Abstract
Preoperative nutrition status is an important determinant of surgical outcomes, yet malnutrition assessment is not integrated into all surgical pathways. Given its importance and the high prevalence of malnutrition in patients undergoing surgical procedures, preoperative nutrition screening, assessment, and intervention are needed to improve postoperative outcomes. This narrative review discusses novel methods to assess malnutrition and frailty in the surgical patient. The Global Leadership Initiative for Malnutrition (GLIM) criteria are increasingly used in surgical settings although further spread and implementation are strongly encouraged to help standardize the diagnosis of malnutrition. The use of body composition (ie, reduced muscle mass) as a phenotypic criterion in GLIM may lead to a greater number of patients identified as having malnutrition, which may otherwise be undetected if screened by other diagnostic tools. Skeletal muscle loss is a defining criterion of malnutrition and frailty. Novel direct and indirect approaches to assess muscle mass in clinical settings may facilitate the identification of patients with or at risk for malnutrition. Selected imaging techniques have the additional advantage of identifying myosteatosis (an independent predictor of morbidity and mortality for surgical patients). Feasible pathways for screening and assessing frailty exist and may determine the cost/benefit of surgery, long‐term independence and productivity, and the value of undertaking targeted interventions. Finally, the evaluation of nutrition risk and status is essential to predict and mitigate surgical outcomes. Nascent to novel approaches are the future of objectively identifying patients at perioperative nutrition risk and guiding therapy toward optimal perioperative standards of care.
Keywords: body composition, frailty, GLIM, imaging, malnutrition, muscle mass, nutritional assessment, nutritional risk, nutritional screening, perioperative nutrition, sarcopenia, surgery
Preoperative nutrition status can be optimized and is a modifiable risk factor for surgical outcomes. 1 The impact of malnutrition on adverse surgical outcomes has been repeatedly demonstrated, yet screening for the presence or risk of malnutrition in this context is not integrated into all surgical pathways. 2 Furthermore, few at‐risk patients receive nutrition therapy preoperatively. 2 , 3 Given that up to 65% of patients admitted to the hospital for surgical procedures present with malnutrition or are at nutrition risk, preoperative nutrition screening and intervention are needed to improve postoperative outcomes. 2 , 4 , 5 , 6 , 7 This narrative review focuses on nascent to novel methods to evaluate nutrition risk, status, and frailty in the surgical patient.
IMPORTANCE OF NUTRITION STATUS ON SURGICAL OUTCOMES
Preoperative nutrition status influences a patient's ability to tolerate surgical stress, rate of wound healing, postoperative physical recovery, length of hospital stay, risk of infection, risk of anemia, gastrointestinal transit time, skeletal muscle health, and overall risk of postoperative complications (Figure 1). 8 , 9 , 10 , 11 Delayed recovery induced by preoperative nutrition risk or malnutrition extends hospital length of stay and incurs added financial cost. 12 Poor nutrition status can adversely affect humoral and cell‐mediated immune responses, which, in turn, impair normal functioning of neutrophils and the ability of inflammatory cells to respond to infection. 8 The humoral agents further promote proinflammatory cytokine generation from the surgical site, which stimulates whole‐body protein catabolism via glycolysis and proteolysis. 13 These immune processes are sensitive to changes in nutrition status, and even a short period of protein‐energy malnutrition induces negative immunological changes in the surgical patient in a prolonged fasting or malnourished state. 8
Figure 1.
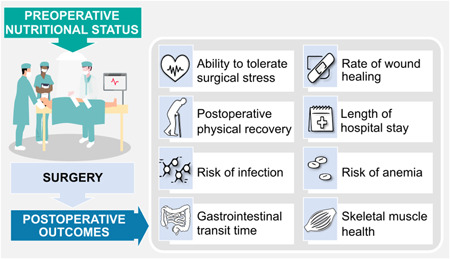
Graphic illustration of the effect of preoperative nutrition status on surgical outcomes. Please see supplementary material for an alternative version of the figure with North American spelling.
The need for optimal nutrition extends beyond the preoperative period and is a critical component of the perioperative care continuum. 14 Widespread integration of Enhanced Recovery After Surgery (ERAS) guidelines has increased awareness of the importance of preoperative nutrition optimization and has reduced the perioperative fasting time for the surgical patient through introduction of oral carbohydrate loading before surgery and early feeding after surgery in addition to other interventions to improve recovery. 15 , 16 These measures have improved postoperative outcomes, 17 but widespread dissemination and adoption of ERAS protocols and other nutrition‐focused protocols are needed to improve surgical outcomes, especially the preservation of muscle mass.
SURGICAL STRESS PROVOKES CATABOLISM, WHICH INFLUENCES POSTOPERATIVE RECOVERY
Preoperative nutrition status is especially important, as it represents the patient's baseline health status before the trauma introduced by surgical stress. 1 The homeostatic state responsible for maintaining body composition is disrupted by the metabolic response to surgery, which causes breakdown of fat and glycogen stores. 13 , 18 Contrary to starvation in the absence of inflammation, in which body fat is mobilized in response to prolonged negative energy balance, surgery induces an inflammatory response that instigates catabolism. 13 In a catabolic state, skeletal muscle is broken down and hepatic uptake of amino acids supports gluconeogenesis and synthesis of acute‐phase proteins. 13 , 19 The metabolic response in combination with the previously mentioned immunologic changes observed in the surgical patient results in loss of fat‐free mass, including skeletal muscle mass. 13 Furthermore, major surgery has also been shown to adversely affect muscle mitochondrial function, which can lead to muscle atrophy. 20
Skeletal muscle is a metabolic organ that accounts for approximately 40% of body mass and serves several functions, including housing the body's largest amino acid reserve, synthesizing and storing glutamine, regulating blood glucose concentrations, and producing myokines. 21 More commonly, skeletal muscle is recognized for its structural functions, including movement, balance, posture, and bodily strength. In a homeostatic state, muscle mass is tightly regulated through muscle protein turnover, but in times of starvation or inadequate protein intake, muscle protein synthesis is downregulated and autophagy pathways upregulated to self‐sacrifice amino acid reserves. 22 , 23 Notably, a patient's preoperative nutrition status determines how acutely critical the catabolic response is (Figure 2). 24 The inflammatory response to surgery and absence of protein and energy intake (common with traditional care) foster the perfect storm for muscle breakdown/loss. This is particularly problematic for older adults and those with preexisting malnutrition or low muscle mass.
Figure 2.
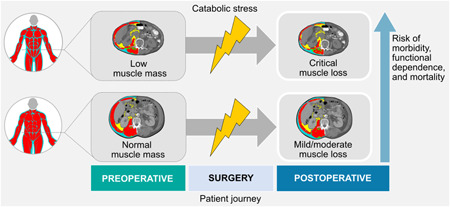
Graphic representation depicting how a patient's preoperative nutrition status determines how critical the catabolic response is. The catabolic stress response is associated with muscle loss (and likely weight loss). However, patients who already have low muscle mass are at greater risk of depleting already compromised reserves, which increases the risk of unfavorable outcomes. Computerized tomography images were used as an example of a method to evaluate changes in body composition (third lumbar vertebra, patients with normal body weight before surgery).
Low muscle mass is a diagnostic criterion for malnutrition. 25 Thus, these conditions are often observed concurrently and share several consequences, including postoperative complications, the need for rehabilitation, increased time to wound healing, greater length of hospital stay, falls and fractures, physical impairment, oncologic treatment toxicity, shorter survival, and poorer quality of life. 26 , 27 The negative impacts incurred from low muscle mass and/or malnutrition can all lead to further health consequences, many of which also promote additional muscle loss and impact functional recovery postoperatively. The interplay between malnutrition, sarcopenia (low muscle mass and function), frailty, and cachexia is a continuum of progressive physiological decline 28 that surgical patients, especially older adults, are at risk of. Therefore, early and continued nutrition assessment and intervention are needed to avoid negative outcomes, including the rapid loss of muscle, in the perioperative period, which can be compared with a wildfire—preventing or ameliorating nutrition decline is better than reversing it. 29
ASSESSING MALNUTRITION: A FOCUS ON NOVEL AND NASCENT METHODS
The first step in assessing malnutrition is nutrition screening. As summarized elsewhere 30 and in Figure 3, screening for nutrition risk can be done using quick and simple methods to assess those at immediate risk for malnutrition. Any frontline personnel can perform nutrition screening. 31 , 32 Nutrition assessment, however, is a more complex and resource‐intensive process, requiring a dietitian/nutrition professional to conduct a detailed evaluation of nutrition status (Figure 4). This step allows for a comprehensive assessment, which will inform the remainder of the nutrition care process (ie, diagnosis, intervention, monitoring, and evaluation). Nutrition assessment can be done using a variety of approaches, such as anthropometric and body composition measurements; food and nutrition‐related history; clinical signs; biochemical data; medical tests, procedures, and diagnosis; and functional assessment.
Figure 3.
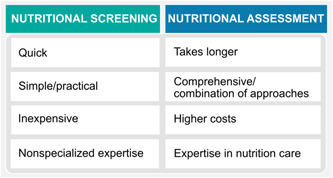
Summary of selected differences between nutrition screening vs assessment. Nutrition assessment can be done using a variety of approaches, such as anthropometric and body composition measurements, food‐ and nutrition‐related history, clinical signs, biochemical data, medical tests, procedures and diagnosis, and functional assessment. Please see supplementary material for an alternative version of the figure with North American spelling.
Figure 4.
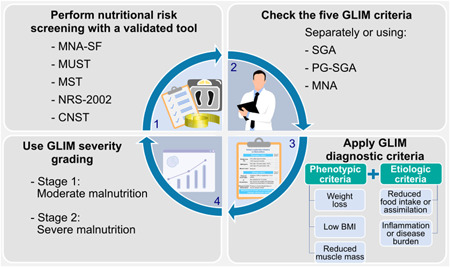
Overview of Global Leadership Initiative on Malnutrition (GLIM) criteria. GLIM is an effort to adopt a global consensus on criteria for malnutrition diagnosis. It does not exclude the use of other nutrition assessment tools to guide individualized care and treatment. As such, GLIM is to be used alongside nutrition screening and assessment. Phenotypic and etiologic criteria were derived from commonly used nutrition screening and assessment tools. Malnutrition diagnosis is based on the identification of one phenotypic and one etiologic criterion. When present, severity of malnutrition is then determined. 35 , 36 BMI, body mass index; CNST, Canadian Nutrition Screening Tool; MNA, Mini Nutritional Assessment; MNA‐SF, Mini Nutritional Assessment—Short‐Form; MST, Malnutrition Screening Tool; MUST, Malnutrition Universal Screening Tool; NRS‐2002, Nutritional Risk Screening‐2002; PG‐SGA, Patient‐Generated Subjective Global Assessment; SGA, Subjective Global Assessment. Please see supplementary material for an alternative version of the figure with North American spelling.
A summary of commonly used nutrition screening and assessment tools to evaluate malnutrition in surgical settings is available elsewhere. 31 Screening/assessment efforts should start at the community level with primary care physicians and healthcare professionals, as preexisting malnutrition and frailty are related to its prevalence in the community. As much as possible, these conditions should be identified and treated before patients need to be admitted to the hospital.
With regard to novel/nascent methods, a targeted perioperative nutrition screening tool, the Perioperative Nutrition Score, has been developed via an international guideline process for perioperative nutrition screening and has demonstrated predictive validity. 2 , 33 This score was devised to be simple to incorporate into the electronic medical record and can be used to rapidly screen for perioperative malnutrition risk. Nonetheless, further studies are needed to compare its performance against validated tools.
In view of the COVID‐19 pandemic, a simple remote nutrition screening tool called the R‐MAPP (Remote – Malnutrition APP) has been made available for primary care practice. 34 The tool involves the use of the Malnutrition Universal Screening Tool (“MUST”) and SARC‐F (five‐item questionnaire: strength, assistance with walking, rise from a chair, climb stairs, and falls), which are simple and validated clinical tools to identify nutrition and sarcopenia risk (SARC‐F being appropriate for older adults).
Another relatively recent advance in diagnosing malnutrition is the development of the Global Leadership Initiative for Malnutrition (GLIM) criteria. 35 , 36 In a two‐step approach, nutrition risk is evaluated, followed by diagnosis, which includes classifying the severity of malnutrition (Figure 4). 35 , 36 A lack of coordinated malnutrition assessment and reporting has made clinically relevant comparisons between trials and the pooling of results for meta‐analyses difficult. As such, use of the GLIM criteria in surgical settings is strongly encouraged to help standardize the diagnosis of malnutrition in clinical practice, as well as the possibility of systematically evaluating malnutrition research worldwide. Notably, GLIM can be applied by any healthcare professional. In settings where a screening/assessment of malnutrition is already collected, information from these tools can be used to conduct the GLIM assessment (eg, Subjective Global Assessment, Patient‐Generated Subjective Global Assessment, Mini Nutritional Assessment) (Figure 4). As such, and importantly, GLIM criteria are an additional approach to be used alongside nutrition screening and assessment tools and are not meant to replace these steps.
The GLIM criteria
The use of the GLIM criteria has increased exponentially since publication, 37 including in surgical settings. 38 , 39 , 40 , 41 , 42 Fiorindi et al. 41 applied the GLIM criteria in a pilot study of patients with inflammatory bowel disease undergoing surgery; patients were evaluated as having Crohn's disease or ulcerative colitis. Forty‐two percent were malnourished according to GLIM, which was higher than the prevalence identified by the other tools. 41 Previous surgery for inflammatory bowel disease, disease recurrence, and presence of ileostomy were factors that most predisposed patients to malnutrition. Kakavas et al. 38 explored the ability of the GLIM criteria to predict postoperative pulmonary complications after abdominal surgery in patients with cancer. In this study, 70% of patients were identified as malnourished. The risk for postoperative pulmonary complications was almost two times higher in malnourished patients than in well‐nourished patients (relative risk [RR] = 1.82; 95% confidence interval [CI], 1.21−2.73); likewise, the risk for 90‐day all‐cause mortality was almost twice as high in those classified as severely malnourished (RR = 1.97; 95% CI, 1.28−2.63).
The GLIM criteria were applied by Boslooper‐Meulenbelt et al. 39 in renal transplant recipients; 14% were malnourished in spite of being stable outpatients. Of these, 91% met the phenotypic criterion of reduced muscle mass within the GLIM framework. Using the GLIM criteria, Emsley et al. 40 showed that 59% of patients who received a lung transplant presented with malnutrition. Similarly, Boslooper‐Meulenbelt et al. 39 showed that a greater proportion of patients were diagnosed as malnourished because of the phenotypic GLIM criterion of reduced muscle mass (47% of all patients), compared with other approaches used in the study to define malnutrition.
Collectively, these findings suggest that GLIM criteria are less conservative, identifying a greater number of patients with malnutrition. This may be explained by the use of body composition (ie, reduced muscle mass) as a phenotypic criterion in GLIM, as this could be undetected by other diagnostic tools. The GLIM guidance for the assessment of the muscle mass phenotypic criterion further promotes the use of this approach. 43 , 44 This publication provides consensus‐based guidance on the assessment of skeletal muscle mass using a variety of tools and techniques. 43 , 44
Laboratory markers
A position paper from the American Society for Parenteral and Enteral Nutrition (ASPEN) provided guidance on the use of visceral proteins as nutrition markers. 45 Serum albumin has been historically considered a marker of surgical risk. However, the authors highlighted that serum albumin and prealbumin (transthyretin) concentrations are not components of updated malnutrition definitions, nor should they be used as a proxy to measure total body protein or muscle mass. 45 Although serum albumin and prealbumin are not nutrition markers, their assessment could be useful as prognostic markers.
A recent study in medical inpatients at nutrition risk suggested that low concentrations of serum albumin at admission had prognostic implications and indicated higher mortality risk. However, this marker was not helpful in selecting patients for nutrition interventions, nor did a change in serum albumin concentration predict a response to nutrition therapy. 46 As such, whether serum albumin and prealbumin concentrations can be used to monitor the delivery and efficacy of nutrition intervention is unclear, as serum albumin concentration may only improve when other inflammatory markers are stable. 45 Improved serum albumin concentrations in a patient's clinical evaluation may indicate a number of possible scenarios such as improved nutrition status (reduced nutrition risk), reduced inflammation, correction of fluid shifts, transition to anabolism, and changes in energy and protein needs. 45 However, in the short‐term, serum concentrations are more affected by inflammation and fluid balance than by malnutrition or nutrition repletion.
Muscle mass
As mentioned earlier, low muscle mass is prevalent in and significant to the surgical patient. Several tools can be used to assess muscle mass or its related compartments (fat‐free mass, lean soft tissue, terminology fully discussed elsewhere 47 ) (Figure 5). Body composition assessment is fundamental for the identification of hidden muscle abnormalities (Figure 6) and, hence, nutrition status. Low muscle mass can occur in spite of adequate, excessive, or stable body weight (ie, weight stability may mask unfavorable shifts in body composition). 31 , 48 , 49
Figure 5.
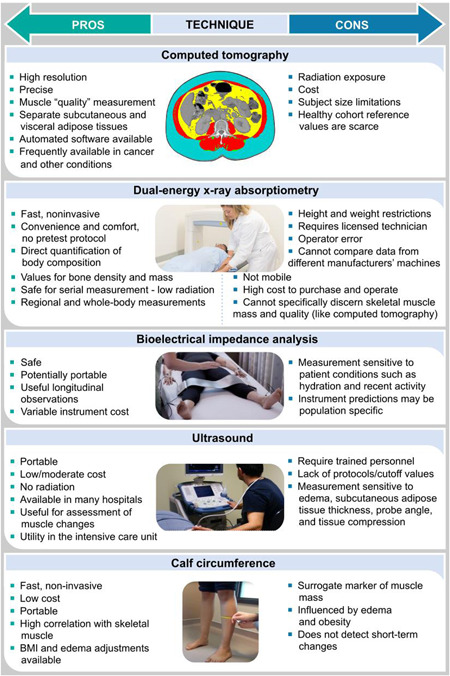
Summary of pros and cons of commonly used anthropometric and body composition approaches to estimate/measure muscle mass. BMI, body mass index.
Figure 6.
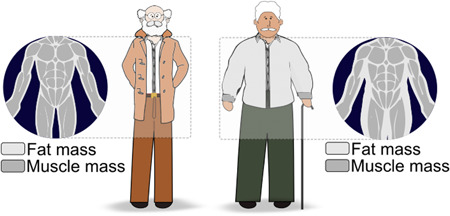
Graphic representation depicting hidden abnormalities in body composition. Low muscle mass may be hidden in individuals with normal body weight and those living with larger body sizes.
When body composition assessment is not available, anthropometry can be used as a surrogate tool. The most recent advancement in the field relates to the measurement of calf circumference. Calf circumference is widely used as a marker of muscle mass in clinical practice, as it is highly correlated with skeletal muscle mass, especially in the context of sarcopenia assessment in the older adult 50 ; it is a simple, accessible, and low‐cost approach. Whereas this measurement by itself is not novel, recently published adjustment factors allow for the elimination of the confounding effects of obesity, therefore improving the use of calf circumference as a marker of low muscle mass. 51 This is important because of the high prevalence of obesity and the fact that excess subcutaneous adipose tissue provides unreliable findings, as it is very difficult to identify low calf circumference in patients with excess body weight. Using a simple approach derived from a population‐representative cohort of adults of all ages, Gonzalez et al. 51 proposed body mass index (BMI)–related adjustment factors. After measuring calf circumference in patients with a BMI beyond the reference range (18.5–24.9 kg/m2), 3, 7, and 12 cm for patients within the overweight, obesity class I, and obesity class II categories, respectively, should be subtracted from the measured calf circumference. After that, the adjusted value can be compared with reference standards. The authors 51 proposed <34 cm for men and <33 cm for women as markers of moderately low calf circumference, and <32 cm for men and <31 cm for women as markers of severely low calf circumference. These were values for White individuals, with small differences in cutoff values by ethnicity (decimal places). For individuals with a low BMI (<18.5 kg/m2), an addition of 4 cm (to the measured value) is suggested. However, most of the participants from that sample were young and healthy, contrary to what is commonly observed among surgical patients. As such, adjustments in patients with low BMI (<18.5 kg/m2) may not be required in clinical settings, and the actual measured calf circumference value should be used (ie, without adjustment) to compare with reference values in this population. Therefore, adjustment factors would only need to be applied for surgical patients with a BMI of ≥25 kg/m2. Of note, correction values for edema have also been proposed by Ishida et al, 52 which include subtracting 2 cm for men and 1.6 cm for women from the measured calf circumference value when edema is present.
Computerized tomography
The use of computerized tomography (CT) images for muscle mass assessment has transformed the importance of body composition in clinical settings, including the surgical patients. Low muscle mass has been associated with surgical complications, increased length of hospital stay, greater need for rehabilitation, and poorer survival, among others. 48 , 53 In addition to muscle mass, muscle radiodensity is reflective of fat infiltration into muscle, or myosteatosis, and is an emerging powerful predictor of patient outcomes. 54 , 55 , 56
Muscle radiodensity has been less studied in the surgical context. Studies exploring myosteatosis in the surgical patient suggest a strong association with postoperative morbidity and mortality. 48 , 55 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 Xiao et al. 48 explored the significance of myosteatosis in patients undergoing colonic resection, using a large database from an integrated healthcare system. Patients with myosteatosis were more likely to remain hospitalized 7 days or longer after surgery, had higher risk of overall mortality, and had higher odds of developing major complications (Figure 7A and B). Murnane et al. 55 studied patients undergoing radical surgery for esophageal and gastric cancer. More than half (56%) of patients had myosteatosis, and this was associated with higher rates of anastomotic leaks. Furthermore, they showed that myosteatosis was an independent predictor of overall and severe complications 55 (Figure 7A). Reduced disease‐free survival was also observed for patients with myosteatosis compared with those without myosteatosis (55.2% vs 87.2%, respectively; P = 0.007). 55
Figure 7.
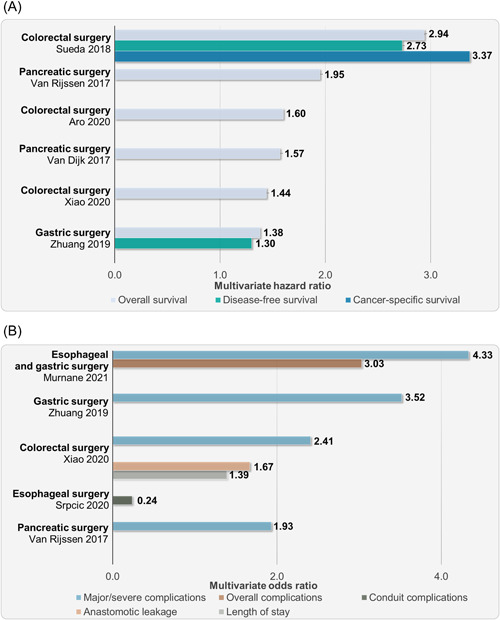
(A) Multivariate odds ratio from studies exploring the association of myosteatosis with poor clinical outcomes (excluding survival) in the surgical context. (B) Multivariate hazard ratio for shorter survival related to myosteatosis from various surgical studies. All studies compared patients with myosteatosis against patients without myosteatosis (reference; odds ratio or hazard ratio: 1). All values presented are significant (P < 0.05). Conduit complications defined in the cited study as clinically silent fistulae, clinically important leaks that required interventions, and frank gastric necroses.
Myosteatosis has been associated with poor survival and surgical complications in other surgical contexts, including pancreatic, gastric, and colorectal surgery, as shown in Figure 7A and B and described by Murnane et al. 55 In patients undergoing pancreaticoduodenectomy, myosteatosis was associated with an increased rate of major complications (P = 0.035) but was not associated with disease‐free or overall survival. 65 However, low muscle radiodensity analyzed as a continuous variable was associated with survival. 65 Notably, the combined phenotype of myosteatosis and low muscle mass has been associated with worse clinical outcomes, as shown previously. 48 , 66
From a methodological perspective, standardized CT protocols, with intravenous contrast in the portal venous phase, are required to ensure that measures of skeletal muscle radiodensity are consistent and comparable between CT images. 67 Contrast enhancement, and the phase of the scan, can significantly influence skeletal muscle density values. On a related note, the use of single‐muscle approaches when evaluating CT images is discouraged and may lead to underestimation of the prevalence of myoesteatosis. 68
The use of CT imaging for body composition analysis is of special importance in surgical oncology, as these images are readily available from medical records of most patients, acquired for the original purpose of cancer diagnosis and surveillance. 47 , 69 However, prospective, single images can be obtained for the purpose of body composition analysis, to minimize radiation exposure. In fact, we are aware of single‐slice CT imaging being specifically acquired in routine clinical practice (coding, billing, and reporting in medical record) in some centers worldwide (including Harvard hospitals [P. Wischmeyer, MD, personal communication]). This technique takes minimal time to perform and exposes patients to less radiation than a chest x‐ray.
Importantly, automated segmentation is modernizing the use of CT images in clinical settings, providing a time‐efficient, clinic‐friendly, and accurate assessment of muscle and adipose tissues. 70 Cespedes‐Feliciano et al. 70 and Beetz et al. 71 mentioned several publications exploring automated and semiautomated software for CT‐derived body composition analysis and the need for their evaluation in large patient data sets. 70 Cespedes‐Feliciano et al. 70 evaluated a commercially available semiautomated software and compared it with manual segmentation in nearly 6000 images of patients with breast or colorectal cancer. Average Jaccard scores (Jaccard similarity coefficient or intersection over union score) and intraclass correlation coefficients exceeded 90%, with only 1%–2% underestimation for muscle mass. 70
The first picture archiving and communication system (PACS)–integrated artificial intelligence–based software has been developed. 71 This approach eliminates the need for manual identification of the landmark of interest for muscle mass analysis (eg, the third lumbar vertebra [L3]), facilitating the process of automated segmentation that was lacking from previously available software and semiautomated programs. As such, the software eliminates the need to anonymize DICOM images during the extraction process from PACS to a second computer where body composition analysis would take place. Time and effort are substantially reduced when automation is used. 71 Machine learning algorithms have been used to automatically detect the L3 vertebra. Using this approach, Kim et al. 72 selected preoperative CT images for body composition analysis in patients with gastric cancer receiving gastrectomy and reported an association between abnormal body composition and long‐term survival.
Finally, a new approach is the fully automated, multiple‐tissue, multiple‐organ, three‐dimensional segmentation and assessment by a commercially available software (Figure 8). 73 In this software program that runs locally on a desktop or laptop computer, all available cross‐sectional axial slices obtained from a CT image are analyzed and segmented, and each slice is annotated by its vertebral level. A complete automated analysis of the volume of all body composition components such as skeletal muscle, visceral adipose tissue, subcutaneous adipose tissue, and intramuscular adipose tissue by each slice is completed. Thus, organs such as liver and spleen can also be quantified. Machine learning algorithms can be used subsequently to predict individual patient outcomes. Not only is this revolutionizing the assessment of body composition beyond one cross‐sectional area and specific compartments (ie, skeletal muscle), but it also makes the use of this technique an accessible and rapid approach for implementation and integration into clinical practice. Consequently, this can substantially accelerate the use of CT body composition imaging for clinical use, including in surgical and nutrition planning. Examples of open‐source software for manual and/or semiautomated segmentation include Horos (Horos Project), 3D Slicer (3D Slicer project), ImageJ (National Institutes of Health), and CoreSlicer and procedures for image analysis using selected software available elsewhere. 74 , 75 , 76
Figure 8.
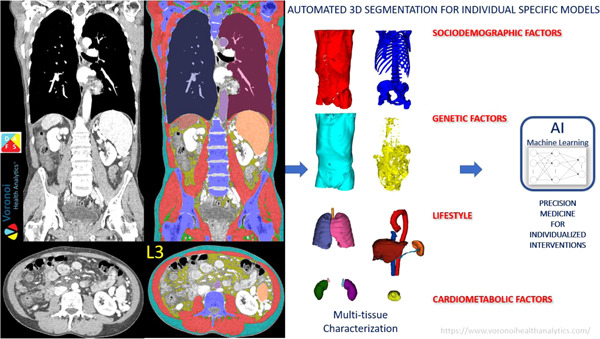
Three‐dimensional, fully automated body composition imaging analysis from computerized tomography (CT) using DAFS—the data analysis facilitation suite by Voronoi Health Analytics Inc, Canada. 73 DAFS can segment any field of view of CT images from head to toe from contrast and noncontrast images and low‐dose and conventional‐dose images in both adults and children. The software is fully automated and provides detailed quality assessment interfaces. AI, artificial intelligence; Adapted with permission from Voronoi Health Analytics Inc, Canada.
Bioelectrical impedance analysis—Phase angle
Phase angle is derived from raw measurements of resistance and reactance obtained from bioelectrical impedance analysis (BIA). It is a measure of the shift that occurs when the electrical current passes through the cell membrane; the shift is smaller in “sick” cell membranes because of a delay in current transmission, leading to a lower phase angle. Therefore, phase angle is reflective of cell membrane integrity, intracellular and extracellular water balance, and consequently, overall health status. Although raw values are most often used, standardized values considering sex, age, and BMI can also be used. 77
Although phase angle has long been recognized as an important and independent predictor of postoperative complications, 78 it has not been widely used in the context of postoperative morbidity in the surgical setting. 79 However, the growing availability of BIA machines providing this measurement and/or raw values for its calculation has revitalized the importance of phase angle.
Phase angle has been more recently associated as a marker of muscle mass and function. 80 These may be due to changes in muscle size, density, architecture, fiber types, mitochondrial function, and abnormal hydration shifts, among others. 80 Its sensitivity to capture changes over a short period of time 81 is especially attractive and should be explored in interventional studies in surgical settings.
The use of phase angle to predict perioperative risk in patients undergoing surgery for cancer has been reviewed by Matthews et al. 79 Twelve studies were included in their analysis that concluded that BIA may be used in the perioperative period to predict risk of complications following elective surgery for cancer. Notably, phase angle was the BIA measurement more consistently associated with predicting outcomes than derived BIA estimates. This could be related to phase angle being a more direct biomarker (ie, raw vs predicted measurement) and more reflective of cell and likely muscle health (including quality). The authors hypothesized that phase angle may be identifying nonobvious cases of malnutrition in which metabolic derangement is present.
Newer studies have explored phase angle in surgical settings. Petrolo et al. 82 used bioimpedance spectroscopy to measure phase angle in a small sample of patients after pancreaticoduodenectomy or total pancreatectomy. They observed a significant reduction in phase angle in early postoperative days, suggestive of cell loss or reduced cell integrity. The concurrent decrease in extracellular water was suggestive of an increase in extracellular volume related to a decrease in body cell mass. 82
In patients undergoing cardiac surgery, low preoperative phase angle was an indicator of poor nutrition status and was associated with higher rates and risk of postoperative morbidity (odds ratio [OR] = 2.50; 95% CI, 1.18–5.29; P = 0.016) after cardiac surgery. Researchers also observed a tendency toward longer length of hospital stay (>14 days) for patients with low phase angle. 83 Previous studies have found similar associations of phase angle as a predictor of in‐hospital mortality (hazard ratio = 1.3; 95% CI, 1.07–1.40; P = 0.003) in patients undergoing cardiac surgery. 84
Ultrasound
Ultrasound is an up‐and‐coming technique with important advantages (Figure 5) for use in surgical settings. The availability of pocket‐size devices is perhaps the most notable advancement of this method. In a small study in healthy participants, measurements of muscle thickness and architecture were similar between standard and pocket‐size ultrasound. 85
Ultrasound has been used in the surgical setting. Bury et al. 86 explored its use to assess short‐term substantial changes in quadriceps muscle layer thickness in critically ill surgical patients. In a study including patients in the surgical and medical ward, a single ultrasound measuring point at the thigh (ie, muscle thickness) plus sex, weight, and height were used in a model to predict CT‐assessed muscle mass (at the L3 level). 87
Notably, although there is a growing interest in using ultrasound as an indirect marker of muscle glycogen concentration (from echogenicity), its validity is questionable. We refer the reader to another publication discussing this issue. 88 Ultrasound echogenicity can nonetheless be used as a surrogate for muscle quality surrogate, similarly to CT radiodensity. 89
Finally, protocols are evolving and so is our understanding of assessment and analytical approaches of this technique. Recent evidence highlights the need for establishing the number and location of measurement sites, and the development of body composition predictive equations, which may be able to bypass the lack of normative data. 90
Additional methods
Additional body composition techniques can be explored for body composition assessment during the perioperative period. For example, most clinical centers have a dual‐energy X‐ray absorptiometry (DXA) scan available for the assessment of bone health (osteoporosis). This can be used for additional assessment of other body composition compartments, including appendicular lean soft tissue (often called appendicular skeletal muscle mass). DXA has also been used in different surgical contexts. 91 , 92 , 93 , 94
The use of D3 creatine dilution 95 can also be explored in these patients, although this method is currently more feasible for research settings owing to the need for isotope data analysis. As such, its relevance and practicality in clinical settings remain to be determined. 96
Finally, magnetic resonance imaging (MRI) is making its way to clinical use on a fee‐for‐service, private‐use basis in selected countries. Rapid, whole‐body, MRI‐based body composition profiling is now available at selected diagnostic facilities in North America and Europe. Quantification of skeletal muscle volume and fat infiltration is possible (in addition to liver fat fraction, as well as visceral and abdominal subcutaneous adipose tissue volumes, Figure 9A). The process involves a specific MR scanning protocol (typical 8 min of scanning time). Thereafter, the acquired images are transferred to a central location using a secure cloud‐based service, in which automated body composition profiling is conducted using a proprietary approach 97 ; the generated body composition profile report is subsequently sent back to the diagnostic imaging facility with the body composition measurements (Figure 9B). The personalized report given to the customer provides information on whether individuals have low or high amounts of the specific body composition compartment, as well as a personalized control group to put the target patient into context of someone with a similar BMI, with references based on the UK Biobank MRI sex‐specific reference database (Figure 9A and B). In addition to research settings, these images can be potentially used in clinical care, although affordability and accessibility are limitations to be considered. Notably, research protocols and analyses with a greater number of body composition variables are also available (Figure 9C). High reproducibility and precision have been reported, 98 as well as clinically relevant outcomes 97 and magnitudes of exercise‐induced increases in muscle volume and reduction of fat infiltration after an 8‐week resistance‐training program. 99
Figure 9.
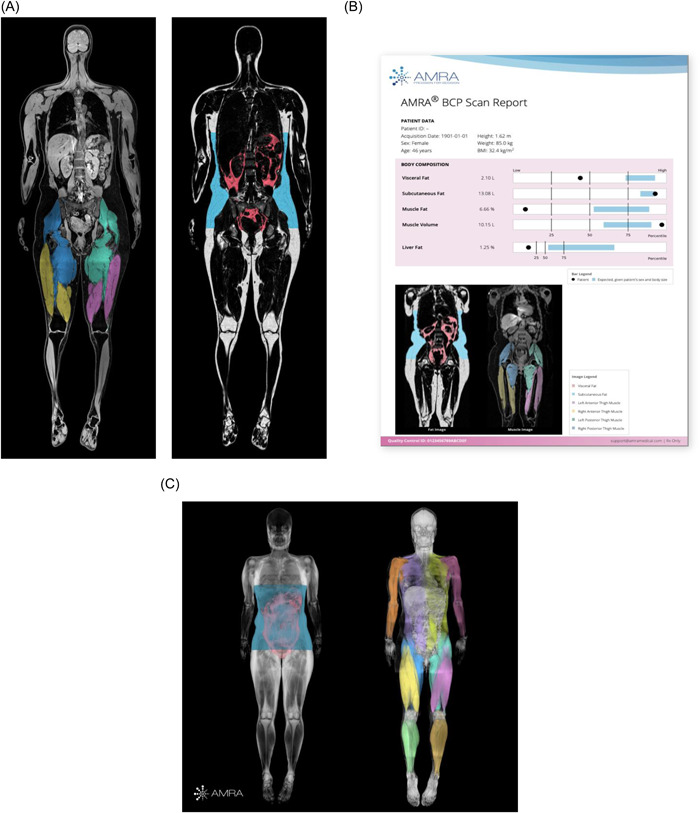
Magnetic resonance imaging (MRI) analysis from AMRA Medical. (A) Whole‐body images separated by water (left) and adipose tissue (right), based on MRI processed and analyzed using AMRA Medical machine learning and automation methods; available at selected diagnostic imaging facilities. (B) AMRA BCP Scan (Body Composition Profile) sample report with body composition measurements quantified and compared with reference values and a personalized control group (blue field in the bar plots). (C) Sample adipose tissue– and water‐separated MR images for research from AMRA Medical. Adapted with permission from AMRA Medical.
Frailty
Frailty is a state of systemic increased vulnerability across multiple organ systems that compromises the ability to respond to stressors. 24 , 100 Assessment of frailty is part of the preoperative care of older surgical patients and an important predictor of morbidity, mortality, or new disability after surgery. 101 , 102 A preoperative diagnosis of frailty can determine the cost/benefit of surgery, long‐term independence and productivity, and the value of undertaking targeted interventions to reduce morbidity and mortality. 100
Commonly used frailty assessment tools have been discussed elsewhere. 28 In the surgical setting, a variety of tools has been used, such as the modified 5‐Item Frailty Index (mFI‐5), 103 the Geriatric Nutritional Risk Index, 104 the Rockwood Clinical Frailty Scale, 105 the Edmonton Frail Scale, 106 and the Fried frailty criteria. 107 In regard to screening tools, newer, easy‐to‐use instruments such as the Sunfrail (http://www.sunfrail.eu/), consistent with the biopsychosocial model, is now available, 108 although its use in surgery is yet to be explored. Practical and valid approaches can facilitate the screening of frailty, identifying the need to observe individuals using the Comprehensive Geriatric Assessment. 108
Ligthart‐Melis et al. 7 discussed the prevalence of prefrailty, frailty, sarcopenia, risk of malnutrition, and malnutrition in older hospitalized patients, which included surgical patients, using a systematic review/meta‐analysis approach. Among the 10 included studies, they reported a higher prevalence of frailty (71%) compared with commonly observed prevalence in community‐dwelling older individuals. The ORs between and the overlapping prevalence of prefrailty and (risk of) malnutrition were 5.8% and 50%, respectively. 7
The Society for Perioperative Assessment and Quality Improvement (SPAQI) proposed practical steps for clinicians to assess and address frailty in older patients who require elective intermediate‐ or high‐risk surgery. 109 In their workflow diagram, screening for frailty may lead to the identification of high‐risk patients, which should subsequently undergo frailty assessment using the Comprehensive Geriatric Assessment tool. Screening and assessment should occur days to weeks before surgery and so should the intervention for those diagnosed with frailty. The proposed intervention pathway highlights the importance of continuing care weeks after surgery (8 weeks), which involves nutrition, physical exercise, and psychological intervention. This is in line with the wildfire analogy 29 mentioned earlier: early and continued assessment and intervention are critical for these patients.
CONCLUSIONS
Evaluating nutrition risk and status is essential to predict and mitigate surgical outcomes. Novel to nascent approaches are the future of diagnosing perioperative malnutrition objectively and guiding perioperative therapy toward optimal standard of care.
Early and continued nutrition and frailty screening and assessment are encouraged to better identify patients who are at extremely high surgical risk. Widespread implementation of malnutrition and frailty screening/assessment will, therefore, help with the shared decision‐making process. These assessments are especially informative in cases when surgery may cause more harm than benefit, such as higher than expected morbidity, mortality, prolonged recovery process, and the unlikelihood of returning to self‐care or near‐normal activities. Furthermore, the assessment of nutrition status can lead to targeted interventions that may improve short‐ and long‐term patient outcomes, including mitigating muscle loss.
AUTHOR CONTRIBUTIONS
Carla M. Prado contributed to the conception and design of the research. Katherine L. Ford, Maria C. Gonzalez, Lisa C. Murnane, Chelsia Gillis, Paul E. Wischmeyer, Chet A. Morrison, and Dileep N. Lobo contributed to the design of the research. Carla M. Prado, Katherine L. Ford, Maria C. Gonzalez, Lisa C. Murnane, Chelsia Gillis, Paul E. Wischmeyer, Chet A. Morrison, and Dileep N. Lobo contributed to the acquisition of the data. Carla M. Prado, Katherine L. Ford, Maria C. Gonzalez, Lisa C. Murnane, Chelsia Gillis, Paul E. Wischmeyer, Chet A. Morrison, and Dileep N. Lobo contributed to the interpretation of the data. Carla M. Prado, Katherine L. Ford, Maria C. Gonzalez, Lisa C. Murnane, Chelsia Gillis, Paul E. Wischmeyer, Chet A. Morrison, and Dileep N. Lobo drafted the manuscript. All authors critically revised the manuscript, agree to be fully accountable for ensuring the integrity and accuracy of the work, and read and approved the final manuscript.
CONFLICT OF INTERESTS
Carla M. Prado reports receiving honoraria and/or paid consultancy from Abbott Nutrition, Nutricia, Nestlé Health Science, Fresenius Kabi, Pfizer, and AMRA Medical. Maria C. Gonzalez reports receiving honoraria for invited educational talks from Abbott Nutrition and Nestlé Health Science. Lisa C. Murnane has received honorarium from Fresenius Kabi and Nestlé Health Science, outside of the submitted work. Chelsia Gillis reports receiving honoraria for invited educational talks from Abbott Nutrition, Nestlé Health Science, and Fresenius Kabi. Paul E. Wischmeyer reports receiving investigator‐initiated grant funding from National Institutes of Health, Department of Defense, Canadian Institutes of Health Research, Abbott Nutrition, Baxter, Cardinal Health, and Fresenius. Paul E. Wischmeyer has served as a consultant to Abbott Nutrition, Fresenius, Baxter, Cardinal Health, and Nutricia, for research related to this work. Paul E. Wischmeyer has received unrestricted gift donations for nutrition research from Musclesound and DSM. Paul E. Wischmeyer has received honoraria or travel expenses for CME lectures on improving nutrition care from Abbott Nutrition, Baxter, Danone‐Nutricia, and Nestlé. Katherine L. Ford, Chet A. Morrison, and Dileep N. Lobo have no conflict of interests to declare. The content of this article was presented during the course, Comprehensive Nutrition Therapy: Tactical Approaches in 2022 (March 25, 2022), which was organized by the ASPEN Physician Engagement Committee and preceded the ASPEN 2022 Nutrition Science & Practice Conference. The author(s) received a modest monetary honorarium. The conference recordings were posted to the ASPEN eLearning Center https://aspen.digitellinc.com/aspen/store/6/index/6.
Supporting information
Supplementary information.
Prado CM, Ford KL, Gonzalez MC, et al. Nascent to novel methods to evaluate malnutrition and frailty in the surgical patient. J Parenter Enteral Nutr. 2023;47:S54‐S68. 10.1002/jpen.2420
Contributor Information
Carla M. Prado, Email: carla.prado@ualberta.ca.
M. Cristina Gonzalez, Email: cristina.gonzalez@ucepl.edu.br.
REFERENCES
- 1. Ford KL, Prado CM, Weimann A, Schuetz P, Lobo DN. Unresolved issues in perioperative nutrition: a narrative review. Clin Nutr. 2022;41(7):1578‐1590. [DOI] [PubMed] [Google Scholar]
- 2. Wischmeyer PE, Carli F, Evans DC, et al. American Society for enhanced recovery and perioperative quality initiative joint consensus statement on nutrition screening and therapy within a surgical enhanced recovery pathway. Anesth Analg. 2018;12(6):1883‐1895. [DOI] [PubMed] [Google Scholar]
- 3. Williams DGA, Ohnuma T, Krishnamoorthy V, et al. Postoperative utilization of oral nutrition supplements in surgical patients in us hospitals. JPEN J Parenter Enteral Nutr. 2021;45(3):596‐606. [DOI] [PubMed] [Google Scholar]
- 4. Bozzetti F, Gianotti L, Braga M, Di Carlo V, Mariani L. Postoperative complications in gastrointestinal cancer patients: the joint role of the nutritional status and the nutritional support. Clin Nutr. 2007;26(6):698‐709. [DOI] [PubMed] [Google Scholar]
- 5. Geurden B, Franck E, Weyler J, Ysebaert D. The risk of malnutrition in community‐living elderly on admission to hospital for major surgery. Acta Chirurgica Belgica. 2015;11(5):341‐347. [DOI] [PubMed] [Google Scholar]
- 6. Thomas MN, Kufeldt J, Kisser U, et al. Effects of malnutrition on complication rates, length of hospital stay, and revenue in elective surgical patients in the G‐DRG‐system. Nutrition. 2016;3(2):249‐254. [DOI] [PubMed] [Google Scholar]
- 7. Ligthart‐Melis GC, Luiking YC, Kakourou A, Cederholm T, Maier AB, De Van Der Schueren MAE. Frailty, sarcopenia, and malnutrition frequently (co‐)occur in hospitalized older adults: a systematic review and meta‐analysis. J Am Med Dir Accoc. 2020;21(9):1216‐1228. [DOI] [PubMed] [Google Scholar]
- 8. Moon M‐S, Kim S‐S, Lee S‐Y, et al. Preoperative nutritional status of the surgical patients in Jeju. Clin Orthop Surg. 2014;6(3):350‐357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wolf JH, Ahuja V, D'Adamo CR, Coleman J, Katlic M, Blumberg D. Preoperative nutritional status predicts major morbidity after primary rectal cancer resection. J Surg Res. 2020;255:325‐331. [DOI] [PubMed] [Google Scholar]
- 10. Kim E, Lee D‐H, Jang J‐Y. Effects of preoperative malnutrition on postoperative surgical outcomes and quality of life of elderly patients with periampullary neoplasms: a single‐center prospective cohort study. Gut Liver. 2019;13(6):690‐697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yamamoto T, Shimoyama T, Umegae S, Kotze PG. Impact of preoperative nutritional status on the incidence rate of surgical complications in patients with inflammatory bowel disease with vs without preoperative biologic therapy: a case‐control study. Clin Transl Gastroenterol. 2019;10(6):e00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Curtis LJ, Bernier P, Jeejeebhoy K, et al. Costs of hospital malnutrition. Clin Nutr. 2017;36(5):1391‐1396. [DOI] [PubMed] [Google Scholar]
- 13. Gillis C, Carli F. Promoting perioperative metabolic and nutritional care. Anesthesiology. 2015;123(6):1455‐1472. [DOI] [PubMed] [Google Scholar]
- 14. Lobo DN, Gianotti L, Adiamah A, et al. Perioperative nutrition: recommendations from the ESPEN expert group. Clin Nutr. 2020;39(11):3211‐3227. [DOI] [PubMed] [Google Scholar]
- 15. Weimann A, Braga M, Carli F, et al. ESPEN practical guideline: clinical nutrition in surgery. Clin Nutr. 2021;40(7):4745‐4761. [DOI] [PubMed] [Google Scholar]
- 16. Gustafsson UO, Scott MJ, Hubner M, et al. Guidelines for perioperative care in elective colorectal surgery: Enhanced Recovery After Surgery (ERAS®) society recommendations: 2018. World J Surg. 2019;43(3):659‐695. [DOI] [PubMed] [Google Scholar]
- 17. Martin L, Gillis C, Atkins M, et al. Implementation of an Enhanced Recovery After Surgery Program can change nutrition care practice: a multicenter experience in elective colorectal surgery. JPEN J Parenter Enteral Nutr. 2019;43(2):206‐219. [DOI] [PubMed] [Google Scholar]
- 18. Kyrou I, Tsigos C. Stress hormones: physiological stress and regulation of metabolism. Curr Opin Pharmacol. 2009;9(6):787‐793. [DOI] [PubMed] [Google Scholar]
- 19. Varadhan KK, Constantin‐Teodosiu D, Constantin D, Greenhaff PL, Lobo DN. Inflammation‐mediated muscle metabolic dysregulation local and remote to the site of major abdominal surgery. Clin Nutr. 2018;37(6):2178‐2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Atkins R, Constantin‐Teodosiu D, Varadhan KK, Constantin D, Lobo DN, Greenhaff PL. Major elective abdominal surgery acutely impairs lower limb muscle pyruvate dehydrogenase complex activity and mitochondrial function. Clin Nutr. 2021;40(3):1046‐1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pant M, Bal NC, Periasamy M. Sarcolipin: a key thermogenic and metabolic regulator in skeletal muscle. Trends Endocrinol Metab. 2016;27(12):881‐892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hardee JP, Montalvo RN, Carson JA. Linking cancer cachexia‐induced anabolic resistance to skeletal muscle oxidative metabolism. Oxid Med Cell Longe. 2017;2017:8018197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lecker SH, Goldberg AL, Mitch WE. Protein degradation by the ubiquitin‐proteasome pathway in normal and disease states. J Am Soc Nephrol. 2006;17(7):1807‐1819. [DOI] [PubMed] [Google Scholar]
- 24. Gillis C, Ljungqvist O, Carli F. Prehabilitation, Enhanced Recovery After Surgery, or both? A narrative review. Br J Anesth. 2022;128(3):434‐448. [DOI] [PubMed] [Google Scholar]
- 25. Deutz NEP, Ashurst I, Ballesteros MD, et al. The underappreciated role of low muscle mass in the management of malnutrition. J Am Med Dir Accoc. 2019;20(1):22‐27. [DOI] [PubMed] [Google Scholar]
- 26. Surov A, Pech M, Gessner D, et al. Low skeletal muscle mass is a predictor of treatment related toxicity in oncologic patients. A meta‐analysis. Clin Nutr. 2021;40(10):5298‐5310. [DOI] [PubMed] [Google Scholar]
- 27. Prado C, Purcell S, Alish C, et al. Implications of low muscle mass across the continuum of care: a narrative review. Ann Med. 2018;50(8):675‐693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Prado CM, Bell JJ, Gonzalez MC. Untangling malnutrition, physical dysfunction, sarcopenia, frailty and cachexia in ageing. In: Geirsdóttir ÓG, Bell, JJ , eds. Interdisciplinary Nutritional Management and Care for Older Adults. Perspectives in Nursing Management and Care for Older Adults. Springer International Publishing; 2021:99‐113. [Google Scholar]
- 29. Prado CM, Anker SD, Coats AJS, Laviano A, von Haehling S. Nutrition in the spotlight in cachexia, sarcopenia and muscle: avoiding the wildfire. J Cachexia Sarcopenia Muscle. 2021;12(1):3‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schuetz P, Seres D, Lobo DN, Gomes F, Kaegi‐Braun N, Stanga Z. Management of disease‐related malnutrition for patients being treated in hospital. Lancet. 2021;398(10314):1927‐1938. [DOI] [PubMed] [Google Scholar]
- 31. Gillis C, Hasil L, Kasvis P, et al. Nutrition care process model approach to surgical prehabilitation in oncology. Front Nutr. 2021;8:644706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Charney P. Nutrition screening vs nutrition assessment: how do they differ? Nutr Clin Pract. 2008;23(4):366‐372. [DOI] [PubMed] [Google Scholar]
- 33. Williams DG, Aronson S, Murray S, et al. Validation of the perioperative nutrition screen for prediction of postoperative outcomes. JPEN J Parenter Enteral Nutr. 2021. [Epub ahead of print]. 10.1002/jpen.2310 [DOI] [PubMed]
- 34. Krznarić Ž, Bender DV, Laviano A, et al. A simple remote nutritional screening tool and practical guidance for nutritional care in primary practice during the COVID‐19 pandemic. Clin Nutr. 2020;39(7):1983‐1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jensen GL, Cederholm T, Correia MITD, et al. GLIM criteria for the diagnosis of malnutrition: a consensus report from the global clinical nutrition community. JPEN J Parenter Enteral Nutr. 2019;43(1):32‐40. [DOI] [PubMed] [Google Scholar]
- 36. Cederholm T, Jensen GL, Correia MITD, et al. GLIM criteria for the diagnosis of malnutrition – a consensus report from the global clinical nutrition community. Clin Nutr. 2019;38(1):1‐9. [DOI] [PubMed] [Google Scholar]
- 37. Correia MITD, Tappenden KA, Malone A, et al. Utilization and validation of the Global Leadership Initiative on Malnutrition (GLIM): a scoping review. Clin Nutr. 2022;41(3):687‐697. [DOI] [PubMed] [Google Scholar]
- 38. Kakavas S, Karayiannis D, Bouloubasi Z, et al. Global Leadership Initiative on Malnutrition criteria predict pulmonary complications and 90‐day mortality after major abdominal surgery in cancer patients. Nutrients. 2020;12(12):3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Boslooper‐Meulenbelt K, Van Vliet IMY, Gomes‐Neto AW, et al. Malnutrition according to GLIM criteria in stable renal transplant recipients: reduced muscle mass as predominant phenotypic criterion. Clin Nutr. 2021;40(5):3522‐3530. [DOI] [PubMed] [Google Scholar]
- 40. Emsley C, King S, Nyulasi I, Snell G. A glimmer of insight into lung transplant nutrition: enhanced detection of malnutrition in lung transplant patients using the GLIM criteria. Clin Nutr. 2021;40(5):2521‐2526. [DOI] [PubMed] [Google Scholar]
- 41. Fiorindi C, Luceri C, Dragoni G, et al. GLIM criteria for malnutrition in surgical IBD patients: a pilot study. Nutrients. 2020;12(8):2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Huang D‐D, Yu D‐Y, Song H‐N, et al. The relationship between the GLIM‐defined malnutrition, body composition and functional parameters, and clinical outcomes in elderly patients undergoing radical gastrectomy for gastric cancer. Eur J Surg Oncol. 2021;47(9):2323‐2331. [DOI] [PubMed] [Google Scholar]
- 43. Barazzoni R, Jensen GL, Correia MITD, et al. Guidance for assessment of the muscle mass phenotypic criterion for the Global Leadership Initiative on Malnutrition (GLIM) diagnosis of malnutrition. Clin Nutr. 2022;41(6):1425‐1433 [DOI] [PubMed] [Google Scholar]
- 44. Compher C, Cederholm T, Correia MITD, et al. Guidance for assessment of the muscle mass phenotypic criterion for the Global Leadership Initiative on Malnutrition diagnosis of malnutrition. JPEN J Parenter Enteral Nutr. 10.1002/jpen.2366 [DOI] [PubMed] [Google Scholar]
- 45. Evans DC, Corkins MR, Malone A, et al; ASPEN Malnutrition Committee . The use of visceral proteins as nutrition markers: an ASPEN position paper. Nutr Clin Pract. 2021;36(1):22‐28. [DOI] [PubMed] [Google Scholar]
- 46. Bretscher C, Boesiger F, Kaegi‐Braun N, et al. Admission serum albumin concentrations and response to nutritional therapy in hospitalised patients at malnutrition risk: secondary analysis of a randomised clinical trial. EClinicalMedicine. 2022;45:101301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Prado CMM, Heymsfield SB. Lean tissue imaging: a new era for nutritional assessment and intervention. JPEN J Parenter Enteral Nutr. 2014;38(8):940‐953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Xiao J, Caan BJ, Cespedes Feliciano EM, et al. Association of low muscle mass and low muscle radiodensity with morbidity and mortality for colon cancer surgery. JAMA Surg. 2020;155(10):942‐959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Brown JC, Caan BJ, Cespedes Feliciano EM, et al. Weight stability masks changes in body composition in colorectal cancer: a retrospective cohort study. Am J Clin Nutr. 2021;113(6):1482‐1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Santos LP, Gonzalez MC, Orlandi SP, Bielemann RM, Barbosa‐Silva TG, Heymsfield SB. New prediction equations to estimate appendicular skeletal muscle mass using calf circumference: results from NHANES 1999–2006. JPEN J Parenter Enteral Nutr. 2019;43(8):998‐1007. [DOI] [PubMed] [Google Scholar]
- 51. Gonzalez MC, Mehrnezhad A, Razaviarab N, Barbosa‐Silva TG, Heymsfield SB. Calf circumference: cutoff values from the NHANES 1999–2006. Am J Clin Nutr. 2021;113(6):1679‐1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ishida Y, Maeda K, Nonogaki T, et al. Impact of edema on length of calf circumference in older adults. Geriatr Gerontol Int. 2019;19(10):993‐998. [DOI] [PubMed] [Google Scholar]
- 53. Elliott JA, Doyle SL, Murphy CF, et al. Sarcopenia: prevalence, and impact on operative and oncologic outcomes in the multimodal management of locally advanced esophageal cancer. Ann Surg. 2017;266(5):822‐830. [DOI] [PubMed] [Google Scholar]
- 54. Aleixo GFP, Shachar SS, Nyrop KA, Muss HB, Malpica L, Williams GR. Myosteatosis and prognosis in cancer: systematic review and meta‐analysis. Crit Rev Oncol Hematol. 2020;145:102839. [DOI] [PubMed] [Google Scholar]
- 55. Murnane LC, Forsyth AK, Koukounaras J, et al. Myosteatosis predicts higher complications and reduced overall survival following radical oesophageal and gastric cancer surgery. Eur J Surg Oncol. 2021;47(9):2295‐2303. [DOI] [PubMed] [Google Scholar]
- 56. Carvalho ALMD, Gonzalez MC, Sousa IMD, et al. Low skeletal muscle radiodensity is the best predictor for short‐term major surgical complications in gastrointestinal surgical cancer: a cohort study. PLOS One. 2021;16(2):e0247322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hopkins JJ, Reif RL, Bigam DL, Baracos VE, Eurich DT, Sawyer MB. The impact of muscle and adipose tissue on long‐term survival in patients with stage I to III colorectal cancer. Dis Colon Rectum. 2019;62(5):549‐560. [DOI] [PubMed] [Google Scholar]
- 58. Malietzis G, Currie AC, Athanasiou T, et al. Influence of body composition profile on outcomes following colorectal cancer surgery. Br J Surg. 2016;103(5):572‐580. [DOI] [PubMed] [Google Scholar]
- 59. Zhuang C‐L, Shen X, Huang Y‐Y, et al. Myosteatosis predicts prognosis after radical gastrectomy for gastric cancer: a propensity score–matched analysis from a large‐scale cohort. Surgery. 2019;166(3):297‐304. [DOI] [PubMed] [Google Scholar]
- 60. Srpcic M, Jordan T, Popuri K, Sok M. Sarcopenia and myosteatosis at presentation adversely affect survival after esophagectomy for esophageal cancer. Radiat Oncol J. 2020;54(2):237‐246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Van Rijssen LB, Van Huijgevoort NCM, Coelen RJS, et al. Skeletal muscle quality is associated with worse survival after pancreatoduodenectomy for periampullary, nonpancreatic cancer. Ann Surg Oncol. 2017;24(1):272‐280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sueda T, Takahasi H, Nishimura J, et al. Impact of low muscularity and myosteatosis on long‐term outcome after curative colorectal cancer surgery: a propensity score‐matched analysis. Dis Colon Rectum. 2018;61(3):364‐374. [DOI] [PubMed] [Google Scholar]
- 63. Aro R, Mäkäräinen‐Uhlbäck E, Ämmälä N, et al. The impact of sarcopenia and myosteatosis on postoperative outcomes and 5‐year survival in curatively operated colorectal cancer patients – a retrospective register study. Eur J Surg Oncol. 2020;46(9):1656‐1662. [DOI] [PubMed] [Google Scholar]
- 64. van Dijk DPJ, Bakens MJAM, Coolsen MME, et al. Low skeletal muscle radiation attenuation and visceral adiposity are associated with overall survival and surgical site infections in patients with pancreatic cancer. J Cachexia Sarcopenia Muscle. 2017;8(2):317‐326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Stretch C, Aubin J‐M, Mickiewicz B, et al. Sarcopenia and myosteatosis are accompanied by distinct biological profiles in patients with pancreatic and periampullary adenocarcinomas. PLOS One. 2018;13(5):e0196235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Martin L, Hopkins J, Malietzis G, et al. Assessment of computed tomography (CT)‐defined muscle and adipose tissue features in relation to short‐term outcomes after elective surgery for colorectal cancer: a multicenter approach. Ann Surg Oncol. 2018;25(9):2669‐2680. [DOI] [PubMed] [Google Scholar]
- 67. Paris MT, Furberg HF, Petruzella S, Akin O, Hötker AM, Mourtzakis M. Influence of contrast administration on computed tomography–based analysis of visceral adipose and skeletal muscle tissue in clear cell renal cell carcinoma. JPEN J Parenter Enteral Nutr. 2018;42(7):1148‐1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Rollins KE, Gopinath A, Awwad A, Macdonald IA, Lobo DN. Computed tomography‐based psoas skeletal muscle area and radiodensity are poor sentinels for whole L3 skeletal muscle values. Clin Nutr. 2020;39(7):2227‐2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Prado CM, Cushen SJ, Orsso CE, Ryan AM. Sarcopenia and cachexia in the era of obesity: clinical and nutritional impact. Proc Nutr Soc. 2016;75(2):188‐198. [DOI] [PubMed] [Google Scholar]
- 70. Cespedes Feliciano EM, Popuri K, Cobzas D, et al. Evaluation of automated computed tomography segmentation to assess body composition and mortality associations in cancer patients. J Cachexia Sarcopenia Muscle. 2020;11(5):1258‐1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Beetz NL, Maier C, Segger L, et al. First PACS‐integrated artificial intelligence‐based software tool for rapid and fully automatic analysis of body composition from CT in clinical routine. JCSM Clinical Reports. 2022;7:3‐11. [Google Scholar]
- 72. Kim J, Han SH, Kim HI. Detection of sarcopenic obesity and prediction of long‐term survival in patients with gastric cancer using preoperative computed tomography and machine learning. J Surg Oncol. 2021;124(8):1347‐1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ma D, Chow V, Popuri K, Beg MF. Comprehensive validation of automated whole body skeletal muscle, adipose tissue, and bone segmentation from 3D CT images for body composition analysis: towards extended body composition. arXiv. Updated July 27, 2021. 10.48550/arXiv.2106.00652 [DOI]
- 74. Gomez‐Perez SL, Haus JM, Sheean P, et al. Measuring abdominal circumference and skeletal muscle from a single cross‐sectional computed tomography image. JPEN J Parenter Enteral Nutr. 2016;40(3):308‐318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Steele S, Lin F, Le T‐L, et al. Segmentation and linear measurement for body composition analysis using Slice‐O‐Matic and Horos. J Vis Exp . 2021;169:e61674. [DOI] [PubMed] [Google Scholar]
- 76. Mullie L, Afilalo J. Coreslicer: a web toolkit for analytic morphomics. BMC Med Imaging. 2019;19(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Barbosa‐Silva MCG, Barros AJD. Bioelectrical impedance analysis in clinical practice: a new perspective on its use beyond body composition equations. Curr Opin Clin Nutr Metab Care. 2005;8(3):311‐317. [DOI] [PubMed] [Google Scholar]
- 78. Barbosa‐Silva MCG, Barros AJD. Bioelectric impedance and individual characteristics as prognostic factors for post‐operative complications. Clin Nutr. 2005;24(5):830‐838. [DOI] [PubMed] [Google Scholar]
- 79. Matthews L, Bates A, Wootton SA, Levett D. The use of bioelectrical impedance analysis to predict post‐operative complications in adult patients having surgery for cancer: a systematic review. Clin Nutr. 2021;40(5):2914‐2922. [DOI] [PubMed] [Google Scholar]
- 80. Souza NC, Avesani CM, Prado CM, et al. Phase angle as a marker for muscle abnormalities and function in patients with colorectal cancer. Clin Nutr. 2021;40(7):4799‐4806. [DOI] [PubMed] [Google Scholar]
- 81. Caccialanza R, Cereda E, Caraccia M, et al. Early 7‐day supplemental parenteral nutrition improves body composition and muscle strength in hypophagic cancer patients at nutritional risk. Support Care Cancer. 2019;27:2497‐2506. [DOI] [PubMed] [Google Scholar]
- 82. Petrolo M, Rangelova E, Toilou M, Hammarqvist F. Body composition, muscle function and biochemical values in patients after pancreatic surgery: an observational study. Clin Nutr. 2021;40(6):4284‐4289. [DOI] [PubMed] [Google Scholar]
- 83. Ringaitiene D, Gineityte D, Vicka V, et al. Malnutrition assessed by phase angle determines outcomes in low‐risk cardiac surgery patients. Clin Nutr. 2016;35(6):1328‐1332. [DOI] [PubMed] [Google Scholar]
- 84. Tsaousi G, Panagidi M, Papakostas P, Grosomanidis V, Stavrou G, Kotzampassi K. Phase angle and handgrip strength as complements to body composition analysis for refining prognostic accuracy in cardiac surgical patients. J Cardiothorac Vasc Anesth. 2021;35(8):2424‐2431. [DOI] [PubMed] [Google Scholar]
- 85. Turton P, Hay R, Welters I. Assessment of peripheral muscle thickness and architecture in healthy volunteers using hand‐held ultrasound devices; a comparison study with standard ultrasound. BMC Med Imaging. 2019;19(1):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Bury C, Dechicco R, Nowak D, et al. Use of bedside ultrasound to assess muscle changes in the critically ill surgical patient. JPEN J Parenter Enteral Nutr. 2021;45(2):394‐402. [DOI] [PubMed] [Google Scholar]
- 87. Fischer A, Hertwig A, Hahn R, et al. Validation of bedside ultrasound to predict lumbar muscle area in the computed tomography in 200 non‐critically ill patients: the USVALID prospective study. Clin Nutr. 2022;41(4):829‐837. [DOI] [PubMed] [Google Scholar]
- 88. Bone JL, Ross ML, Tomcik KA, Jeacocke NA, McKay AKA, Burke LM. The validity of ultrasound technology in providing an indirect estimate of muscle glycogen concentrations is equivocal. Nutrients. 2021;13(7):2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Parry SM, El‐Ansary D, Cartwright MS, et al. Ultrasonography in the intensive care setting can be used to detect changes in the quality and quantity of muscle and is related to muscle strength and function. J Crit Care. 2015;30(5):1151.e9‐1151.e14. [DOI] [PubMed] [Google Scholar]
- 90. Barbosa‐Silva TG, Gonzalez MC, Bielemann RM, Santos LP, Costa CdS, Menezes AMB. 2 + 2 (+2) = 4: a new approach for appendicular muscle mass assessment by ultrasound. Nutrition. 2021;83:111056. [DOI] [PubMed] [Google Scholar]
- 91. Joshi A, Mancini R, Probst S, et al. Sarcopenia in cardiac surgery: dual X‐ray absorptiometry study from the McGill frailty registry. Am Heart J. 2021;239:52‐58. [DOI] [PubMed] [Google Scholar]
- 92. Li W, Zhu L, Mo Z, et al. Effect of laparoscopic roux‐en‐y gastric bypass on body composition and insulin resistance in chinese patients with type 2 diabetes mellitus. Obes Surg. 2014;24(4):578‐583. [DOI] [PubMed] [Google Scholar]
- 93. Pekař M, Pekařová A, Bužga M, Holéczy P, Soltes M. The risk of sarcopenia 24 months after bariatric surgery ‐ assessment by dual energy X‐ray absorptiometry (DEXA): a prospective study. Wideochir Inne Tech Maloinwazyjne. 2020;15(4):583‐587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Dienemann T, Ziolkowski SL, Bender S, et al. Changes in body composition, muscle strength, and fat distribution following kidney transplantation. Am J Kidney Dis. 2021;78(6):816‐825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Shankaran M, Czerwieniec G, Fessler C, et al. Dilution of oral D3‐creatine to measure creatine pool size and estimate skeletal muscle mass: development of a correction algorithm. J Cachexia Sarcopenia Muscle. 2018;9(3):540‐546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Bauer J, Morley JE, Schols AMWJ, et al. Sarcopenia: a time for action. An SCWD position paper. J Cachexia Sarcopenia Muscle. 2019;10(5):956‐961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Linge J, Borga M, West J, et al. Body composition profiling in the UK biobank imaging study. Obesity. 2018;26(11):1785‐1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Borga M, Ahlgren A, Romu T, Widholm P, Dahlqvist Leinhard O, West J. Reproducibility and repeatability of MRI‐based body composition analysis. Magn Reson Med. 2020;84(6):3146‐3156. [DOI] [PubMed] [Google Scholar]
- 99. Mandić M, Rullman E, Widholm P, et al. Automated assessment of regional muscle volume and hypertrophy using MRI. Sci Rep. 2020;10(1):2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Richards SJG, Frizelle FA, Geddes JA, Eglinton TW, Hampton MB. Frailty in surgical patients. Int J Colorectal Dis. 2018;33(12):1657‐1666. [DOI] [PubMed] [Google Scholar]
- 101. McIsaac DI, Taljaard M, Bryson GL, et al. Frailty as a predictor of death or new disability after surgery: a prospective cohort study. Ann Surg. 2020;271(2):283‐289. [DOI] [PubMed] [Google Scholar]
- 102. Panayi AC, Orkaby AR, Sakthivel D, et al. Impact of frailty on outcomes in surgical patients: a systematic review and meta‐analysis. Am J Surg. 2019;218(2):393‐400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Subramaniam S, Aalberg JJ, Soriano RP, Divino CM. New 5‐factor modified frailty index using American College of Surgeons NSQIP data. J Am Coll Surg. 2018;226(2):173‐181. [DOI] [PubMed] [Google Scholar]
- 104. Riveros C, Jazayeri Seyed B, Chalfant V, Ahmed F, Bandyk M, Balaji KC. The geriatric nutritional risk index predicts postoperative outcomes in bladder cancer: a propensity score‐matched analysis. J Urol. 2022;207(4):797‐804. [DOI] [PubMed] [Google Scholar]
- 105. Naganuma M, Kudo Y, Suzuki N, Masuda S, Nagaya K. Effect of malnutrition and frailty status on surgical aortic valve replacement. Gen Thorac Cardiovasc Surg. 2022;70(1):24‐32. [DOI] [PubMed] [Google Scholar]
- 106. Amabili P, Wozolek A, Noirot I, et al. The Edmonton frail scale improves the prediction of 30‐day mortality in elderly patients undergoing cardiac surgery: a prospective observational study. J Cardiothorac Vasc Anesth. 2019;33(4):945‐952. [DOI] [PubMed] [Google Scholar]
- 107. Gillis C, Fenton TR, Gramlich L, et al. Older frail prehabilitated patients who cannot attain a 400 m 6‐min walking distance before colorectal surgery suffer more postoperative complications. Eur J Surg Oncol. 2021;47(4):874‐881. [DOI] [PubMed] [Google Scholar]
- 108. Longobucco Y, Lauretani F, Gionti L, et al. The role of the Sunfrail tool in the screening of frailty and in integrated community‐hospital care pathways: a retrospective observational study. Aging Clin Exp Res. 2022;34(2):419‐427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Alvarez‐Nebreda ML, Bentov N, Urman RD, et al. Recommendations for preoperative management of frailty from the Society for Perioperative Assessment and Quality Improvement (SPAQI). J Clin Anesth. 2018;47:33‐42. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information.


