Abstract
Emerging epidemiological studies suggest that long-term ozone (O3) exposure may increase the risk of mortality, while pre-existing evidence is mixed and has been generated predominantly in North America and Europe. In this study, we investigated the impact of long-term O3 exposure on all-cause mortality in a national cohort in China. A dynamic cohort of 20882 participants aged ≥40 years was recruited between 2011 and 2018 from four waves of the China Health and Retirement Longitudinal Study. A Cox proportional hazard regression model with time-varying exposures on an annual scale was used to estimate the mortality risk associated with warm-season (April–September) O3 exposure. The annual average level of participant exposure to warm-season O3 concentrations was 100 μg m−3 (range: 61–142 μg m−3). An increase of 10 μg m−3 in O3 was associated with a hazard ratio (HR) of 1.18 (95% confidence interval [CI]: 1.13–1.23) for all-cause mortality. Compared with the first exposure quartile of O3, HRs of mortality associated with the second, third, and highest exposure quartiles were 1.09 (95% CI: 0.95–1.25), 1.02 (95% CI: 0.88–1.19), and 1.56 (95% CI: 1.34–1.82), respectively. A J-shaped concentration–response association was observed, revealing a non-significant increase in risk below a concentration of approximately 110 μg m−3. Low-temperature-exposure residents had a higher risk of mortality associated with long-term O3 exposure. This study expands current epidemiological evidence from China and reveals that high-concentration O3 exposure curtails the long-term survival of middle-aged and older adults.
Keywords: Ozone, Long-term exposure, All-cause mortality, Cohort study
Graphical abstract
Highlights
-
•
A national cohort study on O3 exposure and all-cause mortality is conceived in China.
-
•
Long-term O3 exposure is associated with increased mortality risk.
-
•
A J-shaped O3-mortality relationship is identified in middle-aged and older adults.
1. Introduction
In the context of global warming, surface ozone (O3) is an increasingly prominent threat to human health. Prior studies have associated short-term O3 with a variety of multi-cause morbidity (e.g., cardiovascular diseases and asthma [[1], [2], [3]]) and mortality (e.g., all-cause, cardiovascular, and respiratory [[4], [5], [6], [7]]) outcomes globally. A recent multi-country analysis [8] of 406 cities demonstrated a significant increase of 0.18% in all-cause mortality with a 10-μg m−3 increase in 8-h daily maximum O3, and heterogeneity across countries suggested that O3-related mortality could be reduced under stricter air quality standards. These findings emphasized the significance and urgency of quantifying the effect of O3 exposure on long-term survival.
Previous cohort investigations reported associations between long-term O3 exposure and mortality in North America and Europe, while substantial heterogeneities existed across studies [9,10]. The majority of studies suggested positive associations between long-term O3 exposure and the increased risk of all-cause [11,12] and cause-specific [[13], [14], [15]] death. However, non-significant associations were also observed in prior large-scale prospective studies in the United States [16,17]. A recently published pooled analysis in seven large European cohorts, including 28 million participants, revealed a negative association between warm-season O3 exposure and mortality [18]. Given the considerable scarcity of longitudinal evidence in low- and middle-income countries, geographical biases may be introduced when conducting meta-analyses that pool O3–mortality associations estimated from different populations [10]. To address these limitations in knowledge, generating high-quality cohort evidence from low- and middle-income countries such as China, strengthening O3–mortality studies, and developing pollution control policy and public health promotion are indispensable.
The Air Pollution Prevention and Control Action Plan was implemented in 2013 to mitigate severe air pollution in China. The concentrations of other major air pollutants have since decreased, whereas O3 levels have remained high [19,20]. This can be attributed to the nonlinear response of O3 to its precursors (i.e., nitrogen oxides and volatile organic compounds [21]). Most regions with high O3 pollution follow the regulatory regime for volatile organic compounds; reducing vehicle nitrogen oxide emission shows limited effects on O3 control in these regions [21]. High ambient O3 pollution underscores the importance of assessing the health risk to the Chinese population. We designed a national cohort study that aimed to estimate the long-term impact of O3 exposure on all-cause mortality and investigate the concentration–response (C–R) relationship among middle-aged and older Chinese populations across a wide range of exposure levels.
2. Materials and methods
2.1. Study population
The participants of this study were recruited from the China Health and Retirement Longitudinal Study (CHARLS), an ongoing national longitudinal cohort covering 28 provinces in China. Approximately 18000 individuals were involved in the 2011–2012 baseline survey and were generally followed every 2–3 years using a face-to-face computer-assisted interview [22]. Participants were newly recruited in each follow-up wave to avoid attrition due to death and loss to follow-up. We designed a longitudinal dynamic cohort study using 2011, 2013, 2015, and 2018 waves of CHARLS. Data on covariates (e.g., demographics, health status and function, and behavioral habits) for each participant were collected by well-trained professional interviewers using a series of standard questionnaire surveys. Death information for deceased individuals was obtained by interviewers through personal inquiries to family members during follow-up surveys [23].
We ultimately included 20882 eligible respondents with complete data in our study from four waves of the CHARLS. Participants were excluded if they were lost to follow-up at the first follow-up survey (n = 1801), were younger than 40 years of age (n = 160), or were missing information on critical covariates (n = 2111; Fig. S1). The locations of 125 study cities and the number of participants in each province are exhibited in Figs. S2a and b. The average area of prefectural cities included in the CHARLS was 22600 km2, ranging from approximately 2000 km2 (i.e., Shenzhen) to 253000 km2 (i.e., Hulunbuir). More than 80% of surveyed cities had an area of between 5700 km2 and 32500 km2. All participants or their legal representatives provided written informed consent. Ethics approval for the CHARLS was granted by the Ethical Review Committee of Peking University (No. IRB00001052-11015).
2.2. Exposure assessment
Annual warm-season O3 concentrations at a resolution of 0.1° (approximately 10 km) were estimated by fusing data from observations and models widely used by the Global Burden of Disease Study 2019 (https://ghdx.healthdata.org/gbd-2019, accessed on 23 January 2023). Specifically, the warm-season average of 8-h daily maximum O3 concentrations were estimated by the Bayesian maximum entropy method by combining the O3 ground measurement and chemical transport model estimates [24]. In situ O3 measurement data were retrieved from the Tropospheric Ozone Assessment Report and the China National Environmental Monitoring Center Network. Additional details for modeling O3 estimates can be found in prior publication [25]. Given privacy concerns, participants’ residential addresses were not publicly accessible; therefore, we assigned O3 exposure at the city level by linking participants to 125 prefectural cities in our primary analysis. The annual warm-season O3 concentrations of 125 prefectural cities from 2011 to 2018 were calculated by aggregating cell-level concentrations into city-level averages. To quantify the effect of misclassification caused by city-level exposure, we re-estimated the O3 exposure at the residential level with 1000 random simulations using the Monte Carlo simulation approach [26]. We evaluated annual average exposures for each participant for each calendar year according to their survival time during the study period.
Annual particulate matter (PM2.5) concentrations (0.1° × 0.1°) simulated using the Data Integration Model for Air Quality were derived from the Global Burden of Disease Study 2019 and used to evaluate the annual PM2.5 exposure of participants using the same conversion method as that used to estimate O3 exposure [27]. Details of PM2.5 concentration prediction models can be found in published study [28]. Annual nitrogen dioxide (NO2) exposure assessments were calculated using daily NO2 concentrations (0.1° × 0.1°), which were estimated using the robust back-extrapolation with a random forest using the intermediate modeling of scaling factors based on ground-monitoring and satellite-based data [29]. Performing 1000 random simulations for three pollutants simultaneously is an extremely time-consuming process and requires relatively high computer capacity. Therefore, considering the heavy computing burden, we did not apply the Monte Carlo simulation approach for PM2.5 and NO2.
2.3. Statistical analyses
Cox proportional hazard regression models with time-varying exposures on an annual scale were adopted to quantify the association between O3 exposure and all-cause mortality. Person-years of follow-up were calculated from study enrolment to loss to follow-up, the end of the study, or date of death, whichever came first. One observation was created for each person for each year of mortality follow-up, and the corresponding annual average exposures were subsequently assigned to each observation [30]. All models were stratified by age (i.e., 40–49, 50–59, 60–69, 70–79, 80–89, and ≥90 years) and sex. Consistent with previous cohort studies [16,31], we considered a set of potential confounders, including (1) demographic characteristics (i.e., marital status, educational level, residence, and region); (2) behavioral factors (i.e., smoking status, alcohol consumption, and social activities); (3) health status (i.e., body mass index, diabetes, hypertension, and disability status); and (4) ambient temperature. The geographical area was divided into seven regions (i.e., North, Northeast, East, South, Southwest, Northwest, and Central China) based on geographic location. Body mass index was calculated as the participant's weight (in kilograms) divided by height (in meters squared) and classified as underweight or normal (<24 kg m−2), overweight (24–28 kg m−2), or obese (≥28 kg m−2). Education levels were defined as illiterate, primary school, middle school, and high school or above. Diabetes and hypertension were defined based on self-reported history. Social activity was defined by whether individuals participated in social activities such as interacting with friends; playing Mahjong, chess, or cards; going to the community club; or taking part in a community-related organization in the past month. Participants with physical disabilities were defined as those with brain damage, a speech impediment, or vision or hearing problems. Accumulating evidence revealed an approximately U-shaped relationship between temperature and mortality [32], suggesting a continuous metric could better capture the impact of temperature on mortality than metrics of extreme temperature. Thus, in our analysis, the annual average temperature was fitted as a smoothing term using a natural cubic spline with three knots. Annual average temperatures at the city level were aggregated from daily estimates of the European Center for Medium-Range Weather Forecasts atmospheric reanalysis dataset of the global climate at a resolution of 0.1° × 0.1°.
We adopted a sequential adjustment approach to defining the models with varying adjustment levels. Model 1 was stratified by age and sex without additional adjustments. Model 2 was adjusted for covariates of demographic characteristics. Model 3 was adjusted for covariates of demographic characteristics and behavioral factors. Model 4 (fully adjusted model) was adjusted for covariates of demographic characteristics, behavioral factors, health status, and environmental temperature.
Hazard ratios (HRs) of all-cause death and corresponding 95% confidence intervals (CIs) were estimated in relation to a 10-μg m−3 O3 increase. To facilitate the comparability of our results with prior studies, we converted the six-month warm-season mean of the daily maximum 8-h average (6mDMA8) O3 concentrations to several O3 metrics. To be specific, (1) the 6-month warm-season mean of 24-h daily average (6mDA24), (2) the annual mean of daily maximum 8-h average (ADMA8), (3) the annual mean of 24-h daily average (ADA24), (4) the annual mean of daily maximum 1-h average (ADMA1), and (5) the 6-month warm-season mean of daily maximum 1-h average (6mDMA1)—according to cross-metric conversion coefficients [10] and re-estimated the association between O3 exposure and mortality (Table S1). To investigate the C–R relationship between O3 and all-cause mortality, O3 exposure was fitted as a smoothing term using a natural cubic spline function [16,33] with three knots, following the Akaike Information Guidelines in the fully adjusted model. Participants were categorized by O3 concentration quartiles: quartile 1, ≤89.7 μg m−3; quartile 2, >89.7 and ≤ 100.5 μg m−3; quartile 3, >100.5 and ≤ 110.5 μg m−3; and quartile 4, >110.5 μg m−3. We further estimated the effect of changes in quartile O3 concentrations on all-cause mortality, using the first quartiles as a reference to assess HRs and corresponding 95% CIs.
Subgroup analyses were performed to examine potential modifications of effect and were stratified by sex, age (<65 or 65+ years), educational level (illiterate or literate), smoking status (yes or no; former or current smoking was defined as yes), alcohol consumption (yes or no; former or current drinking was defined as yes), residence (urban or rural), and temperature (low: ≤11.7 °C, medium: 11.7–16.8 °C, and high: ≥16.8 °C). Specifically, participants with annual average temperatures lower than the 25th percentile and higher or equal to the 75th percentile were assigned to low- and high-temperature groups, respectively, so that subgroups had enough participants to ensure sufficient power to detect differences between temperature categories. Meta-regression methods were used to determine any differences in HRs between subgroups [34].
Sensitivity analyses were performed to assess the robustness of the results. First, given that city-level exposure could increase misclassification in our exposure assessment, we applied a Monte Carlo simulation approach to explore how this limitation affected the direction and magnitude of the effect of the estimated association. Specifically, we randomly simulated participants' residential addresses within each city and assigned gridded O3 exposure for each individual based on the simulated address. With sufficient simulations, association estimates close to real-world estimates could be included. Considering the computing burden, we only applied the Monte Carlo simulation approach (using 1000 simulations) to Model 4. Second, we re-estimated the association using Model 4 by incorporating random intercepts for surveyed cities to consider the clustering effect caused by city-level exposure. Third, we examined the associations by excluding individuals who died within the first year of the baseline survey (n = 151) to avoid bias from participant selection [35]. Fourth, we adjusted annual province-level gross domestic product as the time-dependent covariate to eliminate the potential confounding effect of area-level socioeconomic status. Fifth, a directed acyclic graph was used to identify a minimal sufficient adjustment variable set (Fig. S3), which was alternatively adopted to select covariates for minimizing confounding bias in epidemiological studies. Sixth, we used single-year lag (lag 1–2) O3 exposure to observe the lag effects of long-term O3 exposure on mortality. In addition, to eliminate the confounding effects of co-pollutants, we tested bi- and tri-pollutant models with consistent time-varying exposures on a one-year time scale [15].
Data analyses were conducted using the R version 4.1.1 (R Foundation for Statistical Computing, Vienna, Austria), using a “survival” package for the Cox model and a “spline” package for the natural cubic spline. Two-sided tests with a P-value <0.05 were considered to be statistically significant.
3. Results
There were 20882 eligible participants included in our study, with 1814 deaths occurring during the entire follow-up period (Table 1). The mean (± standard deviation) age of participants was 57.7 (±10.4) years, and 47.2% were male. Among respondents, 40.8% were recruited from urban areas, and more than half (56.0%) lived in southern cities. Participants who never smoked or drank alcohol accounted for 62.7% and 68.7% of respondents, respectively. Approximately a quarter of participants suffered from hypertension, and only 5.8% were diabetics. Annual warm-season O3 concentrations across China were relatively lower in 2018 than in 2011 (Figs. S2c and d), whereas O3 levels generally showed an increasing annual trend from 2013 to 2018 (Fig. S4). The average air pollution exposures between 2011 and 2018 were 100.7 μg m−3 (range: 60.7–142.4 μg m−3) for warm-season O3, 52.0 μg m−3 (range: 16.1–102.4 μg m−3) for PM2.5, and 25.3 μg m−3 (range: 11.3–72.9 μg m−3) for NO2 (Table S2). Warm-season O3 was moderately correlated with PM2.5 (r = 0.57) and NO2 (r = 0.62).
Table 1.
Descriptive characteristics of the study population at baseline by ozone exposure quartile.
| Characteristics | No. (%) |
||||
|---|---|---|---|---|---|
| Total (60.7–142.4) | Warm-season O3 exposure quartiles (μg m−3) |
||||
| Quartile 1 (60.7–89.7) | Quartile 2 (89.7–100.5) | Quartile 3 (100.5–110.5) | Quartile 4 (110.5–142.4) | ||
| Population, No. | |||||
| Persons | 20882 | 5268 | 5257 | 5215 | 5142 |
| Deaths | 1814 | 356 | 479 | 506 | 473 |
| Demographic characteristics | |||||
| Male sex | 9853 (47.2) | 2522 (47.9) | 2414 (45.9) | 2480 (47.6) | 2437 (47.4) |
| Age (yrs), mean (SD) | 57.7 (10.4) | 55.7 (10.5) | 58.0 (10.5) | 58.3 (10.2) | 58.7 (10.0) |
| 45–50 | 6658 (31.9) | 2242 (42.5) | 1598 (30.4) | 1459 (28.0) | 1359 (26.4) |
| 50–64 | 9585 (45.9) | 2052 (39.0) | 2443 (46.5) | 2541 (48.7) | 2549 (49.6) |
| 65+ | 4639 (22.2) | 974 (18.5) | 1216 (23.1) | 1215 (23.3) | 1234 (24.0) |
| Education level | |||||
| Illiterate | 5315 (25.5) | 1378 (26.2) | 1216 (23.1) | 1260 (24.2) | 1461 (28.4) |
| Elementary school and below | 8291 (39.7) | 2202 (41.8) | 1974 (37.5) | 2183 (41.9) | 1932 (37.6) |
| Middle school | 4594 (22.0) | 1088 (20.7) | 1237 (23.5) | 1097 (21.0) | 1172 (22.8) |
| High school and above | 2682 (12.8) | 600 (11.4) | 830 (15.8) | 675 (12.9) | 577 (11.2) |
| Married status | |||||
| Married | 18553 (88.8) | 4736 (89.9) | 4597 (87.4) | 4645 (89.1) | 4575 (89.0) |
| Never married or Divorced or Widowed | 2329 (11.2) | 532 (10.1) | 660 (12.6) | 570 (10.9) | 567 (11.0) |
| Residence | |||||
| Urban | 8514 (40.8) | 2086 (39.6) | 2506 (47.7) | 2093 (40.1) | 1829 (35.6) |
| Rural | 12368 (59.2) | 3182 (60.4) | 2751 (52.3) | 3122 (59.9) | 3313 (64.4) |
| Region | |||||
| South | 11689 (56.0) | 3766 (71.5) | 3218 (61.2) | 2680 (51.4) | 2025 (39.4) |
| North | 9193 (44.0) | 1502 (28.5) | 2039 (38.8) | 2535 (48.6) | 3117 (60.6) |
| Behavioral factors | |||||
| Smoking status | |||||
| Never | 13028 (62.4) | 3249 (61.7) | 3369 (64.1) | 3143 (60.3) | 3267 (63.5) |
| Former | 1721 (8.2) | 398 (7.6) | 411 (7.8) | 432 (8.3) | 480 (9.3) |
| Current | 6133 (29.4) | 1621 (30.8) | 1477 (28.1) | 1640 (31.4) | 1395 (27.1) |
| Alcohol consumption | |||||
| Never | 14342 (68.7) | 3539 (67.2) | 3741 (71.2) | 3649 (70.0) | 3413 (66.4) |
| Former | 1116 (5.3) | 254 (4.8) | 232 (4.4) | 293 (5.6) | 337 (6.6) |
| Current | 5424 (26.0) | 1475 (28.0) | 1284 (24.4) | 1273 (24.4) | 1392 (27.1) |
| Social activity | 11605 (55.6) | 3151 (59.8) | 2798 (53.2) | 2892 (55.5) | 2764 (53.8) |
| Health status | |||||
| BMI (kg m−2), mean (SD) | 23.5 (3.2) | 23.3 (3.1) | 23.3 (3.2) | 23.5 (3.2) | 23.7 (3.3) |
| Underweight or normal | 12435 (59.5) | 2821 (53.5) | 3322 (63.2) | 3199 (61.3) | 3093 (60.2) |
| Overweight | 6571 (31.5) | 2047 (38.9) | 1517 (28.9) | 1529 (29.3) | 1478 (28.7) |
| Obesity | 1876 (9.0) | 400 (7.6) | 418 (8.0) | 487 (9.3) | 571 (11.1) |
| Hypertension | 4800 (23.0) | 1017 (19.3) | 1184 (22.5) | 1340 (25.7) | 1259 (24.5) |
| Diabetes | 1204 (5.8) | 261 (5.0) | 279 (5.3) | 316 (6.1) | 348 (6.8) |
| Disability | 3485 (16.7) | 891 (16.9) | 741 (14.1) | 838 (16.1) | 1015 (19.7) |
Abbreviations: O3, ozone; SD, standard deviation; BMI, body mass index.
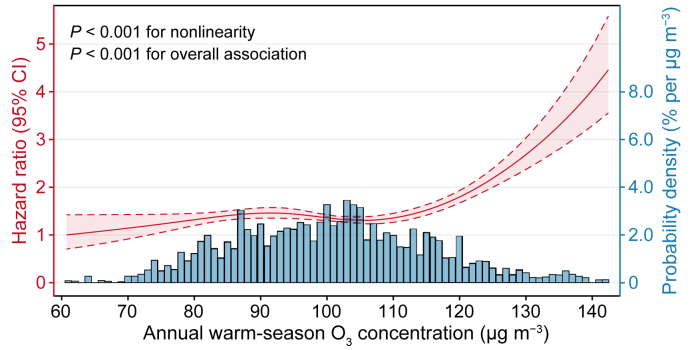
As shown in Fig. 1, the C–R curve for O3 and all-cause mortality was fitted using a natural cubic spline. A nonlinear association between long-term O3 exposure and mortality risk (P for nonlinearity <0.001) was observed at 60.7–142.4 μg m−3. Intuitively, we observed a J-shaped relationship, revealing a relatively flat curve as O3 levels fell below approximately 110 μg m−3, while the slope (i.e., increase in mortality risk) was steeper at higher concentrations.
Fig. 1.
Concentration–response (C–R) curve of the association between ozone exposure and all-cause mortality.
The HRs and 95% CIs of all-cause mortality associated with O3 exposure were presented in Table 2. Sequential adjustment for covariates did not appreciably change the significance and magnitude of estimates, suggesting increased mortality risk associated with high levels of O3 (i.e., in the highest O3 exposure quartile). In the fully adjusted model, the mortality HRs associated with the second, third, and highest O3 exposure quartiles were 1.088 (95% CI: 0.947–1.251), 1.018 (95% CI: 0.875–1.185), and 1.559 (95% CI: 1.338–1.817), respectively, compared with the first exposure quartile. As the O3 exposure was fitted as a linear term in a fully adjusted model, the per 10-μg m−3 increase in annual warm-season O3 was associated with an HR of 1.179 (95% CI: 1.132–1.229) for all-cause mortality risk.
Table 2.
Hazard ratios and 95% confidence intervals of all-cause mortality associated with long-term ozone exposure.
| Model | HR (95% CI) |
|||
|---|---|---|---|---|
| Per 10-μg m−3 increase | Quartile 2∗ (91.7–100.6 μg m−3) | Quartile 3∗ (100.6–110.5 μg m−3) | Quartile 4∗ (110.5–142.4 μg m−3) | |
| Model 1a | 1.116 (1.098–1.135) | 1.070 (1.000–1.146) | 0.977(0.911–1.047) | 1.390 (1.303–1.482) |
| Model 2b | 1.149 (1.108–1.193) | 1.067 (0.931–1.223) | 0.984 (0.851–1.138) | 1.476 (1.285–1.696) |
| Model 3c | 1.148 (1.106–1.191) | 1.065 (0.929–1.222) | 0.985 (0.852–1.139) | 1.470 (1.279–1.689) |
| Model 4d | 1.179 (1.132–1.229) | 1.088 (0.947–1.251) | 1.018 (0.875–1.185) | 1.559 (1.338–1.817) |
∗ Quartile 1 was used as the reference.
Adjusted for age and sex.
Adjusted for covariates in Model 1 plus demographic characteristics including educational level, married status, residence and region.
Adjusted for covariates in Model 2 plus behavioral factors including alcohol consumption, smoking status and social activity.
Adjusted for covariates in Model 3 plus health status including BMI, hypertension, diabetes, disability and temperature. Model 4 was fully adjusted model.
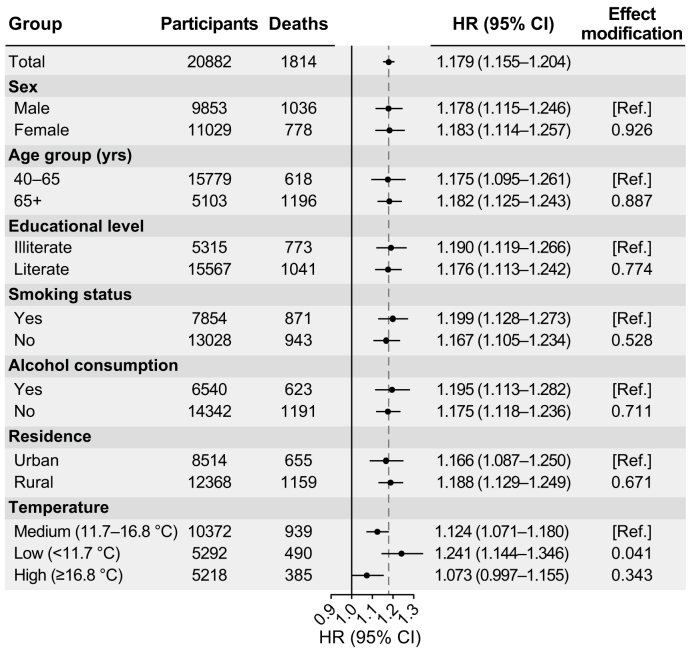
The estimates from subgroup analyses of HR and 95% CI stratified by demographic and behavioral characteristics and temperature were summarized in Fig. 2. A highly similar mortality risk related to O3 exposure was estimated for male (HR = 1.178, 95% CI: 1.115–1.246) and female individuals (HR = 1.183, 95% CI: 1.114–1.257). For a 10-μg m−3 increase in annual warm-season O3 exposure, the associations were more evident in the older group (age ≥65 years), with a corresponding HR of 1.182 (95% CI: 1.125–1.243). Significant and analogous associations were observed between subgroups stratified by smoking and alcohol consumption status. To some extent, O3–mortality associations revealed urban–rural disparity: associations were more pronounced in rural locations (HR = 1.188, 95% CI: 1.129–1.249) than in urban areas (HR = 1.166, 95% CI: 1.087–1.250). Compared with participants exposed to moderate temperatures (11.7–16.8 °C), we observed a significantly higher risk in the low-temperature (≤11.7 °C) group (P = 0.041). In contrast, participants living in warm cities had an insignificant increase in O3-related mortality risk (≥16.8 °C).
Fig. 2.
Subgroup analysis of HRs (with 95% CIs) of all-cause mortality associated with a 10-μg m−3 increase in ozone exposure. HR, hazard ratio; CI, confidence interval.
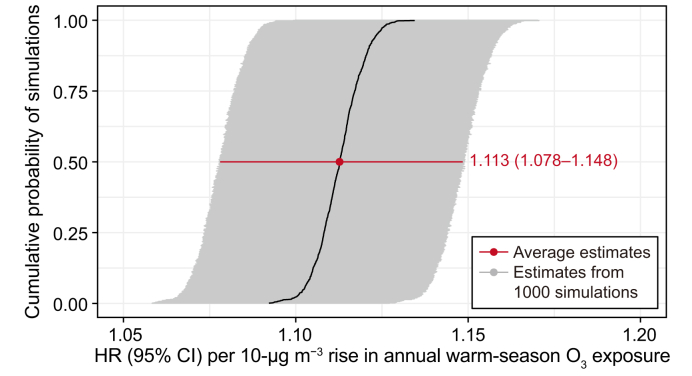
Each estimated association from the 1000 simulations was statistically significant (average HR = 1.113, 95% CI: 1.078–1.148), suggesting that our primary analysis may not have been sensitive to exposure misclassification due to the city-level exposure assessment (Fig. 3). In addition, O3–mortality associations remained largely robust in the sensitivity analyses when the clustering effect of surveyed cities (HR = 1.247, 95% CI: 1.196–1.300) was considered, participants who died within the first year of the baseline survey (HR = 1.182, 95% CI: 1.132–1.234) were excluded, the minimum set of variables based on the directed acyclic graph was adjusted, and bi- and tri-pollutant models additionally adjusted for PM2.5 or/and O3 were used (Table S3).
Fig. 3.
Probability distribution of HRs (with 95% CIs) of all-cause mortality associated with a 10-μg m−3 increase in ozone exposure with 1000 random simulations. HR, hazard ratio; CI, confidence interval.
4. Discussion
To the best of our knowledge, this is the first national study to investigate the association between long-term O3 exposure and all-cause mortality in Chinese men and women. We observed a significantly increased risk of mortality (P < 0.001) associated with O3 exposure at high concentrations among the middle-aged and older population. O3–mortality associations were generally robust after adjusting for co-pollutants (PM2.5 or/and NO2), whereas there was some evidence for effect modification by temperature. These findings provide crucial longitudinal evidence for O3–mortality associations, filling the gap in population-based cohort studies from low- and middle-income countries.
Consistent with findings from prior publications [11,12], we observed a positive association between long-term O3 exposure and mortality risk, with an HR of 1.197 (95% CI: 1.171–1.223) for every 10-μg m−3 increase in annual warm-season O3 at a wide exposure range of 60.7–142.4 μg m−3. The magnitude of the effect size in this study was between the estimated risk for cardiovascular disease mortality (HR = 1.09, 95% CI: 1.05–1.14) from a national cohort study [36] and that (HR = 1.22, 95% CI: 1.13–1.33) from a regional longitudinal study in China [33], although the magnitude we observed was considerably higher than estimates (HRs range:1.01–1.05) from recent cohort studies in Europe [37] and North America [12,38]. Such regional heterogeneity could be partly attributed to the use of differing O3 exposure metrics (e.g., 6mDMA8, 6mDA24, and ADMA8) and differential population susceptibility [10]. In contrast, inverse or non-significant associations were reported by large-scale cohort studies in Europe [18,39,40] and the United States [16,17]. Prior cohort studies have reported the potential existence of a threshold in the C–R curve above which a higher mortality risk is observed [15,16,31]. Hence, a possible explanation for previous studies' lower or inverse estimates is that O3 concentrations in developed countries are generally relatively low. For instance, the O3 exposure ranges reported by cohort studies conducted in Canada [38] and the United States [11] were below 120 μg m−3. In addition, if such a non-linear association does exist, the effect estimation assumed to be linearity might be underestimated at the low range of exposure levels.
Only a few studies have assessed the C–R curve between long-term O3 exposure and mortality [9]. This study explored the C–R relationship for a wide exposure range (60.7–142.4 μg m−3) and observed a J-shaped O3–mortality association among middle-aged and older adults in China (Fig. 2). We observed a consistently increasing trend in the positive association at warm-season O3 levels above approximately 110 μg m−3 (56.1 ppb), suggesting a potential threshold for the impact of long-term O3 exposure on mortality. A comparable estimate (56 ppb) was reported in a prior cohort study based on the American Cancer Society Cancer Prevention Study II [41]. Two large-scale investigations identified potential thresholds of 40 ppb [11,12] in the older cohort (age ≥65 years) enrolled from the Centers for Medicare and Medicaid Services. These discrepancies in potential thresholds may be due to differences in demographic characteristics (e.g., age structure and race) and methodological strategies (e.g., sample size, exposure quantification, and confounding adjustment) [10]. However, given that most studies did not report the threshold when investigating O3–mortality associations, current findings on the potential threshold remain inconclusive in the absence of more relevant evidence, particularly in Asian populations.
Our study did not demonstrate effect modification by demographic and behavioral covariates in subgroup analyses. Comparable findings have been reported in prior epidemiological studies investigating the long- and short-term effects of O3 on death. A prospective cohort study that enrolled more than a half-million Americans ≥50 years of age suggested insignificant discrepancies in mortality risk among subpopulations stratified by a set of covariates (e.g., age, sex, educational level, and smoking status) [16]. Meanwhile, a time-series study of O3–mortality associations using national data from 272 Chinese cities failed to show differential mortality risk in subgroup analyses [7]. However, older adults and less educated populations tended to have higher mortality risks associated with air pollution exposure, which may be partly explained by the vulnerability of the elderly and the disadvantageous living conditions associated with lower socioeconomic status [42,43]. Consequently, more large-scale cohort investigations are warranted to identify differences in O3–mortality associations among subpopulations.
We found that temperature might modify the long-term effect of O3 on mortality. We observed significantly stronger O3–mortality associations in participants enrolled from cold locations (≤11.7 °C) than in those living in locations with moderate temperatures (11.7–16.8 °C). A meta-analysis of associations between short-term O3 exposure and mortality further indicated that a higher O3-induced risk of acute death was only observed at low temperatures among Chinese participants [44]. Nonetheless, national cohort studies in the United States reported stronger O3–mortality associations in areas with low temperatures and the highest temperature category [16,31]. Reducing the use of air conditioning during cool weather and the consequent exposure to more natural ventilation can partially explain the findings [43]. In addition, prior longitudinal evidence revealed that the O3-induced risk of mortality varied spatially by climate zone [13], indicating that O3–mortality associations may be influenced by other climate factors. Therefore, studies investigating the long-term effect of O3 on mortality should consider more climate factors and identify the modification effect in the future.
Our study had several strengths. First, our findings provide a national epidemiologic evidence for the association between long-term O3 exposure and all-cause mortality among middle-aged and older adults in Chinese Mainland. Cohort studies exploring the long-term effects of air pollution exposure on the Chinese population remain rare [33,36]; therefore, our findings appropriately fill the literature gap. Second, our analyses included extensive confounders, including individual lifestyles, and evaluated the C–R relationship in a wide range (60.7–142.4 μg m−3) of O3 exposure. Third, given our comprehensive statistical approach and the validated robustness of effect estimates, our findings can strengthen the evidence for the mortality effects of long-term O3 exposure. Furthermore, our study identified that O3 is an independent risk factor for premature death in China, suggesting that the improved control of ambient O3 could help people live longer and healthier lives and reduce the increased financial burden of aging in China [45].
Our study also had some limitations. First, participants’ O3 exposure was assessed at the city level based on the residential address instead of at the individual level, potentially leading to unavoidable biases in exposure assignment. It might have led to an effect overestimation according to the Monte Carlo simulation results, although the misclassification did not change the direction of the association. Second, given the distinct difference in O3 concentrations between the household and ambient environments [46], the overestimation of individual O3 exposure may be caused by unnoticed indoor O3 exposure. Third, given the lack of clinical diagnoses regarding the cause of death [47], we failed to estimate associations between O3 and cause-specific mortality and excluded accidental deaths from all-cause cases. However, highly similar C–R associations for non-accidental and all-cause mortality were reported in prior cohort studies of air pollution [48,49]. According to the Chinese Statistical Yearbook (http://www.stats.gov.cn/tjsj/ndsj/, accessed on 23 January 2023), the proportion of accidental deaths among the Chinese population between 2011 and 2018 remained low (range: 6.39–7.11%). Therefore, we believe accidental deaths did not considerably influence our results or change the direction of the association. Fourth, unmeasured confounders such as road traffic noise, residential greenness, and other climate factors may have further influenced the estimated association between O3 exposure and long-term survival, despite our consideration of a rich set of covariates. In addition, because the relationship was observed among middle-aged and older adults, our findings may not be generalizable to the younger population.
5. Conclusions
Our study provides longitudinal evidence that long-term exposure to high levels of O3 is associated with increased mortality risk among middle-aged and older Chinese adults. The results are robust to adjustment for co-pollutants and additional covariates. We observe a potential threshold for the O3–mortality associations, indicating that limiting O3 concentrations below the potential threshold could substantially benefit public health. These findings expand the current literature by providing epidemiological evidence on the Chinese population. They suggest the government should stress the synergistic control of ambient O3 with other air pollutants to mitigate premature deaths. The reasons for the considerable heterogeneity between the effect estimates of various studies require further extensive longitudinal investigations.
CRediT authorship contribution statement
Yang Yuan: Writing - original draft, Writing - review & editing, Methodology, Formal analysis; Kai Wang: Writing - review & editing, Validation, Visualization; Haitong Zhe Sun: Writing - review & editing, Validation; Yu Zhan: Resources, Data Curation; Zhiming Yang: Software, Visualization; Kejia Hu: Software, Visualization; Yunquan Zhang: Writing - review & editing, Supervision, Funding acquisition. All authors read and approved the final manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study was supported by the Youth Fund Project of Humanities and Social Sciences Research of the Ministry of Education (Grant No. 21YJCZH229). The funder of the study had no role in study design, data collection, data analysis, data interpretation, or the writing of the report. We sincerely thank the National Development Research Institute of Peking University and the China Social Science Research Center of Peking University for providing CHARLS data. We appreciate the anonymous reviewers whose insightful comments and suggestions that contributed to the considerable improvement in manuscript quality.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ese.2023.100241.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Ensor K.B., Raun L.H., Persse D. A case-crossover analysis of out-of-hospital cardiac arrest and air pollution. Circulation. 2013;127:1192–1199. doi: 10.1161/CIRCULATIONAHA.113.000027. [DOI] [PubMed] [Google Scholar]
- 2.Sheffield P.E., Zhou J., Shmool J.L., Clougherty J.E. Ambient ozone exposure and children's acute asthma in New York city: a case-crossover analysis. Environ. Health. 2015;14:25. doi: 10.1186/s12940-015-0010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zheng X.Y., Orellano P., Lin H.L., Jiang M., Guan W.J. Short-term exposure to ozone, nitrogen dioxide, and sulphur dioxide and emergency department visits and hospital admissions due to asthma: a systematic review and meta-analysis. Environ. Int. 2021;150 doi: 10.1016/j.envint.2021.106435. [DOI] [PubMed] [Google Scholar]
- 4.Niu Z., Liu F., Yu H., Wu S., Xiang H. Association between exposure to ambient air pollution and hospital admission, incidence, and mortality of stroke: an updated systematic review and meta-analysis of more than 23 million participants. Environ. Health Prev. Med. 2021;26:15. doi: 10.1186/s12199-021-00937-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Orellano P., Reynoso J., Quaranta N., Bardach A., Ciapponi A. Short-term exposure to particulate matter (PM10 and PM2.5), nitrogen dioxide (NO2), and ozone (O3) and all-cause and cause-specific mortality: systematic review and meta-analysis. Environ. Int. 2020;142 doi: 10.1016/j.envint.2020.105876. [DOI] [PubMed] [Google Scholar]
- 6.Raza A., Dahlquist M., Lind T., Ljungman P.L.S. Susceptibility to short-term ozone exposure and cardiovascular and respiratory mortality by previous hospitalizations. Environ. Health. 2018;17:37. doi: 10.1186/s12940-018-0384-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yin P., Chen R., Wang L., Meng X., Liu C., Niu Y., et al. Ambient ozone pollution and daily mortality: a nationwide study in 272 Chinese cities. Environ. Health Persp. 2017;125 doi: 10.1289/EHP1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vicedo-Cabrera A.M., Sera F., Liu C., Armstrong B., Milojevic A., Guo Y., et al. Short term association between ozone and mortality: global two stage time series study in 406 locations in 20 countries. BMJ. 2020;368:m108. doi: 10.1136/bmj.m108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huangfu P., Atkinson R. Long-term exposure to NO2 and O3 and all-cause and respiratory mortality: a systematic review and meta-analysis. Environ. Int. 2020;144 doi: 10.1016/j.envint.2020.105998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun H.Z., Yu P., Lan C., Wan M.W.L., Hickman S., Murulitharan J., et al. Cohort-based long-term ozone exposure-associated mortality risks with adjusted metrics: a systematic review and meta-analysis. Innovation. 2022;3 doi: 10.1016/j.xinn.2022.100246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Q., Wang Y., Zanobetti A., Wang Y., Koutrakis P., Choirat C., et al. Air pollution and mortality in the Medicare population. N. Engl. J. Med. 2017;376:2513–2522. doi: 10.1056/NEJMoa1702747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shi L., Rosenberg A., Wang Y., Liu P., Danesh Yazdi M., Requia W., et al. Low-concentration air pollution and mortality in American older adults: a national cohort analysis (2001-2017) Environ. Sci. Technol. 2021;56:7194–7202. doi: 10.1021/acs.est.1c03653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cakmak S., Hebbern C., Pinault L., Lavigne E., Vanos J., Crouse D.L., et al. Associations between long-term PM2.5 and ozone exposure and mortality in the Canadian Census Health and Environment Cohort (CANCHEC), by spatial synoptic classification zone. Environ. Int. 2018;111:200–211. doi: 10.1016/j.envint.2017.11.030. [DOI] [PubMed] [Google Scholar]
- 14.Rush B., McDermid R.C., Celi L.A., Walley K.R., Russell J.A., Boyd J.H. Association between chronic exposure to air pollution and mortality in the acute respiratory distress syndrome. Environ. Pollut. 2017;224:352–356. doi: 10.1016/j.envpol.2017.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weichenthal S., Pinault L.L., Burnett R.T. Impact of oxidant gases on the relationship between outdoor fine particulate air pollution and nonaccidental, cardiovascular, and respiratory mortality. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-16770-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lim C.C., Hayes R.B., Ahn J., Shao Y., Silverman D.T., Jones R.R., et al. Long-term exposure to ozone and cause-specific mortality risk in the United States. Am. J. Respir. Crit. Care Med. 2019;200:1022–1031. doi: 10.1164/rccm.201806-1161OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lipsett M.J., Ostro B.D., Reynolds P., Goldberg D., Hertz A., Jerrett M., et al. Long-term exposure to air pollution and cardiorespiratory disease in the California teachers study cohort. Am. J. Respir. Crit. Care Med. 2011;184:828–835. doi: 10.1164/rccm.201012-2082OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stafoggia M., Oftedal B., Chen J., Rodopoulou S., Renzi M., Atkinson R.W., et al. Long-term exposure to low ambient air pollution concentrations and mortality among 28 million people: results from seven large European cohorts within the ELAPSE project. Lancet Planet. Health. 2022;6:e9–e18. doi: 10.1016/S2542-5196(21)00277-1. [DOI] [PubMed] [Google Scholar]
- 19.Sun Z., Archibald A.T. Multi-stage ensemble-learning-based model fusion for surface ozone simulations: a focus on CMIP6 models. Environ. Sci. Ecotechnol. 2021;8 doi: 10.1016/j.ese.2021.100124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu R., Ma Z., Liu Y., Shao Y., Zhao W., Bi J. Spatiotemporal distributions of surface ozone levels in China from 2005 to 2017: a machine learning approach. Environ. Int. 2020;142 doi: 10.1016/j.envint.2020.105823. [DOI] [PubMed] [Google Scholar]
- 21.Shen H., Sun Z., Chen Y., Russell A.G., Hu Y., Odman M.T., et al. Novel method for ozone isopleth construction and diagnosis for the ozone control strategy of Chinese cities. Environ. Sci. Technol. 2021;55:15625–15636. doi: 10.1021/acs.est.1c01567. [DOI] [PubMed] [Google Scholar]
- 22.Zhao Y., Hu Y., Smith J.P., Strauss J., Yang G. Cohort profile: the China Health and Retirement Longitudinal Study (CHARLS) Int. J. Epidemiol. 2014;43:61–68. doi: 10.1093/ije/dys203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen X., Smith J., Strauss J., Wang Y., Zhao Y. Encyclopedia of Geropsychology. 2015. China Health and Retirement Longitudinal Study (CHARLS) pp. 1–8. [DOI] [Google Scholar]
- 24.DeLang M.N., Becker J.S., Chang K.L., Serre M.L., Cooper O.R., Schultz M.G., et al. Mapping yearly fine resolution global surface ozone through the bayesian maximum entropy data fusion of observations and model output for 1990-2017. Environ. Sci. Technol. 2021;55:4389–4398. doi: 10.1021/acs.est.0c07742. [DOI] [PubMed] [Google Scholar]
- 25.Shaddick G., Thomas M.L., Amini H., Broday D., Cohen A., Frostad J., et al. Data integration for the assessment of population exposure to ambient air pollution for global burden of disease assessment. Environ. Sci. Technol. 2018;52:9069–9078. doi: 10.1021/acs.est.8b02864. [DOI] [PubMed] [Google Scholar]
- 26.Xue T., Han Y., Fan Y., Zheng Y., Geng G., Zhang Q., et al. Association between a rapid reduction in air particle pollution and improved lung function in adults. Ann. Am. Thorac. Soc. 2020;18:247–256. doi: 10.1513/AnnalsATS.202003-246OC. [DOI] [PubMed] [Google Scholar]
- 27.Murray C.J.L., Aravkin A.Y., Zheng P., Abbafati C., Abbas K.M., Abbasi-Kangevari M., et al. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet. 2020;396:1223–1249. doi: 10.1016/S0140-6736(20)30752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Donkelaar A., Martin R.V., Brauer M., Hsu N.C., Kahn R.A., Levy R.C., et al. Global estimates of fine particulate matter using a combined geophysical-statistical method with information from satellites, models, and monitors. Environ. Sci. Technol. 2016;50:3762–3772. doi: 10.1021/acs.est.5b05833. [DOI] [PubMed] [Google Scholar]
- 29.Wu Y., Di B., Luo Y., Grieneisen M.L., Zeng W., Zhang S., et al. A robust approach to deriving long-term daily surface NO2 levels across China: correction to substantial estimation bias in back-extrapolation. Environ. Int. 2021;154 doi: 10.1016/j.envint.2021.106576. [DOI] [PubMed] [Google Scholar]
- 30.Zanobetti A., O'Neill M.S., Gronlund C.J., Schwartz J.D. Summer temperature variability and long-term survival among elderly people with chronic disease. Proc. Natl. Acad. Sci. U. S. A. 2012;109:6608–6613. doi: 10.1073/pnas.1113070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Turner M.C., Jerrett M., Pope C.A., 3rd, Krewski D., Gapstur S.M., Diver W.R., et al. Long-term ozone exposure and mortality in a large prospective study. Am. J. Respir. Crit. Care Med. 2016;193:1134–1142. doi: 10.1164/rccm.201508-1633OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zafeiratou S., Samoli E., Dimakopoulou K., Rodopoulou S., Analitis A., Gasparrini A., et al. A systematic review on the association between total and cardiopulmonary mortality/morbidity or cardiovascular risk factors with long-term exposure to increased or decreased ambient temperature. Sci. Total Environ. 2021;772 doi: 10.1016/j.scitotenv.2021.145383. [DOI] [PubMed] [Google Scholar]
- 33.Liu S., Zhang Y., Ma R., Liu X., Liang J., Lin H., et al. Long-term exposure to ozone and cardiovascular mortality in a large Chinese cohort. Environ. Int. 2022;165 doi: 10.1016/j.envint.2022.107280. [DOI] [PubMed] [Google Scholar]
- 34.Di Q., Dai L., Wang Y., Zanobetti A., Choirat C., Schwartz J.D., et al. Association of short-term exposure to air pollution with mortality in older adults. JAMA. 2017;318:2446–2456. doi: 10.1001/jama.2017.17923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang X., Liang F., Li J., Chen J., Liu F., Huang K., et al. Associations of long-term exposure to ambient PM2.5 with mortality in Chinese adults: a pooled analysis of cohorts in the China-PAR project. Environ. Int. 2020;138 doi: 10.1016/j.envint.2020.105589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Niu Y., Zhou Y., Chen R., Yin P., Meng X., Wang W., et al. Long-term exposure to ozone and cardiovascular mortality in China: a nationwide cohort study. Lancet Planet. Health. 2022;6:e496–e503. doi: 10.1016/S2542-5196(22)00093-6. [DOI] [PubMed] [Google Scholar]
- 37.Bauwelinck M., Chen J., de Hoogh K., Katsouyanni K., Rodopoulou S., Samoli E., et al. Variability in the association between long-term exposure to ambient air pollution and mortality by exposure assessment method and covariate adjustment: a census-based country-wide cohort study. Sci. Total Environ. 2022;804 doi: 10.1016/j.scitotenv.2021.150091. [DOI] [PubMed] [Google Scholar]
- 38.Crouse D.L., Peters P.A., Hystad P., Brook J.R., van Donkelaar A., Martin R.V., et al. Ambient PM2.5, O3, and NO2 exposures and associations with mortality over 16 years of follow-up in the Canadian Census Health and Environment Cohort (CanCHEC) Environ. Health Perspect. 2015;123:1180–1186. doi: 10.1289/ehp.1409276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carey I.M., Atkinson R.W., Kent A.J., van Staa T., Cook D.G., Anderson H.R. Mortality associations with long-term exposure to outdoor air pollution in a national English cohort. Am. J. Respir. Crit. Care Med. 2013;187:1226–1233. doi: 10.1164/rccm.201210-1758OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hvidtfeldt U.A., Sorensen M., Geels C., Ketzel M., Khan J., Tjonneland A., et al. Long-term residential exposure to PM2.5, PM10, black carbon, NO2, and ozone and mortality in a Danish cohort. Environ. Int. 2019;123:265–272. doi: 10.1016/j.envint.2018.12.010. [DOI] [PubMed] [Google Scholar]
- 41.Jerrett M., Burnett R.T., Pope C.A., III, Ito K., Thurston G., et al. Long-term ozone exposure and mortality. N. Engl. J. Med. 2009;360:1085–1095. doi: 10.1056/NEJMoa0803894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bravo M.A., Son J., de Freitas C.U., Gouveia N., Bell M.L. Air pollution and mortality in Sao paulo, Brazil: effects of multiple pollutants and analysis of susceptible populations. J. Expo. Sci. Environ. Epidemiol. 2016;26:150–161. doi: 10.1038/jes.2014.90. [DOI] [PubMed] [Google Scholar]
- 43.Kan H., London S.J., Chen G., Zhang Y., Song G., Zhao N., et al. Season, sex, age, and education as modifiers of the effects of outdoor air pollution on daily mortality in Shanghai, China: the Public Health and Air Pollution in Asia (PAPA) study. Environ. Health Perspect. 2008;116:1183–1188. doi: 10.1289/ehp.10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ye W., Liu M., Bi J. Meta-analysis of the associations between short-term ozone exposure and human mortality in China. Acta Sci. Circumstantiae. 2020;40:2644–2651. [Google Scholar]
- 45.Samarasekera U. Yaohui Zhao: dedicated to delivering healthy ageing in China. Lancet. 2022;400:1916. doi: 10.1016/S0140-6736(22)02360-1. [DOI] [PubMed] [Google Scholar]
- 46.Hu Y., Yao M., Liu Y., Zhao B. Personal exposure to ambient PM2.5, PM10, O3, NO2, and SO2 for different populations in 31 Chinese provinces. Environ. Int. 2020;144 doi: 10.1016/j.envint.2020.106018. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Y.S., Hu P., Strauss J.A., Zhao Y., Wang Y., Crimmins E.M. Ascertaining cause of mortality among middle-aged and older persons using computer-coded and expert review verbal autopsies in the China Health and Retirement Longitudinal Study. Glob. Health Action. 2020;13 doi: 10.1080/16549716.2020.1768502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eum K.-D., Honda T.J., Wang B., Kazemiparkouhi F., Manjourides J., Pun V.C., et al. Long-term nitrogen dioxide exposure and cause-specific mortality in the U.S. Medicare population. Environ. Res. 2022;207 doi: 10.1016/j.envres.2021.112154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Villeneuve P.J., Weichenthal S.A., Crouse D., Miller A.B., To T., Martin R.V., et al. Long-term exposure to fine particulate matter air pollution and mortality among Canadian women. Epidemiology. 2015;26:536–545. doi: 10.1097/EDE.0000000000000294. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.