Abstract
The COVID-19 pandemic has greatly impacted the U.S. food supply and consumer behavior. Food production and processing are being disrupted as illnesses, proactive quarantines, and government-mandated movement restrictions cause labor shortages. In this environment, the food industry has been required to adopt new, additional practices to minimize the risk of COVID-19 cases and outbreaks among its workforce. Successfully overcoming these challenges requires a comprehensive approach that addresses COVID-19 transmission both within and outside the facility. Possible interventions include strategies (i) to vaccinate employees, (ii) to assure that employees practice social distancing, (iii) to assure that employees wear face coverings, (iv) to screen employees for COVID-19, (v) to assure that employees practice frequent hand washing and avoid touching their faces, (vi) to clean frequently touched surfaces, and (vii) to assure proper ventilation. Compliance with these control strategies must be verified, and an overall COVID-19 control culture must be established to implement an effective program. Despite some public misperceptions about the health risk of severe acute respiratory syndrome coronavirus 2 on foods or food packaging, both the virus biology and epidemiological data clearly support a negligible risk of COVID-19 transmission through food and food packing. However, COVID-19 pandemic-related supply chain and workforce disruptions and the shift in resources to protect food industry employees from COVID-19 may increase the actual food safety risks. The goal of this review was to describe the COVID-19 mitigation practices adopted by the food industry and the potential impact of these practices and COVID-19–related disruptions on the industry's food safety mission. A review of these impacts is necessary to ensure that the food industry is prepared to maintain a safe and nutritious food supply in the face of future global disruptions.
Keywords: COVID-19, Food industry, Food safety, Severe acute respiratory syndrome coronavirus 2
COVID-19 has been a true pandemic with an unprecedented global impact, affecting more than 200 countries and regions. According to the Institute for Health Metrics and Evaluation ( 78 ), the death toll is predicted to reach more than 4.5 million by October 2021 ( 57 ). In addition to developing and executing an effective public health response to mitigate the spread of COVID-19, one of the major focuses for the affected countries has been maintaining a steady supply of high-quality and safe food for their citizens despite the social and economic disruptions caused by the pandemic.
The disruptions that have occurred as a result of COVID-19 have affected all parts of the food supply chain, including farms, processors, distributors, retailers, restaurants, and consumers. At the beginning, the stay-at-home orders put in place by local governments resulted in a sudden cessation in the demand for food from restaurants and other food service operations, such as school kitchens. Many farms rely directly and indirectly on demand for fresh produce and meat from food service outlets, and some were initially forced to dump large portions of the food they produced before eventually finding ways to redirect their foods to other food access points. Many of the food processors that depended on the food service customers experienced large losses of manufactured products, with perishable foods being the most affected. Packaging was also an issue as processors had to procure materials to transition from bulk food service–oriented formats to those sized for the at-home consumer. The demand for shelf-stable products, such as canned foods and flour, increased greatly due to consumers shifting to storing and preparing more food at home ( 46 , 55 ).
As countries closed their borders and reduced industry operations to a minimum, the food industry was faced with a challenge of procuring sufficient amounts of high-quality and safe raw materials and other supplies such as packaging and cleaning chemicals. The food industry was also responsible for requisitioning a sufficient supply of face coverings and hand sanitizers to implement the COVID-19 mitigation practices necessary to protect employees. Although local governments and communities eventually helped support face covering and hand sanitizer procurement, the food industry made several changes to production plans to deal with the limited availability of other supplies. These changes included shifting the types of food products made, modifying product formulations, and changing packaging, each change representing its own unique challenge. Some of the changes implemented by the food industry were supported by regulatory changes regarding labeling and end users. For example, the U.S. Food and Drug Administration (FDA) ( 134 ) loosened nutritional labeling information requirements and allowed shelled eggs originally meant for processing to be diverted to retail sales.
In addition to the disruption of the food supply chain, the food industry was also negatively impacted by overall labor shortages. COVID-19 drove labor shortages caused by lengthy isolation of employees due to infection or quarantine because of potential exposure. COVID-19 also impacted the supply of workers in less direct ways. Early in the pandemic, some workers were not showing up to work due to fear of being infected or after an outbreak at their facility. The available pool of workers for the food industry to hire from was also reduced because some individuals that had lost their jobs decided not to look for new work because the temporary federal and state COVID-19 unemployment relief programs provided benefits that were comparable to food industry salaries. Remaining food employees were then required to take on new responsibilities, often adding to their regular responsibilities and working hours. For example, production managers had to operate processing equipment, and quality assurance managers had to develop and implement COVID-19 control plans and mitigation strategies in addition to their food safety and quality programs. As the pool of skilled workers shrank, the food industry was also forced to hire new employees that were less skilled, with no prior food industry experience or no prior training in food safety practices. Training these new employees also represented challenges associated with practices needed to mitigate COVID-19 transmission. For example, shadowing a skilled employee is a common way of training new employees, but the required physical distancing made it difficult for close visual instruction, and face coverings impeded verbal communication in already noisy food processing environments. New employees with limited experience in the food industry were also less likely to commit to staying long term after they have been trained. These labor challenges have further driven the food industry's interest in automation and the use of robotics to reduce the risk of spreading COVID-19 and the reliance on skilled labor ( 17 ).
The aim of this article is to review the practices food companies have implemented to mitigate the risk of COVID-19 transmission for their employees and to discuss the impact of these COVID-19 practices on the food safety mission of these companies. Companies are resource limited by money, time, and personnel, and these unprecedented disruptions and resulting resource demands can challenge the successful operation of even the most well-funded businesses. Although many food companies look forward to a post–COVID-19 return to less strained operations, this pandemic has highlighted the fragility of our global food supply chain. Many experts have tempered their post-COVID optimism, noting that future pandemics of equal or greater severity are possible in our lifetime and perhaps even more likely in our increasingly interconnected world. The food industry must both review the efficacy of the practices they have implemented within their facilities to control COVID-19 and critically assess the potential gaps their implementation has caused in other core business functions to ensure they can successfully deliver consistent, safe, and nutritious foods in the face of future global disruptions.
BACKGROUND ON SARS-CoV-2
Coronaviruses belong to the Coronaviridae family of enveloped, single-stranded, positive-strand RNA viruses that infect numerous animal species, including humans. Human coronaviruses were discovered in the mid-1960s as pathogens associated with cold symptoms, including sore throat, cough, and congestion ( 71 , 129 ). This group of viruses has a characteristic “fringe” of projections (i.e., spike glycoproteins) that are 200 Å long and resemble a solar corona; hence these viruses were named coronavirus ( 3 ). Since their initial discovery, specific coronaviruses associated with respiratory infections have been studied by scientists around the world. Prior to the COVID-19 pandemic, two coronaviruses in particular were associated with large outbreaks of a more serious respiratory infection: severe acute respiratory syndrome coronavirus (SARS-CoV) 1 in 2002 and Middle East respiratory syndrome coronavirus (MERS-CoV) in 2012 ( 113 , 156 ).
The coronavirus structure and genome are important in development of COVID-19 detection assays and vaccines. The coronavirus virion is composed of an internal RNA and protein nucleocapsid surrounded by a host-acquired lipid bilayer envelope with imbedded viral glycoproteins. The coronaviral genome encodes several structural and nonstructural proteins, including proteases and membrane glycoproteins, that are believed to function in virus-host interactions ( 113 , 118 ). The presence of the coronavirus envelope is a critical difference between SARS-CoV-2 and other enteric viruses, such as norovirus and hepatitis A virus, that are likely to be transmitted by contaminated food and water. This envelope is important for host infection but can easily deteriorate, which makes the virus less stable outside of the host, for example, on food and other surfaces ( 92 , 113 ). SARS-CoV-2 is rapidly inactivated outside of the host, with a half-life of 1 to 7 h depending on the surface ( 145 ) and is even more sensitive to acids, heat, detergents, and sanitizers ( 63 ). Although specific data on the stability of SARS-CoV-2 in a frozen state (e.g., at −20°C) is lacking, studies on other coronaviruses have indicated survival for up to 2 years in a frozen state; thus, extended survival of SARS-CoV-2 at temperatures used for storage and transport of frozen foods is possible ( 147 ).
Viruses use fusion proteins to bind to the receptor molecules on host cells. Nonenveloped viruses have fusion proteins on their protein capsids, and enveloped viruses have fusion proteins on their envelopes. This distinction is an important reason why enveloped viruses typically lose their infectivity after disinfection treatment or contact with surfactants because their fusion proteins are lost due to changes in the virus envelopes (e.g., disintegration or conformational changes). Capsids of nonenveloped viruses, like those of norovirus and hepatitis A virus, are more stable outside of the host; therefore, these viruses retain functional fusion proteins and infectivity for longer outside of the host ( 3 , 92 ).
The principal mode by which people are infected with SARS-CoV-2 is through person-to-person contact and exposure to respiratory droplets carrying the virus. Although the virus can be shed in the stool of individuals with COVID-19, which raises the potential of fecal transmission, limited evidence supports this type of transmission in the community. The unlikelihood of fecal transmission is consistent with observed variations in frequency and duration of shedding of replication-competent virus by infected individuals and the complexity of this type of transmission ( 20 , 135 ). Although people can be infected through contact with contaminated fomites, the Centers for Disease Control and Prevention (CDC) ( 34 ) considers this risk to be low. During this pandemic, few reported cases of COVID-19 have been attributed to fomite transmission ( 86 , 97 ), although difficulties associated with confirmation of fomite transmission are exacerbated due to potential asymptomatic individuals ( 9 , 18 , 153 ). In a quantitative microbial risk assessment conducted to better characterize fomite transmission, the risk of SARS-CoV-2 infection via contaminated fomites was estimated as <1 in 10,000 ( 73 , 109 , 144 ). This risk is likely to be significantly lower because in some studies in this assessment exposure risks were estimated from RNA quantification data rather than number of infectious virus particles, and in vitro studies have been criticized for using large amounts of virus that would likely not actually be found on surfaces. This risk assessment issue is compounded by the fact that at this time little is known about the infectious dose of SARS-CoV-2. However, more information is being gathered, including on how the cycle threshold (CT) values from PCR tests for clinical infection are correlated with clinical signs and from in vivo cell culture and animal model studies assessing infectivity of wild-type SARS-CoV-2 and its variants.
STRATEGIES TO CONTROL COVID-19 TRANSMISSION AMONG FOOD INDUSTRY EMPLOYEES
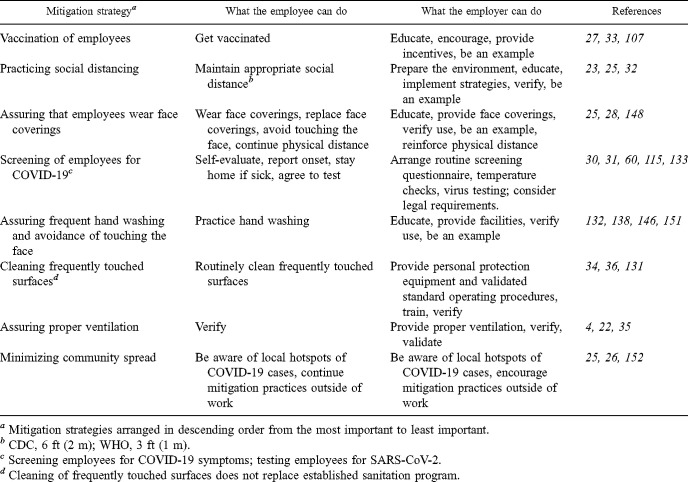
Various COVID-19 control strategies can be implemented in food processing facilities to mitigate potential transmission and assure the safety and health of employees. Organized by priority and effectiveness, these control strategies include (i) vaccinating employees, (ii) practicing social distancing, (iii) assuring employees wear face coverings, (iv) screening of employees for COVID-19, (v) assuring employees practice frequent hand washing and avoid touching their faces, (vi), cleaning frequently touched surfaces, (vii) assuring proper ventilation, and (viii) minimizing community spread (Table 1 ). The hierarchy of these COVID-19 strategies may change as transmission rates change and as new scientific evidence emerges.
TABLE 1.
Priority list of mitigation strategies to control COVID-19 transmission among employees within and outside a food facility
| Mitigation strategya | What the employee can do | What the employer can do | References |
|---|---|---|---|
| Vaccination of employees | Get vaccinated | Educate, encourage, provide incentives, be an example | 27,33,107 |
| Practicing social distancing | Maintain appropriate social distanceb | Prepare the environment, educate, implement strategies, verify, be an example | 23,25,32 |
| Assuring that employees wear face coverings | Wear face coverings, replace face coverings, avoid touching the face, continue physical distance | Educate, provide face coverings, verify use, be an example, reinforce physical distance | 25,28,148 |
| Screening of employees for COVID-19c | Self-evaluate, report onset, stay home if sick, agree to test | Arrange routine screening questionnaire, temperature checks, virus testing; consider legal requirements. | 30,31,60,115,133 |
| Assuring frequent hand washing and avoidance of touching the face | Practice hand washing | Educate, provide facilities, verify use, be an example | 132,138,146,151 |
| Cleaning frequently touched surfacesd | Routinely clean frequently touched surfaces | Provide personal protection equipment and validated standard operating procedures, train, verify | 34,36,131 |
| Assuring proper ventilation | Verify | Provide proper ventilation, verify, validate | 4,22,35 |
| Minimizing community spread | Be aware of local hotspots of COVID-19 cases, continue mitigation practices outside of work | Be aware of local hotspots of COVID-19 cases, encourage mitigation practices outside of work | 25,26,152 |
Mitigation strategies arranged in descending order from the most important to least important.
CDC, 6 ft (2 m); WHO, 3 ft (1 m).
Screening employees for COVID-19 symptoms; testing employees for SARS-CoV-2.
Cleaning of frequently touched surfaces does not replace established sanitation program.
Vaccination of employees
Research on coronaviruses that cause critical illnesses has a long history, even predating the 2002 SARS outbreak. This established body of research on coronaviruses and vaccinology is in part what allowed the rapid development of vaccines in 2020. Researchers currently are pursuing clinical trials of 97 vaccines, according to the New York Times coronavirus vaccine tracker ( 158 ). Similar vaccine development information is available through the Vaccine Centre at the London School of Hygiene & Tropical Medicine ( 121 ). The COVID-19 pandemic has resulted in a remarkable mobilization of resources, skills, and dedication from vaccine researchers ( 108 ). Vaccines train the host's immune system to react and protect the host from a foreign agent (e.g., virus or bacterium) that could cause disease. Although some vaccines utilize whole, attenuated, or inactive virions to elicit an immune response, many of the COVID-19 vaccines initially developed have used only a subunit of SARS-CoV-2 to trigger immunity. Early research indicates that the spike glycoprotein, located on the outer surface of the coronavirus, is a suitable subunit of the virus to be used for vaccine development ( 74 ). The body's immune response includes several mechanisms, one of which is production of T- and B-lymphocytes that will have specific memory of one or several viral subunits, depending on the type of vaccine. This specific memory, which typically takes several weeks to develop, allows the body to identify the virus or cells infected with it in the future and initiate a rapid immune response to stop or slow down the progression of infection and protect the individual from developing the disease. Although some vaccines provide long-term immunity after a single dose, other vaccines require a second dose and sometimes follow-up boosters to maintain the immune system's recognition of the virus. The dosing regimen will be informed by the data from the initial clinical trials on the efficacy and safety of the vaccine. Any changes to the dosing regimen will need to be supported by subsequent clinical trials. The SARS-CoV-2 vaccines in use at this time have undergone extensive review for safety and efficacy before being authorized for early use or emergency use in various countries.
With COVID-19 vaccines becoming increasingly available and their importance in protecting employee health clearly demonstrated, a difficult question emerges: Should food processors require their employees to vaccinate? Although the importance of vaccination to protect individual health and reduce COVID-19 transmission is clear, many businesses are reluctant to require their employees to receive the COVID-19 vaccine. An alternative is to engage employees in ways that encourage and support access to vaccination, with some companies offering incentives and/or time off to get vaccinated. Red Rooster, a U.S. restaurant, offered its employees a vaccination incentive plan that included a monetary bonus plus 16 h of paid time off ( 2 ). Red Rooster reported a successful response to their program, with >85% of their staff vaccinated in a relatively short time. Many larger restaurant and grocery corporations also have been offering at least 2 h of paid time off and financial incentives. To improve vaccine access throughout the early months of 2021, several companies followed guidelines from the CDC ( 27 ) and formed partnerships with local pharmacy chains to hold vaccine clinics for employees. Agencies such as the National Center for Farmworker Health are working with growers to promote migrant health programs, including vaccination sites ( 100 ). Food company and government vaccination policies will likely further evolve following future full approval of vaccines that are currently being used under emergency use authorizations. Company policies will be influenced by the changing political and personal viewpoints on vaccination. Individual employees may have concerns around safety, access, differences between types, and actual need for the COVID-19 vaccine, and many may have concerns around personal freedoms in vaccination requirements. These concerns will evolve as vaccines become more available, and companies will need to continually customize their communication strategies to address reasons for vaccine hesitancy and achieve full vaccination of their workforce.
Practicing social distancing
Social distancing is often used in a broad sense to describe strategies that minimize social interactions between people within a community, for example, closing schools and stopping transit systems ( 47 , 91 , 128 ). The food industry had to adopt social distancing in a stricter sense to continue operations and maintain the food supply. The social distancing practices implemented by the food industry were focused on maintaining the physical distance between individuals occupying the same larger space. Physical distancing prevents transmission by (i) eliminating physical contact with an infected person, (ii) assuring that a healthy individual is exposed to a reduced number of large respiratory droplets generated by an infected person, and by (iii) reducing contact with surfaces that an infected person has recently contaminated through generated respiratory droplets and physical contact. Large respiratory droplets can carry sufficient amounts of active virus to cause an infection; however, due to their size, these droplets drop to the floor relatively quickly and do not travel far from the source ( 7 , 141 ). Recommendations regarding the safe physical distance between individuals currently lacks consensus across countries and agencies. These differences in recommendations are influenced by (i) how available scientific data are interpreted, (ii) how the risk and level of acceptable risk are determined, and (iii) how the level of acceptable uncertainty is determined. For example, the CDC guidelines recommend 2 m (6 ft) of distance, whereas World Health Organization (WHO) guidelines recommend only 1 m (3 ft) ( 23 , 150 ). Time spent within the range of respiratory droplets is an equally important factor for transmission of COVID-19, which is why close contact (i.e., contact likely to result in infection) is defined by both time with and distance from an infected person. As with distance, full consensus has not been reached on minimum time, but most recommendations mention a cumulative time of 10 or 15 min within a 24-h period ( 23 , 60 , 102 , 150 ). Other important factors that influence increased risk of transmission during close interaction are activities and events that increase or decrease the generation of respiratory droplets, for example, talking, coughing, and use of face coverings ( 6 , 56 , 83 ). Scientific data currently are limited on the infectious dose for SARS-CoV-2; however, even the high infectious dose predictions, with the number of virus particles needed to produce infection in 50% of exposed individuals estimated at 280 to 790, suggest that transmission is possible during common exposure to respiratory droplets generated during a single sneeze or face-to-face conversation ( 117 ). Stadnytskyi et al. ( 124 ) estimated that as many as 100,000 viral particles can be emitted during 1 min of talking, and Schijven et al. ( 116 ) estimated that a single sneeze can emit up to 3,200,000 viral particles.
Data on the effectiveness of maintaining physical distance (e.g., 1 to 2 m) for reducing COVID-19 transmission are limited. In a meta-analysis of 44 relevant comparative studies in health care and non–health care settings to determine the effectiveness of different mitigation strategies for preventing transmission of SARS-CoV-2, SARS-CoV-1, or MERS-CoV ( 44 ), the authors concluded that transmission of these respiratory viruses was lower at a physical distance of ≥1 m than at a distance of <1 m, with an odds ratio of 0.18 (95% confidence interval, 0.09 to 0.38). They also concluded that overall protection increased as distance increased, with an approximately twofold increase in protection per added 1 m (for a range of 0 to 2 m). These results are consistent with other reports on mitigating COVID-19 spread in health care settings. In a study from Singapore General Hospital, where for >1,500 COVID-19 patients were cared for, improved physical distancing was one of the key components for mitigating COVID-19 spread, even among COVID-19 cases detected outside isolation ( 140 ). In a separate report, the same researchers highlighted a case in which a COVID-19 patient that had a delayed positive diagnosis and was cared for in the general ward of the hospital did not transmit COVID-19 to 26 others within the same area. The mitigation strategies followed by this COVID-19 patient were physical distancing of 1 m and wearing a surgical mask ( 139 ). These reports indicate that physical distancing of 2 m, as recommended by the CDC and other public health agencies, includes a substantial margin of safety and is, together with wearing a proper face covering, an effective mitigation strategy when complete social separation is not possible, for example, in a food processing environment.
The food processing environment needs to be properly configured so that physical distancing can be successfully implemented and maintained. This process involves three steps: (i) reducing to a minimum the number of individuals present in the facility at any given moment, including employees, visitors, contractors, and inspectors; (ii) preparing the physical environment itself; and (iii) establishing temporal practices that support spatial distancing. Reducing employee density and maintaining production volumes may seem to be diametrically opposed goals, but opportunities exist for food companies willing to challenge traditionally defined roles. One food processor rethought their equipment operator roles and trained these operators to troubleshoot their equipment and resolve minor issues without the need for maintenance staff, thus reducing the number of employees needed in a space and potentially reducing the production time lost to simple problems ( 53 ). Preparing the physical environment can involve finding or repurposing new areas where activities can be performed, organizing spaces to allow one-way foot traffic and prevent crowding, and introducing physical barriers where physical distancing is hard to achieve. Other examples of preparing the physical environment in a food facility are setting up outdoor tents to increase the space where employees can have their lunch breaks, increasing the number of restrooms to reduce the number of people per restroom and prevent crowding, and physical separation of work stations on the line with strip curtains, plexiglass, or similar materials ( 31 ). Managing time is another important strategy for supporting social distancing. Companies could stagger employee arrival times to prevent crowding at shift changes or increase the number of available breaks throughout the day to decrease the number of employees at any given break time. Clear communication and demonstration of spatial and temporal strategies for promoting physical distancing is important to encourage employee adherence to these practices. Physical distancing is an important mitigation strategy implemented by many businesses in the food industry and when combined with the practices subsequently discussed can help create an effective COVID-19 control program.
Assuring that employees wear face coverings
The use of face coverings was initially recommended as a mitigation strategy to complement physical distancing because in certain circumstances and environments, such as the dynamic environment within a food processing facility, physical distancing cannot be maintained consistently. In some instances, physical distancing is consciously not followed by employees, such as during interactions between individuals who may think they are uninfected because they show no clear symptoms even though viral spread has been documented by presymptomatic, paucisymptomatic, and asymptomatic individuals ( 16 ). To address the risk associated with reduced physical distance and a significant number of infected people without observable symptoms spreading COVID-19, the majority of world public health agencies have been recommending the use of face coverings as an important control strategy ( 8 , 28 , 148 ).
Several types of face coverings have been recommended for use, ranging from simple cloth face coverings modified from household items to respirator masks (e.g., N95, KN95, and FFP2). All face coverings have primarily been recommended as source control to prevent transmission of the virus through respiratory droplets from the person wearing the face covering to others. Although different face coverings are somewhat effective for protecting the wearer from respiratory droplets, respirator masks are the only face covings specifically designed for this purpose (e.g., N95 respirators approved in the United States). The use of respirator masks has been recommended for only high-risk environments such as hospitals due to their limited availability, user training needed, and custom fit and thus have not been recommended for use in the food industry. To improve protection of the wearer when using a cloth face covering or a disposable mask, the CDC published recommendations on how to achieve a better fit to a person's face, including advice on double masking and using mask fitters or knotting the ear loops ( 29 , 114 ). Early in the pandemic some food companies opted for face shields as an option because they are easier to wear than face coverings; however, the CDC has advised against the use of these shields, noting that although they are considered good for eye protection they are not designed to be used as source control ( 28 , 93 ). Despite the wide variety of approved face coverings, food companies should feel confident that whichever type they are able to implement, all act as effective source controls that minimize the risk of spreading COVID-19 through large respiratory droplets.
Studies in the laboratory and clinical environment have validated the effectiveness of face coverings for reducing the transmission of COVID-19. Howard et al. ( 75 ) reviewed some of these studies and several population-level studies from various countries and regions (e.g., U.S. states). The authors concluded that although true randomized control trials for investigating the effectiveness of various face coverings have not been conducted for logistical and ethnical reasons, results of observational trials offer some direct evidence of the effectiveness of face coverings. In one cohort study on secondary transmission of SARS-CoV-2 within households, face masks were 79% effective ( 137 ). Population-level studies provide more data, and results suggest that the use of face coverings has a substantial impact on reducing the spread of COVID-19 in the population in agreement with studies in which predictive modeling has been used to test effectiveness of various control strategies ( 75 ). In one study in which the number of COVID-19 cases in the mask-wearing setting inside a workplace environment was compared with the number of cases in the no-mask setting outside the workplace environment, a significant difference (P < 0.05) in the number of cases was found between the two settings ( 39 ). These and other studies highlight the importance of wearing face coverings in work environments such as food processing facilities and the risk that the community environment represents. An important factor in the effectiveness of face coverings as a control strategy is widespread compliance with wearing these coverings in the workplace and the community. Use of face coverings should be communicated as one part of a larger, multipronged strategy to prevent the transmission of COVID-19 within a food processing facility.
Screening employees for COVID-19
People are the main reservoir of viable SARS-CoV-2 and are most likely the only route through which the virus can be introduced into the working environment. Thus, screening people entering the work environment is the first line of defense for preventing introduction and spread of the virus in the working environment. Two general types of screening can be used based on the situation: questionnaires and testing. These screening options may be implemented individually but are more effective when used in combination.
Questionnaire-based screening consists of a set of questions asked daily to anyone entering the facility to determine (i) the presence of any COVID-19–related symptoms, (ii) recent travel to high-risk areas, and (iii) potential exposure to individuals positive for COVID-19. This type of screening relies on an employee's ability to self-assess for COVID-19 symptoms and willingness to self-report the necessary information. Thus, sufficient information, such as a list of symptoms, clear definitions of high-risk travel, and what constitutes a reportable exposure, must be provided so that individuals can accurately fill out the questionnaire. Employee education, training, overall company relationship with the employees, and financial consequences are all factors in the success of a questionnaire-based screening program. For example, a reliable and rapid sick leave policy will contribute to an overall positive relationship with employees by eliminating any negative financial consequences should the employee be required to leave work because of truthfully answering the questions. A common addition to the symptoms screening questions is the direct measurement of each individual's body temperature before they enter the food facility, preferably using a contactless infrared thermometer. Although measuring body temperature has its limitations, such as dependence on ambient temperature and potential use of antipyretic medication ( 11 , 55 , 115 ), this quick and cost-effective approach can complement the overall employee screening program ( 19 , 38 ).
Some food facilities and institutions have implemented SARS-CoV-2 testing to identify individuals infected with the virus and remove the individual from the environment until they no longer represent a risk of spreading the virus. These testing programs are typically implemented in addition to rather than in place of the questionnaire screening. Two general types of virus tests are used to detect a current infection with SARS-CoV-2: nucleic acid amplification tests and antigen tests. Antibody or serological tests are used to determine previous infection with SARS-CoV-2 and are not recommended for screening for individuals who are currently infected and thus can spread COVID-19 ( 30 ). Testing for the current infection has three stages: (i) collect a sample from the tested person (e.g., nasal swab or saliva), (ii) prepare the sample for testing, and (iii) perform the assay on the sample. Some virus tests are appropriate for use at the point of care, whereas others, specifically nucleic acid amplification tests, might require trained individuals, specialized equipment, and materials ( 30 ). These potential requirements and associated costs might limit the ability to use testing for routine daily screening. However, maintaining at least a weekly test-based screening program is still recommended to validate the questionnaire-based screening program and potentially identify any asymptomatic or presymptomatic employees. Frequency of testing should be determined by individual facilities based on factors such as community spread, cases in the facility, employee travel, and risk status of employees and their family members. For example, when an employee has had close contact with an infected person or lives in the same household with a medical worker that works directly with COVID-19 patients, this employee may constitute a higher risk and thus might be monitored through more frequent testing ( 30 ). Employee testing is also subject to regulatory and reporting requirements, including assuring confidentiality, preventing discrimination, and establishing proper record retention protocols ( 11 , 101 , 130 ).
After a questionnaire-based or test-based screening identifies a COVID-19–positive or potentially positive employee, the employer should follow up with contact tracing to identify any potentially exposed employees. Results of contact tracing can be used to perform additional employee testing or initiate quarantine of employees to prevent secondary transmissions of COVID-19 in the workplace ( 11 ). Depending on the circumstances, contact tracing can be performed by the local health department either in parallel or in collaboration with the employer. When the number of new daily cases in the community is high, support from the local health department might be delayed or absent, which is why all food facilities should establish appropriate protocols as part of their COVID-19 control program to be able to perform contact tracing independently when a COVID-19 case is identified among employees.
Currently, no studies have been conducted to specifically evaluate the effectiveness of questionnaire-based and/or test-based screening to mitigate COVID-19 spread within a food production or processing facility. In some studies, computer modeling has been used to evaluate the effectiveness of different types of screening in other community settings. In one study of screening performance standards that would permit the safe return of students to U.S. residential college campuses, questionnaire-based screening was not sufficient as a stand-alone strategy to contain an outbreak under any of the tested scenarios ( 106 ). Although in the same study, test-based screening was identified as a possible cost-effective strategy for limiting the spread of infections, the success of testing depended heavily on strict adherence to other mitigation strategies (e.g., hand washing, indoor masking, and limiting close interactions). The researchers also found that test frequency was more strongly associated with reducing spread of COVID-19 than was test sensitivity. The need for various screening procedures as part of a larger COVID-19 control program rather than as stand-alone strategies is also apparent from some of the studies in which mathematical modeling was used to evaluate travel-related spread of COVID-19 ( 40 , 64 , 65 ). The researchers also recognized the dependence of questionnaire-based screening on people's willingness to report the necessary information, which highlights the importance of creating a work environment where self-reporting is enabled and encouraged. Food companies must critically assess the robustness of any screening model they choose and emphasize to employees that these programs are not 100% effective and adherence to other mitigation practices is necessary to reduce the risk of contracting COVID-19.
Assuring that employees practice frequent hand washing and avoid touching their faces
Hand washing is well established as a key intervention that helps prevent the transmission of respiratory viruses through contact and reduces microbial contamination ( 72 , 80 , 146 , 151 ). SARS-CoV-2 is primarily transmitted from person to person through respiratory droplets, when infectious viral particles come into contact with another person's nose, mouth, or eyes. Although the CDC ( 34 ) has noted that transmission through contact with contaminated surfaces is considered to be lower risk, contact with a recently contaminated surface or direct contact with an infected person (e.g., hand shaking) are sufficiently high risk to require appropriate control strategies to prevent transmission ( 54 ). The two basic control strategies are frequent hand washing and avoiding touching the face. These two strategies are effective for limiting the transfer of active viral particles at several steps along the transfer chain from an infected to a susceptible person. A person should frequently wash hands for at least 20 s with soap and water, especially before preparing and eating food or before and after touching the face ( 24 ). Although hand washing is a more effective control strategy, hand sanitizers with at least 60% alcohol also can be used when soap and water are not available ( 24 , 85 , 122 , 146 ). Hand sanitizers are substantially less effective on soiled hands and should be used in combination with hand washing when hands are visibly soiled ( 62 ).
Food industry workers should be familiar with hand washing procedures as part of employee health and hygiene regulatory requirements as part of the current good manufacturing practices (CGMPs) listed in the FDA's Food Safety Modernization Act (FSMA) Preventive Controls for Human Food (PCHF) rule ( 132 ). Although proper hand washing and CGMPs are targeted to control potential routes of transmission of foodborne viruses and bacterial pathogens, the same principles will also control the minimal, nonfood safety risk presented by potential routes of transmission of SARS-CoV-2 within a food facility. In a food facility, workers should wash their hands in dedicated hand washing stations before starting work, whenever they leave and return to their work station, and any time their hands become dirty or contaminated. Application of hand sanitizer cannot take the place of hand washing in a food facility but can be used as part of the hand hygiene protocol. Gloves are also not a substitute for hand washing. If used, gloves must be maintained to remain intact and in sanitary condition ( 132 ).
Food facilities and farms operating during the COVID-19 pandemic need to communicate and train their employees on updated policies regarding proper hand washing protocols, including frequency. Protocols also should include measures instituted to ensure hand washing facilities provide enough space for employees to maintain physical distance ( 25 , 133 ). Additional hand washing stations may be needed to ensure that employees and farm workers are able to meet updated requirements for the frequency of hand washing. Food facilities also may consider installing touchless devices at hand washing stations (e.g., water faucets, soap and hand sanitizer dispensers, and trash receptacles) ( 21 ).
Cleaning of frequently touched surfaces
Many surfaces in food facilities and on farms, such as door handles, light switches, shared tools, and equipment, are frequently touched by multiple people throughout the day. These frequently touched surfaces have some potential for rapid transfer of active viral particles between infected and susceptible people ( 13 , 45 , 63 , 109 , 144 ). The CDC ( 34 ) recommends at least daily cleaning of these frequently touched surfaces with soap or detergent, because the lipid envelope of SARS-CoV-2 is readily disrupted by these cleaning agents. In response to high-risk events, such as when an infected person is known to have occupied a room for an extended period of time, the CDC recommends a more thorough cleaning procedures with an additional disinfection step. The U.S. Environmental Protection Agency ( 131 ) has developed a list of disinfectants that are active against SARS-CoV-2 for use on frequently touched surfaces, and several of these agents have now been directly tested against SARS-CoV-2 ( 42 ). Some frequently touched surfaces have a higher risk of transmission because of the way they are used close to the mouth, eyes, and nose. These surfaces and devices such as phones, headsets, microphones, and shared personal protective equipment require additional procedures for use and proper cleaning.
Food manufacturing facilities already have established sanitation procedures as required in the CGMPs of the FSMA PCHF rule ( 132 ). In registered food facilities, buildings, fixtures, utensils, and equipment require general maintenance to keep them in a clean and sanitary. Sanitation requirements also exist for all food contact and nonfood contact surfaces for wet processing and for processing, packing, and holding of low-moisture foods. Cleaning of frequently touched surfaces as a mitigation practice to prevent the spread of COVID-19 is not a replacement for these established sanitation procedures.
Currently, no evidence has been found for COVID-19 transmission through food, so SARS-CoV-2 is not considered a biological hazard that would need to be assessed through a facility's environmental monitoring program. Preexisting sanitation programs are effective for inactivating the already highly unstable SARS-CoV-2, which makes persistence of this virus in the environment a highly unlikely scenario and any environmental monitoring unnecessary. Resources should be prioritized for measures such as physical distancing, wearing face coverings, hand washing, and employee screening that will address the main risks in the food facility (e.g., person-to-person transmission).
Assuring proper ventilation
The main routes of SARS-CoV-2 transmission are via person-to-person spread through close contact or respiratory droplets of >5 to 10 μm in diameter released by infected individuals when they talk, laugh, cough, sneeze, or sing. Much discussion has centered on the role of aerosolized droplets (those with a diameter of <5 μm) and their ability to spread infectious virions across larger distances and times and the need for ventilation systems to mitigate this potential route of transmission ( 5 , 98 ). Modeling studies have been conducted on various factors such as droplet size, viral shedding rate, air flow, room dimensions, and types of ventilation systems to predict and highlight the potential risk of aerosolized transmission and infection in indoor environments ( 49 , 89 , 123 , 125 ). In the real world, unambiguous evidence of aerosolized transmission is difficult to obtain, but in a number of outbreak studies poor ventilation and air flow were implicated as contributing factors in SARS-CoV-2 transmission in a wide range of environments, including food processing facilities ( 69 ), restaurants ( 96 ), apartment buildings ( 77 ), nursing homes ( 52 ), and hospitals ( 43 ). Environments such as hospitals, where infected individuals shedding high amounts of virus undergo procedures that would promote aerosolization, are a greater risk for such transmission.
No studies have yet documented a reduction in SARS-CoV-2 via air ventilation and purification systems ( 155 ), but such systems can be used to prevent airborne transmission of other viral disease ( 51 ). Organizations such as the CDC and the American Society of Heating, Refrigerating and Air-Conditioning Engineers (ASHRAE) have published recommendations for ventilation. At the most basic level, the CDC ( 35 ) recommends allowing intake of outdoor air and increased directional airflow for indoor environments. In many food processing facilities, this airflow will require a special heating, ventilation, and air-conditioning system with proper filtration that meets hygienic food safety standards. The ASHRAE ( 4 ) recommends using MERV-13 filters for outdoor air and better filters when using recirculated air. The CDC also mentions the use of UV germicidal irradiation systems as supplemental treatments that can further bring down airborne viral loads ( 12 , 126 ). The appropriateness and cost of ventilation modification must be carefully evaluated by each food facility, with the awareness that COVID-19 transmission primarily occurs over short distances that would not be impacted by these systems ( 56 ). Thus, ventilation systems must be part of a food facility's multilayered approach to COVID-19 mitigation rather than a stand-alone strategy.
Minimizing community spread
Controlling COVID-19 within a food facility is enough of a challenging task for businesses; control beyond those walls might appear to be out of reach by many. However, food facilities and their employees are essential components of communities and can have an important influence on the community spread of COVID-19 through their practices. The influence also goes in the other direction; increase in the community spread is likely to increase the risk of spread within the food facility. Thus, because community spread is the main way the virus is transmitted ( 48 , 58 , 152 ), food businesses must evaluate how their practices can contribute to minimizing community spread.
Food businesses should recognize that COVID-19 mitigation strategies implemented within the facility and the COVID-19 education businesses provide to their employees will have an impact in the greater community. Internal COVID-19 control practices such as social distancing, mask wearing, and frequent hand washing will be the same mitigation practices that employees will need to follow outside of the facility to prevent the spread of COVID-19 ( 58 , 152 ). By educating their employees on COVID-19 and why specific practices are effective for controlling the spread, companies will create COVID-19–cognizant individuals who will then share their COVID-19 knowledge with and model COVID-19 control practices for family and friends in the greater community. This chain of education will help spread safe COVID-19 practices within the community while aiding in reducing community spread of COVID-19.
Food industry employees are essential workers, maintaining the country's food supply, and must travel to and from the workplace while many other workers in the community remain at home. Both public and private transportation have been linked to the community spread of COVID-19 due to the enclosed spatially confined conditions of transportation vehicles that promote person-to-person transmission and the role of travel as a route that can introduce individuals with COVID-19 into communities that were previously free of the virus ( 76 , 119 , 120 , 157 ). Food companies should review how their employees travel to a facility (e.g., personal vehicle, carpool, or public transportation) or between multiple facilities if relevant and then evaluate ways to modify employee travel arrangements to reduce the risk of these employees being nodes of COVID-19 community spread. Some examples of modifying employee travel could include paying for employee car services, providing company vehicles to individual employees, or instituting a policy of no multifamily carpools. The methods chosen to change employee travel will depend on the location of and resources available to a given food facility.
The housing situations of food workers will vary widely by the specific food industry and business location, but companies should be aware that shared housing situations can increase the likelihood of COVID-19 transmission ( 1 , 41 , 70 , 127 ). At a minimum, food companies should review the shared housing situations of their employees. Companies should consider grouping individuals who live in the same dwelling into cohorts and minimizing the contact between cohorts to reduce or mitigate the potential spread of COVID-19. In some cases, food businesses may support employees who choose to move into housing situations that reduce dwelling density and reduce the frequency that employees will come into close contact with nonfamily or work-related individuals who might be sources of COVID-19. How companies address this community risk will depend on their resources and the community housing situations.
MANAGEMENT OF FOOD OPERATION
Developing a COVID-19 control plan
A risk-based methodology, founded on scientific knowledge, is critical to effectively control emerging hazards such as COVID-19 and reduce the business risk. For example, presence of a proper control plan can reduce legal liability by demonstrating that appropriate practices to prevent COVID-19 and protect employees within the facility have been instituted. A well-established plan may also help a company refute the rare case in which a customer or importing country finds a food product that is positive for SARS-CoV-2 RNA. Fortunately for the food industry, such risk-based approaches are commonplace with the widespread adoption of other risked-based programs such as hazard analysis and critical control point systems and PCHF ( 10 , 67 ). The principles of these methodologies can be readily applied to development of a COVID-19 safety plan. As in a food safety plan, individuals responsible for plan development must think through and identify (i) the hazard (SARS-CoV-2), (ii) reasonably foreseeable sources of the hazard (e.g., employees, visitors, and commonly touched surfaces), (iii) unlikely sources of the hazard (e.g., food and packaging), and (iv) effective control measures (e.g., social distancing, barriers, mask wearing, and screening) to prevent the spread of COVID-19 within the facility. When possible, critical limits should be defined for identified controls such as a minimum social distance of 2 m, forehead surface temperature <100.4°F (<38°C), and a face covering worn appropriately at all times. Corrective actions (e.g., contact tracing, cleaning, and quarantine protocols) to be taken when individuals who interact with the facility test positive for COVID-19 should be documented. As with food safety plans, methods for monitoring these controls, such as thermometers for body temperatures or defined time intervals for visual checks of face coverings, will also be necessary to ensure that safety guidelines are followed and documented. Development and implementation of COVID-19 safety plans should be accomplished by a multidisciplinary team of food safety and quality managers and representatives from various business functions, including human resources, maintenance, and the front office. Appropriate training of all employees on COVID-19 and the safety plan is critical for success. Although the details of a COVID-19 safety plan will be specific to an individual food facility and thus cannot be readily copied by other facilities, several guidance documents from the CDC ( 21 ) and the FDA ( 133 ) and templates by state agencies are available to aid food facilities in the development and implementation of effective COVID-19 safety plans ( 103 , 138 ).
Verifying COVID-19 control strategies
As with food safety plans, COVID-19 safety plans must be implemented as intended. Verification activities will differ depending on the controls implemented and resources available to each business. For example, a weekly review of the production floor mask wearing observation logs by a designated person would be sufficient to ensure that observations were being made and no gaps in mask usage were noted. In businesses with sufficient resources, semiroutine surveillance testing of employees for COVID-19 could be a way to verify that the daily screening of employees was being effectively implemented. As with monitoring, these verification activities should be documented and reviewed with appropriate frequency. Multiple positive COVID-19 tests should be a warning to the business that the current COVID-19 safety plan is insufficient or is not being implemented correctly, and the business should review its COVID-19 plan and records.
Building a COVID-19 control culture
Over the last decade, the concept of a food safety culture has generated much discussion ( 154 ). The organizational and behavioral concepts of a successful food safety culture can be readily applied to effectively manage COVID-19 within a food facility. A company's culture is the knowledge, attitudes, and behaviors shared by managers and employees ( 110 ), and a COVID-19 control culture would encompass practices related to mitigating the spread of SARS-CoV-2. A weak food safety culture has been considered a factor that leads to an increased food safety risk for a given food facility ( 66 , 105 ). A weak company COVID-19 control culture will likely increase the risk of a COVID-19 outbreak within a food facility. With an emerging risk such as COVID-19, where there are many unknowns and thus potential changes in scientific guidance as more is learned by public health experts, many managers might find it difficult to develop and implement a robust COVID-19 control plan. Thus, managers must focus on clear, transparent communications with employees that define the risk of COVID-19, explain the uncertainties, and establish the need for and methods of a unified, company-wide approach. Concise and frequent trainings are needed to keep employees within the company informed of changes in knowledge without overwhelming them. The practices and behaviors outlined in the trainings must be consistently modeled by management both within the facility and in the broader community to reenforce and promote behavioral changes across the organization. Metrics that can be tracked and rewarded company wide, similar to what some food facilities do for occupational safety and numbers of days since the last incident, may be useful for creating unified commitment to the COVID-19 control culture. The maturation of an organization's culture occurs in several stages ( 81 ), but as of the time of this writing companies have only had a short time to develop their COVID-19 control cultures. It will be interesting to see how these cultures have matured under the intense pressure of the pandemic, how previously established food safety cultures within a facility influenced the adoption of successful COVID-19 control cultures, and how well the development of a COVID-19 control culture improves the resiliency of food companies to future pandemics.
EVIDENCE FOR NEGLIGIBLE RISK OF COVID-19 TRANSMISSION THROUGH FOOD AND FOOD PACKAGING
Current international consensus is that the risk of COVID-19 transmission through food and food packaging is extremely low ( 61 , 87 , 145 , 149 ). This consensus includes frozen food and is based on strong epidemiological evidence that shows the pattern of spread of COVID-19 to be consistent with community transmission observed for other respiratory viruses (e.g., flu and common cold viruses) ( 104 ). No direct scientific evidence of nonfrozen food, frozen food, or food packaging as a source of SARS-CoV-2 transmission has been presented ( 79 , 145 ) despite >180 million human SARS-CoV-2 infections as of July 2021 ( 82 ).
Epidemiological evidence indicating that nonfrozen food, frozen food, and food packaging are not a likely source of SARS-CoV-2 is also consistent with other observations, including low stability of SARS-CoV-2 outside of the human host ( 92 ) and low likelihood (in virus surrogate studies) of virus transfer from contaminated surfaces to specific receptors in the nose, mouth, and eyes of a susceptible person. The virus must go through several transfer steps from an infected person's face, to their hands, to the food or food packaging, and then to a susceptible person's hands and then face. Because the transfer rate of the virus in each step is relatively low ( 84 , 95 ), the number of viral particles reaching the susceptible person's face is most likely too low to cause an infection. The number of viral particles reaching the susceptible person's face is even lower because of the fragile structure of SARS-CoV-2. Unlike true foodborne viruses (e.g., norovirus and hepatitis A virus) that have a protective protein capsid that makes them highly stable outside of the host ( 92 , 111 ), the lipid envelope of SARS-CoV-2 makes it highly unstable outside of the host ( 42 , 136 ), and the virus is inactivated quickly on the surface of foods and food packaging. Low stability of SARS-CoV-2 has been reported on various porous and nonporous surfaces ( 37 , 94 , 112 , 136 ). Although no studies have been published specifically on the stability on nonfrozen or frozen food, one study with a surrogate coronavirus (e.g., coronavirus 229E) revealed low stability on fresh produce ( 14 ). The authors found a short half-life for this surrogate similar to that previously reported for nonporous surfaces: 2.05, 2.30, and 9.05 h on tomatoes, apples, and cucumbers, respectively. For comparison, Escudero et al. ( 59 ) estimated the half-life of norovirus on lettuce at almost 3 days, whereas in another study in oysters the half-life was 18 days ( 90 ). Based on these observations, the overall chain of events that would have to occur for transmission of SARS-CoV-2 is highly unlikely. Transmission would require (i) contamination of food and food packaging with high levels of SARS-CoV-2 (essentially a person sneezing on a product within a short time frame before customer contact), (ii) contact between food and a person (typically manual contact) soon after a high-level contamination event, (iii) transfer of virus from surface to hand during this contact, and (iv) almost immediate subsequent contact between the hands and a surface that allows for infection (e.g., mucous membranes of the nose). Although survival of SARS-CoV-2 is expected to be longer at temperatures used for storage and transport of frozen foods (e.g., −20°C) and the virus may remain viable longer in fish under refrigeration conditions ( 50 ), the risk of transmission from frozen foods is still considered negligible because the chain of events outlined above is still highly unlikely.
Overall, both the available scientific and epidemiological evidence support the assumption that the risk of COVID-19 transmission through food and food packaging is very low and does not need active management beyond good food safety practices, regular hand washing when handling food. COVID-19 mitigation strategies should continue to focus on the main risks associated with person-to-person contact and direct transmission through respiratory droplets.
IMPACT OF COVID-19 ON FOOD SAFETY
Although SARS-CoV-2 is not a direct food safety hazard, the COVID-19 pandemic had and likely will continue to have an impact on food safety. Although most information on the impact of the COVID-19 pandemic on food safety is anecdotal, the impact can be grouped into several categories: (i) supply chain disruptions, (ii) disruptions of food safety practices in processing facilities and other food-associated establishments, (iii) disruption of private and government food safety audits, inspections, and surveillance systems, and (iv) changes in consumer food safety practices. Food safety consequences of global supply chain disruptions can be due to (i) the need to source from new suppliers, including the fact that travel restrictions may impact the ability to appropriately audit and approve new suppliers, (ii) disruptions forcing suppliers shipping from different nonapproved facilities, and (iii) food fraud due to shortages of certain products (e.g., replacement or cutting of spices with cheaper and more easily sourced ingredients) ( 142 ). Disruptions of practices in processing facilities and other food-associated establishments that may lead to food safety issues include (i) reductions in sanitation crews (e.g., because staff have been reassigned to COVID-19–related tasks), (ii) the need to hire new staff without appropriate food safety training (e.g., because in-person training has been suspended), and (iii) loss of food safety staff (e.g., due to the stress of additional COVID-19–related tasks). Although the importance of audits and inspections for assuring food safety may be controversial, suspension of (in-person) audits and inspections has reduced compliance with food safety practices, at least in some establishments ( 143 ). Reassignment of public health staff and possibly of laboratory space due to COVID-19–associated activities could lead to reduced surveillance capacity, which may have resulted in reduced detection of foodborne disease outbreaks and reduced case reporting ( 15 ). However, the quantification of impacts of possible reduced foodborne disease surveillance is challenging because other changes to the food system and food consumption patterns during COVID-19 may have impacted foodborne disease frequency ( 15 ). Changing food practices may also increase consumer risk of foodborne disease. Although not a microbial food safety issue, an increased use of sanitizers for cleaning foods and food packages may have been prompted by overreactions to unfounded concerns about foodborne transmission of COVID-19, which led to an increase in intoxication reports at U.S. poison control centers ( 36 ). In summary, the COVID-19 pandemic likely has had considerable impacts on food safety, many of which may never be documented or may not be documented until later.
LONG-TERM IMPACT OF THE COVID-19 PANDEMIC ON FOOD SAFETY AND QUALITY SYSTEMS
In addition to the actual food safety impacts during the COVID-19 pandemic (as detailed in the previous section), this pandemic also may have long-term impacts on food safety and quality due to (i) changes in food production and processing practices, (ii) changes in behaviors and attitudes toward food safety and food safety practices, and (iii) long-term changes in regulatory approaches and policies. These COVID-19 impacts likely will be both negative and positive. Changes in food production and processing that will impact food safety and quality may include improved cleaning of frequently touched surfaces, which could reduce transmission of some foodborne diseases, such as norovirus infections ( 99 ). In a recent study with data from nine U.S. states between July 2012 and July 2020, a significant decline in norovirus infection outbreaks began in spring 2020, and this decline probably was not due to seasonality or underreporting of cases ( 88 ). A reduction in 2020 norovirus positivity rates compared with those in 2019 was found in Philadelphia, suggesting that COVID-19 mitigation practices may have had a role in this reduction ( 99 ). However, a reallocation of resources to cleaning frequently touched surfaces may reduce the intensity and thoroughness of cleaning practices for other critical areas in the food processing environment and thus result in greater food safety risks. No published data currently suggest that such incidents have occurred often or that they have resulted in foodborne outbreaks. Relevant changes in behaviors and attitudes may include increased wearing of face masks, which also could reduce food safety risks associated with human carriage and shedding of foodborne pathogens. The COVID-19 pandemic, at least in some cases, may lead to increased availability of paid sick leave and an improved appreciation of hidden factors that may increase the risk of sick food workers reporting to work. A continuing emphasis on assuring that sick workers do not report to work could considerably reduce the risk of transmission of some foodborne diseases, probably most noteworthily norovirus infection and hepatitis A. This pandemic may increase willingness to use vaccinations (e.g., against hepatitis A virus) to improve our ability to prevent foodborne disease transmission and may lead to or accelerate long-term changes in regulatory approaches and policies. Remote audits and inspections may continue to be utilized, at least to some degree ( 68 ), although the impact of this shift on food safety is hard to predict. The use of remote audits and inspections could facilitate increased frequency of these activities, which may positively impact food safety. However, remote audits and inspections may be less effective than in-person audits and inspections, which could negatively affect food safety. The COVID-19 pandemic may have increased our appreciation for the importance of balancing benefits and risks (including economic and business risks) in risk management strategies that address public health issues and could accelerate efforts to implement risk-based food safety approaches rather than a “zero risk” approach ( 159 ). In the long term, the development of an improved public health infrastructure as a consequence of the pandemic will increase our overall ability to detect foodborne disease outbreaks and to translate these findings into improved prevention strategies. Overall, development and implementation of appropriate surveillance systems that can be used to assess and quantify impacts of COVID-19–related changes in food safety and public health practices will allow accurate assessment of positive and negative impacts of changes to our food safety system as a consequence of this pandemic.
CONCLUSIONS
Although the risk of foodborne transmission of COVID-19 is negligible, this pandemic has had a considerable impact on the food industry and most likely on the industry's food safety practices. However, clear quantitative data on the actual impact of this pandemic on food safety are still extremely limited. Some mitigation practices emphasized by the pandemic, such as hand washing and cleaning of surfaces, reinforce industry standard food safety practices, and other practices, such as face coverings and social distancing, may also reduce the transmission of foodborne disease agents. The COVID-19 pandemic is not over and will not be the last pandemic to impact the food industry. Future quantitative assessments of the food safety impacts of the pandemic and internal reviews by food companies of the performance of their food safety systems are essential to minimize long-term negative consequences of this and future pandemics on food safety.
ACKNOWLEDGMENTS
This work was supported by U.S. Department of Agriculture (USDA), National Institute of Food and Agriculture, Agriculture and Food Research Initiative (grant 2020-68006-32875, access 1024254). Any opinions, findings, conclusions, or recommendations expressed in this article are those of the authors and do not necessarily reflect the views of the USDA. This work was also made possible by the generous support of the American people through the U.S. Agency for International Development (USAID) under cooperative agreement 7200AA19LE00003. The contents are the responsibility of the authors and do not necessarily reflect the views of USAID or the U.S. Government. The authors thank all members of Cornell's Institute for Food Safety and all students, faculty, staff, and outside collaborators who contributed to the work of Cornell's Food Industry COVID-19 Emergency Task Force.
REFERENCES
- 1.Accorsi, E.K.,, Samples J.,, McCauley L.A.,, Shadbeh N. Sleeping within six feet: challenging Oregon's labor housing COVID-19 guidelines. J. Agromed. 2020;4:413–416. doi: 10.1080/1059924X.2020.1815622. [DOI] [PubMed] [Google Scholar]
- 2.Alarcón, C. How food and beverage companies are incentivizing employees to get vaccinated. 2021 https://www.forbes.com/sites/claudiaalarcon/2021/03/31/how-food-and-beverage-companies-are-incentivizing-employees-to-get-vaccinated/?sh=7c6fae336f49 Available at: . Accessed 31 March 2021. [Google Scholar]
- 3.Almeida, J.D.,, Berry D.M.,, Cunningham C.H.,, Hamre D.,, Hofstad M.S.,, Mallucci L.,, McIntosh K.,, Tyrrell D.A.J. Virology: coronaviruses. Nature. 1968;220:650. [Google Scholar]
- 4.American Society of Heating, Refrigerating and Air-Conditioning Engineers. Guidance for re-opening buildings. 2020 https://www.ashrae.org/file%20library/technical%20resources/covid-19/guidance-for-re-opening-buildings.pdf Available at: . Accessed 16 April 2021. [Google Scholar]
- 5.Anderson, E.L.,, Turnham P.,, Griffin J.R.,, Clarke C.C. Consideration of the aerosol transmission for COVID-19 and public health. Risk Anal. 2020;40:902–907. doi: 10.1111/risa.13500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asadi, S.,, Wexler A.S.,, Cappa C.D.,, Barreda S.,, Bouvier N.M.,, Ristenpart W.D. Aerosol emission and superemission during human speech increase with voice loudness. Sci. Rep. 2019;9:2348. doi: 10.1038/s41598-019-38808-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atkinson, J.,, Chartier Y.,, Pessoa-Silva C.L.,, Li Y.,, Seto W.-H. In: Atkinson J.,, Chartier Y.,, Pessoa-Silva C.L.,, Jensen P.,, Li Y.,, Seto W.-H., editors. World Health Organization; Geneva: 2009. Respiratory droplets, annex C. (Natural ventilation for infection control in health-care settings). In. [PubMed] [Google Scholar]
- 8.Australian Government, Department of Health. Masks—wearing a mask can help protect you and those around you. 2021 https://www.health.gov.au/news/health-alerts/novel-coronavirus-2019-ncov-health-alert/how-to-protect-yourself-and-others-from-coronavirus-covid-19/masks Available at: . Accessed 29 March 2021. [Google Scholar]
- 9.Bae, S.H.,, Shin H.,, Koo H.Y.,, Lee S.W.,, Yang J.M.,, Yon D.K. Asymptomatic transmission of SARS-CoV-2 on evacuation flight. Emerg. Infect. Dis. 2020;26:2705–2708. doi: 10.3201/eid2611.203353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barlow, S.M.,, Boobis A.R.,, Bridges J.,, Cockburn A.,, Dekant W.,, Hepburn P.,, Houben G.F.,, König J.,, Nauta M.J.,, Schuermans J. The role of hazard- and risk-based approaches in ensuring food safety. Trends Food Sci. Technol. 2015;46:176–188. [Google Scholar]
- 11.Barnes, M.,, Sax P.E. Challenges of “return to work” in an ongoing pandemic. N. Engl. J. Med. 2020;383:779–786. doi: 10.1056/NEJMsr2019953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beggs, C.B.,, Avital E.J. Upper-room ultraviolet air disinfection might help to reduce COVID-19 transmission in buildings: a feasibility study. PeerJ. 2020;8:e10196. doi: 10.7717/peerj.10196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ben-Shmuel, A.,, Brosh-Nissimov T.,, Glinert I.,, Bar-David E.,, Sittner A.,, Poni R.,, Cohen R.,, Achdout H.,, Tamir H.,, Yahalom-Ronen Y.,, Politi B.,, Melamed S.,, Vitner E.,, Cherry L.,, Israeli O.,, Beth-Din A.,, Paran N.,, Israely T.,, Yitzhaki S.,, Levy H.,, Weiss S. Detection and infectivity potential of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) environmental contamination in isolation units and quarantine facilities. Clin. Microbiol. Infect. 2020;26:1658–1662. doi: 10.1016/j.cmi.2020.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blondin-Brosseau, M.,, Harlow J.,, Doctor T.,, Nasheri N. Examining the persistence of human coronavirus 229E on fresh produce. Food Microbiol. 2021;98:103780. doi: 10.1016/j.fm.2021.103780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bright, A.,, Glynn-Robinson A.J.,, Kane S.,, Wright R.,, Saul N. The effect of COVID-19 public health measures on nationally notifiable diseases in Australia: preliminary analysis. Commun. Dis. Intell. (2018) 2020 doi: 10.33321/cdi.2020.44.85. [DOI] [PubMed] [Google Scholar]
- 16.Buitrago-Garcia, D.,, Egli-Gany D.,, Counotte M.J.,, Hossmann S.,, Imeri H.,, Ipekci A.M.,, Salanti G.,, Low N. Occurrence and transmission potential of asymptomatic and presymptomatic SARS-CoV-2 infections: a living systematic review and meta-analysis. PLoS Med. 2020;17:e1003346. doi: 10.1371/journal.pmed.1003346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Byington, L. Meat processors expedite plans to implement robotics as pandemic increases pressure. 2020 https://www.fooddive.com/news/meat-processors-expedite-automation-as-pandemic-increases/588166/ Available at: . Accessed 19 April 2021. [Google Scholar]
- 18.Cai, J.,, Sun W.,, Huang J.,, Gamber M.,, Wu J.,, He G. Indirect virus transmission in cluster of COVID-19 cases, Wenzhou, China, 2020. Emerg. Infect. Dis. 2020;26:1343–1345. doi: 10.3201/eid2606.200412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention. COVID-19 critical infrastructure sector response planning. 2020 https://www.cdc.gov/coronavirus/2019-ncov/community/critical-infrastructure-sectors.html Available at: . Accessed 29 March 2021. [Google Scholar]
- 20.Centers for Disease Control and Prevention. COVID-19 science update: fecal source transmission. 2020 https://www.cdc.gov/library/covid19/pdf/2020-09_08-Science-Update_FINAL_public-v2.pdf Available at: . Accessed 11 July 2021. [Google Scholar]
- 21.Centers for Disease Control and Prevention. Facility assessment checklist for evaluation of coronavirus disease (COVID-19) assessment and control plans for meat and poultry processing facilities: using guidance from the Centers for Disease Control and Prevention (CDC) and Occupational Safety and Health Administration (OSHA) 2020 https://www.cdc.gov/coronavirus/2019-ncov/downloads/php/Meat-And-Poultry-Facility-Assessment-Checklist.pdf Available at: . Accessed 16 April 2021. [Google Scholar]
- 22.Centers for Disease Control and Prevention. Science brief: SARS-CoV-2 and potential airborne transmission. 2020 https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/scientific-brief-sars-cov-2.html Available at: . Accessed 22 April 2021. [Google Scholar]
- 23.Centers for Disease Control and Prevention. Social distancing, quarantine, and isolation. 2020 https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/social-distancing.html Available at: . Accessed 12 January 2021. [Google Scholar]
- 24.Centers for Disease Control and Prevention. When and how to wash your hands. 2020 https://www.cdc.gov/handwashing/when-how-handwashing.html Available at: . Accessed 17 April 2021. [Google Scholar]
- 25.Centers for Disease Control and Prevention. Agriculture workers and employers—interim guidance from CDC and the U.S. Department of Labor. 2021 https://www.cdc.gov/coronavirus/2019-ncov/community/guidance-agricultural-workers.html Available at: . Accessed 17 April 2021. [Google Scholar]
- 26.Centers for Disease Control and Prevention. Community, work, and school—information for where you live, work, learn, and play. 2021 https://www.cdc.gov/coronavirus/2019-ncov/community/index.html Available at: . Accessed 17 April 2021. [Google Scholar]
- 27.Centers for Disease Control and Prevention. COVID-19 vaccination program interim playbook for jurisdictions operations annex. Considerations for increasing COVID-19 vaccination: reaching and increasing uptake in priority populations. 2021 https://www.cdc.gov/vaccines/covid-19/downloads/COVID-19-vaccination-program-playbook-annex.pdf Available at: . Accessed 21 April 2021. [Google Scholar]
- 28.Centers for Disease Control and Prevention. Guidance for wearing masks. 2021 https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/cloth-face-cover-guidance.html Available at: . Accessed 29 March 2021. [Google Scholar]
- 29.Centers for Disease Control and Prevention. Improve how your mask protects you. 2021 https://www.cdc.gov/coronavirus/2019-ncov/your-health/effective-masks.html Available at: . Accessed 29 March 2021. [Google Scholar]
- 30.Centers for Disease Control and Prevention. Interim guidance for SARS-CoV-2 testing in non-healthcare workplaces. 2021 https://www.cdc.gov/coronavirus/2019-ncov/community/organizations/testing-non-healthcare-workplaces.html Available at: . Accessed 29 March 2021. [Google Scholar]
- 31.Centers for Disease Control and Prevention. Interim guidance on developing a COVID-19 case investigation & contact tracing plan: overview. 2021 https://www.cdc.gov/coronavirus/2019-ncov/php/contact-tracing/contact-tracing-plan/overview.html Available at: . Accessed 12 January 2021. [Google Scholar]
- 32.Centers for Disease Control and Prevention. Meat and poultry processing workers and employers—interim guidance from CDC and the Occupational Safety and Health Administration (OSHA) 2021 https://www.cdc.gov/coronavirus/2019-ncov/community/organizations/meat-poultry-processing-workers-employers.html Available at: . Accessed 24 April 2021. [Google Scholar]
- 33.Centers for Disease Control and Prevention. Quick start guide to vaccinating essential workers. 2021 https://www.cdc.gov/vaccines/covid-19/health-departments/essential-workers/index.html Available at: . Accessed 22 April 2021. [Google Scholar]
- 34.Centers for Disease Control and Prevention. Science brief: SARS-CoV-2 and surface (fomite) transmission for indoor community environments. 2021 https://www.cdc.gov/coronavirus/2019-ncov/more/science-and-research/surface-transmission.html Available at: . Accessed 5 April 2021. [PubMed] [Google Scholar]
- 35.Centers for Disease Control and Prevention. Ventilation in buildings. 2021 https://www.cdc.gov/coronavirus/2019-ncov/community/ventilation.html Available at: . Accessed 16 April 2021. [Google Scholar]
- 36.Chang, A.,, Schnall A.H.,, Law R.,, Bronstein A.C.,, Marraffa J.M.,, Spiller H.A.,, Hays H.L.,, Funk A.R.,, Mercurio-Zappala M.,, Calello D.P.,, Aleguas A.,, Borys D.J.,, Boehmer T.,, Svendsen E. Cleaning and disinfectant chemical exposures and temporal associations with COVID-19—National Poison Data System, United States, January 1, 2020–March 31, 2020. Morb. Mortal. Wkly. Rep. 2020;69:496–498. doi: 10.15585/mmwr.mm6916e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chatterjee, S.,, Murallidharan J.S.,, Agrawal A.,, Bhardwaj R. Why coronavirus survives longer on impermeable than porous surfaces. Phys. Fluids (1994) 2021;33:021701. doi: 10.1063/5.0037924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen, H.Y.,, Chen A.,, Chen C. Investigation of the impact of infrared sensors on core body temperature monitoring by comparing measurement sites. Sensors. 2020;20 doi: 10.3390/s20102885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheng, V.C.,, Wong S.C.,, Chuang V.W.,, So S.Y.,, Chen J.H.,, Sridhar S.,, To K.K.,, Chan J.F.,, Hung I.F.,, Ho P.L.,, Yuen K.Y. The role of community-wide wearing of face mask for control of coronavirus disease 2019 (COVID-19) epidemic due to SARS-CoV-2. J. Infect. 2020;81:107–114. doi: 10.1016/j.jinf.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chetty, T.,, Daniels B.B.,, Ngandu N.K.,, Goga A. A rapid review of the effectiveness of screening practices at airports, land borders and ports to reduce the transmission of respiratory infectious diseases such as COVID-19. S. Afr. Med. J. 2020;110:1105–1109. [PubMed] [Google Scholar]
- 41.Chew, M.-H.,, Koh F.H.,, Wu J.-T.,, Ngaserin S.,, Ng A.,, Ong B.-C.,, Lee V.J. Clinical assessment of COVID-19 outbreak among migrant workers residing in a large dormitory in Singapore. J. Hosp. Infect. 2020;106:202–203. doi: 10.1016/j.jhin.2020.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chin, A.W.H.,, Chu J.T.S.,, Perera M.R.A.,, Hui K.P.Y.,, Yen H.L.,, Chan M.C.W.,, Peiris M.,, Poon L.L.M. Stability of SARS-CoV-2 in different environmental conditions. Lancet Microbe. 2020;1:e10. doi: 10.1016/S2666-5247(20)30003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chirico, F.,, Sacco A.,, Bragazzi N.L.,, Magnavita N. Can air-conditioning systems contribute to the spread of SARS/MERS/COVID-19 infection? Insights from a rapid review of the literature. Int. J. Environ. Res. Public Health. 2020;17:6052. doi: 10.3390/ijerph17176052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chu, D.K.,, Akl E.A.,, Duda S.,, Solo K.,, Yaacoub S.,, Schünemann H.J.,, Chu D.K.,, Akl E.A.,, El-harakeh A.,, Bognanni A.,, Lotfi T.,, Loeb M.,, Hajizadeh A.,, Bak A.,, Izcovich A.,, Cuello-Garcia C.A.,, Chen C.,, Harris D.J.,, Borowiack E.,, Chamseddine F.,, Schünemann F.,, Morgano G.P.,, Muti Schünemann G.E.U.,, Chen G.,, Zhao H.,, Neumann I.,, Chan J.,, Khabsa J.,, Hneiny L.,, Harrison L.,, Smith M.,, Rizk N.,, Giorgi Rossi P.,, AbiHanna P.,, El-khoury R.,, Stalteri R.,, Baldeh T.,, Piggott T.,, Zhang Y.,, Saad Z.,, Khamis A.,, Reinap M.,, Duda S.,, Solo K.,, Yaacoub S.,, Schünemann H.J. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet. 2020;395:1973–1987. doi: 10.1016/S0140-6736(20)31142-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Colaneri, M.,, Seminari E.,, Novati S.,, Asperges E.,, Biscarini S.,, Piralla A.,, Percivalle E.,, Cassaniti I.,, Baldanti F.,, Bruno R.,, Mondelli M.U., Severe acute respiratory syndrome coronavirus 2 RNA contamination of inanimate surfaces and virus viability in a health care emergency unit. Clin. Microbiol. Infect. 2020;26:1094.e1–1094.e5. doi: 10.1016/j.cmi.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coluccia, B.,, Agnusdei G.P.,, Miglietta P.P.,, De Leo F. Effects of COVID-19 on the Italian agri-food supply and value chains. Food Control. 2021;123:107839. doi: 10.1016/j.foodcont.2020.107839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Courtemanche, C.,, Garuccio J.,, Le A.,, Pinkston J.,, Yelowitz A. Strong social distancing measures in the United States reduced the COVID-19 growth rate. Health Affairs. 2020;39:1237–1246. doi: 10.1377/hlthaff.2020.00608. [DOI] [PubMed] [Google Scholar]
- 48.Cowling, B.J.,, Aiello A.E. Public health measures to slow community spread of coronavirus disease 2019. J. Infect. Dis. 2020;221:1749–1751. doi: 10.1093/infdis/jiaa123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dai, H.,, Zhao B. Association of the infection probability of COVID-19 with ventilation rates in confined spaces. 2020 doi: 10.1007/s12273-020-0703-5. Build Simul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dai, M.,, Li H.,, Yan N.,, Huang J.,, Zhao L.,, Xu S.,, Wu J.,, Jiang S.,, Pan C.,, Liao M. Long-term survival of SARS-CoV-2 on salmon as a source for international transmission. J. Infect. Dis. 2021;223:537–539. doi: 10.1093/infdis/jiaa712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dee, S.,, Batista L.,, Deen J.,, Pijoan C. Evaluation of an air-filtration system for preventing aerosol transmission of porcine reproductive and respiratory syndrome virus. Can. J. Vet. Res. 2005;69:293–298. [PMC free article] [PubMed] [Google Scholar]
- 52.de Man, P.,, Paltansing S.,, Ong D.S.Y.,, Vaessen N.,, van Nielen G.,, Koeleman J.G.M. Outbreak of coronavirus disease 2019 (COVID-19) in a nursing home associated with aerosol transmission as a result of inadequate ventilation. Clin. Infect. Dis. 2020;73:170–171. doi: 10.1093/cid/ciaa1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Demetrakakes, P. COVID-19 shines light on labor issues in the food and beverage industry. 2020 https://www.foodprocessing.com/articles/2020/covid-labor-issues/ Available at: . Accessed 11 May 2021. [Google Scholar]
- 54.Dhand, R.,, Li J. Coughs and sneezes: their role in transmission of respiratory viral infections, including SARS-CoV-2. Am. J. Resp. Crit. Care Med. 2020;202:651–659. doi: 10.1164/rccm.202004-1263PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Djekic, I.,, Nikolić A.,, Uzunović M.,, Marijke A.,, Liu A.,, Han J.,, Brnčić M.,, Knežević N.,, Papademas P.,, Lemoniati K.,, Witte F.,, Terjung N.,, Papageorgiou M.,, Zinoviadou K.G.,, Dalle Zotte A.,, Pellattiero E.,, Sołowiej B.G.,, Guiné R.P.F.,, Correia P.,, Sirbu A.,, Vasilescu L.,, Semenova A.A.,, Kuznetsova O.A.,, Brodnjak U.V.,, Pateiro M.,, Lorenzo J.M.,, Getya A.,, Kodak T.,, Tomasevic I. COVID-19 pandemic effects on food safety—multi-country survey study. Food Control. 2021;122:107800. doi: 10.1016/j.foodcont.2020.107800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dobramysl, U.,, Sieben C.,, Holcman D. Mean time to infection by small diffusing droplets containing SARS-CoV-2 during close social contacts. medRxiv. 2021 2021.04.01.21254802. [Google Scholar]
- 57.Dong, E.,, Du H.,, Gardner L. An interactive Web-based dashboard to track COVID-19 in real time. Lancet Infect. Dis. 2020;20:533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ebrahim, S.H.,, Ahmed Q.A.,, Gozzer E.,, Schlagenhauf P.,, Memish Z.A. COVID-19 and community mitigation strategies in a pandemic. BMJ. 2020;368 doi: 10.1136/bmj.m1066. m1066. [DOI] [PubMed] [Google Scholar]
- 59.Escudero, B.I.,, Rawsthorne H.,, Gensel C.,, Jaykus L.A. Persistence and transferability of noroviruses on and between common surfaces and foods. J. Food Prot. 2012;75:927–935. doi: 10.4315/0362-028X.JFP-11-460. [DOI] [PubMed] [Google Scholar]
- 60.European Centre for Disease Prevention and Control. Surveillance definitions for COVID-19. 2020 https://www.ecdc.europa.eu/en/covid-19/surveillance/surveillance-definitions Available at: . Accessed 12 January 2021. [Google Scholar]
- 61.European Food Safety Authority. Coronavirus: no evidence that food is a source or transmission route. 2020 https://www.efsa.europa.eu/en/news/coronavirus-no-evidence-food-source-or-transmission-route Available at: . Accessed 21 April 2021. [Google Scholar]
- 62.Foddai, A.C.,, Grant I.R.,, Dean M. Efficacy of instant hand sanitizers against foodborne pathogens compared with hand washing with soap and water in food preparation settings: a systematic review. J. Food Prot. 2016;79:1040–1054. doi: 10.4315/0362-028X.JFP-15-492. [DOI] [PubMed] [Google Scholar]
- 63.Goldman, E. Exaggerated risk of transmission of COVID-19 by fomites. Lancet Infect. Dis. 2020;20:892–893. doi: 10.1016/S1473-3099(20)30561-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gostic, K.,, Gomez A.C.R.,, Mummah R.O.,, Kucharski A.J.,, Lloyd-Smith J.O. Estimated effectiveness of symptom and risk screening to prevent the spread of COVID-19. eLife. 2020;9:e55570. doi: 10.7554/eLife.55570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gostic, K.M.,, Kucharski A.J.,, Lloyd-Smith J.O. Effectiveness of traveller screening for emerging pathogens is shaped by epidemiology and natural history of infection. eLife. 2015;4:e05564. doi: 10.7554/eLife.05564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Griffith, C.J.,, Livesey K.M.,, Clayton D.A. Food safety culture: the evolution of an emerging risk factor? Br. Food J. 2010;112:426–438. [Google Scholar]
- 67.Grover, A.K.,, Chopra S.,, Mosher G.A. Food Safety Modernization Act: a quality management approach to identify and prioritize factors affecting adoption of preventive controls among small food facilities. Food Control. 2016;66:241–249. [Google Scholar]
- 68.Grylls, B. The future of food auditing. 2021 https://www.newfoodmagazine.com/article/142396/food-auditing/ Available at: . Accessed 15 April 2021. [Google Scholar]
- 69.Gunther, T.,, Czech-Sioli M.,, Indenbirken D.,, Robitaille A.,, Tenhaken P.,, Exner M.,, Ottinger M.,, Fischer N.,, Grundhoff A.,, Brinkmann M.M. SARS-CoV-2 outbreak investigation in a German meat processing plant. EMBO Mol. Med. 2020;12:e13296. doi: 10.15252/emmm.202013296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Haley, E.,, Caxaj S.,, George G.,, Hennebry J.,, Martell E.,, McLaughlin J. Migrant farmworkers face heightened vulnerabilities during COVID-19. J. Agric. Food Syst. Community Dev. 2020;9(3):35–39. [Google Scholar]
- 71.Hamre, D.,, Procknow J.J. A new virus isolated from the human respiratory tract. Proc. Soc. Exp. Biol. Med. 1966;121:190–193. doi: 10.3181/00379727-121-30734. [DOI] [PubMed] [Google Scholar]
- 72.Haque, M. Handwashing in averting infectious diseases: relevance to COVID-19. J. Popul. Ther. Clin. Pharmacol. 2020;27:e37–e52. doi: 10.15586/jptcp.v27SP1.711. [DOI] [PubMed] [Google Scholar]
- 73.Harvey, A.P.,, Fuhrmeister E.R.,, Cantrell M.,, Pitol A.K.,, Swarthout J.M.,, Powers J.E.,, Nadimpalli M.L.,, Julian T.R.,, Pickering A.J. Longitudinal monitoring of SARS-CoV-2 RNA on high-touch surfaces in a community setting. Environ. Sci. Technol. Lett. 2020;8:168–175. doi: 10.1021/acs.estlett.0c00875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.He, Y.,, Li J.,, Heck S.,, Lustigman S.,, Jiang S. Antigenic and immunogenic characterization of recombinant baculovirus-expressed severe acute respiratory syndrome coronavirus spike protein: implication for vaccine design. J. Virol. 2006;80:5757–5767. doi: 10.1128/JVI.00083-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Howard, J.,, Huang A.,, Li Z.,, Tufekci Z.,, Zdimal V.,, van der Westhuizen H.-M.,, von Delft A.,, Price A.,, Fridman L.,, Tang L.-H.,, Tang V.,, Watson G.L.,, Bax C.E.,, Shaikh R.,, Questier F.,, Hernandez D.,, Chu L.F.,, Ramirez C.M.,, Rimoin A.W. An evidence review of face masks against COVID-19. Proc. Natl. Acad. Sci. USA. 2021;118 doi: 10.1073/pnas.2014564118. e2014564118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hu, M.,, Lin H.,, Wang J.,, Xu C.,, Tatem A.J.,, Meng B.,, Zhang X.,, Liu Y.,, Wang P.,, Wu G.,, Xie H.,, Lai S. Risk of coronavirus disease 2019 transmission in train passengers: an epidemiological and modeling study. Clin. Infect. Dis. 2021;72:604–610. doi: 10.1093/cid/ciaa1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hwang, S.E.,, Chang J.H.,, Oh B.,, Heo J. Possible aerosol transmission of COVID-19 associated with an outbreak in an apartment in Seoul, South Korea, 2020. Int. J. Infect. Dis. 2021;104:73–76. doi: 10.1016/j.ijid.2020.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Institute for Health Metrics and Evaluation. COVID-19 projections. 2021 https://covid19.healthdata.org/global?view=total-deaths&tab=trend Available at: . Accessed 19 April 2021. [Google Scholar]
- 79.International Commission on Microbiological Specifications for Food. ICMSF opinion on SARS-CoV-2 and its relationship to food safety. 2020 https://www.icmsf.org/wp-content/uploads/2020/09/ICMSF2020-Letterhead-COVID-19-opinion-final-03-Sept-2020.BF_.pdf Available at: . Accessed 21 April 2021. [Google Scholar]
- 80.Jefferson, T.,, Del Mar C.B.,, Dooley L.,, Ferroni E.,, Al-Ansary L.A.,, Bawazeer G.A.,, van Driel M.L.,, Nair N.S.,, Jones M.A.,, Thorning S.,, Conly J.M., Cochrane Acute Respiratory Infections Group. Physical interventions to interrupt or reduce the spread of respiratory virusesCochrane Database Syst. Rev. 2020;2011(7):CD006207. doi: 10.1002/14651858.CD006207.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jespersen, L.,, Griffiths M.,, Maclaurin T.,, Chapman B.,, Wallace C.A. Measurement of food safety culture using survey and maturity profiling tools. Food Control. 2016;66:174–182. [Google Scholar]
- 82.Johns Hopkins University. COVID-19 dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU) 2021 https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6 Available at: . Accessed 1 May 2021. [Google Scholar]
- 83.Johnson, G.R.,, Morawska L.,, Ristovski Z.D.,, Hargreaves M.,, Mengersen K.,, Chao C.Y.H.,, Wan M.P.,, Li Y.,, Xie X.,, Katoshevski D.,, Corbett S. Modality of human expired aerosol size distributions. J. Aerosol Sci. 2011;42:839–851. doi: 10.1016/j.jaerosci.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Julian, T.R.,, Leckie J.O.,, Boehm A.B. Virus transfer between fingerpads and fomites. J. Appl. Microbiol. 2010;109:1868–1874. doi: 10.1111/j.1365-2672.2010.04814.x. [DOI] [PubMed] [Google Scholar]
- 85.Kampf, G. Efficacy of ethanol against viruses in hand disinfection. J. Hosp. Infect. 2018;98:331–338. doi: 10.1016/j.jhin.2017.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kampf, G.,, Bruggemann Y.,, Kaba H.E.J.,, Steinmann J.,, Pfaender S.,, Scheithauer S.,, Steinmann E. Potential sources, modes of transmission and effectiveness of prevention measures against SARS-CoV-2. J. Hosp. Infect. 2020;106:678–697. doi: 10.1016/j.jhin.2020.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kingsbury, J.M.,, Lake R. Potential for foodborne transmission of COVID-19: literature review update. 2020 https://www.unitedfresh.co.nz/assets/COVID-19/United-Fresh—Potential-for-Foodborne-Transmission-of-Covid-19—Literature-Review-Update-19-May-20.pdf Available at: . Accessed 11 May 2021. [Google Scholar]
- 88.Kraay, A.N.M.,, Han P.,, Kambhampati A.K.,, Wikswo M.E.,, Mirza S.A.,, Lopman B.A. Impact of nonpharmaceutical interventions for severe acute respiratory syndrome coronavirus 2 on norovirus outbreaks: an analysis of outbreaks reported by 9 US states. J. Infect. Dis. 2021;224:9–13. doi: 10.1093/infdis/jiab093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lelieveld, J.,, Helleis F.,, Borrmann S.,, Cheng Y.,, Drewnick F.,, Haug G.,, Klimach T.,, Sciare J.,, Su H.,, Pöschl U. Model calculations of aerosol transmission and infection risk of COVID-19 in indoor environments. Int. J. Environ. Res. Public Health. 2020;17(21):8114. doi: 10.3390/ijerph17218114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Le Mennec, C.,, Parnaudeau S.,, Rumebe M.,, Le Saux J.C.,, Piquet J.C.,, Le Guyader S.F. Follow-up of norovirus contamination in an oyster production area linked to repeated outbreaks. Food Environ. Virol. 2017;9:54–61. doi: 10.1007/s12560-016-9260-6. [DOI] [PubMed] [Google Scholar]
- 91.Lewnard, J.A.,, Lo N.C. Scientific and ethical basis for social-distancing interventions against COVID-19. Lancet Infect. Dis. 2020;20:631–633. doi: 10.1016/S1473-3099(20)30190-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li, D.,, Zhao M.Y.,, Tan T.H.M. What makes a foodborne virus: comparing coronaviruses with human noroviruses. Curr. Opin. Food Sci. 2021;42:1–7. doi: 10.1016/j.cofs.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lindsley, W.G.,, Noti J.D.,, Blachere F.M.,, Szalajda J.V.,, Beezhold D.H. Efficacy of face shields against cough aerosol droplets from a cough simulator. J. Occup. Environ. Hyg. 2014;11:509–518. doi: 10.1080/15459624.2013.877591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu, Y.,, Li T.,, Deng Y.,, Liu S.,, Zhang D.,, Li H.,, Wang X.,, Jia L.,, Han J.,, Bei Z.,, Li L.,, Li J. Stability of SARS-CoV-2 on environmental surfaces and in human excreta. J. Hosp. Infect. 2021;107:105–107. doi: 10.1016/j.jhin.2020.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lopez, G.U.,, Gerba C.P.,, Tamimi A.H.,, Kitajima M.,, Maxwell S.L.,, Rose J.B. Transfer efficiency of bacteria and viruses from porous and nonporous fomites to fingers under different relative humidity conditions. Appl. Environ. Microbiol. 2013;79:5728–5734. doi: 10.1128/AEM.01030-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lu, J.,, Gu J.,, Li K.,, Xu C.,, Su W.,, Lai Z.,, Zhou D.,, Yu C.,, Xu B.,, Yang Z. COVID-19 outbreak associated with air conditioning in restaurant, Guangzhou, China, 2020. Emerg. Infect. Dis. 2020;26:1628–1631. doi: 10.3201/eid2607.200764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Meyerowitz, E.A.,, Richterman A.,, Gandhi R.T.,, Sax P.E. Transmission of SARS-CoV-2: a review of viral, host, and environmental factors. Ann. Intern. Med. 2021;174:69–79. doi: 10.7326/M20-5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Morawska, L.,, Milton D.K. It is time to address airborne transmission of coronavirus disease 2019 (COVID-19) Clin. Infect. Dis. 2020;71:2311–2313. doi: 10.1093/cid/ciaa939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nachamkin, I.,, Richard-Greenblatt M.,, Yu M.,, Bui H. Reduction in sporadic norovirus infections following the start of the COVID-19 pandemic, 2019–2020, Philadelphia. Infect. Dis. Ther. 2021;10:1793–1798. doi: 10.1007/s40121-021-00473-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.National Center for Farmworker Health. COVID-19 educational resources for agricultural workers. 2021 http://www.ncfh.org/covid_resources_for_ag_workers.html Available at: . Accessed 21 April 2021. [Google Scholar]
- 101.Neumann, M. The COVID-19 medical record retention conundrum for employers. 2020 https://www.matrixsciences.com/the-covid-19-medical-record-retention-conundrum-for-employers/?mc_cid=790f649b0e&mc_eid=ee117504ae Available at: . Accessed 29 March 2021. [Google Scholar]
- 102.New York State Department of Health. Health advisory: COVID-19 update for local health departments (LHD) contact investigations. 2020 https://coronavirus.health.ny.gov/system/files/documents/2020/04/doh_covid19_lhdcontacttracing_040820.pdf Available at: . Accessed 12 January 2021. [Google Scholar]
- 103.New York State Department of Health. NY forward safety plan template—COVID-19 reopening safety plan. 2020 https://www.governor.ny.gov/sites/default/files/atoms/files/NYS_BusinessReopeningSafetyPlanTemplate.pdf Available at: . Accessed 16 April 2021. [Google Scholar]
- 104.Nickbakhsh, S.,, Ho A.,, Marques D.F.P.,, McMenamin J.,, Gunson R.N.,, Murcia P.R. Epidemiology of seasonal coronaviruses: establishing the context for the emergence of coronavirus disease 2019. J. Infect. Dis. 2020;222:17–25. doi: 10.1093/infdis/jiaa185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nyarugwe, S.P.,, Linnemann A.R.,, Ren Y.,, Bakker E.-J.,, Kussaga J.B.,, Watson D.,, Fogliano V.,, Luning P.A. An intercontinental analysis of food safety culture in view of food safety governance and national values. Food Control. 2020;111:107075. [Google Scholar]
- 106.Paltiel, A.D.,, Zheng A.,, Walensky R.P. Assessment of SARS-CoV-2 screening strategies to permit the safe reopening of college campuses in the United States. JAMA Netw. Open. 2020;3:e2016818. doi: 10.1001/jamanetworkopen.2020.16818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Parker, E.P.K.,, Shrotri M.,, Kampmann B. Keeping track of the SARS-CoV-2 vaccine pipeline. Nat. Rev. Immunol. 2020;20:650. doi: 10.1038/s41577-020-00455-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Parks, C.A.,, Nugent N.B.,, Fleischhacker S.E.,, Yaroch A.L. Food system workers are the unexpected but under protected COVID heroes. J. Nutr. 2020;150:2006–2008. doi: 10.1093/jn/nxaa173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pitol, A.K.,, Julian T.R. Community transmission of SARS-CoV-2 by surfaces: risks and risk reduction strategies. Environ. Sci. Technol. Lett. 2021;8:263–269. doi: 10.1021/acs.estlett.0c00966. [DOI] [PubMed] [Google Scholar]
- 110.Powell, D.A.,, Jacob C.J.,, Chapman B.J. Enhancing food safety culture to reduce rates of foodborne illness. Food Control. 2011;22:817–822. [Google Scholar]
- 111.Randazzo, W.,, Sanchez G. Hepatitis A infections from food. J. Appl. Microbiol. 2020;129:1120–1132. doi: 10.1111/jam.14727. [DOI] [PubMed] [Google Scholar]
- 112.Riddell, S.,, Goldie S.,, Hill A.,, Eagles D.,, Drew T.W. The effect of temperature on persistence of SARS-CoV-2 on common surfaces. Virol. J. 2020;17:145. doi: 10.1186/s12985-020-01418-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rota, P.A.,, Oberste M.S.,, Monroe S.S.,, Nix W.A.,, Campagnoli R.,, Icenogle J.P.,, Penaranda S.,, Bankamp B.,, Maher K.,, Chen M.H.,, Tong S.,, Tamin A.,, Lowe L.,, Frace M.,, DeRisi J.L.,, Chen Q.,, Wang D.,, Erdman D.D.,, Peret T.C.,, Burns C.,, Ksiazek T.G.,, Rollin P.E.,, Sanchez A.,, Liffick S.,, Holloway B.,, Limor J.,, McCaustland K.,, Olsen-Rasmussen M.,, Fouchier R.,, Gunther S.,, Osterhaus A.D.,, Drosten C.,, Pallansch M.A.,, Anderson L.J.,, Bellini W.J. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science. 2003;300:1394–1399. doi: 10.1126/science.1085952. [DOI] [PubMed] [Google Scholar]
- 114.Rothamer, D.A.,, Sanders S.,, Reindl D.,, Bertram T.H. Strategies to minimize SARS-CoV-2 transmission in classroom settings: combined impacts of ventilation and mask effective filtration efficiency. medRxiv. 2021 2020.12.31.20249101. [Google Scholar]
- 115.San Francisco Department of Public Health, Population Health Division. Interim guidance: measuring temperatures when screening for COVID-19 symptoms. 2020 https://www.sfcdcp.org/wp-content/uploads/2020/05/COVID19-Temperature-Measurement-UPDATE-05.26.2020.pdf Available at: . Accessed 19 April 2021. [Google Scholar]
- 116.Schijven, J.,, Vermeulen L.C.,, Swart A.,, Meijer A.,, Duizer E.,, de Roda Husman A.M. Exposure assessment for airborne transmission of SARS-CoV-2 via breathing, speaking, coughing and sneezing. medRxiv. 2020 2020.07.02.20144832. [Google Scholar]
- 117.Schröder, I. COVID-19: a risk assessment perspective. ACS Chem. Health Saf. 2020;27(3):160–169. doi: 10.1021/acs.chas.0c00035. [DOI] [PubMed] [Google Scholar]
- 118.Shamsi, A.,, Mohammad T.,, Anwar S.,, Amani S.,, Khan M.S.,, Husain F.M.,, Rehman M.T.,, Islam A.,, Hassan M.I. Potential drug targets of SARS-CoV-2: from genomics to therapeutics. Int. J. Biol. Macromol. 2021;177:1–9. doi: 10.1016/j.ijbiomac.2021.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shen, J.,, Duan H.,, Zhang B.,, Wang J.,, Ji J.S.,, Wang J.,, Pan L.,, Wang X.,, Zhao K.,, Ying B.,, Tang S.,, Zhang J.,, Liang C.,, Sun H.,, Lv Y.,, Li Y.,, Li T.,, Li L.,, Liu H.,, Zhang L.,, Wang L.,, Shi X. Prevention and control of COVID-19 in public transportation: experience from China. Environ. Pollut. 2020;266:115291. doi: 10.1016/j.envpol.2020.115291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shen, Y.,, Li C.,, Dong H.,, Wang Z.,, Martinez L.,, Sun Z.,, Handel A.,, Chen Z.,, Chen E.,, Ebell M.H. Community outbreak investigation of SARS-CoV-2 transmission among bus riders in eastern China. JAMA Intern. Med. 2020;180:1665–1671. doi: 10.1001/jamainternmed.2020.5225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shrotri, M.,, Swinnen T.,, Kampmann B.,, Parker E.P.K. An interactive website tracking COVID-19 vaccine development. Lancet Glob. Health. 2021;9(5):e590–e592. doi: 10.1016/S2214-109X(21)00043-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Siddharta, A.,, Pfaender S.,, Vielle N.J.,, Dijkman R.,, Friesland M.,, Becker B.,, Yang J.,, Engelmann M.,, Todt D.,, Windisch M.P.,, Brill F.H.,, Steinmann J.,, Steinmann J.,, Becker S.,, Alves M.P.,, Pietschmann T.,, Eickmann M.,, Thiel V.,, Steinmann E. Virucidal activity of World Health Organization–recommended formulations against enveloped viruses, including Zika, Ebola, and emerging coronaviruses. J. Infect. Dis. 2017;215:902–906. doi: 10.1093/infdis/jix046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Somsen, G.A.,, van Rijn C.,, Kooij S.,, Bem R.A.,, Bonn D. Small droplet aerosols in poorly ventilated spaces and SARS-CoV-2 transmission. Lancet Resp. Med. 2020;8:658–659. doi: 10.1016/S2213-2600(20)30245-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Stadnytskyi, V.,, Bax C.E.,, Bax A.,, Anfinrud P. The airborne lifetime of small speech droplets and their potential importance in SARS-CoV-2 transmission. Proc. Natl. Acad. Sci. USA. 2020;117:11875. doi: 10.1073/pnas.2006874117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sun, C.,, Zhai Z. The efficacy of social distance and ventilation effectiveness in preventing COVID-19 transmission. Sustain. Cities Soc. 2020;62:102390. doi: 10.1016/j.scs.2020.102390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sung, M.,, Kato S. Estimating the germicidal effect of upper-room UVGI system on exhaled air of patients based on ventilation efficiency. Build. Environ. 2011;46:2326–2332. doi: 10.1016/j.buildenv.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Teran, R.A.,, Ghinai I.,, Gretsch S.,, Cable T.,, Black S.R.,, Green S.J.,, Perez O.,, Chlipala G.E.,, Maienschein-Cline M.,, Kunstman K.J.,, Bleasdale S.C.,, Fricchione M.J. COVID-19 outbreak among a university's men's and women's soccer teams—Chicago, Illinois, July–August 2020. Morb. Mortal. Wkly. Rep. 2020;69:1591–1594. doi: 10.15585/mmwr.mm6943e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Thu, T.P.B.,, Ngoc P.N.H.,, Hai N.M.,, Tuan L.A. Effect of the social distancing measures on the spread of COVID-19 in 10 highly infected countries. Sci. Total Environ. 2020;742:140430. doi: 10.1016/j.scitotenv.2020.140430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tyrrell, D.A.,, Bynoe M.L. Cultivation of a novel type of common-cold virus in organ cultures. Br. Med. J. 1965;1:1467–1470. doi: 10.1136/bmj.1.5448.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.U.S. Department of Health and Human Services. COVID-19 pandemic response, laboratory data reporting: CARES Act section 18115, public law 116-136, §18115(a) the Coronavirus Aid, Relief, and Economic Security Act. 2021 https://www.hhs.gov/sites/default/files/covid-19-laboratory-data-reporting-guidance.pdf Available at: . Accessed 29 March 2021. [Google Scholar]
- 131.U.S. Environmental Protection Agency. About list N: disinfectants for coronavirus (COVID-19) 2020 https://cfpub.epa.gov/giwiz/disinfectants/index.cfm Available at: . Accessed 17 April 2021. [Google Scholar]
- 132.U.S. Food and Drug Administration. Current good manufacturing practice, hazard analysis and risk-based preventive controls for human food. Code of Federal Regulations, title 21, chap. I, subchap. B, part 117. 2020 https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCFR/CFRSearch.cfm?CFRPart=117 Available at: . Accessed 17 April 2021. [Google Scholar]
- 133.U.S. Food and Drug Administration. Employee health and food safety checklist for human and animal food operations during the COVID-19 pandemic. 2020 https://www.fda.gov/media/141141/download Available at: . Accessed 16 April 2021. [Google Scholar]
- 134.U.S. Food and Drug Administration. Temporary policy regarding packaging and labeling of shell eggs sold by retail food establishments during the COVID-19 public health emergency—guidance for industry. 2020 https://www.fda.gov/media/136671/download Available at: . Accessed 27 January 2021. [Google Scholar]
- 135.van Doorn, A.S.,, Meijer B.,, Frampton C.M.A.,, Barclay M.L.,, de Boer N.K.H. Systematic review with meta-analysis: SARS-CoV-2 stool testing and the potential for faecal-oral transmission. Aliment. Pharmacol. Ther. 2020;52:1276–1288. doi: 10.1111/apt.16036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.van Doremalen, N.,, Bushmaker T.,, Morris D.H.,, Holbrook M.G.,, Gamble A.,, Williamson B.N.,, Tamin A.,, Harcourt J.L.,, Thornburg N.J.,, Gerber S.I.,, Lloyd-Smith J.O.,, de Wit E.,, Munster V.J. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wang, Y.,, Tian H.,, Zhang L.,, Zhang M.,, Guo D.,, Wu W.,, Zhang X.,, Kan G.L.,, Jia L.,, Huo D.,, Liu B.,, Wang X.,, Sun Y.,, Wang Q.,, Yang P.,, MacIntyre C.R. Reduction of secondary transmission of SARS-CoV-2 in households by face mask use, disinfection and social distancing: a cohort study in Beijing, China. BMJ Glob. Health. 2020;5(5):e002794. doi: 10.1136/bmjgh-2020-002794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Washington State Department of Health. COVID-19 safety plan template for critical infrastructure. 2020 https://www.doh.wa.gov/Portals/1/Documents/1600/coronavirus/COVID-19SafetyPlanTemplate04212020.docx Available at: . Accessed 16 April 2021. [Google Scholar]
- 139.Wee, L.E.,, Conceicao E.P.,, Sim X.Y.J.,, Aung M.K.,, Tan K.Y.,, Wong H.M.,, Wijaya L.,, Tan B.H.,, Ling M.L.,, Venkatachalam I. Minimizing intra-hospital transmission of COVID-19: the role of social distancing. J. Hosp. Infect. 2020;105:113–115. doi: 10.1016/j.jhin.2020.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wee, L.E.,, Venkatachalam I.,, Sim X.Y.J.,, Tan K.B.,, Wen R.,, Tham C.K.,, Gan W.H.,, Ko K.K.K.,, Ho W.Q.,, Kwek G.T.C.,, Conceicao E.P.,, Sng C.Y.E.,, Ng X.H.J.,, Ong J.Y.,, Chiang J.L.,, Chua Y.Y.,, Ling M.L.,, Tan T.T.,, Wijaya L. Containment of COVID-19 and reduction in healthcare-associated respiratory viral infections through a multi-tiered infection control strategy. Infect. Dis. Health. 2020;26:123–131. doi: 10.1016/j.idh.2020.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wells, W.F. On air-borne infection. Study II. Droplets and droplet nuclei. Am. J. Epidemiol. 1934;20:611–618. [Google Scholar]
- 142.Whitworth, J. Food fraud rise ‘inevitable' because of COVID-19. 2020 https://www.foodsafetynews.com/2020/05/food-fraud-rise-inevitable-because-of-covid-19/ Available at: . Accessed 15 April 2021. [Google Scholar]
- 143.Whitworth, J. High risk firms may miss an inspection due to COVID-19. 2020 https://www.foodsafetynews.com/2020/12/high-risk-firms-may-miss-an-inspection-due-to-covid-19/ Available at: . Accessed 15 April 2021. [Google Scholar]
- 144.Wilson, A.M.,, Weir M.H.,, Bloomfield S.F.,, Scott E.A.,, Reynolds K.A. Modeling COVID-19 infection risks for a single hand-to-fomite scenario and potential risk reductions offered by surface disinfection. Am. J. Infect. Control. 2020;49:846–848. doi: 10.1016/j.ajic.2020.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Woodcock, J., COVID-19 update: USDA, FDA underscore current epidemiologic and scientific information indicating no transmission of COVID-19 through food or food packaging. 2021 https://www.fda.gov/news-events/press-announcements/covid-19-update-usda-fda-underscore-current-epidemiologic-and-scientific-information-indicating-no Available at: . Accessed 21 April 2021. [Google Scholar]
- 146.World Health Organization. WHO guidelines on hand hygiene in health care. 2009 doi: 10.1086/600379. https://www.who.int/publications/i/item/9789241597906 Available at: . Accessed 17 April 2021. [DOI] [PubMed] [Google Scholar]
- 147.World Health Organization. Coronavirus disease 2019 (COVID-19)—situation report. 2020 https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200221-sitrep-32-covid-19.pdf?sfvrsn=4802d089_2 Available at: . Accessed 9 July 2021. [Google Scholar]
- 148.World Health Organization. Coronavirus disease (COVID-19): masks. 2020 https://www.who.int/news-room/q-a-detail/coronavirus-disease-covid-19-masks Available at: . Accessed 29 March 2021. [Google Scholar]
- 149.World Health Organization. COVID-19 and food safety: guidance for food businesses. 2020 https://apps.who.int/iris/bitstream/handle/10665/331705/WHO-2019-nCoV-Food_Safety-2020.1-eng.pdf Available at: . Accessed 21 April 2021. [Google Scholar]
- 150.World Health Organization. Infection prevention and control guidance for COVID-19. 2020 https://www.who.int/publications-detail/infection-prevention-andcontrol-during-health-care-when-novel-coronavirus-(ncov)-infection-is-suspected20200125 Available at: . Accessed 12 January 2021. [Google Scholar]
- 151.World Health Organization. Recommendations to member states to improve hand hygiene practices to help prevent the transmission of the COVID-19 virus: interim guidance. 2020 https://apps.who.int/iris/bitstream/handle/10665/331661/WHO-2019-nCov-Hand_Hygiene_Stations-2020.1-eng.pdf?sequence=1&isAllowed=y Available at: . Accessed 17 April 2021. [Google Scholar]
- 152.World Health Organization. Responding to community spread of COVID-19: interim guidance. 2020 https://apps.who.int/iris/bitstream/handle/10665/331421/WHO-COVID-19-Community_Transmission-2020.1-eng.pdf?sequence=1&isAllowed=y Available at: . Accessed 16 April 2021. 7 March 2020. [Google Scholar]
- 153.Xie, C.,, Zhao H.,, Li K.,, Zhang Z.,, Lu X.,, Peng H.,, Wang D.,, Chen J.,, Zhang X.,, Wu D.,, Gu Y.,, Yuan J.,, Zhang L.,, Lu J. The evidence of indirect transmission of SARS-CoV-2 reported in Guangzhou, China. BMC Public Health. 2020;20:1202. doi: 10.1186/s12889-020-09296-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yiannas, F. Springer-Verlag; New York: 2008. [Google Scholar]
- 155.Zacharias, N.,, Haag A.,, Brang-Lamprecht R.,, Gebel J.,, Essert S.M.,, Kistemann T.,, Exner M.,, Mutters N.T.,, Engelhart S. Air filtration as a tool for the reduction of viral aerosols. Sci. Total Environ. 2021;772:144956. doi: 10.1016/j.scitotenv.2021.144956. [DOI] [PubMed] [Google Scholar]
- 156.Zaki, A.M.,, van Boheemen S.,, Bestebroer T.M.,, Osterhaus A.D.,, Fouchier R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 157.Zheng, R.,, Xu Y.,, Wang W.,, Ning G.,, Bi Y. Spatial transmission of COVID-19 via public and private transportation in China. Travel Med. Infect. Dis. 2020;34:101626. doi: 10.1016/j.tmaid.2020.101626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zimmer, C.,, Corum J.,, Wee S.L. Coronavirus vaccine tracker. 2021 https://www.nytimes.com/interactive/2020/science/coronavirus-vaccine-tracker.html Available at: . Accessed 8 April 2021. [Google Scholar]
- 159.Zwietering, M.H.,, Garre A.,, Wiedmann M.,, Buchanan R.L. All food processes have a residual risk, some are small, some very small and some are extremely small: zero risk does not exist. Curr. Opin. Food Sci. 2021;39:83–92. [Google Scholar]