Abstract
Background
The relationship between coronavirus disease 2019 (COVID-19) vaccination and long COVID has not been firmly established. We conducted a systematic review and meta-analysis to evaluate the association between COVID-19 vaccination and long COVID.
Methods
PubMed and EMBASE databases were searched on September 2022 without language restrictions (CRD42022360399) to identify prospective trials and observational studies comparing patients with and without vaccination before severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection. We also included studies reporting symptomatic changes of ongoing long COVID following vaccination among those with a history of SARS-CoV-2 infection. Odds ratios (ORs) for each outcome were synthesized using a random-effects model. Symptomatic changes after vaccination were synthesized by a one-group meta-analysis.
Results
Six observational studies involving 536,291 unvaccinated and 84,603 vaccinated (before SARS-CoV-2 infection) patients (mean age, 41.2–66.6; female, 9.0–67.3%) and six observational studies involving 8,199 long COVID patients (mean age, 40.0 to 53.5; female, 22.2–85.9%) who received vaccination after SARS-CoV-2 infection were included. Two-dose vaccination was associated with a lower risk of long COVID compared to no vaccination (OR, 0.64; 95% confidence interval [CI], 0.45–0.92) and one-dose vaccination (OR, 0.60; 95% CI, 0.43–0.83). Two-dose vaccination compared to no vaccination was associated with a lower risk of persistent fatigue (OR, 0.62; 95% CI, 0.41–0.93) and pulmonary disorder (OR, 0.50; 95% CI, 0.47–0.52). Among those with ongoing long COVID symptoms, 54.4% (95% CI, 34.3–73.1%) did not report symptomatic changes following vaccination, while 20.3% (95% CI, 8.1–42.4%) experienced symptomatic improvement after two weeks to six months of COVID-19 vaccination.
Conclusions
COVID-19 vaccination before SARS-CoV-2 infection was associated with a lower risk of long COVID, while most of those with ongoing long COVID did not experience symptomatic changes following vaccination.
Keywords: COVID-19, Vaccine, SARS-CoV-2, Long COVID, Post-acute sequelae of COVID-19
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection has caused over 6.4 million deaths across the globe by August 2022 [1]. This enormous number of death in the acute phase is not the only noteworthy feature of coronavirus disease 2019 (COVID-19) [2]. Long COVID or post-acute COVID-19 syndrome is a condition characterized by various symptoms, including fatigue, dyspnea, cognitive impairment, and mental disorder, that persist four weeks after the acute symptom onset [3]. Studies have demonstrated that 10–55 % of COVID-19 patients, including those with mild or asymptomatic form, suffer from debilitating symptoms [4], [5], [6].
Several risk factors for long COVID have been identified, such as older age [3], [7], [8], female sex [7], pre-existing comorbidities [3], [7], [8], decreased humoral immune responses following vaccinations [9], [10], and severe acute COVID-19 [3], [4], [11]. COVID-19 vaccination has been shown to provide safe and effective protection against SARS-CoV-2 infection and the severe progression of acute COVID-19 [12], [13], [14]. Furthermore, this preventative measure has also been attracting public attention for its potential efficacy against long COVID [15].
The benefit of vaccination before and after SARS-CoV-2 infection has been explored [16], [17], [18]. A recent study involving healthcare workers suggested that two or three doses of the Pfizer-BioNTech mRNA vaccination before SARS-CoV-2 infection were associated with a lower incidence of long COVID than no vaccination [17]. However, another study indicated that vaccination only provided partial protection against long COVID [16]. Meanwhile, a longitudinal survey evaluating the effect of vaccination after SARS-CoV-2 infection, which was hypothesized to modify the immune response, showed an association between vaccination and symptomatic relief [18]. However, due to the limited sample size and generalizability, the association between COVID-19 vaccination and long COVID has not been concluded.
A previous systematic review investigated the effect of vaccination on long COVID [19]. However, their findings should be verified because non-peer-reviewed articles were included, and meta-analyses were not performed. In this study, we conducted a systematic review and meta-analysis to elucidate the relationship between vaccination and long COVID and aid in clear communication between providers, patients, and policymakers.
2. Materials and Methods
This research was conducted under the Preferred Reporting Items for Systematic Reviews and meta-Analyses (PRISMA) guideline and registered in the International prospective register of systematic reviews (CRD42022360399) [20]. An institutional review board exemption was granted for the innocuousness of the study.
2.1. Data sources and search
We used a two-level strategy to search for all prospective trials and observational studies investigating the relationship between COVID-19 vaccination and long COVID. First, a comprehensive literature search was conducted using PubMed and EMBASE databases on September 16, 2022. The search strategy is summarized in eTable 1 in the Supplement. Second, we performed an additional manual search of secondary sources, such as references from initially identified studies, to collect relevant articles comprehensively. No restrictions on language and publication date were applied.
2.2. Eligibility criteria
The eligibility of each study was assessed using the following Population, Intervention, Comparison, and Outcome (PICO) principle;
Population: we included patients of any age diagnosed with COVID-19 either by polymerase chain reaction, serum antibody test, or the development of clinical symptoms after close contact with proven cases.
Intervention: any type of COVID-19 vaccination before or after acute SARS-CoV-2 infections were identified.
Comparison: patients vaccinated before acute SARS-CoV-2 infections were compared to those without vaccination, whereas patients vaccinated after acute SARS-CoV-2 infections did not have a comparator.
Outcome: we set two separate outcomes depending on the vaccination timing (i.e., before or after SARS-CoV-2 infection). For studies investigating vaccination before SARS-CoV-2 infection, the primary outcome was the incidence of long COVID. In this study, we defined long COVID as a condition where patients have persistent or new-onset symptoms and/or conditions four weeks after the acute SARS-CoV-2 infection [3]. When a study reported multiple follow-ups, we extracted the outcomes at the longest follow-up because our interest was the effect of vaccination in the long term. The secondary outcome was each of the persistent symptoms, including fatigue, pulmonary disorder, cardiovascular disorder, gastrointestinal disorder, metabolic disorder, musculoskeletal disorder, neurologic disorder, and mental disorder. The definition of each organ-system disorder followed in each study. For studies investigating vaccination after SARS-CoV-2 infection, the primary outcome was the symptomatic change of long COVID following vaccination.
Preprint articles and studies without original patients’ data (e.g., guidelines, correspondence, and reviews) were excluded. When we identified two or more reports from the same cohort, we included the largest study to avoid duplication. The methodological quality of the included studies was evaluated using the Newcastle-Ottawa Scale [19].
2.3. Data extraction and items
Two reviewers (AW and TK) screened the search results individually to identify the studies based on the inclusion and exclusion criteria and assessed the eligibility for each study. After screening the title and abstract, we retrieved the full texts of potentially eligible articles for further review. Disagreements were resolved through consensus.
Baseline characteristics, such as age, sex, pre-existing comorbidities (obesity, hypertension, diabetes mellitus, chronic lung disease, chronic kidney disease), and ICU admission due to acute COVID-19 were extracted and tabulated. Regarding the COVID-19 vaccine, we collected the type of vaccine (e.g., BNT162b2 by Pfizer-BioNTech, mRNA-1273 by Moderna, and Ad26.COV2.S by AstraZeneca), doses, and timing (before or after SARS-CoV-2 infection).
2.4. Data synthesis and analysis
For studies investigating vaccination before SARS-CoV-2 infection, we extracted unadjusted and adjusted (whenever available) odds ratios (ORs) for each outcome. When a study used propensity score matching, we extracted the outcomes from the matched cohort. The OR with a 95 % confidence interval (CI) for each outcome was synthesized using the Review Manager (RevMan) Version 5.4 (Nordic Cochrane Center, the Cochrane Collaboration, Copenhagen, Denmark) with a random-effects model. Furthermore, we compared one-dose vs no vaccination and two-dose vs one-dose for the primary outcome. For studies investigating vaccination after SARS-CoV-2 infection, we pooled the logarithm of self-reporting symptomatic changes (improved, worsened, and unchanged) and performed a one-group meta-analysis by the Wald method with a random-effects model using OpenMetaAnalyst version 21.11.14 (available from https://www.cebm.brown.edu/openmeta/). Heterogeneity was assessed using I2, with > 50 % indicating substantial heterogeneity. Publication bias was assessed by Egger’s linear regression tests and Funnel plots of the primary outcomes using Comprehensive meta-Analysis version 2 (Biostat, Englewood, NJ, USA) [21]. The overall certainty of each outcome was evaluated using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach.
3. Results
We identified 963 articles through the initial database search and subsequent manual search. After removing 943 items based on the title and abstract, we retrieved the full text of 20 articles. We excluded reports from the same cohort or studies without outcomes of interest. Finally, we included six [16], [17], [22], [23], [24], [25] and six [18], [26], [27], [28], [29], [30] observational studies investigating COVID-19 vaccination before and after SARS-CoV-2 infection, respectively (Fig. 1 ). There were 536,291 unvaccinated and 84,603 vaccinated patients who received vaccination before the SARS-CoV-2 infection, while there were 8,199 patients with long COVID who received vaccination after SARS-CoV-2 infection. The methodological quality assessment is summarized in eTable 2 in the Supplement.
Fig. 1.
Flowchart of study selection.
3.1. Baseline characteristics
The baseline characteristics of the included studies are summarized in Table 1, Table 2 and eTables 3,4. The definitions of organ system impairment are summarized in eTable 5 in the Supplement. In studies investigating COVID-19 vaccination before SARS-CoV-2 infection (Table 1), the mean age ranged from 41.2 to 66.6 years, and the female proportion ranged from 9.0 % to 67.3 %. The proportion of patients admitted to the ICU for acute infection ranged from 0.6 % to 6.0 %. The follow-up duration from acute COVID-19 ranged from 28 days to 6 months. In studies investigating COVID-19 vaccination after SARS-CoV-2 infection (Table 2), the mean age ranged from 40.0 to 53.5 years, and the female proportion ranged from 22.2 % to 85.9 %. The proportion of patients admitted to the ICU for acute infection ranged from 0.8 % to 2.3 %. The median duration between acute COVID-19 and vaccination ranged from 146 to 483 days.
Table 1.
Baseline characteristics of studies investigating vaccination before SARS-CoV-2 infection.
| First author | Study design | Country | Observational period | Vaccine type | Follow-up duration (months) | Patient number, N | Age, median [IQR] or mean ± SD | Female, N (%) | ICU admission, N (%) |
|---|---|---|---|---|---|---|---|---|---|
| Meza-Torres[22] | Retrospective cohort | UK | Mar 2020-Apr 2021 | NA | 6 | No vaccination, 392,324; 1-dose, 15,832; 2-dose, 726 | 45 ± 22 | 232,775 (56)a | 2,852 (0.7)a |
| Emecen[23] | Prospective cohort | Turkey | Dec 2020-May 2021 | CoronaVac, BNT162b2 | 6 | No vaccination, 4,847; 2-dose, 162 | 18–49, 67.7 %; 50–64, 22.4 %; 65-, 9.9 % | 2,908 (52)a | 66 (1.3)a |
| Al-Aly[16] | Retrospective cohort | USA | Jan 2021-Dec 2021 | BNT162b2, mRNA-1273, Ad26.COV2.S | 6 | No vaccination, 113,474; 2-dose, 33,940 | No vaccination, 58 ± 16; 2-dose, 67 ± 14 | No vaccination, 14,842 (13); 2-dose, 3,044 (9) | No vaccination, 2,982 (2.6); 2-dose, 811 (2.4) |
| Zisis[24] | Retrospective cohort | USA | Sep 2020-Dec 2021 | NA | 3 | No vaccination, 25,225; 2-dose, 25,225 | No vaccination, 55 ± 18; 2-dose, 55 ± 18 | No vaccination, 15,129 (60); 2-dose, 15,094 (60) | NA |
| Azzolini[17] | Retrospective cohort | Italy | Mar 2020-Apr 2022 | BNT162b2 | 1 | No vaccination, 421; 1-dose, 10; 2-dose, 46; 3-dose, 262 | 44 ± 11 | 180 (33) | NA |
| Antonelli[25] | Prospective cohort | UK | Dec 2020-Jul 2021 | BNT162b2, mRNA-1273, AZD1222 | 1 | 1-dose, 6,030; 2-dose, 2,370 | 1-dose, 50 ± 14; 2-dose, 53 ± 14 | 1-dose, 3,766 (63); 2-dose, 1,451 (61) | NA |
NA, not available.
Among patients infected with SARS-CoV-2.
Table 2.
Baseline characteristics of studies investigating vaccination after SARS-CoV-2 infection.
| First author | Study design | Country | Observational period | Vaccine type | Time between SARS-CoV-2 infection and vaccination | Patient number | Age, median [IQR] or mean ± SD | Female, N (%) | ICU admission, N (%) |
|---|---|---|---|---|---|---|---|---|---|
| Tsuchida[26] | Prospective cohort | Japan | NA | NA | 196 [110–238] days | 42 | 40 [30–47] | 12 (46) | NA |
| Strain[27] | Prospective cohort | UK | Mar 20201-Apr 2021 | AZD1222, BNT162b2, mRNA-1273 | 1–3-month, 5.4 %; 3–6-month, 15.0 %; 6–9-month, 8.0 %; >9-month, 71.6 % | 812 | −20, 0.4 %; 21–40, 21.9 %; 41–60, 62.3 %; 60-, 15.5 % | 654 (81) | NA |
| Scherlinger[28] | Retrospective cohort | France | Aug-21 | AZD1222, BNT162b2, mRNA-1273, Ad26.COV2.S | 483 [266–506] days | 397 | 44 [37–50] | 327 (86) | 3 (0.8) |
| Ayoubkhani[18] | Retrospective cohort | UK | Apr 2020-Sep 2021 | AZD1222, BNT162b2, mRNA-1273 | 178 [NA] days | 6,729 | 46 ± 14 | NA | NA |
| Peghin[29] | Prospective cohort | Italy | Mar 2020-May 2020 | AZD1222, BNT162b2, mRNA-1273 | 12.4 ± 1.9 months | 132 | 18–40, 25.0 %; 41–60, 48.5 %; 60-, 26.5 % | 94 (71) | 3 (2.3) |
| Wynberg[30] | Prospective cohort | Netherland | May 2020-Jun 2021 | AZD1222, BNT162b2, mRNA-1273, Ad26.COV2.S | 271 [158–387] days | 87 | 54 [35–61] | NA | NA |
NA, not available.
3.2. Vaccination before SARS-CoV-2 infection and long COVID
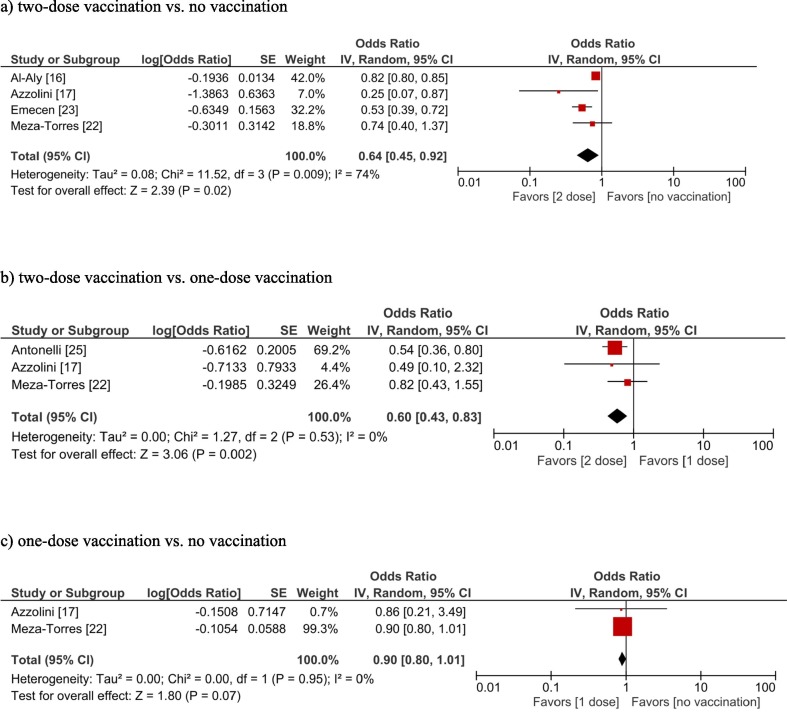
The overall certainty of evidence (GRADE) for each outcome is summarized in eTable 6 in the Supplement. Two-dose COVID-19 vaccination was associated with a lower frequency of long COVID compared to no vaccination (OR, 0.64; 95 % confidence interval [CI], 0.45–0.92; I2 = 74 %; moderate certainty) (Fig. 2 a) and one-dose vaccination (OR, 0.60; 95 % CI, 0.43–0.83; I2 = 0 %; moderate certainty) (Fig. 2b). However, one-dose vaccination compared to no vaccination was not significantly associated with a lower frequency of long COVID (OR, 0.90; 95 % CI, 0.80–1.01; I2 = 0 %; low certainty) (Fig. 2c). Publication bias was not suggested for the primary outcome, although only four studies reported the primary outcome (eTable 1 eFig. 1). Only one study compared (therefore, meta-analyses could not be performed) different types of vaccines (i.e., BNT162b2 vs mRNA-1273 vs Ad26.COV2.S), in which BNT162b2 and mRNA-1273 were associated with lower incidences of long COVID compared to Ad26.COV2.S (OR, 0.86; 95 % CI, 0.80–0.93 and OR, 0.87; 95 % CI, 0.80–0.93, respectively) whereas no difference was seen between BNT162b2 vs mRNA-1273 (OR, 1.00; 95 % CI, 0.95–1.05) [16].
Fig. 2.
Forest plots showing the odds ratio of long COVID (a: two-dose vaccination vs no vaccination, b: two-dose vaccination vs one-dose vaccination, c: one-dose vaccination vs no vaccination).
Two-dose COVID-19 vaccination compared to no vaccination was associated with a lower incidence of persistent fatigue (OR, 0.62; 95 % CI, 0.41–0.93; I2 = 65 %; low certainty) and pulmonary disorder (OR, 0.50; 95 % CI, 0.47–0.52; I2 = 0 %; low certainty) (eFigs. 2a–b). The trend was similar for cardiovascular disorder (OR, 0.59; 95 % CI, 0.23–1.49; I2 = 78 %; low certainty), gastrointestinal disorder (OR, 0.62; 95 % CI, 0.35–1.10; I2 = 78 %; low certainty), metabolic disorder (OR, 0.58; 95 % CI, 0.24–1.41; I2 = 54 %; low certainty), musculoskeletal disorder (OR, 0.63; 95 % CI, 0.26–1.52; I2 = 69 %; low certainty), neurologic disorder (OR, 0.61; 95 % CI, 0.31–1.20; I2 = 72 %; low certainty), and mental disorder (OR, 0.49; 95 % CI, 0.15–1.62; I2 = 87 %; low certainty) (eFigs. 2c–h), though statistically not significant.
3.3. Vaccination after SARS-CoV-2 infection and long COVID
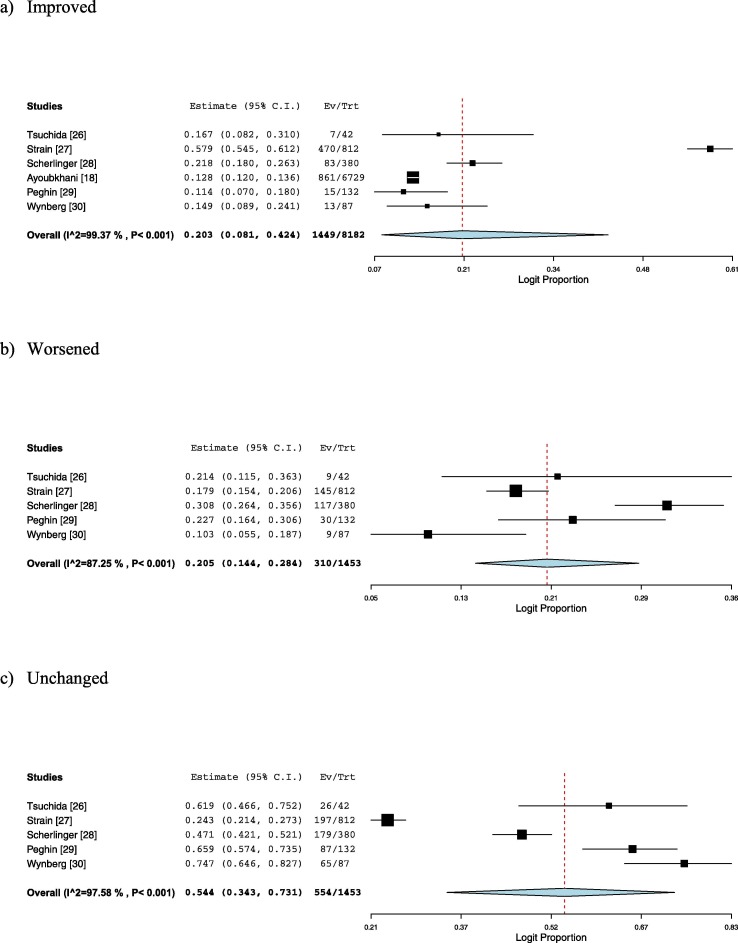
Among those who have a prior infection of SARS-CoV-2, self-reporting symptomatic improvement of long COVID was observed in 20.3 % (95 % CI, 8.1–42.4; I2 = 99 %; very low certainty) two weeks to six months after COVID-19 vaccination, whereas 20.5 % (95 % CI, 14.4–28.4; I2 = 87 %; very low certainty) experienced symptomatic worsening, and 54.4 % (95 % CI, 34.3–73.1; I2 = 98 %; very low certainty) did not report symptomatic change (Fig. 3 a-c). Publication bias was not suggested (eFigs. 3–5). One study showed that more than half of the symptomatic worsening after vaccination resolved within 3–7 days [27]. In another study, the symptomatic worsening following vaccination was mostly driven by fever/chills, fatigue, and muscle/joint pain [28].
Fig. 3.
Forest plots showing the symptomatic change of long COVID after vaccination (a: improved, b: worsened, c: unchanged).
4. Discussion
In this meta-analysis of 12 studies involving 629,093 patients, we demonstrated that COVID-19 vaccination before SARS-CoV-2 infection was associated with a lower incidence of long COVID, especially after the second dose. In contrast, in patients with a prior SARS-CoV-2 infection and ongoing long COVID, vaccination was not associated with symptomatic deterioration.
Among the reported risk factors of developing long COVID, such as older age, female sex, and underlying comorbidities [3], [4], the severity of the acute infection is substantial [31], [32]. Several studies reported that the disease severity correlated with the frequency and duration of sequelae [31], [32], [33]. According to a multicenter retrospective study, hospitalized and ICU-admitted patients were three and five times more likely to have long COVID, respectively [31]. Likewise, patients who required supplemental oxygen or mechanical ventilation were more likely to have residual CT abnormalities at one-year follow-up than those who did not [33]. Since vaccination is a safe and effective measure to prevent the severe progression of COVID-19 [12], [13], [14], it is plausible that vaccinated patients presented a lower incidence of long COVID than unvaccinated patients.
Although the disease severity is a significant risk factor, it is not the sole contributor to developing long COVID. A nationwide database study revealed that vaccinated patients were less likely to develop long COVID than unvaccinated patients, even in those non-hospitalized [16]. In addition, multicenter observational studies that matched vaccinated COVID-19 patients with those unvaccinated using known risk factors for severe infection showed a lower incidence of long COVID among vaccinated patients through 28- to 180-day follow-up [24], [34]. Notably, other studies showed that patients with two-dose vaccination were more likely to have pre-existing comorbidities than those without/one-dose vaccination [16], [24], [25]. Since pre-existing comorbidities, such as asthma, diabetes, obesity, and immunosuppressive conditions, can also be associated with higher risks of long COVID [3], [7], [8], our finding that patients with two-dose vaccination (who were also more likely to have pre-existing comorbidities) were associated with lower incidences of long COVID underscored the importance of vaccination before acute SARS-CoV-2 infection.
The strength of our report lies in the meta-analyses. Since a previous systematic review did not conduct meta-analyses [19], our finding adds to the literature and will aid in clear communication between health professionals and patients. In addition, it should be noted that two-dose vaccination was associated with a lower risk of long COVID even compared to one-dose vaccination. As plenty of articles have demonstrated the efficacy of two-dose vaccination and additional boosters in reducing mortality and severe cases [13], [14], [35], [36], [37], the more effective protection against acute infection may also be a reasonable measure to avoid the debilitating and long-lasting sequelae.
While vaccination before SARS-CoV-2 infection may reduce long COVID, the effects of vaccination in patients with ongoing long COVID are poorly understood. We pooled the reported incidences and showed that vaccination was not associated with symptomatic deteriorations of ongoing long COVID, although the included studies showed varied results. A longitudinal survey demonstrated favorable trajectories of long COVID after vaccination [18]. Symptomatic improvement of long COVID after vaccination might be explained by the additional antibody production due to vaccination [27]. A prospective cohort study involving non-hospitalized patients showed that patients with long COVID had a lower titer of serum spike IgG/micro-neutralizing antibody compared to those without [38]. However, since the population of the included studies was substantially heterogeneous, and the majority reported unchanged symptoms following vaccination, further investigations into specific host factors to predict the course of long COVID after vaccination are warranted.
Our study could not include enough studies to analyze each of the symptomatic changes following vaccination. However, it should be noted that an online survey showed that the majority of the reported symptoms that worsened following vaccination were those commonly observed in the general population, such as fever, chills, and gastrointestinal symptoms [13], while the symptomatic improvement following vaccination was mainly driven by brain fog and anosmia, which has been associated with disability in long COVID [28]. Moreover, as more than half of the symptomatic worsening after vaccination resolved within 3–7 days [27], these deteriorations may simply be temporary reactions rather than long COVID exacerbations [13], [27]. In addition, long COVID patients without confirmed diagnoses of acute SARS-CoV-2 infection (defined by RT-PCR or serology test) were more likely to report symptomatic worsening, possibly suggesting increased nocebo effects in the population without biological proof [28], [39]. As such, although a similar proportion of those with ongoing long COVID reported symptomatic improvement and deterioration in our study, there is a possibility that our findings might have overestimated symptomatic worsening and not have reflected the actual picture of long COVID exacerbations. Taken together, COVID-19 vaccination might safely be injected into patients with ongoing long COVID. Once the safety of vaccination among this population is proven, this could be an essential step to address vaccine hesitancy and promote global health [40].
5. Limitations
This study had several limitations. First, all the included reports were observational studies, which may have affected a range of biases. For example, although the severity of the acute infection appears to be associated with long-term sequelae [33], the proportion of ICU-admitted patients varied in our study. However, as the vaccination itself is effective in reducing the severe progression of COVID-19 [12], the overall benefit of the vaccination should still be highlighted. Second, the definitions of long COVID and its associated symptoms were not universal across the included studies. As the only two studies investigating vaccination before SARS-CoV-2 infection and two studies investigating vaccination after SARS-CoV-2 infection reported each of the organ-specific impairments (the definitions of which were inconsistent) [16], [24], [26], [29], detailed analyses on each condition were not allowed; therefore, our findings should be interpreted with caution. Third, the symptomatic changes after vaccination among patients with long COVID were assessed using one-group meta-analyses, meaning that the pooled analyses were not based on comparative data. Furthermore, since the follow-up durations of the surveys varied from 2 weeks to 6 months, temporary reactions following vaccination may have been included in the effect estimations. Fourth, since the observational periods of the included studies varied widely, we could not evaluate the impact of different variants of concern [41]. Additionally, the effect of three- or four-dose vaccination on long COVID could not be measured. Since booster vaccines seem promising both in preventing infection and severe progression [35], [36], [37], these can also be speculated to be effective in reducing long COVID. Indeed, the effect size of three-dose vaccination on protection against long COVID was larger than the two-dose in the healthcare setting [17]. Future studies on boosters and omicron-specific vaccination are awaited [42].
6. Conclusion
In this systematic review and meta-analysis, COVID-19 vaccination before SARS-CoV-2 infection may be associated with a reduction in long COVID, while the majority of those with ongoing long COVID did not experience symptomatic changes following vaccination. Our findings should be disseminated to patients without a history of SARS-CoV-2 infection to reduce the incidence of long COVID.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
The datasets during and/or analyzed during the current study available from the corresponding author on reasonable request.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2023.02.008.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- 1.World Health Organization. WHO Coronavirus (COVID-19) Dashboard. WHO Coronavirus (COVID-19) Dashboard 2022. https://covid19.who.int/ (accessed March 10, 2022).
- 2.Levine R.L. Addressing the Long-term Effects of COVID-19. JAMA. 2022;328:823–824. doi: 10.1001/jama.2022.14089. [DOI] [PubMed] [Google Scholar]
- 3.Crook H., Raza S., Nowell J., Young M., Edison P. Long covid-mechanisms, risk factors, and management. BMJ. 2021;374 doi: 10.1136/bmj.n1648. [DOI] [PubMed] [Google Scholar]
- 4.Sudre C.H., Murray B., Varsavsky T., Graham M.S., Penfold R.S., Bowyer R.C., et al. Attributes and predictors of long COVID. Nat Med. 2021;27:626–631. doi: 10.1038/s41591-021-01292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sigfrid L., Drake T.M., Pauley E., Jesudason E.C., Olliaro P., Lim W.S., et al. Long Covid in adults discharged from UK hospitals after Covid-19: A prospective, multicentre cohort study using the ISARIC WHO Clinical Characterisation Protocol. Lancet Reg Health Eur. 2021;8 doi: 10.1016/j.lanepe.2021.100186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Kessel S.A.M., Olde Hartman T.C., Lucassen P.L.B.J., van Jaarsveld C.H.M. Post-acute and long-COVID-19 symptoms in patients with mild diseases: a systematic review. Fam Pract. 2022;39:159–167. doi: 10.1093/fampra/cmab076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Notarte K.I., de Oliveira M.H.S., Peligro P.J., Velasco J.V., Macaranas I., Ver A.T., et al. Age, Sex and Previous Comorbidities as Risk Factors Not Associated with SARS-CoV-2 Infection for Long COVID-19: A Systematic Review and Meta-Analysis. J Clin Med Res. 2022;11 doi: 10.3390/jcm11247314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tenforde MW, Kim SS, Lindsell CJ, Billig Rose E, Shapiro NI, Files DC, et al. Symptom Duration and Risk Factors for Delayed Return to Usual Health Among Outpatients with COVID-19 in a Multistate Health Care Systems Network - United States, March-June 2020. MMWR Morb Mortal Wkly Rep 2020;69:993–8. 10.15585/mmwr.mm6930e1. [DOI] [PMC free article] [PubMed]
- 9.Notarte K.I., Ver A.T., Velasco J.V., Pastrana A., Catahay J.A., Salvagno G.L., et al. Effects of age, sex, serostatus, and underlying comorbidities on humoral response post-SARS-CoV-2 Pfizer-BioNTech mRNA vaccination: a systematic review. Crit Rev Clin Lab Sci. 2022;59:373–390. doi: 10.1080/10408363.2022.2038539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Notarte K.I., Guerrero-Arguero I., Velasco J.V., Ver A.T., Santos de Oliveira M.H., Catahay J.A., et al. Characterization of the significant decline in humoral immune response six months post-SARS-CoV-2 mRNA vaccination: A systematic review. J Med Virol. 2022;94:2939–2961. doi: 10.1002/jmv.27688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamal M., Abo Omirah M., Hussein A., Saeed H. Assessment and characterisation of post-COVID-19 manifestations. Int J Clin Pract. 2021;75:e13746. doi: 10.1111/ijcp.13746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lopez Bernal J., Andrews N., Gower C., Robertson C., Stowe J., Tessier E., et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ. 2021;373 doi: 10.1136/bmj.n1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharif N., Alzahrani K.J., Ahmed S.N., Dey S.K. Efficacy, Immunogenicity and Safety of COVID-19 Vaccines: A Systematic Review and Meta-Analysis. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.714170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ledford H. Do vaccines protect against long COVID? What the data say. Nature. 2021;599:546–548. doi: 10.1038/d41586-021-03495-2. [DOI] [PubMed] [Google Scholar]
- 16.Al-Aly Z., Bowe B., Xie Y. Long COVID after breakthrough SARS-CoV-2 infection. Nat Med. 2022;28:1461–1467. doi: 10.1038/s41591-022-01840-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Azzolini E., Levi R., Sarti R., Pozzi C., Mollura M., Mantovani A., et al. Association Between BNT162b2 Vaccination and Long COVID After Infections Not Requiring Hospitalization in Health Care Workers. JAMA. 2022 doi: 10.1001/jama.2022.11691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ayoubkhani D., Bermingham C., Pouwels K.B., Glickman M., Nafilyan V., Zaccardi F., et al. Trajectory of long covid symptoms after covid-19 vaccination: community based cohort study. BMJ. 2022;377:e069676. doi: 10.1136/bmj-2021-069676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Notarte K.I., Catahay J.A., Velasco J.V., Pastrana A., Ver A.T., Pangilinan F.C., et al. Impact of COVID-19 vaccination on the risk of developing long-COVID and on existing long-COVID symptoms: A systematic review. EClinicalMedicine. 2022;53 doi: 10.1016/j.eclinm.2022.101624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Page M.J., Moher D., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372 doi: 10.1136/bmj.n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Egger M., Davey Smith G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meza-Torres B., Delanerolle G., Okusi C., Mayor N., Anand S., Macartney J., et al. Differences in Clinical Presentation With Long COVID After Community and Hospital Infection and Associations With All-Cause Mortality: English Sentinel Network Database Study. JMIR Public Health Surveill. 2022;8:e37668. doi: 10.2196/37668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Emecen A.N., Keskin S., Turunc O., Suner A.F., Siyve N., Basoglu Sensoy E., et al. The presence of symptoms within 6 months after COVID-19: a single-center longitudinal study. Ir J Med Sci. 2022 doi: 10.1007/s11845-022-03072-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zisis SN, Durieux JC, Mouchati C, Perez JA, McComsey GA. The Protective Effect of Coronavirus Disease 2019 (COVID-19) Vaccination on Postacute Sequelae of COVID-19: A Multicenter Study From a Large National Health Research Network. Open Forum Infect Dis 2022;9:ofac228. 10.1093/ofid/ofac228. [DOI] [PMC free article] [PubMed]
- 25.Antonelli M., Penfold R.S., Merino J., Sudre C.H., Molteni E., Berry S., et al. Risk factors and disease profile of post-vaccination SARS-CoV-2 infection in UK users of the COVID Symptom Study app: a prospective, community-based, nested, case-control study. Lancet Infect Dis. 2022;22:43–55. doi: 10.1016/S1473-3099(21)00460-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsuchida T., Hirose M., Inoue Y., Kunishima H., Otsubo T., Matsuda T. Relationship between changes in symptoms and antibody titers after a single vaccination in patients with Long COVID. J Med Virol. 2022;94:3416–3420. doi: 10.1002/jmv.27689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strain WD, Sherwood O, Banerjee A, Van der Togt V, Hishmeh L, Rossman J. The Impact of COVID Vaccination on Symptoms of Long COVID: An International Survey of People with Lived Experience of Long COVID. Vaccines (Basel) 2022;10. 10.3390/vaccines10050652. [DOI] [PMC free article] [PubMed]
- 28.Scherlinger M, Pijnenburg L, Chatelus E, Arnaud L, Gottenberg J-E, Sibilia J, et al. Effect of SARS-CoV-2 Vaccination on Symptoms from Post-Acute Sequelae of COVID-19: Results from the Nationwide VAXILONG Study. Vaccines (Basel) 2021;10. 10.3390/vaccines10010046. [DOI] [PMC free article] [PubMed]
- 29.Peghin M., De Martino M., Palese A., Gerussi V., Bontempo G., Graziano E., et al. Post-COVID-19 syndrome and humoral response association after 1 year in vaccinated and unvaccinated patients. Clin Microbiol Infect. 2022;28:1140–1148. doi: 10.1016/j.cmi.2022.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wynberg E., Han A.X., Boyd A., van Willigen H.D.G., Verveen A., Lebbink R., et al. The effect of SARS-CoV-2 vaccination on post-acute sequelae of COVID-19 (PASC): A prospective cohort study. Vaccine. 2022;40:4424–4431. doi: 10.1016/j.vaccine.2022.05.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mayor N., Meza-Torres B., Okusi C., Delanerolle G., Chapman M., Wang W., et al. Developing a Long COVID Phenotype for Postacute COVID-19 in a National Primary Care Sentinel Cohort: Observational Retrospective Database Analysis. JMIR Public Health Surveill. 2022;8:e36989. doi: 10.2196/36989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chudzik M., Babicki M., Kapusta J., Kałuzińska-Kołat Ż., Kołat D., Jankowski P., et al. Long-COVID Clinical Features and Risk Factors: A Retrospective Analysis of Patients from the STOP-COVID Registry of the PoLoCOV Study. Viruses. 2022;14 doi: 10.3390/v14081755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Watanabe A., So M., Iwagami M., Fukunaga K., Takagi H., Kabata H., et al. One-year follow-up CT findings in COVID-19 patients: A systematic review and meta-analysis. Respirology. 2022;27:605–616. doi: 10.1111/resp.14311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taquet M., Dercon Q., Harrison P.J. Six-month sequelae of post-vaccination SARS-CoV-2 infection: A retrospective cohort study of 10,024 breakthrough infections. Brain Behav Immun. 2022;103:154–162. doi: 10.1016/j.bbi.2022.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Magen O., Waxman J.G., Makov-Assif M., Vered R., Dicker D., Hernán M.A., et al. Fourth Dose of BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Setting. N Engl J Med. 2022;386:1603–1614. doi: 10.1056/NEJMoa2201688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Munro A.P.S., Feng S., Janani L., Cornelius V., Aley P.K., Babbage G., et al. Safety, immunogenicity, and reactogenicity of BNT162b2 and mRNA-1273 COVID-19 vaccines given as fourth-dose boosters following two doses of ChAdOx1 nCoV-19 or BNT162b2 and a third dose of BNT162b2 (COV-BOOST): a multicentre, blinded, phase 2, randomised trial. Lancet Infect Dis. 2022;22:1131–1141. doi: 10.1016/S1473-3099(22)00271-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bar-On Y.M., Goldberg Y., Mandel M., Bodenheimer O., Freedman L., Kalkstein N., et al. Protection of BNT162b2 Vaccine Booster against Covid-19 in Israel. N Engl J Med. 2021;385:1393–1400. doi: 10.1056/NEJMoa2114255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blomberg B., Mohn K.-G.-I., Brokstad K.A., Zhou F., Linchausen D.W., Hansen B.-A., et al. Long COVID in a prospective cohort of home-isolated patients. Nat Med. 2021;27:1607–1613. doi: 10.1038/s41591-021-01433-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matta J., Wiernik E., Robineau O., Carrat F., Touvier M., Severi G., et al. Association of Self-reported COVID-19 Infection and SARS-CoV-2 Serology Test Results With Persistent Physical Symptoms Among French Adults During the COVID-19 Pandemic. JAMA Intern Med. 2022;182:19–25. doi: 10.1001/jamainternmed.2021.6454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Machingaidze S., Wiysonge C.S. Understanding COVID-19 vaccine hesitancy. Nat Med. 2021;27:1338–1339. doi: 10.1038/s41591-021-01459-7. [DOI] [PubMed] [Google Scholar]
- 41.Fernández-de-Las-Peñas C., Notarte K.I., Peligro P.J., Velasco J.V., Ocampo M.J., Henry B.M., et al. Long-COVID Symptoms in Individuals Infected with Different SARS-CoV-2 Variants of Concern: A Systematic Review of the Literature. Viruses. 2022;14 doi: 10.3390/v14122629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Callaway E. New Omicron-specific vaccines offer similar protection to existing boosters. Nature. 2022 doi: 10.1038/d41586-022-02806-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.