psex = 0.20, Pcisplatin = 0.016, and PInteraction = 0.95) (Table 2).
The kidney structure was normal in the male and female control mice with no protein casts or dilated tubules observed (Fig. 1). In contrast, cisplatin-treated mice had multiple protein casts in the cortex, OM and IM, and notable dilated tubules (Fig. 1). Thus, our dosing of 15 mg/kg of cisplatin produced kidney damage in all of the cisplatin-treated mice, regardless of sex.
Fig. 1.
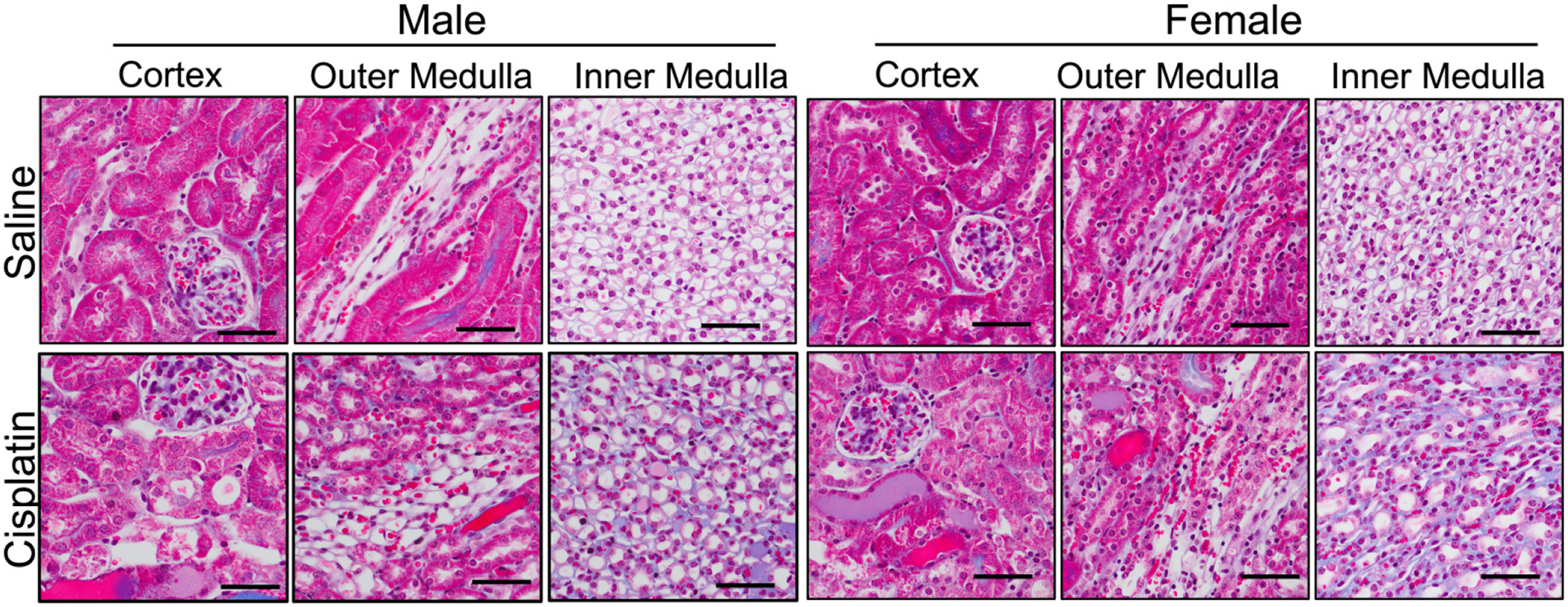
Representative trichrome stained kidney sections from mice that received a single intraperitoneal injection of either saline (vehicle control) or 15 mg/kg of cisplatin 3 days prior. Scale bar is 50 μm.
Cortical kidney endothelin system expression in males and females
Next, the mRNA expression of the ET system was determined in the cortex, OM, and IM. In the cortex, cisplatin led to a threefold increase in Edn1 regardless of sex (Psex = 0.87, Pcisplatin = 0.0047, and PInteraction = 0.93; Fig. 2A). The kidneys also expressed Edn3, and this was 50% greater in the cisplatin-treated mice, regardless of sex (Psex = 0.89, Pcisplatin 0.0072, and PInteraction = 0.44; Fig. 2B). We were unable to detect Edn2 in the cortex. There were no statistically significant differences in cortical Edn1 or Edn3 expression between the sexes (Figs. 2A and 2B). The ET converting enzyme-1 (Ece1) was expressed in the cortex; however, its expression was not significantly affected by sex or cisplatin treatment (Psex = 0.39, Pcisplatin = 0.61, and PInteraction = 0.66; Fig. 2C). Ece2 was not detected in the cortex. Both Ednra and Ednrb were expressed in the cortex and were expressed ~50% more in cisplatin-treated mice (Figs. 2D and 2E). Thus, the cisplatin-treated mice had generally increased transcript abundance of the cortical ET system.
Fig. 2.
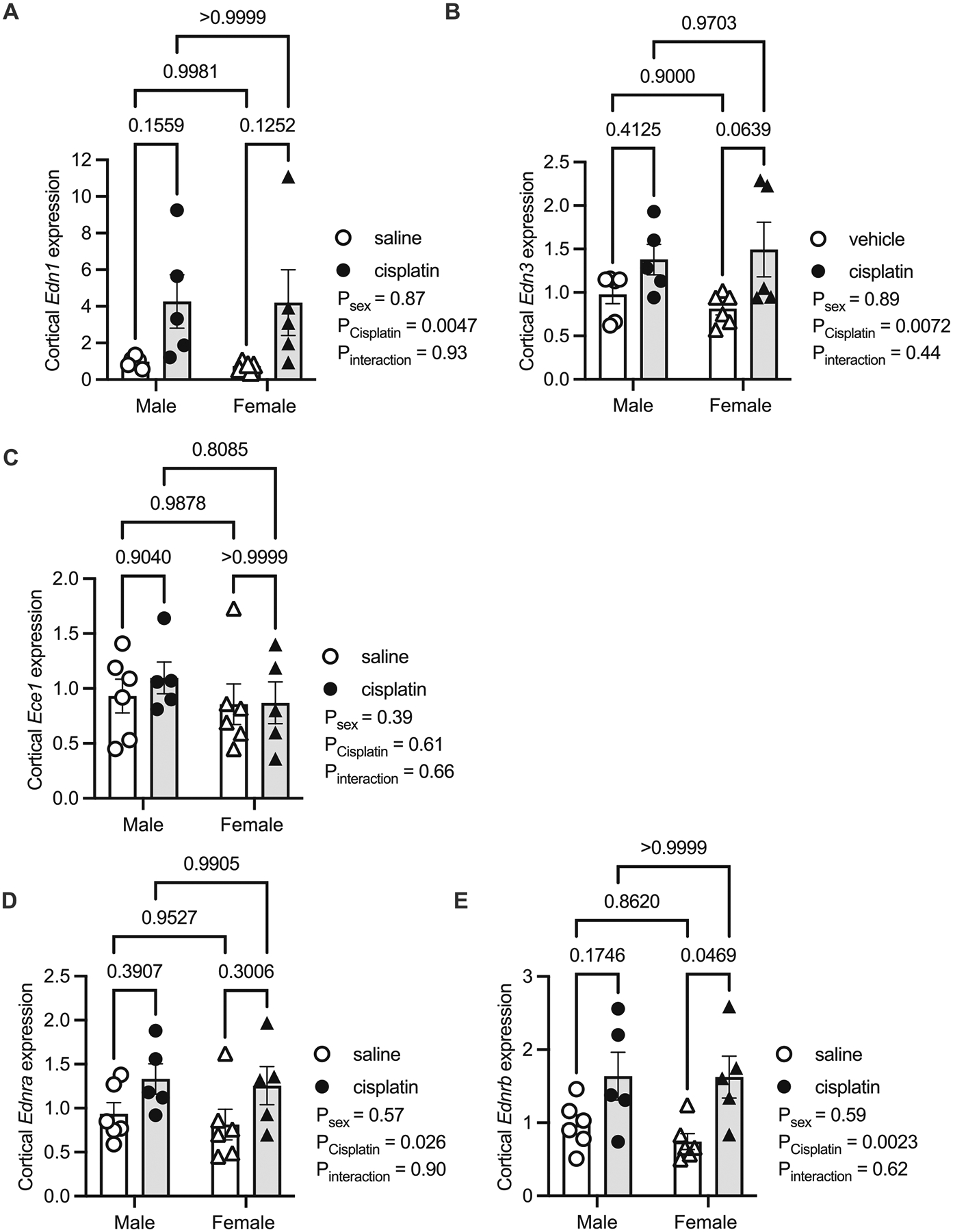
Kidney cortex samples from male and female mice 3 days following a single intraperitoneal injection of saline (vehicle) or 15 mg/kg cisplatin. Real-time quantitative PCR of various endothelin (Edn) related genes from these cortex samples. (A) Edn1, (B) Edn3, (C) Ece1, (D) Ednra, and (E) Ednrb. All values are relative to the male saline group. P values from a two-factor ANOVA reported and results of Tukey’s post hoc are represented by lines with the p value reported. Sample sizes: male saline = 6, male cisplatin = 6, female saline n = 6, and female cisplatin = 5.
Outer medullary kidney endothelin system expression in males and females
Unlike the cortex, the OM ET system had few significant sex or cisplatin-induced differences. Edn1 (Psex = 0.76, Pcisplatin = 0.17, and PInteraction = 0.23; Fig. 3A) and Edn3 (Psex = 0.16, Pcisplatin = 0.60, and PInteraction = 0.47; Fig. 3B) were not significantly different between the sexes or with cisplatin treatment. Edn2 was not detected in the OM. Likewise, Ece1 was not significantly different among the groups (Psex = 0.75, Pcisplatin = 0.21, and PInteraction = 0.25) and Ece2 was not detected (Fig. 3C). In the OM, Ednra was ~25% less expressed in the cisplatin-treated mice than controls in both sexes (Psex = 0.88, Pcisplatin = 0.022, and PInteraction 0.88; Fig. 3D).The Ednrb was expressed ~50% greater in female mice compared to males, regardless of cisplatin (Psex = 0.045, Pcisplatin = 0.53, and PInteraction = 0.75; Fig. 3E).
Fig. 3.
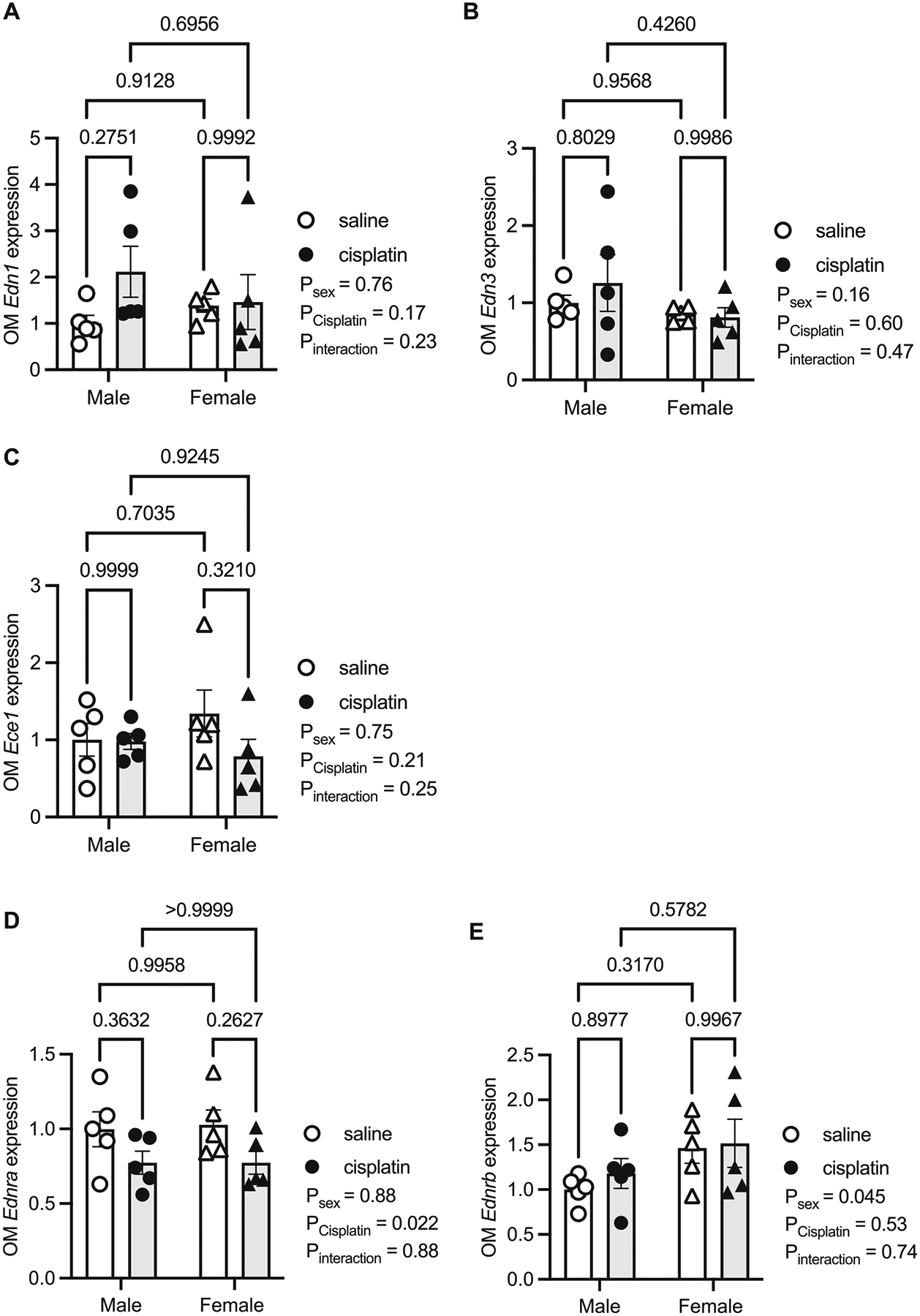
Kidney outer medulla (OM) samples from male and female mice 3 days following a single intraperitoneal injection of saline (vehicle) or 15 mg/kg cisplatin. Real-time quantitative PCR of various endothelin (Edn) related genes from these cortex samples. (A) Edn1, (B) Edn3, (C) Ece1, (D) Ednra, and (E) Ednrb. All values are relative to the male saline group. P values from a two-factor ANOVA reported and results of Tukey’s post hoc are represented by lines with the p value reported. N = 5 per group/sex.
Inner medullary RNA sequencing from male and female mice
Our lab has a standing interest in the molecular mechanisms in the IM, and given that the IMCD produces the most ET-1 in the kidney (Kohan 1991), we performed unbiased RNA-sequencing on the IM. There were more than 25 800 genes sequenced from the IM, but when comparing male and female control mice in the IM, there were only 91 DEGs (adjusted p value <0.05) (Fig. 4A, Table S1, https://doi.org/10.6084/m9.figshare.19222659.v1). Enriched in the male IM were the Slc22a genes (Fig. 4A), which are part of the drug transporter family including the organic anion transporters (OATs) and organic cation transporters (OCTs) (Nigam 2018). Also, not surprisingly, there were Y chromosome-linked genes expressed in the male mice (Uty, Ddx3y, Kdm5d, and Eif2s3y). In the female control mice, there was significantly greater expression of the gene Xist (involved in X-inactivation), the amino acid transporter, Scl7a12, and renin (Ren1) (Fig. 4A). Gene Ontology analyses with these 91 genes highlighted that there are significant sex differences in biological pathways such as sodium-independent organic anion transport (fold enrichment = 61.9, number of genes = 7, and Benjamini p value = 4.6E–7), and oxidation–reduction process (fold enrichment = 4.8, number of genes = 13, and Benjamini p value = 0.0027).
Fig. 4.
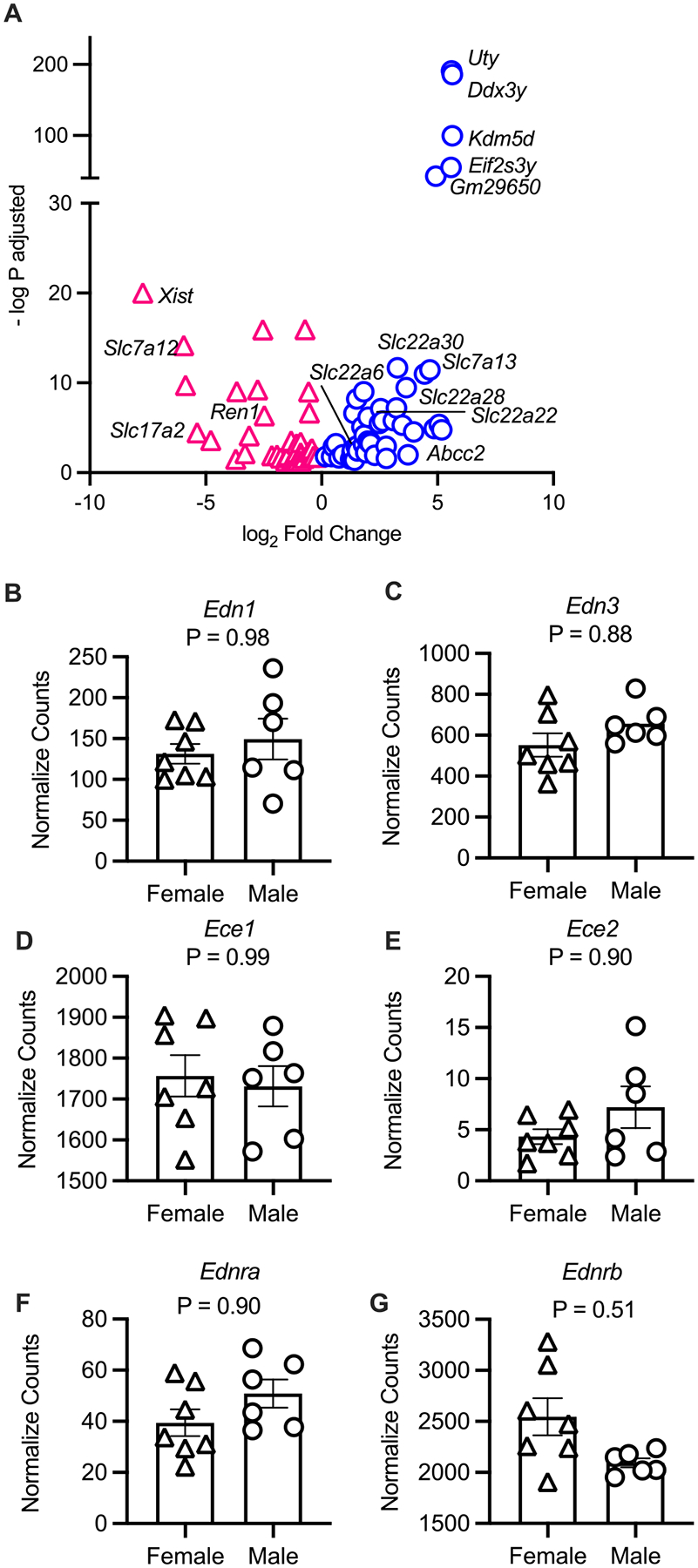
Differentially expressed genes in the inner medulla of male and female saline-injected mice. (A) A volcano plot of the significant (adjusted p value <0.05) differentially expressed genes in males (blue) compared to females (pink). (B–G) Comparisons of the inner medullary endothelin (Edn) related transcripts from female and male saline-injected mice from this study. Normalized counts from the analyses package DESeq2 are reported and p values are adjusted p values from the Wald test with Benjamini and Hochberg correction reported. (B) Edn1, (C) Edn3, (D) Ece1, (E) Ece2, (F) Ednra, and (G) Ednrb. Sample size: females = 7 and males = 6.
When we compared normalized counts of ET-related transcripts, there were no statistically significant differences in expression of Edn1, Edn2, Ece1, Ednra, or Ednrb between control male and female mice in the IM (adjusted p value <0.05, Figs. 4B–G). Similar to the cortex and OM, Edn2 was not detected/sequenced, and Ece2 had low normalized counts (Fig. 4E).
In females, cisplatin-treated mice had twice the IM Edn1 expression than control female mice (p = 0.0009; Fig. 5A). However, there were no statistically significant differences in female IM Edn2, Ece1, Ednra, or Ednrb between cisplatin- and saline-treated mice (Figs. 5B–F). There was a small but statistically significant greater expression of Ece2 in cisplatin-treated female mice (Fig. 5D and Table S1). In males, we likewise detected significantly more Edn1 transcripts in cisplatin-treated mice compared to control (p = 2.03E–8; Fig. 6A) but no statistical differences in the normalized counts of Edn3, Ece1, or Ece2 (Figs. 6B–D). Both Ednra and Ednrb transcripts were significantly fewer in the cisplatin-treated male mice (Figs. 6E and 6F; Table S1).
Fig. 5.
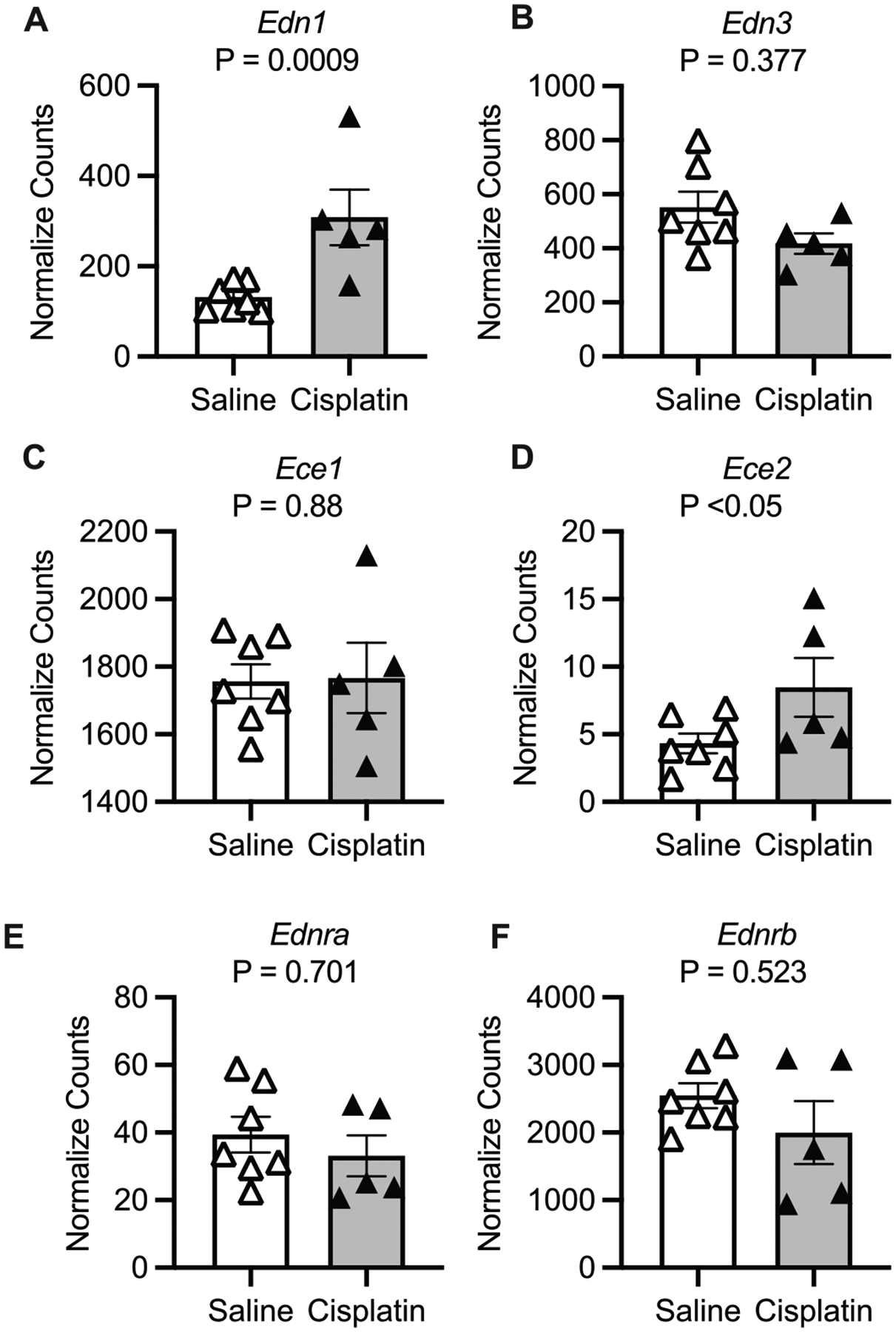
Kidney inner medulla samples from female mice 3 days following a single intraperitoneal injection of saline (vehicle) or 15 mg/kg cisplatin. Normalized counts from the analyses package DESeq2 are reported and p values are adjusted p values from the Wald test with Benjamini and Hochberg correction reported. (A) Edn1, (B) Edn3, (C) Ece1, (D) Ece2, (E) Ednra, and (F) Ednrb. Sample size: saline = 7 and cisplatin = 5.
Fig. 6.
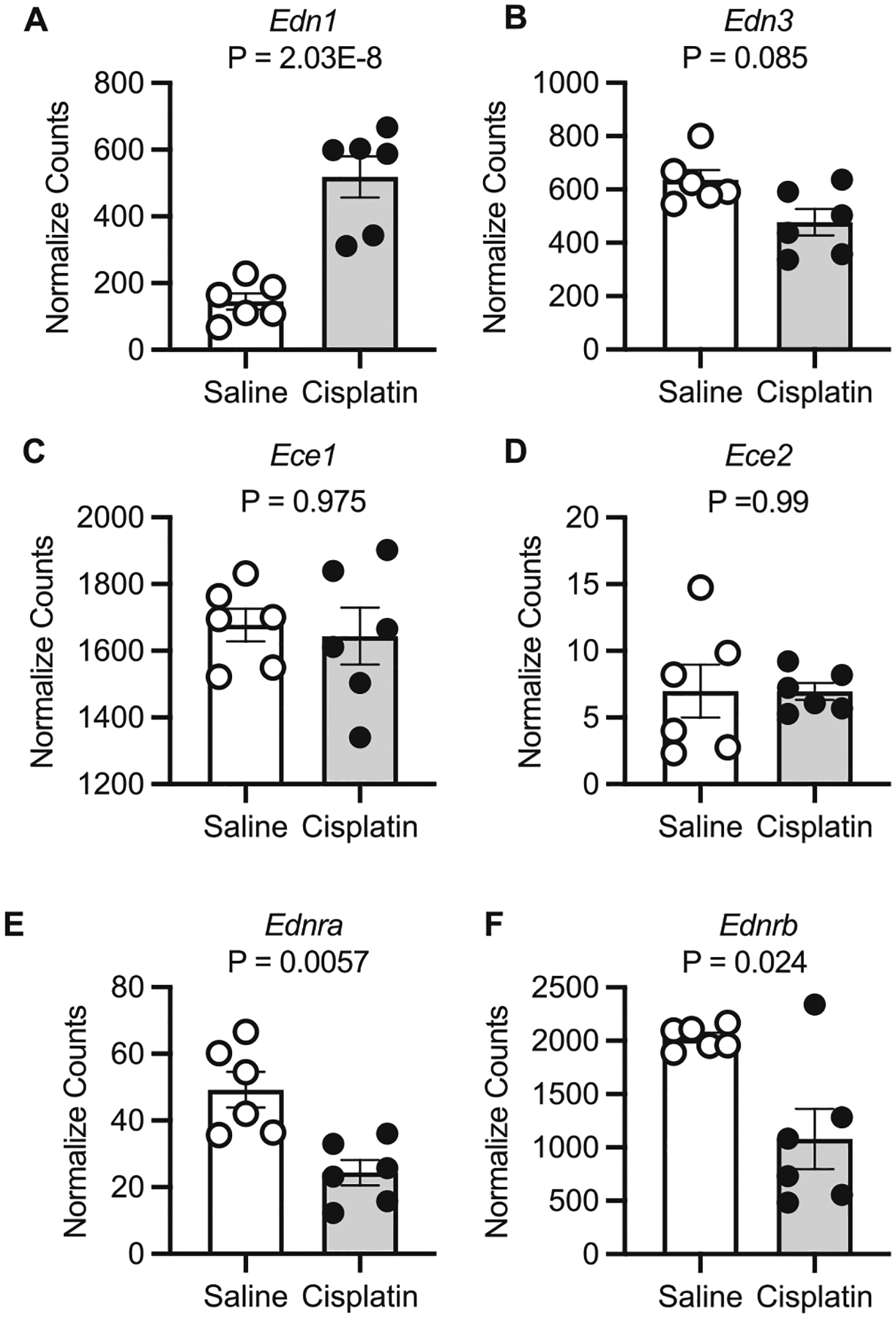
Kidney inner medulla samples from male mice 3 days following a single intraperitoneal injection of saline (vehicle) or 15 mg/kg cisplatin. Normalized counts from the analyses package DESeq2 are reported and p values are adjusted p values from the Wald test with Benjamini and Hochberg correction reported. (A) Edn1, (B) Edn3, (C) Ece1, (D) Ece2, (E) Ednra, and (F) Ednrb. Sample size = 6/group.
Finally, we compared cisplatin-treated male and cisplatin-treated female mice. Although there were 134 DEGs between these mice (Fig. 7A; Table S1), 53 genes lacked official gene symbols (they were gm or rik genes). Thus, although there were sex-specific genes differentially regulated in cisplatin-treated mice, many were novel genes whose function still requires determination. Of the 134 DEGs, 25 are sexually dimorphic as they were also found in the male versus female saline groups (Fig. 4A, e.g., Xist and Uty). Moreover, Gene Ontology analyses failed to detect any statistically significant pathways enriched by these 134 genes. Finally, there were no statistically significant differences between the ET systems of cisplatin-treated female or male mice (Figs. 7B–G).
Fig. 7.
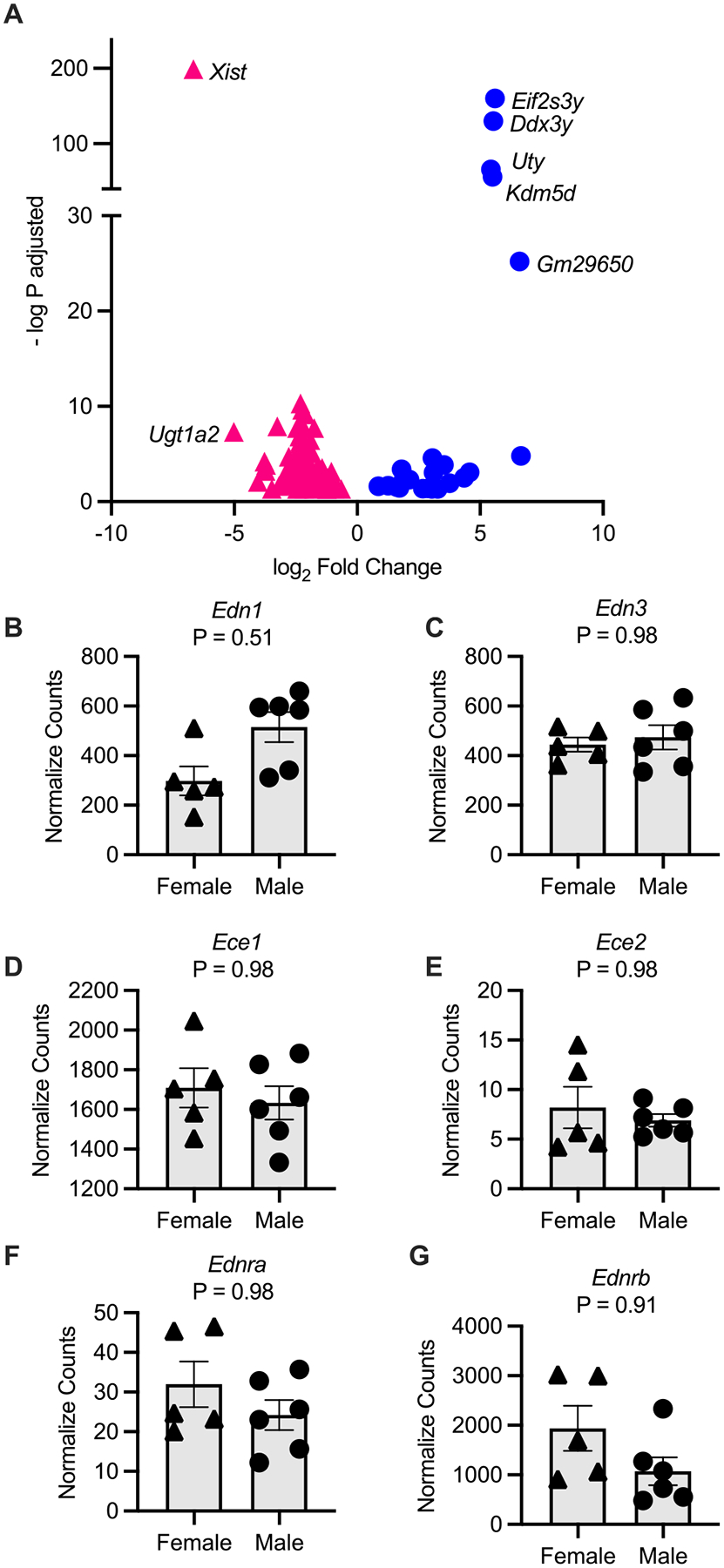
Differentially expressed genes in the inner medulla of male and female cisplatin-injected mice. (A) A volcano plot of the significant (adjusted p value <0.05) differentially expressed genes in males (blue) compared to females (pink). (B–G) Comparisons of the inner medullary endothelin (Edn) related transcripts from female and male cisplatin-injected mice from this study. Normalized counts from the analyses package DESeq2 are reported and p values are adjusted p values from the Wald test with Benjamini and Hochberg correction reported. (B) Edn1, (C) Edn3, (D) Ece1, (E) Ece2, (F) Ednra, and (G) Ednrb. Sample size: females = 5 and males = 6.
Discussion
Although the incidence of cisplatin-AKI has improved with therapeutic approaches such as forced hydration and lower dosing, cisplatin-AKI still affects a significant portion of cancer patients (Perazella et al. 2022). Thus, there is a need to better understand the mechanisms related to cisplatin-AKI to determine alternative therapeutic strategies. One such avenue may be targeting the renal ET system. The Study of Diabetic Nephropathy with atrasentan (SONAR, NCT01858532) was a double-blind, randomized, placebo-controlled large trial that determined that ETA blockade reduced the risk of kidney events in diabetic and CKD (Heerspink et al. 2019, 2021b). This clinical trial solidified the importance of the ET system in kidney health and has led to new hypotheses about how the renal ET system may be affected in disease states, making it a desirable therapeutic target. Thus, the goal of the current study was to determine whether cisplatin-AKI affects the kidney ET system in a preclinical model of both sexes.
As expected, cisplatin resulted in significant kidney injury (e.g., dilated tubules and protein casts) to both young adult male and female mice in this study. Moreover, all cisplatin-AKI mice had a significant reduction in body mass that was associated with a significant increase in plasma urea regardless of sex. This is consistent with the dehydration associated with the cisplatin model (Perse 2021). Biological sex (Chen et al. 2017) and age (Latcha et al. 2016) are important variables that affect kidney outcomes following AKI and generally younger females having some protection from reduced renal function associated with cisplatin-AKI even though they may have kidney injury (Boddu et al. 2017; Hwang et al. 2021). Thus, the phenotype of our cisplatin-AKI model matches well with other preclinical models.
Shortly after the discovery of ET (Yanagisawa et al. 1988), it became apparent that the ET system is heterogeneously expressed throughout the kidney. ET receptors are enriched in areas including the glomerulus, vasculature, interstitial cells, and IMCD (Kohzuki et al. 1989; Yukimura et al. 1996). Unlike previous studies that determined ET system expression in whole kidney homogenates from rodent models with or without cisplatin-AKI (Lee and Ahn 2008; Hwang et al. 2021), we separated the cortex, OM, and IM for our RNA analyses. Making these determinations is important for understanding whether endothelin receptor antagonists (ERAs) will have therapeutic potential in cisplatin-AKI or other kidney disease models. In the cortex, cisplatin-AKI mice had greater Edn1, Edn3, Ednra, and Ednrb expression with no statistically significant sex differences detected. In the OM, cisplatin-AKI mice had significantly reduced Ednra, and female mice had significantly greater Ednrb expression than male mice regardless of cisplatin treatment. Finally, in the IM, all cisplatin-AKI mice had significantly higher Edn1 while only in males did cisplatin-AKI result in less Ednra and Ednrb expression. These findings expand upon previous studies that determined whole kidney Edn1 was increased following cisplatin-AKI in male mice; however, whole kidney Ednra and Ednrb were not statistically different between the groups (Lee and Ahn 2008). Thus, our study provides strong evidence that ET system is differentially affected cisplatin-AKI throughout the whole kidney. For example, in a male rat model of cisplatin-AKI, the ETA receptor antagonist, BQ-123, when administered at the same time as the cisplatin, provided some kidney protection (serum creatinine, urea, and tubular necrosis were improved) compared to control cisplatin rats (Abdel Moneim et al. 2019). However, the dual receptor antagonist, bosentan, did not provide any protection from the cisplatin-induced rise in plasma creatinine or blood urea nitrogen in male (Helmy et al. 2014; Jokar et al. 2015) or female rats (Jokar et al. 2015). These studies suggest that in cisplatin-AKI, it is the ETA receptors and perhaps those that are increased in the cortex that are dysfunctional and contribute to a loss of kidney function. Similar findings have been reported in sickle cell disease nephropathy (Kasztan et al. 2017) and diabetic nephropathy (Saleh et al. 2011; Spires et al. 2018; Heerspink et al. 2019).
Although we did not detect a significant effect of sex on the susceptibility to cisplatin-AKI (no statistical differences in plasma creatinine or urea, and there was evidence of kidney injury in all cisplatin-treated mice), we did find sex-specific differences in the ET system and other genes in our IM transcriptome. Previous studies have clearly demonstrated that although kidney function is similar between the sexes, the mechanisms that are used to maintain homeostasis are distinct (Veiras et al. 2017; Hu et al. 2019, 2020, 2021; Torres-Pinzon et al. 2021). This also may relate to differences in the kidney ET system (Gohar et al. 2016) and susceptibility to cisplatin-AKI (Boddu et al. 2017; Chen et al. 2017; Hwang et al. 2021). Gohar et al. (2022) reported that the expression of Ece2, Ednra, and Ednrb was greater in the IM of healthy female versus male rats. We found no statistically significant sex differences in the IM or cortex mRNA expression of ET systems in healthy mice or cisplatin-AKI mice. Similarly, Hwang et al. (2021) published their kidney transcriptome data from healthy or cisplatin-AKI male and female mice, and in agreement with our study, did not detect a significant difference (>2-fold change, p < 0.05, false discovery rate < 0.01) in the kidney ET system. This suggests that species-specific differences may exist. Unfortunately, studies using human kidney biopsies have been underpowered to test for sex-specific expression or protein abundance (Pupilli et al. 1994; Karet and Davenport 1996; Frank et al. 2006). Healthy women do excrete more ET-1 than healthy, age-matched males (Gohar et al. 2022), and urinary ET-1 excretion likely reflects kidney ET-1 production (Dhaun et al. 2009). Studies rigorously testing potential sex differences in the ET system in the human kidney are warranted.
We found in the IM that multiple Slc22a genes were expressed higher in saline male mice compared to saline female mice. This is the gene family that includes the OATs and OCTs (Nigam 2018). It is well established that there is high expression of OATs/OCTs in the proximal tubules of preclinical models and the human kidney (Saito 2010), and they function in tubular secretion of metabolites, xenobiotics, and drugs like cisplatin (Sekine et al. 2006). Recent single cell/nucleus RNA sequencing has also confirmed that Slc22a genes are expressed in other epithelial cells like the principal cell (Hyndman et al. 2020; Hyndman and Crossman 2022). Slc22a6 (OAT1), for example, was 2.9-fold higher in the male IM than female (adjusted p value = 0.003), and others have also reported that females have only 40% of the Slc22a6 expression of males in the kidney (Cerrutti et al. 2002). Other Slc22a genes have greater expression in the male kidney as well and this may help explain why males (Boddu et al. 2017; Hwang et al. 2021) and men (Latcha et al. 2016) generally have more severe cisplatin-AKI. In our cisplatin male versus female comparison, there were an additional 109 genes differentially expressed (25 overlapped with the saline male and female dataset) and majority were genes whose functions are unknown and are likely unrelated to the ET system.
There are limitations to our study. We reported relative RNA expression, so caution needs to be taken when extrapolating to protein expression or receptor activation. Unfortunately, validated and commercially available antibodies for ETA or ETB are not available. This is a particular problem for studies investigating these and many other G-protein-coupled receptors (Herrera et al. 2013; Baker 2015; Chappell 2016). Often, changes in the mRNA expression of the ET system do correlate and agree with the findings of ERA interventions (e.g., Kasztan et al. 2017; Abdel Moneim et al. 2019). Our design was also completed soon after a single dose of cisplatin, and, therefore, our results may not reflect long-term changes in the kidney or correlate with different cisplatin dosing regimens.
These preclinical findings lead us to conclude that the kidney ET system is differentially affected following cisplatin-AKI and that sex-specific gene differences unrelated to the ET system may explain susceptibility and incidence of cisplatin-AKI in the mouse model. Future studies are needed to determine if there are sex-specific outcomes to ERAs and whether targeting the kidney ET system can prevent cisplatin-induced nephrotoxicity leading to AKI.
Supplementary Material
Funding information
Research reported in this publication was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under Award Number R01DK126664. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
This paper is part of the Collection for the 17th International Conference on Endothelin (ET-17) 2021.
Competing interests
Kelly A.Hyndman is a guest editor of the ET17 Conference Proceedings in CJPP but was not involved in any way in the review process of this manuscript. There are no other conflicts of interest to disclose.
Supplementary material
Supplementary data are available with the article at https://doi.org/10.1139/cjpp-2022-0126.
Data availability
The RNA sequencing data are freely available from GEO as described in the “Materials and methods” section. All supplementary tables are available at figshare: https://doi.org/10.6084/m9.figshare.19222659.
References
- Abdel Moneim LM, Helmy MW, and El-Abhar HS 2019. Co-targeting of endothelin-A and vitamin D receptors: a novel strategy to ameliorate cisplatin-induced nephrotoxicity. Pharmacol. Rep 71: 917–925. doi: 10.1016/j.pharep.2019.04.018. [DOI] [PubMed] [Google Scholar]
- Baker M 2015. Reproducibility crisis: blame it on the antibodies. Nature,521: 274–276. doi: 10.1038/521274a. [DOI] [PubMed] [Google Scholar]
- Boddu R, Fan C, Rangarajan S, Sunil B, Bolisetty S, and Curtis LM 2017. Unique sex- and age-dependent effects in protective pathways in acute kidney injury. Am. J. Physiol. Renal Physiol 313: F740–F755. doi: 10.1152/ajprenal.00049.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerrutti JA, Brandoni A, Quaglia NB, and Torres AM 2002. Sex differences in p-aminohippuric acid transport in rat kidney: role of membrane fluidity and expression of OAT1. Mol. Cell Biochem 233: 175–179. doi: 10.1023/A:1015563021602. [DOI] [PubMed] [Google Scholar]
- Chappell MC 2016. Biochemical evaluation of the renin–angiotensin system: the good, bad, and absolute? Am. J. Physiol. Heart Circ. Physiol 310: H137–H152. doi: 10.1152/ajpheart.00618.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WY, Hsiao CH, Chen YC, Ho CH, Wang JJ Hsing CH, et al. 2017. Cisplatin nephrotoxicity might have a sex difference. An analysis based on women’s sex hormone changes. J. Cancer, 8: 3939–3944. doi: 10.7150/jca.20083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport AP, Hyndman KA, Dhaun N, Southan C, Kohan DE Pollock JS, et al. 2016. Endothelin. Pharmacol. Rev 68: 357–418. 10.1124/pr.115.011833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaun N, Lilitkarntakul P, Macintyre IM, Muilwijk E, Johnston NR Kluth DC, et al. 2009. Urinary endothelin-1 in chronic kidney disease and as a marker of disease activity in lupus nephritis. Am. J. Physiol. Renal Physiol 296: F1477–F1483. doi: 10.1152/ajprenal.90713.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaun N, Webb DJ, and Kluth DC 2012. Endothelin-1 and the kidney——beyond BP. Br. J. Pharmacol 167: 720–731. doi: 10.1111/j.1476-5381.2012.02070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaun N, Melville V, Blackwell S, Talwar DK, Johnston NR, Goddard J, and Webb DJ 2013. Endothelin-A receptor antagonism modifies cardiovascular risk factors in CKD. J. Am. Soc. Nephrol 24: 31–36. doi: 10.1681/ASN.2012040355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan Z, Cai G, Li J, and Chen X 2020. Cisplatin-induced renal toxicity in elderly people. Ther. Adv. Med. Oncol 12: 1758835920923430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank K, Zeier M, Gross ML, Waldherr R, Ritz E, and Amann K 2006. Comprehensive immunohistological analysis of the endothelin system in human kidney grafts. Nephrol. Dial. Transplant 21: 1365–1372. doi: 10.1093/ndt/gfk087. [DOI] [PubMed] [Google Scholar]
- Gohar EY, Giachini FR, Pollock DM, and Tostes RC 2016. Role of the endothelin system in sexual dimorphism in cardiovascular and renal diseases. Life Sci. 159: 20–29. doi: 10.1016/j.lfs.2016.02.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gohar EY, De Miguel C, Obi IE, Daugherty EM, Hyndman KA Becker BK, et al. 2022. Acclimation to a high-salt diet is sex dependent. J. Am. Heart Assoc 11: e020450. doi: 10.1161/JAHA.120.020450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heerspink HJL, Parving HH, Andress DL, Bakris G, Correa-Rotter R Hou FF, et al. 2019. Atrasentan and renal events in patients with type 2 diabetes and chronic kidney disease (SONAR): a double-blind, randomised, placebo-controlled trial. Lancet, 393: 1937–1947. doi: 10.1016/S0140-6736(19)30772-X. [DOI] [PubMed] [Google Scholar]
- Heerspink HJL, Kohan DE, and de Zeeuw D 2021a. New insights from SONAR indicate adding sodium glucose co-transporter 2 inhibitors to an endothelin receptor antagonist mitigates fluid retention and enhances albuminuria reduction. Kidney Int. 99: 346–349. doi: 10.1016/j.kint.2020.09.026. [DOI] [PubMed] [Google Scholar]
- Heerspink HJL, Xie D, Bakris G, Correa-Rotter R, Hou FF Kitzman DW, et al. 2021b. Early response in albuminuria and long-term kidney protection during treatment with an endothelin receptor antagonist: a prespecified analysis from the SONAR trial. J. Am. Soc. Nephrol 32: 2900–2911. doi: 10.1681/ASN.2021030391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimlich JB, Speed JS, O’Connor PM, Pollock JS, Townes TM Meiler SE, et al. 2016. Endothelin-1 contributes to the progression of renal injury in sickle cell disease via reactive oxygen species. Br. J. Pharmacol 173: 386–395. doi: 10.1111/bph.13380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmy MM, Helmy MW, Abd Allah DM, Abo Zaid AM, and Mohy El-Din MM 2014. Selective ETA receptor blockade protects against cisplatin-induced acute renal failure in male rats. Eur. J. Pharmacol 730: 133–139. doi: 10.1016/j.ejphar.2014.03.002. [DOI] [PubMed] [Google Scholar]
- Herrera M, Sparks MA, Alfonso-Pecchio AR, Harrison-Bernard LM, and Coffman TM 2013. Lack of specificity of commercial antibodies leads to misidentification of angiotensin type 1 receptor protein. Hypertension, 61: 253–258. doi: 10.1161/HYPERTENSIONAHA.112.203679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu R, McDonough AA, and Layton AT 2019. Functional implications of the sex differences in transporter abundance along the rat nephron: modeling and analysis. Am. J. Physiol. Renal Physiol 317: F1462–F1474. doi: 10.1152/ajprenal.00352.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu R, McDonough AA, and Layton AT 2020. Sex differences in solute transport along the nephrons: effects of Na+ transport inhibition. Am. J. Physiol. Renal Physiol 319: F487–F505. doi: 10.1152/ajprenal.00240.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu R, McDonough AA, and Layton AT 2021. Sex differences in solute and water handling in the human kidney: modeling and functional implications. iScience, 24: 102667. doi: 10.1016/j.isci.2021.102667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang DB, Cha MH, Won DH, Shin YS, Kim SY Kim C, et al. 2021. Transcriptomic analysis of rat kidney reveals a potential mechanism of sex differences in susceptibility to cisplatin-induced nephrotoxicity. Free Radic. Biol. Med 174: 100–109. doi: 10.1016/j.freeradbiomed.2021.08.008. [DOI] [PubMed] [Google Scholar]
- Hyndman KA, and Crossman DK 2022. Kidney cell type-specific changes in the chromatin and transcriptome landscapes following epithelial Hdac1 and Hdac2 knockdown. Physiol. Genomics, 54: 45–57. doi: 10.1152/physiolgenomics.00102.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyndman KA, Speed JS, Mendoza LD, Allan JM, Colson J Sedaka R, et al. 2020. Fluid–electrolyte homeostasis requires histone deacetylase function. JCI Insight, 5, e137792. doi: 10.1172/jci.insight.137792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao X, Sherman BT, Huang da W, Stephens R, Baseler MW, Lane HC, and Lempicki RA 2012. DAVID-WS: a stateful web service to facilitate gene/protein list analysis. Bioinformatics, 28: 1805–1806. doi: 10.1093/bioinformatics/bts251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokar Z, Nematbakhsh M, Moeini M, and Talebi A 2015. Role of endothelin-1 antagonist; bosentan, against cisplatin-induced nephrotoxicity in male and female rats. Adv. Biomed. Res 4: 83. doi: 10.4103/2277-9175.156642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karet FE, and Davenport AP 1996. Localization of endothelin peptides in human kidney. Kidney Int. 49: 382–387. doi: 10.1038/ki.1996.56. [DOI] [PubMed] [Google Scholar]
- Kasztan M, Fox BM, Speed JS, De Miguel C, Gohar EY Townes TM, et al. 2017. Long-term endothelin-A receptor antagonism provides robust renal protection in humanized sickle cell disease mice. J. Am. Soc. Nephrol 28: 2443–2458. doi: 10.1681/ASN.2016070711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellum JA, Romagnani P, Ashuntantang G, Ronco C, Zarbock A, and Anders HJ 2021. Acute kidney injury. Nat. Rev. Dis. Primers, 7: 52. doi: 10.1038/s41572-021-00284-z. [DOI] [PubMed] [Google Scholar]
- Kitamura K, Tanaka T, Kato J, Eto T, and Tanaka K 1989. Regional distribution of immunoreactive endothelin in porcine tissue: abundance in inner medulla of kidney. Biochem. Biophys. Res. Commun 161: 348–352. doi: 10.1016/0006-291X(89)91603-3. [DOI] [PubMed] [Google Scholar]
- Kohan DE 1991. Endothelin synthesis by rabbit renal tubule cells. Am. J. Physiol 261: F221–F226. [DOI] [PubMed] [Google Scholar]
- Kohan DE 1994. Role of endothelin and tumour necrosis factor in the renal response to sepsis. Nephrol. Dial. Transplant 9(Suppl. 4): 73–77. [PubMed] [Google Scholar]
- Kohan DE 2011. Endothelin and collecting duct sodium and water transport. Contrib. Nephrol 172: 94–106. doi: 10.1159/000328687. [DOI] [PubMed] [Google Scholar]
- Kohzuki M, Johnston CI, Chai SY, Casley DJ, and Mendelsohn FA 1989. Localization of endothelin receptors in rat kidney. Eur. J. Pharmacol 160: 193–194. doi: 10.1016/0014-2999(89)90673-0. [DOI] [PubMed] [Google Scholar]
- Latcha S, Jaimes EA, Patil S, Glezerman IG, Mehta S, and Flombaum CD 2016. Long-term renal outcomes after cisplatin treatment. Clin. J. Am. Soc. Nephrol 11: 1173–1179. doi: 10.2215/CJN.08070715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, and Ahn D 2008. Expression of endothelin-1 and its receptors in cisplatin-induced acute renal failure in mice. Korean J. Physiol. Pharmacol 12: 149–153. doi: 10.4196/kjpp.2008.12.4.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Farre A, Gomez-Garre D, Bernabeu F, and Lopez-Novoa JM 1991. A role for endothelin in the maintenance of post-ischaemic renal failure in the rat. J. Physiol 444: 513–522. doi: 10.1113/jphysiol.1991.sp018891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSweeney KR, Gadanec LK, Qaradakhi T, Ali BA, Zulli A, and Apostolopoulos V 2021. Mechanisms of cisplatin-induced acute kidney injury: pathological mechanisms, pharmacological interventions, and genetic mitigations. Cancers (Basel), 13, 1572. doi: 10.3390/cancers13071572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita S, Kitamura K, Yamamoto Y, Eto T, Osada Y Sumiyoshi A, et al. 1991. Immunoreactive endothelin in human kidney. Ann. Clin. Biochem 28(Pt 3): 267–271. doi: 10.1177/000456329102800312. [DOI] [PubMed] [Google Scholar]
- Nigam SK 2018. The SLC22 transporter family: a paradigm for the impact of drug transporters on metabolic pathways, signaling, and disease. Annu. Rev. Pharmacol. Toxicol 58: 663–687. doi: 10.1146/annurev-pharmtox-010617-052713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perazella MA, Portilla D, and Safar AM 2022. Cisplatin nephrotoxicity. UpToDate, Waltham, MA. [Google Scholar]
- Perse M 2021. Cisplatin mouse models: treatment, toxicity and translatability. Biomedicines, 9, 1406. doi: 10.3390/biomedicines9101406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pupilli C, Brunori M, Misciglia N, Selli C, Ianni L Yanagisawa M, et al. 1994. Presence and distribution of endothelin-1 gene expression in human kidney. Am. J. Physiol 267: F679–F687. [DOI] [PubMed] [Google Scholar]
- Saito H 2010. Pathophysiological regulation of renal SLC22A organic ion transporters in acute kidney injury: pharmacological and toxicological implications. Pharmacol. Ther 125: 79–91. doi: 10.1016/j.pharmthera.2009.09.008. [DOI] [PubMed] [Google Scholar]
- Saleh MA, Boesen EI, Pollock JS, Savin VJ, and Pollock DM 2011. Endothelin receptor A-specific stimulation of glomerular inflammation and injury in a streptozotocin-induced rat model of diabetes. Diabetologia, 54: 979–988. doi: 10.1007/s00125-010-2021-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekine T, Miyazaki H, and Endou H 2006. Molecular physiology of renal organic anion transporters. Am. J. Physiol. Renal. Physiol 290: F251–F261. doi: 10.1152/ajprenal.00439.2004. [DOI] [PubMed] [Google Scholar]
- Spires D, Poudel B, Shields CA, Pennington A, Fizer B Taylor L, et al. 2018. Prevention of the progression of renal injury in diabetic rodent models with preexisting renal disease with chronic endothelin a receptor blockade. Am. J. Physiol. Renal Physiol 315: F977–F985. doi: 10.1152/ajprenal.00182.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Pinzon DL, Ralph DL, Veiras LC, and McDonough AA 2021. Sex-specific adaptations to high-salt diet preserve electrolyte homeostasis with distinct sodium transporter profiles. Am. J. Physiol. Cell Physiol 321: C897–C909. doi: 10.1152/ajpcell.00282.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiras LC, Girardi ACC, Curry J, Pei L, Ralph DL Tran A, et al. 2017. Sexual dimorphic pattern of renal transporters and electrolyte homeostasis. J. Am. Soc. Nephrol 28: 3504–3517. doi: 10.1681/ASN.2017030295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waijer SW, Gansevoort RT, Bakris GL, Correa-Rotter R, Hou FF Kohan DE, et al. 2021. The effect of atrasentan on kidney and heart failure outcomes by baseline albuminuria and kidney function: a post hoc analysis of the SONAR randomized trial. Clin. J. Am. Soc. Nephrol 16: 1824–1832. doi: 10.2215/CJN.07340521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M Mitsui Y, et al. 1988. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature, 332: 411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- Yukimura T, Notoya M, Mizojiri K, Mizuhira V, Matsuura T Ebara T, et al. 1996. High resolution localization of endothelin receptors in rat renal medulla. Kidney Int. 50: 135–147. doi: 10.1038/ki.1996.296. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The RNA sequencing data are freely available from GEO as described in the “Materials and methods” section. All supplementary tables are available at figshare: https://doi.org/10.6084/m9.figshare.19222659.


