Abstract
Background
Immune checkpoint inhibitors (ICI) can cause off-target inflammatory and immune-related adverse events (irAE). Conceivably, COVID-19 vaccination could trigger an inflammatory and immune response that could induce or aggravate irAE.
Methods
The objective of this systematic review is to appraise the efficacy and safety of COVID-19 vaccination in patients with cancer treated with ICI. The literature search was performed in PubMed and Embase in English from December 2019 to February 2022. The review included clinical trials, observational cohort studies, case series, and case reports reporting on the clinical efficacy and safety of COVID-19 vaccines on patients with cancer treated with ICI. Outcomes of interest included seroconversion, SARS-CoV-2 infection rate, severe COVID-19, COVID-19 mortality rate. Incidence of ICI irAEs was also ascertained as well as vaccine adverse events. A meta-analysis was conducted to estimate the pooled effect sizes of the outcomes when possible, using random effects models.
Results
Overall, 19 studies were included for the analysis (n=10 865 with 2477 receiving ICI). We analyzed 15 cohort studies, 1 cross-sectional study, and 3 case reports. There were no statistically significant differences in seroconversion rates after the second dose of the vaccine when comparing patients with cancer receiving ICI with patients without cancer (risk ratio, RR 0.97, 95% CI 0.92 to 1.03) or with patients with cancer without active treatment (RR 1.00, 95% CI 0.96 to 1.04). There was a higher probability of seroconversion in patients with cancer treated with ICI compared with patients with cancer treated with chemotherapy (RR 1.09, 95% CI 1.00 to 1.18). In a single study in patients receiving ICI, no differences were observed in risk of irAE between those receiving inactivated vaccine and those unvaccinated (pneumonitis RR 0.88, 95% CI 0.33 to 2.3; rash RR 1.03, 95% CI 0.66 to 1.62; arthralgia RR 0.94, 95% CI 0.51 to 1.75). There were no studies for other types of vaccines comparing vaccinated vs not vaccinated in patients treated with ICI. The most common vaccine-related adverse events were local pain or fatigue. Overall, the quality of evidence was rated as very low.
Conclusion
COVID-19 vaccination appears to be effective and safe in patients with cancer receiving ICI.
Keywords: COVID-19; Immunotherapy; Vaccination; Immunogenicity, Vaccine; Cytotoxicity, Immunologic
WHAT IS ALREADY KNOWN ON THIS TOPIC.
It has been suggested that COVID-19 vaccines might lead to immune-related adverse event in patients receiving immune checkpoint inhibitor (ICI). Clinical trials that evaluated the efficacy of these vaccines did not include patients with cancer receiving treatment. Several small studies have been published with heterogeneous methods and results.
WHAT THIS STUDY ADDS
COVID-19 vaccination appears to be safe and effective in patients with cancer receiving ICI, although the quality of the evidence is low.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
COVID-19 vaccination can be recommended for patients with cancer receiving ICI. However, additional well-controlled studies are needed to robustly assess the impact of vaccination in this population with respect to clinical outcomes such as incidence and severity of COVID-19.
Background
As we face the third year of the COVID-19 pandemic, vaccination against COVID-19 has exponentially increased, including patient populations with chronic disease. Up to March 2022, 149 COVID-19 vaccines were in clinical development.1 Available vaccines are highly effective for the prevention of severe COVID-19 and mortality. A recent meta-analysis of 35 randomized control trials showed that the efficacy of vaccines to prevent COVID-19 infection was 95% (95% CI 92% to 97%) for mRNA vaccines, 68% (95% CI 61% to 74%) for viral vector vaccines, and 61% (95% CI 52% to 68%) for inactivated vaccines.2 None of the trials included pregnant women or immunocompromised participants such as patients with cancer.3
Patients with cancer are at high risk of COVID-19 severe complications and death. Those who are receiving oncological treatment when they acquire COVID-19 have higher risks of death, hospitalization, and intensive care unit (ICU) admission compared with patients with cancer without recent cancer treatment, or patients without cancer.4 COVID-19 vaccination is highly recommended for patients with cancer, despite a concern about potentially lower efficacy in immunosuppressed patients.5 Moreover, it has been suggested that COVID-19 vaccination may be a risk factor for immune-related adverse event (irAE) in patients with cancer receiving immune checkpoint inhibitor (ICI).6–8 Conceivably, both the COVID-19 vaccination and the ICI can independently stimulate the immune system potentiating adverse events.6 Prior literature on the use of influenza vaccination in patients with cancer receiving ICI, suggests that it is safe.9
However, to the best of our knowledge, there are no prior systematic reviews specifically evaluating COVID-19 vaccination in patients with cancer receiving ICI. The aim of this systematic review and meta-analysis is to determine the efficacy and safety of COVID-19 vaccination in this population.
Methods
This study was registered at PROSPERO (registration number: CRD42022307545; http://www.crd.york.ac.uk/PROSPERO). This study was conducted in accordance with the Preferred Reporting Items of Systematic Reviews and Meta-analyses statement.
Eligibility criteria
We included clinical trials, cohort studies (prospective and retrospective), and cross-sectional studies. We also included case series and case reports to identify unusual adverse events potentially associated with vaccination.
We included studies of adults (≥18 years old) with any type of cancer receiving ICI who underwent COVID-19 vaccination. Immune checkpoint inhibitors included: (1) programmed cell death protein 1 (PD-1) inhibitors (pembrolizumab, nivolumab, cemiplimab); programmed death-ligand 1 (PD-L1) inhibitors (atezolizumab, avelumab, durvalumab); and the cytotoxic T lymphocyte-associated protein 4 inhibitor ipilimumab. We considered any comparison group (eg, chemotherapy, no active treatment, healthy individuals). We excluded studies if insufficient information for analysis was provided, studies where the type of immunotherapy received was not specified, and studies on pediatric populations. We included the following 10 COVID-19 vaccines granted emergency use by the WHO: (1) protein subunit vaccines (Novavax (NVX-CoV2373) and COVOVAX of the Serum Institute of India), (2) mRNA vaccines (Moderna (mRNA-1273) and Pfizer/BioNTech (BNT162b2)), (3) non replicating viral vector vaccines (Janssen (Ad26.COV2.S); Oxford/AstraZeneca (ChAdOx1 nCoV-19); and Covishield of the Serum Institute of India), (4) inactivated vaccines (Bharat Biotech – Covaxin (BBV152)); Sinopharm (BBIBP-CorV); and Sinovac (CoronaVac)). The outcomes of interest were: seroconversion, COVID-19 infection, severe COVID-19, COVID-19 mortality, vaccine adverse events (local and systemic), irAE.
Information sources
An expert librarian searched two electronic databases Ovid Medline and Ovid Embase from December 1, 2019 to February 05, 2022. We also manually reviewed the references in other reviews of COVID-19 vaccination in patients with cancer.
Search strategy
The search included terms related to coronavirus vaccination, cancer, and ICI (online supplemental tables S1 and S2).
jitc-2022-006246supp001.pdf (262.1KB, pdf)
We used EndNote V.X9 (Clarivate) to manage references.
Selection process, data collection process and data items
Two reviewers (JIR and MAL-O) independently screened the citations and reviewed the studies of interest for inclusion. Disagreements were resolved by discussion. Data was extracted by one reviewer (JIR) and cross-checked by a second reviewer (MAL-O). The following information was extracted for each included study: (1) general study information (ie, year of publication, country, study design), (2) population characteristics (ie, age, gender, number of patients), (3) intervention characteristics (ie, number of patients under ICI treatment, number of patients in comparison group, interval between COVID-19 vaccine and outcome assessment, types of ICI, types of comparison (chemotherapy, no treatment, healthy individuals) and (4) outcomes: severe disease (according to each publication authors’ definition), ICU admission, mechanical ventilation, mortality, seroconversion, rate of irAE and type of irAE.
Study risk of bias assessment
The risk of bias was assessed by two reviewers (JIR and MAL-O) and discrepancies were resolved by discussion. We used the Newcastle-Ottawa Scale (NOS) to assess the methodological quality of observational studies. This scale consists of three components: patient selection, study comparability, and outcome assessment, with scores ranging from 0 to 9 (best).
Synthesis methods
We performed the statistical analysis using Review Manager V.5.3 (RevMan).
Effect measures
We presented the measure of association as risk ratios (RRs) and their corresponding 95% CI. If the data were not suitable for pooling, we synthesized the results narratively.
Processes used to decide which studies were eligible for synthesis
In order to decide which studies were eligible for each synthesis we specified and tabulated the study characteristics (population, intervention, the comparisons groups, and the outcomes).
Methods required to prepare the data for synthesis
We calculated the RRs when study provided raw data on frequency of events and sample sizes. We used the Mantel-Haenszel method for meta-analysis of dichotomous raw data. Adjusted estimates were used where possible for primary analyses, to decrease potential confounder bias. Data were pooled using random effects models.
Heterogeneity
We assessed heterogeneity using I2 statistics. We considered that heterogeneity was present when the I2 was higher than 40%.
Methods to explore heterogeneity
We grouped studies by type of vaccine, and type of design (prospective vs retrospective) to determine their potential effect on the results.
Additional analyses
In order to evaluate the occurrence of unusual adverse events of ICIs in patients with COVID-19 immunization, we summarized case reports and case series that identified irAE that may not be detected in longitudinal observational studies.
Reporting bias assessment
We planned a priori to assess and quantify publication bias using funnel plots and Egger’s test if more than 10 studies reported on the primary outcome. However, data were insufficient to perform this analysis.
Certainty assessment
We evaluated the quality of the evidence for each outcome using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) framework, which considers risk of bias, indirectness, inconsistency, imprecision, and publication bias.10 We created summary of findings tables (SoF), which synthesize the most important results of a review in a structured format that is transparent providing information about the quality of evidence and the magnitude of the effects of the outcomes of interest, including the following information for each of the important outcomes (desirable and undesirable)11: (1) the relative effect of the intervention, (2) the baseline risk (control group), (3) the absolute risk of the intervention group, (3) the number of studies and number of participants, and (4) the confidence in the effect estimates or certainty of the evidence.12 The RR with its 95% CI was obtained from the meta-analyses performed for each outcome and comparison. The absolute risks for each comparison groups were obtained from the representative studies of the review or the median comparator group risk across studies. The intervention absolute risk was obtained from the following calculation:
The certainty of the evidence was assessed using the specific grading system of the GRADE working group that considers the following domains: (1) risk of bias (ie, the confidence on the estimate of effect decreases because there are limitations in the study design), (2) inconsistency (ie, when the estimates of effect vary widely from one study to the other and there are no explanations for this heterogeneity), (3) indirectness (ie, the estimate of effect comes from studies with different population, and/or intervention, and/or comparison, and/or outcome from our main review question, (4) imprecision (ie, the studies include few number of participants and/or events or the 95% CI includes both benefits and harms for the patients), and (5) publication bias (ie, when investigators do not report studies because of lack of effect or selecting and non-reporting outcomes). The certainty or quality of the evidence was rated as high (indicating that further research is very unlikely to change our confidence in the estimate of effect), moderate (indicating that further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate), low (indicating that further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate) or very low (indicating that any estimate of effect is very uncertain).
Results
Search results
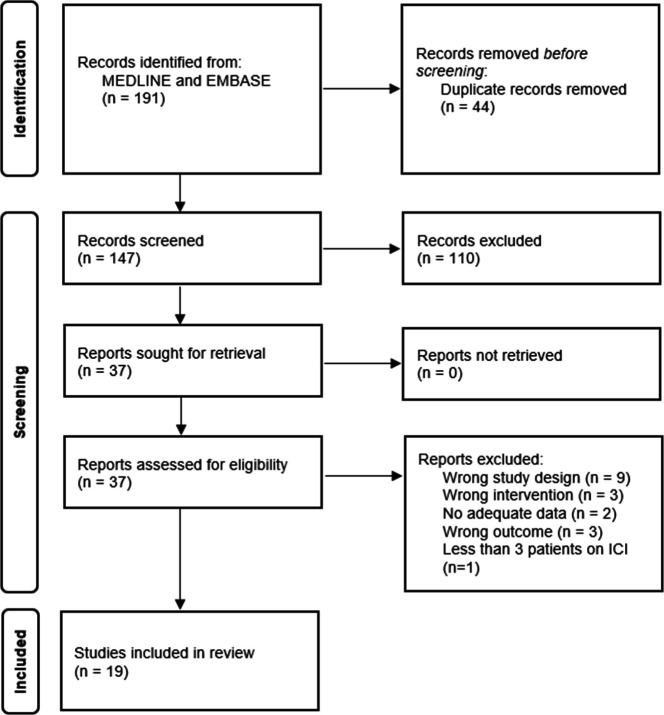
The strategy identified 191 citations (figure 1). After removing duplicated studies, we screened 147 citations. We included 37 studies for full text assessment. Finally, 19 studies were eligible to include for analysis.13–31 A total of 10 865 participants were included in the analysis and 2477 received ICI.
Figure 1.
Flow diagram of study selection.
Description of included studies
Study characteristics are described in table 1. Considering the type of studies included, 3 were case reports, 15 cohort studies, and 1 cross-sectional study. The range of ages included were 16–93 and the average of male participants was 52%. The types of vaccines included were: BNT162b2 (Pfizer) in 16 (84%) studies,13–16 18 20 21 23–25 27–31 mRNA-1273 (Moderna) in 8 (42%) studies,17–19 25–27 29 30 Ad26.COV2S (Janssen) in 3 (16%) studies,25 27 30 Sinovac in 1 (5%) studies,22 Sinopharm in 1 (5%) study,22 ChAdOx1 nCoV-19 (AstraZeneca) in 1 (5%) study.27 With regard to the outcomes, 12 studies reported immunogenicity as humoral response 14–30 days after the second dose of the vaccine,13 16 18–23 25 26 29 30 10 studies reported irAE,14 15 17 20 22 24 26 27 29 31 9 reported vaccine-related adverse events (VrAE),16 20 21 25 26 28 29 31 and 3 reported incidence of COVID-19.20 21 29 The characteristics of the participants included in the studies are in table 2. The types of cancer included and the funding information for each study are shown in online supplemental table S3.
Table 1.
Characteristics of included studies
| Study ID | Country | Health centers n | Period | Vaccine | Total n | Outcomes | Seroconversion cut-off |
| Retrospective cohort studies | |||||||
| Chen et al 202117 | USA | 1 | NR | BNT162b2 (Pfizer) and mRNA-1273 (Moderna) | 81 | irAE, VrAE | – |
| Ligumsky et al 202121 | Israel | 1 | March–April 2021 | BNT162b2 (Pfizer) | 490 | Immunogenicity (humoral response), VrAE | ˃50 AU/mL |
| Ma et al 202122 | China | 4 | NR | CoronoVac (Sinovac) and Beijing Bio-Institute of Biological Products (Sinopharm) | 660 | Immunogenicity (humoral response), irAE | – |
| Strobel et al 202127 | Germany | 1 | March–July 2021 | BNT162b2 (Pfizer), mRNA-1273 (Moderna), ChAdOx1 nCoV-19 (AstraZeneca), and Ad26.COV2S (Janssen) | 130 | irAE | – |
| Svoboda et al 202129 | USA | NR | July 2020–June 2021 | BNT162b2 (Pfizer) and mRNA-1273 (Moderna) | 23 | Immunogenicity (humoral response), VrAE, irAE | Receptor binding domain ˃0.700 AU |
| Prospective cohort studies | |||||||
| Buttiron-Webber et al 202116 | Italy | NR | NR | BNT162b2 (Pfizer) | 320 (291 analyzed) | Immunogenicity (humoral response), VrAE | ˃25 AU/mL |
| Di Giacomo et al 202118 | Italy | NR | NR | BNT162b2 (Pfizer) for healthy hospital personnel and mRNA-1273 (Moderna) for patients with cancer | 173 | Immunogenicity (humoral response) | ˃50 AU/mL |
| Figueiredo et al 202119 | USA | 1 | December 2020–August 2021 | BNT162b2 (Pfizer) and mRNA-1273 (Moderna) | 1697 | Immunogenicity (humoral response) | ˃50 AU/mL |
| Lasagna et al 202120 | Italy | Multi-center | March–April 2021 | BNT162b2 (Pfizer) | 88 | Immunogenicity, incidence of COVID-19, VrAE, irAE | ˃15 AU/mL |
| Massarweh et al 202123 | Israel | 1 | February–March 2021 | BNT162b2 (Pfizer) | 180 | Immunogenicity (humoral response) | ˃50 AU/mL |
| Naranbhai et al 202125 | USA | 1 | April–July 2021 | BNT162b2 (Pfizer), Ad26.COV2S (Janssen), mRNA1273 (Moderna) | 762* | Immunogenicity, VrAE | Index ˃0.8 |
| Oosting et al 2021 (VOICE trial)26 | Netherlands | Multi-center: 3 | February–March 2021 | mRNA-1273 (Moderna) | 750 | Immunogenicity (humoral response), VrAE, irAE | ˃10 AU/mL |
| Subbiah et al 202132 | USA | 1 | NR | BNT162b2 (Pfizer) | 4714 | VrAE (Patient reported outcomes) | – |
| Thakkar et al 202130 | USA | NR | NR | BNT162b2 (Pfizer), mRNA-1273 (Moderna), Ad26.COV2S (Janssen) | 242† | Immunogenicity (humoral response) | ˃50 AU/mL |
| Waissengrin et al 202131 | Israel | 1 | January–February 2021 | BNT162b2 (Pfizer) | 268 | VrAE, IrAE | – |
| Cross-sectional studies | |||||||
| Agbarya et al 202113 | Israel | Multi-center: 2 | February–April 2021 | BNT162b2 (Pfizer) | 355 | Immunogenicity (humoral response) | ˃150 AU/mL |
| Case reports | |||||||
| Au et al 202114 | UK | NR | December 2020 | BNT162b2 (Pfizer) | – | irAE (cytokine release syndrome) | – |
| Blaise et al 202115 | France | NR | January 2021 | BNT162b2 (Pfizer) | – | irAE (necrotizing myopathy – Grade 4) | – |
| Mieczkowska et al 202124 | USA | NR | NR | BNT162b2 (Pfizer) | – | irAE (Psoriasis exacerbation) | – |
*Excluding the healthy controls reported in the literature previously.
†200 analyzed for efficacy.
ICI, immune checkpoint inhibitor; irAE, immune-related adverse events; NA, not applicable; NR, not reported; VrAEs, vaccine-related adverse events.
Table 2.
Characteristics of patients included in the studies
| Study ID | Age | Types of ICI | Non-ICI interventions | Interval between second vaccine and evaluation of outcome | % of patients with prior COVID-19 infection |
| Agbarya et al 202113 | 65.3 (mean) | Pembrolizumab, nivolumab, ipilimumab, durvalumab, avelumab, atezolizumab, cemiplimab (n=43) | Chemotherapy (n=73), biological drugs (24), healthy subjects (n=215)* | 14 days | NR |
| Au et al 202114 | ˃18† | Anti-PD-1 monotherapy (n=1) | NA | 5 days | NR |
| Blaise et al 202115 | ˃18† | Pembrolizumab, nivolumab (1 mg/kg) plus ipilimumab (3 mg/kg) (n=1) | NA | 10 days (after first vaccine) | NR |
| Buttiron Webber et al 202116 | 68 (median) | Unspecified ICI (n=21) | Chemotherapy (n=115), hormone therapy (n=70), targeted therapy (n=23), patients with cancer with no active treatment (n=62) | 21 days | 11.3 |
| Chen et al 202117 | 70 (median) | Pembrolizumab (n=45), nivolumab (n=22), durvalumab (n=6), cemiplimab (n=5), atezolizumab (n=3) | NA | 30 days (at least) | NR |
| Di Giacomo et al 202118 | NR | Unspecified ICI (n=70) | Chemotherapy (n=28), Targeted therapy (n=23), healthy subjects (n=42), | 18 days (median) | 0 |
| Figueiredo et al 202119 | 65 (median) | Unspecified vaccinated ICI (n=74) | healthcare workers (n=1245) Unvaccinated patients with cancer (n=54), vaccinated patients with cancer (n=291) | 42 days (median) | Vaccinated: 6.2 Unvaccinated: 22.2 |
| Lasagna et al 202120 | 68 (median) | PD-1/PD-L1 (n=88) | NA | 21 days | 14.8 |
| Ligumsky et al 202121 | 66 (median) | Unspecified ICI (n=55) | Chemotherapy (n=101), Combination (n=104)‡, targeted therapy (n=38), other treatments (n=28)‡, healthy subjects (n=164)* | 78 days | NR |
| Ma et al 202122 | 50.3 (mean) | Nivolumab (n=51), pembrolizumab (n=49), sintilimab (n=76), toipalimab (n=44), tislelizumab (n=31), camrelizumab (n=39). Patients were divided in PD-1 vaccinated (n=138) and PD1 unvaccinated (n=152) | Patients with cancer without PD-1 (n=164), non-cancer patients (n=206) | 1–3 months | NR |
| Massarweh et al 202123 | 66 (median) | Unspecified ICI (n=22) | Chemotherapy (n=30), chemotherapy plus biological therapy (n=20), biological therapy (n=11), healthy subjects (n=78) | 38 days | NR |
| Mieczkowska et al 202124 | ˃ 18† | Nivolumab (n=1) | NA | 7 days (after first dose) | NR |
| Naranbhai et al 202125 | 66 (median) | Unspecified ICI (n=70) | No systemic treatment (n=205), healthy subjects (n=418), chemotherapy (n=101), targeted therapy (n=149), combination (n=124) | 7 days | |
| Oosting et al 2021 (VOICE trial)26 | 66 (median) | Nivolumab (n=66), pembrolizumab (n=36), cemiplimab (n=7), atezolizumab (n=5), avelumab (n=5), duvalumab (n=2) | Chemotherapy (n=229), chemotherapy plus immunotherapy (n=143), healthy patients (n=247) | 28 days | NR |
| Strobel et al 202127 | 64 (median) | Pembrolizumab (n=45), nivolumab (n=14), cemiplimab (n=4), avelumab (n=6), combination ICI (n=20) | Non-ICI systemic therapies (n=108), unvaccinated patients with cancer (n=19) | 84 days | NR |
| Subbiah et al 202132 | 54 (median) | Unspecified ICI (n=857) | NA | NR | NR |
| Svoboda et al 202129 | 42 (median) | PD-1 (n=23) | NA | – | 26 |
| Thakkar et al 202130 | 67 (median) | Unspecified ICI (n=31) | Non-cancer patients (n=26), non-ICI treatments (n=169) | 7 days | 11 |
| Waissengrin et al 202131 | 72 (median) | Unspecified ICI (n=97) | Healthy subjects (n=134) | 19 days | NR |
*Patients’ relatives, healthcare workers, and volunteers.
†Exact patient age not provided as required by the journal.
‡Includes patients receiving other treatments plus ICI.
ICI, immune checkpoint inhibitor; NA, not applicable; NR, not reported; PD-1, programmed cell death 1; PD-L1, programmed cell death ligand 1.
Observational studies
The reported rates of seroconversion and adverse events of individual studies are shown in online supplemental table S4.
Seroconversion
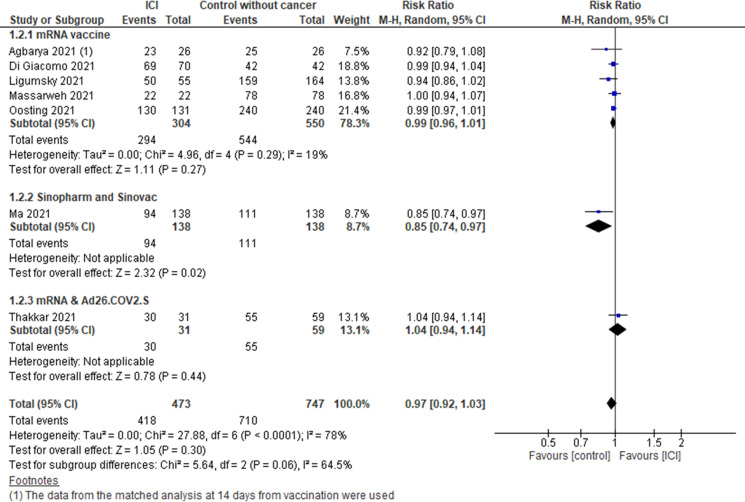
Patients with cancer treated with ICI compared with controls without cancer (figure 2) (7 studies13 18 21–23 26 30 including 473 patients on ICI and 747 controls). No statistically significant differences were observed in the pooled estimate (RR 0.97, 95% CI 0.92 to 1.03). However, when analyzing the subgroups by type of vaccines, the risk of seroconversion with the inactivated vaccines Sinopharm or Sinovac was lower in patients with cancer treated with ICI compared with individuals without cancer (RR 0.85, 95% CI 0.74 to 0.97).
Figure 2.
Risk of seroconversion after COVID-19 vaccination in patients with cancer treated with ICI versus control without cancer. ICI, immune checkpoint inhibitor.
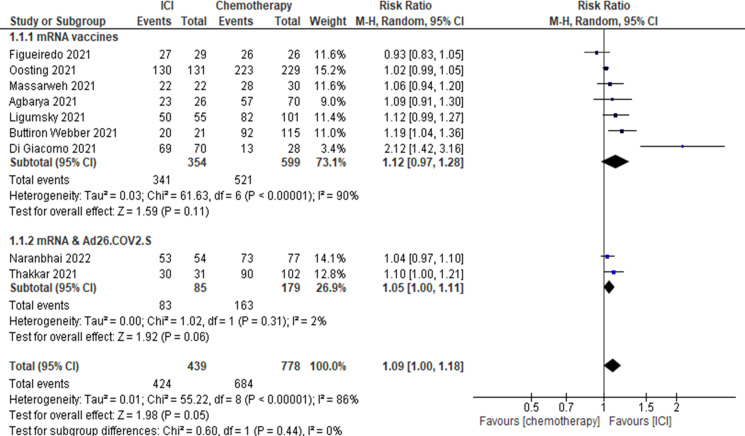
Patients with cancer treated with ICI compared with patients with cancer treated with chemotherapy (figure 3) (9 studies13 16 18 19 21 23 25 26 30 including 439 patients on ICI and 778 patients on chemotherapy). The RR for this comparison was 1.09 (95% CI 1.00 to 1.18) favoring patients treated with ICI.
Figure 3.
Risk of seroconversion after COVID-19 vaccination in patients with cancer treated with ICI versus patients with cancer treated with chemotherapy. ICI, immune checkpoint inhibitor.
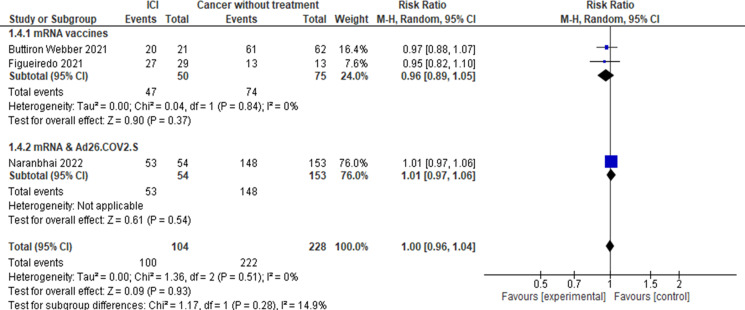
Patients with cancer treated with ICI compared with patients with cancer without active treatment (figure 4). Three studies16 19 25 reported seroconversion for this comparison including 104 patients on ICI and 228 patients with cancer without active treatment. No statistically significant differences were observed in the pooled estimate (RR 1.00, 95% CI 0.96 to 1.04).
Figure 4.
Risk of seroconversion after COVID-19 vaccination in patients with cancer treated with ICI versus patients with cancer without treatment. ICI, immune checkpoint inhibitor.
COVID-19 infection
Three studies20 21 29 evaluated the incidence of COVID-19 infection including 155 patients with cancer treated with ICI. There were no COVID-19 infection cases documented during the period of the studies. As no COVID-19 infections were reported, severity and mortality could not be evaluated.
Vaccine-related adverse events
Ten studies16 20–22 25–29 31 reported the frequency of VrAE in patients with cancer treated with ICI and who received the COVID-19 vaccine. The results for individual studies are shown in online supplemental table S3. Overall, most of the VrAE were mild or moderate, with local pain and fatigue as the most common VrAE. The range of rate of fatigue was from 24% to 59%, the range of local pain was from 6% to 63%. In one study22 that used the inactivated vaccines Sinovac and Sinopharm there was a statistically significant difference in rash comparing patients who received ICI vs those who did not receive ICI (OR 3.5, 95% CI 1.67 to 7.35 p<0.001).
Patients with cancer treated with ICI compared with controls without cancer. One study26 (VOICE trial) including 137 patients on ICI and 240 controls reported VrAE. No statistically significant differences were observed for the following adverse events: fatigue (RR 1.09, 95% CI 0.91 to 1.31 p=0.36), pain (RR 1.60, 95% CI 0.63 to 4.06 p=0.32), VrAE grade 3 or worse (RR 12.22, 95% CI 0.64 to 234.9 p=0.1).
Patients with cancer treated with ICI compared with patients with cancer treated with chemotherapy. One study26 including 137 patients on ICI and 244 on chemotherapy reported VrAE. No statistically significant differences were observed for the following adverse events: fatigue (RR 1.21, 95% CI 0.99 to 1.47 p=0.06), pain (RR 1.25, 95% CI 0.52 to 3.03 p=0.62), VrAE grade 3 or worse (RR 0.89, 95% CI 0.23 to 3.50 p=0.87).
Immune-related adverse events
Six studies17 20 22 26 27 31 reported the frequency of irAE in patients with cancer treated with ICI and who received the COVID-19 vaccine. The results of individual studies are shown in online supplemental table S3. The range of rate of irAE was from 0% to 23.6%. No unusual adverse events were reported.
There was only one study22 that reported the risk of irAE comparing patients treated with ICI who received the vaccine versus those who did not receive it. This study evaluated the inactivated vaccines Sinopharm and Sinovac including 127 patients on the vaccine group and 127 patients on the non-vaccine group performing a propensity score matched analysis. No statistically significant differences were observed for pneumonitis (RR 0.88, 95% CI 0.33 to 2.31 p=0.79), rash (RR 1.03, 95% CI 0.66 to 1.62 p=0.88), diarrhea (RR 0.82, 95% CI 0.35 to 1.91 p=0.64), arthralgia (RR 0.94, 95% CI 0.51 to 1.75 p=0.86), liver function test abnormalities (RR 1.07, 95% CI 0.55 to 2.06 p=0.85).
Risk of bias
We used the NOS to assess the risk of bias of the 16 observational studies, (online supplemental table S5). The scores ranged from 3 to 8 (maximum score 9). Nine (56%) of the studies13 16–18 20 27 29 31 32 had high risk of confounding bias as they did not adjust for potential confounders.
Summary of findings
The summary of findings tables with the certainty of evidence of the different comparisons are shown in online supplemental tables S6–8. The absolute benefit of seroconversion in patients with cancer treated with ICI was: (1) 922 per 1000 (between 874 and 970 per 1000) compared with 950 per 1000 in individuals without cancer, (2) 974 per 1000 (between 935 and 1000 per 1000) compared with 974 per 1000 in patients with cancer without active treatment, and (3) 958 per 1000 (between 879 and 1000 per 1000) compared with 879 per 1000 in patients with cancer treated with chemotherapy. The absolute risk of grade 3 or more VrAE in patients with cancer treated with ICI was 8 per 1000 (between 2 and 29 per 1000) compared with 8 per 1000 in patients with cancer treated with chemotherapy.
For all the comparisons and outcomes, the certainty of the evidence was rated as very low because the risk of bias of the primary studies and downgraded for imprecision. We also downgraded the quality of evidence for indirectness because seroconversion was considered a surrogate outcome.
Case reports
We found three case reports14 15 24 that reported unusual adverse events in patients with cancer treated with ICI who received the COVID-19 vaccine. In the three cases the patients received the BNT162b2 vaccine. One of the patients presented cytokine release syndrome, another patient necrotizing myopathy grade four and the third case exacerbation of psoriasis.
The studies excluded and the reasons for exclusions are presented in online supplemental table S9.
Discussion
To our knowledge, this is the first systematic review evaluating the efficacy and safety of COVID-19 vaccines in patients with cancer receiving ICI. Previous systematic reviews and meta-analyses33 34 have reported on the outcomes of COVID-19 vaccines in patients with cancer at large, but not specifically in those receiving ICI. Becerrill-Gaitan et al showed that patients with cancer were less likely to seroconvert after complete vaccination compared with non-cancer controls (RR 0.69, 95% CI 0.65 to 0.84).33 Nevertheless, they did not analyze the effect of the different cancer treatment on the efficacy of the vaccine. A narrative review included information in patients treated with ICI suggesting that efficacy and safety were similar to that observed in the general population.7
The results of our systematic review suggest that COVID-19 vaccines are effective in patients with cancer treated with ICI, as determined by seroconversion rates. We found no significant differences in the rate of seroconversion after the second dose of the vaccine when comparing patients with cancer receiving ICI versus healthy participants. However, the frequency of seroconversion with the inactivated vaccines Sinopharm or Sinovac was significantly lower in patients with cancer treated with ICI compared with individuals without cancer. Patients with cancer treated with ICI showed a higher probability of seroconversion than patients with cancer treated with chemotherapy. Rates of COVID-19 infection were evaluated in three small studies, and none of the 155 patients receiving ICI developed COVID-19. Therefore, the impact of vaccination on clinically important outcomes such as hospital admissions, use of mechanical ventilation or death, could not be assessed.
As treatment with ICI enhances immune responses, there have been theoretical concerns that concomitant treatment with ICI and receipt of the COVID-19 vaccination, could result in increased risk of irAE.6 Only one study22 reported irAE comparing patients on ICI who received the inactivated vaccines Sinopharm and Sinovac vaccines with those who did not receive vaccination, and found no statistically significant differences between the two groups. Other studies reported rates of irAE with mRNA vaccines, ranging 0%–24%, but they did not have suitable control groups of unvaccinated patients receiving ICI. These rates, however, seem similar to the observed rates of irAE in studies of patients receiving ICI (not related to vaccination).35–37 Moreover, most of the irAE were low grade. No unusual adverse events were reported in these studies.
With regard to the adverse events related to the vaccination, most of them were local pain and fatigue with a range of 6%–63% and 24%–59%, respectively. Most of the VrAE reported were mild or moderate. In the study of patients who received the inactivated vaccines Sinovac and Sinopharm22 there was a higher risk of developing rash in patients treated with ICI compared with those not treated with ICI. The VOICE trial26 that evaluated the safety and efficacy of the mRNA-1273 vaccine in patients with cancer, showed no statistically significant differences on VrAEs in patients treated with ICI compared with participants without cancer, or patients with cancer treated with chemotherapy. Only 3 of 137 patients treated with ICI (2%) in this study had grade 3 or more VrAE. Another study showed that the patients who received ICI reported a higher increase of itch and rash after receiving the second dose of the mRNA vaccine compared with those without cancer treatment.32 However, this was reported in a conference abstract and no frequencies were provided.
This systematic review provides the most recent synthesis of evidence about the efficacy and safety of COVID-19 vaccines in patients with cancer receiving ICI. However, it had limitations inherent to the evidence that was available for review and synthesis. The certainty of the evidence in our systematic review was rated as low or very low for all the outcomes evaluated. We rated down for risk of bias and for imprecision. As we stated before, none of the studies evaluated critical outcomes (ie, mortality, several COVID-19, hospital admission). We consider seroconversion as a surrogate outcome and we rated down the certainty of evidence for indirectness.38 Another limitation, is the information provided regarding to the differences in ICI, regimen, and dose and duration of ICI across the studies. Among the 16 cohort studies included, 11 (69%) did not specify the ICI used or the specific regimen (monotherapy or combination therapy). These factors might have an impact on the outcomes of interest, as it has been shown that dose and duration of ICI might affect the rate of irAE in general.39 Moreover, combination therapy has a higher risk of irAE compare to monotherapy.40 Since the primary studies of this systematic review are not randomized control trials, there are several other known and unknown confounding factors that could have an impact on the results as the compared groups may not be balanced, risk factors were not adjusted for in the analyses. Some potential confounders include demographics, prior comorbidities including history of autoimmune disorders, prior cancer treatment or concurrent medications which could impact the development of irAE.39 41
In summary, the efficacy of the COVID-19 vaccination in patients with cancer treated with ICI, measured by seroconversion, was similar to that of healthy controls and higher than that observed in patients with cancer who received chemotherapy. No increase in VrAE or irAE were reported. Our results suggest that COVID-19 vaccination seems effective and safe in patients with cancer receiving ICI, although higher-quality evidence may be needed to further establish the robustness of these findings, including observational studies with low risk of bias and evaluating clinical important outcomes of vaccination such as COVID-19 incidence and severity, and related hospitalization and mortality.
Footnotes
Twitter: @amlopezo
Contributors: MES-A conceived the idea of the study. MES-A and JIR developed the protocol. YG performed the literature search. JIR and MAL-O reviewed and appraised the data. JIR and MAL-O conducted the analysis. JIR, MAL-O, YG and MES-A participated in writing the manuscript.
Funding: This study was supported by the National Cancer Institute (K08 grant CA237619, Maria A. Lopez-Olivo, MD, PhD, Principal Investigator) and through MD Anderson’s Cancer Center Support Grant, P30 CA016672.
Disclaimer: The study sponsors did not play any role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. MES-A has received consultant fees in the past 12 months from Pfizer, Eli Lilly and Bristol Myers Squibb/Celgene unrelated to this study.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Organization WH . COVID-19 vaccine tracker and landscape: World Health organization; 2022, 2022. Available: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines [Accessed Aug 2022].
- 2.Korang SK, von Rohden E, Veroniki AA, et al. Vaccines to prevent COVID-19: a living systematic review with trial sequential analysis and network meta-analysis of randomized clinical trials. PLoS One 2022;17:e0260733. 10.1371/journal.pone.0260733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corti C, Curigliano G. Commentary: SARS-CoV-2 vaccines and cancer patients. Ann Oncol 2021;32:569–71. 10.1016/j.annonc.2020.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chavez-MacGregor M, Lei X, Zhao H. Evaluation of COVID-19 mortality and adverse outcomes in US patients with or without cancer. JAMA Oncol 2021. 10.1001/jamaoncol.2021.5148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hwang JK, Zhang T, Wang AZ, et al. COVID-19 vaccines for patients with cancer: benefits likely outweigh risks. J Hematol Oncol 2021;14:38. 10.1186/s13045-021-01046-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brest P, Mograbi B, Hofman P, et al. COVID-19 vaccination and cancer immunotherapy: should they stick together? Br J Cancer 2022;126:1–3. 10.1038/s41416-021-01618-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fendler A, de Vries EGE, GeurtsvanKessel CH, et al. COVID-19 vaccines in patients with cancer: immunogenicity, efficacy and safety. Nat Rev Clin Oncol 2022;19:385–401. 10.1038/s41571-022-00610-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yekedüz E, Ayasun R, Köksoy EB, et al. mRNA-Based COVID-19 vaccines appear to not increase immune events in cancer patients receiving immune checkpoint inhibitors. Future Virol 2021;16:583–5. 10.2217/fvl-2021-0166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lopez-Olivo MA, Valerio V, Karpes Matusevich AR, et al. Safety and efficacy of influenza vaccination in patients receiving immune checkpoint inhibitors. systematic review with meta-analysis. Vaccines 2022;10. 10.3390/vaccines10081195. [Epub ahead of print: 27 07 2022]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guyatt GH, Oxman AD, Vist GE, et al. Grade: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924–6. 10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guyatt GH, Oxman AD, Santesso N, et al. Grade guidelines: 12. preparing summary of findings tables-binary outcomes. J Clin Epidemiol 2013;66:158–72. 10.1016/j.jclinepi.2012.01.012 [DOI] [PubMed] [Google Scholar]
- 12.Schünemann HJ, Higgins JP, Vist GE. Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Cochrane Handbook for systematic reviews of interventions, 2019: 375–402. [Google Scholar]
- 13.Agbarya A, Sarel I, Ziv-Baran T, et al. Efficacy of the mRNA-based BNT162b2 COVID-19 vaccine in patients with solid malignancies treated with anti-neoplastic drugs. Cancers 2021;13:4191. 10.3390/cancers13164191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Au L, Fendler A, Shepherd STC, et al. Cytokine release syndrome in a patient with colorectal cancer after vaccination with BNT162b2. Nat Med 2021;27:1362–6. 10.1038/s41591-021-01387-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blaise M, Rocher F, Spittler H, et al. Severe necrotizing myopathy after COVID-19 vaccine with BNT162b2 and regimen with ipilimumab plus nivolumab in a patient with advanced melanoma. J Eur Acad Dermatol Venereol 2022;36:e100–2. 10.1111/jdv.17760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buttiron Webber T, Provinciali N, Musso M, et al. Predictors of poor seroconversion and adverse events to SARS-CoV-2 mRNA BNT162b2 vaccine in cancer patients on active treatment. Eur J Cancer 2021;159:105–12. 10.1016/j.ejca.2021.09.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Y-W, Tucker MD, Beckermann KE, et al. COVID-19 mRNA vaccines and immune-related adverse events in cancer patients treated with immune checkpoint inhibitors. Eur J Cancer 2021;155:291–3. 10.1016/j.ejca.2021.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Di Giacomo AM, Giacobini G, Gandolfo C, et al. Severe acute respiratory syndrome coronavirus 2 vaccination and cancer therapy: a successful but mindful mix. Eur J Cancer 2021;156:119–21. 10.1016/j.ejca.2021.07.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Figueiredo JC, Merin NM, Hamid O, et al. Longitudinal SARS-CoV-2 mRNA vaccine-induced humoral immune responses in patients with cancer. Cancer Res 2021;81:6273–80. 10.1158/0008-5472.CAN-21-3554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lasagna A, Agustoni F, Percivalle E, et al. A snapshot of the immunogenicity, efficacy and safety of a full course of BNT162b2 anti-SARS-CoV-2 vaccine in cancer patients treated with PD-1/PD-L1 inhibitors: a longitudinal cohort study. ESMO Open 2021;6:100272. 10.1016/j.esmoop.2021.100272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ligumsky H, Safadi E, Etan T. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine among actively treated cancer patients. JNCI: Journal of the National Cancer Institute, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma Y, Liu N, Wang Y, et al. Immune checkpoint blocking impact and nomogram prediction of COVID-19 inactivated vaccine seroconversion in patients with cancer: a propensity-score matched analysis. J Immunother Cancer 2021;9:e003712. 10.1136/jitc-2021-003712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Massarweh A, Eliakim-Raz N, Stemmer A, et al. Evaluation of seropositivity following BNT162b2 messenger RNA vaccination for SARS-CoV-2 in patients undergoing treatment for cancer. JAMA Oncol 2021;7:1133–40. 10.1001/jamaoncol.2021.2155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mieczkowska K, Kaubisch A, McLellan BN. Exacerbation of psoriasis following COVID-19 vaccination in a patient previously treated with PD-1 inhibitor. Dermatol Ther 2021;34:e15055. 10.1111/dth.15055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Naranbhai V, Pernat CA, Gavralidis A, et al. Immunogenicity and Reactogenicity of SARS-CoV-2 vaccines in patients with cancer: the CANVAX cohort study. J Clin Oncol 2022;40:12–23. 10.1200/JCO.21.01891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oosting SF, van der Veldt AAM, GeurtsvanKessel CH, et al. mRNA-1273 COVID-19 vaccination in patients receiving chemotherapy, immunotherapy, or chemoimmunotherapy for solid tumours: a prospective, multicentre, non-inferiority trial. Lancet Oncol 2021;22:1681–91. 10.1016/S1470-2045(21)00574-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strobel SB, Machiraju D, Kälber KA, et al. Immune-Related adverse events of COVID-19 vaccination in skin cancer patients receiving immune-checkpoint inhibitor treatment. Cancer Immunol Immunother 2022;71:2051–6. 10.1007/s00262-021-03133-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Subbiah IM, Williams LA, Peek A, et al. Real-World patient-reported and clinical outcomes of BNT162b2 mRNA COVID-19 vaccine in patients with cancer. JCO 2021;39:6510–10. 10.1200/JCO.2021.39.15_suppl.6510 [DOI] [Google Scholar]
- 29.Svoboda J, Ballard HJ, Ho CI, et al. Safety and efficacy of Sars-Cov-2 vaccines in Hodgkin lymphoma patients receiving PD-1 inhibitors. Blood 2021;138:2445–45. 10.1182/blood-2021-153122 [DOI] [Google Scholar]
- 30.Thakkar A, Gonzalez-Lugo JD, Goradia N, et al. Seroconversion rates following COVID-19 vaccination among patients with cancer. Cancer Cell 2021;39:1081–90. 10.1016/j.ccell.2021.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Waissengrin B, Agbarya A, Safadi E, et al. Short-Term safety of the BNT162b2 mRNA COVID-19 vaccine in patients with cancer treated with immune checkpoint inhibitors. Lancet Oncol 2021;22:581–3. 10.1016/S1470-2045(21)00155-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Subbiah IM, Williams LA, Peek A. Real-World patient-reported and clinical outcomes of BNT162b2 mRNA COVID-19 vaccine in patients with cancer. Journal of clinical oncology conference annual meeting of the American Society of clinical oncology. ASCO 2021;39. 10.1200/JCO.2021.39.15 [DOI] [Google Scholar]
- 33.Becerril-Gaitan A, Vaca-Cartagena BF, Ferrigno AS, et al. Immunogenicity and risk of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection after coronavirus disease 2019 (COVID-19) vaccination in patients with cancer: a systematic review and meta-analysis. Eur J Cancer 2022;160:243–60. 10.1016/j.ejca.2021.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cavanna L, Citterio C, Toscani I. COVID-19 vaccines in cancer patients. seropositivity and safety. systematic review and meta-analysis. Vaccines 2021;9. 10.3390/vaccines9091048. [Epub ahead of print: 20 09 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xing P, Zhang F, Wang G, et al. Incidence rates of immune-related adverse events and their correlation with response in advanced solid tumours treated with NIVO or NIVO+IPI: a systematic review and meta-analysis. J Immunother Cancer 2019;7:341. 10.1186/s40425-019-0779-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Y, Zhou S, Yang F, et al. Treatment-Related adverse events of PD-1 and PD-L1 inhibitors in clinical trials: a systematic review and meta-analysis. JAMA Oncol 2019;5:1008–19. 10.1001/jamaoncol.2019.0393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fujii T, Colen RR, Bilen MA, et al. Incidence of immune-related adverse events and its association with treatment outcomes: the MD Anderson cancer center experience. Invest New Drugs 2018;36:638–46. 10.1007/s10637-017-0534-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 8. Rating the quality of evidence--indirectness. J Clin Epidemiol 2011;64:1303–10. 10.1016/j.jclinepi.2011.04.014 [DOI] [PubMed] [Google Scholar]
- 39.Liu X, Shi Y, Zhang D, et al. Risk factors for immune-related adverse events: what have we learned and what lies ahead? Biomark Res 2021;9:79. 10.1186/s40364-021-00314-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med 2015;372:2006–17. 10.1056/NEJMoa1414428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramos-Casals M, Brahmer JR, Callahan MK, et al. Immune-Related adverse events of checkpoint inhibitors. Nat Rev Dis Primers 2020;6:38. 10.1038/s41572-020-0160-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2022-006246supp001.pdf (262.1KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.