Abstract
Approximately 10% of US births deliver preterm before 37 weeks of completed gestation. Premature infants are at risk for life-long debilitating morbidities and death, and spontaneous preterm labor explains 50% of preterm births. In all cases existing treatments are ineffective, and none are FDA approved. The mechanisms that initiate preterm labor are not well understood but may result from dysfunctional regulation of quiescence mechanisms. Human pregnancy is accompanied by large increases in blood flow, and the uterus must enlarge by orders of magnitude to accommodate the growing fetus. This mechanical strain suggests that stretch-activated channels may constitute a mechanism to explain gestational quiescence. Here we identify for the first time that Piezo1, a mechanosensitive cation channel, is present in the uterine smooth muscle and microvascular endothelium of pregnant myometrium. Piezo is downregulated during preterm labor, and stimulation of myometrial Piezo1 in an organ bath with the agonist, Yoda1, relaxes the tissue in a dose-dependent fashion. Further, stimulation of Piezo1 while inhibiting PKA, AKT, or eNOS mutes the negative inotropic effects of Piezo1 activation, intimating that actions on the myocyte and endothelial nitric oxide signaling contributes to Piezo1-mediated contractile dynamics. Taken together, these data highlight the importance of stretch-activated channels in pregnancy maintenance and parturition, and identify Piezo1 as a tocolytic target of interest.
Keywords: Myometrium, Preterm Labor, Pregnancy, Mechanosensitive channels, Stretch-activated channels, Piezo1
Introduction
Preterm labor (PTL) and preterm birth (PTB) have plagued society since time immemorial. PTB is the leading cause of death in children under 5 (Chawanpaiboon et al., 2019), and those who survive commonly suffer from life-long deleterious health disparities (Cooke, 2006). PTL, defined as sustained contractions prior to 37 weeks of gestation, occurs in about 10% of all pregnancies (Martin et al., 2021), with women of African descent being as much as 40% more likely to deliver preterm (Korinek & Ahmmad, 2021), and their infants twice as likely to die as a result (Burris & Parker, 2021). While advances in perinatal care have ameliorated many serious complications if addressed during late preterm (> 32 weeks), each year thirteen million infants are born preterm globally, costing in excess of 38 billion annually (adjusted) in the Unites States alone (Outcomes & Press, 2007). While there are many known correlates to PTL/PTB, such as infection, smoking/drug use, and even race, about half of all PTL is idiopathic, also known as spontaneous preterm labor. After nearly 70 years of active tocolytic development (Abramson & Reid, 1955) there are still no FDA-approved drugs that can significantly delay PTB beyond 48-hours allowing afflicted pregnancies to go to term. Our research seeks to explore distinctive/dysregulated pathways of the uterus to identify novel targets for tocolytic development. Here we investigate Piezo1 (PIEZO1), a stretch-activated cation channel (SAC), to better understand myometrial contractile dynamics.
The myometrium is the smooth muscle of the uterus which contracts to expel the infant during labor. Little is known about the cytoarchitecture of human myometrium in pregnancy (Sweeney et al., 2014). The myometrium is over 90% muscle cells by volume, and the cellular heterogeneity includes blood vessels, fibroblasts, immune and stem cells (Santamaria et al., 2018). Importantly, unlike gastrointestinal smooth muscle that employs both nervous innervations and pacemaker cells (Sanders & Smith, 1986; Sanders et al., 2016), human myometrial muscle fibers are interwoven, do not form distinct layers (Blanks et al., 2007); are not innervated by motor nerves (Tingåker & Irestedt, 2010); and a pacemaker cell type has not been convincingly described (Young, 2018; Wray & Prendergast, 2019). These peculiarities of myometrium, combined with the finding that uterine myocytes preferentially relax to nitric oxide via protein S-nitrosation (Barnett et al., 2018), and not cyclic nucleotide generation (Bradley et al., 1998; Lai et al., 2016), has led us to investigate quiescent-mediated pathways specific to the myometrium.
Wholly unique in human physiology is the demand placed on the uterus to remain quiescent for the entire 40 weeks of gestation. No other muscle must remain functionally dormant for such an extended period. While the pathways that ensure this prolonged quiescence are largely unknown, a logical approach is to investigate actions and stresses unique to the uterus during pregnancy. As such, investigating SACs in the myometrium during gestation is not only reasonable, but may unearth the underlying pathophysiology of PTL. There are several mechanosensitive channels in the myometrium, including the inward rectifying Ca2+ channel, TRPV4 (OTRPC) (Villegas et al., 2021), and the outward rectifying K+ channel, TREK-1 (KCNK2) (Buxton et al., 2011; Heyman et al., 2013), both known to be regulators of membrane polarization during pregnancy. Due to the extraordinary hydrostatic load imposed on the uterus as the pregnancy progresses, here we seek to identify and examine the SAC Piezo1, to determine its contribution to pregnancy maintenance and labor.
Piezo1 ('piesi' meaning pressure in Greek) is a mechanosensitive inward rectifying cation channel (Coste et al., 2012) which is preferential to Ca2+ under physiologic conditions (Romac et al., 2018). Piezo1 was first identified in astrocytes in the mid 2000s (Satoh et al., 2006), and is the subject of the 2021 Nobel Prize in Physiology or Medicine. It has since been found in other tissues (Li et al., 2014; John et al., 2018), including human endometrium (Hennes et al., 2019), where its aberrant expression is thought to contribute to preeclampsia (Arishe et al., 2020). Importantly, until now Piezo1 has not been characterized in human myometrium. Piezo1 assembles in the membrane as a large trimer of ~286 kD (Coste et al., 2010; Gottlieb & Sachs, 2012), with a single channel conductance of ~37 pS (Gottlieb et al., 2012). It is selectively agonized/antagonized by the small molecules Yoda1 and Dooku1, respectively (Evans et al., 2018; Botello-Smith et al., 2019; Lhomme et al., 2019), which allows for precise experimental modulation of the channel. Due to its mechanosensitive property and permeability to Ca2+, Piezo1 is an attractive protein of interest for investigating pregnancy maintenance.
Of all divalent cations, Ca2+ is the preeminent modulator of smooth muscle activity. Ca2+ is a critical agonist and second messenger in both myocytes and microvascular endothelial cells (MECs) of the myometrium. In the myocyte Ca2+ is most notably recognized as an initiator of contraction via calmodulin-mediated myosin light chain kinase activation, but it also activates membrane bound BKCa channels (Maxi-K, KCNMA1), driving K+ efflux (Nardi & Olesen, 2008). Piezo1 activation in human myometrium following TRPV4 stimulation has been found to activate BKCa, resulting in relaxation of the tissue (Villegas et al., 2021), while in human arterial fibroblasts an association was found between Piezo1 stimulation and BKCa activity (Jakob et al., 2021). In vascular smooth muscle BKCa is part of a localized signaling complex that includes the L-type calcium channel, the sarcoplasmic reticulum, and TRPV4, driving polarization of the membrane (Dopico et al., 2018). MECs, on the other hand, which directly interface with the myocytes of the myometrium, are sensitive to mechanical stimuli generated by blood flow (pulsatile stretch and shear stress) within the expanding uterus. These stimuli trigger a physiological response via release of vasodilatory factors such as nucleotides (Buxton et al., 2001; Wang et al., 2016) and nitric oxide (Vanhoutte et al., 2017), which are mediated in part by stimulation of protein kinase A (PKA) (Bir et al., 2012) and AKT, sometime called protein kinase B (Zhang & Hintze, 2006). As such, Ca2+ entry into uterine myocytes and MECs via Piezo1 may act as an important quiescent regulator.
Uterine myocytes and MECs must endure substantial mechanical stress during pregnancy and have evolved to leverage the functionality of SACs to regulate homeostatic function. We have elected to explore Piezo1 expression and function in these cell types, and in whole myometrial tissue, to determine if Piezo1 significantly modulates their function. We posit that Ca2+ influx via Piezo1 in myometrial MECs stimulates nitric oxide production via AKT/PKA activation, promoting quiescence, and that Piezo1 in pregnant human uterine smooth muscle (phUSMC) contributes to quiescence, in part, through Ca2+ mediated BKCa activation.
Materials and Methods
Ethical Approval:
All human tissue collection was obtained in accordance with the Declaration of Helsinki and approved by the Institutional Review Board at the University of Nevada Biomedical Review Committee for the protection of human subjects (approval 509108-19). All experiments were performed in accordance with the NIH guidance on the use of human tissues in research.
Tissue collection:
Human uterine biopsies were obtained with written informed-consent from mothers with singleton pregnancies undergoing Cesarean section as previously described (Barnett et al., 2020). Exclusion criteria in pregnant women include: uterine or generalized infection to include COVID-19, a maternal age < 18 years, any history of drug abuse, co-morbid diagnoses such as HIV infection or AIDS, hepatitis C infection, uncontrolled diabetes, renal disease, and any use of steroids other than betamethasone (including topical use) during pregnancy. Tissues were transported to the laboratory immediately in cold Krebs buffer containing, 118 mM NaCl, 4.75 mM KCl, 2.5 mM CaCl2, 1.2 mM KH2PO4, 25 mM NaHCO3, 1.2 mM MgCl2, 20 mM dextrose, and adjusted to pH 7.4. Tissues were dissected under 4x magnification to isolate smooth muscle, then either immediately employed in contractile experiments or snap frozen in liquid nitrogen and stored in a vapor-phase freezer unit at −150 °C.
Western Blot:
Total protein was collected from whole tissue and cell culture. In all cases protein was isolated in MAPK buffer containing: 60 mM Tris-HCL (pH 6.8), 1% glycerol, 2% SDS, 1 μM leupeptin, 1 mM EGTA, 1 mM EDTA, 1 mM Na3VO4, and protease/phosphatase inhibitors (PPC110: Sigma Aldrich, St. Louis, MO). Tissue samples were frozen and crushed using a liquid nitrogen-cooled mortar and pestle, followed by wet homogenization (gentleMACS™ Dissociator, Miltenyi Biotec Inc., North Rhine-Westphalia, Germany). Cultured cell lysates were collected mechanically (cell scraper) using the buffer described above after (3) washes in sterile PBS. Sample concentrations were determined using EZQ (R33200: Thermo Fischer, Waltham, MA).
Polyacrylamide Gel electrophoresis (PAGE)
For all samples 60 μg of total protein lysate was separated on a 4-15% polyacrylamide gel at 160 V for ~60 minutes, then transferred to a polyvinylidene fluoride (PVDF) membrane and blocked in 5% blotting-grade nonfat milk/TBST (Biorad, 1706404, Hercules, CA) overnight. Western blots were labeled with mouse monoclonal anti-Piezo1 1° antibody (1:500, MA5-32876; Thermo Fischer, Waltham, MA) followed by Goat anti-Mouse IgG (H+L) Cross-Adsorbed 2°, HRP (1:5000, Cat. A16072, Thermo Fischer) with SuperSignal™ West Pico PLUS activator (Cat. 34580, Thermo Fischer). GAPDH was used as a control protein and blots were labeled with anti-GAPDH 1° antibody (1:1000, Cat. sc-47724, Santa Cruz Biotechnology) followed by Goat anti-Rabbit IgG (H+L) 2° Antibody, HRP (1:5000, Cat. 31460; Thermo Fischer). Blots were stripped between each antibody application (Cat. 928-40030, LI-COR Biotechnology, Lincoln, NE). Each data point was from a unique patient and was treated as an individual ‘n.’
Cell culture:
1° cells were generated from TNL human myometrium as previously described (Asif et al. 2022). Cells were detached from flasks using a collagenase (CLS2, Worthington, US) and trypsin (27250-018, Gibco, US) enzyme solution (2:1 collaganse:trypsin) in MACS buffer and Gibco Dulbecco’s Modified Eagle Medium (DMEM, 11995-065, Gibco, Waltham, MA). Using a gentleMACS™ Dissociator, cells were agitated 3x for 90 seconds with 45 minute rest at 37°C between agitations. The digestion was triturated 3x and filtered through a 100 μM sterile mesh. Cells were the cultured to 80% confluency, preincubated with FcR blocking reagent, then separated over CD31+ bead LS columns using a MidiMACS separator (Miltenyi Biotec:130-091-935, Auburn, CA). Cells captured by the beads were deemed CD31+ pregnant human myometrial endothelial cells (phMEC) and CD31− pregnant human uterine smooth muscle cells (phUSMC). phMECs cultured in endothelial basal medium 2 (C-22011, PromoCell, Heidelberg, Germany) containing 10% FBS and 1% penicillin, while the phUSMC cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) with 50 U/ml streptomycin, 50 μg/ml penicillin, and 10% FBS and supplemented with estrogen (15 ng/ml) and progesterone (200 ng/ml). All cells were cultured in a balanced oxygen (95%:5% O2:CO2) incubator at 37°C. Piezo1-deficient HEK293T cells (Piezo1KO) (Lukacs et al., 2015) were kindly provided by Dr. Ardem Patapoutian of the Howard Hughes Medical Institute at Scripps Research and were cultured in DMEM with 10% FBS (without antibiotics).
Immunofluorescence:
phMEC (CD31+), phUSMC (CD31−), and Piezo1KO cells were plated on 35mm glass bottom dishes (MatTek Corporation) and grown to ~80% confluence. Prepared cells were treated for 15 minutes with 4% paraformaldehyde, followed by 0.5% triton-x for 5 minutes and 5% BSA blocking buffer for 1 hour with 3x PBS washes between each step. Cells were then labeled with Piezo1 monoclonal 1° antibody (1:100, MA5-32876, Thermo Fisher Scientific), followed by 2° Alexa Fluor® 594 (1:100, ab150080, Abcam) and imaged at 10x magnification on an inverted fluorescent microscope (ECHO, San Diego, CA). All cells were additionally labeled with wheat germ agglutinin (WGA) conjugated to Alexa Fluor® 488 (1:00, W11261, Thermo Fisher Scientific), followed by DAPI mounting medium (H-1500, Vector Laboratories, Burlingame, CA). Exposure and contrast were adjusted globally to ensure adequate visibility of each channel. All images were taken with negative controls (absence of primary antibody) to verify the absence of nonselective secondary binding (data not shown).
Calbryte™ Ca2+ permeability assay:
1° cultured (p2-p4) phUSMC, phMEC, and Piezo1KO cells were seeded to a density of 4000 cells/well in a half-volume flat bottom 96-well microplate (675076, Greiner Bio One, Kremsmünster, Austria) and left to settle for 24 hours. Just prior to start of experiment media was replaced with Ca2+-free KREBS containing 20 mM HEPES (bufferexp) and cells were incubated for 10 minutes in either 10 nM oxytocin (phUSMC) (Gimpl & Fahrenholz, 2001) or 10 mM caffeine (phMEC/Piezo1KO) (Corda et al., 1995) to deplete the sarcoplasmic/endoplasmic reticulum of Ca2+. Following incubation, 200 nM thapsigargin (Wictome et al., 1992; Xuan et al., 1992) was added for an additional 15 minutes to prevent Ca2+ re-uptake. Cells were then rinsed 2x in bufferexp containing 0.04% Pluronic® F-127 and 1 mM probenecid (anion transporter inhibitor), followed by 5 μM Calbryte™ 520 AM (Cat. 20650 - AAT Bioquest, Sunnyvale, CA) for 1 hour. Following incubation period cells were rinsed 2x in bufferexp containing 0.04% Pluronic® F-127 and 1 mM probenecid, followed by addition of 100 nM amlodipine (Ca2+ channel blocker), Yoda1 ± Dooku1, and 2.5 mM Ca2+. Negative controls were run in either the absence of Calbryte™ 520 AM, or the absence of extracellular Ca2+ (data not shown). Each experimental condition was run 18 times over three plates, with six replicates per condition, per plate. For analysis, ΔCai2+ was calculated as follows: (1) value of blank cell (all reagents minus Calbryte) was subtracted from value of experimental cell; (2) resultant value normalized to the A280 for each condition to account for variations in the number of cells loaded into each well; (3) resultant value subtracted from the average value at t=0 for each condition to provide the ‘delta,’ or change in fluorescence over the experimental period.
Contractile studies:
In a temperature controlled (37°C) organ bath (DMT 820MS, Danish Myo Technology, Hinnerup, Denmark) containing oxygenated (95%:5% O2:CO2) Krebs buffer(Barnett et al., 2018) strips of myometrium (~0.5 × 15 mm) from the superior portion of the transverse incision were clip-mounted to a force transducer and stretched isometrically to L0 then held at 2 grams of final tension. The relationship between experimental stretch to achieve L0 and its impact on the activity of stretch-activated Piezo1 was tested in a set of experiments where tissues were stretched to 1 rather than 2 grams. Lowering the initial tension had no effect on the ability of the Piezo1 agonist Yoda1 to activate the channel. Tissues were challenged with KCL (60 mM replacing NaCl) for 3 minutes, followed by wash-out, then allowed to equilibrate for 1 hour, or until regular spontaneous contractions were observed. Only tissues that responded to KCL-challenge were employed in experiments. Tissues were further challenged with 8 nM oxytocin to mimic endogenous laboring conditions. Control tissues were exposed to a volumetric equivalent of drug solvent. Tissues were pretreated for 15 minutes with the either the PKA inhibitor, ‘PKI 14-22 amide, myristoylated’ (myrPKI14-22 10 μM, Cat. No. 2546 - Tocris, Minneapolis, MN) (Harris et al., 1997), the AKT inhibitor FPA-124 (10 μM, Cat. No. 2926 - Tocris, Minneapolis, MN) (Strittmatter et al., 2012) the eNOS inhibitor Nω-Nitro-L-arginine, (L-NNA 100 μM - N5501 MilliporeSigma, St. Louis, MO), the BKCa inhibitor, Paxilline (10 μM, Cat. No. 2006, Tocris), or the BKCa activator, NS1619 (30 μM, Cat. No. 3804, Tocris), ± 3 μM Yoda1 (~EC50) for 60 minutes. Area under the curve (AUC), peak tension, and contractile frequency were analyzed using LabChart (version 8.1.12, Win10, ADInstruments., Colorado Springs, CO).
Statistical Analysis:
For organ bath experiments each ‘n’ is a unique patient from which the values of 1-3 myometrial strips for each condition were averaged. The last three contractions of each dosing and control period were analyzed to determine AUC and peak tension. Peak tension is defined as the maximum tension at the height of contraction and subtracted from the minimum tension for each given contraction. Each tissue strip was normalized to its own baseline prior to dosing, then to the average of all control strips to account for rundown (controls are vol. equivalents of drug solvent; DMSO for Yoda1, Dooku1, FPA-124, Paxilline, NS1619 and KREBS for myrPKI14-22 and L-NNA). The average of all control strips for each ‘n’ was set to a nominal value of ‘100’ so that data is presented as a “% change from control.” All tissues that did not recover (regain contractions) following washout were rejected for analysis. Contractile frequency is defined the as the number of contractions in the final 15 minutes of the dosing interval divided by the number of contractions during the 15 minutes period just prior to dosing and presented as percentage.
For all experiments, Student’s t-test were unpaired and two-tailed. Normally distributed data were analyzed with a Welch’s correction to account for variable SDs, while non-normal data were subjected to a Mann-Whitney test. Likewise, either an ordinary one-way ANOVA test was employed, or a Kruskal-Wallis test for non-parametric data, as appropriate. All error bars on graphs displayed as SD unless otherwise stated. All data were analyzed using Prism (v. 9.3.1, Graphpad Software, San Diego, CA).
Results
Expression of Piezo1 during pregnancy:
Total protein from full-thickness human myometrium was run on a Western blot (n=5 per sample type). Samples were collected during the following states of pregnancy and labor: non-pregnant (NP), preterm non-laboring (PTNL), term non-laboring (TNL), preterm laboring (PTL) and term laboring (TL). Blots were probed for Piezo1 and normalized to GAPDH, with the average of all NP values set to a nominal value of ‘1.’ Analysis determined that Piezo1 is most highly expressed in TL tissue (Fig. 1), with a 3.57-fold increase in expression over NP (P=0.0117). Piezo1 is also more significantly expressed in TL vs. PTNL (P=0.0427), TL vs. TNL (P=0.0126), PTNL vs. PTL (P=0.0020) and most notably TL vs. PTL (P=0.0028), with a 13.91-fold difference in Piezo1 expression. There was no significant difference in expression of Piezo1 between NP/TNL/PTNL (ANOVA, P=0.1033). Patient demographics and relevant pregnancy data (Table 1) are provided in recognition that health disparities are known correlatives to PTL (de Oliveira et al., 2018), and because better systematic preterm reporting metrics are needed (Chawanpaiboon et al., 2019).
figure 1: Piezo1 protein expression in human myometrium.
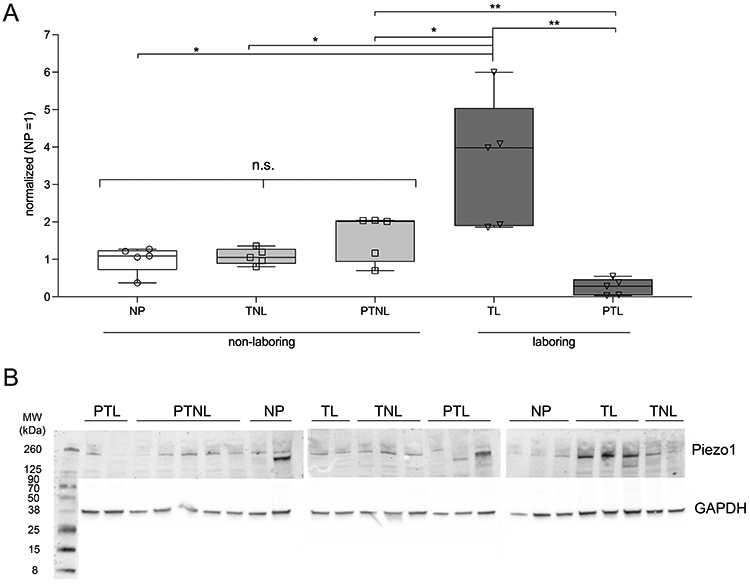
A) Box and whisker plot (min-max) with overlay of individual values of Western blot data using whole human myometrial tissue (n=5 per state). Piezo1 expression does not significantly increase prior to labor (one-way ANOVA, P=0.103); however, it is upregulated ~3.5-fold during TL (P=0.012) vs. NP tissue and downregulated by ~14-fold in PTL relative to TL (P=0.0028). Piezo1 expression is also significantly different in TL vs. PTNL (P=0.0427), TL vs. TNL (P=0.0126), PTNL vs. PTL (P=0.0020) and TL vs. PTL (P=0.0028). Each sample was normalized to GAPDH expression, and the average value of NP tissue was set to a nominal value of ‘1’ for comparative purposes. NP - non pregnant; TNL - term non-laboring; TL - term laboring; PTNL - preterm non-laboring; PTL - preterm laboring. B) Western blots probed for Piezo1 (~260 kDa) and normalized to GAPDH.
Table 1: Pregnancy Data.
Maternal ages, gestational periods, race, and pregnancy/birth complications presented for samples used in Western blots. The difference in gestation between TL and PTL was 4 weeks. Data presented as ± SD.
| Maternal Demographics & Pregnancy Data | ||||
|---|---|---|---|---|
| Sample Type |
Maternal Age (years) |
Gestational Period (weeks) |
Race | Complications |
| NP | 41.8 ± 9.28 | n/a | (5) Caucasian | dysmenorrhea; menorrhagia; abnormal uterine bleeding; omentum mass; endometriosis |
| TNL | 29.0 ± 6.60 | 38.8 ± 1.10 | (5) Caucasian | cervical dilation; low fetal heart rate |
| TL | 34.6 ± 6.19 | 38.6 ± 0.89 | (5) Caucasian | fetal macrosomia; failure to descend; fetal distress; uterine fibroids |
| PTNL | 29.0 ± 6.78 | 32.8 ± 1.30 | (1) Latino (4) Caucasian | placenta previa; cervical intraepithelial neoplasia; preeclampsia; PPROM |
| PTL | 29.6 ± 7.09 | 34.6 ± 1.52 | (1) Latino (1) Hispanic (3) Caucasian | cervical dilation, placenta accreta; placenta previa; breach |
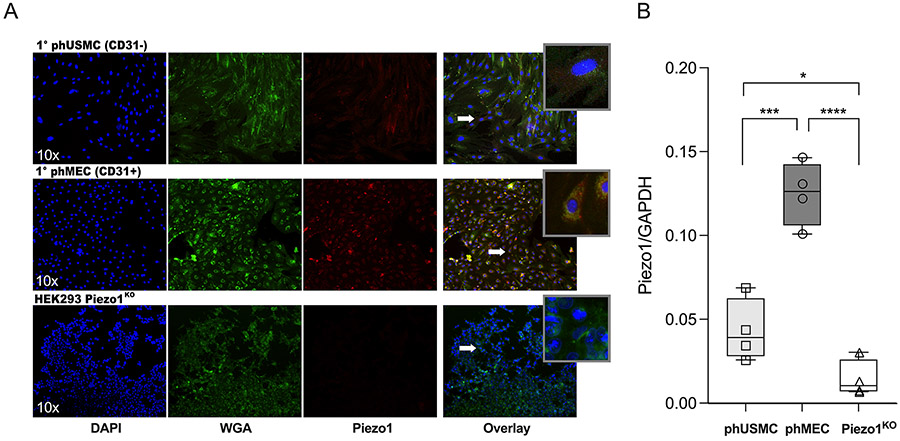
Immunofluorescent imaging and quantification of Piezo1 in myometrial CD31+ and CD31− cells:
To determine distribution of Piezo1 in the two major myometrial cell types, CD31+ (phMEC) and CD31− (phUSMC), immunofluorescence and Western blots were used. Immunofluorescent imaging: Primary cells were plated on 35 mm glass bottom dishes, then labeled with the nuclear stain, 4’,6-diamidino-2-phenylindole, DAPI (blue), wheat germ agglutinin (WGA) to identify cellular boundaries (green), and Piezo1 (red), then imaged at 10x (Fig. 2A). Piezo1KO cells were used as a negative control. Western blot: TNL human myometrial protein was collected from primary phMECs, phUSMCs (p2-4) and Piezo1KO cells (n=4). Total protein was run on a Western blot (as described above) and labeled with Piezo1 antibody and normalized to GAPDH. phMECs exhibited a 2.89-fold increase (P=0.0008) in Piezo1 expression over phUSMCs, while Piezo1KO cells expressed significantly less Piezo1 than either phUSMC (P=0.0185) or phMEC (P<0.0001) cell lines (Fig. 2B).
figure 2: Piezo1 expression in human myometrial phMEC, phUSMC and Piezo1KO cells.
A) Immunofluorescent (IF) imaging of primary phUSMC and phMEC cells from TNL human myometrium and HEK293 Piezo1 KO cells, labelled with DAPI (nuclear), Wheat germ agglutinin (WGA, membrane) and Piezo1-congugated antibodies. Magnified areas of interest (white arrows) presented in the overlay ‘inset’. B) Box and whisker plot (min-max) of Western blot data with overlay of individual values in myometrial phUSMC, phMEC, and Piezo1KO cell lysates (n=4) reveals a ~2.9-fold increase in Piezo1 expression in phMEC cells over phUSMC (P=0.0008). Piezo1KO cells expressed insignificant Piezo1 when compared to either phUSMC (P=0.0185) or phMEC (P<0.0001).
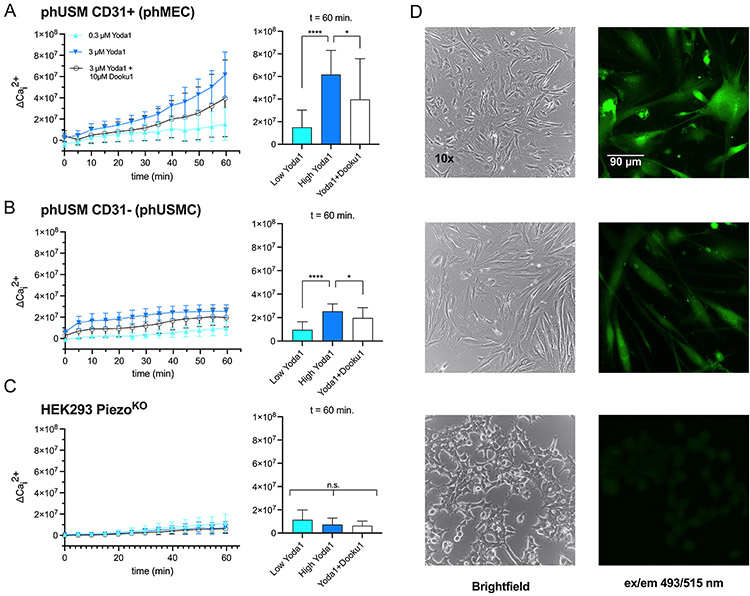
Piezo-mediated Ca2+ influx in CD31+ and CD31− cells:
Piezo1 is selectively permeable to some monovalent and divalent cations (Coste et al., 2010b), primarily Ca2+ (Zhang et al., 2021), with K+ permeability decreasing markedly in the presence of competing extracellular Ca2+ (Gnanasambandam et al., 2015). Because Piezo1 is expressed in both myometrial phMEC and phUSMC, we sought to quantify the relative Ca2+ permeability of Piezo1 in each cell type. To achieve this, we implemented an intracellular calcium flux assay following Piezo1 stimulation using the Piezo1 agonist Yoda1 (Cat. No. 5586 - Tocris, Minneapolis, MN). Calbryte™ 520 AM is a membrane permeable fluorescent Ca2+ indicator that becomes active when hydrolyzed by intracellular esterase. Myometrial-derived primary phUSMC/phMEC, and Piezo1KO cells, were plated to ~80% confluency (Fig. 3D) and incubated with Calbryte™, followed by the addition of 2.5 mM Ca2+, then exposed to either 0.3 or 3 μM Yoda1 ± 10 μM of the Piezo1 antagonist, Dooku1. Fluorescence was recorded for 60 minute (ex/em 493/515 nm) at 5 minute intervals (n=18, Fig. 3A-3C). At the terminal time point (t=60 min), phMECs that had been treated with 3 μM Yoda1 (maximum dose) exhibited 4.09-fold increase in Ca2+ uptake (ΔCai2+) over 0.3 μM treated cells (Fig. 3A, p<0.0001), which decreased by 35.74% when co-treated with Dooku1 (P=0.0327). phUSMC cells under the same conditions experienced a 2.64-fold increase in fluorescence (p<0.0001), with a respective decrease of 22.49% when co-treated with Dooku1 (Fig. 3B, P=0.0326). Conversely, in the Piezo1KO cell line, fluorescent signal did not vary significantly at any dose of Yoda1 or Yoda1 + Dooku1 relative to baseline (Fig. 3C, Kruskal-Wallis one-way ANOVA, P=0.2622).
Figure 3: Piezo1-meidiated Ca2+ influx in CD31+ and CD31− human myometrial cells.
An intracellular calcium flux assay determined Piezo1 activity in phMEC (CD31+) and phUSMC (CD31−) cells. phMEC, phUSMC, and HEK293 Piezo1KO cells were pretreated with the Ca2+ indicator Calbryte® (ex/em 493/515 nm) followed by exposure to Yoda1 (0.3 or 3 μM) ± the Piezo1 antagonist, Dooku1 (10 μM) and the change in fluorescence (ΔCai2+) was measured. A) phMECs treated with 3 μM Yoda1 exhibited 4.09-fold increase in Ca2+ uptake (ΔCai2+) over 0.3 μM treated cells (p<0.0001) which decreased by 35.74% when co-treated with Dooku1 (P=0.0327) at 60 minutes. B) phUSMC experienced a 2.64-fold increase in fluorescence when challenged with 3 μM Yoda1 (p<0.0001), with a respective decrease of 22.49% when co-treated with Dooku1 (P=0.0326). C) Piezo1KO fluorescence did not vary significantly at any dose of Yoda1 or Yoda1 + Dooku1 relative to baseline (Kruskal-Wallis one-way ANOVA, P=0.2622). (D) left panel - 10x bright field images of (top) phMEC, (middle) phUSMC, and (bottom) Piezo1KO. right panel - Calbryte-induced fluorescence after 3 μM Yoda1 stimulation. 20x fluorescent images of (top) phMEC, (middle) phUSMC, and (bottom) Piezo1KO. Data presented as ± SD.
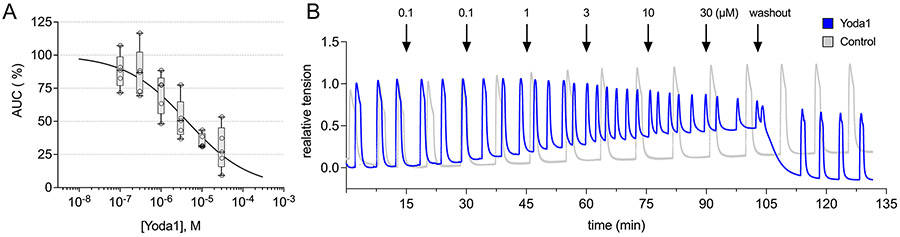
EC50 of Yoda1 in human myometrium:
The role of Ca2+ varies greatly throughout the body. In myometrial tissue Ca2+ entry into endothelial cells initiates a signaling cascade that activates endothelial nitric oxide synthase (eNOS), producing the quiescent-promoting molecule nitric oxide. In the myocyte its actions are more nuanced. While it is primarily known as a depolarizing molecule that triggers smooth muscle myosin phosphorylation (pMYL9) via calmodulin/MLCK activation, it also serves as a ligand to channels such as BKCa, which re-polarizes the cell through potassium efflux. To determine the net inotropic effect on whole myometrial tissue, human TNL myometrium (n=6) was exposed to an accumulative dose of Yoda1 in 15 minute intervals from 100 nM to 30 μM in half-log increments (Fig. 4). Each bath was normalized to itself upon return to normal, phasic contractions following oxytocin addition, then to a control bath at each time point (volumetric DMSO equivalent) to account for tissue rundown. The EC50 of Yoda1 in human myometrium, which we define as a reduction in the area under the curve (AUC) by 50% using the last three contractions per experimental period, was 3.02 μM with a corresponding Hill slope of −1.242 (Fig. 4). Relative to untreated control tissue (100%), the AUC for each concentration of Yoda1 was: 100 nM (88.03%, SD 13.01), 300 nM (86.72%, SD 18.65), 1 μM (70.91%, SD 15.52), 3 μM (52.02%, SD 15.50), 10 μM (35.08%, SD 5.62), 30 μM (29.74%, SD 16.59). Of note, this experiment was also run with the tissue stretched to a final tension of 1 gram (rather than 2 grams) to test for potential variability in Piezo1 activation under different amounts of stretch. No statistical difference in the dose-response curve was observed (data not shown).
figure 4: EC50 of Piezo1 agonist (Yoda1) in human myometrium.
A) TNL human myometrium (n=6) was hung in an organ bath, oxytocin-challenged (8nM), then dosed with the Piezo1 agonist, Yoda1, in 15 minute intervals (0.1, 0.3, 1, 3, 10, 30 μM). Tissue relaxed to Yoda1 exposure in a dose-dependent manner, with an EC50 of 3.02 μM. Relative to untreated control tissue (100%), the AUC for each concentration of Yoda1 was: 100nM (88.03%, SD 13.01), 300nM (86.72%, SD 18.65), 1 μM (70.91%, SD 15.52), 3 μM (52.02%, SD 15.50), 10 μM (35.08%, SD 5.62), 30 μM (29.74%, SD 16.59). Data presented as a box and whisker plot (5-95 percentile) with individual data point overlay. B) Representative traces of Yoda1 (⬤) and control (⬤) tissue.
The effect of PKA/AKT/eNOS/BKCa modulation on Yoda1-induced myometrial quiescence:
Following the derivation of the EC50 for Yoda1 in human myometrium, we next opted to modulate endothelial Ca2+-mediated pathways of nitric oxide generation, as well as the Ca2+-activated BKCa channel, to determine their effects on Piezo1 stimulation by Yoda1. Both protein kinase A (PKA) and protein kinase B (AKT) are known to activate eNOS in MECs and can be stimulated through a rise in intracellular Ca2+(Zhang & Hintze, 2006; Bir et al., 2012). We elected to modulate PKA, AKT, eNOS, to determine whether these treatments would dampen the effects of Piezo-1 stimulation on the tissue, and to inhibit/activate BKCa during Piezo-1 stimulation to determine if BKCa contributes to Yoda1-induced quiescence (Fig. 5).
figure 5: Inotropic effects of Piezo1 agonism on Ca2+-mediated myometrial quiescent pathways.
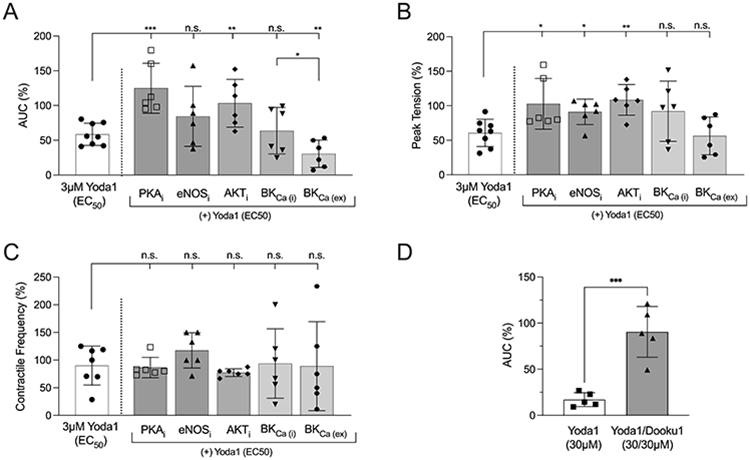
Using an organ bath (n=6), area under the curve (AUC), peak tension, and contractile frequency were determined after co-administration of an EC50 dose (3μM) of Yoda1 + myrPKI14-22 (PKAi), FPA-124 (AKTi), L-NNA (eNOSi), Paxilline (BKCa(i)), or NS1619 (BKCa(ex)). A) AUC: In TNL myometrium, co-treatment with myrPKI14-22 (10 μM) + Yoda1 (P=0.0005) or FPA-124 (10 μM) + Yoda1 (P=0.0067) significantly reduced the effects of Yoda1 returning AUC to baseline. Excitation of BKCa (NS1619, 30 μM) + Yoda1 resulted in an additive effect, decreasing AUC to 28.8% of baseline. B) Peak Tension: Co-treatments with myrPKI14-22 + Yoda1 (P=0.0026), FPA-124 + Yoda1 (P=0.0021), and L-NNA + Yoda1 (P=0.0402) resulted in a significant increase peak tension when compared to treatment with 3 μM Yoda1 alone, while neither NS1619 + Yoda1 (P=0.4770) nor Paxilline + Yoda1 (P=0.1887), significantly altered tension beyond that of 3 μM Yoda1 alone. C) Contractile frequency: None of the treatment conditions, myrPKI14-22 + Yoda1 (P=0.8343), FPA-124 + Yoda1 (P=0.4077), L-NNA + Yoda1 (P=0.1718), NS1619 + Yoda1 (P=0.8074), or Paxilline + Yoda1 (P=0.7084) significantly deviated contractile frequency when compared to a stand-alone EC50 dose of Yoda1. D) Selectivity of Yoda1 was determined by comparing the AUC (n=5) after a maximum dose of Yoda1 (30 μM) relative to co-treatment of Yoda 1 with Piezo1 antagonist, Dooku1 (30 μM/30 μM). Treatment with 30μM Yoda1 decreased AUC to 17.08% of baseline, while co-treatment with Yoda1 + Dooku1 (30 μM/30 μM) recovered AUC to 90.55% of baseline (P=0.0004).
As above, TNL human myometrium (n=6) was hung in an organ bath then pretreated for 15 minutes with the either the PKA inhibitor, ‘PKI 14-22 amide, myristoylated’ (myrPKI14-22 10 μM) , the AKT inhibitor FPA-124 (10 μM) the eNOS inhibitor Nω-Nitro-L-arginine, (L-NNA 100 μM), the BKCa inhibitor, Paxilline (10 μM, Cat. No. 2006, Tocris), or the BKCa activator, NS1619 (30 μM, Cat. No. 3804, Tocris), ± 3 μM Yoda1 (~EC50) for 60 minutes. Area under the curve (AUC), peak tension, and contractile frequency were quantified.
AUC:
Co-treatment of the tissue with myrPKI14-22 + Yoda1 (P=0.0005), FPA-124 + Yoda1 (P=0.0067), but not L-NNA + Yoda1 (P=0.3244) resulted in a significant increase AUC when compared to treatment with 3 μM Yoda1 alone. Stimulation of BKCa with NS1619 + Yoda1 exhibited an additive negative inotropic effect, further decreasing AUC to 28.83% of baseline (P=0.0077), while BKCa inhibition using Paxilline + Yoda1 (P=0.4466) did not significantly alter AUC beyond the effects of Yoda1 alone; however, NS1619/Yoda1 vs. Paxilline/Yoda1 were significantly different (P=0.0311) (Fig. 5A, right most two conditions), and the administration of either Paxilline or NS1619 as individual agents in the absence of Yoda1 produced significantly higher AUCs (105.1% and 82.86% of baseline, respectively) than when coadministered with Yoda1 (Paxilline vs. Paxilline + Yoda1, −41.34% ± 17.36, p=0.0206; NS1619 vs. NS1619 + Yoda1, −52.32% ± 21.27, p=0.0181 - data not shown). AUC was also determined using a maximum dose of Yoda1 (30 μM) and analyzed against co-treatment with the Piezo1 antagonist, Dooku1 (30 μM/30 μM), to ensure the selectivity of Yoda1. Treatment for 60 minutes with 30 μM Yoda1 decreased AUC to 17.08% of baseline, while co-treatment with Yoda1 + Dooku1 (30 μM/30 μM) recovered AUC to 90.55% of baseline (Fig. 5D, P=0.0004).
Peak Tension:
In contrast to AUC, co-treatment of the tissue with myrPKI14-22 + Yoda1 (P=0.0026), FPA-124 + Yoda1 (P=0.0021), and L-NNA + Yoda1 (P=0.0402) resulted in a significant increase peak tension, while neither BKCa stimulation with NS1619 + Yoda1 (P=0.4770), nor inhibition with Paxilline + Yoda1 (P=0.1887), had a significant effect on tension when compared to treatment with 3 μM Yoda1 alone (Fig. 5B).
Contractile frequency:
Co-treatment of the tissue with myrPKI14-22 + Yoda1 (P=0.8343), FPA-124 + Yoda1 (P=0.4077), L-NNA + Yoda1 (P=0.1718), NS1619 + Yoda1 (P=0.8074), or Paxilline + Yoda1 (P=0.7084), did not alter contractile frequency (Fig. 5C).
Discussion
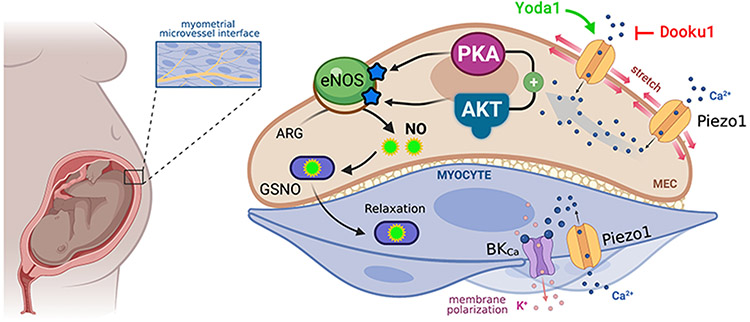
The two primary findings of this research are the novel identification of the mechanosensitive cation channel, Piezo1, in human myometrium, and the discovery that Piezo1 stimulation imparts negative inotropic effects on the tissue, intimating its potential as a tocolytic (Fig. 6).
Figure 6: Proposed pathway for Piezo1-mediated quiescence in human myometrium.
We posit that the stretch experienced by the uterus as the fetus develops activates Piezo1 channels on endothelium and smooth muscle cells of the myometrium. This stretch drives Ca2+ influx into the endothelium where PKA and AKT activate eNOS, generating the quiescence-promoting molecule, nitric oxide, which migrates to the myocyte in the form of S-nitrosoglutathione (GSNO). Concurrently, in the smooth muscle, Piezo1 activation may contribute to BKCa activation, which polarizes the membrane through K+ efflux. Graphic created with BioRender.com.
Spontaneous PTL remains largely an enigma. Advances in perinatal therapeutics bely our failure to better understand the most relevant contributing factors that initiate idiopathic labor. To advance our understanding of myometrial quiescence we posit that it is necessary to view the myometrium simplistically as a two-compartment system. We do not disavow the importance of resident immune cells, nor their role in inflammatory regulation of pregnancy, rather we are exploring relaxation mechanisms that can be demonstrated to prevent PTL in the face of the inflammatory environment.
In many ways it is reasonable to classify pregnancy as a transient pathological state. Following a marked and prolonged increase in proinflammatory mediators during pregnancy (Mor et al., 2011; Cappelletti et al., 2016), intense stretching of the uterine cavity often initiates labor (Waldorf et al., 2015), and it has been theorized that the resulting labor is amplified through the phasic increase in uterine load during subsequent contractions forming a positive feedback loop (Young, 2016). Practically speaking, this is realized by the uptick of PTB rates observed with multi-fetal pregnancies (twins 60.87%, triplets 98.5%) (Martin et al., 2021). Given the unique hydrostatic loads endured by the myocytes and microvascular endothelium of the myometrium during pregnancy, stretch activated channels (SACs) present as a logical investigative target.
Many important contractile proteins are regulated and/or activated by stretch. Cx43, a critical uterine gap junction that imparts cable properties to the myometrium during labor (Pierce et al., 2002; Barnett et al., 2020), is upregulated by mechanical stress (Tellios et al., 2019; Shi, 2021). It has even been suggested that stretch activation of gap junctions in the cervix may initiate crosstalk between the cervix and myometrium to initiate labor (Vink, 2020). TREK-1, an outward rectifying potassium channel is activated by, and upregulated by stretch (Buxton et al., 2010; Heyman et al., 2013), a finding amplified in twin pregnancies (Yin et al., 2018). When considering the role stretch and PTL, it is important to note that stretch alone is most likely not the primary driver of early labor, especially in singleton pregnancies. During ‘extremely preterm’ (less than 28 weeks) and ‘very preterm’ labor (28 to 32 weeks), the fetus and amnion have not developed enough to impart the excessive uterine strain needed to initiate labor, as evidenced by the finding that the ‘maximum uterine wall tension’ in PTL pregnancies (singleton) from weeks 20-30 are equal to or less than in pregnancies that carry to term (Sokolowski et al., 2010). Our finding that Piezo1 is differentially regulated between TL and TNL, and between PTL and PTNL (Fig. 1), indicates the Piezo1 expression is more likely linked to labor state, and not specifically uterine distension. If we consider that during active labor the myometrium must modulate rapidly between contraction and quiescence, it is not surprising that Piezo1 is upregulated to facilitate the phasic nature of the laboring process, and the fact that Piezo1 is significantly downregulated in PTL vs TL suggest that Piezo1 is dysregulated in PTL myometrium. Further, because Piezo1 can be stimulated beyond what stretch alone can achieve using Yoda1 (Fig. 4), it is possible that chemical stimulation of the downregulated channel (PTL myometrium) may compensate for the dearth of Piezo1.
Our finding that Piezo1 is expressed in both the myocyte and microvascular endothelium of the myometrium (Fig. 2) necessitated a more thorough investigation into the role of Piezo1 in each cell type. There is a paucity of data in smooth muscle to determine the role of Piezo1 channel function (Retailleau et al., 2015), and no studies are available (National Library of Medicine, n.d.) examining Piezo1 channels in uterine smooth muscle, or their effect on tension.
Endothelial Piezo1 channels have been found to either relax (Evans et al., 2018), or contract (Rode et al., 2017), underlying smooth muscle depending on their tissue location and the presence of pathology. Ca2+ entry following activation of Piezo1 channels in endothelium leads to increased nitric oxide generation via eNOS activation through cAMP-mediated activation of PKA (Bir et al., 2012) or by phosphoinositide3-kinase (AKT) (Zhang & Hintze, 2006). Piezo1 channels have not yet been studied in the endothelial cells of the myometrium. Their presence, as we demonstrate here, provides an explanation for the physiological origin of nitric oxide as a constitutive quiescence signal during gestation. When investigating expression of Piezo1 and the subsequent Ca2+ entry into phMECs via Piezo1 stimulation, we found that phMECs express Piezo1 at a higher level than phUSMCs (Fig. 2B), and that the expression of Piezo1 correlates to Ca2+ permeability (Fig. 3A/3B). This implies that Piezo1’s relative abundance on phMECs contributes to nitric oxide generation. Our further analysis of Piezo1 activation with Yoda1 using whole tissue in the organ bath supports this hypothesis, as the inhibition of PKA, AKT or eNOS each diminished the effects of Piezo1 stimulation by Yoda1 (Fig. 5).
Although Piezo1 is an inwardly rectifying cation channel, it cannot be assumed to contribute to contraction in myometrial muscle. Ca2+ influx in myometrium, mediated by voltage-dependent L-type Ca2+ channels (Bean, 1989), which is blocked by nifedipine and amlodipine, is expected to determine the final contractile state of myometrium, and ultimately parturition (Wray et al., 2003). However, calcium-channel blockers do not provide adequate tocolysis (Nijman et al., 2016; van Vliet et al., 2016; Songthamwat et al., 2018) at safe doses. Smooth muscle Ca2+ signals can differ in spatial and temporal distribution (Amberg & Navedo, 2013; Brozovich et al., 2016) and include highly localized Ca2+ release events (e.g., sparklets and sparks) (Brozovich et al., 2016) that regulate signaling locally (Mercado et al., 2014); are not necessarily elicited by global increases in [Ca2+]i; are compartmented (Buxton & Brunton, 1983); and do not result in contraction per se, thus, we do not attribute phUSMC Piezo1 activation to providing a Ca2+ source for contraction. This is evidenced most notably by our finding that Piezo1 stimulation by Yoda1 induces relaxation of the myometrium in a dose-dependent manner (Fig. 4), and through our finding that BKCa stimulation amplifies the Yoda-1 response (Fig. 5A). Because the myometrium is ~90% muscle by volume, if Piezo1 stimulation primarily aided in depolarization of the myocyte, we would not expect to see such robust negative inotropic effects on the tissue (Fig. 4, Fig. 6), a finding amplified by our observation that Yoda1 was still effective at reducing AUC when eNOS was inhibited (Fig. 5A). While a shortcoming of this study was the limited availability of PTL myometrium for additional organ bath testing, the TNL myometrium used in lieu of PTL exhibits a comparable expression profile of Piezo1 (Fig. 1), inferring its potential as a suitable analog.
If Ca2+ permeation via Piezo1 in uterine myocytes does not facilitate contraction, how might it mediate quiescent signaling? It has previously been determined that stretch activation of Piezo1 results in stimulation of large conductance Ca2+-activated K+ (BKCa) channels (Hoyer et al., 1994; Jakob et al., 2021). BKCa mRNA (Shi et al., 2015) and protein (Wakle-Prabagaran et al., 2016) have been identified in uterine smooth muscle, and BKCa is regulated by pregnancy state (Gao et al., 2009). In the myometrium, BKCa stimulation via the mechanosensitive Ca2+ channel, TRPV4 (Liedtke, 2006), has already been shown to relax the tissue (Villegas et al., 2021). Our finding that peak tension is significantly reduced following co-administration of L-NNA (eNOS inhibition) + Yoda1 (Piezo1 stimulation) indicates that myocyte-bound Piezo1 likely contributes to BKCa stimulation (Fig. 5B). It is curious that inhibition of BKCa does not significantly alter the contractile dynamics of Yoda1 EC50-dosed myometrium (Fig. 5 A-C); however, BKCa stimulation by NS1619 does further reduce AUC beyond the Yoda1 EC50-dosed myometrium to approximately 25% of baseline. The failure of BKCa inhibition to alter the response to Yoda1 is not entirely unexpected as Aaronson et al.,(Aaronson et al., 2006) examining BKCa inhibition in rat myometrium, noted the failure of BKCa channel inhibitors to significantly affect contractility in strips from either nonpregnant or pregnant animals. Nonetheless, activation of BKCa in the presence of Yoda1 suppresses contractions approximately 50% more that BKCa activation in the absence of Yoda1 (−52.32% ± 21.27 of control AUC). Because inhibition of BKCa in the absence of Yoda1 does not alter the response to oxytocin, it may be that more than one outwardly rectifying K+ channel contributes to contraction/relaxation dynamics and thus limits what can be resolved from BKCa modulation alone. Indeed, such compensation by channels such as TREK-1 are likely(Sanborn, 2000; Heyman et al., 2013).
Taken together, our data imply an interplay between endothelium and muscle to regulate tone, and that disease can influence the expression of, and ability of Piezo1 channels to mediate contraction. We posit that there is a complex relationship between overlapping and compensatory mechanisms that facilitate the phasic contractile pathways studied here, to include pathways beyond the scope of this investigation. Such a notion is consistent with our hypothesis that myometrial Piezo1 normally provides a homeostatic function that modulates stretch-activated membrane hyperpolarization by cation influx without inducing contraction.
Conclusion:
Preterm labor is a confounding obstetric dilemma. The exceptional physiology of the myometrium necessitates its complete quiescence for the 40 weeks of gestation, all while adapting to the increase in uterine strain as the pregnancy progresses. We have determined that the stretch-activated channel, Piezo1, is expressed in the myocytes and microvascular endothelium of the myometrium, that it is dysregulated during PTL, and that its activation via Yoda1 results in myometrial quiescence through endothelial PKA/AKT activation of eNOS. As such, Piezo1 emerges as a novel target for future tocolytic development.
Supplementary Material
Key Points:
Spontaneous preterm labor is a serious obstetric dilemma without a known cause or effective treatments.
Piezo1 is a stretch-activated channel important to muscle contractile dynamics.
Piezo1 is present in the myometrium and is dysregulated in women who experience preterm labor.
Activation of Piezo1 by the agonist, Yoda1, relaxes the myometrium in a dose-dependent fashion, indicating that Piezo1 modulation may have therapeutic benefits to treat preterm labor.
Acknowledgements:
The authors would like to kindly thank Dr. Ardem Patapoutian of the Howard Hughes Medical Institute at Scripps Research for providing the HEK239 Piezo1KO cells, Dylan Saxon for assistance will cell culture, and the physicians and patients at Renown Regional Medical Center in Reno, NV, for their continued support of our research.
Funding:
NIH, R01 HD091114
Biography

Scott Barnett received his Ph.D. in Cellular and Molecular Pharmacology & Physiology from the University of Nevada, Reno, where he studied the pathophysiology of preterm labor. Upon completion of his degree, Dr. Barnett joined the Medical College of Wisconsin as a PhRMA Foundation Postdoctoral Fellow where he investigated the treatment of renal diseases using dual-ligand pharmacology. Dr. Barnett currently resides in the laboratory of Dr. Iain. L.O. Buxton at the University of Nevada, Reno School of Medicine (Pharmacology), where he identifies and explores dysregulated myometrial pathways to aid in novel tocolytic development.
Footnotes
Competing interests: The authors declare no competing interests.
Data availability statement:
All data in a non-identifying format are securely stored at the University of Nevada, Reno. The data are available upon request.
Bibliography
- Aaronson PI, Sarwar U, Gin S, Rockenbauch U, Connolly M, Tillet A, Watson S, Liu B & Tribe RM (2006). A role for voltage-gated, but not Ca 2+-activated, K + channels in regulating spontaneous contractile activity in myometrium from virgin and pregnant rats. Br J Pharmacol 147, 815–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abramson DJ & Reid DE (1955). Use of relaxin in treatment of threatened premature labor. J Clin Endocrinol Metab 15 2, 206–209. [DOI] [PubMed] [Google Scholar]
- Amberg GC & Navedo MF (2013). Calcium Dynamics in Vascular Smooth Muscle. Microcirculation 20, 281–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arishe OO, Ebeigbe AB & Webb RC (2020). Mechanotransduction and Uterine Blood Flow in Preeclampsia: The Role of Mechanosensing Piezo 1 Ion Channels. Am J Hypertens 33, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asif H, Barnett SD, Buxton ILO. β3 Adrenergic Receptor Signaling in the Human Myometrium. Reprod Sci. 2022. Apr 4:1–11. doi: 10.1007/s43032-022-00917-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett SD, Asif H, Anderson MT & Buxton ILO (2020). Novel Tocolytic Strategy: Modulating Cx43 Activity by S-Nitrosation. J Pharmacol Exp TherJPET-AR-2020-000427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett SD, Smith CR, Ulrich CC, Baker JE & Buxton ILO (2018). S-Nitrosoglutathione Reductase Underlies the Dysfunctional Relaxation to Nitric Oxide in Preterm Labor. Sci Rep 8, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean BP (1989). Classes of calcium channels in vertebrate cells. Annu Rev Physiol 51, 367–384. [DOI] [PubMed] [Google Scholar]
- Bir SC, Xiong Y, Kevil CG & Luo J (2012). Emerging role of PKA/eNOS pathway in therapeutic angiogenesis for ischaemic tissue diseases. Cardiovasc Res 95, 7–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanks AM, Shmygol A & Thornton S (2007). Myometrial function in prematurity. Best Pract Res Clin Obstet Gynaecol 21, 807–819. [DOI] [PubMed] [Google Scholar]
- Botello-Smith WM, Jiang W, Zhang H, Ozkan AD, Lin YC, Pham CN, Lacroix JJ & Luo Y (2019). A mechanism for the activation of the mechanosensitive Piezo1 channel by the small molecule Yoda1. Nat Commun; DOI: 10.1038/s41467-019-12501-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley KKK, Buxton ILOL, Barber JEE, McGaw T & Bradley MEE (1998). Nitric oxide relaxes human myometrium by a cGMP-independent mechanism. Am J Physiol 275, C1668–73. [DOI] [PubMed] [Google Scholar]
- Brozovich F V, Nicholson CJ, Degen CV, Gao YZ, Aggarwal M & Morgan KG (2016). Mechanisms of vascular smooth muscle contraction and the basis for pharmacologic treatment of smooth muscle disorders. Pharmacol Rev 68, 476–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burris HH & Parker MG (2021). Racial and ethnic disparities in preterm birth outcomes: a call to action for neonatal providers. J Perinatol 41, 365–366. [DOI] [PubMed] [Google Scholar]
- Buxton IL, Kaiser RA, Oxhorn BC & Cheek DJ (2001). Evidence supporting the Nucleotide Axis Hypothesis: ATP release and metabolism by coronary endothelium. AmJPhysiol Hear CircPhysiol 281, H1657–H1666. [DOI] [PubMed] [Google Scholar]
- Buxton ILO, Heyman N, Wu YY, Barnett S & Ulrich C (2011). A role of stretch-activated potassium currents in the regulation of uterine smooth muscle contraction. Acta Pharmacol Sin 32, 758–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton ILO, Singer CA & Tichenor JN (2010). Expression of stretch-activated two-pore potassium channels in human myometrium in pregnancy and labor. PLoS One 5, e12372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton ILOLO & Brunton LLL (1983). Compartments of cyclic AMP and protein kinase in mammalian cardiomyocytes. J Biol Chem 258, 10233–10239. [PubMed] [Google Scholar]
- Cappelletti M, Della Bella S, Ferrazzi E, Mavilio D & Divanovic S (2016). Inflammation and preterm birth. J Leukoc Biol 99, 67–78. [DOI] [PubMed] [Google Scholar]
- Chawanpaiboon S, Vogel JP, Moller AB, Lumbiganon P, Petzold M, Hogan D, Landoulsi S, Jampathong N, Kongwattanakul K, Laopaiboon M, Lewis C, Rattanakanokchai S, Teng DN, Thinkhamrop J, Watananirun K, Zhang J, Zhou W & Gülmezoglu AM (2019). Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Heal 7, e37–e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke RWI (2006). Preterm mortality and morbidity over 25 years. Arch Dis Child Fetal Neonatal Ed 91, 293–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corda S, Spurgeon HA, Lakatta EG, Capogrossi MC & Ziegelstein RC (1995). Endoplasmic reticulum Ca2+ depletion unmasks a caffeine-induced Ca2+ influx in human aortic endothelial cells. Circ Res 77, 927–935. [DOI] [PubMed] [Google Scholar]
- Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, Dubin AE & Patapoutian A (2010). Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 330, 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coste B, Xiao B, Santos JS, Syeda R, Grandl J, Spencer KS, Kim SE, Schmidt M, Mathur J, Dubin AE, Montal M & Patapoutian A (2012). Piezo proteins are pore-forming subunits of mechanically activated channels. Nat 2012 4837388 483, 176–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dopico AM, Bukiya AN & Jaggar JH (2018). Calcium- and voltage-gated BK channels in vascular smooth muscle. Pflugers Arch 470, 1271–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans EL, Cuthbertson K, Endesh N, Rode B, Blythe NM, Hyman AJ, Hall SJ, Gaunt HJ, Ludlow MJ, Foster R & Beech DJ (2018). Yoda1 analogue (Dooku1) which antagonizes Yoda1-evoked activation of Piezo1 and aortic relaxation. Br J Pharmacol 175, 1744–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L, Cong B, Zhang L & Ni X (2009). Expression of the calcium-activated potassium channel in upper and lower segment human myometrium during pregnancy and parturition. Reprod Biol Endocrinol 2009 71 7, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimpl G & Fahrenholz F (2001). The oxytocin receptor system: Structure, function, and regulation. Physiol Rev 81, 629–683. [DOI] [PubMed] [Google Scholar]
- Gnanasambandam R, Bae C, Gottlieb PA & Sachs F (2015). Ionic selectivity and permeation properties of human PIEZO1 channels. PLoS One 10, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb PA, Bae C & Sachs F (2012). Gating the mechanical channel Piezo1: A comparison between whole-cell and patch recording. Channels 6, 282–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb PA & Sachs F (2012). Piezo1. Channels 6, 214–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris TE, Persaud SJ & Jones PM (1997). Pseudosubstrate inhibition of cyclic AMP-dependent protein kinase in intact pancreatic islets: Effects on cyclic AMP-dependent and glucose-dependent insulin secretion. Biochem Biophys Res Commun 232, 648–651. [DOI] [PubMed] [Google Scholar]
- Hennes A, Held K, Boretto M, De Clercq K, Van den Eynde C, Vanhie A, Van Ranst N, Benoit M, Luyten C, Peeraer K, Tomassetti C, Meuleman C, Voets T, Vankelecom H & Vriens J (2019). Functional expression of the mechanosensitive PIEZO1 channel in primary endometrial epithelial cells and endometrial organoids. Sci Rep 9, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyman NS, Cowles CL, Barnett SD, Wu YY, Cullison C, Singer CA, Leblanc N & Buxton ILO (2013). TREK-1 currents in smooth muscle cells from pregnant human myometrium. Am J Physiol - Cell Physiol 305, 632–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer J, Distler A, Haase W & Gogelein H (1994). Ca2+ influx through stretch-activated cation channels activates maxi K+ channels in porcine endocardial endothelium. Proc Natl Acad Sci U S A 91, 2367–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakob D et al. (2021). Piezo1 and BKCa channels in human atrial fibroblasts: Interplay and remodelling in atrial fibrillation. J Mol Cell Cardiol 158, 49–62. [DOI] [PubMed] [Google Scholar]
- John L, Ko NL, Gokin A, Gokina N, Mandalà M & Osol G (2018). The piezo1 cation channel mediates uterine artery shear stress mechanotransduction and vasodilation during rat pregnancy. Am J Physiol - Hear Circ Physiol 315, H1019–H1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korinek K & Ahmmad Z (2021). The Racial Configuration of Parent Couples and Premature Birth: an Analysis of the Utah Population Database. J Racial Ethn Heal Disparities 655–669. [DOI] [PubMed] [Google Scholar]
- Lai PF, Tribe RM & Johnson MR (2016). Differential impact of acute and prolonged cAMP agonist exposure on protein kinase A activation and human myometrium contractile activity. J Physiol 594, 6369–6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lhomme A, Gilbert G, Pele T, Deweirdt J, Henrion D, Baudrimont I, Campagnac M, Marthan R, Guibert C, Ducret T, Savineau JP & Quignard JF (2019). Stretch-activated piezo1 channel in endothelial cells relaxes mouse intrapulmonary arteries. Am J Respir Cell Mol Biol 60, 650–658. [DOI] [PubMed] [Google Scholar]
- Li J et al. (2014). Piezo1 integration of vascular architecture with physiological force. Nature 515, 279–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedtke WB (2006). TRP ion channel function in sensory transduction and cellular signaling cascades. CRC Press. [PubMed] [Google Scholar]
- Lukacs V, Mathur J, Mao R, Bayrak-Toydemir P, Procter M, Cahalan SM, Kim HJ, Bandell M, Longo N, Day RW, Stevenson DA, Patapoutian A & Krock BL (2015). Impaired PIEZO1 function in patients with a novel autosomal recessive congenital lymphatic dysplasia. Nat Commun 6, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JA, Hamilton BE, Osterman MJK, Driscoll AK, Schwartz S & Horon I (2021). Births: Final data for 2019. Natl Vital Stat Reports. [PubMed] [Google Scholar]
- Mercado J, Baylie R, Navedo MF, Yuan C, Scott JD, Nelson MT, Brayden JE & Santana LF (2014). Local control of TRPV4 channels by akap150-targeted PKC in arterial smooth muscle. J Gen Physiol 143, 559–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mor G, Cardenas I, Abrahams V & Guller S (2011). Inflammation and pregnancy: The role of the immune system at the implantation site. Ann N Y Acad Sci 1221, 80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardi A & Olesen S-P (2008). BK Channel Modulators: A Comprehensive Overview. Curr Med Chem 15, 1126–1146. [DOI] [PubMed] [Google Scholar]
- National Library of Medicine (n.d.). PubMed Literature Search.
- Nijman TAJ, van Vliet EOG, Naaktgeboren CA, Oude Rengerink K, de Lange TS, Bax CJ, Bloemenkamp KWM, van Eyck J, Kok M, Scheepers HCJ, Woiski M, Franx A, Mol BWJ & Oudijk MA (2016). Nifedipine versus placebo in the treatment of preterm prelabor rupture of membranes: a randomized controlled trial: Assessment of perinatal outcome by use of tocolysis in early labor—APOSTEL IV trial. Eur J Obstet Gynecol Reprod Biol 205, 79–84. [DOI] [PubMed] [Google Scholar]
- de Oliveira KA, de Araújo EM, de Oliveira KA, Casotti CA, da Silva CAL & dos Santos DB (2018). Association between race/skin color and premature birth: A systematic review with meta-analysis. Rev Saude Publica 52, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outcomes C on UPB and AH & Press TNA (2007). Preterm Birth: Causes, Consequences, and Preventioned. Behrman A RE and SB. Institute of Medicine of the National Academies, Washington, D.C. [Google Scholar]
- Pierce BT, Calhoun BC, Adolphson KR, Lau AF & Pierce LM (2002). Connexin 43 expression in normal versus dysfunctional labor. Am J Obstet Gynecol 186, 504–511. [DOI] [PubMed] [Google Scholar]
- Retailleau K, Duprat F, Arhatte M, Ranade SS, Peyronnet R, Martins JR, Jodar M, Moro C, Offermanns S, Feng Y, Demolombe S, Patel A & Honoré E (2015). Piezo1 in Smooth Muscle Cells Is Involved in Hypertension-Dependent Arterial Remodeling. Cell Rep 13, 1161–1171. [DOI] [PubMed] [Google Scholar]
- Rode B et al. (2017). Piezo1 channels sense whole body physical activity to reset cardiovascular homeostasis and enhance performance. Nat Commun 8, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romac JMJ, Shahid RA, Swain SM, Vigna SR & Liddle RA (2018). Piezo1 is a mechanically activated ion channel and mediates pressure induced pancreatitis. Nat Commun 9, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanborn BM (2000). Relationship of ion channel activity to control of myometrial calcium. J Soc Gynecol Investig 7, 4–11. [DOI] [PubMed] [Google Scholar]
- Sanders KM, Kito Y, Hwang SJ & Ward SM (2016). Regulation of Gastrointestinal Smooth Muscle Function by Interstitial Cells. Physiology (Bethesda) 31, 316–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders KM & Smith TK (1986). Motoneurones of the submucous plexus regulate electrical activity of the circular muscle of canine proximal colon. J Physiol 380, 293–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santamaria X, Mas A, Cervelló I, Taylor H & Simon C (2018). Uterine stem cells: From basic research to advanced cell therapies. Hum Reprod Update 24, 673–693. [DOI] [PubMed] [Google Scholar]
- Satoh K, Hata M, Takahara S, Tsuzaki H, Yokota H, Akatsu H, Yamamoto T, Kosaka K & Yamada T (2006). A novel membrane protein, encoded by the gene covering KIAA0233, is transcriptionally induced in senile plaque-associated astrocytes. Brain Res 1108, 19–27. [DOI] [PubMed] [Google Scholar]
- Shi J, Jin L, Leng J & Lang J (2015). Response of potassium channels to estrogen and progesterone in the uterine smooth muscle cells of adenomyosis in vitro]. Zhonghua Fu Chan Ke Za Zhi 50, 843–847. [PubMed] [Google Scholar]
- Shi Y (2021). Upregulation of Cx43 Expression Under Stretch Condition is Mediated by TGF Beta1 and Cytoskeletal Network. 1–23. [Google Scholar]
- Sokolowski P, Saison F, Giles W, McGrath S, Smith D, Smith J & Smith R (2010). Human uterine wall tension trajectories and the onset of parturition. PLoS One; DOI: 10.1371/journal.pone.0011037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songthamwat S, Na Nan C & Songthamwat M (2018). Effectiveness of nifedipine in threatened preterm labor: a randomized trial. Int J Womens Health Volume 10, 317–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter F, Walther S, Roosen A, Rutz B, Schlenker B, Limmer S, Waidelich R, Stief CG, Gratzke C & Hennenberg M (2012). Activation of protein kinase B/Akt by alpha1-adrenoceptors in the human prostate. Life Sci 90, 446–453. [DOI] [PubMed] [Google Scholar]
- Sweeney EM, Dockery P, Crankshaw DJ, O’Brien YM, Walsh JM & Morrison JJ (2014). Human uterine lower segment myometrial cell and nuclear volume at term: Influence of maternal age. J Anat 225, 625–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellios N, Feng M, Chen N, Liu H, Tellios V, Wang M, Li X, Chang CA & Hutnik C (2019). Mechanical stretch upregulates connexin43 in human trabecular meshwork cells. Clin Exp Ophthalmol 47, 787–794. [DOI] [PubMed] [Google Scholar]
- Tingåker BK & Irestedt L (2010). Changes in uterine innervation in pregnancy and during labour. Curr Opin Anaesthesiol 23, 300–303. [DOI] [PubMed] [Google Scholar]
- Vanhoutte PM, Shimokawa H, Feletou M & Tang EHC (2017). Endothelial dysfunction and vascular disease – a 30th anniversary update. Acta Physiol 219, 22–96. [DOI] [PubMed] [Google Scholar]
- Villegas D, Giard O, Brochu-Gaudreau K & Rousseau É (2021). Activation of TRPV4 channels leads to a consistent tocolytic effect on human myometrial tissues. Eur J Obstet Gynecol Reprod Biol X 10, 0–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vink J (2020). The potential role of the cervix in myometrial function. Curr Opin Physiol 13, 33–37. [Google Scholar]
- van Vliet E, Dijkema G, Schuit E, Heida K, Roos C, van der Post J, Parry E, McCowan L, Lyell D, El-Sayed Y, Carr D, Clark A, Mahdy Z, Uma M, Sayin N, Varol G, Mol B & Oudijk M (2016). Nifedipine maintenance tocolysis and perinatal outcome: an individual participant data meta-analysis. BJOG An Int J Obstet Gynaecol 123, 1753–1760. [DOI] [PubMed] [Google Scholar]
- Wakle-Prabagaran M, Lorca RA, Ma X, Stamnes SJ, Amazu C, Hsiao JJ, Karch CM, Hyrc KL, Wright ME & England SK (2016). BKCa channel regulates calcium oscillations induced by alpha-2-macroglobulin in human myometrial smooth muscle cells. Proc Natl Acad Sci U S A 113, E2335–E2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldorf KMA et al. (2015). Uterine overdistention induces preterm labor mediated by inflammation: Observations in pregnant women and nonhuman primates. Am J Obstet Gynecol 213, 830.e1–830.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SP, Chennupati R, Kaur H, Iring A, Wettschureck N & Offermanns S (2016). Endothelial cation channel PIEZO1 controls blood pressure by mediating flow-induced ATP release. J Clin Invest 126, 4527–4536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wictome M, Henderson I, Lee AG & East JM (1992). Mechanism of inhibition of the calcium pump of sarcoplasmic reticulum by thapsigargin. Biochem J 283, 525–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray S, Jones K, Kupittayanant S, Li Y, Matthew A, Monir-Bishty E, Noble K, Pierce SJ, Quenby S & Shmygol AV (2003). Calcium signaling and uterine contractility. JSocGynecolInvestig 10, 252–264. [DOI] [PubMed] [Google Scholar]
- Wray S & Prendergast C (2019). The Myometrium: From Excitation to Contractions and Labour. In Advances in Experimental Medicine and Biology, pp. 233–263. Springer; New York LLC. [DOI] [PubMed] [Google Scholar]
- Xuan YT, Wang OL & Whorton AR (1992). Thapsigargin stimulates Ca2+ entry in vascular smooth muscle cells: Nicardipine-sensitive and -insensitive pathways. Am J Physiol - Cell Physiol; DOI: 10.1152/ajpcell.1992.262.5.c1258. [DOI] [PubMed] [Google Scholar]
- Yin Z, He W, Li Y, Li D, Li H, Yang Y, Wei Z, Shen B, Wang X, Cao Y & Khalil RA (2018). Adaptive reduction of human myometrium contractile activity in response to prolonged uterine stretch during term and twin pregnancy. Role of TREK-1 channel. Biochem Pharmacol 152, 252–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RC (2016). Mechanotransduction mechanisms for coordinating uterine contractions in human labor. Reproduction 152, R51–R61. [DOI] [PubMed] [Google Scholar]
- Young RC (2018). The uterine pacemaker of labor. Best Pract Res Clin Obstet Gynaecol 52, 68–87. [DOI] [PubMed] [Google Scholar]
- Zhang XP & Hintze TH (2006). cAMP signal transduction induces eNOS activation by promoting PKB phosphorylation. Am J Physiol - Hear Circ Physiol 290, 2376–2384. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Su SA, Li W, Ma Y, Shen J, Wang Y, Shen Y, Chen J, Ji Y, Xie Y, Ma H & Xiang M (2021). Piezo1-Mediated Mechanotransduction Promotes Cardiac Hypertrophy by Impairing Calcium Homeostasis to Activate Calpain/Calcineurin Signaling. Hypertension 647–660. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data in a non-identifying format are securely stored at the University of Nevada, Reno. The data are available upon request.